User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Children and COVID: Vaccines now available to all ages
The COVID-19 prevention effort in children enters its next phase as June draws to a close, while new pediatric cases continued on a downward trend and hospitalizations continued to rise.
The COVID-19 vaccines from Pfizer-BioNTech and Moderna were approved for use in children as young as 6 months, the Centers for Disease Control and Prevention announced on June 18.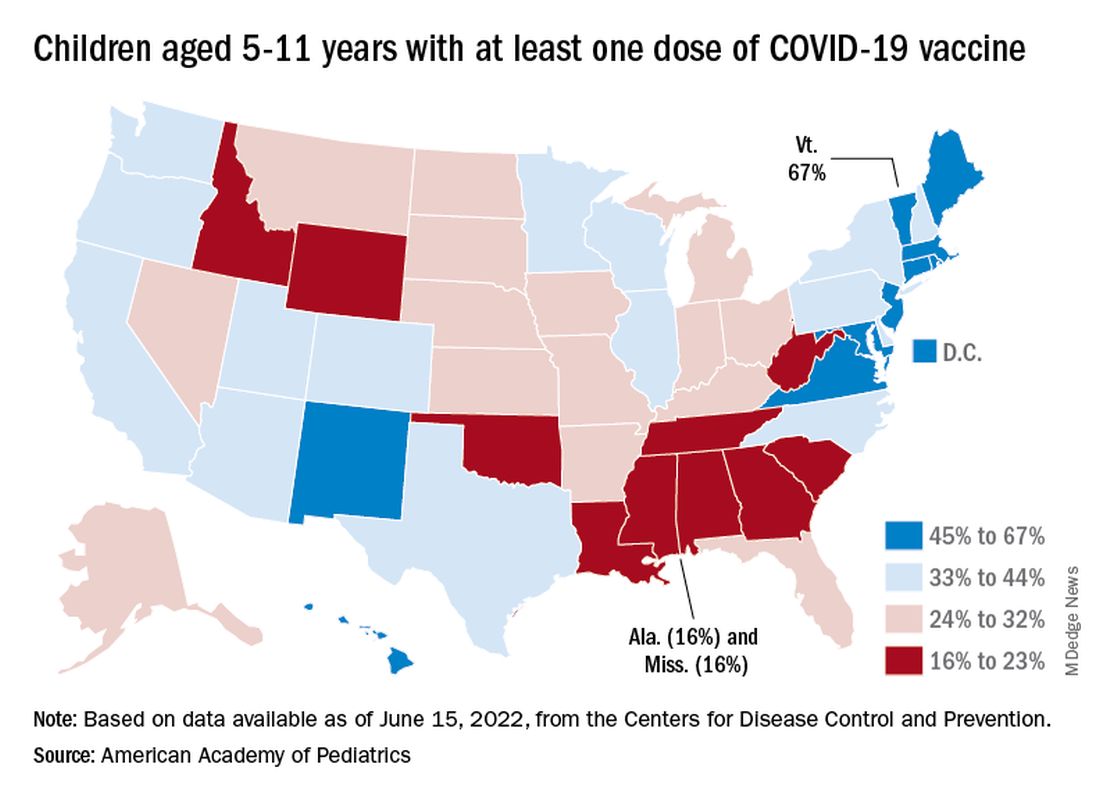
“We know millions of parents and caregivers are eager to get their young children vaccinated. ... I encourage parents and caregivers with questions to talk to their doctor, nurse, or local pharmacist to learn more about the benefits of vaccinations,” CDC Director Rochelle P. Walensky, MD, MPH, said in a written statement.
There are, however, indications that many parents are not that eager. Another 11% said “they will only do so if they are required,” Kaiser noted.
The vaccination experience with children aged 5-11 years seems to agree with those numbers. As of June 16, more than 7 months after the vaccine became available, just over 36% had received at least one dose and about 30% were fully vaccinated, CDC data show.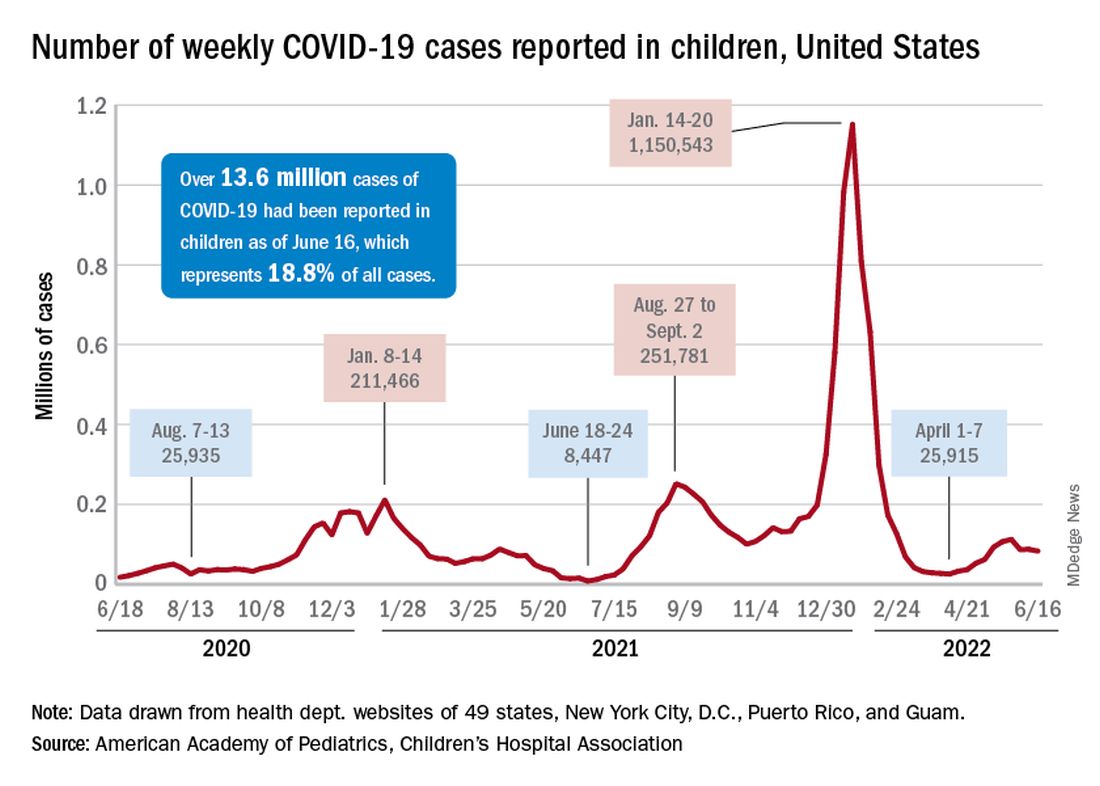
There are, according to the American Academy of Pediatrics, still five states where less than 20% of eligible 5- to 11-year-olds have received an initial vaccination. Among children aged 12-17, uptake has been much higher: 70% have received at least one dose and 60% are fully vaccinated, the CDC said.
Trends for new cases, hospitalizations diverging
COVID incidence in children, meanwhile, dropped for the second time in 3 weeks. There were 83,000 new cases reported during June 10-16, a decline of 4.8% from the previous week, according to the AAP and the Children’s Hospital Association.
New cases had risen by a very slight 0.31% during the week of June 3-9 after dropping 22% the week before (May 27 to June 2). Total cases in children have surpassed 13.6 million, which represents 18.8% of cases in all ages since the start of the pandemic, the AAP and CHA said in their weekly COVID report.
New admissions of children with confirmed COVID-19, however, have continued to climb since early to mid April. On June 16, the rate for children aged 0-17 years was up to 0.31 per 100,000, compared with the 0.13 per 100,000 recorded as late as April 11, the CDC said on its COVID Data Tracker.
The COVID-19 prevention effort in children enters its next phase as June draws to a close, while new pediatric cases continued on a downward trend and hospitalizations continued to rise.
The COVID-19 vaccines from Pfizer-BioNTech and Moderna were approved for use in children as young as 6 months, the Centers for Disease Control and Prevention announced on June 18.
“We know millions of parents and caregivers are eager to get their young children vaccinated. ... I encourage parents and caregivers with questions to talk to their doctor, nurse, or local pharmacist to learn more about the benefits of vaccinations,” CDC Director Rochelle P. Walensky, MD, MPH, said in a written statement.
There are, however, indications that many parents are not that eager. Another 11% said “they will only do so if they are required,” Kaiser noted.
The vaccination experience with children aged 5-11 years seems to agree with those numbers. As of June 16, more than 7 months after the vaccine became available, just over 36% had received at least one dose and about 30% were fully vaccinated, CDC data show.
There are, according to the American Academy of Pediatrics, still five states where less than 20% of eligible 5- to 11-year-olds have received an initial vaccination. Among children aged 12-17, uptake has been much higher: 70% have received at least one dose and 60% are fully vaccinated, the CDC said.
Trends for new cases, hospitalizations diverging
COVID incidence in children, meanwhile, dropped for the second time in 3 weeks. There were 83,000 new cases reported during June 10-16, a decline of 4.8% from the previous week, according to the AAP and the Children’s Hospital Association.
New cases had risen by a very slight 0.31% during the week of June 3-9 after dropping 22% the week before (May 27 to June 2). Total cases in children have surpassed 13.6 million, which represents 18.8% of cases in all ages since the start of the pandemic, the AAP and CHA said in their weekly COVID report.
New admissions of children with confirmed COVID-19, however, have continued to climb since early to mid April. On June 16, the rate for children aged 0-17 years was up to 0.31 per 100,000, compared with the 0.13 per 100,000 recorded as late as April 11, the CDC said on its COVID Data Tracker.
The COVID-19 prevention effort in children enters its next phase as June draws to a close, while new pediatric cases continued on a downward trend and hospitalizations continued to rise.
The COVID-19 vaccines from Pfizer-BioNTech and Moderna were approved for use in children as young as 6 months, the Centers for Disease Control and Prevention announced on June 18.
“We know millions of parents and caregivers are eager to get their young children vaccinated. ... I encourage parents and caregivers with questions to talk to their doctor, nurse, or local pharmacist to learn more about the benefits of vaccinations,” CDC Director Rochelle P. Walensky, MD, MPH, said in a written statement.
There are, however, indications that many parents are not that eager. Another 11% said “they will only do so if they are required,” Kaiser noted.
The vaccination experience with children aged 5-11 years seems to agree with those numbers. As of June 16, more than 7 months after the vaccine became available, just over 36% had received at least one dose and about 30% were fully vaccinated, CDC data show.
There are, according to the American Academy of Pediatrics, still five states where less than 20% of eligible 5- to 11-year-olds have received an initial vaccination. Among children aged 12-17, uptake has been much higher: 70% have received at least one dose and 60% are fully vaccinated, the CDC said.
Trends for new cases, hospitalizations diverging
COVID incidence in children, meanwhile, dropped for the second time in 3 weeks. There were 83,000 new cases reported during June 10-16, a decline of 4.8% from the previous week, according to the AAP and the Children’s Hospital Association.
New cases had risen by a very slight 0.31% during the week of June 3-9 after dropping 22% the week before (May 27 to June 2). Total cases in children have surpassed 13.6 million, which represents 18.8% of cases in all ages since the start of the pandemic, the AAP and CHA said in their weekly COVID report.
New admissions of children with confirmed COVID-19, however, have continued to climb since early to mid April. On June 16, the rate for children aged 0-17 years was up to 0.31 per 100,000, compared with the 0.13 per 100,000 recorded as late as April 11, the CDC said on its COVID Data Tracker.
COVID vaccination in DMT-treated MS patients: New data
NATIONAL HARBOR, MD. – The latest updates on COVID-19 vaccination response among patients with multiple sclerosis (MS) who are treated with disease-modifying therapy (DMT) show that, if patients do contract the virus, cases are mild and serious infections are rare.
However, vaccine antibody response remains lower with anti-CD20 therapies.
One of several late-breaking studies on these issues that were presented at the annual meeting of the Consortium of Multiple Sclerosis Centers included more than 100 patients with MS who were treated with a variety of DMTs.
Results showed that the rate of antibody response was just 55% among those treated with anti-CD20 therapies versus 83% for those treated with other DMTs, including sphingosine-1-phosphate receptor modulators (S1Ps).
Consistent with what has been observed in other studies, “vaccine antibody responses were slightly lower in B cell–depleted patients than with other therapies,” senior author Rahul Dave, MD, director of the INOVA MS and Neuroimmunology Center, Inova Neurosciences Institute, the University of Virginia, Fairfax, said in an interview.
Vaccine response
The investigators sought to assess detailed vaccine responses in 134 patients with MS. Serum COVID antibody measures were conducted approximately 3 weeks to 4 months after vaccination – and mostly after the initial vaccination.
The antibody response rate was significantly lower with anti-CD20 treatments (55%) than with all other DMTs examined (83%), including S1Ps, immunomodulators, immunosuppressive drugs, interferon B, anti-CD52, and natalizumab (P < .01).
The highest prevalence of antibody response was observed among those taking immunomodulators; responses occurred among 91% of patients taking teriflunomide and among 93% of those taking fumarates.
Among those treated with anti-CD20 therapy, antibody responses correlated with higher baseline immunoglobulin levels (P = .01) and shorter durations of therapy.
“We found that longer total duration of therapy and lower immunoglobulin levels tended to correlate with decreases in immune responses,” said Dr. Dave.
“Interestingly, the timing between vaccination versus administration of [anti-CD20 drug] ocrelizumab did not seem to be impactful with regards to antibody responses,” Dr. Dave noted. He added that this is contrary to some past studies that showed benefits if the vaccination could be completed prior to starting ocrelizumab.
Sixteen participants tested polymerase chain reaction positive for COVID during the previous 12 months. Although most infections were described as mild and self-limited, four of the patients received outpatient monoclonal antibody therapy, and one required hospitalization because of COVID.
“I think it is notable and reassuring that, overall, our patients had mild courses. This is consistent with the vaccines ‘working,’ and is true even in patients on high-efficacy immunosuppressants that partially abrogate antibody responses,” Dr. Dave said.
He added that he reassures patients who need high-efficacy therapies that “they should use them.”
That being said, as in the general population, even vaccinated patients can get COVID. “You can be sick and feel terrible, but in general, hospitalization numbers are way down compared to 2 years ago. We are seeing the same trends in MS patients, including the B cell–depleted patients,” he said.
“To get at the question whether B cell–depleted patients behave exactly the same as the general population, or even [with] other DMTs, we will need large, multicenter, prospective datasets,” said Dr. Dave.
Favorable findings
Two other late-breaking posters at the meeting provided updates regarding antibody responses among patients receiving S1Ps. There has been concern that S1Ps may blunt antibody responses to COVID vaccinations.
The concern is in regard to their unique mechanisms of sequestering circulating lymphocytes, particularly the older, nonselective S1P receptor modulator fingolimod, said the author of one of the studies, Daniel Kantor, MD, president emeritus of the Florida Society of Neurology and founding president of the Medical Partnership 4 MS+.
“It appears the issues with fingolimod might relate to the level of white blood cell sequestration, [which is] greater in fingolimod than the newer S1P receptor modulators, and/or the result of S1P4 receptor modulation, which is not seen with the newer, selective medications,” Dr. Kantor said in an interview.
In a prospective observational trial of patients with relapsing MS, among 30 participants who were treated with ozanimod, the mean increase in IgG antibody titer 4 weeks after either of the two available mRNA vaccines was 232.73 AU/mL versus a mean increase of 526.59 AU/mL among 30 non–ozanimod/DMT-treated patients.
To date, only three patients in the study were taking ocrelizumab; for those patients, the mean increase in IgG titers was 0.633.
Despite the lower antibody titers in the ozanimod-treated patients, which Dr. Kantor noted are generally regarded as protective, all but one of the patients had positive results on T-Detect, which was indicative of vaccine protection.
“In this study, [relapsing] MS patients treated with ozanimod had an antibody and T-cell response to the mRNA COVID-19 vaccines,” he reported. “This trial is ongoing, with 48 weeks of follow-up expected in December 2022.”
