User login
Contemporary psychiatry: A SWOT analysis
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at [email protected].
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at [email protected].
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at [email protected].
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
From debate to stalemate and hate: An epidemic of intellectual constipation
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
Positive psychotherapy: Core principles
In a time of great national and global upheaval, increasing social problems, migration, climate crisis, globalization, and increasingly multicultural societies, our patients and their needs are unique, diverse, and changing. We need a new understanding of mental health to be able to adequately meet the demands of an ever-changing world. Treatment exclusively with psychotropic medications or years of psychoanalysis will not meet these needs.
Psychiatrists and psychotherapists feel (and actually have) a social responsibility, particularly in a multifaceted global society. Psychotherapeutic interventions may contribute to a more peaceful society1 by reducing individuals’ inner stress, solving (unconscious) conflicts, and conveying a humanistic worldview. As an integrative and transcultural method, positive psychotherapy has been applied for more than 45 years in more than 60 countries and is an active force within a “positive mental health movement.”2
The term “positive psychotherapy” describes 2 different approaches3: positive psychotherapy (1977) by Nossrat Peseschkian,4 which is a humanistic psychodynamic approach, and positive psychotherapy (2006) by Martin E.P. Seligman, Tayyab Rashid, and Acacia C. Parks,5 which is a more cognitive-behavioral therapy (CBT)–based approach. This article focuses on the first approach.
Why ‘positive’ psychotherapy?
The term “positive” implies that positive psychotherapy focuses on the patient’s possibilities and capacities. Symptoms and disorders are seen as capacities to react to a conflict. The Latin term “positum” or “positivus” is applied in its original meaning—the factual, the given, the actual. Factual and given are not only the disorder, the symptoms, and the problems but also the capacity to become healthy and/or cope with this situation. This positive meaning confronts the patient (and the therapist) with a lesser-known aspect of the illness, but one that is just as important for the understanding and clinical treatment of the affliction: its function, its meaning, and, consequently, its positive aspects.6
Positive psychotherapy is a humanistic psychodynamic psychotherapy approach developed by Nossrat Peseschkian (1933-2010).4,7 Positive psychotherapy has been developed since the 1970s in the clinical setting with neurotic and psychosomatic patients. It integrates approaches of the 4 main modalities of psychotherapy:
- a humanistic view of human beings
- a systemic approach toward culture, work, and environment
- a psychodynamic understanding of disorders
- a practical, goal-oriented approach with some cognitive-behavioral techniques.
The concept of balance
Based on a humanistic view of human beings and the resources every patient possesses, a key concept of positive psychotherapy is the importance of balance in one’s life. The balance model (Figure) is the core of positive psychotherapy and is applied in clinical and nonclinical settings. This model is based on the concept that there are 4 main areas of life in which a human being lives and functions. These areas influence one’s satisfaction in life, one’s feelings of self-worth, and the way one deals with conflicts and challenges. Although all 4 capacities are latent in every human being, depending on one`s education, environment, and zeitgeist, some will be more developed than others. Our life energies, activities, and reactions belong to these 4 areas of life:
- physical: eating, tenderness, sexuality, sleep, relaxation, sports, appearance, clothing
- achievement: work, job, career, money
- relationships: partner, family, friends, acquaintances and strangers, community life
- meaning and future: existential questions, spirituality, religious practices, future plans, fantasy.
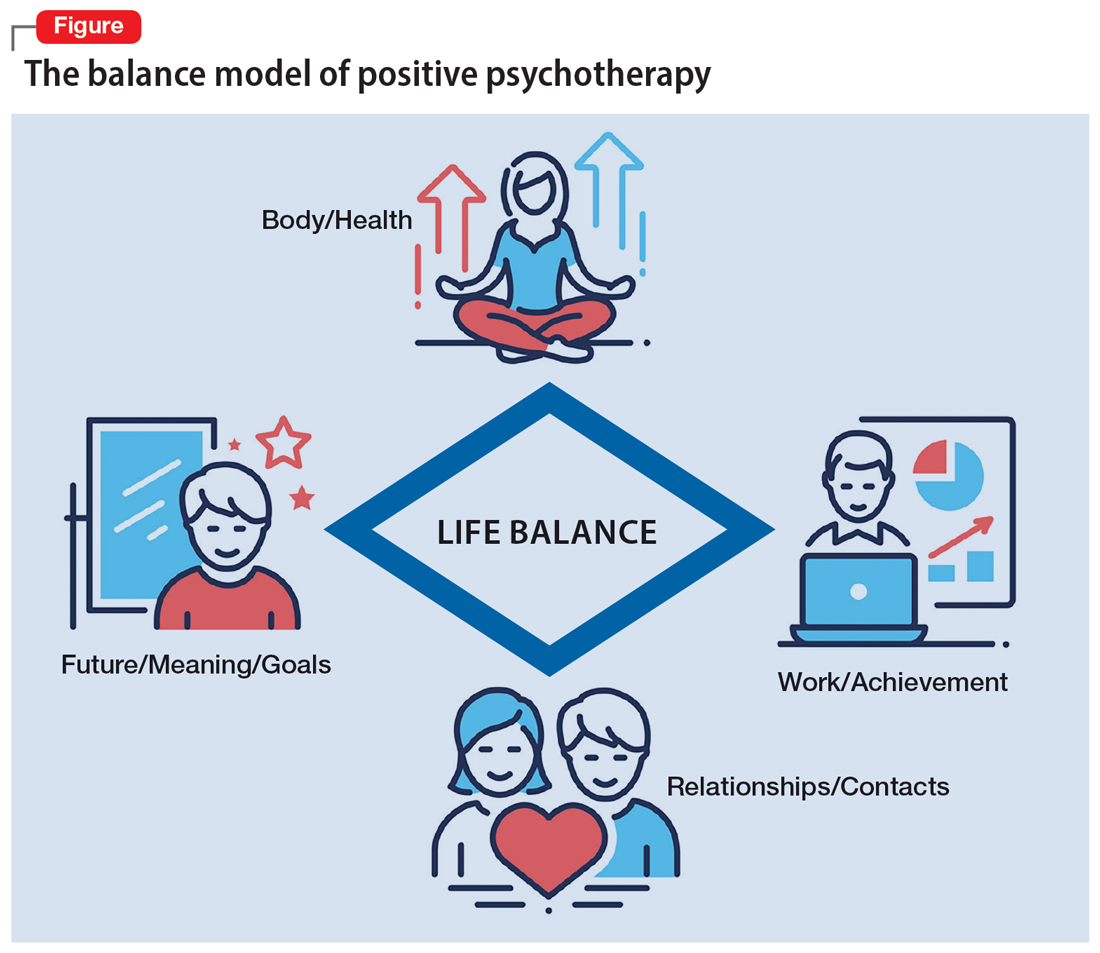
A goal of treatment is to help the patient recognize their own resources and mobilize them with the goal of bringing them into a dynamic equilibrium. This goal places value on a balanced distribution of energy (25% to each area), not of time. According to positive psychotherapy, a person does not become ill because one sphere of life is overemphasized but because of the areas that have been neglected. In the case vignette described in the Box, the problem is not the patient’s work but that his physical health, family and friends, and existential questions are being neglected. That the therapist is not critical from the start of treatment is a constructive experience for the patient and is important and fruitful for building the relationship between the therapist and the patient. Instead of emphasizing the deficits or the disorders, the patient and his family hear that he has neglected other areas of life and not developed them yet.
Box
Mr. M, a 52-year-old manager, is “sent” by his wife to see a psychotherapist. “My wife says I am married to my job, and I should spend more time with her and the children. I understand this, but I love my job. It is no stress for me, but a few minutes at home, and I feel totally stressed out,” he says. During the first interview, the therapist asks Mr. M to draw his energy distribution in the balance model (Figure), and it becomes clear he spends more than 80% of his time and energy on his job.
That is not such a surprise for him. But after some explanation, the therapist tells him that he should continue to do so and that it is an ability to be able to spend so much time every day for his job. Mr. M says, “You are the first person to tell me that it is good that I am working so much. I expected you, like all the others, to tell me I must reduce my working hours immediately, go on vacation, etc.”
Continue to: The balance model...
The balance model also embodies the 4 potential sources of self-esteem. Usually, only 1 or 2 areas provide self-esteem, but in the therapeutic process a patient can learn to uncover the neglected areas so that their self-esteem will have additional pillars of support. By emphasizing how therapy can help to develop one’s self-esteem, many patients can be motivated for the therapeutic process. The balance model, with its concept of devoting 25% of one’s energy to each sphere of life, gives the patient a clear vision about their life and how they can be healthy over the long run by avoiding one-sidedness.8
The transcultural approach
In positive psychotherapy, the term “transcultural” (or cross-cultural) means not only consideration of cultural factors when the therapist and patient come from diverse cultural backgrounds (intercultural psychotherapy or “migrant psychotherapy”) but specifically the consideration of cultural factors in every therapeutic relationship, as a therapeutic attitude and consequently as a sociopolitical dimension of our thinking and behavior. This consideration of the uniqueness of each person, of the relativity of human behavior, and of “unity in diversity” is an essential reason positive psychotherapy is not a “Western” method in the sense of “psychological colonization.”9 Rather, this approach is a culture-sensitive method that can be modified to adapt to particular cultures and life situations.
Transcultural positive psychotherapy begins with answering 2 questions: “How are people different?” and “What do all people have in common?”4 During the therapeutic process, the therapist gives examples from other cultures to the patient to help them relativize their own perspective and broaden their repertoire of behavior.
The use of stories, tales, proverbs, and anecdotes
A special technique of positive psychotherapy is the therapeutic use of stories, tales, proverbs, and anecdotes.10 Often stories from other cultures are used because they offer another perspective when the patient sees none. This has been shown to be highly effective in psychiatric settings, especially in group settings. Psychiatric patients can often easily relate to the images created by stories. In psychiatry and psychotherapy, stories can be a means of changing a patient’s point of view. Such narratives can free up the listener’s feelings and thoughts and often lead to “Aha!” moments. The mirror function of storytelling leads to identification. In the narratives, the reader or listener recognizes themself as well as their needs and situation. They can reflect on the stories without personally becoming the focus of these reflections and remember their own experiences. Stories present solutions that can be models against which one’s own approach can be compared but that also leave room for broader interpretation. Storytelling is particularly useful in bringing about change in patients who are holding fast to old and outworn ideas.
The positive interpretation of disorders
Positive psychotherapy is based on a humanistic view that every human being is good by nature and endowed with unique capacities.11 This positive perspective leads not only to a new quality of relationship between the therapist and patient but also to a new perspective on disorders (Table). Thus, disorders can be “interpreted” in a positive way6: What does the patient unconsciously want to express with their symptoms? What is the function of their disorder? The positive process brings with it a change in perspective to all those concerned: the patient, their family, and the therapist/physician. In this way, one moves from the symptom (which is the disorder and often already has been very thoroughly examined) to the conflict (and the function of the disorder). The positive interpretations are only offered to the patient (“What do you say to this explanation?” “Can you apply this to your own situation?”).

Continue to: This process also helps us...
This process also helps us focus on the “true” patient, who often is not our patient. The patient who comes to us functions as a symptom carrier and can be seen as the “weakest link” in the family chain. The “real patient” is often sitting at home. The positive interpretation of illnesses confronts the patient with the possible function and psychodynamic meaning of their illness for themself and their social milieu, encouraging the patient (and their family) to see their abilities and not merely the pathological aspects.12
Fields of application of positive psychotherapy
As a method positioned between manualized CBT and process-oriented analytical psychotherapy, positive psychotherapy pursues a semi-structured approach in diagnostics (first interview), treatment, posttherapeutic self-help, and training. Positive psychotherapy is applied for the treatment of mood (affective), neurotic, stress-related, and somatoform disorders; behavioral syndromes; and, to some extent, personality disorders. Positive psychotherapy has been employed successfully side-by-side with classical individual therapy as well as in the settings of couple, family, and group therapy.13
What makes positive psychotherapy attractive for mental health professionals?
- As a method that integrates the 4 main modalities of psychotherapy, it does not engage in the conflicts between different schools but combines effective elements into a single approach.
- As an integrative approach, it adjusts to the patient and not vice versa. It gives the therapist the possibility of focusing more on either the actual problems (supportive approach) or the basic conflict (psychodynamic approach).
- It uses vocabulary and terms that can be understood by patients from all strata of society.
- As a culturally sensitive method, it can be applied to patients from different cultures and does not require cultural adaptation.
- As a psychodynamic method, it does not stop after early life conflicts have become more conscious but helps the patient to apply the gained insights using practical techniques.
- It starts with positive affirmations and encouragement but does not later “forget” the unconscious conflicts that have led to disorders. It is not perceived as superficial.
- As a method originally coming from psychiatry and medical practice, it builds a bridge between a scientific basis and psychotherapeutic insights. It favors the biopsychosocial approach.
Bottom Line
Positive psychotherapy combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. It is based on a resource-oriented view of human beings in which disorders are interpreted as capacities to react in a specific and unique way to life events and circumstances. Positive psychotherapy can be applied in psychiatry and psychotherapy. This short-term method is easily understood by patients from diverse cultures and social backgrounds.
Related Resources
- Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32. https://doi.org/10.1007/978-3-030-33264-8_2
- Tritt K, Loew T, Meyer M, et al. Positive psychotherapy: effectiveness of an interdisciplinary approach. Eur J Psychiatry. 1999;13(4):231-241.
- World Association for Positive and Transcultural Psychotherapy. http://www.positum.org
1. Mackenthun G. Passt Psychotherapie an ‚die Gesellschaft’ an? Dynamische Psychiatrie. 1991;24(5-6):326-333.
2. Jeste DV. Foreword: positive mental health. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:vii-xiii.
3. Dobiała E, Winkler P. ‘Positive psychotherapy’ according to Seligman and ‘positive psychotherapy’ according to Peseschkian: a comparison. Int J Psychother. 2016;20(3):5-17.
