User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
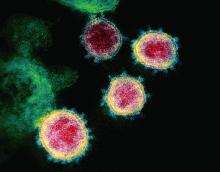
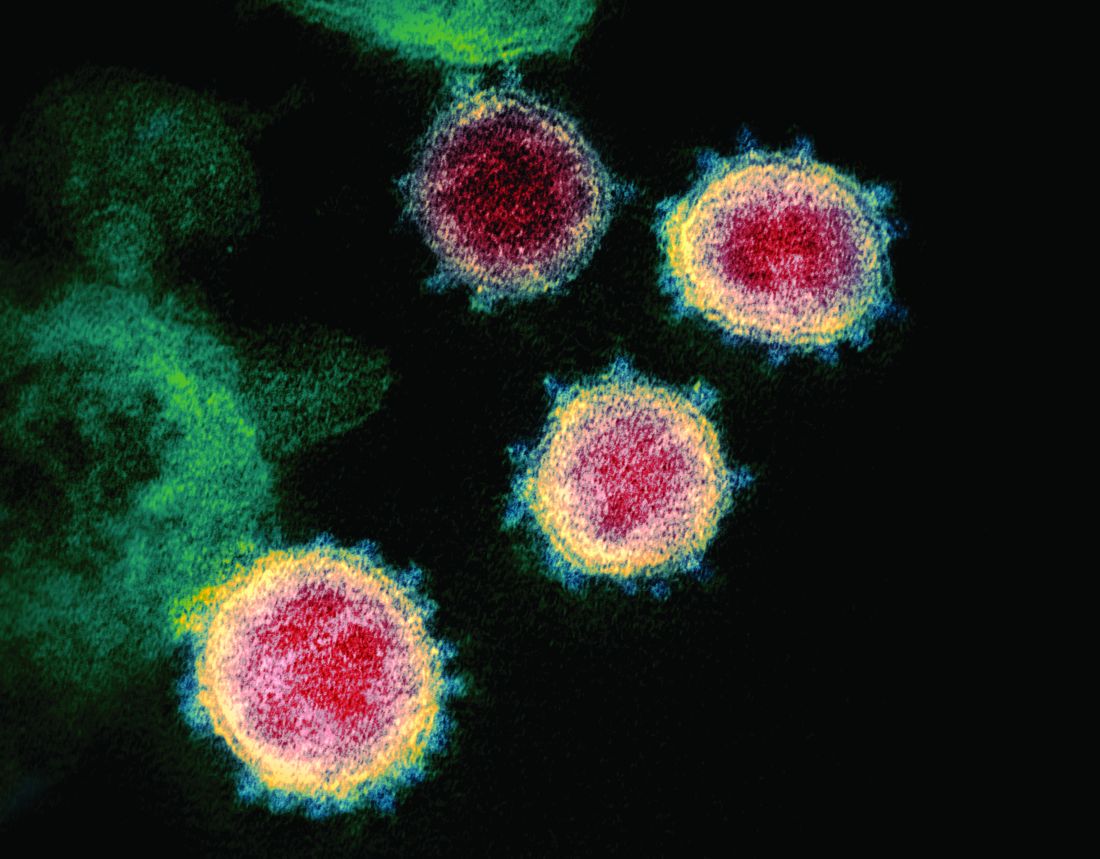
SARS serum neutralizing antibodies may inform the treatment of COVID-19
The immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, raising the likelihood that the similarly behaving SARS-CoV-2 might provoke the same response, according to an online communication published in the Journal of Microbiology, Immunology and Infection.
The authors cited a cohort study of convalescent SARS-CoV patients (56 cases, from the Beijing hospital of the Armed Forces Police, China) that showed that specific IgG antibodies and neutralizing antibodies were highly correlated, peaking at month 4 after the onset of disease and decreasing gradually thereafter.
This and other studies suggest that the immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, according to the authors.
However, of particular concern is the fact that only 11.8% of patients acquire specific SARS-CoV Abs in the early period after recovery at day 7, not reaching 100% until day 90, which highlights the importance of the detection of antibody titers for convalescent COVID-19 patients, according to the authors. “Otherwise, these patients with low titers of antibodies may not be efficient for the clearance of SARS-CoV-2.”
The authors also cited a recent study that showed how neutralizing antibody from a convalescent SARS patient could block the SARS-CoV-2 from entering into target cells in vitro, and suggested that previous experimental SARS-CoV vaccines and neutralizing antibodies could provide novel preventive and therapeutic options for COVID-19.
“These experiences from SARS-CoV are expected to have some implications for the treatment, management and surveillance of SARS-CoV-2 patients,” the authors concluded.
SOURCE: Lin Q et al. J Microbiol Immunol Infect. 2020 Mar 25. https://doi.org/10.1016/j.jmii.2020.03.015.
The immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, raising the likelihood that the similarly behaving SARS-CoV-2 might provoke the same response, according to an online communication published in the Journal of Microbiology, Immunology and Infection.
The authors cited a cohort study of convalescent SARS-CoV patients (56 cases, from the Beijing hospital of the Armed Forces Police, China) that showed that specific IgG antibodies and neutralizing antibodies were highly correlated, peaking at month 4 after the onset of disease and decreasing gradually thereafter.
This and other studies suggest that the immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, according to the authors.
However, of particular concern is the fact that only 11.8% of patients acquire specific SARS-CoV Abs in the early period after recovery at day 7, not reaching 100% until day 90, which highlights the importance of the detection of antibody titers for convalescent COVID-19 patients, according to the authors. “Otherwise, these patients with low titers of antibodies may not be efficient for the clearance of SARS-CoV-2.”
The authors also cited a recent study that showed how neutralizing antibody from a convalescent SARS patient could block the SARS-CoV-2 from entering into target cells in vitro, and suggested that previous experimental SARS-CoV vaccines and neutralizing antibodies could provide novel preventive and therapeutic options for COVID-19.
“These experiences from SARS-CoV are expected to have some implications for the treatment, management and surveillance of SARS-CoV-2 patients,” the authors concluded.
SOURCE: Lin Q et al. J Microbiol Immunol Infect. 2020 Mar 25. https://doi.org/10.1016/j.jmii.2020.03.015.
The immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, raising the likelihood that the similarly behaving SARS-CoV-2 might provoke the same response, according to an online communication published in the Journal of Microbiology, Immunology and Infection.
The authors cited a cohort study of convalescent SARS-CoV patients (56 cases, from the Beijing hospital of the Armed Forces Police, China) that showed that specific IgG antibodies and neutralizing antibodies were highly correlated, peaking at month 4 after the onset of disease and decreasing gradually thereafter.
This and other studies suggest that the immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, according to the authors.
However, of particular concern is the fact that only 11.8% of patients acquire specific SARS-CoV Abs in the early period after recovery at day 7, not reaching 100% until day 90, which highlights the importance of the detection of antibody titers for convalescent COVID-19 patients, according to the authors. “Otherwise, these patients with low titers of antibodies may not be efficient for the clearance of SARS-CoV-2.”
The authors also cited a recent study that showed how neutralizing antibody from a convalescent SARS patient could block the SARS-CoV-2 from entering into target cells in vitro, and suggested that previous experimental SARS-CoV vaccines and neutralizing antibodies could provide novel preventive and therapeutic options for COVID-19.
“These experiences from SARS-CoV are expected to have some implications for the treatment, management and surveillance of SARS-CoV-2 patients,” the authors concluded.
SOURCE: Lin Q et al. J Microbiol Immunol Infect. 2020 Mar 25. https://doi.org/10.1016/j.jmii.2020.03.015.
FROM THE JOURNAL OF MICROBIOLOGY, IMMUNOLOGY AND INFECTION
Liraglutide gives adolescents with obesity an edge in managing weight loss
Prescribing liraglutide plus lifestyle therapy for adolescents with obesity resulted in greater weight loss and greater reduction in body mass index, compared with those prescribed lifestyle therapy alone, according to findings from a new study published in The New England Journal of Medicine.
Liraglutide with lifestyle therapy also “compared favorably in terms of [body mass index] reduction,” compared with other pediatric weight-management programs in the United States and with use of orlistat, wrote Aaron S. Kelly, PhD, of the University of Minnesota, Minneapolis, and colleagues. The study abstract was presented during a virtual news conference held by The Endocrine Society. It had been slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
The study included adolescents aged 12-17 years, who had obesity (BMI, ≥30 kg/m2) and had responded poorly to recommendations involving lifestyle therapy only, as judged by the site investigator and documented in the participant’s medical records. The adolescents participated at one of five sites in Belgium, Mexico, Russia, Sweden, and the United States.
In the randomized, controlled, double-blind trial, 125 participants received 3 mg liraglutide, and 126 received placebo for 56 weeks, during which both groups received lifestyle therapy, “defined as counseling about healthy nutrition and physical activity for weight loss,” the authors wrote.
After 12-weeks of run-in, the treatment period lasted 56 weeks, with a follow-up 26 weeks after treatment ended. The liraglutide group retained 80.8% of its participants, and the placebo group, 79.4%.
At week 56, there were no significant differences between the groups in blood pressure, fasting lipids, fasting plasma glucose, or hemoglobin A1c, the authors noted.
However, in the liraglutide group, 43.3% of participants lost at least 5% of their BMI, compared with 18.7% in the control group. Similarly, 26.1% of those in the liraglutide group had a BMI reduction of at least 10%, compared with 8.1% in the control group.
Participants in the liraglutide group also saw a greater reduction in BMI, compared with those in the placebo group (estimated difference, 4.64 percentage points), and those taking liraglutide lost 9.9 pounds (4.5 kg) more than those receiving placebo – a relative reduction of 5%. The authors noted that a weight loss of 3%-5% “significantly improves some health-related outcomes in adults.”
In addition, the liraglutide group had a BMI standard-deviation score that was 0.22 lower than that in the placebo group (P = .002), but after the participants discontinued with the trial, “a greater increase in the BMI standard-deviation score was observed with liraglutide than with placebo (0.15),” the authors reported.
“Although evidence in children is limited, a change in BMI standard-deviation score of at least 0.20 has been suggested to be clinically meaningful,” they wrote. “Some studies indicate that even temporary weight loss may have long-term benefits, but the extent to which this applies in adolescents and the extent to which long-term adherence to pharmacotherapy can be expected are unknown.”
The researchers added that the reduction in standard-deviation score seen in this study, of 0.22, was a bigger reduction than that seen in lifestyle therapy trials from the U.S. Preventive Services Task Force and from an overview of six Cochrane reviews. Their trial also, however, had a fairly high adherence rate, over 80%.
No notable differences in cardiometabolic markers or in quality of life showed up between the liraglutide and placebo groups. The heterogeneous treatment response in this and past studies suggests the need for future trials to “characterize predictors of treatment response to identify patients who would benefit the most from treatment,” the authors wrote.
About twice as many participants taking liraglutide experienced gastrointestinal adverse events compared with those receiving placebo (64.8% vs. 36.5%, respectively). Those symptoms, a known side effect of this drug type, included nausea, vomiting, and diarrhea and occurred primarily during escalation of the drug dose before then dropping in frequency. Still, the authors note that the high rate of gastrointestinal effects “suggests that this treatment may not be suitable for all patients.”
