User login
Fluctuating and persistent depressive symptoms in patients with atopic dermatitis
Key clinical point: Patients with atopic dermatitis (AD) experience a fluctuation in depression severity over time, with the likelihood of experiencing depressive symptoms being the highest in patients with severe AD.
Major finding: Among patients with ≥2 follow-up visits, most (49.46%) experienced a fluctuation in depression severity, whereas 45.65% experienced a persistent severity of depression. High Eczema Area Severity Index (adjusted odds ratio [aOR] 7.622; 95% CI 3.881-14.968) and itch (aOR 14.745; 95% CI 4.696-46.297) scores were strongly associated with difficulty in concentrating over time.
Study details: Findings are from a longitudinal, dermatology practice-based study including 695 adults with AD who were evaluated at baseline and at every 6-month follow-up visits.
Disclosures: This study was funded by the US Agency for Healthcare Research and Quality, the Dermatology Foundation, and Galderma. R Chavda and S Gabriel declared being employees of Galderma, and JI Silverberg declared serving as a consultant for Galderma.
Source: Chatrath S et al. Longitudinal course and predictors of depressive symptoms in atopic dermatitis. J Am Acad Dermatol. 2022 (May 9). Doi: 10.1016/j.jaad.2022.04.061
Key clinical point: Patients with atopic dermatitis (AD) experience a fluctuation in depression severity over time, with the likelihood of experiencing depressive symptoms being the highest in patients with severe AD.
Major finding: Among patients with ≥2 follow-up visits, most (49.46%) experienced a fluctuation in depression severity, whereas 45.65% experienced a persistent severity of depression. High Eczema Area Severity Index (adjusted odds ratio [aOR] 7.622; 95% CI 3.881-14.968) and itch (aOR 14.745; 95% CI 4.696-46.297) scores were strongly associated with difficulty in concentrating over time.
Study details: Findings are from a longitudinal, dermatology practice-based study including 695 adults with AD who were evaluated at baseline and at every 6-month follow-up visits.
Disclosures: This study was funded by the US Agency for Healthcare Research and Quality, the Dermatology Foundation, and Galderma. R Chavda and S Gabriel declared being employees of Galderma, and JI Silverberg declared serving as a consultant for Galderma.
Source: Chatrath S et al. Longitudinal course and predictors of depressive symptoms in atopic dermatitis. J Am Acad Dermatol. 2022 (May 9). Doi: 10.1016/j.jaad.2022.04.061
Key clinical point: Patients with atopic dermatitis (AD) experience a fluctuation in depression severity over time, with the likelihood of experiencing depressive symptoms being the highest in patients with severe AD.
Major finding: Among patients with ≥2 follow-up visits, most (49.46%) experienced a fluctuation in depression severity, whereas 45.65% experienced a persistent severity of depression. High Eczema Area Severity Index (adjusted odds ratio [aOR] 7.622; 95% CI 3.881-14.968) and itch (aOR 14.745; 95% CI 4.696-46.297) scores were strongly associated with difficulty in concentrating over time.
Study details: Findings are from a longitudinal, dermatology practice-based study including 695 adults with AD who were evaluated at baseline and at every 6-month follow-up visits.
Disclosures: This study was funded by the US Agency for Healthcare Research and Quality, the Dermatology Foundation, and Galderma. R Chavda and S Gabriel declared being employees of Galderma, and JI Silverberg declared serving as a consultant for Galderma.
Source: Chatrath S et al. Longitudinal course and predictors of depressive symptoms in atopic dermatitis. J Am Acad Dermatol. 2022 (May 9). Doi: 10.1016/j.jaad.2022.04.061
Hypertensive pregnancy disorders tied to double hypertension risk
Hypertensive disorders of pregnancy (HDP) are associated with a greater than twofold risk of developing hypertension a decade later, new research suggests.
Investigators prospectively studied patients who had and who had not experienced HDP 10 years earlier; most self-identified as Black. They found that those with a history of HDP had a 2.4-fold higher risk for new hypertension than those without such a history.
Patients who developed hypertension showed greater left ventricular (LV) remodeling (including greater relative wall thickness), worse diastolic function, more abnormal longitudinal strain, and higher effective arterial elastance than those without hypertension, regardless of the presence or absence of an HDP history.
“We know that patients with preeclampsia are at a higher risk for heart disease later in life, and it seems to be driven by the development of new hypertension,” lead author Lisa Levine, MD, MSCE, director, pregnancy and heart disease program, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
It is critically important to “study a more diverse population, including a larger percentage of Black patients, since HDP and CVD both disproportionately affect Black women,” Dr. Levine said. “And it is important to screen patients for hypertension, getting them into primary care for visits, getting them diagnosed sooner, and treating them early for hypertension.”
The study was published in the Journal of the American College of Cardiology.
Understudied population
HDP includes gestational hypertension and preeclampsia, Dr. Levine explained. “We already know that patients who have had preeclampsia are at higher risk for stroke, heart failure [HF], and myocardial infarction later in life,” she said. The goal of this study was to see whether, instead of waiting 20-30 years, they could look only 10 years later to see which patients would be at highest risk for future heart disease, Dr. Levine said.
In particular, it’s known that cardiovascular disease (CVD) and HDP “disproportionately affect Black women,” Dr. Levine continued. “What makes our study different from other studies is that we focused predominantly on the Black African American population, since it’s understudied and also at highest risk for preeclampsia and heart disease,” she said.
They set out to “evaluate differences in CV risk factors as well as subclinical CVD among a well-characterized group of racially diverse patients with and without a history of HDP 10 years earlier,” the authors state.
To investigate the question, the researchers performed a prospective, cross-sectional study between April 2016 and December 2019 of patients with and without a diagnosis of HDP during a previous pregnancy at least 10 years earlier (from 2005 to 2007). Patients were drawn from a parent cohort in a previously performed observational study of patients with preeclampsia or HDP and normotensive control subjects.
The current study focused on 135 patients (85% Black), 84 with a history of HDP and 51 without. Of the Black patients, 91.7% had a history of HDP, compared with 8.3% of the White patients.
During an in-person visit, the researchers assessed participants’ blood pressure and other clinical risk factors for CVD, including fasting glucose and lipids. They also used noninvasive means to measure cardiac and vascular structure and function.
Importance of routine screening
The risk for new hypertension was 2.4 times higher in patients with a history of HDP than in those without HDP, with stage 2 hypertension noted in 56.0% of patients with and in 23.5% without HDP (P < .001). This equates to a relative risk of 2.4 (95% confidence interval, 1.39-4.14), even after adjustment for race, maternal age, body mass index, and history of preterm birth.
“Importantly, 18% of patients with a history of HDP met criteria for a new diagnosis of hypertension identified through the study visit,” the authors report.
There were no differences in many cardiac measures (left ventricular (LV) structure, global longitudinal strain, diastolic function, arterial stiffness, or endothelial function) between patients with and without a history of HDP.
However, patients with chronic hypertension (CHTN), regardless of HDP history, had other cardiac abnormalities, including greater LV remodeling, worse diastolic function, and higher effective arterial elastance.
“The data regarding increased risk of hypertension after HDP is not a novel finding, however our cohort is unique in the high baseline rate of stage 2 hypertension, even among patients without a history of HDP,” the authors comment.
In fact, when they looked at the diagnosis of either stage 1 or stage 2 hypertension, they found that more than 80% of patients with and 60% of patients without a history of HDP had hypertension. Notably, among patients with a history of HDP, only 39% had a formal diagnosis of either stage 1 or stage 2 hypertension, further highlighting “the importance of routine screening for CHTN in this population,” they state.
“Further studies should evaluate the optimal time period to screen for postpartum hypertension and a monitoring plan for these at-risk women,” Dr. Levine added.
‘Opportunity of a lifetime’
Commenting for this news organization, Malamo Countouris, MD, MS, assistant professor of medicine and codirector, postpartum hypertension program, University of Pittsburgh Medical Center, said hypertension is “underrecognized and undertreated among young, premenopausal, Black women.”
Pregnancy “gives us a clue, through HDP, as to who is high risk to develop chronic hypertension and subsequent subclinical structural cardiac changes in the decade after delivery,” said Dr. Countouris, who was not involved with the study.
“The jury is still out on whether HDP contributes independently to cardiovascular changes in the years after delivery. Ongoing research is needed to clarify the unique or compounding contributions of pregnancy complications and hypertension,” she added.
In an accompanying editorial , Josephine Chou, MD, MS, director of cardio-obstetrics and codirector of maternal cardiology, Yale University, New Haven, Conn., called the study a “laudable contribution to understanding of HDP and hypertension within the first decade after pregnancy,” saying that it “paves the way for future efforts to improve postpartum CV care, enabling us to grasp this opportunity of a lifetime to ultimately reduce maternal and pregnancy-related morbidity and mortality.”
This study was supported by the National Institutes of Health, the National Heart, Lung, and Blood Institute, and the American Association of Obstetricians and Gynecologists Foundation. Dr. Levine reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Countouris reports receiving funding from the American Heart Association. Dr. Chou reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hypertensive disorders of pregnancy (HDP) are associated with a greater than twofold risk of developing hypertension a decade later, new research suggests.
Investigators prospectively studied patients who had and who had not experienced HDP 10 years earlier; most self-identified as Black. They found that those with a history of HDP had a 2.4-fold higher risk for new hypertension than those without such a history.
Patients who developed hypertension showed greater left ventricular (LV) remodeling (including greater relative wall thickness), worse diastolic function, more abnormal longitudinal strain, and higher effective arterial elastance than those without hypertension, regardless of the presence or absence of an HDP history.
“We know that patients with preeclampsia are at a higher risk for heart disease later in life, and it seems to be driven by the development of new hypertension,” lead author Lisa Levine, MD, MSCE, director, pregnancy and heart disease program, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
It is critically important to “study a more diverse population, including a larger percentage of Black patients, since HDP and CVD both disproportionately affect Black women,” Dr. Levine said. “And it is important to screen patients for hypertension, getting them into primary care for visits, getting them diagnosed sooner, and treating them early for hypertension.”
The study was published in the Journal of the American College of Cardiology.
Understudied population
HDP includes gestational hypertension and preeclampsia, Dr. Levine explained. “We already know that patients who have had preeclampsia are at higher risk for stroke, heart failure [HF], and myocardial infarction later in life,” she said. The goal of this study was to see whether, instead of waiting 20-30 years, they could look only 10 years later to see which patients would be at highest risk for future heart disease, Dr. Levine said.
In particular, it’s known that cardiovascular disease (CVD) and HDP “disproportionately affect Black women,” Dr. Levine continued. “What makes our study different from other studies is that we focused predominantly on the Black African American population, since it’s understudied and also at highest risk for preeclampsia and heart disease,” she said.
They set out to “evaluate differences in CV risk factors as well as subclinical CVD among a well-characterized group of racially diverse patients with and without a history of HDP 10 years earlier,” the authors state.
To investigate the question, the researchers performed a prospective, cross-sectional study between April 2016 and December 2019 of patients with and without a diagnosis of HDP during a previous pregnancy at least 10 years earlier (from 2005 to 2007). Patients were drawn from a parent cohort in a previously performed observational study of patients with preeclampsia or HDP and normotensive control subjects.
The current study focused on 135 patients (85% Black), 84 with a history of HDP and 51 without. Of the Black patients, 91.7% had a history of HDP, compared with 8.3% of the White patients.
During an in-person visit, the researchers assessed participants’ blood pressure and other clinical risk factors for CVD, including fasting glucose and lipids. They also used noninvasive means to measure cardiac and vascular structure and function.
Importance of routine screening
The risk for new hypertension was 2.4 times higher in patients with a history of HDP than in those without HDP, with stage 2 hypertension noted in 56.0% of patients with and in 23.5% without HDP (P < .001). This equates to a relative risk of 2.4 (95% confidence interval, 1.39-4.14), even after adjustment for race, maternal age, body mass index, and history of preterm birth.
“Importantly, 18% of patients with a history of HDP met criteria for a new diagnosis of hypertension identified through the study visit,” the authors report.
There were no differences in many cardiac measures (left ventricular (LV) structure, global longitudinal strain, diastolic function, arterial stiffness, or endothelial function) between patients with and without a history of HDP.
However, patients with chronic hypertension (CHTN), regardless of HDP history, had other cardiac abnormalities, including greater LV remodeling, worse diastolic function, and higher effective arterial elastance.
“The data regarding increased risk of hypertension after HDP is not a novel finding, however our cohort is unique in the high baseline rate of stage 2 hypertension, even among patients without a history of HDP,” the authors comment.
In fact, when they looked at the diagnosis of either stage 1 or stage 2 hypertension, they found that more than 80% of patients with and 60% of patients without a history of HDP had hypertension. Notably, among patients with a history of HDP, only 39% had a formal diagnosis of either stage 1 or stage 2 hypertension, further highlighting “the importance of routine screening for CHTN in this population,” they state.
“Further studies should evaluate the optimal time period to screen for postpartum hypertension and a monitoring plan for these at-risk women,” Dr. Levine added.
‘Opportunity of a lifetime’
Commenting for this news organization, Malamo Countouris, MD, MS, assistant professor of medicine and codirector, postpartum hypertension program, University of Pittsburgh Medical Center, said hypertension is “underrecognized and undertreated among young, premenopausal, Black women.”
Pregnancy “gives us a clue, through HDP, as to who is high risk to develop chronic hypertension and subsequent subclinical structural cardiac changes in the decade after delivery,” said Dr. Countouris, who was not involved with the study.
“The jury is still out on whether HDP contributes independently to cardiovascular changes in the years after delivery. Ongoing research is needed to clarify the unique or compounding contributions of pregnancy complications and hypertension,” she added.
