User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


‘A good and peaceful death’: Cancer hospice during the pandemic
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
Lillie Shockney, RN, MAS, a two-time breast cancer survivor and adjunct professor at Johns Hopkins School of Nursing in Baltimore, Maryland, mourns the many losses that her patients with advanced cancer now face in the midst of the COVID-19 pandemic. But in the void of the usual support networks and treatment plans, she sees the resurgence of something that has recently been crowded out: hospice.
The pandemic has forced patients and their physicians to reassess the risk/benefit balance of continuing or embarking on yet another cancer treatment.
“It’s one of the pearls that we will get out of this nightmare,” said Ms. Shockney, who recently retired as administrative director of the cancer survivorship programs at the Sidney Kimmel Comprehensive Cancer Center.
“Physicians have been taught to treat the disease – so as long as there’s a treatment they give another treatment,” she told Medscape Medical News during a Zoom call from her home. “But for some patients with advanced disease, those treatments were making them very sick, so they were trading longevity over quality of life.”
Of course, longevity has never been a guarantee with cancer treatment, and even less so now, with the risk of COVID-19.
“This is going to bring them to some hard discussions,” says Brenda Nevidjon, RN, MSN, chief executive officer at the Oncology Nursing Society.
“We’ve known for a long time that there are patients who are on third- and fourth-round treatment options that have very little evidence of prolonging life or quality of life,” she told Medscape Medical News. “Do we bring these people out of their home to a setting where there could be a fair number of COVID-positive patients? Do we expose them to that?”
Across the world, these dilemmas are pushing cancer specialists to initiate discussions of hospice sooner with patients who have advanced disease, and with more clarity than before.
One of the reasons such conversations have often been avoided is that the concept of hospice is generally misunderstood, said Ms. Shockney.
“Patients think ‘you’re giving up on me, you’ve abandoned me’, but hospice is all about preserving the remainder of their quality of life and letting them have time with family and time to fulfill those elements of experiencing a good and peaceful death,” she said.
Indeed, hospice is “a benefit meant for somebody with at least a 6-month horizon,” agrees Ms. Nevidjon. Yet the average length of hospice in the United States is just 5 days. “It’s at the very, very end, and yet for some of these patients the 6 months they could get in hospice might be a better quality of life than the 4 months on another whole plan of chemotherapy. I can’t imagine that on the backside of this pandemic we will not have learned and we won’t start to change practices around initiating more of these conversations.”
Silver lining of this pandemic?
It’s too early into the pandemic to have hard data on whether hospice uptake has increased, but “it’s encouraging to hear that hospice is being discussed and offered sooner as an alternative to that third- or fourth-round chemo,” said Lori Bishop, MHA, RN, vice president of palliative and advanced care at the National Hospice and Palliative Care Organization.
“I agree that improving informed-decision discussions and timely access to hospice is a silver lining of the pandemic,” she told Medscape Medical News.
But she points out that today’s hospice looks quite different than it did before the pandemic, with the immediate and very obvious difference being telehealth, which was not widely utilized previously.
In March, the Centers for Medicare & Medicaid Services expanded telehealth options for hospice providers, something that Ms. Bishop and other hospice providers hope will remain in place after the pandemic passes.
“Telehealth visits are offered to replace some in-home visits both to minimize risk of exposure to COVID-19 and reduce the drain on personal protective equipment,” Bishop explained.
“In-patient hospice programs are also finding unique ways to provide support and connect patients to their loved ones: visitors are allowed but limited to one or two. Music and pet therapy are being provided through the window or virtually and devices such as iPads are being used to help patients connect with loved ones,” she said.
Telehealth links patients out of loneliness, but the one thing it cannot do is provide the comfort of touch – an important part of any hospice program.
“Hand-holding ... I miss that a lot,” says Ms. Shockney, her eyes filling with tears. “When you take somebody’s hand, you don’t even have to speak; that connection, and eye contact, is all you need to help that person emotionally heal.”
This article first appeared on Medscape.com.
‘Promising’ durvalumab results spark phase 3 trial in mesothelioma
Adding durvalumab to first-line pemetrexed and cisplatin improved survival in patients with unresectable malignant pleural mesothelioma (MPM) in a phase 2 trial, compared with historical controls who received only pemetrexed and cisplatin.
The median overall survival was 20.4 months in patients who received durvalumab plus pemetrexed-cisplatin. This is significantly longer than the median overall survival of 12.1 months (P = .0014) observed with pemetrexed-cisplatin in a prior phase 3 study (J Clin Oncol. 2003 Jul 15;21[14]:2636-44).
The new phase 2 results are “promising,” said lead investigator Patrick Forde, MBBCh, director of the thoracic cancer clinical research program at Johns Hopkins University in Baltimore.
He presented the results as part of the American Society of Clinical Oncology virtual scientific program.
Dr. Forde noted that a phase 3 trial directly comparing pemetrexed-cisplatin plus durvalumab to pemetrexed-cisplatin will begin recruiting this year. The trial is a collaboration between U.S. investigators and Australian researchers who reported their own phase 2 results with durvalumab plus pemetrexed-cisplatin in 2018 (J Thorac Oncol. 2018 Oct;13[10]:S338-339).
Study details
Dr. Forde’s phase 2 study enrolled 55 patients with treatment-naive, unresectable MPM. Their median age was 68 years (range, 35-83 years), and 45 (82%) were men. All had an Eastern Cooperative Oncology Group performance status of 0-1.
Epithelioid mesothelioma was the histologic subtype in three-quarters of patients. “It was a fairly typical mesothelioma population,” Dr. Forde said.
The patients received durvalumab at 1,120 mg plus pemetrexed at 500 mg/m2 and cisplatin at 75 mg/m2 every 3 weeks for up to six cycles. Carboplatin was substituted when cisplatin was contraindicated or patients developed toxicities.
All but one patient had stable or responding disease on radiography and went on to durvalumab maintenance, also given at 1,120 mg every 3 weeks, for up to 1 year from study entry.
Results
Dr. Forde said this study had 90% power to detect a 58% improvement in median overall survival, from the 12.1 months seen in historical controls to 19 months, which was the goal of this study.
It was a positive study, he said, as the median overall survival was 20.4 months (P = .0014).
The overall survival rate was 87.2% at 6 months, 70.4% at 12 months, and 44.2% at 24 months. The progression-free survival rate was 69.1% at 6 months, 16.4% at 12 months, and 10.9% at 24 months.
The overall response rate was 56.4%, which comprised 31 partial responses. Forty percent of patients (n = 22) had stable disease. One patient had progressive disease, and one was not evaluable (1.8% each).
To help with future patient selection, the researchers looked for baseline biomarkers that predicted response. Tumor PD-L1 expression, tumor mutation burden, and other potential candidates haven’t worked out so far, but the work continues, Dr. Forde said.
He noted that many of the adverse events in this trial are those typically seen with platinum-based chemotherapy.
Grade 3/4 treatment-emergent adverse events included anemia (n = 14), fatigue (n = 4), decreased appetite (n = 1), and hypomagnesemia (n = 1).
The most common grade 1/2 adverse events of special interest were hypothyroidism (n = 7), rash (n = 5), pruritus (n = 3), AST elevation (n = 3), and hyperthyroidism (n = 3).
Putting the results in context
Given the role of inflammation in MPM, durvalumab is among several immunotherapies under investigation for the disease.
A phase 3 French trial showed MPM patients had a median overall survival of 18.8 months with pemetrexed-cisplatin plus bevacizumab versus 16.1 months with pemetrexed-cisplatin only (Lancet. 2016 Apr 2;387[10026]:1405-1414).
The higher overall survival in the French study’s pemetrexed-cisplatin arm, compared with the 2003 trial results, is likely due to the use of modern second-line options, said Marjorie Zauderer, MD, codirector of the mesothelioma program at Memorial Sloan Kettering Cancer Center in New York, who was the discussant for Dr. Forde’s presentation.
“I think the improvement in overall survival presented by Dr. Forde is potentially clinically meaningful,” she said, but it was “well within the 95% confidence interval” of the bevacizumab trial. Even so, “I look forward” to the phase 3 results, she said.
Dr. Zauderer also pointed out an April press release from Bristol Myers Squibb that reported improved survival over pemetrexed-cisplatin with two of the company’s immunotherapies, nivolumab and ipilimumab, not as additions but as replacement first-line therapy. However, the randomized trial data haven’t been released yet. “We are all eager to evaluate this option further,” she said.
AstraZeneca, maker of durvalumab, funded the current study. Dr. Forde is an adviser for the company and reported research funding. Dr. Zauderer reported a relationship with Roche, which markets bevacizumab through its subsidiary, Genentech. She also disclosed research funding from Bristol Myers Squibb.
SOURCE: Forde PM et al. ASCO 2020, Abstract 9003.
Adding durvalumab to first-line pemetrexed and cisplatin improved survival in patients with unresectable malignant pleural mesothelioma (MPM) in a phase 2 trial, compared with historical controls who received only pemetrexed and cisplatin.
The median overall survival was 20.4 months in patients who received durvalumab plus pemetrexed-cisplatin. This is significantly longer than the median overall survival of 12.1 months (P = .0014) observed with pemetrexed-cisplatin in a prior phase 3 study (J Clin Oncol. 2003 Jul 15;21[14]:2636-44).
The new phase 2 results are “promising,” said lead investigator Patrick Forde, MBBCh, director of the thoracic cancer clinical research program at Johns Hopkins University in Baltimore.
He presented the results as part of the American Society of Clinical Oncology virtual scientific program.
Dr. Forde noted that a phase 3 trial directly comparing pemetrexed-cisplatin plus durvalumab to pemetrexed-cisplatin will begin recruiting this year. The trial is a collaboration between U.S. investigators and Australian researchers who reported their own phase 2 results with durvalumab plus pemetrexed-cisplatin in 2018 (J Thorac Oncol. 2018 Oct;13[10]:S338-339).
Study details
Dr. Forde’s phase 2 study enrolled 55 patients with treatment-naive, unresectable MPM. Their median age was 68 years (range, 35-83 years), and 45 (82%) were men. All had an Eastern Cooperative Oncology Group performance status of 0-1.
Epithelioid mesothelioma was the histologic subtype in three-quarters of patients. “It was a fairly typical mesothelioma population,” Dr. Forde said.
The patients received durvalumab at 1,120 mg plus pemetrexed at 500 mg/m2 and cisplatin at 75 mg/m2 every 3 weeks for up to six cycles. Carboplatin was substituted when cisplatin was contraindicated or patients developed toxicities.
All but one patient had stable or responding disease on radiography and went on to durvalumab maintenance, also given at 1,120 mg every 3 weeks, for up to 1 year from study entry.
