User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Lessons for patients with MS and COVID-19
Two important lessons about managing patients with multiple sclerosis (MS) and COVID-19 have emerged from a hospital clinic in Madrid that managed COVID-infected patients with MS through the peak of the pandemic: Combined polymeric chain reaction and serology testing helped avoid disease reactivation in asymptomatic carriers during the pandemic peak, although after the peak PCR alone proved just as effective; and
Virginia Meca-Lallana, MD, a neurologist and coordinator of the demyelinating diseases unit at the Hospital of the University of the Princess in Madrid, and colleagues presented their findings in two posters at the Joint European Committee for Treatment and Research in Multiple Sclerosis-Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“MS treatments don’t seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors,” Dr. Meca-Lallana said in an interview. “MS treatments prevent the patients’ disability, and it is very important not to stop them if it isn’t necessary.”
The results arose from a multidisciplinary safety protocol involving neurology, microbiology, and preventive medicine that the University of Princess physicians developed to keep MS stable in patients diagnosed with SARS-CoV-2.
The researchers obtained 152 PCR nasopharyngeal swabs and 140 serology tests in 90 patients with MS over 3 months before starting a variety of MS treatments: Natalizumab (96 tests), ocrelizumab (36), rituximab (3), methylprednisolone (7), cladribine (4), and dimethyl fumarate (3). The protocol identified 7 asymptomatic carriers—7.8% of the total population—5 of whom had positive immunoglobulin M and G serology. The study also confirmed 5 patients with positive IgM+IgG serology post-infection, but no COVID-19 reactivations were detected after implementation of the protocol.
“The safety protocol reached its objective of avoiding disease reactivation and clinical activation in asymptomatic carriers,” Dr. Meca-Lallana said.
The second poster she presented reported on the real-world experience with SARS-CoV-2 in the MS unit at her hospital. The observational, prospective study included 41 cases, 38 of which were relapsing-remitting MS and the remainder progressive MS. The patients had MS for an average of 9 years.
“We need more patients to draw more robust conclusions, but in our patients, MS treatments seem safe in this situation,” Dr. Meca-Lallana said. “We did not discontinue treatments, and after our first results, we only delayed treatments in patients with any additional comorbidity or when coming to the hospital was not safe.”
A total of 39 patients were taking disease-modifying therapies (DMTs): 46.3% with oral agents, 39% with monoclonal antibodies, and 10% with injectable agents; 27 patients were previously treated with other DMTs. The median Expanded Disability Status Scale (EDSS) was 2.5, and 11 patients had clinical activity the previous year. Eighteen cases were confirmed by PCR or serology, or both, and 23 were diagnosed clinically.
Among the patients with MS and COVID-19, 17% were admitted to the hospital. Six patients had pneumonia, but none required admission to the intensive care unit, and no deaths occurred. Three patients had other comorbidities. Admitted patients tended to be older and had higher EDSS scores, although the difference was not statistically significant. MS worsened in 7 patients, and 10 patients stopped or paused DMTs because of the infection.
“Multiple sclerosis is a weakening illness,” Dr. Meca-Lallana said. “MS treatments do not seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors.”
The SARS-CoV-2 infection does not seem to result in a more aggressive form of the disease in MS patients, and selective immunosuppression may improve their outcomes, she noted.
“MS treatments avoid the patient’s disability,” the investigator added, “and it is very important not to stop them if it isn’t necessary.”
Dr. Meca-Lallana had no relevant financial disclosures.
Two important lessons about managing patients with multiple sclerosis (MS) and COVID-19 have emerged from a hospital clinic in Madrid that managed COVID-infected patients with MS through the peak of the pandemic: Combined polymeric chain reaction and serology testing helped avoid disease reactivation in asymptomatic carriers during the pandemic peak, although after the peak PCR alone proved just as effective; and
Virginia Meca-Lallana, MD, a neurologist and coordinator of the demyelinating diseases unit at the Hospital of the University of the Princess in Madrid, and colleagues presented their findings in two posters at the Joint European Committee for Treatment and Research in Multiple Sclerosis-Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“MS treatments don’t seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors,” Dr. Meca-Lallana said in an interview. “MS treatments prevent the patients’ disability, and it is very important not to stop them if it isn’t necessary.”
The results arose from a multidisciplinary safety protocol involving neurology, microbiology, and preventive medicine that the University of Princess physicians developed to keep MS stable in patients diagnosed with SARS-CoV-2.
The researchers obtained 152 PCR nasopharyngeal swabs and 140 serology tests in 90 patients with MS over 3 months before starting a variety of MS treatments: Natalizumab (96 tests), ocrelizumab (36), rituximab (3), methylprednisolone (7), cladribine (4), and dimethyl fumarate (3). The protocol identified 7 asymptomatic carriers—7.8% of the total population—5 of whom had positive immunoglobulin M and G serology. The study also confirmed 5 patients with positive IgM+IgG serology post-infection, but no COVID-19 reactivations were detected after implementation of the protocol.
“The safety protocol reached its objective of avoiding disease reactivation and clinical activation in asymptomatic carriers,” Dr. Meca-Lallana said.
The second poster she presented reported on the real-world experience with SARS-CoV-2 in the MS unit at her hospital. The observational, prospective study included 41 cases, 38 of which were relapsing-remitting MS and the remainder progressive MS. The patients had MS for an average of 9 years.
“We need more patients to draw more robust conclusions, but in our patients, MS treatments seem safe in this situation,” Dr. Meca-Lallana said. “We did not discontinue treatments, and after our first results, we only delayed treatments in patients with any additional comorbidity or when coming to the hospital was not safe.”
A total of 39 patients were taking disease-modifying therapies (DMTs): 46.3% with oral agents, 39% with monoclonal antibodies, and 10% with injectable agents; 27 patients were previously treated with other DMTs. The median Expanded Disability Status Scale (EDSS) was 2.5, and 11 patients had clinical activity the previous year. Eighteen cases were confirmed by PCR or serology, or both, and 23 were diagnosed clinically.
Among the patients with MS and COVID-19, 17% were admitted to the hospital. Six patients had pneumonia, but none required admission to the intensive care unit, and no deaths occurred. Three patients had other comorbidities. Admitted patients tended to be older and had higher EDSS scores, although the difference was not statistically significant. MS worsened in 7 patients, and 10 patients stopped or paused DMTs because of the infection.
“Multiple sclerosis is a weakening illness,” Dr. Meca-Lallana said. “MS treatments do not seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors.”
The SARS-CoV-2 infection does not seem to result in a more aggressive form of the disease in MS patients, and selective immunosuppression may improve their outcomes, she noted.
“MS treatments avoid the patient’s disability,” the investigator added, “and it is very important not to stop them if it isn’t necessary.”
Dr. Meca-Lallana had no relevant financial disclosures.
Two important lessons about managing patients with multiple sclerosis (MS) and COVID-19 have emerged from a hospital clinic in Madrid that managed COVID-infected patients with MS through the peak of the pandemic: Combined polymeric chain reaction and serology testing helped avoid disease reactivation in asymptomatic carriers during the pandemic peak, although after the peak PCR alone proved just as effective; and
Virginia Meca-Lallana, MD, a neurologist and coordinator of the demyelinating diseases unit at the Hospital of the University of the Princess in Madrid, and colleagues presented their findings in two posters at the Joint European Committee for Treatment and Research in Multiple Sclerosis-Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
“MS treatments don’t seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors,” Dr. Meca-Lallana said in an interview. “MS treatments prevent the patients’ disability, and it is very important not to stop them if it isn’t necessary.”
The results arose from a multidisciplinary safety protocol involving neurology, microbiology, and preventive medicine that the University of Princess physicians developed to keep MS stable in patients diagnosed with SARS-CoV-2.
The researchers obtained 152 PCR nasopharyngeal swabs and 140 serology tests in 90 patients with MS over 3 months before starting a variety of MS treatments: Natalizumab (96 tests), ocrelizumab (36), rituximab (3), methylprednisolone (7), cladribine (4), and dimethyl fumarate (3). The protocol identified 7 asymptomatic carriers—7.8% of the total population—5 of whom had positive immunoglobulin M and G serology. The study also confirmed 5 patients with positive IgM+IgG serology post-infection, but no COVID-19 reactivations were detected after implementation of the protocol.
“The safety protocol reached its objective of avoiding disease reactivation and clinical activation in asymptomatic carriers,” Dr. Meca-Lallana said.
The second poster she presented reported on the real-world experience with SARS-CoV-2 in the MS unit at her hospital. The observational, prospective study included 41 cases, 38 of which were relapsing-remitting MS and the remainder progressive MS. The patients had MS for an average of 9 years.
“We need more patients to draw more robust conclusions, but in our patients, MS treatments seem safe in this situation,” Dr. Meca-Lallana said. “We did not discontinue treatments, and after our first results, we only delayed treatments in patients with any additional comorbidity or when coming to the hospital was not safe.”
A total of 39 patients were taking disease-modifying therapies (DMTs): 46.3% with oral agents, 39% with monoclonal antibodies, and 10% with injectable agents; 27 patients were previously treated with other DMTs. The median Expanded Disability Status Scale (EDSS) was 2.5, and 11 patients had clinical activity the previous year. Eighteen cases were confirmed by PCR or serology, or both, and 23 were diagnosed clinically.
Among the patients with MS and COVID-19, 17% were admitted to the hospital. Six patients had pneumonia, but none required admission to the intensive care unit, and no deaths occurred. Three patients had other comorbidities. Admitted patients tended to be older and had higher EDSS scores, although the difference was not statistically significant. MS worsened in 7 patients, and 10 patients stopped or paused DMTs because of the infection.
“Multiple sclerosis is a weakening illness,” Dr. Meca-Lallana said. “MS treatments do not seem to make the prognosis of COVID-19 worse, but it is very important to evaluate other risk factors.”