None of the adolescents receiving the placebo stopped treatment, but 10.4% of those taking liraglutide discontinued. One participant in the liraglutide group died by suicide, but the death was determined to be unrelated to the therapy.
Although the 0.22 reduction in the BMI standard-deviation score was for the intent-to-treat population, the authors calculated that the difference would have been 0.26 “if all participants had adhered to the treatment throughout the trial.”
Novo Nordisk funded the research. Several of the authors reported that they are employees of the company.
The abstract will also be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will ost ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Source: Kelly AS et al. NEJM. 2020 Mar 31. doi: 10.1056/NEJMoa1916038.
Prescribing liraglutide plus lifestyle therapy for adolescents with obesity resulted in greater weight loss and greater reduction in body mass index, compared with those prescribed lifestyle therapy alone, according to findings from a new study published in The New England Journal of Medicine.
Liraglutide with lifestyle therapy also “compared favorably in terms of [body mass index] reduction,” compared with other pediatric weight-management programs in the United States and with use of orlistat, wrote Aaron S. Kelly, PhD, of the University of Minnesota, Minneapolis, and colleagues. The study abstract was presented during a virtual news conference held by The Endocrine Society. It had been slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
The study included adolescents aged 12-17 years, who had obesity (BMI, ≥30 kg/m2) and had responded poorly to recommendations involving lifestyle therapy only, as judged by the site investigator and documented in the participant’s medical records. The adolescents participated at one of five sites in Belgium, Mexico, Russia, Sweden, and the United States.
In the randomized, controlled, double-blind trial, 125 participants received 3 mg liraglutide, and 126 received placebo for 56 weeks, during which both groups received lifestyle therapy, “defined as counseling about healthy nutrition and physical activity for weight loss,” the authors wrote.
After 12-weeks of run-in, the treatment period lasted 56 weeks, with a follow-up 26 weeks after treatment ended. The liraglutide group retained 80.8% of its participants, and the placebo group, 79.4%.
At week 56, there were no significant differences between the groups in blood pressure, fasting lipids, fasting plasma glucose, or hemoglobin A1c, the authors noted.
However, in the liraglutide group, 43.3% of participants lost at least 5% of their BMI, compared with 18.7% in the control group. Similarly, 26.1% of those in the liraglutide group had a BMI reduction of at least 10%, compared with 8.1% in the control group.
Participants in the liraglutide group also saw a greater reduction in BMI, compared with those in the placebo group (estimated difference, 4.64 percentage points), and those taking liraglutide lost 9.9 pounds (4.5 kg) more than those receiving placebo – a relative reduction of 5%. The authors noted that a weight loss of 3%-5% “significantly improves some health-related outcomes in adults.”
In addition, the liraglutide group had a BMI standard-deviation score that was 0.22 lower than that in the placebo group (P = .002), but after the participants discontinued with the trial, “a greater increase in the BMI standard-deviation score was observed with liraglutide than with placebo (0.15),” the authors reported.
“Although evidence in children is limited, a change in BMI standard-deviation score of at least 0.20 has been suggested to be clinically meaningful,” they wrote. “Some studies indicate that even temporary weight loss may have long-term benefits, but the extent to which this applies in adolescents and the extent to which long-term adherence to pharmacotherapy can be expected are unknown.”
The researchers added that the reduction in standard-deviation score seen in this study, of 0.22, was a bigger reduction than that seen in lifestyle therapy trials from the U.S. Preventive Services Task Force and from an overview of six Cochrane reviews. Their trial also, however, had a fairly high adherence rate, over 80%.
No notable differences in cardiometabolic markers or in quality of life showed up between the liraglutide and placebo groups. The heterogeneous treatment response in this and past studies suggests the need for future trials to “characterize predictors of treatment response to identify patients who would benefit the most from treatment,” the authors wrote.
About twice as many participants taking liraglutide experienced gastrointestinal adverse events compared with those receiving placebo (64.8% vs. 36.5%, respectively). Those symptoms, a known side effect of this drug type, included nausea, vomiting, and diarrhea and occurred primarily during escalation of the drug dose before then dropping in frequency. Still, the authors note that the high rate of gastrointestinal effects “suggests that this treatment may not be suitable for all patients.”
None of the adolescents receiving the placebo stopped treatment, but 10.4% of those taking liraglutide discontinued. One participant in the liraglutide group died by suicide, but the death was determined to be unrelated to the therapy.
Although the 0.22 reduction in the BMI standard-deviation score was for the intent-to-treat population, the authors calculated that the difference would have been 0.26 “if all participants had adhered to the treatment throughout the trial.”
Novo Nordisk funded the research. Several of the authors reported that they are employees of the company.
The abstract will also be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will ost ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Source: Kelly AS et al. NEJM. 2020 Mar 31. doi: 10.1056/NEJMoa1916038.
Prescribing liraglutide plus lifestyle therapy for adolescents with obesity resulted in greater weight loss and greater reduction in body mass index, compared with those prescribed lifestyle therapy alone, according to findings from a new study published in The New England Journal of Medicine.
Liraglutide with lifestyle therapy also “compared favorably in terms of [body mass index] reduction,” compared with other pediatric weight-management programs in the United States and with use of orlistat, wrote Aaron S. Kelly, PhD, of the University of Minnesota, Minneapolis, and colleagues. The study abstract was presented during a virtual news conference held by The Endocrine Society. It had been slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
The study included adolescents aged 12-17 years, who had obesity (BMI, ≥30 kg/m2) and had responded poorly to recommendations involving lifestyle therapy only, as judged by the site investigator and documented in the participant’s medical records. The adolescents participated at one of five sites in Belgium, Mexico, Russia, Sweden, and the United States.
In the randomized, controlled, double-blind trial, 125 participants received 3 mg liraglutide, and 126 received placebo for 56 weeks, during which both groups received lifestyle therapy, “defined as counseling about healthy nutrition and physical activity for weight loss,” the authors wrote.
After 12-weeks of run-in, the treatment period lasted 56 weeks, with a follow-up 26 weeks after treatment ended. The liraglutide group retained 80.8% of its participants, and the placebo group, 79.4%.
At week 56, there were no significant differences between the groups in blood pressure, fasting lipids, fasting plasma glucose, or hemoglobin A1c, the authors noted.
However, in the liraglutide group, 43.3% of participants lost at least 5% of their BMI, compared with 18.7% in the control group. Similarly, 26.1% of those in the liraglutide group had a BMI reduction of at least 10%, compared with 8.1% in the control group.
Participants in the liraglutide group also saw a greater reduction in BMI, compared with those in the placebo group (estimated difference, 4.64 percentage points), and those taking liraglutide lost 9.9 pounds (4.5 kg) more than those receiving placebo – a relative reduction of 5%. The authors noted that a weight loss of 3%-5% “significantly improves some health-related outcomes in adults.”
In addition, the liraglutide group had a BMI standard-deviation score that was 0.22 lower than that in the placebo group (P = .002), but after the participants discontinued with the trial, “a greater increase in the BMI standard-deviation score was observed with liraglutide than with placebo (0.15),” the authors reported.
“Although evidence in children is limited, a change in BMI standard-deviation score of at least 0.20 has been suggested to be clinically meaningful,” they wrote. “Some studies indicate that even temporary weight loss may have long-term benefits, but the extent to which this applies in adolescents and the extent to which long-term adherence to pharmacotherapy can be expected are unknown.”
The researchers added that the reduction in standard-deviation score seen in this study, of 0.22, was a bigger reduction than that seen in lifestyle therapy trials from the U.S. Preventive Services Task Force and from an overview of six Cochrane reviews. Their trial also, however, had a fairly high adherence rate, over 80%.
No notable differences in cardiometabolic markers or in quality of life showed up between the liraglutide and placebo groups. The heterogeneous treatment response in this and past studies suggests the need for future trials to “characterize predictors of treatment response to identify patients who would benefit the most from treatment,” the authors wrote.
About twice as many participants taking liraglutide experienced gastrointestinal adverse events compared with those receiving placebo (64.8% vs. 36.5%, respectively). Those symptoms, a known side effect of this drug type, included nausea, vomiting, and diarrhea and occurred primarily during escalation of the drug dose before then dropping in frequency. Still, the authors note that the high rate of gastrointestinal effects “suggests that this treatment may not be suitable for all patients.”
None of the adolescents receiving the placebo stopped treatment, but 10.4% of those taking liraglutide discontinued. One participant in the liraglutide group died by suicide, but the death was determined to be unrelated to the therapy.
Although the 0.22 reduction in the BMI standard-deviation score was for the intent-to-treat population, the authors calculated that the difference would have been 0.26 “if all participants had adhered to the treatment throughout the trial.”
Novo Nordisk funded the research. Several of the authors reported that they are employees of the company.
The abstract will also be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will ost ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Source: Kelly AS et al. NEJM. 2020 Mar 31. doi: 10.1056/NEJMoa1916038.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
COVID-19: More hydroxychloroquine data from France, more questions
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
What if a COVID-19 test is negative?
In a physician WhatsApp group, a doctor posted he had fever of 101 °F and muscle ache, gently confessing that it felt like his typical “man flu” which heals with rest and scotch. Nevertheless, he worried that he had coronavirus. When the reverse transcription polymerase chain reaction (RT-PCR) for the virus on his nasal swab came back negative, he jubilantly announced his relief.
Like Twitter, in WhatsApp emotions quickly outstrip facts. After he received a flurry of cheerful emojis, I ruined the party, advising that, despite the negative test, he assume he’s infected and quarantine for 2 weeks, with a bottle of scotch.
It’s conventional wisdom that the secret sauce to fighting the pandemic is testing for the virus. To gauge the breadth of the response against the pandemic we must know who and how many are infected. The depth of the response will be different if 25% of the population is infected than 1%. Testing is the third way, rejecting the false choice between death and economic depression. Without testing, strategy is faith based.
Our reliance on testing has clinical precedence – scarcely any decision in medicine is made without laboratory tests or imaging. Testing is as ingrained in medicine as the GPS is in driving. We use it even when we know our way home. But tests impose a question – what’ll you do differently if the test is negative?
That depends on the test’s performance and the consequences of being wrong. Though coronavirus damages the lungs with reckless abandon, it’s oddly a shy virus. In many patients, it takes 3-4 swabs to get a positive RT-PCR. The Chinese ophthalmologist, Li Wenliang, who originally sounded the alarm about coronavirus, had several negative tests. He died from the infection.
In one Chinese study, the sensitivity of RT-PCR – that’s the proportion of the infected who test positive – was around 70%. To put this in perspective, of 1,000 people infected with coronavirus, 700 will test positive but 300 will test negative.
Is this good enough?
Three hundred “false-negative” people may believe they’re not contagious because they got a clean chit and could infect others. False negatives could undo the hard work of containment.
Surely, better an imperfect test than no test. Isn’t flying with partially accurate weather information safer than no information? Here, aviation analogies aren’t helpful. Better to think of a forest fire.
