User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


PPE shortage crisis continues at most hospitals, survey shows
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
Higher 10-day mortality of lower-acuity patients during times of increased ED crowding
Background: Studies have assessed mortality effect from ED crowding on high-acuity patients, but limited evidence exists for how this affects lower-acuity patients who are discharged home.
Study design: Retrospective cohort study.
Setting: Emergency department, Karolinska University Hospital, Solna, Sweden.
Synopsis: During 2009-2016, 705,813 encounters seen in the ED, triaged to lower-acuity levels 3-5 and discharged without further hospitalization needs were identified. A total of 623 patients died within 10 days of the initial ED visit (0.09%). The study evaluated the association of 10-day mortality with mean ED length of stay and ED-occupancy ratio.
The study demonstrated an increased 10-day mortality for mean ED length of stay of 8 hours or more vs. less than 2 hours (adjusted odds ratio, 5.86; 95% CI, 2.15-15.94). It also found an increased mortality rate for occupancy ratio quartiles with an aOR for quartiles 2, 3, and 4 vs. quartile 1 of 1.48 (95% CI, 1.14-1.92), 1.63 (95% CI, 1.24-2.14), and 1.53 (95% CI, 1.15-2.03), respectively.
While this suggests increased 10-day mortality in this patient population, additional studies should be conducted to determine if this risk is caused by ED crowding and length of stay or by current limitations in triage scoring.
Bottom line: There is an increased 10-day mortality rate for lower-acuity triaged patients who were discharged from the ED without hospitalization experiencing increased ED length of stay and during times of ED crowding.
Citation: Berg L et al. Associations between crowding and 10-day mortality among patients allocated lower triage acuity levels without need of acute hospital care on departure from the emergency department. Ann Emerg Med. 2019 Sep;74(3):345-56.
Dr. Merando is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Studies have assessed mortality effect from ED crowding on high-acuity patients, but limited evidence exists for how this affects lower-acuity patients who are discharged home.
Study design: Retrospective cohort study.
Setting: Emergency department, Karolinska University Hospital, Solna, Sweden.
Synopsis: During 2009-2016, 705,813 encounters seen in the ED, triaged to lower-acuity levels 3-5 and discharged without further hospitalization needs were identified. A total of 623 patients died within 10 days of the initial ED visit (0.09%). The study evaluated the association of 10-day mortality with mean ED length of stay and ED-occupancy ratio.
The study demonstrated an increased 10-day mortality for mean ED length of stay of 8 hours or more vs. less than 2 hours (adjusted odds ratio, 5.86; 95% CI, 2.15-15.94). It also found an increased mortality rate for occupancy ratio quartiles with an aOR for quartiles 2, 3, and 4 vs. quartile 1 of 1.48 (95% CI, 1.14-1.92), 1.63 (95% CI, 1.24-2.14), and 1.53 (95% CI, 1.15-2.03), respectively.
While this suggests increased 10-day mortality in this patient population, additional studies should be conducted to determine if this risk is caused by ED crowding and length of stay or by current limitations in triage scoring.
Bottom line: There is an increased 10-day mortality rate for lower-acuity triaged patients who were discharged from the ED without hospitalization experiencing increased ED length of stay and during times of ED crowding.
Citation: Berg L et al. Associations between crowding and 10-day mortality among patients allocated lower triage acuity levels without need of acute hospital care on departure from the emergency department. Ann Emerg Med. 2019 Sep;74(3):345-56.
Dr. Merando is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Studies have assessed mortality effect from ED crowding on high-acuity patients, but limited evidence exists for how this affects lower-acuity patients who are discharged home.
Study design: Retrospective cohort study.
Setting: Emergency department, Karolinska University Hospital, Solna, Sweden.
Synopsis: During 2009-2016, 705,813 encounters seen in the ED, triaged to lower-acuity levels 3-5 and discharged without further hospitalization needs were identified. A total of 623 patients died within 10 days of the initial ED visit (0.09%). The study evaluated the association of 10-day mortality with mean ED length of stay and ED-occupancy ratio.
The study demonstrated an increased 10-day mortality for mean ED length of stay of 8 hours or more vs. less than 2 hours (adjusted odds ratio, 5.86; 95% CI, 2.15-15.94). It also found an increased mortality rate for occupancy ratio quartiles with an aOR for quartiles 2, 3, and 4 vs. quartile 1 of 1.48 (95% CI, 1.14-1.92), 1.63 (95% CI, 1.24-2.14), and 1.53 (95% CI, 1.15-2.03), respectively.
While this suggests increased 10-day mortality in this patient population, additional studies should be conducted to determine if this risk is caused by ED crowding and length of stay or by current limitations in triage scoring.
Bottom line: There is an increased 10-day mortality rate for lower-acuity triaged patients who were discharged from the ED without hospitalization experiencing increased ED length of stay and during times of ED crowding.
Citation: Berg L et al. Associations between crowding and 10-day mortality among patients allocated lower triage acuity levels without need of acute hospital care on departure from the emergency department. Ann Emerg Med. 2019 Sep;74(3):345-56.
