User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Cancer prevalence among COVID-19 patients may be higher than previously reported
An early report pegged the prevalence of cancer among COVID-19 patients at 1%, but authors of a recent meta-analysis found an overall prevalence of 2% and up to 3% depending on the subset of data they reviewed.
However, those findings are limited by the retrospective nature of the studies published to date, according to the authors of the meta-analysis, led by Aakash Desai, MBBS, of the University of Connecticut, Farmington.
Nevertheless, the results do confirm that cancer patients and survivors are an important at-risk population for COVID-19, according to Dr. Desai and colleagues.
“We hope that additional data from China and Italy will provide information on the characteristics of patients with cancer at risk, types of cancer that confer higher risk, and systemic regimens that may increase COVID-19 infection complications,” the authors wrote in JCO Global Oncology.
More than 15 million individuals with cancer and many more cancer survivors are at increased risk of COVID-19 because of compromised immune systems, according to the authors.
Exactly how many individuals with cancer are among the COVID-19 cases remains unclear, though a previous report suggested the prevalence of cancer was 1% (95% confidence interval, 0.61%-1.65%) among COVID-19 patients in China (Lancet Oncol. 2020 Mar;21[3]:335-7). This “seems to be higher” than the 0.29% prevalence of cancer in the overall Chinese population, the investigators noted at the time.
That study revealed 18 cancer patients among 1,590 COVID-19 cases, though it was “hypothesis generating,” according to Dr. Desai and colleagues, who rolled that data into their meta-analysis of 11 reports including 3,661 COVID-19 cases.
Overall, Dr. Desai and colleagues found the pooled prevalence of cancer was 2.0% (95% CI, 2.0%-3.0%) in that population. In a subgroup analysis of five studies with sample sizes of less than 100 COVID-19 patients, the researchers found a “slightly higher” prevalence of 3.0% (95% CI, 1.0%-6.0%).
However, even that data wasn’t robust enough for Dr. Desai and colleagues to make any pronouncements on cancer prevalence. “Overall, current evidence on the association between cancer and COVID-19 remains inconclusive,” they wrote.
Though inconclusive, the findings raise questions about whether treatments or interventions might need to be postponed in certain patients, whether cancer patients and survivors need stronger personal protection, and how to deal with potential delays in cancer clinical trials, according to Dr. Desai and colleagues.
“As the evidence continues to rise, we must strive to answer the unanswered clinical questions,” the authors wrote.
Dr. Desai and colleagues reported no potential conflicts of interest related to the study.
SOURCE: Desai A et al. JCO Glob Oncol. 2020 Apr 6. doi: 10.1200/GO.20.00097.
An early report pegged the prevalence of cancer among COVID-19 patients at 1%, but authors of a recent meta-analysis found an overall prevalence of 2% and up to 3% depending on the subset of data they reviewed.
However, those findings are limited by the retrospective nature of the studies published to date, according to the authors of the meta-analysis, led by Aakash Desai, MBBS, of the University of Connecticut, Farmington.
Nevertheless, the results do confirm that cancer patients and survivors are an important at-risk population for COVID-19, according to Dr. Desai and colleagues.
“We hope that additional data from China and Italy will provide information on the characteristics of patients with cancer at risk, types of cancer that confer higher risk, and systemic regimens that may increase COVID-19 infection complications,” the authors wrote in JCO Global Oncology.
More than 15 million individuals with cancer and many more cancer survivors are at increased risk of COVID-19 because of compromised immune systems, according to the authors.
Exactly how many individuals with cancer are among the COVID-19 cases remains unclear, though a previous report suggested the prevalence of cancer was 1% (95% confidence interval, 0.61%-1.65%) among COVID-19 patients in China (Lancet Oncol. 2020 Mar;21[3]:335-7). This “seems to be higher” than the 0.29% prevalence of cancer in the overall Chinese population, the investigators noted at the time.
That study revealed 18 cancer patients among 1,590 COVID-19 cases, though it was “hypothesis generating,” according to Dr. Desai and colleagues, who rolled that data into their meta-analysis of 11 reports including 3,661 COVID-19 cases.
Overall, Dr. Desai and colleagues found the pooled prevalence of cancer was 2.0% (95% CI, 2.0%-3.0%) in that population. In a subgroup analysis of five studies with sample sizes of less than 100 COVID-19 patients, the researchers found a “slightly higher” prevalence of 3.0% (95% CI, 1.0%-6.0%).
However, even that data wasn’t robust enough for Dr. Desai and colleagues to make any pronouncements on cancer prevalence. “Overall, current evidence on the association between cancer and COVID-19 remains inconclusive,” they wrote.
Though inconclusive, the findings raise questions about whether treatments or interventions might need to be postponed in certain patients, whether cancer patients and survivors need stronger personal protection, and how to deal with potential delays in cancer clinical trials, according to Dr. Desai and colleagues.
“As the evidence continues to rise, we must strive to answer the unanswered clinical questions,” the authors wrote.
Dr. Desai and colleagues reported no potential conflicts of interest related to the study.
SOURCE: Desai A et al. JCO Glob Oncol. 2020 Apr 6. doi: 10.1200/GO.20.00097.
An early report pegged the prevalence of cancer among COVID-19 patients at 1%, but authors of a recent meta-analysis found an overall prevalence of 2% and up to 3% depending on the subset of data they reviewed.
However, those findings are limited by the retrospective nature of the studies published to date, according to the authors of the meta-analysis, led by Aakash Desai, MBBS, of the University of Connecticut, Farmington.
Nevertheless, the results do confirm that cancer patients and survivors are an important at-risk population for COVID-19, according to Dr. Desai and colleagues.
“We hope that additional data from China and Italy will provide information on the characteristics of patients with cancer at risk, types of cancer that confer higher risk, and systemic regimens that may increase COVID-19 infection complications,” the authors wrote in JCO Global Oncology.
More than 15 million individuals with cancer and many more cancer survivors are at increased risk of COVID-19 because of compromised immune systems, according to the authors.
Exactly how many individuals with cancer are among the COVID-19 cases remains unclear, though a previous report suggested the prevalence of cancer was 1% (95% confidence interval, 0.61%-1.65%) among COVID-19 patients in China (Lancet Oncol. 2020 Mar;21[3]:335-7). This “seems to be higher” than the 0.29% prevalence of cancer in the overall Chinese population, the investigators noted at the time.
That study revealed 18 cancer patients among 1,590 COVID-19 cases, though it was “hypothesis generating,” according to Dr. Desai and colleagues, who rolled that data into their meta-analysis of 11 reports including 3,661 COVID-19 cases.
Overall, Dr. Desai and colleagues found the pooled prevalence of cancer was 2.0% (95% CI, 2.0%-3.0%) in that population. In a subgroup analysis of five studies with sample sizes of less than 100 COVID-19 patients, the researchers found a “slightly higher” prevalence of 3.0% (95% CI, 1.0%-6.0%).
However, even that data wasn’t robust enough for Dr. Desai and colleagues to make any pronouncements on cancer prevalence. “Overall, current evidence on the association between cancer and COVID-19 remains inconclusive,” they wrote.
Though inconclusive, the findings raise questions about whether treatments or interventions might need to be postponed in certain patients, whether cancer patients and survivors need stronger personal protection, and how to deal with potential delays in cancer clinical trials, according to Dr. Desai and colleagues.
“As the evidence continues to rise, we must strive to answer the unanswered clinical questions,” the authors wrote.
Dr. Desai and colleagues reported no potential conflicts of interest related to the study.
SOURCE: Desai A et al. JCO Glob Oncol. 2020 Apr 6. doi: 10.1200/GO.20.00097.
FROM JCO GLOBAL ONCOLOGY
When to treat, delay, or omit breast cancer therapy in the face of COVID-19
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Home-based chemo skyrockets at one U.S. center
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
Major organization opposes concept
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
Managing pediatric heme/onc departments during the pandemic
Given the possibility that children with hematologic malignancies may have increased susceptibility to coronavirus disease 2019 (COVID-19), clinicians from China and the United States have proposed a plan for preventing and managing outbreaks in hospitals’ pediatric hematology and oncology departments.
The plan is focused primarily on infection prevention and control strategies, Yulei He, MD, of Chengdu (China) Women’s and Children’s Central Hospital and colleagues explained in an article published in The Lancet Haematology.
The authors noted that close contact with COVID-19 patients is thought to be the main route of transmission, and a retrospective study indicated that 41.3% of initial COVID-19 cases were caused by hospital-related transmission.
“Children with hematological malignancies might have increased susceptibility to infection with SARS-CoV-2 because of immunodeficiency; therefore, procedures are needed to avoid hospital-related transmission and infection for these patients,” the authors wrote.
Preventing the spread of infection
Dr. He and colleagues advised that medical staff be kept up-to-date with the latest information about COVID-19 and perform assessments regularly to identify cases in their departments.
The authors also recommended establishing a COVID-19 expert committee – consisting of infectious disease physicians, hematologists, oncologists, radiologists, pharmacists, and hospital infection control staff – to make medical decisions in multidisciplinary consultation meetings. In addition, the authors recommended regional management strategies be adopted to minimize cross infection within the hospital. Specifically, the authors proposed creating the following four zones:
1. A surveillance and screening zone for patients potentially infected with SARS-CoV-2
2. A suspected-case quarantine zone where patients thought to have COVID-19 are isolated in single rooms
3. A confirmed-case quarantine zone where patients are treated for COVID-19
4. A hematology/oncology ward for treating non–COVID-19 patients with malignancies.
Dr. He and colleagues also stressed the importance of providing personal protective equipment for all zones, along with instructions for proper use and disposal. The authors recommended developing and following specific protocols for outpatient visits in the hematology/oncology ward, and providing COVID-19 prevention and control information to families and health care workers.
Managing cancer treatment
For patients with acute leukemias who have induction chemotherapy planned, Dr. He and colleagues argued that scheduled chemotherapy should not be interrupted unless COVID-19 is suspected or diagnosed. The authors said treatment should not be delayed more than 7 days during induction, consolidation, or the intermediate phase of chemotherapy because the virus has an incubation period of 2-7 days. This will allow a short period of observation to screen for potential infection.
The authors recommended that patients with lymphoma and solid tumors first undergo COVID-19 screening and then receive treatment in hematology/oncology wards “according to their chemotherapy schedule, and without delay, until they are in complete remission.”
“If the patient is in complete remission, we recommend a treatment delay of no more than 7 days to allow a short period of observation to screen for COVID-19,” the authors added.
Maintenance chemotherapy should not be delayed for more than 14 days, Dr. He and colleagues wrote. “This increase in the maximum delay before chemotherapy strikes a balance between the potential risk of SARS-CoV-2 infection and tumor recurrence, since pediatric patients in this phase of treatment have a reduced risk of tumor recurrence,” the authors added.
