User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


New registry focuses on rheumatic immune-related AEs of cancer therapy
Its first findings were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“We have limited knowledge on the interrelationships between malignant and rheumatic diseases on both the clinical and molecular level, and we have a large unmet need for management guidelines in the case of the coincidence of both disease entities,” noted lead author Karolina Benesova, MD, of the department of hematology, oncology, and rheumatology at University Hospital Heidelberg (Germany).
The TRheuMa registry – Therapy-Induced Rheumatic Symptoms in Patients with Malignancy – is one of three registries in a multicenter observational project exploring various contexts between malignant and rheumatic diseases. Over its first 22 months, the registry recruited 69 patients having rheumatic symptoms as a result of immune checkpoint inhibitor therapy or other cancer therapies.
Registry findings
The largest shares of patients had non–small cell lung cancer (38%) or melanoma (33%), Dr. Benesova reported. The immune checkpoint inhibitors most commonly received were pembrolizumab (Keytruda), nivolumab (Opdivo), and ipilimumab (Yervoy).
The immune-related adverse events usually presented with symptoms of de novo spondyloarthritis or psoriatic arthritis (42%), late-onset RA (17%), or polymyalgia rheumatica (14%). But 16% of the patients were experiencing a flare of a preexisting rheumatic and musculoskeletal disease.
Laboratory findings differed somewhat from those of classical rheumatic and musculoskeletal diseases, according to Dr. Benesova. Specific findings were rare; in particular, most patients did not have detectable autoantibodies. However, 76% had an elevated C-reactive protein level and 39% had an elevated soluble CD25 level. In addition, nearly all patients (96%) undergoing joint ultrasound had pathologic findings.
“Based on our experiences from interdisciplinary care together with our local oncologists, we have developed a therapeutic algorithm for rheumatic immune-related adverse events,” she reported, noting that the algorithm is consistent with recently published recommendations in this area.
The large majority of patients were adequately treated with prednisone at a dose greater than 10 mg (40%) or at a dose of 10 mg or less with or without an NSAID (40%), while some received NSAID monotherapy (14%).
“We have a growing proportion of patients on conventional or biological [disease-modifying antirheumatic drugs],” Dr. Benesova noted. “These are mostly patients with preexisting rheumatic and musculoskeletal disease or highly suspected de novo classical rheumatic and musculoskeletal disease under checkpoint inhibitor therapy.”
Patients with melanoma having a rheumatic immune-related adverse event had a better response to their therapy than historical counterparts who did not have such events: 39% of the former had a complete response, relative to merely 4% of the latter.
Only a small proportion of patients overall (9%) had to discontinue immune checkpoint inhibitor therapy because of their adverse event, and some of them may be eligible for rechallenge if their cancer progresses, Dr. Benesova noted.
“There is still a lot to be done,” she stated, such as better elucidating the nature of these adverse events [whether transient side effects or a triggering of chronic rheumatic and musculoskeletal diseases], the need for a defensive treatment strategy, and the advisability of closer monitoring of high-risk patients given immune checkpoint inhibitors. “We are aiming at solving these questions in the next few years,” she concluded.
Findings in context
“Registries are important to gain prospective data on patient outcomes,” Sabina Sandigursky, MD, an instructor in the division of rheumatology at the Laura and Isaac Perlmutter Cancer Center at New York University, commented in an interview. “One must be careful, while interpreting these data, especially since they are not randomized, controlled trials.”
Patterns may differ at other centers, too, she pointed out. “The German registry reported a predominance of spondyloarthritis-like disease; however, our patients have a predominance of small-joint involvement. It is unclear what accounts for this difference.”
Individual institutions in North America are similarly collecting data on this patient population, with efforts underway to compile those data to provide a larger picture, according to Dr. Sandigursky.
“Many of the syndromes that we consider to be rheumatic immune-related adverse events have been well described by groups from the U.S., Canada, Australia, and European Union,” she concluded. “From this registry, we can observe how patients are being treated in real time since this information is largely consensus based.”
The study did not receive any specific funding. Dr. Benesova disclosed grant/research support from AbbVie, Novartis, Rheumaliga Baden-Wurttemberg, and the University of Heidelberg, and consultancies, speaker fees, and/or travel reimbursements from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Medac, Merck Sharp & Dohme, Mundipharma, Novartis, Pfizer, Roche, and UCB. Some of her coauthors also disclosed financial relationships with industry. Dr. Sandigursky disclosed having no relevant conflicts of interest.
SOURCE: Benesova K et al. Ann Rheum Dis 2020;79[suppl 1]:168-9, Abstract OP0270.
Its first findings were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“We have limited knowledge on the interrelationships between malignant and rheumatic diseases on both the clinical and molecular level, and we have a large unmet need for management guidelines in the case of the coincidence of both disease entities,” noted lead author Karolina Benesova, MD, of the department of hematology, oncology, and rheumatology at University Hospital Heidelberg (Germany).
The TRheuMa registry – Therapy-Induced Rheumatic Symptoms in Patients with Malignancy – is one of three registries in a multicenter observational project exploring various contexts between malignant and rheumatic diseases. Over its first 22 months, the registry recruited 69 patients having rheumatic symptoms as a result of immune checkpoint inhibitor therapy or other cancer therapies.
Registry findings
The largest shares of patients had non–small cell lung cancer (38%) or melanoma (33%), Dr. Benesova reported. The immune checkpoint inhibitors most commonly received were pembrolizumab (Keytruda), nivolumab (Opdivo), and ipilimumab (Yervoy).
The immune-related adverse events usually presented with symptoms of de novo spondyloarthritis or psoriatic arthritis (42%), late-onset RA (17%), or polymyalgia rheumatica (14%). But 16% of the patients were experiencing a flare of a preexisting rheumatic and musculoskeletal disease.
Laboratory findings differed somewhat from those of classical rheumatic and musculoskeletal diseases, according to Dr. Benesova. Specific findings were rare; in particular, most patients did not have detectable autoantibodies. However, 76% had an elevated C-reactive protein level and 39% had an elevated soluble CD25 level. In addition, nearly all patients (96%) undergoing joint ultrasound had pathologic findings.
“Based on our experiences from interdisciplinary care together with our local oncologists, we have developed a therapeutic algorithm for rheumatic immune-related adverse events,” she reported, noting that the algorithm is consistent with recently published recommendations in this area.
The large majority of patients were adequately treated with prednisone at a dose greater than 10 mg (40%) or at a dose of 10 mg or less with or without an NSAID (40%), while some received NSAID monotherapy (14%).
“We have a growing proportion of patients on conventional or biological [disease-modifying antirheumatic drugs],” Dr. Benesova noted. “These are mostly patients with preexisting rheumatic and musculoskeletal disease or highly suspected de novo classical rheumatic and musculoskeletal disease under checkpoint inhibitor therapy.”
Patients with melanoma having a rheumatic immune-related adverse event had a better response to their therapy than historical counterparts who did not have such events: 39% of the former had a complete response, relative to merely 4% of the latter.
Only a small proportion of patients overall (9%) had to discontinue immune checkpoint inhibitor therapy because of their adverse event, and some of them may be eligible for rechallenge if their cancer progresses, Dr. Benesova noted.
“There is still a lot to be done,” she stated, such as better elucidating the nature of these adverse events [whether transient side effects or a triggering of chronic rheumatic and musculoskeletal diseases], the need for a defensive treatment strategy, and the advisability of closer monitoring of high-risk patients given immune checkpoint inhibitors. “We are aiming at solving these questions in the next few years,” she concluded.
Findings in context
“Registries are important to gain prospective data on patient outcomes,” Sabina Sandigursky, MD, an instructor in the division of rheumatology at the Laura and Isaac Perlmutter Cancer Center at New York University, commented in an interview. “One must be careful, while interpreting these data, especially since they are not randomized, controlled trials.”
Patterns may differ at other centers, too, she pointed out. “The German registry reported a predominance of spondyloarthritis-like disease; however, our patients have a predominance of small-joint involvement. It is unclear what accounts for this difference.”
Individual institutions in North America are similarly collecting data on this patient population, with efforts underway to compile those data to provide a larger picture, according to Dr. Sandigursky.
“Many of the syndromes that we consider to be rheumatic immune-related adverse events have been well described by groups from the U.S., Canada, Australia, and European Union,” she concluded. “From this registry, we can observe how patients are being treated in real time since this information is largely consensus based.”
The study did not receive any specific funding. Dr. Benesova disclosed grant/research support from AbbVie, Novartis, Rheumaliga Baden-Wurttemberg, and the University of Heidelberg, and consultancies, speaker fees, and/or travel reimbursements from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Medac, Merck Sharp & Dohme, Mundipharma, Novartis, Pfizer, Roche, and UCB. Some of her coauthors also disclosed financial relationships with industry. Dr. Sandigursky disclosed having no relevant conflicts of interest.
SOURCE: Benesova K et al. Ann Rheum Dis 2020;79[suppl 1]:168-9, Abstract OP0270.
Its first findings were reported at the annual European Congress of Rheumatology, held online this year due to COVID-19.
“We have limited knowledge on the interrelationships between malignant and rheumatic diseases on both the clinical and molecular level, and we have a large unmet need for management guidelines in the case of the coincidence of both disease entities,” noted lead author Karolina Benesova, MD, of the department of hematology, oncology, and rheumatology at University Hospital Heidelberg (Germany).
The TRheuMa registry – Therapy-Induced Rheumatic Symptoms in Patients with Malignancy – is one of three registries in a multicenter observational project exploring various contexts between malignant and rheumatic diseases. Over its first 22 months, the registry recruited 69 patients having rheumatic symptoms as a result of immune checkpoint inhibitor therapy or other cancer therapies.
Registry findings
The largest shares of patients had non–small cell lung cancer (38%) or melanoma (33%), Dr. Benesova reported. The immune checkpoint inhibitors most commonly received were pembrolizumab (Keytruda), nivolumab (Opdivo), and ipilimumab (Yervoy).
The immune-related adverse events usually presented with symptoms of de novo spondyloarthritis or psoriatic arthritis (42%), late-onset RA (17%), or polymyalgia rheumatica (14%). But 16% of the patients were experiencing a flare of a preexisting rheumatic and musculoskeletal disease.
Laboratory findings differed somewhat from those of classical rheumatic and musculoskeletal diseases, according to Dr. Benesova. Specific findings were rare; in particular, most patients did not have detectable autoantibodies. However, 76% had an elevated C-reactive protein level and 39% had an elevated soluble CD25 level. In addition, nearly all patients (96%) undergoing joint ultrasound had pathologic findings.
“Based on our experiences from interdisciplinary care together with our local oncologists, we have developed a therapeutic algorithm for rheumatic immune-related adverse events,” she reported, noting that the algorithm is consistent with recently published recommendations in this area.
The large majority of patients were adequately treated with prednisone at a dose greater than 10 mg (40%) or at a dose of 10 mg or less with or without an NSAID (40%), while some received NSAID monotherapy (14%).
“We have a growing proportion of patients on conventional or biological [disease-modifying antirheumatic drugs],” Dr. Benesova noted. “These are mostly patients with preexisting rheumatic and musculoskeletal disease or highly suspected de novo classical rheumatic and musculoskeletal disease under checkpoint inhibitor therapy.”
Patients with melanoma having a rheumatic immune-related adverse event had a better response to their therapy than historical counterparts who did not have such events: 39% of the former had a complete response, relative to merely 4% of the latter.
Only a small proportion of patients overall (9%) had to discontinue immune checkpoint inhibitor therapy because of their adverse event, and some of them may be eligible for rechallenge if their cancer progresses, Dr. Benesova noted.
“There is still a lot to be done,” she stated, such as better elucidating the nature of these adverse events [whether transient side effects or a triggering of chronic rheumatic and musculoskeletal diseases], the need for a defensive treatment strategy, and the advisability of closer monitoring of high-risk patients given immune checkpoint inhibitors. “We are aiming at solving these questions in the next few years,” she concluded.
Findings in context
“Registries are important to gain prospective data on patient outcomes,” Sabina Sandigursky, MD, an instructor in the division of rheumatology at the Laura and Isaac Perlmutter Cancer Center at New York University, commented in an interview. “One must be careful, while interpreting these data, especially since they are not randomized, controlled trials.”
Patterns may differ at other centers, too, she pointed out. “The German registry reported a predominance of spondyloarthritis-like disease; however, our patients have a predominance of small-joint involvement. It is unclear what accounts for this difference.”
Individual institutions in North America are similarly collecting data on this patient population, with efforts underway to compile those data to provide a larger picture, according to Dr. Sandigursky.
“Many of the syndromes that we consider to be rheumatic immune-related adverse events have been well described by groups from the U.S., Canada, Australia, and European Union,” she concluded. “From this registry, we can observe how patients are being treated in real time since this information is largely consensus based.”
The study did not receive any specific funding. Dr. Benesova disclosed grant/research support from AbbVie, Novartis, Rheumaliga Baden-Wurttemberg, and the University of Heidelberg, and consultancies, speaker fees, and/or travel reimbursements from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Medac, Merck Sharp & Dohme, Mundipharma, Novartis, Pfizer, Roche, and UCB. Some of her coauthors also disclosed financial relationships with industry. Dr. Sandigursky disclosed having no relevant conflicts of interest.
SOURCE: Benesova K et al. Ann Rheum Dis 2020;79[suppl 1]:168-9, Abstract OP0270.
FROM THE EULAR 2020 E-CONGRESS
Vulvar melanoma is increasing in older women
Maia K. Erickson reported in a poster at the virtual annual meeting of the American Academy of Dermatology.
These are often aggressive malignancies. The 5-year survival following diagnosis of vulvar melanoma in women aged 60 years or older was 39.7%, compared with 61.9% in younger women, according to Ms. Erickson, a visiting research fellow in the department of dermatology at Northwestern University, Chicago.
She presented a population-based study of epidemiologic trends in vulvar melanoma based upon analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results database. Vulvar melanoma was rare during the study years 2000-2016, with an overall incidence rate of 0.1 cases per 100,000 women. That worked out to 746 analyzable cases. Of note, the incidence rate ratio was 680% higher in older women (age 60 and older).
One reason for the markedly worse 5-year survival in older women was that the predominant histologic subtype of vulvar melanoma in that population was nodular melanoma, accounting for 48% of the cases where a histologic subtype was specified. In contrast, the less-aggressive superficial spreading melanoma subtype prevailed in patients aged under 60 years, accounting for 63% of cases.
About 93% of vulvar melanomas occurred in whites; 63% were local and 8.7% were metastatic.
Ms. Erickson noted that the vulva is the most common site for gynecologic tract melanomas, accounting for 70% of them. And while the female genitalia make up only 1%-2% of body surface area, that’s the anatomic site of up to 7% of all melanomas in women.
She reported having no financial conflicts regarding her study.
Maia K. Erickson reported in a poster at the virtual annual meeting of the American Academy of Dermatology.
These are often aggressive malignancies. The 5-year survival following diagnosis of vulvar melanoma in women aged 60 years or older was 39.7%, compared with 61.9% in younger women, according to Ms. Erickson, a visiting research fellow in the department of dermatology at Northwestern University, Chicago.
She presented a population-based study of epidemiologic trends in vulvar melanoma based upon analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results database. Vulvar melanoma was rare during the study years 2000-2016, with an overall incidence rate of 0.1 cases per 100,000 women. That worked out to 746 analyzable cases. Of note, the incidence rate ratio was 680% higher in older women (age 60 and older).
One reason for the markedly worse 5-year survival in older women was that the predominant histologic subtype of vulvar melanoma in that population was nodular melanoma, accounting for 48% of the cases where a histologic subtype was specified. In contrast, the less-aggressive superficial spreading melanoma subtype prevailed in patients aged under 60 years, accounting for 63% of cases.
About 93% of vulvar melanomas occurred in whites; 63% were local and 8.7% were metastatic.
Ms. Erickson noted that the vulva is the most common site for gynecologic tract melanomas, accounting for 70% of them. And while the female genitalia make up only 1%-2% of body surface area, that’s the anatomic site of up to 7% of all melanomas in women.
She reported having no financial conflicts regarding her study.
Maia K. Erickson reported in a poster at the virtual annual meeting of the American Academy of Dermatology.
These are often aggressive malignancies. The 5-year survival following diagnosis of vulvar melanoma in women aged 60 years or older was 39.7%, compared with 61.9% in younger women, according to Ms. Erickson, a visiting research fellow in the department of dermatology at Northwestern University, Chicago.
She presented a population-based study of epidemiologic trends in vulvar melanoma based upon analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results database. Vulvar melanoma was rare during the study years 2000-2016, with an overall incidence rate of 0.1 cases per 100,000 women. That worked out to 746 analyzable cases. Of note, the incidence rate ratio was 680% higher in older women (age 60 and older).
