User login
Melanoma
THE COMPARISON
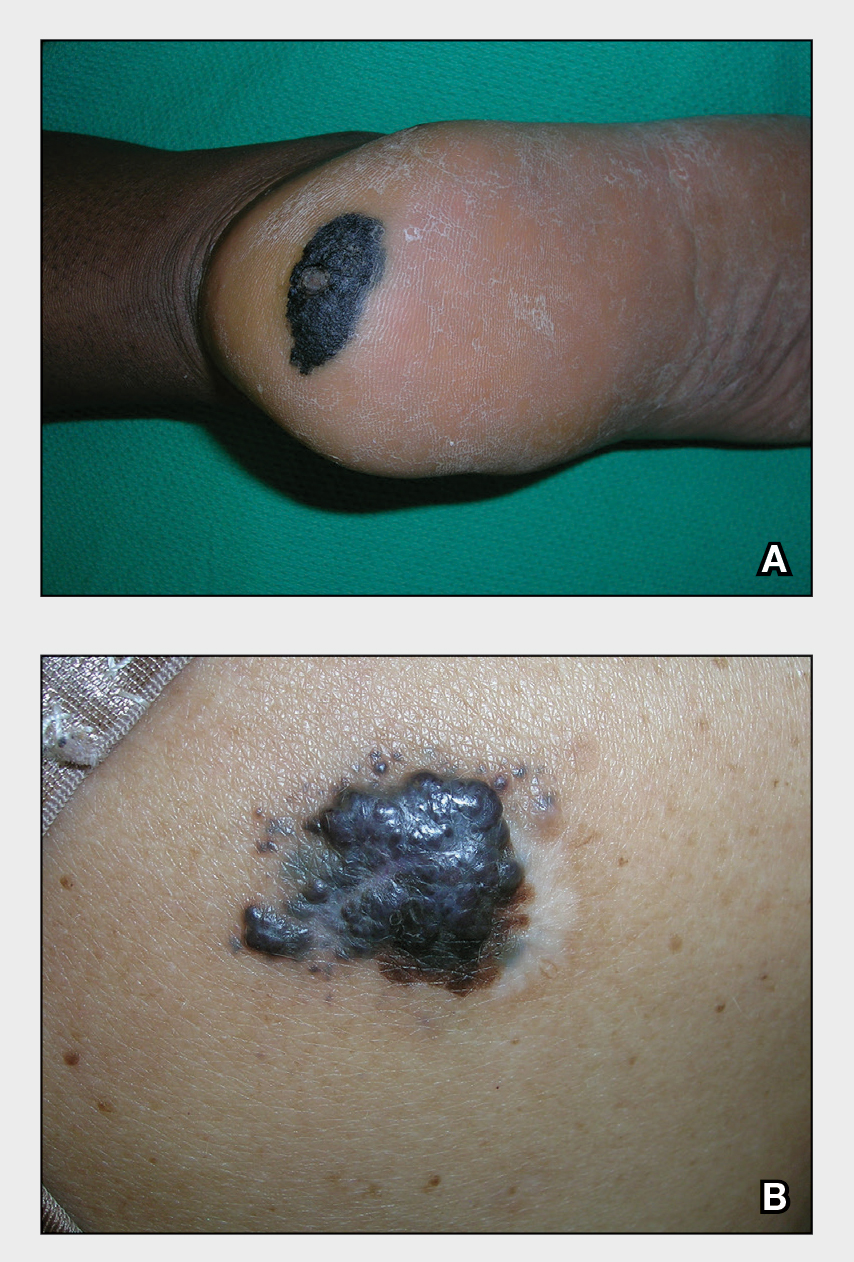
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
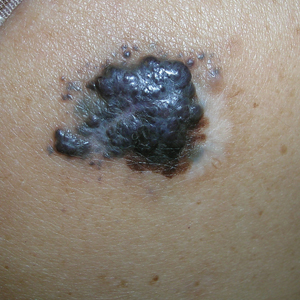
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097

THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.

THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097
Nevus of Ota: Does the 1064-nm Q-switched Nd:YAG laser work in Black patients?
SAN DIEGO – Using a , results from a small single-center study showed.
Nevus of Ota is a benign melanocytic lesion that presents as a unilateral blue-gray to blue-brown facial patch favoring the distribution of the first two branches of the trigeminal nerve. Among Asians, the prevalence of the condition among Asians is estimated to be between 0.03% and 1.113%, while the prevalence among Blacks population is estimated to be between 0.01% and 0.016%, Shelby L. Kubicki, MD, said during a clinical abstract session at the annual meeting of the American Society for Laser Medicine and Surgery.
“Most existing literature describes the characteristics and treatment of Nevus of Ota based on Asian patients with skin types I-IV,” said Dr. Kubicki, a third-year dermatology resident at the University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston. “Special considerations are required when treating [Fitzpatrick skin types] V-VI, which is why it’s important to characterize these patients, to make sure they’re well represented in the literature.”
In what she said is the largest reported case series of its kind, Dr. Kubicki and colleagues identified eight Fitzpatrick skin type V or VI patients who underwent laser treatment for Nevus of Ota from 2016-2021. All were treated with the 1,064-nm Q‐switched Nd:YAG and on average, received 5.4 treatments at 2-10 month intervals. Fluence ranged from 1.8 to 2.4 J/cm2, and total pulse count ranged from 536.8 to 831.1. Two of these patients were additionally treated with 1,550-nm nonablative fractional resurfacing with a mean of six treatments. Primary outcomes were based on improvement of before and after clinical photographs by three independent board-certified dermatologists, who used a 5-point visual analogue scale for grading.
The mean age of patients was 30.4 years and ranged from 9 months to 45 years. Six were females and two were males, two had Fitzpatrick skin type V, and six had Fitzpatrick skin type VI. Of the eight patients, six had blue-gray lesions, one patient had a dark brown lesion, and one patient had “a hybrid lesion that had blue-gray and brown discoloration,” Dr. Kubicki said.
After grading of the clinical photographs, patients demonstrated a mean improvement of 51%-75% at follow-up 5-56 weeks after treatment (a mean of 16.9 weeks). No long-term adverse events were encountered in either group, but three patients developed mild guttate hypopigmentation following laser treatment.
“Lesional color may contribute to outcome, and patients should be educated about the risk of guttate hypopigmentation,” Dr. Kubicki said. “More studies are needed to determine the optimal device and treatment settings in this population.”
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices at AllPhases Dermatology in Alexandria, Va., said that, while the study results impressed her, she speculated that the patients may require more treatments in the future. “What to look out for is the risk of rebound,” Dr. Onwudiwe said. “Because Nevus of Ota is a hamartomatous lesion, it’s very hard to treat, and sometimes it will come back. It will be nice to see how long this treatment can last. If you can use a combination therapy and have ... cases where you’re only needing a touch-up every so often, that’s still a win.”
Another session moderator, Eliot Battle, MD, CEO of Cultura Dermatology and Laser Center in Washington, D.C., said that he wondered what histologic analysis following treatment might show, and if a biopsy after treatment would show “if we really got rid of the nevus, or if we are just cosmetically improving the appearance temporarily.”
Neither Dr. Kubicki nor Dr. Onwudiwe reported having financial disclosures. Dr. Battle disclosed that he conducts research for Cynosure. He has also received discounts from Cynosure, Cutera, Solta Medical, Lumenis, Be Inc., and Sciton.
SAN DIEGO – Using a , results from a small single-center study showed.
Nevus of Ota is a benign melanocytic lesion that presents as a unilateral blue-gray to blue-brown facial patch favoring the distribution of the first two branches of the trigeminal nerve. Among Asians, the prevalence of the condition among Asians is estimated to be between 0.03% and 1.113%, while the prevalence among Blacks population is estimated to be between 0.01% and 0.016%, Shelby L. Kubicki, MD, said during a clinical abstract session at the annual meeting of the American Society for Laser Medicine and Surgery.
“Most existing literature describes the characteristics and treatment of Nevus of Ota based on Asian patients with skin types I-IV,” said Dr. Kubicki, a third-year dermatology resident at the University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston. “Special considerations are required when treating [Fitzpatrick skin types] V-VI, which is why it’s important to characterize these patients, to make sure they’re well represented in the literature.”
In what she said is the largest reported case series of its kind, Dr. Kubicki and colleagues identified eight Fitzpatrick skin type V or VI patients who underwent laser treatment for Nevus of Ota from 2016-2021. All were treated with the 1,064-nm Q‐switched Nd:YAG and on average, received 5.4 treatments at 2-10 month intervals. Fluence ranged from 1.8 to 2.4 J/cm2, and total pulse count ranged from 536.8 to 831.1. Two of these patients were additionally treated with 1,550-nm nonablative fractional resurfacing with a mean of six treatments. Primary outcomes were based on improvement of before and after clinical photographs by three independent board-certified dermatologists, who used a 5-point visual analogue scale for grading.
The mean age of patients was 30.4 years and ranged from 9 months to 45 years. Six were females and two were males, two had Fitzpatrick skin type V, and six had Fitzpatrick skin type VI. Of the eight patients, six had blue-gray lesions, one patient had a dark brown lesion, and one patient had “a hybrid lesion that had blue-gray and brown discoloration,” Dr. Kubicki said.
