User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Guidelines for radiotherapy in prostate cancer during the pandemic
The framework involves using remote visits via telemedicine, avoiding radiotherapy in applicable cases, deferring radiotherapy as appropriate, and shortening the fractionation schedule of treatment based on safety and efficacy parameters.
Nicholas G. Zaorsky, MD, of Penn State Cancer Institute in Hershey, Pennsylvania, and colleagues described the framework and recommendations in Advances in Radiation Oncology.
The authors systematically reviewed the body of literature for evidence pertaining to the safe use of telemedicine, avoidance or deferral of radiotherapy, and optimal use of androgen deprivation therapy for patients with prostate cancer. The team also reviewed best practices for patients undergoing radiotherapy based on disease risk.
Based on their findings, Dr. Zaorsky and colleagues recommended that, during the pandemic, all consultations and return visits become telehealth visits. “Very few prostate cancer patients require an in-person visit during a pandemic,” the authors wrote.
Lower-risk disease
Dr. Zaorsky and colleagues recommended avoiding radiotherapy in patients with very-low-, low-, and favorable intermediate-risk disease. The authors said data suggest that, in general, treatment can be safely deferred in these patients “until after pandemic-related restrictions have been lifted.” However, this recommendation presumes the pandemic will wane over the next 12 months.
“I reassure my patients with very-low- and low-risk prostate cancer that the preferred, evidence-based treatment for patients in these categories is active surveillance,” said study author Amar U. Kishan, MD, of the University of California, Los Angeles.
“If surveillance is an option, then delaying treatment must be reasonable [during the pandemic],” he added. “For favorable intermediate-risk disease, I [review] the data supporting this approach and discuss that short delays are very unlikely to compromise outcomes.”
Higher-risk disease
The authors recommended deferral of radiotherapy for 4-6 months in patients with higher-risk disease, which includes those with unfavorable intermediate-risk, high-risk, very-high-risk, clinical node-positive, oligometastatic, and low-volume M1 disease, as well as patients who have undergone prostatectomy.
The authors noted that in-person consultations and return visits should be converted to “timely remote telehealth visits” for these patients. After these patients have started treatment, androgen deprivation therapy “can allow for further deferral of radiotherapy as necessary based on the nature of the ongoing epidemic.”
In cases where radiotherapy cannot be deferred safely, “the shortest fractionation schedule should be adopted that has evidence of safety and efficacy,” the authors wrote.
They acknowledged that these recommendations are only applicable to patients not infected with COVID-19. In cases of suspected or confirmed COVID-19, local institutional policies and practices should be followed.
The authors further explained that, due to the rapidly evolving nature of the COVID-19 pandemic, state and federal guidelines should be followed when made available.
The authors reported having no conflicts of interest. No funding sources were reported.
SOURCE: Zaorsky NG et al. Adv Radiat Oncol. 2020 Apr 1. doi: 10.1016/j.adro.2020.03.010.
The framework involves using remote visits via telemedicine, avoiding radiotherapy in applicable cases, deferring radiotherapy as appropriate, and shortening the fractionation schedule of treatment based on safety and efficacy parameters.
Nicholas G. Zaorsky, MD, of Penn State Cancer Institute in Hershey, Pennsylvania, and colleagues described the framework and recommendations in Advances in Radiation Oncology.
The authors systematically reviewed the body of literature for evidence pertaining to the safe use of telemedicine, avoidance or deferral of radiotherapy, and optimal use of androgen deprivation therapy for patients with prostate cancer. The team also reviewed best practices for patients undergoing radiotherapy based on disease risk.
Based on their findings, Dr. Zaorsky and colleagues recommended that, during the pandemic, all consultations and return visits become telehealth visits. “Very few prostate cancer patients require an in-person visit during a pandemic,” the authors wrote.
Lower-risk disease
Dr. Zaorsky and colleagues recommended avoiding radiotherapy in patients with very-low-, low-, and favorable intermediate-risk disease. The authors said data suggest that, in general, treatment can be safely deferred in these patients “until after pandemic-related restrictions have been lifted.” However, this recommendation presumes the pandemic will wane over the next 12 months.
“I reassure my patients with very-low- and low-risk prostate cancer that the preferred, evidence-based treatment for patients in these categories is active surveillance,” said study author Amar U. Kishan, MD, of the University of California, Los Angeles.
“If surveillance is an option, then delaying treatment must be reasonable [during the pandemic],” he added. “For favorable intermediate-risk disease, I [review] the data supporting this approach and discuss that short delays are very unlikely to compromise outcomes.”
Higher-risk disease
The authors recommended deferral of radiotherapy for 4-6 months in patients with higher-risk disease, which includes those with unfavorable intermediate-risk, high-risk, very-high-risk, clinical node-positive, oligometastatic, and low-volume M1 disease, as well as patients who have undergone prostatectomy.
The authors noted that in-person consultations and return visits should be converted to “timely remote telehealth visits” for these patients. After these patients have started treatment, androgen deprivation therapy “can allow for further deferral of radiotherapy as necessary based on the nature of the ongoing epidemic.”
In cases where radiotherapy cannot be deferred safely, “the shortest fractionation schedule should be adopted that has evidence of safety and efficacy,” the authors wrote.
They acknowledged that these recommendations are only applicable to patients not infected with COVID-19. In cases of suspected or confirmed COVID-19, local institutional policies and practices should be followed.
The authors further explained that, due to the rapidly evolving nature of the COVID-19 pandemic, state and federal guidelines should be followed when made available.
The authors reported having no conflicts of interest. No funding sources were reported.
SOURCE: Zaorsky NG et al. Adv Radiat Oncol. 2020 Apr 1. doi: 10.1016/j.adro.2020.03.010.
The framework involves using remote visits via telemedicine, avoiding radiotherapy in applicable cases, deferring radiotherapy as appropriate, and shortening the fractionation schedule of treatment based on safety and efficacy parameters.
Nicholas G. Zaorsky, MD, of Penn State Cancer Institute in Hershey, Pennsylvania, and colleagues described the framework and recommendations in Advances in Radiation Oncology.
The authors systematically reviewed the body of literature for evidence pertaining to the safe use of telemedicine, avoidance or deferral of radiotherapy, and optimal use of androgen deprivation therapy for patients with prostate cancer. The team also reviewed best practices for patients undergoing radiotherapy based on disease risk.
Based on their findings, Dr. Zaorsky and colleagues recommended that, during the pandemic, all consultations and return visits become telehealth visits. “Very few prostate cancer patients require an in-person visit during a pandemic,” the authors wrote.
Lower-risk disease
Dr. Zaorsky and colleagues recommended avoiding radiotherapy in patients with very-low-, low-, and favorable intermediate-risk disease. The authors said data suggest that, in general, treatment can be safely deferred in these patients “until after pandemic-related restrictions have been lifted.” However, this recommendation presumes the pandemic will wane over the next 12 months.
“I reassure my patients with very-low- and low-risk prostate cancer that the preferred, evidence-based treatment for patients in these categories is active surveillance,” said study author Amar U. Kishan, MD, of the University of California, Los Angeles.
“If surveillance is an option, then delaying treatment must be reasonable [during the pandemic],” he added. “For favorable intermediate-risk disease, I [review] the data supporting this approach and discuss that short delays are very unlikely to compromise outcomes.”
Higher-risk disease
The authors recommended deferral of radiotherapy for 4-6 months in patients with higher-risk disease, which includes those with unfavorable intermediate-risk, high-risk, very-high-risk, clinical node-positive, oligometastatic, and low-volume M1 disease, as well as patients who have undergone prostatectomy.
The authors noted that in-person consultations and return visits should be converted to “timely remote telehealth visits” for these patients. After these patients have started treatment, androgen deprivation therapy “can allow for further deferral of radiotherapy as necessary based on the nature of the ongoing epidemic.”
In cases where radiotherapy cannot be deferred safely, “the shortest fractionation schedule should be adopted that has evidence of safety and efficacy,” the authors wrote.
They acknowledged that these recommendations are only applicable to patients not infected with COVID-19. In cases of suspected or confirmed COVID-19, local institutional policies and practices should be followed.
The authors further explained that, due to the rapidly evolving nature of the COVID-19 pandemic, state and federal guidelines should be followed when made available.
The authors reported having no conflicts of interest. No funding sources were reported.
SOURCE: Zaorsky NG et al. Adv Radiat Oncol. 2020 Apr 1. doi: 10.1016/j.adro.2020.03.010.
FROM ADVANCES IN RADIATION ONCOLOGY
First report of MM patient successfully treated for COVID-19 with tocilizumab
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
FROM BLOOD ADVANCES
Rethink urologic cancer treatment in the era of COVID-19
according to an editorial set to be published in European Urology.
“Regimens with a clear survival advantage should be prioritized, with curative treatments remaining mandatory,” wrote Silke Gillessen Sommer, MD, of Istituto Oncologico della Svizzera Italiana in Bellizona, Switzerland, and Thomas Powles, MD, of Barts Cancer Institute in London.
However, it may be appropriate to stop or delay therapies with modest or unproven survival benefits. “Delaying the start of therapy ... is an appropriate measure for many of the therapies in urology cancer,” they wrote.
Timely recommendations for oncologists
The COVID-19 pandemic is limiting resources for cancer, noted Zachery Reichert, MD, PhD, a urological oncologist and assistant professor at the University of Michigan, Ann Arbor, who was asked for his thoughts about the editorial.
Oncologists and oncology nurses are being shifted to care for COVID-19 patients, space once devoted to cancer care is being repurposed for the pandemic, and personal protective equipment needed to prepare chemotherapies is in short supply.
