User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Moderna COVID-19 vaccine wins decisive recommendation from FDA panel
The US Food and Drug Administration (FDA) put Moderna’s application before its Vaccines and Related Biological Products Advisory Committee. The panel voted 20-0 on this question: “Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?” There was one abstention.
The FDA is not bound to act on the recommendations of its advisers, but the agency usually takes the panel’s advice. The FDA cleared the similar Pfizer-BioNTech vaccine on December 11 through an emergency use authorization (EUA), following a positive vote for the product at a December 10 advisory committee meeting. In this case, the FDA staff appeared to be pushing for a broad endorsement of the Moderna vaccine, for which the agency appears likely to soon also grant an EUA.
Marion Gruber, PhD, director of the Office of Vaccines Research and Review at FDA’s Center for Biologics Evaluation and Research, earlier rebuffed attempts by some of the panelists to alter the voting question. Some panelists wanted to make tweaks, including a rephrasing to underscore the limited nature of an EUA, compared with a more complete approval through the biologics license application (BLA) process.
FDA panelist Michael Kurilla, MD, PhD, of the National Institutes of Health was the only panelist to abstain from voting. He said he was uncomfortable with the phrasing of the question.
“In the midst of a pandemic and with limited vaccine supply available, a blanket statement for individuals 18 years and older is just too broad,” he said. “I’m not convinced that for all of those age groups the benefits do actually outweigh the risks.”
In general, though, there was strong support for Moderna’s vaccine. FDA panelist James Hildreth Sr, MD, PhD, of Meharry Medical College in Nashville, Tennessee spoke of the “remarkable achievement” seen in having two vaccines ready for clearance by December for a virus that only emerged as a threat this year.
Study data indicate the primary efficacy endpoint demonstrated vaccine efficacy (VE) of 94.1% (95% CI, 89.3% - 96.8%) for the Moderna vaccine, with 11 COVID-19 cases in the vaccine group and 185 COVID-19 cases in the placebo group, the FDA staff noted during the meeting.
The advisers and FDA staff also honed in on several key issues with COVID-19 vaccines, including the challenge of having people in the placebo groups of studies seek to get cleared vaccines. Also of concern to the panel were early reports of allergic reactions seen with the Pfizer product.
Doran L. Fink, MD, PhD, an FDA official who has been closely involved with the COVID-19 vaccines, told the panel that two healthcare workers in Alaska had allergic reactions minutes after receiving the Pfizer vaccine, one of which was a case of anaphylactic reaction that resulted in hospitalization.
In the United Kingdom, there were two cases reported of notable allergic reactions, leading regulators there to issue a warning that people who have a history of significant allergic reactions should not currently receive the Pfizer-BioNTech vaccine.
The people involved in these incidents have recovered or are recovering, Fink said. But the FDA expects there will be additional reports of allergic reactions to COVID-19 vaccines.
“These cases underscores the need to remain vigilant during the early phase of the vaccination campaign,” Fink said. “To this end, FDA is working with Pfizer to further revise factsheets and prescribing information for their vaccine to draw attention to CDC guidelines for post- vaccination monitoring and management of immediate allergic reactions.”
mRNA vaccines in the lead
An FDA emergency clearance for Moderna’s product would be another vote of confidence in a new approach to making vaccines. Both the Pfizer-BioNTech and Moderna vaccines provide the immune system with a kind of blueprint in the form of genetic material, mRNA. The mRNA sets the stage for the synthesis of the signature spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells.
In a December 15 commentary for this news organization Michael E. Pichichero, MD, wrote that the “revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced.”
“This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab — and it can be done incredibly fast,” he wrote.
The FDA allowed one waiver for panelist James K. Hildreth in connection with his personal relationship to a trial participant and his university’s participation in vaccine testing.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) put Moderna’s application before its Vaccines and Related Biological Products Advisory Committee. The panel voted 20-0 on this question: “Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?” There was one abstention.
The FDA is not bound to act on the recommendations of its advisers, but the agency usually takes the panel’s advice. The FDA cleared the similar Pfizer-BioNTech vaccine on December 11 through an emergency use authorization (EUA), following a positive vote for the product at a December 10 advisory committee meeting. In this case, the FDA staff appeared to be pushing for a broad endorsement of the Moderna vaccine, for which the agency appears likely to soon also grant an EUA.
Marion Gruber, PhD, director of the Office of Vaccines Research and Review at FDA’s Center for Biologics Evaluation and Research, earlier rebuffed attempts by some of the panelists to alter the voting question. Some panelists wanted to make tweaks, including a rephrasing to underscore the limited nature of an EUA, compared with a more complete approval through the biologics license application (BLA) process.
FDA panelist Michael Kurilla, MD, PhD, of the National Institutes of Health was the only panelist to abstain from voting. He said he was uncomfortable with the phrasing of the question.
“In the midst of a pandemic and with limited vaccine supply available, a blanket statement for individuals 18 years and older is just too broad,” he said. “I’m not convinced that for all of those age groups the benefits do actually outweigh the risks.”
In general, though, there was strong support for Moderna’s vaccine. FDA panelist James Hildreth Sr, MD, PhD, of Meharry Medical College in Nashville, Tennessee spoke of the “remarkable achievement” seen in having two vaccines ready for clearance by December for a virus that only emerged as a threat this year.
Study data indicate the primary efficacy endpoint demonstrated vaccine efficacy (VE) of 94.1% (95% CI, 89.3% - 96.8%) for the Moderna vaccine, with 11 COVID-19 cases in the vaccine group and 185 COVID-19 cases in the placebo group, the FDA staff noted during the meeting.
The advisers and FDA staff also honed in on several key issues with COVID-19 vaccines, including the challenge of having people in the placebo groups of studies seek to get cleared vaccines. Also of concern to the panel were early reports of allergic reactions seen with the Pfizer product.
Doran L. Fink, MD, PhD, an FDA official who has been closely involved with the COVID-19 vaccines, told the panel that two healthcare workers in Alaska had allergic reactions minutes after receiving the Pfizer vaccine, one of which was a case of anaphylactic reaction that resulted in hospitalization.
In the United Kingdom, there were two cases reported of notable allergic reactions, leading regulators there to issue a warning that people who have a history of significant allergic reactions should not currently receive the Pfizer-BioNTech vaccine.
The people involved in these incidents have recovered or are recovering, Fink said. But the FDA expects there will be additional reports of allergic reactions to COVID-19 vaccines.
“These cases underscores the need to remain vigilant during the early phase of the vaccination campaign,” Fink said. “To this end, FDA is working with Pfizer to further revise factsheets and prescribing information for their vaccine to draw attention to CDC guidelines for post- vaccination monitoring and management of immediate allergic reactions.”
mRNA vaccines in the lead
An FDA emergency clearance for Moderna’s product would be another vote of confidence in a new approach to making vaccines. Both the Pfizer-BioNTech and Moderna vaccines provide the immune system with a kind of blueprint in the form of genetic material, mRNA. The mRNA sets the stage for the synthesis of the signature spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells.
In a December 15 commentary for this news organization Michael E. Pichichero, MD, wrote that the “revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced.”
“This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab — and it can be done incredibly fast,” he wrote.
The FDA allowed one waiver for panelist James K. Hildreth in connection with his personal relationship to a trial participant and his university’s participation in vaccine testing.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) put Moderna’s application before its Vaccines and Related Biological Products Advisory Committee. The panel voted 20-0 on this question: “Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?” There was one abstention.
The FDA is not bound to act on the recommendations of its advisers, but the agency usually takes the panel’s advice. The FDA cleared the similar Pfizer-BioNTech vaccine on December 11 through an emergency use authorization (EUA), following a positive vote for the product at a December 10 advisory committee meeting. In this case, the FDA staff appeared to be pushing for a broad endorsement of the Moderna vaccine, for which the agency appears likely to soon also grant an EUA.
Marion Gruber, PhD, director of the Office of Vaccines Research and Review at FDA’s Center for Biologics Evaluation and Research, earlier rebuffed attempts by some of the panelists to alter the voting question. Some panelists wanted to make tweaks, including a rephrasing to underscore the limited nature of an EUA, compared with a more complete approval through the biologics license application (BLA) process.
FDA panelist Michael Kurilla, MD, PhD, of the National Institutes of Health was the only panelist to abstain from voting. He said he was uncomfortable with the phrasing of the question.
“In the midst of a pandemic and with limited vaccine supply available, a blanket statement for individuals 18 years and older is just too broad,” he said. “I’m not convinced that for all of those age groups the benefits do actually outweigh the risks.”
In general, though, there was strong support for Moderna’s vaccine. FDA panelist James Hildreth Sr, MD, PhD, of Meharry Medical College in Nashville, Tennessee spoke of the “remarkable achievement” seen in having two vaccines ready for clearance by December for a virus that only emerged as a threat this year.
Study data indicate the primary efficacy endpoint demonstrated vaccine efficacy (VE) of 94.1% (95% CI, 89.3% - 96.8%) for the Moderna vaccine, with 11 COVID-19 cases in the vaccine group and 185 COVID-19 cases in the placebo group, the FDA staff noted during the meeting.
The advisers and FDA staff also honed in on several key issues with COVID-19 vaccines, including the challenge of having people in the placebo groups of studies seek to get cleared vaccines. Also of concern to the panel were early reports of allergic reactions seen with the Pfizer product.
Doran L. Fink, MD, PhD, an FDA official who has been closely involved with the COVID-19 vaccines, told the panel that two healthcare workers in Alaska had allergic reactions minutes after receiving the Pfizer vaccine, one of which was a case of anaphylactic reaction that resulted in hospitalization.
In the United Kingdom, there were two cases reported of notable allergic reactions, leading regulators there to issue a warning that people who have a history of significant allergic reactions should not currently receive the Pfizer-BioNTech vaccine.
The people involved in these incidents have recovered or are recovering, Fink said. But the FDA expects there will be additional reports of allergic reactions to COVID-19 vaccines.
“These cases underscores the need to remain vigilant during the early phase of the vaccination campaign,” Fink said. “To this end, FDA is working with Pfizer to further revise factsheets and prescribing information for their vaccine to draw attention to CDC guidelines for post- vaccination monitoring and management of immediate allergic reactions.”
mRNA vaccines in the lead
An FDA emergency clearance for Moderna’s product would be another vote of confidence in a new approach to making vaccines. Both the Pfizer-BioNTech and Moderna vaccines provide the immune system with a kind of blueprint in the form of genetic material, mRNA. The mRNA sets the stage for the synthesis of the signature spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells.
In a December 15 commentary for this news organization Michael E. Pichichero, MD, wrote that the “revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced.”
“This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab — and it can be done incredibly fast,” he wrote.
The FDA allowed one waiver for panelist James K. Hildreth in connection with his personal relationship to a trial participant and his university’s participation in vaccine testing.
This article first appeared on Medscape.com.
COVID-19 ranks as a leading cause of death in United States
Adults over age 45 were more likely to die from COVID-19 than car crashes, respiratory diseases, drug overdoses, and suicide. And those over age 55 faced even higher rates of dying because of the coronavirus.
“The current exponential increase in COVID-19 is reaching a calamitous scale in the U.S.,” the authors wrote. “Putting these numbers in perspective may be difficult.”
Population health researchers at Virginia Commonwealth University put COVID-19 deaths into context by comparing this year’s numbers to the leading causes of death for March through October 2018, sorting by age.
By October 2020, COVID-19 had become the third leading cause of death overall for those between the ages of 45 and 84 years, following after heart disease and cancer. For those over age 85, COVID-19 was the second leading cause of death, surpassing cancer and following behind heart disease.
For people aged 35-44 years, COVID-19 surpassed car crashes and respiratory diseases and was slightly lower than suicide, heart disease, and cancer. For those under age 35, drug overdoses, suicide, and car crashes remained the leading causes of death.
Importantly, the authors wrote, death rates for the two leading causes – heart disease and cancer – are about 1,700 and 1,600 per day, respectively. COVID-19 deaths have surpassed these numbers individually throughout December and, on Wednesday, beat them combined. More than 3,400 deaths were reported, according to the COVID Tracking Project, marking an all-time high that continues to increase. Hospitalizations were also at a new high, with more than 113,000 COVID-19 patients in hospitals across the country, and another 232,000 new cases were reported.
“With COVID-19 mortality rates now exceeding these thresholds, this infectious disease has become deadlier than heart disease and cancer,” the authors wrote. “Its lethality may increase further as transmission increases with holiday travel and gatherings and with the intensified indoor exposure that winter brings.”
The reported number of COVID-19 deaths is likely a 20% underestimate, they wrote, attributable to delays in reporting and an increase in non–COVID-19 deaths that were undetected and untreated because of pandemic-related disruptions. Since the coronavirus is communicable and spreads easily, COVID-19 deaths are particularly unique and worrying, they said.
“Individuals who die from homicide or cancer do not transmit the risk of morbidity and mortality to those nearby,” they wrote. “Every COVID-19 death signals the possibility of more deaths among close contacts.”
The fall surge in cases and deaths is widespread nationally, as compared to the spring, with hot spots on both coasts and in rural areas, according to an accompanying editorial in JAMA from public health researchers at the Harvard T.H. Chan School of Public Health, Boston. People of color have faced twice the death rate as well, with one in 875 Black people and one in 925 Indigenous people dying from COVID-19, as compared with one in 1,625 White people.
“The year 2020 ends with COVID-19 massively surging, as it was in the spring, to be the leading cause of death,” they wrote. “The accelerating numbers of deaths fall far short of fully capturing each devastating human story: Every death represents untold loss for countless families.”
Vaccines offer hope, they said, but won’t prevent the upcoming increase in COVID-19 hospitalizations and deaths this winter. In 2021, containing the pandemic will require national coordination, resources to help overwhelmed health care workers, new support for state and local public health officials, a stimulus package for schools and businesses, and financial aid for people on the brink of eviction. The country needs federal coordination of testing, contact tracing, personal protective equipment, travel precautions, and a face mask mandate, they wrote.
“Ending this crisis will require not only further advances in treatment but also unprecedented commitment to all aspects of prevention, vaccination, and public health,” they wrote. “Only by doing so can future years see this illness revert back to the unfamiliar and unknown condition it once was.”
This article first appeared on WebMD.com.
Adults over age 45 were more likely to die from COVID-19 than car crashes, respiratory diseases, drug overdoses, and suicide. And those over age 55 faced even higher rates of dying because of the coronavirus.
“The current exponential increase in COVID-19 is reaching a calamitous scale in the U.S.,” the authors wrote. “Putting these numbers in perspective may be difficult.”
Population health researchers at Virginia Commonwealth University put COVID-19 deaths into context by comparing this year’s numbers to the leading causes of death for March through October 2018, sorting by age.
By October 2020, COVID-19 had become the third leading cause of death overall for those between the ages of 45 and 84 years, following after heart disease and cancer. For those over age 85, COVID-19 was the second leading cause of death, surpassing cancer and following behind heart disease.
For people aged 35-44 years, COVID-19 surpassed car crashes and respiratory diseases and was slightly lower than suicide, heart disease, and cancer. For those under age 35, drug overdoses, suicide, and car crashes remained the leading causes of death.
Importantly, the authors wrote, death rates for the two leading causes – heart disease and cancer – are about 1,700 and 1,600 per day, respectively. COVID-19 deaths have surpassed these numbers individually throughout December and, on Wednesday, beat them combined. More than 3,400 deaths were reported, according to the COVID Tracking Project, marking an all-time high that continues to increase. Hospitalizations were also at a new high, with more than 113,000 COVID-19 patients in hospitals across the country, and another 232,000 new cases were reported.
“With COVID-19 mortality rates now exceeding these thresholds, this infectious disease has become deadlier than heart disease and cancer,” the authors wrote. “Its lethality may increase further as transmission increases with holiday travel and gatherings and with the intensified indoor exposure that winter brings.”
The reported number of COVID-19 deaths is likely a 20% underestimate, they wrote, attributable to delays in reporting and an increase in non–COVID-19 deaths that were undetected and untreated because of pandemic-related disruptions. Since the coronavirus is communicable and spreads easily, COVID-19 deaths are particularly unique and worrying, they said.
“Individuals who die from homicide or cancer do not transmit the risk of morbidity and mortality to those nearby,” they wrote. “Every COVID-19 death signals the possibility of more deaths among close contacts.”
The fall surge in cases and deaths is widespread nationally, as compared to the spring, with hot spots on both coasts and in rural areas, according to an accompanying editorial in JAMA from public health researchers at the Harvard T.H. Chan School of Public Health, Boston. People of color have faced twice the death rate as well, with one in 875 Black people and one in 925 Indigenous people dying from COVID-19, as compared with one in 1,625 White people.
“The year 2020 ends with COVID-19 massively surging, as it was in the spring, to be the leading cause of death,” they wrote. “The accelerating numbers of deaths fall far short of fully capturing each devastating human story: Every death represents untold loss for countless families.”
Vaccines offer hope, they said, but won’t prevent the upcoming increase in COVID-19 hospitalizations and deaths this winter. In 2021, containing the pandemic will require national coordination, resources to help overwhelmed health care workers, new support for state and local public health officials, a stimulus package for schools and businesses, and financial aid for people on the brink of eviction. The country needs federal coordination of testing, contact tracing, personal protective equipment, travel precautions, and a face mask mandate, they wrote.
“Ending this crisis will require not only further advances in treatment but also unprecedented commitment to all aspects of prevention, vaccination, and public health,” they wrote. “Only by doing so can future years see this illness revert back to the unfamiliar and unknown condition it once was.”
This article first appeared on WebMD.com.
Adults over age 45 were more likely to die from COVID-19 than car crashes, respiratory diseases, drug overdoses, and suicide. And those over age 55 faced even higher rates of dying because of the coronavirus.
“The current exponential increase in COVID-19 is reaching a calamitous scale in the U.S.,” the authors wrote. “Putting these numbers in perspective may be difficult.”
Population health researchers at Virginia Commonwealth University put COVID-19 deaths into context by comparing this year’s numbers to the leading causes of death for March through October 2018, sorting by age.
By October 2020, COVID-19 had become the third leading cause of death overall for those between the ages of 45 and 84 years, following after heart disease and cancer. For those over age 85, COVID-19 was the second leading cause of death, surpassing cancer and following behind heart disease.
For people aged 35-44 years, COVID-19 surpassed car crashes and respiratory diseases and was slightly lower than suicide, heart disease, and cancer. For those under age 35, drug overdoses, suicide, and car crashes remained the leading causes of death.
Importantly, the authors wrote, death rates for the two leading causes – heart disease and cancer – are about 1,700 and 1,600 per day, respectively. COVID-19 deaths have surpassed these numbers individually throughout December and, on Wednesday, beat them combined. More than 3,400 deaths were reported, according to the COVID Tracking Project, marking an all-time high that continues to increase. Hospitalizations were also at a new high, with more than 113,000 COVID-19 patients in hospitals across the country, and another 232,000 new cases were reported.
“With COVID-19 mortality rates now exceeding these thresholds, this infectious disease has become deadlier than heart disease and cancer,” the authors wrote. “Its lethality may increase further as transmission increases with holiday travel and gatherings and with the intensified indoor exposure that winter brings.”
The reported number of COVID-19 deaths is likely a 20% underestimate, they wrote, attributable to delays in reporting and an increase in non–COVID-19 deaths that were undetected and untreated because of pandemic-related disruptions. Since the coronavirus is communicable and spreads easily, COVID-19 deaths are particularly unique and worrying, they said.
“Individuals who die from homicide or cancer do not transmit the risk of morbidity and mortality to those nearby,” they wrote. “Every COVID-19 death signals the possibility of more deaths among close contacts.”
The fall surge in cases and deaths is widespread nationally, as compared to the spring, with hot spots on both coasts and in rural areas, according to an accompanying editorial in JAMA from public health researchers at the Harvard T.H. Chan School of Public Health, Boston. People of color have faced twice the death rate as well, with one in 875 Black people and one in 925 Indigenous people dying from COVID-19, as compared with one in 1,625 White people.
“The year 2020 ends with COVID-19 massively surging, as it was in the spring, to be the leading cause of death,” they wrote. “The accelerating numbers of deaths fall far short of fully capturing each devastating human story: Every death represents untold loss for countless families.”
Vaccines offer hope, they said, but won’t prevent the upcoming increase in COVID-19 hospitalizations and deaths this winter. In 2021, containing the pandemic will require national coordination, resources to help overwhelmed health care workers, new support for state and local public health officials, a stimulus package for schools and businesses, and financial aid for people on the brink of eviction. The country needs federal coordination of testing, contact tracing, personal protective equipment, travel precautions, and a face mask mandate, they wrote.
“Ending this crisis will require not only further advances in treatment but also unprecedented commitment to all aspects of prevention, vaccination, and public health,” they wrote. “Only by doing so can future years see this illness revert back to the unfamiliar and unknown condition it once was.”
This article first appeared on WebMD.com.
13 best practices to increase hospitalist billing efficiency
As an aspiring physician, I like learning about how things work. Since medical students learn very little about the “business” of medicine in school, this led me to pioneer a project on missed billing by hospitalists at a medium-sized hospital in the northeastern US. Although hospitalists do a tremendous amount of work, they do not always bill for what they are doing. The question became: Why are hospitalists missing charges and what can we do to stop it?
Shortly into my study, I recognized there was little daily communication between the administrators and the hospitalists; neither the hospitalists nor administrators understood the different dynamics that the others faced in their own workplace. It became apparent that administrators needed to learn what was important to hospitalists and to address them at their level in order to bring about change.
Some trending themes emerged as I started shadowing the hospitalists. Many of them asked how this project would benefit them. They argued that administrative needs should be dealt with at the administrative level. A major point was made that current incentives, such as the bonuses given for exceeding a certain number of RVUs, were not the motivating force behind their work ethics. From my observations, the motivating factors were the quality of their patient care, the needs of their patients, and teaching. The hospitalists also were eager to teach and continually instructed me on clinical skills and how to be a better medical student.
Bonuses or notoriety didn’t seem to be the main incentives for them. However, efficiency – especially in rounding – was important, and that became the focal point of the project. I found several studies that showed that improvements in aspects of rounding led to increased quality of patient care, decreased burnout, increased patient satisfaction, and decreased workload and discussed some of those findings with the hospitalists.1-10 When the hospitalists felt that their concerns were being heard, they became even more involved in the project, and the administrators and hospitalists started working together as a team.
One hospitalist spent two hours helping me design the platform that would be used for hospitalists to report barriers in their rounding process that may cause them to miss a charge. Once we identified those barriers, we discussed the possibility of standardizing their workflow based off these data. Many hospitalists argued that each physician has unique skills and practices that make them successful; therefore, the disruption of an already established workflow may cause a decrease in efficiency.
The hospitalists and I talked a lot about the importance of them rounding more efficiently and how that could positively affect the time that they have with their patients and themselves. We discussed that due to the additional work missed billing causes, minimizing this burden can possibly help decrease burnout. As a result, seven hospitalists, the administrative staff, and I met and created thirteen best practices, six of which they were able to get approved to use immediately. To note, hospitalists bill differently; some use a software company, fill out paper forms still or have integration within their EMR. Although these solutions were made for a program which has the ability to bill within the EMR, many of the principles will apply to your program too.
The 13 best practices that the seven hospitalists agreed upon are the following:
When a doctor signs a note, it opens a charge option or there is a hard stop.
Charge delinquencies are sent via email to the hospitalist.
Standardize that hospitalists charge directly after writing a note consistently as part of their workflow.*
Prioritize discharges before rounding.*
Standardize the use of the “my prof charges” column, a feature of this hospital’s EMR system that tells them if they had made a charge to a patient or not, in order to remind them to/confirm billing a patient.*
Create reports by the EMR system to provide charge data for individual providers.
Create a report for bill vs note to help providers self-audit. At this hospital, this feature was offered to the administrators as a way to audit their providers and doctors.
Ensure that when a patient is seen by a physician hospitalist as well as an NP/PA hospitalist, the appropriate charge for the physician is entered.
Notifications get sent to the physician hospitalist if a charge gets deleted by another person (e.g., NP/PA hospitalist).
Handoff of daily rounding sheets, or a paper copy of the patients assigned to a hospitalist for his/her shift, at the end of the shift to the project specialist.*
To keep the rounding sheets a complete and accurate account of the patients seen by the hospitalist.*
Hospitalists are to complete and check all billing at the end of their shift at the latest.*
Hospitalists are to participate on Provider Efficiency Training to optimize workflow, by creating more efficient note-writing behavior using Dragon.
*Indicates the practices the hospitalists were able to implement immediately. Practices 1, 2, 6, 7, and 9 request EMR changes. Practice 8 was already an established practice the hospitalists wished to continue. Practice 13 was suggested by the Lean Director for the continuation of a previous project.
Six of the best practices were easier to implement right away because they were at the discretion of the hospitalists. We found that the hospitalists who had the highest billing performances were more likely to start writing notes and charge earlier while rounding. Those who had poorer billing performances were more likely to leave all note writing and billing towards the end of their shift. The few exceptions (hospitalists who left all note writing and charging to the end of their shift yet had high billing performances) were found to have a consistent and standardized workflow. This was unlike the hospitalists who had the lowest billing performances. Having practices that help remind hospitalists to bill will surely help prevent missed billing, but because of the findings from this project, it was important to have consistent and standardized practices to additionally improve missed billing.
When we followed up with the hospitalist division two months later, we learned they were making great progress. Not only were hospitalists using their best practices, but in working with the administrators, they were designing sessions to further educate fellow hospitalists to prevent further missed billing. These sessions outlined shortcuts, resources and ways hospitalists may modify their personal EMR accounts to prevent missed billing. None of the progress could have been made without first understanding and addressing what is truly important to the hospitalists.
In summary, we noted these general observations in this project:
- Hospitalists favor solutions that benefit them or their patients.
- Hospitalists want to be part of the solution process.
- Hospitalists were more likely to accept ideas to improve their rounding if it meant they could keep their routine.
Obstacles exist in our health care system that prevent administrators and hospitalists from working together as a team. The more we are able to communicate and collaborate to fix problems in the health system, the more we can use the system to our mutual advantage. With the ongoing changes in medicine, especially during uncertain times, better communication needs be a major priority to affect positive change.
Ms. Mirabella attends the Frank H. Netter MD School of Medicine at Quinnipiac University, Hamden, Conn., in the class of 2022. She has interests in internal/hospital medicine, primary care, and health management and leadership. Dr. Rosenberg is associate professor at the Frank H. Netter MD School of Medicine at Quinnipiac University where she is director of clinical skills coaching. Dr. Kiassat is associate dean of the School of Engineering and associate clinical professor at Frank H. Netter MD School of Medicine, at Quinnipiac University. His research interests are in process improvement in health care, using Lean Six Sigma.
References
1. Burdick K, et al. Bedside interprofessional rounding. J Patient Exp. 2017;4(1):22-27. doi: 10.1177/2374373517692910.
2. Patel CR. Improving communication between hospitalists and consultants. The Hospital Leader. 2018. https://thehospitalleader.org/improving-communication-between-hospitalists-and-consultants/.
3. Adams TN, et al. Hospitalist perspective of interactions with medicine subspecialty consult services. J Hosp Med. 2018;13(5):318-323. doi: 10.12788/jhm.2882.
4. Michtalik HJ, et al. Impact of attending physician workload on patient care: A survey of hospitalists. JAMA Intern Med. 2013;173(5):375-377. doi: 10.1001/jamainternmed.2013.1864.
5. Chandra R, et al. How hospitalists can improve efficiency on inpatient wards. The Hospitalist. 2014. https://www.the-hospitalist.org/hospitalist/article/126231/how-hospitalists-can-improve-efficiency-inpatient-wards.