The SARS-CoV-2 infection does not seem to result in a more aggressive form of the disease in MS patients, and selective immunosuppression may improve their outcomes, she noted.
“MS treatments avoid the patient’s disability,” the investigator added, “and it is very important not to stop them if it isn’t necessary.”
Dr. Meca-Lallana had no relevant financial disclosures.
FROM MSVirtual2020
New billing code for added COVID practice expense
The American Medical Association on Sept. 8 announced that a new code, 99072, is intended to cover additional supplies, materials, and clinical staff time over and above those usually included in an office visit when performed during a declared public health emergency, as defined by law, attributable to respiratory-transmitted infectious disease, the AMA said in a release.
Fifty national medical specialty societies and other organizations worked with the AMA’s Specialty Society RVS Update Committee over the summer to collect data on the costs of maintaining safe medical offices during the public health emergency. It has submitted recommendations to the Centers for Medicare & Medicaid Services seeking to persuade the federal agencies to recognize the new 99072 payment code.
The intention is to recognize the extra expenses involved in steps now routinely taken to reduce the risk for COVID transmission from office visits, Current Procedural Terminology Editorial Panel Chair Mark S. Synovec, MD, said in an interview. Some practices have adapted by having staff screen patients before they enter offices and making arrangements to keep patients at a safe distance from others during their visits, he said.
“Everyone’s life has significantly changed because of COVID and the health care system has dramatically changed,” Dr. Synovec said. “It was pretty clear that the status quo was not going to work.”
Physician practices will welcome this change, said Veronica Bradley, CPC, a senior industry adviser to the Medical Group Management Association. An office visit that in the past may have involved only basic infection control measures, such as donning a pair of gloves, now may involve clinicians taking the time to put on more extensive protective gear, she said.
“Now they are taking a heck of a lot more precautions, and there’s more time and more supplies being consumed,” Ms. Bradley said in an interview.
Code looks ahead to future use
The AMA explained how this new code differs from CPT code 99070, which is typically reported for supplies and materials that may be used or provided to patients during an office visit.
The new 99072 code applies only during declared public health emergencies and applies only to additional items required to support “a safe in-person provision” of evaluation, treatment, and procedures, the AMA said.
“These items contrast with those typically reported with code 99070, which focuses on additional supplies provided over and above those usually included with a specific service, such as drugs, intravenous catheters, or trays,” the AMA said.
The CPT panel sought to structure the new code for covering COVID practice expenses so that it could not be abused, and also looked ahead to the future, Dr. Synovec said.
“It’s a code that you would put on during a public health emergency as defined by law that would be related to a respiratory-transmitted infectious disease. Obviously we meant it for SARS-CoV-2,” he said. “Hopefully we can go another 100 years before we have another pandemic, but we also wanted to prepare something where if we have another airborne respiratory virus that requires additional practice expenses as seen this time, it would be available for use.”
The AMA also announced a second addition, CPT code 86413, that anticipates greater use of quantitative measurements of SARS-CoV-2 antibodies, as opposed to a qualitative assessment (positive/negative) provided by laboratory tests reported by other CPT codes.
More information is available on the AMA website.
A version of this article originally appeared on Medscape.com.
The American Medical Association on Sept. 8 announced that a new code, 99072, is intended to cover additional supplies, materials, and clinical staff time over and above those usually included in an office visit when performed during a declared public health emergency, as defined by law, attributable to respiratory-transmitted infectious disease, the AMA said in a release.
Fifty national medical specialty societies and other organizations worked with the AMA’s Specialty Society RVS Update Committee over the summer to collect data on the costs of maintaining safe medical offices during the public health emergency. It has submitted recommendations to the Centers for Medicare & Medicaid Services seeking to persuade the federal agencies to recognize the new 99072 payment code.
The intention is to recognize the extra expenses involved in steps now routinely taken to reduce the risk for COVID transmission from office visits, Current Procedural Terminology Editorial Panel Chair Mark S. Synovec, MD, said in an interview. Some practices have adapted by having staff screen patients before they enter offices and making arrangements to keep patients at a safe distance from others during their visits, he said.
“Everyone’s life has significantly changed because of COVID and the health care system has dramatically changed,” Dr. Synovec said. “It was pretty clear that the status quo was not going to work.”
Physician practices will welcome this change, said Veronica Bradley, CPC, a senior industry adviser to the Medical Group Management Association. An office visit that in the past may have involved only basic infection control measures, such as donning a pair of gloves, now may involve clinicians taking the time to put on more extensive protective gear, she said.
“Now they are taking a heck of a lot more precautions, and there’s more time and more supplies being consumed,” Ms. Bradley said in an interview.
Code looks ahead to future use
The AMA explained how this new code differs from CPT code 99070, which is typically reported for supplies and materials that may be used or provided to patients during an office visit.
The new 99072 code applies only during declared public health emergencies and applies only to additional items required to support “a safe in-person provision” of evaluation, treatment, and procedures, the AMA said.
“These items contrast with those typically reported with code 99070, which focuses on additional supplies provided over and above those usually included with a specific service, such as drugs, intravenous catheters, or trays,” the AMA said.
The CPT panel sought to structure the new code for covering COVID practice expenses so that it could not be abused, and also looked ahead to the future, Dr. Synovec said.
“It’s a code that you would put on during a public health emergency as defined by law that would be related to a respiratory-transmitted infectious disease. Obviously we meant it for SARS-CoV-2,” he said. “Hopefully we can go another 100 years before we have another pandemic, but we also wanted to prepare something where if we have another airborne respiratory virus that requires additional practice expenses as seen this time, it would be available for use.”
The AMA also announced a second addition, CPT code 86413, that anticipates greater use of quantitative measurements of SARS-CoV-2 antibodies, as opposed to a qualitative assessment (positive/negative) provided by laboratory tests reported by other CPT codes.
More information is available on the AMA website.
A version of this article originally appeared on Medscape.com.
The American Medical Association on Sept. 8 announced that a new code, 99072, is intended to cover additional supplies, materials, and clinical staff time over and above those usually included in an office visit when performed during a declared public health emergency, as defined by law, attributable to respiratory-transmitted infectious disease, the AMA said in a release.
Fifty national medical specialty societies and other organizations worked with the AMA’s Specialty Society RVS Update Committee over the summer to collect data on the costs of maintaining safe medical offices during the public health emergency. It has submitted recommendations to the Centers for Medicare & Medicaid Services seeking to persuade the federal agencies to recognize the new 99072 payment code.
The intention is to recognize the extra expenses involved in steps now routinely taken to reduce the risk for COVID transmission from office visits, Current Procedural Terminology Editorial Panel Chair Mark S. Synovec, MD, said in an interview. Some practices have adapted by having staff screen patients before they enter offices and making arrangements to keep patients at a safe distance from others during their visits, he said.
“Everyone’s life has significantly changed because of COVID and the health care system has dramatically changed,” Dr. Synovec said. “It was pretty clear that the status quo was not going to work.”
Physician practices will welcome this change, said Veronica Bradley, CPC, a senior industry adviser to the Medical Group Management Association. An office visit that in the past may have involved only basic infection control measures, such as donning a pair of gloves, now may involve clinicians taking the time to put on more extensive protective gear, she said.
“Now they are taking a heck of a lot more precautions, and there’s more time and more supplies being consumed,” Ms. Bradley said in an interview.
Code looks ahead to future use
The AMA explained how this new code differs from CPT code 99070, which is typically reported for supplies and materials that may be used or provided to patients during an office visit.
The new 99072 code applies only during declared public health emergencies and applies only to additional items required to support “a safe in-person provision” of evaluation, treatment, and procedures, the AMA said.
“These items contrast with those typically reported with code 99070, which focuses on additional supplies provided over and above those usually included with a specific service, such as drugs, intravenous catheters, or trays,” the AMA said.
The CPT panel sought to structure the new code for covering COVID practice expenses so that it could not be abused, and also looked ahead to the future, Dr. Synovec said.
“It’s a code that you would put on during a public health emergency as defined by law that would be related to a respiratory-transmitted infectious disease. Obviously we meant it for SARS-CoV-2,” he said. “Hopefully we can go another 100 years before we have another pandemic, but we also wanted to prepare something where if we have another airborne respiratory virus that requires additional practice expenses as seen this time, it would be available for use.”
The AMA also announced a second addition, CPT code 86413, that anticipates greater use of quantitative measurements of SARS-CoV-2 antibodies, as opposed to a qualitative assessment (positive/negative) provided by laboratory tests reported by other CPT codes.
More information is available on the AMA website.
A version of this article originally appeared on Medscape.com.
Obesity-related hypoventilation increased morbidity risk after bariatric surgery
Patients with obesity-associated sleep hypoventilation had a heightened risk of postoperative morbidities after bariatric surgery, according to a retrospective study.
Reena Mehra, MD, director of sleep disorders research for the Sleep Disorders Center at the Cleveland Clinic, led the team and the findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies. Her research team examined the outcomes of 1,665 patients who underwent polysomnography prior to bariatric surgery performed at the Cleveland Clinic from 2011 to 2018.
More than two-thirds – 68.5% – had obesity-associated sleep hypoventilation as defined by body mass index (BMI) of ≥30 kg/m2 and either polysomnography-based end-tidal CO2 ≥45 mm Hg or serum bicarbonate ≥27 mEq/L.
These patients represent “a subset, if you will, of obesity hypoventilation syndrome – a subset that we were able to capture from our sleep studies … [because] we do CO2 monitoring during sleep studies uniformly,” Dr. Mehra said in an interview after the meeting.
Pornprapa Chindamporn, MD, a former fellow at the center and first author on the abstract, presented the findings. Patients in the study had a mean age of 45.2 ± 12.0 years and a BMI of 48.7 ± 9.0. Approximately 20% were male and 63.6% were White.
