User login
-
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
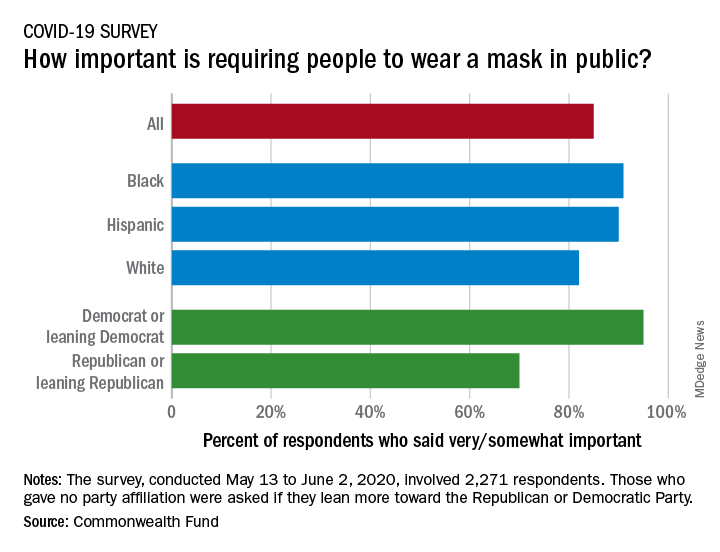
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.

Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.

Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.
Three stages to COVID-19 brain damage, new review suggests
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Daily Recap: Docs are good at saving money; SARS-CoV-2 vaccine trials advance
Here are the stories our MDedge editors across specialties think you need to know about today:
Many physicians live within their means and save
Although about two of five physicians report a net worth of between $1 million and $5 million, about half report that they are living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.
Net worth figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%). Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000. Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
Asked about saving habits, 43% of physicians reported they live below their means. Just 7% said they live above their means. How do they save money? Survey respondents reported putting bonus money into an investment account, putting extra money toward paying down the mortgage, and bringing lunch to work everyday.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic. Read more.
Phase 3 COVID-19 vaccine trials launching in July
There are now 120 Investigational New Drug applications to the Food and Drug Administration for a SARS-CoV-2 vaccine, and researchers at more than 70 companies across the globe are interested in making a vaccine, according to Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.” Read more.
FDA approves in-home breast cancer treatment
The Food and Drug Administration has approved a combination of subcutaneous breast cancer treatments that could be administered at home, following completion of chemotherapy.
The agency gave the green light to pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche), administered subcutaneously rather than intravenously, for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete. Read more.
Could a visual tool aid migraine management?
A new visual tool aims to streamline patient-clinician communication about risk factors for progression from episodic to chronic migraines.
The tool is still just a prototype, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors from depression to insomnia.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” lead researcher Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, said in an interview.
Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Many physicians live within their means and save
Although about two of five physicians report a net worth of between $1 million and $5 million, about half report that they are living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.
Net worth figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%). Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000. Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
Asked about saving habits, 43% of physicians reported they live below their means. Just 7% said they live above their means. How do they save money? Survey respondents reported putting bonus money into an investment account, putting extra money toward paying down the mortgage, and bringing lunch to work everyday.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic. Read more.
Phase 3 COVID-19 vaccine trials launching in July
There are now 120 Investigational New Drug applications to the Food and Drug Administration for a SARS-CoV-2 vaccine, and researchers at more than 70 companies across the globe are interested in making a vaccine, according to Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.” Read more.
FDA approves in-home breast cancer treatment
The Food and Drug Administration has approved a combination of subcutaneous breast cancer treatments that could be administered at home, following completion of chemotherapy.
The agency gave the green light to pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche), administered subcutaneously rather than intravenously, for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete. Read more.
Could a visual tool aid migraine management?
A new visual tool aims to streamline patient-clinician communication about risk factors for progression from episodic to chronic migraines.
The tool is still just a prototype, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors from depression to insomnia.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” lead researcher Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, said in an interview.
Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Many physicians live within their means and save
Although about two of five physicians report a net worth of between $1 million and $5 million, about half report that they are living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.
Net worth figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%). Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000. Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
Asked about saving habits, 43% of physicians reported they live below their means. Just 7% said they live above their means. How do they save money? Survey respondents reported putting bonus money into an investment account, putting extra money toward paying down the mortgage, and bringing lunch to work everyday.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic. Read more.
Phase 3 COVID-19 vaccine trials launching in July
There are now 120 Investigational New Drug applications to the Food and Drug Administration for a SARS-CoV-2 vaccine, and researchers at more than 70 companies across the globe are interested in making a vaccine, according to Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.” Read more.
FDA approves in-home breast cancer treatment
The Food and Drug Administration has approved a combination of subcutaneous breast cancer treatments that could be administered at home, following completion of chemotherapy.
The agency gave the green light to pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche), administered subcutaneously rather than intravenously, for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete. Read more.
Could a visual tool aid migraine management?
A new visual tool aims to streamline patient-clinician communication about risk factors for progression from episodic to chronic migraines.
The tool is still just a prototype, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors from depression to insomnia.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” lead researcher Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, said in an interview.
Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Phase 3 COVID-19 vaccine trials launching in July, expert says
The race to develop a SARS-CoV-2 vaccine is unlike any other global research and development effort in modern medicine.
According to Paul A. Offit, MD, there are now 120 Investigational New Drug applications to the Food and Drug Administration for these vaccines, and researchers at more than 70 companies across the globe are interested in making a vaccine. The Biomedical Advanced Research and Development Authority (BARDA) has awarded $2.5 billion to five different pharmaceutical companies to make a vaccine.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.”
Some research groups are interested in developing a whole, killed virus like those used in the inactivated polio vaccine, and vaccines for hepatitis A virus and rabies, said Dr. Offit, who is a member of Accelerating COVID-19 Technical Innovations And Vaccines, a public-private partnership formed by the National Institutes of Health. Other groups are interested in making a live-attenuated vaccine like those for measles, mumps, and rubella. “Some are interested in using a vectored vaccine, where you take a virus that is relatively weak and doesn’t cause disease in people, like vesicular stomatitis virus, and then clone into that the gene that codes for this coronavirus spike protein, which is the way that we made the Ebola virus vaccine,” Dr. Offit said. “Those approaches have all been used before, with success.”
Novel approaches are also being employed to make this vaccine, including using a replication-defective adenovirus. “That means that the virus can’t reproduce itself, but it can make proteins,” he explained. “There are some proteins that are made, but most aren’t. Therefore, the virus can’t reproduce itself. We’ll see whether or not that [approach] works, but it’s never been used before.”
Another approach is to inject messenger RNA that codes for the coronavirus spike protein, where that genetic material is translated into the spike protein. The other platform being evaluated is a DNA vaccine, in which “you give DNA which is coded for that spike protein, which is transcribed to messenger RNA and then is translated to other proteins.”
Typical vaccine development involves animal models to prove the concept, dose-ranging studies in humans, and progressively larger safety and immunogenicity studies in hundreds of thousands of people. Next come phase 3 studies, “where the proof is in the pudding,” he said. “These are large, prospective placebo-controlled trials to prove that the vaccine is safe. This is the only way whether you can prove or not a vaccine is effective.”
“Some companies may branch out on their own and do smaller studies than that,” he said. “We’ll see how this plays out. Keep your eyes open for that, because you really want to make sure you have a fairly large phase 3 trial. That’s the best way to show whether something works and whether it’s safe.”
The tried and true vaccines that emerge from the effort will not be FDA-licensed products. Rather, they will be approved products under the Emergency Use Authorization program. “Ever since the 1950s, every vaccine that has been used in the U.S. has been under the auspices of FDA licensure,” said Dr. Offit, who is also professor of pediatrics and the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia. “That’s not going to be true here. The FDA is involved every step of the way but here they have a somewhat lighter touch.”
A few candidate vaccines are being mass-produced at risk, “meaning they’re being produced not knowing whether these vaccines are safe and effective yet or not,” he said. “But when they’re shown in a phase 3 trial to be safe and effective, you will have already produced it, and then it’s much easier to roll it out to the general public the minute you’ve shown that it works. This is what we did for the polio vaccine back in the 1950s. We mass-produced that vaccine at risk.”
Dr. Offit emphasized the importance of managing expectations once a COVID-19 vaccine gets approved for use. “Regarding safety, these vaccines will be tested in tens of thousands of people, not tens of millions of people, so although you can disprove a relatively uncommon side effect preapproval, you’re not going to disprove a rare side effect preapproval. You’re only going to know that post approval. I think we need to make people aware of that and to let them know that through groups like the Vaccine Safety Datalink, we’re going to be monitoring these vaccines once they’re approved.”
Regarding efficacy, he continued, “we’re not going know about the rates of immunity initially; we’re only going to know about that after the vaccine [has been administered]. My guess is the protection is going to be short lived and incomplete. By short lived, I mean that protection would last for years but not decades. By incomplete, I mean that protection will be against moderate to severe disease, which is fine. You don’t need protection against all of the disease; it’s hard to do that with respiratory viruses. That means you can keep people out of the hospital, and you can keep them from dying. That’s the main goal.”
Dr. Offit closed his remarks by noting that much is at stake in this effort to develop a vaccine so quickly and that it “could go one of two ways. We could find that the vaccine is a lifesaver, and [that] we can finally end this awful pandemic. Or, if we cut corners and don’t prove that the vaccines are safe and effective as we should before they’re released, we could shake what is a fragile vaccine confidence in this country. Hopefully, it doesn’t play out that way.”
The race to develop a SARS-CoV-2 vaccine is unlike any other global research and development effort in modern medicine.
According to Paul A. Offit, MD, there are now 120 Investigational New Drug applications to the Food and Drug Administration for these vaccines, and researchers at more than 70 companies across the globe are interested in making a vaccine. The Biomedical Advanced Research and Development Authority (BARDA) has awarded $2.5 billion to five different pharmaceutical companies to make a vaccine.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.”
Some research groups are interested in developing a whole, killed virus like those used in the inactivated polio vaccine, and vaccines for hepatitis A virus and rabies, said Dr. Offit, who is a member of Accelerating COVID-19 Technical Innovations And Vaccines, a public-private partnership formed by the National Institutes of Health. Other groups are interested in making a live-attenuated vaccine like those for measles, mumps, and rubella. “Some are interested in using a vectored vaccine, where you take a virus that is relatively weak and doesn’t cause disease in people, like vesicular stomatitis virus, and then clone into that the gene that codes for this coronavirus spike protein, which is the way that we made the Ebola virus vaccine,” Dr. Offit said. “Those approaches have all been used before, with success.”
Novel approaches are also being employed to make this vaccine, including using a replication-defective adenovirus. “That means that the virus can’t reproduce itself, but it can make proteins,” he explained. “There are some proteins that are made, but most aren’t. Therefore, the virus can’t reproduce itself. We’ll see whether or not that [approach] works, but it’s never been used before.”
Another approach is to inject messenger RNA that codes for the coronavirus spike protein, where that genetic material is translated into the spike protein. The other platform being evaluated is a DNA vaccine, in which “you give DNA which is coded for that spike protein, which is transcribed to messenger RNA and then is translated to other proteins.”
Typical vaccine development involves animal models to prove the concept, dose-ranging studies in humans, and progressively larger safety and immunogenicity studies in hundreds of thousands of people. Next come phase 3 studies, “where the proof is in the pudding,” he said. “These are large, prospective placebo-controlled trials to prove that the vaccine is safe. This is the only way whether you can prove or not a vaccine is effective.”
“Some companies may branch out on their own and do smaller studies than that,” he said. “We’ll see how this plays out. Keep your eyes open for that, because you really want to make sure you have a fairly large phase 3 trial. That’s the best way to show whether something works and whether it’s safe.”
The tried and true vaccines that emerge from the effort will not be FDA-licensed products. Rather, they will be approved products under the Emergency Use Authorization program. “Ever since the 1950s, every vaccine that has been used in the U.S. has been under the auspices of FDA licensure,” said Dr. Offit, who is also professor of pediatrics and the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia. “That’s not going to be true here. The FDA is involved every step of the way but here they have a somewhat lighter touch.”
A few candidate vaccines are being mass-produced at risk, “meaning they’re being produced not knowing whether these vaccines are safe and effective yet or not,” he said. “But when they’re shown in a phase 3 trial to be safe and effective, you will have already produced it, and then it’s much easier to roll it out to the general public the minute you’ve shown that it works. This is what we did for the polio vaccine back in the 1950s. We mass-produced that vaccine at risk.”
Dr. Offit emphasized the importance of managing expectations once a COVID-19 vaccine gets approved for use. “Regarding safety, these vaccines will be tested in tens of thousands of people, not tens of millions of people, so although you can disprove a relatively uncommon side effect preapproval, you’re not going to disprove a rare side effect preapproval. You’re only going to know that post approval. I think we need to make people aware of that and to let them know that through groups like the Vaccine Safety Datalink, we’re going to be monitoring these vaccines once they’re approved.”
Regarding efficacy, he continued, “we’re not going know about the rates of immunity initially; we’re only going to know about that after the vaccine [has been administered]. My guess is the protection is going to be short lived and incomplete. By short lived, I mean that protection would last for years but not decades. By incomplete, I mean that protection will be against moderate to severe disease, which is fine. You don’t need protection against all of the disease; it’s hard to do that with respiratory viruses. That means you can keep people out of the hospital, and you can keep them from dying. That’s the main goal.”
Dr. Offit closed his remarks by noting that much is at stake in this effort to develop a vaccine so quickly and that it “could go one of two ways. We could find that the vaccine is a lifesaver, and [that] we can finally end this awful pandemic. Or, if we cut corners and don’t prove that the vaccines are safe and effective as we should before they’re released, we could shake what is a fragile vaccine confidence in this country. Hopefully, it doesn’t play out that way.”
The race to develop a SARS-CoV-2 vaccine is unlike any other global research and development effort in modern medicine.
According to Paul A. Offit, MD, there are now 120 Investigational New Drug applications to the Food and Drug Administration for these vaccines, and researchers at more than 70 companies across the globe are interested in making a vaccine. The Biomedical Advanced Research and Development Authority (BARDA) has awarded $2.5 billion to five different pharmaceutical companies to make a vaccine.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.”
Some research groups are interested in developing a whole, killed virus like those used in the inactivated polio vaccine, and vaccines for hepatitis A virus and rabies, said Dr. Offit, who is a member of Accelerating COVID-19 Technical Innovations And Vaccines, a public-private partnership formed by the National Institutes of Health. Other groups are interested in making a live-attenuated vaccine like those for measles, mumps, and rubella. “Some are interested in using a vectored vaccine, where you take a virus that is relatively weak and doesn’t cause disease in people, like vesicular stomatitis virus, and then clone into that the gene that codes for this coronavirus spike protein, which is the way that we made the Ebola virus vaccine,” Dr. Offit said. “Those approaches have all been used before, with success.”
Novel approaches are also being employed to make this vaccine, including using a replication-defective adenovirus. “That means that the virus can’t reproduce itself, but it can make proteins,” he explained. “There are some proteins that are made, but most aren’t. Therefore, the virus can’t reproduce itself. We’ll see whether or not that [approach] works, but it’s never been used before.”
Another approach is to inject messenger RNA that codes for the coronavirus spike protein, where that genetic material is translated into the spike protein. The other platform being evaluated is a DNA vaccine, in which “you give DNA which is coded for that spike protein, which is transcribed to messenger RNA and then is translated to other proteins.”
Typical vaccine development involves animal models to prove the concept, dose-ranging studies in humans, and progressively larger safety and immunogenicity studies in hundreds of thousands of people. Next come phase 3 studies, “where the proof is in the pudding,” he said. “These are large, prospective placebo-controlled trials to prove that the vaccine is safe. This is the only way whether you can prove or not a vaccine is effective.”
“Some companies may branch out on their own and do smaller studies than that,” he said. “We’ll see how this plays out. Keep your eyes open for that, because you really want to make sure you have a fairly large phase 3 trial. That’s the best way to show whether something works and whether it’s safe.”
The tried and true vaccines that emerge from the effort will not be FDA-licensed products. Rather, they will be approved products under the Emergency Use Authorization program. “Ever since the 1950s, every vaccine that has been used in the U.S. has been under the auspices of FDA licensure,” said Dr. Offit, who is also professor of pediatrics and the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia. “That’s not going to be true here. The FDA is involved every step of the way but here they have a somewhat lighter touch.”
A few candidate vaccines are being mass-produced at risk, “meaning they’re being produced not knowing whether these vaccines are safe and effective yet or not,” he said. “But when they’re shown in a phase 3 trial to be safe and effective, you will have already produced it, and then it’s much easier to roll it out to the general public the minute you’ve shown that it works. This is what we did for the polio vaccine back in the 1950s. We mass-produced that vaccine at risk.”
Dr. Offit emphasized the importance of managing expectations once a COVID-19 vaccine gets approved for use. “Regarding safety, these vaccines will be tested in tens of thousands of people, not tens of millions of people, so although you can disprove a relatively uncommon side effect preapproval, you’re not going to disprove a rare side effect preapproval. You’re only going to know that post approval. I think we need to make people aware of that and to let them know that through groups like the Vaccine Safety Datalink, we’re going to be monitoring these vaccines once they’re approved.”
Regarding efficacy, he continued, “we’re not going know about the rates of immunity initially; we’re only going to know about that after the vaccine [has been administered]. My guess is the protection is going to be short lived and incomplete. By short lived, I mean that protection would last for years but not decades. By incomplete, I mean that protection will be against moderate to severe disease, which is fine. You don’t need protection against all of the disease; it’s hard to do that with respiratory viruses. That means you can keep people out of the hospital, and you can keep them from dying. That’s the main goal.”
Dr. Offit closed his remarks by noting that much is at stake in this effort to develop a vaccine so quickly and that it “could go one of two ways. We could find that the vaccine is a lifesaver, and [that] we can finally end this awful pandemic. Or, if we cut corners and don’t prove that the vaccines are safe and effective as we should before they’re released, we could shake what is a fragile vaccine confidence in this country. Hopefully, it doesn’t play out that way.”
FROM PEDIATRIC DERMATOLOGY 2020
Many physicians live within their means and save, survey shows
Although about two of five physicians report a net worth of between $1 million and $5 million, half are under the million dollars and about half believe in living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.

Along with that somewhat prudent lifestyle comes savings, with
Those habits may help some navigate the financial upheaval in medicine brought about by COVID-19.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic.
The authors of the report note that by some estimates, primary care offices have seen a 55% drop in revenue because of the pandemic, and specialists have been hard hit with the suspension of most elective procedures.
Primary care offices are seeing fewer patients and are limiting hours, and some offices have been forced to close. Others have stemmed the losses by introducing telemedicine options.
Before COVID-19, average incomes had continued to rise – this year to $243,000 (a 2.5% boost from last year’s $237,000) for primary care physicians and $346,000 for specialists (a 1.5% rise from last year’s $341,000).
About half of physicians (42%) reported a net worth of $1 million to $5 million, and 8% reported a net worth of more than $5 million. Fifty percent of physicians had a net worth of less than $1 million.
Those figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%).
Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000.
Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
43% live below their means
Asked about habits regarding saving, 43% of physicians reported they live below their means. Half said they live at their means, and 7% said they live above their means.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., recommends in the report trying to save 20% of annual gross salary.
More than a third of physicians who responded (39%) said they put more than $2,000/month into tax-deferred retirement or college savings, but Dr. Greenwald acknowledged that this may become more challenging.
“Many have seen the employer match in their retirement plans reduced or eliminated through the end of 2020, with what comes in 2021 as yet undefined,” he said.
A smaller percentage (26%) answered that they put more than $2,000 a month into a taxable retirement or college savings account each month.
Home size by specialty
Mortgages on a primary residence were the top reasons for debt (63%), followed by car loans (37%), personal education loans (26%), and credit card balances (25%).
Half of specialists and 61% of primary care physicians live in homes with up to 3,000 square feet. Only 7% of PCPs and 12% of specialists live in homes with 5000 square feet or more.
At 22%, plastic surgeons and orthopedists were the most likely groups to have houses with the largest square footage, according to the survey.
About one in four physicians in five specialties (urology, cardiology, plastic surgery, otolaryngology, and critical care) reported that they had mortgages of more than $500,000.
Standard financial advice, the report authors note, is that a mortgage should take up no more than 28% of monthly gross income.
Another large source of debt came from student loans. Close to 80% of graduating medical students have educational debt. The average balance for graduating students in 2018 was $196,520, the report authors state.
Those in physical medicine/rehabilitation and family medicine were most likely to still be paying off student debt (34% said they were). Conversely, half as many nephrologists and rheumatologists (15%) and gastroenterologists (14%) reported that they were paying off educational debt.
Only 11% of physicians said they were currently free of any debt.
Most physicians in the survey (72%) reported that they had not experienced a significant financial loss in the past year.
For those who did experience such a loss, the top reason given was related to a bad investment or the stock market (9%).
Cost-cutting strategies
Revenue reduction will likely lead to spending less this year as the pandemic challenges continue.
Survey respondents offered their most effective cost-cutting strategies.
A hospitalist said, “Half of every bonus goes into the investment account, no matter how much.”
“We add an extra amount to the principal of our monthly mortgage payment,” an internist said.
A pediatrician offered, “I bring my lunch to work every day and don’t eat in restaurants often.”
This article first appeared on Medscape.com.
Although about two of five physicians report a net worth of between $1 million and $5 million, half are under the million dollars and about half believe in living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.

Along with that somewhat prudent lifestyle comes savings, with
Those habits may help some navigate the financial upheaval in medicine brought about by COVID-19.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic.
The authors of the report note that by some estimates, primary care offices have seen a 55% drop in revenue because of the pandemic, and specialists have been hard hit with the suspension of most elective procedures.
Primary care offices are seeing fewer patients and are limiting hours, and some offices have been forced to close. Others have stemmed the losses by introducing telemedicine options.
Before COVID-19, average incomes had continued to rise – this year to $243,000 (a 2.5% boost from last year’s $237,000) for primary care physicians and $346,000 for specialists (a 1.5% rise from last year’s $341,000).
About half of physicians (42%) reported a net worth of $1 million to $5 million, and 8% reported a net worth of more than $5 million. Fifty percent of physicians had a net worth of less than $1 million.
Those figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%).
Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000.
Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
43% live below their means
Asked about habits regarding saving, 43% of physicians reported they live below their means. Half said they live at their means, and 7% said they live above their means.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., recommends in the report trying to save 20% of annual gross salary.
More than a third of physicians who responded (39%) said they put more than $2,000/month into tax-deferred retirement or college savings, but Dr. Greenwald acknowledged that this may become more challenging.
“Many have seen the employer match in their retirement plans reduced or eliminated through the end of 2020, with what comes in 2021 as yet undefined,” he said.
A smaller percentage (26%) answered that they put more than $2,000 a month into a taxable retirement or college savings account each month.
Home size by specialty
Mortgages on a primary residence were the top reasons for debt (63%), followed by car loans (37%), personal education loans (26%), and credit card balances (25%).
Half of specialists and 61% of primary care physicians live in homes with up to 3,000 square feet. Only 7% of PCPs and 12% of specialists live in homes with 5000 square feet or more.
At 22%, plastic surgeons and orthopedists were the most likely groups to have houses with the largest square footage, according to the survey.
About one in four physicians in five specialties (urology, cardiology, plastic surgery, otolaryngology, and critical care) reported that they had mortgages of more than $500,000.
Standard financial advice, the report authors note, is that a mortgage should take up no more than 28% of monthly gross income.
Another large source of debt came from student loans. Close to 80% of graduating medical students have educational debt. The average balance for graduating students in 2018 was $196,520, the report authors state.
Those in physical medicine/rehabilitation and family medicine were most likely to still be paying off student debt (34% said they were). Conversely, half as many nephrologists and rheumatologists (15%) and gastroenterologists (14%) reported that they were paying off educational debt.
Only 11% of physicians said they were currently free of any debt.
Most physicians in the survey (72%) reported that they had not experienced a significant financial loss in the past year.
For those who did experience such a loss, the top reason given was related to a bad investment or the stock market (9%).
Cost-cutting strategies
Revenue reduction will likely lead to spending less this year as the pandemic challenges continue.
Survey respondents offered their most effective cost-cutting strategies.
A hospitalist said, “Half of every bonus goes into the investment account, no matter how much.”
“We add an extra amount to the principal of our monthly mortgage payment,” an internist said.
A pediatrician offered, “I bring my lunch to work every day and don’t eat in restaurants often.”
This article first appeared on Medscape.com.
Although about two of five physicians report a net worth of between $1 million and $5 million, half are under the million dollars and about half believe in living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.

Along with that somewhat prudent lifestyle comes savings, with
Those habits may help some navigate the financial upheaval in medicine brought about by COVID-19.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic.
The authors of the report note that by some estimates, primary care offices have seen a 55% drop in revenue because of the pandemic, and specialists have been hard hit with the suspension of most elective procedures.
Primary care offices are seeing fewer patients and are limiting hours, and some offices have been forced to close. Others have stemmed the losses by introducing telemedicine options.
Before COVID-19, average incomes had continued to rise – this year to $243,000 (a 2.5% boost from last year’s $237,000) for primary care physicians and $346,000 for specialists (a 1.5% rise from last year’s $341,000).
About half of physicians (42%) reported a net worth of $1 million to $5 million, and 8% reported a net worth of more than $5 million. Fifty percent of physicians had a net worth of less than $1 million.
Those figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%).
Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000.
Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
43% live below their means
Asked about habits regarding saving, 43% of physicians reported they live below their means. Half said they live at their means, and 7% said they live above their means.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., recommends in the report trying to save 20% of annual gross salary.
More than a third of physicians who responded (39%) said they put more than $2,000/month into tax-deferred retirement or college savings, but Dr. Greenwald acknowledged that this may become more challenging.
“Many have seen the employer match in their retirement plans reduced or eliminated through the end of 2020, with what comes in 2021 as yet undefined,” he said.
A smaller percentage (26%) answered that they put more than $2,000 a month into a taxable retirement or college savings account each month.
Home size by specialty
Mortgages on a primary residence were the top reasons for debt (63%), followed by car loans (37%), personal education loans (26%), and credit card balances (25%).
Half of specialists and 61% of primary care physicians live in homes with up to 3,000 square feet. Only 7% of PCPs and 12% of specialists live in homes with 5000 square feet or more.
At 22%, plastic surgeons and orthopedists were the most likely groups to have houses with the largest square footage, according to the survey.
About one in four physicians in five specialties (urology, cardiology, plastic surgery, otolaryngology, and critical care) reported that they had mortgages of more than $500,000.
Standard financial advice, the report authors note, is that a mortgage should take up no more than 28% of monthly gross income.
Another large source of debt came from student loans. Close to 80% of graduating medical students have educational debt. The average balance for graduating students in 2018 was $196,520, the report authors state.
Those in physical medicine/rehabilitation and family medicine were most likely to still be paying off student debt (34% said they were). Conversely, half as many nephrologists and rheumatologists (15%) and gastroenterologists (14%) reported that they were paying off educational debt.
Only 11% of physicians said they were currently free of any debt.
Most physicians in the survey (72%) reported that they had not experienced a significant financial loss in the past year.
For those who did experience such a loss, the top reason given was related to a bad investment or the stock market (9%).
Cost-cutting strategies
Revenue reduction will likely lead to spending less this year as the pandemic challenges continue.
Survey respondents offered their most effective cost-cutting strategies.
A hospitalist said, “Half of every bonus goes into the investment account, no matter how much.”
“We add an extra amount to the principal of our monthly mortgage payment,” an internist said.
A pediatrician offered, “I bring my lunch to work every day and don’t eat in restaurants often.”
This article first appeared on Medscape.com.
Skin patterns of COVID-19 vary widely
according to Christine Ko, MD.
“Things are very fluid,” Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn., said during the virtual annual meeting of the American Academy of Dermatology. “New studies are coming out daily. Due to the need for rapid dissemination, a lot of the studies are case reports, but there are some nice case series. Another caveat for the literature is that a lot of these cases were not necessarily confirmed with testing for SARS-CoV-2, but some were.”
Dr. Ko framed her remarks largely on a case collection survey of images and clinical data from 375 patients in Spain with suspected or confirmed COVID-19 that was published online April 29, 2020, in the British Journal of Dermatology (doi: 10.1111/bjd.19163). Cutaneous manifestations included early vesicular eruptions mainly on the trunk or limbs (9%), maculopapular (47%) to urticarial lesions (19%) mainly on the trunk, and acral areas of erythema sometimes with vesicles or erosion (perniosis-like) (19%) that seemed to be a later manifestation of COVID-19. Retiform purpura or necrosis (6%) was most concerning in terms of skin disease, with an associated with a mortality of 10%.
On histology, the early vesicular eruptions are typically marked by dyskeratotic keratinocytes, Dr. Ko said, while urticarial lesions are characterized by a mixed dermal infiltrate; maculopapular lesions were a broad category. “There are some case reports that show spongiotic dermatitis or parakeratosis with a lymphocytic infiltrate,” she said. “A caveat to keep in mind is that, although these patients may definitely have COVID-19 and be confirmed to have it by testing, hypersensitivity reactions may be due to the multiple medications they’re on.”
Patients can develop a spectrum of lesions that are suggestive of vascular damage or occlusion, Dr. Ko continued. Livedoid lesions may remain static and not eventuate into necrosis or purpura but will self-resolve. Purpuric lesions and acral gangrene have been described, and these lesions correspond to vascular occlusion on biopsy.
A later manifestation are the so-called “COVID toes” with a superficial and deep lymphocytic infiltrate, as published June 1, 2020, in JAAD Case Reports: (doi: 10.1016/j.jdcr.2020.04.011).
“There are patients in the literature that have slightly different pathology, with lymphocytic inflammation as well as occlusion of vessels,” Dr. Ko said. A paper published June 20, 2020, in the British Journal of Dermatology used immunohistochemical staining against the SARS-CoV-2 spike protein, and biopsies of “COVID toes” had positive staining of endothelial cells, supporting the notion that “COVID toes” are a direct manifestation of viral infection (doi: 10.1111/bjd.19327).
“There’s a lot that we still don’t know, and some patterns are going to be outliers,” Dr. Ko concluded. “[As for] determining which skin manifestations are directly from coronavirus infection within the skin, more study is needed and likely time will tell.” She reported having no financial disclosures relevant to her talk.
according to Christine Ko, MD.
“Things are very fluid,” Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn., said during the virtual annual meeting of the American Academy of Dermatology. “New studies are coming out daily. Due to the need for rapid dissemination, a lot of the studies are case reports, but there are some nice case series. Another caveat for the literature is that a lot of these cases were not necessarily confirmed with testing for SARS-CoV-2, but some were.”
Dr. Ko framed her remarks largely on a case collection survey of images and clinical data from 375 patients in Spain with suspected or confirmed COVID-19 that was published online April 29, 2020, in the British Journal of Dermatology (doi: 10.1111/bjd.19163). Cutaneous manifestations included early vesicular eruptions mainly on the trunk or limbs (9%), maculopapular (47%) to urticarial lesions (19%) mainly on the trunk, and acral areas of erythema sometimes with vesicles or erosion (perniosis-like) (19%) that seemed to be a later manifestation of COVID-19. Retiform purpura or necrosis (6%) was most concerning in terms of skin disease, with an associated with a mortality of 10%.
On histology, the early vesicular eruptions are typically marked by dyskeratotic keratinocytes, Dr. Ko said, while urticarial lesions are characterized by a mixed dermal infiltrate; maculopapular lesions were a broad category. “There are some case reports that show spongiotic dermatitis or parakeratosis with a lymphocytic infiltrate,” she said. “A caveat to keep in mind is that, although these patients may definitely have COVID-19 and be confirmed to have it by testing, hypersensitivity reactions may be due to the multiple medications they’re on.”
Patients can develop a spectrum of lesions that are suggestive of vascular damage or occlusion, Dr. Ko continued. Livedoid lesions may remain static and not eventuate into necrosis or purpura but will self-resolve. Purpuric lesions and acral gangrene have been described, and these lesions correspond to vascular occlusion on biopsy.
A later manifestation are the so-called “COVID toes” with a superficial and deep lymphocytic infiltrate, as published June 1, 2020, in JAAD Case Reports: (doi: 10.1016/j.jdcr.2020.04.011).
“There are patients in the literature that have slightly different pathology, with lymphocytic inflammation as well as occlusion of vessels,” Dr. Ko said. A paper published June 20, 2020, in the British Journal of Dermatology used immunohistochemical staining against the SARS-CoV-2 spike protein, and biopsies of “COVID toes” had positive staining of endothelial cells, supporting the notion that “COVID toes” are a direct manifestation of viral infection (doi: 10.1111/bjd.19327).
“There’s a lot that we still don’t know, and some patterns are going to be outliers,” Dr. Ko concluded. “[As for] determining which skin manifestations are directly from coronavirus infection within the skin, more study is needed and likely time will tell.” She reported having no financial disclosures relevant to her talk.
according to Christine Ko, MD.
“Things are very fluid,” Dr. Ko, professor of dermatology and pathology at Yale University, New Haven, Conn., said during the virtual annual meeting of the American Academy of Dermatology. “New studies are coming out daily. Due to the need for rapid dissemination, a lot of the studies are case reports, but there are some nice case series. Another caveat for the literature is that a lot of these cases were not necessarily confirmed with testing for SARS-CoV-2, but some were.”
Dr. Ko framed her remarks largely on a case collection survey of images and clinical data from 375 patients in Spain with suspected or confirmed COVID-19 that was published online April 29, 2020, in the British Journal of Dermatology (doi: 10.1111/bjd.19163). Cutaneous manifestations included early vesicular eruptions mainly on the trunk or limbs (9%), maculopapular (47%) to urticarial lesions (19%) mainly on the trunk, and acral areas of erythema sometimes with vesicles or erosion (perniosis-like) (19%) that seemed to be a later manifestation of COVID-19. Retiform purpura or necrosis (6%) was most concerning in terms of skin disease, with an associated with a mortality of 10%.
On histology, the early vesicular eruptions are typically marked by dyskeratotic keratinocytes, Dr. Ko said, while urticarial lesions are characterized by a mixed dermal infiltrate; maculopapular lesions were a broad category. “There are some case reports that show spongiotic dermatitis or parakeratosis with a lymphocytic infiltrate,” she said. “A caveat to keep in mind is that, although these patients may definitely have COVID-19 and be confirmed to have it by testing, hypersensitivity reactions may be due to the multiple medications they’re on.”
Patients can develop a spectrum of lesions that are suggestive of vascular damage or occlusion, Dr. Ko continued. Livedoid lesions may remain static and not eventuate into necrosis or purpura but will self-resolve. Purpuric lesions and acral gangrene have been described, and these lesions correspond to vascular occlusion on biopsy.
A later manifestation are the so-called “COVID toes” with a superficial and deep lymphocytic infiltrate, as published June 1, 2020, in JAAD Case Reports: (doi: 10.1016/j.jdcr.2020.04.011).
“There are patients in the literature that have slightly different pathology, with lymphocytic inflammation as well as occlusion of vessels,” Dr. Ko said. A paper published June 20, 2020, in the British Journal of Dermatology used immunohistochemical staining against the SARS-CoV-2 spike protein, and biopsies of “COVID toes” had positive staining of endothelial cells, supporting the notion that “COVID toes” are a direct manifestation of viral infection (doi: 10.1111/bjd.19327).
“There’s a lot that we still don’t know, and some patterns are going to be outliers,” Dr. Ko concluded. “[As for] determining which skin manifestations are directly from coronavirus infection within the skin, more study is needed and likely time will tell.” She reported having no financial disclosures relevant to her talk.
FROM AAD 20
Daily Recap: Transgender patients turn to DIY treatments; ACIP plans priority vaccine groups
Here are the stories our MDedge editors across specialties think you need to know about today:
Ignored by doctors, transgender patients turn to DIY treatments
Without access to quality medical care, trans people around the world are seeking hormones from friends or through illegal online markets, even when the cost exceeds what it would through insurance. Although rare, others are resorting to self-surgery by cutting off their own penis and testicles or breasts.
Even with a doctor’s oversight, the health risks of transgender hormone therapy remain unclear, but without formal medical care, the do-it-yourself transition may be downright dangerous. To minimize these risks, some experts suggest health care reforms such as making it easier for primary care physicians to assess trans patients and prescribe hormones or creating specialized clinics where doctors prescribe hormones on demand.
Treating gender dysphoria should be just like treating a patient for any other condition. “It wouldn't be acceptable for someone to come into a primary care provider’s office with diabetes” and for the doctor to say “‘I can't actually treat you. Please leave,’” Zil Goldstein, associate medical director for transgender and gender non-binary health at the Callen-Lorde Community Health Center in New York City. Primary care providers need to see transgender care, she adds, “as a regular part of their practice.” Read more.
ACIP plans priority groups in advance of COVID-19 vaccine
Early plans for prioritizing vaccination when a COVID-19 vaccine becomes available include placing critical health care workers in the first tier, according to Sarah Mbaeyi, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases.
A COVID-19 vaccine work group is developing strategies and identifying priority groups for vaccination to help inform discussions about the use of COVID-19 vaccines, Dr. Mbaeyi said at a virtual meeting of the CDC’s Advisory Committee on Immunization Practices.
Based on current information, the work group has proposed that vaccine priority be given to health care personnel, essential workers, adults aged 65 years and older, long-term care facility residents, and persons with high-risk medical conditions.
Among these groups “a subset of critical health care and other workers should receive initial doses,” Dr. Mbaeyi said. Read more.
‘Nietzsche was wrong’: Past stressors do not create psychological resilience.
The famous quote from the German philosopher Friedrich Nietzsche, “That which does not kill us makes us stronger,” may not be true after all – at least when it comes to mental health.
Results of a new study show that individuals who have a history of a stressful life events are more likely to develop PTSD and/or major depressive disorder (MDD) following a major natural disaster than their counterparts who do not have such a history.
The investigation of more than a thousand Chilean residents – all of whom experienced one of the most powerful earthquakes in the country’s history – showed that the odds of developing postdisaster PTSD or MDD increased according to the number of predisaster stressors participants had experienced.
“At the clinical level, these findings help the clinician know which patients are more likely to need more intensive services,” said Stephen L. Buka, PhD. “And the more trauma and hardship they’ve experienced, the more attention they need and the less likely they’re going to be able to cope and manage on their own.” Read more.
High-impact training can build bone in older women
Older adults, particularly postmenopausal women, are often advised to pursue low-impact, low-intensity exercise as a way to preserve joint health, but that approach might actually contribute to a decline in bone mineral density, researchers report.
Concerns about falls and fracture risk have led many clinicians to advise against higher-impact activities, like jumping, but that is exactly the type of activity that improves bone density and physical function, said Belinda Beck, PhD, professor at the Griffith University School of Allied Health Sciences in Southport, Australia. But new findings show that high-intensity resistance and impact training was a safe and effective way to improve bone mass.
“Once women hit 60, they’re somehow regarded as frail, but that becomes a self-fulfilling prophecy when we take this kinder, gentler approach to exercise,” said Vanessa Yingling, PhD. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Ignored by doctors, transgender patients turn to DIY treatments
Without access to quality medical care, trans people around the world are seeking hormones from friends or through illegal online markets, even when the cost exceeds what it would through insurance. Although rare, others are resorting to self-surgery by cutting off their own penis and testicles or breasts.
Even with a doctor’s oversight, the health risks of transgender hormone therapy remain unclear, but without formal medical care, the do-it-yourself transition may be downright dangerous. To minimize these risks, some experts suggest health care reforms such as making it easier for primary care physicians to assess trans patients and prescribe hormones or creating specialized clinics where doctors prescribe hormones on demand.
Treating gender dysphoria should be just like treating a patient for any other condition. “It wouldn't be acceptable for someone to come into a primary care provider’s office with diabetes” and for the doctor to say “‘I can't actually treat you. Please leave,’” Zil Goldstein, associate medical director for transgender and gender non-binary health at the Callen-Lorde Community Health Center in New York City. Primary care providers need to see transgender care, she adds, “as a regular part of their practice.” Read more.
ACIP plans priority groups in advance of COVID-19 vaccine
Early plans for prioritizing vaccination when a COVID-19 vaccine becomes available include placing critical health care workers in the first tier, according to Sarah Mbaeyi, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases.
A COVID-19 vaccine work group is developing strategies and identifying priority groups for vaccination to help inform discussions about the use of COVID-19 vaccines, Dr. Mbaeyi said at a virtual meeting of the CDC’s Advisory Committee on Immunization Practices.
Based on current information, the work group has proposed that vaccine priority be given to health care personnel, essential workers, adults aged 65 years and older, long-term care facility residents, and persons with high-risk medical conditions.
Among these groups “a subset of critical health care and other workers should receive initial doses,” Dr. Mbaeyi said. Read more.
‘Nietzsche was wrong’: Past stressors do not create psychological resilience.
The famous quote from the German philosopher Friedrich Nietzsche, “That which does not kill us makes us stronger,” may not be true after all – at least when it comes to mental health.
Results of a new study show that individuals who have a history of a stressful life events are more likely to develop PTSD and/or major depressive disorder (MDD) following a major natural disaster than their counterparts who do not have such a history.
The investigation of more than a thousand Chilean residents – all of whom experienced one of the most powerful earthquakes in the country’s history – showed that the odds of developing postdisaster PTSD or MDD increased according to the number of predisaster stressors participants had experienced.
“At the clinical level, these findings help the clinician know which patients are more likely to need more intensive services,” said Stephen L. Buka, PhD. “And the more trauma and hardship they’ve experienced, the more attention they need and the less likely they’re going to be able to cope and manage on their own.” Read more.
High-impact training can build bone in older women
Older adults, particularly postmenopausal women, are often advised to pursue low-impact, low-intensity exercise as a way to preserve joint health, but that approach might actually contribute to a decline in bone mineral density, researchers report.
Concerns about falls and fracture risk have led many clinicians to advise against higher-impact activities, like jumping, but that is exactly the type of activity that improves bone density and physical function, said Belinda Beck, PhD, professor at the Griffith University School of Allied Health Sciences in Southport, Australia. But new findings show that high-intensity resistance and impact training was a safe and effective way to improve bone mass.
“Once women hit 60, they’re somehow regarded as frail, but that becomes a self-fulfilling prophecy when we take this kinder, gentler approach to exercise,” said Vanessa Yingling, PhD. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Ignored by doctors, transgender patients turn to DIY treatments
Without access to quality medical care, trans people around the world are seeking hormones from friends or through illegal online markets, even when the cost exceeds what it would through insurance. Although rare, others are resorting to self-surgery by cutting off their own penis and testicles or breasts.
Even with a doctor’s oversight, the health risks of transgender hormone therapy remain unclear, but without formal medical care, the do-it-yourself transition may be downright dangerous. To minimize these risks, some experts suggest health care reforms such as making it easier for primary care physicians to assess trans patients and prescribe hormones or creating specialized clinics where doctors prescribe hormones on demand.
Treating gender dysphoria should be just like treating a patient for any other condition. “It wouldn't be acceptable for someone to come into a primary care provider’s office with diabetes” and for the doctor to say “‘I can't actually treat you. Please leave,’” Zil Goldstein, associate medical director for transgender and gender non-binary health at the Callen-Lorde Community Health Center in New York City. Primary care providers need to see transgender care, she adds, “as a regular part of their practice.” Read more.
ACIP plans priority groups in advance of COVID-19 vaccine
Early plans for prioritizing vaccination when a COVID-19 vaccine becomes available include placing critical health care workers in the first tier, according to Sarah Mbaeyi, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases.
A COVID-19 vaccine work group is developing strategies and identifying priority groups for vaccination to help inform discussions about the use of COVID-19 vaccines, Dr. Mbaeyi said at a virtual meeting of the CDC’s Advisory Committee on Immunization Practices.
Based on current information, the work group has proposed that vaccine priority be given to health care personnel, essential workers, adults aged 65 years and older, long-term care facility residents, and persons with high-risk medical conditions.
Among these groups “a subset of critical health care and other workers should receive initial doses,” Dr. Mbaeyi said. Read more.
‘Nietzsche was wrong’: Past stressors do not create psychological resilience.
The famous quote from the German philosopher Friedrich Nietzsche, “That which does not kill us makes us stronger,” may not be true after all – at least when it comes to mental health.
Results of a new study show that individuals who have a history of a stressful life events are more likely to develop PTSD and/or major depressive disorder (MDD) following a major natural disaster than their counterparts who do not have such a history.
The investigation of more than a thousand Chilean residents – all of whom experienced one of the most powerful earthquakes in the country’s history – showed that the odds of developing postdisaster PTSD or MDD increased according to the number of predisaster stressors participants had experienced.
“At the clinical level, these findings help the clinician know which patients are more likely to need more intensive services,” said Stephen L. Buka, PhD. “And the more trauma and hardship they’ve experienced, the more attention they need and the less likely they’re going to be able to cope and manage on their own.” Read more.
High-impact training can build bone in older women
Older adults, particularly postmenopausal women, are often advised to pursue low-impact, low-intensity exercise as a way to preserve joint health, but that approach might actually contribute to a decline in bone mineral density, researchers report.
Concerns about falls and fracture risk have led many clinicians to advise against higher-impact activities, like jumping, but that is exactly the type of activity that improves bone density and physical function, said Belinda Beck, PhD, professor at the Griffith University School of Allied Health Sciences in Southport, Australia. But new findings show that high-intensity resistance and impact training was a safe and effective way to improve bone mass.
“Once women hit 60, they’re somehow regarded as frail, but that becomes a self-fulfilling prophecy when we take this kinder, gentler approach to exercise,” said Vanessa Yingling, PhD. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
How racism contributes to the effects of SARS-CoV-2
It’s been about two months since I volunteered in a hospital in Brooklyn, working in an ICU taking care of patients with COVID-19.
Everyone seems to have forgotten the early days of the pandemic – the time when the ICUs were overrun, we were using FEMA ventilators, and endocrinologists and psychiatrists were acting as intensivists.
Even though things are opening up and people are taking summer vacations in a seemingly amnestic state, having witnessed multiple daily deaths remains a part of my daily consciousness. As I see the case numbers climbing juxtaposed against people being out and about without masks, my anxiety level is rising.
A virus doesn’t discriminate. It can fly through the air, landing on the next available surface. If that virus is SARS-CoV-2 and that surface is a human mucosal membrane, the virus makes itself at home. It orders furniture, buys a fancy mattress and a large high definition TV, hangs art on the walls, and settles in for the long haul. It’s not going anywhere anytime soon.
Even as an equal opportunity virus, what SARS-CoV-2 has done is to hold a mirror up to the healthcare system. It has shown us what was here all along. When people first started noticing that underrepresented minorities were more likely to contract the virus and get sick from it, I heard musings that this was likely because of their preexisting health conditions. For example, commentators on cable news were quick to point out that black people are more likely than other people to have hypertension or diabetes. So doesn’t that explain why they are more affected by this virus?
That certainly is part of the story, but it doesn’t entirely explain the discrepancies we’ve seen. For example, in New York 14% of the population is black, and 25% of those who had a COVID-related death were black patients. Similarly, 19% of the population is Hispanic or Latino, and they made up 26% of COVID-related deaths. On the other hand, 55% of the population in New York is white, and white people account for only 34% of COVID-related deaths.
Working in Brooklyn, I didn’t need to be a keen observer to notice that, out of our entire unit of about 20-25 patients, there was only one patient in a 2-week period who was neither black nor Hispanic.
As others have written, there are other factors at play. I’m not sure how many of those commentators back in March stopped to think about why black patients are more likely to have hypertension and diabetes, but the chronic stress of facing racism on a daily basis surely contributes. Beyond those medical problems, minorities are more likely to live in multigenerational housing, which means that it is harder for them to isolate from others. In addition, their living quarters tend to be further from health care centers and grocery stores, which makes it harder for them to access medical care and healthy food.
As if that weren’t enough to put their health at risk, people of color are also affected by environmental racism . Factories with toxic waste are more likely to be built in or near neighborhoods filled with people of color than in other communities. On top of that, black and Hispanic people are also more likely to be under- or uninsured, meaning they often delay seeking care in order to avoid astronomic healthcare costs.
Black and Hispanic people are also more likely than others to be working in the service industry or other essential services, which means they are less likely to be able to work from home. Consequently, they have to risk more exposures to other people and the virus than do those who have the privilege of working safely from home. They also are less likely to have available paid leave and, therefore, are more likely to work while sick.
With the deck completely stacked against them, underrepresented minorities also face systemic bias and racism when interacting with the health care system. Physicians mistakenly believe black patients experience less pain than other patients, according to some research. Black mothers have significantly worse health care outcomes than do their non-black counterparts, and the infant mortality rate for Black infants is much higher as well.
In my limited time in Brooklyn, taking care of almost exclusively black and Hispanic patients, I saw one physician assistant and one nurse who were black; one nurse practitioner was Hispanic. This mismatch is sadly common. Although 13% of the population of the United States is black, only 5% of physicians in the United States are black. Hispanic people, who make up 18% of the US population, are only 6% of physicians. This undoubtedly contributes to poorer outcomes for underrepresented minority patients who have a hard time finding physicians who look like them and understand them.
So while SARS-CoV-2 may not discriminate, the effects it has on patients depends on all of these other factors. If it flies through the air and lands on the mucosal tract of a person who works from home, has effective health insurance and a primary care physician, and lives in a community with no toxic exposures, that person may be more likely to kick it out before it has a chance to settle in. The reason we have such a huge disparity in outcomes related to COVID-19 by race is that a person meeting that description is less likely to be black or Hispanic. Race is not an independent risk factor; structural racism is.
When I drive by the mall that is now open or the restaurants that are now open with indoor dining, my heart rate quickens just a bit with anxiety. The pandemic fatigue people are experiencing is leading them to act in unsafe ways – gathering with more people, not wearing masks, not keeping a safe distance. I worry about everyone, sure, but I really worry about black and Hispanic people who are most vulnerable as a result of everyone else’s refusal to follow guidelines.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University. Find her on Twitter @arghavan_salles.
It’s been about two months since I volunteered in a hospital in Brooklyn, working in an ICU taking care of patients with COVID-19.
Everyone seems to have forgotten the early days of the pandemic – the time when the ICUs were overrun, we were using FEMA ventilators, and endocrinologists and psychiatrists were acting as intensivists.
Even though things are opening up and people are taking summer vacations in a seemingly amnestic state, having witnessed multiple daily deaths remains a part of my daily consciousness. As I see the case numbers climbing juxtaposed against people being out and about without masks, my anxiety level is rising.
A virus doesn’t discriminate. It can fly through the air, landing on the next available surface. If that virus is SARS-CoV-2 and that surface is a human mucosal membrane, the virus makes itself at home. It orders furniture, buys a fancy mattress and a large high definition TV, hangs art on the walls, and settles in for the long haul. It’s not going anywhere anytime soon.
Even as an equal opportunity virus, what SARS-CoV-2 has done is to hold a mirror up to the healthcare system. It has shown us what was here all along. When people first started noticing that underrepresented minorities were more likely to contract the virus and get sick from it, I heard musings that this was likely because of their preexisting health conditions. For example, commentators on cable news were quick to point out that black people are more likely than other people to have hypertension or diabetes. So doesn’t that explain why they are more affected by this virus?
That certainly is part of the story, but it doesn’t entirely explain the discrepancies we’ve seen. For example, in New York 14% of the population is black, and 25% of those who had a COVID-related death were black patients. Similarly, 19% of the population is Hispanic or Latino, and they made up 26% of COVID-related deaths. On the other hand, 55% of the population in New York is white, and white people account for only 34% of COVID-related deaths.
Working in Brooklyn, I didn’t need to be a keen observer to notice that, out of our entire unit of about 20-25 patients, there was only one patient in a 2-week period who was neither black nor Hispanic.
As others have written, there are other factors at play. I’m not sure how many of those commentators back in March stopped to think about why black patients are more likely to have hypertension and diabetes, but the chronic stress of facing racism on a daily basis surely contributes. Beyond those medical problems, minorities are more likely to live in multigenerational housing, which means that it is harder for them to isolate from others. In addition, their living quarters tend to be further from health care centers and grocery stores, which makes it harder for them to access medical care and healthy food.
As if that weren’t enough to put their health at risk, people of color are also affected by environmental racism . Factories with toxic waste are more likely to be built in or near neighborhoods filled with people of color than in other communities. On top of that, black and Hispanic people are also more likely to be under- or uninsured, meaning they often delay seeking care in order to avoid astronomic healthcare costs.
Black and Hispanic people are also more likely than others to be working in the service industry or other essential services, which means they are less likely to be able to work from home. Consequently, they have to risk more exposures to other people and the virus than do those who have the privilege of working safely from home. They also are less likely to have available paid leave and, therefore, are more likely to work while sick.
With the deck completely stacked against them, underrepresented minorities also face systemic bias and racism when interacting with the health care system. Physicians mistakenly believe black patients experience less pain than other patients, according to some research. Black mothers have significantly worse health care outcomes than do their non-black counterparts, and the infant mortality rate for Black infants is much higher as well.
In my limited time in Brooklyn, taking care of almost exclusively black and Hispanic patients, I saw one physician assistant and one nurse who were black; one nurse practitioner was Hispanic. This mismatch is sadly common. Although 13% of the population of the United States is black, only 5% of physicians in the United States are black. Hispanic people, who make up 18% of the US population, are only 6% of physicians. This undoubtedly contributes to poorer outcomes for underrepresented minority patients who have a hard time finding physicians who look like them and understand them.
So while SARS-CoV-2 may not discriminate, the effects it has on patients depends on all of these other factors. If it flies through the air and lands on the mucosal tract of a person who works from home, has effective health insurance and a primary care physician, and lives in a community with no toxic exposures, that person may be more likely to kick it out before it has a chance to settle in. The reason we have such a huge disparity in outcomes related to COVID-19 by race is that a person meeting that description is less likely to be black or Hispanic. Race is not an independent risk factor; structural racism is.
When I drive by the mall that is now open or the restaurants that are now open with indoor dining, my heart rate quickens just a bit with anxiety. The pandemic fatigue people are experiencing is leading them to act in unsafe ways – gathering with more people, not wearing masks, not keeping a safe distance. I worry about everyone, sure, but I really worry about black and Hispanic people who are most vulnerable as a result of everyone else’s refusal to follow guidelines.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University. Find her on Twitter @arghavan_salles.
It’s been about two months since I volunteered in a hospital in Brooklyn, working in an ICU taking care of patients with COVID-19.
Everyone seems to have forgotten the early days of the pandemic – the time when the ICUs were overrun, we were using FEMA ventilators, and endocrinologists and psychiatrists were acting as intensivists.
Even though things are opening up and people are taking summer vacations in a seemingly amnestic state, having witnessed multiple daily deaths remains a part of my daily consciousness. As I see the case numbers climbing juxtaposed against people being out and about without masks, my anxiety level is rising.
A virus doesn’t discriminate. It can fly through the air, landing on the next available surface. If that virus is SARS-CoV-2 and that surface is a human mucosal membrane, the virus makes itself at home. It orders furniture, buys a fancy mattress and a large high definition TV, hangs art on the walls, and settles in for the long haul. It’s not going anywhere anytime soon.
Even as an equal opportunity virus, what SARS-CoV-2 has done is to hold a mirror up to the healthcare system. It has shown us what was here all along. When people first started noticing that underrepresented minorities were more likely to contract the virus and get sick from it, I heard musings that this was likely because of their preexisting health conditions. For example, commentators on cable news were quick to point out that black people are more likely than other people to have hypertension or diabetes. So doesn’t that explain why they are more affected by this virus?
That certainly is part of the story, but it doesn’t entirely explain the discrepancies we’ve seen. For example, in New York 14% of the population is black, and 25% of those who had a COVID-related death were black patients. Similarly, 19% of the population is Hispanic or Latino, and they made up 26% of COVID-related deaths. On the other hand, 55% of the population in New York is white, and white people account for only 34% of COVID-related deaths.
Working in Brooklyn, I didn’t need to be a keen observer to notice that, out of our entire unit of about 20-25 patients, there was only one patient in a 2-week period who was neither black nor Hispanic.
As others have written, there are other factors at play. I’m not sure how many of those commentators back in March stopped to think about why black patients are more likely to have hypertension and diabetes, but the chronic stress of facing racism on a daily basis surely contributes. Beyond those medical problems, minorities are more likely to live in multigenerational housing, which means that it is harder for them to isolate from others. In addition, their living quarters tend to be further from health care centers and grocery stores, which makes it harder for them to access medical care and healthy food.
As if that weren’t enough to put their health at risk, people of color are also affected by environmental racism . Factories with toxic waste are more likely to be built in or near neighborhoods filled with people of color than in other communities. On top of that, black and Hispanic people are also more likely to be under- or uninsured, meaning they often delay seeking care in order to avoid astronomic healthcare costs.
Black and Hispanic people are also more likely than others to be working in the service industry or other essential services, which means they are less likely to be able to work from home. Consequently, they have to risk more exposures to other people and the virus than do those who have the privilege of working safely from home. They also are less likely to have available paid leave and, therefore, are more likely to work while sick.
With the deck completely stacked against them, underrepresented minorities also face systemic bias and racism when interacting with the health care system. Physicians mistakenly believe black patients experience less pain than other patients, according to some research. Black mothers have significantly worse health care outcomes than do their non-black counterparts, and the infant mortality rate for Black infants is much higher as well.
In my limited time in Brooklyn, taking care of almost exclusively black and Hispanic patients, I saw one physician assistant and one nurse who were black; one nurse practitioner was Hispanic. This mismatch is sadly common. Although 13% of the population of the United States is black, only 5% of physicians in the United States are black. Hispanic people, who make up 18% of the US population, are only 6% of physicians. This undoubtedly contributes to poorer outcomes for underrepresented minority patients who have a hard time finding physicians who look like them and understand them.
So while SARS-CoV-2 may not discriminate, the effects it has on patients depends on all of these other factors. If it flies through the air and lands on the mucosal tract of a person who works from home, has effective health insurance and a primary care physician, and lives in a community with no toxic exposures, that person may be more likely to kick it out before it has a chance to settle in. The reason we have such a huge disparity in outcomes related to COVID-19 by race is that a person meeting that description is less likely to be black or Hispanic. Race is not an independent risk factor; structural racism is.
When I drive by the mall that is now open or the restaurants that are now open with indoor dining, my heart rate quickens just a bit with anxiety. The pandemic fatigue people are experiencing is leading them to act in unsafe ways – gathering with more people, not wearing masks, not keeping a safe distance. I worry about everyone, sure, but I really worry about black and Hispanic people who are most vulnerable as a result of everyone else’s refusal to follow guidelines.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University. Find her on Twitter @arghavan_salles.
Endothelial injury may play a major role in COVID-19–associated coagulopathy
A striking clinical feature of illness from SARS-CoV-2 is a marked increase in thrombotic and microvascular complications, or COVID-19–associated coagulopathy (CAC).
A new study suggests endothelial cell injury plays a major role in the pathogenesis of CAC, and blood levels of soluble thrombomodulin correlate with mortality.
George Goshua, MD, of Yale University, New Haven, Conn., presented this study as a late-breaking abstract at the virtual annual congress of the European Hematology Association.
Dr. Goshua cited past research showing CAC to be highly prevalent among hospitalized patients. Venous thromboembolism was found in 17% to 69% of patients, despite thromboprophylaxis.1-4 Arterial thrombosis has been seen in 3.6% to 4.0% of patients,1-3 and autopsy findings have shown microvascular thrombosis in as many as 87% of patients.5-7
For their study, Dr. Goshua and colleagues assessed endothelial cell damage, platelet activation, and hemostatic and fibrinolytic cascade effects of CAC.
The investigators measured markers of endothelial cell injury and platelet activation, plasminogen activation inhibitor 1 (PAI-1), and coagulation factors in stable and critically ill patients hospitalized with COVID-19. In addition, the team sought to identify biomarkers of mortality in hospitalized patients.
Dr. Goshua and colleagues studied 68 adults hospitalized for suspected COVID-19 – 48 in the ICU and 20 outside the ICU. Patients in the ICU received mechanical ventilation, while the non-ICU patients required supplemental oxygen (≤3 L/min per nasal cannula).
There were more men than women (69% vs. 31%) in the ICU population but not in the non-ICU population (40% vs. 60%). There were no statistically significant differences in age or comorbid conditions between the ICU and non-ICU patients.
Results and interpretation
Consistent with augmentation of the coagulation cascade – and as expected – D-dimer and thrombin-antithrombin levels were high in both the ICU and non-ICU populations, but levels were significantly higher (P < .001) among the ICU patients.
Endogenous anticoagulants (antithrombin and proteins C and S) and fibrinolytic enzymes (alpha 2-antiplasmin) were preserved, verifying that CAC is distinct from disseminated intravascular coagulation. Classic fibrinolysis did not occur, as PAI-1 was high in ICU and non-ICU patients, and lysis-30 was normal in nearly all ICU patients (96%).
Von Willebrand factor antigen and activity levels and factor VIII levels were markedly elevated in non-ICU and ICU patients, but they were significantly higher (P < .001) in the ICU cohort. This supports the hypothesis that endothelial cell damage and platelet activation play major roles in CAC.
Similarly, soluble P-selectin, which is shed from endothelial cells and platelets, was dramatically elevated in ICU patients in comparison with controls and non-ICU patients (P < .001 for both comparisons).
Levels of soluble thrombomodulin, which is released from endothelial cells, were not significantly different in ICU patients and controls. However, given thrombomodulin’s significant role in the coagulation cascade, Dr. Goshua and colleagues plotted receiver operating curves to see if soluble thrombomodulin levels were predictive of mortality.
The results showed that soluble thrombomodulin correlated with the probability of survival, both overall and in ICU patients. Soluble thrombomodulin levels greater than 3.26 ng/mL were associated with significantly worse survival in all patients (P = .0087) and ICU patients (P = .0309).
Influence on therapy
Laboratory perturbations were detected in both ICU and non-ICU patients, and otherwise healthy outpatients have exhibited potentially life-threatening CAC, according to Dr. Goshua.
These findings suggest the prothrombotic state occurs early in the pathogenesis of SARS-CoV-2 infection, is driven by platelet activation and endotheliopathy, and becomes more pronounced with worsening severity of infection.
The results of this study prompted a change in how Yale–New Haven Hospital manages COVID-19 patients. Patients without a clinical contraindication now receive aspirin at 81 mg daily in addition to the anticoagulation regimen typically used for all hospitalized COVID-19 patients.
Investigations regarding other medications that can influence platelet-endothelial cell interactions and modulate endothelial cell damage in CAC – such as dipyridamole, defibrotide, and eculizumab – are planned.
Challenges and unanswered questions
Virchow’s triad was described by the eminent German physician, Rudolf Virchow, MD, in the 19th century. It refers to the three broad categories of factors that can predispose patients to thrombosis — circulatory stasis, hypercoagulability, and endothelial injury.
Although all of these elements could be operative in CAC, the current study suggests platelet activation and endothelial cell injury in CAC may be of primary importance.
Because of the limited ability to test critically ill patients and concerns regarding exposure of additional hospital personnel to COVID-19 patients, the current report lacked clarity about the relationship of the detected laboratory abnormalities to confirmed thrombotic events.
It is unknown whether endothelial cells in different organs are damaged uniformly. It is also unclear if the laboratory abnormalities identified in this analysis can be used to monitor response to therapy, to guide follow-up management of discharged patients with CAC, or to identify infected outpatients who should receive prophylactic anticoagulation.
The mechanism by which SARS-CoV-2 injures endothelial cells is not explained by these data. Neutrophil defensins and other prothrombotic peptides or markers of inflammation could play key roles in pathogenesis, assessment of disease severity, or monitoring for therapeutic efficacy.
Today, we have more sophisticated diagnostic tools than Dr. Virchow had. We also have the ability to record and rapidly disseminate information globally. Still, with regard to the COVID-19 pandemic, clinicians face many of the same challenges that confronted Dr. Virchow in his era.
The analysis conducted by Dr. Goshua and colleagues goes a long way toward elucidating some of the mechanisms and therapeutic targets to meet these challenges.
Dr. Goshua disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Goshua G et al. EHA Congress. Abstract LB2605.
References
1. Klok FA et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148-50. doi: 10.1016/j.thromres.2020.04.041.
2. Thomas W et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76-7. doi: 10.1016/j.thromres.2020.04.028
3. Lodigiani C et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. doi: 10.1016/j.thromres.2020.04.024
4. Llitjos JF et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients [published online ahead of print, 2020 Apr 22]. J Thromb Haemost. 2020;10.1111/jth.14869. doi: 10.1111/jth.14869
5. Carsana L et al. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. medRxiv 2020.04.19.20054262; doi: 10.1101/2020.04.19.20054262v1.
6. Menter T et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction [published online ahead of print, 2020 May 4]. Histopathology. 2020;10.1111/his.14134. doi: 10.1111/his.14134
7. Lax SF, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: Results from a prospective, single-center, clinicopathologic case series [published online ahead of print, 2020 May 14]. Ann Intern Med. 2020;M20-2566. doi: 10.7326/M20-2566.
A striking clinical feature of illness from SARS-CoV-2 is a marked increase in thrombotic and microvascular complications, or COVID-19–associated coagulopathy (CAC).
A new study suggests endothelial cell injury plays a major role in the pathogenesis of CAC, and blood levels of soluble thrombomodulin correlate with mortality.
George Goshua, MD, of Yale University, New Haven, Conn., presented this study as a late-breaking abstract at the virtual annual congress of the European Hematology Association.
Dr. Goshua cited past research showing CAC to be highly prevalent among hospitalized patients. Venous thromboembolism was found in 17% to 69% of patients, despite thromboprophylaxis.1-4 Arterial thrombosis has been seen in 3.6% to 4.0% of patients,1-3 and autopsy findings have shown microvascular thrombosis in as many as 87% of patients.5-7
For their study, Dr. Goshua and colleagues assessed endothelial cell damage, platelet activation, and hemostatic and fibrinolytic cascade effects of CAC.
The investigators measured markers of endothelial cell injury and platelet activation, plasminogen activation inhibitor 1 (PAI-1), and coagulation factors in stable and critically ill patients hospitalized with COVID-19. In addition, the team sought to identify biomarkers of mortality in hospitalized patients.
Dr. Goshua and colleagues studied 68 adults hospitalized for suspected COVID-19 – 48 in the ICU and 20 outside the ICU. Patients in the ICU received mechanical ventilation, while the non-ICU patients required supplemental oxygen (≤3 L/min per nasal cannula).
There were more men than women (69% vs. 31%) in the ICU population but not in the non-ICU population (40% vs. 60%). There were no statistically significant differences in age or comorbid conditions between the ICU and non-ICU patients.
Results and interpretation
Consistent with augmentation of the coagulation cascade – and as expected – D-dimer and thrombin-antithrombin levels were high in both the ICU and non-ICU populations, but levels were significantly higher (P < .001) among the ICU patients.
Endogenous anticoagulants (antithrombin and proteins C and S) and fibrinolytic enzymes (alpha 2-antiplasmin) were preserved, verifying that CAC is distinct from disseminated intravascular coagulation. Classic fibrinolysis did not occur, as PAI-1 was high in ICU and non-ICU patients, and lysis-30 was normal in nearly all ICU patients (96%).
Von Willebrand factor antigen and activity levels and factor VIII levels were markedly elevated in non-ICU and ICU patients, but they were significantly higher (P < .001) in the ICU cohort. This supports the hypothesis that endothelial cell damage and platelet activation play major roles in CAC.
Similarly, soluble P-selectin, which is shed from endothelial cells and platelets, was dramatically elevated in ICU patients in comparison with controls and non-ICU patients (P < .001 for both comparisons).
Levels of soluble thrombomodulin, which is released from endothelial cells, were not significantly different in ICU patients and controls. However, given thrombomodulin’s significant role in the coagulation cascade, Dr. Goshua and colleagues plotted receiver operating curves to see if soluble thrombomodulin levels were predictive of mortality.
The results showed that soluble thrombomodulin correlated with the probability of survival, both overall and in ICU patients. Soluble thrombomodulin levels greater than 3.26 ng/mL were associated with significantly worse survival in all patients (P = .0087) and ICU patients (P = .0309).
Influence on therapy
Laboratory perturbations were detected in both ICU and non-ICU patients, and otherwise healthy outpatients have exhibited potentially life-threatening CAC, according to Dr. Goshua.
These findings suggest the prothrombotic state occurs early in the pathogenesis of SARS-CoV-2 infection, is driven by platelet activation and endotheliopathy, and becomes more pronounced with worsening severity of infection.
The results of this study prompted a change in how Yale–New Haven Hospital manages COVID-19 patients. Patients without a clinical contraindication now receive aspirin at 81 mg daily in addition to the anticoagulation regimen typically used for all hospitalized COVID-19 patients.
Investigations regarding other medications that can influence platelet-endothelial cell interactions and modulate endothelial cell damage in CAC – such as dipyridamole, defibrotide, and eculizumab – are planned.
Challenges and unanswered questions
Virchow’s triad was described by the eminent German physician, Rudolf Virchow, MD, in the 19th century. It refers to the three broad categories of factors that can predispose patients to thrombosis — circulatory stasis, hypercoagulability, and endothelial injury.
Although all of these elements could be operative in CAC, the current study suggests platelet activation and endothelial cell injury in CAC may be of primary importance.
Because of the limited ability to test critically ill patients and concerns regarding exposure of additional hospital personnel to COVID-19 patients, the current report lacked clarity about the relationship of the detected laboratory abnormalities to confirmed thrombotic events.
It is unknown whether endothelial cells in different organs are damaged uniformly. It is also unclear if the laboratory abnormalities identified in this analysis can be used to monitor response to therapy, to guide follow-up management of discharged patients with CAC, or to identify infected outpatients who should receive prophylactic anticoagulation.
The mechanism by which SARS-CoV-2 injures endothelial cells is not explained by these data. Neutrophil defensins and other prothrombotic peptides or markers of inflammation could play key roles in pathogenesis, assessment of disease severity, or monitoring for therapeutic efficacy.
Today, we have more sophisticated diagnostic tools than Dr. Virchow had. We also have the ability to record and rapidly disseminate information globally. Still, with regard to the COVID-19 pandemic, clinicians face many of the same challenges that confronted Dr. Virchow in his era.
The analysis conducted by Dr. Goshua and colleagues goes a long way toward elucidating some of the mechanisms and therapeutic targets to meet these challenges.
Dr. Goshua disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Goshua G et al. EHA Congress. Abstract LB2605.
References
1. Klok FA et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148-50. doi: 10.1016/j.thromres.2020.04.041.
2. Thomas W et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76-7. doi: 10.1016/j.thromres.2020.04.028
3. Lodigiani C et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. doi: 10.1016/j.thromres.2020.04.024
4. Llitjos JF et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients [published online ahead of print, 2020 Apr 22]. J Thromb Haemost. 2020;10.1111/jth.14869. doi: 10.1111/jth.14869
5. Carsana L et al. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. medRxiv 2020.04.19.20054262; doi: 10.1101/2020.04.19.20054262v1.
6. Menter T et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction [published online ahead of print, 2020 May 4]. Histopathology. 2020;10.1111/his.14134. doi: 10.1111/his.14134
7. Lax SF, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: Results from a prospective, single-center, clinicopathologic case series [published online ahead of print, 2020 May 14]. Ann Intern Med. 2020;M20-2566. doi: 10.7326/M20-2566.
A striking clinical feature of illness from SARS-CoV-2 is a marked increase in thrombotic and microvascular complications, or COVID-19–associated coagulopathy (CAC).
A new study suggests endothelial cell injury plays a major role in the pathogenesis of CAC, and blood levels of soluble thrombomodulin correlate with mortality.
George Goshua, MD, of Yale University, New Haven, Conn., presented this study as a late-breaking abstract at the virtual annual congress of the European Hematology Association.
Dr. Goshua cited past research showing CAC to be highly prevalent among hospitalized patients. Venous thromboembolism was found in 17% to 69% of patients, despite thromboprophylaxis.1-4 Arterial thrombosis has been seen in 3.6% to 4.0% of patients,1-3 and autopsy findings have shown microvascular thrombosis in as many as 87% of patients.5-7
For their study, Dr. Goshua and colleagues assessed endothelial cell damage, platelet activation, and hemostatic and fibrinolytic cascade effects of CAC.
The investigators measured markers of endothelial cell injury and platelet activation, plasminogen activation inhibitor 1 (PAI-1), and coagulation factors in stable and critically ill patients hospitalized with COVID-19. In addition, the team sought to identify biomarkers of mortality in hospitalized patients.
Dr. Goshua and colleagues studied 68 adults hospitalized for suspected COVID-19 – 48 in the ICU and 20 outside the ICU. Patients in the ICU received mechanical ventilation, while the non-ICU patients required supplemental oxygen (≤3 L/min per nasal cannula).
There were more men than women (69% vs. 31%) in the ICU population but not in the non-ICU population (40% vs. 60%). There were no statistically significant differences in age or comorbid conditions between the ICU and non-ICU patients.
Results and interpretation
Consistent with augmentation of the coagulation cascade – and as expected – D-dimer and thrombin-antithrombin levels were high in both the ICU and non-ICU populations, but levels were significantly higher (P < .001) among the ICU patients.
Endogenous anticoagulants (antithrombin and proteins C and S) and fibrinolytic enzymes (alpha 2-antiplasmin) were preserved, verifying that CAC is distinct from disseminated intravascular coagulation. Classic fibrinolysis did not occur, as PAI-1 was high in ICU and non-ICU patients, and lysis-30 was normal in nearly all ICU patients (96%).
Von Willebrand factor antigen and activity levels and factor VIII levels were markedly elevated in non-ICU and ICU patients, but they were significantly higher (P < .001) in the ICU cohort. This supports the hypothesis that endothelial cell damage and platelet activation play major roles in CAC.
Similarly, soluble P-selectin, which is shed from endothelial cells and platelets, was dramatically elevated in ICU patients in comparison with controls and non-ICU patients (P < .001 for both comparisons).
Levels of soluble thrombomodulin, which is released from endothelial cells, were not significantly different in ICU patients and controls. However, given thrombomodulin’s significant role in the coagulation cascade, Dr. Goshua and colleagues plotted receiver operating curves to see if soluble thrombomodulin levels were predictive of mortality.
The results showed that soluble thrombomodulin correlated with the probability of survival, both overall and in ICU patients. Soluble thrombomodulin levels greater than 3.26 ng/mL were associated with significantly worse survival in all patients (P = .0087) and ICU patients (P = .0309).
Influence on therapy
Laboratory perturbations were detected in both ICU and non-ICU patients, and otherwise healthy outpatients have exhibited potentially life-threatening CAC, according to Dr. Goshua.
These findings suggest the prothrombotic state occurs early in the pathogenesis of SARS-CoV-2 infection, is driven by platelet activation and endotheliopathy, and becomes more pronounced with worsening severity of infection.
The results of this study prompted a change in how Yale–New Haven Hospital manages COVID-19 patients. Patients without a clinical contraindication now receive aspirin at 81 mg daily in addition to the anticoagulation regimen typically used for all hospitalized COVID-19 patients.
Investigations regarding other medications that can influence platelet-endothelial cell interactions and modulate endothelial cell damage in CAC – such as dipyridamole, defibrotide, and eculizumab – are planned.
Challenges and unanswered questions
Virchow’s triad was described by the eminent German physician, Rudolf Virchow, MD, in the 19th century. It refers to the three broad categories of factors that can predispose patients to thrombosis — circulatory stasis, hypercoagulability, and endothelial injury.
Although all of these elements could be operative in CAC, the current study suggests platelet activation and endothelial cell injury in CAC may be of primary importance.
Because of the limited ability to test critically ill patients and concerns regarding exposure of additional hospital personnel to COVID-19 patients, the current report lacked clarity about the relationship of the detected laboratory abnormalities to confirmed thrombotic events.
It is unknown whether endothelial cells in different organs are damaged uniformly. It is also unclear if the laboratory abnormalities identified in this analysis can be used to monitor response to therapy, to guide follow-up management of discharged patients with CAC, or to identify infected outpatients who should receive prophylactic anticoagulation.
The mechanism by which SARS-CoV-2 injures endothelial cells is not explained by these data. Neutrophil defensins and other prothrombotic peptides or markers of inflammation could play key roles in pathogenesis, assessment of disease severity, or monitoring for therapeutic efficacy.
Today, we have more sophisticated diagnostic tools than Dr. Virchow had. We also have the ability to record and rapidly disseminate information globally. Still, with regard to the COVID-19 pandemic, clinicians face many of the same challenges that confronted Dr. Virchow in his era.
The analysis conducted by Dr. Goshua and colleagues goes a long way toward elucidating some of the mechanisms and therapeutic targets to meet these challenges.
Dr. Goshua disclosed no conflicts of interest.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
SOURCE: Goshua G et al. EHA Congress. Abstract LB2605.
References
1. Klok FA et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148-50. doi: 10.1016/j.thromres.2020.04.041.
2. Thomas W et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76-7. doi: 10.1016/j.thromres.2020.04.028
3. Lodigiani C et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. doi: 10.1016/j.thromres.2020.04.024
4. Llitjos JF et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients [published online ahead of print, 2020 Apr 22]. J Thromb Haemost. 2020;10.1111/jth.14869. doi: 10.1111/jth.14869
5. Carsana L et al. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. medRxiv 2020.04.19.20054262; doi: 10.1101/2020.04.19.20054262v1.
6. Menter T et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction [published online ahead of print, 2020 May 4]. Histopathology. 2020;10.1111/his.14134. doi: 10.1111/his.14134
7. Lax SF, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: Results from a prospective, single-center, clinicopathologic case series [published online ahead of print, 2020 May 14]. Ann Intern Med. 2020;M20-2566. doi: 10.7326/M20-2566.
FROM EHA CONGRESS
ACIP plans priority groups in advance of COVID-19 vaccine
according to Sarah Mbaeyi, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases.
A COVID-19 vaccine work group is developing strategies and identifying priority groups for vaccination to help inform discussions about the use of COVID-19 vaccines, Dr. Mbaeyi said at a virtual meeting of the CDC’s Advisory Committee on Immunization Practices.
“Preparing for vaccination during a pandemic has long been a priority of the CDC and the U.S. government,” said Dr. Mbaeyi. The work group is building on a tiered approach to vaccination that was updated in 2018 after the H1N1 flu pandemic, with occupational and high-risk populations placed in the highest-priority groups, Dr. Mbaeyi said.
There are important differences between COVID-19 and influenza, Dr. Mbaeyi said. “Vaccine prioritization is challenging due to incomplete information on COVID-19 epidemiology and vaccines, including characteristics, timing, and number of doses.”
However, guidance for vaccine prioritization developed after the H1N1 outbreak in 2018 can be adapted for COVID-19.
To help inform ACIP deliberations, the work group reviewed the epidemiology of COVID-19. A large proportion of the population remains susceptible, and prioritizations should be based on data to date and continually refined, she said.
The work group defined the objectives of the COVID-19 vaccine program as follows: “Ensure safety and effectiveness of COVID-19 vaccines; reduce transmission, morbidity, and mortality in the population; help minimize disruption to society and economy, including maintaining health care capacity; and ensure equity in vaccine allocation and distribution.”
Based on current information, the work group has proposed that vaccine priority be given to health care personnel, essential workers, adults aged 65 years and older, long-term care facility residents, and persons with high-risk medical conditions.
Among these groups “a subset of critical health care and other workers should receive initial doses,” Dr. Mbaeyi said.
However, vaccines will not be administered until safety and efficacy have been demonstrated, she emphasized. The timing and number of vaccine doses are unknown, and subprioritization may be needed, assuming the vaccine becomes available in incremental quantities over several months.
Next steps for the work group are refinement of priority groups based on ACIP feedback, and assignment of tiers to other groups such as children, pregnant women, and racial/ethnic groups at high risk, Dr. Mbaeyi said.
The goal of the work group is to have a prioritization framework for COVID-19 vaccination to present at the next ACIP meeting.
Committee member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., emphasized that “one of the things we need to know is how is the virus [is] transmitted and who is transmitting,” and that this information will be key to developing strategies for vaccination.
Sarah E. Oliver, MD, an epidemiologist at the National Center for Immunization and Respiratory Diseases, responded that household transmission studies are in progress that will help inform the prioritization process.
Dr. Mbaeyi and Dr. Oliver had no financial conflicts to disclose.
according to Sarah Mbaeyi, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases.
A COVID-19 vaccine work group is developing strategies and identifying priority groups for vaccination to help inform discussions about the use of COVID-19 vaccines, Dr. Mbaeyi said at a virtual meeting of the CDC’s Advisory Committee on Immunization Practices.
“Preparing for vaccination during a pandemic has long been a priority of the CDC and the U.S. government,” said Dr. Mbaeyi. The work group is building on a tiered approach to vaccination that was updated in 2018 after the H1N1 flu pandemic, with occupational and high-risk populations placed in the highest-priority groups, Dr. Mbaeyi said.
There are important differences between COVID-19 and influenza, Dr. Mbaeyi said. “Vaccine prioritization is challenging due to incomplete information on COVID-19 epidemiology and vaccines, including characteristics, timing, and number of doses.”
However, guidance for vaccine prioritization developed after the H1N1 outbreak in 2018 can be adapted for COVID-19.
To help inform ACIP deliberations, the work group reviewed the epidemiology of COVID-19. A large proportion of the population remains susceptible, and prioritizations should be based on data to date and continually refined, she said.
The work group defined the objectives of the COVID-19 vaccine program as follows: “Ensure safety and effectiveness of COVID-19 vaccines; reduce transmission, morbidity, and mortality in the population; help minimize disruption to society and economy, including maintaining health care capacity; and ensure equity in vaccine allocation and distribution.”
Based on current information, the work group has proposed that vaccine priority be given to health care personnel, essential workers, adults aged 65 years and older, long-term care facility residents, and persons with high-risk medical conditions.
Among these groups “a subset of critical health care and other workers should receive initial doses,” Dr. Mbaeyi said.
However, vaccines will not be administered until safety and efficacy have been demonstrated, she emphasized. The timing and number of vaccine doses are unknown, and subprioritization may be needed, assuming the vaccine becomes available in incremental quantities over several months.
Next steps for the work group are refinement of priority groups based on ACIP feedback, and assignment of tiers to other groups such as children, pregnant women, and racial/ethnic groups at high risk, Dr. Mbaeyi said.
The goal of the work group is to have a prioritization framework for COVID-19 vaccination to present at the next ACIP meeting.
Committee member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., emphasized that “one of the things we need to know is how is the virus [is] transmitted and who is transmitting,” and that this information will be key to developing strategies for vaccination.
Sarah E. Oliver, MD, an epidemiologist at the National Center for Immunization and Respiratory Diseases, responded that household transmission studies are in progress that will help inform the prioritization process.
Dr. Mbaeyi and Dr. Oliver had no financial conflicts to disclose.
according to Sarah Mbaeyi, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases.
A COVID-19 vaccine work group is developing strategies and identifying priority groups for vaccination to help inform discussions about the use of COVID-19 vaccines, Dr. Mbaeyi said at a virtual meeting of the CDC’s Advisory Committee on Immunization Practices.
“Preparing for vaccination during a pandemic has long been a priority of the CDC and the U.S. government,” said Dr. Mbaeyi. The work group is building on a tiered approach to vaccination that was updated in 2018 after the H1N1 flu pandemic, with occupational and high-risk populations placed in the highest-priority groups, Dr. Mbaeyi said.
There are important differences between COVID-19 and influenza, Dr. Mbaeyi said. “Vaccine prioritization is challenging due to incomplete information on COVID-19 epidemiology and vaccines, including characteristics, timing, and number of doses.”
However, guidance for vaccine prioritization developed after the H1N1 outbreak in 2018 can be adapted for COVID-19.
To help inform ACIP deliberations, the work group reviewed the epidemiology of COVID-19. A large proportion of the population remains susceptible, and prioritizations should be based on data to date and continually refined, she said.
The work group defined the objectives of the COVID-19 vaccine program as follows: “Ensure safety and effectiveness of COVID-19 vaccines; reduce transmission, morbidity, and mortality in the population; help minimize disruption to society and economy, including maintaining health care capacity; and ensure equity in vaccine allocation and distribution.”
Based on current information, the work group has proposed that vaccine priority be given to health care personnel, essential workers, adults aged 65 years and older, long-term care facility residents, and persons with high-risk medical conditions.
Among these groups “a subset of critical health care and other workers should receive initial doses,” Dr. Mbaeyi said.
However, vaccines will not be administered until safety and efficacy have been demonstrated, she emphasized. The timing and number of vaccine doses are unknown, and subprioritization may be needed, assuming the vaccine becomes available in incremental quantities over several months.
Next steps for the work group are refinement of priority groups based on ACIP feedback, and assignment of tiers to other groups such as children, pregnant women, and racial/ethnic groups at high risk, Dr. Mbaeyi said.
The goal of the work group is to have a prioritization framework for COVID-19 vaccination to present at the next ACIP meeting.
Committee member Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., emphasized that “one of the things we need to know is how is the virus [is] transmitted and who is transmitting,” and that this information will be key to developing strategies for vaccination.
Sarah E. Oliver, MD, an epidemiologist at the National Center for Immunization and Respiratory Diseases, responded that household transmission studies are in progress that will help inform the prioritization process.
Dr. Mbaeyi and Dr. Oliver had no financial conflicts to disclose.







