User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
COVID bivalent booster better vs. recent Omicron subvariants: Pfizer
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
FDA expands tenofovir alafenamide (Vemlidy) use to adolescents with chronic HBV
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
RSV vaccine given during pregnancy protects newborns: Pfizer
New trial data from drugmaker Pfizer shows promising results of a vaccine given to mothers during pregnancy that later protects infants in their first months from the worst effects of respiratory syncytial virus, or RSV.
Pfizer will apply for FDA approval by the end of the year, the company said in a statement Nov. 1.
Trial results are so promising that – after talking with government regulators – the company will stop enrolling new people in the study.
Specifically, the company reported that the vaccine prevented severe illness particularly well during the first 90 days of life, with measurable protection against severe illness continuing through 6 months of age. (That period is when infants are the most fragile if they get sick with RSV.)
RSV is a respiratory illness than can affect anyone, usually resulting in no symptoms or those similar to the common cold. But it can be particularly dangerous – and even deadly – for babies and for people over the age of 65. Pfizer and another drug company, GSK, are developing promising vaccines for older adults, the Washington Post reported.
RSV is the leading cause of hospitalization for infants, the Post noted.
The Pfizer study, called MATISSE, enrolled 7,400 pregnant women in 18 countries worldwide. Those who received the vaccine were given it during the late second to third trimester of pregnancy. Women in the study were monitored for safety through the rest of their pregnancy and 6 months after their children were born. Infants were monitored for at least 1 year for safety and effectiveness; more than half of them were monitored for 2 years.
The Pfizer vaccine works by passing maternal antibodies to the infant during pregnancy, the Post reported, noting that other vaccines transmitted via maternal immunization include those for influenza, diphtheria, tetanus, and pertussis.
Annually, RSV has a devastating impact on young children, hospitalizing tens of thousands and causing up to 300 deaths, data show.
For every 100 children who get RSV under 6 months of age, one or two of them may need to be hospitalized, according to the CDC. Those hospitalized infants may need oxygen, intubation, or even mechanical ventilation to help with breathing.
“Most improve with this type of supportive care and are discharged in a few days,” the CDC said.
“I think this is a big step for protecting babies against RSV and improving overall lung health,” vaccine researcher Barney Graham, PhD, told the Post. “Overall, it’s an exciting time for RSV. It’s also a troubling time, because you see how the patterns of infection have been changed by COVID, and we’re having an earlier, bigger season this year than we have for a couple of years – and it’s causing a lot of hospitalization and misery for people.”
As many as four RSV vaccines may have applications submitted to the FDA in 2022, according to CNN. Also in development is an antibody shot given to infants just after they are born, the news outlet reported.
Pfizer’s data, announced Tuesday, has not yet been published or peer-reviewed, but the company said it is seeking peer-reviewed publication.
“We are thrilled by these data, as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth,” Annaliesa Anderson, PhD, Pfizer chief scientific officer for vaccine research & development, said in a statement. “We look forward to working with the FDA and other regulatory agencies to bring this vaccine candidate to expectant mothers to help protect their infants against severe RSV during their most vulnerable first six months of life, which has the highest burden of RSV illness in infants.”
A version of this article first appeared on WebMD.com.
New trial data from drugmaker Pfizer shows promising results of a vaccine given to mothers during pregnancy that later protects infants in their first months from the worst effects of respiratory syncytial virus, or RSV.
Pfizer will apply for FDA approval by the end of the year, the company said in a statement Nov. 1.
Trial results are so promising that – after talking with government regulators – the company will stop enrolling new people in the study.
Specifically, the company reported that the vaccine prevented severe illness particularly well during the first 90 days of life, with measurable protection against severe illness continuing through 6 months of age. (That period is when infants are the most fragile if they get sick with RSV.)
RSV is a respiratory illness than can affect anyone, usually resulting in no symptoms or those similar to the common cold. But it can be particularly dangerous – and even deadly – for babies and for people over the age of 65. Pfizer and another drug company, GSK, are developing promising vaccines for older adults, the Washington Post reported.
RSV is the leading cause of hospitalization for infants, the Post noted.
The Pfizer study, called MATISSE, enrolled 7,400 pregnant women in 18 countries worldwide. Those who received the vaccine were given it during the late second to third trimester of pregnancy. Women in the study were monitored for safety through the rest of their pregnancy and 6 months after their children were born. Infants were monitored for at least 1 year for safety and effectiveness; more than half of them were monitored for 2 years.
The Pfizer vaccine works by passing maternal antibodies to the infant during pregnancy, the Post reported, noting that other vaccines transmitted via maternal immunization include those for influenza, diphtheria, tetanus, and pertussis.
Annually, RSV has a devastating impact on young children, hospitalizing tens of thousands and causing up to 300 deaths, data show.
For every 100 children who get RSV under 6 months of age, one or two of them may need to be hospitalized, according to the CDC. Those hospitalized infants may need oxygen, intubation, or even mechanical ventilation to help with breathing.
“Most improve with this type of supportive care and are discharged in a few days,” the CDC said.
“I think this is a big step for protecting babies against RSV and improving overall lung health,” vaccine researcher Barney Graham, PhD, told the Post. “Overall, it’s an exciting time for RSV. It’s also a troubling time, because you see how the patterns of infection have been changed by COVID, and we’re having an earlier, bigger season this year than we have for a couple of years – and it’s causing a lot of hospitalization and misery for people.”
As many as four RSV vaccines may have applications submitted to the FDA in 2022, according to CNN. Also in development is an antibody shot given to infants just after they are born, the news outlet reported.
Pfizer’s data, announced Tuesday, has not yet been published or peer-reviewed, but the company said it is seeking peer-reviewed publication.
“We are thrilled by these data, as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth,” Annaliesa Anderson, PhD, Pfizer chief scientific officer for vaccine research & development, said in a statement. “We look forward to working with the FDA and other regulatory agencies to bring this vaccine candidate to expectant mothers to help protect their infants against severe RSV during their most vulnerable first six months of life, which has the highest burden of RSV illness in infants.”
A version of this article first appeared on WebMD.com.
New trial data from drugmaker Pfizer shows promising results of a vaccine given to mothers during pregnancy that later protects infants in their first months from the worst effects of respiratory syncytial virus, or RSV.
Pfizer will apply for FDA approval by the end of the year, the company said in a statement Nov. 1.
Trial results are so promising that – after talking with government regulators – the company will stop enrolling new people in the study.
Specifically, the company reported that the vaccine prevented severe illness particularly well during the first 90 days of life, with measurable protection against severe illness continuing through 6 months of age. (That period is when infants are the most fragile if they get sick with RSV.)
RSV is a respiratory illness than can affect anyone, usually resulting in no symptoms or those similar to the common cold. But it can be particularly dangerous – and even deadly – for babies and for people over the age of 65. Pfizer and another drug company, GSK, are developing promising vaccines for older adults, the Washington Post reported.
RSV is the leading cause of hospitalization for infants, the Post noted.
The Pfizer study, called MATISSE, enrolled 7,400 pregnant women in 18 countries worldwide. Those who received the vaccine were given it during the late second to third trimester of pregnancy. Women in the study were monitored for safety through the rest of their pregnancy and 6 months after their children were born. Infants were monitored for at least 1 year for safety and effectiveness; more than half of them were monitored for 2 years.
The Pfizer vaccine works by passing maternal antibodies to the infant during pregnancy, the Post reported, noting that other vaccines transmitted via maternal immunization include those for influenza, diphtheria, tetanus, and pertussis.
Annually, RSV has a devastating impact on young children, hospitalizing tens of thousands and causing up to 300 deaths, data show.
For every 100 children who get RSV under 6 months of age, one or two of them may need to be hospitalized, according to the CDC. Those hospitalized infants may need oxygen, intubation, or even mechanical ventilation to help with breathing.
“Most improve with this type of supportive care and are discharged in a few days,” the CDC said.
“I think this is a big step for protecting babies against RSV and improving overall lung health,” vaccine researcher Barney Graham, PhD, told the Post. “Overall, it’s an exciting time for RSV. It’s also a troubling time, because you see how the patterns of infection have been changed by COVID, and we’re having an earlier, bigger season this year than we have for a couple of years – and it’s causing a lot of hospitalization and misery for people.”
As many as four RSV vaccines may have applications submitted to the FDA in 2022, according to CNN. Also in development is an antibody shot given to infants just after they are born, the news outlet reported.
Pfizer’s data, announced Tuesday, has not yet been published or peer-reviewed, but the company said it is seeking peer-reviewed publication.
“We are thrilled by these data, as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth,” Annaliesa Anderson, PhD, Pfizer chief scientific officer for vaccine research & development, said in a statement. “We look forward to working with the FDA and other regulatory agencies to bring this vaccine candidate to expectant mothers to help protect their infants against severe RSV during their most vulnerable first six months of life, which has the highest burden of RSV illness in infants.”
A version of this article first appeared on WebMD.com.
Shortage reported of antibiotic commonly used for children
The liquid form of the antibiotic amoxicillin often used to treat ear infections and strep throat in children is in short supply, just as Americans head into the season when they use the bacteria-fighting drug the most.
The FDA officially listed the shortage Oct. 28, but pharmacists, hospitals, and a supply tracking database sounded alarms earlier this month.
“The scary part is, we’re coming into the time of the year where you have the greatest need,” independent pharmacy owner Hugh Chancy, PharmD, of Georgia, told NBC News.
Thus far, reports indicate the impact of the shortages is not widespread but does affect some pharmacies, and at least one hospital has published an algorithm for offering treatment alternatives.
CVS told Bloomberg News that some stores are experiencing shortages of certain doses of amoxicillin, but a Walmart spokesperson said its diverse supply chain meant none of its pharmacies were affected.
“Hypothetically, if amoxicillin doesn’t come into stock for some time, then we’re potentially having to use less effective antibiotics with more side effects,” said Ohio pediatrician Sean Gallagher, MD, according to Bloomberg.
The shortage impacts three of the four largest amoxicillin manufacturers worldwide, according to the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota. The FDA listed the reason for the shortage as “demand increase for drug,” except in the case of manufacturer Sandoz, for which the reason listed read “information pending.”
A company spokesperson told Bloomberg the reasons were complex.
“The combination in rapid succession of the pandemic impact and consequent demand swings, manufacturing capacity constraints, scarcity of raw materials, and the current energy crisis means we face a uniquely difficult situation in the short term,” Sandoz spokesperson Leslie Pott told Bloomberg.
According to Bloomberg, other major manufacturers are still delivering the product, but limiting new orders.
The American Society of Health-System Pharmacists issued an alert for the shortage last week via its real time drug shortage database.
“Amoxicillin comes in many forms – including capsules, powders and chewable tablets – but the most common type children take is the liquid form, which makes up at least 19 products that are part of the” shortage, Becker’s Hospital Review summarized of the database reports.
The pediatric health system Children’s Minnesota told CIDRAP that supplies are low and that alternatives are being prescribed “when appropriate.”
“As a final step, we temporarily discontinued our standard procedure of dispensing the entire bottle of amoxicillin (which comes in multiple sizes),” a spokesperson told CIDRAP. “We are instead mixing and pouring the exact amount for each course of therapy, to eliminate waste.”
The Minnesota pediatric clinic and others are particularly on alert because of the surge nationwide of a respiratory virus that particularly impacts children known as RSV.
“We have certainly observed an increase in recent use most likely correlating with the surge in RSV and other respiratory viruses with concern for superimposed bacterial infection in our critically ill and hospitalized patient population,” Laura Bio, PharmD, a clinical pharmacy specialist at Stanford Medicine Children’s Health told CIDRAP.
A version of this article first appeared on WebMD.com.
The liquid form of the antibiotic amoxicillin often used to treat ear infections and strep throat in children is in short supply, just as Americans head into the season when they use the bacteria-fighting drug the most.
The FDA officially listed the shortage Oct. 28, but pharmacists, hospitals, and a supply tracking database sounded alarms earlier this month.
“The scary part is, we’re coming into the time of the year where you have the greatest need,” independent pharmacy owner Hugh Chancy, PharmD, of Georgia, told NBC News.
Thus far, reports indicate the impact of the shortages is not widespread but does affect some pharmacies, and at least one hospital has published an algorithm for offering treatment alternatives.
CVS told Bloomberg News that some stores are experiencing shortages of certain doses of amoxicillin, but a Walmart spokesperson said its diverse supply chain meant none of its pharmacies were affected.
“Hypothetically, if amoxicillin doesn’t come into stock for some time, then we’re potentially having to use less effective antibiotics with more side effects,” said Ohio pediatrician Sean Gallagher, MD, according to Bloomberg.
The shortage impacts three of the four largest amoxicillin manufacturers worldwide, according to the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota. The FDA listed the reason for the shortage as “demand increase for drug,” except in the case of manufacturer Sandoz, for which the reason listed read “information pending.”
A company spokesperson told Bloomberg the reasons were complex.
“The combination in rapid succession of the pandemic impact and consequent demand swings, manufacturing capacity constraints, scarcity of raw materials, and the current energy crisis means we face a uniquely difficult situation in the short term,” Sandoz spokesperson Leslie Pott told Bloomberg.
According to Bloomberg, other major manufacturers are still delivering the product, but limiting new orders.
The American Society of Health-System Pharmacists issued an alert for the shortage last week via its real time drug shortage database.
“Amoxicillin comes in many forms – including capsules, powders and chewable tablets – but the most common type children take is the liquid form, which makes up at least 19 products that are part of the” shortage, Becker’s Hospital Review summarized of the database reports.
The pediatric health system Children’s Minnesota told CIDRAP that supplies are low and that alternatives are being prescribed “when appropriate.”
“As a final step, we temporarily discontinued our standard procedure of dispensing the entire bottle of amoxicillin (which comes in multiple sizes),” a spokesperson told CIDRAP. “We are instead mixing and pouring the exact amount for each course of therapy, to eliminate waste.”
The Minnesota pediatric clinic and others are particularly on alert because of the surge nationwide of a respiratory virus that particularly impacts children known as RSV.
“We have certainly observed an increase in recent use most likely correlating with the surge in RSV and other respiratory viruses with concern for superimposed bacterial infection in our critically ill and hospitalized patient population,” Laura Bio, PharmD, a clinical pharmacy specialist at Stanford Medicine Children’s Health told CIDRAP.
A version of this article first appeared on WebMD.com.
The liquid form of the antibiotic amoxicillin often used to treat ear infections and strep throat in children is in short supply, just as Americans head into the season when they use the bacteria-fighting drug the most.
The FDA officially listed the shortage Oct. 28, but pharmacists, hospitals, and a supply tracking database sounded alarms earlier this month.
“The scary part is, we’re coming into the time of the year where you have the greatest need,” independent pharmacy owner Hugh Chancy, PharmD, of Georgia, told NBC News.
Thus far, reports indicate the impact of the shortages is not widespread but does affect some pharmacies, and at least one hospital has published an algorithm for offering treatment alternatives.
CVS told Bloomberg News that some stores are experiencing shortages of certain doses of amoxicillin, but a Walmart spokesperson said its diverse supply chain meant none of its pharmacies were affected.
“Hypothetically, if amoxicillin doesn’t come into stock for some time, then we’re potentially having to use less effective antibiotics with more side effects,” said Ohio pediatrician Sean Gallagher, MD, according to Bloomberg.
The shortage impacts three of the four largest amoxicillin manufacturers worldwide, according to the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota. The FDA listed the reason for the shortage as “demand increase for drug,” except in the case of manufacturer Sandoz, for which the reason listed read “information pending.”
A company spokesperson told Bloomberg the reasons were complex.
“The combination in rapid succession of the pandemic impact and consequent demand swings, manufacturing capacity constraints, scarcity of raw materials, and the current energy crisis means we face a uniquely difficult situation in the short term,” Sandoz spokesperson Leslie Pott told Bloomberg.
According to Bloomberg, other major manufacturers are still delivering the product, but limiting new orders.
The American Society of Health-System Pharmacists issued an alert for the shortage last week via its real time drug shortage database.
“Amoxicillin comes in many forms – including capsules, powders and chewable tablets – but the most common type children take is the liquid form, which makes up at least 19 products that are part of the” shortage, Becker’s Hospital Review summarized of the database reports.
The pediatric health system Children’s Minnesota told CIDRAP that supplies are low and that alternatives are being prescribed “when appropriate.”
“As a final step, we temporarily discontinued our standard procedure of dispensing the entire bottle of amoxicillin (which comes in multiple sizes),” a spokesperson told CIDRAP. “We are instead mixing and pouring the exact amount for each course of therapy, to eliminate waste.”
The Minnesota pediatric clinic and others are particularly on alert because of the surge nationwide of a respiratory virus that particularly impacts children known as RSV.
“We have certainly observed an increase in recent use most likely correlating with the surge in RSV and other respiratory viruses with concern for superimposed bacterial infection in our critically ill and hospitalized patient population,” Laura Bio, PharmD, a clinical pharmacy specialist at Stanford Medicine Children’s Health told CIDRAP.
A version of this article first appeared on WebMD.com.
The truth of alcohol consequences
Bad drinking consequence No. 87: Joining the LOTME team
Alcohol and college students go together like peanut butter and jelly. Or peanut butter and chocolate. Or peanut butter and toothpaste. Peanut butter goes with a lot of things.
Naturally, when you combine alcohol and college students, bad decisions are sure to follow. But have you ever wondered just how many bad decisions alcohol causes? A team of researchers from Penn State University, the undisputed champion of poor drinking decisions (trust us, we know), sure has. They’ve even conducted a 4-year study of 1,700 students as they carved a drunken swath through the many fine local drinking establishments, such as East Halls or that one frat house that hosts medieval battle–style ping pong tournaments.
The students were surveyed twice a year throughout the study, and the researchers compiled a list of all the various consequences their subjects experienced. Ultimately, college students will experience an average of 102 consequences from drinking during their 4-year college careers, which is an impressive number. Try thinking up a hundred consequences for anything.
Some consequences are less common than others – we imagine “missing the Renaissance Faire because you felt drunker the morning after than while you were drinking” is pretty low on the list – but more than 96% of students reported that they’d experienced a hangover and that drinking had caused them to say or do embarrassing things. Also, more than 70% said they needed additional alcohol to feel any effect, a potential sign of alcohol use disorder.
Once they had their list, the researchers focused on 12 of the more common and severe consequences, such as blacking out, hangovers, and missing work/class, and asked the study participants how their parents would react to their drinking and those specific consequences. Students who believed their parents would disapprove of alcohol-related consequences actually experienced fewer consequences overall.
College students, it seems, really do care what their parents think, even if they don’t express it, the researchers said. That gives space for parents to offer advice about the consequences of hard drinking, making decisions while drunk, or bringing godawful Fireball whiskey to parties. Seriously, don’t do that. Stuff’s bad, and you should feel bad for bringing it. Your parents raised you better than that.
COVID ‘expert’ discusses data sharing
We interrupt our regularly scheduled programming to bring you this special news event. Elon Musk, the world’s second-most annoying human, is holding a press conference to discuss, of all things, COVID-19.
Reporter: Hey, Mr. Musketeer, what qualifies you to talk about a global pandemic?
EM: As the official king of the Twitterverse, I’m pretty much an expert on any topic.
Reporter: Okay then, Mr. Muskmelon, what can you tell us about the new study in Agricultural Economics, which looked at consumers’ knowledge of local COVID infection rates and their willingness to eat at restaurants?
EM: Well, I know that one of the investigators, Rigoberto Lopez, PhD, of the University of Connecticut, said “no news is bad news.” Restaurants located in cities where local regulations required COVID tracking recovered faster than those in areas that did not, according to data from 87 restaurants in 10 Chinese cities that were gathered between Dec. 1, 2019, and March 27, 2020. Having access to local infection rate data made customers more comfortable going out to eat, the investigators explained.
Second reporter: Interesting, Mr. Muskox, but how about this headline from CNN: “Workers flee China’s biggest iPhone factory over Covid outbreak”? Do you agree with analysts, who said that “the chaos at Zhengzhou could jeopardize Apple and Foxconn’s output in the coming weeks,” as CNN put it?
EM: I did see that a manager at Foxconn, which owns the factory and is known to its friends as Hon Hai Precision Industry, told a Chinese media outlet that “workers are panicking over the spread of the virus at the factory and lack of access to official information.” As we’ve already discussed, no news is bad news.
That’s all the time I have to chat with you today. I’m off to fire some more Twitter employees.
In case you hadn’t already guessed, Vlad Putin is officially more annoying than Elon Musk. We now return to this week’s typical LOTME shenanigans, already in progress.
The deadliest month
With climate change making the world hotter, leading to more heat stroke and organ failure, you would think the summer months would be the most deadly. In reality, though, it’s quite the opposite.
There are multiple factors that make January the most deadly month out of the year, as LiveScience discovered in a recent analysis.
Let’s go through them, shall we?
Respiratory viruses: Robert Glatter, MD, of Lenox Hill Hospital in New York, told LiveScence that winter is the time for illnesses like the flu, bacterial pneumonia, and RSV. Millions of people worldwide die from the flu, according to the CDC. And the World Health Organization reported lower respiratory infections as the fourth-leading cause of death worldwide before COVID came along.
Heart disease: Heart conditions are actually more fatal in the winter months, according to a study published in Circulation. The cold puts more stress on the heart to keep the body warm, which can be a challenge for people who already have preexisting heart conditions.
Space heaters: Dr. Glatter also told Live Science that the use of space heaters could be a factor in the cold winter months since they can lead to carbon monoxide poisoning and even fires. Silent killers.
Holiday season: A time for joy and merriment, certainly, but Christmas et al. have their downsides. By January we’re coming off a 3-month food and alcohol binge, which leads to cardiac stress. There’s also the psychological stress that comes with the season. Sometimes the most wonderful time of the year just isn’t.
So even though summer is hot, fall has hurricanes, and spring tends to have the highest suicide rate, winter still ends up being the deadliest season.
Bad drinking consequence No. 87: Joining the LOTME team
Alcohol and college students go together like peanut butter and jelly. Or peanut butter and chocolate. Or peanut butter and toothpaste. Peanut butter goes with a lot of things.
Naturally, when you combine alcohol and college students, bad decisions are sure to follow. But have you ever wondered just how many bad decisions alcohol causes? A team of researchers from Penn State University, the undisputed champion of poor drinking decisions (trust us, we know), sure has. They’ve even conducted a 4-year study of 1,700 students as they carved a drunken swath through the many fine local drinking establishments, such as East Halls or that one frat house that hosts medieval battle–style ping pong tournaments.
The students were surveyed twice a year throughout the study, and the researchers compiled a list of all the various consequences their subjects experienced. Ultimately, college students will experience an average of 102 consequences from drinking during their 4-year college careers, which is an impressive number. Try thinking up a hundred consequences for anything.
Some consequences are less common than others – we imagine “missing the Renaissance Faire because you felt drunker the morning after than while you were drinking” is pretty low on the list – but more than 96% of students reported that they’d experienced a hangover and that drinking had caused them to say or do embarrassing things. Also, more than 70% said they needed additional alcohol to feel any effect, a potential sign of alcohol use disorder.
Once they had their list, the researchers focused on 12 of the more common and severe consequences, such as blacking out, hangovers, and missing work/class, and asked the study participants how their parents would react to their drinking and those specific consequences. Students who believed their parents would disapprove of alcohol-related consequences actually experienced fewer consequences overall.
College students, it seems, really do care what their parents think, even if they don’t express it, the researchers said. That gives space for parents to offer advice about the consequences of hard drinking, making decisions while drunk, or bringing godawful Fireball whiskey to parties. Seriously, don’t do that. Stuff’s bad, and you should feel bad for bringing it. Your parents raised you better than that.
COVID ‘expert’ discusses data sharing
We interrupt our regularly scheduled programming to bring you this special news event. Elon Musk, the world’s second-most annoying human, is holding a press conference to discuss, of all things, COVID-19.
Reporter: Hey, Mr. Musketeer, what qualifies you to talk about a global pandemic?
EM: As the official king of the Twitterverse, I’m pretty much an expert on any topic.
Reporter: Okay then, Mr. Muskmelon, what can you tell us about the new study in Agricultural Economics, which looked at consumers’ knowledge of local COVID infection rates and their willingness to eat at restaurants?
EM: Well, I know that one of the investigators, Rigoberto Lopez, PhD, of the University of Connecticut, said “no news is bad news.” Restaurants located in cities where local regulations required COVID tracking recovered faster than those in areas that did not, according to data from 87 restaurants in 10 Chinese cities that were gathered between Dec. 1, 2019, and March 27, 2020. Having access to local infection rate data made customers more comfortable going out to eat, the investigators explained.
Second reporter: Interesting, Mr. Muskox, but how about this headline from CNN: “Workers flee China’s biggest iPhone factory over Covid outbreak”? Do you agree with analysts, who said that “the chaos at Zhengzhou could jeopardize Apple and Foxconn’s output in the coming weeks,” as CNN put it?
EM: I did see that a manager at Foxconn, which owns the factory and is known to its friends as Hon Hai Precision Industry, told a Chinese media outlet that “workers are panicking over the spread of the virus at the factory and lack of access to official information.” As we’ve already discussed, no news is bad news.
That’s all the time I have to chat with you today. I’m off to fire some more Twitter employees.
In case you hadn’t already guessed, Vlad Putin is officially more annoying than Elon Musk. We now return to this week’s typical LOTME shenanigans, already in progress.
The deadliest month
With climate change making the world hotter, leading to more heat stroke and organ failure, you would think the summer months would be the most deadly. In reality, though, it’s quite the opposite.
There are multiple factors that make January the most deadly month out of the year, as LiveScience discovered in a recent analysis.
Let’s go through them, shall we?
Respiratory viruses: Robert Glatter, MD, of Lenox Hill Hospital in New York, told LiveScence that winter is the time for illnesses like the flu, bacterial pneumonia, and RSV. Millions of people worldwide die from the flu, according to the CDC. And the World Health Organization reported lower respiratory infections as the fourth-leading cause of death worldwide before COVID came along.
Heart disease: Heart conditions are actually more fatal in the winter months, according to a study published in Circulation. The cold puts more stress on the heart to keep the body warm, which can be a challenge for people who already have preexisting heart conditions.
Space heaters: Dr. Glatter also told Live Science that the use of space heaters could be a factor in the cold winter months since they can lead to carbon monoxide poisoning and even fires. Silent killers.
Holiday season: A time for joy and merriment, certainly, but Christmas et al. have their downsides. By January we’re coming off a 3-month food and alcohol binge, which leads to cardiac stress. There’s also the psychological stress that comes with the season. Sometimes the most wonderful time of the year just isn’t.
So even though summer is hot, fall has hurricanes, and spring tends to have the highest suicide rate, winter still ends up being the deadliest season.
Bad drinking consequence No. 87: Joining the LOTME team
Alcohol and college students go together like peanut butter and jelly. Or peanut butter and chocolate. Or peanut butter and toothpaste. Peanut butter goes with a lot of things.
Naturally, when you combine alcohol and college students, bad decisions are sure to follow. But have you ever wondered just how many bad decisions alcohol causes? A team of researchers from Penn State University, the undisputed champion of poor drinking decisions (trust us, we know), sure has. They’ve even conducted a 4-year study of 1,700 students as they carved a drunken swath through the many fine local drinking establishments, such as East Halls or that one frat house that hosts medieval battle–style ping pong tournaments.
The students were surveyed twice a year throughout the study, and the researchers compiled a list of all the various consequences their subjects experienced. Ultimately, college students will experience an average of 102 consequences from drinking during their 4-year college careers, which is an impressive number. Try thinking up a hundred consequences for anything.
Some consequences are less common than others – we imagine “missing the Renaissance Faire because you felt drunker the morning after than while you were drinking” is pretty low on the list – but more than 96% of students reported that they’d experienced a hangover and that drinking had caused them to say or do embarrassing things. Also, more than 70% said they needed additional alcohol to feel any effect, a potential sign of alcohol use disorder.
Once they had their list, the researchers focused on 12 of the more common and severe consequences, such as blacking out, hangovers, and missing work/class, and asked the study participants how their parents would react to their drinking and those specific consequences. Students who believed their parents would disapprove of alcohol-related consequences actually experienced fewer consequences overall.
College students, it seems, really do care what their parents think, even if they don’t express it, the researchers said. That gives space for parents to offer advice about the consequences of hard drinking, making decisions while drunk, or bringing godawful Fireball whiskey to parties. Seriously, don’t do that. Stuff’s bad, and you should feel bad for bringing it. Your parents raised you better than that.
COVID ‘expert’ discusses data sharing
We interrupt our regularly scheduled programming to bring you this special news event. Elon Musk, the world’s second-most annoying human, is holding a press conference to discuss, of all things, COVID-19.
Reporter: Hey, Mr. Musketeer, what qualifies you to talk about a global pandemic?
EM: As the official king of the Twitterverse, I’m pretty much an expert on any topic.
Reporter: Okay then, Mr. Muskmelon, what can you tell us about the new study in Agricultural Economics, which looked at consumers’ knowledge of local COVID infection rates and their willingness to eat at restaurants?
EM: Well, I know that one of the investigators, Rigoberto Lopez, PhD, of the University of Connecticut, said “no news is bad news.” Restaurants located in cities where local regulations required COVID tracking recovered faster than those in areas that did not, according to data from 87 restaurants in 10 Chinese cities that were gathered between Dec. 1, 2019, and March 27, 2020. Having access to local infection rate data made customers more comfortable going out to eat, the investigators explained.
Second reporter: Interesting, Mr. Muskox, but how about this headline from CNN: “Workers flee China’s biggest iPhone factory over Covid outbreak”? Do you agree with analysts, who said that “the chaos at Zhengzhou could jeopardize Apple and Foxconn’s output in the coming weeks,” as CNN put it?
EM: I did see that a manager at Foxconn, which owns the factory and is known to its friends as Hon Hai Precision Industry, told a Chinese media outlet that “workers are panicking over the spread of the virus at the factory and lack of access to official information.” As we’ve already discussed, no news is bad news.
That’s all the time I have to chat with you today. I’m off to fire some more Twitter employees.
In case you hadn’t already guessed, Vlad Putin is officially more annoying than Elon Musk. We now return to this week’s typical LOTME shenanigans, already in progress.
The deadliest month
With climate change making the world hotter, leading to more heat stroke and organ failure, you would think the summer months would be the most deadly. In reality, though, it’s quite the opposite.
There are multiple factors that make January the most deadly month out of the year, as LiveScience discovered in a recent analysis.
Let’s go through them, shall we?
Respiratory viruses: Robert Glatter, MD, of Lenox Hill Hospital in New York, told LiveScence that winter is the time for illnesses like the flu, bacterial pneumonia, and RSV. Millions of people worldwide die from the flu, according to the CDC. And the World Health Organization reported lower respiratory infections as the fourth-leading cause of death worldwide before COVID came along.
Heart disease: Heart conditions are actually more fatal in the winter months, according to a study published in Circulation. The cold puts more stress on the heart to keep the body warm, which can be a challenge for people who already have preexisting heart conditions.
Space heaters: Dr. Glatter also told Live Science that the use of space heaters could be a factor in the cold winter months since they can lead to carbon monoxide poisoning and even fires. Silent killers.
Holiday season: A time for joy and merriment, certainly, but Christmas et al. have their downsides. By January we’re coming off a 3-month food and alcohol binge, which leads to cardiac stress. There’s also the psychological stress that comes with the season. Sometimes the most wonderful time of the year just isn’t.
So even though summer is hot, fall has hurricanes, and spring tends to have the highest suicide rate, winter still ends up being the deadliest season.
Mid-October flulike illness cases higher than past 5 years
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”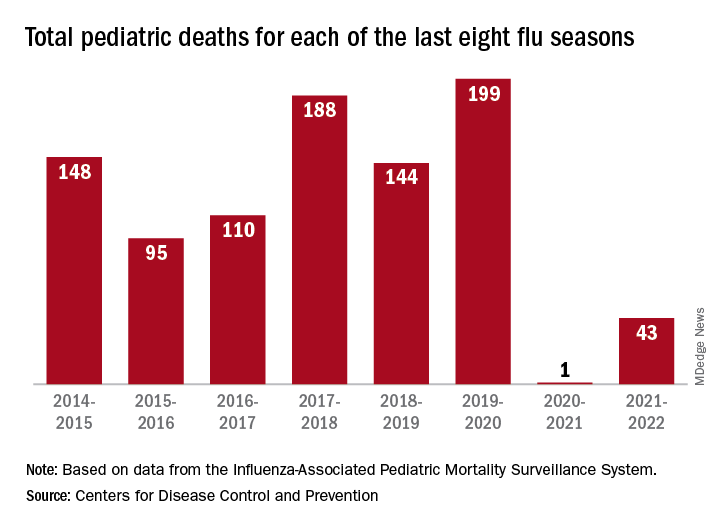
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
FDA rejects bulevirtide for hepatitis D
The U.S. Food and Drug Administration (FDA) has declined to approve bulevirtide, Gilead Sciences’ drug for the treatment of hepatitis delta virus (HDV) infection and compensated liver disease.
In a complete response letter, the FDA voiced concerns over the production and delivery of bulevirtide, an investigational, first-in-class HDV entry-inhibitor that received conditional approved in Europe in 2020.
The FDA did not request new studies to evaluate the safety and efficacy of bulevirtide.
As reported previously by this news organization, data from an ongoing phase 3 trial showed that after 48 weeks of treatment, almost half of those treated with bulevirtide achieved the combined primary endpoint of reduced or undetectable HDV RNA levels and normalized alanine aminotransferase levels.
Chronic HDV infection is the most severe form of viral hepatitis. It is associated with a poor prognosis and high mortality rates.
There are currently no approved treatments for HDV in the United States. Bulevirtide was granted breakthrough therapy and orphan drug designations by the FDA.
Merdad Parsey, MD, PhD, chief medical officer, Gilead Sciences, wrote in a news release that the company looks forward to “continuing our active discussions with FDA so that we may bring bulevirtide to people living with HDV in the U.S. as soon as possible.”
This is the second manufacturing-related complete response letter Gilead has received in the past 8 months. In March, the FDA rejected the long-acting HIV drug lenacapavir. The drug was sanctioned in Europe and the United Kingdom in September.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration (FDA) has declined to approve bulevirtide, Gilead Sciences’ drug for the treatment of hepatitis delta virus (HDV) infection and compensated liver disease.
In a complete response letter, the FDA voiced concerns over the production and delivery of bulevirtide, an investigational, first-in-class HDV entry-inhibitor that received conditional approved in Europe in 2020.
The FDA did not request new studies to evaluate the safety and efficacy of bulevirtide.
As reported previously by this news organization, data from an ongoing phase 3 trial showed that after 48 weeks of treatment, almost half of those treated with bulevirtide achieved the combined primary endpoint of reduced or undetectable HDV RNA levels and normalized alanine aminotransferase levels.
Chronic HDV infection is the most severe form of viral hepatitis. It is associated with a poor prognosis and high mortality rates.
There are currently no approved treatments for HDV in the United States. Bulevirtide was granted breakthrough therapy and orphan drug designations by the FDA.
Merdad Parsey, MD, PhD, chief medical officer, Gilead Sciences, wrote in a news release that the company looks forward to “continuing our active discussions with FDA so that we may bring bulevirtide to people living with HDV in the U.S. as soon as possible.”
This is the second manufacturing-related complete response letter Gilead has received in the past 8 months. In March, the FDA rejected the long-acting HIV drug lenacapavir. The drug was sanctioned in Europe and the United Kingdom in September.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration (FDA) has declined to approve bulevirtide, Gilead Sciences’ drug for the treatment of hepatitis delta virus (HDV) infection and compensated liver disease.
In a complete response letter, the FDA voiced concerns over the production and delivery of bulevirtide, an investigational, first-in-class HDV entry-inhibitor that received conditional approved in Europe in 2020.
The FDA did not request new studies to evaluate the safety and efficacy of bulevirtide.
As reported previously by this news organization, data from an ongoing phase 3 trial showed that after 48 weeks of treatment, almost half of those treated with bulevirtide achieved the combined primary endpoint of reduced or undetectable HDV RNA levels and normalized alanine aminotransferase levels.
Chronic HDV infection is the most severe form of viral hepatitis. It is associated with a poor prognosis and high mortality rates.
There are currently no approved treatments for HDV in the United States. Bulevirtide was granted breakthrough therapy and orphan drug designations by the FDA.
Merdad Parsey, MD, PhD, chief medical officer, Gilead Sciences, wrote in a news release that the company looks forward to “continuing our active discussions with FDA so that we may bring bulevirtide to people living with HDV in the U.S. as soon as possible.”
This is the second manufacturing-related complete response letter Gilead has received in the past 8 months. In March, the FDA rejected the long-acting HIV drug lenacapavir. The drug was sanctioned in Europe and the United Kingdom in September.
A version of this article first appeared on Medscape.com.
‘Unappreciated’ ties between COVID and gut dysbiosis
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Genital HSV shedding declines rapidly in first year post infection
Shedding of genital herpes simplex virus was frequent soon after first-time infection but declined significantly during the first year, based on data from 82 individuals.
Genital herpes simplex virus (HSV) infections remain common and incurable; consequently, the population with residual infection continues to rise, Christine Johnston, MD, of the University of Washington, Seattle, and colleagues wrote. However, data on the viral shedding trajectory of genital HSV-1 are limited, although HSV-1 accounts for an increasing number of infections.
In a study published in JAMA the researchers recruited 82 women with first-episode genital HSV-1 infections from sexual health and primary care clinics in Seattle, between 2013 and 2018. The participants supplied self-collected oral and genital swabs for daily HSV polymerase chain reaction testing for two 30-day periods at 2 months and 11 months after their initial symptoms. The study population was not pregnant and did not have HIV infection. The median age of the participants was 26 years, 54 were women, and 42 had primary HSV-1 infections. Primary HSV-1 infection was defined as the lack of HSV antibody at baseline or an evolving antibody profile, based on the University of Washington HSV Western Blot.
The primary outcome was the rates of genital and oral HSV shedding and lesions at 2 and 11 months and up to 2 years after an initial HSV-1 infection.
At 2 months, approximately two-thirds (64.6%) of the participants had HSV-1 in the genital tract and 29.3% had virus in the mouth. Genital shedding of HSV-1 was detected in 12.1% of 2,264 total testing days at 2 months, but this rate declined to 7.1% of 1,719 testing days at 11 months (relative risk, 0.52).
The researchers identified oral HSV-1 shedding on 3.9% of 2,247 testing days at 2 months, with a slight increase to 5.1% of 1,714 testing days at 11 months.
Both genital and oral lesions were rare, with reports of 2.6% and 0.4%, respectively, at 2 months and 3.8% and 0.5%, respectively, at 11 months.
The risk of genital shedding was significantly higher in individuals with primary HSV-1, compared with those with nonprimary infections (7.9% vs. 2.9%; RR, 2.75). The overall rate of genital shedding was 17.2% for those with primary HSV-1, of which 15.2% was asymptomatic. Oral shedding was similar for individuals with primary and nonprimary HSV in a multivariate analysis.
In addition, HSV-specific CD4+ and CD8+ T-cell responses were identified in all participants, and these remained stable during the study period. No association appeared between rates of genital and oral shedding and the proportion of cells that expressed two, three, or four cytokines.
The current study is the first known to comprehensively assess genital and oral HSV-1 viral shedding using polymerase chain reaction, the researchers wrote. “Characterizing shedding rates is clinically important because patients with genital herpes are often concerned about transmission to sexual partners, which usually occurs in the absence of lesions.”
The study findings were limited by several factors including the 22% loss of participants to follow-up by the end of the first year, and the use of data from a single location with a primarily White population, the researchers noted. Another limitation was reliance on self-reports and the potential underestimation of recurrences because of the possible use of antiviral medications between swabbing periods.
However, the results indicate the early frequency of HSV-1 shedding and suggest that suppressive therapy might benefit individuals with primary HSV-1 during their first year of infection, the researchers said.
Findings may improve HSV management
The current study helps fill a knowledge gap regarding the natural history of genital HSV-1 infections, Richard J. Whitley, MD, and Edward W. Hook III, MD, both of the University of Alabama at Birmingham, wrote in an accompanying editorial. Despite the small study population, the data represent the largest cohort to date of individuals with first-episode infection and up to 2 years’ follow-up.
Although HSV-2 shedding is greater and associated with more symptoms, seroprevalence of HSV-2 in the United States is declining, they noted. Therefore, the findings can inform patient counseling and recommendations for antiviral therapy that may extend to managing HSV-1 in pregnant women as well, although no pregnant women were included in the study.
“For clinicians, these data emphasize the importance of determining the HSV viral type in persons presenting with initial episodes of genital herpes to accurately counsel patients regarding risk of clinical recurrence, the likelihood of asymptomatic shedding of virus and hence transmission, and antiviral prophylaxis,” the editorialists emphasized. For investigators, the results should prompt additional studies of the host defense against HSV and improved serological testing.
Study supports need for attention to HSV-1
“Genital herpes is an extremely common sexually transmitted infection, and often only HSV-2 is measured,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “This study shows that HSV-1 also accounts for a significant amount of genital disease, and should also be considered when determining prevalence of genital herpes.
“I was not surprised to see that viral shedding decreased significantly over the first year after diagnosis, and similarly not surprised that lesions were rare after the initial infection,” said Dr. Prager, who was not involved in the study. “I was somewhat surprised to see that genital HSV-1 shedding was more common than oral shedding.”
Dr. Prager said that she would advise clinicians against serum HSV testing unless someone has an active genital lesion. “Testing after a lesion will often reveal HSV-1, and patients should be counseled that shedding will decrease over the first year. Subsequent genital lesions are uncommon, but certainly possible, and oral lesions and shedding are both rare.” ]
More research is needed in a more diverse population, Dr. Prager emphasized. Following patients for more than a year and learning more about the use of antiviral medications also would be useful.
The study was supported in part by the National Institutes of Health/National Institute of Allergy and Infectious Diseases through grants to several authors, including lead author Dr. Johnston. Dr. Johnston also disclosed personal fees from AbbVie, grants from Gilead, royalties from UpToDate, and personal fees from GlaxoSmithKline unrelated to the current study. Dr. Whitley disclosed personal fees from Virios Therapeutics as a board member and shareholder during the conduct of the study, royalties from Aettis unrelated to the submitted work, and serving on an advisory board for Visby Diagnostics. Dr. Hook disclosed serving on an advisory board for Visby Diagnostics unrelated to the submitted work. Dr. Prager had no conflicts to disclose and serves on the editorial advisory board of Ob.Gyn News.
Shedding of genital herpes simplex virus was frequent soon after first-time infection but declined significantly during the first year, based on data from 82 individuals.
Genital herpes simplex virus (HSV) infections remain common and incurable; consequently, the population with residual infection continues to rise, Christine Johnston, MD, of the University of Washington, Seattle, and colleagues wrote. However, data on the viral shedding trajectory of genital HSV-1 are limited, although HSV-1 accounts for an increasing number of infections.
In a study published in JAMA the researchers recruited 82 women with first-episode genital HSV-1 infections from sexual health and primary care clinics in Seattle, between 2013 and 2018. The participants supplied self-collected oral and genital swabs for daily HSV polymerase chain reaction testing for two 30-day periods at 2 months and 11 months after their initial symptoms. The study population was not pregnant and did not have HIV infection. The median age of the participants was 26 years, 54 were women, and 42 had primary HSV-1 infections. Primary HSV-1 infection was defined as the lack of HSV antibody at baseline or an evolving antibody profile, based on the University of Washington HSV Western Blot.
The primary outcome was the rates of genital and oral HSV shedding and lesions at 2 and 11 months and up to 2 years after an initial HSV-1 infection.
At 2 months, approximately two-thirds (64.6%) of the participants had HSV-1 in the genital tract and 29.3% had virus in the mouth. Genital shedding of HSV-1 was detected in 12.1% of 2,264 total testing days at 2 months, but this rate declined to 7.1% of 1,719 testing days at 11 months (relative risk, 0.52).
The researchers identified oral HSV-1 shedding on 3.9% of 2,247 testing days at 2 months, with a slight increase to 5.1% of 1,714 testing days at 11 months.
Both genital and oral lesions were rare, with reports of 2.6% and 0.4%, respectively, at 2 months and 3.8% and 0.5%, respectively, at 11 months.
The risk of genital shedding was significantly higher in individuals with primary HSV-1, compared with those with nonprimary infections (7.9% vs. 2.9%; RR, 2.75). The overall rate of genital shedding was 17.2% for those with primary HSV-1, of which 15.2% was asymptomatic. Oral shedding was similar for individuals with primary and nonprimary HSV in a multivariate analysis.
In addition, HSV-specific CD4+ and CD8+ T-cell responses were identified in all participants, and these remained stable during the study period. No association appeared between rates of genital and oral shedding and the proportion of cells that expressed two, three, or four cytokines.
The current study is the first known to comprehensively assess genital and oral HSV-1 viral shedding using polymerase chain reaction, the researchers wrote. “Characterizing shedding rates is clinically important because patients with genital herpes are often concerned about transmission to sexual partners, which usually occurs in the absence of lesions.”
The study findings were limited by several factors including the 22% loss of participants to follow-up by the end of the first year, and the use of data from a single location with a primarily White population, the researchers noted. Another limitation was reliance on self-reports and the potential underestimation of recurrences because of the possible use of antiviral medications between swabbing periods.
However, the results indicate the early frequency of HSV-1 shedding and suggest that suppressive therapy might benefit individuals with primary HSV-1 during their first year of infection, the researchers said.
Findings may improve HSV management
The current study helps fill a knowledge gap regarding the natural history of genital HSV-1 infections, Richard J. Whitley, MD, and Edward W. Hook III, MD, both of the University of Alabama at Birmingham, wrote in an accompanying editorial. Despite the small study population, the data represent the largest cohort to date of individuals with first-episode infection and up to 2 years’ follow-up.
Although HSV-2 shedding is greater and associated with more symptoms, seroprevalence of HSV-2 in the United States is declining, they noted. Therefore, the findings can inform patient counseling and recommendations for antiviral therapy that may extend to managing HSV-1 in pregnant women as well, although no pregnant women were included in the study.
“For clinicians, these data emphasize the importance of determining the HSV viral type in persons presenting with initial episodes of genital herpes to accurately counsel patients regarding risk of clinical recurrence, the likelihood of asymptomatic shedding of virus and hence transmission, and antiviral prophylaxis,” the editorialists emphasized. For investigators, the results should prompt additional studies of the host defense against HSV and improved serological testing.
Study supports need for attention to HSV-1
“Genital herpes is an extremely common sexually transmitted infection, and often only HSV-2 is measured,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “This study shows that HSV-1 also accounts for a significant amount of genital disease, and should also be considered when determining prevalence of genital herpes.
“I was not surprised to see that viral shedding decreased significantly over the first year after diagnosis, and similarly not surprised that lesions were rare after the initial infection,” said Dr. Prager, who was not involved in the study. “I was somewhat surprised to see that genital HSV-1 shedding was more common than oral shedding.”
Dr. Prager said that she would advise clinicians against serum HSV testing unless someone has an active genital lesion. “Testing after a lesion will often reveal HSV-1, and patients should be counseled that shedding will decrease over the first year. Subsequent genital lesions are uncommon, but certainly possible, and oral lesions and shedding are both rare.” ]
More research is needed in a more diverse population, Dr. Prager emphasized. Following patients for more than a year and learning more about the use of antiviral medications also would be useful.
The study was supported in part by the National Institutes of Health/National Institute of Allergy and Infectious Diseases through grants to several authors, including lead author Dr. Johnston. Dr. Johnston also disclosed personal fees from AbbVie, grants from Gilead, royalties from UpToDate, and personal fees from GlaxoSmithKline unrelated to the current study. Dr. Whitley disclosed personal fees from Virios Therapeutics as a board member and shareholder during the conduct of the study, royalties from Aettis unrelated to the submitted work, and serving on an advisory board for Visby Diagnostics. Dr. Hook disclosed serving on an advisory board for Visby Diagnostics unrelated to the submitted work. Dr. Prager had no conflicts to disclose and serves on the editorial advisory board of Ob.Gyn News.
Shedding of genital herpes simplex virus was frequent soon after first-time infection but declined significantly during the first year, based on data from 82 individuals.
Genital herpes simplex virus (HSV) infections remain common and incurable; consequently, the population with residual infection continues to rise, Christine Johnston, MD, of the University of Washington, Seattle, and colleagues wrote. However, data on the viral shedding trajectory of genital HSV-1 are limited, although HSV-1 accounts for an increasing number of infections.
In a study published in JAMA the researchers recruited 82 women with first-episode genital HSV-1 infections from sexual health and primary care clinics in Seattle, between 2013 and 2018. The participants supplied self-collected oral and genital swabs for daily HSV polymerase chain reaction testing for two 30-day periods at 2 months and 11 months after their initial symptoms. The study population was not pregnant and did not have HIV infection. The median age of the participants was 26 years, 54 were women, and 42 had primary HSV-1 infections. Primary HSV-1 infection was defined as the lack of HSV antibody at baseline or an evolving antibody profile, based on the University of Washington HSV Western Blot.
The primary outcome was the rates of genital and oral HSV shedding and lesions at 2 and 11 months and up to 2 years after an initial HSV-1 infection.
At 2 months, approximately two-thirds (64.6%) of the participants had HSV-1 in the genital tract and 29.3% had virus in the mouth. Genital shedding of HSV-1 was detected in 12.1% of 2,264 total testing days at 2 months, but this rate declined to 7.1% of 1,719 testing days at 11 months (relative risk, 0.52).
The researchers identified oral HSV-1 shedding on 3.9% of 2,247 testing days at 2 months, with a slight increase to 5.1% of 1,714 testing days at 11 months.
Both genital and oral lesions were rare, with reports of 2.6% and 0.4%, respectively, at 2 months and 3.8% and 0.5%, respectively, at 11 months.
The risk of genital shedding was significantly higher in individuals with primary HSV-1, compared with those with nonprimary infections (7.9% vs. 2.9%; RR, 2.75). The overall rate of genital shedding was 17.2% for those with primary HSV-1, of which 15.2% was asymptomatic. Oral shedding was similar for individuals with primary and nonprimary HSV in a multivariate analysis.
In addition, HSV-specific CD4+ and CD8+ T-cell responses were identified in all participants, and these remained stable during the study period. No association appeared between rates of genital and oral shedding and the proportion of cells that expressed two, three, or four cytokines.
The current study is the first known to comprehensively assess genital and oral HSV-1 viral shedding using polymerase chain reaction, the researchers wrote. “Characterizing shedding rates is clinically important because patients with genital herpes are often concerned about transmission to sexual partners, which usually occurs in the absence of lesions.”
The study findings were limited by several factors including the 22% loss of participants to follow-up by the end of the first year, and the use of data from a single location with a primarily White population, the researchers noted. Another limitation was reliance on self-reports and the potential underestimation of recurrences because of the possible use of antiviral medications between swabbing periods.
However, the results indicate the early frequency of HSV-1 shedding and suggest that suppressive therapy might benefit individuals with primary HSV-1 during their first year of infection, the researchers said.
Findings may improve HSV management
The current study helps fill a knowledge gap regarding the natural history of genital HSV-1 infections, Richard J. Whitley, MD, and Edward W. Hook III, MD, both of the University of Alabama at Birmingham, wrote in an accompanying editorial. Despite the small study population, the data represent the largest cohort to date of individuals with first-episode infection and up to 2 years’ follow-up.
Although HSV-2 shedding is greater and associated with more symptoms, seroprevalence of HSV-2 in the United States is declining, they noted. Therefore, the findings can inform patient counseling and recommendations for antiviral therapy that may extend to managing HSV-1 in pregnant women as well, although no pregnant women were included in the study.
“For clinicians, these data emphasize the importance of determining the HSV viral type in persons presenting with initial episodes of genital herpes to accurately counsel patients regarding risk of clinical recurrence, the likelihood of asymptomatic shedding of virus and hence transmission, and antiviral prophylaxis,” the editorialists emphasized. For investigators, the results should prompt additional studies of the host defense against HSV and improved serological testing.
Study supports need for attention to HSV-1
“Genital herpes is an extremely common sexually transmitted infection, and often only HSV-2 is measured,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “This study shows that HSV-1 also accounts for a significant amount of genital disease, and should also be considered when determining prevalence of genital herpes.
“I was not surprised to see that viral shedding decreased significantly over the first year after diagnosis, and similarly not surprised that lesions were rare after the initial infection,” said Dr. Prager, who was not involved in the study. “I was somewhat surprised to see that genital HSV-1 shedding was more common than oral shedding.”
Dr. Prager said that she would advise clinicians against serum HSV testing unless someone has an active genital lesion. “Testing after a lesion will often reveal HSV-1, and patients should be counseled that shedding will decrease over the first year. Subsequent genital lesions are uncommon, but certainly possible, and oral lesions and shedding are both rare.” ]
More research is needed in a more diverse population, Dr. Prager emphasized. Following patients for more than a year and learning more about the use of antiviral medications also would be useful.
The study was supported in part by the National Institutes of Health/National Institute of Allergy and Infectious Diseases through grants to several authors, including lead author Dr. Johnston. Dr. Johnston also disclosed personal fees from AbbVie, grants from Gilead, royalties from UpToDate, and personal fees from GlaxoSmithKline unrelated to the current study. Dr. Whitley disclosed personal fees from Virios Therapeutics as a board member and shareholder during the conduct of the study, royalties from Aettis unrelated to the submitted work, and serving on an advisory board for Visby Diagnostics. Dr. Hook disclosed serving on an advisory board for Visby Diagnostics unrelated to the submitted work. Dr. Prager had no conflicts to disclose and serves on the editorial advisory board of Ob.Gyn News.
FROM JAMA
Children and COVID: Weekly cases can’t sustain downward trend
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.