One reason for the markedly worse 5-year survival in older women was that the predominant histologic subtype of vulvar melanoma in that population was nodular melanoma, accounting for 48% of the cases where a histologic subtype was specified. In contrast, the less-aggressive superficial spreading melanoma subtype prevailed in patients aged under 60 years, accounting for 63% of cases.
About 93% of vulvar melanomas occurred in whites; 63% were local and 8.7% were metastatic.
Ms. Erickson noted that the vulva is the most common site for gynecologic tract melanomas, accounting for 70% of them. And while the female genitalia make up only 1%-2% of body surface area, that’s the anatomic site of up to 7% of all melanomas in women.
She reported having no financial conflicts regarding her study.
FROM AAD 2020
Gardasil-9 approved for prevention of head and neck cancers
The US Food and Drug Administration (FDA) has expanded the indication for the Gardasil-9 (Merck) vaccine to include prevention of oropharyngeal and other head and neck cancers caused by HPV types 16, 18, 31, 33, 45, 52, and 58.
This new indication is approved under the FDA’s accelerated approval program and is based on the vaccine’s effectiveness in preventing HPV-related anogenital disease. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory clinical trial, which is currently underway.
“At Merck, working to help prevent certain HPV-related cancers has been a priority for more than two decades,” Alain Luxembourg, MD, director, clinical research, Merck Research Laboratories, said in a statement. “Today’s approval for the prevention of HPV-related oropharyngeal and other head and neck cancers represents an important step in Merck’s mission to help reduce the number of men and women affected by certain HPV-related cancers.”
This new indication doesn’t affect the current recommendations that are already in place. In 2018, a supplemental application for Gardasil 9 was approved to include women and men aged 27 through 45 years for preventing a variety of cancers including cervical, vulvar, vaginal, and anal cancer as well as genital warts. But cancers of the head and neck were not included.
The original Gardasil vaccine came on the market in 2006, with an indication to prevent certain cancers and diseases caused by HPV types 6, 11, 16, and 18. It is no longer distributed in the United States.
In 2014, the FDA approved Gardasil 9, which extends the vaccine coverage for the initial four HPV types as five additional types (31, 33, 45, 52, and 58), and its initial indication was for use in both men and women between the ages of 9 through 26 years.
Head and neck cancers surpass cervical cancer
More than 2 decades ago, researchers first found a connection between HPV and a subset of head and neck cancers (Curr Opin Oncol. 1999;11(3):191-199). The cancers associated with HPV also appeared to have a different biology and disease pattern, as well as a better prognosis, compared with those that were unrelated. HPV is now responsible for the majority of oropharyngeal squamous cell cancers diagnosed in the United States.
A study published last year found that oral HPV infections were occurring with significantly less frequency among sexually active female adolescents who had received the quadrivalent vaccine, as compared with those who were unvaccinated.
These findings provided evidence that HPV vaccination was associated with a reduced frequency of HPV infection in the oral cavity, suggesting that vaccination could decrease the future risk of HPV-associated head and neck cancers.
The omission of head and neck cancers from the initial list of indications for the vaccine is notable because, according to data from the Centers for Disease Control and Prevention (CDC), oropharyngeal cancers are now the most common malignancy caused by HPV, surpassing cervical cancer.
Who will benefit?
An estimated 14 million new HPV infections occur every year in the United States, according to the CDC, and about 80% of individuals who are sexually active have been exposed at some point during their lifetime. In most people, however, the virus will clear on its own without causing any illness or symptoms.
In a Medscape videoblog, Sandra Adamson Fryhofer, MD, MACP, FRCP, helped clarify the adult population most likely to benefit from the vaccine. She pointed out that the HPV vaccine doesn’t treat HPV-related disease or help clear infections, and there are currently no clinical antibody tests or titers that can predict immunity.
“Many adults aged 27-45 have already been exposed to HPV early in life,” she said. Those in a long-term mutually monogamous relationship are not likely to get a new HPV infection. Those with multiple prior sex partners are more likely to have already been exposed to vaccine serotypes. For them, the vaccine will be less effective.”
Fryhofer added that individuals who are now at risk for exposure to a new HPV infection from a new sex partner are the ones most likely to benefit from HPV vaccination.
Confirmation needed
The FDA’s accelerated approval is contingent on confirmatory data, and Merck opened a clinical trial this past February to evaluate the efficacy, immunogenicity, and safety of the 9-valent HPV vaccine in men 20 to 45 years of age. The phase 3 multicenter randomized trial will have an estimated enrollment of 6000 men.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has expanded the indication for the Gardasil-9 (Merck) vaccine to include prevention of oropharyngeal and other head and neck cancers caused by HPV types 16, 18, 31, 33, 45, 52, and 58.
This new indication is approved under the FDA’s accelerated approval program and is based on the vaccine’s effectiveness in preventing HPV-related anogenital disease. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory clinical trial, which is currently underway.
“At Merck, working to help prevent certain HPV-related cancers has been a priority for more than two decades,” Alain Luxembourg, MD, director, clinical research, Merck Research Laboratories, said in a statement. “Today’s approval for the prevention of HPV-related oropharyngeal and other head and neck cancers represents an important step in Merck’s mission to help reduce the number of men and women affected by certain HPV-related cancers.”
This new indication doesn’t affect the current recommendations that are already in place. In 2018, a supplemental application for Gardasil 9 was approved to include women and men aged 27 through 45 years for preventing a variety of cancers including cervical, vulvar, vaginal, and anal cancer as well as genital warts. But cancers of the head and neck were not included.
The original Gardasil vaccine came on the market in 2006, with an indication to prevent certain cancers and diseases caused by HPV types 6, 11, 16, and 18. It is no longer distributed in the United States.
In 2014, the FDA approved Gardasil 9, which extends the vaccine coverage for the initial four HPV types as five additional types (31, 33, 45, 52, and 58), and its initial indication was for use in both men and women between the ages of 9 through 26 years.
Head and neck cancers surpass cervical cancer
More than 2 decades ago, researchers first found a connection between HPV and a subset of head and neck cancers (Curr Opin Oncol. 1999;11(3):191-199). The cancers associated with HPV also appeared to have a different biology and disease pattern, as well as a better prognosis, compared with those that were unrelated. HPV is now responsible for the majority of oropharyngeal squamous cell cancers diagnosed in the United States.
A study published last year found that oral HPV infections were occurring with significantly less frequency among sexually active female adolescents who had received the quadrivalent vaccine, as compared with those who were unvaccinated.
These findings provided evidence that HPV vaccination was associated with a reduced frequency of HPV infection in the oral cavity, suggesting that vaccination could decrease the future risk of HPV-associated head and neck cancers.
The omission of head and neck cancers from the initial list of indications for the vaccine is notable because, according to data from the Centers for Disease Control and Prevention (CDC), oropharyngeal cancers are now the most common malignancy caused by HPV, surpassing cervical cancer.
Who will benefit?
An estimated 14 million new HPV infections occur every year in the United States, according to the CDC, and about 80% of individuals who are sexually active have been exposed at some point during their lifetime. In most people, however, the virus will clear on its own without causing any illness or symptoms.
In a Medscape videoblog, Sandra Adamson Fryhofer, MD, MACP, FRCP, helped clarify the adult population most likely to benefit from the vaccine. She pointed out that the HPV vaccine doesn’t treat HPV-related disease or help clear infections, and there are currently no clinical antibody tests or titers that can predict immunity.
“Many adults aged 27-45 have already been exposed to HPV early in life,” she said. Those in a long-term mutually monogamous relationship are not likely to get a new HPV infection. Those with multiple prior sex partners are more likely to have already been exposed to vaccine serotypes. For them, the vaccine will be less effective.”
Fryhofer added that individuals who are now at risk for exposure to a new HPV infection from a new sex partner are the ones most likely to benefit from HPV vaccination.
Confirmation needed
The FDA’s accelerated approval is contingent on confirmatory data, and Merck opened a clinical trial this past February to evaluate the efficacy, immunogenicity, and safety of the 9-valent HPV vaccine in men 20 to 45 years of age. The phase 3 multicenter randomized trial will have an estimated enrollment of 6000 men.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has expanded the indication for the Gardasil-9 (Merck) vaccine to include prevention of oropharyngeal and other head and neck cancers caused by HPV types 16, 18, 31, 33, 45, 52, and 58.
This new indication is approved under the FDA’s accelerated approval program and is based on the vaccine’s effectiveness in preventing HPV-related anogenital disease. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory clinical trial, which is currently underway.
“At Merck, working to help prevent certain HPV-related cancers has been a priority for more than two decades,” Alain Luxembourg, MD, director, clinical research, Merck Research Laboratories, said in a statement. “Today’s approval for the prevention of HPV-related oropharyngeal and other head and neck cancers represents an important step in Merck’s mission to help reduce the number of men and women affected by certain HPV-related cancers.”
This new indication doesn’t affect the current recommendations that are already in place. In 2018, a supplemental application for Gardasil 9 was approved to include women and men aged 27 through 45 years for preventing a variety of cancers including cervical, vulvar, vaginal, and anal cancer as well as genital warts. But cancers of the head and neck were not included.
The original Gardasil vaccine came on the market in 2006, with an indication to prevent certain cancers and diseases caused by HPV types 6, 11, 16, and 18. It is no longer distributed in the United States.
In 2014, the FDA approved Gardasil 9, which extends the vaccine coverage for the initial four HPV types as five additional types (31, 33, 45, 52, and 58), and its initial indication was for use in both men and women between the ages of 9 through 26 years.
Head and neck cancers surpass cervical cancer
More than 2 decades ago, researchers first found a connection between HPV and a subset of head and neck cancers (Curr Opin Oncol. 1999;11(3):191-199). The cancers associated with HPV also appeared to have a different biology and disease pattern, as well as a better prognosis, compared with those that were unrelated. HPV is now responsible for the majority of oropharyngeal squamous cell cancers diagnosed in the United States.
A study published last year found that oral HPV infections were occurring with significantly less frequency among sexually active female adolescents who had received the quadrivalent vaccine, as compared with those who were unvaccinated.
These findings provided evidence that HPV vaccination was associated with a reduced frequency of HPV infection in the oral cavity, suggesting that vaccination could decrease the future risk of HPV-associated head and neck cancers.
The omission of head and neck cancers from the initial list of indications for the vaccine is notable because, according to data from the Centers for Disease Control and Prevention (CDC), oropharyngeal cancers are now the most common malignancy caused by HPV, surpassing cervical cancer.
Who will benefit?
An estimated 14 million new HPV infections occur every year in the United States, according to the CDC, and about 80% of individuals who are sexually active have been exposed at some point during their lifetime. In most people, however, the virus will clear on its own without causing any illness or symptoms.
In a Medscape videoblog, Sandra Adamson Fryhofer, MD, MACP, FRCP, helped clarify the adult population most likely to benefit from the vaccine. She pointed out that the HPV vaccine doesn’t treat HPV-related disease or help clear infections, and there are currently no clinical antibody tests or titers that can predict immunity.
“Many adults aged 27-45 have already been exposed to HPV early in life,” she said. Those in a long-term mutually monogamous relationship are not likely to get a new HPV infection. Those with multiple prior sex partners are more likely to have already been exposed to vaccine serotypes. For them, the vaccine will be less effective.”
Fryhofer added that individuals who are now at risk for exposure to a new HPV infection from a new sex partner are the ones most likely to benefit from HPV vaccination.
Confirmation needed
The FDA’s accelerated approval is contingent on confirmatory data, and Merck opened a clinical trial this past February to evaluate the efficacy, immunogenicity, and safety of the 9-valent HPV vaccine in men 20 to 45 years of age. The phase 3 multicenter randomized trial will have an estimated enrollment of 6000 men.
This article first appeared on Medscape.com.
Three-drug combo promising against high-risk CLL
For patients with high-risk chronic lymphocytic leukemia (CLL), first-line therapy with a triple combination of targeted agents showed encouraging response rates in the phase 2 CLL2-GIVe trial.
Among 41 patients with untreated CLL bearing deleterious TP53 mutations and/or the 17p chromosomal deletion who received the GIVe regimen consisting of obinutuzumab (Gazyva), ibrutinib (Imbruvica), and venetoclax (Venclexta), the complete response rate at final restaging was 58.5%, and 33 patients with a confirmed response were negative for minimal residual disease after a median follow-up of 18.6 months, reported Henriette Huber, MD, of University Hospital Ulm, Germany.
“The GIVe regimen is promising first-line therapy for patients with high-risk CLL,” she said in a presentation during the virtual annual congress of the European Hematology Association.
The overall safety profile of the combination was acceptable, she said, but added that “some higher-grade infections are of concern.” The rate of grade 3 or greater infections/infestations in the study was 19.5%.
Sound rationale (with caveat)
Another adverse event of concern is the rate of atrial fibrillation in the comparatively young patient population (median age 62), noted Alexey Danilov, MD, PhD, of City of Hope in Duarte Calif., who commented on the study for MDedge.
He pointed out that second-generation Bruton’s tyrosine kinase (BTK) inhibitors such as acalabrutinib (Calquence) may pose a lower risk of atrial fibrillation than the BTK inhibitor ibrutinib used in the CLL2-GIVe study.
In general, however, the rationale for the combination is sound, Dr. Danilov said.
“Of all the patient populations that we deal with within CLL, this probably would be most appropriate for this type of therapy. Patients with deletion 17p or TP53 mutations still represent an unmet medical need compared to other patients who don’t have those mutations,” he said.
Patients with CLL bearing the mutations have lower clinical response rates to novel therapies and generally do not respond well to chemoimmunotherapy, he said.
“The question becomes whether using these all at the same time, versus sequential strategies – using one drug and then after that, at relapse, another – is better, and obviously this trial doesn’t address that,” he said.
Three targets
The investigators enrolled 24 men and 17 women with untreated CLL with del(17p) and/or TP53 mutations and adequate organ function (creatinine clearance rate of more than 50 mL/min). The median age was 62 (range 35-85 years); 78% of patients had Binet stage B or C disease. The median Cumulative Illness Rating Scale (CIRS) score was 3 (range 0 to 8).
All patients received treatment with the combination for 6 months. The CD20 inhibitor obinutuzumab was given in a dose of 1,000 mg on days 1, 8 and 15 of cycle 1 and day 1 of cycles 2-6. The BTK inhibitor ibrutinib was given continuously at a dose of 420 mg per day beginning on the first day of the first cycle. Venetoclax, a B-cell lymphoma 2 (BCL-2) inhibitor, was started on day 22 of cycle 1, and was increased to 400 mg per day over 5 weeks until the end of cycle 12.
If patients achieved a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) according to International Workshop on CLL criteria at final restaging (performed with imaging at the end of cycle 12 followed by bone marrow biopsy 2 months later), ibrutinib would be stopped beginning at cycle 15. Patients who did not have a CR or CRi would continue on ibrutinib until cycle 36.
Encouraging results
All but 3 of the 41 patients reached final restaging. Analyses of efficacy and safety included all 41 patients.
The CR/CRi rate at final restaging, the primary endpoint, was accomplished in 24 patients (58.8%), and 14 patients (34.1%) had a partial response.
Of the three patients for whom responses could not be assessed, two died (one from ovarian cancer which was retrospectively determined to have been present at enrollment, and one at cycle 9 from cardiac failure), and the third patient withdrew consent at cycle 10.
In all, 33 patients (80.5%) were MRD-negative in peripheral blood, 4 remained MRD positive, and 4 were not assessed. Per protocol, 22 patients with undetectable MRD and a CR or CRi discontinued therapy at week 15. An additional 13 patients also discontinued therapy because of adverse events or other reasons, and 6 remained on therapy beyond cycle 15.
The most frequent adverse events of any grade through the end of cycle 14 were gastrointestinal disorders in 83%, none higher than grade 2; infections and infestations in 70.7%, of which 19.5% were grade 3 or greater in severity; and blood and lymphatic system disorders in 58.5%, most of which (53.7%) were grade 3 or greater.
Cardiac disorders were reported in 19.5% of all patients, including 12.2% with atrial fibrillation; grade 3 or greater atrial fibrillation occurred in 2.4% of patients.
There was one case each of cerebral aspergillosis, progressive multifocal leukoencephalopathy (without PCR testing), urosepsis, staphylococcal sepsis and febrile infection.
Laboratory confirmed tumor lysis syndrome, all grade 3 or greater, was reported in 9.8% of patients. Infusion-related reactions were reported in 29.3% of patients, with a total of 7.3% being grade 3 or greater.
The trial was supported by Janssen-Cilag and Roche. Dr. Huber disclosed travel reimbursement from Novartis. Dr. Danilov disclosed consulting for AbbVie, Janssen, and Genentech.
SOURCE: Huber H et al. EHA Congress. Abstract S157.
For patients with high-risk chronic lymphocytic leukemia (CLL), first-line therapy with a triple combination of targeted agents showed encouraging response rates in the phase 2 CLL2-GIVe trial.