Dr. Merando is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Infant’s COVID-19–related myocardial injury reversed
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
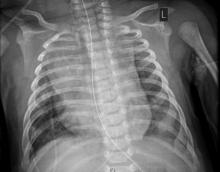
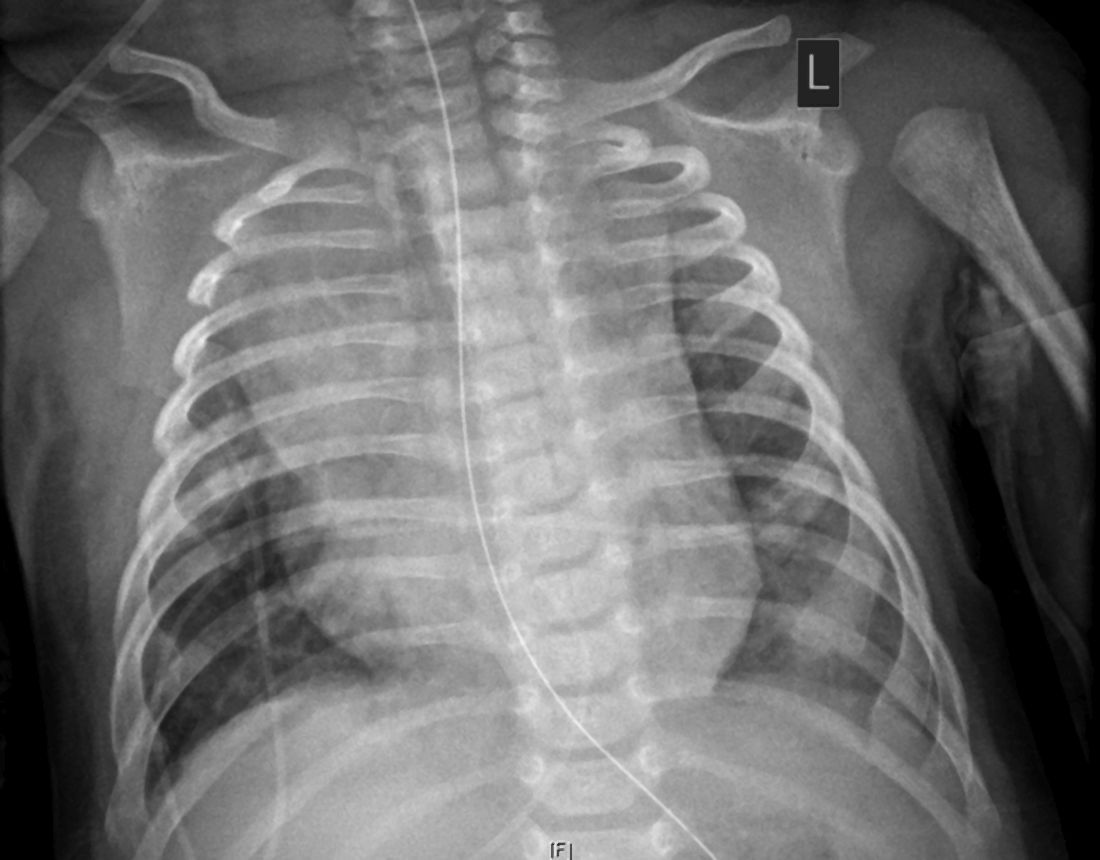
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
FROM JACC CASE REPORTS
Key clinical point: Children presenting with COVID-19 should be tested for heart failure.
Major finding: A 2-month-old infant with COVID-19 had acute but reversible myocardial injury.
Study details: Single case report.
Disclosures: Dr. Sharma, MD, has no relevant financial relationships to disclose.
Source: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Mortality higher in older adults hospitalized for IBD
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Obesity, hypoxia predict severity in children with COVID-19
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
FROM THE JOURNAL OF PEDIATRICS
Risk associated with perioperative atrial fibrillation
Background: New-onset POAF occurs with 10% of noncardiac surgery and 15%-42% of cardiac surgery. POAF is believed to be self-limiting and most patients revert to sinus rhythm before hospital discharge. Previous studies on this topic are both limited and conflicting, but several suggest there is an association of stroke and mortality with POAF.
Study design: Systematic review and meta-analysis. Odds ratios with 95% confidence intervals were used for early outcomes and hazard ratios were used for long-term outcomes.
Setting: Prospective and retrospective cohort studies.
Synopsis: A total of 35 carefully selected studies were analyzed for a total of 2,458,010 patients. Outcomes of interest were early stroke or mortality within 30 days of surgery and long-term stroke or mortality after 30 days. The reference group was patients without POAF at baseline. Subgroup analysis included separating patients into cardiac surgery and noncardiac surgery.
New-onset POAF was associated with increased risk of early stroke (OR, 1.62; 95% CI, 1.47-1.80) and early mortality (OR, 1.44; 95% CI, 1.11-1.88). POAF also was associated with risk for long-term stroke (hazard ratio, 1.37; 95% CI, 1.07-1.77) and long-term mortality (HR, 1.37; 95% CI, 1.27-1.49). The risk of long-term stroke from new-onset POAF was highest among patients who received noncardiac surgery.
Despite identifying high-quality studies with thoughtful analysis, some data had the potential for publication bias. The representative sample did not report paroxysmal vs. persistent atrial fibrillation separately. Furthermore, the study had the potential to be confounded by detection bias of preexisting atrial fibrillation.
Bottom line: New-onset POAF is associated with early and long-term risk of stroke and mortality. Subsequent strategies to reduce this risk have yet to be determined.
Citation: Lin MH et al. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke. 2019 May;50:1364-71.
Dr. Mayer is a hospitalist and assistant professor of medicine at St. Louis University School of Medicine.
Background: New-onset POAF occurs with 10% of noncardiac surgery and 15%-42% of cardiac surgery. POAF is believed to be self-limiting and most patients revert to sinus rhythm before hospital discharge. Previous studies on this topic are both limited and conflicting, but several suggest there is an association of stroke and mortality with POAF.
Study design: Systematic review and meta-analysis. Odds ratios with 95% confidence intervals were used for early outcomes and hazard ratios were used for long-term outcomes.
Setting: Prospective and retrospective cohort studies.
Synopsis: A total of 35 carefully selected studies were analyzed for a total of 2,458,010 patients. Outcomes of interest were early stroke or mortality within 30 days of surgery and long-term stroke or mortality after 30 days. The reference group was patients without POAF at baseline. Subgroup analysis included separating patients into cardiac surgery and noncardiac surgery.
New-onset POAF was associated with increased risk of early stroke (OR, 1.62; 95% CI, 1.47-1.80) and early mortality (OR, 1.44; 95% CI, 1.11-1.88). POAF also was associated with risk for long-term stroke (hazard ratio, 1.37; 95% CI, 1.07-1.77) and long-term mortality (HR, 1.37; 95% CI, 1.27-1.49). The risk of long-term stroke from new-onset POAF was highest among patients who received noncardiac surgery.
Despite identifying high-quality studies with thoughtful analysis, some data had the potential for publication bias. The representative sample did not report paroxysmal vs. persistent atrial fibrillation separately. Furthermore, the study had the potential to be confounded by detection bias of preexisting atrial fibrillation.
Bottom line: New-onset POAF is associated with early and long-term risk of stroke and mortality. Subsequent strategies to reduce this risk have yet to be determined.
Citation: Lin MH et al. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke. 2019 May;50:1364-71.
Dr. Mayer is a hospitalist and assistant professor of medicine at St. Louis University School of Medicine.
Background: New-onset POAF occurs with 10% of noncardiac surgery and 15%-42% of cardiac surgery. POAF is believed to be self-limiting and most patients revert to sinus rhythm before hospital discharge. Previous studies on this topic are both limited and conflicting, but several suggest there is an association of stroke and mortality with POAF.