Ponesimod results
In the other S1P modulator-related late-breaking study, Janssen Research and Development reported on antibody responses of patients who were treated with the S1P drug ponesimod in the phase 2 AC-058B202 study.
The median exposure to ponesimod at time of vaccination was 10.7 years (range, 9.8-11.8 years). There were 134 patients in the study. Of those, both prevaccination and postvaccination blood samples from 49 patients were tested for spike antibody concentrations.
Among those participants, 40 (81.6%) met the definition of response to the COVID-19 vaccination, defined as seroconversion in the case of negative prevaccination antibody testing or a fourfold antibody concentration increase in the case of a positive prevaccination antibody result.
Of the 38 antibody-negative participants, 33 (86.8%) achieved seroconversion post vaccination.
A total of 20 participants reported having had prevaccine COVID, while 17 had postvaccination COVID.
None of the cases were serious, severe, or fatal, and none led to permanent treatment discontinuation.
“In patients with RMS on ponesimod, the majority (> 80%) appear to develop a measurable SARS-CoV-2 humoral response after COVID-19 vaccination,” the authors, led by Janice Wong, of Janssen Research and Development, wrote.
“Further investigations on the efficacy and safety of COVID-19 vaccination in MS patients on ponesimod are warranted,” they added.
In a final study from Genentech, of 4848 patients with MS who were fully vaccinated during the Delta and Omicron waves, 1.3% had a COVID-related hospitalization. In addition, rate of severe SARS-CoV-2 infections was very low (0.6%); there were fewer than 10 infections in each subgroup of DMTs. These patients included 585 (17%) who were treated with ocrelizumab, 238 (7%) who were treated with S1P receptor modulators, 33 (1%) who were treated with interferons, 1,004 (29%) who were treated with other DMTs, and 1,574 (46%) for whom no DMTs were recorded.
“We can conclude from this study that the characteristics of people with MS with more severe COVID-19 outcomes resemble those observed in the general population,” such as in those who are older or have higher rates of comorbidities, Preeti Bajaj, team lead of HEOR, Neuroscience, at Genentech, said in an interview. “We believe [ocrelizumab] treatment decisions should be made between a patient and their treating neurologist or other medical professional based on a benefit-risk assessment specific to the individual patient.”
Concerns remain
In a comment, Bruce A. C. Cree, MD, PhD, professor of clinical neurology and clinical research director at the Weill Institute for Neurosciences, University of California, San Francisco, described the overall data on vaccine efficacy on anti-CD20s as “discouraging” and said he is adjusting his own recommendations for these patients.
“Repeated vaccinations do not seem to stimulate humoral responses in B cell–depleted patients,” said Dr. Cree, who was not involved with the research.
“In my personal practice, I have been suspending dosing in my patients to allow for B-cell reconstitution to occur followed by revaccination,” he added.
Regarding the S1P drugs, he noted that, aside from fingolimod, “the antibody response frequency seems to be better than initial reports. However, the index values are low and may not be protective.”
Overall, the take-home message for patients with MS who are taking DMTs should be, “all patients treated with S1P modulators or anti-C20 antibodies should be vaccinated and boosted,” Dr. Cree said.
“In some cases, temporary interruption of treatment might be useful to help develop robust responses to vaccinations,” he added.
Dr. Dave reported no financial relationships regarding the poster but is a paid speaker/consultant for Novartis, Bristol-Myers Squibb, EMD Serono, Biogen, Alexion, Genentech, Horizon, and Sanofi for their MS & NMO therapies. Dr. Kantor’s research was supported by a grant from BMS; he is a consultant for Biogen, BMS, and Janssen. Dr. Cree reported that he is an unpaid consultant for BMS, the manufacturer of ozanimod.
A version of this article first appeared on Medscape.com.
NATIONAL HARBOR, MD. – The latest updates on COVID-19 vaccination response among patients with multiple sclerosis (MS) who are treated with disease-modifying therapy (DMT) show that, if patients do contract the virus, cases are mild and serious infections are rare.
However, vaccine antibody response remains lower with anti-CD20 therapies.
One of several late-breaking studies on these issues that were presented at the annual meeting of the Consortium of Multiple Sclerosis Centers included more than 100 patients with MS who were treated with a variety of DMTs.
Results showed that the rate of antibody response was just 55% among those treated with anti-CD20 therapies versus 83% for those treated with other DMTs, including sphingosine-1-phosphate receptor modulators (S1Ps).
Consistent with what has been observed in other studies, “vaccine antibody responses were slightly lower in B cell–depleted patients than with other therapies,” senior author Rahul Dave, MD, director of the INOVA MS and Neuroimmunology Center, Inova Neurosciences Institute, the University of Virginia, Fairfax, said in an interview.
Vaccine response
The investigators sought to assess detailed vaccine responses in 134 patients with MS. Serum COVID antibody measures were conducted approximately 3 weeks to 4 months after vaccination – and mostly after the initial vaccination.
The antibody response rate was significantly lower with anti-CD20 treatments (55%) than with all other DMTs examined (83%), including S1Ps, immunomodulators, immunosuppressive drugs, interferon B, anti-CD52, and natalizumab (P < .01).
The highest prevalence of antibody response was observed among those taking immunomodulators; responses occurred among 91% of patients taking teriflunomide and among 93% of those taking fumarates.
Among those treated with anti-CD20 therapy, antibody responses correlated with higher baseline immunoglobulin levels (P = .01) and shorter durations of therapy.
“We found that longer total duration of therapy and lower immunoglobulin levels tended to correlate with decreases in immune responses,” said Dr. Dave.
“Interestingly, the timing between vaccination versus administration of [anti-CD20 drug] ocrelizumab did not seem to be impactful with regards to antibody responses,” Dr. Dave noted. He added that this is contrary to some past studies that showed benefits if the vaccination could be completed prior to starting ocrelizumab.
Sixteen participants tested polymerase chain reaction positive for COVID during the previous 12 months. Although most infections were described as mild and self-limited, four of the patients received outpatient monoclonal antibody therapy, and one required hospitalization because of COVID.
“I think it is notable and reassuring that, overall, our patients had mild courses. This is consistent with the vaccines ‘working,’ and is true even in patients on high-efficacy immunosuppressants that partially abrogate antibody responses,” Dr. Dave said.
He added that he reassures patients who need high-efficacy therapies that “they should use them.”
That being said, as in the general population, even vaccinated patients can get COVID. “You can be sick and feel terrible, but in general, hospitalization numbers are way down compared to 2 years ago. We are seeing the same trends in MS patients, including the B cell–depleted patients,” he said.
“To get at the question whether B cell–depleted patients behave exactly the same as the general population, or even [with] other DMTs, we will need large, multicenter, prospective datasets,” said Dr. Dave.
Favorable findings
Two other late-breaking posters at the meeting provided updates regarding antibody responses among patients receiving S1Ps. There has been concern that S1Ps may blunt antibody responses to COVID vaccinations.
The concern is in regard to their unique mechanisms of sequestering circulating lymphocytes, particularly the older, nonselective S1P receptor modulator fingolimod, said the author of one of the studies, Daniel Kantor, MD, president emeritus of the Florida Society of Neurology and founding president of the Medical Partnership 4 MS+.
“It appears the issues with fingolimod might relate to the level of white blood cell sequestration, [which is] greater in fingolimod than the newer S1P receptor modulators, and/or the result of S1P4 receptor modulation, which is not seen with the newer, selective medications,” Dr. Kantor said in an interview.
In a prospective observational trial of patients with relapsing MS, among 30 participants who were treated with ozanimod, the mean increase in IgG antibody titer 4 weeks after either of the two available mRNA vaccines was 232.73 AU/mL versus a mean increase of 526.59 AU/mL among 30 non–ozanimod/DMT-treated patients.
To date, only three patients in the study were taking ocrelizumab; for those patients, the mean increase in IgG titers was 0.633.
Despite the lower antibody titers in the ozanimod-treated patients, which Dr. Kantor noted are generally regarded as protective, all but one of the patients had positive results on T-Detect, which was indicative of vaccine protection.
“In this study, [relapsing] MS patients treated with ozanimod had an antibody and T-cell response to the mRNA COVID-19 vaccines,” he reported. “This trial is ongoing, with 48 weeks of follow-up expected in December 2022.”
Ponesimod results
In the other S1P modulator-related late-breaking study, Janssen Research and Development reported on antibody responses of patients who were treated with the S1P drug ponesimod in the phase 2 AC-058B202 study.
The median exposure to ponesimod at time of vaccination was 10.7 years (range, 9.8-11.8 years). There were 134 patients in the study. Of those, both prevaccination and postvaccination blood samples from 49 patients were tested for spike antibody concentrations.
Among those participants, 40 (81.6%) met the definition of response to the COVID-19 vaccination, defined as seroconversion in the case of negative prevaccination antibody testing or a fourfold antibody concentration increase in the case of a positive prevaccination antibody result.
Of the 38 antibody-negative participants, 33 (86.8%) achieved seroconversion post vaccination.
A total of 20 participants reported having had prevaccine COVID, while 17 had postvaccination COVID.
None of the cases were serious, severe, or fatal, and none led to permanent treatment discontinuation.
“In patients with RMS on ponesimod, the majority (> 80%) appear to develop a measurable SARS-CoV-2 humoral response after COVID-19 vaccination,” the authors, led by Janice Wong, of Janssen Research and Development, wrote.
“Further investigations on the efficacy and safety of COVID-19 vaccination in MS patients on ponesimod are warranted,” they added.
In a final study from Genentech, of 4848 patients with MS who were fully vaccinated during the Delta and Omicron waves, 1.3% had a COVID-related hospitalization. In addition, rate of severe SARS-CoV-2 infections was very low (0.6%); there were fewer than 10 infections in each subgroup of DMTs. These patients included 585 (17%) who were treated with ocrelizumab, 238 (7%) who were treated with S1P receptor modulators, 33 (1%) who were treated with interferons, 1,004 (29%) who were treated with other DMTs, and 1,574 (46%) for whom no DMTs were recorded.
“We can conclude from this study that the characteristics of people with MS with more severe COVID-19 outcomes resemble those observed in the general population,” such as in those who are older or have higher rates of comorbidities, Preeti Bajaj, team lead of HEOR, Neuroscience, at Genentech, said in an interview. “We believe [ocrelizumab] treatment decisions should be made between a patient and their treating neurologist or other medical professional based on a benefit-risk assessment specific to the individual patient.”
Concerns remain
In a comment, Bruce A. C. Cree, MD, PhD, professor of clinical neurology and clinical research director at the Weill Institute for Neurosciences, University of California, San Francisco, described the overall data on vaccine efficacy on anti-CD20s as “discouraging” and said he is adjusting his own recommendations for these patients.
“Repeated vaccinations do not seem to stimulate humoral responses in B cell–depleted patients,” said Dr. Cree, who was not involved with the research.
“In my personal practice, I have been suspending dosing in my patients to allow for B-cell reconstitution to occur followed by revaccination,” he added.
Regarding the S1P drugs, he noted that, aside from fingolimod, “the antibody response frequency seems to be better than initial reports. However, the index values are low and may not be protective.”
Overall, the take-home message for patients with MS who are taking DMTs should be, “all patients treated with S1P modulators or anti-C20 antibodies should be vaccinated and boosted,” Dr. Cree said.
“In some cases, temporary interruption of treatment might be useful to help develop robust responses to vaccinations,” he added.
Dr. Dave reported no financial relationships regarding the poster but is a paid speaker/consultant for Novartis, Bristol-Myers Squibb, EMD Serono, Biogen, Alexion, Genentech, Horizon, and Sanofi for their MS & NMO therapies. Dr. Kantor’s research was supported by a grant from BMS; he is a consultant for Biogen, BMS, and Janssen. Dr. Cree reported that he is an unpaid consultant for BMS, the manufacturer of ozanimod.
A version of this article first appeared on Medscape.com.
NATIONAL HARBOR, MD. – The latest updates on COVID-19 vaccination response among patients with multiple sclerosis (MS) who are treated with disease-modifying therapy (DMT) show that, if patients do contract the virus, cases are mild and serious infections are rare.
However, vaccine antibody response remains lower with anti-CD20 therapies.
One of several late-breaking studies on these issues that were presented at the annual meeting of the Consortium of Multiple Sclerosis Centers included more than 100 patients with MS who were treated with a variety of DMTs.
Results showed that the rate of antibody response was just 55% among those treated with anti-CD20 therapies versus 83% for those treated with other DMTs, including sphingosine-1-phosphate receptor modulators (S1Ps).
Consistent with what has been observed in other studies, “vaccine antibody responses were slightly lower in B cell–depleted patients than with other therapies,” senior author Rahul Dave, MD, director of the INOVA MS and Neuroimmunology Center, Inova Neurosciences Institute, the University of Virginia, Fairfax, said in an interview.
Vaccine response
The investigators sought to assess detailed vaccine responses in 134 patients with MS. Serum COVID antibody measures were conducted approximately 3 weeks to 4 months after vaccination – and mostly after the initial vaccination.
The antibody response rate was significantly lower with anti-CD20 treatments (55%) than with all other DMTs examined (83%), including S1Ps, immunomodulators, immunosuppressive drugs, interferon B, anti-CD52, and natalizumab (P < .01).
The highest prevalence of antibody response was observed among those taking immunomodulators; responses occurred among 91% of patients taking teriflunomide and among 93% of those taking fumarates.
Among those treated with anti-CD20 therapy, antibody responses correlated with higher baseline immunoglobulin levels (P = .01) and shorter durations of therapy.
“We found that longer total duration of therapy and lower immunoglobulin levels tended to correlate with decreases in immune responses,” said Dr. Dave.
“Interestingly, the timing between vaccination versus administration of [anti-CD20 drug] ocrelizumab did not seem to be impactful with regards to antibody responses,” Dr. Dave noted. He added that this is contrary to some past studies that showed benefits if the vaccination could be completed prior to starting ocrelizumab.
Sixteen participants tested polymerase chain reaction positive for COVID during the previous 12 months. Although most infections were described as mild and self-limited, four of the patients received outpatient monoclonal antibody therapy, and one required hospitalization because of COVID.
“I think it is notable and reassuring that, overall, our patients had mild courses. This is consistent with the vaccines ‘working,’ and is true even in patients on high-efficacy immunosuppressants that partially abrogate antibody responses,” Dr. Dave said.
He added that he reassures patients who need high-efficacy therapies that “they should use them.”
That being said, as in the general population, even vaccinated patients can get COVID. “You can be sick and feel terrible, but in general, hospitalization numbers are way down compared to 2 years ago. We are seeing the same trends in MS patients, including the B cell–depleted patients,” he said.
“To get at the question whether B cell–depleted patients behave exactly the same as the general population, or even [with] other DMTs, we will need large, multicenter, prospective datasets,” said Dr. Dave.
Favorable findings
Two other late-breaking posters at the meeting provided updates regarding antibody responses among patients receiving S1Ps. There has been concern that S1Ps may blunt antibody responses to COVID vaccinations.
The concern is in regard to their unique mechanisms of sequestering circulating lymphocytes, particularly the older, nonselective S1P receptor modulator fingolimod, said the author of one of the studies, Daniel Kantor, MD, president emeritus of the Florida Society of Neurology and founding president of the Medical Partnership 4 MS+.
“It appears the issues with fingolimod might relate to the level of white blood cell sequestration, [which is] greater in fingolimod than the newer S1P receptor modulators, and/or the result of S1P4 receptor modulation, which is not seen with the newer, selective medications,” Dr. Kantor said in an interview.
In a prospective observational trial of patients with relapsing MS, among 30 participants who were treated with ozanimod, the mean increase in IgG antibody titer 4 weeks after either of the two available mRNA vaccines was 232.73 AU/mL versus a mean increase of 526.59 AU/mL among 30 non–ozanimod/DMT-treated patients.
To date, only three patients in the study were taking ocrelizumab; for those patients, the mean increase in IgG titers was 0.633.
Despite the lower antibody titers in the ozanimod-treated patients, which Dr. Kantor noted are generally regarded as protective, all but one of the patients had positive results on T-Detect, which was indicative of vaccine protection.
“In this study, [relapsing] MS patients treated with ozanimod had an antibody and T-cell response to the mRNA COVID-19 vaccines,” he reported. “This trial is ongoing, with 48 weeks of follow-up expected in December 2022.”
Ponesimod results
In the other S1P modulator-related late-breaking study, Janssen Research and Development reported on antibody responses of patients who were treated with the S1P drug ponesimod in the phase 2 AC-058B202 study.
The median exposure to ponesimod at time of vaccination was 10.7 years (range, 9.8-11.8 years). There were 134 patients in the study. Of those, both prevaccination and postvaccination blood samples from 49 patients were tested for spike antibody concentrations.
Among those participants, 40 (81.6%) met the definition of response to the COVID-19 vaccination, defined as seroconversion in the case of negative prevaccination antibody testing or a fourfold antibody concentration increase in the case of a positive prevaccination antibody result.
Of the 38 antibody-negative participants, 33 (86.8%) achieved seroconversion post vaccination.
A total of 20 participants reported having had prevaccine COVID, while 17 had postvaccination COVID.
None of the cases were serious, severe, or fatal, and none led to permanent treatment discontinuation.
“In patients with RMS on ponesimod, the majority (> 80%) appear to develop a measurable SARS-CoV-2 humoral response after COVID-19 vaccination,” the authors, led by Janice Wong, of Janssen Research and Development, wrote.
“Further investigations on the efficacy and safety of COVID-19 vaccination in MS patients on ponesimod are warranted,” they added.
In a final study from Genentech, of 4848 patients with MS who were fully vaccinated during the Delta and Omicron waves, 1.3% had a COVID-related hospitalization. In addition, rate of severe SARS-CoV-2 infections was very low (0.6%); there were fewer than 10 infections in each subgroup of DMTs. These patients included 585 (17%) who were treated with ocrelizumab, 238 (7%) who were treated with S1P receptor modulators, 33 (1%) who were treated with interferons, 1,004 (29%) who were treated with other DMTs, and 1,574 (46%) for whom no DMTs were recorded.
“We can conclude from this study that the characteristics of people with MS with more severe COVID-19 outcomes resemble those observed in the general population,” such as in those who are older or have higher rates of comorbidities, Preeti Bajaj, team lead of HEOR, Neuroscience, at Genentech, said in an interview. “We believe [ocrelizumab] treatment decisions should be made between a patient and their treating neurologist or other medical professional based on a benefit-risk assessment specific to the individual patient.”
Concerns remain
In a comment, Bruce A. C. Cree, MD, PhD, professor of clinical neurology and clinical research director at the Weill Institute for Neurosciences, University of California, San Francisco, described the overall data on vaccine efficacy on anti-CD20s as “discouraging” and said he is adjusting his own recommendations for these patients.
“Repeated vaccinations do not seem to stimulate humoral responses in B cell–depleted patients,” said Dr. Cree, who was not involved with the research.
“In my personal practice, I have been suspending dosing in my patients to allow for B-cell reconstitution to occur followed by revaccination,” he added.
Regarding the S1P drugs, he noted that, aside from fingolimod, “the antibody response frequency seems to be better than initial reports. However, the index values are low and may not be protective.”
Overall, the take-home message for patients with MS who are taking DMTs should be, “all patients treated with S1P modulators or anti-C20 antibodies should be vaccinated and boosted,” Dr. Cree said.
“In some cases, temporary interruption of treatment might be useful to help develop robust responses to vaccinations,” he added.
Dr. Dave reported no financial relationships regarding the poster but is a paid speaker/consultant for Novartis, Bristol-Myers Squibb, EMD Serono, Biogen, Alexion, Genentech, Horizon, and Sanofi for their MS & NMO therapies. Dr. Kantor’s research was supported by a grant from BMS; he is a consultant for Biogen, BMS, and Janssen. Dr. Cree reported that he is an unpaid consultant for BMS, the manufacturer of ozanimod.
A version of this article first appeared on Medscape.com.
AT CMSC 2022
New saliva-based COVID-19 test provides rapid results
A rapid, saliva-based test for COVID-19 could enable testing, diagnosis, and prescribing to take place in a single office visit by immediately confirming whether a patient has the infection and needs to be treated, researchers say. The test has sparked commercial interest and earned additional funding from the Canadian government.
The test uses a DNA aptamer – a short, synthetic oligonucleotide that binds to a specific molecular target – that shows high affinity for the SARS-CoV-2 spike protein and its variants. The approach “can be rapidly adapted to different threats,” as well, Leyla Soleymani, PhD, an associate professor of engineering physics at McMaster University, Hamilton, Ontario, Canada, told this news organization. Her team invented the approach.
Adaptable to other pathogens
Current gold-standard COVID-19 tests are based on reverse transcription-polymerase chain reaction (RT-PCR), which are sensitive but costly, complicated, and require waiting at least a couple of days for results, according to Dr. Soleymani and colleagues. Rapid nucleic acid and antigen tests have only “moderate” sensitivity and specificity, particularly when viral loads are low. None have been shown to work well with saliva samples.
By contrast, the new test “uses a reader and test cartridges, similar to the glucose reader,” said Dr. Soleymani, who is also Canada Research chair in Miniaturized Biomedical Devices. A small sample of saliva is added to a chemical reagent and inserted into the reader, which is attached to a smartphone. Once commercialized, the point-of-care test is expected to be performed quickly in a physician’s office or in a clinic.
“The same reader can be applied to a variety of infectious diseases or infection panels by developing new cartridges,” Dr. Soleymani explained. “Noroviruses and bacteria such as C. difficile are on our list” to examine next.What’s more, she added, “this test is ideally positioned for settings where access to centralized labs is not possible, such as less developed countries.”
The team’s recent studies seem to support the promise. A study published last year in the international edition of Angewandte Chemie documents the development of the test, which at that point could detect wild-type SARS-CoV-2 and its Alpha and Delta variants in unprocessed saliva samples in 10 minutes with 80.5% sensitivity and 100% specificity.
This study was followed in January 2022 by a paper in Chemistry showing that the device also detected Alpha, Gamma, Epsilon, Kappa, and Omicron variants, demonstrating its potential for recognizing rapidly evolving targets such as those found in SARS-CoV-2.
In another demonstration of its versatility, the technology was recently adapted and successfully detected animal viruses from saliva samples.
Commercial and government funding
The findings prompted Zentek, an intellectual property development and commercialization company in Guelph, Ont., to license the technology, with plans to invest more than $1 million in the next 5 years to scale up production of the test components and adapt the technology for other forms of infection.
Furthermore, the collaborative efforts required to develop the test and move it forward gained funding from Canada’s Natural Sciences and Engineering Research Council, which is investing nearly $1.5 million in the form of two grants: $1 million to further streamline the technology development in preparation for the next pandemic and $488,440 (including $140,000 from Zentek) to get the current test to market as quickly as possible.
Meanwhile, Dr. Soleymani is urging clinicians “to be open to nontraditional diagnostic approaches even if the traditional tests do the job. Such tests are more rapid and can be used to enable personalized medicine. Our success relies on collaboration and support from clinicians.”
Further validation needed
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and the Harriet Ryan Albee Professor of Medicine at Harvard Medical School, Boston, commented on the study in response to a request from this news organization.
While “it’s always good to have more testing options available,” he said, “we don’t yet have very much information about performance characteristics of the test – that is, its sensitivity and specificity. I’d like to see the performance characteristics of this test compared to PCR tests and to the current rapid antigen tests using a large number of patient samples with currently circulating variants, and tests over time to see how soon tests become positive after symptom onset and for how long they remain positive.”
“Further validation studies and emergency use authorization or approval by regulatory authorities are needed before we will see this test implemented in the field,” Dr. Kuritzkes concluded.
A version of this article first appeared on Medscape.com.
A rapid, saliva-based test for COVID-19 could enable testing, diagnosis, and prescribing to take place in a single office visit by immediately confirming whether a patient has the infection and needs to be treated, researchers say. The test has sparked commercial interest and earned additional funding from the Canadian government.
The test uses a DNA aptamer – a short, synthetic oligonucleotide that binds to a specific molecular target – that shows high affinity for the SARS-CoV-2 spike protein and its variants. The approach “can be rapidly adapted to different threats,” as well, Leyla Soleymani, PhD, an associate professor of engineering physics at McMaster University, Hamilton, Ontario, Canada, told this news organization. Her team invented the approach.
Adaptable to other pathogens
Current gold-standard COVID-19 tests are based on reverse transcription-polymerase chain reaction (RT-PCR), which are sensitive but costly, complicated, and require waiting at least a couple of days for results, according to Dr. Soleymani and colleagues. Rapid nucleic acid and antigen tests have only “moderate” sensitivity and specificity, particularly when viral loads are low. None have been shown to work well with saliva samples.
By contrast, the new test “uses a reader and test cartridges, similar to the glucose reader,” said Dr. Soleymani, who is also Canada Research chair in Miniaturized Biomedical Devices. A small sample of saliva is added to a chemical reagent and inserted into the reader, which is attached to a smartphone. Once commercialized, the point-of-care test is expected to be performed quickly in a physician’s office or in a clinic.
“The same reader can be applied to a variety of infectious diseases or infection panels by developing new cartridges,” Dr. Soleymani explained. “Noroviruses and bacteria such as C. difficile are on our list” to examine next.What’s more, she added, “this test is ideally positioned for settings where access to centralized labs is not possible, such as less developed countries.”
The team’s recent studies seem to support the promise. A study published last year in the international edition of Angewandte Chemie documents the development of the test, which at that point could detect wild-type SARS-CoV-2 and its Alpha and Delta variants in unprocessed saliva samples in 10 minutes with 80.5% sensitivity and 100% specificity.
This study was followed in January 2022 by a paper in Chemistry showing that the device also detected Alpha, Gamma, Epsilon, Kappa, and Omicron variants, demonstrating its potential for recognizing rapidly evolving targets such as those found in SARS-CoV-2.
In another demonstration of its versatility, the technology was recently adapted and successfully detected animal viruses from saliva samples.
Commercial and government funding
The findings prompted Zentek, an intellectual property development and commercialization company in Guelph, Ont., to license the technology, with plans to invest more than $1 million in the next 5 years to scale up production of the test components and adapt the technology for other forms of infection.
Furthermore, the collaborative efforts required to develop the test and move it forward gained funding from Canada’s Natural Sciences and Engineering Research Council, which is investing nearly $1.5 million in the form of two grants: $1 million to further streamline the technology development in preparation for the next pandemic and $488,440 (including $140,000 from Zentek) to get the current test to market as quickly as possible.
Meanwhile, Dr. Soleymani is urging clinicians “to be open to nontraditional diagnostic approaches even if the traditional tests do the job. Such tests are more rapid and can be used to enable personalized medicine. Our success relies on collaboration and support from clinicians.”
Further validation needed
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and the Harriet Ryan Albee Professor of Medicine at Harvard Medical School, Boston, commented on the study in response to a request from this news organization.
While “it’s always good to have more testing options available,” he said, “we don’t yet have very much information about performance characteristics of the test – that is, its sensitivity and specificity. I’d like to see the performance characteristics of this test compared to PCR tests and to the current rapid antigen tests using a large number of patient samples with currently circulating variants, and tests over time to see how soon tests become positive after symptom onset and for how long they remain positive.”
“Further validation studies and emergency use authorization or approval by regulatory authorities are needed before we will see this test implemented in the field,” Dr. Kuritzkes concluded.
A version of this article first appeared on Medscape.com.
A rapid, saliva-based test for COVID-19 could enable testing, diagnosis, and prescribing to take place in a single office visit by immediately confirming whether a patient has the infection and needs to be treated, researchers say. The test has sparked commercial interest and earned additional funding from the Canadian government.
The test uses a DNA aptamer – a short, synthetic oligonucleotide that binds to a specific molecular target – that shows high affinity for the SARS-CoV-2 spike protein and its variants. The approach “can be rapidly adapted to different threats,” as well, Leyla Soleymani, PhD, an associate professor of engineering physics at McMaster University, Hamilton, Ontario, Canada, told this news organization. Her team invented the approach.
Adaptable to other pathogens
Current gold-standard COVID-19 tests are based on reverse transcription-polymerase chain reaction (RT-PCR), which are sensitive but costly, complicated, and require waiting at least a couple of days for results, according to Dr. Soleymani and colleagues. Rapid nucleic acid and antigen tests have only “moderate” sensitivity and specificity, particularly when viral loads are low. None have been shown to work well with saliva samples.
By contrast, the new test “uses a reader and test cartridges, similar to the glucose reader,” said Dr. Soleymani, who is also Canada Research chair in Miniaturized Biomedical Devices. A small sample of saliva is added to a chemical reagent and inserted into the reader, which is attached to a smartphone. Once commercialized, the point-of-care test is expected to be performed quickly in a physician’s office or in a clinic.
“The same reader can be applied to a variety of infectious diseases or infection panels by developing new cartridges,” Dr. Soleymani explained. “Noroviruses and bacteria such as C. difficile are on our list” to examine next.What’s more, she added, “this test is ideally positioned for settings where access to centralized labs is not possible, such as less developed countries.”
The team’s recent studies seem to support the promise. A study published last year in the international edition of Angewandte Chemie documents the development of the test, which at that point could detect wild-type SARS-CoV-2 and its Alpha and Delta variants in unprocessed saliva samples in 10 minutes with 80.5% sensitivity and 100% specificity.
This study was followed in January 2022 by a paper in Chemistry showing that the device also detected Alpha, Gamma, Epsilon, Kappa, and Omicron variants, demonstrating its potential for recognizing rapidly evolving targets such as those found in SARS-CoV-2.
In another demonstration of its versatility, the technology was recently adapted and successfully detected animal viruses from saliva samples.
Commercial and government funding
The findings prompted Zentek, an intellectual property development and commercialization company in Guelph, Ont., to license the technology, with plans to invest more than $1 million in the next 5 years to scale up production of the test components and adapt the technology for other forms of infection.
Furthermore, the collaborative efforts required to develop the test and move it forward gained funding from Canada’s Natural Sciences and Engineering Research Council, which is investing nearly $1.5 million in the form of two grants: $1 million to further streamline the technology development in preparation for the next pandemic and $488,440 (including $140,000 from Zentek) to get the current test to market as quickly as possible.
Meanwhile, Dr. Soleymani is urging clinicians “to be open to nontraditional diagnostic approaches even if the traditional tests do the job. Such tests are more rapid and can be used to enable personalized medicine. Our success relies on collaboration and support from clinicians.”
Further validation needed
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and the Harriet Ryan Albee Professor of Medicine at Harvard Medical School, Boston, commented on the study in response to a request from this news organization.
While “it’s always good to have more testing options available,” he said, “we don’t yet have very much information about performance characteristics of the test – that is, its sensitivity and specificity. I’d like to see the performance characteristics of this test compared to PCR tests and to the current rapid antigen tests using a large number of patient samples with currently circulating variants, and tests over time to see how soon tests become positive after symptom onset and for how long they remain positive.”
“Further validation studies and emergency use authorization or approval by regulatory authorities are needed before we will see this test implemented in the field,” Dr. Kuritzkes concluded.
A version of this article first appeared on Medscape.com.
What are the signs of post–acute infection syndromes?
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
The long-term health consequences of COVID-19 have refocused our attention on post–acute infection syndromes (PAIS), starting a discussion on the need for a complete understanding of multisystemic pathophysiology, clinical indicators, and the epidemiology of these syndromes, representing a significant blind spot in the field of medicine. A better understanding of these persistent symptom profiles, not only for post-acute sequelae of SARS-CoV-2 infection (PASC), better known as long COVID, but also for other diseases with unexplainable post-acute sequelae, would allow doctors to fine tune the diagnostic criteria. Having a clear definition and better understanding of post–acute infection symptoms is a necessary step toward developing an evidence-based, multidisciplinary management approach.
PAIS, PASC, or long COVID
The observation of unexplained chronic sequelae after SARS-CoV-2 is known as PASC or long COVID.
Long COVID has been reported as a syndrome in survivors of serious and critical disease, but the effects also persist over time for subjects who experienced a mild infection that did not require admission to hospital. This means that PASC, especially when occurring after a mild or moderate COVID-19 infection, shares many of the same characteristics as chronic diseases triggered by other pathogenic organisms, many of which have not been sufficiently clarified.
PAIS are characterized by a set of core symptoms centering on the following:
- Exertion intolerance
- Disproportionate levels of fatigue
- Neurocognitive and sensory impairment
- Flu-like symptoms
- Unrefreshing sleep
- Myalgia/arthralgia
A plethora of nonspecific symptoms are often present to various degrees.
These similarities suggest a unifying pathophysiology that needs to be elucidated to properly understand and manage postinfectious chronic disability.
Overview of PAIS
A detailed revision on what is currently known about PAIS was published in Nature Medicine. It provided various useful pieces of information to assist with the poor recognition of these conditions in clinical practice, a result of which is that patients might experience delayed or a complete lack of clinical care.
The following consolidated postinfection sequelae are mentioned:
- Q fever fatigue syndrome, which follows infection by the intracellular bacterium Coxiella burnetii
- Post-dengue fatigue syndrome, which can follow infection by the mosquito-borne dengue virus
- Fatiguing and rheumatic symptoms in a subset of individuals infected with chikungunya virus, a mosquito-borne virus that causes fever and joint pain in the acute phase
- Post-polio syndrome, which can emerge as many as 15-40 years after an initial poliomyelitis attack (similarly, some other neurotropic microbes, such as West Nile virus, might lead to persistent effects)
- Prolonged, debilitating, chronic symptoms have long been reported in a subset of patients after common and typically nonserious infections. For example, after mononucleosis, a condition generally caused by Epstein-Barr virus (EBV), and after an outbreak of Giardia lamblia, an intestinal parasite that usually causes acute intestinal illness. In fact, several studies identified the association of this outbreak of giardiasis with chronic fatigue, irritable bowel syndrome (IBS), and fibromyalgia persisting for many years.
- Views expressed in the literature regarding the frequency and the validity of posttreatment Lyme disease syndrome are divided. Although substantial evidence points to persistence of arthralgia, fatigue, and subjective neurocognitive impairments in a minority of patients with Lyme disease after the recommended antibiotic treatment, some of the early studies have failed to characterize the initial Lyme disease episode with sufficient rigor.
Symptoms and signs
The symptoms and signs which, based on the evidence available, are seen more frequently in health care checks may be characterized as the following:
- Exertion intolerance, fatigue
- Flu-like and ‘sickness behavior’ symptoms: fever, feverishness, muscle pain, feeling sick, malaise, sweating, irritability
- Neurological/neurocognitive symptoms: brain fog, impaired concentration or memory, trouble finding words
- Rheumatologic symptoms: chronic or recurrent joint pain
- Trigger-specific symptoms: for example, eye problems post Ebola, IBS post Giardia, anosmia and ageusia post COVID-19, motor disturbances post polio and post West Nile virus
Myalgic encephalomyelitis/chronic fatigue syndrome
Patients with this disorder experience worsening of symptoms following physical, cognitive, or emotional exertion above their (very low) tolerated limit. Other prominent features frequently observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) are neurocognitive impairments (colloquially referred to as brain fog), unrefreshing sleep, pain, sensory disturbances, gastrointestinal issues, and various forms of dysautonomia. Up to 75% of ME/CFS cases report an infection-like episode preceding the onset of their illness. Postinfectious and postviral fatigue syndromes were originally postulated as subsets of chronic fatigue syndrome. However, there appears to be no clear consensus at present about whether these terms should be considered synonymous to the ME/CFS label or any of its subsets, or include a wider range of postinfectious fatigue conditions.
Practical diagnostic criteria
From a revision of the available criteria, it emerges that the diagnostic criteria for a PAIS should include not only the presence of symptoms, but ideally also the intensity, course, and constellation of symptoms within an individual, as the individual symptoms and symptom trajectories of PAIS vary over time, rendering a mere comparison of symptom presence at a single time point misleading. Furthermore, when a diagnosis of ME/CFS is made, attention should be given to the choice of diagnostic criteria, with preference given to the more conservative criteria, so as not to run the risk of overestimating the syndrome.
Asthenia is the cornerstone symptom for most epidemiological studies on PAIS, but it would be reductive to concentrate only on this rather than the other characteristics, such as the exacerbation of symptoms following exertion, together with other characteristic symptoms and signs that may allow for better identification of the overall, observable clinical picture in these postinfection syndromes, which have significant impacts on a patient’s quality of life.
This article was translated from Univadis Italy. A version of this article appeared on Medscape.com.
HPV vaccination with Cervarix ‘unmasks’ cervical lesions from non-vax strains
Vaccines against human papillomavirus have been hailed as a success: they have been shown to decrease the incidence of cervical lesions associated with the HPV types that are in the vaccine.
However,
An expert not involved in the research said the new data “tell us to be a little bit careful.” Although the HPV types not included in the vaccine are rarer and less aggressive, they can still cause cancer.
The data come from the Costa Rica HPV Vaccine Trial, which involved more than 10,000 women aged 18-25 years. The HPV vaccine used in the trial was Cervarix, from GlaxoSmithKline. It covers the two leading causes of cervical cancer, HPV-16 and -18, and provides partial protection against three other genotypes.
After a follow-up of 11 years, among vaccinated women, there was an excess of precancerous cervical lesions caused by genotypes not included in the vaccine, resulting in negative vaccine efficacy for those HPV variants.
The increase wasn’t enough to offset the overall benefit of vaccination when all genotypes were considered, said the researchers, led by Jaimie Shing, PhD, a postdoctoral research fellow at the National Cancer Institute in Bethesda, Md.
Vaccinated women “still had long-term absolute reductions in high-grade lesions,” they pointed out.
The net protection “remained considerable, emphasizing the importance of HPV vaccination for cervical cancer prevention,” the team concluded.
The findings were published online in The Lancet Oncology.
The results are likely the first evidence to date of “clinical unmasking” with HPV vaccination, meaning that protection against the strains covered by the vaccine leaves women more prone to attack from other carcinogenic HPV variants.
This phenomenon “could attenuate long-term reductions in high-grade disease following successful implementation of HPV vaccination programs,” the investigators commented.
Highlighting a need for caution
The take-home message from the trial is that “we have to be careful,” said Marc Steben, MD, co-President of HPV Global Action and a professor at the University of Montreal.
He noted that the Cervarix HPV vaccine used in the trial is not the vaccine that is used now in developed nations.
The current standard HPV vaccine is Gardasil 9 (Merck), which offers broader coverage against nine HPV types (types 6, 11, 16, 18, 31, 33, 45, 52, and 58).
There are 12 main carcinogenic HPV genotypes, so unmasking of other strains is still possible with Gardasil 9, he said.
There is another issue, Dr. Steben added. The success of HPV vaccinations - a nearly 90% reduction in invasive cervical cancer in women who are vaccinated at a young age – has led to questions about the future role of routine cervical cancer screening.
“Some people are saying that if we achieve 90% coverage, we might” eliminate community transmission and no longer need to screen, he said.
These trial results “tell us to be a little bit careful,” Dr. Steben continued. Those HPV types that are less aggressive and rarer than HPV-16 and -18 “can still cause cancer and might be there and surprise us. It could take more time than we thought” to get to the point where screening can be eliminated.
“There might be a little problem if we stop too early,” he said.
Study details
During the period 2004-2005, the investigators randomly assigned 3,727 women aged 18-25 years to receive Cervarix and 3,739 to a control group that received the hepatitis A vaccine; after 4 years, the control group also received Cervarix and exited the study. They were replaced by an unvaccinated control group of 2,836 women. The new control group and the original HPV vaccine group were followed for an additional 7 years.
In years 7-11 of the trial, the investigators found 9.2 additional cervical intraepithelial neoplasias of grade 2 or worse (CIN2+) from HPV types not covered by Cervarix per 1,000 vaccinated women in comparison with unvaccinated participants. This corresponds to –71.2% negative vaccine efficacy against CIN2+ lesions of HPV types not covered by the vaccine.
There were 8.3 additional CIN3+ lesions from nontargeted HPV strains per 1,000 vaccinated women in comparison with unvaccinated participants, which corresponds to –135% negative vaccine efficacy.
Overall, however, there was a net benefit of vaccination, with 27 fewer CIN2+ lesions when all HPV genotypes – vaccine covered or not – were considered per 1,000 vaccinated women over the entire 11 years of follow-up.
There were also 8.7 fewer CIN3+ lesions across all genotypes per 1,000 vaccinated women, but the benefit was not statistically significant.
Among the study limits, the team was unable to evaluate the effect of clinical unmasking on cervical cancer, because women were treated for high-grade cervical lesions before cases could progress to cervical cancer.
The trial was funded by the National Cancer Institute and the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline provided the Cervarix vaccine and supported aspects of the trial. Two authors are named inventors on U.S. government–owned HPV vaccine patents with expired licenses to GlaxoSmithKline and Merck. Dr. Steben is an adviser/speaker for many companies, including GlaxoSmithKline and Merck.
A version of this article first appeared on Medscape.com.
Vaccines against human papillomavirus have been hailed as a success: they have been shown to decrease the incidence of cervical lesions associated with the HPV types that are in the vaccine.
However,
An expert not involved in the research said the new data “tell us to be a little bit careful.” Although the HPV types not included in the vaccine are rarer and less aggressive, they can still cause cancer.
The data come from the Costa Rica HPV Vaccine Trial, which involved more than 10,000 women aged 18-25 years. The HPV vaccine used in the trial was Cervarix, from GlaxoSmithKline. It covers the two leading causes of cervical cancer, HPV-16 and -18, and provides partial protection against three other genotypes.
After a follow-up of 11 years, among vaccinated women, there was an excess of precancerous cervical lesions caused by genotypes not included in the vaccine, resulting in negative vaccine efficacy for those HPV variants.
The increase wasn’t enough to offset the overall benefit of vaccination when all genotypes were considered, said the researchers, led by Jaimie Shing, PhD, a postdoctoral research fellow at the National Cancer Institute in Bethesda, Md.
Vaccinated women “still had long-term absolute reductions in high-grade lesions,” they pointed out.
The net protection “remained considerable, emphasizing the importance of HPV vaccination for cervical cancer prevention,” the team concluded.
The findings were published online in The Lancet Oncology.
The results are likely the first evidence to date of “clinical unmasking” with HPV vaccination, meaning that protection against the strains covered by the vaccine leaves women more prone to attack from other carcinogenic HPV variants.
This phenomenon “could attenuate long-term reductions in high-grade disease following successful implementation of HPV vaccination programs,” the investigators commented.
Highlighting a need for caution
The take-home message from the trial is that “we have to be careful,” said Marc Steben, MD, co-President of HPV Global Action and a professor at the University of Montreal.
He noted that the Cervarix HPV vaccine used in the trial is not the vaccine that is used now in developed nations.
The current standard HPV vaccine is Gardasil 9 (Merck), which offers broader coverage against nine HPV types (types 6, 11, 16, 18, 31, 33, 45, 52, and 58).
There are 12 main carcinogenic HPV genotypes, so unmasking of other strains is still possible with Gardasil 9, he said.
There is another issue, Dr. Steben added. The success of HPV vaccinations - a nearly 90% reduction in invasive cervical cancer in women who are vaccinated at a young age – has led to questions about the future role of routine cervical cancer screening.
“Some people are saying that if we achieve 90% coverage, we might” eliminate community transmission and no longer need to screen, he said.
These trial results “tell us to be a little bit careful,” Dr. Steben continued. Those HPV types that are less aggressive and rarer than HPV-16 and -18 “can still cause cancer and might be there and surprise us. It could take more time than we thought” to get to the point where screening can be eliminated.
“There might be a little problem if we stop too early,” he said.
Study details
During the period 2004-2005, the investigators randomly assigned 3,727 women aged 18-25 years to receive Cervarix and 3,739 to a control group that received the hepatitis A vaccine; after 4 years, the control group also received Cervarix and exited the study. They were replaced by an unvaccinated control group of 2,836 women. The new control group and the original HPV vaccine group were followed for an additional 7 years.
In years 7-11 of the trial, the investigators found 9.2 additional cervical intraepithelial neoplasias of grade 2 or worse (CIN2+) from HPV types not covered by Cervarix per 1,000 vaccinated women in comparison with unvaccinated participants. This corresponds to –71.2% negative vaccine efficacy against CIN2+ lesions of HPV types not covered by the vaccine.
There were 8.3 additional CIN3+ lesions from nontargeted HPV strains per 1,000 vaccinated women in comparison with unvaccinated participants, which corresponds to –135% negative vaccine efficacy.
Overall, however, there was a net benefit of vaccination, with 27 fewer CIN2+ lesions when all HPV genotypes – vaccine covered or not – were considered per 1,000 vaccinated women over the entire 11 years of follow-up.
There were also 8.7 fewer CIN3+ lesions across all genotypes per 1,000 vaccinated women, but the benefit was not statistically significant.
Among the study limits, the team was unable to evaluate the effect of clinical unmasking on cervical cancer, because women were treated for high-grade cervical lesions before cases could progress to cervical cancer.
The trial was funded by the National Cancer Institute and the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline provided the Cervarix vaccine and supported aspects of the trial. Two authors are named inventors on U.S. government–owned HPV vaccine patents with expired licenses to GlaxoSmithKline and Merck. Dr. Steben is an adviser/speaker for many companies, including GlaxoSmithKline and Merck.
A version of this article first appeared on Medscape.com.
Vaccines against human papillomavirus have been hailed as a success: they have been shown to decrease the incidence of cervical lesions associated with the HPV types that are in the vaccine.
However,
An expert not involved in the research said the new data “tell us to be a little bit careful.” Although the HPV types not included in the vaccine are rarer and less aggressive, they can still cause cancer.
The data come from the Costa Rica HPV Vaccine Trial, which involved more than 10,000 women aged 18-25 years. The HPV vaccine used in the trial was Cervarix, from GlaxoSmithKline. It covers the two leading causes of cervical cancer, HPV-16 and -18, and provides partial protection against three other genotypes.
After a follow-up of 11 years, among vaccinated women, there was an excess of precancerous cervical lesions caused by genotypes not included in the vaccine, resulting in negative vaccine efficacy for those HPV variants.
The increase wasn’t enough to offset the overall benefit of vaccination when all genotypes were considered, said the researchers, led by Jaimie Shing, PhD, a postdoctoral research fellow at the National Cancer Institute in Bethesda, Md.
Vaccinated women “still had long-term absolute reductions in high-grade lesions,” they pointed out.
The net protection “remained considerable, emphasizing the importance of HPV vaccination for cervical cancer prevention,” the team concluded.
The findings were published online in The Lancet Oncology.
The results are likely the first evidence to date of “clinical unmasking” with HPV vaccination, meaning that protection against the strains covered by the vaccine leaves women more prone to attack from other carcinogenic HPV variants.
This phenomenon “could attenuate long-term reductions in high-grade disease following successful implementation of HPV vaccination programs,” the investigators commented.
Highlighting a need for caution
The take-home message from the trial is that “we have to be careful,” said Marc Steben, MD, co-President of HPV Global Action and a professor at the University of Montreal.
He noted that the Cervarix HPV vaccine used in the trial is not the vaccine that is used now in developed nations.
The current standard HPV vaccine is Gardasil 9 (Merck), which offers broader coverage against nine HPV types (types 6, 11, 16, 18, 31, 33, 45, 52, and 58).
There are 12 main carcinogenic HPV genotypes, so unmasking of other strains is still possible with Gardasil 9, he said.
There is another issue, Dr. Steben added. The success of HPV vaccinations - a nearly 90% reduction in invasive cervical cancer in women who are vaccinated at a young age – has led to questions about the future role of routine cervical cancer screening.
“Some people are saying that if we achieve 90% coverage, we might” eliminate community transmission and no longer need to screen, he said.
These trial results “tell us to be a little bit careful,” Dr. Steben continued. Those HPV types that are less aggressive and rarer than HPV-16 and -18 “can still cause cancer and might be there and surprise us. It could take more time than we thought” to get to the point where screening can be eliminated.
“There might be a little problem if we stop too early,” he said.
Study details
During the period 2004-2005, the investigators randomly assigned 3,727 women aged 18-25 years to receive Cervarix and 3,739 to a control group that received the hepatitis A vaccine; after 4 years, the control group also received Cervarix and exited the study. They were replaced by an unvaccinated control group of 2,836 women. The new control group and the original HPV vaccine group were followed for an additional 7 years.
In years 7-11 of the trial, the investigators found 9.2 additional cervical intraepithelial neoplasias of grade 2 or worse (CIN2+) from HPV types not covered by Cervarix per 1,000 vaccinated women in comparison with unvaccinated participants. This corresponds to –71.2% negative vaccine efficacy against CIN2+ lesions of HPV types not covered by the vaccine.
There were 8.3 additional CIN3+ lesions from nontargeted HPV strains per 1,000 vaccinated women in comparison with unvaccinated participants, which corresponds to –135% negative vaccine efficacy.
Overall, however, there was a net benefit of vaccination, with 27 fewer CIN2+ lesions when all HPV genotypes – vaccine covered or not – were considered per 1,000 vaccinated women over the entire 11 years of follow-up.
There were also 8.7 fewer CIN3+ lesions across all genotypes per 1,000 vaccinated women, but the benefit was not statistically significant.
Among the study limits, the team was unable to evaluate the effect of clinical unmasking on cervical cancer, because women were treated for high-grade cervical lesions before cases could progress to cervical cancer.
The trial was funded by the National Cancer Institute and the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline provided the Cervarix vaccine and supported aspects of the trial. Two authors are named inventors on U.S. government–owned HPV vaccine patents with expired licenses to GlaxoSmithKline and Merck. Dr. Steben is an adviser/speaker for many companies, including GlaxoSmithKline and Merck.
A version of this article first appeared on Medscape.com.
FROM THE LANCET ONCOLOGY
Pandemic public health measures may have mitigated Kawasaki disease
The social behavior associated with the COVID-19 pandemic may have reduced the incidence of Kawasaki disease, according to results of a cohort study of nearly 4,000 children.
The incidence of Kawasaki disease in the United States declined by 28.2% between 2018 and 2020, possibly as a result of factors including school closures, mask mandates, and reduced ambient pollution that might reduce exposure to Kawasaki disease (KD) in the environment, but a potential association has not been explored, wrote Jennifer A. Burney, PhD, of the University of California, San Diego, and colleagues.
KD received greater attention in the public and medical communities because of the emergence of multisystem inflammatory syndrome in children (MIS-C), which is similar to, but distinct from, KD, and because of the noticeable drop in KD cases during the pandemic, the researchers said.
In a multicenter cohort study published in JAMA Network Open , the researchers reviewed data from 2,461 consecutive patients with KD who were diagnosed between Jan. 1, 2018, and Dec. 31, 2020. They conducted a detailed analysis of analysis of 1,461 children with KD who were diagnosed between Jan. 1, 2002, and Nov. 15, 2021, at Rady Children’s Hospital San Diego (RCHSD), using data from before, during, and after the height of the pandemic. The median age of the children in the RCHSD analysis was 2.8 years, 62% were male, and 35% were Hispanic.
Overall, the prevalence of KD declined from 894 in 2018 to 646 in 2020, across the United States, but the decline was uneven, the researchers noted.
In the RCHSD cohort in San Diego, KD cases in children aged 1-5 years decreased significantly from 2020 to 2021 compared to the mean number of cases in previous years (22 vs. 44.9, P = .02). KD cases also decreased significantly among males and Asian children.
Notably, the occurrence of the KD clinical features of strawberry tongue, enlarged cervical lymph node, and subacute periungual desquamation decreased during 2020 compared with the baseline period, although only strawberry tongue reached statistical significance (39% vs. 63%, P = .04). The prevalence of patients with an enlarged lymph node was 21% in 2020 vs. 32% prior to the pandemic (P = .09); the prevalence of periungual desquamation during these periods was 47% vs. 58%, P = .16).
The researchers also used data from Census Block Groups (CBGs) to assess the impact of mobility metrics and environmental exposures on KD during the pandemic for the San Diego patient cohort. They found that KD cases during the pandemic were more likely to occur in neighborhoods of higher socioeconomic status, and that neighborhoods with lower levels of nitrous oxides had fewer KD cases.
Overall, “The reduction in KD case numbers coincided with masking, school closures, reduced circulation of respiratory viruses, and reduced air pollution,” the researchers wrote in their discussion of the findings. “A rebound in KD case numbers to prepandemic levels coincided with the lifting of mask mandates and, subsequently, the return to in-person schooling,” they wrote.
The study findings were limited by several factors including the small sample sizes, which also limit the interpretation of mobility and pollution data, the researchers noted. Other limitations include the high interannual variability of KD and the inclusion of 2021 rebound data from the San Diego region only.
“Although our original hypothesis was that shelter-in-place measures would track with reduced KD cases, this was not borne out by the San Diego region data. Instead, the San Diego case occurrence data suggest that exposures that triggered KD were more likely to occur in the home, with a shift toward households with higher SES during the pandemic,” the researchers noted. However, “The results presented here are consistent with a respiratory portal of entry for the trigger(s) of KD,” they said.
Study fails to validate its conclusions
“This study attempts to test the hypothesis that various social restrictions were associated with a decrease in rate of diagnosed Kawasaki disease cases during portions of the SARS-CoV-2 pandemic,” Mark Gorelik, MD, assistant professor of pediatrics at Columbia University, New York, said in an interview.
“However, it appears that it fails to achieve this conclusion and I disagree with the findings,” said Dr. Gorelik, who was not involved in the study but served as first author on an updated Kawasaki disease treatment guideline published earlier this spring in Arthritis & Rheumatology.
“The study does not find statistically significant associations either with shelter in place orders or with cell phone mobility data, as stated in the conclusion, directly contradicting its own claim,” Dr. Gorelik said. “Secondly, the study makes an assumption that various methods, especially the wearing of masks by children and school closures, had a significant effect on the spread of respiratory viruses. There are no prospective, population based, controlled real world studies that validate this claim, and two prospective controlled real-world studies that dispute this,” he emphasized. “Cloth masks and surgical masks, which were the types of masks worn by school students, are also known to have a nonsignificant and paltry – in the latter, certainly less than 50%, and perhaps as little as 10% – effect on the reduction of respiratory viral spread,” he added.
“Mechanistic studies on mask wearing may suggest some mask efficacy, but these studies are as valid as mechanistic studies showing the effect of various antifungal pharmaceuticals on the replication of SARS-CoV-2 virus in culture, meaning only valid as hypothesis generating, and ultimately the latter hypothesis failed to bear out,” Dr. Gorelik explained. “We do not know the reason why other respiratory viruses and non-SARS-CoV-2 coronaviruses declined during the pandemic, but we do know that despite this, the SARS-CoV-2 coronavirus itself did not appear to suffer the same fate. Thus, it is very possible that another factor was at work, and we know that during other viral pandemics, typically circulating viruses decline, potentially due to induction of interferon responses in hosts, in a general effect known as ‘viral interference,’ ” he said.
“Overall, we must have robust evidence to support benefits of hypotheses that have demonstrated clear damage to children during this pandemic (such as school closures), and this study fails to live up to that requirement,” Dr. Gorelik said.
The study was supported by the Gordon and Marilyn Macklin Foundation and the Patient-Centered Outcomes Research Institute. Dr. Burney and Dr. Gorelik had no financial conflicts to disclose.
The social behavior associated with the COVID-19 pandemic may have reduced the incidence of Kawasaki disease, according to results of a cohort study of nearly 4,000 children.
The incidence of Kawasaki disease in the United States declined by 28.2% between 2018 and 2020, possibly as a result of factors including school closures, mask mandates, and reduced ambient pollution that might reduce exposure to Kawasaki disease (KD) in the environment, but a potential association has not been explored, wrote Jennifer A. Burney, PhD, of the University of California, San Diego, and colleagues.
KD received greater attention in the public and medical communities because of the emergence of multisystem inflammatory syndrome in children (MIS-C), which is similar to, but distinct from, KD, and because of the noticeable drop in KD cases during the pandemic, the researchers said.
In a multicenter cohort study published in JAMA Network Open , the researchers reviewed data from 2,461 consecutive patients with KD who were diagnosed between Jan. 1, 2018, and Dec. 31, 2020. They conducted a detailed analysis of analysis of 1,461 children with KD who were diagnosed between Jan. 1, 2002, and Nov. 15, 2021, at Rady Children’s Hospital San Diego (RCHSD), using data from before, during, and after the height of the pandemic. The median age of the children in the RCHSD analysis was 2.8 years, 62% were male, and 35% were Hispanic.
Overall, the prevalence of KD declined from 894 in 2018 to 646 in 2020, across the United States, but the decline was uneven, the researchers noted.
In the RCHSD cohort in San Diego, KD cases in children aged 1-5 years decreased significantly from 2020 to 2021 compared to the mean number of cases in previous years (22 vs. 44.9, P = .02). KD cases also decreased significantly among males and Asian children.
Notably, the occurrence of the KD clinical features of strawberry tongue, enlarged cervical lymph node, and subacute periungual desquamation decreased during 2020 compared with the baseline period, although only strawberry tongue reached statistical significance (39% vs. 63%, P = .04). The prevalence of patients with an enlarged lymph node was 21% in 2020 vs. 32% prior to the pandemic (P = .09); the prevalence of periungual desquamation during these periods was 47% vs. 58%, P = .16).
The researchers also used data from Census Block Groups (CBGs) to assess the impact of mobility metrics and environmental exposures on KD during the pandemic for the San Diego patient cohort. They found that KD cases during the pandemic were more likely to occur in neighborhoods of higher socioeconomic status, and that neighborhoods with lower levels of nitrous oxides had fewer KD cases.
Overall, “The reduction in KD case numbers coincided with masking, school closures, reduced circulation of respiratory viruses, and reduced air pollution,” the researchers wrote in their discussion of the findings. “A rebound in KD case numbers to prepandemic levels coincided with the lifting of mask mandates and, subsequently, the return to in-person schooling,” they wrote.
The study findings were limited by several factors including the small sample sizes, which also limit the interpretation of mobility and pollution data, the researchers noted. Other limitations include the high interannual variability of KD and the inclusion of 2021 rebound data from the San Diego region only.
“Although our original hypothesis was that shelter-in-place measures would track with reduced KD cases, this was not borne out by the San Diego region data. Instead, the San Diego case occurrence data suggest that exposures that triggered KD were more likely to occur in the home, with a shift toward households with higher SES during the pandemic,” the researchers noted. However, “The results presented here are consistent with a respiratory portal of entry for the trigger(s) of KD,” they said.
Study fails to validate its conclusions
“This study attempts to test the hypothesis that various social restrictions were associated with a decrease in rate of diagnosed Kawasaki disease cases during portions of the SARS-CoV-2 pandemic,” Mark Gorelik, MD, assistant professor of pediatrics at Columbia University, New York, said in an interview.
“However, it appears that it fails to achieve this conclusion and I disagree with the findings,” said Dr. Gorelik, who was not involved in the study but served as first author on an updated Kawasaki disease treatment guideline published earlier this spring in Arthritis & Rheumatology.
“The study does not find statistically significant associations either with shelter in place orders or with cell phone mobility data, as stated in the conclusion, directly contradicting its own claim,” Dr. Gorelik said. “Secondly, the study makes an assumption that various methods, especially the wearing of masks by children and school closures, had a significant effect on the spread of respiratory viruses. There are no prospective, population based, controlled real world studies that validate this claim, and two prospective controlled real-world studies that dispute this,” he emphasized. “Cloth masks and surgical masks, which were the types of masks worn by school students, are also known to have a nonsignificant and paltry – in the latter, certainly less than 50%, and perhaps as little as 10% – effect on the reduction of respiratory viral spread,” he added.
“Mechanistic studies on mask wearing may suggest some mask efficacy, but these studies are as valid as mechanistic studies showing the effect of various antifungal pharmaceuticals on the replication of SARS-CoV-2 virus in culture, meaning only valid as hypothesis generating, and ultimately the latter hypothesis failed to bear out,” Dr. Gorelik explained. “We do not know the reason why other respiratory viruses and non-SARS-CoV-2 coronaviruses declined during the pandemic, but we do know that despite this, the SARS-CoV-2 coronavirus itself did not appear to suffer the same fate. Thus, it is very possible that another factor was at work, and we know that during other viral pandemics, typically circulating viruses decline, potentially due to induction of interferon responses in hosts, in a general effect known as ‘viral interference,’ ” he said.
“Overall, we must have robust evidence to support benefits of hypotheses that have demonstrated clear damage to children during this pandemic (such as school closures), and this study fails to live up to that requirement,” Dr. Gorelik said.
The study was supported by the Gordon and Marilyn Macklin Foundation and the Patient-Centered Outcomes Research Institute. Dr. Burney and Dr. Gorelik had no financial conflicts to disclose.
The social behavior associated with the COVID-19 pandemic may have reduced the incidence of Kawasaki disease, according to results of a cohort study of nearly 4,000 children.
The incidence of Kawasaki disease in the United States declined by 28.2% between 2018 and 2020, possibly as a result of factors including school closures, mask mandates, and reduced ambient pollution that might reduce exposure to Kawasaki disease (KD) in the environment, but a potential association has not been explored, wrote Jennifer A. Burney, PhD, of the University of California, San Diego, and colleagues.
KD received greater attention in the public and medical communities because of the emergence of multisystem inflammatory syndrome in children (MIS-C), which is similar to, but distinct from, KD, and because of the noticeable drop in KD cases during the pandemic, the researchers said.
In a multicenter cohort study published in JAMA Network Open , the researchers reviewed data from 2,461 consecutive patients with KD who were diagnosed between Jan. 1, 2018, and Dec. 31, 2020. They conducted a detailed analysis of analysis of 1,461 children with KD who were diagnosed between Jan. 1, 2002, and Nov. 15, 2021, at Rady Children’s Hospital San Diego (RCHSD), using data from before, during, and after the height of the pandemic. The median age of the children in the RCHSD analysis was 2.8 years, 62% were male, and 35% were Hispanic.
Overall, the prevalence of KD declined from 894 in 2018 to 646 in 2020, across the United States, but the decline was uneven, the researchers noted.
In the RCHSD cohort in San Diego, KD cases in children aged 1-5 years decreased significantly from 2020 to 2021 compared to the mean number of cases in previous years (22 vs. 44.9, P = .02). KD cases also decreased significantly among males and Asian children.
Notably, the occurrence of the KD clinical features of strawberry tongue, enlarged cervical lymph node, and subacute periungual desquamation decreased during 2020 compared with the baseline period, although only strawberry tongue reached statistical significance (39% vs. 63%, P = .04). The prevalence of patients with an enlarged lymph node was 21% in 2020 vs. 32% prior to the pandemic (P = .09); the prevalence of periungual desquamation during these periods was 47% vs. 58%, P = .16).
The researchers also used data from Census Block Groups (CBGs) to assess the impact of mobility metrics and environmental exposures on KD during the pandemic for the San Diego patient cohort. They found that KD cases during the pandemic were more likely to occur in neighborhoods of higher socioeconomic status, and that neighborhoods with lower levels of nitrous oxides had fewer KD cases.
Overall, “The reduction in KD case numbers coincided with masking, school closures, reduced circulation of respiratory viruses, and reduced air pollution,” the researchers wrote in their discussion of the findings. “A rebound in KD case numbers to prepandemic levels coincided with the lifting of mask mandates and, subsequently, the return to in-person schooling,” they wrote.
The study findings were limited by several factors including the small sample sizes, which also limit the interpretation of mobility and pollution data, the researchers noted. Other limitations include the high interannual variability of KD and the inclusion of 2021 rebound data from the San Diego region only.
“Although our original hypothesis was that shelter-in-place measures would track with reduced KD cases, this was not borne out by the San Diego region data. Instead, the San Diego case occurrence data suggest that exposures that triggered KD were more likely to occur in the home, with a shift toward households with higher SES during the pandemic,” the researchers noted. However, “The results presented here are consistent with a respiratory portal of entry for the trigger(s) of KD,” they said.
Study fails to validate its conclusions
“This study attempts to test the hypothesis that various social restrictions were associated with a decrease in rate of diagnosed Kawasaki disease cases during portions of the SARS-CoV-2 pandemic,” Mark Gorelik, MD, assistant professor of pediatrics at Columbia University, New York, said in an interview.
“However, it appears that it fails to achieve this conclusion and I disagree with the findings,” said Dr. Gorelik, who was not involved in the study but served as first author on an updated Kawasaki disease treatment guideline published earlier this spring in Arthritis & Rheumatology.
“The study does not find statistically significant associations either with shelter in place orders or with cell phone mobility data, as stated in the conclusion, directly contradicting its own claim,” Dr. Gorelik said. “Secondly, the study makes an assumption that various methods, especially the wearing of masks by children and school closures, had a significant effect on the spread of respiratory viruses. There are no prospective, population based, controlled real world studies that validate this claim, and two prospective controlled real-world studies that dispute this,” he emphasized. “Cloth masks and surgical masks, which were the types of masks worn by school students, are also known to have a nonsignificant and paltry – in the latter, certainly less than 50%, and perhaps as little as 10% – effect on the reduction of respiratory viral spread,” he added.
“Mechanistic studies on mask wearing may suggest some mask efficacy, but these studies are as valid as mechanistic studies showing the effect of various antifungal pharmaceuticals on the replication of SARS-CoV-2 virus in culture, meaning only valid as hypothesis generating, and ultimately the latter hypothesis failed to bear out,” Dr. Gorelik explained. “We do not know the reason why other respiratory viruses and non-SARS-CoV-2 coronaviruses declined during the pandemic, but we do know that despite this, the SARS-CoV-2 coronavirus itself did not appear to suffer the same fate. Thus, it is very possible that another factor was at work, and we know that during other viral pandemics, typically circulating viruses decline, potentially due to induction of interferon responses in hosts, in a general effect known as ‘viral interference,’ ” he said.
“Overall, we must have robust evidence to support benefits of hypotheses that have demonstrated clear damage to children during this pandemic (such as school closures), and this study fails to live up to that requirement,” Dr. Gorelik said.
The study was supported by the Gordon and Marilyn Macklin Foundation and the Patient-Centered Outcomes Research Institute. Dr. Burney and Dr. Gorelik had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Precision medicine vs. antibiotic resistance
Diversity is an omnipresent element in clinical practice: in the genome, in the environment, in patients’ lifestyles and habits. Precision medicine addresses the variability of the individual to improve diagnosis and treatment. It is increasingly used in specialties such as oncology, neurology, and cardiology. A personalized approach has many objectives, including to optimize treatment, minimize the risk of adverse effects, facilitate early diagnosis, and determine predisposition to disease. Genomic technologies, such as massive sequencing techniques, and tools such as CRISPR-Cas9 are key to the future of personalized medicine.
Jesús Oteo Iglesias, MD, PhD, a specialist in microbiology and director of Spain’s National Center for Microbiology, spoke at the Spanish Association of Infectious Diseases and Clinical Microbiology’s recent conference. He discussed various precision medicine projects aimed at reinforcing the fight against antibiotic resistance.
Infectious diseases are complex because the diversity of the pathogenic microorganism combines with the patient’s own diversity, which influences the interaction between the two, said Dr. Oteo. Thus, the antibiogram and targeted antibiotic treatments (which are chosen according to the species, sensitivity to antimicrobials, type of infection, and patient characteristics) have been established applications of precision medicine for decades. However, multiple tools could further strengthen personalized medicine against multiresistant pathogens.
Therapeutic drug monitoring, in which multiple pharmacokinetic and pharmacodynamic factors are considered, is a strategy with great potential to increase the effectiveness of antibiotics and minimize toxicity. Owing to its costs and the need for trained staff, this tool would be especially indicated in the treatment of patients with more complex conditions, such as those suffering from obesity, complex infections, or infections with multiresistant bacteria, as well as those in critical condition. Multiple computer programs are available to help determine the dosage of antibiotics by estimating drug exposure and to provide recommendations. However, clinical trials are needed to assess the pros and cons of applying therapeutic monitoring for types of antibiotics other than those for which a given type is already used (for example, aminoglycosides and glycopeptides).
One technology that could help in antibiotic use optimization programs is microneedle-based biosensors, which could be implanted in the skin for real-time antibiotic monitoring. This tool “could be the first step in establishing automated antibiotic administration systems, with infusion pumps and feedback systems, like those already used in diabetes for insulin administration,” said Dr. Oteo.
Artificial intelligence could also be a valuable technology for optimization programs. “We should go a step further in the implementation of artificial intelligence through clinical decision support systems,” said Dr. Oteo. This technology would guide the administration of antimicrobials using data extracted from the electronic medical record. However, there are great challenges to overcome in creating these tools, such as the risk of entering erroneous data; the difficulty in entering complex data, such as data relevant to antibiotic resistance; and the variability at the geographic and institutional levels.
Genomics is also a tool with great potential for identifying bacteria’s degree of resistance to antibiotics by studying mutations in chromosomal and acquired genes. A proof-of-concept study evaluated the sensitivity of different Pseudomonas aeruginosa strains to several antibiotics by analyzing genome sequences associated with resistance, said Dr. Otero. The researchers found that this system was effective at predicting the sensitivity of bacteria from genomic data.
In the United States, the PATRIC bioinformatics center, which is financed by the National Institute of Allergy and Infectious Diseases, works with automated learning models to predict the antimicrobial resistance of different species of bacteria, including Staphylococcus aureus, Streptococcus pneumoniae, and Mycobacterium tuberculosis. These models, which work with genomic data associated with antibiotic resistance phenotypes, are able to identify resistance without prior knowledge of the underlying mechanisms.
Another factor to consider with regard to the use of precision medicine for infectious diseases is the microbiota. Dr. Oteo explained that the pathogenic microorganism interacts not only with the host but also with its microbiota, “which can be diverse, is manifold, and can be very different, depending on the circumstances. These interactions can be translated into ecological and evolutionary pressures that may have clinical significance.” One of the best-known examples is the possibility that a beta-lactamase–producing bacterium benefits other bacteria around it by secreting these enzymes. Furthermore, some known forms of bacterial interaction (such as plasmid transfer) are directly related to antibiotic resistance. Metagenomics, which involves the genetic study of communities of microbes, could provide more information for predicting and avoiding infections by multiresistant pathogens by monitoring the microbiome.
The CRISPR-Cas9 gene editing tool could also be an ally in the fight against antibiotic resistance by eliminating resistance genes and thus making bacteria sensitive to certain antibiotics. Several published preliminary studies indicate that this is possible in vitro. The main challenge for the clinical application of CRISPR is in introducing it into the target microbial population. Use of conjugative plasmids and bacteriophages could perhaps be an option for overcoming this obstacle in the future.
Exploiting the possibilities of precision medicine through use of the most innovative tools in addressing antibiotic resistance is a great challenge, said Dr. Oteo, but the situation demands it, and it is necessary to take small steps to achieve this goal.
A version of this article appeared on Medscape.com. This article was translated from Univadis Spain.
Diversity is an omnipresent element in clinical practice: in the genome, in the environment, in patients’ lifestyles and habits. Precision medicine addresses the variability of the individual to improve diagnosis and treatment. It is increasingly used in specialties such as oncology, neurology, and cardiology. A personalized approach has many objectives, including to optimize treatment, minimize the risk of adverse effects, facilitate early diagnosis, and determine predisposition to disease. Genomic technologies, such as massive sequencing techniques, and tools such as CRISPR-Cas9 are key to the future of personalized medicine.
Jesús Oteo Iglesias, MD, PhD, a specialist in microbiology and director of Spain’s National Center for Microbiology, spoke at the Spanish Association of Infectious Diseases and Clinical Microbiology’s recent conference. He discussed various precision medicine projects aimed at reinforcing the fight against antibiotic resistance.
Infectious diseases are complex because the diversity of the pathogenic microorganism combines with the patient’s own diversity, which influences the interaction between the two, said Dr. Oteo. Thus, the antibiogram and targeted antibiotic treatments (which are chosen according to the species, sensitivity to antimicrobials, type of infection, and patient characteristics) have been established applications of precision medicine for decades. However, multiple tools could further strengthen personalized medicine against multiresistant pathogens.
Therapeutic drug monitoring, in which multiple pharmacokinetic and pharmacodynamic factors are considered, is a strategy with great potential to increase the effectiveness of antibiotics and minimize toxicity. Owing to its costs and the need for trained staff, this tool would be especially indicated in the treatment of patients with more complex conditions, such as those suffering from obesity, complex infections, or infections with multiresistant bacteria, as well as those in critical condition. Multiple computer programs are available to help determine the dosage of antibiotics by estimating drug exposure and to provide recommendations. However, clinical trials are needed to assess the pros and cons of applying therapeutic monitoring for types of antibiotics other than those for which a given type is already used (for example, aminoglycosides and glycopeptides).
One technology that could help in antibiotic use optimization programs is microneedle-based biosensors, which could be implanted in the skin for real-time antibiotic monitoring. This tool “could be the first step in establishing automated antibiotic administration systems, with infusion pumps and feedback systems, like those already used in diabetes for insulin administration,” said Dr. Oteo.
Artificial intelligence could also be a valuable technology for optimization programs. “We should go a step further in the implementation of artificial intelligence through clinical decision support systems,” said Dr. Oteo. This technology would guide the administration of antimicrobials using data extracted from the electronic medical record. However, there are great challenges to overcome in creating these tools, such as the risk of entering erroneous data; the difficulty in entering complex data, such as data relevant to antibiotic resistance; and the variability at the geographic and institutional levels.
Genomics is also a tool with great potential for identifying bacteria’s degree of resistance to antibiotics by studying mutations in chromosomal and acquired genes. A proof-of-concept study evaluated the sensitivity of different Pseudomonas aeruginosa strains to several antibiotics by analyzing genome sequences associated with resistance, said Dr. Otero. The researchers found that this system was effective at predicting the sensitivity of bacteria from genomic data.
In the United States, the PATRIC bioinformatics center, which is financed by the National Institute of Allergy and Infectious Diseases, works with automated learning models to predict the antimicrobial resistance of different species of bacteria, including Staphylococcus aureus, Streptococcus pneumoniae, and Mycobacterium tuberculosis. These models, which work with genomic data associated with antibiotic resistance phenotypes, are able to identify resistance without prior knowledge of the underlying mechanisms.
Another factor to consider with regard to the use of precision medicine for infectious diseases is the microbiota. Dr. Oteo explained that the pathogenic microorganism interacts not only with the host but also with its microbiota, “which can be diverse, is manifold, and can be very different, depending on the circumstances. These interactions can be translated into ecological and evolutionary pressures that may have clinical significance.” One of the best-known examples is the possibility that a beta-lactamase–producing bacterium benefits other bacteria around it by secreting these enzymes. Furthermore, some known forms of bacterial interaction (such as plasmid transfer) are directly related to antibiotic resistance. Metagenomics, which involves the genetic study of communities of microbes, could provide more information for predicting and avoiding infections by multiresistant pathogens by monitoring the microbiome.
The CRISPR-Cas9 gene editing tool could also be an ally in the fight against antibiotic resistance by eliminating resistance genes and thus making bacteria sensitive to certain antibiotics. Several published preliminary studies indicate that this is possible in vitro. The main challenge for the clinical application of CRISPR is in introducing it into the target microbial population. Use of conjugative plasmids and bacteriophages could perhaps be an option for overcoming this obstacle in the future.
Exploiting the possibilities of precision medicine through use of the most innovative tools in addressing antibiotic resistance is a great challenge, said Dr. Oteo, but the situation demands it, and it is necessary to take small steps to achieve this goal.
A version of this article appeared on Medscape.com. This article was translated from Univadis Spain.
Diversity is an omnipresent element in clinical practice: in the genome, in the environment, in patients’ lifestyles and habits. Precision medicine addresses the variability of the individual to improve diagnosis and treatment. It is increasingly used in specialties such as oncology, neurology, and cardiology. A personalized approach has many objectives, including to optimize treatment, minimize the risk of adverse effects, facilitate early diagnosis, and determine predisposition to disease. Genomic technologies, such as massive sequencing techniques, and tools such as CRISPR-Cas9 are key to the future of personalized medicine.
Jesús Oteo Iglesias, MD, PhD, a specialist in microbiology and director of Spain’s National Center for Microbiology, spoke at the Spanish Association of Infectious Diseases and Clinical Microbiology’s recent conference. He discussed various precision medicine projects aimed at reinforcing the fight against antibiotic resistance.
Infectious diseases are complex because the diversity of the pathogenic microorganism combines with the patient’s own diversity, which influences the interaction between the two, said Dr. Oteo. Thus, the antibiogram and targeted antibiotic treatments (which are chosen according to the species, sensitivity to antimicrobials, type of infection, and patient characteristics) have been established applications of precision medicine for decades. However, multiple tools could further strengthen personalized medicine against multiresistant pathogens.
Therapeutic drug monitoring, in which multiple pharmacokinetic and pharmacodynamic factors are considered, is a strategy with great potential to increase the effectiveness of antibiotics and minimize toxicity. Owing to its costs and the need for trained staff, this tool would be especially indicated in the treatment of patients with more complex conditions, such as those suffering from obesity, complex infections, or infections with multiresistant bacteria, as well as those in critical condition. Multiple computer programs are available to help determine the dosage of antibiotics by estimating drug exposure and to provide recommendations. However, clinical trials are needed to assess the pros and cons of applying therapeutic monitoring for types of antibiotics other than those for which a given type is already used (for example, aminoglycosides and glycopeptides).
One technology that could help in antibiotic use optimization programs is microneedle-based biosensors, which could be implanted in the skin for real-time antibiotic monitoring. This tool “could be the first step in establishing automated antibiotic administration systems, with infusion pumps and feedback systems, like those already used in diabetes for insulin administration,” said Dr. Oteo.
Artificial intelligence could also be a valuable technology for optimization programs. “We should go a step further in the implementation of artificial intelligence through clinical decision support systems,” said Dr. Oteo. This technology would guide the administration of antimicrobials using data extracted from the electronic medical record. However, there are great challenges to overcome in creating these tools, such as the risk of entering erroneous data; the difficulty in entering complex data, such as data relevant to antibiotic resistance; and the variability at the geographic and institutional levels.
Genomics is also a tool with great potential for identifying bacteria’s degree of resistance to antibiotics by studying mutations in chromosomal and acquired genes. A proof-of-concept study evaluated the sensitivity of different Pseudomonas aeruginosa strains to several antibiotics by analyzing genome sequences associated with resistance, said Dr. Otero. The researchers found that this system was effective at predicting the sensitivity of bacteria from genomic data.
In the United States, the PATRIC bioinformatics center, which is financed by the National Institute of Allergy and Infectious Diseases, works with automated learning models to predict the antimicrobial resistance of different species of bacteria, including Staphylococcus aureus, Streptococcus pneumoniae, and Mycobacterium tuberculosis. These models, which work with genomic data associated with antibiotic resistance phenotypes, are able to identify resistance without prior knowledge of the underlying mechanisms.
Another factor to consider with regard to the use of precision medicine for infectious diseases is the microbiota. Dr. Oteo explained that the pathogenic microorganism interacts not only with the host but also with its microbiota, “which can be diverse, is manifold, and can be very different, depending on the circumstances. These interactions can be translated into ecological and evolutionary pressures that may have clinical significance.” One of the best-known examples is the possibility that a beta-lactamase–producing bacterium benefits other bacteria around it by secreting these enzymes. Furthermore, some known forms of bacterial interaction (such as plasmid transfer) are directly related to antibiotic resistance. Metagenomics, which involves the genetic study of communities of microbes, could provide more information for predicting and avoiding infections by multiresistant pathogens by monitoring the microbiome.
The CRISPR-Cas9 gene editing tool could also be an ally in the fight against antibiotic resistance by eliminating resistance genes and thus making bacteria sensitive to certain antibiotics. Several published preliminary studies indicate that this is possible in vitro. The main challenge for the clinical application of CRISPR is in introducing it into the target microbial population. Use of conjugative plasmids and bacteriophages could perhaps be an option for overcoming this obstacle in the future.
Exploiting the possibilities of precision medicine through use of the most innovative tools in addressing antibiotic resistance is a great challenge, said Dr. Oteo, but the situation demands it, and it is necessary to take small steps to achieve this goal.
A version of this article appeared on Medscape.com. This article was translated from Univadis Spain.
New treatment reduces risk of anal cancer in people with HIV
It all began with the question, “Has your butt been getting enough attention?”
Though that may seem unorthodox, it led researchers to discovering a treatment that may help prevent anal cancer in people with HIV/AIDS. It’s still featured on their study’s website, with this further explanation: “You get your viral load checked, your T-cell count checked, but what about your anus? Did you know that half of HIV+ men have cell changes in their anus caused by HPV?”
The Anal Cancer/HSIL Outcomes Research (ANCHOR) study, led by Joel Palefsky, MD, was published in The New England Journal of Medicine. Dr. Palefsky, an infectious disease expert at the University of California, San Francisco, and his team set out to determine whether a treatment that prevents cervical cancer in people with human papillomavirus (HPV) would benefit people with HIV/AIDS. The new treatment reduced the likelihood of anal cancer by more than 50%.
The team worked over 7 years, during which time they tested 4,459 men, women, transgender, and nonbinary individuals at 25 sites across the United States. The participants were sorted into two groups: Some received treatment for high-grade squamous intraepithelial lesions (HSILs), and some did not but were monitored for signs of disease. These included individuals over 35 who were living with HIV/AIDS and who were found to have patches of abnormal cells in their rectal lining.
HSILs are the cells gynecologists look for in performing a pap smear. They are precancerous cells commonly found in the cervix of persons with HPV. Finding HSILs during a gynecologic examination alerts clinicians to potential problems.
HSILs can also be found in the anal tract of men and women with HIV. Dr. Palefsky therefore hypothesized that, as with HPV and cervical cancer, these anal HSILs may be a precursor of anal cancer.
The scientists decided to treat these cells the same way they would treat them if found in the cervix and to see whether that reduced the risk of cancer. Doctors used lidocaine to numb the area, then removed the HSILs with an electric probe. The team then assessed whether the treatment prevented people from getting cancer.
It turns out that in many cases, it did. The study concluded after 30 of the participants developed anal cancer. Of those, 21 patients had not received HSIL treatment, compared with nine who did receive the treatment. The treatment resulted in a 57% reduction in the rate of anal cancer among patients who received treatment for their HSILs.
These results are encouraging, said Aasma Shaukat, MD, director of outcomes research in the Division of Gastroenterology and Hepatology at NYU Langone Health. Dr. Shaukat was not involved with the study. She believes it’s going to cause ripples across the field.
“The study is likely to change guidelines in favor of active and early treatment for HSIL and away from watchful waiting in individuals living with HIV to reduce the risk of developing anal squamous cell carcinoma, akin to removing polyps during colonoscopy to progression to and incidence of colorectal cancer,” she said in an email interview.
Treatments for this group of patients are more important now than ever. Since the beginning of the AIDS epidemic in the 1980s, the number of people with HIV has increased, Dr. Palefsky detailed in a press conference announcing the ANCHOR results. That’s partially because of new transmissions and partially owing to the fact that new treatments make it possible for people with HIV to live long, healthy lives. So as more people with HIV move into their sunset years, there are more people at risk for developing cancer, which is a disease associated with aging. Anal cancer sits at the intersection of risk for aging people who have HIV.
Any defense we have against the risk of cancer in this growing demographic is a good thing, says Hanna K. Sanoff, MD, a gastrointestinal oncologist at the Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, who was also not involved in the study. Although it’s not ready to be applied in doctors’ offices now, it could be a tool in the future. “Anything we can do to try and decrease the chance of precancerous lesions progressing to a real invasive cancer is of great importance. This kind of prevention work is critical to helping minimize the burden of cancer on our communities,” Dr. Sanoff said in an interview.
The study was funded by the National Cancer Institute of the National Institutes of Health and was conducted through the NCI-supported AIDS Malignancy Consortium. Dr. Shaukat and Dr. Sanoff report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It all began with the question, “Has your butt been getting enough attention?”
Though that may seem unorthodox, it led researchers to discovering a treatment that may help prevent anal cancer in people with HIV/AIDS. It’s still featured on their study’s website, with this further explanation: “You get your viral load checked, your T-cell count checked, but what about your anus? Did you know that half of HIV+ men have cell changes in their anus caused by HPV?”
The Anal Cancer/HSIL Outcomes Research (ANCHOR) study, led by Joel Palefsky, MD, was published in The New England Journal of Medicine. Dr. Palefsky, an infectious disease expert at the University of California, San Francisco, and his team set out to determine whether a treatment that prevents cervical cancer in people with human papillomavirus (HPV) would benefit people with HIV/AIDS. The new treatment reduced the likelihood of anal cancer by more than 50%.
The team worked over 7 years, during which time they tested 4,459 men, women, transgender, and nonbinary individuals at 25 sites across the United States. The participants were sorted into two groups: Some received treatment for high-grade squamous intraepithelial lesions (HSILs), and some did not but were monitored for signs of disease. These included individuals over 35 who were living with HIV/AIDS and who were found to have patches of abnormal cells in their rectal lining.
HSILs are the cells gynecologists look for in performing a pap smear. They are precancerous cells commonly found in the cervix of persons with HPV. Finding HSILs during a gynecologic examination alerts clinicians to potential problems.
HSILs can also be found in the anal tract of men and women with HIV. Dr. Palefsky therefore hypothesized that, as with HPV and cervical cancer, these anal HSILs may be a precursor of anal cancer.
The scientists decided to treat these cells the same way they would treat them if found in the cervix and to see whether that reduced the risk of cancer. Doctors used lidocaine to numb the area, then removed the HSILs with an electric probe. The team then assessed whether the treatment prevented people from getting cancer.
It turns out that in many cases, it did. The study concluded after 30 of the participants developed anal cancer. Of those, 21 patients had not received HSIL treatment, compared with nine who did receive the treatment. The treatment resulted in a 57% reduction in the rate of anal cancer among patients who received treatment for their HSILs.
These results are encouraging, said Aasma Shaukat, MD, director of outcomes research in the Division of Gastroenterology and Hepatology at NYU Langone Health. Dr. Shaukat was not involved with the study. She believes it’s going to cause ripples across the field.
“The study is likely to change guidelines in favor of active and early treatment for HSIL and away from watchful waiting in individuals living with HIV to reduce the risk of developing anal squamous cell carcinoma, akin to removing polyps during colonoscopy to progression to and incidence of colorectal cancer,” she said in an email interview.
Treatments for this group of patients are more important now than ever. Since the beginning of the AIDS epidemic in the 1980s, the number of people with HIV has increased, Dr. Palefsky detailed in a press conference announcing the ANCHOR results. That’s partially because of new transmissions and partially owing to the fact that new treatments make it possible for people with HIV to live long, healthy lives. So as more people with HIV move into their sunset years, there are more people at risk for developing cancer, which is a disease associated with aging. Anal cancer sits at the intersection of risk for aging people who have HIV.
Any defense we have against the risk of cancer in this growing demographic is a good thing, says Hanna K. Sanoff, MD, a gastrointestinal oncologist at the Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, who was also not involved in the study. Although it’s not ready to be applied in doctors’ offices now, it could be a tool in the future. “Anything we can do to try and decrease the chance of precancerous lesions progressing to a real invasive cancer is of great importance. This kind of prevention work is critical to helping minimize the burden of cancer on our communities,” Dr. Sanoff said in an interview.
The study was funded by the National Cancer Institute of the National Institutes of Health and was conducted through the NCI-supported AIDS Malignancy Consortium. Dr. Shaukat and Dr. Sanoff report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It all began with the question, “Has your butt been getting enough attention?”
Though that may seem unorthodox, it led researchers to discovering a treatment that may help prevent anal cancer in people with HIV/AIDS. It’s still featured on their study’s website, with this further explanation: “You get your viral load checked, your T-cell count checked, but what about your anus? Did you know that half of HIV+ men have cell changes in their anus caused by HPV?”
The Anal Cancer/HSIL Outcomes Research (ANCHOR) study, led by Joel Palefsky, MD, was published in The New England Journal of Medicine. Dr. Palefsky, an infectious disease expert at the University of California, San Francisco, and his team set out to determine whether a treatment that prevents cervical cancer in people with human papillomavirus (HPV) would benefit people with HIV/AIDS. The new treatment reduced the likelihood of anal cancer by more than 50%.
The team worked over 7 years, during which time they tested 4,459 men, women, transgender, and nonbinary individuals at 25 sites across the United States. The participants were sorted into two groups: Some received treatment for high-grade squamous intraepithelial lesions (HSILs), and some did not but were monitored for signs of disease. These included individuals over 35 who were living with HIV/AIDS and who were found to have patches of abnormal cells in their rectal lining.
HSILs are the cells gynecologists look for in performing a pap smear. They are precancerous cells commonly found in the cervix of persons with HPV. Finding HSILs during a gynecologic examination alerts clinicians to potential problems.
HSILs can also be found in the anal tract of men and women with HIV. Dr. Palefsky therefore hypothesized that, as with HPV and cervical cancer, these anal HSILs may be a precursor of anal cancer.
The scientists decided to treat these cells the same way they would treat them if found in the cervix and to see whether that reduced the risk of cancer. Doctors used lidocaine to numb the area, then removed the HSILs with an electric probe. The team then assessed whether the treatment prevented people from getting cancer.
It turns out that in many cases, it did. The study concluded after 30 of the participants developed anal cancer. Of those, 21 patients had not received HSIL treatment, compared with nine who did receive the treatment. The treatment resulted in a 57% reduction in the rate of anal cancer among patients who received treatment for their HSILs.
These results are encouraging, said Aasma Shaukat, MD, director of outcomes research in the Division of Gastroenterology and Hepatology at NYU Langone Health. Dr. Shaukat was not involved with the study. She believes it’s going to cause ripples across the field.
“The study is likely to change guidelines in favor of active and early treatment for HSIL and away from watchful waiting in individuals living with HIV to reduce the risk of developing anal squamous cell carcinoma, akin to removing polyps during colonoscopy to progression to and incidence of colorectal cancer,” she said in an email interview.
Treatments for this group of patients are more important now than ever. Since the beginning of the AIDS epidemic in the 1980s, the number of people with HIV has increased, Dr. Palefsky detailed in a press conference announcing the ANCHOR results. That’s partially because of new transmissions and partially owing to the fact that new treatments make it possible for people with HIV to live long, healthy lives. So as more people with HIV move into their sunset years, there are more people at risk for developing cancer, which is a disease associated with aging. Anal cancer sits at the intersection of risk for aging people who have HIV.
Any defense we have against the risk of cancer in this growing demographic is a good thing, says Hanna K. Sanoff, MD, a gastrointestinal oncologist at the Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, who was also not involved in the study. Although it’s not ready to be applied in doctors’ offices now, it could be a tool in the future. “Anything we can do to try and decrease the chance of precancerous lesions progressing to a real invasive cancer is of great importance. This kind of prevention work is critical to helping minimize the burden of cancer on our communities,” Dr. Sanoff said in an interview.
The study was funded by the National Cancer Institute of the National Institutes of Health and was conducted through the NCI-supported AIDS Malignancy Consortium. Dr. Shaukat and Dr. Sanoff report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Disturbed sleep drives poor PrEP adherence in young Black sexual-minority men
Young Black sexual-minority men (YBSMM) who experience sleep disturbance at least 3-4 times a week are much more likely to miss HIV pre-exposure prophylaxis (PrEP) doses than those who rarely report sleep disturbance, according to a new study published online in the journal AIDS and Behavior.
Sleep disturbance, poor cognitive processing, and memory function deficits go hand in hand, especially among people living with HIV.
Data have suggested that poor sleep might be an important factor in common neurocognitive complaints and overall health outcomes, especially among older adults with HIV. But few studies have examined the role that sleep quality might play in driving health behaviors around prevention and PrEP adherence, especially among YBSMM, who are at highest risk for acquiring new HIV infections.
Commonly cited reasons for suboptimal HIV prevention efforts within this population often include stigma, mistrust of the medical system, and a lack of culturally appropriate care.
“We make a lot of assumptions about young people and their brains and their ability to remember things, namely [that] they should be better than older adults at remembering to take medications,” lead study investigator Jade Pagkas-Bather, MD, an infectious disease specialist at University of Chicago Medicine, told this news organization.
“In reality, many young people are not used to taking medications, [especially] for a disease that they do not have.”
Too many pills, too little sleep
The researchers examined data collected from participants in the Neighborhoods and Networks Cohort Study, Chicago, which looked at the role of social, contextual, network, and geospatial factors influencing HIV prevention and care in HIV-negative, cisgender YBSMM between 2018 and 2019.
The investigators included 70 YBSMM participants who reported current PrEP use in the analysis. All were between the ages of 16 and 24 years, self-identified as African American or Black, were assigned male at birth, and reported at least one sexual encounter with a man or transgender woman in the previous 12 months.
Sleep was measured using the Patient Health Questionnaire-9 (PHQ-9) which includes a question on frequency of sleep disturbance (that is, trouble falling asleep, staying asleep, or sleeping too much) categorized as follows: less than 1 day (rarely or none of the time), 1-2 days (some or a little of the time), 3-4 days (occasionally or a moderate amount of time), or 5-7 days (all of the time).
Almost half (47.1%) of participants self-reported some or moderate sleep disturbance, with 8.6% having sleep disturbance all of the time.
“One of the main findings was that poor sleep and having too many pills impacts people’s ability to remember to take their PrEP or is associated with missing PrEP doses,” explained Dr. Pagkas-Bather.
In adjusted models, YBSMM who reported moderate sleep disturbances cited having too many pills to take as the reason for missing or forgetting PrEP doses (adjusted odds ratio, 7.59; 95% confidence interval, 1.05-54.57), compared with peers who did not have sleep issues.
Depression was likewise an important factor. Participants who reported experiencing sleep disturbance all of the time and missing PrEP doses were also highly likely to be depressed (aOR, 11.30; 95% CI, 1.19-107.53).
“The PHQ-9 is a widely accepted measure looking at depression – and sleep as one symptom of depression,” explained Brooke Genkin Rogers, Ph.D., M.P.H., a research scientist and assistant professor of psychiatry and human behavior at the Warren Alpert Medical School of Brown University, Providence, R.I.
Dr. Rogers, who was not involved in the study, noted, “Sleep disturbance is a sign of poor physical or mental health, particularly in an otherwise younger, healthier population.” But Dr. Rogers also had questions about sleep duration (that is, too short or too long) and whether or not it also played a role in poor adherence, information that was not pursued within the study.
“As a clinician, I see quite a few people who are young Black sexual minority men who are on PrEP, or on the converse side, people living with HIV and taking medications for HIV treatment. I would posit a guess that it’s not that people are necessarily sleeping too much, but there are other sorts of factors that interfere with being able to get 8 hours of sleep a night,” explained Dr. Pagkas-Bather.
They include structural issues like greater exposure to housing instability and neighborhood safety.
However, Dr. Pagkas-Bather pointed to an even more critical factor influencing PrEP usage and adherence, one that she refers to as “Trickle Up HIV Care.”
“We can’t just come up with interventions, drugs, and studies and say, we have all of these options, anyone who wants them, come and get them,” she said. “We really need to work very hard at educating and encouraging populations who have no high-level need for prevention and treatment.”
As a clinician who works closely with the YBSMM population, Dr. Pagkas-Bather also shared that her patients have told her that they’ve asked for PrEP and have had providers turn them down because they weren’t comfortable prescribing PrEP or made assumptions about the kind of people who are on PrEP.
“There are sometimes assumptions made about Black men and sexual promiscuity. And the data doesn’t bear that out. It’s not that Black men are having more sex than White men or any other man; it’s that the prevalence of HIV in the Black community is higher overall relative to the population,” noted Dr. Pagkas-Bather.
“We need a nuanced approach to examining these issues ... to take a look at multiple levels of influence on folks’ health and HIV risk,” said Dr. Rogers.
Both clinicians acknowledged that creative solutions have not been exhausted.
“There’s a lot of opportunity if we sit down with communities and share in decisions around HIV treatment and prevention ... if we tap into the wealth and knowledge of the Black communities to prevent HIV,” concluded Dr. Pagkas-Bather.
Dr. Pagkas-Bather reports that she is a Gilead Sciences HIV Research Scholar awardee. Dr. Rogers reports receiving a scientific research grant from Gilead Sciences.
A version of this article first appeared on Medscape.com.
Young Black sexual-minority men (YBSMM) who experience sleep disturbance at least 3-4 times a week are much more likely to miss HIV pre-exposure prophylaxis (PrEP) doses than those who rarely report sleep disturbance, according to a new study published online in the journal AIDS and Behavior.
Sleep disturbance, poor cognitive processing, and memory function deficits go hand in hand, especially among people living with HIV.
Data have suggested that poor sleep might be an important factor in common neurocognitive complaints and overall health outcomes, especially among older adults with HIV. But few studies have examined the role that sleep quality might play in driving health behaviors around prevention and PrEP adherence, especially among YBSMM, who are at highest risk for acquiring new HIV infections.
Commonly cited reasons for suboptimal HIV prevention efforts within this population often include stigma, mistrust of the medical system, and a lack of culturally appropriate care.
“We make a lot of assumptions about young people and their brains and their ability to remember things, namely [that] they should be better than older adults at remembering to take medications,” lead study investigator Jade Pagkas-Bather, MD, an infectious disease specialist at University of Chicago Medicine, told this news organization.
“In reality, many young people are not used to taking medications, [especially] for a disease that they do not have.”
Too many pills, too little sleep
The researchers examined data collected from participants in the Neighborhoods and Networks Cohort Study, Chicago, which looked at the role of social, contextual, network, and geospatial factors influencing HIV prevention and care in HIV-negative, cisgender YBSMM between 2018 and 2019.
The investigators included 70 YBSMM participants who reported current PrEP use in the analysis. All were between the ages of 16 and 24 years, self-identified as African American or Black, were assigned male at birth, and reported at least one sexual encounter with a man or transgender woman in the previous 12 months.
Sleep was measured using the Patient Health Questionnaire-9 (PHQ-9) which includes a question on frequency of sleep disturbance (that is, trouble falling asleep, staying asleep, or sleeping too much) categorized as follows: less than 1 day (rarely or none of the time), 1-2 days (some or a little of the time), 3-4 days (occasionally or a moderate amount of time), or 5-7 days (all of the time).
Almost half (47.1%) of participants self-reported some or moderate sleep disturbance, with 8.6% having sleep disturbance all of the time.
“One of the main findings was that poor sleep and having too many pills impacts people’s ability to remember to take their PrEP or is associated with missing PrEP doses,” explained Dr. Pagkas-Bather.
In adjusted models, YBSMM who reported moderate sleep disturbances cited having too many pills to take as the reason for missing or forgetting PrEP doses (adjusted odds ratio, 7.59; 95% confidence interval, 1.05-54.57), compared with peers who did not have sleep issues.
Depression was likewise an important factor. Participants who reported experiencing sleep disturbance all of the time and missing PrEP doses were also highly likely to be depressed (aOR, 11.30; 95% CI, 1.19-107.53).
“The PHQ-9 is a widely accepted measure looking at depression – and sleep as one symptom of depression,” explained Brooke Genkin Rogers, Ph.D., M.P.H., a research scientist and assistant professor of psychiatry and human behavior at the Warren Alpert Medical School of Brown University, Providence, R.I.
Dr. Rogers, who was not involved in the study, noted, “Sleep disturbance is a sign of poor physical or mental health, particularly in an otherwise younger, healthier population.” But Dr. Rogers also had questions about sleep duration (that is, too short or too long) and whether or not it also played a role in poor adherence, information that was not pursued within the study.
“As a clinician, I see quite a few people who are young Black sexual minority men who are on PrEP, or on the converse side, people living with HIV and taking medications for HIV treatment. I would posit a guess that it’s not that people are necessarily sleeping too much, but there are other sorts of factors that interfere with being able to get 8 hours of sleep a night,” explained Dr. Pagkas-Bather.
They include structural issues like greater exposure to housing instability and neighborhood safety.
However, Dr. Pagkas-Bather pointed to an even more critical factor influencing PrEP usage and adherence, one that she refers to as “Trickle Up HIV Care.”
“We can’t just come up with interventions, drugs, and studies and say, we have all of these options, anyone who wants them, come and get them,” she said. “We really need to work very hard at educating and encouraging populations who have no high-level need for prevention and treatment.”
As a clinician who works closely with the YBSMM population, Dr. Pagkas-Bather also shared that her patients have told her that they’ve asked for PrEP and have had providers turn them down because they weren’t comfortable prescribing PrEP or made assumptions about the kind of people who are on PrEP.
“There are sometimes assumptions made about Black men and sexual promiscuity. And the data doesn’t bear that out. It’s not that Black men are having more sex than White men or any other man; it’s that the prevalence of HIV in the Black community is higher overall relative to the population,” noted Dr. Pagkas-Bather.
“We need a nuanced approach to examining these issues ... to take a look at multiple levels of influence on folks’ health and HIV risk,” said Dr. Rogers.
Both clinicians acknowledged that creative solutions have not been exhausted.
“There’s a lot of opportunity if we sit down with communities and share in decisions around HIV treatment and prevention ... if we tap into the wealth and knowledge of the Black communities to prevent HIV,” concluded Dr. Pagkas-Bather.
Dr. Pagkas-Bather reports that she is a Gilead Sciences HIV Research Scholar awardee. Dr. Rogers reports receiving a scientific research grant from Gilead Sciences.
A version of this article first appeared on Medscape.com.
Young Black sexual-minority men (YBSMM) who experience sleep disturbance at least 3-4 times a week are much more likely to miss HIV pre-exposure prophylaxis (PrEP) doses than those who rarely report sleep disturbance, according to a new study published online in the journal AIDS and Behavior.
Sleep disturbance, poor cognitive processing, and memory function deficits go hand in hand, especially among people living with HIV.
Data have suggested that poor sleep might be an important factor in common neurocognitive complaints and overall health outcomes, especially among older adults with HIV. But few studies have examined the role that sleep quality might play in driving health behaviors around prevention and PrEP adherence, especially among YBSMM, who are at highest risk for acquiring new HIV infections.
Commonly cited reasons for suboptimal HIV prevention efforts within this population often include stigma, mistrust of the medical system, and a lack of culturally appropriate care.
“We make a lot of assumptions about young people and their brains and their ability to remember things, namely [that] they should be better than older adults at remembering to take medications,” lead study investigator Jade Pagkas-Bather, MD, an infectious disease specialist at University of Chicago Medicine, told this news organization.
“In reality, many young people are not used to taking medications, [especially] for a disease that they do not have.”
Too many pills, too little sleep
The researchers examined data collected from participants in the Neighborhoods and Networks Cohort Study, Chicago, which looked at the role of social, contextual, network, and geospatial factors influencing HIV prevention and care in HIV-negative, cisgender YBSMM between 2018 and 2019.
The investigators included 70 YBSMM participants who reported current PrEP use in the analysis. All were between the ages of 16 and 24 years, self-identified as African American or Black, were assigned male at birth, and reported at least one sexual encounter with a man or transgender woman in the previous 12 months.
Sleep was measured using the Patient Health Questionnaire-9 (PHQ-9) which includes a question on frequency of sleep disturbance (that is, trouble falling asleep, staying asleep, or sleeping too much) categorized as follows: less than 1 day (rarely or none of the time), 1-2 days (some or a little of the time), 3-4 days (occasionally or a moderate amount of time), or 5-7 days (all of the time).
Almost half (47.1%) of participants self-reported some or moderate sleep disturbance, with 8.6% having sleep disturbance all of the time.
“One of the main findings was that poor sleep and having too many pills impacts people’s ability to remember to take their PrEP or is associated with missing PrEP doses,” explained Dr. Pagkas-Bather.
In adjusted models, YBSMM who reported moderate sleep disturbances cited having too many pills to take as the reason for missing or forgetting PrEP doses (adjusted odds ratio, 7.59; 95% confidence interval, 1.05-54.57), compared with peers who did not have sleep issues.
Depression was likewise an important factor. Participants who reported experiencing sleep disturbance all of the time and missing PrEP doses were also highly likely to be depressed (aOR, 11.30; 95% CI, 1.19-107.53).
“The PHQ-9 is a widely accepted measure looking at depression – and sleep as one symptom of depression,” explained Brooke Genkin Rogers, Ph.D., M.P.H., a research scientist and assistant professor of psychiatry and human behavior at the Warren Alpert Medical School of Brown University, Providence, R.I.
Dr. Rogers, who was not involved in the study, noted, “Sleep disturbance is a sign of poor physical or mental health, particularly in an otherwise younger, healthier population.” But Dr. Rogers also had questions about sleep duration (that is, too short or too long) and whether or not it also played a role in poor adherence, information that was not pursued within the study.
“As a clinician, I see quite a few people who are young Black sexual minority men who are on PrEP, or on the converse side, people living with HIV and taking medications for HIV treatment. I would posit a guess that it’s not that people are necessarily sleeping too much, but there are other sorts of factors that interfere with being able to get 8 hours of sleep a night,” explained Dr. Pagkas-Bather.
They include structural issues like greater exposure to housing instability and neighborhood safety.
However, Dr. Pagkas-Bather pointed to an even more critical factor influencing PrEP usage and adherence, one that she refers to as “Trickle Up HIV Care.”
“We can’t just come up with interventions, drugs, and studies and say, we have all of these options, anyone who wants them, come and get them,” she said. “We really need to work very hard at educating and encouraging populations who have no high-level need for prevention and treatment.”
As a clinician who works closely with the YBSMM population, Dr. Pagkas-Bather also shared that her patients have told her that they’ve asked for PrEP and have had providers turn them down because they weren’t comfortable prescribing PrEP or made assumptions about the kind of people who are on PrEP.
“There are sometimes assumptions made about Black men and sexual promiscuity. And the data doesn’t bear that out. It’s not that Black men are having more sex than White men or any other man; it’s that the prevalence of HIV in the Black community is higher overall relative to the population,” noted Dr. Pagkas-Bather.
“We need a nuanced approach to examining these issues ... to take a look at multiple levels of influence on folks’ health and HIV risk,” said Dr. Rogers.
Both clinicians acknowledged that creative solutions have not been exhausted.
“There’s a lot of opportunity if we sit down with communities and share in decisions around HIV treatment and prevention ... if we tap into the wealth and knowledge of the Black communities to prevent HIV,” concluded Dr. Pagkas-Bather.
Dr. Pagkas-Bather reports that she is a Gilead Sciences HIV Research Scholar awardee. Dr. Rogers reports receiving a scientific research grant from Gilead Sciences.
A version of this article first appeared on Medscape.com.
Doc’s misdiagnosis causes former firefighter to lose leg from flesh-eating bacterial infection
, as a story in the Pensacola News Journal indicates.
In September 2016, the former firefighter visited a hospital-affiliated urgent care center after he developed an ache and a blue discoloration in his right leg. Prior to this, the story says, he had been “exposed to the waters of Pensacola Bay,” which might have caused the infection.
At the urgent care center, he was examined by a primary care physician, who diagnosed him with an ankle sprain. Instructed to ice and elevate his leg, the former firefighter was given crutches and sent home.
The following day, still in pain, he visited a local podiatrist, who “immediately suspected ... [the patient] was suffering from an ongoing aggressive bacterial infection.” The podiatrist then arranged for the patient to be seen at a nearby hospital emergency department. There, doctors diagnosed a “necrotizing bacterial infection that need[ed] to be aggressively treated with antibodies and the removal of dead tissue.”
But despite their best efforts to control the infection and remove the necrotized tissue, the doctors eventually had to amputate the patient’s right leg above the knee.
The former firefighter and his wife then sued the primary care physician and the hospital where the physician worked.
After an 8-day civil trial, the jury awarded the plaintiff and his wife $6,805,071 and $787,371, respectively.
“What happened to [my clients] should never have happened,” said the attorney representing the plaintiffs.
The hospital declined to comment to the Pensacola News Journal about the case.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
, as a story in the Pensacola News Journal indicates.
In September 2016, the former firefighter visited a hospital-affiliated urgent care center after he developed an ache and a blue discoloration in his right leg. Prior to this, the story says, he had been “exposed to the waters of Pensacola Bay,” which might have caused the infection.
At the urgent care center, he was examined by a primary care physician, who diagnosed him with an ankle sprain. Instructed to ice and elevate his leg, the former firefighter was given crutches and sent home.
The following day, still in pain, he visited a local podiatrist, who “immediately suspected ... [the patient] was suffering from an ongoing aggressive bacterial infection.” The podiatrist then arranged for the patient to be seen at a nearby hospital emergency department. There, doctors diagnosed a “necrotizing bacterial infection that need[ed] to be aggressively treated with antibodies and the removal of dead tissue.”
But despite their best efforts to control the infection and remove the necrotized tissue, the doctors eventually had to amputate the patient’s right leg above the knee.
The former firefighter and his wife then sued the primary care physician and the hospital where the physician worked.
After an 8-day civil trial, the jury awarded the plaintiff and his wife $6,805,071 and $787,371, respectively.
“What happened to [my clients] should never have happened,” said the attorney representing the plaintiffs.
The hospital declined to comment to the Pensacola News Journal about the case.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
, as a story in the Pensacola News Journal indicates.
In September 2016, the former firefighter visited a hospital-affiliated urgent care center after he developed an ache and a blue discoloration in his right leg. Prior to this, the story says, he had been “exposed to the waters of Pensacola Bay,” which might have caused the infection.
At the urgent care center, he was examined by a primary care physician, who diagnosed him with an ankle sprain. Instructed to ice and elevate his leg, the former firefighter was given crutches and sent home.
The following day, still in pain, he visited a local podiatrist, who “immediately suspected ... [the patient] was suffering from an ongoing aggressive bacterial infection.” The podiatrist then arranged for the patient to be seen at a nearby hospital emergency department. There, doctors diagnosed a “necrotizing bacterial infection that need[ed] to be aggressively treated with antibodies and the removal of dead tissue.”
But despite their best efforts to control the infection and remove the necrotized tissue, the doctors eventually had to amputate the patient’s right leg above the knee.
The former firefighter and his wife then sued the primary care physician and the hospital where the physician worked.
After an 8-day civil trial, the jury awarded the plaintiff and his wife $6,805,071 and $787,371, respectively.
“What happened to [my clients] should never have happened,” said the attorney representing the plaintiffs.
The hospital declined to comment to the Pensacola News Journal about the case.
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.

