User login
‘Where does it hurt?’: Primary care tips for common ortho problems
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
Knee and shoulder pain are common complaints for patients in the primary care office.
But identifying the source of the pain can be complicated,
and an accurate diagnosis of the underlying cause of discomfort is key to appropriate management – whether that involves simple home care options of ice and rest or a recommendation for a follow-up with a specialist.
Speaking at the annual meeting of the American College of Physicians, Greg Nakamoto, MD, department of orthopedics, Virginia Mason Medical Center, Seattle, discussed common knee and shoulder problems that patients often present with in the primary care setting, and offered tips on diagnosis and appropriate management.
The most common conditions causing knee pain are osteoarthritis and meniscal tears. “The differential for knee pain is broad,” Dr. Nakamoto said. “You have to have a way to divide it down, such as if it’s acute or chronic.”
The initial workup has several key components. The first steps: Determine the location of the pain – anterior, medial, lateral, posterior – and then whether it stems from an injury or is atraumatic.
“If you have to ask one question – ask where it hurts,” he said. “And is it from an injury or just wear and tear? That helps me when deciding if surgery is needed.”
Pain in the knee generally localizes well to the site of pathology, and knee pain of acute traumatic onset requires more scrutiny for problems best treated with early surgery. “This also helps establish whether radiographic findings are due to injury or degeneration,” Dr. Nakamoto said. “The presence of swelling guides the need for anti-inflammatories or cortisone.”
Palpating for tenderness along the joint line is important, as is palpating above and below the joint line, Dr. Nakamoto said.
“Tenderness limited to the joint line, combined with a meniscal exam maneuver that reproduces joint-line pain, is suggestive of pain from meniscal pathology,” he said.
Imaging is an important component of evaluating knee symptoms, and the question often arises as to when to order an MRI.
Dr. Nakamoto offered the following scenario: If significant osteoarthritis is evident on weight-bearing x-ray, treat the patient for the condition. However, if little or no osteoarthritis appears on x-ray, and if the onset of symptoms was traumatic and both patient history and physical examination suggest a meniscal tear, order an MRI.
An early MRI also is needed if the patient has had either atraumatic or traumatic onset of symptoms and their history and physical exams are suspicious for a mechanically locked or locking meniscus. For suspicion of a ruptured quadriceps or patellar tendon or a stress fracture, an MRI is needed urgently.
An MRI would be ordered later if the patient’s symptoms have not improved significantly after 3 months of conservative management.
Dr. Nakamoto stressed how common undiagnosed meniscus tears are in the general population. A third of men aged 50-59 years and nearly 20% of women in that age group have a tear, he said. “That number goes up to 56% and 51% in men and women aged 70-90 years, and 61% of these tears were in patients who were asymptomatic in the last month.”
In the setting of osteoarthritis, 76% of asymptomatic patients had a meniscus tear, and 91% of patients with symptomatic osteoarthritis had a meniscus tear, he added.
Treating knee pain
Treatment will vary depending on the underlying etiology of pain. For a possible meniscus tear, the recommendation is for a conservative intervention with ice, ibuprofen, knee immobilizer, and crutches, with a follow-up appointment in a week.
Three types of injections also can help:
- Cortisone for osteoarthritis or meniscus tears, swelling, and inflammation, and prophylaxis against inflammation.
- Viscosupplementation (intra‐articular hyaluronic acid) for chronic, baseline osteoarthritis symptoms.
- Regenerative therapies (platelet-rich plasma, stem cells, etc.) are used primarily for osteoarthritis (these do not regrow cartilage, but some patients report decreased pain).
The data on injections are mixed, Dr. Nakamoto said. For example, the results of a 2015 Cochrane review on cortisone injections for osteoarthritis reported that the benefits were small to moderate at 4‐6 weeks, and small to none at 13 weeks.
“There is a lot of controversy for viscosupplementation despite all of the data on it,” he said. “But the recommendations from professional organizations are mixed.”
He noted that he has been using viscosupplementation since the 1990s, and some patients do benefit from it.
Shoulder pain
The most common causes of shoulder pain are adhesive capsulitis, rotator cuff tears and tendinopathy, and impingement.
As with knee pain, the same assessment routine largely applies.
First, pinpoint the location: Is the trouble spot the lateral shoulder and upper arm, the trapezial ridge, or the shoulder blade?
Next, assess pain on movement: Does the patient experience discomfort reaching overhead or behind the back, or moving at the glenohumeral joint/capsule and engaging the rotator cuff? Check for stiffness, weakness, and decreased range of motion in the rotator cuff.
Determine if the cause of the pain is traumatic or atraumatic and stems from an acute injury versus degeneration or overuse.
As with the knee, imaging is a major component of the assessment and typically involves the use of x-ray. An MRI may be required for evaluating full- and partial-thickness tears and when contemplating surgery.
MRI also is necessary for evaluating cases of acute, traumatic shoulder injury, and patients exhibiting disability suggestive of a rotator cuff tear in an otherwise healthy tendon.
Some pain can be treated with cortisone injections or regenerative therapies, which generally are given at the acromioclavicular or glenohumeral joints or in the subacromial space. A 2005 meta-analysis found that subacromial injections of corticosteroids are effective for improvement for rotator cuff tendinitis up to a 9‐month period.
Surgery may be warranted in some cases, Dr. Nakamoto said. These include adhesive capsulitis, rotator cuff tear, acute traumatic injury in an otherwise healthy tendon, and chronic (or acute-on-chronic) tears in a degenerative tendon following a trial of conservative therapy.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2022
How to communicate effectively with patients when tension is high
“At my hospital, it was such a big thing to make sure that families are called,” said Dr. Nwankwo, in an interview following a session on compassionate communication at the annual meeting of the American College of Physicians. “So you have 19 patients, and you have to call almost every family to update them. And then you call, and they say, ‘Call this person as well.’ You feel like you’re at your wit’s end a lot of times.”
Sometimes, she has had to dig deep to find the empathy for patients that she knows her patients deserve.
“You really want to care by thinking about where is this patient coming from? What’s going on in their lives? And not just label them a difficult patient,” she said.
Become curious
Auguste Fortin, MD, MPH, offered advice for handling patient interactions under these kinds of circumstances, while serving as a moderator during the session.
“When the going gets tough, turn to wonder.” Become curious about why a patient might be feeling the way they are, he said.
Dr. Fortin, professor of internal medicine at Yale University, New Haven, Conn., said using the ADOBE acronym, has helped him more effectively communicate with his patients. This tool cues him to keep the following in mind: acknowledge, discover, opportunity, boundary setting, and extend.
He went on to explain to the audience why thinking about these terms is useful when interacting with patients.
First, acknowledge the feelings of the patient. Noting that a patient is angry, perhaps counterintuitively, helps, he said. In fact, not acknowledging the anger “throws gasoline on the fire.”
Then, discover the cause of their emotion. Saying "tell me more" and "help me understand" can be powerful tools, he noted.
Next, take this as an opportunity for empathy – especially important to remember when you’re being verbally attacked.
Boundary setting is important, because it lets the patient know that the conversation won’t continue unless they show the same respect the physician is showing, he said.
Finally, physicians can extend the system of support by asking others – such as colleagues or security – for help.
Use the NURS guide to show empathy
Dr. Fortin said he uses the “NURS” guide or calling to mind “name, express, respect, and support” to show empathy:
This involves naming a patient’s emotion; expressing understanding, with phrases like "I can see how you could be …"; showing respect, acknowledging a patient is going through a lot; and offering support, by saying something like, "Let’s see what we can do together to get to the bottom of this," he explained.
“My lived experience in using [these] in this order is that by the end of it, the patient cannot stay mad at me,” Dr. Fortin said.
“It’s really quite remarkable,” he added.
Steps for nonviolent communication
Rebecca Andrews, MD, MS, another moderator for the session, offered these steps for “nonviolent communication”:
- Observing the situation without blame or judgment.
- Telling the person how this situation makes you feel.
- Connecting with a need of the other person.
- Making a request that is specific and based on action, rather than a request not to do something, such as "Would you be willing to … ?"
Dr. Andrews, who is professor of medicine at the University of Connecticut, Farmington, said this approach has worked well for her, both in interactions with patients and in her personal life.
“It is evidence based that compassion actually makes care better,” she noted.
Varun Jain, MD, a member of the audience, expressed gratitude to the session’s speakers for teaching him something that he had not learned in medical school or residency.
“Every week you will have one or two people who will be labeled as ‘difficult,’ ” and it was nice to have some proven advice on how to handle these tough interactions, said the hospitalist at St. Francis Hospital in Hartford, Conn.
“We never got any actual training on this, and we were expected to know this because we are just physicians, and physicians are expected to be compassionate,” Dr. Jain said. “No one taught us how to have compassion.”
Dr. Fortin and Dr. Andrews disclosed no relevant financial relationships.
“At my hospital, it was such a big thing to make sure that families are called,” said Dr. Nwankwo, in an interview following a session on compassionate communication at the annual meeting of the American College of Physicians. “So you have 19 patients, and you have to call almost every family to update them. And then you call, and they say, ‘Call this person as well.’ You feel like you’re at your wit’s end a lot of times.”
Sometimes, she has had to dig deep to find the empathy for patients that she knows her patients deserve.
“You really want to care by thinking about where is this patient coming from? What’s going on in their lives? And not just label them a difficult patient,” she said.
Become curious
Auguste Fortin, MD, MPH, offered advice for handling patient interactions under these kinds of circumstances, while serving as a moderator during the session.
“When the going gets tough, turn to wonder.” Become curious about why a patient might be feeling the way they are, he said.
Dr. Fortin, professor of internal medicine at Yale University, New Haven, Conn., said using the ADOBE acronym, has helped him more effectively communicate with his patients. This tool cues him to keep the following in mind: acknowledge, discover, opportunity, boundary setting, and extend.
He went on to explain to the audience why thinking about these terms is useful when interacting with patients.
First, acknowledge the feelings of the patient. Noting that a patient is angry, perhaps counterintuitively, helps, he said. In fact, not acknowledging the anger “throws gasoline on the fire.”
Then, discover the cause of their emotion. Saying "tell me more" and "help me understand" can be powerful tools, he noted.
Next, take this as an opportunity for empathy – especially important to remember when you’re being verbally attacked.
Boundary setting is important, because it lets the patient know that the conversation won’t continue unless they show the same respect the physician is showing, he said.
Finally, physicians can extend the system of support by asking others – such as colleagues or security – for help.
Use the NURS guide to show empathy
Dr. Fortin said he uses the “NURS” guide or calling to mind “name, express, respect, and support” to show empathy:
This involves naming a patient’s emotion; expressing understanding, with phrases like "I can see how you could be …"; showing respect, acknowledging a patient is going through a lot; and offering support, by saying something like, "Let’s see what we can do together to get to the bottom of this," he explained.
“My lived experience in using [these] in this order is that by the end of it, the patient cannot stay mad at me,” Dr. Fortin said.
“It’s really quite remarkable,” he added.
Steps for nonviolent communication
Rebecca Andrews, MD, MS, another moderator for the session, offered these steps for “nonviolent communication”:
- Observing the situation without blame or judgment.
- Telling the person how this situation makes you feel.
- Connecting with a need of the other person.
- Making a request that is specific and based on action, rather than a request not to do something, such as "Would you be willing to … ?"
Dr. Andrews, who is professor of medicine at the University of Connecticut, Farmington, said this approach has worked well for her, both in interactions with patients and in her personal life.
“It is evidence based that compassion actually makes care better,” she noted.
Varun Jain, MD, a member of the audience, expressed gratitude to the session’s speakers for teaching him something that he had not learned in medical school or residency.
“Every week you will have one or two people who will be labeled as ‘difficult,’ ” and it was nice to have some proven advice on how to handle these tough interactions, said the hospitalist at St. Francis Hospital in Hartford, Conn.
“We never got any actual training on this, and we were expected to know this because we are just physicians, and physicians are expected to be compassionate,” Dr. Jain said. “No one taught us how to have compassion.”