Caring for patients with COVID-19
For inpatients diagnosed with COVID-19, Dr. He and colleagues recommended the following:
- Prioritize COVID-19 treatment for children with primary disease remission.
- For children not in remission, prioritize treatment for critical patients.
- Isolated patients should be treated for COVID-19, and their chemotherapy should be temporarily suspended or reduced in intensity..
Dr. He and colleagues noted that, by following these recommendations for infection prevention, they had no cases of COVID-19 among children in their hematology/oncology departments. However, the authors said the recommendations “could fail to some extent” based on “differences in medical resources, health care settings, and the policy of the specific government.”
The authors said their recommendations should be updated continuously as new information and clinical evidence emerges.
Dr. He and colleagues reported having no conflicts of interest.
SOURCE: He Y et al. Lancet Haematol. doi: 10/1016/s2352-3026(20)30104-6.
Given the possibility that children with hematologic malignancies may have increased susceptibility to coronavirus disease 2019 (COVID-19), clinicians from China and the United States have proposed a plan for preventing and managing outbreaks in hospitals’ pediatric hematology and oncology departments.
The plan is focused primarily on infection prevention and control strategies, Yulei He, MD, of Chengdu (China) Women’s and Children’s Central Hospital and colleagues explained in an article published in The Lancet Haematology.
The authors noted that close contact with COVID-19 patients is thought to be the main route of transmission, and a retrospective study indicated that 41.3% of initial COVID-19 cases were caused by hospital-related transmission.
“Children with hematological malignancies might have increased susceptibility to infection with SARS-CoV-2 because of immunodeficiency; therefore, procedures are needed to avoid hospital-related transmission and infection for these patients,” the authors wrote.
Preventing the spread of infection
Dr. He and colleagues advised that medical staff be kept up-to-date with the latest information about COVID-19 and perform assessments regularly to identify cases in their departments.
The authors also recommended establishing a COVID-19 expert committee – consisting of infectious disease physicians, hematologists, oncologists, radiologists, pharmacists, and hospital infection control staff – to make medical decisions in multidisciplinary consultation meetings. In addition, the authors recommended regional management strategies be adopted to minimize cross infection within the hospital. Specifically, the authors proposed creating the following four zones:
1. A surveillance and screening zone for patients potentially infected with SARS-CoV-2
2. A suspected-case quarantine zone where patients thought to have COVID-19 are isolated in single rooms
3. A confirmed-case quarantine zone where patients are treated for COVID-19
4. A hematology/oncology ward for treating non–COVID-19 patients with malignancies.
Dr. He and colleagues also stressed the importance of providing personal protective equipment for all zones, along with instructions for proper use and disposal. The authors recommended developing and following specific protocols for outpatient visits in the hematology/oncology ward, and providing COVID-19 prevention and control information to families and health care workers.
Managing cancer treatment
For patients with acute leukemias who have induction chemotherapy planned, Dr. He and colleagues argued that scheduled chemotherapy should not be interrupted unless COVID-19 is suspected or diagnosed. The authors said treatment should not be delayed more than 7 days during induction, consolidation, or the intermediate phase of chemotherapy because the virus has an incubation period of 2-7 days. This will allow a short period of observation to screen for potential infection.
The authors recommended that patients with lymphoma and solid tumors first undergo COVID-19 screening and then receive treatment in hematology/oncology wards “according to their chemotherapy schedule, and without delay, until they are in complete remission.”
“If the patient is in complete remission, we recommend a treatment delay of no more than 7 days to allow a short period of observation to screen for COVID-19,” the authors added.
Maintenance chemotherapy should not be delayed for more than 14 days, Dr. He and colleagues wrote. “This increase in the maximum delay before chemotherapy strikes a balance between the potential risk of SARS-CoV-2 infection and tumor recurrence, since pediatric patients in this phase of treatment have a reduced risk of tumor recurrence,” the authors added.
Caring for patients with COVID-19
For inpatients diagnosed with COVID-19, Dr. He and colleagues recommended the following:
- Prioritize COVID-19 treatment for children with primary disease remission.
- For children not in remission, prioritize treatment for critical patients.
- Isolated patients should be treated for COVID-19, and their chemotherapy should be temporarily suspended or reduced in intensity..
Dr. He and colleagues noted that, by following these recommendations for infection prevention, they had no cases of COVID-19 among children in their hematology/oncology departments. However, the authors said the recommendations “could fail to some extent” based on “differences in medical resources, health care settings, and the policy of the specific government.”
The authors said their recommendations should be updated continuously as new information and clinical evidence emerges.
Dr. He and colleagues reported having no conflicts of interest.
SOURCE: He Y et al. Lancet Haematol. doi: 10/1016/s2352-3026(20)30104-6.
Given the possibility that children with hematologic malignancies may have increased susceptibility to coronavirus disease 2019 (COVID-19), clinicians from China and the United States have proposed a plan for preventing and managing outbreaks in hospitals’ pediatric hematology and oncology departments.
The plan is focused primarily on infection prevention and control strategies, Yulei He, MD, of Chengdu (China) Women’s and Children’s Central Hospital and colleagues explained in an article published in The Lancet Haematology.
The authors noted that close contact with COVID-19 patients is thought to be the main route of transmission, and a retrospective study indicated that 41.3% of initial COVID-19 cases were caused by hospital-related transmission.
“Children with hematological malignancies might have increased susceptibility to infection with SARS-CoV-2 because of immunodeficiency; therefore, procedures are needed to avoid hospital-related transmission and infection for these patients,” the authors wrote.
Preventing the spread of infection
Dr. He and colleagues advised that medical staff be kept up-to-date with the latest information about COVID-19 and perform assessments regularly to identify cases in their departments.
The authors also recommended establishing a COVID-19 expert committee – consisting of infectious disease physicians, hematologists, oncologists, radiologists, pharmacists, and hospital infection control staff – to make medical decisions in multidisciplinary consultation meetings. In addition, the authors recommended regional management strategies be adopted to minimize cross infection within the hospital. Specifically, the authors proposed creating the following four zones:
1. A surveillance and screening zone for patients potentially infected with SARS-CoV-2
2. A suspected-case quarantine zone where patients thought to have COVID-19 are isolated in single rooms
3. A confirmed-case quarantine zone where patients are treated for COVID-19
4. A hematology/oncology ward for treating non–COVID-19 patients with malignancies.
Dr. He and colleagues also stressed the importance of providing personal protective equipment for all zones, along with instructions for proper use and disposal. The authors recommended developing and following specific protocols for outpatient visits in the hematology/oncology ward, and providing COVID-19 prevention and control information to families and health care workers.
Managing cancer treatment
For patients with acute leukemias who have induction chemotherapy planned, Dr. He and colleagues argued that scheduled chemotherapy should not be interrupted unless COVID-19 is suspected or diagnosed. The authors said treatment should not be delayed more than 7 days during induction, consolidation, or the intermediate phase of chemotherapy because the virus has an incubation period of 2-7 days. This will allow a short period of observation to screen for potential infection.
The authors recommended that patients with lymphoma and solid tumors first undergo COVID-19 screening and then receive treatment in hematology/oncology wards “according to their chemotherapy schedule, and without delay, until they are in complete remission.”
“If the patient is in complete remission, we recommend a treatment delay of no more than 7 days to allow a short period of observation to screen for COVID-19,” the authors added.
Maintenance chemotherapy should not be delayed for more than 14 days, Dr. He and colleagues wrote. “This increase in the maximum delay before chemotherapy strikes a balance between the potential risk of SARS-CoV-2 infection and tumor recurrence, since pediatric patients in this phase of treatment have a reduced risk of tumor recurrence,” the authors added.
Caring for patients with COVID-19
For inpatients diagnosed with COVID-19, Dr. He and colleagues recommended the following:
- Prioritize COVID-19 treatment for children with primary disease remission.
- For children not in remission, prioritize treatment for critical patients.
- Isolated patients should be treated for COVID-19, and their chemotherapy should be temporarily suspended or reduced in intensity..
Dr. He and colleagues noted that, by following these recommendations for infection prevention, they had no cases of COVID-19 among children in their hematology/oncology departments. However, the authors said the recommendations “could fail to some extent” based on “differences in medical resources, health care settings, and the policy of the specific government.”
The authors said their recommendations should be updated continuously as new information and clinical evidence emerges.
Dr. He and colleagues reported having no conflicts of interest.
SOURCE: He Y et al. Lancet Haematol. doi: 10/1016/s2352-3026(20)30104-6.
FROM THE LANCET HAEMATOLOGY
Colorectal cancer: Proposed treatment guidelines for the COVID-19 era
In light of the rapid changes affecting cancer clinics due to the COVID-19 pandemic, Dr. David Kerr and Dr. Rachel Kerr, both specialists in gastrointestinal cancers at the University of Oxford in Oxford, United Kingdom, drafted these guidelines for the use of chemotherapy in colorectal cancer patients. Dr. Kerr and Dr. Kerr are putting forth this guidance as a topic for discussion and debate.
Our aim in developing these recommendations for the care of colorectal cancer patients in areas affected by the COVID-19 outbreak is to reduce the comorbidity of chemotherapy and decrease the risk of patients dying from COVID-19, weighed against the potential benefits of receiving chemotherapy. These recommendations are also designed to reduce the burden on chemotherapy units during a time of great pressure.
We have modified the guidelines in such a way that, we believe, will decrease the total number of patients receiving chemotherapy – particularly in the adjuvant setting – and reduce the overall immune impact of chemotherapy on these patients. Specifically, we suggest changing doublet chemotherapy to single-agent chemotherapy for some groups; changing to combinations involving capecitabine rather than bolus and infusional 5-FU for other patients; and, finally, making reasonable dose reductions upfront to reduce the risk for cycle 1 complications.
By changing from push-and-pump 5-FU to capecitabine for the vast majority of patients, we will both reduce the rates of neutropenia and decrease throughput in chemotherapy outpatient units, reducing requirements for weekly line flushing, pump disconnections, and other routine maintenance.
We continue to recommend the use of ToxNav germline genetic testing as a genetic screen for DPYD/ENOSF1 single-nucleotide polymorphisms (SNPs) to identify patients at high risk for fluoropyrimidine toxicity.
Use of biomarkers to sharpen prognosis should also be considered to refine therapeutic decisions.