Imagine only 80% of a burning forest is doused because it’s mistakenly believed that 20% of the forest isn’t burning because we can’t see it burning. It must be extinguished before it relights the whole forest, but to douse it you must know it’s burning – a Catch-22. That “20% of the forest” is a false negative – it’s burning but you think it’s not burning.
Because coronavirus isn’t planning to leave in a hurry and long-term lockdown has grave economic consequences, testing may enable precision quarantining of people, communities, and cities. Rather than applying a one-size-fits-all lockdown on the whole nation, testing could tell us who can work and who should stay home. Why should Austin, if it has a low prevalence of infection, shut shop just because of New York City’s high prevalence?
Testing enables us to think globally but act locally. But it’s the asymptomatic people who drive the epidemic. To emphasize – asymptomatics are yet to have symptoms such as cough and fever. They’re feeling well and don’t know they’ve been colonized by the virus. Theoretically, if we test en masse we can find asymptomatics. If only those who test positive are quarantined, the rest can have some breathing space. Will this approach work?
RT-PCR’s sensitivity, which is low in early illness, is even lower in asymptomatics, likely because of lower viral load, which means even more false negatives. The virus’s average incubation time of 5 days is enough time for false negative asymptomatics – remember they resemble the uninfected – to visit Disney World and infect another four.
Whether false negatives behave like tinder or a controllable fire will determine the testing strategy’s success. The net contagiousness of false negatives depends how many there are, which depends on how many are infected. To know how many are infected we need to test. Or, to know whether to believe a negative test in any person we must test widely – another Catch-22.
Maybe we need a bigger test.
Chest CT is an alternative. It’s rapid – takes less than an hour whereas RT-PCR can take over a day to report. In one study CT had a sensitivity of 97% in symptomatic patients and was often positive before RT-PCR. But there are caveats.
The real sensitivity of CT is likely much lower than 97% because the study has biases which inflate performance. CT, like RT-PCR, has a low sensitivity in early illness and even lower sensitivity in asymptomatic carriers for the same reason – lower viral load. Furthermore, CT has to be disinfected to prevent spread, which limits its access for other patients.
Coronavirus’s signature on CT – white patches in lungs, known as ground glass opacities – doesn’t have the uniqueness of the Mark of Zorro, and looks like lung injury from other rogue actors, which means we can mistake other serious conditions for coronavirus. Imagine hyenas in wolf’s clothing.
No test is perfect. We still use imaging despite its imperfections. But, let’s ask: What would you do differently if the test is negative and you have mild symptoms of cough and fever? Should you not self-isolate? What if you’re falsely negative and still contagious? If the advice dispensed whether the test is positive or negative is the same – i.e. quarantine for 2 weeks – what’s the test’s value?
Perhaps people will more likely comply with voluntary quarantine if they know they’re infected. Information can nudge behavior. But the logical corollary is that to comply with social distancing you need to be tested. People flocking to CT scans to affirm they’re not infected could infect those hitherto uninfected. A pandemic is no time to test nudge theories.
Does that mean testing has no value? Testing is valuable in managing populations. To individuals, the results must be framed wisely, such as by advising those who test positive to quarantine because “you’re infected” and those who test negative to keep social distancing because “you could still be infected.”
Even when policy goals are uniform, messaging can be oppositional. “Get yourself tested now” contradicts “you must hunker down now.” When messages contradict, one must choose which message to amplify.
The calculus of testing can change with new tests such as antibodies. The value of testing depends also on what isolation entails. A couple of weeks watching Netflix on your couch isn’t a big ask. If quarantine means being detained in an isolation center fenced by barbed wires, the cost of frivolous quarantining is higher and testing becomes more valuable.
I knew the doctor with the negative RT-PCR well. He’s heroically nonchalant about his wellbeing, an endearing quality that’s a liability in a contagion. In no time he’d be back in the hospital; or helping his elderly parents with grocery. Not all false negatives are equal. False-negative doctors could infect not just their patients but their colleagues, leaving fewer firefighters to fight fires.
It is better to mistake the man flu for coronavirus than coronavirus for the man flu. All he has to do is hunker down, which is what we should all be doing as much as we can.
Dr. Jha is a contributing editor to The Health Care Blog, where this article first appeared. He can be reached @RogueRad.
This article appeared on Medscape.com.
In a physician WhatsApp group, a doctor posted he had fever of 101 °F and muscle ache, gently confessing that it felt like his typical “man flu” which heals with rest and scotch. Nevertheless, he worried that he had coronavirus. When the reverse transcription polymerase chain reaction (RT-PCR) for the virus on his nasal swab came back negative, he jubilantly announced his relief.
Like Twitter, in WhatsApp emotions quickly outstrip facts. After he received a flurry of cheerful emojis, I ruined the party, advising that, despite the negative test, he assume he’s infected and quarantine for 2 weeks, with a bottle of scotch.
It’s conventional wisdom that the secret sauce to fighting the pandemic is testing for the virus. To gauge the breadth of the response against the pandemic we must know who and how many are infected. The depth of the response will be different if 25% of the population is infected than 1%. Testing is the third way, rejecting the false choice between death and economic depression. Without testing, strategy is faith based.
Our reliance on testing has clinical precedence – scarcely any decision in medicine is made without laboratory tests or imaging. Testing is as ingrained in medicine as the GPS is in driving. We use it even when we know our way home. But tests impose a question – what’ll you do differently if the test is negative?
That depends on the test’s performance and the consequences of being wrong. Though coronavirus damages the lungs with reckless abandon, it’s oddly a shy virus. In many patients, it takes 3-4 swabs to get a positive RT-PCR. The Chinese ophthalmologist, Li Wenliang, who originally sounded the alarm about coronavirus, had several negative tests. He died from the infection.
In one Chinese study, the sensitivity of RT-PCR – that’s the proportion of the infected who test positive – was around 70%. To put this in perspective, of 1,000 people infected with coronavirus, 700 will test positive but 300 will test negative.
Is this good enough?
Three hundred “false-negative” people may believe they’re not contagious because they got a clean chit and could infect others. False negatives could undo the hard work of containment.
Surely, better an imperfect test than no test. Isn’t flying with partially accurate weather information safer than no information? Here, aviation analogies aren’t helpful. Better to think of a forest fire.
Imagine only 80% of a burning forest is doused because it’s mistakenly believed that 20% of the forest isn’t burning because we can’t see it burning. It must be extinguished before it relights the whole forest, but to douse it you must know it’s burning – a Catch-22. That “20% of the forest” is a false negative – it’s burning but you think it’s not burning.
Because coronavirus isn’t planning to leave in a hurry and long-term lockdown has grave economic consequences, testing may enable precision quarantining of people, communities, and cities. Rather than applying a one-size-fits-all lockdown on the whole nation, testing could tell us who can work and who should stay home. Why should Austin, if it has a low prevalence of infection, shut shop just because of New York City’s high prevalence?
Testing enables us to think globally but act locally. But it’s the asymptomatic people who drive the epidemic. To emphasize – asymptomatics are yet to have symptoms such as cough and fever. They’re feeling well and don’t know they’ve been colonized by the virus. Theoretically, if we test en masse we can find asymptomatics. If only those who test positive are quarantined, the rest can have some breathing space. Will this approach work?
RT-PCR’s sensitivity, which is low in early illness, is even lower in asymptomatics, likely because of lower viral load, which means even more false negatives. The virus’s average incubation time of 5 days is enough time for false negative asymptomatics – remember they resemble the uninfected – to visit Disney World and infect another four.
Whether false negatives behave like tinder or a controllable fire will determine the testing strategy’s success. The net contagiousness of false negatives depends how many there are, which depends on how many are infected. To know how many are infected we need to test. Or, to know whether to believe a negative test in any person we must test widely – another Catch-22.
Maybe we need a bigger test.
Chest CT is an alternative. It’s rapid – takes less than an hour whereas RT-PCR can take over a day to report. In one study CT had a sensitivity of 97% in symptomatic patients and was often positive before RT-PCR. But there are caveats.
The real sensitivity of CT is likely much lower than 97% because the study has biases which inflate performance. CT, like RT-PCR, has a low sensitivity in early illness and even lower sensitivity in asymptomatic carriers for the same reason – lower viral load. Furthermore, CT has to be disinfected to prevent spread, which limits its access for other patients.
Coronavirus’s signature on CT – white patches in lungs, known as ground glass opacities – doesn’t have the uniqueness of the Mark of Zorro, and looks like lung injury from other rogue actors, which means we can mistake other serious conditions for coronavirus. Imagine hyenas in wolf’s clothing.
No test is perfect. We still use imaging despite its imperfections. But, let’s ask: What would you do differently if the test is negative and you have mild symptoms of cough and fever? Should you not self-isolate? What if you’re falsely negative and still contagious? If the advice dispensed whether the test is positive or negative is the same – i.e. quarantine for 2 weeks – what’s the test’s value?
Perhaps people will more likely comply with voluntary quarantine if they know they’re infected. Information can nudge behavior. But the logical corollary is that to comply with social distancing you need to be tested. People flocking to CT scans to affirm they’re not infected could infect those hitherto uninfected. A pandemic is no time to test nudge theories.
Does that mean testing has no value? Testing is valuable in managing populations. To individuals, the results must be framed wisely, such as by advising those who test positive to quarantine because “you’re infected” and those who test negative to keep social distancing because “you could still be infected.”
Even when policy goals are uniform, messaging can be oppositional. “Get yourself tested now” contradicts “you must hunker down now.” When messages contradict, one must choose which message to amplify.
The calculus of testing can change with new tests such as antibodies. The value of testing depends also on what isolation entails. A couple of weeks watching Netflix on your couch isn’t a big ask. If quarantine means being detained in an isolation center fenced by barbed wires, the cost of frivolous quarantining is higher and testing becomes more valuable.
I knew the doctor with the negative RT-PCR well. He’s heroically nonchalant about his wellbeing, an endearing quality that’s a liability in a contagion. In no time he’d be back in the hospital; or helping his elderly parents with grocery. Not all false negatives are equal. False-negative doctors could infect not just their patients but their colleagues, leaving fewer firefighters to fight fires.
It is better to mistake the man flu for coronavirus than coronavirus for the man flu. All he has to do is hunker down, which is what we should all be doing as much as we can.
Dr. Jha is a contributing editor to The Health Care Blog, where this article first appeared. He can be reached @RogueRad.
This article appeared on Medscape.com.
In a physician WhatsApp group, a doctor posted he had fever of 101 °F and muscle ache, gently confessing that it felt like his typical “man flu” which heals with rest and scotch. Nevertheless, he worried that he had coronavirus. When the reverse transcription polymerase chain reaction (RT-PCR) for the virus on his nasal swab came back negative, he jubilantly announced his relief.
Like Twitter, in WhatsApp emotions quickly outstrip facts. After he received a flurry of cheerful emojis, I ruined the party, advising that, despite the negative test, he assume he’s infected and quarantine for 2 weeks, with a bottle of scotch.
It’s conventional wisdom that the secret sauce to fighting the pandemic is testing for the virus. To gauge the breadth of the response against the pandemic we must know who and how many are infected. The depth of the response will be different if 25% of the population is infected than 1%. Testing is the third way, rejecting the false choice between death and economic depression. Without testing, strategy is faith based.