4. Peseschkian N. Positive Psychotherapy: Theory and Practice of a New Method. Springer; 1987.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Peseschkian N. Positive Psychosomatics: Clinical Manual of Positive Psychotherapy. AuthorHouse; 2016.
7. Peseschkian N. Positive psychotherapy. In: Pritz A, ed. Globalized Psychotherapy. Facultas Universitätsverlag; 2002.
8. Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32.
9. Moghaddam FM, Harre R. But is it science? Traditional and alternative approaches to the study of social behavior. World Psychol. 1995;1(4):47-78.
10. Peseschkian N. Oriental Stories as Techniques in Positive Psychotherapy. AuthorHouse; 2016.
11. Cope TA. Positive psychotherapy’s theory of the capacity to know as explication of unconscious contents. J Relig Health. 2009;48(1):79-89.
12. Huebner G. Health-illness from the perspective of positive psychotherapy. Global Psychother. 2021;1(1):57-61.
13. Sinici E. A ‘balance model’ for patients with post-traumatic stress disorder. Int J Psychother. 2015;19(3):13-19.
In a time of great national and global upheaval, increasing social problems, migration, climate crisis, globalization, and increasingly multicultural societies, our patients and their needs are unique, diverse, and changing. We need a new understanding of mental health to be able to adequately meet the demands of an ever-changing world. Treatment exclusively with psychotropic medications or years of psychoanalysis will not meet these needs.
Psychiatrists and psychotherapists feel (and actually have) a social responsibility, particularly in a multifaceted global society. Psychotherapeutic interventions may contribute to a more peaceful society1 by reducing individuals’ inner stress, solving (unconscious) conflicts, and conveying a humanistic worldview. As an integrative and transcultural method, positive psychotherapy has been applied for more than 45 years in more than 60 countries and is an active force within a “positive mental health movement.”2
The term “positive psychotherapy” describes 2 different approaches3: positive psychotherapy (1977) by Nossrat Peseschkian,4 which is a humanistic psychodynamic approach, and positive psychotherapy (2006) by Martin E.P. Seligman, Tayyab Rashid, and Acacia C. Parks,5 which is a more cognitive-behavioral therapy (CBT)–based approach. This article focuses on the first approach.
Why ‘positive’ psychotherapy?
The term “positive” implies that positive psychotherapy focuses on the patient’s possibilities and capacities. Symptoms and disorders are seen as capacities to react to a conflict. The Latin term “positum” or “positivus” is applied in its original meaning—the factual, the given, the actual. Factual and given are not only the disorder, the symptoms, and the problems but also the capacity to become healthy and/or cope with this situation. This positive meaning confronts the patient (and the therapist) with a lesser-known aspect of the illness, but one that is just as important for the understanding and clinical treatment of the affliction: its function, its meaning, and, consequently, its positive aspects.6
Positive psychotherapy is a humanistic psychodynamic psychotherapy approach developed by Nossrat Peseschkian (1933-2010).4,7 Positive psychotherapy has been developed since the 1970s in the clinical setting with neurotic and psychosomatic patients. It integrates approaches of the 4 main modalities of psychotherapy:
- a humanistic view of human beings
- a systemic approach toward culture, work, and environment
- a psychodynamic understanding of disorders
- a practical, goal-oriented approach with some cognitive-behavioral techniques.
The concept of balance
Based on a humanistic view of human beings and the resources every patient possesses, a key concept of positive psychotherapy is the importance of balance in one’s life. The balance model (Figure) is the core of positive psychotherapy and is applied in clinical and nonclinical settings. This model is based on the concept that there are 4 main areas of life in which a human being lives and functions. These areas influence one’s satisfaction in life, one’s feelings of self-worth, and the way one deals with conflicts and challenges. Although all 4 capacities are latent in every human being, depending on one`s education, environment, and zeitgeist, some will be more developed than others. Our life energies, activities, and reactions belong to these 4 areas of life:
- physical: eating, tenderness, sexuality, sleep, relaxation, sports, appearance, clothing
- achievement: work, job, career, money
- relationships: partner, family, friends, acquaintances and strangers, community life
- meaning and future: existential questions, spirituality, religious practices, future plans, fantasy.

A goal of treatment is to help the patient recognize their own resources and mobilize them with the goal of bringing them into a dynamic equilibrium. This goal places value on a balanced distribution of energy (25% to each area), not of time. According to positive psychotherapy, a person does not become ill because one sphere of life is overemphasized but because of the areas that have been neglected. In the case vignette described in the Box, the problem is not the patient’s work but that his physical health, family and friends, and existential questions are being neglected. That the therapist is not critical from the start of treatment is a constructive experience for the patient and is important and fruitful for building the relationship between the therapist and the patient. Instead of emphasizing the deficits or the disorders, the patient and his family hear that he has neglected other areas of life and not developed them yet.
Box
Mr. M, a 52-year-old manager, is “sent” by his wife to see a psychotherapist. “My wife says I am married to my job, and I should spend more time with her and the children. I understand this, but I love my job. It is no stress for me, but a few minutes at home, and I feel totally stressed out,” he says. During the first interview, the therapist asks Mr. M to draw his energy distribution in the balance model (Figure), and it becomes clear he spends more than 80% of his time and energy on his job.
That is not such a surprise for him. But after some explanation, the therapist tells him that he should continue to do so and that it is an ability to be able to spend so much time every day for his job. Mr. M says, “You are the first person to tell me that it is good that I am working so much. I expected you, like all the others, to tell me I must reduce my working hours immediately, go on vacation, etc.”
Continue to: The balance model...
The balance model also embodies the 4 potential sources of self-esteem. Usually, only 1 or 2 areas provide self-esteem, but in the therapeutic process a patient can learn to uncover the neglected areas so that their self-esteem will have additional pillars of support. By emphasizing how therapy can help to develop one’s self-esteem, many patients can be motivated for the therapeutic process. The balance model, with its concept of devoting 25% of one’s energy to each sphere of life, gives the patient a clear vision about their life and how they can be healthy over the long run by avoiding one-sidedness.8
The transcultural approach
In positive psychotherapy, the term “transcultural” (or cross-cultural) means not only consideration of cultural factors when the therapist and patient come from diverse cultural backgrounds (intercultural psychotherapy or “migrant psychotherapy”) but specifically the consideration of cultural factors in every therapeutic relationship, as a therapeutic attitude and consequently as a sociopolitical dimension of our thinking and behavior. This consideration of the uniqueness of each person, of the relativity of human behavior, and of “unity in diversity” is an essential reason positive psychotherapy is not a “Western” method in the sense of “psychological colonization.”9 Rather, this approach is a culture-sensitive method that can be modified to adapt to particular cultures and life situations.
Transcultural positive psychotherapy begins with answering 2 questions: “How are people different?” and “What do all people have in common?”4 During the therapeutic process, the therapist gives examples from other cultures to the patient to help them relativize their own perspective and broaden their repertoire of behavior.
The use of stories, tales, proverbs, and anecdotes
A special technique of positive psychotherapy is the therapeutic use of stories, tales, proverbs, and anecdotes.10 Often stories from other cultures are used because they offer another perspective when the patient sees none. This has been shown to be highly effective in psychiatric settings, especially in group settings. Psychiatric patients can often easily relate to the images created by stories. In psychiatry and psychotherapy, stories can be a means of changing a patient’s point of view. Such narratives can free up the listener’s feelings and thoughts and often lead to “Aha!” moments. The mirror function of storytelling leads to identification. In the narratives, the reader or listener recognizes themself as well as their needs and situation. They can reflect on the stories without personally becoming the focus of these reflections and remember their own experiences. Stories present solutions that can be models against which one’s own approach can be compared but that also leave room for broader interpretation. Storytelling is particularly useful in bringing about change in patients who are holding fast to old and outworn ideas.
The positive interpretation of disorders
Positive psychotherapy is based on a humanistic view that every human being is good by nature and endowed with unique capacities.11 This positive perspective leads not only to a new quality of relationship between the therapist and patient but also to a new perspective on disorders (Table). Thus, disorders can be “interpreted” in a positive way6: What does the patient unconsciously want to express with their symptoms? What is the function of their disorder? The positive process brings with it a change in perspective to all those concerned: the patient, their family, and the therapist/physician. In this way, one moves from the symptom (which is the disorder and often already has been very thoroughly examined) to the conflict (and the function of the disorder). The positive interpretations are only offered to the patient (“What do you say to this explanation?” “Can you apply this to your own situation?”).

Continue to: This process also helps us...
This process also helps us focus on the “true” patient, who often is not our patient. The patient who comes to us functions as a symptom carrier and can be seen as the “weakest link” in the family chain. The “real patient” is often sitting at home. The positive interpretation of illnesses confronts the patient with the possible function and psychodynamic meaning of their illness for themself and their social milieu, encouraging the patient (and their family) to see their abilities and not merely the pathological aspects.12
Fields of application of positive psychotherapy
As a method positioned between manualized CBT and process-oriented analytical psychotherapy, positive psychotherapy pursues a semi-structured approach in diagnostics (first interview), treatment, posttherapeutic self-help, and training. Positive psychotherapy is applied for the treatment of mood (affective), neurotic, stress-related, and somatoform disorders; behavioral syndromes; and, to some extent, personality disorders. Positive psychotherapy has been employed successfully side-by-side with classical individual therapy as well as in the settings of couple, family, and group therapy.13
What makes positive psychotherapy attractive for mental health professionals?
- As a method that integrates the 4 main modalities of psychotherapy, it does not engage in the conflicts between different schools but combines effective elements into a single approach.
- As an integrative approach, it adjusts to the patient and not vice versa. It gives the therapist the possibility of focusing more on either the actual problems (supportive approach) or the basic conflict (psychodynamic approach).
- It uses vocabulary and terms that can be understood by patients from all strata of society.
- As a culturally sensitive method, it can be applied to patients from different cultures and does not require cultural adaptation.
- As a psychodynamic method, it does not stop after early life conflicts have become more conscious but helps the patient to apply the gained insights using practical techniques.
- It starts with positive affirmations and encouragement but does not later “forget” the unconscious conflicts that have led to disorders. It is not perceived as superficial.
- As a method originally coming from psychiatry and medical practice, it builds a bridge between a scientific basis and psychotherapeutic insights. It favors the biopsychosocial approach.
Bottom Line
Positive psychotherapy combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. It is based on a resource-oriented view of human beings in which disorders are interpreted as capacities to react in a specific and unique way to life events and circumstances. Positive psychotherapy can be applied in psychiatry and psychotherapy. This short-term method is easily understood by patients from diverse cultures and social backgrounds.
Related Resources
- Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32. https://doi.org/10.1007/978-3-030-33264-8_2
- Tritt K, Loew T, Meyer M, et al. Positive psychotherapy: effectiveness of an interdisciplinary approach. Eur J Psychiatry. 1999;13(4):231-241.
- World Association for Positive and Transcultural Psychotherapy. http://www.positum.org
In a time of great national and global upheaval, increasing social problems, migration, climate crisis, globalization, and increasingly multicultural societies, our patients and their needs are unique, diverse, and changing. We need a new understanding of mental health to be able to adequately meet the demands of an ever-changing world. Treatment exclusively with psychotropic medications or years of psychoanalysis will not meet these needs.
Psychiatrists and psychotherapists feel (and actually have) a social responsibility, particularly in a multifaceted global society. Psychotherapeutic interventions may contribute to a more peaceful society1 by reducing individuals’ inner stress, solving (unconscious) conflicts, and conveying a humanistic worldview. As an integrative and transcultural method, positive psychotherapy has been applied for more than 45 years in more than 60 countries and is an active force within a “positive mental health movement.”2
The term “positive psychotherapy” describes 2 different approaches3: positive psychotherapy (1977) by Nossrat Peseschkian,4 which is a humanistic psychodynamic approach, and positive psychotherapy (2006) by Martin E.P. Seligman, Tayyab Rashid, and Acacia C. Parks,5 which is a more cognitive-behavioral therapy (CBT)–based approach. This article focuses on the first approach.
Why ‘positive’ psychotherapy?
The term “positive” implies that positive psychotherapy focuses on the patient’s possibilities and capacities. Symptoms and disorders are seen as capacities to react to a conflict. The Latin term “positum” or “positivus” is applied in its original meaning—the factual, the given, the actual. Factual and given are not only the disorder, the symptoms, and the problems but also the capacity to become healthy and/or cope with this situation. This positive meaning confronts the patient (and the therapist) with a lesser-known aspect of the illness, but one that is just as important for the understanding and clinical treatment of the affliction: its function, its meaning, and, consequently, its positive aspects.6
Positive psychotherapy is a humanistic psychodynamic psychotherapy approach developed by Nossrat Peseschkian (1933-2010).4,7 Positive psychotherapy has been developed since the 1970s in the clinical setting with neurotic and psychosomatic patients. It integrates approaches of the 4 main modalities of psychotherapy:
- a humanistic view of human beings
- a systemic approach toward culture, work, and environment
- a psychodynamic understanding of disorders
- a practical, goal-oriented approach with some cognitive-behavioral techniques.
The concept of balance
Based on a humanistic view of human beings and the resources every patient possesses, a key concept of positive psychotherapy is the importance of balance in one’s life. The balance model (Figure) is the core of positive psychotherapy and is applied in clinical and nonclinical settings. This model is based on the concept that there are 4 main areas of life in which a human being lives and functions. These areas influence one’s satisfaction in life, one’s feelings of self-worth, and the way one deals with conflicts and challenges. Although all 4 capacities are latent in every human being, depending on one`s education, environment, and zeitgeist, some will be more developed than others. Our life energies, activities, and reactions belong to these 4 areas of life:
- physical: eating, tenderness, sexuality, sleep, relaxation, sports, appearance, clothing
- achievement: work, job, career, money
- relationships: partner, family, friends, acquaintances and strangers, community life
- meaning and future: existential questions, spirituality, religious practices, future plans, fantasy.

