User login
Sotorasib superior to docetaxel in KRAS G12C–mutated NSCLC
PARIS – For patients with non–small cell lung cancers (NSCLC) bearing the KRAS G12C mutation that have progressed on prior therapies, the first-in-class KRAS G12C inhibitor sotorasib (Lumykras) was associated with better progression-free survival (PFS) and objective response rates than docetaxel in the randomized, phase 3 CodeBreaK 200 trial.
Among 345 patients who had experienced disease progression after prior platinum-based chemotherapy and an immune checkpoint inhibitor, 1-year PFS rates at a median follow-up of 17.7 months were 24.8% for patients randomized to receive sotorasib versus 10.1% for patients assigned to docetaxel, reported Melissa L, Johnson, MD, from the Sarah Cannon Research Institute at Tennessee Oncology in Nashville.
“In my opinion, this supports sotorasib as a new second-line standard for patients with KRAS G12C non–small cell lung cancer,” she said in a media briefing prior to her presentation of the data in an oral abstract session at the annual meeting of the European Society for Medical Oncology.
First phase 3, randomized, controlled trial
The trial is the first head-to-head, randomized comparison pitting a KRAS G12C inhibitor against the standard of care in patients with NSCLC.
Approximately 30% of patients with NSCLC have KRAS driver mutations, and KRAS G12C–mutant NSCLC comprises an estimated 13% of all NSCLC cases, Dr. Johnson said.
Sotorasib was hailed as “a triumph of drug discovery” when early results of the trial were reported at the 2020 ESMO annual meeting. It is a small-molecule, specific, and irreversible inhibitor of KRAS that interacts with a “pocket” on the gene’s surface that is present only in an inactive conformation of KRAS. The drug inhibits oncogenic signaling and tumorigenesis by preventing cycling of the oncogene into its active form.
CodeBreaK 200 details
A total of 345 patients from sites in the United States, Europe, Asia and Australia were enrolled and randomly assigned to receive either oral sotorasib 960 mg daily, or intravenous docetaxel 75 mg/m2 every 3 weeks.
As noted before, the trial met its primary endpoint of a statistically significant improvement in PFS with sotorasib as measured by independent central reviewers blinded to study assignment, with a hazard ratio of 0.66 (P = .002). Median PFS with sotorasib was 5.6 months, compared with 4.5 months for docetaxel.
The objective response rate was significantly improved for sotorasib versus docetaxel (28.1% vs. 13.2%, P < .001), as was the disease control rate at 82.5% for sotorasib versus 60.3% for docetaxel. Overall survival was not significantly different between treatment arms, though the study was not powered for this endpoint.
Sotorasib was also superior to docetaxel at forestalling deterioration of patients’ global health status, physical functioning, and cancer-related symptoms such as dyspnea and cough. There was no significant difference between the study arms in reported chest pain, however.
Grade 3 or greater treatment-related adverse events occurred in 33.1% of patients with sotorasib, compared with 40.4% of patients on docetaxel.
‘Tremendous advance’
“I think the conduct of this study is impressive, it’s well designed, it was well run, any imbalances really favored the control arm, and I think that this advance is relevant not just for performance status 0 and 1 KRAS G12C–mutant patients, but even beyond, to performance status 2 and perhaps even performance status 3,” commented Natasha Leighl, MD, MMSc, from the Princess Margaret Cancer Center, Toronto, the invited discussant.
Comparing the drug performance of the respective arms, Dr. Leighl said that “I don’t think I’ve ever seen such good outcomes in a randomized trial with the chemotherapy, but unfortunately sotorasib performed a little bit less well than we had hoped.”
Nonetheless, “I think CodeBreaK 200 is a tremendous advance for patients. It is a confirmatory positive trial, and I think sotorasib is the new standard of care in patients who have received chemo and immunotherapy for KRAS G12C–mutant lung cancer,” she said.
CodeBreaK 200 was supported by Amgen. Dr. Johnson disclosed a consulting and advisory role with payments to her institution from Amgen and others. Dr. Leighl disclosed institutional grant funding and personal honoraria from Amgen and others.
PARIS – For patients with non–small cell lung cancers (NSCLC) bearing the KRAS G12C mutation that have progressed on prior therapies, the first-in-class KRAS G12C inhibitor sotorasib (Lumykras) was associated with better progression-free survival (PFS) and objective response rates than docetaxel in the randomized, phase 3 CodeBreaK 200 trial.
Among 345 patients who had experienced disease progression after prior platinum-based chemotherapy and an immune checkpoint inhibitor, 1-year PFS rates at a median follow-up of 17.7 months were 24.8% for patients randomized to receive sotorasib versus 10.1% for patients assigned to docetaxel, reported Melissa L, Johnson, MD, from the Sarah Cannon Research Institute at Tennessee Oncology in Nashville.
“In my opinion, this supports sotorasib as a new second-line standard for patients with KRAS G12C non–small cell lung cancer,” she said in a media briefing prior to her presentation of the data in an oral abstract session at the annual meeting of the European Society for Medical Oncology.
First phase 3, randomized, controlled trial
The trial is the first head-to-head, randomized comparison pitting a KRAS G12C inhibitor against the standard of care in patients with NSCLC.
Approximately 30% of patients with NSCLC have KRAS driver mutations, and KRAS G12C–mutant NSCLC comprises an estimated 13% of all NSCLC cases, Dr. Johnson said.
Sotorasib was hailed as “a triumph of drug discovery” when early results of the trial were reported at the 2020 ESMO annual meeting. It is a small-molecule, specific, and irreversible inhibitor of KRAS that interacts with a “pocket” on the gene’s surface that is present only in an inactive conformation of KRAS. The drug inhibits oncogenic signaling and tumorigenesis by preventing cycling of the oncogene into its active form.
CodeBreaK 200 details
A total of 345 patients from sites in the United States, Europe, Asia and Australia were enrolled and randomly assigned to receive either oral sotorasib 960 mg daily, or intravenous docetaxel 75 mg/m2 every 3 weeks.
As noted before, the trial met its primary endpoint of a statistically significant improvement in PFS with sotorasib as measured by independent central reviewers blinded to study assignment, with a hazard ratio of 0.66 (P = .002). Median PFS with sotorasib was 5.6 months, compared with 4.5 months for docetaxel.
The objective response rate was significantly improved for sotorasib versus docetaxel (28.1% vs. 13.2%, P < .001), as was the disease control rate at 82.5% for sotorasib versus 60.3% for docetaxel. Overall survival was not significantly different between treatment arms, though the study was not powered for this endpoint.
Sotorasib was also superior to docetaxel at forestalling deterioration of patients’ global health status, physical functioning, and cancer-related symptoms such as dyspnea and cough. There was no significant difference between the study arms in reported chest pain, however.
Grade 3 or greater treatment-related adverse events occurred in 33.1% of patients with sotorasib, compared with 40.4% of patients on docetaxel.
‘Tremendous advance’
“I think the conduct of this study is impressive, it’s well designed, it was well run, any imbalances really favored the control arm, and I think that this advance is relevant not just for performance status 0 and 1 KRAS G12C–mutant patients, but even beyond, to performance status 2 and perhaps even performance status 3,” commented Natasha Leighl, MD, MMSc, from the Princess Margaret Cancer Center, Toronto, the invited discussant.
Comparing the drug performance of the respective arms, Dr. Leighl said that “I don’t think I’ve ever seen such good outcomes in a randomized trial with the chemotherapy, but unfortunately sotorasib performed a little bit less well than we had hoped.”
Nonetheless, “I think CodeBreaK 200 is a tremendous advance for patients. It is a confirmatory positive trial, and I think sotorasib is the new standard of care in patients who have received chemo and immunotherapy for KRAS G12C–mutant lung cancer,” she said.
CodeBreaK 200 was supported by Amgen. Dr. Johnson disclosed a consulting and advisory role with payments to her institution from Amgen and others. Dr. Leighl disclosed institutional grant funding and personal honoraria from Amgen and others.
PARIS – For patients with non–small cell lung cancers (NSCLC) bearing the KRAS G12C mutation that have progressed on prior therapies, the first-in-class KRAS G12C inhibitor sotorasib (Lumykras) was associated with better progression-free survival (PFS) and objective response rates than docetaxel in the randomized, phase 3 CodeBreaK 200 trial.
Among 345 patients who had experienced disease progression after prior platinum-based chemotherapy and an immune checkpoint inhibitor, 1-year PFS rates at a median follow-up of 17.7 months were 24.8% for patients randomized to receive sotorasib versus 10.1% for patients assigned to docetaxel, reported Melissa L, Johnson, MD, from the Sarah Cannon Research Institute at Tennessee Oncology in Nashville.
“In my opinion, this supports sotorasib as a new second-line standard for patients with KRAS G12C non–small cell lung cancer,” she said in a media briefing prior to her presentation of the data in an oral abstract session at the annual meeting of the European Society for Medical Oncology.
First phase 3, randomized, controlled trial
The trial is the first head-to-head, randomized comparison pitting a KRAS G12C inhibitor against the standard of care in patients with NSCLC.
Approximately 30% of patients with NSCLC have KRAS driver mutations, and KRAS G12C–mutant NSCLC comprises an estimated 13% of all NSCLC cases, Dr. Johnson said.
Sotorasib was hailed as “a triumph of drug discovery” when early results of the trial were reported at the 2020 ESMO annual meeting. It is a small-molecule, specific, and irreversible inhibitor of KRAS that interacts with a “pocket” on the gene’s surface that is present only in an inactive conformation of KRAS. The drug inhibits oncogenic signaling and tumorigenesis by preventing cycling of the oncogene into its active form.
CodeBreaK 200 details
A total of 345 patients from sites in the United States, Europe, Asia and Australia were enrolled and randomly assigned to receive either oral sotorasib 960 mg daily, or intravenous docetaxel 75 mg/m2 every 3 weeks.
As noted before, the trial met its primary endpoint of a statistically significant improvement in PFS with sotorasib as measured by independent central reviewers blinded to study assignment, with a hazard ratio of 0.66 (P = .002). Median PFS with sotorasib was 5.6 months, compared with 4.5 months for docetaxel.
The objective response rate was significantly improved for sotorasib versus docetaxel (28.1% vs. 13.2%, P < .001), as was the disease control rate at 82.5% for sotorasib versus 60.3% for docetaxel. Overall survival was not significantly different between treatment arms, though the study was not powered for this endpoint.
Sotorasib was also superior to docetaxel at forestalling deterioration of patients’ global health status, physical functioning, and cancer-related symptoms such as dyspnea and cough. There was no significant difference between the study arms in reported chest pain, however.
Grade 3 or greater treatment-related adverse events occurred in 33.1% of patients with sotorasib, compared with 40.4% of patients on docetaxel.
‘Tremendous advance’
“I think the conduct of this study is impressive, it’s well designed, it was well run, any imbalances really favored the control arm, and I think that this advance is relevant not just for performance status 0 and 1 KRAS G12C–mutant patients, but even beyond, to performance status 2 and perhaps even performance status 3,” commented Natasha Leighl, MD, MMSc, from the Princess Margaret Cancer Center, Toronto, the invited discussant.