Our reliance on testing has clinical precedence – scarcely any decision in medicine is made without laboratory tests or imaging. Testing is as ingrained in medicine as the GPS is in driving. We use it even when we know our way home. But tests impose a question – what’ll you do differently if the test is negative?
That depends on the test’s performance and the consequences of being wrong. Though coronavirus damages the lungs with reckless abandon, it’s oddly a shy virus. In many patients, it takes 3-4 swabs to get a positive RT-PCR. The Chinese ophthalmologist, Li Wenliang, who originally sounded the alarm about coronavirus, had several negative tests. He died from the infection.
In one Chinese study, the sensitivity of RT-PCR – that’s the proportion of the infected who test positive – was around 70%. To put this in perspective, of 1,000 people infected with coronavirus, 700 will test positive but 300 will test negative.
Is this good enough?
Three hundred “false-negative” people may believe they’re not contagious because they got a clean chit and could infect others. False negatives could undo the hard work of containment.
Surely, better an imperfect test than no test. Isn’t flying with partially accurate weather information safer than no information? Here, aviation analogies aren’t helpful. Better to think of a forest fire.
Imagine only 80% of a burning forest is doused because it’s mistakenly believed that 20% of the forest isn’t burning because we can’t see it burning. It must be extinguished before it relights the whole forest, but to douse it you must know it’s burning – a Catch-22. That “20% of the forest” is a false negative – it’s burning but you think it’s not burning.
Because coronavirus isn’t planning to leave in a hurry and long-term lockdown has grave economic consequences, testing may enable precision quarantining of people, communities, and cities. Rather than applying a one-size-fits-all lockdown on the whole nation, testing could tell us who can work and who should stay home. Why should Austin, if it has a low prevalence of infection, shut shop just because of New York City’s high prevalence?
Testing enables us to think globally but act locally. But it’s the asymptomatic people who drive the epidemic. To emphasize – asymptomatics are yet to have symptoms such as cough and fever. They’re feeling well and don’t know they’ve been colonized by the virus. Theoretically, if we test en masse we can find asymptomatics. If only those who test positive are quarantined, the rest can have some breathing space. Will this approach work?
RT-PCR’s sensitivity, which is low in early illness, is even lower in asymptomatics, likely because of lower viral load, which means even more false negatives. The virus’s average incubation time of 5 days is enough time for false negative asymptomatics – remember they resemble the uninfected – to visit Disney World and infect another four.
Whether false negatives behave like tinder or a controllable fire will determine the testing strategy’s success. The net contagiousness of false negatives depends how many there are, which depends on how many are infected. To know how many are infected we need to test. Or, to know whether to believe a negative test in any person we must test widely – another Catch-22.
Maybe we need a bigger test.
Chest CT is an alternative. It’s rapid – takes less than an hour whereas RT-PCR can take over a day to report. In one study CT had a sensitivity of 97% in symptomatic patients and was often positive before RT-PCR. But there are caveats.
The real sensitivity of CT is likely much lower than 97% because the study has biases which inflate performance. CT, like RT-PCR, has a low sensitivity in early illness and even lower sensitivity in asymptomatic carriers for the same reason – lower viral load. Furthermore, CT has to be disinfected to prevent spread, which limits its access for other patients.
Coronavirus’s signature on CT – white patches in lungs, known as ground glass opacities – doesn’t have the uniqueness of the Mark of Zorro, and looks like lung injury from other rogue actors, which means we can mistake other serious conditions for coronavirus. Imagine hyenas in wolf’s clothing.
No test is perfect. We still use imaging despite its imperfections. But, let’s ask: What would you do differently if the test is negative and you have mild symptoms of cough and fever? Should you not self-isolate? What if you’re falsely negative and still contagious? If the advice dispensed whether the test is positive or negative is the same – i.e. quarantine for 2 weeks – what’s the test’s value?
Perhaps people will more likely comply with voluntary quarantine if they know they’re infected. Information can nudge behavior. But the logical corollary is that to comply with social distancing you need to be tested. People flocking to CT scans to affirm they’re not infected could infect those hitherto uninfected. A pandemic is no time to test nudge theories.
Does that mean testing has no value? Testing is valuable in managing populations. To individuals, the results must be framed wisely, such as by advising those who test positive to quarantine because “you’re infected” and those who test negative to keep social distancing because “you could still be infected.”
Even when policy goals are uniform, messaging can be oppositional. “Get yourself tested now” contradicts “you must hunker down now.” When messages contradict, one must choose which message to amplify.
The calculus of testing can change with new tests such as antibodies. The value of testing depends also on what isolation entails. A couple of weeks watching Netflix on your couch isn’t a big ask. If quarantine means being detained in an isolation center fenced by barbed wires, the cost of frivolous quarantining is higher and testing becomes more valuable.
I knew the doctor with the negative RT-PCR well. He’s heroically nonchalant about his wellbeing, an endearing quality that’s a liability in a contagion. In no time he’d be back in the hospital; or helping his elderly parents with grocery. Not all false negatives are equal. False-negative doctors could infect not just their patients but their colleagues, leaving fewer firefighters to fight fires.
It is better to mistake the man flu for coronavirus than coronavirus for the man flu. All he has to do is hunker down, which is what we should all be doing as much as we can.
Dr. Jha is a contributing editor to The Health Care Blog, where this article first appeared. He can be reached @RogueRad.
This article appeared on Medscape.com.
San Diego County CMO vigorously leads COVID-19 response team
SAN DIEGO – On the days family physician Nick Yphantides, MD, announces updates on the COVID-19 epidemic to San Diego County residents, he can’t help but think about his late father.
In June of 2009, 75-year-old George Yphantides, a Steinway-trained piano technician who lived in Escondido, Calif., became the third person in the United States to die from complications of the pandemic H1N1 swine flu – just days before a vaccine became available.
“I loved my dad,” Dr. Yphantides, who has been San Diego County’s Chief Medical Officer since the year of his father’s death, said in an interview. “So, when you take a step back and take into consideration my sense of purpose in serving the 3.3 million residents of San Diego County, my passion based on my personal Christian faith, and my activation in terms of what happened to my dad, I have such a storm of internal sense of urgency right now.”
San Diego County and public health officials got experience with COVID-19 in advance of the country’s widely documented cases of community-based transmission. Around 9 pm on Jan. 31, 2020 – the Friday of Super Bowl weekend – Dr. Yphantides answered a phone call from Eric C. McDonald, MD, the county’s medical director of epidemiology. Dr. McDonald informed him that in a few days, a plane full of American citizens traveling from Wuhan, China, would be landing at Marine Corp Air Station Miramar in San Diego for a 2-week quarantine and that the task of providing medical support to any affected individuals fell on county officials.
“I will never forget that phone call,” he said. “We did have two positive cases. What we experienced with those evacuees was amazing surge preparation, and without exaggeration, I have worked 18-20 hours a day since that day.”
Fast forward to March 31, 2020, the county’s confirmed COVID-19 caseload had grown to 734, up 131 from the day before. As of the final day of March, nine people have died, with an age range between 25 and 87 years. Of confirmed cases, 61% are between the ages of 20 and 49, 43% are female, 19% have required hospitalization, 7% have required admission to intensive care, and the mortality rate has been 1.2%. Data currently show optimal proactive hospital capacity.
In the opinion of Dr. Yphantides, the 734 COVID-19 cases represent a tip of the iceberg. “How big is that iceberg? I can’t tell you yet,” he said.
"I see this as the Super Bowl of public health,” he exclaimed.
At least some of Dr. Yphantides’ vigor seems to be fueled by his pride in his team of professionals who have been helping him respond to the surge of COVID-19 cases.
As the county’s CMO, Dr. Yphantides serves as the liaison for the entire Emergency Medical System, the entire local health care delivery system, the entire physician and medical society network, the payor system, and the proportion of the area population using Medi-Cal.
Dr. Yphantides, who attended medical school at the University of California, San Diego, said that, compared with other regions of the country, San Diego County has made “tremendous progress” in overcoming many chronic lifestyle illnesses. For example, cardiovascular disease is no longer the number one cause of death in the county; it’s bookended by cancer and Alzheimer’s disease.
“In the context of the COVID-19 response, [the county’s health care team established] an entire incident command system in our emergency operations center. Our emergency operations center is activated to the top level,” he said.
Dr. Yphantides shares public communication efforts with Dr. McDonald and Wilma J. Wooten, MD, the county’s public health officer. The San Diego County CMO also engages with policymakers, including the board of supervisors, local mayors, state legislators, and national legislators.
“Because of the relational trust capital that I have in this community, I get pulled into unexpected rooms of discussion,” he said. This included meeting with top executives from the San Diego Padres in early March, putting them on notice that the 2020 Major League Baseball season would likely be postponed. (This was officially announced on March 16.)
“We have made some decisions that have devastated some people economically. Talk about flipping the switch. We are living and making history every day. It is unbelievable,” he said.
“San Diego is a more aged population compared to many other parts of the country. ... [Part] of the reason why I’m so frantically doing everything I can to prepare, to batten down the hatches, and to optimize our health care delivery system is because we have a population that collectively is more at risk [for more serious complications from COVID-19]. A lot of what drives me is advocacy,” Dr. Yphantides noted.
A colleague’s perspective
Kristi L. Koenig, MD, medical director of emergency medical services for the County of San Diego, characterized Dr. Yphantides’ management style as collaborative. “Under his leadership, we have the perspective of ‘just focus on patient care, get it done, be creative, work together as a team,’ ” said Dr. Koenig, who coedited the textbook, “Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices” (Cambridge University Press, 2016). “He’s decisive and he’s responsive. You don’t have to wait a long time to get a decision, which is very important right now because this is so fast moving.”
Dr. Koenig, who has worked with Dr. Yphantides for 3 years, said that she routinely feeds him information that might help the team navigate its response to COVID-19. “For example, if I see an idea for how to get more [personal protective equipment] and feed it to him, he might have a contact somewhere in a factory that could make the PPE,” she said. “We work together by my reminding him to keep it within the incident command system structure, so that we can coordinate all the resources and not duplicate efforts.”
He uses his personal connections in a way to implement ideas that are beneficial to the overall goal of decreasing morbidity and mortality,” Dr. Koenig added.
Predictions for San Diego County
Dr. Yphantides said he considers San Diego to still be in the calm before the storm and that he is working hard to “board up his community.” The county CMO is also trying to prepare the health care delivery system to optimize its capacity, of doing interventions with hopes of lowering the curve and enhancing the capacity, he said.
When the storm hits, “it’s going be brutal, because we’re going to lose life,” Dr. Yphantides said.
“I am praying that maybe by some of our efforts, instead of a Category 5 storm, it’ll be a Category 3 storm,” he remarked.
The future of health care
Dr. Yphantides views the COVID-19 pandemic as “an absolute game-changer” in terms of what the future of health care delivery will look like in the United States. “Whether the right word is the ‘Amazonification’ of health care, or the ‘Uberization’ of health care, I don’t know, but the essence of how we deliver care is radically being transformed literally before our eyes,” he said. “I would encourage my colleagues to embrace that” and take care of their people by doing whatever it takes under this unprecedented paradigm.
