User login
Justice Department task force to fight abortion ban overreach
Department officials announced July 12 that the task force formalizes an existing work group and recent efforts to protect access to reproductive health care considering the Supreme Court’s decision to overturn Roe v. Wade.
The task force will monitor state and local legislation and consider legal action against states that ban abortion medication, out-of-state travel for an abortion, and other measures that try to prevent reproductive health services that are authorized by federal law.
“The Supreme Court’s Dobbs decision is a devastating blow to reproductive freedom in the United States,” Associate Attorney General Vanita Gupta, the task force chair, said in a statement.
“The Court abandoned 50 years of precedent and took away the constitutional right to abortion, preventing women all over the country from being able to make critical decisions about our bodies, our health, and our futures,” she said. “The Justice Department is committed to protecting access to reproductive services.”
The task force includes representatives from the Justice Department’s Civil Division, Civil Rights Division, U.S. attorneys’ offices, Office of the Solicitor General, Office for Access to Justice, Office of Legal Counsel, Office of Legal Policy, Office of Legislative Affairs, Office of the Associate Attorney General, Office of the Deputy Attorney General, and Office of the Attorney General.
The task force is charged with coordinating federal government responses, including proactive and defensive legal action, the department said. Task force members will work with agencies across the federal government to support their work on issues related to reproductive rights and access to reproductive health care.
The Justice Department will also continue to work with external groups, such as reproductive services providers, advocates, and state attorneys general offices. It will also work with the Office of Counsel to the President to hold a meeting with private pro bono attorneys, bar associations, and public interest groups to encourage lawyers to represent patients, providers, and others in reproductive health services cases.
“Recognizing that the best way to protect reproductive freedom is through congressional action, the task force will also coordinate providing technical assistance to Congress in connection with federal legislation to codify reproductive rights and ensure access to comprehensive reproductive services,” the department wrote. “It will also coordinate the provision of technical assistance concerning federal constitutional protections to states seeking to afford legal protection to out-of-state patients and providers who offer legal reproductive health care.”
The announcement comes as some activists and lawmakers have expressed frustration about the White House’s response to changes in abortion law in recent weeks, according to The Washington Post. They’ve called on the Biden administration to do more in the wake of the Supreme Court ruling.
On July 8, President Joe Biden signed an executive order to direct his administration to pursue a variety of measures aimed at protecting abortion access, reproductive health care services, and patient privacy.
On July 11, the Department of Health & Human Services issued guidance to remind hospitals of their duty to comply with the Emergency Medical Treatment and Labor Act (EMTALA), which stands “irrespective of any state laws or mandates that apply to specific procedures.” The law requires health care personnel to provide medical screening and stabilizing treatment to patients in emergency medical situations. In the case of pregnancy, emergencies may include ectopic pregnancy, complications of pregnancy loss, or severe hypertensive disorders. Doctors must terminate a pregnancy if it’s necessary to stabilize the patient.
“When a state law prohibits abortion and does not include an exception for the life and health of the pregnant person – or draws the exception more narrowly than EMTALA’s emergency medical condition definition – that state law is preempted,” the department wrote.
Since the Supreme Court’s ruling to overturn Roe, more than a dozen states have moved to ban or severely restrict abortions, according to a state tracker by The Washington Post. Some of the laws have been temporarily blocked by courts in Kentucky, Louisiana, and Utah.
At the same time, some Republican-led states have moved to ban other reproductive health care services, such as abortion medication and telehealth visits, the newspaper reported. The Food and Drug Administration approved mifepristone in 2000, saying the pill is safe and effective for use during the first 10 weeks of pregnancy.
The Justice Department task force said it will monitor legislation that seeks to ban mifepristone, as well as block people’s ability to inform each other about reproductive care available across the country.
“We’re seeing the intimidation already in states that are making people afraid to share information about legal abortion services in other states,” Nancy Northup, president and chief executive of the Center for Reproductive Rights, told the newspaper.
The center served as the legal counsel for the Jackson Women’s Health Organization in the case that overturned Roe. Ms. Northup said the group is already involved in more than three dozen lawsuits and has filed several more since the Supreme Court’s ruling.
“It is a really frightening time,” she said.
A version of this article first appeared on WebMD.com.
Department officials announced July 12 that the task force formalizes an existing work group and recent efforts to protect access to reproductive health care considering the Supreme Court’s decision to overturn Roe v. Wade.
The task force will monitor state and local legislation and consider legal action against states that ban abortion medication, out-of-state travel for an abortion, and other measures that try to prevent reproductive health services that are authorized by federal law.
“The Supreme Court’s Dobbs decision is a devastating blow to reproductive freedom in the United States,” Associate Attorney General Vanita Gupta, the task force chair, said in a statement.
“The Court abandoned 50 years of precedent and took away the constitutional right to abortion, preventing women all over the country from being able to make critical decisions about our bodies, our health, and our futures,” she said. “The Justice Department is committed to protecting access to reproductive services.”
The task force includes representatives from the Justice Department’s Civil Division, Civil Rights Division, U.S. attorneys’ offices, Office of the Solicitor General, Office for Access to Justice, Office of Legal Counsel, Office of Legal Policy, Office of Legislative Affairs, Office of the Associate Attorney General, Office of the Deputy Attorney General, and Office of the Attorney General.
The task force is charged with coordinating federal government responses, including proactive and defensive legal action, the department said. Task force members will work with agencies across the federal government to support their work on issues related to reproductive rights and access to reproductive health care.
The Justice Department will also continue to work with external groups, such as reproductive services providers, advocates, and state attorneys general offices. It will also work with the Office of Counsel to the President to hold a meeting with private pro bono attorneys, bar associations, and public interest groups to encourage lawyers to represent patients, providers, and others in reproductive health services cases.
“Recognizing that the best way to protect reproductive freedom is through congressional action, the task force will also coordinate providing technical assistance to Congress in connection with federal legislation to codify reproductive rights and ensure access to comprehensive reproductive services,” the department wrote. “It will also coordinate the provision of technical assistance concerning federal constitutional protections to states seeking to afford legal protection to out-of-state patients and providers who offer legal reproductive health care.”
The announcement comes as some activists and lawmakers have expressed frustration about the White House’s response to changes in abortion law in recent weeks, according to The Washington Post. They’ve called on the Biden administration to do more in the wake of the Supreme Court ruling.
On July 8, President Joe Biden signed an executive order to direct his administration to pursue a variety of measures aimed at protecting abortion access, reproductive health care services, and patient privacy.
On July 11, the Department of Health & Human Services issued guidance to remind hospitals of their duty to comply with the Emergency Medical Treatment and Labor Act (EMTALA), which stands “irrespective of any state laws or mandates that apply to specific procedures.” The law requires health care personnel to provide medical screening and stabilizing treatment to patients in emergency medical situations. In the case of pregnancy, emergencies may include ectopic pregnancy, complications of pregnancy loss, or severe hypertensive disorders. Doctors must terminate a pregnancy if it’s necessary to stabilize the patient.
“When a state law prohibits abortion and does not include an exception for the life and health of the pregnant person – or draws the exception more narrowly than EMTALA’s emergency medical condition definition – that state law is preempted,” the department wrote.
Since the Supreme Court’s ruling to overturn Roe, more than a dozen states have moved to ban or severely restrict abortions, according to a state tracker by The Washington Post. Some of the laws have been temporarily blocked by courts in Kentucky, Louisiana, and Utah.
At the same time, some Republican-led states have moved to ban other reproductive health care services, such as abortion medication and telehealth visits, the newspaper reported. The Food and Drug Administration approved mifepristone in 2000, saying the pill is safe and effective for use during the first 10 weeks of pregnancy.
The Justice Department task force said it will monitor legislation that seeks to ban mifepristone, as well as block people’s ability to inform each other about reproductive care available across the country.
“We’re seeing the intimidation already in states that are making people afraid to share information about legal abortion services in other states,” Nancy Northup, president and chief executive of the Center for Reproductive Rights, told the newspaper.