A goal of treatment is to help the patient recognize their own resources and mobilize them with the goal of bringing them into a dynamic equilibrium. This goal places value on a balanced distribution of energy (25% to each area), not of time. According to positive psychotherapy, a person does not become ill because one sphere of life is overemphasized but because of the areas that have been neglected. In the case vignette described in the Box, the problem is not the patient’s work but that his physical health, family and friends, and existential questions are being neglected. That the therapist is not critical from the start of treatment is a constructive experience for the patient and is important and fruitful for building the relationship between the therapist and the patient. Instead of emphasizing the deficits or the disorders, the patient and his family hear that he has neglected other areas of life and not developed them yet.
Box
Mr. M, a 52-year-old manager, is “sent” by his wife to see a psychotherapist. “My wife says I am married to my job, and I should spend more time with her and the children. I understand this, but I love my job. It is no stress for me, but a few minutes at home, and I feel totally stressed out,” he says. During the first interview, the therapist asks Mr. M to draw his energy distribution in the balance model (Figure), and it becomes clear he spends more than 80% of his time and energy on his job.
That is not such a surprise for him. But after some explanation, the therapist tells him that he should continue to do so and that it is an ability to be able to spend so much time every day for his job. Mr. M says, “You are the first person to tell me that it is good that I am working so much. I expected you, like all the others, to tell me I must reduce my working hours immediately, go on vacation, etc.”
Continue to: The balance model...
The balance model also embodies the 4 potential sources of self-esteem. Usually, only 1 or 2 areas provide self-esteem, but in the therapeutic process a patient can learn to uncover the neglected areas so that their self-esteem will have additional pillars of support. By emphasizing how therapy can help to develop one’s self-esteem, many patients can be motivated for the therapeutic process. The balance model, with its concept of devoting 25% of one’s energy to each sphere of life, gives the patient a clear vision about their life and how they can be healthy over the long run by avoiding one-sidedness.8
The transcultural approach
In positive psychotherapy, the term “transcultural” (or cross-cultural) means not only consideration of cultural factors when the therapist and patient come from diverse cultural backgrounds (intercultural psychotherapy or “migrant psychotherapy”) but specifically the consideration of cultural factors in every therapeutic relationship, as a therapeutic attitude and consequently as a sociopolitical dimension of our thinking and behavior. This consideration of the uniqueness of each person, of the relativity of human behavior, and of “unity in diversity” is an essential reason positive psychotherapy is not a “Western” method in the sense of “psychological colonization.”9 Rather, this approach is a culture-sensitive method that can be modified to adapt to particular cultures and life situations.
Transcultural positive psychotherapy begins with answering 2 questions: “How are people different?” and “What do all people have in common?”4 During the therapeutic process, the therapist gives examples from other cultures to the patient to help them relativize their own perspective and broaden their repertoire of behavior.
The use of stories, tales, proverbs, and anecdotes
A special technique of positive psychotherapy is the therapeutic use of stories, tales, proverbs, and anecdotes.10 Often stories from other cultures are used because they offer another perspective when the patient sees none. This has been shown to be highly effective in psychiatric settings, especially in group settings. Psychiatric patients can often easily relate to the images created by stories. In psychiatry and psychotherapy, stories can be a means of changing a patient’s point of view. Such narratives can free up the listener’s feelings and thoughts and often lead to “Aha!” moments. The mirror function of storytelling leads to identification. In the narratives, the reader or listener recognizes themself as well as their needs and situation. They can reflect on the stories without personally becoming the focus of these reflections and remember their own experiences. Stories present solutions that can be models against which one’s own approach can be compared but that also leave room for broader interpretation. Storytelling is particularly useful in bringing about change in patients who are holding fast to old and outworn ideas.
The positive interpretation of disorders
Positive psychotherapy is based on a humanistic view that every human being is good by nature and endowed with unique capacities.11 This positive perspective leads not only to a new quality of relationship between the therapist and patient but also to a new perspective on disorders (Table). Thus, disorders can be “interpreted” in a positive way6: What does the patient unconsciously want to express with their symptoms? What is the function of their disorder? The positive process brings with it a change in perspective to all those concerned: the patient, their family, and the therapist/physician. In this way, one moves from the symptom (which is the disorder and often already has been very thoroughly examined) to the conflict (and the function of the disorder). The positive interpretations are only offered to the patient (“What do you say to this explanation?” “Can you apply this to your own situation?”).

Continue to: This process also helps us...
This process also helps us focus on the “true” patient, who often is not our patient. The patient who comes to us functions as a symptom carrier and can be seen as the “weakest link” in the family chain. The “real patient” is often sitting at home. The positive interpretation of illnesses confronts the patient with the possible function and psychodynamic meaning of their illness for themself and their social milieu, encouraging the patient (and their family) to see their abilities and not merely the pathological aspects.12
Fields of application of positive psychotherapy
As a method positioned between manualized CBT and process-oriented analytical psychotherapy, positive psychotherapy pursues a semi-structured approach in diagnostics (first interview), treatment, posttherapeutic self-help, and training. Positive psychotherapy is applied for the treatment of mood (affective), neurotic, stress-related, and somatoform disorders; behavioral syndromes; and, to some extent, personality disorders. Positive psychotherapy has been employed successfully side-by-side with classical individual therapy as well as in the settings of couple, family, and group therapy.13
What makes positive psychotherapy attractive for mental health professionals?
- As a method that integrates the 4 main modalities of psychotherapy, it does not engage in the conflicts between different schools but combines effective elements into a single approach.
- As an integrative approach, it adjusts to the patient and not vice versa. It gives the therapist the possibility of focusing more on either the actual problems (supportive approach) or the basic conflict (psychodynamic approach).
- It uses vocabulary and terms that can be understood by patients from all strata of society.
- As a culturally sensitive method, it can be applied to patients from different cultures and does not require cultural adaptation.
- As a psychodynamic method, it does not stop after early life conflicts have become more conscious but helps the patient to apply the gained insights using practical techniques.
- It starts with positive affirmations and encouragement but does not later “forget” the unconscious conflicts that have led to disorders. It is not perceived as superficial.
- As a method originally coming from psychiatry and medical practice, it builds a bridge between a scientific basis and psychotherapeutic insights. It favors the biopsychosocial approach.
Bottom Line
Positive psychotherapy combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. It is based on a resource-oriented view of human beings in which disorders are interpreted as capacities to react in a specific and unique way to life events and circumstances. Positive psychotherapy can be applied in psychiatry and psychotherapy. This short-term method is easily understood by patients from diverse cultures and social backgrounds.
Related Resources
- Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32. https://doi.org/10.1007/978-3-030-33264-8_2
- Tritt K, Loew T, Meyer M, et al. Positive psychotherapy: effectiveness of an interdisciplinary approach. Eur J Psychiatry. 1999;13(4):231-241.
- World Association for Positive and Transcultural Psychotherapy. http://www.positum.org
1. Mackenthun G. Passt Psychotherapie an ‚die Gesellschaft’ an? Dynamische Psychiatrie. 1991;24(5-6):326-333.
2. Jeste DV. Foreword: positive mental health. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:vii-xiii.
3. Dobiała E, Winkler P. ‘Positive psychotherapy’ according to Seligman and ‘positive psychotherapy’ according to Peseschkian: a comparison. Int J Psychother. 2016;20(3):5-17.
4. Peseschkian N. Positive Psychotherapy: Theory and Practice of a New Method. Springer; 1987.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Peseschkian N. Positive Psychosomatics: Clinical Manual of Positive Psychotherapy. AuthorHouse; 2016.
7. Peseschkian N. Positive psychotherapy. In: Pritz A, ed. Globalized Psychotherapy. Facultas Universitätsverlag; 2002.
8. Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32.
9. Moghaddam FM, Harre R. But is it science? Traditional and alternative approaches to the study of social behavior. World Psychol. 1995;1(4):47-78.
10. Peseschkian N. Oriental Stories as Techniques in Positive Psychotherapy. AuthorHouse; 2016.
11. Cope TA. Positive psychotherapy’s theory of the capacity to know as explication of unconscious contents. J Relig Health. 2009;48(1):79-89.
12. Huebner G. Health-illness from the perspective of positive psychotherapy. Global Psychother. 2021;1(1):57-61.
13. Sinici E. A ‘balance model’ for patients with post-traumatic stress disorder. Int J Psychother. 2015;19(3):13-19.
1. Mackenthun G. Passt Psychotherapie an ‚die Gesellschaft’ an? Dynamische Psychiatrie. 1991;24(5-6):326-333.
2. Jeste DV. Foreword: positive mental health. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:vii-xiii.
3. Dobiała E, Winkler P. ‘Positive psychotherapy’ according to Seligman and ‘positive psychotherapy’ according to Peseschkian: a comparison. Int J Psychother. 2016;20(3):5-17.
4. Peseschkian N. Positive Psychotherapy: Theory and Practice of a New Method. Springer; 1987.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Peseschkian N. Positive Psychosomatics: Clinical Manual of Positive Psychotherapy. AuthorHouse; 2016.
7. Peseschkian N. Positive psychotherapy. In: Pritz A, ed. Globalized Psychotherapy. Facultas Universitätsverlag; 2002.
8. Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32.
9. Moghaddam FM, Harre R. But is it science? Traditional and alternative approaches to the study of social behavior. World Psychol. 1995;1(4):47-78.
10. Peseschkian N. Oriental Stories as Techniques in Positive Psychotherapy. AuthorHouse; 2016.
11. Cope TA. Positive psychotherapy’s theory of the capacity to know as explication of unconscious contents. J Relig Health. 2009;48(1):79-89.
12. Huebner G. Health-illness from the perspective of positive psychotherapy. Global Psychother. 2021;1(1):57-61.
13. Sinici E. A ‘balance model’ for patients with post-traumatic stress disorder. Int J Psychother. 2015;19(3):13-19.
Problematic alcohol use on the rise among physicians?
However, good data on exactly how common this is and on salient risk factors are lacking.
In a systematic literature review, investigators found the prevalence of self-reported problematic alcohol use varied widely, but could affect up to one third of physicians.
However, all studies were survey-based and self-reported, and definitions of problematic alcohol use were mixed, with inconsistent reporting on differences across sex, age, physician specialty, and career stage.
“Key epidemiologic information of the prevalence of problematic alcohol use in physicians and associated risk factors are unknown, hampering the ability to identify high-risk individuals for targeted interventions,” Manish Sood, MD, University of Ottawa, and colleagues wrote.
The findings were published online in JAMA Network Open.
Serious concern
The researchers noted that physicians are at a higher risk for burnout and mental health conditions, including depression and anxiety, than the general population, which could contribute to problematic drinking.
Problematic drinking among physicians poses a “serious concern” to their health and ability to provide care, the investigators wrote. Understanding the extent and characteristics of the issue is important to guide interventions.
To better characterize problematic drinking among physicians, the investigators reviewed 31 studies from 2006 to 2020 involving 51,680 residents, fellows, or staff physicians in 17 countries.
In the studies, problematic alcohol use was measured by a validated tool: the Alcohol Use Disorders Identification Test, AUDIT Version C (AUDIT-C), or the Cut down, Annoyed, Guilty, and Eye-opener (CAGE) questionnaire.
“Problematic alcohol use” included hazardous, potentially hazardous, risky, at-risk, harmful, problematic, or heavy drinking or alcohol use, as well as alcohol misuse, alcohol dependence, and alcohol use more than low-risk guidelines and alcohol use disorder.
Results showed problematic alcohol use “varied widely” regardless of measurement method used. The rate was 0%-34% with AUDIT, 9%-35% with AUDIT-C, and 4%-22% with CAGE.
The data also showed an increase in reported problematic alcohol use over time, rising from 16.3% between 2006 and 2010 to 26.8% between 2017 and 2020.
True prevalence unknown
“It remains unknown whether this increase is indeed accurate or whether it is due to increased transparency by physicians in self-reporting problematic alcohol use because of a changing culture of medicine,” the investigators wrote.
The data suggest that problematic alcohol use is more common in male than female physicians; but no firm conclusions can be drawn from the data on how problematic alcohol use varies based on physician age, sex, specialty, and career stage, the researchers noted.
True prevalence of problematic alcohol use among physicians remains unknown – and identifying this type of behavior is difficult, they pointed out.
They added that physicians with problematic use may be “high functioning,” making identifying potential impairment a challenge. Also, societal stigma and fear of reprisal from professional colleges for reporting or seeking care for problematic alcohol use may encourage physicians with alcohol problems to keep their problems hidden.
The researchers noted that future population-based studies with longitudinal designs or using health administrative data could help identify the prevalence of and salient risk factors for problematic alcohol use in physicians.
The study was supported by the Canadian Medical Association. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
However, good data on exactly how common this is and on salient risk factors are lacking.
In a systematic literature review, investigators found the prevalence of self-reported problematic alcohol use varied widely, but could affect up to one third of physicians.
However, all studies were survey-based and self-reported, and definitions of problematic alcohol use were mixed, with inconsistent reporting on differences across sex, age, physician specialty, and career stage.
“Key epidemiologic information of the prevalence of problematic alcohol use in physicians and associated risk factors are unknown, hampering the ability to identify high-risk individuals for targeted interventions,” Manish Sood, MD, University of Ottawa, and colleagues wrote.
The findings were published online in JAMA Network Open.
Serious concern
The researchers noted that physicians are at a higher risk for burnout and mental health conditions, including depression and anxiety, than the general population, which could contribute to problematic drinking.
Problematic drinking among physicians poses a “serious concern” to their health and ability to provide care, the investigators wrote. Understanding the extent and characteristics of the issue is important to guide interventions.
To better characterize problematic drinking among physicians, the investigators reviewed 31 studies from 2006 to 2020 involving 51,680 residents, fellows, or staff physicians in 17 countries.
In the studies, problematic alcohol use was measured by a validated tool: the Alcohol Use Disorders Identification Test, AUDIT Version C (AUDIT-C), or the Cut down, Annoyed, Guilty, and Eye-opener (CAGE) questionnaire.
“Problematic alcohol use” included hazardous, potentially hazardous, risky, at-risk, harmful, problematic, or heavy drinking or alcohol use, as well as alcohol misuse, alcohol dependence, and alcohol use more than low-risk guidelines and alcohol use disorder.
