User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


COVID redefines curriculum for hospitalists-in-training
Pandemic brings ‘clarity and urgency’
The coronavirus pandemic has impacted all facets of the education and training of this country’s future hospitalists, including their medical school coursework, elective rotations, clerkships, and residency training – although with variations between settings and localities.
The COVID-19 crisis demanded immediate changes in traditional approaches to medical education. Training programs responded quickly to institute those changes. As hospitals geared up for potential surges in COVID cases starting in mid-March, many onsite training activities for medical students were shut down in order to reserve personal protective equipment for essential personnel and not put learners at risk of catching the virus. A variety of events related to their education were canceled. Didactic presentations and meetings were converted to virtual gatherings on internet platforms such as Zoom. Many of these changes were adopted even in settings with few actual COVID cases.
Medical students on clinical rotations were provided with virtual didactics when in-person clinical experiences were put on hold. In some cases, academic years ended early and fourth-year students graduated early so they might potentially join the hospital work force. Residents’ assignments were also changed, perhaps seeing patients on non–COVID-19 units only or taking different shifts, assignments, or rotations. Public health or research projects replaced elective placements. New electives were created, along with journal clubs, online care conferences, and technology-facilitated, self-directed learning.
But every advancing medical student needs to rotate through an experience of taking care of real patients, said Amy Guiot, MD, MEd, a hospitalist and associate director of medical student education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. “The Liaison Committee of Medical Education, jointly sponsored by the Association of American Medical Colleges and the American Medical Association, will not let you graduate a medical student without actual hands-on encounters with patients,” she explained.
For future doctors, especially those pursuing internal medicine – many of whom will practice as hospitalists – their training can’t duplicate “in the hospital” experiences except in the hospital, said Dr. Guiot, who is involved in pediatric training for medical students from the University of Cincinnati and residents.
For third- and fourth-year medical students, getting that personal contact with patients has been the hardest part, she added. But from March to May 2020, that experience was completely shut down at CCHMC, as at many medical schools, because of precautions aimed at preventing exposure to the novel coronavirus for both students and patients. That meant hospitals had to get creative, reshuffling schedules and the order of learning experiences; converting everything possible to virtual encounters on platforms such as Zoom; and reducing the length of rotations, the total number of in-person encounters, and the number of learners participating in an activity.
“We needed to use shift work for medical students, which hadn’t been done before,” Dr. Guiot said. Having students on different shifts, including nights, created more opportunities to fit clinical experiences into the schedule. The use of standardized patients – actors following a script who are examined by a student as part of learning how to do a physical exam – was also put on hold.
“Now we’re starting to get it back, but maybe not as often,” she said. “The actor wears a mask. The student wears a mask and shield. But it’s been harder for us to find actors – who tend to be older adults who may fear coming to the medical center – to perform their role, teaching medical students the art of examining a patient.”
Back to basics
The COVID-19 pandemic forced medical schools to get back to basics, figuring out the key competencies students needed to learn, said Alison Whelan, MD, AAMC’s chief medical education officer. Both medical schools and residency programs needed to respond quickly and in new ways, including with course content that would teach students about the virus and its management and treatment.
Schools have faced crises before, responding in real time to SARS (severe acute respiratory syndrome), Ebola, HIV, and natural disasters, Dr. Whelan said. “But there was a nimbleness and rapidity of adapting to COVID – with a lot of sharing of curriculums among medical colleges.” Back in late March, AAMC put out guidelines that recommended removing students from direct patient contact – not just for the student’s protection but for the community’s. A subsequent guidance, released Aug. 14, emphasized the need for medical schools to continue medical education – with appropriate attention to safety and local conditions while working closely with clinical partners.
Dr. Guiot, with her colleague Leslie Farrell, MD, and four very creative medical students, developed an online fourth-year elective course for University of Cincinnati medical students, offered asynchronously. It aimed to transmit a comprehensive understanding of COVID-19, its virology, transmission, clinical prevention, diagnosis and treatment, as well as examining national and international responses to the pandemic and their consequences and related issues of race, ethnicity, socioeconomic status, and health disparities. “We used several articles from the Journal of Hospital Medicine for students to read and discuss,” Dr. Guiot said.
Christopher Sankey, MD, SFHM, associate program director of the traditional internal medicine residency program and associate professor of medicine at Yale University, New Haven, Conn., oversees the inpatient educational experience for internal medicine residents at Yale. “As with most programs, there was a lot of trepidation as we made the transition from in-person to virtual education,” he said.
The two principal, non–ward-based educational opportunities for the Yale residents are morning report, which involves a case-based discussion of various medical issues, usually led by a chief resident, and noon conference, which is more didactic and content based. Both made the transition to virtual meetings for residents.
“We wondered, could these still be well-attended, well-liked, and successful learning experiences if offered virtually? What I found when I surveyed our residents was that the virtual conferences were not only well received, but actually preferred,” Dr. Sankey said. “We have a large campus with lots of internal medicine services, so it’s hard to assemble everyone for meetings. There were also situations in which there were so many residents that they couldn’t all fit into the same room.” Zoom, the virtual platform of choice, has actually increased attendance.
Marc Miller, MD, a pediatric hospitalist at the Cleveland Clinic, helped his team develop a virtual curriculum in pediatrics presented to third-year medical students during the month of May, when medical students were being taken off the wards. “Some third-year students still needed to get their pediatric clerkships done. We had to balance clinical exposure with a lot of other things,” he explained.
The curriculum included a focus on interprofessional aspects of interdisciplinary, family-centered bedside rounds; a COVID literature review; and a lot of case-based scenarios. “Most challenging was how to remake family rounds. We tried to incorporate students into table rounds, but that didn’t feel as valuable,” Dr. Miller said. “Because pediatrics is so family centered, talking to patients and families at the bedside is highly valued. So we had virtual sessions talking about how to do that, with videos to illustrate it put out by Cincinnati Children’s Hospital.”
The most interactive sessions got the best feedback, but all the sessions went over very well, Dr. Miller said. “Larger lessons from COVID include things we already knew, but now with extra importance, such as the need to encourage interactivity to get students to buy in and take part in these conversations – whatever the structure.”
Vineet Arora, MD, MHM, an academic hospitalist and chief medical officer for the clinical learning environment at the University of Chicago, said that the changes wrought by COVID have also produced unexpected gains for medical education. “We’ve also had to think differently and more creatively about how to get the same information across in this new environment,” she explained. “In some cases, we saw that it was easier for learners to attend conferences and meetings online, with increased attendance for our events.” That includes participation on quality improvement committees, and attending online medical conferences presented locally and regionally.