After grading of the clinical photographs, patients demonstrated a mean improvement of 51%-75% at follow-up 5-56 weeks after treatment (a mean of 16.9 weeks). No long-term adverse events were encountered in either group, but three patients developed mild guttate hypopigmentation following laser treatment.
“Lesional color may contribute to outcome, and patients should be educated about the risk of guttate hypopigmentation,” Dr. Kubicki said. “More studies are needed to determine the optimal device and treatment settings in this population.”
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices at AllPhases Dermatology in Alexandria, Va., said that, while the study results impressed her, she speculated that the patients may require more treatments in the future. “What to look out for is the risk of rebound,” Dr. Onwudiwe said. “Because Nevus of Ota is a hamartomatous lesion, it’s very hard to treat, and sometimes it will come back. It will be nice to see how long this treatment can last. If you can use a combination therapy and have ... cases where you’re only needing a touch-up every so often, that’s still a win.”
Another session moderator, Eliot Battle, MD, CEO of Cultura Dermatology and Laser Center in Washington, D.C., said that he wondered what histologic analysis following treatment might show, and if a biopsy after treatment would show “if we really got rid of the nevus, or if we are just cosmetically improving the appearance temporarily.”
Neither Dr. Kubicki nor Dr. Onwudiwe reported having financial disclosures. Dr. Battle disclosed that he conducts research for Cynosure. He has also received discounts from Cynosure, Cutera, Solta Medical, Lumenis, Be Inc., and Sciton.
SAN DIEGO – Using a , results from a small single-center study showed.
Nevus of Ota is a benign melanocytic lesion that presents as a unilateral blue-gray to blue-brown facial patch favoring the distribution of the first two branches of the trigeminal nerve. Among Asians, the prevalence of the condition among Asians is estimated to be between 0.03% and 1.113%, while the prevalence among Blacks population is estimated to be between 0.01% and 0.016%, Shelby L. Kubicki, MD, said during a clinical abstract session at the annual meeting of the American Society for Laser Medicine and Surgery.
“Most existing literature describes the characteristics and treatment of Nevus of Ota based on Asian patients with skin types I-IV,” said Dr. Kubicki, a third-year dermatology resident at the University of Texas Health Sciences Center/University of Texas MD Anderson Cancer Center, both in Houston. “Special considerations are required when treating [Fitzpatrick skin types] V-VI, which is why it’s important to characterize these patients, to make sure they’re well represented in the literature.”
In what she said is the largest reported case series of its kind, Dr. Kubicki and colleagues identified eight Fitzpatrick skin type V or VI patients who underwent laser treatment for Nevus of Ota from 2016-2021. All were treated with the 1,064-nm Q‐switched Nd:YAG and on average, received 5.4 treatments at 2-10 month intervals. Fluence ranged from 1.8 to 2.4 J/cm2, and total pulse count ranged from 536.8 to 831.1. Two of these patients were additionally treated with 1,550-nm nonablative fractional resurfacing with a mean of six treatments. Primary outcomes were based on improvement of before and after clinical photographs by three independent board-certified dermatologists, who used a 5-point visual analogue scale for grading.
The mean age of patients was 30.4 years and ranged from 9 months to 45 years. Six were females and two were males, two had Fitzpatrick skin type V, and six had Fitzpatrick skin type VI. Of the eight patients, six had blue-gray lesions, one patient had a dark brown lesion, and one patient had “a hybrid lesion that had blue-gray and brown discoloration,” Dr. Kubicki said.
After grading of the clinical photographs, patients demonstrated a mean improvement of 51%-75% at follow-up 5-56 weeks after treatment (a mean of 16.9 weeks). No long-term adverse events were encountered in either group, but three patients developed mild guttate hypopigmentation following laser treatment.
“Lesional color may contribute to outcome, and patients should be educated about the risk of guttate hypopigmentation,” Dr. Kubicki said. “More studies are needed to determine the optimal device and treatment settings in this population.”
In an interview at the meeting, one of the session moderators, Oge Onwudiwe, MD, a dermatologist who practices at AllPhases Dermatology in Alexandria, Va., said that, while the study results impressed her, she speculated that the patients may require more treatments in the future. “What to look out for is the risk of rebound,” Dr. Onwudiwe said. “Because Nevus of Ota is a hamartomatous lesion, it’s very hard to treat, and sometimes it will come back. It will be nice to see how long this treatment can last. If you can use a combination therapy and have ... cases where you’re only needing a touch-up every so often, that’s still a win.”
Another session moderator, Eliot Battle, MD, CEO of Cultura Dermatology and Laser Center in Washington, D.C., said that he wondered what histologic analysis following treatment might show, and if a biopsy after treatment would show “if we really got rid of the nevus, or if we are just cosmetically improving the appearance temporarily.”
Neither Dr. Kubicki nor Dr. Onwudiwe reported having financial disclosures. Dr. Battle disclosed that he conducts research for Cynosure. He has also received discounts from Cynosure, Cutera, Solta Medical, Lumenis, Be Inc., and Sciton.
AT ASLMS 2022
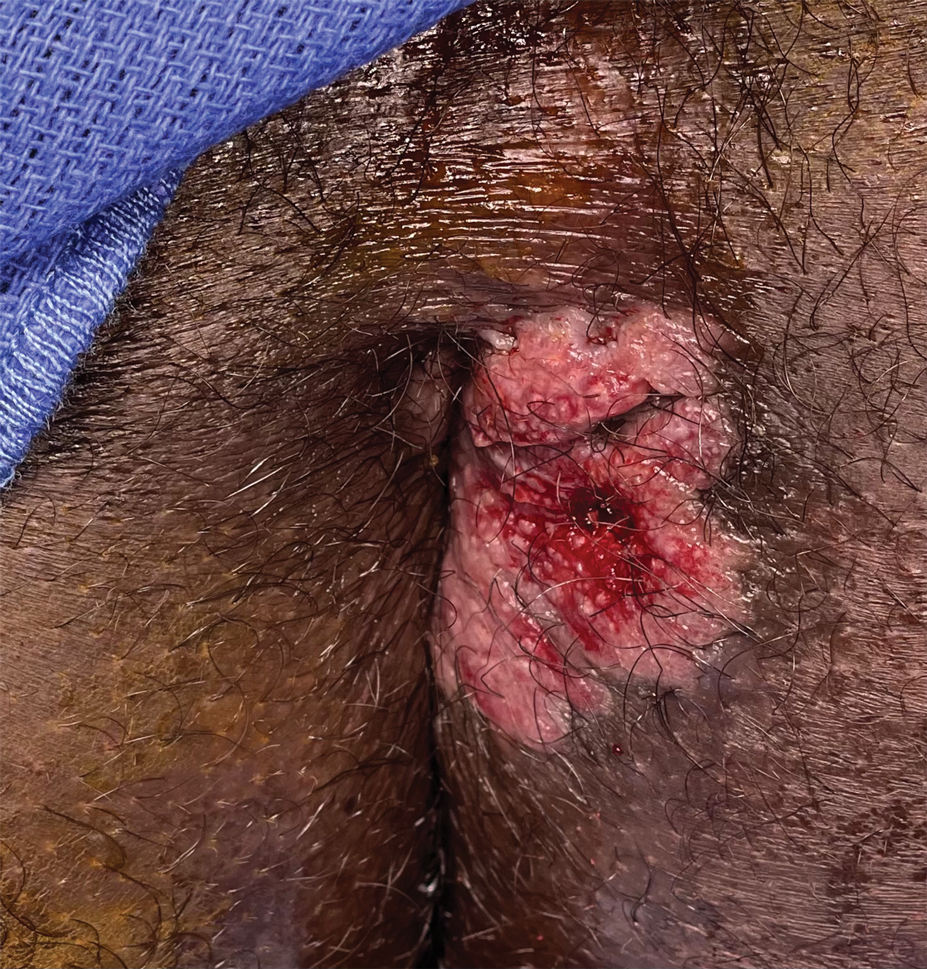
Painful Fungating Perianal Mass
The Diagnosis: Condyloma Latum
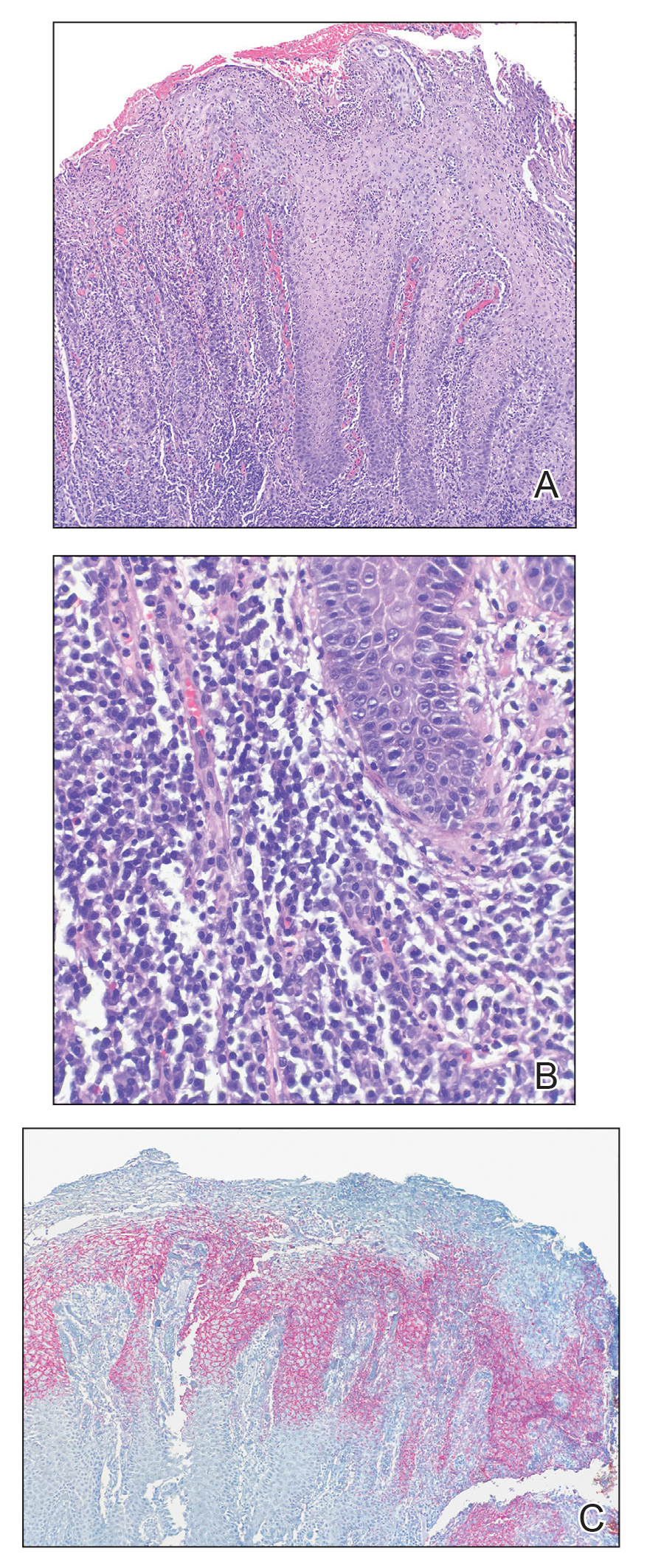
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.
Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
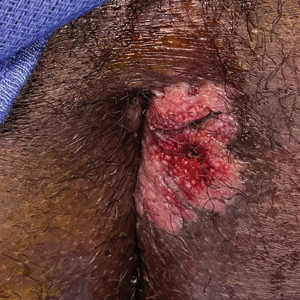
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
The Diagnosis: Condyloma Latum
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.

Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
The Diagnosis: Condyloma Latum
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.

Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
A 21-year-old man presented to our clinic with rectal pain of 2 months’ duration that occurred in association with bowel movements and rectal bleeding in the setting of constipation. The patient’s symptoms had persisted despite multiple clinical encounters and treatment with sulfamethoxazole-trimethoprim, clotrimazole, valacyclovir, topical hydrocortisone and pramoxine, topical lidocaine, imiquimod, and psyllium seed. The patient denied engaging in receptive anal intercourse and had no notable medical or surgical history. Physical examination revealed a 6-cm hypopigmented fungating mass on the left gluteal cleft just external to the anal verge; there were no other abnormal findings. The patient denied any other systemic symptoms.
Supreme Court appears ready to overturn Roe
to the news outlet Politico.
The draft opinion, written by Justice Samuel Alito, outlines ways a presumed majority of the nine justices believes the 1973 ruling in Roe v. Wade was incorrect. If signed by a majority of the court, the ruling would eliminate the protections for abortion rights that Roe provided and give the 50 states the power to legislate abortion.
“We hold that Roe and Casey must be overruled,” Justice Alito writes in the draft. “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
While a final ruling was not expected from the court until June, the leaked draft – a nearly unprecedented breach of the court’s internal workings – gives a strong signal of the court’s five most conservative members’ decisions. During oral arguments in the case in December, conservative justices appeared prepared to undo at least part of the country’s abortion protections.
President Joe Biden said his administration was already preparing for a potential ruling that struck down federal abortion protections.
The White House, he said in a statement, is working on a “response to the continued attack on abortion and reproductive rights, under a variety of possible outcomes in the cases pending before the Supreme Court. We will be ready when any ruling is issued.”
But if the draft opinion becomes final, he said the fight will move to the states.
“It will fall on our nation’s elected officials at all levels of government to protect a woman’s right to choose,” he said. “And it will fall on voters to elect pro-choice officials this November.”
With more pro-abortion rights members of Congress, it would be possible to pass federal legislation protecting abortion rights, “which I will work to pass and sign into law.”
Should the Alito draft become law, its first impact would be to allow a Mississippi law that bans abortions after 15 weeks to take effect.
But quickly after that, abortions would become illegal in many states. Several conservative-leaning states, mostly in the South and Midwest, have already passed laws severely restricting abortions well beyond what Roe allowed. Should Roe be overturned then, those laws would take effect without the threat of lengthy lawsuits or rulings from lower-court judges who have blocked them.
Nearly half of the states, mostly in the Northeast and West, would likely allow abortion to continue in some way. In fact, several states, including Colorado and Vermont, have already passed laws granting the right to an abortion into state law.
The leaked draft, however, is still a draft, meaning it remains possible Roe survives. Anthony Kreis, PhD, a professor of law at Georgia State University, says that could have been the point of whoever leaked the draft.
“It suggests to me that whoever leaked it knew that public outrage was the last resort to stopping the court from overturning Roe v. Wade and letting states ban all abortions,” Dr. Kreis said. “The danger that abortions won’t be legal in most of the country is very real.”
A version of this article first appeared on WebMD.com.
This article was updated 5/3/22.
to the news outlet Politico.
The draft opinion, written by Justice Samuel Alito, outlines ways a presumed majority of the nine justices believes the 1973 ruling in Roe v. Wade was incorrect. If signed by a majority of the court, the ruling would eliminate the protections for abortion rights that Roe provided and give the 50 states the power to legislate abortion.
“We hold that Roe and Casey must be overruled,” Justice Alito writes in the draft. “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
While a final ruling was not expected from the court until June, the leaked draft – a nearly unprecedented breach of the court’s internal workings – gives a strong signal of the court’s five most conservative members’ decisions. During oral arguments in the case in December, conservative justices appeared prepared to undo at least part of the country’s abortion protections.
President Joe Biden said his administration was already preparing for a potential ruling that struck down federal abortion protections.
The White House, he said in a statement, is working on a “response to the continued attack on abortion and reproductive rights, under a variety of possible outcomes in the cases pending before the Supreme Court. We will be ready when any ruling is issued.”
But if the draft opinion becomes final, he said the fight will move to the states.
“It will fall on our nation’s elected officials at all levels of government to protect a woman’s right to choose,” he said. “And it will fall on voters to elect pro-choice officials this November.”
With more pro-abortion rights members of Congress, it would be possible to pass federal legislation protecting abortion rights, “which I will work to pass and sign into law.”
Should the Alito draft become law, its first impact would be to allow a Mississippi law that bans abortions after 15 weeks to take effect.
But quickly after that, abortions would become illegal in many states. Several conservative-leaning states, mostly in the South and Midwest, have already passed laws severely restricting abortions well beyond what Roe allowed. Should Roe be overturned then, those laws would take effect without the threat of lengthy lawsuits or rulings from lower-court judges who have blocked them.
Nearly half of the states, mostly in the Northeast and West, would likely allow abortion to continue in some way. In fact, several states, including Colorado and Vermont, have already passed laws granting the right to an abortion into state law.
The leaked draft, however, is still a draft, meaning it remains possible Roe survives. Anthony Kreis, PhD, a professor of law at Georgia State University, says that could have been the point of whoever leaked the draft.
“It suggests to me that whoever leaked it knew that public outrage was the last resort to stopping the court from overturning Roe v. Wade and letting states ban all abortions,” Dr. Kreis said. “The danger that abortions won’t be legal in most of the country is very real.”
A version of this article first appeared on WebMD.com.
This article was updated 5/3/22.
to the news outlet Politico.
The draft opinion, written by Justice Samuel Alito, outlines ways a presumed majority of the nine justices believes the 1973 ruling in Roe v. Wade was incorrect. If signed by a majority of the court, the ruling would eliminate the protections for abortion rights that Roe provided and give the 50 states the power to legislate abortion.
“We hold that Roe and Casey must be overruled,” Justice Alito writes in the draft. “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
While a final ruling was not expected from the court until June, the leaked draft – a nearly unprecedented breach of the court’s internal workings – gives a strong signal of the court’s five most conservative members’ decisions. During oral arguments in the case in December, conservative justices appeared prepared to undo at least part of the country’s abortion protections.
President Joe Biden said his administration was already preparing for a potential ruling that struck down federal abortion protections.