Results
Dr. Forde said this study had 90% power to detect a 58% improvement in median overall survival, from the 12.1 months seen in historical controls to 19 months, which was the goal of this study.
It was a positive study, he said, as the median overall survival was 20.4 months (P = .0014).
The overall survival rate was 87.2% at 6 months, 70.4% at 12 months, and 44.2% at 24 months. The progression-free survival rate was 69.1% at 6 months, 16.4% at 12 months, and 10.9% at 24 months.
The overall response rate was 56.4%, which comprised 31 partial responses. Forty percent of patients (n = 22) had stable disease. One patient had progressive disease, and one was not evaluable (1.8% each).
To help with future patient selection, the researchers looked for baseline biomarkers that predicted response. Tumor PD-L1 expression, tumor mutation burden, and other potential candidates haven’t worked out so far, but the work continues, Dr. Forde said.
He noted that many of the adverse events in this trial are those typically seen with platinum-based chemotherapy.
Grade 3/4 treatment-emergent adverse events included anemia (n = 14), fatigue (n = 4), decreased appetite (n = 1), and hypomagnesemia (n = 1).
The most common grade 1/2 adverse events of special interest were hypothyroidism (n = 7), rash (n = 5), pruritus (n = 3), AST elevation (n = 3), and hyperthyroidism (n = 3).
Putting the results in context
Given the role of inflammation in MPM, durvalumab is among several immunotherapies under investigation for the disease.
A phase 3 French trial showed MPM patients had a median overall survival of 18.8 months with pemetrexed-cisplatin plus bevacizumab versus 16.1 months with pemetrexed-cisplatin only (Lancet. 2016 Apr 2;387[10026]:1405-1414).
The higher overall survival in the French study’s pemetrexed-cisplatin arm, compared with the 2003 trial results, is likely due to the use of modern second-line options, said Marjorie Zauderer, MD, codirector of the mesothelioma program at Memorial Sloan Kettering Cancer Center in New York, who was the discussant for Dr. Forde’s presentation.
“I think the improvement in overall survival presented by Dr. Forde is potentially clinically meaningful,” she said, but it was “well within the 95% confidence interval” of the bevacizumab trial. Even so, “I look forward” to the phase 3 results, she said.
Dr. Zauderer also pointed out an April press release from Bristol Myers Squibb that reported improved survival over pemetrexed-cisplatin with two of the company’s immunotherapies, nivolumab and ipilimumab, not as additions but as replacement first-line therapy. However, the randomized trial data haven’t been released yet. “We are all eager to evaluate this option further,” she said.
AstraZeneca, maker of durvalumab, funded the current study. Dr. Forde is an adviser for the company and reported research funding. Dr. Zauderer reported a relationship with Roche, which markets bevacizumab through its subsidiary, Genentech. She also disclosed research funding from Bristol Myers Squibb.
SOURCE: Forde PM et al. ASCO 2020, Abstract 9003.
Adding durvalumab to first-line pemetrexed and cisplatin improved survival in patients with unresectable malignant pleural mesothelioma (MPM) in a phase 2 trial, compared with historical controls who received only pemetrexed and cisplatin.
The median overall survival was 20.4 months in patients who received durvalumab plus pemetrexed-cisplatin. This is significantly longer than the median overall survival of 12.1 months (P = .0014) observed with pemetrexed-cisplatin in a prior phase 3 study (J Clin Oncol. 2003 Jul 15;21[14]:2636-44).
The new phase 2 results are “promising,” said lead investigator Patrick Forde, MBBCh, director of the thoracic cancer clinical research program at Johns Hopkins University in Baltimore.
He presented the results as part of the American Society of Clinical Oncology virtual scientific program.
Dr. Forde noted that a phase 3 trial directly comparing pemetrexed-cisplatin plus durvalumab to pemetrexed-cisplatin will begin recruiting this year. The trial is a collaboration between U.S. investigators and Australian researchers who reported their own phase 2 results with durvalumab plus pemetrexed-cisplatin in 2018 (J Thorac Oncol. 2018 Oct;13[10]:S338-339).
Study details
Dr. Forde’s phase 2 study enrolled 55 patients with treatment-naive, unresectable MPM. Their median age was 68 years (range, 35-83 years), and 45 (82%) were men. All had an Eastern Cooperative Oncology Group performance status of 0-1.
Epithelioid mesothelioma was the histologic subtype in three-quarters of patients. “It was a fairly typical mesothelioma population,” Dr. Forde said.
The patients received durvalumab at 1,120 mg plus pemetrexed at 500 mg/m2 and cisplatin at 75 mg/m2 every 3 weeks for up to six cycles. Carboplatin was substituted when cisplatin was contraindicated or patients developed toxicities.
All but one patient had stable or responding disease on radiography and went on to durvalumab maintenance, also given at 1,120 mg every 3 weeks, for up to 1 year from study entry.
Results
Dr. Forde said this study had 90% power to detect a 58% improvement in median overall survival, from the 12.1 months seen in historical controls to 19 months, which was the goal of this study.
It was a positive study, he said, as the median overall survival was 20.4 months (P = .0014).
The overall survival rate was 87.2% at 6 months, 70.4% at 12 months, and 44.2% at 24 months. The progression-free survival rate was 69.1% at 6 months, 16.4% at 12 months, and 10.9% at 24 months.
The overall response rate was 56.4%, which comprised 31 partial responses. Forty percent of patients (n = 22) had stable disease. One patient had progressive disease, and one was not evaluable (1.8% each).
To help with future patient selection, the researchers looked for baseline biomarkers that predicted response. Tumor PD-L1 expression, tumor mutation burden, and other potential candidates haven’t worked out so far, but the work continues, Dr. Forde said.
He noted that many of the adverse events in this trial are those typically seen with platinum-based chemotherapy.
Grade 3/4 treatment-emergent adverse events included anemia (n = 14), fatigue (n = 4), decreased appetite (n = 1), and hypomagnesemia (n = 1).
The most common grade 1/2 adverse events of special interest were hypothyroidism (n = 7), rash (n = 5), pruritus (n = 3), AST elevation (n = 3), and hyperthyroidism (n = 3).
Putting the results in context
Given the role of inflammation in MPM, durvalumab is among several immunotherapies under investigation for the disease.
A phase 3 French trial showed MPM patients had a median overall survival of 18.8 months with pemetrexed-cisplatin plus bevacizumab versus 16.1 months with pemetrexed-cisplatin only (Lancet. 2016 Apr 2;387[10026]:1405-1414).
The higher overall survival in the French study’s pemetrexed-cisplatin arm, compared with the 2003 trial results, is likely due to the use of modern second-line options, said Marjorie Zauderer, MD, codirector of the mesothelioma program at Memorial Sloan Kettering Cancer Center in New York, who was the discussant for Dr. Forde’s presentation.
“I think the improvement in overall survival presented by Dr. Forde is potentially clinically meaningful,” she said, but it was “well within the 95% confidence interval” of the bevacizumab trial. Even so, “I look forward” to the phase 3 results, she said.
Dr. Zauderer also pointed out an April press release from Bristol Myers Squibb that reported improved survival over pemetrexed-cisplatin with two of the company’s immunotherapies, nivolumab and ipilimumab, not as additions but as replacement first-line therapy. However, the randomized trial data haven’t been released yet. “We are all eager to evaluate this option further,” she said.
AstraZeneca, maker of durvalumab, funded the current study. Dr. Forde is an adviser for the company and reported research funding. Dr. Zauderer reported a relationship with Roche, which markets bevacizumab through its subsidiary, Genentech. She also disclosed research funding from Bristol Myers Squibb.
SOURCE: Forde PM et al. ASCO 2020, Abstract 9003.
FROM ASCO 2020
Acute lymphoblastic leukemia can be successfully treated in the frail elderly
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
A treatment schedule of very attenuated chemotherapy using standard drugs is feasible and effective in frail and elderly patients with acute lymphoblastic leukemia (ALL), according to a prospective study published in Clinical Lymphoma, Myeloma & Leukemia.
The study comprised 67 previously untreated patients with B- or T-lineage Philadelphia chromosome–negative ALL from 30 Spanish hospitals who were enrolled in the prospective, multicenter ALL-07FRAIL trial (NCT01358201) from the Spanish PETHEMA (Programa Español de Tratamientos en Hematologia) group from January 2008 to October 2019.
The median patient age in this analysis was 67 years and 51 patients (76%) were older than 70 years. The median Charlson Comorbidity Index was 5, with the main comorbidities being cardiovascular (47 patients), other neoplasia (24), diabetes (17), and very advanced age (>80 years; 12).
The attenuated treatment regimen consisted of a prephase with dexamethasone and intrathecal therapy with methotrexate was given for a maximum of 1 week. Then weekly induction therapy consisted of weekly vincristine (capped at 1 mg/week) and daily dexamethasone with a progressively decreasing dose along 4 weeks, as well as two additional doses of intrathecal methotrexate.
Those patients who achieved complete remission received maintenance therapy with mercaptopurine and methotrexate to complete 2 years of treatment. In addition, reinduction pulses with vincristine and dexamethasone were given every 3 months during the first year, according to Josep-Maria Ribera, MD, of the Universitat Autònoma de Barcelona, Badalona, Spain and colleagues on behalf of the PETHEMA group of the Spanish Society of Hematology.
The complete remission rate was 54% (36/67 patients). The median disease-free survival and overall survival were 6.9 months and 7.6 months, respectively.
Of the 32 patients who initiated maintenance therapy, 5 patients died of infection (2), hemorrhage (2), and acute cognitive impairment (1), and 23 relapsed, with a cumulative incidence of relapse of 74% and a median time to relapse of 12.3 months.
The most frequent toxic events reported were hematologic (neutropenia 77% and thrombocytopenia 54%, of grade III-IV in all cases) followed by infections, metabolic (mainly hyperglycemia), and neurologic, according to the researchers.
“The lack of similar trials specifically directed to this frail population is one of the major strengths of this study, and we consider that this minimal chemotherapy approach could be used as a backbone for addition of immuno/targeted therapy in this subset of infirm patients,” the researchers concluded.
The study was supported by the CERCA Program/Generalitat de Catalunya and the Josep Carreras Leukemia Research Institute. The authors reported having no disclosures.
SOURCE: Ribera J-M et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 5. doi: 10.1016/j.clml.2020.03.011.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Germline testing in advanced cancer can lead to targeted treatment
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
FROM ASCO 2020
Single negative colonoscopy predicts low colorectal cancer risk
a new study concludes.
The population-based study showed a durable reduction in CRC risk over 17.4 years of follow-up.