Recommendations for stage II-III colorectal cancer
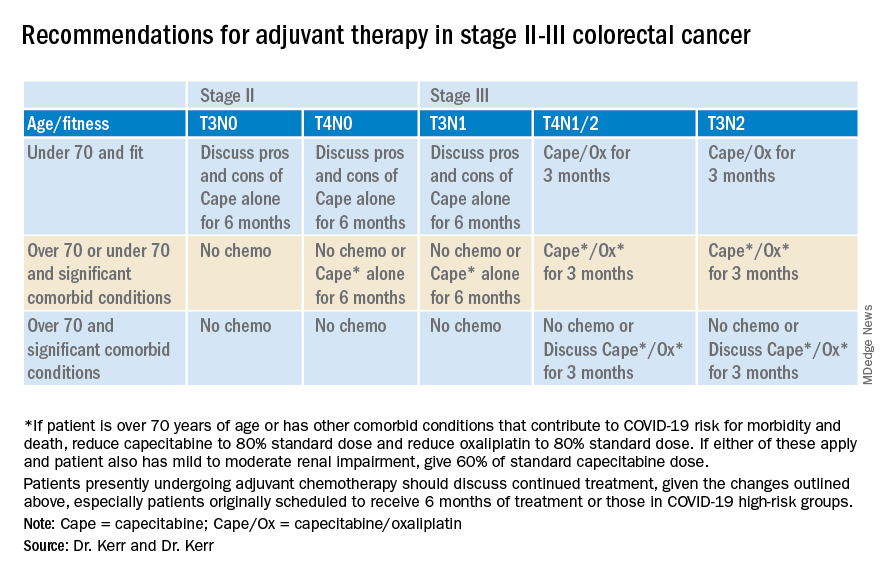
Recommendations for advanced colorectal cancer
Which regimen? Capecitabine/oxaliplatin should be the default backbone chemotherapy (rather than FOLFOX) in order to decrease the stress on infusion units.
Capecitabine plus irinotecan should be considered rather than FOLFIRI. However, in order to increase safety, reduce the dose of the capecitabine and the irinotecan, both to 80%, in all patient groups; and perhaps reduce the capecitabine dose further to 60% in those over the age of 70 or with significant comorbid conditions.
Treatment breaks. Full treatment breaks should be considered after 3 months of treatment in most patients with lower-volume, more indolent disease.
Treatment deintensification to capecitabine alone should be used in those with higher-volume disease (for example, more than 50% of liver replaced by tumor) at the beginning of treatment.
Deferring the start of any chemotherapy. Some older patients, or those with significant other comorbidities (that is, those who will be at increased risk for COVID-19 complications and death); who have low-volume disease, such as a couple of small lung metastases or a single liver metastasis; or who were diagnosed more than 12 months since adjuvant chemotherapy may decide to defer any chemotherapy for a period of time.
In these cases, we suggest rescanning at 3 months and discussing further treatment at that point. Some of these patients will be eligible for other interventions, such as resection, ablation, or stereotactic body radiation therapy. However, it will be important to consider the pressures on these other services during this unprecedented time.
Chemotherapy after resection of metastases. Given the lack of evidence and the present extenuating circumstances, we would not recommend any chemotherapy in this setting.
David J. Kerr, MD, CBE, MD, DSc, is a professor of cancer medicine at the University of Oxford. He is recognized internationally for his work in the research and treatment of colorectal cancer, and has founded three university spin-out companies: COBRA Therapeutics, Celleron Therapeutics, and Oxford Cancer Biomarkers. In 2002, he was appointed Commander of the British Empire by Queen Elizabeth. Rachel S. Kerr, MBChB, is a medical oncologist and associate professor of gastrointestinal oncology at the University of Oxford. She holds a UK Department of Health Fellowship, where she is clinical director of phase 3 trials in the oncology clinical trials office.
This article first appeared on Medscape.com.
In light of the rapid changes affecting cancer clinics due to the COVID-19 pandemic, Dr. David Kerr and Dr. Rachel Kerr, both specialists in gastrointestinal cancers at the University of Oxford in Oxford, United Kingdom, drafted these guidelines for the use of chemotherapy in colorectal cancer patients. Dr. Kerr and Dr. Kerr are putting forth this guidance as a topic for discussion and debate.
Our aim in developing these recommendations for the care of colorectal cancer patients in areas affected by the COVID-19 outbreak is to reduce the comorbidity of chemotherapy and decrease the risk of patients dying from COVID-19, weighed against the potential benefits of receiving chemotherapy. These recommendations are also designed to reduce the burden on chemotherapy units during a time of great pressure.
We have modified the guidelines in such a way that, we believe, will decrease the total number of patients receiving chemotherapy – particularly in the adjuvant setting – and reduce the overall immune impact of chemotherapy on these patients. Specifically, we suggest changing doublet chemotherapy to single-agent chemotherapy for some groups; changing to combinations involving capecitabine rather than bolus and infusional 5-FU for other patients; and, finally, making reasonable dose reductions upfront to reduce the risk for cycle 1 complications.
By changing from push-and-pump 5-FU to capecitabine for the vast majority of patients, we will both reduce the rates of neutropenia and decrease throughput in chemotherapy outpatient units, reducing requirements for weekly line flushing, pump disconnections, and other routine maintenance.
We continue to recommend the use of ToxNav germline genetic testing as a genetic screen for DPYD/ENOSF1 single-nucleotide polymorphisms (SNPs) to identify patients at high risk for fluoropyrimidine toxicity.
Use of biomarkers to sharpen prognosis should also be considered to refine therapeutic decisions.
Recommendations for stage II-III colorectal cancer

Recommendations for advanced colorectal cancer
Which regimen? Capecitabine/oxaliplatin should be the default backbone chemotherapy (rather than FOLFOX) in order to decrease the stress on infusion units.
Capecitabine plus irinotecan should be considered rather than FOLFIRI. However, in order to increase safety, reduce the dose of the capecitabine and the irinotecan, both to 80%, in all patient groups; and perhaps reduce the capecitabine dose further to 60% in those over the age of 70 or with significant comorbid conditions.
Treatment breaks. Full treatment breaks should be considered after 3 months of treatment in most patients with lower-volume, more indolent disease.
Treatment deintensification to capecitabine alone should be used in those with higher-volume disease (for example, more than 50% of liver replaced by tumor) at the beginning of treatment.
Deferring the start of any chemotherapy. Some older patients, or those with significant other comorbidities (that is, those who will be at increased risk for COVID-19 complications and death); who have low-volume disease, such as a couple of small lung metastases or a single liver metastasis; or who were diagnosed more than 12 months since adjuvant chemotherapy may decide to defer any chemotherapy for a period of time.
In these cases, we suggest rescanning at 3 months and discussing further treatment at that point. Some of these patients will be eligible for other interventions, such as resection, ablation, or stereotactic body radiation therapy. However, it will be important to consider the pressures on these other services during this unprecedented time.
Chemotherapy after resection of metastases. Given the lack of evidence and the present extenuating circumstances, we would not recommend any chemotherapy in this setting.
David J. Kerr, MD, CBE, MD, DSc, is a professor of cancer medicine at the University of Oxford. He is recognized internationally for his work in the research and treatment of colorectal cancer, and has founded three university spin-out companies: COBRA Therapeutics, Celleron Therapeutics, and Oxford Cancer Biomarkers. In 2002, he was appointed Commander of the British Empire by Queen Elizabeth. Rachel S. Kerr, MBChB, is a medical oncologist and associate professor of gastrointestinal oncology at the University of Oxford. She holds a UK Department of Health Fellowship, where she is clinical director of phase 3 trials in the oncology clinical trials office.
This article first appeared on Medscape.com.
In light of the rapid changes affecting cancer clinics due to the COVID-19 pandemic, Dr. David Kerr and Dr. Rachel Kerr, both specialists in gastrointestinal cancers at the University of Oxford in Oxford, United Kingdom, drafted these guidelines for the use of chemotherapy in colorectal cancer patients. Dr. Kerr and Dr. Kerr are putting forth this guidance as a topic for discussion and debate.
Our aim in developing these recommendations for the care of colorectal cancer patients in areas affected by the COVID-19 outbreak is to reduce the comorbidity of chemotherapy and decrease the risk of patients dying from COVID-19, weighed against the potential benefits of receiving chemotherapy. These recommendations are also designed to reduce the burden on chemotherapy units during a time of great pressure.
We have modified the guidelines in such a way that, we believe, will decrease the total number of patients receiving chemotherapy – particularly in the adjuvant setting – and reduce the overall immune impact of chemotherapy on these patients. Specifically, we suggest changing doublet chemotherapy to single-agent chemotherapy for some groups; changing to combinations involving capecitabine rather than bolus and infusional 5-FU for other patients; and, finally, making reasonable dose reductions upfront to reduce the risk for cycle 1 complications.
By changing from push-and-pump 5-FU to capecitabine for the vast majority of patients, we will both reduce the rates of neutropenia and decrease throughput in chemotherapy outpatient units, reducing requirements for weekly line flushing, pump disconnections, and other routine maintenance.
We continue to recommend the use of ToxNav germline genetic testing as a genetic screen for DPYD/ENOSF1 single-nucleotide polymorphisms (SNPs) to identify patients at high risk for fluoropyrimidine toxicity.
Use of biomarkers to sharpen prognosis should also be considered to refine therapeutic decisions.
Recommendations for stage II-III colorectal cancer

Recommendations for advanced colorectal cancer
Which regimen? Capecitabine/oxaliplatin should be the default backbone chemotherapy (rather than FOLFOX) in order to decrease the stress on infusion units.
Capecitabine plus irinotecan should be considered rather than FOLFIRI. However, in order to increase safety, reduce the dose of the capecitabine and the irinotecan, both to 80%, in all patient groups; and perhaps reduce the capecitabine dose further to 60% in those over the age of 70 or with significant comorbid conditions.
Treatment breaks. Full treatment breaks should be considered after 3 months of treatment in most patients with lower-volume, more indolent disease.
Treatment deintensification to capecitabine alone should be used in those with higher-volume disease (for example, more than 50% of liver replaced by tumor) at the beginning of treatment.
Deferring the start of any chemotherapy. Some older patients, or those with significant other comorbidities (that is, those who will be at increased risk for COVID-19 complications and death); who have low-volume disease, such as a couple of small lung metastases or a single liver metastasis; or who were diagnosed more than 12 months since adjuvant chemotherapy may decide to defer any chemotherapy for a period of time.
In these cases, we suggest rescanning at 3 months and discussing further treatment at that point. Some of these patients will be eligible for other interventions, such as resection, ablation, or stereotactic body radiation therapy. However, it will be important to consider the pressures on these other services during this unprecedented time.
Chemotherapy after resection of metastases. Given the lack of evidence and the present extenuating circumstances, we would not recommend any chemotherapy in this setting.
David J. Kerr, MD, CBE, MD, DSc, is a professor of cancer medicine at the University of Oxford. He is recognized internationally for his work in the research and treatment of colorectal cancer, and has founded three university spin-out companies: COBRA Therapeutics, Celleron Therapeutics, and Oxford Cancer Biomarkers. In 2002, he was appointed Commander of the British Empire by Queen Elizabeth. Rachel S. Kerr, MBChB, is a medical oncologist and associate professor of gastrointestinal oncology at the University of Oxford. She holds a UK Department of Health Fellowship, where she is clinical director of phase 3 trials in the oncology clinical trials office.
This article first appeared on Medscape.com.