In an accompanying editorial , Josephine Chou, MD, MS, director of cardio-obstetrics and codirector of maternal cardiology, Yale University, New Haven, Conn., called the study a “laudable contribution to understanding of HDP and hypertension within the first decade after pregnancy,” saying that it “paves the way for future efforts to improve postpartum CV care, enabling us to grasp this opportunity of a lifetime to ultimately reduce maternal and pregnancy-related morbidity and mortality.”
This study was supported by the National Institutes of Health, the National Heart, Lung, and Blood Institute, and the American Association of Obstetricians and Gynecologists Foundation. Dr. Levine reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Countouris reports receiving funding from the American Heart Association. Dr. Chou reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hypertensive disorders of pregnancy (HDP) are associated with a greater than twofold risk of developing hypertension a decade later, new research suggests.
Investigators prospectively studied patients who had and who had not experienced HDP 10 years earlier; most self-identified as Black. They found that those with a history of HDP had a 2.4-fold higher risk for new hypertension than those without such a history.
Patients who developed hypertension showed greater left ventricular (LV) remodeling (including greater relative wall thickness), worse diastolic function, more abnormal longitudinal strain, and higher effective arterial elastance than those without hypertension, regardless of the presence or absence of an HDP history.
“We know that patients with preeclampsia are at a higher risk for heart disease later in life, and it seems to be driven by the development of new hypertension,” lead author Lisa Levine, MD, MSCE, director, pregnancy and heart disease program, Hospital of the University of Pennsylvania, Philadelphia, told this news organization.
It is critically important to “study a more diverse population, including a larger percentage of Black patients, since HDP and CVD both disproportionately affect Black women,” Dr. Levine said. “And it is important to screen patients for hypertension, getting them into primary care for visits, getting them diagnosed sooner, and treating them early for hypertension.”
The study was published in the Journal of the American College of Cardiology.
Understudied population
HDP includes gestational hypertension and preeclampsia, Dr. Levine explained. “We already know that patients who have had preeclampsia are at higher risk for stroke, heart failure [HF], and myocardial infarction later in life,” she said. The goal of this study was to see whether, instead of waiting 20-30 years, they could look only 10 years later to see which patients would be at highest risk for future heart disease, Dr. Levine said.
In particular, it’s known that cardiovascular disease (CVD) and HDP “disproportionately affect Black women,” Dr. Levine continued. “What makes our study different from other studies is that we focused predominantly on the Black African American population, since it’s understudied and also at highest risk for preeclampsia and heart disease,” she said.
They set out to “evaluate differences in CV risk factors as well as subclinical CVD among a well-characterized group of racially diverse patients with and without a history of HDP 10 years earlier,” the authors state.
To investigate the question, the researchers performed a prospective, cross-sectional study between April 2016 and December 2019 of patients with and without a diagnosis of HDP during a previous pregnancy at least 10 years earlier (from 2005 to 2007). Patients were drawn from a parent cohort in a previously performed observational study of patients with preeclampsia or HDP and normotensive control subjects.
The current study focused on 135 patients (85% Black), 84 with a history of HDP and 51 without. Of the Black patients, 91.7% had a history of HDP, compared with 8.3% of the White patients.
During an in-person visit, the researchers assessed participants’ blood pressure and other clinical risk factors for CVD, including fasting glucose and lipids. They also used noninvasive means to measure cardiac and vascular structure and function.
Importance of routine screening
The risk for new hypertension was 2.4 times higher in patients with a history of HDP than in those without HDP, with stage 2 hypertension noted in 56.0% of patients with and in 23.5% without HDP (P < .001). This equates to a relative risk of 2.4 (95% confidence interval, 1.39-4.14), even after adjustment for race, maternal age, body mass index, and history of preterm birth.
“Importantly, 18% of patients with a history of HDP met criteria for a new diagnosis of hypertension identified through the study visit,” the authors report.
There were no differences in many cardiac measures (left ventricular (LV) structure, global longitudinal strain, diastolic function, arterial stiffness, or endothelial function) between patients with and without a history of HDP.
However, patients with chronic hypertension (CHTN), regardless of HDP history, had other cardiac abnormalities, including greater LV remodeling, worse diastolic function, and higher effective arterial elastance.
“The data regarding increased risk of hypertension after HDP is not a novel finding, however our cohort is unique in the high baseline rate of stage 2 hypertension, even among patients without a history of HDP,” the authors comment.
In fact, when they looked at the diagnosis of either stage 1 or stage 2 hypertension, they found that more than 80% of patients with and 60% of patients without a history of HDP had hypertension. Notably, among patients with a history of HDP, only 39% had a formal diagnosis of either stage 1 or stage 2 hypertension, further highlighting “the importance of routine screening for CHTN in this population,” they state.
“Further studies should evaluate the optimal time period to screen for postpartum hypertension and a monitoring plan for these at-risk women,” Dr. Levine added.
‘Opportunity of a lifetime’
Commenting for this news organization, Malamo Countouris, MD, MS, assistant professor of medicine and codirector, postpartum hypertension program, University of Pittsburgh Medical Center, said hypertension is “underrecognized and undertreated among young, premenopausal, Black women.”
Pregnancy “gives us a clue, through HDP, as to who is high risk to develop chronic hypertension and subsequent subclinical structural cardiac changes in the decade after delivery,” said Dr. Countouris, who was not involved with the study.
“The jury is still out on whether HDP contributes independently to cardiovascular changes in the years after delivery. Ongoing research is needed to clarify the unique or compounding contributions of pregnancy complications and hypertension,” she added.
In an accompanying editorial , Josephine Chou, MD, MS, director of cardio-obstetrics and codirector of maternal cardiology, Yale University, New Haven, Conn., called the study a “laudable contribution to understanding of HDP and hypertension within the first decade after pregnancy,” saying that it “paves the way for future efforts to improve postpartum CV care, enabling us to grasp this opportunity of a lifetime to ultimately reduce maternal and pregnancy-related morbidity and mortality.”
This study was supported by the National Institutes of Health, the National Heart, Lung, and Blood Institute, and the American Association of Obstetricians and Gynecologists Foundation. Dr. Levine reports no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Countouris reports receiving funding from the American Heart Association. Dr. Chou reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study provides consensus on lab monitoring during isotretinoin therapy
For generally ideally within a month prior to the start of treatment, and a second time at peak dose.
Other tests such as complete blood cell counts and basic metabolic panels as well as specific laboratory tests such as LDL and HDL cholesterol should not be routinely monitored.
Those are key conclusions from a Delphi consensus study that included 22 acne experts from five continents and was published in JAMA Dermatology.
“Our results apply findings from recent literature and are in accordance with recent studies that have recommended against excessive laboratory monitoring,” senior corresponding author Arash Mostaghimi, MD, MPA, MPH, and coauthors wrote. “For instance, several studies in both teenagers and adults have shown that routine complete blood cell count laboratory tests are unnecessary without suspicion of an underlying abnormality and that rare abnormalities, if present, either resolved or remained stable without clinical impact on treatment. Likewise, liver function tests and lipid panels ordered at baseline and after 2 months of therapy were deemed sufficient if the clinical context and results do not suggest potential abnormalities.”
The authors also noted that, while published acne management guidelines exist, such as the American Academy of Dermatology work group guidelines and the National Institute for Health and Care Excellence guideline, “the specific recommendations surrounding laboratory monitoring frequency are nonstandardized and often nonspecific.”
To establish a consensus for isotretinoin laboratory monitoring, Dr. Mostaghimi, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to administer four rounds of electronic surveys to 22 board-certified dermatologists between 2021 and 2022. The primary outcome measured was whether participants could reach consensus on key isotretinoin lab monitoring parameters. Responses that failed to reach a threshold of 70% indicated no consensus.
The surveyed dermatologists had been in practice for a mean of 23.7 years, 54.5% were female, 54.5% practiced in an academic setting, and 63.9% were based in North America. They reached consensus for checking ALT within a month prior to initiation (89.5%) and at peak dose (89.5%), but not checking monthly (76.2%) or after completing treatment (73.7%). They also reached consensus on checking triglycerides within a month prior to initiation (89.5%) and at peak dose (78.9%) but not to check monthly (84.2%) or after completing treatment (73.7%).
Meanwhile, consensus was achieved for not checking complete blood cell count or basic metabolic panel parameters at any point during isotretinoin treatment (all > 70%), as well as not checking gamma-glutamyl transferase (78.9%), bilirubin (81.0%), albumin (72.7%), total protein (72.7%), LDL cholesterol (73.7%), HDL cholesterol (73.7%), or C-reactive protein (77.3%).
“Additional research is required to determine best practices for laboratory measures that did not reach consensus,” the authors wrote. The study results “are intended to guide appropriate clinical decision-making,” they added. “Although our recommendations cannot replace clinical judgment based on the unique circumstances of individual patients, we believe they provide a framework for management of a typical, otherwise healthy patient being treated with isotretinoin for acne. More routine monitoring, or reduced monitoring, should be considered on a case-by-case basis accounting for the unique medical history, circumstances, and baseline abnormalities, if present, of each patient.”
“Practicing dermatologists, including myself, routinely check blood laboratory values during isotretinoin treatment,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “Even though just a small number of U.S.-based and international acne researchers were involved in this Delphi consensus statement, this article still makes us practicing clinicians feel more comfortable in checking fewer lab chemistries and also less frequently checking labs when we use isotretinoin.
“That said, I don’t think most of us are ready, because of legal reasons, to do that infrequent monitoring” during isotretinoin therapy, Dr. Green added. “I think most dermatologists do not routinely perform CBCs anymore, but we still feel obligated to check triglycerides and liver function more frequently” than recommended in the new study.
Dr. Mostaghimi reported receiving grants and personal fees from Pfizer, personal fees from Eli Lilly, personal fees and licensing from Concert, personal fees from Bioniz, holds equity and advisory board membership from Hims & Hers and Figure 1, personal fees from Digital Diagnostics, and personal fees from AbbVie outside the submitted work. Other authors reported serving as an adviser, a speaker consultant, investigator, and/or board member, or having received honoraria from different pharmaceutical companies; several authors had no disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For generally ideally within a month prior to the start of treatment, and a second time at peak dose.
Other tests such as complete blood cell counts and basic metabolic panels as well as specific laboratory tests such as LDL and HDL cholesterol should not be routinely monitored.
Those are key conclusions from a Delphi consensus study that included 22 acne experts from five continents and was published in JAMA Dermatology.
“Our results apply findings from recent literature and are in accordance with recent studies that have recommended against excessive laboratory monitoring,” senior corresponding author Arash Mostaghimi, MD, MPA, MPH, and coauthors wrote. “For instance, several studies in both teenagers and adults have shown that routine complete blood cell count laboratory tests are unnecessary without suspicion of an underlying abnormality and that rare abnormalities, if present, either resolved or remained stable without clinical impact on treatment. Likewise, liver function tests and lipid panels ordered at baseline and after 2 months of therapy were deemed sufficient if the clinical context and results do not suggest potential abnormalities.”
The authors also noted that, while published acne management guidelines exist, such as the American Academy of Dermatology work group guidelines and the National Institute for Health and Care Excellence guideline, “the specific recommendations surrounding laboratory monitoring frequency are nonstandardized and often nonspecific.”
To establish a consensus for isotretinoin laboratory monitoring, Dr. Mostaghimi, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to administer four rounds of electronic surveys to 22 board-certified dermatologists between 2021 and 2022. The primary outcome measured was whether participants could reach consensus on key isotretinoin lab monitoring parameters. Responses that failed to reach a threshold of 70% indicated no consensus.
The surveyed dermatologists had been in practice for a mean of 23.7 years, 54.5% were female, 54.5% practiced in an academic setting, and 63.9% were based in North America. They reached consensus for checking ALT within a month prior to initiation (89.5%) and at peak dose (89.5%), but not checking monthly (76.2%) or after completing treatment (73.7%). They also reached consensus on checking triglycerides within a month prior to initiation (89.5%) and at peak dose (78.9%) but not to check monthly (84.2%) or after completing treatment (73.7%).
Meanwhile, consensus was achieved for not checking complete blood cell count or basic metabolic panel parameters at any point during isotretinoin treatment (all > 70%), as well as not checking gamma-glutamyl transferase (78.9%), bilirubin (81.0%), albumin (72.7%), total protein (72.7%), LDL cholesterol (73.7%), HDL cholesterol (73.7%), or C-reactive protein (77.3%).
“Additional research is required to determine best practices for laboratory measures that did not reach consensus,” the authors wrote. The study results “are intended to guide appropriate clinical decision-making,” they added. “Although our recommendations cannot replace clinical judgment based on the unique circumstances of individual patients, we believe they provide a framework for management of a typical, otherwise healthy patient being treated with isotretinoin for acne. More routine monitoring, or reduced monitoring, should be considered on a case-by-case basis accounting for the unique medical history, circumstances, and baseline abnormalities, if present, of each patient.”
“Practicing dermatologists, including myself, routinely check blood laboratory values during isotretinoin treatment,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “Even though just a small number of U.S.-based and international acne researchers were involved in this Delphi consensus statement, this article still makes us practicing clinicians feel more comfortable in checking fewer lab chemistries and also less frequently checking labs when we use isotretinoin.
“That said, I don’t think most of us are ready, because of legal reasons, to do that infrequent monitoring” during isotretinoin therapy, Dr. Green added. “I think most dermatologists do not routinely perform CBCs anymore, but we still feel obligated to check triglycerides and liver function more frequently” than recommended in the new study.
Dr. Mostaghimi reported receiving grants and personal fees from Pfizer, personal fees from Eli Lilly, personal fees and licensing from Concert, personal fees from Bioniz, holds equity and advisory board membership from Hims & Hers and Figure 1, personal fees from Digital Diagnostics, and personal fees from AbbVie outside the submitted work. Other authors reported serving as an adviser, a speaker consultant, investigator, and/or board member, or having received honoraria from different pharmaceutical companies; several authors had no disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
For generally ideally within a month prior to the start of treatment, and a second time at peak dose.
Other tests such as complete blood cell counts and basic metabolic panels as well as specific laboratory tests such as LDL and HDL cholesterol should not be routinely monitored.