Among 41 patients with untreated CLL bearing deleterious TP53 mutations and/or the 17p chromosomal deletion who received the GIVe regimen consisting of obinutuzumab (Gazyva), ibrutinib (Imbruvica), and venetoclax (Venclexta), the complete response rate at final restaging was 58.5%, and 33 patients with a confirmed response were negative for minimal residual disease after a median follow-up of 18.6 months, reported Henriette Huber, MD, of University Hospital Ulm, Germany.
“The GIVe regimen is promising first-line therapy for patients with high-risk CLL,” she said in a presentation during the virtual annual congress of the European Hematology Association.
The overall safety profile of the combination was acceptable, she said, but added that “some higher-grade infections are of concern.” The rate of grade 3 or greater infections/infestations in the study was 19.5%.
Sound rationale (with caveat)
Another adverse event of concern is the rate of atrial fibrillation in the comparatively young patient population (median age 62), noted Alexey Danilov, MD, PhD, of City of Hope in Duarte Calif., who commented on the study for MDedge.
He pointed out that second-generation Bruton’s tyrosine kinase (BTK) inhibitors such as acalabrutinib (Calquence) may pose a lower risk of atrial fibrillation than the BTK inhibitor ibrutinib used in the CLL2-GIVe study.
In general, however, the rationale for the combination is sound, Dr. Danilov said.
“Of all the patient populations that we deal with within CLL, this probably would be most appropriate for this type of therapy. Patients with deletion 17p or TP53 mutations still represent an unmet medical need compared to other patients who don’t have those mutations,” he said.
Patients with CLL bearing the mutations have lower clinical response rates to novel therapies and generally do not respond well to chemoimmunotherapy, he said.
“The question becomes whether using these all at the same time, versus sequential strategies – using one drug and then after that, at relapse, another – is better, and obviously this trial doesn’t address that,” he said.
Three targets
The investigators enrolled 24 men and 17 women with untreated CLL with del(17p) and/or TP53 mutations and adequate organ function (creatinine clearance rate of more than 50 mL/min). The median age was 62 (range 35-85 years); 78% of patients had Binet stage B or C disease. The median Cumulative Illness Rating Scale (CIRS) score was 3 (range 0 to 8).
All patients received treatment with the combination for 6 months. The CD20 inhibitor obinutuzumab was given in a dose of 1,000 mg on days 1, 8 and 15 of cycle 1 and day 1 of cycles 2-6. The BTK inhibitor ibrutinib was given continuously at a dose of 420 mg per day beginning on the first day of the first cycle. Venetoclax, a B-cell lymphoma 2 (BCL-2) inhibitor, was started on day 22 of cycle 1, and was increased to 400 mg per day over 5 weeks until the end of cycle 12.
If patients achieved a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) according to International Workshop on CLL criteria at final restaging (performed with imaging at the end of cycle 12 followed by bone marrow biopsy 2 months later), ibrutinib would be stopped beginning at cycle 15. Patients who did not have a CR or CRi would continue on ibrutinib until cycle 36.
Encouraging results
All but 3 of the 41 patients reached final restaging. Analyses of efficacy and safety included all 41 patients.
The CR/CRi rate at final restaging, the primary endpoint, was accomplished in 24 patients (58.8%), and 14 patients (34.1%) had a partial response.
Of the three patients for whom responses could not be assessed, two died (one from ovarian cancer which was retrospectively determined to have been present at enrollment, and one at cycle 9 from cardiac failure), and the third patient withdrew consent at cycle 10.
In all, 33 patients (80.5%) were MRD-negative in peripheral blood, 4 remained MRD positive, and 4 were not assessed. Per protocol, 22 patients with undetectable MRD and a CR or CRi discontinued therapy at week 15. An additional 13 patients also discontinued therapy because of adverse events or other reasons, and 6 remained on therapy beyond cycle 15.
The most frequent adverse events of any grade through the end of cycle 14 were gastrointestinal disorders in 83%, none higher than grade 2; infections and infestations in 70.7%, of which 19.5% were grade 3 or greater in severity; and blood and lymphatic system disorders in 58.5%, most of which (53.7%) were grade 3 or greater.
Cardiac disorders were reported in 19.5% of all patients, including 12.2% with atrial fibrillation; grade 3 or greater atrial fibrillation occurred in 2.4% of patients.
There was one case each of cerebral aspergillosis, progressive multifocal leukoencephalopathy (without PCR testing), urosepsis, staphylococcal sepsis and febrile infection.
Laboratory confirmed tumor lysis syndrome, all grade 3 or greater, was reported in 9.8% of patients. Infusion-related reactions were reported in 29.3% of patients, with a total of 7.3% being grade 3 or greater.
The trial was supported by Janssen-Cilag and Roche. Dr. Huber disclosed travel reimbursement from Novartis. Dr. Danilov disclosed consulting for AbbVie, Janssen, and Genentech.
SOURCE: Huber H et al. EHA Congress. Abstract S157.
For patients with high-risk chronic lymphocytic leukemia (CLL), first-line therapy with a triple combination of targeted agents showed encouraging response rates in the phase 2 CLL2-GIVe trial.
Among 41 patients with untreated CLL bearing deleterious TP53 mutations and/or the 17p chromosomal deletion who received the GIVe regimen consisting of obinutuzumab (Gazyva), ibrutinib (Imbruvica), and venetoclax (Venclexta), the complete response rate at final restaging was 58.5%, and 33 patients with a confirmed response were negative for minimal residual disease after a median follow-up of 18.6 months, reported Henriette Huber, MD, of University Hospital Ulm, Germany.
“The GIVe regimen is promising first-line therapy for patients with high-risk CLL,” she said in a presentation during the virtual annual congress of the European Hematology Association.
The overall safety profile of the combination was acceptable, she said, but added that “some higher-grade infections are of concern.” The rate of grade 3 or greater infections/infestations in the study was 19.5%.
Sound rationale (with caveat)
Another adverse event of concern is the rate of atrial fibrillation in the comparatively young patient population (median age 62), noted Alexey Danilov, MD, PhD, of City of Hope in Duarte Calif., who commented on the study for MDedge.
He pointed out that second-generation Bruton’s tyrosine kinase (BTK) inhibitors such as acalabrutinib (Calquence) may pose a lower risk of atrial fibrillation than the BTK inhibitor ibrutinib used in the CLL2-GIVe study.
In general, however, the rationale for the combination is sound, Dr. Danilov said.
“Of all the patient populations that we deal with within CLL, this probably would be most appropriate for this type of therapy. Patients with deletion 17p or TP53 mutations still represent an unmet medical need compared to other patients who don’t have those mutations,” he said.
Patients with CLL bearing the mutations have lower clinical response rates to novel therapies and generally do not respond well to chemoimmunotherapy, he said.
“The question becomes whether using these all at the same time, versus sequential strategies – using one drug and then after that, at relapse, another – is better, and obviously this trial doesn’t address that,” he said.
Three targets
The investigators enrolled 24 men and 17 women with untreated CLL with del(17p) and/or TP53 mutations and adequate organ function (creatinine clearance rate of more than 50 mL/min). The median age was 62 (range 35-85 years); 78% of patients had Binet stage B or C disease. The median Cumulative Illness Rating Scale (CIRS) score was 3 (range 0 to 8).
All patients received treatment with the combination for 6 months. The CD20 inhibitor obinutuzumab was given in a dose of 1,000 mg on days 1, 8 and 15 of cycle 1 and day 1 of cycles 2-6. The BTK inhibitor ibrutinib was given continuously at a dose of 420 mg per day beginning on the first day of the first cycle. Venetoclax, a B-cell lymphoma 2 (BCL-2) inhibitor, was started on day 22 of cycle 1, and was increased to 400 mg per day over 5 weeks until the end of cycle 12.
If patients achieved a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) according to International Workshop on CLL criteria at final restaging (performed with imaging at the end of cycle 12 followed by bone marrow biopsy 2 months later), ibrutinib would be stopped beginning at cycle 15. Patients who did not have a CR or CRi would continue on ibrutinib until cycle 36.
Encouraging results
All but 3 of the 41 patients reached final restaging. Analyses of efficacy and safety included all 41 patients.
The CR/CRi rate at final restaging, the primary endpoint, was accomplished in 24 patients (58.8%), and 14 patients (34.1%) had a partial response.
Of the three patients for whom responses could not be assessed, two died (one from ovarian cancer which was retrospectively determined to have been present at enrollment, and one at cycle 9 from cardiac failure), and the third patient withdrew consent at cycle 10.
In all, 33 patients (80.5%) were MRD-negative in peripheral blood, 4 remained MRD positive, and 4 were not assessed. Per protocol, 22 patients with undetectable MRD and a CR or CRi discontinued therapy at week 15. An additional 13 patients also discontinued therapy because of adverse events or other reasons, and 6 remained on therapy beyond cycle 15.
The most frequent adverse events of any grade through the end of cycle 14 were gastrointestinal disorders in 83%, none higher than grade 2; infections and infestations in 70.7%, of which 19.5% were grade 3 or greater in severity; and blood and lymphatic system disorders in 58.5%, most of which (53.7%) were grade 3 or greater.
Cardiac disorders were reported in 19.5% of all patients, including 12.2% with atrial fibrillation; grade 3 or greater atrial fibrillation occurred in 2.4% of patients.
There was one case each of cerebral aspergillosis, progressive multifocal leukoencephalopathy (without PCR testing), urosepsis, staphylococcal sepsis and febrile infection.
Laboratory confirmed tumor lysis syndrome, all grade 3 or greater, was reported in 9.8% of patients. Infusion-related reactions were reported in 29.3% of patients, with a total of 7.3% being grade 3 or greater.
The trial was supported by Janssen-Cilag and Roche. Dr. Huber disclosed travel reimbursement from Novartis. Dr. Danilov disclosed consulting for AbbVie, Janssen, and Genentech.
SOURCE: Huber H et al. EHA Congress. Abstract S157.
FROM EHA CONGRESS
No OS benefit with gefitinib vs. chemo for EGFR+ NSCLC
The median OS was 75.5 months in patients randomized to adjuvant gefitinib and 62.8 months in patients randomized to vinorelbine plus cisplatin.
Yi-Long Wu, MD, of Guangdong Lung Cancer Institute in Guangzhou, China, reported these results as part of the American Society of Clinical Oncology virtual scientific program.
Prior results from this trial had shown a disease-free survival (DFS) benefit with gefitinib, but this did not translate to an OS benefit at the final analysis, Dr. Wu said.
He noted, however, that the median OS of 75.5 months in the gefitinib arm “was one of the best in resected EGFR-mutant non–small cell lung cancer, compared with historical data.”
The findings also suggest a possible benefit with at least 18 months of gefitinib and show that adjuvant EGFR tyrosine kinase inhibitors (TKIs) should be considered the optimal therapy to improve DFS and achieve potentially better OS in this setting, Dr. Wu said.
Study details and DFS
The ADJUVANT trial (NCT01405079) randomized 222 patients, aged 18-75 years, with EGFR-mutant, stage II-IIIA (N1-N2) NSCLC who had undergone complete resection. Patients were enrolled at 27 sites between September 2011 and April 2014.
The patients were randomized 1:1 to receive 250 mg of gefitinib once daily for 24 months, or 25 mg/m2 of vinorelbine on days 1 and 8 plus 75 mg/m2 of cisplatin on day 1 every 3 weeks for 4 cycles.
The intent-to-treat (ITT) population included 111 patients in each arm. The per-protocol population included 106 patients in the gefitinib arm and 87 patients in the chemotherapy arm.
Primary results from this trial showed a significant improvement in DFS with gefitinib (Lancet Oncol. 2018 Jan;19[1]:139-48). That improvement was maintained in the final analysis.
The median DFS was 30.8 months in the gefitinib arm and 19.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The hazard ratio (HR) was 0.56 (P = .001) in the ITT population and 0.51 (P < .001) in the per-protocol population.
In the ITT population, the 5-year DFS rates were 22.6% in the gefitinib arm and 23.2% in the chemotherapy arm. In the per-protocol population, the 5-year DFS rates were 22.6% and 22.8%, respectively.
OS results
The median OS was 75.5 months in the gefitinib arm and 62.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The HR was 0.92 in both the ITT (P = .674) and per-protocol populations (P = .686).
In the ITT population, the 5-year OS rates were 53.2% in the gefitinib arm and 51.2% in the chemotherapy arm. In the per-protocol population, the 5-year OS rates were 53.2% and 50.7%, respectively.
Subgroup analyses by age, gender, lymph node status, and EGFR mutation showed trends toward improved OS with gefitinib, but the differences were not statistically significant.
The researchers conducted a post hoc analysis to assess the effect of subsequent treatment on patient outcomes. The analysis showed that patients who received gefitinib with subsequent EGFR-TKIs had the best responses and OS.
The median OS was not reached among patients who received gefitinib and subsequent EGFR-TKIs, whereas the median OS ranged from 15.6 months to 62.8 months in other groups. The shortest OS was observed in patients who received adjuvant chemotherapy without subsequent therapy.
The duration of gefitinib treatment also appeared to affect OS. The median OS was 35.7 months in patients who received gefitinib for less than 18 months, and the median OS was not reached in patients who received gefitinib for 18 months or longer (HR, 0.38; P < .001).
Implications and potential next steps
Despite the lack of OS improvement with gefitinib, “all of the patients on this study did much, much better than historical non–small cell lung cancer not specified by the EGFR mutation, with 70 months median survival compared to 35 months median survival for N2-positive disease,” said invited discussant Christopher G. Azzoli, MD, director of thoracic oncology at Lifespan Cancer Institute at Brown University in Providence, R.I.
“But you can’t avoid noticing how the curves come back together in terms of disease-free survival when your effective treatment is limited to 24 months,” he added.
An apparent risk of late brain recurrence in the gefitinib arm is also a concern, Dr. Azzoli said. “So ... longer duration of treatment with a drug that has better control of CNS [central nervous system] disease, such as osimertinib, may improve both DFS and OS,” he added.
Only about 50% of patients in the chemotherapy arm received a TKI at recurrence. The post hoc analysis showing that TKI recipients had the best outcomes raises the question of whether “the survival benefit could be conferred by delivering a superior drug merely at recurrence, or is there benefit to earlier delivery of an effective drug,” Dr. Azzoli said.
Given the high cost of continuous therapy, biomarker refinement could help improve treatment decision-making, he said, noting that “early testing of blood DNA to detect cancer in the body as minimal residual disease is showing promise,” and that many phase 3 studies of EGFR-TKIs are ongoing.
The current trial was sponsored by the Guangdong Association of Clinical Trials. Dr. Wu disclosed relationships with AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/China, Lilly, MSD Oncology, Pfizer, and Roche. Dr. Azzoli reported having no disclosures.
SOURCE: Wu Y et al. ASCO 2020, Abstract 9005.
The median OS was 75.5 months in patients randomized to adjuvant gefitinib and 62.8 months in patients randomized to vinorelbine plus cisplatin.
Yi-Long Wu, MD, of Guangdong Lung Cancer Institute in Guangzhou, China, reported these results as part of the American Society of Clinical Oncology virtual scientific program.
Prior results from this trial had shown a disease-free survival (DFS) benefit with gefitinib, but this did not translate to an OS benefit at the final analysis, Dr. Wu said.
He noted, however, that the median OS of 75.5 months in the gefitinib arm “was one of the best in resected EGFR-mutant non–small cell lung cancer, compared with historical data.”
The findings also suggest a possible benefit with at least 18 months of gefitinib and show that adjuvant EGFR tyrosine kinase inhibitors (TKIs) should be considered the optimal therapy to improve DFS and achieve potentially better OS in this setting, Dr. Wu said.
Study details and DFS
The ADJUVANT trial (NCT01405079) randomized 222 patients, aged 18-75 years, with EGFR-mutant, stage II-IIIA (N1-N2) NSCLC who had undergone complete resection. Patients were enrolled at 27 sites between September 2011 and April 2014.
The patients were randomized 1:1 to receive 250 mg of gefitinib once daily for 24 months, or 25 mg/m2 of vinorelbine on days 1 and 8 plus 75 mg/m2 of cisplatin on day 1 every 3 weeks for 4 cycles.
The intent-to-treat (ITT) population included 111 patients in each arm. The per-protocol population included 106 patients in the gefitinib arm and 87 patients in the chemotherapy arm.
Primary results from this trial showed a significant improvement in DFS with gefitinib (Lancet Oncol. 2018 Jan;19[1]:139-48). That improvement was maintained in the final analysis.
The median DFS was 30.8 months in the gefitinib arm and 19.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The hazard ratio (HR) was 0.56 (P = .001) in the ITT population and 0.51 (P < .001) in the per-protocol population.
In the ITT population, the 5-year DFS rates were 22.6% in the gefitinib arm and 23.2% in the chemotherapy arm. In the per-protocol population, the 5-year DFS rates were 22.6% and 22.8%, respectively.
OS results
The median OS was 75.5 months in the gefitinib arm and 62.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The HR was 0.92 in both the ITT (P = .674) and per-protocol populations (P = .686).