Meanwhile, cancer patients are at increased risk of dying from the virus (Lancet Oncol. 2020;21:335-7), so there’s a need to minimize their contact with the health care system to protect them from nosocomial infection, and a need to keep their immune system as strong as possible to fight it off.
To help cancer patients fight off infection and keep them out of the hospital, the editorialists recommended growth factors and prophylactic antibiotics after chemotherapy, palliative therapies at doses that avoid febrile neutropenia, discontinuing steroids or at least reducing their doses, and avoiding bisphosphonates if they involve potential COVID-19 exposure in medical facilities.
The advice in the editorial mirrors many of the discussions going on right now at the University of Michigan, Dr. Reichert said, and perhaps other oncology services across the United States.
It will come down to how severe the pandemic becomes locally, but he said it seems likely “a lot of us are going to be wearing a different hat for a while.”
Patients who have symptoms from a growing tumor will likely take precedence at the university, but treatment might be postponed until after COVID-19 peaks if tumors don’t affect quality of life. Also, bladder cancer surgery will probably remain urgent “because the longer you wait, the worse the outcomes,” but perhaps not prostate and kidney cancer surgery, where delay is safer, Dr. Reichert said.
Prostate/renal cancers and germ cell tumors
The editorialists noted that oral androgen receptor therapy should be preferred over chemotherapy for prostate cancer. Dr. Reichert explained that’s because androgen blockade is effective, requires less contact with health care providers, and doesn’t suppress the immune system or tie up hospital resources as much as chemotherapy. “In the world we are in right now, oral pills are a better choice,” he said.
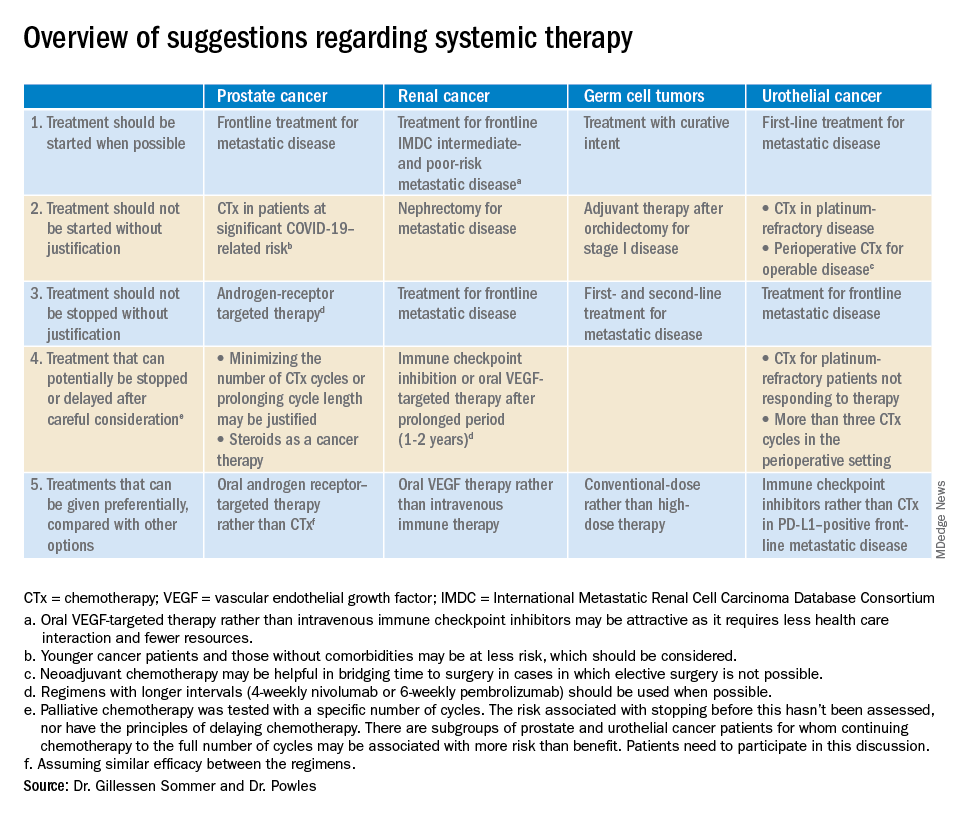
The editorialists recommended against both nephrectomy for metastatic renal cancer and adjuvant therapy after orchidectomy for stage 1 germ cell tumors for similar reasons, and also because there’s minimal evidence of benefit.
Dr. Powles and Dr. Gillessen Sommer suggested considering a break from immune checkpoint inhibitors (ICIs) and oral vascular endothelial growth factors (VEGFs) for renal cancer patients who have been on them a year or two. It’s something that would be considered even under normal circumstances, Dr. Reichert explained, but it’s more urgent now to keep people out of the hospital. VEGFs should also be prioritized over ICIs; they have similar efficacy in renal cancer, but VEGFs are a pill.
They also called for oncologists to favor conventional-dose treatments for germ cell tumors over high-dose treatments, meaning bone marrow transplants or high-intensity chemotherapy. Amid a pandemic, the preference is for options “that don’t require a hospital bed,” Dr. Reichert said.
Urothelial cancer
Dr. Powles and Dr. Gillessen Sommer suggested not starting or continuing second-line chemotherapies in urothelial cancer patients refractory to first-line platinum-based therapies. The chance they will respond to second-line options is low, perhaps around 10%. That might have been enough before the pandemic, but it’s less justified amid resource shortages and the risk of COVID-19 in the infusion suite, Dr. Reichert explained.
Along the same lines, they also suggested reconsidering perioperative chemotherapy for urothelial cancer, and, if it’s still a go, recommended against going past three cycles, as the benefits in both scenarios are likely marginal. However, if COVID-19 cancels surgeries, neoadjuvant therapy might be the right – and only – call, according to the editorialists.
They recommended prioritizing ICIs over chemotherapy in patients with metastatic urothelial cancer who are positive for programmed death-ligand 1 (PD-L1). PD-L1–positive patients have a good chance of responding, and ICIs don’t suppress the immune system.
“Chemotherapy still has a slightly higher percent response, but right now, this is a better choice for” PD-L1-positive patients, Dr. Reichert said.
Dr. Gillessen Sommer and Dr. Powles disclosed ties to Bristol-Myers Squibb, Roche, and numerous other companies. Dr. Reichert has no relevant disclosures.
SOURCE: Gillessen Sommer S, Powles T. “Advice regarding systemic therapy in patients with urological cancers during the COVID-19 pandemic.” Eur Urol. https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb/assets/raw/Health%20Advance/journals/eururo/EURUROL-D-20-00382-1585928967060.pdf.
according to an editorial set to be published in European Urology.
“Regimens with a clear survival advantage should be prioritized, with curative treatments remaining mandatory,” wrote Silke Gillessen Sommer, MD, of Istituto Oncologico della Svizzera Italiana in Bellizona, Switzerland, and Thomas Powles, MD, of Barts Cancer Institute in London.
However, it may be appropriate to stop or delay therapies with modest or unproven survival benefits. “Delaying the start of therapy ... is an appropriate measure for many of the therapies in urology cancer,” they wrote.
Timely recommendations for oncologists
The COVID-19 pandemic is limiting resources for cancer, noted Zachery Reichert, MD, PhD, a urological oncologist and assistant professor at the University of Michigan, Ann Arbor, who was asked for his thoughts about the editorial.
Oncologists and oncology nurses are being shifted to care for COVID-19 patients, space once devoted to cancer care is being repurposed for the pandemic, and personal protective equipment needed to prepare chemotherapies is in short supply.
Meanwhile, cancer patients are at increased risk of dying from the virus (Lancet Oncol. 2020;21:335-7), so there’s a need to minimize their contact with the health care system to protect them from nosocomial infection, and a need to keep their immune system as strong as possible to fight it off.
To help cancer patients fight off infection and keep them out of the hospital, the editorialists recommended growth factors and prophylactic antibiotics after chemotherapy, palliative therapies at doses that avoid febrile neutropenia, discontinuing steroids or at least reducing their doses, and avoiding bisphosphonates if they involve potential COVID-19 exposure in medical facilities.
The advice in the editorial mirrors many of the discussions going on right now at the University of Michigan, Dr. Reichert said, and perhaps other oncology services across the United States.
It will come down to how severe the pandemic becomes locally, but he said it seems likely “a lot of us are going to be wearing a different hat for a while.”
Patients who have symptoms from a growing tumor will likely take precedence at the university, but treatment might be postponed until after COVID-19 peaks if tumors don’t affect quality of life. Also, bladder cancer surgery will probably remain urgent “because the longer you wait, the worse the outcomes,” but perhaps not prostate and kidney cancer surgery, where delay is safer, Dr. Reichert said.
Prostate/renal cancers and germ cell tumors
The editorialists noted that oral androgen receptor therapy should be preferred over chemotherapy for prostate cancer. Dr. Reichert explained that’s because androgen blockade is effective, requires less contact with health care providers, and doesn’t suppress the immune system or tie up hospital resources as much as chemotherapy. “In the world we are in right now, oral pills are a better choice,” he said.

The editorialists recommended against both nephrectomy for metastatic renal cancer and adjuvant therapy after orchidectomy for stage 1 germ cell tumors for similar reasons, and also because there’s minimal evidence of benefit.
Dr. Powles and Dr. Gillessen Sommer suggested considering a break from immune checkpoint inhibitors (ICIs) and oral vascular endothelial growth factors (VEGFs) for renal cancer patients who have been on them a year or two. It’s something that would be considered even under normal circumstances, Dr. Reichert explained, but it’s more urgent now to keep people out of the hospital. VEGFs should also be prioritized over ICIs; they have similar efficacy in renal cancer, but VEGFs are a pill.
They also called for oncologists to favor conventional-dose treatments for germ cell tumors over high-dose treatments, meaning bone marrow transplants or high-intensity chemotherapy. Amid a pandemic, the preference is for options “that don’t require a hospital bed,” Dr. Reichert said.
Urothelial cancer
Dr. Powles and Dr. Gillessen Sommer suggested not starting or continuing second-line chemotherapies in urothelial cancer patients refractory to first-line platinum-based therapies. The chance they will respond to second-line options is low, perhaps around 10%. That might have been enough before the pandemic, but it’s less justified amid resource shortages and the risk of COVID-19 in the infusion suite, Dr. Reichert explained.
Along the same lines, they also suggested reconsidering perioperative chemotherapy for urothelial cancer, and, if it’s still a go, recommended against going past three cycles, as the benefits in both scenarios are likely marginal. However, if COVID-19 cancels surgeries, neoadjuvant therapy might be the right – and only – call, according to the editorialists.
They recommended prioritizing ICIs over chemotherapy in patients with metastatic urothelial cancer who are positive for programmed death-ligand 1 (PD-L1). PD-L1–positive patients have a good chance of responding, and ICIs don’t suppress the immune system.
“Chemotherapy still has a slightly higher percent response, but right now, this is a better choice for” PD-L1-positive patients, Dr. Reichert said.
Dr. Gillessen Sommer and Dr. Powles disclosed ties to Bristol-Myers Squibb, Roche, and numerous other companies. Dr. Reichert has no relevant disclosures.
SOURCE: Gillessen Sommer S, Powles T. “Advice regarding systemic therapy in patients with urological cancers during the COVID-19 pandemic.” Eur Urol. https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb/assets/raw/Health%20Advance/journals/eururo/EURUROL-D-20-00382-1585928967060.pdf.
according to an editorial set to be published in European Urology.
“Regimens with a clear survival advantage should be prioritized, with curative treatments remaining mandatory,” wrote Silke Gillessen Sommer, MD, of Istituto Oncologico della Svizzera Italiana in Bellizona, Switzerland, and Thomas Powles, MD, of Barts Cancer Institute in London.
However, it may be appropriate to stop or delay therapies with modest or unproven survival benefits. “Delaying the start of therapy ... is an appropriate measure for many of the therapies in urology cancer,” they wrote.
Timely recommendations for oncologists
The COVID-19 pandemic is limiting resources for cancer, noted Zachery Reichert, MD, PhD, a urological oncologist and assistant professor at the University of Michigan, Ann Arbor, who was asked for his thoughts about the editorial.
Oncologists and oncology nurses are being shifted to care for COVID-19 patients, space once devoted to cancer care is being repurposed for the pandemic, and personal protective equipment needed to prepare chemotherapies is in short supply.
Meanwhile, cancer patients are at increased risk of dying from the virus (Lancet Oncol. 2020;21:335-7), so there’s a need to minimize their contact with the health care system to protect them from nosocomial infection, and a need to keep their immune system as strong as possible to fight it off.
To help cancer patients fight off infection and keep them out of the hospital, the editorialists recommended growth factors and prophylactic antibiotics after chemotherapy, palliative therapies at doses that avoid febrile neutropenia, discontinuing steroids or at least reducing their doses, and avoiding bisphosphonates if they involve potential COVID-19 exposure in medical facilities.
The advice in the editorial mirrors many of the discussions going on right now at the University of Michigan, Dr. Reichert said, and perhaps other oncology services across the United States.
It will come down to how severe the pandemic becomes locally, but he said it seems likely “a lot of us are going to be wearing a different hat for a while.”
Patients who have symptoms from a growing tumor will likely take precedence at the university, but treatment might be postponed until after COVID-19 peaks if tumors don’t affect quality of life. Also, bladder cancer surgery will probably remain urgent “because the longer you wait, the worse the outcomes,” but perhaps not prostate and kidney cancer surgery, where delay is safer, Dr. Reichert said.
Prostate/renal cancers and germ cell tumors
The editorialists noted that oral androgen receptor therapy should be preferred over chemotherapy for prostate cancer. Dr. Reichert explained that’s because androgen blockade is effective, requires less contact with health care providers, and doesn’t suppress the immune system or tie up hospital resources as much as chemotherapy. “In the world we are in right now, oral pills are a better choice,” he said.

The editorialists recommended against both nephrectomy for metastatic renal cancer and adjuvant therapy after orchidectomy for stage 1 germ cell tumors for similar reasons, and also because there’s minimal evidence of benefit.
Dr. Powles and Dr. Gillessen Sommer suggested considering a break from immune checkpoint inhibitors (ICIs) and oral vascular endothelial growth factors (VEGFs) for renal cancer patients who have been on them a year or two. It’s something that would be considered even under normal circumstances, Dr. Reichert explained, but it’s more urgent now to keep people out of the hospital. VEGFs should also be prioritized over ICIs; they have similar efficacy in renal cancer, but VEGFs are a pill.
They also called for oncologists to favor conventional-dose treatments for germ cell tumors over high-dose treatments, meaning bone marrow transplants or high-intensity chemotherapy. Amid a pandemic, the preference is for options “that don’t require a hospital bed,” Dr. Reichert said.
Urothelial cancer
Dr. Powles and Dr. Gillessen Sommer suggested not starting or continuing second-line chemotherapies in urothelial cancer patients refractory to first-line platinum-based therapies. The chance they will respond to second-line options is low, perhaps around 10%. That might have been enough before the pandemic, but it’s less justified amid resource shortages and the risk of COVID-19 in the infusion suite, Dr. Reichert explained.
Along the same lines, they also suggested reconsidering perioperative chemotherapy for urothelial cancer, and, if it’s still a go, recommended against going past three cycles, as the benefits in both scenarios are likely marginal. However, if COVID-19 cancels surgeries, neoadjuvant therapy might be the right – and only – call, according to the editorialists.
They recommended prioritizing ICIs over chemotherapy in patients with metastatic urothelial cancer who are positive for programmed death-ligand 1 (PD-L1). PD-L1–positive patients have a good chance of responding, and ICIs don’t suppress the immune system.
“Chemotherapy still has a slightly higher percent response, but right now, this is a better choice for” PD-L1-positive patients, Dr. Reichert said.
Dr. Gillessen Sommer and Dr. Powles disclosed ties to Bristol-Myers Squibb, Roche, and numerous other companies. Dr. Reichert has no relevant disclosures.
SOURCE: Gillessen Sommer S, Powles T. “Advice regarding systemic therapy in patients with urological cancers during the COVID-19 pandemic.” Eur Urol. https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb/assets/raw/Health%20Advance/journals/eururo/EURUROL-D-20-00382-1585928967060.pdf.
FROM EUROPEAN UROLOGY
Water-only fasting may reduce chemo modifications, hospital admissions
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
Patients with gynecologic malignancies who consumed only water for 24 hours before and 24 hours after each chemotherapy cycle had fewer dose delays and reductions compared with patients who didn’t fast, results of a small study showed.
The study included 23 women with ovarian, uterine, or cervical cancer, most of whom received platinum-based chemotherapy and taxanes. Fewer treatment modifications were required among the 11 patients randomized to a 24-hour water-only fast before and after each chemotherapy cycle than among the 12 patients randomized to standard care. Furthermore, there were no hospital admissions in the fasting group and two admissions in the control group, according to study author Courtney J. Riedinger, MD, of the University of Tennessee Medical Center in Knoxville.
She and her colleagues detailed the rationale and results of this study in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. Data have been updated from the abstract.
Rationale
“There’s a lot of new research and interest about nonpharmacologic interventions and lifestyle modifications to help patients cope with chemotherapy and even help with treatment, potentially,” Dr. Riedinger said in an interview.
“We decided to test water-only fasting because there’s not much data about the cell-fitness effects of fasting” on chemotherapy outcomes, she said.
Pre-chemotherapy fasting is based on the concept of differential stress resistance intended to protect normal cells but not cancer cells from the effects of chemotherapy. Fasting decreases levels of insulin-like growth factor 1, which leads healthy cells to enter a protective state by decreasing cell growth and proliferation. Cancer cells, in contrast, cannot enter the protective state, and are therefore more vulnerable than healthy, quiescent cells when exposed to drugs that target the cell cycle, Dr. Riedinger and colleagues noted.
The team cited two studies suggesting a benefit from fasting prior to chemotherapy. In the first study, mice that underwent 48-60 hours of short-term fasting were significantly less likely to die after exposure to a high dose of etoposide, compared with mice that did not fast before exposure (PNAS; 105[24]: 8215-822).
The second study showed that breast and ovarian cancer patients had improved quality of life scores and decreased fatigue when they fasted for 36 hours before and 24 hours after a chemotherapy cycle (BMC Cancer;18: article 476).
Study details
Dr. Riedinger and colleagues conducted a nonblinded, randomized trial of fasting in women, aged 34-73 years, who had gynecologic malignancies treated with a planned six cycles of chemotherapy. The patients were instructed to maintain a water-only fast for 24 hours before and 24 hours after each cycle. Controls did not fast.
Patient functional status and quality of life were investigated with the National Comprehensive Cancer Network–Functional Assessment of Cancer Therapy Ovarian Symptom Index (NCCN-FACT FOSI-18). Questionnaires were completed at each chemotherapy visit, and the records were reviewed to evaluate compliance, changes in treatment plan, and hospitalizations.
In all, 92% of chemotherapy cycles were completed with fasting as directed.
There were no significant differences in any of the study measures between patients who fasted and those who did not. However, this study was not powered to detect a difference, according to Dr. Riedinger.
Still, there were trends suggesting a benefit to fasting. Fasting patients had a higher mean change in NCCN-FACT FOSI-18 score compared with controls – increases of 5.11 and .22, respectively.
Five patients in the fasting group required changes to their treatment regimen, compared with eight patients in the control group. In addition, there were no hospital admissions in the fasting group and two admissions in the control group.
Patients tolerated the fast well without significant weight loss, and there were no grade 3 or 4 toxicities among patients who fasted.
The investigators are planning a larger study to further evaluate the effect of fasting on quality of life scores and treatment, and to evaluate the effects of fasting on hematologic toxicities. Future studies will focus on the optimal duration of fasting and the use of fasting-mimicking diets to allow for longer fasting periods, Dr. Riedinger said.
The study was internally funded. The authors reported no conflicts of interest.
SOURCE: Riedinger CJ et al. SGO 2020. Abstract 22.
FROM SGO 2020
Advice from the front lines: How cancer centers can cope with COVID-19
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
20% with cancer on checkpoint inhibitors get thyroid dysfunction
new research suggests.
Immune checkpoint inhibitors have revolutionized the treatment of many different types of cancers, but can also trigger a variety of immune-related adverse effects. As these drugs become more widely used, rates of these events appear to be more common in the real-world compared with clinical trial settings.
In their new study, Zoe Quandt, MD, of the University of California, San Francisco (UCSF), and colleagues specifically looked at thyroid dysfunction in their own institution’s EHR data and found more than double the rate of hypothyroidism and more than triple the rate of hyperthyroidism, compared with rates in published trials.
Moreover, in contrast to previous studies that have found differences in thyroid dysfunction by checkpoint inhibitor type, Dr. Quandt and colleagues instead found significant differences by cancer type.
Dr. Quandt presented the findings during a virtual press briefing held March 31originally scheduled for ENDO 2020.
“Thyroid dysfunction following checkpoint inhibitor therapy appears to be much more common than was previously reported in clinical trials, and this is one of the first studies to show differences by cancer type rather than by checkpoint inhibitor type,” Dr. Quandt said during the presentation.
However, she also cautioned that there’s “a lot more research to be done to validate case definitions and validate these findings.”
Asked to comment, endocrinologist David C. Lieb, MD, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said in an interview, “These drugs are becoming so much more commonly used, so it’s not surprising that we’re seeing more endocrine complications, especially thyroid disease.”
“Endocrinologists need to work closely with oncologists to make sure patients are being screened and followed appropriately.”
‘A much higher percentage than we were expecting’
Dr. Quandt’s study included 1,146 individuals treated with checkpoint inhibitors at UCSF during 2012-2018 who did not have thyroid cancer or preexisting thyroid dysfunction.
Pembrolizumab (Keytruda) was the most common treatment (45%), followed by nivolumab (Opdivo) (20%). Less than 10% of patients received atezolizumab (Tecentriq), durvalumab (Imfizi), ipilimumab (Yervoy) monotherapy, combined ipilimumab/nivolumab, or other combinations of checkpoint inhibitors.
A total of 19.1% developed thyroid disease, with 13.4% having hypothyroidism and 9.5% hyperthyroidism. These figures far exceed those found in a recent meta-analysis of 38 randomized clinical trials of checkpoint inhibitors that included 7551 patients.
“Using this approach, we found a much higher percentage of patients who developed thyroid dysfunction than we were expecting,” Dr. Quandt said.
In both cases, the two categories – hypothyroidism and hyperthyroidism – aren’t mutually exclusive as hypothyroidism can arise de novo or subsequent to hyperthyroidism.
Dr Lieb commented, “It would be interesting to see what the causes of hyperthyroidism are – thyroiditis or Graves disease.”
Dr. Quandt mentioned a possible reason for the large difference between clinical trial and real-world data.
“Once we’re actually using these drugs outside of clinical trials, some of the restrictions about using them in people with other autoimmune diseases have been lifted, so my guess is that as we give them to a broader population we’re seeing more of these [adverse effects],” she suggested.
Also, “In the initial trials, people weren’t quite as aware of the possibilities of these side effects, so now we’re doing many more labs. Patients get thyroid function tests with every infusion, so I think we’re probably catching more patients who develop disease.”
Differences by cancer type, not checkpoint inhibitor type
And in a new twist, Dr. Quandt found that, in contrast to the differences seen by checkpoint inhibitor type in randomized trials, “surprisingly, we found that this difference did not reach statistical significance.”
“Instead, we saw that cancer type was associated with development of thyroid dysfunction, even after taking checkpoint inhibitor type into account.”
The percentages of patients who developed thyroid dysfunction ranged from 9.7% of those with glioblastoma to 40.0% of those with renal cell carcinoma.
The reason for this is not clear, said Dr. Quandt in an interview.
One possibility relates to other treatments patients with cancer also receive. In renal cell carcinoma, for example, patients also are treated with tyrosine kinase inhibitors, which can also cause thyroid dysfunction, so they may be more susceptible. Or there may be shared antigens activating the immune system.
“That’s definitely one of the questions we’re looking at,” she said.
Dr. Quandt and Dr. Lieb have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Immune checkpoint inhibitors have revolutionized the treatment of many different types of cancers, but can also trigger a variety of immune-related adverse effects. As these drugs become more widely used, rates of these events appear to be more common in the real-world compared with clinical trial settings.
In their new study, Zoe Quandt, MD, of the University of California, San Francisco (UCSF), and colleagues specifically looked at thyroid dysfunction in their own institution’s EHR data and found more than double the rate of hypothyroidism and more than triple the rate of hyperthyroidism, compared with rates in published trials.
Moreover, in contrast to previous studies that have found differences in thyroid dysfunction by checkpoint inhibitor type, Dr. Quandt and colleagues instead found significant differences by cancer type.
Dr. Quandt presented the findings during a virtual press briefing held March 31originally scheduled for ENDO 2020.
“Thyroid dysfunction following checkpoint inhibitor therapy appears to be much more common than was previously reported in clinical trials, and this is one of the first studies to show differences by cancer type rather than by checkpoint inhibitor type,” Dr. Quandt said during the presentation.
However, she also cautioned that there’s “a lot more research to be done to validate case definitions and validate these findings.”
Asked to comment, endocrinologist David C. Lieb, MD, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said in an interview, “These drugs are becoming so much more commonly used, so it’s not surprising that we’re seeing more endocrine complications, especially thyroid disease.”
“Endocrinologists need to work closely with oncologists to make sure patients are being screened and followed appropriately.”
‘A much higher percentage than we were expecting’
Dr. Quandt’s study included 1,146 individuals treated with checkpoint inhibitors at UCSF during 2012-2018 who did not have thyroid cancer or preexisting thyroid dysfunction.
Pembrolizumab (Keytruda) was the most common treatment (45%), followed by nivolumab (Opdivo) (20%). Less than 10% of patients received atezolizumab (Tecentriq), durvalumab (Imfizi), ipilimumab (Yervoy) monotherapy, combined ipilimumab/nivolumab, or other combinations of checkpoint inhibitors.
A total of 19.1% developed thyroid disease, with 13.4% having hypothyroidism and 9.5% hyperthyroidism. These figures far exceed those found in a recent meta-analysis of 38 randomized clinical trials of checkpoint inhibitors that included 7551 patients.
“Using this approach, we found a much higher percentage of patients who developed thyroid dysfunction than we were expecting,” Dr. Quandt said.
In both cases, the two categories – hypothyroidism and hyperthyroidism – aren’t mutually exclusive as hypothyroidism can arise de novo or subsequent to hyperthyroidism.
Dr Lieb commented, “It would be interesting to see what the causes of hyperthyroidism are – thyroiditis or Graves disease.”
Dr. Quandt mentioned a possible reason for the large difference between clinical trial and real-world data.
“Once we’re actually using these drugs outside of clinical trials, some of the restrictions about using them in people with other autoimmune diseases have been lifted, so my guess is that as we give them to a broader population we’re seeing more of these [adverse effects],” she suggested.
Also, “In the initial trials, people weren’t quite as aware of the possibilities of these side effects, so now we’re doing many more labs. Patients get thyroid function tests with every infusion, so I think we’re probably catching more patients who develop disease.”
Differences by cancer type, not checkpoint inhibitor type
And in a new twist, Dr. Quandt found that, in contrast to the differences seen by checkpoint inhibitor type in randomized trials, “surprisingly, we found that this difference did not reach statistical significance.”
“Instead, we saw that cancer type was associated with development of thyroid dysfunction, even after taking checkpoint inhibitor type into account.”
The percentages of patients who developed thyroid dysfunction ranged from 9.7% of those with glioblastoma to 40.0% of those with renal cell carcinoma.
The reason for this is not clear, said Dr. Quandt in an interview.
One possibility relates to other treatments patients with cancer also receive. In renal cell carcinoma, for example, patients also are treated with tyrosine kinase inhibitors, which can also cause thyroid dysfunction, so they may be more susceptible. Or there may be shared antigens activating the immune system.
“That’s definitely one of the questions we’re looking at,” she said.
Dr. Quandt and Dr. Lieb have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Immune checkpoint inhibitors have revolutionized the treatment of many different types of cancers, but can also trigger a variety of immune-related adverse effects. As these drugs become more widely used, rates of these events appear to be more common in the real-world compared with clinical trial settings.
In their new study, Zoe Quandt, MD, of the University of California, San Francisco (UCSF), and colleagues specifically looked at thyroid dysfunction in their own institution’s EHR data and found more than double the rate of hypothyroidism and more than triple the rate of hyperthyroidism, compared with rates in published trials.
Moreover, in contrast to previous studies that have found differences in thyroid dysfunction by checkpoint inhibitor type, Dr. Quandt and colleagues instead found significant differences by cancer type.
Dr. Quandt presented the findings during a virtual press briefing held March 31originally scheduled for ENDO 2020.
“Thyroid dysfunction following checkpoint inhibitor therapy appears to be much more common than was previously reported in clinical trials, and this is one of the first studies to show differences by cancer type rather than by checkpoint inhibitor type,” Dr. Quandt said during the presentation.
However, she also cautioned that there’s “a lot more research to be done to validate case definitions and validate these findings.”
Asked to comment, endocrinologist David C. Lieb, MD, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said in an interview, “These drugs are becoming so much more commonly used, so it’s not surprising that we’re seeing more endocrine complications, especially thyroid disease.”
“Endocrinologists need to work closely with oncologists to make sure patients are being screened and followed appropriately.”
‘A much higher percentage than we were expecting’
Dr. Quandt’s study included 1,146 individuals treated with checkpoint inhibitors at UCSF during 2012-2018 who did not have thyroid cancer or preexisting thyroid dysfunction.
Pembrolizumab (Keytruda) was the most common treatment (45%), followed by nivolumab (Opdivo) (20%). Less than 10% of patients received atezolizumab (Tecentriq), durvalumab (Imfizi), ipilimumab (Yervoy) monotherapy, combined ipilimumab/nivolumab, or other combinations of checkpoint inhibitors.
A total of 19.1% developed thyroid disease, with 13.4% having hypothyroidism and 9.5% hyperthyroidism. These figures far exceed those found in a recent meta-analysis of 38 randomized clinical trials of checkpoint inhibitors that included 7551 patients.
“Using this approach, we found a much higher percentage of patients who developed thyroid dysfunction than we were expecting,” Dr. Quandt said.
In both cases, the two categories – hypothyroidism and hyperthyroidism – aren’t mutually exclusive as hypothyroidism can arise de novo or subsequent to hyperthyroidism.
Dr Lieb commented, “It would be interesting to see what the causes of hyperthyroidism are – thyroiditis or Graves disease.”
Dr. Quandt mentioned a possible reason for the large difference between clinical trial and real-world data.
“Once we’re actually using these drugs outside of clinical trials, some of the restrictions about using them in people with other autoimmune diseases have been lifted, so my guess is that as we give them to a broader population we’re seeing more of these [adverse effects],” she suggested.
Also, “In the initial trials, people weren’t quite as aware of the possibilities of these side effects, so now we’re doing many more labs. Patients get thyroid function tests with every infusion, so I think we’re probably catching more patients who develop disease.”
Differences by cancer type, not checkpoint inhibitor type
And in a new twist, Dr. Quandt found that, in contrast to the differences seen by checkpoint inhibitor type in randomized trials, “surprisingly, we found that this difference did not reach statistical significance.”
“Instead, we saw that cancer type was associated with development of thyroid dysfunction, even after taking checkpoint inhibitor type into account.”
The percentages of patients who developed thyroid dysfunction ranged from 9.7% of those with glioblastoma to 40.0% of those with renal cell carcinoma.
The reason for this is not clear, said Dr. Quandt in an interview.
One possibility relates to other treatments patients with cancer also receive. In renal cell carcinoma, for example, patients also are treated with tyrosine kinase inhibitors, which can also cause thyroid dysfunction, so they may be more susceptible. Or there may be shared antigens activating the immune system.
“That’s definitely one of the questions we’re looking at,” she said.
Dr. Quandt and Dr. Lieb have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Maintaining cancer care in the face of COVID-19
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Medical oncologist Anne Chiang, MD, PhD, is scrambling to maintain cancer care in New Haven, Connecticut, while COVID-19 advances unrelentingly. As deputy chief medical officer of the Smilow Cancer Network, the largest cancer care delivery system in Connecticut and Rhode Island, she has no illusions about dodging what’s unfolding just 2 hours down the road in New York City.
“They’re trying their best to continue active cancer treatment but it’s getting harder,” she says of her colleagues in the thick of the pandemic. “We have to be prepared for it here.”
In anticipation of what’s coming, her team has just emptied the top three floors of the Smilow Cancer Hospital, moving 60 patients by ambulance and other medical transport to a different hospital nearby.
The move frees the Smilow Cancer hospital’s negative-pressure wards for the anticipated wave of COVID-19 patients. It will keep the virus sealed off from the rest of the hospital. But in other locations it’s harder to shield patients with cancer from the infection.
Around the state, Smilow Cancer Network’s affiliated hospitals are already treating a growing number of COVID-19 patients, especially at Greenwich Hospital, right on the border with New York state.
To protect patients with cancer, who are among the most vulnerable to the virus, oncologists are embracing telemedicine to allow most patients to stay home.
“We’re really concentrating on decreasing the risk to these patients, with a widespread massive-scale conversion to telehealth,” said Chiang. “This is something that, in the space of about a week, has transformed the care of our patients — it’s a really amazing transformation.”
If anything good comes out of the COVID-19 pandemic, it will be this global adoption of virtual healthcare.
Across the US border in Canada, the medical director of Toronto’s Princess Margaret Cancer Centre is directing a similar transformation.
“We have converted probably about 70% to 80% of our clinic visits to virtual visits,” says radiation oncologist Mary Gospodarowicz, MD.
“We have three priorities: number one, to keep our patients safe; number two, to keep our staff safe, because if staff are sick we won’t be treating anybody; and number three, to treat as many patients with cancer as possible.”
Gospodarowicz woke up last week to a local headline about a woman whose mastectomy had been canceled “because of the coronavirus.” The story exposed the many layers of the COVID-19 crisis. “A lot of hospitals have canceled elective surgeries,” she acknowledged. “For patients who have treatment or surgery deferred, we have a database and we’ll make sure we look after those patients eventually. We have a priority system, so low-risk prostate cancer, very low-risk breast cancer patients are waiting. All the urgent head and neck, breast, and other higher priority surgeries are still being done, but it just depends how it goes. The situation changes every day.”
It’s similar in Los Angeles, at the University of Southern California, says Elizabeth David, MD, a cardiothoracic surgeon with Keck Medicine.
“For thoracic, we just had a conference call with about 30 surgeons around the country going through really nitty-gritty specifics to help with our decision making about what could wait without detriment to the patient – hopefully – and what should be done now,” she told Medscape Medical News.
“There are some hospitals where they are not doing anything but life and death emergency operations, whereas we are still doing our emergent cancer operations in our institution, but we all know – and patients know – that could change from one day to the next. They may think they’re having surgery tomorrow but may get a call saying we can’t do it,” David said.
Many of David’s patients have non–small cell lung cancer, putting them at particular risk with a pulmonary infection like COVID-19. For now, she says delivery of postsurgical chemotherapy and radiotherapy has not been impacted in her area, but her videoconference discussions with patients are much longer – and harder – these days.
“I’ve been in practice a while now and I’ve had numerous conversations with patients this week that I never trained for, and I’ve never known anyone else who has. It’s really hard as a provider to know what to say,” she said.
In cardiothoracic surgery, David said guidance on clinical decision making is coming from the American College of Surgeons, Society of Thoracic Surgery, and American Association of Thoracic Surgeons. Yet, she says each patient is being assessed – and reassessed – individually.
“You have to balance the risk of delaying the intervention with supply issues, hospital exposure issues, the danger to the patient of being in the hospital environment – there’s just so many factors. We’re spending so much time talking through cases, and a lot of times we’re talking about cases we already talked about, but we’re just making sure that based on today’s numbers we should still be moving forward,” she commented.
In Connecticut, Chiang said treatment decisions are also mostly on a case-by-case basis at the moment, although more standardized guidelines are being worked out.
“Our disease teams have been really proactive in terms of offering alternative solutions to patients, creative ways to basically keep them out of the hospital and also reduce the immunosuppressive regimens that we give them,” she said.
Examples include offering endocrine therapy to patients who can’t get breast cancer surgery, or offering alternative drug regimens and dosing schedules. “At this point we haven’t needed to ration actual treatment – patients are continuing to get active therapy if that’s appropriate – it’s more about how can we protect them,” she said. “It’s a complex puzzle of moving pieces.”
In Toronto, Gospodarowicz says newly published medical and radiation oncology guidelines from France are the backbone of her hospital’s policy discussions about treating cancer and protecting patients from COVID-19.
While patients’ concerns are understandable, she says even in the current hot spots of infection, it’s encouraging to know that cancer patients are not being forgotten.
“I recently had email communication with a radiation oncologist in Brescia, one of the worst-affected areas in Italy, and he told me the radiotherapy department has been 60% to 70% capacity, so they still treat 70% these patients, just taking precautions and separating the COVID-positive and negative ones. When we read the stats it looks horrible, but life still goes on and people are still being treated,” she said.
Although telemedicine offers meaningful solutions to the COVID-19 crisis in North America, it may not be possible in other parts of the world.
Web consultations were only just approved in Brazil this week. “We are still discussing how to make it official and reimbursed,” says Rachel Riechelmann, MD, head of clinical oncology at AC Camargo Cancer Center in São Paulo.
To minimize infection risk for patients, Riechelmann says her hospital is doing the following: postponing surgeries in cases where there is good evidence of neoadjuvant treatment, such as total neoadjuvant therapy for rectal cancer; avoiding adjuvant chemo for stage 2 colon cancer; moving to hypofractionated radiotherapy if possible; adopting watchful waiting in grade 1 nonfunctional neuroendocrine tumors; and postponing follow-up visits.
“We do our best,” she wrote in an email. “We keep treating cancer if treatment cannot wait.”
Riechelmann’s center has just launched a trial of hydroxychloroquine and tocilizumab therapy in patients with cancer who have severe COVID-19 and acute respiratory distress syndrome (ARDS).
Meanwhile in New Haven, Chiang says for patients with cancer who are infected with COVID-19, her team is also prognosticating about the fair allocation of limited resources such as ventilators.
“If it ever gets to the point where somebody has to choose between a cancer patient and a noncancer patient in providing life support, it’s really important that people understand that cancer patients are doing very well nowadays and even with a diagnosis of cancer they can potentially live for many years, so that shouldn’t necessarily be a decision-point,” she emphasized.
This article first appeared on Medscape.com.
Barriers to clinical trial participation revealed by gynecologic cancer patients
A survey of gynecologic cancer survivors has revealed why some of these patients don’t participate in clinical trials.
Half of survey respondents with no history of trial participation said their medical team never mentioned the possibility of a trial. About 27% of respondents who never enrolled in a trial said they were interested in trial participation but didn’t qualify, the trial they wanted wasn’t available, their insurance didn’t cover participation, or the trial site was too far away.
Annie Ellis and Mary (Dicey) Jackson Scroggins, who are both ovarian cancer survivors and patient advocates, reported these findings in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“We thought it was important to hear and learn directly from gynecologic cancer survivors,” Ms. Ellis said in an interview. “So we decided to conduct a survey that would expand knowledge about clinical trial participation from a gynecologic cancer patient–specific perspective.”
Ms. Ellis and Ms. Scroggins used survivor networks and social media to distribute a 26-question survey on trial participation. The survey was completed by 189 survivors of gynecologic cancers, 49.19% of whom experienced recurrent disease. The most common diagnoses were ovarian cancer (69.84%) and endometrial or uterine cancer (23.28%).
Perspectives of nonparticipants
Most respondents (65.61%) had never participated in a clinical trial. The most common reason was that the patient’s doctor or medical team never discussed trial participation (50.40%).
There were patients who were interested in trial participation but couldn’t enroll because they didn’t qualify (14.40%), the location was too far away (7.20%), the trial they wanted wasn’t available (4.00%), or their insurance didn’t cover trial participation (1.60%).
Patients who were not interested in trial participation said they didn’t want to receive a placebo (11.20%), they weren’t interested in experimental therapies (3.20%), or they didn’t want to be randomized (2.40%). One patient (1.60%) said she does not trust the medical system.
“Given the frequent conversations about distrust in the medical system, we were surprised that only 1 of the 189 respondents indicated distrust in the medical system as a reason for not participating in a clinical trial,” Ms. Ellis said.
Perspectives of trial participants
Roughly a third of respondents (34.39%) had participated in a clinical trial. Most (86.15%) said they learned about the trial from their doctor. Other sources included the patient’s own research (13.85%), a trial matching service (3.08%), a family member or friend (3.08%), and a support group (1.54%).
The most common reasons patients participated in trials were: “my doctor recommended it,” “to help women in the future,” “to expand my treatment options,” and “to have a chance to benefit personally.”
Additional responses indicated that patients viewed their trial participation in a positive light.
“We were surprised to find that 100% of the respondents who had participated in a clinical trial indicated either that they would participate again (84.62%) or that they were not sure about future participation (15.38%),” Ms. Ellis said. “No respondent indicated that she would not consider another trial. From open comments in the survey, it was clear that even if they did not obtain the result they hoped for or if the experience wasn’t optimal, they maintained the option of participating again.”
Implications and next steps
The survey results suggest there is a need for more discussions about clinical trials with patients who have gynecologic cancers, according to Ms. Ellis and Ms. Scroggins.
“We feel that conversations about clinical trials, with health care team members, should be included at every care decision point, even if – or perhaps especially if – the patient belongs to a group perceived to be unlikely to agree to participate in a trial,” Ms. Ellis said.
“These conversations are necessary with all patients-survivors,” she said, “but they are particularly important and necessary with patients from populations underrepresented in the clinical trial system if we want more representative trial populations, more generalizable results, and the potential for better outcomes for all.”
For their part, Ms. Ellis and Ms. Scroggins plan to conduct more research on this topic to gain additional insights.
“We’d like to conduct a larger survey looking deeper into barriers to and reasons for participation, and to work with medical professionals to develop models of communication to encourage consideration of clinical trials,” Ms. Ellis said. “Additionally, we will work to have a more diverse respondent pool across many dimensions.”
Ms. Ellis is a research advocate on the scientific advisory committee of the Ovarian Cancer National Alliance in Washington. Ms. Scroggins is the director of global outreach and engagement at the International Gynecologic Cancer Society in Louisville, Ken. They have no conflicts of interest.
SOURCE: Ellis A and Scroggins MJ. SGO 2020, Abstract 540.
A survey of gynecologic cancer survivors has revealed why some of these patients don’t participate in clinical trials.
Half of survey respondents with no history of trial participation said their medical team never mentioned the possibility of a trial. About 27% of respondents who never enrolled in a trial said they were interested in trial participation but didn’t qualify, the trial they wanted wasn’t available, their insurance didn’t cover participation, or the trial site was too far away.
Annie Ellis and Mary (Dicey) Jackson Scroggins, who are both ovarian cancer survivors and patient advocates, reported these findings in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“We thought it was important to hear and learn directly from gynecologic cancer survivors,” Ms. Ellis said in an interview. “So we decided to conduct a survey that would expand knowledge about clinical trial participation from a gynecologic cancer patient–specific perspective.”
Ms. Ellis and Ms. Scroggins used survivor networks and social media to distribute a 26-question survey on trial participation. The survey was completed by 189 survivors of gynecologic cancers, 49.19% of whom experienced recurrent disease. The most common diagnoses were ovarian cancer (69.84%) and endometrial or uterine cancer (23.28%).
Perspectives of nonparticipants
Most respondents (65.61%) had never participated in a clinical trial. The most common reason was that the patient’s doctor or medical team never discussed trial participation (50.40%).
There were patients who were interested in trial participation but couldn’t enroll because they didn’t qualify (14.40%), the location was too far away (7.20%), the trial they wanted wasn’t available (4.00%), or their insurance didn’t cover trial participation (1.60%).
Patients who were not interested in trial participation said they didn’t want to receive a placebo (11.20%), they weren’t interested in experimental therapies (3.20%), or they didn’t want to be randomized (2.40%). One patient (1.60%) said she does not trust the medical system.
“Given the frequent conversations about distrust in the medical system, we were surprised that only 1 of the 189 respondents indicated distrust in the medical system as a reason for not participating in a clinical trial,” Ms. Ellis said.
Perspectives of trial participants
Roughly a third of respondents (34.39%) had participated in a clinical trial. Most (86.15%) said they learned about the trial from their doctor. Other sources included the patient’s own research (13.85%), a trial matching service (3.08%), a family member or friend (3.08%), and a support group (1.54%).
The most common reasons patients participated in trials were: “my doctor recommended it,” “to help women in the future,” “to expand my treatment options,” and “to have a chance to benefit personally.”
Additional responses indicated that patients viewed their trial participation in a positive light.
“We were surprised to find that 100% of the respondents who had participated in a clinical trial indicated either that they would participate again (84.62%) or that they were not sure about future participation (15.38%),” Ms. Ellis said. “No respondent indicated that she would not consider another trial. From open comments in the survey, it was clear that even if they did not obtain the result they hoped for or if the experience wasn’t optimal, they maintained the option of participating again.”
Implications and next steps
The survey results suggest there is a need for more discussions about clinical trials with patients who have gynecologic cancers, according to Ms. Ellis and Ms. Scroggins.
“We feel that conversations about clinical trials, with health care team members, should be included at every care decision point, even if – or perhaps especially if – the patient belongs to a group perceived to be unlikely to agree to participate in a trial,” Ms. Ellis said.
“These conversations are necessary with all patients-survivors,” she said, “but they are particularly important and necessary with patients from populations underrepresented in the clinical trial system if we want more representative trial populations, more generalizable results, and the potential for better outcomes for all.”
For their part, Ms. Ellis and Ms. Scroggins plan to conduct more research on this topic to gain additional insights.
“We’d like to conduct a larger survey looking deeper into barriers to and reasons for participation, and to work with medical professionals to develop models of communication to encourage consideration of clinical trials,” Ms. Ellis said. “Additionally, we will work to have a more diverse respondent pool across many dimensions.”
Ms. Ellis is a research advocate on the scientific advisory committee of the Ovarian Cancer National Alliance in Washington. Ms. Scroggins is the director of global outreach and engagement at the International Gynecologic Cancer Society in Louisville, Ken. They have no conflicts of interest.
SOURCE: Ellis A and Scroggins MJ. SGO 2020, Abstract 540.
A survey of gynecologic cancer survivors has revealed why some of these patients don’t participate in clinical trials.
Half of survey respondents with no history of trial participation said their medical team never mentioned the possibility of a trial. About 27% of respondents who never enrolled in a trial said they were interested in trial participation but didn’t qualify, the trial they wanted wasn’t available, their insurance didn’t cover participation, or the trial site was too far away.
Annie Ellis and Mary (Dicey) Jackson Scroggins, who are both ovarian cancer survivors and patient advocates, reported these findings in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“We thought it was important to hear and learn directly from gynecologic cancer survivors,” Ms. Ellis said in an interview. “So we decided to conduct a survey that would expand knowledge about clinical trial participation from a gynecologic cancer patient–specific perspective.”
Ms. Ellis and Ms. Scroggins used survivor networks and social media to distribute a 26-question survey on trial participation. The survey was completed by 189 survivors of gynecologic cancers, 49.19% of whom experienced recurrent disease. The most common diagnoses were ovarian cancer (69.84%) and endometrial or uterine cancer (23.28%).
Perspectives of nonparticipants
Most respondents (65.61%) had never participated in a clinical trial. The most common reason was that the patient’s doctor or medical team never discussed trial participation (50.40%).
There were patients who were interested in trial participation but couldn’t enroll because they didn’t qualify (14.40%), the location was too far away (7.20%), the trial they wanted wasn’t available (4.00%), or their insurance didn’t cover trial participation (1.60%).
Patients who were not interested in trial participation said they didn’t want to receive a placebo (11.20%), they weren’t interested in experimental therapies (3.20%), or they didn’t want to be randomized (2.40%). One patient (1.60%) said she does not trust the medical system.
“Given the frequent conversations about distrust in the medical system, we were surprised that only 1 of the 189 respondents indicated distrust in the medical system as a reason for not participating in a clinical trial,” Ms. Ellis said.
Perspectives of trial participants
Roughly a third of respondents (34.39%) had participated in a clinical trial. Most (86.15%) said they learned about the trial from their doctor. Other sources included the patient’s own research (13.85%), a trial matching service (3.08%), a family member or friend (3.08%), and a support group (1.54%).
The most common reasons patients participated in trials were: “my doctor recommended it,” “to help women in the future,” “to expand my treatment options,” and “to have a chance to benefit personally.”
Additional responses indicated that patients viewed their trial participation in a positive light.
“We were surprised to find that 100% of the respondents who had participated in a clinical trial indicated either that they would participate again (84.62%) or that they were not sure about future participation (15.38%),” Ms. Ellis said. “No respondent indicated that she would not consider another trial. From open comments in the survey, it was clear that even if they did not obtain the result they hoped for or if the experience wasn’t optimal, they maintained the option of participating again.”
Implications and next steps
The survey results suggest there is a need for more discussions about clinical trials with patients who have gynecologic cancers, according to Ms. Ellis and Ms. Scroggins.
“We feel that conversations about clinical trials, with health care team members, should be included at every care decision point, even if – or perhaps especially if – the patient belongs to a group perceived to be unlikely to agree to participate in a trial,” Ms. Ellis said.
“These conversations are necessary with all patients-survivors,” she said, “but they are particularly important and necessary with patients from populations underrepresented in the clinical trial system if we want more representative trial populations, more generalizable results, and the potential for better outcomes for all.”
For their part, Ms. Ellis and Ms. Scroggins plan to conduct more research on this topic to gain additional insights.
“We’d like to conduct a larger survey looking deeper into barriers to and reasons for participation, and to work with medical professionals to develop models of communication to encourage consideration of clinical trials,” Ms. Ellis said. “Additionally, we will work to have a more diverse respondent pool across many dimensions.”
Ms. Ellis is a research advocate on the scientific advisory committee of the Ovarian Cancer National Alliance in Washington. Ms. Scroggins is the director of global outreach and engagement at the International Gynecologic Cancer Society in Louisville, Ken. They have no conflicts of interest.
SOURCE: Ellis A and Scroggins MJ. SGO 2020, Abstract 540.
FROM SGO 2020
Blood test might detect multiple cancer types, study suggests
Investigators led by Minetta C. Liu, MD, a medical oncologist with the Mayo Clinic, Rochester, Minn., studied 6,689 participants – 2,482 with cancers of more than 50 types and 4,207 without cancer – drawn from the Circulating Cell-free Genome Atlas Study and the STRIVE Study populations.
The investigators performed bisulfite sequencing that targeted informative methylation regions of plasma cell-free DNA (cfDNA), and developed and validated a molecular classifier using methylation patterns to detect cancer and determine its tissue of origin.
Test performance was assessed both for cancer overall and for a prespecified set of 12 cancers (anus, bladder, colon/rectum, esophagus, head and neck, liver/bile duct, lung, lymphoma, ovary, pancreas, plasma cell neoplasm, stomach) that account for about 63% of U.S. cancer deaths annually.
Results reported this week in the Annals of Oncology showed that the test had a specificity of 99.3% in the validation cohort, corresponding to a false-positive rate of just 0.7%.
Sensitivity for detecting stage I-III disease was 43.9% for cancer overall and 67.3% for the prespecified set of cancers accounting for the majority of U.S. cancer deaths.
Test sensitivity increased with stage both for cancer overall (18%, 43%, 81%, and 93% for stage I, II, III, and IV disease, respectively) and for the prespecified set of cancers (39%, 69%, 83%, and 92%, respectively).
The test was able to predict a tissue of origin in 96% of samples in which a cancerlike signal was detected, and in 93% of cases, that prediction was accurate.
Some of the patients who had cancer were symptomatic and therefore would not be considered a screening population, Dr. Liu and coinvestigators acknowledged. Also, the test’s potential for reducing mortality remains unknown, and 1-year follow-up to verify cancer-free status was not yet available for all of the individuals without cancer.
“Together, these data provide compelling evidence that targeted methylation analysis of cfDNA can detect and localize a broad range of nonmetastatic and metastatic cancer types including many common and deadly cancers that lack effective screening strategies,” they maintained. The test’s “specificity and sensitivity performance approach ... the goal for population-level screening.”
“Considering the potential value of early detection in deadly malignancies, further evaluation of this test is justified in prospective population-level studies,” the investigators conclude. “Clinical validation in intended use populations is ongoing ... and a study has been initiated that is returning results to health care providers and patients ....”
Dr. Liu disclosed that the Mayo Clinic was compensated for her advisory board activities for GRAIL Inc. The study was supported by GRAIL, and by Princess Margaret Cancer Centre’s McCain Genitourinary BioBank in the department of surgical oncology.
SOURCE: Liu MC et al. Ann Oncol. 2020 Mar 31. doi: 10.1016/j.annonc.2020.02.011.
Investigators led by Minetta C. Liu, MD, a medical oncologist with the Mayo Clinic, Rochester, Minn., studied 6,689 participants – 2,482 with cancers of more than 50 types and 4,207 without cancer – drawn from the Circulating Cell-free Genome Atlas Study and the STRIVE Study populations.
The investigators performed bisulfite sequencing that targeted informative methylation regions of plasma cell-free DNA (cfDNA), and developed and validated a molecular classifier using methylation patterns to detect cancer and determine its tissue of origin.
Test performance was assessed both for cancer overall and for a prespecified set of 12 cancers (anus, bladder, colon/rectum, esophagus, head and neck, liver/bile duct, lung, lymphoma, ovary, pancreas, plasma cell neoplasm, stomach) that account for about 63% of U.S. cancer deaths annually.
Results reported this week in the Annals of Oncology showed that the test had a specificity of 99.3% in the validation cohort, corresponding to a false-positive rate of just 0.7%.
Sensitivity for detecting stage I-III disease was 43.9% for cancer overall and 67.3% for the prespecified set of cancers accounting for the majority of U.S. cancer deaths.
Test sensitivity increased with stage both for cancer overall (18%, 43%, 81%, and 93% for stage I, II, III, and IV disease, respectively) and for the prespecified set of cancers (39%, 69%, 83%, and 92%, respectively).
The test was able to predict a tissue of origin in 96% of samples in which a cancerlike signal was detected, and in 93% of cases, that prediction was accurate.
Some of the patients who had cancer were symptomatic and therefore would not be considered a screening population, Dr. Liu and coinvestigators acknowledged. Also, the test’s potential for reducing mortality remains unknown, and 1-year follow-up to verify cancer-free status was not yet available for all of the individuals without cancer.
“Together, these data provide compelling evidence that targeted methylation analysis of cfDNA can detect and localize a broad range of nonmetastatic and metastatic cancer types including many common and deadly cancers that lack effective screening strategies,” they maintained. The test’s “specificity and sensitivity performance approach ... the goal for population-level screening.”
“Considering the potential value of early detection in deadly malignancies, further evaluation of this test is justified in prospective population-level studies,” the investigators conclude. “Clinical validation in intended use populations is ongoing ... and a study has been initiated that is returning results to health care providers and patients ....”
Dr. Liu disclosed that the Mayo Clinic was compensated for her advisory board activities for GRAIL Inc. The study was supported by GRAIL, and by Princess Margaret Cancer Centre’s McCain Genitourinary BioBank in the department of surgical oncology.
SOURCE: Liu MC et al. Ann Oncol. 2020 Mar 31. doi: 10.1016/j.annonc.2020.02.011.
Investigators led by Minetta C. Liu, MD, a medical oncologist with the Mayo Clinic, Rochester, Minn., studied 6,689 participants – 2,482 with cancers of more than 50 types and 4,207 without cancer – drawn from the Circulating Cell-free Genome Atlas Study and the STRIVE Study populations.
The investigators performed bisulfite sequencing that targeted informative methylation regions of plasma cell-free DNA (cfDNA), and developed and validated a molecular classifier using methylation patterns to detect cancer and determine its tissue of origin.
Test performance was assessed both for cancer overall and for a prespecified set of 12 cancers (anus, bladder, colon/rectum, esophagus, head and neck, liver/bile duct, lung, lymphoma, ovary, pancreas, plasma cell neoplasm, stomach) that account for about 63% of U.S. cancer deaths annually.
Results reported this week in the Annals of Oncology showed that the test had a specificity of 99.3% in the validation cohort, corresponding to a false-positive rate of just 0.7%.
Sensitivity for detecting stage I-III disease was 43.9% for cancer overall and 67.3% for the prespecified set of cancers accounting for the majority of U.S. cancer deaths.
Test sensitivity increased with stage both for cancer overall (18%, 43%, 81%, and 93% for stage I, II, III, and IV disease, respectively) and for the prespecified set of cancers (39%, 69%, 83%, and 92%, respectively).
The test was able to predict a tissue of origin in 96% of samples in which a cancerlike signal was detected, and in 93% of cases, that prediction was accurate.
Some of the patients who had cancer were symptomatic and therefore would not be considered a screening population, Dr. Liu and coinvestigators acknowledged. Also, the test’s potential for reducing mortality remains unknown, and 1-year follow-up to verify cancer-free status was not yet available for all of the individuals without cancer.
“Together, these data provide compelling evidence that targeted methylation analysis of cfDNA can detect and localize a broad range of nonmetastatic and metastatic cancer types including many common and deadly cancers that lack effective screening strategies,” they maintained. The test’s “specificity and sensitivity performance approach ... the goal for population-level screening.”
“Considering the potential value of early detection in deadly malignancies, further evaluation of this test is justified in prospective population-level studies,” the investigators conclude. “Clinical validation in intended use populations is ongoing ... and a study has been initiated that is returning results to health care providers and patients ....”
Dr. Liu disclosed that the Mayo Clinic was compensated for her advisory board activities for GRAIL Inc. The study was supported by GRAIL, and by Princess Margaret Cancer Centre’s McCain Genitourinary BioBank in the department of surgical oncology.
SOURCE: Liu MC et al. Ann Oncol. 2020 Mar 31. doi: 10.1016/j.annonc.2020.02.011.
FROM ANNALS OF ONCOLOGY
Mental Health Support for Self-Isolated Veterans
The message everywhere is “stay home!” But what if staying home threatens your mental health? Veterans are a doubly vulnerable group these days—vulnerable both to the COVID-19 infection and to the mental stress that self-isolation can inflict. To help relieve that pressure and, in particular, to reach veterans who might not otherwise seek counseling and mental health support, the US Department of Veterans Affairs (VA) has been shifting some outpatient care to telehealth and deploying Mobile Vet Center units to coronavirus-crisis areas.
The VA received some money to beef up its telehealth system from the $2 trillion CARES (Coronavirus Aid, Relief, and Economic Security) Act relief package passed and signed last week: $14.4 billion to expand telehealth services and another $2.15 billion to expand coronavirus-related services, including the purchase of mHealth devices.
Several of the provisions in the CARES Act directly address the needs of rural and underserved veterans. For instance, the Act authorizes the VA to expand telemental health services and enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, a critical need among rural residents who may be physically isolated from mental healthcare. The act also allows federally qualified health centers and rural health clinics, 2 types of facilities that serve rural and underserved populations, to be designated as distant sites for telehealth.
Between 2002, when telemental health services were launched, and 2019, veterans have worked with a counselor nearly 3 million times. In 2017, the VA says, psychiatric hospitalizations dropped 31%. Veterans have said they prefer videoconferencing over in-person therapy because they can are more at ease at home.
Using video telehealth, veterans can connect with care teams from anywhere—a safer alternative to traveling to appointments—using the camera on a phone, computer, or Apple or Android devices. Veterans also can use My HealtheVet’s secure messaging feature for non-urgent health questions. VA mental health professionals use both synchronous and asynchronous care: The first to connect patients to providers through a communication link, usually videoconferencing, the second to send data to specialists.
The current pandemic puts a strain on both patients and providers, but the Mobile Vet Centers may help relieve some of that strain. An extension of the VA’s brick-and-mortar Vet Centers, the mobile units provide a range of services, including individual, group, marriage, and family counseling. They also can refer active duty service members, veterans, and their families to VA care or other care facilities.
The mobile units are staffed by Vet Center employees who volunteer to deploy in emergencies, such as hurricanes and wildfires. The first units responding to the COVID-19 pandemic were dispatched to New York City, San Francisco, New Orleans, and Los Angeles.
“In times like this, it’s important to stand shoulder to shoulder with our local communities, support their local needs, and [assure] them they are not alone in navigating this crisis,” said Brooklyn Vet Center Director Gabe Botero.
Although the VA’s top priority remains keeping veterans safe while also making sure they receive the mental and physical healthcare they need , it has been criticized recently for “pausing” the Mission Act, which allows some veterans to get healthcare outside VA centers. The concern was that seeking outside care could expose veterans to the virus and potentially tax private health resources.
Government spokespeople have said the VA is not stopping or pausing the law, but “ensuring the best medical interests of America’s veterans are met.”
The message everywhere is “stay home!” But what if staying home threatens your mental health? Veterans are a doubly vulnerable group these days—vulnerable both to the COVID-19 infection and to the mental stress that self-isolation can inflict. To help relieve that pressure and, in particular, to reach veterans who might not otherwise seek counseling and mental health support, the US Department of Veterans Affairs (VA) has been shifting some outpatient care to telehealth and deploying Mobile Vet Center units to coronavirus-crisis areas.
The VA received some money to beef up its telehealth system from the $2 trillion CARES (Coronavirus Aid, Relief, and Economic Security) Act relief package passed and signed last week: $14.4 billion to expand telehealth services and another $2.15 billion to expand coronavirus-related services, including the purchase of mHealth devices.
Several of the provisions in the CARES Act directly address the needs of rural and underserved veterans. For instance, the Act authorizes the VA to expand telemental health services and enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, a critical need among rural residents who may be physically isolated from mental healthcare. The act also allows federally qualified health centers and rural health clinics, 2 types of facilities that serve rural and underserved populations, to be designated as distant sites for telehealth.
Between 2002, when telemental health services were launched, and 2019, veterans have worked with a counselor nearly 3 million times. In 2017, the VA says, psychiatric hospitalizations dropped 31%. Veterans have said they prefer videoconferencing over in-person therapy because they can are more at ease at home.
Using video telehealth, veterans can connect with care teams from anywhere—a safer alternative to traveling to appointments—using the camera on a phone, computer, or Apple or Android devices. Veterans also can use My HealtheVet’s secure messaging feature for non-urgent health questions. VA mental health professionals use both synchronous and asynchronous care: The first to connect patients to providers through a communication link, usually videoconferencing, the second to send data to specialists.
The current pandemic puts a strain on both patients and providers, but the Mobile Vet Centers may help relieve some of that strain. An extension of the VA’s brick-and-mortar Vet Centers, the mobile units provide a range of services, including individual, group, marriage, and family counseling. They also can refer active duty service members, veterans, and their families to VA care or other care facilities.
The mobile units are staffed by Vet Center employees who volunteer to deploy in emergencies, such as hurricanes and wildfires. The first units responding to the COVID-19 pandemic were dispatched to New York City, San Francisco, New Orleans, and Los Angeles.
“In times like this, it’s important to stand shoulder to shoulder with our local communities, support their local needs, and [assure] them they are not alone in navigating this crisis,” said Brooklyn Vet Center Director Gabe Botero.
Although the VA’s top priority remains keeping veterans safe while also making sure they receive the mental and physical healthcare they need , it has been criticized recently for “pausing” the Mission Act, which allows some veterans to get healthcare outside VA centers. The concern was that seeking outside care could expose veterans to the virus and potentially tax private health resources.
Government spokespeople have said the VA is not stopping or pausing the law, but “ensuring the best medical interests of America’s veterans are met.”
The message everywhere is “stay home!” But what if staying home threatens your mental health? Veterans are a doubly vulnerable group these days—vulnerable both to the COVID-19 infection and to the mental stress that self-isolation can inflict. To help relieve that pressure and, in particular, to reach veterans who might not otherwise seek counseling and mental health support, the US Department of Veterans Affairs (VA) has been shifting some outpatient care to telehealth and deploying Mobile Vet Center units to coronavirus-crisis areas.
The VA received some money to beef up its telehealth system from the $2 trillion CARES (Coronavirus Aid, Relief, and Economic Security) Act relief package passed and signed last week: $14.4 billion to expand telehealth services and another $2.15 billion to expand coronavirus-related services, including the purchase of mHealth devices.
Several of the provisions in the CARES Act directly address the needs of rural and underserved veterans. For instance, the Act authorizes the VA to expand telemental health services and enter into short-term agreements with telecommunications companies to provide temporary broadband services to veterans, a critical need among rural residents who may be physically isolated from mental healthcare. The act also allows federally qualified health centers and rural health clinics, 2 types of facilities that serve rural and underserved populations, to be designated as distant sites for telehealth.
Between 2002, when telemental health services were launched, and 2019, veterans have worked with a counselor nearly 3 million times. In 2017, the VA says, psychiatric hospitalizations dropped 31%. Veterans have said they prefer videoconferencing over in-person therapy because they can are more at ease at home.
Using video telehealth, veterans can connect with care teams from anywhere—a safer alternative to traveling to appointments—using the camera on a phone, computer, or Apple or Android devices. Veterans also can use My HealtheVet’s secure messaging feature for non-urgent health questions. VA mental health professionals use both synchronous and asynchronous care: The first to connect patients to providers through a communication link, usually videoconferencing, the second to send data to specialists.
The current pandemic puts a strain on both patients and providers, but the Mobile Vet Centers may help relieve some of that strain. An extension of the VA’s brick-and-mortar Vet Centers, the mobile units provide a range of services, including individual, group, marriage, and family counseling. They also can refer active duty service members, veterans, and their families to VA care or other care facilities.
The mobile units are staffed by Vet Center employees who volunteer to deploy in emergencies, such as hurricanes and wildfires. The first units responding to the COVID-19 pandemic were dispatched to New York City, San Francisco, New Orleans, and Los Angeles.
“In times like this, it’s important to stand shoulder to shoulder with our local communities, support their local needs, and [assure] them they are not alone in navigating this crisis,” said Brooklyn Vet Center Director Gabe Botero.
Although the VA’s top priority remains keeping veterans safe while also making sure they receive the mental and physical healthcare they need , it has been criticized recently for “pausing” the Mission Act, which allows some veterans to get healthcare outside VA centers. The concern was that seeking outside care could expose veterans to the virus and potentially tax private health resources.
Government spokespeople have said the VA is not stopping or pausing the law, but “ensuring the best medical interests of America’s veterans are met.”