Those are key conclusions from a Delphi consensus study that included 22 acne experts from five continents and was published in JAMA Dermatology.
“Our results apply findings from recent literature and are in accordance with recent studies that have recommended against excessive laboratory monitoring,” senior corresponding author Arash Mostaghimi, MD, MPA, MPH, and coauthors wrote. “For instance, several studies in both teenagers and adults have shown that routine complete blood cell count laboratory tests are unnecessary without suspicion of an underlying abnormality and that rare abnormalities, if present, either resolved or remained stable without clinical impact on treatment. Likewise, liver function tests and lipid panels ordered at baseline and after 2 months of therapy were deemed sufficient if the clinical context and results do not suggest potential abnormalities.”
The authors also noted that, while published acne management guidelines exist, such as the American Academy of Dermatology work group guidelines and the National Institute for Health and Care Excellence guideline, “the specific recommendations surrounding laboratory monitoring frequency are nonstandardized and often nonspecific.”
To establish a consensus for isotretinoin laboratory monitoring, Dr. Mostaghimi, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to administer four rounds of electronic surveys to 22 board-certified dermatologists between 2021 and 2022. The primary outcome measured was whether participants could reach consensus on key isotretinoin lab monitoring parameters. Responses that failed to reach a threshold of 70% indicated no consensus.
The surveyed dermatologists had been in practice for a mean of 23.7 years, 54.5% were female, 54.5% practiced in an academic setting, and 63.9% were based in North America. They reached consensus for checking ALT within a month prior to initiation (89.5%) and at peak dose (89.5%), but not checking monthly (76.2%) or after completing treatment (73.7%). They also reached consensus on checking triglycerides within a month prior to initiation (89.5%) and at peak dose (78.9%) but not to check monthly (84.2%) or after completing treatment (73.7%).
Meanwhile, consensus was achieved for not checking complete blood cell count or basic metabolic panel parameters at any point during isotretinoin treatment (all > 70%), as well as not checking gamma-glutamyl transferase (78.9%), bilirubin (81.0%), albumin (72.7%), total protein (72.7%), LDL cholesterol (73.7%), HDL cholesterol (73.7%), or C-reactive protein (77.3%).
“Additional research is required to determine best practices for laboratory measures that did not reach consensus,” the authors wrote. The study results “are intended to guide appropriate clinical decision-making,” they added. “Although our recommendations cannot replace clinical judgment based on the unique circumstances of individual patients, we believe they provide a framework for management of a typical, otherwise healthy patient being treated with isotretinoin for acne. More routine monitoring, or reduced monitoring, should be considered on a case-by-case basis accounting for the unique medical history, circumstances, and baseline abnormalities, if present, of each patient.”
“Practicing dermatologists, including myself, routinely check blood laboratory values during isotretinoin treatment,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “Even though just a small number of U.S.-based and international acne researchers were involved in this Delphi consensus statement, this article still makes us practicing clinicians feel more comfortable in checking fewer lab chemistries and also less frequently checking labs when we use isotretinoin.
“That said, I don’t think most of us are ready, because of legal reasons, to do that infrequent monitoring” during isotretinoin therapy, Dr. Green added. “I think most dermatologists do not routinely perform CBCs anymore, but we still feel obligated to check triglycerides and liver function more frequently” than recommended in the new study.
Dr. Mostaghimi reported receiving grants and personal fees from Pfizer, personal fees from Eli Lilly, personal fees and licensing from Concert, personal fees from Bioniz, holds equity and advisory board membership from Hims & Hers and Figure 1, personal fees from Digital Diagnostics, and personal fees from AbbVie outside the submitted work. Other authors reported serving as an adviser, a speaker consultant, investigator, and/or board member, or having received honoraria from different pharmaceutical companies; several authors had no disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM JAMA DERMATOLOGY
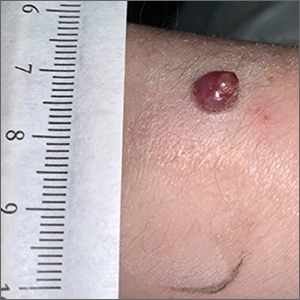
Bleeding arm lesion
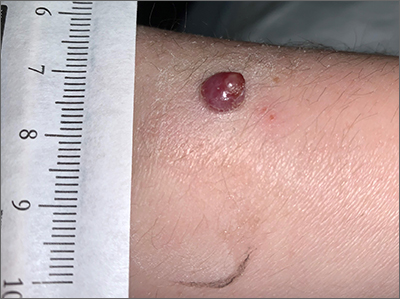
Pyogenic granulomas (PGs), also called lobular capillary hemangiomas, manifest as friable, moist or glistening, papules. PGs are a benign vascular proliferation. They often have a collarette, which is subtle in this lesion, and they bleed with minimal trauma. They are commonly seen on the gingiva during pregnancy, the umbilical area in newborns, or at sites of trauma.
Since PGs often occur during pregnancy, it’s been suggested that their development is related to hormonal changes.1 It’s also been suggested that PGs are the result of an abnormal hypertrophic healing response, as they can occur in men, infants (at the umbilical stump), and even within blood vessels.1
Although benign and painless, PGs are usually hard to ignore due to their raised appearance, tendency to bleed, and the low likelihood that they will resolve on their own. There are multiple physical treatment options available, including excision with primary closure, curettage followed by electrodessication, laser treatment, and cryosurgery. Topical therapies include timolol (a beta-blocker that has been used successfully with congenital hemangiomas), imiquimod, and trichloroacetic acid.1 These topical medications do not require any anesthetic, which may make them an appealing option for children. Unfortunately, topical medications require multiple applications over a period of 2 or more weeks.
In this case, the lesion was shaved off and sent out to pathology to rule out amelanotic melanoma. The pathology for this patient confirmed PG. Immediately following the lesion’s removal, the physician performed 2 cycles of curettage and electrodessication. Thus, the patient’s treatment was completed on the same day as her evaluation.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Plachouri KM, Georgiou S. Therapeutic approaches to pyogenic granuloma: an updated review. Int J Dermatol. 2019;58:642-648. doi: 10.1111/ijd.14268

Pyogenic granulomas (PGs), also called lobular capillary hemangiomas, manifest as friable, moist or glistening, papules. PGs are a benign vascular proliferation. They often have a collarette, which is subtle in this lesion, and they bleed with minimal trauma. They are commonly seen on the gingiva during pregnancy, the umbilical area in newborns, or at sites of trauma.
Since PGs often occur during pregnancy, it’s been suggested that their development is related to hormonal changes.1 It’s also been suggested that PGs are the result of an abnormal hypertrophic healing response, as they can occur in men, infants (at the umbilical stump), and even within blood vessels.1
Although benign and painless, PGs are usually hard to ignore due to their raised appearance, tendency to bleed, and the low likelihood that they will resolve on their own. There are multiple physical treatment options available, including excision with primary closure, curettage followed by electrodessication, laser treatment, and cryosurgery. Topical therapies include timolol (a beta-blocker that has been used successfully with congenital hemangiomas), imiquimod, and trichloroacetic acid.1 These topical medications do not require any anesthetic, which may make them an appealing option for children. Unfortunately, topical medications require multiple applications over a period of 2 or more weeks.
In this case, the lesion was shaved off and sent out to pathology to rule out amelanotic melanoma. The pathology for this patient confirmed PG. Immediately following the lesion’s removal, the physician performed 2 cycles of curettage and electrodessication. Thus, the patient’s treatment was completed on the same day as her evaluation.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Pyogenic granulomas (PGs), also called lobular capillary hemangiomas, manifest as friable, moist or glistening, papules. PGs are a benign vascular proliferation. They often have a collarette, which is subtle in this lesion, and they bleed with minimal trauma. They are commonly seen on the gingiva during pregnancy, the umbilical area in newborns, or at sites of trauma.
Since PGs often occur during pregnancy, it’s been suggested that their development is related to hormonal changes.1 It’s also been suggested that PGs are the result of an abnormal hypertrophic healing response, as they can occur in men, infants (at the umbilical stump), and even within blood vessels.1
Although benign and painless, PGs are usually hard to ignore due to their raised appearance, tendency to bleed, and the low likelihood that they will resolve on their own. There are multiple physical treatment options available, including excision with primary closure, curettage followed by electrodessication, laser treatment, and cryosurgery. Topical therapies include timolol (a beta-blocker that has been used successfully with congenital hemangiomas), imiquimod, and trichloroacetic acid.1 These topical medications do not require any anesthetic, which may make them an appealing option for children. Unfortunately, topical medications require multiple applications over a period of 2 or more weeks.
In this case, the lesion was shaved off and sent out to pathology to rule out amelanotic melanoma. The pathology for this patient confirmed PG. Immediately following the lesion’s removal, the physician performed 2 cycles of curettage and electrodessication. Thus, the patient’s treatment was completed on the same day as her evaluation.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Plachouri KM, Georgiou S. Therapeutic approaches to pyogenic granuloma: an updated review. Int J Dermatol. 2019;58:642-648. doi: 10.1111/ijd.14268
1. Plachouri KM, Georgiou S. Therapeutic approaches to pyogenic granuloma: an updated review. Int J Dermatol. 2019;58:642-648. doi: 10.1111/ijd.14268
This insurance agent thinks disability insurance deserves a rebrand, and he's a doctor
If you already have disability insurance, keep reading as well. I have a great tip for you from personal experience that made a difference in the job I selected.
Let’s start with an important rebrand for “disability insurance.” What does it protect? Income! Car insurance is not called crash insurance. House insurance is not called burnt house insurance. And unlike a car or a house, it protects an asset with 10-20 times as much value as a million-dollar house.
So, let’s call it what it is: “income protection insurance.”
It’s always a bit nerdy when I talk about how much I appreciate insurance that protects lifelong income. I often make an argument that it is simply one of the best products that exists, especially for high-income earners with lots of debt. Many of us doctors are in that category and are not even slightly jealous of our friends whose parents paid for school (I’m looking at you not-her-real-name-Mary).
Disability is not the catchiest name for a product, but it is more pronounceable than “ophthalmology” and way easier to spell. This is my specialty, and I can’t believe we still haven’t gone with “eye surgeon,” but I digress.
So, let’s rebrand “disability insurance” for the sake of clarity:
I personally like to think of it as a monthly subscription for a soft landing in a worst-case scenario. Call me a millennial, but it just goes down smoother in my mind as a subscription a la Netflix ... and the four other streaming services that someone gave me a password to – if you’re a 55-year-old GI specialist, I know you’re on the Spotify family plan, too. No judgment from me.
So, for $15, you get a bunch of movies with Netflix, and, for $150-$300, you cover a lifetime of income. That’s a pretty decent service even without “The Office.”
Disability insurance often covers at least $15-$20 million dollars over a lifetime of earnings for only 1%-2% of your salary per year.
But I’ll pause here. The numbers are irrelevant if you never get the insurance.
I have one goal for this article, and it is simply to try to help you break down that procrastination habit we all have. I will have added immense value to at least one family’s life if you go and get a policy this week that saves your family from substantial loss of income. This is why I love insurance.
Doctors sacrifice essential life steps to get through training. But we are not alone in that.
Tim Kasser, PhD, puts it well when he said: “We live in a machine that is designed to get us to neglect what is important about life.” Here he is talking about relationships, but securing financial protection is loving to those closest to us.
So, what holds us back from taking a seemingly easy step like locking in disability insurance early in training?
Is it the stress of residency? Studying for Step 1? Moving cities and finding a home during a housing crisis? Job change during COVID? Is it because we have already put it off so long that we don’t want to think about it?
Totally fair.
For all of us busy doctors, the necessity and obviousness of buying disability insurance, *ehem*, income protection insurance makes you feel like you can get to it when you get to it because you know you will, so ... what’s the rush?
Or, is it our desire to bet on ourselves, and every month that goes by without insurance is one less payment? Roll the dice! Woo!
The reason to not put off the important things in life
I will give you a few reasons of “the why of” how we can all benefit from disability insurance and the reason there is no benefit in waiting to get a policy.
But, most importantly, I want to talk to you about your life and why you are putting off a lot of important things.
That diet you’ve been wanting to start? Yep.
That ring you haven’t purchased? Maybe that!
That article you’ve been meaning to write for the GI journal? Yes, especially that.
Remember: Take a deep breath in and exhaaaaale.
So, why do we put off the important?
First, even though the “why” of purchasing income protection is a bit basic, I do find it helpful to have discrete reasons for accomplishing an important task.
Why get disability insurance at all?
Let’s look at the value we get out of covering our income.
Reason No. 1. It softens the landing in the event you have an illness. The stats on disability claims are heavily on the side of illness over accidents or trauma. As you know, many autoimmune conditions show up in the 20’s and 30’s, so those are the things your friends will have first.
Unfortunately, if you have a medical issue before you have a disability policy, you will either not have coverage for that specific condition or you will not be approved for insurance. Unlike health insurance, the company can afford to pay out policies because it is picky on who it is willing to cover. It tries to select healthy people, so apply when you are most healthy, if possible.
Reason No. 2. It’s cheap. When you compare with a $2 million policy for life insurance, it might cost $1,000-$2,000 or so per year for a term policy covering about 25 years. With disability insurance, you can cover about 10x as much for the same annual payment. One could easily make a case that if you do not have dependents, disability insurance should be your first stop even before life insurance. You are more likely to be disabled than to die when you’re in your thirties. Act accordingly.
(Please note for obvious reasons they don’t call life insurance “death insurance.” Disability insurance needs that same rebrand – I’m telling you!)
Reason No. 3. Unless you are independently wealthy, it will be nearly impossible to replace your income and live a similar lifestyle. Lock in the benefits of the work you have already accomplished, and lock in the coverage of ALL of your health while you are healthy.
Time to take action
As Elvis famously sang: “A little less conversation, a little more action please.”
Alright, so how do we get ourselves to ACT and get a policy to protect our income?