In the ITT population, the 5-year OS rates were 53.2% in the gefitinib arm and 51.2% in the chemotherapy arm. In the per-protocol population, the 5-year OS rates were 53.2% and 50.7%, respectively.
Subgroup analyses by age, gender, lymph node status, and EGFR mutation showed trends toward improved OS with gefitinib, but the differences were not statistically significant.
The researchers conducted a post hoc analysis to assess the effect of subsequent treatment on patient outcomes. The analysis showed that patients who received gefitinib with subsequent EGFR-TKIs had the best responses and OS.
The median OS was not reached among patients who received gefitinib and subsequent EGFR-TKIs, whereas the median OS ranged from 15.6 months to 62.8 months in other groups. The shortest OS was observed in patients who received adjuvant chemotherapy without subsequent therapy.
The duration of gefitinib treatment also appeared to affect OS. The median OS was 35.7 months in patients who received gefitinib for less than 18 months, and the median OS was not reached in patients who received gefitinib for 18 months or longer (HR, 0.38; P < .001).
Implications and potential next steps
Despite the lack of OS improvement with gefitinib, “all of the patients on this study did much, much better than historical non–small cell lung cancer not specified by the EGFR mutation, with 70 months median survival compared to 35 months median survival for N2-positive disease,” said invited discussant Christopher G. Azzoli, MD, director of thoracic oncology at Lifespan Cancer Institute at Brown University in Providence, R.I.
“But you can’t avoid noticing how the curves come back together in terms of disease-free survival when your effective treatment is limited to 24 months,” he added.
An apparent risk of late brain recurrence in the gefitinib arm is also a concern, Dr. Azzoli said. “So ... longer duration of treatment with a drug that has better control of CNS [central nervous system] disease, such as osimertinib, may improve both DFS and OS,” he added.
Only about 50% of patients in the chemotherapy arm received a TKI at recurrence. The post hoc analysis showing that TKI recipients had the best outcomes raises the question of whether “the survival benefit could be conferred by delivering a superior drug merely at recurrence, or is there benefit to earlier delivery of an effective drug,” Dr. Azzoli said.
Given the high cost of continuous therapy, biomarker refinement could help improve treatment decision-making, he said, noting that “early testing of blood DNA to detect cancer in the body as minimal residual disease is showing promise,” and that many phase 3 studies of EGFR-TKIs are ongoing.
The current trial was sponsored by the Guangdong Association of Clinical Trials. Dr. Wu disclosed relationships with AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/China, Lilly, MSD Oncology, Pfizer, and Roche. Dr. Azzoli reported having no disclosures.
SOURCE: Wu Y et al. ASCO 2020, Abstract 9005.
The median OS was 75.5 months in patients randomized to adjuvant gefitinib and 62.8 months in patients randomized to vinorelbine plus cisplatin.
Yi-Long Wu, MD, of Guangdong Lung Cancer Institute in Guangzhou, China, reported these results as part of the American Society of Clinical Oncology virtual scientific program.
Prior results from this trial had shown a disease-free survival (DFS) benefit with gefitinib, but this did not translate to an OS benefit at the final analysis, Dr. Wu said.
He noted, however, that the median OS of 75.5 months in the gefitinib arm “was one of the best in resected EGFR-mutant non–small cell lung cancer, compared with historical data.”
The findings also suggest a possible benefit with at least 18 months of gefitinib and show that adjuvant EGFR tyrosine kinase inhibitors (TKIs) should be considered the optimal therapy to improve DFS and achieve potentially better OS in this setting, Dr. Wu said.
Study details and DFS
The ADJUVANT trial (NCT01405079) randomized 222 patients, aged 18-75 years, with EGFR-mutant, stage II-IIIA (N1-N2) NSCLC who had undergone complete resection. Patients were enrolled at 27 sites between September 2011 and April 2014.
The patients were randomized 1:1 to receive 250 mg of gefitinib once daily for 24 months, or 25 mg/m2 of vinorelbine on days 1 and 8 plus 75 mg/m2 of cisplatin on day 1 every 3 weeks for 4 cycles.
The intent-to-treat (ITT) population included 111 patients in each arm. The per-protocol population included 106 patients in the gefitinib arm and 87 patients in the chemotherapy arm.
Primary results from this trial showed a significant improvement in DFS with gefitinib (Lancet Oncol. 2018 Jan;19[1]:139-48). That improvement was maintained in the final analysis.
The median DFS was 30.8 months in the gefitinib arm and 19.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The hazard ratio (HR) was 0.56 (P = .001) in the ITT population and 0.51 (P < .001) in the per-protocol population.
In the ITT population, the 5-year DFS rates were 22.6% in the gefitinib arm and 23.2% in the chemotherapy arm. In the per-protocol population, the 5-year DFS rates were 22.6% and 22.8%, respectively.
OS results
The median OS was 75.5 months in the gefitinib arm and 62.8 months in the chemotherapy arm for both the ITT and per-protocol populations. The HR was 0.92 in both the ITT (P = .674) and per-protocol populations (P = .686).
In the ITT population, the 5-year OS rates were 53.2% in the gefitinib arm and 51.2% in the chemotherapy arm. In the per-protocol population, the 5-year OS rates were 53.2% and 50.7%, respectively.
Subgroup analyses by age, gender, lymph node status, and EGFR mutation showed trends toward improved OS with gefitinib, but the differences were not statistically significant.
The researchers conducted a post hoc analysis to assess the effect of subsequent treatment on patient outcomes. The analysis showed that patients who received gefitinib with subsequent EGFR-TKIs had the best responses and OS.
The median OS was not reached among patients who received gefitinib and subsequent EGFR-TKIs, whereas the median OS ranged from 15.6 months to 62.8 months in other groups. The shortest OS was observed in patients who received adjuvant chemotherapy without subsequent therapy.
The duration of gefitinib treatment also appeared to affect OS. The median OS was 35.7 months in patients who received gefitinib for less than 18 months, and the median OS was not reached in patients who received gefitinib for 18 months or longer (HR, 0.38; P < .001).
Implications and potential next steps
Despite the lack of OS improvement with gefitinib, “all of the patients on this study did much, much better than historical non–small cell lung cancer not specified by the EGFR mutation, with 70 months median survival compared to 35 months median survival for N2-positive disease,” said invited discussant Christopher G. Azzoli, MD, director of thoracic oncology at Lifespan Cancer Institute at Brown University in Providence, R.I.
“But you can’t avoid noticing how the curves come back together in terms of disease-free survival when your effective treatment is limited to 24 months,” he added.
An apparent risk of late brain recurrence in the gefitinib arm is also a concern, Dr. Azzoli said. “So ... longer duration of treatment with a drug that has better control of CNS [central nervous system] disease, such as osimertinib, may improve both DFS and OS,” he added.
Only about 50% of patients in the chemotherapy arm received a TKI at recurrence. The post hoc analysis showing that TKI recipients had the best outcomes raises the question of whether “the survival benefit could be conferred by delivering a superior drug merely at recurrence, or is there benefit to earlier delivery of an effective drug,” Dr. Azzoli said.
Given the high cost of continuous therapy, biomarker refinement could help improve treatment decision-making, he said, noting that “early testing of blood DNA to detect cancer in the body as minimal residual disease is showing promise,” and that many phase 3 studies of EGFR-TKIs are ongoing.
The current trial was sponsored by the Guangdong Association of Clinical Trials. Dr. Wu disclosed relationships with AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/China, Lilly, MSD Oncology, Pfizer, and Roche. Dr. Azzoli reported having no disclosures.
SOURCE: Wu Y et al. ASCO 2020, Abstract 9005.
FROM ASCO 2020
Pulmonary Neuroendocrine Tumor Presenting as a Left Pleural Effusion
Neuroendocrine tumors (NETs) account for about 0.5% of all newly diagnosed malignancies.1 Pulmonary NETs are rare, accounting for 1 to 2% of all invasive lung malignancies and involve about 20 to 25% of primary lung malignancies. 2,3 Their prevalence has increased by an estimated 6% per year over the past 30 years.2 Nonetheless, the time of diagnosis is frequently delayed because of nonspecific symptoms that may imitate other pulmonary conditions.
In the normal pleural space, there is a steady state in which there is a roughly equal rate of fluid formation and absorption. Any disequilibrium may produce a pleural effusion. Pleural fluids can be transudates or exudates. Transudates result from imbalances in hydrostatic and oncotic pressures in the pleural space. Exudates result primarily from pleural and/or lung inflammation or from impaired lymphatic drainage of the pleural space. Clinical manifestations include cough, wheezing, recurrent pneumonia, hemoptysis and pleural effusions. We present a case of a man who developed a large left pleural effusion with a pathology report suggesting a pulmonary NET as the etiology. Being aware of this rare entity may help improve prognosis by making an earlier diagnosis and starting treatment sooner.
Case Presentation
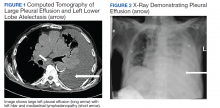
A 90-year-old man with a medical history of arterial hypertension, hyperlipidemia, type 2 diabetes mellitus, coronary artery disease, and vascular dementia presented to the emergency department with hypoactivity, poor appetite, productive cough, and shortness of breath. The patient was a former smoker (unknown pack-years) who quit smoking cigarettes 7 years prior. Vital signs showed sinus tachycardia and peripheral oxygen saturation of 90% at room air. The initial physical examination was remarkable for decreased breath sounds and crackles at the left lung base. Laboratory findings showed leukocytosis with neutrophilia and chronic normocytic anemia. Chest computed tomography (CT) showed a large left-sided pleural effusion occupying most of the left hemithorax with adjacent atelectatic lung, enlarged pretracheal, subcarinal, and left perihilar lymph nodes (Figure 1).
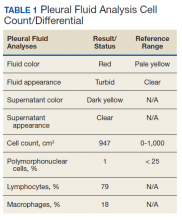
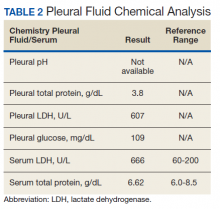
The patient was admitted to the internal medicine ward with the diagnosis of left pneumonic process and started on IV levofloxacin. However, despite 7 days of antibiotic therapy, the patient’s respiratory symptoms worsened. This clinical deterioration prompted pulmonary service consultation. Chest radiography demonstrated an enlarging left pleural effusion (Figure 2). A thoracentesis drained 1.2 L of serosanguineous pleural fluid. Pleural fluid analysis showed a cell count of 947/cm3 with 79% of lymphocytes, total protein 3.8 g/dL, lactic dehydrogenase (LDH) level 607 U/L, and glucose level 109 mg/dL. Serum total protein was 6.62 g/dL, LDH 666 U/L and glucose 92 mg/dL (Tables 1 and 2). Alanine transaminase (ALT) and aspartate aminotransferase (AST) were 11 U/L and 21 U/L, respectively. Using Light criteria, the pleural:serum protein ratio was 0.57, the pleural:serum LDH ratio was 0.91, and the pleural LDH was more than two-thirds of the serum LDH. These calculations were consistent with an exudative effusion. An infectious disease workup, including blood and pleural fluid cultures, was negative.
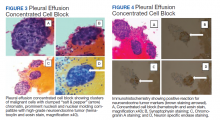
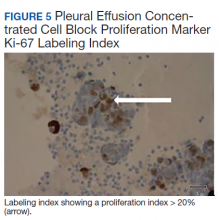
The pleural fluid concentrated cell block hematoxylin and eosin (H&E) staining showed chromatin, prominent nucleoli, and nuclear molding, which was compatible with high-grade lung NET (Figure 3). The cell block immunohistochemistry (IHC) was positive for synaptophysin, chromogranin A, and neuron specific enolase (NSE) also consistent with a high-grade pulmonary NET (Figure 4). The proliferation marker protein Ki-67 labeling index (LI) showed a proliferation index > 20% (Figure 5). The patient did not have decision-making capacity given vascular dementia. Multiple attempts to contact the next of kin or family members were unsuccessful. Risks vs benefits were evaluated, and given the patient’s advanced age and multiple comorbidities, a conservative management approach under palliative care was chosen. For this reason, further genomic studies were not done.
Discussion
NETs are a group of neoplasms that differ in site, amount of cell propagation, and clinical manifestations.4 These tumors are rare with an estimated incidence of 25 to 50 per 100,000.4 The most commonly affected organ systems are the gastroenteropancreatic and the bronchopulmonary tracts, accounting for 60% and 25% of the tumors, respectively.4 The incidence is increasing over the past years in part because of novel diagnostic techniques.
The average age of diagnosis is between the fourth and sixth decades, affecting more women than men.5 Smoking has been identified as a possible culprit for the development of these neoplasms; nonetheless, the association is still not clear.4 For example, poorly differentiated pulmonary NETs have a strong association with smoking but not well-differentiated pulmonary NETs.2
Patients typically present with cough, wheezing, hemoptysis, and recurrent pneumonias, which are in part a consequence of obstruction caused by the mass.2 Sometimes, obstruction may yield persistent pleural effusions. Hemoptysis may be seen secondary to the vascularity of pulmonary NETs.
The diagnosis is often delayed because patients are frequently treated for infection before being diagnosed with the malignancy, such as in our case. Radiologic image findings include round opacities, central masses, and atelectasis. Pulmonary NETs are frequently found incidentally as solitary lung nodules. The CT scan is the most common diagnostic modality and can provide information about the borders of the tumor, the location and surrounding structures, including the presence of atelectasis.5 Pulmonary NETs are usually centrally located in an accessible region for lung biopsy. In cases where the mass is not easily reachable, thoracentesis may provide the only available specimen.
The 2015 World Health Organization classification has identified 4 histologic types of pulmonary NETs, namely, typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC).6 The low-grade pulmonary NET, the typical carcinoid, is slow growing and has lower rates of metastasis. The intermediate-grade NET, the atypical carcinoid, is more aggressive. The highgrade NETs, the LCNEC and the SCLC, are aggressive and spread quickly to other places.6 Consequently, LCNEC and SCLC have higher mortalities with a 5-year survival, ranging from 13 to 57% and 5%, respectively.7
Tumors may be histomorphologically classified by H&E staining. The main characteristics that differentiate the low- and high-grade NETs are the presence of necrosis and the mitotic rate. Both categories form neuropeptides and have dense granular cores when seen with an electron microscopy.6 The TC and AC have welldefined, organized histologic patterns, no necrosis, and scarce mitosis. On the other hand, the LCNEC and SCLC are poorly differentiated tumors with necrosis, atypia, and mitosis.6 LCNEC can be separated from SCLC and other tumors by IHC staining, whereas SCLC is primarily distinguished by morphology.
If the biopsy sample size is small, then IHC morphology and markers are helpful for subclassification.8 IHC is used to discern between neuroendocrine (NE) vs non-NE. The evaluation of pleural fluid includes preparation of cell blocks. Cell block staining is deemed better for IHC because it mimics a small biopsy that enables superior stains.9 The need for a pleural biopsy in cases where the cytology is negative depends on treatment aims, the kind of tumor, and the presence of metastasis.10 In almost 80% of cases, pleural biopsy and cytology are the only specimens obtained for analysis.Therefore, identification of these markers is practical for diagnosis.10 For this reason, pleural effusion samples are appropriate options to lung biopsy for molecular studies.10
Ki-67 LI in samples has the highest specificity and sensitivity for low-tointermediate- grade vs high-grade tumors. It is being used for guiding clinical and treatment decisions.6 In SCLC, the Ki-67 LI is not necessary for diagnosis but will be about 80%.11 The tumor cells will show epithelial characteristics with positive cytokeratin AE1/AE3 and monoclonal antibody CAM5.2 and neuroendocrine markers, including NCAM/CD56, chromogranin A, and synaptophysin.11
Thyroid transcription factor-1 (TTF- 1) is positive in most cases. In LCNEC, the Ki-67 LI is between 40% and 80%. NCAM/ CD56, chromogranin A, and synaptophysin are present in 92 to 100%, 80 to 85%, and 50 to 60%, respectively.11 TTF-1 is identified in half of the tumors. All these tumors express pancytokeratin (AE1/AE3), cytokeratin 7 or low-molecular-weight cytokeratin. Likewise, the carcinoids will show markers, such as chromogranin A, synaptophysin, CD56, and epithelial markers like pancytokeratin.11 However, the high-molecular-weight cytokeratin and TTF-1 are negative. Furthermore, NSE is considered a good tumor marker in the diagnosis and prognosis of SCLC. NSE also has been reported in NSCLC. The level of NSE correlates with tumor burden, number of metastatic sites, and response to treatment. 12 A potentially useful marker is the insulinoma-associated protein 1, which is a nuclear determinant of NE differentiation that stains all types of pulmonary NETs irrespective of the histology but does not stain adenocarcinoma or squamous cell carcinoma (SCC).6
Recently, genomic studies have identified gene alterations that have become standard of care for diagnosis and targeted therapies.8 For example, epidermal growth factor receptor (EGFR) and echinoderm microtubule- associated proteinlike 4, and anaplastic lymphoma kinase (EML4-ALK) mutations have been found in about 25% of lung adenocarcinomas. 8 Other abnormalities in LKB1/STK11, NF1, CDKN2A, SMARCA4 and KEAP1, KRAS, MET, ROS1, and RET have also been identified.8 On the other hand, SCC rarely have derangements in EGFR and EML4-ALK, but do show changes in RTKs, DDR2M, FGGRs, among others.8 In TC and AC, observed molecular alterations include MEN1 mutations, mTOR, and SSTRs pathway activation, and GC/ CEACAM1 and CD44/OTP expression.13 LCNEC and SCLC have shown TP53 and RB1 mutations and CDX2/VIL1/BAI3 expression. DLL3 expression and MET mutations may be present in SCLC.13 Last, chromatin remodeling gene mutations have been identified in all these lung NET types.13
Furthermore, neuropeptides and neuroamines may be measured in the blood and urine.14 Pulmonary NETs may be functional and secrete these substances, leading to systemic symptoms based on the released molecules.15 However, pulmonary NETs produce less serotonin than gastrointestinal NETs; therefore, carcinoid syndrome is less frequent in pulmonary NETs.16 Liver metastasis is often present when it occurs.5 Other possible clinical features include Cushing syndrome and acromegaly depending on the secreted hormones.5
In a recent metanalysis, serum LDH has been found to have a prognostic role in Ewing sarcoma, urologic cancers, malignant mesothelioma, among others.17 It demonstrated that a higher LDH concentration is associated with worse survival in patients with lung cancer.17 Serum LDH is an enzyme that catalyzes the reaction between lactic acid and pyruvic acid that typically takes place in anaerobic conditions.17 LDH levels are elevated in malignancies because tumors have an anaerobic environment. Elevated LDH levels correlate with the anaerobic metabolism in the tumor. Other studies also have noted that patients with high metastatic score have higher LDH levels.17 Therefore, LDH may reflect tumor extension.
In addition, other techniques, such as somatostatin- receptor imaging are specifically beneficial in tumors that express the somatostatin receptor.16 For this reason, this type of study is typically indicated in patients with known metastasis, not in patients with low-grade tumors. Abdominal CT scans are done because the liver is a common site for metastasis.
Our case report demonstrates how biomarkers help diagnose these potentially aggressive and life-threatening tumors that may present as a common condition such as a pleural effusion. Using a less invasive and quicker approach with thoracentesis rather than with lung biopsies is a diagnostic tool in this entity. IHC in cell blocks is a reasonable diagnostic method especially in patients in whom performing a lung biopsy is difficult.
Conclusions
The presence of a symptomatic and recurrent unilateral pleural effusion must urge physicians to consider thoracentesis with mindful use of biomarkers not only for therapeutic purposes, but also for diagnosis of a variety of etiologies, both benign and malignant.
1. Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991-1002. doi: 10.1016/j.neo.2017.09.002
2. Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12(3):425-436. doi: 10.1016/j.jtho.2016.11.2222
3. Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers (Basel). 2012;4(3):777-798. doi: 10.3390/cancers4030777
4. Mandegaran R, David S, Screaton N. Cardiothoracic manifestations of neuroendocrine tumours. Br J Radiol. 2016;89(1060). doi: 10.1259/bjr.20150787
5. Caplin ME, Baudin E, Ferolla P, et al; ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604-1620. doi: 10.1093/annonc/mdv041
6. Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6(5):513-529. doi: 10.21037/tlcr.2017.09.04
7. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394.
8. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(pt 1):103-109. doi: 10.1016/j.semcancer.2017.11.019
9. Kaur G, Nijhawan R, Gupta N, Singh N, Rajwanshi A. Pleural fluid cytology samples in cases of suspected lung cancer: an experience from a tertiary care centre. Diagn Cytopathol. 2017;45(3):195-201.
10. Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis. 2018;12. doi: 10.1177/1753466618808660
11. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. doi: 10.1016/j.anndiagpath.2017.04.005
12. Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125-143. doi: 10.1007/978-94-017-7215-0_9
13. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394
14. Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62(suppl 1):33-38.
15. Melosky B. Low grade neuroendocrine tumors of the lung. Front Oncol. 2017;7:119. doi: 10.3389/fonc.2017.00119
16. Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2001;113(1):5-21. https://doi.org/10.1002/cncr.23542
17. Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (Baltimore). 2018;97(38):e12524
Neuroendocrine tumors (NETs) account for about 0.5% of all newly diagnosed malignancies.1 Pulmonary NETs are rare, accounting for 1 to 2% of all invasive lung malignancies and involve about 20 to 25% of primary lung malignancies. 2,3 Their prevalence has increased by an estimated 6% per year over the past 30 years.2 Nonetheless, the time of diagnosis is frequently delayed because of nonspecific symptoms that may imitate other pulmonary conditions.
In the normal pleural space, there is a steady state in which there is a roughly equal rate of fluid formation and absorption. Any disequilibrium may produce a pleural effusion. Pleural fluids can be transudates or exudates. Transudates result from imbalances in hydrostatic and oncotic pressures in the pleural space. Exudates result primarily from pleural and/or lung inflammation or from impaired lymphatic drainage of the pleural space. Clinical manifestations include cough, wheezing, recurrent pneumonia, hemoptysis and pleural effusions. We present a case of a man who developed a large left pleural effusion with a pathology report suggesting a pulmonary NET as the etiology. Being aware of this rare entity may help improve prognosis by making an earlier diagnosis and starting treatment sooner.
Case Presentation
A 90-year-old man with a medical history of arterial hypertension, hyperlipidemia, type 2 diabetes mellitus, coronary artery disease, and vascular dementia presented to the emergency department with hypoactivity, poor appetite, productive cough, and shortness of breath. The patient was a former smoker (unknown pack-years) who quit smoking cigarettes 7 years prior. Vital signs showed sinus tachycardia and peripheral oxygen saturation of 90% at room air. The initial physical examination was remarkable for decreased breath sounds and crackles at the left lung base. Laboratory findings showed leukocytosis with neutrophilia and chronic normocytic anemia. Chest computed tomography (CT) showed a large left-sided pleural effusion occupying most of the left hemithorax with adjacent atelectatic lung, enlarged pretracheal, subcarinal, and left perihilar lymph nodes (Figure 1).
The patient was admitted to the internal medicine ward with the diagnosis of left pneumonic process and started on IV levofloxacin. However, despite 7 days of antibiotic therapy, the patient’s respiratory symptoms worsened. This clinical deterioration prompted pulmonary service consultation. Chest radiography demonstrated an enlarging left pleural effusion (Figure 2). A thoracentesis drained 1.2 L of serosanguineous pleural fluid. Pleural fluid analysis showed a cell count of 947/cm3 with 79% of lymphocytes, total protein 3.8 g/dL, lactic dehydrogenase (LDH) level 607 U/L, and glucose level 109 mg/dL. Serum total protein was 6.62 g/dL, LDH 666 U/L and glucose 92 mg/dL (Tables 1 and 2). Alanine transaminase (ALT) and aspartate aminotransferase (AST) were 11 U/L and 21 U/L, respectively. Using Light criteria, the pleural:serum protein ratio was 0.57, the pleural:serum LDH ratio was 0.91, and the pleural LDH was more than two-thirds of the serum LDH. These calculations were consistent with an exudative effusion. An infectious disease workup, including blood and pleural fluid cultures, was negative.
The pleural fluid concentrated cell block hematoxylin and eosin (H&E) staining showed chromatin, prominent nucleoli, and nuclear molding, which was compatible with high-grade lung NET (Figure 3). The cell block immunohistochemistry (IHC) was positive for synaptophysin, chromogranin A, and neuron specific enolase (NSE) also consistent with a high-grade pulmonary NET (Figure 4). The proliferation marker protein Ki-67 labeling index (LI) showed a proliferation index > 20% (Figure 5). The patient did not have decision-making capacity given vascular dementia. Multiple attempts to contact the next of kin or family members were unsuccessful. Risks vs benefits were evaluated, and given the patient’s advanced age and multiple comorbidities, a conservative management approach under palliative care was chosen. For this reason, further genomic studies were not done.
Discussion
NETs are a group of neoplasms that differ in site, amount of cell propagation, and clinical manifestations.4 These tumors are rare with an estimated incidence of 25 to 50 per 100,000.4 The most commonly affected organ systems are the gastroenteropancreatic and the bronchopulmonary tracts, accounting for 60% and 25% of the tumors, respectively.4 The incidence is increasing over the past years in part because of novel diagnostic techniques.
The average age of diagnosis is between the fourth and sixth decades, affecting more women than men.5 Smoking has been identified as a possible culprit for the development of these neoplasms; nonetheless, the association is still not clear.4 For example, poorly differentiated pulmonary NETs have a strong association with smoking but not well-differentiated pulmonary NETs.2
Patients typically present with cough, wheezing, hemoptysis, and recurrent pneumonias, which are in part a consequence of obstruction caused by the mass.2 Sometimes, obstruction may yield persistent pleural effusions. Hemoptysis may be seen secondary to the vascularity of pulmonary NETs.
The diagnosis is often delayed because patients are frequently treated for infection before being diagnosed with the malignancy, such as in our case. Radiologic image findings include round opacities, central masses, and atelectasis. Pulmonary NETs are frequently found incidentally as solitary lung nodules. The CT scan is the most common diagnostic modality and can provide information about the borders of the tumor, the location and surrounding structures, including the presence of atelectasis.5 Pulmonary NETs are usually centrally located in an accessible region for lung biopsy. In cases where the mass is not easily reachable, thoracentesis may provide the only available specimen.
The 2015 World Health Organization classification has identified 4 histologic types of pulmonary NETs, namely, typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC).6 The low-grade pulmonary NET, the typical carcinoid, is slow growing and has lower rates of metastasis. The intermediate-grade NET, the atypical carcinoid, is more aggressive. The highgrade NETs, the LCNEC and the SCLC, are aggressive and spread quickly to other places.6 Consequently, LCNEC and SCLC have higher mortalities with a 5-year survival, ranging from 13 to 57% and 5%, respectively.7
Tumors may be histomorphologically classified by H&E staining. The main characteristics that differentiate the low- and high-grade NETs are the presence of necrosis and the mitotic rate. Both categories form neuropeptides and have dense granular cores when seen with an electron microscopy.6 The TC and AC have welldefined, organized histologic patterns, no necrosis, and scarce mitosis. On the other hand, the LCNEC and SCLC are poorly differentiated tumors with necrosis, atypia, and mitosis.6 LCNEC can be separated from SCLC and other tumors by IHC staining, whereas SCLC is primarily distinguished by morphology.
If the biopsy sample size is small, then IHC morphology and markers are helpful for subclassification.8 IHC is used to discern between neuroendocrine (NE) vs non-NE. The evaluation of pleural fluid includes preparation of cell blocks. Cell block staining is deemed better for IHC because it mimics a small biopsy that enables superior stains.9 The need for a pleural biopsy in cases where the cytology is negative depends on treatment aims, the kind of tumor, and the presence of metastasis.10 In almost 80% of cases, pleural biopsy and cytology are the only specimens obtained for analysis.Therefore, identification of these markers is practical for diagnosis.10 For this reason, pleural effusion samples are appropriate options to lung biopsy for molecular studies.10
Ki-67 LI in samples has the highest specificity and sensitivity for low-tointermediate- grade vs high-grade tumors. It is being used for guiding clinical and treatment decisions.6 In SCLC, the Ki-67 LI is not necessary for diagnosis but will be about 80%.11 The tumor cells will show epithelial characteristics with positive cytokeratin AE1/AE3 and monoclonal antibody CAM5.2 and neuroendocrine markers, including NCAM/CD56, chromogranin A, and synaptophysin.11
Thyroid transcription factor-1 (TTF- 1) is positive in most cases. In LCNEC, the Ki-67 LI is between 40% and 80%. NCAM/ CD56, chromogranin A, and synaptophysin are present in 92 to 100%, 80 to 85%, and 50 to 60%, respectively.11 TTF-1 is identified in half of the tumors. All these tumors express pancytokeratin (AE1/AE3), cytokeratin 7 or low-molecular-weight cytokeratin. Likewise, the carcinoids will show markers, such as chromogranin A, synaptophysin, CD56, and epithelial markers like pancytokeratin.11 However, the high-molecular-weight cytokeratin and TTF-1 are negative. Furthermore, NSE is considered a good tumor marker in the diagnosis and prognosis of SCLC. NSE also has been reported in NSCLC. The level of NSE correlates with tumor burden, number of metastatic sites, and response to treatment. 12 A potentially useful marker is the insulinoma-associated protein 1, which is a nuclear determinant of NE differentiation that stains all types of pulmonary NETs irrespective of the histology but does not stain adenocarcinoma or squamous cell carcinoma (SCC).6
Recently, genomic studies have identified gene alterations that have become standard of care for diagnosis and targeted therapies.8 For example, epidermal growth factor receptor (EGFR) and echinoderm microtubule- associated proteinlike 4, and anaplastic lymphoma kinase (EML4-ALK) mutations have been found in about 25% of lung adenocarcinomas. 8 Other abnormalities in LKB1/STK11, NF1, CDKN2A, SMARCA4 and KEAP1, KRAS, MET, ROS1, and RET have also been identified.8 On the other hand, SCC rarely have derangements in EGFR and EML4-ALK, but do show changes in RTKs, DDR2M, FGGRs, among others.8 In TC and AC, observed molecular alterations include MEN1 mutations, mTOR, and SSTRs pathway activation, and GC/ CEACAM1 and CD44/OTP expression.13 LCNEC and SCLC have shown TP53 and RB1 mutations and CDX2/VIL1/BAI3 expression. DLL3 expression and MET mutations may be present in SCLC.13 Last, chromatin remodeling gene mutations have been identified in all these lung NET types.13
Furthermore, neuropeptides and neuroamines may be measured in the blood and urine.14 Pulmonary NETs may be functional and secrete these substances, leading to systemic symptoms based on the released molecules.15 However, pulmonary NETs produce less serotonin than gastrointestinal NETs; therefore, carcinoid syndrome is less frequent in pulmonary NETs.16 Liver metastasis is often present when it occurs.5 Other possible clinical features include Cushing syndrome and acromegaly depending on the secreted hormones.5
In a recent metanalysis, serum LDH has been found to have a prognostic role in Ewing sarcoma, urologic cancers, malignant mesothelioma, among others.17 It demonstrated that a higher LDH concentration is associated with worse survival in patients with lung cancer.17 Serum LDH is an enzyme that catalyzes the reaction between lactic acid and pyruvic acid that typically takes place in anaerobic conditions.17 LDH levels are elevated in malignancies because tumors have an anaerobic environment. Elevated LDH levels correlate with the anaerobic metabolism in the tumor. Other studies also have noted that patients with high metastatic score have higher LDH levels.17 Therefore, LDH may reflect tumor extension.
In addition, other techniques, such as somatostatin- receptor imaging are specifically beneficial in tumors that express the somatostatin receptor.16 For this reason, this type of study is typically indicated in patients with known metastasis, not in patients with low-grade tumors. Abdominal CT scans are done because the liver is a common site for metastasis.
Our case report demonstrates how biomarkers help diagnose these potentially aggressive and life-threatening tumors that may present as a common condition such as a pleural effusion. Using a less invasive and quicker approach with thoracentesis rather than with lung biopsies is a diagnostic tool in this entity. IHC in cell blocks is a reasonable diagnostic method especially in patients in whom performing a lung biopsy is difficult.
Conclusions
The presence of a symptomatic and recurrent unilateral pleural effusion must urge physicians to consider thoracentesis with mindful use of biomarkers not only for therapeutic purposes, but also for diagnosis of a variety of etiologies, both benign and malignant.
Neuroendocrine tumors (NETs) account for about 0.5% of all newly diagnosed malignancies.1 Pulmonary NETs are rare, accounting for 1 to 2% of all invasive lung malignancies and involve about 20 to 25% of primary lung malignancies. 2,3 Their prevalence has increased by an estimated 6% per year over the past 30 years.2 Nonetheless, the time of diagnosis is frequently delayed because of nonspecific symptoms that may imitate other pulmonary conditions.
In the normal pleural space, there is a steady state in which there is a roughly equal rate of fluid formation and absorption. Any disequilibrium may produce a pleural effusion. Pleural fluids can be transudates or exudates. Transudates result from imbalances in hydrostatic and oncotic pressures in the pleural space. Exudates result primarily from pleural and/or lung inflammation or from impaired lymphatic drainage of the pleural space. Clinical manifestations include cough, wheezing, recurrent pneumonia, hemoptysis and pleural effusions. We present a case of a man who developed a large left pleural effusion with a pathology report suggesting a pulmonary NET as the etiology. Being aware of this rare entity may help improve prognosis by making an earlier diagnosis and starting treatment sooner.
Case Presentation
A 90-year-old man with a medical history of arterial hypertension, hyperlipidemia, type 2 diabetes mellitus, coronary artery disease, and vascular dementia presented to the emergency department with hypoactivity, poor appetite, productive cough, and shortness of breath. The patient was a former smoker (unknown pack-years) who quit smoking cigarettes 7 years prior. Vital signs showed sinus tachycardia and peripheral oxygen saturation of 90% at room air. The initial physical examination was remarkable for decreased breath sounds and crackles at the left lung base. Laboratory findings showed leukocytosis with neutrophilia and chronic normocytic anemia. Chest computed tomography (CT) showed a large left-sided pleural effusion occupying most of the left hemithorax with adjacent atelectatic lung, enlarged pretracheal, subcarinal, and left perihilar lymph nodes (Figure 1).
The patient was admitted to the internal medicine ward with the diagnosis of left pneumonic process and started on IV levofloxacin. However, despite 7 days of antibiotic therapy, the patient’s respiratory symptoms worsened. This clinical deterioration prompted pulmonary service consultation. Chest radiography demonstrated an enlarging left pleural effusion (Figure 2). A thoracentesis drained 1.2 L of serosanguineous pleural fluid. Pleural fluid analysis showed a cell count of 947/cm3 with 79% of lymphocytes, total protein 3.8 g/dL, lactic dehydrogenase (LDH) level 607 U/L, and glucose level 109 mg/dL. Serum total protein was 6.62 g/dL, LDH 666 U/L and glucose 92 mg/dL (Tables 1 and 2). Alanine transaminase (ALT) and aspartate aminotransferase (AST) were 11 U/L and 21 U/L, respectively. Using Light criteria, the pleural:serum protein ratio was 0.57, the pleural:serum LDH ratio was 0.91, and the pleural LDH was more than two-thirds of the serum LDH. These calculations were consistent with an exudative effusion. An infectious disease workup, including blood and pleural fluid cultures, was negative.
The pleural fluid concentrated cell block hematoxylin and eosin (H&E) staining showed chromatin, prominent nucleoli, and nuclear molding, which was compatible with high-grade lung NET (Figure 3). The cell block immunohistochemistry (IHC) was positive for synaptophysin, chromogranin A, and neuron specific enolase (NSE) also consistent with a high-grade pulmonary NET (Figure 4). The proliferation marker protein Ki-67 labeling index (LI) showed a proliferation index > 20% (Figure 5). The patient did not have decision-making capacity given vascular dementia. Multiple attempts to contact the next of kin or family members were unsuccessful. Risks vs benefits were evaluated, and given the patient’s advanced age and multiple comorbidities, a conservative management approach under palliative care was chosen. For this reason, further genomic studies were not done.
Discussion
NETs are a group of neoplasms that differ in site, amount of cell propagation, and clinical manifestations.4 These tumors are rare with an estimated incidence of 25 to 50 per 100,000.4 The most commonly affected organ systems are the gastroenteropancreatic and the bronchopulmonary tracts, accounting for 60% and 25% of the tumors, respectively.4 The incidence is increasing over the past years in part because of novel diagnostic techniques.
The average age of diagnosis is between the fourth and sixth decades, affecting more women than men.5 Smoking has been identified as a possible culprit for the development of these neoplasms; nonetheless, the association is still not clear.4 For example, poorly differentiated pulmonary NETs have a strong association with smoking but not well-differentiated pulmonary NETs.2
Patients typically present with cough, wheezing, hemoptysis, and recurrent pneumonias, which are in part a consequence of obstruction caused by the mass.2 Sometimes, obstruction may yield persistent pleural effusions. Hemoptysis may be seen secondary to the vascularity of pulmonary NETs.
The diagnosis is often delayed because patients are frequently treated for infection before being diagnosed with the malignancy, such as in our case. Radiologic image findings include round opacities, central masses, and atelectasis. Pulmonary NETs are frequently found incidentally as solitary lung nodules. The CT scan is the most common diagnostic modality and can provide information about the borders of the tumor, the location and surrounding structures, including the presence of atelectasis.5 Pulmonary NETs are usually centrally located in an accessible region for lung biopsy. In cases where the mass is not easily reachable, thoracentesis may provide the only available specimen.
The 2015 World Health Organization classification has identified 4 histologic types of pulmonary NETs, namely, typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC).6 The low-grade pulmonary NET, the typical carcinoid, is slow growing and has lower rates of metastasis. The intermediate-grade NET, the atypical carcinoid, is more aggressive. The highgrade NETs, the LCNEC and the SCLC, are aggressive and spread quickly to other places.6 Consequently, LCNEC and SCLC have higher mortalities with a 5-year survival, ranging from 13 to 57% and 5%, respectively.7
Tumors may be histomorphologically classified by H&E staining. The main characteristics that differentiate the low- and high-grade NETs are the presence of necrosis and the mitotic rate. Both categories form neuropeptides and have dense granular cores when seen with an electron microscopy.6 The TC and AC have welldefined, organized histologic patterns, no necrosis, and scarce mitosis. On the other hand, the LCNEC and SCLC are poorly differentiated tumors with necrosis, atypia, and mitosis.6 LCNEC can be separated from SCLC and other tumors by IHC staining, whereas SCLC is primarily distinguished by morphology.
If the biopsy sample size is small, then IHC morphology and markers are helpful for subclassification.8 IHC is used to discern between neuroendocrine (NE) vs non-NE. The evaluation of pleural fluid includes preparation of cell blocks. Cell block staining is deemed better for IHC because it mimics a small biopsy that enables superior stains.9 The need for a pleural biopsy in cases where the cytology is negative depends on treatment aims, the kind of tumor, and the presence of metastasis.10 In almost 80% of cases, pleural biopsy and cytology are the only specimens obtained for analysis.Therefore, identification of these markers is practical for diagnosis.10 For this reason, pleural effusion samples are appropriate options to lung biopsy for molecular studies.10
Ki-67 LI in samples has the highest specificity and sensitivity for low-tointermediate- grade vs high-grade tumors. It is being used for guiding clinical and treatment decisions.6 In SCLC, the Ki-67 LI is not necessary for diagnosis but will be about 80%.11 The tumor cells will show epithelial characteristics with positive cytokeratin AE1/AE3 and monoclonal antibody CAM5.2 and neuroendocrine markers, including NCAM/CD56, chromogranin A, and synaptophysin.11
Thyroid transcription factor-1 (TTF- 1) is positive in most cases. In LCNEC, the Ki-67 LI is between 40% and 80%. NCAM/ CD56, chromogranin A, and synaptophysin are present in 92 to 100%, 80 to 85%, and 50 to 60%, respectively.11 TTF-1 is identified in half of the tumors. All these tumors express pancytokeratin (AE1/AE3), cytokeratin 7 or low-molecular-weight cytokeratin. Likewise, the carcinoids will show markers, such as chromogranin A, synaptophysin, CD56, and epithelial markers like pancytokeratin.11 However, the high-molecular-weight cytokeratin and TTF-1 are negative. Furthermore, NSE is considered a good tumor marker in the diagnosis and prognosis of SCLC. NSE also has been reported in NSCLC. The level of NSE correlates with tumor burden, number of metastatic sites, and response to treatment. 12 A potentially useful marker is the insulinoma-associated protein 1, which is a nuclear determinant of NE differentiation that stains all types of pulmonary NETs irrespective of the histology but does not stain adenocarcinoma or squamous cell carcinoma (SCC).6
Recently, genomic studies have identified gene alterations that have become standard of care for diagnosis and targeted therapies.8 For example, epidermal growth factor receptor (EGFR) and echinoderm microtubule- associated proteinlike 4, and anaplastic lymphoma kinase (EML4-ALK) mutations have been found in about 25% of lung adenocarcinomas. 8 Other abnormalities in LKB1/STK11, NF1, CDKN2A, SMARCA4 and KEAP1, KRAS, MET, ROS1, and RET have also been identified.8 On the other hand, SCC rarely have derangements in EGFR and EML4-ALK, but do show changes in RTKs, DDR2M, FGGRs, among others.8 In TC and AC, observed molecular alterations include MEN1 mutations, mTOR, and SSTRs pathway activation, and GC/ CEACAM1 and CD44/OTP expression.13 LCNEC and SCLC have shown TP53 and RB1 mutations and CDX2/VIL1/BAI3 expression. DLL3 expression and MET mutations may be present in SCLC.13 Last, chromatin remodeling gene mutations have been identified in all these lung NET types.13
Furthermore, neuropeptides and neuroamines may be measured in the blood and urine.14 Pulmonary NETs may be functional and secrete these substances, leading to systemic symptoms based on the released molecules.15 However, pulmonary NETs produce less serotonin than gastrointestinal NETs; therefore, carcinoid syndrome is less frequent in pulmonary NETs.16 Liver metastasis is often present when it occurs.5 Other possible clinical features include Cushing syndrome and acromegaly depending on the secreted hormones.5
In a recent metanalysis, serum LDH has been found to have a prognostic role in Ewing sarcoma, urologic cancers, malignant mesothelioma, among others.17 It demonstrated that a higher LDH concentration is associated with worse survival in patients with lung cancer.17 Serum LDH is an enzyme that catalyzes the reaction between lactic acid and pyruvic acid that typically takes place in anaerobic conditions.17 LDH levels are elevated in malignancies because tumors have an anaerobic environment. Elevated LDH levels correlate with the anaerobic metabolism in the tumor. Other studies also have noted that patients with high metastatic score have higher LDH levels.17 Therefore, LDH may reflect tumor extension.
In addition, other techniques, such as somatostatin- receptor imaging are specifically beneficial in tumors that express the somatostatin receptor.16 For this reason, this type of study is typically indicated in patients with known metastasis, not in patients with low-grade tumors. Abdominal CT scans are done because the liver is a common site for metastasis.
Our case report demonstrates how biomarkers help diagnose these potentially aggressive and life-threatening tumors that may present as a common condition such as a pleural effusion. Using a less invasive and quicker approach with thoracentesis rather than with lung biopsies is a diagnostic tool in this entity. IHC in cell blocks is a reasonable diagnostic method especially in patients in whom performing a lung biopsy is difficult.
Conclusions
The presence of a symptomatic and recurrent unilateral pleural effusion must urge physicians to consider thoracentesis with mindful use of biomarkers not only for therapeutic purposes, but also for diagnosis of a variety of etiologies, both benign and malignant.
1. Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991-1002. doi: 10.1016/j.neo.2017.09.002
2. Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12(3):425-436. doi: 10.1016/j.jtho.2016.11.2222
3. Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers (Basel). 2012;4(3):777-798. doi: 10.3390/cancers4030777
4. Mandegaran R, David S, Screaton N. Cardiothoracic manifestations of neuroendocrine tumours. Br J Radiol. 2016;89(1060). doi: 10.1259/bjr.20150787
5. Caplin ME, Baudin E, Ferolla P, et al; ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604-1620. doi: 10.1093/annonc/mdv041
6. Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6(5):513-529. doi: 10.21037/tlcr.2017.09.04
7. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394.
8. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(pt 1):103-109. doi: 10.1016/j.semcancer.2017.11.019
9. Kaur G, Nijhawan R, Gupta N, Singh N, Rajwanshi A. Pleural fluid cytology samples in cases of suspected lung cancer: an experience from a tertiary care centre. Diagn Cytopathol. 2017;45(3):195-201.
10. Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis. 2018;12. doi: 10.1177/1753466618808660
11. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. doi: 10.1016/j.anndiagpath.2017.04.005
12. Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125-143. doi: 10.1007/978-94-017-7215-0_9
13. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394
14. Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62(suppl 1):33-38.
15. Melosky B. Low grade neuroendocrine tumors of the lung. Front Oncol. 2017;7:119. doi: 10.3389/fonc.2017.00119
16. Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2001;113(1):5-21. https://doi.org/10.1002/cncr.23542
17. Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (Baltimore). 2018;97(38):e12524
1. Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991-1002. doi: 10.1016/j.neo.2017.09.002
2. Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12(3):425-436. doi: 10.1016/j.jtho.2016.11.2222
3. Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers (Basel). 2012;4(3):777-798. doi: 10.3390/cancers4030777
4. Mandegaran R, David S, Screaton N. Cardiothoracic manifestations of neuroendocrine tumours. Br J Radiol. 2016;89(1060). doi: 10.1259/bjr.20150787
5. Caplin ME, Baudin E, Ferolla P, et al; ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26(8):1604-1620. doi: 10.1093/annonc/mdv041
6. Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6(5):513-529. doi: 10.21037/tlcr.2017.09.04
7. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394.
8. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(pt 1):103-109. doi: 10.1016/j.semcancer.2017.11.019
9. Kaur G, Nijhawan R, Gupta N, Singh N, Rajwanshi A. Pleural fluid cytology samples in cases of suspected lung cancer: an experience from a tertiary care centre. Diagn Cytopathol. 2017;45(3):195-201.
10. Porcel JM. Biomarkers in the diagnosis of pleural diseases: a 2018 update. Ther Adv Respir Dis. 2018;12. doi: 10.1177/1753466618808660
11. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. doi: 10.1016/j.anndiagpath.2017.04.005
12. Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125-143. doi: 10.1007/978-94-017-7215-0_9
13. Rossi G, Bertero L, Marchiò C, Papotti M. Molecular alterations of neuroendocrine tumours of the lung. Histopathology. 2018;72(1):142-152. doi: 10.1111/his.13394
14. Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion. 2000;62(suppl 1):33-38.
15. Melosky B. Low grade neuroendocrine tumors of the lung. Front Oncol. 2017;7:119. doi: 10.3389/fonc.2017.00119
16. Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2001;113(1):5-21. https://doi.org/10.1002/cncr.23542
17. Deng T, Zhang J, Meng Y, Zhou Y, Li W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine (Baltimore). 2018;97(38):e12524
American Cancer Society update: ‘It is best not to drink alcohol’
In its updated cancer prevention guidelines, the American Cancer Society now recommends that “it is best not to drink alcohol.”
Previously, ACS suggested that, for those who consume alcoholic beverages, intake should be no more than one drink per day for women or two per day for men. That recommendation is still in place, but is now accompanied by this new, stronger directive.
The revised guidelines also place more emphasis on reducing the consumption of processed and red meat and highly processed foods, and on increasing physical activity.
But importantly, there is also a call for action from public, private, and community organizations to work to together to increase access to affordable, nutritious foods and physical activity.
“Making healthy choices can be challenging for many, and there are strategies included in the guidelines that communities can undertake to help reduce barriers to eating well and physical activity,” said Laura Makaroff, DO, American Cancer Society senior vice president. “Individual choice is an important part of a healthy lifestyle, but having the right policies and environmental factors to break down these barriers is also important, and that is something that clinicians can support.”
The guidelines were published in CA: A Cancer Journal for Clinicians.
The link between cancer and lifestyle factors has long been established, and for the past 4 decades, both government and leading nonprofit health organizations, including the ACS and the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR), have released cancer prevention guidelines and recommendations that focus on managing weight, diet, physical activity, and alcohol consumption.
In 2012, the ACS issued guidelines on diet and physical activity, and their current guideline is largely based on the WCRF/AICR systematic reviews and Continuous Update Project reports, which were last updated in 2018. The ACS guidelines also incorporated systematic reviews conducted by the International Agency on Cancer Research (IARC) and the U.S. Department of Agriculture and the Department of Health and Human Services (USDA/HHS) and other analyses that were published since the WCRF/AICR recommendations were released.
Emphasis on three areas
The differences between the old guidelines and the update do not differ dramatically, but Makaroff highlighted a few areas that have increased emphasis.
Time spent being physically active is critical. The recommendation has changed to encourage adults to engage in 150-300 minutes (2.5-5 hours) of moderate-intensity physical activity, or 75-150 minutes (1.25-2.5 hours) of vigorous-intensity physical activity, or an equivalent combination, per week. Achieving or exceeding the upper limit of 300 minutes is optimal.
“That is more than what we have recommended in the past, along with the continued message that children and adolescents engage in at least 1 hour of moderate- or vigorous-intensity activity each day,” she told Medscape Medical News.
The ACS has also increased emphasis on reducing the consumption of processed and red meat. “This is part of a healthy eating pattern and making sure that people are eating food that is high in nutrients that help achieve and maintain a healthy body weight,” said Makaroff.
A healthy diet should include a variety of dark green, red, and orange vegetables; fiber-rich legumes; and fruits with a variety of colors and whole grains, according to the guidelines. Sugar-sweetened beverages, highly processed foods, and refined grain products should be limited or avoided.
The revised dietary recommendations reflect a shift from a “reductionist or nutrient-centric” approach to one that is more “holistic” and that focuses on dietary patterns. In contrast to a focus on individual nutrients and bioactive compounds, the new approach is more consistent with what and how people actually eat, ACS points out.
The third area that Makaroff highlighted is alcohol, where the recommendation is to avoid or limit consumption. “The current update says not to drink alcohol, which is in line with the scientific evidence, but for those people who choose to drink alcohol, to limit it to one drink per day for women and two drinks per day for men.”
Thus, the change here is that the previous guideline only recommended limiting alcohol consumption, while the update suggests that, optimally, it should be avoided completely.
The ACS has also called for community involvement to help implement these goals: “Public, private, and community organizations should work collaboratively at national, state, and local levels to develop, advocate for, and implement policy and environmental changes that increase access to affordable, nutritious foods; provide safe, enjoyable, and accessible opportunities for physical activity; and limit alcohol for all individuals.”
No smoking guns
Commenting on the guidelines, Steven K. Clinton, MD, PhD, associate director of the Center for Advanced Functional Foods Research and Entrepreneurship at the Ohio State University, Columbus, explained that he didn’t view the change in alcohol as that much of an evolution. “It’s been 8 years since they revised their overall guidelines, and during that time frame, there has been an enormous growth in the evidence that has been used by many organizations,” he said.
Clinton noted that the guidelines are consistent with the whole body of current scientific literature. “It’s very easy to go to the document and look for the ‘smoking gun’ – but the smoking gun is really not one thing,” he said. “It’s a pattern, and what dietitians and nutritionists are telling people is that you need to orchestrate a healthy lifestyle and diet, with a diet that has a foundation of fruits, vegetables, whole grains, and modest intake of refined grains and meat. You are orchestrating an entire pattern to get the maximum benefit.”
Makaroff is an employee of the ACS. Clinton has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
In its updated cancer prevention guidelines, the American Cancer Society now recommends that “it is best not to drink alcohol.”
Previously, ACS suggested that, for those who consume alcoholic beverages, intake should be no more than one drink per day for women or two per day for men. That recommendation is still in place, but is now accompanied by this new, stronger directive.
The revised guidelines also place more emphasis on reducing the consumption of processed and red meat and highly processed foods, and on increasing physical activity.
But importantly, there is also a call for action from public, private, and community organizations to work to together to increase access to affordable, nutritious foods and physical activity.
“Making healthy choices can be challenging for many, and there are strategies included in the guidelines that communities can undertake to help reduce barriers to eating well and physical activity,” said Laura Makaroff, DO, American Cancer Society senior vice president. “Individual choice is an important part of a healthy lifestyle, but having the right policies and environmental factors to break down these barriers is also important, and that is something that clinicians can support.”
The guidelines were published in CA: A Cancer Journal for Clinicians.
The link between cancer and lifestyle factors has long been established, and for the past 4 decades, both government and leading nonprofit health organizations, including the ACS and the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR), have released cancer prevention guidelines and recommendations that focus on managing weight, diet, physical activity, and alcohol consumption.
In 2012, the ACS issued guidelines on diet and physical activity, and their current guideline is largely based on the WCRF/AICR systematic reviews and Continuous Update Project reports, which were last updated in 2018. The ACS guidelines also incorporated systematic reviews conducted by the International Agency on Cancer Research (IARC) and the U.S. Department of Agriculture and the Department of Health and Human Services (USDA/HHS) and other analyses that were published since the WCRF/AICR recommendations were released.
Emphasis on three areas
The differences between the old guidelines and the update do not differ dramatically, but Makaroff highlighted a few areas that have increased emphasis.
Time spent being physically active is critical. The recommendation has changed to encourage adults to engage in 150-300 minutes (2.5-5 hours) of moderate-intensity physical activity, or 75-150 minutes (1.25-2.5 hours) of vigorous-intensity physical activity, or an equivalent combination, per week. Achieving or exceeding the upper limit of 300 minutes is optimal.
“That is more than what we have recommended in the past, along with the continued message that children and adolescents engage in at least 1 hour of moderate- or vigorous-intensity activity each day,” she told Medscape Medical News.
The ACS has also increased emphasis on reducing the consumption of processed and red meat. “This is part of a healthy eating pattern and making sure that people are eating food that is high in nutrients that help achieve and maintain a healthy body weight,” said Makaroff.
A healthy diet should include a variety of dark green, red, and orange vegetables; fiber-rich legumes; and fruits with a variety of colors and whole grains, according to the guidelines. Sugar-sweetened beverages, highly processed foods, and refined grain products should be limited or avoided.
The revised dietary recommendations reflect a shift from a “reductionist or nutrient-centric” approach to one that is more “holistic” and that focuses on dietary patterns. In contrast to a focus on individual nutrients and bioactive compounds, the new approach is more consistent with what and how people actually eat, ACS points out.
The third area that Makaroff highlighted is alcohol, where the recommendation is to avoid or limit consumption. “The current update says not to drink alcohol, which is in line with the scientific evidence, but for those people who choose to drink alcohol, to limit it to one drink per day for women and two drinks per day for men.”
Thus, the change here is that the previous guideline only recommended limiting alcohol consumption, while the update suggests that, optimally, it should be avoided completely.
The ACS has also called for community involvement to help implement these goals: “Public, private, and community organizations should work collaboratively at national, state, and local levels to develop, advocate for, and implement policy and environmental changes that increase access to affordable, nutritious foods; provide safe, enjoyable, and accessible opportunities for physical activity; and limit alcohol for all individuals.”
No smoking guns
Commenting on the guidelines, Steven K. Clinton, MD, PhD, associate director of the Center for Advanced Functional Foods Research and Entrepreneurship at the Ohio State University, Columbus, explained that he didn’t view the change in alcohol as that much of an evolution. “It’s been 8 years since they revised their overall guidelines, and during that time frame, there has been an enormous growth in the evidence that has been used by many organizations,” he said.
Clinton noted that the guidelines are consistent with the whole body of current scientific literature. “It’s very easy to go to the document and look for the ‘smoking gun’ – but the smoking gun is really not one thing,” he said. “It’s a pattern, and what dietitians and nutritionists are telling people is that you need to orchestrate a healthy lifestyle and diet, with a diet that has a foundation of fruits, vegetables, whole grains, and modest intake of refined grains and meat. You are orchestrating an entire pattern to get the maximum benefit.”
Makaroff is an employee of the ACS. Clinton has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
In its updated cancer prevention guidelines, the American Cancer Society now recommends that “it is best not to drink alcohol.”
Previously, ACS suggested that, for those who consume alcoholic beverages, intake should be no more than one drink per day for women or two per day for men. That recommendation is still in place, but is now accompanied by this new, stronger directive.
The revised guidelines also place more emphasis on reducing the consumption of processed and red meat and highly processed foods, and on increasing physical activity.
But importantly, there is also a call for action from public, private, and community organizations to work to together to increase access to affordable, nutritious foods and physical activity.
“Making healthy choices can be challenging for many, and there are strategies included in the guidelines that communities can undertake to help reduce barriers to eating well and physical activity,” said Laura Makaroff, DO, American Cancer Society senior vice president. “Individual choice is an important part of a healthy lifestyle, but having the right policies and environmental factors to break down these barriers is also important, and that is something that clinicians can support.”
The guidelines were published in CA: A Cancer Journal for Clinicians.
The link between cancer and lifestyle factors has long been established, and for the past 4 decades, both government and leading nonprofit health organizations, including the ACS and the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR), have released cancer prevention guidelines and recommendations that focus on managing weight, diet, physical activity, and alcohol consumption.
In 2012, the ACS issued guidelines on diet and physical activity, and their current guideline is largely based on the WCRF/AICR systematic reviews and Continuous Update Project reports, which were last updated in 2018. The ACS guidelines also incorporated systematic reviews conducted by the International Agency on Cancer Research (IARC) and the U.S. Department of Agriculture and the Department of Health and Human Services (USDA/HHS) and other analyses that were published since the WCRF/AICR recommendations were released.
Emphasis on three areas
The differences between the old guidelines and the update do not differ dramatically, but Makaroff highlighted a few areas that have increased emphasis.
Time spent being physically active is critical. The recommendation has changed to encourage adults to engage in 150-300 minutes (2.5-5 hours) of moderate-intensity physical activity, or 75-150 minutes (1.25-2.5 hours) of vigorous-intensity physical activity, or an equivalent combination, per week. Achieving or exceeding the upper limit of 300 minutes is optimal.
“That is more than what we have recommended in the past, along with the continued message that children and adolescents engage in at least 1 hour of moderate- or vigorous-intensity activity each day,” she told Medscape Medical News.
The ACS has also increased emphasis on reducing the consumption of processed and red meat. “This is part of a healthy eating pattern and making sure that people are eating food that is high in nutrients that help achieve and maintain a healthy body weight,” said Makaroff.
A healthy diet should include a variety of dark green, red, and orange vegetables; fiber-rich legumes; and fruits with a variety of colors and whole grains, according to the guidelines. Sugar-sweetened beverages, highly processed foods, and refined grain products should be limited or avoided.
The revised dietary recommendations reflect a shift from a “reductionist or nutrient-centric” approach to one that is more “holistic” and that focuses on dietary patterns. In contrast to a focus on individual nutrients and bioactive compounds, the new approach is more consistent with what and how people actually eat, ACS points out.
The third area that Makaroff highlighted is alcohol, where the recommendation is to avoid or limit consumption. “The current update says not to drink alcohol, which is in line with the scientific evidence, but for those people who choose to drink alcohol, to limit it to one drink per day for women and two drinks per day for men.”
Thus, the change here is that the previous guideline only recommended limiting alcohol consumption, while the update suggests that, optimally, it should be avoided completely.
The ACS has also called for community involvement to help implement these goals: “Public, private, and community organizations should work collaboratively at national, state, and local levels to develop, advocate for, and implement policy and environmental changes that increase access to affordable, nutritious foods; provide safe, enjoyable, and accessible opportunities for physical activity; and limit alcohol for all individuals.”
No smoking guns
Commenting on the guidelines, Steven K. Clinton, MD, PhD, associate director of the Center for Advanced Functional Foods Research and Entrepreneurship at the Ohio State University, Columbus, explained that he didn’t view the change in alcohol as that much of an evolution. “It’s been 8 years since they revised their overall guidelines, and during that time frame, there has been an enormous growth in the evidence that has been used by many organizations,” he said.
Clinton noted that the guidelines are consistent with the whole body of current scientific literature. “It’s very easy to go to the document and look for the ‘smoking gun’ – but the smoking gun is really not one thing,” he said. “It’s a pattern, and what dietitians and nutritionists are telling people is that you need to orchestrate a healthy lifestyle and diet, with a diet that has a foundation of fruits, vegetables, whole grains, and modest intake of refined grains and meat. You are orchestrating an entire pattern to get the maximum benefit.”
Makaroff is an employee of the ACS. Clinton has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Can an app guide cancer treatment decisions during the pandemic?
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.
Deciding which cancer patients need immediate treatment and who can safely wait is an uncomfortable assessment for cancer clinicians during the COVID-19 pandemic.
In early April, as the COVID-19 surge was bearing down on New York City, those treatment decisions were “a juggling act every single day,” Jonathan Yang, MD, PhD, a radiation oncologist from New York’s Memorial Sloan Kettering Cancer Center, told Medscape Medical News.
Eventually, a glut of guidelines, recommendations, and expert opinions aimed at helping oncologists emerged. The tools help navigate the complicated risk-benefit analysis of their patient’s risk of infection by SARS-CoV-2 and delaying therapy.
Now, a new tool, which appears to be the first of its kind, quantifies that risk-benefit analysis. But its presence immediately raises the question: can it help?
Three-Tier Systems Are Not Very Sophisticated
OncCOVID, a free tool that was launched May 26 by the University of Michigan, allows physicians to individualize risk estimates for delaying treatment of up to 25 early- to late-stage cancers. It includes more than 45 patient characteristics, such as age, location, cancer type, cancer stage, treatment plan, underlying medical conditions, and proposed length of delay in care.
Combining these personal details with data from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registry and the National Cancer Database, the Michigan app then estimates a patient’s 5- or 10-year survival with immediate vs delayed treatment and weighs that against their risk for COVID-19 using data from the Johns Hopkins Coronavirus Resource Center.
“We thought, isn’t it better to at least provide some evidence-based quantification, rather than a back-of-the-envelope three-tier system that is just sort of ‘made up’?“ explained one of the developers, Daniel Spratt, MD, associate professor of radiation oncology at Michigan Medicine.
Spratt explained that almost every organization, professional society, and government has created something like a three-tier system. Tier 1 represents urgent cases and patients who need immediate treatment. For tier 2, treatment can be delayed weeks or a month, and with tier 3, it can be delayed until the pandemic is over or it’s deemed safe.
“[This system] sounds good at first glance, but in cancer, we’re always talking about personalized medicine, and it’s mind-blowing that these tier systems are only based on urgency and prognosis,” he told Medscape Medical News.
Spratt offered an example. Consider a patient with a very aggressive brain tumor ― that patient is in tier 1 and should undergo treatment immediately. But will the treatment actually help? And how helpful would the procedure be if, say, the patient is 80 years old and, if infected, would have a 30% to 50% chance of dying from the coronavirus?
“If the model says this guy has a 5% harm and this one has 30% harm, you can use that to help prioritize,” summarized Spratt.
The app can generate risk estimates for patients living anywhere in the world and has already been accessed by people from 37 countries. However, Spratt cautions that it is primarily “designed and calibrated for the US.
“The estimates are based on very large US registries, and though it’s probably somewhat similar across much of the world, there’s probably certain cancer types that are more region specific ― especially something like stomach cancer or certain types of head and neck cancer in parts of Asia, for example,” he said.
Although the app’s COVID-19 data are specific to the county level in the United States, elsewhere in the world, it is only country specific.
“We’re using the best data we have for coronavirus, but everyone knows we still have large data gaps,” he acknowledged.
How Accurate?
Asked to comment on the app, Richard Bleicher, MD, leader of the Breast Cancer Program at Fox Chase Cancer Center, Philadelphia, praised the effort and the goal but had some concerns.
“Several questions arise, most important of which is, How accurate is this, and how has this been validated, if at all ― especially as it is too soon to see the outcomes of patients affected in this pandemic?” he told Medscape Medical News.
“We are imposing delays on a broad scale because of the coronavirus, and we are getting continuously changing data as we test more patients. But both situations are novel and may not be accurately represented by the data being pulled, because the datasets use patients from a few years ago, and confounders in these datasets may not apply to this situation,” Bleicher continued.
Although acknowledging the “value in delineating the risk of dying from cancer vs the risk of dying from the SARS-CoV-2 pandemic,” Bleicher urged caution in using the tool to make individual patient decisions.
“We need to remember that the best of modeling ... can be wildly inaccurate and needs to be validated using patients having the circumstances in question. ... This won’t be possible until long after the pandemic is completed, and so the model’s accuracy remains unknown.”
That sentiment was echoed by Giampaolo Bianchini, MD, head of the Breast Cancer Group, Department of Medical Oncology, Ospedale San Raffaele, in Milan, Italy.
“Arbitrarily postponing and modifying treatment strategies including surgery, radiation therapy, and medical therapy without properly balancing the risk/benefit ratio may lead to significantly worse cancer-related outcomes, which largely exceed the actual risks for COVID,” he wrote in an email.
“The OncCOVID app is a remarkable attempt to fill the gap between perception and estimation,” he said. The app provides side by side the COVID-19 risk estimation and the consequences of arbitrary deviation from the standard of care, observed Bianchini.
However, he pointed out weaknesses, including the fact that the “data generated in literature are not always of high quality and do not take into consideration relevant characteristics of the disease and treatment benefit. It should for sure be used, but then also interpreted with caution.”
Another Italian group responded more positively.
“In our opinion, it could be a useful tool for clinicians,” wrote colleagues Alessio Cortelinni and Giampiero Porzio, both medical oncologists at San Salvatore Hospital and the University of L’Aquila, in Italy. “This Web app might assist clinicians in balancing the risk/benefit ratio of being treated and/or access to the outpatient cancer center for each kind of patient (both early and advanced stages), in order to make a more tailored counseling,” they wrote in an email. “Importantly, the Web app might help those clinicians who work ‘alone,’ in peripheral centers, without resources, colleagues, and multidisciplinary tumor boards on whom they can rely.”
Bleicher, who was involved in the COVID-19 Breast Cancer Consortium’s recommendations for prioritizing breast cancer treatment, summarized that the app “may end up being close or accurate, but we won’t know except in hindsight.”
This article first appeared on Medscape.com.
Biologics may carry melanoma risk for patients with immune-mediated inflammatory diseases
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
The in a systematic review and meta-analysis published in JAMA Dermatology.
The studies included in the analysis, however, had limitations, including a lack of those comparing biologic and conventional systemic therapy in psoriasis and inflammatory bowel disease (IBD), according to Shamarke Esse, MRes, of the division of musculoskeletal and dermatological sciences at the University of Manchester (England) and colleagues. “We advocate for more large, well-designed studies of this issue to be performed to help improve certainty” regarding this association, they wrote.