Study design: Systematic review and meta-analysis. Odds ratios with 95% confidence intervals were used for early outcomes and hazard ratios were used for long-term outcomes.
Setting: Prospective and retrospective cohort studies.
Synopsis: A total of 35 carefully selected studies were analyzed for a total of 2,458,010 patients. Outcomes of interest were early stroke or mortality within 30 days of surgery and long-term stroke or mortality after 30 days. The reference group was patients without POAF at baseline. Subgroup analysis included separating patients into cardiac surgery and noncardiac surgery.
New-onset POAF was associated with increased risk of early stroke (OR, 1.62; 95% CI, 1.47-1.80) and early mortality (OR, 1.44; 95% CI, 1.11-1.88). POAF also was associated with risk for long-term stroke (hazard ratio, 1.37; 95% CI, 1.07-1.77) and long-term mortality (HR, 1.37; 95% CI, 1.27-1.49). The risk of long-term stroke from new-onset POAF was highest among patients who received noncardiac surgery.
Despite identifying high-quality studies with thoughtful analysis, some data had the potential for publication bias. The representative sample did not report paroxysmal vs. persistent atrial fibrillation separately. Furthermore, the study had the potential to be confounded by detection bias of preexisting atrial fibrillation.
Bottom line: New-onset POAF is associated with early and long-term risk of stroke and mortality. Subsequent strategies to reduce this risk have yet to be determined.
Citation: Lin MH et al. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke. 2019 May;50:1364-71.
Dr. Mayer is a hospitalist and assistant professor of medicine at St. Louis University School of Medicine.
SHM urges Congress to reverse changes in reimbursement rates under 2021 Medicare Physician Fee Schedule
Approximately 8% reduction in reimbursement for hospitalists
On Dec. 1, the Centers for Medicare & Medicaid Services (CMS) released the 2021 Medicare Physician Fee Schedule, which finalized proposed changes to Medicare reimbursement rates, including a significant negative budget neutrality adjustment. For hospitalists, the Society of Hospital Medicine estimates that the adjustment will amount to an estimated 8% reduction in Medicare reimbursement rates, which will go into effect on Jan. 1, 2021.
“These cuts are coming at the exact wrong time. During the chaos of 2020, when hospitalists have been essential to responding to the COVID-19 pandemic, they should not be met with a significant pay reduction in 2021,” said Eric E. Howell, MD, MHM, chief executive officer of the Society of Hospital Medicine. “While we at SHM support increasing pay for outpatient primary care, which is driving these cuts, we do not believe now is the right time to make significant adjustments to the Medicare Physician Fee Schedule. We now call on Congress to do the right thing for hospitalists and other frontline providers who have otherwise been lauded as heroes.”
SHM will continue to fight for hospitalists and to advocate to reverse these cuts. To send a message of support to your representatives, visit SHM’s Legislative Action Center and click on “Support the Holding Providers Harmless from Medicare Cuts During COVID-19 Act of 2020.” To learn more about and become involved with SHM’s advocacy efforts, visit hospitalmedicine.org/advocacy.
Approximately 8% reduction in reimbursement for hospitalists
Approximately 8% reduction in reimbursement for hospitalists
On Dec. 1, the Centers for Medicare & Medicaid Services (CMS) released the 2021 Medicare Physician Fee Schedule, which finalized proposed changes to Medicare reimbursement rates, including a significant negative budget neutrality adjustment. For hospitalists, the Society of Hospital Medicine estimates that the adjustment will amount to an estimated 8% reduction in Medicare reimbursement rates, which will go into effect on Jan. 1, 2021.
“These cuts are coming at the exact wrong time. During the chaos of 2020, when hospitalists have been essential to responding to the COVID-19 pandemic, they should not be met with a significant pay reduction in 2021,” said Eric E. Howell, MD, MHM, chief executive officer of the Society of Hospital Medicine. “While we at SHM support increasing pay for outpatient primary care, which is driving these cuts, we do not believe now is the right time to make significant adjustments to the Medicare Physician Fee Schedule. We now call on Congress to do the right thing for hospitalists and other frontline providers who have otherwise been lauded as heroes.”
SHM will continue to fight for hospitalists and to advocate to reverse these cuts. To send a message of support to your representatives, visit SHM’s Legislative Action Center and click on “Support the Holding Providers Harmless from Medicare Cuts During COVID-19 Act of 2020.” To learn more about and become involved with SHM’s advocacy efforts, visit hospitalmedicine.org/advocacy.
On Dec. 1, the Centers for Medicare & Medicaid Services (CMS) released the 2021 Medicare Physician Fee Schedule, which finalized proposed changes to Medicare reimbursement rates, including a significant negative budget neutrality adjustment. For hospitalists, the Society of Hospital Medicine estimates that the adjustment will amount to an estimated 8% reduction in Medicare reimbursement rates, which will go into effect on Jan. 1, 2021.
“These cuts are coming at the exact wrong time. During the chaos of 2020, when hospitalists have been essential to responding to the COVID-19 pandemic, they should not be met with a significant pay reduction in 2021,” said Eric E. Howell, MD, MHM, chief executive officer of the Society of Hospital Medicine. “While we at SHM support increasing pay for outpatient primary care, which is driving these cuts, we do not believe now is the right time to make significant adjustments to the Medicare Physician Fee Schedule. We now call on Congress to do the right thing for hospitalists and other frontline providers who have otherwise been lauded as heroes.”
SHM will continue to fight for hospitalists and to advocate to reverse these cuts. To send a message of support to your representatives, visit SHM’s Legislative Action Center and click on “Support the Holding Providers Harmless from Medicare Cuts During COVID-19 Act of 2020.” To learn more about and become involved with SHM’s advocacy efforts, visit hospitalmedicine.org/advocacy.
Leading hospitalists during a pandemic
As I write this, we are entering the third surge of the COVID-19 pandemic, with new cases, hospitalizations, and deaths from COVID-19 skyrocketing around the country. Worst of all, this surge has been most severely affecting areas of the nation least prepared to handle it (rural) and populations already marginalized by the health care system (Latinx and Black). Despite the onslaught of COVID-19, “pandemic fatigue” has begun to set in amongst colleagues, friends, and family, leading to challenges in adhering to social distancing and other infection-control measures, both at work and home.
