User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Coming to a pill near you: The exercise molecule
Exercise in a pill? Sign us up
You just got home from a long shift and you know you should go to the gym, but the bed is calling and you just answered. We know sometimes we have to make sacrifices in the name of fitness, but there just aren’t enough hours in the day. Unless our prayers have been answered. There could be a pill that has the benefits of working out without having to work out.
In a study published in Nature, investigators reported that they have identified a molecule made during exercise and used it on mice, which took in less food after being given the pill, which may open doors to understanding how exercise affects hunger.
In the first part of the study, the researchers found the molecule, known as Lac-Phe – which is synthesized from lactate and phenylalanine – in the blood plasma of mice after they had run on a treadmill.
The investigators then gave a Lac-Phe supplement to mice on high-fat diets and found that their food intake was about 50% of what other mice were eating. The supplement also improved their glucose tolerance.
Because the research also found Lac-Phe in humans who exercised, they hope that this pill will be in our future. “Our next steps include finding more details about how Lac-Phe mediates its effects in the body, including the brain,” Yong Xu, MD, of Baylor College of Medicine, Houston, said in a written statement. “Our goal is to learn to modulate this exercise pathway for therapeutic interventions.”
As always, we are rooting for you, science!
Gonorrhea and grandparents: A match made in prehistoric heaven
*Editorial note: LOTME takes no responsibility for any unfortunate imagery the reader may have experienced from the above headline.
Old people are the greatest. Back pains, cognitive decline, aches in all the diodes down your left side, there’s nothing quite like your golden years. Notably, however, humans are one of the few animals who experience true old age, as most creatures are adapted to maximize reproductive potential. As such, living past menopause is rare in the animal kingdom.
This is where the “grandmother hypothesis” comes in: Back in Ye Olde Stone Age, women who lived into old age could provide child care for younger women, because human babies require a lot more time and attention than other animal offspring. But how did humans end up living so long? Enter a group of Californian researchers, who believe they have an answer. It was gonorrhea.
When compared with the chimpanzee genome (as well as with Neanderthals and Denisovans, our closest ancestors), humans have a unique mutated version of the CD33 gene that lacks a sugar-binding site; the standard version uses the sugar-binding site to protect against autoimmune response in the body, but that same site actually suppresses the brain’s ability to clear away damaged brain cells and amyloid, which eventually leads to diseases like dementia. The mutated version allows microglia (brain immune cells) to attack and clear out this unwanted material. People with higher levels of this mutated CD33 variant actually have higher protection against Alzheimer’s.
Interestingly, gonorrhea bacteria are coated in the same sugar that standard CD33 receptors bind to, thus allowing them to bypass the body’s immune system. According to the researchers, the mutated CD33 version likely emerged as a protection against gonorrhea, depriving the bacteria of their “molecular mimicry” abilities. In one of life’s happy accidents, it turned out this mutation also protects against age-related diseases, thus allowing humans with the mutation to live longer. Obviously, this was a good thing, and we ran with it until the modern day. Now we have senior citizens climbing Everest, and all our politicians keep on politicking into their 70s and 80s ... well, everything has its drawbacks.
Parents raise a glass to children’s food addiction
There can be something pretty addicting about processed foods. Have you ever eaten just one french fry? Or taken just one cookie? If so, your willpower is incredible. For many of us, it can be a struggle to stop.
A recent study from the University of Michigan, which considered the existence of an eating phenotype, suggests our parents’ habits could be to blame.
By administering a series of questionnaires that inquired about food addiction, alcohol use disorders, cannabis use disorder, nicotine/e-cigarette dependence, and their family tree, investigators found that participants with a “paternal history of problematic alcohol use” had higher risk of food addiction but not obesity.
Apparently about one in five people display a clinically significant addiction to highly processed foods. It was noted that foods like ice cream, pizza, and french fries have high amounts of refined carbs and fats, which could trigger an addictive response.
Lindzey Hoover, a graduate student at the university who was the study’s lead author, noted that living in an environment where these foods are cheap and accessible can be really challenging for those with a family history of addiction. The investigators suggested that public health approaches, like restriction of other substances and marketing to kids, should be put in place for highly processed foods.
Maybe french fries should come with a warning label.
A prescription for America’s traffic problems
Nostalgia is a funny thing. Do you ever feel nostalgic about things that really weren’t very pleasant in the first place? Take, for instance, the morning commute. Here in the Washington area, more than 2 years into the COVID era, the traffic is still not what it used to be … and we kind of miss it.
Nah, not really. That was just a way to get everyone thinking about driving, because AAA has something of an explanation for the situation out there on the highways and byways of America. It’s drugs. No, not those kinds of drugs. This time it’s prescription drugs that are the problem. Well, part of the problem, anyway.
AAA did a survey last summer and found that nearly 50% of drivers “used one or more potentially impairing medications in the past 30 days. … The proportion of those choosing to drive is higher among those taking multiple medications.” How much higher? More than 63% of those with two or more prescriptions were driving within 2 hours of taking at least one of those meds, as were 71% of those taking three or more.
The 2,657 respondents also were asked about the types of potentially impairing drugs they were taking: 61% of those using antidepressants had been on the road within 2 hours of use at least once in the past 30 days, as had 73% of those taking an amphetamine, AAA said.
So there you have it. That guy in the BMW who’s been tailgating you for the last 3 miles? He may be a jerk, but there’s a good chance he’s a jerk with a prescription … or two … or three.
Exercise in a pill? Sign us up
You just got home from a long shift and you know you should go to the gym, but the bed is calling and you just answered. We know sometimes we have to make sacrifices in the name of fitness, but there just aren’t enough hours in the day. Unless our prayers have been answered. There could be a pill that has the benefits of working out without having to work out.
In a study published in Nature, investigators reported that they have identified a molecule made during exercise and used it on mice, which took in less food after being given the pill, which may open doors to understanding how exercise affects hunger.
In the first part of the study, the researchers found the molecule, known as Lac-Phe – which is synthesized from lactate and phenylalanine – in the blood plasma of mice after they had run on a treadmill.
The investigators then gave a Lac-Phe supplement to mice on high-fat diets and found that their food intake was about 50% of what other mice were eating. The supplement also improved their glucose tolerance.
Because the research also found Lac-Phe in humans who exercised, they hope that this pill will be in our future. “Our next steps include finding more details about how Lac-Phe mediates its effects in the body, including the brain,” Yong Xu, MD, of Baylor College of Medicine, Houston, said in a written statement. “Our goal is to learn to modulate this exercise pathway for therapeutic interventions.”
As always, we are rooting for you, science!
Gonorrhea and grandparents: A match made in prehistoric heaven
*Editorial note: LOTME takes no responsibility for any unfortunate imagery the reader may have experienced from the above headline.
Old people are the greatest. Back pains, cognitive decline, aches in all the diodes down your left side, there’s nothing quite like your golden years. Notably, however, humans are one of the few animals who experience true old age, as most creatures are adapted to maximize reproductive potential. As such, living past menopause is rare in the animal kingdom.
This is where the “grandmother hypothesis” comes in: Back in Ye Olde Stone Age, women who lived into old age could provide child care for younger women, because human babies require a lot more time and attention than other animal offspring. But how did humans end up living so long? Enter a group of Californian researchers, who believe they have an answer. It was gonorrhea.
When compared with the chimpanzee genome (as well as with Neanderthals and Denisovans, our closest ancestors), humans have a unique mutated version of the CD33 gene that lacks a sugar-binding site; the standard version uses the sugar-binding site to protect against autoimmune response in the body, but that same site actually suppresses the brain’s ability to clear away damaged brain cells and amyloid, which eventually leads to diseases like dementia. The mutated version allows microglia (brain immune cells) to attack and clear out this unwanted material. People with higher levels of this mutated CD33 variant actually have higher protection against Alzheimer’s.
Interestingly, gonorrhea bacteria are coated in the same sugar that standard CD33 receptors bind to, thus allowing them to bypass the body’s immune system. According to the researchers, the mutated CD33 version likely emerged as a protection against gonorrhea, depriving the bacteria of their “molecular mimicry” abilities. In one of life’s happy accidents, it turned out this mutation also protects against age-related diseases, thus allowing humans with the mutation to live longer. Obviously, this was a good thing, and we ran with it until the modern day. Now we have senior citizens climbing Everest, and all our politicians keep on politicking into their 70s and 80s ... well, everything has its drawbacks.
Parents raise a glass to children’s food addiction
There can be something pretty addicting about processed foods. Have you ever eaten just one french fry? Or taken just one cookie? If so, your willpower is incredible. For many of us, it can be a struggle to stop.
A recent study from the University of Michigan, which considered the existence of an eating phenotype, suggests our parents’ habits could be to blame.
By administering a series of questionnaires that inquired about food addiction, alcohol use disorders, cannabis use disorder, nicotine/e-cigarette dependence, and their family tree, investigators found that participants with a “paternal history of problematic alcohol use” had higher risk of food addiction but not obesity.
Apparently about one in five people display a clinically significant addiction to highly processed foods. It was noted that foods like ice cream, pizza, and french fries have high amounts of refined carbs and fats, which could trigger an addictive response.
Lindzey Hoover, a graduate student at the university who was the study’s lead author, noted that living in an environment where these foods are cheap and accessible can be really challenging for those with a family history of addiction. The investigators suggested that public health approaches, like restriction of other substances and marketing to kids, should be put in place for highly processed foods.
Maybe french fries should come with a warning label.
A prescription for America’s traffic problems
Nostalgia is a funny thing. Do you ever feel nostalgic about things that really weren’t very pleasant in the first place? Take, for instance, the morning commute. Here in the Washington area, more than 2 years into the COVID era, the traffic is still not what it used to be … and we kind of miss it.
Nah, not really. That was just a way to get everyone thinking about driving, because AAA has something of an explanation for the situation out there on the highways and byways of America. It’s drugs. No, not those kinds of drugs. This time it’s prescription drugs that are the problem. Well, part of the problem, anyway.
AAA did a survey last summer and found that nearly 50% of drivers “used one or more potentially impairing medications in the past 30 days. … The proportion of those choosing to drive is higher among those taking multiple medications.” How much higher? More than 63% of those with two or more prescriptions were driving within 2 hours of taking at least one of those meds, as were 71% of those taking three or more.
The 2,657 respondents also were asked about the types of potentially impairing drugs they were taking: 61% of those using antidepressants had been on the road within 2 hours of use at least once in the past 30 days, as had 73% of those taking an amphetamine, AAA said.
