User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Routine vaccinations missed by older adults during pandemic
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
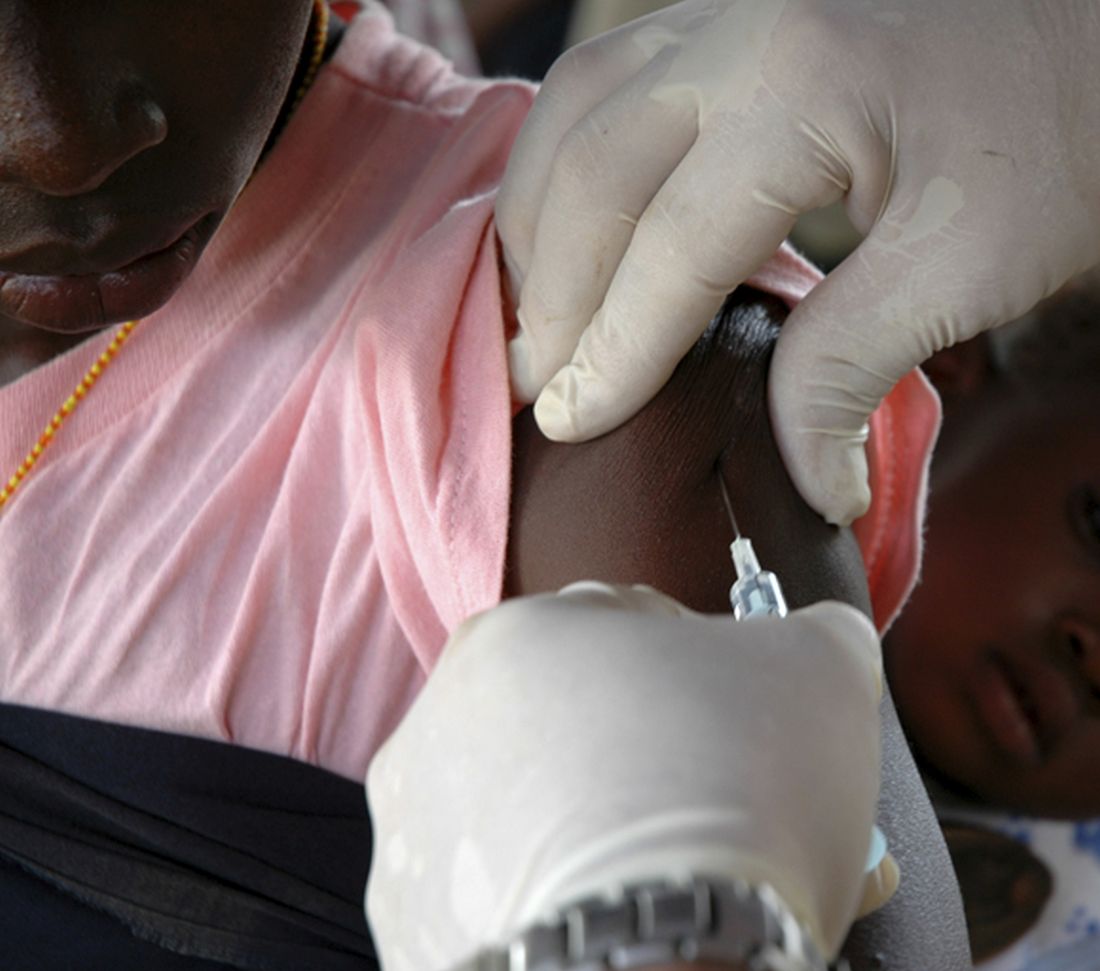
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.
In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
FROM MMWR
BMI, age, and sex affect COVID-19 vaccine antibody response
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
Sleep apnea and cognitive impairment are common bedfellows
“The study shows obstructive sleep apnea is common in patients with cognitive impairment. The results suggest that people with cognitive impairment should be assessed for sleep apnea if they have difficulty with sleep or if they demonstrate sleep-related symptoms,” said study investigator David Colelli, MSc, research coordinator at Sunnybrook Health Sciences Centre in Toronto.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology..
Linked to cognitive impairment
OSA is a common sleep disorder and is associated with an increased risk of developing cognitive impairment. It is also prevalent in the general population, but even more common among patients with dementia.
However, the investigators noted, the frequency and predictors of OSA have not been well established in Alzheimer’s disease and other related conditions such as vascular dementia.
The investigators had conducted a previous feasibility study investigating a home sleep monitor as an OSA screening tool. The current research examined potential correlations between OSA detected by this monitor and cognitive impairment.
The study included 67 patients with cognitive impairment due to neurodegenerative or vascular disease. The range of disorders included Alzheimer’s disease, mild cognitive impairment caused by Alzheimer’s disease, dementia caused by Parkinson’s or Lewy body disease, and vascular conditions.
Participants had a mean age of 72.8 years and 44.8% were male. The mean body mass index (BMI) was 25.6 kg/m2.
These participants completed a home sleep apnea test, which is an alternative to polysomnography for the detection of OSA.
Researchers identified OSA in 52.2% of the study population. This, Mr. Colelli said, “is in the range” of other research investigating sleep and cognitive impairment.
“In the general population, however, this number is a lot lower – in the 10%-20% range depending on the population or country you’re looking at,” Mr. Colelli said.
He emphasized that, without an objective sleep test, some patients may be unaware of their sleep issues. Those with cognitive impairment may “misjudge how they’re sleeping,” especially if they sleep without a partner, so it’s possible that sleep disorder symptoms often go undetected.
Bidirectional relationship?
Participants answered questionnaires on sleep, cognition, and mood. They also completed the 30-point Montreal Cognitive Assessment (MoCA) to assess language, visuospatial abilities, memory and recall, and abstract thinking.
Scores on this test range from 0 to 30, with a score of 26 or higher signifying normal, 18-25 indicating mild cognitive impairment, and 17 or lower indicating moderate to severe cognitive impairment. The average score for study participants with OSA was 20.5, compared with 23.6 for those without the sleep disorder.
Results showed OSA was significantly associated with a lower score on the MoCA scale (odds ratio, 0.40; P = .048). “This demonstrated an association of OSA with lower cognitive scores,” Mr. Colelli said.
The analysis also showed that OSA severity was correlated with actigraphy-derived sleep variables, including lower total sleep time, greater sleep onset latency, lower sleep efficiency, and more awakenings.
The study was too small to determine whether a specific diagnosis of cognitive impairment affected the link to OSA, Mr. Colelli said. “But definitely future research should be directed towards looking at this.”
Obesity is a risk factor for OSA, but the mean BMI in the study was not in the obese range of 30 and over. This, Mr. Colelli said, suggests that sleep apnea may present differently in those with cognitive impairment.
“Sleep apnea in this population might not present with the typical risk factors of obesity or snoring or feeling tired.”
While the new study “adds to the understanding that there’s a link between sleep and cognitive impairment, the direction of that link isn’t entirely clear,” Mr. Colelli said.
“It’s slowly becoming appreciated that the relationship might be bidirectionality, where sleep apnea might be contributing to the cognitive impairment and cognitive impairment could be contributing to the sleep issues.”
The study highlights how essential sleep is to mental health, Mr. Colelli said. “I feel, and I’m sure you do too, that if you don’t get good sleep, you feel tired during the day and you may not have the best concentration or memory.”
Identifying sleep issues in patients with cognitive impairment is important, as treatment and management of these issues could affect outcomes including cognition and quality of life, he added.
“Future research should be directed to see if treatment of sleep disorders with continuous positive airway pressure (CPAP), which is the gold standard, and various other treatments, can improve outcomes.” Future research should also examine OSA prevalence in larger cohorts.
Common, undertreated
Commenting on the resaerch, Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School, Boston, whose areas of expertise include disorders of cognition, sleep, and circadian rhythm, believes the findings are important. “It highlights how common and potentially undertreated OSA is in this age group, and in particular, its link to cognitive impairment.”
OSA is often associated with significant comorbidities, as well as sleep disruption, Dr. Gao noted. One of the study’s strengths was including objective assessment of sleep using actigraphy. “It will be interesting to see to what extent the OSA link to cognitive impairment is via poor sleep or disrupted circadian rest/activity cycles.”
It would also be interesting “to tease out whether OSA is more linked to dementia of vascular etiologies due to common risk factors, or whether it is pervasive to all forms of dementia,” he added.
A version of this article first appeared on Medscape.com.
“The study shows obstructive sleep apnea is common in patients with cognitive impairment. The results suggest that people with cognitive impairment should be assessed for sleep apnea if they have difficulty with sleep or if they demonstrate sleep-related symptoms,” said study investigator David Colelli, MSc, research coordinator at Sunnybrook Health Sciences Centre in Toronto.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology..
Linked to cognitive impairment
OSA is a common sleep disorder and is associated with an increased risk of developing cognitive impairment. It is also prevalent in the general population, but even more common among patients with dementia.
However, the investigators noted, the frequency and predictors of OSA have not been well established in Alzheimer’s disease and other related conditions such as vascular dementia.
The investigators had conducted a previous feasibility study investigating a home sleep monitor as an OSA screening tool. The current research examined potential correlations between OSA detected by this monitor and cognitive impairment.
The study included 67 patients with cognitive impairment due to neurodegenerative or vascular disease. The range of disorders included Alzheimer’s disease, mild cognitive impairment caused by Alzheimer’s disease, dementia caused by Parkinson’s or Lewy body disease, and vascular conditions.
Participants had a mean age of 72.8 years and 44.8% were male. The mean body mass index (BMI) was 25.6 kg/m2.
These participants completed a home sleep apnea test, which is an alternative to polysomnography for the detection of OSA.
Researchers identified OSA in 52.2% of the study population. This, Mr. Colelli said, “is in the range” of other research investigating sleep and cognitive impairment.
“In the general population, however, this number is a lot lower – in the 10%-20% range depending on the population or country you’re looking at,” Mr. Colelli said.
He emphasized that, without an objective sleep test, some patients may be unaware of their sleep issues. Those with cognitive impairment may “misjudge how they’re sleeping,” especially if they sleep without a partner, so it’s possible that sleep disorder symptoms often go undetected.
Bidirectional relationship?
Participants answered questionnaires on sleep, cognition, and mood. They also completed the 30-point Montreal Cognitive Assessment (MoCA) to assess language, visuospatial abilities, memory and recall, and abstract thinking.
Scores on this test range from 0 to 30, with a score of 26 or higher signifying normal, 18-25 indicating mild cognitive impairment, and 17 or lower indicating moderate to severe cognitive impairment. The average score for study participants with OSA was 20.5, compared with 23.6 for those without the sleep disorder.
Results showed OSA was significantly associated with a lower score on the MoCA scale (odds ratio, 0.40; P = .048). “This demonstrated an association of OSA with lower cognitive scores,” Mr. Colelli said.
The analysis also showed that OSA severity was correlated with actigraphy-derived sleep variables, including lower total sleep time, greater sleep onset latency, lower sleep efficiency, and more awakenings.
The study was too small to determine whether a specific diagnosis of cognitive impairment affected the link to OSA, Mr. Colelli said. “But definitely future research should be directed towards looking at this.”
Obesity is a risk factor for OSA, but the mean BMI in the study was not in the obese range of 30 and over. This, Mr. Colelli said, suggests that sleep apnea may present differently in those with cognitive impairment.
“Sleep apnea in this population might not present with the typical risk factors of obesity or snoring or feeling tired.”
While the new study “adds to the understanding that there’s a link between sleep and cognitive impairment, the direction of that link isn’t entirely clear,” Mr. Colelli said.
“It’s slowly becoming appreciated that the relationship might be bidirectionality, where sleep apnea might be contributing to the cognitive impairment and cognitive impairment could be contributing to the sleep issues.”
The study highlights how essential sleep is to mental health, Mr. Colelli said. “I feel, and I’m sure you do too, that if you don’t get good sleep, you feel tired during the day and you may not have the best concentration or memory.”
Identifying sleep issues in patients with cognitive impairment is important, as treatment and management of these issues could affect outcomes including cognition and quality of life, he added.
“Future research should be directed to see if treatment of sleep disorders with continuous positive airway pressure (CPAP), which is the gold standard, and various other treatments, can improve outcomes.” Future research should also examine OSA prevalence in larger cohorts.
Common, undertreated
Commenting on the resaerch, Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School, Boston, whose areas of expertise include disorders of cognition, sleep, and circadian rhythm, believes the findings are important. “It highlights how common and potentially undertreated OSA is in this age group, and in particular, its link to cognitive impairment.”
OSA is often associated with significant comorbidities, as well as sleep disruption, Dr. Gao noted. One of the study’s strengths was including objective assessment of sleep using actigraphy. “It will be interesting to see to what extent the OSA link to cognitive impairment is via poor sleep or disrupted circadian rest/activity cycles.”
It would also be interesting “to tease out whether OSA is more linked to dementia of vascular etiologies due to common risk factors, or whether it is pervasive to all forms of dementia,” he added.
A version of this article first appeared on Medscape.com.
“The study shows obstructive sleep apnea is common in patients with cognitive impairment. The results suggest that people with cognitive impairment should be assessed for sleep apnea if they have difficulty with sleep or if they demonstrate sleep-related symptoms,” said study investigator David Colelli, MSc, research coordinator at Sunnybrook Health Sciences Centre in Toronto.
The findings were released ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology..
Linked to cognitive impairment
OSA is a common sleep disorder and is associated with an increased risk of developing cognitive impairment. It is also prevalent in the general population, but even more common among patients with dementia.
However, the investigators noted, the frequency and predictors of OSA have not been well established in Alzheimer’s disease and other related conditions such as vascular dementia.
The investigators had conducted a previous feasibility study investigating a home sleep monitor as an OSA screening tool. The current research examined potential correlations between OSA detected by this monitor and cognitive impairment.
The study included 67 patients with cognitive impairment due to neurodegenerative or vascular disease. The range of disorders included Alzheimer’s disease, mild cognitive impairment caused by Alzheimer’s disease, dementia caused by Parkinson’s or Lewy body disease, and vascular conditions.
Participants had a mean age of 72.8 years and 44.8% were male. The mean body mass index (BMI) was 25.6 kg/m2.
These participants completed a home sleep apnea test, which is an alternative to polysomnography for the detection of OSA.
Researchers identified OSA in 52.2% of the study population. This, Mr. Colelli said, “is in the range” of other research investigating sleep and cognitive impairment.
“In the general population, however, this number is a lot lower – in the 10%-20% range depending on the population or country you’re looking at,” Mr. Colelli said.
He emphasized that, without an objective sleep test, some patients may be unaware of their sleep issues. Those with cognitive impairment may “misjudge how they’re sleeping,” especially if they sleep without a partner, so it’s possible that sleep disorder symptoms often go undetected.
Bidirectional relationship?
Participants answered questionnaires on sleep, cognition, and mood. They also completed the 30-point Montreal Cognitive Assessment (MoCA) to assess language, visuospatial abilities, memory and recall, and abstract thinking.
Scores on this test range from 0 to 30, with a score of 26 or higher signifying normal, 18-25 indicating mild cognitive impairment, and 17 or lower indicating moderate to severe cognitive impairment. The average score for study participants with OSA was 20.5, compared with 23.6 for those without the sleep disorder.
Results showed OSA was significantly associated with a lower score on the MoCA scale (odds ratio, 0.40; P = .048). “This demonstrated an association of OSA with lower cognitive scores,” Mr. Colelli said.
The analysis also showed that OSA severity was correlated with actigraphy-derived sleep variables, including lower total sleep time, greater sleep onset latency, lower sleep efficiency, and more awakenings.
The study was too small to determine whether a specific diagnosis of cognitive impairment affected the link to OSA, Mr. Colelli said. “But definitely future research should be directed towards looking at this.”
Obesity is a risk factor for OSA, but the mean BMI in the study was not in the obese range of 30 and over. This, Mr. Colelli said, suggests that sleep apnea may present differently in those with cognitive impairment.
“Sleep apnea in this population might not present with the typical risk factors of obesity or snoring or feeling tired.”
While the new study “adds to the understanding that there’s a link between sleep and cognitive impairment, the direction of that link isn’t entirely clear,” Mr. Colelli said.
“It’s slowly becoming appreciated that the relationship might be bidirectionality, where sleep apnea might be contributing to the cognitive impairment and cognitive impairment could be contributing to the sleep issues.”
The study highlights how essential sleep is to mental health, Mr. Colelli said. “I feel, and I’m sure you do too, that if you don’t get good sleep, you feel tired during the day and you may not have the best concentration or memory.”
Identifying sleep issues in patients with cognitive impairment is important, as treatment and management of these issues could affect outcomes including cognition and quality of life, he added.
“Future research should be directed to see if treatment of sleep disorders with continuous positive airway pressure (CPAP), which is the gold standard, and various other treatments, can improve outcomes.” Future research should also examine OSA prevalence in larger cohorts.
Common, undertreated
Commenting on the resaerch, Lei Gao, MD, assistant professor of anesthesia at Harvard Medical School, Boston, whose areas of expertise include disorders of cognition, sleep, and circadian rhythm, believes the findings are important. “It highlights how common and potentially undertreated OSA is in this age group, and in particular, its link to cognitive impairment.”
OSA is often associated with significant comorbidities, as well as sleep disruption, Dr. Gao noted. One of the study’s strengths was including objective assessment of sleep using actigraphy. “It will be interesting to see to what extent the OSA link to cognitive impairment is via poor sleep or disrupted circadian rest/activity cycles.”
It would also be interesting “to tease out whether OSA is more linked to dementia of vascular etiologies due to common risk factors, or whether it is pervasive to all forms of dementia,” he added.
A version of this article first appeared on Medscape.com.
FROM AAN 2021
No vascular benefit of testosterone over exercise in aging men
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).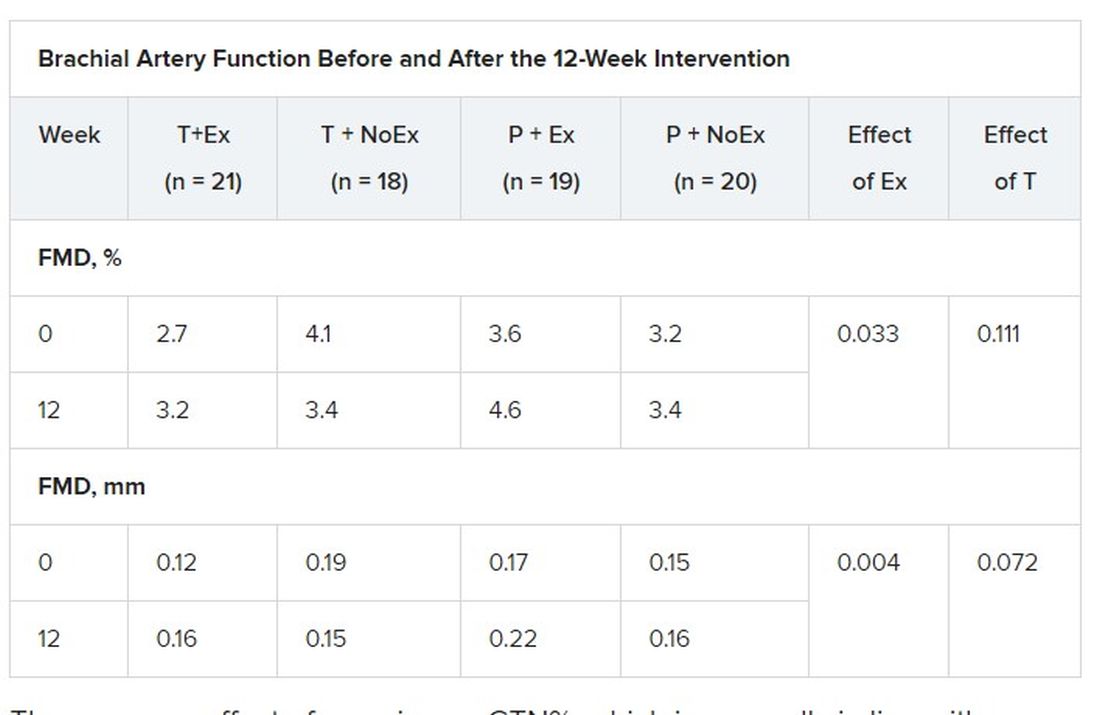
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
‘Landmark’ schizophrenia drug in the wings?
A novel therapy that combines a muscarinic receptor agonist with an anticholinergic agent is associated with a greater reduction in psychosis symptoms, compared with placebo, new research shows.
In a randomized, phase 2 trial composed of nearly 200 participants, xanomeline-trospium (KarXT) was generally well tolerated and had none of the common side effects linked to current antipsychotics, including weight gain and extrapyramidal symptoms such as dystonia, parkinsonism, and tardive dyskinesia.
“The results showing robust therapeutic efficacy of a non–dopamine targeting antipsychotic drug is an important milestone in the advance of the therapeutics of schizophrenia and other psychotic disorders,” coinvestigator Jeffrey A. Lieberman, MD, professor and chairman in the department of psychiatry, Columbia University, New York, said in an interview.
If approved, the new agent will be a “landmark” drug, Dr. Lieberman added.
The study was published in the Feb. 25, 2021, issue of the New England Journal of Medicine.
Long journey
The journey to develop an effective schizophrenia drug that reduces psychosis symptoms without onerous side effects has been a long one full of excitement and disappointment.
First-generation antipsychotics, dating back to the 1950s, targeted the postsynaptic dopamine-2 (D2) receptor. At the time, it was a “breakthrough” similar in scope to insulin for diabetes or antibiotics for infections, said Dr. Lieberman.
That was followed by development of numerous “me too” drugs with the same mechanism of action. However, these drugs had significant side effects, especially neurologic adverse events such as parkinsonism.
In 1989, second-generation antipsychotics were introduced, beginning with clozapine. They still targeted the D2 receptor but were “kinder and gentler,” Dr. Lieberman noted. “They didn’t bind to [the receptor] with such affinity that it shut things down completely, so had fewer neurologic side effects.”
However, these agents had other adverse consequences, such as weight gain and other metabolic effects including hyperglycemia and hyperlipidemia.
Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
“The pharmaceutical industry, biotech industry, and academic psychiatric community have been desperately trying to find novel strategies for antipsychotic drug development and asking, ‘Is D2 the only holy grail or are there other ways of treating psychotic symptoms of schizophrenia?’ ” Dr. Lieberman said.
Enter KarXT – a novel combination of xanomeline with trospium.
An ‘ingenious’ combination
Xanomeline, an oral muscarinic cholinergic receptor agonist, does not have direct effects on the dopamine receptor. Evidence suggests the muscarinic cholinergic system is involved in the pathophysiology of schizophrenia.
However, there may be dose-dependent adverse events with the medication, such as nausea, vomiting, diarrhea, sweating, and hypersalivation from stimulation of peripheral muscarinic cholinergic receptors.
That’s where trospium chloride, an oral panmuscarinic receptor antagonist approved for treating overactive bladder, comes in. It does not reach detectable levels in the cerebrospinal fluid and should avoid adverse central nervous system effects.
Dr. Lieberman said the idea of the drug combination is “ingenious.”
The new phase 2, multisite study included adult patients with a validated diagnosis of schizophrenia who were hospitalized with an acute exacerbation of psychosis, and who were free of antipsychotic medication for at least 2 weeks.
Participants were required to have a baseline Positive and Negative Syndrome Scale (PANSS) total score of 80 points or more.
In addition to seven positive symptom items, including delusions, hallucinations, and conceptual disorganization, the PANSS has seven negative symptom items. These include restricted emotional expression, paucity of speech, and diminished interest, social drive, and activity. Each item is scored from 1 to 7, with higher scores indicating more severe symptoms.
Patients also had to have a score on the Clinical Global Impression–Severity (CGI-S) scale of 4 or higher. Scores on the CGI-S range from 1 to 7, with higher scores indicating greater severity of illness.
The modified intention-to-treat analysis included patients randomly assigned to receive oral xanomeline-trospium (n = 83) or placebo (n = 87).
The dosing schedule was flexible, starting with 50 mg of xanomeline and 20 mg of trospium twice daily. The schedule increased to a maximum of 125 mg of xanomeline and 30 mg of trospium twice daily, with the option of lowering the dose if there were unacceptable side effects.
Mean scores at baseline for the treatment and placebo groups were 97.7 versus 96.6 for the PANSS total score, 26.4 versus 26.3 for the positive subscore, 22.6 versus 22.8 for the negative subscore, and 5.0 versus 4.9 in the CGI-S scale.
‘Impressively robust’ effect size
The primary endpoint was change in the PANSS total score at 5 weeks. Results showed a change of –17.4 points in the treatment group and –5.9 points in the placebo group (least-squares mean difference, –11.6 points; 95% confidence interval, –16.1 to –7.1; P < .001).
The effect size, which was almost 0.8 (0.75), was “impressively robust,” said Dr. Lieberman, adding that a moderate effect size in this patient population might be in the order of 0.4 or 0.5.
“That gives hope that this drug may not just be as effective as other antipsychotics, albeit acting in a novel way and in a way that has a less of side effect burden, but that it may actually have some elements of superior efficacy,” he said.
There were significant benefits on some secondary outcomes, including change in the PANSS positive symptom subscore (–5.6 points in the treatment group vs. –2.4 points in the placebo group; least-squares mean difference, –3.2 points; 95% CI, –4.8 to –1.7; P < .001).
The active treatment also came out on top for CGI-S scores (P < .001), and PANSS negative symptom subscore (P < .001).
Because participants were hospitalized with an acute exacerbation of positive symptoms at time of study, it is difficult to determine “definitive efficacy” for negative symptoms, Dr. Lieberman noted. Negative symptoms may have improved simply because positive symptoms got better, he said.
Although the study included adults only, “there is nothing in the KarXT clinical profile that suggests it would be problematic for younger people,” Dr. Lieberman noted. This could include teenagers with first-episode psychosis.
Safety profile
Adverse events (AEs) were reported in 54% of the treatment group and 43% of the placebo group. AEs that were more common in the active treatment group included constipation (17% vs. 3%), nausea (17% vs. 4%), dry mouth (9% vs. 1%), dyspepsia (9% vs. 4%), and vomiting (9% vs. 4%). All AEs were rated as mild or moderate in severity and none resulted in discontinuation of treatment.
Rates of nausea, vomiting, and dry mouth were highest early in the trial and lower at the end, whereas constipation remained constant throughout the study.
Persistent constipation could affect the drug’s “utility” in elderly patients with cognitive issues but may be more of a “minor nuisance,” compared with other antipsychotics for those with schizophrenia, said Dr. Lieberman. He added that constipation might be mitigated with an over-the-counter treatment such as Metamucil. Importantly, there was no difference between groups in extrapyramidal symptoms.
In addition, participants receiving the active treatment did not have greater weight gain, which was about 3% versus 4% in the placebo group. The mean change in weight was 1.5 kg (3.3 lb) and 1.1 kg (2.4 lb), respectively.
Dr. Lieberman praised the manufacturer for undertaking the study.
“In an era when Big Pharma has retreated to a considerable degree from psychotropic drug development, it’s commendable that some companies have stayed the course and are succeeding in drug development,” he said.
Exciting mechanism
Commenting on the findings in an interview, Thomas Sedlak, MD, PhD, director of the Schizophrenia and Psychosis Consult Clinic and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, called some aspects of the study “exciting.”
What’s especially important is that the novel agent “acts by a different mechanism than what we have been using for maybe 60 years,” said Dr. Sedlak, who was not involved with the research.
“There has been a lot of research in the last few decades into drugs that might act by alternate mechanisms of action, but nothing’s really gotten to market for schizophrenia,” he noted.
Another interesting aspect of the study is that it examined a combination drug that aims to maximize effects on the brain while minimizing periphery effects, Dr. Sedlak said.
In addition, he called the PANSS results “respectable.”
“It’s interesting that you get a big change in PANSS total score, but it’s not as big in the positive symptom score as you might have expected, and it’s not a huge drop in negative symptoms either,” said Dr. Sedlak.
It’s possible, he said, that the drug is targeting PANSS elements not captured in the main positive or negative symptom items; for example, anxiety, depression, guilt, attention, impulse control, disorientation, and judgment.
Dr. Sedlak noted that, while the new results are exciting, the enthusiasm may not be long-lasting. “When a new drug comes out, there’s often a lot of excitement about it, but once you start using it, expectations may deflate..
“More flexible ratios” of the two components of the drug might be useful to treat individual patients, he added.
In addition, using the components by themselves might be preferable for some clinicians. “I think a lot of physicians prefer using two separate drugs so they can alter the dose of each one independently and not be stuck with the ratio the manufacturer is providing,” Dr. Sedlak said.
However, he acknowledged that studying different choices of ratios would require much larger trials.
The study was supported by Karuna Therapeutics and the Wellcome Trust. Dr. Lieberman reported serving on an advisory board for Karuna Therapeutics and Intra-Cellular Therapies. Dr. Sedlak disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel therapy that combines a muscarinic receptor agonist with an anticholinergic agent is associated with a greater reduction in psychosis symptoms, compared with placebo, new research shows.
In a randomized, phase 2 trial composed of nearly 200 participants, xanomeline-trospium (KarXT) was generally well tolerated and had none of the common side effects linked to current antipsychotics, including weight gain and extrapyramidal symptoms such as dystonia, parkinsonism, and tardive dyskinesia.
“The results showing robust therapeutic efficacy of a non–dopamine targeting antipsychotic drug is an important milestone in the advance of the therapeutics of schizophrenia and other psychotic disorders,” coinvestigator Jeffrey A. Lieberman, MD, professor and chairman in the department of psychiatry, Columbia University, New York, said in an interview.
If approved, the new agent will be a “landmark” drug, Dr. Lieberman added.
The study was published in the Feb. 25, 2021, issue of the New England Journal of Medicine.
Long journey
The journey to develop an effective schizophrenia drug that reduces psychosis symptoms without onerous side effects has been a long one full of excitement and disappointment.
First-generation antipsychotics, dating back to the 1950s, targeted the postsynaptic dopamine-2 (D2) receptor. At the time, it was a “breakthrough” similar in scope to insulin for diabetes or antibiotics for infections, said Dr. Lieberman.
That was followed by development of numerous “me too” drugs with the same mechanism of action. However, these drugs had significant side effects, especially neurologic adverse events such as parkinsonism.
In 1989, second-generation antipsychotics were introduced, beginning with clozapine. They still targeted the D2 receptor but were “kinder and gentler,” Dr. Lieberman noted. “They didn’t bind to [the receptor] with such affinity that it shut things down completely, so had fewer neurologic side effects.”
However, these agents had other adverse consequences, such as weight gain and other metabolic effects including hyperglycemia and hyperlipidemia.
Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
“The pharmaceutical industry, biotech industry, and academic psychiatric community have been desperately trying to find novel strategies for antipsychotic drug development and asking, ‘Is D2 the only holy grail or are there other ways of treating psychotic symptoms of schizophrenia?’ ” Dr. Lieberman said.
Enter KarXT – a novel combination of xanomeline with trospium.
An ‘ingenious’ combination
Xanomeline, an oral muscarinic cholinergic receptor agonist, does not have direct effects on the dopamine receptor. Evidence suggests the muscarinic cholinergic system is involved in the pathophysiology of schizophrenia.
However, there may be dose-dependent adverse events with the medication, such as nausea, vomiting, diarrhea, sweating, and hypersalivation from stimulation of peripheral muscarinic cholinergic receptors.
That’s where trospium chloride, an oral panmuscarinic receptor antagonist approved for treating overactive bladder, comes in. It does not reach detectable levels in the cerebrospinal fluid and should avoid adverse central nervous system effects.
Dr. Lieberman said the idea of the drug combination is “ingenious.”
The new phase 2, multisite study included adult patients with a validated diagnosis of schizophrenia who were hospitalized with an acute exacerbation of psychosis, and who were free of antipsychotic medication for at least 2 weeks.
Participants were required to have a baseline Positive and Negative Syndrome Scale (PANSS) total score of 80 points or more.
In addition to seven positive symptom items, including delusions, hallucinations, and conceptual disorganization, the PANSS has seven negative symptom items. These include restricted emotional expression, paucity of speech, and diminished interest, social drive, and activity. Each item is scored from 1 to 7, with higher scores indicating more severe symptoms.
Patients also had to have a score on the Clinical Global Impression–Severity (CGI-S) scale of 4 or higher. Scores on the CGI-S range from 1 to 7, with higher scores indicating greater severity of illness.
The modified intention-to-treat analysis included patients randomly assigned to receive oral xanomeline-trospium (n = 83) or placebo (n = 87).
The dosing schedule was flexible, starting with 50 mg of xanomeline and 20 mg of trospium twice daily. The schedule increased to a maximum of 125 mg of xanomeline and 30 mg of trospium twice daily, with the option of lowering the dose if there were unacceptable side effects.
Mean scores at baseline for the treatment and placebo groups were 97.7 versus 96.6 for the PANSS total score, 26.4 versus 26.3 for the positive subscore, 22.6 versus 22.8 for the negative subscore, and 5.0 versus 4.9 in the CGI-S scale.
‘Impressively robust’ effect size
The primary endpoint was change in the PANSS total score at 5 weeks. Results showed a change of –17.4 points in the treatment group and –5.9 points in the placebo group (least-squares mean difference, –11.6 points; 95% confidence interval, –16.1 to –7.1; P < .001).
The effect size, which was almost 0.8 (0.75), was “impressively robust,” said Dr. Lieberman, adding that a moderate effect size in this patient population might be in the order of 0.4 or 0.5.
“That gives hope that this drug may not just be as effective as other antipsychotics, albeit acting in a novel way and in a way that has a less of side effect burden, but that it may actually have some elements of superior efficacy,” he said.
There were significant benefits on some secondary outcomes, including change in the PANSS positive symptom subscore (–5.6 points in the treatment group vs. –2.4 points in the placebo group; least-squares mean difference, –3.2 points; 95% CI, –4.8 to –1.7; P < .001).
The active treatment also came out on top for CGI-S scores (P < .001), and PANSS negative symptom subscore (P < .001).
Because participants were hospitalized with an acute exacerbation of positive symptoms at time of study, it is difficult to determine “definitive efficacy” for negative symptoms, Dr. Lieberman noted. Negative symptoms may have improved simply because positive symptoms got better, he said.
Although the study included adults only, “there is nothing in the KarXT clinical profile that suggests it would be problematic for younger people,” Dr. Lieberman noted. This could include teenagers with first-episode psychosis.
Safety profile
Adverse events (AEs) were reported in 54% of the treatment group and 43% of the placebo group. AEs that were more common in the active treatment group included constipation (17% vs. 3%), nausea (17% vs. 4%), dry mouth (9% vs. 1%), dyspepsia (9% vs. 4%), and vomiting (9% vs. 4%). All AEs were rated as mild or moderate in severity and none resulted in discontinuation of treatment.
Rates of nausea, vomiting, and dry mouth were highest early in the trial and lower at the end, whereas constipation remained constant throughout the study.
Persistent constipation could affect the drug’s “utility” in elderly patients with cognitive issues but may be more of a “minor nuisance,” compared with other antipsychotics for those with schizophrenia, said Dr. Lieberman. He added that constipation might be mitigated with an over-the-counter treatment such as Metamucil. Importantly, there was no difference between groups in extrapyramidal symptoms.
In addition, participants receiving the active treatment did not have greater weight gain, which was about 3% versus 4% in the placebo group. The mean change in weight was 1.5 kg (3.3 lb) and 1.1 kg (2.4 lb), respectively.
Dr. Lieberman praised the manufacturer for undertaking the study.
“In an era when Big Pharma has retreated to a considerable degree from psychotropic drug development, it’s commendable that some companies have stayed the course and are succeeding in drug development,” he said.
Exciting mechanism
Commenting on the findings in an interview, Thomas Sedlak, MD, PhD, director of the Schizophrenia and Psychosis Consult Clinic and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, called some aspects of the study “exciting.”
What’s especially important is that the novel agent “acts by a different mechanism than what we have been using for maybe 60 years,” said Dr. Sedlak, who was not involved with the research.
“There has been a lot of research in the last few decades into drugs that might act by alternate mechanisms of action, but nothing’s really gotten to market for schizophrenia,” he noted.
Another interesting aspect of the study is that it examined a combination drug that aims to maximize effects on the brain while minimizing periphery effects, Dr. Sedlak said.
In addition, he called the PANSS results “respectable.”
“It’s interesting that you get a big change in PANSS total score, but it’s not as big in the positive symptom score as you might have expected, and it’s not a huge drop in negative symptoms either,” said Dr. Sedlak.
It’s possible, he said, that the drug is targeting PANSS elements not captured in the main positive or negative symptom items; for example, anxiety, depression, guilt, attention, impulse control, disorientation, and judgment.
Dr. Sedlak noted that, while the new results are exciting, the enthusiasm may not be long-lasting. “When a new drug comes out, there’s often a lot of excitement about it, but once you start using it, expectations may deflate..
“More flexible ratios” of the two components of the drug might be useful to treat individual patients, he added.
In addition, using the components by themselves might be preferable for some clinicians. “I think a lot of physicians prefer using two separate drugs so they can alter the dose of each one independently and not be stuck with the ratio the manufacturer is providing,” Dr. Sedlak said.
However, he acknowledged that studying different choices of ratios would require much larger trials.
The study was supported by Karuna Therapeutics and the Wellcome Trust. Dr. Lieberman reported serving on an advisory board for Karuna Therapeutics and Intra-Cellular Therapies. Dr. Sedlak disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A novel therapy that combines a muscarinic receptor agonist with an anticholinergic agent is associated with a greater reduction in psychosis symptoms, compared with placebo, new research shows.
In a randomized, phase 2 trial composed of nearly 200 participants, xanomeline-trospium (KarXT) was generally well tolerated and had none of the common side effects linked to current antipsychotics, including weight gain and extrapyramidal symptoms such as dystonia, parkinsonism, and tardive dyskinesia.
“The results showing robust therapeutic efficacy of a non–dopamine targeting antipsychotic drug is an important milestone in the advance of the therapeutics of schizophrenia and other psychotic disorders,” coinvestigator Jeffrey A. Lieberman, MD, professor and chairman in the department of psychiatry, Columbia University, New York, said in an interview.
If approved, the new agent will be a “landmark” drug, Dr. Lieberman added.
The study was published in the Feb. 25, 2021, issue of the New England Journal of Medicine.
Long journey
The journey to develop an effective schizophrenia drug that reduces psychosis symptoms without onerous side effects has been a long one full of excitement and disappointment.
First-generation antipsychotics, dating back to the 1950s, targeted the postsynaptic dopamine-2 (D2) receptor. At the time, it was a “breakthrough” similar in scope to insulin for diabetes or antibiotics for infections, said Dr. Lieberman.
That was followed by development of numerous “me too” drugs with the same mechanism of action. However, these drugs had significant side effects, especially neurologic adverse events such as parkinsonism.
In 1989, second-generation antipsychotics were introduced, beginning with clozapine. They still targeted the D2 receptor but were “kinder and gentler,” Dr. Lieberman noted. “They didn’t bind to [the receptor] with such affinity that it shut things down completely, so had fewer neurologic side effects.”
However, these agents had other adverse consequences, such as weight gain and other metabolic effects including hyperglycemia and hyperlipidemia.
Many have poor functional status and quality of life despite lifelong treatment with current antipsychotic agents.
“The pharmaceutical industry, biotech industry, and academic psychiatric community have been desperately trying to find novel strategies for antipsychotic drug development and asking, ‘Is D2 the only holy grail or are there other ways of treating psychotic symptoms of schizophrenia?’ ” Dr. Lieberman said.
Enter KarXT – a novel combination of xanomeline with trospium.
An ‘ingenious’ combination
Xanomeline, an oral muscarinic cholinergic receptor agonist, does not have direct effects on the dopamine receptor. Evidence suggests the muscarinic cholinergic system is involved in the pathophysiology of schizophrenia.
However, there may be dose-dependent adverse events with the medication, such as nausea, vomiting, diarrhea, sweating, and hypersalivation from stimulation of peripheral muscarinic cholinergic receptors.
That’s where trospium chloride, an oral panmuscarinic receptor antagonist approved for treating overactive bladder, comes in. It does not reach detectable levels in the cerebrospinal fluid and should avoid adverse central nervous system effects.
Dr. Lieberman said the idea of the drug combination is “ingenious.”
The new phase 2, multisite study included adult patients with a validated diagnosis of schizophrenia who were hospitalized with an acute exacerbation of psychosis, and who were free of antipsychotic medication for at least 2 weeks.
Participants were required to have a baseline Positive and Negative Syndrome Scale (PANSS) total score of 80 points or more.
In addition to seven positive symptom items, including delusions, hallucinations, and conceptual disorganization, the PANSS has seven negative symptom items. These include restricted emotional expression, paucity of speech, and diminished interest, social drive, and activity. Each item is scored from 1 to 7, with higher scores indicating more severe symptoms.
Patients also had to have a score on the Clinical Global Impression–Severity (CGI-S) scale of 4 or higher. Scores on the CGI-S range from 1 to 7, with higher scores indicating greater severity of illness.
The modified intention-to-treat analysis included patients randomly assigned to receive oral xanomeline-trospium (n = 83) or placebo (n = 87).
The dosing schedule was flexible, starting with 50 mg of xanomeline and 20 mg of trospium twice daily. The schedule increased to a maximum of 125 mg of xanomeline and 30 mg of trospium twice daily, with the option of lowering the dose if there were unacceptable side effects.
Mean scores at baseline for the treatment and placebo groups were 97.7 versus 96.6 for the PANSS total score, 26.4 versus 26.3 for the positive subscore, 22.6 versus 22.8 for the negative subscore, and 5.0 versus 4.9 in the CGI-S scale.
‘Impressively robust’ effect size
The primary endpoint was change in the PANSS total score at 5 weeks. Results showed a change of –17.4 points in the treatment group and –5.9 points in the placebo group (least-squares mean difference, –11.6 points; 95% confidence interval, –16.1 to –7.1; P < .001).
The effect size, which was almost 0.8 (0.75), was “impressively robust,” said Dr. Lieberman, adding that a moderate effect size in this patient population might be in the order of 0.4 or 0.5.
“That gives hope that this drug may not just be as effective as other antipsychotics, albeit acting in a novel way and in a way that has a less of side effect burden, but that it may actually have some elements of superior efficacy,” he said.
There were significant benefits on some secondary outcomes, including change in the PANSS positive symptom subscore (–5.6 points in the treatment group vs. –2.4 points in the placebo group; least-squares mean difference, –3.2 points; 95% CI, –4.8 to –1.7; P < .001).
The active treatment also came out on top for CGI-S scores (P < .001), and PANSS negative symptom subscore (P < .001).
Because participants were hospitalized with an acute exacerbation of positive symptoms at time of study, it is difficult to determine “definitive efficacy” for negative symptoms, Dr. Lieberman noted. Negative symptoms may have improved simply because positive symptoms got better, he said.
Although the study included adults only, “there is nothing in the KarXT clinical profile that suggests it would be problematic for younger people,” Dr. Lieberman noted. This could include teenagers with first-episode psychosis.
Safety profile
Adverse events (AEs) were reported in 54% of the treatment group and 43% of the placebo group. AEs that were more common in the active treatment group included constipation (17% vs. 3%), nausea (17% vs. 4%), dry mouth (9% vs. 1%), dyspepsia (9% vs. 4%), and vomiting (9% vs. 4%). All AEs were rated as mild or moderate in severity and none resulted in discontinuation of treatment.
Rates of nausea, vomiting, and dry mouth were highest early in the trial and lower at the end, whereas constipation remained constant throughout the study.
Persistent constipation could affect the drug’s “utility” in elderly patients with cognitive issues but may be more of a “minor nuisance,” compared with other antipsychotics for those with schizophrenia, said Dr. Lieberman. He added that constipation might be mitigated with an over-the-counter treatment such as Metamucil. Importantly, there was no difference between groups in extrapyramidal symptoms.
In addition, participants receiving the active treatment did not have greater weight gain, which was about 3% versus 4% in the placebo group. The mean change in weight was 1.5 kg (3.3 lb) and 1.1 kg (2.4 lb), respectively.
Dr. Lieberman praised the manufacturer for undertaking the study.
“In an era when Big Pharma has retreated to a considerable degree from psychotropic drug development, it’s commendable that some companies have stayed the course and are succeeding in drug development,” he said.
Exciting mechanism
Commenting on the findings in an interview, Thomas Sedlak, MD, PhD, director of the Schizophrenia and Psychosis Consult Clinic and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, called some aspects of the study “exciting.”
What’s especially important is that the novel agent “acts by a different mechanism than what we have been using for maybe 60 years,” said Dr. Sedlak, who was not involved with the research.
“There has been a lot of research in the last few decades into drugs that might act by alternate mechanisms of action, but nothing’s really gotten to market for schizophrenia,” he noted.
Another interesting aspect of the study is that it examined a combination drug that aims to maximize effects on the brain while minimizing periphery effects, Dr. Sedlak said.
In addition, he called the PANSS results “respectable.”
“It’s interesting that you get a big change in PANSS total score, but it’s not as big in the positive symptom score as you might have expected, and it’s not a huge drop in negative symptoms either,” said Dr. Sedlak.
It’s possible, he said, that the drug is targeting PANSS elements not captured in the main positive or negative symptom items; for example, anxiety, depression, guilt, attention, impulse control, disorientation, and judgment.
Dr. Sedlak noted that, while the new results are exciting, the enthusiasm may not be long-lasting. “When a new drug comes out, there’s often a lot of excitement about it, but once you start using it, expectations may deflate..
“More flexible ratios” of the two components of the drug might be useful to treat individual patients, he added.
In addition, using the components by themselves might be preferable for some clinicians. “I think a lot of physicians prefer using two separate drugs so they can alter the dose of each one independently and not be stuck with the ratio the manufacturer is providing,” Dr. Sedlak said.
However, he acknowledged that studying different choices of ratios would require much larger trials.
The study was supported by Karuna Therapeutics and the Wellcome Trust. Dr. Lieberman reported serving on an advisory board for Karuna Therapeutics and Intra-Cellular Therapies. Dr. Sedlak disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe atopic dermatitis often puts a dent in quality of life
In his role as head of the division of pediatric behavioral health at National Jewish Health, Denver, Bruce G. Bender, PhD, helps children and adults navigate the adverse effects of severe atopic dermatitis (AD) on their quality of life.
“There have been many surveys of adults with AD who report impairment of their sleep, reduced activity level, increased work absence, financial burden, emotional distress, and social avoidance,” he said at the Revolutionizing Atopic Dermatitis virtual symposium. “Similarly, children with AD or their parents report emotional distress, reduced activity, and increased school absence, social avoidance, and sleep disturbance. Families report financial burdens, conflict, particularly among the adults, social avoidance, sleep disturbance in the parents, and reduction of well-being in the siblings.”
In an effort to objectively measure sleep change in this population, Dr. Bender and colleagues recruited 14 adults with AD and 14 healthy controls who wore an ActiGraph for 1 week and completed questionnaires about sleep, itch, and quality of life. Patients with AD were awake almost twice as many minutes each night as the healthy controls (a mean of 57.3 vs. 32.3 minutes, respectively; P = .0480). Consequently, their sleep efficiency was significantly reduced based on the Pittsburgh sleep quality index (a mean of 90.6 vs. 95; P = .0305).
In another study, Dr. Bender and colleagues enrolled 20 adults with AD who underwent 2 nights of polysomnography and actigraphy. The lab was set up to measure a scratching event, which was recorded when a burst of electromyographic activity of at least 3 seconds was accompanied by a visible scratching motion. “We learned that sleep efficiency as measured by both PSG and actigraphy correlated with total body surface area and scratching index,” he said. “As we might assume, the more skin involved, the more patients scratch, the less well they sleep.”
Behavioral, neurocognitive effects
In a separate study of AD, sleep, and behavior, the researchers studied 1,041 children with asthma who were enrolled in the Childhood Asthma Management Program at eight North American sites. They used baseline parent ratings on standardized sleep and behavior rating scales and found that increased awakenings were associated with increased school absence and daytime behavior problems. “So, not only do children with AD sleep less well, but this shows up to impair their functioning during the day,” said Dr. Bender, professor of psychiatry at the University of Colorado, Denver.
In a report from Australia, researchers set out to explore the association between sleep and neurocognitive function in 21 children with eczema and 20 healthy controls. Participants underwent cognitive testing and polysomnography. The authors found that the children with eczema demonstrated lower test scores. Reduced scores were correlated with parental reports of sleep problems but not polysomnography.
In a much larger study funded by the Agency for Healthcare Research and Quality, investigators analyzed data on 354,416 children and 34,613 adults from 19 U.S. population surveys including the National Health Interview Survey 1997-2013 and the National Survey of Children’s Health 2003/4 and 2007/8. They found that AD was associated with ADHD in children (adjusted odds ratio, 1.14) and adults (aOR, 1.61). Higher odds of ADHD were found in children who had significant sleep disturbance (aOR, 16.83) and other allergic disease and asthma (aOR, 1.61).
“All of these findings show that AD can impact quality of life, especially sleep, with the result of poorer daytime functioning,” Dr. Bender said. “But those studies don’t answer this question: Are patients with AD at increased risk for psychological disorders such as depression and anxiety?”
Impact on depression, anxiety
Two systematic reviews on the topic suggest that patients with AD are twice as likely to experience depression. One was published in 2018 and the other in 2019. The 2018 review reported a little more than a twofold increase (OR, 2.19), the 2019 review a little bit less (OR, 1.71).
“At the more severe end of the depression continuum, we sometimes see suicidal ideation and suicide attempts,” Dr. Bender said. “A number of studies have asked whether these are increased in patients with AD. Quite a few studies collectively show an increased incidence of suicidal ideation. The question of suicide attempts is reflected in fewer studies. And while the result is small, it is significant. There is a significant increase reported of suicide attempts in AD patients.”
The 2018 review also found an increased incidence of anxiety in AD patients: a little more than twofold in adults (OR, 2.19) and a little less than twofold in children (OR, 1.81).
“It’s a two-way relationship between AD and psychological factors,” Dr. Bender said. “We generally think about AD – the stress that it brings, the burden that it puts on children, adults, and families. But it can work the other way around,” he said, referring to patients who have psychological problems, experience a great deal of stress, have trouble being adherent to their treatment regimen, and find it difficult to resist scratching. “The behavioral/psychological characteristics of the patient also drive the AD. It is well established that acute and chronic stress can result in a worsening of skin conditions in AD patients.”
Behavioral health interventions that have been described in the literature include cognitive therapy, stress management, biofeedback, hypnotherapy, relaxation training, mindfulness, habit reversal, and patient education – some of which have been tested in randomized trials. “All of them report a decrease in scratching as a consequence of the behavioral intervention,” Dr. Bender said.
“Other studies have been reported that look at the impact of behavioral interventions on the severity of the skin condition. Most report an improvement in the skin condition from these behavioral interventions but it’s not a perfect literature.” Critiques of these studies include the fact that there is often not enough detail about the intervention or the framework for the intervention that would allow a clinician to test an intervention in another study or actually pull that intervention into clinical practice (Cochrane Database Syst Rev. 2014 Jan 7;2014[1]:CD004054), (Int Arch Allergy Immunol.2007;144[1]:1-9).
“Some of the studies lack rigorous designs, some have sampling bias, and some have inadequate outcome measurements,” he said. “We really need additional, high-quality studies to look at what is helpful for patients with AD.”
Dr. Bender reported having no financial disclosures.
In his role as head of the division of pediatric behavioral health at National Jewish Health, Denver, Bruce G. Bender, PhD, helps children and adults navigate the adverse effects of severe atopic dermatitis (AD) on their quality of life.
“There have been many surveys of adults with AD who report impairment of their sleep, reduced activity level, increased work absence, financial burden, emotional distress, and social avoidance,” he said at the Revolutionizing Atopic Dermatitis virtual symposium. “Similarly, children with AD or their parents report emotional distress, reduced activity, and increased school absence, social avoidance, and sleep disturbance. Families report financial burdens, conflict, particularly among the adults, social avoidance, sleep disturbance in the parents, and reduction of well-being in the siblings.”
In an effort to objectively measure sleep change in this population, Dr. Bender and colleagues recruited 14 adults with AD and 14 healthy controls who wore an ActiGraph for 1 week and completed questionnaires about sleep, itch, and quality of life. Patients with AD were awake almost twice as many minutes each night as the healthy controls (a mean of 57.3 vs. 32.3 minutes, respectively; P = .0480). Consequently, their sleep efficiency was significantly reduced based on the Pittsburgh sleep quality index (a mean of 90.6 vs. 95; P = .0305).
In another study, Dr. Bender and colleagues enrolled 20 adults with AD who underwent 2 nights of polysomnography and actigraphy. The lab was set up to measure a scratching event, which was recorded when a burst of electromyographic activity of at least 3 seconds was accompanied by a visible scratching motion. “We learned that sleep efficiency as measured by both PSG and actigraphy correlated with total body surface area and scratching index,” he said. “As we might assume, the more skin involved, the more patients scratch, the less well they sleep.”
Behavioral, neurocognitive effects
In a separate study of AD, sleep, and behavior, the researchers studied 1,041 children with asthma who were enrolled in the Childhood Asthma Management Program at eight North American sites. They used baseline parent ratings on standardized sleep and behavior rating scales and found that increased awakenings were associated with increased school absence and daytime behavior problems. “So, not only do children with AD sleep less well, but this shows up to impair their functioning during the day,” said Dr. Bender, professor of psychiatry at the University of Colorado, Denver.
In a report from Australia, researchers set out to explore the association between sleep and neurocognitive function in 21 children with eczema and 20 healthy controls. Participants underwent cognitive testing and polysomnography. The authors found that the children with eczema demonstrated lower test scores. Reduced scores were correlated with parental reports of sleep problems but not polysomnography.
In a much larger study funded by the Agency for Healthcare Research and Quality, investigators analyzed data on 354,416 children and 34,613 adults from 19 U.S. population surveys including the National Health Interview Survey 1997-2013 and the National Survey of Children’s Health 2003/4 and 2007/8. They found that AD was associated with ADHD in children (adjusted odds ratio, 1.14) and adults (aOR, 1.61). Higher odds of ADHD were found in children who had significant sleep disturbance (aOR, 16.83) and other allergic disease and asthma (aOR, 1.61).
“All of these findings show that AD can impact quality of life, especially sleep, with the result of poorer daytime functioning,” Dr. Bender said. “But those studies don’t answer this question: Are patients with AD at increased risk for psychological disorders such as depression and anxiety?”
Impact on depression, anxiety
Two systematic reviews on the topic suggest that patients with AD are twice as likely to experience depression. One was published in 2018 and the other in 2019. The 2018 review reported a little more than a twofold increase (OR, 2.19), the 2019 review a little bit less (OR, 1.71).
“At the more severe end of the depression continuum, we sometimes see suicidal ideation and suicide attempts,” Dr. Bender said. “A number of studies have asked whether these are increased in patients with AD. Quite a few studies collectively show an increased incidence of suicidal ideation. The question of suicide attempts is reflected in fewer studies. And while the result is small, it is significant. There is a significant increase reported of suicide attempts in AD patients.”
The 2018 review also found an increased incidence of anxiety in AD patients: a little more than twofold in adults (OR, 2.19) and a little less than twofold in children (OR, 1.81).
“It’s a two-way relationship between AD and psychological factors,” Dr. Bender said. “We generally think about AD – the stress that it brings, the burden that it puts on children, adults, and families. But it can work the other way around,” he said, referring to patients who have psychological problems, experience a great deal of stress, have trouble being adherent to their treatment regimen, and find it difficult to resist scratching. “The behavioral/psychological characteristics of the patient also drive the AD. It is well established that acute and chronic stress can result in a worsening of skin conditions in AD patients.”
Behavioral health interventions that have been described in the literature include cognitive therapy, stress management, biofeedback, hypnotherapy, relaxation training, mindfulness, habit reversal, and patient education – some of which have been tested in randomized trials. “All of them report a decrease in scratching as a consequence of the behavioral intervention,” Dr. Bender said.
“Other studies have been reported that look at the impact of behavioral interventions on the severity of the skin condition. Most report an improvement in the skin condition from these behavioral interventions but it’s not a perfect literature.” Critiques of these studies include the fact that there is often not enough detail about the intervention or the framework for the intervention that would allow a clinician to test an intervention in another study or actually pull that intervention into clinical practice (Cochrane Database Syst Rev. 2014 Jan 7;2014[1]:CD004054), (Int Arch Allergy Immunol.2007;144[1]:1-9).
“Some of the studies lack rigorous designs, some have sampling bias, and some have inadequate outcome measurements,” he said. “We really need additional, high-quality studies to look at what is helpful for patients with AD.”
Dr. Bender reported having no financial disclosures.
In his role as head of the division of pediatric behavioral health at National Jewish Health, Denver, Bruce G. Bender, PhD, helps children and adults navigate the adverse effects of severe atopic dermatitis (AD) on their quality of life.
“There have been many surveys of adults with AD who report impairment of their sleep, reduced activity level, increased work absence, financial burden, emotional distress, and social avoidance,” he said at the Revolutionizing Atopic Dermatitis virtual symposium. “Similarly, children with AD or their parents report emotional distress, reduced activity, and increased school absence, social avoidance, and sleep disturbance. Families report financial burdens, conflict, particularly among the adults, social avoidance, sleep disturbance in the parents, and reduction of well-being in the siblings.”
In an effort to objectively measure sleep change in this population, Dr. Bender and colleagues recruited 14 adults with AD and 14 healthy controls who wore an ActiGraph for 1 week and completed questionnaires about sleep, itch, and quality of life. Patients with AD were awake almost twice as many minutes each night as the healthy controls (a mean of 57.3 vs. 32.3 minutes, respectively; P = .0480). Consequently, their sleep efficiency was significantly reduced based on the Pittsburgh sleep quality index (a mean of 90.6 vs. 95; P = .0305).
In another study, Dr. Bender and colleagues enrolled 20 adults with AD who underwent 2 nights of polysomnography and actigraphy. The lab was set up to measure a scratching event, which was recorded when a burst of electromyographic activity of at least 3 seconds was accompanied by a visible scratching motion. “We learned that sleep efficiency as measured by both PSG and actigraphy correlated with total body surface area and scratching index,” he said. “As we might assume, the more skin involved, the more patients scratch, the less well they sleep.”
Behavioral, neurocognitive effects
In a separate study of AD, sleep, and behavior, the researchers studied 1,041 children with asthma who were enrolled in the Childhood Asthma Management Program at eight North American sites. They used baseline parent ratings on standardized sleep and behavior rating scales and found that increased awakenings were associated with increased school absence and daytime behavior problems. “So, not only do children with AD sleep less well, but this shows up to impair their functioning during the day,” said Dr. Bender, professor of psychiatry at the University of Colorado, Denver.
In a report from Australia, researchers set out to explore the association between sleep and neurocognitive function in 21 children with eczema and 20 healthy controls. Participants underwent cognitive testing and polysomnography. The authors found that the children with eczema demonstrated lower test scores. Reduced scores were correlated with parental reports of sleep problems but not polysomnography.
In a much larger study funded by the Agency for Healthcare Research and Quality, investigators analyzed data on 354,416 children and 34,613 adults from 19 U.S. population surveys including the National Health Interview Survey 1997-2013 and the National Survey of Children’s Health 2003/4 and 2007/8. They found that AD was associated with ADHD in children (adjusted odds ratio, 1.14) and adults (aOR, 1.61). Higher odds of ADHD were found in children who had significant sleep disturbance (aOR, 16.83) and other allergic disease and asthma (aOR, 1.61).
“All of these findings show that AD can impact quality of life, especially sleep, with the result of poorer daytime functioning,” Dr. Bender said. “But those studies don’t answer this question: Are patients with AD at increased risk for psychological disorders such as depression and anxiety?”
Impact on depression, anxiety
Two systematic reviews on the topic suggest that patients with AD are twice as likely to experience depression. One was published in 2018 and the other in 2019. The 2018 review reported a little more than a twofold increase (OR, 2.19), the 2019 review a little bit less (OR, 1.71).
“At the more severe end of the depression continuum, we sometimes see suicidal ideation and suicide attempts,” Dr. Bender said. “A number of studies have asked whether these are increased in patients with AD. Quite a few studies collectively show an increased incidence of suicidal ideation. The question of suicide attempts is reflected in fewer studies. And while the result is small, it is significant. There is a significant increase reported of suicide attempts in AD patients.”
The 2018 review also found an increased incidence of anxiety in AD patients: a little more than twofold in adults (OR, 2.19) and a little less than twofold in children (OR, 1.81).
“It’s a two-way relationship between AD and psychological factors,” Dr. Bender said. “We generally think about AD – the stress that it brings, the burden that it puts on children, adults, and families. But it can work the other way around,” he said, referring to patients who have psychological problems, experience a great deal of stress, have trouble being adherent to their treatment regimen, and find it difficult to resist scratching. “The behavioral/psychological characteristics of the patient also drive the AD. It is well established that acute and chronic stress can result in a worsening of skin conditions in AD patients.”
Behavioral health interventions that have been described in the literature include cognitive therapy, stress management, biofeedback, hypnotherapy, relaxation training, mindfulness, habit reversal, and patient education – some of which have been tested in randomized trials. “All of them report a decrease in scratching as a consequence of the behavioral intervention,” Dr. Bender said.
“Other studies have been reported that look at the impact of behavioral interventions on the severity of the skin condition. Most report an improvement in the skin condition from these behavioral interventions but it’s not a perfect literature.” Critiques of these studies include the fact that there is often not enough detail about the intervention or the framework for the intervention that would allow a clinician to test an intervention in another study or actually pull that intervention into clinical practice (Cochrane Database Syst Rev. 2014 Jan 7;2014[1]:CD004054), (Int Arch Allergy Immunol.2007;144[1]:1-9).
“Some of the studies lack rigorous designs, some have sampling bias, and some have inadequate outcome measurements,” he said. “We really need additional, high-quality studies to look at what is helpful for patients with AD.”
Dr. Bender reported having no financial disclosures.
FROM REVOLUTIONIZING AD 2020
NfL levels linked to worse disability in real-world MS
according to new findings from a large, diverse population of patients with MS. “This is one of the largest studies to evaluate serum neurofilament light chain levels in people with MS,” said lead author Elias S. Sotirchos, MD, an assistant professor of neurology at Johns Hopkins University, Baltimore.
“An important strength of this cohort is that it is a real-world cohort of patients followed in U.S. and European MS centers,” he said. “The study captures the diversity of the MS population, including demographics, comorbidities, lifestyle factors, and clinical characteristics that may otherwise not be captured in a clinical trial population.”
The research was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Scrutinizing serum neurofilament light chain levels in a real-world cohort
Neurofilaments – neuron-specific proteins that release in response to neuroaxonal injury – have been observed to be elevated in a variety of neurologic disorders, and with a need for biomarkers in MS, there is high interest of their role in the disease. But studies involving real-world, heterogeneous MS populations are lacking, the researchers noted.
To take a broader look at the issue, Dr. Sotirchos and colleagues conducted a cross-sectional evaluation of 6,968 people with MS in the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS), a large network of MS centers in the United States and Europe.
Participants’ baseline serum neurofilament light chain levels were compared with those of 201 healthy controls in the cohort using a novel, high-throughput immunoassay (Siemens Healthineers).
Of those with MS, 1,202 (17.2%) showed elevated serum neurofilament light chain levels, above the age-specific 97.5th percentile derived from the healthy controls.
A look at key factors associated with elevations showed significant links to having progressive MS (odds ratio, 1.63), non-White race (OR, 1.43), type 2 diabetes (OR, 1.89), and smoking (current vs. never smoker; OR, 1.49).
Associations with age and symptom duration were somewhat complex, but overall, younger patients and those with shorter disease duration had the highest frequency of elevated serum neurofilament light chain levels.
Interestingly, those with a higher body mass index (BMI) showed a reduced odds of having elevated serum neurofilament light chain levels (OR, 0.83 per 5 kg/m2 increase in BMI).
Evaluation of neuroperformance measures – including walking speed, manual dexterity and processing speed, and MRI data – showed that those with elevated serum neurofilament light chain levels had worse neurologic function, lower brain parenchymal fraction, lower thalamic volume, and higher T2 lesion volume (P < .001 for all).
Dr. Sotirchos noted that the higher rates of elevations in younger people, also observed in previous clinical trials, may reflect higher early-stage disease activity. “Generally, people who are younger and earlier in the course of disease tend to have more inflammatory disease activity in MS, and that could be what we’re capturing here, but we need to better understand the pathologic correlates of elevated serum neurofilament light chain levels.”
The lower levels of neurofilament light chain with higher BMI, also recently reported in another study, likewise need further investigation, including in healthy controls, Dr. Sotirchos added. “Having lower serum neurofilament light chain levels with increasing BMI could have to do with effects of blood volume and how the serum neurofilament light chain levels is distributed in the body,” he explained.
The findings suggest that interpretation of serum neurofilament light chain levels without accounting for BMI could result in false-negative or false-positive results, Dr. Sotirchos noted. “It will be important to further evaluate this observation in control populations and account for BMI in neurofilament light chain reference ranges.”
Dr. Sotirchos added that the 17% rate of elevated serum neurofilament light chain levels seen in people with MS in the study is likely an underestimate.
“This is a cross-sectional study and represents one sample per patient, so it is a snapshot in time,” he said. “With the nature of MS, we know that people’s levels fluctuate over time.” In addition, most patients were on disease-modifying therapy for MS, so serum neurofilament light chain elevations could have been suppressed.
Applying the findings to individual patients
Commenting on the findings, Jennifer Graves, MD, PhD, director of the neuroimmunology research program at the University of California, San Diego, said the study is an important addition to the ongoing evidence on serum neurofilament light chain in MS.
“The current presented research importantly addresses the gaps we have in understanding how best to apply serum filament light chain levels to individual patients and not just using them to assess group level means of outcome measures,” she said.
“The MS PATHS collaborative is looking at multiple factors (in addition to MS activity) that drive serum neurofilament light chain levels so meaningful and practical cutoffs for what’s abnormal can be created,” said Dr. Graves, who also directs the Rady Children’s Pediatric MS Clinic in San Diego.
Dr. Graves noted that the findings on BMI were unexpected. “Elevated BMI has been shown to be associated with greater brain atrophy and greater relapses and disability in MS participants, so to have an opposite effect with serum neurofilament light chain is interesting.
“My thoughts would be that obesity is somehow affecting measurable blood levels of this marker. I think it less likely BMI has a protective effect against neurodegeneration given the observations with other MS outcome measures,” she added.
Future research
In terms of future directions, Dr. Sotirchos noted that the researchers are following the group longitudinally to further assess changes in neurofilament light chain over time, and will be looking at associations with longitudinal, clinical, and radiologic outcomes.
The current research, meanwhile, offers important insights in terms of developing precision reference ranges, he noted.
“It appears that reference ranges may need to account for sex, race, BMI, and comorbid/lifestyle factors,” Dr. Sotirchos said, “in order to potentially improve the performance of serum neurofilament light chain as a biomarker in MS and other neurological diseases.”
The study received funding from Biogen and the MS PATHS network receives funding from Biogen. Dr. Sotirchos has served on scientific advisory boards for Alexion, Viela Bio, and Genentech, and has received speaker honoraria from Viela Bio and Biogen. Dr. Graves has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new findings from a large, diverse population of patients with MS. “This is one of the largest studies to evaluate serum neurofilament light chain levels in people with MS,” said lead author Elias S. Sotirchos, MD, an assistant professor of neurology at Johns Hopkins University, Baltimore.
“An important strength of this cohort is that it is a real-world cohort of patients followed in U.S. and European MS centers,” he said. “The study captures the diversity of the MS population, including demographics, comorbidities, lifestyle factors, and clinical characteristics that may otherwise not be captured in a clinical trial population.”
The research was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Scrutinizing serum neurofilament light chain levels in a real-world cohort
Neurofilaments – neuron-specific proteins that release in response to neuroaxonal injury – have been observed to be elevated in a variety of neurologic disorders, and with a need for biomarkers in MS, there is high interest of their role in the disease. But studies involving real-world, heterogeneous MS populations are lacking, the researchers noted.
To take a broader look at the issue, Dr. Sotirchos and colleagues conducted a cross-sectional evaluation of 6,968 people with MS in the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS), a large network of MS centers in the United States and Europe.
Participants’ baseline serum neurofilament light chain levels were compared with those of 201 healthy controls in the cohort using a novel, high-throughput immunoassay (Siemens Healthineers).
Of those with MS, 1,202 (17.2%) showed elevated serum neurofilament light chain levels, above the age-specific 97.5th percentile derived from the healthy controls.
A look at key factors associated with elevations showed significant links to having progressive MS (odds ratio, 1.63), non-White race (OR, 1.43), type 2 diabetes (OR, 1.89), and smoking (current vs. never smoker; OR, 1.49).
Associations with age and symptom duration were somewhat complex, but overall, younger patients and those with shorter disease duration had the highest frequency of elevated serum neurofilament light chain levels.
Interestingly, those with a higher body mass index (BMI) showed a reduced odds of having elevated serum neurofilament light chain levels (OR, 0.83 per 5 kg/m2 increase in BMI).
Evaluation of neuroperformance measures – including walking speed, manual dexterity and processing speed, and MRI data – showed that those with elevated serum neurofilament light chain levels had worse neurologic function, lower brain parenchymal fraction, lower thalamic volume, and higher T2 lesion volume (P < .001 for all).
Dr. Sotirchos noted that the higher rates of elevations in younger people, also observed in previous clinical trials, may reflect higher early-stage disease activity. “Generally, people who are younger and earlier in the course of disease tend to have more inflammatory disease activity in MS, and that could be what we’re capturing here, but we need to better understand the pathologic correlates of elevated serum neurofilament light chain levels.”
The lower levels of neurofilament light chain with higher BMI, also recently reported in another study, likewise need further investigation, including in healthy controls, Dr. Sotirchos added. “Having lower serum neurofilament light chain levels with increasing BMI could have to do with effects of blood volume and how the serum neurofilament light chain levels is distributed in the body,” he explained.
The findings suggest that interpretation of serum neurofilament light chain levels without accounting for BMI could result in false-negative or false-positive results, Dr. Sotirchos noted. “It will be important to further evaluate this observation in control populations and account for BMI in neurofilament light chain reference ranges.”
Dr. Sotirchos added that the 17% rate of elevated serum neurofilament light chain levels seen in people with MS in the study is likely an underestimate.
“This is a cross-sectional study and represents one sample per patient, so it is a snapshot in time,” he said. “With the nature of MS, we know that people’s levels fluctuate over time.” In addition, most patients were on disease-modifying therapy for MS, so serum neurofilament light chain elevations could have been suppressed.
Applying the findings to individual patients
Commenting on the findings, Jennifer Graves, MD, PhD, director of the neuroimmunology research program at the University of California, San Diego, said the study is an important addition to the ongoing evidence on serum neurofilament light chain in MS.
“The current presented research importantly addresses the gaps we have in understanding how best to apply serum filament light chain levels to individual patients and not just using them to assess group level means of outcome measures,” she said.
“The MS PATHS collaborative is looking at multiple factors (in addition to MS activity) that drive serum neurofilament light chain levels so meaningful and practical cutoffs for what’s abnormal can be created,” said Dr. Graves, who also directs the Rady Children’s Pediatric MS Clinic in San Diego.
Dr. Graves noted that the findings on BMI were unexpected. “Elevated BMI has been shown to be associated with greater brain atrophy and greater relapses and disability in MS participants, so to have an opposite effect with serum neurofilament light chain is interesting.
“My thoughts would be that obesity is somehow affecting measurable blood levels of this marker. I think it less likely BMI has a protective effect against neurodegeneration given the observations with other MS outcome measures,” she added.
Future research
In terms of future directions, Dr. Sotirchos noted that the researchers are following the group longitudinally to further assess changes in neurofilament light chain over time, and will be looking at associations with longitudinal, clinical, and radiologic outcomes.
The current research, meanwhile, offers important insights in terms of developing precision reference ranges, he noted.
“It appears that reference ranges may need to account for sex, race, BMI, and comorbid/lifestyle factors,” Dr. Sotirchos said, “in order to potentially improve the performance of serum neurofilament light chain as a biomarker in MS and other neurological diseases.”
The study received funding from Biogen and the MS PATHS network receives funding from Biogen. Dr. Sotirchos has served on scientific advisory boards for Alexion, Viela Bio, and Genentech, and has received speaker honoraria from Viela Bio and Biogen. Dr. Graves has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to new findings from a large, diverse population of patients with MS. “This is one of the largest studies to evaluate serum neurofilament light chain levels in people with MS,” said lead author Elias S. Sotirchos, MD, an assistant professor of neurology at Johns Hopkins University, Baltimore.
“An important strength of this cohort is that it is a real-world cohort of patients followed in U.S. and European MS centers,” he said. “The study captures the diversity of the MS population, including demographics, comorbidities, lifestyle factors, and clinical characteristics that may otherwise not be captured in a clinical trial population.”
The research was presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Scrutinizing serum neurofilament light chain levels in a real-world cohort
Neurofilaments – neuron-specific proteins that release in response to neuroaxonal injury – have been observed to be elevated in a variety of neurologic disorders, and with a need for biomarkers in MS, there is high interest of their role in the disease. But studies involving real-world, heterogeneous MS populations are lacking, the researchers noted.
To take a broader look at the issue, Dr. Sotirchos and colleagues conducted a cross-sectional evaluation of 6,968 people with MS in the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS), a large network of MS centers in the United States and Europe.
Participants’ baseline serum neurofilament light chain levels were compared with those of 201 healthy controls in the cohort using a novel, high-throughput immunoassay (Siemens Healthineers).
Of those with MS, 1,202 (17.2%) showed elevated serum neurofilament light chain levels, above the age-specific 97.5th percentile derived from the healthy controls.
A look at key factors associated with elevations showed significant links to having progressive MS (odds ratio, 1.63), non-White race (OR, 1.43), type 2 diabetes (OR, 1.89), and smoking (current vs. never smoker; OR, 1.49).
Associations with age and symptom duration were somewhat complex, but overall, younger patients and those with shorter disease duration had the highest frequency of elevated serum neurofilament light chain levels.
Interestingly, those with a higher body mass index (BMI) showed a reduced odds of having elevated serum neurofilament light chain levels (OR, 0.83 per 5 kg/m2 increase in BMI).
Evaluation of neuroperformance measures – including walking speed, manual dexterity and processing speed, and MRI data – showed that those with elevated serum neurofilament light chain levels had worse neurologic function, lower brain parenchymal fraction, lower thalamic volume, and higher T2 lesion volume (P < .001 for all).
Dr. Sotirchos noted that the higher rates of elevations in younger people, also observed in previous clinical trials, may reflect higher early-stage disease activity. “Generally, people who are younger and earlier in the course of disease tend to have more inflammatory disease activity in MS, and that could be what we’re capturing here, but we need to better understand the pathologic correlates of elevated serum neurofilament light chain levels.”
The lower levels of neurofilament light chain with higher BMI, also recently reported in another study, likewise need further investigation, including in healthy controls, Dr. Sotirchos added. “Having lower serum neurofilament light chain levels with increasing BMI could have to do with effects of blood volume and how the serum neurofilament light chain levels is distributed in the body,” he explained.
The findings suggest that interpretation of serum neurofilament light chain levels without accounting for BMI could result in false-negative or false-positive results, Dr. Sotirchos noted. “It will be important to further evaluate this observation in control populations and account for BMI in neurofilament light chain reference ranges.”
Dr. Sotirchos added that the 17% rate of elevated serum neurofilament light chain levels seen in people with MS in the study is likely an underestimate.
“This is a cross-sectional study and represents one sample per patient, so it is a snapshot in time,” he said. “With the nature of MS, we know that people’s levels fluctuate over time.” In addition, most patients were on disease-modifying therapy for MS, so serum neurofilament light chain elevations could have been suppressed.
Applying the findings to individual patients
Commenting on the findings, Jennifer Graves, MD, PhD, director of the neuroimmunology research program at the University of California, San Diego, said the study is an important addition to the ongoing evidence on serum neurofilament light chain in MS.
“The current presented research importantly addresses the gaps we have in understanding how best to apply serum filament light chain levels to individual patients and not just using them to assess group level means of outcome measures,” she said.
“The MS PATHS collaborative is looking at multiple factors (in addition to MS activity) that drive serum neurofilament light chain levels so meaningful and practical cutoffs for what’s abnormal can be created,” said Dr. Graves, who also directs the Rady Children’s Pediatric MS Clinic in San Diego.
Dr. Graves noted that the findings on BMI were unexpected. “Elevated BMI has been shown to be associated with greater brain atrophy and greater relapses and disability in MS participants, so to have an opposite effect with serum neurofilament light chain is interesting.
“My thoughts would be that obesity is somehow affecting measurable blood levels of this marker. I think it less likely BMI has a protective effect against neurodegeneration given the observations with other MS outcome measures,” she added.
Future research
In terms of future directions, Dr. Sotirchos noted that the researchers are following the group longitudinally to further assess changes in neurofilament light chain over time, and will be looking at associations with longitudinal, clinical, and radiologic outcomes.
The current research, meanwhile, offers important insights in terms of developing precision reference ranges, he noted.
“It appears that reference ranges may need to account for sex, race, BMI, and comorbid/lifestyle factors,” Dr. Sotirchos said, “in order to potentially improve the performance of serum neurofilament light chain as a biomarker in MS and other neurological diseases.”
The study received funding from Biogen and the MS PATHS network receives funding from Biogen. Dr. Sotirchos has served on scientific advisory boards for Alexion, Viela Bio, and Genentech, and has received speaker honoraria from Viela Bio and Biogen. Dr. Graves has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACTRIMS 2021
Study clarifies who gets post–COVID-19 interstitial lung disease
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Opioid use common for pain in multiple sclerosis
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
FROM ACTRIMS FORUM 2021
Decline in children’s COVID-19 cases slows
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
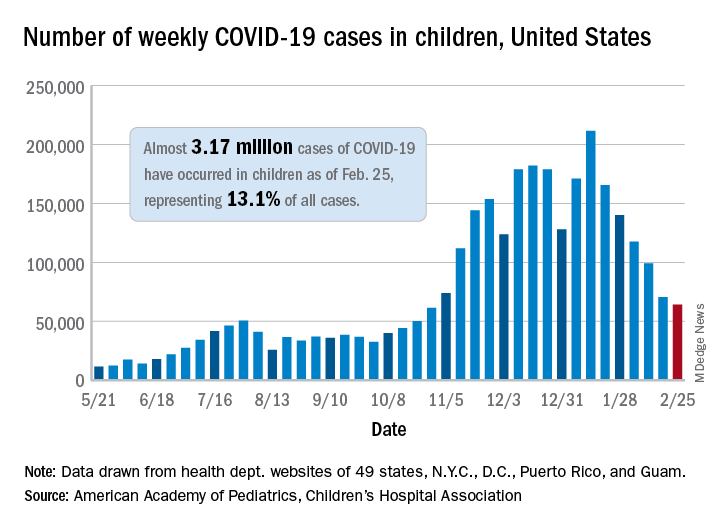
That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.
The number of new COVID-19 cases in children declined for the sixth consecutive week, but the drop was the smallest yet, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That drop of almost 6,400 cases, or 9.0%, falls short of the declines recorded in any the previous 5 weeks, which ranged from 18,000 to 46,000 cases and 15.3% to 28.7%, based on data from the heath departments of 49 states (excluding New York), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of children infected with SARS-CoV-2 is up to almost 3.17 million, which represents 13.1% of cases among all age groups. That cumulative proportion was unchanged from the previous week, which has occurred only three other times over the course of the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Despite the 6-week decline in new cases, however, the cumulative rate continued to climb, rising from 4,124 cases per 100,000 children to 4,209 for the week of Feb. 19-25. The states, not surprisingly, fall on both sides of that national tally. The lowest rates can be found in Hawaii (1,040 per 100,000 children), Vermont (2,111 per 100,000), and Maine (2,394), while the highest rates were recorded in North Dakota (8,580), Tennessee (7,851), and Rhode Island (7,223), the AAP and CHA said.
The number of new child deaths, nine, stayed in single digits for a second consecutive week, although it was up from six deaths reported a week earlier. Total COVID-19–related deaths in children now number 256, which represents just 0.06% of coronavirus deaths for all ages among the 43 states (along with New York City and Guam) reporting such data.
Among those jurisdictions, Texas (40), Arizona (27), and New York City (23) have reported the most deaths in children, while nine states and the District of Columbia have reported no deaths yet, the AAP and CHA noted.