National Watchman registry reports impressive procedural safety
Early results from the massive National Cardiovascular Data Registry Left Atrial Appendage Occlusion Registry indicate that the rollout of the Watchman device into routine clinical practice is going smoothly, with a higher implant success rate and a substantially lower in-hospital complication rate than that seen in the pivotal randomized clinical trials, James V. Freeman, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These real-world results are particularly impressive because the 38,158 registry participants were on average significantly older and sicker than were patients in the clinical trials. They were at higher risk of both stroke and bleeding, yet they fared better in terms of procedural safety, observed Dr. Freeman, an electrophysiologist and director of the Yale University Atrial Fibrillation Center in New Haven, Conn.
“You always worry that once you get outside of the clinical trials setting and you roll out to a large number of centers, including some that are relatively low volume, that you’re going to start to see higher rates of complications. And overall, broadly speaking, the rates of adverse events were quite reassuring,” he said.
The registry, maintained by the ACC, serves as the postmarketing surveillance tool mandated by the Food and Drug Administration and Centers for Medicare & Medicaid Services. The 38,158 participants make this registry the world’s largest patient experience with the Watchman device by many orders of magnitude. Dr. Freeman’s report included patients enrolled during 2016-2018 who were treated at 495 hospitals by 1,318 physician interventionalists. CMS reimbursement requires participation in the registry, which captures more than 95% of all Watchman procedures done in the United States. Although Dr. Freeman presented only the acute in-hospital outcomes, active follow-up for adverse events and medical therapy will be conducted at 45 days, 6 months, and 1 and 2 years.
Participants in the Left Atrial Appendage Occlusion (LAAO) Registry averaged 76.1 years of age, which is 2-4 years older than patients in the pivotal PROTECT-AF and PREVAIL trials or the 1,025-patient EWOLUTION registry. The LAAO Registry participants had a mean CHA2DS2-VASc score of 4.6, compared with 3.4 in PROTECT-AF and 3.8 in PREVAIL. Their mean HAS BLED score was 3.0. Thirty percent had a prior ischemic stroke or transient ischemic attack, 12% had a prior intracranial hemorrhage, and 69% had a history of clinically relevant bleeding. Thirty percent had heart failure, 92% were hypertensive, and 30% had diabetes.
“The take home here is that these patients were at moderate to high risk of stroke and they also carried a high risk of bleeding and therefore had some relative contraindication to anticoagulation,” according to the cardiologist. “The patient population overall is really in accordance with the CMS guidance. We’re not seeing a lot of patients who are getting this device for a lifestyle indication. Most of these patients are really stuck between a rock and a hard place.”
Most hospitals offering the Watchman did 10-40 cases per year. The median annual physician volume was 12 cases. However, there was substantial variation in both hospital and physician volumes.
The device was deployed in 93% of procedures attempted; roughly half of cancellations were cause by LAAO thrombus detected on the day of the procedure. The acute procedural success rate when the device was deployed was 98.3%, compared with 90.9% in PROTECT-AF and 95.1% in PREVAIL. The rate of device margin residual leak of 5 mm or more among registry participants with an acutely successful procedure was 0.2%.
The rate of any major in-hospital complication in the LAAO Registry was 2.16%, the most common of which was pericardial effusion requiring intervention, which occurred in 1.39% of cases. The major bleeding rate was 1.25%. The stroke/transient ischemic attack rate was 0.17%. Systemic arterial embolism was a rare event, occurring in less than 0.01% of patients, as was acute MI, with an incidence of 0.04%. Device embolization occurred in 0.07% of patients.
By comparison, the 7-day rate of pericardial effusion requiring intervention was 4.0% in PROTECT-AF and 1.9% in PREVAIL, with procedure-related stroke rates of 1.1% and 0.7%, respectively, and device embolization rates of 0.4% and 0.7%. The major bleeding rate in PROTECT-AF was 3.5%, nearly triple that in the real-world registry.
Discussant Mark A. Estes, MD, characterized the acute outcomes in the LAAO Registry as “an improvement – a considerable improvement – over some of the early data in PREVAIL and PROTECT-AF.” He credited this to the “very robust validation procedure” the Watchman closure device has undergone, which included the clinical trials, regulatory requirements for training and patient selection, and mandatory reporting of outcomes in the registry.
He noted that a lot is happening now with the Watchman device. There are a couple of dozen prospective clinical trials, including one on the Watchman versus direct oral anticoagulant (DOAC) therapy and another on left atrial ablation plus left atrial appendage closure versus a DOAC. A new-generation Watchman device, the Watchman FLX, is approved in Europe and undergoing an ongoing FDA-mandated approval trial in the United States.
“It has a lot of technical advantages,” according to Dr. Estes, an electrophysiologist and professor of medicine at the University of Pittsburgh.
Current guidelines give LAAO a class IIb rating, meaning it “could be considered” in patients with atrial fibrillation at increased risk of stroke who have a contraindication to long-term anticoagulation. Dr. Estes asked: Does the LAAO Registry data warrant a rating upgrade to a stronger recommendation?
Dr. Freeman replied that the new data should allay the guideline writers’ and government regulators’ concerns regarding acute procedural safety. But that’s only part of the picture. He and his coinvestigators are busy gathering data on intermediate-term outcomes, analyzing the impact of various strategies for periprocedural and long-term management of antiplatelet and anticoagulant medications with an eye toward identifying best practices, and investigating the relationship between procedural volume and outcomes, information, which could have an impact on the next iteration of the guidelines.
Simultaneous with his presentation at ACC 2020, the study was published online (J Am Coll Cardiol. 2020 Mar 13;75[13]1503-18).
In an accompanying editorial, Dhanunjaya Lakkireddy, MD, commented that an important contribution of the LAAO Registry is its inclusion of an enormous number of patients with contraindications to oral anticoagulation, a population excluded from the PROTECT-AF and PREVAIL randomized trials.
The short-term results of the registry suggest a relaxation of the current strict requirement for surgical backup during Watchman procedures is in order, added Dr. Lakkireddy, professor of medicine at the University of Missouri, Columbia, and medical director of the Kansas City Heart Rhythm Institute (J Am Coll Cardiol. 2020 Mar 13;75[13]:1519-22).
Dr. Freeman reported serving as a consultant to Boston Scientific, which markets the Watchman, as well as to Medtronic, Janssen, and Biosense Webster.
SOURCE: Freeman JF. ACC 2020, Abstract 409-10.
Early results from the massive National Cardiovascular Data Registry Left Atrial Appendage Occlusion Registry indicate that the rollout of the Watchman device into routine clinical practice is going smoothly, with a higher implant success rate and a substantially lower in-hospital complication rate than that seen in the pivotal randomized clinical trials, James V. Freeman, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These real-world results are particularly impressive because the 38,158 registry participants were on average significantly older and sicker than were patients in the clinical trials. They were at higher risk of both stroke and bleeding, yet they fared better in terms of procedural safety, observed Dr. Freeman, an electrophysiologist and director of the Yale University Atrial Fibrillation Center in New Haven, Conn.
“You always worry that once you get outside of the clinical trials setting and you roll out to a large number of centers, including some that are relatively low volume, that you’re going to start to see higher rates of complications. And overall, broadly speaking, the rates of adverse events were quite reassuring,” he said.
The registry, maintained by the ACC, serves as the postmarketing surveillance tool mandated by the Food and Drug Administration and Centers for Medicare & Medicaid Services. The 38,158 participants make this registry the world’s largest patient experience with the Watchman device by many orders of magnitude. Dr. Freeman’s report included patients enrolled during 2016-2018 who were treated at 495 hospitals by 1,318 physician interventionalists. CMS reimbursement requires participation in the registry, which captures more than 95% of all Watchman procedures done in the United States. Although Dr. Freeman presented only the acute in-hospital outcomes, active follow-up for adverse events and medical therapy will be conducted at 45 days, 6 months, and 1 and 2 years.
Participants in the Left Atrial Appendage Occlusion (LAAO) Registry averaged 76.1 years of age, which is 2-4 years older than patients in the pivotal PROTECT-AF and PREVAIL trials or the 1,025-patient EWOLUTION registry. The LAAO Registry participants had a mean CHA2DS2-VASc score of 4.6, compared with 3.4 in PROTECT-AF and 3.8 in PREVAIL. Their mean HAS BLED score was 3.0. Thirty percent had a prior ischemic stroke or transient ischemic attack, 12% had a prior intracranial hemorrhage, and 69% had a history of clinically relevant bleeding. Thirty percent had heart failure, 92% were hypertensive, and 30% had diabetes.
“The take home here is that these patients were at moderate to high risk of stroke and they also carried a high risk of bleeding and therefore had some relative contraindication to anticoagulation,” according to the cardiologist. “The patient population overall is really in accordance with the CMS guidance. We’re not seeing a lot of patients who are getting this device for a lifestyle indication. Most of these patients are really stuck between a rock and a hard place.”
Most hospitals offering the Watchman did 10-40 cases per year. The median annual physician volume was 12 cases. However, there was substantial variation in both hospital and physician volumes.
The device was deployed in 93% of procedures attempted; roughly half of cancellations were cause by LAAO thrombus detected on the day of the procedure. The acute procedural success rate when the device was deployed was 98.3%, compared with 90.9% in PROTECT-AF and 95.1% in PREVAIL. The rate of device margin residual leak of 5 mm or more among registry participants with an acutely successful procedure was 0.2%.
The rate of any major in-hospital complication in the LAAO Registry was 2.16%, the most common of which was pericardial effusion requiring intervention, which occurred in 1.39% of cases. The major bleeding rate was 1.25%. The stroke/transient ischemic attack rate was 0.17%. Systemic arterial embolism was a rare event, occurring in less than 0.01% of patients, as was acute MI, with an incidence of 0.04%. Device embolization occurred in 0.07% of patients.
By comparison, the 7-day rate of pericardial effusion requiring intervention was 4.0% in PROTECT-AF and 1.9% in PREVAIL, with procedure-related stroke rates of 1.1% and 0.7%, respectively, and device embolization rates of 0.4% and 0.7%. The major bleeding rate in PROTECT-AF was 3.5%, nearly triple that in the real-world registry.
Discussant Mark A. Estes, MD, characterized the acute outcomes in the LAAO Registry as “an improvement – a considerable improvement – over some of the early data in PREVAIL and PROTECT-AF.” He credited this to the “very robust validation procedure” the Watchman closure device has undergone, which included the clinical trials, regulatory requirements for training and patient selection, and mandatory reporting of outcomes in the registry.
He noted that a lot is happening now with the Watchman device. There are a couple of dozen prospective clinical trials, including one on the Watchman versus direct oral anticoagulant (DOAC) therapy and another on left atrial ablation plus left atrial appendage closure versus a DOAC. A new-generation Watchman device, the Watchman FLX, is approved in Europe and undergoing an ongoing FDA-mandated approval trial in the United States.