The center served as the legal counsel for the Jackson Women’s Health Organization in the case that overturned Roe. Ms. Northup said the group is already involved in more than three dozen lawsuits and has filed several more since the Supreme Court’s ruling.
“It is a really frightening time,” she said.
A version of this article first appeared on WebMD.com.
Department officials announced July 12 that the task force formalizes an existing work group and recent efforts to protect access to reproductive health care considering the Supreme Court’s decision to overturn Roe v. Wade.
The task force will monitor state and local legislation and consider legal action against states that ban abortion medication, out-of-state travel for an abortion, and other measures that try to prevent reproductive health services that are authorized by federal law.
“The Supreme Court’s Dobbs decision is a devastating blow to reproductive freedom in the United States,” Associate Attorney General Vanita Gupta, the task force chair, said in a statement.
“The Court abandoned 50 years of precedent and took away the constitutional right to abortion, preventing women all over the country from being able to make critical decisions about our bodies, our health, and our futures,” she said. “The Justice Department is committed to protecting access to reproductive services.”
The task force includes representatives from the Justice Department’s Civil Division, Civil Rights Division, U.S. attorneys’ offices, Office of the Solicitor General, Office for Access to Justice, Office of Legal Counsel, Office of Legal Policy, Office of Legislative Affairs, Office of the Associate Attorney General, Office of the Deputy Attorney General, and Office of the Attorney General.
The task force is charged with coordinating federal government responses, including proactive and defensive legal action, the department said. Task force members will work with agencies across the federal government to support their work on issues related to reproductive rights and access to reproductive health care.
The Justice Department will also continue to work with external groups, such as reproductive services providers, advocates, and state attorneys general offices. It will also work with the Office of Counsel to the President to hold a meeting with private pro bono attorneys, bar associations, and public interest groups to encourage lawyers to represent patients, providers, and others in reproductive health services cases.
“Recognizing that the best way to protect reproductive freedom is through congressional action, the task force will also coordinate providing technical assistance to Congress in connection with federal legislation to codify reproductive rights and ensure access to comprehensive reproductive services,” the department wrote. “It will also coordinate the provision of technical assistance concerning federal constitutional protections to states seeking to afford legal protection to out-of-state patients and providers who offer legal reproductive health care.”
The announcement comes as some activists and lawmakers have expressed frustration about the White House’s response to changes in abortion law in recent weeks, according to The Washington Post. They’ve called on the Biden administration to do more in the wake of the Supreme Court ruling.
On July 8, President Joe Biden signed an executive order to direct his administration to pursue a variety of measures aimed at protecting abortion access, reproductive health care services, and patient privacy.
On July 11, the Department of Health & Human Services issued guidance to remind hospitals of their duty to comply with the Emergency Medical Treatment and Labor Act (EMTALA), which stands “irrespective of any state laws or mandates that apply to specific procedures.” The law requires health care personnel to provide medical screening and stabilizing treatment to patients in emergency medical situations. In the case of pregnancy, emergencies may include ectopic pregnancy, complications of pregnancy loss, or severe hypertensive disorders. Doctors must terminate a pregnancy if it’s necessary to stabilize the patient.
“When a state law prohibits abortion and does not include an exception for the life and health of the pregnant person – or draws the exception more narrowly than EMTALA’s emergency medical condition definition – that state law is preempted,” the department wrote.
Since the Supreme Court’s ruling to overturn Roe, more than a dozen states have moved to ban or severely restrict abortions, according to a state tracker by The Washington Post. Some of the laws have been temporarily blocked by courts in Kentucky, Louisiana, and Utah.
At the same time, some Republican-led states have moved to ban other reproductive health care services, such as abortion medication and telehealth visits, the newspaper reported. The Food and Drug Administration approved mifepristone in 2000, saying the pill is safe and effective for use during the first 10 weeks of pregnancy.
The Justice Department task force said it will monitor legislation that seeks to ban mifepristone, as well as block people’s ability to inform each other about reproductive care available across the country.
“We’re seeing the intimidation already in states that are making people afraid to share information about legal abortion services in other states,” Nancy Northup, president and chief executive of the Center for Reproductive Rights, told the newspaper.
The center served as the legal counsel for the Jackson Women’s Health Organization in the case that overturned Roe. Ms. Northup said the group is already involved in more than three dozen lawsuits and has filed several more since the Supreme Court’s ruling.
“It is a really frightening time,” she said.
A version of this article first appeared on WebMD.com.
Minimal differences between biologics approved for severe asthma
Differences in the safety and efficacy between the biologics approved for the treatment of severe eosinophilic asthma are so minimal as to not meet clinically important thresholds, a network meta-analysis shows.
“We know relatively little of the comparative effectiveness or safety of biologics approved for the treatment of asthma [but since] the opportunities to use these biologics will only continue to increase, and we need to know more about their comparative effectiveness to optimize their use,” Ayobami Akenroye, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“But the decision to use one biologic or not is complex and goes beyond comparative effectiveness, and factors such as insurance coverage, convenience of self-administration, and comorbidities all play a role in the choice of biologics,” she said, adding that all the outcomes assessed in the study contribute to or reflect a patient’s underlying asthma control.
The study was published online in the Journal of Allergy and Clinical Immunology.
Interleukin pathways
Drugs that target various interleukin signaling pathways involved in the pathogenesis of asthma include mepolizumab (Nucala), benralizumab (Fasenra), and dupilumab (Dupixent), all of which have been shown to decrease exacerbation rates, improve lung function, and enhance quality of life for patients with severe eosinophilic asthma. In a Bayesian network meta-analysis that allows for simultaneous comparisons of these three treatments, investigators analyzed eight randomized, placebo-controlled trials that compared each of the drugs with placebo. In total, the trials involved 6,461 patients; the duration of follow-up was between 24 and 56 weeks.
“In the subgroup of patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics were significantly better than placebo in reducing exacerbations,” Dr. Akenroye and colleagues reported. For example, dupilumab reduced the exacerbation risk by 68% at a risk ratio of 0.32 (95% confidence interval, 0.23-0.45), while mepolizumab reduced it by almost as much at 63% (RR, 0.37; 95% CI, 0.30-0.45).
Benralizumab was slightly less effective than the other two biologics, reducing exacerbation risk by 51% (RR, 0.49; 95% CI, 0.43-0.55). “In patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics had a probability of 1 in improving the exacerbation rate by 20% or more ... in comparison to placebo,” the authors wrote.
Regarding each drug’s effect in improving forced expiratory volume in 1 second (FEV1), the mean difference in milliliters with dupilumab before and after treatment was 230 (95% CI, 160-300), while for benralizumab, the MD was 150 (95% CI, 100-220) before and after treatment. With mepolizumab, the MD in FEV1 before and after treatment was also 150. In the same subgroup of patients with eosinophil counts of at least300 cells/mcL, all three biologics again had a probability of 1 in improving FEV1 by 50 mL or more above the placebo effect. A third endpoint that was analyzed was the potential reduction in asthma control questionnaire (ACQ) scores. With mepolizumab, the MD before and after treatment was –0.65 (95% CI, –0.81 to –0.45); with dupilumab, it was –0.48 (95% CI, –0.83 to –0.14); and with dupilumab, it was –0.32 (95% CI, –0.43 to –0.21).
“Dupilumab was significantly better than benralizumab in improving exacerbations,” the authors noted (RR, 0.66; 95% CI, 0.47-0.94), while mepolizumab was also better than benralizumab (RR, 0.75; 95% CI, 0.60-0.95). On the other hand, both dupilumab and benralizumab led to greater improvements in FEV1 than mepolizumab, although the effects of dupilumab and benralizumab on ACQ scores were not significantly different for patients whose lower eosinophil counts were between 150 and 299 cells/mcL.
As for safety outcomes, both mepolizumab and benralizumab were associated with a lower risk of serious adverse events, but dupilumab was not different from placebo in terms of overall safety, according to the authors. “The ultimate choice of biologic for each patient would ... depend on multiple factors including cost considerations and timing of administration.
“[However], these results may be helpful to clinicians as they optimize patient care,” they concluded. Limitations to the analysis include the fact that indirect comparisons cannot replace randomized trials that compare the three drugs directly.
