User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


The robot comes to mastectomy, but cancer outcomes data not attached
The FDA warning was issued in February 2019 to both the public and physicians. The FDA cautioned that the safety and effectiveness of robotic surgical devices for mastectomy “have not been established” and robots are not approved for the prevention or treatment of breast cancer.
The agency also noted that “diminished long-term survival” was associated with robotic surgery in another women’s cancer, that of hysterectomy for cervical cancer.
The FDA also made a surprising statement. The agency typically approves the robot for surgical use based on 30-day complication rates (compared with standards of care). But it said that going forward it “anticipates” that any evaluation of new use of robots in cancer “would be supported” by cancer outcomes such as progression-free survival and overall survival, which require much longer follow-up.
In short, the FDA hinted that it would change how it regulated medical devices, or at least robots used in women’s cancers. “The FDA takes women’s health very seriously,” said the organization.
Fast forward to 2021, and there are several prospective clinical trials of robot-assisted nipple-sparing mastectomy underway in the United States, including a five-center study sponsored by Intuitive Surgical, the maker of da Vinci robots, the dominant machine on the market. There are also single-center studies at Ohio State and University of Texas Southwestern Medical Center.
However, in each case, the study design either excludes cancer outcomes or does not primarily focus on those measures.
Instead, the primary outcomes are relatively short term and include safety and efficacy measures such as en bloc (in one piece) removal of the breast tissue, conversions to open mastectomy, and the incidence of adverse events during surgery and up to 6 weeks after surgery.
Importantly, none of the studies is a randomized trial; all have single arms.
That’s not what is needed, says breast surgeon Julie A. Margenthaler, MD of Washington University in St. Louis.
“I firmly believe that robotic-assisted mastectomy should only be considered in the context of a well-designed, randomized trial evaluating patient selection, patient safety, surgical complications, and oncologic outcomes with a concomitant cost analysis,” Dr. Margenthaler wrote in an essay published last year in JAMA Surgery.
As with the FDA warning, she cites worse survival with commonly used minimally invasive radical hysterectomy for cervical cancer, saying it “is a stark reminder that the marketing of robotic surgery has its roots in cosmesis and convenience rather than oncologic outcomes.”
In addition, robotic surgery is prohibitively expensive, said Dr. Margenthaler. In fact, cost is her “main criticism regarding robotic-assisted mastectomy.” It costs an additional $6,000 for robot use per procedure, according to a study conducted at a center in Taiwan. “I simply cannot be convinced that this will ever achieve cost-effective or even cost-neutral status,” Dr. Margenthaler wrote.
Not looking at the right outcomes
“They’re not looking at the right outcomes,” said Hooman Noorchashm, MD, PhD, about the current trials in the United States. He is a former surgeon and faculty member at the University of Pennsylvania in Philadelphia, and is now a patient advocate after his wife, Amy Reed, MD, died of uterine cancer in 2017 following a laparoscopic hysterectomy performed with a power morcellator that resulted in the upstaging of an undetected gynecologic cancer.
“You have to look at oncologic outcomes and do randomized, noninferiority trials to demonstrate that those cancer outcomes are at least equivalent to standard of care,” he said in an interview.
The current U.S. trials are “totally inappropriate,” he said.
Are randomized trials forthcoming after this initial set of single-arm trials? This news organization reached out to Intuitive Surgical, maker of the market leader da Vinci robotic surgical equipment to find out.
“Any plans for use of da Vinci Xi surgical system in nipple-sparing mastectomy will be based on these [single-arm] study results as well as other data and evidence,” said a company spokesperson, who did not confirm use of a randomized trial.
What about the FDA? Will the agency change its current approach to approving robots in surgeries for women’s cancers and require – not just anticipate – cancer-related outcomes data? At press time, the FDA did not respond to a request for comment.
Not having a randomized trial with cancer outcomes in any eventual FDA review opens the door for robotic mastectomy to be cleared for use in some mastectomies with short-term, nononcologic data, said Dr. Noorchashm.
Safety concerns with robotic mastectomy
Proponents of robot-assisted nipple-sparing mastectomy, which is coupled with reconstruction to preserve the shape of both the breast and nipple-areola area, suggest that improved patient cosmesis is a significant advantage with the high-tech intervention, said Dr. Margenthaler.
That’s because most robotic mastectomies performed to date (almost exclusively in Europe and Asia) have employed a 3- to 5-cm vertical incision located behind the lateral breast fold, allowing the scar to be hidden under the patient’s arm.
But therein also lies a safety concern, she asserted.
The “oncologic integrity” of the specimen on extraction is in question in some cases, she wrote, because of “such a small opening.”
Dr. Noorchashm agreed: “It all comes down to trying to get a large specimen out of a small incision.”
Traditional open mastectomy optimally yields the en bloc removal of a tumor – in one whole piece – to avoid fragmenting the cancerous tissue and possibly leaving residual disease behind. These undesirable events are associated with a higher risk for recurrence and treatment failure, he explained.
Thus, there is a need for a randomized trial with longer-term oncologic outcomes that compares the new approach with traditional open mastectomy, argued both Dr. Margenthaler and Dr. Noorchashm.
In defense of single-arm trials
“Oncologic safety is what we are concerned about and what we would like to study,” said Ko Un (Clara) Park, MD, a breast surgeon at The Ohio State University in Columbus.
Dr. Park is leading a single-center, single-arm pilot study of robotic nipple-sparing mastectomy enrolling up to 20 women with early-stage breast cancer or inherited genetic risk factors (but no cancer diagnosis). The trial, sponsored by a Pelotonia Idea Grant and Ohio State, recently enrolled its first patient.
The study’s primary outcomes include the feasibility of removal of the breast tissue en bloc; however, none of the outcomes are classic oncologic metrics such as progression-free survival.
The en bloc removal outcome is in direct response to the FDA’s concerns about minimally invasive cancer surgeries in women, Dr. Park said in an interview. The pilot trial has an investigational device exemption (IDE) granted by the FDA.
“The reason why we can’t just open a randomized controlled study (of robot versus open) and measure oncologic outcomes like recurrence-free survival is because, before we get to that point, we have to make sure” basic safety issues are addressed and established, she explained.
But Dr. Noorchashm said that argument is missing the larger, more important point: “They are still doing an oncologic procedure – you are still obliged to do noninferiority [randomized] testing with respect to cancer outcomes.”
Dr. Park sounded a different note: “We are doing it as safely as we can do it.”
Prophylactic use is also a cancer surgery
Intuitive’s five-center trial does not include en bloc removal of the breast gland as a primary outcome. Instead, the two primary outcomes are conversions to open mastectomy (efficacy measure) and the incidence of adverse events during surgery to 42 days after surgery (safety measure).
The company’s trial does not include any women with breast cancer, but is limited to women at increased risk for breast cancer and seeking prophylactic nipple-sparing mastectomy surgery.
Enrollment in the 145-patient single-arm trial began in the last few months and has a primary completion date of December 2022. It also has an IDE from the FDA.
“I do think that things like this need to be done with caution,” said Katherine Kopkash, MD, an investigator in the Intuitive trial and a breast surgeon at NorthShore University HealthSystem in Evanston, Ill., referring to the trial’s FDA exemption.
Dr. Kopkash said in an interview that the researchers in the multisite, single-arm Intuitive trial will also track oncologic outcomes, but the trial description at clinicaltrials.gov does not indicate that.
Both Dr. Kopkash and Dr. Park cited the high-profile missteps that took place in 2018 at Monmouth County Medical Center in Long Branch, N.J., during what was described as the first-ever use of robotic nipple-sparing mastectomy for invasive cancer in the United States, as reported by Medscape Medical News. However, neither the center or surgeon, Stephen Chagares, MD, requested or received an IDE from the FDA, and use of robotic mastectomy was halted after two cases.
It’s conceivable that Intuitive will seek out FDA clearance for use of its da Vinci system in robotic nipple-sparing mastectomy with data in a prophylactic setting and then expand the pool of patients, argued Dr. Noorchashm.
“Even if you introduce a new technology ... for a narrow subset of patients, the application of it eventually occurs on a ‘sliding scale,’ ” he said.
The former surgeon gave an example: The first device used in gastric bypass surgery was cleared for use in 2001 by the FDA for adults who were “severely morbidly obese.” But by the late 2000s, the operation was also being performed on people with lower body mass indexes who hadn’t exhausted traditional weight loss procedures. “It was very lucrative,” Dr. Noorchashm said about the surgery.
Surgeons only get one body
Intuitive has been hugely successful in developing and marketing its da Vinci system around the world for general and oncologic surgeries, with more than 1 million surgeries in 2018, a greater than sevenfold increase in 10 years, according to the authors of a new essay published in the June issue of the Annals of Surgery. The authors include breast surgeon Rosa F. Hwang, MD, of MD Anderson Cancer Center in Houston, who is also an investigator for the Intuitive trial.
However, robotic mastectomy is still a new surgery – only about 150 patients have been treated in the world, mostly in Italy, France, Taiwan, and Korea, the authors noted.
Despite such small numbers, “there’s a lot of interest in bringing this to the United States,” said Dr. Park.
One of the arguments in favor of robotic mastectomy for nipple-sparing procedures has nothing to do with patients. Instead, it is improved ergonomics – the robot makes a tough surgery easier on the surgeon.
Even stalwart robot critic Dr. Margenthaler conceded that this was possibly a winning feature.
“Nipple-sparing mastectomy is a very physically demanding procedure for the surgeon, resulting in higher rates of neck and back pain and fatigue compared with a standard skin-sparing approach,” she noted. She suggested, however, that practitioners of traditional mastectomy ought to first experiment with changes to patient positioning and incision placement to alleviate stress before looking to the robot for change.
When this news organization interviewed NorthShore University’s Dr. Kopkash, she had conducted four nipple-sparing mastectomies in the previous week. “It’s a difficult procedure on our bodies. I just turned 40 and I’m considered young for a surgeon. We get one body for our career and we have to figure out ways to make it work and protect it.”
Intuitive Surgical is funding the five-center clinical trial of robot-assisted nipple-sparing mastectomy, and UT Southwestern is funding its own trial. The Ohio State trial is funded by the university and a Pelotonia Idea Grant. Dr. Noorchashm and Dr. Margenthaler have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
The FDA warning was issued in February 2019 to both the public and physicians. The FDA cautioned that the safety and effectiveness of robotic surgical devices for mastectomy “have not been established” and robots are not approved for the prevention or treatment of breast cancer.
The agency also noted that “diminished long-term survival” was associated with robotic surgery in another women’s cancer, that of hysterectomy for cervical cancer.
The FDA also made a surprising statement. The agency typically approves the robot for surgical use based on 30-day complication rates (compared with standards of care). But it said that going forward it “anticipates” that any evaluation of new use of robots in cancer “would be supported” by cancer outcomes such as progression-free survival and overall survival, which require much longer follow-up.
In short, the FDA hinted that it would change how it regulated medical devices, or at least robots used in women’s cancers. “The FDA takes women’s health very seriously,” said the organization.
Fast forward to 2021, and there are several prospective clinical trials of robot-assisted nipple-sparing mastectomy underway in the United States, including a five-center study sponsored by Intuitive Surgical, the maker of da Vinci robots, the dominant machine on the market. There are also single-center studies at Ohio State and University of Texas Southwestern Medical Center.
However, in each case, the study design either excludes cancer outcomes or does not primarily focus on those measures.
Instead, the primary outcomes are relatively short term and include safety and efficacy measures such as en bloc (in one piece) removal of the breast tissue, conversions to open mastectomy, and the incidence of adverse events during surgery and up to 6 weeks after surgery.
Importantly, none of the studies is a randomized trial; all have single arms.
That’s not what is needed, says breast surgeon Julie A. Margenthaler, MD of Washington University in St. Louis.
“I firmly believe that robotic-assisted mastectomy should only be considered in the context of a well-designed, randomized trial evaluating patient selection, patient safety, surgical complications, and oncologic outcomes with a concomitant cost analysis,” Dr. Margenthaler wrote in an essay published last year in JAMA Surgery.
As with the FDA warning, she cites worse survival with commonly used minimally invasive radical hysterectomy for cervical cancer, saying it “is a stark reminder that the marketing of robotic surgery has its roots in cosmesis and convenience rather than oncologic outcomes.”
In addition, robotic surgery is prohibitively expensive, said Dr. Margenthaler. In fact, cost is her “main criticism regarding robotic-assisted mastectomy.” It costs an additional $6,000 for robot use per procedure, according to a study conducted at a center in Taiwan. “I simply cannot be convinced that this will ever achieve cost-effective or even cost-neutral status,” Dr. Margenthaler wrote.
Not looking at the right outcomes
“They’re not looking at the right outcomes,” said Hooman Noorchashm, MD, PhD, about the current trials in the United States. He is a former surgeon and faculty member at the University of Pennsylvania in Philadelphia, and is now a patient advocate after his wife, Amy Reed, MD, died of uterine cancer in 2017 following a laparoscopic hysterectomy performed with a power morcellator that resulted in the upstaging of an undetected gynecologic cancer.
“You have to look at oncologic outcomes and do randomized, noninferiority trials to demonstrate that those cancer outcomes are at least equivalent to standard of care,” he said in an interview.
The current U.S. trials are “totally inappropriate,” he said.
Are randomized trials forthcoming after this initial set of single-arm trials? This news organization reached out to Intuitive Surgical, maker of the market leader da Vinci robotic surgical equipment to find out.
“Any plans for use of da Vinci Xi surgical system in nipple-sparing mastectomy will be based on these [single-arm] study results as well as other data and evidence,” said a company spokesperson, who did not confirm use of a randomized trial.
What about the FDA? Will the agency change its current approach to approving robots in surgeries for women’s cancers and require – not just anticipate – cancer-related outcomes data? At press time, the FDA did not respond to a request for comment.
Not having a randomized trial with cancer outcomes in any eventual FDA review opens the door for robotic mastectomy to be cleared for use in some mastectomies with short-term, nononcologic data, said Dr. Noorchashm.
Safety concerns with robotic mastectomy
Proponents of robot-assisted nipple-sparing mastectomy, which is coupled with reconstruction to preserve the shape of both the breast and nipple-areola area, suggest that improved patient cosmesis is a significant advantage with the high-tech intervention, said Dr. Margenthaler.
That’s because most robotic mastectomies performed to date (almost exclusively in Europe and Asia) have employed a 3- to 5-cm vertical incision located behind the lateral breast fold, allowing the scar to be hidden under the patient’s arm.
But therein also lies a safety concern, she asserted.
The “oncologic integrity” of the specimen on extraction is in question in some cases, she wrote, because of “such a small opening.”
Dr. Noorchashm agreed: “It all comes down to trying to get a large specimen out of a small incision.”
Traditional open mastectomy optimally yields the en bloc removal of a tumor – in one whole piece – to avoid fragmenting the cancerous tissue and possibly leaving residual disease behind. These undesirable events are associated with a higher risk for recurrence and treatment failure, he explained.
Thus, there is a need for a randomized trial with longer-term oncologic outcomes that compares the new approach with traditional open mastectomy, argued both Dr. Margenthaler and Dr. Noorchashm.