Results showed problematic alcohol use “varied widely” regardless of measurement method used. The rate was 0%-34% with AUDIT, 9%-35% with AUDIT-C, and 4%-22% with CAGE.
The data also showed an increase in reported problematic alcohol use over time, rising from 16.3% between 2006 and 2010 to 26.8% between 2017 and 2020.
True prevalence unknown
“It remains unknown whether this increase is indeed accurate or whether it is due to increased transparency by physicians in self-reporting problematic alcohol use because of a changing culture of medicine,” the investigators wrote.
The data suggest that problematic alcohol use is more common in male than female physicians; but no firm conclusions can be drawn from the data on how problematic alcohol use varies based on physician age, sex, specialty, and career stage, the researchers noted.
True prevalence of problematic alcohol use among physicians remains unknown – and identifying this type of behavior is difficult, they pointed out.
They added that physicians with problematic use may be “high functioning,” making identifying potential impairment a challenge. Also, societal stigma and fear of reprisal from professional colleges for reporting or seeking care for problematic alcohol use may encourage physicians with alcohol problems to keep their problems hidden.
The researchers noted that future population-based studies with longitudinal designs or using health administrative data could help identify the prevalence of and salient risk factors for problematic alcohol use in physicians.
The study was supported by the Canadian Medical Association. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
However, good data on exactly how common this is and on salient risk factors are lacking.
In a systematic literature review, investigators found the prevalence of self-reported problematic alcohol use varied widely, but could affect up to one third of physicians.
However, all studies were survey-based and self-reported, and definitions of problematic alcohol use were mixed, with inconsistent reporting on differences across sex, age, physician specialty, and career stage.
“Key epidemiologic information of the prevalence of problematic alcohol use in physicians and associated risk factors are unknown, hampering the ability to identify high-risk individuals for targeted interventions,” Manish Sood, MD, University of Ottawa, and colleagues wrote.
The findings were published online in JAMA Network Open.
Serious concern
The researchers noted that physicians are at a higher risk for burnout and mental health conditions, including depression and anxiety, than the general population, which could contribute to problematic drinking.
Problematic drinking among physicians poses a “serious concern” to their health and ability to provide care, the investigators wrote. Understanding the extent and characteristics of the issue is important to guide interventions.
To better characterize problematic drinking among physicians, the investigators reviewed 31 studies from 2006 to 2020 involving 51,680 residents, fellows, or staff physicians in 17 countries.
In the studies, problematic alcohol use was measured by a validated tool: the Alcohol Use Disorders Identification Test, AUDIT Version C (AUDIT-C), or the Cut down, Annoyed, Guilty, and Eye-opener (CAGE) questionnaire.
“Problematic alcohol use” included hazardous, potentially hazardous, risky, at-risk, harmful, problematic, or heavy drinking or alcohol use, as well as alcohol misuse, alcohol dependence, and alcohol use more than low-risk guidelines and alcohol use disorder.
Results showed problematic alcohol use “varied widely” regardless of measurement method used. The rate was 0%-34% with AUDIT, 9%-35% with AUDIT-C, and 4%-22% with CAGE.
The data also showed an increase in reported problematic alcohol use over time, rising from 16.3% between 2006 and 2010 to 26.8% between 2017 and 2020.
True prevalence unknown
“It remains unknown whether this increase is indeed accurate or whether it is due to increased transparency by physicians in self-reporting problematic alcohol use because of a changing culture of medicine,” the investigators wrote.
The data suggest that problematic alcohol use is more common in male than female physicians; but no firm conclusions can be drawn from the data on how problematic alcohol use varies based on physician age, sex, specialty, and career stage, the researchers noted.
True prevalence of problematic alcohol use among physicians remains unknown – and identifying this type of behavior is difficult, they pointed out.
They added that physicians with problematic use may be “high functioning,” making identifying potential impairment a challenge. Also, societal stigma and fear of reprisal from professional colleges for reporting or seeking care for problematic alcohol use may encourage physicians with alcohol problems to keep their problems hidden.
The researchers noted that future population-based studies with longitudinal designs or using health administrative data could help identify the prevalence of and salient risk factors for problematic alcohol use in physicians.
The study was supported by the Canadian Medical Association. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Alzheimer’s Association to CMS: Ditch restraints on amyloid drugs
In a letter addressed to CMS administrator Chiquita Brooks-LaSure, MPP, the association has asked the agency to remove the requirements for “coverage with evidence development” in its national coverage determination for FDA-approved anti-amyloid monoclonal antibodies.
The CMS coverage restrictions for anti-amyloid drugs were finalized in April on the basis of data available at the time.
Since then, new data from the CLARITY AD trial “clearly demonstrate a meaningful clinical benefit” from the investigational anti-amyloid agent lecanemab (Eisai/Biogen), Robert Egge, chief public policy officer for the Alzheimer’s Association, told this news organization.
The CLARITY AD results were published in the New England Journal of Medicine. Lecanemab is currently under accelerated review at the FDA.
The Alzheimer’s Association’s letter to the CMS includes a joint statement signed by more than 200 AD researchers and experts. All agree that the lecanemab results represent “significant new evidence” that necessitates reconsidering the restrictions on anti-amyloid agents.
“CMS has said it would look at new evidence, and now that evidence is here. We believe CMS recognizes this evidence for lecanemab is stronger than that for many treatments Medicare routinely covers,” Mr. Egge said.
‘No time to waste’
“With the timing of accelerated approvals for both lecanemab and donanemab in the next few months, the Alzheimer’s Association wants to ensure, if approved, that patients can access these treatments,” Mr. Egge noted.
“Because revisions to National Coverage Determinations can be a lengthy process, CMS needs to act quickly to minimize delays. People living with Alzheimer’s disease don’t have time to waste,” he added.
The Alzheimer’s Association estimates that every day, more than 2,000 individuals aged 65 or older may transition from mild dementia due to AD to a more advanced stage of the disease in which they may no longer be eligible for lecanemab and the other anti-amyloid agents currently being tested.
“Each day matters when it comes to slowing the progression of this disease,” Joanne Pike, DrPH, president and incoming chief executive officer for the Alzheimer’s Association, noted in a news release.
“The current CMS policy to severely limit access to these treatments eliminates people’s options, is resulting in continued irreversible disease progression, and contributes to greater health inequities. That’s not acceptable,” Dr. Pike said.
A version of this article first appeared on Medscape.com.
In a letter addressed to CMS administrator Chiquita Brooks-LaSure, MPP, the association has asked the agency to remove the requirements for “coverage with evidence development” in its national coverage determination for FDA-approved anti-amyloid monoclonal antibodies.
The CMS coverage restrictions for anti-amyloid drugs were finalized in April on the basis of data available at the time.
Since then, new data from the CLARITY AD trial “clearly demonstrate a meaningful clinical benefit” from the investigational anti-amyloid agent lecanemab (Eisai/Biogen), Robert Egge, chief public policy officer for the Alzheimer’s Association, told this news organization.
The CLARITY AD results were published in the New England Journal of Medicine. Lecanemab is currently under accelerated review at the FDA.
The Alzheimer’s Association’s letter to the CMS includes a joint statement signed by more than 200 AD researchers and experts. All agree that the lecanemab results represent “significant new evidence” that necessitates reconsidering the restrictions on anti-amyloid agents.
“CMS has said it would look at new evidence, and now that evidence is here. We believe CMS recognizes this evidence for lecanemab is stronger than that for many treatments Medicare routinely covers,” Mr. Egge said.
‘No time to waste’
“With the timing of accelerated approvals for both lecanemab and donanemab in the next few months, the Alzheimer’s Association wants to ensure, if approved, that patients can access these treatments,” Mr. Egge noted.
“Because revisions to National Coverage Determinations can be a lengthy process, CMS needs to act quickly to minimize delays. People living with Alzheimer’s disease don’t have time to waste,” he added.
The Alzheimer’s Association estimates that every day, more than 2,000 individuals aged 65 or older may transition from mild dementia due to AD to a more advanced stage of the disease in which they may no longer be eligible for lecanemab and the other anti-amyloid agents currently being tested.
“Each day matters when it comes to slowing the progression of this disease,” Joanne Pike, DrPH, president and incoming chief executive officer for the Alzheimer’s Association, noted in a news release.
“The current CMS policy to severely limit access to these treatments eliminates people’s options, is resulting in continued irreversible disease progression, and contributes to greater health inequities. That’s not acceptable,” Dr. Pike said.
A version of this article first appeared on Medscape.com.
In a letter addressed to CMS administrator Chiquita Brooks-LaSure, MPP, the association has asked the agency to remove the requirements for “coverage with evidence development” in its national coverage determination for FDA-approved anti-amyloid monoclonal antibodies.
The CMS coverage restrictions for anti-amyloid drugs were finalized in April on the basis of data available at the time.
Since then, new data from the CLARITY AD trial “clearly demonstrate a meaningful clinical benefit” from the investigational anti-amyloid agent lecanemab (Eisai/Biogen), Robert Egge, chief public policy officer for the Alzheimer’s Association, told this news organization.
The CLARITY AD results were published in the New England Journal of Medicine. Lecanemab is currently under accelerated review at the FDA.
The Alzheimer’s Association’s letter to the CMS includes a joint statement signed by more than 200 AD researchers and experts. All agree that the lecanemab results represent “significant new evidence” that necessitates reconsidering the restrictions on anti-amyloid agents.
“CMS has said it would look at new evidence, and now that evidence is here. We believe CMS recognizes this evidence for lecanemab is stronger than that for many treatments Medicare routinely covers,” Mr. Egge said.
‘No time to waste’
“With the timing of accelerated approvals for both lecanemab and donanemab in the next few months, the Alzheimer’s Association wants to ensure, if approved, that patients can access these treatments,” Mr. Egge noted.
“Because revisions to National Coverage Determinations can be a lengthy process, CMS needs to act quickly to minimize delays. People living with Alzheimer’s disease don’t have time to waste,” he added.
The Alzheimer’s Association estimates that every day, more than 2,000 individuals aged 65 or older may transition from mild dementia due to AD to a more advanced stage of the disease in which they may no longer be eligible for lecanemab and the other anti-amyloid agents currently being tested.
“Each day matters when it comes to slowing the progression of this disease,” Joanne Pike, DrPH, president and incoming chief executive officer for the Alzheimer’s Association, noted in a news release.
“The current CMS policy to severely limit access to these treatments eliminates people’s options, is resulting in continued irreversible disease progression, and contributes to greater health inequities. That’s not acceptable,” Dr. Pike said.
A version of this article first appeared on Medscape.com.
A doctor saves a drowning family in a dangerous river
Is There a Doctor in the House? is a new series telling these stories.
I live on the Maumee River in Ohio, about 50 yards from the water. I had an early quit time and came home to meet my wife for lunch. Afterward, I went up to my barn across the main road to tinker around. It was a nice day out, so my wife had opened some windows. Suddenly, she heard screaming from the river. It did not sound like fun.
She ran down to the river’s edge and saw a dad and three boys struggling in the water. She phoned me screaming: “They’re drowning! They’re drowning!” I jumped in my truck and drove up our driveway through the yard right down to the river.
My wife was on the phone with 911 at that point, and I could see them about 75-100 yards out. The dad had two of the boys clinging around his neck. They were going under the water and coming up and going under again. The other boy was just floating nearby, face down, motionless.
I threw my shoes and scrubs off and started to walk towards the water. My wife screamed at me, “You’re not going in there!” I said, “I’m not going to stand here and watch this. It’s not going to happen.”
I’m not a kid anymore, but I was a high school swimmer, and to this day I work out all the time. I felt like I had to try something. So, I went in the water despite my wife yelling and I swam towards them.
What happens when you get in that deep water is that you panic. You can’t hear anyone because of the rapids, and your instinct is to swim back towards where you went in, which is against the current. Unless you’re a very strong swimmer, you’re just wasting your time, swimming in place.
But these guys weren’t trying to go anywhere. Dad was just trying to stay up and keep the boys alive. He was in about 10 feet of water. What they didn’t see or just didn’t know: About 20 yards upstream from that deep water is a little island.
When I got to them, I yelled at the dad to move towards the island, “Go backwards! Go back!” I flipped the boy over who wasn’t moving. He was the oldest of the three, around 10 or 11 years old. When I turned him over, he was blue and wasn’t breathing. I put my fingers on his neck and didn’t feel a pulse.
So, I’m treading water, holding him. I put an arm behind his back and started doing chest compressions on him. I probably did a dozen to 15 compressions – nothing. I thought, I’ve got to get some air in this kid. So, I gave him two deep breaths and then started doing compressions again. I know ACLS and CPR training would say we don’t do that anymore. But I couldn’t just sit there and give up. Shortly after that, he coughed out a large amount of water and started breathing.
The dad and the other two boys had made it to the island. So, I started moving towards it with the boy. It was a few minutes before he regained consciousness. Of course, he was unaware of what had happened. He started to scream, because here’s this strange man holding him. But he was breathing. That’s all I cared about.
When we got to the island, I saw that my neighbor downstream had launched his canoe. He’s a retired gentleman who lives next to me, a very physically fit man. He started rolling as hard as he could towards us, against the stream. I kind of gave him a thumbs up, like, “we’re safe now. We’re standing.” We loaded the kids and the dad in the canoe and made it back against the stream to the parking lot where they went in.
All this took probably 10 or 15 minutes, and by then the paramedics were there. Life Flight had been dispatched up by my barn where there’s room to land. So, they drove up there in the ambulance. The boy I revived was flown to the hospital. The others went in the ambulance.
I know all the ED docs, so I talked to somebody later who, with permission from the family, said they were all doing fine. They were getting x-rays on the boy’s lungs. And then I heard the dad and two boys were released that night. The other boy I worked on was observed overnight and discharged the following morning.
Four or 5 days later, I heard from their pediatrician, who also had permission to share. He sent me a very nice note through Epic that he had seen the boys. Besides some mental trauma, they were all healthy and doing fine.