The White House, he said in a statement, is working on a “response to the continued attack on abortion and reproductive rights, under a variety of possible outcomes in the cases pending before the Supreme Court. We will be ready when any ruling is issued.”
But if the draft opinion becomes final, he said the fight will move to the states.
“It will fall on our nation’s elected officials at all levels of government to protect a woman’s right to choose,” he said. “And it will fall on voters to elect pro-choice officials this November.”
With more pro-abortion rights members of Congress, it would be possible to pass federal legislation protecting abortion rights, “which I will work to pass and sign into law.”
Should the Alito draft become law, its first impact would be to allow a Mississippi law that bans abortions after 15 weeks to take effect.
But quickly after that, abortions would become illegal in many states. Several conservative-leaning states, mostly in the South and Midwest, have already passed laws severely restricting abortions well beyond what Roe allowed. Should Roe be overturned then, those laws would take effect without the threat of lengthy lawsuits or rulings from lower-court judges who have blocked them.
Nearly half of the states, mostly in the Northeast and West, would likely allow abortion to continue in some way. In fact, several states, including Colorado and Vermont, have already passed laws granting the right to an abortion into state law.
The leaked draft, however, is still a draft, meaning it remains possible Roe survives. Anthony Kreis, PhD, a professor of law at Georgia State University, says that could have been the point of whoever leaked the draft.
“It suggests to me that whoever leaked it knew that public outrage was the last resort to stopping the court from overturning Roe v. Wade and letting states ban all abortions,” Dr. Kreis said. “The danger that abortions won’t be legal in most of the country is very real.”
A version of this article first appeared on WebMD.com.
This article was updated 5/3/22.
Commentary: WHO, UNICEF warn about increased risk of measles outbreaks
The newly released global estimate is now 25 million children (2 million more than in 2020) missing scheduled vaccines. This continues to bode badly for multiple vaccine-preventable infections, but maybe the most for measles in 2022.
Specifically for measles vaccine, global two-dose coverage was only 71%. Coverage was less than 50% in 8 countries: Chad, Guinea, Samoa, North Korea, Central African Republic, Somalia, Angola, and South Sudan. These eight areas seem ripe for outbreaks this year and indeed Somalia is having an outbreak.
Overall, worldwide measles cases increased 79% in early 2022, compared with 2021. The top 10 countries for measles cases from November 2021 to April 2022, per the World Health Organization, include Nigeria, India, Soma Ethiopia, Pakistan, DR Congo, Afghanistan, Liberia, Cameroon, and Ivory Coast.
In the United States, we have been lucky so far with only 55 cases since the start of 2021. However, MMR two-dose coverage has dropped since the pandemic’s start. The list of U.S. areas with the lowest overall two-dose MMR coverage as of 2021 were D.C. (78.9%), Houston (93.7%), Idaho (86.5%), Wisconsin (87.2%), Maryland (87.6%), Georgia (88.5%), Kentucky (88.9%), Ohio (89.6%), and Minnesota (89.8%). Only 14 states had rates over the targeted 95% rate needed for community (herd) immunity against measles (MMWR Morb Mortal Wkly Rep. 2022;71:561-8).
Two bits of good news are that there seems to be some catch-up occurring in vaccine uptake overall (including MMR) and we now have two MMR suppliers in the United States since GlaxoSmithKline’s MMR was recently approved by the Food and Drug Administration for persons over 1 year of age. Let’s all redouble our efforts at adding to the catch-up efforts.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The newly released global estimate is now 25 million children (2 million more than in 2020) missing scheduled vaccines. This continues to bode badly for multiple vaccine-preventable infections, but maybe the most for measles in 2022.
Specifically for measles vaccine, global two-dose coverage was only 71%. Coverage was less than 50% in 8 countries: Chad, Guinea, Samoa, North Korea, Central African Republic, Somalia, Angola, and South Sudan. These eight areas seem ripe for outbreaks this year and indeed Somalia is having an outbreak.
Overall, worldwide measles cases increased 79% in early 2022, compared with 2021. The top 10 countries for measles cases from November 2021 to April 2022, per the World Health Organization, include Nigeria, India, Soma Ethiopia, Pakistan, DR Congo, Afghanistan, Liberia, Cameroon, and Ivory Coast.
In the United States, we have been lucky so far with only 55 cases since the start of 2021. However, MMR two-dose coverage has dropped since the pandemic’s start. The list of U.S. areas with the lowest overall two-dose MMR coverage as of 2021 were D.C. (78.9%), Houston (93.7%), Idaho (86.5%), Wisconsin (87.2%), Maryland (87.6%), Georgia (88.5%), Kentucky (88.9%), Ohio (89.6%), and Minnesota (89.8%). Only 14 states had rates over the targeted 95% rate needed for community (herd) immunity against measles (MMWR Morb Mortal Wkly Rep. 2022;71:561-8).
Two bits of good news are that there seems to be some catch-up occurring in vaccine uptake overall (including MMR) and we now have two MMR suppliers in the United States since GlaxoSmithKline’s MMR was recently approved by the Food and Drug Administration for persons over 1 year of age. Let’s all redouble our efforts at adding to the catch-up efforts.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The newly released global estimate is now 25 million children (2 million more than in 2020) missing scheduled vaccines. This continues to bode badly for multiple vaccine-preventable infections, but maybe the most for measles in 2022.
Specifically for measles vaccine, global two-dose coverage was only 71%. Coverage was less than 50% in 8 countries: Chad, Guinea, Samoa, North Korea, Central African Republic, Somalia, Angola, and South Sudan. These eight areas seem ripe for outbreaks this year and indeed Somalia is having an outbreak.
Overall, worldwide measles cases increased 79% in early 2022, compared with 2021. The top 10 countries for measles cases from November 2021 to April 2022, per the World Health Organization, include Nigeria, India, Soma Ethiopia, Pakistan, DR Congo, Afghanistan, Liberia, Cameroon, and Ivory Coast.
In the United States, we have been lucky so far with only 55 cases since the start of 2021. However, MMR two-dose coverage has dropped since the pandemic’s start. The list of U.S. areas with the lowest overall two-dose MMR coverage as of 2021 were D.C. (78.9%), Houston (93.7%), Idaho (86.5%), Wisconsin (87.2%), Maryland (87.6%), Georgia (88.5%), Kentucky (88.9%), Ohio (89.6%), and Minnesota (89.8%). Only 14 states had rates over the targeted 95% rate needed for community (herd) immunity against measles (MMWR Morb Mortal Wkly Rep. 2022;71:561-8).
Two bits of good news are that there seems to be some catch-up occurring in vaccine uptake overall (including MMR) and we now have two MMR suppliers in the United States since GlaxoSmithKline’s MMR was recently approved by the Food and Drug Administration for persons over 1 year of age. Let’s all redouble our efforts at adding to the catch-up efforts.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Clinical Edge Journal Scan Commentary: Atopic Dermatitis May 2022
- Dupilumab is a subcutaneous injection therapy that inhibits the interleukin 4-receptor alpha subunit. It was approved in the United States for the treatment of adults with moderate to severe AD in 2017 and has since been approved for children and adolescents down to 6 years of age. Ungar and colleagues studied the effects of dupilumab on SARS-CoV-2 antibody responses in patients with moderate to severe AD. They previously found that dupilumab was associated with milder COVID-19 illness. In this study, they similarly found that dupilumab was associated with lower immunoglobulin G (IgG) antibody levels to SARS-CoV-2, consistent with less severe COVID-19 illness. Future studies are needed to confirm these results. However, these results reassure that taking dupilumab does not pose any major harms in regard to COVID-19 outcomes.
- My patients with AD ask me on an almost daily basis about whether they should get vaccinated for SARS-CoV-2. I recommended that my patients get vaccinated, based on data from vaccine studies. However, there has been a dearth of data on the efficacy and safety of SARS-CoV-2 specifically in AD patients. Kridin and colleagues performed a population-based cohort study including 77,682 adults with AD, of which 58,582 patients had completed two doses of the BioNTech-Pfizer SARS-CoV-2 mRNA vaccine. They found that patients with AD who received both vs no vaccine doses had significantly lower risk for COVID-19, hospitalization, and mortality. These are the best data to date in support of SARS-CoV-2 vaccination in patients with AD. Of note, there was no significant impact of immunosuppressive drugs on vaccine efficacy against COVID-19. However, previous studies in other immune-mediated disorders suggest that immunosuppressants may lower vaccine immune responses.1,2 Some authors have advocated for temporarily discontinuing immunosuppressive agents for 1-2 weeks before and after administering SARS-CoV-2 vaccines. Currently, there is insufficient evidence to make strong recommendations.
Numerous in utero and early-life risk factors for AD have been examined over the years. Maternal stress and depression have been considered as potential risk factors for AD in children.
- My research group showed a while back that depression during pregnancy and in the postpartum period was associated with higher likelihood of AD in children.3
- Kawaguchi and colleagues recently analyzed data from the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study in Japan, including 8377 mother-child dyads where the child had not developed AD by the age of 1 year. They found that mothers with vs without psychological distress in both prenatal and postnatal periods or even only in the postnatal period had significantly increased risk of their children developing AD at 1-2 years of age. It seems prudent that mothers try to minimize stress during pregnancy and postpartum, though, understandably, this is not always feasible. Additionally, children of mothers who experience a lot of stress during pregnancy or postpartum may benefit from closer surveillance for the development of AD and other atopic diseases.