It’s estimated that 5%-10% of the 26 million individuals with asthma in the United States have severe disease.
Dr. Akenroye disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Differences in the safety and efficacy between the biologics approved for the treatment of severe eosinophilic asthma are so minimal as to not meet clinically important thresholds, a network meta-analysis shows.
“We know relatively little of the comparative effectiveness or safety of biologics approved for the treatment of asthma [but since] the opportunities to use these biologics will only continue to increase, and we need to know more about their comparative effectiveness to optimize their use,” Ayobami Akenroye, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“But the decision to use one biologic or not is complex and goes beyond comparative effectiveness, and factors such as insurance coverage, convenience of self-administration, and comorbidities all play a role in the choice of biologics,” she said, adding that all the outcomes assessed in the study contribute to or reflect a patient’s underlying asthma control.
The study was published online in the Journal of Allergy and Clinical Immunology.
Interleukin pathways
Drugs that target various interleukin signaling pathways involved in the pathogenesis of asthma include mepolizumab (Nucala), benralizumab (Fasenra), and dupilumab (Dupixent), all of which have been shown to decrease exacerbation rates, improve lung function, and enhance quality of life for patients with severe eosinophilic asthma. In a Bayesian network meta-analysis that allows for simultaneous comparisons of these three treatments, investigators analyzed eight randomized, placebo-controlled trials that compared each of the drugs with placebo. In total, the trials involved 6,461 patients; the duration of follow-up was between 24 and 56 weeks.
“In the subgroup of patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics were significantly better than placebo in reducing exacerbations,” Dr. Akenroye and colleagues reported. For example, dupilumab reduced the exacerbation risk by 68% at a risk ratio of 0.32 (95% confidence interval, 0.23-0.45), while mepolizumab reduced it by almost as much at 63% (RR, 0.37; 95% CI, 0.30-0.45).
Benralizumab was slightly less effective than the other two biologics, reducing exacerbation risk by 51% (RR, 0.49; 95% CI, 0.43-0.55). “In patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics had a probability of 1 in improving the exacerbation rate by 20% or more ... in comparison to placebo,” the authors wrote.
Regarding each drug’s effect in improving forced expiratory volume in 1 second (FEV1), the mean difference in milliliters with dupilumab before and after treatment was 230 (95% CI, 160-300), while for benralizumab, the MD was 150 (95% CI, 100-220) before and after treatment. With mepolizumab, the MD in FEV1 before and after treatment was also 150. In the same subgroup of patients with eosinophil counts of at least300 cells/mcL, all three biologics again had a probability of 1 in improving FEV1 by 50 mL or more above the placebo effect. A third endpoint that was analyzed was the potential reduction in asthma control questionnaire (ACQ) scores. With mepolizumab, the MD before and after treatment was –0.65 (95% CI, –0.81 to –0.45); with dupilumab, it was –0.48 (95% CI, –0.83 to –0.14); and with dupilumab, it was –0.32 (95% CI, –0.43 to –0.21).
“Dupilumab was significantly better than benralizumab in improving exacerbations,” the authors noted (RR, 0.66; 95% CI, 0.47-0.94), while mepolizumab was also better than benralizumab (RR, 0.75; 95% CI, 0.60-0.95). On the other hand, both dupilumab and benralizumab led to greater improvements in FEV1 than mepolizumab, although the effects of dupilumab and benralizumab on ACQ scores were not significantly different for patients whose lower eosinophil counts were between 150 and 299 cells/mcL.
As for safety outcomes, both mepolizumab and benralizumab were associated with a lower risk of serious adverse events, but dupilumab was not different from placebo in terms of overall safety, according to the authors. “The ultimate choice of biologic for each patient would ... depend on multiple factors including cost considerations and timing of administration.
“[However], these results may be helpful to clinicians as they optimize patient care,” they concluded. Limitations to the analysis include the fact that indirect comparisons cannot replace randomized trials that compare the three drugs directly.
It’s estimated that 5%-10% of the 26 million individuals with asthma in the United States have severe disease.
Dr. Akenroye disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Differences in the safety and efficacy between the biologics approved for the treatment of severe eosinophilic asthma are so minimal as to not meet clinically important thresholds, a network meta-analysis shows.
“We know relatively little of the comparative effectiveness or safety of biologics approved for the treatment of asthma [but since] the opportunities to use these biologics will only continue to increase, and we need to know more about their comparative effectiveness to optimize their use,” Ayobami Akenroye, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“But the decision to use one biologic or not is complex and goes beyond comparative effectiveness, and factors such as insurance coverage, convenience of self-administration, and comorbidities all play a role in the choice of biologics,” she said, adding that all the outcomes assessed in the study contribute to or reflect a patient’s underlying asthma control.
The study was published online in the Journal of Allergy and Clinical Immunology.
Interleukin pathways
Drugs that target various interleukin signaling pathways involved in the pathogenesis of asthma include mepolizumab (Nucala), benralizumab (Fasenra), and dupilumab (Dupixent), all of which have been shown to decrease exacerbation rates, improve lung function, and enhance quality of life for patients with severe eosinophilic asthma. In a Bayesian network meta-analysis that allows for simultaneous comparisons of these three treatments, investigators analyzed eight randomized, placebo-controlled trials that compared each of the drugs with placebo. In total, the trials involved 6,461 patients; the duration of follow-up was between 24 and 56 weeks.
“In the subgroup of patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics were significantly better than placebo in reducing exacerbations,” Dr. Akenroye and colleagues reported. For example, dupilumab reduced the exacerbation risk by 68% at a risk ratio of 0.32 (95% confidence interval, 0.23-0.45), while mepolizumab reduced it by almost as much at 63% (RR, 0.37; 95% CI, 0.30-0.45).
Benralizumab was slightly less effective than the other two biologics, reducing exacerbation risk by 51% (RR, 0.49; 95% CI, 0.43-0.55). “In patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics had a probability of 1 in improving the exacerbation rate by 20% or more ... in comparison to placebo,” the authors wrote.
Regarding each drug’s effect in improving forced expiratory volume in 1 second (FEV1), the mean difference in milliliters with dupilumab before and after treatment was 230 (95% CI, 160-300), while for benralizumab, the MD was 150 (95% CI, 100-220) before and after treatment. With mepolizumab, the MD in FEV1 before and after treatment was also 150. In the same subgroup of patients with eosinophil counts of at least300 cells/mcL, all three biologics again had a probability of 1 in improving FEV1 by 50 mL or more above the placebo effect. A third endpoint that was analyzed was the potential reduction in asthma control questionnaire (ACQ) scores. With mepolizumab, the MD before and after treatment was –0.65 (95% CI, –0.81 to –0.45); with dupilumab, it was –0.48 (95% CI, –0.83 to –0.14); and with dupilumab, it was –0.32 (95% CI, –0.43 to –0.21).
“Dupilumab was significantly better than benralizumab in improving exacerbations,” the authors noted (RR, 0.66; 95% CI, 0.47-0.94), while mepolizumab was also better than benralizumab (RR, 0.75; 95% CI, 0.60-0.95). On the other hand, both dupilumab and benralizumab led to greater improvements in FEV1 than mepolizumab, although the effects of dupilumab and benralizumab on ACQ scores were not significantly different for patients whose lower eosinophil counts were between 150 and 299 cells/mcL.
As for safety outcomes, both mepolizumab and benralizumab were associated with a lower risk of serious adverse events, but dupilumab was not different from placebo in terms of overall safety, according to the authors. “The ultimate choice of biologic for each patient would ... depend on multiple factors including cost considerations and timing of administration.
“[However], these results may be helpful to clinicians as they optimize patient care,” they concluded. Limitations to the analysis include the fact that indirect comparisons cannot replace randomized trials that compare the three drugs directly.
It’s estimated that 5%-10% of the 26 million individuals with asthma in the United States have severe disease.
Dr. Akenroye disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
FDA grants emergency authorization for Novavax COVID vaccine
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
Pembrolizumab for melanoma bittersweet, doctor says
CHICAGO – Pembrolizumab has shown promise as adjuvant therapy for stage IIB and IIC melanoma, shows the first interim analysis of the phase 3 KEYNOTE-716 study recently published in The Lancet.
