User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Vast underdiagnosis of monogenic CV disease seen in cath referrals
Monogenic disorders with heart and vascular effects are each pretty rare in clinical practice but collectively can make up a fair proportion of the patients cardiologists see. Still, the diagnosis is missed more often than not, even when the clinical signs are there, suggests an observational study, supporting broader genetic testing in cardiovascular patients.
In a cohort of more than 8,000 patients referred for cardiac catheterization, diagnosis of such a monogenic cardiovascular disease (MCVD) was made in only 35% of those with one related gene variant and signs of phenotypic expression in the electronic health record.
The findings are novel for measuring the field’s “burden of missed diagnoses” in patients with MCVD, which “represent a missed opportunity that could be addressed by genetic screening,” contended the study report, published in the Aug. 18 issue of the Journal of the American College of Cardiology.
“The underrecognition of these diseases underscores the importance of including monogenic diseases in the treating physician’s differential diagnosis,” say the authors, led by Jawan W. Abdulrahim, MD, Duke University, Durham, N.C.
Diagnosis of MCVDs can be important, the group wrote, because many, including familial transthyretin amyloidosis (TTR) and other disorders that pose an increased risk for sudden death, have evidence-based treatment modalities available or are clinically actionable. “Identification of patients with MCVD variants” is also “important for cascade screening of family members who are at risk of inheriting the pathogenic mutations.”
“We tend to ignore these monogenic diseases because they are so rare individually but, in aggregate, monogenic diseases are actually quite common,” senior author Svati H. Shah, MD, MHS, also of Duke University, said in an interview.
The results “support that the cardiology community over time adopt a genotype-forward approach,” one in which every patient presenting to a cardiovascular clinic is genotyped, she said.
One implication of such an approach, Dr. Shah agreed, is that “we would be able to pick these people up earlier in their disease, especially in the context of therapies that could improve certainly their progression, but even their survival.”
For now, she said, the study suggests that “these disorders are more frequent than perhaps all cardiologists are aware of, and we just need to keep our eyes open and know when to refer patients to a cardiovascular genetics clinic, which maybe has more time and can deal with all the nuances that go along with genetic testing.”
In the total cohort, 4.5% were found to carry a gene variant known or believed to cause such diseases. The most frequently represented conditions were familial TTR, hereditary hemochromatosis, heterozygous familial hypercholesterolemia, and various cardiomyopathies.
Of those patients, 52 received a clinical diagnosis of the monogenic disorder after an EHR review. Of the 290 without such a diagnosis, two-thirds showed no evidence in their EHR of the variant’s phenotypic signs. But the records of the other third featured at least some signs that the disease had manifested clinically.
“These data serve as a reminder that monogenic Mendelian disease, including heart and vascular disease, varies in penetrance from individual to individual and may not always present with clinically detectable phenotypes,” noted an editorial accompanying the report.
They also “provide a compelling basis for expanding the role of targeted genetic testing in patients with more traditional forms of heart and vascular disease,” wrote Scott M. Damrauer, MD, University of Pennsylvania, Philadelphia, and William S. Weintraub, MD, Medstar Washington Hospital Center and Georgetown University, Washington.
“Based on the current report, the number needed to screen in a complex cardiovascular patient population to detect 1 case of undiagnosed monogenic cardiovascular disease is 85,” they wrote. “This places targeted genetic testing well within what is considered to be efficacious for most screening programs and in the range of that of other common cardiovascular diseases and cancers.”
Among the 342 patients with a variant predicting a single MCVD – in addition to the 52 who received a diagnosis – 193 had records with no indication of phenotypic expression and so did not receive a diagnosis.
But the 97 patients without a diagnosis who nevertheless had documented signs of some phenotypic expression were deemed, on the basis of extent of expression, to represent a possibly, probably, or definitely missed diagnosis.
Familial TTR made up about 45% of such potentially missed diagnoses, the report noted.
Broader screening of patients with cardiovascular disease, Dr. Shah speculated, “might actually be not only a clinically useful endeavor, but – when we think about the aggregate burden of these monogenic disorders – potentially even cost-effective.”
As the price of genetic sequencing drops, she said, “I think we’ll start to see even more health systems wanting to incorporate the genotype-forward approach.”
Dr. Shah reports serving as primary investigator for research sponsored by Verily Life Sciences and AstraZeneca. Dr. Abdulrahim reports no relevant relationships. Disclosures for the other authors are in the report. Dr. Damrauer discloses receiving research support from RenalytixAI and consulting fees from Calico Labs. Dr. Weintraub had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Monogenic disorders with heart and vascular effects are each pretty rare in clinical practice but collectively can make up a fair proportion of the patients cardiologists see. Still, the diagnosis is missed more often than not, even when the clinical signs are there, suggests an observational study, supporting broader genetic testing in cardiovascular patients.
In a cohort of more than 8,000 patients referred for cardiac catheterization, diagnosis of such a monogenic cardiovascular disease (MCVD) was made in only 35% of those with one related gene variant and signs of phenotypic expression in the electronic health record.
The findings are novel for measuring the field’s “burden of missed diagnoses” in patients with MCVD, which “represent a missed opportunity that could be addressed by genetic screening,” contended the study report, published in the Aug. 18 issue of the Journal of the American College of Cardiology.
“The underrecognition of these diseases underscores the importance of including monogenic diseases in the treating physician’s differential diagnosis,” say the authors, led by Jawan W. Abdulrahim, MD, Duke University, Durham, N.C.
Diagnosis of MCVDs can be important, the group wrote, because many, including familial transthyretin amyloidosis (TTR) and other disorders that pose an increased risk for sudden death, have evidence-based treatment modalities available or are clinically actionable. “Identification of patients with MCVD variants” is also “important for cascade screening of family members who are at risk of inheriting the pathogenic mutations.”
“We tend to ignore these monogenic diseases because they are so rare individually but, in aggregate, monogenic diseases are actually quite common,” senior author Svati H. Shah, MD, MHS, also of Duke University, said in an interview.
The results “support that the cardiology community over time adopt a genotype-forward approach,” one in which every patient presenting to a cardiovascular clinic is genotyped, she said.
One implication of such an approach, Dr. Shah agreed, is that “we would be able to pick these people up earlier in their disease, especially in the context of therapies that could improve certainly their progression, but even their survival.”
For now, she said, the study suggests that “these disorders are more frequent than perhaps all cardiologists are aware of, and we just need to keep our eyes open and know when to refer patients to a cardiovascular genetics clinic, which maybe has more time and can deal with all the nuances that go along with genetic testing.”
In the total cohort, 4.5% were found to carry a gene variant known or believed to cause such diseases. The most frequently represented conditions were familial TTR, hereditary hemochromatosis, heterozygous familial hypercholesterolemia, and various cardiomyopathies.
Of those patients, 52 received a clinical diagnosis of the monogenic disorder after an EHR review. Of the 290 without such a diagnosis, two-thirds showed no evidence in their EHR of the variant’s phenotypic signs. But the records of the other third featured at least some signs that the disease had manifested clinically.
“These data serve as a reminder that monogenic Mendelian disease, including heart and vascular disease, varies in penetrance from individual to individual and may not always present with clinically detectable phenotypes,” noted an editorial accompanying the report.
They also “provide a compelling basis for expanding the role of targeted genetic testing in patients with more traditional forms of heart and vascular disease,” wrote Scott M. Damrauer, MD, University of Pennsylvania, Philadelphia, and William S. Weintraub, MD, Medstar Washington Hospital Center and Georgetown University, Washington.
“Based on the current report, the number needed to screen in a complex cardiovascular patient population to detect 1 case of undiagnosed monogenic cardiovascular disease is 85,” they wrote. “This places targeted genetic testing well within what is considered to be efficacious for most screening programs and in the range of that of other common cardiovascular diseases and cancers.”
Among the 342 patients with a variant predicting a single MCVD – in addition to the 52 who received a diagnosis – 193 had records with no indication of phenotypic expression and so did not receive a diagnosis.
But the 97 patients without a diagnosis who nevertheless had documented signs of some phenotypic expression were deemed, on the basis of extent of expression, to represent a possibly, probably, or definitely missed diagnosis.
Familial TTR made up about 45% of such potentially missed diagnoses, the report noted.
Broader screening of patients with cardiovascular disease, Dr. Shah speculated, “might actually be not only a clinically useful endeavor, but – when we think about the aggregate burden of these monogenic disorders – potentially even cost-effective.”
As the price of genetic sequencing drops, she said, “I think we’ll start to see even more health systems wanting to incorporate the genotype-forward approach.”
Dr. Shah reports serving as primary investigator for research sponsored by Verily Life Sciences and AstraZeneca. Dr. Abdulrahim reports no relevant relationships. Disclosures for the other authors are in the report. Dr. Damrauer discloses receiving research support from RenalytixAI and consulting fees from Calico Labs. Dr. Weintraub had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Monogenic disorders with heart and vascular effects are each pretty rare in clinical practice but collectively can make up a fair proportion of the patients cardiologists see. Still, the diagnosis is missed more often than not, even when the clinical signs are there, suggests an observational study, supporting broader genetic testing in cardiovascular patients.
In a cohort of more than 8,000 patients referred for cardiac catheterization, diagnosis of such a monogenic cardiovascular disease (MCVD) was made in only 35% of those with one related gene variant and signs of phenotypic expression in the electronic health record.
The findings are novel for measuring the field’s “burden of missed diagnoses” in patients with MCVD, which “represent a missed opportunity that could be addressed by genetic screening,” contended the study report, published in the Aug. 18 issue of the Journal of the American College of Cardiology.
“The underrecognition of these diseases underscores the importance of including monogenic diseases in the treating physician’s differential diagnosis,” say the authors, led by Jawan W. Abdulrahim, MD, Duke University, Durham, N.C.
Diagnosis of MCVDs can be important, the group wrote, because many, including familial transthyretin amyloidosis (TTR) and other disorders that pose an increased risk for sudden death, have evidence-based treatment modalities available or are clinically actionable. “Identification of patients with MCVD variants” is also “important for cascade screening of family members who are at risk of inheriting the pathogenic mutations.”
“We tend to ignore these monogenic diseases because they are so rare individually but, in aggregate, monogenic diseases are actually quite common,” senior author Svati H. Shah, MD, MHS, also of Duke University, said in an interview.
The results “support that the cardiology community over time adopt a genotype-forward approach,” one in which every patient presenting to a cardiovascular clinic is genotyped, she said.
One implication of such an approach, Dr. Shah agreed, is that “we would be able to pick these people up earlier in their disease, especially in the context of therapies that could improve certainly their progression, but even their survival.”
For now, she said, the study suggests that “these disorders are more frequent than perhaps all cardiologists are aware of, and we just need to keep our eyes open and know when to refer patients to a cardiovascular genetics clinic, which maybe has more time and can deal with all the nuances that go along with genetic testing.”
In the total cohort, 4.5% were found to carry a gene variant known or believed to cause such diseases. The most frequently represented conditions were familial TTR, hereditary hemochromatosis, heterozygous familial hypercholesterolemia, and various cardiomyopathies.
Of those patients, 52 received a clinical diagnosis of the monogenic disorder after an EHR review. Of the 290 without such a diagnosis, two-thirds showed no evidence in their EHR of the variant’s phenotypic signs. But the records of the other third featured at least some signs that the disease had manifested clinically.
“These data serve as a reminder that monogenic Mendelian disease, including heart and vascular disease, varies in penetrance from individual to individual and may not always present with clinically detectable phenotypes,” noted an editorial accompanying the report.
They also “provide a compelling basis for expanding the role of targeted genetic testing in patients with more traditional forms of heart and vascular disease,” wrote Scott M. Damrauer, MD, University of Pennsylvania, Philadelphia, and William S. Weintraub, MD, Medstar Washington Hospital Center and Georgetown University, Washington.
“Based on the current report, the number needed to screen in a complex cardiovascular patient population to detect 1 case of undiagnosed monogenic cardiovascular disease is 85,” they wrote. “This places targeted genetic testing well within what is considered to be efficacious for most screening programs and in the range of that of other common cardiovascular diseases and cancers.”
Among the 342 patients with a variant predicting a single MCVD – in addition to the 52 who received a diagnosis – 193 had records with no indication of phenotypic expression and so did not receive a diagnosis.
But the 97 patients without a diagnosis who nevertheless had documented signs of some phenotypic expression were deemed, on the basis of extent of expression, to represent a possibly, probably, or definitely missed diagnosis.
Familial TTR made up about 45% of such potentially missed diagnoses, the report noted.
Broader screening of patients with cardiovascular disease, Dr. Shah speculated, “might actually be not only a clinically useful endeavor, but – when we think about the aggregate burden of these monogenic disorders – potentially even cost-effective.”
As the price of genetic sequencing drops, she said, “I think we’ll start to see even more health systems wanting to incorporate the genotype-forward approach.”
Dr. Shah reports serving as primary investigator for research sponsored by Verily Life Sciences and AstraZeneca. Dr. Abdulrahim reports no relevant relationships. Disclosures for the other authors are in the report. Dr. Damrauer discloses receiving research support from RenalytixAI and consulting fees from Calico Labs. Dr. Weintraub had no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Long-lasting COVID-19 symptoms: Patients want answers
Q&A with Dr. Sachin Gupta
For some patients, a bout of COVID-19 may not be over after hospital discharge, acute symptoms subside, or a couple of tests for SARS-CoV-2 come back negative. Those who have reached these milestones of conquering the disease may find that their recovery journey has only begun. Debilitating symptoms such as fatigue, headache, and dyspnea may linger for weeks or longer. Patients with persistent symptoms, often referred to as “long haulers” in reference to the duration of their recovery, are looking for answers about their condition and when their COVID-19 illness will finally resolve.
Long-haul patients organize
What started as an accumulation of anecdotal evidence in social media, blogs, and the mainstream press about slow recovery and long-lasting symptoms of COVID-19 is now the focus of clinical trials in the population of recovering patients. Projects such as the COVID Symptom Study, initiated by the Massachusetts General Hospital, Boston; the Harvard School of Public Health, Boston; King’s College London; and Stanford (Calif.) University, are collecting data on symptoms from millions of patients and will eventually contribute to a better understanding of prolonged recovery.
Patients looking for answers have created groups on social media such as Facebook to exchange information about their experiences (e.g., Survivor Corps, COVID-19 Support Group, COVID-19 Recovered Survivors). Recovering patients have created patient-led research organizations (Body Politic COVID-19 Support Group) to explore persistent symptoms and begin to create data for research.
Some data on lingering symptoms
A small study of 143 previously hospitalized, recovering patients in Italy found that 87.4% of the cohort had at least one persistent symptom 2 months or longer after initial onset and at more than a month after discharge. In this sample, only 5% had been intubated. (JAMA 2020 Jul 9. doi: 10.1001/jama.2020.12603).
One study found that even patients who have had relatively mild symptoms and were not hospitalized can have persistent symptoms. The Centers for Disease Control and Prevention conducted a survey of adults who tested positive for the positive reverse transcription–polymerase chain reaction test for SARS-CoV-2 and found that, among the 292 respondents, 35% were still feeling the impact of the disease 2-3 weeks after testing. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms. The survey found that delayed recovery was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization (MMWR. 2020 Jul 24. doi: 10.15585/mmwr.mm6930e1).
Sachin Gupta, MD, FCCP, ATSF, a pulmonologist and member of the CHEST Physician editorial advisory board, has treated patients with COVID-19 and shared some of his thoughts on the problem of prolonged symptoms of COVID-19.
Q: Should clinicians expect to see COVID-19 patients who have symptoms persisting weeks after they are diagnosed?
Dr. Gupta: I think clinicians, especially in primary care, are already seeing many patients with lingering symptoms, both respiratory and nonrespiratory related, and debility. A few patients here in the San Francisco Bay Area that I have spoken with 4-6 weeks out from their acute illness have complained of persisting, though improving, fatigue and cough. Early studies are confirming this as a topical issue. There may be other long-lasting sequelae of COVID-19 beyond the common mild lingering symptoms. It will also be important to consider (and get more data on) to what degree asymptomatic patients develop some degree of mild inflammatory and subsequent fibrotic changes in organs like the lungs and heart
Q: How does the recovery phase of COVID-19 compare with recovery from severe influenza or ARDS?
Dr. Gupta: Most prior influenza and acute respiratory distress syndrome (ARDS) studies have provided initial follow-up at 3 months and beyond, so technically speaking, it is a little difficult to compare the symptomatology patterns in the JAMA study of 2 months on follow-up. Nevertheless, the key takeaway is that, even though few patients in the study had ARDS requiring intubation (severe disease), many patients with milder disease had significant lingering symptoms (55% with three or more symptoms) at 2 months.
This fits logically with the premise, which we have some limited data on with ARDS (N Engl J Med. 2003;348:683-93. doi: 10.1056/NEJMoa022450) and severe influenza infection survivors (Nature Sci Rep. 2017;7:17275. doi: 10.1038/s41598-017-17497-6) that varying degrees of the inflammation cascade triggered by certain viruses can lead to changes in important patient-reported outcomes, and objective measures such as pulmonary function over the long term.