Additional References
1. Dayam RM, Law JC, Goetbebuer RL, et al. Accelerated waning of immunity to SARS-CoV-2 mRNA vaccines in patients with immune mediated inflammatory diseases. JCI Insight. 2022 (Apr 26). Doi: 10.1172/jci.insight.159721 Source
2. Medeiros-Ribeiro AC, Bonfiglioli KR, Domiciano DS, et al. Distinct impact of DMARD combination and monotherapy in immunogenicity of an inactivated SARS-CoV-2 vaccine in rheumatoid arthritis. Ann Rheum Dis. 2022;81:710-719. Doi: 10.1136/annrheumdis-2021-221735 Source
3. McKenzie C, Silverberg JI. Maternal depression and atopic dermatitis in American children and adolescents. Dermatitis. 2020;31:75-80. Doi: 10.1097/DER.0000000000000548 Source
- Dupilumab is a subcutaneous injection therapy that inhibits the interleukin 4-receptor alpha subunit. It was approved in the United States for the treatment of adults with moderate to severe AD in 2017 and has since been approved for children and adolescents down to 6 years of age. Ungar and colleagues studied the effects of dupilumab on SARS-CoV-2 antibody responses in patients with moderate to severe AD. They previously found that dupilumab was associated with milder COVID-19 illness. In this study, they similarly found that dupilumab was associated with lower immunoglobulin G (IgG) antibody levels to SARS-CoV-2, consistent with less severe COVID-19 illness. Future studies are needed to confirm these results. However, these results reassure that taking dupilumab does not pose any major harms in regard to COVID-19 outcomes.
- My patients with AD ask me on an almost daily basis about whether they should get vaccinated for SARS-CoV-2. I recommended that my patients get vaccinated, based on data from vaccine studies. However, there has been a dearth of data on the efficacy and safety of SARS-CoV-2 specifically in AD patients. Kridin and colleagues performed a population-based cohort study including 77,682 adults with AD, of which 58,582 patients had completed two doses of the BioNTech-Pfizer SARS-CoV-2 mRNA vaccine. They found that patients with AD who received both vs no vaccine doses had significantly lower risk for COVID-19, hospitalization, and mortality. These are the best data to date in support of SARS-CoV-2 vaccination in patients with AD. Of note, there was no significant impact of immunosuppressive drugs on vaccine efficacy against COVID-19. However, previous studies in other immune-mediated disorders suggest that immunosuppressants may lower vaccine immune responses.1,2 Some authors have advocated for temporarily discontinuing immunosuppressive agents for 1-2 weeks before and after administering SARS-CoV-2 vaccines. Currently, there is insufficient evidence to make strong recommendations.
Numerous in utero and early-life risk factors for AD have been examined over the years. Maternal stress and depression have been considered as potential risk factors for AD in children.
- My research group showed a while back that depression during pregnancy and in the postpartum period was associated with higher likelihood of AD in children.3
- Kawaguchi and colleagues recently analyzed data from the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study in Japan, including 8377 mother-child dyads where the child had not developed AD by the age of 1 year. They found that mothers with vs without psychological distress in both prenatal and postnatal periods or even only in the postnatal period had significantly increased risk of their children developing AD at 1-2 years of age. It seems prudent that mothers try to minimize stress during pregnancy and postpartum, though, understandably, this is not always feasible. Additionally, children of mothers who experience a lot of stress during pregnancy or postpartum may benefit from closer surveillance for the development of AD and other atopic diseases.
Additional References
1. Dayam RM, Law JC, Goetbebuer RL, et al. Accelerated waning of immunity to SARS-CoV-2 mRNA vaccines in patients with immune mediated inflammatory diseases. JCI Insight. 2022 (Apr 26). Doi: 10.1172/jci.insight.159721 Source
2. Medeiros-Ribeiro AC, Bonfiglioli KR, Domiciano DS, et al. Distinct impact of DMARD combination and monotherapy in immunogenicity of an inactivated SARS-CoV-2 vaccine in rheumatoid arthritis. Ann Rheum Dis. 2022;81:710-719. Doi: 10.1136/annrheumdis-2021-221735 Source
3. McKenzie C, Silverberg JI. Maternal depression and atopic dermatitis in American children and adolescents. Dermatitis. 2020;31:75-80. Doi: 10.1097/DER.0000000000000548 Source
- Dupilumab is a subcutaneous injection therapy that inhibits the interleukin 4-receptor alpha subunit. It was approved in the United States for the treatment of adults with moderate to severe AD in 2017 and has since been approved for children and adolescents down to 6 years of age. Ungar and colleagues studied the effects of dupilumab on SARS-CoV-2 antibody responses in patients with moderate to severe AD. They previously found that dupilumab was associated with milder COVID-19 illness. In this study, they similarly found that dupilumab was associated with lower immunoglobulin G (IgG) antibody levels to SARS-CoV-2, consistent with less severe COVID-19 illness. Future studies are needed to confirm these results. However, these results reassure that taking dupilumab does not pose any major harms in regard to COVID-19 outcomes.
- My patients with AD ask me on an almost daily basis about whether they should get vaccinated for SARS-CoV-2. I recommended that my patients get vaccinated, based on data from vaccine studies. However, there has been a dearth of data on the efficacy and safety of SARS-CoV-2 specifically in AD patients. Kridin and colleagues performed a population-based cohort study including 77,682 adults with AD, of which 58,582 patients had completed two doses of the BioNTech-Pfizer SARS-CoV-2 mRNA vaccine. They found that patients with AD who received both vs no vaccine doses had significantly lower risk for COVID-19, hospitalization, and mortality. These are the best data to date in support of SARS-CoV-2 vaccination in patients with AD. Of note, there was no significant impact of immunosuppressive drugs on vaccine efficacy against COVID-19. However, previous studies in other immune-mediated disorders suggest that immunosuppressants may lower vaccine immune responses.1,2 Some authors have advocated for temporarily discontinuing immunosuppressive agents for 1-2 weeks before and after administering SARS-CoV-2 vaccines. Currently, there is insufficient evidence to make strong recommendations.
Numerous in utero and early-life risk factors for AD have been examined over the years. Maternal stress and depression have been considered as potential risk factors for AD in children.
- My research group showed a while back that depression during pregnancy and in the postpartum period was associated with higher likelihood of AD in children.3
- Kawaguchi and colleagues recently analyzed data from the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study in Japan, including 8377 mother-child dyads where the child had not developed AD by the age of 1 year. They found that mothers with vs without psychological distress in both prenatal and postnatal periods or even only in the postnatal period had significantly increased risk of their children developing AD at 1-2 years of age. It seems prudent that mothers try to minimize stress during pregnancy and postpartum, though, understandably, this is not always feasible. Additionally, children of mothers who experience a lot of stress during pregnancy or postpartum may benefit from closer surveillance for the development of AD and other atopic diseases.
Additional References
1. Dayam RM, Law JC, Goetbebuer RL, et al. Accelerated waning of immunity to SARS-CoV-2 mRNA vaccines in patients with immune mediated inflammatory diseases. JCI Insight. 2022 (Apr 26). Doi: 10.1172/jci.insight.159721 Source
2. Medeiros-Ribeiro AC, Bonfiglioli KR, Domiciano DS, et al. Distinct impact of DMARD combination and monotherapy in immunogenicity of an inactivated SARS-CoV-2 vaccine in rheumatoid arthritis. Ann Rheum Dis. 2022;81:710-719. Doi: 10.1136/annrheumdis-2021-221735 Source
3. McKenzie C, Silverberg JI. Maternal depression and atopic dermatitis in American children and adolescents. Dermatitis. 2020;31:75-80. Doi: 10.1097/DER.0000000000000548 Source
Paxlovid doesn’t prevent infection in households, Pfizer says
Paxlovid works as a treatment for COVID-19 but not as a preventive measure, particularly if you’ve been exposed to the coronavirus through a household member who is infected, according to a new announcement from Pfizer.
In a clinical trial, the oral antiviral tablets were tested for postexposure prophylactic use, or tested for how well they prevented a coronavirus infection in people exposed to the virus. Paxlovid somewhat reduced the risk of infection, but the results weren’t statistically significant.
“We designed the clinical development program for Paxlovid to be comprehensive and ambitious with the aim of being able to help combat COVID-19 in a very broad population of patients,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the announcement.
“While we are disappointed in the outcome of this particular study, these results do not impact the strong efficacy and safety data we’ve observed in our earlier trial for the treatment of COVID-19 patients at high risk of developing severe illness,” he said.
The trial included nearly 3,000 adults who were living with someone who recently tested positive for COVID-19 and had symptoms. The people in the trial, who tested negative and didn’t have symptoms, were given either Paxlovid twice daily for 5 or 10 days or a placebo. The study recruitment began in September 2021 and was completed during the peak of the Omicron wave.
