User login
-
Seniors face higher risk of other medical conditions after COVID-19
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
FROM BMJ
Endometriosis not linked with preterm birth, new study finds
These new findings, which were published online in JAMA Network Open, suggest that changing monitoring strategies to prevent preterm birth for women with the disease may not be necessary.
The research team, led by Louis Marcellin, MD, PhD, with the department of obstetrics and gynecology at Université de Paris, also found that disease phenotype or whether the preterm birth was induced or spontaneous did not appear to alter the result.
Those results differ from previous research. Data on the phenotypes and their link with preterm birth have been scarce, but previous studies have shown the risk for preterm birth is more pronounced in women who have deep endometriosis than in women with ovarian endometriosis.
Dr. Marcellin said in an interview that “little is known about the impact of endometriosis on obstetric outcomes. In contrast to previous studies, we reported no differences in the risk for preterm delivery between women with endometriosis (34 of 470 [7.2%]) and those without endometriosis (53 of 881 [6.0%]), even when adjusted for multiple factors.”
The authors accounted for mother’s age, body mass index before pregnancy, birth country, number of times the woman had given birth, previous cesarean delivery, and history of preterm birth. After adjusting for potential confounders, endometriosis was not associated with preterm birth (adjusted odds ratio, 1.07; 95% confidence interval, 0.64-1.77).
The researchers found no differences among preterm births based on a mother’s endometriosis phenotype. Those phenotypes include Isolated superficial peritoneal endometriosis, ovarian endometrioma, and deep endometriosis.
“Monitoring pregnancy beyond the normal protocols or changing management strategies may not be warranted in cases of endometriosis,” Dr. Marcellin said.
More research on endometriosis’ potential link to birth outcomes is needed.
An expert not involved with the study said the new paper highlights important new avenues of research but should not be seen as the final word on the connection between endometriosis and preterm birth.
Of the 1,351 study participants (mean age, 32.9 years) who had a singleton delivery after 22 weeks’ gestation, 470 were assigned to the endometriosis group, and 881 were assigned to the control group.
The authors concluded that “pregnant women with endometriosis should not be considered to have an exceptionally high risk for preterm birth. However, further studies are needed to examine the potential for other adverse perinatal outcomes or specific but rare complications.”
Daniela Carusi, MD, said the difficulty with the study’s design is that “premature birth is not one problem or one disease.”
Many very different problems can all end with premature birth. Sometimes it’s an infection or inflammation or bleeding in the uterus or hypertension in the mother, for example, and all those things can lead to a preterm birth, she explained.
“This study inherently lumps all those things together,” said Dr. Carusi, who is director of surgical obstetrics and placental abnormalities in the department of obstetrics and gynecology at Brigham and Women’s Hospital, Boston. “It’s quite possible endometriosis can have a big impact in one of those areas and no impact in the other areas, but the study design wouldn’t be able to pick that up.”
Editorialists: Results challenge findings of previous studies
In an accompanying commentary, Liisu Saavalainen, MD, PhD, and Oskari Heikinheimo, MD, PhD, both with the department of obstetrics and gynecology, Helsinki University Hospital, wrote that several previous studies have suggested that women with endometriosis have a slightly higher risk for preterm birth.
Those studies were mostly retrospective and differed in the way they classified endometriosis and the way they selected patients, the editorialists write. Also, most women in these studies typically had subfertility, they added.
The study by Dr. Marcellin and colleagues differs from previous related research in that was prospective and assessed the risk for preterm delivery in women both with endometriosis and those without endometriosis from several maternity centers in France. The women with endometriosis were classified according to the severity of their disease.
The editorialists wrote: “The novel results by Marcellin et al. challenge the findings of most previous studies on this topic. These results are valuable and comforting. However, they are also likely to trigger new studies on the pregnancy risks associated with different types of endometriosis. That is good news.”
Dr. Carusi said the study was well done and included a notably large size. Further complimenting the research, she said it’s important to talk about this little-discussed pregnancy complication. There’s been much more focus for women with endometriosis and their physicians on getting pregnant and on talking about the length of their term.
The study leaves some things unanswered.
The study was funded by research grants from the French Ministry of Health and was sponsored by the Département de la Recherche Clinique et du Développement de l’Assistance Publique–Hôpitaux de Paris. Dr. Carusi reported no relevant financial relationships. One study coauthor reported receiving personal fees from Bioserinity and Ferring outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
These new findings, which were published online in JAMA Network Open, suggest that changing monitoring strategies to prevent preterm birth for women with the disease may not be necessary.
The research team, led by Louis Marcellin, MD, PhD, with the department of obstetrics and gynecology at Université de Paris, also found that disease phenotype or whether the preterm birth was induced or spontaneous did not appear to alter the result.
Those results differ from previous research. Data on the phenotypes and their link with preterm birth have been scarce, but previous studies have shown the risk for preterm birth is more pronounced in women who have deep endometriosis than in women with ovarian endometriosis.
Dr. Marcellin said in an interview that “little is known about the impact of endometriosis on obstetric outcomes. In contrast to previous studies, we reported no differences in the risk for preterm delivery between women with endometriosis (34 of 470 [7.2%]) and those without endometriosis (53 of 881 [6.0%]), even when adjusted for multiple factors.”
The authors accounted for mother’s age, body mass index before pregnancy, birth country, number of times the woman had given birth, previous cesarean delivery, and history of preterm birth. After adjusting for potential confounders, endometriosis was not associated with preterm birth (adjusted odds ratio, 1.07; 95% confidence interval, 0.64-1.77).
The researchers found no differences among preterm births based on a mother’s endometriosis phenotype. Those phenotypes include Isolated superficial peritoneal endometriosis, ovarian endometrioma, and deep endometriosis.
“Monitoring pregnancy beyond the normal protocols or changing management strategies may not be warranted in cases of endometriosis,” Dr. Marcellin said.
More research on endometriosis’ potential link to birth outcomes is needed.
An expert not involved with the study said the new paper highlights important new avenues of research but should not be seen as the final word on the connection between endometriosis and preterm birth.
Of the 1,351 study participants (mean age, 32.9 years) who had a singleton delivery after 22 weeks’ gestation, 470 were assigned to the endometriosis group, and 881 were assigned to the control group.
The authors concluded that “pregnant women with endometriosis should not be considered to have an exceptionally high risk for preterm birth. However, further studies are needed to examine the potential for other adverse perinatal outcomes or specific but rare complications.”
Daniela Carusi, MD, said the difficulty with the study’s design is that “premature birth is not one problem or one disease.”
Many very different problems can all end with premature birth. Sometimes it’s an infection or inflammation or bleeding in the uterus or hypertension in the mother, for example, and all those things can lead to a preterm birth, she explained.
“This study inherently lumps all those things together,” said Dr. Carusi, who is director of surgical obstetrics and placental abnormalities in the department of obstetrics and gynecology at Brigham and Women’s Hospital, Boston. “It’s quite possible endometriosis can have a big impact in one of those areas and no impact in the other areas, but the study design wouldn’t be able to pick that up.”
Editorialists: Results challenge findings of previous studies
In an accompanying commentary, Liisu Saavalainen, MD, PhD, and Oskari Heikinheimo, MD, PhD, both with the department of obstetrics and gynecology, Helsinki University Hospital, wrote that several previous studies have suggested that women with endometriosis have a slightly higher risk for preterm birth.
Those studies were mostly retrospective and differed in the way they classified endometriosis and the way they selected patients, the editorialists write. Also, most women in these studies typically had subfertility, they added.
The study by Dr. Marcellin and colleagues differs from previous related research in that was prospective and assessed the risk for preterm delivery in women both with endometriosis and those without endometriosis from several maternity centers in France. The women with endometriosis were classified according to the severity of their disease.
The editorialists wrote: “The novel results by Marcellin et al. challenge the findings of most previous studies on this topic. These results are valuable and comforting. However, they are also likely to trigger new studies on the pregnancy risks associated with different types of endometriosis. That is good news.”
Dr. Carusi said the study was well done and included a notably large size. Further complimenting the research, she said it’s important to talk about this little-discussed pregnancy complication. There’s been much more focus for women with endometriosis and their physicians on getting pregnant and on talking about the length of their term.
The study leaves some things unanswered.
The study was funded by research grants from the French Ministry of Health and was sponsored by the Département de la Recherche Clinique et du Développement de l’Assistance Publique–Hôpitaux de Paris. Dr. Carusi reported no relevant financial relationships. One study coauthor reported receiving personal fees from Bioserinity and Ferring outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
These new findings, which were published online in JAMA Network Open, suggest that changing monitoring strategies to prevent preterm birth for women with the disease may not be necessary.
The research team, led by Louis Marcellin, MD, PhD, with the department of obstetrics and gynecology at Université de Paris, also found that disease phenotype or whether the preterm birth was induced or spontaneous did not appear to alter the result.
Those results differ from previous research. Data on the phenotypes and their link with preterm birth have been scarce, but previous studies have shown the risk for preterm birth is more pronounced in women who have deep endometriosis than in women with ovarian endometriosis.
Dr. Marcellin said in an interview that “little is known about the impact of endometriosis on obstetric outcomes. In contrast to previous studies, we reported no differences in the risk for preterm delivery between women with endometriosis (34 of 470 [7.2%]) and those without endometriosis (53 of 881 [6.0%]), even when adjusted for multiple factors.”
The authors accounted for mother’s age, body mass index before pregnancy, birth country, number of times the woman had given birth, previous cesarean delivery, and history of preterm birth. After adjusting for potential confounders, endometriosis was not associated with preterm birth (adjusted odds ratio, 1.07; 95% confidence interval, 0.64-1.77).
The researchers found no differences among preterm births based on a mother’s endometriosis phenotype. Those phenotypes include Isolated superficial peritoneal endometriosis, ovarian endometrioma, and deep endometriosis.
“Monitoring pregnancy beyond the normal protocols or changing management strategies may not be warranted in cases of endometriosis,” Dr. Marcellin said.
More research on endometriosis’ potential link to birth outcomes is needed.
An expert not involved with the study said the new paper highlights important new avenues of research but should not be seen as the final word on the connection between endometriosis and preterm birth.
Of the 1,351 study participants (mean age, 32.9 years) who had a singleton delivery after 22 weeks’ gestation, 470 were assigned to the endometriosis group, and 881 were assigned to the control group.