“Another question: How do we teach interdisciplinary rounds and how to work with other members of the team without having face-to-face interactions?” Dr. Arora said. “Our old interdisciplinary rounding model had to change. It forced us to rethink how to create that kind of learning. We can’t have as many people in the patient’s room at one time. Can there be a physically distanced ‘touch-base’ with the nurse outside the patient’s room after a doctor has gone in to meet the patient?”
Transformational change
In a recent JAMA Viewpoint column, Catherine R. Lucey, MD, and S. Claiborne Johnston, MD, PhD,1 called the impact of COVID-19 “transformational,” in line with changes in medical curriculums recommended by the 2010 Global Independent Commission on Education of Health Professionals for the 21st Century,2 which asserted that the purpose of professional education is to improve the health of communities.
The authors stated that COVID-19 brought clarity and urgency to this purpose, and will someday be viewed as a catalyst for the needed transformation of medical education as medical schools embarked on curriculum redesign to embrace new competencies for current health challenges.
They suggested that medical students not only continued to learn during the COVID crisis “but in many circumstances, accelerated their attainment of the types of competencies that 21st century physicians must master.” Emerging competencies identified by Dr. Lucey and Dr. Johnston include:
- Being able to address population and public health issues
- Designing and continuously improving of the health care system
- Incorporating data and technology in service to patient care, research, and education
- Eliminating health care disparities and discrimination in medicine
- Adapting the curriculum to current issues in real-time
- Engaging in crisis communication and active change leadership
How is the curriculum changing? It’s still a work in progress. “After the disruptions of the spring and summer, schools are now trying to figure which of the changes should stay,” said Dr. Whelan. “The virus has also highlighted other crises, with social determinants of health and racial disparities becoming more front and center. In terms of content, medical educators are rethinking a lot of things – in a good way.”
Another important trend cast in sharper relief by the pandemic is a gradual evolution toward competency-based education and how to assess when someone is ready to be a doctor, Dr. Whelan said. “There’s been an accelerated consideration of how to be sure each student is competent to practice medicine.”3
Many practicing physicians and students were redeployed in the crisis, she said. Pediatric physicians were asked to take care of adult patients, and internists were drafted to work in the ICU. Hospitals quickly developed refresher courses and competency-based assessments to facilitate these redeployments. What can be learned from such on-the-fly assessments? What was needed to make a pediatrician, under the supervision of an internist, able to take good care of adult patients?
And does competency-based assessment point toward some kind of time-variable graduate medical education of the future – with graduation when the competencies are achieved, rather than just tethered to time- and case volume–based requirements? It seems Canada is moving in this direction, and COVID might catalyze a similar transformation in the United States.3
Changing the curriculum
Does the content of the curriculum for preparing future hospitalists need to change significantly? “My honest answer is yes and no,” Dr. Sankey said. “One thing we found in our training program is that it’s possible to become consumed by this pandemic. We need to educate residents about it, but future doctors still need to learn a lot of other things. Heart failure has not gone away.
“It’s okay to stick to the general curriculum, but with a wider variety of learning opportunities. Adding content sessions on population health, social determinants of health, race and bias, and equity is a start, but it’s by no means sufficient to give these topics the importance they deserve. We need to interpolate these subjects into sessions we’re already doing,” he said. “It is not enough to do a couple of lectures on diversity. We need to weave these concepts into the education we provide for residents every day.
“I think the pandemic has posed an opportunity to critically consider what’s the ideal teaching and learning environment. How can we make it better? Societal events around race have demonstrated essential areas for curricular development, and the pandemic had us primed and already thinking about how we educate future doctors – both in terms of medium and content,” he said.
Some medical schools started their new academic year in July; others put it off until September. Patient care at CCHMC is nearly back to where it used to be before COVID-19 began, Dr. Guiot said in a September interview, “but in masks and goggles.” As a result, hospitals are having to get creative all over again to accommodate medical students.
“I am amazed at the camaraderie of hospitals and medical schools, trying to support our learners in the midst of the pandemic,” she said. “I learned that we can be more adaptive than I ever imagined. We were all nervous about the risks, but we learned how to support each other and still provide excellent care in the midst of the pandemic. We’re forever changed. We also learned how to present didactics on Zoom, but that was the easy part.”
References
1. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-4.
2. Bhutta ZA et al. Education of health professionals for the 21st century: A global independent Commission. Lancet. 2010 Apr 3;375(9721):1137-8.
3. Goldhamer MEJ et al. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383:1003-5.
Pandemic brings ‘clarity and urgency’
Pandemic brings ‘clarity and urgency’
The coronavirus pandemic has impacted all facets of the education and training of this country’s future hospitalists, including their medical school coursework, elective rotations, clerkships, and residency training – although with variations between settings and localities.
The COVID-19 crisis demanded immediate changes in traditional approaches to medical education. Training programs responded quickly to institute those changes. As hospitals geared up for potential surges in COVID cases starting in mid-March, many onsite training activities for medical students were shut down in order to reserve personal protective equipment for essential personnel and not put learners at risk of catching the virus. A variety of events related to their education were canceled. Didactic presentations and meetings were converted to virtual gatherings on internet platforms such as Zoom. Many of these changes were adopted even in settings with few actual COVID cases.
Medical students on clinical rotations were provided with virtual didactics when in-person clinical experiences were put on hold. In some cases, academic years ended early and fourth-year students graduated early so they might potentially join the hospital work force. Residents’ assignments were also changed, perhaps seeing patients on non–COVID-19 units only or taking different shifts, assignments, or rotations. Public health or research projects replaced elective placements. New electives were created, along with journal clubs, online care conferences, and technology-facilitated, self-directed learning.
But every advancing medical student needs to rotate through an experience of taking care of real patients, said Amy Guiot, MD, MEd, a hospitalist and associate director of medical student education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. “The Liaison Committee of Medical Education, jointly sponsored by the Association of American Medical Colleges and the American Medical Association, will not let you graduate a medical student without actual hands-on encounters with patients,” she explained.
For future doctors, especially those pursuing internal medicine – many of whom will practice as hospitalists – their training can’t duplicate “in the hospital” experiences except in the hospital, said Dr. Guiot, who is involved in pediatric training for medical students from the University of Cincinnati and residents.
For third- and fourth-year medical students, getting that personal contact with patients has been the hardest part, she added. But from March to May 2020, that experience was completely shut down at CCHMC, as at many medical schools, because of precautions aimed at preventing exposure to the novel coronavirus for both students and patients. That meant hospitals had to get creative, reshuffling schedules and the order of learning experiences; converting everything possible to virtual encounters on platforms such as Zoom; and reducing the length of rotations, the total number of in-person encounters, and the number of learners participating in an activity.
“We needed to use shift work for medical students, which hadn’t been done before,” Dr. Guiot said. Having students on different shifts, including nights, created more opportunities to fit clinical experiences into the schedule. The use of standardized patients – actors following a script who are examined by a student as part of learning how to do a physical exam – was also put on hold.
“Now we’re starting to get it back, but maybe not as often,” she said. “The actor wears a mask. The student wears a mask and shield. But it’s been harder for us to find actors – who tend to be older adults who may fear coming to the medical center – to perform their role, teaching medical students the art of examining a patient.”
Back to basics
The COVID-19 pandemic forced medical schools to get back to basics, figuring out the key competencies students needed to learn, said Alison Whelan, MD, AAMC’s chief medical education officer. Both medical schools and residency programs needed to respond quickly and in new ways, including with course content that would teach students about the virus and its management and treatment.
Schools have faced crises before, responding in real time to SARS (severe acute respiratory syndrome), Ebola, HIV, and natural disasters, Dr. Whelan said. “But there was a nimbleness and rapidity of adapting to COVID – with a lot of sharing of curriculums among medical colleges.” Back in late March, AAMC put out guidelines that recommended removing students from direct patient contact – not just for the student’s protection but for the community’s. A subsequent guidance, released Aug. 14, emphasized the need for medical schools to continue medical education – with appropriate attention to safety and local conditions while working closely with clinical partners.
Dr. Guiot, with her colleague Leslie Farrell, MD, and four very creative medical students, developed an online fourth-year elective course for University of Cincinnati medical students, offered asynchronously. It aimed to transmit a comprehensive understanding of COVID-19, its virology, transmission, clinical prevention, diagnosis and treatment, as well as examining national and international responses to the pandemic and their consequences and related issues of race, ethnicity, socioeconomic status, and health disparities. “We used several articles from the Journal of Hospital Medicine for students to read and discuss,” Dr. Guiot said.
Christopher Sankey, MD, SFHM, associate program director of the traditional internal medicine residency program and associate professor of medicine at Yale University, New Haven, Conn., oversees the inpatient educational experience for internal medicine residents at Yale. “As with most programs, there was a lot of trepidation as we made the transition from in-person to virtual education,” he said.
The two principal, non–ward-based educational opportunities for the Yale residents are morning report, which involves a case-based discussion of various medical issues, usually led by a chief resident, and noon conference, which is more didactic and content based. Both made the transition to virtual meetings for residents.
“We wondered, could these still be well-attended, well-liked, and successful learning experiences if offered virtually? What I found when I surveyed our residents was that the virtual conferences were not only well received, but actually preferred,” Dr. Sankey said. “We have a large campus with lots of internal medicine services, so it’s hard to assemble everyone for meetings. There were also situations in which there were so many residents that they couldn’t all fit into the same room.” Zoom, the virtual platform of choice, has actually increased attendance.
Marc Miller, MD, a pediatric hospitalist at the Cleveland Clinic, helped his team develop a virtual curriculum in pediatrics presented to third-year medical students during the month of May, when medical students were being taken off the wards. “Some third-year students still needed to get their pediatric clerkships done. We had to balance clinical exposure with a lot of other things,” he explained.
The curriculum included a focus on interprofessional aspects of interdisciplinary, family-centered bedside rounds; a COVID literature review; and a lot of case-based scenarios. “Most challenging was how to remake family rounds. We tried to incorporate students into table rounds, but that didn’t feel as valuable,” Dr. Miller said. “Because pediatrics is so family centered, talking to patients and families at the bedside is highly valued. So we had virtual sessions talking about how to do that, with videos to illustrate it put out by Cincinnati Children’s Hospital.”
The most interactive sessions got the best feedback, but all the sessions went over very well, Dr. Miller said. “Larger lessons from COVID include things we already knew, but now with extra importance, such as the need to encourage interactivity to get students to buy in and take part in these conversations – whatever the structure.”
Vineet Arora, MD, MHM, an academic hospitalist and chief medical officer for the clinical learning environment at the University of Chicago, said that the changes wrought by COVID have also produced unexpected gains for medical education. “We’ve also had to think differently and more creatively about how to get the same information across in this new environment,” she explained. “In some cases, we saw that it was easier for learners to attend conferences and meetings online, with increased attendance for our events.” That includes participation on quality improvement committees, and attending online medical conferences presented locally and regionally.
“Another question: How do we teach interdisciplinary rounds and how to work with other members of the team without having face-to-face interactions?” Dr. Arora said. “Our old interdisciplinary rounding model had to change. It forced us to rethink how to create that kind of learning. We can’t have as many people in the patient’s room at one time. Can there be a physically distanced ‘touch-base’ with the nurse outside the patient’s room after a doctor has gone in to meet the patient?”
Transformational change
In a recent JAMA Viewpoint column, Catherine R. Lucey, MD, and S. Claiborne Johnston, MD, PhD,1 called the impact of COVID-19 “transformational,” in line with changes in medical curriculums recommended by the 2010 Global Independent Commission on Education of Health Professionals for the 21st Century,2 which asserted that the purpose of professional education is to improve the health of communities.
The authors stated that COVID-19 brought clarity and urgency to this purpose, and will someday be viewed as a catalyst for the needed transformation of medical education as medical schools embarked on curriculum redesign to embrace new competencies for current health challenges.
They suggested that medical students not only continued to learn during the COVID crisis “but in many circumstances, accelerated their attainment of the types of competencies that 21st century physicians must master.” Emerging competencies identified by Dr. Lucey and Dr. Johnston include:
- Being able to address population and public health issues
- Designing and continuously improving of the health care system
- Incorporating data and technology in service to patient care, research, and education
- Eliminating health care disparities and discrimination in medicine
- Adapting the curriculum to current issues in real-time
- Engaging in crisis communication and active change leadership
How is the curriculum changing? It’s still a work in progress. “After the disruptions of the spring and summer, schools are now trying to figure which of the changes should stay,” said Dr. Whelan. “The virus has also highlighted other crises, with social determinants of health and racial disparities becoming more front and center. In terms of content, medical educators are rethinking a lot of things – in a good way.”
Another important trend cast in sharper relief by the pandemic is a gradual evolution toward competency-based education and how to assess when someone is ready to be a doctor, Dr. Whelan said. “There’s been an accelerated consideration of how to be sure each student is competent to practice medicine.”3
Many practicing physicians and students were redeployed in the crisis, she said. Pediatric physicians were asked to take care of adult patients, and internists were drafted to work in the ICU. Hospitals quickly developed refresher courses and competency-based assessments to facilitate these redeployments. What can be learned from such on-the-fly assessments? What was needed to make a pediatrician, under the supervision of an internist, able to take good care of adult patients?
And does competency-based assessment point toward some kind of time-variable graduate medical education of the future – with graduation when the competencies are achieved, rather than just tethered to time- and case volume–based requirements? It seems Canada is moving in this direction, and COVID might catalyze a similar transformation in the United States.3
Changing the curriculum
Does the content of the curriculum for preparing future hospitalists need to change significantly? “My honest answer is yes and no,” Dr. Sankey said. “One thing we found in our training program is that it’s possible to become consumed by this pandemic. We need to educate residents about it, but future doctors still need to learn a lot of other things. Heart failure has not gone away.
“It’s okay to stick to the general curriculum, but with a wider variety of learning opportunities. Adding content sessions on population health, social determinants of health, race and bias, and equity is a start, but it’s by no means sufficient to give these topics the importance they deserve. We need to interpolate these subjects into sessions we’re already doing,” he said. “It is not enough to do a couple of lectures on diversity. We need to weave these concepts into the education we provide for residents every day.
“I think the pandemic has posed an opportunity to critically consider what’s the ideal teaching and learning environment. How can we make it better? Societal events around race have demonstrated essential areas for curricular development, and the pandemic had us primed and already thinking about how we educate future doctors – both in terms of medium and content,” he said.
Some medical schools started their new academic year in July; others put it off until September. Patient care at CCHMC is nearly back to where it used to be before COVID-19 began, Dr. Guiot said in a September interview, “but in masks and goggles.” As a result, hospitals are having to get creative all over again to accommodate medical students.
“I am amazed at the camaraderie of hospitals and medical schools, trying to support our learners in the midst of the pandemic,” she said. “I learned that we can be more adaptive than I ever imagined. We were all nervous about the risks, but we learned how to support each other and still provide excellent care in the midst of the pandemic. We’re forever changed. We also learned how to present didactics on Zoom, but that was the easy part.”
References
1. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-4.
2. Bhutta ZA et al. Education of health professionals for the 21st century: A global independent Commission. Lancet. 2010 Apr 3;375(9721):1137-8.
3. Goldhamer MEJ et al. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383:1003-5.
The coronavirus pandemic has impacted all facets of the education and training of this country’s future hospitalists, including their medical school coursework, elective rotations, clerkships, and residency training – although with variations between settings and localities.
The COVID-19 crisis demanded immediate changes in traditional approaches to medical education. Training programs responded quickly to institute those changes. As hospitals geared up for potential surges in COVID cases starting in mid-March, many onsite training activities for medical students were shut down in order to reserve personal protective equipment for essential personnel and not put learners at risk of catching the virus. A variety of events related to their education were canceled. Didactic presentations and meetings were converted to virtual gatherings on internet platforms such as Zoom. Many of these changes were adopted even in settings with few actual COVID cases.
Medical students on clinical rotations were provided with virtual didactics when in-person clinical experiences were put on hold. In some cases, academic years ended early and fourth-year students graduated early so they might potentially join the hospital work force. Residents’ assignments were also changed, perhaps seeing patients on non–COVID-19 units only or taking different shifts, assignments, or rotations. Public health or research projects replaced elective placements. New electives were created, along with journal clubs, online care conferences, and technology-facilitated, self-directed learning.
But every advancing medical student needs to rotate through an experience of taking care of real patients, said Amy Guiot, MD, MEd, a hospitalist and associate director of medical student education in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. “The Liaison Committee of Medical Education, jointly sponsored by the Association of American Medical Colleges and the American Medical Association, will not let you graduate a medical student without actual hands-on encounters with patients,” she explained.
For future doctors, especially those pursuing internal medicine – many of whom will practice as hospitalists – their training can’t duplicate “in the hospital” experiences except in the hospital, said Dr. Guiot, who is involved in pediatric training for medical students from the University of Cincinnati and residents.
For third- and fourth-year medical students, getting that personal contact with patients has been the hardest part, she added. But from March to May 2020, that experience was completely shut down at CCHMC, as at many medical schools, because of precautions aimed at preventing exposure to the novel coronavirus for both students and patients. That meant hospitals had to get creative, reshuffling schedules and the order of learning experiences; converting everything possible to virtual encounters on platforms such as Zoom; and reducing the length of rotations, the total number of in-person encounters, and the number of learners participating in an activity.
“We needed to use shift work for medical students, which hadn’t been done before,” Dr. Guiot said. Having students on different shifts, including nights, created more opportunities to fit clinical experiences into the schedule. The use of standardized patients – actors following a script who are examined by a student as part of learning how to do a physical exam – was also put on hold.
“Now we’re starting to get it back, but maybe not as often,” she said. “The actor wears a mask. The student wears a mask and shield. But it’s been harder for us to find actors – who tend to be older adults who may fear coming to the medical center – to perform their role, teaching medical students the art of examining a patient.”
Back to basics
The COVID-19 pandemic forced medical schools to get back to basics, figuring out the key competencies students needed to learn, said Alison Whelan, MD, AAMC’s chief medical education officer. Both medical schools and residency programs needed to respond quickly and in new ways, including with course content that would teach students about the virus and its management and treatment.
Schools have faced crises before, responding in real time to SARS (severe acute respiratory syndrome), Ebola, HIV, and natural disasters, Dr. Whelan said. “But there was a nimbleness and rapidity of adapting to COVID – with a lot of sharing of curriculums among medical colleges.” Back in late March, AAMC put out guidelines that recommended removing students from direct patient contact – not just for the student’s protection but for the community’s. A subsequent guidance, released Aug. 14, emphasized the need for medical schools to continue medical education – with appropriate attention to safety and local conditions while working closely with clinical partners.
Dr. Guiot, with her colleague Leslie Farrell, MD, and four very creative medical students, developed an online fourth-year elective course for University of Cincinnati medical students, offered asynchronously. It aimed to transmit a comprehensive understanding of COVID-19, its virology, transmission, clinical prevention, diagnosis and treatment, as well as examining national and international responses to the pandemic and their consequences and related issues of race, ethnicity, socioeconomic status, and health disparities. “We used several articles from the Journal of Hospital Medicine for students to read and discuss,” Dr. Guiot said.
Christopher Sankey, MD, SFHM, associate program director of the traditional internal medicine residency program and associate professor of medicine at Yale University, New Haven, Conn., oversees the inpatient educational experience for internal medicine residents at Yale. “As with most programs, there was a lot of trepidation as we made the transition from in-person to virtual education,” he said.
The two principal, non–ward-based educational opportunities for the Yale residents are morning report, which involves a case-based discussion of various medical issues, usually led by a chief resident, and noon conference, which is more didactic and content based. Both made the transition to virtual meetings for residents.
“We wondered, could these still be well-attended, well-liked, and successful learning experiences if offered virtually? What I found when I surveyed our residents was that the virtual conferences were not only well received, but actually preferred,” Dr. Sankey said. “We have a large campus with lots of internal medicine services, so it’s hard to assemble everyone for meetings. There were also situations in which there were so many residents that they couldn’t all fit into the same room.” Zoom, the virtual platform of choice, has actually increased attendance.
Marc Miller, MD, a pediatric hospitalist at the Cleveland Clinic, helped his team develop a virtual curriculum in pediatrics presented to third-year medical students during the month of May, when medical students were being taken off the wards. “Some third-year students still needed to get their pediatric clerkships done. We had to balance clinical exposure with a lot of other things,” he explained.
The curriculum included a focus on interprofessional aspects of interdisciplinary, family-centered bedside rounds; a COVID literature review; and a lot of case-based scenarios. “Most challenging was how to remake family rounds. We tried to incorporate students into table rounds, but that didn’t feel as valuable,” Dr. Miller said. “Because pediatrics is so family centered, talking to patients and families at the bedside is highly valued. So we had virtual sessions talking about how to do that, with videos to illustrate it put out by Cincinnati Children’s Hospital.”
The most interactive sessions got the best feedback, but all the sessions went over very well, Dr. Miller said. “Larger lessons from COVID include things we already knew, but now with extra importance, such as the need to encourage interactivity to get students to buy in and take part in these conversations – whatever the structure.”
Vineet Arora, MD, MHM, an academic hospitalist and chief medical officer for the clinical learning environment at the University of Chicago, said that the changes wrought by COVID have also produced unexpected gains for medical education. “We’ve also had to think differently and more creatively about how to get the same information across in this new environment,” she explained. “In some cases, we saw that it was easier for learners to attend conferences and meetings online, with increased attendance for our events.” That includes participation on quality improvement committees, and attending online medical conferences presented locally and regionally.
“Another question: How do we teach interdisciplinary rounds and how to work with other members of the team without having face-to-face interactions?” Dr. Arora said. “Our old interdisciplinary rounding model had to change. It forced us to rethink how to create that kind of learning. We can’t have as many people in the patient’s room at one time. Can there be a physically distanced ‘touch-base’ with the nurse outside the patient’s room after a doctor has gone in to meet the patient?”
Transformational change
In a recent JAMA Viewpoint column, Catherine R. Lucey, MD, and S. Claiborne Johnston, MD, PhD,1 called the impact of COVID-19 “transformational,” in line with changes in medical curriculums recommended by the 2010 Global Independent Commission on Education of Health Professionals for the 21st Century,2 which asserted that the purpose of professional education is to improve the health of communities.
The authors stated that COVID-19 brought clarity and urgency to this purpose, and will someday be viewed as a catalyst for the needed transformation of medical education as medical schools embarked on curriculum redesign to embrace new competencies for current health challenges.
They suggested that medical students not only continued to learn during the COVID crisis “but in many circumstances, accelerated their attainment of the types of competencies that 21st century physicians must master.” Emerging competencies identified by Dr. Lucey and Dr. Johnston include:
- Being able to address population and public health issues
- Designing and continuously improving of the health care system
- Incorporating data and technology in service to patient care, research, and education
- Eliminating health care disparities and discrimination in medicine
- Adapting the curriculum to current issues in real-time
- Engaging in crisis communication and active change leadership
How is the curriculum changing? It’s still a work in progress. “After the disruptions of the spring and summer, schools are now trying to figure which of the changes should stay,” said Dr. Whelan. “The virus has also highlighted other crises, with social determinants of health and racial disparities becoming more front and center. In terms of content, medical educators are rethinking a lot of things – in a good way.”
Another important trend cast in sharper relief by the pandemic is a gradual evolution toward competency-based education and how to assess when someone is ready to be a doctor, Dr. Whelan said. “There’s been an accelerated consideration of how to be sure each student is competent to practice medicine.”3
Many practicing physicians and students were redeployed in the crisis, she said. Pediatric physicians were asked to take care of adult patients, and internists were drafted to work in the ICU. Hospitals quickly developed refresher courses and competency-based assessments to facilitate these redeployments. What can be learned from such on-the-fly assessments? What was needed to make a pediatrician, under the supervision of an internist, able to take good care of adult patients?
And does competency-based assessment point toward some kind of time-variable graduate medical education of the future – with graduation when the competencies are achieved, rather than just tethered to time- and case volume–based requirements? It seems Canada is moving in this direction, and COVID might catalyze a similar transformation in the United States.3
Changing the curriculum
Does the content of the curriculum for preparing future hospitalists need to change significantly? “My honest answer is yes and no,” Dr. Sankey said. “One thing we found in our training program is that it’s possible to become consumed by this pandemic. We need to educate residents about it, but future doctors still need to learn a lot of other things. Heart failure has not gone away.
“It’s okay to stick to the general curriculum, but with a wider variety of learning opportunities. Adding content sessions on population health, social determinants of health, race and bias, and equity is a start, but it’s by no means sufficient to give these topics the importance they deserve. We need to interpolate these subjects into sessions we’re already doing,” he said. “It is not enough to do a couple of lectures on diversity. We need to weave these concepts into the education we provide for residents every day.
“I think the pandemic has posed an opportunity to critically consider what’s the ideal teaching and learning environment. How can we make it better? Societal events around race have demonstrated essential areas for curricular development, and the pandemic had us primed and already thinking about how we educate future doctors – both in terms of medium and content,” he said.
Some medical schools started their new academic year in July; others put it off until September. Patient care at CCHMC is nearly back to where it used to be before COVID-19 began, Dr. Guiot said in a September interview, “but in masks and goggles.” As a result, hospitals are having to get creative all over again to accommodate medical students.
“I am amazed at the camaraderie of hospitals and medical schools, trying to support our learners in the midst of the pandemic,” she said. “I learned that we can be more adaptive than I ever imagined. We were all nervous about the risks, but we learned how to support each other and still provide excellent care in the midst of the pandemic. We’re forever changed. We also learned how to present didactics on Zoom, but that was the easy part.”
References
1. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-4.
2. Bhutta ZA et al. Education of health professionals for the 21st century: A global independent Commission. Lancet. 2010 Apr 3;375(9721):1137-8.
3. Goldhamer MEJ et al. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383:1003-5.
Medicare finalizes 2021 physician pay rule with E/M changes
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Intensive glucose control after acute ischemic stroke does not improve functional outcomes
Background: Higher glucose immediately following acute ischemic stroke is known to be associated with poor outcomes. Patients with elevated glucoses in the aftermath of an acute ischemic stroke are more likely to have expansion of ischemic area and are more likely to have hemorrhagic conversion.
Study design: Randomized, controlled trial, with blinded outcome assessment.
Setting: 63 sites in the United States.
Synopsis: A total of 1,151 patients were randomized to either intensive (goal blood glucose, 80-130 mg/dL) or standard (goal blood glucose, 80-179 mg/dL) glucose control for up to the first 72 hours after presenting with acute ischemic stroke. Patients in the intensive control group were given continuous IV insulin and patients in the standard control group were given subcutaneous sliding. There was no difference between groups (intensive vs. standard) with regards to the primary outcome, which was the percentage of patients who achieved a modified Rankin Score at 90 days of 0-2 (20.5% vs 21.6%; adjusted relative risk, 0.97; 95% confidence interval, 0.87-1.08; P = .55). Severe hypoglycemia (blood glucose of less than 40 mg/dL) occurred in the intensive control group only. The American Heart Association/American Stroke Association guidelines support target blood glucose of 140-180 mg/dL, though limited evidence to support this guideline is noted.
Bottom line: Patients who underwent intensive glucose control regimens following acute ischemic stroke did not have significantly different functional outcomes at 90 days than those who had standard glucose control therapy.
Citation: Johnston KC et al. Intensive vs. standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: The SHINE randomized clinical trial. JAMA. 2019 Jul 23/30;322(4):326-35.
Dr. Fritz is assistant professor of medicine and the director of hospitalist operations at St. Louis University School of Medicine.
Background: Higher glucose immediately following acute ischemic stroke is known to be associated with poor outcomes. Patients with elevated glucoses in the aftermath of an acute ischemic stroke are more likely to have expansion of ischemic area and are more likely to have hemorrhagic conversion.
Study design: Randomized, controlled trial, with blinded outcome assessment.
Setting: 63 sites in the United States.
Synopsis: A total of 1,151 patients were randomized to either intensive (goal blood glucose, 80-130 mg/dL) or standard (goal blood glucose, 80-179 mg/dL) glucose control for up to the first 72 hours after presenting with acute ischemic stroke. Patients in the intensive control group were given continuous IV insulin and patients in the standard control group were given subcutaneous sliding. There was no difference between groups (intensive vs. standard) with regards to the primary outcome, which was the percentage of patients who achieved a modified Rankin Score at 90 days of 0-2 (20.5% vs 21.6%; adjusted relative risk, 0.97; 95% confidence interval, 0.87-1.08; P = .55). Severe hypoglycemia (blood glucose of less than 40 mg/dL) occurred in the intensive control group only. The American Heart Association/American Stroke Association guidelines support target blood glucose of 140-180 mg/dL, though limited evidence to support this guideline is noted.
Bottom line: Patients who underwent intensive glucose control regimens following acute ischemic stroke did not have significantly different functional outcomes at 90 days than those who had standard glucose control therapy.
Citation: Johnston KC et al. Intensive vs. standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: The SHINE randomized clinical trial. JAMA. 2019 Jul 23/30;322(4):326-35.
Dr. Fritz is assistant professor of medicine and the director of hospitalist operations at St. Louis University School of Medicine.
Background: Higher glucose immediately following acute ischemic stroke is known to be associated with poor outcomes. Patients with elevated glucoses in the aftermath of an acute ischemic stroke are more likely to have expansion of ischemic area and are more likely to have hemorrhagic conversion.
Study design: Randomized, controlled trial, with blinded outcome assessment.
Setting: 63 sites in the United States.
Synopsis: A total of 1,151 patients were randomized to either intensive (goal blood glucose, 80-130 mg/dL) or standard (goal blood glucose, 80-179 mg/dL) glucose control for up to the first 72 hours after presenting with acute ischemic stroke. Patients in the intensive control group were given continuous IV insulin and patients in the standard control group were given subcutaneous sliding. There was no difference between groups (intensive vs. standard) with regards to the primary outcome, which was the percentage of patients who achieved a modified Rankin Score at 90 days of 0-2 (20.5% vs 21.6%; adjusted relative risk, 0.97; 95% confidence interval, 0.87-1.08; P = .55). Severe hypoglycemia (blood glucose of less than 40 mg/dL) occurred in the intensive control group only. The American Heart Association/American Stroke Association guidelines support target blood glucose of 140-180 mg/dL, though limited evidence to support this guideline is noted.
Bottom line: Patients who underwent intensive glucose control regimens following acute ischemic stroke did not have significantly different functional outcomes at 90 days than those who had standard glucose control therapy.
Citation: Johnston KC et al. Intensive vs. standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: The SHINE randomized clinical trial. JAMA. 2019 Jul 23/30;322(4):326-35.
Dr. Fritz is assistant professor of medicine and the director of hospitalist operations at St. Louis University School of Medicine.
CDC shortens COVID-19 quarantine time to 10 or 7 days, with conditions
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
CRP testing in acute COPD exacerbations cuts antibiotic use without compromising outcomes
Background: A previous study has shown that patients with acute COPD exacerbations had little difference in rate of clinical cure with placebo or antibiotics when CRP is less than 40 mg/L.
Study design: Multicenter, open-label, randomized controlled trial.
Setting: 86 general medical practices in the United Kingdom from January 2015 through September 2017.
Synopsis: More than 600 patients who presented to a primary care physician with an acute COPD exacerbation were randomized to point of care CRP testing vs. usual care. Clinicians in the CRP testing group were provided with a point-of-care testing unit along with an algorithm for results. If the CRP was greater than 40 mg/L, antibiotics were thought to be beneficial; but they were urged not to prescribe antibiotics if the level was less than 20 mg/L. For levels between 20 mg/L and 40 mg/L, it was suggested that antibiotics might be beneficial if the sputum is purulent.
The primary outcomes were patient-reported use of antibiotics for an acute COPD exacerbation within 4 weeks of randomization along with measurement of COPD-related health status on the Clinical COPD Questionnaire at 2 weeks of randomization. Fewer antibiotics were prescribed in the CRP testing group over the usual care group (57% vs. 77%). The adjusted mean difference in the Clinical COPD Questionnaire total score at 2 weeks was –0.19 points, in favor of the CRP-guided group.
Bottom line: The use of point-of-care testing CRP as an adjunctive guide to antibiotic use in acute COPD exacerbations may lower the amount of antibiotic prescribing without compromising clinical outcomes.
Citation: Butler CC et al. C-reactive protein testing to guide antibiotics prescribing for COPD exacerbations. N Engl J Med. 2019 Jul 11; 381:111-120.
Dr. Choksi is a hospitalist and associate professor of internal medicine at Saint Louis University, where she is assistant dean of admissions. She is president of the SHM St. Louis Chapter.
Background: A previous study has shown that patients with acute COPD exacerbations had little difference in rate of clinical cure with placebo or antibiotics when CRP is less than 40 mg/L.
Study design: Multicenter, open-label, randomized controlled trial.
Setting: 86 general medical practices in the United Kingdom from January 2015 through September 2017.
Synopsis: More than 600 patients who presented to a primary care physician with an acute COPD exacerbation were randomized to point of care CRP testing vs. usual care. Clinicians in the CRP testing group were provided with a point-of-care testing unit along with an algorithm for results. If the CRP was greater than 40 mg/L, antibiotics were thought to be beneficial; but they were urged not to prescribe antibiotics if the level was less than 20 mg/L. For levels between 20 mg/L and 40 mg/L, it was suggested that antibiotics might be beneficial if the sputum is purulent.
The primary outcomes were patient-reported use of antibiotics for an acute COPD exacerbation within 4 weeks of randomization along with measurement of COPD-related health status on the Clinical COPD Questionnaire at 2 weeks of randomization. Fewer antibiotics were prescribed in the CRP testing group over the usual care group (57% vs. 77%). The adjusted mean difference in the Clinical COPD Questionnaire total score at 2 weeks was –0.19 points, in favor of the CRP-guided group.
Bottom line: The use of point-of-care testing CRP as an adjunctive guide to antibiotic use in acute COPD exacerbations may lower the amount of antibiotic prescribing without compromising clinical outcomes.
Citation: Butler CC et al. C-reactive protein testing to guide antibiotics prescribing for COPD exacerbations. N Engl J Med. 2019 Jul 11; 381:111-120.
Dr. Choksi is a hospitalist and associate professor of internal medicine at Saint Louis University, where she is assistant dean of admissions. She is president of the SHM St. Louis Chapter.
Background: A previous study has shown that patients with acute COPD exacerbations had little difference in rate of clinical cure with placebo or antibiotics when CRP is less than 40 mg/L.
Study design: Multicenter, open-label, randomized controlled trial.
Setting: 86 general medical practices in the United Kingdom from January 2015 through September 2017.
Synopsis: More than 600 patients who presented to a primary care physician with an acute COPD exacerbation were randomized to point of care CRP testing vs. usual care. Clinicians in the CRP testing group were provided with a point-of-care testing unit along with an algorithm for results. If the CRP was greater than 40 mg/L, antibiotics were thought to be beneficial; but they were urged not to prescribe antibiotics if the level was less than 20 mg/L. For levels between 20 mg/L and 40 mg/L, it was suggested that antibiotics might be beneficial if the sputum is purulent.
The primary outcomes were patient-reported use of antibiotics for an acute COPD exacerbation within 4 weeks of randomization along with measurement of COPD-related health status on the Clinical COPD Questionnaire at 2 weeks of randomization. Fewer antibiotics were prescribed in the CRP testing group over the usual care group (57% vs. 77%). The adjusted mean difference in the Clinical COPD Questionnaire total score at 2 weeks was –0.19 points, in favor of the CRP-guided group.
Bottom line: The use of point-of-care testing CRP as an adjunctive guide to antibiotic use in acute COPD exacerbations may lower the amount of antibiotic prescribing without compromising clinical outcomes.
Citation: Butler CC et al. C-reactive protein testing to guide antibiotics prescribing for COPD exacerbations. N Engl J Med. 2019 Jul 11; 381:111-120.
Dr. Choksi is a hospitalist and associate professor of internal medicine at Saint Louis University, where she is assistant dean of admissions. She is president of the SHM St. Louis Chapter.
Three pillars of a successful coronavirus vaccine program in minorities
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
U.S. passes 1.3 million COVID-19 cases in children
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.
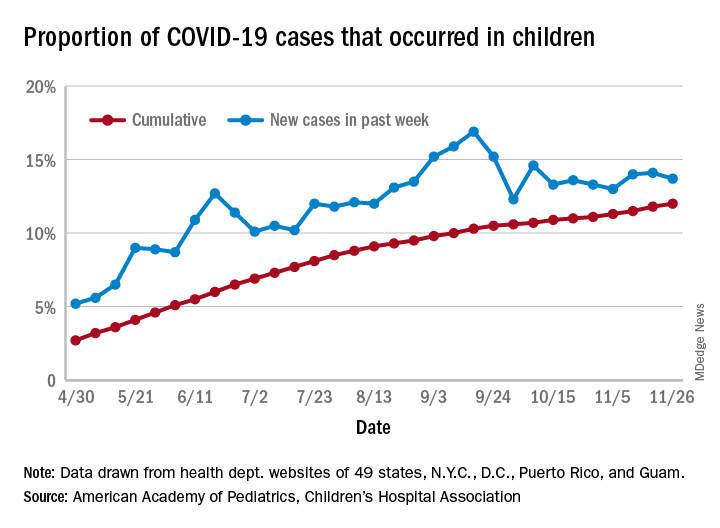
the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
ACIP: Health workers, long-term care residents first tier for COVID-19 vaccine
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
What to do when anticoagulation fails cancer patients
When a patient with cancer develops venous thromboembolism despite anticoagulation, how to help them comes down to clinical judgment, according to hematologist Neil Zakai, MD, associate professor at the University of Vermont, Burlington.
“Unfortunately,” when it comes to “anticoagulation failure, we are entering an evidence free-zone,” with no large trials to guide management and only a few guiding principles, he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The first thing is to check if there was an inciting incident, such as medical noncompliance, an infection, or an interruption of anticoagulation. Dr. Zakai said he’s even had cancer patients develop heparin-induced thrombocytopenia when switched to enoxaparin from a direct oral anticoagulants (DOAC) for a procedure.
Once the underlying problem is addressed, patients may be able to continue with their original anticoagulant.
However, cancer progression is the main reason anticoagulation fails. “In general, it is very difficult to control cancer thrombosis if you can’t control cancer progression,” Dr. Zakai said.
In those cases, he steps up anticoagulation. Prophylactic dosing is increased to full treatment dosing, and patients on a DOAC are generally switched to a low molecular weight heparin (LMWH).
If patients are already on LMWH once daily, they will be bumped up to twice daily dosing; for instance, enoxaparin 1 mg/kg b.i.d. instead of 1.5 mg/kg q.d. Dr. Zakai said he’s gone as high at 2 or even 2.5 mg/kg to control thrombosis, without excessive bleeding.
In general, anticoagulation for thrombosis prophylaxis continues as long as the cancer is active, and certainly while patients are on hormonal treatments such as tamoxifen, which increases the risk.
Dr. Zakai stressed that both thrombosis and bleeding risk change for cancer patients over time, and treatment needs to keep up.
“I continuously assess the risk and benefit of anticoagulation. At certain times” such as during and for a few months after hospitalization, thrombosis risk increases; at other times, bleeding risk is higher. “You need to actively change your anticoagulation during those periods,” and tailor therapy based on transient risk factors. “People with cancer have peaks and troughs for their risk that we don’t take advantage of,” he said.
Dr. Zakai generally favors apixaban or enoxaparin for prophylaxis, carefully monitoring patients for bleeding and, for the DOAC, drug interactions with antiemetics, dexamethasone, and certain chemotherapy drugs.
He noted a recent trial that found a 59% reduction in venous thromboembolism risk in ambulatory cancer patients with apixaban 2.5 mg twice daily over 6 months, versus placebo, and a 6% absolute reduction, but at the cost of a twofold increase in bleeding risk, with an absolute 1.7% increase.
Dr. Zakai cautioned that patients in trials are selected for higher VTE and lower bleeding risks, so outcomes might “poorly reflect real world populations.” Dr. Zakai did not have any industry disclosures. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
When a patient with cancer develops venous thromboembolism despite anticoagulation, how to help them comes down to clinical judgment, according to hematologist Neil Zakai, MD, associate professor at the University of Vermont, Burlington.
“Unfortunately,” when it comes to “anticoagulation failure, we are entering an evidence free-zone,” with no large trials to guide management and only a few guiding principles, he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The first thing is to check if there was an inciting incident, such as medical noncompliance, an infection, or an interruption of anticoagulation. Dr. Zakai said he’s even had cancer patients develop heparin-induced thrombocytopenia when switched to enoxaparin from a direct oral anticoagulants (DOAC) for a procedure.
Once the underlying problem is addressed, patients may be able to continue with their original anticoagulant.
However, cancer progression is the main reason anticoagulation fails. “In general, it is very difficult to control cancer thrombosis if you can’t control cancer progression,” Dr. Zakai said.
In those cases, he steps up anticoagulation. Prophylactic dosing is increased to full treatment dosing, and patients on a DOAC are generally switched to a low molecular weight heparin (LMWH).
If patients are already on LMWH once daily, they will be bumped up to twice daily dosing; for instance, enoxaparin 1 mg/kg b.i.d. instead of 1.5 mg/kg q.d. Dr. Zakai said he’s gone as high at 2 or even 2.5 mg/kg to control thrombosis, without excessive bleeding.
In general, anticoagulation for thrombosis prophylaxis continues as long as the cancer is active, and certainly while patients are on hormonal treatments such as tamoxifen, which increases the risk.
Dr. Zakai stressed that both thrombosis and bleeding risk change for cancer patients over time, and treatment needs to keep up.
“I continuously assess the risk and benefit of anticoagulation. At certain times” such as during and for a few months after hospitalization, thrombosis risk increases; at other times, bleeding risk is higher. “You need to actively change your anticoagulation during those periods,” and tailor therapy based on transient risk factors. “People with cancer have peaks and troughs for their risk that we don’t take advantage of,” he said.
Dr. Zakai generally favors apixaban or enoxaparin for prophylaxis, carefully monitoring patients for bleeding and, for the DOAC, drug interactions with antiemetics, dexamethasone, and certain chemotherapy drugs.
He noted a recent trial that found a 59% reduction in venous thromboembolism risk in ambulatory cancer patients with apixaban 2.5 mg twice daily over 6 months, versus placebo, and a 6% absolute reduction, but at the cost of a twofold increase in bleeding risk, with an absolute 1.7% increase.
Dr. Zakai cautioned that patients in trials are selected for higher VTE and lower bleeding risks, so outcomes might “poorly reflect real world populations.” Dr. Zakai did not have any industry disclosures. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
When a patient with cancer develops venous thromboembolism despite anticoagulation, how to help them comes down to clinical judgment, according to hematologist Neil Zakai, MD, associate professor at the University of Vermont, Burlington.
“Unfortunately,” when it comes to “anticoagulation failure, we are entering an evidence free-zone,” with no large trials to guide management and only a few guiding principles, he said during his presentation at the 2020 Update in Nonneoplastic Hematology virtual conference.
The first thing is to check if there was an inciting incident, such as medical noncompliance, an infection, or an interruption of anticoagulation. Dr. Zakai said he’s even had cancer patients develop heparin-induced thrombocytopenia when switched to enoxaparin from a direct oral anticoagulants (DOAC) for a procedure.
Once the underlying problem is addressed, patients may be able to continue with their original anticoagulant.
However, cancer progression is the main reason anticoagulation fails. “In general, it is very difficult to control cancer thrombosis if you can’t control cancer progression,” Dr. Zakai said.
In those cases, he steps up anticoagulation. Prophylactic dosing is increased to full treatment dosing, and patients on a DOAC are generally switched to a low molecular weight heparin (LMWH).
If patients are already on LMWH once daily, they will be bumped up to twice daily dosing; for instance, enoxaparin 1 mg/kg b.i.d. instead of 1.5 mg/kg q.d. Dr. Zakai said he’s gone as high at 2 or even 2.5 mg/kg to control thrombosis, without excessive bleeding.
In general, anticoagulation for thrombosis prophylaxis continues as long as the cancer is active, and certainly while patients are on hormonal treatments such as tamoxifen, which increases the risk.
Dr. Zakai stressed that both thrombosis and bleeding risk change for cancer patients over time, and treatment needs to keep up.
“I continuously assess the risk and benefit of anticoagulation. At certain times” such as during and for a few months after hospitalization, thrombosis risk increases; at other times, bleeding risk is higher. “You need to actively change your anticoagulation during those periods,” and tailor therapy based on transient risk factors. “People with cancer have peaks and troughs for their risk that we don’t take advantage of,” he said.
Dr. Zakai generally favors apixaban or enoxaparin for prophylaxis, carefully monitoring patients for bleeding and, for the DOAC, drug interactions with antiemetics, dexamethasone, and certain chemotherapy drugs.
He noted a recent trial that found a 59% reduction in venous thromboembolism risk in ambulatory cancer patients with apixaban 2.5 mg twice daily over 6 months, versus placebo, and a 6% absolute reduction, but at the cost of a twofold increase in bleeding risk, with an absolute 1.7% increase.
Dr. Zakai cautioned that patients in trials are selected for higher VTE and lower bleeding risks, so outcomes might “poorly reflect real world populations.” Dr. Zakai did not have any industry disclosures. The conference was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
FROM 2020 UNNH
CMS launches hospital-at-home program to free up hospital capacity
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.