The family lives in the area and the kids go to school 5 miles from my house. So, the following weekend they came over. It was Father’s Day, which was kind of cool. They brought me some flowers and candy and a card the boys had drawn to thank me.
I learned that the dad had brought the boys to the fishing site. They were horsing around in knee deep water. One of the boys walked off a little way and didn’t realize there was a drop off. He went in, and of course the dad went after him, and the other two followed.
I said to the parents: “Look, things like this happen for a reason. People like your son are saved and go on in this world because they’ve got special things to do. I can’t wait to see what kind of man he becomes.”
Two or 3 months later, it was football season, and I got at a message from the dad saying their son was playing football on Saturday at the school. He wondered if I could drop by. So, I kind of snuck over and watched, but I didn’t go say hi. There’s trauma there, and I didn’t want them to have to relive that.
I’m very fortunate that I exercise every day and I know how to do CPR and swim. And thank God the boy was floating when I got to him, or I never would’ve found him. The Maumee River is known as the “muddy Maumee.” You can’t see anything under the water.
Depending on the time of year, the river can be almost dry or overflowing into the parking lot with the current rushing hard. If it had been like that, I wouldn’t have considered going in. And they wouldn’t they have been there in the first place. They’d have been a mile downstream.
I took a risk. I could have gone out there and had the dad and two other kids jump on top of me. Then we all would have been in trouble. But like I told my wife, I couldn’t stand there and watch it. I’m just not that person.
I think it was also about being a dad myself and having grandkids now. Doctor or no doctor, I felt like I was in reasonably good shape and I had to go in there to help. This dad was trying his butt off, but three little kids is too many. You can’t do that by yourself. They were not going to make it.
I go to the hospital and I save lives as part of my job, and I don’t even come home and talk about it. But this is a whole different thing. Being able to save someone’s life when put in this situation is very gratifying. It’s a tremendous feeling. There’s a reason that young man is here today, and I’ll be watching for great things from him.
A version of this article first appeared on Medscape.com.
Daniel Cassavar, MD, is a cardiologist with ProMedica in Perrysburg, Ohio.
Is There a Doctor in the House? is a new series telling these stories.
I live on the Maumee River in Ohio, about 50 yards from the water. I had an early quit time and came home to meet my wife for lunch. Afterward, I went up to my barn across the main road to tinker around. It was a nice day out, so my wife had opened some windows. Suddenly, she heard screaming from the river. It did not sound like fun.
She ran down to the river’s edge and saw a dad and three boys struggling in the water. She phoned me screaming: “They’re drowning! They’re drowning!” I jumped in my truck and drove up our driveway through the yard right down to the river.
My wife was on the phone with 911 at that point, and I could see them about 75-100 yards out. The dad had two of the boys clinging around his neck. They were going under the water and coming up and going under again. The other boy was just floating nearby, face down, motionless.
I threw my shoes and scrubs off and started to walk towards the water. My wife screamed at me, “You’re not going in there!” I said, “I’m not going to stand here and watch this. It’s not going to happen.”
I’m not a kid anymore, but I was a high school swimmer, and to this day I work out all the time. I felt like I had to try something. So, I went in the water despite my wife yelling and I swam towards them.
What happens when you get in that deep water is that you panic. You can’t hear anyone because of the rapids, and your instinct is to swim back towards where you went in, which is against the current. Unless you’re a very strong swimmer, you’re just wasting your time, swimming in place.
But these guys weren’t trying to go anywhere. Dad was just trying to stay up and keep the boys alive. He was in about 10 feet of water. What they didn’t see or just didn’t know: About 20 yards upstream from that deep water is a little island.
When I got to them, I yelled at the dad to move towards the island, “Go backwards! Go back!” I flipped the boy over who wasn’t moving. He was the oldest of the three, around 10 or 11 years old. When I turned him over, he was blue and wasn’t breathing. I put my fingers on his neck and didn’t feel a pulse.
So, I’m treading water, holding him. I put an arm behind his back and started doing chest compressions on him. I probably did a dozen to 15 compressions – nothing. I thought, I’ve got to get some air in this kid. So, I gave him two deep breaths and then started doing compressions again. I know ACLS and CPR training would say we don’t do that anymore. But I couldn’t just sit there and give up. Shortly after that, he coughed out a large amount of water and started breathing.
The dad and the other two boys had made it to the island. So, I started moving towards it with the boy. It was a few minutes before he regained consciousness. Of course, he was unaware of what had happened. He started to scream, because here’s this strange man holding him. But he was breathing. That’s all I cared about.
When we got to the island, I saw that my neighbor downstream had launched his canoe. He’s a retired gentleman who lives next to me, a very physically fit man. He started rolling as hard as he could towards us, against the stream. I kind of gave him a thumbs up, like, “we’re safe now. We’re standing.” We loaded the kids and the dad in the canoe and made it back against the stream to the parking lot where they went in.
All this took probably 10 or 15 minutes, and by then the paramedics were there. Life Flight had been dispatched up by my barn where there’s room to land. So, they drove up there in the ambulance. The boy I revived was flown to the hospital. The others went in the ambulance.
I know all the ED docs, so I talked to somebody later who, with permission from the family, said they were all doing fine. They were getting x-rays on the boy’s lungs. And then I heard the dad and two boys were released that night. The other boy I worked on was observed overnight and discharged the following morning.
Four or 5 days later, I heard from their pediatrician, who also had permission to share. He sent me a very nice note through Epic that he had seen the boys. Besides some mental trauma, they were all healthy and doing fine.
The family lives in the area and the kids go to school 5 miles from my house. So, the following weekend they came over. It was Father’s Day, which was kind of cool. They brought me some flowers and candy and a card the boys had drawn to thank me.
I learned that the dad had brought the boys to the fishing site. They were horsing around in knee deep water. One of the boys walked off a little way and didn’t realize there was a drop off. He went in, and of course the dad went after him, and the other two followed.
I said to the parents: “Look, things like this happen for a reason. People like your son are saved and go on in this world because they’ve got special things to do. I can’t wait to see what kind of man he becomes.”
Two or 3 months later, it was football season, and I got at a message from the dad saying their son was playing football on Saturday at the school. He wondered if I could drop by. So, I kind of snuck over and watched, but I didn’t go say hi. There’s trauma there, and I didn’t want them to have to relive that.
I’m very fortunate that I exercise every day and I know how to do CPR and swim. And thank God the boy was floating when I got to him, or I never would’ve found him. The Maumee River is known as the “muddy Maumee.” You can’t see anything under the water.
Depending on the time of year, the river can be almost dry or overflowing into the parking lot with the current rushing hard. If it had been like that, I wouldn’t have considered going in. And they wouldn’t they have been there in the first place. They’d have been a mile downstream.
I took a risk. I could have gone out there and had the dad and two other kids jump on top of me. Then we all would have been in trouble. But like I told my wife, I couldn’t stand there and watch it. I’m just not that person.
I think it was also about being a dad myself and having grandkids now. Doctor or no doctor, I felt like I was in reasonably good shape and I had to go in there to help. This dad was trying his butt off, but three little kids is too many. You can’t do that by yourself. They were not going to make it.
I go to the hospital and I save lives as part of my job, and I don’t even come home and talk about it. But this is a whole different thing. Being able to save someone’s life when put in this situation is very gratifying. It’s a tremendous feeling. There’s a reason that young man is here today, and I’ll be watching for great things from him.
A version of this article first appeared on Medscape.com.
Daniel Cassavar, MD, is a cardiologist with ProMedica in Perrysburg, Ohio.
Is There a Doctor in the House? is a new series telling these stories.
I live on the Maumee River in Ohio, about 50 yards from the water. I had an early quit time and came home to meet my wife for lunch. Afterward, I went up to my barn across the main road to tinker around. It was a nice day out, so my wife had opened some windows. Suddenly, she heard screaming from the river. It did not sound like fun.
She ran down to the river’s edge and saw a dad and three boys struggling in the water. She phoned me screaming: “They’re drowning! They’re drowning!” I jumped in my truck and drove up our driveway through the yard right down to the river.
My wife was on the phone with 911 at that point, and I could see them about 75-100 yards out. The dad had two of the boys clinging around his neck. They were going under the water and coming up and going under again. The other boy was just floating nearby, face down, motionless.
I threw my shoes and scrubs off and started to walk towards the water. My wife screamed at me, “You’re not going in there!” I said, “I’m not going to stand here and watch this. It’s not going to happen.”
I’m not a kid anymore, but I was a high school swimmer, and to this day I work out all the time. I felt like I had to try something. So, I went in the water despite my wife yelling and I swam towards them.
What happens when you get in that deep water is that you panic. You can’t hear anyone because of the rapids, and your instinct is to swim back towards where you went in, which is against the current. Unless you’re a very strong swimmer, you’re just wasting your time, swimming in place.
But these guys weren’t trying to go anywhere. Dad was just trying to stay up and keep the boys alive. He was in about 10 feet of water. What they didn’t see or just didn’t know: About 20 yards upstream from that deep water is a little island.
When I got to them, I yelled at the dad to move towards the island, “Go backwards! Go back!” I flipped the boy over who wasn’t moving. He was the oldest of the three, around 10 or 11 years old. When I turned him over, he was blue and wasn’t breathing. I put my fingers on his neck and didn’t feel a pulse.
So, I’m treading water, holding him. I put an arm behind his back and started doing chest compressions on him. I probably did a dozen to 15 compressions – nothing. I thought, I’ve got to get some air in this kid. So, I gave him two deep breaths and then started doing compressions again. I know ACLS and CPR training would say we don’t do that anymore. But I couldn’t just sit there and give up. Shortly after that, he coughed out a large amount of water and started breathing.
The dad and the other two boys had made it to the island. So, I started moving towards it with the boy. It was a few minutes before he regained consciousness. Of course, he was unaware of what had happened. He started to scream, because here’s this strange man holding him. But he was breathing. That’s all I cared about.
When we got to the island, I saw that my neighbor downstream had launched his canoe. He’s a retired gentleman who lives next to me, a very physically fit man. He started rolling as hard as he could towards us, against the stream. I kind of gave him a thumbs up, like, “we’re safe now. We’re standing.” We loaded the kids and the dad in the canoe and made it back against the stream to the parking lot where they went in.
All this took probably 10 or 15 minutes, and by then the paramedics were there. Life Flight had been dispatched up by my barn where there’s room to land. So, they drove up there in the ambulance. The boy I revived was flown to the hospital. The others went in the ambulance.
I know all the ED docs, so I talked to somebody later who, with permission from the family, said they were all doing fine. They were getting x-rays on the boy’s lungs. And then I heard the dad and two boys were released that night. The other boy I worked on was observed overnight and discharged the following morning.
Four or 5 days later, I heard from their pediatrician, who also had permission to share. He sent me a very nice note through Epic that he had seen the boys. Besides some mental trauma, they were all healthy and doing fine.
The family lives in the area and the kids go to school 5 miles from my house. So, the following weekend they came over. It was Father’s Day, which was kind of cool. They brought me some flowers and candy and a card the boys had drawn to thank me.
I learned that the dad had brought the boys to the fishing site. They were horsing around in knee deep water. One of the boys walked off a little way and didn’t realize there was a drop off. He went in, and of course the dad went after him, and the other two followed.
I said to the parents: “Look, things like this happen for a reason. People like your son are saved and go on in this world because they’ve got special things to do. I can’t wait to see what kind of man he becomes.”
Two or 3 months later, it was football season, and I got at a message from the dad saying their son was playing football on Saturday at the school. He wondered if I could drop by. So, I kind of snuck over and watched, but I didn’t go say hi. There’s trauma there, and I didn’t want them to have to relive that.
I’m very fortunate that I exercise every day and I know how to do CPR and swim. And thank God the boy was floating when I got to him, or I never would’ve found him. The Maumee River is known as the “muddy Maumee.” You can’t see anything under the water.
Depending on the time of year, the river can be almost dry or overflowing into the parking lot with the current rushing hard. If it had been like that, I wouldn’t have considered going in. And they wouldn’t they have been there in the first place. They’d have been a mile downstream.
I took a risk. I could have gone out there and had the dad and two other kids jump on top of me. Then we all would have been in trouble. But like I told my wife, I couldn’t stand there and watch it. I’m just not that person.
I think it was also about being a dad myself and having grandkids now. Doctor or no doctor, I felt like I was in reasonably good shape and I had to go in there to help. This dad was trying his butt off, but three little kids is too many. You can’t do that by yourself. They were not going to make it.
I go to the hospital and I save lives as part of my job, and I don’t even come home and talk about it. But this is a whole different thing. Being able to save someone’s life when put in this situation is very gratifying. It’s a tremendous feeling. There’s a reason that young man is here today, and I’ll be watching for great things from him.
A version of this article first appeared on Medscape.com.
Daniel Cassavar, MD, is a cardiologist with ProMedica in Perrysburg, Ohio.
Increasing fatigue and dry cough
This patient's clinical presentation is consistent with a diagnosis of superior vena cava syndrome (SVCS), secondary to SCLC.
SCLC is an aggressive, poorly differentiated, high-grade neuroendocrine carcinoma that accounts for approximately 13%-15% of all new lung cancer cases in the United States. SCLC has a propensity for early dissemination; as such, 80%-85% of patients are diagnosed with extensive disease (ES-SCLC). This is common in heavy smokers. Most SCLC tumors are found in hilar or perihilar areas; <5% present in peripheral locations. In many cases, invasion into the peribronchial tissue and lymph node can be clearly identified, with a typical circumferential spread along the submucosa of the bronchi.
Up to 10% of patients with SCLC develop SVCS, which comprises an array of signs and symptoms that result from the obstruction of blood flow through the thin-walled superior vena cava. Clinical symptoms may include cough, dyspnea, and orthopnea; facial edema and plethora, upper extremity swelling, and venous distension of the chest wall and neck are the most commonly encountered signs. Most cases of SVCS occur in patients with mediastinal tumors, although noncancerous causes (eg, thrombosis and fibrosing mediastinitis) can also give rise to it. The diagnosis of SVCS is usually made clinically and then confirmed with imaging (chest radiography, contrast-enhanced CT, duplex ultrasound, conventional venography, and/or magnetic resonance venography).
