User login
The right indoor relative humidity could ward off COVID
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF THE ROYAL SOCIETY INTERFACE
Discontinuing immunotherapy: Is the infusion bag half empty or half full?
It’s a “champagne problem” many of us have encountered over the past few years in the clinic.
A patient with advanced non–small cell lung cancer (NSCLC) is fortunate enough to continue to do well for 2 years on ongoing pembrolizumab or perhaps pemetrexed and pembrolizumab as maintenance therapy. The latest CT shows a residual but far smaller primary tumor than what she started with.
In this instance, you may be considering stopping treatment but are concerned about doing so with evidence of disease still present.
Clinical trials of immunotherapy or chemoimmunotherapy have generally terminated treatment in nonprogressing patients after 2 years. We also know that some patients in early trials of immunotherapy stopped treatment after a fixed period of 1 or 2 years and continued to show no evidence of progression many years later.
The reason some patients experience this kind of success: Unlike the mechanism of action of conventional chemotherapy or targeted therapies, where ongoing treatment would be important to continue to exert an inhibitory effect, the active substrate of immunotherapy is the patient’s immune system, which can potentially have a self-sustaining efficacy beyond the stimulatory effect of the checkpoint inhibitor.
One trial directly addressed this question of stopping vs. continuing treatment in patients on immunotherapy. The CheckMate 153 trial, published in 2020, randomly assigned 252 previously treated patients who hadn’t demonstrated progression after 1 year on nivolumab to either discontinue nivolumab or continue nivolumab on an ongoing basis. The results were strongly in favor of ongoing therapy. Both progression-free survival (PFS) and overall survival (OS) were significantly longer in patients who continued therapy: PFS of 24.7 months vs. 9.4 months and OS not reached vs. 32.5 months.
This finding is important, but there’s an important caveat. The study population included many heavily pretreated patients, but, in practice, immunotherapy has generally moved into the first-line setting, where we see dramatic responses in a significant subset of patients.
Even more recent data are emerging that may help us evaluate who will do well off therapy and who should continue treatment.
We now have a growing collection of long-term data on patients who are more likely to have good outcomes with immunotherapy, specifically those with high tumor programmed death-ligand 1 (PD-L1) expression (≥ 50%), from the KEYNOTE-024 trial. In this study, 39 of 151 (25.8%) patients assigned to pembrolizumab completed the planned maximum of 2 years of treatment, among whom 82.1% achieved an objective response; but, only 10% (4 patients) achieved a complete response. The proportion of patients without progression and remaining off therapy wasn’t reported, but the OS rate 3 years after completing treatment was 81.4%.
In addition, restarting immunotherapy after discontinuing appears to be a moderately effective strategy. In the KEYNOTE-024 trial, 12 patients received a second course of pembrolizumab because of disease progression a median of 15.2 months after discontinuing pembrolizumab. In this small cohort, eight of these patients (66.7%) were alive at the data cutoff, and six (50%) achieved stable disease.
Recently, we received additional insight in the follow-up from two chemoimmunotherapy trials that have most shaped my practice for patients with advanced NSCLC and any level of PD-L1 expression. These are the KEYNOTE-189 trial of platinum-pemetrexed with pembrolizumab vs. placebo in those with nonsquamous NSCLC, and the KEYNOTE-407 trial of carboplatin-taxane with pembrolizumab vs. placebo in patients with advanced squamous NSCLC. The National Comprehensive Cancer Network has designated each as a “preferred regimen” for patients with advanced NSCLC.
Both regimens have demonstrated sustained efficacy benefits with prolonged follow-up, including significantly superior objective response rate, PFS, and OS with the addition of pembrolizumab. These findings merely cemented the role of these regimens in our practice, but the trials also reported on the cohort of patients who completed 35 cycles of treatment over 2 years then discontinued therapy. In both, the majority of patients showed an objective response (86% in KEYNOTE-189 and 90% in KEYNOTE-407), with most patients alive at 3 years after 2 years of treatment (71.9% in KEYNOTE-189 and 69.5% in KEYNOTE-407). In addition, the proportion of patients alive without disease progression or subsequent therapy was notable – 40.4% in KEYNOTE-189 and 43.6% KEYNOTE-407.
How should we interpret these data for the patient who is in the exam room with us?
The short answer is that we don’t know. I see this as a half-empty, half-full conundrum.
I’m disappointed that more patients who responded for 2 years will experience disease progression in the 1-3 years that follow. This signals that their immune systems have not perpetuated their initial response over the long-term. But these patients may have demonstrated disease progression even if they had continued therapy.
We also know that some patients can be rechallenged and will respond again. Some of these patients will show stable disease, whereas others will progress with repeat treatment. I would love to be able to better predict which patients are destined to do well without treatment vs. those who benefit from treatment beyond 2 years.
Might the level of PD-L1 expression tell us? Can PET imaging discriminate those with residual hypermetabolism who may need continued treatment from those with no residual uptake who could be spared it? Would serial measurement of circulating tumor DNA (ctDNA) in responding patients identify when they have achieved a point of diminishing returns, potentially indicating that some can safely discontinue treatment after 2 years, whereas others need to continue to suppress on prolonged maintenance therapy?
These questions have yet to be studied systematically. In the meantime, I take an individualized approach with my patients facing this decision. Some have experienced escalating arthralgias and myalgias, cost concerns, or other issues related to immunotherapy that may dissuade us from continuing treatment. But several others have been grateful to continue with their treatment, hesitant to do anything that could change the path of their disease.
In my patients who tolerate therapy well, I’m more worried about potential undertreatment than overtreatment. I tend to favor having my patients continue therapy in the absence of problematic toxicity or practical challenges. There is certainly room for debate here while we await data to better guide these decisions. How do you approach these patients?
Dr. West is Clinical Associate Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Care, Duarte, Calif. He reported conflicts of interest with Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, Merck, and Eli Lilly.
A version of this article first appeared on Medscape.com.
It’s a “champagne problem” many of us have encountered over the past few years in the clinic.
A patient with advanced non–small cell lung cancer (NSCLC) is fortunate enough to continue to do well for 2 years on ongoing pembrolizumab or perhaps pemetrexed and pembrolizumab as maintenance therapy. The latest CT shows a residual but far smaller primary tumor than what she started with.
In this instance, you may be considering stopping treatment but are concerned about doing so with evidence of disease still present.
Clinical trials of immunotherapy or chemoimmunotherapy have generally terminated treatment in nonprogressing patients after 2 years. We also know that some patients in early trials of immunotherapy stopped treatment after a fixed period of 1 or 2 years and continued to show no evidence of progression many years later.
The reason some patients experience this kind of success: Unlike the mechanism of action of conventional chemotherapy or targeted therapies, where ongoing treatment would be important to continue to exert an inhibitory effect, the active substrate of immunotherapy is the patient’s immune system, which can potentially have a self-sustaining efficacy beyond the stimulatory effect of the checkpoint inhibitor.
One trial directly addressed this question of stopping vs. continuing treatment in patients on immunotherapy. The CheckMate 153 trial, published in 2020, randomly assigned 252 previously treated patients who hadn’t demonstrated progression after 1 year on nivolumab to either discontinue nivolumab or continue nivolumab on an ongoing basis. The results were strongly in favor of ongoing therapy. Both progression-free survival (PFS) and overall survival (OS) were significantly longer in patients who continued therapy: PFS of 24.7 months vs. 9.4 months and OS not reached vs. 32.5 months.
This finding is important, but there’s an important caveat. The study population included many heavily pretreated patients, but, in practice, immunotherapy has generally moved into the first-line setting, where we see dramatic responses in a significant subset of patients.
Even more recent data are emerging that may help us evaluate who will do well off therapy and who should continue treatment.
We now have a growing collection of long-term data on patients who are more likely to have good outcomes with immunotherapy, specifically those with high tumor programmed death-ligand 1 (PD-L1) expression (≥ 50%), from the KEYNOTE-024 trial. In this study, 39 of 151 (25.8%) patients assigned to pembrolizumab completed the planned maximum of 2 years of treatment, among whom 82.1% achieved an objective response; but, only 10% (4 patients) achieved a complete response. The proportion of patients without progression and remaining off therapy wasn’t reported, but the OS rate 3 years after completing treatment was 81.4%.
In addition, restarting immunotherapy after discontinuing appears to be a moderately effective strategy. In the KEYNOTE-024 trial, 12 patients received a second course of pembrolizumab because of disease progression a median of 15.2 months after discontinuing pembrolizumab. In this small cohort, eight of these patients (66.7%) were alive at the data cutoff, and six (50%) achieved stable disease.
Recently, we received additional insight in the follow-up from two chemoimmunotherapy trials that have most shaped my practice for patients with advanced NSCLC and any level of PD-L1 expression. These are the KEYNOTE-189 trial of platinum-pemetrexed with pembrolizumab vs. placebo in those with nonsquamous NSCLC, and the KEYNOTE-407 trial of carboplatin-taxane with pembrolizumab vs. placebo in patients with advanced squamous NSCLC. The National Comprehensive Cancer Network has designated each as a “preferred regimen” for patients with advanced NSCLC.
Both regimens have demonstrated sustained efficacy benefits with prolonged follow-up, including significantly superior objective response rate, PFS, and OS with the addition of pembrolizumab. These findings merely cemented the role of these regimens in our practice, but the trials also reported on the cohort of patients who completed 35 cycles of treatment over 2 years then discontinued therapy. In both, the majority of patients showed an objective response (86% in KEYNOTE-189 and 90% in KEYNOTE-407), with most patients alive at 3 years after 2 years of treatment (71.9% in KEYNOTE-189 and 69.5% in KEYNOTE-407). In addition, the proportion of patients alive without disease progression or subsequent therapy was notable – 40.4% in KEYNOTE-189 and 43.6% KEYNOTE-407.
How should we interpret these data for the patient who is in the exam room with us?
The short answer is that we don’t know. I see this as a half-empty, half-full conundrum.
I’m disappointed that more patients who responded for 2 years will experience disease progression in the 1-3 years that follow. This signals that their immune systems have not perpetuated their initial response over the long-term. But these patients may have demonstrated disease progression even if they had continued therapy.
We also know that some patients can be rechallenged and will respond again. Some of these patients will show stable disease, whereas others will progress with repeat treatment. I would love to be able to better predict which patients are destined to do well without treatment vs. those who benefit from treatment beyond 2 years.
Might the level of PD-L1 expression tell us? Can PET imaging discriminate those with residual hypermetabolism who may need continued treatment from those with no residual uptake who could be spared it? Would serial measurement of circulating tumor DNA (ctDNA) in responding patients identify when they have achieved a point of diminishing returns, potentially indicating that some can safely discontinue treatment after 2 years, whereas others need to continue to suppress on prolonged maintenance therapy?
These questions have yet to be studied systematically. In the meantime, I take an individualized approach with my patients facing this decision. Some have experienced escalating arthralgias and myalgias, cost concerns, or other issues related to immunotherapy that may dissuade us from continuing treatment. But several others have been grateful to continue with their treatment, hesitant to do anything that could change the path of their disease.
In my patients who tolerate therapy well, I’m more worried about potential undertreatment than overtreatment. I tend to favor having my patients continue therapy in the absence of problematic toxicity or practical challenges. There is certainly room for debate here while we await data to better guide these decisions. How do you approach these patients?
Dr. West is Clinical Associate Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Care, Duarte, Calif. He reported conflicts of interest with Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, Merck, and Eli Lilly.
A version of this article first appeared on Medscape.com.
It’s a “champagne problem” many of us have encountered over the past few years in the clinic.
A patient with advanced non–small cell lung cancer (NSCLC) is fortunate enough to continue to do well for 2 years on ongoing pembrolizumab or perhaps pemetrexed and pembrolizumab as maintenance therapy. The latest CT shows a residual but far smaller primary tumor than what she started with.
In this instance, you may be considering stopping treatment but are concerned about doing so with evidence of disease still present.
Clinical trials of immunotherapy or chemoimmunotherapy have generally terminated treatment in nonprogressing patients after 2 years. We also know that some patients in early trials of immunotherapy stopped treatment after a fixed period of 1 or 2 years and continued to show no evidence of progression many years later.
The reason some patients experience this kind of success: Unlike the mechanism of action of conventional chemotherapy or targeted therapies, where ongoing treatment would be important to continue to exert an inhibitory effect, the active substrate of immunotherapy is the patient’s immune system, which can potentially have a self-sustaining efficacy beyond the stimulatory effect of the checkpoint inhibitor.
One trial directly addressed this question of stopping vs. continuing treatment in patients on immunotherapy. The CheckMate 153 trial, published in 2020, randomly assigned 252 previously treated patients who hadn’t demonstrated progression after 1 year on nivolumab to either discontinue nivolumab or continue nivolumab on an ongoing basis. The results were strongly in favor of ongoing therapy. Both progression-free survival (PFS) and overall survival (OS) were significantly longer in patients who continued therapy: PFS of 24.7 months vs. 9.4 months and OS not reached vs. 32.5 months.
This finding is important, but there’s an important caveat. The study population included many heavily pretreated patients, but, in practice, immunotherapy has generally moved into the first-line setting, where we see dramatic responses in a significant subset of patients.
Even more recent data are emerging that may help us evaluate who will do well off therapy and who should continue treatment.
We now have a growing collection of long-term data on patients who are more likely to have good outcomes with immunotherapy, specifically those with high tumor programmed death-ligand 1 (PD-L1) expression (≥ 50%), from the KEYNOTE-024 trial. In this study, 39 of 151 (25.8%) patients assigned to pembrolizumab completed the planned maximum of 2 years of treatment, among whom 82.1% achieved an objective response; but, only 10% (4 patients) achieved a complete response. The proportion of patients without progression and remaining off therapy wasn’t reported, but the OS rate 3 years after completing treatment was 81.4%.
In addition, restarting immunotherapy after discontinuing appears to be a moderately effective strategy. In the KEYNOTE-024 trial, 12 patients received a second course of pembrolizumab because of disease progression a median of 15.2 months after discontinuing pembrolizumab. In this small cohort, eight of these patients (66.7%) were alive at the data cutoff, and six (50%) achieved stable disease.
Recently, we received additional insight in the follow-up from two chemoimmunotherapy trials that have most shaped my practice for patients with advanced NSCLC and any level of PD-L1 expression. These are the KEYNOTE-189 trial of platinum-pemetrexed with pembrolizumab vs. placebo in those with nonsquamous NSCLC, and the KEYNOTE-407 trial of carboplatin-taxane with pembrolizumab vs. placebo in patients with advanced squamous NSCLC. The National Comprehensive Cancer Network has designated each as a “preferred regimen” for patients with advanced NSCLC.
Both regimens have demonstrated sustained efficacy benefits with prolonged follow-up, including significantly superior objective response rate, PFS, and OS with the addition of pembrolizumab. These findings merely cemented the role of these regimens in our practice, but the trials also reported on the cohort of patients who completed 35 cycles of treatment over 2 years then discontinued therapy. In both, the majority of patients showed an objective response (86% in KEYNOTE-189 and 90% in KEYNOTE-407), with most patients alive at 3 years after 2 years of treatment (71.9% in KEYNOTE-189 and 69.5% in KEYNOTE-407). In addition, the proportion of patients alive without disease progression or subsequent therapy was notable – 40.4% in KEYNOTE-189 and 43.6% KEYNOTE-407.
How should we interpret these data for the patient who is in the exam room with us?
The short answer is that we don’t know. I see this as a half-empty, half-full conundrum.
I’m disappointed that more patients who responded for 2 years will experience disease progression in the 1-3 years that follow. This signals that their immune systems have not perpetuated their initial response over the long-term. But these patients may have demonstrated disease progression even if they had continued therapy.
We also know that some patients can be rechallenged and will respond again. Some of these patients will show stable disease, whereas others will progress with repeat treatment. I would love to be able to better predict which patients are destined to do well without treatment vs. those who benefit from treatment beyond 2 years.
Might the level of PD-L1 expression tell us? Can PET imaging discriminate those with residual hypermetabolism who may need continued treatment from those with no residual uptake who could be spared it? Would serial measurement of circulating tumor DNA (ctDNA) in responding patients identify when they have achieved a point of diminishing returns, potentially indicating that some can safely discontinue treatment after 2 years, whereas others need to continue to suppress on prolonged maintenance therapy?
These questions have yet to be studied systematically. In the meantime, I take an individualized approach with my patients facing this decision. Some have experienced escalating arthralgias and myalgias, cost concerns, or other issues related to immunotherapy that may dissuade us from continuing treatment. But several others have been grateful to continue with their treatment, hesitant to do anything that could change the path of their disease.
In my patients who tolerate therapy well, I’m more worried about potential undertreatment than overtreatment. I tend to favor having my patients continue therapy in the absence of problematic toxicity or practical challenges. There is certainly room for debate here while we await data to better guide these decisions. How do you approach these patients?
Dr. West is Clinical Associate Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Care, Duarte, Calif. He reported conflicts of interest with Ariad/Takeda, Bristol-Myers Squibb, Boehringer Ingelheim, Spectrum, AstraZeneca, Celgene, Genentech/Roche, Pfizer, Merck, and Eli Lilly.
A version of this article first appeared on Medscape.com.
Could this computer help you beat cancer?
The 1960s marked the arrival of computers in medicine. Expensive, cumbersome hunks of plastic and metal that could (maybe) get test results to a doctor faster. The 1980s saw the first real difference-making functions computers could offer – clinical, financial, administrative – and in 1991, the Institute of Medicine published the first manifesto on what electronic health records could (and would) be.
Since then, we’ve seen computer breakthroughs across all areas of medicine, with artificial intelligence (AI), virtual reality, and telemedicine brought to the fore. But something else is brewing that not a lot of people know about yet:
“Think of it as transitioning from getting light through fire and candles and now having electricity, and there’s a light bulb that is lighting it all,” said Lara Jehi, MD, Cleveland Clinic’s chief research information officer.
What is quantum computing?
Classical computers (aka binary computers), which are the foundation of today’s devices, including artificial intelligence and machine learning, work by using information known as bits. These appear as 0 or 1 (sometimes defined as off/on or false/true).
Quantum computers, on the other hand, use quantum bits known as qubits. And yes, the definition of “quantum” – as in: very, very small – applies.
International Business Machines, more commonly known as IBM, is currently leading this new tech. A common misconception about quantum computers is that they are “a next evolution of computers that will get faster,” said Frederik Flöther, PhD, life sciences and health care lead with IBM Quantum Industry Consulting. Instead, he wants us to look at quantum computing as something completely new “because it is fundamentally a different hardware, a different software, not just an evolution of the same.”
How does it work differently from existing computers? Quantum computing deals in nature. Therefore, qubits have to be based on the natural world. What does that mean? Nobel Prize-winning physicist Richard Feynman was famously quoted as saying: “Nature isn’t classical, dammit, and if you want to make a simulation of nature, you’d better make it quantum mechanical, and by golly it’s a wonderful problem, because it doesn’t look so easy.”
Nature, said Dr. Jehi, doesn’t work in black and white or fit into boxes.
“We have to convert it to zeros and ones because that’s what computers speak,” she explained. But quantum computing uses the principles of quantum mechanics. “It’s exactly how nature works, because it is based on the fundamental unit of everything in nature, which is atomic structure.”
Very, very small indeed. And that’s why quantum computing could be game-changing tech in medicine.
“Quantum computers can be used to represent a bunch of different solutions to a problem all at the same time, and then collapse down to the optimal solution, the one that actually works,” said Tony Uttley, president and chief operating officer with Quantinuum, a collaboration between Cambridge Quantum and Honeywell Quantum Solutions that is working to drive the future of quantum computing. “And the reason it does that is because of some fabulous properties of quantum physics.”
Establishing a quantum computing beachhead
Scientists around the globe are studying quantum computers looking for ways to harness this technology to make big gains in medicine.
IBM has created the IBM Quantum Network and is partnering with different organizations, from startups to Fortune 500 companies, to develop and test technology in various settings. One of these partnerships with the Cleveland Clinic is set to establish the “Discovery Accelerator,” focused on advancing health care through high-performance computing on the hybrid cloud, quantum computing technologies, and artificial intelligence.
Many people around the country are now using this technology on existing computers by tapping into the cloud, but with limited qubit access. IBM has researchers in places like Germany and Japan working on quantum computers and will be installing the country’s first of IBM’s next-generation 1,000+ qubit quantum systems on the Cleveland Clinic campus, which they are planning to use to help further investigate quantum computing’s many predicted benefits.
But what are those benefits?
Drug discovery and development
Quantum chemistry is one main area quantum computing is poised to help.
“The immediate application of that would be in drug discovery,” said Dr. Jehi. When scientists make drugs, they sit in a lab and develop different chemical formulas for what might constitute that drug.
“But for us to really know if it’s going to work, we need to be able to imagine how that chemical composition will translate into a structure,” she said.
Even in their most powerful form, today’s supercomputers are slow in their ability to change this chemical formula on paper to a simulation of what the chemical compound will look like. And in many cases, they can’t do this type of analysis.
“So, we end up making the drugs without knowing exactly how they’re going to look, which is not really the optimal way of creating a drug you expect to work” explained Dr. Jehi. “It’s a waste of time creating compounds that aren’t going to have any effect.”
Quantum computers will allow researchers to create and see these molecular structures and know how they bind and interact with the human body. In effect, they’ll know if a potential drug will work before ever having to physically make it.
Because of its differences from classic computing, quantum computers are not limited in their ability to simulate how different compounds can appear. Being able to simulate the compounds that drugs are made of can lead to a faster discovery of medications to treat a wide range of conditions.
Disease analysis
Eventually, this technology could assist with disease analysis, working on a molecular level to allow computers/AI to contemplate, for example, cancer molecules and gain a deeper understanding of how they function.
Dr. Jehi said quantum computing can also be used to study things like chronic illnesses. These are conditions that people must live with and manage, and how a person is feeling in this instance can vary day to day, based on things like what a person is eating, the weather, or medications they are taking.
“There are so many different possibilities for what could change a patient’s trajectory in one way versus another,” said Dr. Jehi.
She stressed that, if one has a group of patients, and everything that’s happened to them along their disease journey has been captured, it’s very challenging to mimic what that group looks like, and then study the effects of these different interventions on it using traditional computing.
“It just gets way too complicated, and the computers that we have can’t keep up with analyzing the effects of the different possibilities. It gets jumbled up,” Dr. Jehi said.
But quantum computing can offer quantum machine learning, meaning you use this special quantum ability to handle different simulations and different possibilities.
The Cleveland Clinic, for instance, is looking at how some patients who undergo general surgeries have heart complications after their procedures.
“It would be transformative if we could identify ahead of time who is at highest risk of having a heart attack after surgery, as so we could take care of those people better,” she said.
The clinic’s current data set includes records for 450,000 patients, and current AI/machine learning makes sifting through this very slow and complex. The clinic is using machine learning approaches to create a synthetic data set, a smaller group that is a replica of the much larger one. Quantum technology could improve and speed this analysis to produce models that better perform.
Disease detection
“Imagine you go get a CT scan,” said Mr. Uttley. “There are already AI solutions that you can run that set of images through and ask: ‘Does this look like something that would be cancer?’ ” This existing technology works well on things that are typical and have been identified before, because that’s how machine learning works. If AI has seen something 100,000 times, it can often find something else that looks like it.
But today’s classical computers aren’t equipped to identify something unfamiliar. “Those are places where quantum computers can be much better at thinking of images and being able to say: ‘I can detect rare cancers or rare conditions that you don’t have a huge library of things that look like that,’ ” Mr. Uttley said.
This is also where researchers can use a quantum computer to be able to figure out what things could look like.
“The beauty of quantum computing is that it is a bias formation in quantum physics, this more probabilistic design. And so you can take advantage of that probabilistic design to help them think about this,” Mr. Uttley said.
How far out are we?
Mr. Uttley said we’re in an emergent era of quantum computing. Quantum computers exist and that’s a big deal, but a lot of this technology is still in fairly early stages.
“It’s a little bit like we’re at the beginning of the internet and saying, how are things going to play out,” he explained.
Right now, companies like Quantinuum are striving to perform computations on both a quantum and classic computer, compare the results, and say: “We’re getting the same answer.”
“So, this is the era where we’re able to build trust and say these quantum computers are actually working correctly,” Mr. Uttley explained.
In the future, he said, we can possibly imagine something like a quantum MRI that is able to understand your body in a way that transmits that data to a quantum computer to detect what’s wrong, and be able to tell the difference between cancerous and noncancerous. That will allow faster treatments and tailoring them to specific patient populations.
“What we’re doing today might seem slightly less sexy than that, but is maybe even equally important,” said Mr. Uttley.
This is using quantum computers to make the best encryption keys that can be made. The medical community, which is already using quantum computing to execute this, is excited about this being a better means of keeping patient data as secure as possible.
In June, Quantinuum launched InQuanto, which is quantum computing software that is allowing computational chemists, who, until now, only had classical computers at their fingertips. The move created an opportunity to start thinking about the problems that they worked on and what they would do with a quantum computer. As quantum computers become higher performing over the years, Mr. Uttley said the software will go from tasks like isolating one molecule to solving larger problems.
“That will happen over this next decade, where I think we’ll see the first kind of real use cases come out in the next likely 2 to 3 years,” he said. For now, this technology will likely be used in tandem with classical computers.
Mr. Uttley said that progress in the quantum world and medicine will continue to grow at a slow and steady pace, and in years to come, we’ll likely see things start to click and then eventually take off “full force.”
A version of this article first appeared on WebMD.com.
The 1960s marked the arrival of computers in medicine. Expensive, cumbersome hunks of plastic and metal that could (maybe) get test results to a doctor faster. The 1980s saw the first real difference-making functions computers could offer – clinical, financial, administrative – and in 1991, the Institute of Medicine published the first manifesto on what electronic health records could (and would) be.
Since then, we’ve seen computer breakthroughs across all areas of medicine, with artificial intelligence (AI), virtual reality, and telemedicine brought to the fore. But something else is brewing that not a lot of people know about yet:
“Think of it as transitioning from getting light through fire and candles and now having electricity, and there’s a light bulb that is lighting it all,” said Lara Jehi, MD, Cleveland Clinic’s chief research information officer.
What is quantum computing?
Classical computers (aka binary computers), which are the foundation of today’s devices, including artificial intelligence and machine learning, work by using information known as bits. These appear as 0 or 1 (sometimes defined as off/on or false/true).
Quantum computers, on the other hand, use quantum bits known as qubits. And yes, the definition of “quantum” – as in: very, very small – applies.
International Business Machines, more commonly known as IBM, is currently leading this new tech. A common misconception about quantum computers is that they are “a next evolution of computers that will get faster,” said Frederik Flöther, PhD, life sciences and health care lead with IBM Quantum Industry Consulting. Instead, he wants us to look at quantum computing as something completely new “because it is fundamentally a different hardware, a different software, not just an evolution of the same.”
How does it work differently from existing computers? Quantum computing deals in nature. Therefore, qubits have to be based on the natural world. What does that mean? Nobel Prize-winning physicist Richard Feynman was famously quoted as saying: “Nature isn’t classical, dammit, and if you want to make a simulation of nature, you’d better make it quantum mechanical, and by golly it’s a wonderful problem, because it doesn’t look so easy.”
Nature, said Dr. Jehi, doesn’t work in black and white or fit into boxes.
“We have to convert it to zeros and ones because that’s what computers speak,” she explained. But quantum computing uses the principles of quantum mechanics. “It’s exactly how nature works, because it is based on the fundamental unit of everything in nature, which is atomic structure.”
Very, very small indeed. And that’s why quantum computing could be game-changing tech in medicine.
“Quantum computers can be used to represent a bunch of different solutions to a problem all at the same time, and then collapse down to the optimal solution, the one that actually works,” said Tony Uttley, president and chief operating officer with Quantinuum, a collaboration between Cambridge Quantum and Honeywell Quantum Solutions that is working to drive the future of quantum computing. “And the reason it does that is because of some fabulous properties of quantum physics.”
Establishing a quantum computing beachhead
Scientists around the globe are studying quantum computers looking for ways to harness this technology to make big gains in medicine.
IBM has created the IBM Quantum Network and is partnering with different organizations, from startups to Fortune 500 companies, to develop and test technology in various settings. One of these partnerships with the Cleveland Clinic is set to establish the “Discovery Accelerator,” focused on advancing health care through high-performance computing on the hybrid cloud, quantum computing technologies, and artificial intelligence.
Many people around the country are now using this technology on existing computers by tapping into the cloud, but with limited qubit access. IBM has researchers in places like Germany and Japan working on quantum computers and will be installing the country’s first of IBM’s next-generation 1,000+ qubit quantum systems on the Cleveland Clinic campus, which they are planning to use to help further investigate quantum computing’s many predicted benefits.
But what are those benefits?
Drug discovery and development
Quantum chemistry is one main area quantum computing is poised to help.
“The immediate application of that would be in drug discovery,” said Dr. Jehi. When scientists make drugs, they sit in a lab and develop different chemical formulas for what might constitute that drug.
“But for us to really know if it’s going to work, we need to be able to imagine how that chemical composition will translate into a structure,” she said.
Even in their most powerful form, today’s supercomputers are slow in their ability to change this chemical formula on paper to a simulation of what the chemical compound will look like. And in many cases, they can’t do this type of analysis.
“So, we end up making the drugs without knowing exactly how they’re going to look, which is not really the optimal way of creating a drug you expect to work” explained Dr. Jehi. “It’s a waste of time creating compounds that aren’t going to have any effect.”
Quantum computers will allow researchers to create and see these molecular structures and know how they bind and interact with the human body. In effect, they’ll know if a potential drug will work before ever having to physically make it.
Because of its differences from classic computing, quantum computers are not limited in their ability to simulate how different compounds can appear. Being able to simulate the compounds that drugs are made of can lead to a faster discovery of medications to treat a wide range of conditions.
Disease analysis
Eventually, this technology could assist with disease analysis, working on a molecular level to allow computers/AI to contemplate, for example, cancer molecules and gain a deeper understanding of how they function.
Dr. Jehi said quantum computing can also be used to study things like chronic illnesses. These are conditions that people must live with and manage, and how a person is feeling in this instance can vary day to day, based on things like what a person is eating, the weather, or medications they are taking.
“There are so many different possibilities for what could change a patient’s trajectory in one way versus another,” said Dr. Jehi.
She stressed that, if one has a group of patients, and everything that’s happened to them along their disease journey has been captured, it’s very challenging to mimic what that group looks like, and then study the effects of these different interventions on it using traditional computing.
“It just gets way too complicated, and the computers that we have can’t keep up with analyzing the effects of the different possibilities. It gets jumbled up,” Dr. Jehi said.
But quantum computing can offer quantum machine learning, meaning you use this special quantum ability to handle different simulations and different possibilities.
The Cleveland Clinic, for instance, is looking at how some patients who undergo general surgeries have heart complications after their procedures.
“It would be transformative if we could identify ahead of time who is at highest risk of having a heart attack after surgery, as so we could take care of those people better,” she said.
The clinic’s current data set includes records for 450,000 patients, and current AI/machine learning makes sifting through this very slow and complex. The clinic is using machine learning approaches to create a synthetic data set, a smaller group that is a replica of the much larger one. Quantum technology could improve and speed this analysis to produce models that better perform.
Disease detection
“Imagine you go get a CT scan,” said Mr. Uttley. “There are already AI solutions that you can run that set of images through and ask: ‘Does this look like something that would be cancer?’ ” This existing technology works well on things that are typical and have been identified before, because that’s how machine learning works. If AI has seen something 100,000 times, it can often find something else that looks like it.
But today’s classical computers aren’t equipped to identify something unfamiliar. “Those are places where quantum computers can be much better at thinking of images and being able to say: ‘I can detect rare cancers or rare conditions that you don’t have a huge library of things that look like that,’ ” Mr. Uttley said.
This is also where researchers can use a quantum computer to be able to figure out what things could look like.
“The beauty of quantum computing is that it is a bias formation in quantum physics, this more probabilistic design. And so you can take advantage of that probabilistic design to help them think about this,” Mr. Uttley said.
How far out are we?
Mr. Uttley said we’re in an emergent era of quantum computing. Quantum computers exist and that’s a big deal, but a lot of this technology is still in fairly early stages.
“It’s a little bit like we’re at the beginning of the internet and saying, how are things going to play out,” he explained.
Right now, companies like Quantinuum are striving to perform computations on both a quantum and classic computer, compare the results, and say: “We’re getting the same answer.”
“So, this is the era where we’re able to build trust and say these quantum computers are actually working correctly,” Mr. Uttley explained.
In the future, he said, we can possibly imagine something like a quantum MRI that is able to understand your body in a way that transmits that data to a quantum computer to detect what’s wrong, and be able to tell the difference between cancerous and noncancerous. That will allow faster treatments and tailoring them to specific patient populations.
“What we’re doing today might seem slightly less sexy than that, but is maybe even equally important,” said Mr. Uttley.
This is using quantum computers to make the best encryption keys that can be made. The medical community, which is already using quantum computing to execute this, is excited about this being a better means of keeping patient data as secure as possible.
In June, Quantinuum launched InQuanto, which is quantum computing software that is allowing computational chemists, who, until now, only had classical computers at their fingertips. The move created an opportunity to start thinking about the problems that they worked on and what they would do with a quantum computer. As quantum computers become higher performing over the years, Mr. Uttley said the software will go from tasks like isolating one molecule to solving larger problems.
“That will happen over this next decade, where I think we’ll see the first kind of real use cases come out in the next likely 2 to 3 years,” he said. For now, this technology will likely be used in tandem with classical computers.
Mr. Uttley said that progress in the quantum world and medicine will continue to grow at a slow and steady pace, and in years to come, we’ll likely see things start to click and then eventually take off “full force.”
A version of this article first appeared on WebMD.com.
The 1960s marked the arrival of computers in medicine. Expensive, cumbersome hunks of plastic and metal that could (maybe) get test results to a doctor faster. The 1980s saw the first real difference-making functions computers could offer – clinical, financial, administrative – and in 1991, the Institute of Medicine published the first manifesto on what electronic health records could (and would) be.
Since then, we’ve seen computer breakthroughs across all areas of medicine, with artificial intelligence (AI), virtual reality, and telemedicine brought to the fore. But something else is brewing that not a lot of people know about yet:
“Think of it as transitioning from getting light through fire and candles and now having electricity, and there’s a light bulb that is lighting it all,” said Lara Jehi, MD, Cleveland Clinic’s chief research information officer.
What is quantum computing?
Classical computers (aka binary computers), which are the foundation of today’s devices, including artificial intelligence and machine learning, work by using information known as bits. These appear as 0 or 1 (sometimes defined as off/on or false/true).
Quantum computers, on the other hand, use quantum bits known as qubits. And yes, the definition of “quantum” – as in: very, very small – applies.
International Business Machines, more commonly known as IBM, is currently leading this new tech. A common misconception about quantum computers is that they are “a next evolution of computers that will get faster,” said Frederik Flöther, PhD, life sciences and health care lead with IBM Quantum Industry Consulting. Instead, he wants us to look at quantum computing as something completely new “because it is fundamentally a different hardware, a different software, not just an evolution of the same.”
How does it work differently from existing computers? Quantum computing deals in nature. Therefore, qubits have to be based on the natural world. What does that mean? Nobel Prize-winning physicist Richard Feynman was famously quoted as saying: “Nature isn’t classical, dammit, and if you want to make a simulation of nature, you’d better make it quantum mechanical, and by golly it’s a wonderful problem, because it doesn’t look so easy.”
Nature, said Dr. Jehi, doesn’t work in black and white or fit into boxes.
“We have to convert it to zeros and ones because that’s what computers speak,” she explained. But quantum computing uses the principles of quantum mechanics. “It’s exactly how nature works, because it is based on the fundamental unit of everything in nature, which is atomic structure.”
Very, very small indeed. And that’s why quantum computing could be game-changing tech in medicine.
“Quantum computers can be used to represent a bunch of different solutions to a problem all at the same time, and then collapse down to the optimal solution, the one that actually works,” said Tony Uttley, president and chief operating officer with Quantinuum, a collaboration between Cambridge Quantum and Honeywell Quantum Solutions that is working to drive the future of quantum computing. “And the reason it does that is because of some fabulous properties of quantum physics.”
Establishing a quantum computing beachhead
Scientists around the globe are studying quantum computers looking for ways to harness this technology to make big gains in medicine.
IBM has created the IBM Quantum Network and is partnering with different organizations, from startups to Fortune 500 companies, to develop and test technology in various settings. One of these partnerships with the Cleveland Clinic is set to establish the “Discovery Accelerator,” focused on advancing health care through high-performance computing on the hybrid cloud, quantum computing technologies, and artificial intelligence.
Many people around the country are now using this technology on existing computers by tapping into the cloud, but with limited qubit access. IBM has researchers in places like Germany and Japan working on quantum computers and will be installing the country’s first of IBM’s next-generation 1,000+ qubit quantum systems on the Cleveland Clinic campus, which they are planning to use to help further investigate quantum computing’s many predicted benefits.
But what are those benefits?
Drug discovery and development
Quantum chemistry is one main area quantum computing is poised to help.
“The immediate application of that would be in drug discovery,” said Dr. Jehi. When scientists make drugs, they sit in a lab and develop different chemical formulas for what might constitute that drug.
“But for us to really know if it’s going to work, we need to be able to imagine how that chemical composition will translate into a structure,” she said.
Even in their most powerful form, today’s supercomputers are slow in their ability to change this chemical formula on paper to a simulation of what the chemical compound will look like. And in many cases, they can’t do this type of analysis.
“So, we end up making the drugs without knowing exactly how they’re going to look, which is not really the optimal way of creating a drug you expect to work” explained Dr. Jehi. “It’s a waste of time creating compounds that aren’t going to have any effect.”
Quantum computers will allow researchers to create and see these molecular structures and know how they bind and interact with the human body. In effect, they’ll know if a potential drug will work before ever having to physically make it.
Because of its differences from classic computing, quantum computers are not limited in their ability to simulate how different compounds can appear. Being able to simulate the compounds that drugs are made of can lead to a faster discovery of medications to treat a wide range of conditions.
Disease analysis
Eventually, this technology could assist with disease analysis, working on a molecular level to allow computers/AI to contemplate, for example, cancer molecules and gain a deeper understanding of how they function.
Dr. Jehi said quantum computing can also be used to study things like chronic illnesses. These are conditions that people must live with and manage, and how a person is feeling in this instance can vary day to day, based on things like what a person is eating, the weather, or medications they are taking.
“There are so many different possibilities for what could change a patient’s trajectory in one way versus another,” said Dr. Jehi.
She stressed that, if one has a group of patients, and everything that’s happened to them along their disease journey has been captured, it’s very challenging to mimic what that group looks like, and then study the effects of these different interventions on it using traditional computing.
“It just gets way too complicated, and the computers that we have can’t keep up with analyzing the effects of the different possibilities. It gets jumbled up,” Dr. Jehi said.
But quantum computing can offer quantum machine learning, meaning you use this special quantum ability to handle different simulations and different possibilities.
The Cleveland Clinic, for instance, is looking at how some patients who undergo general surgeries have heart complications after their procedures.
“It would be transformative if we could identify ahead of time who is at highest risk of having a heart attack after surgery, as so we could take care of those people better,” she said.
The clinic’s current data set includes records for 450,000 patients, and current AI/machine learning makes sifting through this very slow and complex. The clinic is using machine learning approaches to create a synthetic data set, a smaller group that is a replica of the much larger one. Quantum technology could improve and speed this analysis to produce models that better perform.
Disease detection
“Imagine you go get a CT scan,” said Mr. Uttley. “There are already AI solutions that you can run that set of images through and ask: ‘Does this look like something that would be cancer?’ ” This existing technology works well on things that are typical and have been identified before, because that’s how machine learning works. If AI has seen something 100,000 times, it can often find something else that looks like it.
But today’s classical computers aren’t equipped to identify something unfamiliar. “Those are places where quantum computers can be much better at thinking of images and being able to say: ‘I can detect rare cancers or rare conditions that you don’t have a huge library of things that look like that,’ ” Mr. Uttley said.
This is also where researchers can use a quantum computer to be able to figure out what things could look like.
“The beauty of quantum computing is that it is a bias formation in quantum physics, this more probabilistic design. And so you can take advantage of that probabilistic design to help them think about this,” Mr. Uttley said.
How far out are we?
Mr. Uttley said we’re in an emergent era of quantum computing. Quantum computers exist and that’s a big deal, but a lot of this technology is still in fairly early stages.
“It’s a little bit like we’re at the beginning of the internet and saying, how are things going to play out,” he explained.
Right now, companies like Quantinuum are striving to perform computations on both a quantum and classic computer, compare the results, and say: “We’re getting the same answer.”
“So, this is the era where we’re able to build trust and say these quantum computers are actually working correctly,” Mr. Uttley explained.
In the future, he said, we can possibly imagine something like a quantum MRI that is able to understand your body in a way that transmits that data to a quantum computer to detect what’s wrong, and be able to tell the difference between cancerous and noncancerous. That will allow faster treatments and tailoring them to specific patient populations.
“What we’re doing today might seem slightly less sexy than that, but is maybe even equally important,” said Mr. Uttley.
This is using quantum computers to make the best encryption keys that can be made. The medical community, which is already using quantum computing to execute this, is excited about this being a better means of keeping patient data as secure as possible.
In June, Quantinuum launched InQuanto, which is quantum computing software that is allowing computational chemists, who, until now, only had classical computers at their fingertips. The move created an opportunity to start thinking about the problems that they worked on and what they would do with a quantum computer. As quantum computers become higher performing over the years, Mr. Uttley said the software will go from tasks like isolating one molecule to solving larger problems.
“That will happen over this next decade, where I think we’ll see the first kind of real use cases come out in the next likely 2 to 3 years,” he said. For now, this technology will likely be used in tandem with classical computers.
Mr. Uttley said that progress in the quantum world and medicine will continue to grow at a slow and steady pace, and in years to come, we’ll likely see things start to click and then eventually take off “full force.”
A version of this article first appeared on WebMD.com.
Are nurses who pick up extra shifts at risk of harming themselves or others?
on a nurse’s physical and mental health. Plus, it can diminish quality of care and lead to patient errors.
Medscape’s RN/LPN Compensation Report 2022 found that more than half of RNs and LPNs don’t think they get paid enough. Even though many nurses saw pay increases over the past 2 years, many were still dissatisfied with their earnings. They blamed job stress, staffing shortages, and benefits that cut into their wages.
Why do nurses pick up extra shifts?
Most nurses work extra hours for the money. Incentives like getting paid time and a half or scoring a $200 bonus are hard to pass up.
“I’m a single mother with two kids,” said Cynthia West, a critical care nurse in Atlanta. “I want to be able to pay my bills and enjoy my life, too.” So, Ms. West picks up two to three extra shifts a month. She also works on-call for a sexual assault center, earning $350 per exam.
But money isn’t the only reason for some nurses. Trang Robinson travels from her home in Atlanta to Palo Alto, Calif., every other week for her job as a labor and delivery RN.
“If my unit needs extra help, I want to help,” she said. “It’s not about the extra money, although that helps my family; it’s that we’ve been so short-staffed. My colleagues are burned out. Staff members are burned out. When I’m there, I work as much as I can to help out my unit.”
Leslie Wysong, an Atlanta postanesthesia nurse, worked in intensive care during much of COVID. She said the chance to make level 3 pay was rewarding for many nurses, but most weren’t doing it for the money.
“We were doing it to alleviate the strain on our fellow nurses, to get closer to a 2:1 patient/nurse ratio rather than the 3:1 we were dealing with over the pandemic,” she said. “It was to help out our colleagues during a desperate situation.”
What are the risks?
The U.S. Occupational Safety and Health Administration states that a work shift that lasts more than 8 hours can disrupt the body’s sleep/wake cycle. It can also lead to physical and mental fatigue resulting in errors, injuries, and accidents.
And a study published in the American Association of Occupational Health Nurses found that extended shifts or shift work impacted nurses in many ways, including more medication errors, falling asleep during work hours, decreased productivity in the last 4 shift hours (of a 12-hour shift), increased risk of mistakes and near-errors associated with decreased vigilance, critical thinking impairment, and more needlestick injuries.
Another study, published in Rehabilitation Nursing Journal, found even more adverse effects, such as sleep disorders like insomnia and excessive sleepiness; cognitive impairment such as the reduced ability to concentrate, slower reactions times, and reduced ability to remember information; higher rates of injury while on the job; being more likely to engage in overeating and alcohol misuse; GI issues such as abdominal pain, constipation, and heartburn; higher rates of heart disease and high blood pressure; higher risk for breast and prostate cancers, and higher rates of depression and anxiety.
These are risks some nurses aren’t willing to take. For example, Caitlin Riley, a pediatric ED nurse in Ocala, Fla., only picks up extra shifts when she must, like when Hurricane Ian swept through Central Florida.
“I think working extra hours can compromise your quality of care,” she said. “You may make mistakes with things like math calculations or not catch something if you’re not totally ‘in’ it mentally. At the end of the day, it’s your nursing license. Sure, the money is great, but I won’t do anything to compromise losing my license or patient care.”
How can nurses boost pay without working extra shifts?
Instead, Ms. Riley returned to school and earned an MSN in health care leadership/management, knowing that an advanced degree could lead to higher-paying work. According to the Medscape report, RNs with master’s and doctoral degrees earned over $10,000 more than those with bachelor’s, associate’s, or RN diplomas.
The report also compiled the following earnings data. The data may help nurses find other ways to raise their salaries without taking on extra shifts.
- Salaried RNs and LPNs made more than hourly paid nurses.
- In-patient hospital RNs and skilled nursing facility LPNs got paid more than nurses in other settings.
- Specialty certifications helped RNs earn more money than nurses without specialty certificates.
- Union RNs and LPNs earned more than nonunion nurses.
- RNs and LPNs who work in big cities or suburbs make more money than those in rural areas.
How to prevent burnout and exhaustion when you work extra shifts
While burnout can happen in any profession, an investigation published in JAMA Network Open suggests it’s prevalent among US nurses. The study found that nurses who worked over 40 hours a week were more likely to experience burnout. However, researchers say that adequate staffing and limiting shift hours may alleviate the problem. Here’s how the nurses in the survey dealt with battle burnout:
- Change departments. Ms. Wysong stepped away from the ICU after COVID and switched to postanesthesia. “The move has made my work life much less stressful,” said Ms. Wysong. “They are all happy endings in postanesthesia.”
- Leave work at work. Ms. Riley said she mentally clocks out as she leaves the hospital. “When I put my papers in my shredder at the end of my shift, I let it go. I walk away knowing I did the best for my patients. Once I’m home, it’s time for me to be with the people I love and to refuel my own sense of happiness with the people that mean the most to me.”
- Take time off. “When I’m burned out, I just don’t come in,” said Ms. Robinson. “If I’m mentally or emotionally drained, I give myself a shift off to decompress, or I don’t pick up extra shifts.”
- Engage in relaxing hobbies. Kris Coleman, an ED nurse in Hardeeville, S.C., typically works three 12-hours shifts and only picks up an extra 4-hour shift once a week. When he’s off, he takes advantage of his time away from work. He said: “Do the things that help you relax on your time off. For me, it’s golfing, fishing, and spending time with my family.”
- Build a support system. “I have a group of friends at work,” said Ms. West. “We talk to each other and vent. Having a good support system, people that are in it with you who get what you’re going through is a helpful way to manage burnout.”
A version of this article first appeared on Medscape.com.
on a nurse’s physical and mental health. Plus, it can diminish quality of care and lead to patient errors.
Medscape’s RN/LPN Compensation Report 2022 found that more than half of RNs and LPNs don’t think they get paid enough. Even though many nurses saw pay increases over the past 2 years, many were still dissatisfied with their earnings. They blamed job stress, staffing shortages, and benefits that cut into their wages.
Why do nurses pick up extra shifts?
Most nurses work extra hours for the money. Incentives like getting paid time and a half or scoring a $200 bonus are hard to pass up.
“I’m a single mother with two kids,” said Cynthia West, a critical care nurse in Atlanta. “I want to be able to pay my bills and enjoy my life, too.” So, Ms. West picks up two to three extra shifts a month. She also works on-call for a sexual assault center, earning $350 per exam.
But money isn’t the only reason for some nurses. Trang Robinson travels from her home in Atlanta to Palo Alto, Calif., every other week for her job as a labor and delivery RN.
“If my unit needs extra help, I want to help,” she said. “It’s not about the extra money, although that helps my family; it’s that we’ve been so short-staffed. My colleagues are burned out. Staff members are burned out. When I’m there, I work as much as I can to help out my unit.”
Leslie Wysong, an Atlanta postanesthesia nurse, worked in intensive care during much of COVID. She said the chance to make level 3 pay was rewarding for many nurses, but most weren’t doing it for the money.
“We were doing it to alleviate the strain on our fellow nurses, to get closer to a 2:1 patient/nurse ratio rather than the 3:1 we were dealing with over the pandemic,” she said. “It was to help out our colleagues during a desperate situation.”
What are the risks?
The U.S. Occupational Safety and Health Administration states that a work shift that lasts more than 8 hours can disrupt the body’s sleep/wake cycle. It can also lead to physical and mental fatigue resulting in errors, injuries, and accidents.
And a study published in the American Association of Occupational Health Nurses found that extended shifts or shift work impacted nurses in many ways, including more medication errors, falling asleep during work hours, decreased productivity in the last 4 shift hours (of a 12-hour shift), increased risk of mistakes and near-errors associated with decreased vigilance, critical thinking impairment, and more needlestick injuries.
Another study, published in Rehabilitation Nursing Journal, found even more adverse effects, such as sleep disorders like insomnia and excessive sleepiness; cognitive impairment such as the reduced ability to concentrate, slower reactions times, and reduced ability to remember information; higher rates of injury while on the job; being more likely to engage in overeating and alcohol misuse; GI issues such as abdominal pain, constipation, and heartburn; higher rates of heart disease and high blood pressure; higher risk for breast and prostate cancers, and higher rates of depression and anxiety.
These are risks some nurses aren’t willing to take. For example, Caitlin Riley, a pediatric ED nurse in Ocala, Fla., only picks up extra shifts when she must, like when Hurricane Ian swept through Central Florida.
“I think working extra hours can compromise your quality of care,” she said. “You may make mistakes with things like math calculations or not catch something if you’re not totally ‘in’ it mentally. At the end of the day, it’s your nursing license. Sure, the money is great, but I won’t do anything to compromise losing my license or patient care.”
How can nurses boost pay without working extra shifts?
Instead, Ms. Riley returned to school and earned an MSN in health care leadership/management, knowing that an advanced degree could lead to higher-paying work. According to the Medscape report, RNs with master’s and doctoral degrees earned over $10,000 more than those with bachelor’s, associate’s, or RN diplomas.
The report also compiled the following earnings data. The data may help nurses find other ways to raise their salaries without taking on extra shifts.
- Salaried RNs and LPNs made more than hourly paid nurses.
- In-patient hospital RNs and skilled nursing facility LPNs got paid more than nurses in other settings.
- Specialty certifications helped RNs earn more money than nurses without specialty certificates.
- Union RNs and LPNs earned more than nonunion nurses.
- RNs and LPNs who work in big cities or suburbs make more money than those in rural areas.
How to prevent burnout and exhaustion when you work extra shifts
While burnout can happen in any profession, an investigation published in JAMA Network Open suggests it’s prevalent among US nurses. The study found that nurses who worked over 40 hours a week were more likely to experience burnout. However, researchers say that adequate staffing and limiting shift hours may alleviate the problem. Here’s how the nurses in the survey dealt with battle burnout:
- Change departments. Ms. Wysong stepped away from the ICU after COVID and switched to postanesthesia. “The move has made my work life much less stressful,” said Ms. Wysong. “They are all happy endings in postanesthesia.”
- Leave work at work. Ms. Riley said she mentally clocks out as she leaves the hospital. “When I put my papers in my shredder at the end of my shift, I let it go. I walk away knowing I did the best for my patients. Once I’m home, it’s time for me to be with the people I love and to refuel my own sense of happiness with the people that mean the most to me.”
- Take time off. “When I’m burned out, I just don’t come in,” said Ms. Robinson. “If I’m mentally or emotionally drained, I give myself a shift off to decompress, or I don’t pick up extra shifts.”
- Engage in relaxing hobbies. Kris Coleman, an ED nurse in Hardeeville, S.C., typically works three 12-hours shifts and only picks up an extra 4-hour shift once a week. When he’s off, he takes advantage of his time away from work. He said: “Do the things that help you relax on your time off. For me, it’s golfing, fishing, and spending time with my family.”
- Build a support system. “I have a group of friends at work,” said Ms. West. “We talk to each other and vent. Having a good support system, people that are in it with you who get what you’re going through is a helpful way to manage burnout.”
A version of this article first appeared on Medscape.com.
on a nurse’s physical and mental health. Plus, it can diminish quality of care and lead to patient errors.
Medscape’s RN/LPN Compensation Report 2022 found that more than half of RNs and LPNs don’t think they get paid enough. Even though many nurses saw pay increases over the past 2 years, many were still dissatisfied with their earnings. They blamed job stress, staffing shortages, and benefits that cut into their wages.
Why do nurses pick up extra shifts?
Most nurses work extra hours for the money. Incentives like getting paid time and a half or scoring a $200 bonus are hard to pass up.
“I’m a single mother with two kids,” said Cynthia West, a critical care nurse in Atlanta. “I want to be able to pay my bills and enjoy my life, too.” So, Ms. West picks up two to three extra shifts a month. She also works on-call for a sexual assault center, earning $350 per exam.
But money isn’t the only reason for some nurses. Trang Robinson travels from her home in Atlanta to Palo Alto, Calif., every other week for her job as a labor and delivery RN.
“If my unit needs extra help, I want to help,” she said. “It’s not about the extra money, although that helps my family; it’s that we’ve been so short-staffed. My colleagues are burned out. Staff members are burned out. When I’m there, I work as much as I can to help out my unit.”
Leslie Wysong, an Atlanta postanesthesia nurse, worked in intensive care during much of COVID. She said the chance to make level 3 pay was rewarding for many nurses, but most weren’t doing it for the money.
“We were doing it to alleviate the strain on our fellow nurses, to get closer to a 2:1 patient/nurse ratio rather than the 3:1 we were dealing with over the pandemic,” she said. “It was to help out our colleagues during a desperate situation.”
What are the risks?
The U.S. Occupational Safety and Health Administration states that a work shift that lasts more than 8 hours can disrupt the body’s sleep/wake cycle. It can also lead to physical and mental fatigue resulting in errors, injuries, and accidents.
And a study published in the American Association of Occupational Health Nurses found that extended shifts or shift work impacted nurses in many ways, including more medication errors, falling asleep during work hours, decreased productivity in the last 4 shift hours (of a 12-hour shift), increased risk of mistakes and near-errors associated with decreased vigilance, critical thinking impairment, and more needlestick injuries.
Another study, published in Rehabilitation Nursing Journal, found even more adverse effects, such as sleep disorders like insomnia and excessive sleepiness; cognitive impairment such as the reduced ability to concentrate, slower reactions times, and reduced ability to remember information; higher rates of injury while on the job; being more likely to engage in overeating and alcohol misuse; GI issues such as abdominal pain, constipation, and heartburn; higher rates of heart disease and high blood pressure; higher risk for breast and prostate cancers, and higher rates of depression and anxiety.
These are risks some nurses aren’t willing to take. For example, Caitlin Riley, a pediatric ED nurse in Ocala, Fla., only picks up extra shifts when she must, like when Hurricane Ian swept through Central Florida.
“I think working extra hours can compromise your quality of care,” she said. “You may make mistakes with things like math calculations or not catch something if you’re not totally ‘in’ it mentally. At the end of the day, it’s your nursing license. Sure, the money is great, but I won’t do anything to compromise losing my license or patient care.”
How can nurses boost pay without working extra shifts?
Instead, Ms. Riley returned to school and earned an MSN in health care leadership/management, knowing that an advanced degree could lead to higher-paying work. According to the Medscape report, RNs with master’s and doctoral degrees earned over $10,000 more than those with bachelor’s, associate’s, or RN diplomas.
The report also compiled the following earnings data. The data may help nurses find other ways to raise their salaries without taking on extra shifts.
- Salaried RNs and LPNs made more than hourly paid nurses.
- In-patient hospital RNs and skilled nursing facility LPNs got paid more than nurses in other settings.
- Specialty certifications helped RNs earn more money than nurses without specialty certificates.
- Union RNs and LPNs earned more than nonunion nurses.
- RNs and LPNs who work in big cities or suburbs make more money than those in rural areas.
How to prevent burnout and exhaustion when you work extra shifts
While burnout can happen in any profession, an investigation published in JAMA Network Open suggests it’s prevalent among US nurses. The study found that nurses who worked over 40 hours a week were more likely to experience burnout. However, researchers say that adequate staffing and limiting shift hours may alleviate the problem. Here’s how the nurses in the survey dealt with battle burnout:
- Change departments. Ms. Wysong stepped away from the ICU after COVID and switched to postanesthesia. “The move has made my work life much less stressful,” said Ms. Wysong. “They are all happy endings in postanesthesia.”
- Leave work at work. Ms. Riley said she mentally clocks out as she leaves the hospital. “When I put my papers in my shredder at the end of my shift, I let it go. I walk away knowing I did the best for my patients. Once I’m home, it’s time for me to be with the people I love and to refuel my own sense of happiness with the people that mean the most to me.”
- Take time off. “When I’m burned out, I just don’t come in,” said Ms. Robinson. “If I’m mentally or emotionally drained, I give myself a shift off to decompress, or I don’t pick up extra shifts.”
- Engage in relaxing hobbies. Kris Coleman, an ED nurse in Hardeeville, S.C., typically works three 12-hours shifts and only picks up an extra 4-hour shift once a week. When he’s off, he takes advantage of his time away from work. He said: “Do the things that help you relax on your time off. For me, it’s golfing, fishing, and spending time with my family.”
- Build a support system. “I have a group of friends at work,” said Ms. West. “We talk to each other and vent. Having a good support system, people that are in it with you who get what you’re going through is a helpful way to manage burnout.”
A version of this article first appeared on Medscape.com.
Lupus Nephritis Highlights From ASN Kidney Week 2022
Dr Gregg Silverman of New York University Langone Medical Center highlights four key studies on lupus nephritis (LN) presented at ASN Kidney Week 2022.
First, he focuses on a follow-up study of voclosporin after the successful phase 3 trial of the medication. According to the study, persistent proteinuria increases risk for comorbidities in lupus nephritis and rapid reductions in protein are predictive of improved long-term renal health. Voclosporin may be beneficial in limiting the negative long-term effects of proteinuria for patients with LN.
Next, Dr Silverman discusses a study that investigates the safety and tolerability of a first-in-class selective proteasome inhibitor for the treatment of LN. Use of this type of proteasome may improve autoimmunity for these patients.
The third abstract he discusses is a study of an investigational agent, VIB 4920, that was first explored over 20 years ago and that may have activity in LN.
Finally, Dr Silverman examines a phase 2b study that evaluated the efficacy and safety of telitacicept vs placebo in combination with standard therapy in patients with lupus. Early results were encouraging, but more mature results are needed.
--
Highlights in lupus nephritis (LN) from ASN Kidney Week 2022 focus on results on voclosporin, repurposing of telitacicept, promising agent VIB 4920, and other novel treatments for patients with LN.
Gregg J. Silverman, MD, has disclosed no relevant financial relationships.
Dr Gregg Silverman of New York University Langone Medical Center highlights four key studies on lupus nephritis (LN) presented at ASN Kidney Week 2022.
First, he focuses on a follow-up study of voclosporin after the successful phase 3 trial of the medication. According to the study, persistent proteinuria increases risk for comorbidities in lupus nephritis and rapid reductions in protein are predictive of improved long-term renal health. Voclosporin may be beneficial in limiting the negative long-term effects of proteinuria for patients with LN.
Next, Dr Silverman discusses a study that investigates the safety and tolerability of a first-in-class selective proteasome inhibitor for the treatment of LN. Use of this type of proteasome may improve autoimmunity for these patients.
The third abstract he discusses is a study of an investigational agent, VIB 4920, that was first explored over 20 years ago and that may have activity in LN.
Finally, Dr Silverman examines a phase 2b study that evaluated the efficacy and safety of telitacicept vs placebo in combination with standard therapy in patients with lupus. Early results were encouraging, but more mature results are needed.
--
Highlights in lupus nephritis (LN) from ASN Kidney Week 2022 focus on results on voclosporin, repurposing of telitacicept, promising agent VIB 4920, and other novel treatments for patients with LN.
Gregg J. Silverman, MD, has disclosed no relevant financial relationships.
Dr Gregg Silverman of New York University Langone Medical Center highlights four key studies on lupus nephritis (LN) presented at ASN Kidney Week 2022.
First, he focuses on a follow-up study of voclosporin after the successful phase 3 trial of the medication. According to the study, persistent proteinuria increases risk for comorbidities in lupus nephritis and rapid reductions in protein are predictive of improved long-term renal health. Voclosporin may be beneficial in limiting the negative long-term effects of proteinuria for patients with LN.
Next, Dr Silverman discusses a study that investigates the safety and tolerability of a first-in-class selective proteasome inhibitor for the treatment of LN. Use of this type of proteasome may improve autoimmunity for these patients.
The third abstract he discusses is a study of an investigational agent, VIB 4920, that was first explored over 20 years ago and that may have activity in LN.
Finally, Dr Silverman examines a phase 2b study that evaluated the efficacy and safety of telitacicept vs placebo in combination with standard therapy in patients with lupus. Early results were encouraging, but more mature results are needed.
--
Highlights in lupus nephritis (LN) from ASN Kidney Week 2022 focus on results on voclosporin, repurposing of telitacicept, promising agent VIB 4920, and other novel treatments for patients with LN.
Gregg J. Silverman, MD, has disclosed no relevant financial relationships.

Laser and light devices for acne treatment continue to advance
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Patient safety in hospitals improved in past decade: Report
, according to the 10th annual report from nonprofit the Leapfrog Group, a national nonprofit organization focused on health care safety and quality.
For five outcome measures, the safety improvements saved an estimated 16,000 lives during the 10-year period, the report said. These included two “never” events that both declined by approximately 25%: incidents of falls and trauma and incidents of objects unintentionally left in a body after surgery.
There were also decreases in three health care–associated infections, including methicillin-resistant Staphylococcus aureus (MRSA), which decreased by 22%; central line–associated bloodstream infection (CLABSI), which fell by 43%; and Clostridioides difficile infection (C. Diff), which declined by 8%.
The patient safety record of U.S. hospitals improved over the past decade, according to the report.
“Never in history have we seen across-the-board improvement in patient safety until this last decade, coinciding with the history of the [Leapfrog] Hospital Safety Grade,” said Leah Binder, president and CEO of the Leapfrog Group, in a news release. “We salute hospitals for this milestone and encourage them to accelerate their hard work saving patient lives.”
During the past decade, the report noted, hospitals have widely adopted technology and staffing strategies that can protect patients from preventable harm and death. Leapfrog cited a nearly sevenfold increase in the adoption of computerized provider order entry, which can reduce medication errors by more than 40%.
However, federal health officials separately have reported that the pandemic may have eroded some of those gains.
The Leapfrog report also cited a recent study, published in JAMA, that found that the rates of preventable adverse events in hospitalized patients – including adverse drug events, hospital-acquired infections, postprocedure events, and hospital-acquired pressure ulcers and falls – significantly declined between 2010 and 2019.
That study pointed to specific decreases in the rates of adverse events for patients admitted for myocardial infarction, heart failure, pneumonia, and major surgical procedures. There were also significant drops in adverse events for all other conditions, the study found.
Quality improvement efforts targeting those four conditions might have partly accounted for the lower rates of adverse events in patients with the conditions, the study observed. But “similar interventions did not occur for most of the conditions represented in the ‘all other conditions’ group,” it said.
In a 2019 report by the U.S. Agency for Healthcare Research and Quality (AHRQ), the agency noted that from 2000 to 2017, there had been gains in nearly two-thirds of patient-safety measures in acute, post-acute, and ambulatory care. Hospital safety improved on nine metrics and was unchanged on three. For example, from 2014 to 2017, the number of some hospital-acquired conditions, including adverse drug events and C. Diff infections, dropped about 20%.
However, in an article this past February, officials of the Centers for Medicare & Medicaid Services (CMS) said they had observed deterioration on multiple patient-safety metrics since the start of the pandemic. For example, central line infections, which had dropped by 31% in the five years before the COVID-19 outbreak, jumped 28% in the second quarter of 2020, compared with the prior-year period.
Commenting on these developments, the CMS authors said “the fact that the pandemic degraded patient safety so quickly and severely suggests that our health care system lacks a sufficiently resilient safety culture and infrastructure.”
A version of this article first appeared on Medscape.com.
, according to the 10th annual report from nonprofit the Leapfrog Group, a national nonprofit organization focused on health care safety and quality.
For five outcome measures, the safety improvements saved an estimated 16,000 lives during the 10-year period, the report said. These included two “never” events that both declined by approximately 25%: incidents of falls and trauma and incidents of objects unintentionally left in a body after surgery.
There were also decreases in three health care–associated infections, including methicillin-resistant Staphylococcus aureus (MRSA), which decreased by 22%; central line–associated bloodstream infection (CLABSI), which fell by 43%; and Clostridioides difficile infection (C. Diff), which declined by 8%.
The patient safety record of U.S. hospitals improved over the past decade, according to the report.
“Never in history have we seen across-the-board improvement in patient safety until this last decade, coinciding with the history of the [Leapfrog] Hospital Safety Grade,” said Leah Binder, president and CEO of the Leapfrog Group, in a news release. “We salute hospitals for this milestone and encourage them to accelerate their hard work saving patient lives.”
During the past decade, the report noted, hospitals have widely adopted technology and staffing strategies that can protect patients from preventable harm and death. Leapfrog cited a nearly sevenfold increase in the adoption of computerized provider order entry, which can reduce medication errors by more than 40%.
However, federal health officials separately have reported that the pandemic may have eroded some of those gains.
The Leapfrog report also cited a recent study, published in JAMA, that found that the rates of preventable adverse events in hospitalized patients – including adverse drug events, hospital-acquired infections, postprocedure events, and hospital-acquired pressure ulcers and falls – significantly declined between 2010 and 2019.
That study pointed to specific decreases in the rates of adverse events for patients admitted for myocardial infarction, heart failure, pneumonia, and major surgical procedures. There were also significant drops in adverse events for all other conditions, the study found.
Quality improvement efforts targeting those four conditions might have partly accounted for the lower rates of adverse events in patients with the conditions, the study observed. But “similar interventions did not occur for most of the conditions represented in the ‘all other conditions’ group,” it said.
In a 2019 report by the U.S. Agency for Healthcare Research and Quality (AHRQ), the agency noted that from 2000 to 2017, there had been gains in nearly two-thirds of patient-safety measures in acute, post-acute, and ambulatory care. Hospital safety improved on nine metrics and was unchanged on three. For example, from 2014 to 2017, the number of some hospital-acquired conditions, including adverse drug events and C. Diff infections, dropped about 20%.
However, in an article this past February, officials of the Centers for Medicare & Medicaid Services (CMS) said they had observed deterioration on multiple patient-safety metrics since the start of the pandemic. For example, central line infections, which had dropped by 31% in the five years before the COVID-19 outbreak, jumped 28% in the second quarter of 2020, compared with the prior-year period.
Commenting on these developments, the CMS authors said “the fact that the pandemic degraded patient safety so quickly and severely suggests that our health care system lacks a sufficiently resilient safety culture and infrastructure.”
A version of this article first appeared on Medscape.com.
, according to the 10th annual report from nonprofit the Leapfrog Group, a national nonprofit organization focused on health care safety and quality.
For five outcome measures, the safety improvements saved an estimated 16,000 lives during the 10-year period, the report said. These included two “never” events that both declined by approximately 25%: incidents of falls and trauma and incidents of objects unintentionally left in a body after surgery.
There were also decreases in three health care–associated infections, including methicillin-resistant Staphylococcus aureus (MRSA), which decreased by 22%; central line–associated bloodstream infection (CLABSI), which fell by 43%; and Clostridioides difficile infection (C. Diff), which declined by 8%.
The patient safety record of U.S. hospitals improved over the past decade, according to the report.
“Never in history have we seen across-the-board improvement in patient safety until this last decade, coinciding with the history of the [Leapfrog] Hospital Safety Grade,” said Leah Binder, president and CEO of the Leapfrog Group, in a news release. “We salute hospitals for this milestone and encourage them to accelerate their hard work saving patient lives.”
During the past decade, the report noted, hospitals have widely adopted technology and staffing strategies that can protect patients from preventable harm and death. Leapfrog cited a nearly sevenfold increase in the adoption of computerized provider order entry, which can reduce medication errors by more than 40%.
However, federal health officials separately have reported that the pandemic may have eroded some of those gains.
The Leapfrog report also cited a recent study, published in JAMA, that found that the rates of preventable adverse events in hospitalized patients – including adverse drug events, hospital-acquired infections, postprocedure events, and hospital-acquired pressure ulcers and falls – significantly declined between 2010 and 2019.
That study pointed to specific decreases in the rates of adverse events for patients admitted for myocardial infarction, heart failure, pneumonia, and major surgical procedures. There were also significant drops in adverse events for all other conditions, the study found.
Quality improvement efforts targeting those four conditions might have partly accounted for the lower rates of adverse events in patients with the conditions, the study observed. But “similar interventions did not occur for most of the conditions represented in the ‘all other conditions’ group,” it said.
In a 2019 report by the U.S. Agency for Healthcare Research and Quality (AHRQ), the agency noted that from 2000 to 2017, there had been gains in nearly two-thirds of patient-safety measures in acute, post-acute, and ambulatory care. Hospital safety improved on nine metrics and was unchanged on three. For example, from 2014 to 2017, the number of some hospital-acquired conditions, including adverse drug events and C. Diff infections, dropped about 20%.
However, in an article this past February, officials of the Centers for Medicare & Medicaid Services (CMS) said they had observed deterioration on multiple patient-safety metrics since the start of the pandemic. For example, central line infections, which had dropped by 31% in the five years before the COVID-19 outbreak, jumped 28% in the second quarter of 2020, compared with the prior-year period.
Commenting on these developments, the CMS authors said “the fact that the pandemic degraded patient safety so quickly and severely suggests that our health care system lacks a sufficiently resilient safety culture and infrastructure.”
A version of this article first appeared on Medscape.com.
Meet the JCOM Author with Dr. Barkoudah: Neurosurgery Operating Room Efficiency During the COVID-19 Era
Meet the JCOM Author with Dr. Barkoudah: Quality of Life and Population Health in Behavioral Health Care



Surgical management of early pregnancy loss
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
Hysteroscopic management
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).
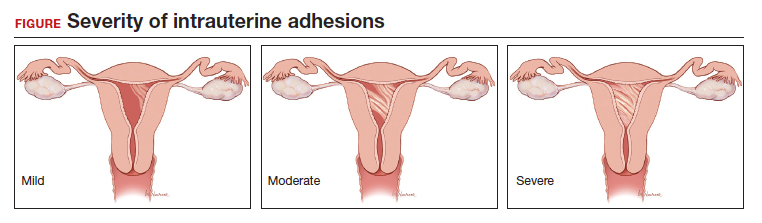
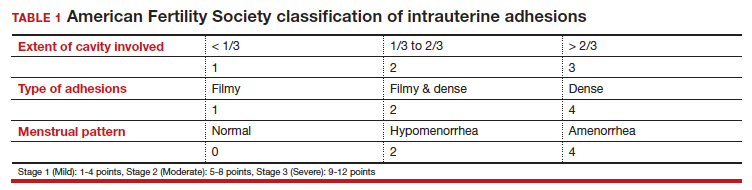
Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.
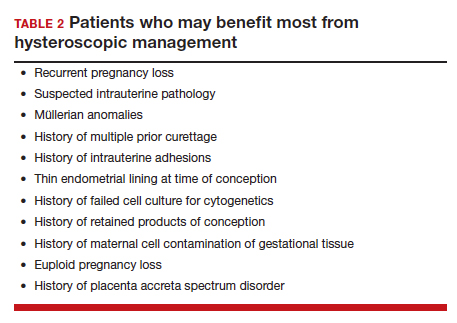
CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
Hysteroscopic management
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).


Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.

CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
CASE Concern for surgical management after repeat miscarriage
A 34-year-old woman (G3P0030) with a history of recurrent pregnancy loss was recently diagnosed with a 7-week missed abortion. After her second miscarriage, she had an evaluation for recurrent pregnancy loss which was unremarkable. Both prior miscarriages were managed with dilation & curettage (D&C), but cytogenetic testing of the tissue did not yield a result in either case. The karyotype from the first pregnancy resulted as 46, XX but was confirmed to be due to maternal cell contamination, and the karyotype from the second pregnancy resulted in cell culture failure. The patient is interested in surgical management for her current missed abortion to help with tissue collection for cytogenetic testing, she but is concerned about her risk of intrauterine adhesions with repeated uterine instrumentation given 2 prior D&Cs, one of which was complicated by retained products of conception.
How do you approach the surgical management of this patient with recurrent pregnancy loss?
Approximately 1 in every 8 recognized pregnancies results in miscarriage. The risk of loss is lowest in women with no history of miscarriage (11%), and increases by about 10% for each additional miscarriage, reaching 42% in women with 3 or more previous losses. The population prevalence of women who have had 1 miscarriage is 11%, 2 miscarriages is 2%, and 3 or more is <1%.1 While 90% of miscarriages occur in the first trimester, their etiology can be quite varied.2 A woman’s age is the most strongly associated risk factor, with both very young (<20 years) and older age (>35 years) groups at highest risk. This association is largely attributed to an age-related increase in embryonic chromosomal aneuploidies, of which trisomies, particularly trisomy 16, are the most common.3 Maternal anatomic anomalies such as leiomyomas, intrauterine adhesions, Müllerian anomalies, and adenomyosis have been linked to an increased risk of miscarriage in addition to several lifestyle and environmental factor exposures.1
Regardless of the etiology, women with recurrent miscarriage are exposed to the potential for iatrogenic harm from the management of their pregnancy loss, including intrauterine adhesions and retained products, which may negatively impact future reproductive attempts. The management of patients with recurrent miscarriages demands special attention to reduce the risk of iatrogenic harm, maximize diagnostic evaluation of the products of conception, and improve future reproductive outcomes.
Management strategies
First trimester pregnancy loss may be managed expectantly, medically, or surgically. Approximately 76% of women who opt for expectant management will successfully pass pregnancy tissue, but for 1 out of every 6 women it may take longer than 14 days.4 For patients who prefer to expedite this process, medication abortion is a highly effective and safe option. According to Schreiber and colleagues, a combination of mifepristone and misoprostol together resulted in expulsion in approximately 91% of 148 patients, although 9% still required surgical intervention for incomplete passage of tissue.5 Both expectant management and medical management strategies are associated with the potential for retained products of conception requiring subsequent instrumentation as well as tissue that is often unsuitable or contaminated for cytogenetic analysis.
The most definitive treatment option is surgical management via manual or electric vacuum aspiration or curettage, with efficacy approaching 99.6% in some series.6 While highly effective, even ultrasound-guided evacuation carries with it procedure-related risks that are of particular consequence for patients of reproductive age, including adhesion formation and retained products of conception.
In 1997, Goldenberg and colleagues reported on the use of hysteroscopy for the management of retained products of conception as a strategy to minimize trauma to the uterus and maximize excision of retained tissue, both of which reduce potential for adhesion formation.7 Based on these data, several groups have extended the use of hysteroscopic resection for retained tissue to upfront evacuation following pregnancy loss, in lieu of D&C.8,9 This approach allows for the direct visualization of the focal removal of the implanted pregnancy tissue, which can:
- decrease the risk of intrauterine adhesion formation
- decrease the risk of retained products of conception
- allow for directed tissue sampling to improve the accuracy of cytogenetic testing
- allow for detection of embryo anatomic anomalies that often go undetected on traditional cytogenetic analysis.
For the remainder of this article, we will discuss the advantages of hysteroscopic management of a missed abortion in greater detail.
Continue to: Hysteroscopic management...
Hysteroscopic management
Like aspiration or curettage, hysteroscopic management may be offered once the diagnosis of fetal demise is confirmed on ultrasonography. The procedure may be accomplished in the office setting or in the operative room with either morcellation or resectoscopic instruments. Morcellation allows for improved visibility during the procedure given the ability of continuous suction to manage tissue fragments in the surgical field, while resectoscopic instruments offer the added benefit of electrosurgery should bleeding that is unresponsive to increased distention pressure be encountered. Use of the cold loop of the resectoscope to accomplish evacuation is advocated to avoid the thermal damage to the endometrium with electrosurgery. Regardless of the chosen instrument, there are several potential benefits for a hysteroscopic approach over the traditional ultrasound-guided or blind D&C.
Reducing risk of iatrogenic harm
Intrauterine adhesions form secondary to trauma to the endometrial basalis layer, where a population of adult progenitor stem cells continuously work to regenerate the overlying functionalis layer. Once damaged, adhesions may form and range from thin, filmy adhesions to dense, cavity obliterating bands of scar tissue (FIGURE). The degree of severity and location of the adhesions account for the variable presentation that range from menstrual abnormalities to infertility and recurrent pregnancy loss. While several classification systems exist for scoring severity of adhesions, the American Fertility Society (now American Society for Reproductive Medicine) Classification system from 1988 is still commonly utilized (TABLE 1).


Intrauterine adhesions from D&C after pregnancy loss are not uncommon. A 2014 meta-analysis of 10 prospective studies including 912 women reported a pooled prevalence for intrauterine adhesions of 19.1% (95% confidence interval [CI], 12.8–27.5) on hysteroscopic evaluation within 12 months following curettage.10 Once formed, these adhesions are associated with long-term impairment in reproductive outcomes, regardless of if they were treated or not. In a long-term follow-up study of women with and without adhesions after recurrent D&C for miscarriage, women with treated adhesions reported lower live birth rates, longer time to pregnancy, higher rates of preterm birth and higher rates of peripartum complications compared with those without adhesions.11
Compared with curettage, hysteroscopy affords the surgeon complete visualization of the uterine cavity and tissue to be resected. This, in turn, minimizes trauma to the surrounding uterine cavity, minimizes the potential for post-procedural adhesion formation and their associated sequelae, and maximizes complete resection of tissue. Those treated with D&C appear to be significantly more likely to have adhesions than those treated via a hysteroscopic approach (30% vs 13%).12
Retained products of conception. Classically, a “gritty” sensation of the endometrium following evacuation of the uterus with a sharp curette has been used to indicate complete removal of tissue. The evolution from a nonvisualized procedure to ultrasound-guided vacuum aspiration of 1st trimester pregnancy tissue has been associated with a decreased risk of procedural complications and retained products of conception.13 However, even with intraoperative imaging, the risk of retained products of conception remains because it can be difficult to distinguish a small blood clot from retained pregnancy tissue on ultrasonography.
Retained pregnancy tissue can result in abnormal or heavy bleeding, require additional medical or surgical intervention, and is associated with endometrial inflammation and infection. Approximately 1 in every 4 women undergoing hysteroscopic resection of retained products are found to have evidence of endometritis in the resected tissue.14 This number is even higher in women with a diagnosis of recurrent pregnancy loss (62%).15
These complications from retained products of conception can be avoided with the hysteroscopic approach due to the direct visualization of the tissue removal. This benefit may be particularly beneficial in patients with known abnormal uterine cavities, such as those with Müllerian anomalies, uterine leiomyomas, preexisting adhesions, and history of placenta accreta spectrum disorder.
Continue to: Maximizing diagnostic yield...
Maximizing diagnostic yield
Many patients prefer surgical management of a missed abortion not for the procedural advantages, but to assist with tissue collection for cytogenetic testing of the pregnancy tissue. Given that embryonic chromosomal aneuploidy is implicated in 70% of miscarriages prior to 20 weeks’ gestation, genetic evaluation of the products of conception is commonly performed to identify a potential cause for the miscarriage.16 G-band karyotype is the most commonly performed genetic evaluation. Karyotype requires culturing of pregnancy tissue for 7-14 days to produce metaphase cells that are then chemically treated to arrest them at their maximally contracted stage. Cytogenetic evaluation is often curtailed when nonviable cells from products of conception fail to culture due to either time elapsed from diagnosis to demise or damage from tissue handling. Careful, directly observed tissue handling via a hysteroscopic approach may alleviate culture failure secondary to tissue damage.
Another concern with cultures of products of conception is the potential for maternal cell contamination. Early studies from the 1970s noted a significant skew toward 46, XX karyotype results in miscarried tissue as compared with 46, XY results. It was not until microsatellite analysis technology was available that it was determined that the result was due to analysis of maternal cells instead of products of conception.17 A 2014 study by Levy and colleagues and another by Lathi and colleagues that utilized single-nucleotide polymorphism (SNP) microarray found that maternal cell contamination affected 22% of all miscarriage samples analyzed and over half of karyotypes with a 46, XX result.18,19
Traditional “blind” suction and curettage may inadvertently collect maternal endometrial tissue and contaminate the culture of fetal cells, limiting the validity of karyotype for products of conception.20 The hysteroscopic approach may provide a higher diagnostic yield for karyotype analysis of fetal tissue by the nature of targeted tissue sampling under direct visualization, minimizing maternal cell contamination. One retrospective study by Cholkeri-Singh and colleagues evaluated rates of fetal chromosome detection without maternal contamination in a total of 264 patients undergoing either suction curettage or hysteroscopic resection. They found that fetal chromosomal detection without contamination was significantly higher in the hysteroscopy group compared with the suction curettage group (88.5 vs 64.8%, P< .001).21 Additionally, biopsies of tissue under direct visualization may enable the diagnosis of a true placental mosaicism and the study of the individual karyotype of each embryo in dizygotic twin missed abortions.
Finally, a hysteroscopic approach may afford the opportunity to also perform morphologic evaluation of the intact early fetus furthering the diagnostic utility of the procedure. With hysteroscopy, the gestational sac is identified and carefully entered, allowing for complete visualization of the early fetus and assessment of anatomic malformations that may provide insight into the pregnancy loss (ie, embryoscopy). In one series of 272 patients with missed abortions, while nearly 75% of conceptuses had abnormal karyotypes, 18% were found to have gross morphologic defects with a normal karyotype.22
Bottom line
When faced with a patient with an early pregnancy loss, physicians should consider the decreased iatrogenic risks and improved diagnostic yield when deciding between D&C versus hysteroscopy for surgical management. There are certain patients with pre-existing risk factors that may stand to benefit the most (TABLE 2). Much like the opening case, those at risk for intrauterine adhesions, retained products of conception, or in whom a successful and accurate cytogenetic analysis is essential are the most likely to benefit from a hysteroscopic approach. The hysteroscopic approach also affords concurrent diagnosis and treatment of intrauterine pathology, such as leiomyomas and uterine septum, which are encountered approximately 12.5% of the time after one miscarriage and 29.4% of the time in patients with a history of more than one miscarriage.10 In the appropriately counseled patient and clinical setting, clinicians could also perform definitive surgical management during the same hysteroscopy. Finally, evaluation of the morphology of the demised fetus may provide additional information for patient counseling in those with euploid pregnancy losses.

CASE Resolved
Ultimately, our patient underwent complete hysteroscopic resection of the pregnancy tissue, which confirmed both a morphologically abnormal fetus and a 45, X karyotype of the products of conception. ●
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.
- Quenby S, Gallos ID, Dhillon-Smith RK, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658-1667.
- Kolte AM, Westergaard D, Lidegaard Ø, et al. Chance of live birth: a nationwide, registry-based cohort study. Hum Reprod Oxf Engl. 2021;36:1065-1073.
- Magnus MC, Wilcox AJ, Morken N-H, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Ireland LD, Gatter M, Chen AY. Medical compared with surgical abortion for effective pregnancy termination in the first trimester. Obstet Gynecol. 2015;126:22-28.
- Goldenberg M, Schiff E, Achiron R, et al. Managing residual trophoblastic tissue. Hysteroscopy for directing curettage. J Reprod Med. 1997;42:26-28.
- Weinberg S, Pansky M, Burshtein I, et al. A pilot study of guided conservative hysteroscopic evacuation of early miscarriage. J Minim Invasive Gynecol. 2021;28:1860-1867.
- Young S, Miller CE. Hysteroscopic resection for management of early pregnancy loss: a case report and literature review. FS Rep. 2022;3:163-167.
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20:262-278.
- Hooker AB, de Leeuw RA, Twisk JWR, et al. Reproductive performance of women with and without intrauterine adhesions following recurrent dilatation and curettage for miscarriage: long-term follow-up of a randomized controlled trial. Hum Reprod. 2021;36:70-81.
- Hooker AB, Aydin H, Brölmann HAM, et al. Longterm complications and reproductive outcome after the management of retained products of conception: a systematic review. Fertil Steril. 2016;105:156-164.e1-e2.
- Debby A, Malinger G, Harow E, et al. Transvaginal ultrasound after first-trimester uterine evacuation reduces the incidence of retained products of conception. Ultrasound Obstet Gynecol. 2006;27:61-64.
- Elder S, Bortoletto P, Romanski PA, et al. Chronic endometritis in women with suspected retained products of conception and their reproductive outcomes. Am J Reprod Immunol N Y N 1989. 2021;86:e13410.
- McQueen DB, Maniar KP, Hutchinson A, et al. Retained pregnancy tissue after miscarriage is associated with high rate of chronic endometritis. J Obstet Gynaecol J Inst Obstet Gynaecol. 2022;1-5.
- Soler A, Morales C, Mademont-Soler I, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152:81-89.
- Jarrett KL, Michaelis RC, Phelan MC, et al. Microsatellite analysis reveals a high incidence of maternal cell contamination in 46, XX products of conception consisting of villi or a combination of villi and membranous material. Am J Obstet Gynecol. 2001;185:198-203.
- Levy B, Sigurjonsson S, Pettersen B, et al. Genomic imbalance in products of conception: single-nucleotide polymorphism chromosomal microarray analysis. Obstet Gynecol. 2014;124:202-209.
- Lathi RB, Gustin SLF, Keller J, et al. Reliability of 46, XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101:178-182.
- Chung JPW, Li Y, Law TSM, et al. Ultrasound-guided manual vacuum aspiration is an optimal method for obtaining products of conception from early pregnancy loss for cytogenetic testing. Int J Biochem Cell Biol. 2022;147:106226.
- Cholkeri-Singh A, Zamfirova I, Miller CE. Increased fetal chromosome detection with the use of operative hysteroscopy during evacuation of products of conception for diagnosed miscarriage. J Minim Invasive Gynecol. 2020;27:160-165.
- Philipp T, Philipp K, Reiner A, et al. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003;18:1724-1732.



