User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
The best exercises for BP control? European statement sorts it out
Recommendations for prescribing exercise to control high blood pressure have been put forward by various medical organizations and expert panels, but finding the bandwidth to craft personalized exercise training for their patients poses a challenge for clinicians.
Now, European cardiology societies have issued a consensus statement that offers an algorithm of sorts for developing personalized exercise programs as part of overall management approach for patients with or at risk of high BP.
The statement, published in the European Journal of Preventive Cardiology and issued by the European Association of Preventive Cardiology and the European Society of Cardiology Council on Hypertension, claims to be the first document to focus on personalized exercise for BP.
The statement draws on a systematic review, including meta-analyses, to produce guidance on how to lower BP in three specific types of patients: Those with hypertension (>140/90 mm Hg), high-normal blood pressure (130-139/85-89 mm Hg), and normal blood pressure (<130/84 mm Hg).
By making recommendations for these three specific groups, along with providing guidance for combined exercise – that is, blending aerobic exercise with resistance training (RT) – the consensus statement goes one step further than recommendations other organizations have issued, Matthew W. Martinez, MD, said in an interview.
“What it adds is an algorithmic approach, if you will,” said Dr. Martinez, a sports medicine cardiologist at Morristown (N.J.) Medical Center. “There are some recommendations to help the clinicians to decide what they’re going to offer individuals, but what’s a challenge for us when seeing patients is finding the time to deliver the message and explain how valuable nutrition and exercise are.”
Guidelines, updates, and statements that include the role of exercise in BP control have been issued by the European Society of Cardiology, American Heart Association, and American College of Sports Medicine (Med Sci Sports Exercise. 2019;51:1314-23).
The European consensus statement includes the expected range of BP lowering for each activity. For example, aerobic exercise for patients with hypertension should lead to a reduction from –4.9 to –12 mm Hg systolic and –3.4 to –5.8 mm Hg diastolic.
The consensus statement recommends the following exercise priorities based on a patient’s blood pressure:
- Hypertension: Aerobic training (AT) as a first-line exercise therapy; and low- to moderate-intensity RT – equally using dynamic and isometric RT – as second-line therapy. In non-White patients, dynamic RT should be considered as a first-line therapy. RT can be combined with aerobic exercise on an individual basis if the clinician determines either form of RT would provide a metabolic benefit.
- High-to-normal BP: Dynamic RT as a first-line exercise, which the systematic review determined led to greater BP reduction than that of aerobic training. “Isometric RT is likely to elicit similar if not superior BP-lowering effects as [dynamic RT], but the level of evidence is low and the available data are scarce,” wrote first author Henner Hanssen, MD, of the University of Basel, Switzerland, and coauthors. Combining dynamic resistance training with aerobic training “may be preferable” to dynamic RT alone in patients with a combination of cardiovascular risk factors.
- Normal BP: Isometric RT may be indicated as a first-line intervention in individuals with a family or gestational history or obese or overweight people currently with normal BP. This advice includes a caveat: “The number of studies is limited and the 95% confidence intervals are large,” Dr. Hanssen and coauthors noted. AT is also an option in these patients, with more high-quality meta-analyses than the recommendation for isometric RT. “Hence, the BP-lowering effects of [isometric RT] as compared to AT may be overestimated and both exercise modalities may have similar BP-lowering effects in individuals with normotension,” wrote the consensus statement authors.
They note that more research is needed to validate the BP-lowering effects of combined exercise.
The statement acknowledges the difficulty clinicians face in managing patients with high blood pressure. “From a socioeconomic health perspective, it is a major challenge to develop, promote, and implement individually tailored exercise programs for patients with hypertension under consideration of sustainable costs,” wrote Dr. Hanssen and coauthors.
Dr. Martinez noted that one strength of the consensus statement is that it addresses the impact exercise can have on vascular health and metabolic function. And, it points out existing knowledge gaps.
“Are we going to see greater applicability of this as we use IT health technology?” he asked. “Are wearables and telehealth going to help deliver this message more easily, more frequently? Is there work to be done in terms of differences in gender? Do men and women respond differently, and is there a different exercise prescription based on that as well as ethnicity? We well know there’s a different treatment for African Americans compared to other ethnic groups.”
The statement also raises the stakes for using exercise as part of a multifaceted, integrated approach to hypertension management, he said.
“It’s not enough to talk just about exercise or nutrition, or to just give an antihypertension medicine,” Dr. Martinez said. “Perhaps the sweet spot is in integrating an approach that includes all three.”
Consensus statement coauthor Antonio Coca, MD, reported financial relationships with Abbott, Berlin-Chemie, Biolab, Boehringer-Ingelheim, Ferrer, Menarini, Merck, Novartis and Sanofi-Aventis. Coauthor Maria Simonenko, MD, reported financial relationships with Novartis and Sanofi-Aventis. Linda Pescatello, PhD, is lead author of the American College of Sports Medicine 2019 statement. Dr. Hanssen and all other authors have no disclosures. Dr. Martinez has no relevant relationships to disclose.
Recommendations for prescribing exercise to control high blood pressure have been put forward by various medical organizations and expert panels, but finding the bandwidth to craft personalized exercise training for their patients poses a challenge for clinicians.
Now, European cardiology societies have issued a consensus statement that offers an algorithm of sorts for developing personalized exercise programs as part of overall management approach for patients with or at risk of high BP.
The statement, published in the European Journal of Preventive Cardiology and issued by the European Association of Preventive Cardiology and the European Society of Cardiology Council on Hypertension, claims to be the first document to focus on personalized exercise for BP.
The statement draws on a systematic review, including meta-analyses, to produce guidance on how to lower BP in three specific types of patients: Those with hypertension (>140/90 mm Hg), high-normal blood pressure (130-139/85-89 mm Hg), and normal blood pressure (<130/84 mm Hg).
By making recommendations for these three specific groups, along with providing guidance for combined exercise – that is, blending aerobic exercise with resistance training (RT) – the consensus statement goes one step further than recommendations other organizations have issued, Matthew W. Martinez, MD, said in an interview.
“What it adds is an algorithmic approach, if you will,” said Dr. Martinez, a sports medicine cardiologist at Morristown (N.J.) Medical Center. “There are some recommendations to help the clinicians to decide what they’re going to offer individuals, but what’s a challenge for us when seeing patients is finding the time to deliver the message and explain how valuable nutrition and exercise are.”
Guidelines, updates, and statements that include the role of exercise in BP control have been issued by the European Society of Cardiology, American Heart Association, and American College of Sports Medicine (Med Sci Sports Exercise. 2019;51:1314-23).
The European consensus statement includes the expected range of BP lowering for each activity. For example, aerobic exercise for patients with hypertension should lead to a reduction from –4.9 to –12 mm Hg systolic and –3.4 to –5.8 mm Hg diastolic.
The consensus statement recommends the following exercise priorities based on a patient’s blood pressure:
- Hypertension: Aerobic training (AT) as a first-line exercise therapy; and low- to moderate-intensity RT – equally using dynamic and isometric RT – as second-line therapy. In non-White patients, dynamic RT should be considered as a first-line therapy. RT can be combined with aerobic exercise on an individual basis if the clinician determines either form of RT would provide a metabolic benefit.
- High-to-normal BP: Dynamic RT as a first-line exercise, which the systematic review determined led to greater BP reduction than that of aerobic training. “Isometric RT is likely to elicit similar if not superior BP-lowering effects as [dynamic RT], but the level of evidence is low and the available data are scarce,” wrote first author Henner Hanssen, MD, of the University of Basel, Switzerland, and coauthors. Combining dynamic resistance training with aerobic training “may be preferable” to dynamic RT alone in patients with a combination of cardiovascular risk factors.
- Normal BP: Isometric RT may be indicated as a first-line intervention in individuals with a family or gestational history or obese or overweight people currently with normal BP. This advice includes a caveat: “The number of studies is limited and the 95% confidence intervals are large,” Dr. Hanssen and coauthors noted. AT is also an option in these patients, with more high-quality meta-analyses than the recommendation for isometric RT. “Hence, the BP-lowering effects of [isometric RT] as compared to AT may be overestimated and both exercise modalities may have similar BP-lowering effects in individuals with normotension,” wrote the consensus statement authors.
They note that more research is needed to validate the BP-lowering effects of combined exercise.
The statement acknowledges the difficulty clinicians face in managing patients with high blood pressure. “From a socioeconomic health perspective, it is a major challenge to develop, promote, and implement individually tailored exercise programs for patients with hypertension under consideration of sustainable costs,” wrote Dr. Hanssen and coauthors.
Dr. Martinez noted that one strength of the consensus statement is that it addresses the impact exercise can have on vascular health and metabolic function. And, it points out existing knowledge gaps.
“Are we going to see greater applicability of this as we use IT health technology?” he asked. “Are wearables and telehealth going to help deliver this message more easily, more frequently? Is there work to be done in terms of differences in gender? Do men and women respond differently, and is there a different exercise prescription based on that as well as ethnicity? We well know there’s a different treatment for African Americans compared to other ethnic groups.”
The statement also raises the stakes for using exercise as part of a multifaceted, integrated approach to hypertension management, he said.
“It’s not enough to talk just about exercise or nutrition, or to just give an antihypertension medicine,” Dr. Martinez said. “Perhaps the sweet spot is in integrating an approach that includes all three.”
Consensus statement coauthor Antonio Coca, MD, reported financial relationships with Abbott, Berlin-Chemie, Biolab, Boehringer-Ingelheim, Ferrer, Menarini, Merck, Novartis and Sanofi-Aventis. Coauthor Maria Simonenko, MD, reported financial relationships with Novartis and Sanofi-Aventis. Linda Pescatello, PhD, is lead author of the American College of Sports Medicine 2019 statement. Dr. Hanssen and all other authors have no disclosures. Dr. Martinez has no relevant relationships to disclose.
Recommendations for prescribing exercise to control high blood pressure have been put forward by various medical organizations and expert panels, but finding the bandwidth to craft personalized exercise training for their patients poses a challenge for clinicians.
Now, European cardiology societies have issued a consensus statement that offers an algorithm of sorts for developing personalized exercise programs as part of overall management approach for patients with or at risk of high BP.
The statement, published in the European Journal of Preventive Cardiology and issued by the European Association of Preventive Cardiology and the European Society of Cardiology Council on Hypertension, claims to be the first document to focus on personalized exercise for BP.
The statement draws on a systematic review, including meta-analyses, to produce guidance on how to lower BP in three specific types of patients: Those with hypertension (>140/90 mm Hg), high-normal blood pressure (130-139/85-89 mm Hg), and normal blood pressure (<130/84 mm Hg).
By making recommendations for these three specific groups, along with providing guidance for combined exercise – that is, blending aerobic exercise with resistance training (RT) – the consensus statement goes one step further than recommendations other organizations have issued, Matthew W. Martinez, MD, said in an interview.
“What it adds is an algorithmic approach, if you will,” said Dr. Martinez, a sports medicine cardiologist at Morristown (N.J.) Medical Center. “There are some recommendations to help the clinicians to decide what they’re going to offer individuals, but what’s a challenge for us when seeing patients is finding the time to deliver the message and explain how valuable nutrition and exercise are.”
Guidelines, updates, and statements that include the role of exercise in BP control have been issued by the European Society of Cardiology, American Heart Association, and American College of Sports Medicine (Med Sci Sports Exercise. 2019;51:1314-23).
The European consensus statement includes the expected range of BP lowering for each activity. For example, aerobic exercise for patients with hypertension should lead to a reduction from –4.9 to –12 mm Hg systolic and –3.4 to –5.8 mm Hg diastolic.
The consensus statement recommends the following exercise priorities based on a patient’s blood pressure:
- Hypertension: Aerobic training (AT) as a first-line exercise therapy; and low- to moderate-intensity RT – equally using dynamic and isometric RT – as second-line therapy. In non-White patients, dynamic RT should be considered as a first-line therapy. RT can be combined with aerobic exercise on an individual basis if the clinician determines either form of RT would provide a metabolic benefit.
- High-to-normal BP: Dynamic RT as a first-line exercise, which the systematic review determined led to greater BP reduction than that of aerobic training. “Isometric RT is likely to elicit similar if not superior BP-lowering effects as [dynamic RT], but the level of evidence is low and the available data are scarce,” wrote first author Henner Hanssen, MD, of the University of Basel, Switzerland, and coauthors. Combining dynamic resistance training with aerobic training “may be preferable” to dynamic RT alone in patients with a combination of cardiovascular risk factors.
- Normal BP: Isometric RT may be indicated as a first-line intervention in individuals with a family or gestational history or obese or overweight people currently with normal BP. This advice includes a caveat: “The number of studies is limited and the 95% confidence intervals are large,” Dr. Hanssen and coauthors noted. AT is also an option in these patients, with more high-quality meta-analyses than the recommendation for isometric RT. “Hence, the BP-lowering effects of [isometric RT] as compared to AT may be overestimated and both exercise modalities may have similar BP-lowering effects in individuals with normotension,” wrote the consensus statement authors.
They note that more research is needed to validate the BP-lowering effects of combined exercise.
The statement acknowledges the difficulty clinicians face in managing patients with high blood pressure. “From a socioeconomic health perspective, it is a major challenge to develop, promote, and implement individually tailored exercise programs for patients with hypertension under consideration of sustainable costs,” wrote Dr. Hanssen and coauthors.
Dr. Martinez noted that one strength of the consensus statement is that it addresses the impact exercise can have on vascular health and metabolic function. And, it points out existing knowledge gaps.
“Are we going to see greater applicability of this as we use IT health technology?” he asked. “Are wearables and telehealth going to help deliver this message more easily, more frequently? Is there work to be done in terms of differences in gender? Do men and women respond differently, and is there a different exercise prescription based on that as well as ethnicity? We well know there’s a different treatment for African Americans compared to other ethnic groups.”
The statement also raises the stakes for using exercise as part of a multifaceted, integrated approach to hypertension management, he said.
“It’s not enough to talk just about exercise or nutrition, or to just give an antihypertension medicine,” Dr. Martinez said. “Perhaps the sweet spot is in integrating an approach that includes all three.”
Consensus statement coauthor Antonio Coca, MD, reported financial relationships with Abbott, Berlin-Chemie, Biolab, Boehringer-Ingelheim, Ferrer, Menarini, Merck, Novartis and Sanofi-Aventis. Coauthor Maria Simonenko, MD, reported financial relationships with Novartis and Sanofi-Aventis. Linda Pescatello, PhD, is lead author of the American College of Sports Medicine 2019 statement. Dr. Hanssen and all other authors have no disclosures. Dr. Martinez has no relevant relationships to disclose.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
Cardiologist forks out $2M to resolve unnecessary testing claims
Michigan cardiologist Dinesh M. Shah, MD, has paid the United States $2 million to resolve claims he violated the False Claims Act by knowingly billing federal health care programs for diagnostic tests that were unnecessary or not performed, the Department of Justice announced.
The settlement resolves allegations that, from 2006 to 2017, Dr. Shah and his practice, Michigan Physicians Group (MPG), of which he is sole owner, billed Medicare, Medicaid, and TRICARE for unnecessary diagnostic tests, including ankle brachial index and toe brachial index tests that were routinely performed on patients without first being ordered by a physician and without regard to medical necessity.
The prosecutors also alleged that Dr. Shah was routinely ordering, and MPG was providing, unnecessary nuclear stress tests to some patients.
“Subjecting patients to unnecessary testing in order to fill one’s pockets with taxpayer funds will not be tolerated. Such practices are particularly concerning because overuse of some tests can be harmful to patients,” acting U.S. Attorney Saima Mohsin said in the news release. “With these lawsuits and the accompanying resolution, Dr. Shah and Michigan Physicians Group are being held to account for these exploitative and improper past practices.”
In addition to the settlement, Dr. Shah and MPG entered into an Integrity Agreement with the Office of Inspector General for the Department of Health & Human Services, which will provide oversight of Dr. Shah and MPG’s billing practices for a 3-year period.
There was “no determination of liability” with the settlement, according to the Department of Justice. Dr. Shah’s case was sparked by two whistleblower lawsuits filed by Arlene Klinke and Khrystyna Malva, both former MPG employees.
The settlement comes after a years-long investigation by the HHS acting on behalf of TRICARE, a health care program for active and retired military members. Allegations that William Beaumont Hospital in Royal Oak, Mich., paid eight physicians excessive compensation to increase patient referrals led to an $84.5 million settlement in 2018.
Dr. Shah was one of three private practice cardiologists who denied involvement in the scheme but were named in the settlement, according to Crain’s Detroit Business.
A version of this article first appeared on Medscape.com.
Michigan cardiologist Dinesh M. Shah, MD, has paid the United States $2 million to resolve claims he violated the False Claims Act by knowingly billing federal health care programs for diagnostic tests that were unnecessary or not performed, the Department of Justice announced.
The settlement resolves allegations that, from 2006 to 2017, Dr. Shah and his practice, Michigan Physicians Group (MPG), of which he is sole owner, billed Medicare, Medicaid, and TRICARE for unnecessary diagnostic tests, including ankle brachial index and toe brachial index tests that were routinely performed on patients without first being ordered by a physician and without regard to medical necessity.
The prosecutors also alleged that Dr. Shah was routinely ordering, and MPG was providing, unnecessary nuclear stress tests to some patients.
“Subjecting patients to unnecessary testing in order to fill one’s pockets with taxpayer funds will not be tolerated. Such practices are particularly concerning because overuse of some tests can be harmful to patients,” acting U.S. Attorney Saima Mohsin said in the news release. “With these lawsuits and the accompanying resolution, Dr. Shah and Michigan Physicians Group are being held to account for these exploitative and improper past practices.”
In addition to the settlement, Dr. Shah and MPG entered into an Integrity Agreement with the Office of Inspector General for the Department of Health & Human Services, which will provide oversight of Dr. Shah and MPG’s billing practices for a 3-year period.
There was “no determination of liability” with the settlement, according to the Department of Justice. Dr. Shah’s case was sparked by two whistleblower lawsuits filed by Arlene Klinke and Khrystyna Malva, both former MPG employees.
The settlement comes after a years-long investigation by the HHS acting on behalf of TRICARE, a health care program for active and retired military members. Allegations that William Beaumont Hospital in Royal Oak, Mich., paid eight physicians excessive compensation to increase patient referrals led to an $84.5 million settlement in 2018.
Dr. Shah was one of three private practice cardiologists who denied involvement in the scheme but were named in the settlement, according to Crain’s Detroit Business.
A version of this article first appeared on Medscape.com.
Michigan cardiologist Dinesh M. Shah, MD, has paid the United States $2 million to resolve claims he violated the False Claims Act by knowingly billing federal health care programs for diagnostic tests that were unnecessary or not performed, the Department of Justice announced.
The settlement resolves allegations that, from 2006 to 2017, Dr. Shah and his practice, Michigan Physicians Group (MPG), of which he is sole owner, billed Medicare, Medicaid, and TRICARE for unnecessary diagnostic tests, including ankle brachial index and toe brachial index tests that were routinely performed on patients without first being ordered by a physician and without regard to medical necessity.
The prosecutors also alleged that Dr. Shah was routinely ordering, and MPG was providing, unnecessary nuclear stress tests to some patients.
“Subjecting patients to unnecessary testing in order to fill one’s pockets with taxpayer funds will not be tolerated. Such practices are particularly concerning because overuse of some tests can be harmful to patients,” acting U.S. Attorney Saima Mohsin said in the news release. “With these lawsuits and the accompanying resolution, Dr. Shah and Michigan Physicians Group are being held to account for these exploitative and improper past practices.”
In addition to the settlement, Dr. Shah and MPG entered into an Integrity Agreement with the Office of Inspector General for the Department of Health & Human Services, which will provide oversight of Dr. Shah and MPG’s billing practices for a 3-year period.
There was “no determination of liability” with the settlement, according to the Department of Justice. Dr. Shah’s case was sparked by two whistleblower lawsuits filed by Arlene Klinke and Khrystyna Malva, both former MPG employees.
The settlement comes after a years-long investigation by the HHS acting on behalf of TRICARE, a health care program for active and retired military members. Allegations that William Beaumont Hospital in Royal Oak, Mich., paid eight physicians excessive compensation to increase patient referrals led to an $84.5 million settlement in 2018.
Dr. Shah was one of three private practice cardiologists who denied involvement in the scheme but were named in the settlement, according to Crain’s Detroit Business.
A version of this article first appeared on Medscape.com.
FDA okays transcatheter pulmonary valve for congenital heart disease
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
Encephalopathy common, often lethal in hospitalized patients with COVID-19
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.
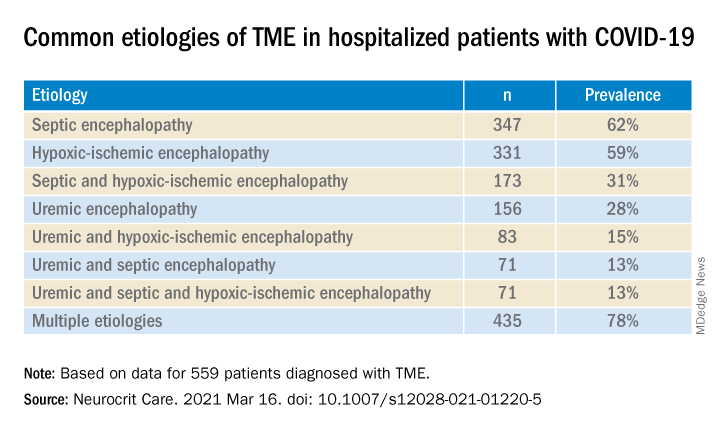
Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROCRITICAL CARE
Experts highlight recent breakthroughs in psoriatic arthritis
Apremilast (Otezla) monotherapy may be an effective option in oligoarticular psoriatic arthritis, Alexis R. Ogdie, MD, reported at the 2021 Rheumatology Winter Clinical Symposium.
Her analysis of apremilast data from the CORRONA Registry was among several recent highlights in psoriatic arthritis (PsA) cited by speakers at the meeting. Other significant developments included a large pan-Scandinavian study that reassuringly found no increased risk of solid cancers in tumor necrosis factor (TNF) inhibitor–treated patients with PsA, and evidence to suggest a sex difference in the efficacy of both secukinumab (Cosentyx) and adalimumab (Humira), with men responding better than women to two biologics with differing mechanisms of action.
A role for apremilast in oligoarticular disease?
Dr. Ogdie presented an analysis of 150 patients in the U.S. observational CORRONA Registry who initiated monotherapy for oligoarticular PsA and were followed for 6 months. Thirty-four started on apremilast, 15 on methotrexate, and 101 on a biologic. Even though the apremilast group had higher baseline disease activity than did those who started on methotrexate, at 6 months a swollen joint count of 1 or 0 was present in 41% of the apremilast-treated patients, compared with none on methotrexate and 15% on a biologic agent.
A tender joint count of 0-1 was documented at 6 months in 24% of patients on apremilast, 13% with methotrexate, and 21% on a biologic agent. Apremilast’s numeric superiority in outcomes compared to methotrexate in this exploratory study wasn’t subjected to statistical analysis because of the small sample size. However, the ongoing phase 4, double-blind, placebo-controlled, multicenter FOREMOST trial in 330 patients with early oligoarticular PsA should provide more definitive efficacy data, noted Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
RWCS program director Arthur Kavanaugh, MD, said, “The most recent EULAR [European Alliance of Associations for Rheumatology] PsA guidelines totally discount apremilast, and I think mostly on the basis of cost, but then they also say that in groups of people it’s not as effective as methotrexate.”
“This study shows to me that, even though it’s a registry, with all the caveats about getting data from registries, apremilast certainly can be an effective drug,” said Dr. Kavanaugh, a rheumatologist and professor of medicine at the University of California, San Diego.
Another valuable piece of information from the CORRONA analysis is that it zeros in on patients with oligoarticular PsA.
“Almost all of our PsA studies are focused on people with polyarticular disease. What about those who have lesser involvement? That, of course, is important in the clinic,” he noted.
Dr. Ogdie concurred.
“If we study only polyarticular disease and we make all of our assumptions based on polyarticular disease, we might be leaving out at least half of the patients with PsA. And those patients may not need a bigger gun. Apremilast and methotrexate are kind of in the same group for that mild oligoarticular disease, and they probably work just fine,” she said.
A final point: “We really don’t have good outcome measures to study oligoarticular disease well. The ACR20 is not good because a 20% improvement in three joints is not readily measurable. That’s why trialists enroll patients with high joint count numbers,” according to the rheumatologist.
No increased risk of solid cancers in PsA patients treated with TNF inhibitors
A new analysis of clinical rheumatology registries in five Nordic countries finally puts to rest any concerns that treatment of PsA with TNF inhibitors is associated with increased risk of solid cancers. The same group previously reported no link between TNF inhibitors and lymphoma in PsA.
The solid cancers study included 9,655 PsA patients who started a first TNF inhibitor during 2001-2017, 14,809 others not treated with biologics, and 31,350 matched general population controls. Linkage to Swedish, Norwegian, Danish, Icelandic, and Finnish national cancer registries showed that the adjusted risk for solid cancer in TNF inhibitor–treated, compared with biologic-naive PsA patients, was 1.0. Similarly, the pooled standardized incidence ratio of solid cancer in TNF inhibitor–treated PsA patients compared to the general population was 1.0. There was no signal of a differential risk for incident cancer for any of the eight malignancies studied: lung, colorectal, breast, prostate, uterine, brain, liver, and pancreatic cancer.
“I like this study a lot because it’s specific to PsA rather than extrapolating from rheumatoid arthritis data, where we have a bunch more information for a much longer period of time, but it’s a different population,” Dr. Kavanaugh said.
Dr. Ogdie said, “I talk to my patients about this particular study or the same group’s earlier lymphoma study all the time.”
“I have to say, these are important data for the dermatology world because there are dermatologists who are still not convinced that TNF inhibitors don’t have an increased risk of malignancy. This kind of information is going to be helpful,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Greater efficacy for biologics in males than females with PsA?
A secondary analysis of the phase 3b EXCEED trial raised the intriguing possibility that both secukinumab, an interleukin-17A inhibitor, and adalimumab, a TNF inhibitor, have greater efficacy in men than in women with PsA. In this randomized trial of 853 biologic-naive patients with PsA, the ACR20 response rate to secukinumab at week 52 was 61% in females versus 74% in males, with ACR50 rates of 43% in females and 55.3% in males. The ACR20 rate with adalimumab was 51.5% in females and 70.2% in males. Similarly, the corresponding ACR50s were 32.6% and 55.3%, respectively. Minimal disease activity was achieved in 36.2% of women and 51% of men on secukinumab, and in 24.2% of women and 49.8% of men on adalimumab.
“These are randomized patients, so you really shouldn’t see these big differences in minimal disease activity,” Dr. Ogdie noted. “The question is why do men seem to respond better to therapy than women? I don’t think it’s the fibromyalgia-ness. There’s probably some biologic rationale for this that we just don’t understand yet. Maybe hormonal interactions.”
This gender difference in response is an important issue because it can potentially distort outcomes in head-to-head drug trials, Dr. Ruderman added.
“That gender difference is not likely to be huge if you’re looking at a placebo-controlled trial because the difference between the active drug and placebo is going to outweigh it. But when you have two active drugs, if there’s an imbalance in terms of how many men or women there are on each of the two drugs, you may end up with an efficacy difference that’s not real but is based on gender and not response to the drug,” he explained.
Roy M. Fleischmann, MD, a rheumatologist and clinical trialist at the University of Texas, Dallas, rose from the audience to pronounce the EXCEED male-versus-female analysis “very interesting.”
“We should go back and look at other trials and see if that occurred, and if it did, then we have to think about that going forward,” he proposed.
Dr. Ogdie, Dr. Kavanaugh, and Dr. Ruderman reported having financial relationships with numerous pharmaceutical companies.
Apremilast (Otezla) monotherapy may be an effective option in oligoarticular psoriatic arthritis, Alexis R. Ogdie, MD, reported at the 2021 Rheumatology Winter Clinical Symposium.
Her analysis of apremilast data from the CORRONA Registry was among several recent highlights in psoriatic arthritis (PsA) cited by speakers at the meeting. Other significant developments included a large pan-Scandinavian study that reassuringly found no increased risk of solid cancers in tumor necrosis factor (TNF) inhibitor–treated patients with PsA, and evidence to suggest a sex difference in the efficacy of both secukinumab (Cosentyx) and adalimumab (Humira), with men responding better than women to two biologics with differing mechanisms of action.
A role for apremilast in oligoarticular disease?
Dr. Ogdie presented an analysis of 150 patients in the U.S. observational CORRONA Registry who initiated monotherapy for oligoarticular PsA and were followed for 6 months. Thirty-four started on apremilast, 15 on methotrexate, and 101 on a biologic. Even though the apremilast group had higher baseline disease activity than did those who started on methotrexate, at 6 months a swollen joint count of 1 or 0 was present in 41% of the apremilast-treated patients, compared with none on methotrexate and 15% on a biologic agent.
A tender joint count of 0-1 was documented at 6 months in 24% of patients on apremilast, 13% with methotrexate, and 21% on a biologic agent. Apremilast’s numeric superiority in outcomes compared to methotrexate in this exploratory study wasn’t subjected to statistical analysis because of the small sample size. However, the ongoing phase 4, double-blind, placebo-controlled, multicenter FOREMOST trial in 330 patients with early oligoarticular PsA should provide more definitive efficacy data, noted Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
RWCS program director Arthur Kavanaugh, MD, said, “The most recent EULAR [European Alliance of Associations for Rheumatology] PsA guidelines totally discount apremilast, and I think mostly on the basis of cost, but then they also say that in groups of people it’s not as effective as methotrexate.”
“This study shows to me that, even though it’s a registry, with all the caveats about getting data from registries, apremilast certainly can be an effective drug,” said Dr. Kavanaugh, a rheumatologist and professor of medicine at the University of California, San Diego.
Another valuable piece of information from the CORRONA analysis is that it zeros in on patients with oligoarticular PsA.
“Almost all of our PsA studies are focused on people with polyarticular disease. What about those who have lesser involvement? That, of course, is important in the clinic,” he noted.
Dr. Ogdie concurred.
“If we study only polyarticular disease and we make all of our assumptions based on polyarticular disease, we might be leaving out at least half of the patients with PsA. And those patients may not need a bigger gun. Apremilast and methotrexate are kind of in the same group for that mild oligoarticular disease, and they probably work just fine,” she said.
A final point: “We really don’t have good outcome measures to study oligoarticular disease well. The ACR20 is not good because a 20% improvement in three joints is not readily measurable. That’s why trialists enroll patients with high joint count numbers,” according to the rheumatologist.
No increased risk of solid cancers in PsA patients treated with TNF inhibitors
A new analysis of clinical rheumatology registries in five Nordic countries finally puts to rest any concerns that treatment of PsA with TNF inhibitors is associated with increased risk of solid cancers. The same group previously reported no link between TNF inhibitors and lymphoma in PsA.
The solid cancers study included 9,655 PsA patients who started a first TNF inhibitor during 2001-2017, 14,809 others not treated with biologics, and 31,350 matched general population controls. Linkage to Swedish, Norwegian, Danish, Icelandic, and Finnish national cancer registries showed that the adjusted risk for solid cancer in TNF inhibitor–treated, compared with biologic-naive PsA patients, was 1.0. Similarly, the pooled standardized incidence ratio of solid cancer in TNF inhibitor–treated PsA patients compared to the general population was 1.0. There was no signal of a differential risk for incident cancer for any of the eight malignancies studied: lung, colorectal, breast, prostate, uterine, brain, liver, and pancreatic cancer.
“I like this study a lot because it’s specific to PsA rather than extrapolating from rheumatoid arthritis data, where we have a bunch more information for a much longer period of time, but it’s a different population,” Dr. Kavanaugh said.
Dr. Ogdie said, “I talk to my patients about this particular study or the same group’s earlier lymphoma study all the time.”
“I have to say, these are important data for the dermatology world because there are dermatologists who are still not convinced that TNF inhibitors don’t have an increased risk of malignancy. This kind of information is going to be helpful,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Greater efficacy for biologics in males than females with PsA?
A secondary analysis of the phase 3b EXCEED trial raised the intriguing possibility that both secukinumab, an interleukin-17A inhibitor, and adalimumab, a TNF inhibitor, have greater efficacy in men than in women with PsA. In this randomized trial of 853 biologic-naive patients with PsA, the ACR20 response rate to secukinumab at week 52 was 61% in females versus 74% in males, with ACR50 rates of 43% in females and 55.3% in males. The ACR20 rate with adalimumab was 51.5% in females and 70.2% in males. Similarly, the corresponding ACR50s were 32.6% and 55.3%, respectively. Minimal disease activity was achieved in 36.2% of women and 51% of men on secukinumab, and in 24.2% of women and 49.8% of men on adalimumab.
“These are randomized patients, so you really shouldn’t see these big differences in minimal disease activity,” Dr. Ogdie noted. “The question is why do men seem to respond better to therapy than women? I don’t think it’s the fibromyalgia-ness. There’s probably some biologic rationale for this that we just don’t understand yet. Maybe hormonal interactions.”
This gender difference in response is an important issue because it can potentially distort outcomes in head-to-head drug trials, Dr. Ruderman added.
“That gender difference is not likely to be huge if you’re looking at a placebo-controlled trial because the difference between the active drug and placebo is going to outweigh it. But when you have two active drugs, if there’s an imbalance in terms of how many men or women there are on each of the two drugs, you may end up with an efficacy difference that’s not real but is based on gender and not response to the drug,” he explained.
Roy M. Fleischmann, MD, a rheumatologist and clinical trialist at the University of Texas, Dallas, rose from the audience to pronounce the EXCEED male-versus-female analysis “very interesting.”
“We should go back and look at other trials and see if that occurred, and if it did, then we have to think about that going forward,” he proposed.
Dr. Ogdie, Dr. Kavanaugh, and Dr. Ruderman reported having financial relationships with numerous pharmaceutical companies.
Apremilast (Otezla) monotherapy may be an effective option in oligoarticular psoriatic arthritis, Alexis R. Ogdie, MD, reported at the 2021 Rheumatology Winter Clinical Symposium.
Her analysis of apremilast data from the CORRONA Registry was among several recent highlights in psoriatic arthritis (PsA) cited by speakers at the meeting. Other significant developments included a large pan-Scandinavian study that reassuringly found no increased risk of solid cancers in tumor necrosis factor (TNF) inhibitor–treated patients with PsA, and evidence to suggest a sex difference in the efficacy of both secukinumab (Cosentyx) and adalimumab (Humira), with men responding better than women to two biologics with differing mechanisms of action.
A role for apremilast in oligoarticular disease?
Dr. Ogdie presented an analysis of 150 patients in the U.S. observational CORRONA Registry who initiated monotherapy for oligoarticular PsA and were followed for 6 months. Thirty-four started on apremilast, 15 on methotrexate, and 101 on a biologic. Even though the apremilast group had higher baseline disease activity than did those who started on methotrexate, at 6 months a swollen joint count of 1 or 0 was present in 41% of the apremilast-treated patients, compared with none on methotrexate and 15% on a biologic agent.
A tender joint count of 0-1 was documented at 6 months in 24% of patients on apremilast, 13% with methotrexate, and 21% on a biologic agent. Apremilast’s numeric superiority in outcomes compared to methotrexate in this exploratory study wasn’t subjected to statistical analysis because of the small sample size. However, the ongoing phase 4, double-blind, placebo-controlled, multicenter FOREMOST trial in 330 patients with early oligoarticular PsA should provide more definitive efficacy data, noted Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia.
RWCS program director Arthur Kavanaugh, MD, said, “The most recent EULAR [European Alliance of Associations for Rheumatology] PsA guidelines totally discount apremilast, and I think mostly on the basis of cost, but then they also say that in groups of people it’s not as effective as methotrexate.”
“This study shows to me that, even though it’s a registry, with all the caveats about getting data from registries, apremilast certainly can be an effective drug,” said Dr. Kavanaugh, a rheumatologist and professor of medicine at the University of California, San Diego.
Another valuable piece of information from the CORRONA analysis is that it zeros in on patients with oligoarticular PsA.
“Almost all of our PsA studies are focused on people with polyarticular disease. What about those who have lesser involvement? That, of course, is important in the clinic,” he noted.
Dr. Ogdie concurred.
“If we study only polyarticular disease and we make all of our assumptions based on polyarticular disease, we might be leaving out at least half of the patients with PsA. And those patients may not need a bigger gun. Apremilast and methotrexate are kind of in the same group for that mild oligoarticular disease, and they probably work just fine,” she said.
A final point: “We really don’t have good outcome measures to study oligoarticular disease well. The ACR20 is not good because a 20% improvement in three joints is not readily measurable. That’s why trialists enroll patients with high joint count numbers,” according to the rheumatologist.
No increased risk of solid cancers in PsA patients treated with TNF inhibitors
A new analysis of clinical rheumatology registries in five Nordic countries finally puts to rest any concerns that treatment of PsA with TNF inhibitors is associated with increased risk of solid cancers. The same group previously reported no link between TNF inhibitors and lymphoma in PsA.
The solid cancers study included 9,655 PsA patients who started a first TNF inhibitor during 2001-2017, 14,809 others not treated with biologics, and 31,350 matched general population controls. Linkage to Swedish, Norwegian, Danish, Icelandic, and Finnish national cancer registries showed that the adjusted risk for solid cancer in TNF inhibitor–treated, compared with biologic-naive PsA patients, was 1.0. Similarly, the pooled standardized incidence ratio of solid cancer in TNF inhibitor–treated PsA patients compared to the general population was 1.0. There was no signal of a differential risk for incident cancer for any of the eight malignancies studied: lung, colorectal, breast, prostate, uterine, brain, liver, and pancreatic cancer.
“I like this study a lot because it’s specific to PsA rather than extrapolating from rheumatoid arthritis data, where we have a bunch more information for a much longer period of time, but it’s a different population,” Dr. Kavanaugh said.
Dr. Ogdie said, “I talk to my patients about this particular study or the same group’s earlier lymphoma study all the time.”
“I have to say, these are important data for the dermatology world because there are dermatologists who are still not convinced that TNF inhibitors don’t have an increased risk of malignancy. This kind of information is going to be helpful,” observed Eric M. Ruderman, MD, professor of medicine (rheumatology) at Northwestern University, Chicago.
Greater efficacy for biologics in males than females with PsA?
A secondary analysis of the phase 3b EXCEED trial raised the intriguing possibility that both secukinumab, an interleukin-17A inhibitor, and adalimumab, a TNF inhibitor, have greater efficacy in men than in women with PsA. In this randomized trial of 853 biologic-naive patients with PsA, the ACR20 response rate to secukinumab at week 52 was 61% in females versus 74% in males, with ACR50 rates of 43% in females and 55.3% in males. The ACR20 rate with adalimumab was 51.5% in females and 70.2% in males. Similarly, the corresponding ACR50s were 32.6% and 55.3%, respectively. Minimal disease activity was achieved in 36.2% of women and 51% of men on secukinumab, and in 24.2% of women and 49.8% of men on adalimumab.
“These are randomized patients, so you really shouldn’t see these big differences in minimal disease activity,” Dr. Ogdie noted. “The question is why do men seem to respond better to therapy than women? I don’t think it’s the fibromyalgia-ness. There’s probably some biologic rationale for this that we just don’t understand yet. Maybe hormonal interactions.”
This gender difference in response is an important issue because it can potentially distort outcomes in head-to-head drug trials, Dr. Ruderman added.
“That gender difference is not likely to be huge if you’re looking at a placebo-controlled trial because the difference between the active drug and placebo is going to outweigh it. But when you have two active drugs, if there’s an imbalance in terms of how many men or women there are on each of the two drugs, you may end up with an efficacy difference that’s not real but is based on gender and not response to the drug,” he explained.
Roy M. Fleischmann, MD, a rheumatologist and clinical trialist at the University of Texas, Dallas, rose from the audience to pronounce the EXCEED male-versus-female analysis “very interesting.”
“We should go back and look at other trials and see if that occurred, and if it did, then we have to think about that going forward,” he proposed.
Dr. Ogdie, Dr. Kavanaugh, and Dr. Ruderman reported having financial relationships with numerous pharmaceutical companies.
FROM RWCS 2021
COVID-19 vaccination in RMD patients: Safety data “reassuring”
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
FROM ANNALS OF THE RHEUMATIC DISEASES
Arcalyst gets FDA nod as first therapy for recurrent pericarditis
The Food and Drug Administration has approved rilonacept (Arcalyst) to treat recurrent pericarditis and reduce the risk for recurrence in adults and children 12 years and older.
Approval of the weekly subcutaneous injection offers patients the first and only FDA-approved therapy for recurrent pericarditis, the agency said in a release.
Recurrent pericarditis is characterized by a remitting relapsing inflammation of the pericardium, and therapeutic options have been limited to NSAIDs, colchicine, and corticosteroids.
Rilonacept is a recombinant fusion protein that blocks interleukin-1 alpha and interleukin-1 beta signaling. It is already approved by the FDA to treat a group of rare inherited inflammatory diseases called cryopyrin-associated periodic syndromes.
The new indication is based on the pivotal phase 3 RHAPSODY trial in 86 patients with acute symptoms of recurrent pericarditis and systemic inflammation. After randomization, pericarditis recurred in 2 of 30 patients (7%) treated with rilonacept and in 23 of 31 patients (74%) treated with placebo, representing a 96% reduction in the relative risk for recurrence with rilonacept.
Patients who received rilonacept were also pain free or had minimal pain on 98% of trial days, whereas those who received placebo had minimal or no pain on 46% of trial days.
The most common adverse effects of rilonacept are injection-site reactions and upper-respiratory tract infections.
Serious, life-threatening infections have been reported in patients taking rilonacept, according to the FDA. Patients with active or chronic infections should not take the drug.
The FDA label also advises that patients should avoid live vaccines while taking rilonacept and that it should be discontinued if a hypersensitivity reaction occurs.
The commercial launch is expected in April, according to the company.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved rilonacept (Arcalyst) to treat recurrent pericarditis and reduce the risk for recurrence in adults and children 12 years and older.
Approval of the weekly subcutaneous injection offers patients the first and only FDA-approved therapy for recurrent pericarditis, the agency said in a release.
Recurrent pericarditis is characterized by a remitting relapsing inflammation of the pericardium, and therapeutic options have been limited to NSAIDs, colchicine, and corticosteroids.
Rilonacept is a recombinant fusion protein that blocks interleukin-1 alpha and interleukin-1 beta signaling. It is already approved by the FDA to treat a group of rare inherited inflammatory diseases called cryopyrin-associated periodic syndromes.
The new indication is based on the pivotal phase 3 RHAPSODY trial in 86 patients with acute symptoms of recurrent pericarditis and systemic inflammation. After randomization, pericarditis recurred in 2 of 30 patients (7%) treated with rilonacept and in 23 of 31 patients (74%) treated with placebo, representing a 96% reduction in the relative risk for recurrence with rilonacept.
Patients who received rilonacept were also pain free or had minimal pain on 98% of trial days, whereas those who received placebo had minimal or no pain on 46% of trial days.
The most common adverse effects of rilonacept are injection-site reactions and upper-respiratory tract infections.
Serious, life-threatening infections have been reported in patients taking rilonacept, according to the FDA. Patients with active or chronic infections should not take the drug.
The FDA label also advises that patients should avoid live vaccines while taking rilonacept and that it should be discontinued if a hypersensitivity reaction occurs.
The commercial launch is expected in April, according to the company.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved rilonacept (Arcalyst) to treat recurrent pericarditis and reduce the risk for recurrence in adults and children 12 years and older.
Approval of the weekly subcutaneous injection offers patients the first and only FDA-approved therapy for recurrent pericarditis, the agency said in a release.
Recurrent pericarditis is characterized by a remitting relapsing inflammation of the pericardium, and therapeutic options have been limited to NSAIDs, colchicine, and corticosteroids.
Rilonacept is a recombinant fusion protein that blocks interleukin-1 alpha and interleukin-1 beta signaling. It is already approved by the FDA to treat a group of rare inherited inflammatory diseases called cryopyrin-associated periodic syndromes.
The new indication is based on the pivotal phase 3 RHAPSODY trial in 86 patients with acute symptoms of recurrent pericarditis and systemic inflammation. After randomization, pericarditis recurred in 2 of 30 patients (7%) treated with rilonacept and in 23 of 31 patients (74%) treated with placebo, representing a 96% reduction in the relative risk for recurrence with rilonacept.
Patients who received rilonacept were also pain free or had minimal pain on 98% of trial days, whereas those who received placebo had minimal or no pain on 46% of trial days.
The most common adverse effects of rilonacept are injection-site reactions and upper-respiratory tract infections.
Serious, life-threatening infections have been reported in patients taking rilonacept, according to the FDA. Patients with active or chronic infections should not take the drug.
The FDA label also advises that patients should avoid live vaccines while taking rilonacept and that it should be discontinued if a hypersensitivity reaction occurs.
The commercial launch is expected in April, according to the company.
A version of this article first appeared on Medscape.com.
Drug-resistant TB trial stopped early after successful results
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
Médecins Sans Frontières (MSF/Doctors Without Borders) announced early closure of its phase 2/3 trial of a 6-month multidrug regimen for multidrug-resistant tuberculosis (MDR-TB) because an independent data safety and monitoring board (DSMB) determined that the drug combination in the study regimen was superior to current therapy, according to a press release.
The trial, called TB PRACTECAL, compared the current local standard of care with a 6-month regimen of bedaquiline, pretomanid, linezolid, and moxifloxacin. The interim analysis included 242 patients and the randomized, controlled trial was conducted in sites in Belarus, South Africa, and Uzbekistan.
The preliminary data will be shared with the World Health Organization soon and will also be submitted to a peer-reviewed journal. If it withstands further reviews, as is anticipated, the trial would support the first solely oral regimen for MDR-TB.
In 2019, an estimated 465,000 people developed MDR-TB and 182,000 died. The global burden of TB at that time was about 10 million new cases, many with coexisting HIV.
Current treatment for MDR-TB lasts 9-20 months and is complicated by the need for painful shots and toxic antibiotics. Side effects can include psychiatric problems from quinolones, isoniazid, ethambutol, or cycloserine; deafness from aminoglycosides; and bone marrow suppression from linezolid, among other toxicities.
It’s hoped that the shorter regimen will reduce toxicity and improve patient compliance. Poor adherence to treatment is a major driver of further drug resistance. Current regimens require up to 20 pills per day as well as daily injections.
In a prepared statement from MSF, David Moore, MD, MSc, London School of Hygiene and Tropical Medicine, a member of the TB-PRACTECAL trial’s steering committee, concluded: “The findings could transform the way we treat patients with drug-resistant forms of TB worldwide, who have been neglected for too long.”
This good news is particularly welcome as, in the time of COVID-19, “an estimated 1.4 million fewer people received care for tuberculosis in 2020 than in 2019,” according to the WHO. The drop, an overall 21% reduction in patients beginning treatment, ranged as high as 42% in Indonesia.
Although awaiting complete data, Madhukar Pai, MD, PhD, associate director of the McGill International TB Centre, McGill University, Montreal, shares Dr. Moore’s enthusiasm. In an interview, Dr. Pai compared MDR-TB with extensively drug-resistant TB (XDR-TB).
“I’m excited about the possibility that these trial results might help shorten MDR-TB treatment to 6 months,” said Dr. Pai. “That will be a huge relief to all patients battling drug-resistant disease. The 6-month BPaL regimen (bedaquiline, pretomanid, and linezolid) regimen works well in XDR-TB. So, I would expect the TB PRACTECAL regimen with one added drug (moxifloxacin) to work well in MDR-TB, which is less severe than XDR-TB. Between these two regimens, if we can bring down MDR and XDR treatment to 6 months, all oral, that would be a huge advance.”
The expense of bedaquiline has been a long-standing concern in the global health community. Janssen, a subsidiary of Johnson & Johnson, has reduced the price to $340 per 6-month treatment course for more than 135 eligible low- and middle-income countries.
Previously, the tiered pricing structure was different for low-, middle-, and high-income countries (U.S. $900, $3,000, and $30,000, respectively). “The global TB community has asked Janssen to drop the price of bedaquiline to a level no higher than $32 per month – double the price at which researchers estimated bedaquiline could be sold for a profit,” according to the Treatment Action Group A major source of contention over pricing has been that there has been considerable public investment in the drug›s development.
Dr. Pai concluded: “Bedaquiline is likely the most important drug in both 6-month regimens. We need to work harder to make bedaquiline, an excellent drug, more affordable and accessible.”
While the full data is not yet publicly available, TB PRACTECAL was a randomized, controlled, multicenter study. The fact that enrollment was discontinued early by the DSMB suggests the efficacy data was compelling and that this completely oral regimen will become the standard of care.
Dr. Stone is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. A version of this article first appeared on Medscape.com.
Vitamin D may protect against COVID-19, especially in Black patients
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher levels of vitamin D than traditionally considered sufficient may help prevent COVID-19 infection – particularly in Black patients, shows a new single-center, retrospective study looking at the role of vitamin D in prevention of infection.
The study, published recently in JAMA Network Open, noted that expert opinion varies as to what “sufficient” levels of vitamin D are, some define this as 30 ng/mL, while others cite 40 ng/mL or greater.
In their discussion, the authors also noted that their results showed the “risk of positive COVID-19 test results decreased significantly with increased vitamin D level of 30 ng/mL or greater when measured as a continuous variable.”
“These new results tell us that having vitamin D levels above those normally considered sufficient is associated with decreased risk of testing positive for COVID-19, at least in Black individuals,” lead author, David Meltzer, MD, chief of hospital medicine at the University of Chicago, said in a press release from his institution.
“These findings suggest that randomized clinical trials to determine whether increasing vitamin D levels to greater than 30-40 ng/mL affect COVID-19 risk are warranted, especially in Black individuals,” he and his coauthors said.
Vitamin D at time of testing most strongly associated with COVID risk
An earlier study by the same researchers found that vitamin D deficiency (less than 20 ng/mL) may raise the risk of testing positive for COVID-19 in people from various ethnicities, as reported by this news organization.
Data for this latest study were drawn from electronic health records for 4,638 individuals at the University of Chicago Medicine and were used to examine whether the likelihood of a positive COVID-19 test was associated with a person’s most recent vitamin D level (within the previous year), and whether there was any effect of ethnicity on this outcome.
Mean age was 52.8 years, 69% were women, 49% were Black, 43% White, and 8% were another race/ethnicity. A total of 27% of the individuals were deficient in vitamin D (less than 20 ng/mL), 27% had insufficient levels (20-30 ng/mL), 22% had sufficient levels (30-40 ng/mL), and the remaining 24% had levels of 40 ng/mL or greater.
In total, 333 (7%) of people tested positive for COVID-19, including 102 (5%) Whites and 211 (9%) Blacks. And 36% of Black individuals who tested positive for COVID-19 were classified as vitamin D deficient, compared with 16% of Whites.
A positive test result for COVID-19 was not significantly associated with vitamin D levels in white individuals but was in Black individuals.
In Black people, compared with levels of at least 40 ng/mL, vitamin D levels of 30-40 ng/mL were associated with an incidence rate ratio of 2.64 for COVID-19 positivity (P = .01). For levels of 20-30 ng/mL, the IRR was 1.69 (P = 0.21); and for less than 20 ng/mL the IRR was 2.55 (P = .009).
The researchers also found that the risk of positive test results with lower vitamin D levels increased when those levels were lower just prior to the positive COVID-19 test, lending “support [to] the idea that vitamin D level at the time of testing is most strongly associated with COVID-19 risk,” they wrote.
Try upping vitamin D levels to 40 ng/mL or greater to prevent COVID?
In their discussion, the authors noted that significant association of vitamin D levels with COVID-19 risk in Blacks but not in Whites, “could reflect their higher COVID-19 risk, to which socioeconomic factors and structural inequities clearly contribute.
“Biological susceptibility to vitamin D deficiency may also be less frequent in White than Black individuals, since lighter skin increases vitamin D production in response to sunlight, and vitamin D binding proteins may vary by race and affect vitamin D bioavailability.”
Given less than 10% of U.S. adults have a vitamin D level greater than 40 ng/mL, the study findings increase the urgency to consider whether increased sun exposure or supplementation could reduce COVID-19 risk, according to the authors.
“When increased sun exposure is impractical, achieving vitamin D levels of 40 ng/mL or greater typically requires greater supplementation than currently recommended for most individuals of 600-800 IU/d vitamin D3,” they added.
However, Dr. Meltzer also acknowledged that “this is an observational study. We can see that there’s an association between vitamin D levels and likelihood of a COVID-19 diagnosis, but we don’t know exactly why that is, or whether these results are due to the vitamin D directly or other related biological factors.”
All in all, the authors suggested that randomized clinical trials are needed to understand if vitamin D can reduce COVID-19 risk, and as such they should include doses of supplements likely to increase vitamin D to at least 40 ng/mL, and perhaps even higher, although they pointed out that the latter must be achieved safely.
“Studies should also consider the role of vitamin D testing, loading doses, dose adjustments for individuals who are obese or overweight, risks for hypercalcemia, and strategies to monitor for and mitigate hypercalcemia, and that non-White populations, such as Black individuals, may have greater needs for supplementation,” they outlined.
They are now recruiting participants for two separate clinical trials testing the efficacy of vitamin D supplements for preventing COVID-19.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Poor survival with COVID in patients who have had HSCT
Among individuals who have received a hematopoietic stem cell transplant (HSCT), often used in the treatment of blood cancers, rates of survival are poor for those who develop COVID-19.
The probability of survival 30 days after being diagnosed with COVID-19 is only 68% for persons who have received an allogeneic HSCT and 67% for autologous HSCT recipients, according to new data from the Center for International Blood and Marrow Transplant Research.
These findings underscore the need for “stringent surveillance and aggressive treatment measures” in this population, Akshay Sharma, MBBS, of St. Jude Children’s Research Hospital, Memphis, and colleagues wrote.
The findings were published online March 1, 2021, in The Lancet Haematology.
The study is “of importance for physicians caring for HSCT recipients worldwide,” Mathieu Leclerc, MD, and Sébastien Maury, MD, Hôpital Henri Mondor, Créteil, France, commented in an accompanying editorial.
Study details
For their study, Dr. Sharma and colleagues analyzed outcomes for all HSCT recipients who developed COVID-19 and whose cases were reported to the CIBMTR. Of 318 such patients, 184 had undergone allogeneic HSCT, and 134 had undergone autologous HSCT.
Overall, about half of these patients (49%) had mild COVID-19.
Severe COVID-19 that required mechanical ventilation developed in 15% and 13% of the allogeneic and autologous HSCT recipients, respectively.
About one-fifth of patients died: 22% and 19% of allogeneic and autologous HSCT recipients, respectively.
Factors associated with greater mortality risk included age of 50 years or older (hazard ratio, 2.53), male sex (HR, 3.53), and development of COVID-19 within 12 months of undergoing HSCT (HR, 2.67).
Among autologous HSCT recipients, lymphoma was associated with higher mortality risk in comparison with a plasma cell disorder or myeloma (HR, 2.41), the authors noted.
“Two important messages can be drawn from the results reported by Sharma and colleagues,” Dr. Leclerc and Dr. Maury wrote in their editorial. “The first is the confirmation that the prognosis of COVID-19 is particularly poor in HSCT recipients, and that its prevention, in the absence of any specific curative treatment with sufficient efficacy, should be at the forefront of concerns.”
The second relates to the risk factors for death among HSCT recipients who develop COVID-19. In addition to previously known risk factors, such as age and gender, the investigators identified transplant-specific factors potentially associated with prognosis – namely, the nearly threefold increase in death among allogeneic HSCT recipients who develop COVID-19 within 12 months of transplant, they explained.
However, the findings are limited by a substantial amount of missing data, short follow-up, and the possibility of selection bias, they noted.
“Further large and well-designed studies with longer follow-up are needed to confirm and refine the results,” the editorialists wrote.
“[A] better understanding of the distinctive features of COVID-19 infection in HSCT recipients will be a necessary and essential step toward improvement of the remarkably poor prognosis observed in this setting,” they added.
The study was funded by the American Society of Hematology; the Leukemia and Lymphoma Society; the National Cancer Institute; the National Heart, Lung and Blood Institute; the National Institute of Allergy and Infectious Diseases; the National Institutes of Health; the Health Resources and Services Administration; and the Office of Naval Research. Dr. Sharma receives support for the conduct of industry-sponsored trials from Vertex Pharmaceuticals, CRISPR Therapeutics, and Novartis and consulting fees from Spotlight Therapeutics. Dr. Leclerc and Dr. Maury disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among individuals who have received a hematopoietic stem cell transplant (HSCT), often used in the treatment of blood cancers, rates of survival are poor for those who develop COVID-19.
The probability of survival 30 days after being diagnosed with COVID-19 is only 68% for persons who have received an allogeneic HSCT and 67% for autologous HSCT recipients, according to new data from the Center for International Blood and Marrow Transplant Research.
These findings underscore the need for “stringent surveillance and aggressive treatment measures” in this population, Akshay Sharma, MBBS, of St. Jude Children’s Research Hospital, Memphis, and colleagues wrote.
The findings were published online March 1, 2021, in The Lancet Haematology.
The study is “of importance for physicians caring for HSCT recipients worldwide,” Mathieu Leclerc, MD, and Sébastien Maury, MD, Hôpital Henri Mondor, Créteil, France, commented in an accompanying editorial.
Study details
For their study, Dr. Sharma and colleagues analyzed outcomes for all HSCT recipients who developed COVID-19 and whose cases were reported to the CIBMTR. Of 318 such patients, 184 had undergone allogeneic HSCT, and 134 had undergone autologous HSCT.
Overall, about half of these patients (49%) had mild COVID-19.
Severe COVID-19 that required mechanical ventilation developed in 15% and 13% of the allogeneic and autologous HSCT recipients, respectively.
About one-fifth of patients died: 22% and 19% of allogeneic and autologous HSCT recipients, respectively.
Factors associated with greater mortality risk included age of 50 years or older (hazard ratio, 2.53), male sex (HR, 3.53), and development of COVID-19 within 12 months of undergoing HSCT (HR, 2.67).
Among autologous HSCT recipients, lymphoma was associated with higher mortality risk in comparison with a plasma cell disorder or myeloma (HR, 2.41), the authors noted.
“Two important messages can be drawn from the results reported by Sharma and colleagues,” Dr. Leclerc and Dr. Maury wrote in their editorial. “The first is the confirmation that the prognosis of COVID-19 is particularly poor in HSCT recipients, and that its prevention, in the absence of any specific curative treatment with sufficient efficacy, should be at the forefront of concerns.”
The second relates to the risk factors for death among HSCT recipients who develop COVID-19. In addition to previously known risk factors, such as age and gender, the investigators identified transplant-specific factors potentially associated with prognosis – namely, the nearly threefold increase in death among allogeneic HSCT recipients who develop COVID-19 within 12 months of transplant, they explained.
However, the findings are limited by a substantial amount of missing data, short follow-up, and the possibility of selection bias, they noted.
“Further large and well-designed studies with longer follow-up are needed to confirm and refine the results,” the editorialists wrote.
“[A] better understanding of the distinctive features of COVID-19 infection in HSCT recipients will be a necessary and essential step toward improvement of the remarkably poor prognosis observed in this setting,” they added.
The study was funded by the American Society of Hematology; the Leukemia and Lymphoma Society; the National Cancer Institute; the National Heart, Lung and Blood Institute; the National Institute of Allergy and Infectious Diseases; the National Institutes of Health; the Health Resources and Services Administration; and the Office of Naval Research. Dr. Sharma receives support for the conduct of industry-sponsored trials from Vertex Pharmaceuticals, CRISPR Therapeutics, and Novartis and consulting fees from Spotlight Therapeutics. Dr. Leclerc and Dr. Maury disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among individuals who have received a hematopoietic stem cell transplant (HSCT), often used in the treatment of blood cancers, rates of survival are poor for those who develop COVID-19.
The probability of survival 30 days after being diagnosed with COVID-19 is only 68% for persons who have received an allogeneic HSCT and 67% for autologous HSCT recipients, according to new data from the Center for International Blood and Marrow Transplant Research.
These findings underscore the need for “stringent surveillance and aggressive treatment measures” in this population, Akshay Sharma, MBBS, of St. Jude Children’s Research Hospital, Memphis, and colleagues wrote.
The findings were published online March 1, 2021, in The Lancet Haematology.
The study is “of importance for physicians caring for HSCT recipients worldwide,” Mathieu Leclerc, MD, and Sébastien Maury, MD, Hôpital Henri Mondor, Créteil, France, commented in an accompanying editorial.
Study details
For their study, Dr. Sharma and colleagues analyzed outcomes for all HSCT recipients who developed COVID-19 and whose cases were reported to the CIBMTR. Of 318 such patients, 184 had undergone allogeneic HSCT, and 134 had undergone autologous HSCT.
Overall, about half of these patients (49%) had mild COVID-19.
Severe COVID-19 that required mechanical ventilation developed in 15% and 13% of the allogeneic and autologous HSCT recipients, respectively.
About one-fifth of patients died: 22% and 19% of allogeneic and autologous HSCT recipients, respectively.
Factors associated with greater mortality risk included age of 50 years or older (hazard ratio, 2.53), male sex (HR, 3.53), and development of COVID-19 within 12 months of undergoing HSCT (HR, 2.67).
Among autologous HSCT recipients, lymphoma was associated with higher mortality risk in comparison with a plasma cell disorder or myeloma (HR, 2.41), the authors noted.
“Two important messages can be drawn from the results reported by Sharma and colleagues,” Dr. Leclerc and Dr. Maury wrote in their editorial. “The first is the confirmation that the prognosis of COVID-19 is particularly poor in HSCT recipients, and that its prevention, in the absence of any specific curative treatment with sufficient efficacy, should be at the forefront of concerns.”
The second relates to the risk factors for death among HSCT recipients who develop COVID-19. In addition to previously known risk factors, such as age and gender, the investigators identified transplant-specific factors potentially associated with prognosis – namely, the nearly threefold increase in death among allogeneic HSCT recipients who develop COVID-19 within 12 months of transplant, they explained.
However, the findings are limited by a substantial amount of missing data, short follow-up, and the possibility of selection bias, they noted.
“Further large and well-designed studies with longer follow-up are needed to confirm and refine the results,” the editorialists wrote.
“[A] better understanding of the distinctive features of COVID-19 infection in HSCT recipients will be a necessary and essential step toward improvement of the remarkably poor prognosis observed in this setting,” they added.
The study was funded by the American Society of Hematology; the Leukemia and Lymphoma Society; the National Cancer Institute; the National Heart, Lung and Blood Institute; the National Institute of Allergy and Infectious Diseases; the National Institutes of Health; the Health Resources and Services Administration; and the Office of Naval Research. Dr. Sharma receives support for the conduct of industry-sponsored trials from Vertex Pharmaceuticals, CRISPR Therapeutics, and Novartis and consulting fees from Spotlight Therapeutics. Dr. Leclerc and Dr. Maury disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.