Meanwhile, Dr. Yphantides braces for a potential surge of COVID-19 cases in San Diego County in the coming weeks. He honors the memory of his dad, and he expresses thanks for his mom, who cares for his two teenaged daughters while he helps steward the region’s response to the pandemic.
“Without my mom I could not function in the way that I’m currently functioning,” he said. “So, when you add all of those factors up, and wrap it with a bowtie of sincere love and passion for my community, there’s a fire that’s burning inside of me right now.”
SAN DIEGO – On the days family physician Nick Yphantides, MD, announces updates on the COVID-19 epidemic to San Diego County residents, he can’t help but think about his late father.
In June of 2009, 75-year-old George Yphantides, a Steinway-trained piano technician who lived in Escondido, Calif., became the third person in the United States to die from complications of the pandemic H1N1 swine flu – just days before a vaccine became available.
“I loved my dad,” Dr. Yphantides, who has been San Diego County’s Chief Medical Officer since the year of his father’s death, said in an interview. “So, when you take a step back and take into consideration my sense of purpose in serving the 3.3 million residents of San Diego County, my passion based on my personal Christian faith, and my activation in terms of what happened to my dad, I have such a storm of internal sense of urgency right now.”
San Diego County and public health officials got experience with COVID-19 in advance of the country’s widely documented cases of community-based transmission. Around 9 pm on Jan. 31, 2020 – the Friday of Super Bowl weekend – Dr. Yphantides answered a phone call from Eric C. McDonald, MD, the county’s medical director of epidemiology. Dr. McDonald informed him that in a few days, a plane full of American citizens traveling from Wuhan, China, would be landing at Marine Corp Air Station Miramar in San Diego for a 2-week quarantine and that the task of providing medical support to any affected individuals fell on county officials.
“I will never forget that phone call,” he said. “We did have two positive cases. What we experienced with those evacuees was amazing surge preparation, and without exaggeration, I have worked 18-20 hours a day since that day.”
Fast forward to March 31, 2020, the county’s confirmed COVID-19 caseload had grown to 734, up 131 from the day before. As of the final day of March, nine people have died, with an age range between 25 and 87 years. Of confirmed cases, 61% are between the ages of 20 and 49, 43% are female, 19% have required hospitalization, 7% have required admission to intensive care, and the mortality rate has been 1.2%. Data currently show optimal proactive hospital capacity.
In the opinion of Dr. Yphantides, the 734 COVID-19 cases represent a tip of the iceberg. “How big is that iceberg? I can’t tell you yet,” he said.
"I see this as the Super Bowl of public health,” he exclaimed.
At least some of Dr. Yphantides’ vigor seems to be fueled by his pride in his team of professionals who have been helping him respond to the surge of COVID-19 cases.
As the county’s CMO, Dr. Yphantides serves as the liaison for the entire Emergency Medical System, the entire local health care delivery system, the entire physician and medical society network, the payor system, and the proportion of the area population using Medi-Cal.
Dr. Yphantides, who attended medical school at the University of California, San Diego, said that, compared with other regions of the country, San Diego County has made “tremendous progress” in overcoming many chronic lifestyle illnesses. For example, cardiovascular disease is no longer the number one cause of death in the county; it’s bookended by cancer and Alzheimer’s disease.
“In the context of the COVID-19 response, [the county’s health care team established] an entire incident command system in our emergency operations center. Our emergency operations center is activated to the top level,” he said.
Dr. Yphantides shares public communication efforts with Dr. McDonald and Wilma J. Wooten, MD, the county’s public health officer. The San Diego County CMO also engages with policymakers, including the board of supervisors, local mayors, state legislators, and national legislators.
“Because of the relational trust capital that I have in this community, I get pulled into unexpected rooms of discussion,” he said. This included meeting with top executives from the San Diego Padres in early March, putting them on notice that the 2020 Major League Baseball season would likely be postponed. (This was officially announced on March 16.)
“We have made some decisions that have devastated some people economically. Talk about flipping the switch. We are living and making history every day. It is unbelievable,” he said.
“San Diego is a more aged population compared to many other parts of the country. ... [Part] of the reason why I’m so frantically doing everything I can to prepare, to batten down the hatches, and to optimize our health care delivery system is because we have a population that collectively is more at risk [for more serious complications from COVID-19]. A lot of what drives me is advocacy,” Dr. Yphantides noted.
A colleague’s perspective
Kristi L. Koenig, MD, medical director of emergency medical services for the County of San Diego, characterized Dr. Yphantides’ management style as collaborative. “Under his leadership, we have the perspective of ‘just focus on patient care, get it done, be creative, work together as a team,’ ” said Dr. Koenig, who coedited the textbook, “Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices” (Cambridge University Press, 2016). “He’s decisive and he’s responsive. You don’t have to wait a long time to get a decision, which is very important right now because this is so fast moving.”
Dr. Koenig, who has worked with Dr. Yphantides for 3 years, said that she routinely feeds him information that might help the team navigate its response to COVID-19. “For example, if I see an idea for how to get more [personal protective equipment] and feed it to him, he might have a contact somewhere in a factory that could make the PPE,” she said. “We work together by my reminding him to keep it within the incident command system structure, so that we can coordinate all the resources and not duplicate efforts.”
He uses his personal connections in a way to implement ideas that are beneficial to the overall goal of decreasing morbidity and mortality,” Dr. Koenig added.
Predictions for San Diego County
Dr. Yphantides said he considers San Diego to still be in the calm before the storm and that he is working hard to “board up his community.” The county CMO is also trying to prepare the health care delivery system to optimize its capacity, of doing interventions with hopes of lowering the curve and enhancing the capacity, he said.
When the storm hits, “it’s going be brutal, because we’re going to lose life,” Dr. Yphantides said.
“I am praying that maybe by some of our efforts, instead of a Category 5 storm, it’ll be a Category 3 storm,” he remarked.
The future of health care
Dr. Yphantides views the COVID-19 pandemic as “an absolute game-changer” in terms of what the future of health care delivery will look like in the United States. “Whether the right word is the ‘Amazonification’ of health care, or the ‘Uberization’ of health care, I don’t know, but the essence of how we deliver care is radically being transformed literally before our eyes,” he said. “I would encourage my colleagues to embrace that” and take care of their people by doing whatever it takes under this unprecedented paradigm.
Meanwhile, Dr. Yphantides braces for a potential surge of COVID-19 cases in San Diego County in the coming weeks. He honors the memory of his dad, and he expresses thanks for his mom, who cares for his two teenaged daughters while he helps steward the region’s response to the pandemic.
“Without my mom I could not function in the way that I’m currently functioning,” he said. “So, when you add all of those factors up, and wrap it with a bowtie of sincere love and passion for my community, there’s a fire that’s burning inside of me right now.”
SAN DIEGO – On the days family physician Nick Yphantides, MD, announces updates on the COVID-19 epidemic to San Diego County residents, he can’t help but think about his late father.
In June of 2009, 75-year-old George Yphantides, a Steinway-trained piano technician who lived in Escondido, Calif., became the third person in the United States to die from complications of the pandemic H1N1 swine flu – just days before a vaccine became available.
“I loved my dad,” Dr. Yphantides, who has been San Diego County’s Chief Medical Officer since the year of his father’s death, said in an interview. “So, when you take a step back and take into consideration my sense of purpose in serving the 3.3 million residents of San Diego County, my passion based on my personal Christian faith, and my activation in terms of what happened to my dad, I have such a storm of internal sense of urgency right now.”
San Diego County and public health officials got experience with COVID-19 in advance of the country’s widely documented cases of community-based transmission. Around 9 pm on Jan. 31, 2020 – the Friday of Super Bowl weekend – Dr. Yphantides answered a phone call from Eric C. McDonald, MD, the county’s medical director of epidemiology. Dr. McDonald informed him that in a few days, a plane full of American citizens traveling from Wuhan, China, would be landing at Marine Corp Air Station Miramar in San Diego for a 2-week quarantine and that the task of providing medical support to any affected individuals fell on county officials.
“I will never forget that phone call,” he said. “We did have two positive cases. What we experienced with those evacuees was amazing surge preparation, and without exaggeration, I have worked 18-20 hours a day since that day.”
Fast forward to March 31, 2020, the county’s confirmed COVID-19 caseload had grown to 734, up 131 from the day before. As of the final day of March, nine people have died, with an age range between 25 and 87 years. Of confirmed cases, 61% are between the ages of 20 and 49, 43% are female, 19% have required hospitalization, 7% have required admission to intensive care, and the mortality rate has been 1.2%. Data currently show optimal proactive hospital capacity.
In the opinion of Dr. Yphantides, the 734 COVID-19 cases represent a tip of the iceberg. “How big is that iceberg? I can’t tell you yet,” he said.
"I see this as the Super Bowl of public health,” he exclaimed.
At least some of Dr. Yphantides’ vigor seems to be fueled by his pride in his team of professionals who have been helping him respond to the surge of COVID-19 cases.
As the county’s CMO, Dr. Yphantides serves as the liaison for the entire Emergency Medical System, the entire local health care delivery system, the entire physician and medical society network, the payor system, and the proportion of the area population using Medi-Cal.
Dr. Yphantides, who attended medical school at the University of California, San Diego, said that, compared with other regions of the country, San Diego County has made “tremendous progress” in overcoming many chronic lifestyle illnesses. For example, cardiovascular disease is no longer the number one cause of death in the county; it’s bookended by cancer and Alzheimer’s disease.
“In the context of the COVID-19 response, [the county’s health care team established] an entire incident command system in our emergency operations center. Our emergency operations center is activated to the top level,” he said.
Dr. Yphantides shares public communication efforts with Dr. McDonald and Wilma J. Wooten, MD, the county’s public health officer. The San Diego County CMO also engages with policymakers, including the board of supervisors, local mayors, state legislators, and national legislators.
“Because of the relational trust capital that I have in this community, I get pulled into unexpected rooms of discussion,” he said. This included meeting with top executives from the San Diego Padres in early March, putting them on notice that the 2020 Major League Baseball season would likely be postponed. (This was officially announced on March 16.)
“We have made some decisions that have devastated some people economically. Talk about flipping the switch. We are living and making history every day. It is unbelievable,” he said.
“San Diego is a more aged population compared to many other parts of the country. ... [Part] of the reason why I’m so frantically doing everything I can to prepare, to batten down the hatches, and to optimize our health care delivery system is because we have a population that collectively is more at risk [for more serious complications from COVID-19]. A lot of what drives me is advocacy,” Dr. Yphantides noted.
A colleague’s perspective
Kristi L. Koenig, MD, medical director of emergency medical services for the County of San Diego, characterized Dr. Yphantides’ management style as collaborative. “Under his leadership, we have the perspective of ‘just focus on patient care, get it done, be creative, work together as a team,’ ” said Dr. Koenig, who coedited the textbook, “Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices” (Cambridge University Press, 2016). “He’s decisive and he’s responsive. You don’t have to wait a long time to get a decision, which is very important right now because this is so fast moving.”
Dr. Koenig, who has worked with Dr. Yphantides for 3 years, said that she routinely feeds him information that might help the team navigate its response to COVID-19. “For example, if I see an idea for how to get more [personal protective equipment] and feed it to him, he might have a contact somewhere in a factory that could make the PPE,” she said. “We work together by my reminding him to keep it within the incident command system structure, so that we can coordinate all the resources and not duplicate efforts.”
He uses his personal connections in a way to implement ideas that are beneficial to the overall goal of decreasing morbidity and mortality,” Dr. Koenig added.
Predictions for San Diego County
Dr. Yphantides said he considers San Diego to still be in the calm before the storm and that he is working hard to “board up his community.” The county CMO is also trying to prepare the health care delivery system to optimize its capacity, of doing interventions with hopes of lowering the curve and enhancing the capacity, he said.
When the storm hits, “it’s going be brutal, because we’re going to lose life,” Dr. Yphantides said.
“I am praying that maybe by some of our efforts, instead of a Category 5 storm, it’ll be a Category 3 storm,” he remarked.
The future of health care
Dr. Yphantides views the COVID-19 pandemic as “an absolute game-changer” in terms of what the future of health care delivery will look like in the United States. “Whether the right word is the ‘Amazonification’ of health care, or the ‘Uberization’ of health care, I don’t know, but the essence of how we deliver care is radically being transformed literally before our eyes,” he said. “I would encourage my colleagues to embrace that” and take care of their people by doing whatever it takes under this unprecedented paradigm.
Meanwhile, Dr. Yphantides braces for a potential surge of COVID-19 cases in San Diego County in the coming weeks. He honors the memory of his dad, and he expresses thanks for his mom, who cares for his two teenaged daughters while he helps steward the region’s response to the pandemic.
“Without my mom I could not function in the way that I’m currently functioning,” he said. “So, when you add all of those factors up, and wrap it with a bowtie of sincere love and passion for my community, there’s a fire that’s burning inside of me right now.”
Case fatality rate for COVID-19 near 1.4%, increases with age
The risk for death from COVID-19 is 1.38% overall, according to a new study. However, the fatality rate rises with age, from well below 1% among children aged 9 years or younger to nearly 8% for seniors aged 80 years or older, the latest statistics show.
“These early estimates give an indication of the fatality ratio across the spectrum of COVID-19 disease and show a strong age gradient in risk of death,” Robert Verity, PhD, of University College London, and colleagues, wrote in a study published online in the Lancet Infectious Diseases.
Among those infected with SARS-CoV-2, the virus that causes COVID-19, the risk for hospitalization also increases with age. Specifically, 11.8% of people in their 60s require admission, as do 16.6% of people in their 70s and 18.4% for those in their 80s or older.
The case fatality estimates are based on data regarding individual patients who died from COVID-19 in Hubei, China, through Feb. 8, as well as those who died in Hong Kong, Macau, and 37 countries outside China through Feb. 25.
“It is clear from the data that have emerged from China that case fatality ratio increases substantially with age. Our results suggest a very low fatality ratio in those under the age of 20 years. As there are very few cases in this age group, it remains unclear whether this reflects a low risk of death or a difference in susceptibility, although early results indicate young people are not at lower risk of infection than adults,” Dr. Verity and colleagues wrote.
The authors emphasized that serologic testing of adolescents and children will be vital to understanding how individuals younger than 20 years may be driving viral transmission.
In an accompanying editorial Shigui Ruan, PhD, of the department of mathematics at the University of Miami in Coral Gables, Fla., wrote that early detection, diagnosis, isolation, and treatment, as practiced in China, may help to prevent more deaths
“Even though the fatality rate is low for younger people, it is very clear that any suggestion of COVID-19 being just like influenza is false: Even for those aged 20-29 years, once infected with SARS-CoV-2, the mortality rate is 33 times higher than that from seasonal influenza,” he noted.
Dr. Ruan, who uses applied mathematics to model disease transmission, noted that otherwise healthy people stand a good chance – approximately 95% – of surviving COVID-19, but the odds of survival for people with comorbidities will be “considerably decreased.”
Time to death or discharge
Dr. Verity and colleagues first used data on deaths of 24 patients in mainland China and on 165 persons who recovered from infection outside of China to estimate the time between onset of symptoms and either death or discharge from the hospital. They estimated that the mean duration from symptom onset to death is 17.8 days, and the mean duration to discharge is 24.7 days.
They then estimated age-stratified case fatality ratios among all clinically diagnosed and laboratory-confirmed cases in mainland China to the end of the study period (70,117 cases).
The estimated crude case fatality ratio, adjusted for censoring, was 3.67%. With further adjustment for demographic characteristics and under-ascertainment, the authors’ best estimate of a case fatality ratio in China is 1.38%.
The following figure shows adjusted fatality infection rates by age group.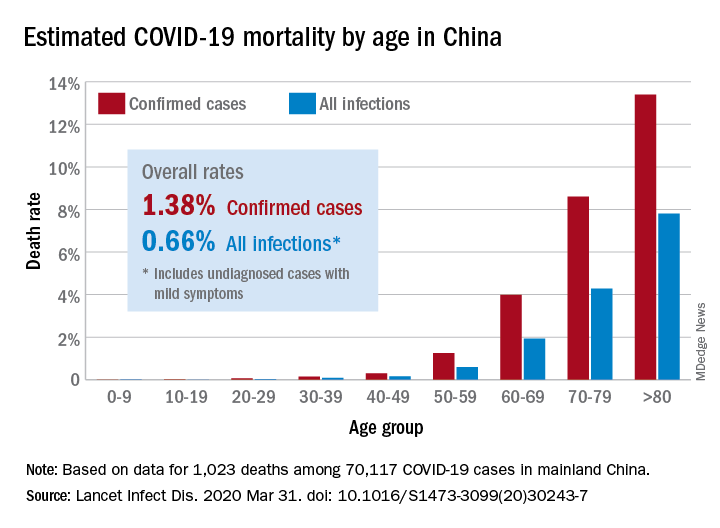
The investigators noted that the case fatality estimate is lower than the estimates for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) outbreaks, both caused by coronaviruses, but “is substantially higher than estimates from the 2009 H1N1 influenza pandemic.”
Earlier reports suggested that the overall fatality rate in China through Feb. 11 was 2.3%. The rate in Hubei province, which is believed to be where the infection started, was 2.9%.
Hospitalizations rise with age
The investigators also estimated the proportion of infected patients who require hospitalization. Their estimation was based on data from a subset of cases reported in mainland China. The hospitalization estimates range from zero among the youngest patients to 18% among the oldest.
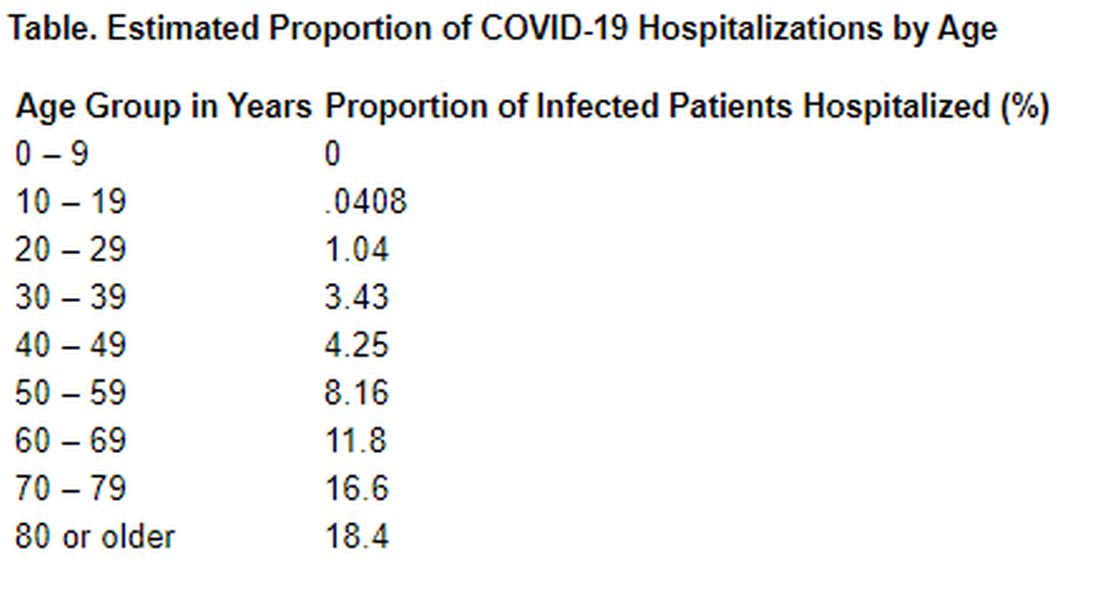
“Although China has succeeded in containing the disease spread for 2 months, such containment is unlikely to be achievable in most countries. Thus, much of the world will experience very large community epidemics of COVID-19 over the coming weeks and months. Our estimates of the underlying infection fatality ratio of this virus will inform assessments of health effects likely to be experienced in different countries, and thus decisions around appropriate mitigation policies to be adopted,” Dr. Verity and colleagues concluded.
In his editorial, Dr. Ruan agreed with that assessment. “Although China seems to be out of the woods now, many other countries are facing tremendous pressure from the COVID-19 pandemic,” he wrote. “The strategies of early detection, early diagnosis, early isolation, and early treatment that were practiced in China are likely to be not only useful in controlling the outbreak but also contribute to decreasing the case fatality ratio of the disease.”
The study was supported by the UK Medical Research Council. Dr. Verity and Dr. Ruan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for death from COVID-19 is 1.38% overall, according to a new study. However, the fatality rate rises with age, from well below 1% among children aged 9 years or younger to nearly 8% for seniors aged 80 years or older, the latest statistics show.
“These early estimates give an indication of the fatality ratio across the spectrum of COVID-19 disease and show a strong age gradient in risk of death,” Robert Verity, PhD, of University College London, and colleagues, wrote in a study published online in the Lancet Infectious Diseases.
Among those infected with SARS-CoV-2, the virus that causes COVID-19, the risk for hospitalization also increases with age. Specifically, 11.8% of people in their 60s require admission, as do 16.6% of people in their 70s and 18.4% for those in their 80s or older.
The case fatality estimates are based on data regarding individual patients who died from COVID-19 in Hubei, China, through Feb. 8, as well as those who died in Hong Kong, Macau, and 37 countries outside China through Feb. 25.
“It is clear from the data that have emerged from China that case fatality ratio increases substantially with age. Our results suggest a very low fatality ratio in those under the age of 20 years. As there are very few cases in this age group, it remains unclear whether this reflects a low risk of death or a difference in susceptibility, although early results indicate young people are not at lower risk of infection than adults,” Dr. Verity and colleagues wrote.
The authors emphasized that serologic testing of adolescents and children will be vital to understanding how individuals younger than 20 years may be driving viral transmission.
In an accompanying editorial Shigui Ruan, PhD, of the department of mathematics at the University of Miami in Coral Gables, Fla., wrote that early detection, diagnosis, isolation, and treatment, as practiced in China, may help to prevent more deaths
“Even though the fatality rate is low for younger people, it is very clear that any suggestion of COVID-19 being just like influenza is false: Even for those aged 20-29 years, once infected with SARS-CoV-2, the mortality rate is 33 times higher than that from seasonal influenza,” he noted.
Dr. Ruan, who uses applied mathematics to model disease transmission, noted that otherwise healthy people stand a good chance – approximately 95% – of surviving COVID-19, but the odds of survival for people with comorbidities will be “considerably decreased.”
Time to death or discharge
Dr. Verity and colleagues first used data on deaths of 24 patients in mainland China and on 165 persons who recovered from infection outside of China to estimate the time between onset of symptoms and either death or discharge from the hospital. They estimated that the mean duration from symptom onset to death is 17.8 days, and the mean duration to discharge is 24.7 days.
They then estimated age-stratified case fatality ratios among all clinically diagnosed and laboratory-confirmed cases in mainland China to the end of the study period (70,117 cases).
The estimated crude case fatality ratio, adjusted for censoring, was 3.67%. With further adjustment for demographic characteristics and under-ascertainment, the authors’ best estimate of a case fatality ratio in China is 1.38%.
The following figure shows adjusted fatality infection rates by age group.
The investigators noted that the case fatality estimate is lower than the estimates for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) outbreaks, both caused by coronaviruses, but “is substantially higher than estimates from the 2009 H1N1 influenza pandemic.”
Earlier reports suggested that the overall fatality rate in China through Feb. 11 was 2.3%. The rate in Hubei province, which is believed to be where the infection started, was 2.9%.
Hospitalizations rise with age
The investigators also estimated the proportion of infected patients who require hospitalization. Their estimation was based on data from a subset of cases reported in mainland China. The hospitalization estimates range from zero among the youngest patients to 18% among the oldest.

“Although China has succeeded in containing the disease spread for 2 months, such containment is unlikely to be achievable in most countries. Thus, much of the world will experience very large community epidemics of COVID-19 over the coming weeks and months. Our estimates of the underlying infection fatality ratio of this virus will inform assessments of health effects likely to be experienced in different countries, and thus decisions around appropriate mitigation policies to be adopted,” Dr. Verity and colleagues concluded.
In his editorial, Dr. Ruan agreed with that assessment. “Although China seems to be out of the woods now, many other countries are facing tremendous pressure from the COVID-19 pandemic,” he wrote. “The strategies of early detection, early diagnosis, early isolation, and early treatment that were practiced in China are likely to be not only useful in controlling the outbreak but also contribute to decreasing the case fatality ratio of the disease.”
The study was supported by the UK Medical Research Council. Dr. Verity and Dr. Ruan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for death from COVID-19 is 1.38% overall, according to a new study. However, the fatality rate rises with age, from well below 1% among children aged 9 years or younger to nearly 8% for seniors aged 80 years or older, the latest statistics show.
“These early estimates give an indication of the fatality ratio across the spectrum of COVID-19 disease and show a strong age gradient in risk of death,” Robert Verity, PhD, of University College London, and colleagues, wrote in a study published online in the Lancet Infectious Diseases.
Among those infected with SARS-CoV-2, the virus that causes COVID-19, the risk for hospitalization also increases with age. Specifically, 11.8% of people in their 60s require admission, as do 16.6% of people in their 70s and 18.4% for those in their 80s or older.
The case fatality estimates are based on data regarding individual patients who died from COVID-19 in Hubei, China, through Feb. 8, as well as those who died in Hong Kong, Macau, and 37 countries outside China through Feb. 25.
“It is clear from the data that have emerged from China that case fatality ratio increases substantially with age. Our results suggest a very low fatality ratio in those under the age of 20 years. As there are very few cases in this age group, it remains unclear whether this reflects a low risk of death or a difference in susceptibility, although early results indicate young people are not at lower risk of infection than adults,” Dr. Verity and colleagues wrote.
The authors emphasized that serologic testing of adolescents and children will be vital to understanding how individuals younger than 20 years may be driving viral transmission.
In an accompanying editorial Shigui Ruan, PhD, of the department of mathematics at the University of Miami in Coral Gables, Fla., wrote that early detection, diagnosis, isolation, and treatment, as practiced in China, may help to prevent more deaths
“Even though the fatality rate is low for younger people, it is very clear that any suggestion of COVID-19 being just like influenza is false: Even for those aged 20-29 years, once infected with SARS-CoV-2, the mortality rate is 33 times higher than that from seasonal influenza,” he noted.
Dr. Ruan, who uses applied mathematics to model disease transmission, noted that otherwise healthy people stand a good chance – approximately 95% – of surviving COVID-19, but the odds of survival for people with comorbidities will be “considerably decreased.”
Time to death or discharge
Dr. Verity and colleagues first used data on deaths of 24 patients in mainland China and on 165 persons who recovered from infection outside of China to estimate the time between onset of symptoms and either death or discharge from the hospital. They estimated that the mean duration from symptom onset to death is 17.8 days, and the mean duration to discharge is 24.7 days.
They then estimated age-stratified case fatality ratios among all clinically diagnosed and laboratory-confirmed cases in mainland China to the end of the study period (70,117 cases).
The estimated crude case fatality ratio, adjusted for censoring, was 3.67%. With further adjustment for demographic characteristics and under-ascertainment, the authors’ best estimate of a case fatality ratio in China is 1.38%.
The following figure shows adjusted fatality infection rates by age group.
The investigators noted that the case fatality estimate is lower than the estimates for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) outbreaks, both caused by coronaviruses, but “is substantially higher than estimates from the 2009 H1N1 influenza pandemic.”
Earlier reports suggested that the overall fatality rate in China through Feb. 11 was 2.3%. The rate in Hubei province, which is believed to be where the infection started, was 2.9%.
Hospitalizations rise with age
The investigators also estimated the proportion of infected patients who require hospitalization. Their estimation was based on data from a subset of cases reported in mainland China. The hospitalization estimates range from zero among the youngest patients to 18% among the oldest.

“Although China has succeeded in containing the disease spread for 2 months, such containment is unlikely to be achievable in most countries. Thus, much of the world will experience very large community epidemics of COVID-19 over the coming weeks and months. Our estimates of the underlying infection fatality ratio of this virus will inform assessments of health effects likely to be experienced in different countries, and thus decisions around appropriate mitigation policies to be adopted,” Dr. Verity and colleagues concluded.
In his editorial, Dr. Ruan agreed with that assessment. “Although China seems to be out of the woods now, many other countries are facing tremendous pressure from the COVID-19 pandemic,” he wrote. “The strategies of early detection, early diagnosis, early isolation, and early treatment that were practiced in China are likely to be not only useful in controlling the outbreak but also contribute to decreasing the case fatality ratio of the disease.”
The study was supported by the UK Medical Research Council. Dr. Verity and Dr. Ruan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19: Adjusting practice in acute leukemia care
The SARS-CoV-2 pandemic poses significant risks to leukemia patients and their providers, impacting every aspect of care from diagnosis through therapy, according to an editorial letter published online in Leukemia Research.
One key concern to be considered is the risk of missed or delayed diagnosis due to the pandemic conditions. An estimated 50%-75% of patients with acute leukemia are febrile at diagnosis and this puts them at high risk of a misdiagnosis of COVID-19 upon initial evaluation. As with other oncological conditions (primary mediastinal lymphoma or lung cancer, for example), which often present with a cough with or without fever, their symptoms “are likely to be considered trivial after a negative SARS-CoV-2 test,” with patients then being sent home without further assessment. In a rapidly progressing disease such as acute leukemia, this could lead to critical delays in therapeutic intervention.
The authors, from the Service and Central Laboratory of Hematology, Lausanne (Switzerland) University Hospital, also discussed the problems that might occur with regard to most standard forms of therapy. In particular, they addressed potential impacts of the pandemic on chemotherapy, bone marrow transplantation, maintenance treatments, supportive measures, and targeted therapies.
Of particular concern, “most patients may suffer from postponed chemotherapy, due to a shortage of isolation beds and blood products or the wish to avoid immunosuppressive treatments,” the authors noted, warning that “delay in chemotherapy initiation may negatively affect prognosis, [particularly in patients under age 60] with favorable- or intermediate-risk disease.”
With regard to stem cell transplantation, the authors detail the many potential difficulties with regard to procedures involving both donors and recipients, and warn that in some cases, delay in transplant could result in the reappearance of a significant minimal residual disease, which has a well-established negative impact on survival.
The authors also noted that blood product shortages have already begun in most affected countries, and how, in response, transfusion societies have called for conservative transfusion policies in strict adherence to evidence-based guidelines for patient’s blood management.
“COVID-19 will result in numerous casualties. Acute leukemia patients are at a higher risk of severe complications,” the authors stated. In particular, physicians should especially be aware of how treatment for acute leukemia may have “interactions with other drugs used to treat SARS-CoV-2–related infections/complications such as antibiotics, antiviral drugs, and various other drugs that prolong QTc or impact targeted-therapy pharmacokinetics,” they concluded.
The authors reported that they received no government or private funding for this research, and that they had no conflicts of interest.
SOURCE: Gavillet M et al. Leuk. Res. 2020. doi.org/10.1016/j.leukres.2020.106353.
The SARS-CoV-2 pandemic poses significant risks to leukemia patients and their providers, impacting every aspect of care from diagnosis through therapy, according to an editorial letter published online in Leukemia Research.
One key concern to be considered is the risk of missed or delayed diagnosis due to the pandemic conditions. An estimated 50%-75% of patients with acute leukemia are febrile at diagnosis and this puts them at high risk of a misdiagnosis of COVID-19 upon initial evaluation. As with other oncological conditions (primary mediastinal lymphoma or lung cancer, for example), which often present with a cough with or without fever, their symptoms “are likely to be considered trivial after a negative SARS-CoV-2 test,” with patients then being sent home without further assessment. In a rapidly progressing disease such as acute leukemia, this could lead to critical delays in therapeutic intervention.
The authors, from the Service and Central Laboratory of Hematology, Lausanne (Switzerland) University Hospital, also discussed the problems that might occur with regard to most standard forms of therapy. In particular, they addressed potential impacts of the pandemic on chemotherapy, bone marrow transplantation, maintenance treatments, supportive measures, and targeted therapies.
Of particular concern, “most patients may suffer from postponed chemotherapy, due to a shortage of isolation beds and blood products or the wish to avoid immunosuppressive treatments,” the authors noted, warning that “delay in chemotherapy initiation may negatively affect prognosis, [particularly in patients under age 60] with favorable- or intermediate-risk disease.”
With regard to stem cell transplantation, the authors detail the many potential difficulties with regard to procedures involving both donors and recipients, and warn that in some cases, delay in transplant could result in the reappearance of a significant minimal residual disease, which has a well-established negative impact on survival.
The authors also noted that blood product shortages have already begun in most affected countries, and how, in response, transfusion societies have called for conservative transfusion policies in strict adherence to evidence-based guidelines for patient’s blood management.
“COVID-19 will result in numerous casualties. Acute leukemia patients are at a higher risk of severe complications,” the authors stated. In particular, physicians should especially be aware of how treatment for acute leukemia may have “interactions with other drugs used to treat SARS-CoV-2–related infections/complications such as antibiotics, antiviral drugs, and various other drugs that prolong QTc or impact targeted-therapy pharmacokinetics,” they concluded.
The authors reported that they received no government or private funding for this research, and that they had no conflicts of interest.
SOURCE: Gavillet M et al. Leuk. Res. 2020. doi.org/10.1016/j.leukres.2020.106353.
The SARS-CoV-2 pandemic poses significant risks to leukemia patients and their providers, impacting every aspect of care from diagnosis through therapy, according to an editorial letter published online in Leukemia Research.
One key concern to be considered is the risk of missed or delayed diagnosis due to the pandemic conditions. An estimated 50%-75% of patients with acute leukemia are febrile at diagnosis and this puts them at high risk of a misdiagnosis of COVID-19 upon initial evaluation. As with other oncological conditions (primary mediastinal lymphoma or lung cancer, for example), which often present with a cough with or without fever, their symptoms “are likely to be considered trivial after a negative SARS-CoV-2 test,” with patients then being sent home without further assessment. In a rapidly progressing disease such as acute leukemia, this could lead to critical delays in therapeutic intervention.
The authors, from the Service and Central Laboratory of Hematology, Lausanne (Switzerland) University Hospital, also discussed the problems that might occur with regard to most standard forms of therapy. In particular, they addressed potential impacts of the pandemic on chemotherapy, bone marrow transplantation, maintenance treatments, supportive measures, and targeted therapies.
Of particular concern, “most patients may suffer from postponed chemotherapy, due to a shortage of isolation beds and blood products or the wish to avoid immunosuppressive treatments,” the authors noted, warning that “delay in chemotherapy initiation may negatively affect prognosis, [particularly in patients under age 60] with favorable- or intermediate-risk disease.”
With regard to stem cell transplantation, the authors detail the many potential difficulties with regard to procedures involving both donors and recipients, and warn that in some cases, delay in transplant could result in the reappearance of a significant minimal residual disease, which has a well-established negative impact on survival.
The authors also noted that blood product shortages have already begun in most affected countries, and how, in response, transfusion societies have called for conservative transfusion policies in strict adherence to evidence-based guidelines for patient’s blood management.
“COVID-19 will result in numerous casualties. Acute leukemia patients are at a higher risk of severe complications,” the authors stated. In particular, physicians should especially be aware of how treatment for acute leukemia may have “interactions with other drugs used to treat SARS-CoV-2–related infections/complications such as antibiotics, antiviral drugs, and various other drugs that prolong QTc or impact targeted-therapy pharmacokinetics,” they concluded.
The authors reported that they received no government or private funding for this research, and that they had no conflicts of interest.
SOURCE: Gavillet M et al. Leuk. Res. 2020. doi.org/10.1016/j.leukres.2020.106353.
FROM LEUKEMIA RESEARCH
AASLD: Liver transplants should proceed despite COVID-19
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
In liver transplant recipients or patients with autoimmune hepatitis on immunosuppressive therapy, acute cellular rejection or disease flare should not be presumed in the face of active coronavirus disease 2019 (COVID-19), according to the American Association for the Study of Liver Diseases (AASLD).
Signs that would normally be interpreted as flare or rejection need to be considered more cautiously now because the virus attacks the liver, and elevated aspartate aminotransferase, alanine aminotransferase, and slightly elevated bilirubin are common, ranging from a prevalence of 14% to 53% in COVID-19 patients. Acute liver injury is possible, especially in more severe cases, the group said.
The advice comes from a recently released document from AASLD, called “Clinical Insights for Hepatology and Liver Transplant Providers During the Covid-19 Pandemic,” to help hepatologists and liver transplant providers negotiate the pandemic, according to the latest data. It’s a far-ranging work that contains a lot of now familiar steps for providers to take to protect themselves and patients from the virus, but also much advice specific to liver medicine.
For instance, the group said it’s important to keep in mind that experimental treatments for the infection, including statins, remdesivir, and tocilizumab, can be hepatotoxic. Abnormal liver biochemistries are not a contraindication, but liver biochemistries need to be followed regularly in COVID-19 patients, especially those treated with remdesivir or tocilizumab, regardless of baseline values.
Also, lopinavir/ritonavir is a potent inhibitor of cytochrome P450 enzymes involved with calcineurin inhibitor metabolism, so if it’s used, AASLD said to reduce tacrolimus dosages to 1/20–1/50 of baseline.
The group cautioned against anticipatory adjustments to immunosuppressive drugs or dosages in patients without COVID-19, but if immunosuppressed liver disease patients do get the infection, prednisone doses should be reduced but kept above 10 mg/day to avoid adrenal insufficiency. In the setting of lymphopenia, fever, or worsening COVID-19 pneumonia, it advised reduction of azathioprine and mycophenolate dosages and reduction of, but not stopping, calcineurin inhibitors.
Liver transplants should not be postponed. However, to minimize exposure to the hospital environment, AASLD advised to “consider evaluating only patients with HCC [hepatocellular carcinoma] or those patients with severe disease and high MELD [model for end-stage liver disease] scores who are likely to benefit from immediate liver transplant.”
“An argument that has been put forward to justify deferring some transplants is concern about immunosuppressing patients during the COVID-19 pandemic,” the group said, but “data suggest the innate immune response may be the main driver for pulmonary injury due to COVID-19 and [that] immunosuppression may be protective. ... Posttransplant immunosuppression was not a risk factor for mortality associated with” the severe acute respiratory syndrome pandemic in 2003-2004 or the ongoing Middle East respiratory syndrome pandemic, both also caused by coronaviruses.
AASLD advised against reducing immunosuppression or stopping mycophenolate for asymptomatic patients after transplant, but COVID-19 prevention measures should be emphasized, including frequent hand washing and staying away from large crowds.
People who test positive for COVID-19 are ineligible for organ donation. Bronchoalveolar lavage is the most sensitive test (93%), followed by nasal swabs (63%) and pharyngeal swabs (32%).
In general, the group said elective procedures should be postponed, but urgent ones, such as biliary surgery and transjugular intrahepatic portosystemic shunts for bleeding varices, in addition to liver transplants, should not.
Also, HCC patients “should not wait until the pandemic abates to undergo [surveillance] imaging because the prospective duration of the pandemic is unknown. ... An arbitrary delay of 2 months is reasonable” for imaging based on patient and facility circumstances, but otherwise, “proceed with HCC treatments rather than delaying them due to the pandemic,” the group said.
As for who to bring into the office for an initial consult, “consider seeing in person only new adult and pediatric patients with urgent issues and clinically significant liver disease (e.g., jaundice, elevated ALT or AST above 500 U/L, recent onset of hepatic decompensation),” AASLD said.
Patients with preexisting diabetes benefit less from bariatric surgery
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
according to a retrospective review of patients receiving both sleeve gastrectomy and gastric bypass.
The difference was particularly pronounced and persistent for patients who had gastric bypass, Yingying Luo, MD, said during a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“Our findings demonstrated that having bariatric surgery before developing diabetes may result in greater weight loss from the surgery, especially within the first 3 years after surgery and in patients undergoing gastric bypass surgery,” said Dr. Luo.
More than a third of U.S. adults have obesity, and more than half the population is overweight or has obesity, said Dr. Luo, citing data from the Centers for Disease Control and Prevention.
Bariatric surgery not only reduces body weight, but also “can lead to remission of many metabolic disorders, including diabetes, hypertension, and dyslipidemia,” said Dr. Luo, a visiting scholar at the University of Michigan’s division of metabolism, endocrinology, and diabetes. However, until now, it has not been known how diabetes interacts with bariatric surgery to affect weight loss outcomes.
To address that question, Dr. Luo and her colleagues looked at patients in the Michigan Bariatric Surgery Cohort who were at least 18 years old and had a body mass index (BMI) of more than 40 kg/m2, or of more than 35 kg/m2 with comorbidities.
The researchers followed 380 patients who received gastric bypass and 334 who received sleeve gastrectomy for at least 5 years. Over time, sleeve gastrectomy became the predominant type of surgery conducted, noted Dr. Luo.
At baseline, and yearly for 5 years thereafter, the researchers recorded participants’ BMI as well as their lipid levels and other laboratory values. Medication use was also tracked. Patients with a diagnosis of diabetes also had their hemoglobin A1c levels recorded at each visit.
Overall, patients in the sleeve gastrectomy group were more overweight, and those in the gastric bypass group had higher HbA1c and total cholesterol levels. The mean baseline weight for the sleeve gastrectomy recipients was 141.5 kg, compared with 133.5 kg for those receiving gastric bypass (BMI, 49.9 vs. 47.3 kg/m2, respectively; P < .01 for both measures). Mean HbA1c was 6.5% for the gastric bypass group, compared with 6.3% for the sleeve gastrectomy group (P = .03).
At baseline, 149 (39.2%) of the gastric bypass patients had diabetes, compared with 108 (32.3%) of the sleeve gastrectomy patients, but the difference was not statistically significant.
About two-thirds of the full cohort were tracked for at least 5 years, which is still considered “a good follow-up rate in a real-world study,” said Dr. Luo.
Total weight loss was defined as the difference between initial weight and postoperative weight at a given point in time. Excess weight was the difference between initial weight and an individual’s ideal weight, that is, what their weight would have been if they had a BMI of 25 kg/m2.
“The probability of achieving a BMI of less than 30 kg/m2 or excess weight loss of 50% or more was higher in patients who did not have diabetes diagnosis at baseline. We found that the presence of diabetes at baseline substantially impacted the probability of achieving both indicators,” said Dr. Luo. “Individuals without diabetes had a 1.5 times higher chance of achieving a BMI of under 30 kg/m2, and … [they also] had a 1.6 times higher chance of achieving excess body weight loss of 50%, or more.” Both of those differences were statistically significant on univariate analysis (P = .0249 and .0021, respectively).
The researchers conducted further statistical analysis – adjusted for age, gender, surgery type, and baseline weight – to examine whether diabetes still predicted future weight loss after bariatric surgery. After those adjustments, they still found that “the presence of diabetes before surgery is an indicator of future weight loss outcomes,” said Dr. Luo.
The differences in outcomes for those with and without diabetes tended to diminish over time in looking at the cohort as a whole. However, greater BMI reduction for those without diabetes persisted for the full 5 years of follow-up for the gastric bypass recipients. Those trends held when the researchers looked at the proportion of patients whose BMI dropped to below 30 kg/m2, and those who achieved excess weight loss of more than 50%.
Dr. Luo acknowledged that an ideal study would track patients for longer than 5 years and that studies involving more patients would also be useful. Still, she said, “our study opens the door for further research to understand why diabetes diminishes the weight loss effect of bariatric surgery.”
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
Dr. Luo reported no outside sources of funding and no conflicts of interest.
SOURCE: Luo Y et al. ENDO 2020, Abstract 590.
FROM ENDO 2020
Maintaining cancer care in the face of COVID-19
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.