User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Hospitalist movers and shakers – September 2021
Chi-Cheng Huang, MD, SFHM, was recently was named one of the Notable Asian/Pacific American Physicians in U.S. History by the American Board of Internal Medicine. May was Asian/Pacific American Heritage Month. Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System (Winston-Salem, N.C.) and associate professor at Wake Forest School of Medicine.
Dr. Huang is a board-certified hospitalist and pediatrician, and he is the founder of the Bolivian Children Project, a non-profit organization that focuses on sheltering street children in La Paz and other areas of Bolivia. Dr. Huang was inspired to start the project during a year sabbatical from medical school. He worked at an orphanage and cared for children who were victims of physical abuse. The Bolivian Children Project supports those children, and Dr. Huang’s book, When Invisible Children Sing, tells their story.
Joshua Lenchus, DO, RPh, SFHM, has been elected president of the Florida Medical Association. It is the first time in its history that the FMA will have a DO as its president. Dr. Lenchus is a hospitalist and chief medical officer at the Broward Health Medical Center in Fort Lauderdale, Fla.
Mark V. Williams, MD, MHM, will join Washington University School of Medicine and BJC HealthCare, both in St. Louis, as professor and chief for the Division of Hospital Medicine in October 2021. Dr. Williams is currently professor and director of the Center for Health Services Research at the University of Kentucky and chief quality officer at UK HealthCare, both in Lexington.
Dr. Williams was a founding member of the Society of Hospital Medicine, one of the first two elected members of the Board of SHM, its former president, founding editor of the Journal of Hospital Medicine, and principal investigator for Project BOOST. He established the first hospitalist program at a public hospital (Grady Memorial in Atlanta) in 1998, and later became the founding chief of the Division of Hospital Medicine in 2007 at Northwestern University School of Medicine in Chicago. At the University of Kentucky, he established the Center for Health Services Research and the Division of Hospital Medicine in 2014.
At Washington University, Dr. Williams will be tasked with translating the division of hospital medicine’s scholarly work, innovation, and research into practice improvement, focusing on developing new systems of health care delivery that are patient-centered, cost effective, and provide outstanding value.
Jordan Messler, MD, SFHM, has been named the new chief medical officer at Glytec (Waltham, Mass.), where he has worked as executive director of clinical practice since 2018. Dr. Messler will be tasked with leading strategy and product development while also supporting efforts in quality care, customer relations, and delivery of products.
Glytec provides insulin management software across the care continuum and is touted as the only cloud-based software provider of its kind. Dr. Messler’s background includes expertise in glycemic management. In addition, he still works as a hospitalist at Morton Plant Hospitalist Group (Clearwater, Fla.).
Dr. Messler is a senior fellow with SHM and is physician editor of SHM’s official blog The Hospital Leader.
Tiffani Maycock, DO, recently was named to the Board of Directors for the American Board of Family Medicine. Dr. Maycock is director of the Selma Family Medicine Residency Program at the University of Alabama at Birmingham, where she is an assistant professor in the department of family medicine.
Dr. Maycock helped create hospitalist services at Vaughan Regional Medical Center (Selma, Ala.) – Selma Family Medicine’s primary teaching site – and currently serves as its hospitalist director and on its Medical Executive Committee. She has worked at the facility since 2017.
Preetham Talari, MD, SFHM, has been named associate chief of quality safety for the Division of Hospital Medicine at the University of Kentucky’s UK HealthCare (Lexington, Ky.). Dr. Talari is an associate professor of internal medicine in the Division of Hospital Medicine at the UK College of Medicine.
Over the last decade, Dr. Talari’s work in quality, safety, and health care leadership has positioned him as a leader in several UK Healthcare committees and transformation projects. In his role as associate chief, Dr. Talari collaborates with hospital medicine directors, enterprise leadership, and medical education leadership to improve the system’s quality of care.
Dr. Talari is the president of the Kentucky chapter of SHM and is a member of SHM’s Hospital Quality and Patient Safety Committee.
Adrian Paraschiv, MD, FHM, is being recognized by Continental Who’s Who as a Trusted Internist and Hospitalist in the field of Medicine in acknowledgment of his commitment to providing quality health care services.
Dr. Paraschiv is a board-certified Internist at Garnet Health Medical Center in Middletown, N.Y. He also serves in an administrative capacity as the Garnet Health Doctors Hospitalist Division’s Associate Program Director. He is also the Director of Clinical Informatics. Dr. Paraschiv is certified as the Epic physician builder in analytics, information technology, and improved documentation.
DCH Health System (Tuscaloosa, Ala.) recently selected Capstone Health Services Foundation (Tuscaloosa) and IN Compass Health Inc. (Alpharetta, Ga.) as its joint hospitalist service provider for facilities in Northport and Tuscaloosa. Capstone will provide the physicians, while IN Compass will handle staffing management of the hospitalists, as well as day-to-day operations and calculating quality care metrics. The agreement is slated to begin on Oct. 1, 2021, at Northport Medical Center, and on Nov. 1, 2021, at DCH Regional Medical Center.
Capstone is an affiliate of the University of Alabama and oversees University Hospitalist Group, which currently provides hospitalists at DCH Regional Medical Center. Its partnership with IN Compass includes working together in recruiting and hiring physicians for both facilities.
UPMC Kane Medical Center (Kane, Pa.) recently announced the creation of a virtual telemedicine hospitalist program. UPMC Kane is partnering with the UPMC Center for Community Hospitalist Medicine to create this new mode of care.
Telehospitalists will care for UPMC Kane patients using advanced diagnostic technique and high-definition cameras. The physicians will bring expert service to Kane 24 hours per day utilizing physicians and specialists based in Pittsburgh. Those hospitalists will work with local nurse practitioners and support staff and deliver care to Kane patients.
Wake Forest Baptist Health (Winston-Salem, N.C.) has launched a Hospitalist at Home program with hopes of keeping patients safe while also reducing time they spend in the hospital. The telehealth initiative kicked into gear at the start of 2021 and considered the first of its kind in the region.
Patients who qualify for the program establish a plan before they leave the hospital. Wake Forest Baptist Health paramedics makes home visits and conducts care with a hospitalist reviewing the visit virtually. Those appointments continue until the patient does not require monitoring.
The impetus of creating the program was the COVID-19 pandemic, however, Wake Forest said it expects to care for between 75-100 patients through Hospitalist at Home at any one time.
Chi-Cheng Huang, MD, SFHM, was recently was named one of the Notable Asian/Pacific American Physicians in U.S. History by the American Board of Internal Medicine. May was Asian/Pacific American Heritage Month. Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System (Winston-Salem, N.C.) and associate professor at Wake Forest School of Medicine.
Dr. Huang is a board-certified hospitalist and pediatrician, and he is the founder of the Bolivian Children Project, a non-profit organization that focuses on sheltering street children in La Paz and other areas of Bolivia. Dr. Huang was inspired to start the project during a year sabbatical from medical school. He worked at an orphanage and cared for children who were victims of physical abuse. The Bolivian Children Project supports those children, and Dr. Huang’s book, When Invisible Children Sing, tells their story.
Joshua Lenchus, DO, RPh, SFHM, has been elected president of the Florida Medical Association. It is the first time in its history that the FMA will have a DO as its president. Dr. Lenchus is a hospitalist and chief medical officer at the Broward Health Medical Center in Fort Lauderdale, Fla.
Mark V. Williams, MD, MHM, will join Washington University School of Medicine and BJC HealthCare, both in St. Louis, as professor and chief for the Division of Hospital Medicine in October 2021. Dr. Williams is currently professor and director of the Center for Health Services Research at the University of Kentucky and chief quality officer at UK HealthCare, both in Lexington.
Dr. Williams was a founding member of the Society of Hospital Medicine, one of the first two elected members of the Board of SHM, its former president, founding editor of the Journal of Hospital Medicine, and principal investigator for Project BOOST. He established the first hospitalist program at a public hospital (Grady Memorial in Atlanta) in 1998, and later became the founding chief of the Division of Hospital Medicine in 2007 at Northwestern University School of Medicine in Chicago. At the University of Kentucky, he established the Center for Health Services Research and the Division of Hospital Medicine in 2014.
At Washington University, Dr. Williams will be tasked with translating the division of hospital medicine’s scholarly work, innovation, and research into practice improvement, focusing on developing new systems of health care delivery that are patient-centered, cost effective, and provide outstanding value.
Jordan Messler, MD, SFHM, has been named the new chief medical officer at Glytec (Waltham, Mass.), where he has worked as executive director of clinical practice since 2018. Dr. Messler will be tasked with leading strategy and product development while also supporting efforts in quality care, customer relations, and delivery of products.
Glytec provides insulin management software across the care continuum and is touted as the only cloud-based software provider of its kind. Dr. Messler’s background includes expertise in glycemic management. In addition, he still works as a hospitalist at Morton Plant Hospitalist Group (Clearwater, Fla.).
Dr. Messler is a senior fellow with SHM and is physician editor of SHM’s official blog The Hospital Leader.
Tiffani Maycock, DO, recently was named to the Board of Directors for the American Board of Family Medicine. Dr. Maycock is director of the Selma Family Medicine Residency Program at the University of Alabama at Birmingham, where she is an assistant professor in the department of family medicine.
Dr. Maycock helped create hospitalist services at Vaughan Regional Medical Center (Selma, Ala.) – Selma Family Medicine’s primary teaching site – and currently serves as its hospitalist director and on its Medical Executive Committee. She has worked at the facility since 2017.
Preetham Talari, MD, SFHM, has been named associate chief of quality safety for the Division of Hospital Medicine at the University of Kentucky’s UK HealthCare (Lexington, Ky.). Dr. Talari is an associate professor of internal medicine in the Division of Hospital Medicine at the UK College of Medicine.
Over the last decade, Dr. Talari’s work in quality, safety, and health care leadership has positioned him as a leader in several UK Healthcare committees and transformation projects. In his role as associate chief, Dr. Talari collaborates with hospital medicine directors, enterprise leadership, and medical education leadership to improve the system’s quality of care.
Dr. Talari is the president of the Kentucky chapter of SHM and is a member of SHM’s Hospital Quality and Patient Safety Committee.
Adrian Paraschiv, MD, FHM, is being recognized by Continental Who’s Who as a Trusted Internist and Hospitalist in the field of Medicine in acknowledgment of his commitment to providing quality health care services.
Dr. Paraschiv is a board-certified Internist at Garnet Health Medical Center in Middletown, N.Y. He also serves in an administrative capacity as the Garnet Health Doctors Hospitalist Division’s Associate Program Director. He is also the Director of Clinical Informatics. Dr. Paraschiv is certified as the Epic physician builder in analytics, information technology, and improved documentation.
DCH Health System (Tuscaloosa, Ala.) recently selected Capstone Health Services Foundation (Tuscaloosa) and IN Compass Health Inc. (Alpharetta, Ga.) as its joint hospitalist service provider for facilities in Northport and Tuscaloosa. Capstone will provide the physicians, while IN Compass will handle staffing management of the hospitalists, as well as day-to-day operations and calculating quality care metrics. The agreement is slated to begin on Oct. 1, 2021, at Northport Medical Center, and on Nov. 1, 2021, at DCH Regional Medical Center.
Capstone is an affiliate of the University of Alabama and oversees University Hospitalist Group, which currently provides hospitalists at DCH Regional Medical Center. Its partnership with IN Compass includes working together in recruiting and hiring physicians for both facilities.
UPMC Kane Medical Center (Kane, Pa.) recently announced the creation of a virtual telemedicine hospitalist program. UPMC Kane is partnering with the UPMC Center for Community Hospitalist Medicine to create this new mode of care.
Telehospitalists will care for UPMC Kane patients using advanced diagnostic technique and high-definition cameras. The physicians will bring expert service to Kane 24 hours per day utilizing physicians and specialists based in Pittsburgh. Those hospitalists will work with local nurse practitioners and support staff and deliver care to Kane patients.
Wake Forest Baptist Health (Winston-Salem, N.C.) has launched a Hospitalist at Home program with hopes of keeping patients safe while also reducing time they spend in the hospital. The telehealth initiative kicked into gear at the start of 2021 and considered the first of its kind in the region.
Patients who qualify for the program establish a plan before they leave the hospital. Wake Forest Baptist Health paramedics makes home visits and conducts care with a hospitalist reviewing the visit virtually. Those appointments continue until the patient does not require monitoring.
The impetus of creating the program was the COVID-19 pandemic, however, Wake Forest said it expects to care for between 75-100 patients through Hospitalist at Home at any one time.
Chi-Cheng Huang, MD, SFHM, was recently was named one of the Notable Asian/Pacific American Physicians in U.S. History by the American Board of Internal Medicine. May was Asian/Pacific American Heritage Month. Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System (Winston-Salem, N.C.) and associate professor at Wake Forest School of Medicine.
Dr. Huang is a board-certified hospitalist and pediatrician, and he is the founder of the Bolivian Children Project, a non-profit organization that focuses on sheltering street children in La Paz and other areas of Bolivia. Dr. Huang was inspired to start the project during a year sabbatical from medical school. He worked at an orphanage and cared for children who were victims of physical abuse. The Bolivian Children Project supports those children, and Dr. Huang’s book, When Invisible Children Sing, tells their story.
Joshua Lenchus, DO, RPh, SFHM, has been elected president of the Florida Medical Association. It is the first time in its history that the FMA will have a DO as its president. Dr. Lenchus is a hospitalist and chief medical officer at the Broward Health Medical Center in Fort Lauderdale, Fla.
Mark V. Williams, MD, MHM, will join Washington University School of Medicine and BJC HealthCare, both in St. Louis, as professor and chief for the Division of Hospital Medicine in October 2021. Dr. Williams is currently professor and director of the Center for Health Services Research at the University of Kentucky and chief quality officer at UK HealthCare, both in Lexington.
Dr. Williams was a founding member of the Society of Hospital Medicine, one of the first two elected members of the Board of SHM, its former president, founding editor of the Journal of Hospital Medicine, and principal investigator for Project BOOST. He established the first hospitalist program at a public hospital (Grady Memorial in Atlanta) in 1998, and later became the founding chief of the Division of Hospital Medicine in 2007 at Northwestern University School of Medicine in Chicago. At the University of Kentucky, he established the Center for Health Services Research and the Division of Hospital Medicine in 2014.
At Washington University, Dr. Williams will be tasked with translating the division of hospital medicine’s scholarly work, innovation, and research into practice improvement, focusing on developing new systems of health care delivery that are patient-centered, cost effective, and provide outstanding value.
Jordan Messler, MD, SFHM, has been named the new chief medical officer at Glytec (Waltham, Mass.), where he has worked as executive director of clinical practice since 2018. Dr. Messler will be tasked with leading strategy and product development while also supporting efforts in quality care, customer relations, and delivery of products.
Glytec provides insulin management software across the care continuum and is touted as the only cloud-based software provider of its kind. Dr. Messler’s background includes expertise in glycemic management. In addition, he still works as a hospitalist at Morton Plant Hospitalist Group (Clearwater, Fla.).
Dr. Messler is a senior fellow with SHM and is physician editor of SHM’s official blog The Hospital Leader.
Tiffani Maycock, DO, recently was named to the Board of Directors for the American Board of Family Medicine. Dr. Maycock is director of the Selma Family Medicine Residency Program at the University of Alabama at Birmingham, where she is an assistant professor in the department of family medicine.
Dr. Maycock helped create hospitalist services at Vaughan Regional Medical Center (Selma, Ala.) – Selma Family Medicine’s primary teaching site – and currently serves as its hospitalist director and on its Medical Executive Committee. She has worked at the facility since 2017.
Preetham Talari, MD, SFHM, has been named associate chief of quality safety for the Division of Hospital Medicine at the University of Kentucky’s UK HealthCare (Lexington, Ky.). Dr. Talari is an associate professor of internal medicine in the Division of Hospital Medicine at the UK College of Medicine.
Over the last decade, Dr. Talari’s work in quality, safety, and health care leadership has positioned him as a leader in several UK Healthcare committees and transformation projects. In his role as associate chief, Dr. Talari collaborates with hospital medicine directors, enterprise leadership, and medical education leadership to improve the system’s quality of care.
Dr. Talari is the president of the Kentucky chapter of SHM and is a member of SHM’s Hospital Quality and Patient Safety Committee.
Adrian Paraschiv, MD, FHM, is being recognized by Continental Who’s Who as a Trusted Internist and Hospitalist in the field of Medicine in acknowledgment of his commitment to providing quality health care services.
Dr. Paraschiv is a board-certified Internist at Garnet Health Medical Center in Middletown, N.Y. He also serves in an administrative capacity as the Garnet Health Doctors Hospitalist Division’s Associate Program Director. He is also the Director of Clinical Informatics. Dr. Paraschiv is certified as the Epic physician builder in analytics, information technology, and improved documentation.
DCH Health System (Tuscaloosa, Ala.) recently selected Capstone Health Services Foundation (Tuscaloosa) and IN Compass Health Inc. (Alpharetta, Ga.) as its joint hospitalist service provider for facilities in Northport and Tuscaloosa. Capstone will provide the physicians, while IN Compass will handle staffing management of the hospitalists, as well as day-to-day operations and calculating quality care metrics. The agreement is slated to begin on Oct. 1, 2021, at Northport Medical Center, and on Nov. 1, 2021, at DCH Regional Medical Center.
Capstone is an affiliate of the University of Alabama and oversees University Hospitalist Group, which currently provides hospitalists at DCH Regional Medical Center. Its partnership with IN Compass includes working together in recruiting and hiring physicians for both facilities.
UPMC Kane Medical Center (Kane, Pa.) recently announced the creation of a virtual telemedicine hospitalist program. UPMC Kane is partnering with the UPMC Center for Community Hospitalist Medicine to create this new mode of care.
Telehospitalists will care for UPMC Kane patients using advanced diagnostic technique and high-definition cameras. The physicians will bring expert service to Kane 24 hours per day utilizing physicians and specialists based in Pittsburgh. Those hospitalists will work with local nurse practitioners and support staff and deliver care to Kane patients.
Wake Forest Baptist Health (Winston-Salem, N.C.) has launched a Hospitalist at Home program with hopes of keeping patients safe while also reducing time they spend in the hospital. The telehealth initiative kicked into gear at the start of 2021 and considered the first of its kind in the region.
Patients who qualify for the program establish a plan before they leave the hospital. Wake Forest Baptist Health paramedics makes home visits and conducts care with a hospitalist reviewing the visit virtually. Those appointments continue until the patient does not require monitoring.
The impetus of creating the program was the COVID-19 pandemic, however, Wake Forest said it expects to care for between 75-100 patients through Hospitalist at Home at any one time.
POCUS in hospital pediatrics
PHM 2021 Session
Safe and (Ultra)sound: Why you should use POCUS in your Pediatric Practice
Presenter
Ria Dancel, MD, FAAP, FACP
Session summary
Dr. Ria Dancel and her colleagues from the University of North Carolina at Chapel Hill presented a broad overview of point-of-care ultrasound (POCUS) applications in the field of pediatric hospital medicine. They discussed its advantages and potential uses, ranging from common scenarios to critical care to procedural guidance. Using illustrative scenarios and interactive cases, she discussed the bedside applications to improve care of hospitalized children. The benefits and risks of radiography and POCUS were reviewed.
The session highlighted the use of POCUS in SSTI (skin and soft tissue infection) to help with differentiating cellulitis from abscesses. Use of POCUS for safer incision and drainages and making day-to-day changes in management was discussed. The ease and benefits of performing real-time lung ultrasound in different pathologies (like pneumonia, effusion, COVID-19) was presented. The speakers discussed the use of POCUS in emergency situations like hypotension and different types of shock. The use of ultrasound in common bedside procedures (bladder catheterization, lumbar ultrasound, peripheral IV placement) were also highlighted. Current literature and evidence were reviewed.
Key takeaways
- Pediatric POCUS is an extremely valuable bedside tool in pediatric hospital medicine.
- It can be used to guide clinical care for many conditions including SSTI, pneumonia, and shock.
- It can be used for procedural guidance for bladder catheterization, lumbar puncture, and intravenous access.
Dr. Patra is a pediatric hospitalist at West Virginia University Children’s Hospital, Morgantown, and associate professor at West Virginia University School of Medicine. He is interested in medical education, quality improvement and clinical research. He is a member of the Executive Council of the Pediatric Special Interest Group of the Society of Hospital Medicine.
PHM 2021 Session
Safe and (Ultra)sound: Why you should use POCUS in your Pediatric Practice
Presenter
Ria Dancel, MD, FAAP, FACP
Session summary
Dr. Ria Dancel and her colleagues from the University of North Carolina at Chapel Hill presented a broad overview of point-of-care ultrasound (POCUS) applications in the field of pediatric hospital medicine. They discussed its advantages and potential uses, ranging from common scenarios to critical care to procedural guidance. Using illustrative scenarios and interactive cases, she discussed the bedside applications to improve care of hospitalized children. The benefits and risks of radiography and POCUS were reviewed.
The session highlighted the use of POCUS in SSTI (skin and soft tissue infection) to help with differentiating cellulitis from abscesses. Use of POCUS for safer incision and drainages and making day-to-day changes in management was discussed. The ease and benefits of performing real-time lung ultrasound in different pathologies (like pneumonia, effusion, COVID-19) was presented. The speakers discussed the use of POCUS in emergency situations like hypotension and different types of shock. The use of ultrasound in common bedside procedures (bladder catheterization, lumbar ultrasound, peripheral IV placement) were also highlighted. Current literature and evidence were reviewed.
Key takeaways
- Pediatric POCUS is an extremely valuable bedside tool in pediatric hospital medicine.
- It can be used to guide clinical care for many conditions including SSTI, pneumonia, and shock.
- It can be used for procedural guidance for bladder catheterization, lumbar puncture, and intravenous access.
Dr. Patra is a pediatric hospitalist at West Virginia University Children’s Hospital, Morgantown, and associate professor at West Virginia University School of Medicine. He is interested in medical education, quality improvement and clinical research. He is a member of the Executive Council of the Pediatric Special Interest Group of the Society of Hospital Medicine.
PHM 2021 Session
Safe and (Ultra)sound: Why you should use POCUS in your Pediatric Practice
Presenter
Ria Dancel, MD, FAAP, FACP
Session summary
Dr. Ria Dancel and her colleagues from the University of North Carolina at Chapel Hill presented a broad overview of point-of-care ultrasound (POCUS) applications in the field of pediatric hospital medicine. They discussed its advantages and potential uses, ranging from common scenarios to critical care to procedural guidance. Using illustrative scenarios and interactive cases, she discussed the bedside applications to improve care of hospitalized children. The benefits and risks of radiography and POCUS were reviewed.
The session highlighted the use of POCUS in SSTI (skin and soft tissue infection) to help with differentiating cellulitis from abscesses. Use of POCUS for safer incision and drainages and making day-to-day changes in management was discussed. The ease and benefits of performing real-time lung ultrasound in different pathologies (like pneumonia, effusion, COVID-19) was presented. The speakers discussed the use of POCUS in emergency situations like hypotension and different types of shock. The use of ultrasound in common bedside procedures (bladder catheterization, lumbar ultrasound, peripheral IV placement) were also highlighted. Current literature and evidence were reviewed.
Key takeaways
- Pediatric POCUS is an extremely valuable bedside tool in pediatric hospital medicine.
- It can be used to guide clinical care for many conditions including SSTI, pneumonia, and shock.
- It can be used for procedural guidance for bladder catheterization, lumbar puncture, and intravenous access.
Dr. Patra is a pediatric hospitalist at West Virginia University Children’s Hospital, Morgantown, and associate professor at West Virginia University School of Medicine. He is interested in medical education, quality improvement and clinical research. He is a member of the Executive Council of the Pediatric Special Interest Group of the Society of Hospital Medicine.
Heart failure hospitalization risk lower with SGLT2 inhibitors than GLP-1 RAs
Around a 30% reduction in the risk for being hospitalized for heart failure was achieved in people with type 2 diabetes who were treated with a SGLT2 inhibitor over a GLP-1 RA regardless of whether the patients had a preexisting heart condition.
The findings, published in the Annals of Internal Medicine, also showed a 10% lower risk for myocardial infarction or stroke among those treated with a SGLT2 inhibitor who had preexisting cardiovascular disease (CVD), although there was no difference in risk between the two classes of drugs in those without preexisting CVD.
“These findings are important as they suggest that SGLT2 [inhibitors] and GLP-1 RA offer similar benefits in preventing myocardial infarction and stroke in patients with diabetes,” said study investigator Elisabetta Patorno, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School in Boston, in an interview.
They also show “that SGLT2 [inhibitors] offer greater efficacy in preventing heart failure, which supports the existing guidelines,” she added.
Paul S. Jellinger, MD, MACE, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said these data were likely to be “additive to guidelines but not transformative.” The overall analysis results were “not surprising.” It was not unexpected that SGLT2 inhibitors provided a robust chronic heart failure (CHF) benefit, particularly in individuals with history of CVD, he said.
Dr. Jellinger, a clinical endocrinologist and professor of clinical medicine on the voluntary faculty at the University of Miami, observed, however, that “the similar CVD benefit in both drug classes in patients without known CVD adds to our knowledge in this somewhat controversial area and may be useful to the clinician in evaluating therapy in a diabetic individual without evidence of or at high risk for CHF.”
Furthermore, “the study also reminds us that, as demonstrated in published meta-analysis, there is also a modest CHF benefit associated with GLP-1 RA treatment particularly in patients with a history of CVD.”
Addressing the knowledge gap
Thanks to the results of many large-scale, prospective, cardiovascular outcomes studies, both SGLT2 inhibitors and GLP1 RA have been recommended as treatment for people with diabetes who have established CVD. But with no direct head-to-head trials having been conducted, there is a gap in knowledge and there is currently little guidance for physicians on which drug class to choose for an individual patient.
To try to clarify things, Dr. Patorno and associates looked at data from more than 370,000 people with type 2 diabetes who had been treated between April 2013 and December 2017 with either a SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or GLP-1 RA (albiglutide, dulaglutide, exenatide, or liraglutide).
One-to-one propensity score matching was used to create the study groups: participants were first grouped according to their history of CVD, and then by the class of drug they had been prescribed. The primary outcomes were hospitalization for MI, stroke, or heart failure.
Comparing the initiation of a SGLT2 inhibitor with GLP-1 RA therapy, the hazard ratios (HRs) for MI or stroke in patients with and without a history of CVD were a respective 0.90 (95% CI, 0.82 to 0.98) and 1.07 (0.97 to 1.18).
The corresponding hazard ratios for heart failure hospitalizations were 0.71 (0.64 to 0.79) and 0.69 (0.56 to 0.85).
Real-world studies are of ‘increasing value’
“As in other not-randomized studies based on real-world data, residual confounding cannot be completely ruled out,” Dr. Patorno acknowledged. She added, however that “state-of-the-art methodological strategies were implemented to minimize this possibility.”
Limitations notwithstanding, “real world studies are demonstrating increasing value,” observed Dr. Jellinger. Further large-scale cardiovascular outcomes trials that directly compare these two drug classes “are unlikely given the depth of information available now,” Dr. Jellinger suggested.
“This head-to-head retrospective study may be as close as we get and does represent the first effort at a comparison of these two classes.”
Dr. Patorno said of the potential clinical implications: “Because the two classes are equally effective for stroke and myocardial infarction, but the SGLT2 inhibitors are superior for heart failure, when considered in aggregate, SGLT2 inhibitors are likely to prevent more of these adverse cardiovascular events than GLP-1 RA.”
The study received no commercial funding and was supported by the Brigham and Women’s Hospital and Harvard Medical School Division of Pharmacoepidemiology and Pharmacoeconomics.
Dr. Patorno reported no conflicts of interest. Dr. Jellinger is on the speaker’s bureau for Astra Zeneca, Amgen, and Esperion, and has served on advisory boards for Corcept and Regeneron.
Around a 30% reduction in the risk for being hospitalized for heart failure was achieved in people with type 2 diabetes who were treated with a SGLT2 inhibitor over a GLP-1 RA regardless of whether the patients had a preexisting heart condition.
The findings, published in the Annals of Internal Medicine, also showed a 10% lower risk for myocardial infarction or stroke among those treated with a SGLT2 inhibitor who had preexisting cardiovascular disease (CVD), although there was no difference in risk between the two classes of drugs in those without preexisting CVD.
“These findings are important as they suggest that SGLT2 [inhibitors] and GLP-1 RA offer similar benefits in preventing myocardial infarction and stroke in patients with diabetes,” said study investigator Elisabetta Patorno, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School in Boston, in an interview.
They also show “that SGLT2 [inhibitors] offer greater efficacy in preventing heart failure, which supports the existing guidelines,” she added.
Paul S. Jellinger, MD, MACE, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said these data were likely to be “additive to guidelines but not transformative.” The overall analysis results were “not surprising.” It was not unexpected that SGLT2 inhibitors provided a robust chronic heart failure (CHF) benefit, particularly in individuals with history of CVD, he said.
Dr. Jellinger, a clinical endocrinologist and professor of clinical medicine on the voluntary faculty at the University of Miami, observed, however, that “the similar CVD benefit in both drug classes in patients without known CVD adds to our knowledge in this somewhat controversial area and may be useful to the clinician in evaluating therapy in a diabetic individual without evidence of or at high risk for CHF.”
Furthermore, “the study also reminds us that, as demonstrated in published meta-analysis, there is also a modest CHF benefit associated with GLP-1 RA treatment particularly in patients with a history of CVD.”
Addressing the knowledge gap
Thanks to the results of many large-scale, prospective, cardiovascular outcomes studies, both SGLT2 inhibitors and GLP1 RA have been recommended as treatment for people with diabetes who have established CVD. But with no direct head-to-head trials having been conducted, there is a gap in knowledge and there is currently little guidance for physicians on which drug class to choose for an individual patient.
To try to clarify things, Dr. Patorno and associates looked at data from more than 370,000 people with type 2 diabetes who had been treated between April 2013 and December 2017 with either a SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or GLP-1 RA (albiglutide, dulaglutide, exenatide, or liraglutide).
One-to-one propensity score matching was used to create the study groups: participants were first grouped according to their history of CVD, and then by the class of drug they had been prescribed. The primary outcomes were hospitalization for MI, stroke, or heart failure.
Comparing the initiation of a SGLT2 inhibitor with GLP-1 RA therapy, the hazard ratios (HRs) for MI or stroke in patients with and without a history of CVD were a respective 0.90 (95% CI, 0.82 to 0.98) and 1.07 (0.97 to 1.18).
The corresponding hazard ratios for heart failure hospitalizations were 0.71 (0.64 to 0.79) and 0.69 (0.56 to 0.85).
Real-world studies are of ‘increasing value’
“As in other not-randomized studies based on real-world data, residual confounding cannot be completely ruled out,” Dr. Patorno acknowledged. She added, however that “state-of-the-art methodological strategies were implemented to minimize this possibility.”
Limitations notwithstanding, “real world studies are demonstrating increasing value,” observed Dr. Jellinger. Further large-scale cardiovascular outcomes trials that directly compare these two drug classes “are unlikely given the depth of information available now,” Dr. Jellinger suggested.
“This head-to-head retrospective study may be as close as we get and does represent the first effort at a comparison of these two classes.”
Dr. Patorno said of the potential clinical implications: “Because the two classes are equally effective for stroke and myocardial infarction, but the SGLT2 inhibitors are superior for heart failure, when considered in aggregate, SGLT2 inhibitors are likely to prevent more of these adverse cardiovascular events than GLP-1 RA.”
The study received no commercial funding and was supported by the Brigham and Women’s Hospital and Harvard Medical School Division of Pharmacoepidemiology and Pharmacoeconomics.
Dr. Patorno reported no conflicts of interest. Dr. Jellinger is on the speaker’s bureau for Astra Zeneca, Amgen, and Esperion, and has served on advisory boards for Corcept and Regeneron.
Around a 30% reduction in the risk for being hospitalized for heart failure was achieved in people with type 2 diabetes who were treated with a SGLT2 inhibitor over a GLP-1 RA regardless of whether the patients had a preexisting heart condition.
The findings, published in the Annals of Internal Medicine, also showed a 10% lower risk for myocardial infarction or stroke among those treated with a SGLT2 inhibitor who had preexisting cardiovascular disease (CVD), although there was no difference in risk between the two classes of drugs in those without preexisting CVD.
“These findings are important as they suggest that SGLT2 [inhibitors] and GLP-1 RA offer similar benefits in preventing myocardial infarction and stroke in patients with diabetes,” said study investigator Elisabetta Patorno, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School in Boston, in an interview.
They also show “that SGLT2 [inhibitors] offer greater efficacy in preventing heart failure, which supports the existing guidelines,” she added.
Paul S. Jellinger, MD, MACE, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said these data were likely to be “additive to guidelines but not transformative.” The overall analysis results were “not surprising.” It was not unexpected that SGLT2 inhibitors provided a robust chronic heart failure (CHF) benefit, particularly in individuals with history of CVD, he said.
Dr. Jellinger, a clinical endocrinologist and professor of clinical medicine on the voluntary faculty at the University of Miami, observed, however, that “the similar CVD benefit in both drug classes in patients without known CVD adds to our knowledge in this somewhat controversial area and may be useful to the clinician in evaluating therapy in a diabetic individual without evidence of or at high risk for CHF.”
Furthermore, “the study also reminds us that, as demonstrated in published meta-analysis, there is also a modest CHF benefit associated with GLP-1 RA treatment particularly in patients with a history of CVD.”
Addressing the knowledge gap
Thanks to the results of many large-scale, prospective, cardiovascular outcomes studies, both SGLT2 inhibitors and GLP1 RA have been recommended as treatment for people with diabetes who have established CVD. But with no direct head-to-head trials having been conducted, there is a gap in knowledge and there is currently little guidance for physicians on which drug class to choose for an individual patient.
To try to clarify things, Dr. Patorno and associates looked at data from more than 370,000 people with type 2 diabetes who had been treated between April 2013 and December 2017 with either a SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or GLP-1 RA (albiglutide, dulaglutide, exenatide, or liraglutide).
One-to-one propensity score matching was used to create the study groups: participants were first grouped according to their history of CVD, and then by the class of drug they had been prescribed. The primary outcomes were hospitalization for MI, stroke, or heart failure.
Comparing the initiation of a SGLT2 inhibitor with GLP-1 RA therapy, the hazard ratios (HRs) for MI or stroke in patients with and without a history of CVD were a respective 0.90 (95% CI, 0.82 to 0.98) and 1.07 (0.97 to 1.18).
The corresponding hazard ratios for heart failure hospitalizations were 0.71 (0.64 to 0.79) and 0.69 (0.56 to 0.85).
Real-world studies are of ‘increasing value’
“As in other not-randomized studies based on real-world data, residual confounding cannot be completely ruled out,” Dr. Patorno acknowledged. She added, however that “state-of-the-art methodological strategies were implemented to minimize this possibility.”
Limitations notwithstanding, “real world studies are demonstrating increasing value,” observed Dr. Jellinger. Further large-scale cardiovascular outcomes trials that directly compare these two drug classes “are unlikely given the depth of information available now,” Dr. Jellinger suggested.
“This head-to-head retrospective study may be as close as we get and does represent the first effort at a comparison of these two classes.”
Dr. Patorno said of the potential clinical implications: “Because the two classes are equally effective for stroke and myocardial infarction, but the SGLT2 inhibitors are superior for heart failure, when considered in aggregate, SGLT2 inhibitors are likely to prevent more of these adverse cardiovascular events than GLP-1 RA.”
The study received no commercial funding and was supported by the Brigham and Women’s Hospital and Harvard Medical School Division of Pharmacoepidemiology and Pharmacoeconomics.
Dr. Patorno reported no conflicts of interest. Dr. Jellinger is on the speaker’s bureau for Astra Zeneca, Amgen, and Esperion, and has served on advisory boards for Corcept and Regeneron.
FROM ANNALS OF INTERNAL MEDICINE
New fellowship, no problem
Using growth mindset to tackle fellowship in a new program
Growth mindset is a well-established phenomenon in childhood education that is now starting to appear in health care education literature.1 This concept emphasizes the capacity of individuals to change and grow through experience and that an individual’s basic qualities can be cultivated through hard work, open-mindedness, and help from others.2
Growth mindset opposes the concept of fixed mindset, which implies intelligence or other personal traits are set in stone, unable to be fundamentally changed.2 Individuals with fixed mindsets are less adept at coping with perceived failures and critical feedback because they view these as attacks on their own abilities.2 This oftentimes leads these individuals to avoid potential challenges and feedback because of fear of being exposed as incompetent or feeling inadequate. Conversely, individuals with a growth mindset embrace challenges and failures as learning opportunities and identify feedback as a critical element of growth.2 These individuals maintain a sense of resilience in the face of adversity and strive to become lifelong learners.
As the field of pediatric hospital medicine (PHM) continues to rapidly evolve, so too does the landscape of PHM fellowships. New programs are opening at a torrid pace to accommodate the increasing demand of residents looking to enter the field with new subspecialty accreditation. Most first-year PHM fellows in established programs enter with a clear precedent to follow, set forth by fellows who have come before them. For PHM fellows in new programs, however, there is often no beaten path to follow.
Entering fellowship as a first-year PHM fellow in a new program and blazing one’s own trail can be intriguing and exhilarating given the unique opportunities available. However, the potential challenges for both fellows and program directors during this transition cannot be understated. The role of new PHM fellows within the institutional framework may initially be unclear to others, which can lead to ambiguous expectations and disruptions to normal workflows. Furthermore, assessing and evaluating new fellows may prove difficult as a result of these unclear expectations and general uncertainties. Using the growth mindset can help both PHM fellows and program directors take a deliberate approach to the challenges and uncertainty that may accompany the creation of a new fellowship program.
One of the challenges new PHM fellows may encounter lies within the structure of the care team. Resident and medical student learners may express consternation that the new fellow role may limit their own autonomy. In addition, finding the right balance of autonomy and supervision between the attending-fellow dyad may prove to be difficult. However, using the growth mindset may allow fellows to see the inherent benefits of this new role.
Fellows should seize the opportunity to discuss the nuances and differing approaches to difficult clinical questions, managing a team and interpersonal dynamics, and balancing clinical and nonclinical responsibilities with an experienced supervising clinician; issues that are often less pressing as a resident. The fellow role also affords the opportunity to more carefully observe different clinical styles of practice to subsequently shape one’s own preferred style.
Finally, fellows should employ a growth mindset to optimize clinical time by discussing expectations with involved stakeholders prior to rotations and explicitly identifying goals for feedback and improvement. Program directors can also help stakeholders including faculty, residency programs, medical schools, and other health care professionals on the clinical teams prepare for this transition by providing expectations for the fellow role and by soliciting questions and feedback before and after fellows begin.
One of the key tenets of the growth mindset is actively seeking out constructive feedback and learning from failures to grow and improve. This can be a particularly useful practice for fellows during the course of their scholarly pursuits in clinical research, quality improvement, and medical education. From initial stages of idea development through the final steps of manuscript submission and peer review, fellows will undoubtedly navigate a plethora of challenges and setbacks along the way. Program directors and other core faculty members can promote a growth mindset culture by honestly discussing their own challenges and failures in career endeavors in addition to giving thoughtful constructive feedback.
Fellows should routinely practice explicitly identifying knowledge and skills gaps that represent areas for potential improvement. But perhaps most importantly, fellows must strive to see all feedback and perceived failures not as personal affronts or as commentaries on personal abilities, but rather as opportunities to strengthen their scholarly products and gain valuable experience for future endeavors.
Not all learners will come equipped with a growth mindset. So, what can fellows and program directors in new programs do to develop this practice and mitigate some of the inevitable uncertainty? To begin, program directors should think about how to create cultures of growth and development as the fixed and growth mindsets are not just limited to individuals.3 Program directors can strive to augment this process by committing to solicit feedback for themselves and acknowledging their own vulnerabilities and perceived weaknesses.
Fellows must have early, honest discussions with program directors and other stakeholders about expectations and goals. Program directors should consider creating lists of “must meet” individuals within the institution that can help fellows begin to carve out their roles in the clinical, educational, and research realms. Deliberately crafting a mentorship team that will encourage a commitment to growth and improvement is critical. Seeking out growth feedback, particularly in areas that prove challenging, should become common practice for fellows from the onset.
Most importantly, fellows should reframe uncertainty as opportunity for growth and progression. Seeing oneself as a work in progress provides a new perspective that prioritizes learning and emphasizes improvement potential.
Embodying this approach requires patience and practice. Being part of a newly created fellowship represents an opportunity to learn from personal challenges rather than leaning on the precedent set by previous fellows. And although fellows will often face uncertainty as a part of the novelty within a new program, they can ultimately succeed by practicing the principles of Dweck’s Growth Mindset: embracing challenges and failure as learning experiences, seeking out feedback, and pursuing the opportunities among ambiguity.
Dr. Herchline is a pediatric hospitalist at Cincinnati Children’s Hospital Medical Center and recent fellow graduate of the Children’s Hospital of Philadelphia. During fellowship, he completed a master’s degree in medical education at the University of Pennsylvania. His academic interests include graduate medical education, interprofessional collaboration and teamwork, and quality improvement.
References
1. Klein J et al. A growth mindset approach to preparing trainees for medical error. BMJ Qual Saf. 2017 Sep;26(9):771-4. doi: 10.1136/bmjqs-2016-006416.
2. Dweck C. Mindset: The new psychology of success. New York: Ballantine Books; 2006.
3. Murphy MC, Dweck CS. A culture of genius: How an organization’s lay theory shapes people’s cognition, affect, and behavior. Pers Soc Psychol Bull. 2010 Mar;36(3):283-96. doi: 10.1177/0146167209347380.
Using growth mindset to tackle fellowship in a new program
Using growth mindset to tackle fellowship in a new program
Growth mindset is a well-established phenomenon in childhood education that is now starting to appear in health care education literature.1 This concept emphasizes the capacity of individuals to change and grow through experience and that an individual’s basic qualities can be cultivated through hard work, open-mindedness, and help from others.2
Growth mindset opposes the concept of fixed mindset, which implies intelligence or other personal traits are set in stone, unable to be fundamentally changed.2 Individuals with fixed mindsets are less adept at coping with perceived failures and critical feedback because they view these as attacks on their own abilities.2 This oftentimes leads these individuals to avoid potential challenges and feedback because of fear of being exposed as incompetent or feeling inadequate. Conversely, individuals with a growth mindset embrace challenges and failures as learning opportunities and identify feedback as a critical element of growth.2 These individuals maintain a sense of resilience in the face of adversity and strive to become lifelong learners.
As the field of pediatric hospital medicine (PHM) continues to rapidly evolve, so too does the landscape of PHM fellowships. New programs are opening at a torrid pace to accommodate the increasing demand of residents looking to enter the field with new subspecialty accreditation. Most first-year PHM fellows in established programs enter with a clear precedent to follow, set forth by fellows who have come before them. For PHM fellows in new programs, however, there is often no beaten path to follow.
Entering fellowship as a first-year PHM fellow in a new program and blazing one’s own trail can be intriguing and exhilarating given the unique opportunities available. However, the potential challenges for both fellows and program directors during this transition cannot be understated. The role of new PHM fellows within the institutional framework may initially be unclear to others, which can lead to ambiguous expectations and disruptions to normal workflows. Furthermore, assessing and evaluating new fellows may prove difficult as a result of these unclear expectations and general uncertainties. Using the growth mindset can help both PHM fellows and program directors take a deliberate approach to the challenges and uncertainty that may accompany the creation of a new fellowship program.
One of the challenges new PHM fellows may encounter lies within the structure of the care team. Resident and medical student learners may express consternation that the new fellow role may limit their own autonomy. In addition, finding the right balance of autonomy and supervision between the attending-fellow dyad may prove to be difficult. However, using the growth mindset may allow fellows to see the inherent benefits of this new role.
Fellows should seize the opportunity to discuss the nuances and differing approaches to difficult clinical questions, managing a team and interpersonal dynamics, and balancing clinical and nonclinical responsibilities with an experienced supervising clinician; issues that are often less pressing as a resident. The fellow role also affords the opportunity to more carefully observe different clinical styles of practice to subsequently shape one’s own preferred style.
Finally, fellows should employ a growth mindset to optimize clinical time by discussing expectations with involved stakeholders prior to rotations and explicitly identifying goals for feedback and improvement. Program directors can also help stakeholders including faculty, residency programs, medical schools, and other health care professionals on the clinical teams prepare for this transition by providing expectations for the fellow role and by soliciting questions and feedback before and after fellows begin.
One of the key tenets of the growth mindset is actively seeking out constructive feedback and learning from failures to grow and improve. This can be a particularly useful practice for fellows during the course of their scholarly pursuits in clinical research, quality improvement, and medical education. From initial stages of idea development through the final steps of manuscript submission and peer review, fellows will undoubtedly navigate a plethora of challenges and setbacks along the way. Program directors and other core faculty members can promote a growth mindset culture by honestly discussing their own challenges and failures in career endeavors in addition to giving thoughtful constructive feedback.
Fellows should routinely practice explicitly identifying knowledge and skills gaps that represent areas for potential improvement. But perhaps most importantly, fellows must strive to see all feedback and perceived failures not as personal affronts or as commentaries on personal abilities, but rather as opportunities to strengthen their scholarly products and gain valuable experience for future endeavors.
Not all learners will come equipped with a growth mindset. So, what can fellows and program directors in new programs do to develop this practice and mitigate some of the inevitable uncertainty? To begin, program directors should think about how to create cultures of growth and development as the fixed and growth mindsets are not just limited to individuals.3 Program directors can strive to augment this process by committing to solicit feedback for themselves and acknowledging their own vulnerabilities and perceived weaknesses.
Fellows must have early, honest discussions with program directors and other stakeholders about expectations and goals. Program directors should consider creating lists of “must meet” individuals within the institution that can help fellows begin to carve out their roles in the clinical, educational, and research realms. Deliberately crafting a mentorship team that will encourage a commitment to growth and improvement is critical. Seeking out growth feedback, particularly in areas that prove challenging, should become common practice for fellows from the onset.
Most importantly, fellows should reframe uncertainty as opportunity for growth and progression. Seeing oneself as a work in progress provides a new perspective that prioritizes learning and emphasizes improvement potential.
Embodying this approach requires patience and practice. Being part of a newly created fellowship represents an opportunity to learn from personal challenges rather than leaning on the precedent set by previous fellows. And although fellows will often face uncertainty as a part of the novelty within a new program, they can ultimately succeed by practicing the principles of Dweck’s Growth Mindset: embracing challenges and failure as learning experiences, seeking out feedback, and pursuing the opportunities among ambiguity.
Dr. Herchline is a pediatric hospitalist at Cincinnati Children’s Hospital Medical Center and recent fellow graduate of the Children’s Hospital of Philadelphia. During fellowship, he completed a master’s degree in medical education at the University of Pennsylvania. His academic interests include graduate medical education, interprofessional collaboration and teamwork, and quality improvement.
References
1. Klein J et al. A growth mindset approach to preparing trainees for medical error. BMJ Qual Saf. 2017 Sep;26(9):771-4. doi: 10.1136/bmjqs-2016-006416.
2. Dweck C. Mindset: The new psychology of success. New York: Ballantine Books; 2006.
3. Murphy MC, Dweck CS. A culture of genius: How an organization’s lay theory shapes people’s cognition, affect, and behavior. Pers Soc Psychol Bull. 2010 Mar;36(3):283-96. doi: 10.1177/0146167209347380.
Growth mindset is a well-established phenomenon in childhood education that is now starting to appear in health care education literature.1 This concept emphasizes the capacity of individuals to change and grow through experience and that an individual’s basic qualities can be cultivated through hard work, open-mindedness, and help from others.2
Growth mindset opposes the concept of fixed mindset, which implies intelligence or other personal traits are set in stone, unable to be fundamentally changed.2 Individuals with fixed mindsets are less adept at coping with perceived failures and critical feedback because they view these as attacks on their own abilities.2 This oftentimes leads these individuals to avoid potential challenges and feedback because of fear of being exposed as incompetent or feeling inadequate. Conversely, individuals with a growth mindset embrace challenges and failures as learning opportunities and identify feedback as a critical element of growth.2 These individuals maintain a sense of resilience in the face of adversity and strive to become lifelong learners.
As the field of pediatric hospital medicine (PHM) continues to rapidly evolve, so too does the landscape of PHM fellowships. New programs are opening at a torrid pace to accommodate the increasing demand of residents looking to enter the field with new subspecialty accreditation. Most first-year PHM fellows in established programs enter with a clear precedent to follow, set forth by fellows who have come before them. For PHM fellows in new programs, however, there is often no beaten path to follow.
Entering fellowship as a first-year PHM fellow in a new program and blazing one’s own trail can be intriguing and exhilarating given the unique opportunities available. However, the potential challenges for both fellows and program directors during this transition cannot be understated. The role of new PHM fellows within the institutional framework may initially be unclear to others, which can lead to ambiguous expectations and disruptions to normal workflows. Furthermore, assessing and evaluating new fellows may prove difficult as a result of these unclear expectations and general uncertainties. Using the growth mindset can help both PHM fellows and program directors take a deliberate approach to the challenges and uncertainty that may accompany the creation of a new fellowship program.
One of the challenges new PHM fellows may encounter lies within the structure of the care team. Resident and medical student learners may express consternation that the new fellow role may limit their own autonomy. In addition, finding the right balance of autonomy and supervision between the attending-fellow dyad may prove to be difficult. However, using the growth mindset may allow fellows to see the inherent benefits of this new role.
Fellows should seize the opportunity to discuss the nuances and differing approaches to difficult clinical questions, managing a team and interpersonal dynamics, and balancing clinical and nonclinical responsibilities with an experienced supervising clinician; issues that are often less pressing as a resident. The fellow role also affords the opportunity to more carefully observe different clinical styles of practice to subsequently shape one’s own preferred style.
Finally, fellows should employ a growth mindset to optimize clinical time by discussing expectations with involved stakeholders prior to rotations and explicitly identifying goals for feedback and improvement. Program directors can also help stakeholders including faculty, residency programs, medical schools, and other health care professionals on the clinical teams prepare for this transition by providing expectations for the fellow role and by soliciting questions and feedback before and after fellows begin.
One of the key tenets of the growth mindset is actively seeking out constructive feedback and learning from failures to grow and improve. This can be a particularly useful practice for fellows during the course of their scholarly pursuits in clinical research, quality improvement, and medical education. From initial stages of idea development through the final steps of manuscript submission and peer review, fellows will undoubtedly navigate a plethora of challenges and setbacks along the way. Program directors and other core faculty members can promote a growth mindset culture by honestly discussing their own challenges and failures in career endeavors in addition to giving thoughtful constructive feedback.
Fellows should routinely practice explicitly identifying knowledge and skills gaps that represent areas for potential improvement. But perhaps most importantly, fellows must strive to see all feedback and perceived failures not as personal affronts or as commentaries on personal abilities, but rather as opportunities to strengthen their scholarly products and gain valuable experience for future endeavors.
Not all learners will come equipped with a growth mindset. So, what can fellows and program directors in new programs do to develop this practice and mitigate some of the inevitable uncertainty? To begin, program directors should think about how to create cultures of growth and development as the fixed and growth mindsets are not just limited to individuals.3 Program directors can strive to augment this process by committing to solicit feedback for themselves and acknowledging their own vulnerabilities and perceived weaknesses.
Fellows must have early, honest discussions with program directors and other stakeholders about expectations and goals. Program directors should consider creating lists of “must meet” individuals within the institution that can help fellows begin to carve out their roles in the clinical, educational, and research realms. Deliberately crafting a mentorship team that will encourage a commitment to growth and improvement is critical. Seeking out growth feedback, particularly in areas that prove challenging, should become common practice for fellows from the onset.
Most importantly, fellows should reframe uncertainty as opportunity for growth and progression. Seeing oneself as a work in progress provides a new perspective that prioritizes learning and emphasizes improvement potential.
Embodying this approach requires patience and practice. Being part of a newly created fellowship represents an opportunity to learn from personal challenges rather than leaning on the precedent set by previous fellows. And although fellows will often face uncertainty as a part of the novelty within a new program, they can ultimately succeed by practicing the principles of Dweck’s Growth Mindset: embracing challenges and failure as learning experiences, seeking out feedback, and pursuing the opportunities among ambiguity.
Dr. Herchline is a pediatric hospitalist at Cincinnati Children’s Hospital Medical Center and recent fellow graduate of the Children’s Hospital of Philadelphia. During fellowship, he completed a master’s degree in medical education at the University of Pennsylvania. His academic interests include graduate medical education, interprofessional collaboration and teamwork, and quality improvement.
References
1. Klein J et al. A growth mindset approach to preparing trainees for medical error. BMJ Qual Saf. 2017 Sep;26(9):771-4. doi: 10.1136/bmjqs-2016-006416.
2. Dweck C. Mindset: The new psychology of success. New York: Ballantine Books; 2006.
3. Murphy MC, Dweck CS. A culture of genius: How an organization’s lay theory shapes people’s cognition, affect, and behavior. Pers Soc Psychol Bull. 2010 Mar;36(3):283-96. doi: 10.1177/0146167209347380.
New virus causing ‘Alaskapox’ detected in two more cases
Both people were diagnosed after receiving urgent care in a Fairbanks-area clinic. One was a child with a sore on the left elbow, along with fever and swollen lymph nodes. And the other was an unrelated middle-aged woman with a pox mark on her leg, swollen lymph nodes, and joint pain. In both cases, symptoms improved within 3 weeks.
This isn’t the first time the so-called Alaskapox virus has been detected in the region. In 2015, a woman living near Fairbanks turned up at her doctor’s office with a single reddened pox-like mark on her upper arm and a feeling of fatigue.
Sampling of the pox mark showed that it was caused by a previously unidentified virus of the same family as smallpox and cowpox.
Five years later, another woman showed up with similar signs and symptoms, and her pox also proved to be the result of what public health experts started calling the Alaskapox virus.
In both cases, the women recovered completely.
Smallpox-like illness
Public health sleuths figured out that in three of the four cases, the patients lived in a home with a cat or cats, and one of these cats was known to hunt small animals.
Experts already knew that cats mingling in cow pastures and sickened by cattle virus had helped cowpox make the leap from bovines to humans. And just as in the case of cowpox, they suspected that cats might again be spreading this new virus to people, too.
All four of the infected people lived in sparsely populated areas amid forests. Officials laid animal traps where some of the affected people lived and identified the virus in several species of small wild animals.
The animals that turned up most often with Alaskapox were small mouse-like voles. The rodents with rounded muzzles are known for burrowing in the region. And scientists suspect the Alaskapox virus makes its way from these wild animals to humans through their pet cats or possibly by direct exposure outdoors.
None of the four people identified so far with Alaskapox knew each other or interacted, so officials also suspect that there are more cases going unrecognized, possibly because the symptoms are mild or nonexistent.
There are no documented cases of person-to-person transmission of Alaskapox, according to public health officials monitoring the small number of cases. But other pox viruses can spread by direct contact with skin lesions, so clinicians are recommending that people cover wounds with bandages. Three of the people with Alaskapox mistook their lesions at first for a bite from a spider or insect.
A version of this article first appeared on WebMD.com.
Both people were diagnosed after receiving urgent care in a Fairbanks-area clinic. One was a child with a sore on the left elbow, along with fever and swollen lymph nodes. And the other was an unrelated middle-aged woman with a pox mark on her leg, swollen lymph nodes, and joint pain. In both cases, symptoms improved within 3 weeks.
This isn’t the first time the so-called Alaskapox virus has been detected in the region. In 2015, a woman living near Fairbanks turned up at her doctor’s office with a single reddened pox-like mark on her upper arm and a feeling of fatigue.
Sampling of the pox mark showed that it was caused by a previously unidentified virus of the same family as smallpox and cowpox.
Five years later, another woman showed up with similar signs and symptoms, and her pox also proved to be the result of what public health experts started calling the Alaskapox virus.
In both cases, the women recovered completely.
Smallpox-like illness
Public health sleuths figured out that in three of the four cases, the patients lived in a home with a cat or cats, and one of these cats was known to hunt small animals.
Experts already knew that cats mingling in cow pastures and sickened by cattle virus had helped cowpox make the leap from bovines to humans. And just as in the case of cowpox, they suspected that cats might again be spreading this new virus to people, too.
All four of the infected people lived in sparsely populated areas amid forests. Officials laid animal traps where some of the affected people lived and identified the virus in several species of small wild animals.
The animals that turned up most often with Alaskapox were small mouse-like voles. The rodents with rounded muzzles are known for burrowing in the region. And scientists suspect the Alaskapox virus makes its way from these wild animals to humans through their pet cats or possibly by direct exposure outdoors.
None of the four people identified so far with Alaskapox knew each other or interacted, so officials also suspect that there are more cases going unrecognized, possibly because the symptoms are mild or nonexistent.
There are no documented cases of person-to-person transmission of Alaskapox, according to public health officials monitoring the small number of cases. But other pox viruses can spread by direct contact with skin lesions, so clinicians are recommending that people cover wounds with bandages. Three of the people with Alaskapox mistook their lesions at first for a bite from a spider or insect.
A version of this article first appeared on WebMD.com.
Both people were diagnosed after receiving urgent care in a Fairbanks-area clinic. One was a child with a sore on the left elbow, along with fever and swollen lymph nodes. And the other was an unrelated middle-aged woman with a pox mark on her leg, swollen lymph nodes, and joint pain. In both cases, symptoms improved within 3 weeks.
This isn’t the first time the so-called Alaskapox virus has been detected in the region. In 2015, a woman living near Fairbanks turned up at her doctor’s office with a single reddened pox-like mark on her upper arm and a feeling of fatigue.
Sampling of the pox mark showed that it was caused by a previously unidentified virus of the same family as smallpox and cowpox.
Five years later, another woman showed up with similar signs and symptoms, and her pox also proved to be the result of what public health experts started calling the Alaskapox virus.
In both cases, the women recovered completely.
Smallpox-like illness
Public health sleuths figured out that in three of the four cases, the patients lived in a home with a cat or cats, and one of these cats was known to hunt small animals.
Experts already knew that cats mingling in cow pastures and sickened by cattle virus had helped cowpox make the leap from bovines to humans. And just as in the case of cowpox, they suspected that cats might again be spreading this new virus to people, too.
All four of the infected people lived in sparsely populated areas amid forests. Officials laid animal traps where some of the affected people lived and identified the virus in several species of small wild animals.
The animals that turned up most often with Alaskapox were small mouse-like voles. The rodents with rounded muzzles are known for burrowing in the region. And scientists suspect the Alaskapox virus makes its way from these wild animals to humans through their pet cats or possibly by direct exposure outdoors.
None of the four people identified so far with Alaskapox knew each other or interacted, so officials also suspect that there are more cases going unrecognized, possibly because the symptoms are mild or nonexistent.
There are no documented cases of person-to-person transmission of Alaskapox, according to public health officials monitoring the small number of cases. But other pox viruses can spread by direct contact with skin lesions, so clinicians are recommending that people cover wounds with bandages. Three of the people with Alaskapox mistook their lesions at first for a bite from a spider or insect.
A version of this article first appeared on WebMD.com.
COVID-19 a rare trigger for Guillain-Barré syndrome
Although Guillain-Barré syndrome may rarely follow a recent infection with SARS-CoV-2, a strong relationship of GBS with the novel coronavirus is unlikely, say researchers with the International GBS Outcome Study (IGOS) consortium.
“Our study shows that COVID-19 may precede Guillain-Barré syndrome in rare cases, but the existence of a true association or causal relation still needs to be established,” Bart Jacobs, MD, PhD, department of neurology and immunology, Erasmus Medical Center and University Medical Center, both in Rotterdam, the Netherlands, said in a statement.
The study was published online in the journal Brain.
No uptick in pandemic cases
Since the beginning of the pandemic, there are reports of more than 90 GBS diagnoses following a possible COVID-19 infection. However, it remains unclear whether COVID-19 is another potential infectious trigger or whether the reported cases are coincidental.
To investigate further, Dr. Jacobs and the IGOS consortium reviewed 49 patients (median age, 56 years) with GBS who were added to their ongoing prospective observational cohort study between Jan. 30 and May 30, 2020.
The patients came from China, Denmark, France, Greece, Italy, Japan, the Netherlands, Spain, Switzerland, and the United Kingdom.
Of the 49 GBS patients, 8 (16%) had a confirmed and 3 (6%) had a probable SARS-CoV-2 infection; 15 had possible SARS-CoV-2 infection, 21 had no suspicion of SARS-CoV-2 infection, and 2 were “unclassifiable.”
Of the 11 patients with confirmed/probable SARS-CoV-2 infection, 9 had no serological evidence of any other recent preceding infection known to be associated with GBS.
The other two had serological evidence of a recent Campylobacter jejuni infection, which could have played a role in GBS onset, the researchers noted.
Most patients with a confirmed/probable SARS-CoV-2 infection had a sensorimotor GBS variant (73%), although Miller Fisher syndrome–GBS overlap (18%) and an ataxic variant (9%) were also found.
All patients with a confirmed/probable SARS-CoV-2 infection had a severe form of GBS. Common early neurologic features were facial weakness (64%), sensory deficits (82%), and autonomic dysfunction (64%), although not significantly different, compared with the other patients.
All eight patients who underwent nerve conduction study had a demyelinating subtype, which was more frequent than in the other GBS patients (47%; P = .012) as well as historical region and age-matched controls included in the IGOS cohort before the pandemic (52%, P = .016).
The median time from the onset of SARS-CoV-2 infection to neurologic symptoms was 16 days and ranged from 12 to 22 days.
More research needed
The researchers noted that the 22% frequency of a preceding SARS-CoV-2 infection in this study population was “higher than estimates of the contemporaneous background prevalence of SARS-CoV-2, which may be a result of recruitment bias during the pandemic, but could also indicate that GBS may rarely follow a recent SARS-CoV-2 infection.”
Importantly, however, they did not find more patients diagnosed with GBS during the first 4 months of the pandemic, compared with previous years, “suggesting that a strong association between SARS-CoV-2 and GBS is unlikely.”
“Should SARS-CoV-2 indeed be able to trigger GBS, our data are consistent with a postinfectious disease mechanism rather than direct viral invasion,” they noted, adding that the study was not designed to quantify a causative link between GBS and SARS-CoV-2.
“An unbiased multicenter, international, case-control study is needed to determine whether there is an association or not,” they wrote.
The IGOS is financially supported by the GBS-CIDP Foundation International, Gain, Erasmus MC University Medical Center Rotterdam, Glasgow University, CSL Behring, Grifols, Annexon and Hansa Biopharma. Dr. Jacobs received grants from Grifols, CSL-Behring, Annexon, Prinses Beatrix Spierfonds, Hansa Biopharma, and GBS-CIDP Foundation International and is on the global medical advisory board of the GBS CIDP Foundation International.
A version of this article first appeared on Medscape.com.
Although Guillain-Barré syndrome may rarely follow a recent infection with SARS-CoV-2, a strong relationship of GBS with the novel coronavirus is unlikely, say researchers with the International GBS Outcome Study (IGOS) consortium.
“Our study shows that COVID-19 may precede Guillain-Barré syndrome in rare cases, but the existence of a true association or causal relation still needs to be established,” Bart Jacobs, MD, PhD, department of neurology and immunology, Erasmus Medical Center and University Medical Center, both in Rotterdam, the Netherlands, said in a statement.
The study was published online in the journal Brain.
No uptick in pandemic cases
Since the beginning of the pandemic, there are reports of more than 90 GBS diagnoses following a possible COVID-19 infection. However, it remains unclear whether COVID-19 is another potential infectious trigger or whether the reported cases are coincidental.
To investigate further, Dr. Jacobs and the IGOS consortium reviewed 49 patients (median age, 56 years) with GBS who were added to their ongoing prospective observational cohort study between Jan. 30 and May 30, 2020.
The patients came from China, Denmark, France, Greece, Italy, Japan, the Netherlands, Spain, Switzerland, and the United Kingdom.
Of the 49 GBS patients, 8 (16%) had a confirmed and 3 (6%) had a probable SARS-CoV-2 infection; 15 had possible SARS-CoV-2 infection, 21 had no suspicion of SARS-CoV-2 infection, and 2 were “unclassifiable.”
Of the 11 patients with confirmed/probable SARS-CoV-2 infection, 9 had no serological evidence of any other recent preceding infection known to be associated with GBS.
The other two had serological evidence of a recent Campylobacter jejuni infection, which could have played a role in GBS onset, the researchers noted.
Most patients with a confirmed/probable SARS-CoV-2 infection had a sensorimotor GBS variant (73%), although Miller Fisher syndrome–GBS overlap (18%) and an ataxic variant (9%) were also found.
All patients with a confirmed/probable SARS-CoV-2 infection had a severe form of GBS. Common early neurologic features were facial weakness (64%), sensory deficits (82%), and autonomic dysfunction (64%), although not significantly different, compared with the other patients.
All eight patients who underwent nerve conduction study had a demyelinating subtype, which was more frequent than in the other GBS patients (47%; P = .012) as well as historical region and age-matched controls included in the IGOS cohort before the pandemic (52%, P = .016).
The median time from the onset of SARS-CoV-2 infection to neurologic symptoms was 16 days and ranged from 12 to 22 days.
More research needed
The researchers noted that the 22% frequency of a preceding SARS-CoV-2 infection in this study population was “higher than estimates of the contemporaneous background prevalence of SARS-CoV-2, which may be a result of recruitment bias during the pandemic, but could also indicate that GBS may rarely follow a recent SARS-CoV-2 infection.”
Importantly, however, they did not find more patients diagnosed with GBS during the first 4 months of the pandemic, compared with previous years, “suggesting that a strong association between SARS-CoV-2 and GBS is unlikely.”
“Should SARS-CoV-2 indeed be able to trigger GBS, our data are consistent with a postinfectious disease mechanism rather than direct viral invasion,” they noted, adding that the study was not designed to quantify a causative link between GBS and SARS-CoV-2.
“An unbiased multicenter, international, case-control study is needed to determine whether there is an association or not,” they wrote.
The IGOS is financially supported by the GBS-CIDP Foundation International, Gain, Erasmus MC University Medical Center Rotterdam, Glasgow University, CSL Behring, Grifols, Annexon and Hansa Biopharma. Dr. Jacobs received grants from Grifols, CSL-Behring, Annexon, Prinses Beatrix Spierfonds, Hansa Biopharma, and GBS-CIDP Foundation International and is on the global medical advisory board of the GBS CIDP Foundation International.
A version of this article first appeared on Medscape.com.
Although Guillain-Barré syndrome may rarely follow a recent infection with SARS-CoV-2, a strong relationship of GBS with the novel coronavirus is unlikely, say researchers with the International GBS Outcome Study (IGOS) consortium.
“Our study shows that COVID-19 may precede Guillain-Barré syndrome in rare cases, but the existence of a true association or causal relation still needs to be established,” Bart Jacobs, MD, PhD, department of neurology and immunology, Erasmus Medical Center and University Medical Center, both in Rotterdam, the Netherlands, said in a statement.
The study was published online in the journal Brain.
No uptick in pandemic cases
Since the beginning of the pandemic, there are reports of more than 90 GBS diagnoses following a possible COVID-19 infection. However, it remains unclear whether COVID-19 is another potential infectious trigger or whether the reported cases are coincidental.
To investigate further, Dr. Jacobs and the IGOS consortium reviewed 49 patients (median age, 56 years) with GBS who were added to their ongoing prospective observational cohort study between Jan. 30 and May 30, 2020.
The patients came from China, Denmark, France, Greece, Italy, Japan, the Netherlands, Spain, Switzerland, and the United Kingdom.
Of the 49 GBS patients, 8 (16%) had a confirmed and 3 (6%) had a probable SARS-CoV-2 infection; 15 had possible SARS-CoV-2 infection, 21 had no suspicion of SARS-CoV-2 infection, and 2 were “unclassifiable.”
Of the 11 patients with confirmed/probable SARS-CoV-2 infection, 9 had no serological evidence of any other recent preceding infection known to be associated with GBS.
The other two had serological evidence of a recent Campylobacter jejuni infection, which could have played a role in GBS onset, the researchers noted.
Most patients with a confirmed/probable SARS-CoV-2 infection had a sensorimotor GBS variant (73%), although Miller Fisher syndrome–GBS overlap (18%) and an ataxic variant (9%) were also found.
All patients with a confirmed/probable SARS-CoV-2 infection had a severe form of GBS. Common early neurologic features were facial weakness (64%), sensory deficits (82%), and autonomic dysfunction (64%), although not significantly different, compared with the other patients.
All eight patients who underwent nerve conduction study had a demyelinating subtype, which was more frequent than in the other GBS patients (47%; P = .012) as well as historical region and age-matched controls included in the IGOS cohort before the pandemic (52%, P = .016).
The median time from the onset of SARS-CoV-2 infection to neurologic symptoms was 16 days and ranged from 12 to 22 days.
More research needed
The researchers noted that the 22% frequency of a preceding SARS-CoV-2 infection in this study population was “higher than estimates of the contemporaneous background prevalence of SARS-CoV-2, which may be a result of recruitment bias during the pandemic, but could also indicate that GBS may rarely follow a recent SARS-CoV-2 infection.”
Importantly, however, they did not find more patients diagnosed with GBS during the first 4 months of the pandemic, compared with previous years, “suggesting that a strong association between SARS-CoV-2 and GBS is unlikely.”
“Should SARS-CoV-2 indeed be able to trigger GBS, our data are consistent with a postinfectious disease mechanism rather than direct viral invasion,” they noted, adding that the study was not designed to quantify a causative link between GBS and SARS-CoV-2.
“An unbiased multicenter, international, case-control study is needed to determine whether there is an association or not,” they wrote.
The IGOS is financially supported by the GBS-CIDP Foundation International, Gain, Erasmus MC University Medical Center Rotterdam, Glasgow University, CSL Behring, Grifols, Annexon and Hansa Biopharma. Dr. Jacobs received grants from Grifols, CSL-Behring, Annexon, Prinses Beatrix Spierfonds, Hansa Biopharma, and GBS-CIDP Foundation International and is on the global medical advisory board of the GBS CIDP Foundation International.
A version of this article first appeared on Medscape.com.
CDC chief overrules panel, OKs boosters for health care workers
The CDC’s Advisory Committee on Immunization Practices earlier Thursday voted to allow several groups of Americans to get a booster shot, but voted not to recommend it for adults age 18 to 64 who live or work in a place where the risk of COVID-19 is high. That would have included health care workers and other frontline employees.
But CDC Director Rochelle Walensky, MD, decided to reverse that recommendation and include the 18-to-64-year-olds in her final decision.
“As CDC Director, it is my job to recognize where our actions can have the greatest impact,” Dr. Walensky said in a statement late Thursday night, according to published reports. “At CDC, we are tasked with analyzing complex, often imperfect data to make concrete recommendations that optimize health. In a pandemic, even with uncertainty, we must take actions that we anticipate will do the greatest good.”
Dr. Walensky agreed with the rest of the advisory committee's decisions, which included recommendations that the following groups also be eligible for a booster shot:
- Adults ages 65 and up and residents of long-term care facilities
- Adults ages 50 to 64 who have an underlying medical condition that may increase their risk from a COVID infection
- Adults ages 18 to 49 who may be at increased risk from a COVID-19 infection because of an underlying medical condition, if a person feels like they need one based on a consideration of their individual benefit and risks.
About 26 million Americans are at least 6 months past the last dose of the Pfizer vaccines, making them eligible to receive a third dose. About 13.6 million of them are over the age of 65. Another 5.3 million are ages 50 to 64.
In making the recommendations, the committee left out healthcare workers. This was a departure from the Food and Drug Administration’s authorization which included boosters for those 65 and over, and for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers – such as those in healthcare -- whose jobs increase their risk for infection.
This is the group Dr. Walensky added to the eligible list on her own.
Committee members “did not buy the need in occupational or institutional settings,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville. Dr. Schaffner sits on the ACIP workgroup that considered the evidence behind boosters. He said that he would have voted yes to offer boosters to healthcare and other essential workers.
“There was a real split in the committee,” he said.
The vote on boosters for healthcare and other high-risk workers was rejected 9 to 6.
“I think that there is ample evidence that people such as healthcare workers do not have repeated exposure in the workplace,” said Beth Bell, MD, a clinical professor at the University of Washington. “They’re using PPE as they should and they’re following the other policies within the healthcare setting. There’s lots of evidence that suggest that health care workers who become infected become infected because of exposures in the community.”
She was not alone in feeling cautious.
“I think this is an extremely slippery slope,” said Sarah Long, MD, a pediatric infectious disease specialist at Drexel University in Philadelphia, before her vote to reject boosters for healthcare and other high-risk workers.
“We might as well just say, ‘Give it to everybody 18 and over.’ We have an extremely effective vaccine. It’s like saying it’s not working, and it is working.”
The committee saw data showing that all of the vaccines remain highly protective against hospitalization and death for all age groups, though protection against getting sick with COVID has waned slightly over time and with the dominance of the more contagious Delta variant. Those at highest risk for a severe breakthrough infection — those that cause hospitalization or death — are older adults.
How much will the U.S. benefit from boosters?
Some felt squeamish about broadly recommending boosters at all.
“We have too much hope on the line with these boosters,” said James Loehr, MD, who is a family physician in Ithaca, N.Y. Dr. Loehr said he felt the goal of giving boosters in the United States should be to decrease hospitalizations, and he felt they would, but that the impact would likely be smaller than appreciated.
Based on his calculations of the benefits of boosters for each age group, Dr. Loehr said if boosters were given to all 13 million seniors previously vaccinated with the Pfizer vaccine, we might prevent 200 hospitalizations a day, “which would be a lot,” he noted. But, he said, “considering that we have 10,000 hospitalizations a day now, it’s probably not that much.”
Others agreed.
“I really think this is a solution looking for a problem,” said Jason Goldman, MD, an associate professor at Florida Atlantic University who was representing the American College of Physicians. “You know, I don’t think it’s going to address the issue of the pandemic. I really think it’s just going to create more confusion on the provider from the position of implementation, and I really think it’s going really far afield of the data.”
ACIP Chair Grace Lee, MD, a pediatric infectious disease specialist at Stanford, said she had cared for children who had died of COVID.
“I can tell you that their family members really wished they had extra protection for their kids, because they weren’t symptomatic. Nobody else was sick at home,” she said.
Dr. Lee said for her, access was paramount, and she was in favor of expanding access to boosters for as many people as possible.
Next steps
People who were initially vaccinated with either Moderna or Johnson & Johnson vaccines are excluded from booster recommendations, something many on the committee were uncomfortable with.
The FDA is still considering Moderna’s application to market booster doses. Johnson & Johnson hasn’t yet applied to the FDA for permission to offer second doses in the United States.
While the ACIP’s recommendations are important, in this case, they may not have a huge practical effect, said Schaffner. The CDC has already approved third shots for people who are immunocompromised, and no proof of a medical condition is required to get one.
More than 2 million people have already gotten a third dose, he noted, and not all of them are immunocompromised.
“They have heard the president say that, you know, everybody should get a booster, and they’ve taken that at face value,” he said.
A version of this article first appeared on WebMD.com.
The CDC’s Advisory Committee on Immunization Practices earlier Thursday voted to allow several groups of Americans to get a booster shot, but voted not to recommend it for adults age 18 to 64 who live or work in a place where the risk of COVID-19 is high. That would have included health care workers and other frontline employees.
But CDC Director Rochelle Walensky, MD, decided to reverse that recommendation and include the 18-to-64-year-olds in her final decision.
“As CDC Director, it is my job to recognize where our actions can have the greatest impact,” Dr. Walensky said in a statement late Thursday night, according to published reports. “At CDC, we are tasked with analyzing complex, often imperfect data to make concrete recommendations that optimize health. In a pandemic, even with uncertainty, we must take actions that we anticipate will do the greatest good.”
Dr. Walensky agreed with the rest of the advisory committee's decisions, which included recommendations that the following groups also be eligible for a booster shot:
- Adults ages 65 and up and residents of long-term care facilities
- Adults ages 50 to 64 who have an underlying medical condition that may increase their risk from a COVID infection
- Adults ages 18 to 49 who may be at increased risk from a COVID-19 infection because of an underlying medical condition, if a person feels like they need one based on a consideration of their individual benefit and risks.
About 26 million Americans are at least 6 months past the last dose of the Pfizer vaccines, making them eligible to receive a third dose. About 13.6 million of them are over the age of 65. Another 5.3 million are ages 50 to 64.
In making the recommendations, the committee left out healthcare workers. This was a departure from the Food and Drug Administration’s authorization which included boosters for those 65 and over, and for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers – such as those in healthcare -- whose jobs increase their risk for infection.
This is the group Dr. Walensky added to the eligible list on her own.
Committee members “did not buy the need in occupational or institutional settings,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville. Dr. Schaffner sits on the ACIP workgroup that considered the evidence behind boosters. He said that he would have voted yes to offer boosters to healthcare and other essential workers.
“There was a real split in the committee,” he said.
The vote on boosters for healthcare and other high-risk workers was rejected 9 to 6.
“I think that there is ample evidence that people such as healthcare workers do not have repeated exposure in the workplace,” said Beth Bell, MD, a clinical professor at the University of Washington. “They’re using PPE as they should and they’re following the other policies within the healthcare setting. There’s lots of evidence that suggest that health care workers who become infected become infected because of exposures in the community.”
She was not alone in feeling cautious.
“I think this is an extremely slippery slope,” said Sarah Long, MD, a pediatric infectious disease specialist at Drexel University in Philadelphia, before her vote to reject boosters for healthcare and other high-risk workers.
“We might as well just say, ‘Give it to everybody 18 and over.’ We have an extremely effective vaccine. It’s like saying it’s not working, and it is working.”
The committee saw data showing that all of the vaccines remain highly protective against hospitalization and death for all age groups, though protection against getting sick with COVID has waned slightly over time and with the dominance of the more contagious Delta variant. Those at highest risk for a severe breakthrough infection — those that cause hospitalization or death — are older adults.
How much will the U.S. benefit from boosters?
Some felt squeamish about broadly recommending boosters at all.
“We have too much hope on the line with these boosters,” said James Loehr, MD, who is a family physician in Ithaca, N.Y. Dr. Loehr said he felt the goal of giving boosters in the United States should be to decrease hospitalizations, and he felt they would, but that the impact would likely be smaller than appreciated.
Based on his calculations of the benefits of boosters for each age group, Dr. Loehr said if boosters were given to all 13 million seniors previously vaccinated with the Pfizer vaccine, we might prevent 200 hospitalizations a day, “which would be a lot,” he noted. But, he said, “considering that we have 10,000 hospitalizations a day now, it’s probably not that much.”
Others agreed.
“I really think this is a solution looking for a problem,” said Jason Goldman, MD, an associate professor at Florida Atlantic University who was representing the American College of Physicians. “You know, I don’t think it’s going to address the issue of the pandemic. I really think it’s just going to create more confusion on the provider from the position of implementation, and I really think it’s going really far afield of the data.”
ACIP Chair Grace Lee, MD, a pediatric infectious disease specialist at Stanford, said she had cared for children who had died of COVID.
“I can tell you that their family members really wished they had extra protection for their kids, because they weren’t symptomatic. Nobody else was sick at home,” she said.
Dr. Lee said for her, access was paramount, and she was in favor of expanding access to boosters for as many people as possible.
Next steps
People who were initially vaccinated with either Moderna or Johnson & Johnson vaccines are excluded from booster recommendations, something many on the committee were uncomfortable with.
The FDA is still considering Moderna’s application to market booster doses. Johnson & Johnson hasn’t yet applied to the FDA for permission to offer second doses in the United States.
While the ACIP’s recommendations are important, in this case, they may not have a huge practical effect, said Schaffner. The CDC has already approved third shots for people who are immunocompromised, and no proof of a medical condition is required to get one.
More than 2 million people have already gotten a third dose, he noted, and not all of them are immunocompromised.
“They have heard the president say that, you know, everybody should get a booster, and they’ve taken that at face value,” he said.
A version of this article first appeared on WebMD.com.
The CDC’s Advisory Committee on Immunization Practices earlier Thursday voted to allow several groups of Americans to get a booster shot, but voted not to recommend it for adults age 18 to 64 who live or work in a place where the risk of COVID-19 is high. That would have included health care workers and other frontline employees.
But CDC Director Rochelle Walensky, MD, decided to reverse that recommendation and include the 18-to-64-year-olds in her final decision.
“As CDC Director, it is my job to recognize where our actions can have the greatest impact,” Dr. Walensky said in a statement late Thursday night, according to published reports. “At CDC, we are tasked with analyzing complex, often imperfect data to make concrete recommendations that optimize health. In a pandemic, even with uncertainty, we must take actions that we anticipate will do the greatest good.”
Dr. Walensky agreed with the rest of the advisory committee's decisions, which included recommendations that the following groups also be eligible for a booster shot:
- Adults ages 65 and up and residents of long-term care facilities
- Adults ages 50 to 64 who have an underlying medical condition that may increase their risk from a COVID infection
- Adults ages 18 to 49 who may be at increased risk from a COVID-19 infection because of an underlying medical condition, if a person feels like they need one based on a consideration of their individual benefit and risks.
About 26 million Americans are at least 6 months past the last dose of the Pfizer vaccines, making them eligible to receive a third dose. About 13.6 million of them are over the age of 65. Another 5.3 million are ages 50 to 64.
In making the recommendations, the committee left out healthcare workers. This was a departure from the Food and Drug Administration’s authorization which included boosters for those 65 and over, and for people 18 through 64 years of age who are at high risk for severe illness from the coronavirus, including essential workers – such as those in healthcare -- whose jobs increase their risk for infection.
This is the group Dr. Walensky added to the eligible list on her own.
Committee members “did not buy the need in occupational or institutional settings,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University in Nashville. Dr. Schaffner sits on the ACIP workgroup that considered the evidence behind boosters. He said that he would have voted yes to offer boosters to healthcare and other essential workers.
“There was a real split in the committee,” he said.
The vote on boosters for healthcare and other high-risk workers was rejected 9 to 6.
“I think that there is ample evidence that people such as healthcare workers do not have repeated exposure in the workplace,” said Beth Bell, MD, a clinical professor at the University of Washington. “They’re using PPE as they should and they’re following the other policies within the healthcare setting. There’s lots of evidence that suggest that health care workers who become infected become infected because of exposures in the community.”
She was not alone in feeling cautious.
“I think this is an extremely slippery slope,” said Sarah Long, MD, a pediatric infectious disease specialist at Drexel University in Philadelphia, before her vote to reject boosters for healthcare and other high-risk workers.
“We might as well just say, ‘Give it to everybody 18 and over.’ We have an extremely effective vaccine. It’s like saying it’s not working, and it is working.”
The committee saw data showing that all of the vaccines remain highly protective against hospitalization and death for all age groups, though protection against getting sick with COVID has waned slightly over time and with the dominance of the more contagious Delta variant. Those at highest risk for a severe breakthrough infection — those that cause hospitalization or death — are older adults.
How much will the U.S. benefit from boosters?
Some felt squeamish about broadly recommending boosters at all.
“We have too much hope on the line with these boosters,” said James Loehr, MD, who is a family physician in Ithaca, N.Y. Dr. Loehr said he felt the goal of giving boosters in the United States should be to decrease hospitalizations, and he felt they would, but that the impact would likely be smaller than appreciated.
Based on his calculations of the benefits of boosters for each age group, Dr. Loehr said if boosters were given to all 13 million seniors previously vaccinated with the Pfizer vaccine, we might prevent 200 hospitalizations a day, “which would be a lot,” he noted. But, he said, “considering that we have 10,000 hospitalizations a day now, it’s probably not that much.”
Others agreed.
“I really think this is a solution looking for a problem,” said Jason Goldman, MD, an associate professor at Florida Atlantic University who was representing the American College of Physicians. “You know, I don’t think it’s going to address the issue of the pandemic. I really think it’s just going to create more confusion on the provider from the position of implementation, and I really think it’s going really far afield of the data.”
ACIP Chair Grace Lee, MD, a pediatric infectious disease specialist at Stanford, said she had cared for children who had died of COVID.
“I can tell you that their family members really wished they had extra protection for their kids, because they weren’t symptomatic. Nobody else was sick at home,” she said.
Dr. Lee said for her, access was paramount, and she was in favor of expanding access to boosters for as many people as possible.
Next steps
People who were initially vaccinated with either Moderna or Johnson & Johnson vaccines are excluded from booster recommendations, something many on the committee were uncomfortable with.
The FDA is still considering Moderna’s application to market booster doses. Johnson & Johnson hasn’t yet applied to the FDA for permission to offer second doses in the United States.
While the ACIP’s recommendations are important, in this case, they may not have a huge practical effect, said Schaffner. The CDC has already approved third shots for people who are immunocompromised, and no proof of a medical condition is required to get one.
More than 2 million people have already gotten a third dose, he noted, and not all of them are immunocompromised.
“They have heard the president say that, you know, everybody should get a booster, and they’ve taken that at face value,” he said.
A version of this article first appeared on WebMD.com.
Remdesivir sharply cuts COVID hospitalization risk, Gilead says
Remdesivir (Veklury, Gilead) was found to reduce some COVID-19 patients’ risk of hospitalization by 87% in a phase 3 trial, the drug’s manufacturer announced Sept. 22 in a press release.
The randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of a 3-day course of intravenous remdesivir in an analysis of 562 nonhospitalized patients at high risk for disease progression.
Remdesivir demonstrated a statistically significant 87% reduction in risk for COVID-19–related hospitalization or all-cause death by Day 28 (0.7% [2/279]) compared with placebo (5.3% [15/283]) P = .008. Participants were assigned 1:1 to remdesivir or the placebo group.
Researchers also found an 81% reduction in risk for the composite secondary endpoint – medical visits due to COVID-19 or all-cause death by Day 28. Only 1.6% had COVID-19 medical visits ([4/246]) compared with those in the placebo group (8.3% [21/252]) P = .002. No deaths were observed in either arm by Day 28.
“These latest data show remdesivir’s potential to help high-risk patients recover before they get sicker and stay out of the hospital altogether,” coauthor Robert L. Gottlieb, MD, PhD, from Baylor University Medical Center, Houston, said in the press release.
Remdesivir is the only drug approved by the U.S. Food and Drug Administration for hospitalized COVID-19 patients at least 12 years old. Its treatment of nonhospitalized patients with 3 days of dosing is investigational, and the safety and efficacy for this use and dosing duration have not been established or approved by any regulatory agency, the Gilead press release notes.
The patients in this study were considered high-risk for disease progression based on comorbidities – commonly obesity, hypertension, and diabetes – and age, but had not recently been hospitalized due to COVID-19.
A third of the participants were at least 60 years old. Participants in the study must have received a positive diagnosis within 4 days of starting treatment and experienced symptoms for 7 days or less.
Use of remdesivir controversial
Results from the Adaptive COVID-19 Treatment Trial (ACTT-1) showed remdesivir was superior to placebo in shortening time to recovery in adults hospitalized with COVID-19 with evidence of lower respiratory tract infection.
However, a large trial of more than 11,000 people in 30 countries, sponsored by the World Health Organization, did not show any benefit for the drug in reducing COVID deaths.
The WHO has conditionally recommended against using remdesivir in hospitalized patients, regardless of disease severity, “as there is currently no evidence that remdesivir improves survival and other outcomes in these patients.”
The drug also is given intravenously, and this study tested three infusions over 3 days, a difficult treatment for nonhospitalized patients.
The study results were released ahead of IDWeek, where the late-breaking abstract will be presented at the virtual conference in full at the end of next week.
A version of this article first appeared on Medscape.com.
Remdesivir (Veklury, Gilead) was found to reduce some COVID-19 patients’ risk of hospitalization by 87% in a phase 3 trial, the drug’s manufacturer announced Sept. 22 in a press release.
The randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of a 3-day course of intravenous remdesivir in an analysis of 562 nonhospitalized patients at high risk for disease progression.
Remdesivir demonstrated a statistically significant 87% reduction in risk for COVID-19–related hospitalization or all-cause death by Day 28 (0.7% [2/279]) compared with placebo (5.3% [15/283]) P = .008. Participants were assigned 1:1 to remdesivir or the placebo group.
Researchers also found an 81% reduction in risk for the composite secondary endpoint – medical visits due to COVID-19 or all-cause death by Day 28. Only 1.6% had COVID-19 medical visits ([4/246]) compared with those in the placebo group (8.3% [21/252]) P = .002. No deaths were observed in either arm by Day 28.
“These latest data show remdesivir’s potential to help high-risk patients recover before they get sicker and stay out of the hospital altogether,” coauthor Robert L. Gottlieb, MD, PhD, from Baylor University Medical Center, Houston, said in the press release.
Remdesivir is the only drug approved by the U.S. Food and Drug Administration for hospitalized COVID-19 patients at least 12 years old. Its treatment of nonhospitalized patients with 3 days of dosing is investigational, and the safety and efficacy for this use and dosing duration have not been established or approved by any regulatory agency, the Gilead press release notes.
The patients in this study were considered high-risk for disease progression based on comorbidities – commonly obesity, hypertension, and diabetes – and age, but had not recently been hospitalized due to COVID-19.
A third of the participants were at least 60 years old. Participants in the study must have received a positive diagnosis within 4 days of starting treatment and experienced symptoms for 7 days or less.
Use of remdesivir controversial
Results from the Adaptive COVID-19 Treatment Trial (ACTT-1) showed remdesivir was superior to placebo in shortening time to recovery in adults hospitalized with COVID-19 with evidence of lower respiratory tract infection.
However, a large trial of more than 11,000 people in 30 countries, sponsored by the World Health Organization, did not show any benefit for the drug in reducing COVID deaths.
The WHO has conditionally recommended against using remdesivir in hospitalized patients, regardless of disease severity, “as there is currently no evidence that remdesivir improves survival and other outcomes in these patients.”
The drug also is given intravenously, and this study tested three infusions over 3 days, a difficult treatment for nonhospitalized patients.
The study results were released ahead of IDWeek, where the late-breaking abstract will be presented at the virtual conference in full at the end of next week.
A version of this article first appeared on Medscape.com.
Remdesivir (Veklury, Gilead) was found to reduce some COVID-19 patients’ risk of hospitalization by 87% in a phase 3 trial, the drug’s manufacturer announced Sept. 22 in a press release.
The randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of a 3-day course of intravenous remdesivir in an analysis of 562 nonhospitalized patients at high risk for disease progression.
Remdesivir demonstrated a statistically significant 87% reduction in risk for COVID-19–related hospitalization or all-cause death by Day 28 (0.7% [2/279]) compared with placebo (5.3% [15/283]) P = .008. Participants were assigned 1:1 to remdesivir or the placebo group.
Researchers also found an 81% reduction in risk for the composite secondary endpoint – medical visits due to COVID-19 or all-cause death by Day 28. Only 1.6% had COVID-19 medical visits ([4/246]) compared with those in the placebo group (8.3% [21/252]) P = .002. No deaths were observed in either arm by Day 28.
“These latest data show remdesivir’s potential to help high-risk patients recover before they get sicker and stay out of the hospital altogether,” coauthor Robert L. Gottlieb, MD, PhD, from Baylor University Medical Center, Houston, said in the press release.
Remdesivir is the only drug approved by the U.S. Food and Drug Administration for hospitalized COVID-19 patients at least 12 years old. Its treatment of nonhospitalized patients with 3 days of dosing is investigational, and the safety and efficacy for this use and dosing duration have not been established or approved by any regulatory agency, the Gilead press release notes.
The patients in this study were considered high-risk for disease progression based on comorbidities – commonly obesity, hypertension, and diabetes – and age, but had not recently been hospitalized due to COVID-19.
A third of the participants were at least 60 years old. Participants in the study must have received a positive diagnosis within 4 days of starting treatment and experienced symptoms for 7 days or less.
Use of remdesivir controversial
Results from the Adaptive COVID-19 Treatment Trial (ACTT-1) showed remdesivir was superior to placebo in shortening time to recovery in adults hospitalized with COVID-19 with evidence of lower respiratory tract infection.
However, a large trial of more than 11,000 people in 30 countries, sponsored by the World Health Organization, did not show any benefit for the drug in reducing COVID deaths.
The WHO has conditionally recommended against using remdesivir in hospitalized patients, regardless of disease severity, “as there is currently no evidence that remdesivir improves survival and other outcomes in these patients.”
The drug also is given intravenously, and this study tested three infusions over 3 days, a difficult treatment for nonhospitalized patients.
The study results were released ahead of IDWeek, where the late-breaking abstract will be presented at the virtual conference in full at the end of next week.
A version of this article first appeared on Medscape.com.
New COVID-19 strain has reached the U.S.
Deadline, citing a Centers for Disease Control and Prevention report, said 26 residents and 20 workers tested positive for COVID-19 at a skilled care nursing home. The facility has 83 residents and 116 employees.
On March 1, 28 specimens that had been subjected to whole genome sequencing were found to have “mutations aligning with the R.1 lineage,” Deadline said.
About 90% of the facility’s residents and 52% of the staff had received two COVID vaccine doses, the CDC said. Because of the high vaccination rate, the finding raises concerns about “reduced protective immunity” in relation to the R.1 variant, the CDC said.
However, the nursing home case appears to show that the vaccine keeps most people from getting extremely sick, the CDC said. The vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective for employees.
“Compared with unvaccinated persons, vaccinated persons had reduced risk for SARS-CoV-2 infection and symptomatic COVID-19,” the CDC said. The vaccination of nursing home residents and health care workers “is essential to reduce the risk for symptomatic COVID-19, as is continued focus on infection prevention and control practices,” the CDC said.
Since being reported in Kentucky, R.1 has been detected more than 10,000 times in the United States, Forbes reported, basing that number on entries in the GISAID SARS-CoV-2 database.
Overall, more than 42 million cases of COVID have been reported since the start of the pandemic.
Deadline reported that the R.1 strain was first detected in Japan in January among three members of one family. The family members had no history of traveling abroad, Deadline said, citing an National Institutes of Health report.
The CDC has not classified R.1 as a variant of concern yet but noted it has “several mutations of importance” and “demonstrates evidence of increasing virus transmissibility.”
A version of this article first appeared on WebMD.com.
Deadline, citing a Centers for Disease Control and Prevention report, said 26 residents and 20 workers tested positive for COVID-19 at a skilled care nursing home. The facility has 83 residents and 116 employees.
On March 1, 28 specimens that had been subjected to whole genome sequencing were found to have “mutations aligning with the R.1 lineage,” Deadline said.
About 90% of the facility’s residents and 52% of the staff had received two COVID vaccine doses, the CDC said. Because of the high vaccination rate, the finding raises concerns about “reduced protective immunity” in relation to the R.1 variant, the CDC said.
However, the nursing home case appears to show that the vaccine keeps most people from getting extremely sick, the CDC said. The vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective for employees.
“Compared with unvaccinated persons, vaccinated persons had reduced risk for SARS-CoV-2 infection and symptomatic COVID-19,” the CDC said. The vaccination of nursing home residents and health care workers “is essential to reduce the risk for symptomatic COVID-19, as is continued focus on infection prevention and control practices,” the CDC said.
Since being reported in Kentucky, R.1 has been detected more than 10,000 times in the United States, Forbes reported, basing that number on entries in the GISAID SARS-CoV-2 database.
Overall, more than 42 million cases of COVID have been reported since the start of the pandemic.
Deadline reported that the R.1 strain was first detected in Japan in January among three members of one family. The family members had no history of traveling abroad, Deadline said, citing an National Institutes of Health report.
The CDC has not classified R.1 as a variant of concern yet but noted it has “several mutations of importance” and “demonstrates evidence of increasing virus transmissibility.”
A version of this article first appeared on WebMD.com.
Deadline, citing a Centers for Disease Control and Prevention report, said 26 residents and 20 workers tested positive for COVID-19 at a skilled care nursing home. The facility has 83 residents and 116 employees.
On March 1, 28 specimens that had been subjected to whole genome sequencing were found to have “mutations aligning with the R.1 lineage,” Deadline said.
About 90% of the facility’s residents and 52% of the staff had received two COVID vaccine doses, the CDC said. Because of the high vaccination rate, the finding raises concerns about “reduced protective immunity” in relation to the R.1 variant, the CDC said.
However, the nursing home case appears to show that the vaccine keeps most people from getting extremely sick, the CDC said. The vaccine was 86.5% protective against symptomatic illness among residents and 87.1% protective for employees.
“Compared with unvaccinated persons, vaccinated persons had reduced risk for SARS-CoV-2 infection and symptomatic COVID-19,” the CDC said. The vaccination of nursing home residents and health care workers “is essential to reduce the risk for symptomatic COVID-19, as is continued focus on infection prevention and control practices,” the CDC said.
Since being reported in Kentucky, R.1 has been detected more than 10,000 times in the United States, Forbes reported, basing that number on entries in the GISAID SARS-CoV-2 database.
Overall, more than 42 million cases of COVID have been reported since the start of the pandemic.
Deadline reported that the R.1 strain was first detected in Japan in January among three members of one family. The family members had no history of traveling abroad, Deadline said, citing an National Institutes of Health report.
The CDC has not classified R.1 as a variant of concern yet but noted it has “several mutations of importance” and “demonstrates evidence of increasing virus transmissibility.”
A version of this article first appeared on WebMD.com.
Mean leadership
The differences between the mean and median of leadership data
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.
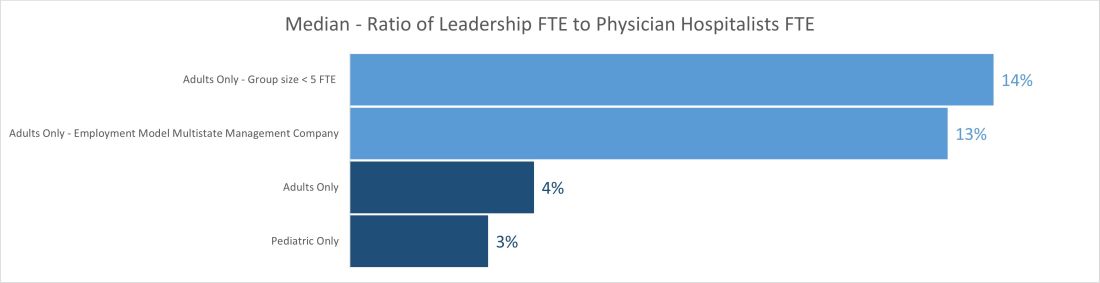
The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.
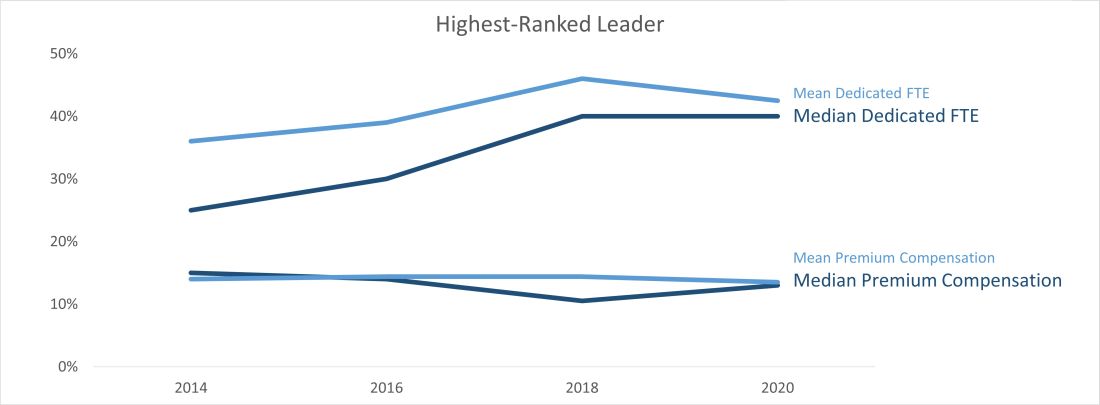
At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
The differences between the mean and median of leadership data
The differences between the mean and median of leadership data
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.

The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.

At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
Let me apologize for misleading all of you; this is not an article about malignant physician leaders; instead, it goes over the numbers and trends uncovered by the 2020 State of Hospital Medicine report (SoHM).1 The hospital medicine leader ends up doing many tasks like planning, growth, collaboration, finance, recruiting, scheduling, onboarding, coaching, and most near and dear to our hearts, putting out the fires and conflict resolution.
Ratio of leadership FTE to physician hospitalists FTE
If my pun has already put you off, you can avoid reading the rest of the piece and go to the 2020 SoHM to look at pages 52 (Table 3.7c), 121 (Table 4.7c), and 166 (Table 5.7c). It has a newly added table (3.7c), and it is phenomenal; it is the ratio of leadership FTE to physician hospitalists FTE. As an avid user of SoHM, I always ended up doing a makeshift calculation to “guesstimate” this number. Now that we have it calculated for us and the ultimate revelation lies in its narrow range across all groups. We might differ in the region, employment type, academics, teaching, or size, but this range is relatively narrow.

The median ratio of leadership FTE to total FTE lies between 2% and 5% in pediatric groups and between 3% and 6% for most adult groups. The only two outliers are on the adult side, with less than 5 FTE and multistate management companies. The higher median for the less than 5 FTE group size is understandable because of the small number of hospitalist FTEs that the leader’s time must be spread over. Even a small amount of dedicated leadership time will result in a high ratio of leader time to hospitalist clinical time if the group is very small. The multistate management company is probably a result of multiple layers of physician leadership (for example, regional medical directors) and travel-related time adjustments. Still, it raises the question of why the local leadership is not developed to decrease the leadership cost and better access.
Another helpful pattern is the decrease in standard deviation with the increase in group size. The hospital medicine leaders and CEOs of the hospital need to watch this number closely; any extremes on high or low side would be indicators for a deep dive in leadership structure and health.
Total number and total dedicated FTE for all physician leaders
Once we start seeing the differences between the mean and median of leadership data, we can see the median is relatively static while the mean has increased year after year and took a big jump in the 2020 SoHM. The chart below shows trends for the number of individuals in leadership positions (“Total No” and total FTEs allocated to leadership (“Total FTE”) over the last several surveys. The data is heavily skewed toward the right (positive); so, it makes sense to use the median in this case rather than mean. A few factors could explain the right skew of data.
- Large groups of 30 or more hospitalists are increasing, and so is their leadership need.
- There is more recognition of the need for dedicated leadership individuals and FTE.
- The leadership is getting less concentrated among just one or a few leaders.
- Outliers on the high side.
- Lower bounds of 0 or 0.1 FTE.
Highest-ranked leader dedicated FTE and premium compensation
Another pleasing trend is an increase in dedicated FTE for the highest-paid leader. Like any skill-set development, leadership requires the investment of deliberate practice, financial acumen, negotiation skills, and increased vulnerability. Time helps way more in developing these skill sets than money. SoHM trends show increase in dedicated FTE for the highest physician leader over the years and static premium compensation.

At last, we can say median leadership is always better than “mean” leadership in skewed data. Pun apart, every group needs leadership, and SoHM offers a nice window to the trends in leadership amongst many practice groups. It is a valuable resource for every group.
Dr. Chadha is chief of the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, practice analysis, and operation of the group. He is finishing his first tenure in the Practice Analysis Committee. He is often found spending a lot more than required time with spreadsheets and graphs.
Reference
1. 2020 State of Hospital Medicine. www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/