In defense of single-arm trials
“Oncologic safety is what we are concerned about and what we would like to study,” said Ko Un (Clara) Park, MD, a breast surgeon at The Ohio State University in Columbus.
Dr. Park is leading a single-center, single-arm pilot study of robotic nipple-sparing mastectomy enrolling up to 20 women with early-stage breast cancer or inherited genetic risk factors (but no cancer diagnosis). The trial, sponsored by a Pelotonia Idea Grant and Ohio State, recently enrolled its first patient.
The study’s primary outcomes include the feasibility of removal of the breast tissue en bloc; however, none of the outcomes are classic oncologic metrics such as progression-free survival.
The en bloc removal outcome is in direct response to the FDA’s concerns about minimally invasive cancer surgeries in women, Dr. Park said in an interview. The pilot trial has an investigational device exemption (IDE) granted by the FDA.
“The reason why we can’t just open a randomized controlled study (of robot versus open) and measure oncologic outcomes like recurrence-free survival is because, before we get to that point, we have to make sure” basic safety issues are addressed and established, she explained.
But Dr. Noorchashm said that argument is missing the larger, more important point: “They are still doing an oncologic procedure – you are still obliged to do noninferiority [randomized] testing with respect to cancer outcomes.”
Dr. Park sounded a different note: “We are doing it as safely as we can do it.”
Prophylactic use is also a cancer surgery
Intuitive’s five-center trial does not include en bloc removal of the breast gland as a primary outcome. Instead, the two primary outcomes are conversions to open mastectomy (efficacy measure) and the incidence of adverse events during surgery to 42 days after surgery (safety measure).
The company’s trial does not include any women with breast cancer, but is limited to women at increased risk for breast cancer and seeking prophylactic nipple-sparing mastectomy surgery.
Enrollment in the 145-patient single-arm trial began in the last few months and has a primary completion date of December 2022. It also has an IDE from the FDA.
“I do think that things like this need to be done with caution,” said Katherine Kopkash, MD, an investigator in the Intuitive trial and a breast surgeon at NorthShore University HealthSystem in Evanston, Ill., referring to the trial’s FDA exemption.
Dr. Kopkash said in an interview that the researchers in the multisite, single-arm Intuitive trial will also track oncologic outcomes, but the trial description at clinicaltrials.gov does not indicate that.
Both Dr. Kopkash and Dr. Park cited the high-profile missteps that took place in 2018 at Monmouth County Medical Center in Long Branch, N.J., during what was described as the first-ever use of robotic nipple-sparing mastectomy for invasive cancer in the United States, as reported by Medscape Medical News. However, neither the center or surgeon, Stephen Chagares, MD, requested or received an IDE from the FDA, and use of robotic mastectomy was halted after two cases.
It’s conceivable that Intuitive will seek out FDA clearance for use of its da Vinci system in robotic nipple-sparing mastectomy with data in a prophylactic setting and then expand the pool of patients, argued Dr. Noorchashm.
“Even if you introduce a new technology ... for a narrow subset of patients, the application of it eventually occurs on a ‘sliding scale,’ ” he said.
The former surgeon gave an example: The first device used in gastric bypass surgery was cleared for use in 2001 by the FDA for adults who were “severely morbidly obese.” But by the late 2000s, the operation was also being performed on people with lower body mass indexes who hadn’t exhausted traditional weight loss procedures. “It was very lucrative,” Dr. Noorchashm said about the surgery.
Surgeons only get one body
Intuitive has been hugely successful in developing and marketing its da Vinci system around the world for general and oncologic surgeries, with more than 1 million surgeries in 2018, a greater than sevenfold increase in 10 years, according to the authors of a new essay published in the June issue of the Annals of Surgery. The authors include breast surgeon Rosa F. Hwang, MD, of MD Anderson Cancer Center in Houston, who is also an investigator for the Intuitive trial.
However, robotic mastectomy is still a new surgery – only about 150 patients have been treated in the world, mostly in Italy, France, Taiwan, and Korea, the authors noted.
Despite such small numbers, “there’s a lot of interest in bringing this to the United States,” said Dr. Park.
One of the arguments in favor of robotic mastectomy for nipple-sparing procedures has nothing to do with patients. Instead, it is improved ergonomics – the robot makes a tough surgery easier on the surgeon.
Even stalwart robot critic Dr. Margenthaler conceded that this was possibly a winning feature.
“Nipple-sparing mastectomy is a very physically demanding procedure for the surgeon, resulting in higher rates of neck and back pain and fatigue compared with a standard skin-sparing approach,” she noted. She suggested, however, that practitioners of traditional mastectomy ought to first experiment with changes to patient positioning and incision placement to alleviate stress before looking to the robot for change.
When this news organization interviewed NorthShore University’s Dr. Kopkash, she had conducted four nipple-sparing mastectomies in the previous week. “It’s a difficult procedure on our bodies. I just turned 40 and I’m considered young for a surgeon. We get one body for our career and we have to figure out ways to make it work and protect it.”
Intuitive Surgical is funding the five-center clinical trial of robot-assisted nipple-sparing mastectomy, and UT Southwestern is funding its own trial. The Ohio State trial is funded by the university and a Pelotonia Idea Grant. Dr. Noorchashm and Dr. Margenthaler have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
The FDA warning was issued in February 2019 to both the public and physicians. The FDA cautioned that the safety and effectiveness of robotic surgical devices for mastectomy “have not been established” and robots are not approved for the prevention or treatment of breast cancer.
The agency also noted that “diminished long-term survival” was associated with robotic surgery in another women’s cancer, that of hysterectomy for cervical cancer.
The FDA also made a surprising statement. The agency typically approves the robot for surgical use based on 30-day complication rates (compared with standards of care). But it said that going forward it “anticipates” that any evaluation of new use of robots in cancer “would be supported” by cancer outcomes such as progression-free survival and overall survival, which require much longer follow-up.
In short, the FDA hinted that it would change how it regulated medical devices, or at least robots used in women’s cancers. “The FDA takes women’s health very seriously,” said the organization.
Fast forward to 2021, and there are several prospective clinical trials of robot-assisted nipple-sparing mastectomy underway in the United States, including a five-center study sponsored by Intuitive Surgical, the maker of da Vinci robots, the dominant machine on the market. There are also single-center studies at Ohio State and University of Texas Southwestern Medical Center.
However, in each case, the study design either excludes cancer outcomes or does not primarily focus on those measures.
Instead, the primary outcomes are relatively short term and include safety and efficacy measures such as en bloc (in one piece) removal of the breast tissue, conversions to open mastectomy, and the incidence of adverse events during surgery and up to 6 weeks after surgery.
Importantly, none of the studies is a randomized trial; all have single arms.
That’s not what is needed, says breast surgeon Julie A. Margenthaler, MD of Washington University in St. Louis.
“I firmly believe that robotic-assisted mastectomy should only be considered in the context of a well-designed, randomized trial evaluating patient selection, patient safety, surgical complications, and oncologic outcomes with a concomitant cost analysis,” Dr. Margenthaler wrote in an essay published last year in JAMA Surgery.
As with the FDA warning, she cites worse survival with commonly used minimally invasive radical hysterectomy for cervical cancer, saying it “is a stark reminder that the marketing of robotic surgery has its roots in cosmesis and convenience rather than oncologic outcomes.”
In addition, robotic surgery is prohibitively expensive, said Dr. Margenthaler. In fact, cost is her “main criticism regarding robotic-assisted mastectomy.” It costs an additional $6,000 for robot use per procedure, according to a study conducted at a center in Taiwan. “I simply cannot be convinced that this will ever achieve cost-effective or even cost-neutral status,” Dr. Margenthaler wrote.
Not looking at the right outcomes
“They’re not looking at the right outcomes,” said Hooman Noorchashm, MD, PhD, about the current trials in the United States. He is a former surgeon and faculty member at the University of Pennsylvania in Philadelphia, and is now a patient advocate after his wife, Amy Reed, MD, died of uterine cancer in 2017 following a laparoscopic hysterectomy performed with a power morcellator that resulted in the upstaging of an undetected gynecologic cancer.
“You have to look at oncologic outcomes and do randomized, noninferiority trials to demonstrate that those cancer outcomes are at least equivalent to standard of care,” he said in an interview.
The current U.S. trials are “totally inappropriate,” he said.
Are randomized trials forthcoming after this initial set of single-arm trials? This news organization reached out to Intuitive Surgical, maker of the market leader da Vinci robotic surgical equipment to find out.
“Any plans for use of da Vinci Xi surgical system in nipple-sparing mastectomy will be based on these [single-arm] study results as well as other data and evidence,” said a company spokesperson, who did not confirm use of a randomized trial.
What about the FDA? Will the agency change its current approach to approving robots in surgeries for women’s cancers and require – not just anticipate – cancer-related outcomes data? At press time, the FDA did not respond to a request for comment.
Not having a randomized trial with cancer outcomes in any eventual FDA review opens the door for robotic mastectomy to be cleared for use in some mastectomies with short-term, nononcologic data, said Dr. Noorchashm.
Safety concerns with robotic mastectomy
Proponents of robot-assisted nipple-sparing mastectomy, which is coupled with reconstruction to preserve the shape of both the breast and nipple-areola area, suggest that improved patient cosmesis is a significant advantage with the high-tech intervention, said Dr. Margenthaler.
That’s because most robotic mastectomies performed to date (almost exclusively in Europe and Asia) have employed a 3- to 5-cm vertical incision located behind the lateral breast fold, allowing the scar to be hidden under the patient’s arm.
But therein also lies a safety concern, she asserted.
The “oncologic integrity” of the specimen on extraction is in question in some cases, she wrote, because of “such a small opening.”
Dr. Noorchashm agreed: “It all comes down to trying to get a large specimen out of a small incision.”
Traditional open mastectomy optimally yields the en bloc removal of a tumor – in one whole piece – to avoid fragmenting the cancerous tissue and possibly leaving residual disease behind. These undesirable events are associated with a higher risk for recurrence and treatment failure, he explained.
Thus, there is a need for a randomized trial with longer-term oncologic outcomes that compares the new approach with traditional open mastectomy, argued both Dr. Margenthaler and Dr. Noorchashm.
In defense of single-arm trials
“Oncologic safety is what we are concerned about and what we would like to study,” said Ko Un (Clara) Park, MD, a breast surgeon at The Ohio State University in Columbus.
Dr. Park is leading a single-center, single-arm pilot study of robotic nipple-sparing mastectomy enrolling up to 20 women with early-stage breast cancer or inherited genetic risk factors (but no cancer diagnosis). The trial, sponsored by a Pelotonia Idea Grant and Ohio State, recently enrolled its first patient.
The study’s primary outcomes include the feasibility of removal of the breast tissue en bloc; however, none of the outcomes are classic oncologic metrics such as progression-free survival.
The en bloc removal outcome is in direct response to the FDA’s concerns about minimally invasive cancer surgeries in women, Dr. Park said in an interview. The pilot trial has an investigational device exemption (IDE) granted by the FDA.
“The reason why we can’t just open a randomized controlled study (of robot versus open) and measure oncologic outcomes like recurrence-free survival is because, before we get to that point, we have to make sure” basic safety issues are addressed and established, she explained.
But Dr. Noorchashm said that argument is missing the larger, more important point: “They are still doing an oncologic procedure – you are still obliged to do noninferiority [randomized] testing with respect to cancer outcomes.”
Dr. Park sounded a different note: “We are doing it as safely as we can do it.”
Prophylactic use is also a cancer surgery
Intuitive’s five-center trial does not include en bloc removal of the breast gland as a primary outcome. Instead, the two primary outcomes are conversions to open mastectomy (efficacy measure) and the incidence of adverse events during surgery to 42 days after surgery (safety measure).
The company’s trial does not include any women with breast cancer, but is limited to women at increased risk for breast cancer and seeking prophylactic nipple-sparing mastectomy surgery.
Enrollment in the 145-patient single-arm trial began in the last few months and has a primary completion date of December 2022. It also has an IDE from the FDA.
“I do think that things like this need to be done with caution,” said Katherine Kopkash, MD, an investigator in the Intuitive trial and a breast surgeon at NorthShore University HealthSystem in Evanston, Ill., referring to the trial’s FDA exemption.
Dr. Kopkash said in an interview that the researchers in the multisite, single-arm Intuitive trial will also track oncologic outcomes, but the trial description at clinicaltrials.gov does not indicate that.
Both Dr. Kopkash and Dr. Park cited the high-profile missteps that took place in 2018 at Monmouth County Medical Center in Long Branch, N.J., during what was described as the first-ever use of robotic nipple-sparing mastectomy for invasive cancer in the United States, as reported by Medscape Medical News. However, neither the center or surgeon, Stephen Chagares, MD, requested or received an IDE from the FDA, and use of robotic mastectomy was halted after two cases.
It’s conceivable that Intuitive will seek out FDA clearance for use of its da Vinci system in robotic nipple-sparing mastectomy with data in a prophylactic setting and then expand the pool of patients, argued Dr. Noorchashm.
“Even if you introduce a new technology ... for a narrow subset of patients, the application of it eventually occurs on a ‘sliding scale,’ ” he said.
The former surgeon gave an example: The first device used in gastric bypass surgery was cleared for use in 2001 by the FDA for adults who were “severely morbidly obese.” But by the late 2000s, the operation was also being performed on people with lower body mass indexes who hadn’t exhausted traditional weight loss procedures. “It was very lucrative,” Dr. Noorchashm said about the surgery.
Surgeons only get one body
Intuitive has been hugely successful in developing and marketing its da Vinci system around the world for general and oncologic surgeries, with more than 1 million surgeries in 2018, a greater than sevenfold increase in 10 years, according to the authors of a new essay published in the June issue of the Annals of Surgery. The authors include breast surgeon Rosa F. Hwang, MD, of MD Anderson Cancer Center in Houston, who is also an investigator for the Intuitive trial.
However, robotic mastectomy is still a new surgery – only about 150 patients have been treated in the world, mostly in Italy, France, Taiwan, and Korea, the authors noted.
Despite such small numbers, “there’s a lot of interest in bringing this to the United States,” said Dr. Park.
One of the arguments in favor of robotic mastectomy for nipple-sparing procedures has nothing to do with patients. Instead, it is improved ergonomics – the robot makes a tough surgery easier on the surgeon.
Even stalwart robot critic Dr. Margenthaler conceded that this was possibly a winning feature.
“Nipple-sparing mastectomy is a very physically demanding procedure for the surgeon, resulting in higher rates of neck and back pain and fatigue compared with a standard skin-sparing approach,” she noted. She suggested, however, that practitioners of traditional mastectomy ought to first experiment with changes to patient positioning and incision placement to alleviate stress before looking to the robot for change.
When this news organization interviewed NorthShore University’s Dr. Kopkash, she had conducted four nipple-sparing mastectomies in the previous week. “It’s a difficult procedure on our bodies. I just turned 40 and I’m considered young for a surgeon. We get one body for our career and we have to figure out ways to make it work and protect it.”