Though it was traditionally considered a virtual emergency, patients seldom experience life-threatening complications from SVCS. The goals of treatment are to alleviate the symptoms of SVC obstruction and treat the underlying disease process. Treatment approaches include radiation therapy, chemotherapy, open surgery, and endovenous recanalization; however, patients with clinical SVCS often achieve significant improvement in symptoms from conservative treatment approaches, including elevation of the head of the bed and supplemental oxygen. Systemic chemotherapy can effectively relieve the symptoms of SVCS obstruction, typically within 1-2 weeks of treatment initiation. Up to 80% of patients with SCLC and non-Hodgkin lymphoma may experience complete relief of SVCS symptoms with chemotherapy treatment.
Radiation therapy was once considered the standard approach to the management of SVCS in patients with cancer; however, endovenous recanalization can alleviate symptoms faster than radiation therapy — usually within 72 hours, whereas radiation therapy can take up to 2 weeks to provide relief. Endovascular therapy is also associated with higher efficacy rates than is radiation therapy.
Open surgery plays a limited role in the management of SVC obstruction, although it may be the best approach in select cases.
In cases involving brain edema, decreased cardiac output, or upper airway edema, emergency treatment is indicated.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation is consistent with a diagnosis of superior vena cava syndrome (SVCS), secondary to SCLC.
SCLC is an aggressive, poorly differentiated, high-grade neuroendocrine carcinoma that accounts for approximately 13%-15% of all new lung cancer cases in the United States. SCLC has a propensity for early dissemination; as such, 80%-85% of patients are diagnosed with extensive disease (ES-SCLC). This is common in heavy smokers. Most SCLC tumors are found in hilar or perihilar areas; <5% present in peripheral locations. In many cases, invasion into the peribronchial tissue and lymph node can be clearly identified, with a typical circumferential spread along the submucosa of the bronchi.
Up to 10% of patients with SCLC develop SVCS, which comprises an array of signs and symptoms that result from the obstruction of blood flow through the thin-walled superior vena cava. Clinical symptoms may include cough, dyspnea, and orthopnea; facial edema and plethora, upper extremity swelling, and venous distension of the chest wall and neck are the most commonly encountered signs. Most cases of SVCS occur in patients with mediastinal tumors, although noncancerous causes (eg, thrombosis and fibrosing mediastinitis) can also give rise to it. The diagnosis of SVCS is usually made clinically and then confirmed with imaging (chest radiography, contrast-enhanced CT, duplex ultrasound, conventional venography, and/or magnetic resonance venography).
Though it was traditionally considered a virtual emergency, patients seldom experience life-threatening complications from SVCS. The goals of treatment are to alleviate the symptoms of SVC obstruction and treat the underlying disease process. Treatment approaches include radiation therapy, chemotherapy, open surgery, and endovenous recanalization; however, patients with clinical SVCS often achieve significant improvement in symptoms from conservative treatment approaches, including elevation of the head of the bed and supplemental oxygen. Systemic chemotherapy can effectively relieve the symptoms of SVCS obstruction, typically within 1-2 weeks of treatment initiation. Up to 80% of patients with SCLC and non-Hodgkin lymphoma may experience complete relief of SVCS symptoms with chemotherapy treatment.
Radiation therapy was once considered the standard approach to the management of SVCS in patients with cancer; however, endovenous recanalization can alleviate symptoms faster than radiation therapy — usually within 72 hours, whereas radiation therapy can take up to 2 weeks to provide relief. Endovascular therapy is also associated with higher efficacy rates than is radiation therapy.
Open surgery plays a limited role in the management of SVC obstruction, although it may be the best approach in select cases.
In cases involving brain edema, decreased cardiac output, or upper airway edema, emergency treatment is indicated.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation is consistent with a diagnosis of superior vena cava syndrome (SVCS), secondary to SCLC.
SCLC is an aggressive, poorly differentiated, high-grade neuroendocrine carcinoma that accounts for approximately 13%-15% of all new lung cancer cases in the United States. SCLC has a propensity for early dissemination; as such, 80%-85% of patients are diagnosed with extensive disease (ES-SCLC). This is common in heavy smokers. Most SCLC tumors are found in hilar or perihilar areas; <5% present in peripheral locations. In many cases, invasion into the peribronchial tissue and lymph node can be clearly identified, with a typical circumferential spread along the submucosa of the bronchi.
Up to 10% of patients with SCLC develop SVCS, which comprises an array of signs and symptoms that result from the obstruction of blood flow through the thin-walled superior vena cava. Clinical symptoms may include cough, dyspnea, and orthopnea; facial edema and plethora, upper extremity swelling, and venous distension of the chest wall and neck are the most commonly encountered signs. Most cases of SVCS occur in patients with mediastinal tumors, although noncancerous causes (eg, thrombosis and fibrosing mediastinitis) can also give rise to it. The diagnosis of SVCS is usually made clinically and then confirmed with imaging (chest radiography, contrast-enhanced CT, duplex ultrasound, conventional venography, and/or magnetic resonance venography).
Though it was traditionally considered a virtual emergency, patients seldom experience life-threatening complications from SVCS. The goals of treatment are to alleviate the symptoms of SVC obstruction and treat the underlying disease process. Treatment approaches include radiation therapy, chemotherapy, open surgery, and endovenous recanalization; however, patients with clinical SVCS often achieve significant improvement in symptoms from conservative treatment approaches, including elevation of the head of the bed and supplemental oxygen. Systemic chemotherapy can effectively relieve the symptoms of SVCS obstruction, typically within 1-2 weeks of treatment initiation. Up to 80% of patients with SCLC and non-Hodgkin lymphoma may experience complete relief of SVCS symptoms with chemotherapy treatment.
Radiation therapy was once considered the standard approach to the management of SVCS in patients with cancer; however, endovenous recanalization can alleviate symptoms faster than radiation therapy — usually within 72 hours, whereas radiation therapy can take up to 2 weeks to provide relief. Endovascular therapy is also associated with higher efficacy rates than is radiation therapy.
Open surgery plays a limited role in the management of SVC obstruction, although it may be the best approach in select cases.
In cases involving brain edema, decreased cardiac output, or upper airway edema, emergency treatment is indicated.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 66-year-old African American man was diagnosed with small cell lung cancer (SCLC) after the discovery of an endobronchial tumor on bronchoscopy. A biopsy of the tumor was positive for SCLC and CT revealed multiple pulmonary nodules and extensive mediastinal nodal metastases. The patient completed his first cycle of carboplatin-based chemotherapy about 1 month ago. At today's visit, he presents with complaints of worsening symptoms over the past week or so; specifically, he reports increasing fatigue and shortness of breath, a dry cough, light-headedness, difficulty swallowing, and facial swelling. Physical examination reveals facial edema and venous distension of the neck and chest wall; blood pressure is 140/70 mm Hg, respiratory rate is 19 breaths/min, and pulse is 84 beats/min. The patient has a 45-pack-year smoking history and reports having two or three alcoholic drinks per day. His previous medical history is positive for hypertension, which is treated with enalapril 20 mg/day and metoprolol 200 mg/day. Complete blood cell count findings are all within normal range.
Commentary: Early Breast Cancer Treatment Strategies and Acupuncture, January 2023
The risk for disease recurrence, and specifically distant relapse, for women with high-risk early breast cancer highlights the need for novel therapies in this population.2,3 The phase 3 randomized monarchE trial investigated the role of the CDK4/6 inhibitor abemaciclib combined with endocrine therapy vs standard endocrine therapy alone in 5637 patients with high-risk (≥ 4 positive axillary nodes or 1-3 positive nodes and either grade 3 tumor, tumor size ≥ 5 cm or Ki-67 ≥ 20%) hormone receptor–positive/HER2-negative early breast cancer. At a median follow-up of 42 months, the median invasive disease-free survival (iDFS) benefit was sustained with abemaciclib + endocrine therapy vs endocrine therapy alone (HR 0.664; nominal P < .0001); the absolute 4-year iDFS benefit was 6.4% (85.8% in the abemaciclib + endocrine therapy group vs 79.4% in the endocrine therapy–alone group). Furthermore, this effect appeared to deepen over time, as the previous absolute iDFS differences were 2.8% (2 years) and 4.8% (3 years). Abemaciclib was associated with a higher rate of grade 3 or higher adverse events (49.9% vs 16.9%), the most common being neutropenia, leukopenia, and diarrhea (Johnston et al). Although adjuvant palbociclib trials (PALLAS4 and PENELOPE-B5) did not meet their primary endpoint, longer follow-up of monarchE and results from NATALEE with ribociclib are anxiously awaited to further define the role of CDK4/6 inhibitors in this space.
Aromatase inhibitors (AI) are an integral component of treatment for hormone receptor–positive breast cancer for many women. However, joint pain and stiffness associated with these agents can affect compliance. Various management strategies, including trials of alternative AI or endocrine therapies and pharmacologic (duloxetine) and non-pharmacologic (acupuncture,6 exercise) modalities, have been investigated. A randomized trial including 226 women with early-stage breast cancer receiving AI therapy with baseline joint pain (Brief Pain Inventory Worst Pain [BPI-WP] item score of ≥ 3) evaluated whether true acupuncture (TA) provided a sustained reduction in pain symptoms compared with sham acupuncture (SA) or waiting-list control (WC). Acupuncture protocols consisted of 6 weeks of intervention (2 sessions per week) followed by 1 session per week for another 6 weeks. At 52 weeks, mean BPI-WP scores were 1.08 points lower in the TA group compared with the SA group (P = .01) and were 0.99 points lower in the TA group compared with the WC group (P = .03) (Hershman et al). These data support consideration of acupuncture as a mechanism to help maintain patients on aromatase inhibitors, particularly for patients who wish to avoid or have not received benefit from pharmacologic therapy.
Additional References
- Puglisi F, Gerratana L, Lambertini M, et al. Composite risk and benefit from adjuvant dose-dense chemotherapy in hormone receptor-positive breast cancer. NPJ Breast Cancer. 2021;7:82. Doi: 10.1038/s41523-021-00286-w
- Salvo EM, Ramirez AO, Cueto J, et al. Risk of recurrence among patients with HR-positive, HER2-negative, early breast cancer receiving adjuvant endocrine therapy: A systematic review and meta-analysis. Breast. 2021;57:5-17. Doi: 10.1016/j.breast.2021.02.009
- Sheffield KM, Peachey JR, Method M, et al. A real-world US study of recurrence risks using combined clinicopathological features in HR-positive, HER2-negative early breast cancer. Future Oncol.2022;18:2667-2682. Doi: 10.2217/fon-2022-0310
- Mayer EL, Dueck AC, Martin M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212-222. Doi: Loibl S, Marmé F, Martin M, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-The Penelope-B trial. J Clin Oncol. 2021;39(14):1518-1530. Doi: Liu X, Lu J, Wang G, et al. Acupuncture for arthralgia induced by aromatase inhibitors in patients with breast cancer: A systematic review and meta-analysis. Integr Cancer Ther. 2021;20:1534735420980811. Doi: 10.1177/1534735420980811
The risk for disease recurrence, and specifically distant relapse, for women with high-risk early breast cancer highlights the need for novel therapies in this population.2,3 The phase 3 randomized monarchE trial investigated the role of the CDK4/6 inhibitor abemaciclib combined with endocrine therapy vs standard endocrine therapy alone in 5637 patients with high-risk (≥ 4 positive axillary nodes or 1-3 positive nodes and either grade 3 tumor, tumor size ≥ 5 cm or Ki-67 ≥ 20%) hormone receptor–positive/HER2-negative early breast cancer. At a median follow-up of 42 months, the median invasive disease-free survival (iDFS) benefit was sustained with abemaciclib + endocrine therapy vs endocrine therapy alone (HR 0.664; nominal P < .0001); the absolute 4-year iDFS benefit was 6.4% (85.8% in the abemaciclib + endocrine therapy group vs 79.4% in the endocrine therapy–alone group). Furthermore, this effect appeared to deepen over time, as the previous absolute iDFS differences were 2.8% (2 years) and 4.8% (3 years). Abemaciclib was associated with a higher rate of grade 3 or higher adverse events (49.9% vs 16.9%), the most common being neutropenia, leukopenia, and diarrhea (Johnston et al). Although adjuvant palbociclib trials (PALLAS4 and PENELOPE-B5) did not meet their primary endpoint, longer follow-up of monarchE and results from NATALEE with ribociclib are anxiously awaited to further define the role of CDK4/6 inhibitors in this space.
Aromatase inhibitors (AI) are an integral component of treatment for hormone receptor–positive breast cancer for many women. However, joint pain and stiffness associated with these agents can affect compliance. Various management strategies, including trials of alternative AI or endocrine therapies and pharmacologic (duloxetine) and non-pharmacologic (acupuncture,6 exercise) modalities, have been investigated. A randomized trial including 226 women with early-stage breast cancer receiving AI therapy with baseline joint pain (Brief Pain Inventory Worst Pain [BPI-WP] item score of ≥ 3) evaluated whether true acupuncture (TA) provided a sustained reduction in pain symptoms compared with sham acupuncture (SA) or waiting-list control (WC). Acupuncture protocols consisted of 6 weeks of intervention (2 sessions per week) followed by 1 session per week for another 6 weeks. At 52 weeks, mean BPI-WP scores were 1.08 points lower in the TA group compared with the SA group (P = .01) and were 0.99 points lower in the TA group compared with the WC group (P = .03) (Hershman et al). These data support consideration of acupuncture as a mechanism to help maintain patients on aromatase inhibitors, particularly for patients who wish to avoid or have not received benefit from pharmacologic therapy.