In the face of the pandemic’s onslaught, hospitalists – who have faced the brunt of caring for patients with COVID-19, despite the absence of reporting about the subspecialty’s role – are faced with mustering the grit to respond with resolve, coordinated action, and empathy. Luckily, hospitalists are equipped with the very characteristics needed to lead teams, groups, and hospitals through the crisis of this pandemic. Ask yourself, why did you become a hospitalist? If you wanted steady predictability and control, there were many office-based specialties you could have chosen. You chose to become a hospitalist because you seek the challenges of clinical variety, problem-solving, systems improvement, and you are a natural team leader, whether you have been designated as such or not. In the words of John Quincy Adams, “if your actions inspire others to dream more, learn more, do more, and become more, you are a leader.”
As a leader, how can you lead your team through the series of trials and tribulations that this year has thrown at you? From COVID-19 to racism directed against Black and Latinx people to the behavioral health crisis, 2020 has likely made you feel as if you’re stuck in a ghoulish carnival fun house without an exit.
Yet this is where some leaders hit their stride, in what Bennis and Thomas describe as the “crucible of leadership.”1 There are many types of “crucibles of leadership,” according to Bennis and Thomas, and this year has thrown most of these at us: prejudice/bias, physical fatigue and illness, sudden elevation of responsibility to lead new processes, not to mention family stressors. Leaders who succeed in guiding their colleagues through these challenges have manifested critical skills: engaging others in shared meaning, having a distinctive and compelling voice, displaying integrity, and having adaptive capacity.
What exactly is adaptive capacity, the most important of these, in my opinion? Adaptive capacity requires understanding the new context of a crisis and how it has shifted team members’ needs and perceptions. It also requires what Bennis and Thomas call hardiness and what I call grit – the ability to face adversity, get knocked down, get up, and do it again.
There is probably no better example of a crisis leader with extraordinary adaptive capacity than Anglo-Irish explorer Sir Ernest Shackleton. Bitten by the bug of exploration, Shackleton failed at reaching the South Pole (1908-1909) but subsequently attempted to cross the Antarctic, departing South Georgia Island on Dec. 5, 1914. Depressingly for Shackleton, his ship, the Endurance, became stuck in sea ice on Jan. 19, 1915 before even reaching the continent. Drifting with the ice floe, his crew had set up a winter station hoping to be released from the ice later, but the Endurance was crushed by the pressure of sea ice and sank on Nov. 21, 1915. From there, Shackleton hoped to drift north to Paulet Island, 250 miles away, but eventually was forced to take his crew on lifeboats to the nearest land, Elephant Island, 346 miles from where the Endurance sank. He then took five of his men on an open boat, 828-mile journey to South Georgia Island. Encountering hurricane-force winds, the team landed on South Georgia Island 15 days later, only to face a climb of 32 miles over mountainous terrain to reach a whaling station. Shackleton eventually organized his men’s rescue on Elephant Island, reaching them on Aug. 30, 1916, 4½ months after he had set out for South Georgia Island. His entire crew survived, only to have two of them killed later in World War I.
You might consider Shackleton a failure for not even coming close to his original goal, but his success in saving his crew is regarded as the epitome of crisis leadership. As Harvard Business School professor Nancy F. Koehn, PhD, whose case study of Shackleton is one of the most popular at HBS, stated, “He thought he was going to be an entrepreneur of exploration, but he became an entrepreneur of survival.”2 Upon realizing the futility of his original mission, he pivoted immediately to the survival of his crew. “A man must shape himself to a new mark directly the old one goes to ground,” wrote Shackleton in his diary.3
Realizing that preserving his crew’s morale was critical, he maintained the crew’s everyday activities, despite the prospect of dying on the ice. He realized that he needed to keep up his own courage and confidence as well as that of his crew. Despite his ability to share the strategic focus of getting to safety with his men, he didn’t lose sight of day-to-day needs, such as keeping the crew entertained. When he encountered crew members who seemed problematic to his mission goals, he assigned them to his own tent.
Despite the extreme cold, his decision-making did not freeze – he acted decisively. He took risks when he thought appropriate, twice needing to abandon his efforts to drag a lifeboat full of supplies with his men toward the sea. “You can’t be afraid to make smart mistakes,” says Dr. Koehn. “That’s something we have no training in.”4 Most importantly, Shackleton took ultimate responsibility for his men’s survival, never resting until they had all been rescued. And he modeled a culture of shared responsibility for one another5 – he had once offered his only biscuit of the day on a prior expedition to his fellow explorer Frank Wild.
As winter arrives in 2020 and deepens into 2021, we will all be faced with leading our teams across the ice and to the safety of spring, and hopefully a vaccine. Whether we can get there with our entire crew depends on effective crisis leadership. But we can draw on the lessons provided by Shackleton and other crisis leaders in the past to guide us in the present.
Author disclosure: I studied the HBS case study “Leadership in Crisis: Ernest Shackleton and the Epic Voyage of the Endurance” as part of a 12-month certificate course in Safety, Quality, Informatics, and Leadership (SQIL) offered by Harvard Medical School.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. HBR’s 10 must reads on leadership. Boston: Harvard Business Review Press, 2011.
2. Lagace M. Shackleton: An entrepreneur of survival. Harvard Business School. Working Knowledge website. Published 2003. Accessed 2020 Nov 19.
3. Koehn N. Leadership lessons from the Shackleton Expedition. The New York Times. 2011 Dec 25.
4. Potier B. Shackleton in business school. Harvard Public Affairs and Communications. The Harvard Gazette website. Published 2004. Accessed 2020 Nov 19.
5. Perkins D. 4 Lessons in crisis leadership from Shackleton’s expedition. In Leadership Essentials by HarpersCollins Leadership. Vol 2020. New York: HarpersCollins, 2020.
As I write this, we are entering the third surge of the COVID-19 pandemic, with new cases, hospitalizations, and deaths from COVID-19 skyrocketing around the country. Worst of all, this surge has been most severely affecting areas of the nation least prepared to handle it (rural) and populations already marginalized by the health care system (Latinx and Black). Despite the onslaught of COVID-19, “pandemic fatigue” has begun to set in amongst colleagues, friends, and family, leading to challenges in adhering to social distancing and other infection-control measures, both at work and home.