Intuitive Surgical is funding the five-center clinical trial of robot-assisted nipple-sparing mastectomy, and UT Southwestern is funding its own trial. The Ohio State trial is funded by the university and a Pelotonia Idea Grant. Dr. Noorchashm and Dr. Margenthaler have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
MDs rebut claims of toxic culture after resident suicides
The tragic loss of three medical residents in our beloved South Bronx hospital shook us to the core. They were our colleagues and friends – promising young physicians whose lives and contributions to our hospital family will never be forgotten. We miss them and we grieve them.
We have been keenly aware of the growing trend of physician suicides across the country. That’s one of the reasons why, years ago, we established the nationally recognized Helping Healers Heal program across our health system and more recently expanded other mental health counseling and support to our frontline clinicians.
Our focus is wellness and prevention, as well as helping address the sadness, anxiety, and depression that so many of us experience after a traumatic event. During the surge of the COVID pandemic, these programs proved to be essential, as we expanded these services to all staff, not just those on the frontlines of patient care.
We share Dr. Pamela Wible’s concerns about the physician suicide crisis in this country. However, she misrepresented our residency program and made numerous statements that are false and simply hurtful.
Out of respect for our colleagues and their families, we cannot share everything that we know about this tragic and irreparable loss. But we must set the record straight about a number of incorrect references made by Dr. Wible:
1. We lost two residents to suicide. Though no less horrific, the third death was investigated and declared an accident by the police department.
2. Resident work hours and workload are closely monitored to follow guidance set by the New York State Department of Health and by ACGME. In fact, at the peak of the COVID pandemic, when we were caring for nearly 130 intubated patients at a time, we adopted a strict residency program schedule with built-in breaks and reduced shifts and hours. Even at that tasking time, no one worked more than 80 hours. Although the maximum number of patients assigned to an intern allowed by ACGME is 10, we rarely have more than five or six patients assigned to each of our interns.
3. We swiftly investigate any allegation and do not hesitate to take the appropriate action against anyone who does not honor our values of professionalism and respect.
4. Our ACGME survey results are close to the mean of all internal medicine residency programs in the country. The fact that the results range from 75% to 95% clearly indicates that residents respond independently, and there is no coaching.
5. No resident has ever been threatened to have their visa canceled or withdrawn. Never. And the implication that we were intolerant because of their nationality is reprehensible. At NYC Health + Hospitals, we celebrate diversity. We are deeply committed to serving everyone, regardless of where they come from, what language they speak, what religion they practice. If you spend one day, or one hour, in our facility, you will see and feel our pride and commitment to this mission. We take pride in the fact that our staff and residents reflect the diversity of the community we serve.
6. As for the allegations of “toxic culture at Lincoln” – many of our graduates chose to stay on as attendings, serve the local community, and train new residents. Out of the 67 attendings in our department, 24 are former graduates. They are being joined by another five graduates from this year’s graduating class. There is no better testament to how our graduates feel about our residency program, Department of Medicine, and Lincoln Hospital.
Dr. Wible poses a legitimate question: How to prevent another suicide. No one has the exact answer. But it is a question we will keep asking ourselves as we continue to do all we can to meet our residents’ needs, extend the social and mental health support they need to thrive, and provide the learning and training they need to offer the best care to our patients.
A version of this article first appeared on Medscape.com.
The tragic loss of three medical residents in our beloved South Bronx hospital shook us to the core. They were our colleagues and friends – promising young physicians whose lives and contributions to our hospital family will never be forgotten. We miss them and we grieve them.
We have been keenly aware of the growing trend of physician suicides across the country. That’s one of the reasons why, years ago, we established the nationally recognized Helping Healers Heal program across our health system and more recently expanded other mental health counseling and support to our frontline clinicians.
Our focus is wellness and prevention, as well as helping address the sadness, anxiety, and depression that so many of us experience after a traumatic event. During the surge of the COVID pandemic, these programs proved to be essential, as we expanded these services to all staff, not just those on the frontlines of patient care.
We share Dr. Pamela Wible’s concerns about the physician suicide crisis in this country. However, she misrepresented our residency program and made numerous statements that are false and simply hurtful.
Out of respect for our colleagues and their families, we cannot share everything that we know about this tragic and irreparable loss. But we must set the record straight about a number of incorrect references made by Dr. Wible:
1. We lost two residents to suicide. Though no less horrific, the third death was investigated and declared an accident by the police department.
2. Resident work hours and workload are closely monitored to follow guidance set by the New York State Department of Health and by ACGME. In fact, at the peak of the COVID pandemic, when we were caring for nearly 130 intubated patients at a time, we adopted a strict residency program schedule with built-in breaks and reduced shifts and hours. Even at that tasking time, no one worked more than 80 hours. Although the maximum number of patients assigned to an intern allowed by ACGME is 10, we rarely have more than five or six patients assigned to each of our interns.
3. We swiftly investigate any allegation and do not hesitate to take the appropriate action against anyone who does not honor our values of professionalism and respect.
4. Our ACGME survey results are close to the mean of all internal medicine residency programs in the country. The fact that the results range from 75% to 95% clearly indicates that residents respond independently, and there is no coaching.
5. No resident has ever been threatened to have their visa canceled or withdrawn. Never. And the implication that we were intolerant because of their nationality is reprehensible. At NYC Health + Hospitals, we celebrate diversity. We are deeply committed to serving everyone, regardless of where they come from, what language they speak, what religion they practice. If you spend one day, or one hour, in our facility, you will see and feel our pride and commitment to this mission. We take pride in the fact that our staff and residents reflect the diversity of the community we serve.
6. As for the allegations of “toxic culture at Lincoln” – many of our graduates chose to stay on as attendings, serve the local community, and train new residents. Out of the 67 attendings in our department, 24 are former graduates. They are being joined by another five graduates from this year’s graduating class. There is no better testament to how our graduates feel about our residency program, Department of Medicine, and Lincoln Hospital.
Dr. Wible poses a legitimate question: How to prevent another suicide. No one has the exact answer. But it is a question we will keep asking ourselves as we continue to do all we can to meet our residents’ needs, extend the social and mental health support they need to thrive, and provide the learning and training they need to offer the best care to our patients.
A version of this article first appeared on Medscape.com.
The tragic loss of three medical residents in our beloved South Bronx hospital shook us to the core. They were our colleagues and friends – promising young physicians whose lives and contributions to our hospital family will never be forgotten. We miss them and we grieve them.
We have been keenly aware of the growing trend of physician suicides across the country. That’s one of the reasons why, years ago, we established the nationally recognized Helping Healers Heal program across our health system and more recently expanded other mental health counseling and support to our frontline clinicians.
Our focus is wellness and prevention, as well as helping address the sadness, anxiety, and depression that so many of us experience after a traumatic event. During the surge of the COVID pandemic, these programs proved to be essential, as we expanded these services to all staff, not just those on the frontlines of patient care.
We share Dr. Pamela Wible’s concerns about the physician suicide crisis in this country. However, she misrepresented our residency program and made numerous statements that are false and simply hurtful.
Out of respect for our colleagues and their families, we cannot share everything that we know about this tragic and irreparable loss. But we must set the record straight about a number of incorrect references made by Dr. Wible:
1. We lost two residents to suicide. Though no less horrific, the third death was investigated and declared an accident by the police department.
2. Resident work hours and workload are closely monitored to follow guidance set by the New York State Department of Health and by ACGME. In fact, at the peak of the COVID pandemic, when we were caring for nearly 130 intubated patients at a time, we adopted a strict residency program schedule with built-in breaks and reduced shifts and hours. Even at that tasking time, no one worked more than 80 hours. Although the maximum number of patients assigned to an intern allowed by ACGME is 10, we rarely have more than five or six patients assigned to each of our interns.
3. We swiftly investigate any allegation and do not hesitate to take the appropriate action against anyone who does not honor our values of professionalism and respect.
4. Our ACGME survey results are close to the mean of all internal medicine residency programs in the country. The fact that the results range from 75% to 95% clearly indicates that residents respond independently, and there is no coaching.
5. No resident has ever been threatened to have their visa canceled or withdrawn. Never. And the implication that we were intolerant because of their nationality is reprehensible. At NYC Health + Hospitals, we celebrate diversity. We are deeply committed to serving everyone, regardless of where they come from, what language they speak, what religion they practice. If you spend one day, or one hour, in our facility, you will see and feel our pride and commitment to this mission. We take pride in the fact that our staff and residents reflect the diversity of the community we serve.
6. As for the allegations of “toxic culture at Lincoln” – many of our graduates chose to stay on as attendings, serve the local community, and train new residents. Out of the 67 attendings in our department, 24 are former graduates. They are being joined by another five graduates from this year’s graduating class. There is no better testament to how our graduates feel about our residency program, Department of Medicine, and Lincoln Hospital.
Dr. Wible poses a legitimate question: How to prevent another suicide. No one has the exact answer. But it is a question we will keep asking ourselves as we continue to do all we can to meet our residents’ needs, extend the social and mental health support they need to thrive, and provide the learning and training they need to offer the best care to our patients.
A version of this article first appeared on Medscape.com.
The VA, California, and NYC requiring employee vaccinations
-- or, in the case of California and New York City, undergo regular testing.
The VA becomes the first federal agency to mandate COVID vaccinations for workers. In a news release, VA Secretary Denis McDonough said the mandate is “the best way to keep Veterans safe, especially as the Delta variant spreads across the country.”
VA health care personnel -- including doctors, dentists, podiatrists, optometrists, registered nurses, physician assistants, and chiropractors -- have 8 weeks to become fully vaccinated, the news release said. The New York Times reported that about 115,000 workers will be affected.
The trifecta of federal-state-municipal vaccine requirements arrived as the nation searches for ways to get more people vaccinated to tamp down the Delta variant.
Some organizations, including the military, have already said vaccinations will be required as soon as the Food and Drug Administration formally approves the vaccines, which are now given under emergency use authorizations. The FDA has said the Pfizer vaccine could receive full approval within months.
California Gov. Gavin Newsom said the requirements he announced July 27 were the first in the nation on the state level.
“As the state’s largest employer, we are leading by example and requiring all state and health care workers to show proof of vaccination or be tested regularly, and we are encouraging local governments and businesses to do the same,” he said in a news release.
California employees must provide proof of vaccination or get tested at least once a week. The policy starts Aug. 2 for state employees and Aug. 9 for state health care workers and employees of congregate facilities, such as jails or homeless shelters.
California, especially the southern part of the state, is grappling with a COVID-19 surge. The state’s daily case rate more than quadrupled, from a low of 1.9 cases per 100,000 in May to at least 9.5 cases per 100,000 today, the release said.
In New York City, Mayor Bill de Blasio had previously announced that city health and hospital employees and those working in Department of Health and Mental Hygiene clinical settings would be required to provide proof of vaccination or have regular testing.
On July 27 he expanded the rule to cover all city employees, with a Sept. 13 deadline for most of them, according to a news release.
“This is what it takes to continue our recovery for all of us while fighting back the Delta variant,” Mayor de Blasio said. “It’s going to take all of us to finally end the fight against COVID-19.”
“We have a moral responsibility to take every precaution possible to ensure we keep ourselves, our colleagues and loved ones safe,” NYC Health + Hospitals President and CEO Mitchell Katz, MD, said in the release. “Our city’s new testing requirement for city workers provides more [peace] of mind until more people get their safe and effective COVID-19 vaccine.”
NBC News reported the plan would affect about 340,000 employees.
A version of this article first appeared on WebMD.com.
-- or, in the case of California and New York City, undergo regular testing.
The VA becomes the first federal agency to mandate COVID vaccinations for workers. In a news release, VA Secretary Denis McDonough said the mandate is “the best way to keep Veterans safe, especially as the Delta variant spreads across the country.”
VA health care personnel -- including doctors, dentists, podiatrists, optometrists, registered nurses, physician assistants, and chiropractors -- have 8 weeks to become fully vaccinated, the news release said. The New York Times reported that about 115,000 workers will be affected.
The trifecta of federal-state-municipal vaccine requirements arrived as the nation searches for ways to get more people vaccinated to tamp down the Delta variant.
Some organizations, including the military, have already said vaccinations will be required as soon as the Food and Drug Administration formally approves the vaccines, which are now given under emergency use authorizations. The FDA has said the Pfizer vaccine could receive full approval within months.
California Gov. Gavin Newsom said the requirements he announced July 27 were the first in the nation on the state level.
“As the state’s largest employer, we are leading by example and requiring all state and health care workers to show proof of vaccination or be tested regularly, and we are encouraging local governments and businesses to do the same,” he said in a news release.
California employees must provide proof of vaccination or get tested at least once a week. The policy starts Aug. 2 for state employees and Aug. 9 for state health care workers and employees of congregate facilities, such as jails or homeless shelters.
California, especially the southern part of the state, is grappling with a COVID-19 surge. The state’s daily case rate more than quadrupled, from a low of 1.9 cases per 100,000 in May to at least 9.5 cases per 100,000 today, the release said.
In New York City, Mayor Bill de Blasio had previously announced that city health and hospital employees and those working in Department of Health and Mental Hygiene clinical settings would be required to provide proof of vaccination or have regular testing.
On July 27 he expanded the rule to cover all city employees, with a Sept. 13 deadline for most of them, according to a news release.
“This is what it takes to continue our recovery for all of us while fighting back the Delta variant,” Mayor de Blasio said. “It’s going to take all of us to finally end the fight against COVID-19.”
“We have a moral responsibility to take every precaution possible to ensure we keep ourselves, our colleagues and loved ones safe,” NYC Health + Hospitals President and CEO Mitchell Katz, MD, said in the release. “Our city’s new testing requirement for city workers provides more [peace] of mind until more people get their safe and effective COVID-19 vaccine.”
NBC News reported the plan would affect about 340,000 employees.
A version of this article first appeared on WebMD.com.
-- or, in the case of California and New York City, undergo regular testing.
The VA becomes the first federal agency to mandate COVID vaccinations for workers. In a news release, VA Secretary Denis McDonough said the mandate is “the best way to keep Veterans safe, especially as the Delta variant spreads across the country.”
VA health care personnel -- including doctors, dentists, podiatrists, optometrists, registered nurses, physician assistants, and chiropractors -- have 8 weeks to become fully vaccinated, the news release said. The New York Times reported that about 115,000 workers will be affected.
The trifecta of federal-state-municipal vaccine requirements arrived as the nation searches for ways to get more people vaccinated to tamp down the Delta variant.
Some organizations, including the military, have already said vaccinations will be required as soon as the Food and Drug Administration formally approves the vaccines, which are now given under emergency use authorizations. The FDA has said the Pfizer vaccine could receive full approval within months.
California Gov. Gavin Newsom said the requirements he announced July 27 were the first in the nation on the state level.
“As the state’s largest employer, we are leading by example and requiring all state and health care workers to show proof of vaccination or be tested regularly, and we are encouraging local governments and businesses to do the same,” he said in a news release.
California employees must provide proof of vaccination or get tested at least once a week. The policy starts Aug. 2 for state employees and Aug. 9 for state health care workers and employees of congregate facilities, such as jails or homeless shelters.