Those who took the 5-day course of Paxlovid were found to be 32% less likely to become infected than the placebo group. Those who took the 10-day treatment had a 37% risk reduction. But the results weren’t statistically significant and may have been because of chance.
“Traditionally, it’s been difficult to use small-molecule antivirals for true prophylaxis because the biology of treating infection is different from the biology of preventing infection,” Daniel Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, told STAT News.
He also noted that the Omicron variant could have played a role.
“That hyperinfectiousness probably makes it more difficult to prevent infections,” Dr. Barouch said.
The safety data was consistent with that of previous studies, Pfizer said, which found that the treatment was about 90% effective at preventing hospitalization or death in COVID-19 patients with a high risk of severe illness if the pills were taken for 5 days soon after symptoms started.
Paxlovid is approved or authorized for conditional or emergency use in more than 60 countries to treat high-risk COVID-19 patients, Pfizer said. In the United States, the drug is authorized for emergency use for the treatment of mild to moderate COVID-19 in those aged 12 and older who face high risks for severe disease, hospitalization, or death.
The full study data will be released in coming months and submitted to a peer-reviewed publication, the company said. More details are on the ClinicalTrials.gov website (NCT05047601).
A version of this article first appeared on WebMD.com.
Paxlovid works as a treatment for COVID-19 but not as a preventive measure, particularly if you’ve been exposed to the coronavirus through a household member who is infected, according to a new announcement from Pfizer.
In a clinical trial, the oral antiviral tablets were tested for postexposure prophylactic use, or tested for how well they prevented a coronavirus infection in people exposed to the virus. Paxlovid somewhat reduced the risk of infection, but the results weren’t statistically significant.
“We designed the clinical development program for Paxlovid to be comprehensive and ambitious with the aim of being able to help combat COVID-19 in a very broad population of patients,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the announcement.
“While we are disappointed in the outcome of this particular study, these results do not impact the strong efficacy and safety data we’ve observed in our earlier trial for the treatment of COVID-19 patients at high risk of developing severe illness,” he said.
The trial included nearly 3,000 adults who were living with someone who recently tested positive for COVID-19 and had symptoms. The people in the trial, who tested negative and didn’t have symptoms, were given either Paxlovid twice daily for 5 or 10 days or a placebo. The study recruitment began in September 2021 and was completed during the peak of the Omicron wave.
Those who took the 5-day course of Paxlovid were found to be 32% less likely to become infected than the placebo group. Those who took the 10-day treatment had a 37% risk reduction. But the results weren’t statistically significant and may have been because of chance.
“Traditionally, it’s been difficult to use small-molecule antivirals for true prophylaxis because the biology of treating infection is different from the biology of preventing infection,” Daniel Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, told STAT News.
He also noted that the Omicron variant could have played a role.
“That hyperinfectiousness probably makes it more difficult to prevent infections,” Dr. Barouch said.
The safety data was consistent with that of previous studies, Pfizer said, which found that the treatment was about 90% effective at preventing hospitalization or death in COVID-19 patients with a high risk of severe illness if the pills were taken for 5 days soon after symptoms started.
Paxlovid is approved or authorized for conditional or emergency use in more than 60 countries to treat high-risk COVID-19 patients, Pfizer said. In the United States, the drug is authorized for emergency use for the treatment of mild to moderate COVID-19 in those aged 12 and older who face high risks for severe disease, hospitalization, or death.
The full study data will be released in coming months and submitted to a peer-reviewed publication, the company said. More details are on the ClinicalTrials.gov website (NCT05047601).
A version of this article first appeared on WebMD.com.
Paxlovid works as a treatment for COVID-19 but not as a preventive measure, particularly if you’ve been exposed to the coronavirus through a household member who is infected, according to a new announcement from Pfizer.
In a clinical trial, the oral antiviral tablets were tested for postexposure prophylactic use, or tested for how well they prevented a coronavirus infection in people exposed to the virus. Paxlovid somewhat reduced the risk of infection, but the results weren’t statistically significant.
“We designed the clinical development program for Paxlovid to be comprehensive and ambitious with the aim of being able to help combat COVID-19 in a very broad population of patients,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the announcement.
“While we are disappointed in the outcome of this particular study, these results do not impact the strong efficacy and safety data we’ve observed in our earlier trial for the treatment of COVID-19 patients at high risk of developing severe illness,” he said.
The trial included nearly 3,000 adults who were living with someone who recently tested positive for COVID-19 and had symptoms. The people in the trial, who tested negative and didn’t have symptoms, were given either Paxlovid twice daily for 5 or 10 days or a placebo. The study recruitment began in September 2021 and was completed during the peak of the Omicron wave.
Those who took the 5-day course of Paxlovid were found to be 32% less likely to become infected than the placebo group. Those who took the 10-day treatment had a 37% risk reduction. But the results weren’t statistically significant and may have been because of chance.
“Traditionally, it’s been difficult to use small-molecule antivirals for true prophylaxis because the biology of treating infection is different from the biology of preventing infection,” Daniel Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, told STAT News.
He also noted that the Omicron variant could have played a role.
“That hyperinfectiousness probably makes it more difficult to prevent infections,” Dr. Barouch said.
The safety data was consistent with that of previous studies, Pfizer said, which found that the treatment was about 90% effective at preventing hospitalization or death in COVID-19 patients with a high risk of severe illness if the pills were taken for 5 days soon after symptoms started.
Paxlovid is approved or authorized for conditional or emergency use in more than 60 countries to treat high-risk COVID-19 patients, Pfizer said. In the United States, the drug is authorized for emergency use for the treatment of mild to moderate COVID-19 in those aged 12 and older who face high risks for severe disease, hospitalization, or death.
The full study data will be released in coming months and submitted to a peer-reviewed publication, the company said. More details are on the ClinicalTrials.gov website (NCT05047601).
A version of this article first appeared on WebMD.com.
Higher ‘chemical restraint’ rates in Black psych patients in the ED
Black patients presenting with psychiatric disorders to hospital emergency departments across the United States have significantly higher rates of chemical restraint than their White counterparts, new research shows.
The investigators also found White patients were more likely to receive chemical sedation at hospitals with a higher proportion of Black patients – a finding that suggests hospital demographics influence practice patterns and that structural racism may be a root cause.
“There is a large disparity in the rates at which patients who presented to EDs nationally in the United States are restrained by race. You are 63% more likely, for the same set of chief complaints, to be chemically sedated if you are Black versus if you’re White,” senior investigator Ari Friedman, MD, PhD, an assistant professor of emergency medicine, and medical ethics and health policy, University of Pennsylvania, Philadelphia, told this news organization.
“The major mediator of that difference is the institution you are at – hospitals that primarily serve Black patients are more likely to chemically sedate their patients for these chief complaints – including White patients. So, it’s mediated by the practice pattern and environment,” Dr. Friedman added.
The study was published in the May issue of Annals of Epidemiology.
First large-scale study
Chemical sedation, also known as chemical restraint, is used to calm and help protect patients from harming themselves or others. Previous research on racial differences in the care of ED psychiatric patients with agitation suggests that there may be treatment disparities.
“Previous research from single institutions [has] shown that Black patients are more likely than White patients to be physically restrained, and this has been shown to be true among adult patients and pediatric patients,” lead author Utsha Khatri, MD, assistant professor of emergency medicine at the Icahn School of Medicine, New York, told this news organization.
Specifically, two single-institution studies within the last year revealed similar disparities, with higher rates of physical restraint for Black and Hispanic psychiatric patients in the ED. Another recent study showed an association with race, ethnicity, and pharmacological restraint use among pediatric patients presenting to the ED for mental health concerns.
“There has been work in psychiatry on disparities in this context, although there is less work in emergency departments,” said Dr. Friedman. “We looked across all U.S. EDs as opposed to within a single health system. The major trade-offs for us were that we weren’t able to observe restraint orders, which don’t find their way into national datasets, so we had to make some inferences based on the type of medications given.”
For the study the investigators analyzed data from 2008-2018 through the National Hospital Ambulatory Medical Survey (NHAMCS) database. They examined the association of race and the administration of chemical sedation, with either an antipsychotic or ketamine, in ED visits for psychiatric disorders. These were any visit where the reason for the visit was “symptoms referable to psychological and mental disorders.”
Of the 76.2 million total ED visits evaluated, the researchers found that Black patients presenting with a psychiatric disorder were significantly more likely to receive chemical sedation with antipsychotics or ketamine than White patients presenting with the same conditions (5.3% vs. 3.0%; P < .01). This difference remained significant when accounting for admission or transfer to psychiatric facilities.
Combatting the forces of racism
When researchers accounted for the percent of hospital population that was Black, they found that patient race no longer affected the likelihood of chemical restraint.
“We found the key source of this racial disparity in use of chemical sedation is accounted for by the fact that hospitals that treat a higher proportion of Black patients tend to use more sedation,” said Dr. Khatri.
“Our findings suggest that patients who present to hospitals that serve a patient population that is 60% Black would have [a] roughly 1.8 times likelihood of getting chemically sedated, compared with a hospital that serves a population that is 10% Black,” she added.