Those with obesity-associated sleep hypoventilation were more likely to be male and have a higher BMI and higher hemoglobin A1c than those without the condition. They also had a significantly higher apnea-hypopnea index (17.0 vs. 13.8) in those without the condition, she reported.
A number of outcomes (ICU stay, intubation, tracheostomy, discharge disposition, and 30-day readmission) were compared individually and as a composite outcome between those with and without obesity-associated sleep hypoventilation. While some of these postoperative morbidities were more common in patients with the condition, the differences between those with and without OHS were not statistically significant for intubation (1.5% vs. 1.3%, P = .81) and 30-day readmission (13.8% vs. 11.3%, P = .16). However, the composite outcome was significantly higher: 18.9% vs. 14.3% (P = .021), including in multivariable analysis that considered age, gender, BMI, Apnea Hypopnea Index, and diabetes.
All-cause mortality was not significantly different between the groups, likely because of its low overall rate (hazard ratio, 1.39; 95% confidence interval, 0.56-3.42).
“In this largest sample to date of systematically phenotyped obesity-associated sleep hypoventilation in patients undergoing bariatric surgery, we identified increased postoperative morbidity,” said Dr. Chindamporn, now a pulmonologist and sleep specialist practicing in Bangkok.
Dr. Mehra said in the interview that patients considering bariatric surgery are typically assessed for obstructive sleep apnea, but “not so much obesity hypoventilation syndrome or obesity-associated sleep-related hypoventilation syndrome.” The findings, “support the notion that we should be closely examining sleep-related hypoventilation in these patients.”
At the Cleveland Clinic, “clinically, we make sure we’re identifying these individuals and communicating the findings to bariatric surgery colleagues and to anesthesia,” said Dr. Mehra, also professor of medicine at Case Western Reserve University, Cleveland.
OHS is defined, according to the 2019 American Thoracic Society clinical practice guideline on evaluation and management of OHS, by the combination of obesity, sleep-disordered breathing, and awake daytime hypercapnia, after excluding other causes for hypoventilation (Am J Respir Crit Care Med. 2019;200[3]:e6-24).
A European Respiratory Society task force has proposed severity grading for OHS, with early stages defined by sleep-related hypoventilation and the highest grade of severity defined by morbidity-associated daytime hypercapnia (Eur Respir Rev. 2019;28:180097). However, Dr. Mehra said she is “not sure that we know enough [from long-term studies of OHS] to say definitively that there’s such an evolution.”
Certainly, she said, future research on OHS should consider its heterogeneity. It is possible that a subset of patients with OHS, “maybe these individuals with sleep-related hypoventilation,” are most likely to have adverse postsurgical outcomes.
Atul Malhotra, MD, professor of medicine at the University of California, San Diego, who was asked to comment on the study, said that OHS is understudied in general and particularly in the perioperative setting. “With the obesity pandemic, issues around OHS are likely to be [increasingly] important. And with increasing [use of] bariatric surgery, strategies to minimize risks are clearly needed,” he said, adding that the potential risks of nonbariatric surgery in patients with OHS require further study.
He noted that mortality rates in good hospitals “have become quite low for many elective surgeries, making it hard to show mortality benefit to most interventions.”
The ATS guideline on OHS states that it is the most severe form of obesity-induced respiratory compromise and leads to serious sequelae, including increased rates of mortality, chronic heart failure, pulmonary hypertension, and hospitalization caused by acute-on-chronic hypercapnic respiratory failure.
Dr. Chindamporn said in her presentation that she had no disclosures. Dr. Mehra’s research program is funded by the National Institute of Health, but she has also procured funding from the American College of Chest Physicians, American Heart Association, Clinical Translational Science Collaborative, and Central Society of Clinical Research. Dr. Malhotra disclosed that he is funded by the NIH and has received income from Merck and LIvanova related to medical education.
CORRECTION 9/15/2020: The original story misstated the presenter of the study. Dr. Chindamporn presented the findings.
Patients with obesity-associated sleep hypoventilation had a heightened risk of postoperative morbidities after bariatric surgery, according to a retrospective study.
Reena Mehra, MD, director of sleep disorders research for the Sleep Disorders Center at the Cleveland Clinic, led the team and the findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies. Her research team examined the outcomes of 1,665 patients who underwent polysomnography prior to bariatric surgery performed at the Cleveland Clinic from 2011 to 2018.
More than two-thirds – 68.5% – had obesity-associated sleep hypoventilation as defined by body mass index (BMI) of ≥30 kg/m2 and either polysomnography-based end-tidal CO2 ≥45 mm Hg or serum bicarbonate ≥27 mEq/L.
These patients represent “a subset, if you will, of obesity hypoventilation syndrome – a subset that we were able to capture from our sleep studies … [because] we do CO2 monitoring during sleep studies uniformly,” Dr. Mehra said in an interview after the meeting.
Pornprapa Chindamporn, MD, a former fellow at the center and first author on the abstract, presented the findings. Patients in the study had a mean age of 45.2 ± 12.0 years and a BMI of 48.7 ± 9.0. Approximately 20% were male and 63.6% were White.
Those with obesity-associated sleep hypoventilation were more likely to be male and have a higher BMI and higher hemoglobin A1c than those without the condition. They also had a significantly higher apnea-hypopnea index (17.0 vs. 13.8) in those without the condition, she reported.
A number of outcomes (ICU stay, intubation, tracheostomy, discharge disposition, and 30-day readmission) were compared individually and as a composite outcome between those with and without obesity-associated sleep hypoventilation. While some of these postoperative morbidities were more common in patients with the condition, the differences between those with and without OHS were not statistically significant for intubation (1.5% vs. 1.3%, P = .81) and 30-day readmission (13.8% vs. 11.3%, P = .16). However, the composite outcome was significantly higher: 18.9% vs. 14.3% (P = .021), including in multivariable analysis that considered age, gender, BMI, Apnea Hypopnea Index, and diabetes.
All-cause mortality was not significantly different between the groups, likely because of its low overall rate (hazard ratio, 1.39; 95% confidence interval, 0.56-3.42).
“In this largest sample to date of systematically phenotyped obesity-associated sleep hypoventilation in patients undergoing bariatric surgery, we identified increased postoperative morbidity,” said Dr. Chindamporn, now a pulmonologist and sleep specialist practicing in Bangkok.
Dr. Mehra said in the interview that patients considering bariatric surgery are typically assessed for obstructive sleep apnea, but “not so much obesity hypoventilation syndrome or obesity-associated sleep-related hypoventilation syndrome.” The findings, “support the notion that we should be closely examining sleep-related hypoventilation in these patients.”
At the Cleveland Clinic, “clinically, we make sure we’re identifying these individuals and communicating the findings to bariatric surgery colleagues and to anesthesia,” said Dr. Mehra, also professor of medicine at Case Western Reserve University, Cleveland.
OHS is defined, according to the 2019 American Thoracic Society clinical practice guideline on evaluation and management of OHS, by the combination of obesity, sleep-disordered breathing, and awake daytime hypercapnia, after excluding other causes for hypoventilation (Am J Respir Crit Care Med. 2019;200[3]:e6-24).
A European Respiratory Society task force has proposed severity grading for OHS, with early stages defined by sleep-related hypoventilation and the highest grade of severity defined by morbidity-associated daytime hypercapnia (Eur Respir Rev. 2019;28:180097). However, Dr. Mehra said she is “not sure that we know enough [from long-term studies of OHS] to say definitively that there’s such an evolution.”
Certainly, she said, future research on OHS should consider its heterogeneity. It is possible that a subset of patients with OHS, “maybe these individuals with sleep-related hypoventilation,” are most likely to have adverse postsurgical outcomes.
Atul Malhotra, MD, professor of medicine at the University of California, San Diego, who was asked to comment on the study, said that OHS is understudied in general and particularly in the perioperative setting. “With the obesity pandemic, issues around OHS are likely to be [increasingly] important. And with increasing [use of] bariatric surgery, strategies to minimize risks are clearly needed,” he said, adding that the potential risks of nonbariatric surgery in patients with OHS require further study.
He noted that mortality rates in good hospitals “have become quite low for many elective surgeries, making it hard to show mortality benefit to most interventions.”
The ATS guideline on OHS states that it is the most severe form of obesity-induced respiratory compromise and leads to serious sequelae, including increased rates of mortality, chronic heart failure, pulmonary hypertension, and hospitalization caused by acute-on-chronic hypercapnic respiratory failure.
Dr. Chindamporn said in her presentation that she had no disclosures. Dr. Mehra’s research program is funded by the National Institute of Health, but she has also procured funding from the American College of Chest Physicians, American Heart Association, Clinical Translational Science Collaborative, and Central Society of Clinical Research. Dr. Malhotra disclosed that he is funded by the NIH and has received income from Merck and LIvanova related to medical education.
CORRECTION 9/15/2020: The original story misstated the presenter of the study. Dr. Chindamporn presented the findings.
Patients with obesity-associated sleep hypoventilation had a heightened risk of postoperative morbidities after bariatric surgery, according to a retrospective study.
Reena Mehra, MD, director of sleep disorders research for the Sleep Disorders Center at the Cleveland Clinic, led the team and the findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies. Her research team examined the outcomes of 1,665 patients who underwent polysomnography prior to bariatric surgery performed at the Cleveland Clinic from 2011 to 2018.
More than two-thirds – 68.5% – had obesity-associated sleep hypoventilation as defined by body mass index (BMI) of ≥30 kg/m2 and either polysomnography-based end-tidal CO2 ≥45 mm Hg or serum bicarbonate ≥27 mEq/L.