Comparing the drug performance of the respective arms, Dr. Leighl said that “I don’t think I’ve ever seen such good outcomes in a randomized trial with the chemotherapy, but unfortunately sotorasib performed a little bit less well than we had hoped.”
Nonetheless, “I think CodeBreaK 200 is a tremendous advance for patients. It is a confirmatory positive trial, and I think sotorasib is the new standard of care in patients who have received chemo and immunotherapy for KRAS G12C–mutant lung cancer,” she said.
CodeBreaK 200 was supported by Amgen. Dr. Johnson disclosed a consulting and advisory role with payments to her institution from Amgen and others. Dr. Leighl disclosed institutional grant funding and personal honoraria from Amgen and others.
AT ESMO CONGRESS 2022
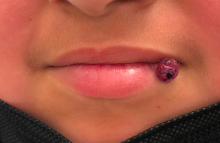
A 10-year-old with a red bump on her lower lip
The patient’s history and examination are consistent with a diagnosis of pyogenic granuloma. Specifically, the history of rapid growth, friable nature, associated bleeding, and hemorrhagic crusting point to pyogenic granuloma as the most likely diagnosis.
Pyogenic granuloma is an acquired benign vascular growth of the skin or mucous membranes.1 It most frequently occurs in children and young adults and most commonly affects the skin of the head, trunk, and extremities.2 Common mucosal sites include the gingiva, lips, and tongue.2 The etiology of pyogenic granuloma is unknown, though it is thought to be a process akin to the overgrowth of granulation tissue.3,4 Expression of angiogenic factors and subsequent vascular hyperplasia are also implicated as key players in the pathogenesis of pyogenic granuloma.1,4 In addition, several associated factors and inciting triggers have been proposed including trauma, infections, and hormonal fluctuations.3-5 However, the majority of patients do not report predisposing factors or a history of prior trauma at the site.3,6
Clinically, pyogenic granuloma usually presents as a painless, erythematous, dome-shaped friable papule or nodule that easily bleeds and may ulcerate. It typically undergoes a period of growth over weeks to months followed by stabilization. Occasionally, pyogenic granulomas will spontaneously involute, though most do not.7 Pyogenic granuloma may occur within an existing capillary malformation, such as a port wine stain, spontaneously or as a sequela of laser treatment.8,9 Diagnosis of pyogenic granuloma can typically be made clinically on the basis of history and exam. Dermoscopic evaluation of pyogenic granuloma will reveal a homogeneous papule with a surrounding white-brown collarette, and potentially white intersecting lines.10 Histopathologic evaluation may be necessary to differentiate lesions from conditions that may mimic pyogenic granuloma.
What’s on the differential?
The differential diagnosis for pyogenic granuloma consists of Spitz nevus, cherry hemangioma, amelanotic melanoma, and glomus tumor.
Spitz nevus
Spitz nevus (spindle and epithelial cell nevus) is a benign melanocytic lesion that classically appears as a sharply circumscribed, smooth, dome-shaped, pink-red, or brown papule or plaque. There is typically a history of rapid growth over several months followed by stabilization. It usually presents in childhood or adolescence and is most commonly located on the face and extremities. While there are similarities in the appearance of Spitz nevi and pyogenic granuloma, Spitz nevi are not usually friable nor associated with bleeding as in our patient. Furthermore, on dermoscopy, Spitz nevus typically exhibits a starburst pattern with regularly distributed dotted vessels, or a peripheral globular pattern with reticular depigmentation. The definitive diagnosis of Spitz nevi relies on histopathologic evaluation, which is critical for discriminating Spitz nevi from melanoma.
Cherry hemangioma
Cherry angiomas are the most common type of acquired benign vascular proliferation. They present as small, bright red or violaceous macules or papules. However, they typically appear in early to midadulthood and increase in number with age. The age of our patient and solitary presentation of the lesion make this diagnosis unlikely. In addition, cherry angiomas are not usually associated with bleeding. It is important to note that, depending on the age of the patient, pyogenic granuloma may also be confused with infantile hemangioma. Infantile hemangiomas may become bright red papules, nodules, or plaques that appear in early infancy. They characteristically involute, which does not typically happen with pyogenic granuloma.
Amelanotic melanoma
Amelanotic melanoma is an uncommon variant of melanoma with little to no pigmentation. It may appear as a skin-colored to light-brown, pink, or red macule, papule, or nodule. The lesion may be asymmetric with irregular and well-defined borders. The variable and uncharacteristic appearance of this melanoma variant makes it diagnostically challenging and it is often confused with benign lesions including pyogenic granuloma. Dermoscopy can help distinguish amelanotic melanoma from other benign conditions, and will reveal areas of pink to white, polymorphous vessels and crystalline structures. However, ultimately biopsy and histopathological evaluation is necessary for accurate diagnosis.
Glomus tumor
Glomus tumors are rare, benign neoplasms originating from cells of the glomus body that presents as a red-purple, vascular papule or nodule. They are usually found in areas rich in glomus bodies, such as the subungual regions, fingertips, palms, wrists, and forearms. Glomus tumors are typically associated with tenderness, paroxysmal pain, and cold sensitivity. They do not bleed or ulcerate. While pyogenic granuloma may be confused for glomus tumor when present on the fingers or extremities, the location of the lesion in our patient is not consistent with a diagnosis of glomus tumor.
Management and disease course
Management with procedural or topical interventions is usually pursued for pyogenic granuloma because of frequent bleeding and ulceration of lesions. The most common approach is simple excision by a scoop or shave technique, with or without curettage and most commonly with electrocautery of the base. Other options include full-thickness excision, destruction with laser therapy, cryotherapy, or topical treatments (for example, timolol).11 Lesion recurrence can occur with both surgical and nonsurgical management.11 Regardless of management technique, it is useful to obtain histopathologic evaluation of tissue for accurate diagnosis.
Our patient underwent surgical destruction of her lower-lip lesion with shave excision followed by electrocautery. The surgical specimen was sent for pathology, which confirmed the diagnosis of pyogenic granuloma. The patient experienced no complications from the procedure and did not have recurrence of the lesion.
Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Ms. Sui nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Lin RL and Janniger CK. Cutis. 2004 Oct;74(4):229-33.
2. Harris MN et al. J Am Acad Dermatol. 2000 Jun;42(6):1012-6.
3. Pagliai KA and Cohen BA. Pediatr Dermatol. 2004 Jan-Feb;21(1):10-3.
4. Kamal R et al. J Oral Maxillofac Pathol. 2012 Jan;16(1):79-82.
5. Requena L and Sangueza OP. J Am Acad Dermatol. 1997 Dec;37(6):887-919.
6. Patrice SJ et al. Pediatr Dermatol. 1991 Dec;8(4):267-76.
7. Luba MC et al. Am Fam Physician. 2003 Feb 15;67(4):729-38.
8. Swerlick RA and Cooper PH. J Am Acad Dermatol. 1983 May;8(5):627-30.
9. Sheehan DJ and Lesher JL Jr. Cutis. 2004 Mar;73(3):175-80.
10. Zaballos P et al. Br J Dermatol. 2006 Jun;154(6):1108-11.
11. Lee J et al. J Plast Reconstr Aesthet Surg. 2011 Sep;64(9):1216-20. .
The patient’s history and examination are consistent with a diagnosis of pyogenic granuloma. Specifically, the history of rapid growth, friable nature, associated bleeding, and hemorrhagic crusting point to pyogenic granuloma as the most likely diagnosis.
Pyogenic granuloma is an acquired benign vascular growth of the skin or mucous membranes.1 It most frequently occurs in children and young adults and most commonly affects the skin of the head, trunk, and extremities.2 Common mucosal sites include the gingiva, lips, and tongue.2 The etiology of pyogenic granuloma is unknown, though it is thought to be a process akin to the overgrowth of granulation tissue.3,4 Expression of angiogenic factors and subsequent vascular hyperplasia are also implicated as key players in the pathogenesis of pyogenic granuloma.1,4 In addition, several associated factors and inciting triggers have been proposed including trauma, infections, and hormonal fluctuations.3-5 However, the majority of patients do not report predisposing factors or a history of prior trauma at the site.3,6
Clinically, pyogenic granuloma usually presents as a painless, erythematous, dome-shaped friable papule or nodule that easily bleeds and may ulcerate. It typically undergoes a period of growth over weeks to months followed by stabilization. Occasionally, pyogenic granulomas will spontaneously involute, though most do not.7 Pyogenic granuloma may occur within an existing capillary malformation, such as a port wine stain, spontaneously or as a sequela of laser treatment.8,9 Diagnosis of pyogenic granuloma can typically be made clinically on the basis of history and exam. Dermoscopic evaluation of pyogenic granuloma will reveal a homogeneous papule with a surrounding white-brown collarette, and potentially white intersecting lines.10 Histopathologic evaluation may be necessary to differentiate lesions from conditions that may mimic pyogenic granuloma.
What’s on the differential?
The differential diagnosis for pyogenic granuloma consists of Spitz nevus, cherry hemangioma, amelanotic melanoma, and glomus tumor.
Spitz nevus
Spitz nevus (spindle and epithelial cell nevus) is a benign melanocytic lesion that classically appears as a sharply circumscribed, smooth, dome-shaped, pink-red, or brown papule or plaque. There is typically a history of rapid growth over several months followed by stabilization. It usually presents in childhood or adolescence and is most commonly located on the face and extremities. While there are similarities in the appearance of Spitz nevi and pyogenic granuloma, Spitz nevi are not usually friable nor associated with bleeding as in our patient. Furthermore, on dermoscopy, Spitz nevus typically exhibits a starburst pattern with regularly distributed dotted vessels, or a peripheral globular pattern with reticular depigmentation. The definitive diagnosis of Spitz nevi relies on histopathologic evaluation, which is critical for discriminating Spitz nevi from melanoma.
Cherry hemangioma
Cherry angiomas are the most common type of acquired benign vascular proliferation. They present as small, bright red or violaceous macules or papules. However, they typically appear in early to midadulthood and increase in number with age. The age of our patient and solitary presentation of the lesion make this diagnosis unlikely. In addition, cherry angiomas are not usually associated with bleeding. It is important to note that, depending on the age of the patient, pyogenic granuloma may also be confused with infantile hemangioma. Infantile hemangiomas may become bright red papules, nodules, or plaques that appear in early infancy. They characteristically involute, which does not typically happen with pyogenic granuloma.
Amelanotic melanoma
Amelanotic melanoma is an uncommon variant of melanoma with little to no pigmentation. It may appear as a skin-colored to light-brown, pink, or red macule, papule, or nodule. The lesion may be asymmetric with irregular and well-defined borders. The variable and uncharacteristic appearance of this melanoma variant makes it diagnostically challenging and it is often confused with benign lesions including pyogenic granuloma. Dermoscopy can help distinguish amelanotic melanoma from other benign conditions, and will reveal areas of pink to white, polymorphous vessels and crystalline structures. However, ultimately biopsy and histopathological evaluation is necessary for accurate diagnosis.
