User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Physicians’ trust in health care leadership drops in pandemic
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.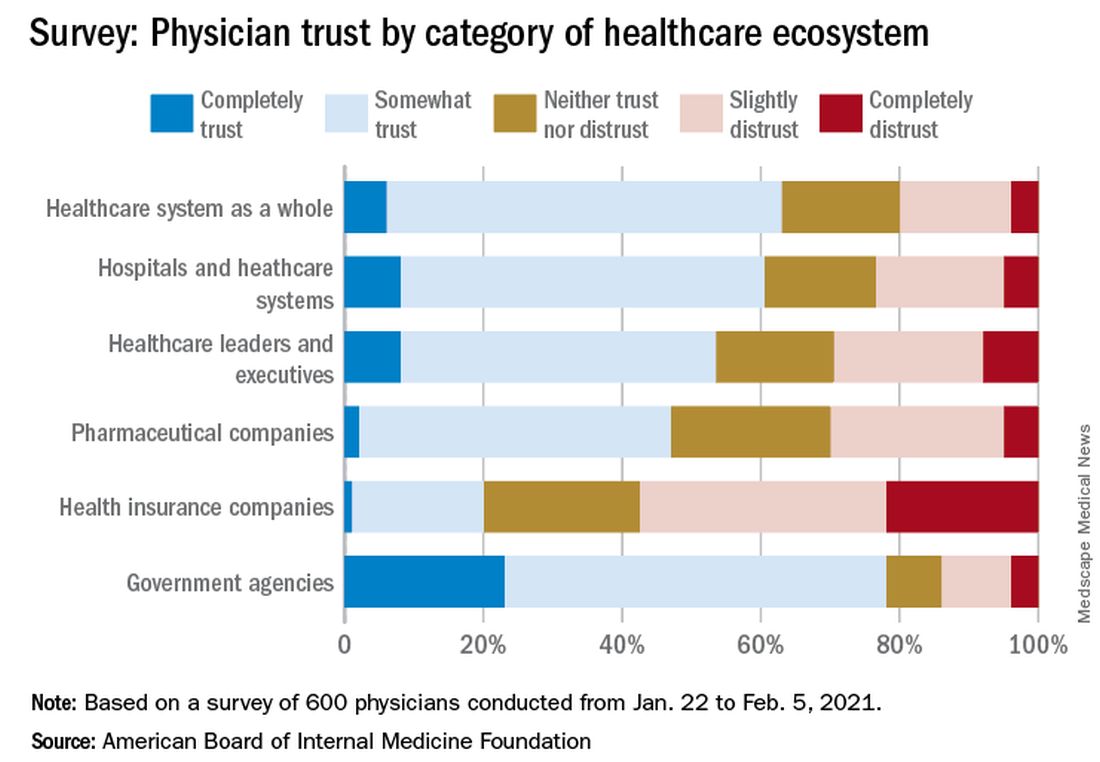
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity hope as neuropeptide Y blocker turns white fat to brown
A peripherally-acting substance that boosts energy expenditure and reduces fat mass has the potential to become an obesity treatment that doesn’t produce cardiovascular or psychiatric side effects, scientists say.
The agent, BIBO3304, is a selective antagonist of the neuropeptide Y1 receptor, which is elevated in the fat tissue of individuals with obesity, resulting in reduced fat accumulation. It was originally developed more than 25 years ago by scientists at Boehringer Ingelheim, who had thought that it would reduce appetite by targeting Y1 receptors in the brain. But when it didn’t cross the blood-brain barrier as an oral drug, the company abandoned it.
Now a series of experiments by Chenxu Yan, of the Garvan Institute of Medical Research, St. Vincent’s Hospital, Sydney, and colleagues have shown that “BIBO” works directly on Y1 receptors in the periphery to turn fat-storing white fat cells into heat-generating brown-like fat tissue, thereby enhancing energy expenditure.
The data were published online May 11 in Nature Communications.
Drug’s lack of effect on the brain turns out to be a positive
“Rather than just having the cells store fat, we change their characteristics so that most of the excess energy gets burned and produces heat instead of being stored as fat. BIBO programs the cell toward a more heat-producing cell rather than a fat-storing cell,” study coauthor Herbert Herzog, PhD, of the Garvan Institute, said in an interview.
Importantly, he said, the lack of effect on the brain that caused the drug’s initial developer to abandon it turns out to be a positive.
“As we looked at fat specifically, and we didn’t want to have any interference with the brain, this seems to work out as a real advantage … It has the desired effect of blocking fat accumulation but has the enormous benefit of not interfering with any brain function. That’s why so many of the obesity drugs that were on the market were taken off, because of the side effects they caused in the brain on mood and cardiovascular control. It’s a completely different ball game.”
The problem now, he said, is that because BIBO is off-patent, no pharmaceutical company is currently willing to invest in its development as a peripherally acting weight-loss drug, despite its potential advantages.
“We’re trying to find some interested party to help us get this to the clinical setting. We’re basic scientists. We need big money. We can do small-scale studies to get proof of principle. Hopefully, if that’s interesting, some bigger company will come along,” said Dr. Herzog.
Experiments in mice, human tissues demonstrate principle
In the series of studies, investigators fed genetically inbred mice a high-fat, high-sugar diet while giving BIBO to half of them. Over 8 weeks, the mice given BIBO had 40% less gain in fat mass compared to those overfed without the drug, despite them all eating the same amount.
Using a noninvasive infrared camera to measure skin surface temperature above brown adipose tissue, they found that the temperature was significantly increased with BIBO, independent of the weight of the brown fat.
This suggests that the thermogenesis of the brown fat is significantly contributing to whole-body energy expenditure. “With the drug, the mice have far greater energy expenditure measured by heat production,” Dr. Herzog explained.
In vitro experiments showed that Y1R blockade by BIBO induced “beigeing” of white fat deposits into more heat-producing brown fat. The body temperature increase is about 0.1-0.2ºC. “That’s a tiny amount, but it actually requires quite a lot of energy,” he said.
Experiments using fat tissue taken from obese and normal-weight humans showed the same thermogenesis with BIBO. “It’s such a fundamental process [that] you wouldn’t expect it to differ. The same mechanism is even found in flies and primitive worms,” he noted.
Neuropeptide Y receptor blockage: A treatment for many ills?
Previously, Dr. Herzog and colleagues found that blockade of the neuropeptide Y1 receptor also increases bone mass in mice.
“It’s a modest effect, but there’s nothing out there at the moment that really improves bone mass. If you can stop osteoporosis, that’s a benefit on its own,” he said.
Now they hope to study BIBO’s vasodilatory properties as a potential treatment for hypertension, if they get the funding.
Dr. Herzog is hopeful, as obesity, osteoporosis, and hypertension are all chronic conditions. “Having one drug that benefits them all would surely be of interest to clinicians and drug companies,” he observed.
Dr. Yan and Dr. Herzog have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A peripherally-acting substance that boosts energy expenditure and reduces fat mass has the potential to become an obesity treatment that doesn’t produce cardiovascular or psychiatric side effects, scientists say.
The agent, BIBO3304, is a selective antagonist of the neuropeptide Y1 receptor, which is elevated in the fat tissue of individuals with obesity, resulting in reduced fat accumulation. It was originally developed more than 25 years ago by scientists at Boehringer Ingelheim, who had thought that it would reduce appetite by targeting Y1 receptors in the brain. But when it didn’t cross the blood-brain barrier as an oral drug, the company abandoned it.
Now a series of experiments by Chenxu Yan, of the Garvan Institute of Medical Research, St. Vincent’s Hospital, Sydney, and colleagues have shown that “BIBO” works directly on Y1 receptors in the periphery to turn fat-storing white fat cells into heat-generating brown-like fat tissue, thereby enhancing energy expenditure.
The data were published online May 11 in Nature Communications.
Drug’s lack of effect on the brain turns out to be a positive
“Rather than just having the cells store fat, we change their characteristics so that most of the excess energy gets burned and produces heat instead of being stored as fat. BIBO programs the cell toward a more heat-producing cell rather than a fat-storing cell,” study coauthor Herbert Herzog, PhD, of the Garvan Institute, said in an interview.
Importantly, he said, the lack of effect on the brain that caused the drug’s initial developer to abandon it turns out to be a positive.
“As we looked at fat specifically, and we didn’t want to have any interference with the brain, this seems to work out as a real advantage … It has the desired effect of blocking fat accumulation but has the enormous benefit of not interfering with any brain function. That’s why so many of the obesity drugs that were on the market were taken off, because of the side effects they caused in the brain on mood and cardiovascular control. It’s a completely different ball game.”
The problem now, he said, is that because BIBO is off-patent, no pharmaceutical company is currently willing to invest in its development as a peripherally acting weight-loss drug, despite its potential advantages.
“We’re trying to find some interested party to help us get this to the clinical setting. We’re basic scientists. We need big money. We can do small-scale studies to get proof of principle. Hopefully, if that’s interesting, some bigger company will come along,” said Dr. Herzog.
Experiments in mice, human tissues demonstrate principle
In the series of studies, investigators fed genetically inbred mice a high-fat, high-sugar diet while giving BIBO to half of them. Over 8 weeks, the mice given BIBO had 40% less gain in fat mass compared to those overfed without the drug, despite them all eating the same amount.
Using a noninvasive infrared camera to measure skin surface temperature above brown adipose tissue, they found that the temperature was significantly increased with BIBO, independent of the weight of the brown fat.
This suggests that the thermogenesis of the brown fat is significantly contributing to whole-body energy expenditure. “With the drug, the mice have far greater energy expenditure measured by heat production,” Dr. Herzog explained.
In vitro experiments showed that Y1R blockade by BIBO induced “beigeing” of white fat deposits into more heat-producing brown fat. The body temperature increase is about 0.1-0.2ºC. “That’s a tiny amount, but it actually requires quite a lot of energy,” he said.
Experiments using fat tissue taken from obese and normal-weight humans showed the same thermogenesis with BIBO. “It’s such a fundamental process [that] you wouldn’t expect it to differ. The same mechanism is even found in flies and primitive worms,” he noted.
Neuropeptide Y receptor blockage: A treatment for many ills?
Previously, Dr. Herzog and colleagues found that blockade of the neuropeptide Y1 receptor also increases bone mass in mice.
“It’s a modest effect, but there’s nothing out there at the moment that really improves bone mass. If you can stop osteoporosis, that’s a benefit on its own,” he said.
Now they hope to study BIBO’s vasodilatory properties as a potential treatment for hypertension, if they get the funding.
Dr. Herzog is hopeful, as obesity, osteoporosis, and hypertension are all chronic conditions. “Having one drug that benefits them all would surely be of interest to clinicians and drug companies,” he observed.
Dr. Yan and Dr. Herzog have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A peripherally-acting substance that boosts energy expenditure and reduces fat mass has the potential to become an obesity treatment that doesn’t produce cardiovascular or psychiatric side effects, scientists say.
The agent, BIBO3304, is a selective antagonist of the neuropeptide Y1 receptor, which is elevated in the fat tissue of individuals with obesity, resulting in reduced fat accumulation. It was originally developed more than 25 years ago by scientists at Boehringer Ingelheim, who had thought that it would reduce appetite by targeting Y1 receptors in the brain. But when it didn’t cross the blood-brain barrier as an oral drug, the company abandoned it.
Now a series of experiments by Chenxu Yan, of the Garvan Institute of Medical Research, St. Vincent’s Hospital, Sydney, and colleagues have shown that “BIBO” works directly on Y1 receptors in the periphery to turn fat-storing white fat cells into heat-generating brown-like fat tissue, thereby enhancing energy expenditure.
The data were published online May 11 in Nature Communications.
Drug’s lack of effect on the brain turns out to be a positive
“Rather than just having the cells store fat, we change their characteristics so that most of the excess energy gets burned and produces heat instead of being stored as fat. BIBO programs the cell toward a more heat-producing cell rather than a fat-storing cell,” study coauthor Herbert Herzog, PhD, of the Garvan Institute, said in an interview.
Importantly, he said, the lack of effect on the brain that caused the drug’s initial developer to abandon it turns out to be a positive.
“As we looked at fat specifically, and we didn’t want to have any interference with the brain, this seems to work out as a real advantage … It has the desired effect of blocking fat accumulation but has the enormous benefit of not interfering with any brain function. That’s why so many of the obesity drugs that were on the market were taken off, because of the side effects they caused in the brain on mood and cardiovascular control. It’s a completely different ball game.”
The problem now, he said, is that because BIBO is off-patent, no pharmaceutical company is currently willing to invest in its development as a peripherally acting weight-loss drug, despite its potential advantages.
“We’re trying to find some interested party to help us get this to the clinical setting. We’re basic scientists. We need big money. We can do small-scale studies to get proof of principle. Hopefully, if that’s interesting, some bigger company will come along,” said Dr. Herzog.
Experiments in mice, human tissues demonstrate principle
In the series of studies, investigators fed genetically inbred mice a high-fat, high-sugar diet while giving BIBO to half of them. Over 8 weeks, the mice given BIBO had 40% less gain in fat mass compared to those overfed without the drug, despite them all eating the same amount.
Using a noninvasive infrared camera to measure skin surface temperature above brown adipose tissue, they found that the temperature was significantly increased with BIBO, independent of the weight of the brown fat.
This suggests that the thermogenesis of the brown fat is significantly contributing to whole-body energy expenditure. “With the drug, the mice have far greater energy expenditure measured by heat production,” Dr. Herzog explained.
In vitro experiments showed that Y1R blockade by BIBO induced “beigeing” of white fat deposits into more heat-producing brown fat. The body temperature increase is about 0.1-0.2ºC. “That’s a tiny amount, but it actually requires quite a lot of energy,” he said.
Experiments using fat tissue taken from obese and normal-weight humans showed the same thermogenesis with BIBO. “It’s such a fundamental process [that] you wouldn’t expect it to differ. The same mechanism is even found in flies and primitive worms,” he noted.
Neuropeptide Y receptor blockage: A treatment for many ills?
Previously, Dr. Herzog and colleagues found that blockade of the neuropeptide Y1 receptor also increases bone mass in mice.
“It’s a modest effect, but there’s nothing out there at the moment that really improves bone mass. If you can stop osteoporosis, that’s a benefit on its own,” he said.
Now they hope to study BIBO’s vasodilatory properties as a potential treatment for hypertension, if they get the funding.
Dr. Herzog is hopeful, as obesity, osteoporosis, and hypertension are all chronic conditions. “Having one drug that benefits them all would surely be of interest to clinicians and drug companies,” he observed.
Dr. Yan and Dr. Herzog have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA OKs nivolumab after surgery for esophageal or GEJ cancer: Practice-changing use of immunotherapy
The Food and Drug Administration has approved the adjuvant use of nivolumab (Opdivo) in patients with resected esophageal or gastroesophageal junction (GEJ) cancer who have received neoadjuvant chemoradiotherapy and have residual pathological disease following surgery.
The approval addresses an unmet need among these patients, who have a high risk of recurrence but for whom surveillance is the only current management option after the above-described standard treatment, according to experts.
The FDA’s approval is based on results from the CheckMate 577 study, which showed a significant improvement in disease-free survival compared with placebo.
This was described as “a practice-changing trial in the treatment of esophageal cancer” by David H. Ilson, MD, PhD, Memorial Sloan Kettering Cancer Center, New York, in an editorial in The New England Journal of Medicine that accompanied the published study results.
“The trial shows the first true advance in the adjuvant therapy of esophageal cancer in recent years,” wrote Dr. Ilson.
In the randomized, double-blind, phase 3 trial, patients with resected stage II or III esophageal or GEJ cancer were randomly assigned in a 2:1 ratio to receive nivolumab (at a dose of 240 mg every 2 weeks for 16 weeks, followed by a dose of 480 mg every 4 weeks) or placebo. The maximum duration of the intervention period was 1 year.
All of these patients had received neoadjuvant chemoradiotherapy and had residual pathological disease, as noted in the new indication. Patients were enrolled regardless of programmed death ligand 1 (PD-L1) expression.
For the primary endpoint of disease-free survival, the median was 22.4 months for the nivolumab group (n = 532) versus 11.0 months for the placebo group (n = 262; hazard ratio [HR] for disease recurrence or death, 0.69; 96.4% confidence interval [CI], 0.56-0.86; P < .001).
The median follow-up was 24.4 months.
Disease-free survival favored nivolumab across multiple preplanned subgroups.
However, as Dr. Ilson noted in the editorial, the efficacy of nivolumab varied, with more benefit seen for patients with squamous cell cancer (HR, 0.61) than for those with adenocarcinoma (HR, 0.75). Patients with esophageal tumors also had greater benefit (HR, 0.61) than did those with GEJ tumors (HR, 0.87).
There was benefit in patients with node-negative disease (HR, 0.74) and node-positive disease (HR, 0.67) and benefit in patients with tumors that were PD-L1–negative (HR, 0.73) and PD-L1–positive (HR, 0.75).
There were fewer distant recurrences in the nivolumab group than in the placebo group (29% vs. 39%) and fewer locoregional recurrences (12% vs. 17%).
No new safety signals were observed, and 9% of the nivolumab patients discontinued the drug because of treatment-related adverse events versus 3% of placebo patients. In addition, a 1-year course of adjuvant nivolumab did not negatively impact patient-reported quality of life, the trialists reported.
Grade 3 or 4 adverse events of any cause were more frequent in the nivolumab group versus the placebo group (34% vs. 32%) as were those related to the intervention (13% vs. 6%).
Although overall survival data are not mature, “the doubling of median disease-free survival will almost certainly translate into an overall survival benefit,” Dr. Ilson wrote.
Notably, the trial’s original co-primary endpoint was overall survival, but was dropped to a secondary endpoint after enrollment “challenges.”
When the now-published data were first presented at the 2020 annual meeting of the European Society for Medical Oncology, the invited discussant, Andrés Cervantes, MD, PhD, University of Valencia, Spain, raised several issues with the trial.
Preoperative chemoradiation is not “universally accepted” as the standard of care in this setting, said Dr. Cervantes, president-elect of ESMO.
Dr. Ilson acknowledged as much. “A debate is ongoing about whether chemotherapy alone or combined chemoradiotherapy is the preferred treatment for esophageal cancer before surgery,” he wrote.
In addition, Dr. Cervantes noted that disease-free survival is not currently validated as a major endpoint in gastroesophageal cancers, and the median follow-up was short.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the adjuvant use of nivolumab (Opdivo) in patients with resected esophageal or gastroesophageal junction (GEJ) cancer who have received neoadjuvant chemoradiotherapy and have residual pathological disease following surgery.
The approval addresses an unmet need among these patients, who have a high risk of recurrence but for whom surveillance is the only current management option after the above-described standard treatment, according to experts.
The FDA’s approval is based on results from the CheckMate 577 study, which showed a significant improvement in disease-free survival compared with placebo.
This was described as “a practice-changing trial in the treatment of esophageal cancer” by David H. Ilson, MD, PhD, Memorial Sloan Kettering Cancer Center, New York, in an editorial in The New England Journal of Medicine that accompanied the published study results.
“The trial shows the first true advance in the adjuvant therapy of esophageal cancer in recent years,” wrote Dr. Ilson.
In the randomized, double-blind, phase 3 trial, patients with resected stage II or III esophageal or GEJ cancer were randomly assigned in a 2:1 ratio to receive nivolumab (at a dose of 240 mg every 2 weeks for 16 weeks, followed by a dose of 480 mg every 4 weeks) or placebo. The maximum duration of the intervention period was 1 year.
All of these patients had received neoadjuvant chemoradiotherapy and had residual pathological disease, as noted in the new indication. Patients were enrolled regardless of programmed death ligand 1 (PD-L1) expression.
For the primary endpoint of disease-free survival, the median was 22.4 months for the nivolumab group (n = 532) versus 11.0 months for the placebo group (n = 262; hazard ratio [HR] for disease recurrence or death, 0.69; 96.4% confidence interval [CI], 0.56-0.86; P < .001).
The median follow-up was 24.4 months.
Disease-free survival favored nivolumab across multiple preplanned subgroups.
However, as Dr. Ilson noted in the editorial, the efficacy of nivolumab varied, with more benefit seen for patients with squamous cell cancer (HR, 0.61) than for those with adenocarcinoma (HR, 0.75). Patients with esophageal tumors also had greater benefit (HR, 0.61) than did those with GEJ tumors (HR, 0.87).
There was benefit in patients with node-negative disease (HR, 0.74) and node-positive disease (HR, 0.67) and benefit in patients with tumors that were PD-L1–negative (HR, 0.73) and PD-L1–positive (HR, 0.75).
There were fewer distant recurrences in the nivolumab group than in the placebo group (29% vs. 39%) and fewer locoregional recurrences (12% vs. 17%).
No new safety signals were observed, and 9% of the nivolumab patients discontinued the drug because of treatment-related adverse events versus 3% of placebo patients. In addition, a 1-year course of adjuvant nivolumab did not negatively impact patient-reported quality of life, the trialists reported.
Grade 3 or 4 adverse events of any cause were more frequent in the nivolumab group versus the placebo group (34% vs. 32%) as were those related to the intervention (13% vs. 6%).
Although overall survival data are not mature, “the doubling of median disease-free survival will almost certainly translate into an overall survival benefit,” Dr. Ilson wrote.
Notably, the trial’s original co-primary endpoint was overall survival, but was dropped to a secondary endpoint after enrollment “challenges.”
When the now-published data were first presented at the 2020 annual meeting of the European Society for Medical Oncology, the invited discussant, Andrés Cervantes, MD, PhD, University of Valencia, Spain, raised several issues with the trial.
Preoperative chemoradiation is not “universally accepted” as the standard of care in this setting, said Dr. Cervantes, president-elect of ESMO.
Dr. Ilson acknowledged as much. “A debate is ongoing about whether chemotherapy alone or combined chemoradiotherapy is the preferred treatment for esophageal cancer before surgery,” he wrote.
In addition, Dr. Cervantes noted that disease-free survival is not currently validated as a major endpoint in gastroesophageal cancers, and the median follow-up was short.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the adjuvant use of nivolumab (Opdivo) in patients with resected esophageal or gastroesophageal junction (GEJ) cancer who have received neoadjuvant chemoradiotherapy and have residual pathological disease following surgery.
The approval addresses an unmet need among these patients, who have a high risk of recurrence but for whom surveillance is the only current management option after the above-described standard treatment, according to experts.
The FDA’s approval is based on results from the CheckMate 577 study, which showed a significant improvement in disease-free survival compared with placebo.
This was described as “a practice-changing trial in the treatment of esophageal cancer” by David H. Ilson, MD, PhD, Memorial Sloan Kettering Cancer Center, New York, in an editorial in The New England Journal of Medicine that accompanied the published study results.
“The trial shows the first true advance in the adjuvant therapy of esophageal cancer in recent years,” wrote Dr. Ilson.
In the randomized, double-blind, phase 3 trial, patients with resected stage II or III esophageal or GEJ cancer were randomly assigned in a 2:1 ratio to receive nivolumab (at a dose of 240 mg every 2 weeks for 16 weeks, followed by a dose of 480 mg every 4 weeks) or placebo. The maximum duration of the intervention period was 1 year.
All of these patients had received neoadjuvant chemoradiotherapy and had residual pathological disease, as noted in the new indication. Patients were enrolled regardless of programmed death ligand 1 (PD-L1) expression.
For the primary endpoint of disease-free survival, the median was 22.4 months for the nivolumab group (n = 532) versus 11.0 months for the placebo group (n = 262; hazard ratio [HR] for disease recurrence or death, 0.69; 96.4% confidence interval [CI], 0.56-0.86; P < .001).
The median follow-up was 24.4 months.
Disease-free survival favored nivolumab across multiple preplanned subgroups.
However, as Dr. Ilson noted in the editorial, the efficacy of nivolumab varied, with more benefit seen for patients with squamous cell cancer (HR, 0.61) than for those with adenocarcinoma (HR, 0.75). Patients with esophageal tumors also had greater benefit (HR, 0.61) than did those with GEJ tumors (HR, 0.87).
There was benefit in patients with node-negative disease (HR, 0.74) and node-positive disease (HR, 0.67) and benefit in patients with tumors that were PD-L1–negative (HR, 0.73) and PD-L1–positive (HR, 0.75).
There were fewer distant recurrences in the nivolumab group than in the placebo group (29% vs. 39%) and fewer locoregional recurrences (12% vs. 17%).
No new safety signals were observed, and 9% of the nivolumab patients discontinued the drug because of treatment-related adverse events versus 3% of placebo patients. In addition, a 1-year course of adjuvant nivolumab did not negatively impact patient-reported quality of life, the trialists reported.
Grade 3 or 4 adverse events of any cause were more frequent in the nivolumab group versus the placebo group (34% vs. 32%) as were those related to the intervention (13% vs. 6%).
Although overall survival data are not mature, “the doubling of median disease-free survival will almost certainly translate into an overall survival benefit,” Dr. Ilson wrote.
Notably, the trial’s original co-primary endpoint was overall survival, but was dropped to a secondary endpoint after enrollment “challenges.”
When the now-published data were first presented at the 2020 annual meeting of the European Society for Medical Oncology, the invited discussant, Andrés Cervantes, MD, PhD, University of Valencia, Spain, raised several issues with the trial.
Preoperative chemoradiation is not “universally accepted” as the standard of care in this setting, said Dr. Cervantes, president-elect of ESMO.
Dr. Ilson acknowledged as much. “A debate is ongoing about whether chemotherapy alone or combined chemoradiotherapy is the preferred treatment for esophageal cancer before surgery,” he wrote.
In addition, Dr. Cervantes noted that disease-free survival is not currently validated as a major endpoint in gastroesophageal cancers, and the median follow-up was short.
A version of this article first appeared on Medscape.com.
GALACTIC-HF: Novel drug most effective in sickest HFrEF patients
The greatest relative benefit from omecamtiv mecarbil, a member of the novel myotropic drug class that improves cardiac performance, is produced in heart failure patients with the lowest left ventricular ejection fraction (LVEF), a new analysis of the recently published phase 3 GALACTIC-HF trial has found.
The findings reinforce the potential for this drug to be helpful in the management of the most advanced stages of heart failure with reduced ejection fraction (HFrEF), reported John R. Teerlink, MD, director of heart failure at San Francisco Veterans Affairs Medical Center, at the annual scientific sessions of the American College of Cardiology.
The phase 3 multinational GALACTIC-HF trial, published earlier this year, linked omecamtiv mecarbil with an 8% reduction in the risk of a heart failure–related events or cardiovascular death, relative to placebo, which was the primary outcome. For entry, HFrEF patients were required to have a LVEF of 35% or less.
Drilling down on ejection fraction
The new analysis divided participants into quartiles of baseline LVEF and then compared relative outcomes and safety.
In the lowest quartile, defined by a LVEF of 22% or lower, the reduction in risk of events reached 17% (hazard ratio, 0.83; 95% confidence interval, 0.73-0.95) for omecamtiv mecarbil relative to placebo. In the highest, defined by a LVEF of 33% or greater, the benefit fell short of significance (HR 0.99; 95% CI, 0.84-1.16). Across quartiles, LVEF was the “strongest modifier of the treatment effect,” emerging in this analysis as a statistically significant (P = .004) continuous variable.
The comparison by LVEF quartiles also provided an opportunity to show that omecamtiv mecarbil was as safe and well tolerated in those with the most advanced disease as in those less sick. At the lowest levels of LVEF, like the higher levels, omecamtiv mecarbil did not produce any adverse effects on blood pressure, heart rate, potassium homeostasis, or renal function.
In GALACTIC-HF, 8,256 HFrEF patients with LVEF 35% or less were randomized to omecamtiv mecarbil or placebo. The primary composite outcome of hospitalization or urgent visit for heart failure or death from cardiovascular causes was evaluated after a median of 21.8 months on therapy.
When incidence rate per 100 patient years was graphed against the range of LVEF, the relative advantage of omecamtiv mecarbil became visible just below an LVEF of 30%, climbing steadily even to the lowest LVEF, which reached 10%.
Perhaps relevant to the reduction in events, there were also greater relative reductions in NT-proBNP (NT-proB-type natriuretic peptide) for omecamtiv mecarbil at lower relative to higher LVEF. Although omecamtiv mecarbil is not associated with any direct vascular, electrophysiologic, or neurohormonal effects, according to Dr. Teerlink, the indirect effects of selective binding to cardiac myosin has been associated with lower NT-proBNP and other biomarkers of cardiac remodeling in prior clinical studies.
Although Dr. Teerlink acknowledged that relatively few patients in GALACTIC-HF received an angiotensin-receptor neprilysin inhibitor (ARNI) or a sodium glucose cotransporter-2 (SGLT2) inhibitor, he said there is “every reason to believe that omecamtiv mecarbil would be complementary to these therapies.” He said the mechanism of action of omecamtiv mecarbil, which improves systolic function, has no overlap with these drugs.
Importantly, there is a particular need for new treatment options in patients with advanced LVEF, according to Dr. Teerlink, who cited evidence, for example, that “the beneficial effect of [the ARNI] sacubitril valsartan, while still significant, decreases in patients with LVEF less than 35%.”
Overall, based on these results, “we believe that omecamtiv mecarbil represents a novel therapy that holds the promise of improving clinical outcomes in patients with severely reduced ejection fraction, which are the very patients that are most challenging for us to treat,” Dr. Teerlink said.
Omecamtiv mecarbil may ‘buy you some time’
Ileana Piña, MD, clinical professor of medicine, Central Michigan University, Mount Pleasant, Mich., agreed. She said that omecamtiv mecarbil, if approved, will be an option for the type of HFrEF patients who are being considered for heart transplant or mechanical-assist devices.
“We are very loath to use inotropes in this population, because we know that ultimately the inotrope is not going to do well,” said Dr. Piña, calling these therapies a “Band-Aid.” Based on the evidence from GALACTIC-HF, she thinks that omecamtiv mecarbil will be more versatile.
“This drug does not increase myocardial oxygen demand as do the inotropes, and it can be given in the outpatient setting if need be, so I see this as a real advance,” Dr. Piña said. Although Dr. Piña acknowledged that omecamtiv mecarbil did not reduce mortality in the GALACTIC-HF trial, “at least it will buy you some time.”
Dr. Teerlink has financial relationships with multiple pharmaceutical companies, including Amgen, Cytogenetics, and Servier, which provided funding for the GALACTIC-HF trial. Dr. Piña reports no potential conflicts of interest.
The greatest relative benefit from omecamtiv mecarbil, a member of the novel myotropic drug class that improves cardiac performance, is produced in heart failure patients with the lowest left ventricular ejection fraction (LVEF), a new analysis of the recently published phase 3 GALACTIC-HF trial has found.
The findings reinforce the potential for this drug to be helpful in the management of the most advanced stages of heart failure with reduced ejection fraction (HFrEF), reported John R. Teerlink, MD, director of heart failure at San Francisco Veterans Affairs Medical Center, at the annual scientific sessions of the American College of Cardiology.
The phase 3 multinational GALACTIC-HF trial, published earlier this year, linked omecamtiv mecarbil with an 8% reduction in the risk of a heart failure–related events or cardiovascular death, relative to placebo, which was the primary outcome. For entry, HFrEF patients were required to have a LVEF of 35% or less.
Drilling down on ejection fraction
The new analysis divided participants into quartiles of baseline LVEF and then compared relative outcomes and safety.
In the lowest quartile, defined by a LVEF of 22% or lower, the reduction in risk of events reached 17% (hazard ratio, 0.83; 95% confidence interval, 0.73-0.95) for omecamtiv mecarbil relative to placebo. In the highest, defined by a LVEF of 33% or greater, the benefit fell short of significance (HR 0.99; 95% CI, 0.84-1.16). Across quartiles, LVEF was the “strongest modifier of the treatment effect,” emerging in this analysis as a statistically significant (P = .004) continuous variable.
The comparison by LVEF quartiles also provided an opportunity to show that omecamtiv mecarbil was as safe and well tolerated in those with the most advanced disease as in those less sick. At the lowest levels of LVEF, like the higher levels, omecamtiv mecarbil did not produce any adverse effects on blood pressure, heart rate, potassium homeostasis, or renal function.
In GALACTIC-HF, 8,256 HFrEF patients with LVEF 35% or less were randomized to omecamtiv mecarbil or placebo. The primary composite outcome of hospitalization or urgent visit for heart failure or death from cardiovascular causes was evaluated after a median of 21.8 months on therapy.
When incidence rate per 100 patient years was graphed against the range of LVEF, the relative advantage of omecamtiv mecarbil became visible just below an LVEF of 30%, climbing steadily even to the lowest LVEF, which reached 10%.
Perhaps relevant to the reduction in events, there were also greater relative reductions in NT-proBNP (NT-proB-type natriuretic peptide) for omecamtiv mecarbil at lower relative to higher LVEF. Although omecamtiv mecarbil is not associated with any direct vascular, electrophysiologic, or neurohormonal effects, according to Dr. Teerlink, the indirect effects of selective binding to cardiac myosin has been associated with lower NT-proBNP and other biomarkers of cardiac remodeling in prior clinical studies.
Although Dr. Teerlink acknowledged that relatively few patients in GALACTIC-HF received an angiotensin-receptor neprilysin inhibitor (ARNI) or a sodium glucose cotransporter-2 (SGLT2) inhibitor, he said there is “every reason to believe that omecamtiv mecarbil would be complementary to these therapies.” He said the mechanism of action of omecamtiv mecarbil, which improves systolic function, has no overlap with these drugs.
Importantly, there is a particular need for new treatment options in patients with advanced LVEF, according to Dr. Teerlink, who cited evidence, for example, that “the beneficial effect of [the ARNI] sacubitril valsartan, while still significant, decreases in patients with LVEF less than 35%.”
Overall, based on these results, “we believe that omecamtiv mecarbil represents a novel therapy that holds the promise of improving clinical outcomes in patients with severely reduced ejection fraction, which are the very patients that are most challenging for us to treat,” Dr. Teerlink said.
Omecamtiv mecarbil may ‘buy you some time’
Ileana Piña, MD, clinical professor of medicine, Central Michigan University, Mount Pleasant, Mich., agreed. She said that omecamtiv mecarbil, if approved, will be an option for the type of HFrEF patients who are being considered for heart transplant or mechanical-assist devices.
“We are very loath to use inotropes in this population, because we know that ultimately the inotrope is not going to do well,” said Dr. Piña, calling these therapies a “Band-Aid.” Based on the evidence from GALACTIC-HF, she thinks that omecamtiv mecarbil will be more versatile.
“This drug does not increase myocardial oxygen demand as do the inotropes, and it can be given in the outpatient setting if need be, so I see this as a real advance,” Dr. Piña said. Although Dr. Piña acknowledged that omecamtiv mecarbil did not reduce mortality in the GALACTIC-HF trial, “at least it will buy you some time.”
Dr. Teerlink has financial relationships with multiple pharmaceutical companies, including Amgen, Cytogenetics, and Servier, which provided funding for the GALACTIC-HF trial. Dr. Piña reports no potential conflicts of interest.
The greatest relative benefit from omecamtiv mecarbil, a member of the novel myotropic drug class that improves cardiac performance, is produced in heart failure patients with the lowest left ventricular ejection fraction (LVEF), a new analysis of the recently published phase 3 GALACTIC-HF trial has found.
The findings reinforce the potential for this drug to be helpful in the management of the most advanced stages of heart failure with reduced ejection fraction (HFrEF), reported John R. Teerlink, MD, director of heart failure at San Francisco Veterans Affairs Medical Center, at the annual scientific sessions of the American College of Cardiology.
The phase 3 multinational GALACTIC-HF trial, published earlier this year, linked omecamtiv mecarbil with an 8% reduction in the risk of a heart failure–related events or cardiovascular death, relative to placebo, which was the primary outcome. For entry, HFrEF patients were required to have a LVEF of 35% or less.
Drilling down on ejection fraction
The new analysis divided participants into quartiles of baseline LVEF and then compared relative outcomes and safety.
In the lowest quartile, defined by a LVEF of 22% or lower, the reduction in risk of events reached 17% (hazard ratio, 0.83; 95% confidence interval, 0.73-0.95) for omecamtiv mecarbil relative to placebo. In the highest, defined by a LVEF of 33% or greater, the benefit fell short of significance (HR 0.99; 95% CI, 0.84-1.16). Across quartiles, LVEF was the “strongest modifier of the treatment effect,” emerging in this analysis as a statistically significant (P = .004) continuous variable.
The comparison by LVEF quartiles also provided an opportunity to show that omecamtiv mecarbil was as safe and well tolerated in those with the most advanced disease as in those less sick. At the lowest levels of LVEF, like the higher levels, omecamtiv mecarbil did not produce any adverse effects on blood pressure, heart rate, potassium homeostasis, or renal function.
In GALACTIC-HF, 8,256 HFrEF patients with LVEF 35% or less were randomized to omecamtiv mecarbil or placebo. The primary composite outcome of hospitalization or urgent visit for heart failure or death from cardiovascular causes was evaluated after a median of 21.8 months on therapy.
When incidence rate per 100 patient years was graphed against the range of LVEF, the relative advantage of omecamtiv mecarbil became visible just below an LVEF of 30%, climbing steadily even to the lowest LVEF, which reached 10%.
Perhaps relevant to the reduction in events, there were also greater relative reductions in NT-proBNP (NT-proB-type natriuretic peptide) for omecamtiv mecarbil at lower relative to higher LVEF. Although omecamtiv mecarbil is not associated with any direct vascular, electrophysiologic, or neurohormonal effects, according to Dr. Teerlink, the indirect effects of selective binding to cardiac myosin has been associated with lower NT-proBNP and other biomarkers of cardiac remodeling in prior clinical studies.
Although Dr. Teerlink acknowledged that relatively few patients in GALACTIC-HF received an angiotensin-receptor neprilysin inhibitor (ARNI) or a sodium glucose cotransporter-2 (SGLT2) inhibitor, he said there is “every reason to believe that omecamtiv mecarbil would be complementary to these therapies.” He said the mechanism of action of omecamtiv mecarbil, which improves systolic function, has no overlap with these drugs.
Importantly, there is a particular need for new treatment options in patients with advanced LVEF, according to Dr. Teerlink, who cited evidence, for example, that “the beneficial effect of [the ARNI] sacubitril valsartan, while still significant, decreases in patients with LVEF less than 35%.”
Overall, based on these results, “we believe that omecamtiv mecarbil represents a novel therapy that holds the promise of improving clinical outcomes in patients with severely reduced ejection fraction, which are the very patients that are most challenging for us to treat,” Dr. Teerlink said.
Omecamtiv mecarbil may ‘buy you some time’
Ileana Piña, MD, clinical professor of medicine, Central Michigan University, Mount Pleasant, Mich., agreed. She said that omecamtiv mecarbil, if approved, will be an option for the type of HFrEF patients who are being considered for heart transplant or mechanical-assist devices.
“We are very loath to use inotropes in this population, because we know that ultimately the inotrope is not going to do well,” said Dr. Piña, calling these therapies a “Band-Aid.” Based on the evidence from GALACTIC-HF, she thinks that omecamtiv mecarbil will be more versatile.
“This drug does not increase myocardial oxygen demand as do the inotropes, and it can be given in the outpatient setting if need be, so I see this as a real advance,” Dr. Piña said. Although Dr. Piña acknowledged that omecamtiv mecarbil did not reduce mortality in the GALACTIC-HF trial, “at least it will buy you some time.”
Dr. Teerlink has financial relationships with multiple pharmaceutical companies, including Amgen, Cytogenetics, and Servier, which provided funding for the GALACTIC-HF trial. Dr. Piña reports no potential conflicts of interest.
FROM ACC 2021
Healthy lifestyle can reduce dementia risk despite family history
Individuals at increased risk for dementia because of family history can reduce that risk by adopting healthy lifestyle behaviors, data from more than 300,000 adults aged 50-73 years suggest.
Having a parent or sibling with dementia can increase a person’s risk of developing dementia themselves by nearly 75%, compared with someone with no first-degree family history of dementia, according to Angelique Brellenthin, PhD, of Iowa State University, Ames, and colleagues.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting sponsored by the American Heart Association, the researchers reviewed information for 302,239 men and women who were enrolled in the U.K. Biobank, a population-based study of more than 500,000 individuals in the United Kingdom, between 2006 and 2010.
The study participants had no evidence of dementia at baseline, and completed questionnaires about family history and lifestyle. The questions included details about six healthy lifestyle behaviors: eating a healthy diet, engaging in at least 150 minutes of moderate to vigorous physical activity per week, sleeping 6-9 hours each night, drinking alcohol in moderation, not smoking, and maintaining a body mass index below the obese level (less than 30 kg/m2).
The researchers identified 1,698 participants (0.6%) who developed dementia over an average follow-up period of 8 years. Those with a family history (first-degree relative) of dementia had a 70% increased risk of dementia, compared with those who had no such family history.
Overall, individuals who engaged in all six healthy behaviors reduced their risk of dementia by about half, compared with those who engaged in two or fewer healthy behaviors. Engaging in three healthy behaviors reduced the risk of dementia by 30%, compared with engaging in two or fewer healthy behaviors, and this association held after controlling not only for family history of dementia, but also for other dementia risk factors such as age, sex, race, and education level, as well as high blood pressure, high cholesterol, and the presence of type 2 diabetes.
Similarly, among participants with a family history of dementia, those who engaged in three healthy lifestyle behaviors showed a 25%-35% reduction in dementia risk, compared with those who engaged in two or fewer healthy behaviors.
The study findings were limited by several factors including the inability to prove that lifestyle can cause or prevent dementia, only to show an association, the researchers noted. Also, the findings were limited by the reliance on self-reports, rather than genetic data, to confirm familial dementia.
However, the findings were strengthened by the large sample size, and the results suggest that a healthy lifestyle can impact cognitive health, and support the value of encouraging healthy behaviors in general, and especially among individuals with a family history of dementia, they said.
Small changes may promote prevention
The study is important now because, as the population ages, many individuals have a family member who has had dementia, said lead author Dr. Brellenthin, in an interview. “It’s important to understand how lifestyle behaviors affect the risk of dementia when it runs in families,” she said.
Dr. Brellenthin said she was surprised by some of the findings. “It was surprising to see that the risk of dementia was reduced with just three healthy behaviors [but was further reduced as you added more behaviors] compared to two or fewer behaviors. However, it was not surprising to see that these same lifestyle behaviors that tend to be good for the heart and body are also good for the brain.”
The evidence that following just three healthy behaviors can reduce the risk of dementia by 25%-35% for individuals with a familial history of dementia has clinical implications, Dr. Brellenthin said. “Many people are already following some of these behaviors like not smoking, so it might be possible to focus on adding just one more behavior, like getting enough sleep, and going from there.”
Commenting on the study, AHA President Mitchell S. V. Elkind, MD, said that the study “tells us that, yes, family history is important [in determining the risk of dementia], and much of that may be driven by genetic factors, but some of that impact can be mitigated or decreased by engaging in those important behaviors that we know are good to maintain brain health.
“The tricky thing, of course, is getting people to engage in these behaviors. That’s where a lot of work in the future will be: changing people’s behavior to become more healthy, and figuring out exactly which behaviors may be the easiest to engage in and be most likely to have public health impact,” added Dr. Elkind, professor of neurology and epidemiology at Columbia University and attending neurologist at New York–Presbyterian/Columbia University Irving Medical Center, New York.
The study received no outside funding, but the was research was conducted using the U.K. Biobank resources. The researchers had no financial conflicts to disclose.
Individuals at increased risk for dementia because of family history can reduce that risk by adopting healthy lifestyle behaviors, data from more than 300,000 adults aged 50-73 years suggest.
Having a parent or sibling with dementia can increase a person’s risk of developing dementia themselves by nearly 75%, compared with someone with no first-degree family history of dementia, according to Angelique Brellenthin, PhD, of Iowa State University, Ames, and colleagues.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting sponsored by the American Heart Association, the researchers reviewed information for 302,239 men and women who were enrolled in the U.K. Biobank, a population-based study of more than 500,000 individuals in the United Kingdom, between 2006 and 2010.
The study participants had no evidence of dementia at baseline, and completed questionnaires about family history and lifestyle. The questions included details about six healthy lifestyle behaviors: eating a healthy diet, engaging in at least 150 minutes of moderate to vigorous physical activity per week, sleeping 6-9 hours each night, drinking alcohol in moderation, not smoking, and maintaining a body mass index below the obese level (less than 30 kg/m2).
The researchers identified 1,698 participants (0.6%) who developed dementia over an average follow-up period of 8 years. Those with a family history (first-degree relative) of dementia had a 70% increased risk of dementia, compared with those who had no such family history.
Overall, individuals who engaged in all six healthy behaviors reduced their risk of dementia by about half, compared with those who engaged in two or fewer healthy behaviors. Engaging in three healthy behaviors reduced the risk of dementia by 30%, compared with engaging in two or fewer healthy behaviors, and this association held after controlling not only for family history of dementia, but also for other dementia risk factors such as age, sex, race, and education level, as well as high blood pressure, high cholesterol, and the presence of type 2 diabetes.
Similarly, among participants with a family history of dementia, those who engaged in three healthy lifestyle behaviors showed a 25%-35% reduction in dementia risk, compared with those who engaged in two or fewer healthy behaviors.
The study findings were limited by several factors including the inability to prove that lifestyle can cause or prevent dementia, only to show an association, the researchers noted. Also, the findings were limited by the reliance on self-reports, rather than genetic data, to confirm familial dementia.
However, the findings were strengthened by the large sample size, and the results suggest that a healthy lifestyle can impact cognitive health, and support the value of encouraging healthy behaviors in general, and especially among individuals with a family history of dementia, they said.
Small changes may promote prevention
The study is important now because, as the population ages, many individuals have a family member who has had dementia, said lead author Dr. Brellenthin, in an interview. “It’s important to understand how lifestyle behaviors affect the risk of dementia when it runs in families,” she said.
Dr. Brellenthin said she was surprised by some of the findings. “It was surprising to see that the risk of dementia was reduced with just three healthy behaviors [but was further reduced as you added more behaviors] compared to two or fewer behaviors. However, it was not surprising to see that these same lifestyle behaviors that tend to be good for the heart and body are also good for the brain.”
The evidence that following just three healthy behaviors can reduce the risk of dementia by 25%-35% for individuals with a familial history of dementia has clinical implications, Dr. Brellenthin said. “Many people are already following some of these behaviors like not smoking, so it might be possible to focus on adding just one more behavior, like getting enough sleep, and going from there.”
Commenting on the study, AHA President Mitchell S. V. Elkind, MD, said that the study “tells us that, yes, family history is important [in determining the risk of dementia], and much of that may be driven by genetic factors, but some of that impact can be mitigated or decreased by engaging in those important behaviors that we know are good to maintain brain health.
“The tricky thing, of course, is getting people to engage in these behaviors. That’s where a lot of work in the future will be: changing people’s behavior to become more healthy, and figuring out exactly which behaviors may be the easiest to engage in and be most likely to have public health impact,” added Dr. Elkind, professor of neurology and epidemiology at Columbia University and attending neurologist at New York–Presbyterian/Columbia University Irving Medical Center, New York.
The study received no outside funding, but the was research was conducted using the U.K. Biobank resources. The researchers had no financial conflicts to disclose.
Individuals at increased risk for dementia because of family history can reduce that risk by adopting healthy lifestyle behaviors, data from more than 300,000 adults aged 50-73 years suggest.
Having a parent or sibling with dementia can increase a person’s risk of developing dementia themselves by nearly 75%, compared with someone with no first-degree family history of dementia, according to Angelique Brellenthin, PhD, of Iowa State University, Ames, and colleagues.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting sponsored by the American Heart Association, the researchers reviewed information for 302,239 men and women who were enrolled in the U.K. Biobank, a population-based study of more than 500,000 individuals in the United Kingdom, between 2006 and 2010.
The study participants had no evidence of dementia at baseline, and completed questionnaires about family history and lifestyle. The questions included details about six healthy lifestyle behaviors: eating a healthy diet, engaging in at least 150 minutes of moderate to vigorous physical activity per week, sleeping 6-9 hours each night, drinking alcohol in moderation, not smoking, and maintaining a body mass index below the obese level (less than 30 kg/m2).
The researchers identified 1,698 participants (0.6%) who developed dementia over an average follow-up period of 8 years. Those with a family history (first-degree relative) of dementia had a 70% increased risk of dementia, compared with those who had no such family history.
Overall, individuals who engaged in all six healthy behaviors reduced their risk of dementia by about half, compared with those who engaged in two or fewer healthy behaviors. Engaging in three healthy behaviors reduced the risk of dementia by 30%, compared with engaging in two or fewer healthy behaviors, and this association held after controlling not only for family history of dementia, but also for other dementia risk factors such as age, sex, race, and education level, as well as high blood pressure, high cholesterol, and the presence of type 2 diabetes.
Similarly, among participants with a family history of dementia, those who engaged in three healthy lifestyle behaviors showed a 25%-35% reduction in dementia risk, compared with those who engaged in two or fewer healthy behaviors.
The study findings were limited by several factors including the inability to prove that lifestyle can cause or prevent dementia, only to show an association, the researchers noted. Also, the findings were limited by the reliance on self-reports, rather than genetic data, to confirm familial dementia.
However, the findings were strengthened by the large sample size, and the results suggest that a healthy lifestyle can impact cognitive health, and support the value of encouraging healthy behaviors in general, and especially among individuals with a family history of dementia, they said.
Small changes may promote prevention
The study is important now because, as the population ages, many individuals have a family member who has had dementia, said lead author Dr. Brellenthin, in an interview. “It’s important to understand how lifestyle behaviors affect the risk of dementia when it runs in families,” she said.
Dr. Brellenthin said she was surprised by some of the findings. “It was surprising to see that the risk of dementia was reduced with just three healthy behaviors [but was further reduced as you added more behaviors] compared to two or fewer behaviors. However, it was not surprising to see that these same lifestyle behaviors that tend to be good for the heart and body are also good for the brain.”
The evidence that following just three healthy behaviors can reduce the risk of dementia by 25%-35% for individuals with a familial history of dementia has clinical implications, Dr. Brellenthin said. “Many people are already following some of these behaviors like not smoking, so it might be possible to focus on adding just one more behavior, like getting enough sleep, and going from there.”
Commenting on the study, AHA President Mitchell S. V. Elkind, MD, said that the study “tells us that, yes, family history is important [in determining the risk of dementia], and much of that may be driven by genetic factors, but some of that impact can be mitigated or decreased by engaging in those important behaviors that we know are good to maintain brain health.
“The tricky thing, of course, is getting people to engage in these behaviors. That’s where a lot of work in the future will be: changing people’s behavior to become more healthy, and figuring out exactly which behaviors may be the easiest to engage in and be most likely to have public health impact,” added Dr. Elkind, professor of neurology and epidemiology at Columbia University and attending neurologist at New York–Presbyterian/Columbia University Irving Medical Center, New York.
The study received no outside funding, but the was research was conducted using the U.K. Biobank resources. The researchers had no financial conflicts to disclose.
FROM EPI/LIFESTYLE 2021
ID experts dole out practical advice to help with mask confusion
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
HHS to inject billions into mental health, substance use disorders
The U.S. Department of Health and Human Services will inject billions of dollars into programs designed to address mental health and substance use disorders, including $3 billion released to states as of May 18, said federal officials.
The American Rescue Plan, a COVID-relief package signed into law in March, contained the money, which will be divided equally between the Community Mental Health Services Block Grant Program and the Substance Abuse Prevention and Treatment Block Grant Program, said Tom Coderre, Acting Assistant Secretary for Mental Health and Substance Use, in a call with reporters.
The award amounts will vary by state.
The mental health program helps states and territories provide services for children with serious emotional issues and adults with serious mental illness.
The substance use program provides money to plan, implement, and evaluate prevention, intervention, treatment, and recovery services.
, which fueled an increase in anxiety, depression, and overdose, said Assistant Secretary for Health Rachel Levine, MD, on the call.
“We know multiple stressors during the pandemic – isolation, sickness, grief, job loss, food instability, and loss of routines – have devastated many Americans and presented the unprecedented behavioral health challenges across the nation,” said Dr. Levine.
The HHS also announced that it is re-establishing a Behavioral Health Coordinating Council (BHCC). Dr. Levine and Mr. Coderre will serve as cochairs of the Council, which will coordinate action-oriented approaches to addressing the HHS’s behavioral health efforts.
However, in 2014, the U.S. Government Accountability Office criticized the BHCC for only focusing on the HHS, and noted the lack of coordination across the federal government’s various efforts to address mental health.
‘A huge step forward’
The American Psychiatric Association welcomed the new money and the return of the council.
“In the wake of the pandemic an unprecedented, and as of yet untold, number of Americans are faced with mental health and substance use disorders, particularly in communities impacted by structural racism,” said APA President Vivian Pender, MD, in a statement. “With the creation of this Council and this investment in mental health, the administration is taking a huge step forward.”
APA CEO and Medical Director Saul Levin, MD, MPA, added: “This Council has great potential to ease the challenges we face as we begin to recover from the pandemic’s impact on our society, and [the] APA looks forward to assisting in their efforts.”
HHS Secretary Xavier Becerra noted in a statement that the COVID-19 pandemic “has made clear the need to invest resources in our nation’s mental health and address the inequities that still exist around behavioral health care.” He added, “This national problem calls for department-wide coordination to address the issue.”
Dr. Levine said the Council “will assure the right prioritization and guidelines are in place to provide pathways to prevention, intervention, treatment, and recovery services.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Health and Human Services will inject billions of dollars into programs designed to address mental health and substance use disorders, including $3 billion released to states as of May 18, said federal officials.
The American Rescue Plan, a COVID-relief package signed into law in March, contained the money, which will be divided equally between the Community Mental Health Services Block Grant Program and the Substance Abuse Prevention and Treatment Block Grant Program, said Tom Coderre, Acting Assistant Secretary for Mental Health and Substance Use, in a call with reporters.
The award amounts will vary by state.
The mental health program helps states and territories provide services for children with serious emotional issues and adults with serious mental illness.
The substance use program provides money to plan, implement, and evaluate prevention, intervention, treatment, and recovery services.
, which fueled an increase in anxiety, depression, and overdose, said Assistant Secretary for Health Rachel Levine, MD, on the call.
“We know multiple stressors during the pandemic – isolation, sickness, grief, job loss, food instability, and loss of routines – have devastated many Americans and presented the unprecedented behavioral health challenges across the nation,” said Dr. Levine.
The HHS also announced that it is re-establishing a Behavioral Health Coordinating Council (BHCC). Dr. Levine and Mr. Coderre will serve as cochairs of the Council, which will coordinate action-oriented approaches to addressing the HHS’s behavioral health efforts.
However, in 2014, the U.S. Government Accountability Office criticized the BHCC for only focusing on the HHS, and noted the lack of coordination across the federal government’s various efforts to address mental health.
‘A huge step forward’
The American Psychiatric Association welcomed the new money and the return of the council.
“In the wake of the pandemic an unprecedented, and as of yet untold, number of Americans are faced with mental health and substance use disorders, particularly in communities impacted by structural racism,” said APA President Vivian Pender, MD, in a statement. “With the creation of this Council and this investment in mental health, the administration is taking a huge step forward.”
APA CEO and Medical Director Saul Levin, MD, MPA, added: “This Council has great potential to ease the challenges we face as we begin to recover from the pandemic’s impact on our society, and [the] APA looks forward to assisting in their efforts.”
HHS Secretary Xavier Becerra noted in a statement that the COVID-19 pandemic “has made clear the need to invest resources in our nation’s mental health and address the inequities that still exist around behavioral health care.” He added, “This national problem calls for department-wide coordination to address the issue.”
Dr. Levine said the Council “will assure the right prioritization and guidelines are in place to provide pathways to prevention, intervention, treatment, and recovery services.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Health and Human Services will inject billions of dollars into programs designed to address mental health and substance use disorders, including $3 billion released to states as of May 18, said federal officials.
The American Rescue Plan, a COVID-relief package signed into law in March, contained the money, which will be divided equally between the Community Mental Health Services Block Grant Program and the Substance Abuse Prevention and Treatment Block Grant Program, said Tom Coderre, Acting Assistant Secretary for Mental Health and Substance Use, in a call with reporters.
The award amounts will vary by state.
The mental health program helps states and territories provide services for children with serious emotional issues and adults with serious mental illness.
The substance use program provides money to plan, implement, and evaluate prevention, intervention, treatment, and recovery services.
, which fueled an increase in anxiety, depression, and overdose, said Assistant Secretary for Health Rachel Levine, MD, on the call.
“We know multiple stressors during the pandemic – isolation, sickness, grief, job loss, food instability, and loss of routines – have devastated many Americans and presented the unprecedented behavioral health challenges across the nation,” said Dr. Levine.
The HHS also announced that it is re-establishing a Behavioral Health Coordinating Council (BHCC). Dr. Levine and Mr. Coderre will serve as cochairs of the Council, which will coordinate action-oriented approaches to addressing the HHS’s behavioral health efforts.
However, in 2014, the U.S. Government Accountability Office criticized the BHCC for only focusing on the HHS, and noted the lack of coordination across the federal government’s various efforts to address mental health.
‘A huge step forward’
The American Psychiatric Association welcomed the new money and the return of the council.
“In the wake of the pandemic an unprecedented, and as of yet untold, number of Americans are faced with mental health and substance use disorders, particularly in communities impacted by structural racism,” said APA President Vivian Pender, MD, in a statement. “With the creation of this Council and this investment in mental health, the administration is taking a huge step forward.”
APA CEO and Medical Director Saul Levin, MD, MPA, added: “This Council has great potential to ease the challenges we face as we begin to recover from the pandemic’s impact on our society, and [the] APA looks forward to assisting in their efforts.”
HHS Secretary Xavier Becerra noted in a statement that the COVID-19 pandemic “has made clear the need to invest resources in our nation’s mental health and address the inequities that still exist around behavioral health care.” He added, “This national problem calls for department-wide coordination to address the issue.”
Dr. Levine said the Council “will assure the right prioritization and guidelines are in place to provide pathways to prevention, intervention, treatment, and recovery services.”
A version of this article first appeared on Medscape.com.
Family physicians’ compensation levels stable in pandemic
to $236,000, up from $234,000 last year, even as many practices saw a decrease in hours and patient visits during the pandemic.
Only pediatricians earned less ($221,000) according to the Medscape Family Physician Compensation Report 2021. Plastic surgeons topped this year’s list, at $526,000, followed by orthopedists, at $511,000, and cardiologists, at $459,000.
Family physicians ranked in the middle of specialties in terms of the percentages of physicians who thought they were fairly compensated: 57% of family physicians said they were fairly paid, and 79% of oncologists said they were. Only 44% of infectious disease physicians said they were fairly compensated.
Survey answers indicate, though, that pay isn’t driving family physicians’ satisfaction.
Only 10% of family physicians in the survey said that “making good money at a job I like” was the most rewarding aspect of the job. The top two answers by far were “gratitude/relationships with patients” (chosen by 34%) and “knowing I’m making the world a better place” (27%). Respondents could choose more than one answer.
Despite the small uptick in earnings overall in the specialty, more than one-third of family physicians (36%) reported a decline in compensation in this year’s survey, which included 18,000 responses from physicians in 29 specialties.
Male family physicians continue to be paid much more than their female colleagues, this year 29% more, widening the gap from 26% last year. Overall, men in primary care earned 27% more than their female colleagues, and male specialists earned 33% more.
As for decline in patients seen in some specialties, family physicians are holding their own.
Whereas pediatricians have seen a drop of 18% in patient visits, family physicians saw a decline of just 5%, from an average of 81 to 77 patients per week.
Most expect return to normal pay within 3 years
Most family physicians (83%) who incurred financial losses this year said they expect that income will return to normal within 3 years. More than one-third of that group (38%) said they expect compensation to get back to normal in the next year.
Almost all of the family physicians who lost income (91%) pointed the finger at COVID-19. Respondents could choose more than one answer, and 18% said other factors were also to blame.
Family physicians averaged $27,000 in incentive bonuses, higher than those in internal medicine, pediatrics, and psychiatry. Orthopedists had by far the highest bonuses, at $116,000.
For family physicians who received a bonus this year, the amount equaled about 12% of their salary, up from 10% last year. Bonuses are usually based on productivity but can also be tied to patient satisfaction, clinical processes, and other factors.
The number of family physicians who achieved more than three-quarters of their potential annual bonus rose to 61% this year, up from 55%.
17 hours a week on administrative tasks
The survey also ranked specialties by the amount of time physicians spent on paperwork and administrative tasks, including participation in professional organizations and clinical reading.
Family physicians fell squarely in the middle, with 17 hours per week spent on such tasks. Infectious disease physicians spent the most time, at 24.2 hours a week, and anesthesiologists spent the least, at 10.1.
Work hours declined for many physicians during the pandemic, and some were furloughed.
But, like most physicians, family physicians are once more working normal hours. They average 49 hours per week, which is slightly more than before the pandemic.
Specialists whose weekly hours are above normal are infectious disease physicians, intensivists, and public health and preventive medicine physicians; all are working 6 to 7 hours a week more than usual, according to the survey responses.
Responses also turned up some uncertainty on the future makeup of patient panels.
Most family physicians (69%) said they would continue to take new and current Medicare/Medicaid patients.
However, close to one-third of family physicians said they would stop treating at least some patients they already have and will not take new ones or haven’t decided yet.
A version of this article first appeared on Medscape.com.
to $236,000, up from $234,000 last year, even as many practices saw a decrease in hours and patient visits during the pandemic.

Only pediatricians earned less ($221,000) according to the Medscape Family Physician Compensation Report 2021. Plastic surgeons topped this year’s list, at $526,000, followed by orthopedists, at $511,000, and cardiologists, at $459,000.
Family physicians ranked in the middle of specialties in terms of the percentages of physicians who thought they were fairly compensated: 57% of family physicians said they were fairly paid, and 79% of oncologists said they were. Only 44% of infectious disease physicians said they were fairly compensated.
Survey answers indicate, though, that pay isn’t driving family physicians’ satisfaction.
Only 10% of family physicians in the survey said that “making good money at a job I like” was the most rewarding aspect of the job. The top two answers by far were “gratitude/relationships with patients” (chosen by 34%) and “knowing I’m making the world a better place” (27%). Respondents could choose more than one answer.
Despite the small uptick in earnings overall in the specialty, more than one-third of family physicians (36%) reported a decline in compensation in this year’s survey, which included 18,000 responses from physicians in 29 specialties.
Male family physicians continue to be paid much more than their female colleagues, this year 29% more, widening the gap from 26% last year. Overall, men in primary care earned 27% more than their female colleagues, and male specialists earned 33% more.
As for decline in patients seen in some specialties, family physicians are holding their own.
Whereas pediatricians have seen a drop of 18% in patient visits, family physicians saw a decline of just 5%, from an average of 81 to 77 patients per week.
Most expect return to normal pay within 3 years
Most family physicians (83%) who incurred financial losses this year said they expect that income will return to normal within 3 years. More than one-third of that group (38%) said they expect compensation to get back to normal in the next year.
Almost all of the family physicians who lost income (91%) pointed the finger at COVID-19. Respondents could choose more than one answer, and 18% said other factors were also to blame.
Family physicians averaged $27,000 in incentive bonuses, higher than those in internal medicine, pediatrics, and psychiatry. Orthopedists had by far the highest bonuses, at $116,000.
For family physicians who received a bonus this year, the amount equaled about 12% of their salary, up from 10% last year. Bonuses are usually based on productivity but can also be tied to patient satisfaction, clinical processes, and other factors.
The number of family physicians who achieved more than three-quarters of their potential annual bonus rose to 61% this year, up from 55%.
17 hours a week on administrative tasks
The survey also ranked specialties by the amount of time physicians spent on paperwork and administrative tasks, including participation in professional organizations and clinical reading.
Family physicians fell squarely in the middle, with 17 hours per week spent on such tasks. Infectious disease physicians spent the most time, at 24.2 hours a week, and anesthesiologists spent the least, at 10.1.
Work hours declined for many physicians during the pandemic, and some were furloughed.
But, like most physicians, family physicians are once more working normal hours. They average 49 hours per week, which is slightly more than before the pandemic.
Specialists whose weekly hours are above normal are infectious disease physicians, intensivists, and public health and preventive medicine physicians; all are working 6 to 7 hours a week more than usual, according to the survey responses.
Responses also turned up some uncertainty on the future makeup of patient panels.
Most family physicians (69%) said they would continue to take new and current Medicare/Medicaid patients.
However, close to one-third of family physicians said they would stop treating at least some patients they already have and will not take new ones or haven’t decided yet.
A version of this article first appeared on Medscape.com.
to $236,000, up from $234,000 last year, even as many practices saw a decrease in hours and patient visits during the pandemic.

Only pediatricians earned less ($221,000) according to the Medscape Family Physician Compensation Report 2021. Plastic surgeons topped this year’s list, at $526,000, followed by orthopedists, at $511,000, and cardiologists, at $459,000.
Family physicians ranked in the middle of specialties in terms of the percentages of physicians who thought they were fairly compensated: 57% of family physicians said they were fairly paid, and 79% of oncologists said they were. Only 44% of infectious disease physicians said they were fairly compensated.
Survey answers indicate, though, that pay isn’t driving family physicians’ satisfaction.
Only 10% of family physicians in the survey said that “making good money at a job I like” was the most rewarding aspect of the job. The top two answers by far were “gratitude/relationships with patients” (chosen by 34%) and “knowing I’m making the world a better place” (27%). Respondents could choose more than one answer.
Despite the small uptick in earnings overall in the specialty, more than one-third of family physicians (36%) reported a decline in compensation in this year’s survey, which included 18,000 responses from physicians in 29 specialties.
Male family physicians continue to be paid much more than their female colleagues, this year 29% more, widening the gap from 26% last year. Overall, men in primary care earned 27% more than their female colleagues, and male specialists earned 33% more.
As for decline in patients seen in some specialties, family physicians are holding their own.
Whereas pediatricians have seen a drop of 18% in patient visits, family physicians saw a decline of just 5%, from an average of 81 to 77 patients per week.
Most expect return to normal pay within 3 years
Most family physicians (83%) who incurred financial losses this year said they expect that income will return to normal within 3 years. More than one-third of that group (38%) said they expect compensation to get back to normal in the next year.
Almost all of the family physicians who lost income (91%) pointed the finger at COVID-19. Respondents could choose more than one answer, and 18% said other factors were also to blame.
Family physicians averaged $27,000 in incentive bonuses, higher than those in internal medicine, pediatrics, and psychiatry. Orthopedists had by far the highest bonuses, at $116,000.
For family physicians who received a bonus this year, the amount equaled about 12% of their salary, up from 10% last year. Bonuses are usually based on productivity but can also be tied to patient satisfaction, clinical processes, and other factors.
The number of family physicians who achieved more than three-quarters of their potential annual bonus rose to 61% this year, up from 55%.
17 hours a week on administrative tasks
The survey also ranked specialties by the amount of time physicians spent on paperwork and administrative tasks, including participation in professional organizations and clinical reading.
Family physicians fell squarely in the middle, with 17 hours per week spent on such tasks. Infectious disease physicians spent the most time, at 24.2 hours a week, and anesthesiologists spent the least, at 10.1.
Work hours declined for many physicians during the pandemic, and some were furloughed.
But, like most physicians, family physicians are once more working normal hours. They average 49 hours per week, which is slightly more than before the pandemic.
Specialists whose weekly hours are above normal are infectious disease physicians, intensivists, and public health and preventive medicine physicians; all are working 6 to 7 hours a week more than usual, according to the survey responses.
Responses also turned up some uncertainty on the future makeup of patient panels.
Most family physicians (69%) said they would continue to take new and current Medicare/Medicaid patients.
However, close to one-third of family physicians said they would stop treating at least some patients they already have and will not take new ones or haven’t decided yet.
A version of this article first appeared on Medscape.com.
Pressure on primary care expected to intensify with long-COVID
, experts say.
“It could be as many as 5% to 10% who are still having symptoms at 12 weeks. Those numbers are higher if you’re talking about patients who had been hospitalized with COVID-19,” Russ Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston, said in an interview.
A recent study from the Centers for Disease Control and Prevention and Kaiser Permanente Georgia found that among 3,171 nonhospitalized adult patients with COVID-19, 69% had one or more outpatient visits 28 to 180 days after the diagnosis. Two-thirds had a visit for a new primary diagnosis, and about one-third had a new specialist visit. Symptom diagnoses included cough, shortness of breath, chest or throat pain, and fatigue.
These visits have come while cases of acute COVID continue to occur, and there has been an increase in patients returning to primary care after avoiding it while the pandemic surged. For these patients, delay in seeking care has often led a worsening of chronic conditions.
Dr. Phillips pointed to a shortcoming in primary care that will need to be addressed with regard to long-COVID: “We don’t have good systems to follow patients and their symptoms over time.”
Long-COVID will require that kind of care, but current payment systems don’t support proactively reaching out to patients to track them over time, he noted.
“We do a good job of identifying these issues for patients who come in, but it’s the patients who don’t that we worry about the most,” he said.
Dr. Phillips provided examples of the kind of management plans needed to improve outcomes for patients with long-COVID. In anticoagulation clinics, patients who receive blood thinners are monitored closely, and in mental health care, patients with depression are linked with social workers and are monitored regularly.
“Around COVID, those management plans are in their infancy,” he said.
John Brooks, MD, chief medical officer for the CDC’s COVID-19 response, testified in a congressional hearing at the end of April that interim guidance concerning protocols for long-COVID in primary care are forthcoming. He also noted that the CDC is working closely with the Centers for Medicare & Medicaid Services to develop medical coding for long-COVID.
In the meantime, Dr. Phillips said, one strategy is to have patients self-monitor their condition and relay results to primary care physicians electronically.
As an example, Dr. Phillips described a patient with long-COVID who was receiving supplemental oxygen and who wanted to resume her exercise regimen.
She checked her own oxygen saturation levels before and during exercise and reported the levels every few days through their patient portal.
“Very slowly we were able to cut down on her oxygen and increase her exercise capacity until she no longer needed oxygen and could go back to her usual activities of daily living,” he said.
Nurse practitioners, social workers, and other nonphysician care team members may be increasingly relied upon to provide care for long-COVID patients as well, he said.
Additionally, telehealth, which is currently reimbursed the same way as in-person visits are, enables easier access for checking in with patients, he said.
Empathy and listening needed
Sabrina Assoumou, MD, MPH, assistant professor of medicine at Boston University, told this news organization that it will be crucial to address health care disparities as long-COVID cases mount.
COVID disproportionately affects communities of color, and it stands to reason that this will be the case for long-COVID as well, she said. Diversifying the workforce will be vital, inasmuch as diagnosis may depend on how well a physician listens to patients as they describe their symptoms, continued Dr. Assoumou, whose primary care practice centers on HIV patients.
The symptoms of long-COVID are vague, she explained, and include brain fog, fatigue, and shortness of breath, and it takes longer to diagnose than many conditions.
Dr. Assoumou said some people were never tested for COVID and never received a diagnosis, yet they are now experiencing the extended effects.
“Long-COVID will force us to go back to the basics – like really listening to our patients,” she said. “We’re definitely going to need to be more empathetic.”
No large influx yet
Charles Vega, MD, health sciences clinical professor of family medicine at the University of California, Irvine, said he is skeptical that the primary care system will be overwhelmed with long-COVID cases.
Dr. Vega is a family physician working in the largest safety net clinic in Orange County, California. About 90% of his patients are LatinX, a population disproportionately burdened by COVID, yet he hasn’t seen a surge in long-COVID cases.
He said that may be because patients know there isn’t a treatment for long-COVID. They are well connected through online forums such as Body Politic COVID-19 Support Group and may not feel they need to see a doctor.
“It wasn’t scientists finding [long-COVID], it was patients who developed this disease model themselves,” he said. “That’s where most of the data sharing is.”
Yet, for long-COVID patients who do need care, primary care is the best home for them, Dr. Vega said.
He said the most common symptoms he sees are fatigue and poor activity tolerance. “They get winded going to the bathroom,” he said.
The most difficult symptom is dyspnea, he said. Patients describe being breathless, but it’s not bad enough to qualify for supplemental oxygen.
“Being breathless is a pretty desperate thing and hurts quality of life,” he said.
Most patients describe general malaise.
Care for long-COVID will require medical care and mental health care, Dr. Vega notes. Primary care is already set up to screen and to coordinate care with the appropriate provider.
“I think there’s a role for specialists, but primary care has to be involved,” he said.
Dr. Phillips, Dr. Assoumou, and Dr. Vega report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, experts say.
“It could be as many as 5% to 10% who are still having symptoms at 12 weeks. Those numbers are higher if you’re talking about patients who had been hospitalized with COVID-19,” Russ Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston, said in an interview.
A recent study from the Centers for Disease Control and Prevention and Kaiser Permanente Georgia found that among 3,171 nonhospitalized adult patients with COVID-19, 69% had one or more outpatient visits 28 to 180 days after the diagnosis. Two-thirds had a visit for a new primary diagnosis, and about one-third had a new specialist visit. Symptom diagnoses included cough, shortness of breath, chest or throat pain, and fatigue.
These visits have come while cases of acute COVID continue to occur, and there has been an increase in patients returning to primary care after avoiding it while the pandemic surged. For these patients, delay in seeking care has often led a worsening of chronic conditions.
Dr. Phillips pointed to a shortcoming in primary care that will need to be addressed with regard to long-COVID: “We don’t have good systems to follow patients and their symptoms over time.”
Long-COVID will require that kind of care, but current payment systems don’t support proactively reaching out to patients to track them over time, he noted.
“We do a good job of identifying these issues for patients who come in, but it’s the patients who don’t that we worry about the most,” he said.
Dr. Phillips provided examples of the kind of management plans needed to improve outcomes for patients with long-COVID. In anticoagulation clinics, patients who receive blood thinners are monitored closely, and in mental health care, patients with depression are linked with social workers and are monitored regularly.
“Around COVID, those management plans are in their infancy,” he said.
John Brooks, MD, chief medical officer for the CDC’s COVID-19 response, testified in a congressional hearing at the end of April that interim guidance concerning protocols for long-COVID in primary care are forthcoming. He also noted that the CDC is working closely with the Centers for Medicare & Medicaid Services to develop medical coding for long-COVID.
In the meantime, Dr. Phillips said, one strategy is to have patients self-monitor their condition and relay results to primary care physicians electronically.
As an example, Dr. Phillips described a patient with long-COVID who was receiving supplemental oxygen and who wanted to resume her exercise regimen.
She checked her own oxygen saturation levels before and during exercise and reported the levels every few days through their patient portal.
“Very slowly we were able to cut down on her oxygen and increase her exercise capacity until she no longer needed oxygen and could go back to her usual activities of daily living,” he said.
Nurse practitioners, social workers, and other nonphysician care team members may be increasingly relied upon to provide care for long-COVID patients as well, he said.
Additionally, telehealth, which is currently reimbursed the same way as in-person visits are, enables easier access for checking in with patients, he said.
Empathy and listening needed
Sabrina Assoumou, MD, MPH, assistant professor of medicine at Boston University, told this news organization that it will be crucial to address health care disparities as long-COVID cases mount.
COVID disproportionately affects communities of color, and it stands to reason that this will be the case for long-COVID as well, she said. Diversifying the workforce will be vital, inasmuch as diagnosis may depend on how well a physician listens to patients as they describe their symptoms, continued Dr. Assoumou, whose primary care practice centers on HIV patients.
The symptoms of long-COVID are vague, she explained, and include brain fog, fatigue, and shortness of breath, and it takes longer to diagnose than many conditions.
Dr. Assoumou said some people were never tested for COVID and never received a diagnosis, yet they are now experiencing the extended effects.
“Long-COVID will force us to go back to the basics – like really listening to our patients,” she said. “We’re definitely going to need to be more empathetic.”
No large influx yet
Charles Vega, MD, health sciences clinical professor of family medicine at the University of California, Irvine, said he is skeptical that the primary care system will be overwhelmed with long-COVID cases.
Dr. Vega is a family physician working in the largest safety net clinic in Orange County, California. About 90% of his patients are LatinX, a population disproportionately burdened by COVID, yet he hasn’t seen a surge in long-COVID cases.
He said that may be because patients know there isn’t a treatment for long-COVID. They are well connected through online forums such as Body Politic COVID-19 Support Group and may not feel they need to see a doctor.
“It wasn’t scientists finding [long-COVID], it was patients who developed this disease model themselves,” he said. “That’s where most of the data sharing is.”
Yet, for long-COVID patients who do need care, primary care is the best home for them, Dr. Vega said.
He said the most common symptoms he sees are fatigue and poor activity tolerance. “They get winded going to the bathroom,” he said.
The most difficult symptom is dyspnea, he said. Patients describe being breathless, but it’s not bad enough to qualify for supplemental oxygen.
“Being breathless is a pretty desperate thing and hurts quality of life,” he said.
Most patients describe general malaise.
Care for long-COVID will require medical care and mental health care, Dr. Vega notes. Primary care is already set up to screen and to coordinate care with the appropriate provider.
“I think there’s a role for specialists, but primary care has to be involved,” he said.
Dr. Phillips, Dr. Assoumou, and Dr. Vega report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, experts say.
“It could be as many as 5% to 10% who are still having symptoms at 12 weeks. Those numbers are higher if you’re talking about patients who had been hospitalized with COVID-19,” Russ Phillips, MD, director of the Center for Primary Care at Harvard Medical School, Boston, said in an interview.
A recent study from the Centers for Disease Control and Prevention and Kaiser Permanente Georgia found that among 3,171 nonhospitalized adult patients with COVID-19, 69% had one or more outpatient visits 28 to 180 days after the diagnosis. Two-thirds had a visit for a new primary diagnosis, and about one-third had a new specialist visit. Symptom diagnoses included cough, shortness of breath, chest or throat pain, and fatigue.
These visits have come while cases of acute COVID continue to occur, and there has been an increase in patients returning to primary care after avoiding it while the pandemic surged. For these patients, delay in seeking care has often led a worsening of chronic conditions.
Dr. Phillips pointed to a shortcoming in primary care that will need to be addressed with regard to long-COVID: “We don’t have good systems to follow patients and their symptoms over time.”
Long-COVID will require that kind of care, but current payment systems don’t support proactively reaching out to patients to track them over time, he noted.
“We do a good job of identifying these issues for patients who come in, but it’s the patients who don’t that we worry about the most,” he said.
Dr. Phillips provided examples of the kind of management plans needed to improve outcomes for patients with long-COVID. In anticoagulation clinics, patients who receive blood thinners are monitored closely, and in mental health care, patients with depression are linked with social workers and are monitored regularly.
“Around COVID, those management plans are in their infancy,” he said.
John Brooks, MD, chief medical officer for the CDC’s COVID-19 response, testified in a congressional hearing at the end of April that interim guidance concerning protocols for long-COVID in primary care are forthcoming. He also noted that the CDC is working closely with the Centers for Medicare & Medicaid Services to develop medical coding for long-COVID.
In the meantime, Dr. Phillips said, one strategy is to have patients self-monitor their condition and relay results to primary care physicians electronically.
As an example, Dr. Phillips described a patient with long-COVID who was receiving supplemental oxygen and who wanted to resume her exercise regimen.
She checked her own oxygen saturation levels before and during exercise and reported the levels every few days through their patient portal.
“Very slowly we were able to cut down on her oxygen and increase her exercise capacity until she no longer needed oxygen and could go back to her usual activities of daily living,” he said.
Nurse practitioners, social workers, and other nonphysician care team members may be increasingly relied upon to provide care for long-COVID patients as well, he said.
Additionally, telehealth, which is currently reimbursed the same way as in-person visits are, enables easier access for checking in with patients, he said.
Empathy and listening needed
Sabrina Assoumou, MD, MPH, assistant professor of medicine at Boston University, told this news organization that it will be crucial to address health care disparities as long-COVID cases mount.
COVID disproportionately affects communities of color, and it stands to reason that this will be the case for long-COVID as well, she said. Diversifying the workforce will be vital, inasmuch as diagnosis may depend on how well a physician listens to patients as they describe their symptoms, continued Dr. Assoumou, whose primary care practice centers on HIV patients.
The symptoms of long-COVID are vague, she explained, and include brain fog, fatigue, and shortness of breath, and it takes longer to diagnose than many conditions.
Dr. Assoumou said some people were never tested for COVID and never received a diagnosis, yet they are now experiencing the extended effects.
“Long-COVID will force us to go back to the basics – like really listening to our patients,” she said. “We’re definitely going to need to be more empathetic.”
No large influx yet
Charles Vega, MD, health sciences clinical professor of family medicine at the University of California, Irvine, said he is skeptical that the primary care system will be overwhelmed with long-COVID cases.
Dr. Vega is a family physician working in the largest safety net clinic in Orange County, California. About 90% of his patients are LatinX, a population disproportionately burdened by COVID, yet he hasn’t seen a surge in long-COVID cases.
He said that may be because patients know there isn’t a treatment for long-COVID. They are well connected through online forums such as Body Politic COVID-19 Support Group and may not feel they need to see a doctor.
“It wasn’t scientists finding [long-COVID], it was patients who developed this disease model themselves,” he said. “That’s where most of the data sharing is.”
Yet, for long-COVID patients who do need care, primary care is the best home for them, Dr. Vega said.
He said the most common symptoms he sees are fatigue and poor activity tolerance. “They get winded going to the bathroom,” he said.
The most difficult symptom is dyspnea, he said. Patients describe being breathless, but it’s not bad enough to qualify for supplemental oxygen.
“Being breathless is a pretty desperate thing and hurts quality of life,” he said.
Most patients describe general malaise.
Care for long-COVID will require medical care and mental health care, Dr. Vega notes. Primary care is already set up to screen and to coordinate care with the appropriate provider.
“I think there’s a role for specialists, but primary care has to be involved,” he said.
Dr. Phillips, Dr. Assoumou, and Dr. Vega report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.








