User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Many children with COVID-19 present without classic symptoms
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
Most children who tested positive for SARS-CoV-2 had no respiratory illness, according to data from a retrospective study of 22 patients at a single center.
To date, children account for less than 5% of COVID-19 cases in the United States, but details of the clinical presentations in children are limited, wrote Rabia Agha, MD, and colleagues of Maimonides Children’s Hospital, Brooklyn, N.Y.
In a study published in Hospital Pediatrics, the researchers reviewed data from 22 children aged 0-18 years who tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) and were admitted to a single hospital over a 4-week period from March 18, 2020, to April 15, 2020.
Of four patients requiring mechanical ventilation, two had underlying pulmonary disease. The other two patients who required intubation were one with cerebral palsy and status epilepticus and one who presented in a state of cardiac arrest.
The study population ranged from 11 days to 18 years of age, but 45% were infants younger than 1 year. None of the children had a travel history that might increase their risk for SARS-CoV-2 infection; 27% had confirmed exposure to the virus.
Most of the children (82%) were hospitalized within 3 days of the onset of symptoms, and no deaths occurred during the study period. The most common symptom was fever without a source in five (23%) otherwise healthy infants aged 11-35 days. All five of these children underwent a sepsis evaluation, received empiric antibiotics, and were discharged home with negative bacterial cultures within 48-72 hours. Another 10 children had fever in combination with other symptoms.
Other presenting symptoms were respiratory (9), fatigue (6), seizures (2), and headache (1).
Most children with respiratory illness were treated with supportive therapy and antibiotics, but three of those on mechanical ventilation also were treated with remdesivir; all three were ultimately extubated.
Neurological abnormalities occurred in two patients: an 11-year-old otherwise healthy boy who presented with fever, headache, confusion, and seizure but ultimately improved without short-term sequelae; and a 12-year-old girl with cerebral palsy who developed new onset seizures and required mechanical ventilation, but ultimately improved to baseline.
Positive PCR results were identified in seven patients (32%) during the second half of the study period who were initially hospitalized for non-COVID related symptoms; four with bacterial infections, two with illnesses of unknown etiology, and one with cardiac arrest. Another two children were completely asymptomatic at the time of admission but then tested positive by PCR; one child had been admitted for routine chemotherapy and the other for social reasons, Dr. Agha and associates said.
The study findings contrast with early data from China in which respiratory illness of varying severity was the major presentation in children with COVID-19, but support a more recent meta-analysis of 551 cases, the researchers noted. The findings also highlight the value of universal testing for children.
“Our initial testing strategy was according to the federal and local guidelines that recommended PCR testing for the symptoms of fever, cough and shortness of breath, or travel to certain countries or close contact with a confirmed case,” Dr. Agha and colleagues said.
“With the implementation of our universal screening strategy of all admitted pediatric patients, we identified 9 (41%) patients with COVID-19 that would have been missed, as they did not meet the then-recommended criteria for testing,” they wrote.
The results suggest the need for broader guidelines to test pediatric patients because children presenting with other illnesses may be positive for SARS-CoV-2 as well, the researchers said.
“Testing of all hospitalized patients will not only identify cases early in the course of their admission process, but will also help prevent inadvertent exposure of other patients and health care workers, assist in cohorting infected patients, and aid in conservation of personal protective equipment,” Dr. Agha and associates concluded.
The current study is important as clinicians continue to learn about how infection with SARS-CoV-2 presents in different populations, Diana Lee, MD, of the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“Understanding how it can present in the pediatric population is important in identifying children who may have the infection and developing strategies for testing,” she said.
“I was not surprised by the finding that most children did not present with the classic symptoms of COVID-19 in adults based on other published studies and my personal clinical experience taking care of hospitalized children in New York City,” said Dr. Lee. “Studies from the U.S. and other countries have reported that fewer children experience fever, cough, and shortness of breath [compared with] adults, and that most children have a milder clinical course, though there is a small percentage of children who can have severe or critical illness,” she said.
“A multisystem inflammatory syndrome in children associated with COVID-19 has also emerged and appears to be a postinfectious process with a presentation that often differs from classic COVID-19 infection in adults,” she added.
The take-home message for clinicians is the reminder that SARS-CoV-2 infection often presents differently in children than in adults, said Dr. Lee.