California, especially the southern part of the state, is grappling with a COVID-19 surge. The state’s daily case rate more than quadrupled, from a low of 1.9 cases per 100,000 in May to at least 9.5 cases per 100,000 today, the release said.
In New York City, Mayor Bill de Blasio had previously announced that city health and hospital employees and those working in Department of Health and Mental Hygiene clinical settings would be required to provide proof of vaccination or have regular testing.
On July 27 he expanded the rule to cover all city employees, with a Sept. 13 deadline for most of them, according to a news release.
“This is what it takes to continue our recovery for all of us while fighting back the Delta variant,” Mayor de Blasio said. “It’s going to take all of us to finally end the fight against COVID-19.”
“We have a moral responsibility to take every precaution possible to ensure we keep ourselves, our colleagues and loved ones safe,” NYC Health + Hospitals President and CEO Mitchell Katz, MD, said in the release. “Our city’s new testing requirement for city workers provides more [peace] of mind until more people get their safe and effective COVID-19 vaccine.”
NBC News reported the plan would affect about 340,000 employees.
A version of this article first appeared on WebMD.com.
Mayo, Cleveland Clinics top latest U.S. News & World Report hospital rankings
This year’s expanded report debuts new ratings for seven “important procedures and conditions to help patients, in consultation with their doctors, narrow down their choice of hospital based on the specific type of care they need,” Ben Harder, managing editor and chief of health analysis, said in a news release.
With new ratings for myocardial infarction, stroke, hip fracture, and back surgery (spinal fusion), the report now ranks 17 procedures and conditions.
Also new to the 2021 report, which marks the 32nd edition, is a look at racial disparities in health care and the inclusion of health equity measures alongside the hospital rankings.
The new measures examine whether the patients each hospital has treated reflect the racial and ethnic diversity of the surrounding community, among other aspects of health equity.
“At roughly four out of five hospitals, we found that the community’s minority residents were underrepresented among patients receiving services such as joint replacement, cancer surgery and common heart procedures,” Mr. Harder said.
“Against this backdrop, however, we found important exceptions – hospitals that provide care to a disproportionate share of their community’s minority residents. These metrics are just a beginning; we aim to expand on our measurement of health equity in the future,” Mr. Harder added.
Mayo and Cleveland Clinic remain tops
Following the Mayo Clinic, the Cleveland Clinic once again takes the No. 2 spot in the magazine’s latest annual honor roll of best hospitals, which highlights hospitals that deliver exceptional treatment across multiple areas of care.
UCLA Medical Center, Los Angeles, holds the No. 3 spot in 2021. In 2020, UCLA Medical Center and New York–Presbyterian Hospital–Columbia and Cornell, New York, sat in a tie at No. 4.
In 2021, Johns Hopkins Hospital, Baltimore, which held the No. 3 spot in 2020, drops to No. 4, while Massachusetts General Hospital in Boston takes the No. 5 spot, up from No. 6 in 2020.
Rounding out the top 10 (in order) are Cedars-Sinai Medical Center, Los Angeles; New York–Presbyterian Hospital–Columbia and Cornell, New York; NYU Langone Hospitals, New York; UCSF Medical Center, San Francisco; and Northwestern Memorial Hospital, Chicago.
2021-2022 Best Hospitals honor roll
1. Mayo Clinic, Rochester, Minn.
2. Cleveland Clinic, Cleveland
3. UCLA Medical Center, Los Angeles
4. Johns Hopkins Hospital, Baltimore
5. Massachusetts General Hospital, Boston
6. Cedars-Sinai Medical Center, San Francisco
7. New York–Presbyterian Hospital–Columbia and Cornell, New York
8. NYU Langone Hospitals, New York
9. UCSF Medical Center, San Francisco
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor.
12. Stanford Health Care–Stanford Hospital, Palo Alto, Calif.
13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
14. Brigham and Women’s Hospital, Boston
15. Mayo Clinic–Phoenix, Phoenix
16. Houston Methodist Hospital, Houston
17. (tie) Barnes-Jewish Hospital, St. Louis
17. (tie) Mount Sinai Hospital, New York Rush University Medical Center, Chicago
19. Rush University Medical Center, Chicago
20. Vanderbilt University Medical Center, Nashville, Tenn.
For the 2021-2022 rankings and ratings, the magazine compared more than 4,750 hospitals nationwide in 15 specialties and 17 procedures and conditions.
At least 2,039 hospitals received a high performance rating in at least one of the services rated; 11 hospitals received high performance in all 17. A total of 175 hospitals were nationally ranked in at least one specialty
For specialty rankings, the University of Texas MD Anderson Cancer Center continues to hold the No. 1 spot in cancer care, the Hospital for Special Surgery continues to be No. 1 in orthopedics, and the Cleveland Clinic continues to be No. 1 in cardiology and heart surgery.
Top five for cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York
3. Mayo Clinic, Rochester, Minn.
4. Dana-Farber/Brigham & Women’s Cancer Center, Boston
5. Cleveland Clinic, Cleveland
Top five for cardiology and heart surgery
1. Cleveland Clinic, Cleveland
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, New York
5. NYU Langone Hospitals, New York
Top five for orthopedics
1. Hospital for Special Surgery, New York
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York
5. UCLA Medical Center, Los Angeles
The magazine noted that data for the 2021-2022 Best Hospitals rankings and ratings were not affected by the COVID-19 pandemic, which began after the end of the data collection period.
The methodologies used in determining the rankings are based largely on objective measures, such as risk-adjusted survival, discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report is available online.
A version of this article first appeared on Medscape.com.
This year’s expanded report debuts new ratings for seven “important procedures and conditions to help patients, in consultation with their doctors, narrow down their choice of hospital based on the specific type of care they need,” Ben Harder, managing editor and chief of health analysis, said in a news release.
With new ratings for myocardial infarction, stroke, hip fracture, and back surgery (spinal fusion), the report now ranks 17 procedures and conditions.
Also new to the 2021 report, which marks the 32nd edition, is a look at racial disparities in health care and the inclusion of health equity measures alongside the hospital rankings.
The new measures examine whether the patients each hospital has treated reflect the racial and ethnic diversity of the surrounding community, among other aspects of health equity.
“At roughly four out of five hospitals, we found that the community’s minority residents were underrepresented among patients receiving services such as joint replacement, cancer surgery and common heart procedures,” Mr. Harder said.
“Against this backdrop, however, we found important exceptions – hospitals that provide care to a disproportionate share of their community’s minority residents. These metrics are just a beginning; we aim to expand on our measurement of health equity in the future,” Mr. Harder added.
Mayo and Cleveland Clinic remain tops
Following the Mayo Clinic, the Cleveland Clinic once again takes the No. 2 spot in the magazine’s latest annual honor roll of best hospitals, which highlights hospitals that deliver exceptional treatment across multiple areas of care.
UCLA Medical Center, Los Angeles, holds the No. 3 spot in 2021. In 2020, UCLA Medical Center and New York–Presbyterian Hospital–Columbia and Cornell, New York, sat in a tie at No. 4.
In 2021, Johns Hopkins Hospital, Baltimore, which held the No. 3 spot in 2020, drops to No. 4, while Massachusetts General Hospital in Boston takes the No. 5 spot, up from No. 6 in 2020.
Rounding out the top 10 (in order) are Cedars-Sinai Medical Center, Los Angeles; New York–Presbyterian Hospital–Columbia and Cornell, New York; NYU Langone Hospitals, New York; UCSF Medical Center, San Francisco; and Northwestern Memorial Hospital, Chicago.
2021-2022 Best Hospitals honor roll
1. Mayo Clinic, Rochester, Minn.
2. Cleveland Clinic, Cleveland
3. UCLA Medical Center, Los Angeles
4. Johns Hopkins Hospital, Baltimore
5. Massachusetts General Hospital, Boston
6. Cedars-Sinai Medical Center, San Francisco
7. New York–Presbyterian Hospital–Columbia and Cornell, New York
8. NYU Langone Hospitals, New York
9. UCSF Medical Center, San Francisco
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor.
12. Stanford Health Care–Stanford Hospital, Palo Alto, Calif.
13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
14. Brigham and Women’s Hospital, Boston
15. Mayo Clinic–Phoenix, Phoenix
16. Houston Methodist Hospital, Houston
17. (tie) Barnes-Jewish Hospital, St. Louis
17. (tie) Mount Sinai Hospital, New York Rush University Medical Center, Chicago
19. Rush University Medical Center, Chicago
20. Vanderbilt University Medical Center, Nashville, Tenn.
For the 2021-2022 rankings and ratings, the magazine compared more than 4,750 hospitals nationwide in 15 specialties and 17 procedures and conditions.
At least 2,039 hospitals received a high performance rating in at least one of the services rated; 11 hospitals received high performance in all 17. A total of 175 hospitals were nationally ranked in at least one specialty
For specialty rankings, the University of Texas MD Anderson Cancer Center continues to hold the No. 1 spot in cancer care, the Hospital for Special Surgery continues to be No. 1 in orthopedics, and the Cleveland Clinic continues to be No. 1 in cardiology and heart surgery.
Top five for cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York
3. Mayo Clinic, Rochester, Minn.
4. Dana-Farber/Brigham & Women’s Cancer Center, Boston
5. Cleveland Clinic, Cleveland
Top five for cardiology and heart surgery
1. Cleveland Clinic, Cleveland
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, New York
5. NYU Langone Hospitals, New York
Top five for orthopedics
1. Hospital for Special Surgery, New York
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York
5. UCLA Medical Center, Los Angeles
The magazine noted that data for the 2021-2022 Best Hospitals rankings and ratings were not affected by the COVID-19 pandemic, which began after the end of the data collection period.
The methodologies used in determining the rankings are based largely on objective measures, such as risk-adjusted survival, discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report is available online.
A version of this article first appeared on Medscape.com.
This year’s expanded report debuts new ratings for seven “important procedures and conditions to help patients, in consultation with their doctors, narrow down their choice of hospital based on the specific type of care they need,” Ben Harder, managing editor and chief of health analysis, said in a news release.
With new ratings for myocardial infarction, stroke, hip fracture, and back surgery (spinal fusion), the report now ranks 17 procedures and conditions.
Also new to the 2021 report, which marks the 32nd edition, is a look at racial disparities in health care and the inclusion of health equity measures alongside the hospital rankings.
The new measures examine whether the patients each hospital has treated reflect the racial and ethnic diversity of the surrounding community, among other aspects of health equity.
“At roughly four out of five hospitals, we found that the community’s minority residents were underrepresented among patients receiving services such as joint replacement, cancer surgery and common heart procedures,” Mr. Harder said.
“Against this backdrop, however, we found important exceptions – hospitals that provide care to a disproportionate share of their community’s minority residents. These metrics are just a beginning; we aim to expand on our measurement of health equity in the future,” Mr. Harder added.
Mayo and Cleveland Clinic remain tops
Following the Mayo Clinic, the Cleveland Clinic once again takes the No. 2 spot in the magazine’s latest annual honor roll of best hospitals, which highlights hospitals that deliver exceptional treatment across multiple areas of care.
UCLA Medical Center, Los Angeles, holds the No. 3 spot in 2021. In 2020, UCLA Medical Center and New York–Presbyterian Hospital–Columbia and Cornell, New York, sat in a tie at No. 4.
In 2021, Johns Hopkins Hospital, Baltimore, which held the No. 3 spot in 2020, drops to No. 4, while Massachusetts General Hospital in Boston takes the No. 5 spot, up from No. 6 in 2020.
Rounding out the top 10 (in order) are Cedars-Sinai Medical Center, Los Angeles; New York–Presbyterian Hospital–Columbia and Cornell, New York; NYU Langone Hospitals, New York; UCSF Medical Center, San Francisco; and Northwestern Memorial Hospital, Chicago.
2021-2022 Best Hospitals honor roll
1. Mayo Clinic, Rochester, Minn.
2. Cleveland Clinic, Cleveland
3. UCLA Medical Center, Los Angeles
4. Johns Hopkins Hospital, Baltimore
5. Massachusetts General Hospital, Boston
6. Cedars-Sinai Medical Center, San Francisco
7. New York–Presbyterian Hospital–Columbia and Cornell, New York
8. NYU Langone Hospitals, New York
9. UCSF Medical Center, San Francisco
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor.
12. Stanford Health Care–Stanford Hospital, Palo Alto, Calif.
13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
14. Brigham and Women’s Hospital, Boston
15. Mayo Clinic–Phoenix, Phoenix
16. Houston Methodist Hospital, Houston
17. (tie) Barnes-Jewish Hospital, St. Louis
17. (tie) Mount Sinai Hospital, New York Rush University Medical Center, Chicago
19. Rush University Medical Center, Chicago
20. Vanderbilt University Medical Center, Nashville, Tenn.
For the 2021-2022 rankings and ratings, the magazine compared more than 4,750 hospitals nationwide in 15 specialties and 17 procedures and conditions.
At least 2,039 hospitals received a high performance rating in at least one of the services rated; 11 hospitals received high performance in all 17. A total of 175 hospitals were nationally ranked in at least one specialty
For specialty rankings, the University of Texas MD Anderson Cancer Center continues to hold the No. 1 spot in cancer care, the Hospital for Special Surgery continues to be No. 1 in orthopedics, and the Cleveland Clinic continues to be No. 1 in cardiology and heart surgery.
Top five for cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York
3. Mayo Clinic, Rochester, Minn.
4. Dana-Farber/Brigham & Women’s Cancer Center, Boston
5. Cleveland Clinic, Cleveland
Top five for cardiology and heart surgery
1. Cleveland Clinic, Cleveland
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, New York
5. NYU Langone Hospitals, New York
Top five for orthopedics
1. Hospital for Special Surgery, New York
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York
5. UCLA Medical Center, Los Angeles
The magazine noted that data for the 2021-2022 Best Hospitals rankings and ratings were not affected by the COVID-19 pandemic, which began after the end of the data collection period.
The methodologies used in determining the rankings are based largely on objective measures, such as risk-adjusted survival, discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report is available online.
A version of this article first appeared on Medscape.com.
AMA, 55 other groups urge health care vax mandate
As COVID-19 cases, hospitalizations, and deaths mount again across the country, the American Medical Association (AMA), the American Nursing Association, and 54 other
This injunction, issued July 26, covers everyone in healthcare, Emanuel Ezekiel, MD, PhD, chair of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and the organizer of the joint statement, said in an interview.
That includes not only hospitals, but also physician offices, ambulatory surgery centers, home care agencies, skilled nursing facilities, pharmacies, laboratories, and imaging centers, he said.
The exhortation to get vaccinated also extends to federal and state healthcare facilities, including those of the military health system — TRICARE and the Department of Veterans Affairs — which instituted a mandate the same day.
The American Hospital Association (AHA) and other hospital groups recently said they supported hospitals and health systems that required their personnel to get vaccinated. Several dozen healthcare organizations have already done so, including some of the nation’s largest health systems.
A substantial fraction of U.S. healthcare workers have not yet gotten vaccinated, although how many are unvaccinated is unclear. An analysis by WebMD and Medscape Medical News estimated that 25% of hospital workers who had contact with patients were unvaccinated at the end of May.