These patients represent “a subset, if you will, of obesity hypoventilation syndrome – a subset that we were able to capture from our sleep studies … [because] we do CO2 monitoring during sleep studies uniformly,” Dr. Mehra said in an interview after the meeting.
Pornprapa Chindamporn, MD, a former fellow at the center and first author on the abstract, presented the findings. Patients in the study had a mean age of 45.2 ± 12.0 years and a BMI of 48.7 ± 9.0. Approximately 20% were male and 63.6% were White.
Those with obesity-associated sleep hypoventilation were more likely to be male and have a higher BMI and higher hemoglobin A1c than those without the condition. They also had a significantly higher apnea-hypopnea index (17.0 vs. 13.8) in those without the condition, she reported.
A number of outcomes (ICU stay, intubation, tracheostomy, discharge disposition, and 30-day readmission) were compared individually and as a composite outcome between those with and without obesity-associated sleep hypoventilation. While some of these postoperative morbidities were more common in patients with the condition, the differences between those with and without OHS were not statistically significant for intubation (1.5% vs. 1.3%, P = .81) and 30-day readmission (13.8% vs. 11.3%, P = .16). However, the composite outcome was significantly higher: 18.9% vs. 14.3% (P = .021), including in multivariable analysis that considered age, gender, BMI, Apnea Hypopnea Index, and diabetes.
All-cause mortality was not significantly different between the groups, likely because of its low overall rate (hazard ratio, 1.39; 95% confidence interval, 0.56-3.42).
“In this largest sample to date of systematically phenotyped obesity-associated sleep hypoventilation in patients undergoing bariatric surgery, we identified increased postoperative morbidity,” said Dr. Chindamporn, now a pulmonologist and sleep specialist practicing in Bangkok.
Dr. Mehra said in the interview that patients considering bariatric surgery are typically assessed for obstructive sleep apnea, but “not so much obesity hypoventilation syndrome or obesity-associated sleep-related hypoventilation syndrome.” The findings, “support the notion that we should be closely examining sleep-related hypoventilation in these patients.”
At the Cleveland Clinic, “clinically, we make sure we’re identifying these individuals and communicating the findings to bariatric surgery colleagues and to anesthesia,” said Dr. Mehra, also professor of medicine at Case Western Reserve University, Cleveland.
OHS is defined, according to the 2019 American Thoracic Society clinical practice guideline on evaluation and management of OHS, by the combination of obesity, sleep-disordered breathing, and awake daytime hypercapnia, after excluding other causes for hypoventilation (Am J Respir Crit Care Med. 2019;200[3]:e6-24).
A European Respiratory Society task force has proposed severity grading for OHS, with early stages defined by sleep-related hypoventilation and the highest grade of severity defined by morbidity-associated daytime hypercapnia (Eur Respir Rev. 2019;28:180097). However, Dr. Mehra said she is “not sure that we know enough [from long-term studies of OHS] to say definitively that there’s such an evolution.”
Certainly, she said, future research on OHS should consider its heterogeneity. It is possible that a subset of patients with OHS, “maybe these individuals with sleep-related hypoventilation,” are most likely to have adverse postsurgical outcomes.
Atul Malhotra, MD, professor of medicine at the University of California, San Diego, who was asked to comment on the study, said that OHS is understudied in general and particularly in the perioperative setting. “With the obesity pandemic, issues around OHS are likely to be [increasingly] important. And with increasing [use of] bariatric surgery, strategies to minimize risks are clearly needed,” he said, adding that the potential risks of nonbariatric surgery in patients with OHS require further study.
He noted that mortality rates in good hospitals “have become quite low for many elective surgeries, making it hard to show mortality benefit to most interventions.”
The ATS guideline on OHS states that it is the most severe form of obesity-induced respiratory compromise and leads to serious sequelae, including increased rates of mortality, chronic heart failure, pulmonary hypertension, and hospitalization caused by acute-on-chronic hypercapnic respiratory failure.
Dr. Chindamporn said in her presentation that she had no disclosures. Dr. Mehra’s research program is funded by the National Institute of Health, but she has also procured funding from the American College of Chest Physicians, American Heart Association, Clinical Translational Science Collaborative, and Central Society of Clinical Research. Dr. Malhotra disclosed that he is funded by the NIH and has received income from Merck and LIvanova related to medical education.
CORRECTION 9/15/2020: The original story misstated the presenter of the study. Dr. Chindamporn presented the findings.
FROM SLEEP 2020
Dangers behind antimaskers and antivaxxers: How to combat both
Niket Sonpal, MD, thought he’d heard most of the myths about wearing masks during the pandemic, but the recent claim from a patient was a new one for the New York City gastroenterologist.
The patient refused to wear a mask because she heard inhaling bad breath through a mask could be toxic. The woman said the rumor was circulating on Facebook. Sonpal calmly explained that breathing your own breath is not going to cause health problems, he said.
“There’s a lot of controversy on masks,” he said. “Unfortunately, it’s really just a lack of education and buy-in. Social media is the primary source of all this misinformation. These kinds of over-the-top hyperbole has basically led to a disbelief that masks are effective. The disbelief is hard to break up.”
As mask requirements have tightened amid the ongoing pandemic, debates about face coverings have emerged front and center, with a growing number of people opposing mask usage. So-called antimaskers dispute the benefits of wearing masks and many contend that face coverings decrease oxygen flow and can lead to illness. Sentiment against masks have led to protests nationwide, ignited public conflicts in some areas, and even generated lawsuits over mask mandates.
The issue presents an ongoing challenge for physicians as they strive to educate patients about the significance of masking against the flood of antimask messages on social media and beyond. Opposition to masks is particularly frustrating for health professionals who have witnessed patients, family, or friends become ill or die from the virus. Refusing to mask and failing to social distance have been linked to the rapid spread of the coronavirus and subsequent deaths.
“I have had colleagues pass away, and it’s extremely disheartening and frustrating to see science so easily disregarded,” Sonpal said. “Masks save lives and protect people and not wearing them is simply a lack of respect, not just for your fellow colleagues, but for a member of your species.”
Michael Rebresh, who helped create the antimask group Million Unmasked Patriots, says his group’s objections to masks are rational and reasonable. The group, which has more than 8,000 members, formed in response to guidance by Illinois state officials that children would only be allowed to return to school wearing a mask.
“Our objections are to the fact that masks on children in school have a greater propensity to make children sick from breathing in bacteria that forms on the inner layer of a mask worn for hours on end,” Rebresh said. “We have an objection to the increase of CO2 intake and a decrease in oxygen flow for kids who need all the oxygen they can get during a learning environment. We recognized the masking of ourselves and kids for what it is: A political move to separate the two parties in our November election and define and create division between the two.”
Million Unmasked Patriots is one of dozens of antimask groups on social media platforms such as Facebook, Instagram, and TikTok. In July, Facebook suspended one such group, Unmasking America, which boasts 9,600 members, for posting repeated claims that face masks obstruct oxygen flow and have negative mental health effects.
Experts say the antiscience rhetoric is far from new. The antimask movement in many ways, shares similarities with that of the anti-vaccine movement, says Todd Wolynn, MD, a Pittsburgh pediatrician and cofounder of Shots Heard Round the World, an organization that defends vaccine advocates against coordinated online attacks by antivaxxers.
“A lot of it is conspiracy-laden,” said Wolynn of the disinformation. “That Dr. [Anthony] Fauci somehow helped construct the pandemic and that it’s not real. That Bill Gates is funding the vaccine so he can inject people with microchips. All sorts of really out-there, ungrounded conspiracy theories. If you had Venn diagram of antimask and antivaxx, I would say there’s clearly overlap.”
Parallels between antimaskers, antivaxxers
Opponents to masks fall on a spectrum, explains Vineet Arora, MD, a hospitalist and associate chief medical officer–clinical learning environment at University of Chicago Medicine. People who believe conspiracy theories and push misinformation are on one end, she said. There are also those who generally don’t believe the seriousness of the pandemic, feel their risk is minimal, or doubt the benefits of masks.
The two trains of thought resemble the distinction among parents who are antivaccine and those who are simply “vaccine hesitant,” says Arora, who co-authored a recent article about masking and misinformation that addresses antivaccine attitudes.
“While the antimask sentiment gets a lot of attention, I think it’s important to highlight there’s a lot of vocal anti-mask sentiment since most people are supportive of masks,” she said. “There might be people sitting on the fence who are just unsure about wearing a mask. That’s understandable because the science and the communication has evolved. There was a lot of early mixed messages about masking. Anytime you have confusion about the science or the science is evolving, it’s easy to have misinformation and then have that take off as myth.”
Just as antivaxxers work to swing the opinion of the vaccine hesitant, antimaskers are vying with public health advocates for the support of the mask hesitant, she said. Creating doubt in public health authorities is one way they are gaining followers. Anti-maskers often question and scrutinize past messaging about masks by public health officials, claiming that because guidance on masks has changed over time, the science behind masks and current guidance can’t be trusted, Wolynn said. Similarly, antivaxxers frequently question past actions by public health officials, such as the Tuskegee Experiment (which began in 1932), to try to poke holes in the credibility of public health officials and their advice.
Both the antimask and antivaccine movements also tend to base their resistance on a personal liberties argument, adds Jacqueline Winfield Fincher, MD, president for the American College of Physicians and an internist based in Thomson, Georgia. Antimaskers contend they should be free to decide whether to wear face coverings and that rules requiring masks infringe upon their civil liberties. Similarly, antivaxxers argue they should be free to decide whether to vaccinate their children and contend vaccine mandates violate their personal liberties.
Taking a deeper look, fear and control are two likely drivers of antimasking and antivaccine attitudes, Fincher said. Those refusing to wear masks may feel they have no control over the pandemic or its impacts, but they can control how they respond to mask-wearing requirements, she said.