Glomus tumor
Glomus tumors are rare, benign neoplasms originating from cells of the glomus body that presents as a red-purple, vascular papule or nodule. They are usually found in areas rich in glomus bodies, such as the subungual regions, fingertips, palms, wrists, and forearms. Glomus tumors are typically associated with tenderness, paroxysmal pain, and cold sensitivity. They do not bleed or ulcerate. While pyogenic granuloma may be confused for glomus tumor when present on the fingers or extremities, the location of the lesion in our patient is not consistent with a diagnosis of glomus tumor.
Management and disease course
Management with procedural or topical interventions is usually pursued for pyogenic granuloma because of frequent bleeding and ulceration of lesions. The most common approach is simple excision by a scoop or shave technique, with or without curettage and most commonly with electrocautery of the base. Other options include full-thickness excision, destruction with laser therapy, cryotherapy, or topical treatments (for example, timolol).11 Lesion recurrence can occur with both surgical and nonsurgical management.11 Regardless of management technique, it is useful to obtain histopathologic evaluation of tissue for accurate diagnosis.
Our patient underwent surgical destruction of her lower-lip lesion with shave excision followed by electrocautery. The surgical specimen was sent for pathology, which confirmed the diagnosis of pyogenic granuloma. The patient experienced no complications from the procedure and did not have recurrence of the lesion.
Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Ms. Sui nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Lin RL and Janniger CK. Cutis. 2004 Oct;74(4):229-33.
2. Harris MN et al. J Am Acad Dermatol. 2000 Jun;42(6):1012-6.
3. Pagliai KA and Cohen BA. Pediatr Dermatol. 2004 Jan-Feb;21(1):10-3.
4. Kamal R et al. J Oral Maxillofac Pathol. 2012 Jan;16(1):79-82.
5. Requena L and Sangueza OP. J Am Acad Dermatol. 1997 Dec;37(6):887-919.
6. Patrice SJ et al. Pediatr Dermatol. 1991 Dec;8(4):267-76.
7. Luba MC et al. Am Fam Physician. 2003 Feb 15;67(4):729-38.
8. Swerlick RA and Cooper PH. J Am Acad Dermatol. 1983 May;8(5):627-30.
9. Sheehan DJ and Lesher JL Jr. Cutis. 2004 Mar;73(3):175-80.
10. Zaballos P et al. Br J Dermatol. 2006 Jun;154(6):1108-11.
11. Lee J et al. J Plast Reconstr Aesthet Surg. 2011 Sep;64(9):1216-20. .
The patient’s history and examination are consistent with a diagnosis of pyogenic granuloma. Specifically, the history of rapid growth, friable nature, associated bleeding, and hemorrhagic crusting point to pyogenic granuloma as the most likely diagnosis.
Pyogenic granuloma is an acquired benign vascular growth of the skin or mucous membranes.1 It most frequently occurs in children and young adults and most commonly affects the skin of the head, trunk, and extremities.2 Common mucosal sites include the gingiva, lips, and tongue.2 The etiology of pyogenic granuloma is unknown, though it is thought to be a process akin to the overgrowth of granulation tissue.3,4 Expression of angiogenic factors and subsequent vascular hyperplasia are also implicated as key players in the pathogenesis of pyogenic granuloma.1,4 In addition, several associated factors and inciting triggers have been proposed including trauma, infections, and hormonal fluctuations.3-5 However, the majority of patients do not report predisposing factors or a history of prior trauma at the site.3,6
Clinically, pyogenic granuloma usually presents as a painless, erythematous, dome-shaped friable papule or nodule that easily bleeds and may ulcerate. It typically undergoes a period of growth over weeks to months followed by stabilization. Occasionally, pyogenic granulomas will spontaneously involute, though most do not.7 Pyogenic granuloma may occur within an existing capillary malformation, such as a port wine stain, spontaneously or as a sequela of laser treatment.8,9 Diagnosis of pyogenic granuloma can typically be made clinically on the basis of history and exam. Dermoscopic evaluation of pyogenic granuloma will reveal a homogeneous papule with a surrounding white-brown collarette, and potentially white intersecting lines.10 Histopathologic evaluation may be necessary to differentiate lesions from conditions that may mimic pyogenic granuloma.
What’s on the differential?
The differential diagnosis for pyogenic granuloma consists of Spitz nevus, cherry hemangioma, amelanotic melanoma, and glomus tumor.
Spitz nevus
Spitz nevus (spindle and epithelial cell nevus) is a benign melanocytic lesion that classically appears as a sharply circumscribed, smooth, dome-shaped, pink-red, or brown papule or plaque. There is typically a history of rapid growth over several months followed by stabilization. It usually presents in childhood or adolescence and is most commonly located on the face and extremities. While there are similarities in the appearance of Spitz nevi and pyogenic granuloma, Spitz nevi are not usually friable nor associated with bleeding as in our patient. Furthermore, on dermoscopy, Spitz nevus typically exhibits a starburst pattern with regularly distributed dotted vessels, or a peripheral globular pattern with reticular depigmentation. The definitive diagnosis of Spitz nevi relies on histopathologic evaluation, which is critical for discriminating Spitz nevi from melanoma.
Cherry hemangioma
Cherry angiomas are the most common type of acquired benign vascular proliferation. They present as small, bright red or violaceous macules or papules. However, they typically appear in early to midadulthood and increase in number with age. The age of our patient and solitary presentation of the lesion make this diagnosis unlikely. In addition, cherry angiomas are not usually associated with bleeding. It is important to note that, depending on the age of the patient, pyogenic granuloma may also be confused with infantile hemangioma. Infantile hemangiomas may become bright red papules, nodules, or plaques that appear in early infancy. They characteristically involute, which does not typically happen with pyogenic granuloma.
Amelanotic melanoma
Amelanotic melanoma is an uncommon variant of melanoma with little to no pigmentation. It may appear as a skin-colored to light-brown, pink, or red macule, papule, or nodule. The lesion may be asymmetric with irregular and well-defined borders. The variable and uncharacteristic appearance of this melanoma variant makes it diagnostically challenging and it is often confused with benign lesions including pyogenic granuloma. Dermoscopy can help distinguish amelanotic melanoma from other benign conditions, and will reveal areas of pink to white, polymorphous vessels and crystalline structures. However, ultimately biopsy and histopathological evaluation is necessary for accurate diagnosis.
Glomus tumor
Glomus tumors are rare, benign neoplasms originating from cells of the glomus body that presents as a red-purple, vascular papule or nodule. They are usually found in areas rich in glomus bodies, such as the subungual regions, fingertips, palms, wrists, and forearms. Glomus tumors are typically associated with tenderness, paroxysmal pain, and cold sensitivity. They do not bleed or ulcerate. While pyogenic granuloma may be confused for glomus tumor when present on the fingers or extremities, the location of the lesion in our patient is not consistent with a diagnosis of glomus tumor.
Management and disease course
Management with procedural or topical interventions is usually pursued for pyogenic granuloma because of frequent bleeding and ulceration of lesions. The most common approach is simple excision by a scoop or shave technique, with or without curettage and most commonly with electrocautery of the base. Other options include full-thickness excision, destruction with laser therapy, cryotherapy, or topical treatments (for example, timolol).11 Lesion recurrence can occur with both surgical and nonsurgical management.11 Regardless of management technique, it is useful to obtain histopathologic evaluation of tissue for accurate diagnosis.
Our patient underwent surgical destruction of her lower-lip lesion with shave excision followed by electrocautery. The surgical specimen was sent for pathology, which confirmed the diagnosis of pyogenic granuloma. The patient experienced no complications from the procedure and did not have recurrence of the lesion.
Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Neither Ms. Sui nor Dr. Eichenfield have any relevant financial disclosures.
References
1. Lin RL and Janniger CK. Cutis. 2004 Oct;74(4):229-33.
2. Harris MN et al. J Am Acad Dermatol. 2000 Jun;42(6):1012-6.
3. Pagliai KA and Cohen BA. Pediatr Dermatol. 2004 Jan-Feb;21(1):10-3.
4. Kamal R et al. J Oral Maxillofac Pathol. 2012 Jan;16(1):79-82.
5. Requena L and Sangueza OP. J Am Acad Dermatol. 1997 Dec;37(6):887-919.
6. Patrice SJ et al. Pediatr Dermatol. 1991 Dec;8(4):267-76.
7. Luba MC et al. Am Fam Physician. 2003 Feb 15;67(4):729-38.
8. Swerlick RA and Cooper PH. J Am Acad Dermatol. 1983 May;8(5):627-30.
9. Sheehan DJ and Lesher JL Jr. Cutis. 2004 Mar;73(3):175-80.
10. Zaballos P et al. Br J Dermatol. 2006 Jun;154(6):1108-11.
11. Lee J et al. J Plast Reconstr Aesthet Surg. 2011 Sep;64(9):1216-20. .
Night owls may have greater risks of T2D and CVD
In the study involving 51 people, night owls metabolized fat less efficiently, showed less insulin sensitivity, and demonstrated lower physical fitness than early birds, lead author Steven K. Malin, PhD, of Rutgers University, New Brunswick, N.J., and colleagues reported.
Prior publications have suggested that night owls, formally known as “late chronotypes,” have an increased risk of obesity, type 2 diabetes, and cardiovascular disease, Dr. Malin said in an interview. But no previous research involved the gold-standard measurement tools used in this study, including euglycemic clamp and indirect calorimetry to quantify fat metabolism.
Dr. Malin also noted that this is the first study of its kind to characterize metabolism during both rest and exercise.
The study, published in Experimental Physiology, involved 24 early birds and 27 night owls classified by the Morning-Eveningness Questionnaire. All participants were sedentary, reporting less than one hour of structured exercise per week, and had metabolic syndrome according to Adult Treatment Panel III report criteria. Groups were otherwise demographically similar, with average ages in each group of approximately 54-55 years.
Compared with night owls, early birds were more physically active during the morning into midday. During exercise, they metabolized more fat and demonstrated greater physical fitness based on VO2max readings. At rest, early birds also came out ahead – they had higher fat oxidation and non–oxidative glucose disposal, suggesting more sensitivity to insulin.
“Collectively, this work highlights and supports chronotype as a potential risk factor related to type 2 diabetes and cardiovascular disease risk,” the investigators concluded.
Night owls have less metabolic control
Jed Friedman, PhD, director of OU Health Harold Hamm Diabetes Center at the University of Oklahoma Health Sciences Center, Oklahoma City, praised the study for the size of the groups the researchers compared with each other and how well matched those groups were, as well as the “state-of-the-art” measurement tools employed.
The findings show that night owls have “less metabolic control,” Dr. Friedman said in an interview.
“That’s a term that’s frequently invoked in [regard to] prediabetes,” he said. “Blood sugar goes up, because when you’re eating a high carbohydrate diet, your cells aren’t metabolizing sugar properly. That tends to raise your risk for a lot of diseases.”
Dr. Friedman added that the findings align with those of previous studies that have linked less sleep with changes in brain biology, and therefore behavior, especially in dietary choices.
“When you’re tired, the mechanisms for appetite control go haywire,” Dr. Friedman explained. “The evidence suggests that sugar is the primary driver for what people eat when they’re tired. That obviously has implications for diabetes and metabolic syndrome. So sleeping more really can help you control cravings.”