More than 38% of nursing workers were not fully vaccinated by July 11, according to an analysis of Centers for Medicare & Medicaid Services data by LeadingAge, which was cited by the Washington Post. And more than 40% of nursing home employees have not been fully vaccinated, according to the Centers for Disease Control and Prevention.
The joint statement did not give any indication of how many employees of physician practices have failed to get COVID shots. However, a recent AMA survey shows that 96% of physicians have been fully vaccinated.
Ethical commitment
The main reason for vaccine mandates, according to the healthcare associations’ statement, is “the ethical commitment to put patients as well as residents of long-term care facilities first and take all steps necessary to ensure their health and well-being.”
In addition, the statement noted, vaccination can protect healthcare workers and their families from getting COVID-19.
The statement also pointed out that many healthcare and long-term care organizations already require vaccinations for influenza, hepatitis B, and pertussis.
Workers who have certain medical conditions should be exempt from the vaccination mandates, the statement added.
While recognizing the “historical mistrust of health care institutions” among some healthcare workers, the statement said, “We must continue to address workers’ concerns, engage with marginalized populations, and work with trusted messengers to improve vaccine acceptance.”
There has been some skepticism about the legality of requiring healthcare workers to get vaccinated as a condition of employment, partly because the U.S. Food and Drug Administration has not yet fully authorized any of the COVID-19 vaccines.
But in June, a federal judge turned down a legal challenge to Houston Methodist’s vaccination mandate.
“It is critical that all people in the health care workforce get vaccinated against COVID-19 for the safety of our patients and our colleagues. With more than 300 million doses administered in the United States and nearly 4 billion doses administered worldwide, we know the vaccines are safe and highly effective at preventing severe illness and death from COVID-19.
“Increased vaccinations among health care personnel will not only reduce the spread of COVID-19 but also reduce the harmful toll this virus is taking within the health care workforce and those we are striving to serve,” Susan Bailey, MD, immediate past president of the AMA, said in a news release.
A version of this article first appeared on Medscape.com.
As COVID-19 cases, hospitalizations, and deaths mount again across the country, the American Medical Association (AMA), the American Nursing Association, and 54 other
This injunction, issued July 26, covers everyone in healthcare, Emanuel Ezekiel, MD, PhD, chair of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and the organizer of the joint statement, said in an interview.
That includes not only hospitals, but also physician offices, ambulatory surgery centers, home care agencies, skilled nursing facilities, pharmacies, laboratories, and imaging centers, he said.
The exhortation to get vaccinated also extends to federal and state healthcare facilities, including those of the military health system — TRICARE and the Department of Veterans Affairs — which instituted a mandate the same day.
The American Hospital Association (AHA) and other hospital groups recently said they supported hospitals and health systems that required their personnel to get vaccinated. Several dozen healthcare organizations have already done so, including some of the nation’s largest health systems.
A substantial fraction of U.S. healthcare workers have not yet gotten vaccinated, although how many are unvaccinated is unclear. An analysis by WebMD and Medscape Medical News estimated that 25% of hospital workers who had contact with patients were unvaccinated at the end of May.
More than 38% of nursing workers were not fully vaccinated by July 11, according to an analysis of Centers for Medicare & Medicaid Services data by LeadingAge, which was cited by the Washington Post. And more than 40% of nursing home employees have not been fully vaccinated, according to the Centers for Disease Control and Prevention.
The joint statement did not give any indication of how many employees of physician practices have failed to get COVID shots. However, a recent AMA survey shows that 96% of physicians have been fully vaccinated.
Ethical commitment
The main reason for vaccine mandates, according to the healthcare associations’ statement, is “the ethical commitment to put patients as well as residents of long-term care facilities first and take all steps necessary to ensure their health and well-being.”
In addition, the statement noted, vaccination can protect healthcare workers and their families from getting COVID-19.
The statement also pointed out that many healthcare and long-term care organizations already require vaccinations for influenza, hepatitis B, and pertussis.
Workers who have certain medical conditions should be exempt from the vaccination mandates, the statement added.
While recognizing the “historical mistrust of health care institutions” among some healthcare workers, the statement said, “We must continue to address workers’ concerns, engage with marginalized populations, and work with trusted messengers to improve vaccine acceptance.”
There has been some skepticism about the legality of requiring healthcare workers to get vaccinated as a condition of employment, partly because the U.S. Food and Drug Administration has not yet fully authorized any of the COVID-19 vaccines.
But in June, a federal judge turned down a legal challenge to Houston Methodist’s vaccination mandate.
“It is critical that all people in the health care workforce get vaccinated against COVID-19 for the safety of our patients and our colleagues. With more than 300 million doses administered in the United States and nearly 4 billion doses administered worldwide, we know the vaccines are safe and highly effective at preventing severe illness and death from COVID-19.
“Increased vaccinations among health care personnel will not only reduce the spread of COVID-19 but also reduce the harmful toll this virus is taking within the health care workforce and those we are striving to serve,” Susan Bailey, MD, immediate past president of the AMA, said in a news release.
A version of this article first appeared on Medscape.com.
As COVID-19 cases, hospitalizations, and deaths mount again across the country, the American Medical Association (AMA), the American Nursing Association, and 54 other
This injunction, issued July 26, covers everyone in healthcare, Emanuel Ezekiel, MD, PhD, chair of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and the organizer of the joint statement, said in an interview.
That includes not only hospitals, but also physician offices, ambulatory surgery centers, home care agencies, skilled nursing facilities, pharmacies, laboratories, and imaging centers, he said.
The exhortation to get vaccinated also extends to federal and state healthcare facilities, including those of the military health system — TRICARE and the Department of Veterans Affairs — which instituted a mandate the same day.
The American Hospital Association (AHA) and other hospital groups recently said they supported hospitals and health systems that required their personnel to get vaccinated. Several dozen healthcare organizations have already done so, including some of the nation’s largest health systems.
A substantial fraction of U.S. healthcare workers have not yet gotten vaccinated, although how many are unvaccinated is unclear. An analysis by WebMD and Medscape Medical News estimated that 25% of hospital workers who had contact with patients were unvaccinated at the end of May.
More than 38% of nursing workers were not fully vaccinated by July 11, according to an analysis of Centers for Medicare & Medicaid Services data by LeadingAge, which was cited by the Washington Post. And more than 40% of nursing home employees have not been fully vaccinated, according to the Centers for Disease Control and Prevention.
The joint statement did not give any indication of how many employees of physician practices have failed to get COVID shots. However, a recent AMA survey shows that 96% of physicians have been fully vaccinated.
Ethical commitment
The main reason for vaccine mandates, according to the healthcare associations’ statement, is “the ethical commitment to put patients as well as residents of long-term care facilities first and take all steps necessary to ensure their health and well-being.”
In addition, the statement noted, vaccination can protect healthcare workers and their families from getting COVID-19.
The statement also pointed out that many healthcare and long-term care organizations already require vaccinations for influenza, hepatitis B, and pertussis.
Workers who have certain medical conditions should be exempt from the vaccination mandates, the statement added.
While recognizing the “historical mistrust of health care institutions” among some healthcare workers, the statement said, “We must continue to address workers’ concerns, engage with marginalized populations, and work with trusted messengers to improve vaccine acceptance.”
There has been some skepticism about the legality of requiring healthcare workers to get vaccinated as a condition of employment, partly because the U.S. Food and Drug Administration has not yet fully authorized any of the COVID-19 vaccines.
But in June, a federal judge turned down a legal challenge to Houston Methodist’s vaccination mandate.
“It is critical that all people in the health care workforce get vaccinated against COVID-19 for the safety of our patients and our colleagues. With more than 300 million doses administered in the United States and nearly 4 billion doses administered worldwide, we know the vaccines are safe and highly effective at preventing severe illness and death from COVID-19.
“Increased vaccinations among health care personnel will not only reduce the spread of COVID-19 but also reduce the harmful toll this virus is taking within the health care workforce and those we are striving to serve,” Susan Bailey, MD, immediate past president of the AMA, said in a news release.
A version of this article first appeared on Medscape.com.
Sharp decrease in opioid access for dying U.S. cancer patients
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
There has been a sharp decrease in access to opioids during the past decade, and many patients are going to emergency departments for pain treatment.
Overall, during the study period (2007-2017), there was a 34% reduction in the number of opioid prescriptions filled per patient and a 38% reduction in the total dose of opioids filled near the end of life.
There was a dramatic drop in the use of long-acting opioids, which can provide patients with more consistent pain relief and are important for managing severe cancer pain. The investigators’ results show that during the study period, the number of long-acting opioid prescriptions filled per patient fell by 50%.
“We do believe that the decline in cancer patients’ access to opioids near the end of life is likely attributable to the efforts to curtail opioid misuse,” commented lead author Andrea Enzinger, MD, a medical oncologist at Dana-Farber Cancer Institute, Boston.
The study was published online July 22 in the Journal of Clinical Oncology.
“The study provides fascinating data that support our clinical observations,” said Marcin Chwistek, MD, FAAHPM, director of the supportive oncology and palliative care program at Fox Chase Cancer Center, Philadelphia, who was asked for comment. “Primarily, we have noticed a heightened reluctance on the parts of patients with cancer, including those with advanced cancer, to take opioids in general.”
Many factors involved
The crisis of opioid misuse and abuse led to the implementation of regulations to curb inappropriate prescribing. But these restrictions on opioid prescribing may have unintended consequences for patients with advanced, incurable malignancies who are experiencing pain.
“Many but not all opioid regulations specifically exclude cancer patients,” said Dr. Enzinger. “However, the cumulative effect of these regulations may have had a chilling effect on providers’ comfort or willingness to prescribe opioids, even for cancer pain.”
She said in an interview that the prescribing of opioids has become much more difficult. Prescribers are often required to sign an opioid agreement with patients prior to providing them with opioids. Health care professionals may need to use a two-factor authentication to prescribe, and prescribers in 49 of 50 U.S. states are required to check electronic prescription drug monitoring programs prior to providing the prescription.
“After the medications are prescribed, insurance companies require prior-authorization paperwork before filling the medications, particularly for long-acting opioids or high-dose opioids,” Dr. Enzinger said. “These barriers pile up and make the whole process onerous and time consuming.”
Patient factors may also have contributed to the decline in use.
“Cancer patients are often very hesitant to use opioids to treat their pain, as they worry about becoming addicted or being labeled a ‘pill seeker,’” she explained. “Also, the added regulations, such as requirements for prior authorization paperwork, signing opioid agreements, and so on, may add to the stigma of opioid therapy and send a message to patients that these medications are inherently dangerous.”
Dr. Enzinger added that there are legitimate reasons why patients may not want to use opioids and that these should be respected. “But addiction risk should really not weigh into the decisions about pain management for patients who are dying from cancer,” she said.
Decline in opioid dose and prescriptions
Dr. Enzinger and colleagues used administrative data from the Centers for Medicare & Medicaid Services to identify 270,632 Medicare fee-for-service patients who had cancers that were associated with poor prognoses and who died from 2007 to 2017. During this period, the opioid crisis was first recognized. There followed legislative reforms and subsequent declines in population-based opioid prescribing.
Among the patients in the study, the most common cancers were lung, colorectal, pancreatic, prostate, and breast cancers; 166,962 patients (61.7%) were enrolled in hospice before death. This percentage increased from 57.1% in 2007 to 66.2% in 2017 (P for trend < .001).
From 2007 to 2017, the proportion of patients filling greater than or equal to 1 opioid prescriptions declined from 42.0% to 35.5%. The proportion declined faster from 2012-2017 than from 2007-2011.
The proportion of patients who filled prescriptions for long-acting opioids dropped from 18.1% to 11.5%. Here again, the decline was faster from 2012-2017 than from 2007-2011. Prescriptions for strong short-acting opioids declined from 31.7% to 28.5%. Prescribing was initially stable from 2007-2011 and began to decline in 2012. Conversely, prescriptions for weak short-acting opioids dropped from 8.4% to 6.5% from 2007-2011 and then stabilized after 2012.
The mean daily dose fell 24.5%, from 85.6 morphine milligram equivalents per day (MMED) to 64.6 MMED. Overall, the total amount of opioids prescribed per decedent fell 38.0%, from 1,075 MMEs per person to 666 MMEs.
At the same time, the proportion of patients who visited EDs increased 50.8%, from 13.2% to 19.9%.
Experts weigh in
Approached for an independent comment, Amit Barochia, MD, a hematologist/oncologist with Health First Medical Group, Titusville, Fla., commented that the decline could be due, in part, to greater vigilance and awareness by physicians in light of more stringent requirements and of federal and state regulations. “Some physicians are avoiding prescribing opioids due to more regulations and requirements as well, which is routing patients to the ER for pain relief,” he said.
Dr. Barochia agreed that some of the decline could be due to patient factors. “I do think that some of the patients are hesitant about considering opioid use for better pain relief, in part due to fear of addiction as well as complications arising from their use,” he said. “This is likely resulting from more awareness in the community about their adverse effects.
“That awareness could come from aggressive media coverage as well as social media,” he continued. “It is also true that there is a difficulty in getting authorization for certain opioid products, which is delaying the onset of a proper pain regimen that would help to provide adequate pain relief early on.”
For patients with advanced cancer, earlier referral to palliative care would be beneficial, Dr. Barochia pointed out, because this would allow for a more in-depth discussion about pain in addition to addressing the physical and mental symptoms associated with cancer.
Fox Chase Cancer Center’s Dr. Chwistek noted that patients and their caregivers are often apprehensive about the potential adverse effects of opioids, because they often hear about community-based opioid overdoses and are fearful of taking the medications. “Additionally, it has become increasingly challenging to fill opioid prescriptions at local pharmacies, due to quantity limitations, ubiquitous need for prior authorizations, and stigma,” he said.
The fear of addiction is often brought up by the patients during clinic visits, and insurers and pharmacies have imposed many limits on opioid prescribing. “Most of these can be overcome with prior authorizations, but not always, and prior authorizations are time consuming, confusing, and very frustrating for patients,” he said in an interview.
These findings suggest that not enough patients are getting optimal palliative care. “One of the primary tenets of palliative care is optimal symptom control, including pain,” said Dr. Chwistek. “Palliative care teams have the experience and insight needed to help patients overcome the barriers to appropriate pain control. Education, support, and advocacy are critical to ensure that patients’ pain is appropriately addressed.”
The study was funded by a grant from the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
A version of this article first appeared on Medscape.com.
Hematologic cancer increases risk of delivery complications
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
The risk of in-hospital complications and poor birth outcomes were greater in pregnant women with current or historical cancer diagnoses, new research suggests.
The study, published in Mayo Clinic Proceedings, found that women with current and historical cancer diagnoses had an increased risk of death, kidney injury, and stroke during delivery hospitalizations, compared with those with no cancer. When it came to delivery outcomes, this group also had a higher risk for preterm birth and postpartum hemorrhage. Those with a current cancer diagnoses had a 1.7-fold increase in odds for a preterm birth, compared with women without cancer.
“Our study found that metastases increased the odds of mortality, cesarean delivery, preterm birth, and stillbirth,” the researchers noted. “Coupled with previous research reporting that pregnant women are more likely to be diagnosed with advanced disease, this implies that pregnant women with newly diagnosed cancer have poor prognoses.”