Antivaccine parents often want more control over their children’s healthcare and falsely believe that vaccines are injecting something harmful into their children or may lead to harmful reactions.
“It’s a control issue and a defense mechanism,” she said. “Some people may feel helpless to deal with the pandemic or believe since it is not affecting them or their family, that it is not real. ‘If I just deny it and I don’t acknowledge facts, I don’t have to worry about it or do anything about it, and therefore I will have more control over my day-to-day life.’”
Groups fueling each other
In some cases, antimask and antivaxx groups are joining forces or adopting dual causes.
In California for instance, longtime opponents to vaccines are now objecting to mask policies as similar infringement to their bodily autonomy. Demonstrations in Texas, Idaho, and Michigan against mask mandates and other COVID-19 requirements have drawn support from anti-vaccine activists and incorporated antivaccine propaganda.
In Illinois, Million Unmasked Patriots, formally the Million Unmasked March, has received widespread attention for protesting both masks for returning schoolchildren and a future COVID-19 vaccine requirement.
A July protest planned by the antimask group triggered a letter by Arora and 500 other healthcare professionals to Illinois lawmakers decrying the group’s views and urging the state to move forward with universal masking in schools.
“What’s happening is those who are distrustful of government and public health and science are joining together,” said Arora, who coauthored a piece about the problem on KevinMD.com. “It’s important to address both movements together because they can quickly feed off each other and build in momentum. At the heart of both is really this deep skepticism of science.”
Rebresh of Million Unmasked Patriots said most of his members are not opposed to all vaccines, but rather they are opposed to “untested vaccines.” The primary concern is the inability to research long-term effects of a COVID-19 vaccine before its approval, he said.
Rebresh disagrees with the antimask movement being compared with the antivaccine movement. The two groups are “motivated by different things and a different set of circumstances drive their opinions,” he said. However, Rebresh believes that potential harm resulting from “mass vaccinations” is a valid concern. For this reason, he and his wife chose for their children to receive their vaccinations individually over a series of weeks, rather than the “kiddie cocktail of vaccines,” at a single visit, he said.
Vaccine scientist Peter Hotez, MD, PhD, said the antivaccine movement appears to have grown stronger from the pandemic fueled by fresh conspiracies and new alliances. Antivaccine sentiment has been gaining steam over the last several years and collecting more allies from the far-right, said Hotez, dean for the National School of Tropical Medicine and codirector for the Texas Children’s Hospital Center for Vaccine Development.
“Now what you’re seeing is yet another expansion this year, with antivaccine groups, under the banner of ‘health freedom,’ campaigning against social distancing and wearing masks and contact tracing,” he said. “What was an antivaccine movement has now become a full-blown antiscience movement and an anti-public health movement. It’s causing a lot of damage and I believe costing a lot of American lives.”
Neil F. Johnson, PhD, who has studied the antivaccine movement and its social media proliferation during the pandemic, said online comments by antivaxxers frequently condemn mask usage and showcase memes making fun of masks.
“In those same narratives about opposing vaccines for COVID, we see a lot of discussion against masks,” said Johnson, a physics professor at George Washington University in Washington, D.C. “If you don’t believe in the official picture of COVID, you don’t believe the policies or the advice that’s given about COVID.”
An analysis by Johnson that examined 1,300 Facebook pages found that, while antivaxxers have fewer followers than provaccine pages, antivaccine pages are more numerous, faster growing, and are more often connected to unrelated, undecided pages. Conversely, pages that advocate the benefits of vaccinations and explain the science behind immunizations are largely disconnected from such undecided communities, according to the study, published May 13 in Nature.
The study suggests the antivaccine movement is making influential strides during the pandemic and connecting with people who are undecided, while public health advocates are not building the same bridges, Johnson said.
“I think it’s hugely dangerous, because I don’t know any other moment in science or in public health when there was so much uncertainty in something affecting everybody,” he said. “Every policy that will be coming, everything depends on people buying into the official message. Once you have the seeds of doubt, that’s a very difficult thing to overcome. It’s an unprecedented challenge.”
How physicians and clinicians can help
A more aggressive approach is necessary when it comes to taking down antiscience content on social media, says Hotez. Too often, misinformation and antiscience rhetoric is allowed to linger on popular sites such as Facebook and Amazon.
Wolynn agrees. On personal or business platforms, it’s crucial to ban, hide, and delete such comments as quickly as possible, he said. On public sites, purposeful disinformation should be immediately reported to the platform.
At the same time, Wolynn said it’s essential to support those who make sound, science-based comments in social media forums.
“If you see someone who is pushing accurate, evidence-based information, and they come under attack, they should be supported and defended and empowered,” Wolynn said. “Shots Heard Round the World is doing all of those things, including galvanizing and recruiting more people to help get their voices out there.”
Expanded visibility by physicians and scientists would greatly help counter the spread of antiscience sentiment, adds Hotez.
“Too often, antiscience movements are able to flourish because scientists and physicians are invisible,” he said. “They’re too focused on either clinical practices or in the case of physician scientists, on grants and papers and not enough attention to public engagement. We’re going to have to change that around. We need to hear more from scientists directly.”
To that end, Wolynn said health care professionals, including medical students and residents, need to have formal training in communications, media, and social media as part of their education – and more support from employers to engage through social media.
“That’s where the fight is,” Wolynn said. “You can be the best diagnostician, the best clinician. You can make the right diagnosis and prescribe the right medication, but if families don’t hear what you’re saying, you’re not going to be effective. If you can’t be on the platform where they’re being influenced, we’re losing the battle.”
Speaking to your mask-hesitant patients
Concentrating on those who are uncertain about masks is particularly key for physicians and public health advocates as the pandemic continues, says Arora.
“It’s important for us to focus on the mask-hesitant who often don’t get the attention they need,” she said.
She suggests bringing up the subject of masks with patients during visits, asking about mask usage, discussing rumors they’ve heard, and emphasizing why masks are important. Be a role model by wearing a mask in your community and on social media, she added.
Some patients have real concerns about not being able to breathe through masks or anxiety disorders that can be aggravated even by the thought of wearing a mask, noted Susan R. Bailey, MD, president for the American Medical Association. Bailey, an immunologist, recently counseled a patient with a deviated nasal septum in addition to a panic disorder who was worried about wearing a mask, she said. Bailey listened to the patient’s concerns, discussed his health conditions, and proposed an alternative face covering that might make him more comfortable.
“Every patient is different,” Bailey said. “It’s important for us to remember that each person who is reluctant to wear a mask has their own reasons. It’s important for us to express some empathy – to agree with them, yes, masks are hot and inconvenient – and help understand their questions, which you may be able to answer to their satisfaction. There are patients that have legitimate questions and a physician caring about how they feel, can make all the difference.”
Physicians can also get involved with the AMA’s #MaskUp campaign, an effort to normalize mask wearing and debunk myths associated with masks. The campaign includes social media materials, slogans doctors can tweet, and profile pictures they can use on social media. The campaign’s toolkit includes images, videos, and information that physicians can share with patients and the public.
Enforcing strong mask policies at your practice and ensuring all staff are modeling appropriate mask behavior is also important, adds Fincher of the ACP. The college recently issued a policy supporting mask usage in community settings.
If a patient conveys an antimask belief, Fincher suggests not directly challenging the person’s views, but listening to them and offering objective data, discussing the science behind masks, and directing them to credible sources.
“Doctors are used to this. We recommend a lot of things to patients that they don’t want to do,” Fincher said. “If a patient feels attacked, they act defensively. But if you base your explanation in more objective terms with data, numbers, and personalize the risks and benefits of a vaccine, a healthy change in behavior, or a medication, then patients are more likely to hear your concerns and do the right thing. Having a long-term relationship with a trusted physician makes all of these issues much easier to discuss and to implement the best plan for the individual patient.”
This article first appeared on Medscape.com.
Niket Sonpal, MD, thought he’d heard most of the myths about wearing masks during the pandemic, but the recent claim from a patient was a new one for the New York City gastroenterologist.
The patient refused to wear a mask because she heard inhaling bad breath through a mask could be toxic. The woman said the rumor was circulating on Facebook. Sonpal calmly explained that breathing your own breath is not going to cause health problems, he said.
“There’s a lot of controversy on masks,” he said. “Unfortunately, it’s really just a lack of education and buy-in. Social media is the primary source of all this misinformation. These kinds of over-the-top hyperbole has basically led to a disbelief that masks are effective. The disbelief is hard to break up.”
As mask requirements have tightened amid the ongoing pandemic, debates about face coverings have emerged front and center, with a growing number of people opposing mask usage. So-called antimaskers dispute the benefits of wearing masks and many contend that face coverings decrease oxygen flow and can lead to illness. Sentiment against masks have led to protests nationwide, ignited public conflicts in some areas, and even generated lawsuits over mask mandates.
The issue presents an ongoing challenge for physicians as they strive to educate patients about the significance of masking against the flood of antimask messages on social media and beyond. Opposition to masks is particularly frustrating for health professionals who have witnessed patients, family, or friends become ill or die from the virus. Refusing to mask and failing to social distance have been linked to the rapid spread of the coronavirus and subsequent deaths.
“I have had colleagues pass away, and it’s extremely disheartening and frustrating to see science so easily disregarded,” Sonpal said. “Masks save lives and protect people and not wearing them is simply a lack of respect, not just for your fellow colleagues, but for a member of your species.”
Michael Rebresh, who helped create the antimask group Million Unmasked Patriots, says his group’s objections to masks are rational and reasonable. The group, which has more than 8,000 members, formed in response to guidance by Illinois state officials that children would only be allowed to return to school wearing a mask.