“Children who present to the hospital with non-classic COVID-19 symptoms or with other diagnoses may be positive for SARS-CoV-2 on testing. Broadly testing hospitalized children for SARS-CoV-2 and instituting appropriate isolation precautions may help to protect other individuals from being exposed to the virus,” she said.
“Further research is needed to understand which individuals are contagious and how to accurately distinguish those who are infectious versus those who are not,” said Dr. Lee. “There have been individuals who persistently test positive for SARS-CoV-2 RNA (the genetic material of the virus), but were not found to have virus in their bodies that can replicate and thereby infect others,” she emphasized. “Further study is needed regarding the likelihood of household exposures in children with SARS-CoV-2 infection given that this study was done early in the epidemic in New York City when testing and contact tracing was less established,” she said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Lee had no financial conflicts to disclose.
SOURCE: Agha R et al. Hosp Pediatr. 2020 July. doi: 10.1542/hpeds.2020-000257.
FROM HOSPITAL PEDIATRICS
Diabetic amputations soared amid Italian pandemic lockdown
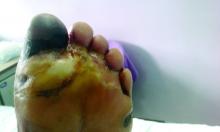
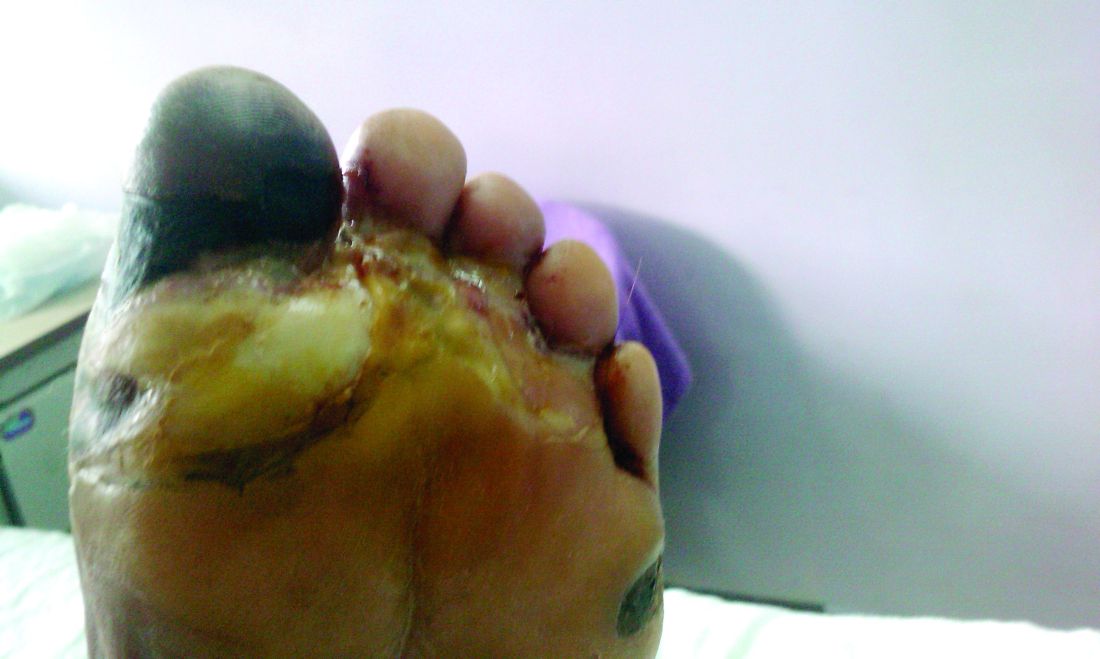
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
Amid a mandatory national lockdown, the rates of amputations skyrocketed at a hospital far from the hardest-hit region as many patients developed gangrene.
The findings offer critical lessons for the United States, said wound care specialist William H. Tettelbach, MD, of Western Peaks Specialty Hospital near Salt Lake City. “It’s become more obvious that outpatient wound care is a critical care need for the community because of the risk of ignoring these chronic wounds and letting them remain open. We cannot let these services be closed down like some were when the pandemic started.”
The study, led by Paola Caruso, MD, of the University of Campania Luigi Vanvitelli in Naples, appeared in Diabetes Care.
The researchers launched the study to understand how patients with diabetes and DFU fared during the height of the pandemic in Italy, where tens of thousands of people died, mainly in the northern region of the country. They focused on patients in the southern region who were admitted to the division of endocrinology and metabolic diseases at the Teaching Hospital at the University of Campania Luigi Vanvitelli.