Additional References
- Puglisi F, Gerratana L, Lambertini M, et al. Composite risk and benefit from adjuvant dose-dense chemotherapy in hormone receptor-positive breast cancer. NPJ Breast Cancer. 2021;7:82. Doi: 10.1038/s41523-021-00286-w
- Salvo EM, Ramirez AO, Cueto J, et al. Risk of recurrence among patients with HR-positive, HER2-negative, early breast cancer receiving adjuvant endocrine therapy: A systematic review and meta-analysis. Breast. 2021;57:5-17. Doi: 10.1016/j.breast.2021.02.009
- Sheffield KM, Peachey JR, Method M, et al. A real-world US study of recurrence risks using combined clinicopathological features in HR-positive, HER2-negative early breast cancer. Future Oncol.2022;18:2667-2682. Doi: 10.2217/fon-2022-0310
- Mayer EL, Dueck AC, Martin M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212-222. Doi: Loibl S, Marmé F, Martin M, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-The Penelope-B trial. J Clin Oncol. 2021;39(14):1518-1530. Doi: Liu X, Lu J, Wang G, et al. Acupuncture for arthralgia induced by aromatase inhibitors in patients with breast cancer: A systematic review and meta-analysis. Integr Cancer Ther. 2021;20:1534735420980811. Doi: 10.1177/1534735420980811
The risk for disease recurrence, and specifically distant relapse, for women with high-risk early breast cancer highlights the need for novel therapies in this population.2,3 The phase 3 randomized monarchE trial investigated the role of the CDK4/6 inhibitor abemaciclib combined with endocrine therapy vs standard endocrine therapy alone in 5637 patients with high-risk (≥ 4 positive axillary nodes or 1-3 positive nodes and either grade 3 tumor, tumor size ≥ 5 cm or Ki-67 ≥ 20%) hormone receptor–positive/HER2-negative early breast cancer. At a median follow-up of 42 months, the median invasive disease-free survival (iDFS) benefit was sustained with abemaciclib + endocrine therapy vs endocrine therapy alone (HR 0.664; nominal P < .0001); the absolute 4-year iDFS benefit was 6.4% (85.8% in the abemaciclib + endocrine therapy group vs 79.4% in the endocrine therapy–alone group). Furthermore, this effect appeared to deepen over time, as the previous absolute iDFS differences were 2.8% (2 years) and 4.8% (3 years). Abemaciclib was associated with a higher rate of grade 3 or higher adverse events (49.9% vs 16.9%), the most common being neutropenia, leukopenia, and diarrhea (Johnston et al). Although adjuvant palbociclib trials (PALLAS4 and PENELOPE-B5) did not meet their primary endpoint, longer follow-up of monarchE and results from NATALEE with ribociclib are anxiously awaited to further define the role of CDK4/6 inhibitors in this space.
Aromatase inhibitors (AI) are an integral component of treatment for hormone receptor–positive breast cancer for many women. However, joint pain and stiffness associated with these agents can affect compliance. Various management strategies, including trials of alternative AI or endocrine therapies and pharmacologic (duloxetine) and non-pharmacologic (acupuncture,6 exercise) modalities, have been investigated. A randomized trial including 226 women with early-stage breast cancer receiving AI therapy with baseline joint pain (Brief Pain Inventory Worst Pain [BPI-WP] item score of ≥ 3) evaluated whether true acupuncture (TA) provided a sustained reduction in pain symptoms compared with sham acupuncture (SA) or waiting-list control (WC). Acupuncture protocols consisted of 6 weeks of intervention (2 sessions per week) followed by 1 session per week for another 6 weeks. At 52 weeks, mean BPI-WP scores were 1.08 points lower in the TA group compared with the SA group (P = .01) and were 0.99 points lower in the TA group compared with the WC group (P = .03) (Hershman et al). These data support consideration of acupuncture as a mechanism to help maintain patients on aromatase inhibitors, particularly for patients who wish to avoid or have not received benefit from pharmacologic therapy.
Additional References
- Puglisi F, Gerratana L, Lambertini M, et al. Composite risk and benefit from adjuvant dose-dense chemotherapy in hormone receptor-positive breast cancer. NPJ Breast Cancer. 2021;7:82. Doi: 10.1038/s41523-021-00286-w
- Salvo EM, Ramirez AO, Cueto J, et al. Risk of recurrence among patients with HR-positive, HER2-negative, early breast cancer receiving adjuvant endocrine therapy: A systematic review and meta-analysis. Breast. 2021;57:5-17. Doi: 10.1016/j.breast.2021.02.009
- Sheffield KM, Peachey JR, Method M, et al. A real-world US study of recurrence risks using combined clinicopathological features in HR-positive, HER2-negative early breast cancer. Future Oncol.2022;18:2667-2682. Doi: 10.2217/fon-2022-0310
- Mayer EL, Dueck AC, Martin M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212-222. Doi: Loibl S, Marmé F, Martin M, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-The Penelope-B trial. J Clin Oncol. 2021;39(14):1518-1530. Doi: Liu X, Lu J, Wang G, et al. Acupuncture for arthralgia induced by aromatase inhibitors in patients with breast cancer: A systematic review and meta-analysis. Integr Cancer Ther. 2021;20:1534735420980811. Doi: 10.1177/1534735420980811
FMT doesn’t appear to affect weight loss after bariatric surgery
according to results of a randomized controlled trial.
The small study by Perttu Lahtinen, MD, with Päijät-Häme Central Hospital in Lahti, Finland, and colleagues was published online in JAMA Network Open.
Bariatric surgery remains the most effective strategy for treating severe obesity. Yet some patients achieve only minimal weight loss or regain weight after surgery, the researchers noted.
There is much interest in the gut microbiota as a potential target for the treatment of obesity. FMT from a lean donor has shown promise in treating obesity in mouse models (Science. 2013 Sep 6. doi: 10.1126/science.1241214).
The Finnish trial, however, does not support that conclusion.
The study included 41 adults (71% women; mean age, 48.7 years) with severe obesity (mean body mass index, 42.5 kg/m2). Twenty-one received FMT from a lean donor, and 20 received FMT from their own feces (autologous placebo). FMT was administered by gastroscopy into the duodenum 6 months before laparoscopic Roux-en-Y gastric bypass or sleeve gastrectomy. All patients also consumed a very-low-calorie diet approximately 4 weeks before the surgery.
Bariatric surgery led to equal weight reductions for both groups, but there was no additive benefit in terms of weight loss with FMT.
Six months after the administration of FMT, and before the surgery was performed, the percentage of total weight loss, the main outcome, was 4.8% (P < .001) in the FMT group and 4.6% (P = .006) in the placebo group. There was no statistically significant difference between the groups (absolute difference, 0.2%).
At 18 months (12 months after surgery), the percentage of total weight loss was 25.3% (P < .001) in the FMT group and 25.2% (P < .001) in the placebo group – an absolute difference of 0.1%.
The researchers said the main limitation of their study is the small number of patients. Because there were few patients, the study may be inadequate to show possible minor effects of FMT on weight; it’s unclear whether a much larger sample size would have yielded any differences between the groups.
Nonetheless, the study suggests that FMT does not affect weight loss for patients who undergo bariatric surgery, the researchers said.
The study was supported by governmental research grants and the Sigrid Juselius Foundation. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a randomized controlled trial.
The small study by Perttu Lahtinen, MD, with Päijät-Häme Central Hospital in Lahti, Finland, and colleagues was published online in JAMA Network Open.
Bariatric surgery remains the most effective strategy for treating severe obesity. Yet some patients achieve only minimal weight loss or regain weight after surgery, the researchers noted.
There is much interest in the gut microbiota as a potential target for the treatment of obesity. FMT from a lean donor has shown promise in treating obesity in mouse models (Science. 2013 Sep 6. doi: 10.1126/science.1241214).
The Finnish trial, however, does not support that conclusion.
The study included 41 adults (71% women; mean age, 48.7 years) with severe obesity (mean body mass index, 42.5 kg/m2). Twenty-one received FMT from a lean donor, and 20 received FMT from their own feces (autologous placebo). FMT was administered by gastroscopy into the duodenum 6 months before laparoscopic Roux-en-Y gastric bypass or sleeve gastrectomy. All patients also consumed a very-low-calorie diet approximately 4 weeks before the surgery.
Bariatric surgery led to equal weight reductions for both groups, but there was no additive benefit in terms of weight loss with FMT.
Six months after the administration of FMT, and before the surgery was performed, the percentage of total weight loss, the main outcome, was 4.8% (P < .001) in the FMT group and 4.6% (P = .006) in the placebo group. There was no statistically significant difference between the groups (absolute difference, 0.2%).
At 18 months (12 months after surgery), the percentage of total weight loss was 25.3% (P < .001) in the FMT group and 25.2% (P < .001) in the placebo group – an absolute difference of 0.1%.
The researchers said the main limitation of their study is the small number of patients. Because there were few patients, the study may be inadequate to show possible minor effects of FMT on weight; it’s unclear whether a much larger sample size would have yielded any differences between the groups.
Nonetheless, the study suggests that FMT does not affect weight loss for patients who undergo bariatric surgery, the researchers said.
The study was supported by governmental research grants and the Sigrid Juselius Foundation. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a randomized controlled trial.
The small study by Perttu Lahtinen, MD, with Päijät-Häme Central Hospital in Lahti, Finland, and colleagues was published online in JAMA Network Open.
Bariatric surgery remains the most effective strategy for treating severe obesity. Yet some patients achieve only minimal weight loss or regain weight after surgery, the researchers noted.
There is much interest in the gut microbiota as a potential target for the treatment of obesity. FMT from a lean donor has shown promise in treating obesity in mouse models (Science. 2013 Sep 6. doi: 10.1126/science.1241214).
The Finnish trial, however, does not support that conclusion.
The study included 41 adults (71% women; mean age, 48.7 years) with severe obesity (mean body mass index, 42.5 kg/m2). Twenty-one received FMT from a lean donor, and 20 received FMT from their own feces (autologous placebo). FMT was administered by gastroscopy into the duodenum 6 months before laparoscopic Roux-en-Y gastric bypass or sleeve gastrectomy. All patients also consumed a very-low-calorie diet approximately 4 weeks before the surgery.
Bariatric surgery led to equal weight reductions for both groups, but there was no additive benefit in terms of weight loss with FMT.
Six months after the administration of FMT, and before the surgery was performed, the percentage of total weight loss, the main outcome, was 4.8% (P < .001) in the FMT group and 4.6% (P = .006) in the placebo group. There was no statistically significant difference between the groups (absolute difference, 0.2%).
At 18 months (12 months after surgery), the percentage of total weight loss was 25.3% (P < .001) in the FMT group and 25.2% (P < .001) in the placebo group – an absolute difference of 0.1%.
The researchers said the main limitation of their study is the small number of patients. Because there were few patients, the study may be inadequate to show possible minor effects of FMT on weight; it’s unclear whether a much larger sample size would have yielded any differences between the groups.
Nonetheless, the study suggests that FMT does not affect weight loss for patients who undergo bariatric surgery, the researchers said.
The study was supported by governmental research grants and the Sigrid Juselius Foundation. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Nonheavy alcohol use associated with liver fibrosis, NASH
according to a new report.
An analysis of current drinkers in the Framingham Heart Study found that a higher number of drinks per week and higher frequency of drinking were associated with increased odds of fibrosis among patients whose consumption fell below the threshold for heavy alcohol use.
“Although the detrimental effects of heavy alcohol use are well accepted, there is no consensus guideline on how to counsel patients about how nonheavy alcohol use may affect liver health,” Brooke Rice, MD, an internal medicine resident at Boston University, said in an interview.
“Current terminology classifies fatty liver disease as either alcoholic or nonalcoholic,” she said. “Our results call this strict categorization into question, suggesting that even nonheavy alcohol use should be considered as a factor contributing to more advanced nonalcoholic fatty liver disease [NAFLD] phenotypes.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing associations
NAFLD and alcohol-related liver disease, which are the most common causes of chronic liver disease worldwide, are histologically identical but distinguished by the presence of significant alcohol use, the study authors wrote.
Heavy alcohol use, based on guidelines from the American Association for the Study of Liver Diseases, is defined as more than 14 drinks per week for women or more than 21 drinks per week for men.
Although heavy alcohol use is consistently associated with cirrhosis and steatohepatitis, studies of nonheavy alcohol use have shown conflicting results, the authors wrote. However, evidence suggests that the pattern of alcohol consumption – particularly increased weekly drinking and binge drinking – may be an important predictor.
Dr. Rice and colleagues conducted a cross-sectional study of 2,629 current drinkers in the Framingham Heart Study who completed alcohol-use questionnaires and vibration-controlled transient elastography between April 2016 and April 2019. They analyzed the association between fibrosis and several alcohol-use measures, including total consumption and drinking patterns, among nonheavy alcohol users whose liver disease would be classified as “nonalcoholic” by current nomenclature.
The research team defined clinically significant fibrosis as a liver stiffness measurement of 8.2 kPa or higher. For at-risk NASH, the researchers used two FibroScan-AST (FAST) score thresholds – greater than 0.35 or 0.67 and higher. They also considered additional metabolic factors such as physical activity, body mass index, blood pressure, glucose measures, and metabolic syndrome.
Participants were asked to estimate the frequency of alcohol use (average number of drinking days per week during the past year) and the usual quantity of alcohol consumed (average number of drinks on a typical drinking day during the past year). Researchers multiplied the figures to estimate the average total number of drinks per week.
Among the 2,629 current drinkers (53% women, 47% men), the average age was 54 years, 7.2% had diabetes, and 26.9% met the criteria for metabolic syndrome. Participants drank about 3 days per week on average with a usual consumption of two drinks per drinking day, averaging a total weekly alcohol consumption of six drinks.
The average liver stiffness measurement was 5.6 kPa, and 8.2% had significant fibrosis.
At the FAST score threshold of 0.67 or greater, 1.9% of participants were likely to have at-risk NASH, with a higher prevalence in those with obesity (4.5%) or diabetes (9.5%). At the FAST score threshold of greater than 0.35, the prevalence of at-risk NASH was 12.4%, which was higher in those with obesity (26.3%) or diabetes (34.4%).
Overall, an increased total number of drinks per week and higher frequency of drinking days were associated with increased odds of fibrosis.