“When a hospital has fewer resources, they often don’t have the staff or time to de-escalate a patient in distress and can have to resort to chemical sedation more quickly than a hospital with ample staff and resources,” said Dr. Friedman in a release.
Dr. Khatri added that the study highlights the need to combat the forces of racism by focusing not just on provider bias but by addressing the “underlying structural issues that lead to Black patients getting worse care based on where they live.”
“Hospitals have unequal distribution of resources and quality, largely patterned on the racial makeup of their patients. Dedicated training and funding for de-escalation techniques as well as sufficient staffing and availability of outpatient mental health care may help keep both patients and staff safe by reducing the use of physical restraint and chemical sedation in appropriate circumstances,” said Dr. Khatri.
Dr. Friedman noted that there will always be a need for restraint use to facilitate rapid medical evaluation and stabilization of patients, but “we want to make it as humane, thoughtful, and rare as possible, and to have a large armamentarium of alternative strategies that can be equitably applied across emergency departments.”
Need for widespread, systemic change
Commenting on the findings, Regina James, MD, the American Psychiatric Association’s chief of Diversity and Health Equity and deputy medical director, said the large-scale study confirms the widespread existence of racial and ethnic disparities in patients with psychiatric disorders.
“This study and previous studies, not only in psychiatry but in other areas of medicine, all bring to light that there continues to be evidence of racial and ethnic disparities in health care, and this is consistent across a range of illnesses and health care services,” said Dr. James.
“It’s important that as we think about the solution, we also think about the etiology of the problem and the layers that have contributed to it – understanding, embracing, and recognizing that these differences didn’t just come up de novo. It’s policies, practices, and behaviors that got us to this point, and it’s going to be policies, practices, and behaviors that are going to move us away from this point,” noted Dr. James.
She added that future research should focus on further understanding which factors exacerbate agitation among patients and what resources directed at the hospital level, including de-escalation training, nursing staff, and waiting room crowding, may be effective at reducing the use of chemical sedation when clinically appropriate.
The authors and Dr. James report no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Black patients presenting with psychiatric disorders to hospital emergency departments across the United States have significantly higher rates of chemical restraint than their White counterparts, new research shows.
The investigators also found White patients were more likely to receive chemical sedation at hospitals with a higher proportion of Black patients – a finding that suggests hospital demographics influence practice patterns and that structural racism may be a root cause.
“There is a large disparity in the rates at which patients who presented to EDs nationally in the United States are restrained by race. You are 63% more likely, for the same set of chief complaints, to be chemically sedated if you are Black versus if you’re White,” senior investigator Ari Friedman, MD, PhD, an assistant professor of emergency medicine, and medical ethics and health policy, University of Pennsylvania, Philadelphia, told this news organization.
“The major mediator of that difference is the institution you are at – hospitals that primarily serve Black patients are more likely to chemically sedate their patients for these chief complaints – including White patients. So, it’s mediated by the practice pattern and environment,” Dr. Friedman added.
The study was published in the May issue of Annals of Epidemiology.
First large-scale study
Chemical sedation, also known as chemical restraint, is used to calm and help protect patients from harming themselves or others. Previous research on racial differences in the care of ED psychiatric patients with agitation suggests that there may be treatment disparities.
“Previous research from single institutions [has] shown that Black patients are more likely than White patients to be physically restrained, and this has been shown to be true among adult patients and pediatric patients,” lead author Utsha Khatri, MD, assistant professor of emergency medicine at the Icahn School of Medicine, New York, told this news organization.
Specifically, two single-institution studies within the last year revealed similar disparities, with higher rates of physical restraint for Black and Hispanic psychiatric patients in the ED. Another recent study showed an association with race, ethnicity, and pharmacological restraint use among pediatric patients presenting to the ED for mental health concerns.
“There has been work in psychiatry on disparities in this context, although there is less work in emergency departments,” said Dr. Friedman. “We looked across all U.S. EDs as opposed to within a single health system. The major trade-offs for us were that we weren’t able to observe restraint orders, which don’t find their way into national datasets, so we had to make some inferences based on the type of medications given.”
For the study the investigators analyzed data from 2008-2018 through the National Hospital Ambulatory Medical Survey (NHAMCS) database. They examined the association of race and the administration of chemical sedation, with either an antipsychotic or ketamine, in ED visits for psychiatric disorders. These were any visit where the reason for the visit was “symptoms referable to psychological and mental disorders.”
Of the 76.2 million total ED visits evaluated, the researchers found that Black patients presenting with a psychiatric disorder were significantly more likely to receive chemical sedation with antipsychotics or ketamine than White patients presenting with the same conditions (5.3% vs. 3.0%; P < .01). This difference remained significant when accounting for admission or transfer to psychiatric facilities.
Combatting the forces of racism
When researchers accounted for the percent of hospital population that was Black, they found that patient race no longer affected the likelihood of chemical restraint.
“We found the key source of this racial disparity in use of chemical sedation is accounted for by the fact that hospitals that treat a higher proportion of Black patients tend to use more sedation,” said Dr. Khatri.
“Our findings suggest that patients who present to hospitals that serve a patient population that is 60% Black would have [a] roughly 1.8 times likelihood of getting chemically sedated, compared with a hospital that serves a population that is 10% Black,” she added.
“When a hospital has fewer resources, they often don’t have the staff or time to de-escalate a patient in distress and can have to resort to chemical sedation more quickly than a hospital with ample staff and resources,” said Dr. Friedman in a release.
Dr. Khatri added that the study highlights the need to combat the forces of racism by focusing not just on provider bias but by addressing the “underlying structural issues that lead to Black patients getting worse care based on where they live.”
“Hospitals have unequal distribution of resources and quality, largely patterned on the racial makeup of their patients. Dedicated training and funding for de-escalation techniques as well as sufficient staffing and availability of outpatient mental health care may help keep both patients and staff safe by reducing the use of physical restraint and chemical sedation in appropriate circumstances,” said Dr. Khatri.
Dr. Friedman noted that there will always be a need for restraint use to facilitate rapid medical evaluation and stabilization of patients, but “we want to make it as humane, thoughtful, and rare as possible, and to have a large armamentarium of alternative strategies that can be equitably applied across emergency departments.”
Need for widespread, systemic change
Commenting on the findings, Regina James, MD, the American Psychiatric Association’s chief of Diversity and Health Equity and deputy medical director, said the large-scale study confirms the widespread existence of racial and ethnic disparities in patients with psychiatric disorders.
“This study and previous studies, not only in psychiatry but in other areas of medicine, all bring to light that there continues to be evidence of racial and ethnic disparities in health care, and this is consistent across a range of illnesses and health care services,” said Dr. James.
“It’s important that as we think about the solution, we also think about the etiology of the problem and the layers that have contributed to it – understanding, embracing, and recognizing that these differences didn’t just come up de novo. It’s policies, practices, and behaviors that got us to this point, and it’s going to be policies, practices, and behaviors that are going to move us away from this point,” noted Dr. James.
She added that future research should focus on further understanding which factors exacerbate agitation among patients and what resources directed at the hospital level, including de-escalation training, nursing staff, and waiting room crowding, may be effective at reducing the use of chemical sedation when clinically appropriate.
The authors and Dr. James report no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Black patients presenting with psychiatric disorders to hospital emergency departments across the United States have significantly higher rates of chemical restraint than their White counterparts, new research shows.
The investigators also found White patients were more likely to receive chemical sedation at hospitals with a higher proportion of Black patients – a finding that suggests hospital demographics influence practice patterns and that structural racism may be a root cause.
“There is a large disparity in the rates at which patients who presented to EDs nationally in the United States are restrained by race. You are 63% more likely, for the same set of chief complaints, to be chemically sedated if you are Black versus if you’re White,” senior investigator Ari Friedman, MD, PhD, an assistant professor of emergency medicine, and medical ethics and health policy, University of Pennsylvania, Philadelphia, told this news organization.
“The major mediator of that difference is the institution you are at – hospitals that primarily serve Black patients are more likely to chemically sedate their patients for these chief complaints – including White patients. So, it’s mediated by the practice pattern and environment,” Dr. Friedman added.
The study was published in the May issue of Annals of Epidemiology.
First large-scale study
Chemical sedation, also known as chemical restraint, is used to calm and help protect patients from harming themselves or others. Previous research on racial differences in the care of ED psychiatric patients with agitation suggests that there may be treatment disparities.
“Previous research from single institutions [has] shown that Black patients are more likely than White patients to be physically restrained, and this has been shown to be true among adult patients and pediatric patients,” lead author Utsha Khatri, MD, assistant professor of emergency medicine at the Icahn School of Medicine, New York, told this news organization.
Specifically, two single-institution studies within the last year revealed similar disparities, with higher rates of physical restraint for Black and Hispanic psychiatric patients in the ED. Another recent study showed an association with race, ethnicity, and pharmacological restraint use among pediatric patients presenting to the ED for mental health concerns.
“There has been work in psychiatry on disparities in this context, although there is less work in emergency departments,” said Dr. Friedman. “We looked across all U.S. EDs as opposed to within a single health system. The major trade-offs for us were that we weren’t able to observe restraint orders, which don’t find their way into national datasets, so we had to make some inferences based on the type of medications given.”