The findings meet an unmet need as the recurrence risk in stage IIB and IIC melanoma is “underrecognized,” said author Georgina Long, MD, comedical director of the Melanoma Institute Australia, University of Sydney.
In fact, their risk of recurrence is similar to patients with stage IIIB disease, wrote David Killock, PhD, in a related commentary published in Nature Reviews.
The adjuvant treatment resulted in an 89% recurrence-free survival in patients who received pembrolizumab, compared with 83% of patients in the placebo group (hazard ratio, 0.65; P = .0066). These findings were used as the basis for Food and Drug Administration approval of pembrolizumab (Keytruda, Merck) for this patient population in December 2021.
Despite the positive findings, Dr. Killock called for more research on distant metastasis-free survival, overall survival, and quality of life data to “establish the true clinical benefit of adjuvant pembrolizumab.”
At the annual meeting of the American Society of Clinical Oncology, Dr. Long presented the third interim analysis which showed pembrolizumab reduced recurrence and distant metastases at 24 months, although the clinical benefit was relatively small at an approximately 8% improvement in recurrence-free survival and about a 6% improvement in distant metastasis-free survival. About 83% in the pembrolizumab group had treatment-related toxicities versus 64% in the placebo group. There were no deaths caused by treatment. About 90% of pembrolizumab-related endocrinopathies led to long-term hormone replacement.
In a discussion that followed the presentation at ASCO, Charlotte Eielson Ariyan, MD, PhD, said the results are bittersweet. Higher-risk stage IIC patients have a risk of recurrence of about 40%. “It’s high, but the absolute risk reduction is about 8%. This is a very personalized discussion with the patient and the physician in understanding their risk of toxicity is about 17% and higher than their absolute risk reduction with the treatment. For me, this is a bitter pill to swallow because you’re treating people longer and you’re not sure if you’re really helping them. Until we can further define who the highest-risk patients are, I think it’s hard to give it to everyone,” said Dr. Ariyan, who is a surgeon with Memorial Sloan Kettering Cancer Center, New York.
In addition to weighing short-term benefits and toxicity, there are longer-term concerns. Toxicity experienced from PD-1 inhibitors in the adjuvant setting could impact future treatment decisions. “We’re very lucky here in melanoma to know that systemic therapies are effective and we can cure people who recur. I would argue this is why we probably will never really see a difference in the survival benefit in this group because people who cross over will probably do well,” Dr. Ariyan said.
During the Q&A session, Vernon Sondek, MD, Moffitt Cancer Center, Tampa, encouraged physician colleagues to have an open mind about treatments. “Beware of dogma. We thought that adjuvant immunotherapy works much better in patients with ulcerated primary tumors. That’s a dogma in some parts of the world. Yet the T4a patients in KEYNOTE-716 dramatically outperformed the ulcerated T3b and T4b [patients]. We still don’t know what we don’t know.”
The study details
KEYNOTE-716 included 976 patients 12 years or older with newly diagnosed completely resected stage IIB or IIC melanoma with a negative sentinel lymph node. Patients were randomized to placebo or 200 mg pembrolizumab every 3 weeks, or 2 mg/kg in pediatric patients, over 17 cycles. Almost 40% of patients were age 65 or older. T3b and T4b were the most common melanoma subcategories at 41% and 35%, respectively.
The planned third interim analysis occurred after the occurrence of 146 distant metastases. After a median follow-up of 27.4 months, distant metastasis-free survival favored the pembrolizumab group (HR, 0.64; P = .0029). At 24 months, the pembrolizumab group had a higher distant metastasis-free survival at 88.1% versus 82.2% and a lower recurrence rate at 81.2% versus 72.8% (HR, 0.64; 95% confidence interval, 0.50-0.84).
At 24 months, only the T4a patients had a statistically significant reduction in distant metastases at 58% (HR, 0.42; 95% CI, 0.19-0.96), although there were numerical reductions in T3a (HR, 0.71; 95% CI, 0.41-1.22) and T4b (HR, 0.70; 95% CI, 0.44-1.33) patients. Of patients experiencing a distant metastasis, 73% of the placebo group had a first distant metastasis to the lung compared with 49% of the pembrolizumab group.
Dr. Long has held consulting or advisory roles for Merck Sharpe & Dohme, which funded this study.
CHICAGO – Pembrolizumab has shown promise as adjuvant therapy for stage IIB and IIC melanoma, shows the first interim analysis of the phase 3 KEYNOTE-716 study recently published in The Lancet.
The findings meet an unmet need as the recurrence risk in stage IIB and IIC melanoma is “underrecognized,” said author Georgina Long, MD, comedical director of the Melanoma Institute Australia, University of Sydney.
In fact, their risk of recurrence is similar to patients with stage IIIB disease, wrote David Killock, PhD, in a related commentary published in Nature Reviews.
The adjuvant treatment resulted in an 89% recurrence-free survival in patients who received pembrolizumab, compared with 83% of patients in the placebo group (hazard ratio, 0.65; P = .0066). These findings were used as the basis for Food and Drug Administration approval of pembrolizumab (Keytruda, Merck) for this patient population in December 2021.
Despite the positive findings, Dr. Killock called for more research on distant metastasis-free survival, overall survival, and quality of life data to “establish the true clinical benefit of adjuvant pembrolizumab.”
At the annual meeting of the American Society of Clinical Oncology, Dr. Long presented the third interim analysis which showed pembrolizumab reduced recurrence and distant metastases at 24 months, although the clinical benefit was relatively small at an approximately 8% improvement in recurrence-free survival and about a 6% improvement in distant metastasis-free survival. About 83% in the pembrolizumab group had treatment-related toxicities versus 64% in the placebo group. There were no deaths caused by treatment. About 90% of pembrolizumab-related endocrinopathies led to long-term hormone replacement.
In a discussion that followed the presentation at ASCO, Charlotte Eielson Ariyan, MD, PhD, said the results are bittersweet. Higher-risk stage IIC patients have a risk of recurrence of about 40%. “It’s high, but the absolute risk reduction is about 8%. This is a very personalized discussion with the patient and the physician in understanding their risk of toxicity is about 17% and higher than their absolute risk reduction with the treatment. For me, this is a bitter pill to swallow because you’re treating people longer and you’re not sure if you’re really helping them. Until we can further define who the highest-risk patients are, I think it’s hard to give it to everyone,” said Dr. Ariyan, who is a surgeon with Memorial Sloan Kettering Cancer Center, New York.
In addition to weighing short-term benefits and toxicity, there are longer-term concerns. Toxicity experienced from PD-1 inhibitors in the adjuvant setting could impact future treatment decisions. “We’re very lucky here in melanoma to know that systemic therapies are effective and we can cure people who recur. I would argue this is why we probably will never really see a difference in the survival benefit in this group because people who cross over will probably do well,” Dr. Ariyan said.
During the Q&A session, Vernon Sondek, MD, Moffitt Cancer Center, Tampa, encouraged physician colleagues to have an open mind about treatments. “Beware of dogma. We thought that adjuvant immunotherapy works much better in patients with ulcerated primary tumors. That’s a dogma in some parts of the world. Yet the T4a patients in KEYNOTE-716 dramatically outperformed the ulcerated T3b and T4b [patients]. We still don’t know what we don’t know.”
The study details
KEYNOTE-716 included 976 patients 12 years or older with newly diagnosed completely resected stage IIB or IIC melanoma with a negative sentinel lymph node. Patients were randomized to placebo or 200 mg pembrolizumab every 3 weeks, or 2 mg/kg in pediatric patients, over 17 cycles. Almost 40% of patients were age 65 or older. T3b and T4b were the most common melanoma subcategories at 41% and 35%, respectively.
The planned third interim analysis occurred after the occurrence of 146 distant metastases. After a median follow-up of 27.4 months, distant metastasis-free survival favored the pembrolizumab group (HR, 0.64; P = .0029). At 24 months, the pembrolizumab group had a higher distant metastasis-free survival at 88.1% versus 82.2% and a lower recurrence rate at 81.2% versus 72.8% (HR, 0.64; 95% confidence interval, 0.50-0.84).