“Our objections are to the fact that masks on children in school have a greater propensity to make children sick from breathing in bacteria that forms on the inner layer of a mask worn for hours on end,” Rebresh said. “We have an objection to the increase of CO2 intake and a decrease in oxygen flow for kids who need all the oxygen they can get during a learning environment. We recognized the masking of ourselves and kids for what it is: A political move to separate the two parties in our November election and define and create division between the two.”
Million Unmasked Patriots is one of dozens of antimask groups on social media platforms such as Facebook, Instagram, and TikTok. In July, Facebook suspended one such group, Unmasking America, which boasts 9,600 members, for posting repeated claims that face masks obstruct oxygen flow and have negative mental health effects.
Experts say the antiscience rhetoric is far from new. The antimask movement in many ways, shares similarities with that of the anti-vaccine movement, says Todd Wolynn, MD, a Pittsburgh pediatrician and cofounder of Shots Heard Round the World, an organization that defends vaccine advocates against coordinated online attacks by antivaxxers.
“A lot of it is conspiracy-laden,” said Wolynn of the disinformation. “That Dr. [Anthony] Fauci somehow helped construct the pandemic and that it’s not real. That Bill Gates is funding the vaccine so he can inject people with microchips. All sorts of really out-there, ungrounded conspiracy theories. If you had Venn diagram of antimask and antivaxx, I would say there’s clearly overlap.”
Parallels between antimaskers, antivaxxers
Opponents to masks fall on a spectrum, explains Vineet Arora, MD, a hospitalist and associate chief medical officer–clinical learning environment at University of Chicago Medicine. People who believe conspiracy theories and push misinformation are on one end, she said. There are also those who generally don’t believe the seriousness of the pandemic, feel their risk is minimal, or doubt the benefits of masks.
The two trains of thought resemble the distinction among parents who are antivaccine and those who are simply “vaccine hesitant,” says Arora, who co-authored a recent article about masking and misinformation that addresses antivaccine attitudes.
“While the antimask sentiment gets a lot of attention, I think it’s important to highlight there’s a lot of vocal anti-mask sentiment since most people are supportive of masks,” she said. “There might be people sitting on the fence who are just unsure about wearing a mask. That’s understandable because the science and the communication has evolved. There was a lot of early mixed messages about masking. Anytime you have confusion about the science or the science is evolving, it’s easy to have misinformation and then have that take off as myth.”
Just as antivaxxers work to swing the opinion of the vaccine hesitant, antimaskers are vying with public health advocates for the support of the mask hesitant, she said. Creating doubt in public health authorities is one way they are gaining followers. Anti-maskers often question and scrutinize past messaging about masks by public health officials, claiming that because guidance on masks has changed over time, the science behind masks and current guidance can’t be trusted, Wolynn said. Similarly, antivaxxers frequently question past actions by public health officials, such as the Tuskegee Experiment (which began in 1932), to try to poke holes in the credibility of public health officials and their advice.
Both the antimask and antivaccine movements also tend to base their resistance on a personal liberties argument, adds Jacqueline Winfield Fincher, MD, president for the American College of Physicians and an internist based in Thomson, Georgia. Antimaskers contend they should be free to decide whether to wear face coverings and that rules requiring masks infringe upon their civil liberties. Similarly, antivaxxers argue they should be free to decide whether to vaccinate their children and contend vaccine mandates violate their personal liberties.
Taking a deeper look, fear and control are two likely drivers of antimasking and antivaccine attitudes, Fincher said. Those refusing to wear masks may feel they have no control over the pandemic or its impacts, but they can control how they respond to mask-wearing requirements, she said.
Antivaccine parents often want more control over their children’s healthcare and falsely believe that vaccines are injecting something harmful into their children or may lead to harmful reactions.
“It’s a control issue and a defense mechanism,” she said. “Some people may feel helpless to deal with the pandemic or believe since it is not affecting them or their family, that it is not real. ‘If I just deny it and I don’t acknowledge facts, I don’t have to worry about it or do anything about it, and therefore I will have more control over my day-to-day life.’”
Groups fueling each other
In some cases, antimask and antivaxx groups are joining forces or adopting dual causes.
In California for instance, longtime opponents to vaccines are now objecting to mask policies as similar infringement to their bodily autonomy. Demonstrations in Texas, Idaho, and Michigan against mask mandates and other COVID-19 requirements have drawn support from anti-vaccine activists and incorporated antivaccine propaganda.
In Illinois, Million Unmasked Patriots, formally the Million Unmasked March, has received widespread attention for protesting both masks for returning schoolchildren and a future COVID-19 vaccine requirement.
A July protest planned by the antimask group triggered a letter by Arora and 500 other healthcare professionals to Illinois lawmakers decrying the group’s views and urging the state to move forward with universal masking in schools.
“What’s happening is those who are distrustful of government and public health and science are joining together,” said Arora, who coauthored a piece about the problem on KevinMD.com. “It’s important to address both movements together because they can quickly feed off each other and build in momentum. At the heart of both is really this deep skepticism of science.”
Rebresh of Million Unmasked Patriots said most of his members are not opposed to all vaccines, but rather they are opposed to “untested vaccines.” The primary concern is the inability to research long-term effects of a COVID-19 vaccine before its approval, he said.
Rebresh disagrees with the antimask movement being compared with the antivaccine movement. The two groups are “motivated by different things and a different set of circumstances drive their opinions,” he said. However, Rebresh believes that potential harm resulting from “mass vaccinations” is a valid concern. For this reason, he and his wife chose for their children to receive their vaccinations individually over a series of weeks, rather than the “kiddie cocktail of vaccines,” at a single visit, he said.
Vaccine scientist Peter Hotez, MD, PhD, said the antivaccine movement appears to have grown stronger from the pandemic fueled by fresh conspiracies and new alliances. Antivaccine sentiment has been gaining steam over the last several years and collecting more allies from the far-right, said Hotez, dean for the National School of Tropical Medicine and codirector for the Texas Children’s Hospital Center for Vaccine Development.
“Now what you’re seeing is yet another expansion this year, with antivaccine groups, under the banner of ‘health freedom,’ campaigning against social distancing and wearing masks and contact tracing,” he said. “What was an antivaccine movement has now become a full-blown antiscience movement and an anti-public health movement. It’s causing a lot of damage and I believe costing a lot of American lives.”
Neil F. Johnson, PhD, who has studied the antivaccine movement and its social media proliferation during the pandemic, said online comments by antivaxxers frequently condemn mask usage and showcase memes making fun of masks.
“In those same narratives about opposing vaccines for COVID, we see a lot of discussion against masks,” said Johnson, a physics professor at George Washington University in Washington, D.C. “If you don’t believe in the official picture of COVID, you don’t believe the policies or the advice that’s given about COVID.”
An analysis by Johnson that examined 1,300 Facebook pages found that, while antivaxxers have fewer followers than provaccine pages, antivaccine pages are more numerous, faster growing, and are more often connected to unrelated, undecided pages. Conversely, pages that advocate the benefits of vaccinations and explain the science behind immunizations are largely disconnected from such undecided communities, according to the study, published May 13 in Nature.
The study suggests the antivaccine movement is making influential strides during the pandemic and connecting with people who are undecided, while public health advocates are not building the same bridges, Johnson said.
“I think it’s hugely dangerous, because I don’t know any other moment in science or in public health when there was so much uncertainty in something affecting everybody,” he said. “Every policy that will be coming, everything depends on people buying into the official message. Once you have the seeds of doubt, that’s a very difficult thing to overcome. It’s an unprecedented challenge.”
How physicians and clinicians can help
A more aggressive approach is necessary when it comes to taking down antiscience content on social media, says Hotez. Too often, misinformation and antiscience rhetoric is allowed to linger on popular sites such as Facebook and Amazon.
Wolynn agrees. On personal or business platforms, it’s crucial to ban, hide, and delete such comments as quickly as possible, he said. On public sites, purposeful disinformation should be immediately reported to the platform.
At the same time, Wolynn said it’s essential to support those who make sound, science-based comments in social media forums.
“If you see someone who is pushing accurate, evidence-based information, and they come under attack, they should be supported and defended and empowered,” Wolynn said. “Shots Heard Round the World is doing all of those things, including galvanizing and recruiting more people to help get their voices out there.”
Expanded visibility by physicians and scientists would greatly help counter the spread of antiscience sentiment, adds Hotez.
“Too often, antiscience movements are able to flourish because scientists and physicians are invisible,” he said. “They’re too focused on either clinical practices or in the case of physician scientists, on grants and papers and not enough attention to public engagement. We’re going to have to change that around. We need to hear more from scientists directly.”
To that end, Wolynn said health care professionals, including medical students and residents, need to have formal training in communications, media, and social media as part of their education – and more support from employers to engage through social media.
“That’s where the fight is,” Wolynn said. “You can be the best diagnostician, the best clinician. You can make the right diagnosis and prescribe the right medication, but if families don’t hear what you’re saying, you’re not going to be effective. If you can’t be on the platform where they’re being influenced, we’re losing the battle.”
Speaking to your mask-hesitant patients
Concentrating on those who are uncertain about masks is particularly key for physicians and public health advocates as the pandemic continues, says Arora.
“It’s important for us to focus on the mask-hesitant who often don’t get the attention they need,” she said.
She suggests bringing up the subject of masks with patients during visits, asking about mask usage, discussing rumors they’ve heard, and emphasizing why masks are important. Be a role model by wearing a mask in your community and on social media, she added.
Some patients have real concerns about not being able to breathe through masks or anxiety disorders that can be aggravated even by the thought of wearing a mask, noted Susan R. Bailey, MD, president for the American Medical Association. Bailey, an immunologist, recently counseled a patient with a deviated nasal septum in addition to a panic disorder who was worried about wearing a mask, she said. Bailey listened to the patient’s concerns, discussed his health conditions, and proposed an alternative face covering that might make him more comfortable.