In the face of the pandemic’s onslaught, hospitalists – who have faced the brunt of caring for patients with COVID-19, despite the absence of reporting about the subspecialty’s role – are faced with mustering the grit to respond with resolve, coordinated action, and empathy. Luckily, hospitalists are equipped with the very characteristics needed to lead teams, groups, and hospitals through the crisis of this pandemic. Ask yourself, why did you become a hospitalist? If you wanted steady predictability and control, there were many office-based specialties you could have chosen. You chose to become a hospitalist because you seek the challenges of clinical variety, problem-solving, systems improvement, and you are a natural team leader, whether you have been designated as such or not. In the words of John Quincy Adams, “if your actions inspire others to dream more, learn more, do more, and become more, you are a leader.”
As a leader, how can you lead your team through the series of trials and tribulations that this year has thrown at you? From COVID-19 to racism directed against Black and Latinx people to the behavioral health crisis, 2020 has likely made you feel as if you’re stuck in a ghoulish carnival fun house without an exit.
Yet this is where some leaders hit their stride, in what Bennis and Thomas describe as the “crucible of leadership.”1 There are many types of “crucibles of leadership,” according to Bennis and Thomas, and this year has thrown most of these at us: prejudice/bias, physical fatigue and illness, sudden elevation of responsibility to lead new processes, not to mention family stressors. Leaders who succeed in guiding their colleagues through these challenges have manifested critical skills: engaging others in shared meaning, having a distinctive and compelling voice, displaying integrity, and having adaptive capacity.
What exactly is adaptive capacity, the most important of these, in my opinion? Adaptive capacity requires understanding the new context of a crisis and how it has shifted team members’ needs and perceptions. It also requires what Bennis and Thomas call hardiness and what I call grit – the ability to face adversity, get knocked down, get up, and do it again.
There is probably no better example of a crisis leader with extraordinary adaptive capacity than Anglo-Irish explorer Sir Ernest Shackleton. Bitten by the bug of exploration, Shackleton failed at reaching the South Pole (1908-1909) but subsequently attempted to cross the Antarctic, departing South Georgia Island on Dec. 5, 1914. Depressingly for Shackleton, his ship, the Endurance, became stuck in sea ice on Jan. 19, 1915 before even reaching the continent. Drifting with the ice floe, his crew had set up a winter station hoping to be released from the ice later, but the Endurance was crushed by the pressure of sea ice and sank on Nov. 21, 1915. From there, Shackleton hoped to drift north to Paulet Island, 250 miles away, but eventually was forced to take his crew on lifeboats to the nearest land, Elephant Island, 346 miles from where the Endurance sank. He then took five of his men on an open boat, 828-mile journey to South Georgia Island. Encountering hurricane-force winds, the team landed on South Georgia Island 15 days later, only to face a climb of 32 miles over mountainous terrain to reach a whaling station. Shackleton eventually organized his men’s rescue on Elephant Island, reaching them on Aug. 30, 1916, 4½ months after he had set out for South Georgia Island. His entire crew survived, only to have two of them killed later in World War I.
You might consider Shackleton a failure for not even coming close to his original goal, but his success in saving his crew is regarded as the epitome of crisis leadership. As Harvard Business School professor Nancy F. Koehn, PhD, whose case study of Shackleton is one of the most popular at HBS, stated, “He thought he was going to be an entrepreneur of exploration, but he became an entrepreneur of survival.”2 Upon realizing the futility of his original mission, he pivoted immediately to the survival of his crew. “A man must shape himself to a new mark directly the old one goes to ground,” wrote Shackleton in his diary.3
Realizing that preserving his crew’s morale was critical, he maintained the crew’s everyday activities, despite the prospect of dying on the ice. He realized that he needed to keep up his own courage and confidence as well as that of his crew. Despite his ability to share the strategic focus of getting to safety with his men, he didn’t lose sight of day-to-day needs, such as keeping the crew entertained. When he encountered crew members who seemed problematic to his mission goals, he assigned them to his own tent.
Despite the extreme cold, his decision-making did not freeze – he acted decisively. He took risks when he thought appropriate, twice needing to abandon his efforts to drag a lifeboat full of supplies with his men toward the sea. “You can’t be afraid to make smart mistakes,” says Dr. Koehn. “That’s something we have no training in.”4 Most importantly, Shackleton took ultimate responsibility for his men’s survival, never resting until they had all been rescued. And he modeled a culture of shared responsibility for one another5 – he had once offered his only biscuit of the day on a prior expedition to his fellow explorer Frank Wild.
As winter arrives in 2020 and deepens into 2021, we will all be faced with leading our teams across the ice and to the safety of spring, and hopefully a vaccine. Whether we can get there with our entire crew depends on effective crisis leadership. But we can draw on the lessons provided by Shackleton and other crisis leaders in the past to guide us in the present.
Author disclosure: I studied the HBS case study “Leadership in Crisis: Ernest Shackleton and the Epic Voyage of the Endurance” as part of a 12-month certificate course in Safety, Quality, Informatics, and Leadership (SQIL) offered by Harvard Medical School.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. HBR’s 10 must reads on leadership. Boston: Harvard Business Review Press, 2011.
2. Lagace M. Shackleton: An entrepreneur of survival. Harvard Business School. Working Knowledge website. Published 2003. Accessed 2020 Nov 19.
3. Koehn N. Leadership lessons from the Shackleton Expedition. The New York Times. 2011 Dec 25.
4. Potier B. Shackleton in business school. Harvard Public Affairs and Communications. The Harvard Gazette website. Published 2004. Accessed 2020 Nov 19.
5. Perkins D. 4 Lessons in crisis leadership from Shackleton’s expedition. In Leadership Essentials by HarpersCollins Leadership. Vol 2020. New York: HarpersCollins, 2020.
As I write this, we are entering the third surge of the COVID-19 pandemic, with new cases, hospitalizations, and deaths from COVID-19 skyrocketing around the country. Worst of all, this surge has been most severely affecting areas of the nation least prepared to handle it (rural) and populations already marginalized by the health care system (Latinx and Black). Despite the onslaught of COVID-19, “pandemic fatigue” has begun to set in amongst colleagues, friends, and family, leading to challenges in adhering to social distancing and other infection-control measures, both at work and home.