However, although women with prior cancer had increased odds of mortality, the researchers said it was not statistically significant.
“The study really did not show an increase of mortality [for women with prior cancer diagnosis],” said Justin Chura, MD, a specialist in gynecologic oncology who was not involved in the study. “And the reason might be because there is not or the reason might be because it’s such a rare event. You would need 100 million births to assess that. So I would actually use caution in that interpretation.”
Researchers analyzed more than 43 million delivery hospitalizations of women with or without current or historical cancer diagnoses between January 2004 and December 2014. They found that the most common cancer diagnoses were hematologic, thyroid, cervical, skin, and breast.
Of the five most common cancers, the prevalence of all maternal complications and negative delivery outcomes was the highest among women with hematologic cancers. They were more likely to experience peripartum cardiomyopathy, acute kidney injury, and arrhythmia, compared with other cancers. Postpartum hemorrhage, maternal mortality, and placental abruption was also more likely to occur in those with this type of cancer.
“I was surprised that it was the hematologic cancers that were worse when they did it by cancer type,” said Dr. Chura, who is the chief of surgery and the director of gynecologic oncology and robotic surgery at the Cancer Treatment Centers of America’s Eastern Regional Medical Center in Philadelphia. “I think this is a useful bit of information for counseling our patients and also to identify the cohort with the highest risk.”
The findings also suggested that those with skin cancer had the highest odds for stroke, while women with cervical and breast cancers were more likely to experience acute kidney injury and preterm birth.
Dr. Chura said cancer treatments can have an impact on a woman’s health when she’s giving birth. For example, if a woman is diagnosed with cervical cancer, doctors may perform a cone biopsy on her where they remove a large portion of the cervix and still leave them with the ability to conceive and become pregnant. However, those patients are left with a higher risk of a preterm delivery.
For women with a hematologic cancer like non-Hodgkin’s lymphoma, chest radiation may cause some subsequent damage to their heart muscles “and now the stress of pregnancy puts more demand on the heart that can lead to cardiac complications for that patient,” Dr. Chura said.
“There are potential long-term effects from radiation and chemotherapy,” Dr. Chura said.
Previous studies have shown that chemotherapy may affect pregnancy and delivery. A 2019 study published in the Journal of Cancer also found that 59 pregnant women with cancer had increased mortality compared with those without the long-term illness. Meanwhile, another 2018 study published in Cancer found that women who conceived less than a year after starting chemotherapy had higher risks of preterm birth in comparison with those who conceived more than a year after starting chemotherapy. The study also found that cancer survivors who conceived more than a year after finishing chemotherapy with or without radiation had no higher risk of a preterm birth than those without cancer.
Dr. Chura said the new study could force doctors to think about the long-term effects of their cancer therapies and make them more apt to think about how to make cancer therapy less toxic with less long-term health consequences, while still curing patients.
“Most oncologists, when dealing with younger patients, are very focused on curing the cancer at hand, but not necessarily thinking 5 or 10 years down the road,” Dr. Chura said. “[This study] could help inform or at least make us aware of the long-term consequences of our cancer therapies.”
Dr. Chura had no relevant financial disclosures.
FROM MAYO CLINIC PROCEEDINGS
Recent trend: Melanoma mortality declining rapidly
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.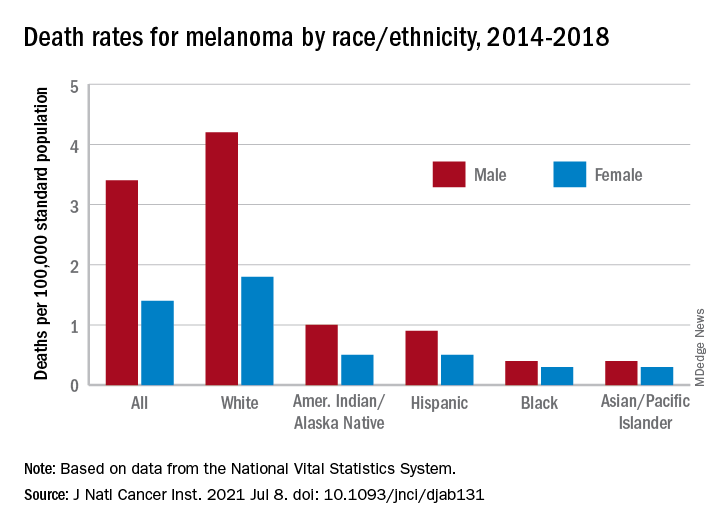
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
‘I did nothing wrong’: MDs used their own sperm for fertility patients
Martin D. Greenberg, MD, was sued in May for secretly using his own sperm to inseminate one of his infertility patients 38 years earlier. The patient’s daughter found out last year when she used a DNA test from 23andme to learn about her family history. The 77-year-old New York gynecologist is retired in Florida.
All but one of the cases took place before 1990. Most of them came to light in the past few years, when biological offspring found out from home DNA tests.
“It is a gross betrayal of the trust that a patient puts in her doctor. It is an absolute perversion of the practice of medicine,” said Dev Sethi, a plaintiff attorney who sued a Tucson, Ariz., physician who inseminated at least 10 patients with his own sperm. “The hubris of a doctor to impregnate his own patient, in some effort to either save money or populate the world with his offspring, is striking.”
Why would these physicians use their own sperm and then keep it secret? Why were there so many of them? When their offspring now try to communicate with them, do they want to have a relationship? And how do they react when they’re found out?
The doctors’ behavior mystifies Sigal Klipstein, MD, a reproductive endocrinologist in Hoffman Estates, Ill., who is chair of the ethics committee of the American Society for Reproductive Medicine.
“These doctors lived with secrets for many years. How do you live with that as a doctor?” said Dr. Klipstein, who was still in high school when most of these cases occurred. “It surprises me that anybody would do this.”
Lack of training and lots of secrecy
Were these physicians particularly selfish or egotistical? Or was expedience the prime motivation?
At the time, there was little training in the techniques and ethics of infertility care, said Jody Madeira, JD, PhD, a law professor at Indiana University, Bloomington, who has closely studied the doctors.
“Many of them were ob.gyns., but they did not take CME courses for this work,” she said. The subspecialty of reproductive endocrinology and infertility was just beginning in the early 1970s, according an ASRM spokesman.
Treatment of infertility was a rather hush-hush topic at that time, which made it easier to be deceptive. In 1955, an Illinois court held that artificial insemination constituted adultery. “The social stigma resulting from the practice forces the parents to keep secret the infant’s origin,” a law review article from 1955 stated.
“In the 1950s and 1960s and even into the 1970s and 1980s, infertility treatments were considered shameful, and patients were often advised to keep their treatment to themselves,” Dr. Madeira said. “With everything so secret, it was easy to be deceptive.”
The field has become more sophisticated since then, Dr. Klipstein said. “For known donors, there is a legal contract between the recipient and donor. And it is no longer possible to be an anonymous donor. People can find you through DNA tests.”
Owing to changes in the field as well as the growing likelihood of being caught through DNA tests, most experts believe that rampant infertility fraud ended long ago.
How they were found out
When the doctors were active, there was little risk of being exposed. In those times, paternity tests were based on broad factors such as blood type and were unreliable. More accurate DNA tests were underway, but the doctors’ offspring did not think of using them because they suspected nothing.
Most of the doctors’ deeds only came to light with the rise of a new industry – home DNA testing for people who are curious about their family background. First came 23andme in 2007, then Ancestry.com in 2015. The number of people being tested reached almost 2 million in 2016, 7 million in 2017, and 30 million in 2020.
As more people entered company databases, it became easier to pinpoint biological fathers through other relatives. This explains how doctors who had not taken a home DNA test were identified.
The home tests have been shown to be highly accurate. None of the results for doctors accused of using their own sperm have proven to be false, and courts recognize similar DNA tests as proof of paternity.
But when found out, many of the physicians disputed the results and acted as if they could still keep their secret. “I don’t deny it; I don’t admit it,” Paul Brennan Jones, MD, a Colorado doctor, said when he was accused of siring eight children through his infertility patients decades before. Asked whether he would provide a DNA sample, the 80-year-old doctor responded: “No ... because I don’t want to have any incriminating evidence against me.”
How often did it happen?
Donor Deceived, a website that monitors these cases, reports 32 cases of physicians surreptitiously providing sperm to their patients. Eleven of the doctors are linked to 1 known offspring, two are linked to more than 75 offspring, one to 15, one to 10, three to 9, three to 7, and two to 5.
“It’s unlikely that any of the doctors did it just once,” said Adam B. Wolf, a San Francisco attorney who is representing the plaintiff in the Greenberg case. “It’s happened before. When doctors get the idea to do something crazy, they do it multiple times.”
Mr. Wolf believes that, because most people haven’t taken a DNA test, there are many more biological children of infertility doctors who have yet to come forward.
Many of the doctors who were found out have negotiated settlements with patients, under which they pay undisclosed sums of money in exchange for the patient’s keeping silent. Mr. Wolf said that, of the two dozen victims of sperm-donor doctors his law firm has represented, all but three have settled.
“We give an opportunity to the doctor to resolve the claims without having to publicly out this person for using his own sperm in his patients,” Mr. Wolf said. “Most doctors jump at the opportunity to not be known as the kind of person who would do that.”
Cases about to go to trial have been withdrawn because of being settled. In May, a case against Gerald E. Mortimer, MD, in Idaho, was dismissed after 3 years of litigation. The judge had made some key decisions that made it less likely that Dr. Mortimer would win. Dr. Mortimer’s biological daughter filed the initial case. She alleged medical negligence, failure to obtain informed consent, fraud, battery, intentional infliction of emotional distress, and several other causes of action.
Dr. Madeira objects to the use of confidential settlements, because other offspring cannot be alerted. But she also believes that, as more people find out about their parentage through DNA tests, it will be harder for accused doctors to make confidential settlements with all of them, and the doctors will eventually be identified.
In settlements, offspring ask for the medical histories of these doctors. So far, offspring have linked the development of Tay-Sachs disease, cystic fibrosis, and ovarian cancer with these doctors.
Denial: Physicians’ most frequent reaction
Once identified, most of the doctors denied the charge. When Gary Phillip Wood, MD, of Arkansas, was tracked down by his biological son, Dr. Wood insisted he had had a vasectomy years before the man was born but still would not agree to a DNA test. He died in April 2021.
None of the identified sperm doctors were interested in having a relationship with their newly identified offspring. When Gary Vandenberg, MD, of California, was contacted by his biological daughter, he abruptly ended the conversation, wishing her “good luck in life,” she recalled. “When I first found out, I was very suicidal. I did not want this existence. I still have those days. My husband had to take off work and stay home quite a bit to make sure I didn’t do anything to myself.”
When Gary Don Davis, MD, of Idaho, was asked about his paternity, he replied: “Let me check on that. Goodbye.” He could not be reached after that, and he died a few months later.
The accused doctors often have no medical records of their work. Dr. Wood said that all his records had been destroyed, and Dr. Greenberg said he did not have any records on his accuser and doubted that he had ever treated her. A 1977 survey found that more than half of infertility doctors did not keep any medical records so as to preserve the donor’s anonymity.
Many of the accused doctors said they used their own sperm because they were deeply committed to helping their patients. At one physician’s trial, his defense attorney said: “If Cecil made any mistakes, it was in losing his objectivity and trying so hard to get patients pregnant.”
Was it really ethically wrong?
Many of the doctors don’t accept that they did any harm, says Julie D. Cantor, MD, JD, a former adjunct professor at the University of California, Los Angeles. “These doctors seemed to be thinking: ‘The patient wanted to get pregnant and have a baby, and that’s what happened, so no harm done.’ But the entire interaction is based on a lie.”
The doctors also had the problem of having to use fresh sperm rather than frozen sperm, as is used today. Sperm had to be used within hours of being produced. If the donor did not show up at the time of the appointment, the doctor might decide to keep the appointment with the patient anyway and provide his own sperm.
However, “these doctors didn’t have to use their own sperm,” Mr. Wolf said. “They could have rescheduled the appointment until a new donor could be found.”
Some say that the doctors seemed to have had a very high opinion of themselves and their own sperm. “Some of them had savior complexes,” Dr. Madeira said. “They seemed to be thinking: ‘I’m giving the gift of life, and I’m the only one who can really do it, because I have great genes.’ ”
When Kim McMorries, MD, of Texas, was confronted with the fact that he had donated sperm 33 years before, he insisted that it was ethical at the time. “When this occurred, it was not considered wrong,” he wrote in an email to his biological daughter.
Today, doctors are bound by the doctrine of informed consent, which holds that patients should be informed about all steps taken in their care. The term was coined by a judge in 1960, and it took some time for some in the medical world to fully accept informed consent. Still, Dr. Madeira asserts it was always unethical to secretly fertilize patients.
“Even in the more paternalistic era of the 1970s and 1980s, it was not right to lie to your patients about such an important part of their lives,” she said.
Some sperm doctors insisted that they had received informed consent when the patient agreed to use an anonymous donor. “Dr. Kiken did that which he was asked to do,” wrote the attorneys for Michael S. Kiken, MD, of Virginia. “Anonymous donor meant that the patient would not know the donor’s identity, he would be anonymous to her.”
Dr. Madeira does not accept this argument either. “The doctor may have thought it was understood that he could be the anonymous person, but the patients did not see it that way,” she said. “They were not expecting the anonymous donor would be their own doctor.”
“I think what happened is a crime,” said Dr. Klipstein. “It’s an ethical violation, a fracture in the trust between doctor and patient.”
Existing laws, however, don’t make it easy to prosecute the doctors. When lawsuits are filed against these doctors, “you have to shoehorn existing statutes to fit the facts, and that may not be a terrific fit,” Dr. Cantor said.
The doctors have been charged with battery, fraud, negligence, breach of duty, unjust enrichment, and rape. But none of them have been found guilty specifically of secretly using their own sperm. Two of the doctors were convicted, but for other offenses, such as perjury for denying their involvement.
Since 2019, five states – Arizona, Colorado, Florida, Indiana, and Texas – have passed statutes specifically outlawing infertility fraud. In addition, a 1995 California law requires identifying the sperm donor.
It may be difficult, however, to apply these new laws to offenses by aging sperm doctors that happened decades ago. “Some states have inflexible limits on the amount of time in which you can sue, even if you didn’t know about the problem until recently,” Dr. Madeira said. “Texas, for example, allows civil lawsuits only up to 10 years after commission.”
Before the fertility fraud physicians can be brought to justice, many of them might just fade away.
A version of this article first appeared on Medscape.com.
Martin D. Greenberg, MD, was sued in May for secretly using his own sperm to inseminate one of his infertility patients 38 years earlier. The patient’s daughter found out last year when she used a DNA test from 23andme to learn about her family history. The 77-year-old New York gynecologist is retired in Florida.
All but one of the cases took place before 1990. Most of them came to light in the past few years, when biological offspring found out from home DNA tests.