“Our findings confirm that a 10-year interval between high-quality screening colonoscopies [as is currently recommended] is safe and that there is no benefit from more frequent screening,” lead author Nastazja Pilonis, MD, from the Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw, Poland, told Medscape Medical News.
“Furthermore, our findings suggest that this interval could even be prolonged, provided the baseline colonoscopy is of high quality,” she added.
However, she emphasized that “only high-quality colonoscopy provided a durable reduction in mortality risk,” and noted that “low-quality colonoscopy was associated with a significantly increased risk of CRC death after the first 5 years following the examination.”
The study was published online May 25 in the Annals of Internal Medicine.
Polish Colonoscopy Screening Program
The study included 165,887 average-risk patients enrolled in the Polish Colonoscopy Screening Program who had a single negative screening colonoscopy between October 2000 and December 2011.
Negative colonoscopy was defined as an examination where no evidence of any neoplastic lesion was found.
A high-quality screening colonoscopy was defined by three key properties: cecal intubation, adequate bowel preparation, and an endoscopist’s adenoma detection rate (ADR) of 20% or greater calculated on a yearly basis.
A total of 505 different endoscopists performed the colonoscopies over a median follow-up of 10.1 years.
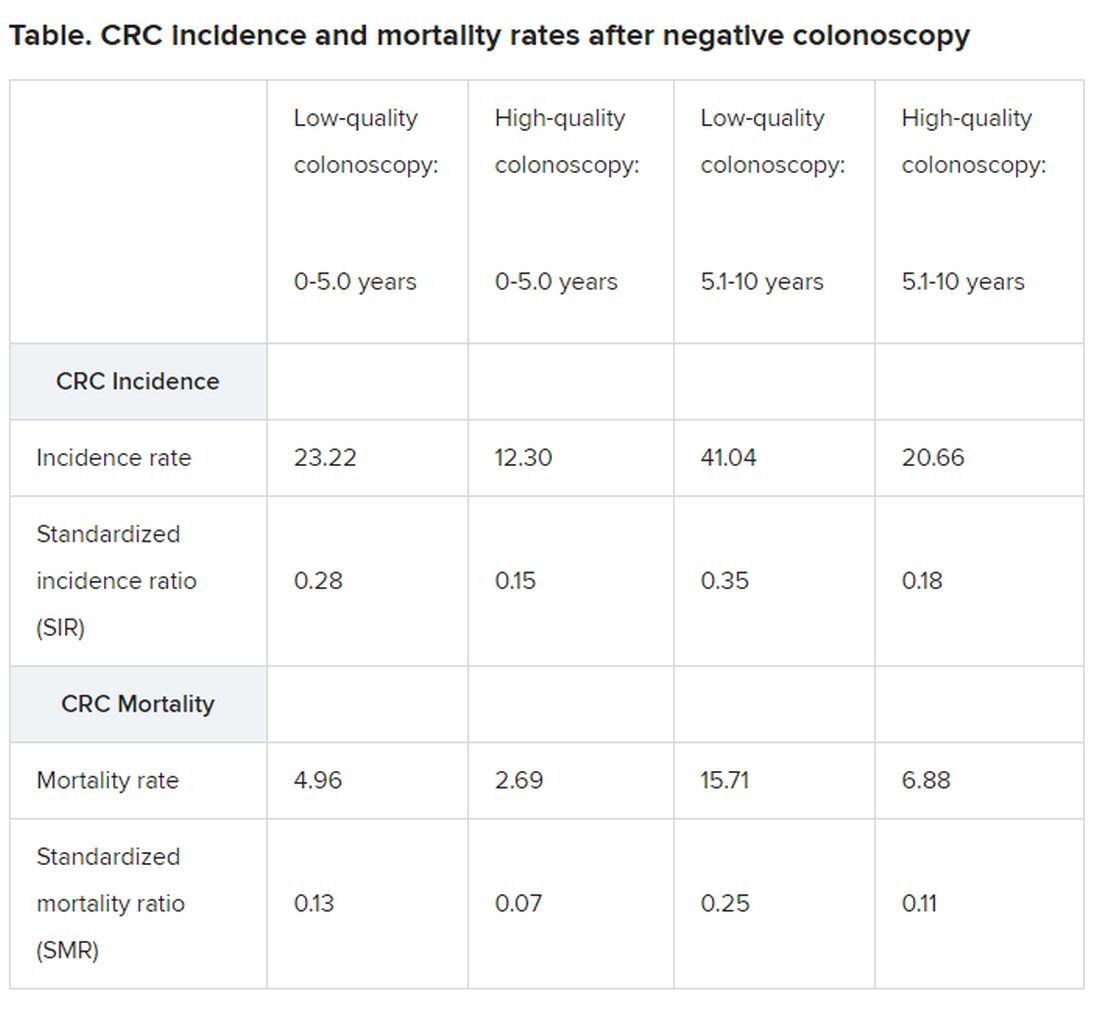
Compared with the general population, among individuals with a negative colonoscopy, the incidence of CRC was 72% lower and CRC mortality was 81% lower over a period of 5.1 to 10 years, Pilonis and colleagues report.
“This was mainly driven by long-lasting reductions in CRC incidence and mortality (by 84% and 90%, respectively) after high-quality screening colonoscopies,” the investigators emphasize.
Beyond 10 years of follow-up, reductions in CRC incidence and mortality were similar to those observed for the earlier period of 5.1 to 10 years but only for participants who had had a high-quality screening colonoscopy, they emphasize.
Subgroup analyses
In addition, subgroup analyses showed that high-quality colonoscopy – although not those of low-quality – effectively reduced the incidence of, and mortality from, CRC in women and in the proximal colon.
As Pilonis pointed out, previous studies have suggested that women may not benefit from screening colonoscopy to the same extent as men. Plus previous research suggests a reduced CRC risk in the proximal colon relative to that in the distal colon.
Overall, standardized incidence ratios (SIRs) and standardized mortality ratios (SMRs) significantly differed between men and women in the current study, but this difference was not observed after high-quality examinations, the investigators report.
“This is an extremely important finding because, for the first time, we showed that when you have high-quality colonoscopy, women benefit from screening colonoscopy as much as men,” Pilonis emphasized.
Similarly, high-quality screening colonoscopy was associated with a 50% reduction in mortality in the proximal colon throughout the 17.4-year follow-up, whereas there was no decrease in mortality from CRC in the proximal colon with low-quality colonoscopies.
As Pilonis noted, lesions in the proximal colon are more subtle and are harder to detect.”It’s also easier to achieve good bowel preparation in the distal colon than in the proximal colon,” she added.
Women are also more prone to develop lesions in the right (proximal) side of the colon and appear to have more pain with colonoscopy than men, all of which could have contributed to previous reports of colonoscopy not being very effective in women or for the detection of lesions in the proximal colon, as Pilonis suggested.
As the authors explain, current guidelines recommend a 10-year screening interval for the average-risk patient when colonoscopy results are negative.
This interval was partially based on the estimated time it was thought to take an adenoma to progress to a carcinoma and partially on the estimated sensitivity of screening colonoscopy.
“We showed that high-quality is a prerequisite for safe intervals between colonoscopies, Pilonis said. “So I would say that if, at a certain age, a patient has a negative colonoscopy of high-quality, a negative colonoscopy is highly predictive of a very low future risk of CRC,” she added.
The study was funded by the Polish Ministry of Health.
This article first appeared on Medscape.com.
a new study concludes.
The population-based study showed a durable reduction in CRC risk over 17.4 years of follow-up.
“Our findings confirm that a 10-year interval between high-quality screening colonoscopies [as is currently recommended] is safe and that there is no benefit from more frequent screening,” lead author Nastazja Pilonis, MD, from the Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw, Poland, told Medscape Medical News.
“Furthermore, our findings suggest that this interval could even be prolonged, provided the baseline colonoscopy is of high quality,” she added.
However, she emphasized that “only high-quality colonoscopy provided a durable reduction in mortality risk,” and noted that “low-quality colonoscopy was associated with a significantly increased risk of CRC death after the first 5 years following the examination.”
The study was published online May 25 in the Annals of Internal Medicine.
Polish Colonoscopy Screening Program
The study included 165,887 average-risk patients enrolled in the Polish Colonoscopy Screening Program who had a single negative screening colonoscopy between October 2000 and December 2011.
Negative colonoscopy was defined as an examination where no evidence of any neoplastic lesion was found.
A high-quality screening colonoscopy was defined by three key properties: cecal intubation, adequate bowel preparation, and an endoscopist’s adenoma detection rate (ADR) of 20% or greater calculated on a yearly basis.
A total of 505 different endoscopists performed the colonoscopies over a median follow-up of 10.1 years.
Compared with the general population, among individuals with a negative colonoscopy, the incidence of CRC was 72% lower and CRC mortality was 81% lower over a period of 5.1 to 10 years, Pilonis and colleagues report.
“This was mainly driven by long-lasting reductions in CRC incidence and mortality (by 84% and 90%, respectively) after high-quality screening colonoscopies,” the investigators emphasize.
Beyond 10 years of follow-up, reductions in CRC incidence and mortality were similar to those observed for the earlier period of 5.1 to 10 years but only for participants who had had a high-quality screening colonoscopy, they emphasize.
Subgroup analyses
In addition, subgroup analyses showed that high-quality colonoscopy – although not those of low-quality – effectively reduced the incidence of, and mortality from, CRC in women and in the proximal colon.
As Pilonis pointed out, previous studies have suggested that women may not benefit from screening colonoscopy to the same extent as men. Plus previous research suggests a reduced CRC risk in the proximal colon relative to that in the distal colon.
Overall, standardized incidence ratios (SIRs) and standardized mortality ratios (SMRs) significantly differed between men and women in the current study, but this difference was not observed after high-quality examinations, the investigators report.
“This is an extremely important finding because, for the first time, we showed that when you have high-quality colonoscopy, women benefit from screening colonoscopy as much as men,” Pilonis emphasized.
Similarly, high-quality screening colonoscopy was associated with a 50% reduction in mortality in the proximal colon throughout the 17.4-year follow-up, whereas there was no decrease in mortality from CRC in the proximal colon with low-quality colonoscopies.
As Pilonis noted, lesions in the proximal colon are more subtle and are harder to detect.”It’s also easier to achieve good bowel preparation in the distal colon than in the proximal colon,” she added.
Women are also more prone to develop lesions in the right (proximal) side of the colon and appear to have more pain with colonoscopy than men, all of which could have contributed to previous reports of colonoscopy not being very effective in women or for the detection of lesions in the proximal colon, as Pilonis suggested.
As the authors explain, current guidelines recommend a 10-year screening interval for the average-risk patient when colonoscopy results are negative.
This interval was partially based on the estimated time it was thought to take an adenoma to progress to a carcinoma and partially on the estimated sensitivity of screening colonoscopy.
“We showed that high-quality is a prerequisite for safe intervals between colonoscopies, Pilonis said. “So I would say that if, at a certain age, a patient has a negative colonoscopy of high-quality, a negative colonoscopy is highly predictive of a very low future risk of CRC,” she added.
The study was funded by the Polish Ministry of Health.
This article first appeared on Medscape.com.
a new study concludes.
The population-based study showed a durable reduction in CRC risk over 17.4 years of follow-up.
“Our findings confirm that a 10-year interval between high-quality screening colonoscopies [as is currently recommended] is safe and that there is no benefit from more frequent screening,” lead author Nastazja Pilonis, MD, from the Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw, Poland, told Medscape Medical News.
“Furthermore, our findings suggest that this interval could even be prolonged, provided the baseline colonoscopy is of high quality,” she added.
However, she emphasized that “only high-quality colonoscopy provided a durable reduction in mortality risk,” and noted that “low-quality colonoscopy was associated with a significantly increased risk of CRC death after the first 5 years following the examination.”
The study was published online May 25 in the Annals of Internal Medicine.
Polish Colonoscopy Screening Program
The study included 165,887 average-risk patients enrolled in the Polish Colonoscopy Screening Program who had a single negative screening colonoscopy between October 2000 and December 2011.
Negative colonoscopy was defined as an examination where no evidence of any neoplastic lesion was found.
A high-quality screening colonoscopy was defined by three key properties: cecal intubation, adequate bowel preparation, and an endoscopist’s adenoma detection rate (ADR) of 20% or greater calculated on a yearly basis.
A total of 505 different endoscopists performed the colonoscopies over a median follow-up of 10.1 years.
Compared with the general population, among individuals with a negative colonoscopy, the incidence of CRC was 72% lower and CRC mortality was 81% lower over a period of 5.1 to 10 years, Pilonis and colleagues report.
“This was mainly driven by long-lasting reductions in CRC incidence and mortality (by 84% and 90%, respectively) after high-quality screening colonoscopies,” the investigators emphasize.
Beyond 10 years of follow-up, reductions in CRC incidence and mortality were similar to those observed for the earlier period of 5.1 to 10 years but only for participants who had had a high-quality screening colonoscopy, they emphasize.
Subgroup analyses
In addition, subgroup analyses showed that high-quality colonoscopy – although not those of low-quality – effectively reduced the incidence of, and mortality from, CRC in women and in the proximal colon.
As Pilonis pointed out, previous studies have suggested that women may not benefit from screening colonoscopy to the same extent as men. Plus previous research suggests a reduced CRC risk in the proximal colon relative to that in the distal colon.
Overall, standardized incidence ratios (SIRs) and standardized mortality ratios (SMRs) significantly differed between men and women in the current study, but this difference was not observed after high-quality examinations, the investigators report.
“This is an extremely important finding because, for the first time, we showed that when you have high-quality colonoscopy, women benefit from screening colonoscopy as much as men,” Pilonis emphasized.
Similarly, high-quality screening colonoscopy was associated with a 50% reduction in mortality in the proximal colon throughout the 17.4-year follow-up, whereas there was no decrease in mortality from CRC in the proximal colon with low-quality colonoscopies.
As Pilonis noted, lesions in the proximal colon are more subtle and are harder to detect.”It’s also easier to achieve good bowel preparation in the distal colon than in the proximal colon,” she added.
Women are also more prone to develop lesions in the right (proximal) side of the colon and appear to have more pain with colonoscopy than men, all of which could have contributed to previous reports of colonoscopy not being very effective in women or for the detection of lesions in the proximal colon, as Pilonis suggested.
As the authors explain, current guidelines recommend a 10-year screening interval for the average-risk patient when colonoscopy results are negative.
This interval was partially based on the estimated time it was thought to take an adenoma to progress to a carcinoma and partially on the estimated sensitivity of screening colonoscopy.
“We showed that high-quality is a prerequisite for safe intervals between colonoscopies, Pilonis said. “So I would say that if, at a certain age, a patient has a negative colonoscopy of high-quality, a negative colonoscopy is highly predictive of a very low future risk of CRC,” she added.
The study was funded by the Polish Ministry of Health.
This article first appeared on Medscape.com.
Convalescent plasma: ‘Flavor of the month’ or valid COVID-19 treatment?
On March 31, soon after the Food and Drug Administration authorized emergency use of antibody-packed plasma from recovered patients with COVID-19, Marisa Leuzzi became the first donor at an American Red Cross center. She hoped it could help her aunt, Renee Bannister, who was failing after 3 weeks on a ventilator at Virtua Hospital in Voorhees, N.J.
It may have worked; 11 days after receiving the plasma, Ms. Bannister was weaned off the ventilator and she is now awake and speaking, said Red Cross spokesperson Stephanie Rendon.
This kind of anecdote is fueling demand for the therapy, which can be provided through an expanded access program led by the Mayo Clinic, backed by the FDA, and the plasma paid for by the U.S. Department of Health & Human Services. But while this program is collecting safety and outcomes data, it’s not a randomized, controlled trial.
Others, however, are pursuing that data.
“One of the things I don’t want this to be is the flavor of the month,” Shmuel Shoham, MD, associate professor of medicine at Johns Hopkins University, said in an interview.
Dr. Shoham, principal investigator for a study evaluating convalescent plasma to prevent the infection in high-risk individuals, said some clinicians, desperate for any treatment, have tried potential therapies such as hydroxychloroquine and remdesivir without evidence of safety or efficacy in COVID-19.
The National Institutes of Health recently said something similar for convalescent plasma, that “there are insufficient clinical data to recommend either for or against” its use for COVID-19.
But plasma has promise, according to a Johns Hopkins School of Medicine’s Bloomberg Distinguished Professor, Arturo Casadevall, MD, PhD, in Baltimore, and Liise-anne Pirofski, MD, a professor at Albert Einstein College of Medicine, New York. They lay out the case for convalescent plasma in an article published online March 13 in the Journal of Clinical Investigation. Passive antibody therapy, they wrote, has been used to stem polio, measles, mumps, and influenza, and more recently has shown some success against SARS-CoV-1 and Middle East respiratory syndrome (MERS).
“The special attraction of this modality of treatment is that, unlike vaccines or newly developed drugs, it could, in principle, be made available very rapidly,” said researchers with the National COVID-19 Convalescent Plasma Project, which includes physicians and scientists from 57 institutions in 46 states. But where principle veers from reality is in availability of the plasma itself, and donors are in short supply.
Aiming to prevent infection
So far, the FDA has approved 12 plasma trials – including Dr. Shoham’s – and the NIH’s clinicaltrials.gov lists more than two dozen convalescent plasma studies in the United States and elsewhere.
Most are single-arm trials to determine if one infusion can decrease the need for intubation or help those on a ventilator improve. Two others, one at Johns Hopkins and one at Stanford (Calif.) Hospital are investigating whether convalescent plasma might be used before severe disease sets in.
“A general principle of passive antibody therapy is that it is more effective when used for prophylaxis than for treatment of disease,” Dr. Casadevall and Dr. Pirofski wrote.
Stanford’s randomized, double-blind study will evaluate regular versus convalescent plasma in ED patients who are not sick enough to require hospitalization.
The Johns Hopkins trial, which aims to protect against infection in the first place, will begin at Johns Hopkins, Baltimore, and at Hopkins-affiliated hospitals throughout Maryland, Dr. Shoham said. He hopes it will expand nationwide eventually, and said that they expect to enroll the first patients soon.
To start, the prevention study will enroll only 150 patients, each of whom must have had close contact with someone who has COVID-19 within the previous 120 hours and be asymptomatic. The number of subjects is small, compared with the trial size of other potential therapies, and an issue, Shoham said, “that keeps me up at night.” But finding thousands of enrollees for plasma studies is hard, in part because it’s so difficult to recruit donors.
Participants will receive normal plasma (which will act as a placebo) or convalescent plasma.
The primary endpoint is cumulative incidence of COVID-19, defined as symptoms and a polymerase chain reaction–positive test; participants will be tracked for 90 days. Hospitals and health care workers could then decide if they want to use the therapy, he said.
The study will not answer whether participants will continue to have antibodies beyond the 90 days. Convalescent plasma is given as a rapid response to an emergent pathogen – a short-term boost of immunity rather than a long-term therapeutic.
What can we learn from expanded access?
Meanwhile, some 2,200 hospitals are participating in the expanded access program being led by the Mayo Clinic nationwide; more than 9,000 patients had received infusions at press time.
One participant is Northwell Health, a 23-hospital system that sprawls across the U.S. COVID epicenter: four of the five boroughs of New York City and Long Island.
Convalescent plasma is an in-demand therapy, said Christina Brennan, MD, vice president of clinical research at Northwell. “We get patients, family members, they say my family member is at X hospital – if it’s not being offered there, can you have them transferred?” she said in an interview.
When Northwell – through the New York Blood Bank – opened up donor registration, 800 people signed up in the first 24 hours, Dr. Brennan said. As of mid-May, 527 patients had received a transfusion.
Who’s the best donor and when should donation occur?
The Red Cross, hospitals, and independent blood banks are all soliciting donors, who can sign up at the Red Cross website. The FDA recommends that donors have a history of COVID-19 as confirmed by molecular or antibody testing, be symptom free for 14 days, have a negative follow-up molecular test, and be virus free at the time of collection. The FDA also suggests measuring a donor’s SARS-CoV-2 neutralizing antibody titers, if available, with a recommendation of at least 1:160.
But questions remain, such as whether there is a theoretical risk for antibody-dependent enhancement (ADE) of infection with SARS-CoV-2. “Antibodies to one type of coronavirus could enhance infection to another viral strain,” of coronavirus, Dr. Casadevall wrote. ADE has been observed in both severe acute respiratory syndrome (SARS) and MERS.
The other risk is that donors may still be shedding active virus. While the FDA suggests that donors are unlikely to still be infectious 14 days after infection, that is as of yet unproven. Both COVID-19 diagnostics and antibody tests have high rates of false negatives, which raises the specter that infection could be spread via the plasma donation.
Daniele Focosi, MD, PhD, from Pisa (Italy) University Hospital and colleagues raise that concern in a preprint review on convalescent plasma in COVID-19. “Although the recipient is already infected, theoretically transmission of more infectious particles could worsen clinical conditions,” they wrote, noting that “such a concern can be somewhat reduced by treatment with modern pathogen inactivation techniques.”
No evidence exists that SARS-CoV-2 can be transmitted through blood, but “we don’t know for sure,” Dr. Shoham said in an interview. A reassuring point: Even those with severe infection do not have viral RNA in their blood, he said, adding, “We don’t think there’s going to be viral transmission of this particular virus with transfusion.”