The authors concluded that “pregnant women with endometriosis should not be considered to have an exceptionally high risk for preterm birth. However, further studies are needed to examine the potential for other adverse perinatal outcomes or specific but rare complications.”
Daniela Carusi, MD, said the difficulty with the study’s design is that “premature birth is not one problem or one disease.”
Many very different problems can all end with premature birth. Sometimes it’s an infection or inflammation or bleeding in the uterus or hypertension in the mother, for example, and all those things can lead to a preterm birth, she explained.
“This study inherently lumps all those things together,” said Dr. Carusi, who is director of surgical obstetrics and placental abnormalities in the department of obstetrics and gynecology at Brigham and Women’s Hospital, Boston. “It’s quite possible endometriosis can have a big impact in one of those areas and no impact in the other areas, but the study design wouldn’t be able to pick that up.”
Editorialists: Results challenge findings of previous studies
In an accompanying commentary, Liisu Saavalainen, MD, PhD, and Oskari Heikinheimo, MD, PhD, both with the department of obstetrics and gynecology, Helsinki University Hospital, wrote that several previous studies have suggested that women with endometriosis have a slightly higher risk for preterm birth.
Those studies were mostly retrospective and differed in the way they classified endometriosis and the way they selected patients, the editorialists write. Also, most women in these studies typically had subfertility, they added.
The study by Dr. Marcellin and colleagues differs from previous related research in that was prospective and assessed the risk for preterm delivery in women both with endometriosis and those without endometriosis from several maternity centers in France. The women with endometriosis were classified according to the severity of their disease.
The editorialists wrote: “The novel results by Marcellin et al. challenge the findings of most previous studies on this topic. These results are valuable and comforting. However, they are also likely to trigger new studies on the pregnancy risks associated with different types of endometriosis. That is good news.”
Dr. Carusi said the study was well done and included a notably large size. Further complimenting the research, she said it’s important to talk about this little-discussed pregnancy complication. There’s been much more focus for women with endometriosis and their physicians on getting pregnant and on talking about the length of their term.
The study leaves some things unanswered.
The study was funded by research grants from the French Ministry of Health and was sponsored by the Département de la Recherche Clinique et du Développement de l’Assistance Publique–Hôpitaux de Paris. Dr. Carusi reported no relevant financial relationships. One study coauthor reported receiving personal fees from Bioserinity and Ferring outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
If you’ve got 3 seconds, then you’ve got time to work out
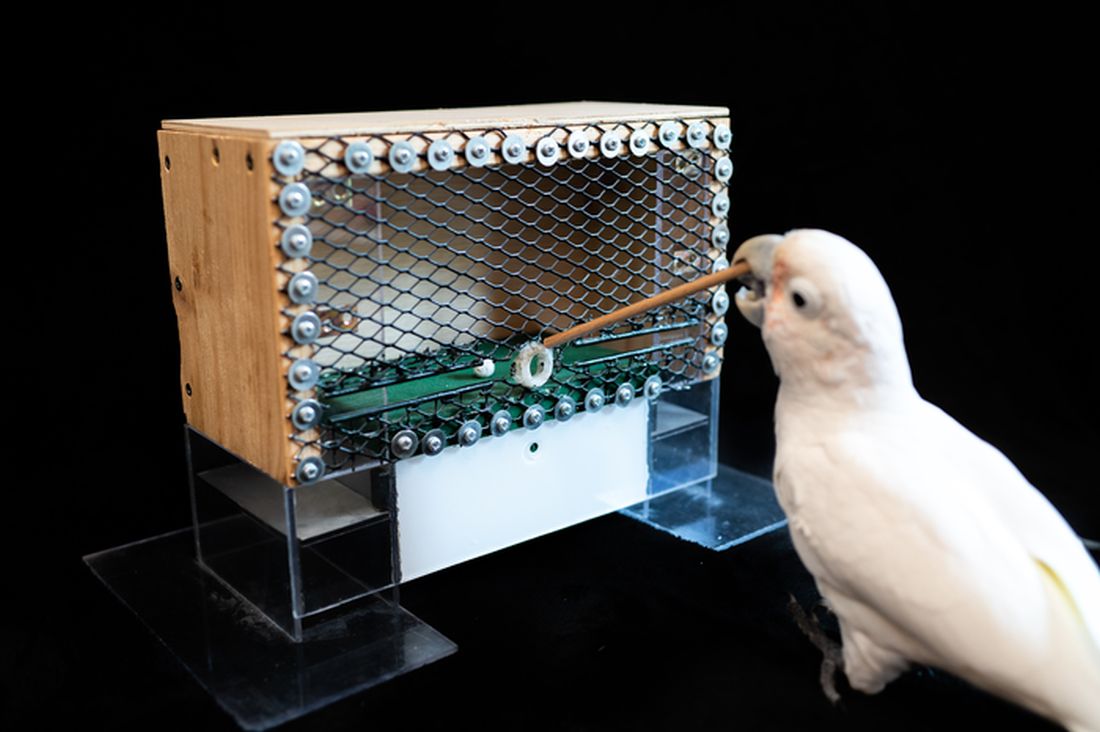
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Stopping venetoclax treatment early reduces CLL survival outcomes
“There’s not a lot of awareness about the fact that you’re probably better off not permanently discontinuing treatment,” Anthony R. Mato, first author of the research published in Haematologica, said in an interview.
“Instead, attempting dose reductions with later resumption to complete the planned schedule for treatment probably could improve outcomes,” said Dr. Mato, who is director of the CLL Program at Memorial Sloan Kettering Cancer Center in New York.
Venetoclax, a potent B-cell lymphoma-2 (BCL2) inhibitor, provides a novel, chemotherapy-free treatment option for first-line and r/r CLL. While its safety profile is manageable, treatment interruptions are very common, and premature discontinuations are reported in about a third of patients, often because of adverse events.
Lacking data on the effects of those interruptions on survival outcomes, Dr. Mato and colleagues conducted a post hoc analysis of the phase 3 MURANO trial. In this open-label study, treatment with six cycles of venetoclax in combination with rituximab followed by venetoclax once daily for a total of 2 years showed superior progression-free survival, compared with six cycles of bendamustine plus rituximab in patients with r/r CLL (P < .0001).
The current analysis involved 194 intention-to-treat patients from the trial’s venetoclax arm, among whom 140 (72%) completed 2 years of therapy, and 54 (28%) prematurely discontinued treatment. The most common reasons for discontinuation were adverse events (53.7%) and disease progression (22.2%).
Among those with early discontinuation for any reason except disease progression, the rate of progression-free survival was significantly inferior, compared with those who completed the treatment (hazard ratio, 5.98; P < .0001), as was the rate or discontinuation caused specifically by adverse events, which most commonly involved neutropenia or thrombocytopenia (HR, 5.82; P < .0001).
Those who discontinued had a mean duration of venetoclax therapy of 11.3 months, compared with 24.4 months for all patients. For each additional month of venetoclax therapy, there was a significantly lower risk of a progression-free survival event (P = .0263) and of an overall survival event (P < .0001).
The treatment interruption rate was much higher, at 69% (134), involving neutropenia in 43% (84) of instances and requiring dose reductions in 23% (45) of cases.
However, in contrast to permanent discontinuations, the temporary interruptions and dose reductions had no significant effect on progression-free or overall survival, regardless of the duration.
“Improved progression-free and overall survival were associated with greater cumulative venetoclax treatment exposure,” the authors wrote.
“The results of these analyses highlight the importance of appropriately managing treatment modifications to ensure optimal outcomes for patients receiving targeted treatment for CLL,” they said.
Key measures including “better supportive care, use of growth factors, and more aggressive strategies for dose reduction could potentially help to improve or decrease the number of patients discontinuing due to an adverse event,” Dr. Mato added.“We can’t say definitively because this is not a randomized study – it’s a retrospective analysis from a randomized study – but those measures likely could have a positive impact on patient outcomes.”
The study received support from Genentech and AbbVie. Dr. Mato reported consulting or other relationships with AbbVie, AstraZeneca, Celgene, DTRM, Genentech, Janssen, Loxo, PCYC, Sunesis, and TG Therapeutics.
“There’s not a lot of awareness about the fact that you’re probably better off not permanently discontinuing treatment,” Anthony R. Mato, first author of the research published in Haematologica, said in an interview.
“Instead, attempting dose reductions with later resumption to complete the planned schedule for treatment probably could improve outcomes,” said Dr. Mato, who is director of the CLL Program at Memorial Sloan Kettering Cancer Center in New York.
Venetoclax, a potent B-cell lymphoma-2 (BCL2) inhibitor, provides a novel, chemotherapy-free treatment option for first-line and r/r CLL. While its safety profile is manageable, treatment interruptions are very common, and premature discontinuations are reported in about a third of patients, often because of adverse events.
Lacking data on the effects of those interruptions on survival outcomes, Dr. Mato and colleagues conducted a post hoc analysis of the phase 3 MURANO trial. In this open-label study, treatment with six cycles of venetoclax in combination with rituximab followed by venetoclax once daily for a total of 2 years showed superior progression-free survival, compared with six cycles of bendamustine plus rituximab in patients with r/r CLL (P < .0001).
The current analysis involved 194 intention-to-treat patients from the trial’s venetoclax arm, among whom 140 (72%) completed 2 years of therapy, and 54 (28%) prematurely discontinued treatment. The most common reasons for discontinuation were adverse events (53.7%) and disease progression (22.2%).
Among those with early discontinuation for any reason except disease progression, the rate of progression-free survival was significantly inferior, compared with those who completed the treatment (hazard ratio, 5.98; P < .0001), as was the rate or discontinuation caused specifically by adverse events, which most commonly involved neutropenia or thrombocytopenia (HR, 5.82; P < .0001).
Those who discontinued had a mean duration of venetoclax therapy of 11.3 months, compared with 24.4 months for all patients. For each additional month of venetoclax therapy, there was a significantly lower risk of a progression-free survival event (P = .0263) and of an overall survival event (P < .0001).
The treatment interruption rate was much higher, at 69% (134), involving neutropenia in 43% (84) of instances and requiring dose reductions in 23% (45) of cases.
However, in contrast to permanent discontinuations, the temporary interruptions and dose reductions had no significant effect on progression-free or overall survival, regardless of the duration.
“Improved progression-free and overall survival were associated with greater cumulative venetoclax treatment exposure,” the authors wrote.
“The results of these analyses highlight the importance of appropriately managing treatment modifications to ensure optimal outcomes for patients receiving targeted treatment for CLL,” they said.
Key measures including “better supportive care, use of growth factors, and more aggressive strategies for dose reduction could potentially help to improve or decrease the number of patients discontinuing due to an adverse event,” Dr. Mato added.“We can’t say definitively because this is not a randomized study – it’s a retrospective analysis from a randomized study – but those measures likely could have a positive impact on patient outcomes.”
The study received support from Genentech and AbbVie. Dr. Mato reported consulting or other relationships with AbbVie, AstraZeneca, Celgene, DTRM, Genentech, Janssen, Loxo, PCYC, Sunesis, and TG Therapeutics.
“There’s not a lot of awareness about the fact that you’re probably better off not permanently discontinuing treatment,” Anthony R. Mato, first author of the research published in Haematologica, said in an interview.
“Instead, attempting dose reductions with later resumption to complete the planned schedule for treatment probably could improve outcomes,” said Dr. Mato, who is director of the CLL Program at Memorial Sloan Kettering Cancer Center in New York.
Venetoclax, a potent B-cell lymphoma-2 (BCL2) inhibitor, provides a novel, chemotherapy-free treatment option for first-line and r/r CLL. While its safety profile is manageable, treatment interruptions are very common, and premature discontinuations are reported in about a third of patients, often because of adverse events.
Lacking data on the effects of those interruptions on survival outcomes, Dr. Mato and colleagues conducted a post hoc analysis of the phase 3 MURANO trial. In this open-label study, treatment with six cycles of venetoclax in combination with rituximab followed by venetoclax once daily for a total of 2 years showed superior progression-free survival, compared with six cycles of bendamustine plus rituximab in patients with r/r CLL (P < .0001).
The current analysis involved 194 intention-to-treat patients from the trial’s venetoclax arm, among whom 140 (72%) completed 2 years of therapy, and 54 (28%) prematurely discontinued treatment. The most common reasons for discontinuation were adverse events (53.7%) and disease progression (22.2%).
Among those with early discontinuation for any reason except disease progression, the rate of progression-free survival was significantly inferior, compared with those who completed the treatment (hazard ratio, 5.98; P < .0001), as was the rate or discontinuation caused specifically by adverse events, which most commonly involved neutropenia or thrombocytopenia (HR, 5.82; P < .0001).
Those who discontinued had a mean duration of venetoclax therapy of 11.3 months, compared with 24.4 months for all patients. For each additional month of venetoclax therapy, there was a significantly lower risk of a progression-free survival event (P = .0263) and of an overall survival event (P < .0001).
The treatment interruption rate was much higher, at 69% (134), involving neutropenia in 43% (84) of instances and requiring dose reductions in 23% (45) of cases.
However, in contrast to permanent discontinuations, the temporary interruptions and dose reductions had no significant effect on progression-free or overall survival, regardless of the duration.
“Improved progression-free and overall survival were associated with greater cumulative venetoclax treatment exposure,” the authors wrote.
“The results of these analyses highlight the importance of appropriately managing treatment modifications to ensure optimal outcomes for patients receiving targeted treatment for CLL,” they said.
Key measures including “better supportive care, use of growth factors, and more aggressive strategies for dose reduction could potentially help to improve or decrease the number of patients discontinuing due to an adverse event,” Dr. Mato added.“We can’t say definitively because this is not a randomized study – it’s a retrospective analysis from a randomized study – but those measures likely could have a positive impact on patient outcomes.”
The study received support from Genentech and AbbVie. Dr. Mato reported consulting or other relationships with AbbVie, AstraZeneca, Celgene, DTRM, Genentech, Janssen, Loxo, PCYC, Sunesis, and TG Therapeutics.
Enough is enough: the pandemic and loss of female oncologists
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Aspirin use risk for postpartum bleeding unclear
Low-dose aspirin may increase risk of postpartum bleeding if patients don’t discontinue its use at least 7 days before delivery, but it’s otherwise unclear whether its use increases bleeding risk, according to research presented Feb. 5 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“These findings were a little surprising to me because we have generally been taught that aspirin is safe to continue up until delivery with minimal risk,” Jenny Mei, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, said in an interview after attending the presentation. “Theoretically it makes sense that it may increase bleeding risk, but multiple studies in the past analyzing its use with gynecological surgery show minimal risk, which was conferred to the obstetrical population as well.”
She noted, however, that patients prescribed low-dose aspirin already have risk factors that may increase their risk of postpartum bleeding, and the study’s finding of possible increased risk was not statistically significant after accounting for those confounders. “I wouldn’t change my practice management over it, but it does raise awareness that all interventions likely come with some risk,” Dr. Mei said.
Hypertensive disorders of pregnancy are responsible for 6.6% of U.S. pregnancy-related deaths. The SMFM currently recommends low-dose aspirin starting at 12 weeks’ gestation in patients at high risk for preeclampsia, which includes people with multifetal gestation, chronic hypertension, pregestational diabetes, renal disease, autoimmune disease, or a history of preeclampsia. However, previous research has shown mixed results on the safety of low-dose aspirin in terms of bleeding risk, Kelsey White, MD, a second-year maternal-fetal medicine fellow of the Yale University, New Haven, Conn., told attendees.
This retrospective study compared a bleeding composite endpoint among those who did and did not take low-dose aspirin between January 2018 and April 2021. The composite included an estimated blood loss of greater than 1,000 mL, postpartum hemorrhage based on ICD-9/10 code diagnosis, and red blood cell transfusion. The researchers also compared bleeding risk within the aspirin group based on discontinuation at greater or less than 7 days before delivery.
Among 16,980 patients, 11.3% were prescribed low-dose aspirin. The patients prescribed low-dose aspirin significantly differed from those not prescribed it in all demographic and clinical characteristics except placenta accreta spectrum. The average age of the aspirin group was 39 years, compared with 24 years in the nonaspirin group (P < .01). More of the aspirin group patients were Hispanic and Black, and 52.3% of patients taking aspirin had a body mass index greater than 30 kg/m2, compared with 22.9% of the nonaspirin group. Rates of diabetes, lupus, fibroids, nonaspirin anticoagulation use, cesarean delivery, and preterm delivery were all greater in the aspirin group.
In addition, 43.9% of the patients in the aspirin group had a hypertensive disorder, including 20.2% with preeclampsia, compared with 17.1% with hypertensive disorders, including 6.2% with preeclampsia, in the group not taking aspirin (P < .0001). “This shows that a high-risk population was prescribed aspirin, which correlates to the recommended prescription guidelines,” Dr. White said.
The postpartum bleeding composite outcome occurred in 14.7% of patients in the low-dose aspirin group, compared with 9.2% of patients in the nonaspirin group, for an unadjusted 1.7 times greater risk of bleeding (95% confidence interval, 1.49-1.96). After adjustment for confounders, the risk declined and was no longer statistically significant (aOR = 1.15; 95% CI, 0.98-1.34).
Meanwhile, 15% of those who discontinued aspirin within 7 days of delivery had postpartum bleeding, compared with 9% of those who discontinued aspirin at 7 or more days before delivery (P = .03).
Therefore, while the study found only a possible, nonsignificant association between low-dose aspirin and postpartum bleeding, risk of bleeding was significantly greater among those who discontinued aspirin only in the last week before delivery.
”Our study is timely and supports a recent Swedish study [that] found an increased risk of intrapartum bleeding, postpartum hemorrhage, and postpartum hematoma,” Dr. White said. She also noted that the United States Preventive Services Task Force changed their recommendation in 2021 for low-dose aspirin prophylaxis for cardiovascular disease.
“They now recommend against the use of low-dose aspirin for prevention in adults without a history of cardiovascular disease,” Dr. White said. “The change in recommendations came after recent randomized control trials showed that low-dose aspirin had very little benefit and may increase the risk of bleeding.”
However, Dr. White added that they “do not believe this study should be used to make any clinical decisions.” While the study had a large sample size, it was limited by its retrospective reliance on EMR data, including the EMR medication list, and the researchers couldn’t assess patient compliance or patient use of over-the-counter aspirin not recorded in the EMR.
Deirdre Lyell, MD, a professor of maternal-fetal medicine at Stanford (Calif.) University, agreed that the findings should not impact clinical practice given its limitations.
“The investigators could not entirely identify who stopped low-dose aspirin and when. When they estimated timing of stoppage of low-dose aspirin, their data suggested a small benefit among those who discontinued it at least 7 days before delivery, though this should be interpreted with caution, given the potential inaccuracy in these data,” Dr. Lyell, who was not involved in the study, said in an interview. “Their study did not examine factors that should be used to confirm if there are real differences in blood loss, such as changes in blood counts before and after delivery, or more use of medications that we use to stop heavy bleeding.”
In fact, Dr. Lyell noted, other research at the SMFM meeting found ”that low-dose aspirin is not used frequently enough in patients who might benefit, such as those at high risk for preeclampsia,” she said. ”Low-dose aspirin among those at increased risk has been shown to reduce rates of preeclampsia, reducing the likelihood of risky situations for moms and babies.”
The authors had no disclosures. Dr. Lyell has consulted for Bloomlife, a uterine contraction and fetal monitor. Dr. White and Dr. Mei had no disclosures.
Low-dose aspirin may increase risk of postpartum bleeding if patients don’t discontinue its use at least 7 days before delivery, but it’s otherwise unclear whether its use increases bleeding risk, according to research presented Feb. 5 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“These findings were a little surprising to me because we have generally been taught that aspirin is safe to continue up until delivery with minimal risk,” Jenny Mei, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, said in an interview after attending the presentation. “Theoretically it makes sense that it may increase bleeding risk, but multiple studies in the past analyzing its use with gynecological surgery show minimal risk, which was conferred to the obstetrical population as well.”
She noted, however, that patients prescribed low-dose aspirin already have risk factors that may increase their risk of postpartum bleeding, and the study’s finding of possible increased risk was not statistically significant after accounting for those confounders. “I wouldn’t change my practice management over it, but it does raise awareness that all interventions likely come with some risk,” Dr. Mei said.
Hypertensive disorders of pregnancy are responsible for 6.6% of U.S. pregnancy-related deaths. The SMFM currently recommends low-dose aspirin starting at 12 weeks’ gestation in patients at high risk for preeclampsia, which includes people with multifetal gestation, chronic hypertension, pregestational diabetes, renal disease, autoimmune disease, or a history of preeclampsia. However, previous research has shown mixed results on the safety of low-dose aspirin in terms of bleeding risk, Kelsey White, MD, a second-year maternal-fetal medicine fellow of the Yale University, New Haven, Conn., told attendees.
This retrospective study compared a bleeding composite endpoint among those who did and did not take low-dose aspirin between January 2018 and April 2021. The composite included an estimated blood loss of greater than 1,000 mL, postpartum hemorrhage based on ICD-9/10 code diagnosis, and red blood cell transfusion. The researchers also compared bleeding risk within the aspirin group based on discontinuation at greater or less than 7 days before delivery.
Among 16,980 patients, 11.3% were prescribed low-dose aspirin. The patients prescribed low-dose aspirin significantly differed from those not prescribed it in all demographic and clinical characteristics except placenta accreta spectrum. The average age of the aspirin group was 39 years, compared with 24 years in the nonaspirin group (P < .01). More of the aspirin group patients were Hispanic and Black, and 52.3% of patients taking aspirin had a body mass index greater than 30 kg/m2, compared with 22.9% of the nonaspirin group. Rates of diabetes, lupus, fibroids, nonaspirin anticoagulation use, cesarean delivery, and preterm delivery were all greater in the aspirin group.
In addition, 43.9% of the patients in the aspirin group had a hypertensive disorder, including 20.2% with preeclampsia, compared with 17.1% with hypertensive disorders, including 6.2% with preeclampsia, in the group not taking aspirin (P < .0001). “This shows that a high-risk population was prescribed aspirin, which correlates to the recommended prescription guidelines,” Dr. White said.
The postpartum bleeding composite outcome occurred in 14.7% of patients in the low-dose aspirin group, compared with 9.2% of patients in the nonaspirin group, for an unadjusted 1.7 times greater risk of bleeding (95% confidence interval, 1.49-1.96). After adjustment for confounders, the risk declined and was no longer statistically significant (aOR = 1.15; 95% CI, 0.98-1.34).
Meanwhile, 15% of those who discontinued aspirin within 7 days of delivery had postpartum bleeding, compared with 9% of those who discontinued aspirin at 7 or more days before delivery (P = .03).
Therefore, while the study found only a possible, nonsignificant association between low-dose aspirin and postpartum bleeding, risk of bleeding was significantly greater among those who discontinued aspirin only in the last week before delivery.
”Our study is timely and supports a recent Swedish study [that] found an increased risk of intrapartum bleeding, postpartum hemorrhage, and postpartum hematoma,” Dr. White said. She also noted that the United States Preventive Services Task Force changed their recommendation in 2021 for low-dose aspirin prophylaxis for cardiovascular disease.
“They now recommend against the use of low-dose aspirin for prevention in adults without a history of cardiovascular disease,” Dr. White said. “The change in recommendations came after recent randomized control trials showed that low-dose aspirin had very little benefit and may increase the risk of bleeding.”
However, Dr. White added that they “do not believe this study should be used to make any clinical decisions.” While the study had a large sample size, it was limited by its retrospective reliance on EMR data, including the EMR medication list, and the researchers couldn’t assess patient compliance or patient use of over-the-counter aspirin not recorded in the EMR.
Deirdre Lyell, MD, a professor of maternal-fetal medicine at Stanford (Calif.) University, agreed that the findings should not impact clinical practice given its limitations.
“The investigators could not entirely identify who stopped low-dose aspirin and when. When they estimated timing of stoppage of low-dose aspirin, their data suggested a small benefit among those who discontinued it at least 7 days before delivery, though this should be interpreted with caution, given the potential inaccuracy in these data,” Dr. Lyell, who was not involved in the study, said in an interview. “Their study did not examine factors that should be used to confirm if there are real differences in blood loss, such as changes in blood counts before and after delivery, or more use of medications that we use to stop heavy bleeding.”
In fact, Dr. Lyell noted, other research at the SMFM meeting found ”that low-dose aspirin is not used frequently enough in patients who might benefit, such as those at high risk for preeclampsia,” she said. ”Low-dose aspirin among those at increased risk has been shown to reduce rates of preeclampsia, reducing the likelihood of risky situations for moms and babies.”
The authors had no disclosures. Dr. Lyell has consulted for Bloomlife, a uterine contraction and fetal monitor. Dr. White and Dr. Mei had no disclosures.
Low-dose aspirin may increase risk of postpartum bleeding if patients don’t discontinue its use at least 7 days before delivery, but it’s otherwise unclear whether its use increases bleeding risk, according to research presented Feb. 5 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“These findings were a little surprising to me because we have generally been taught that aspirin is safe to continue up until delivery with minimal risk,” Jenny Mei, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, said in an interview after attending the presentation. “Theoretically it makes sense that it may increase bleeding risk, but multiple studies in the past analyzing its use with gynecological surgery show minimal risk, which was conferred to the obstetrical population as well.”
She noted, however, that patients prescribed low-dose aspirin already have risk factors that may increase their risk of postpartum bleeding, and the study’s finding of possible increased risk was not statistically significant after accounting for those confounders. “I wouldn’t change my practice management over it, but it does raise awareness that all interventions likely come with some risk,” Dr. Mei said.
Hypertensive disorders of pregnancy are responsible for 6.6% of U.S. pregnancy-related deaths. The SMFM currently recommends low-dose aspirin starting at 12 weeks’ gestation in patients at high risk for preeclampsia, which includes people with multifetal gestation, chronic hypertension, pregestational diabetes, renal disease, autoimmune disease, or a history of preeclampsia. However, previous research has shown mixed results on the safety of low-dose aspirin in terms of bleeding risk, Kelsey White, MD, a second-year maternal-fetal medicine fellow of the Yale University, New Haven, Conn., told attendees.
This retrospective study compared a bleeding composite endpoint among those who did and did not take low-dose aspirin between January 2018 and April 2021. The composite included an estimated blood loss of greater than 1,000 mL, postpartum hemorrhage based on ICD-9/10 code diagnosis, and red blood cell transfusion. The researchers also compared bleeding risk within the aspirin group based on discontinuation at greater or less than 7 days before delivery.
Among 16,980 patients, 11.3% were prescribed low-dose aspirin. The patients prescribed low-dose aspirin significantly differed from those not prescribed it in all demographic and clinical characteristics except placenta accreta spectrum. The average age of the aspirin group was 39 years, compared with 24 years in the nonaspirin group (P < .01). More of the aspirin group patients were Hispanic and Black, and 52.3% of patients taking aspirin had a body mass index greater than 30 kg/m2, compared with 22.9% of the nonaspirin group. Rates of diabetes, lupus, fibroids, nonaspirin anticoagulation use, cesarean delivery, and preterm delivery were all greater in the aspirin group.
In addition, 43.9% of the patients in the aspirin group had a hypertensive disorder, including 20.2% with preeclampsia, compared with 17.1% with hypertensive disorders, including 6.2% with preeclampsia, in the group not taking aspirin (P < .0001). “This shows that a high-risk population was prescribed aspirin, which correlates to the recommended prescription guidelines,” Dr. White said.
The postpartum bleeding composite outcome occurred in 14.7% of patients in the low-dose aspirin group, compared with 9.2% of patients in the nonaspirin group, for an unadjusted 1.7 times greater risk of bleeding (95% confidence interval, 1.49-1.96). After adjustment for confounders, the risk declined and was no longer statistically significant (aOR = 1.15; 95% CI, 0.98-1.34).
Meanwhile, 15% of those who discontinued aspirin within 7 days of delivery had postpartum bleeding, compared with 9% of those who discontinued aspirin at 7 or more days before delivery (P = .03).
Therefore, while the study found only a possible, nonsignificant association between low-dose aspirin and postpartum bleeding, risk of bleeding was significantly greater among those who discontinued aspirin only in the last week before delivery.
”Our study is timely and supports a recent Swedish study [that] found an increased risk of intrapartum bleeding, postpartum hemorrhage, and postpartum hematoma,” Dr. White said. She also noted that the United States Preventive Services Task Force changed their recommendation in 2021 for low-dose aspirin prophylaxis for cardiovascular disease.
“They now recommend against the use of low-dose aspirin for prevention in adults without a history of cardiovascular disease,” Dr. White said. “The change in recommendations came after recent randomized control trials showed that low-dose aspirin had very little benefit and may increase the risk of bleeding.”
However, Dr. White added that they “do not believe this study should be used to make any clinical decisions.” While the study had a large sample size, it was limited by its retrospective reliance on EMR data, including the EMR medication list, and the researchers couldn’t assess patient compliance or patient use of over-the-counter aspirin not recorded in the EMR.
Deirdre Lyell, MD, a professor of maternal-fetal medicine at Stanford (Calif.) University, agreed that the findings should not impact clinical practice given its limitations.
“The investigators could not entirely identify who stopped low-dose aspirin and when. When they estimated timing of stoppage of low-dose aspirin, their data suggested a small benefit among those who discontinued it at least 7 days before delivery, though this should be interpreted with caution, given the potential inaccuracy in these data,” Dr. Lyell, who was not involved in the study, said in an interview. “Their study did not examine factors that should be used to confirm if there are real differences in blood loss, such as changes in blood counts before and after delivery, or more use of medications that we use to stop heavy bleeding.”
In fact, Dr. Lyell noted, other research at the SMFM meeting found ”that low-dose aspirin is not used frequently enough in patients who might benefit, such as those at high risk for preeclampsia,” she said. ”Low-dose aspirin among those at increased risk has been shown to reduce rates of preeclampsia, reducing the likelihood of risky situations for moms and babies.”
The authors had no disclosures. Dr. Lyell has consulted for Bloomlife, a uterine contraction and fetal monitor. Dr. White and Dr. Mei had no disclosures.
AT THE PREGNANCY MEETING
FDA approves first-ever drug for cold agglutinin disease
A new drug has become the first and only treatment for people with cold agglutinin disease (CAD) that is approved by the U.S. Food and Drug Administration.
CAD is a rare autoimmune hemolytic anemia, affecting about 5,000 people in the United States. It is caused by antibodies binding to the surface of red blood cells, which starts a process that causes the body’s immune system to mistakenly attack healthy red blood cells and cause their rupture (hemolysis). This can lead to severe anemia, which is often treated by blood transfusions.
“For people living with cold agglutinin disease, it is as if their body’s immune system is waging a war on itself. The relentless destruction of healthy red blood cells is a daily, silent reality for people with CAD. For the first time, we have a treatment that targets complement-mediated hemolysis, which is the underlying cause of the red blood cell destruction in many CAD patients,” commented Catherine Broome, MD, associate professor of medicine at Georgetown University Lombardi Comprehensive Cancer Center, in a company press release.
Dr. Broome was the principal investigator in the CARDINAL study, which was the basis for the new approval. In this pivotal study, patients treated with sutimlimab had an improvement in anemia as measured by hemoglobin (Hgb) and bilirubin levels, she commented in the company statement.
The CARDINAL study was a 26-week open-label, single-arm phase 3 study conducted in 24 patients with CAD who had recent history of blood transfusion.
The primary efficacy endpoint was a composite defined as the proportion of patients who achieved normalization of Hgb level greater than or equal to 12 g/dL or demonstrated an increase from baseline in Hgb level greater than or equal to 2 g/dL at the treatment assessment time point (mean value from weeks 23, 25, and 26) and no blood transfusion from weeks 5 through 26.
More than half of the patients (13 of 24, 54%) met the composite primary endpoint criteria, with 17 of 24 (71%) patients remaining transfusion-free after week 5. Most patients (22 of 24, 92%) did not use other CAD-related treatments.
For the secondary measures on disease process, patients enrolled in the trial experienced a mean increase in Hgb level of 2.29 g/dL at week 3 and 3.18 g/dL at the 26-week treatment assessment time points (increasing from the mean baseline level of 8.6 g/dL). In addition, there was a mean reduction in bilirubin levels (n = 14) of -2.23 mg/dL from a mean baseline level of 3.23 mg/dL.
The most common adverse reactions occurring in 10% or more of patients were respiratory tract infection, viral infection, diarrhea, dyspepsia, cough, arthralgia, arthritis, and peripheral edema. Serious adverse reactions were reported in 3 of 24 (13%) patients, and these included streptococcal sepsis and staphylococcal wound infection (n = 1), arthralgia (n = 1), and respiratory tract infection (n = 1).
None of the adverse reactions led to discontinuation of the drug, the company noted. Dosage interruptions due to an adverse reaction occurred in 4 of 24 (17%) patients who received the drug.
The recommended dose is based on body weight (6,500 mg for people 39-75 kg and 7,500 mg for people greater than 75 kg). The drug is administered intravenously weekly for the first 2 weeks with administration every 2 weeks thereafter.
Full prescribing information is available here.
The product is a humanized monoclonal antibody that is designed to selectively target and inhibit C1s in the classical complement pathway, which is part of the innate immune system. By blocking C1s, Enjaymo inhibits the activation of the complement cascade in the immune system and inhibits C1-activated hemolysis in CAD to prevent the abnormal destruction of healthy red blood cells. Enjaymo does not inhibit the lectin and alternative pathways, the company noted.
In the U.S., Enjaymo received FDA breakthrough therapy and orphan drug designation, as well as priority review. The product is awaiting approval in Europe and Japan.
Sanofi says the product is expected to be available in the United States in the coming weeks, with a list price, or wholesale acquisition cost, of $1,800 per vial. Actual costs to patients are generally anticipated to be lower, as the list price does not reflect insurance coverage, copay support, or financial assistance from patient support programs. The company offers support for eligible patients on 1-833-223-2428.
A version of this article first appeared on Medscape.com.
A new drug has become the first and only treatment for people with cold agglutinin disease (CAD) that is approved by the U.S. Food and Drug Administration.
CAD is a rare autoimmune hemolytic anemia, affecting about 5,000 people in the United States. It is caused by antibodies binding to the surface of red blood cells, which starts a process that causes the body’s immune system to mistakenly attack healthy red blood cells and cause their rupture (hemolysis). This can lead to severe anemia, which is often treated by blood transfusions.
“For people living with cold agglutinin disease, it is as if their body’s immune system is waging a war on itself. The relentless destruction of healthy red blood cells is a daily, silent reality for people with CAD. For the first time, we have a treatment that targets complement-mediated hemolysis, which is the underlying cause of the red blood cell destruction in many CAD patients,” commented Catherine Broome, MD, associate professor of medicine at Georgetown University Lombardi Comprehensive Cancer Center, in a company press release.
Dr. Broome was the principal investigator in the CARDINAL study, which was the basis for the new approval. In this pivotal study, patients treated with sutimlimab had an improvement in anemia as measured by hemoglobin (Hgb) and bilirubin levels, she commented in the company statement.
The CARDINAL study was a 26-week open-label, single-arm phase 3 study conducted in 24 patients with CAD who had recent history of blood transfusion.
The primary efficacy endpoint was a composite defined as the proportion of patients who achieved normalization of Hgb level greater than or equal to 12 g/dL or demonstrated an increase from baseline in Hgb level greater than or equal to 2 g/dL at the treatment assessment time point (mean value from weeks 23, 25, and 26) and no blood transfusion from weeks 5 through 26.
More than half of the patients (13 of 24, 54%) met the composite primary endpoint criteria, with 17 of 24 (71%) patients remaining transfusion-free after week 5. Most patients (22 of 24, 92%) did not use other CAD-related treatments.
For the secondary measures on disease process, patients enrolled in the trial experienced a mean increase in Hgb level of 2.29 g/dL at week 3 and 3.18 g/dL at the 26-week treatment assessment time points (increasing from the mean baseline level of 8.6 g/dL). In addition, there was a mean reduction in bilirubin levels (n = 14) of -2.23 mg/dL from a mean baseline level of 3.23 mg/dL.
The most common adverse reactions occurring in 10% or more of patients were respiratory tract infection, viral infection, diarrhea, dyspepsia, cough, arthralgia, arthritis, and peripheral edema. Serious adverse reactions were reported in 3 of 24 (13%) patients, and these included streptococcal sepsis and staphylococcal wound infection (n = 1), arthralgia (n = 1), and respiratory tract infection (n = 1).
None of the adverse reactions led to discontinuation of the drug, the company noted. Dosage interruptions due to an adverse reaction occurred in 4 of 24 (17%) patients who received the drug.
The recommended dose is based on body weight (6,500 mg for people 39-75 kg and 7,500 mg for people greater than 75 kg). The drug is administered intravenously weekly for the first 2 weeks with administration every 2 weeks thereafter.
Full prescribing information is available here.
The product is a humanized monoclonal antibody that is designed to selectively target and inhibit C1s in the classical complement pathway, which is part of the innate immune system. By blocking C1s, Enjaymo inhibits the activation of the complement cascade in the immune system and inhibits C1-activated hemolysis in CAD to prevent the abnormal destruction of healthy red blood cells. Enjaymo does not inhibit the lectin and alternative pathways, the company noted.
In the U.S., Enjaymo received FDA breakthrough therapy and orphan drug designation, as well as priority review. The product is awaiting approval in Europe and Japan.
Sanofi says the product is expected to be available in the United States in the coming weeks, with a list price, or wholesale acquisition cost, of $1,800 per vial. Actual costs to patients are generally anticipated to be lower, as the list price does not reflect insurance coverage, copay support, or financial assistance from patient support programs. The company offers support for eligible patients on 1-833-223-2428.
A version of this article first appeared on Medscape.com.
A new drug has become the first and only treatment for people with cold agglutinin disease (CAD) that is approved by the U.S. Food and Drug Administration.
CAD is a rare autoimmune hemolytic anemia, affecting about 5,000 people in the United States. It is caused by antibodies binding to the surface of red blood cells, which starts a process that causes the body’s immune system to mistakenly attack healthy red blood cells and cause their rupture (hemolysis). This can lead to severe anemia, which is often treated by blood transfusions.
“For people living with cold agglutinin disease, it is as if their body’s immune system is waging a war on itself. The relentless destruction of healthy red blood cells is a daily, silent reality for people with CAD. For the first time, we have a treatment that targets complement-mediated hemolysis, which is the underlying cause of the red blood cell destruction in many CAD patients,” commented Catherine Broome, MD, associate professor of medicine at Georgetown University Lombardi Comprehensive Cancer Center, in a company press release.
Dr. Broome was the principal investigator in the CARDINAL study, which was the basis for the new approval. In this pivotal study, patients treated with sutimlimab had an improvement in anemia as measured by hemoglobin (Hgb) and bilirubin levels, she commented in the company statement.
The CARDINAL study was a 26-week open-label, single-arm phase 3 study conducted in 24 patients with CAD who had recent history of blood transfusion.
The primary efficacy endpoint was a composite defined as the proportion of patients who achieved normalization of Hgb level greater than or equal to 12 g/dL or demonstrated an increase from baseline in Hgb level greater than or equal to 2 g/dL at the treatment assessment time point (mean value from weeks 23, 25, and 26) and no blood transfusion from weeks 5 through 26.
More than half of the patients (13 of 24, 54%) met the composite primary endpoint criteria, with 17 of 24 (71%) patients remaining transfusion-free after week 5. Most patients (22 of 24, 92%) did not use other CAD-related treatments.
For the secondary measures on disease process, patients enrolled in the trial experienced a mean increase in Hgb level of 2.29 g/dL at week 3 and 3.18 g/dL at the 26-week treatment assessment time points (increasing from the mean baseline level of 8.6 g/dL). In addition, there was a mean reduction in bilirubin levels (n = 14) of -2.23 mg/dL from a mean baseline level of 3.23 mg/dL.
The most common adverse reactions occurring in 10% or more of patients were respiratory tract infection, viral infection, diarrhea, dyspepsia, cough, arthralgia, arthritis, and peripheral edema. Serious adverse reactions were reported in 3 of 24 (13%) patients, and these included streptococcal sepsis and staphylococcal wound infection (n = 1), arthralgia (n = 1), and respiratory tract infection (n = 1).
None of the adverse reactions led to discontinuation of the drug, the company noted. Dosage interruptions due to an adverse reaction occurred in 4 of 24 (17%) patients who received the drug.
The recommended dose is based on body weight (6,500 mg for people 39-75 kg and 7,500 mg for people greater than 75 kg). The drug is administered intravenously weekly for the first 2 weeks with administration every 2 weeks thereafter.
Full prescribing information is available here.
The product is a humanized monoclonal antibody that is designed to selectively target and inhibit C1s in the classical complement pathway, which is part of the innate immune system. By blocking C1s, Enjaymo inhibits the activation of the complement cascade in the immune system and inhibits C1-activated hemolysis in CAD to prevent the abnormal destruction of healthy red blood cells. Enjaymo does not inhibit the lectin and alternative pathways, the company noted.
In the U.S., Enjaymo received FDA breakthrough therapy and orphan drug designation, as well as priority review. The product is awaiting approval in Europe and Japan.
Sanofi says the product is expected to be available in the United States in the coming weeks, with a list price, or wholesale acquisition cost, of $1,800 per vial. Actual costs to patients are generally anticipated to be lower, as the list price does not reflect insurance coverage, copay support, or financial assistance from patient support programs. The company offers support for eligible patients on 1-833-223-2428.
A version of this article first appeared on Medscape.com.
Oncologists in malpractice suits: Less than other specialties
, notes the latest Medscape Malpractice Report.
Over a third (39%) of oncologists who were surveyed said that they had been named in at least one malpractice suit, according to the Medscape Oncologist Malpractice Report 2021.
This number is considerably lower than that reported by physicians across all specialties (51%), and it is also much lower than that reported by oncologists in past years. In the 2019 report, 54% of oncologists surveyed said they had been named in a malpractice suit, while in the 2017 report, the figure was 53%.
The dramatic decline in malpractice suits may have much to do with the COVID-19 pandemic, when oncology care was in a state of flux.
“Fewer people were seeking cancer care during the COVID pandemic, which might have impacted the number of lawsuits brought against oncologists,” says Paul Walker, a New York–based malpractice attorney at Walker Medical Law, who represents physicians and other healthcare professionals.
“Additionally, a fair number of people who died of COVID were also older,” he pointed out, and it is often older people who get cancer, so there were fewer older people who consulted an oncologist or were treated by one, he added.
However, the pandemic may be storing up trouble for future years. “Patient fears of contracting COVID-19 have led many to avoid seeking or resuming care, so delays in diagnosing new cancer cases could mean that more patients are diagnosed at a later stage of their disease, leading to potential adverse events and malpractice claims,” commented David L. Feldman, MD, MBA, chief medical officer of The Doctors Company Group.
This latest 2021 Medscape Malpractice Report was compiled from an online survey that included more than 4,300 physicians from 29 specialties. It included 106 oncologists. More than half of respondents (56%) had been in practice for more than 25 years, and 54% were aged 60 years or older. The survey was available from May 21 to August 28, 2021.
Similar to findings in previous years, complications from treatment/surgery were the most common reason for the lawsuits (31%). Failure to make a correct diagnosis or a delay in diagnosis was the second most common reason (23%), while 20% of patients sued because of a poor outcome or disease progression.
Surprise at being sued
Among the oncologists who reported involvement in a lawsuit in 2021, the majority (86%) said they were “very surprised” or “somewhat surprised” by the malpractice suit, which is similar to that of other physicians surveyed. However, fewer were surprised this year as compared to 2019 and 2017 (90% and 94%).
One reason for the surprise over the litigation was that it concerned a patient who had been treated a long time ago. One oncologist wrote that “the patient had not seen me for over 7 years and during that time, he did not call me with his new symptomatology. I was only named in the suit because I had previously been involved.”
Another common scenario reported by oncologists was being named in a lawsuit which was brought by another clinician’s patient. “I was the chairperson of the department, and one of the doctors in the practice was involved in the suit,” wrote one respondent. “I was named as an accomplice.”
More than half of surveyed oncologists said that they were able to identify the patient who bought the suit, and these figures are again comparable to those of other physicians. One oncologist commented that in the case he was involved with, the family did not understand or accept the nature of cancer and the different ways that a patient could die of complications. This patient had died of sepsis and pneumonia related to decubitus ulcers that were completely unrelated to her radiation therapy.
As in the case above, sometimes it is the family who filed the lawsuit, not the patient.
“The patient may even recognize that you did your best and be grateful for your skill and efforts, but the family can’t accept that grandma died of cancer and brings a lawsuit,” said Dennis Hursh, an attorney with Physicians Agreement Health Law in Pennsylvania.
When looking at outcomes of the lawsuit, 40% of oncologists were dismissed from the suit within the first few months, or the case was settled before going to trial. This trend is also consistent with the results from the 2019 and 2017 surveys. When the case did go to trial, 10% received a favorable verdict, which was the same in 2019.
“It seems that most of my clients end up being released from lawsuits, and many lawsuits are dismissed prior to proceeding to trial,” Mr. Hursh commented.
Murdering psychopath
Some oncologists weighed in on what they felt was the worst experience of being sued.
“Mental anguish, knowing that I did nothing wrong,” said one physician. Another reported that it was a feeling of being “inadequate and totally alone.”
Another oncologist commented that the “depositions from lawyers implied that I was worse than a murdering psychopath. My reputation was permanently damaged.”
However, the vast majority of oncologists (88%) did not believe that the lawsuit negatively affected their career, which was similar to physicians in general. That said, many did complain about the ongoing requirement to report the lawsuit to the credentialing committee, even if it was dismissed, and then having to pay increased malpractice premiums. “I still need to document this episode every single time I apply for any medical position, even more than 29 years after I was dismissed from the case,” said one respondent.
When asked if they would do anything differently, many oncologists (42%) said no, they would not have done anything differently. This is similar to the responses from physicians in general and with 2019 responses from oncologists. However, 15% of the respondents said that in retrospect, they would not have taken on that patient to begin with.
Some oncologists noted that they would have been more conscientious in relaying the information to the referring physician. Evan Lyman, an associate attorney at Voute, Lohrfink, McAndrew, Meisner & Roberts, LLP, in White Plains, N.Y., pointed out that a common reason for lawsuits is a slip-up of communication between the specialist and the referring physician.
Oncologists who had been sued have some insights to offer to colleagues, should they find themselves in a similar situation.
“Only answer with short and precise statements,” wrote one oncologist. “Attend all the depositions as much as you can; they are more likely to fabricate or exaggerate if you are not sitting in the room.”
Another physician said to base “everything on the medical record and do not answer hypothetical questions.”
“Document all interactions with patients as if a jury will be reading them, word by word,” said one respondent.
As for the public or patients, oncologists had this message: “malpractice suits should be rarely launched and only when gross errors can be absolutely proven.”
Another oncologist pointed out that communication is key. “Speak to the physicians against whom you have distrust. Lots of things could be cleared by good communication.”
A version of this article first appeared on Medscape.com.
, notes the latest Medscape Malpractice Report.
Over a third (39%) of oncologists who were surveyed said that they had been named in at least one malpractice suit, according to the Medscape Oncologist Malpractice Report 2021.
This number is considerably lower than that reported by physicians across all specialties (51%), and it is also much lower than that reported by oncologists in past years. In the 2019 report, 54% of oncologists surveyed said they had been named in a malpractice suit, while in the 2017 report, the figure was 53%.
The dramatic decline in malpractice suits may have much to do with the COVID-19 pandemic, when oncology care was in a state of flux.
“Fewer people were seeking cancer care during the COVID pandemic, which might have impacted the number of lawsuits brought against oncologists,” says Paul Walker, a New York–based malpractice attorney at Walker Medical Law, who represents physicians and other healthcare professionals.
“Additionally, a fair number of people who died of COVID were also older,” he pointed out, and it is often older people who get cancer, so there were fewer older people who consulted an oncologist or were treated by one, he added.
However, the pandemic may be storing up trouble for future years. “Patient fears of contracting COVID-19 have led many to avoid seeking or resuming care, so delays in diagnosing new cancer cases could mean that more patients are diagnosed at a later stage of their disease, leading to potential adverse events and malpractice claims,” commented David L. Feldman, MD, MBA, chief medical officer of The Doctors Company Group.
This latest 2021 Medscape Malpractice Report was compiled from an online survey that included more than 4,300 physicians from 29 specialties. It included 106 oncologists. More than half of respondents (56%) had been in practice for more than 25 years, and 54% were aged 60 years or older. The survey was available from May 21 to August 28, 2021.
Similar to findings in previous years, complications from treatment/surgery were the most common reason for the lawsuits (31%). Failure to make a correct diagnosis or a delay in diagnosis was the second most common reason (23%), while 20% of patients sued because of a poor outcome or disease progression.
Surprise at being sued
Among the oncologists who reported involvement in a lawsuit in 2021, the majority (86%) said they were “very surprised” or “somewhat surprised” by the malpractice suit, which is similar to that of other physicians surveyed. However, fewer were surprised this year as compared to 2019 and 2017 (90% and 94%).
One reason for the surprise over the litigation was that it concerned a patient who had been treated a long time ago. One oncologist wrote that “the patient had not seen me for over 7 years and during that time, he did not call me with his new symptomatology. I was only named in the suit because I had previously been involved.”
Another common scenario reported by oncologists was being named in a lawsuit which was brought by another clinician’s patient. “I was the chairperson of the department, and one of the doctors in the practice was involved in the suit,” wrote one respondent. “I was named as an accomplice.”
More than half of surveyed oncologists said that they were able to identify the patient who bought the suit, and these figures are again comparable to those of other physicians. One oncologist commented that in the case he was involved with, the family did not understand or accept the nature of cancer and the different ways that a patient could die of complications. This patient had died of sepsis and pneumonia related to decubitus ulcers that were completely unrelated to her radiation therapy.
As in the case above, sometimes it is the family who filed the lawsuit, not the patient.
“The patient may even recognize that you did your best and be grateful for your skill and efforts, but the family can’t accept that grandma died of cancer and brings a lawsuit,” said Dennis Hursh, an attorney with Physicians Agreement Health Law in Pennsylvania.
When looking at outcomes of the lawsuit, 40% of oncologists were dismissed from the suit within the first few months, or the case was settled before going to trial. This trend is also consistent with the results from the 2019 and 2017 surveys. When the case did go to trial, 10% received a favorable verdict, which was the same in 2019.
“It seems that most of my clients end up being released from lawsuits, and many lawsuits are dismissed prior to proceeding to trial,” Mr. Hursh commented.
Murdering psychopath
Some oncologists weighed in on what they felt was the worst experience of being sued.
“Mental anguish, knowing that I did nothing wrong,” said one physician. Another reported that it was a feeling of being “inadequate and totally alone.”
Another oncologist commented that the “depositions from lawyers implied that I was worse than a murdering psychopath. My reputation was permanently damaged.”
However, the vast majority of oncologists (88%) did not believe that the lawsuit negatively affected their career, which was similar to physicians in general. That said, many did complain about the ongoing requirement to report the lawsuit to the credentialing committee, even if it was dismissed, and then having to pay increased malpractice premiums. “I still need to document this episode every single time I apply for any medical position, even more than 29 years after I was dismissed from the case,” said one respondent.
When asked if they would do anything differently, many oncologists (42%) said no, they would not have done anything differently. This is similar to the responses from physicians in general and with 2019 responses from oncologists. However, 15% of the respondents said that in retrospect, they would not have taken on that patient to begin with.
Some oncologists noted that they would have been more conscientious in relaying the information to the referring physician. Evan Lyman, an associate attorney at Voute, Lohrfink, McAndrew, Meisner & Roberts, LLP, in White Plains, N.Y., pointed out that a common reason for lawsuits is a slip-up of communication between the specialist and the referring physician.
Oncologists who had been sued have some insights to offer to colleagues, should they find themselves in a similar situation.
“Only answer with short and precise statements,” wrote one oncologist. “Attend all the depositions as much as you can; they are more likely to fabricate or exaggerate if you are not sitting in the room.”
Another physician said to base “everything on the medical record and do not answer hypothetical questions.”
“Document all interactions with patients as if a jury will be reading them, word by word,” said one respondent.
As for the public or patients, oncologists had this message: “malpractice suits should be rarely launched and only when gross errors can be absolutely proven.”
Another oncologist pointed out that communication is key. “Speak to the physicians against whom you have distrust. Lots of things could be cleared by good communication.”
A version of this article first appeared on Medscape.com.
, notes the latest Medscape Malpractice Report.
Over a third (39%) of oncologists who were surveyed said that they had been named in at least one malpractice suit, according to the Medscape Oncologist Malpractice Report 2021.
This number is considerably lower than that reported by physicians across all specialties (51%), and it is also much lower than that reported by oncologists in past years. In the 2019 report, 54% of oncologists surveyed said they had been named in a malpractice suit, while in the 2017 report, the figure was 53%.
The dramatic decline in malpractice suits may have much to do with the COVID-19 pandemic, when oncology care was in a state of flux.
“Fewer people were seeking cancer care during the COVID pandemic, which might have impacted the number of lawsuits brought against oncologists,” says Paul Walker, a New York–based malpractice attorney at Walker Medical Law, who represents physicians and other healthcare professionals.
“Additionally, a fair number of people who died of COVID were also older,” he pointed out, and it is often older people who get cancer, so there were fewer older people who consulted an oncologist or were treated by one, he added.
However, the pandemic may be storing up trouble for future years. “Patient fears of contracting COVID-19 have led many to avoid seeking or resuming care, so delays in diagnosing new cancer cases could mean that more patients are diagnosed at a later stage of their disease, leading to potential adverse events and malpractice claims,” commented David L. Feldman, MD, MBA, chief medical officer of The Doctors Company Group.
This latest 2021 Medscape Malpractice Report was compiled from an online survey that included more than 4,300 physicians from 29 specialties. It included 106 oncologists. More than half of respondents (56%) had been in practice for more than 25 years, and 54% were aged 60 years or older. The survey was available from May 21 to August 28, 2021.
Similar to findings in previous years, complications from treatment/surgery were the most common reason for the lawsuits (31%). Failure to make a correct diagnosis or a delay in diagnosis was the second most common reason (23%), while 20% of patients sued because of a poor outcome or disease progression.
Surprise at being sued
Among the oncologists who reported involvement in a lawsuit in 2021, the majority (86%) said they were “very surprised” or “somewhat surprised” by the malpractice suit, which is similar to that of other physicians surveyed. However, fewer were surprised this year as compared to 2019 and 2017 (90% and 94%).
One reason for the surprise over the litigation was that it concerned a patient who had been treated a long time ago. One oncologist wrote that “the patient had not seen me for over 7 years and during that time, he did not call me with his new symptomatology. I was only named in the suit because I had previously been involved.”
Another common scenario reported by oncologists was being named in a lawsuit which was brought by another clinician’s patient. “I was the chairperson of the department, and one of the doctors in the practice was involved in the suit,” wrote one respondent. “I was named as an accomplice.”
More than half of surveyed oncologists said that they were able to identify the patient who bought the suit, and these figures are again comparable to those of other physicians. One oncologist commented that in the case he was involved with, the family did not understand or accept the nature of cancer and the different ways that a patient could die of complications. This patient had died of sepsis and pneumonia related to decubitus ulcers that were completely unrelated to her radiation therapy.
As in the case above, sometimes it is the family who filed the lawsuit, not the patient.
“The patient may even recognize that you did your best and be grateful for your skill and efforts, but the family can’t accept that grandma died of cancer and brings a lawsuit,” said Dennis Hursh, an attorney with Physicians Agreement Health Law in Pennsylvania.
When looking at outcomes of the lawsuit, 40% of oncologists were dismissed from the suit within the first few months, or the case was settled before going to trial. This trend is also consistent with the results from the 2019 and 2017 surveys. When the case did go to trial, 10% received a favorable verdict, which was the same in 2019.
“It seems that most of my clients end up being released from lawsuits, and many lawsuits are dismissed prior to proceeding to trial,” Mr. Hursh commented.
Murdering psychopath
Some oncologists weighed in on what they felt was the worst experience of being sued.
“Mental anguish, knowing that I did nothing wrong,” said one physician. Another reported that it was a feeling of being “inadequate and totally alone.”
Another oncologist commented that the “depositions from lawyers implied that I was worse than a murdering psychopath. My reputation was permanently damaged.”
However, the vast majority of oncologists (88%) did not believe that the lawsuit negatively affected their career, which was similar to physicians in general. That said, many did complain about the ongoing requirement to report the lawsuit to the credentialing committee, even if it was dismissed, and then having to pay increased malpractice premiums. “I still need to document this episode every single time I apply for any medical position, even more than 29 years after I was dismissed from the case,” said one respondent.
When asked if they would do anything differently, many oncologists (42%) said no, they would not have done anything differently. This is similar to the responses from physicians in general and with 2019 responses from oncologists. However, 15% of the respondents said that in retrospect, they would not have taken on that patient to begin with.
Some oncologists noted that they would have been more conscientious in relaying the information to the referring physician. Evan Lyman, an associate attorney at Voute, Lohrfink, McAndrew, Meisner & Roberts, LLP, in White Plains, N.Y., pointed out that a common reason for lawsuits is a slip-up of communication between the specialist and the referring physician.
Oncologists who had been sued have some insights to offer to colleagues, should they find themselves in a similar situation.
“Only answer with short and precise statements,” wrote one oncologist. “Attend all the depositions as much as you can; they are more likely to fabricate or exaggerate if you are not sitting in the room.”
Another physician said to base “everything on the medical record and do not answer hypothetical questions.”
“Document all interactions with patients as if a jury will be reading them, word by word,” said one respondent.
As for the public or patients, oncologists had this message: “malpractice suits should be rarely launched and only when gross errors can be absolutely proven.”
Another oncologist pointed out that communication is key. “Speak to the physicians against whom you have distrust. Lots of things could be cleared by good communication.”
A version of this article first appeared on Medscape.com.
FDA investigates possible increased risk of death with lymphoma drug
The FDA granted accelerated approval to umbralisib in February 2021 for patients with two types of lymphoma: Adults with relapsed or refractory marginal zone lymphoma who received at least one prior therapy, and those with relapsed or refractory follicular lymphoma who received at least three prior therapies.
According to the FDA, the possible increased risk of death arose from early findings in a phase 3 trial evaluating the drug in a related type of cancer: chronic lymphocytic leukemia.
“Because of the seriousness of this safety concern and the similarities between the two types of cancer for which this drug is approved and the type of cancer that was studied in the clinical trial, we are alerting patients and health care professionals that we are reevaluating this risk against the benefits of Ukoniq [umbralisib] for its approved uses,” the FDA safety communication states.
The FDA said it performed an initial review of data from the phase 3, randomized controlled UNITY trial, which is evaluating the efficacy of umbralisib plus a monoclonal antibody in patients with chronic lymphocytic leukemia.
“The results showed a possible increased risk of death in patients receiving the combination of Ukoniq [umbralisib] and the monoclonal antibody compared to the control arm,” according to the FDA. “Those receiving the combination of Ukoniq [umbralisib] and the monoclonal antibody also experienced more serious adverse events than those in the control arm.”
Although the drug has not been approved for patients with chronic lymphocytic leukemia, the FDA believes the findings could “have implications for its approved uses” in marginal zone lymphoma and follicular lymphoma.
However, the phase 2 trial that led to February 2021 approvals found the drug’s safety profile to be “manageable,” with serious adverse reactions reported in 18% of patients receiving the dual oral inhibitor of phosphoinositide 3 kinase delta and casein kinase 1 epsilon. These adverse reactions included diarrhea-colitis (4%), pneumonia (3%), sepsis (2%), and urinary tract infection (2%); however, no elevated risk of death was indicated in that analysis.
The FDA noted it will continue to evaluate the results from the phase 3 UNITY trial in chronic lymphocytic leukemia and has suspended enrollment of new patients in other ongoing clinical trials of the drug.
The FDA stated that it would communicate its “final conclusions and recommendations when we have completed our review.” In the meantime, the agency asks health care professionals to review how patients receiving umbralisib are faring and discuss “the risks and benefits of continuing” versus switching to other treatments.
The FDA also asks clinicians and patients to report side effects involving the drug to the FDA MedWatch program.
A version of this article first appeared on Medscape.com.
The FDA granted accelerated approval to umbralisib in February 2021 for patients with two types of lymphoma: Adults with relapsed or refractory marginal zone lymphoma who received at least one prior therapy, and those with relapsed or refractory follicular lymphoma who received at least three prior therapies.
According to the FDA, the possible increased risk of death arose from early findings in a phase 3 trial evaluating the drug in a related type of cancer: chronic lymphocytic leukemia.
“Because of the seriousness of this safety concern and the similarities between the two types of cancer for which this drug is approved and the type of cancer that was studied in the clinical trial, we are alerting patients and health care professionals that we are reevaluating this risk against the benefits of Ukoniq [umbralisib] for its approved uses,” the FDA safety communication states.
The FDA said it performed an initial review of data from the phase 3, randomized controlled UNITY trial, which is evaluating the efficacy of umbralisib plus a monoclonal antibody in patients with chronic lymphocytic leukemia.
“The results showed a possible increased risk of death in patients receiving the combination of Ukoniq [umbralisib] and the monoclonal antibody compared to the control arm,” according to the FDA. “Those receiving the combination of Ukoniq [umbralisib] and the monoclonal antibody also experienced more serious adverse events than those in the control arm.”
Although the drug has not been approved for patients with chronic lymphocytic leukemia, the FDA believes the findings could “have implications for its approved uses” in marginal zone lymphoma and follicular lymphoma.
However, the phase 2 trial that led to February 2021 approvals found the drug’s safety profile to be “manageable,” with serious adverse reactions reported in 18% of patients receiving the dual oral inhibitor of phosphoinositide 3 kinase delta and casein kinase 1 epsilon. These adverse reactions included diarrhea-colitis (4%), pneumonia (3%), sepsis (2%), and urinary tract infection (2%); however, no elevated risk of death was indicated in that analysis.
The FDA noted it will continue to evaluate the results from the phase 3 UNITY trial in chronic lymphocytic leukemia and has suspended enrollment of new patients in other ongoing clinical trials of the drug.
The FDA stated that it would communicate its “final conclusions and recommendations when we have completed our review.” In the meantime, the agency asks health care professionals to review how patients receiving umbralisib are faring and discuss “the risks and benefits of continuing” versus switching to other treatments.
The FDA also asks clinicians and patients to report side effects involving the drug to the FDA MedWatch program.
A version of this article first appeared on Medscape.com.
The FDA granted accelerated approval to umbralisib in February 2021 for patients with two types of lymphoma: Adults with relapsed or refractory marginal zone lymphoma who received at least one prior therapy, and those with relapsed or refractory follicular lymphoma who received at least three prior therapies.
According to the FDA, the possible increased risk of death arose from early findings in a phase 3 trial evaluating the drug in a related type of cancer: chronic lymphocytic leukemia.
“Because of the seriousness of this safety concern and the similarities between the two types of cancer for which this drug is approved and the type of cancer that was studied in the clinical trial, we are alerting patients and health care professionals that we are reevaluating this risk against the benefits of Ukoniq [umbralisib] for its approved uses,” the FDA safety communication states.
The FDA said it performed an initial review of data from the phase 3, randomized controlled UNITY trial, which is evaluating the efficacy of umbralisib plus a monoclonal antibody in patients with chronic lymphocytic leukemia.
“The results showed a possible increased risk of death in patients receiving the combination of Ukoniq [umbralisib] and the monoclonal antibody compared to the control arm,” according to the FDA. “Those receiving the combination of Ukoniq [umbralisib] and the monoclonal antibody also experienced more serious adverse events than those in the control arm.”
Although the drug has not been approved for patients with chronic lymphocytic leukemia, the FDA believes the findings could “have implications for its approved uses” in marginal zone lymphoma and follicular lymphoma.
However, the phase 2 trial that led to February 2021 approvals found the drug’s safety profile to be “manageable,” with serious adverse reactions reported in 18% of patients receiving the dual oral inhibitor of phosphoinositide 3 kinase delta and casein kinase 1 epsilon. These adverse reactions included diarrhea-colitis (4%), pneumonia (3%), sepsis (2%), and urinary tract infection (2%); however, no elevated risk of death was indicated in that analysis.
The FDA noted it will continue to evaluate the results from the phase 3 UNITY trial in chronic lymphocytic leukemia and has suspended enrollment of new patients in other ongoing clinical trials of the drug.
The FDA stated that it would communicate its “final conclusions and recommendations when we have completed our review.” In the meantime, the agency asks health care professionals to review how patients receiving umbralisib are faring and discuss “the risks and benefits of continuing” versus switching to other treatments.
The FDA also asks clinicians and patients to report side effects involving the drug to the FDA MedWatch program.
A version of this article first appeared on Medscape.com.
Boosted Americans 97 times less likely to die of COVID-19 than unvaccinated
according to a new update from the CDC.
In addition, fully vaccinated Americans — meaning those with up to two doses, but no booster — are 14 times less likely to die from COVID-19 than unvaccinated people.
“These data confirm that vaccination and boosting continues to protect against severe illness and hospitalization, even during the Omicron surge,” Rochelle Walensky, MD, director of the CDC, said during a briefing by the White House COVID-19 Response Team.
“If you are not up to date on your COVID-19 vaccinations, you have not optimized your protection against severe disease and death, and you should get vaccinated and boosted if you are eligible,” she said.
Dr. Walensky presented the latest numbers on Feb. 2 based on reports from 25 jurisdictions in early December. The number of average weekly deaths for those who were unvaccinated was 9.7 per 100,000 people, as compared with 0.7 of those who were vaccinated and 0.1 of those who had received a booster.
“The data are really stunningly obvious why a booster is really very important,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during the briefing.
Dr. Fauci also encouraged vaccination for those who are pregnant and couples who may want to conceive in the near feature. He highlighted two recent studies that found vaccination in either partner didn’t affect fertility, including in vitro fertilization.
Meanwhile, fertility fell temporarily among men who were infected with the coronavirus. Couples were 18% less likely to conceive if the male partner had contracted the coronavirus within 60 days before a menstrual cycle.
“New data adds to previous studies that indicate that COVID-19 vaccination does not negatively impact fertility,” Dr. Fauci said. “Vaccination is recommended for people who are trying to get pregnant now or might become pregnant in the future, as well as their partners.”
About 80% of eligible Americans have received at least one vaccine dose, and 68% are fully vaccinated, according to the latest CDC data. About 51% of those who are eligible for a booster dose have received one.
The FDA could authorize the Pfizer vaccine for children under age 5 later this month. When that happens, about 18 million children will qualify for a shot, Jeff Zients, coordinator of the White House COVID-19 Response Team, said during the briefing. The Biden administration is already working on distribution plans for the shot for young kids, he added.
“We’ll be ready to start getting shots in arms soon after FDA and CDC make their decisions,” he said.
A version of this article first appeared on WebMD.com.
according to a new update from the CDC.
In addition, fully vaccinated Americans — meaning those with up to two doses, but no booster — are 14 times less likely to die from COVID-19 than unvaccinated people.
“These data confirm that vaccination and boosting continues to protect against severe illness and hospitalization, even during the Omicron surge,” Rochelle Walensky, MD, director of the CDC, said during a briefing by the White House COVID-19 Response Team.
“If you are not up to date on your COVID-19 vaccinations, you have not optimized your protection against severe disease and death, and you should get vaccinated and boosted if you are eligible,” she said.
Dr. Walensky presented the latest numbers on Feb. 2 based on reports from 25 jurisdictions in early December. The number of average weekly deaths for those who were unvaccinated was 9.7 per 100,000 people, as compared with 0.7 of those who were vaccinated and 0.1 of those who had received a booster.
“The data are really stunningly obvious why a booster is really very important,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during the briefing.
Dr. Fauci also encouraged vaccination for those who are pregnant and couples who may want to conceive in the near feature. He highlighted two recent studies that found vaccination in either partner didn’t affect fertility, including in vitro fertilization.
Meanwhile, fertility fell temporarily among men who were infected with the coronavirus. Couples were 18% less likely to conceive if the male partner had contracted the coronavirus within 60 days before a menstrual cycle.
“New data adds to previous studies that indicate that COVID-19 vaccination does not negatively impact fertility,” Dr. Fauci said. “Vaccination is recommended for people who are trying to get pregnant now or might become pregnant in the future, as well as their partners.”
About 80% of eligible Americans have received at least one vaccine dose, and 68% are fully vaccinated, according to the latest CDC data. About 51% of those who are eligible for a booster dose have received one.
The FDA could authorize the Pfizer vaccine for children under age 5 later this month. When that happens, about 18 million children will qualify for a shot, Jeff Zients, coordinator of the White House COVID-19 Response Team, said during the briefing. The Biden administration is already working on distribution plans for the shot for young kids, he added.
“We’ll be ready to start getting shots in arms soon after FDA and CDC make their decisions,” he said.
A version of this article first appeared on WebMD.com.
according to a new update from the CDC.
In addition, fully vaccinated Americans — meaning those with up to two doses, but no booster — are 14 times less likely to die from COVID-19 than unvaccinated people.
“These data confirm that vaccination and boosting continues to protect against severe illness and hospitalization, even during the Omicron surge,” Rochelle Walensky, MD, director of the CDC, said during a briefing by the White House COVID-19 Response Team.
“If you are not up to date on your COVID-19 vaccinations, you have not optimized your protection against severe disease and death, and you should get vaccinated and boosted if you are eligible,” she said.
Dr. Walensky presented the latest numbers on Feb. 2 based on reports from 25 jurisdictions in early December. The number of average weekly deaths for those who were unvaccinated was 9.7 per 100,000 people, as compared with 0.7 of those who were vaccinated and 0.1 of those who had received a booster.
“The data are really stunningly obvious why a booster is really very important,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during the briefing.
Dr. Fauci also encouraged vaccination for those who are pregnant and couples who may want to conceive in the near feature. He highlighted two recent studies that found vaccination in either partner didn’t affect fertility, including in vitro fertilization.
Meanwhile, fertility fell temporarily among men who were infected with the coronavirus. Couples were 18% less likely to conceive if the male partner had contracted the coronavirus within 60 days before a menstrual cycle.
“New data adds to previous studies that indicate that COVID-19 vaccination does not negatively impact fertility,” Dr. Fauci said. “Vaccination is recommended for people who are trying to get pregnant now or might become pregnant in the future, as well as their partners.”
About 80% of eligible Americans have received at least one vaccine dose, and 68% are fully vaccinated, according to the latest CDC data. About 51% of those who are eligible for a booster dose have received one.
The FDA could authorize the Pfizer vaccine for children under age 5 later this month. When that happens, about 18 million children will qualify for a shot, Jeff Zients, coordinator of the White House COVID-19 Response Team, said during the briefing. The Biden administration is already working on distribution plans for the shot for young kids, he added.
“We’ll be ready to start getting shots in arms soon after FDA and CDC make their decisions,” he said.
A version of this article first appeared on WebMD.com.