User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Pandemic colonoscopy restrictions may lead to worse CRC outcomes
For veterans, changes in colonoscopy screening caused by the COVID-19 pandemic may have increased risks of delayed colorectal cancer (CRC) diagnosis and could lead to worse CRC outcomes, based on data from more than 33,000 patients in the Veterans Health Administration.
After COVID-19 screening policies were implemented, a significantly lower rate of veterans with red-flag signs or symptoms for CRC underwent colonoscopy, lead author Joshua Demb, PhD, a cancer epidemiologist at the University of California, San Diego, reported at the annual Digestive Disease Week® (DDW).
“As a result of the COVID-19 pandemic, the Veterans Health Administration enacted risk mitigation and management strategies in March 2020, including postponement of nearly all colonoscopies,” the investigators reported. “Notably, this included veterans with red flag signs or symptoms for CRC, among whom delays in workup could increase risk for later-stage and fatal CRC, if present.”
To measure the effects of this policy change, Dr. Demb and colleagues performed a cohort study involving 33,804 veterans with red-flag signs or symptoms for CRC, including hematochezia, iron deficiency anemia, or abnormal guaiac fecal occult blood test or fecal immunochemical test (FIT). Veterans were divided into two cohorts based on date of first red flag diagnosis: either before the COVID-19 policy was implemented (April to October 2019; n = 19,472) or after (April to October 2020; n = 14,332), with an intervening 6-month washout period.
Primary outcomes were proportion completing colonoscopy and time to colonoscopy completion. Multivariable logistic regression incorporated a number of demographic and medical covariates, including race/ethnicity, sex, age, number of red-flag signs/symptoms, first red-flag sign/symptom, and others.
Before the COVID-19 policy change, 44% of individuals with red-flag signs or symptoms received a colonoscopy, compared with 32% after the policy was introduced (P < .01). Adjusted models showed that veterans in the COVID policy group were 42% less likely to receive a diagnostic colonoscopy than those in the prepolicy group (odds ratio, 0.58; 95% confidence interval, 0.55-0.61). While these findings showed greater likelihood of receiving a screening before the pandemic, postpolicy colonoscopies were conducted sooner, with a median time to procedure of 41 days, compared with 65 days before the pandemic (P < .01). Similar differences in screening rates between pre- and postpandemic groups were observed across all types of red flag signs and symptoms.
“Lower colonoscopy uptake was observed among individuals with red-flag signs/symptoms for CRC post- versus preimplementation of COVID-19 policies, suggesting increased future risk for delayed CRC diagnosis and adverse CRC outcomes,” the investigators concluded.
Prioritization may be needed to overcome backlog of colonoscopies
Jill Tinmouth, MD, PhD, lead scientist for ColonCancerCheck, Ontario’s organized colorectal cancer screening program, and a gastroenterologist and scientist at Sunnybrook Health Sciences Centre, Toronto, shared similar concerns about delayed diagnoses.
“We might expect these cancers to present ... at a more advanced stage, and that, as a result, the outcomes from these cancers could be worse,” Dr. Tinmouth said in an interview.
She also noted the change in colonoscopy timing.
“A particularly interesting finding was that, when a colonoscopy occurred, the time to colonoscopy was shorter during the COVID era than in the pre-COVID era,” Dr. Tinmouth said. “The authors suggested that this might be as a result of Veterans Health Administration policies implemented as a result of the pandemic that led to prioritization of more urgent procedures.”
According to Dr. Tinmouth, similar prioritization may be needed to catch up with the backlog of colonoscopies created by pandemic-related policy changes. In a recent study comparing two backlog management techniques, Dr. Tinmouth and colleagues concluded that redirecting low-yield colonoscopies to FIT without increasing hospital colonoscopy capacity could reduce time to recovery by more than half.
Even so, screening programs may be facing a long road to recovery.
“Recovery of the colonoscopy backlog is going to be a challenge that will take a while – maybe even years – to resolve,” Dr. Tinmouth said. “Jurisdictions/institutions that have a strong centralized intake or triage will likely be most successful in resolving the backlog quickly as they will be able to prioritize the most urgent cases, such as persons with an abnormal FIT or with symptoms, and to redirect persons scheduled for a ‘low-yield’ colonoscopy to have a FIT instead.” Ontario defines low-yield colonoscopies as primary screening for average-risk individuals and follow-up colonoscopies for patients with low-risk adenomas at baseline.
When asked about strategies to address future pandemics, Dr. Tinmouth said, “I think that two key learnings for me from this [pandemic] are: one, not to let our guard down, and to remain vigilant and prepared – in terms of monitoring, supply chain, equipment, etc.] ... and two to create a nimble and agile health system so that we are able to assess the challenges that the next pandemic brings and address them as quickly as possible.”The investigators and Dr. Tinmouth reported no conflicts of interest.
For veterans, changes in colonoscopy screening caused by the COVID-19 pandemic may have increased risks of delayed colorectal cancer (CRC) diagnosis and could lead to worse CRC outcomes, based on data from more than 33,000 patients in the Veterans Health Administration.
After COVID-19 screening policies were implemented, a significantly lower rate of veterans with red-flag signs or symptoms for CRC underwent colonoscopy, lead author Joshua Demb, PhD, a cancer epidemiologist at the University of California, San Diego, reported at the annual Digestive Disease Week® (DDW).
“As a result of the COVID-19 pandemic, the Veterans Health Administration enacted risk mitigation and management strategies in March 2020, including postponement of nearly all colonoscopies,” the investigators reported. “Notably, this included veterans with red flag signs or symptoms for CRC, among whom delays in workup could increase risk for later-stage and fatal CRC, if present.”
To measure the effects of this policy change, Dr. Demb and colleagues performed a cohort study involving 33,804 veterans with red-flag signs or symptoms for CRC, including hematochezia, iron deficiency anemia, or abnormal guaiac fecal occult blood test or fecal immunochemical test (FIT). Veterans were divided into two cohorts based on date of first red flag diagnosis: either before the COVID-19 policy was implemented (April to October 2019; n = 19,472) or after (April to October 2020; n = 14,332), with an intervening 6-month washout period.
Primary outcomes were proportion completing colonoscopy and time to colonoscopy completion. Multivariable logistic regression incorporated a number of demographic and medical covariates, including race/ethnicity, sex, age, number of red-flag signs/symptoms, first red-flag sign/symptom, and others.
Before the COVID-19 policy change, 44% of individuals with red-flag signs or symptoms received a colonoscopy, compared with 32% after the policy was introduced (P < .01). Adjusted models showed that veterans in the COVID policy group were 42% less likely to receive a diagnostic colonoscopy than those in the prepolicy group (odds ratio, 0.58; 95% confidence interval, 0.55-0.61). While these findings showed greater likelihood of receiving a screening before the pandemic, postpolicy colonoscopies were conducted sooner, with a median time to procedure of 41 days, compared with 65 days before the pandemic (P < .01). Similar differences in screening rates between pre- and postpandemic groups were observed across all types of red flag signs and symptoms.
“Lower colonoscopy uptake was observed among individuals with red-flag signs/symptoms for CRC post- versus preimplementation of COVID-19 policies, suggesting increased future risk for delayed CRC diagnosis and adverse CRC outcomes,” the investigators concluded.
Prioritization may be needed to overcome backlog of colonoscopies
Jill Tinmouth, MD, PhD, lead scientist for ColonCancerCheck, Ontario’s organized colorectal cancer screening program, and a gastroenterologist and scientist at Sunnybrook Health Sciences Centre, Toronto, shared similar concerns about delayed diagnoses.
“We might expect these cancers to present ... at a more advanced stage, and that, as a result, the outcomes from these cancers could be worse,” Dr. Tinmouth said in an interview.
She also noted the change in colonoscopy timing.
“A particularly interesting finding was that, when a colonoscopy occurred, the time to colonoscopy was shorter during the COVID era than in the pre-COVID era,” Dr. Tinmouth said. “The authors suggested that this might be as a result of Veterans Health Administration policies implemented as a result of the pandemic that led to prioritization of more urgent procedures.”
According to Dr. Tinmouth, similar prioritization may be needed to catch up with the backlog of colonoscopies created by pandemic-related policy changes. In a recent study comparing two backlog management techniques, Dr. Tinmouth and colleagues concluded that redirecting low-yield colonoscopies to FIT without increasing hospital colonoscopy capacity could reduce time to recovery by more than half.
Even so, screening programs may be facing a long road to recovery.
“Recovery of the colonoscopy backlog is going to be a challenge that will take a while – maybe even years – to resolve,” Dr. Tinmouth said. “Jurisdictions/institutions that have a strong centralized intake or triage will likely be most successful in resolving the backlog quickly as they will be able to prioritize the most urgent cases, such as persons with an abnormal FIT or with symptoms, and to redirect persons scheduled for a ‘low-yield’ colonoscopy to have a FIT instead.” Ontario defines low-yield colonoscopies as primary screening for average-risk individuals and follow-up colonoscopies for patients with low-risk adenomas at baseline.
When asked about strategies to address future pandemics, Dr. Tinmouth said, “I think that two key learnings for me from this [pandemic] are: one, not to let our guard down, and to remain vigilant and prepared – in terms of monitoring, supply chain, equipment, etc.] ... and two to create a nimble and agile health system so that we are able to assess the challenges that the next pandemic brings and address them as quickly as possible.”The investigators and Dr. Tinmouth reported no conflicts of interest.
For veterans, changes in colonoscopy screening caused by the COVID-19 pandemic may have increased risks of delayed colorectal cancer (CRC) diagnosis and could lead to worse CRC outcomes, based on data from more than 33,000 patients in the Veterans Health Administration.
After COVID-19 screening policies were implemented, a significantly lower rate of veterans with red-flag signs or symptoms for CRC underwent colonoscopy, lead author Joshua Demb, PhD, a cancer epidemiologist at the University of California, San Diego, reported at the annual Digestive Disease Week® (DDW).
“As a result of the COVID-19 pandemic, the Veterans Health Administration enacted risk mitigation and management strategies in March 2020, including postponement of nearly all colonoscopies,” the investigators reported. “Notably, this included veterans with red flag signs or symptoms for CRC, among whom delays in workup could increase risk for later-stage and fatal CRC, if present.”
To measure the effects of this policy change, Dr. Demb and colleagues performed a cohort study involving 33,804 veterans with red-flag signs or symptoms for CRC, including hematochezia, iron deficiency anemia, or abnormal guaiac fecal occult blood test or fecal immunochemical test (FIT). Veterans were divided into two cohorts based on date of first red flag diagnosis: either before the COVID-19 policy was implemented (April to October 2019; n = 19,472) or after (April to October 2020; n = 14,332), with an intervening 6-month washout period.
Primary outcomes were proportion completing colonoscopy and time to colonoscopy completion. Multivariable logistic regression incorporated a number of demographic and medical covariates, including race/ethnicity, sex, age, number of red-flag signs/symptoms, first red-flag sign/symptom, and others.
Before the COVID-19 policy change, 44% of individuals with red-flag signs or symptoms received a colonoscopy, compared with 32% after the policy was introduced (P < .01). Adjusted models showed that veterans in the COVID policy group were 42% less likely to receive a diagnostic colonoscopy than those in the prepolicy group (odds ratio, 0.58; 95% confidence interval, 0.55-0.61). While these findings showed greater likelihood of receiving a screening before the pandemic, postpolicy colonoscopies were conducted sooner, with a median time to procedure of 41 days, compared with 65 days before the pandemic (P < .01). Similar differences in screening rates between pre- and postpandemic groups were observed across all types of red flag signs and symptoms.
“Lower colonoscopy uptake was observed among individuals with red-flag signs/symptoms for CRC post- versus preimplementation of COVID-19 policies, suggesting increased future risk for delayed CRC diagnosis and adverse CRC outcomes,” the investigators concluded.
Prioritization may be needed to overcome backlog of colonoscopies
Jill Tinmouth, MD, PhD, lead scientist for ColonCancerCheck, Ontario’s organized colorectal cancer screening program, and a gastroenterologist and scientist at Sunnybrook Health Sciences Centre, Toronto, shared similar concerns about delayed diagnoses.
“We might expect these cancers to present ... at a more advanced stage, and that, as a result, the outcomes from these cancers could be worse,” Dr. Tinmouth said in an interview.
She also noted the change in colonoscopy timing.
“A particularly interesting finding was that, when a colonoscopy occurred, the time to colonoscopy was shorter during the COVID era than in the pre-COVID era,” Dr. Tinmouth said. “The authors suggested that this might be as a result of Veterans Health Administration policies implemented as a result of the pandemic that led to prioritization of more urgent procedures.”
According to Dr. Tinmouth, similar prioritization may be needed to catch up with the backlog of colonoscopies created by pandemic-related policy changes. In a recent study comparing two backlog management techniques, Dr. Tinmouth and colleagues concluded that redirecting low-yield colonoscopies to FIT without increasing hospital colonoscopy capacity could reduce time to recovery by more than half.
Even so, screening programs may be facing a long road to recovery.
“Recovery of the colonoscopy backlog is going to be a challenge that will take a while – maybe even years – to resolve,” Dr. Tinmouth said. “Jurisdictions/institutions that have a strong centralized intake or triage will likely be most successful in resolving the backlog quickly as they will be able to prioritize the most urgent cases, such as persons with an abnormal FIT or with symptoms, and to redirect persons scheduled for a ‘low-yield’ colonoscopy to have a FIT instead.” Ontario defines low-yield colonoscopies as primary screening for average-risk individuals and follow-up colonoscopies for patients with low-risk adenomas at baseline.
When asked about strategies to address future pandemics, Dr. Tinmouth said, “I think that two key learnings for me from this [pandemic] are: one, not to let our guard down, and to remain vigilant and prepared – in terms of monitoring, supply chain, equipment, etc.] ... and two to create a nimble and agile health system so that we are able to assess the challenges that the next pandemic brings and address them as quickly as possible.”The investigators and Dr. Tinmouth reported no conflicts of interest.
FROM DDW 2021
Lower SARS-CoV-2 vaccine responses seen in patients with immune-mediated inflammatory diseases
Ten percent of patients with immune-mediated inflammatory diseases (IMIDs) fail to respond properly to COVID-19 vaccinations regardless of medication, researchers report, and small new studies suggest those on methotrexate and rituximab may be especially vulnerable to vaccine failure.
Even so, it’s still crucially vital for patients with IMIDs to get vaccinated and for clinicians to follow recommendations to temporarily withhold certain medications around the time of vaccination, rheumatologist Anne R. Bass, MD, of Weill Cornell Medicine and the Hospital for Special Surgery, New York, said in an interview. “We’re not making any significant adjustments,” added Dr. Bass, a coauthor of the American College of Rheumatology’s COVID-19 vaccination guidelines for patients with rheumatic and musculoskeletal diseases.
The findings appear in a trio of studies in Annals of the Rheumatic Diseases. The most recent study, which appeared May 25, 2021, found that more than one-third of patients with IMIDs who took methotrexate didn’t produce adequate antibody levels after vaccination versus 10% of those in other groups. (P < .001) A May 11 study found that 20 of 30 patients with rheumatic diseases on rituximab failed to respond to vaccination. And a May 6 study reported that immune responses against SARS-CoV-2 are “somewhat delayed and reduced” in patients with IMID, with 99.5% of a control group developing neutralizing antibody activity after vaccination versus 90% of those with IMID (P = .0008).
Development of neutralizing antibodies somewhat delayed and reduced
Team members were surprised by the high number of vaccine nonresponders in the May 6 IMID study, coauthor Georg Schett, MD, of Germany’s Friedrich-Alexander University Erlangen-Nuremberg and University Hospital Erlangen, said in an interview.
The researchers compared two groups of patients who had no history of COVID-19 and received COVID-19 vaccinations, mostly two shots of the Pfizer-BioNTech vaccine (96%): 84 with IMID (mean age, 53.1 years; 65.5% females) and 182 healthy controls (mean age, 40.8 years; 57.1% females).
The patients with IMID most commonly had spondyloarthritis (32.1%), RA (29.8%), inflammatory bowel disease (9.5%), and psoriasis (9.5%). Nearly 43% of the patients were treated with biologic and targeted synthetic disease-modifying antirheumatic drugs and 23.9% with conventional synthetic DMARDSs. Another 29% were not treated.
All of the controls developed anti–SARS-CoV-2 IgG, but 6% of the patients with IMID did not (P = .003). The gap in development of neutralizing antibodies was even higher: 99.5% of the controls developed neutralizing antibody activity versus 90% of the IMID group. “Neutralizing antibodies are more relevant because the test shows how much the antibodies interfere with the binding of SARS-CoV-2 proteins to the receptor,” Dr. Schett said.
The study authors concluded that “our study provides evidence that, while vaccination against SARS-CoV-2 is well tolerated and even associated with lower incidence of side effects in patients with IMID, its efficacy is somewhat delayed and reduced. Nonetheless, the data also show that, in principle, patients with IMID respond to SARS-CoV-2 vaccination, supporting an aggressive vaccination strategy.”
Lowered antibody response to vaccination for some methotrexate users
In the newer study, led by Rebecca H. Haberman, MD, of New York University Langone Health, researchers examined COVID-19 vaccine response in cohorts in New York City and Erlangen, Germany.
The New York cohort included 25 patients with IMID who were taking methotrexate by itself or with other immunomodulatory medications (mean age, 63.2 years), 26 with IMID who were on anticytokine therapy and/or other oral immunomodulators (mean age, 49.1 years) and 26 healthy controls (mean age, 49.2 years). Most patients with IMID had psoriasis/psoriatic arthritis or RA.
The German validation cohort included 182 healthy subjects (mean age, 45.0 years), 11 subjects with IMID who received TNF inhibitor monotherapy (mean age, 40.8 years), and 20 subjects with IMID on methotrexate monotherapy (mean age, 54.5 years).
In the New York cohort, 96.1% of healthy controls showed “adequate humoral immune response,” along with 92.3% of patients with IMID who weren’t taking methotrexate. However, those on methotrexate had a lower rate of adequate response (72.0%), and the gap persisted even after researchers removed those who showed signs of previous COVID-19 infection (P = .045).
In the German cohort, 98.3% of healthy cohorts and 90.9% of patients with IMID who didn’t receive methotrexate reached an “adequate” humoral response versus just half (50.0%) of those who were taking methotrexate.
When both cohorts are combined, over 90% of the healthy subjects and the patients with IMID on biologic treatments (mainly TNF blockers, n = 37) showed “robust” antibody response. However, only 62% of patients with IMID who took methotrexate (n = 45) reached an “adequate” level of response. The methotrexate gap remained after researchers accounted for differences in age among the cohorts.
What’s going on? “We think that the underlying chronic immune stimulation in autoimmune patients may cause T-cell exhaustion and thus blunts the immune response,” said Dr. Schett, who’s also a coauthor of this study. “In addition, specific drugs such as methotrexate could additionally impair the immune response.”
Still, the findings “reiterate that vaccinations are safe and effective, which is what the recommendations state,” he said, adding that more testing of vaccination immune response is wise.
Insights into vaccine response while on rituximab
Two more reports, also published in Annals of the Rheumatic Diseases, offer insight into vaccine response in patients with IMID who take rituximab.
In one report, published May 11, U.S. researchers retrospectively tracked 89 rheumatic disease patients (76% female; mean age, 61) at a single clinic who’d received at least one dose of a COVID-19 vaccine. Of those, 21 patients showed no sign of vaccine antibody response, and 20 of them were in the group taking rituximab. (The other patient was taking belimumab.) Another 10 patients taking rituximab did show a response.
“Longer duration from most recent rituximab exposure was associated with a greater likelihood of response,” the report’s authors wrote. “The results suggest that time from last rituximab exposure is an important consideration in maximizing the likelihood of a serological response, but this likely is related to the substantial variation in the period of B-cell depletion following rituximab.”
Finally, an Austrian report published May 6 examined COVID-19 vaccine immune response in five patients who were taking rituximab (four with other drugs such as methotrexate and prednisone). Researchers compared them with eight healthy controls, half who’d been vaccinated.
The researchers found evidence that rituximab “may not have to preclude SARS-CoV-2 vaccination, since a cellular immune response will be mounted even in the absence of circulating B cells. Alternatively, in patients with stable disease, delaying [rituximab] treatment until after the second vaccination may be warranted and, therefore, vaccines with a short interval between first and second vaccination or those showing full protection after a single vaccination may be preferable. Importantly, in the presence of circulating B cells also a humoral immune response may be expected despite prior [rituximab] therapy.”
Dr. Bass said the findings reflect growing awareness that “patients with autoimmune disease, especially when they’re on immunosuppressant medications, don’t quite have as optimal responses to the vaccinations.” However, she said, the vaccines are so potent that they’re likely to still have significant efficacy in these patients even if there’s a reduction in response.
What’s next? Dr. Schett said “testing immune response to vaccination is important for patients with autoimmune disease. Some of them may need a third vaccination.”
The American College of Rheumatology’s COVID-19 vaccination guidelines do not recommend third vaccinations or postvaccination immune testing at this time. However, Dr. Bass, one of the coauthors of the recommendations, said it’s likely that postvaccination immune testing and booster shots will become routine.
Dr. Bass reported no relevant disclosures. Dr. Schett reported receiving consulting fees from AbbVie. The May 6 German vaccine study was funded by Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, the ERC Synergy grant 4D Nanoscope, the IMI funded project RTCure, the Emerging Fields Initiative MIRACLE of the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Schreiber Stiftung, and the Else Kröner-Memorial Scholarship. The study authors reported no disclosures. The May 25 study of German and American cohorts was funded by the National Institute of Arthritis and Musculoskletal and Skin Diseases, National Institute of Allergy and Infectious Diseases, Rheumatology Research Foundation, Bloomberg Philanthropies COVID-19 Initiative, Pfizer COVID-19 Competitive Grant Program, Beatrice Snyder Foundation, Riley Family Foundation, National Psoriasis Foundation, and Deutsche Forschungsgemeinschaft. The authors reported a range of financial relationships with pharmaceutical companies. No specific funding was reported for the other two studies mentioned.
Ten percent of patients with immune-mediated inflammatory diseases (IMIDs) fail to respond properly to COVID-19 vaccinations regardless of medication, researchers report, and small new studies suggest those on methotrexate and rituximab may be especially vulnerable to vaccine failure.
Even so, it’s still crucially vital for patients with IMIDs to get vaccinated and for clinicians to follow recommendations to temporarily withhold certain medications around the time of vaccination, rheumatologist Anne R. Bass, MD, of Weill Cornell Medicine and the Hospital for Special Surgery, New York, said in an interview. “We’re not making any significant adjustments,” added Dr. Bass, a coauthor of the American College of Rheumatology’s COVID-19 vaccination guidelines for patients with rheumatic and musculoskeletal diseases.
The findings appear in a trio of studies in Annals of the Rheumatic Diseases. The most recent study, which appeared May 25, 2021, found that more than one-third of patients with IMIDs who took methotrexate didn’t produce adequate antibody levels after vaccination versus 10% of those in other groups. (P < .001) A May 11 study found that 20 of 30 patients with rheumatic diseases on rituximab failed to respond to vaccination. And a May 6 study reported that immune responses against SARS-CoV-2 are “somewhat delayed and reduced” in patients with IMID, with 99.5% of a control group developing neutralizing antibody activity after vaccination versus 90% of those with IMID (P = .0008).
Development of neutralizing antibodies somewhat delayed and reduced
Team members were surprised by the high number of vaccine nonresponders in the May 6 IMID study, coauthor Georg Schett, MD, of Germany’s Friedrich-Alexander University Erlangen-Nuremberg and University Hospital Erlangen, said in an interview.
The researchers compared two groups of patients who had no history of COVID-19 and received COVID-19 vaccinations, mostly two shots of the Pfizer-BioNTech vaccine (96%): 84 with IMID (mean age, 53.1 years; 65.5% females) and 182 healthy controls (mean age, 40.8 years; 57.1% females).
The patients with IMID most commonly had spondyloarthritis (32.1%), RA (29.8%), inflammatory bowel disease (9.5%), and psoriasis (9.5%). Nearly 43% of the patients were treated with biologic and targeted synthetic disease-modifying antirheumatic drugs and 23.9% with conventional synthetic DMARDSs. Another 29% were not treated.
All of the controls developed anti–SARS-CoV-2 IgG, but 6% of the patients with IMID did not (P = .003). The gap in development of neutralizing antibodies was even higher: 99.5% of the controls developed neutralizing antibody activity versus 90% of the IMID group. “Neutralizing antibodies are more relevant because the test shows how much the antibodies interfere with the binding of SARS-CoV-2 proteins to the receptor,” Dr. Schett said.
The study authors concluded that “our study provides evidence that, while vaccination against SARS-CoV-2 is well tolerated and even associated with lower incidence of side effects in patients with IMID, its efficacy is somewhat delayed and reduced. Nonetheless, the data also show that, in principle, patients with IMID respond to SARS-CoV-2 vaccination, supporting an aggressive vaccination strategy.”
Lowered antibody response to vaccination for some methotrexate users
In the newer study, led by Rebecca H. Haberman, MD, of New York University Langone Health, researchers examined COVID-19 vaccine response in cohorts in New York City and Erlangen, Germany.
The New York cohort included 25 patients with IMID who were taking methotrexate by itself or with other immunomodulatory medications (mean age, 63.2 years), 26 with IMID who were on anticytokine therapy and/or other oral immunomodulators (mean age, 49.1 years) and 26 healthy controls (mean age, 49.2 years). Most patients with IMID had psoriasis/psoriatic arthritis or RA.
The German validation cohort included 182 healthy subjects (mean age, 45.0 years), 11 subjects with IMID who received TNF inhibitor monotherapy (mean age, 40.8 years), and 20 subjects with IMID on methotrexate monotherapy (mean age, 54.5 years).
In the New York cohort, 96.1% of healthy controls showed “adequate humoral immune response,” along with 92.3% of patients with IMID who weren’t taking methotrexate. However, those on methotrexate had a lower rate of adequate response (72.0%), and the gap persisted even after researchers removed those who showed signs of previous COVID-19 infection (P = .045).
In the German cohort, 98.3% of healthy cohorts and 90.9% of patients with IMID who didn’t receive methotrexate reached an “adequate” humoral response versus just half (50.0%) of those who were taking methotrexate.
When both cohorts are combined, over 90% of the healthy subjects and the patients with IMID on biologic treatments (mainly TNF blockers, n = 37) showed “robust” antibody response. However, only 62% of patients with IMID who took methotrexate (n = 45) reached an “adequate” level of response. The methotrexate gap remained after researchers accounted for differences in age among the cohorts.
What’s going on? “We think that the underlying chronic immune stimulation in autoimmune patients may cause T-cell exhaustion and thus blunts the immune response,” said Dr. Schett, who’s also a coauthor of this study. “In addition, specific drugs such as methotrexate could additionally impair the immune response.”
Still, the findings “reiterate that vaccinations are safe and effective, which is what the recommendations state,” he said, adding that more testing of vaccination immune response is wise.
Insights into vaccine response while on rituximab
Two more reports, also published in Annals of the Rheumatic Diseases, offer insight into vaccine response in patients with IMID who take rituximab.
In one report, published May 11, U.S. researchers retrospectively tracked 89 rheumatic disease patients (76% female; mean age, 61) at a single clinic who’d received at least one dose of a COVID-19 vaccine. Of those, 21 patients showed no sign of vaccine antibody response, and 20 of them were in the group taking rituximab. (The other patient was taking belimumab.) Another 10 patients taking rituximab did show a response.
“Longer duration from most recent rituximab exposure was associated with a greater likelihood of response,” the report’s authors wrote. “The results suggest that time from last rituximab exposure is an important consideration in maximizing the likelihood of a serological response, but this likely is related to the substantial variation in the period of B-cell depletion following rituximab.”
Finally, an Austrian report published May 6 examined COVID-19 vaccine immune response in five patients who were taking rituximab (four with other drugs such as methotrexate and prednisone). Researchers compared them with eight healthy controls, half who’d been vaccinated.
The researchers found evidence that rituximab “may not have to preclude SARS-CoV-2 vaccination, since a cellular immune response will be mounted even in the absence of circulating B cells. Alternatively, in patients with stable disease, delaying [rituximab] treatment until after the second vaccination may be warranted and, therefore, vaccines with a short interval between first and second vaccination or those showing full protection after a single vaccination may be preferable. Importantly, in the presence of circulating B cells also a humoral immune response may be expected despite prior [rituximab] therapy.”
Dr. Bass said the findings reflect growing awareness that “patients with autoimmune disease, especially when they’re on immunosuppressant medications, don’t quite have as optimal responses to the vaccinations.” However, she said, the vaccines are so potent that they’re likely to still have significant efficacy in these patients even if there’s a reduction in response.
What’s next? Dr. Schett said “testing immune response to vaccination is important for patients with autoimmune disease. Some of them may need a third vaccination.”
The American College of Rheumatology’s COVID-19 vaccination guidelines do not recommend third vaccinations or postvaccination immune testing at this time. However, Dr. Bass, one of the coauthors of the recommendations, said it’s likely that postvaccination immune testing and booster shots will become routine.
Dr. Bass reported no relevant disclosures. Dr. Schett reported receiving consulting fees from AbbVie. The May 6 German vaccine study was funded by Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, the ERC Synergy grant 4D Nanoscope, the IMI funded project RTCure, the Emerging Fields Initiative MIRACLE of the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Schreiber Stiftung, and the Else Kröner-Memorial Scholarship. The study authors reported no disclosures. The May 25 study of German and American cohorts was funded by the National Institute of Arthritis and Musculoskletal and Skin Diseases, National Institute of Allergy and Infectious Diseases, Rheumatology Research Foundation, Bloomberg Philanthropies COVID-19 Initiative, Pfizer COVID-19 Competitive Grant Program, Beatrice Snyder Foundation, Riley Family Foundation, National Psoriasis Foundation, and Deutsche Forschungsgemeinschaft. The authors reported a range of financial relationships with pharmaceutical companies. No specific funding was reported for the other two studies mentioned.
Ten percent of patients with immune-mediated inflammatory diseases (IMIDs) fail to respond properly to COVID-19 vaccinations regardless of medication, researchers report, and small new studies suggest those on methotrexate and rituximab may be especially vulnerable to vaccine failure.
Even so, it’s still crucially vital for patients with IMIDs to get vaccinated and for clinicians to follow recommendations to temporarily withhold certain medications around the time of vaccination, rheumatologist Anne R. Bass, MD, of Weill Cornell Medicine and the Hospital for Special Surgery, New York, said in an interview. “We’re not making any significant adjustments,” added Dr. Bass, a coauthor of the American College of Rheumatology’s COVID-19 vaccination guidelines for patients with rheumatic and musculoskeletal diseases.
The findings appear in a trio of studies in Annals of the Rheumatic Diseases. The most recent study, which appeared May 25, 2021, found that more than one-third of patients with IMIDs who took methotrexate didn’t produce adequate antibody levels after vaccination versus 10% of those in other groups. (P < .001) A May 11 study found that 20 of 30 patients with rheumatic diseases on rituximab failed to respond to vaccination. And a May 6 study reported that immune responses against SARS-CoV-2 are “somewhat delayed and reduced” in patients with IMID, with 99.5% of a control group developing neutralizing antibody activity after vaccination versus 90% of those with IMID (P = .0008).
Development of neutralizing antibodies somewhat delayed and reduced
Team members were surprised by the high number of vaccine nonresponders in the May 6 IMID study, coauthor Georg Schett, MD, of Germany’s Friedrich-Alexander University Erlangen-Nuremberg and University Hospital Erlangen, said in an interview.
The researchers compared two groups of patients who had no history of COVID-19 and received COVID-19 vaccinations, mostly two shots of the Pfizer-BioNTech vaccine (96%): 84 with IMID (mean age, 53.1 years; 65.5% females) and 182 healthy controls (mean age, 40.8 years; 57.1% females).
The patients with IMID most commonly had spondyloarthritis (32.1%), RA (29.8%), inflammatory bowel disease (9.5%), and psoriasis (9.5%). Nearly 43% of the patients were treated with biologic and targeted synthetic disease-modifying antirheumatic drugs and 23.9% with conventional synthetic DMARDSs. Another 29% were not treated.
All of the controls developed anti–SARS-CoV-2 IgG, but 6% of the patients with IMID did not (P = .003). The gap in development of neutralizing antibodies was even higher: 99.5% of the controls developed neutralizing antibody activity versus 90% of the IMID group. “Neutralizing antibodies are more relevant because the test shows how much the antibodies interfere with the binding of SARS-CoV-2 proteins to the receptor,” Dr. Schett said.
The study authors concluded that “our study provides evidence that, while vaccination against SARS-CoV-2 is well tolerated and even associated with lower incidence of side effects in patients with IMID, its efficacy is somewhat delayed and reduced. Nonetheless, the data also show that, in principle, patients with IMID respond to SARS-CoV-2 vaccination, supporting an aggressive vaccination strategy.”
Lowered antibody response to vaccination for some methotrexate users
In the newer study, led by Rebecca H. Haberman, MD, of New York University Langone Health, researchers examined COVID-19 vaccine response in cohorts in New York City and Erlangen, Germany.
The New York cohort included 25 patients with IMID who were taking methotrexate by itself or with other immunomodulatory medications (mean age, 63.2 years), 26 with IMID who were on anticytokine therapy and/or other oral immunomodulators (mean age, 49.1 years) and 26 healthy controls (mean age, 49.2 years). Most patients with IMID had psoriasis/psoriatic arthritis or RA.
The German validation cohort included 182 healthy subjects (mean age, 45.0 years), 11 subjects with IMID who received TNF inhibitor monotherapy (mean age, 40.8 years), and 20 subjects with IMID on methotrexate monotherapy (mean age, 54.5 years).
In the New York cohort, 96.1% of healthy controls showed “adequate humoral immune response,” along with 92.3% of patients with IMID who weren’t taking methotrexate. However, those on methotrexate had a lower rate of adequate response (72.0%), and the gap persisted even after researchers removed those who showed signs of previous COVID-19 infection (P = .045).
In the German cohort, 98.3% of healthy cohorts and 90.9% of patients with IMID who didn’t receive methotrexate reached an “adequate” humoral response versus just half (50.0%) of those who were taking methotrexate.
When both cohorts are combined, over 90% of the healthy subjects and the patients with IMID on biologic treatments (mainly TNF blockers, n = 37) showed “robust” antibody response. However, only 62% of patients with IMID who took methotrexate (n = 45) reached an “adequate” level of response. The methotrexate gap remained after researchers accounted for differences in age among the cohorts.
What’s going on? “We think that the underlying chronic immune stimulation in autoimmune patients may cause T-cell exhaustion and thus blunts the immune response,” said Dr. Schett, who’s also a coauthor of this study. “In addition, specific drugs such as methotrexate could additionally impair the immune response.”
Still, the findings “reiterate that vaccinations are safe and effective, which is what the recommendations state,” he said, adding that more testing of vaccination immune response is wise.
Insights into vaccine response while on rituximab
Two more reports, also published in Annals of the Rheumatic Diseases, offer insight into vaccine response in patients with IMID who take rituximab.
In one report, published May 11, U.S. researchers retrospectively tracked 89 rheumatic disease patients (76% female; mean age, 61) at a single clinic who’d received at least one dose of a COVID-19 vaccine. Of those, 21 patients showed no sign of vaccine antibody response, and 20 of them were in the group taking rituximab. (The other patient was taking belimumab.) Another 10 patients taking rituximab did show a response.
“Longer duration from most recent rituximab exposure was associated with a greater likelihood of response,” the report’s authors wrote. “The results suggest that time from last rituximab exposure is an important consideration in maximizing the likelihood of a serological response, but this likely is related to the substantial variation in the period of B-cell depletion following rituximab.”
Finally, an Austrian report published May 6 examined COVID-19 vaccine immune response in five patients who were taking rituximab (four with other drugs such as methotrexate and prednisone). Researchers compared them with eight healthy controls, half who’d been vaccinated.
The researchers found evidence that rituximab “may not have to preclude SARS-CoV-2 vaccination, since a cellular immune response will be mounted even in the absence of circulating B cells. Alternatively, in patients with stable disease, delaying [rituximab] treatment until after the second vaccination may be warranted and, therefore, vaccines with a short interval between first and second vaccination or those showing full protection after a single vaccination may be preferable. Importantly, in the presence of circulating B cells also a humoral immune response may be expected despite prior [rituximab] therapy.”
Dr. Bass said the findings reflect growing awareness that “patients with autoimmune disease, especially when they’re on immunosuppressant medications, don’t quite have as optimal responses to the vaccinations.” However, she said, the vaccines are so potent that they’re likely to still have significant efficacy in these patients even if there’s a reduction in response.
What’s next? Dr. Schett said “testing immune response to vaccination is important for patients with autoimmune disease. Some of them may need a third vaccination.”
The American College of Rheumatology’s COVID-19 vaccination guidelines do not recommend third vaccinations or postvaccination immune testing at this time. However, Dr. Bass, one of the coauthors of the recommendations, said it’s likely that postvaccination immune testing and booster shots will become routine.
Dr. Bass reported no relevant disclosures. Dr. Schett reported receiving consulting fees from AbbVie. The May 6 German vaccine study was funded by Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung, the ERC Synergy grant 4D Nanoscope, the IMI funded project RTCure, the Emerging Fields Initiative MIRACLE of the Friedrich-Alexander-Universität Erlangen-Nürnberg, the Schreiber Stiftung, and the Else Kröner-Memorial Scholarship. The study authors reported no disclosures. The May 25 study of German and American cohorts was funded by the National Institute of Arthritis and Musculoskletal and Skin Diseases, National Institute of Allergy and Infectious Diseases, Rheumatology Research Foundation, Bloomberg Philanthropies COVID-19 Initiative, Pfizer COVID-19 Competitive Grant Program, Beatrice Snyder Foundation, Riley Family Foundation, National Psoriasis Foundation, and Deutsche Forschungsgemeinschaft. The authors reported a range of financial relationships with pharmaceutical companies. No specific funding was reported for the other two studies mentioned.
FROM ANNALS OF THE RHEUMATIC DISEASES
COVID-19 vaccination rate rising quickly among adolescents
With nearly half of all Americans having received at least one dose of a COVID-19 vaccine, the youngest eligible group is beginning to overcome its late start, according to data from the Centers for Disease Control and Prevention.
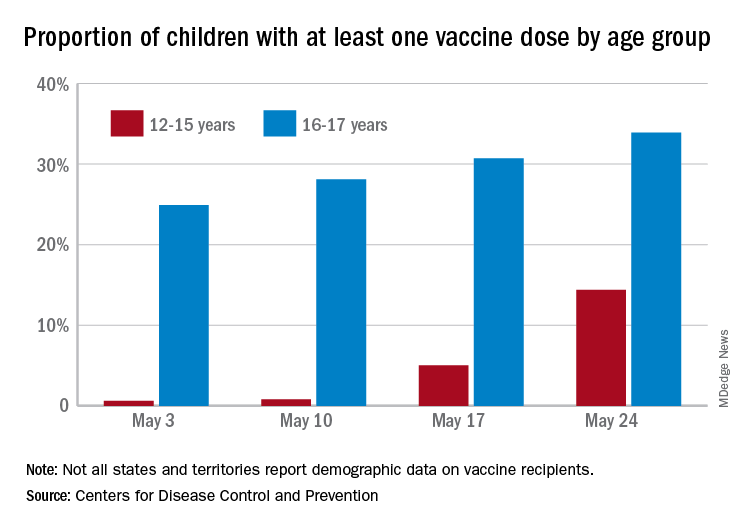
As of May 24, 49.4% of the U.S. population – that’s almost 164 million people – has received at least one dose of vaccine. The corresponding figure for children aged 12-15 years is 14.4%, but that’s up from only 0.6% just 3 weeks before. Among children aged 16-17, who’ve been getting vaccinated since early April in some states, the proportion receiving at least one dose went from 24.9% to 33.9% over those same 3 weeks, the CDC said on its COVID Data Tracker site.
The comparatively rapid increase among the younger group of eligible children can be seen over the last 14 days. To put that into perspective, only those aged 25-39 years were higher at 21.9%, while 18-24 (12.1%), 40-49 (13.4%), 50-64 (18.2%), 65-74 (5.3%), and ≥75 (2.9%) were all lower.
The 12- to 15-year-olds are further behind when it comes to full vaccination status, however, with just 0.6% having received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 21.6% for those aged 16-17 years. Children aged 12-15 make up 5% of the total U.S. population but just 0.1% of all those who have been fully vaccinated versus 2.5% and 1.4%, respectively, for those aged 16-17, the CDC reported.
With nearly half of all Americans having received at least one dose of a COVID-19 vaccine, the youngest eligible group is beginning to overcome its late start, according to data from the Centers for Disease Control and Prevention.

As of May 24, 49.4% of the U.S. population – that’s almost 164 million people – has received at least one dose of vaccine. The corresponding figure for children aged 12-15 years is 14.4%, but that’s up from only 0.6% just 3 weeks before. Among children aged 16-17, who’ve been getting vaccinated since early April in some states, the proportion receiving at least one dose went from 24.9% to 33.9% over those same 3 weeks, the CDC said on its COVID Data Tracker site.
The comparatively rapid increase among the younger group of eligible children can be seen over the last 14 days. To put that into perspective, only those aged 25-39 years were higher at 21.9%, while 18-24 (12.1%), 40-49 (13.4%), 50-64 (18.2%), 65-74 (5.3%), and ≥75 (2.9%) were all lower.
The 12- to 15-year-olds are further behind when it comes to full vaccination status, however, with just 0.6% having received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 21.6% for those aged 16-17 years. Children aged 12-15 make up 5% of the total U.S. population but just 0.1% of all those who have been fully vaccinated versus 2.5% and 1.4%, respectively, for those aged 16-17, the CDC reported.
With nearly half of all Americans having received at least one dose of a COVID-19 vaccine, the youngest eligible group is beginning to overcome its late start, according to data from the Centers for Disease Control and Prevention.

As of May 24, 49.4% of the U.S. population – that’s almost 164 million people – has received at least one dose of vaccine. The corresponding figure for children aged 12-15 years is 14.4%, but that’s up from only 0.6% just 3 weeks before. Among children aged 16-17, who’ve been getting vaccinated since early April in some states, the proportion receiving at least one dose went from 24.9% to 33.9% over those same 3 weeks, the CDC said on its COVID Data Tracker site.
The comparatively rapid increase among the younger group of eligible children can be seen over the last 14 days. To put that into perspective, only those aged 25-39 years were higher at 21.9%, while 18-24 (12.1%), 40-49 (13.4%), 50-64 (18.2%), 65-74 (5.3%), and ≥75 (2.9%) were all lower.
The 12- to 15-year-olds are further behind when it comes to full vaccination status, however, with just 0.6% having received both doses of a two-dose vaccine or one dose of the single-shot variety, compared with 21.6% for those aged 16-17 years. Children aged 12-15 make up 5% of the total U.S. population but just 0.1% of all those who have been fully vaccinated versus 2.5% and 1.4%, respectively, for those aged 16-17, the CDC reported.
USPSTF final recommendation on CRC screening: 45 is the new 50
Screening for colorectal cancer (CRC) should now begin at the age of 45 and not 50 for average-risk individuals in the United States, notes the final recommendation from the U.S. Preventive Services Task Force.
The recommendation finalizes draft guidelines issued in October 2020 and mandates insurance coverage to ensure equal access to CRC screening regardless of a patient’s insurance status.
The USPSTF’s final recommendations also now align with those of the American Cancer Society, which lowered the age for initiation of CRC screening to 45 years in 2018.
“New statistics project an alarming rise in the incidence of young-onset colorectal cancer, projected to be the leading cause of cancer death in patients aged 20-49 by 2040,” commented Kimmie Ng, MD, MPH, director, Young-Onset Colorectal Cancer Center, Dana-Farber Cancer Institute, Boston, and lead author of a JAMA editorial about the new guideline.
“We must take bold steps to translate the lowered age of beginning screening into meaningful decreases in CRC incidence and mortality,” she emphasized.
The USPSTF recommendations and substantial evidence supporting them were published online May 18, 2021, in JAMA.
Risk factors for CRC
As the USPSTF authors noted, age is one of the most important risk factors for CRC, with nearly 94% of all new cases of CRC occurring in adults 45 years of age and older. Justification for the lower age of CRC screening initiation was based on simulation models showing that initiation of screening at the age of 45 was associated with an estimated additional 22-27 life-years gained, compared with starting at the age of 50.
The USPSTF continues to recommend screening for CRC in all adults aged between 50 and 75 years, lowering the age for screening to 45 years in recognition of the fact that, in 2020, 11% of colon cancers and 15% of rectal cancers occurred in patients under the age of 50.
The USPSTF also continues to conclude that there is a “small net benefit” of screening for CRC in adults aged between 76 and 85 years who have been previously screened.
However, the decision to screen patients in this age group should be based on individual risk factors for CRC, a patient’s overall health status, and personal preference. Perhaps self-evidently, adults in this age group who have never been screened for CRC are more likely to benefit from CRC screening than those who have been previously screened.
Similar to the previous guidelines released in 2016, the updated USPSTF recommendations continue to offer a menu of screening strategies, although the frequency of screening for each of the screening strategies varies. Recommended screening strategies include:
- High-sensitivity guaiac fecal occult blood test or fecal immunochemical test (FIT) every year
- Stool DNA-FIT every 1-3 years
- CT colonography every 5 years
- every 5 years
- Flexible sigmoidoscopy every 10 years plus annual FIT
- screening every 10 years
“Based on the evidence, there are many tests available that can effectively screen for colorectal cancer and the right test is the one that gets done,” USPSTF member Martha Kubik, PhD, RN, said in a statement.
“To encourage screening and help patients select the best test for them, we urge primary care clinicians to talk about the pros and cons of the various recommended options with their patients,” she added.
An accompanying review of the effectiveness, accuracy, and potential harms of CRC screening methods underscores how different screening tests have different levels of evidence demonstrating their ability to detect cancer, precursor lesions, or both, as well as their ability to reduce mortality from cancer.
Eligible patients
Currently, fewer than 70% of eligible patients in the United States undergo CRC screening, Dr. Ng pointed out in the editorial. In addition, CRC disproportionately affects African American patients, who are about 20% more likely to get CRC and about 40% more likely to die from it, compared with other patient groups. Modeling studies published along with the USPSTF recommendations showed equal benefit for screening regardless of race and gender, underscoring the importance of screening adherence, especially in patient populations disproportionately affected by CRC.
“Far too many people in the U.S. are not receiving this lifesaving preventive service,” USPSTF vice chair Michael Barry, MD, said in a statement.
“We hope that this new recommendation to screen people ages 45-49, coupled with our long-standing recommendation to screen people 50-75, will prevent more people from dying from colorectal cancer,” he added.
Dr. Ng echoed this sentiment in her editorial: “The USPSTF recommendation for beginning colorectal cancer screening for average-risk adults at age 45 years has moved the field one step forward and indicates that ‘45 is the new 50,’ ” she observed.
“Lowering the recommended age to initiate screening will make colorectal cancer screening available to millions more people in the United States and, hopefully, many more lives will be saved by catching colorectal cancer earlier as well as by preventing colorectal cancer,” Dr. Ng affirmed.
All members of the USPSTF received travel reimbursement and an honorarium for participating in USPSTF meetings.
Dr. Ng reported receiving nonfinancial support from Pharmavite as well as grants from the Evergrande Group, Janssen, Revolution Medicines, Genentech, and Gilead Sciences. She has also reported receiving personal fees from Seattle Genetics, Array Biopharma, BiomX, and X-Biotix Therapeutics.
A version of this article first appeared on Medscape.com.
Screening for colorectal cancer (CRC) should now begin at the age of 45 and not 50 for average-risk individuals in the United States, notes the final recommendation from the U.S. Preventive Services Task Force.
The recommendation finalizes draft guidelines issued in October 2020 and mandates insurance coverage to ensure equal access to CRC screening regardless of a patient’s insurance status.
The USPSTF’s final recommendations also now align with those of the American Cancer Society, which lowered the age for initiation of CRC screening to 45 years in 2018.
“New statistics project an alarming rise in the incidence of young-onset colorectal cancer, projected to be the leading cause of cancer death in patients aged 20-49 by 2040,” commented Kimmie Ng, MD, MPH, director, Young-Onset Colorectal Cancer Center, Dana-Farber Cancer Institute, Boston, and lead author of a JAMA editorial about the new guideline.
“We must take bold steps to translate the lowered age of beginning screening into meaningful decreases in CRC incidence and mortality,” she emphasized.
The USPSTF recommendations and substantial evidence supporting them were published online May 18, 2021, in JAMA.
Risk factors for CRC
As the USPSTF authors noted, age is one of the most important risk factors for CRC, with nearly 94% of all new cases of CRC occurring in adults 45 years of age and older. Justification for the lower age of CRC screening initiation was based on simulation models showing that initiation of screening at the age of 45 was associated with an estimated additional 22-27 life-years gained, compared with starting at the age of 50.
The USPSTF continues to recommend screening for CRC in all adults aged between 50 and 75 years, lowering the age for screening to 45 years in recognition of the fact that, in 2020, 11% of colon cancers and 15% of rectal cancers occurred in patients under the age of 50.
The USPSTF also continues to conclude that there is a “small net benefit” of screening for CRC in adults aged between 76 and 85 years who have been previously screened.
However, the decision to screen patients in this age group should be based on individual risk factors for CRC, a patient’s overall health status, and personal preference. Perhaps self-evidently, adults in this age group who have never been screened for CRC are more likely to benefit from CRC screening than those who have been previously screened.
Similar to the previous guidelines released in 2016, the updated USPSTF recommendations continue to offer a menu of screening strategies, although the frequency of screening for each of the screening strategies varies. Recommended screening strategies include:
- High-sensitivity guaiac fecal occult blood test or fecal immunochemical test (FIT) every year
- Stool DNA-FIT every 1-3 years
- CT colonography every 5 years
- every 5 years
- Flexible sigmoidoscopy every 10 years plus annual FIT
- screening every 10 years
“Based on the evidence, there are many tests available that can effectively screen for colorectal cancer and the right test is the one that gets done,” USPSTF member Martha Kubik, PhD, RN, said in a statement.
“To encourage screening and help patients select the best test for them, we urge primary care clinicians to talk about the pros and cons of the various recommended options with their patients,” she added.
An accompanying review of the effectiveness, accuracy, and potential harms of CRC screening methods underscores how different screening tests have different levels of evidence demonstrating their ability to detect cancer, precursor lesions, or both, as well as their ability to reduce mortality from cancer.
Eligible patients
Currently, fewer than 70% of eligible patients in the United States undergo CRC screening, Dr. Ng pointed out in the editorial. In addition, CRC disproportionately affects African American patients, who are about 20% more likely to get CRC and about 40% more likely to die from it, compared with other patient groups. Modeling studies published along with the USPSTF recommendations showed equal benefit for screening regardless of race and gender, underscoring the importance of screening adherence, especially in patient populations disproportionately affected by CRC.
“Far too many people in the U.S. are not receiving this lifesaving preventive service,” USPSTF vice chair Michael Barry, MD, said in a statement.
“We hope that this new recommendation to screen people ages 45-49, coupled with our long-standing recommendation to screen people 50-75, will prevent more people from dying from colorectal cancer,” he added.
Dr. Ng echoed this sentiment in her editorial: “The USPSTF recommendation for beginning colorectal cancer screening for average-risk adults at age 45 years has moved the field one step forward and indicates that ‘45 is the new 50,’ ” she observed.
“Lowering the recommended age to initiate screening will make colorectal cancer screening available to millions more people in the United States and, hopefully, many more lives will be saved by catching colorectal cancer earlier as well as by preventing colorectal cancer,” Dr. Ng affirmed.
All members of the USPSTF received travel reimbursement and an honorarium for participating in USPSTF meetings.
Dr. Ng reported receiving nonfinancial support from Pharmavite as well as grants from the Evergrande Group, Janssen, Revolution Medicines, Genentech, and Gilead Sciences. She has also reported receiving personal fees from Seattle Genetics, Array Biopharma, BiomX, and X-Biotix Therapeutics.
A version of this article first appeared on Medscape.com.
Screening for colorectal cancer (CRC) should now begin at the age of 45 and not 50 for average-risk individuals in the United States, notes the final recommendation from the U.S. Preventive Services Task Force.
The recommendation finalizes draft guidelines issued in October 2020 and mandates insurance coverage to ensure equal access to CRC screening regardless of a patient’s insurance status.
The USPSTF’s final recommendations also now align with those of the American Cancer Society, which lowered the age for initiation of CRC screening to 45 years in 2018.
“New statistics project an alarming rise in the incidence of young-onset colorectal cancer, projected to be the leading cause of cancer death in patients aged 20-49 by 2040,” commented Kimmie Ng, MD, MPH, director, Young-Onset Colorectal Cancer Center, Dana-Farber Cancer Institute, Boston, and lead author of a JAMA editorial about the new guideline.
“We must take bold steps to translate the lowered age of beginning screening into meaningful decreases in CRC incidence and mortality,” she emphasized.
The USPSTF recommendations and substantial evidence supporting them were published online May 18, 2021, in JAMA.
Risk factors for CRC
As the USPSTF authors noted, age is one of the most important risk factors for CRC, with nearly 94% of all new cases of CRC occurring in adults 45 years of age and older. Justification for the lower age of CRC screening initiation was based on simulation models showing that initiation of screening at the age of 45 was associated with an estimated additional 22-27 life-years gained, compared with starting at the age of 50.
The USPSTF continues to recommend screening for CRC in all adults aged between 50 and 75 years, lowering the age for screening to 45 years in recognition of the fact that, in 2020, 11% of colon cancers and 15% of rectal cancers occurred in patients under the age of 50.
The USPSTF also continues to conclude that there is a “small net benefit” of screening for CRC in adults aged between 76 and 85 years who have been previously screened.
However, the decision to screen patients in this age group should be based on individual risk factors for CRC, a patient’s overall health status, and personal preference. Perhaps self-evidently, adults in this age group who have never been screened for CRC are more likely to benefit from CRC screening than those who have been previously screened.
Similar to the previous guidelines released in 2016, the updated USPSTF recommendations continue to offer a menu of screening strategies, although the frequency of screening for each of the screening strategies varies. Recommended screening strategies include:
- High-sensitivity guaiac fecal occult blood test or fecal immunochemical test (FIT) every year
- Stool DNA-FIT every 1-3 years
- CT colonography every 5 years
- every 5 years
- Flexible sigmoidoscopy every 10 years plus annual FIT
- screening every 10 years
“Based on the evidence, there are many tests available that can effectively screen for colorectal cancer and the right test is the one that gets done,” USPSTF member Martha Kubik, PhD, RN, said in a statement.
“To encourage screening and help patients select the best test for them, we urge primary care clinicians to talk about the pros and cons of the various recommended options with their patients,” she added.
An accompanying review of the effectiveness, accuracy, and potential harms of CRC screening methods underscores how different screening tests have different levels of evidence demonstrating their ability to detect cancer, precursor lesions, or both, as well as their ability to reduce mortality from cancer.
Eligible patients
Currently, fewer than 70% of eligible patients in the United States undergo CRC screening, Dr. Ng pointed out in the editorial. In addition, CRC disproportionately affects African American patients, who are about 20% more likely to get CRC and about 40% more likely to die from it, compared with other patient groups. Modeling studies published along with the USPSTF recommendations showed equal benefit for screening regardless of race and gender, underscoring the importance of screening adherence, especially in patient populations disproportionately affected by CRC.
“Far too many people in the U.S. are not receiving this lifesaving preventive service,” USPSTF vice chair Michael Barry, MD, said in a statement.
“We hope that this new recommendation to screen people ages 45-49, coupled with our long-standing recommendation to screen people 50-75, will prevent more people from dying from colorectal cancer,” he added.
Dr. Ng echoed this sentiment in her editorial: “The USPSTF recommendation for beginning colorectal cancer screening for average-risk adults at age 45 years has moved the field one step forward and indicates that ‘45 is the new 50,’ ” she observed.
“Lowering the recommended age to initiate screening will make colorectal cancer screening available to millions more people in the United States and, hopefully, many more lives will be saved by catching colorectal cancer earlier as well as by preventing colorectal cancer,” Dr. Ng affirmed.
All members of the USPSTF received travel reimbursement and an honorarium for participating in USPSTF meetings.
Dr. Ng reported receiving nonfinancial support from Pharmavite as well as grants from the Evergrande Group, Janssen, Revolution Medicines, Genentech, and Gilead Sciences. She has also reported receiving personal fees from Seattle Genetics, Array Biopharma, BiomX, and X-Biotix Therapeutics.
A version of this article first appeared on Medscape.com.
Cardiologists’ pay increases, despite COVID-19 impacts
Despite the huge challenges of COVID-19, including a drop in patient visits, cardiologists reported an average increase in income in 2020 and remain among the top earners in medicine, according to the 2021 Medscape Cardiologist Compensation Report.
Although 46% of cardiologists reported some decline in compensation, average cardiologist income was $459,000 in 2020 – up from $438,000 in 2019.
Cardiologist pay is the third highest of all specialties in the overall 2021 Medscape Physician Compensation Report, which covers U.S. physicians as a whole and almost 18,000 physicians in 29 specialties.
Only plastic surgeons ($526,000) and orthopedists ($511,000) earned more than cardiologists in 2020.
On average among cardiologists, self-employment yields a somewhat higher paycheck than does being employed ($477,000 vs. $450,000).
Just like in last year’s report, nearly two-thirds (61%) of cardiologists overall say they feel fairly compensated.
The average incentive bonus payment for cardiologists in 2020 was 14% of total salary, about the same as last year. Two-thirds of cardiologists who earn an incentive bonus achieve more than three-quarters of their potential annual payment, up from 55% the prior year.
COVID challenges and the road back
The vast majority (92%) of cardiologists who saw a drop in income last year cited COVID-related issues such as job loss, working fewer hours, and seeing fewer patients.
Close to half (48%) of cardiologists who suffered financial or practice-related ill effects as a result of the pandemic expect their income to return to normal this year; 38% believe it will take 2 to 3 years. Notably, 45% of physicians overall said the pandemic did not cause them financial or practice-related harm.
Physician work hours generally declined for at least some time during the pandemic – and some physicians were furloughed – but most are now working about the same number of hours they did prior to COVID-19.
Cardiologists are back working an average of 57 hours per week. Perhaps not surprising, intensivists, infectious disease physicians, and public health/preventive medicine physicians are pulling longer hours now, about 6 or 7 more per week than before.
Although working about the same number of hours per week now as they did before the pandemic, physicians overall are typically seeing fewer patients because of time spent on medical office safety protocols, answering COVID-19–related questions and other factors.
Cardiologists are seeing an average decline in weekly patient visits of about 6% – from 77 to 72 patients. Pediatricians are experiencing the largest average declines – from 78 patients per week prior to 64 now, an 18% drop.
Among self-employed cardiologists, 43% believe that a drop in patient volume of up to one-quarter is permanent.
Most cardiologists remain happy at work
Despite COVID-19 and other professional challenges, most cardiologists (and physicians overall) continue to find their work rewarding.
Cardiologists say the most rewarding aspect of their profession is “being good at what I do/finding answers and diagnoses” (27%), followed by relationships with and gratitude from patients (26%), making the world a better place (23%) and making good money at a job they like (12%). A few cited pride in their profession (6%) and teaching (2%). These figures are in line with last year’s responses.
The most challenging part of practicing cardiology is having so many rules and regulations (22%), followed by having to work long hours (16%), working with electronic health records (13%), trouble getting fair reimbursement (11%), danger/risk associated with treating COVID-19 patients (11%), dealing with difficult patients (8%) and worry about being sued (7%).
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, cardiologists spend 17.4 hours per week on paperwork and administration, similar to last year (16.9 hours per week) and to physicians overall (16.3 hours).
Despite the challenges, 86% of cardiologists said they would choose medicine again, and 92% would choose cardiology again, about the same as last year.
Most cardiologists (83%) plan to keep Medicare and/or Medicaid patients; only 1% say they won’t take new Medicare or Medicaid patients; and 16% are undecided.
Thirty-nine percent of cardiologists plan to participate in the Merit-based Incentive Payment System (MIPS) in 2021.
“The stakes of the Quality Payment Program – the program that incorporates MIPS – are high, with a 9% penalty applied to all Medicare reimbursement for failure to participate,” said Elizabeth Woodcock, MBA, CPC, president of physician practice consulting firm Woodcock & Associates, Atlanta.
“With margins already slim, most physicians can’t afford this massive penalty. It makes sense to protect your revenue by complying with at least the bare minimum,” she noted.
A version of this article first appeared on Medscape.com.
Despite the huge challenges of COVID-19, including a drop in patient visits, cardiologists reported an average increase in income in 2020 and remain among the top earners in medicine, according to the 2021 Medscape Cardiologist Compensation Report.
Although 46% of cardiologists reported some decline in compensation, average cardiologist income was $459,000 in 2020 – up from $438,000 in 2019.
Cardiologist pay is the third highest of all specialties in the overall 2021 Medscape Physician Compensation Report, which covers U.S. physicians as a whole and almost 18,000 physicians in 29 specialties.
Only plastic surgeons ($526,000) and orthopedists ($511,000) earned more than cardiologists in 2020.
On average among cardiologists, self-employment yields a somewhat higher paycheck than does being employed ($477,000 vs. $450,000).
Just like in last year’s report, nearly two-thirds (61%) of cardiologists overall say they feel fairly compensated.
The average incentive bonus payment for cardiologists in 2020 was 14% of total salary, about the same as last year. Two-thirds of cardiologists who earn an incentive bonus achieve more than three-quarters of their potential annual payment, up from 55% the prior year.
COVID challenges and the road back
The vast majority (92%) of cardiologists who saw a drop in income last year cited COVID-related issues such as job loss, working fewer hours, and seeing fewer patients.
Close to half (48%) of cardiologists who suffered financial or practice-related ill effects as a result of the pandemic expect their income to return to normal this year; 38% believe it will take 2 to 3 years. Notably, 45% of physicians overall said the pandemic did not cause them financial or practice-related harm.
Physician work hours generally declined for at least some time during the pandemic – and some physicians were furloughed – but most are now working about the same number of hours they did prior to COVID-19.
Cardiologists are back working an average of 57 hours per week. Perhaps not surprising, intensivists, infectious disease physicians, and public health/preventive medicine physicians are pulling longer hours now, about 6 or 7 more per week than before.
Although working about the same number of hours per week now as they did before the pandemic, physicians overall are typically seeing fewer patients because of time spent on medical office safety protocols, answering COVID-19–related questions and other factors.
Cardiologists are seeing an average decline in weekly patient visits of about 6% – from 77 to 72 patients. Pediatricians are experiencing the largest average declines – from 78 patients per week prior to 64 now, an 18% drop.
Among self-employed cardiologists, 43% believe that a drop in patient volume of up to one-quarter is permanent.
Most cardiologists remain happy at work
Despite COVID-19 and other professional challenges, most cardiologists (and physicians overall) continue to find their work rewarding.
Cardiologists say the most rewarding aspect of their profession is “being good at what I do/finding answers and diagnoses” (27%), followed by relationships with and gratitude from patients (26%), making the world a better place (23%) and making good money at a job they like (12%). A few cited pride in their profession (6%) and teaching (2%). These figures are in line with last year’s responses.
The most challenging part of practicing cardiology is having so many rules and regulations (22%), followed by having to work long hours (16%), working with electronic health records (13%), trouble getting fair reimbursement (11%), danger/risk associated with treating COVID-19 patients (11%), dealing with difficult patients (8%) and worry about being sued (7%).
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, cardiologists spend 17.4 hours per week on paperwork and administration, similar to last year (16.9 hours per week) and to physicians overall (16.3 hours).
Despite the challenges, 86% of cardiologists said they would choose medicine again, and 92% would choose cardiology again, about the same as last year.
Most cardiologists (83%) plan to keep Medicare and/or Medicaid patients; only 1% say they won’t take new Medicare or Medicaid patients; and 16% are undecided.
Thirty-nine percent of cardiologists plan to participate in the Merit-based Incentive Payment System (MIPS) in 2021.
“The stakes of the Quality Payment Program – the program that incorporates MIPS – are high, with a 9% penalty applied to all Medicare reimbursement for failure to participate,” said Elizabeth Woodcock, MBA, CPC, president of physician practice consulting firm Woodcock & Associates, Atlanta.
“With margins already slim, most physicians can’t afford this massive penalty. It makes sense to protect your revenue by complying with at least the bare minimum,” she noted.
A version of this article first appeared on Medscape.com.
Despite the huge challenges of COVID-19, including a drop in patient visits, cardiologists reported an average increase in income in 2020 and remain among the top earners in medicine, according to the 2021 Medscape Cardiologist Compensation Report.
Although 46% of cardiologists reported some decline in compensation, average cardiologist income was $459,000 in 2020 – up from $438,000 in 2019.
Cardiologist pay is the third highest of all specialties in the overall 2021 Medscape Physician Compensation Report, which covers U.S. physicians as a whole and almost 18,000 physicians in 29 specialties.
Only plastic surgeons ($526,000) and orthopedists ($511,000) earned more than cardiologists in 2020.
On average among cardiologists, self-employment yields a somewhat higher paycheck than does being employed ($477,000 vs. $450,000).
Just like in last year’s report, nearly two-thirds (61%) of cardiologists overall say they feel fairly compensated.
The average incentive bonus payment for cardiologists in 2020 was 14% of total salary, about the same as last year. Two-thirds of cardiologists who earn an incentive bonus achieve more than three-quarters of their potential annual payment, up from 55% the prior year.
COVID challenges and the road back
The vast majority (92%) of cardiologists who saw a drop in income last year cited COVID-related issues such as job loss, working fewer hours, and seeing fewer patients.
Close to half (48%) of cardiologists who suffered financial or practice-related ill effects as a result of the pandemic expect their income to return to normal this year; 38% believe it will take 2 to 3 years. Notably, 45% of physicians overall said the pandemic did not cause them financial or practice-related harm.
Physician work hours generally declined for at least some time during the pandemic – and some physicians were furloughed – but most are now working about the same number of hours they did prior to COVID-19.
Cardiologists are back working an average of 57 hours per week. Perhaps not surprising, intensivists, infectious disease physicians, and public health/preventive medicine physicians are pulling longer hours now, about 6 or 7 more per week than before.
Although working about the same number of hours per week now as they did before the pandemic, physicians overall are typically seeing fewer patients because of time spent on medical office safety protocols, answering COVID-19–related questions and other factors.
Cardiologists are seeing an average decline in weekly patient visits of about 6% – from 77 to 72 patients. Pediatricians are experiencing the largest average declines – from 78 patients per week prior to 64 now, an 18% drop.
Among self-employed cardiologists, 43% believe that a drop in patient volume of up to one-quarter is permanent.
Most cardiologists remain happy at work
Despite COVID-19 and other professional challenges, most cardiologists (and physicians overall) continue to find their work rewarding.
Cardiologists say the most rewarding aspect of their profession is “being good at what I do/finding answers and diagnoses” (27%), followed by relationships with and gratitude from patients (26%), making the world a better place (23%) and making good money at a job they like (12%). A few cited pride in their profession (6%) and teaching (2%). These figures are in line with last year’s responses.
The most challenging part of practicing cardiology is having so many rules and regulations (22%), followed by having to work long hours (16%), working with electronic health records (13%), trouble getting fair reimbursement (11%), danger/risk associated with treating COVID-19 patients (11%), dealing with difficult patients (8%) and worry about being sued (7%).
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, cardiologists spend 17.4 hours per week on paperwork and administration, similar to last year (16.9 hours per week) and to physicians overall (16.3 hours).
Despite the challenges, 86% of cardiologists said they would choose medicine again, and 92% would choose cardiology again, about the same as last year.
Most cardiologists (83%) plan to keep Medicare and/or Medicaid patients; only 1% say they won’t take new Medicare or Medicaid patients; and 16% are undecided.
Thirty-nine percent of cardiologists plan to participate in the Merit-based Incentive Payment System (MIPS) in 2021.
“The stakes of the Quality Payment Program – the program that incorporates MIPS – are high, with a 9% penalty applied to all Medicare reimbursement for failure to participate,” said Elizabeth Woodcock, MBA, CPC, president of physician practice consulting firm Woodcock & Associates, Atlanta.
“With margins already slim, most physicians can’t afford this massive penalty. It makes sense to protect your revenue by complying with at least the bare minimum,” she noted.
A version of this article first appeared on Medscape.com.
The more drinking, the higher the risk of heart disease, especially in those genetically predisposed
Cardiovascular disease risk is associated with alcohol intake in general, but variations in risk exist with levels of intake, based on data from a genetic-based assessment of more than 300,000 individuals.
Previous studies have identified the “J-shaped model” of alcohol intake and cardiovascular disease, Kiran J. Biddinger of the Broad Institute, Cambridge, Mass., and colleagues said. The J-shaped model suggests that light alcohol intake, defined as one to two drinks per day, appears to reduce cardiovascular disease risk, while heavy alcohol intake, defined as about five drinks per day, increases cardiovascular disease risk, Mr. Biddenger said. However, most studies of the association between alcohol and cardiovascular disease risk are observational, and subject to confounders such as the impact of healthy lifestyle behaviors.
To better assess causality, the researchers used a genetics technique known as Mendelian randomization.
“Some individuals are genetically predisposed to drink more alcohol than others, based on the random allocation of alleles,” he explained. This genetic risk should not be associated with confounding variables such as vegetable consumption or physical activity.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, sponsored by the American Heart Association, the researchers analyzed genetic and lifestyle data from 371,463 participants in the U.K. Biobank, a population-based study of more than 500,000 individuals in the United Kingdom. The researchers used traditional and nonlinear genetic approaches to assess causality between alcohol consumption and cardiovascular disease.
Overall, study participants averaged 9.2 drinks per week. A total of 121,708 (32.8%) had hypertension, and 27,667 (7.5%) had coronary artery disease. The researchers found that individuals who consumed light to moderate amounts of alcohol also lived healthier lifestyles, and had a lower body mass index and higher levels of physical activity than did those who abstained from alcohol. Light to moderate drinkers also had higher vegetable consumption, lower red meat consumption, were less likely to smoke, and had higher self-reported overall health ratings, compared with abstainers.
The researchers then applied Mendelian randomization analyses, creating a genetic proxy and finding that individuals who were predisposed to drink more alcohol had a higher risk of cardiovascular disease.
Traditional and nonlinear Mendelian randomization using quadratic associations showed consistently increased risk of cardiovascular disease with increased alcohol consumption, and this risk increased dramatically for the heaviest drinkers. Compared with individuals who abstained, alcohol consumption of 7, 14, 21, and 28 drinks per week was associated, respectively, with 1.2-, 1.7-, 3.4-, and 8.9-fold odds of hypertension, and 1.2-, 2.3-, 6.2-, and 25.9-fold odds of coronary artery disease.
Notably, an increase of one standard deviation in genetic predisposition for alcohol consumption was associated with a 1.28-fold increase in hypertension, as well as significantly increased risk of coronary artery disease (odds ratio, 1.38), MI (OR, 1.37), stroke (OR, 1.26), heart failure (OR, 1.34), and atrial fibrillation (OR, 1.24).
The study findings were limited by several factors, including the inability to detect specific benefits associated with moderate alcohol consumption. However, the results suggest that, although all amounts of alcohol intake convey some increase in cardiovascular disease risk, “recommendations around alcohol use should reflect this nuanced relationship,” Mr. Biddinger said.
Distinctive study design supports association
Studies examining the association of alcohol consumption with cardiovascular (CVD) outcomes have been mostly observational in nature because of ethical considerations, Anna Kucharska-Newton, PhD, of the University of North Carolina at Chapel Hill, said in an interview. “Results of those studies have not been conclusive, and more research is needed. This study takes advantage of the ‘natural experiment’ of the randomized distribution of genetic variants associated with alcohol consumption,” said Dr. Kucharska-Newton, who served as moderator for the session at the meeting when the study was presented. “This method is similar to a randomized clinical trial and as such is less subject to confounding and potential reverse causality than an observational study..
“The findings confirm data from previous studies, including published data based on the UK Biobank study and the FinnGen registry of genetic data,” said Dr. Kucharska-Newton. “Findings from that study are largely supportive, suggesting that alcohol intake is associated with increased risk of coronary artery disease, an association that is sustained following adjustment for smoking.
“What the present study adds is an elegant presentation of the nonlinearity in that association. However, in contrast to the earlier study that included participants who reported drinking 1-2 drinks per week, Mr. Biddinger and colleagues examined effects among those drinking 7-28 drinks per week, making generalization to light to moderate drinkers [the majority] difficult,” she noted.
As for clinical implications, “assessment of habitual drinking is an important element in routine clinical care.” Dr. Kucharska-Newton noted. “Alcohol intake of seven or more drinks per week is associated exponentially with increased risk of coronary artery disease and, as other data suggest, increased levels of CVD risk factors. Therefore, CVD risk factor control is of particular importance in this population.
“Additional research in populations of ancestry other than White European is very much needed,” Dr. Kucharska-Newton emphasized. “Replication of the analyses presented by Mr. Biddinger and colleagues in different cohorts would strengthen inferences from this study. Extension of study findings to clinically manifest CVD would provide more relevant take-home messages. However, prior studies, based on Mendelian randomization protocols, suggest that adjustment for lifestyle factors attenuates the association of alcohol intake with adverse clinical CVD outcomes.”
Mr. Biddinger had no financial conflicts to disclose, but several coauthors disclosed relationships with companies including Novartis, Regeneron, Bayer, Quest Diagnostics, Corvidia, Pfizer, Verve Therapeutics, and Medgenome. Dr. Kucharska-Newton had no financial conflicts to disclose.
Cardiovascular disease risk is associated with alcohol intake in general, but variations in risk exist with levels of intake, based on data from a genetic-based assessment of more than 300,000 individuals.
Previous studies have identified the “J-shaped model” of alcohol intake and cardiovascular disease, Kiran J. Biddinger of the Broad Institute, Cambridge, Mass., and colleagues said. The J-shaped model suggests that light alcohol intake, defined as one to two drinks per day, appears to reduce cardiovascular disease risk, while heavy alcohol intake, defined as about five drinks per day, increases cardiovascular disease risk, Mr. Biddenger said. However, most studies of the association between alcohol and cardiovascular disease risk are observational, and subject to confounders such as the impact of healthy lifestyle behaviors.
To better assess causality, the researchers used a genetics technique known as Mendelian randomization.
“Some individuals are genetically predisposed to drink more alcohol than others, based on the random allocation of alleles,” he explained. This genetic risk should not be associated with confounding variables such as vegetable consumption or physical activity.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, sponsored by the American Heart Association, the researchers analyzed genetic and lifestyle data from 371,463 participants in the U.K. Biobank, a population-based study of more than 500,000 individuals in the United Kingdom. The researchers used traditional and nonlinear genetic approaches to assess causality between alcohol consumption and cardiovascular disease.
Overall, study participants averaged 9.2 drinks per week. A total of 121,708 (32.8%) had hypertension, and 27,667 (7.5%) had coronary artery disease. The researchers found that individuals who consumed light to moderate amounts of alcohol also lived healthier lifestyles, and had a lower body mass index and higher levels of physical activity than did those who abstained from alcohol. Light to moderate drinkers also had higher vegetable consumption, lower red meat consumption, were less likely to smoke, and had higher self-reported overall health ratings, compared with abstainers.
The researchers then applied Mendelian randomization analyses, creating a genetic proxy and finding that individuals who were predisposed to drink more alcohol had a higher risk of cardiovascular disease.
Traditional and nonlinear Mendelian randomization using quadratic associations showed consistently increased risk of cardiovascular disease with increased alcohol consumption, and this risk increased dramatically for the heaviest drinkers. Compared with individuals who abstained, alcohol consumption of 7, 14, 21, and 28 drinks per week was associated, respectively, with 1.2-, 1.7-, 3.4-, and 8.9-fold odds of hypertension, and 1.2-, 2.3-, 6.2-, and 25.9-fold odds of coronary artery disease.
Notably, an increase of one standard deviation in genetic predisposition for alcohol consumption was associated with a 1.28-fold increase in hypertension, as well as significantly increased risk of coronary artery disease (odds ratio, 1.38), MI (OR, 1.37), stroke (OR, 1.26), heart failure (OR, 1.34), and atrial fibrillation (OR, 1.24).
The study findings were limited by several factors, including the inability to detect specific benefits associated with moderate alcohol consumption. However, the results suggest that, although all amounts of alcohol intake convey some increase in cardiovascular disease risk, “recommendations around alcohol use should reflect this nuanced relationship,” Mr. Biddinger said.
Distinctive study design supports association
Studies examining the association of alcohol consumption with cardiovascular (CVD) outcomes have been mostly observational in nature because of ethical considerations, Anna Kucharska-Newton, PhD, of the University of North Carolina at Chapel Hill, said in an interview. “Results of those studies have not been conclusive, and more research is needed. This study takes advantage of the ‘natural experiment’ of the randomized distribution of genetic variants associated with alcohol consumption,” said Dr. Kucharska-Newton, who served as moderator for the session at the meeting when the study was presented. “This method is similar to a randomized clinical trial and as such is less subject to confounding and potential reverse causality than an observational study..
“The findings confirm data from previous studies, including published data based on the UK Biobank study and the FinnGen registry of genetic data,” said Dr. Kucharska-Newton. “Findings from that study are largely supportive, suggesting that alcohol intake is associated with increased risk of coronary artery disease, an association that is sustained following adjustment for smoking.
“What the present study adds is an elegant presentation of the nonlinearity in that association. However, in contrast to the earlier study that included participants who reported drinking 1-2 drinks per week, Mr. Biddinger and colleagues examined effects among those drinking 7-28 drinks per week, making generalization to light to moderate drinkers [the majority] difficult,” she noted.
As for clinical implications, “assessment of habitual drinking is an important element in routine clinical care.” Dr. Kucharska-Newton noted. “Alcohol intake of seven or more drinks per week is associated exponentially with increased risk of coronary artery disease and, as other data suggest, increased levels of CVD risk factors. Therefore, CVD risk factor control is of particular importance in this population.
“Additional research in populations of ancestry other than White European is very much needed,” Dr. Kucharska-Newton emphasized. “Replication of the analyses presented by Mr. Biddinger and colleagues in different cohorts would strengthen inferences from this study. Extension of study findings to clinically manifest CVD would provide more relevant take-home messages. However, prior studies, based on Mendelian randomization protocols, suggest that adjustment for lifestyle factors attenuates the association of alcohol intake with adverse clinical CVD outcomes.”
Mr. Biddinger had no financial conflicts to disclose, but several coauthors disclosed relationships with companies including Novartis, Regeneron, Bayer, Quest Diagnostics, Corvidia, Pfizer, Verve Therapeutics, and Medgenome. Dr. Kucharska-Newton had no financial conflicts to disclose.
Cardiovascular disease risk is associated with alcohol intake in general, but variations in risk exist with levels of intake, based on data from a genetic-based assessment of more than 300,000 individuals.
Previous studies have identified the “J-shaped model” of alcohol intake and cardiovascular disease, Kiran J. Biddinger of the Broad Institute, Cambridge, Mass., and colleagues said. The J-shaped model suggests that light alcohol intake, defined as one to two drinks per day, appears to reduce cardiovascular disease risk, while heavy alcohol intake, defined as about five drinks per day, increases cardiovascular disease risk, Mr. Biddenger said. However, most studies of the association between alcohol and cardiovascular disease risk are observational, and subject to confounders such as the impact of healthy lifestyle behaviors.
To better assess causality, the researchers used a genetics technique known as Mendelian randomization.
“Some individuals are genetically predisposed to drink more alcohol than others, based on the random allocation of alleles,” he explained. This genetic risk should not be associated with confounding variables such as vegetable consumption or physical activity.
In a study presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting, sponsored by the American Heart Association, the researchers analyzed genetic and lifestyle data from 371,463 participants in the U.K. Biobank, a population-based study of more than 500,000 individuals in the United Kingdom. The researchers used traditional and nonlinear genetic approaches to assess causality between alcohol consumption and cardiovascular disease.
Overall, study participants averaged 9.2 drinks per week. A total of 121,708 (32.8%) had hypertension, and 27,667 (7.5%) had coronary artery disease. The researchers found that individuals who consumed light to moderate amounts of alcohol also lived healthier lifestyles, and had a lower body mass index and higher levels of physical activity than did those who abstained from alcohol. Light to moderate drinkers also had higher vegetable consumption, lower red meat consumption, were less likely to smoke, and had higher self-reported overall health ratings, compared with abstainers.
The researchers then applied Mendelian randomization analyses, creating a genetic proxy and finding that individuals who were predisposed to drink more alcohol had a higher risk of cardiovascular disease.
Traditional and nonlinear Mendelian randomization using quadratic associations showed consistently increased risk of cardiovascular disease with increased alcohol consumption, and this risk increased dramatically for the heaviest drinkers. Compared with individuals who abstained, alcohol consumption of 7, 14, 21, and 28 drinks per week was associated, respectively, with 1.2-, 1.7-, 3.4-, and 8.9-fold odds of hypertension, and 1.2-, 2.3-, 6.2-, and 25.9-fold odds of coronary artery disease.
Notably, an increase of one standard deviation in genetic predisposition for alcohol consumption was associated with a 1.28-fold increase in hypertension, as well as significantly increased risk of coronary artery disease (odds ratio, 1.38), MI (OR, 1.37), stroke (OR, 1.26), heart failure (OR, 1.34), and atrial fibrillation (OR, 1.24).
The study findings were limited by several factors, including the inability to detect specific benefits associated with moderate alcohol consumption. However, the results suggest that, although all amounts of alcohol intake convey some increase in cardiovascular disease risk, “recommendations around alcohol use should reflect this nuanced relationship,” Mr. Biddinger said.
Distinctive study design supports association
Studies examining the association of alcohol consumption with cardiovascular (CVD) outcomes have been mostly observational in nature because of ethical considerations, Anna Kucharska-Newton, PhD, of the University of North Carolina at Chapel Hill, said in an interview. “Results of those studies have not been conclusive, and more research is needed. This study takes advantage of the ‘natural experiment’ of the randomized distribution of genetic variants associated with alcohol consumption,” said Dr. Kucharska-Newton, who served as moderator for the session at the meeting when the study was presented. “This method is similar to a randomized clinical trial and as such is less subject to confounding and potential reverse causality than an observational study..
“The findings confirm data from previous studies, including published data based on the UK Biobank study and the FinnGen registry of genetic data,” said Dr. Kucharska-Newton. “Findings from that study are largely supportive, suggesting that alcohol intake is associated with increased risk of coronary artery disease, an association that is sustained following adjustment for smoking.
“What the present study adds is an elegant presentation of the nonlinearity in that association. However, in contrast to the earlier study that included participants who reported drinking 1-2 drinks per week, Mr. Biddinger and colleagues examined effects among those drinking 7-28 drinks per week, making generalization to light to moderate drinkers [the majority] difficult,” she noted.
As for clinical implications, “assessment of habitual drinking is an important element in routine clinical care.” Dr. Kucharska-Newton noted. “Alcohol intake of seven or more drinks per week is associated exponentially with increased risk of coronary artery disease and, as other data suggest, increased levels of CVD risk factors. Therefore, CVD risk factor control is of particular importance in this population.
“Additional research in populations of ancestry other than White European is very much needed,” Dr. Kucharska-Newton emphasized. “Replication of the analyses presented by Mr. Biddinger and colleagues in different cohorts would strengthen inferences from this study. Extension of study findings to clinically manifest CVD would provide more relevant take-home messages. However, prior studies, based on Mendelian randomization protocols, suggest that adjustment for lifestyle factors attenuates the association of alcohol intake with adverse clinical CVD outcomes.”
Mr. Biddinger had no financial conflicts to disclose, but several coauthors disclosed relationships with companies including Novartis, Regeneron, Bayer, Quest Diagnostics, Corvidia, Pfizer, Verve Therapeutics, and Medgenome. Dr. Kucharska-Newton had no financial conflicts to disclose.
FROM EPI LIFESTYLE
COPD in younger adults deadlier than expected
Adults in their 30s, 40s and 50s with chronic obstructive pulmonary disease (COPD) experience significant morbidity and excess mortality from the disease, results of a population-based study show.
Among adults aged 35-55 years with COPD in Ontario in a longitudinal population cohort study, the overall mortality rate was fivefold higher, compared with other adults in the same age range without COPD.
In contrast, the mortality rate among adults 65 years and older with COPD was 2.5-fold higher than that of their peers without COPD, reported Alina J. Blazer, MSc, MD, a clinical and research fellow at the University of Toronto.
“Overall, our study has shown that younger adults with COPD experience significant morbidity, as evidence by their elevated rates of health care use and excess mortality from their disease. This study provides further evidence that so-called ‘early’ COPD is not a benign disease, and suggests that we should focus clinical efforts on identifying COPD in younger patients, in the hopes that earlier intervention may improve their current health, reduce resource utilization, and prevent further disease progression,” she said during a minisymposium at the American Thoracic Society’s virtual international conference (Abstract A1131).
COPD is widely regarded as a disease affecting only older adults, but it can also occur in those younger than 65, and although it is commonly assumed that COPD diagnosed earlier in life will be milder in severity, this assumption has not been fully explored in real-world settings, Dr. Blazer said.
She and her colleagues conducted a study to examine disease burden as measured by health services utilization and mortality among younger adults with COPD, and compared the rates with those of older adults with COPD.
The sample for this study included 194,759 adults with COPD aged 35-55 years in Ontario in 2016. COPD was identified from health administrative data for three or more outpatient claims or one or more hospitalization claims for COPD over a 2-year period.
For context, the data were compared with those for 496,2113 COPD patients aged 65 years and older.
They found that, compared with their peers without the disease, younger adults had a 3.1-fold higher rate of hospitalization for any cause, a 2.2-fold higher rate of all-cause ED visits, and a 1.7-fold higher rate of outpatient visits for any cause.
In contrast, the comparative rates for seniors with versus without COPD were 2.1-fold, 1.8-fold, and 1.4-fold, respectively.
As noted before, the mortality rate for younger adults with COPD was 5-fold higher than for those without COPD, compared with 2.5-fold among older adults with COPD versus those without.
Earlier diagnosis, follow-up
“A very important talk,” commented session comoderator Valerie Press, MD, MPH, from the University of Chicago. “I know that there’s a lot of work to be done in earlier diagnosis in general, and I think starting with the younger population is a really important area.”
She asked Dr. Blazer about the possibility of asthma codiagnosis or misdiagnosis in the younger patients.
“We use a very specific, validated case definition in the study that our group has used before, and the specificity is over 96% for physician-diagnosed COPD, at the expense of sensitivity, so if anything we probably underestimated the rate of COPD in our study,” Dr. Blazer said.
Audience member Sherry Rogers, MD, an allergist and immunologist in private practice in Syracuse, N.Y., asked whether the investigators could determine what proportion of the excess mortality they saw was attributable to COPD.
“This was looking at all-cause mortality, so we don’t know that it’s necessarily all attributable to COPD per se but perhaps also to COPD-attributable comorbidities,” Dr. Blazer said. “It would be important to piece out the actual causes of mortality that are contributing to that elevated [morality] in that population.”
She added that the next step could include examining rates of specialty referrals and pharmacotherapy to see whether younger patients with COPD are receiving appropriate care, and to ascertain how they are being followed.
The study was supported by the University of Toronto and Sunnybrook Research Institute. Dr. Blazer reported no conflicts of interest to disclose.
Adults in their 30s, 40s and 50s with chronic obstructive pulmonary disease (COPD) experience significant morbidity and excess mortality from the disease, results of a population-based study show.
Among adults aged 35-55 years with COPD in Ontario in a longitudinal population cohort study, the overall mortality rate was fivefold higher, compared with other adults in the same age range without COPD.
In contrast, the mortality rate among adults 65 years and older with COPD was 2.5-fold higher than that of their peers without COPD, reported Alina J. Blazer, MSc, MD, a clinical and research fellow at the University of Toronto.
“Overall, our study has shown that younger adults with COPD experience significant morbidity, as evidence by their elevated rates of health care use and excess mortality from their disease. This study provides further evidence that so-called ‘early’ COPD is not a benign disease, and suggests that we should focus clinical efforts on identifying COPD in younger patients, in the hopes that earlier intervention may improve their current health, reduce resource utilization, and prevent further disease progression,” she said during a minisymposium at the American Thoracic Society’s virtual international conference (Abstract A1131).
COPD is widely regarded as a disease affecting only older adults, but it can also occur in those younger than 65, and although it is commonly assumed that COPD diagnosed earlier in life will be milder in severity, this assumption has not been fully explored in real-world settings, Dr. Blazer said.
She and her colleagues conducted a study to examine disease burden as measured by health services utilization and mortality among younger adults with COPD, and compared the rates with those of older adults with COPD.
The sample for this study included 194,759 adults with COPD aged 35-55 years in Ontario in 2016. COPD was identified from health administrative data for three or more outpatient claims or one or more hospitalization claims for COPD over a 2-year period.
For context, the data were compared with those for 496,2113 COPD patients aged 65 years and older.
They found that, compared with their peers without the disease, younger adults had a 3.1-fold higher rate of hospitalization for any cause, a 2.2-fold higher rate of all-cause ED visits, and a 1.7-fold higher rate of outpatient visits for any cause.
In contrast, the comparative rates for seniors with versus without COPD were 2.1-fold, 1.8-fold, and 1.4-fold, respectively.
As noted before, the mortality rate for younger adults with COPD was 5-fold higher than for those without COPD, compared with 2.5-fold among older adults with COPD versus those without.
Earlier diagnosis, follow-up
“A very important talk,” commented session comoderator Valerie Press, MD, MPH, from the University of Chicago. “I know that there’s a lot of work to be done in earlier diagnosis in general, and I think starting with the younger population is a really important area.”
She asked Dr. Blazer about the possibility of asthma codiagnosis or misdiagnosis in the younger patients.
“We use a very specific, validated case definition in the study that our group has used before, and the specificity is over 96% for physician-diagnosed COPD, at the expense of sensitivity, so if anything we probably underestimated the rate of COPD in our study,” Dr. Blazer said.
Audience member Sherry Rogers, MD, an allergist and immunologist in private practice in Syracuse, N.Y., asked whether the investigators could determine what proportion of the excess mortality they saw was attributable to COPD.
“This was looking at all-cause mortality, so we don’t know that it’s necessarily all attributable to COPD per se but perhaps also to COPD-attributable comorbidities,” Dr. Blazer said. “It would be important to piece out the actual causes of mortality that are contributing to that elevated [morality] in that population.”
She added that the next step could include examining rates of specialty referrals and pharmacotherapy to see whether younger patients with COPD are receiving appropriate care, and to ascertain how they are being followed.
The study was supported by the University of Toronto and Sunnybrook Research Institute. Dr. Blazer reported no conflicts of interest to disclose.
Adults in their 30s, 40s and 50s with chronic obstructive pulmonary disease (COPD) experience significant morbidity and excess mortality from the disease, results of a population-based study show.
Among adults aged 35-55 years with COPD in Ontario in a longitudinal population cohort study, the overall mortality rate was fivefold higher, compared with other adults in the same age range without COPD.
In contrast, the mortality rate among adults 65 years and older with COPD was 2.5-fold higher than that of their peers without COPD, reported Alina J. Blazer, MSc, MD, a clinical and research fellow at the University of Toronto.
“Overall, our study has shown that younger adults with COPD experience significant morbidity, as evidence by their elevated rates of health care use and excess mortality from their disease. This study provides further evidence that so-called ‘early’ COPD is not a benign disease, and suggests that we should focus clinical efforts on identifying COPD in younger patients, in the hopes that earlier intervention may improve their current health, reduce resource utilization, and prevent further disease progression,” she said during a minisymposium at the American Thoracic Society’s virtual international conference (Abstract A1131).
COPD is widely regarded as a disease affecting only older adults, but it can also occur in those younger than 65, and although it is commonly assumed that COPD diagnosed earlier in life will be milder in severity, this assumption has not been fully explored in real-world settings, Dr. Blazer said.
She and her colleagues conducted a study to examine disease burden as measured by health services utilization and mortality among younger adults with COPD, and compared the rates with those of older adults with COPD.
The sample for this study included 194,759 adults with COPD aged 35-55 years in Ontario in 2016. COPD was identified from health administrative data for three or more outpatient claims or one or more hospitalization claims for COPD over a 2-year period.
For context, the data were compared with those for 496,2113 COPD patients aged 65 years and older.
They found that, compared with their peers without the disease, younger adults had a 3.1-fold higher rate of hospitalization for any cause, a 2.2-fold higher rate of all-cause ED visits, and a 1.7-fold higher rate of outpatient visits for any cause.
In contrast, the comparative rates for seniors with versus without COPD were 2.1-fold, 1.8-fold, and 1.4-fold, respectively.
As noted before, the mortality rate for younger adults with COPD was 5-fold higher than for those without COPD, compared with 2.5-fold among older adults with COPD versus those without.
Earlier diagnosis, follow-up
“A very important talk,” commented session comoderator Valerie Press, MD, MPH, from the University of Chicago. “I know that there’s a lot of work to be done in earlier diagnosis in general, and I think starting with the younger population is a really important area.”
She asked Dr. Blazer about the possibility of asthma codiagnosis or misdiagnosis in the younger patients.
“We use a very specific, validated case definition in the study that our group has used before, and the specificity is over 96% for physician-diagnosed COPD, at the expense of sensitivity, so if anything we probably underestimated the rate of COPD in our study,” Dr. Blazer said.
Audience member Sherry Rogers, MD, an allergist and immunologist in private practice in Syracuse, N.Y., asked whether the investigators could determine what proportion of the excess mortality they saw was attributable to COPD.
“This was looking at all-cause mortality, so we don’t know that it’s necessarily all attributable to COPD per se but perhaps also to COPD-attributable comorbidities,” Dr. Blazer said. “It would be important to piece out the actual causes of mortality that are contributing to that elevated [morality] in that population.”
She added that the next step could include examining rates of specialty referrals and pharmacotherapy to see whether younger patients with COPD are receiving appropriate care, and to ascertain how they are being followed.
The study was supported by the University of Toronto and Sunnybrook Research Institute. Dr. Blazer reported no conflicts of interest to disclose.
FROM ATS 2021
Patients with moderate COPD also benefit from triple therapy
The benefits of a triple fixed-dose inhaled corticosteroid, long-acting muscarinic antagonist, and long-acting beta2 agonist combination extend to patients with moderate as well as severe chronic obstructive pulmonary disease (COPD).
That’s according to investigators in the ETHOS (Efficacy and Safety of Triple Therapy in Obstructive Lung Disease) trial (NCT02465567).
In a subanalysis of data on patients with moderate COPD who were enrolled in the comparison trial, the single-inhaler combination of the inhaled corticosteroid (ICS) budesonide, the long-acting muscarinic antagonist (LAMA) glycopyrrolate, and the long-acting beta2 agonist (LABA) formoterol fumarate (BGF) showed benefits in terms of COPD exacerbations, lung function, symptoms, and quality-of-life compared with either of two dual therapy combinations (glycopyrrolate or budesonide with formoterol [GFF/BFF]).
“A moderate benefit:risk ratio was demonstrated in patients with moderate COPD, consistent with the results of the overall ETHOS population, indicating the results of the ETHOS study were not driven by patients with severe or very severe COPD,” wrote Gary T. Ferguson, MD, from the Pulmonary Research Institute of Southeast Michigan in Farmington Hills, and colleagues. Their poster was presented during the American Thoracic Society’s virtual international conference. (Abstract A2244).
As reported at ATS 2020, in the overall ETHOS population of 8,509 patients with moderate to very severe COPD the annual rate of moderate or severe COPD exacerbations was 1.08 and 1.07 for the triple combinations with 320-mcg and 160-mcg doses of budesonide, respectively, compared with 1.42 for glycopyrrolate-formoterol, and 1.24 for budesonide-formoterol.
, Klaus F. Rabe, MD, PhD, of LungenClinic Grosshansdorf and Christian-Albrechts University Kiel (Germany), and colleagues found.
Subanalysis details
At the 2021 iteration of ATS, ETHOS investigator Dr. Ferguson and colleagues reported results for 613 patients with moderate COPD assigned to BGF 320 mcg, 604 assigned to BGF 160 mcg, 596 assigned to GFF, and 614 randomized to BFF.
Baseline demographic and clinical characteristics were similar among the groups, including age, sex, smoking status, mean COPD Assessment Test (CAT) score, mean blood eosinophil count, ICS use at screening, exacerbations in the previous year, mean postbronchodilator forced expiratory volume in 1 second (FEV1) percentage of predicted, and mean postbronchodilator percentage reversibility.
A modified intention-to-treat (ITT) analysis showed that the rate of moderate or severe exacerbations over 52 weeks with BGF 320 mcg was 21% lower than with GFF (P = .0123), but only 4% lower than with BFF, a difference that was not statistically significant.
The BGF 160-mg dose was associated with a 30% reduction in exacerbations vs. GFF (P = .0002), and with a nonsignificant reduction of 15% compared with BFF.
There was a numerical but not statistically significant improvement from baseline at week 24 in morning pre-dose trough FEV1 between the BGF 320-mcg dose and GFF (difference 47 mL), and a significant improvement (90 mL) with BGF compared with BFF (P = .0006). The BGF 160-mcg dose was associated with a larger improvement (89 mL) compared with BFF (P = .0004) but not with GFF.
The FEV1 area under the curve (AUC) of receiver operating characteristics from 0 to 4 hours was superior with BGF at both doses compared with both GFF and BFF.
Patients who used BGF 320 mcg also used significantly less rescue medication over 24 weeks compared with patients who used GFF (P < .0001) or BFF (P = .0001). There were no significant differences in rescue medication use between the BGF 160-mg dose and either of the dual therapy combinations.
Time to clinically important deterioration – defined as a greater than 100 mL decrease in trough FEV1, or a 4 units increase in St. George’s Respiratory Questionnaire total score, or a treatment-emergent moderate/severe COPD exacerbation occurring up to week 52 – was significantly longer with the 320-mcg but not 160-mcg BGF dose compared with GFF (P = .0295) or BFF (P = .0172).
Safety
Treatment-emergent adverse events (TEAEs) occurred in about two-thirds of patients in each trial arm, although TEAEs related to study treatment were more common with the two triple-therapy combinations and with BFF than with GFF.
TEAEs leading to study discontinuation occurred in 5.5% of patients on BGF 320 mcg, 4% on BGF 160 mcg, 4.5% on GFF, and 3.2% on BFF.
Confirmed major adverse cardiovascular events occurred in 0.8% and 1.5% in the BGF 320- and 160-mcg groups, respectively, in 1.8% of patients in the GFF arm, and 1.5% in the BFF arm.
Confirmed pneumonia was seen in 2.6% of patients in each BGF arm, 2.2% in the GFF arm, and 3.6% in the BFF arm.
Selected population
In a comment, David Mannino, MD, medical director of the COPD Foundation, who was not involved in the study, noted that the enrollment criteria for ETHOS tended to skew the population toward patients with severe disease.
In the trial, all patients were receiving at least two inhaled maintenance therapies at the time of screening, and had a postbronchodilator ratio of FEV1 to forced vital capacity of less than 0.7, with a postbronchodilator FEV1 of 25%-65% of the predicted normal value. The patients all had a smoking history of at least 10 pack-years and a documented history of at least one moderate or severe COPD exacerbation in the year before screening.
“The question was whether they would see the same results in people with more moderate impairment, and the answer in this subanalysis is ‘yes.’ The findings weren’t identical between patients with severe and moderate disease, but there were similarities with what was seen in the overall ETHOS study,” he said.
The ETHOS Trial was supported by Pearl Therapeutics. Dr. Ferguson reported grants, personal fees, and nonfinancial support from AstraZeneca during the conduct of the study; and grants, fees, and nonfinancial support from Pearl and others. Dr. Mannino reports recruitment to an advisory board for AstraZeneca.
The benefits of a triple fixed-dose inhaled corticosteroid, long-acting muscarinic antagonist, and long-acting beta2 agonist combination extend to patients with moderate as well as severe chronic obstructive pulmonary disease (COPD).
That’s according to investigators in the ETHOS (Efficacy and Safety of Triple Therapy in Obstructive Lung Disease) trial (NCT02465567).
In a subanalysis of data on patients with moderate COPD who were enrolled in the comparison trial, the single-inhaler combination of the inhaled corticosteroid (ICS) budesonide, the long-acting muscarinic antagonist (LAMA) glycopyrrolate, and the long-acting beta2 agonist (LABA) formoterol fumarate (BGF) showed benefits in terms of COPD exacerbations, lung function, symptoms, and quality-of-life compared with either of two dual therapy combinations (glycopyrrolate or budesonide with formoterol [GFF/BFF]).
“A moderate benefit:risk ratio was demonstrated in patients with moderate COPD, consistent with the results of the overall ETHOS population, indicating the results of the ETHOS study were not driven by patients with severe or very severe COPD,” wrote Gary T. Ferguson, MD, from the Pulmonary Research Institute of Southeast Michigan in Farmington Hills, and colleagues. Their poster was presented during the American Thoracic Society’s virtual international conference. (Abstract A2244).
As reported at ATS 2020, in the overall ETHOS population of 8,509 patients with moderate to very severe COPD the annual rate of moderate or severe COPD exacerbations was 1.08 and 1.07 for the triple combinations with 320-mcg and 160-mcg doses of budesonide, respectively, compared with 1.42 for glycopyrrolate-formoterol, and 1.24 for budesonide-formoterol.
, Klaus F. Rabe, MD, PhD, of LungenClinic Grosshansdorf and Christian-Albrechts University Kiel (Germany), and colleagues found.
Subanalysis details
At the 2021 iteration of ATS, ETHOS investigator Dr. Ferguson and colleagues reported results for 613 patients with moderate COPD assigned to BGF 320 mcg, 604 assigned to BGF 160 mcg, 596 assigned to GFF, and 614 randomized to BFF.
Baseline demographic and clinical characteristics were similar among the groups, including age, sex, smoking status, mean COPD Assessment Test (CAT) score, mean blood eosinophil count, ICS use at screening, exacerbations in the previous year, mean postbronchodilator forced expiratory volume in 1 second (FEV1) percentage of predicted, and mean postbronchodilator percentage reversibility.
A modified intention-to-treat (ITT) analysis showed that the rate of moderate or severe exacerbations over 52 weeks with BGF 320 mcg was 21% lower than with GFF (P = .0123), but only 4% lower than with BFF, a difference that was not statistically significant.
The BGF 160-mg dose was associated with a 30% reduction in exacerbations vs. GFF (P = .0002), and with a nonsignificant reduction of 15% compared with BFF.
There was a numerical but not statistically significant improvement from baseline at week 24 in morning pre-dose trough FEV1 between the BGF 320-mcg dose and GFF (difference 47 mL), and a significant improvement (90 mL) with BGF compared with BFF (P = .0006). The BGF 160-mcg dose was associated with a larger improvement (89 mL) compared with BFF (P = .0004) but not with GFF.
The FEV1 area under the curve (AUC) of receiver operating characteristics from 0 to 4 hours was superior with BGF at both doses compared with both GFF and BFF.
Patients who used BGF 320 mcg also used significantly less rescue medication over 24 weeks compared with patients who used GFF (P < .0001) or BFF (P = .0001). There were no significant differences in rescue medication use between the BGF 160-mg dose and either of the dual therapy combinations.
Time to clinically important deterioration – defined as a greater than 100 mL decrease in trough FEV1, or a 4 units increase in St. George’s Respiratory Questionnaire total score, or a treatment-emergent moderate/severe COPD exacerbation occurring up to week 52 – was significantly longer with the 320-mcg but not 160-mcg BGF dose compared with GFF (P = .0295) or BFF (P = .0172).
Safety
Treatment-emergent adverse events (TEAEs) occurred in about two-thirds of patients in each trial arm, although TEAEs related to study treatment were more common with the two triple-therapy combinations and with BFF than with GFF.
TEAEs leading to study discontinuation occurred in 5.5% of patients on BGF 320 mcg, 4% on BGF 160 mcg, 4.5% on GFF, and 3.2% on BFF.
Confirmed major adverse cardiovascular events occurred in 0.8% and 1.5% in the BGF 320- and 160-mcg groups, respectively, in 1.8% of patients in the GFF arm, and 1.5% in the BFF arm.
Confirmed pneumonia was seen in 2.6% of patients in each BGF arm, 2.2% in the GFF arm, and 3.6% in the BFF arm.
Selected population
In a comment, David Mannino, MD, medical director of the COPD Foundation, who was not involved in the study, noted that the enrollment criteria for ETHOS tended to skew the population toward patients with severe disease.
In the trial, all patients were receiving at least two inhaled maintenance therapies at the time of screening, and had a postbronchodilator ratio of FEV1 to forced vital capacity of less than 0.7, with a postbronchodilator FEV1 of 25%-65% of the predicted normal value. The patients all had a smoking history of at least 10 pack-years and a documented history of at least one moderate or severe COPD exacerbation in the year before screening.
“The question was whether they would see the same results in people with more moderate impairment, and the answer in this subanalysis is ‘yes.’ The findings weren’t identical between patients with severe and moderate disease, but there were similarities with what was seen in the overall ETHOS study,” he said.
The ETHOS Trial was supported by Pearl Therapeutics. Dr. Ferguson reported grants, personal fees, and nonfinancial support from AstraZeneca during the conduct of the study; and grants, fees, and nonfinancial support from Pearl and others. Dr. Mannino reports recruitment to an advisory board for AstraZeneca.
The benefits of a triple fixed-dose inhaled corticosteroid, long-acting muscarinic antagonist, and long-acting beta2 agonist combination extend to patients with moderate as well as severe chronic obstructive pulmonary disease (COPD).
That’s according to investigators in the ETHOS (Efficacy and Safety of Triple Therapy in Obstructive Lung Disease) trial (NCT02465567).
In a subanalysis of data on patients with moderate COPD who were enrolled in the comparison trial, the single-inhaler combination of the inhaled corticosteroid (ICS) budesonide, the long-acting muscarinic antagonist (LAMA) glycopyrrolate, and the long-acting beta2 agonist (LABA) formoterol fumarate (BGF) showed benefits in terms of COPD exacerbations, lung function, symptoms, and quality-of-life compared with either of two dual therapy combinations (glycopyrrolate or budesonide with formoterol [GFF/BFF]).
“A moderate benefit:risk ratio was demonstrated in patients with moderate COPD, consistent with the results of the overall ETHOS population, indicating the results of the ETHOS study were not driven by patients with severe or very severe COPD,” wrote Gary T. Ferguson, MD, from the Pulmonary Research Institute of Southeast Michigan in Farmington Hills, and colleagues. Their poster was presented during the American Thoracic Society’s virtual international conference. (Abstract A2244).
As reported at ATS 2020, in the overall ETHOS population of 8,509 patients with moderate to very severe COPD the annual rate of moderate or severe COPD exacerbations was 1.08 and 1.07 for the triple combinations with 320-mcg and 160-mcg doses of budesonide, respectively, compared with 1.42 for glycopyrrolate-formoterol, and 1.24 for budesonide-formoterol.
, Klaus F. Rabe, MD, PhD, of LungenClinic Grosshansdorf and Christian-Albrechts University Kiel (Germany), and colleagues found.
Subanalysis details
At the 2021 iteration of ATS, ETHOS investigator Dr. Ferguson and colleagues reported results for 613 patients with moderate COPD assigned to BGF 320 mcg, 604 assigned to BGF 160 mcg, 596 assigned to GFF, and 614 randomized to BFF.
Baseline demographic and clinical characteristics were similar among the groups, including age, sex, smoking status, mean COPD Assessment Test (CAT) score, mean blood eosinophil count, ICS use at screening, exacerbations in the previous year, mean postbronchodilator forced expiratory volume in 1 second (FEV1) percentage of predicted, and mean postbronchodilator percentage reversibility.
A modified intention-to-treat (ITT) analysis showed that the rate of moderate or severe exacerbations over 52 weeks with BGF 320 mcg was 21% lower than with GFF (P = .0123), but only 4% lower than with BFF, a difference that was not statistically significant.
The BGF 160-mg dose was associated with a 30% reduction in exacerbations vs. GFF (P = .0002), and with a nonsignificant reduction of 15% compared with BFF.
There was a numerical but not statistically significant improvement from baseline at week 24 in morning pre-dose trough FEV1 between the BGF 320-mcg dose and GFF (difference 47 mL), and a significant improvement (90 mL) with BGF compared with BFF (P = .0006). The BGF 160-mcg dose was associated with a larger improvement (89 mL) compared with BFF (P = .0004) but not with GFF.
The FEV1 area under the curve (AUC) of receiver operating characteristics from 0 to 4 hours was superior with BGF at both doses compared with both GFF and BFF.
Patients who used BGF 320 mcg also used significantly less rescue medication over 24 weeks compared with patients who used GFF (P < .0001) or BFF (P = .0001). There were no significant differences in rescue medication use between the BGF 160-mg dose and either of the dual therapy combinations.
Time to clinically important deterioration – defined as a greater than 100 mL decrease in trough FEV1, or a 4 units increase in St. George’s Respiratory Questionnaire total score, or a treatment-emergent moderate/severe COPD exacerbation occurring up to week 52 – was significantly longer with the 320-mcg but not 160-mcg BGF dose compared with GFF (P = .0295) or BFF (P = .0172).
Safety
Treatment-emergent adverse events (TEAEs) occurred in about two-thirds of patients in each trial arm, although TEAEs related to study treatment were more common with the two triple-therapy combinations and with BFF than with GFF.
TEAEs leading to study discontinuation occurred in 5.5% of patients on BGF 320 mcg, 4% on BGF 160 mcg, 4.5% on GFF, and 3.2% on BFF.
Confirmed major adverse cardiovascular events occurred in 0.8% and 1.5% in the BGF 320- and 160-mcg groups, respectively, in 1.8% of patients in the GFF arm, and 1.5% in the BFF arm.
Confirmed pneumonia was seen in 2.6% of patients in each BGF arm, 2.2% in the GFF arm, and 3.6% in the BFF arm.
Selected population
In a comment, David Mannino, MD, medical director of the COPD Foundation, who was not involved in the study, noted that the enrollment criteria for ETHOS tended to skew the population toward patients with severe disease.
In the trial, all patients were receiving at least two inhaled maintenance therapies at the time of screening, and had a postbronchodilator ratio of FEV1 to forced vital capacity of less than 0.7, with a postbronchodilator FEV1 of 25%-65% of the predicted normal value. The patients all had a smoking history of at least 10 pack-years and a documented history of at least one moderate or severe COPD exacerbation in the year before screening.
“The question was whether they would see the same results in people with more moderate impairment, and the answer in this subanalysis is ‘yes.’ The findings weren’t identical between patients with severe and moderate disease, but there were similarities with what was seen in the overall ETHOS study,” he said.
The ETHOS Trial was supported by Pearl Therapeutics. Dr. Ferguson reported grants, personal fees, and nonfinancial support from AstraZeneca during the conduct of the study; and grants, fees, and nonfinancial support from Pearl and others. Dr. Mannino reports recruitment to an advisory board for AstraZeneca.
FROM ATS 2021
Benefit from cooling temps for cardiac arrest does not differ in randomized trial
The first randomized controlled trial to compare specific temperatures for therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest showed no differences in major outcomes, according to a single-center, double-blind study.
In the CAPITAL-CHILL trial, cooling temperatures of 31° C and 34° C were compared to explore the hypothesis that a lower temperature would improve major outcomes, explained Michel Le May, MD.
No differences for the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days were observed, he reported at the annual scientific sessions of the American College of Cardiology.
The study was completed over a period of almost 7 years in patients presumed to have had an out-of-hospital cardiac arrest and who were unconscious when they reached a center affiliated with the Ottawa Heart Institute, where Dr. Le May directs the regional STEMI (ST-elevation myocardial infarction) program. The initial rhythm at the time of the cardiac arrest was not an entry criterion.
Of 389 patients enrolled, the intention-to-treat analysis included 184 randomized to a cooling temperature of 31° C group and 183 to a temperature of 34° C. The assigned target temperature, reached with an endovascular device, was known only by the managing nurses.
31° C and 34° C are equivalent
There was a small numerical disadvantage for the lower temperature assignment, but none reached statistical significance. This was true of the primary outcome (48.4% vs. 45.4% for the higher temperature) and its components of mortality (43.5% vs. 41.0%) and poor neurologic outcome (4.9% vs. 4.4%). Poor neurologic outcome was defined as a Disability Rating Scale score of greater than 5.
Deaths were most common in the early part of the 180-day follow-up in both arms. On a Kaplan-Meier survival graph, Dr. Le May showed curves that he characterized as “almost superimposable.”
There were no significant differences for any subgroup stratifications, such as age 75 years or older versus younger, males versus females, presence versus absence or an initial shockable rhythm, percutaneous coronary intervention (PCI) within 24 hours versus later, and STEMI versus non-STEMI. In these analyses, the higher temperature was associated with a potential trend for benefit among females and those with a shockable rhythm.
There was no signal for a difference in neurologic outcomes on the Disability Rating Scale or the Modified Rankin Scale. On the latter, for example, 46% of those in the 31° C group and 44% of these in the 34° C group had a score of four or greater at the end of follow-up.
The baseline characteristics of the two groups were similar. About 80% were male; the average age was roughly 62 years. More than 80% of the cardiac arrests were witnessed with CPR being administered by bystanders in nearly 70%. Nearly 40% had a STEMI.
Interventions were similar. Almost all patients underwent coronary angiography, of which nearly 60% received a percutaneous coronary intervention. More than 50% received a stent. The time from arrest to randomization was slightly longer in the 31° C group (228 vs. 204 minutes). The time to balloon inflation from arrival at the cardiac center was also slightly longer (73 vs. 60 minutes).
There was a trend for an increased rate of seizures in the 31° C group (12.5% vs. 7.1%; P = .08), but other secondary outcomes, including pneumonia (67.8% vs. 63.4%), renal replacement therapy (9.2% vs. 9.3%), and stroke (4.4% vs. 1.6%), were similar in the 31° C and 34° C groups, respectively.
Bleeding, whether measured by transfusion (19.6% vs. 22.4%) or TIMI major bleed (23.4% vs. 19.7%) were similar in the 31° C and 34° C groups, respectively. Thrombosis, whether measured by stent thrombosis (1.2% vs. 2.2%) or deep venous thrombosis (11.4% vs. 10.9%) were similar in these two groups, respectively.
The length of stay in the cardiac intensive care unit was significantly greater in the 31° C group (10 vs. 7 days; P = .004). Some of this increased length of stay can be attributed to the longer rewarming process required for the greater cooling, according to Dr. Le May, but he acknowledged that it is not clear this provides a full explanation.
More trials like CAPITAL-CHILL needed
The validity of these findings is supported by several strengths of the methodology, according to Jeanne E. Poole, MD, director of the arrhythmia service and electrophysiology laboratory, University of Washington, Seattle. This includes the reliance of an endovascular device, which can accelerate the time to the target temperature and assure the precision with which it is reached and maintained.
Dr. Poole did note that many of the primary and secondary measures, including the rates of stroke, seizures, and major bleeds, even though not significantly different, favored the higher temperature. The slightly longer door-to-balloon times might have been a factor. For the higher rate of pneumonia in the 31° C group, she questioned whether the longer period of ventilation linked to a longer period of rewarming might have been a factor.
However, Dr. Poole praised the CAPITAL-CHILL trial for drawing attention to a group of patients for whom survival rates remain “dismally low.” She indicated that these types of high-level trials are needed to look for strategies to improve outcomes.
Dr. Le May and Dr. Poole report no potential conflicts of interest.
The first randomized controlled trial to compare specific temperatures for therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest showed no differences in major outcomes, according to a single-center, double-blind study.
In the CAPITAL-CHILL trial, cooling temperatures of 31° C and 34° C were compared to explore the hypothesis that a lower temperature would improve major outcomes, explained Michel Le May, MD.
No differences for the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days were observed, he reported at the annual scientific sessions of the American College of Cardiology.
The study was completed over a period of almost 7 years in patients presumed to have had an out-of-hospital cardiac arrest and who were unconscious when they reached a center affiliated with the Ottawa Heart Institute, where Dr. Le May directs the regional STEMI (ST-elevation myocardial infarction) program. The initial rhythm at the time of the cardiac arrest was not an entry criterion.
Of 389 patients enrolled, the intention-to-treat analysis included 184 randomized to a cooling temperature of 31° C group and 183 to a temperature of 34° C. The assigned target temperature, reached with an endovascular device, was known only by the managing nurses.
31° C and 34° C are equivalent
There was a small numerical disadvantage for the lower temperature assignment, but none reached statistical significance. This was true of the primary outcome (48.4% vs. 45.4% for the higher temperature) and its components of mortality (43.5% vs. 41.0%) and poor neurologic outcome (4.9% vs. 4.4%). Poor neurologic outcome was defined as a Disability Rating Scale score of greater than 5.
Deaths were most common in the early part of the 180-day follow-up in both arms. On a Kaplan-Meier survival graph, Dr. Le May showed curves that he characterized as “almost superimposable.”
There were no significant differences for any subgroup stratifications, such as age 75 years or older versus younger, males versus females, presence versus absence or an initial shockable rhythm, percutaneous coronary intervention (PCI) within 24 hours versus later, and STEMI versus non-STEMI. In these analyses, the higher temperature was associated with a potential trend for benefit among females and those with a shockable rhythm.
There was no signal for a difference in neurologic outcomes on the Disability Rating Scale or the Modified Rankin Scale. On the latter, for example, 46% of those in the 31° C group and 44% of these in the 34° C group had a score of four or greater at the end of follow-up.
The baseline characteristics of the two groups were similar. About 80% were male; the average age was roughly 62 years. More than 80% of the cardiac arrests were witnessed with CPR being administered by bystanders in nearly 70%. Nearly 40% had a STEMI.
Interventions were similar. Almost all patients underwent coronary angiography, of which nearly 60% received a percutaneous coronary intervention. More than 50% received a stent. The time from arrest to randomization was slightly longer in the 31° C group (228 vs. 204 minutes). The time to balloon inflation from arrival at the cardiac center was also slightly longer (73 vs. 60 minutes).
There was a trend for an increased rate of seizures in the 31° C group (12.5% vs. 7.1%; P = .08), but other secondary outcomes, including pneumonia (67.8% vs. 63.4%), renal replacement therapy (9.2% vs. 9.3%), and stroke (4.4% vs. 1.6%), were similar in the 31° C and 34° C groups, respectively.
Bleeding, whether measured by transfusion (19.6% vs. 22.4%) or TIMI major bleed (23.4% vs. 19.7%) were similar in the 31° C and 34° C groups, respectively. Thrombosis, whether measured by stent thrombosis (1.2% vs. 2.2%) or deep venous thrombosis (11.4% vs. 10.9%) were similar in these two groups, respectively.
The length of stay in the cardiac intensive care unit was significantly greater in the 31° C group (10 vs. 7 days; P = .004). Some of this increased length of stay can be attributed to the longer rewarming process required for the greater cooling, according to Dr. Le May, but he acknowledged that it is not clear this provides a full explanation.
More trials like CAPITAL-CHILL needed
The validity of these findings is supported by several strengths of the methodology, according to Jeanne E. Poole, MD, director of the arrhythmia service and electrophysiology laboratory, University of Washington, Seattle. This includes the reliance of an endovascular device, which can accelerate the time to the target temperature and assure the precision with which it is reached and maintained.
Dr. Poole did note that many of the primary and secondary measures, including the rates of stroke, seizures, and major bleeds, even though not significantly different, favored the higher temperature. The slightly longer door-to-balloon times might have been a factor. For the higher rate of pneumonia in the 31° C group, she questioned whether the longer period of ventilation linked to a longer period of rewarming might have been a factor.
However, Dr. Poole praised the CAPITAL-CHILL trial for drawing attention to a group of patients for whom survival rates remain “dismally low.” She indicated that these types of high-level trials are needed to look for strategies to improve outcomes.
Dr. Le May and Dr. Poole report no potential conflicts of interest.
The first randomized controlled trial to compare specific temperatures for therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest showed no differences in major outcomes, according to a single-center, double-blind study.
In the CAPITAL-CHILL trial, cooling temperatures of 31° C and 34° C were compared to explore the hypothesis that a lower temperature would improve major outcomes, explained Michel Le May, MD.
No differences for the primary composite outcome of all-cause mortality or poor neurologic outcome at 180 days were observed, he reported at the annual scientific sessions of the American College of Cardiology.
The study was completed over a period of almost 7 years in patients presumed to have had an out-of-hospital cardiac arrest and who were unconscious when they reached a center affiliated with the Ottawa Heart Institute, where Dr. Le May directs the regional STEMI (ST-elevation myocardial infarction) program. The initial rhythm at the time of the cardiac arrest was not an entry criterion.
Of 389 patients enrolled, the intention-to-treat analysis included 184 randomized to a cooling temperature of 31° C group and 183 to a temperature of 34° C. The assigned target temperature, reached with an endovascular device, was known only by the managing nurses.
31° C and 34° C are equivalent
There was a small numerical disadvantage for the lower temperature assignment, but none reached statistical significance. This was true of the primary outcome (48.4% vs. 45.4% for the higher temperature) and its components of mortality (43.5% vs. 41.0%) and poor neurologic outcome (4.9% vs. 4.4%). Poor neurologic outcome was defined as a Disability Rating Scale score of greater than 5.
Deaths were most common in the early part of the 180-day follow-up in both arms. On a Kaplan-Meier survival graph, Dr. Le May showed curves that he characterized as “almost superimposable.”
There were no significant differences for any subgroup stratifications, such as age 75 years or older versus younger, males versus females, presence versus absence or an initial shockable rhythm, percutaneous coronary intervention (PCI) within 24 hours versus later, and STEMI versus non-STEMI. In these analyses, the higher temperature was associated with a potential trend for benefit among females and those with a shockable rhythm.
There was no signal for a difference in neurologic outcomes on the Disability Rating Scale or the Modified Rankin Scale. On the latter, for example, 46% of those in the 31° C group and 44% of these in the 34° C group had a score of four or greater at the end of follow-up.
The baseline characteristics of the two groups were similar. About 80% were male; the average age was roughly 62 years. More than 80% of the cardiac arrests were witnessed with CPR being administered by bystanders in nearly 70%. Nearly 40% had a STEMI.
Interventions were similar. Almost all patients underwent coronary angiography, of which nearly 60% received a percutaneous coronary intervention. More than 50% received a stent. The time from arrest to randomization was slightly longer in the 31° C group (228 vs. 204 minutes). The time to balloon inflation from arrival at the cardiac center was also slightly longer (73 vs. 60 minutes).
There was a trend for an increased rate of seizures in the 31° C group (12.5% vs. 7.1%; P = .08), but other secondary outcomes, including pneumonia (67.8% vs. 63.4%), renal replacement therapy (9.2% vs. 9.3%), and stroke (4.4% vs. 1.6%), were similar in the 31° C and 34° C groups, respectively.
Bleeding, whether measured by transfusion (19.6% vs. 22.4%) or TIMI major bleed (23.4% vs. 19.7%) were similar in the 31° C and 34° C groups, respectively. Thrombosis, whether measured by stent thrombosis (1.2% vs. 2.2%) or deep venous thrombosis (11.4% vs. 10.9%) were similar in these two groups, respectively.
The length of stay in the cardiac intensive care unit was significantly greater in the 31° C group (10 vs. 7 days; P = .004). Some of this increased length of stay can be attributed to the longer rewarming process required for the greater cooling, according to Dr. Le May, but he acknowledged that it is not clear this provides a full explanation.
More trials like CAPITAL-CHILL needed
The validity of these findings is supported by several strengths of the methodology, according to Jeanne E. Poole, MD, director of the arrhythmia service and electrophysiology laboratory, University of Washington, Seattle. This includes the reliance of an endovascular device, which can accelerate the time to the target temperature and assure the precision with which it is reached and maintained.
Dr. Poole did note that many of the primary and secondary measures, including the rates of stroke, seizures, and major bleeds, even though not significantly different, favored the higher temperature. The slightly longer door-to-balloon times might have been a factor. For the higher rate of pneumonia in the 31° C group, she questioned whether the longer period of ventilation linked to a longer period of rewarming might have been a factor.
However, Dr. Poole praised the CAPITAL-CHILL trial for drawing attention to a group of patients for whom survival rates remain “dismally low.” She indicated that these types of high-level trials are needed to look for strategies to improve outcomes.
Dr. Le May and Dr. Poole report no potential conflicts of interest.
FROM ACC 2021
AHA reassures myocarditis rare after COVID vaccination, benefits overwhelm risks
The benefits of COVID-19 vaccination “enormously outweigh” the rare possible risk for heart-related complications, including myocarditis, the American Heart Association/American Stroke Association (ASA) says in new statement.
The message follows a Centers for Disease Control and Prevention report that the agency is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
The “relatively few” reported cases myocarditis in adolescents or young adults have involved males more often than females, more often followed the second dose rather than the first, and were usually seen in the 4 days after vaccination, the CDC’s COVID-19 Vaccine Safety Technical Work Group (VaST) found.
“Most cases appear to be mild, and follow-up of cases is ongoing,” the CDC says. “Within CDC safety monitoring systems, rates of myocarditis reports in the window following COVID-19 vaccination have not differed from expected baseline rates.”
In their statement, the AHA/ASA “strongly urge” all adults and children 12 years and older to receive a COVID-19 vaccine as soon as possible.
“The evidence continues to indicate that the COVID-19 vaccines are nearly 100% effective at preventing death and hospitalization due to COVID-19 infection,” the groups say.
Although the investigation of cases of myocarditis related to COVID-19 vaccination is ongoing, the AHA/ASA notes that myocarditis is typically the result of an actual viral infection, “and it is yet to be determined if these cases have any correlation to receiving a COVID-19 vaccine.”
“We’ve lost hundreds of children, and there have been thousands who have been hospitalized, thousands who developed an inflammatory syndrome, and one of the pieces of that can be myocarditis,” Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation (RWJF), said today on ABC’s Good Morning America.
Still, “from my perspective, the risk of COVID is so much greater than any theoretical risk from the vaccine,” said Dr. Besser, former acting director of the CDC.
The symptoms that can occur after COVID-19 vaccination include tiredness, headache, muscle pain, chills, fever, and nausea, reminds the AHA/ASA statement. Such symptoms would “typically appear within 24-48 hours and usually pass within 36-48 hours after receiving the vaccine.”
All health care providers should be aware of the “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation, it says.
“Health care professionals should strongly consider inquiring about the timing of any recent COVID vaccination among patients presenting with these conditions, as needed, in order to provide appropriate treatment quickly,” the statement advises.
A version of this article first appeared on Medscape.com.
The benefits of COVID-19 vaccination “enormously outweigh” the rare possible risk for heart-related complications, including myocarditis, the American Heart Association/American Stroke Association (ASA) says in new statement.
The message follows a Centers for Disease Control and Prevention report that the agency is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
The “relatively few” reported cases myocarditis in adolescents or young adults have involved males more often than females, more often followed the second dose rather than the first, and were usually seen in the 4 days after vaccination, the CDC’s COVID-19 Vaccine Safety Technical Work Group (VaST) found.
“Most cases appear to be mild, and follow-up of cases is ongoing,” the CDC says. “Within CDC safety monitoring systems, rates of myocarditis reports in the window following COVID-19 vaccination have not differed from expected baseline rates.”
In their statement, the AHA/ASA “strongly urge” all adults and children 12 years and older to receive a COVID-19 vaccine as soon as possible.
“The evidence continues to indicate that the COVID-19 vaccines are nearly 100% effective at preventing death and hospitalization due to COVID-19 infection,” the groups say.
Although the investigation of cases of myocarditis related to COVID-19 vaccination is ongoing, the AHA/ASA notes that myocarditis is typically the result of an actual viral infection, “and it is yet to be determined if these cases have any correlation to receiving a COVID-19 vaccine.”
“We’ve lost hundreds of children, and there have been thousands who have been hospitalized, thousands who developed an inflammatory syndrome, and one of the pieces of that can be myocarditis,” Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation (RWJF), said today on ABC’s Good Morning America.
Still, “from my perspective, the risk of COVID is so much greater than any theoretical risk from the vaccine,” said Dr. Besser, former acting director of the CDC.
The symptoms that can occur after COVID-19 vaccination include tiredness, headache, muscle pain, chills, fever, and nausea, reminds the AHA/ASA statement. Such symptoms would “typically appear within 24-48 hours and usually pass within 36-48 hours after receiving the vaccine.”
All health care providers should be aware of the “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation, it says.
“Health care professionals should strongly consider inquiring about the timing of any recent COVID vaccination among patients presenting with these conditions, as needed, in order to provide appropriate treatment quickly,” the statement advises.
A version of this article first appeared on Medscape.com.
The benefits of COVID-19 vaccination “enormously outweigh” the rare possible risk for heart-related complications, including myocarditis, the American Heart Association/American Stroke Association (ASA) says in new statement.
The message follows a Centers for Disease Control and Prevention report that the agency is monitoring the Vaccine Adverse Events Reporting System (VAERS) and the Vaccine Safety Datalink (VSD) for cases of myocarditis that have been associated with the mRNA vaccines against SARS-CoV-2 from Pfizer and Moderna.
The “relatively few” reported cases myocarditis in adolescents or young adults have involved males more often than females, more often followed the second dose rather than the first, and were usually seen in the 4 days after vaccination, the CDC’s COVID-19 Vaccine Safety Technical Work Group (VaST) found.
“Most cases appear to be mild, and follow-up of cases is ongoing,” the CDC says. “Within CDC safety monitoring systems, rates of myocarditis reports in the window following COVID-19 vaccination have not differed from expected baseline rates.”
In their statement, the AHA/ASA “strongly urge” all adults and children 12 years and older to receive a COVID-19 vaccine as soon as possible.
“The evidence continues to indicate that the COVID-19 vaccines are nearly 100% effective at preventing death and hospitalization due to COVID-19 infection,” the groups say.
Although the investigation of cases of myocarditis related to COVID-19 vaccination is ongoing, the AHA/ASA notes that myocarditis is typically the result of an actual viral infection, “and it is yet to be determined if these cases have any correlation to receiving a COVID-19 vaccine.”
“We’ve lost hundreds of children, and there have been thousands who have been hospitalized, thousands who developed an inflammatory syndrome, and one of the pieces of that can be myocarditis,” Richard Besser, MD, president and CEO of the Robert Wood Johnson Foundation (RWJF), said today on ABC’s Good Morning America.
Still, “from my perspective, the risk of COVID is so much greater than any theoretical risk from the vaccine,” said Dr. Besser, former acting director of the CDC.
The symptoms that can occur after COVID-19 vaccination include tiredness, headache, muscle pain, chills, fever, and nausea, reminds the AHA/ASA statement. Such symptoms would “typically appear within 24-48 hours and usually pass within 36-48 hours after receiving the vaccine.”
All health care providers should be aware of the “very rare” adverse events that could be related to a COVID-19 vaccine, including myocarditis, blood clots, low platelets, and symptoms of severe inflammation, it says.
“Health care professionals should strongly consider inquiring about the timing of any recent COVID vaccination among patients presenting with these conditions, as needed, in order to provide appropriate treatment quickly,” the statement advises.
A version of this article first appeared on Medscape.com.