Dr. Fortin and Dr. Andrews disclosed no relevant financial relationships.
“At my hospital, it was such a big thing to make sure that families are called,” said Dr. Nwankwo, in an interview following a session on compassionate communication at the annual meeting of the American College of Physicians. “So you have 19 patients, and you have to call almost every family to update them. And then you call, and they say, ‘Call this person as well.’ You feel like you’re at your wit’s end a lot of times.”
Sometimes, she has had to dig deep to find the empathy for patients that she knows her patients deserve.
“You really want to care by thinking about where is this patient coming from? What’s going on in their lives? And not just label them a difficult patient,” she said.
Become curious
Auguste Fortin, MD, MPH, offered advice for handling patient interactions under these kinds of circumstances, while serving as a moderator during the session.
“When the going gets tough, turn to wonder.” Become curious about why a patient might be feeling the way they are, he said.
Dr. Fortin, professor of internal medicine at Yale University, New Haven, Conn., said using the ADOBE acronym, has helped him more effectively communicate with his patients. This tool cues him to keep the following in mind: acknowledge, discover, opportunity, boundary setting, and extend.
He went on to explain to the audience why thinking about these terms is useful when interacting with patients.
First, acknowledge the feelings of the patient. Noting that a patient is angry, perhaps counterintuitively, helps, he said. In fact, not acknowledging the anger “throws gasoline on the fire.”
Then, discover the cause of their emotion. Saying "tell me more" and "help me understand" can be powerful tools, he noted.
Next, take this as an opportunity for empathy – especially important to remember when you’re being verbally attacked.
Boundary setting is important, because it lets the patient know that the conversation won’t continue unless they show the same respect the physician is showing, he said.
Finally, physicians can extend the system of support by asking others – such as colleagues or security – for help.
Use the NURS guide to show empathy
Dr. Fortin said he uses the “NURS” guide or calling to mind “name, express, respect, and support” to show empathy:
This involves naming a patient’s emotion; expressing understanding, with phrases like "I can see how you could be …"; showing respect, acknowledging a patient is going through a lot; and offering support, by saying something like, "Let’s see what we can do together to get to the bottom of this," he explained.
“My lived experience in using [these] in this order is that by the end of it, the patient cannot stay mad at me,” Dr. Fortin said.
“It’s really quite remarkable,” he added.
Steps for nonviolent communication
Rebecca Andrews, MD, MS, another moderator for the session, offered these steps for “nonviolent communication”:
- Observing the situation without blame or judgment.
- Telling the person how this situation makes you feel.
- Connecting with a need of the other person.
- Making a request that is specific and based on action, rather than a request not to do something, such as "Would you be willing to … ?"
Dr. Andrews, who is professor of medicine at the University of Connecticut, Farmington, said this approach has worked well for her, both in interactions with patients and in her personal life.
“It is evidence based that compassion actually makes care better,” she noted.
Varun Jain, MD, a member of the audience, expressed gratitude to the session’s speakers for teaching him something that he had not learned in medical school or residency.
“Every week you will have one or two people who will be labeled as ‘difficult,’ ” and it was nice to have some proven advice on how to handle these tough interactions, said the hospitalist at St. Francis Hospital in Hartford, Conn.
“We never got any actual training on this, and we were expected to know this because we are just physicians, and physicians are expected to be compassionate,” Dr. Jain said. “No one taught us how to have compassion.”
Dr. Fortin and Dr. Andrews disclosed no relevant financial relationships.
AT INTERNAL MEDICINE 2022
Homelessness seems to have greater link to death than common diseases, says physician
On a return visit about 10 years later, Dr. Perri went to the park and inquired about the men.
“I came to the horrible realization that all of these people were dead. All of them in 10 years,” he continued, speaking to an audience at the annual meeting of the American College of Physicians.
People experiencing homelessness don’t have to have such a grim health outlook, said Dr. Perri, who is medical director of the Center for Inclusion Health at the Allegheny Health Network in Pittsburgh.
During his talk, filled with jarring statistics on the health plight of those who struggle to stay sheltered, Dr. Perri said that many of the things that sicken and kill these people are the same things that sicken and kill others – liver disease, congestive heart failure, substance abuse. But the system isn’t equipped to handle the problems.
“Their needs are actually straightforward, they’re easy to describe,” he declared. “They’re known quantities. But the way that our systems respond, or don’t respond, to that creates the complexity. It’s the systems that are complex.”
Morbidity, mortality rates ‘go off a cliff’
A 2017 study in The Lancet compared morbidity and mortality in high-income countries, grouping people by their “level of deprivation.” The morbidity and mortality ticked higher with each deprivation level, but skyrocketed – nearly 10 times higher – for the group that included those experiencing homelessness or imprisonment, sex workers, and those with substance use disorders. As Dr. Perri put it, the rates “go off a cliff.”
Studies by the Boston Healthcare for the Homeless program have tracked mortality, and from 1988 to 1993 the average age at death was 47, so, “if you died while homeless, you probably died young.” Moreover, from their first contact to receive care through the program, to their death, only 25 months had elapsed.
“If there’s going to be an effective health care intervention, an acute one at least, you’ve got to get cracking,” Dr. Perri said.
Age at death has improved somewhat over time but drug overdose has become a much more common cause, Dr. Perri noted.
“There is utilitarian value in learning from people experiencing homelessness,” he said.
The same program looked at a high-risk cohort of 199 – those who went unsheltered for more than 6 months,were age 60 or older, or had certain serious health conditions, such as cirrhosis, substance abuse, and AIDS. A third of these people died within 5 years.
“There aren’t any other common diseases that I’m aware of that have statistics like that,” he said.
These people had an average of 31 emergency department visits a year and accounted for 871 hospitalizations. The estimated cost per-person, per-year was $22,000, while the average annual rent for a one-bedroom in Boston was $10,000.
“We’re hemorrhaging utilization around this population,” Dr. Perri said. “Maybe it makes sense to invest in something else other than acute health care. It’s not really yielding very much return on investment.”
Street medicine could be the answer
Housing First, a program to provide housing without the need to meet preconditions such as sobriety or passing background checks, has had a nonsignificant effect on mortality, substance use disorders, and mental health but has improved self-reported health status and quality of life. Analyses of the program suggest that better interventions are needed, Dr. Perri said.
Street medicine could be an answer, he said. Teams of medical staff go to where the people are, and the concept is intended as a continuous, cost-effective, flexible approach to care. Lehigh Valley Street Medicine in Pennsylvania has reported a reduction in emergency department visits and hospitalizations, Dr. Perri said. The programs are still too new to gauge the effect on actual health outcomes, but they hold the promise of being able to do so, he continued.
Curiosity about those experiencing homeless is a key first step in improving care, he said. The HOUSED BEDS tool, developed in Los Angeles, can help guide clinicians through their interactions with patients who do not have homes.
Dr. Perri said it is “enlightening” when you “express interest, genuine curiosity, about other people’s experiences.”
Catherine Kiley, MD, a retired internal medicine physician who volunteers as a preceptor for medical students in Cincinnati, said there is a void when it comes to teaching students about those experiencing homelessness.
“I don’t think there’s much of this type of discussion that they’re exposed to as part of medical education,” Dr. Kiley said. “Their experiences over time, as with most of medicine, will inform them.”
But the findings shared in the session show “how great the need is to speak out, speak up, about patients as people, and what they have to teach us.”
Dr. Perri disclosed no relevant financial relationships.
On a return visit about 10 years later, Dr. Perri went to the park and inquired about the men.
“I came to the horrible realization that all of these people were dead. All of them in 10 years,” he continued, speaking to an audience at the annual meeting of the American College of Physicians.
People experiencing homelessness don’t have to have such a grim health outlook, said Dr. Perri, who is medical director of the Center for Inclusion Health at the Allegheny Health Network in Pittsburgh.
During his talk, filled with jarring statistics on the health plight of those who struggle to stay sheltered, Dr. Perri said that many of the things that sicken and kill these people are the same things that sicken and kill others – liver disease, congestive heart failure, substance abuse. But the system isn’t equipped to handle the problems.
“Their needs are actually straightforward, they’re easy to describe,” he declared. “They’re known quantities. But the way that our systems respond, or don’t respond, to that creates the complexity. It’s the systems that are complex.”
Morbidity, mortality rates ‘go off a cliff’
A 2017 study in The Lancet compared morbidity and mortality in high-income countries, grouping people by their “level of deprivation.” The morbidity and mortality ticked higher with each deprivation level, but skyrocketed – nearly 10 times higher – for the group that included those experiencing homelessness or imprisonment, sex workers, and those with substance use disorders. As Dr. Perri put it, the rates “go off a cliff.”
Studies by the Boston Healthcare for the Homeless program have tracked mortality, and from 1988 to 1993 the average age at death was 47, so, “if you died while homeless, you probably died young.” Moreover, from their first contact to receive care through the program, to their death, only 25 months had elapsed.
“If there’s going to be an effective health care intervention, an acute one at least, you’ve got to get cracking,” Dr. Perri said.
Age at death has improved somewhat over time but drug overdose has become a much more common cause, Dr. Perri noted.
“There is utilitarian value in learning from people experiencing homelessness,” he said.
The same program looked at a high-risk cohort of 199 – those who went unsheltered for more than 6 months,were age 60 or older, or had certain serious health conditions, such as cirrhosis, substance abuse, and AIDS. A third of these people died within 5 years.
“There aren’t any other common diseases that I’m aware of that have statistics like that,” he said.
These people had an average of 31 emergency department visits a year and accounted for 871 hospitalizations. The estimated cost per-person, per-year was $22,000, while the average annual rent for a one-bedroom in Boston was $10,000.
“We’re hemorrhaging utilization around this population,” Dr. Perri said. “Maybe it makes sense to invest in something else other than acute health care. It’s not really yielding very much return on investment.”
Street medicine could be the answer
Housing First, a program to provide housing without the need to meet preconditions such as sobriety or passing background checks, has had a nonsignificant effect on mortality, substance use disorders, and mental health but has improved self-reported health status and quality of life. Analyses of the program suggest that better interventions are needed, Dr. Perri said.
Street medicine could be an answer, he said. Teams of medical staff go to where the people are, and the concept is intended as a continuous, cost-effective, flexible approach to care. Lehigh Valley Street Medicine in Pennsylvania has reported a reduction in emergency department visits and hospitalizations, Dr. Perri said. The programs are still too new to gauge the effect on actual health outcomes, but they hold the promise of being able to do so, he continued.
Curiosity about those experiencing homeless is a key first step in improving care, he said. The HOUSED BEDS tool, developed in Los Angeles, can help guide clinicians through their interactions with patients who do not have homes.
Dr. Perri said it is “enlightening” when you “express interest, genuine curiosity, about other people’s experiences.”
Catherine Kiley, MD, a retired internal medicine physician who volunteers as a preceptor for medical students in Cincinnati, said there is a void when it comes to teaching students about those experiencing homelessness.
“I don’t think there’s much of this type of discussion that they’re exposed to as part of medical education,” Dr. Kiley said. “Their experiences over time, as with most of medicine, will inform them.”
But the findings shared in the session show “how great the need is to speak out, speak up, about patients as people, and what they have to teach us.”
Dr. Perri disclosed no relevant financial relationships.
On a return visit about 10 years later, Dr. Perri went to the park and inquired about the men.
“I came to the horrible realization that all of these people were dead. All of them in 10 years,” he continued, speaking to an audience at the annual meeting of the American College of Physicians.
People experiencing homelessness don’t have to have such a grim health outlook, said Dr. Perri, who is medical director of the Center for Inclusion Health at the Allegheny Health Network in Pittsburgh.
During his talk, filled with jarring statistics on the health plight of those who struggle to stay sheltered, Dr. Perri said that many of the things that sicken and kill these people are the same things that sicken and kill others – liver disease, congestive heart failure, substance abuse. But the system isn’t equipped to handle the problems.