Q: What can you do for patients with lingering symptoms of COVID-19 or what can you tell them about their symptoms?
Dr. Gupta: For many patients, there is fear, given the novel nature of the virus/pandemic, that their symptoms may persist long term. Acknowledgment of their symptoms is validating and important for us to recognize as we learn more about the virus. As we are finding, many patients are going online to find answers, after sometimes feeling rushed or dismissed initially in the clinical setting.
In my experience, the bar is fairly high for most patients to reach out to their physicians with complaints of lingering symptoms after acute infection. For the ones who do reach out, they tend to have either a greater constellation of symptoms or higher severity of one or two key symptoms. After assessing and, when appropriate, ruling out secondary infections or newly developed conditions, I shift toward symptom management. I encourage such patients to build up slowly. I suggest they work first on their activities of daily living (bathing, grooming), then their instrumental activities of daily living (cooking, cleaning, checking the mail), and then to engage, based on their tolerance of symptoms, to light purposeful exercise. There are many online resources for at-home exercise activities that I recommend to patients who are more debilitated; some larger centers are beginning to offer some forms of telepulmonary rehab.
Based on what we know about other causes of viral pneumonitis and ARDS, I ask such symptomatic patients to expect a slow, gradual, and in most cases a complete recovery, and depending on the individual case, I recommend pulmonary function tests and imaging that may be helpful to track that progress.
I remind myself, and patients, that our understanding may change as we learn more over time. Checking in at set intervals, even if not in person but through a phone call, can go a long way in a setting where we do not have a specific therapy, other than gradual exercise training, to help these patients recover faster. Reassurance and encouragement are vital for patients who are struggling with the lingering burden of disease and who may find it difficult to return to work or function as usual at home. The final point is to be mindful of our patient’s mental health and, where our reassurance is not enough, to consider appropriate mental health referrals.
Q: Can you handle this kind of problem with telemedicine or which patients with lingering symptoms need to come into the office – or failing that, the ED?
Dr. Gupta: Telemedicine in the outpatient setting provides a helpful tool to assess and manage patients, in my experience, with limited and straightforward complaints. Its scope is limited diagnostically (assessing symptoms and signs) as is its reach (ability to connect with elderly, disabled, or patients without/limited telemedicine access). In many instances, telemedicine limits our ability to connect with patients emotionally and build trust. Many patients who have gone through the acute illness that we see in pulmonary clinic on follow-up are older in age, and for many, video visits are not a practical solution. Telemedicine visits can sometimes present challenges for me as well in terms of thoroughly conveying lifestyle and symptom management strategies. Health literacy is typically easier to gauge and address in person.
For patients with any degree of enduring dyspnea, more so in the acute phase, I recommend home pulse oximetry for monitoring their oxygen saturation if it is financially and technically feasible for them to obtain one. Sending a patient to the ED is an option of last resort, but one that is necessary in some cases. I expect patients with lingering symptoms to tell me that symptoms may be persisting, hopefully gradually improving, and not getting worse. If post–COVID-19 symptoms such as fever, dyspnea, fatigue, or lightheadedness are new or worsening, particularly rapidly, the safest and best option I advise patients is to go to the ED for further assessment and testing. Postviral bacterial pneumonia is something we should consider, and there is some potential for aspergillosis as well.
Q: Do you have any concerns about patients with asthma, chronic obstructive pulmonary disease, or other pulmonary issues having lingering symptoms that may mask exacerbations or may cause exacerbation of their disease?
Dr. Gupta: So far, patients with chronic lung conditions do not appear to have not been disproportionately affected by the pandemic in terms of absolute numbers or percentage wise compared to the general public. I think that sheltering in place has been readily followed by many of these patients, and in addition, I assume better adherence to their maintenance therapies has likely helped. The very few cases of patients with underlying chronic obstructive pulmonary disease and interstitial lung disease that I have seen have fared very poorly when they were diagnosed with COVID-19 in the hospital. There are emerging data about short-term outcomes from severe COVID-19 infection in patients with interstitial lung disease in Europe (medRxiv. 2020 Jul 17. doi: 10.1101/2020.07.15.20152967), and from physicians treating pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (Ann Am Thorac Soc. 2020 Jul 29. doi: 10.1513/AnnalsATS.202005-521OC). But so far, little has been published on the outcomes of mild disease in these patients with chronic lung disease.
Q: It’s still early days to know the significance of lingering symptoms. But at what point do you begin to consider the possibility of some kind of relapse? And what is your next move if the symptoms get worse?
Dr. Gupta: COVID-19 recurrence, whether because of reinfection or relapse, is a potential concern but not one that is very commonly seen so far in my purview. Generally, symptoms of post–COVID-19 infection that are lingering trend toward getting better, even if slowly. If post–COVID-19 infection symptoms are progressing (particularly if rapidly), that would be a strong indication to evaluate that patient in the ED (less likely in clinic), reswab them for SARS-CoV-2, and obtain further testing such as blood work and imaging. A significant challenge from a research perspective will be determining if coinfection with another virus is playing a role as we move closer to the fall season.
Q&A with Dr. Sachin Gupta
Q&A with Dr. Sachin Gupta
For some patients, a bout of COVID-19 may not be over after hospital discharge, acute symptoms subside, or a couple of tests for SARS-CoV-2 come back negative. Those who have reached these milestones of conquering the disease may find that their recovery journey has only begun. Debilitating symptoms such as fatigue, headache, and dyspnea may linger for weeks or longer. Patients with persistent symptoms, often referred to as “long haulers” in reference to the duration of their recovery, are looking for answers about their condition and when their COVID-19 illness will finally resolve.
Long-haul patients organize
What started as an accumulation of anecdotal evidence in social media, blogs, and the mainstream press about slow recovery and long-lasting symptoms of COVID-19 is now the focus of clinical trials in the population of recovering patients. Projects such as the COVID Symptom Study, initiated by the Massachusetts General Hospital, Boston; the Harvard School of Public Health, Boston; King’s College London; and Stanford (Calif.) University, are collecting data on symptoms from millions of patients and will eventually contribute to a better understanding of prolonged recovery.
Patients looking for answers have created groups on social media such as Facebook to exchange information about their experiences (e.g., Survivor Corps, COVID-19 Support Group, COVID-19 Recovered Survivors). Recovering patients have created patient-led research organizations (Body Politic COVID-19 Support Group) to explore persistent symptoms and begin to create data for research.
Some data on lingering symptoms
A small study of 143 previously hospitalized, recovering patients in Italy found that 87.4% of the cohort had at least one persistent symptom 2 months or longer after initial onset and at more than a month after discharge. In this sample, only 5% had been intubated. (JAMA 2020 Jul 9. doi: 10.1001/jama.2020.12603).
One study found that even patients who have had relatively mild symptoms and were not hospitalized can have persistent symptoms. The Centers for Disease Control and Prevention conducted a survey of adults who tested positive for the positive reverse transcription–polymerase chain reaction test for SARS-CoV-2 and found that, among the 292 respondents, 35% were still feeling the impact of the disease 2-3 weeks after testing. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms. The survey found that delayed recovery was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization (MMWR. 2020 Jul 24. doi: 10.15585/mmwr.mm6930e1).
Sachin Gupta, MD, FCCP, ATSF, a pulmonologist and member of the CHEST Physician editorial advisory board, has treated patients with COVID-19 and shared some of his thoughts on the problem of prolonged symptoms of COVID-19.
Q: Should clinicians expect to see COVID-19 patients who have symptoms persisting weeks after they are diagnosed?
Dr. Gupta: I think clinicians, especially in primary care, are already seeing many patients with lingering symptoms, both respiratory and nonrespiratory related, and debility. A few patients here in the San Francisco Bay Area that I have spoken with 4-6 weeks out from their acute illness have complained of persisting, though improving, fatigue and cough. Early studies are confirming this as a topical issue. There may be other long-lasting sequelae of COVID-19 beyond the common mild lingering symptoms. It will also be important to consider (and get more data on) to what degree asymptomatic patients develop some degree of mild inflammatory and subsequent fibrotic changes in organs like the lungs and heart
Q: How does the recovery phase of COVID-19 compare with recovery from severe influenza or ARDS?
Dr. Gupta: Most prior influenza and acute respiratory distress syndrome (ARDS) studies have provided initial follow-up at 3 months and beyond, so technically speaking, it is a little difficult to compare the symptomatology patterns in the JAMA study of 2 months on follow-up. Nevertheless, the key takeaway is that, even though few patients in the study had ARDS requiring intubation (severe disease), many patients with milder disease had significant lingering symptoms (55% with three or more symptoms) at 2 months.
This fits logically with the premise, which we have some limited data on with ARDS (N Engl J Med. 2003;348:683-93. doi: 10.1056/NEJMoa022450) and severe influenza infection survivors (Nature Sci Rep. 2017;7:17275. doi: 10.1038/s41598-017-17497-6) that varying degrees of the inflammation cascade triggered by certain viruses can lead to changes in important patient-reported outcomes, and objective measures such as pulmonary function over the long term.
Q: What can you do for patients with lingering symptoms of COVID-19 or what can you tell them about their symptoms?
Dr. Gupta: For many patients, there is fear, given the novel nature of the virus/pandemic, that their symptoms may persist long term. Acknowledgment of their symptoms is validating and important for us to recognize as we learn more about the virus. As we are finding, many patients are going online to find answers, after sometimes feeling rushed or dismissed initially in the clinical setting.
In my experience, the bar is fairly high for most patients to reach out to their physicians with complaints of lingering symptoms after acute infection. For the ones who do reach out, they tend to have either a greater constellation of symptoms or higher severity of one or two key symptoms. After assessing and, when appropriate, ruling out secondary infections or newly developed conditions, I shift toward symptom management. I encourage such patients to build up slowly. I suggest they work first on their activities of daily living (bathing, grooming), then their instrumental activities of daily living (cooking, cleaning, checking the mail), and then to engage, based on their tolerance of symptoms, to light purposeful exercise. There are many online resources for at-home exercise activities that I recommend to patients who are more debilitated; some larger centers are beginning to offer some forms of telepulmonary rehab.
Based on what we know about other causes of viral pneumonitis and ARDS, I ask such symptomatic patients to expect a slow, gradual, and in most cases a complete recovery, and depending on the individual case, I recommend pulmonary function tests and imaging that may be helpful to track that progress.
I remind myself, and patients, that our understanding may change as we learn more over time. Checking in at set intervals, even if not in person but through a phone call, can go a long way in a setting where we do not have a specific therapy, other than gradual exercise training, to help these patients recover faster. Reassurance and encouragement are vital for patients who are struggling with the lingering burden of disease and who may find it difficult to return to work or function as usual at home. The final point is to be mindful of our patient’s mental health and, where our reassurance is not enough, to consider appropriate mental health referrals.
Q: Can you handle this kind of problem with telemedicine or which patients with lingering symptoms need to come into the office – or failing that, the ED?
Dr. Gupta: Telemedicine in the outpatient setting provides a helpful tool to assess and manage patients, in my experience, with limited and straightforward complaints. Its scope is limited diagnostically (assessing symptoms and signs) as is its reach (ability to connect with elderly, disabled, or patients without/limited telemedicine access). In many instances, telemedicine limits our ability to connect with patients emotionally and build trust. Many patients who have gone through the acute illness that we see in pulmonary clinic on follow-up are older in age, and for many, video visits are not a practical solution. Telemedicine visits can sometimes present challenges for me as well in terms of thoroughly conveying lifestyle and symptom management strategies. Health literacy is typically easier to gauge and address in person.
For patients with any degree of enduring dyspnea, more so in the acute phase, I recommend home pulse oximetry for monitoring their oxygen saturation if it is financially and technically feasible for them to obtain one. Sending a patient to the ED is an option of last resort, but one that is necessary in some cases. I expect patients with lingering symptoms to tell me that symptoms may be persisting, hopefully gradually improving, and not getting worse. If post–COVID-19 symptoms such as fever, dyspnea, fatigue, or lightheadedness are new or worsening, particularly rapidly, the safest and best option I advise patients is to go to the ED for further assessment and testing. Postviral bacterial pneumonia is something we should consider, and there is some potential for aspergillosis as well.
Q: Do you have any concerns about patients with asthma, chronic obstructive pulmonary disease, or other pulmonary issues having lingering symptoms that may mask exacerbations or may cause exacerbation of their disease?
Dr. Gupta: So far, patients with chronic lung conditions do not appear to have not been disproportionately affected by the pandemic in terms of absolute numbers or percentage wise compared to the general public. I think that sheltering in place has been readily followed by many of these patients, and in addition, I assume better adherence to their maintenance therapies has likely helped. The very few cases of patients with underlying chronic obstructive pulmonary disease and interstitial lung disease that I have seen have fared very poorly when they were diagnosed with COVID-19 in the hospital. There are emerging data about short-term outcomes from severe COVID-19 infection in patients with interstitial lung disease in Europe (medRxiv. 2020 Jul 17. doi: 10.1101/2020.07.15.20152967), and from physicians treating pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (Ann Am Thorac Soc. 2020 Jul 29. doi: 10.1513/AnnalsATS.202005-521OC). But so far, little has been published on the outcomes of mild disease in these patients with chronic lung disease.
Q: It’s still early days to know the significance of lingering symptoms. But at what point do you begin to consider the possibility of some kind of relapse? And what is your next move if the symptoms get worse?
Dr. Gupta: COVID-19 recurrence, whether because of reinfection or relapse, is a potential concern but not one that is very commonly seen so far in my purview. Generally, symptoms of post–COVID-19 infection that are lingering trend toward getting better, even if slowly. If post–COVID-19 infection symptoms are progressing (particularly if rapidly), that would be a strong indication to evaluate that patient in the ED (less likely in clinic), reswab them for SARS-CoV-2, and obtain further testing such as blood work and imaging. A significant challenge from a research perspective will be determining if coinfection with another virus is playing a role as we move closer to the fall season.
For some patients, a bout of COVID-19 may not be over after hospital discharge, acute symptoms subside, or a couple of tests for SARS-CoV-2 come back negative. Those who have reached these milestones of conquering the disease may find that their recovery journey has only begun. Debilitating symptoms such as fatigue, headache, and dyspnea may linger for weeks or longer. Patients with persistent symptoms, often referred to as “long haulers” in reference to the duration of their recovery, are looking for answers about their condition and when their COVID-19 illness will finally resolve.
Long-haul patients organize
What started as an accumulation of anecdotal evidence in social media, blogs, and the mainstream press about slow recovery and long-lasting symptoms of COVID-19 is now the focus of clinical trials in the population of recovering patients. Projects such as the COVID Symptom Study, initiated by the Massachusetts General Hospital, Boston; the Harvard School of Public Health, Boston; King’s College London; and Stanford (Calif.) University, are collecting data on symptoms from millions of patients and will eventually contribute to a better understanding of prolonged recovery.
Patients looking for answers have created groups on social media such as Facebook to exchange information about their experiences (e.g., Survivor Corps, COVID-19 Support Group, COVID-19 Recovered Survivors). Recovering patients have created patient-led research organizations (Body Politic COVID-19 Support Group) to explore persistent symptoms and begin to create data for research.
Some data on lingering symptoms
A small study of 143 previously hospitalized, recovering patients in Italy found that 87.4% of the cohort had at least one persistent symptom 2 months or longer after initial onset and at more than a month after discharge. In this sample, only 5% had been intubated. (JAMA 2020 Jul 9. doi: 10.1001/jama.2020.12603).
One study found that even patients who have had relatively mild symptoms and were not hospitalized can have persistent symptoms. The Centers for Disease Control and Prevention conducted a survey of adults who tested positive for the positive reverse transcription–polymerase chain reaction test for SARS-CoV-2 and found that, among the 292 respondents, 35% were still feeling the impact of the disease 2-3 weeks after testing. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms. The survey found that delayed recovery was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization (MMWR. 2020 Jul 24. doi: 10.15585/mmwr.mm6930e1).
Sachin Gupta, MD, FCCP, ATSF, a pulmonologist and member of the CHEST Physician editorial advisory board, has treated patients with COVID-19 and shared some of his thoughts on the problem of prolonged symptoms of COVID-19.
Q: Should clinicians expect to see COVID-19 patients who have symptoms persisting weeks after they are diagnosed?
Dr. Gupta: I think clinicians, especially in primary care, are already seeing many patients with lingering symptoms, both respiratory and nonrespiratory related, and debility. A few patients here in the San Francisco Bay Area that I have spoken with 4-6 weeks out from their acute illness have complained of persisting, though improving, fatigue and cough. Early studies are confirming this as a topical issue. There may be other long-lasting sequelae of COVID-19 beyond the common mild lingering symptoms. It will also be important to consider (and get more data on) to what degree asymptomatic patients develop some degree of mild inflammatory and subsequent fibrotic changes in organs like the lungs and heart
Q: How does the recovery phase of COVID-19 compare with recovery from severe influenza or ARDS?