6. Chand DV. Observational study using the tools of lean six sigma to improve the efficiency of the resident rounding process. J Grad Med Educ. 2011;3(2):144-150. doi: 10.4300/JGME-D-10-00116.1.
7. O’Leary KJ, et al. How hospitalists spend their time: Insights on efficiency and safety. J Hosp Med. 2006;1(2):88-93. doi: 10.1002/jhm.88.
8. Wachter RM. Hospitalist workload: The search for the magic number. JAMA Intern Med. 2014;174(5):794-795. doi: 10.1001/jamainternmed.2014.18.
9. Bryson C, et al. Geographical assignment of hospitalists in an urban teaching hospital: Feasibility and impact on efficiency and provider satisfaction. Hospital Practice. 2017;45(4):135-142. doi: 10.1080/21548331.2017.1353884.
10. Calderon AS, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014 Dec;6(4):750-5. doi: 10.4300/JGME-D-13-00324.1.
As an aspiring physician, I like learning about how things work. Since medical students learn very little about the “business” of medicine in school, this led me to pioneer a project on missed billing by hospitalists at a medium-sized hospital in the northeastern US. Although hospitalists do a tremendous amount of work, they do not always bill for what they are doing. The question became: Why are hospitalists missing charges and what can we do to stop it?
Shortly into my study, I recognized there was little daily communication between the administrators and the hospitalists; neither the hospitalists nor administrators understood the different dynamics that the others faced in their own workplace. It became apparent that administrators needed to learn what was important to hospitalists and to address them at their level in order to bring about change.
Some trending themes emerged as I started shadowing the hospitalists. Many of them asked how this project would benefit them. They argued that administrative needs should be dealt with at the administrative level. A major point was made that current incentives, such as the bonuses given for exceeding a certain number of RVUs, were not the motivating force behind their work ethics. From my observations, the motivating factors were the quality of their patient care, the needs of their patients, and teaching. The hospitalists also were eager to teach and continually instructed me on clinical skills and how to be a better medical student.
Bonuses or notoriety didn’t seem to be the main incentives for them. However, efficiency – especially in rounding – was important, and that became the focal point of the project. I found several studies that showed that improvements in aspects of rounding led to increased quality of patient care, decreased burnout, increased patient satisfaction, and decreased workload and discussed some of those findings with the hospitalists.1-10 When the hospitalists felt that their concerns were being heard, they became even more involved in the project, and the administrators and hospitalists started working together as a team.
One hospitalist spent two hours helping me design the platform that would be used for hospitalists to report barriers in their rounding process that may cause them to miss a charge. Once we identified those barriers, we discussed the possibility of standardizing their workflow based off these data. Many hospitalists argued that each physician has unique skills and practices that make them successful; therefore, the disruption of an already established workflow may cause a decrease in efficiency.
The hospitalists and I talked a lot about the importance of them rounding more efficiently and how that could positively affect the time that they have with their patients and themselves. We discussed that due to the additional work missed billing causes, minimizing this burden can possibly help decrease burnout. As a result, seven hospitalists, the administrative staff, and I met and created thirteen best practices, six of which they were able to get approved to use immediately. To note, hospitalists bill differently; some use a software company, fill out paper forms still or have integration within their EMR. Although these solutions were made for a program which has the ability to bill within the EMR, many of the principles will apply to your program too.
The 13 best practices that the seven hospitalists agreed upon are the following:
When a doctor signs a note, it opens a charge option or there is a hard stop.
Charge delinquencies are sent via email to the hospitalist.
Standardize that hospitalists charge directly after writing a note consistently as part of their workflow.*
Prioritize discharges before rounding.*
Standardize the use of the “my prof charges” column, a feature of this hospital’s EMR system that tells them if they had made a charge to a patient or not, in order to remind them to/confirm billing a patient.*
Create reports by the EMR system to provide charge data for individual providers.
Create a report for bill vs note to help providers self-audit. At this hospital, this feature was offered to the administrators as a way to audit their providers and doctors.
Ensure that when a patient is seen by a physician hospitalist as well as an NP/PA hospitalist, the appropriate charge for the physician is entered.
Notifications get sent to the physician hospitalist if a charge gets deleted by another person (e.g., NP/PA hospitalist).
Handoff of daily rounding sheets, or a paper copy of the patients assigned to a hospitalist for his/her shift, at the end of the shift to the project specialist.*
To keep the rounding sheets a complete and accurate account of the patients seen by the hospitalist.*
Hospitalists are to complete and check all billing at the end of their shift at the latest.*
Hospitalists are to participate on Provider Efficiency Training to optimize workflow, by creating more efficient note-writing behavior using Dragon.
*Indicates the practices the hospitalists were able to implement immediately. Practices 1, 2, 6, 7, and 9 request EMR changes. Practice 8 was already an established practice the hospitalists wished to continue. Practice 13 was suggested by the Lean Director for the continuation of a previous project.
Six of the best practices were easier to implement right away because they were at the discretion of the hospitalists. We found that the hospitalists who had the highest billing performances were more likely to start writing notes and charge earlier while rounding. Those who had poorer billing performances were more likely to leave all note writing and billing towards the end of their shift. The few exceptions (hospitalists who left all note writing and charging to the end of their shift yet had high billing performances) were found to have a consistent and standardized workflow. This was unlike the hospitalists who had the lowest billing performances. Having practices that help remind hospitalists to bill will surely help prevent missed billing, but because of the findings from this project, it was important to have consistent and standardized practices to additionally improve missed billing.
When we followed up with the hospitalist division two months later, we learned they were making great progress. Not only were hospitalists using their best practices, but in working with the administrators, they were designing sessions to further educate fellow hospitalists to prevent further missed billing. These sessions outlined shortcuts, resources and ways hospitalists may modify their personal EMR accounts to prevent missed billing. None of the progress could have been made without first understanding and addressing what is truly important to the hospitalists.
In summary, we noted these general observations in this project:
- Hospitalists favor solutions that benefit them or their patients.
- Hospitalists want to be part of the solution process.
- Hospitalists were more likely to accept ideas to improve their rounding if it meant they could keep their routine.
Obstacles exist in our health care system that prevent administrators and hospitalists from working together as a team. The more we are able to communicate and collaborate to fix problems in the health system, the more we can use the system to our mutual advantage. With the ongoing changes in medicine, especially during uncertain times, better communication needs be a major priority to affect positive change.
Ms. Mirabella attends the Frank H. Netter MD School of Medicine at Quinnipiac University, Hamden, Conn., in the class of 2022. She has interests in internal/hospital medicine, primary care, and health management and leadership. Dr. Rosenberg is associate professor at the Frank H. Netter MD School of Medicine at Quinnipiac University where she is director of clinical skills coaching. Dr. Kiassat is associate dean of the School of Engineering and associate clinical professor at Frank H. Netter MD School of Medicine, at Quinnipiac University. His research interests are in process improvement in health care, using Lean Six Sigma.
References
1. Burdick K, et al. Bedside interprofessional rounding. J Patient Exp. 2017;4(1):22-27. doi: 10.1177/2374373517692910.
2. Patel CR. Improving communication between hospitalists and consultants. The Hospital Leader. 2018. https://thehospitalleader.org/improving-communication-between-hospitalists-and-consultants/.
3. Adams TN, et al. Hospitalist perspective of interactions with medicine subspecialty consult services. J Hosp Med. 2018;13(5):318-323. doi: 10.12788/jhm.2882.
4. Michtalik HJ, et al. Impact of attending physician workload on patient care: A survey of hospitalists. JAMA Intern Med. 2013;173(5):375-377. doi: 10.1001/jamainternmed.2013.1864.
5. Chandra R, et al. How hospitalists can improve efficiency on inpatient wards. The Hospitalist. 2014. https://www.the-hospitalist.org/hospitalist/article/126231/how-hospitalists-can-improve-efficiency-inpatient-wards.
6. Chand DV. Observational study using the tools of lean six sigma to improve the efficiency of the resident rounding process. J Grad Med Educ. 2011;3(2):144-150. doi: 10.4300/JGME-D-10-00116.1.
7. O’Leary KJ, et al. How hospitalists spend their time: Insights on efficiency and safety. J Hosp Med. 2006;1(2):88-93. doi: 10.1002/jhm.88.
8. Wachter RM. Hospitalist workload: The search for the magic number. JAMA Intern Med. 2014;174(5):794-795. doi: 10.1001/jamainternmed.2014.18.
9. Bryson C, et al. Geographical assignment of hospitalists in an urban teaching hospital: Feasibility and impact on efficiency and provider satisfaction. Hospital Practice. 2017;45(4):135-142. doi: 10.1080/21548331.2017.1353884.
10. Calderon AS, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014 Dec;6(4):750-5. doi: 10.4300/JGME-D-13-00324.1.
As an aspiring physician, I like learning about how things work. Since medical students learn very little about the “business” of medicine in school, this led me to pioneer a project on missed billing by hospitalists at a medium-sized hospital in the northeastern US. Although hospitalists do a tremendous amount of work, they do not always bill for what they are doing. The question became: Why are hospitalists missing charges and what can we do to stop it?
Shortly into my study, I recognized there was little daily communication between the administrators and the hospitalists; neither the hospitalists nor administrators understood the different dynamics that the others faced in their own workplace. It became apparent that administrators needed to learn what was important to hospitalists and to address them at their level in order to bring about change.
Some trending themes emerged as I started shadowing the hospitalists. Many of them asked how this project would benefit them. They argued that administrative needs should be dealt with at the administrative level. A major point was made that current incentives, such as the bonuses given for exceeding a certain number of RVUs, were not the motivating force behind their work ethics. From my observations, the motivating factors were the quality of their patient care, the needs of their patients, and teaching. The hospitalists also were eager to teach and continually instructed me on clinical skills and how to be a better medical student.
Bonuses or notoriety didn’t seem to be the main incentives for them. However, efficiency – especially in rounding – was important, and that became the focal point of the project. I found several studies that showed that improvements in aspects of rounding led to increased quality of patient care, decreased burnout, increased patient satisfaction, and decreased workload and discussed some of those findings with the hospitalists.1-10 When the hospitalists felt that their concerns were being heard, they became even more involved in the project, and the administrators and hospitalists started working together as a team.
One hospitalist spent two hours helping me design the platform that would be used for hospitalists to report barriers in their rounding process that may cause them to miss a charge. Once we identified those barriers, we discussed the possibility of standardizing their workflow based off these data. Many hospitalists argued that each physician has unique skills and practices that make them successful; therefore, the disruption of an already established workflow may cause a decrease in efficiency.
The hospitalists and I talked a lot about the importance of them rounding more efficiently and how that could positively affect the time that they have with their patients and themselves. We discussed that due to the additional work missed billing causes, minimizing this burden can possibly help decrease burnout. As a result, seven hospitalists, the administrative staff, and I met and created thirteen best practices, six of which they were able to get approved to use immediately. To note, hospitalists bill differently; some use a software company, fill out paper forms still or have integration within their EMR. Although these solutions were made for a program which has the ability to bill within the EMR, many of the principles will apply to your program too.
The 13 best practices that the seven hospitalists agreed upon are the following:
When a doctor signs a note, it opens a charge option or there is a hard stop.
Charge delinquencies are sent via email to the hospitalist.
Standardize that hospitalists charge directly after writing a note consistently as part of their workflow.*
Prioritize discharges before rounding.*
Standardize the use of the “my prof charges” column, a feature of this hospital’s EMR system that tells them if they had made a charge to a patient or not, in order to remind them to/confirm billing a patient.*
Create reports by the EMR system to provide charge data for individual providers.
Create a report for bill vs note to help providers self-audit. At this hospital, this feature was offered to the administrators as a way to audit their providers and doctors.
Ensure that when a patient is seen by a physician hospitalist as well as an NP/PA hospitalist, the appropriate charge for the physician is entered.
Notifications get sent to the physician hospitalist if a charge gets deleted by another person (e.g., NP/PA hospitalist).
Handoff of daily rounding sheets, or a paper copy of the patients assigned to a hospitalist for his/her shift, at the end of the shift to the project specialist.*
To keep the rounding sheets a complete and accurate account of the patients seen by the hospitalist.*
Hospitalists are to complete and check all billing at the end of their shift at the latest.*
Hospitalists are to participate on Provider Efficiency Training to optimize workflow, by creating more efficient note-writing behavior using Dragon.
*Indicates the practices the hospitalists were able to implement immediately. Practices 1, 2, 6, 7, and 9 request EMR changes. Practice 8 was already an established practice the hospitalists wished to continue. Practice 13 was suggested by the Lean Director for the continuation of a previous project.
Six of the best practices were easier to implement right away because they were at the discretion of the hospitalists. We found that the hospitalists who had the highest billing performances were more likely to start writing notes and charge earlier while rounding. Those who had poorer billing performances were more likely to leave all note writing and billing towards the end of their shift. The few exceptions (hospitalists who left all note writing and charging to the end of their shift yet had high billing performances) were found to have a consistent and standardized workflow. This was unlike the hospitalists who had the lowest billing performances. Having practices that help remind hospitalists to bill will surely help prevent missed billing, but because of the findings from this project, it was important to have consistent and standardized practices to additionally improve missed billing.
When we followed up with the hospitalist division two months later, we learned they were making great progress. Not only were hospitalists using their best practices, but in working with the administrators, they were designing sessions to further educate fellow hospitalists to prevent further missed billing. These sessions outlined shortcuts, resources and ways hospitalists may modify their personal EMR accounts to prevent missed billing. None of the progress could have been made without first understanding and addressing what is truly important to the hospitalists.
In summary, we noted these general observations in this project:
- Hospitalists favor solutions that benefit them or their patients.
- Hospitalists want to be part of the solution process.
- Hospitalists were more likely to accept ideas to improve their rounding if it meant they could keep their routine.
Obstacles exist in our health care system that prevent administrators and hospitalists from working together as a team. The more we are able to communicate and collaborate to fix problems in the health system, the more we can use the system to our mutual advantage. With the ongoing changes in medicine, especially during uncertain times, better communication needs be a major priority to affect positive change.
Ms. Mirabella attends the Frank H. Netter MD School of Medicine at Quinnipiac University, Hamden, Conn., in the class of 2022. She has interests in internal/hospital medicine, primary care, and health management and leadership. Dr. Rosenberg is associate professor at the Frank H. Netter MD School of Medicine at Quinnipiac University where she is director of clinical skills coaching. Dr. Kiassat is associate dean of the School of Engineering and associate clinical professor at Frank H. Netter MD School of Medicine, at Quinnipiac University. His research interests are in process improvement in health care, using Lean Six Sigma.
References
1. Burdick K, et al. Bedside interprofessional rounding. J Patient Exp. 2017;4(1):22-27. doi: 10.1177/2374373517692910.
2. Patel CR. Improving communication between hospitalists and consultants. The Hospital Leader. 2018. https://thehospitalleader.org/improving-communication-between-hospitalists-and-consultants/.
3. Adams TN, et al. Hospitalist perspective of interactions with medicine subspecialty consult services. J Hosp Med. 2018;13(5):318-323. doi: 10.12788/jhm.2882.
4. Michtalik HJ, et al. Impact of attending physician workload on patient care: A survey of hospitalists. JAMA Intern Med. 2013;173(5):375-377. doi: 10.1001/jamainternmed.2013.1864.
5. Chandra R, et al. How hospitalists can improve efficiency on inpatient wards. The Hospitalist. 2014. https://www.the-hospitalist.org/hospitalist/article/126231/how-hospitalists-can-improve-efficiency-inpatient-wards.
6. Chand DV. Observational study using the tools of lean six sigma to improve the efficiency of the resident rounding process. J Grad Med Educ. 2011;3(2):144-150. doi: 10.4300/JGME-D-10-00116.1.
7. O’Leary KJ, et al. How hospitalists spend their time: Insights on efficiency and safety. J Hosp Med. 2006;1(2):88-93. doi: 10.1002/jhm.88.
8. Wachter RM. Hospitalist workload: The search for the magic number. JAMA Intern Med. 2014;174(5):794-795. doi: 10.1001/jamainternmed.2014.18.
9. Bryson C, et al. Geographical assignment of hospitalists in an urban teaching hospital: Feasibility and impact on efficiency and provider satisfaction. Hospital Practice. 2017;45(4):135-142. doi: 10.1080/21548331.2017.1353884.
10. Calderon AS, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014 Dec;6(4):750-5. doi: 10.4300/JGME-D-13-00324.1.
COVID-19 vaccines: Safe for immunocompromised patients?
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
FDA clears first OTC rapid at-home COVID diagnostic test
The Food and Drug Administration has issued an emergency-use authorization (EUA) for the first COVID-19 diagnostic test that can be completed at home without a prescription.
Authorization of the Ellume COVID-19 Home Test is “a major milestone in diagnostic testing for COVID-19,” FDA Commissioner Stephen M. Hahn, MD, said in a news release.
“By authorizing a test for over-the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test, and find out their results in as little as 20 minutes,” said Dr. Hahn.
The Ellume COVID-19 Home Test is a rapid antigen test that detects fragments of the SARS-CoV-2 virus from a nasal swab sample taken from anyone aged 2 years and older, including those not showing any symptoms.
In testing, the Ellume COVID-19 Home Test correctly identified 96% of positive samples and 100% of negative samples in individuals with symptoms.
In people without symptoms, the test correctly identified 91% of positive samples and 96% of negative samples, the FDA said.
The test includes a sterile nasal swab, a dropper, processing fluid, and a Bluetooth-connected analyzer for use with an app on the user’s smartphone. The sample is analyzed and results are automatically transmitted to the user’s smartphone.
“The Ellume COVID-19 home test’s core technology combines ultra-sensitive optics, electronics, and proprietary software to leverage best-in-class digital immunoassay technology with next-generation multi-quantum dot fluorescence technology,” the company said in a news release.
The mobile app requires individuals to input their ZIP code and date of birth, with optional fields including name and email address. The app automatically reports the results as appropriate to public health authorities to monitor disease prevalence.
Ellume expects to produce more than 3 million tests in January 2021. The company said the test will cost around $30.
FDA authorization of this first fully at-home nonprescription COVID-19 diagnostic test follows last month’s EUA for the first prescription COVID-19 test for home use, as reported this news organization.
Since the start of the pandemic, the FDA has authorized more than 225 diagnostic tests for COVID-19, including more than 25 tests that allow for home collection of samples, which are then sent to a lab for testing.
“As we continue to authorize additional tests for home use, we are helping expand Americans’ access to testing, reducing the burden on laboratories and test supplies, and giving Americans more testing options from the comfort and safety of their own homes,” Dr. Hahn said.
“This test, like other antigen tests, is less sensitive and less specific than typical molecular tests run in a lab,” said Jeffrey Shuren, MD, JD, director of FDA’s Center for Devices and Radiological Health, in the release. “However, the fact that it can be used completely at home and return results quickly means that it can play an important role in response to the pandemic.”
As with other antigen tests, a small percentage of positive and negative results from the Ellume test may be false. In patients without symptoms, positive results should be treated as presumptively positive until confirmed by another test as soon as possible, the FDA advised.
This is especially true if there are fewer infections in a particular community, as false-positive results can be more common when antigen tests are used in populations where there is a low prevalence of COVID-19, the agency said.
Because all tests can give false-negative and false-positive results, individuals with positive results should self-isolate and seek additional care from their health care provider.
Individuals who test negative and have symptoms of COVID-19 should follow up with their health care provider, as negative results don’t preclude an individual from SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued an emergency-use authorization (EUA) for the first COVID-19 diagnostic test that can be completed at home without a prescription.
Authorization of the Ellume COVID-19 Home Test is “a major milestone in diagnostic testing for COVID-19,” FDA Commissioner Stephen M. Hahn, MD, said in a news release.
“By authorizing a test for over-the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test, and find out their results in as little as 20 minutes,” said Dr. Hahn.
The Ellume COVID-19 Home Test is a rapid antigen test that detects fragments of the SARS-CoV-2 virus from a nasal swab sample taken from anyone aged 2 years and older, including those not showing any symptoms.
In testing, the Ellume COVID-19 Home Test correctly identified 96% of positive samples and 100% of negative samples in individuals with symptoms.
In people without symptoms, the test correctly identified 91% of positive samples and 96% of negative samples, the FDA said.
The test includes a sterile nasal swab, a dropper, processing fluid, and a Bluetooth-connected analyzer for use with an app on the user’s smartphone. The sample is analyzed and results are automatically transmitted to the user’s smartphone.
“The Ellume COVID-19 home test’s core technology combines ultra-sensitive optics, electronics, and proprietary software to leverage best-in-class digital immunoassay technology with next-generation multi-quantum dot fluorescence technology,” the company said in a news release.
The mobile app requires individuals to input their ZIP code and date of birth, with optional fields including name and email address. The app automatically reports the results as appropriate to public health authorities to monitor disease prevalence.
Ellume expects to produce more than 3 million tests in January 2021. The company said the test will cost around $30.
FDA authorization of this first fully at-home nonprescription COVID-19 diagnostic test follows last month’s EUA for the first prescription COVID-19 test for home use, as reported this news organization.
Since the start of the pandemic, the FDA has authorized more than 225 diagnostic tests for COVID-19, including more than 25 tests that allow for home collection of samples, which are then sent to a lab for testing.
“As we continue to authorize additional tests for home use, we are helping expand Americans’ access to testing, reducing the burden on laboratories and test supplies, and giving Americans more testing options from the comfort and safety of their own homes,” Dr. Hahn said.
“This test, like other antigen tests, is less sensitive and less specific than typical molecular tests run in a lab,” said Jeffrey Shuren, MD, JD, director of FDA’s Center for Devices and Radiological Health, in the release. “However, the fact that it can be used completely at home and return results quickly means that it can play an important role in response to the pandemic.”
As with other antigen tests, a small percentage of positive and negative results from the Ellume test may be false. In patients without symptoms, positive results should be treated as presumptively positive until confirmed by another test as soon as possible, the FDA advised.
This is especially true if there are fewer infections in a particular community, as false-positive results can be more common when antigen tests are used in populations where there is a low prevalence of COVID-19, the agency said.
Because all tests can give false-negative and false-positive results, individuals with positive results should self-isolate and seek additional care from their health care provider.
Individuals who test negative and have symptoms of COVID-19 should follow up with their health care provider, as negative results don’t preclude an individual from SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has issued an emergency-use authorization (EUA) for the first COVID-19 diagnostic test that can be completed at home without a prescription.
Authorization of the Ellume COVID-19 Home Test is “a major milestone in diagnostic testing for COVID-19,” FDA Commissioner Stephen M. Hahn, MD, said in a news release.
“By authorizing a test for over-the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test, and find out their results in as little as 20 minutes,” said Dr. Hahn.
The Ellume COVID-19 Home Test is a rapid antigen test that detects fragments of the SARS-CoV-2 virus from a nasal swab sample taken from anyone aged 2 years and older, including those not showing any symptoms.
In testing, the Ellume COVID-19 Home Test correctly identified 96% of positive samples and 100% of negative samples in individuals with symptoms.
In people without symptoms, the test correctly identified 91% of positive samples and 96% of negative samples, the FDA said.
The test includes a sterile nasal swab, a dropper, processing fluid, and a Bluetooth-connected analyzer for use with an app on the user’s smartphone. The sample is analyzed and results are automatically transmitted to the user’s smartphone.
“The Ellume COVID-19 home test’s core technology combines ultra-sensitive optics, electronics, and proprietary software to leverage best-in-class digital immunoassay technology with next-generation multi-quantum dot fluorescence technology,” the company said in a news release.
The mobile app requires individuals to input their ZIP code and date of birth, with optional fields including name and email address. The app automatically reports the results as appropriate to public health authorities to monitor disease prevalence.
Ellume expects to produce more than 3 million tests in January 2021. The company said the test will cost around $30.
FDA authorization of this first fully at-home nonprescription COVID-19 diagnostic test follows last month’s EUA for the first prescription COVID-19 test for home use, as reported this news organization.
Since the start of the pandemic, the FDA has authorized more than 225 diagnostic tests for COVID-19, including more than 25 tests that allow for home collection of samples, which are then sent to a lab for testing.
“As we continue to authorize additional tests for home use, we are helping expand Americans’ access to testing, reducing the burden on laboratories and test supplies, and giving Americans more testing options from the comfort and safety of their own homes,” Dr. Hahn said.
“This test, like other antigen tests, is less sensitive and less specific than typical molecular tests run in a lab,” said Jeffrey Shuren, MD, JD, director of FDA’s Center for Devices and Radiological Health, in the release. “However, the fact that it can be used completely at home and return results quickly means that it can play an important role in response to the pandemic.”
As with other antigen tests, a small percentage of positive and negative results from the Ellume test may be false. In patients without symptoms, positive results should be treated as presumptively positive until confirmed by another test as soon as possible, the FDA advised.
This is especially true if there are fewer infections in a particular community, as false-positive results can be more common when antigen tests are used in populations where there is a low prevalence of COVID-19, the agency said.
Because all tests can give false-negative and false-positive results, individuals with positive results should self-isolate and seek additional care from their health care provider.
Individuals who test negative and have symptoms of COVID-19 should follow up with their health care provider, as negative results don’t preclude an individual from SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
COVID-19 and patient safety in the medical office
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
As the pandemic hits its third nationwide surge, families are gathering for the holidays, and medical practices are preparing for a potential increase in cases. Medical offices in states that were not strongly affected by the first and second waves of the virus may now be facing an influx of COVID-19 patients. Therefore, medical offices must remain very attentive to the widespread outbreak of COVID-19, continuing to proactively take steps to safely manage patients while protecting clinical staff.
Here are tips and resources for this season of the pandemic:
- Documentation: Maintain administrative records of how you have adapted to the evolving crisis, including the challenges you faced. For details, see Keep a COVID-19 Diary: Document Now in Case of Future Lawsuits.
- Legislation and Guidance: Reference the CDC; your state medical board; professional societies; and federal, state, and local authorities daily for public health guidance and new legislation, as this continues to be a fluid situation.
- Screening Criteria: Follow the CDC’s patient assessment protocol for early disease detection for patients presenting to your practice. Patients should be screened using these guidelines: Overview of Testing for SARS-CoV-2 (COVID-19). Essential visitors to your facility should also be assessed for symptoms of coronavirus and contact exposure and redirected to remain outside if suspect.
- Accepting Patients: Do not turn patients away simply because a patient calls with acute respiratory symptoms. Refusing assessment/care may lead to concerns of patient abandonment.
- Designated Triage Location: Check with your local public health authorities for locations designated to triage suspected patients, so exposure is limited in general medical offices.
- Telehealth Triage: Licensed staff should be trained in triage protocol to determine which patients can be managed safely at home. See Healthcare Facilities: Managing Operations During the COVID-19 Pandemic. The Doctors Company offers resources on telemedicine in our COVID-19 Telehealth Resource Center.
- Patient Testing: When there is a reasonable presumption that a patient may have been exposed to COVID-19, contact the local or state health department to coordinate testing using available community resources. See the CDC’s Testing for COVID-19 , the COVID-19 Testing Overview, and the Clinician Call Center.
- Elective Services: Check with regional governmental and health authorities on the provision of nonessential and elective health care visits and group-related activities. Many states continue or have reinstated restrictions on the provision of nonurgent, elective surgeries and procedures.
- Patient Precautions: Educational resources, including posters for use in the medical office, are available from the WHO and for health care workers from the CDC (Contact Precautions, Droplet Precautions, and Airborne Precautions). Reference the CDC’s and Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19) for patient management guidance.
- Provider/Staff Precautions: Follow Standard Precautions and Transmission-Based Precautions, including gloves, gowns, protective eyewear, and NIOSH-certified N95 respirators that have been properly fit-tested. If there is a shortage of N95 respirators in your facility, access current CDC respirator recommendations and review Optimizing Personal Protective Equipment (PPE) Supplies.
- Limit Exposure: Limit staff exposure to suspected patients, with the exam room door kept closed. Ideally, the designated exam room should be at the back of the office, far away from other staff and patients.
- Surface Disinfection: Once the patient exits the room, conduct surface disinfection while staff continues to wear PPE.
- Patient Education: Provide up-to-date, factual information on the virus to suspected COVID-19–positive patients and their close contacts.
- Provider/Staff Exposure: Screen health care personnel daily for symptoms/contacts relevant to COVID-19. Any unprotected occupational exposure by staff members should be assessed and monitored. See Interim U.S. Guidance for Risk Assessment and Work Restrictions for Healthcare Personnel with Potential Exposure to COVID-19. Should providers and/or staff test positive within your facility, conduct and document a risk assessment identifying contacts, type of interaction, and PPE in use, then contact local health authorities for additional instruction. The CDC provides guidance here under the section “Infection Control.” The health department may assist with patient notification if determined to be necessary. For return-to-work guidance, review the Criteria for Return to Work for Healthcare Personnel with SARS-CoV-2 Infection (Interim Guidance).
- Staff Training: Provide and document additional staff training as protocols change. Maintain training records in administrative files.
Ms. Hill is senior patient safety risk manager at The Doctors Company. The guidelines suggested here are not rules, do not constitute legal advice, and do not ensure a successful outcome. The ultimate decision regarding the appropriateness of any treatment must be made by each health care provider considering the circumstances of the individual situation and in accordance with the laws of the jurisdiction in which the care is rendered.
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
As the pandemic hits its third nationwide surge, families are gathering for the holidays, and medical practices are preparing for a potential increase in cases. Medical offices in states that were not strongly affected by the first and second waves of the virus may now be facing an influx of COVID-19 patients. Therefore, medical offices must remain very attentive to the widespread outbreak of COVID-19, continuing to proactively take steps to safely manage patients while protecting clinical staff.
Here are tips and resources for this season of the pandemic:
- Documentation: Maintain administrative records of how you have adapted to the evolving crisis, including the challenges you faced. For details, see Keep a COVID-19 Diary: Document Now in Case of Future Lawsuits.
- Legislation and Guidance: Reference the CDC; your state medical board; professional societies; and federal, state, and local authorities daily for public health guidance and new legislation, as this continues to be a fluid situation.
- Screening Criteria: Follow the CDC’s patient assessment protocol for early disease detection for patients presenting to your practice. Patients should be screened using these guidelines: Overview of Testing for SARS-CoV-2 (COVID-19). Essential visitors to your facility should also be assessed for symptoms of coronavirus and contact exposure and redirected to remain outside if suspect.
- Accepting Patients: Do not turn patients away simply because a patient calls with acute respiratory symptoms. Refusing assessment/care may lead to concerns of patient abandonment.
- Designated Triage Location: Check with your local public health authorities for locations designated to triage suspected patients, so exposure is limited in general medical offices.
- Telehealth Triage: Licensed staff should be trained in triage protocol to determine which patients can be managed safely at home. See Healthcare Facilities: Managing Operations During the COVID-19 Pandemic. The Doctors Company offers resources on telemedicine in our COVID-19 Telehealth Resource Center.
- Patient Testing: When there is a reasonable presumption that a patient may have been exposed to COVID-19, contact the local or state health department to coordinate testing using available community resources. See the CDC’s Testing for COVID-19 , the COVID-19 Testing Overview, and the Clinician Call Center.
- Elective Services: Check with regional governmental and health authorities on the provision of nonessential and elective health care visits and group-related activities. Many states continue or have reinstated restrictions on the provision of nonurgent, elective surgeries and procedures.
- Patient Precautions: Educational resources, including posters for use in the medical office, are available from the WHO and for health care workers from the CDC (Contact Precautions, Droplet Precautions, and Airborne Precautions). Reference the CDC’s and Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19) for patient management guidance.
- Provider/Staff Precautions: Follow Standard Precautions and Transmission-Based Precautions, including gloves, gowns, protective eyewear, and NIOSH-certified N95 respirators that have been properly fit-tested. If there is a shortage of N95 respirators in your facility, access current CDC respirator recommendations and review Optimizing Personal Protective Equipment (PPE) Supplies.
- Limit Exposure: Limit staff exposure to suspected patients, with the exam room door kept closed. Ideally, the designated exam room should be at the back of the office, far away from other staff and patients.
- Surface Disinfection: Once the patient exits the room, conduct surface disinfection while staff continues to wear PPE.
- Patient Education: Provide up-to-date, factual information on the virus to suspected COVID-19–positive patients and their close contacts.
- Provider/Staff Exposure: Screen health care personnel daily for symptoms/contacts relevant to COVID-19. Any unprotected occupational exposure by staff members should be assessed and monitored. See Interim U.S. Guidance for Risk Assessment and Work Restrictions for Healthcare Personnel with Potential Exposure to COVID-19. Should providers and/or staff test positive within your facility, conduct and document a risk assessment identifying contacts, type of interaction, and PPE in use, then contact local health authorities for additional instruction. The CDC provides guidance here under the section “Infection Control.” The health department may assist with patient notification if determined to be necessary. For return-to-work guidance, review the Criteria for Return to Work for Healthcare Personnel with SARS-CoV-2 Infection (Interim Guidance).
- Staff Training: Provide and document additional staff training as protocols change. Maintain training records in administrative files.
Ms. Hill is senior patient safety risk manager at The Doctors Company. The guidelines suggested here are not rules, do not constitute legal advice, and do not ensure a successful outcome. The ultimate decision regarding the appropriateness of any treatment must be made by each health care provider considering the circumstances of the individual situation and in accordance with the laws of the jurisdiction in which the care is rendered.
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
As the pandemic hits its third nationwide surge, families are gathering for the holidays, and medical practices are preparing for a potential increase in cases. Medical offices in states that were not strongly affected by the first and second waves of the virus may now be facing an influx of COVID-19 patients. Therefore, medical offices must remain very attentive to the widespread outbreak of COVID-19, continuing to proactively take steps to safely manage patients while protecting clinical staff.
Here are tips and resources for this season of the pandemic:
- Documentation: Maintain administrative records of how you have adapted to the evolving crisis, including the challenges you faced. For details, see Keep a COVID-19 Diary: Document Now in Case of Future Lawsuits.
- Legislation and Guidance: Reference the CDC; your state medical board; professional societies; and federal, state, and local authorities daily for public health guidance and new legislation, as this continues to be a fluid situation.
- Screening Criteria: Follow the CDC’s patient assessment protocol for early disease detection for patients presenting to your practice. Patients should be screened using these guidelines: Overview of Testing for SARS-CoV-2 (COVID-19). Essential visitors to your facility should also be assessed for symptoms of coronavirus and contact exposure and redirected to remain outside if suspect.
- Accepting Patients: Do not turn patients away simply because a patient calls with acute respiratory symptoms. Refusing assessment/care may lead to concerns of patient abandonment.
- Designated Triage Location: Check with your local public health authorities for locations designated to triage suspected patients, so exposure is limited in general medical offices.
- Telehealth Triage: Licensed staff should be trained in triage protocol to determine which patients can be managed safely at home. See Healthcare Facilities: Managing Operations During the COVID-19 Pandemic. The Doctors Company offers resources on telemedicine in our COVID-19 Telehealth Resource Center.
- Patient Testing: When there is a reasonable presumption that a patient may have been exposed to COVID-19, contact the local or state health department to coordinate testing using available community resources. See the CDC’s Testing for COVID-19 , the COVID-19 Testing Overview, and the Clinician Call Center.
- Elective Services: Check with regional governmental and health authorities on the provision of nonessential and elective health care visits and group-related activities. Many states continue or have reinstated restrictions on the provision of nonurgent, elective surgeries and procedures.
- Patient Precautions: Educational resources, including posters for use in the medical office, are available from the WHO and for health care workers from the CDC (Contact Precautions, Droplet Precautions, and Airborne Precautions). Reference the CDC’s and Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19) for patient management guidance.
- Provider/Staff Precautions: Follow Standard Precautions and Transmission-Based Precautions, including gloves, gowns, protective eyewear, and NIOSH-certified N95 respirators that have been properly fit-tested. If there is a shortage of N95 respirators in your facility, access current CDC respirator recommendations and review Optimizing Personal Protective Equipment (PPE) Supplies.
- Limit Exposure: Limit staff exposure to suspected patients, with the exam room door kept closed. Ideally, the designated exam room should be at the back of the office, far away from other staff and patients.
- Surface Disinfection: Once the patient exits the room, conduct surface disinfection while staff continues to wear PPE.
- Patient Education: Provide up-to-date, factual information on the virus to suspected COVID-19–positive patients and their close contacts.
- Provider/Staff Exposure: Screen health care personnel daily for symptoms/contacts relevant to COVID-19. Any unprotected occupational exposure by staff members should be assessed and monitored. See Interim U.S. Guidance for Risk Assessment and Work Restrictions for Healthcare Personnel with Potential Exposure to COVID-19. Should providers and/or staff test positive within your facility, conduct and document a risk assessment identifying contacts, type of interaction, and PPE in use, then contact local health authorities for additional instruction. The CDC provides guidance here under the section “Infection Control.” The health department may assist with patient notification if determined to be necessary. For return-to-work guidance, review the Criteria for Return to Work for Healthcare Personnel with SARS-CoV-2 Infection (Interim Guidance).
- Staff Training: Provide and document additional staff training as protocols change. Maintain training records in administrative files.
Ms. Hill is senior patient safety risk manager at The Doctors Company. The guidelines suggested here are not rules, do not constitute legal advice, and do not ensure a successful outcome. The ultimate decision regarding the appropriateness of any treatment must be made by each health care provider considering the circumstances of the individual situation and in accordance with the laws of the jurisdiction in which the care is rendered.
COVID-related harm to HCWs must be tracked more rigorously: NAS panel
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
Coronavirus has infected over 2% of U.S. children
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.
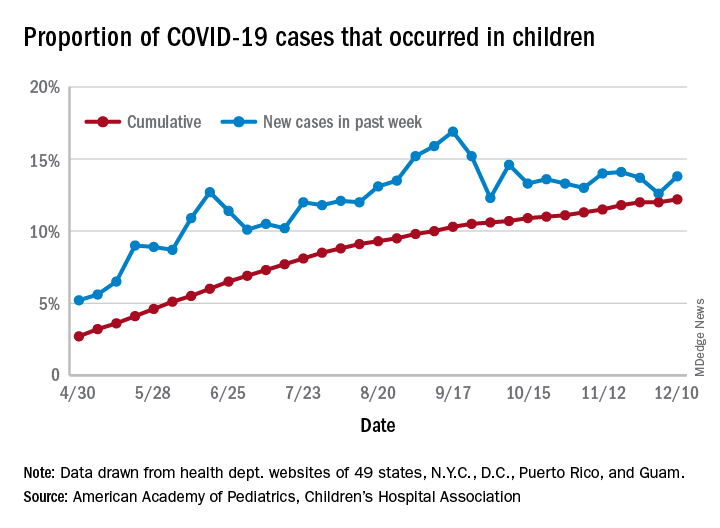
For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.

For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.

For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
Bias against hiring hospitalists trained in family medicine still persists
Outdated perceptions of family medicine
A family medicine trained doctor, fresh out of residency, visits a career website to scout out prospective hospitalist jobs in their region. As they scroll through the job listings, they come across one opportunity at a nearby hospital system that seems like a good fit. The listing offers a competitive salary and comprehensive benefits for the position, and mentions hospitalists in the department will have the opportunity to teach medical students.
The only problem? The position is for internal medicine trained doctors only. After searching through several more listings with the same internal medicine requirement, the pool of jobs available to the family medicine doctor seems much smaller.
When Robert M. Wachter, MD, MHM, and Lee Goldman, MD coined the term “hospitalist” in a 1996 New England Journal of Medicine article, hospitalists were primarily clinicians with an internal medicine background, filling the gap created by family medicine doctors who increasingly devoted their time to patients in their practice and spent less time rounding in the hospital.
As family medicine doctors have returned to hospital medicine, it has become difficult to find positions as hospitalists due to a preference by some recruiters and employers that favors internal medicine physicians over those who are trained in family medicine. The preference for internal medicine physicians is sometimes overt, such as a requirement on a job application. But the preference can also surface after a physician has already applied for a position, and they will then discover a recruiter is actually looking for someone with a background in internal medicine. In other cases, family medicine physicians find out after applying that applicants with a background in family medicine are considered, but they’re expected to have additional training or certification not listed on the job application.
The situation can even be as stark as a hospital system hiring an internal medicine doctor just out of residency over a family medicine doctor with years of experience as a board-certified physician. Hiring practices in large systems across multiple states sometimes don’t just favor internal medicine, they are entirely focused on internal medicine hospitalists, said experts who spoke with The Hospitalist.
Outdated perceptions of family medicine
Victoria McCurry, MD, current chair of the Society of Hospital Medicine’s family medicine Special Interest Group (SIG) Executive Committee and Faculty Director of Inpatient Services at UPMC McKeesport (Pa.) Family Medicine Residency, said hearsay inside the family medicine community influenced her first job search looking for hospitalist positions as a family medicine physician.
“I was intentional about choosing places that I assumed would be open to family medicine,” she said. “I avoided the downtown urban academic hospitals, the ones that had a large internal medicine residency and fellowship presence, because I assumed that they would not hire me.
“There’s a recognition that depending on the system that you’re in and their history with family medicine trained hospitalists, it can be difficult as a family physician to seek employment,” Dr. McCurry said.
“When I graduated from my residency in 2014, I did not have the same opportunities to be a hospitalist as an internal medicine resident would have,” said Shyam Odeti, MD, a family-practice-trained hospitalist who works at Ballad Health in Johnson City, Tenn. “The perception is family medicine physicians are not trained for hospitalist practice. It’s an old perception.”
