User login
-
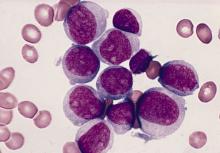
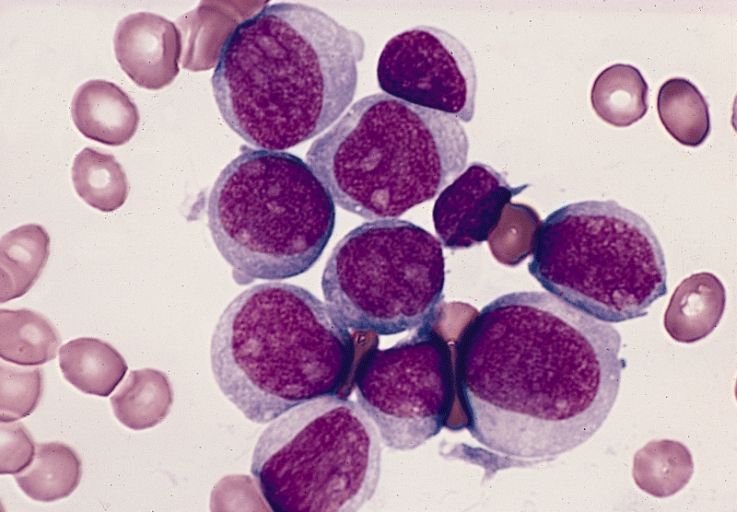
FDA approves loncastuximab for diffuse large B-cell lymphomas
The Food and Drug Administration granted an accelerated approval April 24, 2021, for a new drug for use in patients with relapsed/refractory diffuse large B-cell lymphomas (DLBCL) who have tried at least two prior systemic therapies.
The new product, loncastuximab tesirine-lpyl (Zynlonta, ADC Therapeutics), is the first and only CD19-targeted antibody-drug conjugate approved for this disease.
DLBCL is the most common type of non-Hodgkin lymphoma in the United States, but the indication also includes DLBCL not otherwise specified, DLBCL arising from low grade lymphoma, and high-grade B-cell lymphoma.
“There is a significant unmet need for treatment options for patients with [relapsed or refractory] DLBCL, including those who have been heavily pretreated and have difficult-to-treat disease,” Paolo F. Caimi, MD, University Hospitals Cleveland Medical Center and Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, said in a company press release.
The company also cited data from previous clinical trials showing that more than 40% of first-line DLBCL treatments fail, and that these patients have a poor prognosis, worsening with each line of therapy that is tried.
Accelerated approval based on ORR
The accelerated approval was based on overall response rate data from the single-arm LOTIS-2 trial. All patients received the new drug, administered as a 30-minute infusion once every 3 weeks for 1 year.
The trial was conducted in 145 patients with relapsed/refractory DLBCL who had already tried at least two lines of systemic therapy. Dr. Caimi noted that this included patients who had been heavily pretreated, as the population included patients who previously received stem cell transplant or chimeric antigen receptor T-cell therapy.
The ORR was 48.3% (70/145 patients), which included a complete response rate of 24.1% (35/145 patients) and a partial response rate of 24.1% (35/145 patients).
Patients had a median time to response of 1.3 months and the median duration of response for the 70 responders was 10.3 months.
“Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial,” the company noted.
A phase 3 confirmatory is underway: the LOTIS 5 trial (NCT04384484) compares the combination of loncastuximab tesirine and rituximab versus chemoimmunotherapy in patients with relapsed/refractory DLBCL.
The company also noted that in a pooled safety population the most common adverse reactions (≥20%) were thrombocytopenia, an increase in levels of the liver enzyme gamma-glutamyltransferase, neutropenia, anemia, hyperglycemia, transaminase elevation, fatigue, hypoalbuminemia, rash, edema, nausea, and musculoskeletal pain.
In the LOTIS-2 trial, the most common (≥10%) grade 3 or higher treatment-emergent adverse events were neutropenia (26.2%), thrombocytopenia (17.9%), GGT increase (17.2%) and anemia (10.3%).
Permanent treatment discontinuation as the result of an adverse reaction occurred in 19% of patients, and these included a GGT increase, edema, and effusion.
Dose reductions because of an adverse reaction occurred in 8% of patients, and most were the result of a GGT increase. Dosage interruptions because of an adverse reaction occurred in 49% of patients, and these included a GGT increase, neutropenia, thrombocytopenia, and edema.
Warnings on effusions, infections, and skin reactions
The product carries a warning that serious effusion and edema has been reported. Grade 3 edema occurred in 3% (primarily peripheral edema or ascites), grade 3 pleural effusion occurred in 3%, and grade 3 or 4 pericardial effusion occurred in 1%.
Prescribers are recommended to monitor patients for new or worsening edema or effusions, and to consider diagnostic imaging in patients who develop symptoms of pleural effusion or pericardial effusion, such as new or worsened dyspnea, chest pain, and/or ascites such as swelling in the abdomen and bloating.
The product also carries a warning about fatal and serious infections, including opportunistic infections, and serious cutaneous reactions, including photosensitivity reaction, rash (including exfoliative and maculopapular), and erythema.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration granted an accelerated approval April 24, 2021, for a new drug for use in patients with relapsed/refractory diffuse large B-cell lymphomas (DLBCL) who have tried at least two prior systemic therapies.
The new product, loncastuximab tesirine-lpyl (Zynlonta, ADC Therapeutics), is the first and only CD19-targeted antibody-drug conjugate approved for this disease.
DLBCL is the most common type of non-Hodgkin lymphoma in the United States, but the indication also includes DLBCL not otherwise specified, DLBCL arising from low grade lymphoma, and high-grade B-cell lymphoma.
“There is a significant unmet need for treatment options for patients with [relapsed or refractory] DLBCL, including those who have been heavily pretreated and have difficult-to-treat disease,” Paolo F. Caimi, MD, University Hospitals Cleveland Medical Center and Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, said in a company press release.
The company also cited data from previous clinical trials showing that more than 40% of first-line DLBCL treatments fail, and that these patients have a poor prognosis, worsening with each line of therapy that is tried.
Accelerated approval based on ORR
The accelerated approval was based on overall response rate data from the single-arm LOTIS-2 trial. All patients received the new drug, administered as a 30-minute infusion once every 3 weeks for 1 year.
The trial was conducted in 145 patients with relapsed/refractory DLBCL who had already tried at least two lines of systemic therapy. Dr. Caimi noted that this included patients who had been heavily pretreated, as the population included patients who previously received stem cell transplant or chimeric antigen receptor T-cell therapy.
The ORR was 48.3% (70/145 patients), which included a complete response rate of 24.1% (35/145 patients) and a partial response rate of 24.1% (35/145 patients).
Patients had a median time to response of 1.3 months and the median duration of response for the 70 responders was 10.3 months.
“Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial,” the company noted.
A phase 3 confirmatory is underway: the LOTIS 5 trial (NCT04384484) compares the combination of loncastuximab tesirine and rituximab versus chemoimmunotherapy in patients with relapsed/refractory DLBCL.
The company also noted that in a pooled safety population the most common adverse reactions (≥20%) were thrombocytopenia, an increase in levels of the liver enzyme gamma-glutamyltransferase, neutropenia, anemia, hyperglycemia, transaminase elevation, fatigue, hypoalbuminemia, rash, edema, nausea, and musculoskeletal pain.
In the LOTIS-2 trial, the most common (≥10%) grade 3 or higher treatment-emergent adverse events were neutropenia (26.2%), thrombocytopenia (17.9%), GGT increase (17.2%) and anemia (10.3%).
Permanent treatment discontinuation as the result of an adverse reaction occurred in 19% of patients, and these included a GGT increase, edema, and effusion.
Dose reductions because of an adverse reaction occurred in 8% of patients, and most were the result of a GGT increase. Dosage interruptions because of an adverse reaction occurred in 49% of patients, and these included a GGT increase, neutropenia, thrombocytopenia, and edema.
Warnings on effusions, infections, and skin reactions
The product carries a warning that serious effusion and edema has been reported. Grade 3 edema occurred in 3% (primarily peripheral edema or ascites), grade 3 pleural effusion occurred in 3%, and grade 3 or 4 pericardial effusion occurred in 1%.
Prescribers are recommended to monitor patients for new or worsening edema or effusions, and to consider diagnostic imaging in patients who develop symptoms of pleural effusion or pericardial effusion, such as new or worsened dyspnea, chest pain, and/or ascites such as swelling in the abdomen and bloating.
The product also carries a warning about fatal and serious infections, including opportunistic infections, and serious cutaneous reactions, including photosensitivity reaction, rash (including exfoliative and maculopapular), and erythema.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration granted an accelerated approval April 24, 2021, for a new drug for use in patients with relapsed/refractory diffuse large B-cell lymphomas (DLBCL) who have tried at least two prior systemic therapies.
The new product, loncastuximab tesirine-lpyl (Zynlonta, ADC Therapeutics), is the first and only CD19-targeted antibody-drug conjugate approved for this disease.
DLBCL is the most common type of non-Hodgkin lymphoma in the United States, but the indication also includes DLBCL not otherwise specified, DLBCL arising from low grade lymphoma, and high-grade B-cell lymphoma.
“There is a significant unmet need for treatment options for patients with [relapsed or refractory] DLBCL, including those who have been heavily pretreated and have difficult-to-treat disease,” Paolo F. Caimi, MD, University Hospitals Cleveland Medical Center and Case Comprehensive Cancer Center, Case Western Reserve University, Cleveland, said in a company press release.
The company also cited data from previous clinical trials showing that more than 40% of first-line DLBCL treatments fail, and that these patients have a poor prognosis, worsening with each line of therapy that is tried.
Accelerated approval based on ORR
The accelerated approval was based on overall response rate data from the single-arm LOTIS-2 trial. All patients received the new drug, administered as a 30-minute infusion once every 3 weeks for 1 year.
The trial was conducted in 145 patients with relapsed/refractory DLBCL who had already tried at least two lines of systemic therapy. Dr. Caimi noted that this included patients who had been heavily pretreated, as the population included patients who previously received stem cell transplant or chimeric antigen receptor T-cell therapy.
The ORR was 48.3% (70/145 patients), which included a complete response rate of 24.1% (35/145 patients) and a partial response rate of 24.1% (35/145 patients).
Patients had a median time to response of 1.3 months and the median duration of response for the 70 responders was 10.3 months.
“Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial,” the company noted.
A phase 3 confirmatory is underway: the LOTIS 5 trial (NCT04384484) compares the combination of loncastuximab tesirine and rituximab versus chemoimmunotherapy in patients with relapsed/refractory DLBCL.
The company also noted that in a pooled safety population the most common adverse reactions (≥20%) were thrombocytopenia, an increase in levels of the liver enzyme gamma-glutamyltransferase, neutropenia, anemia, hyperglycemia, transaminase elevation, fatigue, hypoalbuminemia, rash, edema, nausea, and musculoskeletal pain.
In the LOTIS-2 trial, the most common (≥10%) grade 3 or higher treatment-emergent adverse events were neutropenia (26.2%), thrombocytopenia (17.9%), GGT increase (17.2%) and anemia (10.3%).
Permanent treatment discontinuation as the result of an adverse reaction occurred in 19% of patients, and these included a GGT increase, edema, and effusion.
Dose reductions because of an adverse reaction occurred in 8% of patients, and most were the result of a GGT increase. Dosage interruptions because of an adverse reaction occurred in 49% of patients, and these included a GGT increase, neutropenia, thrombocytopenia, and edema.
Warnings on effusions, infections, and skin reactions
The product carries a warning that serious effusion and edema has been reported. Grade 3 edema occurred in 3% (primarily peripheral edema or ascites), grade 3 pleural effusion occurred in 3%, and grade 3 or 4 pericardial effusion occurred in 1%.
Prescribers are recommended to monitor patients for new or worsening edema or effusions, and to consider diagnostic imaging in patients who develop symptoms of pleural effusion or pericardial effusion, such as new or worsened dyspnea, chest pain, and/or ascites such as swelling in the abdomen and bloating.
The product also carries a warning about fatal and serious infections, including opportunistic infections, and serious cutaneous reactions, including photosensitivity reaction, rash (including exfoliative and maculopapular), and erythema.
A version of this article first appeared on Medscape.com.
Commentary: Functional assessment developed for older adults with sickle cell disease
As individuals with sickle cell disease (SCD) are living longer than ever before there is a greater need to focus on maintaining and improving function and independence in this growing population. In the general population, impairments in functional measures such as usual gait speed, grip strength, Timed Up and Go, and cognition are associated with adverse health outcomes such as falls, fractures, loss of independence, and death.
Adults with SCD experience multiple complications such as avascular necrosis of the joints, retinopathy, and strokes that lead to functional limitations similar to those experienced by geriatric populations. However, functional assessments are not routinely performed during clinic visits with older adults with SCD.
In order to address this gap in care, my colleagues and I developed the first functional assessment for older adults with SCD, called the Sickle Cell Disease Functional Assessment (SCD-FA). This assessment will allow providers to evaluate the capabilities and vulnerabilities of older adults with SCD.
We assessed the feasibility of administering the SCD-FA in a prospective cohort pilot study. We enrolled 40 adults with SCD (20 older adults aged at least 50 years and 20 younger adults aged 18-49 years as a comparison group). All participants were assessed at steady-state.
For the SCD-FA, we selected geriatric assessment measures across seven domains: functional status, comorbid medical conditions, psychological state, social support, nutritional status, cognition, and medications. Several of these measures were previously validated in an oncology geriatric assessment and enriched with additional physical and cognitive measures to evaluate conditions at the intersection of SCD and geriatrics.
In September 2020, we published a protocol describing the methods and rationale for selecting measures for the SCD-FA in Pilot and Feasibility Studies.1 The preliminary data was presented at the annual meeting of the American Society of Hematology in December 2020 and was included in the annual Hematology and Aging Poster Walk.
The results of this pilot study showed that the SCD-FA is feasible (91% of participants who consented completed the SCD-FA), acceptable (95% reported the length as appropriate and had no difficulty understanding the measures), and safe with no adverse events.2 On physical performance testing, both younger and older participants had results consistent with accelerated aging with a functional age at least 20-30 years older than their chronological age.2
The majority of the participants (63%) had a usual gait speed slower than the speed required to safely cross the street at an intersection, and 25% had a gait speed slower than 1 m/s, which has been associated with increased mortality in the general population.3,4
Benefits to management
The SCD-FA can improve management of adults with SCD by:
- Characterizing their capabilities and physiological age, identifying individuals at high risk for functional decline and death early identifying targets for interventions that have been successful in geriatrics,5 assessing risk of toxicity from curative therapies, and evaluating functional response to SCD-specific therapies.
The SCD-FA provides a framework for developing exercise interventions to target functional impairments. This work supports our goal of improving the quality of life and longevity for people with SCD.
Dr. Oyedeji is a senior hematology Fellow at the department of medicine, division of hematology, Duke University, Durham, N.C. She reported that she has no conflicts of interest.
References
1. Pilot Feasibility Stud. 2020;6:131.
2. Blood. 2020;136(Supplement 1):26-7.
3. J Rehabil Res Dev. 2005;42(4):535-46.
4. JAMA. 2011;305(1):50-8.
5. South Med J. 1994;87(5):S83-7.
As individuals with sickle cell disease (SCD) are living longer than ever before there is a greater need to focus on maintaining and improving function and independence in this growing population. In the general population, impairments in functional measures such as usual gait speed, grip strength, Timed Up and Go, and cognition are associated with adverse health outcomes such as falls, fractures, loss of independence, and death.
Adults with SCD experience multiple complications such as avascular necrosis of the joints, retinopathy, and strokes that lead to functional limitations similar to those experienced by geriatric populations. However, functional assessments are not routinely performed during clinic visits with older adults with SCD.
In order to address this gap in care, my colleagues and I developed the first functional assessment for older adults with SCD, called the Sickle Cell Disease Functional Assessment (SCD-FA). This assessment will allow providers to evaluate the capabilities and vulnerabilities of older adults with SCD.
We assessed the feasibility of administering the SCD-FA in a prospective cohort pilot study. We enrolled 40 adults with SCD (20 older adults aged at least 50 years and 20 younger adults aged 18-49 years as a comparison group). All participants were assessed at steady-state.
For the SCD-FA, we selected geriatric assessment measures across seven domains: functional status, comorbid medical conditions, psychological state, social support, nutritional status, cognition, and medications. Several of these measures were previously validated in an oncology geriatric assessment and enriched with additional physical and cognitive measures to evaluate conditions at the intersection of SCD and geriatrics.
In September 2020, we published a protocol describing the methods and rationale for selecting measures for the SCD-FA in Pilot and Feasibility Studies.1 The preliminary data was presented at the annual meeting of the American Society of Hematology in December 2020 and was included in the annual Hematology and Aging Poster Walk.
The results of this pilot study showed that the SCD-FA is feasible (91% of participants who consented completed the SCD-FA), acceptable (95% reported the length as appropriate and had no difficulty understanding the measures), and safe with no adverse events.2 On physical performance testing, both younger and older participants had results consistent with accelerated aging with a functional age at least 20-30 years older than their chronological age.2
The majority of the participants (63%) had a usual gait speed slower than the speed required to safely cross the street at an intersection, and 25% had a gait speed slower than 1 m/s, which has been associated with increased mortality in the general population.3,4
Benefits to management
The SCD-FA can improve management of adults with SCD by:
- Characterizing their capabilities and physiological age, identifying individuals at high risk for functional decline and death early identifying targets for interventions that have been successful in geriatrics,5 assessing risk of toxicity from curative therapies, and evaluating functional response to SCD-specific therapies.
The SCD-FA provides a framework for developing exercise interventions to target functional impairments. This work supports our goal of improving the quality of life and longevity for people with SCD.
Dr. Oyedeji is a senior hematology Fellow at the department of medicine, division of hematology, Duke University, Durham, N.C. She reported that she has no conflicts of interest.
References
1. Pilot Feasibility Stud. 2020;6:131.
2. Blood. 2020;136(Supplement 1):26-7.
3. J Rehabil Res Dev. 2005;42(4):535-46.
4. JAMA. 2011;305(1):50-8.
5. South Med J. 1994;87(5):S83-7.
As individuals with sickle cell disease (SCD) are living longer than ever before there is a greater need to focus on maintaining and improving function and independence in this growing population. In the general population, impairments in functional measures such as usual gait speed, grip strength, Timed Up and Go, and cognition are associated with adverse health outcomes such as falls, fractures, loss of independence, and death.
Adults with SCD experience multiple complications such as avascular necrosis of the joints, retinopathy, and strokes that lead to functional limitations similar to those experienced by geriatric populations. However, functional assessments are not routinely performed during clinic visits with older adults with SCD.
In order to address this gap in care, my colleagues and I developed the first functional assessment for older adults with SCD, called the Sickle Cell Disease Functional Assessment (SCD-FA). This assessment will allow providers to evaluate the capabilities and vulnerabilities of older adults with SCD.
We assessed the feasibility of administering the SCD-FA in a prospective cohort pilot study. We enrolled 40 adults with SCD (20 older adults aged at least 50 years and 20 younger adults aged 18-49 years as a comparison group). All participants were assessed at steady-state.
For the SCD-FA, we selected geriatric assessment measures across seven domains: functional status, comorbid medical conditions, psychological state, social support, nutritional status, cognition, and medications. Several of these measures were previously validated in an oncology geriatric assessment and enriched with additional physical and cognitive measures to evaluate conditions at the intersection of SCD and geriatrics.
In September 2020, we published a protocol describing the methods and rationale for selecting measures for the SCD-FA in Pilot and Feasibility Studies.1 The preliminary data was presented at the annual meeting of the American Society of Hematology in December 2020 and was included in the annual Hematology and Aging Poster Walk.
The results of this pilot study showed that the SCD-FA is feasible (91% of participants who consented completed the SCD-FA), acceptable (95% reported the length as appropriate and had no difficulty understanding the measures), and safe with no adverse events.2 On physical performance testing, both younger and older participants had results consistent with accelerated aging with a functional age at least 20-30 years older than their chronological age.2
The majority of the participants (63%) had a usual gait speed slower than the speed required to safely cross the street at an intersection, and 25% had a gait speed slower than 1 m/s, which has been associated with increased mortality in the general population.3,4
Benefits to management
The SCD-FA can improve management of adults with SCD by:
- Characterizing their capabilities and physiological age, identifying individuals at high risk for functional decline and death early identifying targets for interventions that have been successful in geriatrics,5 assessing risk of toxicity from curative therapies, and evaluating functional response to SCD-specific therapies.
The SCD-FA provides a framework for developing exercise interventions to target functional impairments. This work supports our goal of improving the quality of life and longevity for people with SCD.
Dr. Oyedeji is a senior hematology Fellow at the department of medicine, division of hematology, Duke University, Durham, N.C. She reported that she has no conflicts of interest.
References
1. Pilot Feasibility Stud. 2020;6:131.
2. Blood. 2020;136(Supplement 1):26-7.
3. J Rehabil Res Dev. 2005;42(4):535-46.
4. JAMA. 2011;305(1):50-8.
5. South Med J. 1994;87(5):S83-7.
Feds lift pause of J&J COVID vaccine, add new warning
Use of the Johnson & Johnson COVID-19 vaccine should resume in the United States for all adults, the Food and Drug Administration and Centers for Disease Contol and Prevention said April 23, although health care providers should warn patients of the risk of developing the rare and serious blood clots that caused the agencies to pause the vaccine’s distribution earlier this month.
“What we are seeing is the overall rate of events was 1.9 cases per million people. In women 18 to 29 years there was an approximate 7 cases per million. The risk is even lower in women over the age of 50 at .9 cases per million,” CDC Director Rochelle Walensky, MD, said in a news briefing the same day.
In the end, the potential benefits of the vaccine far outweighed its risks.
“In terms of benefits, we found that for every 1 million doses of this vaccine, the J&J vaccine could prevent over 650 hospitalizations and 12 deaths among women ages 18-49,” Dr. Walensky said. The potential benefits to women over 50 were even greater: It could prevent 4,700 hospitalizations and 650 deaths.
“In the end, this vaccine was shown to be safe and effective for the vast majority of people,” Dr. Walensky said.
The recommendation to continue the vaccine’s rollout came barely 2 hours after a CDC Advisory Committee on Immunization Practices voted to recommend the pause be lifted. The vote was 10-4 with one abstention.
The decision also includes instructions for the warning directed at women under 50 who have an increased risk of a rare but serious blood clot disorder called thrombosis with thrombocytopenia syndrome (TTS).
As of April 21, 15 cases of TTS, all in women and 13 of them in women under 50, have been confirmed among 7.98 million doses of the J&J vaccine administered in the United States. Three women have died.
The FDA and CDC recommended the pause on April 13 after reports that 6 women developed a blood clotting disorder 6 to 13 days after they received the J&J vaccine.
William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville, and a non-voting ACIP member, said in an interview the panel made the right recommendation.
He applauded both the decision to restart the vaccine and the updated warning information that “will explain [TTS] more fully to people, particularly women, who are coming to be vaccinated.”
As to women in the risk group needing to have a choice of vaccines, Dr. Schaffner said that will be addressed differently across the country.
“Every provider will not have alternative vaccines in their location so there will be many different ways to do this. You may have to get this information and select which site you’re going to depending on which vaccine is available if this matter is important to you,” he noted.
ACIP made the decision after a 6-hour emergency meeting to hear evidence on the Johnson & Johnson vaccine's protective benefits against COVID-19 vs. risk of TTS.
In the CDC-FDA press briefing, Dr. Walensky pointed out that over the past few days, as regulators have reviewed the rare events, newly identified patients had been treated appropriately, without the use of heparin, which is not advised for treating TTS.
As a result, regulators felt as if their messages had gotten out to doctors who now knew how to take special precautions when treating patients with the disorder.
She said the Johnson & Johnson shot remained an important option because it was convenient to give and easier to store than the other vaccines currently authorized in the United States.
Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the agency had already added information describing the risk of the rare clotting disorder to its fact sheets for patients and doctors.
Janet Woodcock, MD, acting commissioner of the FDA, said vaccination centers could resume giving the “one and done” shots as early as April 24.
This article was updated April 24, 2021, and first appeared on WebMD.com.
Use of the Johnson & Johnson COVID-19 vaccine should resume in the United States for all adults, the Food and Drug Administration and Centers for Disease Contol and Prevention said April 23, although health care providers should warn patients of the risk of developing the rare and serious blood clots that caused the agencies to pause the vaccine’s distribution earlier this month.
“What we are seeing is the overall rate of events was 1.9 cases per million people. In women 18 to 29 years there was an approximate 7 cases per million. The risk is even lower in women over the age of 50 at .9 cases per million,” CDC Director Rochelle Walensky, MD, said in a news briefing the same day.
In the end, the potential benefits of the vaccine far outweighed its risks.
“In terms of benefits, we found that for every 1 million doses of this vaccine, the J&J vaccine could prevent over 650 hospitalizations and 12 deaths among women ages 18-49,” Dr. Walensky said. The potential benefits to women over 50 were even greater: It could prevent 4,700 hospitalizations and 650 deaths.
“In the end, this vaccine was shown to be safe and effective for the vast majority of people,” Dr. Walensky said.
The recommendation to continue the vaccine’s rollout came barely 2 hours after a CDC Advisory Committee on Immunization Practices voted to recommend the pause be lifted. The vote was 10-4 with one abstention.
The decision also includes instructions for the warning directed at women under 50 who have an increased risk of a rare but serious blood clot disorder called thrombosis with thrombocytopenia syndrome (TTS).
As of April 21, 15 cases of TTS, all in women and 13 of them in women under 50, have been confirmed among 7.98 million doses of the J&J vaccine administered in the United States. Three women have died.
The FDA and CDC recommended the pause on April 13 after reports that 6 women developed a blood clotting disorder 6 to 13 days after they received the J&J vaccine.
William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville, and a non-voting ACIP member, said in an interview the panel made the right recommendation.
He applauded both the decision to restart the vaccine and the updated warning information that “will explain [TTS] more fully to people, particularly women, who are coming to be vaccinated.”
As to women in the risk group needing to have a choice of vaccines, Dr. Schaffner said that will be addressed differently across the country.
“Every provider will not have alternative vaccines in their location so there will be many different ways to do this. You may have to get this information and select which site you’re going to depending on which vaccine is available if this matter is important to you,” he noted.
ACIP made the decision after a 6-hour emergency meeting to hear evidence on the Johnson & Johnson vaccine's protective benefits against COVID-19 vs. risk of TTS.
In the CDC-FDA press briefing, Dr. Walensky pointed out that over the past few days, as regulators have reviewed the rare events, newly identified patients had been treated appropriately, without the use of heparin, which is not advised for treating TTS.
As a result, regulators felt as if their messages had gotten out to doctors who now knew how to take special precautions when treating patients with the disorder.
She said the Johnson & Johnson shot remained an important option because it was convenient to give and easier to store than the other vaccines currently authorized in the United States.
Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the agency had already added information describing the risk of the rare clotting disorder to its fact sheets for patients and doctors.
Janet Woodcock, MD, acting commissioner of the FDA, said vaccination centers could resume giving the “one and done” shots as early as April 24.
This article was updated April 24, 2021, and first appeared on WebMD.com.
Use of the Johnson & Johnson COVID-19 vaccine should resume in the United States for all adults, the Food and Drug Administration and Centers for Disease Contol and Prevention said April 23, although health care providers should warn patients of the risk of developing the rare and serious blood clots that caused the agencies to pause the vaccine’s distribution earlier this month.
“What we are seeing is the overall rate of events was 1.9 cases per million people. In women 18 to 29 years there was an approximate 7 cases per million. The risk is even lower in women over the age of 50 at .9 cases per million,” CDC Director Rochelle Walensky, MD, said in a news briefing the same day.
In the end, the potential benefits of the vaccine far outweighed its risks.
“In terms of benefits, we found that for every 1 million doses of this vaccine, the J&J vaccine could prevent over 650 hospitalizations and 12 deaths among women ages 18-49,” Dr. Walensky said. The potential benefits to women over 50 were even greater: It could prevent 4,700 hospitalizations and 650 deaths.
“In the end, this vaccine was shown to be safe and effective for the vast majority of people,” Dr. Walensky said.
The recommendation to continue the vaccine’s rollout came barely 2 hours after a CDC Advisory Committee on Immunization Practices voted to recommend the pause be lifted. The vote was 10-4 with one abstention.
The decision also includes instructions for the warning directed at women under 50 who have an increased risk of a rare but serious blood clot disorder called thrombosis with thrombocytopenia syndrome (TTS).
As of April 21, 15 cases of TTS, all in women and 13 of them in women under 50, have been confirmed among 7.98 million doses of the J&J vaccine administered in the United States. Three women have died.
The FDA and CDC recommended the pause on April 13 after reports that 6 women developed a blood clotting disorder 6 to 13 days after they received the J&J vaccine.
William Schaffner, MD, an infectious disease expert at Vanderbilt University in Nashville, and a non-voting ACIP member, said in an interview the panel made the right recommendation.
He applauded both the decision to restart the vaccine and the updated warning information that “will explain [TTS] more fully to people, particularly women, who are coming to be vaccinated.”
As to women in the risk group needing to have a choice of vaccines, Dr. Schaffner said that will be addressed differently across the country.
“Every provider will not have alternative vaccines in their location so there will be many different ways to do this. You may have to get this information and select which site you’re going to depending on which vaccine is available if this matter is important to you,” he noted.
ACIP made the decision after a 6-hour emergency meeting to hear evidence on the Johnson & Johnson vaccine's protective benefits against COVID-19 vs. risk of TTS.
In the CDC-FDA press briefing, Dr. Walensky pointed out that over the past few days, as regulators have reviewed the rare events, newly identified patients had been treated appropriately, without the use of heparin, which is not advised for treating TTS.
As a result, regulators felt as if their messages had gotten out to doctors who now knew how to take special precautions when treating patients with the disorder.
She said the Johnson & Johnson shot remained an important option because it was convenient to give and easier to store than the other vaccines currently authorized in the United States.
Peter Marks, MD, the director of FDA’s Center for Biologics Evaluation and Research, said the agency had already added information describing the risk of the rare clotting disorder to its fact sheets for patients and doctors.
Janet Woodcock, MD, acting commissioner of the FDA, said vaccination centers could resume giving the “one and done” shots as early as April 24.
This article was updated April 24, 2021, and first appeared on WebMD.com.
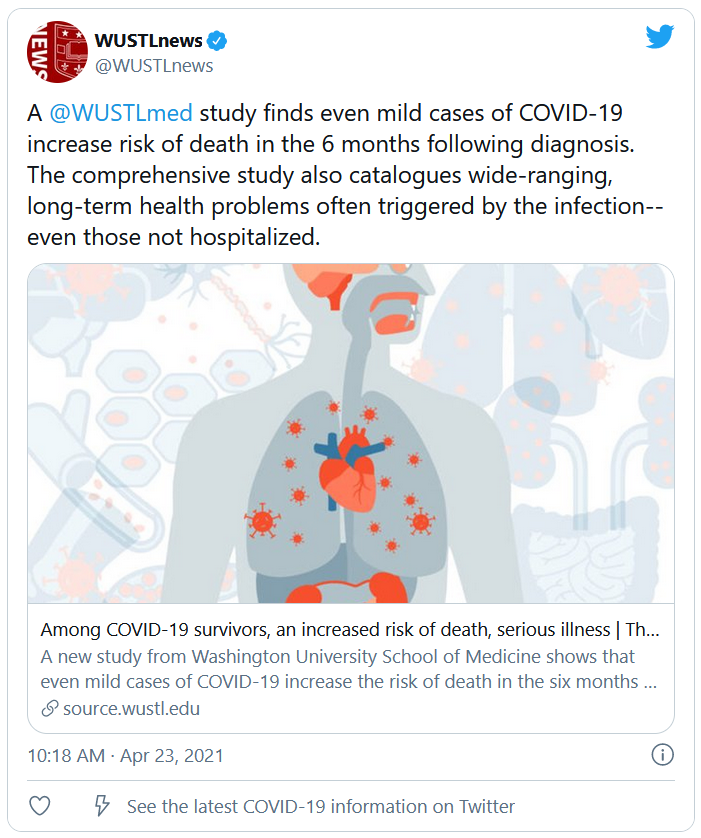
Study: COVID-19 can kill months after infection
Long-haul COVID-19 patients face many health threats – including a higher chance of dying – up to 6 months after they catch the virus, according to a massive study published in the journal Nature.
Researchers examined more than 87,000 COVID-19 patients and nearly 5 million control patients in a federal database. They found COVID-19 patients had a 59% higher risk of death up to 6 months after infection, compared with noninfected people.
Those findings translate into about 8 extra deaths per 1,000 patients over 6 months, because many deaths caused by long-term COVID complications are not recorded as COVID-19 deaths, the researchers said. Among patients who were hospitalized and died after more than 30 days, there were 29 excess deaths per 1,000 patients over 6 months.
“As far as total pandemic death toll, these numbers suggest that the deaths we’re counting due to the immediate viral infection are only the tip of the iceberg,” Ziyad Al-Aly, MD, the senior author of the study and a director of the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System, said in a news release from the Washington University, St. Louis.
Johns Hopkins University in Baltimore says more than 3 million people worldwide and about 570,000 people in the United States have died of coronavirus-related reasons.
Long-haul COVID patients also had a much higher chance of getting sick, and not just in the respiratory system, according to the study.
The patients had a high rate of stroke and other nervous system ailments, mental health problems such as depression, the onset of diabetes, heart disease and other coronary problems, diarrhea and digestive disorders, kidney disease, blood clots, joint pain, hair loss, and general fatigue.
Patients often had clusters of these ailments. And the more severe the case of COVID-19, the higher the chance of long-term health problems, the study said.
Researchers based their study on health care databases of the U.S. Department of Veterans Affairs. Besides the 87,000 COVID patients, the database included about 5 million patients who didn’t catch COVID. The veterans in the study were about 88% men, but the large sample size included 8,880 women with confirmed cases, the news release said.
Dr. Al-Aly, an assistant professor at Washington University, said the study shows that long-haul COVID-19 could be “America’s next big health crisis.”
“Our study demonstrates that, up to 6 months after diagnosis, the risk of death following even a mild case of COVID-19 is not trivial and increases with disease severity,” he said. “Given that more than 30 million Americans have been infected with this virus, and given that the burden of long COVID-19 is substantial, the lingering effects of this disease will reverberate for many years and even decades.”
A version of this article first appeared on WebMD.com.
Long-haul COVID-19 patients face many health threats – including a higher chance of dying – up to 6 months after they catch the virus, according to a massive study published in the journal Nature.
Researchers examined more than 87,000 COVID-19 patients and nearly 5 million control patients in a federal database. They found COVID-19 patients had a 59% higher risk of death up to 6 months after infection, compared with noninfected people.
Those findings translate into about 8 extra deaths per 1,000 patients over 6 months, because many deaths caused by long-term COVID complications are not recorded as COVID-19 deaths, the researchers said. Among patients who were hospitalized and died after more than 30 days, there were 29 excess deaths per 1,000 patients over 6 months.
“As far as total pandemic death toll, these numbers suggest that the deaths we’re counting due to the immediate viral infection are only the tip of the iceberg,” Ziyad Al-Aly, MD, the senior author of the study and a director of the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System, said in a news release from the Washington University, St. Louis.
Johns Hopkins University in Baltimore says more than 3 million people worldwide and about 570,000 people in the United States have died of coronavirus-related reasons.
Long-haul COVID patients also had a much higher chance of getting sick, and not just in the respiratory system, according to the study.
The patients had a high rate of stroke and other nervous system ailments, mental health problems such as depression, the onset of diabetes, heart disease and other coronary problems, diarrhea and digestive disorders, kidney disease, blood clots, joint pain, hair loss, and general fatigue.
Patients often had clusters of these ailments. And the more severe the case of COVID-19, the higher the chance of long-term health problems, the study said.
Researchers based their study on health care databases of the U.S. Department of Veterans Affairs. Besides the 87,000 COVID patients, the database included about 5 million patients who didn’t catch COVID. The veterans in the study were about 88% men, but the large sample size included 8,880 women with confirmed cases, the news release said.
Dr. Al-Aly, an assistant professor at Washington University, said the study shows that long-haul COVID-19 could be “America’s next big health crisis.”
“Our study demonstrates that, up to 6 months after diagnosis, the risk of death following even a mild case of COVID-19 is not trivial and increases with disease severity,” he said. “Given that more than 30 million Americans have been infected with this virus, and given that the burden of long COVID-19 is substantial, the lingering effects of this disease will reverberate for many years and even decades.”
A version of this article first appeared on WebMD.com.
Long-haul COVID-19 patients face many health threats – including a higher chance of dying – up to 6 months after they catch the virus, according to a massive study published in the journal Nature.
Researchers examined more than 87,000 COVID-19 patients and nearly 5 million control patients in a federal database. They found COVID-19 patients had a 59% higher risk of death up to 6 months after infection, compared with noninfected people.
Those findings translate into about 8 extra deaths per 1,000 patients over 6 months, because many deaths caused by long-term COVID complications are not recorded as COVID-19 deaths, the researchers said. Among patients who were hospitalized and died after more than 30 days, there were 29 excess deaths per 1,000 patients over 6 months.
“As far as total pandemic death toll, these numbers suggest that the deaths we’re counting due to the immediate viral infection are only the tip of the iceberg,” Ziyad Al-Aly, MD, the senior author of the study and a director of the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System, said in a news release from the Washington University, St. Louis.
Johns Hopkins University in Baltimore says more than 3 million people worldwide and about 570,000 people in the United States have died of coronavirus-related reasons.
Long-haul COVID patients also had a much higher chance of getting sick, and not just in the respiratory system, according to the study.
The patients had a high rate of stroke and other nervous system ailments, mental health problems such as depression, the onset of diabetes, heart disease and other coronary problems, diarrhea and digestive disorders, kidney disease, blood clots, joint pain, hair loss, and general fatigue.
Patients often had clusters of these ailments. And the more severe the case of COVID-19, the higher the chance of long-term health problems, the study said.
Researchers based their study on health care databases of the U.S. Department of Veterans Affairs. Besides the 87,000 COVID patients, the database included about 5 million patients who didn’t catch COVID. The veterans in the study were about 88% men, but the large sample size included 8,880 women with confirmed cases, the news release said.
Dr. Al-Aly, an assistant professor at Washington University, said the study shows that long-haul COVID-19 could be “America’s next big health crisis.”
“Our study demonstrates that, up to 6 months after diagnosis, the risk of death following even a mild case of COVID-19 is not trivial and increases with disease severity,” he said. “Given that more than 30 million Americans have been infected with this virus, and given that the burden of long COVID-19 is substantial, the lingering effects of this disease will reverberate for many years and even decades.”
A version of this article first appeared on WebMD.com.
Can we get to ‘COVID zero’? Experts predict the next 8 months
COVID-19 is likely to follow a seasonal pattern – similar to some other respiratory viruses – with fewer cases come summer 2021 followed by a jump next winter, experts predicted in a Thursday briefing.
If that pattern holds, it could mean a need to reinforce the mask-wearing message as the weather gets colder and people once again congregate indoors.
“Right now, we are projecting the United States all the way to Aug. 1 [will have] 619,000 deaths from COVID-19, with 4.7 million globally,” said Ali H. Mokdad, PhD, professor of health metrics sciences at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle, during today’s media briefing sponsored by the Infectious Diseases Society of America and IHME.
The encouraging news is the vaccines appear to be working, and more Americans are getting them. “If you look at the data for these vaccines, they are extremely safe, they are extremely efficacious, and they make you basically impervious – for the most part – to getting serious disease, hospitalization, or death,” said Amesh Adalja, MD, senior scholar at Johns Hopkins University Center for Health Security in Baltimore.
“These vaccines do what they were meant to do: defang this virus,” said Dr. Adalja, who is an IDSA Fellow and adjunct assistant professor at Johns Hopkins Bloomberg School of Public Health. Emerging data out of Israel and other countries suggest a vaccinated person is less likely to transmit the virus as well, he added.
Still aiming for herd immunity
Furthermore, the U.S. Food and Drug Administration is likely to approve emergency use authorization (EUA) among teenagers 12-15 years old “imminently,” thereby expanding the pool of people potentially protected by vaccines.
Such authorization could help with overall public health efforts. “That’s simply a mathematical formula,” Dr. Adalja said. “The more people that are vaccinated, including children, the quicker we’ll get to herd immunity.”
In addition, with lower case numbers expected this summer, herd immunity might become more achievable, said Dr. Mokdad, who is also chief strategy officer for population health at the University of Washington.
As important as herd immunity is, so-called decoupling is “more important to me,” Dr. Adalja said. Decoupling refers to separating infections from the more severe outcomes, so people who get COVID-19 are less likely to need hospitalization or die from it.
Vaccines get the credit here, he added, including with the variants. “Even if you get a breakthrough infection with a variant, it’s not likely to land you in the hospital or cause serious disease or death,” Dr. Adalja said.
Masks and the uncommon cold
Wearing a mask until we reach herd immunity is important because it’s not possible to tell who is vaccinated and who isn’t, Dr. Mokdad said. “Remember, as many people are waiting to get a vaccine, all of us have access to a mask,” he said.
Dr. Adalja agreed, adding that public health guidance on masks will likely stay in place until we cross that herd immunity threshold and community circulation of the virus goes down.
“People are probably going to want to continue wearing masks, at least some proportion, because they see the benefit for other respiratory viruses,” Dr. Adalja said. “How many of you had a common cold this year?”
Variants: Some good news?
Experts are monitoring the spread of variants of concern in the United States and abroad. On a positive note, the B.1.1.7 variant first identified in the United Kingdom appears to be dominant in the United States at this time, which is potentially good for two reasons. One is that the available COVID-19 vaccines show sufficient efficacy against the strain, Dr. Mokdad said.
Second, a predominance of B.1.1.7 makes it more difficult for other emerging variants of concern like P1 [Brazil] or B.1.351 [South Africa] to gain control, Dr. Adalja said.
“B.1.1.7 is such an efficient transmitter,” he said. “That’s kind of an advantage … because the more B.1.1.7, you have the less opportunity B.1.351 and P1 have to set up shop.”
Hesitancy from misinformation
Vaccine hesitancy remains a concern, particularly at a time when some predict a drop in the number of Americans seeking vaccination. Although needle phobia plays a role in dissuading some from vaccination, the bigger issue is vaccine misinformation, Dr. Adalja said.
“Some people are just terrified when they see the needle. That’s a small part of the proportion of people who don’t want to get vaccinated,” Dr. Adalja said. In contrast, he attributed most hesitancy to misinformation about the vaccine, including reports that the vaccines are fake.
Even celebrities are getting drawn into the misinformation.
“I just had to answer something about Mariah Carey’s vaccination,” he said. Someone believed “that it was done with a retractable needle that didn’t really go into her arm.”
Vaccine hesitancy is more about people not understanding the risk-benefit analysis, taking side effects out of out of context if there are side effects, or being influenced by “arbitrary statements about microchips, infertility, or whatever it might be,” Dr. Adalja said.
The future is subject to change
“We’re expecting another rise in cases and more mortality in our winter season here in the United States,” Dr. Mokdad said, adding that the efficacy of the vaccines is likely to attenuate the mortality rate in particular.
However, as the epidemiology of the pandemic evolves, so too will the long-term predictions. Factors that could influence future numbers include the expansion of vaccination to teens 12-15 years old and (eventually) younger children, a need for booster vaccines, emerging variants, and the changing proportion of the population who are fully vaccinated or were previously infected.
Again, getting people to adhere to mask wearing come winter could be challenging if the scenario over the summer is “close to normal with less than 200 deaths a day in the United States,” he added. Asking people to wear masks again will be like “swimming upstream.”
“I think it’s a mistake to think that we’re going to get to ‘COVID zero,’ ” Dr. Adalja said. “This is not an eradicable disease. There’s only been one human infectious disease eradicated from the planet, and that’s smallpox, and it had very different characteristics.”
A version of this article first appeared on Medscape.com.
COVID-19 is likely to follow a seasonal pattern – similar to some other respiratory viruses – with fewer cases come summer 2021 followed by a jump next winter, experts predicted in a Thursday briefing.
If that pattern holds, it could mean a need to reinforce the mask-wearing message as the weather gets colder and people once again congregate indoors.
“Right now, we are projecting the United States all the way to Aug. 1 [will have] 619,000 deaths from COVID-19, with 4.7 million globally,” said Ali H. Mokdad, PhD, professor of health metrics sciences at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle, during today’s media briefing sponsored by the Infectious Diseases Society of America and IHME.
The encouraging news is the vaccines appear to be working, and more Americans are getting them. “If you look at the data for these vaccines, they are extremely safe, they are extremely efficacious, and they make you basically impervious – for the most part – to getting serious disease, hospitalization, or death,” said Amesh Adalja, MD, senior scholar at Johns Hopkins University Center for Health Security in Baltimore.
“These vaccines do what they were meant to do: defang this virus,” said Dr. Adalja, who is an IDSA Fellow and adjunct assistant professor at Johns Hopkins Bloomberg School of Public Health. Emerging data out of Israel and other countries suggest a vaccinated person is less likely to transmit the virus as well, he added.
Still aiming for herd immunity
Furthermore, the U.S. Food and Drug Administration is likely to approve emergency use authorization (EUA) among teenagers 12-15 years old “imminently,” thereby expanding the pool of people potentially protected by vaccines.
Such authorization could help with overall public health efforts. “That’s simply a mathematical formula,” Dr. Adalja said. “The more people that are vaccinated, including children, the quicker we’ll get to herd immunity.”
In addition, with lower case numbers expected this summer, herd immunity might become more achievable, said Dr. Mokdad, who is also chief strategy officer for population health at the University of Washington.
As important as herd immunity is, so-called decoupling is “more important to me,” Dr. Adalja said. Decoupling refers to separating infections from the more severe outcomes, so people who get COVID-19 are less likely to need hospitalization or die from it.
Vaccines get the credit here, he added, including with the variants. “Even if you get a breakthrough infection with a variant, it’s not likely to land you in the hospital or cause serious disease or death,” Dr. Adalja said.
Masks and the uncommon cold
Wearing a mask until we reach herd immunity is important because it’s not possible to tell who is vaccinated and who isn’t, Dr. Mokdad said. “Remember, as many people are waiting to get a vaccine, all of us have access to a mask,” he said.
Dr. Adalja agreed, adding that public health guidance on masks will likely stay in place until we cross that herd immunity threshold and community circulation of the virus goes down.
“People are probably going to want to continue wearing masks, at least some proportion, because they see the benefit for other respiratory viruses,” Dr. Adalja said. “How many of you had a common cold this year?”
Variants: Some good news?
Experts are monitoring the spread of variants of concern in the United States and abroad. On a positive note, the B.1.1.7 variant first identified in the United Kingdom appears to be dominant in the United States at this time, which is potentially good for two reasons. One is that the available COVID-19 vaccines show sufficient efficacy against the strain, Dr. Mokdad said.
Second, a predominance of B.1.1.7 makes it more difficult for other emerging variants of concern like P1 [Brazil] or B.1.351 [South Africa] to gain control, Dr. Adalja said.
“B.1.1.7 is such an efficient transmitter,” he said. “That’s kind of an advantage … because the more B.1.1.7, you have the less opportunity B.1.351 and P1 have to set up shop.”
Hesitancy from misinformation
Vaccine hesitancy remains a concern, particularly at a time when some predict a drop in the number of Americans seeking vaccination. Although needle phobia plays a role in dissuading some from vaccination, the bigger issue is vaccine misinformation, Dr. Adalja said.
“Some people are just terrified when they see the needle. That’s a small part of the proportion of people who don’t want to get vaccinated,” Dr. Adalja said. In contrast, he attributed most hesitancy to misinformation about the vaccine, including reports that the vaccines are fake.
Even celebrities are getting drawn into the misinformation.
“I just had to answer something about Mariah Carey’s vaccination,” he said. Someone believed “that it was done with a retractable needle that didn’t really go into her arm.”
Vaccine hesitancy is more about people not understanding the risk-benefit analysis, taking side effects out of out of context if there are side effects, or being influenced by “arbitrary statements about microchips, infertility, or whatever it might be,” Dr. Adalja said.
The future is subject to change
“We’re expecting another rise in cases and more mortality in our winter season here in the United States,” Dr. Mokdad said, adding that the efficacy of the vaccines is likely to attenuate the mortality rate in particular.
However, as the epidemiology of the pandemic evolves, so too will the long-term predictions. Factors that could influence future numbers include the expansion of vaccination to teens 12-15 years old and (eventually) younger children, a need for booster vaccines, emerging variants, and the changing proportion of the population who are fully vaccinated or were previously infected.
Again, getting people to adhere to mask wearing come winter could be challenging if the scenario over the summer is “close to normal with less than 200 deaths a day in the United States,” he added. Asking people to wear masks again will be like “swimming upstream.”
“I think it’s a mistake to think that we’re going to get to ‘COVID zero,’ ” Dr. Adalja said. “This is not an eradicable disease. There’s only been one human infectious disease eradicated from the planet, and that’s smallpox, and it had very different characteristics.”
A version of this article first appeared on Medscape.com.
COVID-19 is likely to follow a seasonal pattern – similar to some other respiratory viruses – with fewer cases come summer 2021 followed by a jump next winter, experts predicted in a Thursday briefing.
If that pattern holds, it could mean a need to reinforce the mask-wearing message as the weather gets colder and people once again congregate indoors.
“Right now, we are projecting the United States all the way to Aug. 1 [will have] 619,000 deaths from COVID-19, with 4.7 million globally,” said Ali H. Mokdad, PhD, professor of health metrics sciences at the Institute for Health Metrics and Evaluation at the University of Washington, Seattle, during today’s media briefing sponsored by the Infectious Diseases Society of America and IHME.
The encouraging news is the vaccines appear to be working, and more Americans are getting them. “If you look at the data for these vaccines, they are extremely safe, they are extremely efficacious, and they make you basically impervious – for the most part – to getting serious disease, hospitalization, or death,” said Amesh Adalja, MD, senior scholar at Johns Hopkins University Center for Health Security in Baltimore.
“These vaccines do what they were meant to do: defang this virus,” said Dr. Adalja, who is an IDSA Fellow and adjunct assistant professor at Johns Hopkins Bloomberg School of Public Health. Emerging data out of Israel and other countries suggest a vaccinated person is less likely to transmit the virus as well, he added.
Still aiming for herd immunity
Furthermore, the U.S. Food and Drug Administration is likely to approve emergency use authorization (EUA) among teenagers 12-15 years old “imminently,” thereby expanding the pool of people potentially protected by vaccines.
Such authorization could help with overall public health efforts. “That’s simply a mathematical formula,” Dr. Adalja said. “The more people that are vaccinated, including children, the quicker we’ll get to herd immunity.”
In addition, with lower case numbers expected this summer, herd immunity might become more achievable, said Dr. Mokdad, who is also chief strategy officer for population health at the University of Washington.
As important as herd immunity is, so-called decoupling is “more important to me,” Dr. Adalja said. Decoupling refers to separating infections from the more severe outcomes, so people who get COVID-19 are less likely to need hospitalization or die from it.
Vaccines get the credit here, he added, including with the variants. “Even if you get a breakthrough infection with a variant, it’s not likely to land you in the hospital or cause serious disease or death,” Dr. Adalja said.
Masks and the uncommon cold
Wearing a mask until we reach herd immunity is important because it’s not possible to tell who is vaccinated and who isn’t, Dr. Mokdad said. “Remember, as many people are waiting to get a vaccine, all of us have access to a mask,” he said.
Dr. Adalja agreed, adding that public health guidance on masks will likely stay in place until we cross that herd immunity threshold and community circulation of the virus goes down.
“People are probably going to want to continue wearing masks, at least some proportion, because they see the benefit for other respiratory viruses,” Dr. Adalja said. “How many of you had a common cold this year?”
Variants: Some good news?
Experts are monitoring the spread of variants of concern in the United States and abroad. On a positive note, the B.1.1.7 variant first identified in the United Kingdom appears to be dominant in the United States at this time, which is potentially good for two reasons. One is that the available COVID-19 vaccines show sufficient efficacy against the strain, Dr. Mokdad said.
Second, a predominance of B.1.1.7 makes it more difficult for other emerging variants of concern like P1 [Brazil] or B.1.351 [South Africa] to gain control, Dr. Adalja said.
“B.1.1.7 is such an efficient transmitter,” he said. “That’s kind of an advantage … because the more B.1.1.7, you have the less opportunity B.1.351 and P1 have to set up shop.”
Hesitancy from misinformation
Vaccine hesitancy remains a concern, particularly at a time when some predict a drop in the number of Americans seeking vaccination. Although needle phobia plays a role in dissuading some from vaccination, the bigger issue is vaccine misinformation, Dr. Adalja said.
“Some people are just terrified when they see the needle. That’s a small part of the proportion of people who don’t want to get vaccinated,” Dr. Adalja said. In contrast, he attributed most hesitancy to misinformation about the vaccine, including reports that the vaccines are fake.
Even celebrities are getting drawn into the misinformation.
“I just had to answer something about Mariah Carey’s vaccination,” he said. Someone believed “that it was done with a retractable needle that didn’t really go into her arm.”
Vaccine hesitancy is more about people not understanding the risk-benefit analysis, taking side effects out of out of context if there are side effects, or being influenced by “arbitrary statements about microchips, infertility, or whatever it might be,” Dr. Adalja said.
The future is subject to change
“We’re expecting another rise in cases and more mortality in our winter season here in the United States,” Dr. Mokdad said, adding that the efficacy of the vaccines is likely to attenuate the mortality rate in particular.
However, as the epidemiology of the pandemic evolves, so too will the long-term predictions. Factors that could influence future numbers include the expansion of vaccination to teens 12-15 years old and (eventually) younger children, a need for booster vaccines, emerging variants, and the changing proportion of the population who are fully vaccinated or were previously infected.
Again, getting people to adhere to mask wearing come winter could be challenging if the scenario over the summer is “close to normal with less than 200 deaths a day in the United States,” he added. Asking people to wear masks again will be like “swimming upstream.”
“I think it’s a mistake to think that we’re going to get to ‘COVID zero,’ ” Dr. Adalja said. “This is not an eradicable disease. There’s only been one human infectious disease eradicated from the planet, and that’s smallpox, and it had very different characteristics.”
A version of this article first appeared on Medscape.com.
Percentage of doctors who are Black barely changed in 120 years
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 infection conveys imperfect immunity in young adults
Do your patients think that getting COVID-19 is fully protective against subsequent reinfection? Tell it to the Marines.
A study of U.S. Marine recruits on their way to boot camp at Parris Island, S.C., showed that those who were seropositive at baseline, indicating prior exposure to SARS-CoV-2, remained at some risk for reinfection. They had about one-fifth the risk of subsequent infection, compared with seronegative recruits during basic training, but reinfections did occur.
The study, by Stuart C. Sealfon, MD, of Icahn School of Medicine at Mount Sinai in New York, and colleagues, was published in The Lancet Respiratory Medicine.
“Although antibodies induced by initial infection are largely protective, they do not guarantee effective SARS-CoV-2 neutralization activity or immunity against subsequent infection,” they wrote.
An infectious disease specialist who was not involved in the study said that the findings provide further evidence about the level of immunity acquired after an infection.
“It’s quite clear that reinfections do occur, they are of public health importance, and they’re something we need to be mindful of in terms of advising patients about whether a prior infection protects them from reinfection,” Mark Siedner, MD, MPH, a clinician and researcher in the division of infectious diseases at Massachusetts General Hospital, Boston, said in an interview.
The study results reinforce that “not all antibodies are the same,” said Sachin Gupta, MD, an attending physician in pulmonary and critical care medicine at Alameda Health System in Oakland, Calif. “We’re seeing still that 10% of folks who have antibodies can get infected again,” he said in an interview.
CHARM initiative
Dr. Sealfon and colleagues presented an analysis of data from the ironically named CHARM (COVID-19 Health Action Response for Marines) prospective study.
CHARM included U.S. Marine recruits, most of them male, aged 18-20 years, who were instructed to follow a 2-week unsupervised quarantine at home, after which they reported to a Marine-supervised facility for an additional 2-week quarantine.
At baseline, participants were tested for SARS-CoV-2 immunoglobulin G (IgG) seropositivity, defined as a dilution of 1:150 or more on receptor-binding domain and full-length spike protein enzyme-linked immunosorbent assay (ELISA).
The recruits filled out questionnaires asking them to report any of 14 specific COVID-19–related symptoms or any other unspecified symptom, as well as demographic information, risk factors, and a brief medical history.
Investigators tested recruits for SARS-CoV-2 infection by polymerase chain reaction (PCR) assay at weeks 0, 1, and 2 of quarantine, and any who had positive PCR results during quarantine were excluded.
Participants who had three negative swab PCR results during quarantine and a baseline serology test at the beginning of the supervised quarantine period – either seronegative or seropositive – then went on to enjoy their basic training at the Marine Corps Recruit Depot, Parris Island, S.C.
The participants were followed prospectively with PCR tests at weeks 2, 4, and 6 in both the seropositive and seronegative groups, and sera were obtained at the same time.
Holes in immunologic armor
Full data were available for a total of 189 participants who were seropositive and 2,247 who were seronegative at enrollment.
In all, 19 of 189 seropositive recruits (10%) had at least one PCR test positive for SARS-CoV-2 infection during the 6-week follow-up period. This translated into an incidence of 1.1 cases per person-year.
Of the 2,247 participants seronegative at baseline, 1,079 tested positive (6.2 cases per person-year; incidence rate ratio 0.18).
It appeared that antibodies provided some protection for seropositive recruits, as evidenced by a higher likelihood of infection among those with lower baseline full-length spike protein IgG titers than in those with higher baseline titers (hazard ratio 0.4, P < .001).
Among the seropositive participants who did acquire a second SARS-CoV-2 infection, viral loads in mid-turbinate nasal swabs were about 10-fold lower than in seronegative recruits who acquired infections during follow-up.
“This finding suggests that some reinfected individuals could have a similar capacity to transmit infection as those who are infected for the first time. The rate at which reinfection occurs after vaccines and natural immunity is important for estimating the proportion of the population that needs to be vaccinated to suppress the pandemic,” the investigators wrote.
Baseline neutralizing antibody titers were detected in 45 of the first 54 seropositive recruits who remained PCR negative throughout follow-up, but also in 6 of 19 seropositive participants who became infected during the 6 weeks of observation.
Lessons
Both Dr. Siedner and Dr. Gupta agreed with the authors that the risks for reinfection that were observed in young, physically fit people may differ for other populations, such as women (only 10% of seropositive recruits and 8% of seronegative recruits were female), older patients, or those who are immunocompromised.
Given that the adjusted odds ratio for reinfection in this study was nearly identical to that of a recent British study comparing infection rates between seropositive and seronegative health care workers, the risk of reinfection for other young adults and for the general population may be similar, Dr. Sealfon and colleagues wrote.
Adding to the challenge of reaching herd immunity is the observation that some patients who have recovered from COVID-19 are skeptical about the need for further protection.
“There are patients who feel like vaccination is of low benefit to them, and I think these are the same people who would be hesitant to get the vaccine anyway,” Dr. Gupta said.
Although no vaccine is perfect – the vaccine failure rate from the mRNA-based vaccines from Moderna and Pfizer/Biontech is about 5% – the protections they afford are unmistakable, Dr. Siedner said.
“I think it’s important to make the distinction that most postvaccination infections by and large have been very mild,” he said. “In people with normal immune systems, we have not seen an onslaught of postvaccination infections requiring hospitalization. Even if people do get infected after vaccination, the vaccines protect people from severe infection, and that’s what we want them to do.”
The investigators stated, “Young adults, of whom a high proportion are asymptomatically infected and become seropositive in the absence of known infection, can be an important source of transmission to more vulnerable populations. Evaluating the protection against subsequent SARS-CoV-2 infection conferred by seropositivity in young adults is important for determining the need for vaccinating previously infected individuals in this age group.”
The study was funded by the Defense Health Agency and Defense Advanced Research Projects Agency. Dr. Sealfon, Dr. Siedner, and Dr. Gupta have no conflicts of interest to report. Dr. Gupta is a member of the editorial advisory board for this publication.
Do your patients think that getting COVID-19 is fully protective against subsequent reinfection? Tell it to the Marines.
A study of U.S. Marine recruits on their way to boot camp at Parris Island, S.C., showed that those who were seropositive at baseline, indicating prior exposure to SARS-CoV-2, remained at some risk for reinfection. They had about one-fifth the risk of subsequent infection, compared with seronegative recruits during basic training, but reinfections did occur.
The study, by Stuart C. Sealfon, MD, of Icahn School of Medicine at Mount Sinai in New York, and colleagues, was published in The Lancet Respiratory Medicine.
“Although antibodies induced by initial infection are largely protective, they do not guarantee effective SARS-CoV-2 neutralization activity or immunity against subsequent infection,” they wrote.
An infectious disease specialist who was not involved in the study said that the findings provide further evidence about the level of immunity acquired after an infection.
“It’s quite clear that reinfections do occur, they are of public health importance, and they’re something we need to be mindful of in terms of advising patients about whether a prior infection protects them from reinfection,” Mark Siedner, MD, MPH, a clinician and researcher in the division of infectious diseases at Massachusetts General Hospital, Boston, said in an interview.
The study results reinforce that “not all antibodies are the same,” said Sachin Gupta, MD, an attending physician in pulmonary and critical care medicine at Alameda Health System in Oakland, Calif. “We’re seeing still that 10% of folks who have antibodies can get infected again,” he said in an interview.
CHARM initiative
Dr. Sealfon and colleagues presented an analysis of data from the ironically named CHARM (COVID-19 Health Action Response for Marines) prospective study.
CHARM included U.S. Marine recruits, most of them male, aged 18-20 years, who were instructed to follow a 2-week unsupervised quarantine at home, after which they reported to a Marine-supervised facility for an additional 2-week quarantine.
At baseline, participants were tested for SARS-CoV-2 immunoglobulin G (IgG) seropositivity, defined as a dilution of 1:150 or more on receptor-binding domain and full-length spike protein enzyme-linked immunosorbent assay (ELISA).
The recruits filled out questionnaires asking them to report any of 14 specific COVID-19–related symptoms or any other unspecified symptom, as well as demographic information, risk factors, and a brief medical history.
Investigators tested recruits for SARS-CoV-2 infection by polymerase chain reaction (PCR) assay at weeks 0, 1, and 2 of quarantine, and any who had positive PCR results during quarantine were excluded.
Participants who had three negative swab PCR results during quarantine and a baseline serology test at the beginning of the supervised quarantine period – either seronegative or seropositive – then went on to enjoy their basic training at the Marine Corps Recruit Depot, Parris Island, S.C.
The participants were followed prospectively with PCR tests at weeks 2, 4, and 6 in both the seropositive and seronegative groups, and sera were obtained at the same time.
Holes in immunologic armor
Full data were available for a total of 189 participants who were seropositive and 2,247 who were seronegative at enrollment.
In all, 19 of 189 seropositive recruits (10%) had at least one PCR test positive for SARS-CoV-2 infection during the 6-week follow-up period. This translated into an incidence of 1.1 cases per person-year.
Of the 2,247 participants seronegative at baseline, 1,079 tested positive (6.2 cases per person-year; incidence rate ratio 0.18).
It appeared that antibodies provided some protection for seropositive recruits, as evidenced by a higher likelihood of infection among those with lower baseline full-length spike protein IgG titers than in those with higher baseline titers (hazard ratio 0.4, P < .001).
Among the seropositive participants who did acquire a second SARS-CoV-2 infection, viral loads in mid-turbinate nasal swabs were about 10-fold lower than in seronegative recruits who acquired infections during follow-up.
“This finding suggests that some reinfected individuals could have a similar capacity to transmit infection as those who are infected for the first time. The rate at which reinfection occurs after vaccines and natural immunity is important for estimating the proportion of the population that needs to be vaccinated to suppress the pandemic,” the investigators wrote.
Baseline neutralizing antibody titers were detected in 45 of the first 54 seropositive recruits who remained PCR negative throughout follow-up, but also in 6 of 19 seropositive participants who became infected during the 6 weeks of observation.
Lessons
Both Dr. Siedner and Dr. Gupta agreed with the authors that the risks for reinfection that were observed in young, physically fit people may differ for other populations, such as women (only 10% of seropositive recruits and 8% of seronegative recruits were female), older patients, or those who are immunocompromised.
Given that the adjusted odds ratio for reinfection in this study was nearly identical to that of a recent British study comparing infection rates between seropositive and seronegative health care workers, the risk of reinfection for other young adults and for the general population may be similar, Dr. Sealfon and colleagues wrote.
Adding to the challenge of reaching herd immunity is the observation that some patients who have recovered from COVID-19 are skeptical about the need for further protection.
“There are patients who feel like vaccination is of low benefit to them, and I think these are the same people who would be hesitant to get the vaccine anyway,” Dr. Gupta said.
Although no vaccine is perfect – the vaccine failure rate from the mRNA-based vaccines from Moderna and Pfizer/Biontech is about 5% – the protections they afford are unmistakable, Dr. Siedner said.
“I think it’s important to make the distinction that most postvaccination infections by and large have been very mild,” he said. “In people with normal immune systems, we have not seen an onslaught of postvaccination infections requiring hospitalization. Even if people do get infected after vaccination, the vaccines protect people from severe infection, and that’s what we want them to do.”
The investigators stated, “Young adults, of whom a high proportion are asymptomatically infected and become seropositive in the absence of known infection, can be an important source of transmission to more vulnerable populations. Evaluating the protection against subsequent SARS-CoV-2 infection conferred by seropositivity in young adults is important for determining the need for vaccinating previously infected individuals in this age group.”
The study was funded by the Defense Health Agency and Defense Advanced Research Projects Agency. Dr. Sealfon, Dr. Siedner, and Dr. Gupta have no conflicts of interest to report. Dr. Gupta is a member of the editorial advisory board for this publication.
Do your patients think that getting COVID-19 is fully protective against subsequent reinfection? Tell it to the Marines.
A study of U.S. Marine recruits on their way to boot camp at Parris Island, S.C., showed that those who were seropositive at baseline, indicating prior exposure to SARS-CoV-2, remained at some risk for reinfection. They had about one-fifth the risk of subsequent infection, compared with seronegative recruits during basic training, but reinfections did occur.
The study, by Stuart C. Sealfon, MD, of Icahn School of Medicine at Mount Sinai in New York, and colleagues, was published in The Lancet Respiratory Medicine.
“Although antibodies induced by initial infection are largely protective, they do not guarantee effective SARS-CoV-2 neutralization activity or immunity against subsequent infection,” they wrote.
An infectious disease specialist who was not involved in the study said that the findings provide further evidence about the level of immunity acquired after an infection.
“It’s quite clear that reinfections do occur, they are of public health importance, and they’re something we need to be mindful of in terms of advising patients about whether a prior infection protects them from reinfection,” Mark Siedner, MD, MPH, a clinician and researcher in the division of infectious diseases at Massachusetts General Hospital, Boston, said in an interview.
The study results reinforce that “not all antibodies are the same,” said Sachin Gupta, MD, an attending physician in pulmonary and critical care medicine at Alameda Health System in Oakland, Calif. “We’re seeing still that 10% of folks who have antibodies can get infected again,” he said in an interview.
CHARM initiative
Dr. Sealfon and colleagues presented an analysis of data from the ironically named CHARM (COVID-19 Health Action Response for Marines) prospective study.
CHARM included U.S. Marine recruits, most of them male, aged 18-20 years, who were instructed to follow a 2-week unsupervised quarantine at home, after which they reported to a Marine-supervised facility for an additional 2-week quarantine.
At baseline, participants were tested for SARS-CoV-2 immunoglobulin G (IgG) seropositivity, defined as a dilution of 1:150 or more on receptor-binding domain and full-length spike protein enzyme-linked immunosorbent assay (ELISA).
The recruits filled out questionnaires asking them to report any of 14 specific COVID-19–related symptoms or any other unspecified symptom, as well as demographic information, risk factors, and a brief medical history.
Investigators tested recruits for SARS-CoV-2 infection by polymerase chain reaction (PCR) assay at weeks 0, 1, and 2 of quarantine, and any who had positive PCR results during quarantine were excluded.
Participants who had three negative swab PCR results during quarantine and a baseline serology test at the beginning of the supervised quarantine period – either seronegative or seropositive – then went on to enjoy their basic training at the Marine Corps Recruit Depot, Parris Island, S.C.
The participants were followed prospectively with PCR tests at weeks 2, 4, and 6 in both the seropositive and seronegative groups, and sera were obtained at the same time.
Holes in immunologic armor
Full data were available for a total of 189 participants who were seropositive and 2,247 who were seronegative at enrollment.
In all, 19 of 189 seropositive recruits (10%) had at least one PCR test positive for SARS-CoV-2 infection during the 6-week follow-up period. This translated into an incidence of 1.1 cases per person-year.
Of the 2,247 participants seronegative at baseline, 1,079 tested positive (6.2 cases per person-year; incidence rate ratio 0.18).
It appeared that antibodies provided some protection for seropositive recruits, as evidenced by a higher likelihood of infection among those with lower baseline full-length spike protein IgG titers than in those with higher baseline titers (hazard ratio 0.4, P < .001).
Among the seropositive participants who did acquire a second SARS-CoV-2 infection, viral loads in mid-turbinate nasal swabs were about 10-fold lower than in seronegative recruits who acquired infections during follow-up.
“This finding suggests that some reinfected individuals could have a similar capacity to transmit infection as those who are infected for the first time. The rate at which reinfection occurs after vaccines and natural immunity is important for estimating the proportion of the population that needs to be vaccinated to suppress the pandemic,” the investigators wrote.
Baseline neutralizing antibody titers were detected in 45 of the first 54 seropositive recruits who remained PCR negative throughout follow-up, but also in 6 of 19 seropositive participants who became infected during the 6 weeks of observation.
Lessons
Both Dr. Siedner and Dr. Gupta agreed with the authors that the risks for reinfection that were observed in young, physically fit people may differ for other populations, such as women (only 10% of seropositive recruits and 8% of seronegative recruits were female), older patients, or those who are immunocompromised.
Given that the adjusted odds ratio for reinfection in this study was nearly identical to that of a recent British study comparing infection rates between seropositive and seronegative health care workers, the risk of reinfection for other young adults and for the general population may be similar, Dr. Sealfon and colleagues wrote.
Adding to the challenge of reaching herd immunity is the observation that some patients who have recovered from COVID-19 are skeptical about the need for further protection.
“There are patients who feel like vaccination is of low benefit to them, and I think these are the same people who would be hesitant to get the vaccine anyway,” Dr. Gupta said.
Although no vaccine is perfect – the vaccine failure rate from the mRNA-based vaccines from Moderna and Pfizer/Biontech is about 5% – the protections they afford are unmistakable, Dr. Siedner said.
“I think it’s important to make the distinction that most postvaccination infections by and large have been very mild,” he said. “In people with normal immune systems, we have not seen an onslaught of postvaccination infections requiring hospitalization. Even if people do get infected after vaccination, the vaccines protect people from severe infection, and that’s what we want them to do.”
The investigators stated, “Young adults, of whom a high proportion are asymptomatically infected and become seropositive in the absence of known infection, can be an important source of transmission to more vulnerable populations. Evaluating the protection against subsequent SARS-CoV-2 infection conferred by seropositivity in young adults is important for determining the need for vaccinating previously infected individuals in this age group.”
The study was funded by the Defense Health Agency and Defense Advanced Research Projects Agency. Dr. Sealfon, Dr. Siedner, and Dr. Gupta have no conflicts of interest to report. Dr. Gupta is a member of the editorial advisory board for this publication.
FROM THE LANCET RESPIRATORY MEDICINE
Mitochondrial DNA predicts survival in pediatric acute myeloid leukemia
Mitochondrial DNA (mtDNA) copy number alterations are known to occur in acute myeloid leukemia (AML), however their biological significance has not been well studied. Pediatric AML has a distinct biology, different from adults, and with heterogeneous clinical outcomes, the biological basis of which are not well understood, according to researchers Shilpi Chaudhary, PhD, of the All India Institute of Medical Sciences, New Delhi, and colleagues.
Their analysis of 123 pediatric patients with AML found that mtDNA copy number was an independent predictor of aggressive disease, lower event-free survival, and overall survival, according to a report published in Mitochondrion.
In an attempt to find the biological factors involved in the increased mtDNA copy numbers and their effect on the development and aggressiveness of pediatric AML, the researchers studied the regulation and significance of mtDNA copy number in pediatric AML patients using quantitative real time–polymerase chain reaction, as well as in vitro studies. For patients, results were correlated with clinical outcomes.
Mitochondrial biogenesis genes (TFAM, POLG, POLRMT) and two regulator of mitochondrial biogenesis, MYC and PGC1A, were also assessed, according to Dr. Chaudhary and colleagues.
Predictive results
MtDNA copy number was significantly higher in patients, compared with controls (P < .001) and was found to be an independent predictor of aggressive disease (P = .006), lower event-free survival (P = .033), and overall survival (P = .007).
TFAM, POLG & POLRMT and ND3 were also found to be significantly up-regulated in patients, compared with controls as was the expression of the mitochondrial biogenesis regulator MYC (P < .001). However, correlation analysis showed that mtDNA copy number was not associated with the expression of these genes.
In contrast, PGC1A expression was not significantly different in patients, compared with controls overall, although there was a subset of patients whose PGC1A expression was extremely high, according to the researchers.
Importantly, however, in the subset of patients with high PGC1A expression (n = 28), mtDNA copy number had a positive correlation with PGC1A expression (P = .013). On the other hand, among patients with low MYC expression (n = 27), there was no correlation of mtDNA copy number with either PGC1A or MYC expression.
These results were corroborated in in vitro studies, where treatment with the inhibitor tigecycline led to a significant decrease in expression of MYC (P < .001), TFAM (P = .037) and ND3 (P = .010) but resulted in no significant change in mtDNA copy number (P = .23) or expression of PGC1A (P = .10).
Therapeutic candidate?
In contrast to the case of MYC, in vitro PGC1A inhibition significantly reduced mtDNA copy number in along with expression of TFAM and even expression of POLG and POLRMT at higher concentration.
“This observation is in line with our finding in patient samples as well that PGC1A expression positively correlated with mtDNA copy number, more so in patients with higher PGC1A expression,” the researchers stated.
“This makes it plausible to infer that PGC1A may have a possible role in enhancing mtDNA copy number in AML patients, likely independent of MYC,” they added. “Therefore, a strategy of designing therapeutics using already approved inhibitors targeting PGC1A may be an exciting area of therapeutic intervention.”
The authors reported that they have no competing financial conflicts of interests.
Mitochondrial DNA (mtDNA) copy number alterations are known to occur in acute myeloid leukemia (AML), however their biological significance has not been well studied. Pediatric AML has a distinct biology, different from adults, and with heterogeneous clinical outcomes, the biological basis of which are not well understood, according to researchers Shilpi Chaudhary, PhD, of the All India Institute of Medical Sciences, New Delhi, and colleagues.
Their analysis of 123 pediatric patients with AML found that mtDNA copy number was an independent predictor of aggressive disease, lower event-free survival, and overall survival, according to a report published in Mitochondrion.
In an attempt to find the biological factors involved in the increased mtDNA copy numbers and their effect on the development and aggressiveness of pediatric AML, the researchers studied the regulation and significance of mtDNA copy number in pediatric AML patients using quantitative real time–polymerase chain reaction, as well as in vitro studies. For patients, results were correlated with clinical outcomes.
Mitochondrial biogenesis genes (TFAM, POLG, POLRMT) and two regulator of mitochondrial biogenesis, MYC and PGC1A, were also assessed, according to Dr. Chaudhary and colleagues.
Predictive results
MtDNA copy number was significantly higher in patients, compared with controls (P < .001) and was found to be an independent predictor of aggressive disease (P = .006), lower event-free survival (P = .033), and overall survival (P = .007).
TFAM, POLG & POLRMT and ND3 were also found to be significantly up-regulated in patients, compared with controls as was the expression of the mitochondrial biogenesis regulator MYC (P < .001). However, correlation analysis showed that mtDNA copy number was not associated with the expression of these genes.
In contrast, PGC1A expression was not significantly different in patients, compared with controls overall, although there was a subset of patients whose PGC1A expression was extremely high, according to the researchers.
Importantly, however, in the subset of patients with high PGC1A expression (n = 28), mtDNA copy number had a positive correlation with PGC1A expression (P = .013). On the other hand, among patients with low MYC expression (n = 27), there was no correlation of mtDNA copy number with either PGC1A or MYC expression.
These results were corroborated in in vitro studies, where treatment with the inhibitor tigecycline led to a significant decrease in expression of MYC (P < .001), TFAM (P = .037) and ND3 (P = .010) but resulted in no significant change in mtDNA copy number (P = .23) or expression of PGC1A (P = .10).
Therapeutic candidate?
In contrast to the case of MYC, in vitro PGC1A inhibition significantly reduced mtDNA copy number in along with expression of TFAM and even expression of POLG and POLRMT at higher concentration.
“This observation is in line with our finding in patient samples as well that PGC1A expression positively correlated with mtDNA copy number, more so in patients with higher PGC1A expression,” the researchers stated.
“This makes it plausible to infer that PGC1A may have a possible role in enhancing mtDNA copy number in AML patients, likely independent of MYC,” they added. “Therefore, a strategy of designing therapeutics using already approved inhibitors targeting PGC1A may be an exciting area of therapeutic intervention.”
The authors reported that they have no competing financial conflicts of interests.
Mitochondrial DNA (mtDNA) copy number alterations are known to occur in acute myeloid leukemia (AML), however their biological significance has not been well studied. Pediatric AML has a distinct biology, different from adults, and with heterogeneous clinical outcomes, the biological basis of which are not well understood, according to researchers Shilpi Chaudhary, PhD, of the All India Institute of Medical Sciences, New Delhi, and colleagues.
Their analysis of 123 pediatric patients with AML found that mtDNA copy number was an independent predictor of aggressive disease, lower event-free survival, and overall survival, according to a report published in Mitochondrion.
In an attempt to find the biological factors involved in the increased mtDNA copy numbers and their effect on the development and aggressiveness of pediatric AML, the researchers studied the regulation and significance of mtDNA copy number in pediatric AML patients using quantitative real time–polymerase chain reaction, as well as in vitro studies. For patients, results were correlated with clinical outcomes.
Mitochondrial biogenesis genes (TFAM, POLG, POLRMT) and two regulator of mitochondrial biogenesis, MYC and PGC1A, were also assessed, according to Dr. Chaudhary and colleagues.
Predictive results
MtDNA copy number was significantly higher in patients, compared with controls (P < .001) and was found to be an independent predictor of aggressive disease (P = .006), lower event-free survival (P = .033), and overall survival (P = .007).
TFAM, POLG & POLRMT and ND3 were also found to be significantly up-regulated in patients, compared with controls as was the expression of the mitochondrial biogenesis regulator MYC (P < .001). However, correlation analysis showed that mtDNA copy number was not associated with the expression of these genes.
In contrast, PGC1A expression was not significantly different in patients, compared with controls overall, although there was a subset of patients whose PGC1A expression was extremely high, according to the researchers.
Importantly, however, in the subset of patients with high PGC1A expression (n = 28), mtDNA copy number had a positive correlation with PGC1A expression (P = .013). On the other hand, among patients with low MYC expression (n = 27), there was no correlation of mtDNA copy number with either PGC1A or MYC expression.
These results were corroborated in in vitro studies, where treatment with the inhibitor tigecycline led to a significant decrease in expression of MYC (P < .001), TFAM (P = .037) and ND3 (P = .010) but resulted in no significant change in mtDNA copy number (P = .23) or expression of PGC1A (P = .10).
Therapeutic candidate?
In contrast to the case of MYC, in vitro PGC1A inhibition significantly reduced mtDNA copy number in along with expression of TFAM and even expression of POLG and POLRMT at higher concentration.
“This observation is in line with our finding in patient samples as well that PGC1A expression positively correlated with mtDNA copy number, more so in patients with higher PGC1A expression,” the researchers stated.
“This makes it plausible to infer that PGC1A may have a possible role in enhancing mtDNA copy number in AML patients, likely independent of MYC,” they added. “Therefore, a strategy of designing therapeutics using already approved inhibitors targeting PGC1A may be an exciting area of therapeutic intervention.”
The authors reported that they have no competing financial conflicts of interests.
FROM MITOCHONDRION
Vaccinating homebound patients is an uphill battle

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.
“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.

“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.

There are about 2 million to 4 million homebound patients in the United States, according to a webinar from The Trust for America’s Health, which was broadcast in March. But many of these individuals have not been vaccinated yet because of logistical challenges.
Some homebound COVID-19 immunization programs are administering Moderna and Pfizer vaccines to their patients, but many state, city, and local programs administered the Johnson & Johnson vaccine after it was cleared for use by the Food and Drug Administration in February 2021. The efficacy of the one-shot vaccine, as well as it being easier to store and ship than the Moderna and Pfizer vaccines, makes getting it to homebound patients less challenging.
“With Pfizer and Moderna, transportation is a challenge because the temperature demands and the fragility of [messenger] RNA–based vaccines,” Brent Feorene, executive director of the American Academy of Home Care Medicine, said in an interview. That’s why [the Johnson & Johnson] vaccine held such promise – it’s less fragile, [can be stored in] higher temperatures, and was a one shot.”
Other hurdles to getting homebound patients vaccinated had already been in place prior to the 10-day-pause on using the J&J vaccine that occurred for federal agencies to consider possible serious side effects linked to it.
Many roadblocks to vaccination
Although many homebound patients can’t readily go out into the community and be exposed to the COVID-19 virus themselves, they are dependent on caregivers and family members who do go out into the community.
“Their friends, family, neighbors, home health aides, and other kinds of health care workers come into the home,” said Shawn Amer, clinical program director at Central Ohio Primary Care in Columbus.
Nurses from Ms. Amer’s practice vaccinated approximately ten homebound patients with the J&J vaccine through a pilot program in March. Then on April 24, nurses from Central Ohio Primary Care vaccinated just under 40 homebound patients and about a handful of their caregivers who were not able to get their vaccines elsewhere, according to Ms. Amer. This time they used the Pfizer vaccine and will be returning to these patients’ homes on May 15 to administer the second dose.

“Any time you are getting in the car and adding miles, it adds complexity,” Ms. Amer said.
“We called patients 24 to 36 hours before coming to their homes to make sure they were ready, but we learned that just because the healthcare power of attorney agrees to a patient getting vaccinated does not mean that patient will be willing to get the vaccine when the nurse shows up," she noted.
Ms. Amer elaborated that three patients with dementia refused the vaccine when nurses arrived at their home on April 24.
“We had to pivot and find other people,” Ms. Amer. Her practice ended up having to waste one shot.
Expenses are greater
The higher costs of getting homebound patients vaccinated is an additional hurdle to getting these vulnerable individuals protected by COVID-19 shots.
Vaccinating patients in their homes “doesn’t require a lot of technology, but it does require a lot of time” and the staffing expense becomes part of the challenge, Ms. Amer noted.
For each of the two days that Central Ohio Primary Care provides the Pfizer vaccine to homebound patients, the practice needs to pay seven nurses to administer the vaccine, Ms. Amer explained.
There have also been reports of organizations that administer the vaccines – which are free for patients because the federal government is paying for them – not being paid enough by Medicare to cover staff time and efforts to vaccinate patients in their homes, Kaiser Health News reported. According to the Centers for Medicare & Medicaid Services, they pay $40 for the administration of a single-dose COVID-19 vaccine and, for COVID-19 vaccines requiring multiple doses, Medicare pays approximately $40 for each dose in the series. These rates were implemented after March 15. Before that date, the rates were even lower, with the Medicare reimbursement rates for initial doses of COVID-19 vaccines being $16.94 and final doses being $28.39.
William Dombi, president of the National Association for Home Care & Hospice, told Kaiser Health News that the actual cost of these homebound visits are closer to $150 or $160.
“The reimbursement for the injection is pretty minimal,” Mr. Feorene said. “So unless you’re a larger organization and able to have staff to deploy some of your smaller practices, just couldn’t afford to do it.”
Many homebound patients have also been unable to get the lifesaving shots because of logistical roadblocks and many practices not being able to do home visits.
“I think that initially when the [Centers for Disease Control and Prevention] came out with vaccine guidance for medical providers, they offered no guidance for in-home medical providers and we had to go back and ask for that, which they did produce,” Mr. Feorene said. “And we’re grateful for that. But I think just this general understanding that there is a population of folks that are [limited to their home], that they do receive medical care and other care in the home, and that we have to remember that the medical providers who provide care in the home are also primary care providers.”
Furthermore, trying to navigate or find programs delivering vaccines to the homebound can be difficult depending on where a patient lives.
While some programs have been launched on the country or city level – the New York Fire Department launched a pilot program to bring the Johnson & Johnson vaccine to homebound seniors – other programs have been spearheaded by hospital networks like Northwell and Mount Sinai. However, many of these hospital networks only reach out to people who already have a relationship with the hospital.
Ms Amer said identifying homebound patients and reaching out to them can be tough and can contribute to the logistics and time involved in setting patients up for the vaccine.
“Reaching some of these patients is difficult,” Ms. Amer noted. “Sometimes the best way to reach them or get a hold of them is through their caregiver. And so do you have the right phone number? Do you have the right name?”
Overcoming the challenges
With the absence of a national plan targeting homebound patients, many local initiatives were launched to help these individuals get vaccinated. Local fire department paramedics have gone door to door to administer the COVID-19 vaccine in cities like Chicago, New York, and Miami. The suspension of the Johnson & Johnson vaccine resulted in the suspension of in-home vaccinations for some people in New York City. However, the program resumed after the FDA and CDC lifted the pause on April 24.

Health systems like Mount Sinai vaccinated approximately 530 people through the Mount Sinai Visiting Doctors Program, including patients and their caregivers, according to Peter Gliatto, MD, associate director of the Mount Sinai Visiting Doctors Program.
“In different cities, townships, and jurisdictions, different health departments and different provider groups are approaching [the distribution of the COVID-19 vaccine] slightly differently,” Ms. Amer said. So a lot of the decisions surrounding the distribution of shots are local or dependent on local resourcing.
People who live in rural areas present a unique challenge, but Mr. Feorene said reaching out to local emergency medical services or the local health departments can provide some insight on what their town is doing to vaccinate homebound patients.
“I think understanding what a [public health department] is doing would be the very first place to start,” Mr. Feorene said in an interview.
If a patient is bedridden and is mobile enough to sit in a car, Mr. Feorene also recommends finding out if there are vaccine fairs “within a reasonable driving distance.”
Ms. Amer said continuing this mission of getting homebound patients vaccinated is necessary for public health.
“Even if it’s going to take longer to vaccinate these homebound patients, we still have to make an effort. So much of the country’s vaccine efforts have been focused on getting as many shots in as many arms as quickly as possible. And that is definitely super important,” she said.
Ms. Amer is working with her practice’s primary care physicians to try to identify all of those patients who are functionally debilitated or unable to leave their home to get vaccinated and that Central Ohio Primary Care will vaccinate more homebound patients, she added.
The experts interviewed in this article have no conflicts.
Katie Lennon contributed to this report.
This article was updated 4/29/21.
Predicting outcomes in therapy-related AML
Therapy-related acute myeloid leukemia (t-AML) occurs as a complication of chemotherapy and/or radiotherapy for previous cancer or for nonmalignant disorders, with an estimated prevalence of 10%-15% of all AML cases, according to Ram Vasudevan Nampoothiri, MD, and colleagues at the Princess Margaret Cancer Center, University of Toronto.
Dr. Nampoothiri and colleagues performed a retrospective study of 68 patients with t-AML who underwent hematopoietic stem cell transplantation (HSCT) at their institution. They found significant predictors of reduced overall survival, including chromosomal rearrangements, induction regimens, donor type, patient performance status, and the type of graft-versus-host disease (GVHD) prophylaxis the patients received, as reported in Hematology/Oncology and Stem Cell Therapy.
Some populations benefit
Among the 68 patients studied, a total of 59.9% were women; and the median age was 56.5 years. All patients were analyzed for prior malignancy, therapy, time to diagnosis of t-AML, transplant details, relapse-free survival, overall survival, and predictors of outcomes.
At 2 years, the cumulative incidence of relapse, nonrelapse mortality, relapse-free survival, and overall survival were 17.9%, 34.5%, 47.6%, and 49.3%, respectively. Overall, acute and chronic GVHD occurred in 39 (57.4%) and 23 (33.8%) patients, respectively, according to the researchers.
The significant predictors of reduced overall survival were the presence of the 11q23 chromosomal rearrangement (hazard ratio, 3.24), use of induction regimens other than fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor or 7 + 3 (HR, 3.65), use of haploidentical donors (HR, 3.48), an Eastern Cooperative Oncology Group performance status of 2 or higher (HR, 5.83), and use of cyclosporine A–methotrexate as GVHD prophylaxis (HR, 2.41).
The researchers also found that a significant decrease in survival was seen with an increasing number of any of these prognostic factors.
A growing need
The incidence of t-AML is increasing because of longer life expectancy of the general population and also because of the improved survival of patients treated with chemotherapy and/or radiation for prior malignancies, according to the researchers.
They concluded that, even with this increasing prevalence and normally poor prognosis, “patients of t-AML having good-risk karyotypes, good performance status, and having HLA-matched donors have favorable outcomes after allo-HSCT.”
The authors reported that they had no competing financial interests.
Therapy-related acute myeloid leukemia (t-AML) occurs as a complication of chemotherapy and/or radiotherapy for previous cancer or for nonmalignant disorders, with an estimated prevalence of 10%-15% of all AML cases, according to Ram Vasudevan Nampoothiri, MD, and colleagues at the Princess Margaret Cancer Center, University of Toronto.
Dr. Nampoothiri and colleagues performed a retrospective study of 68 patients with t-AML who underwent hematopoietic stem cell transplantation (HSCT) at their institution. They found significant predictors of reduced overall survival, including chromosomal rearrangements, induction regimens, donor type, patient performance status, and the type of graft-versus-host disease (GVHD) prophylaxis the patients received, as reported in Hematology/Oncology and Stem Cell Therapy.
Some populations benefit
Among the 68 patients studied, a total of 59.9% were women; and the median age was 56.5 years. All patients were analyzed for prior malignancy, therapy, time to diagnosis of t-AML, transplant details, relapse-free survival, overall survival, and predictors of outcomes.
At 2 years, the cumulative incidence of relapse, nonrelapse mortality, relapse-free survival, and overall survival were 17.9%, 34.5%, 47.6%, and 49.3%, respectively. Overall, acute and chronic GVHD occurred in 39 (57.4%) and 23 (33.8%) patients, respectively, according to the researchers.
The significant predictors of reduced overall survival were the presence of the 11q23 chromosomal rearrangement (hazard ratio, 3.24), use of induction regimens other than fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor or 7 + 3 (HR, 3.65), use of haploidentical donors (HR, 3.48), an Eastern Cooperative Oncology Group performance status of 2 or higher (HR, 5.83), and use of cyclosporine A–methotrexate as GVHD prophylaxis (HR, 2.41).
The researchers also found that a significant decrease in survival was seen with an increasing number of any of these prognostic factors.
A growing need
The incidence of t-AML is increasing because of longer life expectancy of the general population and also because of the improved survival of patients treated with chemotherapy and/or radiation for prior malignancies, according to the researchers.
They concluded that, even with this increasing prevalence and normally poor prognosis, “patients of t-AML having good-risk karyotypes, good performance status, and having HLA-matched donors have favorable outcomes after allo-HSCT.”
The authors reported that they had no competing financial interests.
Therapy-related acute myeloid leukemia (t-AML) occurs as a complication of chemotherapy and/or radiotherapy for previous cancer or for nonmalignant disorders, with an estimated prevalence of 10%-15% of all AML cases, according to Ram Vasudevan Nampoothiri, MD, and colleagues at the Princess Margaret Cancer Center, University of Toronto.
Dr. Nampoothiri and colleagues performed a retrospective study of 68 patients with t-AML who underwent hematopoietic stem cell transplantation (HSCT) at their institution. They found significant predictors of reduced overall survival, including chromosomal rearrangements, induction regimens, donor type, patient performance status, and the type of graft-versus-host disease (GVHD) prophylaxis the patients received, as reported in Hematology/Oncology and Stem Cell Therapy.
Some populations benefit
Among the 68 patients studied, a total of 59.9% were women; and the median age was 56.5 years. All patients were analyzed for prior malignancy, therapy, time to diagnosis of t-AML, transplant details, relapse-free survival, overall survival, and predictors of outcomes.
At 2 years, the cumulative incidence of relapse, nonrelapse mortality, relapse-free survival, and overall survival were 17.9%, 34.5%, 47.6%, and 49.3%, respectively. Overall, acute and chronic GVHD occurred in 39 (57.4%) and 23 (33.8%) patients, respectively, according to the researchers.
The significant predictors of reduced overall survival were the presence of the 11q23 chromosomal rearrangement (hazard ratio, 3.24), use of induction regimens other than fludarabine, cytarabine, idarubicin, and granulocyte colony-stimulating factor or 7 + 3 (HR, 3.65), use of haploidentical donors (HR, 3.48), an Eastern Cooperative Oncology Group performance status of 2 or higher (HR, 5.83), and use of cyclosporine A–methotrexate as GVHD prophylaxis (HR, 2.41).
The researchers also found that a significant decrease in survival was seen with an increasing number of any of these prognostic factors.
A growing need
The incidence of t-AML is increasing because of longer life expectancy of the general population and also because of the improved survival of patients treated with chemotherapy and/or radiation for prior malignancies, according to the researchers.
They concluded that, even with this increasing prevalence and normally poor prognosis, “patients of t-AML having good-risk karyotypes, good performance status, and having HLA-matched donors have favorable outcomes after allo-HSCT.”
The authors reported that they had no competing financial interests.
FROM HEMATOLOGY/ONCOLOGY AND STEM CELL THERAPY