User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Upfront asymptomatic primary tumor resection: No benefit in advanced CRC
Upfront resection of an asymptomatic primary tumor does not improve survival over chemotherapy alone in stage IV colorectal cancer with unresectable synchronous metastases, according to a randomized trial in Japan with 165 patients.
Median overall survival was slightly more than 2 years with or without primary resection, plus upfront surgery delayed systemic treatment and had a 4% risk of fatal complications. The trial was terminated early because of futility.
“PTR [primary tumor resection] should no longer be considered a standard of care for patients with CRC with asymptomatic primary tumors and synchronous unresectable metastases,” said investigators led by Yukihide Kanemitsu, MD, a colorectal surgeon at National Cancer Center Hospital in Tokyo.
“This paper is important for establishing solid evidence-based decisions. Why perform an invasive procedure on a patient that introduces additional risks if it won’t change their disease course?” said colorectal surgeon Deborah Keller, MD, clinical assistant professor of surgery at the University of California at Davis Medical Center, when asked for comment.
She explained that, in general, when the primary tumor is not obstructing, the standard of care is upfront chemotherapy, as supported by National Comprehensive Cancer Network guidelines.
“However, there was a change in practice over the last few years” after several retrospective studies reported better overall survival with surgery before chemotherapy, but “the studies were not the highest quality of evidence,” she said.
To bring better data to bear, the Japanese team randomized 84 patients to chemotherapy alone and 81 to PTR followed by chemotherapy. Primary tumors were asymptomatic, and patients had no more than three unresectable metastases in the liver, lungs, distant lymph nodes, or peritoneum. Chemotherapy was either mFOLFOX6 plus bevacizumab or CapeOX plus bevacizumab at investigator discretion.
The trial was halted at interim analysis in September 2019. With a median follow-up of 22 months, median overall survival – the primary endpoint – was 25.9 months in the surgery-first arm and 26.7 months in the chemotherapy-alone group (P = .69). Subgroup analysis found no populations that benefited from PTR. Median progression free survival was 10.4 months with PTR first and 12.1 months with chemotherapy alone (P = .48)
Twenty-seven patients in the PTR arm had grade 3 or worse surgery-related adverse events, including anastomotic leakage in 3 and increased aspartate aminotransferase in 13. Three patients (4%) died within 30 days of surgery. Chemotherapy-related grade 3 or worse adverse events were more frequent and severe in the PTR arm (48% PTR versus 34% chemotherapy alone).
“For those who had been performing resections, this could push the paradigm back to chemotherapy alone,” Dr. Keller said.
Eleven patients (13%) in the chemotherapy-alone arm required surgery for intestinal obstruction or other primary tumor symptoms that developed after randomization, which means that 87% avoided surgery entirely, the investigators noted.
In the PTR group, surgery was performed within 21 days of enrollment, and chemotherapy started a median of 34 days after PTR. Chemotherapy was started within 14 days of enrollment in the chemotherapy-alone arm.
The groups were well balanced, including colon and rectosigmoid tumor locations in 93% of both arms and liver metastases in 73% of both. A bit over half the subjects were men and the median age was 65 years.
The team noted that early termination meant that the planned sample size was not achieved, which in turn limited the statistical power of the conclusions. “It is hoped that the comprehensive results of [ongoing trials] will clearly demonstrate the role of PTR for these patients,” they said.
The study was conducted by the Japan Clinical Oncology Group at 38 cancer centers in Japan. The work was funded by the Ministry of Health of Japan. The investigators had numerous industry ties, including Dr. Kanemitsu, who reported honoraria from Chugai Pharma, Ethicon, Covidien, and Intuitive Surgical, and being a Covidien adviser.
Upfront resection of an asymptomatic primary tumor does not improve survival over chemotherapy alone in stage IV colorectal cancer with unresectable synchronous metastases, according to a randomized trial in Japan with 165 patients.
Median overall survival was slightly more than 2 years with or without primary resection, plus upfront surgery delayed systemic treatment and had a 4% risk of fatal complications. The trial was terminated early because of futility.
“PTR [primary tumor resection] should no longer be considered a standard of care for patients with CRC with asymptomatic primary tumors and synchronous unresectable metastases,” said investigators led by Yukihide Kanemitsu, MD, a colorectal surgeon at National Cancer Center Hospital in Tokyo.
“This paper is important for establishing solid evidence-based decisions. Why perform an invasive procedure on a patient that introduces additional risks if it won’t change their disease course?” said colorectal surgeon Deborah Keller, MD, clinical assistant professor of surgery at the University of California at Davis Medical Center, when asked for comment.
She explained that, in general, when the primary tumor is not obstructing, the standard of care is upfront chemotherapy, as supported by National Comprehensive Cancer Network guidelines.
“However, there was a change in practice over the last few years” after several retrospective studies reported better overall survival with surgery before chemotherapy, but “the studies were not the highest quality of evidence,” she said.
To bring better data to bear, the Japanese team randomized 84 patients to chemotherapy alone and 81 to PTR followed by chemotherapy. Primary tumors were asymptomatic, and patients had no more than three unresectable metastases in the liver, lungs, distant lymph nodes, or peritoneum. Chemotherapy was either mFOLFOX6 plus bevacizumab or CapeOX plus bevacizumab at investigator discretion.