“It is a gross betrayal of the trust that a patient puts in her doctor. It is an absolute perversion of the practice of medicine,” said Dev Sethi, a plaintiff attorney who sued a Tucson, Ariz., physician who inseminated at least 10 patients with his own sperm. “The hubris of a doctor to impregnate his own patient, in some effort to either save money or populate the world with his offspring, is striking.”
Why would these physicians use their own sperm and then keep it secret? Why were there so many of them? When their offspring now try to communicate with them, do they want to have a relationship? And how do they react when they’re found out?
The doctors’ behavior mystifies Sigal Klipstein, MD, a reproductive endocrinologist in Hoffman Estates, Ill., who is chair of the ethics committee of the American Society for Reproductive Medicine.
“These doctors lived with secrets for many years. How do you live with that as a doctor?” said Dr. Klipstein, who was still in high school when most of these cases occurred. “It surprises me that anybody would do this.”
Lack of training and lots of secrecy
Were these physicians particularly selfish or egotistical? Or was expedience the prime motivation?
At the time, there was little training in the techniques and ethics of infertility care, said Jody Madeira, JD, PhD, a law professor at Indiana University, Bloomington, who has closely studied the doctors.
“Many of them were ob.gyns., but they did not take CME courses for this work,” she said. The subspecialty of reproductive endocrinology and infertility was just beginning in the early 1970s, according an ASRM spokesman.
Treatment of infertility was a rather hush-hush topic at that time, which made it easier to be deceptive. In 1955, an Illinois court held that artificial insemination constituted adultery. “The social stigma resulting from the practice forces the parents to keep secret the infant’s origin,” a law review article from 1955 stated.
“In the 1950s and 1960s and even into the 1970s and 1980s, infertility treatments were considered shameful, and patients were often advised to keep their treatment to themselves,” Dr. Madeira said. “With everything so secret, it was easy to be deceptive.”
The field has become more sophisticated since then, Dr. Klipstein said. “For known donors, there is a legal contract between the recipient and donor. And it is no longer possible to be an anonymous donor. People can find you through DNA tests.”
Owing to changes in the field as well as the growing likelihood of being caught through DNA tests, most experts believe that rampant infertility fraud ended long ago.
How they were found out
When the doctors were active, there was little risk of being exposed. In those times, paternity tests were based on broad factors such as blood type and were unreliable. More accurate DNA tests were underway, but the doctors’ offspring did not think of using them because they suspected nothing.
Most of the doctors’ deeds only came to light with the rise of a new industry – home DNA testing for people who are curious about their family background. First came 23andme in 2007, then Ancestry.com in 2015. The number of people being tested reached almost 2 million in 2016, 7 million in 2017, and 30 million in 2020.
As more people entered company databases, it became easier to pinpoint biological fathers through other relatives. This explains how doctors who had not taken a home DNA test were identified.
The home tests have been shown to be highly accurate. None of the results for doctors accused of using their own sperm have proven to be false, and courts recognize similar DNA tests as proof of paternity.
But when found out, many of the physicians disputed the results and acted as if they could still keep their secret. “I don’t deny it; I don’t admit it,” Paul Brennan Jones, MD, a Colorado doctor, said when he was accused of siring eight children through his infertility patients decades before. Asked whether he would provide a DNA sample, the 80-year-old doctor responded: “No ... because I don’t want to have any incriminating evidence against me.”
How often did it happen?
Donor Deceived, a website that monitors these cases, reports 32 cases of physicians surreptitiously providing sperm to their patients. Eleven of the doctors are linked to 1 known offspring, two are linked to more than 75 offspring, one to 15, one to 10, three to 9, three to 7, and two to 5.
“It’s unlikely that any of the doctors did it just once,” said Adam B. Wolf, a San Francisco attorney who is representing the plaintiff in the Greenberg case. “It’s happened before. When doctors get the idea to do something crazy, they do it multiple times.”
Mr. Wolf believes that, because most people haven’t taken a DNA test, there are many more biological children of infertility doctors who have yet to come forward.
Many of the doctors who were found out have negotiated settlements with patients, under which they pay undisclosed sums of money in exchange for the patient’s keeping silent. Mr. Wolf said that, of the two dozen victims of sperm-donor doctors his law firm has represented, all but three have settled.
“We give an opportunity to the doctor to resolve the claims without having to publicly out this person for using his own sperm in his patients,” Mr. Wolf said. “Most doctors jump at the opportunity to not be known as the kind of person who would do that.”
Cases about to go to trial have been withdrawn because of being settled. In May, a case against Gerald E. Mortimer, MD, in Idaho, was dismissed after 3 years of litigation. The judge had made some key decisions that made it less likely that Dr. Mortimer would win. Dr. Mortimer’s biological daughter filed the initial case. She alleged medical negligence, failure to obtain informed consent, fraud, battery, intentional infliction of emotional distress, and several other causes of action.
Dr. Madeira objects to the use of confidential settlements, because other offspring cannot be alerted. But she also believes that, as more people find out about their parentage through DNA tests, it will be harder for accused doctors to make confidential settlements with all of them, and the doctors will eventually be identified.
In settlements, offspring ask for the medical histories of these doctors. So far, offspring have linked the development of Tay-Sachs disease, cystic fibrosis, and ovarian cancer with these doctors.
Denial: Physicians’ most frequent reaction
Once identified, most of the doctors denied the charge. When Gary Phillip Wood, MD, of Arkansas, was tracked down by his biological son, Dr. Wood insisted he had had a vasectomy years before the man was born but still would not agree to a DNA test. He died in April 2021.
None of the identified sperm doctors were interested in having a relationship with their newly identified offspring. When Gary Vandenberg, MD, of California, was contacted by his biological daughter, he abruptly ended the conversation, wishing her “good luck in life,” she recalled. “When I first found out, I was very suicidal. I did not want this existence. I still have those days. My husband had to take off work and stay home quite a bit to make sure I didn’t do anything to myself.”
When Gary Don Davis, MD, of Idaho, was asked about his paternity, he replied: “Let me check on that. Goodbye.” He could not be reached after that, and he died a few months later.
The accused doctors often have no medical records of their work. Dr. Wood said that all his records had been destroyed, and Dr. Greenberg said he did not have any records on his accuser and doubted that he had ever treated her. A 1977 survey found that more than half of infertility doctors did not keep any medical records so as to preserve the donor’s anonymity.
Many of the accused doctors said they used their own sperm because they were deeply committed to helping their patients. At one physician’s trial, his defense attorney said: “If Cecil made any mistakes, it was in losing his objectivity and trying so hard to get patients pregnant.”
Was it really ethically wrong?
Many of the doctors don’t accept that they did any harm, says Julie D. Cantor, MD, JD, a former adjunct professor at the University of California, Los Angeles. “These doctors seemed to be thinking: ‘The patient wanted to get pregnant and have a baby, and that’s what happened, so no harm done.’ But the entire interaction is based on a lie.”
The doctors also had the problem of having to use fresh sperm rather than frozen sperm, as is used today. Sperm had to be used within hours of being produced. If the donor did not show up at the time of the appointment, the doctor might decide to keep the appointment with the patient anyway and provide his own sperm.
However, “these doctors didn’t have to use their own sperm,” Mr. Wolf said. “They could have rescheduled the appointment until a new donor could be found.”
Some say that the doctors seemed to have had a very high opinion of themselves and their own sperm. “Some of them had savior complexes,” Dr. Madeira said. “They seemed to be thinking: ‘I’m giving the gift of life, and I’m the only one who can really do it, because I have great genes.’ ”
When Kim McMorries, MD, of Texas, was confronted with the fact that he had donated sperm 33 years before, he insisted that it was ethical at the time. “When this occurred, it was not considered wrong,” he wrote in an email to his biological daughter.
Today, doctors are bound by the doctrine of informed consent, which holds that patients should be informed about all steps taken in their care. The term was coined by a judge in 1960, and it took some time for some in the medical world to fully accept informed consent. Still, Dr. Madeira asserts it was always unethical to secretly fertilize patients.
“Even in the more paternalistic era of the 1970s and 1980s, it was not right to lie to your patients about such an important part of their lives,” she said.
Some sperm doctors insisted that they had received informed consent when the patient agreed to use an anonymous donor. “Dr. Kiken did that which he was asked to do,” wrote the attorneys for Michael S. Kiken, MD, of Virginia. “Anonymous donor meant that the patient would not know the donor’s identity, he would be anonymous to her.”
Dr. Madeira does not accept this argument either. “The doctor may have thought it was understood that he could be the anonymous person, but the patients did not see it that way,” she said. “They were not expecting the anonymous donor would be their own doctor.”
“I think what happened is a crime,” said Dr. Klipstein. “It’s an ethical violation, a fracture in the trust between doctor and patient.”
Existing laws, however, don’t make it easy to prosecute the doctors. When lawsuits are filed against these doctors, “you have to shoehorn existing statutes to fit the facts, and that may not be a terrific fit,” Dr. Cantor said.
The doctors have been charged with battery, fraud, negligence, breach of duty, unjust enrichment, and rape. But none of them have been found guilty specifically of secretly using their own sperm. Two of the doctors were convicted, but for other offenses, such as perjury for denying their involvement.
Since 2019, five states – Arizona, Colorado, Florida, Indiana, and Texas – have passed statutes specifically outlawing infertility fraud. In addition, a 1995 California law requires identifying the sperm donor.
It may be difficult, however, to apply these new laws to offenses by aging sperm doctors that happened decades ago. “Some states have inflexible limits on the amount of time in which you can sue, even if you didn’t know about the problem until recently,” Dr. Madeira said. “Texas, for example, allows civil lawsuits only up to 10 years after commission.”
Before the fertility fraud physicians can be brought to justice, many of them might just fade away.
A version of this article first appeared on Medscape.com.
Martin D. Greenberg, MD, was sued in May for secretly using his own sperm to inseminate one of his infertility patients 38 years earlier. The patient’s daughter found out last year when she used a DNA test from 23andme to learn about her family history. The 77-year-old New York gynecologist is retired in Florida.
All but one of the cases took place before 1990. Most of them came to light in the past few years, when biological offspring found out from home DNA tests.
“It is a gross betrayal of the trust that a patient puts in her doctor. It is an absolute perversion of the practice of medicine,” said Dev Sethi, a plaintiff attorney who sued a Tucson, Ariz., physician who inseminated at least 10 patients with his own sperm. “The hubris of a doctor to impregnate his own patient, in some effort to either save money or populate the world with his offspring, is striking.”
Why would these physicians use their own sperm and then keep it secret? Why were there so many of them? When their offspring now try to communicate with them, do they want to have a relationship? And how do they react when they’re found out?
The doctors’ behavior mystifies Sigal Klipstein, MD, a reproductive endocrinologist in Hoffman Estates, Ill., who is chair of the ethics committee of the American Society for Reproductive Medicine.
“These doctors lived with secrets for many years. How do you live with that as a doctor?” said Dr. Klipstein, who was still in high school when most of these cases occurred. “It surprises me that anybody would do this.”
Lack of training and lots of secrecy
Were these physicians particularly selfish or egotistical? Or was expedience the prime motivation?
At the time, there was little training in the techniques and ethics of infertility care, said Jody Madeira, JD, PhD, a law professor at Indiana University, Bloomington, who has closely studied the doctors.
“Many of them were ob.gyns., but they did not take CME courses for this work,” she said. The subspecialty of reproductive endocrinology and infertility was just beginning in the early 1970s, according an ASRM spokesman.
Treatment of infertility was a rather hush-hush topic at that time, which made it easier to be deceptive. In 1955, an Illinois court held that artificial insemination constituted adultery. “The social stigma resulting from the practice forces the parents to keep secret the infant’s origin,” a law review article from 1955 stated.
“In the 1950s and 1960s and even into the 1970s and 1980s, infertility treatments were considered shameful, and patients were often advised to keep their treatment to themselves,” Dr. Madeira said. “With everything so secret, it was easy to be deceptive.”
The field has become more sophisticated since then, Dr. Klipstein said. “For known donors, there is a legal contract between the recipient and donor. And it is no longer possible to be an anonymous donor. People can find you through DNA tests.”
Owing to changes in the field as well as the growing likelihood of being caught through DNA tests, most experts believe that rampant infertility fraud ended long ago.
How they were found out
When the doctors were active, there was little risk of being exposed. In those times, paternity tests were based on broad factors such as blood type and were unreliable. More accurate DNA tests were underway, but the doctors’ offspring did not think of using them because they suspected nothing.
Most of the doctors’ deeds only came to light with the rise of a new industry – home DNA testing for people who are curious about their family background. First came 23andme in 2007, then Ancestry.com in 2015. The number of people being tested reached almost 2 million in 2016, 7 million in 2017, and 30 million in 2020.
As more people entered company databases, it became easier to pinpoint biological fathers through other relatives. This explains how doctors who had not taken a home DNA test were identified.
The home tests have been shown to be highly accurate. None of the results for doctors accused of using their own sperm have proven to be false, and courts recognize similar DNA tests as proof of paternity.
But when found out, many of the physicians disputed the results and acted as if they could still keep their secret. “I don’t deny it; I don’t admit it,” Paul Brennan Jones, MD, a Colorado doctor, said when he was accused of siring eight children through his infertility patients decades before. Asked whether he would provide a DNA sample, the 80-year-old doctor responded: “No ... because I don’t want to have any incriminating evidence against me.”
How often did it happen?
Donor Deceived, a website that monitors these cases, reports 32 cases of physicians surreptitiously providing sperm to their patients. Eleven of the doctors are linked to 1 known offspring, two are linked to more than 75 offspring, one to 15, one to 10, three to 9, three to 7, and two to 5.
“It’s unlikely that any of the doctors did it just once,” said Adam B. Wolf, a San Francisco attorney who is representing the plaintiff in the Greenberg case. “It’s happened before. When doctors get the idea to do something crazy, they do it multiple times.”
Mr. Wolf believes that, because most people haven’t taken a DNA test, there are many more biological children of infertility doctors who have yet to come forward.
Many of the doctors who were found out have negotiated settlements with patients, under which they pay undisclosed sums of money in exchange for the patient’s keeping silent. Mr. Wolf said that, of the two dozen victims of sperm-donor doctors his law firm has represented, all but three have settled.
“We give an opportunity to the doctor to resolve the claims without having to publicly out this person for using his own sperm in his patients,” Mr. Wolf said. “Most doctors jump at the opportunity to not be known as the kind of person who would do that.”
Cases about to go to trial have been withdrawn because of being settled. In May, a case against Gerald E. Mortimer, MD, in Idaho, was dismissed after 3 years of litigation. The judge had made some key decisions that made it less likely that Dr. Mortimer would win. Dr. Mortimer’s biological daughter filed the initial case. She alleged medical negligence, failure to obtain informed consent, fraud, battery, intentional infliction of emotional distress, and several other causes of action.
Dr. Madeira objects to the use of confidential settlements, because other offspring cannot be alerted. But she also believes that, as more people find out about their parentage through DNA tests, it will be harder for accused doctors to make confidential settlements with all of them, and the doctors will eventually be identified.
In settlements, offspring ask for the medical histories of these doctors. So far, offspring have linked the development of Tay-Sachs disease, cystic fibrosis, and ovarian cancer with these doctors.