Almost 17.5% of participants engaged in risky weekly drinking, which was defined as 8 or more drinks per week for women and 15 or more drinks per week for men. Risky weekly drinking was also associated with higher odds of fibrosis.
After excluding 158 heavy drinkers, the prevalence of fibrosis was unchanged at 8%, and an increased total of drinks per week remained significantly associated with fibrosis.
In addition, multiple alcohol-use measures were positively associated with a FAST score greater than 0.35 and were similar after excluding heavy alcohol users. These measures include the number of drinks per week, the frequency of drinking days, and binge drinking.
“We showed that nonheavy alcohol use is associated with fibrosis and at-risk NASH, which are both predictors of long-term liver-related morbidity and mortality,” Dr. Rice said.
Implications for patient care
The findings have important implications for both NAFLD clinical trials and patient care, the study authors wrote. For instance, the U.S. Dietary Guidelines for Americans recommend limiting alcohol use to one drink per day for women and two drinks per day for men.
“Our results reinforce the importance of encouraging all patients to reduce alcohol intake as much as possible and to at least adhere to current U.S. Dietary Guidelines recommended limits,” Dr. Rice said. “Almost half of participants in our study consumed in excess of these limits, which strongly associated with at-risk NASH.”
Additional long-term studies are needed to determine the benefits of limiting alcohol consumption to reduce liver-related morbidity and mortality, the authors wrote.
The effect of alcohol consumption on liver health “has been controversial, since some studies have suggested that nonheavy alcohol use can even have some beneficial metabolic effects and has been associated with reduced risk of fatty liver disease, while other studies have found that nonheavy alcohol use is associated with increased risk for liver-related clinical outcomes,” Fredrik Åberg, MD, PhD, a hepatologist and liver transplant specialist at Helsinki University Hospital, said in an interview.
Dr. Åberg wasn’t involved with this study but has researched alcohol consumption and liver disease. Among non–heavy alcohol users, drinking more alcohol per week is associated with increased hospitalization for liver disease, hepatocellular carcinoma, and liver-related death, he and his colleagues have found.
“We concluded that the net effect of non-heavy drinking on the liver is harm,” he said. “Overall, this study by Rice and colleagues supports the recommendation that persons with mild liver disease should reduce their drinking, and persons with severe liver disease (cirrhosis and advanced fibrosis) should abstain from alcohol use.”
The study authors are supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases, a Doris Duke Charitable Foundation Grant, a Gilead Sciences Research Scholars Award, the Boston University Department of Medicine Career Investment Award, and the Boston University Clinical Translational Science Institute. The Framingham Heart Study is supported in part by the National Heart, Lung, and Blood Institute. The authors and Dr. Åberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new report.
An analysis of current drinkers in the Framingham Heart Study found that a higher number of drinks per week and higher frequency of drinking were associated with increased odds of fibrosis among patients whose consumption fell below the threshold for heavy alcohol use.
“Although the detrimental effects of heavy alcohol use are well accepted, there is no consensus guideline on how to counsel patients about how nonheavy alcohol use may affect liver health,” Brooke Rice, MD, an internal medicine resident at Boston University, said in an interview.
“Current terminology classifies fatty liver disease as either alcoholic or nonalcoholic,” she said. “Our results call this strict categorization into question, suggesting that even nonheavy alcohol use should be considered as a factor contributing to more advanced nonalcoholic fatty liver disease [NAFLD] phenotypes.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing associations
NAFLD and alcohol-related liver disease, which are the most common causes of chronic liver disease worldwide, are histologically identical but distinguished by the presence of significant alcohol use, the study authors wrote.
Heavy alcohol use, based on guidelines from the American Association for the Study of Liver Diseases, is defined as more than 14 drinks per week for women or more than 21 drinks per week for men.
Although heavy alcohol use is consistently associated with cirrhosis and steatohepatitis, studies of nonheavy alcohol use have shown conflicting results, the authors wrote. However, evidence suggests that the pattern of alcohol consumption – particularly increased weekly drinking and binge drinking – may be an important predictor.
Dr. Rice and colleagues conducted a cross-sectional study of 2,629 current drinkers in the Framingham Heart Study who completed alcohol-use questionnaires and vibration-controlled transient elastography between April 2016 and April 2019. They analyzed the association between fibrosis and several alcohol-use measures, including total consumption and drinking patterns, among nonheavy alcohol users whose liver disease would be classified as “nonalcoholic” by current nomenclature.
The research team defined clinically significant fibrosis as a liver stiffness measurement of 8.2 kPa or higher. For at-risk NASH, the researchers used two FibroScan-AST (FAST) score thresholds – greater than 0.35 or 0.67 and higher. They also considered additional metabolic factors such as physical activity, body mass index, blood pressure, glucose measures, and metabolic syndrome.
Participants were asked to estimate the frequency of alcohol use (average number of drinking days per week during the past year) and the usual quantity of alcohol consumed (average number of drinks on a typical drinking day during the past year). Researchers multiplied the figures to estimate the average total number of drinks per week.
Among the 2,629 current drinkers (53% women, 47% men), the average age was 54 years, 7.2% had diabetes, and 26.9% met the criteria for metabolic syndrome. Participants drank about 3 days per week on average with a usual consumption of two drinks per drinking day, averaging a total weekly alcohol consumption of six drinks.
The average liver stiffness measurement was 5.6 kPa, and 8.2% had significant fibrosis.
At the FAST score threshold of 0.67 or greater, 1.9% of participants were likely to have at-risk NASH, with a higher prevalence in those with obesity (4.5%) or diabetes (9.5%). At the FAST score threshold of greater than 0.35, the prevalence of at-risk NASH was 12.4%, which was higher in those with obesity (26.3%) or diabetes (34.4%).
Overall, an increased total number of drinks per week and higher frequency of drinking days were associated with increased odds of fibrosis.
Almost 17.5% of participants engaged in risky weekly drinking, which was defined as 8 or more drinks per week for women and 15 or more drinks per week for men. Risky weekly drinking was also associated with higher odds of fibrosis.
After excluding 158 heavy drinkers, the prevalence of fibrosis was unchanged at 8%, and an increased total of drinks per week remained significantly associated with fibrosis.
In addition, multiple alcohol-use measures were positively associated with a FAST score greater than 0.35 and were similar after excluding heavy alcohol users. These measures include the number of drinks per week, the frequency of drinking days, and binge drinking.
“We showed that nonheavy alcohol use is associated with fibrosis and at-risk NASH, which are both predictors of long-term liver-related morbidity and mortality,” Dr. Rice said.
Implications for patient care
The findings have important implications for both NAFLD clinical trials and patient care, the study authors wrote. For instance, the U.S. Dietary Guidelines for Americans recommend limiting alcohol use to one drink per day for women and two drinks per day for men.
“Our results reinforce the importance of encouraging all patients to reduce alcohol intake as much as possible and to at least adhere to current U.S. Dietary Guidelines recommended limits,” Dr. Rice said. “Almost half of participants in our study consumed in excess of these limits, which strongly associated with at-risk NASH.”
Additional long-term studies are needed to determine the benefits of limiting alcohol consumption to reduce liver-related morbidity and mortality, the authors wrote.
The effect of alcohol consumption on liver health “has been controversial, since some studies have suggested that nonheavy alcohol use can even have some beneficial metabolic effects and has been associated with reduced risk of fatty liver disease, while other studies have found that nonheavy alcohol use is associated with increased risk for liver-related clinical outcomes,” Fredrik Åberg, MD, PhD, a hepatologist and liver transplant specialist at Helsinki University Hospital, said in an interview.
Dr. Åberg wasn’t involved with this study but has researched alcohol consumption and liver disease. Among non–heavy alcohol users, drinking more alcohol per week is associated with increased hospitalization for liver disease, hepatocellular carcinoma, and liver-related death, he and his colleagues have found.
“We concluded that the net effect of non-heavy drinking on the liver is harm,” he said. “Overall, this study by Rice and colleagues supports the recommendation that persons with mild liver disease should reduce their drinking, and persons with severe liver disease (cirrhosis and advanced fibrosis) should abstain from alcohol use.”
The study authors are supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases, a Doris Duke Charitable Foundation Grant, a Gilead Sciences Research Scholars Award, the Boston University Department of Medicine Career Investment Award, and the Boston University Clinical Translational Science Institute. The Framingham Heart Study is supported in part by the National Heart, Lung, and Blood Institute. The authors and Dr. Åberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new report.
An analysis of current drinkers in the Framingham Heart Study found that a higher number of drinks per week and higher frequency of drinking were associated with increased odds of fibrosis among patients whose consumption fell below the threshold for heavy alcohol use.
“Although the detrimental effects of heavy alcohol use are well accepted, there is no consensus guideline on how to counsel patients about how nonheavy alcohol use may affect liver health,” Brooke Rice, MD, an internal medicine resident at Boston University, said in an interview.
“Current terminology classifies fatty liver disease as either alcoholic or nonalcoholic,” she said. “Our results call this strict categorization into question, suggesting that even nonheavy alcohol use should be considered as a factor contributing to more advanced nonalcoholic fatty liver disease [NAFLD] phenotypes.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing associations
NAFLD and alcohol-related liver disease, which are the most common causes of chronic liver disease worldwide, are histologically identical but distinguished by the presence of significant alcohol use, the study authors wrote.
Heavy alcohol use, based on guidelines from the American Association for the Study of Liver Diseases, is defined as more than 14 drinks per week for women or more than 21 drinks per week for men.
Although heavy alcohol use is consistently associated with cirrhosis and steatohepatitis, studies of nonheavy alcohol use have shown conflicting results, the authors wrote. However, evidence suggests that the pattern of alcohol consumption – particularly increased weekly drinking and binge drinking – may be an important predictor.
Dr. Rice and colleagues conducted a cross-sectional study of 2,629 current drinkers in the Framingham Heart Study who completed alcohol-use questionnaires and vibration-controlled transient elastography between April 2016 and April 2019. They analyzed the association between fibrosis and several alcohol-use measures, including total consumption and drinking patterns, among nonheavy alcohol users whose liver disease would be classified as “nonalcoholic” by current nomenclature.
The research team defined clinically significant fibrosis as a liver stiffness measurement of 8.2 kPa or higher. For at-risk NASH, the researchers used two FibroScan-AST (FAST) score thresholds – greater than 0.35 or 0.67 and higher. They also considered additional metabolic factors such as physical activity, body mass index, blood pressure, glucose measures, and metabolic syndrome.
Participants were asked to estimate the frequency of alcohol use (average number of drinking days per week during the past year) and the usual quantity of alcohol consumed (average number of drinks on a typical drinking day during the past year). Researchers multiplied the figures to estimate the average total number of drinks per week.
Among the 2,629 current drinkers (53% women, 47% men), the average age was 54 years, 7.2% had diabetes, and 26.9% met the criteria for metabolic syndrome. Participants drank about 3 days per week on average with a usual consumption of two drinks per drinking day, averaging a total weekly alcohol consumption of six drinks.
The average liver stiffness measurement was 5.6 kPa, and 8.2% had significant fibrosis.
At the FAST score threshold of 0.67 or greater, 1.9% of participants were likely to have at-risk NASH, with a higher prevalence in those with obesity (4.5%) or diabetes (9.5%). At the FAST score threshold of greater than 0.35, the prevalence of at-risk NASH was 12.4%, which was higher in those with obesity (26.3%) or diabetes (34.4%).
Overall, an increased total number of drinks per week and higher frequency of drinking days were associated with increased odds of fibrosis.
Almost 17.5% of participants engaged in risky weekly drinking, which was defined as 8 or more drinks per week for women and 15 or more drinks per week for men. Risky weekly drinking was also associated with higher odds of fibrosis.
After excluding 158 heavy drinkers, the prevalence of fibrosis was unchanged at 8%, and an increased total of drinks per week remained significantly associated with fibrosis.
In addition, multiple alcohol-use measures were positively associated with a FAST score greater than 0.35 and were similar after excluding heavy alcohol users. These measures include the number of drinks per week, the frequency of drinking days, and binge drinking.
“We showed that nonheavy alcohol use is associated with fibrosis and at-risk NASH, which are both predictors of long-term liver-related morbidity and mortality,” Dr. Rice said.
Implications for patient care
The findings have important implications for both NAFLD clinical trials and patient care, the study authors wrote. For instance, the U.S. Dietary Guidelines for Americans recommend limiting alcohol use to one drink per day for women and two drinks per day for men.
“Our results reinforce the importance of encouraging all patients to reduce alcohol intake as much as possible and to at least adhere to current U.S. Dietary Guidelines recommended limits,” Dr. Rice said. “Almost half of participants in our study consumed in excess of these limits, which strongly associated with at-risk NASH.”
Additional long-term studies are needed to determine the benefits of limiting alcohol consumption to reduce liver-related morbidity and mortality, the authors wrote.
The effect of alcohol consumption on liver health “has been controversial, since some studies have suggested that nonheavy alcohol use can even have some beneficial metabolic effects and has been associated with reduced risk of fatty liver disease, while other studies have found that nonheavy alcohol use is associated with increased risk for liver-related clinical outcomes,” Fredrik Åberg, MD, PhD, a hepatologist and liver transplant specialist at Helsinki University Hospital, said in an interview.
Dr. Åberg wasn’t involved with this study but has researched alcohol consumption and liver disease. Among non–heavy alcohol users, drinking more alcohol per week is associated with increased hospitalization for liver disease, hepatocellular carcinoma, and liver-related death, he and his colleagues have found.
“We concluded that the net effect of non-heavy drinking on the liver is harm,” he said. “Overall, this study by Rice and colleagues supports the recommendation that persons with mild liver disease should reduce their drinking, and persons with severe liver disease (cirrhosis and advanced fibrosis) should abstain from alcohol use.”
The study authors are supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases, a Doris Duke Charitable Foundation Grant, a Gilead Sciences Research Scholars Award, the Boston University Department of Medicine Career Investment Award, and the Boston University Clinical Translational Science Institute. The Framingham Heart Study is supported in part by the National Heart, Lung, and Blood Institute. The authors and Dr. Åberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY