User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
COVID-19 in children: New cases back on the decline
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
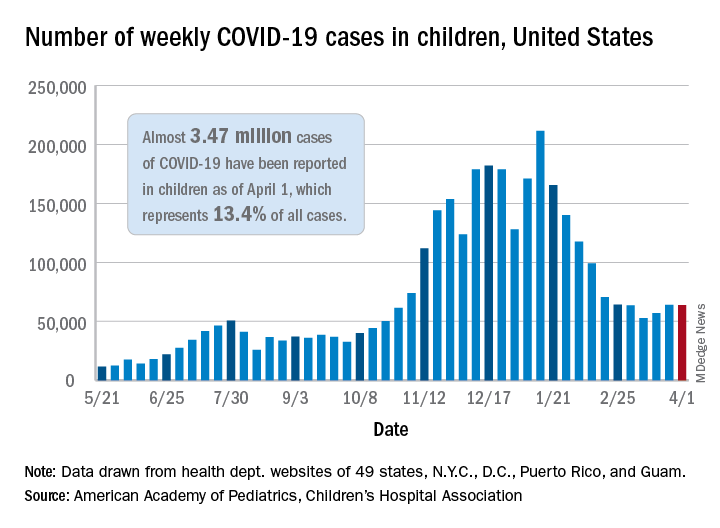
the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
New cases of COVID-19 in children in the United States fell slightly, but even that small dip was enough to reverse 2 straight weeks of increases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and the CHA said in their weekly COVID-19 report. For the week ending April 1, children represented 18.1% of all new cases reported in the United States, down from a pandemic-high 19.1% the week before.
COVID-19 cases in children now total just under 3.47 million, which works out to 13.4% of reported cases for all ages and 4,610 cases per 100,000 children since the beginning of the pandemic, the AAP and the CHA said based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Among those jurisdictions, Vermont has the highest proportion of its cases occurring in children at 21.0%, and North Dakota has the highest cumulative rate at 8,958 cases per 100,000 children. Looking at those states from the bottoms of their respective lists are Florida, where children aged 0-14 years represent 8.4% of all cases, and Hawaii, with 1,133 cases per 100,000 children aged 0-17 years, the AAP/CHA report shows.
The data on more serious illness show that Minnesota has the highest proportion of hospitalizations occurring in children at 3.1%, while New York City has the highest hospitalization rate among infected children, 2.0%. Among the other 23 states reporting on such admissions, children make up only 1.3% of hospitalizations in Florida and in New Hampshire, which also has the lowest hospitalization rate at 0.1%, the AAP and CHA said.
Five more deaths were reported in children during the week ending April 1, bringing the total to 284 in the 43 states, along with New York City, Puerto Rico, and Guam, that are sharing age-distribution data on mortality.
Cardiovascular disease remains leading cause of type 2 diabetes mortality
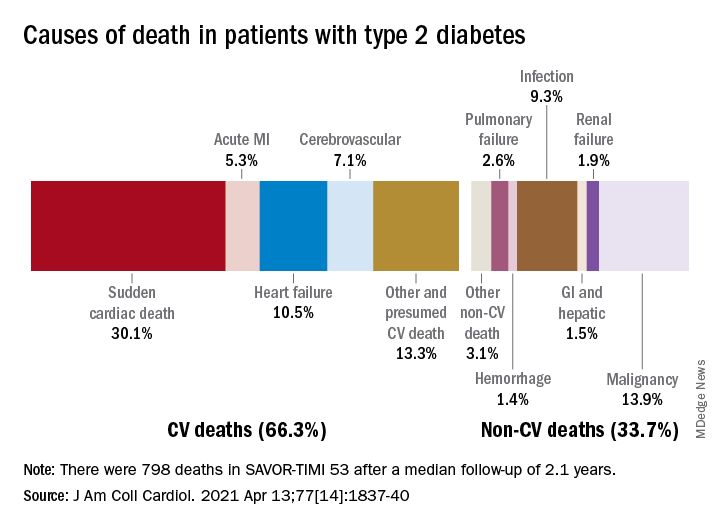
Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Excess deaths jump 23% in U.S. in 2020, mostly because of COVID-19
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
Clinically important deterioration predicts poor future outcomes in COPD
Patients with COPD may benefit from stepped-up treatment of short-term disease progression with triple therapy to stave off longer-term exacerbations and all-cause mortality.
, a study based on data from more than 10,000 patients has shown.
For this study, clinically important deterioration (CID) as a measure of COPD is defined as a combination of change in lung function and/or health status, or a first acute moderate to severe COPD exacerbation, wrote MeiLan K. Han, MD, of the University of Michigan, Ann Arbor, and colleagues.
The study was published in ERJ Open Research The investigators analyzed data from the IMPACT trial, a phase III, double-blind, multicenter, 52-week study of symptomatic COPD patients aged 40 years and older.
In the intent-to-treat population, patients with symptomatic COPD and at least one moderate or severe exacerbation in the past year were randomized to a once-daily dose of fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) 100/62.5/25 mcg (4,151 patients); FF/VI 100/25 mcg (4,134 patients); or UMEC/VI 62.5/25 mcg using a single dry-power inhaler (2,070 patients).
The researchers explored both the prognostic value of a CID event on future clinical outcomes and the impact of single-inhaler triple versus dual therapy on reducing CID risk. CID was defined as any of the following: moderate/severe exacerbation; deterioration in lung function (defined as a decrease of 100 mL or more from baseline in trough forced expiratory volume per second); or deterioration in health status based on increases of 4.0 units or more on the St George’s Respiratory Questionnaire (SGRQ) total score or 2.0 units or more on the COPD Assessment Test (CAT) score.
Overall, patients with a CID by 28 weeks had significantly increased exacerbation rates after week 28, as well as smaller improvements in lung function and health status at week 52 (P < .001 for all). In addition, CID patients had an increased risk of all-cause mortality after 28 weeks, compared with patients without CID. However, FF/UMEC/VI significantly reduced CID risk, compared with dual therapies, the researchers noted.
Based on the CID SGRQ definition, patients with CID had a 75% increase in moderate to severe exacerbations by week 28 and a 96% in severe exacerbations over weeks 29-52. The increases were similar using the CID CAT definition (72% and 91%, respectively).
Patients with CID also showed significantly reduced improvements in both lung function and health status after 1 year, and a significantly increased risk of all-cause mortality compared to patients without CID.
In comparing triple vs. double therapies, FF/UMEC/VI patients showed significant reductions in CID risk by 52 weeks, compared with patients treated with FF/VI and UMEC/VI. This difference was true across all subgroups, except for the subgroup of patients who were on long-acting beta2-agonist (LABA) and long-acting muscarinic antagonist (LAMA) therapy prior to screening, the researchers said.
In addition, “treatment effect was greater at higher blood eosinophil counts for FF/UMEC/VI versus UMEC/VI,” the researchers noted.
The study findings were limited by several factors including the lack of CID as a primary endpoint, the relatively short 5-month follow-up period, and the use of a symptomatic patient population with an established risk of exacerbation, which could limit generalizability, the researchers noted. However, the findings support the value of preventing short-term CID and adding inhaled corticosteroids (ICS) or bronchodilation for patients in this study population, they said.
Data may help drive tailored treatments
“This study is a post hoc analysis of data from the IMPACT trial, an RCT examining triple therapy vs ICS/LABA vs LABA/LAMA,” Dr. Han, lead and corresponding author, said in an interview. “In this particular paper, we conducted a treatment independent analysis examining individuals who experienced clinically important deteriorations at week 28 and then compared outcomes at week 52 based on CID status at week 28. Patients with a CID by week 28 had significantly increased exacerbation rates after week 28, smaller improvements in lung function and health status at week 52, and increased risk of all-cause mortality after week 28 versus patients who were CID free,” she emphasized. “We also saw that FF/UMEC/VI significantly reduced CID risk versus dual therapies.” These data suggest that shorter-term changes are associated with longer term outcomes, and provide important information both for the purposes of clinical trials design as well as patient clinical assessments, she added.
Dr. Han said she was not surprised by the findings. “I think these results are consistent with prior analyses but suggest that short-term outcomes relate to longer-term ones,” she said. However, she stressed the need for individualized treatment.
“While there are relationships between symptoms, lung function, and exacerbations as demonstrated by these analyses, in any individual patient sometimes these three disease axes do not perfectly align,” she explained. Dr. Han’s main message for clinicians in practice is that optimization of triple therapy in patients with severe disease and high risk for exacerbations was associated not only with short-term improvements in symptoms and lung function, but also with longer-term reductions in exacerbations and mortality.
As for additional research, prospective studies using CID as a primary or secondary outcome would help validate the composite outcome in this study, as regulatory agencies have been slow to adopt composite outcomes, Dr. Han said.
Dr. Han disclosed relationships with GlaxoSmithKline, which funded the study, as well as AstraZeneca, Boehringer Ingelheim, Novartis, Sunovion, Mylan, Merck, and Verona.
Patients with COPD may benefit from stepped-up treatment of short-term disease progression with triple therapy to stave off longer-term exacerbations and all-cause mortality.
, a study based on data from more than 10,000 patients has shown.
For this study, clinically important deterioration (CID) as a measure of COPD is defined as a combination of change in lung function and/or health status, or a first acute moderate to severe COPD exacerbation, wrote MeiLan K. Han, MD, of the University of Michigan, Ann Arbor, and colleagues.
The study was published in ERJ Open Research The investigators analyzed data from the IMPACT trial, a phase III, double-blind, multicenter, 52-week study of symptomatic COPD patients aged 40 years and older.
In the intent-to-treat population, patients with symptomatic COPD and at least one moderate or severe exacerbation in the past year were randomized to a once-daily dose of fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) 100/62.5/25 mcg (4,151 patients); FF/VI 100/25 mcg (4,134 patients); or UMEC/VI 62.5/25 mcg using a single dry-power inhaler (2,070 patients).
The researchers explored both the prognostic value of a CID event on future clinical outcomes and the impact of single-inhaler triple versus dual therapy on reducing CID risk. CID was defined as any of the following: moderate/severe exacerbation; deterioration in lung function (defined as a decrease of 100 mL or more from baseline in trough forced expiratory volume per second); or deterioration in health status based on increases of 4.0 units or more on the St George’s Respiratory Questionnaire (SGRQ) total score or 2.0 units or more on the COPD Assessment Test (CAT) score.
Overall, patients with a CID by 28 weeks had significantly increased exacerbation rates after week 28, as well as smaller improvements in lung function and health status at week 52 (P < .001 for all). In addition, CID patients had an increased risk of all-cause mortality after 28 weeks, compared with patients without CID. However, FF/UMEC/VI significantly reduced CID risk, compared with dual therapies, the researchers noted.
Based on the CID SGRQ definition, patients with CID had a 75% increase in moderate to severe exacerbations by week 28 and a 96% in severe exacerbations over weeks 29-52. The increases were similar using the CID CAT definition (72% and 91%, respectively).
Patients with CID also showed significantly reduced improvements in both lung function and health status after 1 year, and a significantly increased risk of all-cause mortality compared to patients without CID.
In comparing triple vs. double therapies, FF/UMEC/VI patients showed significant reductions in CID risk by 52 weeks, compared with patients treated with FF/VI and UMEC/VI. This difference was true across all subgroups, except for the subgroup of patients who were on long-acting beta2-agonist (LABA) and long-acting muscarinic antagonist (LAMA) therapy prior to screening, the researchers said.
In addition, “treatment effect was greater at higher blood eosinophil counts for FF/UMEC/VI versus UMEC/VI,” the researchers noted.
The study findings were limited by several factors including the lack of CID as a primary endpoint, the relatively short 5-month follow-up period, and the use of a symptomatic patient population with an established risk of exacerbation, which could limit generalizability, the researchers noted. However, the findings support the value of preventing short-term CID and adding inhaled corticosteroids (ICS) or bronchodilation for patients in this study population, they said.
Data may help drive tailored treatments
“This study is a post hoc analysis of data from the IMPACT trial, an RCT examining triple therapy vs ICS/LABA vs LABA/LAMA,” Dr. Han, lead and corresponding author, said in an interview. “In this particular paper, we conducted a treatment independent analysis examining individuals who experienced clinically important deteriorations at week 28 and then compared outcomes at week 52 based on CID status at week 28. Patients with a CID by week 28 had significantly increased exacerbation rates after week 28, smaller improvements in lung function and health status at week 52, and increased risk of all-cause mortality after week 28 versus patients who were CID free,” she emphasized. “We also saw that FF/UMEC/VI significantly reduced CID risk versus dual therapies.” These data suggest that shorter-term changes are associated with longer term outcomes, and provide important information both for the purposes of clinical trials design as well as patient clinical assessments, she added.
Dr. Han said she was not surprised by the findings. “I think these results are consistent with prior analyses but suggest that short-term outcomes relate to longer-term ones,” she said. However, she stressed the need for individualized treatment.
“While there are relationships between symptoms, lung function, and exacerbations as demonstrated by these analyses, in any individual patient sometimes these three disease axes do not perfectly align,” she explained. Dr. Han’s main message for clinicians in practice is that optimization of triple therapy in patients with severe disease and high risk for exacerbations was associated not only with short-term improvements in symptoms and lung function, but also with longer-term reductions in exacerbations and mortality.
As for additional research, prospective studies using CID as a primary or secondary outcome would help validate the composite outcome in this study, as regulatory agencies have been slow to adopt composite outcomes, Dr. Han said.
Dr. Han disclosed relationships with GlaxoSmithKline, which funded the study, as well as AstraZeneca, Boehringer Ingelheim, Novartis, Sunovion, Mylan, Merck, and Verona.
Patients with COPD may benefit from stepped-up treatment of short-term disease progression with triple therapy to stave off longer-term exacerbations and all-cause mortality.
, a study based on data from more than 10,000 patients has shown.
For this study, clinically important deterioration (CID) as a measure of COPD is defined as a combination of change in lung function and/or health status, or a first acute moderate to severe COPD exacerbation, wrote MeiLan K. Han, MD, of the University of Michigan, Ann Arbor, and colleagues.
The study was published in ERJ Open Research The investigators analyzed data from the IMPACT trial, a phase III, double-blind, multicenter, 52-week study of symptomatic COPD patients aged 40 years and older.
In the intent-to-treat population, patients with symptomatic COPD and at least one moderate or severe exacerbation in the past year were randomized to a once-daily dose of fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) 100/62.5/25 mcg (4,151 patients); FF/VI 100/25 mcg (4,134 patients); or UMEC/VI 62.5/25 mcg using a single dry-power inhaler (2,070 patients).
The researchers explored both the prognostic value of a CID event on future clinical outcomes and the impact of single-inhaler triple versus dual therapy on reducing CID risk. CID was defined as any of the following: moderate/severe exacerbation; deterioration in lung function (defined as a decrease of 100 mL or more from baseline in trough forced expiratory volume per second); or deterioration in health status based on increases of 4.0 units or more on the St George’s Respiratory Questionnaire (SGRQ) total score or 2.0 units or more on the COPD Assessment Test (CAT) score.
Overall, patients with a CID by 28 weeks had significantly increased exacerbation rates after week 28, as well as smaller improvements in lung function and health status at week 52 (P < .001 for all). In addition, CID patients had an increased risk of all-cause mortality after 28 weeks, compared with patients without CID. However, FF/UMEC/VI significantly reduced CID risk, compared with dual therapies, the researchers noted.
Based on the CID SGRQ definition, patients with CID had a 75% increase in moderate to severe exacerbations by week 28 and a 96% in severe exacerbations over weeks 29-52. The increases were similar using the CID CAT definition (72% and 91%, respectively).
Patients with CID also showed significantly reduced improvements in both lung function and health status after 1 year, and a significantly increased risk of all-cause mortality compared to patients without CID.
In comparing triple vs. double therapies, FF/UMEC/VI patients showed significant reductions in CID risk by 52 weeks, compared with patients treated with FF/VI and UMEC/VI. This difference was true across all subgroups, except for the subgroup of patients who were on long-acting beta2-agonist (LABA) and long-acting muscarinic antagonist (LAMA) therapy prior to screening, the researchers said.
In addition, “treatment effect was greater at higher blood eosinophil counts for FF/UMEC/VI versus UMEC/VI,” the researchers noted.
The study findings were limited by several factors including the lack of CID as a primary endpoint, the relatively short 5-month follow-up period, and the use of a symptomatic patient population with an established risk of exacerbation, which could limit generalizability, the researchers noted. However, the findings support the value of preventing short-term CID and adding inhaled corticosteroids (ICS) or bronchodilation for patients in this study population, they said.
Data may help drive tailored treatments
“This study is a post hoc analysis of data from the IMPACT trial, an RCT examining triple therapy vs ICS/LABA vs LABA/LAMA,” Dr. Han, lead and corresponding author, said in an interview. “In this particular paper, we conducted a treatment independent analysis examining individuals who experienced clinically important deteriorations at week 28 and then compared outcomes at week 52 based on CID status at week 28. Patients with a CID by week 28 had significantly increased exacerbation rates after week 28, smaller improvements in lung function and health status at week 52, and increased risk of all-cause mortality after week 28 versus patients who were CID free,” she emphasized. “We also saw that FF/UMEC/VI significantly reduced CID risk versus dual therapies.” These data suggest that shorter-term changes are associated with longer term outcomes, and provide important information both for the purposes of clinical trials design as well as patient clinical assessments, she added.
Dr. Han said she was not surprised by the findings. “I think these results are consistent with prior analyses but suggest that short-term outcomes relate to longer-term ones,” she said. However, she stressed the need for individualized treatment.
“While there are relationships between symptoms, lung function, and exacerbations as demonstrated by these analyses, in any individual patient sometimes these three disease axes do not perfectly align,” she explained. Dr. Han’s main message for clinicians in practice is that optimization of triple therapy in patients with severe disease and high risk for exacerbations was associated not only with short-term improvements in symptoms and lung function, but also with longer-term reductions in exacerbations and mortality.
As for additional research, prospective studies using CID as a primary or secondary outcome would help validate the composite outcome in this study, as regulatory agencies have been slow to adopt composite outcomes, Dr. Han said.
Dr. Han disclosed relationships with GlaxoSmithKline, which funded the study, as well as AstraZeneca, Boehringer Ingelheim, Novartis, Sunovion, Mylan, Merck, and Verona.
FROM ERJ OPEN RESEARCH
Children likely the ‘leading edge’ in spread of COVID-19 variants
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
Public health officials in the Midwest and Northeast are sounding the alarm about steep new increases in COVID-19 cases in children.
The increases seem to be driven by greater circulation of more contagious variants, just as children and teens have returned to in-person activities such as sports, parties, and classes.
“I can just tell you from my 46 years in the business, I’ve never seen dynamic transmission in kids like we’re seeing right now, younger kids,” said Michael Osterholm, PhD, who directs the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis.
In earlier surges, children – especially younger children – played only minor roles in transmitting the infection. When they were diagnosed with COVID-19, their symptoms tended to be mild or even absent, and for reasons that aren’t well understood, they haven’t usually been the first cases in households or clusters.
Now, as more SARS-CoV-2 variants have begun to dominate, and seniors gain protection from vaccines, that pattern may be changing. Infectious disease experts are watching to see if COVID-19 will start to spread in a pattern more similar to influenza, with children becoming infected first and bringing the infection home to their parents.
Michigan sees jump in cases
Governors in some hard-hit states are pleading with a pandemic-weary public to keep up mask-wearing and social distancing and avoid unnecessary travel and large gatherings in order to protect in-person classes.
In Michigan, many schools reopened and youth sports resumed just as the more contagious B.1.1.7 variant spread widely. There, cases are rising among all age groups, but the largest number of new COVID-19 cases is among children aged 10-19, the first time that’s happened since the start of the pandemic.
Over the month of March, incidence in this age group had more than doubled in the state. Cases among younger children – infants through 9-year-olds – are also going up, increasing by more than 230% since Feb. 19, according to data from the Michigan Department of Health and Human Services.
The increases have prompted some schools to pause in-person learning for a time after spring break to slow transmission, according to Natasha Bagdasarian, MD, senior public health physician with the Michigan health department in Ann Arbor.
In Minnesota, on a recent call with reporters, Ruth Lynfield, MD, state epidemiologist, said the B.1.1.7 variant, which has rapidly risen in the state, has a higher attack rate among children than that of earlier versions of the virus, meaning they’re more likely to be infected when exposed.
“We certainly get the sense that youth are what we might refer to as the leading edge of the spread of variants,” she said.
Dr. Lynfield said they were tracking cases spreading through youth sports, classrooms, and daycare centers.
In Massachusetts, the largest number of new COVID-19 infections in the last 2 weeks of March was among children and teens. Massachusetts has the fifth-highest number of recorded B.1.1.7 cases in the United States, according to CDC data.
Although most COVID-19 cases in children and teens are mild, the disease can be severe for those who have underlying medical conditions. Even in healthy children, it can trigger a serious postviral syndrome called MIS-C that requires hospitalization.
Emerging studies show that children, like adults, can develop the lingering symptoms of long COVID-19. Recent data from the United Kingdom show 10%-15% of children younger than 16 infected with COVID-19 still had at least one symptom 5 weeks later.
Dr. Osterholm said it remains to be seen whether more cases in children will also mean a rise in more serious outcomes for children, as it has in Europe and Israel.
In Israel, the B.1.1.7 variant arrived at the end of December and became dominant in January. By the end of January, Hadassah Ein Kerem Medical Center in Jerusalem had four patients in its newly opened pediatric COVID-19 ICU unit. They ranged in age from 13 days to 2 years.
By early February, the Ministry of Health warned the country’s doctors to prepare for an “imminent upward trend” in pediatric COVID-19 cases. They notified hospitals to be ready to open more ICU beds for children with COVID-19, according to Cyrille Cohen, PhD, head of the laboratory of immunotherapy at Bar-Ilan University in Ramat Gan, Israel.
On March 31, French President Emmanuel Macron ordered France into its third national lockdown and closed schools for 3 weeks to try to hold off a third wave of COVID-19. President Macron had been a staunch defender of keeping schools open, but said the closure was necessary.
“It is the best solution to slow down the virus,” he said, according to Reuters.
German Chancellor Angela Merkel recently announced a new lockdown for Germany as the spread of the variants has led to rising cases there.
“I think what we’re seeing here is this is going to play out over the country,” said Dr. Osterholm. “Before this time, we didn’t see major transmission in younger kids particularly K through eighth grade, and now we’re seeing that happening with many school outbreaks, particularly in the Northeast and in the Midwest.” He added that it will spread through southern states as well.
Fall surge all over again
“It’s starting to feel an awful lot like déjà vu, where the hospitalization numbers, the positivity rate, all of the metrics that we track are trending up significantly, and it’s feeling like the fall surge,” said Brian Peters, CEO of the Michigan Hospital Association. “It’s feeling in many ways like the initial surge a year ago.”
Mr. Peters said that in January and February, COVID-19 hospitalizations in Michigan were less than 1,000 a day. Recently, he said, there were 2,558 people hospitalized with COVID-19 in Michigan.
About half of adults aged 65 and older have been fully vaccinated in Michigan. That’s led to a dramatic drop in cases and hospitalizations among seniors, who are at highest risk of death. At the same time, Gov. Gretchen Whitmer and health officials with the Biden administration have encouraged schools to reopen for in-person learning, and extracurricular activities have largely resumed.
The same circumstances – students in classrooms, combined with the arrival of the variants – resulted in COVID-19 cases caused by the B.1.1.7 variant increasing among younger age groups in the United Kingdom.
When schools were locked down again, however, cases caused by variant and wild type viruses both dropped in children, suggesting that there wasn’t anything that made B.1.1.7 extra risky for children, but that the strain is more contagious for everyone. Sports, extracurricular activities, and classrooms offered the virus plenty of opportunities to spread.
In Michigan, Dr. Bagdasarian said the outbreaks in children started with winter sports.
“Not necessarily transmission on the field, but we’re really talking about social gatherings that were happening in and around sports,” like the pizza party to celebrate a team win, she said, “and I think those social gatherings were a big driver.”
“Outbreaks are trickling over into teams and trickling over into schools, which is exactly what we want to avoid,” she added.
Thus far, Michigan has been reserving vaccine doses for older adults but will open eligibility to anyone age 16 and older starting on April 6.
Until younger age groups can be vaccinated, Mr. Peters said people need to continue to be careful.
“We see people letting their guard down and it’s to be expected,” Mr. Peters said. “People have COVID fatigue, and they are eager to get together with their friends. We’re not out of the woods yet.”
Children ‘heavily impacted’
In Nebraska, Alice Sato, MD, PhD, hospital epidemiologist at Children’s Hospital and Medical Center in Omaha, said they saw an increase in MIS-C cases after the winter surges, and she’s watching the data carefully as COVID-19 cases tick up in other midwestern states.
Dr. Sato got so tired of hearing people compare COVID-19 to the flu that she pulled some numbers on pediatric deaths.
While COVID-19 fatality rates in children are much lower than they are for adults, at least 279 children have died across the United States since the start of the pandemic. The highest number of confirmed pediatric deaths recorded during any of the previous 10 flu seasons was 188, according to the CDC.
“So while children are relatively spared, they’re still heavily impacted,” said Dr. Sato.
She was thrilled to hear the recent news that the Pfizer vaccine works well in children aged 12-15, but because Pfizer’s cold-chain requirements make it one the trickiest to store, the Food and Drug Administration hasn’t given the go-ahead yet. She said it will be months before she has any to offer to teens in her state.
In the meantime, genetic testing has shown that the variants are already circulating there.
“We really want parents and family members who are eligible to be vaccinated because that is a great way to protect children that I cannot vaccinate yet,” Dr. Sato said. “The best way for me to protect children is to prevent the adults around them from being infected.”
A version of this article first appeared on Medscape.com.
Six pregnancy complications flag later heart disease risk
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
AstraZeneca COVID vaccine: Clotting disorder mechanism revealed?
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
FDA approves new ready-to-inject glucagon product
The Food and Drug Administration has approved dasiglucagon (Zegalogue 0.6 mg/0.6 mL, Zealand Pharma) autoinjector and prefilled syringe for the treatment of severe hypoglycemia in people with diabetes aged 6 years and older.
The product has a shelf-life of 36 months at refrigerated temperatures and is stable for up to 12 months at room temperature.
“This approval will help enable appropriate children and adults with diabetes to be able to address sudden and severe hypoglycemia, which can quickly progress from a mild event to an emergency,” Jeremy Pettus, MD, assistant professor of medicine at the University of California, San Diego, said in a company statement.
The approval marks the latest step in the development of newer glucagon formulations that are easier to use in hypoglycemic emergencies than the traditional formulation that requires several steps for reconstitution.
The first intranasal glucagon (Baqsimi, Eli Lilly) was approved in the United States in July 2019 for people with diabetes age 4 years and older.
In September 2019, the FDA approved another prefilled glucagon rescue pen (Gvoke HypoPen, Xeris Pharmaceuticals) for the treatment of severe hypoglycemia in adult and pediatric patients age 2 years and older with diabetes.
Dasiglucagon is currently in phase 3 trials as a subcutaneous infusion for treating congenital hyperinsulinemia, and in phase 2 trials as part of a bihormonal artificial pancreas pump system.
The FDA approval was based on results from three randomized, double-blind, placebo-controlled, phase 3 studies of dasiglucagon in children age 6-17 years and adults with type 1 diabetes.
The primary endpoint was time to achieving an increase in blood glucose of 20 mg/dL or greater from time of administration without additional intervention within 45 minutes. That endpoint was achieved in all three studies, with a median time to blood glucose recovery of 10 minutes overall, with 99% of adults recovering within 15 minutes.
The most common adverse events reported in 2% or more of study participants were nausea, vomiting, headache, and injection-site pain in both children and adults. Diarrhea was also reported in adults.
Full launch is expected in late June 2021.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved dasiglucagon (Zegalogue 0.6 mg/0.6 mL, Zealand Pharma) autoinjector and prefilled syringe for the treatment of severe hypoglycemia in people with diabetes aged 6 years and older.
The product has a shelf-life of 36 months at refrigerated temperatures and is stable for up to 12 months at room temperature.
“This approval will help enable appropriate children and adults with diabetes to be able to address sudden and severe hypoglycemia, which can quickly progress from a mild event to an emergency,” Jeremy Pettus, MD, assistant professor of medicine at the University of California, San Diego, said in a company statement.
The approval marks the latest step in the development of newer glucagon formulations that are easier to use in hypoglycemic emergencies than the traditional formulation that requires several steps for reconstitution.
The first intranasal glucagon (Baqsimi, Eli Lilly) was approved in the United States in July 2019 for people with diabetes age 4 years and older.
In September 2019, the FDA approved another prefilled glucagon rescue pen (Gvoke HypoPen, Xeris Pharmaceuticals) for the treatment of severe hypoglycemia in adult and pediatric patients age 2 years and older with diabetes.
Dasiglucagon is currently in phase 3 trials as a subcutaneous infusion for treating congenital hyperinsulinemia, and in phase 2 trials as part of a bihormonal artificial pancreas pump system.
The FDA approval was based on results from three randomized, double-blind, placebo-controlled, phase 3 studies of dasiglucagon in children age 6-17 years and adults with type 1 diabetes.
The primary endpoint was time to achieving an increase in blood glucose of 20 mg/dL or greater from time of administration without additional intervention within 45 minutes. That endpoint was achieved in all three studies, with a median time to blood glucose recovery of 10 minutes overall, with 99% of adults recovering within 15 minutes.
The most common adverse events reported in 2% or more of study participants were nausea, vomiting, headache, and injection-site pain in both children and adults. Diarrhea was also reported in adults.
Full launch is expected in late June 2021.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved dasiglucagon (Zegalogue 0.6 mg/0.6 mL, Zealand Pharma) autoinjector and prefilled syringe for the treatment of severe hypoglycemia in people with diabetes aged 6 years and older.
The product has a shelf-life of 36 months at refrigerated temperatures and is stable for up to 12 months at room temperature.
“This approval will help enable appropriate children and adults with diabetes to be able to address sudden and severe hypoglycemia, which can quickly progress from a mild event to an emergency,” Jeremy Pettus, MD, assistant professor of medicine at the University of California, San Diego, said in a company statement.
The approval marks the latest step in the development of newer glucagon formulations that are easier to use in hypoglycemic emergencies than the traditional formulation that requires several steps for reconstitution.
The first intranasal glucagon (Baqsimi, Eli Lilly) was approved in the United States in July 2019 for people with diabetes age 4 years and older.
In September 2019, the FDA approved another prefilled glucagon rescue pen (Gvoke HypoPen, Xeris Pharmaceuticals) for the treatment of severe hypoglycemia in adult and pediatric patients age 2 years and older with diabetes.
Dasiglucagon is currently in phase 3 trials as a subcutaneous infusion for treating congenital hyperinsulinemia, and in phase 2 trials as part of a bihormonal artificial pancreas pump system.
The FDA approval was based on results from three randomized, double-blind, placebo-controlled, phase 3 studies of dasiglucagon in children age 6-17 years and adults with type 1 diabetes.
The primary endpoint was time to achieving an increase in blood glucose of 20 mg/dL or greater from time of administration without additional intervention within 45 minutes. That endpoint was achieved in all three studies, with a median time to blood glucose recovery of 10 minutes overall, with 99% of adults recovering within 15 minutes.
The most common adverse events reported in 2% or more of study participants were nausea, vomiting, headache, and injection-site pain in both children and adults. Diarrhea was also reported in adults.
Full launch is expected in late June 2021.
A version of this article first appeared on Medscape.com.
FDA okays new indication for alirocumab in homozygous FH
The Food and Drug Administration has approved alirocumab (Praluent, Regeneron Pharmaceuticals) injection as add-on therapy for adults with homozygous familial hypercholesterolemia, the agency announced.
The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor was originally approved in the United States in 2015 as an adjunct to diet, alone or in combination with other lipid-lowering therapies, to reduce LDL cholesterol in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (FH).
Heterozygous FH is one of the most common genetic disorders, affecting 1 in every 200-500 people worldwide, whereas homozygous FH is very rare, affecting about 1 in 1 million people worldwide.
Alirocumab is also approved to reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with cardiovascular disease.
The new indication is based on a 12-week randomized trial in 45 adults who received 150 mg alirocumab every 2 weeks and 24 patients who received placebo, both on top of other therapies to reduce LDL cholesterol. At week 12, patients receiving alirocumab had an average 27% decrease in LDL cholesterol, compared with an average 9% increase among patients on placebo.
Common side effects of alirocumab are nasopharyngitis, injection-site reactions, and influenza. Serious hypersensitivity reactions have occurred among people taking alirocumab.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved alirocumab (Praluent, Regeneron Pharmaceuticals) injection as add-on therapy for adults with homozygous familial hypercholesterolemia, the agency announced.
The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor was originally approved in the United States in 2015 as an adjunct to diet, alone or in combination with other lipid-lowering therapies, to reduce LDL cholesterol in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (FH).
Heterozygous FH is one of the most common genetic disorders, affecting 1 in every 200-500 people worldwide, whereas homozygous FH is very rare, affecting about 1 in 1 million people worldwide.
Alirocumab is also approved to reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with cardiovascular disease.
The new indication is based on a 12-week randomized trial in 45 adults who received 150 mg alirocumab every 2 weeks and 24 patients who received placebo, both on top of other therapies to reduce LDL cholesterol. At week 12, patients receiving alirocumab had an average 27% decrease in LDL cholesterol, compared with an average 9% increase among patients on placebo.
Common side effects of alirocumab are nasopharyngitis, injection-site reactions, and influenza. Serious hypersensitivity reactions have occurred among people taking alirocumab.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved alirocumab (Praluent, Regeneron Pharmaceuticals) injection as add-on therapy for adults with homozygous familial hypercholesterolemia, the agency announced.
The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor was originally approved in the United States in 2015 as an adjunct to diet, alone or in combination with other lipid-lowering therapies, to reduce LDL cholesterol in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (FH).
Heterozygous FH is one of the most common genetic disorders, affecting 1 in every 200-500 people worldwide, whereas homozygous FH is very rare, affecting about 1 in 1 million people worldwide.
Alirocumab is also approved to reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with cardiovascular disease.
The new indication is based on a 12-week randomized trial in 45 adults who received 150 mg alirocumab every 2 weeks and 24 patients who received placebo, both on top of other therapies to reduce LDL cholesterol. At week 12, patients receiving alirocumab had an average 27% decrease in LDL cholesterol, compared with an average 9% increase among patients on placebo.
Common side effects of alirocumab are nasopharyngitis, injection-site reactions, and influenza. Serious hypersensitivity reactions have occurred among people taking alirocumab.
A version of this article first appeared on Medscape.com.
COVID-19 in 2020: Deaths and disparities
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
FROM MMWR