Tip No. 1. As doctors we often shoot for perfection. It’s no surprise, therefore, that we have an illusion that we need to find the “perfect policy.”
One of my friends is a great financial adviser, and he often tells me about first meetings with clients to create a long-lasting plan. Often, somewhere along the way when discussing risks of stocks going down and up, someone will ask, “Why don’t we pick one that is low risk but tends to go up in value?” Of course, the reality is that if it were that easy ... everyone would do it!
Fortunately, with disability insurance, the policies are fairly straight forward. You can skip the analysis paralysis with disability insurance by talking with an agent who consistently works with physicians. I enjoy talking policies and helping doctors protect their financial health, so I started selling policies shortly after residency because so many of my co-residents were making me nervous putting it off. Some I helped, and some put it off and are unable to get policies after health issues even just 3 years after residency.
Tip No. 2. Having a policy is better than not having one, and if you’re worried about getting the wrong one, just get two! Seriously, some companies let you split coverage between two and this can even increase the maximum coverage you can get later in life, too. Does it add cost? Surprisingly, it typically does not, and it does not make the agent more money either. In most cases it’s actually more work for them for the same amount of commission. Don’t be afraid to ask about this.
Tip No. 3. This is my hot tip for current policy owners: ask for the full version of your policy, and read the entire policy. I recently asked for my policy because I was doing some international work abroad and wanted to know if I could reside abroad if I made a disability claim. My policy stated that I would need to reside in the United States within 12 months of disability. I likely would do this in the event of disability, but it is quite important to know these aspects.
While reading the fine print, I found that a minimum number of work-hours per week (35 for my policy) was required to qualify for my physician-specific coverage. This was an important part of my job criteria when looking for a new position and is worth investigating for anyone considering part-time employment.
Tip No. 4. The obvious tip: The fear of failure gets a lot of perfectionists from even starting a task unless they know everything about it.
Just start.
That’s my go-to for overcoming fear of failure. You won’t fail. You just won’t. You will learn!
Pretend you are curious about it and try with any of these actionable steps:
- Google disability insurance.
- Email me at [email protected].
- Read an article on a doctor-based blog.
I personally geeked out on insurance so much in residency that I became an insurance agent. I am an independent broker, so I have no bias toward any particular policies (email me anytime even if just with questions). Personally, I believe in this product and the value of this type of insurance, and I would hate for anyone to not have coverage of their most valuable asset: lifelong income!
The steps of applying for disability insurance
Now you know all the great reasons to get going! What are the next steps?
No matter where you get your policy, you can expect the process to be fairly simple. If it’s not then shoot me an email and I’m happy to help chat and discuss further.
The general process is:
Step 1. Initial phone call or email: Chat with an agent to discuss your needs and situation. Immediately after, you can sign initial application documents with DocuSign. (20 minutes).
Step 2. Complete health questionnaire on the phone with the insurance company. (20-40 minutes).
Step 3. Sign the final documents and confirm physician-specific language in their policies. (20 minutes).
The whole application period typically lasts only 2-4 weeks from start to finish and, if you pay up front, you are covered from the moment you send in the check. If you don’t accept the policy, you even get the money back.
I genuinely enjoy talking with my colleagues from all over the world and learning about their lives and plans, so, if you have any questions, please do not hesitate to email me at [email protected]. Also, feel free to check out my mini-blog at curiousmd.com or listen to me chat with Jon Solitro, CFP, on his FinancialMD.com podcast. Similar to this article, it is fairly informal and covers real life, tough career decisions, and actionable financial planning tips.
If you made it to the end of this article, you are a perfectionist and should go back and read Tip No. 1.
Reference
The Context of Things. “We live in a machine that is designed to get us to neglect what’s important about life,” 2021 Aug 24.
Dr. Smith is an ophthalmologist and consultant with Advanced Eyecare Professionals, Grand Rapids, Mich., and founder of DigitalGlaucoma.com. He is cohost of The FinancialMD Show podcast. He is an insurance producer and assists clients with advising and decision-making related to disability insurance at FinancialMD.
If you already have disability insurance, keep reading as well. I have a great tip for you from personal experience that made a difference in the job I selected.
Let’s start with an important rebrand for “disability insurance.” What does it protect? Income! Car insurance is not called crash insurance. House insurance is not called burnt house insurance. And unlike a car or a house, it protects an asset with 10-20 times as much value as a million-dollar house.
So, let’s call it what it is: “income protection insurance.”
It’s always a bit nerdy when I talk about how much I appreciate insurance that protects lifelong income. I often make an argument that it is simply one of the best products that exists, especially for high-income earners with lots of debt. Many of us doctors are in that category and are not even slightly jealous of our friends whose parents paid for school (I’m looking at you not-her-real-name-Mary).
Disability is not the catchiest name for a product, but it is more pronounceable than “ophthalmology” and way easier to spell. This is my specialty, and I can’t believe we still haven’t gone with “eye surgeon,” but I digress.
So, let’s rebrand “disability insurance” for the sake of clarity:
I personally like to think of it as a monthly subscription for a soft landing in a worst-case scenario. Call me a millennial, but it just goes down smoother in my mind as a subscription a la Netflix ... and the four other streaming services that someone gave me a password to – if you’re a 55-year-old GI specialist, I know you’re on the Spotify family plan, too. No judgment from me.
So, for $15, you get a bunch of movies with Netflix, and, for $150-$300, you cover a lifetime of income. That’s a pretty decent service even without “The Office.”
Disability insurance often covers at least $15-$20 million dollars over a lifetime of earnings for only 1%-2% of your salary per year.
But I’ll pause here. The numbers are irrelevant if you never get the insurance.
I have one goal for this article, and it is simply to try to help you break down that procrastination habit we all have. I will have added immense value to at least one family’s life if you go and get a policy this week that saves your family from substantial loss of income. This is why I love insurance.
Doctors sacrifice essential life steps to get through training. But we are not alone in that.
Tim Kasser, PhD, puts it well when he said: “We live in a machine that is designed to get us to neglect what is important about life.” Here he is talking about relationships, but securing financial protection is loving to those closest to us.
So, what holds us back from taking a seemingly easy step like locking in disability insurance early in training?
Is it the stress of residency? Studying for Step 1? Moving cities and finding a home during a housing crisis? Job change during COVID? Is it because we have already put it off so long that we don’t want to think about it?
Totally fair.
For all of us busy doctors, the necessity and obviousness of buying disability insurance, *ehem*, income protection insurance makes you feel like you can get to it when you get to it because you know you will, so ... what’s the rush?
Or, is it our desire to bet on ourselves, and every month that goes by without insurance is one less payment? Roll the dice! Woo!
The reason to not put off the important things in life
I will give you a few reasons of “the why of” how we can all benefit from disability insurance and the reason there is no benefit in waiting to get a policy.
But, most importantly, I want to talk to you about your life and why you are putting off a lot of important things.
That diet you’ve been wanting to start? Yep.
That ring you haven’t purchased? Maybe that!
That article you’ve been meaning to write for the GI journal? Yes, especially that.
Remember: Take a deep breath in and exhaaaaale.
So, why do we put off the important?
First, even though the “why” of purchasing income protection is a bit basic, I do find it helpful to have discrete reasons for accomplishing an important task.
Why get disability insurance at all?
Let’s look at the value we get out of covering our income.
Reason No. 1. It softens the landing in the event you have an illness. The stats on disability claims are heavily on the side of illness over accidents or trauma. As you know, many autoimmune conditions show up in the 20’s and 30’s, so those are the things your friends will have first.
Unfortunately, if you have a medical issue before you have a disability policy, you will either not have coverage for that specific condition or you will not be approved for insurance. Unlike health insurance, the company can afford to pay out policies because it is picky on who it is willing to cover. It tries to select healthy people, so apply when you are most healthy, if possible.
Reason No. 2. It’s cheap. When you compare with a $2 million policy for life insurance, it might cost $1,000-$2,000 or so per year for a term policy covering about 25 years. With disability insurance, you can cover about 10x as much for the same annual payment. One could easily make a case that if you do not have dependents, disability insurance should be your first stop even before life insurance. You are more likely to be disabled than to die when you’re in your thirties. Act accordingly.
(Please note for obvious reasons they don’t call life insurance “death insurance.” Disability insurance needs that same rebrand – I’m telling you!)
Reason No. 3. Unless you are independently wealthy, it will be nearly impossible to replace your income and live a similar lifestyle. Lock in the benefits of the work you have already accomplished, and lock in the coverage of ALL of your health while you are healthy.
Time to take action
As Elvis famously sang: “A little less conversation, a little more action please.”
Alright, so how do we get ourselves to ACT and get a policy to protect our income?
Tip No. 1. As doctors we often shoot for perfection. It’s no surprise, therefore, that we have an illusion that we need to find the “perfect policy.”
One of my friends is a great financial adviser, and he often tells me about first meetings with clients to create a long-lasting plan. Often, somewhere along the way when discussing risks of stocks going down and up, someone will ask, “Why don’t we pick one that is low risk but tends to go up in value?” Of course, the reality is that if it were that easy ... everyone would do it!
Fortunately, with disability insurance, the policies are fairly straight forward. You can skip the analysis paralysis with disability insurance by talking with an agent who consistently works with physicians. I enjoy talking policies and helping doctors protect their financial health, so I started selling policies shortly after residency because so many of my co-residents were making me nervous putting it off. Some I helped, and some put it off and are unable to get policies after health issues even just 3 years after residency.
Tip No. 2. Having a policy is better than not having one, and if you’re worried about getting the wrong one, just get two! Seriously, some companies let you split coverage between two and this can even increase the maximum coverage you can get later in life, too. Does it add cost? Surprisingly, it typically does not, and it does not make the agent more money either. In most cases it’s actually more work for them for the same amount of commission. Don’t be afraid to ask about this.
Tip No. 3. This is my hot tip for current policy owners: ask for the full version of your policy, and read the entire policy. I recently asked for my policy because I was doing some international work abroad and wanted to know if I could reside abroad if I made a disability claim. My policy stated that I would need to reside in the United States within 12 months of disability. I likely would do this in the event of disability, but it is quite important to know these aspects.
While reading the fine print, I found that a minimum number of work-hours per week (35 for my policy) was required to qualify for my physician-specific coverage. This was an important part of my job criteria when looking for a new position and is worth investigating for anyone considering part-time employment.
Tip No. 4. The obvious tip: The fear of failure gets a lot of perfectionists from even starting a task unless they know everything about it.
Just start.
That’s my go-to for overcoming fear of failure. You won’t fail. You just won’t. You will learn!
Pretend you are curious about it and try with any of these actionable steps:
- Google disability insurance.
- Email me at [email protected].
- Read an article on a doctor-based blog.
I personally geeked out on insurance so much in residency that I became an insurance agent. I am an independent broker, so I have no bias toward any particular policies (email me anytime even if just with questions). Personally, I believe in this product and the value of this type of insurance, and I would hate for anyone to not have coverage of their most valuable asset: lifelong income!
The steps of applying for disability insurance
Now you know all the great reasons to get going! What are the next steps?
No matter where you get your policy, you can expect the process to be fairly simple. If it’s not then shoot me an email and I’m happy to help chat and discuss further.
The general process is:
Step 1. Initial phone call or email: Chat with an agent to discuss your needs and situation. Immediately after, you can sign initial application documents with DocuSign. (20 minutes).
Step 2. Complete health questionnaire on the phone with the insurance company. (20-40 minutes).
Step 3. Sign the final documents and confirm physician-specific language in their policies. (20 minutes).
The whole application period typically lasts only 2-4 weeks from start to finish and, if you pay up front, you are covered from the moment you send in the check. If you don’t accept the policy, you even get the money back.
I genuinely enjoy talking with my colleagues from all over the world and learning about their lives and plans, so, if you have any questions, please do not hesitate to email me at [email protected]. Also, feel free to check out my mini-blog at curiousmd.com or listen to me chat with Jon Solitro, CFP, on his FinancialMD.com podcast. Similar to this article, it is fairly informal and covers real life, tough career decisions, and actionable financial planning tips.
If you made it to the end of this article, you are a perfectionist and should go back and read Tip No. 1.
Reference
The Context of Things. “We live in a machine that is designed to get us to neglect what’s important about life,” 2021 Aug 24.
Dr. Smith is an ophthalmologist and consultant with Advanced Eyecare Professionals, Grand Rapids, Mich., and founder of DigitalGlaucoma.com. He is cohost of The FinancialMD Show podcast. He is an insurance producer and assists clients with advising and decision-making related to disability insurance at FinancialMD.
If you already have disability insurance, keep reading as well. I have a great tip for you from personal experience that made a difference in the job I selected.
Let’s start with an important rebrand for “disability insurance.” What does it protect? Income! Car insurance is not called crash insurance. House insurance is not called burnt house insurance. And unlike a car or a house, it protects an asset with 10-20 times as much value as a million-dollar house.
So, let’s call it what it is: “income protection insurance.”
It’s always a bit nerdy when I talk about how much I appreciate insurance that protects lifelong income. I often make an argument that it is simply one of the best products that exists, especially for high-income earners with lots of debt. Many of us doctors are in that category and are not even slightly jealous of our friends whose parents paid for school (I’m looking at you not-her-real-name-Mary).
Disability is not the catchiest name for a product, but it is more pronounceable than “ophthalmology” and way easier to spell. This is my specialty, and I can’t believe we still haven’t gone with “eye surgeon,” but I digress.
So, let’s rebrand “disability insurance” for the sake of clarity:
I personally like to think of it as a monthly subscription for a soft landing in a worst-case scenario. Call me a millennial, but it just goes down smoother in my mind as a subscription a la Netflix ... and the four other streaming services that someone gave me a password to – if you’re a 55-year-old GI specialist, I know you’re on the Spotify family plan, too. No judgment from me.
So, for $15, you get a bunch of movies with Netflix, and, for $150-$300, you cover a lifetime of income. That’s a pretty decent service even without “The Office.”
Disability insurance often covers at least $15-$20 million dollars over a lifetime of earnings for only 1%-2% of your salary per year.
But I’ll pause here. The numbers are irrelevant if you never get the insurance.
I have one goal for this article, and it is simply to try to help you break down that procrastination habit we all have. I will have added immense value to at least one family’s life if you go and get a policy this week that saves your family from substantial loss of income. This is why I love insurance.
Doctors sacrifice essential life steps to get through training. But we are not alone in that.
Tim Kasser, PhD, puts it well when he said: “We live in a machine that is designed to get us to neglect what is important about life.” Here he is talking about relationships, but securing financial protection is loving to those closest to us.
So, what holds us back from taking a seemingly easy step like locking in disability insurance early in training?
Is it the stress of residency? Studying for Step 1? Moving cities and finding a home during a housing crisis? Job change during COVID? Is it because we have already put it off so long that we don’t want to think about it?
Totally fair.
For all of us busy doctors, the necessity and obviousness of buying disability insurance, *ehem*, income protection insurance makes you feel like you can get to it when you get to it because you know you will, so ... what’s the rush?
Or, is it our desire to bet on ourselves, and every month that goes by without insurance is one less payment? Roll the dice! Woo!
The reason to not put off the important things in life
I will give you a few reasons of “the why of” how we can all benefit from disability insurance and the reason there is no benefit in waiting to get a policy.
But, most importantly, I want to talk to you about your life and why you are putting off a lot of important things.
That diet you’ve been wanting to start? Yep.
That ring you haven’t purchased? Maybe that!
That article you’ve been meaning to write for the GI journal? Yes, especially that.
Remember: Take a deep breath in and exhaaaaale.
So, why do we put off the important?
First, even though the “why” of purchasing income protection is a bit basic, I do find it helpful to have discrete reasons for accomplishing an important task.
Why get disability insurance at all?
Let’s look at the value we get out of covering our income.
Reason No. 1. It softens the landing in the event you have an illness. The stats on disability claims are heavily on the side of illness over accidents or trauma. As you know, many autoimmune conditions show up in the 20’s and 30’s, so those are the things your friends will have first.
Unfortunately, if you have a medical issue before you have a disability policy, you will either not have coverage for that specific condition or you will not be approved for insurance. Unlike health insurance, the company can afford to pay out policies because it is picky on who it is willing to cover. It tries to select healthy people, so apply when you are most healthy, if possible.
Reason No. 2. It’s cheap. When you compare with a $2 million policy for life insurance, it might cost $1,000-$2,000 or so per year for a term policy covering about 25 years. With disability insurance, you can cover about 10x as much for the same annual payment. One could easily make a case that if you do not have dependents, disability insurance should be your first stop even before life insurance. You are more likely to be disabled than to die when you’re in your thirties. Act accordingly.
(Please note for obvious reasons they don’t call life insurance “death insurance.” Disability insurance needs that same rebrand – I’m telling you!)
Reason No. 3. Unless you are independently wealthy, it will be nearly impossible to replace your income and live a similar lifestyle. Lock in the benefits of the work you have already accomplished, and lock in the coverage of ALL of your health while you are healthy.
Time to take action
As Elvis famously sang: “A little less conversation, a little more action please.”
Alright, so how do we get ourselves to ACT and get a policy to protect our income?
Tip No. 1. As doctors we often shoot for perfection. It’s no surprise, therefore, that we have an illusion that we need to find the “perfect policy.”
One of my friends is a great financial adviser, and he often tells me about first meetings with clients to create a long-lasting plan. Often, somewhere along the way when discussing risks of stocks going down and up, someone will ask, “Why don’t we pick one that is low risk but tends to go up in value?” Of course, the reality is that if it were that easy ... everyone would do it!
Fortunately, with disability insurance, the policies are fairly straight forward. You can skip the analysis paralysis with disability insurance by talking with an agent who consistently works with physicians. I enjoy talking policies and helping doctors protect their financial health, so I started selling policies shortly after residency because so many of my co-residents were making me nervous putting it off. Some I helped, and some put it off and are unable to get policies after health issues even just 3 years after residency.
Tip No. 2. Having a policy is better than not having one, and if you’re worried about getting the wrong one, just get two! Seriously, some companies let you split coverage between two and this can even increase the maximum coverage you can get later in life, too. Does it add cost? Surprisingly, it typically does not, and it does not make the agent more money either. In most cases it’s actually more work for them for the same amount of commission. Don’t be afraid to ask about this.
Tip No. 3. This is my hot tip for current policy owners: ask for the full version of your policy, and read the entire policy. I recently asked for my policy because I was doing some international work abroad and wanted to know if I could reside abroad if I made a disability claim. My policy stated that I would need to reside in the United States within 12 months of disability. I likely would do this in the event of disability, but it is quite important to know these aspects.
While reading the fine print, I found that a minimum number of work-hours per week (35 for my policy) was required to qualify for my physician-specific coverage. This was an important part of my job criteria when looking for a new position and is worth investigating for anyone considering part-time employment.
Tip No. 4. The obvious tip: The fear of failure gets a lot of perfectionists from even starting a task unless they know everything about it.
Just start.
That’s my go-to for overcoming fear of failure. You won’t fail. You just won’t. You will learn!
Pretend you are curious about it and try with any of these actionable steps:
- Google disability insurance.
- Email me at [email protected].
- Read an article on a doctor-based blog.
I personally geeked out on insurance so much in residency that I became an insurance agent. I am an independent broker, so I have no bias toward any particular policies (email me anytime even if just with questions). Personally, I believe in this product and the value of this type of insurance, and I would hate for anyone to not have coverage of their most valuable asset: lifelong income!
The steps of applying for disability insurance
Now you know all the great reasons to get going! What are the next steps?
No matter where you get your policy, you can expect the process to be fairly simple. If it’s not then shoot me an email and I’m happy to help chat and discuss further.
The general process is:
Step 1. Initial phone call or email: Chat with an agent to discuss your needs and situation. Immediately after, you can sign initial application documents with DocuSign. (20 minutes).
Step 2. Complete health questionnaire on the phone with the insurance company. (20-40 minutes).
Step 3. Sign the final documents and confirm physician-specific language in their policies. (20 minutes).
The whole application period typically lasts only 2-4 weeks from start to finish and, if you pay up front, you are covered from the moment you send in the check. If you don’t accept the policy, you even get the money back.
I genuinely enjoy talking with my colleagues from all over the world and learning about their lives and plans, so, if you have any questions, please do not hesitate to email me at [email protected]. Also, feel free to check out my mini-blog at curiousmd.com or listen to me chat with Jon Solitro, CFP, on his FinancialMD.com podcast. Similar to this article, it is fairly informal and covers real life, tough career decisions, and actionable financial planning tips.
If you made it to the end of this article, you are a perfectionist and should go back and read Tip No. 1.
Reference
The Context of Things. “We live in a machine that is designed to get us to neglect what’s important about life,” 2021 Aug 24.
Dr. Smith is an ophthalmologist and consultant with Advanced Eyecare Professionals, Grand Rapids, Mich., and founder of DigitalGlaucoma.com. He is cohost of The FinancialMD Show podcast. He is an insurance producer and assists clients with advising and decision-making related to disability insurance at FinancialMD.
Milk allergy frequently overdiagnosed
According to a consensus study, many infants in some countries are misdiagnosed with allergy to cow, sheep, or goat milk, and they’re prescribed specialized formulas they don’t need.
“Milk allergy overdiagnosis is common in some regions and can potentially harm mothers and infants,” the authors write in Clinical & Experimental Allergy. “These new consensus recommendations on the safe detection and management of milk allergy in children under 2 years aim to reduce harms associated with milk allergy overdiagnosis.”
“This guidance, developed by experts without commercial ties to the formula industry, aims to reduce milk allergy overdiagnosis and [to] support ... breastfeeding and less use of specialized formula, compared with current guidelines,” they add.
Up to 1% of European infants 2 years of age and younger are considered allergic to cow’s milk. Prescriptions for specialized formula for bottle-fed infants allergic to cow’s milk in Australia, England, and Norway have grown to over 10 times the expected volumes.
Lead study author Hilary I. Allen, National Heart and Lung Institute, Imperial College London, and her colleagues on several continents developed practical guidance for providers on safely detecting and managing milk allergy in infants.
Due to lack of high-certainty research evidence in this area, they used the Delphi consensus method.
The study involved two rounds of anonymous consensus-building surveys and one formal meeting in 2021.
The team identified experts from diverse geographic and cultural settings by searching medical databases for the term “milk hypersensitivity.” They asked those experts to recommend colleagues. The researchers also contacted experts with ties to international professional organizations, such as the International Board of Lactation Consultant Examiners, as well as societies associated with the World Allergy Organization.
The 17 study participants included clinicians and researchers in general practice, health visiting, lactation support, midwifery, nutrition, and relevant areas of pediatrics from Africa, Asia, Australia, Europe, the Middle East, and North America. Experts with recent conflicts of interest with the breastmilk substitute (formula) industry were excluded from the study. Five authors of earlier milk allergy guidelines and seven parents contributed feedback.
In each survey round, participants used a nine-point scale to rank the importance of each proposed statement that addressed prevention of overdiagnosis or underdiagnosis, support of breastfeeding women, and the role of specialized formula products.
Based on the number of total points participants assigned, each statement was classified as “essential,” “recommended,” “no consensus,” or “excluded” due to lack of relevance.
The experts agreed on 38 essential statements in several categories, including:
- Maternal dietary restriction is often not necessary to manage milk allergy
- In infants with chronic symptoms who are exclusively breastfed, milk allergy diagnosis should be considered only in specific, rare circumstances
- Milk allergy diagnosis does not usually need to be considered for stool changes, aversive feeding, or occasional spots of blood in stool, if not related in time with milk protein ingestion
The consensus recommendations provide more restrictive criteria than earlier guidelines for detecting milk allergy, fewer maternal dietary exclusions, and less use of specialized formula.
During an infant formula shortage in the U.S., a timely study
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program, Children’s Mercy Kansas City, Missouri, welcomed the study’s engagement of specialists in various fields and avoidance of bias from formula companies.
“Food allergies have received a lot of attention, especially through websites and social media,” Ms. Shroba, who was not involved in the study, told this news organization in an email. “Unfortunately, a lot of that information is incorrect and can lead to misunderstanding and misdiagnosis.”
“This article helps guide practitioners through identifying the concerning symptoms of milk allergy versus normal infant symptoms,” she said. “It can help providers discern when testing, elimination diets, and changes in formula are warranted.
“This guidance emphasizes the reproducibility and specificity of symptoms, which are key elements of a food allergy diagnosis,” she explained. “By eliminating unnecessary milk allergy labeling, we can keep infants on appropriate diets for their age, such as breastfeeding or milk-based formulas. Proper diagnosis can also reduce unnecessary financial strain of specialty formulas, stress to the family regarding feedings, and a restrictive diet for the breastfeeding mother.”
The study will be useful to a wide range of health care providers, Jennifer Anne Dantzer, MD, assistant professor of pediatrics, Johns Hopkins Medicine, Baltimore, said in an email.
“With the current formula shortage, there has perhaps never been a more important time to do this study and provide additional guidance on who does or does not need special formula,” noted Dr. Dantzer, who also was not involved in the study. “A milk allergy diagnosis impacts the child and the family, so it is very important to avoid overdiagnosis and to support the breastfeeding mother.”
“These findings should provide reassurance that dietary exclusions for the breastfeeding mother are not needed for most children with milk allergy,” she said. “If a milk allergy is suspected, the child should be referred to an allergist.”
The authors recommend further related research into the safety and effectiveness of using the guidance in practice.
One coauthor reports financial relationships with a biotech company. Ms. Allen and her remaining coauthors, as well as Ms. Shroba and Dr. Dantzer, report no relevant financial relationships. The study was funded through fellowships.
A version of this article first appeared on Medscape.com.
According to a consensus study, many infants in some countries are misdiagnosed with allergy to cow, sheep, or goat milk, and they’re prescribed specialized formulas they don’t need.
“Milk allergy overdiagnosis is common in some regions and can potentially harm mothers and infants,” the authors write in Clinical & Experimental Allergy. “These new consensus recommendations on the safe detection and management of milk allergy in children under 2 years aim to reduce harms associated with milk allergy overdiagnosis.”
“This guidance, developed by experts without commercial ties to the formula industry, aims to reduce milk allergy overdiagnosis and [to] support ... breastfeeding and less use of specialized formula, compared with current guidelines,” they add.
Up to 1% of European infants 2 years of age and younger are considered allergic to cow’s milk. Prescriptions for specialized formula for bottle-fed infants allergic to cow’s milk in Australia, England, and Norway have grown to over 10 times the expected volumes.
Lead study author Hilary I. Allen, National Heart and Lung Institute, Imperial College London, and her colleagues on several continents developed practical guidance for providers on safely detecting and managing milk allergy in infants.
Due to lack of high-certainty research evidence in this area, they used the Delphi consensus method.
The study involved two rounds of anonymous consensus-building surveys and one formal meeting in 2021.
The team identified experts from diverse geographic and cultural settings by searching medical databases for the term “milk hypersensitivity.” They asked those experts to recommend colleagues. The researchers also contacted experts with ties to international professional organizations, such as the International Board of Lactation Consultant Examiners, as well as societies associated with the World Allergy Organization.
The 17 study participants included clinicians and researchers in general practice, health visiting, lactation support, midwifery, nutrition, and relevant areas of pediatrics from Africa, Asia, Australia, Europe, the Middle East, and North America. Experts with recent conflicts of interest with the breastmilk substitute (formula) industry were excluded from the study. Five authors of earlier milk allergy guidelines and seven parents contributed feedback.
In each survey round, participants used a nine-point scale to rank the importance of each proposed statement that addressed prevention of overdiagnosis or underdiagnosis, support of breastfeeding women, and the role of specialized formula products.
Based on the number of total points participants assigned, each statement was classified as “essential,” “recommended,” “no consensus,” or “excluded” due to lack of relevance.
The experts agreed on 38 essential statements in several categories, including:
- Maternal dietary restriction is often not necessary to manage milk allergy
- In infants with chronic symptoms who are exclusively breastfed, milk allergy diagnosis should be considered only in specific, rare circumstances
- Milk allergy diagnosis does not usually need to be considered for stool changes, aversive feeding, or occasional spots of blood in stool, if not related in time with milk protein ingestion
The consensus recommendations provide more restrictive criteria than earlier guidelines for detecting milk allergy, fewer maternal dietary exclusions, and less use of specialized formula.
During an infant formula shortage in the U.S., a timely study
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program, Children’s Mercy Kansas City, Missouri, welcomed the study’s engagement of specialists in various fields and avoidance of bias from formula companies.
“Food allergies have received a lot of attention, especially through websites and social media,” Ms. Shroba, who was not involved in the study, told this news organization in an email. “Unfortunately, a lot of that information is incorrect and can lead to misunderstanding and misdiagnosis.”
“This article helps guide practitioners through identifying the concerning symptoms of milk allergy versus normal infant symptoms,” she said. “It can help providers discern when testing, elimination diets, and changes in formula are warranted.
“This guidance emphasizes the reproducibility and specificity of symptoms, which are key elements of a food allergy diagnosis,” she explained. “By eliminating unnecessary milk allergy labeling, we can keep infants on appropriate diets for their age, such as breastfeeding or milk-based formulas. Proper diagnosis can also reduce unnecessary financial strain of specialty formulas, stress to the family regarding feedings, and a restrictive diet for the breastfeeding mother.”
The study will be useful to a wide range of health care providers, Jennifer Anne Dantzer, MD, assistant professor of pediatrics, Johns Hopkins Medicine, Baltimore, said in an email.
“With the current formula shortage, there has perhaps never been a more important time to do this study and provide additional guidance on who does or does not need special formula,” noted Dr. Dantzer, who also was not involved in the study. “A milk allergy diagnosis impacts the child and the family, so it is very important to avoid overdiagnosis and to support the breastfeeding mother.”
“These findings should provide reassurance that dietary exclusions for the breastfeeding mother are not needed for most children with milk allergy,” she said. “If a milk allergy is suspected, the child should be referred to an allergist.”
The authors recommend further related research into the safety and effectiveness of using the guidance in practice.
One coauthor reports financial relationships with a biotech company. Ms. Allen and her remaining coauthors, as well as Ms. Shroba and Dr. Dantzer, report no relevant financial relationships. The study was funded through fellowships.
A version of this article first appeared on Medscape.com.
According to a consensus study, many infants in some countries are misdiagnosed with allergy to cow, sheep, or goat milk, and they’re prescribed specialized formulas they don’t need.
“Milk allergy overdiagnosis is common in some regions and can potentially harm mothers and infants,” the authors write in Clinical & Experimental Allergy. “These new consensus recommendations on the safe detection and management of milk allergy in children under 2 years aim to reduce harms associated with milk allergy overdiagnosis.”
“This guidance, developed by experts without commercial ties to the formula industry, aims to reduce milk allergy overdiagnosis and [to] support ... breastfeeding and less use of specialized formula, compared with current guidelines,” they add.
Up to 1% of European infants 2 years of age and younger are considered allergic to cow’s milk. Prescriptions for specialized formula for bottle-fed infants allergic to cow’s milk in Australia, England, and Norway have grown to over 10 times the expected volumes.
Lead study author Hilary I. Allen, National Heart and Lung Institute, Imperial College London, and her colleagues on several continents developed practical guidance for providers on safely detecting and managing milk allergy in infants.
Due to lack of high-certainty research evidence in this area, they used the Delphi consensus method.
The study involved two rounds of anonymous consensus-building surveys and one formal meeting in 2021.
The team identified experts from diverse geographic and cultural settings by searching medical databases for the term “milk hypersensitivity.” They asked those experts to recommend colleagues. The researchers also contacted experts with ties to international professional organizations, such as the International Board of Lactation Consultant Examiners, as well as societies associated with the World Allergy Organization.
The 17 study participants included clinicians and researchers in general practice, health visiting, lactation support, midwifery, nutrition, and relevant areas of pediatrics from Africa, Asia, Australia, Europe, the Middle East, and North America. Experts with recent conflicts of interest with the breastmilk substitute (formula) industry were excluded from the study. Five authors of earlier milk allergy guidelines and seven parents contributed feedback.
In each survey round, participants used a nine-point scale to rank the importance of each proposed statement that addressed prevention of overdiagnosis or underdiagnosis, support of breastfeeding women, and the role of specialized formula products.
Based on the number of total points participants assigned, each statement was classified as “essential,” “recommended,” “no consensus,” or “excluded” due to lack of relevance.
The experts agreed on 38 essential statements in several categories, including:
- Maternal dietary restriction is often not necessary to manage milk allergy
- In infants with chronic symptoms who are exclusively breastfed, milk allergy diagnosis should be considered only in specific, rare circumstances
- Milk allergy diagnosis does not usually need to be considered for stool changes, aversive feeding, or occasional spots of blood in stool, if not related in time with milk protein ingestion
The consensus recommendations provide more restrictive criteria than earlier guidelines for detecting milk allergy, fewer maternal dietary exclusions, and less use of specialized formula.
During an infant formula shortage in the U.S., a timely study
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program, Children’s Mercy Kansas City, Missouri, welcomed the study’s engagement of specialists in various fields and avoidance of bias from formula companies.
“Food allergies have received a lot of attention, especially through websites and social media,” Ms. Shroba, who was not involved in the study, told this news organization in an email. “Unfortunately, a lot of that information is incorrect and can lead to misunderstanding and misdiagnosis.”
“This article helps guide practitioners through identifying the concerning symptoms of milk allergy versus normal infant symptoms,” she said. “It can help providers discern when testing, elimination diets, and changes in formula are warranted.
“This guidance emphasizes the reproducibility and specificity of symptoms, which are key elements of a food allergy diagnosis,” she explained. “By eliminating unnecessary milk allergy labeling, we can keep infants on appropriate diets for their age, such as breastfeeding or milk-based formulas. Proper diagnosis can also reduce unnecessary financial strain of specialty formulas, stress to the family regarding feedings, and a restrictive diet for the breastfeeding mother.”
The study will be useful to a wide range of health care providers, Jennifer Anne Dantzer, MD, assistant professor of pediatrics, Johns Hopkins Medicine, Baltimore, said in an email.
“With the current formula shortage, there has perhaps never been a more important time to do this study and provide additional guidance on who does or does not need special formula,” noted Dr. Dantzer, who also was not involved in the study. “A milk allergy diagnosis impacts the child and the family, so it is very important to avoid overdiagnosis and to support the breastfeeding mother.”
“These findings should provide reassurance that dietary exclusions for the breastfeeding mother are not needed for most children with milk allergy,” she said. “If a milk allergy is suspected, the child should be referred to an allergist.”
The authors recommend further related research into the safety and effectiveness of using the guidance in practice.
One coauthor reports financial relationships with a biotech company. Ms. Allen and her remaining coauthors, as well as Ms. Shroba and Dr. Dantzer, report no relevant financial relationships. The study was funded through fellowships.
A version of this article first appeared on Medscape.com.
Pediatric obesity disparities widen
Lower levels of household income and education in the United States are associated with higher rates of adolescent obesity. These socioeconomic disparities “have widened during the last two decades,” new research shows.
Because obesity in adolescence has immediate and long-term health consequences, this phenomenon “may exacerbate socioeconomic disparities in chronic diseases into adulthood,” study author Ryunosuke Goto, MD, of University of Tokyo Hospital, and colleagues reported in JAMA Pediatrics.
Groups with higher rates of obesity may also be less likely to access treatment, said Kyung E. Rhee, MD, professor of pediatrics at University of California, San Diego School of Medicine, who was not involved in the new analysis.
“These are the families who have a harder time getting to the doctor’s office or getting to programs because they are working multiple jobs, or they don’t have as much flexibility,” Dr. Rhee told this news organization.
20 years of data
A recent study showed a relationship between socioeconomic status (SES) and weight in adults. Research examining current trends in adolescents has been limited, however, according to the authors of the new study.
To address this gap, Dr. Goto and colleagues looked at obesity trends among approximately 20,000 U.S. children aged 10-19 years using cross-sectional data from the 1999-2018 National Health and Nutrition Examination Surveys.
They compared the prevalence of obesity among participants whose household income was 138% of the federal poverty level or less versus those with higher levels of household income. They also examined obesity prevalence according to whether the head of household had graduated college.
Relative to higher-income households, adolescents from lower-income households were more likely to be non-Hispanic Black (21.7% vs. 10.4%) or Hispanic (30.6% vs. 13.4%) and to have an unmarried parent (54.5% vs. 23%). They were also more likely to have obesity (22.8% vs. 17.3%).
The prevalence of obesity likewise was higher among adolescents whose head of household did not have a college degree (21.8% vs. 11.6%).
In an analysis that adjusted for race, ethnicity, height, and marital status of the head of household, the prevalence of obesity increased over 20 years, particularly among adolescents from lower-income homes, the researchers reported.
Lower income was associated with an increase in obesity prevalence of 4.2 percentage points, and less education was associated with an increase in obesity prevalence of 9 percentage points.
By 2015-2018, the gap in obesity prevalence between low-income households and higher-income households was 6.4 percentage points more than it had been during 1999-2002 (95% confidence interval, 1.5-11.4). “When we assessed linear trends, the gap in obesity prevalence by income and education increased by an average of 1.5 (95% CI, 0.4-2.6) and 1.1 (95% CI, 0.0-2.3) percentage points every 4 years, respectively,” according to the researchers.
How to treat
Separately, researchers are studying ways to help treat patients with obesity and increase access to treatment. To that end, Dr. Rhee and colleagues developed a new program called Guided Self-Help Obesity Treatment in the Doctor’s Office (GOT Doc).
The guided self-help program was designed to provide similar resources as a leading treatment approach – family-based treatment – but in a less intensive, more accessible way.
Results from a randomized trial comparing this guided self-help approach with family-based treatment were published in Pediatrics.
The trial included 159 children and their parents. The children had an average age of 9.6 years and body mass index z-score of 2.1. Participants were primarily Latinx and from lower income neighborhoods.
Whereas family-based treatment included hour-long sessions at an academic center, the guided self-help program featured a 20-minute session in the office where patients typically see their primary care physician.
Both programs covered how to self-monitor food intake, set healthy goals, and modify the home environment to promote behavioral change. They also discussed body image, bullying, and emotional health. The program is framed around developing a healthy lifestyle rather than weight loss itself, Dr. Rhee said.
Children in both groups had significant reductions in their body mass index percentiles after the 6-month treatment programs. The reductions were largely maintained at 6-month follow-up.
Families in the guided self-help program, however, had a 67% lower risk of dropping out of the study and reported greater satisfaction and convenience. They attended more than half of the treatment sessions, whereas participants assigned to family-based treatment attended 1 in 5 sessions, on average.
The trial was conducted before the COVID-19 pandemic. Next, the researchers plan to test delivery of a guided self-help program via video calls, Dr. Rhee said.
Having options readily available for families who are interested in treatment for obesity proved valuable to clinicians, Dr. Rhee said. “They could then just refer them down the hall to the interventionist who was there, who was going to then work with the family to make these changes,” she said.
The study by Dr. Goto and colleagues was supported by grants from the Japan Society for the Promotion of Science. The trial by Dr. Rhee et al. was supported by a grant from the Health Resources and Services Administration. Neither research team had conflict of interest disclosures.
A version of this article first appeared on Medscape.com.
Lower levels of household income and education in the United States are associated with higher rates of adolescent obesity. These socioeconomic disparities “have widened during the last two decades,” new research shows.
Because obesity in adolescence has immediate and long-term health consequences, this phenomenon “may exacerbate socioeconomic disparities in chronic diseases into adulthood,” study author Ryunosuke Goto, MD, of University of Tokyo Hospital, and colleagues reported in JAMA Pediatrics.
Groups with higher rates of obesity may also be less likely to access treatment, said Kyung E. Rhee, MD, professor of pediatrics at University of California, San Diego School of Medicine, who was not involved in the new analysis.
“These are the families who have a harder time getting to the doctor’s office or getting to programs because they are working multiple jobs, or they don’t have as much flexibility,” Dr. Rhee told this news organization.
20 years of data
A recent study showed a relationship between socioeconomic status (SES) and weight in adults. Research examining current trends in adolescents has been limited, however, according to the authors of the new study.
To address this gap, Dr. Goto and colleagues looked at obesity trends among approximately 20,000 U.S. children aged 10-19 years using cross-sectional data from the 1999-2018 National Health and Nutrition Examination Surveys.
They compared the prevalence of obesity among participants whose household income was 138% of the federal poverty level or less versus those with higher levels of household income. They also examined obesity prevalence according to whether the head of household had graduated college.
Relative to higher-income households, adolescents from lower-income households were more likely to be non-Hispanic Black (21.7% vs. 10.4%) or Hispanic (30.6% vs. 13.4%) and to have an unmarried parent (54.5% vs. 23%). They were also more likely to have obesity (22.8% vs. 17.3%).
The prevalence of obesity likewise was higher among adolescents whose head of household did not have a college degree (21.8% vs. 11.6%).
In an analysis that adjusted for race, ethnicity, height, and marital status of the head of household, the prevalence of obesity increased over 20 years, particularly among adolescents from lower-income homes, the researchers reported.
Lower income was associated with an increase in obesity prevalence of 4.2 percentage points, and less education was associated with an increase in obesity prevalence of 9 percentage points.
By 2015-2018, the gap in obesity prevalence between low-income households and higher-income households was 6.4 percentage points more than it had been during 1999-2002 (95% confidence interval, 1.5-11.4). “When we assessed linear trends, the gap in obesity prevalence by income and education increased by an average of 1.5 (95% CI, 0.4-2.6) and 1.1 (95% CI, 0.0-2.3) percentage points every 4 years, respectively,” according to the researchers.
How to treat
Separately, researchers are studying ways to help treat patients with obesity and increase access to treatment. To that end, Dr. Rhee and colleagues developed a new program called Guided Self-Help Obesity Treatment in the Doctor’s Office (GOT Doc).
The guided self-help program was designed to provide similar resources as a leading treatment approach – family-based treatment – but in a less intensive, more accessible way.
Results from a randomized trial comparing this guided self-help approach with family-based treatment were published in Pediatrics.
The trial included 159 children and their parents. The children had an average age of 9.6 years and body mass index z-score of 2.1. Participants were primarily Latinx and from lower income neighborhoods.
Whereas family-based treatment included hour-long sessions at an academic center, the guided self-help program featured a 20-minute session in the office where patients typically see their primary care physician.
Both programs covered how to self-monitor food intake, set healthy goals, and modify the home environment to promote behavioral change. They also discussed body image, bullying, and emotional health. The program is framed around developing a healthy lifestyle rather than weight loss itself, Dr. Rhee said.
Children in both groups had significant reductions in their body mass index percentiles after the 6-month treatment programs. The reductions were largely maintained at 6-month follow-up.
Families in the guided self-help program, however, had a 67% lower risk of dropping out of the study and reported greater satisfaction and convenience. They attended more than half of the treatment sessions, whereas participants assigned to family-based treatment attended 1 in 5 sessions, on average.
The trial was conducted before the COVID-19 pandemic. Next, the researchers plan to test delivery of a guided self-help program via video calls, Dr. Rhee said.
Having options readily available for families who are interested in treatment for obesity proved valuable to clinicians, Dr. Rhee said. “They could then just refer them down the hall to the interventionist who was there, who was going to then work with the family to make these changes,” she said.
The study by Dr. Goto and colleagues was supported by grants from the Japan Society for the Promotion of Science. The trial by Dr. Rhee et al. was supported by a grant from the Health Resources and Services Administration. Neither research team had conflict of interest disclosures.
A version of this article first appeared on Medscape.com.
Lower levels of household income and education in the United States are associated with higher rates of adolescent obesity. These socioeconomic disparities “have widened during the last two decades,” new research shows.
Because obesity in adolescence has immediate and long-term health consequences, this phenomenon “may exacerbate socioeconomic disparities in chronic diseases into adulthood,” study author Ryunosuke Goto, MD, of University of Tokyo Hospital, and colleagues reported in JAMA Pediatrics.
Groups with higher rates of obesity may also be less likely to access treatment, said Kyung E. Rhee, MD, professor of pediatrics at University of California, San Diego School of Medicine, who was not involved in the new analysis.
“These are the families who have a harder time getting to the doctor’s office or getting to programs because they are working multiple jobs, or they don’t have as much flexibility,” Dr. Rhee told this news organization.
20 years of data
A recent study showed a relationship between socioeconomic status (SES) and weight in adults. Research examining current trends in adolescents has been limited, however, according to the authors of the new study.
To address this gap, Dr. Goto and colleagues looked at obesity trends among approximately 20,000 U.S. children aged 10-19 years using cross-sectional data from the 1999-2018 National Health and Nutrition Examination Surveys.
They compared the prevalence of obesity among participants whose household income was 138% of the federal poverty level or less versus those with higher levels of household income. They also examined obesity prevalence according to whether the head of household had graduated college.
Relative to higher-income households, adolescents from lower-income households were more likely to be non-Hispanic Black (21.7% vs. 10.4%) or Hispanic (30.6% vs. 13.4%) and to have an unmarried parent (54.5% vs. 23%). They were also more likely to have obesity (22.8% vs. 17.3%).
The prevalence of obesity likewise was higher among adolescents whose head of household did not have a college degree (21.8% vs. 11.6%).
In an analysis that adjusted for race, ethnicity, height, and marital status of the head of household, the prevalence of obesity increased over 20 years, particularly among adolescents from lower-income homes, the researchers reported.
Lower income was associated with an increase in obesity prevalence of 4.2 percentage points, and less education was associated with an increase in obesity prevalence of 9 percentage points.
By 2015-2018, the gap in obesity prevalence between low-income households and higher-income households was 6.4 percentage points more than it had been during 1999-2002 (95% confidence interval, 1.5-11.4). “When we assessed linear trends, the gap in obesity prevalence by income and education increased by an average of 1.5 (95% CI, 0.4-2.6) and 1.1 (95% CI, 0.0-2.3) percentage points every 4 years, respectively,” according to the researchers.
How to treat
Separately, researchers are studying ways to help treat patients with obesity and increase access to treatment. To that end, Dr. Rhee and colleagues developed a new program called Guided Self-Help Obesity Treatment in the Doctor’s Office (GOT Doc).
The guided self-help program was designed to provide similar resources as a leading treatment approach – family-based treatment – but in a less intensive, more accessible way.
Results from a randomized trial comparing this guided self-help approach with family-based treatment were published in Pediatrics.
The trial included 159 children and their parents. The children had an average age of 9.6 years and body mass index z-score of 2.1. Participants were primarily Latinx and from lower income neighborhoods.
Whereas family-based treatment included hour-long sessions at an academic center, the guided self-help program featured a 20-minute session in the office where patients typically see their primary care physician.
Both programs covered how to self-monitor food intake, set healthy goals, and modify the home environment to promote behavioral change. They also discussed body image, bullying, and emotional health. The program is framed around developing a healthy lifestyle rather than weight loss itself, Dr. Rhee said.
Children in both groups had significant reductions in their body mass index percentiles after the 6-month treatment programs. The reductions were largely maintained at 6-month follow-up.
Families in the guided self-help program, however, had a 67% lower risk of dropping out of the study and reported greater satisfaction and convenience. They attended more than half of the treatment sessions, whereas participants assigned to family-based treatment attended 1 in 5 sessions, on average.
The trial was conducted before the COVID-19 pandemic. Next, the researchers plan to test delivery of a guided self-help program via video calls, Dr. Rhee said.
Having options readily available for families who are interested in treatment for obesity proved valuable to clinicians, Dr. Rhee said. “They could then just refer them down the hall to the interventionist who was there, who was going to then work with the family to make these changes,” she said.
The study by Dr. Goto and colleagues was supported by grants from the Japan Society for the Promotion of Science. The trial by Dr. Rhee et al. was supported by a grant from the Health Resources and Services Administration. Neither research team had conflict of interest disclosures.
A version of this article first appeared on Medscape.com.
iLet system simplifies insulin delivery for type 1 diabetes
This transcript has been edited for clarity.
Today, I’m going to discuss the results of a new automated insulin delivery system that I think can really help many people with type 1 diabetes.
Dr. Steven Russell presented the results at the Advanced Technologies & Treatments for Diabetes meeting. The study focused on the iLet system, which is made by Beta Bionics and has been under development for a while. This was the single-hormone study, so it just looked at their algorithm using insulin alone. Eventually they’re going to study this, looking at the use of insulin plus glucagon together to see if that further improves outcomes.
One of the main reasons I think this study was so cool is because it included over 25% minority individuals who aren’t routinely studied in these insulin device trials. The study also included people who had a wide range of hemoglobin A1c levels; there was no high cut-point here. Over 30% of participants had an A1c greater than 8%. They also studied both children and adults and combined the results together.
Before I talk about the results, let me tell you about the pump. This is a tubed pump that has a sensor that it communicates with – it’s the Dexcom sensor – and it has an algorithm so it does automated insulin delivery. Instead of having to enter all sorts of information into the system, this thing requires that you put in only the patient’s weight. That’s it. From there, the system begins to figure out what the patient needs in terms of automated insulin delivery.
There are several different target settings that can be entered, and they can differ by time of day. There’s basically the time of day that one is eating a meal, so breakfast, lunch, or dinner, and there is the meal size, basically small, medium, and large. The individual enters this in real time so the system knows they’re eating, but other than that, the system just works.
It does this in a way that doesn’t allow for the individual using the pump to fidget with it. They can’t override the system and they can’t put in other insulin doses. The system is just there to take care of their diabetes.
They compared this system with people on any other system, including other automated insulin delivery systems, and put them into this trial. People were randomized to this system vs. whatever they’d been on (that was the control group) and they followed them for 13 weeks, which is not all that long.
There was a 0.5% reduction in A1c between the two groups. There was also an increase in the time in range, and this improvement in time in range happened almost immediately – within the first day or two of people being on the system. In terms of actual numbers, the adult patients started out with a time in range of 56% and this increased to 69% by the end of the study. The biggest improvement was time in range overnight, as is seen with other automated insulin delivery systems.
There was no reduction in time below a glucose level of 54 and there was an increase in the number of episodes of severe hypoglycemia in the group treated with the iLet system, but this was not statistically significant between the two groups.
I think these results are hard to compare with other pivotal trials investigating automated insulin delivery systems. The Tandem pivotal trial was a randomized controlled trial similar to this one, but the Medtronic and Omnipod studies were single-arm trials where patients were compared before and after they used the device.
More than anything, I think what’s important about this system is that it may allow for greater use of automated insulin delivery systems. It may allow primary care providers to use these systems without needing all sorts of support, and patients may be able to use these devices more simply than a device where they have to do carb counting and adjusting in ways that I think tend to be pretty complicated and require higher numeracy and literacy skills.
I couldn’t be happier. I love what they’re doing at Beta Bionics, and I look forward to more results, and in particular, to see if these results improve further when they do a study of insulin and glucagon in their dual-hormone pump system.
Thank you very much. This has been Dr Anne Peters for Medscape.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations. She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Today, I’m going to discuss the results of a new automated insulin delivery system that I think can really help many people with type 1 diabetes.
Dr. Steven Russell presented the results at the Advanced Technologies & Treatments for Diabetes meeting. The study focused on the iLet system, which is made by Beta Bionics and has been under development for a while. This was the single-hormone study, so it just looked at their algorithm using insulin alone. Eventually they’re going to study this, looking at the use of insulin plus glucagon together to see if that further improves outcomes.
One of the main reasons I think this study was so cool is because it included over 25% minority individuals who aren’t routinely studied in these insulin device trials. The study also included people who had a wide range of hemoglobin A1c levels; there was no high cut-point here. Over 30% of participants had an A1c greater than 8%. They also studied both children and adults and combined the results together.
Before I talk about the results, let me tell you about the pump. This is a tubed pump that has a sensor that it communicates with – it’s the Dexcom sensor – and it has an algorithm so it does automated insulin delivery. Instead of having to enter all sorts of information into the system, this thing requires that you put in only the patient’s weight. That’s it. From there, the system begins to figure out what the patient needs in terms of automated insulin delivery.
There are several different target settings that can be entered, and they can differ by time of day. There’s basically the time of day that one is eating a meal, so breakfast, lunch, or dinner, and there is the meal size, basically small, medium, and large. The individual enters this in real time so the system knows they’re eating, but other than that, the system just works.
It does this in a way that doesn’t allow for the individual using the pump to fidget with it. They can’t override the system and they can’t put in other insulin doses. The system is just there to take care of their diabetes.
They compared this system with people on any other system, including other automated insulin delivery systems, and put them into this trial. People were randomized to this system vs. whatever they’d been on (that was the control group) and they followed them for 13 weeks, which is not all that long.
There was a 0.5% reduction in A1c between the two groups. There was also an increase in the time in range, and this improvement in time in range happened almost immediately – within the first day or two of people being on the system. In terms of actual numbers, the adult patients started out with a time in range of 56% and this increased to 69% by the end of the study. The biggest improvement was time in range overnight, as is seen with other automated insulin delivery systems.
There was no reduction in time below a glucose level of 54 and there was an increase in the number of episodes of severe hypoglycemia in the group treated with the iLet system, but this was not statistically significant between the two groups.
I think these results are hard to compare with other pivotal trials investigating automated insulin delivery systems. The Tandem pivotal trial was a randomized controlled trial similar to this one, but the Medtronic and Omnipod studies were single-arm trials where patients were compared before and after they used the device.
More than anything, I think what’s important about this system is that it may allow for greater use of automated insulin delivery systems. It may allow primary care providers to use these systems without needing all sorts of support, and patients may be able to use these devices more simply than a device where they have to do carb counting and adjusting in ways that I think tend to be pretty complicated and require higher numeracy and literacy skills.
I couldn’t be happier. I love what they’re doing at Beta Bionics, and I look forward to more results, and in particular, to see if these results improve further when they do a study of insulin and glucagon in their dual-hormone pump system.
Thank you very much. This has been Dr Anne Peters for Medscape.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations. She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Today, I’m going to discuss the results of a new automated insulin delivery system that I think can really help many people with type 1 diabetes.
Dr. Steven Russell presented the results at the Advanced Technologies & Treatments for Diabetes meeting. The study focused on the iLet system, which is made by Beta Bionics and has been under development for a while. This was the single-hormone study, so it just looked at their algorithm using insulin alone. Eventually they’re going to study this, looking at the use of insulin plus glucagon together to see if that further improves outcomes.
One of the main reasons I think this study was so cool is because it included over 25% minority individuals who aren’t routinely studied in these insulin device trials. The study also included people who had a wide range of hemoglobin A1c levels; there was no high cut-point here. Over 30% of participants had an A1c greater than 8%. They also studied both children and adults and combined the results together.
Before I talk about the results, let me tell you about the pump. This is a tubed pump that has a sensor that it communicates with – it’s the Dexcom sensor – and it has an algorithm so it does automated insulin delivery. Instead of having to enter all sorts of information into the system, this thing requires that you put in only the patient’s weight. That’s it. From there, the system begins to figure out what the patient needs in terms of automated insulin delivery.
There are several different target settings that can be entered, and they can differ by time of day. There’s basically the time of day that one is eating a meal, so breakfast, lunch, or dinner, and there is the meal size, basically small, medium, and large. The individual enters this in real time so the system knows they’re eating, but other than that, the system just works.
It does this in a way that doesn’t allow for the individual using the pump to fidget with it. They can’t override the system and they can’t put in other insulin doses. The system is just there to take care of their diabetes.
They compared this system with people on any other system, including other automated insulin delivery systems, and put them into this trial. People were randomized to this system vs. whatever they’d been on (that was the control group) and they followed them for 13 weeks, which is not all that long.
There was a 0.5% reduction in A1c between the two groups. There was also an increase in the time in range, and this improvement in time in range happened almost immediately – within the first day or two of people being on the system. In terms of actual numbers, the adult patients started out with a time in range of 56% and this increased to 69% by the end of the study. The biggest improvement was time in range overnight, as is seen with other automated insulin delivery systems.
There was no reduction in time below a glucose level of 54 and there was an increase in the number of episodes of severe hypoglycemia in the group treated with the iLet system, but this was not statistically significant between the two groups.
I think these results are hard to compare with other pivotal trials investigating automated insulin delivery systems. The Tandem pivotal trial was a randomized controlled trial similar to this one, but the Medtronic and Omnipod studies were single-arm trials where patients were compared before and after they used the device.
More than anything, I think what’s important about this system is that it may allow for greater use of automated insulin delivery systems. It may allow primary care providers to use these systems without needing all sorts of support, and patients may be able to use these devices more simply than a device where they have to do carb counting and adjusting in ways that I think tend to be pretty complicated and require higher numeracy and literacy skills.
I couldn’t be happier. I love what they’re doing at Beta Bionics, and I look forward to more results, and in particular, to see if these results improve further when they do a study of insulin and glucagon in their dual-hormone pump system.
Thank you very much. This has been Dr Anne Peters for Medscape.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations. She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen.
A version of this article first appeared on Medscape.com.
Moderate-to-severe atopic dermatitis: Rapid improvement in itch with baricitinib vs. dupilumab
Key clinical point: Baricitinib vs. dupilumab demonstrated similar efficacy in reducing atopic dermatitis (AD) severity and improving quality of life (QoL), with more rapid improvement in itch.
Major finding: A dose of 4 mg baricitinib vs. dupilumab as monotherapy or with topical corticosteroids (TCS) was more likely to show ≥4-point improvement in itch scores at 4 weeks among patients with inadequate response or intolerance to topical treatments (dupliumab: relative risk [RR] 2.62; P = .013; TCS: RR 2.16; P = .029). However, both drugs showed similar efficacy across Eczema Area and Severity Index 75, itch, and QoL scores at 16 weeks.
Study details: This data comes from an indirect treatment comparison analysis of nine placebo-controlled trials included 3364 adults with moderate-to-severe AD and inadequate response or intolerance to topical treatments or cyclosporine who received dupilumab ± TCS or baricitinib ± TCS.
Disclosures: This study was supported by Eli Lilly and Company. Five authors declared being employees or shareholders of Eli Lilly. The other authors reported ties with various sources, including Eli Lilly.
Source: de Bruin-Weller MS et al. Indirect treatment comparison of baricitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis. Dermatol Ther (Heidelb). 2022 (May 11). Doi: 10.1007/s13555-022-00734-w
Key clinical point: Baricitinib vs. dupilumab demonstrated similar efficacy in reducing atopic dermatitis (AD) severity and improving quality of life (QoL), with more rapid improvement in itch.
Major finding: A dose of 4 mg baricitinib vs. dupilumab as monotherapy or with topical corticosteroids (TCS) was more likely to show ≥4-point improvement in itch scores at 4 weeks among patients with inadequate response or intolerance to topical treatments (dupliumab: relative risk [RR] 2.62; P = .013; TCS: RR 2.16; P = .029). However, both drugs showed similar efficacy across Eczema Area and Severity Index 75, itch, and QoL scores at 16 weeks.
Study details: This data comes from an indirect treatment comparison analysis of nine placebo-controlled trials included 3364 adults with moderate-to-severe AD and inadequate response or intolerance to topical treatments or cyclosporine who received dupilumab ± TCS or baricitinib ± TCS.
Disclosures: This study was supported by Eli Lilly and Company. Five authors declared being employees or shareholders of Eli Lilly. The other authors reported ties with various sources, including Eli Lilly.
Source: de Bruin-Weller MS et al. Indirect treatment comparison of baricitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis. Dermatol Ther (Heidelb). 2022 (May 11). Doi: 10.1007/s13555-022-00734-w
Key clinical point: Baricitinib vs. dupilumab demonstrated similar efficacy in reducing atopic dermatitis (AD) severity and improving quality of life (QoL), with more rapid improvement in itch.
Major finding: A dose of 4 mg baricitinib vs. dupilumab as monotherapy or with topical corticosteroids (TCS) was more likely to show ≥4-point improvement in itch scores at 4 weeks among patients with inadequate response or intolerance to topical treatments (dupliumab: relative risk [RR] 2.62; P = .013; TCS: RR 2.16; P = .029). However, both drugs showed similar efficacy across Eczema Area and Severity Index 75, itch, and QoL scores at 16 weeks.
Study details: This data comes from an indirect treatment comparison analysis of nine placebo-controlled trials included 3364 adults with moderate-to-severe AD and inadequate response or intolerance to topical treatments or cyclosporine who received dupilumab ± TCS or baricitinib ± TCS.
Disclosures: This study was supported by Eli Lilly and Company. Five authors declared being employees or shareholders of Eli Lilly. The other authors reported ties with various sources, including Eli Lilly.
Source: de Bruin-Weller MS et al. Indirect treatment comparison of baricitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis. Dermatol Ther (Heidelb). 2022 (May 11). Doi: 10.1007/s13555-022-00734-w
Repeat endoscopy for deliberate foreign body ingestions
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9
A 35-year-old female with a complex psychiatric history and polysubstance use presents to the emergency department following ingestion of three sewing needles. The patient has a long history of multiple suicide attempts and foreign-body ingestions requiring repeated endoscopy. Prior ingestions include, but are not limited to, razor blades, screws, toothbrushes, batteries, plastic cutlery, and shower curtain rings. The patient has had over 50 upper endoscopies within the past year in addition to a laryngoscopy and bronchoscopy for retrieval of foreign bodies. Despite intensive inpatient psychiatric treatment and outpatient behavioral therapy, the patient continues to present with recurrent ingestions, creating frustration among multiple health care providers. Are gastroenterologists obligated to perform repeated endoscopies for recurrent foreign-body ingestions? Is there a point at which it would be medically and ethically appropriate to defer endoscopy in this clinical scenario?
Deliberate foreign-body ingestion (DFBI) is a psychological disorder in which patients swallow nonnutritive objects. The disorder is commonly seen in young female patients with psychiatric disorders.1 It is also associated with substance abuse, intellectual disabilities, and malingering (such as external motivation to avoid jail). Of those with psychiatric disorders, repeat ingestions are primarily seen in patients with borderline personality disorder (BPD) or part of a syndrome of self-mutilation or attention-seeking behavior.2 Patients with BPD are thought to have atrophic changes in the brain causing neurocognitive dysfunction accounting for such behaviors.1 Self-injurious behavior is also associated with a history of abandonment and childhood abuse.3 Studies show that 85% of patients evaluated for DFBI have a prior psychiatric diagnosis and 84% of these patients have a history of prior ingestions.4
In this case, the patient’s needles were successfully removed endoscopically. The psychiatry service adjusted her medication regimen and conducted a prolonged behavioral therapy session focused on coping strategies and impulse control. The following morning, the patient managed to overpower her 24-hour 1:1 sitter to ingest a pen. Endoscopy was performed again, with successful removal of the pen.
Although intentional ingestions occur in a small subset of patients, DFBI utilizes significant hospital and fiscal resources. The startling economic impact of caring for these patients was demonstrated in a cost analysis at a large academic center in Rhode Island. It found 33 patients with repeated ingestions accounted for over 300 endoscopies in an 8-year period culminating in a total hospitalization cost of 2 million dollars per year.5 Another study estimated the average cost of a patient with DFBI per hospital visit to be $6,616 in the United States with an average length of stay of 5.6 days.6 The cost burden is largely caused by the repetitive nature of the clinical presentation and involvement of multiple disciplines, including emergency medicine, gastroenterology, anesthesia, psychiatry, social work, security services, and in some cases, otolaryngology, pulmonology, and surgery.
In addition to endoscopy, an inpatient admission for DFBI centers around preventing repeated ingestions. This entails constant observation by security or a sitter, limiting access to objects through restraints or room modifications, and psychiatry consultation for management of the underlying psychiatric disorder. Studies show this management approach rarely succeeds in preventing recurrent ingestions.6 Interestingly, data also shows inpatient psychiatric admission is not beneficial in preventing recurrent DFBI and can paradoxically increase the frequency of swallowing behavior in some patients.6 This patient failed multiple inpatient treatment programs and was noncompliant with outpatient therapies. Given the costly burden to the health care system and propensity of repeated behavior, should this patient continue to receive endoscopies? Would it ever be justifiable to forgo endoscopic retrieval?
One of the fundamental principles of medical ethics is beneficence, supporting the notion that all providers should act in the best interest of the patient. Adults may make poor or self-destructive choices, but that does not preclude our moral obligation to treat them. Patients with substance abuse disorders may repeatedly use emergency room services for acute intoxication and overdose treatment. An emergency department physician would not withhold Narcan from a patient simply because of the frequency of repeated overdoses. A similar rationale could be applied to patients with DFBI – they should undergo endoscopy if they are accepting of the risks/benefits of repeated procedures. Given that this patient’s repeated ingestions are suicide attempts, it could be argued that not removing the object would make a clinician complicit with a patient’s suicide attempt or intent of self-harm.
From an alternative vantage point, patients with repeated DFBI have an increased risk of complications with repeated endoscopy, especially when performed emergently. Patients may have an increased risk of aspiration because of insufficient preoperative fasting, and attempted removal of ingested needles and other sharp objects carries a high risk of penetrating trauma, bleeding, and perforation. The patient’s swallowing history predicts a high likelihood of repeat ingestion which, over time, makes subsequent endoscopies seem futile. Endoscopic treatment does not address the underlying problem and only serves as a temporary fix to bridge the patient to their next ingestion. Furthermore, the utilization of resources is substantial – namely, the repeated emergency use of anesthesia and operating room and endoscopy staff, as well as the psychiatry, surgical, internal medicine, and gastroenterology services. Inevitably, treatment of a patient such as this diverts limited health care resources away from other patients who may have equally or more pressing medical needs.
Despite the seemingly futile nature of these procedures and strain on resources, it would be difficult from a medicolegal perspective to justify withholding endoscopy. In 1986, the Emergency Medical Treatment and Labor Act was enacted that requires anyone presenting to an emergency department to be stabilized and treated.7 In this particular patient case, an ethics consultation was obtained and recommended that the patient continue to undergo endoscopy. However, the team also suggested that a multidisciplinary meeting with ethics, the primary and procedural teams, and the hospital’s medicolegal department be held to further elucidate a plan for future admissions and to decide if or when it may be appropriate to withhold invasive procedures. This case was presented at our weekly gastroenterology grand rounds, and procedural guidelines were reviewed. Given the size and nature of most of the objects the patient ingests, we reviewed that it would be safe in the majority of scenarios to wait until the morning for removal if called overnight – providing some relief to those on call while minimizing utilization of emergency anesthesia resources as well as operating room and endoscopy staff.
Caring for these patients is challenging as providers may feel frustrated and angry after repeated admissions. The patient may sense the low morale from providers and feel judged for their actions. It is theorized that this leads to repeated ingestions as a defense mechanism and a means of acting out.1 Additionally, friction can develop between teams as there is a common perception that psychiatry is not “doing enough” to treat the psychiatric disorder to prevent recurrences.8
In conclusion, DFBIs occur in a small number of patients with psychiatric disorders, but account for a large utilization of health care recourses. Gastroenterologists have an ethical and legal obligation to provide treatment including repeat endoscopies as long as the therapeutic benefit of the procedure outweighs risks. A multidisciplinary approach with individualized care plans can help prevent recurrent hospitalizations and procedures which may, in turn, improve outcomes and reduce health care costs.1 Until the patient and clinicians can successfully mitigate the psychiatric and social factors perpetuating repeated ingestions, gastroenterologists will continue to provide endoscopic management. Individual cases should be discussed with the hospital’s ethics and medicolegal teams for further guidance on deferring endoscopic treatment in cases of medically refractory psychological disease.
Dr. Sims is a gastroenterology fellow in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. Dr. Rao is assistant professor in the section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine. They had no conflicts of interest to disclose.
References
1. Bangash F et al. Cureus. 2021 Feb;13(2):e13179. doi: 10.7759/cureus.13179
2. Palese C et al. Gastroenterol Hepatol (N Y). 2012 July;8(7):485-6
3. Gitlin GF et al. Psychosomatics, 2007 March;48(2):162-6. doi: 10.1176/appi.psy.48.2.162
4. Palta R et al. Gastrointest Endosc. 2009 March;69(3):426-33. doi: 10.1016/j.gie.2008.05.072
5. Huang BL et al. Clin Gastroenterol Hepatol. 2010 Nov;8(11):941-6. doi: 10.1016/j.cgh.2010.07.013
6. Poynter BA et al. Gen Hosp Psychiatry. 2011 Sep-Oct;33(5):518-24. doi: 10.1016/j.genhosppsych.2011.06.011
7. American College of Emergency Physicians, EMTALA Fact Sheet. https://www.acep.org/life-as-a-physician/ethics--legal/emtala/emtala-fact-sheet/
8. Grzenda A. Carlat Hosp Psych Report. 2021 Jan;1(1 ):5-9