“It has a lot of technical advantages,” according to Dr. Estes, an electrophysiologist and professor of medicine at the University of Pittsburgh.
Current guidelines give LAAO a class IIb rating, meaning it “could be considered” in patients with atrial fibrillation at increased risk of stroke who have a contraindication to long-term anticoagulation. Dr. Estes asked: Does the LAAO Registry data warrant a rating upgrade to a stronger recommendation?
Dr. Freeman replied that the new data should allay the guideline writers’ and government regulators’ concerns regarding acute procedural safety. But that’s only part of the picture. He and his coinvestigators are busy gathering data on intermediate-term outcomes, analyzing the impact of various strategies for periprocedural and long-term management of antiplatelet and anticoagulant medications with an eye toward identifying best practices, and investigating the relationship between procedural volume and outcomes, information, which could have an impact on the next iteration of the guidelines.
Simultaneous with his presentation at ACC 2020, the study was published online (J Am Coll Cardiol. 2020 Mar 13;75[13]1503-18).
In an accompanying editorial, Dhanunjaya Lakkireddy, MD, commented that an important contribution of the LAAO Registry is its inclusion of an enormous number of patients with contraindications to oral anticoagulation, a population excluded from the PROTECT-AF and PREVAIL randomized trials.
The short-term results of the registry suggest a relaxation of the current strict requirement for surgical backup during Watchman procedures is in order, added Dr. Lakkireddy, professor of medicine at the University of Missouri, Columbia, and medical director of the Kansas City Heart Rhythm Institute (J Am Coll Cardiol. 2020 Mar 13;75[13]:1519-22).
Dr. Freeman reported serving as a consultant to Boston Scientific, which markets the Watchman, as well as to Medtronic, Janssen, and Biosense Webster.
SOURCE: Freeman JF. ACC 2020, Abstract 409-10.
Early results from the massive National Cardiovascular Data Registry Left Atrial Appendage Occlusion Registry indicate that the rollout of the Watchman device into routine clinical practice is going smoothly, with a higher implant success rate and a substantially lower in-hospital complication rate than that seen in the pivotal randomized clinical trials, James V. Freeman, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These real-world results are particularly impressive because the 38,158 registry participants were on average significantly older and sicker than were patients in the clinical trials. They were at higher risk of both stroke and bleeding, yet they fared better in terms of procedural safety, observed Dr. Freeman, an electrophysiologist and director of the Yale University Atrial Fibrillation Center in New Haven, Conn.
“You always worry that once you get outside of the clinical trials setting and you roll out to a large number of centers, including some that are relatively low volume, that you’re going to start to see higher rates of complications. And overall, broadly speaking, the rates of adverse events were quite reassuring,” he said.
The registry, maintained by the ACC, serves as the postmarketing surveillance tool mandated by the Food and Drug Administration and Centers for Medicare & Medicaid Services. The 38,158 participants make this registry the world’s largest patient experience with the Watchman device by many orders of magnitude. Dr. Freeman’s report included patients enrolled during 2016-2018 who were treated at 495 hospitals by 1,318 physician interventionalists. CMS reimbursement requires participation in the registry, which captures more than 95% of all Watchman procedures done in the United States. Although Dr. Freeman presented only the acute in-hospital outcomes, active follow-up for adverse events and medical therapy will be conducted at 45 days, 6 months, and 1 and 2 years.
Participants in the Left Atrial Appendage Occlusion (LAAO) Registry averaged 76.1 years of age, which is 2-4 years older than patients in the pivotal PROTECT-AF and PREVAIL trials or the 1,025-patient EWOLUTION registry. The LAAO Registry participants had a mean CHA2DS2-VASc score of 4.6, compared with 3.4 in PROTECT-AF and 3.8 in PREVAIL. Their mean HAS BLED score was 3.0. Thirty percent had a prior ischemic stroke or transient ischemic attack, 12% had a prior intracranial hemorrhage, and 69% had a history of clinically relevant bleeding. Thirty percent had heart failure, 92% were hypertensive, and 30% had diabetes.
“The take home here is that these patients were at moderate to high risk of stroke and they also carried a high risk of bleeding and therefore had some relative contraindication to anticoagulation,” according to the cardiologist. “The patient population overall is really in accordance with the CMS guidance. We’re not seeing a lot of patients who are getting this device for a lifestyle indication. Most of these patients are really stuck between a rock and a hard place.”
Most hospitals offering the Watchman did 10-40 cases per year. The median annual physician volume was 12 cases. However, there was substantial variation in both hospital and physician volumes.
The device was deployed in 93% of procedures attempted; roughly half of cancellations were cause by LAAO thrombus detected on the day of the procedure. The acute procedural success rate when the device was deployed was 98.3%, compared with 90.9% in PROTECT-AF and 95.1% in PREVAIL. The rate of device margin residual leak of 5 mm or more among registry participants with an acutely successful procedure was 0.2%.
The rate of any major in-hospital complication in the LAAO Registry was 2.16%, the most common of which was pericardial effusion requiring intervention, which occurred in 1.39% of cases. The major bleeding rate was 1.25%. The stroke/transient ischemic attack rate was 0.17%. Systemic arterial embolism was a rare event, occurring in less than 0.01% of patients, as was acute MI, with an incidence of 0.04%. Device embolization occurred in 0.07% of patients.
By comparison, the 7-day rate of pericardial effusion requiring intervention was 4.0% in PROTECT-AF and 1.9% in PREVAIL, with procedure-related stroke rates of 1.1% and 0.7%, respectively, and device embolization rates of 0.4% and 0.7%. The major bleeding rate in PROTECT-AF was 3.5%, nearly triple that in the real-world registry.
Discussant Mark A. Estes, MD, characterized the acute outcomes in the LAAO Registry as “an improvement – a considerable improvement – over some of the early data in PREVAIL and PROTECT-AF.” He credited this to the “very robust validation procedure” the Watchman closure device has undergone, which included the clinical trials, regulatory requirements for training and patient selection, and mandatory reporting of outcomes in the registry.
He noted that a lot is happening now with the Watchman device. There are a couple of dozen prospective clinical trials, including one on the Watchman versus direct oral anticoagulant (DOAC) therapy and another on left atrial ablation plus left atrial appendage closure versus a DOAC. A new-generation Watchman device, the Watchman FLX, is approved in Europe and undergoing an ongoing FDA-mandated approval trial in the United States.
“It has a lot of technical advantages,” according to Dr. Estes, an electrophysiologist and professor of medicine at the University of Pittsburgh.
Current guidelines give LAAO a class IIb rating, meaning it “could be considered” in patients with atrial fibrillation at increased risk of stroke who have a contraindication to long-term anticoagulation. Dr. Estes asked: Does the LAAO Registry data warrant a rating upgrade to a stronger recommendation?
Dr. Freeman replied that the new data should allay the guideline writers’ and government regulators’ concerns regarding acute procedural safety. But that’s only part of the picture. He and his coinvestigators are busy gathering data on intermediate-term outcomes, analyzing the impact of various strategies for periprocedural and long-term management of antiplatelet and anticoagulant medications with an eye toward identifying best practices, and investigating the relationship between procedural volume and outcomes, information, which could have an impact on the next iteration of the guidelines.
Simultaneous with his presentation at ACC 2020, the study was published online (J Am Coll Cardiol. 2020 Mar 13;75[13]1503-18).
In an accompanying editorial, Dhanunjaya Lakkireddy, MD, commented that an important contribution of the LAAO Registry is its inclusion of an enormous number of patients with contraindications to oral anticoagulation, a population excluded from the PROTECT-AF and PREVAIL randomized trials.
The short-term results of the registry suggest a relaxation of the current strict requirement for surgical backup during Watchman procedures is in order, added Dr. Lakkireddy, professor of medicine at the University of Missouri, Columbia, and medical director of the Kansas City Heart Rhythm Institute (J Am Coll Cardiol. 2020 Mar 13;75[13]:1519-22).
Dr. Freeman reported serving as a consultant to Boston Scientific, which markets the Watchman, as well as to Medtronic, Janssen, and Biosense Webster.
SOURCE: Freeman JF. ACC 2020, Abstract 409-10.
FROM ACC 2020
Treating lung cancer in COVID-19 times: Update from experts
Lung cancer experts in Europe issued highly considered recommendations for the management of lung cancer during the COVID-19 crisis, the main intention of which is to minimize the risk of patients getting infected by SARS-CoV-2 while in hospital receiving treatment.
The recommendations were published online April 3 in ESMO Open.
“We know that having cancer increases the risk of dying of COVID-19, although not necessarily the risk of getting the virus, and we also know that having lung cancer could increase the risk of pulmonary complications from SARS-CoV-2,” lead author Alfredo Addeo, MD, University Hospital of Geneva, Switzerland, told Medscape Medical News.
“But patients who are often in the hospital have a higher risk of catching the virus. So this paper is not about not giving necessary treatment, it’s about treating patients the best you can based on the area where you live and the resources you have and keeping patients away from the hospital as much as possible,” he added.
“The main message is, try to personalize the care you deliver,” Addeo said. “Rather than remain rigid about how you’ve been treating patients thus far, try to think outside the box and find a way to minimize the risk of infection, and, if you have to limit treatment, discuss the pros and cons of your treatment plan with the patient and make sure the message is given clearly.”
How much benefit?
The first general concept to keep in mind is: How likely is a patient to benefit from treatment?
“All regimens with a survival benefit should be maintained and prioritised whenever possible,” Addeo and colleagues observe. The other co-authors of the paper are Giuseppe Banna, MD, Ospedale Cannizzaro, Catania, Italy; Alessandra Curioni-Fontecedro, MD, University Hospital Zurich, Switzerland; and Alex Friedlaender, MD, University Hospital of Geneva.
For non–small cell lung cancer (NSCLC), neoadjuvant chemotherapy for locally advanced resectable disease and sequential/concurrent chemotherapy/radiation therapy for patients with stage III lung cancer – provided they have adequate respiratory function – should be started when possible and should not be stopped without justification, the authors point out.
This is also true for first-line therapy in patients with metastatic disease. Treatment should also not be stopped without good reason among patients already receiving maintenance immune checkpoint inhibitor therapy.
For small cell lung cancer (SCLC), both first-line treatment for extensive-stage disease as well as concurrent chemotherapy/radiotherapy for patients with limited-stage disease should be started when possible, again provided they have adequate respiratory function.
Palliative or stereotactic body radiotherapy (SBRT) delivered outside the lung should also be initiated when possible in SCLC patients.
The authors caution, however, that if palliative or SBRT outside the lung requires multiple visits to the hospital, treatment to the lung should be limited to cases with compression of airways or bleeding.
Oncologists should also try to start radiotherapy on day 1 of chemotherapy because then only 2 cycles will be needed; if radiotherapy is started with cycle 2 or is given sequentially, 3 cycles of treatment will be required.
“Fractions of SBRT could be reduced, depending on organ at risk (8 fractions to 5 or 3) while palliative RT [given] as a single fraction or two (8-10 Gy or 17 Gy, respectively) should be used where possible,” the authors observe.
Concurrent chemotherapy with radiotherapy for limited-stage disease should not be stopped without justification and nor should first-line treatment for metastatic SCLC, the authors continue.
Again, however, patients must have adequate respiratory function to receive or continue with concurrent chemotherapy and radiotherapy, they add.
For patients with stage III NSCLC, concurrent chemotherapy plus radiotherapy may be considered and given preferentially or not.
Similarly, oral rather than intravenous chemotherapy may be preferred for elderly NSCLC patients or for those with an ECOG performance status of 2 as well as for SCLC patients.
Delaying surgery
As a general principle, the use of neoadjuvant chemotherapy instead of adjuvant therapy following surgery can delay the need for immediate surgery. If surgery can be delayed, “the risk of a patient catching the virus several months from now might be less,” Addeo noted. Thus, treating patients upfront with chemotherapy is one tactic to consider in appropriate patients.
For NSCLC patients at high risk for COVID-19, adjuvant chemotherapy should be discussed and potentially withheld, the authors observe.
NSCLC patients at high risk for COVID-19 include those with comorbidities, such as cardiovascular or pulmonary disease, as well as patients who are 70 years of age and older.
Immunotherapy should also be discussed and possibly delayed for stage III NSCLC patients following concurrent chemotherapy and radiation, they add.
Maintenance pemetrexed also may be withheld for NSCLC patients, and intervals of immunotherapy may be prolonged (e.g., nivolumab every 4 weeks and pembrolizumab every 6 weeks).
Intervals of immunotherapy should be similarly prolonged for SCLC patients, they continue.
“Shorter duration of chemotherapy (e.g., four cycles of chemotherapy instead of six) should be discussed with patients and maintenance chemotherapy can be withheld,” the authors note.
Furthermore, “given the pandemic, it is highly likely that metastatic cancer patients will be less likely to be intubated or to be heavily ventilated compared to patients without any comorbidity,” Addeo explained.
“So we have to acknowledge that metastatic lung cancer patients will be at higher risk of dying due to severe pulmonary COVID-19 complications,” he added.
Therefore, third and further lines of chemotherapy in both NSCLC and SCLC patients at significant COVID-19 risk should not be initiated without having a good reason to do so.
“Prophylactic cranial irradiation (PCI) is still a matter of debate [in SCLC patients],” Addeo noted. “So the reasonable alternative is to do surveillance MRI, and, in 6 or 8 months, we can probably offer PCI more safely at that point,” he suggested, adding that radiation therapy to the brain should only be considered if a patient develops brain metastases.
The authors also suggest that thoracic consolidation radiotherapy for extensive stage SCLC should not be initiated unless there is good reason to do so.
Patients with family members or caregivers who have tested positive for COVID-19 should themselves be tested before or during any cancer treatment.
If patients themselves then test positive and are asymptomatic, “28 days of delay should be considered before (re)starting the treatment,” the authors advise.
However, two negative tests done 1 week apart should be carried out before starting or restarting treatment, they note.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Lung cancer experts in Europe issued highly considered recommendations for the management of lung cancer during the COVID-19 crisis, the main intention of which is to minimize the risk of patients getting infected by SARS-CoV-2 while in hospital receiving treatment.
The recommendations were published online April 3 in ESMO Open.
“We know that having cancer increases the risk of dying of COVID-19, although not necessarily the risk of getting the virus, and we also know that having lung cancer could increase the risk of pulmonary complications from SARS-CoV-2,” lead author Alfredo Addeo, MD, University Hospital of Geneva, Switzerland, told Medscape Medical News.
“But patients who are often in the hospital have a higher risk of catching the virus. So this paper is not about not giving necessary treatment, it’s about treating patients the best you can based on the area where you live and the resources you have and keeping patients away from the hospital as much as possible,” he added.
“The main message is, try to personalize the care you deliver,” Addeo said. “Rather than remain rigid about how you’ve been treating patients thus far, try to think outside the box and find a way to minimize the risk of infection, and, if you have to limit treatment, discuss the pros and cons of your treatment plan with the patient and make sure the message is given clearly.”
How much benefit?
The first general concept to keep in mind is: How likely is a patient to benefit from treatment?
“All regimens with a survival benefit should be maintained and prioritised whenever possible,” Addeo and colleagues observe. The other co-authors of the paper are Giuseppe Banna, MD, Ospedale Cannizzaro, Catania, Italy; Alessandra Curioni-Fontecedro, MD, University Hospital Zurich, Switzerland; and Alex Friedlaender, MD, University Hospital of Geneva.
For non–small cell lung cancer (NSCLC), neoadjuvant chemotherapy for locally advanced resectable disease and sequential/concurrent chemotherapy/radiation therapy for patients with stage III lung cancer – provided they have adequate respiratory function – should be started when possible and should not be stopped without justification, the authors point out.
This is also true for first-line therapy in patients with metastatic disease. Treatment should also not be stopped without good reason among patients already receiving maintenance immune checkpoint inhibitor therapy.
For small cell lung cancer (SCLC), both first-line treatment for extensive-stage disease as well as concurrent chemotherapy/radiotherapy for patients with limited-stage disease should be started when possible, again provided they have adequate respiratory function.
Palliative or stereotactic body radiotherapy (SBRT) delivered outside the lung should also be initiated when possible in SCLC patients.
The authors caution, however, that if palliative or SBRT outside the lung requires multiple visits to the hospital, treatment to the lung should be limited to cases with compression of airways or bleeding.
Oncologists should also try to start radiotherapy on day 1 of chemotherapy because then only 2 cycles will be needed; if radiotherapy is started with cycle 2 or is given sequentially, 3 cycles of treatment will be required.
“Fractions of SBRT could be reduced, depending on organ at risk (8 fractions to 5 or 3) while palliative RT [given] as a single fraction or two (8-10 Gy or 17 Gy, respectively) should be used where possible,” the authors observe.
Concurrent chemotherapy with radiotherapy for limited-stage disease should not be stopped without justification and nor should first-line treatment for metastatic SCLC, the authors continue.
Again, however, patients must have adequate respiratory function to receive or continue with concurrent chemotherapy and radiotherapy, they add.
For patients with stage III NSCLC, concurrent chemotherapy plus radiotherapy may be considered and given preferentially or not.
Similarly, oral rather than intravenous chemotherapy may be preferred for elderly NSCLC patients or for those with an ECOG performance status of 2 as well as for SCLC patients.
Delaying surgery
As a general principle, the use of neoadjuvant chemotherapy instead of adjuvant therapy following surgery can delay the need for immediate surgery. If surgery can be delayed, “the risk of a patient catching the virus several months from now might be less,” Addeo noted. Thus, treating patients upfront with chemotherapy is one tactic to consider in appropriate patients.
For NSCLC patients at high risk for COVID-19, adjuvant chemotherapy should be discussed and potentially withheld, the authors observe.
NSCLC patients at high risk for COVID-19 include those with comorbidities, such as cardiovascular or pulmonary disease, as well as patients who are 70 years of age and older.
Immunotherapy should also be discussed and possibly delayed for stage III NSCLC patients following concurrent chemotherapy and radiation, they add.
Maintenance pemetrexed also may be withheld for NSCLC patients, and intervals of immunotherapy may be prolonged (e.g., nivolumab every 4 weeks and pembrolizumab every 6 weeks).
Intervals of immunotherapy should be similarly prolonged for SCLC patients, they continue.
“Shorter duration of chemotherapy (e.g., four cycles of chemotherapy instead of six) should be discussed with patients and maintenance chemotherapy can be withheld,” the authors note.
Furthermore, “given the pandemic, it is highly likely that metastatic cancer patients will be less likely to be intubated or to be heavily ventilated compared to patients without any comorbidity,” Addeo explained.
“So we have to acknowledge that metastatic lung cancer patients will be at higher risk of dying due to severe pulmonary COVID-19 complications,” he added.
Therefore, third and further lines of chemotherapy in both NSCLC and SCLC patients at significant COVID-19 risk should not be initiated without having a good reason to do so.
“Prophylactic cranial irradiation (PCI) is still a matter of debate [in SCLC patients],” Addeo noted. “So the reasonable alternative is to do surveillance MRI, and, in 6 or 8 months, we can probably offer PCI more safely at that point,” he suggested, adding that radiation therapy to the brain should only be considered if a patient develops brain metastases.
The authors also suggest that thoracic consolidation radiotherapy for extensive stage SCLC should not be initiated unless there is good reason to do so.
Patients with family members or caregivers who have tested positive for COVID-19 should themselves be tested before or during any cancer treatment.
If patients themselves then test positive and are asymptomatic, “28 days of delay should be considered before (re)starting the treatment,” the authors advise.
However, two negative tests done 1 week apart should be carried out before starting or restarting treatment, they note.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Lung cancer experts in Europe issued highly considered recommendations for the management of lung cancer during the COVID-19 crisis, the main intention of which is to minimize the risk of patients getting infected by SARS-CoV-2 while in hospital receiving treatment.
The recommendations were published online April 3 in ESMO Open.
“We know that having cancer increases the risk of dying of COVID-19, although not necessarily the risk of getting the virus, and we also know that having lung cancer could increase the risk of pulmonary complications from SARS-CoV-2,” lead author Alfredo Addeo, MD, University Hospital of Geneva, Switzerland, told Medscape Medical News.
“But patients who are often in the hospital have a higher risk of catching the virus. So this paper is not about not giving necessary treatment, it’s about treating patients the best you can based on the area where you live and the resources you have and keeping patients away from the hospital as much as possible,” he added.
“The main message is, try to personalize the care you deliver,” Addeo said. “Rather than remain rigid about how you’ve been treating patients thus far, try to think outside the box and find a way to minimize the risk of infection, and, if you have to limit treatment, discuss the pros and cons of your treatment plan with the patient and make sure the message is given clearly.”
How much benefit?
The first general concept to keep in mind is: How likely is a patient to benefit from treatment?
“All regimens with a survival benefit should be maintained and prioritised whenever possible,” Addeo and colleagues observe. The other co-authors of the paper are Giuseppe Banna, MD, Ospedale Cannizzaro, Catania, Italy; Alessandra Curioni-Fontecedro, MD, University Hospital Zurich, Switzerland; and Alex Friedlaender, MD, University Hospital of Geneva.
For non–small cell lung cancer (NSCLC), neoadjuvant chemotherapy for locally advanced resectable disease and sequential/concurrent chemotherapy/radiation therapy for patients with stage III lung cancer – provided they have adequate respiratory function – should be started when possible and should not be stopped without justification, the authors point out.
This is also true for first-line therapy in patients with metastatic disease. Treatment should also not be stopped without good reason among patients already receiving maintenance immune checkpoint inhibitor therapy.
For small cell lung cancer (SCLC), both first-line treatment for extensive-stage disease as well as concurrent chemotherapy/radiotherapy for patients with limited-stage disease should be started when possible, again provided they have adequate respiratory function.
Palliative or stereotactic body radiotherapy (SBRT) delivered outside the lung should also be initiated when possible in SCLC patients.
The authors caution, however, that if palliative or SBRT outside the lung requires multiple visits to the hospital, treatment to the lung should be limited to cases with compression of airways or bleeding.
Oncologists should also try to start radiotherapy on day 1 of chemotherapy because then only 2 cycles will be needed; if radiotherapy is started with cycle 2 or is given sequentially, 3 cycles of treatment will be required.
“Fractions of SBRT could be reduced, depending on organ at risk (8 fractions to 5 or 3) while palliative RT [given] as a single fraction or two (8-10 Gy or 17 Gy, respectively) should be used where possible,” the authors observe.
Concurrent chemotherapy with radiotherapy for limited-stage disease should not be stopped without justification and nor should first-line treatment for metastatic SCLC, the authors continue.
Again, however, patients must have adequate respiratory function to receive or continue with concurrent chemotherapy and radiotherapy, they add.
For patients with stage III NSCLC, concurrent chemotherapy plus radiotherapy may be considered and given preferentially or not.
Similarly, oral rather than intravenous chemotherapy may be preferred for elderly NSCLC patients or for those with an ECOG performance status of 2 as well as for SCLC patients.
Delaying surgery
As a general principle, the use of neoadjuvant chemotherapy instead of adjuvant therapy following surgery can delay the need for immediate surgery. If surgery can be delayed, “the risk of a patient catching the virus several months from now might be less,” Addeo noted. Thus, treating patients upfront with chemotherapy is one tactic to consider in appropriate patients.
For NSCLC patients at high risk for COVID-19, adjuvant chemotherapy should be discussed and potentially withheld, the authors observe.
NSCLC patients at high risk for COVID-19 include those with comorbidities, such as cardiovascular or pulmonary disease, as well as patients who are 70 years of age and older.
Immunotherapy should also be discussed and possibly delayed for stage III NSCLC patients following concurrent chemotherapy and radiation, they add.
Maintenance pemetrexed also may be withheld for NSCLC patients, and intervals of immunotherapy may be prolonged (e.g., nivolumab every 4 weeks and pembrolizumab every 6 weeks).
Intervals of immunotherapy should be similarly prolonged for SCLC patients, they continue.
“Shorter duration of chemotherapy (e.g., four cycles of chemotherapy instead of six) should be discussed with patients and maintenance chemotherapy can be withheld,” the authors note.
Furthermore, “given the pandemic, it is highly likely that metastatic cancer patients will be less likely to be intubated or to be heavily ventilated compared to patients without any comorbidity,” Addeo explained.
“So we have to acknowledge that metastatic lung cancer patients will be at higher risk of dying due to severe pulmonary COVID-19 complications,” he added.
Therefore, third and further lines of chemotherapy in both NSCLC and SCLC patients at significant COVID-19 risk should not be initiated without having a good reason to do so.
“Prophylactic cranial irradiation (PCI) is still a matter of debate [in SCLC patients],” Addeo noted. “So the reasonable alternative is to do surveillance MRI, and, in 6 or 8 months, we can probably offer PCI more safely at that point,” he suggested, adding that radiation therapy to the brain should only be considered if a patient develops brain metastases.
The authors also suggest that thoracic consolidation radiotherapy for extensive stage SCLC should not be initiated unless there is good reason to do so.
Patients with family members or caregivers who have tested positive for COVID-19 should themselves be tested before or during any cancer treatment.
If patients themselves then test positive and are asymptomatic, “28 days of delay should be considered before (re)starting the treatment,” the authors advise.
However, two negative tests done 1 week apart should be carried out before starting or restarting treatment, they note.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
One-third of high-risk CLL patients received treatment counter to recommendations
Approximately , according to a study based upon data from the informCLL registry. In addition, low levels of prognostic marker testing in these patients was a concern.
Researchers assessed data from 840 enrolled CLL patients, of whom 459 (55%) were previously untreated, and 381 (45%) had relapsed/refractory disease. In terms of therapy, chemoimmunotherapy was more common in previously untreated patients, compared with relapsed/refractory patients (42% vs. 23%), whereas ibrutinib was more frequently used in relapsed/refractory vs. previously untreated patients (51% vs. 39%), according to the researchers.
Fluorescent in situ hybridization (FISH) testing, TP53 mutation, and immunoglobulin heavy chain somatic hypermutation biomarker testing were performed infrequently across all patients at registry enrollment, according to the authors.
Among patients who were tested, the rate of mutated TP53 was the same for previously untreated (14/54; 26%) and relapsed/refractory patients (9/35; 26%). In those patients who were tested, 34% with del(17p), a chromosomal deletion, and 26% of mutated TP53 patients received chemoimmunotherapy combinations. The authors stated that this was concerning in that it contradicts consensus guidelines based on data from several clinical studies. Chemoimmunotherapy is not recommended for these high-risk patients because of poor disease and survival outcomes with this treatment strategy, according to the authors.
“Current clinical practice is not keeping pace with recommendations and guidelines for prognostic
marker testing and subsequent selection of appropriate therapy,” the authors stated.
“Even with the approval of novel agents and updated guidelines, low rates of prognostic biomarker testing may lead to suboptimal therapy choices for patients with unknown risk status. In addition, we note that the presence of high-risk features (del(17p) and TP53) is unfortunately not translating to choosing the optimal therapy for these patients,” the researchers concluded.
The study was sponsored by an AbbVie Company and Janssen. The authors reported consulting and grants from these and other pharmaceutical companies.
SOURCE: Mato AR et al. Clin Lymphoma Myeloma Leuk. 2020;20(3):174-83.
Approximately , according to a study based upon data from the informCLL registry. In addition, low levels of prognostic marker testing in these patients was a concern.
Researchers assessed data from 840 enrolled CLL patients, of whom 459 (55%) were previously untreated, and 381 (45%) had relapsed/refractory disease. In terms of therapy, chemoimmunotherapy was more common in previously untreated patients, compared with relapsed/refractory patients (42% vs. 23%), whereas ibrutinib was more frequently used in relapsed/refractory vs. previously untreated patients (51% vs. 39%), according to the researchers.
Fluorescent in situ hybridization (FISH) testing, TP53 mutation, and immunoglobulin heavy chain somatic hypermutation biomarker testing were performed infrequently across all patients at registry enrollment, according to the authors.
Among patients who were tested, the rate of mutated TP53 was the same for previously untreated (14/54; 26%) and relapsed/refractory patients (9/35; 26%). In those patients who were tested, 34% with del(17p), a chromosomal deletion, and 26% of mutated TP53 patients received chemoimmunotherapy combinations. The authors stated that this was concerning in that it contradicts consensus guidelines based on data from several clinical studies. Chemoimmunotherapy is not recommended for these high-risk patients because of poor disease and survival outcomes with this treatment strategy, according to the authors.
“Current clinical practice is not keeping pace with recommendations and guidelines for prognostic
marker testing and subsequent selection of appropriate therapy,” the authors stated.
“Even with the approval of novel agents and updated guidelines, low rates of prognostic biomarker testing may lead to suboptimal therapy choices for patients with unknown risk status. In addition, we note that the presence of high-risk features (del(17p) and TP53) is unfortunately not translating to choosing the optimal therapy for these patients,” the researchers concluded.
The study was sponsored by an AbbVie Company and Janssen. The authors reported consulting and grants from these and other pharmaceutical companies.
SOURCE: Mato AR et al. Clin Lymphoma Myeloma Leuk. 2020;20(3):174-83.
Approximately , according to a study based upon data from the informCLL registry. In addition, low levels of prognostic marker testing in these patients was a concern.
Researchers assessed data from 840 enrolled CLL patients, of whom 459 (55%) were previously untreated, and 381 (45%) had relapsed/refractory disease. In terms of therapy, chemoimmunotherapy was more common in previously untreated patients, compared with relapsed/refractory patients (42% vs. 23%), whereas ibrutinib was more frequently used in relapsed/refractory vs. previously untreated patients (51% vs. 39%), according to the researchers.
Fluorescent in situ hybridization (FISH) testing, TP53 mutation, and immunoglobulin heavy chain somatic hypermutation biomarker testing were performed infrequently across all patients at registry enrollment, according to the authors.
Among patients who were tested, the rate of mutated TP53 was the same for previously untreated (14/54; 26%) and relapsed/refractory patients (9/35; 26%). In those patients who were tested, 34% with del(17p), a chromosomal deletion, and 26% of mutated TP53 patients received chemoimmunotherapy combinations. The authors stated that this was concerning in that it contradicts consensus guidelines based on data from several clinical studies. Chemoimmunotherapy is not recommended for these high-risk patients because of poor disease and survival outcomes with this treatment strategy, according to the authors.
“Current clinical practice is not keeping pace with recommendations and guidelines for prognostic
marker testing and subsequent selection of appropriate therapy,” the authors stated.
“Even with the approval of novel agents and updated guidelines, low rates of prognostic biomarker testing may lead to suboptimal therapy choices for patients with unknown risk status. In addition, we note that the presence of high-risk features (del(17p) and TP53) is unfortunately not translating to choosing the optimal therapy for these patients,” the researchers concluded.
The study was sponsored by an AbbVie Company and Janssen. The authors reported consulting and grants from these and other pharmaceutical companies.
SOURCE: Mato AR et al. Clin Lymphoma Myeloma Leuk. 2020;20(3):174-83.
Conducting cancer trials amid the COVID-19 pandemic
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Genotyping improves accuracy of pancreatic cancer tumor markers
Stratifying diagnostic cut-off values of tumor markers based on genetic variants may improve detection of pancreatic cancer, according to investigators.
Stratification had the greatest positive impact on accuracy of carbohydrate antigen 19-9 (CA19-9), reported lead author Toshiya Abe, MD, PhD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“Despite the evidence that genetic factors influence tumor marker levels, the potential utility of using a genetic test to improve the interpretation of tumor markers has drawn limited attention,” the investigators wrote in Clinical Gastroenterology and Hepatology.
And improvements are needed, the investigators noted, particularly for early cancer detection in high-risk individuals.
“[T]he toughest hurdle for a pancreatic cancer detection blood test is the detection of stage I disease,” the investigators wrote. “Cancers generally shed biomarkers in proportion to their size, and small stage I pancreatic cancers shed fewer diagnostic biomarkers into the circulation, making diagnosis more difficult.”
Although a 2016 study by Dr. Guopei Luo and colleagues demonstrated that diagnostic accuracy of CA19-9 could be improved via genotyping, tumor marker performance was not characterized by high-specificity cut-off values, which the present study aimed to do.
The control group included 504 high-risk individuals who were prospectively enrolled in the Cancer of the Pancreas Screening (CAPS) studies from 2002 to 2018, while the case group included 245 patients with pancreatic ductal adenocarcinoma (PDAC) who underwent resection at Johns Hopkins from 2010 to 2017.
The control group was randomly divided into discovery and validation sets in order to achieve 99% specificity cut-off values, which were used to measure sensitivity in the case group. According to the investigators, high-specificity cut-off values are necessary for surveillance of asymptomatic high-risk individuals in order to minimize false-positive results.
In all patients, tumor markers and genotype were analyzed. Tumor markers included carcinoembryonic antigen (CEA), CA19-9, and cancer antigen 125 (CA-125). Genotyping included 16 single-nucleotide polymorphisms (SNPs) in 9 genes, including FUT2 and FUT3, which are known to influence levels of CA19-9.
In contrast with previous findings, which identified three relevant subgroups of FUT2/FUT3, the present study found that four distinct subgroups were significantly associated with CA19-9 levels: FUT3-null, FUT3+/-, FUT3+/+, and FUT2-null.
When CA19-9 cut-off levels were stratified by these four subgroups and applied to the 245 patients with pancreatic cancer, the investigators achieved a sensitivity of 60.8%, compared with 52.7% without stratification. The new cut-off values led to reclassification of 28 (11.4%) patients with pancreatic cancer, including 24 who switched from negative to positive, and 4 who switched from positive to negative.
Sensitivity of the SNP-adjusted CA19-9 test was improved to 66.4% when used exclusively in patients with functional FUT3 genes. Conversely, sensitivity was markedly lower, at 36.7%, when the test was used for patients with stage I disease.
While CA19-9 testing was notably improved by SNP-based stratification, results from CEA and CA-125 testing were more modest. Standard CEA testing had a sensitivity of 13.8%, compared with 15.9% when cut-off values were stratified by FUT2 status and ABO blood group. Similarly, modifying CA-125 values based on SNPs in GAL3ST2 raised sensitivity from 15.5% to 17.6%.
Although combining SNP-modified tumor marker results did increase overall sensitivity to as high as 66.1%, this also reduced specificity to as low as 95.4%
Still, Dr. Abe and colleagues suggested that the findings demonstrate proof of concept.
“Our results show that a tumor marker SNP test can improve the diagnostic accuracy of CA19-9 and, to a lesser extent, CEA and CA-125, but further work is needed to improve the diagnostic accuracy of our panel for the detection of early-stage pancreatic cancer,” they concluded.
The investigators also suggested that the technique could have value for surveillance of ovarian cancer; however, again, they emphasized the need for more research.The study was funded by the National Institutes of Health, Susan Wojcicki and Dennis Troper, the Pancreatic Cancer Action Network, and others. The investigators reported no conflicts of interest.
SOURCE: Abe T et al. Clin Gastro Hepatol. 2019 Oct 29. doi: 10.1016/j.cgh.2019.10.036.
Stratifying diagnostic cut-off values of tumor markers based on genetic variants may improve detection of pancreatic cancer, according to investigators.
Stratification had the greatest positive impact on accuracy of carbohydrate antigen 19-9 (CA19-9), reported lead author Toshiya Abe, MD, PhD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“Despite the evidence that genetic factors influence tumor marker levels, the potential utility of using a genetic test to improve the interpretation of tumor markers has drawn limited attention,” the investigators wrote in Clinical Gastroenterology and Hepatology.
And improvements are needed, the investigators noted, particularly for early cancer detection in high-risk individuals.
“[T]he toughest hurdle for a pancreatic cancer detection blood test is the detection of stage I disease,” the investigators wrote. “Cancers generally shed biomarkers in proportion to their size, and small stage I pancreatic cancers shed fewer diagnostic biomarkers into the circulation, making diagnosis more difficult.”
Although a 2016 study by Dr. Guopei Luo and colleagues demonstrated that diagnostic accuracy of CA19-9 could be improved via genotyping, tumor marker performance was not characterized by high-specificity cut-off values, which the present study aimed to do.
The control group included 504 high-risk individuals who were prospectively enrolled in the Cancer of the Pancreas Screening (CAPS) studies from 2002 to 2018, while the case group included 245 patients with pancreatic ductal adenocarcinoma (PDAC) who underwent resection at Johns Hopkins from 2010 to 2017.
The control group was randomly divided into discovery and validation sets in order to achieve 99% specificity cut-off values, which were used to measure sensitivity in the case group. According to the investigators, high-specificity cut-off values are necessary for surveillance of asymptomatic high-risk individuals in order to minimize false-positive results.
In all patients, tumor markers and genotype were analyzed. Tumor markers included carcinoembryonic antigen (CEA), CA19-9, and cancer antigen 125 (CA-125). Genotyping included 16 single-nucleotide polymorphisms (SNPs) in 9 genes, including FUT2 and FUT3, which are known to influence levels of CA19-9.
In contrast with previous findings, which identified three relevant subgroups of FUT2/FUT3, the present study found that four distinct subgroups were significantly associated with CA19-9 levels: FUT3-null, FUT3+/-, FUT3+/+, and FUT2-null.
When CA19-9 cut-off levels were stratified by these four subgroups and applied to the 245 patients with pancreatic cancer, the investigators achieved a sensitivity of 60.8%, compared with 52.7% without stratification. The new cut-off values led to reclassification of 28 (11.4%) patients with pancreatic cancer, including 24 who switched from negative to positive, and 4 who switched from positive to negative.
Sensitivity of the SNP-adjusted CA19-9 test was improved to 66.4% when used exclusively in patients with functional FUT3 genes. Conversely, sensitivity was markedly lower, at 36.7%, when the test was used for patients with stage I disease.
While CA19-9 testing was notably improved by SNP-based stratification, results from CEA and CA-125 testing were more modest. Standard CEA testing had a sensitivity of 13.8%, compared with 15.9% when cut-off values were stratified by FUT2 status and ABO blood group. Similarly, modifying CA-125 values based on SNPs in GAL3ST2 raised sensitivity from 15.5% to 17.6%.
Although combining SNP-modified tumor marker results did increase overall sensitivity to as high as 66.1%, this also reduced specificity to as low as 95.4%
Still, Dr. Abe and colleagues suggested that the findings demonstrate proof of concept.
“Our results show that a tumor marker SNP test can improve the diagnostic accuracy of CA19-9 and, to a lesser extent, CEA and CA-125, but further work is needed to improve the diagnostic accuracy of our panel for the detection of early-stage pancreatic cancer,” they concluded.
The investigators also suggested that the technique could have value for surveillance of ovarian cancer; however, again, they emphasized the need for more research.The study was funded by the National Institutes of Health, Susan Wojcicki and Dennis Troper, the Pancreatic Cancer Action Network, and others. The investigators reported no conflicts of interest.
SOURCE: Abe T et al. Clin Gastro Hepatol. 2019 Oct 29. doi: 10.1016/j.cgh.2019.10.036.
Stratifying diagnostic cut-off values of tumor markers based on genetic variants may improve detection of pancreatic cancer, according to investigators.
Stratification had the greatest positive impact on accuracy of carbohydrate antigen 19-9 (CA19-9), reported lead author Toshiya Abe, MD, PhD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“Despite the evidence that genetic factors influence tumor marker levels, the potential utility of using a genetic test to improve the interpretation of tumor markers has drawn limited attention,” the investigators wrote in Clinical Gastroenterology and Hepatology.
And improvements are needed, the investigators noted, particularly for early cancer detection in high-risk individuals.
“[T]he toughest hurdle for a pancreatic cancer detection blood test is the detection of stage I disease,” the investigators wrote. “Cancers generally shed biomarkers in proportion to their size, and small stage I pancreatic cancers shed fewer diagnostic biomarkers into the circulation, making diagnosis more difficult.”
Although a 2016 study by Dr. Guopei Luo and colleagues demonstrated that diagnostic accuracy of CA19-9 could be improved via genotyping, tumor marker performance was not characterized by high-specificity cut-off values, which the present study aimed to do.
The control group included 504 high-risk individuals who were prospectively enrolled in the Cancer of the Pancreas Screening (CAPS) studies from 2002 to 2018, while the case group included 245 patients with pancreatic ductal adenocarcinoma (PDAC) who underwent resection at Johns Hopkins from 2010 to 2017.
The control group was randomly divided into discovery and validation sets in order to achieve 99% specificity cut-off values, which were used to measure sensitivity in the case group. According to the investigators, high-specificity cut-off values are necessary for surveillance of asymptomatic high-risk individuals in order to minimize false-positive results.
In all patients, tumor markers and genotype were analyzed. Tumor markers included carcinoembryonic antigen (CEA), CA19-9, and cancer antigen 125 (CA-125). Genotyping included 16 single-nucleotide polymorphisms (SNPs) in 9 genes, including FUT2 and FUT3, which are known to influence levels of CA19-9.
In contrast with previous findings, which identified three relevant subgroups of FUT2/FUT3, the present study found that four distinct subgroups were significantly associated with CA19-9 levels: FUT3-null, FUT3+/-, FUT3+/+, and FUT2-null.
When CA19-9 cut-off levels were stratified by these four subgroups and applied to the 245 patients with pancreatic cancer, the investigators achieved a sensitivity of 60.8%, compared with 52.7% without stratification. The new cut-off values led to reclassification of 28 (11.4%) patients with pancreatic cancer, including 24 who switched from negative to positive, and 4 who switched from positive to negative.
Sensitivity of the SNP-adjusted CA19-9 test was improved to 66.4% when used exclusively in patients with functional FUT3 genes. Conversely, sensitivity was markedly lower, at 36.7%, when the test was used for patients with stage I disease.
While CA19-9 testing was notably improved by SNP-based stratification, results from CEA and CA-125 testing were more modest. Standard CEA testing had a sensitivity of 13.8%, compared with 15.9% when cut-off values were stratified by FUT2 status and ABO blood group. Similarly, modifying CA-125 values based on SNPs in GAL3ST2 raised sensitivity from 15.5% to 17.6%.
Although combining SNP-modified tumor marker results did increase overall sensitivity to as high as 66.1%, this also reduced specificity to as low as 95.4%
Still, Dr. Abe and colleagues suggested that the findings demonstrate proof of concept.
“Our results show that a tumor marker SNP test can improve the diagnostic accuracy of CA19-9 and, to a lesser extent, CEA and CA-125, but further work is needed to improve the diagnostic accuracy of our panel for the detection of early-stage pancreatic cancer,” they concluded.
The investigators also suggested that the technique could have value for surveillance of ovarian cancer; however, again, they emphasized the need for more research.The study was funded by the National Institutes of Health, Susan Wojcicki and Dennis Troper, the Pancreatic Cancer Action Network, and others. The investigators reported no conflicts of interest.
SOURCE: Abe T et al. Clin Gastro Hepatol. 2019 Oct 29. doi: 10.1016/j.cgh.2019.10.036.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY






