User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Procalcitonin-guided antibiotic stewardship for lower respiratory tract infection
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 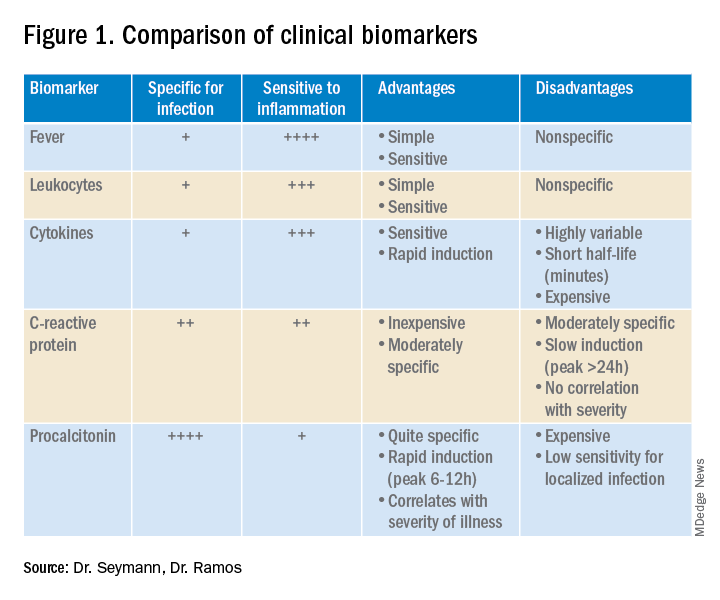
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)
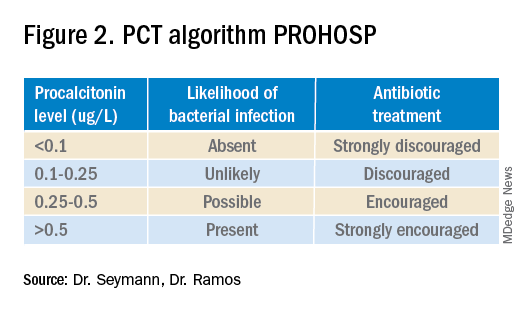
For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.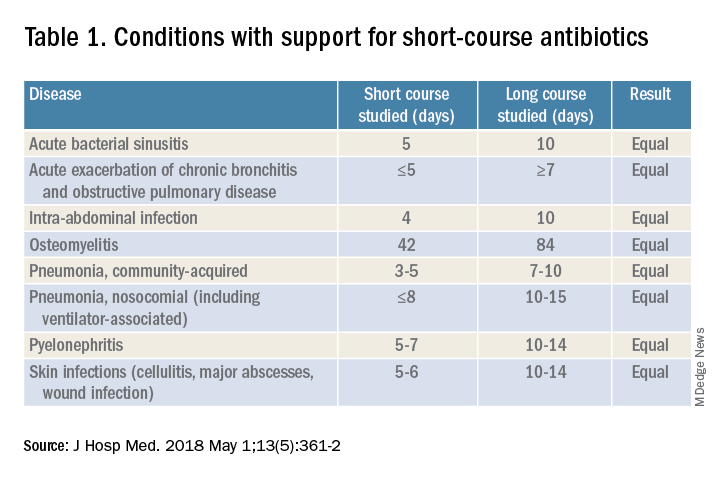
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.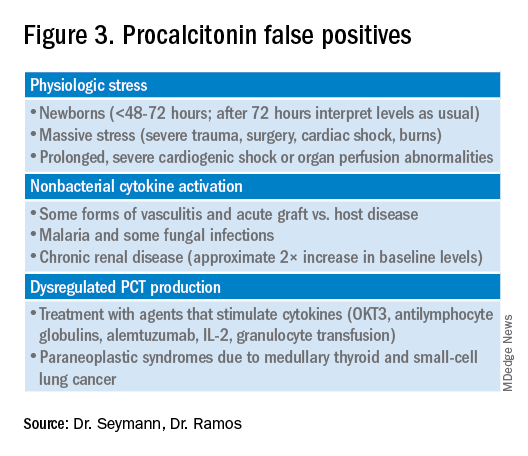
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Dynamics of the assay must be considered
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
COVID-19 in children: Weekly cases drop to 6-month low
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
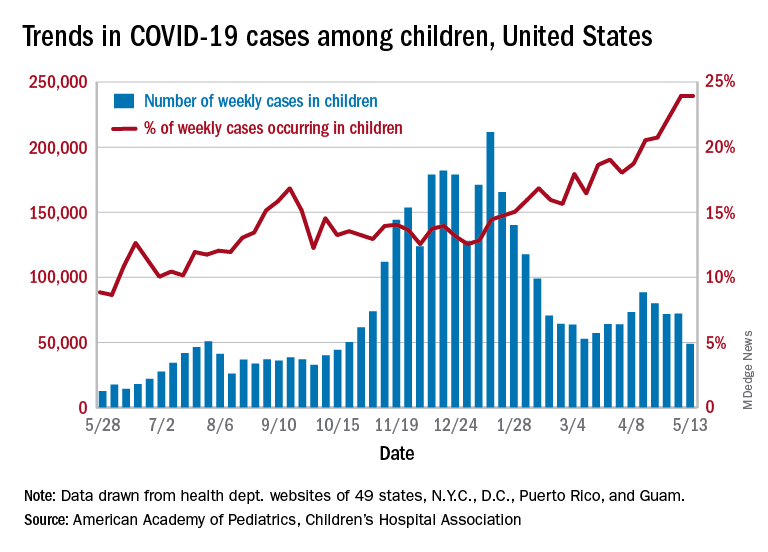
the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Dr. Fauci: Extraordinary challenges, scientific triumphs with COVID-19
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
FIDELIO-DKD: Finerenone cuts new-onset AFib in patients with type 2 diabetes and CKD
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
FROM ACC 2021
Dapagliflozin misses as treatment for COVID-19 but leaves intriguing signal for benefit
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
FROM ACC 2021
Planning for SHM Converge 2022 now underway
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
Omics analysis links blood type to COVID-19
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PARADISE-MI: Sacubitril/valsartan can’t beat ramipril in patients with acute MI
Treatment with sacubitril/valsartan, a pillar of therapy for patients with chronic heart failure with below-normal ejection fraction, came suggestively close to showing efficacy for preventing cardiovascular death or heart failure events in patients who have just had an MI but have no history of heart failure in a controlled trial with more than 5,600 patients.
Although sacubitril/valsartan (Entresto) fell short of producing a significant benefit, it did show good safety that was similar to the study’s comparator treatment, ramipril, an agent from the angiotensin-converting enzyme inhibitor class that is a mainstay of treatment in these patients.
