User login
In Case You Missed It: COVID
FDA grants emergency use authorization to Lilly’s antibody COVID-19 therapy
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
Great Barrington coauthor backs off strict reliance on herd immunity
A coauthor of the Great Barrington Declaration says that he and colleagues have never argued against using mitigation strategies to keep COVID-19 from spreading, and that critics have mischaracterized the document as a “let it rip” strategy.
Jay Bhattacharya, MD, PhD, a professor and public health policy expert in infectious diseases at Stanford University in California, spoke on a JAMA Livestream debate on November 6. Marc Lipsitch, MD, an epidemiology professor at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, represented the 6900 signatories of the John Snow Memorandum, a rebuttal to the Great Barrington document.
The Great Barrington approach of “Focused Protection” advocates isolation and protection of people who are most vulnerable to COVID-19 while avoiding what they characterize as lockdowns. “The most compassionate approach that balances the risks and benefits of reaching herd immunity, is to allow those who are at minimal risk of death to live their lives normally to build up immunity to the virus through natural infection, while better protecting those who are at highest risk,” the document reads.
The Infectious Diseases Society of America (IDSA) and its HIV Medicine Association denounced the declaration, as reported by Medscape Medical News, and the World Health Organization (WHO) Director General Tedros Adhanom Ghebreyesus called the proposal “unethical.” But the idea has gained some traction at the White House, where Coronavirus Task Force Member and Stanford professor Scott Atlas, MD, has been advising President Donald J. Trump.
On the JAMA debate, Bhattacharya said, “I think all of the mitigation measures are really important,” listing social distancing, hand washing, and masks when distancing is not possible as chief among those strategies for the less vulnerable. “I don’t want to create infections intentionally, but I want us to allow people to go back to their lives as best they can, understanding of the risks they are taking when they do it,” he said, claiming that 99.95% of the population will survive infection.
“The harmful lockdowns are worse for many, many people,” Bhattacharya said.
“I think Jay is moving towards a middle ground which is not really what the Great Barrington Declaration seems to promote,” countered Lipsitch. The declaration does not say use masks or social distance, he said. “It just says we need to go back to a normal life.”
Bhattacharya’s statements to JAMA mean that “maybe we are approaching some common ground,” Lipsitch said.
Definition of a lockdown
Both men were asked to give their definition of a “lockdown.” To Lipsitch, it means people are not allowed out except for essential services and that most businesses are closed, with exceptions for those deemed essential.
Bhattacharya, however, said he views that as a quarantine. Lockdowns “are what we’re currently doing,” he said. Schools, churches, businesses, and arts and culture organizations are shuttered, and “almost every aspect of society is restricted in some way,” Bhattacharya said.
He blamed these lockdowns for most of the excess deaths over and above the COVID-19 deaths and said they had failed to control the pandemic.
Lipsitch said that “it feels to me that Jay is describing as lockdown everything that causes harm, even when it’s not locked down.” He noted that the country was truly closed down for 2 months or so in the spring.
“All of these harms I agree are real,” said Lipsitch. “But they are because the normal life of our society is being interfered with by viral transmission and by people’s inability to live their normal lives.”
Closures and lockdowns are essential to delaying cases and deaths, said Lipsitch. “A case today is worse than a case tomorrow and a lot worse than a case 6 months from now,” he said, noting that a vaccine or improved therapeutics could evolve.
“Delay is not nothing,” Lipsitch added. “It’s actually the goal as I see it, and as the John Snow memo says, we want to keep the virus under control in such a way as that the vulnerable people are not at risk.”
He predicted that cases will continue to grow exponentially because the nation is “not even close to herd immunity.” And, if intensive care units fill up, “there will be a responsive lockdown,” he said, adding that he did not endorse that as a general matter or favor it as a default position.
Bhattacharya claimed that Sweden has tallied only 1800 excess deaths since the pandemic began. “That’s lockdown harm avoided,” he said, advocating a similar strategy for the United States. But, infections have been on the rise in Sweden, and the nation has a higher COVID-19 death rate — with 6000 deaths — than other Nordic countries.
“If we keep this policy of lockdown we will have the same kind of outcomes we’ve already had — high excess deaths and sort of indifferent control of COVID,” Bhattacharya said.
“We’re still going to have misery and death going forward until we reach a point where there’s sufficient immunity either though a vaccine or through natural infection,” he said.
This article first appeared on Medscape.com.
A coauthor of the Great Barrington Declaration says that he and colleagues have never argued against using mitigation strategies to keep COVID-19 from spreading, and that critics have mischaracterized the document as a “let it rip” strategy.
Jay Bhattacharya, MD, PhD, a professor and public health policy expert in infectious diseases at Stanford University in California, spoke on a JAMA Livestream debate on November 6. Marc Lipsitch, MD, an epidemiology professor at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, represented the 6900 signatories of the John Snow Memorandum, a rebuttal to the Great Barrington document.
The Great Barrington approach of “Focused Protection” advocates isolation and protection of people who are most vulnerable to COVID-19 while avoiding what they characterize as lockdowns. “The most compassionate approach that balances the risks and benefits of reaching herd immunity, is to allow those who are at minimal risk of death to live their lives normally to build up immunity to the virus through natural infection, while better protecting those who are at highest risk,” the document reads.
The Infectious Diseases Society of America (IDSA) and its HIV Medicine Association denounced the declaration, as reported by Medscape Medical News, and the World Health Organization (WHO) Director General Tedros Adhanom Ghebreyesus called the proposal “unethical.” But the idea has gained some traction at the White House, where Coronavirus Task Force Member and Stanford professor Scott Atlas, MD, has been advising President Donald J. Trump.
On the JAMA debate, Bhattacharya said, “I think all of the mitigation measures are really important,” listing social distancing, hand washing, and masks when distancing is not possible as chief among those strategies for the less vulnerable. “I don’t want to create infections intentionally, but I want us to allow people to go back to their lives as best they can, understanding of the risks they are taking when they do it,” he said, claiming that 99.95% of the population will survive infection.
“The harmful lockdowns are worse for many, many people,” Bhattacharya said.
“I think Jay is moving towards a middle ground which is not really what the Great Barrington Declaration seems to promote,” countered Lipsitch. The declaration does not say use masks or social distance, he said. “It just says we need to go back to a normal life.”
Bhattacharya’s statements to JAMA mean that “maybe we are approaching some common ground,” Lipsitch said.
Definition of a lockdown
Both men were asked to give their definition of a “lockdown.” To Lipsitch, it means people are not allowed out except for essential services and that most businesses are closed, with exceptions for those deemed essential.
Bhattacharya, however, said he views that as a quarantine. Lockdowns “are what we’re currently doing,” he said. Schools, churches, businesses, and arts and culture organizations are shuttered, and “almost every aspect of society is restricted in some way,” Bhattacharya said.
He blamed these lockdowns for most of the excess deaths over and above the COVID-19 deaths and said they had failed to control the pandemic.
Lipsitch said that “it feels to me that Jay is describing as lockdown everything that causes harm, even when it’s not locked down.” He noted that the country was truly closed down for 2 months or so in the spring.
“All of these harms I agree are real,” said Lipsitch. “But they are because the normal life of our society is being interfered with by viral transmission and by people’s inability to live their normal lives.”
Closures and lockdowns are essential to delaying cases and deaths, said Lipsitch. “A case today is worse than a case tomorrow and a lot worse than a case 6 months from now,” he said, noting that a vaccine or improved therapeutics could evolve.
“Delay is not nothing,” Lipsitch added. “It’s actually the goal as I see it, and as the John Snow memo says, we want to keep the virus under control in such a way as that the vulnerable people are not at risk.”
He predicted that cases will continue to grow exponentially because the nation is “not even close to herd immunity.” And, if intensive care units fill up, “there will be a responsive lockdown,” he said, adding that he did not endorse that as a general matter or favor it as a default position.
Bhattacharya claimed that Sweden has tallied only 1800 excess deaths since the pandemic began. “That’s lockdown harm avoided,” he said, advocating a similar strategy for the United States. But, infections have been on the rise in Sweden, and the nation has a higher COVID-19 death rate — with 6000 deaths — than other Nordic countries.
“If we keep this policy of lockdown we will have the same kind of outcomes we’ve already had — high excess deaths and sort of indifferent control of COVID,” Bhattacharya said.
“We’re still going to have misery and death going forward until we reach a point where there’s sufficient immunity either though a vaccine or through natural infection,” he said.
This article first appeared on Medscape.com.
A coauthor of the Great Barrington Declaration says that he and colleagues have never argued against using mitigation strategies to keep COVID-19 from spreading, and that critics have mischaracterized the document as a “let it rip” strategy.
Jay Bhattacharya, MD, PhD, a professor and public health policy expert in infectious diseases at Stanford University in California, spoke on a JAMA Livestream debate on November 6. Marc Lipsitch, MD, an epidemiology professor at the Harvard T.H. Chan School of Public Health in Boston, Massachusetts, represented the 6900 signatories of the John Snow Memorandum, a rebuttal to the Great Barrington document.
The Great Barrington approach of “Focused Protection” advocates isolation and protection of people who are most vulnerable to COVID-19 while avoiding what they characterize as lockdowns. “The most compassionate approach that balances the risks and benefits of reaching herd immunity, is to allow those who are at minimal risk of death to live their lives normally to build up immunity to the virus through natural infection, while better protecting those who are at highest risk,” the document reads.
The Infectious Diseases Society of America (IDSA) and its HIV Medicine Association denounced the declaration, as reported by Medscape Medical News, and the World Health Organization (WHO) Director General Tedros Adhanom Ghebreyesus called the proposal “unethical.” But the idea has gained some traction at the White House, where Coronavirus Task Force Member and Stanford professor Scott Atlas, MD, has been advising President Donald J. Trump.
On the JAMA debate, Bhattacharya said, “I think all of the mitigation measures are really important,” listing social distancing, hand washing, and masks when distancing is not possible as chief among those strategies for the less vulnerable. “I don’t want to create infections intentionally, but I want us to allow people to go back to their lives as best they can, understanding of the risks they are taking when they do it,” he said, claiming that 99.95% of the population will survive infection.
“The harmful lockdowns are worse for many, many people,” Bhattacharya said.
“I think Jay is moving towards a middle ground which is not really what the Great Barrington Declaration seems to promote,” countered Lipsitch. The declaration does not say use masks or social distance, he said. “It just says we need to go back to a normal life.”
Bhattacharya’s statements to JAMA mean that “maybe we are approaching some common ground,” Lipsitch said.
Definition of a lockdown
Both men were asked to give their definition of a “lockdown.” To Lipsitch, it means people are not allowed out except for essential services and that most businesses are closed, with exceptions for those deemed essential.
Bhattacharya, however, said he views that as a quarantine. Lockdowns “are what we’re currently doing,” he said. Schools, churches, businesses, and arts and culture organizations are shuttered, and “almost every aspect of society is restricted in some way,” Bhattacharya said.
He blamed these lockdowns for most of the excess deaths over and above the COVID-19 deaths and said they had failed to control the pandemic.
Lipsitch said that “it feels to me that Jay is describing as lockdown everything that causes harm, even when it’s not locked down.” He noted that the country was truly closed down for 2 months or so in the spring.
“All of these harms I agree are real,” said Lipsitch. “But they are because the normal life of our society is being interfered with by viral transmission and by people’s inability to live their normal lives.”
Closures and lockdowns are essential to delaying cases and deaths, said Lipsitch. “A case today is worse than a case tomorrow and a lot worse than a case 6 months from now,” he said, noting that a vaccine or improved therapeutics could evolve.
“Delay is not nothing,” Lipsitch added. “It’s actually the goal as I see it, and as the John Snow memo says, we want to keep the virus under control in such a way as that the vulnerable people are not at risk.”
He predicted that cases will continue to grow exponentially because the nation is “not even close to herd immunity.” And, if intensive care units fill up, “there will be a responsive lockdown,” he said, adding that he did not endorse that as a general matter or favor it as a default position.
Bhattacharya claimed that Sweden has tallied only 1800 excess deaths since the pandemic began. “That’s lockdown harm avoided,” he said, advocating a similar strategy for the United States. But, infections have been on the rise in Sweden, and the nation has a higher COVID-19 death rate — with 6000 deaths — than other Nordic countries.
“If we keep this policy of lockdown we will have the same kind of outcomes we’ve already had — high excess deaths and sort of indifferent control of COVID,” Bhattacharya said.
“We’re still going to have misery and death going forward until we reach a point where there’s sufficient immunity either though a vaccine or through natural infection,” he said.
This article first appeared on Medscape.com.
Hospitals poised to launch first COVID-19 vaccines in clinicians
At first, when news spread of a 28-year-old doctor on the COVID-19 front lines in Brazil who died after receiving an experimental vaccine, doubts arose about the safety of one of the most promising coronavirus vaccine candidates. But then the story flipped. Although the vaccine maker wouldn’t confirm it, the doctor appeared to have been in the control group and had received a dose of an established meningitis vaccine. The danger came from exposure to the coronavirus itself.
That tragedy underscores the ongoing risk of COVID-19 to healthcare workers, who have been designated by US advisory panels as part of phase 1A – the first to receive doses of any approved vaccine. The Centers for Disease Control and Prevention (CDC) recently reported that 6% of adults hospitalized with COVID from March to May were healthcare workers. The report was based on surveillance data from 13 states. The average age of the patients was 49 years. The agency set a November 15 vaccination “readiness date” for jurisdictions, such as state health departments, even though a vaccine isn’t likely to be authorized by then.
As hospitals scramble to prepare, their watchword is flexibility. They don’t yet know how many initial doses they will get, of which vaccine, or in what time frame. They have a sophisticated infrastructure to deliver flu vaccines each fall, but that framework doesn’t align with the likely scenarios of limited supply, additional reporting requirements, two-dose regimens, and differing storage needs.
“Healthcare organizations have consistently risen to the challenge. I wholeheartedly believe in their potential to do this,” Anna Legreid Dopp, PharmD, senior director of quality improvement and guidelines for the American Society of Health-System Pharmacists, told Medscape Medical News.
Healthcare workers won’t face a vaccine mandate
Even after months of caring for COVID patients, most clinicians remain vulnerable to infection – at work and in their communities. That was what occupational medicine physician Kevin Smith, MD, realized when his health system, Toledo, Ohio–based ProMedica, offered antibody testing to all its 50,000 employees. About 2% of the 6933 tests given came back positive, he says.
Yet many physicians, nurses, and other healthcare workers share the public’s skepticism about the safety and effectiveness of a vaccine that receives swift US Food and Drug Administration (FDA) approval for emergency use. About half of nurses (47%) and almost 1 in 3 physicians (30%) say that they don’t want to get the vaccine when it first becomes available or that they’re unsure about vaccination, according to a Medscape survey.
Because vaccination of healthcare workers will set the stage for public acceptance of the vaccine, hospital epidemiologists are concerned. “We know that there will be some hesitancy in the healthcare workforce, just as there will be in the broader public,” said Marci Drees, MD, chief infection prevention officer and hospital epidemiologist for ChristianaCare in Newark, Delaware, and liaison from the Society for Healthcare Epidemiology of America to the CDC’s Advisory Committee on Immunization Practices.* “I do not think we can expect anyone to be vaccinated if we’re not willing to vaccinate ourselves.”
Healthcare workers are typically required to receive a range of vaccines, including measles, mumps, and rubella (MMR) and pertussis shots. Each year, close to half of US healthcare workers receive a flu vaccine under a workplace mandate. But COVID-19 will be different. The FDA requires anyone given products under an emergency use authorization (EUA) to receive information about risks and benefits and to have the option to decline. Hospitals instead will rely on education as they offer a novel vaccine (or more than one) that will have a minimum effectiveness of 50%.
ProMedica doesn’t require employees to be vaccinated against flu, but employees who decline must get a note from a doctor indicating that they have talked about the risks and benefits of the vaccine. A similar approach may be used with a COVID-19 vaccine, in which employees may be required to learn about the vaccine before they decline, Smith says. “I do believe some people will say they don’t want to get it,” he added.
Like colleagues across the country, Smith is identifying healthcare workers who are involved in direct care of COVID-19 patients and are at highest risk for exposure. Even within the top tier, those performing the riskiest tasks, such as respiratory therapists who provide breathing treatments that spread aerosols and droplets, will be tagged as a priority group, he says. Healthcare workers who spend the most time in proximity to COVID patients, such as nurses in a COVID unit, also are likely to get the first doses, he says.
Swirl, don’t shake, the vaccine
Hospitals are adept at ramping up vaccination campaigns. For example, last year, Vanderbilt University Medical Center, in Nashville, Tennessee, vaccinated nearly 16,000 employees against influenza in their 1-day “Flulapalooza” event. The medical center even earned a Guinness world record in 2011 at the first Flulapalooza for giving the most vaccinations ever within 8 hours.
The 10th anniversary of the event was canceled this year because of COVID restrictions. Instead, nurses, pharmacists, and other clinicians pitched in to vaccinate their coworkers against influenza. Now, plans for COVID-19 vaccination move forward amid uncertainty.
Instead of holding a mass event, “the delivery mechanisms will need to be more targeted and focused,” said Lori Rolando, MD, MPH, director of the Vanderbilt Occupational Health Clinic. In the CDC’s most recent version of its vaccination program “playbook,” the agency recommends giving the vaccines in an area that allows people to remain 6 feet apart and for them to wait for 15 minutes after receiving the shot to make sure they don’t faint, a potential risk common to almost all vaccines.
That’s the easy part. Planning becomes more complex, given the uncertainty as to which vaccines will receive approval and which one a hospital will receive.
If the Pfizer/BioNTech vaccine receives EUA in 2020, about 10 to 20 million doses could be available in November and 20 to 30 million doses in December. The ultracold containers used to ship the vaccines have to be replenished with dry ice within 24 hours of receipt and every 5 days thereafter. Hospitals will need temperature probes to monitor storage in the containers. The five-dose vials can be refrigerated before administering, but only for 5 days. The product must be diluted, and it then must be used within 6 hours.
The Moderna vaccine will be somewhat less plentiful at first. About 10 million doses are expected in November and 15 million doses by the end of December. The 10-dose vials are stored in a freezer. Once they are placed in a refrigerator to thaw, they have to be used within 7 days, and once they’re removed from the refrigerator, they have to be used within 12 hours. The pharmacist or other vaccinator must swirl – but not shake! – the vial before delivering a dose, according to the CDC playbook.
As more information emerges about the vaccines, instructions may change, and Smith is steeled for shifting scenarios. “These are all draft plans. We’re going to modify as we go along,” he says.
The Pfizer vaccine requires a second dose at 21 days, and the Moderna vaccine targets the second dose at 28 days. In addition to using information systems to track vaccinations and any adverse effects, hospitals will give employees a card indicating what vaccine they received, the date it was administered, and the date on which they need to return. (At this point, the time frame for the second dose doesn’t appear to be flexible.)
Regardless of the vaccine, one message stays the same: COVID precautions must continue. That means mask wearing, social distancing, and hand washing – practices that also must be followed by healthcare workers who test positive for naturally acquired antibodies.
“I don’t think anyone expects the COVID vaccine to be 100% effective at preventing COVID,” says Rolando. “So all of the other tools in our toolbox are going to need to be continued to be used as well.”
*Correction, 11/12/20: An earlier version of this article misstated the name of Dr. Drees' institution.
This article first appeared on Medscape.com.
At first, when news spread of a 28-year-old doctor on the COVID-19 front lines in Brazil who died after receiving an experimental vaccine, doubts arose about the safety of one of the most promising coronavirus vaccine candidates. But then the story flipped. Although the vaccine maker wouldn’t confirm it, the doctor appeared to have been in the control group and had received a dose of an established meningitis vaccine. The danger came from exposure to the coronavirus itself.
That tragedy underscores the ongoing risk of COVID-19 to healthcare workers, who have been designated by US advisory panels as part of phase 1A – the first to receive doses of any approved vaccine. The Centers for Disease Control and Prevention (CDC) recently reported that 6% of adults hospitalized with COVID from March to May were healthcare workers. The report was based on surveillance data from 13 states. The average age of the patients was 49 years. The agency set a November 15 vaccination “readiness date” for jurisdictions, such as state health departments, even though a vaccine isn’t likely to be authorized by then.
As hospitals scramble to prepare, their watchword is flexibility. They don’t yet know how many initial doses they will get, of which vaccine, or in what time frame. They have a sophisticated infrastructure to deliver flu vaccines each fall, but that framework doesn’t align with the likely scenarios of limited supply, additional reporting requirements, two-dose regimens, and differing storage needs.
“Healthcare organizations have consistently risen to the challenge. I wholeheartedly believe in their potential to do this,” Anna Legreid Dopp, PharmD, senior director of quality improvement and guidelines for the American Society of Health-System Pharmacists, told Medscape Medical News.
Healthcare workers won’t face a vaccine mandate
Even after months of caring for COVID patients, most clinicians remain vulnerable to infection – at work and in their communities. That was what occupational medicine physician Kevin Smith, MD, realized when his health system, Toledo, Ohio–based ProMedica, offered antibody testing to all its 50,000 employees. About 2% of the 6933 tests given came back positive, he says.
Yet many physicians, nurses, and other healthcare workers share the public’s skepticism about the safety and effectiveness of a vaccine that receives swift US Food and Drug Administration (FDA) approval for emergency use. About half of nurses (47%) and almost 1 in 3 physicians (30%) say that they don’t want to get the vaccine when it first becomes available or that they’re unsure about vaccination, according to a Medscape survey.
Because vaccination of healthcare workers will set the stage for public acceptance of the vaccine, hospital epidemiologists are concerned. “We know that there will be some hesitancy in the healthcare workforce, just as there will be in the broader public,” said Marci Drees, MD, chief infection prevention officer and hospital epidemiologist for ChristianaCare in Newark, Delaware, and liaison from the Society for Healthcare Epidemiology of America to the CDC’s Advisory Committee on Immunization Practices.* “I do not think we can expect anyone to be vaccinated if we’re not willing to vaccinate ourselves.”
Healthcare workers are typically required to receive a range of vaccines, including measles, mumps, and rubella (MMR) and pertussis shots. Each year, close to half of US healthcare workers receive a flu vaccine under a workplace mandate. But COVID-19 will be different. The FDA requires anyone given products under an emergency use authorization (EUA) to receive information about risks and benefits and to have the option to decline. Hospitals instead will rely on education as they offer a novel vaccine (or more than one) that will have a minimum effectiveness of 50%.
ProMedica doesn’t require employees to be vaccinated against flu, but employees who decline must get a note from a doctor indicating that they have talked about the risks and benefits of the vaccine. A similar approach may be used with a COVID-19 vaccine, in which employees may be required to learn about the vaccine before they decline, Smith says. “I do believe some people will say they don’t want to get it,” he added.
Like colleagues across the country, Smith is identifying healthcare workers who are involved in direct care of COVID-19 patients and are at highest risk for exposure. Even within the top tier, those performing the riskiest tasks, such as respiratory therapists who provide breathing treatments that spread aerosols and droplets, will be tagged as a priority group, he says. Healthcare workers who spend the most time in proximity to COVID patients, such as nurses in a COVID unit, also are likely to get the first doses, he says.
Swirl, don’t shake, the vaccine
Hospitals are adept at ramping up vaccination campaigns. For example, last year, Vanderbilt University Medical Center, in Nashville, Tennessee, vaccinated nearly 16,000 employees against influenza in their 1-day “Flulapalooza” event. The medical center even earned a Guinness world record in 2011 at the first Flulapalooza for giving the most vaccinations ever within 8 hours.
The 10th anniversary of the event was canceled this year because of COVID restrictions. Instead, nurses, pharmacists, and other clinicians pitched in to vaccinate their coworkers against influenza. Now, plans for COVID-19 vaccination move forward amid uncertainty.
Instead of holding a mass event, “the delivery mechanisms will need to be more targeted and focused,” said Lori Rolando, MD, MPH, director of the Vanderbilt Occupational Health Clinic. In the CDC’s most recent version of its vaccination program “playbook,” the agency recommends giving the vaccines in an area that allows people to remain 6 feet apart and for them to wait for 15 minutes after receiving the shot to make sure they don’t faint, a potential risk common to almost all vaccines.
That’s the easy part. Planning becomes more complex, given the uncertainty as to which vaccines will receive approval and which one a hospital will receive.
If the Pfizer/BioNTech vaccine receives EUA in 2020, about 10 to 20 million doses could be available in November and 20 to 30 million doses in December. The ultracold containers used to ship the vaccines have to be replenished with dry ice within 24 hours of receipt and every 5 days thereafter. Hospitals will need temperature probes to monitor storage in the containers. The five-dose vials can be refrigerated before administering, but only for 5 days. The product must be diluted, and it then must be used within 6 hours.
The Moderna vaccine will be somewhat less plentiful at first. About 10 million doses are expected in November and 15 million doses by the end of December. The 10-dose vials are stored in a freezer. Once they are placed in a refrigerator to thaw, they have to be used within 7 days, and once they’re removed from the refrigerator, they have to be used within 12 hours. The pharmacist or other vaccinator must swirl – but not shake! – the vial before delivering a dose, according to the CDC playbook.
As more information emerges about the vaccines, instructions may change, and Smith is steeled for shifting scenarios. “These are all draft plans. We’re going to modify as we go along,” he says.
The Pfizer vaccine requires a second dose at 21 days, and the Moderna vaccine targets the second dose at 28 days. In addition to using information systems to track vaccinations and any adverse effects, hospitals will give employees a card indicating what vaccine they received, the date it was administered, and the date on which they need to return. (At this point, the time frame for the second dose doesn’t appear to be flexible.)
Regardless of the vaccine, one message stays the same: COVID precautions must continue. That means mask wearing, social distancing, and hand washing – practices that also must be followed by healthcare workers who test positive for naturally acquired antibodies.
“I don’t think anyone expects the COVID vaccine to be 100% effective at preventing COVID,” says Rolando. “So all of the other tools in our toolbox are going to need to be continued to be used as well.”
*Correction, 11/12/20: An earlier version of this article misstated the name of Dr. Drees' institution.
This article first appeared on Medscape.com.
At first, when news spread of a 28-year-old doctor on the COVID-19 front lines in Brazil who died after receiving an experimental vaccine, doubts arose about the safety of one of the most promising coronavirus vaccine candidates. But then the story flipped. Although the vaccine maker wouldn’t confirm it, the doctor appeared to have been in the control group and had received a dose of an established meningitis vaccine. The danger came from exposure to the coronavirus itself.
That tragedy underscores the ongoing risk of COVID-19 to healthcare workers, who have been designated by US advisory panels as part of phase 1A – the first to receive doses of any approved vaccine. The Centers for Disease Control and Prevention (CDC) recently reported that 6% of adults hospitalized with COVID from March to May were healthcare workers. The report was based on surveillance data from 13 states. The average age of the patients was 49 years. The agency set a November 15 vaccination “readiness date” for jurisdictions, such as state health departments, even though a vaccine isn’t likely to be authorized by then.
As hospitals scramble to prepare, their watchword is flexibility. They don’t yet know how many initial doses they will get, of which vaccine, or in what time frame. They have a sophisticated infrastructure to deliver flu vaccines each fall, but that framework doesn’t align with the likely scenarios of limited supply, additional reporting requirements, two-dose regimens, and differing storage needs.
“Healthcare organizations have consistently risen to the challenge. I wholeheartedly believe in their potential to do this,” Anna Legreid Dopp, PharmD, senior director of quality improvement and guidelines for the American Society of Health-System Pharmacists, told Medscape Medical News.
Healthcare workers won’t face a vaccine mandate
Even after months of caring for COVID patients, most clinicians remain vulnerable to infection – at work and in their communities. That was what occupational medicine physician Kevin Smith, MD, realized when his health system, Toledo, Ohio–based ProMedica, offered antibody testing to all its 50,000 employees. About 2% of the 6933 tests given came back positive, he says.
Yet many physicians, nurses, and other healthcare workers share the public’s skepticism about the safety and effectiveness of a vaccine that receives swift US Food and Drug Administration (FDA) approval for emergency use. About half of nurses (47%) and almost 1 in 3 physicians (30%) say that they don’t want to get the vaccine when it first becomes available or that they’re unsure about vaccination, according to a Medscape survey.
Because vaccination of healthcare workers will set the stage for public acceptance of the vaccine, hospital epidemiologists are concerned. “We know that there will be some hesitancy in the healthcare workforce, just as there will be in the broader public,” said Marci Drees, MD, chief infection prevention officer and hospital epidemiologist for ChristianaCare in Newark, Delaware, and liaison from the Society for Healthcare Epidemiology of America to the CDC’s Advisory Committee on Immunization Practices.* “I do not think we can expect anyone to be vaccinated if we’re not willing to vaccinate ourselves.”
Healthcare workers are typically required to receive a range of vaccines, including measles, mumps, and rubella (MMR) and pertussis shots. Each year, close to half of US healthcare workers receive a flu vaccine under a workplace mandate. But COVID-19 will be different. The FDA requires anyone given products under an emergency use authorization (EUA) to receive information about risks and benefits and to have the option to decline. Hospitals instead will rely on education as they offer a novel vaccine (or more than one) that will have a minimum effectiveness of 50%.
ProMedica doesn’t require employees to be vaccinated against flu, but employees who decline must get a note from a doctor indicating that they have talked about the risks and benefits of the vaccine. A similar approach may be used with a COVID-19 vaccine, in which employees may be required to learn about the vaccine before they decline, Smith says. “I do believe some people will say they don’t want to get it,” he added.
Like colleagues across the country, Smith is identifying healthcare workers who are involved in direct care of COVID-19 patients and are at highest risk for exposure. Even within the top tier, those performing the riskiest tasks, such as respiratory therapists who provide breathing treatments that spread aerosols and droplets, will be tagged as a priority group, he says. Healthcare workers who spend the most time in proximity to COVID patients, such as nurses in a COVID unit, also are likely to get the first doses, he says.
Swirl, don’t shake, the vaccine
Hospitals are adept at ramping up vaccination campaigns. For example, last year, Vanderbilt University Medical Center, in Nashville, Tennessee, vaccinated nearly 16,000 employees against influenza in their 1-day “Flulapalooza” event. The medical center even earned a Guinness world record in 2011 at the first Flulapalooza for giving the most vaccinations ever within 8 hours.
The 10th anniversary of the event was canceled this year because of COVID restrictions. Instead, nurses, pharmacists, and other clinicians pitched in to vaccinate their coworkers against influenza. Now, plans for COVID-19 vaccination move forward amid uncertainty.
Instead of holding a mass event, “the delivery mechanisms will need to be more targeted and focused,” said Lori Rolando, MD, MPH, director of the Vanderbilt Occupational Health Clinic. In the CDC’s most recent version of its vaccination program “playbook,” the agency recommends giving the vaccines in an area that allows people to remain 6 feet apart and for them to wait for 15 minutes after receiving the shot to make sure they don’t faint, a potential risk common to almost all vaccines.
That’s the easy part. Planning becomes more complex, given the uncertainty as to which vaccines will receive approval and which one a hospital will receive.
If the Pfizer/BioNTech vaccine receives EUA in 2020, about 10 to 20 million doses could be available in November and 20 to 30 million doses in December. The ultracold containers used to ship the vaccines have to be replenished with dry ice within 24 hours of receipt and every 5 days thereafter. Hospitals will need temperature probes to monitor storage in the containers. The five-dose vials can be refrigerated before administering, but only for 5 days. The product must be diluted, and it then must be used within 6 hours.
The Moderna vaccine will be somewhat less plentiful at first. About 10 million doses are expected in November and 15 million doses by the end of December. The 10-dose vials are stored in a freezer. Once they are placed in a refrigerator to thaw, they have to be used within 7 days, and once they’re removed from the refrigerator, they have to be used within 12 hours. The pharmacist or other vaccinator must swirl – but not shake! – the vial before delivering a dose, according to the CDC playbook.
As more information emerges about the vaccines, instructions may change, and Smith is steeled for shifting scenarios. “These are all draft plans. We’re going to modify as we go along,” he says.
The Pfizer vaccine requires a second dose at 21 days, and the Moderna vaccine targets the second dose at 28 days. In addition to using information systems to track vaccinations and any adverse effects, hospitals will give employees a card indicating what vaccine they received, the date it was administered, and the date on which they need to return. (At this point, the time frame for the second dose doesn’t appear to be flexible.)
Regardless of the vaccine, one message stays the same: COVID precautions must continue. That means mask wearing, social distancing, and hand washing – practices that also must be followed by healthcare workers who test positive for naturally acquired antibodies.
“I don’t think anyone expects the COVID vaccine to be 100% effective at preventing COVID,” says Rolando. “So all of the other tools in our toolbox are going to need to be continued to be used as well.”
*Correction, 11/12/20: An earlier version of this article misstated the name of Dr. Drees' institution.
This article first appeared on Medscape.com.
United States adds nearly 74,000 more children with COVID-19
The new weekly high for COVID-19 cases in children announced last week has been surpassed already, as the United States experienced almost 74,000 new pediatric cases for the week ending Nov. 5, according to the American Academy of Pediatrics and the Children’s Hospital Association.
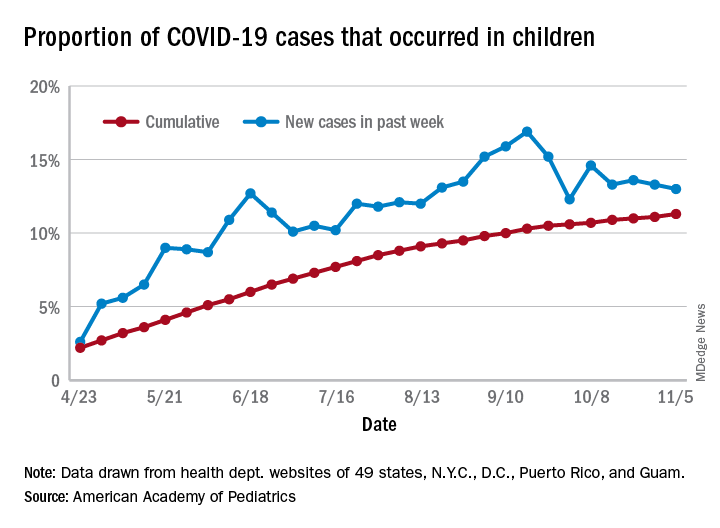
The total number of COVID-19 cases in children is now 927,518 in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly report.
Cumulatively, children represent 11.3% of all COVID-19 cases in those jurisdictions, up from 11.1% a week ago. For just the past week, those 73,883 children represent 13.0% of the 567,672 new cases reported among all ages. That proportion peaked at 16.9% in mid-September, the AAP/CHA data show.
Dropping down to the state level, cumulative proportions as of Nov. 5 range from 5.2% in New Jersey to 23.3% in Wyoming, with 11 other states over 15%. California has had more cases, 100,856, than any other state, and Vermont the fewest at 329, the AAP and CHA said.
The national rate per 100,000 children is now 1,232, up from 1,134 the previous week and more than doubled since mid-August (582.2 per 100,000 on Aug. 20). North Dakota’s rate of 3,990 per 100,000 children is the highest of any state (South Dakota is next at 2,779), while Vermont is again the lowest at 245 per 100,000, based on data collected from state health department websites.
Two COVID-19–related deaths in children were reported during the week ending Nov. 5, bringing the total to 123 but leaving the overall proportion of deaths in children unchanged at 0.06% of all deaths. Texas has reported the most COVID-19 deaths in children with 29, while 15 states have recorded no deaths so far (mortality data in children reported by 42 states and New York City), the AAP and CHA said.
The new weekly high for COVID-19 cases in children announced last week has been surpassed already, as the United States experienced almost 74,000 new pediatric cases for the week ending Nov. 5, according to the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases in children is now 927,518 in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly report.
Cumulatively, children represent 11.3% of all COVID-19 cases in those jurisdictions, up from 11.1% a week ago. For just the past week, those 73,883 children represent 13.0% of the 567,672 new cases reported among all ages. That proportion peaked at 16.9% in mid-September, the AAP/CHA data show.
Dropping down to the state level, cumulative proportions as of Nov. 5 range from 5.2% in New Jersey to 23.3% in Wyoming, with 11 other states over 15%. California has had more cases, 100,856, than any other state, and Vermont the fewest at 329, the AAP and CHA said.
The national rate per 100,000 children is now 1,232, up from 1,134 the previous week and more than doubled since mid-August (582.2 per 100,000 on Aug. 20). North Dakota’s rate of 3,990 per 100,000 children is the highest of any state (South Dakota is next at 2,779), while Vermont is again the lowest at 245 per 100,000, based on data collected from state health department websites.
Two COVID-19–related deaths in children were reported during the week ending Nov. 5, bringing the total to 123 but leaving the overall proportion of deaths in children unchanged at 0.06% of all deaths. Texas has reported the most COVID-19 deaths in children with 29, while 15 states have recorded no deaths so far (mortality data in children reported by 42 states and New York City), the AAP and CHA said.
The new weekly high for COVID-19 cases in children announced last week has been surpassed already, as the United States experienced almost 74,000 new pediatric cases for the week ending Nov. 5, according to the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of COVID-19 cases in children is now 927,518 in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA said in their weekly report.
Cumulatively, children represent 11.3% of all COVID-19 cases in those jurisdictions, up from 11.1% a week ago. For just the past week, those 73,883 children represent 13.0% of the 567,672 new cases reported among all ages. That proportion peaked at 16.9% in mid-September, the AAP/CHA data show.
Dropping down to the state level, cumulative proportions as of Nov. 5 range from 5.2% in New Jersey to 23.3% in Wyoming, with 11 other states over 15%. California has had more cases, 100,856, than any other state, and Vermont the fewest at 329, the AAP and CHA said.
The national rate per 100,000 children is now 1,232, up from 1,134 the previous week and more than doubled since mid-August (582.2 per 100,000 on Aug. 20). North Dakota’s rate of 3,990 per 100,000 children is the highest of any state (South Dakota is next at 2,779), while Vermont is again the lowest at 245 per 100,000, based on data collected from state health department websites.
Two COVID-19–related deaths in children were reported during the week ending Nov. 5, bringing the total to 123 but leaving the overall proportion of deaths in children unchanged at 0.06% of all deaths. Texas has reported the most COVID-19 deaths in children with 29, while 15 states have recorded no deaths so far (mortality data in children reported by 42 states and New York City), the AAP and CHA said.
Food insecurity called urgent issue you must address
and advocate on behalf of those experiencing or at risk of food insecurity, according to Kofi Essel, MD, MPH, a pediatrician at Children’s National Hospital in Washington.
More than one in four adults are dealing with food access hardships during the pandemic, Dr. Essel said at the virtual annual meeting of the American Academy of Pediatrics. Food insecurity is often interchangeable with hunger and refers to limited or uncertain availability of foods that are nutritious and safe.
“Food insecurity is as much about the threat of deprivation as it is about deprivation itself: A food-insecure life means a life lived in fear of hunger, and the psychological toll that takes,” according to a 2020 New York Times photo feature on food insecurity by Brenda Ann Kenneally that Dr. Essel quoted.
The lived experience of food insecure households includes food anxiety, a preoccupation with being able to get enough food that takes up cognitive bandwidth and prevents people from being able to focus on other important things. Another feature of food-insecure homes is a monotony of diet, which often involves an increase in caloric density and decrease in nutritional quality. As food insecurity grows more dire, adults’ food intake decreases, and then children’s intake decreases as adults seek out any way to get food, including “socially unacceptable” ways, which can include food pantries and bartering for food.
Food insecurity is associated with a wide range of negative outcomes even after accounting for other confounders, including decreased overall health, mental health, and educational outcomes. It’s also associated with an increase in developmental delays, hospitalizations, iron deficiency, asthma, and birth defects, among other problems. Somewhat paradoxically, it’s associated with both an increase and a decrease in obesity in the research.
Megan J. Gray, MD, MPH, assistant professor of pediatrics and population health at Dell Medical School at the The University of Texas at Austin, attended Dr. Essel’s session because food insecurity during COVID-19 now affects about half her patients, according to screening research she’s conducted.
“I wanted to learn more about the nuances of screening and using language and talking points that are helpful with families and with staff in building a culture of discussing food insecurity in our clinics,” Dr. Gray said in an interview. “What I’ve learned in my clinic is that if we don’t ask about it, families aren’t telling us – food insecurity is hiding in plain sight.”
She particularly appreciated Dr. Essel’s slides on the progression of food insecurity and how they acknowledged the mental health burden of food insecurity among parents.
“Right now during COVID-19, I see more patients I would call ‘socially complex’ rather than ‘medically complex,’ ” she said. “We all need to get a crash course in social work and Dr. Essel’s presentation is a great starting place.”
Screening for food insecurity
Beginning in 2015, an AAP policy statement charged pediatricians to “screen and intervene” with regard to food insecurity and their patients, Dr. Essel said. The statement also called for pediatricians to advocate for programs and policies that end childhood food insecurity.
The policy statement recommended a validated two-question screening tool called the Hunger Vital Sign:
1. “Within the past 12 months, we worried whether our food would run out before we got money to buy more.”
2. “Within the past 12 months, the food that we bought just didn’t last and we didn’t have money to get more.”
But in screening, you need to be conscious of how dignity intersects with food insecurity concerns, Dr. Essel said.
“We need to create dignity for our families,” he said. “We need to create a safe environment for our families and use appropriate tools when necessary to be able to identify families that are struggling with food insecurity.”
That need is seen in research on food screening. The Hunger Vital Signs questions can be asked with a dichotomous variable, as a yes/no question, or on a Likert scale, though the latter is a more complex way to ask.
A 2017 study found, however, that asking with “yes/no” answers missed more than a quarter of at-risk families. In the AAP survey using “yes/no” answers, 31% of families screened positive for being at risk of food insecurity, compared with 46% when the same question was asked on a Likert scale. It seems the ability to answer with “sometimes” feels “safer” than answering “yes,” Dr. Essel said.
Another factor that potentially affects answers is how doctors ask. In a March 2020 study at a single primary care practice, 16% of families screened positive with yes/no responses to a food insecurity screen when the questions were written, compared with 10% of positive screens with verbal responses (P < .001).
Epidemiology of food insecurity
The most updated United States Department of Agriculture report on food insecurity released in September shows the United States finally reached prerecession levels in 2019, with 11% of families designated as “food insecure.” But 2019 data cannot show what has occurred since the pandemic.
Further, the numbers are higher in households with children: Fourteen percent, or one in seven households with children, are experiencing food insecurity. Racial and ethnic disparities in food insecurity have remained consistent over the past 2 decades, with about twice as many Black and Hispanic homes experiencing food insecurity as White homes.
More recent research using Census Household Pulse Surveys has found a tremendous increase in food insecurity for children in 2020. One in three Black children and one in four Hispanic children are food insecure, according to these surveys. The rates are one in six for Asian households and one in ten for White households.
“The disparity is consistent,” Dr. Essel said. “We see what COVID has done. We once may have described it as a great equalizer – everyone is touched in the same way – but the reality is, this is actually a great magnifier. It’s revealing to us and magnifying disparities that have existed for far too long and has really allowed us to see it in a new way.”
A big part of disparities in food insecurity is disparities in wealth, “the safety net or cushion for families when things go wrong,” Dr. Essel said. The median wealth of White Americans in 2016 was $171,000, compared to $20,700 among Latinx Americans and $17,600 among Black Americans, according to the Federal Reserve Board Survey of Consumer Finances.
Food insecurity interventions
Federal nutrition programs – such as Supplemental Nutrition Assistance Program (SNAP), the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), and school meal programs – are key to addressing food insecurity, Dr. Essel said.
“They have a long track record of rescuing families out of poverty, of rescuing families from food security and improving overall health of families,” he said.
But emergency food relief programs are important as well. Four in 10 families currently coming into food pantries are new recipients, and these resources have seen a 60% increase in clients, he said.
“This is utterly unreasonable for them to be able to manage,” he said. “Food pantries are essential but inadequate to compensate for large numbers of families,” even while they also may be the only option for families unable or unwilling to access federal programs. For example, for every one meal that food banks can provide, SNAP can provide nine meals, Dr. Essel said. Further, during times of economic downtown, every SNAP $1 spent generates $1.50 to $2 in economic activity.
Currently, the Pandemic Electronic Benefit Transfer (P-EBT) program provides benefits to families for school breakfast and lunch and has been extended through December 2021. Another federal pandemic response was to increase SNAP to the maximum household benefit for families, about $646 for a family of four, although 40% of households were already receiving the maximum benefit.
Food insecurity advocacy
You can advocate for any one of multiple pillars when it comes to food insecurity, Dr. Essel said. “Food cannot solve food insecurity by itself,” he said. “We have to think about root causes – systemic causes – and think about unemployment, livable wage, systemic racism, oppression, an inequitable food system. All of these things are pillars that any of you can advocate for when recognizing a family that is struggling with food insecurity.”
He offered several suggestions for advocacy:
- Join your local AAP chapter and prioritize food insecurity.
- Join a local antihunger task force.
- Make your clinical environment as safe as possible for families to respond to questions about food insecurity.
- Know what’s happening in your community immigrant populations.
- Provide up-to-date information to families about eligibility for federal programs.
- Share stories through op-eds and letters to the editor, and by contacting congressional representatives and providing expert testimony to school boards and city councils.
- Educate others about food insecurity through the above channels and on social media.
Jessica Lazerov, MD, a general pediatrician at Children’s National Anacostia and assistant professor of pediatrics at George Washington University, Washington, said the session was fantastic.
“Dr. Essel went beyond the basics of food insecurity, delving into the root causes, potential solutions, and important considerations when screening for food insecurity in practice,” Dr. Lazerov said in an interview. “I enjoyed his focus on advocacy, as well as the fact that he spent a bit of time reviewing how the COVID pandemic has affected food insecurity. I truly felt empowered to take my advocacy efforts a step further as Dr. Essel laid out concrete, actionable next steps, as well as a review of the most relevant and current information about food insecurity.”
Dr. Essel, Dr. Lazerov, and Dr. Gray have no relevant financial disclosures.
and advocate on behalf of those experiencing or at risk of food insecurity, according to Kofi Essel, MD, MPH, a pediatrician at Children’s National Hospital in Washington.
More than one in four adults are dealing with food access hardships during the pandemic, Dr. Essel said at the virtual annual meeting of the American Academy of Pediatrics. Food insecurity is often interchangeable with hunger and refers to limited or uncertain availability of foods that are nutritious and safe.
“Food insecurity is as much about the threat of deprivation as it is about deprivation itself: A food-insecure life means a life lived in fear of hunger, and the psychological toll that takes,” according to a 2020 New York Times photo feature on food insecurity by Brenda Ann Kenneally that Dr. Essel quoted.
The lived experience of food insecure households includes food anxiety, a preoccupation with being able to get enough food that takes up cognitive bandwidth and prevents people from being able to focus on other important things. Another feature of food-insecure homes is a monotony of diet, which often involves an increase in caloric density and decrease in nutritional quality. As food insecurity grows more dire, adults’ food intake decreases, and then children’s intake decreases as adults seek out any way to get food, including “socially unacceptable” ways, which can include food pantries and bartering for food.
Food insecurity is associated with a wide range of negative outcomes even after accounting for other confounders, including decreased overall health, mental health, and educational outcomes. It’s also associated with an increase in developmental delays, hospitalizations, iron deficiency, asthma, and birth defects, among other problems. Somewhat paradoxically, it’s associated with both an increase and a decrease in obesity in the research.
Megan J. Gray, MD, MPH, assistant professor of pediatrics and population health at Dell Medical School at the The University of Texas at Austin, attended Dr. Essel’s session because food insecurity during COVID-19 now affects about half her patients, according to screening research she’s conducted.
“I wanted to learn more about the nuances of screening and using language and talking points that are helpful with families and with staff in building a culture of discussing food insecurity in our clinics,” Dr. Gray said in an interview. “What I’ve learned in my clinic is that if we don’t ask about it, families aren’t telling us – food insecurity is hiding in plain sight.”
She particularly appreciated Dr. Essel’s slides on the progression of food insecurity and how they acknowledged the mental health burden of food insecurity among parents.
“Right now during COVID-19, I see more patients I would call ‘socially complex’ rather than ‘medically complex,’ ” she said. “We all need to get a crash course in social work and Dr. Essel’s presentation is a great starting place.”
Screening for food insecurity
Beginning in 2015, an AAP policy statement charged pediatricians to “screen and intervene” with regard to food insecurity and their patients, Dr. Essel said. The statement also called for pediatricians to advocate for programs and policies that end childhood food insecurity.
The policy statement recommended a validated two-question screening tool called the Hunger Vital Sign:
1. “Within the past 12 months, we worried whether our food would run out before we got money to buy more.”
2. “Within the past 12 months, the food that we bought just didn’t last and we didn’t have money to get more.”
But in screening, you need to be conscious of how dignity intersects with food insecurity concerns, Dr. Essel said.
“We need to create dignity for our families,” he said. “We need to create a safe environment for our families and use appropriate tools when necessary to be able to identify families that are struggling with food insecurity.”
That need is seen in research on food screening. The Hunger Vital Signs questions can be asked with a dichotomous variable, as a yes/no question, or on a Likert scale, though the latter is a more complex way to ask.
A 2017 study found, however, that asking with “yes/no” answers missed more than a quarter of at-risk families. In the AAP survey using “yes/no” answers, 31% of families screened positive for being at risk of food insecurity, compared with 46% when the same question was asked on a Likert scale. It seems the ability to answer with “sometimes” feels “safer” than answering “yes,” Dr. Essel said.
Another factor that potentially affects answers is how doctors ask. In a March 2020 study at a single primary care practice, 16% of families screened positive with yes/no responses to a food insecurity screen when the questions were written, compared with 10% of positive screens with verbal responses (P < .001).
Epidemiology of food insecurity
The most updated United States Department of Agriculture report on food insecurity released in September shows the United States finally reached prerecession levels in 2019, with 11% of families designated as “food insecure.” But 2019 data cannot show what has occurred since the pandemic.
Further, the numbers are higher in households with children: Fourteen percent, or one in seven households with children, are experiencing food insecurity. Racial and ethnic disparities in food insecurity have remained consistent over the past 2 decades, with about twice as many Black and Hispanic homes experiencing food insecurity as White homes.
More recent research using Census Household Pulse Surveys has found a tremendous increase in food insecurity for children in 2020. One in three Black children and one in four Hispanic children are food insecure, according to these surveys. The rates are one in six for Asian households and one in ten for White households.
“The disparity is consistent,” Dr. Essel said. “We see what COVID has done. We once may have described it as a great equalizer – everyone is touched in the same way – but the reality is, this is actually a great magnifier. It’s revealing to us and magnifying disparities that have existed for far too long and has really allowed us to see it in a new way.”
A big part of disparities in food insecurity is disparities in wealth, “the safety net or cushion for families when things go wrong,” Dr. Essel said. The median wealth of White Americans in 2016 was $171,000, compared to $20,700 among Latinx Americans and $17,600 among Black Americans, according to the Federal Reserve Board Survey of Consumer Finances.
Food insecurity interventions
Federal nutrition programs – such as Supplemental Nutrition Assistance Program (SNAP), the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), and school meal programs – are key to addressing food insecurity, Dr. Essel said.
“They have a long track record of rescuing families out of poverty, of rescuing families from food security and improving overall health of families,” he said.
But emergency food relief programs are important as well. Four in 10 families currently coming into food pantries are new recipients, and these resources have seen a 60% increase in clients, he said.
“This is utterly unreasonable for them to be able to manage,” he said. “Food pantries are essential but inadequate to compensate for large numbers of families,” even while they also may be the only option for families unable or unwilling to access federal programs. For example, for every one meal that food banks can provide, SNAP can provide nine meals, Dr. Essel said. Further, during times of economic downtown, every SNAP $1 spent generates $1.50 to $2 in economic activity.
Currently, the Pandemic Electronic Benefit Transfer (P-EBT) program provides benefits to families for school breakfast and lunch and has been extended through December 2021. Another federal pandemic response was to increase SNAP to the maximum household benefit for families, about $646 for a family of four, although 40% of households were already receiving the maximum benefit.
Food insecurity advocacy
You can advocate for any one of multiple pillars when it comes to food insecurity, Dr. Essel said. “Food cannot solve food insecurity by itself,” he said. “We have to think about root causes – systemic causes – and think about unemployment, livable wage, systemic racism, oppression, an inequitable food system. All of these things are pillars that any of you can advocate for when recognizing a family that is struggling with food insecurity.”
He offered several suggestions for advocacy:
- Join your local AAP chapter and prioritize food insecurity.
- Join a local antihunger task force.
- Make your clinical environment as safe as possible for families to respond to questions about food insecurity.
- Know what’s happening in your community immigrant populations.
- Provide up-to-date information to families about eligibility for federal programs.
- Share stories through op-eds and letters to the editor, and by contacting congressional representatives and providing expert testimony to school boards and city councils.
- Educate others about food insecurity through the above channels and on social media.
Jessica Lazerov, MD, a general pediatrician at Children’s National Anacostia and assistant professor of pediatrics at George Washington University, Washington, said the session was fantastic.
“Dr. Essel went beyond the basics of food insecurity, delving into the root causes, potential solutions, and important considerations when screening for food insecurity in practice,” Dr. Lazerov said in an interview. “I enjoyed his focus on advocacy, as well as the fact that he spent a bit of time reviewing how the COVID pandemic has affected food insecurity. I truly felt empowered to take my advocacy efforts a step further as Dr. Essel laid out concrete, actionable next steps, as well as a review of the most relevant and current information about food insecurity.”
Dr. Essel, Dr. Lazerov, and Dr. Gray have no relevant financial disclosures.
and advocate on behalf of those experiencing or at risk of food insecurity, according to Kofi Essel, MD, MPH, a pediatrician at Children’s National Hospital in Washington.
More than one in four adults are dealing with food access hardships during the pandemic, Dr. Essel said at the virtual annual meeting of the American Academy of Pediatrics. Food insecurity is often interchangeable with hunger and refers to limited or uncertain availability of foods that are nutritious and safe.
“Food insecurity is as much about the threat of deprivation as it is about deprivation itself: A food-insecure life means a life lived in fear of hunger, and the psychological toll that takes,” according to a 2020 New York Times photo feature on food insecurity by Brenda Ann Kenneally that Dr. Essel quoted.
The lived experience of food insecure households includes food anxiety, a preoccupation with being able to get enough food that takes up cognitive bandwidth and prevents people from being able to focus on other important things. Another feature of food-insecure homes is a monotony of diet, which often involves an increase in caloric density and decrease in nutritional quality. As food insecurity grows more dire, adults’ food intake decreases, and then children’s intake decreases as adults seek out any way to get food, including “socially unacceptable” ways, which can include food pantries and bartering for food.
Food insecurity is associated with a wide range of negative outcomes even after accounting for other confounders, including decreased overall health, mental health, and educational outcomes. It’s also associated with an increase in developmental delays, hospitalizations, iron deficiency, asthma, and birth defects, among other problems. Somewhat paradoxically, it’s associated with both an increase and a decrease in obesity in the research.
Megan J. Gray, MD, MPH, assistant professor of pediatrics and population health at Dell Medical School at the The University of Texas at Austin, attended Dr. Essel’s session because food insecurity during COVID-19 now affects about half her patients, according to screening research she’s conducted.
“I wanted to learn more about the nuances of screening and using language and talking points that are helpful with families and with staff in building a culture of discussing food insecurity in our clinics,” Dr. Gray said in an interview. “What I’ve learned in my clinic is that if we don’t ask about it, families aren’t telling us – food insecurity is hiding in plain sight.”
She particularly appreciated Dr. Essel’s slides on the progression of food insecurity and how they acknowledged the mental health burden of food insecurity among parents.
“Right now during COVID-19, I see more patients I would call ‘socially complex’ rather than ‘medically complex,’ ” she said. “We all need to get a crash course in social work and Dr. Essel’s presentation is a great starting place.”
Screening for food insecurity
Beginning in 2015, an AAP policy statement charged pediatricians to “screen and intervene” with regard to food insecurity and their patients, Dr. Essel said. The statement also called for pediatricians to advocate for programs and policies that end childhood food insecurity.
The policy statement recommended a validated two-question screening tool called the Hunger Vital Sign:
1. “Within the past 12 months, we worried whether our food would run out before we got money to buy more.”
2. “Within the past 12 months, the food that we bought just didn’t last and we didn’t have money to get more.”
But in screening, you need to be conscious of how dignity intersects with food insecurity concerns, Dr. Essel said.
“We need to create dignity for our families,” he said. “We need to create a safe environment for our families and use appropriate tools when necessary to be able to identify families that are struggling with food insecurity.”
That need is seen in research on food screening. The Hunger Vital Signs questions can be asked with a dichotomous variable, as a yes/no question, or on a Likert scale, though the latter is a more complex way to ask.
A 2017 study found, however, that asking with “yes/no” answers missed more than a quarter of at-risk families. In the AAP survey using “yes/no” answers, 31% of families screened positive for being at risk of food insecurity, compared with 46% when the same question was asked on a Likert scale. It seems the ability to answer with “sometimes” feels “safer” than answering “yes,” Dr. Essel said.
Another factor that potentially affects answers is how doctors ask. In a March 2020 study at a single primary care practice, 16% of families screened positive with yes/no responses to a food insecurity screen when the questions were written, compared with 10% of positive screens with verbal responses (P < .001).
Epidemiology of food insecurity
The most updated United States Department of Agriculture report on food insecurity released in September shows the United States finally reached prerecession levels in 2019, with 11% of families designated as “food insecure.” But 2019 data cannot show what has occurred since the pandemic.
Further, the numbers are higher in households with children: Fourteen percent, or one in seven households with children, are experiencing food insecurity. Racial and ethnic disparities in food insecurity have remained consistent over the past 2 decades, with about twice as many Black and Hispanic homes experiencing food insecurity as White homes.
More recent research using Census Household Pulse Surveys has found a tremendous increase in food insecurity for children in 2020. One in three Black children and one in four Hispanic children are food insecure, according to these surveys. The rates are one in six for Asian households and one in ten for White households.
“The disparity is consistent,” Dr. Essel said. “We see what COVID has done. We once may have described it as a great equalizer – everyone is touched in the same way – but the reality is, this is actually a great magnifier. It’s revealing to us and magnifying disparities that have existed for far too long and has really allowed us to see it in a new way.”
A big part of disparities in food insecurity is disparities in wealth, “the safety net or cushion for families when things go wrong,” Dr. Essel said. The median wealth of White Americans in 2016 was $171,000, compared to $20,700 among Latinx Americans and $17,600 among Black Americans, according to the Federal Reserve Board Survey of Consumer Finances.
Food insecurity interventions
Federal nutrition programs – such as Supplemental Nutrition Assistance Program (SNAP), the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), and school meal programs – are key to addressing food insecurity, Dr. Essel said.
“They have a long track record of rescuing families out of poverty, of rescuing families from food security and improving overall health of families,” he said.
But emergency food relief programs are important as well. Four in 10 families currently coming into food pantries are new recipients, and these resources have seen a 60% increase in clients, he said.
“This is utterly unreasonable for them to be able to manage,” he said. “Food pantries are essential but inadequate to compensate for large numbers of families,” even while they also may be the only option for families unable or unwilling to access federal programs. For example, for every one meal that food banks can provide, SNAP can provide nine meals, Dr. Essel said. Further, during times of economic downtown, every SNAP $1 spent generates $1.50 to $2 in economic activity.
Currently, the Pandemic Electronic Benefit Transfer (P-EBT) program provides benefits to families for school breakfast and lunch and has been extended through December 2021. Another federal pandemic response was to increase SNAP to the maximum household benefit for families, about $646 for a family of four, although 40% of households were already receiving the maximum benefit.
Food insecurity advocacy
You can advocate for any one of multiple pillars when it comes to food insecurity, Dr. Essel said. “Food cannot solve food insecurity by itself,” he said. “We have to think about root causes – systemic causes – and think about unemployment, livable wage, systemic racism, oppression, an inequitable food system. All of these things are pillars that any of you can advocate for when recognizing a family that is struggling with food insecurity.”
He offered several suggestions for advocacy:
- Join your local AAP chapter and prioritize food insecurity.
- Join a local antihunger task force.
- Make your clinical environment as safe as possible for families to respond to questions about food insecurity.
- Know what’s happening in your community immigrant populations.
- Provide up-to-date information to families about eligibility for federal programs.
- Share stories through op-eds and letters to the editor, and by contacting congressional representatives and providing expert testimony to school boards and city councils.
- Educate others about food insecurity through the above channels and on social media.
Jessica Lazerov, MD, a general pediatrician at Children’s National Anacostia and assistant professor of pediatrics at George Washington University, Washington, said the session was fantastic.
“Dr. Essel went beyond the basics of food insecurity, delving into the root causes, potential solutions, and important considerations when screening for food insecurity in practice,” Dr. Lazerov said in an interview. “I enjoyed his focus on advocacy, as well as the fact that he spent a bit of time reviewing how the COVID pandemic has affected food insecurity. I truly felt empowered to take my advocacy efforts a step further as Dr. Essel laid out concrete, actionable next steps, as well as a review of the most relevant and current information about food insecurity.”
Dr. Essel, Dr. Lazerov, and Dr. Gray have no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 2020
Whales, seals, and dolphins: Will SARS-CoV-2–contaminated wastewater prove a killer?
Zoonoses are no respecter of biological boundaries and are notorious for crossing genus and even higher taxonomic boundaries. SARS-CoV-2 is no exception, the current outbreak most probably having originated in bats, a common source of human-affecting zoonoses throughout history. But it is not a one-way street, and the virus has been shown to spread from infected humans to a variety of other land mammals, including our domesticated animals and kept zoo species.
A recent troubling report, however, has indicated that sea mammals may be part of a next wave of likely candidates for infection, put at risk by the current human pandemic and environmental degradation on a global scale, according to a the results of a genomic analysis of four major groups of sea mammals.
Researchers Sabateeshan Mathavarajah and colleagues from Dalhousie University, Halifax, N.S., examined the sequences of the ACE2 receptors in the various marine mammal species. The ACE2 receptor has recently been identified as the SARS-CoV-2 receptor, which allows for infection.
The researchers examined genomic databases of the marine species to determine if their ACE2 receptor sequences indicated the potential for high, medium, or low susceptibility to infection, as reported in Science of the Total Environment. Database analysis was performed for four groups: Cetacea (whales and dolphins), Pinnepidia (seals), Sirenia (sea cows), and Fissipedia (sea otters and polar bears).
The researchers defined susceptibility values based on comparable binding with the receptor and came up with the following subgroups: higher than human, high (resembles human ACE2), medium (resembles cat ACE2), and low (resembles dog ACE2). It has yet to be established if these marine mammals actually are infected with SARS-CoV-2 and what the impact of such an infection might have on animal health or humans who come in contact with infected animals.
They also cross-referenced for the level of species endangerment and with maps of potential wastewater contamination for certain areas that species came in contact with, using Alaska as the model.
Populations in danger
The researchers found 15 species that are already at risk globally that fall under the categories of near threatened, vulnerable, endangered, and critically endangered that were predicted to be medium to higher susceptibility to the SARS-CoV-2 virus than humans. Cross infection is of particular concern because other coronaviruses have been shown to have severe and lethal effects among many of these species.
Among the potentially impacted species were the near threatened–status Antarctic Mink whale and the stellar sea lion; the vulnerable sperm whale, northern fur seal, and Atlantic walrus; the endangered northern and southern sea otters, the North Pacific right whale, and the Amazon River dolphin; and the critically threatened Baiji and Vaquita dolphin species.
Pollution risks
In Alaska, as of Aug. 7th, 2020, there were 4,221 confirmed cases of COVID-19 and this number continues to rise, according to the researchers. Since there is a diversity of marine mammals in Alaska and their populations are well documented, they compared this information with available data on the wastewater treatment plants in the state. They were thus able to determine the potential geographic locations and species at high risk for transmission of SARS-CoV-2 via wastewater effluent.
Among their findings, the city of Cold Bay discharges wastewater into Cold Bay, where there are Northern sea otter populations that are predicted to be highly susceptible to the virus. Beluga whales are also predicted to have high susceptibility and they can be found in Bristol Bay near Naknek, a city which relies only on lagoon treatment prior to the discharge of wastewater effluent; the city of Dillingham discharges wastewater into the Nushagak River where beluga whales are found. In Palmer, wastewater effluent flows into the Talkeetna River, which is a tributary to the Susitna River and home to two species predicted to have high susceptibility, beluga whales and harbor seals, the authors added.
Based on these results, the researchers predicted that there was likely a significant risk to sea mammals across the globe, especially where less-adequate treatment facilities and high population densities may lead to greater wastewater contamination.
“Given the proximity of marine animals to high-risk environments where viral spill over is likely, we must act with foresight to protect marine mammal species predicted to be at risk and mitigate the environmental impact of the COVID-19 pandemic,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Mathavarajah S et al. Sci Total Environ. 2020 Oct 29. doi: 10.1016/j.scitotenv.2020.143346.
Zoonoses are no respecter of biological boundaries and are notorious for crossing genus and even higher taxonomic boundaries. SARS-CoV-2 is no exception, the current outbreak most probably having originated in bats, a common source of human-affecting zoonoses throughout history. But it is not a one-way street, and the virus has been shown to spread from infected humans to a variety of other land mammals, including our domesticated animals and kept zoo species.
A recent troubling report, however, has indicated that sea mammals may be part of a next wave of likely candidates for infection, put at risk by the current human pandemic and environmental degradation on a global scale, according to a the results of a genomic analysis of four major groups of sea mammals.
Researchers Sabateeshan Mathavarajah and colleagues from Dalhousie University, Halifax, N.S., examined the sequences of the ACE2 receptors in the various marine mammal species. The ACE2 receptor has recently been identified as the SARS-CoV-2 receptor, which allows for infection.
The researchers examined genomic databases of the marine species to determine if their ACE2 receptor sequences indicated the potential for high, medium, or low susceptibility to infection, as reported in Science of the Total Environment. Database analysis was performed for four groups: Cetacea (whales and dolphins), Pinnepidia (seals), Sirenia (sea cows), and Fissipedia (sea otters and polar bears).
The researchers defined susceptibility values based on comparable binding with the receptor and came up with the following subgroups: higher than human, high (resembles human ACE2), medium (resembles cat ACE2), and low (resembles dog ACE2). It has yet to be established if these marine mammals actually are infected with SARS-CoV-2 and what the impact of such an infection might have on animal health or humans who come in contact with infected animals.
They also cross-referenced for the level of species endangerment and with maps of potential wastewater contamination for certain areas that species came in contact with, using Alaska as the model.
Populations in danger
The researchers found 15 species that are already at risk globally that fall under the categories of near threatened, vulnerable, endangered, and critically endangered that were predicted to be medium to higher susceptibility to the SARS-CoV-2 virus than humans. Cross infection is of particular concern because other coronaviruses have been shown to have severe and lethal effects among many of these species.
Among the potentially impacted species were the near threatened–status Antarctic Mink whale and the stellar sea lion; the vulnerable sperm whale, northern fur seal, and Atlantic walrus; the endangered northern and southern sea otters, the North Pacific right whale, and the Amazon River dolphin; and the critically threatened Baiji and Vaquita dolphin species.
Pollution risks
In Alaska, as of Aug. 7th, 2020, there were 4,221 confirmed cases of COVID-19 and this number continues to rise, according to the researchers. Since there is a diversity of marine mammals in Alaska and their populations are well documented, they compared this information with available data on the wastewater treatment plants in the state. They were thus able to determine the potential geographic locations and species at high risk for transmission of SARS-CoV-2 via wastewater effluent.
Among their findings, the city of Cold Bay discharges wastewater into Cold Bay, where there are Northern sea otter populations that are predicted to be highly susceptible to the virus. Beluga whales are also predicted to have high susceptibility and they can be found in Bristol Bay near Naknek, a city which relies only on lagoon treatment prior to the discharge of wastewater effluent; the city of Dillingham discharges wastewater into the Nushagak River where beluga whales are found. In Palmer, wastewater effluent flows into the Talkeetna River, which is a tributary to the Susitna River and home to two species predicted to have high susceptibility, beluga whales and harbor seals, the authors added.
Based on these results, the researchers predicted that there was likely a significant risk to sea mammals across the globe, especially where less-adequate treatment facilities and high population densities may lead to greater wastewater contamination.
“Given the proximity of marine animals to high-risk environments where viral spill over is likely, we must act with foresight to protect marine mammal species predicted to be at risk and mitigate the environmental impact of the COVID-19 pandemic,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Mathavarajah S et al. Sci Total Environ. 2020 Oct 29. doi: 10.1016/j.scitotenv.2020.143346.
Zoonoses are no respecter of biological boundaries and are notorious for crossing genus and even higher taxonomic boundaries. SARS-CoV-2 is no exception, the current outbreak most probably having originated in bats, a common source of human-affecting zoonoses throughout history. But it is not a one-way street, and the virus has been shown to spread from infected humans to a variety of other land mammals, including our domesticated animals and kept zoo species.
A recent troubling report, however, has indicated that sea mammals may be part of a next wave of likely candidates for infection, put at risk by the current human pandemic and environmental degradation on a global scale, according to a the results of a genomic analysis of four major groups of sea mammals.
Researchers Sabateeshan Mathavarajah and colleagues from Dalhousie University, Halifax, N.S., examined the sequences of the ACE2 receptors in the various marine mammal species. The ACE2 receptor has recently been identified as the SARS-CoV-2 receptor, which allows for infection.
The researchers examined genomic databases of the marine species to determine if their ACE2 receptor sequences indicated the potential for high, medium, or low susceptibility to infection, as reported in Science of the Total Environment. Database analysis was performed for four groups: Cetacea (whales and dolphins), Pinnepidia (seals), Sirenia (sea cows), and Fissipedia (sea otters and polar bears).
The researchers defined susceptibility values based on comparable binding with the receptor and came up with the following subgroups: higher than human, high (resembles human ACE2), medium (resembles cat ACE2), and low (resembles dog ACE2). It has yet to be established if these marine mammals actually are infected with SARS-CoV-2 and what the impact of such an infection might have on animal health or humans who come in contact with infected animals.
They also cross-referenced for the level of species endangerment and with maps of potential wastewater contamination for certain areas that species came in contact with, using Alaska as the model.
Populations in danger
The researchers found 15 species that are already at risk globally that fall under the categories of near threatened, vulnerable, endangered, and critically endangered that were predicted to be medium to higher susceptibility to the SARS-CoV-2 virus than humans. Cross infection is of particular concern because other coronaviruses have been shown to have severe and lethal effects among many of these species.
Among the potentially impacted species were the near threatened–status Antarctic Mink whale and the stellar sea lion; the vulnerable sperm whale, northern fur seal, and Atlantic walrus; the endangered northern and southern sea otters, the North Pacific right whale, and the Amazon River dolphin; and the critically threatened Baiji and Vaquita dolphin species.
Pollution risks
In Alaska, as of Aug. 7th, 2020, there were 4,221 confirmed cases of COVID-19 and this number continues to rise, according to the researchers. Since there is a diversity of marine mammals in Alaska and their populations are well documented, they compared this information with available data on the wastewater treatment plants in the state. They were thus able to determine the potential geographic locations and species at high risk for transmission of SARS-CoV-2 via wastewater effluent.
Among their findings, the city of Cold Bay discharges wastewater into Cold Bay, where there are Northern sea otter populations that are predicted to be highly susceptible to the virus. Beluga whales are also predicted to have high susceptibility and they can be found in Bristol Bay near Naknek, a city which relies only on lagoon treatment prior to the discharge of wastewater effluent; the city of Dillingham discharges wastewater into the Nushagak River where beluga whales are found. In Palmer, wastewater effluent flows into the Talkeetna River, which is a tributary to the Susitna River and home to two species predicted to have high susceptibility, beluga whales and harbor seals, the authors added.
Based on these results, the researchers predicted that there was likely a significant risk to sea mammals across the globe, especially where less-adequate treatment facilities and high population densities may lead to greater wastewater contamination.
“Given the proximity of marine animals to high-risk environments where viral spill over is likely, we must act with foresight to protect marine mammal species predicted to be at risk and mitigate the environmental impact of the COVID-19 pandemic,” the researchers concluded.
The authors reported that they had no disclosures.
SOURCE: Mathavarajah S et al. Sci Total Environ. 2020 Oct 29. doi: 10.1016/j.scitotenv.2020.143346.
FROM SCIENCE OF THE TOTAL ENVIRONMENT
Pfizer vaccine data show 90% efficacy in early results
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
A vaccine candidate against SARS-CoV-2 has been found to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus, results from an interim analysis of a phase 3 study demonstrated.
BTN162b2, a messenger RNA–based vaccine candidate that requires two doses, is being developed by Pfizer and BioNTech SE independently of the Trump administration’s Operation Warp Speed. A global phase 3 clinical trial of BTN162b2 began on July 27 and has enrolled 43,538 participants to date; 42% of enrollees have racially and ethnically diverse backgrounds.
According to a press release issued by the two companies, 38,955 trial volunteers had received a second dose of either vaccine or placebo as of Nov. 8. An interim analysis of 94 individuals conducted by an independent data monitoring committee (DMC) found that the vaccine efficacy rate was above 90% 7 days after the second dose. This means that protection was achieved 28 days after the first vaccine dose.
“It’s promising in that it validates the genetic strategy – whether it’s mRNA vaccines or DNA vaccines,” Paul A. Offit, MD, told Medscape Medical News. Offit is a member of the US Food and Drug Administraiton’s COVID-19 Vaccine Advisory Committee. “All of them have the same approach, which is that they introduce the gene that codes for the coronavirus spike protein into the cell. Your cell makes the spike protein, and your immune system makes antibodies to the spike protein. At least in these preliminary data, which involved 94 people getting sick, it looks like it’s effective. That’s good. We knew that it seemed to work in experimental animals, but you never know until you put it into people.”
According to Pfizer and BioNTech SE, a final data analysis is planned once 164 confirmed COVID-19 cases have accrued. So far, the DMC has not reported any serious safety concerns. It recommends that the study continue to collect safety and efficacy data as planned. The companies plan to apply to the FDA for emergency use authorization soon after the required safety milestone is achieved.
Pfizer CEO Albert Bourla, DVM, PhD, added in a separate press release, “It’s important to note that we cannot apply for FDA Emergency Use Authorization based on these efficacy results alone. More data on safety is also needed, and we are continuing to accumulate that safety data as part of our ongoing clinical study.
“We estimate that a median of two months of safety data following the second and final dose of the vaccine candidate – required by FDA’s guidance for potential Emergency Use Authorization – will be available by the third week of November.”
Offit, professor of pediatrics in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, said that, if BTN162b2 is approved, administering it will be tricky. “This particular vaccine has to be shipped and stored at –70° C or –80° C, which we’ve never done before in this country,” he said. “That means maintaining the product on dry ice. That’s going to be a challenge for distribution, I think.”
Good news, but…
In the press release, BioNTech SE’s cofounder and CEO, Ugur Sahin, MD, characterized the findings as “a victory for innovation, science and a global collaborative effort. When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality.”
President-elect Joe Biden also weighed in, calling the results “excellent news” in a news release.
“At the same time, it is also important to understand that the end of the battle against COVID-19 is still months away,” he said. “This news follows a previously announced timeline by industry officials that forecast vaccine approval by late November. Even if that is achieved, and some Americans are vaccinated later this year, it will be many more months before there is widespread vaccination in this country.
“Today’s news does not change this urgent reality. Americans will have to rely on masking, distancing, contact tracing, hand washing, and other measures to keep themselves safe well into next year,” Biden added.
This article first appeared on Medscape.com.
VA joins Pentagon in recruiting volunteers for COVID vaccine trials
according to officials with the VA and Operation Warp Speed, the Trump administration’s initiative to fast-track a coronavirus vaccine.
The largely unpublicized effort follows a Department of Defense announcement in September that it has partnered with AstraZeneca to recruit volunteers at five of its medical facilities, which are separate from the VA system. DOD is also is in talks with developers of other vaccine candidates, although officials won’t say which ones.
Both federal departments have long experience in medical research and diverse populations – a crucial component of effective clinical trials, said J. Stephen Morrison, senior vice president and director of global health policy at the Center for Strategic and International Studies, a bipartisan think tank in Washington.
Since active troops are essential to national security, and veterans are extremely vulnerable to COVID-19, both departments have a vested interest in supporting the development of safe, effective vaccines, Mr. Morrison said.
“On the DOD active servicemen and -women side, it’s a question of making sure they’re ready, they are protected,” Mr. Morrison said. “With VA, their population, all elderly and infirm with underlying conditions, they could really be suffering if we don’t get a vaccine.”
According to a VA website, of its 20 medical centers involved, 17 would be part of the Johnson & Johnson vaccine trial, while the three others are recruiting – or have completed recruitment – for advanced-stage trials for Moderna, AstraZeneca, and Pfizer vaccines.
Matthew Hepburn, MD, head of vaccine development at Operation Warp Speed, said the VA effort lets veterans contribute to the overall well-being of the country.
“This is another way they can continue to serve in this way, fighting the pandemic as a volunteer,” Dr. Hepburn said during a discussion of vaccine and therapeutics development hosted by the Heritage Foundation on Oct. 27.
It’s not unusual for the military to participate in multicenter trials for treatments of ailments as diverse as cancer and trauma. Historically, many vaccines have been tested first by the military.
In the general population, clinicians often have difficulty recruiting African Americans and other minorities for medical research, and “the military provides a rich opportunity to find volunteers for those groups,” said retired Rear Adm. Thomas Cullison, MD, a doctor and former deputy surgeon general for the Navy.
Military health facilities are held to the same standards as private research facilities, he said.
No service members will be required to participate in the COVID vaccine trials. All volunteers will be paid by the developer.
Support for routine vaccinations runs high in the military, but some have expressed concerns about new vaccines and mandatory inoculations, especially for anthrax. In a 2002 federal study, 85% of those who received that vaccine reported an adverse reaction, with just under half noticing minor redness at the injection site. But nearly a quarter of the side effects reported were more systemic, including fevers, chills, fatigue and joint pain.
That survey of a small group of National Guard and Reserve members found that, while 73% said they believe immunizations are effective, two-thirds said they did not support the mandatory anthrax program, and 6 in 10 said they were not satisfied with the information they were given on the vaccines.
To quell concerns over the military’s role in supporting COVID vaccine development, the Pentagon has reiterated that troops or their dependents interested in participating in the research must provide voluntary written consent, and they will be allowed to take part only if they will be in the same location for the length of the research, expected to last at least 2 years.
In addition, active-duty members such as new recruits and boot camp participants will not be allowed to volunteer because they are “considered vulnerable from an ethical and regulatory standpoint,” an official said.
At the VA, officials are seeking to recruit healthy veterans aged 18-65 years old who are not pregnant and may be at risk for exposure. As with trials conducted in civilian facilities, participants will be paid by the developer, VA spokesperson Christina Noel said.
Also, VA nurses and caseworkers also are being asked to identify their sickest, highest-risk patients to determine who should be at the top of the list once a vaccine is approved, according to a VA nurse and other health officials who asked not to be identified because they were not authorized to speak with the press.
The U.S. military has a long history of contributing to research on vaccines, including a key role in developing inoculations against yellow fever and adenovirus, and the Walter Reed Army Institute of Research is developing its own vaccine against the coronavirus.
Some segments of the population remain skeptical of federal medical experiments. A survey by AP-NORC in May found that Black people are particularly reluctant to get the coronavirus vaccine. Many have concerns about federal research in part because of associations with the infamous Tuskegee Institute syphilis experiments, in which U.S. Public Health Service officials intentionally withheld a cure from Black men infected with the disease.
But Mr. Morrison, of the Center for Strategic and International Studies, said the Defense Department and VA are a “natural fit” for the COVID vaccine trials.
“DOD has lots of expertise. They know how to vaccinate; they know how to reach communities. They have a whole science infrastructure and research-and-development infrastructure. And when you are thinking what the mission of VA is, [VA] sees this is part of their mission,” Mr. Morrison said.
The Defense Department announced its agreement with AstraZeneca in September, shortly before the drugmaker’s vaccine trial was put on hold to study a serious medical condition that one participant reported. That research was approved by the Food and Drug Administration to begin again Oct. 23. The military plans to restart its efforts to recruit 3,000 volunteers.
The Pentagon has also signed an agreement with another vaccine developer, the head of the Defense Health Agency, Army Lt. Gen. Ronald Place, told reporters Oct. 8. He wouldn’t provide the company’s name.
Senator Elizabeth Warren (D-Mass.) and Senator Mazie Hirono (D-Hawaii) have called, unsuccessfully, for the Senate Armed Services Committee to investigate what they say is a lack of Pentagon transparency on its role in vaccine development and distribution. The Defense Department has awarded more than $6 billion in Operation Warp Speed contracts through an intermediary, Advanced Technology International, and the two senators want more information about those contracts.
“There may well be a valuable role for DoD officials in [Operation Warp Speed] – particularly given the department’s logistical capacity,” they wrote to the committee chair and ranking member. “But it is important that Congress conduct appropriate oversight of, and understand, DoD’s activities in this area.”
Neither department has disclosed the financial arrangements they have made with developers to support the vaccine research.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
according to officials with the VA and Operation Warp Speed, the Trump administration’s initiative to fast-track a coronavirus vaccine.
The largely unpublicized effort follows a Department of Defense announcement in September that it has partnered with AstraZeneca to recruit volunteers at five of its medical facilities, which are separate from the VA system. DOD is also is in talks with developers of other vaccine candidates, although officials won’t say which ones.
Both federal departments have long experience in medical research and diverse populations – a crucial component of effective clinical trials, said J. Stephen Morrison, senior vice president and director of global health policy at the Center for Strategic and International Studies, a bipartisan think tank in Washington.
Since active troops are essential to national security, and veterans are extremely vulnerable to COVID-19, both departments have a vested interest in supporting the development of safe, effective vaccines, Mr. Morrison said.
“On the DOD active servicemen and -women side, it’s a question of making sure they’re ready, they are protected,” Mr. Morrison said. “With VA, their population, all elderly and infirm with underlying conditions, they could really be suffering if we don’t get a vaccine.”
According to a VA website, of its 20 medical centers involved, 17 would be part of the Johnson & Johnson vaccine trial, while the three others are recruiting – or have completed recruitment – for advanced-stage trials for Moderna, AstraZeneca, and Pfizer vaccines.
Matthew Hepburn, MD, head of vaccine development at Operation Warp Speed, said the VA effort lets veterans contribute to the overall well-being of the country.
“This is another way they can continue to serve in this way, fighting the pandemic as a volunteer,” Dr. Hepburn said during a discussion of vaccine and therapeutics development hosted by the Heritage Foundation on Oct. 27.
It’s not unusual for the military to participate in multicenter trials for treatments of ailments as diverse as cancer and trauma. Historically, many vaccines have been tested first by the military.
In the general population, clinicians often have difficulty recruiting African Americans and other minorities for medical research, and “the military provides a rich opportunity to find volunteers for those groups,” said retired Rear Adm. Thomas Cullison, MD, a doctor and former deputy surgeon general for the Navy.
Military health facilities are held to the same standards as private research facilities, he said.
No service members will be required to participate in the COVID vaccine trials. All volunteers will be paid by the developer.
Support for routine vaccinations runs high in the military, but some have expressed concerns about new vaccines and mandatory inoculations, especially for anthrax. In a 2002 federal study, 85% of those who received that vaccine reported an adverse reaction, with just under half noticing minor redness at the injection site. But nearly a quarter of the side effects reported were more systemic, including fevers, chills, fatigue and joint pain.
That survey of a small group of National Guard and Reserve members found that, while 73% said they believe immunizations are effective, two-thirds said they did not support the mandatory anthrax program, and 6 in 10 said they were not satisfied with the information they were given on the vaccines.
To quell concerns over the military’s role in supporting COVID vaccine development, the Pentagon has reiterated that troops or their dependents interested in participating in the research must provide voluntary written consent, and they will be allowed to take part only if they will be in the same location for the length of the research, expected to last at least 2 years.
In addition, active-duty members such as new recruits and boot camp participants will not be allowed to volunteer because they are “considered vulnerable from an ethical and regulatory standpoint,” an official said.
At the VA, officials are seeking to recruit healthy veterans aged 18-65 years old who are not pregnant and may be at risk for exposure. As with trials conducted in civilian facilities, participants will be paid by the developer, VA spokesperson Christina Noel said.
Also, VA nurses and caseworkers also are being asked to identify their sickest, highest-risk patients to determine who should be at the top of the list once a vaccine is approved, according to a VA nurse and other health officials who asked not to be identified because they were not authorized to speak with the press.
The U.S. military has a long history of contributing to research on vaccines, including a key role in developing inoculations against yellow fever and adenovirus, and the Walter Reed Army Institute of Research is developing its own vaccine against the coronavirus.
Some segments of the population remain skeptical of federal medical experiments. A survey by AP-NORC in May found that Black people are particularly reluctant to get the coronavirus vaccine. Many have concerns about federal research in part because of associations with the infamous Tuskegee Institute syphilis experiments, in which U.S. Public Health Service officials intentionally withheld a cure from Black men infected with the disease.
But Mr. Morrison, of the Center for Strategic and International Studies, said the Defense Department and VA are a “natural fit” for the COVID vaccine trials.
“DOD has lots of expertise. They know how to vaccinate; they know how to reach communities. They have a whole science infrastructure and research-and-development infrastructure. And when you are thinking what the mission of VA is, [VA] sees this is part of their mission,” Mr. Morrison said.
The Defense Department announced its agreement with AstraZeneca in September, shortly before the drugmaker’s vaccine trial was put on hold to study a serious medical condition that one participant reported. That research was approved by the Food and Drug Administration to begin again Oct. 23. The military plans to restart its efforts to recruit 3,000 volunteers.
The Pentagon has also signed an agreement with another vaccine developer, the head of the Defense Health Agency, Army Lt. Gen. Ronald Place, told reporters Oct. 8. He wouldn’t provide the company’s name.
Senator Elizabeth Warren (D-Mass.) and Senator Mazie Hirono (D-Hawaii) have called, unsuccessfully, for the Senate Armed Services Committee to investigate what they say is a lack of Pentagon transparency on its role in vaccine development and distribution. The Defense Department has awarded more than $6 billion in Operation Warp Speed contracts through an intermediary, Advanced Technology International, and the two senators want more information about those contracts.
“There may well be a valuable role for DoD officials in [Operation Warp Speed] – particularly given the department’s logistical capacity,” they wrote to the committee chair and ranking member. “But it is important that Congress conduct appropriate oversight of, and understand, DoD’s activities in this area.”
Neither department has disclosed the financial arrangements they have made with developers to support the vaccine research.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
according to officials with the VA and Operation Warp Speed, the Trump administration’s initiative to fast-track a coronavirus vaccine.
The largely unpublicized effort follows a Department of Defense announcement in September that it has partnered with AstraZeneca to recruit volunteers at five of its medical facilities, which are separate from the VA system. DOD is also is in talks with developers of other vaccine candidates, although officials won’t say which ones.
Both federal departments have long experience in medical research and diverse populations – a crucial component of effective clinical trials, said J. Stephen Morrison, senior vice president and director of global health policy at the Center for Strategic and International Studies, a bipartisan think tank in Washington.
Since active troops are essential to national security, and veterans are extremely vulnerable to COVID-19, both departments have a vested interest in supporting the development of safe, effective vaccines, Mr. Morrison said.
“On the DOD active servicemen and -women side, it’s a question of making sure they’re ready, they are protected,” Mr. Morrison said. “With VA, their population, all elderly and infirm with underlying conditions, they could really be suffering if we don’t get a vaccine.”
According to a VA website, of its 20 medical centers involved, 17 would be part of the Johnson & Johnson vaccine trial, while the three others are recruiting – or have completed recruitment – for advanced-stage trials for Moderna, AstraZeneca, and Pfizer vaccines.
Matthew Hepburn, MD, head of vaccine development at Operation Warp Speed, said the VA effort lets veterans contribute to the overall well-being of the country.
“This is another way they can continue to serve in this way, fighting the pandemic as a volunteer,” Dr. Hepburn said during a discussion of vaccine and therapeutics development hosted by the Heritage Foundation on Oct. 27.
It’s not unusual for the military to participate in multicenter trials for treatments of ailments as diverse as cancer and trauma. Historically, many vaccines have been tested first by the military.
In the general population, clinicians often have difficulty recruiting African Americans and other minorities for medical research, and “the military provides a rich opportunity to find volunteers for those groups,” said retired Rear Adm. Thomas Cullison, MD, a doctor and former deputy surgeon general for the Navy.
Military health facilities are held to the same standards as private research facilities, he said.
No service members will be required to participate in the COVID vaccine trials. All volunteers will be paid by the developer.
Support for routine vaccinations runs high in the military, but some have expressed concerns about new vaccines and mandatory inoculations, especially for anthrax. In a 2002 federal study, 85% of those who received that vaccine reported an adverse reaction, with just under half noticing minor redness at the injection site. But nearly a quarter of the side effects reported were more systemic, including fevers, chills, fatigue and joint pain.
That survey of a small group of National Guard and Reserve members found that, while 73% said they believe immunizations are effective, two-thirds said they did not support the mandatory anthrax program, and 6 in 10 said they were not satisfied with the information they were given on the vaccines.
To quell concerns over the military’s role in supporting COVID vaccine development, the Pentagon has reiterated that troops or their dependents interested in participating in the research must provide voluntary written consent, and they will be allowed to take part only if they will be in the same location for the length of the research, expected to last at least 2 years.
In addition, active-duty members such as new recruits and boot camp participants will not be allowed to volunteer because they are “considered vulnerable from an ethical and regulatory standpoint,” an official said.
At the VA, officials are seeking to recruit healthy veterans aged 18-65 years old who are not pregnant and may be at risk for exposure. As with trials conducted in civilian facilities, participants will be paid by the developer, VA spokesperson Christina Noel said.
Also, VA nurses and caseworkers also are being asked to identify their sickest, highest-risk patients to determine who should be at the top of the list once a vaccine is approved, according to a VA nurse and other health officials who asked not to be identified because they were not authorized to speak with the press.
The U.S. military has a long history of contributing to research on vaccines, including a key role in developing inoculations against yellow fever and adenovirus, and the Walter Reed Army Institute of Research is developing its own vaccine against the coronavirus.
Some segments of the population remain skeptical of federal medical experiments. A survey by AP-NORC in May found that Black people are particularly reluctant to get the coronavirus vaccine. Many have concerns about federal research in part because of associations with the infamous Tuskegee Institute syphilis experiments, in which U.S. Public Health Service officials intentionally withheld a cure from Black men infected with the disease.
But Mr. Morrison, of the Center for Strategic and International Studies, said the Defense Department and VA are a “natural fit” for the COVID vaccine trials.
“DOD has lots of expertise. They know how to vaccinate; they know how to reach communities. They have a whole science infrastructure and research-and-development infrastructure. And when you are thinking what the mission of VA is, [VA] sees this is part of their mission,” Mr. Morrison said.
The Defense Department announced its agreement with AstraZeneca in September, shortly before the drugmaker’s vaccine trial was put on hold to study a serious medical condition that one participant reported. That research was approved by the Food and Drug Administration to begin again Oct. 23. The military plans to restart its efforts to recruit 3,000 volunteers.
The Pentagon has also signed an agreement with another vaccine developer, the head of the Defense Health Agency, Army Lt. Gen. Ronald Place, told reporters Oct. 8. He wouldn’t provide the company’s name.
Senator Elizabeth Warren (D-Mass.) and Senator Mazie Hirono (D-Hawaii) have called, unsuccessfully, for the Senate Armed Services Committee to investigate what they say is a lack of Pentagon transparency on its role in vaccine development and distribution. The Defense Department has awarded more than $6 billion in Operation Warp Speed contracts through an intermediary, Advanced Technology International, and the two senators want more information about those contracts.
“There may well be a valuable role for DoD officials in [Operation Warp Speed] – particularly given the department’s logistical capacity,” they wrote to the committee chair and ranking member. “But it is important that Congress conduct appropriate oversight of, and understand, DoD’s activities in this area.”
Neither department has disclosed the financial arrangements they have made with developers to support the vaccine research.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
COVID-19 risks in rheumatic disease remain unclear
ACR 2020 studies offer conflicting findings.
Among people with COVID-19, those with systemic autoimmune rheumatic diseases had an elevated 30-day risk of hospitalization, ICU admission, need for mechanical ventilation, and acute kidney injury, compared to a group without rheumatic diseases at 4 months in a match-controlled study.
When investigators expanded the study to 6 months, the difference in need for mechanical ventilation disappeared. However, relative risk for venous thromboembolism (VTE) emerged as 74% higher among people with COVID-19 and with rheumatic disease, said Kristin D’Silva, MD, who presented the findings during a plenary session at the virtual annual meeting of the American College of Rheumatology. She noted that rheumatic disease itself could contribute to VTE risk.
Comorbidities including hypertension, diabetes, and asthma were more common among people with systemic autoimmune rheumatic diseases (SARDs). After adjustment for comorbidities, “the risks of hospitalization and ICU admission were attenuated, suggesting comorbidities are likely key mediators of the increased risk of severe COVID-19 outcomes observed in SARDs patients versus comparators,” Dr. D’Silva, a rheumatology fellow at Massachusetts General Hospital in Boston, said in an interview.
“The risk of venous thromboembolism persisted even after adjusting for comorbidities,” Dr. D’Silva said. Patients with SARDs should be closely monitored for VTE during COVID-19 infection, she added. “Patients with significant cardiovascular, pulmonary, and metabolic comorbidities should be closely monitored for severe COVID-19.”
At the same time, a systematic review of 15 published studies revealed a low incidence of COVID-19 infection among people with rheumatic disease. Furthermore, most experienced a mild clinical course and low mortality, Akhil Sood, MD, said when presenting results of his poster at the meeting.
Underlying immunosuppression, chronic inflammation, comorbidities, and disparities based on racial, ethnic, and socioeconomic status could predispose people with rheumatic disease to poorer COVID-19 outcomes. However, the risks and outcomes of COVID-19 infection among this population “are not well understood,” said Dr. Sood, a second-year resident in internal medicine at the University of Texas Medical Branch in Galveston.
Elevated risks in match-controlled study
Dr. D’Silva and colleagues examined a COVID-19 population and compared 716 people with SARDs and another 716 people from the general public at 4 months, as well as 2,379 people each in similar groups at 6 months. They used real-time electronic medical record data from the TriNetX research network to identify ICD-10 codes for inflammatory arthritis, connective tissue diseases, and systemic vasculitis. They also used ICD-10 codes and positive PCR tests to identify people with COVID-19.
Mean age was 57 years and women accounted for 79% of both groups evaluated at 4 months. Those with SARDs were 23% more likely to be hospitalized (relative risk, 1.23; 95% confidence interval, 1.01-1.50). This group was 75% more likely to be admitted to the ICU (RR, 1.75; 95% CI, 1.11-2.75), 77% more likely to require mechanical ventilation (RR, 1.77; 95% CI, 1.06-2.96), and 83% more likely to experience acute kidney injury (RR, 1.83; 95% CI, 1.11-3.00).
Risk of death was not significantly higher in the SARDs group (RR, 1.16; 95% CI, 0.73-1.86).
When Dr. D’Silva expanded the study to more people at 6 months, they added additional 30-day outcomes of interest: renal replacement therapy, VTE, and ischemic stroke. Risk of need for renal replacement therapy, for example, was 81% higher in the SARDs group (RR, 1.81; 95% CI, 1.07-3.07). Risk of stroke was not significantly different between groups.The improvement in mechanical ventilation risk between 4 and 6 months was not completely unexpected, Dr. D’Silva said. The relative risk dropped from 1.77 to 1.05. “This is not particularly surprising given national trends in the general population reporting decreased severe outcomes of COVID-19 including mortality as the pandemic progresses. This is likely multifactorial including changes in COVID-19 management (such as increasing use of nonintubated prone positioning rather than early intubation and treatments such as dexamethasone and remdesivir), decreased strain on hospitals and staffing compared to the early crisis phase of the pandemic, and higher testing capacity leading to detection of milder cases.”
When the 6-month analysis was further adjusted for comorbidities and a history of prior hospitalization within 1 year, only risk for acute kidney injury and VTE remained significant with relative risks of 1.33 and 1.60, respectively, likely because comorbidities are causal intermediates of COVID-19 30-day outcomes rather than confounders.
When asked to comment on the results, session comoderator Victoria K. Shanmugam, MD, said in an interview that the study “is of great interest both to rheumatologists and to patients with rheumatic disease.”
The higher risk of hospitalization, ICU admission, mechanical ventilation, acute kidney injury, and heart failure “is an important finding with implications for how our patients navigate risk during this pandemic,” said Dr. Shanmugam, director of the division of rheumatology at George Washington University in Washington.
Lower risks emerge in systematic review
The 15 observational studies in the systematic review included 11,815 participants. A total of 179, or 1.5%, tested positive for COVID-19.
“The incidence of COVID-19 infection among patients with rheumatic disease was low,” Dr. Sood said.
Within the COVID-19-positive group, almost 50% required hospitalization, 10% required ICU admission, and 8% died. The pooled event rate for hospitalization was 0.440 (95% CI, 0.296-0.596), while for ICU admission it was 0.132 (95% CI, 0.087-0.194) and for death it was 0.125 (95% CI, 0.082-0.182).
Different calculations of risk
The two studies seem to offer contradictory findings, but the disparities could be explained by study design differences. For example, Dr. D’Silva’s study evaluated a population with COVID-19 and compared those with SARDs versus a matched group from the general public. Dr. Sood and colleagues assessed study populations with rheumatic disease and assessed incidence of SARS-CoV-2 infection and difference in outcomes.
“We are asking very different questions,” Dr. D’Silva said.
“The study by D’Silva et al. was able to account for different factors to reduce confounding,” Dr. Sood said, adding that Dr. D’Silva and colleagues included a high proportion of minorities, compared with a less diverse population in the systematic review, which featured a large number of studies from Italy.
The authors of the two studies had no relevant financial disclosures to report.
SOURCES: D’Silva K et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 0430, and Sood A et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 0008.
ACR 2020 studies offer conflicting findings.
ACR 2020 studies offer conflicting findings.
Among people with COVID-19, those with systemic autoimmune rheumatic diseases had an elevated 30-day risk of hospitalization, ICU admission, need for mechanical ventilation, and acute kidney injury, compared to a group without rheumatic diseases at 4 months in a match-controlled study.
When investigators expanded the study to 6 months, the difference in need for mechanical ventilation disappeared. However, relative risk for venous thromboembolism (VTE) emerged as 74% higher among people with COVID-19 and with rheumatic disease, said Kristin D’Silva, MD, who presented the findings during a plenary session at the virtual annual meeting of the American College of Rheumatology. She noted that rheumatic disease itself could contribute to VTE risk.
Comorbidities including hypertension, diabetes, and asthma were more common among people with systemic autoimmune rheumatic diseases (SARDs). After adjustment for comorbidities, “the risks of hospitalization and ICU admission were attenuated, suggesting comorbidities are likely key mediators of the increased risk of severe COVID-19 outcomes observed in SARDs patients versus comparators,” Dr. D’Silva, a rheumatology fellow at Massachusetts General Hospital in Boston, said in an interview.
“The risk of venous thromboembolism persisted even after adjusting for comorbidities,” Dr. D’Silva said. Patients with SARDs should be closely monitored for VTE during COVID-19 infection, she added. “Patients with significant cardiovascular, pulmonary, and metabolic comorbidities should be closely monitored for severe COVID-19.”
At the same time, a systematic review of 15 published studies revealed a low incidence of COVID-19 infection among people with rheumatic disease. Furthermore, most experienced a mild clinical course and low mortality, Akhil Sood, MD, said when presenting results of his poster at the meeting.
Underlying immunosuppression, chronic inflammation, comorbidities, and disparities based on racial, ethnic, and socioeconomic status could predispose people with rheumatic disease to poorer COVID-19 outcomes. However, the risks and outcomes of COVID-19 infection among this population “are not well understood,” said Dr. Sood, a second-year resident in internal medicine at the University of Texas Medical Branch in Galveston.
Elevated risks in match-controlled study
Dr. D’Silva and colleagues examined a COVID-19 population and compared 716 people with SARDs and another 716 people from the general public at 4 months, as well as 2,379 people each in similar groups at 6 months. They used real-time electronic medical record data from the TriNetX research network to identify ICD-10 codes for inflammatory arthritis, connective tissue diseases, and systemic vasculitis. They also used ICD-10 codes and positive PCR tests to identify people with COVID-19.
Mean age was 57 years and women accounted for 79% of both groups evaluated at 4 months. Those with SARDs were 23% more likely to be hospitalized (relative risk, 1.23; 95% confidence interval, 1.01-1.50). This group was 75% more likely to be admitted to the ICU (RR, 1.75; 95% CI, 1.11-2.75), 77% more likely to require mechanical ventilation (RR, 1.77; 95% CI, 1.06-2.96), and 83% more likely to experience acute kidney injury (RR, 1.83; 95% CI, 1.11-3.00).
Risk of death was not significantly higher in the SARDs group (RR, 1.16; 95% CI, 0.73-1.86).
When Dr. D’Silva expanded the study to more people at 6 months, they added additional 30-day outcomes of interest: renal replacement therapy, VTE, and ischemic stroke. Risk of need for renal replacement therapy, for example, was 81% higher in the SARDs group (RR, 1.81; 95% CI, 1.07-3.07). Risk of stroke was not significantly different between groups.The improvement in mechanical ventilation risk between 4 and 6 months was not completely unexpected, Dr. D’Silva said. The relative risk dropped from 1.77 to 1.05. “This is not particularly surprising given national trends in the general population reporting decreased severe outcomes of COVID-19 including mortality as the pandemic progresses. This is likely multifactorial including changes in COVID-19 management (such as increasing use of nonintubated prone positioning rather than early intubation and treatments such as dexamethasone and remdesivir), decreased strain on hospitals and staffing compared to the early crisis phase of the pandemic, and higher testing capacity leading to detection of milder cases.”
When the 6-month analysis was further adjusted for comorbidities and a history of prior hospitalization within 1 year, only risk for acute kidney injury and VTE remained significant with relative risks of 1.33 and 1.60, respectively, likely because comorbidities are causal intermediates of COVID-19 30-day outcomes rather than confounders.
When asked to comment on the results, session comoderator Victoria K. Shanmugam, MD, said in an interview that the study “is of great interest both to rheumatologists and to patients with rheumatic disease.”
The higher risk of hospitalization, ICU admission, mechanical ventilation, acute kidney injury, and heart failure “is an important finding with implications for how our patients navigate risk during this pandemic,” said Dr. Shanmugam, director of the division of rheumatology at George Washington University in Washington.
Lower risks emerge in systematic review
The 15 observational studies in the systematic review included 11,815 participants. A total of 179, or 1.5%, tested positive for COVID-19.
“The incidence of COVID-19 infection among patients with rheumatic disease was low,” Dr. Sood said.
Within the COVID-19-positive group, almost 50% required hospitalization, 10% required ICU admission, and 8% died. The pooled event rate for hospitalization was 0.440 (95% CI, 0.296-0.596), while for ICU admission it was 0.132 (95% CI, 0.087-0.194) and for death it was 0.125 (95% CI, 0.082-0.182).
Different calculations of risk
The two studies seem to offer contradictory findings, but the disparities could be explained by study design differences. For example, Dr. D’Silva’s study evaluated a population with COVID-19 and compared those with SARDs versus a matched group from the general public. Dr. Sood and colleagues assessed study populations with rheumatic disease and assessed incidence of SARS-CoV-2 infection and difference in outcomes.
“We are asking very different questions,” Dr. D’Silva said.
“The study by D’Silva et al. was able to account for different factors to reduce confounding,” Dr. Sood said, adding that Dr. D’Silva and colleagues included a high proportion of minorities, compared with a less diverse population in the systematic review, which featured a large number of studies from Italy.
The authors of the two studies had no relevant financial disclosures to report.
SOURCES: D’Silva K et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 0430, and Sood A et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 0008.
Among people with COVID-19, those with systemic autoimmune rheumatic diseases had an elevated 30-day risk of hospitalization, ICU admission, need for mechanical ventilation, and acute kidney injury, compared to a group without rheumatic diseases at 4 months in a match-controlled study.
When investigators expanded the study to 6 months, the difference in need for mechanical ventilation disappeared. However, relative risk for venous thromboembolism (VTE) emerged as 74% higher among people with COVID-19 and with rheumatic disease, said Kristin D’Silva, MD, who presented the findings during a plenary session at the virtual annual meeting of the American College of Rheumatology. She noted that rheumatic disease itself could contribute to VTE risk.
Comorbidities including hypertension, diabetes, and asthma were more common among people with systemic autoimmune rheumatic diseases (SARDs). After adjustment for comorbidities, “the risks of hospitalization and ICU admission were attenuated, suggesting comorbidities are likely key mediators of the increased risk of severe COVID-19 outcomes observed in SARDs patients versus comparators,” Dr. D’Silva, a rheumatology fellow at Massachusetts General Hospital in Boston, said in an interview.
“The risk of venous thromboembolism persisted even after adjusting for comorbidities,” Dr. D’Silva said. Patients with SARDs should be closely monitored for VTE during COVID-19 infection, she added. “Patients with significant cardiovascular, pulmonary, and metabolic comorbidities should be closely monitored for severe COVID-19.”
At the same time, a systematic review of 15 published studies revealed a low incidence of COVID-19 infection among people with rheumatic disease. Furthermore, most experienced a mild clinical course and low mortality, Akhil Sood, MD, said when presenting results of his poster at the meeting.
Underlying immunosuppression, chronic inflammation, comorbidities, and disparities based on racial, ethnic, and socioeconomic status could predispose people with rheumatic disease to poorer COVID-19 outcomes. However, the risks and outcomes of COVID-19 infection among this population “are not well understood,” said Dr. Sood, a second-year resident in internal medicine at the University of Texas Medical Branch in Galveston.
Elevated risks in match-controlled study
Dr. D’Silva and colleagues examined a COVID-19 population and compared 716 people with SARDs and another 716 people from the general public at 4 months, as well as 2,379 people each in similar groups at 6 months. They used real-time electronic medical record data from the TriNetX research network to identify ICD-10 codes for inflammatory arthritis, connective tissue diseases, and systemic vasculitis. They also used ICD-10 codes and positive PCR tests to identify people with COVID-19.
Mean age was 57 years and women accounted for 79% of both groups evaluated at 4 months. Those with SARDs were 23% more likely to be hospitalized (relative risk, 1.23; 95% confidence interval, 1.01-1.50). This group was 75% more likely to be admitted to the ICU (RR, 1.75; 95% CI, 1.11-2.75), 77% more likely to require mechanical ventilation (RR, 1.77; 95% CI, 1.06-2.96), and 83% more likely to experience acute kidney injury (RR, 1.83; 95% CI, 1.11-3.00).
Risk of death was not significantly higher in the SARDs group (RR, 1.16; 95% CI, 0.73-1.86).
When Dr. D’Silva expanded the study to more people at 6 months, they added additional 30-day outcomes of interest: renal replacement therapy, VTE, and ischemic stroke. Risk of need for renal replacement therapy, for example, was 81% higher in the SARDs group (RR, 1.81; 95% CI, 1.07-3.07). Risk of stroke was not significantly different between groups.The improvement in mechanical ventilation risk between 4 and 6 months was not completely unexpected, Dr. D’Silva said. The relative risk dropped from 1.77 to 1.05. “This is not particularly surprising given national trends in the general population reporting decreased severe outcomes of COVID-19 including mortality as the pandemic progresses. This is likely multifactorial including changes in COVID-19 management (such as increasing use of nonintubated prone positioning rather than early intubation and treatments such as dexamethasone and remdesivir), decreased strain on hospitals and staffing compared to the early crisis phase of the pandemic, and higher testing capacity leading to detection of milder cases.”
When the 6-month analysis was further adjusted for comorbidities and a history of prior hospitalization within 1 year, only risk for acute kidney injury and VTE remained significant with relative risks of 1.33 and 1.60, respectively, likely because comorbidities are causal intermediates of COVID-19 30-day outcomes rather than confounders.
When asked to comment on the results, session comoderator Victoria K. Shanmugam, MD, said in an interview that the study “is of great interest both to rheumatologists and to patients with rheumatic disease.”
The higher risk of hospitalization, ICU admission, mechanical ventilation, acute kidney injury, and heart failure “is an important finding with implications for how our patients navigate risk during this pandemic,” said Dr. Shanmugam, director of the division of rheumatology at George Washington University in Washington.
Lower risks emerge in systematic review
The 15 observational studies in the systematic review included 11,815 participants. A total of 179, or 1.5%, tested positive for COVID-19.
“The incidence of COVID-19 infection among patients with rheumatic disease was low,” Dr. Sood said.
Within the COVID-19-positive group, almost 50% required hospitalization, 10% required ICU admission, and 8% died. The pooled event rate for hospitalization was 0.440 (95% CI, 0.296-0.596), while for ICU admission it was 0.132 (95% CI, 0.087-0.194) and for death it was 0.125 (95% CI, 0.082-0.182).
Different calculations of risk
The two studies seem to offer contradictory findings, but the disparities could be explained by study design differences. For example, Dr. D’Silva’s study evaluated a population with COVID-19 and compared those with SARDs versus a matched group from the general public. Dr. Sood and colleagues assessed study populations with rheumatic disease and assessed incidence of SARS-CoV-2 infection and difference in outcomes.
“We are asking very different questions,” Dr. D’Silva said.
“The study by D’Silva et al. was able to account for different factors to reduce confounding,” Dr. Sood said, adding that Dr. D’Silva and colleagues included a high proportion of minorities, compared with a less diverse population in the systematic review, which featured a large number of studies from Italy.
The authors of the two studies had no relevant financial disclosures to report.
SOURCES: D’Silva K et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 0430, and Sood A et al. Arthritis Rheumatol. 2020;72(suppl 10): Abstract 0008.
FROM ACR 2020
Biden victory: What it means for COVID, health care
The former vice president has sketched out a big health agenda: ramping up the federal response to COVID-19, boosting the Affordable Care Act, creating a new “public option” to cover uninsured Americans, and expanding Medicare and Medicaid.
But the president-elect’s long to-do list on health is likely to face significant roadblocks in Congress and the courts, experts say.
For instance, Biden’s ambitious proposals on COVID-19 -- including his recent call for a national mask mandate -- could be waylaid by legal challenges and run into political hurdles on Capitol Hill, where he may face a divided Congress.
Joseph Antos, PhD, a health policy expert with the conservative American Enterprise Institute, predicts Biden will encounter the same type of congressional “gridlock situation” that President Barack Obama ran into during his second term.
“We have a situation that has been like this for a very, very long time -- lack of cooperation, lack of recognition that either party is capable of rising above their own electoral views to deal with problems that the country actually has.”
Antos also suggests that Biden may also face enormous political pressure to address the economic fallout from the coronavirus, including record unemployment and business closures, before anything else.
“I think it’s really going to be efforts that are intended to promote economic development and promote the economy,” he says.
In addition, Biden’s plans to expand Obamacare might face a new challenge from the Supreme Court in the year ahead. This month, the high court will take up a new case seeking to overturn the law.
Even so, experts say Biden’s plans on COVID-19 and expanding health care are likely to define his tenure in the White House as a central focus of his presidency.
“Health care will be at the very top of the list of the president’s priorities,” says Sabrina Corlette, JD, co-director of the Center on Health Insurance Reforms at Georgetown University’s McCourt School of Public Policy. “I do think, however, that the administration is going to be very preoccupied with the response to COVID-19 and the economic fallout … particularly in the first year.”
Here’s a closer look at what we can expect from a Biden presidency.
COVID-19: Federalizing response efforts
Biden will move to federalize the response to COVID-19. He has said he will take back major responsibilities from the states -- such as setting national policies on mask wearing, social distancing, and the reopening of schools and businesses, based on CDC guidance. In the days leading up to the election, Biden called for a national mask mandate, after waffling on the issue throughout the summer.
He has said he will let public health science drive political policy. Biden is also planning to create his own task force to advise officials during the transition on managing the new surge in COVID-19 cases, vaccine safety and protecting at-risk populations, Politico reported this week. He received a virtual briefing on the pandemic from a panel of experts as he awaited the election’s outcome.
“I think we will no longer have this confused and contradictory public messaging,” Corlette says, “but I also think there will be humility and the recognition that the evidence is evolving -- that we don’t have all the answers, but we’re learning as we go.”
But national mandates on masks and social distancing will be challenging to enforce, experts say. They are also likely to face pushback from business interests, opposition from public officials in GOP-led states, and even legal challenges.
Biden’s ability to work with Congress -- or not -- may determine whether he is able to implement some of the key components of his coronavirus action plan, which includes:
- Providing free COVID-19 testing for all Americans
- Hiring 100,000 contact tracers
- Eliminating out-of-pocket expenses for coronavirus treatment
- Delivering “sufficient” PPE for essential workers
- Supporting science-backed vaccines and medical treatments being developed
- Requiring the reopening of businesses, workplaces, and schools only after “sufficient” reductions in community transmission -- under evidence-based protocols put forward by the CDC
- Giving emergency paid leave for workers dislocated by the pandemic and more financial aid for workers, families, and small businesses
- Shoring up safeguards to protect at-risk Americans, including older people
- Boosting pay for health care workers on the front lines
Biden has not detailed how he would pay for many of these, beyond promising to force wealthy Americans to “pay their fair share” of taxes to help. He has proposed a tax increase on Americans making more than $400,000 a year, which would require congressional approval.
Antos says he expects Biden’s proposed COVID-19 action plan to be virtually the same as Trump’s in two areas: efforts to develop a vaccine and antiviral treatments.
The administration has spent some $225 million on COVID-19 testing efforts, with a particular focus on rural areas.
Trump launched Operation Warp Speed to fast-track a vaccine. As part of that, the federal government has contracted with six drug companies, spending nearly $11 billion. The operation aims to provide at least 300 million doses of a coronavirus vaccine by January 2021.
Antos would like to see “a more sophisticated approach to social distancing” from the president-elect that takes into account the different challenges facing Americans depending on their income, work situation, and other factors during the pandemic.
“There are a lot of people in this country where working from home is fine and their jobs are secure,” he notes. “It’s the person who used to work at a restaurant that closed, it’s the line worker at a factory that has severely cut back its hours. It’s basically lower-middle-class people, low-income people, middle-class people, and it’s not the elite.
“And the policies have not given enough consideration to the fact that their circumstances and their tradeoffs would differ from the tradeoffs of somebody who doesn’t have anything to worry about economically.
“So, what we need is a more supple policy [that] will give people the information they need and give them the financial support that they also need … so they can make good decisions for themselves and their families. And we basically haven’t done that.”
Obamacare on the blocks?
The Supreme Court’s decision to take up another case seeking to overturn the Affordable Care Act could hand Biden’s health agenda a major setback -- and put the medical care for millions of Americans in jeopardy.
On Nov. 10, the high court will hear oral arguments on a lawsuit that would strike down all of Obamacare. A decision is not expected until next year.
The court has previously upheld the 2010 law, which Biden helped usher through Congress as vice president. But the addition of right-leaning Supreme Court Justice Amy Coney Barrett to the bench last month gives the court a clear conservative majority that could mean the end of Obamacare, legal experts say.
Republicans have opposed the law since its passage, but they have been unable to muster the votes to repeal it, or to pass an alternative
Antos, from the American Enterprise Institute, notes conservatives believe the law has increased costs for health care and insurance over the past decade, in part because of its protections for Americans with preexisting conditions and requiring insurers to provide comprehensive “gold-plated” policies.
“It’s driven up costs, offers plans that are not very strong, put high-risk folks into the same [insurance pool], which has increased costs for everyone, the employer mandate … these are all the reasons,” he says.
The Supreme Court isn’t expected to deliver a decision on the Affordable Care Act before the middle of next year. But the uncertainty will likely push back Biden’s proposals to expand on the law.
Overturning Obamacare would have huge impacts on millions of Americans:
- As many as 133 million Americans -- roughly half the U.S. population -- with preexisting conditions could find it harder, if not impossible, to find affordable health insurance. That figure does not include Americans infected with COVID-19.
- About 165 million who require expensive treatments -- for cancer and other conditions -- would no longer be protected from huge costs for care by federal caps on out-of-pocket expenditures the Affordable Care Act requires.
- An estimated 21 million who now buy insurance through the Obamacare Marketplaces could lose their coverage.
- Another 12 million on Medicaid could find themselves without insurance.
- At least 2 million young adults ages 26 and under, now on their parents’ health policies, could be kicked off.
- Millions of people who use Medicare could face higher costs.
- Federal subsidies for lower-income Americans to buy policies would disappear.
Throughout the campaign, Biden repeatedly stressed the need to preserve the law’s provision barring insurance companies from refusing coverage for Americans with preexisting conditions, such as diabetes, cancer, and heart disease. It also outlaws charging higher premiums on the basis of health status, age, or gender.
Biden has also pledged to bolster the law as president.
He has proposed a variety of add-ons to the Affordable Care Act he says will “insure more than an estimated 97% of Americans,” according to the Biden campaign site.
Biden’s proposals include offering larger federal subsidies to help low- and middle-income Americans pay for policies purchased through Obamacare insurance Marketplaces.
The boldest of Biden’s proposals is the creation of a “public option” for insurance -- a Medicare-like program that small businesses and individuals could choose if they do not have coverage, cannot afford it, or don’t like their employer-based coverage.
It would also automatically enroll millions of uninsured Americans living in the 14 states that have not expanded Medicaid, which covers low-income people.
But such a plan would require congressional approval -- including a “super majority” of 60 Senate votes to block a likely GOP filibuster. That will be a significant challenge Biden will have to overcome, with Congress so evenly divided.
The White House would also have to defeat heavy lobbying from some of the most influential industry interest groups in Washington, Corlette says.
“I’m not even confident they would get all the Democrat votes,” she says.
“So, it’s a going to be an uphill battle to get a public option passed.”
Taken together, Biden’s plans for expanding Obamacare are projected to cost $750 billion over 10 years. He has said much of that financing would come from increasing taxes on the wealthy.
That means it would likely require congressional approval, which Antos suggests is unlikely given the polarization on Capitol Hill.
Medicare, Medicaid, and drug costs
Biden has called for a host of reforms targeting Medicare, Medicaid, and rising drug costs.
On Medicare, which primarily covers seniors 65 and older, Biden has proposed lowering the eligibility age from 65 to 60. That could extend Medicare to up to 20 million more Americans.
On Medicaid, the health care safety net for low-income and disabled Americans, the president-elect supports increased federal funding to states during the current economic crisis, and potentially beyond.
Medicare is likely to become a key focus of the new administration, in light of the pressures the pandemic is placing on Medicare funding.
In April, Medicare’s trustees said that the Part A trust fund for the program, which pays for hospital and inpatient care, could start to run dry in 2026.
But those projections did not include the impact of COVID-19. Some economists have since projected that Medicare Part A could become insolvent as early as 2022.
Medicare Part B, which pays for doctor and outpatient costs, is funded by general tax funding and beneficiary insurance premiums, so it is not in danger of drying up.
Adding to those pressures is an executive order Trump signed in August temporarily deferring payroll taxes, a primary funding vehicle for Medicare and Social Security.
Under these taxes, employees pay 6.2% of their earnings (on annual income up to $137,700) toward Social Security and 1.45% for Medicare taxes each pay period. Employers pay the same rate per paycheck, adding up to a combined 12.4% Social Security tax and 2.9% Medicare tax.
Biden has said he would reverse the tax cut when he takes office.
But to get a handle on Medicare and Medicaid funding issues, he is likely to need congressional support. Corlette and other experts say that could be a challenge while the nation remains in the grip of the coronavirus pandemic.
In addition to his Medicare and Medicaid reforms, Biden has proposed several plans to lower drug prices, a subset of rising health care and insurance costs.
U.S. spending on prescription drugs has increased nearly 42% over the past decade -- from $253.1 billion in 2010 to $358.7 billion in 2020 (projected) -- according to the Centers for Medicare & Medicaid Services.
In 2020, retail prices for 460 commonly prescribed drugs have spiked an average of 5.2%, according to new analysis by 3 Axis Advisors, a health research firm.
That’s more than double the projected rate of inflation.
To control drug costs, Biden supports legislation approved by the Democratic-led House of Representatives last year that would empower Medicare to negotiate drug prices with drug companies, as private insurers do.
Federal law now bars Medicare from negotiating prices on behalf of the 67.7 million Americans who use it. Drug companies and many GOP leaders argue that the current law is necessary to allow them to spend more on research and development of new medications.
In addition, Biden supports the idea of lifting bans on importing drugs from foreign countries with lower costs.
He also backs creating an independent review board to set price caps for new medications with no competitors; making high-quality generics more available; ending tax breaks for drug company advertising; and limiting their leeway in raising prices.
All of these proposals would likely require congressional approval and could face legal challenges in the courts.
This article first appeared on WebMD.com.
The former vice president has sketched out a big health agenda: ramping up the federal response to COVID-19, boosting the Affordable Care Act, creating a new “public option” to cover uninsured Americans, and expanding Medicare and Medicaid.
But the president-elect’s long to-do list on health is likely to face significant roadblocks in Congress and the courts, experts say.
For instance, Biden’s ambitious proposals on COVID-19 -- including his recent call for a national mask mandate -- could be waylaid by legal challenges and run into political hurdles on Capitol Hill, where he may face a divided Congress.
Joseph Antos, PhD, a health policy expert with the conservative American Enterprise Institute, predicts Biden will encounter the same type of congressional “gridlock situation” that President Barack Obama ran into during his second term.
“We have a situation that has been like this for a very, very long time -- lack of cooperation, lack of recognition that either party is capable of rising above their own electoral views to deal with problems that the country actually has.”
Antos also suggests that Biden may also face enormous political pressure to address the economic fallout from the coronavirus, including record unemployment and business closures, before anything else.
“I think it’s really going to be efforts that are intended to promote economic development and promote the economy,” he says.
In addition, Biden’s plans to expand Obamacare might face a new challenge from the Supreme Court in the year ahead. This month, the high court will take up a new case seeking to overturn the law.
Even so, experts say Biden’s plans on COVID-19 and expanding health care are likely to define his tenure in the White House as a central focus of his presidency.
“Health care will be at the very top of the list of the president’s priorities,” says Sabrina Corlette, JD, co-director of the Center on Health Insurance Reforms at Georgetown University’s McCourt School of Public Policy. “I do think, however, that the administration is going to be very preoccupied with the response to COVID-19 and the economic fallout … particularly in the first year.”
Here’s a closer look at what we can expect from a Biden presidency.
COVID-19: Federalizing response efforts
Biden will move to federalize the response to COVID-19. He has said he will take back major responsibilities from the states -- such as setting national policies on mask wearing, social distancing, and the reopening of schools and businesses, based on CDC guidance. In the days leading up to the election, Biden called for a national mask mandate, after waffling on the issue throughout the summer.
He has said he will let public health science drive political policy. Biden is also planning to create his own task force to advise officials during the transition on managing the new surge in COVID-19 cases, vaccine safety and protecting at-risk populations, Politico reported this week. He received a virtual briefing on the pandemic from a panel of experts as he awaited the election’s outcome.
“I think we will no longer have this confused and contradictory public messaging,” Corlette says, “but I also think there will be humility and the recognition that the evidence is evolving -- that we don’t have all the answers, but we’re learning as we go.”
But national mandates on masks and social distancing will be challenging to enforce, experts say. They are also likely to face pushback from business interests, opposition from public officials in GOP-led states, and even legal challenges.
Biden’s ability to work with Congress -- or not -- may determine whether he is able to implement some of the key components of his coronavirus action plan, which includes:
- Providing free COVID-19 testing for all Americans
- Hiring 100,000 contact tracers
- Eliminating out-of-pocket expenses for coronavirus treatment
- Delivering “sufficient” PPE for essential workers
- Supporting science-backed vaccines and medical treatments being developed
- Requiring the reopening of businesses, workplaces, and schools only after “sufficient” reductions in community transmission -- under evidence-based protocols put forward by the CDC
- Giving emergency paid leave for workers dislocated by the pandemic and more financial aid for workers, families, and small businesses
- Shoring up safeguards to protect at-risk Americans, including older people
- Boosting pay for health care workers on the front lines
Biden has not detailed how he would pay for many of these, beyond promising to force wealthy Americans to “pay their fair share” of taxes to help. He has proposed a tax increase on Americans making more than $400,000 a year, which would require congressional approval.
Antos says he expects Biden’s proposed COVID-19 action plan to be virtually the same as Trump’s in two areas: efforts to develop a vaccine and antiviral treatments.
The administration has spent some $225 million on COVID-19 testing efforts, with a particular focus on rural areas.
Trump launched Operation Warp Speed to fast-track a vaccine. As part of that, the federal government has contracted with six drug companies, spending nearly $11 billion. The operation aims to provide at least 300 million doses of a coronavirus vaccine by January 2021.
Antos would like to see “a more sophisticated approach to social distancing” from the president-elect that takes into account the different challenges facing Americans depending on their income, work situation, and other factors during the pandemic.
“There are a lot of people in this country where working from home is fine and their jobs are secure,” he notes. “It’s the person who used to work at a restaurant that closed, it’s the line worker at a factory that has severely cut back its hours. It’s basically lower-middle-class people, low-income people, middle-class people, and it’s not the elite.
“And the policies have not given enough consideration to the fact that their circumstances and their tradeoffs would differ from the tradeoffs of somebody who doesn’t have anything to worry about economically.
“So, what we need is a more supple policy [that] will give people the information they need and give them the financial support that they also need … so they can make good decisions for themselves and their families. And we basically haven’t done that.”
Obamacare on the blocks?
The Supreme Court’s decision to take up another case seeking to overturn the Affordable Care Act could hand Biden’s health agenda a major setback -- and put the medical care for millions of Americans in jeopardy.
On Nov. 10, the high court will hear oral arguments on a lawsuit that would strike down all of Obamacare. A decision is not expected until next year.
The court has previously upheld the 2010 law, which Biden helped usher through Congress as vice president. But the addition of right-leaning Supreme Court Justice Amy Coney Barrett to the bench last month gives the court a clear conservative majority that could mean the end of Obamacare, legal experts say.
Republicans have opposed the law since its passage, but they have been unable to muster the votes to repeal it, or to pass an alternative
Antos, from the American Enterprise Institute, notes conservatives believe the law has increased costs for health care and insurance over the past decade, in part because of its protections for Americans with preexisting conditions and requiring insurers to provide comprehensive “gold-plated” policies.
“It’s driven up costs, offers plans that are not very strong, put high-risk folks into the same [insurance pool], which has increased costs for everyone, the employer mandate … these are all the reasons,” he says.
The Supreme Court isn’t expected to deliver a decision on the Affordable Care Act before the middle of next year. But the uncertainty will likely push back Biden’s proposals to expand on the law.
Overturning Obamacare would have huge impacts on millions of Americans:
- As many as 133 million Americans -- roughly half the U.S. population -- with preexisting conditions could find it harder, if not impossible, to find affordable health insurance. That figure does not include Americans infected with COVID-19.
- About 165 million who require expensive treatments -- for cancer and other conditions -- would no longer be protected from huge costs for care by federal caps on out-of-pocket expenditures the Affordable Care Act requires.
- An estimated 21 million who now buy insurance through the Obamacare Marketplaces could lose their coverage.
- Another 12 million on Medicaid could find themselves without insurance.
- At least 2 million young adults ages 26 and under, now on their parents’ health policies, could be kicked off.
- Millions of people who use Medicare could face higher costs.
- Federal subsidies for lower-income Americans to buy policies would disappear.
Throughout the campaign, Biden repeatedly stressed the need to preserve the law’s provision barring insurance companies from refusing coverage for Americans with preexisting conditions, such as diabetes, cancer, and heart disease. It also outlaws charging higher premiums on the basis of health status, age, or gender.
Biden has also pledged to bolster the law as president.
He has proposed a variety of add-ons to the Affordable Care Act he says will “insure more than an estimated 97% of Americans,” according to the Biden campaign site.
Biden’s proposals include offering larger federal subsidies to help low- and middle-income Americans pay for policies purchased through Obamacare insurance Marketplaces.
The boldest of Biden’s proposals is the creation of a “public option” for insurance -- a Medicare-like program that small businesses and individuals could choose if they do not have coverage, cannot afford it, or don’t like their employer-based coverage.
It would also automatically enroll millions of uninsured Americans living in the 14 states that have not expanded Medicaid, which covers low-income people.
But such a plan would require congressional approval -- including a “super majority” of 60 Senate votes to block a likely GOP filibuster. That will be a significant challenge Biden will have to overcome, with Congress so evenly divided.
The White House would also have to defeat heavy lobbying from some of the most influential industry interest groups in Washington, Corlette says.
“I’m not even confident they would get all the Democrat votes,” she says.
“So, it’s a going to be an uphill battle to get a public option passed.”
Taken together, Biden’s plans for expanding Obamacare are projected to cost $750 billion over 10 years. He has said much of that financing would come from increasing taxes on the wealthy.
That means it would likely require congressional approval, which Antos suggests is unlikely given the polarization on Capitol Hill.
Medicare, Medicaid, and drug costs
Biden has called for a host of reforms targeting Medicare, Medicaid, and rising drug costs.
On Medicare, which primarily covers seniors 65 and older, Biden has proposed lowering the eligibility age from 65 to 60. That could extend Medicare to up to 20 million more Americans.
On Medicaid, the health care safety net for low-income and disabled Americans, the president-elect supports increased federal funding to states during the current economic crisis, and potentially beyond.
Medicare is likely to become a key focus of the new administration, in light of the pressures the pandemic is placing on Medicare funding.
In April, Medicare’s trustees said that the Part A trust fund for the program, which pays for hospital and inpatient care, could start to run dry in 2026.
But those projections did not include the impact of COVID-19. Some economists have since projected that Medicare Part A could become insolvent as early as 2022.
Medicare Part B, which pays for doctor and outpatient costs, is funded by general tax funding and beneficiary insurance premiums, so it is not in danger of drying up.
Adding to those pressures is an executive order Trump signed in August temporarily deferring payroll taxes, a primary funding vehicle for Medicare and Social Security.
Under these taxes, employees pay 6.2% of their earnings (on annual income up to $137,700) toward Social Security and 1.45% for Medicare taxes each pay period. Employers pay the same rate per paycheck, adding up to a combined 12.4% Social Security tax and 2.9% Medicare tax.
Biden has said he would reverse the tax cut when he takes office.
But to get a handle on Medicare and Medicaid funding issues, he is likely to need congressional support. Corlette and other experts say that could be a challenge while the nation remains in the grip of the coronavirus pandemic.
In addition to his Medicare and Medicaid reforms, Biden has proposed several plans to lower drug prices, a subset of rising health care and insurance costs.
U.S. spending on prescription drugs has increased nearly 42% over the past decade -- from $253.1 billion in 2010 to $358.7 billion in 2020 (projected) -- according to the Centers for Medicare & Medicaid Services.
In 2020, retail prices for 460 commonly prescribed drugs have spiked an average of 5.2%, according to new analysis by 3 Axis Advisors, a health research firm.
That’s more than double the projected rate of inflation.
To control drug costs, Biden supports legislation approved by the Democratic-led House of Representatives last year that would empower Medicare to negotiate drug prices with drug companies, as private insurers do.
Federal law now bars Medicare from negotiating prices on behalf of the 67.7 million Americans who use it. Drug companies and many GOP leaders argue that the current law is necessary to allow them to spend more on research and development of new medications.
In addition, Biden supports the idea of lifting bans on importing drugs from foreign countries with lower costs.
He also backs creating an independent review board to set price caps for new medications with no competitors; making high-quality generics more available; ending tax breaks for drug company advertising; and limiting their leeway in raising prices.
All of these proposals would likely require congressional approval and could face legal challenges in the courts.
This article first appeared on WebMD.com.
The former vice president has sketched out a big health agenda: ramping up the federal response to COVID-19, boosting the Affordable Care Act, creating a new “public option” to cover uninsured Americans, and expanding Medicare and Medicaid.
But the president-elect’s long to-do list on health is likely to face significant roadblocks in Congress and the courts, experts say.
For instance, Biden’s ambitious proposals on COVID-19 -- including his recent call for a national mask mandate -- could be waylaid by legal challenges and run into political hurdles on Capitol Hill, where he may face a divided Congress.
Joseph Antos, PhD, a health policy expert with the conservative American Enterprise Institute, predicts Biden will encounter the same type of congressional “gridlock situation” that President Barack Obama ran into during his second term.
“We have a situation that has been like this for a very, very long time -- lack of cooperation, lack of recognition that either party is capable of rising above their own electoral views to deal with problems that the country actually has.”
Antos also suggests that Biden may also face enormous political pressure to address the economic fallout from the coronavirus, including record unemployment and business closures, before anything else.
“I think it’s really going to be efforts that are intended to promote economic development and promote the economy,” he says.
In addition, Biden’s plans to expand Obamacare might face a new challenge from the Supreme Court in the year ahead. This month, the high court will take up a new case seeking to overturn the law.
Even so, experts say Biden’s plans on COVID-19 and expanding health care are likely to define his tenure in the White House as a central focus of his presidency.
“Health care will be at the very top of the list of the president’s priorities,” says Sabrina Corlette, JD, co-director of the Center on Health Insurance Reforms at Georgetown University’s McCourt School of Public Policy. “I do think, however, that the administration is going to be very preoccupied with the response to COVID-19 and the economic fallout … particularly in the first year.”
Here’s a closer look at what we can expect from a Biden presidency.
COVID-19: Federalizing response efforts
Biden will move to federalize the response to COVID-19. He has said he will take back major responsibilities from the states -- such as setting national policies on mask wearing, social distancing, and the reopening of schools and businesses, based on CDC guidance. In the days leading up to the election, Biden called for a national mask mandate, after waffling on the issue throughout the summer.
He has said he will let public health science drive political policy. Biden is also planning to create his own task force to advise officials during the transition on managing the new surge in COVID-19 cases, vaccine safety and protecting at-risk populations, Politico reported this week. He received a virtual briefing on the pandemic from a panel of experts as he awaited the election’s outcome.
“I think we will no longer have this confused and contradictory public messaging,” Corlette says, “but I also think there will be humility and the recognition that the evidence is evolving -- that we don’t have all the answers, but we’re learning as we go.”
But national mandates on masks and social distancing will be challenging to enforce, experts say. They are also likely to face pushback from business interests, opposition from public officials in GOP-led states, and even legal challenges.
Biden’s ability to work with Congress -- or not -- may determine whether he is able to implement some of the key components of his coronavirus action plan, which includes:
- Providing free COVID-19 testing for all Americans
- Hiring 100,000 contact tracers
- Eliminating out-of-pocket expenses for coronavirus treatment
- Delivering “sufficient” PPE for essential workers
- Supporting science-backed vaccines and medical treatments being developed
- Requiring the reopening of businesses, workplaces, and schools only after “sufficient” reductions in community transmission -- under evidence-based protocols put forward by the CDC
- Giving emergency paid leave for workers dislocated by the pandemic and more financial aid for workers, families, and small businesses
- Shoring up safeguards to protect at-risk Americans, including older people
- Boosting pay for health care workers on the front lines
Biden has not detailed how he would pay for many of these, beyond promising to force wealthy Americans to “pay their fair share” of taxes to help. He has proposed a tax increase on Americans making more than $400,000 a year, which would require congressional approval.
Antos says he expects Biden’s proposed COVID-19 action plan to be virtually the same as Trump’s in two areas: efforts to develop a vaccine and antiviral treatments.
The administration has spent some $225 million on COVID-19 testing efforts, with a particular focus on rural areas.
Trump launched Operation Warp Speed to fast-track a vaccine. As part of that, the federal government has contracted with six drug companies, spending nearly $11 billion. The operation aims to provide at least 300 million doses of a coronavirus vaccine by January 2021.
Antos would like to see “a more sophisticated approach to social distancing” from the president-elect that takes into account the different challenges facing Americans depending on their income, work situation, and other factors during the pandemic.
“There are a lot of people in this country where working from home is fine and their jobs are secure,” he notes. “It’s the person who used to work at a restaurant that closed, it’s the line worker at a factory that has severely cut back its hours. It’s basically lower-middle-class people, low-income people, middle-class people, and it’s not the elite.
“And the policies have not given enough consideration to the fact that their circumstances and their tradeoffs would differ from the tradeoffs of somebody who doesn’t have anything to worry about economically.
“So, what we need is a more supple policy [that] will give people the information they need and give them the financial support that they also need … so they can make good decisions for themselves and their families. And we basically haven’t done that.”
Obamacare on the blocks?
The Supreme Court’s decision to take up another case seeking to overturn the Affordable Care Act could hand Biden’s health agenda a major setback -- and put the medical care for millions of Americans in jeopardy.
On Nov. 10, the high court will hear oral arguments on a lawsuit that would strike down all of Obamacare. A decision is not expected until next year.
The court has previously upheld the 2010 law, which Biden helped usher through Congress as vice president. But the addition of right-leaning Supreme Court Justice Amy Coney Barrett to the bench last month gives the court a clear conservative majority that could mean the end of Obamacare, legal experts say.
Republicans have opposed the law since its passage, but they have been unable to muster the votes to repeal it, or to pass an alternative
Antos, from the American Enterprise Institute, notes conservatives believe the law has increased costs for health care and insurance over the past decade, in part because of its protections for Americans with preexisting conditions and requiring insurers to provide comprehensive “gold-plated” policies.
“It’s driven up costs, offers plans that are not very strong, put high-risk folks into the same [insurance pool], which has increased costs for everyone, the employer mandate … these are all the reasons,” he says.
The Supreme Court isn’t expected to deliver a decision on the Affordable Care Act before the middle of next year. But the uncertainty will likely push back Biden’s proposals to expand on the law.
Overturning Obamacare would have huge impacts on millions of Americans:
- As many as 133 million Americans -- roughly half the U.S. population -- with preexisting conditions could find it harder, if not impossible, to find affordable health insurance. That figure does not include Americans infected with COVID-19.
- About 165 million who require expensive treatments -- for cancer and other conditions -- would no longer be protected from huge costs for care by federal caps on out-of-pocket expenditures the Affordable Care Act requires.
- An estimated 21 million who now buy insurance through the Obamacare Marketplaces could lose their coverage.
- Another 12 million on Medicaid could find themselves without insurance.
- At least 2 million young adults ages 26 and under, now on their parents’ health policies, could be kicked off.
- Millions of people who use Medicare could face higher costs.
- Federal subsidies for lower-income Americans to buy policies would disappear.
Throughout the campaign, Biden repeatedly stressed the need to preserve the law’s provision barring insurance companies from refusing coverage for Americans with preexisting conditions, such as diabetes, cancer, and heart disease. It also outlaws charging higher premiums on the basis of health status, age, or gender.
Biden has also pledged to bolster the law as president.
He has proposed a variety of add-ons to the Affordable Care Act he says will “insure more than an estimated 97% of Americans,” according to the Biden campaign site.
Biden’s proposals include offering larger federal subsidies to help low- and middle-income Americans pay for policies purchased through Obamacare insurance Marketplaces.
The boldest of Biden’s proposals is the creation of a “public option” for insurance -- a Medicare-like program that small businesses and individuals could choose if they do not have coverage, cannot afford it, or don’t like their employer-based coverage.
It would also automatically enroll millions of uninsured Americans living in the 14 states that have not expanded Medicaid, which covers low-income people.
But such a plan would require congressional approval -- including a “super majority” of 60 Senate votes to block a likely GOP filibuster. That will be a significant challenge Biden will have to overcome, with Congress so evenly divided.
The White House would also have to defeat heavy lobbying from some of the most influential industry interest groups in Washington, Corlette says.
“I’m not even confident they would get all the Democrat votes,” she says.
“So, it’s a going to be an uphill battle to get a public option passed.”
Taken together, Biden’s plans for expanding Obamacare are projected to cost $750 billion over 10 years. He has said much of that financing would come from increasing taxes on the wealthy.
That means it would likely require congressional approval, which Antos suggests is unlikely given the polarization on Capitol Hill.
Medicare, Medicaid, and drug costs
Biden has called for a host of reforms targeting Medicare, Medicaid, and rising drug costs.
On Medicare, which primarily covers seniors 65 and older, Biden has proposed lowering the eligibility age from 65 to 60. That could extend Medicare to up to 20 million more Americans.
On Medicaid, the health care safety net for low-income and disabled Americans, the president-elect supports increased federal funding to states during the current economic crisis, and potentially beyond.
Medicare is likely to become a key focus of the new administration, in light of the pressures the pandemic is placing on Medicare funding.
In April, Medicare’s trustees said that the Part A trust fund for the program, which pays for hospital and inpatient care, could start to run dry in 2026.
But those projections did not include the impact of COVID-19. Some economists have since projected that Medicare Part A could become insolvent as early as 2022.
Medicare Part B, which pays for doctor and outpatient costs, is funded by general tax funding and beneficiary insurance premiums, so it is not in danger of drying up.
Adding to those pressures is an executive order Trump signed in August temporarily deferring payroll taxes, a primary funding vehicle for Medicare and Social Security.
Under these taxes, employees pay 6.2% of their earnings (on annual income up to $137,700) toward Social Security and 1.45% for Medicare taxes each pay period. Employers pay the same rate per paycheck, adding up to a combined 12.4% Social Security tax and 2.9% Medicare tax.
Biden has said he would reverse the tax cut when he takes office.
But to get a handle on Medicare and Medicaid funding issues, he is likely to need congressional support. Corlette and other experts say that could be a challenge while the nation remains in the grip of the coronavirus pandemic.
In addition to his Medicare and Medicaid reforms, Biden has proposed several plans to lower drug prices, a subset of rising health care and insurance costs.
U.S. spending on prescription drugs has increased nearly 42% over the past decade -- from $253.1 billion in 2010 to $358.7 billion in 2020 (projected) -- according to the Centers for Medicare & Medicaid Services.
In 2020, retail prices for 460 commonly prescribed drugs have spiked an average of 5.2%, according to new analysis by 3 Axis Advisors, a health research firm.
That’s more than double the projected rate of inflation.
To control drug costs, Biden supports legislation approved by the Democratic-led House of Representatives last year that would empower Medicare to negotiate drug prices with drug companies, as private insurers do.
Federal law now bars Medicare from negotiating prices on behalf of the 67.7 million Americans who use it. Drug companies and many GOP leaders argue that the current law is necessary to allow them to spend more on research and development of new medications.
In addition, Biden supports the idea of lifting bans on importing drugs from foreign countries with lower costs.
He also backs creating an independent review board to set price caps for new medications with no competitors; making high-quality generics more available; ending tax breaks for drug company advertising; and limiting their leeway in raising prices.
All of these proposals would likely require congressional approval and could face legal challenges in the courts.
This article first appeared on WebMD.com.