In the face of the pandemic’s onslaught, hospitalists – who have faced the brunt of caring for patients with COVID-19, despite the absence of reporting about the subspecialty’s role – are faced with mustering the grit to respond with resolve, coordinated action, and empathy. Luckily, hospitalists are equipped with the very characteristics needed to lead teams, groups, and hospitals through the crisis of this pandemic. Ask yourself, why did you become a hospitalist? If you wanted steady predictability and control, there were many office-based specialties you could have chosen. You chose to become a hospitalist because you seek the challenges of clinical variety, problem-solving, systems improvement, and you are a natural team leader, whether you have been designated as such or not. In the words of John Quincy Adams, “if your actions inspire others to dream more, learn more, do more, and become more, you are a leader.”
As a leader, how can you lead your team through the series of trials and tribulations that this year has thrown at you? From COVID-19 to racism directed against Black and Latinx people to the behavioral health crisis, 2020 has likely made you feel as if you’re stuck in a ghoulish carnival fun house without an exit.
Yet this is where some leaders hit their stride, in what Bennis and Thomas describe as the “crucible of leadership.”1 There are many types of “crucibles of leadership,” according to Bennis and Thomas, and this year has thrown most of these at us: prejudice/bias, physical fatigue and illness, sudden elevation of responsibility to lead new processes, not to mention family stressors. Leaders who succeed in guiding their colleagues through these challenges have manifested critical skills: engaging others in shared meaning, having a distinctive and compelling voice, displaying integrity, and having adaptive capacity.
What exactly is adaptive capacity, the most important of these, in my opinion? Adaptive capacity requires understanding the new context of a crisis and how it has shifted team members’ needs and perceptions. It also requires what Bennis and Thomas call hardiness and what I call grit – the ability to face adversity, get knocked down, get up, and do it again.
There is probably no better example of a crisis leader with extraordinary adaptive capacity than Anglo-Irish explorer Sir Ernest Shackleton. Bitten by the bug of exploration, Shackleton failed at reaching the South Pole (1908-1909) but subsequently attempted to cross the Antarctic, departing South Georgia Island on Dec. 5, 1914. Depressingly for Shackleton, his ship, the Endurance, became stuck in sea ice on Jan. 19, 1915 before even reaching the continent. Drifting with the ice floe, his crew had set up a winter station hoping to be released from the ice later, but the Endurance was crushed by the pressure of sea ice and sank on Nov. 21, 1915. From there, Shackleton hoped to drift north to Paulet Island, 250 miles away, but eventually was forced to take his crew on lifeboats to the nearest land, Elephant Island, 346 miles from where the Endurance sank. He then took five of his men on an open boat, 828-mile journey to South Georgia Island. Encountering hurricane-force winds, the team landed on South Georgia Island 15 days later, only to face a climb of 32 miles over mountainous terrain to reach a whaling station. Shackleton eventually organized his men’s rescue on Elephant Island, reaching them on Aug. 30, 1916, 4½ months after he had set out for South Georgia Island. His entire crew survived, only to have two of them killed later in World War I.
You might consider Shackleton a failure for not even coming close to his original goal, but his success in saving his crew is regarded as the epitome of crisis leadership. As Harvard Business School professor Nancy F. Koehn, PhD, whose case study of Shackleton is one of the most popular at HBS, stated, “He thought he was going to be an entrepreneur of exploration, but he became an entrepreneur of survival.”2 Upon realizing the futility of his original mission, he pivoted immediately to the survival of his crew. “A man must shape himself to a new mark directly the old one goes to ground,” wrote Shackleton in his diary.3
Realizing that preserving his crew’s morale was critical, he maintained the crew’s everyday activities, despite the prospect of dying on the ice. He realized that he needed to keep up his own courage and confidence as well as that of his crew. Despite his ability to share the strategic focus of getting to safety with his men, he didn’t lose sight of day-to-day needs, such as keeping the crew entertained. When he encountered crew members who seemed problematic to his mission goals, he assigned them to his own tent.
Despite the extreme cold, his decision-making did not freeze – he acted decisively. He took risks when he thought appropriate, twice needing to abandon his efforts to drag a lifeboat full of supplies with his men toward the sea. “You can’t be afraid to make smart mistakes,” says Dr. Koehn. “That’s something we have no training in.”4 Most importantly, Shackleton took ultimate responsibility for his men’s survival, never resting until they had all been rescued. And he modeled a culture of shared responsibility for one another5 – he had once offered his only biscuit of the day on a prior expedition to his fellow explorer Frank Wild.
As winter arrives in 2020 and deepens into 2021, we will all be faced with leading our teams across the ice and to the safety of spring, and hopefully a vaccine. Whether we can get there with our entire crew depends on effective crisis leadership. But we can draw on the lessons provided by Shackleton and other crisis leaders in the past to guide us in the present.
Author disclosure: I studied the HBS case study “Leadership in Crisis: Ernest Shackleton and the Epic Voyage of the Endurance” as part of a 12-month certificate course in Safety, Quality, Informatics, and Leadership (SQIL) offered by Harvard Medical School.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
References
1. HBR’s 10 must reads on leadership. Boston: Harvard Business Review Press, 2011.
2. Lagace M. Shackleton: An entrepreneur of survival. Harvard Business School. Working Knowledge website. Published 2003. Accessed 2020 Nov 19.
3. Koehn N. Leadership lessons from the Shackleton Expedition. The New York Times. 2011 Dec 25.
4. Potier B. Shackleton in business school. Harvard Public Affairs and Communications. The Harvard Gazette website. Published 2004. Accessed 2020 Nov 19.
5. Perkins D. 4 Lessons in crisis leadership from Shackleton’s expedition. In Leadership Essentials by HarpersCollins Leadership. Vol 2020. New York: HarpersCollins, 2020.
Two consecutive negative FUBC results clear S. aureus bacteremia
reported Caitlin Cardenas-Comfort, MD, of the section of pediatric infectious diseases at Baylor College of Medicine, Houston, and colleagues.
In a retrospective cohort study of 122 pediatric patients with documented Staphylococcus aureus bacteremia (SAB) that were hospitalized at one of three hospitals in the Texas Children’s Hospital network in Houston, Dr. Cardenas-Comfort and colleagues sought to determine whether specific recommendations can be made on the number of follow-up blood cultures (FUBC) needed to document clearance of SAB. Patients included in the study were under 18 years of age and had confirmed diagnosis of SAB between Jan. 1, and Dec. 31, 2018.