“To say that, with no run-in, sacubitril/valsartan is as well tolerated and as safe as one of the best-studied ACE inhibitors – ramipril – in acutely ill MI patients, is a big statement,” said Marc A. Pfeffer, MD, at the annual scientific sessions of the American College of Cardiology. This high level of safety without gradual uptitration of sacubitril/valsartan (Entresto) “should lower barriers” to broader use of the dual-drug formulation for its approved indication in patients with chronic heart failure, especially patients with a left ventricular ejection fraction that is below normal. In addition, results from the PARADISE-MI trial suggested that “patients seemed to benefit before they develop heart failure. We couldn’t prove that, but we should build on this, and make it easier for patients to use this treatment,” Dr. Pfeffer said during a press briefing following his talk at the sessions.
Preventing heart failures to come
Treatment with sacubitril/valsartan in acute MI patients within a few days of their event “is perhaps addressing prevention of the heart failure that’s to come,” commented Lynne W. Stevenson, MD, designated discussant for the report and professor of medicine at Vanderbilt University Medical Center in Nashville. “Patients who are destined to develop heart failure are beginning their treatment early. The subgroup analyses suggest that it’s the sicker patients who benefited the most,” she said.
But Dr. Pfeffer stressed that “I don’t think this is a subgroup discussion. I would like to pursue this, but that’s up to the sponsor,” Novartis, the company that markets sacubitril/valsartan.
‘Exceedingly reassuring’ safety
The safety data that Dr. Pfeffer reported “are exceedingly reassuring. We didn’t see a signal of harm, and in some of the exploratory endpoints there was some evidence of benefit, so we need to encourage you to continue,” commented Mary N. Walsh, MD, medical director of the heart failure and cardiac transplantation program at Ascension St. Vincent Heart Center of Indiana in Indianapolis.
The PARADISE-MI (Prospective ARNI vs. ACE Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After MI) trial enrolled 5,669 patients with no history of heart failure within an average of 4 days following an acute MI at 495 sites in 41 countries during 2016-2020, with 8% of enrolled patients from the United States. Patients averaged 64 years of age, about three-quarters were men, about 43% had a history of diabetes, and only 1% were Black; Dr. Pfeffer noted that this is because most patients came from countries with low Black populations. The enrollment criteria required a left ventricular ejection fraction no greater than 40%, and among the enrolled patients this averaged about 37%.
A 10% nonsignificant relative risk reduction for the primary endpoint
The study’s primary endpoint was the combined first-event rate of cardiovascular death, hospitalization for heart failure, or an outpatient visit for heart failure. During a median follow-up of 23 months, this occurred at a rate of 7.4/100 patient years in the ramipril arm and 6.7/100 patient years in the sacubitril/valsartan arm, a 10% relative risk reduction with sacubitril/valsartan that was not significant, which meant all other efficacy analyses were exploratory, Dr. Pfeffer stressed.
Several secondary efficacy analyses showed significant benefits from sacubitril/valsartan, compared with ramipril, including the total number of events that comprised the primary endpoint, with a 21% relative risk reduction associated with sacubitril/valsartan, as well as investigator-reported events. The primary-endpoint benefit from sacubitril/valsartan was also significant in two subgroup analyses: patients aged 65 years or older (roughly half the study cohort), who had a 24% relative risk reduction on sacubitril/valsartan, compared with ramipril, and the 88% of patients who received treatment with percutaneous coronary intervention for their acute MI, who had a 19% relative risk reduction on sacubitril/valsartan, compared with patients who received ramipril.
The study’s safety data showed nearly identical rates in the two treatment arms for total adverse events, serious adverse events, adverse events that led to stopping the study drug, as well as in laboratory measures. The biggest between-treatment differences were a modest excess of hypotension on sacubitril valsartan, 28%, compared with 22% on ramipril, and a modest excess rate of cough on ramipril, 13%, compared with 9% on sacubitril/valsartan.
The added insight the results provide about sacubitril/valsartan comes at a time when U.S. patients continue to struggle to get health insurance coverage for an agent that has been approved for U.S. use in treating heart failure since 2015.
“Our patients do not have access to this important treatment,” declared Dr. Walsh during the press briefing. “The prior authorization process is unbelievable, and some patients have no access unless they pay the full cost on their own. This is an important, real-world problem that we face with this drug.”
PARADISE-MI was sponsored by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Pfeffer has received research funding from and is a consultant to Novartis. He is also a consultant to AstraZeneca, Boehringer Ingelheim, Corvidia, DalCor, Eli Lilly, GlaxoSmithKline, Novo Nordisk, Peerbridge, and Sanofi, and he holds equity in DalCor and Peerbridge. Dr. Stevenson has received honoraria from LivaNova and has received research support from Abbott. Dr. Walsh had no disclosures.
Treatment with sacubitril/valsartan, a pillar of therapy for patients with chronic heart failure with below-normal ejection fraction, came suggestively close to showing efficacy for preventing cardiovascular death or heart failure events in patients who have just had an MI but have no history of heart failure in a controlled trial with more than 5,600 patients.
Although sacubitril/valsartan (Entresto) fell short of producing a significant benefit, it did show good safety that was similar to the study’s comparator treatment, ramipril, an agent from the angiotensin-converting enzyme inhibitor class that is a mainstay of treatment in these patients.
“To say that, with no run-in, sacubitril/valsartan is as well tolerated and as safe as one of the best-studied ACE inhibitors – ramipril – in acutely ill MI patients, is a big statement,” said Marc A. Pfeffer, MD, at the annual scientific sessions of the American College of Cardiology. This high level of safety without gradual uptitration of sacubitril/valsartan (Entresto) “should lower barriers” to broader use of the dual-drug formulation for its approved indication in patients with chronic heart failure, especially patients with a left ventricular ejection fraction that is below normal. In addition, results from the PARADISE-MI trial suggested that “patients seemed to benefit before they develop heart failure. We couldn’t prove that, but we should build on this, and make it easier for patients to use this treatment,” Dr. Pfeffer said during a press briefing following his talk at the sessions.
Preventing heart failures to come
Treatment with sacubitril/valsartan in acute MI patients within a few days of their event “is perhaps addressing prevention of the heart failure that’s to come,” commented Lynne W. Stevenson, MD, designated discussant for the report and professor of medicine at Vanderbilt University Medical Center in Nashville. “Patients who are destined to develop heart failure are beginning their treatment early. The subgroup analyses suggest that it’s the sicker patients who benefited the most,” she said.
But Dr. Pfeffer stressed that “I don’t think this is a subgroup discussion. I would like to pursue this, but that’s up to the sponsor,” Novartis, the company that markets sacubitril/valsartan.
‘Exceedingly reassuring’ safety
The safety data that Dr. Pfeffer reported “are exceedingly reassuring. We didn’t see a signal of harm, and in some of the exploratory endpoints there was some evidence of benefit, so we need to encourage you to continue,” commented Mary N. Walsh, MD, medical director of the heart failure and cardiac transplantation program at Ascension St. Vincent Heart Center of Indiana in Indianapolis.
The PARADISE-MI (Prospective ARNI vs. ACE Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After MI) trial enrolled 5,669 patients with no history of heart failure within an average of 4 days following an acute MI at 495 sites in 41 countries during 2016-2020, with 8% of enrolled patients from the United States. Patients averaged 64 years of age, about three-quarters were men, about 43% had a history of diabetes, and only 1% were Black; Dr. Pfeffer noted that this is because most patients came from countries with low Black populations. The enrollment criteria required a left ventricular ejection fraction no greater than 40%, and among the enrolled patients this averaged about 37%.
A 10% nonsignificant relative risk reduction for the primary endpoint
The study’s primary endpoint was the combined first-event rate of cardiovascular death, hospitalization for heart failure, or an outpatient visit for heart failure. During a median follow-up of 23 months, this occurred at a rate of 7.4/100 patient years in the ramipril arm and 6.7/100 patient years in the sacubitril/valsartan arm, a 10% relative risk reduction with sacubitril/valsartan that was not significant, which meant all other efficacy analyses were exploratory, Dr. Pfeffer stressed.
Several secondary efficacy analyses showed significant benefits from sacubitril/valsartan, compared with ramipril, including the total number of events that comprised the primary endpoint, with a 21% relative risk reduction associated with sacubitril/valsartan, as well as investigator-reported events. The primary-endpoint benefit from sacubitril/valsartan was also significant in two subgroup analyses: patients aged 65 years or older (roughly half the study cohort), who had a 24% relative risk reduction on sacubitril/valsartan, compared with ramipril, and the 88% of patients who received treatment with percutaneous coronary intervention for their acute MI, who had a 19% relative risk reduction on sacubitril/valsartan, compared with patients who received ramipril.
The study’s safety data showed nearly identical rates in the two treatment arms for total adverse events, serious adverse events, adverse events that led to stopping the study drug, as well as in laboratory measures. The biggest between-treatment differences were a modest excess of hypotension on sacubitril valsartan, 28%, compared with 22% on ramipril, and a modest excess rate of cough on ramipril, 13%, compared with 9% on sacubitril/valsartan.
The added insight the results provide about sacubitril/valsartan comes at a time when U.S. patients continue to struggle to get health insurance coverage for an agent that has been approved for U.S. use in treating heart failure since 2015.
“Our patients do not have access to this important treatment,” declared Dr. Walsh during the press briefing. “The prior authorization process is unbelievable, and some patients have no access unless they pay the full cost on their own. This is an important, real-world problem that we face with this drug.”
PARADISE-MI was sponsored by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Pfeffer has received research funding from and is a consultant to Novartis. He is also a consultant to AstraZeneca, Boehringer Ingelheim, Corvidia, DalCor, Eli Lilly, GlaxoSmithKline, Novo Nordisk, Peerbridge, and Sanofi, and he holds equity in DalCor and Peerbridge. Dr. Stevenson has received honoraria from LivaNova and has received research support from Abbott. Dr. Walsh had no disclosures.
Treatment with sacubitril/valsartan, a pillar of therapy for patients with chronic heart failure with below-normal ejection fraction, came suggestively close to showing efficacy for preventing cardiovascular death or heart failure events in patients who have just had an MI but have no history of heart failure in a controlled trial with more than 5,600 patients.
Although sacubitril/valsartan (Entresto) fell short of producing a significant benefit, it did show good safety that was similar to the study’s comparator treatment, ramipril, an agent from the angiotensin-converting enzyme inhibitor class that is a mainstay of treatment in these patients.
“To say that, with no run-in, sacubitril/valsartan is as well tolerated and as safe as one of the best-studied ACE inhibitors – ramipril – in acutely ill MI patients, is a big statement,” said Marc A. Pfeffer, MD, at the annual scientific sessions of the American College of Cardiology. This high level of safety without gradual uptitration of sacubitril/valsartan (Entresto) “should lower barriers” to broader use of the dual-drug formulation for its approved indication in patients with chronic heart failure, especially patients with a left ventricular ejection fraction that is below normal. In addition, results from the PARADISE-MI trial suggested that “patients seemed to benefit before they develop heart failure. We couldn’t prove that, but we should build on this, and make it easier for patients to use this treatment,” Dr. Pfeffer said during a press briefing following his talk at the sessions.
Preventing heart failures to come
Treatment with sacubitril/valsartan in acute MI patients within a few days of their event “is perhaps addressing prevention of the heart failure that’s to come,” commented Lynne W. Stevenson, MD, designated discussant for the report and professor of medicine at Vanderbilt University Medical Center in Nashville. “Patients who are destined to develop heart failure are beginning their treatment early. The subgroup analyses suggest that it’s the sicker patients who benefited the most,” she said.
But Dr. Pfeffer stressed that “I don’t think this is a subgroup discussion. I would like to pursue this, but that’s up to the sponsor,” Novartis, the company that markets sacubitril/valsartan.
‘Exceedingly reassuring’ safety
The safety data that Dr. Pfeffer reported “are exceedingly reassuring. We didn’t see a signal of harm, and in some of the exploratory endpoints there was some evidence of benefit, so we need to encourage you to continue,” commented Mary N. Walsh, MD, medical director of the heart failure and cardiac transplantation program at Ascension St. Vincent Heart Center of Indiana in Indianapolis.
The PARADISE-MI (Prospective ARNI vs. ACE Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After MI) trial enrolled 5,669 patients with no history of heart failure within an average of 4 days following an acute MI at 495 sites in 41 countries during 2016-2020, with 8% of enrolled patients from the United States. Patients averaged 64 years of age, about three-quarters were men, about 43% had a history of diabetes, and only 1% were Black; Dr. Pfeffer noted that this is because most patients came from countries with low Black populations. The enrollment criteria required a left ventricular ejection fraction no greater than 40%, and among the enrolled patients this averaged about 37%.
A 10% nonsignificant relative risk reduction for the primary endpoint
The study’s primary endpoint was the combined first-event rate of cardiovascular death, hospitalization for heart failure, or an outpatient visit for heart failure. During a median follow-up of 23 months, this occurred at a rate of 7.4/100 patient years in the ramipril arm and 6.7/100 patient years in the sacubitril/valsartan arm, a 10% relative risk reduction with sacubitril/valsartan that was not significant, which meant all other efficacy analyses were exploratory, Dr. Pfeffer stressed.
Several secondary efficacy analyses showed significant benefits from sacubitril/valsartan, compared with ramipril, including the total number of events that comprised the primary endpoint, with a 21% relative risk reduction associated with sacubitril/valsartan, as well as investigator-reported events. The primary-endpoint benefit from sacubitril/valsartan was also significant in two subgroup analyses: patients aged 65 years or older (roughly half the study cohort), who had a 24% relative risk reduction on sacubitril/valsartan, compared with ramipril, and the 88% of patients who received treatment with percutaneous coronary intervention for their acute MI, who had a 19% relative risk reduction on sacubitril/valsartan, compared with patients who received ramipril.
The study’s safety data showed nearly identical rates in the two treatment arms for total adverse events, serious adverse events, adverse events that led to stopping the study drug, as well as in laboratory measures. The biggest between-treatment differences were a modest excess of hypotension on sacubitril valsartan, 28%, compared with 22% on ramipril, and a modest excess rate of cough on ramipril, 13%, compared with 9% on sacubitril/valsartan.
The added insight the results provide about sacubitril/valsartan comes at a time when U.S. patients continue to struggle to get health insurance coverage for an agent that has been approved for U.S. use in treating heart failure since 2015.
“Our patients do not have access to this important treatment,” declared Dr. Walsh during the press briefing. “The prior authorization process is unbelievable, and some patients have no access unless they pay the full cost on their own. This is an important, real-world problem that we face with this drug.”
PARADISE-MI was sponsored by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Pfeffer has received research funding from and is a consultant to Novartis. He is also a consultant to AstraZeneca, Boehringer Ingelheim, Corvidia, DalCor, Eli Lilly, GlaxoSmithKline, Novo Nordisk, Peerbridge, and Sanofi, and he holds equity in DalCor and Peerbridge. Dr. Stevenson has received honoraria from LivaNova and has received research support from Abbott. Dr. Walsh had no disclosures.
FROM ACC 2021
PHM groups issue Choosing Wisely® recommendations
SHM members involved from the start
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
SHM members involved from the start
SHM members involved from the start
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
Among asymptomatic, 2% may harbor 90% of community’s viral load: Study
About 2% of asymptomatic college students carried 90% of COVID-19 viral load levels on a Colorado campus last year, new research reveals. Furthermore, the viral loads in these students were as elevated as those seen in hospitalized patients.
“College campuses were one of the few places where people without any symptoms or suspicions of exposure were being screened for the virus. This allowed us to make some powerful comparisons between symptomatic vs healthy carriers of the virus,” senior study author Sara Sawyer, PhD, professor of virology at the University of Colorado, Boulder, said in an interview.
“It turns out, walking around a college campus can be as dangerous as walking through a COVID ward in the hospital, in that you will experience these viral ‘super carriers’ equally in both settings,” she said.
“This is an important study in advancing our understanding of how SARS-CoV-2 is distributed in the population,” Thomas Giordano, MD, MPH, professor and section chief of infectious diseases at Baylor College of Medicine, Houston, said in an interview.
The study “adds to the evidence that viral load is not too tightly correlated with symptoms.” In fact, Dr. Giordano added, “this study suggests viral load is not at all correlated with symptoms.”
Viral load may not be correlated with transmissibility either, said Raphael Viscidi, MD, when asked to comment. “This is not a transmissibility study. They did not show that viral load is the factor related to transmission.”
“It’s true that 2% of the population they studied carried 90% of the virus, but it does not establish any biological importance to that 2%,” added Dr. Viscidi, professor of pediatrics and oncology at Johns Hopkins University, Baltimore,.
The 2% could just be the upper tail end of a normal bell-shaped distribution curve, Dr. Viscidi said, or there could be something biologically unique about that group. But the study does not make that distinction, he said.
The study was published online May 10, 2021, in PNAS, the official journal of the National Academy of Sciences.
A similar picture in hospitalized patients
Out of more than 72,500 saliva samples taken during COVID-19 screening at the University of Colorado Boulder between Aug. 27 and Dec. 11, 2020, 1,405 were positive for SARS-CoV-2.
The investigators also compared viral loads from students with those of hospitalized patients based on published data. They found the distribution of viral loads between these groups “indistinguishable.”
“Strikingly, these datasets demonstrate dramatic differences in viral levels between individuals, with a very small minority of the infected individuals harboring the vast majority of the infectious virions,” the researchers wrote. The comparison “really represents two extremes: One group is mostly hospitalized, while the other group represents a mostly young and healthy (but infected) college population.”
“It would be interesting to adjust public health recommendations based on a person’s viral load,” Dr. Giordano said. “One could speculate that a person with a very high viral load could be isolated longer or more thoroughly, while someone with a very low viral load could be minimally isolated.
“This is speculation, and more data are needed to test this concept,” he added. Also, quantitative viral load testing would need to be standardized before it could be used to guide such decision-making
Preceding the COVID-19 vaccine era
It should be noted that the research was conducted in fall 2020, before access to COVID-19 immunization.
“The study was performed prior to vaccine availability in a cohort of young people. It adds further data to support prior observations that the majority of infections are spread by a much smaller group of individuals,” David Hirschwerk, MD, said in an interview.
“Now that vaccines are available, I think it is very likely that a repeat study of this type would show diminished transmission from vaccinated people who were infected yet asymptomatic,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in New Hyde Park, N.Y., who was not affiliated with the research.
Mechanism still a mystery
“This finding has been in the literature in piecemeal fashion since the beginning of the pandemic,” Dr. Sawyer said. “I just think we were the first to realize the bigger implications of these plots of viral load that we have all been seeing over and over again.”
How a minority of people walk around asymptomatic with a majority of virus remains unanswered. Are there special people who can harbor these extremely high viral loads? Or do many infected individuals experience a short period of time when they carry such elevated levels?
The highest observed viral load in the current study was more than 6 trillion virions per mL. “It is remarkable to consider that this individual was on campus and reported no symptoms at our testing site,” the researchers wrote.
In contrast, the lowest viral load detected was 8 virions per mL.
Although more research is needed, the investigators noted that “a strong implication is that these individuals who are viral ‘super carriers’ may also be ‘superspreaders.’ ”
Some of the study authors have financial ties to companies that offer commercial SARS-CoV-2 testing, including Darwin Biosciences, TUMI Genomics, Faze Medicines, and Arpeggio Biosciences.
A version of this article first appeared on Medscape.com.
About 2% of asymptomatic college students carried 90% of COVID-19 viral load levels on a Colorado campus last year, new research reveals. Furthermore, the viral loads in these students were as elevated as those seen in hospitalized patients.
“College campuses were one of the few places where people without any symptoms or suspicions of exposure were being screened for the virus. This allowed us to make some powerful comparisons between symptomatic vs healthy carriers of the virus,” senior study author Sara Sawyer, PhD, professor of virology at the University of Colorado, Boulder, said in an interview.
“It turns out, walking around a college campus can be as dangerous as walking through a COVID ward in the hospital, in that you will experience these viral ‘super carriers’ equally in both settings,” she said.
“This is an important study in advancing our understanding of how SARS-CoV-2 is distributed in the population,” Thomas Giordano, MD, MPH, professor and section chief of infectious diseases at Baylor College of Medicine, Houston, said in an interview.
The study “adds to the evidence that viral load is not too tightly correlated with symptoms.” In fact, Dr. Giordano added, “this study suggests viral load is not at all correlated with symptoms.”
Viral load may not be correlated with transmissibility either, said Raphael Viscidi, MD, when asked to comment. “This is not a transmissibility study. They did not show that viral load is the factor related to transmission.”
“It’s true that 2% of the population they studied carried 90% of the virus, but it does not establish any biological importance to that 2%,” added Dr. Viscidi, professor of pediatrics and oncology at Johns Hopkins University, Baltimore,.
The 2% could just be the upper tail end of a normal bell-shaped distribution curve, Dr. Viscidi said, or there could be something biologically unique about that group. But the study does not make that distinction, he said.
The study was published online May 10, 2021, in PNAS, the official journal of the National Academy of Sciences.
A similar picture in hospitalized patients
Out of more than 72,500 saliva samples taken during COVID-19 screening at the University of Colorado Boulder between Aug. 27 and Dec. 11, 2020, 1,405 were positive for SARS-CoV-2.
The investigators also compared viral loads from students with those of hospitalized patients based on published data. They found the distribution of viral loads between these groups “indistinguishable.”
“Strikingly, these datasets demonstrate dramatic differences in viral levels between individuals, with a very small minority of the infected individuals harboring the vast majority of the infectious virions,” the researchers wrote. The comparison “really represents two extremes: One group is mostly hospitalized, while the other group represents a mostly young and healthy (but infected) college population.”
“It would be interesting to adjust public health recommendations based on a person’s viral load,” Dr. Giordano said. “One could speculate that a person with a very high viral load could be isolated longer or more thoroughly, while someone with a very low viral load could be minimally isolated.
“This is speculation, and more data are needed to test this concept,” he added. Also, quantitative viral load testing would need to be standardized before it could be used to guide such decision-making
Preceding the COVID-19 vaccine era
It should be noted that the research was conducted in fall 2020, before access to COVID-19 immunization.
“The study was performed prior to vaccine availability in a cohort of young people. It adds further data to support prior observations that the majority of infections are spread by a much smaller group of individuals,” David Hirschwerk, MD, said in an interview.
“Now that vaccines are available, I think it is very likely that a repeat study of this type would show diminished transmission from vaccinated people who were infected yet asymptomatic,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in New Hyde Park, N.Y., who was not affiliated with the research.
Mechanism still a mystery
“This finding has been in the literature in piecemeal fashion since the beginning of the pandemic,” Dr. Sawyer said. “I just think we were the first to realize the bigger implications of these plots of viral load that we have all been seeing over and over again.”
How a minority of people walk around asymptomatic with a majority of virus remains unanswered. Are there special people who can harbor these extremely high viral loads? Or do many infected individuals experience a short period of time when they carry such elevated levels?
The highest observed viral load in the current study was more than 6 trillion virions per mL. “It is remarkable to consider that this individual was on campus and reported no symptoms at our testing site,” the researchers wrote.
In contrast, the lowest viral load detected was 8 virions per mL.
Although more research is needed, the investigators noted that “a strong implication is that these individuals who are viral ‘super carriers’ may also be ‘superspreaders.’ ”
Some of the study authors have financial ties to companies that offer commercial SARS-CoV-2 testing, including Darwin Biosciences, TUMI Genomics, Faze Medicines, and Arpeggio Biosciences.
A version of this article first appeared on Medscape.com.
About 2% of asymptomatic college students carried 90% of COVID-19 viral load levels on a Colorado campus last year, new research reveals. Furthermore, the viral loads in these students were as elevated as those seen in hospitalized patients.
“College campuses were one of the few places where people without any symptoms or suspicions of exposure were being screened for the virus. This allowed us to make some powerful comparisons between symptomatic vs healthy carriers of the virus,” senior study author Sara Sawyer, PhD, professor of virology at the University of Colorado, Boulder, said in an interview.
“It turns out, walking around a college campus can be as dangerous as walking through a COVID ward in the hospital, in that you will experience these viral ‘super carriers’ equally in both settings,” she said.
“This is an important study in advancing our understanding of how SARS-CoV-2 is distributed in the population,” Thomas Giordano, MD, MPH, professor and section chief of infectious diseases at Baylor College of Medicine, Houston, said in an interview.
The study “adds to the evidence that viral load is not too tightly correlated with symptoms.” In fact, Dr. Giordano added, “this study suggests viral load is not at all correlated with symptoms.”
Viral load may not be correlated with transmissibility either, said Raphael Viscidi, MD, when asked to comment. “This is not a transmissibility study. They did not show that viral load is the factor related to transmission.”
“It’s true that 2% of the population they studied carried 90% of the virus, but it does not establish any biological importance to that 2%,” added Dr. Viscidi, professor of pediatrics and oncology at Johns Hopkins University, Baltimore,.
The 2% could just be the upper tail end of a normal bell-shaped distribution curve, Dr. Viscidi said, or there could be something biologically unique about that group. But the study does not make that distinction, he said.
The study was published online May 10, 2021, in PNAS, the official journal of the National Academy of Sciences.
A similar picture in hospitalized patients
Out of more than 72,500 saliva samples taken during COVID-19 screening at the University of Colorado Boulder between Aug. 27 and Dec. 11, 2020, 1,405 were positive for SARS-CoV-2.
The investigators also compared viral loads from students with those of hospitalized patients based on published data. They found the distribution of viral loads between these groups “indistinguishable.”
“Strikingly, these datasets demonstrate dramatic differences in viral levels between individuals, with a very small minority of the infected individuals harboring the vast majority of the infectious virions,” the researchers wrote. The comparison “really represents two extremes: One group is mostly hospitalized, while the other group represents a mostly young and healthy (but infected) college population.”
“It would be interesting to adjust public health recommendations based on a person’s viral load,” Dr. Giordano said. “One could speculate that a person with a very high viral load could be isolated longer or more thoroughly, while someone with a very low viral load could be minimally isolated.
“This is speculation, and more data are needed to test this concept,” he added. Also, quantitative viral load testing would need to be standardized before it could be used to guide such decision-making
Preceding the COVID-19 vaccine era
It should be noted that the research was conducted in fall 2020, before access to COVID-19 immunization.
“The study was performed prior to vaccine availability in a cohort of young people. It adds further data to support prior observations that the majority of infections are spread by a much smaller group of individuals,” David Hirschwerk, MD, said in an interview.
“Now that vaccines are available, I think it is very likely that a repeat study of this type would show diminished transmission from vaccinated people who were infected yet asymptomatic,” added Dr. Hirschwerk, an infectious disease specialist at Northwell Health in New Hyde Park, N.Y., who was not affiliated with the research.
Mechanism still a mystery
“This finding has been in the literature in piecemeal fashion since the beginning of the pandemic,” Dr. Sawyer said. “I just think we were the first to realize the bigger implications of these plots of viral load that we have all been seeing over and over again.”
How a minority of people walk around asymptomatic with a majority of virus remains unanswered. Are there special people who can harbor these extremely high viral loads? Or do many infected individuals experience a short period of time when they carry such elevated levels?
The highest observed viral load in the current study was more than 6 trillion virions per mL. “It is remarkable to consider that this individual was on campus and reported no symptoms at our testing site,” the researchers wrote.
In contrast, the lowest viral load detected was 8 virions per mL.
Although more research is needed, the investigators noted that “a strong implication is that these individuals who are viral ‘super carriers’ may also be ‘superspreaders.’ ”
Some of the study authors have financial ties to companies that offer commercial SARS-CoV-2 testing, including Darwin Biosciences, TUMI Genomics, Faze Medicines, and Arpeggio Biosciences.
A version of this article first appeared on Medscape.com.




















