User login
Commentary: Something for Everyone in AD Treatment, September 2022
Let's start with tralokinumab. I previously had the privilege of being lead author on a study of the efficacy of tralokinumab beyond week 16 — the ECZTRA3 study.1 ECZTRA3 studied tralokinumab at the approved dose (600 mg loading dose followed by 300 mg every other week) vs placebo with concomitant topical corticosteroids (TCS) for an initial 16-week treatment period. Patients who achieved an Investigator's Global Assessment (IGA) score of 0 or 1 or 75% improvement in the Eczema Area and Severity Index (EASI-75) response were then randomly assigned again to receive 300 mg tralokinumab either continuously at every-other-week intervals or a prolonged interval of every 4 weeks (again with concomitant TCS).
With ECZTRA3, we found that patients continued to improve on tralokinumab + TCS well beyond week 16, with increased EASI-75 responses (week 16: 56%; week 32: 70.2%) and sustained or increased improvement across multiple patient-reported outcomes. Together, these results indicate that clinical responses may take more than 16 weeks to achieve with tralokinumab. In addition, some patients may be able to maintain clinical responses using fewer injections at 4-week intervals. This may allow tailoring dosing to individual patient needs. In fact, tralokinumab is approved in the United States and other regions with the option of every-2-week or every-4-week maintenance dosing in patients who have a good clinical response at week 16.
Since AD can be a lifelong disease, we expect that some patients will need to remain on various therapies for extended periods of time, perhaps many years, in order to maintain long-term control. It is imperative that any long-term treatment demonstrate a good long-term safety and efficacy profile. Blauvelt and colleagues published 2-year interim results from the ongoing ECZTEND long-term, open-label extension study of tralokinumab. They showed no new safety signals and stable rates of adverse events compared with earlier time points. Additionally, they showed that 82.5% of patients treated with open-label tralokinumab + TCS for 2 years maintained EASI-75 responses. These data are reassuring and support the potential use of tralokinumab as a long-term treatment option in AD.
While dupilumab is not approved for every-4-week maintenance dosing, a recent study by Spekhorst and colleagues confirmed that dupilumab can also be safely and effectively administered at intervals of every 4 weeks or every 6-8 weeks. Analyzing data from the BioDay real-world observational registry, they found that among patients who achieved good clinical responses (EASI scores ≤ 7) after 52 weeks of treatment with dupilumab administered every 2 weeks, many patients were able to maintain those responses at 3 months after the interval of administration was increased to every 4 weeks (> 80%) or 6-8 weeks (93.3%). These real-world data confirm the results previously observed in the phase 3 SOLO-CONTINUE study2 and support the use of maintenance dosing of dupilumab at prolonged intervals, though such use would technically be considered off-label.
Let’s also review some new data for abrocitinib, a once-daily oral preferential Janus kinase (JAK) 1 inhibitor. Reich and colleagues reported results from a phase 3 trial of adults with moderate-to-severe AD that compared the safety and efficacy of oral abrocitinib at the higher 200 mg dose vs subcutaneous dupilumab over 26 weeks. They found that more patients achieved ≥ 4-point improvement in the Peak Pruritus Numerical Rating Scale score at week 2 with 200 mg abrocitinib compared with 300 mg dupilumab every other week (48% vs 26%). There were also improved EASI-90 responses at week 4 (29% vs 15%). A dose of 200 mg abrocitinib was also significantly more effective than dupilumab for a number of additional investigator- and patient-reported outcomes. In general, abrocitinib had a faster onset of treatment benefit than dupilumab. However, treatment-emergent adverse events were more common with abrocitinib compared with dupilumab (74% vs 65%). Dupilumab was associated with more ocular adverse events (eg, conjunctivitis), whereas abrocitinib was associated with more headaches, nausea, and herpes zoster infections. These results provide important insights into the comparative effectiveness of treatments in moderate-to-severe AD. Of note, this study compared the higher dose of abrocitinib (200 mg) vs dupilumab. However, in the United States, the FDA-approved label recommends initiating abrocitinib therapy with the lower 100 mg dose and increasing to 200 mg only in those who had an inadequate response to 100 mg.
Additional References
1. Silverberg JI, Toth D, Bieber T, et al, for the ECZTRA 3 study investigators. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450-463. Doi: 10.1111/bjd.19573
2. Worm M, Simpson EL, Thaçi D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020;156(2):131-143. Doi: 10.1001/jamadermatol.2019.3617
Let's start with tralokinumab. I previously had the privilege of being lead author on a study of the efficacy of tralokinumab beyond week 16 — the ECZTRA3 study.1 ECZTRA3 studied tralokinumab at the approved dose (600 mg loading dose followed by 300 mg every other week) vs placebo with concomitant topical corticosteroids (TCS) for an initial 16-week treatment period. Patients who achieved an Investigator's Global Assessment (IGA) score of 0 or 1 or 75% improvement in the Eczema Area and Severity Index (EASI-75) response were then randomly assigned again to receive 300 mg tralokinumab either continuously at every-other-week intervals or a prolonged interval of every 4 weeks (again with concomitant TCS).
With ECZTRA3, we found that patients continued to improve on tralokinumab + TCS well beyond week 16, with increased EASI-75 responses (week 16: 56%; week 32: 70.2%) and sustained or increased improvement across multiple patient-reported outcomes. Together, these results indicate that clinical responses may take more than 16 weeks to achieve with tralokinumab. In addition, some patients may be able to maintain clinical responses using fewer injections at 4-week intervals. This may allow tailoring dosing to individual patient needs. In fact, tralokinumab is approved in the United States and other regions with the option of every-2-week or every-4-week maintenance dosing in patients who have a good clinical response at week 16.
Since AD can be a lifelong disease, we expect that some patients will need to remain on various therapies for extended periods of time, perhaps many years, in order to maintain long-term control. It is imperative that any long-term treatment demonstrate a good long-term safety and efficacy profile. Blauvelt and colleagues published 2-year interim results from the ongoing ECZTEND long-term, open-label extension study of tralokinumab. They showed no new safety signals and stable rates of adverse events compared with earlier time points. Additionally, they showed that 82.5% of patients treated with open-label tralokinumab + TCS for 2 years maintained EASI-75 responses. These data are reassuring and support the potential use of tralokinumab as a long-term treatment option in AD.
While dupilumab is not approved for every-4-week maintenance dosing, a recent study by Spekhorst and colleagues confirmed that dupilumab can also be safely and effectively administered at intervals of every 4 weeks or every 6-8 weeks. Analyzing data from the BioDay real-world observational registry, they found that among patients who achieved good clinical responses (EASI scores ≤ 7) after 52 weeks of treatment with dupilumab administered every 2 weeks, many patients were able to maintain those responses at 3 months after the interval of administration was increased to every 4 weeks (> 80%) or 6-8 weeks (93.3%). These real-world data confirm the results previously observed in the phase 3 SOLO-CONTINUE study2 and support the use of maintenance dosing of dupilumab at prolonged intervals, though such use would technically be considered off-label.
Let’s also review some new data for abrocitinib, a once-daily oral preferential Janus kinase (JAK) 1 inhibitor. Reich and colleagues reported results from a phase 3 trial of adults with moderate-to-severe AD that compared the safety and efficacy of oral abrocitinib at the higher 200 mg dose vs subcutaneous dupilumab over 26 weeks. They found that more patients achieved ≥ 4-point improvement in the Peak Pruritus Numerical Rating Scale score at week 2 with 200 mg abrocitinib compared with 300 mg dupilumab every other week (48% vs 26%). There were also improved EASI-90 responses at week 4 (29% vs 15%). A dose of 200 mg abrocitinib was also significantly more effective than dupilumab for a number of additional investigator- and patient-reported outcomes. In general, abrocitinib had a faster onset of treatment benefit than dupilumab. However, treatment-emergent adverse events were more common with abrocitinib compared with dupilumab (74% vs 65%). Dupilumab was associated with more ocular adverse events (eg, conjunctivitis), whereas abrocitinib was associated with more headaches, nausea, and herpes zoster infections. These results provide important insights into the comparative effectiveness of treatments in moderate-to-severe AD. Of note, this study compared the higher dose of abrocitinib (200 mg) vs dupilumab. However, in the United States, the FDA-approved label recommends initiating abrocitinib therapy with the lower 100 mg dose and increasing to 200 mg only in those who had an inadequate response to 100 mg.
Additional References
1. Silverberg JI, Toth D, Bieber T, et al, for the ECZTRA 3 study investigators. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450-463. Doi: 10.1111/bjd.19573
2. Worm M, Simpson EL, Thaçi D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020;156(2):131-143. Doi: 10.1001/jamadermatol.2019.3617
Let's start with tralokinumab. I previously had the privilege of being lead author on a study of the efficacy of tralokinumab beyond week 16 — the ECZTRA3 study.1 ECZTRA3 studied tralokinumab at the approved dose (600 mg loading dose followed by 300 mg every other week) vs placebo with concomitant topical corticosteroids (TCS) for an initial 16-week treatment period. Patients who achieved an Investigator's Global Assessment (IGA) score of 0 or 1 or 75% improvement in the Eczema Area and Severity Index (EASI-75) response were then randomly assigned again to receive 300 mg tralokinumab either continuously at every-other-week intervals or a prolonged interval of every 4 weeks (again with concomitant TCS).
With ECZTRA3, we found that patients continued to improve on tralokinumab + TCS well beyond week 16, with increased EASI-75 responses (week 16: 56%; week 32: 70.2%) and sustained or increased improvement across multiple patient-reported outcomes. Together, these results indicate that clinical responses may take more than 16 weeks to achieve with tralokinumab. In addition, some patients may be able to maintain clinical responses using fewer injections at 4-week intervals. This may allow tailoring dosing to individual patient needs. In fact, tralokinumab is approved in the United States and other regions with the option of every-2-week or every-4-week maintenance dosing in patients who have a good clinical response at week 16.
Since AD can be a lifelong disease, we expect that some patients will need to remain on various therapies for extended periods of time, perhaps many years, in order to maintain long-term control. It is imperative that any long-term treatment demonstrate a good long-term safety and efficacy profile. Blauvelt and colleagues published 2-year interim results from the ongoing ECZTEND long-term, open-label extension study of tralokinumab. They showed no new safety signals and stable rates of adverse events compared with earlier time points. Additionally, they showed that 82.5% of patients treated with open-label tralokinumab + TCS for 2 years maintained EASI-75 responses. These data are reassuring and support the potential use of tralokinumab as a long-term treatment option in AD.