Most cases of bacteremia resolve in under 48 hours
In the majority of cases, patients had bacteremia for less than 48 hours and few to no complications. Only 16% of patients experienced bacteremia lasting 3 or more days, and they had either central line-associated bloodstream infection, endocarditis, or osteomyelitis. In such cases, “patients with endovascular and closed-space infections are at an increased risk of persistent bacteremia,” warranting more conservative monitoring and follow-up, cautioned the researchers.
Although Dr. Cardenas-Comfort and colleagues did note an association between the duration of bacteremia and a diagnosis of infectious disease, increased risk for persistent SAB did not appear to be tied to an underlying medical condition, including immunosuppression.
Fewer than 5% of patients with SAB had intermittent positive cultures and fewer than 1% had repeat positive cultures following two negative FUBC results. For those patients with intermittent positive cultures, the risk of being diagnosed with endocarditis or osteomyelitis is more than double. The authors suggested that “source control could be a critical variable” increasing the risk for intermittent positive cultures, noting that surgical debridement occurred more than 24 hours following initial blood draw for every patient in the osteomyelitis group. In contrast, of those who had consistently negative FUBC results, only 2 of 33 (6%) had debridement in the same period, and only 6 of 33 (18%) required more than one debridement.
Children are less likely to have intermittent positive cultures
Dr. Cardenas-Comfort and colleagues also observed that intermittent positive cultures may appear less frequently in children than adults, consistent with a recent study of adults in which intermittent cultures were found in 13% of 1.071 SAB cases. In just 4% of the cases in that study, more than 2 days of negative blood cultures preceded a repeat positive culture.
The researchers noted several study limitations in their own research. Because more than half (61%) of patients had two or less FUBCs collected, and 21% one or less, they acknowledged that their conclusions are based on the presumption that the 61% of patients would not have any further positive cultures if they had been drawn. Relying on provider documentation also suggested that cases of bacteremia without an identified source also likely were overrepresented. The retrospective nature of the study only allowed for limited collection of standardized follow-up metrics with the limited patient sample available. Patient characteristics also may have affected the quality of study results because a large number of patients had underlying medical conditions or were premature infants.
Look for ongoing hemodynamic instability before third FUBC
Dr. Cardenas-Comfort and colleagues only recommend a third FUBC in cases where patients demonstrate ongoing hemodynamic instability. Applying this to their study population, in retrospect, the authors noted that unnecessary FUBCs could have been prevented in 26% of patients included in the study. They further recommend a thorough clinical evaluation for any patients with SAB lasting 3 or more days with an unidentified infection source. Further research could be beneficial in evaluating cost savings that come from eliminating unnecessary cultures. Additionally, performing a powered analysis would help to determine the probability of an increase in complications based on implementation of these recommendations.
In a separate interview, Tina Q. Tan, MD, infectious disease specialist at Ann & Robert H. Lurie Children’s Hospital of Chicago noted: “This study provides some importance evidence-based guidance on deciding how many blood cultures are needed to demonstrate clearance of S. aureus bacteremia, even in children who have intermittent positive cultures after having negative FUBCs. The recommendation that additional blood cultures to document sterility are not needed after 2 FUBC results are negative in well-appearing children is one that has the potential to decrease cost and unnecessary discomfort in patients. The recommendation currently is for well-appearing children; children who are ill appearing may require further blood cultures to document sterility. Even though this is a single-center study with a relatively small number of patients (n = 122), the information provided is a very useful guide to all clinicians who deal with this issue. Further studies are needed to determine the impact on cost reduction by the elimination of unnecessary blood cultures and whether the rate of complications would increase as a result of not obtaining further cultures in well-appearing children who have two negative follow up blood cultures.”
Dr. Cardenas-Comfort and colleagues as well as Dr. Tan had no conflicts of interest and no relevant financial disclosures. There was no external funding for the study.
SOURCE: Cardenas-Comfort C et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1821.
reported Caitlin Cardenas-Comfort, MD, of the section of pediatric infectious diseases at Baylor College of Medicine, Houston, and colleagues.

In a retrospective cohort study of 122 pediatric patients with documented Staphylococcus aureus bacteremia (SAB) that were hospitalized at one of three hospitals in the Texas Children’s Hospital network in Houston, Dr. Cardenas-Comfort and colleagues sought to determine whether specific recommendations can be made on the number of follow-up blood cultures (FUBC) needed to document clearance of SAB. Patients included in the study were under 18 years of age and had confirmed diagnosis of SAB between Jan. 1, and Dec. 31, 2018.
Most cases of bacteremia resolve in under 48 hours
In the majority of cases, patients had bacteremia for less than 48 hours and few to no complications. Only 16% of patients experienced bacteremia lasting 3 or more days, and they had either central line-associated bloodstream infection, endocarditis, or osteomyelitis. In such cases, “patients with endovascular and closed-space infections are at an increased risk of persistent bacteremia,” warranting more conservative monitoring and follow-up, cautioned the researchers.
Although Dr. Cardenas-Comfort and colleagues did note an association between the duration of bacteremia and a diagnosis of infectious disease, increased risk for persistent SAB did not appear to be tied to an underlying medical condition, including immunosuppression.
Fewer than 5% of patients with SAB had intermittent positive cultures and fewer than 1% had repeat positive cultures following two negative FUBC results. For those patients with intermittent positive cultures, the risk of being diagnosed with endocarditis or osteomyelitis is more than double. The authors suggested that “source control could be a critical variable” increasing the risk for intermittent positive cultures, noting that surgical debridement occurred more than 24 hours following initial blood draw for every patient in the osteomyelitis group. In contrast, of those who had consistently negative FUBC results, only 2 of 33 (6%) had debridement in the same period, and only 6 of 33 (18%) required more than one debridement.
Children are less likely to have intermittent positive cultures
Dr. Cardenas-Comfort and colleagues also observed that intermittent positive cultures may appear less frequently in children than adults, consistent with a recent study of adults in which intermittent cultures were found in 13% of 1.071 SAB cases. In just 4% of the cases in that study, more than 2 days of negative blood cultures preceded a repeat positive culture.
The researchers noted several study limitations in their own research. Because more than half (61%) of patients had two or less FUBCs collected, and 21% one or less, they acknowledged that their conclusions are based on the presumption that the 61% of patients would not have any further positive cultures if they had been drawn. Relying on provider documentation also suggested that cases of bacteremia without an identified source also likely were overrepresented. The retrospective nature of the study only allowed for limited collection of standardized follow-up metrics with the limited patient sample available. Patient characteristics also may have affected the quality of study results because a large number of patients had underlying medical conditions or were premature infants.