For another highly infectious pathogen, the Ebola virus, the World Health Organization recommended in 2014 that potential plasma donors wait at least 28 days after infection.
It’s also not known how long SARS-CoV-2 antibodies persist in the blood; longer viability could mean a longer donation window. Dr. Focosi noted that a previous Chinese study had shown that SARS-specific antibodies in people infected with the first SARS virus, SARS-CoV-1, persisted for 2 years.
Dr. Casadevall and Dr. Pirofski have disclosed no relevant financial relationships. Shoham has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
On March 31, soon after the Food and Drug Administration authorized emergency use of antibody-packed plasma from recovered patients with COVID-19, Marisa Leuzzi became the first donor at an American Red Cross center. She hoped it could help her aunt, Renee Bannister, who was failing after 3 weeks on a ventilator at Virtua Hospital in Voorhees, N.J.
It may have worked; 11 days after receiving the plasma, Ms. Bannister was weaned off the ventilator and she is now awake and speaking, said Red Cross spokesperson Stephanie Rendon.
This kind of anecdote is fueling demand for the therapy, which can be provided through an expanded access program led by the Mayo Clinic, backed by the FDA, and the plasma paid for by the U.S. Department of Health & Human Services. But while this program is collecting safety and outcomes data, it’s not a randomized, controlled trial.
Others, however, are pursuing that data.
“One of the things I don’t want this to be is the flavor of the month,” Shmuel Shoham, MD, associate professor of medicine at Johns Hopkins University, said in an interview.
Dr. Shoham, principal investigator for a study evaluating convalescent plasma to prevent the infection in high-risk individuals, said some clinicians, desperate for any treatment, have tried potential therapies such as hydroxychloroquine and remdesivir without evidence of safety or efficacy in COVID-19.
The National Institutes of Health recently said something similar for convalescent plasma, that “there are insufficient clinical data to recommend either for or against” its use for COVID-19.
But plasma has promise, according to a Johns Hopkins School of Medicine’s Bloomberg Distinguished Professor, Arturo Casadevall, MD, PhD, in Baltimore, and Liise-anne Pirofski, MD, a professor at Albert Einstein College of Medicine, New York. They lay out the case for convalescent plasma in an article published online March 13 in the Journal of Clinical Investigation. Passive antibody therapy, they wrote, has been used to stem polio, measles, mumps, and influenza, and more recently has shown some success against SARS-CoV-1 and Middle East respiratory syndrome (MERS).
“The special attraction of this modality of treatment is that, unlike vaccines or newly developed drugs, it could, in principle, be made available very rapidly,” said researchers with the National COVID-19 Convalescent Plasma Project, which includes physicians and scientists from 57 institutions in 46 states. But where principle veers from reality is in availability of the plasma itself, and donors are in short supply.
Aiming to prevent infection
So far, the FDA has approved 12 plasma trials – including Dr. Shoham’s – and the NIH’s clinicaltrials.gov lists more than two dozen convalescent plasma studies in the United States and elsewhere.
Most are single-arm trials to determine if one infusion can decrease the need for intubation or help those on a ventilator improve. Two others, one at Johns Hopkins and one at Stanford (Calif.) Hospital are investigating whether convalescent plasma might be used before severe disease sets in.
“A general principle of passive antibody therapy is that it is more effective when used for prophylaxis than for treatment of disease,” Dr. Casadevall and Dr. Pirofski wrote.
Stanford’s randomized, double-blind study will evaluate regular versus convalescent plasma in ED patients who are not sick enough to require hospitalization.
The Johns Hopkins trial, which aims to protect against infection in the first place, will begin at Johns Hopkins, Baltimore, and at Hopkins-affiliated hospitals throughout Maryland, Dr. Shoham said. He hopes it will expand nationwide eventually, and said that they expect to enroll the first patients soon.
To start, the prevention study will enroll only 150 patients, each of whom must have had close contact with someone who has COVID-19 within the previous 120 hours and be asymptomatic. The number of subjects is small, compared with the trial size of other potential therapies, and an issue, Shoham said, “that keeps me up at night.” But finding thousands of enrollees for plasma studies is hard, in part because it’s so difficult to recruit donors.
Participants will receive normal plasma (which will act as a placebo) or convalescent plasma.
The primary endpoint is cumulative incidence of COVID-19, defined as symptoms and a polymerase chain reaction–positive test; participants will be tracked for 90 days. Hospitals and health care workers could then decide if they want to use the therapy, he said.
The study will not answer whether participants will continue to have antibodies beyond the 90 days. Convalescent plasma is given as a rapid response to an emergent pathogen – a short-term boost of immunity rather than a long-term therapeutic.
What can we learn from expanded access?
Meanwhile, some 2,200 hospitals are participating in the expanded access program being led by the Mayo Clinic nationwide; more than 9,000 patients had received infusions at press time.
One participant is Northwell Health, a 23-hospital system that sprawls across the U.S. COVID epicenter: four of the five boroughs of New York City and Long Island.
Convalescent plasma is an in-demand therapy, said Christina Brennan, MD, vice president of clinical research at Northwell. “We get patients, family members, they say my family member is at X hospital – if it’s not being offered there, can you have them transferred?” she said in an interview.
When Northwell – through the New York Blood Bank – opened up donor registration, 800 people signed up in the first 24 hours, Dr. Brennan said. As of mid-May, 527 patients had received a transfusion.
Who’s the best donor and when should donation occur?
The Red Cross, hospitals, and independent blood banks are all soliciting donors, who can sign up at the Red Cross website. The FDA recommends that donors have a history of COVID-19 as confirmed by molecular or antibody testing, be symptom free for 14 days, have a negative follow-up molecular test, and be virus free at the time of collection. The FDA also suggests measuring a donor’s SARS-CoV-2 neutralizing antibody titers, if available, with a recommendation of at least 1:160.
But questions remain, such as whether there is a theoretical risk for antibody-dependent enhancement (ADE) of infection with SARS-CoV-2. “Antibodies to one type of coronavirus could enhance infection to another viral strain,” of coronavirus, Dr. Casadevall wrote. ADE has been observed in both severe acute respiratory syndrome (SARS) and MERS.
The other risk is that donors may still be shedding active virus. While the FDA suggests that donors are unlikely to still be infectious 14 days after infection, that is as of yet unproven. Both COVID-19 diagnostics and antibody tests have high rates of false negatives, which raises the specter that infection could be spread via the plasma donation.
Daniele Focosi, MD, PhD, from Pisa (Italy) University Hospital and colleagues raise that concern in a preprint review on convalescent plasma in COVID-19. “Although the recipient is already infected, theoretically transmission of more infectious particles could worsen clinical conditions,” they wrote, noting that “such a concern can be somewhat reduced by treatment with modern pathogen inactivation techniques.”
No evidence exists that SARS-CoV-2 can be transmitted through blood, but “we don’t know for sure,” Dr. Shoham said in an interview. A reassuring point: Even those with severe infection do not have viral RNA in their blood, he said, adding, “We don’t think there’s going to be viral transmission of this particular virus with transfusion.”
For another highly infectious pathogen, the Ebola virus, the World Health Organization recommended in 2014 that potential plasma donors wait at least 28 days after infection.
It’s also not known how long SARS-CoV-2 antibodies persist in the blood; longer viability could mean a longer donation window. Dr. Focosi noted that a previous Chinese study had shown that SARS-specific antibodies in people infected with the first SARS virus, SARS-CoV-1, persisted for 2 years.
Dr. Casadevall and Dr. Pirofski have disclosed no relevant financial relationships. Shoham has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
On March 31, soon after the Food and Drug Administration authorized emergency use of antibody-packed plasma from recovered patients with COVID-19, Marisa Leuzzi became the first donor at an American Red Cross center. She hoped it could help her aunt, Renee Bannister, who was failing after 3 weeks on a ventilator at Virtua Hospital in Voorhees, N.J.
It may have worked; 11 days after receiving the plasma, Ms. Bannister was weaned off the ventilator and she is now awake and speaking, said Red Cross spokesperson Stephanie Rendon.
This kind of anecdote is fueling demand for the therapy, which can be provided through an expanded access program led by the Mayo Clinic, backed by the FDA, and the plasma paid for by the U.S. Department of Health & Human Services. But while this program is collecting safety and outcomes data, it’s not a randomized, controlled trial.
Others, however, are pursuing that data.
“One of the things I don’t want this to be is the flavor of the month,” Shmuel Shoham, MD, associate professor of medicine at Johns Hopkins University, said in an interview.
Dr. Shoham, principal investigator for a study evaluating convalescent plasma to prevent the infection in high-risk individuals, said some clinicians, desperate for any treatment, have tried potential therapies such as hydroxychloroquine and remdesivir without evidence of safety or efficacy in COVID-19.
The National Institutes of Health recently said something similar for convalescent plasma, that “there are insufficient clinical data to recommend either for or against” its use for COVID-19.
But plasma has promise, according to a Johns Hopkins School of Medicine’s Bloomberg Distinguished Professor, Arturo Casadevall, MD, PhD, in Baltimore, and Liise-anne Pirofski, MD, a professor at Albert Einstein College of Medicine, New York. They lay out the case for convalescent plasma in an article published online March 13 in the Journal of Clinical Investigation. Passive antibody therapy, they wrote, has been used to stem polio, measles, mumps, and influenza, and more recently has shown some success against SARS-CoV-1 and Middle East respiratory syndrome (MERS).
“The special attraction of this modality of treatment is that, unlike vaccines or newly developed drugs, it could, in principle, be made available very rapidly,” said researchers with the National COVID-19 Convalescent Plasma Project, which includes physicians and scientists from 57 institutions in 46 states. But where principle veers from reality is in availability of the plasma itself, and donors are in short supply.
Aiming to prevent infection
So far, the FDA has approved 12 plasma trials – including Dr. Shoham’s – and the NIH’s clinicaltrials.gov lists more than two dozen convalescent plasma studies in the United States and elsewhere.
Most are single-arm trials to determine if one infusion can decrease the need for intubation or help those on a ventilator improve. Two others, one at Johns Hopkins and one at Stanford (Calif.) Hospital are investigating whether convalescent plasma might be used before severe disease sets in.
“A general principle of passive antibody therapy is that it is more effective when used for prophylaxis than for treatment of disease,” Dr. Casadevall and Dr. Pirofski wrote.
Stanford’s randomized, double-blind study will evaluate regular versus convalescent plasma in ED patients who are not sick enough to require hospitalization.
The Johns Hopkins trial, which aims to protect against infection in the first place, will begin at Johns Hopkins, Baltimore, and at Hopkins-affiliated hospitals throughout Maryland, Dr. Shoham said. He hopes it will expand nationwide eventually, and said that they expect to enroll the first patients soon.
To start, the prevention study will enroll only 150 patients, each of whom must have had close contact with someone who has COVID-19 within the previous 120 hours and be asymptomatic. The number of subjects is small, compared with the trial size of other potential therapies, and an issue, Shoham said, “that keeps me up at night.” But finding thousands of enrollees for plasma studies is hard, in part because it’s so difficult to recruit donors.
Participants will receive normal plasma (which will act as a placebo) or convalescent plasma.
The primary endpoint is cumulative incidence of COVID-19, defined as symptoms and a polymerase chain reaction–positive test; participants will be tracked for 90 days. Hospitals and health care workers could then decide if they want to use the therapy, he said.
The study will not answer whether participants will continue to have antibodies beyond the 90 days. Convalescent plasma is given as a rapid response to an emergent pathogen – a short-term boost of immunity rather than a long-term therapeutic.
What can we learn from expanded access?
Meanwhile, some 2,200 hospitals are participating in the expanded access program being led by the Mayo Clinic nationwide; more than 9,000 patients had received infusions at press time.
One participant is Northwell Health, a 23-hospital system that sprawls across the U.S. COVID epicenter: four of the five boroughs of New York City and Long Island.
Convalescent plasma is an in-demand therapy, said Christina Brennan, MD, vice president of clinical research at Northwell. “We get patients, family members, they say my family member is at X hospital – if it’s not being offered there, can you have them transferred?” she said in an interview.
When Northwell – through the New York Blood Bank – opened up donor registration, 800 people signed up in the first 24 hours, Dr. Brennan said. As of mid-May, 527 patients had received a transfusion.
Who’s the best donor and when should donation occur?
The Red Cross, hospitals, and independent blood banks are all soliciting donors, who can sign up at the Red Cross website. The FDA recommends that donors have a history of COVID-19 as confirmed by molecular or antibody testing, be symptom free for 14 days, have a negative follow-up molecular test, and be virus free at the time of collection. The FDA also suggests measuring a donor’s SARS-CoV-2 neutralizing antibody titers, if available, with a recommendation of at least 1:160.
But questions remain, such as whether there is a theoretical risk for antibody-dependent enhancement (ADE) of infection with SARS-CoV-2. “Antibodies to one type of coronavirus could enhance infection to another viral strain,” of coronavirus, Dr. Casadevall wrote. ADE has been observed in both severe acute respiratory syndrome (SARS) and MERS.
The other risk is that donors may still be shedding active virus. While the FDA suggests that donors are unlikely to still be infectious 14 days after infection, that is as of yet unproven. Both COVID-19 diagnostics and antibody tests have high rates of false negatives, which raises the specter that infection could be spread via the plasma donation.
Daniele Focosi, MD, PhD, from Pisa (Italy) University Hospital and colleagues raise that concern in a preprint review on convalescent plasma in COVID-19. “Although the recipient is already infected, theoretically transmission of more infectious particles could worsen clinical conditions,” they wrote, noting that “such a concern can be somewhat reduced by treatment with modern pathogen inactivation techniques.”
No evidence exists that SARS-CoV-2 can be transmitted through blood, but “we don’t know for sure,” Dr. Shoham said in an interview. A reassuring point: Even those with severe infection do not have viral RNA in their blood, he said, adding, “We don’t think there’s going to be viral transmission of this particular virus with transfusion.”
For another highly infectious pathogen, the Ebola virus, the World Health Organization recommended in 2014 that potential plasma donors wait at least 28 days after infection.
It’s also not known how long SARS-CoV-2 antibodies persist in the blood; longer viability could mean a longer donation window. Dr. Focosi noted that a previous Chinese study had shown that SARS-specific antibodies in people infected with the first SARS virus, SARS-CoV-1, persisted for 2 years.
Dr. Casadevall and Dr. Pirofski have disclosed no relevant financial relationships. Shoham has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patient-focused precautions, testing help blunt pandemic effects on heme-onc unit
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Low-dose erlotinib seems feasible for frail, elderly patients with NSCLC
, according to researchers.
They conducted a phase 2 trial to investigate whether one-third of the maximum tolerated dose of erlotinib could maintain sufficient plasma concentration of the drug while avoiding the adverse effects of higher doses. The results were published in JAMA Oncology.
Erlotinib and other epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have demonstrated efficacy in elderly patients with EGFR-positive NSCLC, according to study author Shingo Miyamoto, MD, of Japanese Red Cross Medical Center in Tokyo, and colleagues.
“With the increasing number of elderly patients with cancer, many of whom also have significant comorbidities, there is a considerable value in investigating whether EGFR-TKIs are effective for the frail population,” the authors wrote. They also noted that it is “difficult to identify the appropriate dose of molecular-targeted drugs.”
With this in mind, Dr. Miyamoto and colleagues conducted a single-arm, phase 2 trial of low-dose erlotinib in 80 chemotherapy-naive frail or elderly patients with EGFR-positive NSCLC. Frailty was defined by age and the Charlson Comorbidity Index. The patients’ median age was 80 years (range, 49-90 years).
Patients received erlotinib at 50 mg per day, which is one-third of the established maximum tolerated dose, for 4 weeks. Then, they were evaluated with radiologic imaging. Treatment continued until disease progression or unacceptable adverse events. Dosing was modified by treatment response or by adverse events.
Results
At last follow-up, 7 of the 80 patients were still receiving low-dose erlotinib. Reasons for discontinuation were disease progression (n = 60), patient request (n = 6), adverse events (n = 4), and death (n = 3).
The overall response rate was 60%, and the disease control rate was 90%. The researchers measured plasma erlotinib concentration in 48 patients and found it did not correlate with response.
The median progression-free survival was 9.3 months, and the median overall survival was 26.2 months.
Ten patients had erlotinib temporarily suspended because of adverse events. Five patients had their dose reduced to 25 mg because of adverse events, including oral mucositis, paronychia, erythema multiforme, diarrhea, and anorexia.
Two patients discontinued treatment because of adverse events. One patient had a cutaneous ulcer and bone infection. The other had oral mucositis.
Dr. Miyamoto and colleagues concluded that, “low-dose erlotinib was associated with efficacy and safety in frail patients with EGFR mutation–positive lung cancer. More research on the dosing strategy of target-based drugs is warranted, especially in frail patients in the real-world setting.”
Less is more
Sometimes, less can be more, said Mellar P. Davis, MD, an oncologist and section head of the palliative care department at Geisinger Medical System in Danville, Penn., who was not involved in this study.
“Why do patients benefit from small doses? It may be that there are fewer drug interruptions over time and patients are able to stay on schedule,” Dr. Davis said. “It may also be that erlotinib clearance is reduced in the elderly and comorbid patient. The reduced dose may, in fact, be the ‘therapeutic’ dose in this special population.”
Plasma levels were frequently in therapeutic ranges in this study, but patients who had subtherapeutic plasma levels also responded to therapy, Dr. Davis pointed out. The lower dose was shown to maintain sufficient concentrations of the treatment while reducing adverse effects.
However, Dr. Davis noted, this was not a randomized trial. “It is always a risk hedging bets on single-arm trials,” he said. “Randomized trials often prove phase 2 single-arm trials wrong.”
He added that quality-of-life measures are absent from the study. Erlotinib is a palliative drug with side effects, Dr. Davis noted.
“Control of cancer and cancer regression should improve symptoms and quality of life when balanced against treatment toxicity,” he said. “In this study, I would have thought that symptom improvement, performance score, and quality of life would have been the primary outcome or the co-primary outcome with disease control.”
Should a randomized, controlled trial of low-dose erlotinib be conducted in the frail/elderly population? “If one believes trials should be quantitatively based, the answer would be no,” Dr. Davis said. “Responses may be the same, and it would be expensive to prove that low-dose erlotinib is the same as standard doses when comparing survival.”
However, if one is interested in quality of life, particularly in this growing population, a trial that incorporated quality-of-life measures would make more sense, according to Dr. Davis. “For if one can achieve less toxicity and treat more patients and get the same duration of clinical benefit, then less will be more,” he concluded.
Dr. Davis reported having no conflicts of interest. Study authors disclosed relationships with Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, and many other companies. Erlotinib is manufactured for OSI Pharmaceuticals, an affiliate of Astellas Pharma, and distributed by Genentech, a member of the Roche Group.
The study was supported by the Japan Agency for Medical Research and Development.
SOURCE: Miyamoto S et al. JAMA Oncol. 2020 May 14; e201250. doi: 10.1001/jamaoncol.2020.1250.
, according to researchers.
They conducted a phase 2 trial to investigate whether one-third of the maximum tolerated dose of erlotinib could maintain sufficient plasma concentration of the drug while avoiding the adverse effects of higher doses. The results were published in JAMA Oncology.
Erlotinib and other epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have demonstrated efficacy in elderly patients with EGFR-positive NSCLC, according to study author Shingo Miyamoto, MD, of Japanese Red Cross Medical Center in Tokyo, and colleagues.
“With the increasing number of elderly patients with cancer, many of whom also have significant comorbidities, there is a considerable value in investigating whether EGFR-TKIs are effective for the frail population,” the authors wrote. They also noted that it is “difficult to identify the appropriate dose of molecular-targeted drugs.”
With this in mind, Dr. Miyamoto and colleagues conducted a single-arm, phase 2 trial of low-dose erlotinib in 80 chemotherapy-naive frail or elderly patients with EGFR-positive NSCLC. Frailty was defined by age and the Charlson Comorbidity Index. The patients’ median age was 80 years (range, 49-90 years).
Patients received erlotinib at 50 mg per day, which is one-third of the established maximum tolerated dose, for 4 weeks. Then, they were evaluated with radiologic imaging. Treatment continued until disease progression or unacceptable adverse events. Dosing was modified by treatment response or by adverse events.
Results
At last follow-up, 7 of the 80 patients were still receiving low-dose erlotinib. Reasons for discontinuation were disease progression (n = 60), patient request (n = 6), adverse events (n = 4), and death (n = 3).
The overall response rate was 60%, and the disease control rate was 90%. The researchers measured plasma erlotinib concentration in 48 patients and found it did not correlate with response.
The median progression-free survival was 9.3 months, and the median overall survival was 26.2 months.
Ten patients had erlotinib temporarily suspended because of adverse events. Five patients had their dose reduced to 25 mg because of adverse events, including oral mucositis, paronychia, erythema multiforme, diarrhea, and anorexia.
Two patients discontinued treatment because of adverse events. One patient had a cutaneous ulcer and bone infection. The other had oral mucositis.