While dupilumab is not approved for every-4-week maintenance dosing, a recent study by Spekhorst and colleagues confirmed that dupilumab can also be safely and effectively administered at intervals of every 4 weeks or every 6-8 weeks. Analyzing data from the BioDay real-world observational registry, they found that among patients who achieved good clinical responses (EASI scores ≤ 7) after 52 weeks of treatment with dupilumab administered every 2 weeks, many patients were able to maintain those responses at 3 months after the interval of administration was increased to every 4 weeks (> 80%) or 6-8 weeks (93.3%). These real-world data confirm the results previously observed in the phase 3 SOLO-CONTINUE study2 and support the use of maintenance dosing of dupilumab at prolonged intervals, though such use would technically be considered off-label.
Let’s also review some new data for abrocitinib, a once-daily oral preferential Janus kinase (JAK) 1 inhibitor. Reich and colleagues reported results from a phase 3 trial of adults with moderate-to-severe AD that compared the safety and efficacy of oral abrocitinib at the higher 200 mg dose vs subcutaneous dupilumab over 26 weeks. They found that more patients achieved ≥ 4-point improvement in the Peak Pruritus Numerical Rating Scale score at week 2 with 200 mg abrocitinib compared with 300 mg dupilumab every other week (48% vs 26%). There were also improved EASI-90 responses at week 4 (29% vs 15%). A dose of 200 mg abrocitinib was also significantly more effective than dupilumab for a number of additional investigator- and patient-reported outcomes. In general, abrocitinib had a faster onset of treatment benefit than dupilumab. However, treatment-emergent adverse events were more common with abrocitinib compared with dupilumab (74% vs 65%). Dupilumab was associated with more ocular adverse events (eg, conjunctivitis), whereas abrocitinib was associated with more headaches, nausea, and herpes zoster infections. These results provide important insights into the comparative effectiveness of treatments in moderate-to-severe AD. Of note, this study compared the higher dose of abrocitinib (200 mg) vs dupilumab. However, in the United States, the FDA-approved label recommends initiating abrocitinib therapy with the lower 100 mg dose and increasing to 200 mg only in those who had an inadequate response to 100 mg.
Additional References
1. Silverberg JI, Toth D, Bieber T, et al, for the ECZTRA 3 study investigators. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450-463. Doi: 10.1111/bjd.19573
2. Worm M, Simpson EL, Thaçi D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2020;156(2):131-143. Doi: 10.1001/jamadermatol.2019.3617
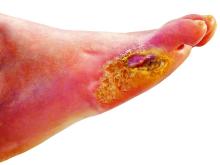
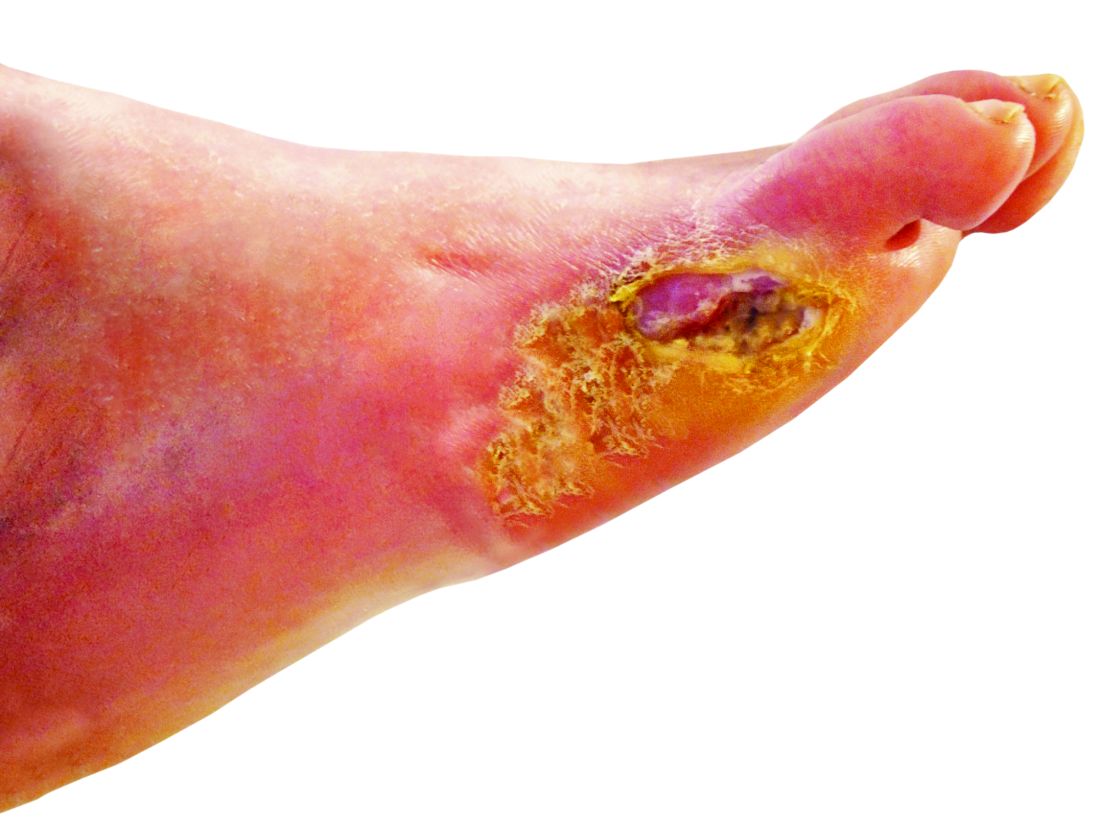
‘Amazing’ data for cheap beta-blocker gel for diabetic foot ulcers
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EASD 2022
Early trial supports hypofractionated radiotherapy in uterine cancer
Postoperative radiotherapy is a mainstay in the treatment of uterine cancer, but the typical 5-week regimen can be time-consuming and expensive. A pilot study found that delivery of approximately the same dose over just 2.5 weeks, known as hypofractionation, had good short-term toxicity outcomes.
Nevertheless, shortening the duration of radiotherapy could have benefits, especially in advanced uterine cancer, where chemotherapy is employed against distant metastases. Following surgery, there is a risk of both local recurrence and distant metastasis, complicating the choice of initial treatment. “Chemo can be several months long and radiation is typically several weeks. Therefore a shortened radiation schedule may have potential benefits, especially if there is an opportunity for this to be delivered earlier without delaying or interrupting chemotherapy, for example,” said lead study author Eric Leung, MD, who is an associate professor of radiation oncology at the University of Toronto’s Sunnybrook Health Sciences Centre.
The research was published in JAMA Oncology.
Delivery of hypofractionation is tricky, according to Dr. Leung. “Gynecological cancer patients were treated with hypofractionation radiation to the pelvis which included the vagina, paravaginal tissues, and pelvic lymph nodes. With this relatively large pelvic volume with surrounding normal tissues, this requires a highly focused radiation treatment with advanced technology,” said Dr. Leung. The study protocol employed stereotactic technique to deliver 30 Gy in 5 fractions.
Hypofractionation could be beneficial in reduction of travel time and time spent in the hospital, as well as reducing financial burden and increasing quality of life. These benefits have taken on a larger role in the context of the COVID-19 pandemic.
Although the findings are encouraging, they are preliminary, according to Vonetta Williams, MD, PhD, who wrote an accompanying editorial. “I would caution that all they’ve done is presented preliminary toxicity data, so we don’t have any proof yet that it is equally effective [compared to standard protocol], and their study cannot answer that at any rate because it was not designed to answer that question,” said Dr. Williams in an interview. She also noted that long-term follow-up is needed to measure bowel dysfunction, sexual dysfunction, vaginal stenosis, and other side effects.
It is also uncertain whether hypofractionated doses are actually equivalent to the standard dose. “We know that they’re roughly equivalent, but that is very much a question if they are equivalent in terms of efficacy. I don’t know that I would be confident that they are. That’s probably what would give most radiation oncologists pause, because we don’t have any data to say that it is [equivalent]. Although it would be nice to shorten treatment, and I think it would certainly be better for patients, I want to caution that we want to do so once we know what the toxicity and the outcomes really are,” Dr. Williams said.
The study’s findings
The researchers enrolled 61 patients with a median age of 66 years. Thirty-nine had endometrioid adenocarcinoma, 15 serous or clear cell, 3 carcinosarcoma, and 4 had dedifferentiated disease. Sixteen patients underwent sequential chemotherapy, and 9 underwent additional vault brachytherapy. Over a median follow-up of 9 months, 54% had a worst gastrointestinal side effect of grade 1, while 13% had a worst side effect of grade 2. Among worst genitourinary side effects, 41% had grade 1 and 3% had grade 2. One patient had acute grade 3 diarrhea at fraction 5, but this resolved at follow-up. One patient had diarrhea scores that were both clinically and statistically significantly worse than baseline at fraction 5, and this improved at follow-up.
Patient-reported quality of life outcomes were generally good. Of all measures, only diarrhea was clinically and statistically worse by fraction 5, and improvement was seen at 6 weeks and 3 months. Global health status was consistent throughout treatment and follow-up. There was no change in sexual and vaginal symptoms.
Postoperative radiotherapy is a mainstay in the treatment of uterine cancer, but the typical 5-week regimen can be time-consuming and expensive. A pilot study found that delivery of approximately the same dose over just 2.5 weeks, known as hypofractionation, had good short-term toxicity outcomes.
Nevertheless, shortening the duration of radiotherapy could have benefits, especially in advanced uterine cancer, where chemotherapy is employed against distant metastases. Following surgery, there is a risk of both local recurrence and distant metastasis, complicating the choice of initial treatment. “Chemo can be several months long and radiation is typically several weeks. Therefore a shortened radiation schedule may have potential benefits, especially if there is an opportunity for this to be delivered earlier without delaying or interrupting chemotherapy, for example,” said lead study author Eric Leung, MD, who is an associate professor of radiation oncology at the University of Toronto’s Sunnybrook Health Sciences Centre.
The research was published in JAMA Oncology.
Delivery of hypofractionation is tricky, according to Dr. Leung. “Gynecological cancer patients were treated with hypofractionation radiation to the pelvis which included the vagina, paravaginal tissues, and pelvic lymph nodes. With this relatively large pelvic volume with surrounding normal tissues, this requires a highly focused radiation treatment with advanced technology,” said Dr. Leung. The study protocol employed stereotactic technique to deliver 30 Gy in 5 fractions.
Hypofractionation could be beneficial in reduction of travel time and time spent in the hospital, as well as reducing financial burden and increasing quality of life. These benefits have taken on a larger role in the context of the COVID-19 pandemic.
Although the findings are encouraging, they are preliminary, according to Vonetta Williams, MD, PhD, who wrote an accompanying editorial. “I would caution that all they’ve done is presented preliminary toxicity data, so we don’t have any proof yet that it is equally effective [compared to standard protocol], and their study cannot answer that at any rate because it was not designed to answer that question,” said Dr. Williams in an interview. She also noted that long-term follow-up is needed to measure bowel dysfunction, sexual dysfunction, vaginal stenosis, and other side effects.