This perception may have to do with the mindset of the leadership where a doctor has had residency training, according to Usman Chaudhry, MD, a family medicine hospitalist with Texas Health Physicians Group and leader of the National Advocacy subcommittee for the Family Medicine Executive Council in SHM. Residents trained in bigger university hospital systems where internal medicine (IM) residents do mostly inpatient – in addition to outpatient services – and family medicine (FM) residents do mostly outpatient – including pediatrics and ob/gyn clinics in addition to inpatient services – may believe that to be the case in other systems too, Dr. Chaudhry explained.
“When you go to community hospital residency programs, it’s totally different,” he said. “It all depends. If you have only family medicine residency in a community hospital, they tend to do all training of inpatient clinical medicine, as IM training would in any other program”
Dr. McCurry noted that there seems to be a persisting, mental assumption that as a family medicine doctor, you’re only going to be practicing outpatient only or maybe urgent care, which is historically just not the case. “If that’s ingrained within the local hospital system, then it will be difficult for that system to hire a family medicine-trained hospitalist,” she said.
Another source of outdated perceptions of family medicine come from hospital and institutional bylaws that have written internal medicine training in as a requirement for hospitalists. “In many bigger systems, and even in the smaller hospital community and regional hospitals, the bylaws of the hospitals were written approximately 20 years ago,” Dr. Chaudhry said.
Unless someone has advocated for updating a hospital or institution’s bylaws, they may have outdated requirements for hospitalists. “The situation right now is, in a lot of urban hospitals, they would be able to give a hospitalist position to internal medicine residents who just graduated, not even board certified, but they cannot give it to a hospitalist trained in family medicine who has worked for 10 years and is board certified, just because of the bylaws,” said Dr. Odeti who is also co-chair for the SHM National Advocacy subcommittee of hospitalists trained in family medicine. “There is no good rhyme or reason to it. It is just there and they haven’t changed it.”
Dr. Chaudhry added that no one provides an adequate reason for the bias during the hiring process. “If you ask the recruiter, they would say ‘the employer asked me [to do it this way].’ If you ask the employers, they say ‘the hospital’s bylaws say that.’ And then, we request changes to the hospital bylaws because you don’t have access to them. So the burden of responsibility falls on the shoulders of hospitalists in leadership positions to request equal privileges from the hospital boards for FM-trained hospitalists.”
Experience, education closes some gaps
Over the years, the American Board of Family Medicine (ABFM) and SHM have offered several opportunities for family medicine doctors to demonstrate their experience and training in hospital medicine. In 2010, ABFM began offering the Focused Recognition of Hospital Medicine board examination, together with the American Board of Internal Medicine. SHM also offers hospitalist fellowships and a designation of Fellow in Hospital Medicine (FHM) for health care professionals. In 2015, ABFM and SHM released a joint statement encouraging the growth of hospitalists trained in family medicine (HTFM) and outlining these opportunities.
These measures help fill a gap in both IM and FM training, but also appear to have some effect in convincing recruiters and employers to consider family medicine doctors for hospitalist positions. An abstract published at Hospital Medicine 2014 reviewed 252 hospitalist positions listed in journals and search engines attempted to document the disparities in job listings, the perceptions of physician recruiters, and how factors like experience, training, and certification impacted a family medicine physician’s likelihood to be considered for a position. HTFMs were explicitly mentioned as being eligible in 119 of 252 positions (47%). The investigators then sent surveys out to physician recruiters of the remaining 133 positions asking whether HTFMs were being considered for the position. The results of the survey showed 66% of the recruiters were open to HTFMs, while 34% of recruiters said they did not have a willingness to hire HTFMs.
That willingness to hire changed based on the level of experience, training, and certification. More than one-fourth (29%) of physician recruiters said institutional bylaws prevented hiring of HTFMs. If respondents earned a Recognition of Focused Practice in Hospital Medicine (RFPHM) board examination, 78% of physician recruiters would reconsider hiring the candidate. If the HTFM applicant had prior experience in hospital medicine, 87% of physician recruiters said they would consider the candidate. HTFMs who earned a Designation of Fellow in Hospital Medicine (FHM) from SHM would be reconsidered by 93% of physician recruiters who initially refused the HTFM candidate. All physician recruiters said they would reconsider if the candidate had a fellowship in hospital medicine.
However, to date, there is no official American College of Graduate Medical Education (ACGME)-recognized hospitalist board certification or designated specialty credentialing. This can lead to situations where family medicine trained physicians are applying for jobs without the necessary requirements for the position, because those requirements may not be immediately obvious when first applying to a position. “There’s often no specification until you apply and then are informed that you don’t qualify – ‘Oh, no, you haven’t completed a fellowship,’ or the added qualification in hospital medicine,” Dr. McCurry said.
The 2015 joint statement from AAFP and SHM asserts that “more than two-thirds of HTFMs are also involved in the training of residents and medical students, enhancing the skills of our future physicians.” But when HTFMs do find positions, they may be limited in other ways, such as being prohibited from serving on the faculty of internal medicine residency programs and teaching internal medicine residents. When Dr. Odeti was medical director for Johnston Memorial Hospital in Abingdon, Va., he said he encountered this issue.
“If you are a hospitalist who is internal medicine trained, then you can teach FM or IM, whereas if you’re family medicine trained, you cannot teach internal medicine residents,” he said. “What happened with me, I had to prioritize recruiting internal medicine residents over FM residents to be able to staff IM teaching faculty.”
A rule change has been lobbied by SHM, under the direction of SHM family medicine SIG former chair David Goldstein, MD, to address this issue that would allow HTFMs with a FPHM designation to teach IM residents. The change was quietly made by the ACGME Review Committee for Internal Medicine in 2017, Dr. McCurry said, but implementation of the change has been slow.
“Essentially, the change was made in 2017 to allow for family medicine trainied physicians who have the FPHM designation to teach IM residents, but this knowledge has not been widely dispersed or policies updated to clearly reflect this change,” Dr. McCurry said. “It is a significant change, however, because prior to that, there were explicit policies preventing a family medicine hospitalist from teaching internal medicine residents even if they were experienced.”
FM physicians uniquely suited for HM
Requirements aside, it is “arguably not the case” that family medicine physicians need these extra certifications and fellowships to serve as hospitalists, Dr. McCurry said. It is difficult to quantify IM and FM hospitalist quality outcomes due to challenges with attribution, Dr. Odeti noted. One 2007 study published in the New England Journal of Medicine looked at patient quality and cost of care across the hospitalist model, and family medicine practitioners providing inpatients care. The investigators found similar outcomes in the internist model and with family practitioners providing inpatient care. Dr. Odeti said this research supports “the fact that family medicine physicians are equally competent as internists in providing inpatient care.”
Dr. Odeti argued that family medicine training is valuable for work as a hospitalist. “Hospital medicine is a team sport. You have a quarterback, you have a wide receiver, you have a running back. Everybody has a role to play and everybody has their own strength,” he said.
Family medicine hospitalists are uniquely positioned to handle the shift within hospital medicine from volume to value-based care. “That does not depend solely on what we do within the hospital. It depends a lot on what we do for the patients as they get out of the hospital into the community,” he explained.
Family medicine hospitalists are also well prepared to handle the continuum of care for patients in the hospital. “In their training, FM hospitalists have their own patient panels and they have complete ownership of their patient in their training, so they are prepared because they know how to set up things for outpatients,” Dr. Odeti explained.
“Every hospitalist group needs to use the family medicine doctors to their advantage,” he said. “A family medicine trained hospitalist should be part of every good hospitalist group, is what I would say.”
HTFMs are growing within SHM
HTFMs are “all over,” being represented in smaller hospitals, larger hospitals, and university hospitals in every state. “But to reach those positions, they probably have to go over more hurdles and have fewer opportunities,” Dr. Chaudhry said.
There isn’t a completely accurate count of family medicine hospitalists in the United States. Out of an estimated 50,000 hospitalists in the U. S., about 16,000 hospitalists are members of SHM. A number of family medicine hospitalists may also take AAFP membership instead of SHM, Dr. Odeti explained.
However, there are a growing number of hospitalists within SHM with a family medicine background. In the 2007-2008 Society of Hospital Medicine Annual Survey, 3.7% of U.S. hospitalists claimed family medicine training. That number increased to 6.9% of physicians who answered the SHM membership data report in 2010.
A Medscape Hospitalist Lifestyle, Happiness & Burnout Report from 2019 estimates 17% of hospitalists are trained in family medicine. In the latest State of Hospital Medicine Report published in 2020, 38.6% of hospital medicine groups containing family medicine trained physicians were part of a university, medical school, or faculty practice; 79.6% did not have academic status; 83.8% were at a non-teaching hospital; 60.7% were in a group in a non-teaching service at a teaching hospital; and 52.8% were in a group at a combination teaching/non-teaching service at a teaching hospital.
Although the Report did not specify whether family medicine hospitalists were mainly in rural or urban areas, “some of us do practice in underserved area hospitals where you have the smaller ICU model, critical access hospitals, potentially dealing with a whole gamut of inpatient medicine from ER, to the hospital inpatient adult cases, to critical care level,” Dr. McCurry said.
“But then, there are a large number of us who practice in private groups or at large hospitals, academic centers around the country,” she added. “There’s a range of family medicine trained hospitalist practice areas.”
Equal recognition for HTFM in HM
The SHM family medicine SIG has been working to highlight the issue of hiring practices for HTFMs, and is taking a number of actions to bring greater awareness and recognition to family medicine hospitalists.
The family medicine SIG is looking at steps for requesting a new joint statement from ABFM and SHM focused on hiring practices for family medicine physicians as hospitalists. “I think it’s worth considering now that we’re at a point where we comprise about one-fifth of hospitalists as family medicine docs,” Dr. McCurry said. “Is it time to take that joint statement to the next step, and seek a review of how we can improve the balance of hiring in terms of favoring more balanced consideration now that there are a lot more family medicine trained hospitalists than historically?
“I think the call is really to help us all move to that next step in terms of identifying any of the lingering vestiges of expectation that are really no longer applicable to the hiring practices, or shouldn’t be,” she said.
The next step will be to ask hospitals with internal medicine only requirements for hospitalists to update their bylaws to include family medicine physicians when considering candidates for hospitalist positions. If SHM does not make a distinction to grant Fellow in Hospital Medicine status between internal medicine and family medicine trained hospitalists, “then there should not be any distinction, or there should not be any hindrance by the recruiters, by the bigger systems, as well as by the employers” in hiring a family medicine trained physician for a hospitalist position, Dr. Chaudhry said.
Dr. Odeti, who serves in several leadership roles within Ballad Health, describes the system as being friendly to HTFMs. About one-fourth of the hospitalists in Ballad Health are trained in family medicine. But when Dr. Odeti started his hospitalist practice, he was only one of a handful of HTFMs. He sees a future where the accomplishments and contributions of HTFMs will pave the way for future hospitalists. “Access into the urban hospitals is key, and I hope that SHM and the HTFM SIG will act as a catalyst for this change,” he said.
Colleagues of family medicine hospitalists, especially those in leadership positions at hospitals, can help by raising awareness, as can “those of our colleagues who sit on medical executive committees within their hospitals to review their bylaws, to see what the policies are, and encourage more competitiveness,” Dr. McCurry said. “Truly, the best candidate for the position, regardless of background and training, is what you want. You want the best colleagues for your fellow hospitalists. You want the best physician for your patients in the hospital.”
If training and all other things are equal, family medicine physicians should be evaluated on a case-by-case basis, she said. “I think that that puts the burden back on any good medical committee, and a good medical committee member who is an SHM member as well, to say, ‘If we are committed to quality patient care, we want to encourage the recruitment of all physicians that are truly the best physicians to reduce that distinction between FM and IM in order to allow those best candidates to present, whether they are FM or IM.’ That’s all that we’re asking.”
Dr. Chaudhry emphasized that the preference for internal medicine trained physicians isn’t intentional. “It’s not as if the systems are trying to do it,” he said. “I think it is more like everybody needs to be educated. And through the platform of the Society of Hospital Medicine, I think we can make a difference. It will be a slow change, but we’ll have to keep on working on it.”
Dr. Odeti, Dr. McCurry, and Dr. Chaudhry have no relevant financial disclosures.
Outdated perceptions of family medicine
Outdated perceptions of family medicine
A family medicine trained doctor, fresh out of residency, visits a career website to scout out prospective hospitalist jobs in their region. As they scroll through the job listings, they come across one opportunity at a nearby hospital system that seems like a good fit. The listing offers a competitive salary and comprehensive benefits for the position, and mentions hospitalists in the department will have the opportunity to teach medical students.
The only problem? The position is for internal medicine trained doctors only. After searching through several more listings with the same internal medicine requirement, the pool of jobs available to the family medicine doctor seems much smaller.
When Robert M. Wachter, MD, MHM, and Lee Goldman, MD coined the term “hospitalist” in a 1996 New England Journal of Medicine article, hospitalists were primarily clinicians with an internal medicine background, filling the gap created by family medicine doctors who increasingly devoted their time to patients in their practice and spent less time rounding in the hospital.
As family medicine doctors have returned to hospital medicine, it has become difficult to find positions as hospitalists due to a preference by some recruiters and employers that favors internal medicine physicians over those who are trained in family medicine. The preference for internal medicine physicians is sometimes overt, such as a requirement on a job application. But the preference can also surface after a physician has already applied for a position, and they will then discover a recruiter is actually looking for someone with a background in internal medicine. In other cases, family medicine physicians find out after applying that applicants with a background in family medicine are considered, but they’re expected to have additional training or certification not listed on the job application.
The situation can even be as stark as a hospital system hiring an internal medicine doctor just out of residency over a family medicine doctor with years of experience as a board-certified physician. Hiring practices in large systems across multiple states sometimes don’t just favor internal medicine, they are entirely focused on internal medicine hospitalists, said experts who spoke with The Hospitalist.
Outdated perceptions of family medicine
Victoria McCurry, MD, current chair of the Society of Hospital Medicine’s family medicine Special Interest Group (SIG) Executive Committee and Faculty Director of Inpatient Services at UPMC McKeesport (Pa.) Family Medicine Residency, said hearsay inside the family medicine community influenced her first job search looking for hospitalist positions as a family medicine physician.
“I was intentional about choosing places that I assumed would be open to family medicine,” she said. “I avoided the downtown urban academic hospitals, the ones that had a large internal medicine residency and fellowship presence, because I assumed that they would not hire me.
“There’s a recognition that depending on the system that you’re in and their history with family medicine trained hospitalists, it can be difficult as a family physician to seek employment,” Dr. McCurry said.
“When I graduated from my residency in 2014, I did not have the same opportunities to be a hospitalist as an internal medicine resident would have,” said Shyam Odeti, MD, a family-practice-trained hospitalist who works at Ballad Health in Johnson City, Tenn. “The perception is family medicine physicians are not trained for hospitalist practice. It’s an old perception.”
This perception may have to do with the mindset of the leadership where a doctor has had residency training, according to Usman Chaudhry, MD, a family medicine hospitalist with Texas Health Physicians Group and leader of the National Advocacy subcommittee for the Family Medicine Executive Council in SHM. Residents trained in bigger university hospital systems where internal medicine (IM) residents do mostly inpatient – in addition to outpatient services – and family medicine (FM) residents do mostly outpatient – including pediatrics and ob/gyn clinics in addition to inpatient services – may believe that to be the case in other systems too, Dr. Chaudhry explained.
“When you go to community hospital residency programs, it’s totally different,” he said. “It all depends. If you have only family medicine residency in a community hospital, they tend to do all training of inpatient clinical medicine, as IM training would in any other program”
Dr. McCurry noted that there seems to be a persisting, mental assumption that as a family medicine doctor, you’re only going to be practicing outpatient only or maybe urgent care, which is historically just not the case. “If that’s ingrained within the local hospital system, then it will be difficult for that system to hire a family medicine-trained hospitalist,” she said.
Another source of outdated perceptions of family medicine come from hospital and institutional bylaws that have written internal medicine training in as a requirement for hospitalists. “In many bigger systems, and even in the smaller hospital community and regional hospitals, the bylaws of the hospitals were written approximately 20 years ago,” Dr. Chaudhry said.
Unless someone has advocated for updating a hospital or institution’s bylaws, they may have outdated requirements for hospitalists. “The situation right now is, in a lot of urban hospitals, they would be able to give a hospitalist position to internal medicine residents who just graduated, not even board certified, but they cannot give it to a hospitalist trained in family medicine who has worked for 10 years and is board certified, just because of the bylaws,” said Dr. Odeti who is also co-chair for the SHM National Advocacy subcommittee of hospitalists trained in family medicine. “There is no good rhyme or reason to it. It is just there and they haven’t changed it.”
Dr. Chaudhry added that no one provides an adequate reason for the bias during the hiring process. “If you ask the recruiter, they would say ‘the employer asked me [to do it this way].’ If you ask the employers, they say ‘the hospital’s bylaws say that.’ And then, we request changes to the hospital bylaws because you don’t have access to them. So the burden of responsibility falls on the shoulders of hospitalists in leadership positions to request equal privileges from the hospital boards for FM-trained hospitalists.”
Experience, education closes some gaps
Over the years, the American Board of Family Medicine (ABFM) and SHM have offered several opportunities for family medicine doctors to demonstrate their experience and training in hospital medicine. In 2010, ABFM began offering the Focused Recognition of Hospital Medicine board examination, together with the American Board of Internal Medicine. SHM also offers hospitalist fellowships and a designation of Fellow in Hospital Medicine (FHM) for health care professionals. In 2015, ABFM and SHM released a joint statement encouraging the growth of hospitalists trained in family medicine (HTFM) and outlining these opportunities.
These measures help fill a gap in both IM and FM training, but also appear to have some effect in convincing recruiters and employers to consider family medicine doctors for hospitalist positions. An abstract published at Hospital Medicine 2014 reviewed 252 hospitalist positions listed in journals and search engines attempted to document the disparities in job listings, the perceptions of physician recruiters, and how factors like experience, training, and certification impacted a family medicine physician’s likelihood to be considered for a position. HTFMs were explicitly mentioned as being eligible in 119 of 252 positions (47%). The investigators then sent surveys out to physician recruiters of the remaining 133 positions asking whether HTFMs were being considered for the position. The results of the survey showed 66% of the recruiters were open to HTFMs, while 34% of recruiters said they did not have a willingness to hire HTFMs.
That willingness to hire changed based on the level of experience, training, and certification. More than one-fourth (29%) of physician recruiters said institutional bylaws prevented hiring of HTFMs. If respondents earned a Recognition of Focused Practice in Hospital Medicine (RFPHM) board examination, 78% of physician recruiters would reconsider hiring the candidate. If the HTFM applicant had prior experience in hospital medicine, 87% of physician recruiters said they would consider the candidate. HTFMs who earned a Designation of Fellow in Hospital Medicine (FHM) from SHM would be reconsidered by 93% of physician recruiters who initially refused the HTFM candidate. All physician recruiters said they would reconsider if the candidate had a fellowship in hospital medicine.
However, to date, there is no official American College of Graduate Medical Education (ACGME)-recognized hospitalist board certification or designated specialty credentialing. This can lead to situations where family medicine trained physicians are applying for jobs without the necessary requirements for the position, because those requirements may not be immediately obvious when first applying to a position. “There’s often no specification until you apply and then are informed that you don’t qualify – ‘Oh, no, you haven’t completed a fellowship,’ or the added qualification in hospital medicine,” Dr. McCurry said.
The 2015 joint statement from AAFP and SHM asserts that “more than two-thirds of HTFMs are also involved in the training of residents and medical students, enhancing the skills of our future physicians.” But when HTFMs do find positions, they may be limited in other ways, such as being prohibited from serving on the faculty of internal medicine residency programs and teaching internal medicine residents. When Dr. Odeti was medical director for Johnston Memorial Hospital in Abingdon, Va., he said he encountered this issue.
“If you are a hospitalist who is internal medicine trained, then you can teach FM or IM, whereas if you’re family medicine trained, you cannot teach internal medicine residents,” he said. “What happened with me, I had to prioritize recruiting internal medicine residents over FM residents to be able to staff IM teaching faculty.”
A rule change has been lobbied by SHM, under the direction of SHM family medicine SIG former chair David Goldstein, MD, to address this issue that would allow HTFMs with a FPHM designation to teach IM residents. The change was quietly made by the ACGME Review Committee for Internal Medicine in 2017, Dr. McCurry said, but implementation of the change has been slow.
“Essentially, the change was made in 2017 to allow for family medicine trainied physicians who have the FPHM designation to teach IM residents, but this knowledge has not been widely dispersed or policies updated to clearly reflect this change,” Dr. McCurry said. “It is a significant change, however, because prior to that, there were explicit policies preventing a family medicine hospitalist from teaching internal medicine residents even if they were experienced.”
FM physicians uniquely suited for HM
Requirements aside, it is “arguably not the case” that family medicine physicians need these extra certifications and fellowships to serve as hospitalists, Dr. McCurry said. It is difficult to quantify IM and FM hospitalist quality outcomes due to challenges with attribution, Dr. Odeti noted. One 2007 study published in the New England Journal of Medicine looked at patient quality and cost of care across the hospitalist model, and family medicine practitioners providing inpatients care. The investigators found similar outcomes in the internist model and with family practitioners providing inpatient care. Dr. Odeti said this research supports “the fact that family medicine physicians are equally competent as internists in providing inpatient care.”
Dr. Odeti argued that family medicine training is valuable for work as a hospitalist. “Hospital medicine is a team sport. You have a quarterback, you have a wide receiver, you have a running back. Everybody has a role to play and everybody has their own strength,” he said.
Family medicine hospitalists are uniquely positioned to handle the shift within hospital medicine from volume to value-based care. “That does not depend solely on what we do within the hospital. It depends a lot on what we do for the patients as they get out of the hospital into the community,” he explained.
Family medicine hospitalists are also well prepared to handle the continuum of care for patients in the hospital. “In their training, FM hospitalists have their own patient panels and they have complete ownership of their patient in their training, so they are prepared because they know how to set up things for outpatients,” Dr. Odeti explained.
“Every hospitalist group needs to use the family medicine doctors to their advantage,” he said. “A family medicine trained hospitalist should be part of every good hospitalist group, is what I would say.”
HTFMs are growing within SHM
HTFMs are “all over,” being represented in smaller hospitals, larger hospitals, and university hospitals in every state. “But to reach those positions, they probably have to go over more hurdles and have fewer opportunities,” Dr. Chaudhry said.
There isn’t a completely accurate count of family medicine hospitalists in the United States. Out of an estimated 50,000 hospitalists in the U. S., about 16,000 hospitalists are members of SHM. A number of family medicine hospitalists may also take AAFP membership instead of SHM, Dr. Odeti explained.
However, there are a growing number of hospitalists within SHM with a family medicine background. In the 2007-2008 Society of Hospital Medicine Annual Survey, 3.7% of U.S. hospitalists claimed family medicine training. That number increased to 6.9% of physicians who answered the SHM membership data report in 2010.
A Medscape Hospitalist Lifestyle, Happiness & Burnout Report from 2019 estimates 17% of hospitalists are trained in family medicine. In the latest State of Hospital Medicine Report published in 2020, 38.6% of hospital medicine groups containing family medicine trained physicians were part of a university, medical school, or faculty practice; 79.6% did not have academic status; 83.8% were at a non-teaching hospital; 60.7% were in a group in a non-teaching service at a teaching hospital; and 52.8% were in a group at a combination teaching/non-teaching service at a teaching hospital.
Although the Report did not specify whether family medicine hospitalists were mainly in rural or urban areas, “some of us do practice in underserved area hospitals where you have the smaller ICU model, critical access hospitals, potentially dealing with a whole gamut of inpatient medicine from ER, to the hospital inpatient adult cases, to critical care level,” Dr. McCurry said.
“But then, there are a large number of us who practice in private groups or at large hospitals, academic centers around the country,” she added. “There’s a range of family medicine trained hospitalist practice areas.”
Equal recognition for HTFM in HM
The SHM family medicine SIG has been working to highlight the issue of hiring practices for HTFMs, and is taking a number of actions to bring greater awareness and recognition to family medicine hospitalists.
The family medicine SIG is looking at steps for requesting a new joint statement from ABFM and SHM focused on hiring practices for family medicine physicians as hospitalists. “I think it’s worth considering now that we’re at a point where we comprise about one-fifth of hospitalists as family medicine docs,” Dr. McCurry said. “Is it time to take that joint statement to the next step, and seek a review of how we can improve the balance of hiring in terms of favoring more balanced consideration now that there are a lot more family medicine trained hospitalists than historically?
“I think the call is really to help us all move to that next step in terms of identifying any of the lingering vestiges of expectation that are really no longer applicable to the hiring practices, or shouldn’t be,” she said.
The next step will be to ask hospitals with internal medicine only requirements for hospitalists to update their bylaws to include family medicine physicians when considering candidates for hospitalist positions. If SHM does not make a distinction to grant Fellow in Hospital Medicine status between internal medicine and family medicine trained hospitalists, “then there should not be any distinction, or there should not be any hindrance by the recruiters, by the bigger systems, as well as by the employers” in hiring a family medicine trained physician for a hospitalist position, Dr. Chaudhry said.
Dr. Odeti, who serves in several leadership roles within Ballad Health, describes the system as being friendly to HTFMs. About one-fourth of the hospitalists in Ballad Health are trained in family medicine. But when Dr. Odeti started his hospitalist practice, he was only one of a handful of HTFMs. He sees a future where the accomplishments and contributions of HTFMs will pave the way for future hospitalists. “Access into the urban hospitals is key, and I hope that SHM and the HTFM SIG will act as a catalyst for this change,” he said.
Colleagues of family medicine hospitalists, especially those in leadership positions at hospitals, can help by raising awareness, as can “those of our colleagues who sit on medical executive committees within their hospitals to review their bylaws, to see what the policies are, and encourage more competitiveness,” Dr. McCurry said. “Truly, the best candidate for the position, regardless of background and training, is what you want. You want the best colleagues for your fellow hospitalists. You want the best physician for your patients in the hospital.”
If training and all other things are equal, family medicine physicians should be evaluated on a case-by-case basis, she said. “I think that that puts the burden back on any good medical committee, and a good medical committee member who is an SHM member as well, to say, ‘If we are committed to quality patient care, we want to encourage the recruitment of all physicians that are truly the best physicians to reduce that distinction between FM and IM in order to allow those best candidates to present, whether they are FM or IM.’ That’s all that we’re asking.”
Dr. Chaudhry emphasized that the preference for internal medicine trained physicians isn’t intentional. “It’s not as if the systems are trying to do it,” he said. “I think it is more like everybody needs to be educated. And through the platform of the Society of Hospital Medicine, I think we can make a difference. It will be a slow change, but we’ll have to keep on working on it.”
Dr. Odeti, Dr. McCurry, and Dr. Chaudhry have no relevant financial disclosures.
A family medicine trained doctor, fresh out of residency, visits a career website to scout out prospective hospitalist jobs in their region. As they scroll through the job listings, they come across one opportunity at a nearby hospital system that seems like a good fit. The listing offers a competitive salary and comprehensive benefits for the position, and mentions hospitalists in the department will have the opportunity to teach medical students.
The only problem? The position is for internal medicine trained doctors only. After searching through several more listings with the same internal medicine requirement, the pool of jobs available to the family medicine doctor seems much smaller.
When Robert M. Wachter, MD, MHM, and Lee Goldman, MD coined the term “hospitalist” in a 1996 New England Journal of Medicine article, hospitalists were primarily clinicians with an internal medicine background, filling the gap created by family medicine doctors who increasingly devoted their time to patients in their practice and spent less time rounding in the hospital.
As family medicine doctors have returned to hospital medicine, it has become difficult to find positions as hospitalists due to a preference by some recruiters and employers that favors internal medicine physicians over those who are trained in family medicine. The preference for internal medicine physicians is sometimes overt, such as a requirement on a job application. But the preference can also surface after a physician has already applied for a position, and they will then discover a recruiter is actually looking for someone with a background in internal medicine. In other cases, family medicine physicians find out after applying that applicants with a background in family medicine are considered, but they’re expected to have additional training or certification not listed on the job application.
The situation can even be as stark as a hospital system hiring an internal medicine doctor just out of residency over a family medicine doctor with years of experience as a board-certified physician. Hiring practices in large systems across multiple states sometimes don’t just favor internal medicine, they are entirely focused on internal medicine hospitalists, said experts who spoke with The Hospitalist.
Outdated perceptions of family medicine
Victoria McCurry, MD, current chair of the Society of Hospital Medicine’s family medicine Special Interest Group (SIG) Executive Committee and Faculty Director of Inpatient Services at UPMC McKeesport (Pa.) Family Medicine Residency, said hearsay inside the family medicine community influenced her first job search looking for hospitalist positions as a family medicine physician.
“I was intentional about choosing places that I assumed would be open to family medicine,” she said. “I avoided the downtown urban academic hospitals, the ones that had a large internal medicine residency and fellowship presence, because I assumed that they would not hire me.
“There’s a recognition that depending on the system that you’re in and their history with family medicine trained hospitalists, it can be difficult as a family physician to seek employment,” Dr. McCurry said.
“When I graduated from my residency in 2014, I did not have the same opportunities to be a hospitalist as an internal medicine resident would have,” said Shyam Odeti, MD, a family-practice-trained hospitalist who works at Ballad Health in Johnson City, Tenn. “The perception is family medicine physicians are not trained for hospitalist practice. It’s an old perception.”
This perception may have to do with the mindset of the leadership where a doctor has had residency training, according to Usman Chaudhry, MD, a family medicine hospitalist with Texas Health Physicians Group and leader of the National Advocacy subcommittee for the Family Medicine Executive Council in SHM. Residents trained in bigger university hospital systems where internal medicine (IM) residents do mostly inpatient – in addition to outpatient services – and family medicine (FM) residents do mostly outpatient – including pediatrics and ob/gyn clinics in addition to inpatient services – may believe that to be the case in other systems too, Dr. Chaudhry explained.
“When you go to community hospital residency programs, it’s totally different,” he said. “It all depends. If you have only family medicine residency in a community hospital, they tend to do all training of inpatient clinical medicine, as IM training would in any other program”
Dr. McCurry noted that there seems to be a persisting, mental assumption that as a family medicine doctor, you’re only going to be practicing outpatient only or maybe urgent care, which is historically just not the case. “If that’s ingrained within the local hospital system, then it will be difficult for that system to hire a family medicine-trained hospitalist,” she said.
Another source of outdated perceptions of family medicine come from hospital and institutional bylaws that have written internal medicine training in as a requirement for hospitalists. “In many bigger systems, and even in the smaller hospital community and regional hospitals, the bylaws of the hospitals were written approximately 20 years ago,” Dr. Chaudhry said.
Unless someone has advocated for updating a hospital or institution’s bylaws, they may have outdated requirements for hospitalists. “The situation right now is, in a lot of urban hospitals, they would be able to give a hospitalist position to internal medicine residents who just graduated, not even board certified, but they cannot give it to a hospitalist trained in family medicine who has worked for 10 years and is board certified, just because of the bylaws,” said Dr. Odeti who is also co-chair for the SHM National Advocacy subcommittee of hospitalists trained in family medicine. “There is no good rhyme or reason to it. It is just there and they haven’t changed it.”
Dr. Chaudhry added that no one provides an adequate reason for the bias during the hiring process. “If you ask the recruiter, they would say ‘the employer asked me [to do it this way].’ If you ask the employers, they say ‘the hospital’s bylaws say that.’ And then, we request changes to the hospital bylaws because you don’t have access to them. So the burden of responsibility falls on the shoulders of hospitalists in leadership positions to request equal privileges from the hospital boards for FM-trained hospitalists.”
Experience, education closes some gaps
Over the years, the American Board of Family Medicine (ABFM) and SHM have offered several opportunities for family medicine doctors to demonstrate their experience and training in hospital medicine. In 2010, ABFM began offering the Focused Recognition of Hospital Medicine board examination, together with the American Board of Internal Medicine. SHM also offers hospitalist fellowships and a designation of Fellow in Hospital Medicine (FHM) for health care professionals. In 2015, ABFM and SHM released a joint statement encouraging the growth of hospitalists trained in family medicine (HTFM) and outlining these opportunities.
These measures help fill a gap in both IM and FM training, but also appear to have some effect in convincing recruiters and employers to consider family medicine doctors for hospitalist positions. An abstract published at Hospital Medicine 2014 reviewed 252 hospitalist positions listed in journals and search engines attempted to document the disparities in job listings, the perceptions of physician recruiters, and how factors like experience, training, and certification impacted a family medicine physician’s likelihood to be considered for a position. HTFMs were explicitly mentioned as being eligible in 119 of 252 positions (47%). The investigators then sent surveys out to physician recruiters of the remaining 133 positions asking whether HTFMs were being considered for the position. The results of the survey showed 66% of the recruiters were open to HTFMs, while 34% of recruiters said they did not have a willingness to hire HTFMs.
That willingness to hire changed based on the level of experience, training, and certification. More than one-fourth (29%) of physician recruiters said institutional bylaws prevented hiring of HTFMs. If respondents earned a Recognition of Focused Practice in Hospital Medicine (RFPHM) board examination, 78% of physician recruiters would reconsider hiring the candidate. If the HTFM applicant had prior experience in hospital medicine, 87% of physician recruiters said they would consider the candidate. HTFMs who earned a Designation of Fellow in Hospital Medicine (FHM) from SHM would be reconsidered by 93% of physician recruiters who initially refused the HTFM candidate. All physician recruiters said they would reconsider if the candidate had a fellowship in hospital medicine.
However, to date, there is no official American College of Graduate Medical Education (ACGME)-recognized hospitalist board certification or designated specialty credentialing. This can lead to situations where family medicine trained physicians are applying for jobs without the necessary requirements for the position, because those requirements may not be immediately obvious when first applying to a position. “There’s often no specification until you apply and then are informed that you don’t qualify – ‘Oh, no, you haven’t completed a fellowship,’ or the added qualification in hospital medicine,” Dr. McCurry said.
The 2015 joint statement from AAFP and SHM asserts that “more than two-thirds of HTFMs are also involved in the training of residents and medical students, enhancing the skills of our future physicians.” But when HTFMs do find positions, they may be limited in other ways, such as being prohibited from serving on the faculty of internal medicine residency programs and teaching internal medicine residents. When Dr. Odeti was medical director for Johnston Memorial Hospital in Abingdon, Va., he said he encountered this issue.
“If you are a hospitalist who is internal medicine trained, then you can teach FM or IM, whereas if you’re family medicine trained, you cannot teach internal medicine residents,” he said. “What happened with me, I had to prioritize recruiting internal medicine residents over FM residents to be able to staff IM teaching faculty.”
A rule change has been lobbied by SHM, under the direction of SHM family medicine SIG former chair David Goldstein, MD, to address this issue that would allow HTFMs with a FPHM designation to teach IM residents. The change was quietly made by the ACGME Review Committee for Internal Medicine in 2017, Dr. McCurry said, but implementation of the change has been slow.
“Essentially, the change was made in 2017 to allow for family medicine trainied physicians who have the FPHM designation to teach IM residents, but this knowledge has not been widely dispersed or policies updated to clearly reflect this change,” Dr. McCurry said. “It is a significant change, however, because prior to that, there were explicit policies preventing a family medicine hospitalist from teaching internal medicine residents even if they were experienced.”
FM physicians uniquely suited for HM
Requirements aside, it is “arguably not the case” that family medicine physicians need these extra certifications and fellowships to serve as hospitalists, Dr. McCurry said. It is difficult to quantify IM and FM hospitalist quality outcomes due to challenges with attribution, Dr. Odeti noted. One 2007 study published in the New England Journal of Medicine looked at patient quality and cost of care across the hospitalist model, and family medicine practitioners providing inpatients care. The investigators found similar outcomes in the internist model and with family practitioners providing inpatient care. Dr. Odeti said this research supports “the fact that family medicine physicians are equally competent as internists in providing inpatient care.”
Dr. Odeti argued that family medicine training is valuable for work as a hospitalist. “Hospital medicine is a team sport. You have a quarterback, you have a wide receiver, you have a running back. Everybody has a role to play and everybody has their own strength,” he said.
Family medicine hospitalists are uniquely positioned to handle the shift within hospital medicine from volume to value-based care. “That does not depend solely on what we do within the hospital. It depends a lot on what we do for the patients as they get out of the hospital into the community,” he explained.
Family medicine hospitalists are also well prepared to handle the continuum of care for patients in the hospital. “In their training, FM hospitalists have their own patient panels and they have complete ownership of their patient in their training, so they are prepared because they know how to set up things for outpatients,” Dr. Odeti explained.
“Every hospitalist group needs to use the family medicine doctors to their advantage,” he said. “A family medicine trained hospitalist should be part of every good hospitalist group, is what I would say.”
HTFMs are growing within SHM
HTFMs are “all over,” being represented in smaller hospitals, larger hospitals, and university hospitals in every state. “But to reach those positions, they probably have to go over more hurdles and have fewer opportunities,” Dr. Chaudhry said.
There isn’t a completely accurate count of family medicine hospitalists in the United States. Out of an estimated 50,000 hospitalists in the U. S., about 16,000 hospitalists are members of SHM. A number of family medicine hospitalists may also take AAFP membership instead of SHM, Dr. Odeti explained.
However, there are a growing number of hospitalists within SHM with a family medicine background. In the 2007-2008 Society of Hospital Medicine Annual Survey, 3.7% of U.S. hospitalists claimed family medicine training. That number increased to 6.9% of physicians who answered the SHM membership data report in 2010.
A Medscape Hospitalist Lifestyle, Happiness & Burnout Report from 2019 estimates 17% of hospitalists are trained in family medicine. In the latest State of Hospital Medicine Report published in 2020, 38.6% of hospital medicine groups containing family medicine trained physicians were part of a university, medical school, or faculty practice; 79.6% did not have academic status; 83.8% were at a non-teaching hospital; 60.7% were in a group in a non-teaching service at a teaching hospital; and 52.8% were in a group at a combination teaching/non-teaching service at a teaching hospital.
Although the Report did not specify whether family medicine hospitalists were mainly in rural or urban areas, “some of us do practice in underserved area hospitals where you have the smaller ICU model, critical access hospitals, potentially dealing with a whole gamut of inpatient medicine from ER, to the hospital inpatient adult cases, to critical care level,” Dr. McCurry said.
“But then, there are a large number of us who practice in private groups or at large hospitals, academic centers around the country,” she added. “There’s a range of family medicine trained hospitalist practice areas.”
Equal recognition for HTFM in HM
The SHM family medicine SIG has been working to highlight the issue of hiring practices for HTFMs, and is taking a number of actions to bring greater awareness and recognition to family medicine hospitalists.
The family medicine SIG is looking at steps for requesting a new joint statement from ABFM and SHM focused on hiring practices for family medicine physicians as hospitalists. “I think it’s worth considering now that we’re at a point where we comprise about one-fifth of hospitalists as family medicine docs,” Dr. McCurry said. “Is it time to take that joint statement to the next step, and seek a review of how we can improve the balance of hiring in terms of favoring more balanced consideration now that there are a lot more family medicine trained hospitalists than historically?
“I think the call is really to help us all move to that next step in terms of identifying any of the lingering vestiges of expectation that are really no longer applicable to the hiring practices, or shouldn’t be,” she said.
The next step will be to ask hospitals with internal medicine only requirements for hospitalists to update their bylaws to include family medicine physicians when considering candidates for hospitalist positions. If SHM does not make a distinction to grant Fellow in Hospital Medicine status between internal medicine and family medicine trained hospitalists, “then there should not be any distinction, or there should not be any hindrance by the recruiters, by the bigger systems, as well as by the employers” in hiring a family medicine trained physician for a hospitalist position, Dr. Chaudhry said.
Dr. Odeti, who serves in several leadership roles within Ballad Health, describes the system as being friendly to HTFMs. About one-fourth of the hospitalists in Ballad Health are trained in family medicine. But when Dr. Odeti started his hospitalist practice, he was only one of a handful of HTFMs. He sees a future where the accomplishments and contributions of HTFMs will pave the way for future hospitalists. “Access into the urban hospitals is key, and I hope that SHM and the HTFM SIG will act as a catalyst for this change,” he said.
Colleagues of family medicine hospitalists, especially those in leadership positions at hospitals, can help by raising awareness, as can “those of our colleagues who sit on medical executive committees within their hospitals to review their bylaws, to see what the policies are, and encourage more competitiveness,” Dr. McCurry said. “Truly, the best candidate for the position, regardless of background and training, is what you want. You want the best colleagues for your fellow hospitalists. You want the best physician for your patients in the hospital.”
If training and all other things are equal, family medicine physicians should be evaluated on a case-by-case basis, she said. “I think that that puts the burden back on any good medical committee, and a good medical committee member who is an SHM member as well, to say, ‘If we are committed to quality patient care, we want to encourage the recruitment of all physicians that are truly the best physicians to reduce that distinction between FM and IM in order to allow those best candidates to present, whether they are FM or IM.’ That’s all that we’re asking.”
Dr. Chaudhry emphasized that the preference for internal medicine trained physicians isn’t intentional. “It’s not as if the systems are trying to do it,” he said. “I think it is more like everybody needs to be educated. And through the platform of the Society of Hospital Medicine, I think we can make a difference. It will be a slow change, but we’ll have to keep on working on it.”
Dr. Odeti, Dr. McCurry, and Dr. Chaudhry have no relevant financial disclosures.
Understanding messenger RNA and other SARS-CoV-2 vaccines
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.