Dr. Gupta: Most prior influenza and acute respiratory distress syndrome (ARDS) studies have provided initial follow-up at 3 months and beyond, so technically speaking, it is a little difficult to compare the symptomatology patterns in the JAMA study of 2 months on follow-up. Nevertheless, the key takeaway is that, even though few patients in the study had ARDS requiring intubation (severe disease), many patients with milder disease had significant lingering symptoms (55% with three or more symptoms) at 2 months.
This fits logically with the premise, which we have some limited data on with ARDS (N Engl J Med. 2003;348:683-93. doi: 10.1056/NEJMoa022450) and severe influenza infection survivors (Nature Sci Rep. 2017;7:17275. doi: 10.1038/s41598-017-17497-6) that varying degrees of the inflammation cascade triggered by certain viruses can lead to changes in important patient-reported outcomes, and objective measures such as pulmonary function over the long term.
Q: What can you do for patients with lingering symptoms of COVID-19 or what can you tell them about their symptoms?
Dr. Gupta: For many patients, there is fear, given the novel nature of the virus/pandemic, that their symptoms may persist long term. Acknowledgment of their symptoms is validating and important for us to recognize as we learn more about the virus. As we are finding, many patients are going online to find answers, after sometimes feeling rushed or dismissed initially in the clinical setting.
In my experience, the bar is fairly high for most patients to reach out to their physicians with complaints of lingering symptoms after acute infection. For the ones who do reach out, they tend to have either a greater constellation of symptoms or higher severity of one or two key symptoms. After assessing and, when appropriate, ruling out secondary infections or newly developed conditions, I shift toward symptom management. I encourage such patients to build up slowly. I suggest they work first on their activities of daily living (bathing, grooming), then their instrumental activities of daily living (cooking, cleaning, checking the mail), and then to engage, based on their tolerance of symptoms, to light purposeful exercise. There are many online resources for at-home exercise activities that I recommend to patients who are more debilitated; some larger centers are beginning to offer some forms of telepulmonary rehab.
Based on what we know about other causes of viral pneumonitis and ARDS, I ask such symptomatic patients to expect a slow, gradual, and in most cases a complete recovery, and depending on the individual case, I recommend pulmonary function tests and imaging that may be helpful to track that progress.
I remind myself, and patients, that our understanding may change as we learn more over time. Checking in at set intervals, even if not in person but through a phone call, can go a long way in a setting where we do not have a specific therapy, other than gradual exercise training, to help these patients recover faster. Reassurance and encouragement are vital for patients who are struggling with the lingering burden of disease and who may find it difficult to return to work or function as usual at home. The final point is to be mindful of our patient’s mental health and, where our reassurance is not enough, to consider appropriate mental health referrals.
Q: Can you handle this kind of problem with telemedicine or which patients with lingering symptoms need to come into the office – or failing that, the ED?
Dr. Gupta: Telemedicine in the outpatient setting provides a helpful tool to assess and manage patients, in my experience, with limited and straightforward complaints. Its scope is limited diagnostically (assessing symptoms and signs) as is its reach (ability to connect with elderly, disabled, or patients without/limited telemedicine access). In many instances, telemedicine limits our ability to connect with patients emotionally and build trust. Many patients who have gone through the acute illness that we see in pulmonary clinic on follow-up are older in age, and for many, video visits are not a practical solution. Telemedicine visits can sometimes present challenges for me as well in terms of thoroughly conveying lifestyle and symptom management strategies. Health literacy is typically easier to gauge and address in person.
For patients with any degree of enduring dyspnea, more so in the acute phase, I recommend home pulse oximetry for monitoring their oxygen saturation if it is financially and technically feasible for them to obtain one. Sending a patient to the ED is an option of last resort, but one that is necessary in some cases. I expect patients with lingering symptoms to tell me that symptoms may be persisting, hopefully gradually improving, and not getting worse. If post–COVID-19 symptoms such as fever, dyspnea, fatigue, or lightheadedness are new or worsening, particularly rapidly, the safest and best option I advise patients is to go to the ED for further assessment and testing. Postviral bacterial pneumonia is something we should consider, and there is some potential for aspergillosis as well.
Q: Do you have any concerns about patients with asthma, chronic obstructive pulmonary disease, or other pulmonary issues having lingering symptoms that may mask exacerbations or may cause exacerbation of their disease?
Dr. Gupta: So far, patients with chronic lung conditions do not appear to have not been disproportionately affected by the pandemic in terms of absolute numbers or percentage wise compared to the general public. I think that sheltering in place has been readily followed by many of these patients, and in addition, I assume better adherence to their maintenance therapies has likely helped. The very few cases of patients with underlying chronic obstructive pulmonary disease and interstitial lung disease that I have seen have fared very poorly when they were diagnosed with COVID-19 in the hospital. There are emerging data about short-term outcomes from severe COVID-19 infection in patients with interstitial lung disease in Europe (medRxiv. 2020 Jul 17. doi: 10.1101/2020.07.15.20152967), and from physicians treating pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (Ann Am Thorac Soc. 2020 Jul 29. doi: 10.1513/AnnalsATS.202005-521OC). But so far, little has been published on the outcomes of mild disease in these patients with chronic lung disease.
Q: It’s still early days to know the significance of lingering symptoms. But at what point do you begin to consider the possibility of some kind of relapse? And what is your next move if the symptoms get worse?
Dr. Gupta: COVID-19 recurrence, whether because of reinfection or relapse, is a potential concern but not one that is very commonly seen so far in my purview. Generally, symptoms of post–COVID-19 infection that are lingering trend toward getting better, even if slowly. If post–COVID-19 infection symptoms are progressing (particularly if rapidly), that would be a strong indication to evaluate that patient in the ED (less likely in clinic), reswab them for SARS-CoV-2, and obtain further testing such as blood work and imaging. A significant challenge from a research perspective will be determining if coinfection with another virus is playing a role as we move closer to the fall season.
FDA approves first oral treatment for spinal muscular atrophy
This marks the first approval of an oral therapy for the rare and devastating condition.
Risdiplam, marketed by Roche and PTC Therapeutics, provides “an important treatment option for patients with SMA, following the approval of the first treatment for this devastating disease less than 4 years ago,” Billy Dunn, MD, director of the Office of Neuroscience in the Center for Drug Evaluation and Research at the FDA, said in a release from the agency.
The approval was based on the results from two trials. In the open-label FIREFISH study of infantile-onset SMA, 7 (41%) of the 17 participants (mean baseline age, 6.7 months) were able to sit independently for more than 5 seconds after 12 months of treatment with risdiplam. This was a “meaningful difference from the natural progression of the disease because all untreated infants with infantile-onset SMA cannot sit independently,” the FDA noted. In addition, 81% of the participants were alive after 23 or more months of treatment – and without need of permanent ventilation.
The second study was the randomized controlled trial known as SUNFISH and included 180 patients with SMA between the ages of 2 and 25 years. Those who received the study drug had an average 1.36 increase from baseline on a motor function measure versus a 0.19 decrease in function for those who received placebo.
The FDA noted that the most common treatment-related adverse events (AEs) include fever, diarrhea, rash, ulcers of the mouth, arthralgia, and urinary tract infections. Additional AEs reported in some patients with infantile-onset SMA included upper respiratory tract infection, pneumonia, constipation, and vomiting.
The drug received fast track designation and priority review from the FDA, as well as orphan drug designation.
‘Eagerly awaited’
“Today marks an incredibly important moment for the broader SMA patient community that had been in dire need of safe and effective treatment options,” Stuart W. Peltz, PhD, chief executive officer of PTC Therapeutics, said in a company statement.
“Given [that] the majority of people with SMA in the U.S. remain untreated, we believe Evrysdi, with its favorable clinical profile and oral administration, may offer meaningful benefits for many living with this rare neurological disease,” Levi Garraway, MD, PhD, chief medical officer and head of global product development for Genentech, added in the company’s press release. Genentech is a member of the Roche Group.
The drug is continuing to be studied in more than 450 individuals as part of a “large and robust clinical trial program in SMA,” the company reports. These participants are between the ages of 2 months and 60 years.
“The approval of Evrysdi is an eagerly awaited milestone for our community. We appreciate Genentech’s commitment to … developing a treatment that can be administered at home,” Kenneth Hobby, president of the nonprofit Cure SMA, said in the same release.
In May 2019, the FDA approved the first gene therapy for SMA – the infusion drug onasemnogene abeparvovec-xioi (Zolgensma, AveXis Inc).
Genentech announced that the new oral drug will be available in the United States within 2 weeks “for direct delivery to patients’ homes.”
A version of this article originally appeared on Medscape.com.
This marks the first approval of an oral therapy for the rare and devastating condition.
Risdiplam, marketed by Roche and PTC Therapeutics, provides “an important treatment option for patients with SMA, following the approval of the first treatment for this devastating disease less than 4 years ago,” Billy Dunn, MD, director of the Office of Neuroscience in the Center for Drug Evaluation and Research at the FDA, said in a release from the agency.
The approval was based on the results from two trials. In the open-label FIREFISH study of infantile-onset SMA, 7 (41%) of the 17 participants (mean baseline age, 6.7 months) were able to sit independently for more than 5 seconds after 12 months of treatment with risdiplam. This was a “meaningful difference from the natural progression of the disease because all untreated infants with infantile-onset SMA cannot sit independently,” the FDA noted. In addition, 81% of the participants were alive after 23 or more months of treatment – and without need of permanent ventilation.
The second study was the randomized controlled trial known as SUNFISH and included 180 patients with SMA between the ages of 2 and 25 years. Those who received the study drug had an average 1.36 increase from baseline on a motor function measure versus a 0.19 decrease in function for those who received placebo.
The FDA noted that the most common treatment-related adverse events (AEs) include fever, diarrhea, rash, ulcers of the mouth, arthralgia, and urinary tract infections. Additional AEs reported in some patients with infantile-onset SMA included upper respiratory tract infection, pneumonia, constipation, and vomiting.
The drug received fast track designation and priority review from the FDA, as well as orphan drug designation.
‘Eagerly awaited’
“Today marks an incredibly important moment for the broader SMA patient community that had been in dire need of safe and effective treatment options,” Stuart W. Peltz, PhD, chief executive officer of PTC Therapeutics, said in a company statement.
“Given [that] the majority of people with SMA in the U.S. remain untreated, we believe Evrysdi, with its favorable clinical profile and oral administration, may offer meaningful benefits for many living with this rare neurological disease,” Levi Garraway, MD, PhD, chief medical officer and head of global product development for Genentech, added in the company’s press release. Genentech is a member of the Roche Group.
The drug is continuing to be studied in more than 450 individuals as part of a “large and robust clinical trial program in SMA,” the company reports. These participants are between the ages of 2 months and 60 years.
“The approval of Evrysdi is an eagerly awaited milestone for our community. We appreciate Genentech’s commitment to … developing a treatment that can be administered at home,” Kenneth Hobby, president of the nonprofit Cure SMA, said in the same release.
In May 2019, the FDA approved the first gene therapy for SMA – the infusion drug onasemnogene abeparvovec-xioi (Zolgensma, AveXis Inc).
Genentech announced that the new oral drug will be available in the United States within 2 weeks “for direct delivery to patients’ homes.”
A version of this article originally appeared on Medscape.com.
This marks the first approval of an oral therapy for the rare and devastating condition.
Risdiplam, marketed by Roche and PTC Therapeutics, provides “an important treatment option for patients with SMA, following the approval of the first treatment for this devastating disease less than 4 years ago,” Billy Dunn, MD, director of the Office of Neuroscience in the Center for Drug Evaluation and Research at the FDA, said in a release from the agency.
The approval was based on the results from two trials. In the open-label FIREFISH study of infantile-onset SMA, 7 (41%) of the 17 participants (mean baseline age, 6.7 months) were able to sit independently for more than 5 seconds after 12 months of treatment with risdiplam. This was a “meaningful difference from the natural progression of the disease because all untreated infants with infantile-onset SMA cannot sit independently,” the FDA noted. In addition, 81% of the participants were alive after 23 or more months of treatment – and without need of permanent ventilation.
The second study was the randomized controlled trial known as SUNFISH and included 180 patients with SMA between the ages of 2 and 25 years. Those who received the study drug had an average 1.36 increase from baseline on a motor function measure versus a 0.19 decrease in function for those who received placebo.
The FDA noted that the most common treatment-related adverse events (AEs) include fever, diarrhea, rash, ulcers of the mouth, arthralgia, and urinary tract infections. Additional AEs reported in some patients with infantile-onset SMA included upper respiratory tract infection, pneumonia, constipation, and vomiting.
The drug received fast track designation and priority review from the FDA, as well as orphan drug designation.
‘Eagerly awaited’
“Today marks an incredibly important moment for the broader SMA patient community that had been in dire need of safe and effective treatment options,” Stuart W. Peltz, PhD, chief executive officer of PTC Therapeutics, said in a company statement.
“Given [that] the majority of people with SMA in the U.S. remain untreated, we believe Evrysdi, with its favorable clinical profile and oral administration, may offer meaningful benefits for many living with this rare neurological disease,” Levi Garraway, MD, PhD, chief medical officer and head of global product development for Genentech, added in the company’s press release. Genentech is a member of the Roche Group.
The drug is continuing to be studied in more than 450 individuals as part of a “large and robust clinical trial program in SMA,” the company reports. These participants are between the ages of 2 months and 60 years.
“The approval of Evrysdi is an eagerly awaited milestone for our community. We appreciate Genentech’s commitment to … developing a treatment that can be administered at home,” Kenneth Hobby, president of the nonprofit Cure SMA, said in the same release.
In May 2019, the FDA approved the first gene therapy for SMA – the infusion drug onasemnogene abeparvovec-xioi (Zolgensma, AveXis Inc).
Genentech announced that the new oral drug will be available in the United States within 2 weeks “for direct delivery to patients’ homes.”
A version of this article originally appeared on Medscape.com.
AHA statement recommends dietary screening at routine checkups
A new scientific statement from the American Heart Association recommends incorporating a rapid diet-screening tool into routine primary care visits to inform dietary counseling and integrating the tool into patients’ electronic health record platforms across all healthcare settings.
The statement authors evaluated 15 existing screening tools and, although they did not recommend a specific tool, they did present advantages and disadvantages of some of the tools and encouraged “critical conversations” among clinicians and other specialists to arrive at a tool that would be most appropriate for use in a particular health care setting.
“The key takeaway is for clinicians to incorporate discussion of dietary patterns into routine preventive care appointments because a suboptimal diet is the No. 1 risk factor for cardiovascular disease,” Maya Vadiveloo, PhD, RD, chair of the statement group, said in an interview.
“We also wanted to touch on the fact the screening tool could be incorporated into the EHR and then used for clinical support and for tracking and monitoring the patient’s dietary patterns over time,” said Dr. Vadiveloo, assistant professor of nutrition and food sciences in the College of Health Science, University of Rhode Island, Kingston.
The statement was published online Aug. 7 in Circulation: Cardiovascular Quality and Outcomes.
Competing demands
Poor dietary quality has “surpassed all other mortality risk factors, accounting for 11 million deaths and about 50% of cardiovascular disease (CVD) deaths globally,” the authors wrote.
Diets deficient in fruits, vegetables, and whole grains and high in red and processed meat, added sugars, sodium, and total energy are the “leading determinants” of the risks for CVD and other conditions, so “strategies that promote holistically healthier dietary patterns to reduce chronic disease risk are of contemporary importance.”
Most clinicians and other members of health care teams “do not currently assess or counsel patients about their food and beverage intake during routine clinical care,” the authors observed.
Reasons for this may include lack of training and knowledge, insufficient time, insufficient integration of nutrition services into health care settings, insufficient reimbursement, and “competing demands during the visit,” they noted.
Dr. Vadiveloo said that an evidence-based rapid screening tool can go a long way toward helping to overcome these barriers.
“Research shows that when primary care practitioners discuss diet with patients, the patients are receptive, but we also know that clinical workloads are already very compressed, and adding another thing to a routine preventive care appointment is challenging,” she said. “So we wanted to look and see if there were already screening tools that showed promise as valid, reliable, reflective of the best science, and easy to incorporate into various types of practice settings.”
Top picks
The authors established “theoretical and practice-based criteria” for an optimal diet screening tool for use in the adult population (aged 20 to 75 years). The tool had to:
- Be developed or used within clinical practice in the past 10 years.
- Be evidence-based, reliable, and valid.
- Assess total dietary pattern rather than focusing on a single food or nutrient.
- Be able to be completed and scored at administration without special knowledge or software.
- Give actionable next steps and support to patients.
- Be able track and monitor dietary change over time.
- Be brief.
- Be useful for chronic disease management.
Of the 15 tools reviewed, the three that met the most theoretical and practice-based validity criteria were the Mediterranean Diet Adherence Screener (MEDAS) and its variations; the modified, shortened Rapid Eating Assessment for Participants (REAP), and the modified version of the Starting the Conversation Tool. However, the authors noted that the Powell and Greenberg Screening Tool was the “least time-intensive.”