It is also uncertain whether hypofractionated doses are actually equivalent to the standard dose. “We know that they’re roughly equivalent, but that is very much a question if they are equivalent in terms of efficacy. I don’t know that I would be confident that they are. That’s probably what would give most radiation oncologists pause, because we don’t have any data to say that it is [equivalent]. Although it would be nice to shorten treatment, and I think it would certainly be better for patients, I want to caution that we want to do so once we know what the toxicity and the outcomes really are,” Dr. Williams said.
The study’s findings
The researchers enrolled 61 patients with a median age of 66 years. Thirty-nine had endometrioid adenocarcinoma, 15 serous or clear cell, 3 carcinosarcoma, and 4 had dedifferentiated disease. Sixteen patients underwent sequential chemotherapy, and 9 underwent additional vault brachytherapy. Over a median follow-up of 9 months, 54% had a worst gastrointestinal side effect of grade 1, while 13% had a worst side effect of grade 2. Among worst genitourinary side effects, 41% had grade 1 and 3% had grade 2. One patient had acute grade 3 diarrhea at fraction 5, but this resolved at follow-up. One patient had diarrhea scores that were both clinically and statistically significantly worse than baseline at fraction 5, and this improved at follow-up.
Patient-reported quality of life outcomes were generally good. Of all measures, only diarrhea was clinically and statistically worse by fraction 5, and improvement was seen at 6 weeks and 3 months. Global health status was consistent throughout treatment and follow-up. There was no change in sexual and vaginal symptoms.
Postoperative radiotherapy is a mainstay in the treatment of uterine cancer, but the typical 5-week regimen can be time-consuming and expensive. A pilot study found that delivery of approximately the same dose over just 2.5 weeks, known as hypofractionation, had good short-term toxicity outcomes.
Nevertheless, shortening the duration of radiotherapy could have benefits, especially in advanced uterine cancer, where chemotherapy is employed against distant metastases. Following surgery, there is a risk of both local recurrence and distant metastasis, complicating the choice of initial treatment. “Chemo can be several months long and radiation is typically several weeks. Therefore a shortened radiation schedule may have potential benefits, especially if there is an opportunity for this to be delivered earlier without delaying or interrupting chemotherapy, for example,” said lead study author Eric Leung, MD, who is an associate professor of radiation oncology at the University of Toronto’s Sunnybrook Health Sciences Centre.
The research was published in JAMA Oncology.
Delivery of hypofractionation is tricky, according to Dr. Leung. “Gynecological cancer patients were treated with hypofractionation radiation to the pelvis which included the vagina, paravaginal tissues, and pelvic lymph nodes. With this relatively large pelvic volume with surrounding normal tissues, this requires a highly focused radiation treatment with advanced technology,” said Dr. Leung. The study protocol employed stereotactic technique to deliver 30 Gy in 5 fractions.
Hypofractionation could be beneficial in reduction of travel time and time spent in the hospital, as well as reducing financial burden and increasing quality of life. These benefits have taken on a larger role in the context of the COVID-19 pandemic.
Although the findings are encouraging, they are preliminary, according to Vonetta Williams, MD, PhD, who wrote an accompanying editorial. “I would caution that all they’ve done is presented preliminary toxicity data, so we don’t have any proof yet that it is equally effective [compared to standard protocol], and their study cannot answer that at any rate because it was not designed to answer that question,” said Dr. Williams in an interview. She also noted that long-term follow-up is needed to measure bowel dysfunction, sexual dysfunction, vaginal stenosis, and other side effects.
It is also uncertain whether hypofractionated doses are actually equivalent to the standard dose. “We know that they’re roughly equivalent, but that is very much a question if they are equivalent in terms of efficacy. I don’t know that I would be confident that they are. That’s probably what would give most radiation oncologists pause, because we don’t have any data to say that it is [equivalent]. Although it would be nice to shorten treatment, and I think it would certainly be better for patients, I want to caution that we want to do so once we know what the toxicity and the outcomes really are,” Dr. Williams said.
The study’s findings
The researchers enrolled 61 patients with a median age of 66 years. Thirty-nine had endometrioid adenocarcinoma, 15 serous or clear cell, 3 carcinosarcoma, and 4 had dedifferentiated disease. Sixteen patients underwent sequential chemotherapy, and 9 underwent additional vault brachytherapy. Over a median follow-up of 9 months, 54% had a worst gastrointestinal side effect of grade 1, while 13% had a worst side effect of grade 2. Among worst genitourinary side effects, 41% had grade 1 and 3% had grade 2. One patient had acute grade 3 diarrhea at fraction 5, but this resolved at follow-up. One patient had diarrhea scores that were both clinically and statistically significantly worse than baseline at fraction 5, and this improved at follow-up.
Patient-reported quality of life outcomes were generally good. Of all measures, only diarrhea was clinically and statistically worse by fraction 5, and improvement was seen at 6 weeks and 3 months. Global health status was consistent throughout treatment and follow-up. There was no change in sexual and vaginal symptoms.
FROM JAMA ONCOLOGY
Formula may be right for infants, but experts warn that toddlers don’t need it
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Could exercise improve bone health in youth with type 1 diabetes?
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2022
Eat more dairy, less red meat to prevent type 2 diabetes
STOCKHOLM – Among animal protein foods, low-fat dairy consumption may minimize the risk of developing type 2 diabetes while red meat raises that risk, a new analysis finds.
“A plant-based dietary pattern with limited intake of meat, moderate intake of fish, eggs, and full-fat dairy, and habitual consumption of yogurt, milk, or low-fat dairy, might represent the most feasible, sustainable, and successful population strategy to optimize the prevention of type 2 diabetes,” lead author Annalisa Giosuè, MD, of the University of Naples (Italy) Federico II, told this news organization.
She presented the findings from an umbrella review of 13 dose-response meta-analyses of prospective cohort studies at the annual meeting of the European Association for the Study of Diabetes.
The study is believed to be the first comprehensive overview of the available evidence from all published meta-analyses on the relationship between well-defined amounts of animal-origin foods and the risk of type 2 diabetes.
Dr. Giosuè and colleagues focused on animal-based foods because they represent a gap in most guidelines for type 2 diabetes prevention, she explained.
“The existing evidence and dietary recommendations for type 2 diabetes prevention are mainly based on the appropriate consumption of plant foods: high amounts of the fiber-rich ones and low consumption of the refined ones as well as those rich in free sugars. And also on the adequate choice among fat sources – reduction of saturated fat sources like butter and cream and replacement with plant-based poly- and monounsaturated fat sources like nontropical vegetable oils. But not on the most suitable choices among different animal foods for the prevention of type 2 diabetes,” she explained.
The new findings are in line with the Mediterranean diet in that, while plant based, it also limits red-meat consumption, but not all animal-based foods, and has consistently been associated with a reduced risk of type 2 diabetes. Vegetarian diets have also been associated with a reduced risk of type 2 diabetes, but far less evidence is available for that, she said.
Asked for comment, session moderator Matthias Schulze, MD, head of the department of molecular epidemiology at the German Institute of Human Nutrition, Berlin, said: “Decreasing intake of red and processed meat is already a strong recommendation, and these data support that. You have to make choices for and against [certain] foods. So, if you decide to eat less red meat, then the question is what do you eat instead? This study shows that specifically other animal products, like dairy and ... fish or white meat sources ... are healthy among the animal-based foods. But you could also obviously look at plant-based foods as protein sources as well.”
And Dr. Schulze noted that the data suggest another dimension to type 2 diabetes prevention beyond simply focusing on weight loss.
“You can achieve weight loss with very different diets. Diet quality plays an important role. These data support that if you look at diabetes prevention, then you would focus on people with high intakes of specific animal-based foods, besides looking at overweight and obesity. Then you could intervene to reduce this intake, with potential substitutions with other animal foods like fish or white meat, or plant-based sources of proteins.”
Red meat damages, dairy protects
The 13 meta-analyses included 175 summary risk ratios for type 2 diabetes incidence for the consumption of total meat, red meat, white meat, processed meats, fish, total dairy, full-fat dairy, low-fat dairy, milk, cheese, yogurt, or eggs.
Significant increases in the risk of developing type 2 diabetes were found for consumption of 100 g/day of total meat (SRR, 1.20; 20% increase) and red meat (SRR, 1.22, 22% increase) and with 50 g/day of processed meats (SRR, 1.30; 30% increase). A borderline increased risk was also seen for 50 g/day of white meat (SRR, 1.04; 4% increase).
The opposite was found for dairy foods. Inverse associations for type 2 diabetes development were found for an intake of 200 g/day of total dairy (SRR, 0.95; 5% reduction), low-fat dairy (SRR, 0.96; 4% reduction), milk (SRR, 0.90; 10% reduction), and for 100 g/day of yogurt (SRR, 0.94, 6% reduction).
Neutral (nonsignificant) effects were found for 200 g/day of full-fat dairy (SRR, 0.98) and for 30 g/day of cheese (SRR, 0.97). Fish consumption also had a neutral association with type 2 diabetes risk (SRR, 1.04 for 100 g/day) as did one egg per day (SRR, 1.07), but evidence quality was low.
And, Dr. Giosuè noted during her presentation, these relationships could change with alterations in the amounts consumed.
Dr. Schulze commented: “Fish is more clearly related to reduced cardiovascular risk than for preventing type 2 diabetes, where we’ve had mixed results. They might not always be the same.”
What are the mechanisms?
The reasons for these positive and negative associations aren’t entirely clear, but Dr. Giosuè noted that dairy products contain several nutrients, vitamins, and other components, such as calcium and vitamin D, that have potential beneficial effects on glucose metabolism.
In particular, she said, “Whey proteins in milk have a well-known beneficial effect on the regulation of the rise of glucose levels in the blood after meals, and also on the control of appetite and body weight.”
Moreover, probiotics found in yogurt have been linked to protective effects against weight gain and obesity, which “may in part [explain] the beneficial role of yogurt in type 2 diabetes prevention.”
Meat, in contrast, is full of cholesterol, saturated fatty acids, and heme iron, which can promote subclinical inflammation and oxidative stress, which may in turn, affect insulin sensitivity, Dr. Giosuè explained. What’s more, “processed meats also contain nitrates, nitrites, and sodium that can contribute to pancreatic cell damage and vascular dysfunction, thus affecting insulin sensitivity.”
And white meat (poultry) has a lower fat content than red meats such as beef, lamb, and pork, as well as a more favorable fatty acid profile and a lower heme-iron content, she said in an interview.
What about vegan diets? The devil is in the details
Asked about the relative health benefits of diets that completely eliminate animal-based foods, Dr. Giosuè replied: “What is important to keep in mind when hearing about the potential of vegan diets to prevent, or manage, or induce the remission of type 2 diabetes, is that the inclusion in the diet of solely foods of plant origin does not mean ‘automatically’ to eat only foods that are good for diabetes prevention.”
“Just like the exclusion of all foods of animal origin is not equivalent to reduce the risk of type 2 diabetes ... Solid evidence has demonstrated that plant foods which are refined and/or rich in free sugars like white bread, biscuits, and sweetened beverages are as harmful as red and processed meats for diabetes incidence and progression.”
Dr. Giosuè and Dr. Schulze have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Among animal protein foods, low-fat dairy consumption may minimize the risk of developing type 2 diabetes while red meat raises that risk, a new analysis finds.
“A plant-based dietary pattern with limited intake of meat, moderate intake of fish, eggs, and full-fat dairy, and habitual consumption of yogurt, milk, or low-fat dairy, might represent the most feasible, sustainable, and successful population strategy to optimize the prevention of type 2 diabetes,” lead author Annalisa Giosuè, MD, of the University of Naples (Italy) Federico II, told this news organization.
She presented the findings from an umbrella review of 13 dose-response meta-analyses of prospective cohort studies at the annual meeting of the European Association for the Study of Diabetes.
The study is believed to be the first comprehensive overview of the available evidence from all published meta-analyses on the relationship between well-defined amounts of animal-origin foods and the risk of type 2 diabetes.
Dr. Giosuè and colleagues focused on animal-based foods because they represent a gap in most guidelines for type 2 diabetes prevention, she explained.
“The existing evidence and dietary recommendations for type 2 diabetes prevention are mainly based on the appropriate consumption of plant foods: high amounts of the fiber-rich ones and low consumption of the refined ones as well as those rich in free sugars. And also on the adequate choice among fat sources – reduction of saturated fat sources like butter and cream and replacement with plant-based poly- and monounsaturated fat sources like nontropical vegetable oils. But not on the most suitable choices among different animal foods for the prevention of type 2 diabetes,” she explained.
The new findings are in line with the Mediterranean diet in that, while plant based, it also limits red-meat consumption, but not all animal-based foods, and has consistently been associated with a reduced risk of type 2 diabetes. Vegetarian diets have also been associated with a reduced risk of type 2 diabetes, but far less evidence is available for that, she said.
Asked for comment, session moderator Matthias Schulze, MD, head of the department of molecular epidemiology at the German Institute of Human Nutrition, Berlin, said: “Decreasing intake of red and processed meat is already a strong recommendation, and these data support that. You have to make choices for and against [certain] foods. So, if you decide to eat less red meat, then the question is what do you eat instead? This study shows that specifically other animal products, like dairy and ... fish or white meat sources ... are healthy among the animal-based foods. But you could also obviously look at plant-based foods as protein sources as well.”
And Dr. Schulze noted that the data suggest another dimension to type 2 diabetes prevention beyond simply focusing on weight loss.
“You can achieve weight loss with very different diets. Diet quality plays an important role. These data support that if you look at diabetes prevention, then you would focus on people with high intakes of specific animal-based foods, besides looking at overweight and obesity. Then you could intervene to reduce this intake, with potential substitutions with other animal foods like fish or white meat, or plant-based sources of proteins.”
Red meat damages, dairy protects
The 13 meta-analyses included 175 summary risk ratios for type 2 diabetes incidence for the consumption of total meat, red meat, white meat, processed meats, fish, total dairy, full-fat dairy, low-fat dairy, milk, cheese, yogurt, or eggs.
Significant increases in the risk of developing type 2 diabetes were found for consumption of 100 g/day of total meat (SRR, 1.20; 20% increase) and red meat (SRR, 1.22, 22% increase) and with 50 g/day of processed meats (SRR, 1.30; 30% increase). A borderline increased risk was also seen for 50 g/day of white meat (SRR, 1.04; 4% increase).
The opposite was found for dairy foods. Inverse associations for type 2 diabetes development were found for an intake of 200 g/day of total dairy (SRR, 0.95; 5% reduction), low-fat dairy (SRR, 0.96; 4% reduction), milk (SRR, 0.90; 10% reduction), and for 100 g/day of yogurt (SRR, 0.94, 6% reduction).
Neutral (nonsignificant) effects were found for 200 g/day of full-fat dairy (SRR, 0.98) and for 30 g/day of cheese (SRR, 0.97). Fish consumption also had a neutral association with type 2 diabetes risk (SRR, 1.04 for 100 g/day) as did one egg per day (SRR, 1.07), but evidence quality was low.
And, Dr. Giosuè noted during her presentation, these relationships could change with alterations in the amounts consumed.
Dr. Schulze commented: “Fish is more clearly related to reduced cardiovascular risk than for preventing type 2 diabetes, where we’ve had mixed results. They might not always be the same.”
What are the mechanisms?
The reasons for these positive and negative associations aren’t entirely clear, but Dr. Giosuè noted that dairy products contain several nutrients, vitamins, and other components, such as calcium and vitamin D, that have potential beneficial effects on glucose metabolism.
In particular, she said, “Whey proteins in milk have a well-known beneficial effect on the regulation of the rise of glucose levels in the blood after meals, and also on the control of appetite and body weight.”
Moreover, probiotics found in yogurt have been linked to protective effects against weight gain and obesity, which “may in part [explain] the beneficial role of yogurt in type 2 diabetes prevention.”
Meat, in contrast, is full of cholesterol, saturated fatty acids, and heme iron, which can promote subclinical inflammation and oxidative stress, which may in turn, affect insulin sensitivity, Dr. Giosuè explained. What’s more, “processed meats also contain nitrates, nitrites, and sodium that can contribute to pancreatic cell damage and vascular dysfunction, thus affecting insulin sensitivity.”
And white meat (poultry) has a lower fat content than red meats such as beef, lamb, and pork, as well as a more favorable fatty acid profile and a lower heme-iron content, she said in an interview.
What about vegan diets? The devil is in the details
Asked about the relative health benefits of diets that completely eliminate animal-based foods, Dr. Giosuè replied: “What is important to keep in mind when hearing about the potential of vegan diets to prevent, or manage, or induce the remission of type 2 diabetes, is that the inclusion in the diet of solely foods of plant origin does not mean ‘automatically’ to eat only foods that are good for diabetes prevention.”
“Just like the exclusion of all foods of animal origin is not equivalent to reduce the risk of type 2 diabetes ... Solid evidence has demonstrated that plant foods which are refined and/or rich in free sugars like white bread, biscuits, and sweetened beverages are as harmful as red and processed meats for diabetes incidence and progression.”
Dr. Giosuè and Dr. Schulze have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Among animal protein foods, low-fat dairy consumption may minimize the risk of developing type 2 diabetes while red meat raises that risk, a new analysis finds.
“A plant-based dietary pattern with limited intake of meat, moderate intake of fish, eggs, and full-fat dairy, and habitual consumption of yogurt, milk, or low-fat dairy, might represent the most feasible, sustainable, and successful population strategy to optimize the prevention of type 2 diabetes,” lead author Annalisa Giosuè, MD, of the University of Naples (Italy) Federico II, told this news organization.
She presented the findings from an umbrella review of 13 dose-response meta-analyses of prospective cohort studies at the annual meeting of the European Association for the Study of Diabetes.
The study is believed to be the first comprehensive overview of the available evidence from all published meta-analyses on the relationship between well-defined amounts of animal-origin foods and the risk of type 2 diabetes.
Dr. Giosuè and colleagues focused on animal-based foods because they represent a gap in most guidelines for type 2 diabetes prevention, she explained.
“The existing evidence and dietary recommendations for type 2 diabetes prevention are mainly based on the appropriate consumption of plant foods: high amounts of the fiber-rich ones and low consumption of the refined ones as well as those rich in free sugars. And also on the adequate choice among fat sources – reduction of saturated fat sources like butter and cream and replacement with plant-based poly- and monounsaturated fat sources like nontropical vegetable oils. But not on the most suitable choices among different animal foods for the prevention of type 2 diabetes,” she explained.
The new findings are in line with the Mediterranean diet in that, while plant based, it also limits red-meat consumption, but not all animal-based foods, and has consistently been associated with a reduced risk of type 2 diabetes. Vegetarian diets have also been associated with a reduced risk of type 2 diabetes, but far less evidence is available for that, she said.
Asked for comment, session moderator Matthias Schulze, MD, head of the department of molecular epidemiology at the German Institute of Human Nutrition, Berlin, said: “Decreasing intake of red and processed meat is already a strong recommendation, and these data support that. You have to make choices for and against [certain] foods. So, if you decide to eat less red meat, then the question is what do you eat instead? This study shows that specifically other animal products, like dairy and ... fish or white meat sources ... are healthy among the animal-based foods. But you could also obviously look at plant-based foods as protein sources as well.”
And Dr. Schulze noted that the data suggest another dimension to type 2 diabetes prevention beyond simply focusing on weight loss.
“You can achieve weight loss with very different diets. Diet quality plays an important role. These data support that if you look at diabetes prevention, then you would focus on people with high intakes of specific animal-based foods, besides looking at overweight and obesity. Then you could intervene to reduce this intake, with potential substitutions with other animal foods like fish or white meat, or plant-based sources of proteins.”
Red meat damages, dairy protects
The 13 meta-analyses included 175 summary risk ratios for type 2 diabetes incidence for the consumption of total meat, red meat, white meat, processed meats, fish, total dairy, full-fat dairy, low-fat dairy, milk, cheese, yogurt, or eggs.
Significant increases in the risk of developing type 2 diabetes were found for consumption of 100 g/day of total meat (SRR, 1.20; 20% increase) and red meat (SRR, 1.22, 22% increase) and with 50 g/day of processed meats (SRR, 1.30; 30% increase). A borderline increased risk was also seen for 50 g/day of white meat (SRR, 1.04; 4% increase).
The opposite was found for dairy foods. Inverse associations for type 2 diabetes development were found for an intake of 200 g/day of total dairy (SRR, 0.95; 5% reduction), low-fat dairy (SRR, 0.96; 4% reduction), milk (SRR, 0.90; 10% reduction), and for 100 g/day of yogurt (SRR, 0.94, 6% reduction).
Neutral (nonsignificant) effects were found for 200 g/day of full-fat dairy (SRR, 0.98) and for 30 g/day of cheese (SRR, 0.97). Fish consumption also had a neutral association with type 2 diabetes risk (SRR, 1.04 for 100 g/day) as did one egg per day (SRR, 1.07), but evidence quality was low.
And, Dr. Giosuè noted during her presentation, these relationships could change with alterations in the amounts consumed.
Dr. Schulze commented: “Fish is more clearly related to reduced cardiovascular risk than for preventing type 2 diabetes, where we’ve had mixed results. They might not always be the same.”
What are the mechanisms?
The reasons for these positive and negative associations aren’t entirely clear, but Dr. Giosuè noted that dairy products contain several nutrients, vitamins, and other components, such as calcium and vitamin D, that have potential beneficial effects on glucose metabolism.
In particular, she said, “Whey proteins in milk have a well-known beneficial effect on the regulation of the rise of glucose levels in the blood after meals, and also on the control of appetite and body weight.”
Moreover, probiotics found in yogurt have been linked to protective effects against weight gain and obesity, which “may in part [explain] the beneficial role of yogurt in type 2 diabetes prevention.”
Meat, in contrast, is full of cholesterol, saturated fatty acids, and heme iron, which can promote subclinical inflammation and oxidative stress, which may in turn, affect insulin sensitivity, Dr. Giosuè explained. What’s more, “processed meats also contain nitrates, nitrites, and sodium that can contribute to pancreatic cell damage and vascular dysfunction, thus affecting insulin sensitivity.”
And white meat (poultry) has a lower fat content than red meats such as beef, lamb, and pork, as well as a more favorable fatty acid profile and a lower heme-iron content, she said in an interview.
What about vegan diets? The devil is in the details
Asked about the relative health benefits of diets that completely eliminate animal-based foods, Dr. Giosuè replied: “What is important to keep in mind when hearing about the potential of vegan diets to prevent, or manage, or induce the remission of type 2 diabetes, is that the inclusion in the diet of solely foods of plant origin does not mean ‘automatically’ to eat only foods that are good for diabetes prevention.”
“Just like the exclusion of all foods of animal origin is not equivalent to reduce the risk of type 2 diabetes ... Solid evidence has demonstrated that plant foods which are refined and/or rich in free sugars like white bread, biscuits, and sweetened beverages are as harmful as red and processed meats for diabetes incidence and progression.”
Dr. Giosuè and Dr. Schulze have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
High iron levels predict greater fracture risk, more so in men
than matched control patients, in a large study.
Compared with control patients, those with iron overload had a roughly twofold increased risk of a vertebral fracture, as well as an increased risk of a hip or humerus fracture, but not a forearm fracture.
The increased risk of fracture in men with iron overload (compared with other matched men) was greater than the increased risk of fracture in women with iron overload (compared with other matched women).
Andrea Burden, PhD, presented the findings during a late-breaking clinical science session at the annual meeting of the American Society of Bone and Mineral Research.
‘We should worry about the bones as well as the liver’
Based on these results, clinicians should probably do earlier bone mineral density (BMD) determinations to screen for osteoporosis and perhaps consider prophylaxis with vitamin D and calcium, said Dr. Burden, assistant professor, Institute of Pharmaceutical Sciences, ETH Zürich.
“However, I say that with a bunch of caution,” she added, “because we actually don’t have much evidence of the impact of these treatment differences on fracture risk.”
“This is the first large population study on this topic,” although there have been a few case reports, Dr. Burden explained in an interview.
However, “the high iron overload of greater than 1,000 mcg/L is not common, and hereditary hemochromatosis or thalassemia also are very rare,” she noted.
“The study shows that, once patients have an iron overload of more than 1,000 mcg/L, we need to be doing regular checks for their BMD and figuring how to best minimize their fracture risk,” she said.
“A twofold risk for a vertebral fracture” in patients with iron overload “is really high,” she noted. It is known that men with iron overload have loss of testosterone, but it may be less well known that they have an increased fracture risk.
“We worry about the liver,” she said, “not so much about the bones, and this shows us that we really should.”
Session comoderator Michael J. Econs, MD, who was not involved with the research, agreed. “Iron overload does occur, and it is a clinically important problem and can lead to hemochromatosis, which can lead to a whole host of diseases, but the most common is liver disease,” he told this news organization.
“So, it is a clinically important problem, not only in people who are genetically predisposed but in people who get frequent transfusion,” said Dr. Econs, distinguished professor of medicine and medical and molecular genetics at Indiana University, Indianapolis.
Now this new study has found an increase in fractures in such people, he noted.
Large case-control study used U.K. database
Using data from the IQVIA Medical Research Database, researchers identified 21,166 iron overload patients aged 18 years and older who saw a general practitioner in the United Kingdom between 2010 and 2020 and had a serum ferritin level above 1,000 mcg/L or a diagnostic code for hemochromatosis or nonanemic thalassemia.
They matched each iron overload patient with up to 10 control patients based on age, sex, year, and general practitioner, for a total of 198,037 control patients.
Patients were a mean age of 59 years and 59% were men.
During follow-up there were 777 fractures in the iron-overload patients (9.61 fractures per 1,000 patient-years) and 4,344 fractures in the control group (4.68 fractures per 1,000 patient-years).
In adjusted hazard ratio models, researchers adjusted for age, sex, body mass index, alcohol, smoking, history of fractures earlier than 365 days prior to study entry, hypogonadism, osteoporosis, medications, and comorbidities.
Overall, patients in the iron overload group had a 60% higher risk of an osteoporotic fracture (aHR, 1.60).
Among women, the incidence of osteoporotic fracture was 12.63 per 1,000 patient-years in the iron overload group and 7.09 per 1,000 patient-years in the control group.
Women with iron overload had a 48% higher risk of osteoporotic fracture, compared with other women (aHR, 1.48).
Among men, the incidence of osteoporotic fracture was 6.71 per 1,000 patient-years in the iron overload group and 3.01 per 1,000 patient-years in the control group.
Men with iron overload therefore had an 82% higher risk of osteoporotic fracture, compared with other men (aHR, 1.82).
Compared with patients without iron overload, patients with iron overload had an increased risk of a vertebral (aHR, 2.18), hip (aHR, 1.60), and humerus (aHR, 1.82) fracture but not a forearm fracture.
The researchers acknowledge that study limitations include they did not look at phlebotomy or changes in ferritin levels, and they excluded patients with hereditary hemochromatosis diagnosed before age 18.
The work was funded by the German Research Foundation. One of the researchers has reported receiving an independent grant from Pharmacosmos. The other researchers as well as Dr. Econs have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than matched control patients, in a large study.
Compared with control patients, those with iron overload had a roughly twofold increased risk of a vertebral fracture, as well as an increased risk of a hip or humerus fracture, but not a forearm fracture.
The increased risk of fracture in men with iron overload (compared with other matched men) was greater than the increased risk of fracture in women with iron overload (compared with other matched women).
Andrea Burden, PhD, presented the findings during a late-breaking clinical science session at the annual meeting of the American Society of Bone and Mineral Research.
‘We should worry about the bones as well as the liver’
Based on these results, clinicians should probably do earlier bone mineral density (BMD) determinations to screen for osteoporosis and perhaps consider prophylaxis with vitamin D and calcium, said Dr. Burden, assistant professor, Institute of Pharmaceutical Sciences, ETH Zürich.
“However, I say that with a bunch of caution,” she added, “because we actually don’t have much evidence of the impact of these treatment differences on fracture risk.”
“This is the first large population study on this topic,” although there have been a few case reports, Dr. Burden explained in an interview.
However, “the high iron overload of greater than 1,000 mcg/L is not common, and hereditary hemochromatosis or thalassemia also are very rare,” she noted.
“The study shows that, once patients have an iron overload of more than 1,000 mcg/L, we need to be doing regular checks for their BMD and figuring how to best minimize their fracture risk,” she said.
“A twofold risk for a vertebral fracture” in patients with iron overload “is really high,” she noted. It is known that men with iron overload have loss of testosterone, but it may be less well known that they have an increased fracture risk.
“We worry about the liver,” she said, “not so much about the bones, and this shows us that we really should.”
Session comoderator Michael J. Econs, MD, who was not involved with the research, agreed. “Iron overload does occur, and it is a clinically important problem and can lead to hemochromatosis, which can lead to a whole host of diseases, but the most common is liver disease,” he told this news organization.
“So, it is a clinically important problem, not only in people who are genetically predisposed but in people who get frequent transfusion,” said Dr. Econs, distinguished professor of medicine and medical and molecular genetics at Indiana University, Indianapolis.
Now this new study has found an increase in fractures in such people, he noted.
Large case-control study used U.K. database
Using data from the IQVIA Medical Research Database, researchers identified 21,166 iron overload patients aged 18 years and older who saw a general practitioner in the United Kingdom between 2010 and 2020 and had a serum ferritin level above 1,000 mcg/L or a diagnostic code for hemochromatosis or nonanemic thalassemia.
They matched each iron overload patient with up to 10 control patients based on age, sex, year, and general practitioner, for a total of 198,037 control patients.
Patients were a mean age of 59 years and 59% were men.
During follow-up there were 777 fractures in the iron-overload patients (9.61 fractures per 1,000 patient-years) and 4,344 fractures in the control group (4.68 fractures per 1,000 patient-years).
In adjusted hazard ratio models, researchers adjusted for age, sex, body mass index, alcohol, smoking, history of fractures earlier than 365 days prior to study entry, hypogonadism, osteoporosis, medications, and comorbidities.
Overall, patients in the iron overload group had a 60% higher risk of an osteoporotic fracture (aHR, 1.60).
Among women, the incidence of osteoporotic fracture was 12.63 per 1,000 patient-years in the iron overload group and 7.09 per 1,000 patient-years in the control group.
Women with iron overload had a 48% higher risk of osteoporotic fracture, compared with other women (aHR, 1.48).
Among men, the incidence of osteoporotic fracture was 6.71 per 1,000 patient-years in the iron overload group and 3.01 per 1,000 patient-years in the control group.
Men with iron overload therefore had an 82% higher risk of osteoporotic fracture, compared with other men (aHR, 1.82).
Compared with patients without iron overload, patients with iron overload had an increased risk of a vertebral (aHR, 2.18), hip (aHR, 1.60), and humerus (aHR, 1.82) fracture but not a forearm fracture.
The researchers acknowledge that study limitations include they did not look at phlebotomy or changes in ferritin levels, and they excluded patients with hereditary hemochromatosis diagnosed before age 18.
The work was funded by the German Research Foundation. One of the researchers has reported receiving an independent grant from Pharmacosmos. The other researchers as well as Dr. Econs have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than matched control patients, in a large study.
Compared with control patients, those with iron overload had a roughly twofold increased risk of a vertebral fracture, as well as an increased risk of a hip or humerus fracture, but not a forearm fracture.
The increased risk of fracture in men with iron overload (compared with other matched men) was greater than the increased risk of fracture in women with iron overload (compared with other matched women).
Andrea Burden, PhD, presented the findings during a late-breaking clinical science session at the annual meeting of the American Society of Bone and Mineral Research.
‘We should worry about the bones as well as the liver’
Based on these results, clinicians should probably do earlier bone mineral density (BMD) determinations to screen for osteoporosis and perhaps consider prophylaxis with vitamin D and calcium, said Dr. Burden, assistant professor, Institute of Pharmaceutical Sciences, ETH Zürich.
“However, I say that with a bunch of caution,” she added, “because we actually don’t have much evidence of the impact of these treatment differences on fracture risk.”
“This is the first large population study on this topic,” although there have been a few case reports, Dr. Burden explained in an interview.
However, “the high iron overload of greater than 1,000 mcg/L is not common, and hereditary hemochromatosis or thalassemia also are very rare,” she noted.
“The study shows that, once patients have an iron overload of more than 1,000 mcg/L, we need to be doing regular checks for their BMD and figuring how to best minimize their fracture risk,” she said.
“A twofold risk for a vertebral fracture” in patients with iron overload “is really high,” she noted. It is known that men with iron overload have loss of testosterone, but it may be less well known that they have an increased fracture risk.
“We worry about the liver,” she said, “not so much about the bones, and this shows us that we really should.”
Session comoderator Michael J. Econs, MD, who was not involved with the research, agreed. “Iron overload does occur, and it is a clinically important problem and can lead to hemochromatosis, which can lead to a whole host of diseases, but the most common is liver disease,” he told this news organization.
“So, it is a clinically important problem, not only in people who are genetically predisposed but in people who get frequent transfusion,” said Dr. Econs, distinguished professor of medicine and medical and molecular genetics at Indiana University, Indianapolis.
Now this new study has found an increase in fractures in such people, he noted.
Large case-control study used U.K. database
Using data from the IQVIA Medical Research Database, researchers identified 21,166 iron overload patients aged 18 years and older who saw a general practitioner in the United Kingdom between 2010 and 2020 and had a serum ferritin level above 1,000 mcg/L or a diagnostic code for hemochromatosis or nonanemic thalassemia.
They matched each iron overload patient with up to 10 control patients based on age, sex, year, and general practitioner, for a total of 198,037 control patients.
Patients were a mean age of 59 years and 59% were men.
During follow-up there were 777 fractures in the iron-overload patients (9.61 fractures per 1,000 patient-years) and 4,344 fractures in the control group (4.68 fractures per 1,000 patient-years).
In adjusted hazard ratio models, researchers adjusted for age, sex, body mass index, alcohol, smoking, history of fractures earlier than 365 days prior to study entry, hypogonadism, osteoporosis, medications, and comorbidities.
Overall, patients in the iron overload group had a 60% higher risk of an osteoporotic fracture (aHR, 1.60).
Among women, the incidence of osteoporotic fracture was 12.63 per 1,000 patient-years in the iron overload group and 7.09 per 1,000 patient-years in the control group.
Women with iron overload had a 48% higher risk of osteoporotic fracture, compared with other women (aHR, 1.48).
Among men, the incidence of osteoporotic fracture was 6.71 per 1,000 patient-years in the iron overload group and 3.01 per 1,000 patient-years in the control group.
Men with iron overload therefore had an 82% higher risk of osteoporotic fracture, compared with other men (aHR, 1.82).
Compared with patients without iron overload, patients with iron overload had an increased risk of a vertebral (aHR, 2.18), hip (aHR, 1.60), and humerus (aHR, 1.82) fracture but not a forearm fracture.
The researchers acknowledge that study limitations include they did not look at phlebotomy or changes in ferritin levels, and they excluded patients with hereditary hemochromatosis diagnosed before age 18.
The work was funded by the German Research Foundation. One of the researchers has reported receiving an independent grant from Pharmacosmos. The other researchers as well as Dr. Econs have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2022
Children born from frozen embryos may have increased cancer risk
, a large registry study suggests.
The results, however, “should be interpreted cautiously,” the authors noted, given the low number of cancer cases reported among children born using FET.
Still, the findings do “raise concerns considering the increasing use of FET, in particular freeze-all strategies without clear medical indications,” the authors concluded.
The study was published online in PLOS Medicine.
The number of children born after FET has increased globally and even exceeds the number of those born after fresh embryo transfer in many countries. In the United States, for instance, the FET rate has doubled since 2015; FETs constituted almost 80% of all embryo transfers using assisted reproductive technology (ART) without a donor in 2019.
Despite the benefits associated with FET, which include improved embryo survival and higher live birth rates, some previous research has hinted at a higher risk of childhood cancer in this population.
In the current study, researchers from the University of Gothenburg, Sweden, wanted to better understand the risk of childhood cancer following FET. The investigators analyzed data from 171,774 children born via ART, including 22,630 born after FET, as well as roughly 7.7 million children born after spontaneous conception in Denmark, Finland, Norway, and Sweden.
After a mean follow-up of about 10 years, the incidence rate of cancer diagnosed before age 18 years was 16.7 per 100,000 person-years for children born after spontaneous conception (16,184 cases) and 19.3 per 100,000 person-years for children born after ART (329 cases).
The researchers found no increased risk of cancer before age 18 years in the group of children conceived via ART compared with those conceived spontaneously.
However, children born after FET had a significantly higher risk of cancer compared with children born after fresh embryo transfer (adjusted hazard ratio [aHR], 1.59) and spontaneous conception (aHR, 1.65). Specifically with regard to ART, the incidence rate for those born after FET was 30.1 per 100,000 person-years – 48 total cases – compared with 18.8 per 1000,000 person-years after fresh embryo transfer.
Adjustment for macrosomia, birth weight, or major birth defects influenced the association only marginally.
For specific cancer types, children born after FET had more than a twofold higher risk for leukemia in comparison with those born after fresh embryo transfer (aHR, 2.25) and spontaneous conception (aHR, 2.22).
Still, the authors said these results should be interpreted “cautiously,” given the small number of children diagnosed with cancer after FET. The researchers also acknowledged that they do not know why children born after FET would face a higher risk of cancer.
These findings, however, do align with those from a 2019 Dutch population-based study. In the Dutch study, which included more than 24,000 ART-conceived children and more than 23,000 naturally conceived children, the risk of cancer after ART was not higher overall, but it was greater when only those conceived after FET were considered (aHR 1.80); this increased risk, however, was not statistically significant.
“Since the use of FET is substantially increasing, it is important to tease out whether the increased cancer risk is a true risk increase due to the ART procedures using FET, or due to chance or confounding by other factors,” authors of the 2019 Dutch study, Mandy Spaan, PhD, and Flora E. van Leeuwen, PhD, said in an interview.
“But, as childhood cancer is (fortunately) a rare disease, it is very difficult to study this research question among ART children due to limited numbers,” said Dr. Spaan and Dr. van Leeuwen, who are with the Netherlands Cancer Institute.
Given this, the two experts call for additional large population-based cohort studies to investigate the risk of cancer after ART, especially FET, and for a subsequent analysis that pools these data. They hope this strategy “will lead to reliable estimates” and provide information on the risks of FET in comparison with approaches that involve fresh embryos.
The current study had no commercial funding. The study authors as well as Dr. Spaan and Dr. van Leeuwen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large registry study suggests.
The results, however, “should be interpreted cautiously,” the authors noted, given the low number of cancer cases reported among children born using FET.
Still, the findings do “raise concerns considering the increasing use of FET, in particular freeze-all strategies without clear medical indications,” the authors concluded.
The study was published online in PLOS Medicine.
The number of children born after FET has increased globally and even exceeds the number of those born after fresh embryo transfer in many countries. In the United States, for instance, the FET rate has doubled since 2015; FETs constituted almost 80% of all embryo transfers using assisted reproductive technology (ART) without a donor in 2019.
Despite the benefits associated with FET, which include improved embryo survival and higher live birth rates, some previous research has hinted at a higher risk of childhood cancer in this population.
In the current study, researchers from the University of Gothenburg, Sweden, wanted to better understand the risk of childhood cancer following FET. The investigators analyzed data from 171,774 children born via ART, including 22,630 born after FET, as well as roughly 7.7 million children born after spontaneous conception in Denmark, Finland, Norway, and Sweden.
After a mean follow-up of about 10 years, the incidence rate of cancer diagnosed before age 18 years was 16.7 per 100,000 person-years for children born after spontaneous conception (16,184 cases) and 19.3 per 100,000 person-years for children born after ART (329 cases).
The researchers found no increased risk of cancer before age 18 years in the group of children conceived via ART compared with those conceived spontaneously.
However, children born after FET had a significantly higher risk of cancer compared with children born after fresh embryo transfer (adjusted hazard ratio [aHR], 1.59) and spontaneous conception (aHR, 1.65). Specifically with regard to ART, the incidence rate for those born after FET was 30.1 per 100,000 person-years – 48 total cases – compared with 18.8 per 1000,000 person-years after fresh embryo transfer.
Adjustment for macrosomia, birth weight, or major birth defects influenced the association only marginally.
For specific cancer types, children born after FET had more than a twofold higher risk for leukemia in comparison with those born after fresh embryo transfer (aHR, 2.25) and spontaneous conception (aHR, 2.22).
Still, the authors said these results should be interpreted “cautiously,” given the small number of children diagnosed with cancer after FET. The researchers also acknowledged that they do not know why children born after FET would face a higher risk of cancer.
These findings, however, do align with those from a 2019 Dutch population-based study. In the Dutch study, which included more than 24,000 ART-conceived children and more than 23,000 naturally conceived children, the risk of cancer after ART was not higher overall, but it was greater when only those conceived after FET were considered (aHR 1.80); this increased risk, however, was not statistically significant.
“Since the use of FET is substantially increasing, it is important to tease out whether the increased cancer risk is a true risk increase due to the ART procedures using FET, or due to chance or confounding by other factors,” authors of the 2019 Dutch study, Mandy Spaan, PhD, and Flora E. van Leeuwen, PhD, said in an interview.
“But, as childhood cancer is (fortunately) a rare disease, it is very difficult to study this research question among ART children due to limited numbers,” said Dr. Spaan and Dr. van Leeuwen, who are with the Netherlands Cancer Institute.
Given this, the two experts call for additional large population-based cohort studies to investigate the risk of cancer after ART, especially FET, and for a subsequent analysis that pools these data. They hope this strategy “will lead to reliable estimates” and provide information on the risks of FET in comparison with approaches that involve fresh embryos.
The current study had no commercial funding. The study authors as well as Dr. Spaan and Dr. van Leeuwen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large registry study suggests.
The results, however, “should be interpreted cautiously,” the authors noted, given the low number of cancer cases reported among children born using FET.
Still, the findings do “raise concerns considering the increasing use of FET, in particular freeze-all strategies without clear medical indications,” the authors concluded.
The study was published online in PLOS Medicine.
The number of children born after FET has increased globally and even exceeds the number of those born after fresh embryo transfer in many countries. In the United States, for instance, the FET rate has doubled since 2015; FETs constituted almost 80% of all embryo transfers using assisted reproductive technology (ART) without a donor in 2019.
Despite the benefits associated with FET, which include improved embryo survival and higher live birth rates, some previous research has hinted at a higher risk of childhood cancer in this population.
In the current study, researchers from the University of Gothenburg, Sweden, wanted to better understand the risk of childhood cancer following FET. The investigators analyzed data from 171,774 children born via ART, including 22,630 born after FET, as well as roughly 7.7 million children born after spontaneous conception in Denmark, Finland, Norway, and Sweden.
After a mean follow-up of about 10 years, the incidence rate of cancer diagnosed before age 18 years was 16.7 per 100,000 person-years for children born after spontaneous conception (16,184 cases) and 19.3 per 100,000 person-years for children born after ART (329 cases).
The researchers found no increased risk of cancer before age 18 years in the group of children conceived via ART compared with those conceived spontaneously.
However, children born after FET had a significantly higher risk of cancer compared with children born after fresh embryo transfer (adjusted hazard ratio [aHR], 1.59) and spontaneous conception (aHR, 1.65). Specifically with regard to ART, the incidence rate for those born after FET was 30.1 per 100,000 person-years – 48 total cases – compared with 18.8 per 1000,000 person-years after fresh embryo transfer.
Adjustment for macrosomia, birth weight, or major birth defects influenced the association only marginally.
For specific cancer types, children born after FET had more than a twofold higher risk for leukemia in comparison with those born after fresh embryo transfer (aHR, 2.25) and spontaneous conception (aHR, 2.22).
Still, the authors said these results should be interpreted “cautiously,” given the small number of children diagnosed with cancer after FET. The researchers also acknowledged that they do not know why children born after FET would face a higher risk of cancer.
These findings, however, do align with those from a 2019 Dutch population-based study. In the Dutch study, which included more than 24,000 ART-conceived children and more than 23,000 naturally conceived children, the risk of cancer after ART was not higher overall, but it was greater when only those conceived after FET were considered (aHR 1.80); this increased risk, however, was not statistically significant.
“Since the use of FET is substantially increasing, it is important to tease out whether the increased cancer risk is a true risk increase due to the ART procedures using FET, or due to chance or confounding by other factors,” authors of the 2019 Dutch study, Mandy Spaan, PhD, and Flora E. van Leeuwen, PhD, said in an interview.
“But, as childhood cancer is (fortunately) a rare disease, it is very difficult to study this research question among ART children due to limited numbers,” said Dr. Spaan and Dr. van Leeuwen, who are with the Netherlands Cancer Institute.
Given this, the two experts call for additional large population-based cohort studies to investigate the risk of cancer after ART, especially FET, and for a subsequent analysis that pools these data. They hope this strategy “will lead to reliable estimates” and provide information on the risks of FET in comparison with approaches that involve fresh embryos.
The current study had no commercial funding. The study authors as well as Dr. Spaan and Dr. van Leeuwen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS MEDICINE
USPSTF recommends anxiety screening in adults younger than 65
For the first time, the task force is recommending screening all adults aged 64 and younger for anxiety – including pregnant and postpartum women.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in this population has a moderate net benefit, the task force notes in a draft recommendation statement posted on its website.
The recommendation applies to adults aged 19-64 years who do not have a diagnosed mental health disorder or are not showing recognized signs or symptoms of anxiety.
Anxiety disorders are common and often go unrecognized in primary care, leading to long delays in treatment, the task force writes. They add that more evidence is needed to identify ideal screening intervals for all populations.
“A pragmatic approach in the absence of data might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” they write.
For adults aged 65 and older, the task force found “insufficient” evidence on the benefits and potential harms of screening for anxiety.
“Evidence on the accuracy of screening tools and the benefits and harms of screening and treatment of screen-detected anxiety in older adults is lacking, and the balance of benefits and harms cannot be determined,” they write.
Jury out on screening for suicide risk
The task force is continuing to recommend screening all adults for depression. This “B” recommendation reflects moderate-certainty evidence that screening for major depression in adults has a moderate net benefit.
However, they note there is not enough evidence to recommend for or against screening for suicide risk in all adults.
They therefore issued an “I” statement, indicating that the balance of benefits and harms cannot be determined at present.
“To address the critical need for supporting the mental health of adults in primary care, the Task Force reviewed the evidence on screening for anxiety, depression, and suicide risk,” task force member Lori Pbert, PhD, University of Massachusetts, Worcester, said in a news release.
“The good news is that screening all adults for depression, including those who are pregnant and postpartum, and screening adults younger than 65 for anxiety can help identify these conditions early so people can be connected to care,” Dr. Pbert said.
“Unfortunately, evidence is limited on screening adults 65 or older for anxiety and screening all adults for suicide risk, so we are urgently calling for more research,” added task force member Gbenga Ogedegbe, MD, MPH, founding director of the Institute for Excellence in Health Equity at NYU Langone Health.
Dr. Ogedegbe, also a professor at New York University, noted that “in the absence of evidence, health care professionals should use their judgment based on individual patient circumstances when determining whether or not to screen.”
The public comment period for the draft recommendations runs until Oct. 17.
A version of this article first appeared on Medscape.com.
For the first time, the task force is recommending screening all adults aged 64 and younger for anxiety – including pregnant and postpartum women.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in this population has a moderate net benefit, the task force notes in a draft recommendation statement posted on its website.
The recommendation applies to adults aged 19-64 years who do not have a diagnosed mental health disorder or are not showing recognized signs or symptoms of anxiety.
Anxiety disorders are common and often go unrecognized in primary care, leading to long delays in treatment, the task force writes. They add that more evidence is needed to identify ideal screening intervals for all populations.
“A pragmatic approach in the absence of data might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” they write.
For adults aged 65 and older, the task force found “insufficient” evidence on the benefits and potential harms of screening for anxiety.
“Evidence on the accuracy of screening tools and the benefits and harms of screening and treatment of screen-detected anxiety in older adults is lacking, and the balance of benefits and harms cannot be determined,” they write.
Jury out on screening for suicide risk
The task force is continuing to recommend screening all adults for depression. This “B” recommendation reflects moderate-certainty evidence that screening for major depression in adults has a moderate net benefit.
However, they note there is not enough evidence to recommend for or against screening for suicide risk in all adults.
They therefore issued an “I” statement, indicating that the balance of benefits and harms cannot be determined at present.
“To address the critical need for supporting the mental health of adults in primary care, the Task Force reviewed the evidence on screening for anxiety, depression, and suicide risk,” task force member Lori Pbert, PhD, University of Massachusetts, Worcester, said in a news release.
“The good news is that screening all adults for depression, including those who are pregnant and postpartum, and screening adults younger than 65 for anxiety can help identify these conditions early so people can be connected to care,” Dr. Pbert said.
“Unfortunately, evidence is limited on screening adults 65 or older for anxiety and screening all adults for suicide risk, so we are urgently calling for more research,” added task force member Gbenga Ogedegbe, MD, MPH, founding director of the Institute for Excellence in Health Equity at NYU Langone Health.
Dr. Ogedegbe, also a professor at New York University, noted that “in the absence of evidence, health care professionals should use their judgment based on individual patient circumstances when determining whether or not to screen.”
The public comment period for the draft recommendations runs until Oct. 17.
A version of this article first appeared on Medscape.com.
For the first time, the task force is recommending screening all adults aged 64 and younger for anxiety – including pregnant and postpartum women.
This “B” recommendation reflects “moderate certainty” evidence that screening for anxiety in this population has a moderate net benefit, the task force notes in a draft recommendation statement posted on its website.
The recommendation applies to adults aged 19-64 years who do not have a diagnosed mental health disorder or are not showing recognized signs or symptoms of anxiety.
Anxiety disorders are common and often go unrecognized in primary care, leading to long delays in treatment, the task force writes. They add that more evidence is needed to identify ideal screening intervals for all populations.
“A pragmatic approach in the absence of data might include screening all adults who have not been screened previously and using clinical judgment in consideration of risk factors, comorbid conditions, and life events to determine if additional screening of high-risk patients is warranted,” they write.
For adults aged 65 and older, the task force found “insufficient” evidence on the benefits and potential harms of screening for anxiety.
“Evidence on the accuracy of screening tools and the benefits and harms of screening and treatment of screen-detected anxiety in older adults is lacking, and the balance of benefits and harms cannot be determined,” they write.
Jury out on screening for suicide risk
The task force is continuing to recommend screening all adults for depression. This “B” recommendation reflects moderate-certainty evidence that screening for major depression in adults has a moderate net benefit.
However, they note there is not enough evidence to recommend for or against screening for suicide risk in all adults.
They therefore issued an “I” statement, indicating that the balance of benefits and harms cannot be determined at present.
“To address the critical need for supporting the mental health of adults in primary care, the Task Force reviewed the evidence on screening for anxiety, depression, and suicide risk,” task force member Lori Pbert, PhD, University of Massachusetts, Worcester, said in a news release.
“The good news is that screening all adults for depression, including those who are pregnant and postpartum, and screening adults younger than 65 for anxiety can help identify these conditions early so people can be connected to care,” Dr. Pbert said.
“Unfortunately, evidence is limited on screening adults 65 or older for anxiety and screening all adults for suicide risk, so we are urgently calling for more research,” added task force member Gbenga Ogedegbe, MD, MPH, founding director of the Institute for Excellence in Health Equity at NYU Langone Health.
Dr. Ogedegbe, also a professor at New York University, noted that “in the absence of evidence, health care professionals should use their judgment based on individual patient circumstances when determining whether or not to screen.”
The public comment period for the draft recommendations runs until Oct. 17.
A version of this article first appeared on Medscape.com.
Sugary drinks linked to obesity-related cancer deaths
The study, which included more than 900,000 participants, contributes to previous research suggesting that sugary beverages increase the risk of cancer and cancer-related mortality.
A more surprising finding is that consuming artificially sweetened beverages was linked to an increased risk of death from pancreatic cancer.
“This finding is very interesting,” said Marjorie McCullough, ScD, RD, senior scientific director of epidemiology research, American Cancer Society. She noted that other studies that examined an association between artificially sweetened beverages and pancreatic cancer did not reveal a statistically significant association.
“Our study is the first, to our knowledge, that has found a statistically significant positive association, and it will be important to replicate this finding,” said Dr. McCullough.
The study was published online in Cancer, Epidemiology, Biomarkers, and Prevention.
In the study, Dr. McCullough and colleagues examined associations between drinking sugar-sweetened and artificially sweetened beverages and dying from any cancer or any obesity-related cancers. The researchers also examined this association for 20 individual cancer types.
Participants included 934,777 cancer-free adults from the Cancer Prevention Study-II (CPS-II) prospective cohort. At baseline, adults completed a questionnaire on their medical history, lifestyle exposures, and habits, including how many sugar-sweetened or artificially sweetened drinks they typically consumed each day.
Over a median 28-year follow-up, 135,093 participants died from cancer.
Overall, the researchers determined that consuming two or more sugar-sweetened beverages daily (vs. consuming none) was not associated with all-cancer mortality.
Regarding obesity-related cancers, Dr. McCullough and colleagues found a significant 5% increased risk of death from these cancer (hazard ratio, 1.05); however, this association disappeared after controlling for body mass index (BMI). According to Dr. McCullough, this finding may signal that the association between sugary drinks and obesity-related cancer deaths is at least partly mediated by higher BMI, or excess body fat.
“Weight control is key to cancer prevention,” noted Linda Van Horn, RD, chief of the nutrition division at the Feinberg School of Medicine, Northwestern University, Chicago, who wasn’t involved in the study.
However, with regard to individual cancers, consuming two or more sugar-sweetened drinks each day was associated with an increased risk of dying from colorectal cancer (HR, 1.09) and kidney cancer (HR, 1.17) after adjusting for BMI.
Unexpectedly, sugary beverage intake was associated with a lower risk of esophageal and lung cancer mortality. This association held for lung cancer but not esophageal cancer after restricting the analysis to never-smoking participants (HR, 0.81; 95% confidence interval, 0.70-0.94).
Artificial sweetener and pancreatic cancer?
With respect to artificially sweetened drinks, consuming two or more beverages daily was associated with a 5% increased risk of death from obesity-related cancers (HR, 1.05), but that association became null after controlling for BMI.
However, the link to pancreatic cancer mortality remained after adjusting for BMI (HR, 1.11). This association should be studied further, the researchers say. They say there is a possibility that undiagnosed diabetes influenced the results.
“Continued research on the impact of both beverage types with cancer risk and mortality is warranted to determine whether these associations are causal or confounded by other lifestyle factors and whether they are mediated through BMI,” the researchers write.
Reached for comment, Marcus DaSilva Goncalves, MD, PhD, with Weill Cornell Medicine, New York, noted that the association with colorectal cancer has been previously reported, and he agreed that these “findings strengthen the available evidence of an association between sugar-sweetened beverages and colorectal cancer mortality.”
“Data from my lab in mice have shown that sugar-sweetened beverages deliver fructose directly to colon tumors, which stimulates the survival of cancer cells and growth of tumors,” Dr. Goncalves said.
There are also recent clinical data suggesting that exposure to sugar-sweetened beverages during adolescence and adulthood promotes adenoma formation, the precursor to colorectal cancer, he said.
Regarding artificially sweetened beverage intake, Dr. Goncalves said the effect with pancreatic cancer is “surprising” and that he is not aware of other data, including data from several large studies, that support this relationship.
No specific funding for study has been reported. Dr. McCullough, Ms. Van Horn, and Dr. Goncalves have disclosed no relevant disclosures relationships.
A version of this article first appeared on Medscape.com.
The study, which included more than 900,000 participants, contributes to previous research suggesting that sugary beverages increase the risk of cancer and cancer-related mortality.
A more surprising finding is that consuming artificially sweetened beverages was linked to an increased risk of death from pancreatic cancer.
“This finding is very interesting,” said Marjorie McCullough, ScD, RD, senior scientific director of epidemiology research, American Cancer Society. She noted that other studies that examined an association between artificially sweetened beverages and pancreatic cancer did not reveal a statistically significant association.
“Our study is the first, to our knowledge, that has found a statistically significant positive association, and it will be important to replicate this finding,” said Dr. McCullough.
The study was published online in Cancer, Epidemiology, Biomarkers, and Prevention.
In the study, Dr. McCullough and colleagues examined associations between drinking sugar-sweetened and artificially sweetened beverages and dying from any cancer or any obesity-related cancers. The researchers also examined this association for 20 individual cancer types.
Participants included 934,777 cancer-free adults from the Cancer Prevention Study-II (CPS-II) prospective cohort. At baseline, adults completed a questionnaire on their medical history, lifestyle exposures, and habits, including how many sugar-sweetened or artificially sweetened drinks they typically consumed each day.
Over a median 28-year follow-up, 135,093 participants died from cancer.
Overall, the researchers determined that consuming two or more sugar-sweetened beverages daily (vs. consuming none) was not associated with all-cancer mortality.
Regarding obesity-related cancers, Dr. McCullough and colleagues found a significant 5% increased risk of death from these cancer (hazard ratio, 1.05); however, this association disappeared after controlling for body mass index (BMI). According to Dr. McCullough, this finding may signal that the association between sugary drinks and obesity-related cancer deaths is at least partly mediated by higher BMI, or excess body fat.
“Weight control is key to cancer prevention,” noted Linda Van Horn, RD, chief of the nutrition division at the Feinberg School of Medicine, Northwestern University, Chicago, who wasn’t involved in the study.
However, with regard to individual cancers, consuming two or more sugar-sweetened drinks each day was associated with an increased risk of dying from colorectal cancer (HR, 1.09) and kidney cancer (HR, 1.17) after adjusting for BMI.
Unexpectedly, sugary beverage intake was associated with a lower risk of esophageal and lung cancer mortality. This association held for lung cancer but not esophageal cancer after restricting the analysis to never-smoking participants (HR, 0.81; 95% confidence interval, 0.70-0.94).
Artificial sweetener and pancreatic cancer?
With respect to artificially sweetened drinks, consuming two or more beverages daily was associated with a 5% increased risk of death from obesity-related cancers (HR, 1.05), but that association became null after controlling for BMI.
However, the link to pancreatic cancer mortality remained after adjusting for BMI (HR, 1.11). This association should be studied further, the researchers say. They say there is a possibility that undiagnosed diabetes influenced the results.
“Continued research on the impact of both beverage types with cancer risk and mortality is warranted to determine whether these associations are causal or confounded by other lifestyle factors and whether they are mediated through BMI,” the researchers write.
Reached for comment, Marcus DaSilva Goncalves, MD, PhD, with Weill Cornell Medicine, New York, noted that the association with colorectal cancer has been previously reported, and he agreed that these “findings strengthen the available evidence of an association between sugar-sweetened beverages and colorectal cancer mortality.”
“Data from my lab in mice have shown that sugar-sweetened beverages deliver fructose directly to colon tumors, which stimulates the survival of cancer cells and growth of tumors,” Dr. Goncalves said.
There are also recent clinical data suggesting that exposure to sugar-sweetened beverages during adolescence and adulthood promotes adenoma formation, the precursor to colorectal cancer, he said.
Regarding artificially sweetened beverage intake, Dr. Goncalves said the effect with pancreatic cancer is “surprising” and that he is not aware of other data, including data from several large studies, that support this relationship.
No specific funding for study has been reported. Dr. McCullough, Ms. Van Horn, and Dr. Goncalves have disclosed no relevant disclosures relationships.
A version of this article first appeared on Medscape.com.
The study, which included more than 900,000 participants, contributes to previous research suggesting that sugary beverages increase the risk of cancer and cancer-related mortality.
A more surprising finding is that consuming artificially sweetened beverages was linked to an increased risk of death from pancreatic cancer.
“This finding is very interesting,” said Marjorie McCullough, ScD, RD, senior scientific director of epidemiology research, American Cancer Society. She noted that other studies that examined an association between artificially sweetened beverages and pancreatic cancer did not reveal a statistically significant association.
“Our study is the first, to our knowledge, that has found a statistically significant positive association, and it will be important to replicate this finding,” said Dr. McCullough.
The study was published online in Cancer, Epidemiology, Biomarkers, and Prevention.
In the study, Dr. McCullough and colleagues examined associations between drinking sugar-sweetened and artificially sweetened beverages and dying from any cancer or any obesity-related cancers. The researchers also examined this association for 20 individual cancer types.
Participants included 934,777 cancer-free adults from the Cancer Prevention Study-II (CPS-II) prospective cohort. At baseline, adults completed a questionnaire on their medical history, lifestyle exposures, and habits, including how many sugar-sweetened or artificially sweetened drinks they typically consumed each day.
Over a median 28-year follow-up, 135,093 participants died from cancer.
Overall, the researchers determined that consuming two or more sugar-sweetened beverages daily (vs. consuming none) was not associated with all-cancer mortality.
Regarding obesity-related cancers, Dr. McCullough and colleagues found a significant 5% increased risk of death from these cancer (hazard ratio, 1.05); however, this association disappeared after controlling for body mass index (BMI). According to Dr. McCullough, this finding may signal that the association between sugary drinks and obesity-related cancer deaths is at least partly mediated by higher BMI, or excess body fat.
“Weight control is key to cancer prevention,” noted Linda Van Horn, RD, chief of the nutrition division at the Feinberg School of Medicine, Northwestern University, Chicago, who wasn’t involved in the study.
However, with regard to individual cancers, consuming two or more sugar-sweetened drinks each day was associated with an increased risk of dying from colorectal cancer (HR, 1.09) and kidney cancer (HR, 1.17) after adjusting for BMI.
Unexpectedly, sugary beverage intake was associated with a lower risk of esophageal and lung cancer mortality. This association held for lung cancer but not esophageal cancer after restricting the analysis to never-smoking participants (HR, 0.81; 95% confidence interval, 0.70-0.94).
Artificial sweetener and pancreatic cancer?
With respect to artificially sweetened drinks, consuming two or more beverages daily was associated with a 5% increased risk of death from obesity-related cancers (HR, 1.05), but that association became null after controlling for BMI.
However, the link to pancreatic cancer mortality remained after adjusting for BMI (HR, 1.11). This association should be studied further, the researchers say. They say there is a possibility that undiagnosed diabetes influenced the results.
“Continued research on the impact of both beverage types with cancer risk and mortality is warranted to determine whether these associations are causal or confounded by other lifestyle factors and whether they are mediated through BMI,” the researchers write.
Reached for comment, Marcus DaSilva Goncalves, MD, PhD, with Weill Cornell Medicine, New York, noted that the association with colorectal cancer has been previously reported, and he agreed that these “findings strengthen the available evidence of an association between sugar-sweetened beverages and colorectal cancer mortality.”
“Data from my lab in mice have shown that sugar-sweetened beverages deliver fructose directly to colon tumors, which stimulates the survival of cancer cells and growth of tumors,” Dr. Goncalves said.
There are also recent clinical data suggesting that exposure to sugar-sweetened beverages during adolescence and adulthood promotes adenoma formation, the precursor to colorectal cancer, he said.
Regarding artificially sweetened beverage intake, Dr. Goncalves said the effect with pancreatic cancer is “surprising” and that he is not aware of other data, including data from several large studies, that support this relationship.
No specific funding for study has been reported. Dr. McCullough, Ms. Van Horn, and Dr. Goncalves have disclosed no relevant disclosures relationships.
A version of this article first appeared on Medscape.com.
FROM CANCER, EPIDEMIOLOGY, BIOMARKERS, AND PREVENTION