So there you have it. That guy in the BMW who’s been tailgating you for the last 3 miles? He may be a jerk, but there’s a good chance he’s a jerk with a prescription … or two … or three.
Exercise in a pill? Sign us up
You just got home from a long shift and you know you should go to the gym, but the bed is calling and you just answered. We know sometimes we have to make sacrifices in the name of fitness, but there just aren’t enough hours in the day. Unless our prayers have been answered. There could be a pill that has the benefits of working out without having to work out.
In a study published in Nature, investigators reported that they have identified a molecule made during exercise and used it on mice, which took in less food after being given the pill, which may open doors to understanding how exercise affects hunger.
In the first part of the study, the researchers found the molecule, known as Lac-Phe – which is synthesized from lactate and phenylalanine – in the blood plasma of mice after they had run on a treadmill.
The investigators then gave a Lac-Phe supplement to mice on high-fat diets and found that their food intake was about 50% of what other mice were eating. The supplement also improved their glucose tolerance.
Because the research also found Lac-Phe in humans who exercised, they hope that this pill will be in our future. “Our next steps include finding more details about how Lac-Phe mediates its effects in the body, including the brain,” Yong Xu, MD, of Baylor College of Medicine, Houston, said in a written statement. “Our goal is to learn to modulate this exercise pathway for therapeutic interventions.”
As always, we are rooting for you, science!
Gonorrhea and grandparents: A match made in prehistoric heaven
*Editorial note: LOTME takes no responsibility for any unfortunate imagery the reader may have experienced from the above headline.
Old people are the greatest. Back pains, cognitive decline, aches in all the diodes down your left side, there’s nothing quite like your golden years. Notably, however, humans are one of the few animals who experience true old age, as most creatures are adapted to maximize reproductive potential. As such, living past menopause is rare in the animal kingdom.
This is where the “grandmother hypothesis” comes in: Back in Ye Olde Stone Age, women who lived into old age could provide child care for younger women, because human babies require a lot more time and attention than other animal offspring. But how did humans end up living so long? Enter a group of Californian researchers, who believe they have an answer. It was gonorrhea.
When compared with the chimpanzee genome (as well as with Neanderthals and Denisovans, our closest ancestors), humans have a unique mutated version of the CD33 gene that lacks a sugar-binding site; the standard version uses the sugar-binding site to protect against autoimmune response in the body, but that same site actually suppresses the brain’s ability to clear away damaged brain cells and amyloid, which eventually leads to diseases like dementia. The mutated version allows microglia (brain immune cells) to attack and clear out this unwanted material. People with higher levels of this mutated CD33 variant actually have higher protection against Alzheimer’s.
Interestingly, gonorrhea bacteria are coated in the same sugar that standard CD33 receptors bind to, thus allowing them to bypass the body’s immune system. According to the researchers, the mutated CD33 version likely emerged as a protection against gonorrhea, depriving the bacteria of their “molecular mimicry” abilities. In one of life’s happy accidents, it turned out this mutation also protects against age-related diseases, thus allowing humans with the mutation to live longer. Obviously, this was a good thing, and we ran with it until the modern day. Now we have senior citizens climbing Everest, and all our politicians keep on politicking into their 70s and 80s ... well, everything has its drawbacks.
Parents raise a glass to children’s food addiction
There can be something pretty addicting about processed foods. Have you ever eaten just one french fry? Or taken just one cookie? If so, your willpower is incredible. For many of us, it can be a struggle to stop.
A recent study from the University of Michigan, which considered the existence of an eating phenotype, suggests our parents’ habits could be to blame.
By administering a series of questionnaires that inquired about food addiction, alcohol use disorders, cannabis use disorder, nicotine/e-cigarette dependence, and their family tree, investigators found that participants with a “paternal history of problematic alcohol use” had higher risk of food addiction but not obesity.
Apparently about one in five people display a clinically significant addiction to highly processed foods. It was noted that foods like ice cream, pizza, and french fries have high amounts of refined carbs and fats, which could trigger an addictive response.
Lindzey Hoover, a graduate student at the university who was the study’s lead author, noted that living in an environment where these foods are cheap and accessible can be really challenging for those with a family history of addiction. The investigators suggested that public health approaches, like restriction of other substances and marketing to kids, should be put in place for highly processed foods.
Maybe french fries should come with a warning label.
A prescription for America’s traffic problems
Nostalgia is a funny thing. Do you ever feel nostalgic about things that really weren’t very pleasant in the first place? Take, for instance, the morning commute. Here in the Washington area, more than 2 years into the COVID era, the traffic is still not what it used to be … and we kind of miss it.
Nah, not really. That was just a way to get everyone thinking about driving, because AAA has something of an explanation for the situation out there on the highways and byways of America. It’s drugs. No, not those kinds of drugs. This time it’s prescription drugs that are the problem. Well, part of the problem, anyway.
AAA did a survey last summer and found that nearly 50% of drivers “used one or more potentially impairing medications in the past 30 days. … The proportion of those choosing to drive is higher among those taking multiple medications.” How much higher? More than 63% of those with two or more prescriptions were driving within 2 hours of taking at least one of those meds, as were 71% of those taking three or more.
The 2,657 respondents also were asked about the types of potentially impairing drugs they were taking: 61% of those using antidepressants had been on the road within 2 hours of use at least once in the past 30 days, as had 73% of those taking an amphetamine, AAA said.
So there you have it. That guy in the BMW who’s been tailgating you for the last 3 miles? He may be a jerk, but there’s a good chance he’s a jerk with a prescription … or two … or three.
A hypogastric nerve-focused approach to nerve-sparing endometriosis surgery
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.
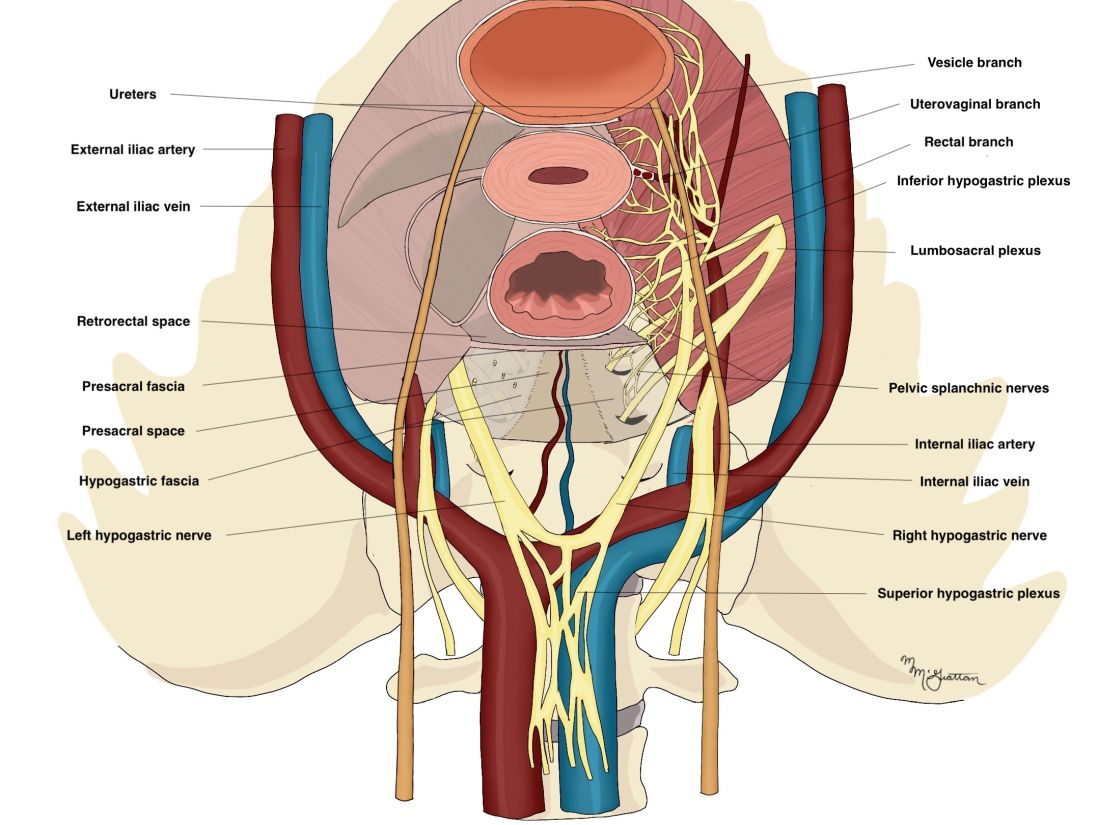
The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7
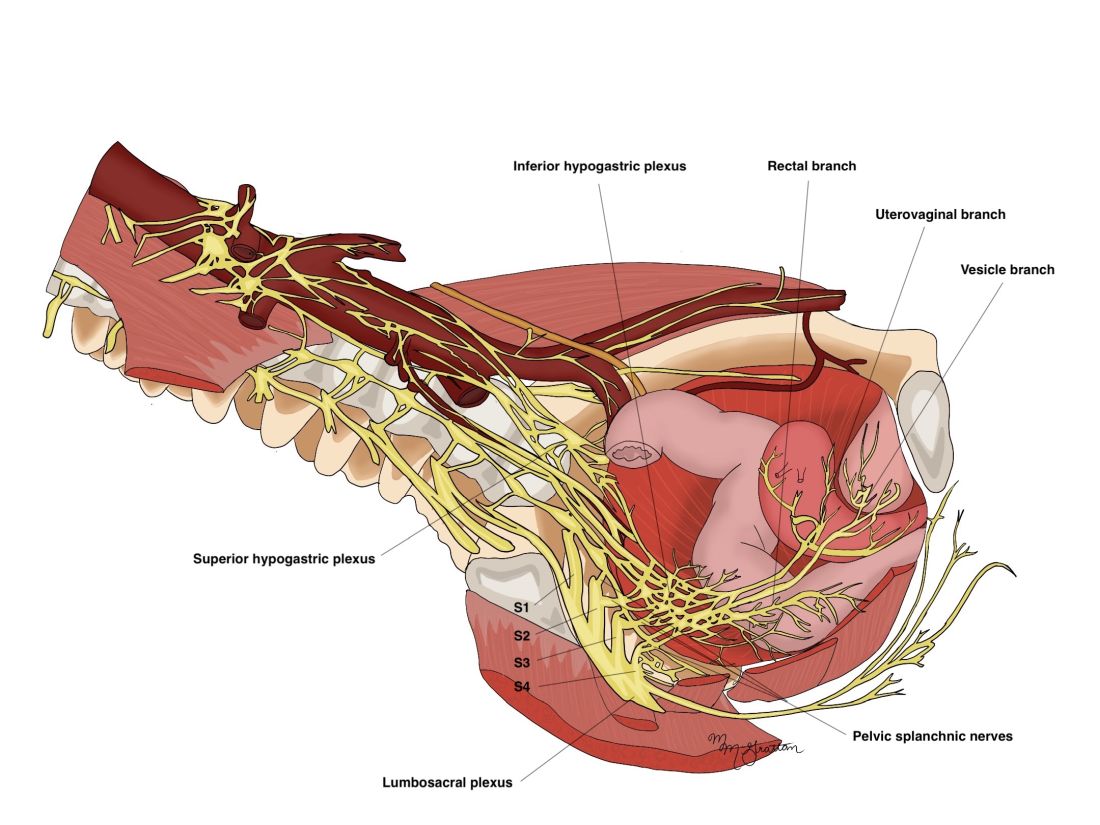
The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.