One size does not fit all
No single tool will be appropriate for all practice settings, so “we would like clinicians to discuss what will work in their particular setting,” Dr. Vadiveloo emphasized.
For example, should the screening tool be completed by the clinician, a member of the health care team, or the patient? Advantages of a tool completed by clinicians or team members include collection of the information in real time, where it can be used in shared decision-making during the encounter and increased reliability because the screen has been completed by a clinician. On the other hand, the clinician might not be able to prioritize administering the screening tool during a short clinical encounter.
Advantages of a tool completed by the patient via an EHR portal is that the patient may feel less risk of judgment by the clinician or health care professional and patients can complete the screen at their convenience. Disadvantages are limited reach into underserved populations and, potentially, less reliability than clinician-administered tools.
“It is advantageous to have tools that can be administered by multiple members of health care teams to ease the demand on clinicians, if such staff is available, but in other settings, self-administration might be better, so we tried to leave it open-ended,” Dr. Vadiveloo explained.
‘Ideal platform’
“The EHR is the ideal platform to prompt clinicians and other members of the health care team to capture dietary data and deliver dietary advice to patients,” the authors observed.
EHRs allow secure storage of data and also enable access to these data when needed at the point of care. They are also important for documentation purposes.
The authors noted that the use of “myriad EHR platforms and versions of platforms” have created “technical challenges.” They recommended “standardized approaches” for transmitting health data that will “more seamlessly allow rapid diet screeners to be implemented in the EHR.”
They also recommended that the prototypes of rapid diet screeners be tested by end users prior to implementation within particular clinics. “Gathering these data ahead of time can improve the uptake of the application in the real world,” they stated.
Dr. Vadiveloo added that dietary counseling can be conducted by several members of a health care team, such as a dietitian, not just by the physician. Or the patient may need to be referred to a dietitian for counseling and follow-up.
The authors concluded by characterizing the AHA statement as “a call to action ... designed to accelerate efforts to make diet quality assessment an integral part of office-based care delivery by encouraging critical conversations among clinicians, individuals with diet/lifestyle expertise, and specialists in information technology.”
Dr. Vadiveloo has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article originally appeared on Medscape.com.
A new scientific statement from the American Heart Association recommends incorporating a rapid diet-screening tool into routine primary care visits to inform dietary counseling and integrating the tool into patients’ electronic health record platforms across all healthcare settings.
The statement authors evaluated 15 existing screening tools and, although they did not recommend a specific tool, they did present advantages and disadvantages of some of the tools and encouraged “critical conversations” among clinicians and other specialists to arrive at a tool that would be most appropriate for use in a particular health care setting.
“The key takeaway is for clinicians to incorporate discussion of dietary patterns into routine preventive care appointments because a suboptimal diet is the No. 1 risk factor for cardiovascular disease,” Maya Vadiveloo, PhD, RD, chair of the statement group, said in an interview.
“We also wanted to touch on the fact the screening tool could be incorporated into the EHR and then used for clinical support and for tracking and monitoring the patient’s dietary patterns over time,” said Dr. Vadiveloo, assistant professor of nutrition and food sciences in the College of Health Science, University of Rhode Island, Kingston.
The statement was published online Aug. 7 in Circulation: Cardiovascular Quality and Outcomes.
Competing demands
Poor dietary quality has “surpassed all other mortality risk factors, accounting for 11 million deaths and about 50% of cardiovascular disease (CVD) deaths globally,” the authors wrote.
Diets deficient in fruits, vegetables, and whole grains and high in red and processed meat, added sugars, sodium, and total energy are the “leading determinants” of the risks for CVD and other conditions, so “strategies that promote holistically healthier dietary patterns to reduce chronic disease risk are of contemporary importance.”
Most clinicians and other members of health care teams “do not currently assess or counsel patients about their food and beverage intake during routine clinical care,” the authors observed.
Reasons for this may include lack of training and knowledge, insufficient time, insufficient integration of nutrition services into health care settings, insufficient reimbursement, and “competing demands during the visit,” they noted.
Dr. Vadiveloo said that an evidence-based rapid screening tool can go a long way toward helping to overcome these barriers.
“Research shows that when primary care practitioners discuss diet with patients, the patients are receptive, but we also know that clinical workloads are already very compressed, and adding another thing to a routine preventive care appointment is challenging,” she said. “So we wanted to look and see if there were already screening tools that showed promise as valid, reliable, reflective of the best science, and easy to incorporate into various types of practice settings.”
Top picks
The authors established “theoretical and practice-based criteria” for an optimal diet screening tool for use in the adult population (aged 20 to 75 years). The tool had to:
- Be developed or used within clinical practice in the past 10 years.
- Be evidence-based, reliable, and valid.
- Assess total dietary pattern rather than focusing on a single food or nutrient.
- Be able to be completed and scored at administration without special knowledge or software.
- Give actionable next steps and support to patients.
- Be able track and monitor dietary change over time.
- Be brief.
- Be useful for chronic disease management.
Of the 15 tools reviewed, the three that met the most theoretical and practice-based validity criteria were the Mediterranean Diet Adherence Screener (MEDAS) and its variations; the modified, shortened Rapid Eating Assessment for Participants (REAP), and the modified version of the Starting the Conversation Tool. However, the authors noted that the Powell and Greenberg Screening Tool was the “least time-intensive.”
One size does not fit all
No single tool will be appropriate for all practice settings, so “we would like clinicians to discuss what will work in their particular setting,” Dr. Vadiveloo emphasized.
For example, should the screening tool be completed by the clinician, a member of the health care team, or the patient? Advantages of a tool completed by clinicians or team members include collection of the information in real time, where it can be used in shared decision-making during the encounter and increased reliability because the screen has been completed by a clinician. On the other hand, the clinician might not be able to prioritize administering the screening tool during a short clinical encounter.
Advantages of a tool completed by the patient via an EHR portal is that the patient may feel less risk of judgment by the clinician or health care professional and patients can complete the screen at their convenience. Disadvantages are limited reach into underserved populations and, potentially, less reliability than clinician-administered tools.
“It is advantageous to have tools that can be administered by multiple members of health care teams to ease the demand on clinicians, if such staff is available, but in other settings, self-administration might be better, so we tried to leave it open-ended,” Dr. Vadiveloo explained.
‘Ideal platform’
“The EHR is the ideal platform to prompt clinicians and other members of the health care team to capture dietary data and deliver dietary advice to patients,” the authors observed.
EHRs allow secure storage of data and also enable access to these data when needed at the point of care. They are also important for documentation purposes.
The authors noted that the use of “myriad EHR platforms and versions of platforms” have created “technical challenges.” They recommended “standardized approaches” for transmitting health data that will “more seamlessly allow rapid diet screeners to be implemented in the EHR.”
They also recommended that the prototypes of rapid diet screeners be tested by end users prior to implementation within particular clinics. “Gathering these data ahead of time can improve the uptake of the application in the real world,” they stated.
Dr. Vadiveloo added that dietary counseling can be conducted by several members of a health care team, such as a dietitian, not just by the physician. Or the patient may need to be referred to a dietitian for counseling and follow-up.
The authors concluded by characterizing the AHA statement as “a call to action ... designed to accelerate efforts to make diet quality assessment an integral part of office-based care delivery by encouraging critical conversations among clinicians, individuals with diet/lifestyle expertise, and specialists in information technology.”
Dr. Vadiveloo has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article originally appeared on Medscape.com.
A new scientific statement from the American Heart Association recommends incorporating a rapid diet-screening tool into routine primary care visits to inform dietary counseling and integrating the tool into patients’ electronic health record platforms across all healthcare settings.
The statement authors evaluated 15 existing screening tools and, although they did not recommend a specific tool, they did present advantages and disadvantages of some of the tools and encouraged “critical conversations” among clinicians and other specialists to arrive at a tool that would be most appropriate for use in a particular health care setting.
“The key takeaway is for clinicians to incorporate discussion of dietary patterns into routine preventive care appointments because a suboptimal diet is the No. 1 risk factor for cardiovascular disease,” Maya Vadiveloo, PhD, RD, chair of the statement group, said in an interview.
“We also wanted to touch on the fact the screening tool could be incorporated into the EHR and then used for clinical support and for tracking and monitoring the patient’s dietary patterns over time,” said Dr. Vadiveloo, assistant professor of nutrition and food sciences in the College of Health Science, University of Rhode Island, Kingston.
The statement was published online Aug. 7 in Circulation: Cardiovascular Quality and Outcomes.
Competing demands
Poor dietary quality has “surpassed all other mortality risk factors, accounting for 11 million deaths and about 50% of cardiovascular disease (CVD) deaths globally,” the authors wrote.
Diets deficient in fruits, vegetables, and whole grains and high in red and processed meat, added sugars, sodium, and total energy are the “leading determinants” of the risks for CVD and other conditions, so “strategies that promote holistically healthier dietary patterns to reduce chronic disease risk are of contemporary importance.”
Most clinicians and other members of health care teams “do not currently assess or counsel patients about their food and beverage intake during routine clinical care,” the authors observed.
Reasons for this may include lack of training and knowledge, insufficient time, insufficient integration of nutrition services into health care settings, insufficient reimbursement, and “competing demands during the visit,” they noted.
Dr. Vadiveloo said that an evidence-based rapid screening tool can go a long way toward helping to overcome these barriers.
“Research shows that when primary care practitioners discuss diet with patients, the patients are receptive, but we also know that clinical workloads are already very compressed, and adding another thing to a routine preventive care appointment is challenging,” she said. “So we wanted to look and see if there were already screening tools that showed promise as valid, reliable, reflective of the best science, and easy to incorporate into various types of practice settings.”
Top picks
The authors established “theoretical and practice-based criteria” for an optimal diet screening tool for use in the adult population (aged 20 to 75 years). The tool had to:
- Be developed or used within clinical practice in the past 10 years.
- Be evidence-based, reliable, and valid.
- Assess total dietary pattern rather than focusing on a single food or nutrient.
- Be able to be completed and scored at administration without special knowledge or software.
- Give actionable next steps and support to patients.
- Be able track and monitor dietary change over time.
- Be brief.
- Be useful for chronic disease management.
Of the 15 tools reviewed, the three that met the most theoretical and practice-based validity criteria were the Mediterranean Diet Adherence Screener (MEDAS) and its variations; the modified, shortened Rapid Eating Assessment for Participants (REAP), and the modified version of the Starting the Conversation Tool. However, the authors noted that the Powell and Greenberg Screening Tool was the “least time-intensive.”
One size does not fit all
No single tool will be appropriate for all practice settings, so “we would like clinicians to discuss what will work in their particular setting,” Dr. Vadiveloo emphasized.
For example, should the screening tool be completed by the clinician, a member of the health care team, or the patient? Advantages of a tool completed by clinicians or team members include collection of the information in real time, where it can be used in shared decision-making during the encounter and increased reliability because the screen has been completed by a clinician. On the other hand, the clinician might not be able to prioritize administering the screening tool during a short clinical encounter.
Advantages of a tool completed by the patient via an EHR portal is that the patient may feel less risk of judgment by the clinician or health care professional and patients can complete the screen at their convenience. Disadvantages are limited reach into underserved populations and, potentially, less reliability than clinician-administered tools.
“It is advantageous to have tools that can be administered by multiple members of health care teams to ease the demand on clinicians, if such staff is available, but in other settings, self-administration might be better, so we tried to leave it open-ended,” Dr. Vadiveloo explained.
‘Ideal platform’
“The EHR is the ideal platform to prompt clinicians and other members of the health care team to capture dietary data and deliver dietary advice to patients,” the authors observed.
EHRs allow secure storage of data and also enable access to these data when needed at the point of care. They are also important for documentation purposes.
The authors noted that the use of “myriad EHR platforms and versions of platforms” have created “technical challenges.” They recommended “standardized approaches” for transmitting health data that will “more seamlessly allow rapid diet screeners to be implemented in the EHR.”
They also recommended that the prototypes of rapid diet screeners be tested by end users prior to implementation within particular clinics. “Gathering these data ahead of time can improve the uptake of the application in the real world,” they stated.
Dr. Vadiveloo added that dietary counseling can be conducted by several members of a health care team, such as a dietitian, not just by the physician. Or the patient may need to be referred to a dietitian for counseling and follow-up.
The authors concluded by characterizing the AHA statement as “a call to action ... designed to accelerate efforts to make diet quality assessment an integral part of office-based care delivery by encouraging critical conversations among clinicians, individuals with diet/lifestyle expertise, and specialists in information technology.”
Dr. Vadiveloo has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article originally appeared on Medscape.com.
COVID-19 cases in children nearly doubled in just 4 weeks
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.
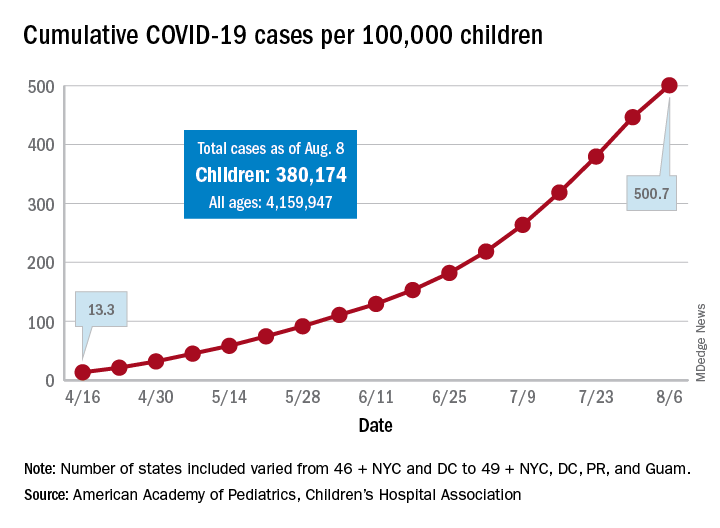
“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.

“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.
The cumulative number of new COVID-19 cases among children in the United States jumped by 90% during a recent 4-week period, according to a report that confirms children are not immune to the coronavirus.

“In areas with rapid community spread, it’s likely that more children will also be infected, and these data show that,” Sally Goza, MD, president of the American Academy of Pediatrics, said in a written statement. “I urge people to wear cloth face coverings and be diligent in social distancing and hand-washing. It is up to us to make the difference, community by community.”
The joint report from the AAP and the Children’s Hospital Association draws on data from state and local health departments in 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
The cumulative number of COVID-19 cases in children as of Aug. 6, 2020, was 380,174, and that number is 90% higher – an increase of 179,990 cases – than the total on July 9, just 4 weeks earlier, the two organizations said in the report.
and 27 states out of 47 with available data now report that over 10% of their cases were children, with Wyoming the highest at 16.5% and New Jersey the lowest at 2.9%, the report data show.
Alabama has a higher percentage of 22.5%, but the state has been reporting cases in individuals aged 0-24 years as child cases since May 7. The report’s findings are somewhat limited by differences in reporting among the states and by “gaps in the data they are reporting [that affect] how the data can be interpreted,” the AAP said in its statement.
The cumulative number of cases per 100,000 children has risen from 13.3 in mid-April, when the total number was 9,259 cases, to 500.7 per 100,000 as of Aug. 6, and there are now 21 states, along with the District of Columbia, reporting a rate of over 500 cases per 100,000 children. Arizona has the highest rate at 1,206.4, followed by South Carolina (1,074.4) and Tennessee (1,050.8), the AAP and the CHA said.
In New York City, the early epicenter of the pandemic, the 390.5 cases per 100,000 children have been reported, and in New Jersey, which joined New York in the initial surge of cases, the number is 269.5. As of Aug. 6, Hawaii had the fewest cases of any state at 91.2 per 100,000, according to the report.
Children continue to represent a very low proportion of COVID-19 deaths, “but as case counts rise across the board, that is likely to impact more children with severe illness as well,” Sean O’Leary, MD, MPH, vice chair of the AAP’s committee on infectious diseases, said in the AAP statement.
It is possible that “some of the increase in numbers of cases in children could be due to more testing. Early in the pandemic, testing only occurred for the sickest individuals. Now that there is more testing capacity … the numbers reflect a broader slice of the population, including children who may have mild or few symptoms,” the AAP suggested.
This article was updated on 8/17/2020.
Sintilimab scintillates in first-line nonsquamous NSCLC
The investigational anti-PD-1 antibody sintilimab (Tyvyt, Innovent Biologics and Eli Lilly) has shown that it improves the efficacy of platinum-based chemotherapy in the first-line treatment of patients with advanced nonsquamous non–small cell lung cancer (NSCLC) in a phase 3 trial dubbed ORIENT-11.
The study was presented at the World Congress on Lung Cancer 2020 Virtual Presidential Symposium, held virtually due to the COVID-19 pandemic, on August 8. It was also published simultaneously in the Journal of Thoracic Oncology.
Sintilimab is a fully human IgG4 monoclonal antibody that blocks the binding of programmed death (PD)-1 to PD-ligand 1 (PD-L1) or PD-L2 with high affinity, and has received market authorization in China for the treatment of Hodgkin lymphoma.
For ORIENT-11, almost 400 patients with advanced nonsquamous NSCLC were randomly assigned to sintilimab or placebo plus pemetrexed and platinum-based chemotherapy in a 2:1 ratio.
“The addition of sintilimab to pemetrexed and platinum significantly improved PFS [progression-free survival], compared to placebo,” reducing progression rates by 52%, noted lead investigator Li Zhang, MD, professor of medical oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China.
Crucially, this benefit “was seen across key clinical subgroups,” he added.
He noted that the overall response rate “was also improved, with a durable response,” while the results, which are not yet mature, suggest the experimental arm was associated with an overall survival (OS) benefit.
Study discussant Misako Nagasaka, MD, a thoracic oncologist and clinical investigator at Karmanos Cancer Institute, Detroit, said that the PFS benefit seen in the study is “certainly encouraging.”
Adding a note of caution, she continued: “But we have seen studies with PFS improvement which did not translate into OS improvement.
“Longer follow-up would allow events to mature and we will ultimately see if there would be a significant OS benefit,” she said.
Dr. Nagasaka also pointed out that the greater benefit with sintilimab seen in patients with high PD-L1 begs the question as to what would be the preferred regimen in those with higher or lower expression.
And, she said, this is not just about what regimen to choose but “more importantly, why?”
“Perhaps you’d like to use something with the best response rate, perhaps you’re sticking to a single agent immunotherapy because the toxicity profile is more favorable, or perhaps you [are] convinced with a certain regimen because of the robust PFS and OS data,” she said.
“Whatever you chose, there was a reason for your choice,” she said, adding that the sintilimab combination would have to “fulfill those reasons for you to consider choosing this regimen.”
Study details
Dr. Zhang began his presentation by noting that previous phase 1b studies have shown that sintilimab plus pemetrexed and platinum-based chemotherapy has a “tolerable safety profile and promising efficacy” in previously untreated non-squamous NSCLC.
They therefore conducted ORIENT-11, a randomized, double-blind, phase 3 study involving 397 patients with untreated stage IIIB/C or IV nonsquamous NSCLC who had neither EGFR nor ALK gene alterations.
The patients were randomly assigned in a 2:1 fashion to sintilimab plus pemetrexed and platinum-based chemotherapy (n = 266) or placebo plus pemetrexed and chemo (n = 131) for four cycles, followed by sintilimab or placebo plus pemetrexed for up to 24 months.
Thirty-five patients in the placebo arm crossed over to sintilimab monotherapy, representing 31.3% of the intention-to-treat population.
At the data cutoff of Nov. 15, 2019, 198 events had occurred, at a median follow-up of 8.9 months.
The team found that median PFS was significantly higher with sintilimab than placebo combination therapy, at 8.9 months vs. 5.0 months, or a hazard ratio of 0.482 (P < .00001).
Dr. Zhang noted that the benefit with sintilimab plus pemetrexed and platinum-based chemotherapy was seen across all subgroups.
However, it was notable that the impact of adding sintilimab on PFS was greater in patients with a tumor proportion score (TPS) ≥50%.
The HR for progression vs. the placebo treatment arm was 0.310, with median PFS not reached, which decreased to 0.503 in patients with a TPS of 1%-49% and 0.664 among those with a TPS <1%.
The results also showed that there was a “nominally significant improvement” in overall survival with sintilimab versus placebo, at a HR of 0.609 (P = 0.01921).
The ORR was markedly different between the sintilimab and placebo groups, at 51.9% vs. 29.8%, with the duration of response not reached in the sintilimab arm compared with 5.5 months in the placebo arm.
The sintilimab arm included three (1.1%) complete responses, which was not observed with pemetrexed and platinum-based chemotherapy alone.
Finally, Dr. Zhang observed that the safety profiles of the sintilimab and placebo arms were similar, with comparable rates of any, grade 3-5, and serious adverse events largely driven by high rates of chemotherapy-related events.
While there were fewer adverse events that led to death with sintilimab, at 2.3% vs. 6.9% with placebo, there were, as expected, more immune-related adverse events, at 43.2% vs. 36.6%, respectively.
Comparison with pembrolizumab
In her discussion, Dr. Nagasaka said that the first question that came to mind when she saw the results was: “How does the ORIENT-11 data compare with KEYNOTE-189?”
For that study, pembrolizumab (Keytruda, Merck) was added to pemetrexed plus carboplatin chemotherapy and compared with standard of care alone in patients with untreated metastatic nonsquamous NSCLC.
As reported by Medscape Medical News, pembrolizumab was associated with a 48% reduced risk of disease progression, as well as improved overall survival.
Dr. Nagasaka said that ORIENT-11 “had patients that tended to be younger, there were more males, more with performance status 1, and those who had never smoked” than those in KEYNOTE-189.
“But most importantly, KEYNOTE-189 had a very small number of patients from East Asia, only 1% in the pembro arm and 2.9% in the placebo arm.”
In contrast, all the patients included in ORIENT-11 were from East Asia, making the study of “high importance.”
She added that, “while across-trial comparisons must be taken with caution, the medium PFS of ORIENT-11 ... appears comparable to those of KEYNOTE-189,” while the HR “appears identical.”
This is despite median follow-up time in ORIENT-11 of “only” 8.9 months vs. a median of 23.1 months in the updated KEYNOTE-189 data.
There are plans to register the sintilimab combination therapy in China for the treatment of nonsquamous NSCLC, where it will go up against pembrolizumab as well as, potentially, tislelizumab (BeiGene).
The study was sponsored by Innovent Biologics and Eli Lilly. Dr. Zhang disclosed research grants from Eli Lilly and Pfizer. Dr. Nagasaka disclosed serving on the advisory boards of AstraZeneca, Daiichi Sankyo, Takeda, Novartis, and EMD Serono; as a consultant for Caris Life Sciences; and receiving travel support from An Hearts Therapeutics.
This article first appeared on Medscape.com.
The investigational anti-PD-1 antibody sintilimab (Tyvyt, Innovent Biologics and Eli Lilly) has shown that it improves the efficacy of platinum-based chemotherapy in the first-line treatment of patients with advanced nonsquamous non–small cell lung cancer (NSCLC) in a phase 3 trial dubbed ORIENT-11.
The study was presented at the World Congress on Lung Cancer 2020 Virtual Presidential Symposium, held virtually due to the COVID-19 pandemic, on August 8. It was also published simultaneously in the Journal of Thoracic Oncology.
Sintilimab is a fully human IgG4 monoclonal antibody that blocks the binding of programmed death (PD)-1 to PD-ligand 1 (PD-L1) or PD-L2 with high affinity, and has received market authorization in China for the treatment of Hodgkin lymphoma.
For ORIENT-11, almost 400 patients with advanced nonsquamous NSCLC were randomly assigned to sintilimab or placebo plus pemetrexed and platinum-based chemotherapy in a 2:1 ratio.
“The addition of sintilimab to pemetrexed and platinum significantly improved PFS [progression-free survival], compared to placebo,” reducing progression rates by 52%, noted lead investigator Li Zhang, MD, professor of medical oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China.
Crucially, this benefit “was seen across key clinical subgroups,” he added.
He noted that the overall response rate “was also improved, with a durable response,” while the results, which are not yet mature, suggest the experimental arm was associated with an overall survival (OS) benefit.
Study discussant Misako Nagasaka, MD, a thoracic oncologist and clinical investigator at Karmanos Cancer Institute, Detroit, said that the PFS benefit seen in the study is “certainly encouraging.”
Adding a note of caution, she continued: “But we have seen studies with PFS improvement which did not translate into OS improvement.
“Longer follow-up would allow events to mature and we will ultimately see if there would be a significant OS benefit,” she said.
Dr. Nagasaka also pointed out that the greater benefit with sintilimab seen in patients with high PD-L1 begs the question as to what would be the preferred regimen in those with higher or lower expression.
And, she said, this is not just about what regimen to choose but “more importantly, why?”
“Perhaps you’d like to use something with the best response rate, perhaps you’re sticking to a single agent immunotherapy because the toxicity profile is more favorable, or perhaps you [are] convinced with a certain regimen because of the robust PFS and OS data,” she said.
“Whatever you chose, there was a reason for your choice,” she said, adding that the sintilimab combination would have to “fulfill those reasons for you to consider choosing this regimen.”
Study details
Dr. Zhang began his presentation by noting that previous phase 1b studies have shown that sintilimab plus pemetrexed and platinum-based chemotherapy has a “tolerable safety profile and promising efficacy” in previously untreated non-squamous NSCLC.
They therefore conducted ORIENT-11, a randomized, double-blind, phase 3 study involving 397 patients with untreated stage IIIB/C or IV nonsquamous NSCLC who had neither EGFR nor ALK gene alterations.
The patients were randomly assigned in a 2:1 fashion to sintilimab plus pemetrexed and platinum-based chemotherapy (n = 266) or placebo plus pemetrexed and chemo (n = 131) for four cycles, followed by sintilimab or placebo plus pemetrexed for up to 24 months.
Thirty-five patients in the placebo arm crossed over to sintilimab monotherapy, representing 31.3% of the intention-to-treat population.
At the data cutoff of Nov. 15, 2019, 198 events had occurred, at a median follow-up of 8.9 months.
The team found that median PFS was significantly higher with sintilimab than placebo combination therapy, at 8.9 months vs. 5.0 months, or a hazard ratio of 0.482 (P < .00001).
Dr. Zhang noted that the benefit with sintilimab plus pemetrexed and platinum-based chemotherapy was seen across all subgroups.
However, it was notable that the impact of adding sintilimab on PFS was greater in patients with a tumor proportion score (TPS) ≥50%.
The HR for progression vs. the placebo treatment arm was 0.310, with median PFS not reached, which decreased to 0.503 in patients with a TPS of 1%-49% and 0.664 among those with a TPS <1%.
The results also showed that there was a “nominally significant improvement” in overall survival with sintilimab versus placebo, at a HR of 0.609 (P = 0.01921).
The ORR was markedly different between the sintilimab and placebo groups, at 51.9% vs. 29.8%, with the duration of response not reached in the sintilimab arm compared with 5.5 months in the placebo arm.
The sintilimab arm included three (1.1%) complete responses, which was not observed with pemetrexed and platinum-based chemotherapy alone.
Finally, Dr. Zhang observed that the safety profiles of the sintilimab and placebo arms were similar, with comparable rates of any, grade 3-5, and serious adverse events largely driven by high rates of chemotherapy-related events.
While there were fewer adverse events that led to death with sintilimab, at 2.3% vs. 6.9% with placebo, there were, as expected, more immune-related adverse events, at 43.2% vs. 36.6%, respectively.
Comparison with pembrolizumab
In her discussion, Dr. Nagasaka said that the first question that came to mind when she saw the results was: “How does the ORIENT-11 data compare with KEYNOTE-189?”
For that study, pembrolizumab (Keytruda, Merck) was added to pemetrexed plus carboplatin chemotherapy and compared with standard of care alone in patients with untreated metastatic nonsquamous NSCLC.
As reported by Medscape Medical News, pembrolizumab was associated with a 48% reduced risk of disease progression, as well as improved overall survival.
Dr. Nagasaka said that ORIENT-11 “had patients that tended to be younger, there were more males, more with performance status 1, and those who had never smoked” than those in KEYNOTE-189.
“But most importantly, KEYNOTE-189 had a very small number of patients from East Asia, only 1% in the pembro arm and 2.9% in the placebo arm.”
In contrast, all the patients included in ORIENT-11 were from East Asia, making the study of “high importance.”
She added that, “while across-trial comparisons must be taken with caution, the medium PFS of ORIENT-11 ... appears comparable to those of KEYNOTE-189,” while the HR “appears identical.”
This is despite median follow-up time in ORIENT-11 of “only” 8.9 months vs. a median of 23.1 months in the updated KEYNOTE-189 data.
There are plans to register the sintilimab combination therapy in China for the treatment of nonsquamous NSCLC, where it will go up against pembrolizumab as well as, potentially, tislelizumab (BeiGene).
The study was sponsored by Innovent Biologics and Eli Lilly. Dr. Zhang disclosed research grants from Eli Lilly and Pfizer. Dr. Nagasaka disclosed serving on the advisory boards of AstraZeneca, Daiichi Sankyo, Takeda, Novartis, and EMD Serono; as a consultant for Caris Life Sciences; and receiving travel support from An Hearts Therapeutics.
This article first appeared on Medscape.com.
The investigational anti-PD-1 antibody sintilimab (Tyvyt, Innovent Biologics and Eli Lilly) has shown that it improves the efficacy of platinum-based chemotherapy in the first-line treatment of patients with advanced nonsquamous non–small cell lung cancer (NSCLC) in a phase 3 trial dubbed ORIENT-11.
The study was presented at the World Congress on Lung Cancer 2020 Virtual Presidential Symposium, held virtually due to the COVID-19 pandemic, on August 8. It was also published simultaneously in the Journal of Thoracic Oncology.
Sintilimab is a fully human IgG4 monoclonal antibody that blocks the binding of programmed death (PD)-1 to PD-ligand 1 (PD-L1) or PD-L2 with high affinity, and has received market authorization in China for the treatment of Hodgkin lymphoma.
For ORIENT-11, almost 400 patients with advanced nonsquamous NSCLC were randomly assigned to sintilimab or placebo plus pemetrexed and platinum-based chemotherapy in a 2:1 ratio.
“The addition of sintilimab to pemetrexed and platinum significantly improved PFS [progression-free survival], compared to placebo,” reducing progression rates by 52%, noted lead investigator Li Zhang, MD, professor of medical oncology, Sun Yat-Sen University Cancer Center, Guangzhou, China.
Crucially, this benefit “was seen across key clinical subgroups,” he added.
He noted that the overall response rate “was also improved, with a durable response,” while the results, which are not yet mature, suggest the experimental arm was associated with an overall survival (OS) benefit.
Study discussant Misako Nagasaka, MD, a thoracic oncologist and clinical investigator at Karmanos Cancer Institute, Detroit, said that the PFS benefit seen in the study is “certainly encouraging.”
Adding a note of caution, she continued: “But we have seen studies with PFS improvement which did not translate into OS improvement.
“Longer follow-up would allow events to mature and we will ultimately see if there would be a significant OS benefit,” she said.
Dr. Nagasaka also pointed out that the greater benefit with sintilimab seen in patients with high PD-L1 begs the question as to what would be the preferred regimen in those with higher or lower expression.
And, she said, this is not just about what regimen to choose but “more importantly, why?”
“Perhaps you’d like to use something with the best response rate, perhaps you’re sticking to a single agent immunotherapy because the toxicity profile is more favorable, or perhaps you [are] convinced with a certain regimen because of the robust PFS and OS data,” she said.
“Whatever you chose, there was a reason for your choice,” she said, adding that the sintilimab combination would have to “fulfill those reasons for you to consider choosing this regimen.”
Study details
Dr. Zhang began his presentation by noting that previous phase 1b studies have shown that sintilimab plus pemetrexed and platinum-based chemotherapy has a “tolerable safety profile and promising efficacy” in previously untreated non-squamous NSCLC.
They therefore conducted ORIENT-11, a randomized, double-blind, phase 3 study involving 397 patients with untreated stage IIIB/C or IV nonsquamous NSCLC who had neither EGFR nor ALK gene alterations.
The patients were randomly assigned in a 2:1 fashion to sintilimab plus pemetrexed and platinum-based chemotherapy (n = 266) or placebo plus pemetrexed and chemo (n = 131) for four cycles, followed by sintilimab or placebo plus pemetrexed for up to 24 months.
Thirty-five patients in the placebo arm crossed over to sintilimab monotherapy, representing 31.3% of the intention-to-treat population.
At the data cutoff of Nov. 15, 2019, 198 events had occurred, at a median follow-up of 8.9 months.
The team found that median PFS was significantly higher with sintilimab than placebo combination therapy, at 8.9 months vs. 5.0 months, or a hazard ratio of 0.482 (P < .00001).
Dr. Zhang noted that the benefit with sintilimab plus pemetrexed and platinum-based chemotherapy was seen across all subgroups.
However, it was notable that the impact of adding sintilimab on PFS was greater in patients with a tumor proportion score (TPS) ≥50%.
The HR for progression vs. the placebo treatment arm was 0.310, with median PFS not reached, which decreased to 0.503 in patients with a TPS of 1%-49% and 0.664 among those with a TPS <1%.
The results also showed that there was a “nominally significant improvement” in overall survival with sintilimab versus placebo, at a HR of 0.609 (P = 0.01921).
The ORR was markedly different between the sintilimab and placebo groups, at 51.9% vs. 29.8%, with the duration of response not reached in the sintilimab arm compared with 5.5 months in the placebo arm.
The sintilimab arm included three (1.1%) complete responses, which was not observed with pemetrexed and platinum-based chemotherapy alone.
Finally, Dr. Zhang observed that the safety profiles of the sintilimab and placebo arms were similar, with comparable rates of any, grade 3-5, and serious adverse events largely driven by high rates of chemotherapy-related events.
While there were fewer adverse events that led to death with sintilimab, at 2.3% vs. 6.9% with placebo, there were, as expected, more immune-related adverse events, at 43.2% vs. 36.6%, respectively.
Comparison with pembrolizumab
In her discussion, Dr. Nagasaka said that the first question that came to mind when she saw the results was: “How does the ORIENT-11 data compare with KEYNOTE-189?”
For that study, pembrolizumab (Keytruda, Merck) was added to pemetrexed plus carboplatin chemotherapy and compared with standard of care alone in patients with untreated metastatic nonsquamous NSCLC.
As reported by Medscape Medical News, pembrolizumab was associated with a 48% reduced risk of disease progression, as well as improved overall survival.
Dr. Nagasaka said that ORIENT-11 “had patients that tended to be younger, there were more males, more with performance status 1, and those who had never smoked” than those in KEYNOTE-189.
“But most importantly, KEYNOTE-189 had a very small number of patients from East Asia, only 1% in the pembro arm and 2.9% in the placebo arm.”
In contrast, all the patients included in ORIENT-11 were from East Asia, making the study of “high importance.”
She added that, “while across-trial comparisons must be taken with caution, the medium PFS of ORIENT-11 ... appears comparable to those of KEYNOTE-189,” while the HR “appears identical.”
This is despite median follow-up time in ORIENT-11 of “only” 8.9 months vs. a median of 23.1 months in the updated KEYNOTE-189 data.
There are plans to register the sintilimab combination therapy in China for the treatment of nonsquamous NSCLC, where it will go up against pembrolizumab as well as, potentially, tislelizumab (BeiGene).
The study was sponsored by Innovent Biologics and Eli Lilly. Dr. Zhang disclosed research grants from Eli Lilly and Pfizer. Dr. Nagasaka disclosed serving on the advisory boards of AstraZeneca, Daiichi Sankyo, Takeda, Novartis, and EMD Serono; as a consultant for Caris Life Sciences; and receiving travel support from An Hearts Therapeutics.
This article first appeared on Medscape.com.
Chemo-free management of mesothelioma on horizon
Patients with untreated mesothelioma may be able to avoid chemotherapy, say researchers reporting new survival data with the immunotherapy combination of nivolumab (Opdivo) and ipilimumab (Yervoy).
The two approaches were compared in more than 600 patients with treatment-naive mesothelioma in the phase 3 CheckMate 743 trial, which was supported by the manufacturer of both immunotherapies, Bristol-Myers Squibb.
The trial “met its primary endpoint of statistically improving overall survival for the experimental arm vs chemotherapy in a prespecified interim analysis,” reported Paul Baas, MD, PhD, Netherlands Cancer Institute, Amsterdam, The Netherlands,
The combined nivo+ipi immunotherapy regimen was associated with a 26% improvement in overall survival. At 2 years, 41% of patients in the immunotherapy arm were still alive, vs 27% in the chemotherapy group.
“This is the first positive randomized trial of dual immunotherapy in the first-line treatment of patients with mesothelioma,” he said. He suggested that it should therefore “be considered as a new standard of care.”
The data were presented on August 8 in the presidential symposium of the World Congress on Lung Cancer 2020, which was held online because of the COVID-19 pandemic.
A key analysis for the study was by histologic subgroup. It is known that standard-of-care chemotherapy performs better in patients with epithelioid as opposed to nonepithelioid tumor subtypes.
Bass highlighted that the performance of nivo+ipi was “almost the same” in patients with epithelioid and nonepithelioid tumors, at a median overall survival of 18.7 months and 18.1 months, respectively.
In contrast, overall survival in the chemotherapy arm was markedly lower in patients with nonepithelioid tumors, at 8.8 months vs 16.5 months among those with epithelioid tumors.
This was reflected in the hazard ratios for overall survival vs nivo+ipi, at 0.46 and 0.86, respectively, the latter nonsignificantly different from combination immunotherapy.
For study discussant Dean A. Fennell, MD, PhD, professor and consultant in thoracic medical oncology, University of Leicester, United Kingdom, the epithet of a “new standard of care” for nivo+ipi should be reserved for nonepithelioid disease.
In this setting, he described the overall survival improvement as “transformative,” considering the “marked chemo resistance” of nonepithelioid tumors, which is “almost certainly” associated with epithelial-to-mesenchymal transition (EMT).
In the future, he suggested, combinations of chemotherapy and immunotherapy involving all histologies or selective targeting of nonepithelioid mesothelioma “could further extend the benefit for patients.”
Improving survival in mesothelioma
“We have been trying to improve the overall survival of patients with mesothelioma now for many decades,” Bass commented. Platinum-based chemotherapy plus pemetrexed is a standard of care, although the 5-year survival rate «is still below 10%,” he noted.
Randomized trials of single-agent immune checkpoint inhibitor therapy in the second-line treatment of patients with mesothelioma have not shown any significant benefits.
However, nivolumab and ipilimumab have a “complementary mechanism of action,” and two previous reports have indicated that together, they have clinical activity in the second-line setting.
The team conducted CheckMate 743 to determine the efficacy of the combination in the first-line setting.
The study involved 605 patients with pleural mesothelioma who had received no prior systemic therapy and had good performance status.
They were randomly assigned in a 1:1 ratio to receive nivo+ipi for up to 2 years or six cycles of pemetrexed plus cisplatin or carboplatin until disease progression or unacceptable toxicity occurred.
“Patients could have a subsequent therapy,” Bass noted; 44.0% of patients in the experimental arm received subsequent therapy, vs 44.1% of those in the chemotherapy arm.
Of the latter, 20% received an immune checkpoint inhibitor as subsequent therapy.
The minimum follow-up for overall survival was 22.1 months; the median follow-up was 29.7 months.
Nivo+ipi was associated with a significant improvement in overall survival vs standard-of-care chemotherapy, at a median overall survival of 18.1 months vs 14.1 months, with a hazard ratio of 0.74 (P = .0020).
The results indicated that overall survival was similar across key subgroups, which suggests that “no subgroup was harmed” by nivo+ipi, Bass said.
Stratification by PD-LI expression
Stratifying the patients by the absence or presence of programmed cell death–ligand-1 (PD-L1) expression, the team found that the performance of nivo+ipi was “the same” as that of chemotherapy, Bass said.
“But in cases where there is any expression of PD-L1, the experimental arm performs better,” at an overall survival 18.0 months vs 13.3 months for chemotherapy and a hazard ratio of 0.69, he said.
There was no difference between the two treatment arms in progression-free survival. Chemotherapy performed better in the first 6 months of treatment, after which the nivo+ipi arm had lower event rates.
Nivo+ipi was also associated with a greater duration of response, at a median of 11.0 months vs 6.7 months for standard-of-care chemotherapy.
Moreover, at 24 months, 32% of nivo+ipi patients were still experiencing a response, whereas 8% of those in the chemotherapy arm were.
Treatment-related adverse events rates were almost identical between the two treatment groups, although treatment with nivo+ipi was associated with more grade 3/4 serious treatment-related adverse events, at 15 vs six for chemotherapy.
Choosing immunotherapy vs. chemotherapy
In his discussion of the new study, Fennell compared the current results with those from two studies, INITIATE and MAPS2. “What’s very clear is the response rate is slightly higher,” as is the disease control rate, he said.
This, he explained, “is perhaps not surprising, given that these two previous trials were in the relapse setting.”
He pointed out, however, that the progression-free survival data from those previous trials were “not a million miles away” from results seen in CheckMate 743, “suggesting that this immunotherapy does have significant activity in the relapse setting.”
For Fennell, the “pivotal data” are in patients with nonepithelioid tumors, particularly inasmuch as chemotherapy performed “poorly” in this setting, whereas it performs “as expected” in epithelioid mesothelioma.
He believes that the driver for this is the poor prognosis associated with sarcomatoid biphasic disease, a subtype characterized by increased expression of vimentin and ZEB1, proteins both associated with EMT.
“What does this mean?” Fennell asked.
“If you have have enrichment of EMT, what you see is increased drug resistance, increased invasiveness, something we know well with sarcomatoid mesotheliomas in particular, and this drug-resistance phenotype may account for the drug resistance that we see in CheckMate 743 with chemotherapy.
“This does not appear, however, to impact in any way the efficacy of the immunotherapy,” he noted.
Fennell believes that, with regard to both efficacy and safety, the balance is “very much in favor” of nivo+ipi in epithelioid mesothelioma, although there is less to choose between immunotherapy and chemotherapy in the nonepithelioid setting.
Indeed, the choice is “possible tilting slightly towards chemotherapy” in patients with the nonepithelioid tumors, owing to the lower rates of grade 3/4 serious treatment-related adverse events in comparison with combination immunotherapy.
The study was supported by Bristol-Myers Squibb. Bass has served on the advisory boards of MSD, AstraZeneca, and Takeda. Fennell has received research support from AstraZeneca, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly, MSD, and Roche; research funding from Astex Therapeutics, Bayer, and Boehringer Ingelheim; has served on the speaker bureau of AstraZeneca, Boehringer Ingelheim, and Roche; has acted as a consultant for Bayer and Lab 21; and has served on the advisory board of Atara Biotherapeutics, Boehringer Ingelheim, and Inventiva.
This article first appeared on Medscape.com.
Patients with untreated mesothelioma may be able to avoid chemotherapy, say researchers reporting new survival data with the immunotherapy combination of nivolumab (Opdivo) and ipilimumab (Yervoy).
The two approaches were compared in more than 600 patients with treatment-naive mesothelioma in the phase 3 CheckMate 743 trial, which was supported by the manufacturer of both immunotherapies, Bristol-Myers Squibb.
The trial “met its primary endpoint of statistically improving overall survival for the experimental arm vs chemotherapy in a prespecified interim analysis,” reported Paul Baas, MD, PhD, Netherlands Cancer Institute, Amsterdam, The Netherlands,
The combined nivo+ipi immunotherapy regimen was associated with a 26% improvement in overall survival. At 2 years, 41% of patients in the immunotherapy arm were still alive, vs 27% in the chemotherapy group.
“This is the first positive randomized trial of dual immunotherapy in the first-line treatment of patients with mesothelioma,” he said. He suggested that it should therefore “be considered as a new standard of care.”
The data were presented on August 8 in the presidential symposium of the World Congress on Lung Cancer 2020, which was held online because of the COVID-19 pandemic.
A key analysis for the study was by histologic subgroup. It is known that standard-of-care chemotherapy performs better in patients with epithelioid as opposed to nonepithelioid tumor subtypes.
Bass highlighted that the performance of nivo+ipi was “almost the same” in patients with epithelioid and nonepithelioid tumors, at a median overall survival of 18.7 months and 18.1 months, respectively.
In contrast, overall survival in the chemotherapy arm was markedly lower in patients with nonepithelioid tumors, at 8.8 months vs 16.5 months among those with epithelioid tumors.
This was reflected in the hazard ratios for overall survival vs nivo+ipi, at 0.46 and 0.86, respectively, the latter nonsignificantly different from combination immunotherapy.
For study discussant Dean A. Fennell, MD, PhD, professor and consultant in thoracic medical oncology, University of Leicester, United Kingdom, the epithet of a “new standard of care” for nivo+ipi should be reserved for nonepithelioid disease.
In this setting, he described the overall survival improvement as “transformative,” considering the “marked chemo resistance” of nonepithelioid tumors, which is “almost certainly” associated with epithelial-to-mesenchymal transition (EMT).
In the future, he suggested, combinations of chemotherapy and immunotherapy involving all histologies or selective targeting of nonepithelioid mesothelioma “could further extend the benefit for patients.”
Improving survival in mesothelioma
“We have been trying to improve the overall survival of patients with mesothelioma now for many decades,” Bass commented. Platinum-based chemotherapy plus pemetrexed is a standard of care, although the 5-year survival rate «is still below 10%,” he noted.
Randomized trials of single-agent immune checkpoint inhibitor therapy in the second-line treatment of patients with mesothelioma have not shown any significant benefits.
However, nivolumab and ipilimumab have a “complementary mechanism of action,” and two previous reports have indicated that together, they have clinical activity in the second-line setting.
The team conducted CheckMate 743 to determine the efficacy of the combination in the first-line setting.
The study involved 605 patients with pleural mesothelioma who had received no prior systemic therapy and had good performance status.
They were randomly assigned in a 1:1 ratio to receive nivo+ipi for up to 2 years or six cycles of pemetrexed plus cisplatin or carboplatin until disease progression or unacceptable toxicity occurred.
“Patients could have a subsequent therapy,” Bass noted; 44.0% of patients in the experimental arm received subsequent therapy, vs 44.1% of those in the chemotherapy arm.
Of the latter, 20% received an immune checkpoint inhibitor as subsequent therapy.
The minimum follow-up for overall survival was 22.1 months; the median follow-up was 29.7 months.
Nivo+ipi was associated with a significant improvement in overall survival vs standard-of-care chemotherapy, at a median overall survival of 18.1 months vs 14.1 months, with a hazard ratio of 0.74 (P = .0020).
The results indicated that overall survival was similar across key subgroups, which suggests that “no subgroup was harmed” by nivo+ipi, Bass said.
Stratification by PD-LI expression
Stratifying the patients by the absence or presence of programmed cell death–ligand-1 (PD-L1) expression, the team found that the performance of nivo+ipi was “the same” as that of chemotherapy, Bass said.
“But in cases where there is any expression of PD-L1, the experimental arm performs better,” at an overall survival 18.0 months vs 13.3 months for chemotherapy and a hazard ratio of 0.69, he said.
There was no difference between the two treatment arms in progression-free survival. Chemotherapy performed better in the first 6 months of treatment, after which the nivo+ipi arm had lower event rates.
Nivo+ipi was also associated with a greater duration of response, at a median of 11.0 months vs 6.7 months for standard-of-care chemotherapy.
Moreover, at 24 months, 32% of nivo+ipi patients were still experiencing a response, whereas 8% of those in the chemotherapy arm were.
Treatment-related adverse events rates were almost identical between the two treatment groups, although treatment with nivo+ipi was associated with more grade 3/4 serious treatment-related adverse events, at 15 vs six for chemotherapy.
Choosing immunotherapy vs. chemotherapy
In his discussion of the new study, Fennell compared the current results with those from two studies, INITIATE and MAPS2. “What’s very clear is the response rate is slightly higher,” as is the disease control rate, he said.
This, he explained, “is perhaps not surprising, given that these two previous trials were in the relapse setting.”
He pointed out, however, that the progression-free survival data from those previous trials were “not a million miles away” from results seen in CheckMate 743, “suggesting that this immunotherapy does have significant activity in the relapse setting.”
For Fennell, the “pivotal data” are in patients with nonepithelioid tumors, particularly inasmuch as chemotherapy performed “poorly” in this setting, whereas it performs “as expected” in epithelioid mesothelioma.
He believes that the driver for this is the poor prognosis associated with sarcomatoid biphasic disease, a subtype characterized by increased expression of vimentin and ZEB1, proteins both associated with EMT.
“What does this mean?” Fennell asked.
“If you have have enrichment of EMT, what you see is increased drug resistance, increased invasiveness, something we know well with sarcomatoid mesotheliomas in particular, and this drug-resistance phenotype may account for the drug resistance that we see in CheckMate 743 with chemotherapy.
“This does not appear, however, to impact in any way the efficacy of the immunotherapy,” he noted.
Fennell believes that, with regard to both efficacy and safety, the balance is “very much in favor” of nivo+ipi in epithelioid mesothelioma, although there is less to choose between immunotherapy and chemotherapy in the nonepithelioid setting.
Indeed, the choice is “possible tilting slightly towards chemotherapy” in patients with the nonepithelioid tumors, owing to the lower rates of grade 3/4 serious treatment-related adverse events in comparison with combination immunotherapy.
The study was supported by Bristol-Myers Squibb. Bass has served on the advisory boards of MSD, AstraZeneca, and Takeda. Fennell has received research support from AstraZeneca, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly, MSD, and Roche; research funding from Astex Therapeutics, Bayer, and Boehringer Ingelheim; has served on the speaker bureau of AstraZeneca, Boehringer Ingelheim, and Roche; has acted as a consultant for Bayer and Lab 21; and has served on the advisory board of Atara Biotherapeutics, Boehringer Ingelheim, and Inventiva.
This article first appeared on Medscape.com.
Patients with untreated mesothelioma may be able to avoid chemotherapy, say researchers reporting new survival data with the immunotherapy combination of nivolumab (Opdivo) and ipilimumab (Yervoy).
The two approaches were compared in more than 600 patients with treatment-naive mesothelioma in the phase 3 CheckMate 743 trial, which was supported by the manufacturer of both immunotherapies, Bristol-Myers Squibb.
The trial “met its primary endpoint of statistically improving overall survival for the experimental arm vs chemotherapy in a prespecified interim analysis,” reported Paul Baas, MD, PhD, Netherlands Cancer Institute, Amsterdam, The Netherlands,
The combined nivo+ipi immunotherapy regimen was associated with a 26% improvement in overall survival. At 2 years, 41% of patients in the immunotherapy arm were still alive, vs 27% in the chemotherapy group.