“Their needs are actually straightforward, they’re easy to describe,” he declared. “They’re known quantities. But the way that our systems respond, or don’t respond, to that creates the complexity. It’s the systems that are complex.”
Morbidity, mortality rates ‘go off a cliff’
A 2017 study in The Lancet compared morbidity and mortality in high-income countries, grouping people by their “level of deprivation.” The morbidity and mortality ticked higher with each deprivation level, but skyrocketed – nearly 10 times higher – for the group that included those experiencing homelessness or imprisonment, sex workers, and those with substance use disorders. As Dr. Perri put it, the rates “go off a cliff.”
Studies by the Boston Healthcare for the Homeless program have tracked mortality, and from 1988 to 1993 the average age at death was 47, so, “if you died while homeless, you probably died young.” Moreover, from their first contact to receive care through the program, to their death, only 25 months had elapsed.
“If there’s going to be an effective health care intervention, an acute one at least, you’ve got to get cracking,” Dr. Perri said.
Age at death has improved somewhat over time but drug overdose has become a much more common cause, Dr. Perri noted.
“There is utilitarian value in learning from people experiencing homelessness,” he said.
The same program looked at a high-risk cohort of 199 – those who went unsheltered for more than 6 months,were age 60 or older, or had certain serious health conditions, such as cirrhosis, substance abuse, and AIDS. A third of these people died within 5 years.
“There aren’t any other common diseases that I’m aware of that have statistics like that,” he said.
These people had an average of 31 emergency department visits a year and accounted for 871 hospitalizations. The estimated cost per-person, per-year was $22,000, while the average annual rent for a one-bedroom in Boston was $10,000.
“We’re hemorrhaging utilization around this population,” Dr. Perri said. “Maybe it makes sense to invest in something else other than acute health care. It’s not really yielding very much return on investment.”
Street medicine could be the answer
Housing First, a program to provide housing without the need to meet preconditions such as sobriety or passing background checks, has had a nonsignificant effect on mortality, substance use disorders, and mental health but has improved self-reported health status and quality of life. Analyses of the program suggest that better interventions are needed, Dr. Perri said.
Street medicine could be an answer, he said. Teams of medical staff go to where the people are, and the concept is intended as a continuous, cost-effective, flexible approach to care. Lehigh Valley Street Medicine in Pennsylvania has reported a reduction in emergency department visits and hospitalizations, Dr. Perri said. The programs are still too new to gauge the effect on actual health outcomes, but they hold the promise of being able to do so, he continued.
Curiosity about those experiencing homeless is a key first step in improving care, he said. The HOUSED BEDS tool, developed in Los Angeles, can help guide clinicians through their interactions with patients who do not have homes.
Dr. Perri said it is “enlightening” when you “express interest, genuine curiosity, about other people’s experiences.”
Catherine Kiley, MD, a retired internal medicine physician who volunteers as a preceptor for medical students in Cincinnati, said there is a void when it comes to teaching students about those experiencing homelessness.
“I don’t think there’s much of this type of discussion that they’re exposed to as part of medical education,” Dr. Kiley said. “Their experiences over time, as with most of medicine, will inform them.”
But the findings shared in the session show “how great the need is to speak out, speak up, about patients as people, and what they have to teach us.”
Dr. Perri disclosed no relevant financial relationships.
REPORTING FROM INTERNAL MEDICINE 2022
Clinical Edge Journal Scan Commentary: Multiple Sclerosis May 2022
Portaccio and colleagues explored this issue and concluded that disease progression independent of relapse activity (PIRA) was a major contributor of confirmed disability accrual (CDA) in early relapse after the onset of MS, with age being a major determinant in the way that CDA occurs. In a retrospective cohort analysis of 5169 patients with either clinically isolated syndrome or early relapsing-remitting MS who were assessed within 1 year of onset and followed-up for ≥ 5 years, PIRA accounted for 27.6% of disability-worsening events, whereas relapse-associated worsening accounted for 17.8% of events, with relapse-associated worsening being more frequent in younger (hazard ratio [HR] 0.87) and PIRA in older (HR 1.19; both P < .001) patients. Recognition of disease relapse or progression is not always simple in a complex disease, but failure to recognize these issues can result in long-term accumulation of economically important disability. Multiple other issues related to effective disease management also require effective juggling in routine care. Adherence to treatment, timing of DMT change, and interval between discontinuing a DMT and starting a different one continue to be critical concerns as well. Malpas and associates explored this issue and noted that the importance of adherence impact on disease control also relates to treatment interruption and timing of duration between stopping one DMT and starting another agent. The annualized relapse rate (ARR) and the rate of severe relapses was explored in a cohort of 685 people with MS and did not increase significantly after discontinuation of fingolimod in this population, but delaying the commencement of immunotherapy increased the risk for relapse. The ARR was not significantly different during and after fingolimod cessation (mean difference −0.06; 95% CI −0.14 to 0.01), with no severe relapses reported in the year prior to and after fingolimod cessation. However, delaying the recommencement of DMT, or if change in DMT was delayed from 2 to 4 months, vs beginning within 2 months (odds ratio 1.67; 95% CI 1.22-2.27) was associated with a higher risk for relapse. Discontinuation of DMT or treatment interruption also relates to planned or unplanned pregnancy in people with MS. In another study Portaccio and colleagues continued treatment with natalizumab until conception and then restarted treatment within 1 month after delivery. This reduced the risk for disease activity more than natalizumab cessation before conception or restarting 1 month after delivery in women with MS. No major developmental abnormalities were noted in the infants born of 72 pregnancies in 70 women with MS who were treated for at least 2 years. Specifically, relapses occurred in 29.4% of people with MS treated until conception vs 70.2% in those who discontinued prior to conception, after a mean follow-up of 6.1 years (P = .001), with timing of treatment cessation being the only predictor of relapses (HR 4.1; P = .003). No developmental abnormalities were observed in the infants.
Practical points for the treating clinician are many and continue to highlight the complexity of managing people with MS in the real world, with real issues from COVID-19 vaccination, SARS-CoV-2 infection, recognition or awareness of relapse, and the increased challenges of adherence and timing of DMT change and of DMT use relative to pregnancy. Data-driven, reliable, relevant information is critical to incorporate into routine care to enhance and optimize decision-making.
Portaccio and colleagues explored this issue and concluded that disease progression independent of relapse activity (PIRA) was a major contributor of confirmed disability accrual (CDA) in early relapse after the onset of MS, with age being a major determinant in the way that CDA occurs. In a retrospective cohort analysis of 5169 patients with either clinically isolated syndrome or early relapsing-remitting MS who were assessed within 1 year of onset and followed-up for ≥ 5 years, PIRA accounted for 27.6% of disability-worsening events, whereas relapse-associated worsening accounted for 17.8% of events, with relapse-associated worsening being more frequent in younger (hazard ratio [HR] 0.87) and PIRA in older (HR 1.19; both P < .001) patients. Recognition of disease relapse or progression is not always simple in a complex disease, but failure to recognize these issues can result in long-term accumulation of economically important disability. Multiple other issues related to effective disease management also require effective juggling in routine care. Adherence to treatment, timing of DMT change, and interval between discontinuing a DMT and starting a different one continue to be critical concerns as well. Malpas and associates explored this issue and noted that the importance of adherence impact on disease control also relates to treatment interruption and timing of duration between stopping one DMT and starting another agent. The annualized relapse rate (ARR) and the rate of severe relapses was explored in a cohort of 685 people with MS and did not increase significantly after discontinuation of fingolimod in this population, but delaying the commencement of immunotherapy increased the risk for relapse. The ARR was not significantly different during and after fingolimod cessation (mean difference −0.06; 95% CI −0.14 to 0.01), with no severe relapses reported in the year prior to and after fingolimod cessation. However, delaying the recommencement of DMT, or if change in DMT was delayed from 2 to 4 months, vs beginning within 2 months (odds ratio 1.67; 95% CI 1.22-2.27) was associated with a higher risk for relapse. Discontinuation of DMT or treatment interruption also relates to planned or unplanned pregnancy in people with MS. In another study Portaccio and colleagues continued treatment with natalizumab until conception and then restarted treatment within 1 month after delivery. This reduced the risk for disease activity more than natalizumab cessation before conception or restarting 1 month after delivery in women with MS. No major developmental abnormalities were noted in the infants born of 72 pregnancies in 70 women with MS who were treated for at least 2 years. Specifically, relapses occurred in 29.4% of people with MS treated until conception vs 70.2% in those who discontinued prior to conception, after a mean follow-up of 6.1 years (P = .001), with timing of treatment cessation being the only predictor of relapses (HR 4.1; P = .003). No developmental abnormalities were observed in the infants.
Practical points for the treating clinician are many and continue to highlight the complexity of managing people with MS in the real world, with real issues from COVID-19 vaccination, SARS-CoV-2 infection, recognition or awareness of relapse, and the increased challenges of adherence and timing of DMT change and of DMT use relative to pregnancy. Data-driven, reliable, relevant information is critical to incorporate into routine care to enhance and optimize decision-making.
Portaccio and colleagues explored this issue and concluded that disease progression independent of relapse activity (PIRA) was a major contributor of confirmed disability accrual (CDA) in early relapse after the onset of MS, with age being a major determinant in the way that CDA occurs. In a retrospective cohort analysis of 5169 patients with either clinically isolated syndrome or early relapsing-remitting MS who were assessed within 1 year of onset and followed-up for ≥ 5 years, PIRA accounted for 27.6% of disability-worsening events, whereas relapse-associated worsening accounted for 17.8% of events, with relapse-associated worsening being more frequent in younger (hazard ratio [HR] 0.87) and PIRA in older (HR 1.19; both P < .001) patients. Recognition of disease relapse or progression is not always simple in a complex disease, but failure to recognize these issues can result in long-term accumulation of economically important disability. Multiple other issues related to effective disease management also require effective juggling in routine care. Adherence to treatment, timing of DMT change, and interval between discontinuing a DMT and starting a different one continue to be critical concerns as well. Malpas and associates explored this issue and noted that the importance of adherence impact on disease control also relates to treatment interruption and timing of duration between stopping one DMT and starting another agent. The annualized relapse rate (ARR) and the rate of severe relapses was explored in a cohort of 685 people with MS and did not increase significantly after discontinuation of fingolimod in this population, but delaying the commencement of immunotherapy increased the risk for relapse. The ARR was not significantly different during and after fingolimod cessation (mean difference −0.06; 95% CI −0.14 to 0.01), with no severe relapses reported in the year prior to and after fingolimod cessation. However, delaying the recommencement of DMT, or if change in DMT was delayed from 2 to 4 months, vs beginning within 2 months (odds ratio 1.67; 95% CI 1.22-2.27) was associated with a higher risk for relapse. Discontinuation of DMT or treatment interruption also relates to planned or unplanned pregnancy in people with MS. In another study Portaccio and colleagues continued treatment with natalizumab until conception and then restarted treatment within 1 month after delivery. This reduced the risk for disease activity more than natalizumab cessation before conception or restarting 1 month after delivery in women with MS. No major developmental abnormalities were noted in the infants born of 72 pregnancies in 70 women with MS who were treated for at least 2 years. Specifically, relapses occurred in 29.4% of people with MS treated until conception vs 70.2% in those who discontinued prior to conception, after a mean follow-up of 6.1 years (P = .001), with timing of treatment cessation being the only predictor of relapses (HR 4.1; P = .003). No developmental abnormalities were observed in the infants.
Practical points for the treating clinician are many and continue to highlight the complexity of managing people with MS in the real world, with real issues from COVID-19 vaccination, SARS-CoV-2 infection, recognition or awareness of relapse, and the increased challenges of adherence and timing of DMT change and of DMT use relative to pregnancy. Data-driven, reliable, relevant information is critical to incorporate into routine care to enhance and optimize decision-making.
Clinical Edge Journal Scan Commentary: Breast Cancer May 2022
A meta-analysis including over 5000 patients with metastatic hormone receptor–positive (HR+) and HER2- breast cancer showed a significant overall survival (OS) benefit with the addition of cyclin-dependent kinase (CDK) 4/6 inhibitors to endocrine therapy (hazard ratio 1.33; P < .001), albeit with higher rates of toxicities, including neutropenia, leukopenia, and diarrhea.3 The MONALEESA-2 study randomly assigned 668 postmenopausal women with metastatic HR+/HER2- breast cancer, treatment-naive in the advanced setting, to either ribociclib or placebo plus letrozole. Updated results with a median follow-up of 6.6 years demonstrated a significant OS benefit with ribociclib + letrozole compared with placebo + letrozole (median OS 63.9 months vs 51.4 months; hazard ratio 0.76; P = .008) (Hortobagyi and colleagues). An OS > 5 years with ribociclib plus endocrine therapy is certainly impressive, and efficacy as well as respective toxicities of the various CDK 4/6 inhibitors are factors taken into consideration when choosing the appropriate therapy for an individual patient.
The optimization of adjuvant endocrine therapy (ET) for HR+ early breast cancer, including use of ovarian suppression and extended adjuvant therapy, has improved outcomes for these women. However, there is a high-risk subset for whom the risk for distant recurrence persists. The phase 3 monarchE trial, which included 5637 patients with high-risk early breast cancer (≥ 4 positive nodes, or 1-3 nodes and either tumor size ≥ 5 cm, histologic grade 3, or central Ki-67 ≥ 20%), demonstrated benefits in invasive disease-free and distant-relapse-free survival with the addition of abemaciclib for 2 years to ET. A safety analysis of the monarchE study among patients who had received at least one dose of the study drug (n = 5591) demonstrated an overall manageable side-effect profile, with the majority of these toxicities addressed via dose holds/reductions or supportive medications (Rugo and colleagues). Abemaciclib + ET led to higher incidence of grade ≥ 3 adverse events vs ET alone (49.7% vs 16.3%), with neutropenia being the most frequent (grade 3 = 19.6%) although without significant clinical implications. Diarrhea was common (83.5%), although the majority was low grade (grade 1/2 = 75.7%), with grade 2/3 events characterized by early onset and short duration. Discontinuation of abemaciclib occurred in 18.5%, with two thirds due to grade 1/2 events and in over half without dose reduction.4 These findings show an acceptable safety profile with abemaciclib in the curative setting and highlight the importance of education, recognition, and early management of side effects to maintain patients on treatment.
The heterogeneity of tumor biology within the HR+ breast cancer subtype indicates the need to refine treatment regimens for an individual patient. Genomic assays (70-gene signature and 21-gene recurrence score) have helped tailor adjuvant systemic therapy and in many cases have identified women for whom chemotherapy can be omitted. CDK 4/6 inhibitors have shown impressive activity in the metastatic/advanced setting, although results from trials in the adjuvant setting have produced mixed results. The phase 2 NEOPAL trial evaluated the combination of letrozole + palbociclib vs chemotherapy (sequential anthracycline-taxane) among 106 postmenopausal women with high-risk, HR+/HER2- early breast cancer (luminal B or luminal A with nodal involvement). At a median follow-up of 40.4 months, 3-year PFS (hazard ratio 1.01; P = .98) and invasive disease-free survival (hazard ratio 0.83; P = .71) were similar in the letrozole + palbociclib and chemotherapy arms (Delaloge and colleagues). The phase 2 CORALLEEN trial,5 which investigated neoadjuvant letrozole + ribociclib vs chemotherapy in HR+/HER2- luminal B early breast cancer, demonstrated similar percentages of patients achieving downstaging via molecular assessment at the time of surgery. The neoadjuvant space represents a valuable setting to further study CDK 4/6 inhibitors as well as other novel therapies; endpoints including pathologic complete response and residual cancer burden correlating with long-term outcomes can provide a more rapid means to identify effective therapies. Translational biomarkers can be gathered and adjuvant strategies can be tailored based on response.
Additional References
- Modi S, Saura C, Yamashita T, et al; DESTINY-Breast01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610-621. Doi: 10.1056/NEJMoa1914510 Source
- Hurvitz S, Kim S-B, Chung W-P, et al. Trastuzumab deruxtecan (T-DXd; DS-8201a) versus trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): Subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Presented at 2021 San Antonio Breast Cancer Symposium; December 7-10, 2021;General Session, GS3-01. Source
- Li J, Huo X, Zhao F, et al. Association of cyclin-dependent kinases 4 and 6 inhibitors with survival in patients with hormone receptor-positive metastatic breast cancer: A systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2020312. Doi: 10.1001/jamanetworkopen.2020.20312 Source
- Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571-1581. Doi: 10.1016/j.annonc.2021.09.015 Source
- Prat A, Saura C, Pascual T, et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2- negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21:33-43. Doi: 10.1016/S1470-2045(19)30786-7 Source
A meta-analysis including over 5000 patients with metastatic hormone receptor–positive (HR+) and HER2- breast cancer showed a significant overall survival (OS) benefit with the addition of cyclin-dependent kinase (CDK) 4/6 inhibitors to endocrine therapy (hazard ratio 1.33; P < .001), albeit with higher rates of toxicities, including neutropenia, leukopenia, and diarrhea.3 The MONALEESA-2 study randomly assigned 668 postmenopausal women with metastatic HR+/HER2- breast cancer, treatment-naive in the advanced setting, to either ribociclib or placebo plus letrozole. Updated results with a median follow-up of 6.6 years demonstrated a significant OS benefit with ribociclib + letrozole compared with placebo + letrozole (median OS 63.9 months vs 51.4 months; hazard ratio 0.76; P = .008) (Hortobagyi and colleagues). An OS > 5 years with ribociclib plus endocrine therapy is certainly impressive, and efficacy as well as respective toxicities of the various CDK 4/6 inhibitors are factors taken into consideration when choosing the appropriate therapy for an individual patient.
The optimization of adjuvant endocrine therapy (ET) for HR+ early breast cancer, including use of ovarian suppression and extended adjuvant therapy, has improved outcomes for these women. However, there is a high-risk subset for whom the risk for distant recurrence persists. The phase 3 monarchE trial, which included 5637 patients with high-risk early breast cancer (≥ 4 positive nodes, or 1-3 nodes and either tumor size ≥ 5 cm, histologic grade 3, or central Ki-67 ≥ 20%), demonstrated benefits in invasive disease-free and distant-relapse-free survival with the addition of abemaciclib for 2 years to ET. A safety analysis of the monarchE study among patients who had received at least one dose of the study drug (n = 5591) demonstrated an overall manageable side-effect profile, with the majority of these toxicities addressed via dose holds/reductions or supportive medications (Rugo and colleagues). Abemaciclib + ET led to higher incidence of grade ≥ 3 adverse events vs ET alone (49.7% vs 16.3%), with neutropenia being the most frequent (grade 3 = 19.6%) although without significant clinical implications. Diarrhea was common (83.5%), although the majority was low grade (grade 1/2 = 75.7%), with grade 2/3 events characterized by early onset and short duration. Discontinuation of abemaciclib occurred in 18.5%, with two thirds due to grade 1/2 events and in over half without dose reduction.4 These findings show an acceptable safety profile with abemaciclib in the curative setting and highlight the importance of education, recognition, and early management of side effects to maintain patients on treatment.
The heterogeneity of tumor biology within the HR+ breast cancer subtype indicates the need to refine treatment regimens for an individual patient. Genomic assays (70-gene signature and 21-gene recurrence score) have helped tailor adjuvant systemic therapy and in many cases have identified women for whom chemotherapy can be omitted. CDK 4/6 inhibitors have shown impressive activity in the metastatic/advanced setting, although results from trials in the adjuvant setting have produced mixed results. The phase 2 NEOPAL trial evaluated the combination of letrozole + palbociclib vs chemotherapy (sequential anthracycline-taxane) among 106 postmenopausal women with high-risk, HR+/HER2- early breast cancer (luminal B or luminal A with nodal involvement). At a median follow-up of 40.4 months, 3-year PFS (hazard ratio 1.01; P = .98) and invasive disease-free survival (hazard ratio 0.83; P = .71) were similar in the letrozole + palbociclib and chemotherapy arms (Delaloge and colleagues). The phase 2 CORALLEEN trial,5 which investigated neoadjuvant letrozole + ribociclib vs chemotherapy in HR+/HER2- luminal B early breast cancer, demonstrated similar percentages of patients achieving downstaging via molecular assessment at the time of surgery. The neoadjuvant space represents a valuable setting to further study CDK 4/6 inhibitors as well as other novel therapies; endpoints including pathologic complete response and residual cancer burden correlating with long-term outcomes can provide a more rapid means to identify effective therapies. Translational biomarkers can be gathered and adjuvant strategies can be tailored based on response.
Additional References
- Modi S, Saura C, Yamashita T, et al; DESTINY-Breast01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610-621. Doi: 10.1056/NEJMoa1914510 Source
- Hurvitz S, Kim S-B, Chung W-P, et al. Trastuzumab deruxtecan (T-DXd; DS-8201a) versus trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): Subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Presented at 2021 San Antonio Breast Cancer Symposium; December 7-10, 2021;General Session, GS3-01. Source
- Li J, Huo X, Zhao F, et al. Association of cyclin-dependent kinases 4 and 6 inhibitors with survival in patients with hormone receptor-positive metastatic breast cancer: A systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2020312. Doi: 10.1001/jamanetworkopen.2020.20312 Source
- Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571-1581. Doi: 10.1016/j.annonc.2021.09.015 Source
- Prat A, Saura C, Pascual T, et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2- negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21:33-43. Doi: 10.1016/S1470-2045(19)30786-7 Source
A meta-analysis including over 5000 patients with metastatic hormone receptor–positive (HR+) and HER2- breast cancer showed a significant overall survival (OS) benefit with the addition of cyclin-dependent kinase (CDK) 4/6 inhibitors to endocrine therapy (hazard ratio 1.33; P < .001), albeit with higher rates of toxicities, including neutropenia, leukopenia, and diarrhea.3 The MONALEESA-2 study randomly assigned 668 postmenopausal women with metastatic HR+/HER2- breast cancer, treatment-naive in the advanced setting, to either ribociclib or placebo plus letrozole. Updated results with a median follow-up of 6.6 years demonstrated a significant OS benefit with ribociclib + letrozole compared with placebo + letrozole (median OS 63.9 months vs 51.4 months; hazard ratio 0.76; P = .008) (Hortobagyi and colleagues). An OS > 5 years with ribociclib plus endocrine therapy is certainly impressive, and efficacy as well as respective toxicities of the various CDK 4/6 inhibitors are factors taken into consideration when choosing the appropriate therapy for an individual patient.
The optimization of adjuvant endocrine therapy (ET) for HR+ early breast cancer, including use of ovarian suppression and extended adjuvant therapy, has improved outcomes for these women. However, there is a high-risk subset for whom the risk for distant recurrence persists. The phase 3 monarchE trial, which included 5637 patients with high-risk early breast cancer (≥ 4 positive nodes, or 1-3 nodes and either tumor size ≥ 5 cm, histologic grade 3, or central Ki-67 ≥ 20%), demonstrated benefits in invasive disease-free and distant-relapse-free survival with the addition of abemaciclib for 2 years to ET. A safety analysis of the monarchE study among patients who had received at least one dose of the study drug (n = 5591) demonstrated an overall manageable side-effect profile, with the majority of these toxicities addressed via dose holds/reductions or supportive medications (Rugo and colleagues). Abemaciclib + ET led to higher incidence of grade ≥ 3 adverse events vs ET alone (49.7% vs 16.3%), with neutropenia being the most frequent (grade 3 = 19.6%) although without significant clinical implications. Diarrhea was common (83.5%), although the majority was low grade (grade 1/2 = 75.7%), with grade 2/3 events characterized by early onset and short duration. Discontinuation of abemaciclib occurred in 18.5%, with two thirds due to grade 1/2 events and in over half without dose reduction.4 These findings show an acceptable safety profile with abemaciclib in the curative setting and highlight the importance of education, recognition, and early management of side effects to maintain patients on treatment.
The heterogeneity of tumor biology within the HR+ breast cancer subtype indicates the need to refine treatment regimens for an individual patient. Genomic assays (70-gene signature and 21-gene recurrence score) have helped tailor adjuvant systemic therapy and in many cases have identified women for whom chemotherapy can be omitted. CDK 4/6 inhibitors have shown impressive activity in the metastatic/advanced setting, although results from trials in the adjuvant setting have produced mixed results. The phase 2 NEOPAL trial evaluated the combination of letrozole + palbociclib vs chemotherapy (sequential anthracycline-taxane) among 106 postmenopausal women with high-risk, HR+/HER2- early breast cancer (luminal B or luminal A with nodal involvement). At a median follow-up of 40.4 months, 3-year PFS (hazard ratio 1.01; P = .98) and invasive disease-free survival (hazard ratio 0.83; P = .71) were similar in the letrozole + palbociclib and chemotherapy arms (Delaloge and colleagues). The phase 2 CORALLEEN trial,5 which investigated neoadjuvant letrozole + ribociclib vs chemotherapy in HR+/HER2- luminal B early breast cancer, demonstrated similar percentages of patients achieving downstaging via molecular assessment at the time of surgery. The neoadjuvant space represents a valuable setting to further study CDK 4/6 inhibitors as well as other novel therapies; endpoints including pathologic complete response and residual cancer burden correlating with long-term outcomes can provide a more rapid means to identify effective therapies. Translational biomarkers can be gathered and adjuvant strategies can be tailored based on response.
Additional References
- Modi S, Saura C, Yamashita T, et al; DESTINY-Breast01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610-621. Doi: 10.1056/NEJMoa1914510 Source
- Hurvitz S, Kim S-B, Chung W-P, et al. Trastuzumab deruxtecan (T-DXd; DS-8201a) versus trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): Subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Presented at 2021 San Antonio Breast Cancer Symposium; December 7-10, 2021;General Session, GS3-01. Source
- Li J, Huo X, Zhao F, et al. Association of cyclin-dependent kinases 4 and 6 inhibitors with survival in patients with hormone receptor-positive metastatic breast cancer: A systematic review and meta-analysis. JAMA Netw Open. 2020;3:e2020312. Doi: 10.1001/jamanetworkopen.2020.20312 Source
- Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571-1581. Doi: 10.1016/j.annonc.2021.09.015 Source
- Prat A, Saura C, Pascual T, et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2- negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21:33-43. Doi: 10.1016/S1470-2045(19)30786-7 Source
What's your diagnosis?
Answer: Colonic Malakoplakia.
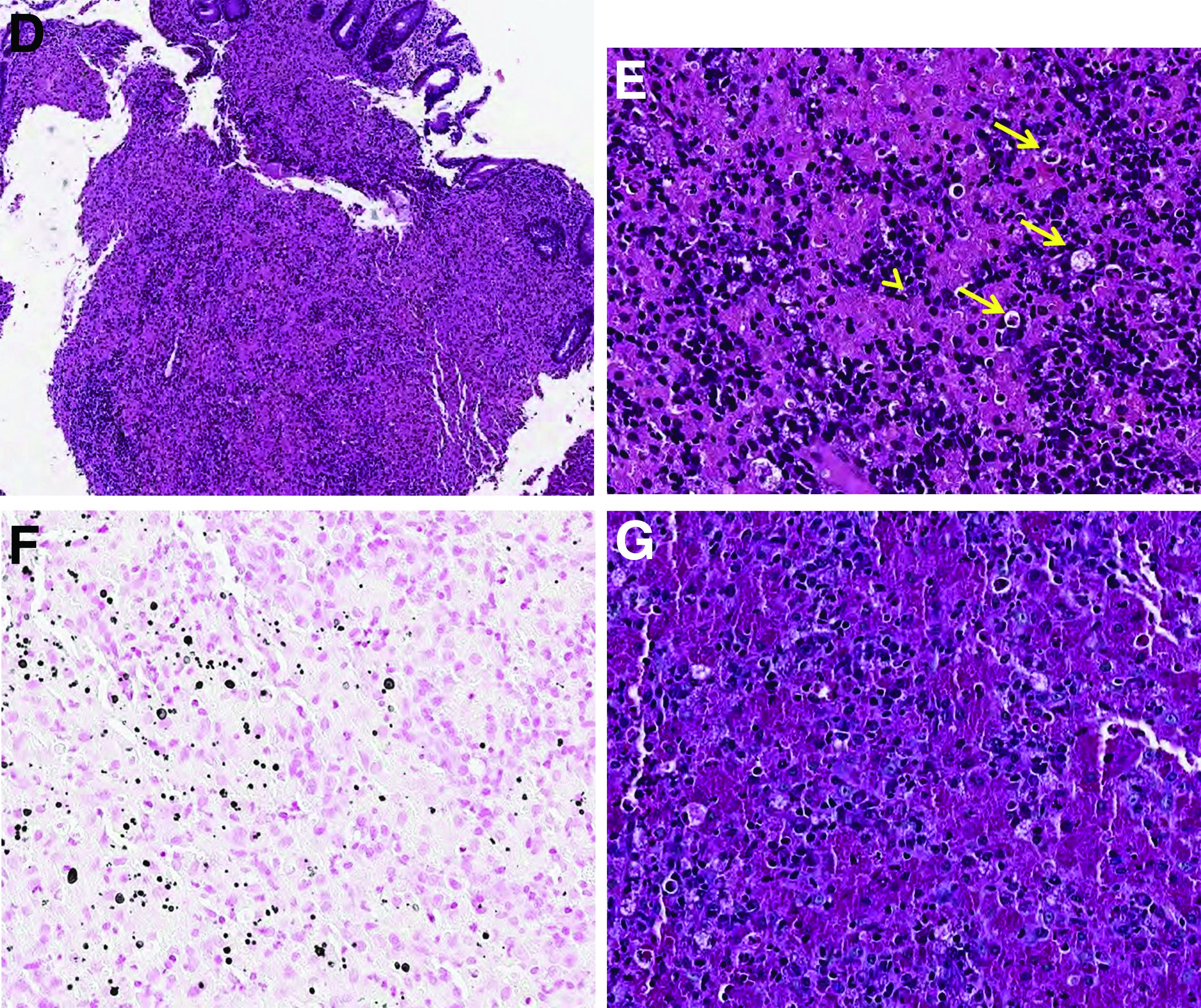
Histopathologic examination of the biopsy specimens revealed nodular mixed inflammatory cells and infiltration of the epithelioid histiocytes in lamina propria (Figure D; stain: hematoxylin and eosin; original magnification 40×). The histiocytes showed foamy and eosinophilic cytoplasm (Figure E, arrow) and some of them had a targetoid appearance (Figure E, arrow head; stain: hematoxylin and eosin; original magnification 200×). Von Kossa stains highlighted the targetoid structures in the histiocytes (Figure F, Michaelis-Gutmann bodies). The granular cytoplasm of the histiocytes was positive on periodic acid-Schiff stain (Figure G). Based on these findings, the patient was diagnosed with colonic malakoplakia.
Malakoplakia is an uncommon, chronic, granulomatous inflammatory disease. It most commonly affects the urinary tract and gastrointestinal tract, but may occur at any anatomic site. Malakoplakia of the gastrointestinal tract are seen most frequently in the rectum, sigmoid, and right colon.1 It is diagnosed by the characteristic histologic feature of accumulated histiocytes with abundant eosinophilic granular cytoplasm containing basophilic inclusions, consistent with Michaelis-Gutmann bodies. Although the exact etiology and pathogenesis of malakoplakia are unclear, it seems to originate from an acquired defect in the intracellular destruction of phagocytosed bacteria, usually associated with Escherichia coli, Klebsiella, and Mycobacterium.2 It can have various causes, such as immunosuppression, malignant neoplasms, systemic diseases, and genetic diseases. Clinical manifestation of colonic malakoplakia is diverse, ranging from asymptomatic to malaise, fever, abdominal pain, diarrhea, hematochezia, and intestinal obstruction. Granulomatous reaction of malakoplakia generates the endoscopic appearance of lesions, which ranges from plaques to nodules and yellow-brown masses. In the early stage, malakoplakia commonly presents as soft yellow to tan mucosal plaques endoscopically, as seen in our case (Figure A). As the disease progresses in the later stage, malakoplakia presents as raised, grey to tan polypoid lesions of various sizes with peripheral hyperemia and a central depressed area, as seen in our case (Figure B).3 Owing to this endoscopic morphology, colonic malakoplakia may be misdiagnosed as atypical lymphoma, familial adenomatous polyposis, and metastatic carcinoma. To date, the natural course of malakoplakia of the colon is unclear, and no guidelines for treatment, treatment methods, duration of treatment, or surveillance are currently available. However, treatment of malakoplakia is essential to reduce immunosuppression and includes antibiotics with intracellular action and choline agonists that replenish the decreased cyclic 3’, 5’-guanosine monophosphate levels. In summary, although malakoplakia of the colon is very rare, it should be considered in the differential diagnosis of polypoid colonic lesions, especially in immunocompromised or malnourished patients.
References
1. Cipolletta L et al. Gastrointest Endosc. 1995 Mar;41(3):255-8.
2. Berney T et al. Transpl Int. 1999;12(4):293-6.
3. Weinrach DM et al. Arch Pathol Lab Med. 2004 Oct;128(10):e133-4.
Answer: Colonic Malakoplakia.
Histopathologic examination of the biopsy specimens revealed nodular mixed inflammatory cells and infiltration of the epithelioid histiocytes in lamina propria (Figure D; stain: hematoxylin and eosin; original magnification 40×). The histiocytes showed foamy and eosinophilic cytoplasm (Figure E, arrow) and some of them had a targetoid appearance (Figure E, arrow head; stain: hematoxylin and eosin; original magnification 200×). Von Kossa stains highlighted the targetoid structures in the histiocytes (Figure F, Michaelis-Gutmann bodies). The granular cytoplasm of the histiocytes was positive on periodic acid-Schiff stain (Figure G). Based on these findings, the patient was diagnosed with colonic malakoplakia.

Malakoplakia is an uncommon, chronic, granulomatous inflammatory disease. It most commonly affects the urinary tract and gastrointestinal tract, but may occur at any anatomic site. Malakoplakia of the gastrointestinal tract are seen most frequently in the rectum, sigmoid, and right colon.1 It is diagnosed by the characteristic histologic feature of accumulated histiocytes with abundant eosinophilic granular cytoplasm containing basophilic inclusions, consistent with Michaelis-Gutmann bodies. Although the exact etiology and pathogenesis of malakoplakia are unclear, it seems to originate from an acquired defect in the intracellular destruction of phagocytosed bacteria, usually associated with Escherichia coli, Klebsiella, and Mycobacterium.2 It can have various causes, such as immunosuppression, malignant neoplasms, systemic diseases, and genetic diseases. Clinical manifestation of colonic malakoplakia is diverse, ranging from asymptomatic to malaise, fever, abdominal pain, diarrhea, hematochezia, and intestinal obstruction. Granulomatous reaction of malakoplakia generates the endoscopic appearance of lesions, which ranges from plaques to nodules and yellow-brown masses. In the early stage, malakoplakia commonly presents as soft yellow to tan mucosal plaques endoscopically, as seen in our case (Figure A). As the disease progresses in the later stage, malakoplakia presents as raised, grey to tan polypoid lesions of various sizes with peripheral hyperemia and a central depressed area, as seen in our case (Figure B).3 Owing to this endoscopic morphology, colonic malakoplakia may be misdiagnosed as atypical lymphoma, familial adenomatous polyposis, and metastatic carcinoma. To date, the natural course of malakoplakia of the colon is unclear, and no guidelines for treatment, treatment methods, duration of treatment, or surveillance are currently available. However, treatment of malakoplakia is essential to reduce immunosuppression and includes antibiotics with intracellular action and choline agonists that replenish the decreased cyclic 3’, 5’-guanosine monophosphate levels. In summary, although malakoplakia of the colon is very rare, it should be considered in the differential diagnosis of polypoid colonic lesions, especially in immunocompromised or malnourished patients.
References
1. Cipolletta L et al. Gastrointest Endosc. 1995 Mar;41(3):255-8.
2. Berney T et al. Transpl Int. 1999;12(4):293-6.
3. Weinrach DM et al. Arch Pathol Lab Med. 2004 Oct;128(10):e133-4.
Answer: Colonic Malakoplakia.
Histopathologic examination of the biopsy specimens revealed nodular mixed inflammatory cells and infiltration of the epithelioid histiocytes in lamina propria (Figure D; stain: hematoxylin and eosin; original magnification 40×). The histiocytes showed foamy and eosinophilic cytoplasm (Figure E, arrow) and some of them had a targetoid appearance (Figure E, arrow head; stain: hematoxylin and eosin; original magnification 200×). Von Kossa stains highlighted the targetoid structures in the histiocytes (Figure F, Michaelis-Gutmann bodies). The granular cytoplasm of the histiocytes was positive on periodic acid-Schiff stain (Figure G). Based on these findings, the patient was diagnosed with colonic malakoplakia.

Malakoplakia is an uncommon, chronic, granulomatous inflammatory disease. It most commonly affects the urinary tract and gastrointestinal tract, but may occur at any anatomic site. Malakoplakia of the gastrointestinal tract are seen most frequently in the rectum, sigmoid, and right colon.1 It is diagnosed by the characteristic histologic feature of accumulated histiocytes with abundant eosinophilic granular cytoplasm containing basophilic inclusions, consistent with Michaelis-Gutmann bodies. Although the exact etiology and pathogenesis of malakoplakia are unclear, it seems to originate from an acquired defect in the intracellular destruction of phagocytosed bacteria, usually associated with Escherichia coli, Klebsiella, and Mycobacterium.2 It can have various causes, such as immunosuppression, malignant neoplasms, systemic diseases, and genetic diseases. Clinical manifestation of colonic malakoplakia is diverse, ranging from asymptomatic to malaise, fever, abdominal pain, diarrhea, hematochezia, and intestinal obstruction. Granulomatous reaction of malakoplakia generates the endoscopic appearance of lesions, which ranges from plaques to nodules and yellow-brown masses. In the early stage, malakoplakia commonly presents as soft yellow to tan mucosal plaques endoscopically, as seen in our case (Figure A). As the disease progresses in the later stage, malakoplakia presents as raised, grey to tan polypoid lesions of various sizes with peripheral hyperemia and a central depressed area, as seen in our case (Figure B).3 Owing to this endoscopic morphology, colonic malakoplakia may be misdiagnosed as atypical lymphoma, familial adenomatous polyposis, and metastatic carcinoma. To date, the natural course of malakoplakia of the colon is unclear, and no guidelines for treatment, treatment methods, duration of treatment, or surveillance are currently available. However, treatment of malakoplakia is essential to reduce immunosuppression and includes antibiotics with intracellular action and choline agonists that replenish the decreased cyclic 3’, 5’-guanosine monophosphate levels. In summary, although malakoplakia of the colon is very rare, it should be considered in the differential diagnosis of polypoid colonic lesions, especially in immunocompromised or malnourished patients.
References
1. Cipolletta L et al. Gastrointest Endosc. 1995 Mar;41(3):255-8.
2. Berney T et al. Transpl Int. 1999;12(4):293-6.
3. Weinrach DM et al. Arch Pathol Lab Med. 2004 Oct;128(10):e133-4.
A 60-year-old man with C3 tetraplegia was referred to our department for evaluation of abdominal pain and hematochezia. He was diagnosed with adrenal insufficiency 5 years prior and has been taking low-dose prednisolone (7.5 mg) once a day. One year before presentation, he complained of intermittent loose, mucoid stool and abdominal pain. Sigmoidoscopy revealed multiple small yellowish plaques in the sigmoid colon (Figure A). However, symptoms improved without any treatment, and he was discharged from the rehabilitation department. He was readmitted for respiratory rehabilitation owing to dyspnea. On hospital day 4, he complained of abdominal pain and passing loose stool with foul odor 4-5 times a day. On hospital day 7, the abdominal pain worsened, and hematochezia occurred.
On physical examination, he was hemodynamically stable and afebrile. The abdomen was soft with mild tenderness on palpation in the periumbilical area without peritoneal signs. Laboratory studies were notable with a hemoglobin level of 10.7 g/dL, total protein of 4.09 g/dL, and albumin of 2.21 g/dL. Inflammatory marker (C-reactive protein) was mildly elevated to 1.83 mg/dL. Serology for human immunodeficiency virus was negative. Tumor markers, such as carcinoembryonic antigen, carbohydrate antigenic determinant, and alpha-fetoprotein, were within the normal range. Antineutrophil cytoplasmic antibody was negative, and rheumatic factor was within the normal range. Findings from stool for acid-fast bacillus and Clostridioides difficile toxin were negative; no pathogens were cultured, and no parasites were identified.
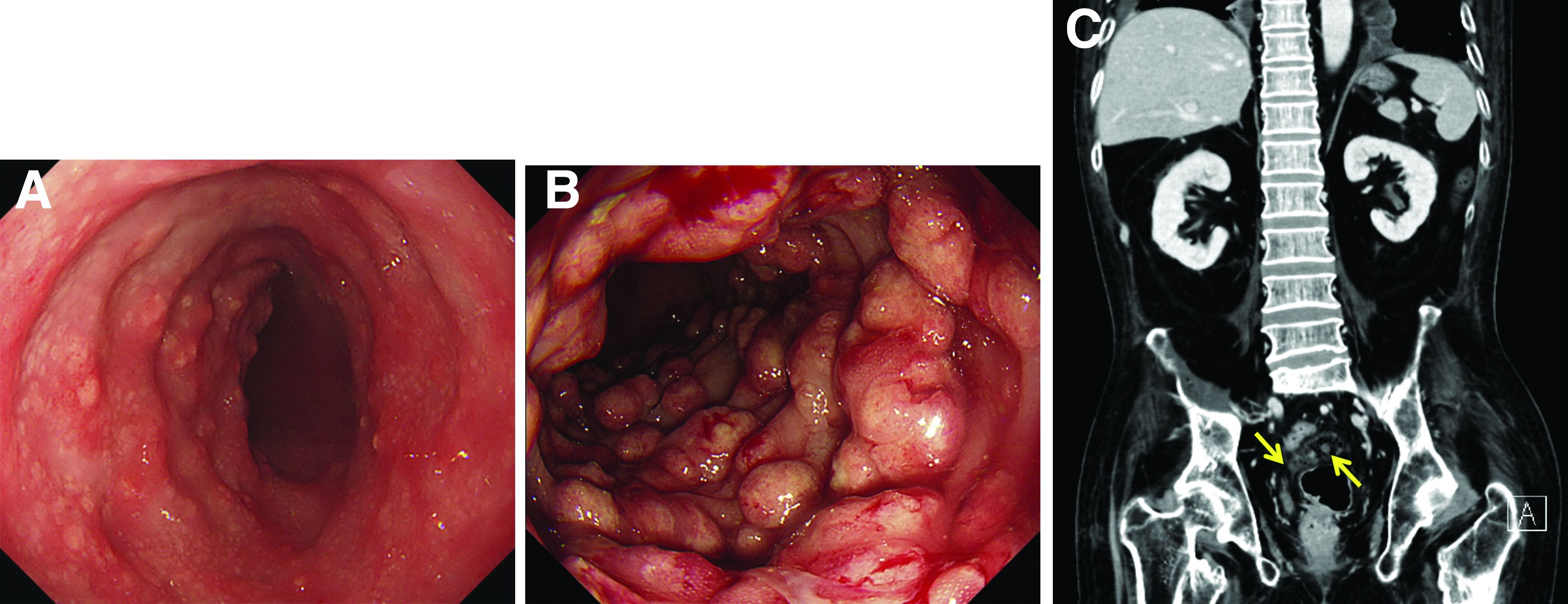
Sigmoidoscopy revealed diverse, multiple polypoid lesions (3-10 mm) with erythema, edema, and friability surrounding the entire lumen on the sigmoid colon (Figure B). The number and size of the polypoid lesions increased compared with the endoscopic findings obtained 1 year prior. The lesions easily bled on contact. Multiple biopsies of different sites were taken. An abdominal computed tomography scan showed multiple polyps of <1 cm that were confined to the sigmoid colon (Figure C, arrow).
Based on this information, what is the most likely diagnosis?
Previously published in Gastroenterology (2020 Feb;158[3]:482-4).
Undertreated hypothyroidism may worsen hospital outcomes
Suboptimal treatment of primary hypothyroidism may increase the risk of worse hospital outcomes, new research suggests.
The risks, including longer length of stay (LOS) and higher readmission rates, were no longer present in patients with adequately treated hypothyroidism, and in fact, appeared better than among those without hypothyroidism.
“Unfortunately, suboptimal treatment is common amongst the patient population with hypothyroidism,” wrote Matthew D. Ettleson, MD, of the Section of Endocrinology, Diabetes, and Metabolism at the University of Chicago, and colleagues.
“It is important for both patients and physicians to know that maintaining optimal thyroid hormone replacement is important to minimize length of hospital stays and hospital readmission. It is particularly important for planned admissions where thyroid hormone replacement can be adjusted if needed prior to admission,” said Dr. Ettleson in a press release from the Endocrine Society.
More evidence of adverse effects of suboptimal treatment
The findings, from a large U.S. claims database, “add to the growing body of evidence demonstrating the serious adverse short- and long-term health effects associated with suboptimal treatment of hypothyroidism,” the authors write in their article, published online in the Journal of Clinical Endocrinology and Metabolism. Dr. Ettleson will also present the data on June 11 at the ENDO 2022 meeting.
Thyroid hormone replacement therapy – generally levothyroxine – is given for primary hypothyroidism with the aim of maintaining serum thyroid-stimulating hormone (TSH) within the normal reference range.
TSH is inversely related to the level of circulating thyroid hormone, so low levels of TSH indicate overtreatment of thyroid disease and high levels indicate undertreatment.
Worse hospital outcomes associated with high TSH
In their study, Dr. Ettleson and colleagues retrospectively examined IBM MarketScan claims for 43,478 privately insured patients younger than age 65 years and hospitalized for medical or surgical reasons in 2008-2015.
Of those, 8,873 met the criteria for primary hypothyroidism based on a pre-admission prescription claim for levothyroxine, TSH > 10.00 mIU/L, confirmed diagnosis of hypothyroidism during hospitalization, or chronic lymphocytic thyroiditis. Of those, 4,770 (53.8%) had a prescription claim for levothyroxine.
Patients who met the clinical criteria for hypothyroidism were divided into four subgroups based on prehospitalization TSH level: low (< 0.40 mIU/L), normal (0.40-4.50 mIU/L), intermediate (4.51-10.00 mIU/L), and high (> 10.00 mIU/L).
The median length of time between TSH collection and hospital admission was 56 days in the hypothyroidism group and 63 days in the control group.
There were no differences in hospital outcomes between those with and without hypothyroidism among those who had low or intermediate TSH levels, in a multivariate analysis that used propensity-score matching.
In those with normal TSH levels, those with hypothyroidism actually had a lower risk of in-hospital death (risk ratio, 0.46; P = .004) and 90-day readmission rate (RR, 0.92; P = .02) than controls.
And those in the high TSH level subgroup had longer length of stay (+1.2 days; P = .003) and higher risk of 30-day readmission (RR, 1.49; P < .001) and 90-day readmission (RR, 1.43; P < .001), compared with balanced controls.