“Every patient is different,” Bailey said. “It’s important for us to remember that each person who is reluctant to wear a mask has their own reasons. It’s important for us to express some empathy – to agree with them, yes, masks are hot and inconvenient – and help understand their questions, which you may be able to answer to their satisfaction. There are patients that have legitimate questions and a physician caring about how they feel, can make all the difference.”
Physicians can also get involved with the AMA’s #MaskUp campaign, an effort to normalize mask wearing and debunk myths associated with masks. The campaign includes social media materials, slogans doctors can tweet, and profile pictures they can use on social media. The campaign’s toolkit includes images, videos, and information that physicians can share with patients and the public.
Enforcing strong mask policies at your practice and ensuring all staff are modeling appropriate mask behavior is also important, adds Fincher of the ACP. The college recently issued a policy supporting mask usage in community settings.
If a patient conveys an antimask belief, Fincher suggests not directly challenging the person’s views, but listening to them and offering objective data, discussing the science behind masks, and directing them to credible sources.
“Doctors are used to this. We recommend a lot of things to patients that they don’t want to do,” Fincher said. “If a patient feels attacked, they act defensively. But if you base your explanation in more objective terms with data, numbers, and personalize the risks and benefits of a vaccine, a healthy change in behavior, or a medication, then patients are more likely to hear your concerns and do the right thing. Having a long-term relationship with a trusted physician makes all of these issues much easier to discuss and to implement the best plan for the individual patient.”
This article first appeared on Medscape.com.
Niket Sonpal, MD, thought he’d heard most of the myths about wearing masks during the pandemic, but the recent claim from a patient was a new one for the New York City gastroenterologist.
The patient refused to wear a mask because she heard inhaling bad breath through a mask could be toxic. The woman said the rumor was circulating on Facebook. Sonpal calmly explained that breathing your own breath is not going to cause health problems, he said.
“There’s a lot of controversy on masks,” he said. “Unfortunately, it’s really just a lack of education and buy-in. Social media is the primary source of all this misinformation. These kinds of over-the-top hyperbole has basically led to a disbelief that masks are effective. The disbelief is hard to break up.”
As mask requirements have tightened amid the ongoing pandemic, debates about face coverings have emerged front and center, with a growing number of people opposing mask usage. So-called antimaskers dispute the benefits of wearing masks and many contend that face coverings decrease oxygen flow and can lead to illness. Sentiment against masks have led to protests nationwide, ignited public conflicts in some areas, and even generated lawsuits over mask mandates.
The issue presents an ongoing challenge for physicians as they strive to educate patients about the significance of masking against the flood of antimask messages on social media and beyond. Opposition to masks is particularly frustrating for health professionals who have witnessed patients, family, or friends become ill or die from the virus. Refusing to mask and failing to social distance have been linked to the rapid spread of the coronavirus and subsequent deaths.
“I have had colleagues pass away, and it’s extremely disheartening and frustrating to see science so easily disregarded,” Sonpal said. “Masks save lives and protect people and not wearing them is simply a lack of respect, not just for your fellow colleagues, but for a member of your species.”
Michael Rebresh, who helped create the antimask group Million Unmasked Patriots, says his group’s objections to masks are rational and reasonable. The group, which has more than 8,000 members, formed in response to guidance by Illinois state officials that children would only be allowed to return to school wearing a mask.
“Our objections are to the fact that masks on children in school have a greater propensity to make children sick from breathing in bacteria that forms on the inner layer of a mask worn for hours on end,” Rebresh said. “We have an objection to the increase of CO2 intake and a decrease in oxygen flow for kids who need all the oxygen they can get during a learning environment. We recognized the masking of ourselves and kids for what it is: A political move to separate the two parties in our November election and define and create division between the two.”
Million Unmasked Patriots is one of dozens of antimask groups on social media platforms such as Facebook, Instagram, and TikTok. In July, Facebook suspended one such group, Unmasking America, which boasts 9,600 members, for posting repeated claims that face masks obstruct oxygen flow and have negative mental health effects.
Experts say the antiscience rhetoric is far from new. The antimask movement in many ways, shares similarities with that of the anti-vaccine movement, says Todd Wolynn, MD, a Pittsburgh pediatrician and cofounder of Shots Heard Round the World, an organization that defends vaccine advocates against coordinated online attacks by antivaxxers.
“A lot of it is conspiracy-laden,” said Wolynn of the disinformation. “That Dr. [Anthony] Fauci somehow helped construct the pandemic and that it’s not real. That Bill Gates is funding the vaccine so he can inject people with microchips. All sorts of really out-there, ungrounded conspiracy theories. If you had Venn diagram of antimask and antivaxx, I would say there’s clearly overlap.”
Parallels between antimaskers, antivaxxers
Opponents to masks fall on a spectrum, explains Vineet Arora, MD, a hospitalist and associate chief medical officer–clinical learning environment at University of Chicago Medicine. People who believe conspiracy theories and push misinformation are on one end, she said. There are also those who generally don’t believe the seriousness of the pandemic, feel their risk is minimal, or doubt the benefits of masks.
The two trains of thought resemble the distinction among parents who are antivaccine and those who are simply “vaccine hesitant,” says Arora, who co-authored a recent article about masking and misinformation that addresses antivaccine attitudes.
“While the antimask sentiment gets a lot of attention, I think it’s important to highlight there’s a lot of vocal anti-mask sentiment since most people are supportive of masks,” she said. “There might be people sitting on the fence who are just unsure about wearing a mask. That’s understandable because the science and the communication has evolved. There was a lot of early mixed messages about masking. Anytime you have confusion about the science or the science is evolving, it’s easy to have misinformation and then have that take off as myth.”
Just as antivaxxers work to swing the opinion of the vaccine hesitant, antimaskers are vying with public health advocates for the support of the mask hesitant, she said. Creating doubt in public health authorities is one way they are gaining followers. Anti-maskers often question and scrutinize past messaging about masks by public health officials, claiming that because guidance on masks has changed over time, the science behind masks and current guidance can’t be trusted, Wolynn said. Similarly, antivaxxers frequently question past actions by public health officials, such as the Tuskegee Experiment (which began in 1932), to try to poke holes in the credibility of public health officials and their advice.
Both the antimask and antivaccine movements also tend to base their resistance on a personal liberties argument, adds Jacqueline Winfield Fincher, MD, president for the American College of Physicians and an internist based in Thomson, Georgia. Antimaskers contend they should be free to decide whether to wear face coverings and that rules requiring masks infringe upon their civil liberties. Similarly, antivaxxers argue they should be free to decide whether to vaccinate their children and contend vaccine mandates violate their personal liberties.
Taking a deeper look, fear and control are two likely drivers of antimasking and antivaccine attitudes, Fincher said. Those refusing to wear masks may feel they have no control over the pandemic or its impacts, but they can control how they respond to mask-wearing requirements, she said.
Antivaccine parents often want more control over their children’s healthcare and falsely believe that vaccines are injecting something harmful into their children or may lead to harmful reactions.
“It’s a control issue and a defense mechanism,” she said. “Some people may feel helpless to deal with the pandemic or believe since it is not affecting them or their family, that it is not real. ‘If I just deny it and I don’t acknowledge facts, I don’t have to worry about it or do anything about it, and therefore I will have more control over my day-to-day life.’”
Groups fueling each other
In some cases, antimask and antivaxx groups are joining forces or adopting dual causes.
In California for instance, longtime opponents to vaccines are now objecting to mask policies as similar infringement to their bodily autonomy. Demonstrations in Texas, Idaho, and Michigan against mask mandates and other COVID-19 requirements have drawn support from anti-vaccine activists and incorporated antivaccine propaganda.
In Illinois, Million Unmasked Patriots, formally the Million Unmasked March, has received widespread attention for protesting both masks for returning schoolchildren and a future COVID-19 vaccine requirement.
A July protest planned by the antimask group triggered a letter by Arora and 500 other healthcare professionals to Illinois lawmakers decrying the group’s views and urging the state to move forward with universal masking in schools.
“What’s happening is those who are distrustful of government and public health and science are joining together,” said Arora, who coauthored a piece about the problem on KevinMD.com. “It’s important to address both movements together because they can quickly feed off each other and build in momentum. At the heart of both is really this deep skepticism of science.”
Rebresh of Million Unmasked Patriots said most of his members are not opposed to all vaccines, but rather they are opposed to “untested vaccines.” The primary concern is the inability to research long-term effects of a COVID-19 vaccine before its approval, he said.
Rebresh disagrees with the antimask movement being compared with the antivaccine movement. The two groups are “motivated by different things and a different set of circumstances drive their opinions,” he said. However, Rebresh believes that potential harm resulting from “mass vaccinations” is a valid concern. For this reason, he and his wife chose for their children to receive their vaccinations individually over a series of weeks, rather than the “kiddie cocktail of vaccines,” at a single visit, he said.
Vaccine scientist Peter Hotez, MD, PhD, said the antivaccine movement appears to have grown stronger from the pandemic fueled by fresh conspiracies and new alliances. Antivaccine sentiment has been gaining steam over the last several years and collecting more allies from the far-right, said Hotez, dean for the National School of Tropical Medicine and codirector for the Texas Children’s Hospital Center for Vaccine Development.
“Now what you’re seeing is yet another expansion this year, with antivaccine groups, under the banner of ‘health freedom,’ campaigning against social distancing and wearing masks and contact tracing,” he said. “What was an antivaccine movement has now become a full-blown antiscience movement and an anti-public health movement. It’s causing a lot of damage and I believe costing a lot of American lives.”
Neil F. Johnson, PhD, who has studied the antivaccine movement and its social media proliferation during the pandemic, said online comments by antivaxxers frequently condemn mask usage and showcase memes making fun of masks.