At 24 months, only the T4a patients had a statistically significant reduction in distant metastases at 58% (HR, 0.42; 95% CI, 0.19-0.96), although there were numerical reductions in T3a (HR, 0.71; 95% CI, 0.41-1.22) and T4b (HR, 0.70; 95% CI, 0.44-1.33) patients. Of patients experiencing a distant metastasis, 73% of the placebo group had a first distant metastasis to the lung compared with 49% of the pembrolizumab group.
Dr. Long has held consulting or advisory roles for Merck Sharpe & Dohme, which funded this study.
CHICAGO – Pembrolizumab has shown promise as adjuvant therapy for stage IIB and IIC melanoma, shows the first interim analysis of the phase 3 KEYNOTE-716 study recently published in The Lancet.
The findings meet an unmet need as the recurrence risk in stage IIB and IIC melanoma is “underrecognized,” said author Georgina Long, MD, comedical director of the Melanoma Institute Australia, University of Sydney.
In fact, their risk of recurrence is similar to patients with stage IIIB disease, wrote David Killock, PhD, in a related commentary published in Nature Reviews.
The adjuvant treatment resulted in an 89% recurrence-free survival in patients who received pembrolizumab, compared with 83% of patients in the placebo group (hazard ratio, 0.65; P = .0066). These findings were used as the basis for Food and Drug Administration approval of pembrolizumab (Keytruda, Merck) for this patient population in December 2021.
Despite the positive findings, Dr. Killock called for more research on distant metastasis-free survival, overall survival, and quality of life data to “establish the true clinical benefit of adjuvant pembrolizumab.”
At the annual meeting of the American Society of Clinical Oncology, Dr. Long presented the third interim analysis which showed pembrolizumab reduced recurrence and distant metastases at 24 months, although the clinical benefit was relatively small at an approximately 8% improvement in recurrence-free survival and about a 6% improvement in distant metastasis-free survival. About 83% in the pembrolizumab group had treatment-related toxicities versus 64% in the placebo group. There were no deaths caused by treatment. About 90% of pembrolizumab-related endocrinopathies led to long-term hormone replacement.
In a discussion that followed the presentation at ASCO, Charlotte Eielson Ariyan, MD, PhD, said the results are bittersweet. Higher-risk stage IIC patients have a risk of recurrence of about 40%. “It’s high, but the absolute risk reduction is about 8%. This is a very personalized discussion with the patient and the physician in understanding their risk of toxicity is about 17% and higher than their absolute risk reduction with the treatment. For me, this is a bitter pill to swallow because you’re treating people longer and you’re not sure if you’re really helping them. Until we can further define who the highest-risk patients are, I think it’s hard to give it to everyone,” said Dr. Ariyan, who is a surgeon with Memorial Sloan Kettering Cancer Center, New York.
In addition to weighing short-term benefits and toxicity, there are longer-term concerns. Toxicity experienced from PD-1 inhibitors in the adjuvant setting could impact future treatment decisions. “We’re very lucky here in melanoma to know that systemic therapies are effective and we can cure people who recur. I would argue this is why we probably will never really see a difference in the survival benefit in this group because people who cross over will probably do well,” Dr. Ariyan said.
During the Q&A session, Vernon Sondek, MD, Moffitt Cancer Center, Tampa, encouraged physician colleagues to have an open mind about treatments. “Beware of dogma. We thought that adjuvant immunotherapy works much better in patients with ulcerated primary tumors. That’s a dogma in some parts of the world. Yet the T4a patients in KEYNOTE-716 dramatically outperformed the ulcerated T3b and T4b [patients]. We still don’t know what we don’t know.”
The study details
KEYNOTE-716 included 976 patients 12 years or older with newly diagnosed completely resected stage IIB or IIC melanoma with a negative sentinel lymph node. Patients were randomized to placebo or 200 mg pembrolizumab every 3 weeks, or 2 mg/kg in pediatric patients, over 17 cycles. Almost 40% of patients were age 65 or older. T3b and T4b were the most common melanoma subcategories at 41% and 35%, respectively.
The planned third interim analysis occurred after the occurrence of 146 distant metastases. After a median follow-up of 27.4 months, distant metastasis-free survival favored the pembrolizumab group (HR, 0.64; P = .0029). At 24 months, the pembrolizumab group had a higher distant metastasis-free survival at 88.1% versus 82.2% and a lower recurrence rate at 81.2% versus 72.8% (HR, 0.64; 95% confidence interval, 0.50-0.84).
At 24 months, only the T4a patients had a statistically significant reduction in distant metastases at 58% (HR, 0.42; 95% CI, 0.19-0.96), although there were numerical reductions in T3a (HR, 0.71; 95% CI, 0.41-1.22) and T4b (HR, 0.70; 95% CI, 0.44-1.33) patients. Of patients experiencing a distant metastasis, 73% of the placebo group had a first distant metastasis to the lung compared with 49% of the pembrolizumab group.
Dr. Long has held consulting or advisory roles for Merck Sharpe & Dohme, which funded this study.
AT ASCO 2022
What influences a trainee’s decision to choose pediatric dermatology as a career?
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
INDIANAPOLIS – Three during and after fellowship.
Those are key findings from a survey of current and prior pediatric dermatology fellows, which sought to investigate what factors influence their career decisions.
According to the study’s principal investigator, Lucia Z. Diaz, MD, pediatric dermatology suffers from workforce shortages and geographic maldistribution as a subspecialty in the United States. She also noted that, from 2016 to 2021, 100% of pediatric dermatology applicants matched, yet about 15 of every 31 positions remained unfilled during each of those years. This suggests that there may be a lack of trainee mentorship secondary to a lack of available pediatric dermatologists.
“Somewhere along the way, we lose trainees to general dermatology, or they may go through a pediatric dermatology fellowship but not actually see children upon completion of their training,” Dr. Diaz, chief of pediatric dermatology at the University of Texas at Austin, said in an interview at the annual meeting of the Society for Pediatric Dermatology, where the study was presented during a poster session. “We wanted to find out factors influencing this.”
For the study, Dr. Diaz, Courtney N. Haller, MD, a first-year dermatology resident at the University of Texas at Austin, and their colleagues emailed a 37-item survey to 59 current and prior pediatric dermatology fellows who trained in the United States in the past 4 years (classes of 2019-2022). Current fellows were asked to share their future plans, and past fellows were asked to share details about their current practice situation including practice type (such as academics, private practice, and a mix of adult and pediatrics), and the researchers used descriptive statistics and chi-square analyses to evaluate qualitative data.
In all, 41 survey participants gave complete responses, and 3 gave partial responses. Of these, 8 were current fellows, 36 were past fellows, and 38 were female. The researchers found that 67% of survey respondents first became interested in pediatric dermatology in medical school, while the decision to pursue a fellowship occurred then (33%) or during their third year of dermatology residency (33%). Early exposure to pediatric dermatology, from medical school through dermatology PGY-2, was significantly associated with an early decision to pursue a pediatric dermatology career (P = .004).
In addition, respondents at institutions with two or more pediatric dermatology faculty were significantly more likely to cite home institution mentorship as an influencing factor in their career decision (P = .035).
“I thought that the interest in pediatric dermatology would peak early on during dermatology residency, but it primarily happens during medical school,” said Dr. Diaz, who is also associate director of the dermatology residency program at the medical school. “Mentorship and early exposure to pediatric dermatology during medical school are really important.”
The top three factors that discouraged respondents from pursuing a pediatric dermatology fellowship included a lack of salary benefit with additional training (83%), additional time required to complete training (73%), and geographic relocation (20%). After fellowship, 51% of respondents said they plan to or currently work in academic settings, while 88% said they plan to work full time or currently were working full time.
Interestingly, fellows with additional pediatric training such as an internship or residency were not more likely to see a greater percentage of pediatric patients in practice than those without this training (P = .14). The top 3 reasons for not seeing pediatric patients 100% of the clinical time were interest in seeing adult patients (67%), financial factors (56%), and interest in performing more procedures (56%).