Previous studies that have found an increased risk of melanoma in patients on biologics for psoriasis, rheumatoid arthritis, and IBD have “typically used the general population as the comparator,” they noted. There is a large amount of evidence that has established short-term efficacy and safety of biologics, compared with conventional systemic treatments, but concerns about longer-term cancer risk associated with biologics remains a concern. Moreover, they added, “melanoma is a highly immunogenic skin cancer and therefore of concern to patients treated with TNFIs [tumor necrosis factor inhibitors] because melanoma risk increases with suppression of the immune system and TNF-alpha plays an important role in the immune surveillance of tumors.12,13
In their review, the researchers identified seven cohort studies from MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases published between January 1995 and February 2019 that evaluated melanoma risk in about 34,000 patients receiving biologics and 135,370 patients who had never been treated with biologics, and were receiving conventional systemic therapy for psoriasis, RA, or IBD. Of these, four studies were in patients with RA, two studies were in patients with IBD, and a single study was in patients with psoriasis. Six studies examined patients taking TNF inhibitors, but only one of six studies had information on specific TNF inhibitors (adalimumab, etanercept, and infliximab) in patients with RA. One study evaluated abatacept and rituximab in RA patients.
The researchers analyzed the pooled relative risk across all studies. Compared with patients who received conventional systemic therapy, there was a nonsignificant association with risk of melanoma in patients with psoriasis (hazard ratio, 1.57; 95% confidence interval, 0.61-4.09), RA (pooled relative risk, 1.20; 95% CI, 0.83-1.74), and IBD (pRR, 1.20; 95% CI, 0.60-2.40).
Among RA patients who received TNF inhibitors only, there was a slightly elevated nonsignificant risk of melanoma (pRR, 1.08; 95% CI, 0.81-1.43). Patients receiving rituximab had a pRR of 0.73 (95% CI, 0.38-1.39), and patients taking abatacept had a pRR of 1.43 (95% CI, 0.66-3.09), compared with RA patients receiving conventional systemic therapy. When excluding two major studies in the RA subgroup of patients in a sensitivity analysis, pooled risk estimates varied from 0.91 (95% CI, 0.69-1.18) to 1.95 (95% CI, 1.16- 3.30). There were no significant between-study heterogeneity or publication bias among the IBD and RA studies.
Mr. Esse and colleagues acknowledged the small number of IBD and psoriasis studies in the meta-analysis, which could affect pooled risk estimates. “Any future update of our study through the inclusion of newly published studies may produce significantly different pooled risk estimates than those reported in our meta-analysis,” they said. In addition, the use of health insurance databases, lack of risk factors for melanoma, and inconsistent information about treatment duration for patients receiving conventional systemic therapy were also limitations.
“Prospective cohort studies using an active comparator, new-user study design providing detailed information on treatment history, concomitant treatments, biologic and conventional systemic treatment duration, recreational and treatment-related UV exposure, skin color, and date of melanoma diagnosis are required to help improve certainty. These studies would also need to account for key risk factors and the latency period of melanoma,” the researchers said.
Mr. Esse disclosed being funded by a PhD studentship from the Psoriasis Association. One author disclosed receiving personal fees from Janssen, LEO Pharma, Lilly, and Novartis outside the study; another disclosed receiving grants and personal fees from those and several other pharmaceutical companies during the study, and personal fees from several pharmaceutical companies outside of the submitted work; the fourth author had no disclosures.
SOURCE: Esse S et al. JAMA Dermatol. 2020 May 20;e201300.
FROM JAMA DERMATOLOGY
Risk index stratifies pediatric leukemia patients undergoing HSCT
A disease risk index is now available for pediatric patients with acute myeloid leukemia or acute lymphoblastic leukemia who undergo allogeneic hematopoietic stem cell transplantation.
The model, which was developed and validated using data from more than 2,000 patients, stratifies probabilities of leukemia-free survival (LFS) into four risk groups for acute myeloid leukemia (AML) and three risk groups for acute lymphoblastic leukemia (ALL), reported lead author Muna Qayed, MD, of Emory University, Atlanta, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“The outcome of stem cell transplantation for hematologic malignancy is influenced by disease type, cytogenetics, and disease status at transplantation,” Dr. Qayed said. “In adults, these attributes were used to develop the disease risk index, or DRI, that can stratify patients for overall survival for purposes such as prognostication or clinical trial entry.”
But no such model exists for pediatric patients, Dr. Qayed said, noting that the adult DRI was found to be inaccurate when applied to children.
“[T]he [adult] DRI did not differentiate [pediatric] patients by overall survival,” Dr. Qayed said. “Therefore, knowing that pediatric AML and ALL differ biologically from adult leukemia, and further, treatment strategies differ between adults and children, we aimed to develop a pediatric-specific DRI.”
This involved analysis of data from 1,135 children with AML and 1,228 children with ALL who underwent transplantation between 2008 and 2017. All patients had myeloablative conditioning, and 75% received an unrelated donor graft. Haploidentical transplants were excluded because of small sample size.
Analyses were conducted in AML and ALL cohorts, with patients in each population randomized to training and validation subgroups in a 1:1 ratio. The primary outcome was LFS. Cox regression models were used to identify significant characteristics, which were then integrated into a prognostic scoring system for the training groups. These scoring systems were then tested in the validation subgroups. Maximum likelihood was used to identify age cutoffs, which were 3 years for AML and 2 years for ALL.
In both cohorts, disease status at transplantation was characterized by complete remission and minimal residual disease status.
In the AML cohort, approximately one-third of patients were in first complete remission with negative minimal residual disease. Risk was stratified into four groups, including good, intermediate, high, and very high risk, with respective 5-year LFS probabilities of 81%, 56%, 44%, and 21%. Independent predictors of poorer outcome included unfavorable cytogenetics, first or second complete remission with minimal residual disease positivity, relapse at transplantation, and age less than 3 years.
In the ALL cohort, risk was stratified into three risk tiers: good, intermediate, and high, with 5-year LFS probabilities of 68%, 50%, and 15%, respectively. Independent predictors of poorer outcome included age less than 2 years, relapse at transplantation, and second complete remission regardless of minimal residual disease status.
The models for each disease also predicted overall survival.
For AML, hazard ratios, ascending from good to very-high-risk tiers, were 1.00, 3.52, 4.67, and 8.62. For ALL risk tiers, ascending hazard ratios were 1.00, 2.16, and 3.86.
“In summary, the pediatric disease risk index validated for leukemia-free survival and overall survival successfully stratifies children with acute leukemia at the time of transplantation,” Dr. Qayed said.
She concluded her presentation by highlighting the practicality and relevance of the new scoring system.
“The components included in the scoring system used information that is readily available pretransplantation, lending support to the deliverability of the prognostic scoring system,” Dr. Qayed said. “It can further be used for improved interpretation of multicenter data and in clinical trials for risk stratification.”
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., first emphasized the clinical importance of an accurate disease risk index for pediatric patients.
“When going into transplant, the No. 1 question that all parents will ask is: ‘Will my child be cured?’ ” she said.
According to Dr. Shah, the risk model developed by Dr. Qayed and colleagues is built on a strong foundation, including adequate sample size, comprehensive disease characterization, exclusion of patients that did not undergo myeloablative conditioning, and use of minimal residual disease status.
Still, more work is needed, Dr. Shah said.
“This DRI will need to be prospectively tested and compared to other established risk factors. For instance, minimal residual disease alone can be further stratified and has a significant role in establishing risk for posttransplant relapse. And the development of acute graft-versus-host disease also plays an important role in posttransplant relapse.”
Dr. Shah went on to outline potential areas of improvement.
“[F]uture directions for this study could include incorporation of early posttransplant events like graft-versus-host disease, potential stratification of the minimal residual disease results among those patients in complete remission, and potential application of this DRI to the adolescent and young adult population, which may have slight variation even from the adult DRI.”The study was funded by the National Institutes of Health. The investigators disclosed no conflicts of interest
SOURCE: Qayed M et al. ASCO 2020, Abstract 7503.
A disease risk index is now available for pediatric patients with acute myeloid leukemia or acute lymphoblastic leukemia who undergo allogeneic hematopoietic stem cell transplantation.
The model, which was developed and validated using data from more than 2,000 patients, stratifies probabilities of leukemia-free survival (LFS) into four risk groups for acute myeloid leukemia (AML) and three risk groups for acute lymphoblastic leukemia (ALL), reported lead author Muna Qayed, MD, of Emory University, Atlanta, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“The outcome of stem cell transplantation for hematologic malignancy is influenced by disease type, cytogenetics, and disease status at transplantation,” Dr. Qayed said. “In adults, these attributes were used to develop the disease risk index, or DRI, that can stratify patients for overall survival for purposes such as prognostication or clinical trial entry.”
But no such model exists for pediatric patients, Dr. Qayed said, noting that the adult DRI was found to be inaccurate when applied to children.
“[T]he [adult] DRI did not differentiate [pediatric] patients by overall survival,” Dr. Qayed said. “Therefore, knowing that pediatric AML and ALL differ biologically from adult leukemia, and further, treatment strategies differ between adults and children, we aimed to develop a pediatric-specific DRI.”
This involved analysis of data from 1,135 children with AML and 1,228 children with ALL who underwent transplantation between 2008 and 2017. All patients had myeloablative conditioning, and 75% received an unrelated donor graft. Haploidentical transplants were excluded because of small sample size.
Analyses were conducted in AML and ALL cohorts, with patients in each population randomized to training and validation subgroups in a 1:1 ratio. The primary outcome was LFS. Cox regression models were used to identify significant characteristics, which were then integrated into a prognostic scoring system for the training groups. These scoring systems were then tested in the validation subgroups. Maximum likelihood was used to identify age cutoffs, which were 3 years for AML and 2 years for ALL.
In both cohorts, disease status at transplantation was characterized by complete remission and minimal residual disease status.
In the AML cohort, approximately one-third of patients were in first complete remission with negative minimal residual disease. Risk was stratified into four groups, including good, intermediate, high, and very high risk, with respective 5-year LFS probabilities of 81%, 56%, 44%, and 21%. Independent predictors of poorer outcome included unfavorable cytogenetics, first or second complete remission with minimal residual disease positivity, relapse at transplantation, and age less than 3 years.
In the ALL cohort, risk was stratified into three risk tiers: good, intermediate, and high, with 5-year LFS probabilities of 68%, 50%, and 15%, respectively. Independent predictors of poorer outcome included age less than 2 years, relapse at transplantation, and second complete remission regardless of minimal residual disease status.
The models for each disease also predicted overall survival.
For AML, hazard ratios, ascending from good to very-high-risk tiers, were 1.00, 3.52, 4.67, and 8.62. For ALL risk tiers, ascending hazard ratios were 1.00, 2.16, and 3.86.
“In summary, the pediatric disease risk index validated for leukemia-free survival and overall survival successfully stratifies children with acute leukemia at the time of transplantation,” Dr. Qayed said.
She concluded her presentation by highlighting the practicality and relevance of the new scoring system.
“The components included in the scoring system used information that is readily available pretransplantation, lending support to the deliverability of the prognostic scoring system,” Dr. Qayed said. “It can further be used for improved interpretation of multicenter data and in clinical trials for risk stratification.”
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., first emphasized the clinical importance of an accurate disease risk index for pediatric patients.
“When going into transplant, the No. 1 question that all parents will ask is: ‘Will my child be cured?’ ” she said.
According to Dr. Shah, the risk model developed by Dr. Qayed and colleagues is built on a strong foundation, including adequate sample size, comprehensive disease characterization, exclusion of patients that did not undergo myeloablative conditioning, and use of minimal residual disease status.
Still, more work is needed, Dr. Shah said.
“This DRI will need to be prospectively tested and compared to other established risk factors. For instance, minimal residual disease alone can be further stratified and has a significant role in establishing risk for posttransplant relapse. And the development of acute graft-versus-host disease also plays an important role in posttransplant relapse.”
Dr. Shah went on to outline potential areas of improvement.
“[F]uture directions for this study could include incorporation of early posttransplant events like graft-versus-host disease, potential stratification of the minimal residual disease results among those patients in complete remission, and potential application of this DRI to the adolescent and young adult population, which may have slight variation even from the adult DRI.”The study was funded by the National Institutes of Health. The investigators disclosed no conflicts of interest
SOURCE: Qayed M et al. ASCO 2020, Abstract 7503.
A disease risk index is now available for pediatric patients with acute myeloid leukemia or acute lymphoblastic leukemia who undergo allogeneic hematopoietic stem cell transplantation.
The model, which was developed and validated using data from more than 2,000 patients, stratifies probabilities of leukemia-free survival (LFS) into four risk groups for acute myeloid leukemia (AML) and three risk groups for acute lymphoblastic leukemia (ALL), reported lead author Muna Qayed, MD, of Emory University, Atlanta, who presented findings as part of the American Society of Clinical Oncology virtual scientific program.
“The outcome of stem cell transplantation for hematologic malignancy is influenced by disease type, cytogenetics, and disease status at transplantation,” Dr. Qayed said. “In adults, these attributes were used to develop the disease risk index, or DRI, that can stratify patients for overall survival for purposes such as prognostication or clinical trial entry.”
But no such model exists for pediatric patients, Dr. Qayed said, noting that the adult DRI was found to be inaccurate when applied to children.
“[T]he [adult] DRI did not differentiate [pediatric] patients by overall survival,” Dr. Qayed said. “Therefore, knowing that pediatric AML and ALL differ biologically from adult leukemia, and further, treatment strategies differ between adults and children, we aimed to develop a pediatric-specific DRI.”
This involved analysis of data from 1,135 children with AML and 1,228 children with ALL who underwent transplantation between 2008 and 2017. All patients had myeloablative conditioning, and 75% received an unrelated donor graft. Haploidentical transplants were excluded because of small sample size.
Analyses were conducted in AML and ALL cohorts, with patients in each population randomized to training and validation subgroups in a 1:1 ratio. The primary outcome was LFS. Cox regression models were used to identify significant characteristics, which were then integrated into a prognostic scoring system for the training groups. These scoring systems were then tested in the validation subgroups. Maximum likelihood was used to identify age cutoffs, which were 3 years for AML and 2 years for ALL.
In both cohorts, disease status at transplantation was characterized by complete remission and minimal residual disease status.
In the AML cohort, approximately one-third of patients were in first complete remission with negative minimal residual disease. Risk was stratified into four groups, including good, intermediate, high, and very high risk, with respective 5-year LFS probabilities of 81%, 56%, 44%, and 21%. Independent predictors of poorer outcome included unfavorable cytogenetics, first or second complete remission with minimal residual disease positivity, relapse at transplantation, and age less than 3 years.
In the ALL cohort, risk was stratified into three risk tiers: good, intermediate, and high, with 5-year LFS probabilities of 68%, 50%, and 15%, respectively. Independent predictors of poorer outcome included age less than 2 years, relapse at transplantation, and second complete remission regardless of minimal residual disease status.
The models for each disease also predicted overall survival.
For AML, hazard ratios, ascending from good to very-high-risk tiers, were 1.00, 3.52, 4.67, and 8.62. For ALL risk tiers, ascending hazard ratios were 1.00, 2.16, and 3.86.
“In summary, the pediatric disease risk index validated for leukemia-free survival and overall survival successfully stratifies children with acute leukemia at the time of transplantation,” Dr. Qayed said.
She concluded her presentation by highlighting the practicality and relevance of the new scoring system.
“The components included in the scoring system used information that is readily available pretransplantation, lending support to the deliverability of the prognostic scoring system,” Dr. Qayed said. “It can further be used for improved interpretation of multicenter data and in clinical trials for risk stratification.”
In a virtual presentation, invited discussant Nirali N. Shah, MD, of the National Cancer Institute, Bethesda, Md., first emphasized the clinical importance of an accurate disease risk index for pediatric patients.
“When going into transplant, the No. 1 question that all parents will ask is: ‘Will my child be cured?’ ” she said.
According to Dr. Shah, the risk model developed by Dr. Qayed and colleagues is built on a strong foundation, including adequate sample size, comprehensive disease characterization, exclusion of patients that did not undergo myeloablative conditioning, and use of minimal residual disease status.
Still, more work is needed, Dr. Shah said.
“This DRI will need to be prospectively tested and compared to other established risk factors. For instance, minimal residual disease alone can be further stratified and has a significant role in establishing risk for posttransplant relapse. And the development of acute graft-versus-host disease also plays an important role in posttransplant relapse.”
Dr. Shah went on to outline potential areas of improvement.
“[F]uture directions for this study could include incorporation of early posttransplant events like graft-versus-host disease, potential stratification of the minimal residual disease results among those patients in complete remission, and potential application of this DRI to the adolescent and young adult population, which may have slight variation even from the adult DRI.”The study was funded by the National Institutes of Health. The investigators disclosed no conflicts of interest
SOURCE: Qayed M et al. ASCO 2020, Abstract 7503.
FROM ASCO 2020