Public health effort needed to improve quality of care
There are multiple reasons why those with undertreated or undiagnosed hypothyroidism might have worse hospital outcomes, the authors say.
A bit more puzzling is why those with well-controlled hypothyroidism appeared to do better than those without hypothyroidism, given that thyroid hormone replacement isn’t likely to provide an advantage over normal, endogenous thyroid hormone production.
Dr. Ettleson and colleagues speculate that in-range TSH values may be a surrogate for regular health care and adherence to medical therapy, which likely leads to better hospital outcomes.
“The long- and short-term adverse health effects associated with off-target treatment of hypothyroidism, coupled with the high frequency of off-target treatment amongst the millions of patients in the United States on thyroid hormone, suggest that a public health effort to improve the quality of care of hypothyroidism is necessary,” Dr. Ettleson and colleagues write.
However, they note that there is currently no quality measure regarding appropriate treatment of hypothyroidism within the Merit-Based Incentive Payment System of the Centers for Medicare & Medicaid Services.
“The presence of guidelines alone may not be sufficient, as demonstrated by the inadequate application of guidelines for the use of levothyroxine in the treatment of thyroid cancer, a serious but much less common disease than clinical hypothyroidism,” the authors add.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Dr. Ettleson has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Suboptimal treatment of primary hypothyroidism may increase the risk of worse hospital outcomes, new research suggests.
The risks, including longer length of stay (LOS) and higher readmission rates, were no longer present in patients with adequately treated hypothyroidism, and in fact, appeared better than among those without hypothyroidism.
“Unfortunately, suboptimal treatment is common amongst the patient population with hypothyroidism,” wrote Matthew D. Ettleson, MD, of the Section of Endocrinology, Diabetes, and Metabolism at the University of Chicago, and colleagues.
“It is important for both patients and physicians to know that maintaining optimal thyroid hormone replacement is important to minimize length of hospital stays and hospital readmission. It is particularly important for planned admissions where thyroid hormone replacement can be adjusted if needed prior to admission,” said Dr. Ettleson in a press release from the Endocrine Society.
More evidence of adverse effects of suboptimal treatment
The findings, from a large U.S. claims database, “add to the growing body of evidence demonstrating the serious adverse short- and long-term health effects associated with suboptimal treatment of hypothyroidism,” the authors write in their article, published online in the Journal of Clinical Endocrinology and Metabolism. Dr. Ettleson will also present the data on June 11 at the ENDO 2022 meeting.
Thyroid hormone replacement therapy – generally levothyroxine – is given for primary hypothyroidism with the aim of maintaining serum thyroid-stimulating hormone (TSH) within the normal reference range.
TSH is inversely related to the level of circulating thyroid hormone, so low levels of TSH indicate overtreatment of thyroid disease and high levels indicate undertreatment.
Worse hospital outcomes associated with high TSH
In their study, Dr. Ettleson and colleagues retrospectively examined IBM MarketScan claims for 43,478 privately insured patients younger than age 65 years and hospitalized for medical or surgical reasons in 2008-2015.
Of those, 8,873 met the criteria for primary hypothyroidism based on a pre-admission prescription claim for levothyroxine, TSH > 10.00 mIU/L, confirmed diagnosis of hypothyroidism during hospitalization, or chronic lymphocytic thyroiditis. Of those, 4,770 (53.8%) had a prescription claim for levothyroxine.
Patients who met the clinical criteria for hypothyroidism were divided into four subgroups based on prehospitalization TSH level: low (< 0.40 mIU/L), normal (0.40-4.50 mIU/L), intermediate (4.51-10.00 mIU/L), and high (> 10.00 mIU/L).
The median length of time between TSH collection and hospital admission was 56 days in the hypothyroidism group and 63 days in the control group.
There were no differences in hospital outcomes between those with and without hypothyroidism among those who had low or intermediate TSH levels, in a multivariate analysis that used propensity-score matching.
In those with normal TSH levels, those with hypothyroidism actually had a lower risk of in-hospital death (risk ratio, 0.46; P = .004) and 90-day readmission rate (RR, 0.92; P = .02) than controls.
And those in the high TSH level subgroup had longer length of stay (+1.2 days; P = .003) and higher risk of 30-day readmission (RR, 1.49; P < .001) and 90-day readmission (RR, 1.43; P < .001), compared with balanced controls.
Public health effort needed to improve quality of care
There are multiple reasons why those with undertreated or undiagnosed hypothyroidism might have worse hospital outcomes, the authors say.
A bit more puzzling is why those with well-controlled hypothyroidism appeared to do better than those without hypothyroidism, given that thyroid hormone replacement isn’t likely to provide an advantage over normal, endogenous thyroid hormone production.
Dr. Ettleson and colleagues speculate that in-range TSH values may be a surrogate for regular health care and adherence to medical therapy, which likely leads to better hospital outcomes.
“The long- and short-term adverse health effects associated with off-target treatment of hypothyroidism, coupled with the high frequency of off-target treatment amongst the millions of patients in the United States on thyroid hormone, suggest that a public health effort to improve the quality of care of hypothyroidism is necessary,” Dr. Ettleson and colleagues write.
However, they note that there is currently no quality measure regarding appropriate treatment of hypothyroidism within the Merit-Based Incentive Payment System of the Centers for Medicare & Medicaid Services.
“The presence of guidelines alone may not be sufficient, as demonstrated by the inadequate application of guidelines for the use of levothyroxine in the treatment of thyroid cancer, a serious but much less common disease than clinical hypothyroidism,” the authors add.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Dr. Ettleson has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Suboptimal treatment of primary hypothyroidism may increase the risk of worse hospital outcomes, new research suggests.
The risks, including longer length of stay (LOS) and higher readmission rates, were no longer present in patients with adequately treated hypothyroidism, and in fact, appeared better than among those without hypothyroidism.
“Unfortunately, suboptimal treatment is common amongst the patient population with hypothyroidism,” wrote Matthew D. Ettleson, MD, of the Section of Endocrinology, Diabetes, and Metabolism at the University of Chicago, and colleagues.
“It is important for both patients and physicians to know that maintaining optimal thyroid hormone replacement is important to minimize length of hospital stays and hospital readmission. It is particularly important for planned admissions where thyroid hormone replacement can be adjusted if needed prior to admission,” said Dr. Ettleson in a press release from the Endocrine Society.
More evidence of adverse effects of suboptimal treatment
The findings, from a large U.S. claims database, “add to the growing body of evidence demonstrating the serious adverse short- and long-term health effects associated with suboptimal treatment of hypothyroidism,” the authors write in their article, published online in the Journal of Clinical Endocrinology and Metabolism. Dr. Ettleson will also present the data on June 11 at the ENDO 2022 meeting.
Thyroid hormone replacement therapy – generally levothyroxine – is given for primary hypothyroidism with the aim of maintaining serum thyroid-stimulating hormone (TSH) within the normal reference range.
TSH is inversely related to the level of circulating thyroid hormone, so low levels of TSH indicate overtreatment of thyroid disease and high levels indicate undertreatment.
Worse hospital outcomes associated with high TSH
In their study, Dr. Ettleson and colleagues retrospectively examined IBM MarketScan claims for 43,478 privately insured patients younger than age 65 years and hospitalized for medical or surgical reasons in 2008-2015.
Of those, 8,873 met the criteria for primary hypothyroidism based on a pre-admission prescription claim for levothyroxine, TSH > 10.00 mIU/L, confirmed diagnosis of hypothyroidism during hospitalization, or chronic lymphocytic thyroiditis. Of those, 4,770 (53.8%) had a prescription claim for levothyroxine.
Patients who met the clinical criteria for hypothyroidism were divided into four subgroups based on prehospitalization TSH level: low (< 0.40 mIU/L), normal (0.40-4.50 mIU/L), intermediate (4.51-10.00 mIU/L), and high (> 10.00 mIU/L).
The median length of time between TSH collection and hospital admission was 56 days in the hypothyroidism group and 63 days in the control group.
There were no differences in hospital outcomes between those with and without hypothyroidism among those who had low or intermediate TSH levels, in a multivariate analysis that used propensity-score matching.
In those with normal TSH levels, those with hypothyroidism actually had a lower risk of in-hospital death (risk ratio, 0.46; P = .004) and 90-day readmission rate (RR, 0.92; P = .02) than controls.
And those in the high TSH level subgroup had longer length of stay (+1.2 days; P = .003) and higher risk of 30-day readmission (RR, 1.49; P < .001) and 90-day readmission (RR, 1.43; P < .001), compared with balanced controls.
Public health effort needed to improve quality of care
There are multiple reasons why those with undertreated or undiagnosed hypothyroidism might have worse hospital outcomes, the authors say.
A bit more puzzling is why those with well-controlled hypothyroidism appeared to do better than those without hypothyroidism, given that thyroid hormone replacement isn’t likely to provide an advantage over normal, endogenous thyroid hormone production.
Dr. Ettleson and colleagues speculate that in-range TSH values may be a surrogate for regular health care and adherence to medical therapy, which likely leads to better hospital outcomes.
“The long- and short-term adverse health effects associated with off-target treatment of hypothyroidism, coupled with the high frequency of off-target treatment amongst the millions of patients in the United States on thyroid hormone, suggest that a public health effort to improve the quality of care of hypothyroidism is necessary,” Dr. Ettleson and colleagues write.
However, they note that there is currently no quality measure regarding appropriate treatment of hypothyroidism within the Merit-Based Incentive Payment System of the Centers for Medicare & Medicaid Services.
“The presence of guidelines alone may not be sufficient, as demonstrated by the inadequate application of guidelines for the use of levothyroxine in the treatment of thyroid cancer, a serious but much less common disease than clinical hypothyroidism,” the authors add.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Dr. Ettleson has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinical Edge Journal Scan Commentary: HCC May 2022
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
Clinical Edge Journal Scan Commentary: HCC May 2022
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
Clinical trials for unresectable HCC (uHCC) have mandated excellent underlying liver function. Patients with Child-Pugh (CP) A cirrhosis do not have cirrhosis as their most life-limiting disease. In clinical practice, there are many patients with uHCC who are functionally well yet have CP-B cirrhosis. D'Alessio and colleagues undertook a retrospective evaluation of 202 patients with either CP-A or CP-B cirrhosis who received atezolizumab and bevacizumab as first-line treatment of uHCC. The majority, 154 patients (76%), had CP-A cirrhosis, whereas 48 (24%) had CP-B, including 21 B7, 21 B8, and 6 B9. The authors found that in the overall population, median overall survival (mOS) was 14.9 months (95% CI 13.6-16.3), with patients with CP-A mOS of 16.8 months (95% CI 14.1-23.9), and CP-B mOS of 6.7 months (95% CI 4.3-15.6; P = .0003). Overall response rates (ORR) were comparable, with an ORR of 26% in CP-A and 21% in CP-B, not influenced by Barcelona Clinic Liver Cancer (BCLC) stage, performance status, etiology (viral vs nonviral), portal vein thrombosis (PVT), or extrahepatic spread (P > .05 for all associations). The investigators concluded that atezolizumab and bevacizumab in patients with CP-B was well tolerated, with no relevant difference in terms of clinically significant treatment-related adverse events compared with patients with CP-A.
Shi and colleagues reported a randomized controlled trial of patients with HCC and microvascular invasion (MVI) who underwent suboptimal resection (distance from tumor edge to the cut surface < 1 mm), followed by either stereotactic body radiotherapy (SBRT) or observation. From August 2015 to December 2016, 76 patients with BCLC stage 0/A liver disease, MVI, and no macroscopic vascular invasion were randomized after partial hepatectomy to either observation or SBRT (35 Gy delivered in a week). The 1-, 3-, and 5-year disease free survival (DFS) rates were 92.1%, 65.8%, and 56.1% in the SBRT group vs 76.3%, 36.8%, and 26.3% in the surgery alone group, respectively (P = .005). The 1-, 3-, and 5-year overall survival (OS) rates were 100%, 89.5%, and 75.0% in SBRT group vs 100.0%, 68.4%, and 53.7% in the surgery alone group, respectively (P = .053). The authors concluded that SBRT eradicates residual tumor cells present at the margin and improves surgical outcomes.