Look for ongoing hemodynamic instability before third FUBC
Dr. Cardenas-Comfort and colleagues only recommend a third FUBC in cases where patients demonstrate ongoing hemodynamic instability. Applying this to their study population, in retrospect, the authors noted that unnecessary FUBCs could have been prevented in 26% of patients included in the study. They further recommend a thorough clinical evaluation for any patients with SAB lasting 3 or more days with an unidentified infection source. Further research could be beneficial in evaluating cost savings that come from eliminating unnecessary cultures. Additionally, performing a powered analysis would help to determine the probability of an increase in complications based on implementation of these recommendations.
In a separate interview, Tina Q. Tan, MD, infectious disease specialist at Ann & Robert H. Lurie Children’s Hospital of Chicago noted: “This study provides some importance evidence-based guidance on deciding how many blood cultures are needed to demonstrate clearance of S. aureus bacteremia, even in children who have intermittent positive cultures after having negative FUBCs. The recommendation that additional blood cultures to document sterility are not needed after 2 FUBC results are negative in well-appearing children is one that has the potential to decrease cost and unnecessary discomfort in patients. The recommendation currently is for well-appearing children; children who are ill appearing may require further blood cultures to document sterility. Even though this is a single-center study with a relatively small number of patients (n = 122), the information provided is a very useful guide to all clinicians who deal with this issue. Further studies are needed to determine the impact on cost reduction by the elimination of unnecessary blood cultures and whether the rate of complications would increase as a result of not obtaining further cultures in well-appearing children who have two negative follow up blood cultures.”
Dr. Cardenas-Comfort and colleagues as well as Dr. Tan had no conflicts of interest and no relevant financial disclosures. There was no external funding for the study.
SOURCE: Cardenas-Comfort C et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1821.
reported Caitlin Cardenas-Comfort, MD, of the section of pediatric infectious diseases at Baylor College of Medicine, Houston, and colleagues.

In a retrospective cohort study of 122 pediatric patients with documented Staphylococcus aureus bacteremia (SAB) that were hospitalized at one of three hospitals in the Texas Children’s Hospital network in Houston, Dr. Cardenas-Comfort and colleagues sought to determine whether specific recommendations can be made on the number of follow-up blood cultures (FUBC) needed to document clearance of SAB. Patients included in the study were under 18 years of age and had confirmed diagnosis of SAB between Jan. 1, and Dec. 31, 2018.
Most cases of bacteremia resolve in under 48 hours
In the majority of cases, patients had bacteremia for less than 48 hours and few to no complications. Only 16% of patients experienced bacteremia lasting 3 or more days, and they had either central line-associated bloodstream infection, endocarditis, or osteomyelitis. In such cases, “patients with endovascular and closed-space infections are at an increased risk of persistent bacteremia,” warranting more conservative monitoring and follow-up, cautioned the researchers.
Although Dr. Cardenas-Comfort and colleagues did note an association between the duration of bacteremia and a diagnosis of infectious disease, increased risk for persistent SAB did not appear to be tied to an underlying medical condition, including immunosuppression.
Fewer than 5% of patients with SAB had intermittent positive cultures and fewer than 1% had repeat positive cultures following two negative FUBC results. For those patients with intermittent positive cultures, the risk of being diagnosed with endocarditis or osteomyelitis is more than double. The authors suggested that “source control could be a critical variable” increasing the risk for intermittent positive cultures, noting that surgical debridement occurred more than 24 hours following initial blood draw for every patient in the osteomyelitis group. In contrast, of those who had consistently negative FUBC results, only 2 of 33 (6%) had debridement in the same period, and only 6 of 33 (18%) required more than one debridement.
Children are less likely to have intermittent positive cultures
Dr. Cardenas-Comfort and colleagues also observed that intermittent positive cultures may appear less frequently in children than adults, consistent with a recent study of adults in which intermittent cultures were found in 13% of 1.071 SAB cases. In just 4% of the cases in that study, more than 2 days of negative blood cultures preceded a repeat positive culture.
The researchers noted several study limitations in their own research. Because more than half (61%) of patients had two or less FUBCs collected, and 21% one or less, they acknowledged that their conclusions are based on the presumption that the 61% of patients would not have any further positive cultures if they had been drawn. Relying on provider documentation also suggested that cases of bacteremia without an identified source also likely were overrepresented. The retrospective nature of the study only allowed for limited collection of standardized follow-up metrics with the limited patient sample available. Patient characteristics also may have affected the quality of study results because a large number of patients had underlying medical conditions or were premature infants.
Look for ongoing hemodynamic instability before third FUBC
Dr. Cardenas-Comfort and colleagues only recommend a third FUBC in cases where patients demonstrate ongoing hemodynamic instability. Applying this to their study population, in retrospect, the authors noted that unnecessary FUBCs could have been prevented in 26% of patients included in the study. They further recommend a thorough clinical evaluation for any patients with SAB lasting 3 or more days with an unidentified infection source. Further research could be beneficial in evaluating cost savings that come from eliminating unnecessary cultures. Additionally, performing a powered analysis would help to determine the probability of an increase in complications based on implementation of these recommendations.
In a separate interview, Tina Q. Tan, MD, infectious disease specialist at Ann & Robert H. Lurie Children’s Hospital of Chicago noted: “This study provides some importance evidence-based guidance on deciding how many blood cultures are needed to demonstrate clearance of S. aureus bacteremia, even in children who have intermittent positive cultures after having negative FUBCs. The recommendation that additional blood cultures to document sterility are not needed after 2 FUBC results are negative in well-appearing children is one that has the potential to decrease cost and unnecessary discomfort in patients. The recommendation currently is for well-appearing children; children who are ill appearing may require further blood cultures to document sterility. Even though this is a single-center study with a relatively small number of patients (n = 122), the information provided is a very useful guide to all clinicians who deal with this issue. Further studies are needed to determine the impact on cost reduction by the elimination of unnecessary blood cultures and whether the rate of complications would increase as a result of not obtaining further cultures in well-appearing children who have two negative follow up blood cultures.”
Dr. Cardenas-Comfort and colleagues as well as Dr. Tan had no conflicts of interest and no relevant financial disclosures. There was no external funding for the study.
SOURCE: Cardenas-Comfort C et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1821.
FROM PEDIATRICS
Diabetic retinopathy may predict greater risk of COVID-19 severity
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.