“This is the first positive randomized trial of dual immunotherapy in the first-line treatment of patients with mesothelioma,” he said. He suggested that it should therefore “be considered as a new standard of care.”
The data were presented on August 8 in the presidential symposium of the World Congress on Lung Cancer 2020, which was held online because of the COVID-19 pandemic.
A key analysis for the study was by histologic subgroup. It is known that standard-of-care chemotherapy performs better in patients with epithelioid as opposed to nonepithelioid tumor subtypes.
Bass highlighted that the performance of nivo+ipi was “almost the same” in patients with epithelioid and nonepithelioid tumors, at a median overall survival of 18.7 months and 18.1 months, respectively.
In contrast, overall survival in the chemotherapy arm was markedly lower in patients with nonepithelioid tumors, at 8.8 months vs 16.5 months among those with epithelioid tumors.
This was reflected in the hazard ratios for overall survival vs nivo+ipi, at 0.46 and 0.86, respectively, the latter nonsignificantly different from combination immunotherapy.
For study discussant Dean A. Fennell, MD, PhD, professor and consultant in thoracic medical oncology, University of Leicester, United Kingdom, the epithet of a “new standard of care” for nivo+ipi should be reserved for nonepithelioid disease.
In this setting, he described the overall survival improvement as “transformative,” considering the “marked chemo resistance” of nonepithelioid tumors, which is “almost certainly” associated with epithelial-to-mesenchymal transition (EMT).
In the future, he suggested, combinations of chemotherapy and immunotherapy involving all histologies or selective targeting of nonepithelioid mesothelioma “could further extend the benefit for patients.”
Improving survival in mesothelioma
“We have been trying to improve the overall survival of patients with mesothelioma now for many decades,” Bass commented. Platinum-based chemotherapy plus pemetrexed is a standard of care, although the 5-year survival rate «is still below 10%,” he noted.
Randomized trials of single-agent immune checkpoint inhibitor therapy in the second-line treatment of patients with mesothelioma have not shown any significant benefits.
However, nivolumab and ipilimumab have a “complementary mechanism of action,” and two previous reports have indicated that together, they have clinical activity in the second-line setting.
The team conducted CheckMate 743 to determine the efficacy of the combination in the first-line setting.
The study involved 605 patients with pleural mesothelioma who had received no prior systemic therapy and had good performance status.
They were randomly assigned in a 1:1 ratio to receive nivo+ipi for up to 2 years or six cycles of pemetrexed plus cisplatin or carboplatin until disease progression or unacceptable toxicity occurred.
“Patients could have a subsequent therapy,” Bass noted; 44.0% of patients in the experimental arm received subsequent therapy, vs 44.1% of those in the chemotherapy arm.
Of the latter, 20% received an immune checkpoint inhibitor as subsequent therapy.
The minimum follow-up for overall survival was 22.1 months; the median follow-up was 29.7 months.
Nivo+ipi was associated with a significant improvement in overall survival vs standard-of-care chemotherapy, at a median overall survival of 18.1 months vs 14.1 months, with a hazard ratio of 0.74 (P = .0020).
The results indicated that overall survival was similar across key subgroups, which suggests that “no subgroup was harmed” by nivo+ipi, Bass said.
Stratification by PD-LI expression
Stratifying the patients by the absence or presence of programmed cell death–ligand-1 (PD-L1) expression, the team found that the performance of nivo+ipi was “the same” as that of chemotherapy, Bass said.
“But in cases where there is any expression of PD-L1, the experimental arm performs better,” at an overall survival 18.0 months vs 13.3 months for chemotherapy and a hazard ratio of 0.69, he said.
There was no difference between the two treatment arms in progression-free survival. Chemotherapy performed better in the first 6 months of treatment, after which the nivo+ipi arm had lower event rates.
Nivo+ipi was also associated with a greater duration of response, at a median of 11.0 months vs 6.7 months for standard-of-care chemotherapy.
Moreover, at 24 months, 32% of nivo+ipi patients were still experiencing a response, whereas 8% of those in the chemotherapy arm were.
Treatment-related adverse events rates were almost identical between the two treatment groups, although treatment with nivo+ipi was associated with more grade 3/4 serious treatment-related adverse events, at 15 vs six for chemotherapy.
Choosing immunotherapy vs. chemotherapy
In his discussion of the new study, Fennell compared the current results with those from two studies, INITIATE and MAPS2. “What’s very clear is the response rate is slightly higher,” as is the disease control rate, he said.
This, he explained, “is perhaps not surprising, given that these two previous trials were in the relapse setting.”
He pointed out, however, that the progression-free survival data from those previous trials were “not a million miles away” from results seen in CheckMate 743, “suggesting that this immunotherapy does have significant activity in the relapse setting.”
For Fennell, the “pivotal data” are in patients with nonepithelioid tumors, particularly inasmuch as chemotherapy performed “poorly” in this setting, whereas it performs “as expected” in epithelioid mesothelioma.
He believes that the driver for this is the poor prognosis associated with sarcomatoid biphasic disease, a subtype characterized by increased expression of vimentin and ZEB1, proteins both associated with EMT.
“What does this mean?” Fennell asked.
“If you have have enrichment of EMT, what you see is increased drug resistance, increased invasiveness, something we know well with sarcomatoid mesotheliomas in particular, and this drug-resistance phenotype may account for the drug resistance that we see in CheckMate 743 with chemotherapy.
“This does not appear, however, to impact in any way the efficacy of the immunotherapy,” he noted.
Fennell believes that, with regard to both efficacy and safety, the balance is “very much in favor” of nivo+ipi in epithelioid mesothelioma, although there is less to choose between immunotherapy and chemotherapy in the nonepithelioid setting.
Indeed, the choice is “possible tilting slightly towards chemotherapy” in patients with the nonepithelioid tumors, owing to the lower rates of grade 3/4 serious treatment-related adverse events in comparison with combination immunotherapy.
The study was supported by Bristol-Myers Squibb. Bass has served on the advisory boards of MSD, AstraZeneca, and Takeda. Fennell has received research support from AstraZeneca, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly, MSD, and Roche; research funding from Astex Therapeutics, Bayer, and Boehringer Ingelheim; has served on the speaker bureau of AstraZeneca, Boehringer Ingelheim, and Roche; has acted as a consultant for Bayer and Lab 21; and has served on the advisory board of Atara Biotherapeutics, Boehringer Ingelheim, and Inventiva.
This article first appeared on Medscape.com.
Artificial intelligence matches cancer genotypes to patient phenotypes
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Precision medicine is driven by technologies such as rapid genome sequencing and artificial intelligence (AI), according to a presentation at the AACR virtual meeting II.
AI can be applied to the sequencing information derived from advanced cancers to make highly personalized treatment recommendations for patients, said Olivier Elemento, PhD, of Weill Cornell Medicine, New York.
Dr. Elemento described such work during the opening plenary session of the meeting.
Dr. Elemento advocated for whole-genome sequencing (WGS) of metastatic sites, as it can reveal “branched evolution” as tumors progress from localized to metastatic (Nat Genet. 2016 Dec;48[12]:1490-9).
The metastases share common mutations with the primaries from which they arise but also develop their own mutational profiles, which facilitate site-of-origin-agnostic, predictive treatment choices.
As examples, Dr. Elemento mentioned HER2 amplification found in a patient with urothelial cancer (J Natl Compr Canc Netw. 2019 Mar 1;17[3]:194-200) and a patient with uterine serous carcinoma (Gynecol Oncol Rep. 2019 Feb 21;28:54-7), both of whom experienced long-lasting remissions to HER2-targeted therapy.
Dr. Elemento also noted that WGS can reveal complex structural variants in lung adenocarcinomas that lack alterations in the RTK/RAS/RAF pathway (unpublished data).
Application of machine learning
One study suggested that microRNA expression and machine learning can be used to identify malignant thyroid lesions (Clin Cancer Res. 2012 Apr 1;18[7]:2032-8). The approach diagnosed malignant lesions with 90% accuracy, 100% sensitivity, and 86% specificity.
Dr. Elemento and colleagues used a similar approach to predict response to immunotherapy in melanoma (unpublished data).
The idea was to mine the cancer genome and transcriptome, allowing for identification of signals from neoantigens, immune gene expression, immune cell composition, and T-cell receptor repertoires, Dr. Elemento said. Integrating these signals with clinical outcome data via machine learning technology enabled the researchers to predict immunotherapy response in malignant melanoma with nearly 90% accuracy.
AI and image analysis
Studies have indicated that AI can be applied to medical images to improve diagnosis and treatment. The approach has been shown to:
- Facilitate correct diagnoses of malignant skin lesions (Nature. 2017 Feb 2;542[7639]:115-8).
- Distinguish lung adenocarcinoma from squamous cell cancer with 100% accuracy (EBioMedicine. 2018 Jan;27:317-28).
- Recognize distinct breast cancer subtypes (ductal, lobular, mucinous, papillary) and biomarkers (bioRxiv 242818. doi: 10.1101/242818; EBioMedicine. 2018 Jan;27:317-28)
- Predict mesothelioma prognosis (Nat Med. 2019 Oct;25[10]:1519-25).
- Predict prostate biopsy results (unpublished data) and calculate Gleason scores that can predict survival in prostate cancer patients (AACR 2020, Abstract 867).
Drug development through applied AI
In another study, Dr. Elemento and colleagues used a Bayesian machine learning approach to predict targets of molecules without a known mechanism of action (Nat Commun. 2019 Nov 19;10[1]:5221).
The method involved using data on gene expression profiles, cell line viability, side effects in animals, and structures of the molecules. The researchers applied this method to a large library of orphan small molecules and found it could predict targets in about 40% of cases.
Of 24 AI-predicted microtubule-targeting molecules, 14 depolymerized microtubules in the lab. Five of these molecules were effective in cell lines that were resistant to other microtubule-targeted drugs.
Dr. Elemento went on to describe how Oncoceutics was developing an antineoplastic agent called ONC201, but the company lacked information about the agent’s target. Using AI, the target was identified as dopamine receptor 2 (DRD2; Clin Cancer Res. 2019 Apr 1;25[7]:2305-13).
With that information, Oncoceutics initiated trials of ONC201 in tumors expressing high levels of DRD2, including a highly resistant glioma (J Neurooncol. 2019 Oct;145[1]:97-105). Responses were seen, and ONC201 is now being tested against other DRD2-expressing cancers.
Challenges to acknowledge
Potential benefits of AI in the clinic are exciting, but there are many bench-to-bedside challenges.
A clinically obvious example of AI’s applications is radiographic image analysis. There is no biologic rationale for our RECIST “cut values” for partial response, minimal response, and stable disease.
If AI can measure subtle changes on imaging that correlate with tumor biology (i.e., radiomics), we stand a better chance of predicting treatment outcomes than we can with conventional measurements of shrinkage of arbitrarily selected “target lesions.”
A tremendous amount of work is needed to build the required large image banks. During that time, AI will only improve – and without the human risks of fatigue, inconsistency, or burnout.
Those human frailties notwithstanding, AI cannot substitute for the key discussions between patient and clinician regarding goals of care, trade-offs of risks and benefits, and shared decision-making regarding management options.
At least initially (but painfully), complex technologies like WGS and digital image analysis via AI may further disadvantage patients who are medically disadvantaged by geography or socioeconomic circumstances.
In the discussion period, AACR President Antoni Ribas, MD, of University of California, Los Angeles, asked whether AI can simulate crosstalk between gene pathways so that unique treatment combinations can be identified. Dr. Elemento said those simulations are the subject of ongoing investigation.
The theme of the opening plenary session at the AACR virtual meeting II was “Turning Science into Life-Saving Care.” Applications of AI to optimize personalized use of genomics, digital image analysis, and drug development show great promise for being among the technologies that can help to realize AACR’s thematic vision.
Dr. Elemento disclosed relationships with Volastra Therapeutics, OneThree Biotech, Owkin, Freenome, Genetic Intelligence, Acuamark Diagnostics, Eli Lilly, Janssen, and Sanofi.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR 2020
Antibiotic resistance: Personal responsibility in somewhat short supply
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.

Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.

Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
Most primary care physicians agree that antibiotic resistance and inappropriate prescribing are problems in the United States, but they are much less inclined to recognize these issues in their own practices, according to the results of a nationwide survey.

Rachel M. Zetts, MPH, of the Pew Charitable Trusts, Washington, D.C., and associates wrote in Open Forum Infectious Diseases.
Almost all (94%) of the 1,550 internists, family physicians, and pediatricians who responded to the survey said that antibiotic resistance is a national problem, and nearly that many (91%) agreed that “inappropriate antibiotic prescribing is a problem in outpatient health care settings,” the investigators acknowledged.
Narrowing the focus to their own practices, however, changed some opinions. At that level, only 55% of the respondents said that resistance was a problem for their practices, and just 37% said that there any sort of inappropriate prescribing going on, based on data from the survey, which was conducted from August to October 2018 by Pew and the American Medical Association.
Antibiotic stewardship, defined as activities meant to ensure appropriate prescribing of antibiotics, should include “staff and patient education, clinician-level antibiotic prescribing feedback, and communications training on how to discuss antibiotic prescribing with patients,” Ms. Zetts and associates explained.
The need for such stewardship in health care settings was acknowledged by 72% of respondents, but 53% of those surveyed also said that all they need to do to support such efforts “is to talk with their patients about the value of an antibiotic for their symptoms,” they noted.
The bacteria, it seems, are not the only ones with some resistance. Half of the primary care physicians believe that it would be difficult to fairly and accurately track the appropriate use of antibiotics, and 52% agreed with the statement that “practice-based reporting requirements for antibiotic use would be too onerous,” the researchers pointed out.
“Antibiotic resistance is an impending public health crisis. We are seeing today, as we respond to the COVID-19 pandemic, what our health system looks like with no or limited treatments available to tackle an outbreak. … We must all remain vigilant in combating the spread of antibiotic resistant bacteria and be prudent when prescribing antibiotics,” AMA President Susan R. Bailey, MD, said in a written statement.
SOURCE: Zetts RM et al. Open Forum Infect Dis. 2020 July;7(7). doi: 10.1093/ofid/ofaa244.
FROM OPEN FORUM INFECTIOUS DISEASES
Pandemic hampers reopening of joint replacement gold mine
Dr. Ira Weintraub, a recently retired orthopedic surgeon who now works at a medical billing consultancy, saw a hip replacement bill for over $400,000 earlier this year.
“The patient stayed in the hospital 17 days, which is only 17 times normal. The bill got paid,” mused Weintraub, chief medical officer of Portland, Oregon-based WellRithms, which helps self-funded employers and workers’ compensation insurers make sense of large, complex medical bills and ensure they pay the fair amount.
Charges like that go a long way toward explaining why hospitals are eager to restore joint replacements to pre-COVID levels as quickly as possible – an eagerness tempered only by safety concerns amid a resurgence of the coronavirus in some regions of the country. Revenue losses at hospitals and outpatient surgery centers may have exceeded $5 billion from canceled knee and hip replacements alone during a roughly two-month hiatus on elective procedures earlier this year.
The cost of joint replacement surgery varies widely – though, on average, it is in the tens, not hundreds, of thousands of dollars. Still, given the high and rapidly growing volume, it’s easy to see why joint replacement operations have become a vital chunk of revenue at most U.S. hospitals.
The rate of knee and hip replacements more than doubled from 2000 to 2015, according to inpatient discharge data from the Agency for Healthcare Research and Quality. And that growth is likely to continue: Knee replacements are expected to triple between now and 2040, with hip replacements not far behind, according to projections published last year in the Journal of Rheumatology.
Joint procedures are usually not emergencies, and they were among the first to be scrubbed or delayed when hospitals froze elective surgeries in March – and again in July in some areas plagued by renewed COVID outbreaks.
“Without orthopedic volumes returning to something near their pre-pandemic levels, it will make it difficult for health systems to get back to anywhere near break-even from a bottom-line perspective,” said Stephen Thome, a principal in health care consulting at Grant Thornton, an advisory, audit and tax firm.
It’s impossible to know exactly how much knee and hip replacements are worth to hospitals, because no definitive data on total volume or price exists.
But using published estimates of volume, extrapolating average commercial payments from published Medicare rates based on a study, and making an educated guess of patient coinsurance, Thome helped KHN arrive at an annual market value for American hospitals and surgery centers of between $15.5 billion and $21.5 billion for knee replacements alone.
That suggests a revenue loss of $1.3 billion to $1.8 billion per month for the period the surgeries were shut down. These figures include ambulatory surgery centers not owned by hospitals, which also suspended most operations in late March, all of April and into May.
If you add hip replacements, which account for about half the volume of knees and are paid at similar rates, the total annual value rises to a range of $23 billion to $32 billion, with monthly revenue losses from $1.9 billion to $2.7 billion.
The American Hospital Association projects total revenue lost at U.S. hospitals will reach $323 billion by year’s end, not counting additional losses from surgeries canceled during the current coronavirus spike. That amount is partially offset by $69 billion in federal relief dollars hospitals have received so far, according to the association. The California Hospital Association puts the net revenue loss for hospitals in that state at about $10.5 billion, said spokesperson Jan Emerson-Shea.
Hospitals resumed joint replacement surgeries in early to mid-May, with the timing and ramp-up speed varying by region and hospital. Some hospitals restored volume quickly; others took a more cautious route and continue to lose revenue. Still others have had to shut down again.
At the NYU Langone Orthopedic Hospital in New York City, “people are starting to come in and you see the operating rooms full again,” said Dr. Claudette Lajam, chief orthopedic safety officer.
At St. Jude Medical Center in Fullerton, California, where the coronavirus is raging, inpatient joint replacements resumed in the second or third week of May – cautiously at first, but volume is “very close to pre-pandemic levels at this point,” said Dr. Kevin Khajavi, chairman of the hospital’s orthopedic surgery department. However, “we are constantly monitoring the situation to determine if we have to scale back once again,” he said.
In large swaths of Texas, elective surgeries were once again suspended in July because of the COVID-19 resurgence. The same is true at many hospitals in Florida, Alabama, South Carolina and Nevada.
The Mayo Clinic in Phoenix suspended nonemergency joint replacement surgeries in early July. It resumed outpatient replacement procedures the week of July 27, but still has not resumed nonemergency inpatient procedures, said Dr. Mark Spangehl, an orthopedic surgeon there. In terms of medical urgency, joint replacements are “at the bottom of the totem pole,” Spangehl said.
In terms of cash flow, however, joint replacements are decidedly not at the bottom of the totem pole. They have become a cash cow as the number of patients undergoing them has skyrocketed in recent decades.
The volume is being driven by an aging population, an epidemic of obesity and a significant rise in the number of younger people replacing joints worn out by years of sports and exercise.
It’s also being driven by the cash. Once only done in hospitals, the operations are now increasingly performed at ambulatory surgery centers – especially on younger, healthier patients who don’t require hospitalization.
The surgery centers are often physician-owned, but private equity groups such as Bain Capital and KKR & Co. have taken an interest in them, drawn by their high growth potential, robust financial returns and ability to offer competitive prices.
“[G]enerally the savings should be very good – but I do see a lot of outlier surgery centers where they are charging exorbitant amounts of money – $100,000 wouldn’t be too much,” said WellRithm’s Weintraub, who co-owned such a surgery center in Portland.
Fear of catching the coronavirus in a hospital is reinforcing the outpatient trend. Matthew Davis, a 58-year-old resident of Washington, was scheduled for a hip replacement on March 30 but got cold feet because of COVID-19, and canceled just before all elective surgeries were halted. When it came time to reschedule in June, he overcame his reservations in large part because the surgeon planned to perform the procedure at a free-standing surgery center.
“That was key to me – avoiding an overnight hospital stay to minimize my exposure,” Davis said. “These joint replacements are almost industrial-scale. They are cranking out joint replacements 9 to 5. I went in at 6:30 a.m. and I was walking out the door at 11:30.”
Acutely aware of the financial benefits, hospitals and surgery clinics have been marketing joint replacements for years, competing for coveted rankings and running ads that show healthy aging people, all smiles, engaged in vigorous activity.
However, a 2014 study concluded that one-third of knee replacements were not warranted, mainly because the symptoms of the patients were not severe enough to justify the procedures.
“The whole marketing of health care is so manipulative to the consuming public,” said Lisa McGiffert, a longtime consumer advocate and co-founder of the Patient Safety Action Network. “People might be encouraged to get a knee replacement, when in reality something less invasive could have improved their condition.”
McGiffert recounted a conversation with an orthopedic surgeon in Washington state who told her about a patient who requested a knee replacement, even though he had not tried any lower-impact treatments to fix the problem. “I asked the surgeon, ‘You didn’t do it, did you?’ And he said, ‘Of course I did. He would just have gone to somebody else.’ ”
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation.
Dr. Ira Weintraub, a recently retired orthopedic surgeon who now works at a medical billing consultancy, saw a hip replacement bill for over $400,000 earlier this year.
“The patient stayed in the hospital 17 days, which is only 17 times normal. The bill got paid,” mused Weintraub, chief medical officer of Portland, Oregon-based WellRithms, which helps self-funded employers and workers’ compensation insurers make sense of large, complex medical bills and ensure they pay the fair amount.
Charges like that go a long way toward explaining why hospitals are eager to restore joint replacements to pre-COVID levels as quickly as possible – an eagerness tempered only by safety concerns amid a resurgence of the coronavirus in some regions of the country. Revenue losses at hospitals and outpatient surgery centers may have exceeded $5 billion from canceled knee and hip replacements alone during a roughly two-month hiatus on elective procedures earlier this year.
The cost of joint replacement surgery varies widely – though, on average, it is in the tens, not hundreds, of thousands of dollars. Still, given the high and rapidly growing volume, it’s easy to see why joint replacement operations have become a vital chunk of revenue at most U.S. hospitals.
The rate of knee and hip replacements more than doubled from 2000 to 2015, according to inpatient discharge data from the Agency for Healthcare Research and Quality. And that growth is likely to continue: Knee replacements are expected to triple between now and 2040, with hip replacements not far behind, according to projections published last year in the Journal of Rheumatology.
Joint procedures are usually not emergencies, and they were among the first to be scrubbed or delayed when hospitals froze elective surgeries in March – and again in July in some areas plagued by renewed COVID outbreaks.
“Without orthopedic volumes returning to something near their pre-pandemic levels, it will make it difficult for health systems to get back to anywhere near break-even from a bottom-line perspective,” said Stephen Thome, a principal in health care consulting at Grant Thornton, an advisory, audit and tax firm.
It’s impossible to know exactly how much knee and hip replacements are worth to hospitals, because no definitive data on total volume or price exists.
But using published estimates of volume, extrapolating average commercial payments from published Medicare rates based on a study, and making an educated guess of patient coinsurance, Thome helped KHN arrive at an annual market value for American hospitals and surgery centers of between $15.5 billion and $21.5 billion for knee replacements alone.
That suggests a revenue loss of $1.3 billion to $1.8 billion per month for the period the surgeries were shut down. These figures include ambulatory surgery centers not owned by hospitals, which also suspended most operations in late March, all of April and into May.
If you add hip replacements, which account for about half the volume of knees and are paid at similar rates, the total annual value rises to a range of $23 billion to $32 billion, with monthly revenue losses from $1.9 billion to $2.7 billion.
The American Hospital Association projects total revenue lost at U.S. hospitals will reach $323 billion by year’s end, not counting additional losses from surgeries canceled during the current coronavirus spike. That amount is partially offset by $69 billion in federal relief dollars hospitals have received so far, according to the association. The California Hospital Association puts the net revenue loss for hospitals in that state at about $10.5 billion, said spokesperson Jan Emerson-Shea.
Hospitals resumed joint replacement surgeries in early to mid-May, with the timing and ramp-up speed varying by region and hospital. Some hospitals restored volume quickly; others took a more cautious route and continue to lose revenue. Still others have had to shut down again.
At the NYU Langone Orthopedic Hospital in New York City, “people are starting to come in and you see the operating rooms full again,” said Dr. Claudette Lajam, chief orthopedic safety officer.
At St. Jude Medical Center in Fullerton, California, where the coronavirus is raging, inpatient joint replacements resumed in the second or third week of May – cautiously at first, but volume is “very close to pre-pandemic levels at this point,” said Dr. Kevin Khajavi, chairman of the hospital’s orthopedic surgery department. However, “we are constantly monitoring the situation to determine if we have to scale back once again,” he said.
In large swaths of Texas, elective surgeries were once again suspended in July because of the COVID-19 resurgence. The same is true at many hospitals in Florida, Alabama, South Carolina and Nevada.
The Mayo Clinic in Phoenix suspended nonemergency joint replacement surgeries in early July. It resumed outpatient replacement procedures the week of July 27, but still has not resumed nonemergency inpatient procedures, said Dr. Mark Spangehl, an orthopedic surgeon there. In terms of medical urgency, joint replacements are “at the bottom of the totem pole,” Spangehl said.
In terms of cash flow, however, joint replacements are decidedly not at the bottom of the totem pole. They have become a cash cow as the number of patients undergoing them has skyrocketed in recent decades.
The volume is being driven by an aging population, an epidemic of obesity and a significant rise in the number of younger people replacing joints worn out by years of sports and exercise.
It’s also being driven by the cash. Once only done in hospitals, the operations are now increasingly performed at ambulatory surgery centers – especially on younger, healthier patients who don’t require hospitalization.
The surgery centers are often physician-owned, but private equity groups such as Bain Capital and KKR & Co. have taken an interest in them, drawn by their high growth potential, robust financial returns and ability to offer competitive prices.
“[G]enerally the savings should be very good – but I do see a lot of outlier surgery centers where they are charging exorbitant amounts of money – $100,000 wouldn’t be too much,” said WellRithm’s Weintraub, who co-owned such a surgery center in Portland.
Fear of catching the coronavirus in a hospital is reinforcing the outpatient trend. Matthew Davis, a 58-year-old resident of Washington, was scheduled for a hip replacement on March 30 but got cold feet because of COVID-19, and canceled just before all elective surgeries were halted. When it came time to reschedule in June, he overcame his reservations in large part because the surgeon planned to perform the procedure at a free-standing surgery center.
“That was key to me – avoiding an overnight hospital stay to minimize my exposure,” Davis said. “These joint replacements are almost industrial-scale. They are cranking out joint replacements 9 to 5. I went in at 6:30 a.m. and I was walking out the door at 11:30.”
Acutely aware of the financial benefits, hospitals and surgery clinics have been marketing joint replacements for years, competing for coveted rankings and running ads that show healthy aging people, all smiles, engaged in vigorous activity.
However, a 2014 study concluded that one-third of knee replacements were not warranted, mainly because the symptoms of the patients were not severe enough to justify the procedures.
“The whole marketing of health care is so manipulative to the consuming public,” said Lisa McGiffert, a longtime consumer advocate and co-founder of the Patient Safety Action Network. “People might be encouraged to get a knee replacement, when in reality something less invasive could have improved their condition.”
McGiffert recounted a conversation with an orthopedic surgeon in Washington state who told her about a patient who requested a knee replacement, even though he had not tried any lower-impact treatments to fix the problem. “I asked the surgeon, ‘You didn’t do it, did you?’ And he said, ‘Of course I did. He would just have gone to somebody else.’ ”
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation.
Dr. Ira Weintraub, a recently retired orthopedic surgeon who now works at a medical billing consultancy, saw a hip replacement bill for over $400,000 earlier this year.
“The patient stayed in the hospital 17 days, which is only 17 times normal. The bill got paid,” mused Weintraub, chief medical officer of Portland, Oregon-based WellRithms, which helps self-funded employers and workers’ compensation insurers make sense of large, complex medical bills and ensure they pay the fair amount.
Charges like that go a long way toward explaining why hospitals are eager to restore joint replacements to pre-COVID levels as quickly as possible – an eagerness tempered only by safety concerns amid a resurgence of the coronavirus in some regions of the country. Revenue losses at hospitals and outpatient surgery centers may have exceeded $5 billion from canceled knee and hip replacements alone during a roughly two-month hiatus on elective procedures earlier this year.
The cost of joint replacement surgery varies widely – though, on average, it is in the tens, not hundreds, of thousands of dollars. Still, given the high and rapidly growing volume, it’s easy to see why joint replacement operations have become a vital chunk of revenue at most U.S. hospitals.
The rate of knee and hip replacements more than doubled from 2000 to 2015, according to inpatient discharge data from the Agency for Healthcare Research and Quality. And that growth is likely to continue: Knee replacements are expected to triple between now and 2040, with hip replacements not far behind, according to projections published last year in the Journal of Rheumatology.
Joint procedures are usually not emergencies, and they were among the first to be scrubbed or delayed when hospitals froze elective surgeries in March – and again in July in some areas plagued by renewed COVID outbreaks.
“Without orthopedic volumes returning to something near their pre-pandemic levels, it will make it difficult for health systems to get back to anywhere near break-even from a bottom-line perspective,” said Stephen Thome, a principal in health care consulting at Grant Thornton, an advisory, audit and tax firm.
It’s impossible to know exactly how much knee and hip replacements are worth to hospitals, because no definitive data on total volume or price exists.
But using published estimates of volume, extrapolating average commercial payments from published Medicare rates based on a study, and making an educated guess of patient coinsurance, Thome helped KHN arrive at an annual market value for American hospitals and surgery centers of between $15.5 billion and $21.5 billion for knee replacements alone.
That suggests a revenue loss of $1.3 billion to $1.8 billion per month for the period the surgeries were shut down. These figures include ambulatory surgery centers not owned by hospitals, which also suspended most operations in late March, all of April and into May.
If you add hip replacements, which account for about half the volume of knees and are paid at similar rates, the total annual value rises to a range of $23 billion to $32 billion, with monthly revenue losses from $1.9 billion to $2.7 billion.
The American Hospital Association projects total revenue lost at U.S. hospitals will reach $323 billion by year’s end, not counting additional losses from surgeries canceled during the current coronavirus spike. That amount is partially offset by $69 billion in federal relief dollars hospitals have received so far, according to the association. The California Hospital Association puts the net revenue loss for hospitals in that state at about $10.5 billion, said spokesperson Jan Emerson-Shea.
Hospitals resumed joint replacement surgeries in early to mid-May, with the timing and ramp-up speed varying by region and hospital. Some hospitals restored volume quickly; others took a more cautious route and continue to lose revenue. Still others have had to shut down again.
At the NYU Langone Orthopedic Hospital in New York City, “people are starting to come in and you see the operating rooms full again,” said Dr. Claudette Lajam, chief orthopedic safety officer.
At St. Jude Medical Center in Fullerton, California, where the coronavirus is raging, inpatient joint replacements resumed in the second or third week of May – cautiously at first, but volume is “very close to pre-pandemic levels at this point,” said Dr. Kevin Khajavi, chairman of the hospital’s orthopedic surgery department. However, “we are constantly monitoring the situation to determine if we have to scale back once again,” he said.
In large swaths of Texas, elective surgeries were once again suspended in July because of the COVID-19 resurgence. The same is true at many hospitals in Florida, Alabama, South Carolina and Nevada.
The Mayo Clinic in Phoenix suspended nonemergency joint replacement surgeries in early July. It resumed outpatient replacement procedures the week of July 27, but still has not resumed nonemergency inpatient procedures, said Dr. Mark Spangehl, an orthopedic surgeon there. In terms of medical urgency, joint replacements are “at the bottom of the totem pole,” Spangehl said.
In terms of cash flow, however, joint replacements are decidedly not at the bottom of the totem pole. They have become a cash cow as the number of patients undergoing them has skyrocketed in recent decades.
The volume is being driven by an aging population, an epidemic of obesity and a significant rise in the number of younger people replacing joints worn out by years of sports and exercise.
It’s also being driven by the cash. Once only done in hospitals, the operations are now increasingly performed at ambulatory surgery centers – especially on younger, healthier patients who don’t require hospitalization.
The surgery centers are often physician-owned, but private equity groups such as Bain Capital and KKR & Co. have taken an interest in them, drawn by their high growth potential, robust financial returns and ability to offer competitive prices.
“[G]enerally the savings should be very good – but I do see a lot of outlier surgery centers where they are charging exorbitant amounts of money – $100,000 wouldn’t be too much,” said WellRithm’s Weintraub, who co-owned such a surgery center in Portland.
Fear of catching the coronavirus in a hospital is reinforcing the outpatient trend. Matthew Davis, a 58-year-old resident of Washington, was scheduled for a hip replacement on March 30 but got cold feet because of COVID-19, and canceled just before all elective surgeries were halted. When it came time to reschedule in June, he overcame his reservations in large part because the surgeon planned to perform the procedure at a free-standing surgery center.
“That was key to me – avoiding an overnight hospital stay to minimize my exposure,” Davis said. “These joint replacements are almost industrial-scale. They are cranking out joint replacements 9 to 5. I went in at 6:30 a.m. and I was walking out the door at 11:30.”
Acutely aware of the financial benefits, hospitals and surgery clinics have been marketing joint replacements for years, competing for coveted rankings and running ads that show healthy aging people, all smiles, engaged in vigorous activity.
However, a 2014 study concluded that one-third of knee replacements were not warranted, mainly because the symptoms of the patients were not severe enough to justify the procedures.
“The whole marketing of health care is so manipulative to the consuming public,” said Lisa McGiffert, a longtime consumer advocate and co-founder of the Patient Safety Action Network. “People might be encouraged to get a knee replacement, when in reality something less invasive could have improved their condition.”
McGiffert recounted a conversation with an orthopedic surgeon in Washington state who told her about a patient who requested a knee replacement, even though he had not tried any lower-impact treatments to fix the problem. “I asked the surgeon, ‘You didn’t do it, did you?’ And he said, ‘Of course I did. He would just have gone to somebody else.’ ”
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation.