Dr. Miyamoto and colleagues concluded that, “low-dose erlotinib was associated with efficacy and safety in frail patients with EGFR mutation–positive lung cancer. More research on the dosing strategy of target-based drugs is warranted, especially in frail patients in the real-world setting.”
Less is more
Sometimes, less can be more, said Mellar P. Davis, MD, an oncologist and section head of the palliative care department at Geisinger Medical System in Danville, Penn., who was not involved in this study.
“Why do patients benefit from small doses? It may be that there are fewer drug interruptions over time and patients are able to stay on schedule,” Dr. Davis said. “It may also be that erlotinib clearance is reduced in the elderly and comorbid patient. The reduced dose may, in fact, be the ‘therapeutic’ dose in this special population.”
Plasma levels were frequently in therapeutic ranges in this study, but patients who had subtherapeutic plasma levels also responded to therapy, Dr. Davis pointed out. The lower dose was shown to maintain sufficient concentrations of the treatment while reducing adverse effects.
However, Dr. Davis noted, this was not a randomized trial. “It is always a risk hedging bets on single-arm trials,” he said. “Randomized trials often prove phase 2 single-arm trials wrong.”
He added that quality-of-life measures are absent from the study. Erlotinib is a palliative drug with side effects, Dr. Davis noted.
“Control of cancer and cancer regression should improve symptoms and quality of life when balanced against treatment toxicity,” he said. “In this study, I would have thought that symptom improvement, performance score, and quality of life would have been the primary outcome or the co-primary outcome with disease control.”
Should a randomized, controlled trial of low-dose erlotinib be conducted in the frail/elderly population? “If one believes trials should be quantitatively based, the answer would be no,” Dr. Davis said. “Responses may be the same, and it would be expensive to prove that low-dose erlotinib is the same as standard doses when comparing survival.”
However, if one is interested in quality of life, particularly in this growing population, a trial that incorporated quality-of-life measures would make more sense, according to Dr. Davis. “For if one can achieve less toxicity and treat more patients and get the same duration of clinical benefit, then less will be more,” he concluded.
Dr. Davis reported having no conflicts of interest. Study authors disclosed relationships with Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, and many other companies. Erlotinib is manufactured for OSI Pharmaceuticals, an affiliate of Astellas Pharma, and distributed by Genentech, a member of the Roche Group.
The study was supported by the Japan Agency for Medical Research and Development.
SOURCE: Miyamoto S et al. JAMA Oncol. 2020 May 14; e201250. doi: 10.1001/jamaoncol.2020.1250.
, according to researchers.
They conducted a phase 2 trial to investigate whether one-third of the maximum tolerated dose of erlotinib could maintain sufficient plasma concentration of the drug while avoiding the adverse effects of higher doses. The results were published in JAMA Oncology.
Erlotinib and other epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have demonstrated efficacy in elderly patients with EGFR-positive NSCLC, according to study author Shingo Miyamoto, MD, of Japanese Red Cross Medical Center in Tokyo, and colleagues.
“With the increasing number of elderly patients with cancer, many of whom also have significant comorbidities, there is a considerable value in investigating whether EGFR-TKIs are effective for the frail population,” the authors wrote. They also noted that it is “difficult to identify the appropriate dose of molecular-targeted drugs.”
With this in mind, Dr. Miyamoto and colleagues conducted a single-arm, phase 2 trial of low-dose erlotinib in 80 chemotherapy-naive frail or elderly patients with EGFR-positive NSCLC. Frailty was defined by age and the Charlson Comorbidity Index. The patients’ median age was 80 years (range, 49-90 years).
Patients received erlotinib at 50 mg per day, which is one-third of the established maximum tolerated dose, for 4 weeks. Then, they were evaluated with radiologic imaging. Treatment continued until disease progression or unacceptable adverse events. Dosing was modified by treatment response or by adverse events.
Results
At last follow-up, 7 of the 80 patients were still receiving low-dose erlotinib. Reasons for discontinuation were disease progression (n = 60), patient request (n = 6), adverse events (n = 4), and death (n = 3).
The overall response rate was 60%, and the disease control rate was 90%. The researchers measured plasma erlotinib concentration in 48 patients and found it did not correlate with response.
The median progression-free survival was 9.3 months, and the median overall survival was 26.2 months.
Ten patients had erlotinib temporarily suspended because of adverse events. Five patients had their dose reduced to 25 mg because of adverse events, including oral mucositis, paronychia, erythema multiforme, diarrhea, and anorexia.
Two patients discontinued treatment because of adverse events. One patient had a cutaneous ulcer and bone infection. The other had oral mucositis.
Dr. Miyamoto and colleagues concluded that, “low-dose erlotinib was associated with efficacy and safety in frail patients with EGFR mutation–positive lung cancer. More research on the dosing strategy of target-based drugs is warranted, especially in frail patients in the real-world setting.”
Less is more
Sometimes, less can be more, said Mellar P. Davis, MD, an oncologist and section head of the palliative care department at Geisinger Medical System in Danville, Penn., who was not involved in this study.
“Why do patients benefit from small doses? It may be that there are fewer drug interruptions over time and patients are able to stay on schedule,” Dr. Davis said. “It may also be that erlotinib clearance is reduced in the elderly and comorbid patient. The reduced dose may, in fact, be the ‘therapeutic’ dose in this special population.”
Plasma levels were frequently in therapeutic ranges in this study, but patients who had subtherapeutic plasma levels also responded to therapy, Dr. Davis pointed out. The lower dose was shown to maintain sufficient concentrations of the treatment while reducing adverse effects.
However, Dr. Davis noted, this was not a randomized trial. “It is always a risk hedging bets on single-arm trials,” he said. “Randomized trials often prove phase 2 single-arm trials wrong.”
He added that quality-of-life measures are absent from the study. Erlotinib is a palliative drug with side effects, Dr. Davis noted.
“Control of cancer and cancer regression should improve symptoms and quality of life when balanced against treatment toxicity,” he said. “In this study, I would have thought that symptom improvement, performance score, and quality of life would have been the primary outcome or the co-primary outcome with disease control.”
Should a randomized, controlled trial of low-dose erlotinib be conducted in the frail/elderly population? “If one believes trials should be quantitatively based, the answer would be no,” Dr. Davis said. “Responses may be the same, and it would be expensive to prove that low-dose erlotinib is the same as standard doses when comparing survival.”
However, if one is interested in quality of life, particularly in this growing population, a trial that incorporated quality-of-life measures would make more sense, according to Dr. Davis. “For if one can achieve less toxicity and treat more patients and get the same duration of clinical benefit, then less will be more,” he concluded.
Dr. Davis reported having no conflicts of interest. Study authors disclosed relationships with Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, and many other companies. Erlotinib is manufactured for OSI Pharmaceuticals, an affiliate of Astellas Pharma, and distributed by Genentech, a member of the Roche Group.
The study was supported by the Japan Agency for Medical Research and Development.
SOURCE: Miyamoto S et al. JAMA Oncol. 2020 May 14; e201250. doi: 10.1001/jamaoncol.2020.1250.
Vaccination regimen effective in preventing pneumonia in MM patients
Patients with hematological malignancies are at high risk of invasive Staphylococcus pneumoniae. Multiple myeloma (MM) patients, in particular, have been found to have one of the highest incidences of invasive pneumococcal disease. However, researchers found that a full three-dose vaccination regimen by 13-valent pneumococcal conjugate (PCV13) vaccine was protective in MM patients when provided between treatment courses, according to a study reported in Vaccine.
The researchers performed a prospective study of 18 adult patients who were vaccinated with PCV13, compared with 18 control-matched patients from 2017 to 2020. The three-dose vaccination regimen was provided between treatment courses with novel target agents (bortezomib, lenalidomide, ixazomib) with a minimum of a 1-month interval. They used the incidence of pneumonias during the one-year observation period as the primary outcome.
Totally there were 12 cases (33.3%) of clinically and radiologically confirmed pneumonias in the entire study group (n = 36), with a distribution between the vaccinated and nonvaccinated groups of 3 (16.7%) and 9 (50%). respectively (P = .037).
The absolute risk reduction seen with vaccination was 33.3%, and the number needed to treat with PCV13 vaccination in MM patients receiving novel agents was 3.0; (95% confidence interval 1.61-22.1). In addition, there were no adverse effects seen from vaccination, according to the authors.
“Despite the expected decrease in immunological response to vaccination during the chemotherapy, we have shown the clinical effectiveness of a PCV13 vaccination schedule based on 3 doses given with a minimum 1 month interval between the courses of novel agents,” the investigators concluded.
The authors reported that they had no relevant disclosures.
SOURCE: Stoma I et al. Vaccine. 2020 May 14; doi.org/10.1016/j.vaccine.2020.05.024.
Patients with hematological malignancies are at high risk of invasive Staphylococcus pneumoniae. Multiple myeloma (MM) patients, in particular, have been found to have one of the highest incidences of invasive pneumococcal disease. However, researchers found that a full three-dose vaccination regimen by 13-valent pneumococcal conjugate (PCV13) vaccine was protective in MM patients when provided between treatment courses, according to a study reported in Vaccine.
The researchers performed a prospective study of 18 adult patients who were vaccinated with PCV13, compared with 18 control-matched patients from 2017 to 2020. The three-dose vaccination regimen was provided between treatment courses with novel target agents (bortezomib, lenalidomide, ixazomib) with a minimum of a 1-month interval. They used the incidence of pneumonias during the one-year observation period as the primary outcome.
Totally there were 12 cases (33.3%) of clinically and radiologically confirmed pneumonias in the entire study group (n = 36), with a distribution between the vaccinated and nonvaccinated groups of 3 (16.7%) and 9 (50%). respectively (P = .037).
The absolute risk reduction seen with vaccination was 33.3%, and the number needed to treat with PCV13 vaccination in MM patients receiving novel agents was 3.0; (95% confidence interval 1.61-22.1). In addition, there were no adverse effects seen from vaccination, according to the authors.
“Despite the expected decrease in immunological response to vaccination during the chemotherapy, we have shown the clinical effectiveness of a PCV13 vaccination schedule based on 3 doses given with a minimum 1 month interval between the courses of novel agents,” the investigators concluded.
The authors reported that they had no relevant disclosures.
SOURCE: Stoma I et al. Vaccine. 2020 May 14; doi.org/10.1016/j.vaccine.2020.05.024.
Patients with hematological malignancies are at high risk of invasive Staphylococcus pneumoniae. Multiple myeloma (MM) patients, in particular, have been found to have one of the highest incidences of invasive pneumococcal disease. However, researchers found that a full three-dose vaccination regimen by 13-valent pneumococcal conjugate (PCV13) vaccine was protective in MM patients when provided between treatment courses, according to a study reported in Vaccine.