The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7

The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Radical resection of deep infiltrating endometriosis (DIE) or pelvic malignancies can lead to inadvertent damage to the pelvic autonomic nerve bundles, causing urinary dysfunction in up to 41% of cases, as well as anorectal and sexual dysfunction.1 Each of these sequelae can significantly affect the patient’s quality of life.
Nerve-sparing techniques have therefore been a trending topic in gynecologic surgery in the 21st century, starting with papers by Marc Possover, MD, of Switzerland, on the laparoscopic neuronavigation (LANN) technique. In an important 2005 publication, he described how the LANN technique can significantly reduce postoperative functional morbidity in laparoscopic radical pelvic surgery.2
The LANN method utilizes intraoperative neurostimulation to identify and dissect the intrapelvic nerve bundles away from surrounding tissue prior to dissection of the DIE or pelvic malignancies. The nerves are exposed and preserved under direct visualization in a fashion similar to that used to expose and preserve the ureters. Pelvic dissection using the LANN technique is extensive and occurs down to the level of the sacral nerve roots.
Dr. Possover’s 2005 paper and others like it spurred increased awareness of the intrapelvic part of the autonomic nervous system – in particular, the hypogastric nerves, the pelvic splanchnic nerves, and the inferior hypogastric plexus. Across additional published studies, nerve-sparing techniques were shown to be effective in preserving neurologic pelvic functions, with significantly less urinary retention and rectal/sexual dysfunction than seen with traditional laparoscopy techniques.
For example, in a single-center prospective clinical trial reported in 2012, 56 of 65 (86.2%) patients treated with a classical laparoscopic technique for excision of DIE reported neurologic pelvic dysfunctions, compared with 1 of 61 (1.6%) patients treated with a nerve-sparing approach.3
While research has confirmed the importance of nerve-sparing techniques, it also shone light on the reality that the LANN technique is extremely technically challenging and requires a high level of surgical expertise and advanced training. In my teaching of the technique, I also saw that few gynecologic surgeons were able to incorporate the advanced nerve-sparing technique into their practices.
A group consisting of myself and collaborators at the University of Bologna, Italy, and the University of Cambridge, England, recently developed an alternative to the LANN approach that uses the hypogastric nerves as landmarks. The technique requires less dissection and should be technically achievable when the pelvic neuroanatomy and anatomy of the presacral fascia are well understood. The hypogastric nerve is identified and used as a landmark to preserve the deeper autonomic nerve bundles in the pelvis without exposure and without more extensive dissection to the level of the sacral nerve roots.4,5
This hypogastric nerve-based technique will cover the vast majority of radical surgeries for DIE. When more advanced nerve sparing and more extensive dissection is needed for the very deepest levels of disease infiltration, patients can be referred to surgeons with advanced training, comfort, and experience with the LANN technique.
The pelvic neuroanatomy
As described in our video articles published in 2015 in Fertility and Sterility6 and 2019 in the Journal of Minimally Invasive Gynecology,5 the left and right hypogastric nerves are the main sympathetic nerves of the autonomic nervous system in the pelvis. They originate from the superior hypogastric plexus and, at the level of the middle rectal vessels, they join the pelvic sacral splanchnic nerves to form the inferior hypogastric plexus. They are easily identifiable at their origin and are the most superficial and readily identifiable component of the inferior hypogastric plexus.

The sympathetic input from the hypogastric nerves causes the internal urethral and anal sphincters to contract, as well as detrusor relaxation and a reduction of peristalsis of the descending colon, sigmoid, and rectum; thus, hypogastric nerve input promotes continence.
The hypogastric nerves also carry afferent signals for pelvic visceral proprioception. Lesion to the hypogastric nerves will usually be subclinical and will put the patient at risk for unnoticeable bladder distension, which usually becomes symptomatic about 7 years after the procedure.7

The thin pelvic splanchnic nerves – which merge with the hypogastric nerves into the pararectal fossae to form the inferior hypogastric plexus – arise from nerve roots S2 and S4 and carry all parasympathetic signals to the bladder, rectum, and the sigmoid and left colons. Lesions to these bundles are the main cause of neurogenic urinary retention.
The inferior hypogastric plexi split into the vesical, uterine, and rectal branches, which carry the sympathetic, parasympathetic, and sensory fibers from both the splanchnic and hypogastric nerves. Damage to the inferior hypogastric plexi and/or its branches may induce severe dysfunction to the target organs of the injured fibers.
A focus on the hypogastric nerve
Our approach was developed after we studied the anatomic reliability of the hypogastric nerves through a prospective observational study consisting of measurements during five cadaveric dissections and 10 in-vivo laparoscopic surgeries for rectosigmoid endometriosis.4 We took an interfascial approach to dissection.
Our goal was to clarify the distances between the hypogastric nerves and the ureters, the midsagittal plane, the midcervical plane, and the uterosacral ligaments in each hemipelvis, and in doing so, enable identification of the hypogastric nerves and establish recognizable limits for dissection.
We found quite a bit of variance in the anatomic position and appearance of the hypogastric nerves, but the variances were not very broad. Most notably, the right hypogastric nerve was significantly farther toward the ureter (mean, 14.5 mm; range, 10-25 mm) than the left one (mean, 8.6 mm; range, 7-12 mm).
The ureters were a good landmark for identification of the hypogastric nerves because the nerves were consistently found medially and posteriorly to the ureter at a mean distance of 11.6 mm. Overall, we demonstrated reproducibility in the identification and dissection of the hypogastric nerves using recognizable interfascial planes and anatomic landmarks.4
With good anatomic understanding, a stepwise approach can be taken to identify and preserve the hypogastric nerve and the deeper inferior hypogastric plexus without the need for more extensive dissection.
As shown in our 2019 video, the right hypogastric nerves can be identified transperitoneally in most cases.5 For confirmation, a gentle anterior pulling on the hypogastric nerve causes a caudal movement of the peritoneum overlying the superior hypogastric plexus. (Intermittent pulling on the nerve can also be helpful in localizing the left hypogastric nerve.)
To dissect a hypogastric nerve, the retroperitoneum is opened at the level of the pelvic brim, just inferomedially to the external iliac vessels, and the incision is extended anteriorly, with gentle dissection of the underlying tissue until the ureter is identified.
Once the ureter is identified and lateralized, dissection along the peritoneum is carried deeper and medially into the pelvis until the hypogastric nerve is identified. Lateral to this area are the internal iliac artery, the branching uterine artery, and the obliterated umbilical ligament. In the left hemipelvis, the hypogastric nerve can reliably be found at a mean distance of 8.6 mm from the ureter, while the right one will be found on average 14.5 mm away.
The hypogastric nerves form the posteromedial limit for a safe and simple nerve-sparing dissection. Any dissection posteriorly and laterally to these landmarks should start with the identification of sacral nerve roots and hypogastric nerves.
Dr. Lemos reported that he has no relevant disclosures.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto.
References
1. Imboden S et al. J Minim Invasive Gynecol. 2021 Aug;28(8):1544-51. doi: 10.1016/j.jmig.2021.01.009.
2. Possover M et al. J Am Coll Surg. 2005;201(6):913-7. doi: 10.1016/j.jamcollsurg.2005.07.006.
3. Ceccaroni M et al. Surg Endosc. 2012;26(7):2029-45. doi: 10.1007/s00464-012-2153-3.
4. Seracchioli R et al. J Minim Invasive Gynecol. 2019;26(7):1340-5. doi: 10.1016/j.jmig.2019.01.010.
5. Zakhari A et al. J Minim Invasive Gynecol. 2020;27(4):813-4. doi: 10.1016/j.jmig.2019.08.001
6. Lemos N et al. Fertil Steril. 2015 Nov;104(5):e11-2. doi: 10.1016/j.fertnstert.2015.07.1138.
7. Possover M. Fertil Steril. 2014 Mar;101(3):754-8. doi: 10.1016/j.fertnstert.2013.12.019.
Spare the nerves in deep infiltrative endometriosis surgery
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
The pelvic autonomic nerves are responsible for the neurogenic control of the rectum and bladder and for sexual arousal. Over the past 30 years, different nerve-sparing techniques have been recommended and adopted to minimize risk of urinary or rectal dysfunction and incontinence, as well as sexual dysfunction, in radical surgery for rectal and early cervical cancer without compromising surgical outcome.
As the treatment of deep infiltrative endometriosis has become more aggressive and radical, it is certainly feasible to consider nerve-sparing techniques at the time of dissection and endometriosis excision to minimize the known risk of urinary, rectal, and sexual dysfunction. Interestingly, because endometriosis generally follows an asymmetric distribution, effect on bladder function is not as problematic as it is in the case of cancer surgery.
Early innovators include Dr. Marc Possover from Switzerland and Dr. Marcello Ceccaroni from Italy. Both physicians are superior pelvic neuroanatomists. Both describe meticulous and extensive dissection of the nerves of the pelvis at the time of excision of deep infiltrative endometriosis. Unfortunately, their techniques would appear to be beyond the scope of even the most experienced excisional surgeons.
A simplified approach to nerve sparing at the time of excision of deep infiltrative endometriosis has been developed by our guest author, Dr. Nucelio Lemos, in collaboration with physicians at the University of Bologna and the University of Cambridge. By using the hypogastric nerves as the landmark, they have developed a more surgeon friendly and less radical approach to nerve sparing at the time of deep infiltrative endometriosis surgery.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of both Dr. Lemos and his fellow in advanced gynecologic surgery, Dr. Meghan McGrattan, from Mount Sinai and Women’s College Hospital in Toronto. Dr. McGrattan drew the anatomic illustrations that accompany Dr. Lemos’ description of the new technique.
Dr. Lemos is associate professor in the department of obstetrics and gynecology at the University of Toronto. He specializes in pelvic pain, pelvic floor dysfunction, pelvic organ prolapse, endometriosis, and neuropelveology. Dr. Lemos is a founding member and second vice president of the International Society of Neuropelveology. In addition, Dr. Lemos started the Pelvic Functional Surgery and Neuropelveology Clinic in the department of obstetrics and gynecology of Mount Sinai and Women’s College Hospitals, Toronto.
It is a pleasure and honor to welcome Dr. Lemos and Dr. McGrattan to this addition of the Master Class in Gynecologic Surgery.
Dr. Miller is a professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago, Ill. He has no conflicts of interest to report.
U.S. News issues top hospitals list, now with expanded health equity measures
For the seventh consecutive year, the Mayo Clinic in Rochester, Minn., took the top spot in the annual honor roll of best hospitals, published July 26 by U.S. News & World Report.
The 2022 rankings, which marks the 33rd edition, showcase several methodology changes, including new ratings for ovarian, prostate, and uterine cancer surgeries that “provide patients ... with previously unavailable information to assist them in making a critical health care decision,” a news release from the publication explains.
said the release. Finally, a new metric called “home time” determines how successfully each hospital helps patients return home.
Mayo Clinic remains No. 1
For the 2022-2023 rankings and ratings, U.S. News compared more than 4,500 medical centers across the country in 15 specialties and 20 procedures and conditions. Of these, 493 were recognized as Best Regional Hospitals as a result of their overall strong performance.
The list was then narrowed to the top 20 hospitals, outlined in the honor roll below, that deliver “exceptional treatment across multiple areas of care.”
Following Mayo Clinic in the annual ranking’s top spot, Cedars-Sinai Medical Center in Los Angeles rises from No. 6 to No. 2, and New York University Langone Hospitals finish third, up from eighth in 2021.
Cleveland Clinic in Ohio holds the No. 4 spot, down two from 2021, while Johns Hopkins Hospital in Baltimore and UCLA Medical Center in Los Angeles tie for fifth place. Rounding out the top 10, in order, are: New York–Presbyterian Hospital–Columbia and Cornell, New York; Massachusetts General Hospital, Boston; Northwestern Memorial Hospital, Chicago; Stanford (Calif.) Health Care–Stanford Hospital.
The following hospitals complete the top 20 in the United States:
- 11. Barnes-Jewish Hospital, St. Louis
- 12. UCSF Medical Center, San Francisco
- 13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
- 14. Brigham and Women’s Hospital, Boston
- 15. Houston Methodist Hospital
- 16. Mount Sinai Hospital, New York
- 17. University of Michigan Health–Michigan Medicine, Ann Arbor
- 18. Mayo Clinic–Phoenix
- 19. Vanderbilt University Medical Center, Nashville, Tenn.
- 20. Rush University Medical Center, Chicago
For the specialty rankings, the University of Texas MD Anderson Cancer Center, Houston, remains No. 1 in cancer care, the Cleveland Clinic is No. 1 in cardiology and heart surgery, and the Hospital for Special Surgery in New York is No. 1 in orthopedics.
Top five for cancer
- 1. University of Texas MD Anderson Cancer Center, Houston
- 2. Memorial Sloan Kettering Cancer Center, New York
- 3. Mayo Clinic, Rochester, Minn.
- 4. Dana-Farber/Brigham and Women’s Cancer Center, Boston
- 5. UCLA Medical Center, Los Angeles
Top five for cardiology and heart surgery
- 1. Cleveland Clinic
- 2. Mayo Clinic, Rochester, Minn.
- 3. Cedars-Sinai Medical Center, Los Angeles
- 4. New York–Presbyterian Hospital–Columbia and Cornell, New York
- 5. New York University Langone Hospitals
Top five for orthopedics
- 1. Hospital for Special Surgery, New York
- 2. Mayo Clinic, Rochester, Minn.
- 3. Cedars-Sinai Medical Center, Los Angeles
- 4. New York University Langone Hospitals
- 5. (tie) Rush University Medical Center, Chicago
- 5. (tie) UCLA Medical Center, Los Angeles
According to the news release, the procedures and conditions ratings are based entirely on objective patient care measures like survival rates, patient experience, home time, and level of nursing care. The Best Hospitals rankings consider a variety of data provided by the Centers for Medicare & Medicaid Services, American Hospital Association, professional organizations, and medical specialists.
The full report is available online.
A version of this article first appeared on Medscape.com.
For the seventh consecutive year, the Mayo Clinic in Rochester, Minn., took the top spot in the annual honor roll of best hospitals, published July 26 by U.S. News & World Report.
The 2022 rankings, which marks the 33rd edition, showcase several methodology changes, including new ratings for ovarian, prostate, and uterine cancer surgeries that “provide patients ... with previously unavailable information to assist them in making a critical health care decision,” a news release from the publication explains.
said the release. Finally, a new metric called “home time” determines how successfully each hospital helps patients return home.
Mayo Clinic remains No. 1
For the 2022-2023 rankings and ratings, U.S. News compared more than 4,500 medical centers across the country in 15 specialties and 20 procedures and conditions. Of these, 493 were recognized as Best Regional Hospitals as a result of their overall strong performance.
The list was then narrowed to the top 20 hospitals, outlined in the honor roll below, that deliver “exceptional treatment across multiple areas of care.”
Following Mayo Clinic in the annual ranking’s top spot, Cedars-Sinai Medical Center in Los Angeles rises from No. 6 to No. 2, and New York University Langone Hospitals finish third, up from eighth in 2021.
Cleveland Clinic in Ohio holds the No. 4 spot, down two from 2021, while Johns Hopkins Hospital in Baltimore and UCLA Medical Center in Los Angeles tie for fifth place. Rounding out the top 10, in order, are: New York–Presbyterian Hospital–Columbia and Cornell, New York; Massachusetts General Hospital, Boston; Northwestern Memorial Hospital, Chicago; Stanford (Calif.) Health Care–Stanford Hospital.
The following hospitals complete the top 20 in the United States:
- 11. Barnes-Jewish Hospital, St. Louis
- 12. UCSF Medical Center, San Francisco
- 13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
- 14. Brigham and Women’s Hospital, Boston
- 15. Houston Methodist Hospital
- 16. Mount Sinai Hospital, New York
- 17. University of Michigan Health–Michigan Medicine, Ann Arbor
- 18. Mayo Clinic–Phoenix
- 19. Vanderbilt University Medical Center, Nashville, Tenn.
- 20. Rush University Medical Center, Chicago
For the specialty rankings, the University of Texas MD Anderson Cancer Center, Houston, remains No. 1 in cancer care, the Cleveland Clinic is No. 1 in cardiology and heart surgery, and the Hospital for Special Surgery in New York is No. 1 in orthopedics.
Top five for cancer
- 1. University of Texas MD Anderson Cancer Center, Houston
- 2. Memorial Sloan Kettering Cancer Center, New York
- 3. Mayo Clinic, Rochester, Minn.
- 4. Dana-Farber/Brigham and Women’s Cancer Center, Boston
- 5. UCLA Medical Center, Los Angeles
Top five for cardiology and heart surgery
- 1. Cleveland Clinic
- 2. Mayo Clinic, Rochester, Minn.
- 3. Cedars-Sinai Medical Center, Los Angeles
- 4. New York–Presbyterian Hospital–Columbia and Cornell, New York
- 5. New York University Langone Hospitals
Top five for orthopedics
- 1. Hospital for Special Surgery, New York
- 2. Mayo Clinic, Rochester, Minn.
- 3. Cedars-Sinai Medical Center, Los Angeles
- 4. New York University Langone Hospitals
- 5. (tie) Rush University Medical Center, Chicago
- 5. (tie) UCLA Medical Center, Los Angeles
According to the news release, the procedures and conditions ratings are based entirely on objective patient care measures like survival rates, patient experience, home time, and level of nursing care. The Best Hospitals rankings consider a variety of data provided by the Centers for Medicare & Medicaid Services, American Hospital Association, professional organizations, and medical specialists.
The full report is available online.
A version of this article first appeared on Medscape.com.
For the seventh consecutive year, the Mayo Clinic in Rochester, Minn., took the top spot in the annual honor roll of best hospitals, published July 26 by U.S. News & World Report.
The 2022 rankings, which marks the 33rd edition, showcase several methodology changes, including new ratings for ovarian, prostate, and uterine cancer surgeries that “provide patients ... with previously unavailable information to assist them in making a critical health care decision,” a news release from the publication explains.
said the release. Finally, a new metric called “home time” determines how successfully each hospital helps patients return home.
Mayo Clinic remains No. 1
For the 2022-2023 rankings and ratings, U.S. News compared more than 4,500 medical centers across the country in 15 specialties and 20 procedures and conditions. Of these, 493 were recognized as Best Regional Hospitals as a result of their overall strong performance.
The list was then narrowed to the top 20 hospitals, outlined in the honor roll below, that deliver “exceptional treatment across multiple areas of care.”
Following Mayo Clinic in the annual ranking’s top spot, Cedars-Sinai Medical Center in Los Angeles rises from No. 6 to No. 2, and New York University Langone Hospitals finish third, up from eighth in 2021.
Cleveland Clinic in Ohio holds the No. 4 spot, down two from 2021, while Johns Hopkins Hospital in Baltimore and UCLA Medical Center in Los Angeles tie for fifth place. Rounding out the top 10, in order, are: New York–Presbyterian Hospital–Columbia and Cornell, New York; Massachusetts General Hospital, Boston; Northwestern Memorial Hospital, Chicago; Stanford (Calif.) Health Care–Stanford Hospital.
The following hospitals complete the top 20 in the United States:
- 11. Barnes-Jewish Hospital, St. Louis
- 12. UCSF Medical Center, San Francisco
- 13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
- 14. Brigham and Women’s Hospital, Boston
- 15. Houston Methodist Hospital
- 16. Mount Sinai Hospital, New York
- 17. University of Michigan Health–Michigan Medicine, Ann Arbor
- 18. Mayo Clinic–Phoenix
- 19. Vanderbilt University Medical Center, Nashville, Tenn.
- 20. Rush University Medical Center, Chicago
For the specialty rankings, the University of Texas MD Anderson Cancer Center, Houston, remains No. 1 in cancer care, the Cleveland Clinic is No. 1 in cardiology and heart surgery, and the Hospital for Special Surgery in New York is No. 1 in orthopedics.
Top five for cancer
- 1. University of Texas MD Anderson Cancer Center, Houston
- 2. Memorial Sloan Kettering Cancer Center, New York
- 3. Mayo Clinic, Rochester, Minn.
- 4. Dana-Farber/Brigham and Women’s Cancer Center, Boston
- 5. UCLA Medical Center, Los Angeles
Top five for cardiology and heart surgery
- 1. Cleveland Clinic
- 2. Mayo Clinic, Rochester, Minn.
- 3. Cedars-Sinai Medical Center, Los Angeles
- 4. New York–Presbyterian Hospital–Columbia and Cornell, New York
- 5. New York University Langone Hospitals
Top five for orthopedics
- 1. Hospital for Special Surgery, New York
- 2. Mayo Clinic, Rochester, Minn.
- 3. Cedars-Sinai Medical Center, Los Angeles
- 4. New York University Langone Hospitals
- 5. (tie) Rush University Medical Center, Chicago
- 5. (tie) UCLA Medical Center, Los Angeles
According to the news release, the procedures and conditions ratings are based entirely on objective patient care measures like survival rates, patient experience, home time, and level of nursing care. The Best Hospitals rankings consider a variety of data provided by the Centers for Medicare & Medicaid Services, American Hospital Association, professional organizations, and medical specialists.
The full report is available online.
A version of this article first appeared on Medscape.com.
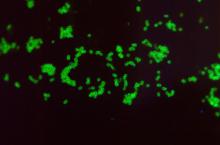
Two distinct phenotypes of COVID-related myocarditis emerge
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
‘Stunning variation’ in CV test, procedure costs revealed at top U.S. hospitals
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Prior decompensation in alcohol-associated hepatitis not an ‘absolute contraindication’ for early liver transplant
Past decompensation in alcohol-associated hepatitis may be linked with worse survival following liver transplantation, but it’s not all bad news, according to a retrospective study.
Traditionally, patients with alcoholic liver disease were asked to be alcohol free for 6 months before consideration for a liver transplantation. In recent years, there’s been a loosening of that policy, with physicians considering “early” liver transplantation (early LT) instead of waiting 6 months. “It became obvious that a lot of patients do resume alcohol use after transplant, and most of them don’t appear to suffer too much in the way of adverse consequences,” said Paul Martin, MD, chief of hepatology at the University of Miami, who was not involved in the current research.
In 2011, a study confirmed that suspicion, finding that 6-month survival was 77% among carefully selected patients with alcohol-associated hepatitis for whom the 6-month sobriety requirement was waived; 6-month survival in those who did not receive a transplant was 22%. The selection criteria included the presence of supportive family members, the absence of severe coexisting conditions, and a commitment to abstaining from alcohol.
However, authors of the current study, published in the American Journal of Gastroenterology sought nuance: The appropriateness of prior decompensation as exclusion criteria in published studies is unknown, so the researchers compared outcomes of patients with prior versus first-time liver decompensation in alcohol-associated hepatitis.
Not all bad news
The study included 241 patients from six sites who consecutively received early LT between 2007 and 2020. Among these, 210 were identified as having a first-time liver decompensation event and 31 as having had a prior history of liver decompensation, defined as being diagnosed with ascites, hepatic encephalopathy, variceal bleeding, or jaundice.
There was no significant difference in median age, Model for End-Stage Liver Disease (MELD) scores, or post–liver transplant follow-up time between those with first-time liver decompensation or a prior history. The unadjusted 1-year survival rate was 93% in the first decompensation group (95% confidence interval, 89%-96%) and 86% in the prior decompensation group (95% CI, 66%-94%). The unadjusted 3-year survival rates were 85% (95% CI, 79%-90%) and 78% (95% CI, 57%-89%), respectively.
Importantly, the researchers found an association between prior decompensation and higher adjusted post–liver transplantation mortality (adjusted hazard ratio, 2.72; 95% CI, 1.61-4.59) and harmful alcohol use (aHR, 1.77; 95% CI, 1.07-2.92).
However, the researchers noted that these patients, who had MELD scores of 39 and previous decompensation, were at exceptionally high risk of short-term mortality, but still had 1- and 3-year survival rates above 85% and 75%, respectively, with early LT. “While longer follow-up is desirable as graft failure related to alcohol is most apparent after 5-years post LT, these results suggest that prior decompensation alone should not be considered an absolute contraindication to early LT.”
Limitations of the study included its retrospective data and small sample size for patients with prior decompensation.
“These findings validate the value of the ‘first decompensation’ criteria in published experiences regarding early LT for [alcoholic hepatitis],” the investigators concluded. “Further larger and prospective studies with longer-term follow-up will be needed to assess ways to optimally select patients in this cohort who may benefit most from early LT, and ways to manage patients at highest risk for worse outcomes post LT.”
A note of caution for early LT
About half of all liver mortality is attributable to alcoholic-associated liver disease. Corticosteroids can improve short-term survival, but there are no medications proven to increase long-term survival. That leaves liver transplant as the sole alternative for patients who don’t respond to corticosteroids.
“Programs in North America have liberalized their acceptance criteria for patients with alcoholic liver disease, and that’s resulted in large numbers of patients being transplanted who have less than 6 months abstinence. And overall, the results seem good, but I think this paper strikes an appropriate note of caution. In essence, if a patient had at least one prior episode of liver failure related to alcoholic excess and had recovered from that, and continued to drink and got into trouble again, [and then] presented for consideration for liver transplantation, the fact that they resumed alcohol use after prior episodes of decompensation suggests that they may be less-than-ideal candidates [for liver transplantation],” said Dr. Martin.
He pointed out important caveats to the study, including its retrospective nature and its inclusion of a relatively small number of patients with a history of liver decompensation. But it reinforces what physicians generally know, which is that some patients with severe alcohol use disorder also have liver failure, and they tend to fare worse than others after a liver transplant.
Still, physicians also face a conundrum because there are increasing numbers of younger patients who won’t survive if they don’t get a liver transplant. “The challenge is picking out patients who are going to be good candidates from a purely medical point of view, but have a low likelihood of resuming alcohol use after transplantation [which could injure] the new liver,” said Dr. Martin. The new study has the potential to provide some additional guidance in patient selection.
The study authors disclosed no relevant conflicts of interest. Dr. Martin has no relevant financial disclosures.
Past decompensation in alcohol-associated hepatitis may be linked with worse survival following liver transplantation, but it’s not all bad news, according to a retrospective study.
Traditionally, patients with alcoholic liver disease were asked to be alcohol free for 6 months before consideration for a liver transplantation. In recent years, there’s been a loosening of that policy, with physicians considering “early” liver transplantation (early LT) instead of waiting 6 months. “It became obvious that a lot of patients do resume alcohol use after transplant, and most of them don’t appear to suffer too much in the way of adverse consequences,” said Paul Martin, MD, chief of hepatology at the University of Miami, who was not involved in the current research.
In 2011, a study confirmed that suspicion, finding that 6-month survival was 77% among carefully selected patients with alcohol-associated hepatitis for whom the 6-month sobriety requirement was waived; 6-month survival in those who did not receive a transplant was 22%. The selection criteria included the presence of supportive family members, the absence of severe coexisting conditions, and a commitment to abstaining from alcohol.
However, authors of the current study, published in the American Journal of Gastroenterology sought nuance: The appropriateness of prior decompensation as exclusion criteria in published studies is unknown, so the researchers compared outcomes of patients with prior versus first-time liver decompensation in alcohol-associated hepatitis.
Not all bad news
The study included 241 patients from six sites who consecutively received early LT between 2007 and 2020. Among these, 210 were identified as having a first-time liver decompensation event and 31 as having had a prior history of liver decompensation, defined as being diagnosed with ascites, hepatic encephalopathy, variceal bleeding, or jaundice.
There was no significant difference in median age, Model for End-Stage Liver Disease (MELD) scores, or post–liver transplant follow-up time between those with first-time liver decompensation or a prior history. The unadjusted 1-year survival rate was 93% in the first decompensation group (95% confidence interval, 89%-96%) and 86% in the prior decompensation group (95% CI, 66%-94%). The unadjusted 3-year survival rates were 85% (95% CI, 79%-90%) and 78% (95% CI, 57%-89%), respectively.
Importantly, the researchers found an association between prior decompensation and higher adjusted post–liver transplantation mortality (adjusted hazard ratio, 2.72; 95% CI, 1.61-4.59) and harmful alcohol use (aHR, 1.77; 95% CI, 1.07-2.92).
However, the researchers noted that these patients, who had MELD scores of 39 and previous decompensation, were at exceptionally high risk of short-term mortality, but still had 1- and 3-year survival rates above 85% and 75%, respectively, with early LT. “While longer follow-up is desirable as graft failure related to alcohol is most apparent after 5-years post LT, these results suggest that prior decompensation alone should not be considered an absolute contraindication to early LT.”
Limitations of the study included its retrospective data and small sample size for patients with prior decompensation.
“These findings validate the value of the ‘first decompensation’ criteria in published experiences regarding early LT for [alcoholic hepatitis],” the investigators concluded. “Further larger and prospective studies with longer-term follow-up will be needed to assess ways to optimally select patients in this cohort who may benefit most from early LT, and ways to manage patients at highest risk for worse outcomes post LT.”
A note of caution for early LT
About half of all liver mortality is attributable to alcoholic-associated liver disease. Corticosteroids can improve short-term survival, but there are no medications proven to increase long-term survival. That leaves liver transplant as the sole alternative for patients who don’t respond to corticosteroids.
“Programs in North America have liberalized their acceptance criteria for patients with alcoholic liver disease, and that’s resulted in large numbers of patients being transplanted who have less than 6 months abstinence. And overall, the results seem good, but I think this paper strikes an appropriate note of caution. In essence, if a patient had at least one prior episode of liver failure related to alcoholic excess and had recovered from that, and continued to drink and got into trouble again, [and then] presented for consideration for liver transplantation, the fact that they resumed alcohol use after prior episodes of decompensation suggests that they may be less-than-ideal candidates [for liver transplantation],” said Dr. Martin.
He pointed out important caveats to the study, including its retrospective nature and its inclusion of a relatively small number of patients with a history of liver decompensation. But it reinforces what physicians generally know, which is that some patients with severe alcohol use disorder also have liver failure, and they tend to fare worse than others after a liver transplant.
Still, physicians also face a conundrum because there are increasing numbers of younger patients who won’t survive if they don’t get a liver transplant. “The challenge is picking out patients who are going to be good candidates from a purely medical point of view, but have a low likelihood of resuming alcohol use after transplantation [which could injure] the new liver,” said Dr. Martin. The new study has the potential to provide some additional guidance in patient selection.
The study authors disclosed no relevant conflicts of interest. Dr. Martin has no relevant financial disclosures.
Past decompensation in alcohol-associated hepatitis may be linked with worse survival following liver transplantation, but it’s not all bad news, according to a retrospective study.
Traditionally, patients with alcoholic liver disease were asked to be alcohol free for 6 months before consideration for a liver transplantation. In recent years, there’s been a loosening of that policy, with physicians considering “early” liver transplantation (early LT) instead of waiting 6 months. “It became obvious that a lot of patients do resume alcohol use after transplant, and most of them don’t appear to suffer too much in the way of adverse consequences,” said Paul Martin, MD, chief of hepatology at the University of Miami, who was not involved in the current research.
In 2011, a study confirmed that suspicion, finding that 6-month survival was 77% among carefully selected patients with alcohol-associated hepatitis for whom the 6-month sobriety requirement was waived; 6-month survival in those who did not receive a transplant was 22%. The selection criteria included the presence of supportive family members, the absence of severe coexisting conditions, and a commitment to abstaining from alcohol.
However, authors of the current study, published in the American Journal of Gastroenterology sought nuance: The appropriateness of prior decompensation as exclusion criteria in published studies is unknown, so the researchers compared outcomes of patients with prior versus first-time liver decompensation in alcohol-associated hepatitis.
Not all bad news
The study included 241 patients from six sites who consecutively received early LT between 2007 and 2020. Among these, 210 were identified as having a first-time liver decompensation event and 31 as having had a prior history of liver decompensation, defined as being diagnosed with ascites, hepatic encephalopathy, variceal bleeding, or jaundice.
There was no significant difference in median age, Model for End-Stage Liver Disease (MELD) scores, or post–liver transplant follow-up time between those with first-time liver decompensation or a prior history. The unadjusted 1-year survival rate was 93% in the first decompensation group (95% confidence interval, 89%-96%) and 86% in the prior decompensation group (95% CI, 66%-94%). The unadjusted 3-year survival rates were 85% (95% CI, 79%-90%) and 78% (95% CI, 57%-89%), respectively.
Importantly, the researchers found an association between prior decompensation and higher adjusted post–liver transplantation mortality (adjusted hazard ratio, 2.72; 95% CI, 1.61-4.59) and harmful alcohol use (aHR, 1.77; 95% CI, 1.07-2.92).
However, the researchers noted that these patients, who had MELD scores of 39 and previous decompensation, were at exceptionally high risk of short-term mortality, but still had 1- and 3-year survival rates above 85% and 75%, respectively, with early LT. “While longer follow-up is desirable as graft failure related to alcohol is most apparent after 5-years post LT, these results suggest that prior decompensation alone should not be considered an absolute contraindication to early LT.”
Limitations of the study included its retrospective data and small sample size for patients with prior decompensation.
“These findings validate the value of the ‘first decompensation’ criteria in published experiences regarding early LT for [alcoholic hepatitis],” the investigators concluded. “Further larger and prospective studies with longer-term follow-up will be needed to assess ways to optimally select patients in this cohort who may benefit most from early LT, and ways to manage patients at highest risk for worse outcomes post LT.”
A note of caution for early LT
About half of all liver mortality is attributable to alcoholic-associated liver disease. Corticosteroids can improve short-term survival, but there are no medications proven to increase long-term survival. That leaves liver transplant as the sole alternative for patients who don’t respond to corticosteroids.
“Programs in North America have liberalized their acceptance criteria for patients with alcoholic liver disease, and that’s resulted in large numbers of patients being transplanted who have less than 6 months abstinence. And overall, the results seem good, but I think this paper strikes an appropriate note of caution. In essence, if a patient had at least one prior episode of liver failure related to alcoholic excess and had recovered from that, and continued to drink and got into trouble again, [and then] presented for consideration for liver transplantation, the fact that they resumed alcohol use after prior episodes of decompensation suggests that they may be less-than-ideal candidates [for liver transplantation],” said Dr. Martin.
He pointed out important caveats to the study, including its retrospective nature and its inclusion of a relatively small number of patients with a history of liver decompensation. But it reinforces what physicians generally know, which is that some patients with severe alcohol use disorder also have liver failure, and they tend to fare worse than others after a liver transplant.
Still, physicians also face a conundrum because there are increasing numbers of younger patients who won’t survive if they don’t get a liver transplant. “The challenge is picking out patients who are going to be good candidates from a purely medical point of view, but have a low likelihood of resuming alcohol use after transplantation [which could injure] the new liver,” said Dr. Martin. The new study has the potential to provide some additional guidance in patient selection.
The study authors disclosed no relevant conflicts of interest. Dr. Martin has no relevant financial disclosures.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
One thing is certain, says survey: Doctors hate taxes
For the Medscape Physicians and Taxes Report 2022, physicians shared information about their tax debt as well as how they feel about the U.S. tax code, audits, and the prospects for the future.
Even though it may not always seem that way to physicians, their family tax bills – around $75,406 on average – are in line with the other top 10% of U.S. taxpayers, according to an examination of IRS data by the Tax Foundation. However, when it comes to local taxes, the Tax Foundation found that physicians pay more than average. (Forty-three states collect tax on individual incomes.)
The average physician’s family pays a 35% marginal tax rate, compared with the top marginal tax rate in the United States of 37%. (The marginal tax rate is the highest amount of tax charged on each additional dollar after the IRS bracket rates are applied to your income.)
According to Alexis Gallati, founder of Cerebral Tax Advisors, a Knoxville, Tenn.–based firm that caters to medical professionals, doctors also should pay attention to their effective tax rate, or the percentage of income they pay in taxes. It takes into account differing tax rates on ordinary income, capital gains, and other income sources, she says. “It gives a better 30,000-foot view of your tax situation.”
Some high-income families are required to pay the Alternative Minimum Tax (AMT), though in 2019 that applied to only one-tenth of U.S. households. The AMT is designed to make sure that high earners with many options for exemptions and deductions still contribute a minimum amount of tax. Only 13% of physicians surveyed said they paid the AMT, though 29% were unsure.
Filing taxes as painful as paying them
According to a 2021 Gallup poll, 50% of Americans think they pay too much tax. (About 44% think their tax bill is about right, and a kindhearted 4% think they pay too little.) Doctors are outliers on this one, with 75% saying they pay too much in taxes.
When asked what they would do to fix the tax system, the physicians in the Medscape survey had a wide array of proposed solutions, from “drop the corporate tax rate to nearly nothing to stimulate the economy” to “everyone should pay equitably. There are too many loopholes for the very wealthy.”
Some of the complaints were less about tax rates than the process of filing. One respondent said: “I would love for this system to not be our personal responsibility. Why should it be my duty to pay someone every year to do my taxes?”
About 48% of physicians prepare their own taxes (about the same percentage as the rest of the population), with most of those filing electronically, primarily because it saves time and the software is easy to use. Intuit TurboTax was the most popular online software, with 22% of respondents saying they currently used this product.
Of those who did pay someone to prepare their taxes, the complexity of their taxes cost them; the average respondent paid about three times the average rate for the service. In the long run, the cost might have been recouped.
Navjeet Chahal, managing partner and CEO of Chahal and Associates, a San Francisco–area firm specializing in working with physicians, points out that tax advisors don’t just fill out the forms; they proactively advise physicians about how they can limit their taxes. And indeed, most respondents feel that they got their money’s worth, with 70% saying their tax preparers charged a fair fee.
Though the physicians surveyed tended to think they pay too much tax, and several mentioned particular gripes with the system, the complexity of the tax code didn’t seem to be a big issue. While 82% of Americans polled in 2021 by Pew Research said they were bothered “a lot” or “some” by the complexity of the tax system, 68% of physicians agreed or slightly agreed that the U.S. tax system “makes sense.”
Gimme a break
Physicians are the beneficiaries of several types of tax breaks. Contributing to a pretax 401(k) account was the most common exemption, with 60% of physicians surveyed using this plan. Other tax breaks cited by respondents were: contributing to charity (54%), home mortgage interest (46%), and writing off business expenses (39%).
About one in five physicians has experienced an audit, but that risk has declined significantly in recent years, thanks to tighter IRS budgets. Overall, only about 1 in 167 U.S. taxpayers were audited in 2020, according to the IRS. Even for taxpayers reporting $5 million or more in income, the audit rate is only about 0.25%, the Government Accountability Office says.
The odds of a physician being summoned to a meeting with an auditor probably won’t increase for a few years, Mr. Gallati said. But the good news for doctors is bad news for lower-income Americans. “The IRS is woefully understaffed and underfunded, with the result that the agency is going for lower-hanging fruit and auditing more people in lower income brackets,” she said in an interview.
While one respondent described his experience with the IRS as “the audit from hell,” others thought it not so bad, with 72% saying the auditors treated them fairly. One respondent described the audit as “boring, short, and successful for me. The IRS owed me money.”
When it comes to taxes, physician respondents, on the whole, did not seem to be optimistic about the future. About 61% expect an increase in their tax rate because of Biden administration policies. One respondent veered into hyperbole with the comment: “I believe taxes will increase for physicians until they have no more money!”
Mr. Chahal doesn’t see it that way. He pointed out that recent attempts to raise taxes completely failed. “I personally don’t see that happening unless there’s a significant shift in the House and the Senate.”
A version of this article first appeared on Medscape.com.
For the Medscape Physicians and Taxes Report 2022, physicians shared information about their tax debt as well as how they feel about the U.S. tax code, audits, and the prospects for the future.
Even though it may not always seem that way to physicians, their family tax bills – around $75,406 on average – are in line with the other top 10% of U.S. taxpayers, according to an examination of IRS data by the Tax Foundation. However, when it comes to local taxes, the Tax Foundation found that physicians pay more than average. (Forty-three states collect tax on individual incomes.)
The average physician’s family pays a 35% marginal tax rate, compared with the top marginal tax rate in the United States of 37%. (The marginal tax rate is the highest amount of tax charged on each additional dollar after the IRS bracket rates are applied to your income.)
According to Alexis Gallati, founder of Cerebral Tax Advisors, a Knoxville, Tenn.–based firm that caters to medical professionals, doctors also should pay attention to their effective tax rate, or the percentage of income they pay in taxes. It takes into account differing tax rates on ordinary income, capital gains, and other income sources, she says. “It gives a better 30,000-foot view of your tax situation.”
Some high-income families are required to pay the Alternative Minimum Tax (AMT), though in 2019 that applied to only one-tenth of U.S. households. The AMT is designed to make sure that high earners with many options for exemptions and deductions still contribute a minimum amount of tax. Only 13% of physicians surveyed said they paid the AMT, though 29% were unsure.
Filing taxes as painful as paying them
According to a 2021 Gallup poll, 50% of Americans think they pay too much tax. (About 44% think their tax bill is about right, and a kindhearted 4% think they pay too little.) Doctors are outliers on this one, with 75% saying they pay too much in taxes.
When asked what they would do to fix the tax system, the physicians in the Medscape survey had a wide array of proposed solutions, from “drop the corporate tax rate to nearly nothing to stimulate the economy” to “everyone should pay equitably. There are too many loopholes for the very wealthy.”
Some of the complaints were less about tax rates than the process of filing. One respondent said: “I would love for this system to not be our personal responsibility. Why should it be my duty to pay someone every year to do my taxes?”
About 48% of physicians prepare their own taxes (about the same percentage as the rest of the population), with most of those filing electronically, primarily because it saves time and the software is easy to use. Intuit TurboTax was the most popular online software, with 22% of respondents saying they currently used this product.
Of those who did pay someone to prepare their taxes, the complexity of their taxes cost them; the average respondent paid about three times the average rate for the service. In the long run, the cost might have been recouped.
Navjeet Chahal, managing partner and CEO of Chahal and Associates, a San Francisco–area firm specializing in working with physicians, points out that tax advisors don’t just fill out the forms; they proactively advise physicians about how they can limit their taxes. And indeed, most respondents feel that they got their money’s worth, with 70% saying their tax preparers charged a fair fee.
Though the physicians surveyed tended to think they pay too much tax, and several mentioned particular gripes with the system, the complexity of the tax code didn’t seem to be a big issue. While 82% of Americans polled in 2021 by Pew Research said they were bothered “a lot” or “some” by the complexity of the tax system, 68% of physicians agreed or slightly agreed that the U.S. tax system “makes sense.”
Gimme a break
Physicians are the beneficiaries of several types of tax breaks. Contributing to a pretax 401(k) account was the most common exemption, with 60% of physicians surveyed using this plan. Other tax breaks cited by respondents were: contributing to charity (54%), home mortgage interest (46%), and writing off business expenses (39%).
About one in five physicians has experienced an audit, but that risk has declined significantly in recent years, thanks to tighter IRS budgets. Overall, only about 1 in 167 U.S. taxpayers were audited in 2020, according to the IRS. Even for taxpayers reporting $5 million or more in income, the audit rate is only about 0.25%, the Government Accountability Office says.
The odds of a physician being summoned to a meeting with an auditor probably won’t increase for a few years, Mr. Gallati said. But the good news for doctors is bad news for lower-income Americans. “The IRS is woefully understaffed and underfunded, with the result that the agency is going for lower-hanging fruit and auditing more people in lower income brackets,” she said in an interview.
While one respondent described his experience with the IRS as “the audit from hell,” others thought it not so bad, with 72% saying the auditors treated them fairly. One respondent described the audit as “boring, short, and successful for me. The IRS owed me money.”
When it comes to taxes, physician respondents, on the whole, did not seem to be optimistic about the future. About 61% expect an increase in their tax rate because of Biden administration policies. One respondent veered into hyperbole with the comment: “I believe taxes will increase for physicians until they have no more money!”
Mr. Chahal doesn’t see it that way. He pointed out that recent attempts to raise taxes completely failed. “I personally don’t see that happening unless there’s a significant shift in the House and the Senate.”
A version of this article first appeared on Medscape.com.
For the Medscape Physicians and Taxes Report 2022, physicians shared information about their tax debt as well as how they feel about the U.S. tax code, audits, and the prospects for the future.
Even though it may not always seem that way to physicians, their family tax bills – around $75,406 on average – are in line with the other top 10% of U.S. taxpayers, according to an examination of IRS data by the Tax Foundation. However, when it comes to local taxes, the Tax Foundation found that physicians pay more than average. (Forty-three states collect tax on individual incomes.)
The average physician’s family pays a 35% marginal tax rate, compared with the top marginal tax rate in the United States of 37%. (The marginal tax rate is the highest amount of tax charged on each additional dollar after the IRS bracket rates are applied to your income.)
According to Alexis Gallati, founder of Cerebral Tax Advisors, a Knoxville, Tenn.–based firm that caters to medical professionals, doctors also should pay attention to their effective tax rate, or the percentage of income they pay in taxes. It takes into account differing tax rates on ordinary income, capital gains, and other income sources, she says. “It gives a better 30,000-foot view of your tax situation.”
Some high-income families are required to pay the Alternative Minimum Tax (AMT), though in 2019 that applied to only one-tenth of U.S. households. The AMT is designed to make sure that high earners with many options for exemptions and deductions still contribute a minimum amount of tax. Only 13% of physicians surveyed said they paid the AMT, though 29% were unsure.
Filing taxes as painful as paying them
According to a 2021 Gallup poll, 50% of Americans think they pay too much tax. (About 44% think their tax bill is about right, and a kindhearted 4% think they pay too little.) Doctors are outliers on this one, with 75% saying they pay too much in taxes.
When asked what they would do to fix the tax system, the physicians in the Medscape survey had a wide array of proposed solutions, from “drop the corporate tax rate to nearly nothing to stimulate the economy” to “everyone should pay equitably. There are too many loopholes for the very wealthy.”
Some of the complaints were less about tax rates than the process of filing. One respondent said: “I would love for this system to not be our personal responsibility. Why should it be my duty to pay someone every year to do my taxes?”
About 48% of physicians prepare their own taxes (about the same percentage as the rest of the population), with most of those filing electronically, primarily because it saves time and the software is easy to use. Intuit TurboTax was the most popular online software, with 22% of respondents saying they currently used this product.
Of those who did pay someone to prepare their taxes, the complexity of their taxes cost them; the average respondent paid about three times the average rate for the service. In the long run, the cost might have been recouped.
Navjeet Chahal, managing partner and CEO of Chahal and Associates, a San Francisco–area firm specializing in working with physicians, points out that tax advisors don’t just fill out the forms; they proactively advise physicians about how they can limit their taxes. And indeed, most respondents feel that they got their money’s worth, with 70% saying their tax preparers charged a fair fee.
Though the physicians surveyed tended to think they pay too much tax, and several mentioned particular gripes with the system, the complexity of the tax code didn’t seem to be a big issue. While 82% of Americans polled in 2021 by Pew Research said they were bothered “a lot” or “some” by the complexity of the tax system, 68% of physicians agreed or slightly agreed that the U.S. tax system “makes sense.”
Gimme a break
Physicians are the beneficiaries of several types of tax breaks. Contributing to a pretax 401(k) account was the most common exemption, with 60% of physicians surveyed using this plan. Other tax breaks cited by respondents were: contributing to charity (54%), home mortgage interest (46%), and writing off business expenses (39%).
About one in five physicians has experienced an audit, but that risk has declined significantly in recent years, thanks to tighter IRS budgets. Overall, only about 1 in 167 U.S. taxpayers were audited in 2020, according to the IRS. Even for taxpayers reporting $5 million or more in income, the audit rate is only about 0.25%, the Government Accountability Office says.
The odds of a physician being summoned to a meeting with an auditor probably won’t increase for a few years, Mr. Gallati said. But the good news for doctors is bad news for lower-income Americans. “The IRS is woefully understaffed and underfunded, with the result that the agency is going for lower-hanging fruit and auditing more people in lower income brackets,” she said in an interview.
While one respondent described his experience with the IRS as “the audit from hell,” others thought it not so bad, with 72% saying the auditors treated them fairly. One respondent described the audit as “boring, short, and successful for me. The IRS owed me money.”
When it comes to taxes, physician respondents, on the whole, did not seem to be optimistic about the future. About 61% expect an increase in their tax rate because of Biden administration policies. One respondent veered into hyperbole with the comment: “I believe taxes will increase for physicians until they have no more money!”
Mr. Chahal doesn’t see it that way. He pointed out that recent attempts to raise taxes completely failed. “I personally don’t see that happening unless there’s a significant shift in the House and the Senate.”
A version of this article first appeared on Medscape.com.
FDA clears endoscopic devices for sleeve gastroplasty, bariatric revision
The Food and Drug Administration has cleared for marketing the first devices indicated for endoscopic sleeve gastroplasty (ESG) and endoscopic bariatric revision, according to the manufacturer.
The Apollo ESG, Apollo ESG Sx, Apollo Revise, and Apollo Revise Sx systems made by Apollo Endosurgery were reviewed through the de novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type.
“The Apollo ESG and Apollo Revise systems offer a compelling mix of effectiveness, safety, durability, and convenience for treatment of patients with obesity,” Chas McKhann, president and CEO of the company, said in a news release.
“The authorization of these new endoscopic systems represents a major step forward in addressing the global obesity epidemic,” Mr. McKhann added.
The Apollo ESG and Apollo ESG Sx systems are intended for use by trained gastroenterologists or surgeons to facilitate weight loss in adults with obesity who have failed to lose weight or maintain weight loss through more conservative measures, the company says.
The Apollo Revise and Apollo Revise Sx systems allow gastroenterologists or surgeons to perform transoral outlet reduction (TORe) as a revision to a previous bariatric procedure.
Studies have shown that 10 years after bariatric surgery, patients have regained an average of 20%-30% of weight they initially lost. Bariatric revision procedures are the fastest growing segment of the bariatric surgery market.
TORe is an endoscopic procedure performed to revise a previous gastric bypass and like ESG, can be performed as a same-day procedure without incisions or scars.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared for marketing the first devices indicated for endoscopic sleeve gastroplasty (ESG) and endoscopic bariatric revision, according to the manufacturer.
The Apollo ESG, Apollo ESG Sx, Apollo Revise, and Apollo Revise Sx systems made by Apollo Endosurgery were reviewed through the de novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type.
“The Apollo ESG and Apollo Revise systems offer a compelling mix of effectiveness, safety, durability, and convenience for treatment of patients with obesity,” Chas McKhann, president and CEO of the company, said in a news release.
“The authorization of these new endoscopic systems represents a major step forward in addressing the global obesity epidemic,” Mr. McKhann added.
The Apollo ESG and Apollo ESG Sx systems are intended for use by trained gastroenterologists or surgeons to facilitate weight loss in adults with obesity who have failed to lose weight or maintain weight loss through more conservative measures, the company says.
The Apollo Revise and Apollo Revise Sx systems allow gastroenterologists or surgeons to perform transoral outlet reduction (TORe) as a revision to a previous bariatric procedure.
Studies have shown that 10 years after bariatric surgery, patients have regained an average of 20%-30% of weight they initially lost. Bariatric revision procedures are the fastest growing segment of the bariatric surgery market.
TORe is an endoscopic procedure performed to revise a previous gastric bypass and like ESG, can be performed as a same-day procedure without incisions or scars.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared for marketing the first devices indicated for endoscopic sleeve gastroplasty (ESG) and endoscopic bariatric revision, according to the manufacturer.
The Apollo ESG, Apollo ESG Sx, Apollo Revise, and Apollo Revise Sx systems made by Apollo Endosurgery were reviewed through the de novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type.
“The Apollo ESG and Apollo Revise systems offer a compelling mix of effectiveness, safety, durability, and convenience for treatment of patients with obesity,” Chas McKhann, president and CEO of the company, said in a news release.
“The authorization of these new endoscopic systems represents a major step forward in addressing the global obesity epidemic,” Mr. McKhann added.
The Apollo ESG and Apollo ESG Sx systems are intended for use by trained gastroenterologists or surgeons to facilitate weight loss in adults with obesity who have failed to lose weight or maintain weight loss through more conservative measures, the company says.
The Apollo Revise and Apollo Revise Sx systems allow gastroenterologists or surgeons to perform transoral outlet reduction (TORe) as a revision to a previous bariatric procedure.
Studies have shown that 10 years after bariatric surgery, patients have regained an average of 20%-30% of weight they initially lost. Bariatric revision procedures are the fastest growing segment of the bariatric surgery market.
TORe is an endoscopic procedure performed to revise a previous gastric bypass and like ESG, can be performed as a same-day procedure without incisions or scars.
A version of this article first appeared on Medscape.com.
Colonic Crohn’s: Segmental vs. total colectomy
Segmental rather than total colectomy may be a safe and effective choice for some patients with colonic Crohn’s disease (cCD), showing significantly lower rates of repeat surgery and reduced need for stoma, according to long-term data.
Gianluca Pellino, MD, with the department of advanced medical and surgical sciences, Università degli Studi della Campania “Luigi Vanvitelli” in Naples, Italy, led the study, which was published in the Journal of Crohn’s and Colitis.
CD of the colon has gotten less attention than the more prevalent small bowel disease, according to the authors, but it can be debilitating and permanently reduce quality of life. Isolated cCD incidence ranges between 14% and 32% of all CD cases from the start of disease. Historically, extensive resection has been linked with longer disease-free intervals, and reduced repeat surgeries compared with segmental resections. However, most of the data have included low-quality evidence and reports typically have not adequately considered the role of biologics or advances in perioperative management of patients with cCD, the authors wrote.
The Segmental Colectomy for Crohn’s disease (SCOTCH) international study included a retrospective analysis of data from six European Inflammatory Bowel Disease referral centers on patients operated on between 2000 and 2019 who had either segmental or total colectomy for cCD.
Among 687 patients (301 male; 386 female), segmental colectomy was performed in 285 (41.5%) of cases and total colectomy in 402 (58.5%). The 15-year surgical recurrence rate was 44% among patients who had TC and 27% for patients with segmental colectomy (P = .006).
The SCOTCH study found that segmental colectomy may be performed safely and effectively and reduce the need for stoma in cCD patients without increasing risk of repeat surgeries compared with total colectomy, which was the primary measure investigators studied.
The findings of this study also suggest that biologics, when used early and correctly, may allow more conservative options for cCD, with a fivefold reduction in surgical recurrence risk in patients who have one to three large bowel locations.
Morbidity and mortality were similar in the SC and TC groups.
Among the limitations of the study are that the total colectomy patients in the study had indications for total colectomy that were also higher risk factors for recurrence – for instance, perianal disease.
The authors wrote, “The differences between patients who underwent SC vs TC might have accounted for the choice of one treatment over the other. It is however difficult to obtain a homogenous population of cCD patients.” They also cite the difficulties in gathering enough patients for randomized trials.
“These findings need to be discussed with the patients, and the choice of operation should be individualised,” they concluded. “Multidisciplinary management of patients with cCD is of critical importance to achieve optimal long-term results of bowel-sparing approaches.”
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic, who was not part of the study, told this publication the findings should be considered confirmatory rather than suggestive of practice change.
“If a patient has a limited segment of Crohn’s, for example ileocecal Crohn’s – a common phenotype – then the standard of care is a segmental resection and primary anastomosis,” he said. “If the patient has more extensive CD – perianal fistula, colonic-only CD – they’re more likely to undergo a total colectomy. This study confirms that.“
The authors and Dr. Regueiro declared no relevant financial relationships.
Segmental rather than total colectomy may be a safe and effective choice for some patients with colonic Crohn’s disease (cCD), showing significantly lower rates of repeat surgery and reduced need for stoma, according to long-term data.
Gianluca Pellino, MD, with the department of advanced medical and surgical sciences, Università degli Studi della Campania “Luigi Vanvitelli” in Naples, Italy, led the study, which was published in the Journal of Crohn’s and Colitis.
CD of the colon has gotten less attention than the more prevalent small bowel disease, according to the authors, but it can be debilitating and permanently reduce quality of life. Isolated cCD incidence ranges between 14% and 32% of all CD cases from the start of disease. Historically, extensive resection has been linked with longer disease-free intervals, and reduced repeat surgeries compared with segmental resections. However, most of the data have included low-quality evidence and reports typically have not adequately considered the role of biologics or advances in perioperative management of patients with cCD, the authors wrote.
The Segmental Colectomy for Crohn’s disease (SCOTCH) international study included a retrospective analysis of data from six European Inflammatory Bowel Disease referral centers on patients operated on between 2000 and 2019 who had either segmental or total colectomy for cCD.
Among 687 patients (301 male; 386 female), segmental colectomy was performed in 285 (41.5%) of cases and total colectomy in 402 (58.5%). The 15-year surgical recurrence rate was 44% among patients who had TC and 27% for patients with segmental colectomy (P = .006).
The SCOTCH study found that segmental colectomy may be performed safely and effectively and reduce the need for stoma in cCD patients without increasing risk of repeat surgeries compared with total colectomy, which was the primary measure investigators studied.
The findings of this study also suggest that biologics, when used early and correctly, may allow more conservative options for cCD, with a fivefold reduction in surgical recurrence risk in patients who have one to three large bowel locations.
Morbidity and mortality were similar in the SC and TC groups.
Among the limitations of the study are that the total colectomy patients in the study had indications for total colectomy that were also higher risk factors for recurrence – for instance, perianal disease.
The authors wrote, “The differences between patients who underwent SC vs TC might have accounted for the choice of one treatment over the other. It is however difficult to obtain a homogenous population of cCD patients.” They also cite the difficulties in gathering enough patients for randomized trials.
“These findings need to be discussed with the patients, and the choice of operation should be individualised,” they concluded. “Multidisciplinary management of patients with cCD is of critical importance to achieve optimal long-term results of bowel-sparing approaches.”
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic, who was not part of the study, told this publication the findings should be considered confirmatory rather than suggestive of practice change.
“If a patient has a limited segment of Crohn’s, for example ileocecal Crohn’s – a common phenotype – then the standard of care is a segmental resection and primary anastomosis,” he said. “If the patient has more extensive CD – perianal fistula, colonic-only CD – they’re more likely to undergo a total colectomy. This study confirms that.“
The authors and Dr. Regueiro declared no relevant financial relationships.
Segmental rather than total colectomy may be a safe and effective choice for some patients with colonic Crohn’s disease (cCD), showing significantly lower rates of repeat surgery and reduced need for stoma, according to long-term data.
Gianluca Pellino, MD, with the department of advanced medical and surgical sciences, Università degli Studi della Campania “Luigi Vanvitelli” in Naples, Italy, led the study, which was published in the Journal of Crohn’s and Colitis.
CD of the colon has gotten less attention than the more prevalent small bowel disease, according to the authors, but it can be debilitating and permanently reduce quality of life. Isolated cCD incidence ranges between 14% and 32% of all CD cases from the start of disease. Historically, extensive resection has been linked with longer disease-free intervals, and reduced repeat surgeries compared with segmental resections. However, most of the data have included low-quality evidence and reports typically have not adequately considered the role of biologics or advances in perioperative management of patients with cCD, the authors wrote.
The Segmental Colectomy for Crohn’s disease (SCOTCH) international study included a retrospective analysis of data from six European Inflammatory Bowel Disease referral centers on patients operated on between 2000 and 2019 who had either segmental or total colectomy for cCD.
Among 687 patients (301 male; 386 female), segmental colectomy was performed in 285 (41.5%) of cases and total colectomy in 402 (58.5%). The 15-year surgical recurrence rate was 44% among patients who had TC and 27% for patients with segmental colectomy (P = .006).
The SCOTCH study found that segmental colectomy may be performed safely and effectively and reduce the need for stoma in cCD patients without increasing risk of repeat surgeries compared with total colectomy, which was the primary measure investigators studied.
The findings of this study also suggest that biologics, when used early and correctly, may allow more conservative options for cCD, with a fivefold reduction in surgical recurrence risk in patients who have one to three large bowel locations.
Morbidity and mortality were similar in the SC and TC groups.
Among the limitations of the study are that the total colectomy patients in the study had indications for total colectomy that were also higher risk factors for recurrence – for instance, perianal disease.
The authors wrote, “The differences between patients who underwent SC vs TC might have accounted for the choice of one treatment over the other. It is however difficult to obtain a homogenous population of cCD patients.” They also cite the difficulties in gathering enough patients for randomized trials.
“These findings need to be discussed with the patients, and the choice of operation should be individualised,” they concluded. “Multidisciplinary management of patients with cCD is of critical importance to achieve optimal long-term results of bowel-sparing approaches.”
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic, who was not part of the study, told this publication the findings should be considered confirmatory rather than suggestive of practice change.
“If a patient has a limited segment of Crohn’s, for example ileocecal Crohn’s – a common phenotype – then the standard of care is a segmental resection and primary anastomosis,” he said. “If the patient has more extensive CD – perianal fistula, colonic-only CD – they’re more likely to undergo a total colectomy. This study confirms that.“
The authors and Dr. Regueiro declared no relevant financial relationships.
FROM JOURNAL OF CROHN’S AND COLITIS