Dr. Friedman also noted that people who are tired tend to engage in less physical activity, further increasing their risk of metabolic issues. To control this risk, he advised people to return to their circadian rhythms, which could mean forgetting the midnight snack.
“Having a daily pattern that’s in sync with chronicity, or these daily rhythms, is associated with greater health,” Dr. Friedman said. “We’re not really made to eat at night. I think this [study] kind of reinforces that.”
Can a night owl become an early bird?
When asked if a person’s natural circadian rhythm can be later, Dr. Malin responded that chronotypes may be dictated by genetics and age, as well as external drivers like work schedule. For these reasons, it’s “tricky” to answer whether night owls can turn into early birds and reap the potential health benefits of making that shift.
“Given that so many life factors can influence what our routine entails, it’s hard to know if we [can] truly change our chronotype or if rather we [can] learn to manage,” Dr. Malin said. “In either case, there is some work that suggests people can adopt earlier bedtimes and waketimes through practical recommendations.”
Specifically, he suggested increasing physical activity during the day, and adjusting bedtimes gradually by 15-minute increments.
“Go to bed 15 minutes earlier then wake up 15 minutes earlier,” Dr. Malin said. “In time, and depending on how things are going, this can expand to another 15-minute window. Then, during the earlier time waking up, a person can engage in light physical activity to help with promoting general fitness. If they can get outside with sunlight, that would be great too, as the natural sunlight would provide cues to the circadian system to adjust.”
The study was supported by the National Institutes of Health. The investigators and Dr. Friedman disclosed no conflicts of interest.
In the study involving 51 people, night owls metabolized fat less efficiently, showed less insulin sensitivity, and demonstrated lower physical fitness than early birds, lead author Steven K. Malin, PhD, of Rutgers University, New Brunswick, N.J., and colleagues reported.
Prior publications have suggested that night owls, formally known as “late chronotypes,” have an increased risk of obesity, type 2 diabetes, and cardiovascular disease, Dr. Malin said in an interview. But no previous research involved the gold-standard measurement tools used in this study, including euglycemic clamp and indirect calorimetry to quantify fat metabolism.
Dr. Malin also noted that this is the first study of its kind to characterize metabolism during both rest and exercise.
The study, published in Experimental Physiology, involved 24 early birds and 27 night owls classified by the Morning-Eveningness Questionnaire. All participants were sedentary, reporting less than one hour of structured exercise per week, and had metabolic syndrome according to Adult Treatment Panel III report criteria. Groups were otherwise demographically similar, with average ages in each group of approximately 54-55 years.
Compared with night owls, early birds were more physically active during the morning into midday. During exercise, they metabolized more fat and demonstrated greater physical fitness based on VO2max readings. At rest, early birds also came out ahead – they had higher fat oxidation and non–oxidative glucose disposal, suggesting more sensitivity to insulin.
“Collectively, this work highlights and supports chronotype as a potential risk factor related to type 2 diabetes and cardiovascular disease risk,” the investigators concluded.
Night owls have less metabolic control
Jed Friedman, PhD, director of OU Health Harold Hamm Diabetes Center at the University of Oklahoma Health Sciences Center, Oklahoma City, praised the study for the size of the groups the researchers compared with each other and how well matched those groups were, as well as the “state-of-the-art” measurement tools employed.
The findings show that night owls have “less metabolic control,” Dr. Friedman said in an interview.
“That’s a term that’s frequently invoked in [regard to] prediabetes,” he said. “Blood sugar goes up, because when you’re eating a high carbohydrate diet, your cells aren’t metabolizing sugar properly. That tends to raise your risk for a lot of diseases.”
Dr. Friedman added that the findings align with those of previous studies that have linked less sleep with changes in brain biology, and therefore behavior, especially in dietary choices.
“When you’re tired, the mechanisms for appetite control go haywire,” Dr. Friedman explained. “The evidence suggests that sugar is the primary driver for what people eat when they’re tired. That obviously has implications for diabetes and metabolic syndrome. So sleeping more really can help you control cravings.”
Dr. Friedman also noted that people who are tired tend to engage in less physical activity, further increasing their risk of metabolic issues. To control this risk, he advised people to return to their circadian rhythms, which could mean forgetting the midnight snack.
“Having a daily pattern that’s in sync with chronicity, or these daily rhythms, is associated with greater health,” Dr. Friedman said. “We’re not really made to eat at night. I think this [study] kind of reinforces that.”
Can a night owl become an early bird?
When asked if a person’s natural circadian rhythm can be later, Dr. Malin responded that chronotypes may be dictated by genetics and age, as well as external drivers like work schedule. For these reasons, it’s “tricky” to answer whether night owls can turn into early birds and reap the potential health benefits of making that shift.
“Given that so many life factors can influence what our routine entails, it’s hard to know if we [can] truly change our chronotype or if rather we [can] learn to manage,” Dr. Malin said. “In either case, there is some work that suggests people can adopt earlier bedtimes and waketimes through practical recommendations.”
Specifically, he suggested increasing physical activity during the day, and adjusting bedtimes gradually by 15-minute increments.
“Go to bed 15 minutes earlier then wake up 15 minutes earlier,” Dr. Malin said. “In time, and depending on how things are going, this can expand to another 15-minute window. Then, during the earlier time waking up, a person can engage in light physical activity to help with promoting general fitness. If they can get outside with sunlight, that would be great too, as the natural sunlight would provide cues to the circadian system to adjust.”
The study was supported by the National Institutes of Health. The investigators and Dr. Friedman disclosed no conflicts of interest.
In the study involving 51 people, night owls metabolized fat less efficiently, showed less insulin sensitivity, and demonstrated lower physical fitness than early birds, lead author Steven K. Malin, PhD, of Rutgers University, New Brunswick, N.J., and colleagues reported.
Prior publications have suggested that night owls, formally known as “late chronotypes,” have an increased risk of obesity, type 2 diabetes, and cardiovascular disease, Dr. Malin said in an interview. But no previous research involved the gold-standard measurement tools used in this study, including euglycemic clamp and indirect calorimetry to quantify fat metabolism.
Dr. Malin also noted that this is the first study of its kind to characterize metabolism during both rest and exercise.
The study, published in Experimental Physiology, involved 24 early birds and 27 night owls classified by the Morning-Eveningness Questionnaire. All participants were sedentary, reporting less than one hour of structured exercise per week, and had metabolic syndrome according to Adult Treatment Panel III report criteria. Groups were otherwise demographically similar, with average ages in each group of approximately 54-55 years.
Compared with night owls, early birds were more physically active during the morning into midday. During exercise, they metabolized more fat and demonstrated greater physical fitness based on VO2max readings. At rest, early birds also came out ahead – they had higher fat oxidation and non–oxidative glucose disposal, suggesting more sensitivity to insulin.
“Collectively, this work highlights and supports chronotype as a potential risk factor related to type 2 diabetes and cardiovascular disease risk,” the investigators concluded.
Night owls have less metabolic control
Jed Friedman, PhD, director of OU Health Harold Hamm Diabetes Center at the University of Oklahoma Health Sciences Center, Oklahoma City, praised the study for the size of the groups the researchers compared with each other and how well matched those groups were, as well as the “state-of-the-art” measurement tools employed.
The findings show that night owls have “less metabolic control,” Dr. Friedman said in an interview.
“That’s a term that’s frequently invoked in [regard to] prediabetes,” he said. “Blood sugar goes up, because when you’re eating a high carbohydrate diet, your cells aren’t metabolizing sugar properly. That tends to raise your risk for a lot of diseases.”
Dr. Friedman added that the findings align with those of previous studies that have linked less sleep with changes in brain biology, and therefore behavior, especially in dietary choices.
“When you’re tired, the mechanisms for appetite control go haywire,” Dr. Friedman explained. “The evidence suggests that sugar is the primary driver for what people eat when they’re tired. That obviously has implications for diabetes and metabolic syndrome. So sleeping more really can help you control cravings.”
Dr. Friedman also noted that people who are tired tend to engage in less physical activity, further increasing their risk of metabolic issues. To control this risk, he advised people to return to their circadian rhythms, which could mean forgetting the midnight snack.
“Having a daily pattern that’s in sync with chronicity, or these daily rhythms, is associated with greater health,” Dr. Friedman said. “We’re not really made to eat at night. I think this [study] kind of reinforces that.”
Can a night owl become an early bird?
When asked if a person’s natural circadian rhythm can be later, Dr. Malin responded that chronotypes may be dictated by genetics and age, as well as external drivers like work schedule. For these reasons, it’s “tricky” to answer whether night owls can turn into early birds and reap the potential health benefits of making that shift.
“Given that so many life factors can influence what our routine entails, it’s hard to know if we [can] truly change our chronotype or if rather we [can] learn to manage,” Dr. Malin said. “In either case, there is some work that suggests people can adopt earlier bedtimes and waketimes through practical recommendations.”
Specifically, he suggested increasing physical activity during the day, and adjusting bedtimes gradually by 15-minute increments.
“Go to bed 15 minutes earlier then wake up 15 minutes earlier,” Dr. Malin said. “In time, and depending on how things are going, this can expand to another 15-minute window. Then, during the earlier time waking up, a person can engage in light physical activity to help with promoting general fitness. If they can get outside with sunlight, that would be great too, as the natural sunlight would provide cues to the circadian system to adjust.”
The study was supported by the National Institutes of Health. The investigators and Dr. Friedman disclosed no conflicts of interest.
FROM EXPERIMENTAL PHYSIOLOGY
Medical cannabis appears safe for patients with movement disorders
, two Israeli research teams reported.
The practice calls for careful monitoring of patients and additional study, said the researchers, who presented their findings at the International Congress of Parkinson’s Disease and Movement Disorders.
Cannabis for Parkinson’s disease
One retrospective study focused on Parkinson’s disease, evaluating the safety and effects of long-term treatment with medical cannabis, which has become a widely available treatment for controlling symptoms in Parkinson’s disease and other pain disorders. Studies have demonstrated its efficacy in patients with Parkinson’s disease, but long-term safety has never been examined in Parkinson’s disease compared with untreated patients.
Their study included 152 patients with idiopathic Parkinson’s disease (mean age at diagnosis: 55.6 plus or minus 9.5 years) from the Sheba Medical Center Movement Disorders Institute who had been issued a license for medical cannabis. Seventy-six patients treated with cannabis were compared with 76 patients with similar characteristics who were not treated with cannabis.
Investigators collected data on patients who were followed at the institute between 2008 and 2022. Average follow-up period was 3.6 years.
Specifically, they collected data on levodopa equivalent daily dose (LEDD), Hoehn and Yahr scale progression, and patient-reported outcome measures on cognitive impairment, depressive, and psychotic symptoms, at baseline and at follow-up.
The Hoehn and Yahr scale allows for the quantification of different disease stages and LEDD provides a summary of the total daily medication a patient is receiving, explained Tomer Goldberg, BSc, the study’s lead author. Both are widely accepted motor severity and progression measures for Parkinson’s disease. “We wanted to check whether cannabis treatment influences these two motor parameters,” said Mr. Goldberg, who is affiliated with Tel Aviv University and the Movement Disorders Institute at Sheba Medical Center.
The medical cannabis–treated group and the untreated group had no significant differences in the mean annual change in LEDD or Hoehn and Yahr score. At 1, 2, and 3 years of follow-up, the treated group showed no signs of psychotic, depressive, or cognitive deterioration (P = .10-.68). The groups in Kaplan-Meier analyses also exhibited no differences in these nonmotor symptoms over time (P = .27-.93).
The findings suggest that cannabis treatment appears to be safe and has no negative effect on disease progression, said Mr. Goldberg. “It is important to note that we did not investigate all of the potential side effects of this treatment, and that prescribing medical cannabis for patients with Parkinson’s disease should be done with careful monitoring of each patient’s individual response to the treatment,” he added.
Cannabis for Huntington’s disease
Another study, targeting Huntington’s disease, drew similar conclusions. Psychiatric symptoms and cognitive decline are often present in Huntington’s disease patients, who have few treatment options. “An overall improvement in chorea and in neuropsychiatric symptoms was reported following cannabis treatment in several studies both in humans and in murine models,” wrote the study authors.
In this study, a certified Huntington’s disease specialist reviewed the medical records of 150 patients who were being followed in an Huntington’s disease clinic. Study metrics included the Unified Huntington’s Disease Rating Scale and Montreal Cognitive Assessment scores, indications for treatment, and adverse events related to treatment. Among the 150 patients, 19 had received cannabis treatment for indications such as sleep disorders, behavioral anomalies, and chorea. All but one patient reported an improvement in symptoms (94%). No adverse events were recorded, although one patient died from a COVID-19 infection.
Overall, medical cannabis appeared to safely relieve symptoms in patients with Huntington’s disease. A double-blind randomized controlled trial should further examine efficacy of these findings, the study authors recommended.
Mr. Goldberg had no disclosures or conflicts of interest in reporting his research.
, two Israeli research teams reported.
The practice calls for careful monitoring of patients and additional study, said the researchers, who presented their findings at the International Congress of Parkinson’s Disease and Movement Disorders.
Cannabis for Parkinson’s disease
One retrospective study focused on Parkinson’s disease, evaluating the safety and effects of long-term treatment with medical cannabis, which has become a widely available treatment for controlling symptoms in Parkinson’s disease and other pain disorders. Studies have demonstrated its efficacy in patients with Parkinson’s disease, but long-term safety has never been examined in Parkinson’s disease compared with untreated patients.
Their study included 152 patients with idiopathic Parkinson’s disease (mean age at diagnosis: 55.6 plus or minus 9.5 years) from the Sheba Medical Center Movement Disorders Institute who had been issued a license for medical cannabis. Seventy-six patients treated with cannabis were compared with 76 patients with similar characteristics who were not treated with cannabis.
Investigators collected data on patients who were followed at the institute between 2008 and 2022. Average follow-up period was 3.6 years.
Specifically, they collected data on levodopa equivalent daily dose (LEDD), Hoehn and Yahr scale progression, and patient-reported outcome measures on cognitive impairment, depressive, and psychotic symptoms, at baseline and at follow-up.
The Hoehn and Yahr scale allows for the quantification of different disease stages and LEDD provides a summary of the total daily medication a patient is receiving, explained Tomer Goldberg, BSc, the study’s lead author. Both are widely accepted motor severity and progression measures for Parkinson’s disease. “We wanted to check whether cannabis treatment influences these two motor parameters,” said Mr. Goldberg, who is affiliated with Tel Aviv University and the Movement Disorders Institute at Sheba Medical Center.
The medical cannabis–treated group and the untreated group had no significant differences in the mean annual change in LEDD or Hoehn and Yahr score. At 1, 2, and 3 years of follow-up, the treated group showed no signs of psychotic, depressive, or cognitive deterioration (P = .10-.68). The groups in Kaplan-Meier analyses also exhibited no differences in these nonmotor symptoms over time (P = .27-.93).
The findings suggest that cannabis treatment appears to be safe and has no negative effect on disease progression, said Mr. Goldberg. “It is important to note that we did not investigate all of the potential side effects of this treatment, and that prescribing medical cannabis for patients with Parkinson’s disease should be done with careful monitoring of each patient’s individual response to the treatment,” he added.
Cannabis for Huntington’s disease
Another study, targeting Huntington’s disease, drew similar conclusions. Psychiatric symptoms and cognitive decline are often present in Huntington’s disease patients, who have few treatment options. “An overall improvement in chorea and in neuropsychiatric symptoms was reported following cannabis treatment in several studies both in humans and in murine models,” wrote the study authors.
In this study, a certified Huntington’s disease specialist reviewed the medical records of 150 patients who were being followed in an Huntington’s disease clinic. Study metrics included the Unified Huntington’s Disease Rating Scale and Montreal Cognitive Assessment scores, indications for treatment, and adverse events related to treatment. Among the 150 patients, 19 had received cannabis treatment for indications such as sleep disorders, behavioral anomalies, and chorea. All but one patient reported an improvement in symptoms (94%). No adverse events were recorded, although one patient died from a COVID-19 infection.
Overall, medical cannabis appeared to safely relieve symptoms in patients with Huntington’s disease. A double-blind randomized controlled trial should further examine efficacy of these findings, the study authors recommended.
Mr. Goldberg had no disclosures or conflicts of interest in reporting his research.
, two Israeli research teams reported.
The practice calls for careful monitoring of patients and additional study, said the researchers, who presented their findings at the International Congress of Parkinson’s Disease and Movement Disorders.
Cannabis for Parkinson’s disease
One retrospective study focused on Parkinson’s disease, evaluating the safety and effects of long-term treatment with medical cannabis, which has become a widely available treatment for controlling symptoms in Parkinson’s disease and other pain disorders. Studies have demonstrated its efficacy in patients with Parkinson’s disease, but long-term safety has never been examined in Parkinson’s disease compared with untreated patients.
Their study included 152 patients with idiopathic Parkinson’s disease (mean age at diagnosis: 55.6 plus or minus 9.5 years) from the Sheba Medical Center Movement Disorders Institute who had been issued a license for medical cannabis. Seventy-six patients treated with cannabis were compared with 76 patients with similar characteristics who were not treated with cannabis.
Investigators collected data on patients who were followed at the institute between 2008 and 2022. Average follow-up period was 3.6 years.
Specifically, they collected data on levodopa equivalent daily dose (LEDD), Hoehn and Yahr scale progression, and patient-reported outcome measures on cognitive impairment, depressive, and psychotic symptoms, at baseline and at follow-up.
The Hoehn and Yahr scale allows for the quantification of different disease stages and LEDD provides a summary of the total daily medication a patient is receiving, explained Tomer Goldberg, BSc, the study’s lead author. Both are widely accepted motor severity and progression measures for Parkinson’s disease. “We wanted to check whether cannabis treatment influences these two motor parameters,” said Mr. Goldberg, who is affiliated with Tel Aviv University and the Movement Disorders Institute at Sheba Medical Center.
The medical cannabis–treated group and the untreated group had no significant differences in the mean annual change in LEDD or Hoehn and Yahr score. At 1, 2, and 3 years of follow-up, the treated group showed no signs of psychotic, depressive, or cognitive deterioration (P = .10-.68). The groups in Kaplan-Meier analyses also exhibited no differences in these nonmotor symptoms over time (P = .27-.93).
The findings suggest that cannabis treatment appears to be safe and has no negative effect on disease progression, said Mr. Goldberg. “It is important to note that we did not investigate all of the potential side effects of this treatment, and that prescribing medical cannabis for patients with Parkinson’s disease should be done with careful monitoring of each patient’s individual response to the treatment,” he added.
Cannabis for Huntington’s disease
Another study, targeting Huntington’s disease, drew similar conclusions. Psychiatric symptoms and cognitive decline are often present in Huntington’s disease patients, who have few treatment options. “An overall improvement in chorea and in neuropsychiatric symptoms was reported following cannabis treatment in several studies both in humans and in murine models,” wrote the study authors.
In this study, a certified Huntington’s disease specialist reviewed the medical records of 150 patients who were being followed in an Huntington’s disease clinic. Study metrics included the Unified Huntington’s Disease Rating Scale and Montreal Cognitive Assessment scores, indications for treatment, and adverse events related to treatment. Among the 150 patients, 19 had received cannabis treatment for indications such as sleep disorders, behavioral anomalies, and chorea. All but one patient reported an improvement in symptoms (94%). No adverse events were recorded, although one patient died from a COVID-19 infection.
Overall, medical cannabis appeared to safely relieve symptoms in patients with Huntington’s disease. A double-blind randomized controlled trial should further examine efficacy of these findings, the study authors recommended.
Mr. Goldberg had no disclosures or conflicts of interest in reporting his research.
FROM MDS 2022
Early bird gets the worm, night owl gets the diabetes
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
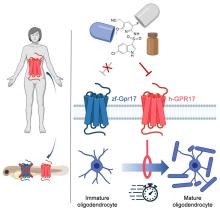
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Metabolism a player in circadian rhythm section
Are you an early bird, or do you wake up and stare at your phone, wondering why you were up watching “The Crown” until 3 a.m.? Recent research suggests that people who wake up earlier tend to be more active during the day and burn more fat than those who sleep in. Fat builds up in the night owls, putting them at higher risk of type 2 diabetes and heart disease.
The study gives physicians something to think about when assessing a patient’s risk factors. “This could help medical professionals consider another behavioral factor contributing to disease risk,” Steven Malin, PhD, lead author of the study and expert in metabolism at Rutgers University in New Brunswick, N.J., said in The Guardian.
For the research, 51 participants were divided into night owls and early birds, depending on their answers to a questionnaire. They were examined, monitored for a week, and assessed while doing various activities. Those who woke up early tended to be more sensitive to insulin and burned off fat faster than those who woke up late, the researchers explained.
“Night owls are reported to have a higher risk of obesity, type 2 diabetes, and cardiovascular disease when compared with early birds,” Dr. Malin said. “A potential explanation is they become misaligned with their circadian rhythm for various reasons, but most notably among adults would be work.”
We all know that we may not be at our best when we throw off our internal clocks by going to sleep late and waking up early. Think about that next time you start another episode on Netflix at 2:57 a.m.
Mosquitoes, chemical cocktails, and glass sock beads
We all know that mosquitoes are annoying little disease vectors with a taste for human blood. One of the less-known things about mosquitoes is what attracts them to humans in the first place. It’s so less known that, until now, it was unknown. Oh sure, we knew that odor was involved, and that lactic acid was part of the odor equation, but what are the specific chemicals? Well, there’s carbon dioxide … and ammonia. Those were already known.
Ring Cardé, PhD, an entomologist at the University of California, Riverside, wasn’t convinced. “I suspected there was something undiscovered about the chemistry of odors luring the yellow fever mosquito. I wanted to nail down the exact blend,” he said in a statement from the university.
Dr. Cardé and his associates eventually figured out that the exact chemical cocktail attracting female Aedes aegypti mosquitoes was a combination of carbon dioxide plus two chemicals, 2-ketoglutaric acid and lactic acid. The odor from these chemicals enables mosquitoes to locate and land on their victim and “also encourages probing, the use of piercing mouthparts to find blood,” the university said.
This amazing destination of science is important, but we have to acknowledge the journey as well. To do that we turn to one of Dr. Cardé’s associates, Jan Bello, PhD, formerly of Cal-Riverside and now with insect pest control company Provivi. Turns out that 2-ketoglutaric acid is tricky stuff because the methods typically used to identify chemicals don’t work on it.
Dr. Bello employed a somewhat unorthodox chemical extraction method: He filled his socks with glass beads and walked around with the beads in his socks.
“Wearing the beads felt almost like a massage, like squeezing stress balls full of sand, but with your feet,” Dr. Bello said. “The most frustrating part of doing it for a long time is that they would get stuck in between your toes, so it would be uncomfortable after a while.”
We hate when science gets stuck between our toes, but we love it when scientists write their own punchlines.
The MS drugs are better down where it’s wetter, take it from me
The myth of the mermaid is one with hundreds, if not thousands, of years of history. The ancient Greeks had the mythological siren, while the Babylonians depicted kulullû (which were mermen – never let the Babylonians be known as noninclusive) in artwork as far back as 1600 BC. Cultures as far flung as Japan, southern Africa, and New Zealand have folkloric figures similar to the mermaid. It is most decidedly not a creation of western Europe, Hans Christian Andersen, or Disney.
With that mild rant out of the way, let’s move to Germany and a group of researchers from the University of Bonn, who have not created a mermaid. They did, however, add human genes to a zebrafish for research purposes, which feels uncomfortably close. Nothing better than unholy animal-human hybrids, right?
Stick with us here, because the researchers did have a good reason for their gene splicing. Zebrafish and humans both have the GPR17 receptor, which is highly active in nerve tissue. When GPR17 is overactivated, diseases such as multiple sclerosis can develop. Because the zebrafish has this receptor, which performs the same function in its body as in ours, it’s a prime candidate for replacement. Also, zebrafish larvae are transparent, which makes it very easy to observe a drug working.
That said, fish and humans are very far apart, genetically speaking. Big shock right there. But by replacing their GPR17 receptor with ours, the scientists have created a fish that we could test drug candidates on and be assured that they would also work on humans. Actually testing drugs for MS on these humanized zebrafish was beyond the scope of the study, but the researchers said that the new genes function normally in the fish larvae, making them a promising new avenue for MS drug development.
Can we all promise not to tell Disney that human DNA can be spliced into a fish without consequence? Otherwise, we’re just going to have to sit through another “Little Mermaid” adaptation in 30 years, this one in super live-action featuring actual, real-life mermaids. And we’re not ready for that level of man-made horror just yet.
Beware of the fly vomit
Picture this: You’re outside at a picnic or barbecue, loading a plate with food. In a brief moment of conversation a fly lands right on top of your sandwich. You shoo it away and think nothing more of it, eating the sandwich anyway. We’ve all been there.
A recent study is making us think again.
John Stoffolano, an entomology professor at the University of Massachusetts, Amherst, claims that too much attention has been focused on pathogen transmission by the biting, blood-feeding flies when really we should be taking note of the nonbiting, or synanthropic, flies we live with, which may have a greater impact on the transmission of pathogens right in our own homes.
Sure, blood-feeding flies can spread pathogens directly, but house flies vomit every time they land on something. Think about that.
The fly that sneakily swooped into your house from a tear in your window screen has just been outside in the neighbor’s garbage or sitting on dog poop and now has who knows what filling its crop, the tank in their body that serves as “a place to store food before it makes its way into the digestive tract where it will get turned into energy for the fly,” Dr. Stoffolano explained in a written statement.
Did that fly land right on the baked potato you were prepping for dinner before you shooed it away? Guess what? Before flying off it emitted excess water that has pathogens from whatever was in its crop. We don’t want to say your potato might have dog poop on it, but you get the idea. The crop doesn’t have a ton of digestive enzymes that would help neutralize pathogens, so whatever that fly regurgitated before buzzing off is still around for you to ingest and there’s not much you can do about it.
More research needs to be done about flies, but at the very least this study should make you think twice before eating that baked potato after a fly has been there.
Angiography in patients with prior CABG does better when planned with CT
BOSTON – Coronary angiography in patients who have previously undergone cardiac artery bypass grafting (CABG) is challenging, but the procedure can be streamlined and made safer when preprocedural CT coronary angiography (CTCA) is performed to plan the intervention, according to a randomized controlled trial.
In this study, all three endpoints, including a reduction in the incidence of contrast-induced nephropathy (CIN) and duration of the procedure, were met, according to Daniel Jones, MBBS, PhD.
Preprocedural CTCA was also associated with about a 40% improvement in patient satisfaction.
“When logistically possible, CTCA should be considered for any stable postbypass patient undergoing coronary angiography,” said Dr. Jones, who supported this assertion with data presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
In this study, called BYPASS-CTCA, 688 patients with a prior CABG scheduled for invasive coronary angiography were randomized to a preprocedural CTCA or no preprocedural CTCA. Patients with stable angina and those with a non–ST elevated acute coronary syndrome were eligible. Those with ST-segment elevated MI or severe renal impairment (eGFR < 20 mL/min) were excluded.
All three co–primary endpoints favor CTCA
CTCA relative to no CTCA provided a significant advantage for all three of the coprimary endpoints, which were procedure duration, CIN as defined by KDIGO criteria, and patient satisfaction as measured by questionnaire.
The procedure duration was reduced by almost 21 minutes, cutting the time from nearly 39 minutes to less than 18 minutes (P < .001). This relative reduction was of similar magnitude across groups, such as those with or without acute coronary syndrome and procedures performed by a senior or a junior operator.
“Even when you include the preprocedural CTCA evaluation time, there was still a significant reduction [P < .001] in duration for those in the CTCA arm,” reported Dr. Jones, honorary consultant cardiologist, Barts Heart Centre, Queen Mary University, London.
The rates of CIN following the procedure in this study, which had a follow-up of 12 months, were 3.4% versus 27.9% (P < .0001) in the preprocedural CTCA and non-CTCA groups, respectively. Again, a sensitivity analysis showed a similar magnitude of risk reduction across all subgroups evaluated.
CTCA planning reduced contrast exposure
The reduced risk of CIN was consistent with a large reduction in contrast exposure for those in the CTCA group (77.4 vs. 173.0 mL; P < .001). The advantage narrowed substantially when adding in contrast exposure from CTCA, but still remained statistically significant (148.9 vs. 173.0 mL; P < .001).
Dr. Jones did not speculate about the specific reasons for the 40% improvement in patient satisfaction among those who underwent preprocedural CTCA relative to those who did not, but, again, a sensitivity analysis showed consistency across subgroups defined by race, operator experience, and underlying diagnosis.
Numerous secondary endpoints also favored CTCA over no CTCA. This included fewer catheters used to complete the procedure (three vs. four; P < .001), a greater likelihood that the procedure was performed with radial access (76.9% vs. 56.7%), and lower rates of procedural complications (2.3% vs. 10.8%; P < .001). This latter category included fewer vascular access complications such as bleeding (0.6 % vs. 4.4%; P = .007) and periprocedural MI (0.6% vs. 6.4%; P < 0.001).
In a graph of time to first major adverse cardiovascular event (MACE), the curves separated almost immediately with a consistently lower rate maintained in the CTCA arm over the 12 months of follow-up, but this is observational. Dr. Jones acknowledged that this trial was not powered to show a difference in MACE.
Study intriguing but not definitive
In a panel discussion that followed the presentation of these results at the meeting, sponsored by the Cardiovascular Research Foundation, some reservations with this study were expressed. In particular, several of the panelists, including Jeffrey W. Moses, MD, director of interventional cardiovascular therapeutics, Columbia University Medical Center, New York, expressed surprise at the 27% rate of CIN, which he considered uncommonly high even in a high-risk population.
The unusual rate of CIN was also considered problematic given that it was the most significant clinical outcome among the three co–primary endpoints. Procedural times and patient satisfaction, while valid endpoints, are important subjects of study, but Dr. Moses was not alone in suggesting this study deserves validation.
In particular, there appeared to be a consensus among panelists that a larger multicenter study looking at hard endpoints, such as MACE, would be more compelling. They indicated that even if CTCA poses a very low risk of meaningful complications, it does add expense and an extra step.
Dr. Jones reported no potential conflicts of interest. Dr. Moses reported financial relationships with Covanos, Orchestra Biomed, Ostial, and Xenter.
BOSTON – Coronary angiography in patients who have previously undergone cardiac artery bypass grafting (CABG) is challenging, but the procedure can be streamlined and made safer when preprocedural CT coronary angiography (CTCA) is performed to plan the intervention, according to a randomized controlled trial.
In this study, all three endpoints, including a reduction in the incidence of contrast-induced nephropathy (CIN) and duration of the procedure, were met, according to Daniel Jones, MBBS, PhD.
Preprocedural CTCA was also associated with about a 40% improvement in patient satisfaction.
“When logistically possible, CTCA should be considered for any stable postbypass patient undergoing coronary angiography,” said Dr. Jones, who supported this assertion with data presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
In this study, called BYPASS-CTCA, 688 patients with a prior CABG scheduled for invasive coronary angiography were randomized to a preprocedural CTCA or no preprocedural CTCA. Patients with stable angina and those with a non–ST elevated acute coronary syndrome were eligible. Those with ST-segment elevated MI or severe renal impairment (eGFR < 20 mL/min) were excluded.
All three co–primary endpoints favor CTCA
CTCA relative to no CTCA provided a significant advantage for all three of the coprimary endpoints, which were procedure duration, CIN as defined by KDIGO criteria, and patient satisfaction as measured by questionnaire.
The procedure duration was reduced by almost 21 minutes, cutting the time from nearly 39 minutes to less than 18 minutes (P < .001). This relative reduction was of similar magnitude across groups, such as those with or without acute coronary syndrome and procedures performed by a senior or a junior operator.
“Even when you include the preprocedural CTCA evaluation time, there was still a significant reduction [P < .001] in duration for those in the CTCA arm,” reported Dr. Jones, honorary consultant cardiologist, Barts Heart Centre, Queen Mary University, London.
The rates of CIN following the procedure in this study, which had a follow-up of 12 months, were 3.4% versus 27.9% (P < .0001) in the preprocedural CTCA and non-CTCA groups, respectively. Again, a sensitivity analysis showed a similar magnitude of risk reduction across all subgroups evaluated.
CTCA planning reduced contrast exposure
The reduced risk of CIN was consistent with a large reduction in contrast exposure for those in the CTCA group (77.4 vs. 173.0 mL; P < .001). The advantage narrowed substantially when adding in contrast exposure from CTCA, but still remained statistically significant (148.9 vs. 173.0 mL; P < .001).
Dr. Jones did not speculate about the specific reasons for the 40% improvement in patient satisfaction among those who underwent preprocedural CTCA relative to those who did not, but, again, a sensitivity analysis showed consistency across subgroups defined by race, operator experience, and underlying diagnosis.
Numerous secondary endpoints also favored CTCA over no CTCA. This included fewer catheters used to complete the procedure (three vs. four; P < .001), a greater likelihood that the procedure was performed with radial access (76.9% vs. 56.7%), and lower rates of procedural complications (2.3% vs. 10.8%; P < .001). This latter category included fewer vascular access complications such as bleeding (0.6 % vs. 4.4%; P = .007) and periprocedural MI (0.6% vs. 6.4%; P < 0.001).
In a graph of time to first major adverse cardiovascular event (MACE), the curves separated almost immediately with a consistently lower rate maintained in the CTCA arm over the 12 months of follow-up, but this is observational. Dr. Jones acknowledged that this trial was not powered to show a difference in MACE.
Study intriguing but not definitive
In a panel discussion that followed the presentation of these results at the meeting, sponsored by the Cardiovascular Research Foundation, some reservations with this study were expressed. In particular, several of the panelists, including Jeffrey W. Moses, MD, director of interventional cardiovascular therapeutics, Columbia University Medical Center, New York, expressed surprise at the 27% rate of CIN, which he considered uncommonly high even in a high-risk population.
The unusual rate of CIN was also considered problematic given that it was the most significant clinical outcome among the three co–primary endpoints. Procedural times and patient satisfaction, while valid endpoints, are important subjects of study, but Dr. Moses was not alone in suggesting this study deserves validation.
In particular, there appeared to be a consensus among panelists that a larger multicenter study looking at hard endpoints, such as MACE, would be more compelling. They indicated that even if CTCA poses a very low risk of meaningful complications, it does add expense and an extra step.
Dr. Jones reported no potential conflicts of interest. Dr. Moses reported financial relationships with Covanos, Orchestra Biomed, Ostial, and Xenter.
BOSTON – Coronary angiography in patients who have previously undergone cardiac artery bypass grafting (CABG) is challenging, but the procedure can be streamlined and made safer when preprocedural CT coronary angiography (CTCA) is performed to plan the intervention, according to a randomized controlled trial.
In this study, all three endpoints, including a reduction in the incidence of contrast-induced nephropathy (CIN) and duration of the procedure, were met, according to Daniel Jones, MBBS, PhD.
Preprocedural CTCA was also associated with about a 40% improvement in patient satisfaction.
“When logistically possible, CTCA should be considered for any stable postbypass patient undergoing coronary angiography,” said Dr. Jones, who supported this assertion with data presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
In this study, called BYPASS-CTCA, 688 patients with a prior CABG scheduled for invasive coronary angiography were randomized to a preprocedural CTCA or no preprocedural CTCA. Patients with stable angina and those with a non–ST elevated acute coronary syndrome were eligible. Those with ST-segment elevated MI or severe renal impairment (eGFR < 20 mL/min) were excluded.
All three co–primary endpoints favor CTCA
CTCA relative to no CTCA provided a significant advantage for all three of the coprimary endpoints, which were procedure duration, CIN as defined by KDIGO criteria, and patient satisfaction as measured by questionnaire.
The procedure duration was reduced by almost 21 minutes, cutting the time from nearly 39 minutes to less than 18 minutes (P < .001). This relative reduction was of similar magnitude across groups, such as those with or without acute coronary syndrome and procedures performed by a senior or a junior operator.
“Even when you include the preprocedural CTCA evaluation time, there was still a significant reduction [P < .001] in duration for those in the CTCA arm,” reported Dr. Jones, honorary consultant cardiologist, Barts Heart Centre, Queen Mary University, London.
The rates of CIN following the procedure in this study, which had a follow-up of 12 months, were 3.4% versus 27.9% (P < .0001) in the preprocedural CTCA and non-CTCA groups, respectively. Again, a sensitivity analysis showed a similar magnitude of risk reduction across all subgroups evaluated.
CTCA planning reduced contrast exposure
The reduced risk of CIN was consistent with a large reduction in contrast exposure for those in the CTCA group (77.4 vs. 173.0 mL; P < .001). The advantage narrowed substantially when adding in contrast exposure from CTCA, but still remained statistically significant (148.9 vs. 173.0 mL; P < .001).
Dr. Jones did not speculate about the specific reasons for the 40% improvement in patient satisfaction among those who underwent preprocedural CTCA relative to those who did not, but, again, a sensitivity analysis showed consistency across subgroups defined by race, operator experience, and underlying diagnosis.
Numerous secondary endpoints also favored CTCA over no CTCA. This included fewer catheters used to complete the procedure (three vs. four; P < .001), a greater likelihood that the procedure was performed with radial access (76.9% vs. 56.7%), and lower rates of procedural complications (2.3% vs. 10.8%; P < .001). This latter category included fewer vascular access complications such as bleeding (0.6 % vs. 4.4%; P = .007) and periprocedural MI (0.6% vs. 6.4%; P < 0.001).
In a graph of time to first major adverse cardiovascular event (MACE), the curves separated almost immediately with a consistently lower rate maintained in the CTCA arm over the 12 months of follow-up, but this is observational. Dr. Jones acknowledged that this trial was not powered to show a difference in MACE.
Study intriguing but not definitive
In a panel discussion that followed the presentation of these results at the meeting, sponsored by the Cardiovascular Research Foundation, some reservations with this study were expressed. In particular, several of the panelists, including Jeffrey W. Moses, MD, director of interventional cardiovascular therapeutics, Columbia University Medical Center, New York, expressed surprise at the 27% rate of CIN, which he considered uncommonly high even in a high-risk population.
The unusual rate of CIN was also considered problematic given that it was the most significant clinical outcome among the three co–primary endpoints. Procedural times and patient satisfaction, while valid endpoints, are important subjects of study, but Dr. Moses was not alone in suggesting this study deserves validation.
In particular, there appeared to be a consensus among panelists that a larger multicenter study looking at hard endpoints, such as MACE, would be more compelling. They indicated that even if CTCA poses a very low risk of meaningful complications, it does add expense and an extra step.
Dr. Jones reported no potential conflicts of interest. Dr. Moses reported financial relationships with Covanos, Orchestra Biomed, Ostial, and Xenter.
AT TCT 2022
Community-level actions could mitigate maternal mortality
Maternal mortality in the United States has been rising for several decades, but actions taken at the community level, as well as larger public health initiatives, have the potential to slow this trend, according to experts at a webinar sponsored by the National Institute for Health Care Management.
Maternal mortality in the United States increased by 14% from 2018 to 2020, according to data from the Centers for Disease Control and Prevention’s National Center for Health Statistics.
However, more than 80% of pregnancy-related deaths are preventable, according to 2017-2019 data from the Maternal Mortality Review Committees published online by the CDC. MMRCs include representatives of diverse clinical and nonclinical backgrounds who review the circumstances of pregnancy-related deaths.
In a webinar presented on Sept. 20, the NIHCM enlisted a panel of experts to discuss maternal mortality, the effect of changes to reproductive rights, and potential strategies to improve maternal health outcomes.
Maternal mortality is defined as “death while pregnant or within 42 days of the end of pregnancy, irrespective of the duration and site of pregnancy, from any cause related to pregnancy or its management,” according to the CDC.
Importantly, mortality rates in the United States are approximately three times higher in Black women compared with White women, said Ndidiamaka Amutah-Onukagha, PhD, MPH, of the Tufts University Center for Black Maternal Health & Reproductive Justice. Dr. Amutah-Onukagha addressed some of the potential issues that appear to drive the disparity in care.
The lack of diversity in the health care workforce has a significant effect on patient outcomes, Dr. Amutah-Onukagha said. Overall, Black newborns are more than twice as likely as White newborns to die during their first year of life, but this number is cut in half when Black infants are cared for by Black physicians, she emphasized.
Other factors that may affect disparities in maternal health care include limited access to prenatal care, discriminatory hospital protocols, and mistreatment by health care professionals, said Dr. Amutah-Onukagha. She cited data showing that maternal mortality rates were higher in rural compared with urban areas. “According to the American Hospital Association, half of rural hospitals have no obstetric care, leaving mothers in maternity care deserts; this exacerbates existing disparities,” she said.
In the webinar, Sindhu Srinivas, MD, a maternal-fetal medicine specialist at the University of Pennsylvania, explained how patient, community, and system factors play a role in the disparities in maternal care.
Overall, Black women have to travel further to receive care, which has implications for high-risk pregnancies, and patients on Medicaid have to wait longer for care, and are less likely to be referred, she added. Black women also have higher rates of preexisting conditions compared with other populations that put them in the high-risk category, such as high blood pressure, diabetes, obesity, or being HIV positive, she said.
Other factors contributing to persistent disparities in maternal care include sociodemographics, patient beliefs and knowledge, and psychological issues including stress, said Dr. Srinivas. Community factors, such as social networks, safety, and poverty, also play a role, as do clinician factors of implicit bias and communication skills, she said.
Strategies to reduce disparity
Dr. Srinivas presented several strategies to reduce disparities at various levels. At the policy level, interventions such as establishing a Maternal Mortality Review Committee, establishing a perinatal quality collaborative, and extending Medicaid for a full year postpartum could help improve outcomes, she said. Dr. Srinivas also encouraged clinicians to report maternal mortality data stratified by race and ethnicity, and to participate in the Alliance for Innovation on Maternal Health program (AIM), an initiative in partnership with the American College of Obstetrics and Gynecology.
Dr. Srinivas also proposed maternal health policies to develop payment models “to sustain and scale innovative solutions, and “preserve access to contraception and abortion care.”
For clinicians looking to have an immediate impact, the panelists agreed that working with community health centers can make a significant difference by improving access to maternal care. Consider opportunities for partnership between hospitals and health care delivery centers in the community, said Dr. Srinivas.
Also, don’t underestimate the value of doulas in the birthing process, Dr. Amutah-Onukagha said. She urged clinicians to advocate for doula reimbursement and to take advantage of opportunities for doulas to work with pregnant individuals at the community levels. Data suggest that doulas are associated with increased maternal care visits and with breastfeeding, she noted.
Adam Myers, MD, of the Blue Cross Blue Shield Association, also contributed to the webinar discussion with a key point: Having financial means and commercial coverage is not a buffer against adverse maternal outcomes for racial minorities.
Dr. Myers cited the latest Health of America Report, which included data up to April 2021 with surveys of Medicaid members and their experiences. According to the report, rates of severe maternal mortality (SMM) increased by 9% for commercially and Medicaid-insured women between 2018 and 2020.
Among commercially insured women, SMM was 53% higher among Black women than White women; among Medicaid-insured women, Black women had a 73% higher rate of SMM, compared with White women.
In addition, the report showed that significantly more mothers of color were not able to complete the recommended series of prenatal visits, mainly for reasons of scheduling and transportation, which were greater barriers than COVID-19, Dr. Myers said.
Based on the data, one specific risk profile rose to the top: “We believe women of color aged 35 or higher with comorbid conditions should be treated as very high risk for SMM,” Dr. Myers emphasized. He stressed the need to focus on transportation and scheduling barriers and expressed support for partnerships and health care delivery centers in the community to mitigate these issues.
Finally, Dr. Srinivas encouraged clinicians to have confidence in their expertise and make themselves heard to help their patients and improve maternal health for all. “Use your voice,” said Dr. Srinivas, “As physicians we don’t think of that as an important aspect of our work, or that we can’t articulate, but remember that we are experts, and sharing stories of patients who are impacted is incredibly powerful,” she said.
The presenters had no relevant financial conflicts to disclose.
Maternal mortality in the United States has been rising for several decades, but actions taken at the community level, as well as larger public health initiatives, have the potential to slow this trend, according to experts at a webinar sponsored by the National Institute for Health Care Management.
Maternal mortality in the United States increased by 14% from 2018 to 2020, according to data from the Centers for Disease Control and Prevention’s National Center for Health Statistics.
However, more than 80% of pregnancy-related deaths are preventable, according to 2017-2019 data from the Maternal Mortality Review Committees published online by the CDC. MMRCs include representatives of diverse clinical and nonclinical backgrounds who review the circumstances of pregnancy-related deaths.
In a webinar presented on Sept. 20, the NIHCM enlisted a panel of experts to discuss maternal mortality, the effect of changes to reproductive rights, and potential strategies to improve maternal health outcomes.
Maternal mortality is defined as “death while pregnant or within 42 days of the end of pregnancy, irrespective of the duration and site of pregnancy, from any cause related to pregnancy or its management,” according to the CDC.
Importantly, mortality rates in the United States are approximately three times higher in Black women compared with White women, said Ndidiamaka Amutah-Onukagha, PhD, MPH, of the Tufts University Center for Black Maternal Health & Reproductive Justice. Dr. Amutah-Onukagha addressed some of the potential issues that appear to drive the disparity in care.
The lack of diversity in the health care workforce has a significant effect on patient outcomes, Dr. Amutah-Onukagha said. Overall, Black newborns are more than twice as likely as White newborns to die during their first year of life, but this number is cut in half when Black infants are cared for by Black physicians, she emphasized.
Other factors that may affect disparities in maternal health care include limited access to prenatal care, discriminatory hospital protocols, and mistreatment by health care professionals, said Dr. Amutah-Onukagha. She cited data showing that maternal mortality rates were higher in rural compared with urban areas. “According to the American Hospital Association, half of rural hospitals have no obstetric care, leaving mothers in maternity care deserts; this exacerbates existing disparities,” she said.
In the webinar, Sindhu Srinivas, MD, a maternal-fetal medicine specialist at the University of Pennsylvania, explained how patient, community, and system factors play a role in the disparities in maternal care.
Overall, Black women have to travel further to receive care, which has implications for high-risk pregnancies, and patients on Medicaid have to wait longer for care, and are less likely to be referred, she added. Black women also have higher rates of preexisting conditions compared with other populations that put them in the high-risk category, such as high blood pressure, diabetes, obesity, or being HIV positive, she said.
Other factors contributing to persistent disparities in maternal care include sociodemographics, patient beliefs and knowledge, and psychological issues including stress, said Dr. Srinivas. Community factors, such as social networks, safety, and poverty, also play a role, as do clinician factors of implicit bias and communication skills, she said.
Strategies to reduce disparity
Dr. Srinivas presented several strategies to reduce disparities at various levels. At the policy level, interventions such as establishing a Maternal Mortality Review Committee, establishing a perinatal quality collaborative, and extending Medicaid for a full year postpartum could help improve outcomes, she said. Dr. Srinivas also encouraged clinicians to report maternal mortality data stratified by race and ethnicity, and to participate in the Alliance for Innovation on Maternal Health program (AIM), an initiative in partnership with the American College of Obstetrics and Gynecology.
Dr. Srinivas also proposed maternal health policies to develop payment models “to sustain and scale innovative solutions, and “preserve access to contraception and abortion care.”
For clinicians looking to have an immediate impact, the panelists agreed that working with community health centers can make a significant difference by improving access to maternal care. Consider opportunities for partnership between hospitals and health care delivery centers in the community, said Dr. Srinivas.
Also, don’t underestimate the value of doulas in the birthing process, Dr. Amutah-Onukagha said. She urged clinicians to advocate for doula reimbursement and to take advantage of opportunities for doulas to work with pregnant individuals at the community levels. Data suggest that doulas are associated with increased maternal care visits and with breastfeeding, she noted.
Adam Myers, MD, of the Blue Cross Blue Shield Association, also contributed to the webinar discussion with a key point: Having financial means and commercial coverage is not a buffer against adverse maternal outcomes for racial minorities.
Dr. Myers cited the latest Health of America Report, which included data up to April 2021 with surveys of Medicaid members and their experiences. According to the report, rates of severe maternal mortality (SMM) increased by 9% for commercially and Medicaid-insured women between 2018 and 2020.
Among commercially insured women, SMM was 53% higher among Black women than White women; among Medicaid-insured women, Black women had a 73% higher rate of SMM, compared with White women.
In addition, the report showed that significantly more mothers of color were not able to complete the recommended series of prenatal visits, mainly for reasons of scheduling and transportation, which were greater barriers than COVID-19, Dr. Myers said.
Based on the data, one specific risk profile rose to the top: “We believe women of color aged 35 or higher with comorbid conditions should be treated as very high risk for SMM,” Dr. Myers emphasized. He stressed the need to focus on transportation and scheduling barriers and expressed support for partnerships and health care delivery centers in the community to mitigate these issues.
Finally, Dr. Srinivas encouraged clinicians to have confidence in their expertise and make themselves heard to help their patients and improve maternal health for all. “Use your voice,” said Dr. Srinivas, “As physicians we don’t think of that as an important aspect of our work, or that we can’t articulate, but remember that we are experts, and sharing stories of patients who are impacted is incredibly powerful,” she said.
The presenters had no relevant financial conflicts to disclose.
Maternal mortality in the United States has been rising for several decades, but actions taken at the community level, as well as larger public health initiatives, have the potential to slow this trend, according to experts at a webinar sponsored by the National Institute for Health Care Management.
Maternal mortality in the United States increased by 14% from 2018 to 2020, according to data from the Centers for Disease Control and Prevention’s National Center for Health Statistics.
However, more than 80% of pregnancy-related deaths are preventable, according to 2017-2019 data from the Maternal Mortality Review Committees published online by the CDC. MMRCs include representatives of diverse clinical and nonclinical backgrounds who review the circumstances of pregnancy-related deaths.
In a webinar presented on Sept. 20, the NIHCM enlisted a panel of experts to discuss maternal mortality, the effect of changes to reproductive rights, and potential strategies to improve maternal health outcomes.
Maternal mortality is defined as “death while pregnant or within 42 days of the end of pregnancy, irrespective of the duration and site of pregnancy, from any cause related to pregnancy or its management,” according to the CDC.
Importantly, mortality rates in the United States are approximately three times higher in Black women compared with White women, said Ndidiamaka Amutah-Onukagha, PhD, MPH, of the Tufts University Center for Black Maternal Health & Reproductive Justice. Dr. Amutah-Onukagha addressed some of the potential issues that appear to drive the disparity in care.
The lack of diversity in the health care workforce has a significant effect on patient outcomes, Dr. Amutah-Onukagha said. Overall, Black newborns are more than twice as likely as White newborns to die during their first year of life, but this number is cut in half when Black infants are cared for by Black physicians, she emphasized.
Other factors that may affect disparities in maternal health care include limited access to prenatal care, discriminatory hospital protocols, and mistreatment by health care professionals, said Dr. Amutah-Onukagha. She cited data showing that maternal mortality rates were higher in rural compared with urban areas. “According to the American Hospital Association, half of rural hospitals have no obstetric care, leaving mothers in maternity care deserts; this exacerbates existing disparities,” she said.
In the webinar, Sindhu Srinivas, MD, a maternal-fetal medicine specialist at the University of Pennsylvania, explained how patient, community, and system factors play a role in the disparities in maternal care.
Overall, Black women have to travel further to receive care, which has implications for high-risk pregnancies, and patients on Medicaid have to wait longer for care, and are less likely to be referred, she added. Black women also have higher rates of preexisting conditions compared with other populations that put them in the high-risk category, such as high blood pressure, diabetes, obesity, or being HIV positive, she said.
Other factors contributing to persistent disparities in maternal care include sociodemographics, patient beliefs and knowledge, and psychological issues including stress, said Dr. Srinivas. Community factors, such as social networks, safety, and poverty, also play a role, as do clinician factors of implicit bias and communication skills, she said.
Strategies to reduce disparity
Dr. Srinivas presented several strategies to reduce disparities at various levels. At the policy level, interventions such as establishing a Maternal Mortality Review Committee, establishing a perinatal quality collaborative, and extending Medicaid for a full year postpartum could help improve outcomes, she said. Dr. Srinivas also encouraged clinicians to report maternal mortality data stratified by race and ethnicity, and to participate in the Alliance for Innovation on Maternal Health program (AIM), an initiative in partnership with the American College of Obstetrics and Gynecology.
Dr. Srinivas also proposed maternal health policies to develop payment models “to sustain and scale innovative solutions, and “preserve access to contraception and abortion care.”
For clinicians looking to have an immediate impact, the panelists agreed that working with community health centers can make a significant difference by improving access to maternal care. Consider opportunities for partnership between hospitals and health care delivery centers in the community, said Dr. Srinivas.
Also, don’t underestimate the value of doulas in the birthing process, Dr. Amutah-Onukagha said. She urged clinicians to advocate for doula reimbursement and to take advantage of opportunities for doulas to work with pregnant individuals at the community levels. Data suggest that doulas are associated with increased maternal care visits and with breastfeeding, she noted.
Adam Myers, MD, of the Blue Cross Blue Shield Association, also contributed to the webinar discussion with a key point: Having financial means and commercial coverage is not a buffer against adverse maternal outcomes for racial minorities.
Dr. Myers cited the latest Health of America Report, which included data up to April 2021 with surveys of Medicaid members and their experiences. According to the report, rates of severe maternal mortality (SMM) increased by 9% for commercially and Medicaid-insured women between 2018 and 2020.
Among commercially insured women, SMM was 53% higher among Black women than White women; among Medicaid-insured women, Black women had a 73% higher rate of SMM, compared with White women.
In addition, the report showed that significantly more mothers of color were not able to complete the recommended series of prenatal visits, mainly for reasons of scheduling and transportation, which were greater barriers than COVID-19, Dr. Myers said.
Based on the data, one specific risk profile rose to the top: “We believe women of color aged 35 or higher with comorbid conditions should be treated as very high risk for SMM,” Dr. Myers emphasized. He stressed the need to focus on transportation and scheduling barriers and expressed support for partnerships and health care delivery centers in the community to mitigate these issues.
Finally, Dr. Srinivas encouraged clinicians to have confidence in their expertise and make themselves heard to help their patients and improve maternal health for all. “Use your voice,” said Dr. Srinivas, “As physicians we don’t think of that as an important aspect of our work, or that we can’t articulate, but remember that we are experts, and sharing stories of patients who are impacted is incredibly powerful,” she said.
The presenters had no relevant financial conflicts to disclose.