“In those same narratives about opposing vaccines for COVID, we see a lot of discussion against masks,” said Johnson, a physics professor at George Washington University in Washington, D.C. “If you don’t believe in the official picture of COVID, you don’t believe the policies or the advice that’s given about COVID.”
An analysis by Johnson that examined 1,300 Facebook pages found that, while antivaxxers have fewer followers than provaccine pages, antivaccine pages are more numerous, faster growing, and are more often connected to unrelated, undecided pages. Conversely, pages that advocate the benefits of vaccinations and explain the science behind immunizations are largely disconnected from such undecided communities, according to the study, published May 13 in Nature.
The study suggests the antivaccine movement is making influential strides during the pandemic and connecting with people who are undecided, while public health advocates are not building the same bridges, Johnson said.
“I think it’s hugely dangerous, because I don’t know any other moment in science or in public health when there was so much uncertainty in something affecting everybody,” he said. “Every policy that will be coming, everything depends on people buying into the official message. Once you have the seeds of doubt, that’s a very difficult thing to overcome. It’s an unprecedented challenge.”
How physicians and clinicians can help
A more aggressive approach is necessary when it comes to taking down antiscience content on social media, says Hotez. Too often, misinformation and antiscience rhetoric is allowed to linger on popular sites such as Facebook and Amazon.
Wolynn agrees. On personal or business platforms, it’s crucial to ban, hide, and delete such comments as quickly as possible, he said. On public sites, purposeful disinformation should be immediately reported to the platform.
At the same time, Wolynn said it’s essential to support those who make sound, science-based comments in social media forums.
“If you see someone who is pushing accurate, evidence-based information, and they come under attack, they should be supported and defended and empowered,” Wolynn said. “Shots Heard Round the World is doing all of those things, including galvanizing and recruiting more people to help get their voices out there.”
Expanded visibility by physicians and scientists would greatly help counter the spread of antiscience sentiment, adds Hotez.
“Too often, antiscience movements are able to flourish because scientists and physicians are invisible,” he said. “They’re too focused on either clinical practices or in the case of physician scientists, on grants and papers and not enough attention to public engagement. We’re going to have to change that around. We need to hear more from scientists directly.”
To that end, Wolynn said health care professionals, including medical students and residents, need to have formal training in communications, media, and social media as part of their education – and more support from employers to engage through social media.
“That’s where the fight is,” Wolynn said. “You can be the best diagnostician, the best clinician. You can make the right diagnosis and prescribe the right medication, but if families don’t hear what you’re saying, you’re not going to be effective. If you can’t be on the platform where they’re being influenced, we’re losing the battle.”
Speaking to your mask-hesitant patients
Concentrating on those who are uncertain about masks is particularly key for physicians and public health advocates as the pandemic continues, says Arora.
“It’s important for us to focus on the mask-hesitant who often don’t get the attention they need,” she said.
She suggests bringing up the subject of masks with patients during visits, asking about mask usage, discussing rumors they’ve heard, and emphasizing why masks are important. Be a role model by wearing a mask in your community and on social media, she added.
Some patients have real concerns about not being able to breathe through masks or anxiety disorders that can be aggravated even by the thought of wearing a mask, noted Susan R. Bailey, MD, president for the American Medical Association. Bailey, an immunologist, recently counseled a patient with a deviated nasal septum in addition to a panic disorder who was worried about wearing a mask, she said. Bailey listened to the patient’s concerns, discussed his health conditions, and proposed an alternative face covering that might make him more comfortable.
“Every patient is different,” Bailey said. “It’s important for us to remember that each person who is reluctant to wear a mask has their own reasons. It’s important for us to express some empathy – to agree with them, yes, masks are hot and inconvenient – and help understand their questions, which you may be able to answer to their satisfaction. There are patients that have legitimate questions and a physician caring about how they feel, can make all the difference.”
Physicians can also get involved with the AMA’s #MaskUp campaign, an effort to normalize mask wearing and debunk myths associated with masks. The campaign includes social media materials, slogans doctors can tweet, and profile pictures they can use on social media. The campaign’s toolkit includes images, videos, and information that physicians can share with patients and the public.
Enforcing strong mask policies at your practice and ensuring all staff are modeling appropriate mask behavior is also important, adds Fincher of the ACP. The college recently issued a policy supporting mask usage in community settings.
If a patient conveys an antimask belief, Fincher suggests not directly challenging the person’s views, but listening to them and offering objective data, discussing the science behind masks, and directing them to credible sources.
“Doctors are used to this. We recommend a lot of things to patients that they don’t want to do,” Fincher said. “If a patient feels attacked, they act defensively. But if you base your explanation in more objective terms with data, numbers, and personalize the risks and benefits of a vaccine, a healthy change in behavior, or a medication, then patients are more likely to hear your concerns and do the right thing. Having a long-term relationship with a trusted physician makes all of these issues much easier to discuss and to implement the best plan for the individual patient.”
This article first appeared on Medscape.com.
U.S. tops 500,000 COVID-19 cases in children
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
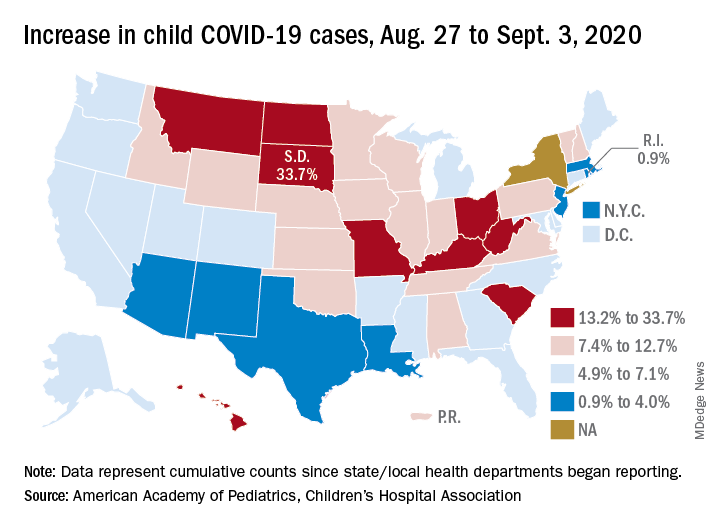
States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
Deaths sky high in hospitalized COVID patients with kidney injury
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
Could these old drugs help fight COVID-19 and save lives?
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Unexpected results in new COVID-19 ‘cytokine storm’ data
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
HHS plan to improve rural health focuses on better broadband, telehealth services
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Even before the coronavirus pandemic reached into the nation’s less-populated regions, rural Americans were sicker, poorer, and older than the rest of the country. Hospitals are shuttering at record rates, and health care experts have long called for changes.
The new plan, released by the Department of Health & Human Services Secretary Alex M. Azar, II, acknowledges the gaps in health care and other problems facing rural America. It lists a litany of projects and directives, with many already underway or announced within federal agencies.
“We cannot just tinker around the edges of a rural healthcare system that has struggled for too long,” Azar said in a prepared statement.
Yet, that is exactly what experts say the administration continues to do.
“They tinker around the edges,” said Tommy Barnhart, former president of the National Rural Health Association. And he added, “there’s a lot of political hype” that has happened under President Trump, as well as previous presidents.
In the past few months, rural health care has increasingly become a focus for Mr. Trump, whose polling numbers are souring as COVID-19 kills hundreds of Americans every day, drives down restaurant demand for some farm products, and spreads through meatpacking plants. Rural states including Iowa and the Dakotas are reporting the latest surges in cases.
This announcement comes in response to Mr. Trump’s executive order last month calling for improved rural health and telehealth access. Earlier this week, three federal agencies also announced they would team up to address gaps in rural broadband service – a key need because large portions of the plan seek to expand telehealth.
The plan is more than 70 pages long and the word “telehealth” appears more than 90 times, with a focus on projects across HHS, including the Health Resources and Services Administration and the Centers for Medicare & Medicaid Services.
Mr. Barnhart said CMS has passed some public health emergency waivers since the beginning of the pandemic that helped rural facilities get more funding, including one that specifically was designed to provide additional money for telehealth services. However, those waivers are set to expire when the coronavirus emergency ends. Officials have not yet set a date for when the federal emergency will end.
Andrew Jay Schwartzman, senior counselor to the Benton Institute for Broadband & Society, a private foundation that works to ensure greater Internet access, said there are multiple challenges with implementing telehealth across the nation. Many initiatives for robust telehealth programs need fast bandwidth, yet getting the money and setting up the necessary infrastructure is very difficult, he said.
“It will be a long time before this kind of technology will be readily available to much of the country,” he said.
Ge Bai, associate professor of accounting and health policy at Johns Hopkins University in Baltimore, noted that telehealth was short on funding in the HHS initiative. However, she said, the focus on telehealth, as well as a proposed shift in payment for small rural hospitals and changing workforce licensing requirements, had good potential.
“We are so close to the election that this is probably more of a messaging issue to cater to rural residents,” Ms. Bai said. “But it doesn’t matter who will be president. This report will give the next administration useful guidance.”
The American Hospital Association, representing 5,000 hospitals nationwide, sent a letter to Mr. Trump last week recommending a host of steps the administration could take. As of late Thursday, AHA was still reviewing the HHS plan but said it was “encouraged by the increased attention on rural health care.”
Buried within the HHS announcement are technical initiatives, such as a contract to help clinics and hospitals integrate care, and detailed efforts to address gaps in care, including a proposal to increase funding for school-based mental health programs in the president’s 2021 budget.
A senior HHS official said that, while some actions have been taken in recent months to improve rural health — such as the $11 billion provided to rural hospitals through coronavirus relief funding — more is needed.
“We’re putting our stake in the ground that the time for talk is over,” he said. “We’re going to move forward.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Study: 10% of pregnant women test positive for COVID-19, with most asymptomatic
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
FROM BMJ