Roth and colleagues evaluated the safety and efficacy of transarterial chemoembolization (TACE) in older (> 70 years) patients with intermediate HCC. Out of 271 patients evaluated, 88 were older patients. 20.5% of older patients experienced serious adverse events vs 21.3% of younger patients (P = .87). The predictive factors of serious adverse events were CP stage ≥ B7 (P < .0001), Eastern Cooperative Oncology Group (ECOG) scale ≥ 1 (P = .0019), and Model for End-stage Liver Disease (MELD) score ≥ 9 (P = .0415). The serious adverse event rate was not increased with age (P = .87). The authors concluded that age should not be an exclusionary factor when considering TACE.
New blood biomarker to detect early dementia?
Investigators found that plasma concentrations of 2-aminoethyl dihydrogen phosphate and taurine could distinguish adults with early-stage Alzheimer’s disease from cognitively normal adults.
“Our biomarker for early-stage Alzheimer’s disease represents new thinking and is unique from the amyloid-beta and p-tau molecules that are currently being investigated to diagnose AD,” Sandra Banack, PhD, senior scientist, Brain Chemistry Labs, Jackson, Wyoming, told this news organization.
If further studies pan out, Dr. Banack said this biomarker could “easily be transformed into a test to aid clinical evaluations for Alzheimer’s disease.”
The study was published online in PLOS ONE.
New drug target?
The researchers measured concentrations of 2-aminoethyl dihydrogen phosphate and taurine in blood plasma samples in 25 patients (21 men; mean age, 71) with a clinical diagnosis of early-stage Alzheimer’s based on a Clinical Dementia Rating (CDR) score of 0.5, suggesting very mild cognitive impairment, and 25 healthy controls (20 men; mean age, 39).
The concentration of 2-aminoethyl dihydrogen phosphate, normalized by the concentration of taurine, reliably distinguished blood samples of early-stage Alzheimer’s patients from controls in a blinded analysis.
This biomarker “could lead to new understanding of [AD] and lead to new drug candidates,” Dr. Banack told this news organization.
The researchers note that 2-aminoethyl dihydrogen phosphate plays an important role in the structure and function of cellular membranes.
Physiologic effects of increased 2-aminoethyl dihydrogen phosphate concentrations in the blood are not known. However, in one study, concentrations of this molecule were found to be significantly lower in the temporal cortex, frontal cortex, and hippocampus (40%) in patients with Alzheimer’s disease, compared with controls.
“New biomarkers take time before they can be implemented in the clinic. The next step will be to repeat the experiments using a large sample size of AD patient blood samples,” Dr. Banack told this news organization.
The study team is looking to source a larger sample size of AD blood samples to replicate these findings. They are also examining this biomarker relative to other neurodegenerative diseases.
“If verified with larger sample sizes, the quantification of 2-aminoethyl dihydrogen phosphate could potentially assist in the diagnosis of early-stage Alzheimer’s disease when used in conjunction with the patient’s CDR score and other potential AD biomarkers,” Dr. Banack and colleagues say.
Caveats, cautionary notes
Commenting on the findings, Rebecca M. Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, said the study is “interesting, though very small-scale and very preliminary.”
Dr. Edelmayer said one “major limitation” is that participants did not have their Alzheimer’s diagnosis confirmed with “gold standard biomarkers. They have been diagnosed based only on their cognitive and behavioral symptoms.”
She also cautioned that the study population is not representative – either of the general public or people living with Alzheimer’s disease.
For example, 41 out of all 50 samples are from men, “though we know women are disproportionately impacted by Alzheimer’s.”
“There is a mismatch in the age of the study groups,” Dr. Edelmayer noted. The mean age of controls in the study was 39 and the mean age of people with dementia was 71. Race or ethnicity and other demographic information is also unclear from the article.
“There is an urgent need for simple, inexpensive, noninvasive and easily available diagnostic tools for Alzheimer’s, such as a blood test. A simple blood test for Alzheimer’s would be a great advance for individuals with – and at risk for – the disease, families, doctors, and researchers,” Dr. Edelmayer said.
“Bottom line,” Dr. Edelmayer continued, “these results need to be further tested and verified in long-term, large-scale studies with diverse populations that are representative of those living with Alzheimer’s disease.”
This research was supported by the William Stamps Farish Fund and the Josephine P. & John J. Louis Foundation. Brain Chemistry Labs has applied for a patent related to this research. Dr. Edelmayer has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators found that plasma concentrations of 2-aminoethyl dihydrogen phosphate and taurine could distinguish adults with early-stage Alzheimer’s disease from cognitively normal adults.
“Our biomarker for early-stage Alzheimer’s disease represents new thinking and is unique from the amyloid-beta and p-tau molecules that are currently being investigated to diagnose AD,” Sandra Banack, PhD, senior scientist, Brain Chemistry Labs, Jackson, Wyoming, told this news organization.
If further studies pan out, Dr. Banack said this biomarker could “easily be transformed into a test to aid clinical evaluations for Alzheimer’s disease.”
The study was published online in PLOS ONE.
New drug target?
The researchers measured concentrations of 2-aminoethyl dihydrogen phosphate and taurine in blood plasma samples in 25 patients (21 men; mean age, 71) with a clinical diagnosis of early-stage Alzheimer’s based on a Clinical Dementia Rating (CDR) score of 0.5, suggesting very mild cognitive impairment, and 25 healthy controls (20 men; mean age, 39).
The concentration of 2-aminoethyl dihydrogen phosphate, normalized by the concentration of taurine, reliably distinguished blood samples of early-stage Alzheimer’s patients from controls in a blinded analysis.
This biomarker “could lead to new understanding of [AD] and lead to new drug candidates,” Dr. Banack told this news organization.
The researchers note that 2-aminoethyl dihydrogen phosphate plays an important role in the structure and function of cellular membranes.
Physiologic effects of increased 2-aminoethyl dihydrogen phosphate concentrations in the blood are not known. However, in one study, concentrations of this molecule were found to be significantly lower in the temporal cortex, frontal cortex, and hippocampus (40%) in patients with Alzheimer’s disease, compared with controls.
“New biomarkers take time before they can be implemented in the clinic. The next step will be to repeat the experiments using a large sample size of AD patient blood samples,” Dr. Banack told this news organization.
The study team is looking to source a larger sample size of AD blood samples to replicate these findings. They are also examining this biomarker relative to other neurodegenerative diseases.
“If verified with larger sample sizes, the quantification of 2-aminoethyl dihydrogen phosphate could potentially assist in the diagnosis of early-stage Alzheimer’s disease when used in conjunction with the patient’s CDR score and other potential AD biomarkers,” Dr. Banack and colleagues say.
Caveats, cautionary notes
Commenting on the findings, Rebecca M. Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, said the study is “interesting, though very small-scale and very preliminary.”
Dr. Edelmayer said one “major limitation” is that participants did not have their Alzheimer’s diagnosis confirmed with “gold standard biomarkers. They have been diagnosed based only on their cognitive and behavioral symptoms.”
She also cautioned that the study population is not representative – either of the general public or people living with Alzheimer’s disease.
For example, 41 out of all 50 samples are from men, “though we know women are disproportionately impacted by Alzheimer’s.”
“There is a mismatch in the age of the study groups,” Dr. Edelmayer noted. The mean age of controls in the study was 39 and the mean age of people with dementia was 71. Race or ethnicity and other demographic information is also unclear from the article.
“There is an urgent need for simple, inexpensive, noninvasive and easily available diagnostic tools for Alzheimer’s, such as a blood test. A simple blood test for Alzheimer’s would be a great advance for individuals with – and at risk for – the disease, families, doctors, and researchers,” Dr. Edelmayer said.
“Bottom line,” Dr. Edelmayer continued, “these results need to be further tested and verified in long-term, large-scale studies with diverse populations that are representative of those living with Alzheimer’s disease.”
This research was supported by the William Stamps Farish Fund and the Josephine P. & John J. Louis Foundation. Brain Chemistry Labs has applied for a patent related to this research. Dr. Edelmayer has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators found that plasma concentrations of 2-aminoethyl dihydrogen phosphate and taurine could distinguish adults with early-stage Alzheimer’s disease from cognitively normal adults.
“Our biomarker for early-stage Alzheimer’s disease represents new thinking and is unique from the amyloid-beta and p-tau molecules that are currently being investigated to diagnose AD,” Sandra Banack, PhD, senior scientist, Brain Chemistry Labs, Jackson, Wyoming, told this news organization.
If further studies pan out, Dr. Banack said this biomarker could “easily be transformed into a test to aid clinical evaluations for Alzheimer’s disease.”
The study was published online in PLOS ONE.
New drug target?
The researchers measured concentrations of 2-aminoethyl dihydrogen phosphate and taurine in blood plasma samples in 25 patients (21 men; mean age, 71) with a clinical diagnosis of early-stage Alzheimer’s based on a Clinical Dementia Rating (CDR) score of 0.5, suggesting very mild cognitive impairment, and 25 healthy controls (20 men; mean age, 39).
The concentration of 2-aminoethyl dihydrogen phosphate, normalized by the concentration of taurine, reliably distinguished blood samples of early-stage Alzheimer’s patients from controls in a blinded analysis.
This biomarker “could lead to new understanding of [AD] and lead to new drug candidates,” Dr. Banack told this news organization.
The researchers note that 2-aminoethyl dihydrogen phosphate plays an important role in the structure and function of cellular membranes.
Physiologic effects of increased 2-aminoethyl dihydrogen phosphate concentrations in the blood are not known. However, in one study, concentrations of this molecule were found to be significantly lower in the temporal cortex, frontal cortex, and hippocampus (40%) in patients with Alzheimer’s disease, compared with controls.
“New biomarkers take time before they can be implemented in the clinic. The next step will be to repeat the experiments using a large sample size of AD patient blood samples,” Dr. Banack told this news organization.
The study team is looking to source a larger sample size of AD blood samples to replicate these findings. They are also examining this biomarker relative to other neurodegenerative diseases.
“If verified with larger sample sizes, the quantification of 2-aminoethyl dihydrogen phosphate could potentially assist in the diagnosis of early-stage Alzheimer’s disease when used in conjunction with the patient’s CDR score and other potential AD biomarkers,” Dr. Banack and colleagues say.
Caveats, cautionary notes
Commenting on the findings, Rebecca M. Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, said the study is “interesting, though very small-scale and very preliminary.”
Dr. Edelmayer said one “major limitation” is that participants did not have their Alzheimer’s diagnosis confirmed with “gold standard biomarkers. They have been diagnosed based only on their cognitive and behavioral symptoms.”
She also cautioned that the study population is not representative – either of the general public or people living with Alzheimer’s disease.
For example, 41 out of all 50 samples are from men, “though we know women are disproportionately impacted by Alzheimer’s.”
“There is a mismatch in the age of the study groups,” Dr. Edelmayer noted. The mean age of controls in the study was 39 and the mean age of people with dementia was 71. Race or ethnicity and other demographic information is also unclear from the article.
“There is an urgent need for simple, inexpensive, noninvasive and easily available diagnostic tools for Alzheimer’s, such as a blood test. A simple blood test for Alzheimer’s would be a great advance for individuals with – and at risk for – the disease, families, doctors, and researchers,” Dr. Edelmayer said.
“Bottom line,” Dr. Edelmayer continued, “these results need to be further tested and verified in long-term, large-scale studies with diverse populations that are representative of those living with Alzheimer’s disease.”
This research was supported by the William Stamps Farish Fund and the Josephine P. & John J. Louis Foundation. Brain Chemistry Labs has applied for a patent related to this research. Dr. Edelmayer has no relevant disclosures.
A version of this article first appeared on Medscape.com.