In other findings, the top three factors in deciding practice location were proximity to extended family (63%), practice type (59%), and income (51%).
Adelaide A. Hebert, MD, who was asked to comment on the study, said that the lack of salary benefit from additional training is a sticking point for many fellows. “The market trends of supply and demand do not work in pediatric dermatology,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “You would think that, because there are fewer of us, we should be paid more, but it does not work that way.”
She characterized the overall study findings as “a real testament to what the challenges are” in recruiting trainees to pediatric dermatology. “The influence of mentors resonates in this assessment, but influences that are somewhat beyond our control also play a role, such as lack of salary benefit from additional training, interest in seeing adult patients, and financial factors.”
Neither the researchers nor Dr. Hebert reported having relevant financial disclosures.
AT SPD 2022
Chest pain and difficulty swallowing
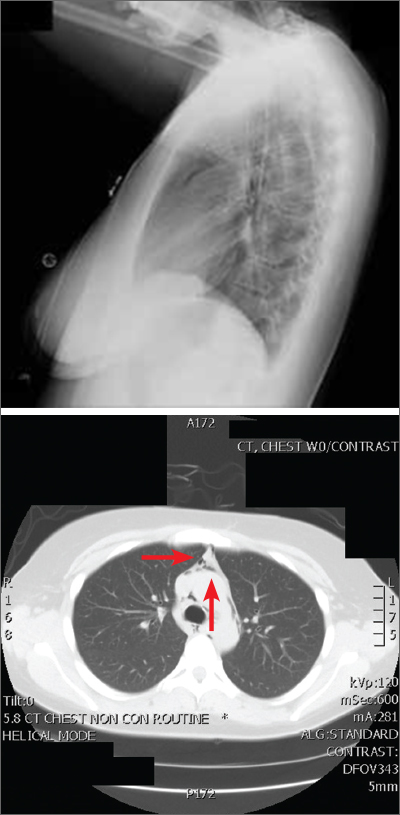
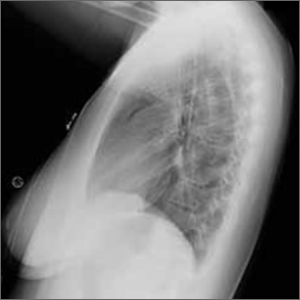
The posteroanterior (PA) and lateral view CXRs, as well as the CT scan, revealed the presence of retrosternal air and confirmed the patient’s diagnosis of pneumomediastinum.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by the Hamman sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
Pneumomediastinum is generally a self-limiting condition. Thus, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home. If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation.3 If the result of this test is abnormal, a follow-up study with barium is recommended.3
The patient underwent both swallow studies, and they were negative. This patient received subsequent serial CXRs that showed improvement in the pneumomediastinum. Once the patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged (after a 3-day hospitalization) with close follow-up. One week later, her pain had almost entirely resolved.
This case was adapted from: Gawrys B, Shaha D. Pleuritic chest pain and globus pharyngeus. J Fam Pract. 2015;64:305-307.
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Elsevier Health Sciences; 2005:2039-2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057. doi: 10.1148/radiographics.20.4.g00jl13104
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334. doi: 10.1016/j.rmed.2008.03.023

The posteroanterior (PA) and lateral view CXRs, as well as the CT scan, revealed the presence of retrosternal air and confirmed the patient’s diagnosis of pneumomediastinum.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by the Hamman sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
Pneumomediastinum is generally a self-limiting condition. Thus, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home. If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation.3 If the result of this test is abnormal, a follow-up study with barium is recommended.3
The patient underwent both swallow studies, and they were negative. This patient received subsequent serial CXRs that showed improvement in the pneumomediastinum. Once the patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged (after a 3-day hospitalization) with close follow-up. One week later, her pain had almost entirely resolved.
This case was adapted from: Gawrys B, Shaha D. Pleuritic chest pain and globus pharyngeus. J Fam Pract. 2015;64:305-307.

The posteroanterior (PA) and lateral view CXRs, as well as the CT scan, revealed the presence of retrosternal air and confirmed the patient’s diagnosis of pneumomediastinum.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by the Hamman sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
Pneumomediastinum is generally a self-limiting condition. Thus, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home. If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation.3 If the result of this test is abnormal, a follow-up study with barium is recommended.3
The patient underwent both swallow studies, and they were negative. This patient received subsequent serial CXRs that showed improvement in the pneumomediastinum. Once the patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged (after a 3-day hospitalization) with close follow-up. One week later, her pain had almost entirely resolved.
This case was adapted from: Gawrys B, Shaha D. Pleuritic chest pain and globus pharyngeus. J Fam Pract. 2015;64:305-307.
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Elsevier Health Sciences; 2005:2039-2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057. doi: 10.1148/radiographics.20.4.g00jl13104
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334. doi: 10.1016/j.rmed.2008.03.023
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Elsevier Health Sciences; 2005:2039-2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057. doi: 10.1148/radiographics.20.4.g00jl13104
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334. doi: 10.1016/j.rmed.2008.03.023
Inflammatory profiles impact major depressive disorder
Early onset of disease in patients with major depressive disorder may be linked to a specific inflammatory profile, based on data from 234 individuals.
Major depressive disorder (MDD) remains common, and evidence suggests that it is increasing among younger individuals, but data on early-onset MDD in adults are limited, Ana Paula Anzolin, a graduate student at the Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, and colleagues wrote.
Although previous studies have shown abnormal cytokine production in patients with MDD, the impact of inflammation on MDD and disease onset and progression remains unclear, they said.
In a study published in Psychiatry Research, the authors identified outpatients aged 18-85 years with confirmed MDD and scores of at least 8 on the HAM-D scale who were undergoing treatment at a single center. Early onset was defined as a diagnosis of MDD before age 30 years (99 patients) and late onset was defined as a diagnosis at age 30 years and older (135 patients). The researchers measured levels of interleukin-6, IL-1 beta, IL-10, and tumor necrosis factor alpha (TNF-alpha).
Overall, the level of cytokine profiles in early- versus late-onset disease was significantly higher for IL-1B and TNF-alpha (P < .001 for both). The significant difference between early- and late-onset disease remained regardless of comorbidity with autoimmune diseases, the researchers noted.
IL-6 levels were higher in the early-onset group and IL-10 levels were higher in the late-onset group, but these differences were not significant.
the researchers wrote.
The results also support findings from previous studies that suggest a divergence between early- and late adult–onset depression, they said. More research on early-onset MDD in adults is needed, as these patients tend to have more severe symptoms, more medical and psychiatric comorbidities, and an increased risk of depressive episodes and suicide attempts.
The study findings were limited by several factors including the lack of a control group, the retrospective assessment of disease onset, and the limited cytokines studied, which do not reflect changes in the entire immune network response, the researchers noted.
However, the study is the first known to examine the association of serum cytokines and early- and late-onset MDD in adults, and the results support the use of IL-1B and TNF-alpha as potential treatment targets in the development of new therapies for MDD, they concluded.
The study was supported by the Fundo de Incentivo à Pesquisa – Hospital de Clínicas de Porto Alegre, the Conselho Nacional de Desenvolvimento Científico e Tecnológico, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. The researchers had no financial conflicts to disclose.
Early onset of disease in patients with major depressive disorder may be linked to a specific inflammatory profile, based on data from 234 individuals.
Major depressive disorder (MDD) remains common, and evidence suggests that it is increasing among younger individuals, but data on early-onset MDD in adults are limited, Ana Paula Anzolin, a graduate student at the Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, and colleagues wrote.
Although previous studies have shown abnormal cytokine production in patients with MDD, the impact of inflammation on MDD and disease onset and progression remains unclear, they said.
In a study published in Psychiatry Research, the authors identified outpatients aged 18-85 years with confirmed MDD and scores of at least 8 on the HAM-D scale who were undergoing treatment at a single center. Early onset was defined as a diagnosis of MDD before age 30 years (99 patients) and late onset was defined as a diagnosis at age 30 years and older (135 patients). The researchers measured levels of interleukin-6, IL-1 beta, IL-10, and tumor necrosis factor alpha (TNF-alpha).
Overall, the level of cytokine profiles in early- versus late-onset disease was significantly higher for IL-1B and TNF-alpha (P < .001 for both). The significant difference between early- and late-onset disease remained regardless of comorbidity with autoimmune diseases, the researchers noted.
IL-6 levels were higher in the early-onset group and IL-10 levels were higher in the late-onset group, but these differences were not significant.
the researchers wrote.
The results also support findings from previous studies that suggest a divergence between early- and late adult–onset depression, they said. More research on early-onset MDD in adults is needed, as these patients tend to have more severe symptoms, more medical and psychiatric comorbidities, and an increased risk of depressive episodes and suicide attempts.
The study findings were limited by several factors including the lack of a control group, the retrospective assessment of disease onset, and the limited cytokines studied, which do not reflect changes in the entire immune network response, the researchers noted.
However, the study is the first known to examine the association of serum cytokines and early- and late-onset MDD in adults, and the results support the use of IL-1B and TNF-alpha as potential treatment targets in the development of new therapies for MDD, they concluded.
The study was supported by the Fundo de Incentivo à Pesquisa – Hospital de Clínicas de Porto Alegre, the Conselho Nacional de Desenvolvimento Científico e Tecnológico, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. The researchers had no financial conflicts to disclose.
Early onset of disease in patients with major depressive disorder may be linked to a specific inflammatory profile, based on data from 234 individuals.
Major depressive disorder (MDD) remains common, and evidence suggests that it is increasing among younger individuals, but data on early-onset MDD in adults are limited, Ana Paula Anzolin, a graduate student at the Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, and colleagues wrote.
Although previous studies have shown abnormal cytokine production in patients with MDD, the impact of inflammation on MDD and disease onset and progression remains unclear, they said.
In a study published in Psychiatry Research, the authors identified outpatients aged 18-85 years with confirmed MDD and scores of at least 8 on the HAM-D scale who were undergoing treatment at a single center. Early onset was defined as a diagnosis of MDD before age 30 years (99 patients) and late onset was defined as a diagnosis at age 30 years and older (135 patients). The researchers measured levels of interleukin-6, IL-1 beta, IL-10, and tumor necrosis factor alpha (TNF-alpha).
Overall, the level of cytokine profiles in early- versus late-onset disease was significantly higher for IL-1B and TNF-alpha (P < .001 for both). The significant difference between early- and late-onset disease remained regardless of comorbidity with autoimmune diseases, the researchers noted.
IL-6 levels were higher in the early-onset group and IL-10 levels were higher in the late-onset group, but these differences were not significant.
the researchers wrote.
The results also support findings from previous studies that suggest a divergence between early- and late adult–onset depression, they said. More research on early-onset MDD in adults is needed, as these patients tend to have more severe symptoms, more medical and psychiatric comorbidities, and an increased risk of depressive episodes and suicide attempts.
The study findings were limited by several factors including the lack of a control group, the retrospective assessment of disease onset, and the limited cytokines studied, which do not reflect changes in the entire immune network response, the researchers noted.
However, the study is the first known to examine the association of serum cytokines and early- and late-onset MDD in adults, and the results support the use of IL-1B and TNF-alpha as potential treatment targets in the development of new therapies for MDD, they concluded.
The study was supported by the Fundo de Incentivo à Pesquisa – Hospital de Clínicas de Porto Alegre, the Conselho Nacional de Desenvolvimento Científico e Tecnológico, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRY RESEARCH
2022 Update on menopause
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
Should treatment be initiated for mild chronic hypertension in pregnancy to improve outcomes?
Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792. doi: 10.1056/NEJMoa2201295.
Expert Commentary
In the nonpregnant population, medical management of hypertension >140/90 mm Hg is standard practice. By contrast, much higher blood pressures (BPs; up to 160/110 mm Hg) traditionally have been tolerated in pregnant patients with chronic hypertension without initiating treatment, and existing medications are often discontinued during pregnancy. Concern for impaired fetal growth as well as lack of data on improved outcomes have led to different recommendations for the management of mild chronic hypertension in pregnancy. However, chronic hypertension affects a substantial number of pregnant patients and is known to be a risk factor for severe short-term pregnancy and long-term health complications. With preliminary data suggesting that BPs >140/90 mm Hg prior to 20 weeks’ gestation are associated with an increase in adverse outcomes, Tita and colleagues sought to determine the effects of decreasing BP in pregnant patients with mild chronic hypertension.
Details about the study
This is an investigator-initiated, multicenter, pragmatic, open-label, randomized control trial of 2,408 patients with mild chronic hypertension. The active treatment group was treated with antihypertensive medication (including titration of existing medication), targeting a BP of <140/90 mm Hg. The control group only received medication for severe hypertension (≥160 mm Hg systolic or ≥105 mm Hg diastolic). The primary outcome of the study was a composite of preeclampsia with severe features, medically indicated preterm delivery prior to 35 weeks’ gestation (not spontaneous labor or rupture of membranes), placental abruption, and fetal or neonatal death. Birthweight that was less than the 10th percentile was used as a safety outcome. The hypothesis was that treatment would decrease the rate of adverse pregnancy and fetal/neonatal outcomes.
The patient population of singleton pregnancies at a gestational age of less than 23 weeks included 56% with known chronic hypertension on medications, 22% with known chronic hypertension without medications, and 22% with newly diagnosed (during pregnancy) chronic hypertension. The treatment group primarily received labetalol (61.7%) or nifedipine (35.6%); the maximum dose of a single agent was used as tolerated prior to adding a second agent. The control group only received an antihypertensive medication for severe hypertension.
Treatment of chronic hypertension demonstrated a decreased risk of the composite adverse outcome with an adjusted risk ratio of 0.82 (95% confidence interval [CI], 0.74 to 0.92; P<.001) and a number needed to treat (NNT) of 14.7. When analyzed separately, a similar risk reduction was noted for both preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation. There was no statistical difference between the groups for birth weight <10th percentile and <5th percentile (adjusted risk ratio, 1.04 [0.82–1.31] vs 0.89 [0.62–1.26], respectively).
Planned subgroup analysis by type of chronic hypertension, race/ethnic group, diabetes status, gestational age at baseline, and body mass index (BMI) demonstrated a similar treatment effect to the overall composite primary outcome, with the exception of patients with newly diagn
Study strengths and weaknesses
The study strengths cited are a large sample size, multiple study sites, an independent data and safety monitoring board with close oversight, and centralized blinded confirmation of outcomes. Another strength is that the patient population of the study was similar to the overall population of pregnant patients in the United States with chronic hypertension in terms of age, race, and ethnicity.
The weaknesses of the study include the open-label design and the high ratio of screened to enrolled patients. Both of these issues appear related to the study design (ethics and logistics of a blinded treatment and gestational age cutoff) and the physiology of pregnancy (expected decrease in BP in the second trimester rendering patients ineligible due to lower BP). The study was also not powered to assess treatment effect in all of the subgroups, and further evaluation of patients with newly diagnosed chronic hypertension and BMI ≥ 40 kg/m2 is needed. ●
Pregnant patients with chronic hypertension should continue or initiate antihypertensive medication to target a BP goal of <140/90 mm Hg. This substantial practice change is supported by the significant decrease demonstrated in this study in adverse outcomes such as preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation without an increase in small-for-gestational-age newborns.
Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792. doi: 10.1056/NEJMoa2201295.
Expert Commentary
In the nonpregnant population, medical management of hypertension >140/90 mm Hg is standard practice. By contrast, much higher blood pressures (BPs; up to 160/110 mm Hg) traditionally have been tolerated in pregnant patients with chronic hypertension without initiating treatment, and existing medications are often discontinued during pregnancy. Concern for impaired fetal growth as well as lack of data on improved outcomes have led to different recommendations for the management of mild chronic hypertension in pregnancy. However, chronic hypertension affects a substantial number of pregnant patients and is known to be a risk factor for severe short-term pregnancy and long-term health complications. With preliminary data suggesting that BPs >140/90 mm Hg prior to 20 weeks’ gestation are associated with an increase in adverse outcomes, Tita and colleagues sought to determine the effects of decreasing BP in pregnant patients with mild chronic hypertension.
Details about the study
This is an investigator-initiated, multicenter, pragmatic, open-label, randomized control trial of 2,408 patients with mild chronic hypertension. The active treatment group was treated with antihypertensive medication (including titration of existing medication), targeting a BP of <140/90 mm Hg. The control group only received medication for severe hypertension (≥160 mm Hg systolic or ≥105 mm Hg diastolic). The primary outcome of the study was a composite of preeclampsia with severe features, medically indicated preterm delivery prior to 35 weeks’ gestation (not spontaneous labor or rupture of membranes), placental abruption, and fetal or neonatal death. Birthweight that was less than the 10th percentile was used as a safety outcome. The hypothesis was that treatment would decrease the rate of adverse pregnancy and fetal/neonatal outcomes.
The patient population of singleton pregnancies at a gestational age of less than 23 weeks included 56% with known chronic hypertension on medications, 22% with known chronic hypertension without medications, and 22% with newly diagnosed (during pregnancy) chronic hypertension. The treatment group primarily received labetalol (61.7%) or nifedipine (35.6%); the maximum dose of a single agent was used as tolerated prior to adding a second agent. The control group only received an antihypertensive medication for severe hypertension.
Treatment of chronic hypertension demonstrated a decreased risk of the composite adverse outcome with an adjusted risk ratio of 0.82 (95% confidence interval [CI], 0.74 to 0.92; P<.001) and a number needed to treat (NNT) of 14.7. When analyzed separately, a similar risk reduction was noted for both preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation. There was no statistical difference between the groups for birth weight <10th percentile and <5th percentile (adjusted risk ratio, 1.04 [0.82–1.31] vs 0.89 [0.62–1.26], respectively).
Planned subgroup analysis by type of chronic hypertension, race/ethnic group, diabetes status, gestational age at baseline, and body mass index (BMI) demonstrated a similar treatment effect to the overall composite primary outcome, with the exception of patients with newly diagn
Study strengths and weaknesses
The study strengths cited are a large sample size, multiple study sites, an independent data and safety monitoring board with close oversight, and centralized blinded confirmation of outcomes. Another strength is that the patient population of the study was similar to the overall population of pregnant patients in the United States with chronic hypertension in terms of age, race, and ethnicity.
The weaknesses of the study include the open-label design and the high ratio of screened to enrolled patients. Both of these issues appear related to the study design (ethics and logistics of a blinded treatment and gestational age cutoff) and the physiology of pregnancy (expected decrease in BP in the second trimester rendering patients ineligible due to lower BP). The study was also not powered to assess treatment effect in all of the subgroups, and further evaluation of patients with newly diagnosed chronic hypertension and BMI ≥ 40 kg/m2 is needed. ●
Pregnant patients with chronic hypertension should continue or initiate antihypertensive medication to target a BP goal of <140/90 mm Hg. This substantial practice change is supported by the significant decrease demonstrated in this study in adverse outcomes such as preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation without an increase in small-for-gestational-age newborns.
Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781-1792. doi: 10.1056/NEJMoa2201295.
Expert Commentary
In the nonpregnant population, medical management of hypertension >140/90 mm Hg is standard practice. By contrast, much higher blood pressures (BPs; up to 160/110 mm Hg) traditionally have been tolerated in pregnant patients with chronic hypertension without initiating treatment, and existing medications are often discontinued during pregnancy. Concern for impaired fetal growth as well as lack of data on improved outcomes have led to different recommendations for the management of mild chronic hypertension in pregnancy. However, chronic hypertension affects a substantial number of pregnant patients and is known to be a risk factor for severe short-term pregnancy and long-term health complications. With preliminary data suggesting that BPs >140/90 mm Hg prior to 20 weeks’ gestation are associated with an increase in adverse outcomes, Tita and colleagues sought to determine the effects of decreasing BP in pregnant patients with mild chronic hypertension.
Details about the study
This is an investigator-initiated, multicenter, pragmatic, open-label, randomized control trial of 2,408 patients with mild chronic hypertension. The active treatment group was treated with antihypertensive medication (including titration of existing medication), targeting a BP of <140/90 mm Hg. The control group only received medication for severe hypertension (≥160 mm Hg systolic or ≥105 mm Hg diastolic). The primary outcome of the study was a composite of preeclampsia with severe features, medically indicated preterm delivery prior to 35 weeks’ gestation (not spontaneous labor or rupture of membranes), placental abruption, and fetal or neonatal death. Birthweight that was less than the 10th percentile was used as a safety outcome. The hypothesis was that treatment would decrease the rate of adverse pregnancy and fetal/neonatal outcomes.
The patient population of singleton pregnancies at a gestational age of less than 23 weeks included 56% with known chronic hypertension on medications, 22% with known chronic hypertension without medications, and 22% with newly diagnosed (during pregnancy) chronic hypertension. The treatment group primarily received labetalol (61.7%) or nifedipine (35.6%); the maximum dose of a single agent was used as tolerated prior to adding a second agent. The control group only received an antihypertensive medication for severe hypertension.
Treatment of chronic hypertension demonstrated a decreased risk of the composite adverse outcome with an adjusted risk ratio of 0.82 (95% confidence interval [CI], 0.74 to 0.92; P<.001) and a number needed to treat (NNT) of 14.7. When analyzed separately, a similar risk reduction was noted for both preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation. There was no statistical difference between the groups for birth weight <10th percentile and <5th percentile (adjusted risk ratio, 1.04 [0.82–1.31] vs 0.89 [0.62–1.26], respectively).
Planned subgroup analysis by type of chronic hypertension, race/ethnic group, diabetes status, gestational age at baseline, and body mass index (BMI) demonstrated a similar treatment effect to the overall composite primary outcome, with the exception of patients with newly diagn
Study strengths and weaknesses
The study strengths cited are a large sample size, multiple study sites, an independent data and safety monitoring board with close oversight, and centralized blinded confirmation of outcomes. Another strength is that the patient population of the study was similar to the overall population of pregnant patients in the United States with chronic hypertension in terms of age, race, and ethnicity.
The weaknesses of the study include the open-label design and the high ratio of screened to enrolled patients. Both of these issues appear related to the study design (ethics and logistics of a blinded treatment and gestational age cutoff) and the physiology of pregnancy (expected decrease in BP in the second trimester rendering patients ineligible due to lower BP). The study was also not powered to assess treatment effect in all of the subgroups, and further evaluation of patients with newly diagnosed chronic hypertension and BMI ≥ 40 kg/m2 is needed. ●
Pregnant patients with chronic hypertension should continue or initiate antihypertensive medication to target a BP goal of <140/90 mm Hg. This substantial practice change is supported by the significant decrease demonstrated in this study in adverse outcomes such as preeclampsia with severe features and medically indicated preterm birth <35 weeks’ gestation without an increase in small-for-gestational-age newborns.
Best practices for evaluating pelvic pain in patients with Essure tubal occlusion devices
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.
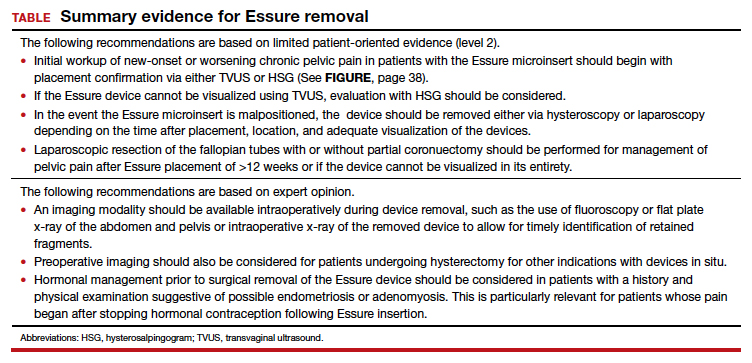
Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.
Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.