The trial was halted at interim analysis in September 2019. With a median follow-up of 22 months, median overall survival – the primary endpoint – was 25.9 months in the surgery-first arm and 26.7 months in the chemotherapy-alone group (P = .69). Subgroup analysis found no populations that benefited from PTR. Median progression free survival was 10.4 months with PTR first and 12.1 months with chemotherapy alone (P = .48)
Twenty-seven patients in the PTR arm had grade 3 or worse surgery-related adverse events, including anastomotic leakage in 3 and increased aspartate aminotransferase in 13. Three patients (4%) died within 30 days of surgery. Chemotherapy-related grade 3 or worse adverse events were more frequent and severe in the PTR arm (48% PTR versus 34% chemotherapy alone).
“For those who had been performing resections, this could push the paradigm back to chemotherapy alone,” Dr. Keller said.
Eleven patients (13%) in the chemotherapy-alone arm required surgery for intestinal obstruction or other primary tumor symptoms that developed after randomization, which means that 87% avoided surgery entirely, the investigators noted.
In the PTR group, surgery was performed within 21 days of enrollment, and chemotherapy started a median of 34 days after PTR. Chemotherapy was started within 14 days of enrollment in the chemotherapy-alone arm.
The groups were well balanced, including colon and rectosigmoid tumor locations in 93% of both arms and liver metastases in 73% of both. A bit over half the subjects were men and the median age was 65 years.
The team noted that early termination meant that the planned sample size was not achieved, which in turn limited the statistical power of the conclusions. “It is hoped that the comprehensive results of [ongoing trials] will clearly demonstrate the role of PTR for these patients,” they said.
The study was conducted by the Japan Clinical Oncology Group at 38 cancer centers in Japan. The work was funded by the Ministry of Health of Japan. The investigators had numerous industry ties, including Dr. Kanemitsu, who reported honoraria from Chugai Pharma, Ethicon, Covidien, and Intuitive Surgical, and being a Covidien adviser.
Upfront resection of an asymptomatic primary tumor does not improve survival over chemotherapy alone in stage IV colorectal cancer with unresectable synchronous metastases, according to a randomized trial in Japan with 165 patients.
Median overall survival was slightly more than 2 years with or without primary resection, plus upfront surgery delayed systemic treatment and had a 4% risk of fatal complications. The trial was terminated early because of futility.
“PTR [primary tumor resection] should no longer be considered a standard of care for patients with CRC with asymptomatic primary tumors and synchronous unresectable metastases,” said investigators led by Yukihide Kanemitsu, MD, a colorectal surgeon at National Cancer Center Hospital in Tokyo.
“This paper is important for establishing solid evidence-based decisions. Why perform an invasive procedure on a patient that introduces additional risks if it won’t change their disease course?” said colorectal surgeon Deborah Keller, MD, clinical assistant professor of surgery at the University of California at Davis Medical Center, when asked for comment.
She explained that, in general, when the primary tumor is not obstructing, the standard of care is upfront chemotherapy, as supported by National Comprehensive Cancer Network guidelines.
“However, there was a change in practice over the last few years” after several retrospective studies reported better overall survival with surgery before chemotherapy, but “the studies were not the highest quality of evidence,” she said.
To bring better data to bear, the Japanese team randomized 84 patients to chemotherapy alone and 81 to PTR followed by chemotherapy. Primary tumors were asymptomatic, and patients had no more than three unresectable metastases in the liver, lungs, distant lymph nodes, or peritoneum. Chemotherapy was either mFOLFOX6 plus bevacizumab or CapeOX plus bevacizumab at investigator discretion.
The trial was halted at interim analysis in September 2019. With a median follow-up of 22 months, median overall survival – the primary endpoint – was 25.9 months in the surgery-first arm and 26.7 months in the chemotherapy-alone group (P = .69). Subgroup analysis found no populations that benefited from PTR. Median progression free survival was 10.4 months with PTR first and 12.1 months with chemotherapy alone (P = .48)
Twenty-seven patients in the PTR arm had grade 3 or worse surgery-related adverse events, including anastomotic leakage in 3 and increased aspartate aminotransferase in 13. Three patients (4%) died within 30 days of surgery. Chemotherapy-related grade 3 or worse adverse events were more frequent and severe in the PTR arm (48% PTR versus 34% chemotherapy alone).
“For those who had been performing resections, this could push the paradigm back to chemotherapy alone,” Dr. Keller said.
Eleven patients (13%) in the chemotherapy-alone arm required surgery for intestinal obstruction or other primary tumor symptoms that developed after randomization, which means that 87% avoided surgery entirely, the investigators noted.
In the PTR group, surgery was performed within 21 days of enrollment, and chemotherapy started a median of 34 days after PTR. Chemotherapy was started within 14 days of enrollment in the chemotherapy-alone arm.
The groups were well balanced, including colon and rectosigmoid tumor locations in 93% of both arms and liver metastases in 73% of both. A bit over half the subjects were men and the median age was 65 years.
The team noted that early termination meant that the planned sample size was not achieved, which in turn limited the statistical power of the conclusions. “It is hoped that the comprehensive results of [ongoing trials] will clearly demonstrate the role of PTR for these patients,” they said.
The study was conducted by the Japan Clinical Oncology Group at 38 cancer centers in Japan. The work was funded by the Ministry of Health of Japan. The investigators had numerous industry ties, including Dr. Kanemitsu, who reported honoraria from Chugai Pharma, Ethicon, Covidien, and Intuitive Surgical, and being a Covidien adviser.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
COVID vaccines could lose their punch within a year, experts say
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a survey of 77 epidemiologists from 28 countries by the People’s Vaccine Alliance, 66.2% predicted that the world has a year or less before variants make current vaccines ineffective. The People’s Vaccine Alliance is a coalition of more than 50 organizations, including the African Alliance, Oxfam, Public Citizen, and UNAIDS (the Joint United Nations Programme on HIV/AIDS).
Almost a third (32.5%) of those surveyed said ineffectiveness would happen in 9 months or less; 18.2% said 6 months or less.
Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said in an interview that, while it’s hard to say whether vaccines could become ineffective in that time frame, “It’s perfectly reasonable to think it could happen.”
The good news, said Dr. Offit, who was not involved with the survey, is that SARS-CoV-2 mutates slowly, compared with other viruses such as influenza.
“To date,” he said, “the mutations that have occurred are not far enough away from the immunity induced by your natural infection or immunization such that one isn’t protected at least against severe and critical disease.”
That’s the goal of vaccines, he noted: “to keep people from suffering mightily.”
A line may be crossed
“And so far that’s happening, even with the variants,” Dr. Offit said. “That line has not been crossed. But I think we should assume that it might be.”
Dr. Offit said it will be critical to monitor anyone who gets hospitalized who is known to have been infected or fully vaccinated. Then countries need to get really good at sequencing those viruses.
The great majority of those surveyed (88%) said that persistently low vaccine coverage in many countries would make it more likely that vaccine-resistant mutations will appear.
Coverage comparisons between countries are stark.
Many countries haven’t given a single vaccine dose
While rich countries are giving COVID-19 vaccinations at the rate of a person a second, many of the poorest countries have given hardly any vaccines, the People’s Vaccine Alliance says.
Additionally, according to researchers at the Global Health Innovation Center at Duke University, Durham, N.C., high- and upper-middle–income countries, which represent one-fifth of the world’s population, have bought about 6 billion doses. But low- and lower-middle–income countries, which make up four-fifths of the population, have bought only about 2.6 billion, an article in Nature reports.
“You’re only as strong as your weakest country,” Dr. Offit said. “If we haven’t learned that what happens in other countries can [affect the global population], we haven’t been paying attention.”
Gregg Gonsalves, PhD, associate professor of epidemiology at Yale University, New Haven, Conn., one of the academic centers surveyed, didn’t specify a timeline for when vaccines would become ineffective, but said in a press release that the urgency for widespread global vaccination is real.
“Unless we vaccinate the world,” he said, “we leave the playing field open to more and more mutations, which could churn out variants that could evade our current vaccines and require booster shots to deal with them.”
“Dire, but not surprising”
Panagis Galiatsatos, MD, MHS, a pulmonologist at John Hopkins University, Baltimore, whose research focuses on health care disparities, said the survey findings were “dire, but not surprising.”
Johns Hopkins was another of the centers surveyed, but Dr. Galiatsatos wasn’t personally involved with the survey.
COVID-19, Dr. Galiatsatos pointed out, has laid bare disparities, both in who gets the vaccine and who’s involved in trials to develop the vaccines.
“It’s morally concerning and an ethical reckoning,” he said in an interview.
Recognition of the borderless swath of destruction the virus is exacting is critical, he said.
The United States “has to realize this can’t be a U.S.-centric issue,” he said. “We’re going to be back to the beginning if we don’t make sure that every country is doing well. We haven’t seen that level of uniform approach.”
He noted that scientists have always known that viruses mutate, but now the race is on to find the parts of SARS-CoV-2 that don’t mutate as much.
“My suspicion is we’ll probably need boosters instead of a whole different vaccine,” Dr. Galiatsatos said.
Among the strategies sought by the People’s Vaccine Alliance is for all pharmaceutical companies working on COVID-19 vaccines to openly share technology and intellectual property through the World Health Organization COVID-19 Technology Access Pool, to speed production and rollout of vaccines to all countries.
In the survey, 74% said that open sharing of technology and intellectual property could boost global vaccine coverage; 23% said maybe and 3% said it wouldn’t help.
The survey was carried out between Feb. 17 and March 25, 2021. Respondents included epidemiologists, virologists, and infection disease specialists from the following countries: Algeria, Argentina, Australia, Belgium, Bolivia, Canada, Denmark, Ethiopia, France, Guatemala, India, Italy, Kenya, Lebanon, Norway, Philippines, Senegal, Somalia, South Africa, South Sudan, Spain, United Arab Emirates, Uganda, United Kingdom, United States, Vietnam, Zambia, and Zimbabwe.
Dr. Offit and Dr. Galiatsatos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
National Psoriasis Foundation recommends some stop methotrexate for 2 weeks after J&J vaccine
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
FDA okays transcatheter pulmonary valve for congenital heart disease
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
New analysis eyes the surgical landscape for hidradenitis suppurativa
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
yet these options should be balanced against potentially higher morbidity of extensive procedures.
Those are among the key findings of a systematic review and meta-analysis published online in Dermatologic Surgery.
“There is a major need to better understand the best surgical approaches to HS,” one of the study authors, Christopher Sayed, MD, associate professor of dermatology at the University of North Carolina at Chapel Hill, said in an interview. Previous studies have mostly reviewed outcomes for procedure types in individual cohorts, “but no recent reports have combined and analyzed data from recent studies.”
When Dr. Sayed and colleagues set out to summarize the literature on HS surgery regarding patient characteristics, surgical approaches, and study quality, as well as compare postsurgical recurrence rates, the most recent meta-analysis on postoperative recurrence rates of HS included studies published between 1990 and 2015. “In the past few years, surgical management of HS has become an increasingly popular area of study,” corresponding author Ashley Riddle, MD, MPH, who is currently an internal medicine resident at the Carolinas Medical Center, Charlotte, said in an interview. “We sought to provide an updated picture of the HS surgical landscape by analyzing studies published between 2004 to 2019. We also limited our analysis to studies with follow-up periods of greater than 1 year and included information on disease severity, adverse events, and patient satisfaction when available.”
Of 715 relevant studies identified in the medical literature, the researchers included 59 in the review and 33 in the meta-analysis. Of these 59 studies, 56 were case series, 2 were randomized, controlled trials, and one was a retrospective cohort study.
Of the 50 studies reporting gender and age at time of surgery, 61% of patients were female and their average age was 37 years. Of the 25 studies that reported Hurley scores, 73% had Hurley stage 3 HS. Of the 38 studies reporting the number of procedures per anatomic region, the most commonly operated on regions were the axilla (59%) and the inguinal region (20%).
The researchers found that 22 studies of wide excision had the lowest pooled recurrence rate at 8%, while local excision had the highest pooled recurrence rate at 34%. Meanwhile, among studies of wide/radical excision, flap repair had a pooled recurrence rate of 0%, while delayed primary closure had the highest pooled recurrence rate at 38%.
“Extensive excisions of HS seem to portend a lower risk of postoperative recurrence, but there are many approaches available that may be more appropriate for certain patients,” Dr. Riddle said. “The influence of patient factors such as comorbidities and disease severity on surgical outcomes is unclear and is a potential area of future study.”
Dr. Sayed, an author of the 2019 North American guidelines for the clinical management of HS, pointed out that most studies in the review and meta-analysis included patients who had diabetes, were on biologics or other therapy, were actively smoking, or had other comorbidities that sometimes influence surgeons to delay surgical treatment because they consider it elective. “Most studies indicated minimal or no risk of significant complications relating to these factors, so they should ideally not become obstacles for patients interested in surgical care,” he said.
Dr. Riddle said that she was surprised by how relatively few studies had been published on more conservative surgical approaches such as skin tissue–sparing excision with electrosurgical peeling, deroofing, local excision, and CO2 laser–based evaporation.
The researchers acknowledged certain limitations of their work, including the high risk of bias for most included studies. “Almost all studies were retrospective with substantial methodological limitations, and there were no head-to-head comparisons of different surgical approaches,” Dr. Riddle said. “Patient comorbidities and postoperative complications were variably reported.”
Dr. Sayed disclosed that he is a speaker for AbbVie and Novartis; an investigator for AbbVie, Novartis, InflaRx, and UCB; and on the advisory board of AbbVie and InflaRx. The remaining authors reported having no financial disclosures.
FROM DERMATOLOGIC SURGERY
Candida Esophagitis Associated With Adalimumab for Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.
Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
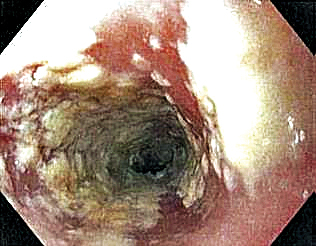
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.

Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease characterized by the development of painful abscesses, fistulous tracts, and scars. It most commonly affects the apocrine gland–bearing areas of the body such as the axillary, inguinal, and anogenital regions. With a prevalence of approximately 1%, HS can lead to notable morbidity.1 The pathogenesis is thought to be due to occlusion of terminal hair follicles that subsequently stimulates release of proinflammatory cytokines from nearby keratinocytes. The mechanism of initial occlusion is not well understood but may be due to friction or trauma. An inflammatory mechanism of disease also has been hypothesized; however, the exact cytokine profile is not known. Treatment of HS consists of several different modalities, including oral retinoids, antibiotics, antiandrogenic therapy, and surgery.1,2 Adalimumab is a well-known biologic that has been approved by the US Food and Drug Administration for the treatment of HS.
Adalimumab is a human monoclonal antibody against tumor necrosis factor (TNF) α and is thought to improve HS by several mechanisms. Inhibition of TNF-α and other proinflammatory cytokines found in inflammatory lesions and apocrine glands directly decreases the severity of lesion size and the frequency of recurrence.3 Adalimumab also is thought to downregulate expression of keratin 6 and prevent the hyperkeratinization seen in HS.4 Additionally, TNF-α inhibition decreases production of IL-1, which has been shown to cause hypercornification of follicles and perpetuate HS pathogenesis.5
A 41-year-old woman with a history of endometriosis, adenomyosis, polycystic ovary syndrome, interstitial cystitis, asthma, fibromyalgia, depression, and Hashimoto thyroiditis presented to our dermatology clinic with active draining lesions and sinus tracts in the perivaginal area that were consistent with HS, which initially was treated with doxycycline 100 mg twice daily. She experienced minimal improvement of the HS lesions at 2-month follow-up.
Due to disease severity, adalimumab was started. The patient received a loading dose of 4 injections totaling 160 mg and 80 mg on day 15, followed by a maintenance dose of 40 mg/0.4 mL weekly. The patient reported substantial improvement of pain, and complete resolution of active lesions was noted on physical examination after 4 weeks of treatment with adalimumab.
Six weeks after adalimumab was started, the patient developed severe dysphagia. She was evaluated by a gastroenterologist and underwent endoscopy (Figure), which led to a diagnosis of esophageal candidiasis. Adalimumab was discontinued immediately thereafter. The patient started treatment with nystatin oral rinse 4 times daily and oral fluconazole 200 mg daily. The candidiasis resolved within 2 weeks; however, she experienced recurrence of HS with draining lesions in the perivaginal area approximately 8 weeks after discontinuation of adalimumab. The patient requested to restart adalimumab treatment despite the recent history of esophagitis. Adalimumab 40 mg/0.4 mL weekly was restarted along with oral fluconazole 200 mg twice weekly and nystatin oral rinse 4 times daily. This regimen resulted in complete resolution of HS symptoms within 6 weeks with no recurrence of esophageal candidiasis during 6 months of follow-up.

Although the side effect of Candida esophagitis associated with adalimumab treatment in our patient may be logical given the medication’s mechanism of action and side-effect profile, this case warrants additional attention. An increase in fungal infections occurs from treatment with adalimumab because TNF-α is involved in many immune regulatory steps that counteract infection. Candida typically activates the innate immune system through macrophages via pathogen-associated molecular pattern stimulation, subsequently stimulating the release of inflammatory cytokines such as TNF-α. The cellular immune system also is activated. Helper T cells (TH1) release TNF-α along with other proinflammatory cytokines to increase phagocytosis in polymorphonuclear cells and macrophages.6 Thus, inhibition of TNF-α compromises innate and cellular immunity, thereby increasing susceptibility to fungal organisms.
A PubMed search of articles indexed for MEDLINE using the terms Candida, candidiasis, esophageal, adalimumab, anti-TNF, and TNF revealed no reports of esophageal candidiasis in patients receiving adalimumab or any of the TNF inhibitors. Candida laryngitis was reported in a patient receiving adalimumab for treatment of rheumatoid arthritis.7 Other studies have demonstrated an incidence of mucocutaneous candidiasis, most notably oropharyngeal and vaginal candidiasis.8-10 One study found that anti-TNF medications were associated with an increased risk for candidiasis by a hazard ratio of 2.7 in patients with Crohn disease.8 Other studies have shown that the highest incidence of fungal infection is seen with the use of infliximab, while adalimumab is associated with lower rates of fungal infection.9,10 Although it is known that anti-TNF therapy predisposes patients to fungal infection, the dose of medication known to preclude the highest risk has not been studied. Furthermore, most studies assess rates of Candida infection in individuals receiving anti-TNF therapy in addition to several other immunosuppressant agents (ie, corticosteroids), which confounds the interpretation of results. Additional studies assessing rates of Candida and other opportunistic infections associated with use of adalimumab alone are needed to better guide clinical practices in dermatology.
Patients receiving adalimumab for dermatologic or other conditions should be closely monitored for opportunistic infections. Although immunomodulatory medications offer promising therapeutic benefits in patients with HS, larger studies regarding treatment with anti-TNF agents in HS are warranted to prevent complications from treatment and promote long-term efficacy and safety.
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
- Kurayev A, Ashkar H, Saraiya A, et al. Hidradenitis suppurativa: review of the pathogenesis and treatment. J Drugs Dermatol. 2016;15:1107-1022.
- Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439-446.
- van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br J Dermatol. 2011;164:1292-1298.
- Shuja F, Chan CS, Rosen T. Biologic drugs for the treatment of hidradenitis suppurativa: an evidence-based review. Dermatol Clin. 2010;28:511-521, 523-514.
- Kutsch CL, Norris DA, Arend WP. Tumor necrosis factor-alpha induces interleukin-1 alpha and interleukin-1 receptor antagonist production by cultured human keratinocytes. J Invest Dermatol. 1993;101:79-85.
- Senet JM. Risk factors and physiopathology of candidiasis. Rev Iberoam Micol. 1997;14:6-13.
- Kobak S, Yilmaz H, Guclu O, et al. Severe candida laryngitis in a patient with rheumatoid arthritis treated with adalimumab. Eur J Rheumatol. 2014;1:167-169.
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2009;104:2524-2533.
- Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181-194.
- Aikawa NE, Rosa DT, Del Negro GM, et al. Systemic and localized infection by Candida species in patients with rheumatic diseases receiving anti-TNF therapy [in Portuguese]. Rev Bras Reumatol. doi:10.1016/j.rbr.2015.03.010
Practice Points
- Adalimumab is an effective treatment for patients with hidradenitis suppurativa.
- There is risk for opportunistic infections with adalimumab, and patients should be monitored closely.
2021 match sets records: Who matched and who didn’t?
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
We’re all vaccinated: Can we go back to the office (unmasked) now?
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Congratulations, you’ve been vaccinated!
It’s been a year like no other, and outpatient psychiatrists turned to Zoom and other telemental health platforms to provide treatment for our patients. Offices sit empty as the dust lands and the plants wilt. Perhaps a few patients are seen in person, masked and carefully distanced, after health screening and temperature checks, with surfaces sanitized between visits, all in accordance with health department regulations. But now the vaccine offers both safety and the promise of a return to a new normal, one that is certain to look different from the normal that was left behind.
I have been vaccinated and many of my patients have also been vaccinated. I began to wonder if it was safe to start seeing patients in person; could I see fully vaccinated patients, unmasked and without temperature checks and sanitizing? I started asking this question in February, and the response I got then was that it was too soon to tell; we did not have any data on whether vaccinated people could transmit the novel coronavirus. Two vaccinated people might be at risk of transmitting the virus and then infecting others, and the question of whether the vaccines would protect against illness caused by variants remained. Preliminary data out of Israel indicated that the vaccine did reduce transmission, but no one was saying that it was fine to see patients without masks, and video-conferencing remained the safest option.
On Monday, March 8, 2021, the Centers for Disease Control and Prevention released long-awaited interim public health guidelines for fully vaccinated people. The guidelines allowed for two vaccinated people to be in a room together unmasked, and for a fully-vaccinated person to be in a room unmasked with an unvaccinated person who did not have risk factors for becoming severely ill with COVID. Was this the green light that psychiatrists were waiting for? Was there new data about transmission, or was this part of the CDC’s effort to make vaccines more desirable?
Michael Chang, MD, is a pediatric infectious disease specialist at the University of Texas Health Science Center at Houston. We spoke 2 days after the CDC interim guidelines were released. Dr. Chang was optimistic.
“, including data about variants and about transmission. At some point, however, the risk is low enough, and we should probably start thinking about going back to in-person visits,” Dr. Chang said. He said he personally would feel safe meeting unmasked with a vaccinated patient, but noted that his institution still requires doctors to wear masks. “Most vaccinations reduce transmission of illness,” Dr. Chang said, “but SARS-CoV-2 continues to surprise us in many ways.”
Katelyn Jetelina, PhD, MPH, an epidemiologist at the University of Texas School of Public Health in Dallas, distributes a newsletter, “Your Local Epidemiologist,” where she discusses data pertaining to the pandemic. In her newsletter dated March 14, 2021, Dr. Jetelina wrote, “There are now 7 sub-studies/press releases that confirm a 50-95% reduced transmission after vaccination. This is a big range, which is typical for such drastically different scientific studies. Variability is likely due to different sample sizes, locations, vaccines, genetics, cultures, etc. It will be a while until we know the ‘true’ percentage for each vaccine.”
Leslie Walker, MD, is a fully vaccinated psychiatrist in private practice in Shaker Heights, Ohio. She has recently started seeing fully vaccinated patients in person.
“So far it’s only 1 or 2 patients a day. I’m leaving it up to the patient. If they prefer masks, we stay masked. I may reverse course, depending on what information comes out.” She went on to note, “There are benefits to being able to see someone’s full facial expressions and whether they match someone’s words and body language, so the benefit of “unmasking” extends beyond comfort and convenience and must be balanced against the theoretical risk of COVID exposure in the room.”
While the CDC has now said it is safe to meet, the state health departments also have guidelines for medical practices, and everyone is still worried about vulnerable people in their households and potential spread to the community at large.
In Maryland, where I work, Aliya Jones, MD, MBA, is the head of the Behavioral Health Administration (BHA) for the Maryland Department of Health. “It remains risky to not wear masks, however, the risk is low when both individuals are vaccinated,” Dr. Jones wrote. “BHA is not recommending that providers see clients without both parties wearing a mask. All of our general practice recommendations for infection control are unchanged. People should be screened before entering clinical practices and persons who are symptomatic, whether vaccinated or not, should not be seen face-to-face, except in cases of an emergency, in which case additional precautions should be taken.”
So is it safe for a fully-vaccinated psychiatrist to have a session with a fully-vaccinated patient sitting 8 feet apart without masks? I’m left with the idea that it is for those two people, but when it comes to unvaccinated people in their households, we want more certainty than we currently have. The messaging remains unclear. The CDC’s interim guidelines offer hope for a future, but the science is still catching up, and to feel safe enough, we may want to wait a little longer for more definitive data – or herd immunity – before we reveal our smiles.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
BASILICA technique prevents TAVR-related coronary obstruction in registry study
For patients undergoing transcatheter aortic valve replacement (TAVR), the intentional laceration technique of diseased valve leaflets called BASILICA is effective and reasonably safe for preventing coronary artery obstruction, according to a late-breaking study presented at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
In a series of 214 patients entered into a registry over a recent 30-month period, leaflets posing risk were effectively traversed with the technique in 95% of cases, and complication rates were reasonably low with 30-day stroke and death rate of 3.4%, reported Jaffar M. Khan, BMBCH, PhD, cardiovascular branch of the National Heart, Lung, and Blood Institute.
The rate of complications is acceptable given the large potential risk, according to Dr. Khan. If coronary obstruction occurs, reported mortality rates have been as high as 50%. The 1-year survival rate in the registry following BASILICA was 84%.
Results should ‘push people toward BASILICA’
The acronym BASILICA stands for bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction. In the procedure, performed immediately before TAVR, guidewires are introduced to first traverse and then lacerate aortic leaflets threatening obstruction of a coronary artery.
In cases where diseased valve leaflets pose a risk of coronary obstruction, most interventionalists “are comfortable with surgery when patients are at low or intermediate risk, but the choices for high-risk patients are a snorkel stent or BASILICA. Given the limits of snorkel stenting, these data should be reassuring and push people toward BASILICA,” Dr. Khan said.
The 214 patients were entered into the registry from June 2015 to December 2020. The mean age was 74.9 years. Of valves treated, 73% were failed bioprosthetic devices. The remaining were native aortic valves. Solo BASILICA was performed in most patients, but 21.5% underwent a doppio procedure, meaning the laceration of two leaflets.
Despite BASILICA, 10 patients (4.7%) had some degree of coronary obstruction, including 5 with partial obstruction of the main coronary artery and 1 with partial obstruction of the right coronary artery. All of these partial obstructions were successfully treated with orthotopic stents.
An obstruction of the right coronary artery was successfully treated with balloon angioplasty. Another patient with significant left main coronary artery obstruction required cardiopulmonary bypass but was successfully treated with snorkel stenting. Of two patients with complete obstruction of the left main coronary artery caused by the skirt of the TAVR device, one died in hospital despite several maneuvers to restore perfusion.
Procedural complications included a mitral chord laceration, which subsequently led to valve replacement, and three guidewire transversals into surrounding tissue that did not result in serious sequelae. Hypotension requiring pressors occurred in 8.5%.
There was a “slight trend” for worse outcomes in those undergoing doppio rather than solo BASILICA, but the difference did not reach statistical significance. Cerebral embolic protection was offered to a minority of patients in this series. The trend for a lower risk of stroke in this group did not reach significance, Dr. Khan reported.
Best for high-volume centers, for now
Although these data support the conclusion that BASILICA “is feasible in a real-world setting,” Dr. Khan acknowledged that BASILICA might not be appropriate at low-volume centers. Dr. Khan cited data that indicates obstruction of a coronary artery by a diseased leaflet occurs in less than 1% of TAVR cases.
“Not every site doing a handful of TAVRs is going to want to tackle these cases, but those working in a high-volume center will from time to time encounter patients with coronary obstruction or who are at increased risk,” Dr. Khan said.
In North America, there has been a proctoring program to disseminate the skills required to perform BASILICA, according to Dr. Khan, who explained that proctors typically participate in two or three cases before these are performed without supervision.
So far, the uptake of BASILICA has been limited.
“BASILICA has not been catching on in EUROPE,” said Didier F. Loulmet, MD, chief of cardiac surgery at Tisch Hospital, New York University Langone Health. There might be several reasons, but Dr. Loulmet said that lack of a comparable proctoring program is one factor.”
“This is a relatively complex procedure performed in a small number of patients, so building up expertise is quite a challenge, particularly in small centers,” he added. He encouraged proctoring as “the way that it has to be propagated.”
The results presented by Dr. Khan on March 6 at CRT 2021 were simultaneously published in JACC: Cardiovascular Interventions.
Dr. Khan has patents on several devices, including catheters to lacerate valve leaflet. Dr. Loulmet reported no potential conflicts of interest.
For patients undergoing transcatheter aortic valve replacement (TAVR), the intentional laceration technique of diseased valve leaflets called BASILICA is effective and reasonably safe for preventing coronary artery obstruction, according to a late-breaking study presented at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
In a series of 214 patients entered into a registry over a recent 30-month period, leaflets posing risk were effectively traversed with the technique in 95% of cases, and complication rates were reasonably low with 30-day stroke and death rate of 3.4%, reported Jaffar M. Khan, BMBCH, PhD, cardiovascular branch of the National Heart, Lung, and Blood Institute.
The rate of complications is acceptable given the large potential risk, according to Dr. Khan. If coronary obstruction occurs, reported mortality rates have been as high as 50%. The 1-year survival rate in the registry following BASILICA was 84%.
Results should ‘push people toward BASILICA’
The acronym BASILICA stands for bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction. In the procedure, performed immediately before TAVR, guidewires are introduced to first traverse and then lacerate aortic leaflets threatening obstruction of a coronary artery.
In cases where diseased valve leaflets pose a risk of coronary obstruction, most interventionalists “are comfortable with surgery when patients are at low or intermediate risk, but the choices for high-risk patients are a snorkel stent or BASILICA. Given the limits of snorkel stenting, these data should be reassuring and push people toward BASILICA,” Dr. Khan said.
The 214 patients were entered into the registry from June 2015 to December 2020. The mean age was 74.9 years. Of valves treated, 73% were failed bioprosthetic devices. The remaining were native aortic valves. Solo BASILICA was performed in most patients, but 21.5% underwent a doppio procedure, meaning the laceration of two leaflets.
Despite BASILICA, 10 patients (4.7%) had some degree of coronary obstruction, including 5 with partial obstruction of the main coronary artery and 1 with partial obstruction of the right coronary artery. All of these partial obstructions were successfully treated with orthotopic stents.
An obstruction of the right coronary artery was successfully treated with balloon angioplasty. Another patient with significant left main coronary artery obstruction required cardiopulmonary bypass but was successfully treated with snorkel stenting. Of two patients with complete obstruction of the left main coronary artery caused by the skirt of the TAVR device, one died in hospital despite several maneuvers to restore perfusion.
Procedural complications included a mitral chord laceration, which subsequently led to valve replacement, and three guidewire transversals into surrounding tissue that did not result in serious sequelae. Hypotension requiring pressors occurred in 8.5%.
There was a “slight trend” for worse outcomes in those undergoing doppio rather than solo BASILICA, but the difference did not reach statistical significance. Cerebral embolic protection was offered to a minority of patients in this series. The trend for a lower risk of stroke in this group did not reach significance, Dr. Khan reported.
Best for high-volume centers, for now
Although these data support the conclusion that BASILICA “is feasible in a real-world setting,” Dr. Khan acknowledged that BASILICA might not be appropriate at low-volume centers. Dr. Khan cited data that indicates obstruction of a coronary artery by a diseased leaflet occurs in less than 1% of TAVR cases.
“Not every site doing a handful of TAVRs is going to want to tackle these cases, but those working in a high-volume center will from time to time encounter patients with coronary obstruction or who are at increased risk,” Dr. Khan said.
In North America, there has been a proctoring program to disseminate the skills required to perform BASILICA, according to Dr. Khan, who explained that proctors typically participate in two or three cases before these are performed without supervision.
So far, the uptake of BASILICA has been limited.
“BASILICA has not been catching on in EUROPE,” said Didier F. Loulmet, MD, chief of cardiac surgery at Tisch Hospital, New York University Langone Health. There might be several reasons, but Dr. Loulmet said that lack of a comparable proctoring program is one factor.”
“This is a relatively complex procedure performed in a small number of patients, so building up expertise is quite a challenge, particularly in small centers,” he added. He encouraged proctoring as “the way that it has to be propagated.”
The results presented by Dr. Khan on March 6 at CRT 2021 were simultaneously published in JACC: Cardiovascular Interventions.
Dr. Khan has patents on several devices, including catheters to lacerate valve leaflet. Dr. Loulmet reported no potential conflicts of interest.
For patients undergoing transcatheter aortic valve replacement (TAVR), the intentional laceration technique of diseased valve leaflets called BASILICA is effective and reasonably safe for preventing coronary artery obstruction, according to a late-breaking study presented at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
In a series of 214 patients entered into a registry over a recent 30-month period, leaflets posing risk were effectively traversed with the technique in 95% of cases, and complication rates were reasonably low with 30-day stroke and death rate of 3.4%, reported Jaffar M. Khan, BMBCH, PhD, cardiovascular branch of the National Heart, Lung, and Blood Institute.
The rate of complications is acceptable given the large potential risk, according to Dr. Khan. If coronary obstruction occurs, reported mortality rates have been as high as 50%. The 1-year survival rate in the registry following BASILICA was 84%.
Results should ‘push people toward BASILICA’
The acronym BASILICA stands for bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction. In the procedure, performed immediately before TAVR, guidewires are introduced to first traverse and then lacerate aortic leaflets threatening obstruction of a coronary artery.
In cases where diseased valve leaflets pose a risk of coronary obstruction, most interventionalists “are comfortable with surgery when patients are at low or intermediate risk, but the choices for high-risk patients are a snorkel stent or BASILICA. Given the limits of snorkel stenting, these data should be reassuring and push people toward BASILICA,” Dr. Khan said.
The 214 patients were entered into the registry from June 2015 to December 2020. The mean age was 74.9 years. Of valves treated, 73% were failed bioprosthetic devices. The remaining were native aortic valves. Solo BASILICA was performed in most patients, but 21.5% underwent a doppio procedure, meaning the laceration of two leaflets.
Despite BASILICA, 10 patients (4.7%) had some degree of coronary obstruction, including 5 with partial obstruction of the main coronary artery and 1 with partial obstruction of the right coronary artery. All of these partial obstructions were successfully treated with orthotopic stents.
An obstruction of the right coronary artery was successfully treated with balloon angioplasty. Another patient with significant left main coronary artery obstruction required cardiopulmonary bypass but was successfully treated with snorkel stenting. Of two patients with complete obstruction of the left main coronary artery caused by the skirt of the TAVR device, one died in hospital despite several maneuvers to restore perfusion.
Procedural complications included a mitral chord laceration, which subsequently led to valve replacement, and three guidewire transversals into surrounding tissue that did not result in serious sequelae. Hypotension requiring pressors occurred in 8.5%.
There was a “slight trend” for worse outcomes in those undergoing doppio rather than solo BASILICA, but the difference did not reach statistical significance. Cerebral embolic protection was offered to a minority of patients in this series. The trend for a lower risk of stroke in this group did not reach significance, Dr. Khan reported.
Best for high-volume centers, for now
Although these data support the conclusion that BASILICA “is feasible in a real-world setting,” Dr. Khan acknowledged that BASILICA might not be appropriate at low-volume centers. Dr. Khan cited data that indicates obstruction of a coronary artery by a diseased leaflet occurs in less than 1% of TAVR cases.
“Not every site doing a handful of TAVRs is going to want to tackle these cases, but those working in a high-volume center will from time to time encounter patients with coronary obstruction or who are at increased risk,” Dr. Khan said.
In North America, there has been a proctoring program to disseminate the skills required to perform BASILICA, according to Dr. Khan, who explained that proctors typically participate in two or three cases before these are performed without supervision.
So far, the uptake of BASILICA has been limited.
“BASILICA has not been catching on in EUROPE,” said Didier F. Loulmet, MD, chief of cardiac surgery at Tisch Hospital, New York University Langone Health. There might be several reasons, but Dr. Loulmet said that lack of a comparable proctoring program is one factor.”
“This is a relatively complex procedure performed in a small number of patients, so building up expertise is quite a challenge, particularly in small centers,” he added. He encouraged proctoring as “the way that it has to be propagated.”
The results presented by Dr. Khan on March 6 at CRT 2021 were simultaneously published in JACC: Cardiovascular Interventions.
Dr. Khan has patents on several devices, including catheters to lacerate valve leaflet. Dr. Loulmet reported no potential conflicts of interest.
FROM CRT 2021
Delay surgery by 7 weeks after COVID-19 diagnosis, study shows
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.