For the study the investigators analyzed data from 2008-2018 through the National Hospital Ambulatory Medical Survey (NHAMCS) database. They examined the association of race and the administration of chemical sedation, with either an antipsychotic or ketamine, in ED visits for psychiatric disorders. These were any visit where the reason for the visit was “symptoms referable to psychological and mental disorders.”
Of the 76.2 million total ED visits evaluated, the researchers found that Black patients presenting with a psychiatric disorder were significantly more likely to receive chemical sedation with antipsychotics or ketamine than White patients presenting with the same conditions (5.3% vs. 3.0%; P < .01). This difference remained significant when accounting for admission or transfer to psychiatric facilities.
Combatting the forces of racism
When researchers accounted for the percent of hospital population that was Black, they found that patient race no longer affected the likelihood of chemical restraint.
“We found the key source of this racial disparity in use of chemical sedation is accounted for by the fact that hospitals that treat a higher proportion of Black patients tend to use more sedation,” said Dr. Khatri.
“Our findings suggest that patients who present to hospitals that serve a patient population that is 60% Black would have [a] roughly 1.8 times likelihood of getting chemically sedated, compared with a hospital that serves a population that is 10% Black,” she added.
“When a hospital has fewer resources, they often don’t have the staff or time to de-escalate a patient in distress and can have to resort to chemical sedation more quickly than a hospital with ample staff and resources,” said Dr. Friedman in a release.
Dr. Khatri added that the study highlights the need to combat the forces of racism by focusing not just on provider bias but by addressing the “underlying structural issues that lead to Black patients getting worse care based on where they live.”
“Hospitals have unequal distribution of resources and quality, largely patterned on the racial makeup of their patients. Dedicated training and funding for de-escalation techniques as well as sufficient staffing and availability of outpatient mental health care may help keep both patients and staff safe by reducing the use of physical restraint and chemical sedation in appropriate circumstances,” said Dr. Khatri.
Dr. Friedman noted that there will always be a need for restraint use to facilitate rapid medical evaluation and stabilization of patients, but “we want to make it as humane, thoughtful, and rare as possible, and to have a large armamentarium of alternative strategies that can be equitably applied across emergency departments.”
Need for widespread, systemic change
Commenting on the findings, Regina James, MD, the American Psychiatric Association’s chief of Diversity and Health Equity and deputy medical director, said the large-scale study confirms the widespread existence of racial and ethnic disparities in patients with psychiatric disorders.
“This study and previous studies, not only in psychiatry but in other areas of medicine, all bring to light that there continues to be evidence of racial and ethnic disparities in health care, and this is consistent across a range of illnesses and health care services,” said Dr. James.
“It’s important that as we think about the solution, we also think about the etiology of the problem and the layers that have contributed to it – understanding, embracing, and recognizing that these differences didn’t just come up de novo. It’s policies, practices, and behaviors that got us to this point, and it’s going to be policies, practices, and behaviors that are going to move us away from this point,” noted Dr. James.
She added that future research should focus on further understanding which factors exacerbate agitation among patients and what resources directed at the hospital level, including de-escalation training, nursing staff, and waiting room crowding, may be effective at reducing the use of chemical sedation when clinically appropriate.
The authors and Dr. James report no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Cutting dementia risk in atrial fibrillation: Does rhythm control strategy matter?
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. But a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD alone at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings at the annual scientific sessions of the Heart Rhythm Society.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ont., who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler disclosed consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. But a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD alone at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings at the annual scientific sessions of the Heart Rhythm Society.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ont., who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler disclosed consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. But a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD alone at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., said in an interview. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings at the annual scientific sessions of the Heart Rhythm Society.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ont., who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler disclosed consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
HEART RHYTHM 2022
CDC reports first human case of H5 bird flu in the U.S.
A man who worked on a commercial poultry farm in Colorado has tested positive for avian influenza A(H5) virus, better known as H5 bird flu, the CDC announced on April 28.
This is the first case of H5 bird flu in humans in the United States and only the second case in the world, the CDC said in a news release. The first case was detected last December in a man who raised birds in the United Kingdom. That man had no symptoms.
The only symptom the man in Colorado reported was fatigue, the Colorado Department of Public Health and Environment (CDPHE) reported. He has recovered and is isolating and being treated with oseltamivir, an antiviral drug.
The CDC said the man was helping kill poultry that likely had the H5N1 bird flu.
He is a state prison inmate who was working on a commercial poultry farm in Montrose County in a prerelease employment program, the CDPHE said. The flock he was working with has been euthanized, and the response team and other inmates working on the farm were given protective equipment, the CDPHE said.
“Repeat testing on the person was negative for influenza,” the department said. “Because the person was in close contact with infected poultry, the virus may have been in the person’s nose without causing infection.”
This CDC said the case does not change the risk of bird flu for the general public, which is considered low. People who work with birds should continue to take safety precautions, such as wearing gloves when handling birds and avoiding birds that appear to be dead or ill, the CDC said.
“We want to reassure Coloradans that the risk to them is low,” said Rachel Herlihy, MD, state epidemiologist with the CDPHE. “I am grateful for the seamless collaboration between CDC, Department of Corrections, Department of Agriculture, and CDPHE, as we continue to monitor this virus and protect all Coloradans.”
The federal government says the H5N1 virus has been found in commercial and backyard birds in 29 states and in wild birds in 34 states since the first cases were detected in late 2021.
The CDC says it has tracked the health of 2,500 people exposed to birds infected with H5N1 and only found one case of human infection, in Colorado.
A version of this article first appeared on WebMD.com.
A man who worked on a commercial poultry farm in Colorado has tested positive for avian influenza A(H5) virus, better known as H5 bird flu, the CDC announced on April 28.
This is the first case of H5 bird flu in humans in the United States and only the second case in the world, the CDC said in a news release. The first case was detected last December in a man who raised birds in the United Kingdom. That man had no symptoms.
The only symptom the man in Colorado reported was fatigue, the Colorado Department of Public Health and Environment (CDPHE) reported. He has recovered and is isolating and being treated with oseltamivir, an antiviral drug.
The CDC said the man was helping kill poultry that likely had the H5N1 bird flu.
He is a state prison inmate who was working on a commercial poultry farm in Montrose County in a prerelease employment program, the CDPHE said. The flock he was working with has been euthanized, and the response team and other inmates working on the farm were given protective equipment, the CDPHE said.
“Repeat testing on the person was negative for influenza,” the department said. “Because the person was in close contact with infected poultry, the virus may have been in the person’s nose without causing infection.”
This CDC said the case does not change the risk of bird flu for the general public, which is considered low. People who work with birds should continue to take safety precautions, such as wearing gloves when handling birds and avoiding birds that appear to be dead or ill, the CDC said.
“We want to reassure Coloradans that the risk to them is low,” said Rachel Herlihy, MD, state epidemiologist with the CDPHE. “I am grateful for the seamless collaboration between CDC, Department of Corrections, Department of Agriculture, and CDPHE, as we continue to monitor this virus and protect all Coloradans.”
The federal government says the H5N1 virus has been found in commercial and backyard birds in 29 states and in wild birds in 34 states since the first cases were detected in late 2021.
The CDC says it has tracked the health of 2,500 people exposed to birds infected with H5N1 and only found one case of human infection, in Colorado.
A version of this article first appeared on WebMD.com.
A man who worked on a commercial poultry farm in Colorado has tested positive for avian influenza A(H5) virus, better known as H5 bird flu, the CDC announced on April 28.
This is the first case of H5 bird flu in humans in the United States and only the second case in the world, the CDC said in a news release. The first case was detected last December in a man who raised birds in the United Kingdom. That man had no symptoms.
The only symptom the man in Colorado reported was fatigue, the Colorado Department of Public Health and Environment (CDPHE) reported. He has recovered and is isolating and being treated with oseltamivir, an antiviral drug.
The CDC said the man was helping kill poultry that likely had the H5N1 bird flu.
He is a state prison inmate who was working on a commercial poultry farm in Montrose County in a prerelease employment program, the CDPHE said. The flock he was working with has been euthanized, and the response team and other inmates working on the farm were given protective equipment, the CDPHE said.
“Repeat testing on the person was negative for influenza,” the department said. “Because the person was in close contact with infected poultry, the virus may have been in the person’s nose without causing infection.”
This CDC said the case does not change the risk of bird flu for the general public, which is considered low. People who work with birds should continue to take safety precautions, such as wearing gloves when handling birds and avoiding birds that appear to be dead or ill, the CDC said.
“We want to reassure Coloradans that the risk to them is low,” said Rachel Herlihy, MD, state epidemiologist with the CDPHE. “I am grateful for the seamless collaboration between CDC, Department of Corrections, Department of Agriculture, and CDPHE, as we continue to monitor this virus and protect all Coloradans.”
The federal government says the H5N1 virus has been found in commercial and backyard birds in 29 states and in wild birds in 34 states since the first cases were detected in late 2021.
The CDC says it has tracked the health of 2,500 people exposed to birds infected with H5N1 and only found one case of human infection, in Colorado.
A version of this article first appeared on WebMD.com.