Denial: Physicians’ most frequent reaction
Once identified, most of the doctors denied the charge. When Gary Phillip Wood, MD, of Arkansas, was tracked down by his biological son, Dr. Wood insisted he had had a vasectomy years before the man was born but still would not agree to a DNA test. He died in April 2021.
None of the identified sperm doctors were interested in having a relationship with their newly identified offspring. When Gary Vandenberg, MD, of California, was contacted by his biological daughter, he abruptly ended the conversation, wishing her “good luck in life,” she recalled. “When I first found out, I was very suicidal. I did not want this existence. I still have those days. My husband had to take off work and stay home quite a bit to make sure I didn’t do anything to myself.”
When Gary Don Davis, MD, of Idaho, was asked about his paternity, he replied: “Let me check on that. Goodbye.” He could not be reached after that, and he died a few months later.
The accused doctors often have no medical records of their work. Dr. Wood said that all his records had been destroyed, and Dr. Greenberg said he did not have any records on his accuser and doubted that he had ever treated her. A 1977 survey found that more than half of infertility doctors did not keep any medical records so as to preserve the donor’s anonymity.
Many of the accused doctors said they used their own sperm because they were deeply committed to helping their patients. At one physician’s trial, his defense attorney said: “If Cecil made any mistakes, it was in losing his objectivity and trying so hard to get patients pregnant.”
Was it really ethically wrong?
Many of the doctors don’t accept that they did any harm, says Julie D. Cantor, MD, JD, a former adjunct professor at the University of California, Los Angeles. “These doctors seemed to be thinking: ‘The patient wanted to get pregnant and have a baby, and that’s what happened, so no harm done.’ But the entire interaction is based on a lie.”
The doctors also had the problem of having to use fresh sperm rather than frozen sperm, as is used today. Sperm had to be used within hours of being produced. If the donor did not show up at the time of the appointment, the doctor might decide to keep the appointment with the patient anyway and provide his own sperm.
However, “these doctors didn’t have to use their own sperm,” Mr. Wolf said. “They could have rescheduled the appointment until a new donor could be found.”
Some say that the doctors seemed to have had a very high opinion of themselves and their own sperm. “Some of them had savior complexes,” Dr. Madeira said. “They seemed to be thinking: ‘I’m giving the gift of life, and I’m the only one who can really do it, because I have great genes.’ ”
When Kim McMorries, MD, of Texas, was confronted with the fact that he had donated sperm 33 years before, he insisted that it was ethical at the time. “When this occurred, it was not considered wrong,” he wrote in an email to his biological daughter.
Today, doctors are bound by the doctrine of informed consent, which holds that patients should be informed about all steps taken in their care. The term was coined by a judge in 1960, and it took some time for some in the medical world to fully accept informed consent. Still, Dr. Madeira asserts it was always unethical to secretly fertilize patients.
“Even in the more paternalistic era of the 1970s and 1980s, it was not right to lie to your patients about such an important part of their lives,” she said.
Some sperm doctors insisted that they had received informed consent when the patient agreed to use an anonymous donor. “Dr. Kiken did that which he was asked to do,” wrote the attorneys for Michael S. Kiken, MD, of Virginia. “Anonymous donor meant that the patient would not know the donor’s identity, he would be anonymous to her.”
Dr. Madeira does not accept this argument either. “The doctor may have thought it was understood that he could be the anonymous person, but the patients did not see it that way,” she said. “They were not expecting the anonymous donor would be their own doctor.”
“I think what happened is a crime,” said Dr. Klipstein. “It’s an ethical violation, a fracture in the trust between doctor and patient.”
Existing laws, however, don’t make it easy to prosecute the doctors. When lawsuits are filed against these doctors, “you have to shoehorn existing statutes to fit the facts, and that may not be a terrific fit,” Dr. Cantor said.
The doctors have been charged with battery, fraud, negligence, breach of duty, unjust enrichment, and rape. But none of them have been found guilty specifically of secretly using their own sperm. Two of the doctors were convicted, but for other offenses, such as perjury for denying their involvement.
Since 2019, five states – Arizona, Colorado, Florida, Indiana, and Texas – have passed statutes specifically outlawing infertility fraud. In addition, a 1995 California law requires identifying the sperm donor.
It may be difficult, however, to apply these new laws to offenses by aging sperm doctors that happened decades ago. “Some states have inflexible limits on the amount of time in which you can sue, even if you didn’t know about the problem until recently,” Dr. Madeira said. “Texas, for example, allows civil lawsuits only up to 10 years after commission.”
Before the fertility fraud physicians can be brought to justice, many of them might just fade away.
A version of this article first appeared on Medscape.com.
Homeopath arrested for fake COVID immunization, vaccine card scheme
A homeopathic doctor licensed in California was arrested July 14 and charged with a scheme to sell homeoprophylaxis immunization pellets and to falsify COVID-19 vaccination cards by making it appear that her customers had received the Moderna vaccine, according to the U.S. Department of Justice.
Juli A. Mazi, 41, of Napa, is charged with one count of wire fraud and one count of false statements related to health care matters. The case is the first federal criminal fraud prosecution related to homeoprophylaxis immunizations and fraudulent vaccination record cards, the DOJ said in a news release.
In April, according to federal authorities, an individual submitted a complaint to the Department of Health and Human Services Office of Inspector General, stating that family members had purchased the immunization pellets from Ms. Mazi. The complainant stated that the family members had told her/him that Ms. Mazi had said the pellets contained the COVID-19 virus and would create an antibody response in the immune system.
The affidavit noted that none of the family members had received injections of any of the COVID-19 vaccines authorized by the Food and Drug Administration.
However, the complainant said, Ms. Mazi sent COVID-19 vaccination cards listing Moderna to the complainant family. Ms. Mazi allegedly instructed the family members to mark the cards to falsely state that they had received the Moderna vaccine on the date that they ingested the homeoprophylaxis immunization pellets.
She also allegedly provided instructions on how to fraudulently complete the cards to make it appear that a customer had received two doses of the Moderna vaccine. She even supplied Moderna lot numbers to enter on the cards.
In addition, Ms. Mazi allegedly offered homeoprophylaxis immunizations for childhood illnesses that she falsely claimed would satisfy the immunization requirements for California schools, and falsified immunization cards that were submitted by parents to California schools.
Ms. Mazi further stated that her customers could provide the pellets to children for COVID-19 immunity, and that “the dose is actually the same for babies,” the news release said.
Ms. Mazi is alleged to have falsely claimed that ingesting the pellets would result in full lifelong immunity from COVID-19. In addition, she exploited the disinformation and fear surrounding COVID-19 vaccination by falsely claiming that the FDA-authorized vaccines contain “toxic ingredients,” the DOJ said.
Homeopathic preparations
According to the DOJ, “Homeophrophylaxis involves the exposure of an individual to dilute amounts of a disease, purportedly to stimulate the immune system and confer immunity.”
According to Australia’s National Centre for Immunisation Research & Surveillance (NCIRS), a private organization funded by the Australian and New South Wales governments, there is no high-quality research showing that homeopathic preparations are effective in preventing infectious disease.
Typical homeopathic preparations dilute a disease, tissue, or plant extract in water “to the point where none of the original material is contained within the preparation by the end of the process,” an NCIRS fact sheet says.
Referring to Ms. Mazi, Deputy Attorney General Lisa Monaco said in the news release, “This defendant allegedly defrauded and endangered the public by preying on fears and spreading misinformation about FDA-authorized vaccinations, while also peddling fake treatments that put people’s lives at risk.
“Even worse, the defendant allegedly created counterfeit COVID-19 vaccination cards and instructed her customers to falsely mark that they had received a vaccine, allowing them to circumvent efforts to contain the spread of the disease.”
The case against Ms. Mazi was brought in coordination with the DOJ Health Care Fraud Unit’s COVID-19 Interagency Working Group, which organizes efforts to address illegal activity involving health care programs during the pandemic.
The fraud unit leads the department’s Health Care Fraud Strike Force, which has existed since 2007. In May, U.S. Attorney General Merrick Garland established the COVID-19 Fraud Enforcement Task Force in partnership with other government agencies to combat and prevent pandemic-related fraud.
A version of this article first appeared on Medscape.com.
A homeopathic doctor licensed in California was arrested July 14 and charged with a scheme to sell homeoprophylaxis immunization pellets and to falsify COVID-19 vaccination cards by making it appear that her customers had received the Moderna vaccine, according to the U.S. Department of Justice.
Juli A. Mazi, 41, of Napa, is charged with one count of wire fraud and one count of false statements related to health care matters. The case is the first federal criminal fraud prosecution related to homeoprophylaxis immunizations and fraudulent vaccination record cards, the DOJ said in a news release.
In April, according to federal authorities, an individual submitted a complaint to the Department of Health and Human Services Office of Inspector General, stating that family members had purchased the immunization pellets from Ms. Mazi. The complainant stated that the family members had told her/him that Ms. Mazi had said the pellets contained the COVID-19 virus and would create an antibody response in the immune system.
The affidavit noted that none of the family members had received injections of any of the COVID-19 vaccines authorized by the Food and Drug Administration.
However, the complainant said, Ms. Mazi sent COVID-19 vaccination cards listing Moderna to the complainant family. Ms. Mazi allegedly instructed the family members to mark the cards to falsely state that they had received the Moderna vaccine on the date that they ingested the homeoprophylaxis immunization pellets.
She also allegedly provided instructions on how to fraudulently complete the cards to make it appear that a customer had received two doses of the Moderna vaccine. She even supplied Moderna lot numbers to enter on the cards.
In addition, Ms. Mazi allegedly offered homeoprophylaxis immunizations for childhood illnesses that she falsely claimed would satisfy the immunization requirements for California schools, and falsified immunization cards that were submitted by parents to California schools.
Ms. Mazi further stated that her customers could provide the pellets to children for COVID-19 immunity, and that “the dose is actually the same for babies,” the news release said.
Ms. Mazi is alleged to have falsely claimed that ingesting the pellets would result in full lifelong immunity from COVID-19. In addition, she exploited the disinformation and fear surrounding COVID-19 vaccination by falsely claiming that the FDA-authorized vaccines contain “toxic ingredients,” the DOJ said.
Homeopathic preparations
According to the DOJ, “Homeophrophylaxis involves the exposure of an individual to dilute amounts of a disease, purportedly to stimulate the immune system and confer immunity.”
According to Australia’s National Centre for Immunisation Research & Surveillance (NCIRS), a private organization funded by the Australian and New South Wales governments, there is no high-quality research showing that homeopathic preparations are effective in preventing infectious disease.
Typical homeopathic preparations dilute a disease, tissue, or plant extract in water “to the point where none of the original material is contained within the preparation by the end of the process,” an NCIRS fact sheet says.
Referring to Ms. Mazi, Deputy Attorney General Lisa Monaco said in the news release, “This defendant allegedly defrauded and endangered the public by preying on fears and spreading misinformation about FDA-authorized vaccinations, while also peddling fake treatments that put people’s lives at risk.
“Even worse, the defendant allegedly created counterfeit COVID-19 vaccination cards and instructed her customers to falsely mark that they had received a vaccine, allowing them to circumvent efforts to contain the spread of the disease.”
The case against Ms. Mazi was brought in coordination with the DOJ Health Care Fraud Unit’s COVID-19 Interagency Working Group, which organizes efforts to address illegal activity involving health care programs during the pandemic.
The fraud unit leads the department’s Health Care Fraud Strike Force, which has existed since 2007. In May, U.S. Attorney General Merrick Garland established the COVID-19 Fraud Enforcement Task Force in partnership with other government agencies to combat and prevent pandemic-related fraud.
A version of this article first appeared on Medscape.com.
A homeopathic doctor licensed in California was arrested July 14 and charged with a scheme to sell homeoprophylaxis immunization pellets and to falsify COVID-19 vaccination cards by making it appear that her customers had received the Moderna vaccine, according to the U.S. Department of Justice.
Juli A. Mazi, 41, of Napa, is charged with one count of wire fraud and one count of false statements related to health care matters. The case is the first federal criminal fraud prosecution related to homeoprophylaxis immunizations and fraudulent vaccination record cards, the DOJ said in a news release.
In April, according to federal authorities, an individual submitted a complaint to the Department of Health and Human Services Office of Inspector General, stating that family members had purchased the immunization pellets from Ms. Mazi. The complainant stated that the family members had told her/him that Ms. Mazi had said the pellets contained the COVID-19 virus and would create an antibody response in the immune system.
The affidavit noted that none of the family members had received injections of any of the COVID-19 vaccines authorized by the Food and Drug Administration.
However, the complainant said, Ms. Mazi sent COVID-19 vaccination cards listing Moderna to the complainant family. Ms. Mazi allegedly instructed the family members to mark the cards to falsely state that they had received the Moderna vaccine on the date that they ingested the homeoprophylaxis immunization pellets.
She also allegedly provided instructions on how to fraudulently complete the cards to make it appear that a customer had received two doses of the Moderna vaccine. She even supplied Moderna lot numbers to enter on the cards.
In addition, Ms. Mazi allegedly offered homeoprophylaxis immunizations for childhood illnesses that she falsely claimed would satisfy the immunization requirements for California schools, and falsified immunization cards that were submitted by parents to California schools.
Ms. Mazi further stated that her customers could provide the pellets to children for COVID-19 immunity, and that “the dose is actually the same for babies,” the news release said.
Ms. Mazi is alleged to have falsely claimed that ingesting the pellets would result in full lifelong immunity from COVID-19. In addition, she exploited the disinformation and fear surrounding COVID-19 vaccination by falsely claiming that the FDA-authorized vaccines contain “toxic ingredients,” the DOJ said.
Homeopathic preparations
According to the DOJ, “Homeophrophylaxis involves the exposure of an individual to dilute amounts of a disease, purportedly to stimulate the immune system and confer immunity.”
According to Australia’s National Centre for Immunisation Research & Surveillance (NCIRS), a private organization funded by the Australian and New South Wales governments, there is no high-quality research showing that homeopathic preparations are effective in preventing infectious disease.
Typical homeopathic preparations dilute a disease, tissue, or plant extract in water “to the point where none of the original material is contained within the preparation by the end of the process,” an NCIRS fact sheet says.
Referring to Ms. Mazi, Deputy Attorney General Lisa Monaco said in the news release, “This defendant allegedly defrauded and endangered the public by preying on fears and spreading misinformation about FDA-authorized vaccinations, while also peddling fake treatments that put people’s lives at risk.
“Even worse, the defendant allegedly created counterfeit COVID-19 vaccination cards and instructed her customers to falsely mark that they had received a vaccine, allowing them to circumvent efforts to contain the spread of the disease.”
The case against Ms. Mazi was brought in coordination with the DOJ Health Care Fraud Unit’s COVID-19 Interagency Working Group, which organizes efforts to address illegal activity involving health care programs during the pandemic.
The fraud unit leads the department’s Health Care Fraud Strike Force, which has existed since 2007. In May, U.S. Attorney General Merrick Garland established the COVID-19 Fraud Enforcement Task Force in partnership with other government agencies to combat and prevent pandemic-related fraud.
A version of this article first appeared on Medscape.com.