The study compared 25 patients who were admitted from March 9 to May 18, 2020, with 38 patients who were admitted from a longer period between January and May 2019. The demographics of the groups are similar, with average ages in the early 60s and more men than women (21:4, respectively, in 2020 and 23:15, respectively, in 2019.)
The results reveal high numbers of emergent and serious cases in 2020. Compared with 2019, fewer were outpatients (16% vs. 45%, P = .028) and more were emergency patients (76% vs. 26%, P < .001).
Clinically, gangrene was much more common in the 2020 group, compared with the 2019 group (64% vs. 29%, P = .009), as was amputation (60% vs. 18%, P = .001).
The researchers determined that amputation was more than three times more likely in the 2020 versus the 2019 group (relative risk, 3.26; 95% confidence interval, 1.55-6.84) even though the 2019 period was longer. After adjustment for gender, the heightened risk in 2020 was 2.50 (95% CI, 1.18-5.29).
There was no statistically significant increase in the risk of revascularization.
“The COVID-19 lockdown may have had a detrimental impact on amputation risk because of the sudden interruption of DFU care and lower-limb preservation pathways, resulting in delayed diagnosis and treatment,” the researchers wrote. “DFU is often characterized by progressive clinical course, which can rapidly lead patients to critical worsening of their ulcers.”
They added that “the higher risk of amputation observed during COVID-19 lockdown confirms the need for proper and timely management of DFU patients to prevent dramatic outcomes responsible for a reduction of quality of life and increased morbidity and mortality.”
The study authors didn’t discuss why more patients seemed to have stayed home and not gotten proper care. It’s not clear if they were scared to get treatment or couldn’t obtain it because of the national shutdown.
Both have been factors affecting diabetic foot care in the United States during the pandemic, said Dr. Tettelbach. He called the study “timely and pertinent,” and said it highlights how wound care is “a critical need” that must remain available even when other medical services such as elective surgeries are shut down.
Infection-control protocols such as allowing patients to wait for appointments in their cars instead of waiting rooms will alleviate the fears of certain patients about seeking in-person care during the pandemic, he said. But some patients will be afraid to come in no matter what, he said, and home health may be the best solution for their care.
Several of the study authors reported various disclosures. Dr. Tettelbach reported no relevant disclosures.
SOURCE: Caruso P et al. Diabetes Care. 2020 Jul 23. doi:10.2337/dc20-1347.
FROM DIABETES CARE
Appendix may be common site of endometriosis
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
FROM SGS 2020
Cutaneous clues linked to COVID-19 coagulation risk
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
, new evidence suggests.
Researchers at Weill Cornell Medicine NewYork–Presbyterian Medical Center in New York linked livedoid and purpuric skin eruptions to a greater likelihood for occlusive vascular disease associated with SARS-CoV-2 infection in a small case series.
These skin signs could augment coagulation assays in this patient population. “Physicians should consider a hematology consult for potential anticoagulation in patients with these skin presentations and severe COVID-19,” senior author Joanna Harp, MD, said in an interview.
“Physicians should also consider D-dimer, fibrinogen, coagulation studies, and a skin biopsy given that there are other diagnoses on the differential as well.”
The research letter was published online on Aug. 5 in JAMA Dermatology.
The findings build on multiple previous reports of skin manifestations associated with COVID-19, including a study of 375 patients in Spain. Among people with suspected or confirmed SARS-CoV-2 infection, senior author of the Spanish research, Ignacio Garcia-Doval, MD, PhD, also observed livedoid and necrotic skin eruptions more commonly in severe disease.
“I think that this case series [from Harp and colleagues] confirms the findings of our previous paper – that patients with livedoid or necrotic lesions have a worse prognosis, as these are markers of vascular occlusion,” he said in an interview.
Dr. Harp and colleagues reported their observations with four patients aged 40-80 years. Each had severe COVID-19 with acute respiratory distress syndrome and required intubation. Treating clinicians requested a dermatology consult to assess acral fixed livedo racemosa and retiform purpura presentations.
D-dimer levels exceeded 3 mcg/mL in each case. All four patients had a suspected pulmonary embolism within 1-5 days of the dermatologic findings. Prophylactic anticoagulation at admission was changed to therapeutic anticoagulation because of increasing D-dimer levels and the suspected thrombotic events.
“I think that the paper is interesting because it shows the associated histopathological findings and has important clinical implications due to the association with pulmonary embolism,” said Dr. Garcia-Doval, a researcher at the Spanish Academy of Dermatology in Madrid. “These patients should probably be anticoagulated.”
Skin biopsy results
Punch biopsies revealed pauci-inflammatory thrombogenic vasculopathy involving capillaries, venules, arterioles, or small arteries.
Livedo racemosa skin findings point to partial occlusion of cutaneous blood vessels, whereas retiform purpura indicate full occlusion of cutaneous blood vessels.
An inability to confirm the exact timing of the onset of the skin rash was a limitation of the study.
“The findings suggest that clinicians caring for patients with COVID-19 should be aware of livedoid and purpuric rashes as potential manifestations of an underlying hypercoagulable state,” the authors noted. “If these skin findings are identified, a skin biopsy should be considered because the result may guide anticoagulation management.”
Observations during an outbreak
The researchers observed these cases between March 13 and April 3, during the peak of the COVID-19 outbreak in New York.
“We did see additional cases since our study period. However, it has decreased significantly with the falling number of COVID-19 cases in the city,” said Dr. Harp, a dermatologist at NewYork–Presbyterian.
Another contributing factor in the drop in cases was “implementation of earlier, more aggressive anticoagulation in many of these patients at our institution,” she added.
The investigators plan to continue the research. “We are working on a more formalized study,” lead author Caren Droesch, MD, said in an interview.
“But given very low patient numbers in our area we have not started recruiting patients,” said Dr. Droesch, a resident at Weill Cornell Medicine and NewYork–Presbyterian at the time of the study. She is now a dermatologist at Mass General Brigham in Wellesley, Mass.
Consider a dermatology consult
“This is a small case series of four patients, but mirrors what we have seen at our institution and what others have reported about individual patients around the world,” Anthony Fernandez, MD, PhD, a dermatologist at Cleveland Clinic, said in an interview. “The skin, like many other organ systems, can be affected by thrombotic events within the setting of COVID-19 disease.”
As in the current study, Dr. Fernandez observed skin manifestations in people with severe COVID-19 with elevated D-dimer levels. These patients typically require mechanical ventilation in the intensive care unit, he added.
“As these authors point out, it is important for all clinicians caring for COVID-19 patients to look for these rashes,” said Dr. Fernandez, who coauthored a report on skin manifestations in this patient population. “We also agree that clinicians should have a low threshold for consulting dermatology. A skin biopsy is minimally invasive and can be important in confirming or refuting that such rashes are truly reflective of thrombotic vasculopathy.”
Dr. Harp, Dr. Droesch and Dr. Garcia-Doval have disclosed no relevant financial relationships. Dr. Fernandez received funding from the Clinical and Translational Science Collaborative at Case Western Reserve University to study skin manifestations of COVID-19.
A version of this article originally appeared on Medscape.com.
FROM JAMA DERMATOLOGY
When you see something ...
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Does stirrup choice influence vaginal surgery outcome?
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
FROM OBSTETRICS & GYNECOLOGY
Study finds no link between platelet count, surgery bleed risk in cirrhosis
The findings raise questions about current recommendations that call for transfusing platelet concentrates to reduce bleeding risk during surgery in cirrhosis patients with extremely low platelet counts, Gian Marco Podda, MD, PhD, said at the International Society on Thrombosis and Haemostasis virtual congress.
The overall rate of perioperative bleeding was 8.9% in 996 patients who underwent excision of hepatocellular carcinoma by resection (42%) or radiofrequency ablation (58%) without platelet transfusion between 1998 and 2018. The rates were slightly higher among 65 patients with platelet count of fewer than 50 × 109/L indicating severe thrombocytopenia, and in 292 patients with counts of 50-100 × 109/L, indicating moderate thrombocytopenia (10.8% and 10.2%, respectively), compared with those with a platelet count of higher than 100 × 109/L (8.1%), but the differences were not statistically significant, said Dr. Podda of the University of Milan (Italy).
The corresponding rates among those who underwent radiofrequency ablation were 8.6%, 5.9%, and 5%, and among those who underwent resection, they were 18.8%, 17.7%, and 15.9%.
On multivariate analysis, factors associated with an increased incidence of major bleeding were low hemoglobin level (odds ratio, 0.57), age over 65 years (OR, 1.19), aspartate aminotransferase level greater than twice the upper limit of normal (OR, 2.12), hepatitis B or C cirrhosis versus cryptogenic cirrhosis (OR, 0.08), and resection versus radiofrequency ablation (OR, 3.74), he noted. Logistic regression analysis showed no significant association between platelet count and major bleeding events.
Mortality, a secondary outcome measure, was significantly higher among those with moderate or severe thrombocytopenia (rate of 5.5% for each), compared with those with mild or no thrombocytopenia (2.4%), Dr. Podda said.
Factors associated with mortality on multivariate analysis were severe liver dysfunction as demonstrated by Model for End-Stage Liver Disease score of 10 or greater versus less than 10 (OR, 3.13) and Child-Pugh B and C score versus Child-Pugh A score (OR, 16.72), advanced tumor status as measured by Barcelona-Clínic Liver Cancer staging greater than A4 versus A1 (OR, 5.78), major bleeding (OR, 4.59), and resection versus radiofrequency ablation (OR, 3.31).
“Low platelet count was associated with an increased risk of mortality at 3 months. However, this association disappeared at the multivariate analysis, which took into account markers of severity of liver cirrhosis,” he said.
Dr. Podda and his colleagues conducted the study in light of a recommendation from a consensus conference of the Italian Association for the Study of Liver Disease and the Italian Society of Internal Medicine that called for increasing platelet count by platelet transfusions in patients with cirrhosis who undergo an invasive procedure and who have a platelet count lower than 50 × 109/L.
“This recommendation mostly stemmed from consideration of biological plausibility prospects rather than being based on hard experimental evidence,” he explained, noting that such severe thrombocytopenia affects about 10% of patients with liver cirrhosis.
Based on the findings of this study, the practice is not supported, he concluded.
Dr. Podda reported honoraria from Sanofi, Boehringer Ingelheim.
SOURCE: Ronca V et al. ISTH 2020, Abstract OC 13.4.
The findings raise questions about current recommendations that call for transfusing platelet concentrates to reduce bleeding risk during surgery in cirrhosis patients with extremely low platelet counts, Gian Marco Podda, MD, PhD, said at the International Society on Thrombosis and Haemostasis virtual congress.
The overall rate of perioperative bleeding was 8.9% in 996 patients who underwent excision of hepatocellular carcinoma by resection (42%) or radiofrequency ablation (58%) without platelet transfusion between 1998 and 2018. The rates were slightly higher among 65 patients with platelet count of fewer than 50 × 109/L indicating severe thrombocytopenia, and in 292 patients with counts of 50-100 × 109/L, indicating moderate thrombocytopenia (10.8% and 10.2%, respectively), compared with those with a platelet count of higher than 100 × 109/L (8.1%), but the differences were not statistically significant, said Dr. Podda of the University of Milan (Italy).
The corresponding rates among those who underwent radiofrequency ablation were 8.6%, 5.9%, and 5%, and among those who underwent resection, they were 18.8%, 17.7%, and 15.9%.
On multivariate analysis, factors associated with an increased incidence of major bleeding were low hemoglobin level (odds ratio, 0.57), age over 65 years (OR, 1.19), aspartate aminotransferase level greater than twice the upper limit of normal (OR, 2.12), hepatitis B or C cirrhosis versus cryptogenic cirrhosis (OR, 0.08), and resection versus radiofrequency ablation (OR, 3.74), he noted. Logistic regression analysis showed no significant association between platelet count and major bleeding events.
Mortality, a secondary outcome measure, was significantly higher among those with moderate or severe thrombocytopenia (rate of 5.5% for each), compared with those with mild or no thrombocytopenia (2.4%), Dr. Podda said.
Factors associated with mortality on multivariate analysis were severe liver dysfunction as demonstrated by Model for End-Stage Liver Disease score of 10 or greater versus less than 10 (OR, 3.13) and Child-Pugh B and C score versus Child-Pugh A score (OR, 16.72), advanced tumor status as measured by Barcelona-Clínic Liver Cancer staging greater than A4 versus A1 (OR, 5.78), major bleeding (OR, 4.59), and resection versus radiofrequency ablation (OR, 3.31).
“Low platelet count was associated with an increased risk of mortality at 3 months. However, this association disappeared at the multivariate analysis, which took into account markers of severity of liver cirrhosis,” he said.
Dr. Podda and his colleagues conducted the study in light of a recommendation from a consensus conference of the Italian Association for the Study of Liver Disease and the Italian Society of Internal Medicine that called for increasing platelet count by platelet transfusions in patients with cirrhosis who undergo an invasive procedure and who have a platelet count lower than 50 × 109/L.
“This recommendation mostly stemmed from consideration of biological plausibility prospects rather than being based on hard experimental evidence,” he explained, noting that such severe thrombocytopenia affects about 10% of patients with liver cirrhosis.
Based on the findings of this study, the practice is not supported, he concluded.
Dr. Podda reported honoraria from Sanofi, Boehringer Ingelheim.
SOURCE: Ronca V et al. ISTH 2020, Abstract OC 13.4.
The findings raise questions about current recommendations that call for transfusing platelet concentrates to reduce bleeding risk during surgery in cirrhosis patients with extremely low platelet counts, Gian Marco Podda, MD, PhD, said at the International Society on Thrombosis and Haemostasis virtual congress.
The overall rate of perioperative bleeding was 8.9% in 996 patients who underwent excision of hepatocellular carcinoma by resection (42%) or radiofrequency ablation (58%) without platelet transfusion between 1998 and 2018. The rates were slightly higher among 65 patients with platelet count of fewer than 50 × 109/L indicating severe thrombocytopenia, and in 292 patients with counts of 50-100 × 109/L, indicating moderate thrombocytopenia (10.8% and 10.2%, respectively), compared with those with a platelet count of higher than 100 × 109/L (8.1%), but the differences were not statistically significant, said Dr. Podda of the University of Milan (Italy).
The corresponding rates among those who underwent radiofrequency ablation were 8.6%, 5.9%, and 5%, and among those who underwent resection, they were 18.8%, 17.7%, and 15.9%.
On multivariate analysis, factors associated with an increased incidence of major bleeding were low hemoglobin level (odds ratio, 0.57), age over 65 years (OR, 1.19), aspartate aminotransferase level greater than twice the upper limit of normal (OR, 2.12), hepatitis B or C cirrhosis versus cryptogenic cirrhosis (OR, 0.08), and resection versus radiofrequency ablation (OR, 3.74), he noted. Logistic regression analysis showed no significant association between platelet count and major bleeding events.
Mortality, a secondary outcome measure, was significantly higher among those with moderate or severe thrombocytopenia (rate of 5.5% for each), compared with those with mild or no thrombocytopenia (2.4%), Dr. Podda said.
Factors associated with mortality on multivariate analysis were severe liver dysfunction as demonstrated by Model for End-Stage Liver Disease score of 10 or greater versus less than 10 (OR, 3.13) and Child-Pugh B and C score versus Child-Pugh A score (OR, 16.72), advanced tumor status as measured by Barcelona-Clínic Liver Cancer staging greater than A4 versus A1 (OR, 5.78), major bleeding (OR, 4.59), and resection versus radiofrequency ablation (OR, 3.31).
“Low platelet count was associated with an increased risk of mortality at 3 months. However, this association disappeared at the multivariate analysis, which took into account markers of severity of liver cirrhosis,” he said.
Dr. Podda and his colleagues conducted the study in light of a recommendation from a consensus conference of the Italian Association for the Study of Liver Disease and the Italian Society of Internal Medicine that called for increasing platelet count by platelet transfusions in patients with cirrhosis who undergo an invasive procedure and who have a platelet count lower than 50 × 109/L.
“This recommendation mostly stemmed from consideration of biological plausibility prospects rather than being based on hard experimental evidence,” he explained, noting that such severe thrombocytopenia affects about 10% of patients with liver cirrhosis.
Based on the findings of this study, the practice is not supported, he concluded.
Dr. Podda reported honoraria from Sanofi, Boehringer Ingelheim.
SOURCE: Ronca V et al. ISTH 2020, Abstract OC 13.4.
REPORTING FROM THE 2020 ISTH CONGRESS
Value of palliative care shines clearly in a crisis
Hospitalists have played a key role
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
Hospitalists have played a key role
Hospitalists have played a key role
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
Postpartum tubal ligation safe in obese women
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
FROM OBSTETRICS & GYNECOLOGY
The best and worst states for health care in 2020
according to the personal finance website WalletHub.
The Bay State finds itself at the top of the company’s annual ranking of state health care systems this year after finishing second in 2019 to Minnesota, which is now ranked second. Rhode Island is third this year, followed by Washington, D.C., and North Dakota, WalletHub reported Aug. 3.
The inclusion of Washington, D.C., allowed Georgia to finish 51st out of 50 states, just below the quartet of Louisiana (50th), Alabama (49th), North Carolina (48th), and Mississippi (47th). Alaska, which occupied the bottom spot in 2019, moved up to 42nd this year, the analysis showed.
The rankings are based on 44 (up from 43 last year) metrics that are grouped into three broad categories: cost (6 metrics), access (24 metrics), and outcomes (14 metrics). The one new measure added for 2020? That would be health infrastructure for coronavirus, which is itself based on a different WalletHub ranking.
Massachusetts’ top finish this year was driven by strong showings in such metrics as average monthly insurance premium (first), physicians per capita (second), insured children (first) and adults (first), and infant mortality rate (fourth). The state was 1st overall in outcomes and 4th in access but only 20th in cost, the company said.
Positive signs among the lowest-ranked states include Louisiana’s 18th-place finish in access, ahead of such top 10 states as Iowa and Hawaii, and Mississippi’s 17th in cost, which is higher than four of the states in the top 10, including Massachusetts, WalletHub said in the report.
Data for the analysis came from 22 different sources, including the Institute for Health Metrics and Evaluation, Centers for Medicare & Medicaid Services, Association of American Medical Colleges, and the American Telemedicine Association.
according to the personal finance website WalletHub.

The Bay State finds itself at the top of the company’s annual ranking of state health care systems this year after finishing second in 2019 to Minnesota, which is now ranked second. Rhode Island is third this year, followed by Washington, D.C., and North Dakota, WalletHub reported Aug. 3.
The inclusion of Washington, D.C., allowed Georgia to finish 51st out of 50 states, just below the quartet of Louisiana (50th), Alabama (49th), North Carolina (48th), and Mississippi (47th). Alaska, which occupied the bottom spot in 2019, moved up to 42nd this year, the analysis showed.
The rankings are based on 44 (up from 43 last year) metrics that are grouped into three broad categories: cost (6 metrics), access (24 metrics), and outcomes (14 metrics). The one new measure added for 2020? That would be health infrastructure for coronavirus, which is itself based on a different WalletHub ranking.
Massachusetts’ top finish this year was driven by strong showings in such metrics as average monthly insurance premium (first), physicians per capita (second), insured children (first) and adults (first), and infant mortality rate (fourth). The state was 1st overall in outcomes and 4th in access but only 20th in cost, the company said.
Positive signs among the lowest-ranked states include Louisiana’s 18th-place finish in access, ahead of such top 10 states as Iowa and Hawaii, and Mississippi’s 17th in cost, which is higher than four of the states in the top 10, including Massachusetts, WalletHub said in the report.
Data for the analysis came from 22 different sources, including the Institute for Health Metrics and Evaluation, Centers for Medicare & Medicaid Services, Association of American Medical Colleges, and the American Telemedicine Association.
according to the personal finance website WalletHub.

The Bay State finds itself at the top of the company’s annual ranking of state health care systems this year after finishing second in 2019 to Minnesota, which is now ranked second. Rhode Island is third this year, followed by Washington, D.C., and North Dakota, WalletHub reported Aug. 3.
The inclusion of Washington, D.C., allowed Georgia to finish 51st out of 50 states, just below the quartet of Louisiana (50th), Alabama (49th), North Carolina (48th), and Mississippi (47th). Alaska, which occupied the bottom spot in 2019, moved up to 42nd this year, the analysis showed.
The rankings are based on 44 (up from 43 last year) metrics that are grouped into three broad categories: cost (6 metrics), access (24 metrics), and outcomes (14 metrics). The one new measure added for 2020? That would be health infrastructure for coronavirus, which is itself based on a different WalletHub ranking.
Massachusetts’ top finish this year was driven by strong showings in such metrics as average monthly insurance premium (first), physicians per capita (second), insured children (first) and adults (first), and infant mortality rate (fourth). The state was 1st overall in outcomes and 4th in access but only 20th in cost, the company said.
Positive signs among the lowest-ranked states include Louisiana’s 18th-place finish in access, ahead of such top 10 states as Iowa and Hawaii, and Mississippi’s 17th in cost, which is higher than four of the states in the top 10, including Massachusetts, WalletHub said in the report.
Data for the analysis came from 22 different sources, including the Institute for Health Metrics and Evaluation, Centers for Medicare & Medicaid Services, Association of American Medical Colleges, and the American Telemedicine Association.