The researchers performed a prospective study of 18 adult patients who were vaccinated with PCV13, compared with 18 control-matched patients from 2017 to 2020. The three-dose vaccination regimen was provided between treatment courses with novel target agents (bortezomib, lenalidomide, ixazomib) with a minimum of a 1-month interval. They used the incidence of pneumonias during the one-year observation period as the primary outcome.
Totally there were 12 cases (33.3%) of clinically and radiologically confirmed pneumonias in the entire study group (n = 36), with a distribution between the vaccinated and nonvaccinated groups of 3 (16.7%) and 9 (50%). respectively (P = .037).
The absolute risk reduction seen with vaccination was 33.3%, and the number needed to treat with PCV13 vaccination in MM patients receiving novel agents was 3.0; (95% confidence interval 1.61-22.1). In addition, there were no adverse effects seen from vaccination, according to the authors.
“Despite the expected decrease in immunological response to vaccination during the chemotherapy, we have shown the clinical effectiveness of a PCV13 vaccination schedule based on 3 doses given with a minimum 1 month interval between the courses of novel agents,” the investigators concluded.
The authors reported that they had no relevant disclosures.
SOURCE: Stoma I et al. Vaccine. 2020 May 14; doi.org/10.1016/j.vaccine.2020.05.024.
FROM VACCINE
FDA approves olaparib for certain metastatic prostate cancers
The Food and Drug Administration approved olaparib (Lynparza, AstraZeneca) for deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC).
The drug is limited to use in men who have progressed following prior treatment with enzalutamide or abiraterone.
Olaparib becomes the second PARP inhibitor approved by the FDA for use in prostate cancer this week. Earlier, rucaparib (Rubraca, Clovis Oncology) was approved for use in patients with mCRPC that harbor deleterious BRCA mutations (germline and/or somatic).
Olaparib is also indicated for use in ovarian, breast, and pancreatic cancers.
The FDA also approved two companion diagnostic devices for treatment with olaparib: the FoundationOne CDx test (Foundation Medicine) for the selection of patients carrying HRR gene alterations and the BRACAnalysis CDx test (Myriad Genetic Laboratories) for the selection of patients carrying germline BRCA1/2 alterations.
The approval was based on results from the open-label, multicenter PROfound trial, which randomly assigned 387 patients to olaparib 300 mg twice daily and to investigator’s choice of enzalutamide or abiraterone acetate. All patients received a GnRH analogue or had prior bilateral orchiectomy.
The study involved two cohorts. Patients with mutations in either BRCA1, BRCA2, or ATM were randomly assigned in cohort A (n = 245); patients with mutations among 12 other genes involved in the HRR pathway were randomly assigned in cohort B (n = 142); those with co-mutations were assigned to cohort A.
The major efficacy outcome of the trial was radiological progression-free survival (rPFS) (cohort A).
In cohort A, patients receiving olaparib had a median rPFS of 7.4 months vs 3.6 months among patients receiving investigator’s choice (hazard ratio [HR], 0.34; P < .0001). Median overall survival was 19.1 months vs 14.7 months (HR, 0.69; P = .0175) and the overall response rate was 33% vs 2% (P < .0001).
In cohort A+B, patients receiving olaparib had a median rPFS of 5.8 months vs 3.5 months among patients receiving investigator’s choice (HR, 0.49; P < .0001).
The study results were first presented at the 2019 annual meeting of the European Society for Medical Oncology. At that time, study investigator Maha Hussain, MD, Northwestern University, Chicago, said the rPFS result and other outcomes were a “remarkable achievement” in such heavily pretreated patients with prostate cancer.
Patients with prostate cancer should now undergo genetic testing of tumor tissue to identify the roughly 30% of patients who can benefit – as is already routinely being done for breast, ovarian, and lung cancer, said experts at ESMO.
The most common adverse reactions with olaparib (≥10% of patients) were anemia, nausea, fatigue (including asthenia), decreased appetite, diarrhea, vomiting, thrombocytopenia, cough, and dyspnea. Venous thromboembolic events, including pulmonary embolism, occurred in 7% of patients randomly assigned to olaparib, compared with 3.1% of those receiving investigator’s choice of enzalutamide or abiraterone.
Olaparib carries the warning that myelodysplastic syndrome/acute myeloid leukemia (MDS/AML) occurred in <1.5% of patients exposed to it as a monotherapy, and that the majority of events had a fatal outcome.
The recommended olaparib dose is 300 mg taken orally twice daily, with or without food.
This article first appeared on Medscape.com.
The Food and Drug Administration approved olaparib (Lynparza, AstraZeneca) for deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC).
The drug is limited to use in men who have progressed following prior treatment with enzalutamide or abiraterone.
Olaparib becomes the second PARP inhibitor approved by the FDA for use in prostate cancer this week. Earlier, rucaparib (Rubraca, Clovis Oncology) was approved for use in patients with mCRPC that harbor deleterious BRCA mutations (germline and/or somatic).
Olaparib is also indicated for use in ovarian, breast, and pancreatic cancers.
The FDA also approved two companion diagnostic devices for treatment with olaparib: the FoundationOne CDx test (Foundation Medicine) for the selection of patients carrying HRR gene alterations and the BRACAnalysis CDx test (Myriad Genetic Laboratories) for the selection of patients carrying germline BRCA1/2 alterations.
The approval was based on results from the open-label, multicenter PROfound trial, which randomly assigned 387 patients to olaparib 300 mg twice daily and to investigator’s choice of enzalutamide or abiraterone acetate. All patients received a GnRH analogue or had prior bilateral orchiectomy.
The study involved two cohorts. Patients with mutations in either BRCA1, BRCA2, or ATM were randomly assigned in cohort A (n = 245); patients with mutations among 12 other genes involved in the HRR pathway were randomly assigned in cohort B (n = 142); those with co-mutations were assigned to cohort A.
The major efficacy outcome of the trial was radiological progression-free survival (rPFS) (cohort A).
In cohort A, patients receiving olaparib had a median rPFS of 7.4 months vs 3.6 months among patients receiving investigator’s choice (hazard ratio [HR], 0.34; P < .0001). Median overall survival was 19.1 months vs 14.7 months (HR, 0.69; P = .0175) and the overall response rate was 33% vs 2% (P < .0001).
In cohort A+B, patients receiving olaparib had a median rPFS of 5.8 months vs 3.5 months among patients receiving investigator’s choice (HR, 0.49; P < .0001).
The study results were first presented at the 2019 annual meeting of the European Society for Medical Oncology. At that time, study investigator Maha Hussain, MD, Northwestern University, Chicago, said the rPFS result and other outcomes were a “remarkable achievement” in such heavily pretreated patients with prostate cancer.
Patients with prostate cancer should now undergo genetic testing of tumor tissue to identify the roughly 30% of patients who can benefit – as is already routinely being done for breast, ovarian, and lung cancer, said experts at ESMO.
The most common adverse reactions with olaparib (≥10% of patients) were anemia, nausea, fatigue (including asthenia), decreased appetite, diarrhea, vomiting, thrombocytopenia, cough, and dyspnea. Venous thromboembolic events, including pulmonary embolism, occurred in 7% of patients randomly assigned to olaparib, compared with 3.1% of those receiving investigator’s choice of enzalutamide or abiraterone.
Olaparib carries the warning that myelodysplastic syndrome/acute myeloid leukemia (MDS/AML) occurred in <1.5% of patients exposed to it as a monotherapy, and that the majority of events had a fatal outcome.
The recommended olaparib dose is 300 mg taken orally twice daily, with or without food.
This article first appeared on Medscape.com.
The Food and Drug Administration approved olaparib (Lynparza, AstraZeneca) for deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC).
The drug is limited to use in men who have progressed following prior treatment with enzalutamide or abiraterone.
Olaparib becomes the second PARP inhibitor approved by the FDA for use in prostate cancer this week. Earlier, rucaparib (Rubraca, Clovis Oncology) was approved for use in patients with mCRPC that harbor deleterious BRCA mutations (germline and/or somatic).
Olaparib is also indicated for use in ovarian, breast, and pancreatic cancers.
The FDA also approved two companion diagnostic devices for treatment with olaparib: the FoundationOne CDx test (Foundation Medicine) for the selection of patients carrying HRR gene alterations and the BRACAnalysis CDx test (Myriad Genetic Laboratories) for the selection of patients carrying germline BRCA1/2 alterations.
The approval was based on results from the open-label, multicenter PROfound trial, which randomly assigned 387 patients to olaparib 300 mg twice daily and to investigator’s choice of enzalutamide or abiraterone acetate. All patients received a GnRH analogue or had prior bilateral orchiectomy.
The study involved two cohorts. Patients with mutations in either BRCA1, BRCA2, or ATM were randomly assigned in cohort A (n = 245); patients with mutations among 12 other genes involved in the HRR pathway were randomly assigned in cohort B (n = 142); those with co-mutations were assigned to cohort A.
The major efficacy outcome of the trial was radiological progression-free survival (rPFS) (cohort A).
In cohort A, patients receiving olaparib had a median rPFS of 7.4 months vs 3.6 months among patients receiving investigator’s choice (hazard ratio [HR], 0.34; P < .0001). Median overall survival was 19.1 months vs 14.7 months (HR, 0.69; P = .0175) and the overall response rate was 33% vs 2% (P < .0001).
In cohort A+B, patients receiving olaparib had a median rPFS of 5.8 months vs 3.5 months among patients receiving investigator’s choice (HR, 0.49; P < .0001).
The study results were first presented at the 2019 annual meeting of the European Society for Medical Oncology. At that time, study investigator Maha Hussain, MD, Northwestern University, Chicago, said the rPFS result and other outcomes were a “remarkable achievement” in such heavily pretreated patients with prostate cancer.
Patients with prostate cancer should now undergo genetic testing of tumor tissue to identify the roughly 30% of patients who can benefit – as is already routinely being done for breast, ovarian, and lung cancer, said experts at ESMO.
The most common adverse reactions with olaparib (≥10% of patients) were anemia, nausea, fatigue (including asthenia), decreased appetite, diarrhea, vomiting, thrombocytopenia, cough, and dyspnea. Venous thromboembolic events, including pulmonary embolism, occurred in 7% of patients randomly assigned to olaparib, compared with 3.1% of those receiving investigator’s choice of enzalutamide or abiraterone.
Olaparib carries the warning that myelodysplastic syndrome/acute myeloid leukemia (MDS/AML) occurred in <1.5% of patients exposed to it as a monotherapy, and that the majority of events had a fatal outcome.
The recommended olaparib dose is 300 mg taken orally twice daily, with or without food.
This article first appeared on Medscape.com.