User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Clinicians slow to implement lipid-lowering guidelines: GOULD registry
Among patients with atherosclerotic cardiovascular disease (ASCVD), 2 years after release of treat-to-target guidelines from the American Heart Association and the European Society of Cardiology and European Atherosclerosis Society, most patients with LDL cholesterol higher than 70 mg/dL did not receive intensification of therapy, and two-thirds continued to have LDL levels above that level, according to a prospective registry study.
Both guidelines recommend driving LDL-C levels to 50% or below of baseline levels; results from the Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD) registry suggest this is rarely achieved. “Unfortunately it’s not a total surprise, but it’s disappointing,” said Christopher Cannon, MD, the study’s lead author.
“Therapeutic inertia seems to be the rule in clinical practice,” said Jennifer G. Robinson, MD, MPH, who was asked to comment on the study. Dr. Robinson is professor epidemiology and cardiology at the University of Iowa, Iowa City.
“This is yet another disappointing reminder of how we are failing our patients. Lipid lowering is one of the safest, most effective ways to prevent cardiovascular disease, and yet we are falling short. We have the tools in our toolkit to achieve guideline-based lipid lowering goals, but we just aren’t using them,” said Ann Marie Navar, MD, PhD, associate professor of cardiology at the University of Texas, Dallas.
Patients hesitant
Changes in practice following guidelines can often be slow, but in this case may have been complicated by the fact that statins have a reputation for causing side effects, so some patients may be refusing treatment based on what they’ve seen on the Internet. Even though the study looked at all lipid-lowering agents, the misinformation around statins may be spilling over, according to Dr. Cannon. “There’s in general so much misinformation around COVID and every other topic in the world. That makes people question what is real [about] anything,” said Dr. Cannon, a cardiologist at Brigham and Women’s hospital and professor of medicine at Harvard Medical School, both in Boston.
Patient characteristics may partly explain slow uptake. “Clinicians may not think further LDL-C lowering is a high enough priority in terms of potential benefit for a given patient in light of the effort being expended to take care of all their other issues and chronic health problems. If the clinician does bring it up to the patient, there may be barriers in terms of additional medication burden, cost, or acquisition issues,” said Dr. Robinson.
The answer may be better evidence and a more personalized approach. Clinical trials that explore defined patient populations could convince patients of a benefit, and payers to reimburse, according to Dr. Robinson.
Changing guidance
Another complication is that both the guidelines and the field are rapidly changing. The 2013 AHA guidelines did not include a treatment to goal and focused instead on use of high-dose statins. But the 2018 update reversed course after randomized studies demonstrated a benefit to treating to target. The researchers found no increase in the frequency of treating to target after the release of the 2018 guidelines. “Publication and announcement of guidelines doesn’t mean that people are getting treated better. We really have to implement them,” said Dr. Cannon.
On a positive note, the GOULD researchers found high acceptance of the new proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) inhibitors, with over 90% of patients continuing those medications after 2 years. “That’s nice and high. If people do get onto the very intensive lipid-lowing therapies, they tend to stay on them,” said Dr. Cannon.
What’s next
Still, the lack of intensification is concerning, and the findings led to some consternation in Twitter exchanges, said Dr. Cannon. “People posted ‘Well, what do we do now?’ ” Dr. Cannon’s team is addressing the issue with an algorithm-based risk management program with prospective enrollment. They have conducted educational webinars and provided site-specific reports on LDL status among patients at each center compared to others, and hope that information will improve compliance. In 2020, the group published an interim analysis of the first 5,000 enrollees, and Dr. Cannon expects to finish that study by the end of the year.
Dr. Navar agreed that physicians need to do a better job of testing LDL-C levels after treatment to identify patients who require more aggressive therapy. That can be deferred in some primary prevention patients with high LDL-C but normal particle numbers as measured with ApoB. “But in those at high risk for disease and those with established CVD who are not at goal, as long as they don’t have a life-limiting condition, we should always up-titrate therapy. It’s one of the safest, most effective ways to lower cardiovascular risk,” said Dr. Navar.
Key study results
The prospective study included 5,006 patients at 119 centers with a mean age of 68 years. About 40% were women, and 86.1% were White. All had ASCVD and LDL levels of at least 70 mg/dL. After 2 years, 17% had undergone intensification of lipid-lowering therapy (LLT). Among patients with LDL-C levels ≥ 100 mg/dL, 22% underwent LLT intensification, compared with 14% of patients with LDL-C levels of 70-99 mg/dL.
The vast majority, 92%, of patients who underwent LLT via addition of PCSK9 inhibitors were still taking the drug after 2 years.
Three-quarters (3,768) had lipid level measurements at least once during follow-up, and median LDL-C levels dropped from 120 to 95 mg/dL in the ≥100-mg/dL cohort (P < .001), and from 82 to 77 mg/dL in the 70- to 99-mg/dL cohort (P <. 001). There was no significant difference in the median values in the patients on PCSK9 inhibitors.
In all, 21% of the ≥100-mg/dL cohort achieved LDL-C levels <70 mg/dL at 2 years, versus 34% in the 77- to 99-mg/dL cohort and 52% of patients taking PCSK9 inhibitors.
Patients seen at teaching hospitals were more likely to undergo LLT intensification compared to nonteaching hospitals (25% versus 17%; P < .001), as were those where lipid protocols were in place (22% versus 15%; P < .001), and those treated in cardiology (22%) compared to treatment in internal or family medicine (12%; P <.001). The study was published online June 16 in JAMA Cardiology.
Dr. Cannon, Dr. Navar, and Dr. Robinson disclosed ties with Amgen, which funded the study, and other companies.
Among patients with atherosclerotic cardiovascular disease (ASCVD), 2 years after release of treat-to-target guidelines from the American Heart Association and the European Society of Cardiology and European Atherosclerosis Society, most patients with LDL cholesterol higher than 70 mg/dL did not receive intensification of therapy, and two-thirds continued to have LDL levels above that level, according to a prospective registry study.
Both guidelines recommend driving LDL-C levels to 50% or below of baseline levels; results from the Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD) registry suggest this is rarely achieved. “Unfortunately it’s not a total surprise, but it’s disappointing,” said Christopher Cannon, MD, the study’s lead author.
“Therapeutic inertia seems to be the rule in clinical practice,” said Jennifer G. Robinson, MD, MPH, who was asked to comment on the study. Dr. Robinson is professor epidemiology and cardiology at the University of Iowa, Iowa City.
“This is yet another disappointing reminder of how we are failing our patients. Lipid lowering is one of the safest, most effective ways to prevent cardiovascular disease, and yet we are falling short. We have the tools in our toolkit to achieve guideline-based lipid lowering goals, but we just aren’t using them,” said Ann Marie Navar, MD, PhD, associate professor of cardiology at the University of Texas, Dallas.
Patients hesitant
Changes in practice following guidelines can often be slow, but in this case may have been complicated by the fact that statins have a reputation for causing side effects, so some patients may be refusing treatment based on what they’ve seen on the Internet. Even though the study looked at all lipid-lowering agents, the misinformation around statins may be spilling over, according to Dr. Cannon. “There’s in general so much misinformation around COVID and every other topic in the world. That makes people question what is real [about] anything,” said Dr. Cannon, a cardiologist at Brigham and Women’s hospital and professor of medicine at Harvard Medical School, both in Boston.
Patient characteristics may partly explain slow uptake. “Clinicians may not think further LDL-C lowering is a high enough priority in terms of potential benefit for a given patient in light of the effort being expended to take care of all their other issues and chronic health problems. If the clinician does bring it up to the patient, there may be barriers in terms of additional medication burden, cost, or acquisition issues,” said Dr. Robinson.
The answer may be better evidence and a more personalized approach. Clinical trials that explore defined patient populations could convince patients of a benefit, and payers to reimburse, according to Dr. Robinson.
Changing guidance
Another complication is that both the guidelines and the field are rapidly changing. The 2013 AHA guidelines did not include a treatment to goal and focused instead on use of high-dose statins. But the 2018 update reversed course after randomized studies demonstrated a benefit to treating to target. The researchers found no increase in the frequency of treating to target after the release of the 2018 guidelines. “Publication and announcement of guidelines doesn’t mean that people are getting treated better. We really have to implement them,” said Dr. Cannon.
On a positive note, the GOULD researchers found high acceptance of the new proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) inhibitors, with over 90% of patients continuing those medications after 2 years. “That’s nice and high. If people do get onto the very intensive lipid-lowing therapies, they tend to stay on them,” said Dr. Cannon.
What’s next
Still, the lack of intensification is concerning, and the findings led to some consternation in Twitter exchanges, said Dr. Cannon. “People posted ‘Well, what do we do now?’ ” Dr. Cannon’s team is addressing the issue with an algorithm-based risk management program with prospective enrollment. They have conducted educational webinars and provided site-specific reports on LDL status among patients at each center compared to others, and hope that information will improve compliance. In 2020, the group published an interim analysis of the first 5,000 enrollees, and Dr. Cannon expects to finish that study by the end of the year.
Dr. Navar agreed that physicians need to do a better job of testing LDL-C levels after treatment to identify patients who require more aggressive therapy. That can be deferred in some primary prevention patients with high LDL-C but normal particle numbers as measured with ApoB. “But in those at high risk for disease and those with established CVD who are not at goal, as long as they don’t have a life-limiting condition, we should always up-titrate therapy. It’s one of the safest, most effective ways to lower cardiovascular risk,” said Dr. Navar.
Key study results
The prospective study included 5,006 patients at 119 centers with a mean age of 68 years. About 40% were women, and 86.1% were White. All had ASCVD and LDL levels of at least 70 mg/dL. After 2 years, 17% had undergone intensification of lipid-lowering therapy (LLT). Among patients with LDL-C levels ≥ 100 mg/dL, 22% underwent LLT intensification, compared with 14% of patients with LDL-C levels of 70-99 mg/dL.
The vast majority, 92%, of patients who underwent LLT via addition of PCSK9 inhibitors were still taking the drug after 2 years.
Three-quarters (3,768) had lipid level measurements at least once during follow-up, and median LDL-C levels dropped from 120 to 95 mg/dL in the ≥100-mg/dL cohort (P < .001), and from 82 to 77 mg/dL in the 70- to 99-mg/dL cohort (P <. 001). There was no significant difference in the median values in the patients on PCSK9 inhibitors.
In all, 21% of the ≥100-mg/dL cohort achieved LDL-C levels <70 mg/dL at 2 years, versus 34% in the 77- to 99-mg/dL cohort and 52% of patients taking PCSK9 inhibitors.
Patients seen at teaching hospitals were more likely to undergo LLT intensification compared to nonteaching hospitals (25% versus 17%; P < .001), as were those where lipid protocols were in place (22% versus 15%; P < .001), and those treated in cardiology (22%) compared to treatment in internal or family medicine (12%; P <.001). The study was published online June 16 in JAMA Cardiology.
Dr. Cannon, Dr. Navar, and Dr. Robinson disclosed ties with Amgen, which funded the study, and other companies.
Among patients with atherosclerotic cardiovascular disease (ASCVD), 2 years after release of treat-to-target guidelines from the American Heart Association and the European Society of Cardiology and European Atherosclerosis Society, most patients with LDL cholesterol higher than 70 mg/dL did not receive intensification of therapy, and two-thirds continued to have LDL levels above that level, according to a prospective registry study.
Both guidelines recommend driving LDL-C levels to 50% or below of baseline levels; results from the Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD) registry suggest this is rarely achieved. “Unfortunately it’s not a total surprise, but it’s disappointing,” said Christopher Cannon, MD, the study’s lead author.
“Therapeutic inertia seems to be the rule in clinical practice,” said Jennifer G. Robinson, MD, MPH, who was asked to comment on the study. Dr. Robinson is professor epidemiology and cardiology at the University of Iowa, Iowa City.
“This is yet another disappointing reminder of how we are failing our patients. Lipid lowering is one of the safest, most effective ways to prevent cardiovascular disease, and yet we are falling short. We have the tools in our toolkit to achieve guideline-based lipid lowering goals, but we just aren’t using them,” said Ann Marie Navar, MD, PhD, associate professor of cardiology at the University of Texas, Dallas.
Patients hesitant
Changes in practice following guidelines can often be slow, but in this case may have been complicated by the fact that statins have a reputation for causing side effects, so some patients may be refusing treatment based on what they’ve seen on the Internet. Even though the study looked at all lipid-lowering agents, the misinformation around statins may be spilling over, according to Dr. Cannon. “There’s in general so much misinformation around COVID and every other topic in the world. That makes people question what is real [about] anything,” said Dr. Cannon, a cardiologist at Brigham and Women’s hospital and professor of medicine at Harvard Medical School, both in Boston.
Patient characteristics may partly explain slow uptake. “Clinicians may not think further LDL-C lowering is a high enough priority in terms of potential benefit for a given patient in light of the effort being expended to take care of all their other issues and chronic health problems. If the clinician does bring it up to the patient, there may be barriers in terms of additional medication burden, cost, or acquisition issues,” said Dr. Robinson.
The answer may be better evidence and a more personalized approach. Clinical trials that explore defined patient populations could convince patients of a benefit, and payers to reimburse, according to Dr. Robinson.
Changing guidance
Another complication is that both the guidelines and the field are rapidly changing. The 2013 AHA guidelines did not include a treatment to goal and focused instead on use of high-dose statins. But the 2018 update reversed course after randomized studies demonstrated a benefit to treating to target. The researchers found no increase in the frequency of treating to target after the release of the 2018 guidelines. “Publication and announcement of guidelines doesn’t mean that people are getting treated better. We really have to implement them,” said Dr. Cannon.
On a positive note, the GOULD researchers found high acceptance of the new proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) inhibitors, with over 90% of patients continuing those medications after 2 years. “That’s nice and high. If people do get onto the very intensive lipid-lowing therapies, they tend to stay on them,” said Dr. Cannon.
What’s next
Still, the lack of intensification is concerning, and the findings led to some consternation in Twitter exchanges, said Dr. Cannon. “People posted ‘Well, what do we do now?’ ” Dr. Cannon’s team is addressing the issue with an algorithm-based risk management program with prospective enrollment. They have conducted educational webinars and provided site-specific reports on LDL status among patients at each center compared to others, and hope that information will improve compliance. In 2020, the group published an interim analysis of the first 5,000 enrollees, and Dr. Cannon expects to finish that study by the end of the year.
Dr. Navar agreed that physicians need to do a better job of testing LDL-C levels after treatment to identify patients who require more aggressive therapy. That can be deferred in some primary prevention patients with high LDL-C but normal particle numbers as measured with ApoB. “But in those at high risk for disease and those with established CVD who are not at goal, as long as they don’t have a life-limiting condition, we should always up-titrate therapy. It’s one of the safest, most effective ways to lower cardiovascular risk,” said Dr. Navar.
Key study results
The prospective study included 5,006 patients at 119 centers with a mean age of 68 years. About 40% were women, and 86.1% were White. All had ASCVD and LDL levels of at least 70 mg/dL. After 2 years, 17% had undergone intensification of lipid-lowering therapy (LLT). Among patients with LDL-C levels ≥ 100 mg/dL, 22% underwent LLT intensification, compared with 14% of patients with LDL-C levels of 70-99 mg/dL.
The vast majority, 92%, of patients who underwent LLT via addition of PCSK9 inhibitors were still taking the drug after 2 years.
Three-quarters (3,768) had lipid level measurements at least once during follow-up, and median LDL-C levels dropped from 120 to 95 mg/dL in the ≥100-mg/dL cohort (P < .001), and from 82 to 77 mg/dL in the 70- to 99-mg/dL cohort (P <. 001). There was no significant difference in the median values in the patients on PCSK9 inhibitors.
In all, 21% of the ≥100-mg/dL cohort achieved LDL-C levels <70 mg/dL at 2 years, versus 34% in the 77- to 99-mg/dL cohort and 52% of patients taking PCSK9 inhibitors.
Patients seen at teaching hospitals were more likely to undergo LLT intensification compared to nonteaching hospitals (25% versus 17%; P < .001), as were those where lipid protocols were in place (22% versus 15%; P < .001), and those treated in cardiology (22%) compared to treatment in internal or family medicine (12%; P <.001). The study was published online June 16 in JAMA Cardiology.
Dr. Cannon, Dr. Navar, and Dr. Robinson disclosed ties with Amgen, which funded the study, and other companies.
FROM JAMA CARDIOLOGY
No overall statin effect seen on dementia, cognition in ASPREE analysis
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”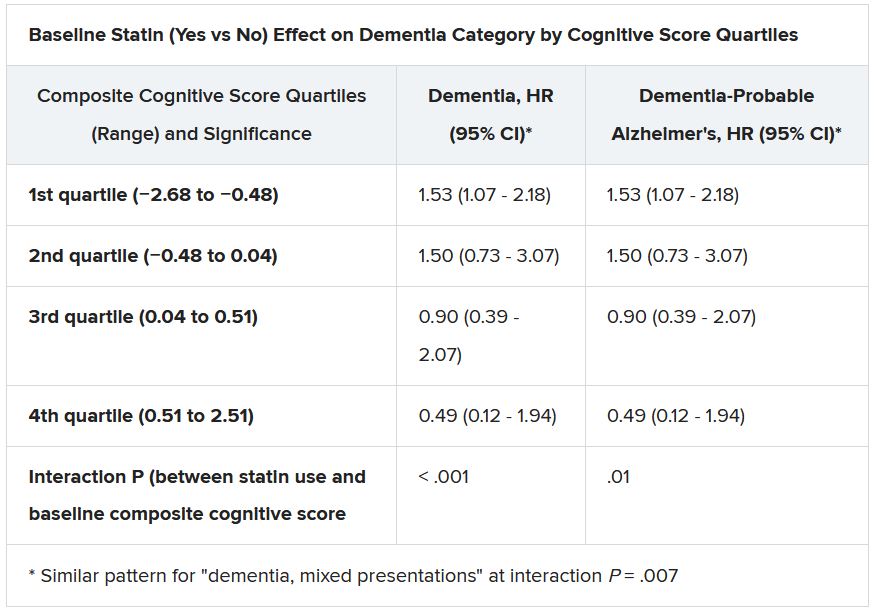
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Sotagliflozin use in T2D patients linked with posthospitalization benefits in analysis
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
The outcome measure –days alive and out of the hospital – may be a meaningful, patient-centered way of capturing disease burden, the researchers wrote in their paper, published in Annals of Internal Medicine.
“The question was: Can we keep patients alive and out of the hospital for any reason, accounting for the duration of each hospitalization?” author Michael Szarek, PhD, a visiting professor in the division of cardiology at the University of Colorado at Denver, Aurora, said in an interview.
“For every 100 days of follow-up, patients in the sotagliflozin group were alive and out of the hospital 3% more days in relative terms or 2.9 days in absolute terms than those in the placebo group (91.8 vs. 88.9 days),” the researchers reported in their analysis of data from the SOLOIST-WHF trial.
“If you translate that to over the course of a year, that’s more than 10 days,” said Dr. Szarek, who is also a faculty member of CPC Clinical Research, an academic research organization affiliated with the University of Colorado.
Most patients in both groups survived to the end of the study without hospitalization, according to the paper.
Sotagliflozin, a sodium-glucose cotransporter 1 and SGLT2 inhibitor, is not approved in the United States. In 2019, the Food and Drug Administration rejected sotagliflozin as an adjunct to insulin for the treatment of type 1 diabetes after members of an advisory committee expressed concerns about an increased risk for diabetic ketoacidosis with the drug.
Methods and results
To examine whether sotagliflozin increased days alive and out of the hospital in the SOLOIST-WHF trial, Dr. Szarek and colleagues analyzed data from this randomized, double-blind, placebo-controlled study. The trial’s primary results were published in the New England Journal of Medicine in January 2021. Researchers conducted SOLOIST-WHF at more than 300 sites in 32 countries. The trial included 1,222 patients with type 2 diabetes and reduced or preserved ejection fraction who were recently hospitalized for worsening heart failure.
In the new analysis the researchers looked at hospitalizations for any reason and the duration of hospital admissions after randomization. They analyzed days alive and out of the hospital using prespecified models.
Similar proportions of patients who received sotagliflozin and placebo were hospitalized at least once (38.5% vs. 41.4%) during a median follow-up of 9 months. Fewer patients who received sotagliflozin were hospitalized more than once (16.3% vs. 22.1%). In all, 64 patients in the sotagliflozin group and 76 patients in the placebo group died.
The reason for each hospitalization was unspecified, except for cases of heart failure, the authors noted. About 62% of hospitalizations during the trial were for reasons other than heart failure.
Outside expert cites similarities to initial trial
The results for days alive and out of the hospital are “not particularly surprising given the previous publication” of the trial’s primary results, but the new analysis provides a “different view of outcomes that might be clinically meaningful for patients,” commented Frank Brosius, MD, a professor of medicine at the University of Arizona, Tucson.
The SOLOIST-WHF trial indicated that doctors may be able to effectively treat patients with relatively new heart failure with sotagliflozin as long as patients are relatively stable, said Dr. Brosius, who coauthored an editorial in the New England Journal of Medicine that accompanied the initial results from the SOLOIST-WHF trial. It appears that previously reported benefits with regard to heart failure outcomes “showed up in these other indicators” in the secondary analysis.
Still, the effect sizes in the new analysis were relatively small and “probably more studies will be necessary” to examine these end points, he added.
SOLOIST-WHF was funded by Sanofi at initiation and by Lexicon Pharmaceuticals at completion. Dr. Szarek disclosed grants from Lexicon and grants and personal fees from Sanofi, as well as personal fees from other companies. His coauthors included employees of Lexicon and other researchers with financial ties to Lexicon and other pharmaceutical companies. Dr. Brosius disclosed personal fees from the American Diabetes Association and is a member of the Diabetic Kidney Disease Collaborative task force for the American Society of Nephrology that is broadly advocating the use of SGLT2 inhibitors by patients with diabetic kidney diseases. He also has participated in an advisory group for treatment of diabetic kidney disease for Gilead.
FROM ANNALS OF INTERNAL MEDICINE
Ten killer steps to writing a great medical thriller
For many physicians and other professionals, aspirations of crafting a work of fiction are not uncommon — and with good reason. We are, after all, a generally well-disciplined bunch capable of completing complex tasks, and there is certainly no shortage of excitement and drama in medicine and surgery — ample fodder for thrilling stories. Nonetheless, writing a novel is a major commitment, and it requires persistence, patience, and dedicated time, especially for one with a busy medical career.
Getting started is not easy. Writing workshops are helpful, and in my case, I tried to mentor with some of the best. Before writing my novel, I attended workshops for aspiring novelists, given by noted physician authors Tess Gerritsen (Body Double, The Surgeon) and the late Michael Palmer (The Society, The Fifth Vial).
Writers are often advised to “write about what you know.” In my case, I combined my knowledge of medicine and my experience with the thoroughbred racing world to craft a thriller that one reviewer described as “Dick Francis meets Robin Cook.” For those who have never read the Dick Francis series, he was a renowned crime writer whose novels centered on horse racing in England. Having been an avid reader of both authors, that comparison was the ultimate compliment.
So against that backdrop, the novel Shedrow, along with some shared wisdom from a few legendary writers.
1. Start with the big “what if.” Any great story starts with that simple “what if” question. What if a series of high-profile executives in the managed care industry are serially murdered (Michael Palmer’s The Society)? What if a multimillion-dollar stallion dies suddenly under very mysterious circumstances on a supposedly secure farm in Kentucky (Dean DeLuke’s Shedrow)?
2. Put a MacGuffin to work in your story. Popularized by Alfred Hitchcock, the MacGuffin is that essential plot element that drives virtually all characters in the story, although it may be rather vague and meaningless to the story itself. In the iconic movie Pulp Fiction, the MacGuffin is the briefcase — everyone wants it, and we never do find out what’s in it.
3. Pacing is critical. Plot out the timeline of emotional highs and lows in a story. It should look like a rolling pattern of highs and lows that crescendo upward to the ultimate crisis. Take advantage of the fact that following any of those emotional peaks, you probably have the reader’s undivided attention. That would be a good time to provide backstory or fill in needed information for the reader – information that may be critical but perhaps not as exciting as what just transpired.
4. Torture your protagonists. Just when the reader thinks that the hero is finally home free, throw in another obstacle. Readers will empathize with the character and be drawn in by the unexpected hurdle.
5. Be original and surprise your readers. Create twists and turns that are totally unexpected, yet believable. This is easier said than done but will go a long way toward making your novel original, gripping, and unpredictable.
6. As a general rule, consider short sentences and short chapters. This is strictly a personal preference, but who can argue with James Patterson’s short chapters or with Robert Parker’s short and engaging sentences? Sentence length can be varied for effect, too, with shorter sentences serving to heighten action or increase tension.
7. Avoid the passive voice. Your readers want action. This is an important rule in almost any type of writing.
8. Keep descriptions brief. Long, drawn-out descriptions of the way characters look, or even setting descriptions, are easily overdone in a thriller. The thriller genre is very different from literary fiction in this regard. Stephen King advises writers to “just say what they see, then get on with the story.”
9. Sustain the reader’s interest throughout. Assess each chapter ending and determine whether the reader has been given enough reason to want to continue reading. Pose a question, end with a minor cliffhanger, or at least ensure that there is enough accumulated tension in the story.
10. Edit aggressively and cut out the fluff. Ernest Hemingway once confided to F. Scott Fitzgerald, “I write one page of masterpiece to 91 pages of shit. I try to put the shit in the wastebasket.”
Dr. DeLuke is professor emeritus of oral and facial surgery at Virginia Commonwealth University and author of the novel Shedrow.
A version of this article first appeared on Medscape.com.
For many physicians and other professionals, aspirations of crafting a work of fiction are not uncommon — and with good reason. We are, after all, a generally well-disciplined bunch capable of completing complex tasks, and there is certainly no shortage of excitement and drama in medicine and surgery — ample fodder for thrilling stories. Nonetheless, writing a novel is a major commitment, and it requires persistence, patience, and dedicated time, especially for one with a busy medical career.
Getting started is not easy. Writing workshops are helpful, and in my case, I tried to mentor with some of the best. Before writing my novel, I attended workshops for aspiring novelists, given by noted physician authors Tess Gerritsen (Body Double, The Surgeon) and the late Michael Palmer (The Society, The Fifth Vial).
Writers are often advised to “write about what you know.” In my case, I combined my knowledge of medicine and my experience with the thoroughbred racing world to craft a thriller that one reviewer described as “Dick Francis meets Robin Cook.” For those who have never read the Dick Francis series, he was a renowned crime writer whose novels centered on horse racing in England. Having been an avid reader of both authors, that comparison was the ultimate compliment.
So against that backdrop, the novel Shedrow, along with some shared wisdom from a few legendary writers.
1. Start with the big “what if.” Any great story starts with that simple “what if” question. What if a series of high-profile executives in the managed care industry are serially murdered (Michael Palmer’s The Society)? What if a multimillion-dollar stallion dies suddenly under very mysterious circumstances on a supposedly secure farm in Kentucky (Dean DeLuke’s Shedrow)?
2. Put a MacGuffin to work in your story. Popularized by Alfred Hitchcock, the MacGuffin is that essential plot element that drives virtually all characters in the story, although it may be rather vague and meaningless to the story itself. In the iconic movie Pulp Fiction, the MacGuffin is the briefcase — everyone wants it, and we never do find out what’s in it.
3. Pacing is critical. Plot out the timeline of emotional highs and lows in a story. It should look like a rolling pattern of highs and lows that crescendo upward to the ultimate crisis. Take advantage of the fact that following any of those emotional peaks, you probably have the reader’s undivided attention. That would be a good time to provide backstory or fill in needed information for the reader – information that may be critical but perhaps not as exciting as what just transpired.
4. Torture your protagonists. Just when the reader thinks that the hero is finally home free, throw in another obstacle. Readers will empathize with the character and be drawn in by the unexpected hurdle.
5. Be original and surprise your readers. Create twists and turns that are totally unexpected, yet believable. This is easier said than done but will go a long way toward making your novel original, gripping, and unpredictable.
6. As a general rule, consider short sentences and short chapters. This is strictly a personal preference, but who can argue with James Patterson’s short chapters or with Robert Parker’s short and engaging sentences? Sentence length can be varied for effect, too, with shorter sentences serving to heighten action or increase tension.
7. Avoid the passive voice. Your readers want action. This is an important rule in almost any type of writing.
8. Keep descriptions brief. Long, drawn-out descriptions of the way characters look, or even setting descriptions, are easily overdone in a thriller. The thriller genre is very different from literary fiction in this regard. Stephen King advises writers to “just say what they see, then get on with the story.”
9. Sustain the reader’s interest throughout. Assess each chapter ending and determine whether the reader has been given enough reason to want to continue reading. Pose a question, end with a minor cliffhanger, or at least ensure that there is enough accumulated tension in the story.
10. Edit aggressively and cut out the fluff. Ernest Hemingway once confided to F. Scott Fitzgerald, “I write one page of masterpiece to 91 pages of shit. I try to put the shit in the wastebasket.”
Dr. DeLuke is professor emeritus of oral and facial surgery at Virginia Commonwealth University and author of the novel Shedrow.
A version of this article first appeared on Medscape.com.
For many physicians and other professionals, aspirations of crafting a work of fiction are not uncommon — and with good reason. We are, after all, a generally well-disciplined bunch capable of completing complex tasks, and there is certainly no shortage of excitement and drama in medicine and surgery — ample fodder for thrilling stories. Nonetheless, writing a novel is a major commitment, and it requires persistence, patience, and dedicated time, especially for one with a busy medical career.
Getting started is not easy. Writing workshops are helpful, and in my case, I tried to mentor with some of the best. Before writing my novel, I attended workshops for aspiring novelists, given by noted physician authors Tess Gerritsen (Body Double, The Surgeon) and the late Michael Palmer (The Society, The Fifth Vial).
Writers are often advised to “write about what you know.” In my case, I combined my knowledge of medicine and my experience with the thoroughbred racing world to craft a thriller that one reviewer described as “Dick Francis meets Robin Cook.” For those who have never read the Dick Francis series, he was a renowned crime writer whose novels centered on horse racing in England. Having been an avid reader of both authors, that comparison was the ultimate compliment.
So against that backdrop, the novel Shedrow, along with some shared wisdom from a few legendary writers.
1. Start with the big “what if.” Any great story starts with that simple “what if” question. What if a series of high-profile executives in the managed care industry are serially murdered (Michael Palmer’s The Society)? What if a multimillion-dollar stallion dies suddenly under very mysterious circumstances on a supposedly secure farm in Kentucky (Dean DeLuke’s Shedrow)?
2. Put a MacGuffin to work in your story. Popularized by Alfred Hitchcock, the MacGuffin is that essential plot element that drives virtually all characters in the story, although it may be rather vague and meaningless to the story itself. In the iconic movie Pulp Fiction, the MacGuffin is the briefcase — everyone wants it, and we never do find out what’s in it.
3. Pacing is critical. Plot out the timeline of emotional highs and lows in a story. It should look like a rolling pattern of highs and lows that crescendo upward to the ultimate crisis. Take advantage of the fact that following any of those emotional peaks, you probably have the reader’s undivided attention. That would be a good time to provide backstory or fill in needed information for the reader – information that may be critical but perhaps not as exciting as what just transpired.
4. Torture your protagonists. Just when the reader thinks that the hero is finally home free, throw in another obstacle. Readers will empathize with the character and be drawn in by the unexpected hurdle.
5. Be original and surprise your readers. Create twists and turns that are totally unexpected, yet believable. This is easier said than done but will go a long way toward making your novel original, gripping, and unpredictable.
6. As a general rule, consider short sentences and short chapters. This is strictly a personal preference, but who can argue with James Patterson’s short chapters or with Robert Parker’s short and engaging sentences? Sentence length can be varied for effect, too, with shorter sentences serving to heighten action or increase tension.
7. Avoid the passive voice. Your readers want action. This is an important rule in almost any type of writing.
8. Keep descriptions brief. Long, drawn-out descriptions of the way characters look, or even setting descriptions, are easily overdone in a thriller. The thriller genre is very different from literary fiction in this regard. Stephen King advises writers to “just say what they see, then get on with the story.”
9. Sustain the reader’s interest throughout. Assess each chapter ending and determine whether the reader has been given enough reason to want to continue reading. Pose a question, end with a minor cliffhanger, or at least ensure that there is enough accumulated tension in the story.
10. Edit aggressively and cut out the fluff. Ernest Hemingway once confided to F. Scott Fitzgerald, “I write one page of masterpiece to 91 pages of shit. I try to put the shit in the wastebasket.”
Dr. DeLuke is professor emeritus of oral and facial surgery at Virginia Commonwealth University and author of the novel Shedrow.
A version of this article first appeared on Medscape.com.
Telemedicine is poised to drive new models of care
Telemedicine has been proposed as a solution for an array of health care access problems over decades of gradual growth. The vast ramping up of , according to an update at the annual health policy and advocacy conference sponsored by the American College of Chest Physicians.
“The cat is out of the bag,” said Jaspal Singh, MD, professor of medicine, Atrium Health, Charlotte, N.C. Due to changes in access and reimbursement to telemedicine driven by the pandemic, he said, “we now have permission to explore new models of care.”
Prior to February 2020, telemedicine was crawling forward at a leisurely pace, according to Dr. Singh. After March 2020, it broke into a run due to enormous demand and was met by a rapid response from the U.S. Congress. The first of four legislative bills that directly or indirectly supported telemedicine was passed on March 6, 2020.
The Centers for Medicare and Medicaid Services responded in kind, making modifications in a number of rules that removed obstacles to telehealth. One modification on April 6, 2020, for example, removed the requirement for a preexisting relationship between the clinician and patient, Dr. Singh said. The CMS also subsequently modified reimbursement policies in order to make telemedicine more tenable for physicians.
Given the risk of contagion from face-to-face encounters, telemedicine in the early days of the pandemic was not just attractive but the only practical and safe approach to medical care in many circumstances. Physicians and patients were anxious for health care that did not require in-office visits even though many critical issues for telemedicine, including its relative effectiveness, had not yet been fully evaluated.
Much has been learned regarding the feasibility and acceptability of telemedicine during the pandemic, but Dr. Singh noted that quality of care relative to in-person visits remains weakly supported for most indications. Indeed, he outlined sizable list of incompletely resolved issues, including optimal payment models, management of privacy concerns, and how to balance advantages to disadvantages.
For patients and physicians, the strengths of telemedicine include greater convenience made possible by the elimination of travel and waiting rooms. For the health care system, it can include less infrastructure and overhead. For many physicians, telemedicine might be perceived as more efficient.
On the other hand, some patients might feel that a clinical encounter is incomplete without a physical examination even when the physician does not feel the physical examination is needed, according to Dr. Singh. He cited a survey suggesting nearly half of patients expressed concern about a lack of connection to health care providers following a virtual visit.
In the same 2020 National Poll on Healthy Aging 2020 survey conducted by the University of Michigan 67% of respondents reported that the quality of care was not as good as that provided by in-patient visits, and 24% expressed concern about privacy. However, at the time the poll was taken in May 2020, experience with telemedicine among many of the respondents may have been limited. As telemedicine is integrated into routine care, perceptions might change as experience increases.
A distinction between telemedicine in routine care and telemedicine as a strategy to respond to a pandemic is important, Dr. Singh indicated. Dr. Singh was the lead author for a position paper on telemedicine for the diagnosis and treatment of sleep disorders from the American Academy of Sleep Medicine 5 years ago, but he acknowledged that models of care might differ when responding to abnormal surges in health care demand.
The surge in demand for COVID-19–related care engendered numerous innovative solutions. As examples, Dr. Singh recounted how a virtual hospital was created at his own institution. In a published study, 1,477 patients diagnosed with COVID-19 over a 6-week period remained at home and received care in a virtual observation unit (VCU) or a virtual acute care unit (VACU) . Only a small percentage required eventual hospital admission. In the VACU, patients were able to receive advanced care including IV fluids and some form of respiratory support .
It is unclear how the COVID-19 pandemic will change telemedicine. Now, with declining cases of the infection, telemedicine is back to a walk after the sprint required during the height of the pandemic, according to Dr. Singh. However, Dr. Singh thinks many physicians and patients will have a different perception of telemedicine after the widespread exposure to this type of care.
In terms of the relative role of in-patient and virtual visits across indications, “we do not know how this will play out, but we will probably end up toggling between the two,” Dr. Singh said.
This is an area that is being followed closely by the CHEST Health Policy and Advocacy Committee, according to Kathleen Sarmiento, MD, director, VISN 21 Sleep Clinical Resource Hub for the San Francisco VA Health Care System. A member of that committee and moderator of the session in which Dr. Singh spoke,
Dr. Sarmiento called the effort to bring permanent coverage of telehealth services “the shared responsibility of every medical society engaged in advocacy.”
However, she cautioned that there might be intended and unintended consequences from telehealth that require analysis to develop policies that are in the best interests of effective care. She said, the “ACCP, along with its sister societies, does have a role in supporting the evaluation of the impact of these changes on both patients and providers in the fields of pulmonary medicine, critical care, and sleep medicine.”
Dr. Singh reports a financial relationship with AstraZeneca. Dr. Sarmiento reports no relevant financial relationship with AstraZeneca.
Telemedicine has been proposed as a solution for an array of health care access problems over decades of gradual growth. The vast ramping up of , according to an update at the annual health policy and advocacy conference sponsored by the American College of Chest Physicians.
“The cat is out of the bag,” said Jaspal Singh, MD, professor of medicine, Atrium Health, Charlotte, N.C. Due to changes in access and reimbursement to telemedicine driven by the pandemic, he said, “we now have permission to explore new models of care.”
Prior to February 2020, telemedicine was crawling forward at a leisurely pace, according to Dr. Singh. After March 2020, it broke into a run due to enormous demand and was met by a rapid response from the U.S. Congress. The first of four legislative bills that directly or indirectly supported telemedicine was passed on March 6, 2020.
The Centers for Medicare and Medicaid Services responded in kind, making modifications in a number of rules that removed obstacles to telehealth. One modification on April 6, 2020, for example, removed the requirement for a preexisting relationship between the clinician and patient, Dr. Singh said. The CMS also subsequently modified reimbursement policies in order to make telemedicine more tenable for physicians.
Given the risk of contagion from face-to-face encounters, telemedicine in the early days of the pandemic was not just attractive but the only practical and safe approach to medical care in many circumstances. Physicians and patients were anxious for health care that did not require in-office visits even though many critical issues for telemedicine, including its relative effectiveness, had not yet been fully evaluated.
Much has been learned regarding the feasibility and acceptability of telemedicine during the pandemic, but Dr. Singh noted that quality of care relative to in-person visits remains weakly supported for most indications. Indeed, he outlined sizable list of incompletely resolved issues, including optimal payment models, management of privacy concerns, and how to balance advantages to disadvantages.
For patients and physicians, the strengths of telemedicine include greater convenience made possible by the elimination of travel and waiting rooms. For the health care system, it can include less infrastructure and overhead. For many physicians, telemedicine might be perceived as more efficient.
On the other hand, some patients might feel that a clinical encounter is incomplete without a physical examination even when the physician does not feel the physical examination is needed, according to Dr. Singh. He cited a survey suggesting nearly half of patients expressed concern about a lack of connection to health care providers following a virtual visit.
In the same 2020 National Poll on Healthy Aging 2020 survey conducted by the University of Michigan 67% of respondents reported that the quality of care was not as good as that provided by in-patient visits, and 24% expressed concern about privacy. However, at the time the poll was taken in May 2020, experience with telemedicine among many of the respondents may have been limited. As telemedicine is integrated into routine care, perceptions might change as experience increases.
A distinction between telemedicine in routine care and telemedicine as a strategy to respond to a pandemic is important, Dr. Singh indicated. Dr. Singh was the lead author for a position paper on telemedicine for the diagnosis and treatment of sleep disorders from the American Academy of Sleep Medicine 5 years ago, but he acknowledged that models of care might differ when responding to abnormal surges in health care demand.
The surge in demand for COVID-19–related care engendered numerous innovative solutions. As examples, Dr. Singh recounted how a virtual hospital was created at his own institution. In a published study, 1,477 patients diagnosed with COVID-19 over a 6-week period remained at home and received care in a virtual observation unit (VCU) or a virtual acute care unit (VACU) . Only a small percentage required eventual hospital admission. In the VACU, patients were able to receive advanced care including IV fluids and some form of respiratory support .
It is unclear how the COVID-19 pandemic will change telemedicine. Now, with declining cases of the infection, telemedicine is back to a walk after the sprint required during the height of the pandemic, according to Dr. Singh. However, Dr. Singh thinks many physicians and patients will have a different perception of telemedicine after the widespread exposure to this type of care.
In terms of the relative role of in-patient and virtual visits across indications, “we do not know how this will play out, but we will probably end up toggling between the two,” Dr. Singh said.
This is an area that is being followed closely by the CHEST Health Policy and Advocacy Committee, according to Kathleen Sarmiento, MD, director, VISN 21 Sleep Clinical Resource Hub for the San Francisco VA Health Care System. A member of that committee and moderator of the session in which Dr. Singh spoke,
Dr. Sarmiento called the effort to bring permanent coverage of telehealth services “the shared responsibility of every medical society engaged in advocacy.”
However, she cautioned that there might be intended and unintended consequences from telehealth that require analysis to develop policies that are in the best interests of effective care. She said, the “ACCP, along with its sister societies, does have a role in supporting the evaluation of the impact of these changes on both patients and providers in the fields of pulmonary medicine, critical care, and sleep medicine.”
Dr. Singh reports a financial relationship with AstraZeneca. Dr. Sarmiento reports no relevant financial relationship with AstraZeneca.
Telemedicine has been proposed as a solution for an array of health care access problems over decades of gradual growth. The vast ramping up of , according to an update at the annual health policy and advocacy conference sponsored by the American College of Chest Physicians.
“The cat is out of the bag,” said Jaspal Singh, MD, professor of medicine, Atrium Health, Charlotte, N.C. Due to changes in access and reimbursement to telemedicine driven by the pandemic, he said, “we now have permission to explore new models of care.”
Prior to February 2020, telemedicine was crawling forward at a leisurely pace, according to Dr. Singh. After March 2020, it broke into a run due to enormous demand and was met by a rapid response from the U.S. Congress. The first of four legislative bills that directly or indirectly supported telemedicine was passed on March 6, 2020.
The Centers for Medicare and Medicaid Services responded in kind, making modifications in a number of rules that removed obstacles to telehealth. One modification on April 6, 2020, for example, removed the requirement for a preexisting relationship between the clinician and patient, Dr. Singh said. The CMS also subsequently modified reimbursement policies in order to make telemedicine more tenable for physicians.
Given the risk of contagion from face-to-face encounters, telemedicine in the early days of the pandemic was not just attractive but the only practical and safe approach to medical care in many circumstances. Physicians and patients were anxious for health care that did not require in-office visits even though many critical issues for telemedicine, including its relative effectiveness, had not yet been fully evaluated.
Much has been learned regarding the feasibility and acceptability of telemedicine during the pandemic, but Dr. Singh noted that quality of care relative to in-person visits remains weakly supported for most indications. Indeed, he outlined sizable list of incompletely resolved issues, including optimal payment models, management of privacy concerns, and how to balance advantages to disadvantages.
For patients and physicians, the strengths of telemedicine include greater convenience made possible by the elimination of travel and waiting rooms. For the health care system, it can include less infrastructure and overhead. For many physicians, telemedicine might be perceived as more efficient.
On the other hand, some patients might feel that a clinical encounter is incomplete without a physical examination even when the physician does not feel the physical examination is needed, according to Dr. Singh. He cited a survey suggesting nearly half of patients expressed concern about a lack of connection to health care providers following a virtual visit.
In the same 2020 National Poll on Healthy Aging 2020 survey conducted by the University of Michigan 67% of respondents reported that the quality of care was not as good as that provided by in-patient visits, and 24% expressed concern about privacy. However, at the time the poll was taken in May 2020, experience with telemedicine among many of the respondents may have been limited. As telemedicine is integrated into routine care, perceptions might change as experience increases.
A distinction between telemedicine in routine care and telemedicine as a strategy to respond to a pandemic is important, Dr. Singh indicated. Dr. Singh was the lead author for a position paper on telemedicine for the diagnosis and treatment of sleep disorders from the American Academy of Sleep Medicine 5 years ago, but he acknowledged that models of care might differ when responding to abnormal surges in health care demand.
The surge in demand for COVID-19–related care engendered numerous innovative solutions. As examples, Dr. Singh recounted how a virtual hospital was created at his own institution. In a published study, 1,477 patients diagnosed with COVID-19 over a 6-week period remained at home and received care in a virtual observation unit (VCU) or a virtual acute care unit (VACU) . Only a small percentage required eventual hospital admission. In the VACU, patients were able to receive advanced care including IV fluids and some form of respiratory support .
It is unclear how the COVID-19 pandemic will change telemedicine. Now, with declining cases of the infection, telemedicine is back to a walk after the sprint required during the height of the pandemic, according to Dr. Singh. However, Dr. Singh thinks many physicians and patients will have a different perception of telemedicine after the widespread exposure to this type of care.
In terms of the relative role of in-patient and virtual visits across indications, “we do not know how this will play out, but we will probably end up toggling between the two,” Dr. Singh said.
This is an area that is being followed closely by the CHEST Health Policy and Advocacy Committee, according to Kathleen Sarmiento, MD, director, VISN 21 Sleep Clinical Resource Hub for the San Francisco VA Health Care System. A member of that committee and moderator of the session in which Dr. Singh spoke,
Dr. Sarmiento called the effort to bring permanent coverage of telehealth services “the shared responsibility of every medical society engaged in advocacy.”
However, she cautioned that there might be intended and unintended consequences from telehealth that require analysis to develop policies that are in the best interests of effective care. She said, the “ACCP, along with its sister societies, does have a role in supporting the evaluation of the impact of these changes on both patients and providers in the fields of pulmonary medicine, critical care, and sleep medicine.”
Dr. Singh reports a financial relationship with AstraZeneca. Dr. Sarmiento reports no relevant financial relationship with AstraZeneca.
FROM A HEALTH POLICY AND ADVOCACY CONFERENCE
Bariatric surgery tied to 22% lower 5-year stroke risk
Patients with obesity who underwent bariatric surgery had 46% lower odds of stroke 1 year later, similar odds of stroke 3 years later, and 22% lower odds of stroke 5 years later, compared with matched control patients, in new research.
Michael D. Williams, MD, presented the study findings (abstract A002) at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings are “very good news,” even though the protection against stroke declined further out from the surgery, John D. Scott, MD, scientific program chair of the ASMBS meeting, told this news organization.
The investigators matched more than 56,000 patients with obesity who had bariatric surgery with an equal number of similar patients who did not have this surgery, from a large national insurance database, in what they believe is the largest study of this to date.
“Any intervention that decreases your risk of [cardiovascular] events is good news,” said Dr. Scott, a clinical professor of surgery at the University of South Carolina, Greenville, and metabolic and bariatric surgery director at Prisma Health in Greenville, S.C. “And having a 22%-45% chance of reduction in stroke risk is a very worthwhile intervention.”
Asked how this would change the way clinicians inform patients of what to expect from bariatric surgery, he said: “I would advise patients that studies like this show that surgery would not increase your risk of having a stroke.
“This is consistent with many studies that show that the risks of all macrovascular events decrease after the comorbidity reductions seen after surgery.”
According to Dr. Scott, “the next steps might include a prospective randomized trial of medical treatment versus surgery alone for [cardiovascular]/stroke outcomes, but this is unlikely.”
Similarly, Dr. Williams told this news organization that “I would tell [patients] that surgery is an effective and durable method for weight loss. It also can improve comorbid conditions, particularly diabetes and hypertension.”
Even with this study, “I’m not sure it’s appropriate to say that bariatric surgery will reduce the risk of stroke,” he cautioned.
“However, as we continue to investigate the effects of bariatric surgery, this study contributes to the greater body of knowledge that suggests that reduction in ischemic stroke risk is yet another benefit of bariatric surgery.”
The assigned discussant, Corrigan L. McBride, MD, MBA wanted to know if the lower odds ratio at 1 year might be because preoperative patient selection might eliminate patients at high risk of poor cardiovascular outcomes.
Dr. Williams, a resident at Rush Medical College, Chicago, replied that it is difficult to eliminate potential selection bias, despite best efforts, but this study shows that he can tell patients: “Having surgery is not going to increases your risk of stroke.”
“This is an important study,” Dr. McBride, professor and chief of minimally invasive surgery and bariatric surgery, University of Nebraska Medical Center, Omaha, told this news organization.
“It is the first large study to show a decreased [or no increased] risk of stroke 1, 3, and 5 years after bariatric surgery compared to matched patients, and it had enough data to look at stroke as a standalone endpoint,” Dr. McBride said. “It is important too, for patients and their physicians to understand that there is a lower chance of them having a stroke if they have surgery than if they do not.”
‘Important,’ ‘good news’ for stroke risk after bariatric surgery
The impact of bariatric surgery on remission of type 2 diabetes is well known, Dr. Williams noted, and other studies have reported how bariatric surgery affects the risk of major adverse cardiovascular events – a composite of stroke, myocardial infarction, coronary artery disease, and all-cause death – including a study presented in the same meeting session.
However, a very large sample size is needed to be able to demonstrate the effect of bariatric surgery on stroke, since stroke is a rare event.
The researchers analyzed data from the Mariner (PearlDiver.) all-payer insurance national claims database of patients in the United States.
They matched 56,514 patients with a body mass index over 35 kg/m2 and comorbidities or a BMI of more than 40 who underwent sleeve gastrectomy or Roux-en-Y gastric bypass during 2010-2019 with 56,514 control patients who did not undergo bariatric surgery.
A year after bariatric surgery, patients in that group had a lower stroke rate than patients in the control group (0.6% vs. 1.2%), and they had close to 50% lower odds of having a stroke (odds ratio, 0.54; 95% CI, 0.47-0.61).
Three years after bariatric surgery, there were 44,948 patients in each group; the rate of stroke was 2.1% in the surgery group and 2.2% in the control group, and there was no significant difference in the odds of having a stroke (OR, 0.96; 95% CI, 0.91-1.00).
Five years after bariatric surgery, there were 27,619 patients in each group; the stroke rate was lower in the bariatric surgery group than in the control group (2.8% vs 3.6%), but reduced odds of stroke was not as great as after 1 year (OR, 0.78; 95% CI, 0.65-0.90).
Dr. Williams has no relevant financial disclosures. Dr. McBride and Dr. Scott disclosed that they are speakers/trainers/faculty advisers for Gore. Dr. Scott is also a consultant for C-SATS (part of Johnson & Johnson).
Patients with obesity who underwent bariatric surgery had 46% lower odds of stroke 1 year later, similar odds of stroke 3 years later, and 22% lower odds of stroke 5 years later, compared with matched control patients, in new research.
Michael D. Williams, MD, presented the study findings (abstract A002) at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings are “very good news,” even though the protection against stroke declined further out from the surgery, John D. Scott, MD, scientific program chair of the ASMBS meeting, told this news organization.
The investigators matched more than 56,000 patients with obesity who had bariatric surgery with an equal number of similar patients who did not have this surgery, from a large national insurance database, in what they believe is the largest study of this to date.
“Any intervention that decreases your risk of [cardiovascular] events is good news,” said Dr. Scott, a clinical professor of surgery at the University of South Carolina, Greenville, and metabolic and bariatric surgery director at Prisma Health in Greenville, S.C. “And having a 22%-45% chance of reduction in stroke risk is a very worthwhile intervention.”
Asked how this would change the way clinicians inform patients of what to expect from bariatric surgery, he said: “I would advise patients that studies like this show that surgery would not increase your risk of having a stroke.
“This is consistent with many studies that show that the risks of all macrovascular events decrease after the comorbidity reductions seen after surgery.”
According to Dr. Scott, “the next steps might include a prospective randomized trial of medical treatment versus surgery alone for [cardiovascular]/stroke outcomes, but this is unlikely.”
Similarly, Dr. Williams told this news organization that “I would tell [patients] that surgery is an effective and durable method for weight loss. It also can improve comorbid conditions, particularly diabetes and hypertension.”
Even with this study, “I’m not sure it’s appropriate to say that bariatric surgery will reduce the risk of stroke,” he cautioned.
“However, as we continue to investigate the effects of bariatric surgery, this study contributes to the greater body of knowledge that suggests that reduction in ischemic stroke risk is yet another benefit of bariatric surgery.”
The assigned discussant, Corrigan L. McBride, MD, MBA wanted to know if the lower odds ratio at 1 year might be because preoperative patient selection might eliminate patients at high risk of poor cardiovascular outcomes.
Dr. Williams, a resident at Rush Medical College, Chicago, replied that it is difficult to eliminate potential selection bias, despite best efforts, but this study shows that he can tell patients: “Having surgery is not going to increases your risk of stroke.”
“This is an important study,” Dr. McBride, professor and chief of minimally invasive surgery and bariatric surgery, University of Nebraska Medical Center, Omaha, told this news organization.
“It is the first large study to show a decreased [or no increased] risk of stroke 1, 3, and 5 years after bariatric surgery compared to matched patients, and it had enough data to look at stroke as a standalone endpoint,” Dr. McBride said. “It is important too, for patients and their physicians to understand that there is a lower chance of them having a stroke if they have surgery than if they do not.”
‘Important,’ ‘good news’ for stroke risk after bariatric surgery
The impact of bariatric surgery on remission of type 2 diabetes is well known, Dr. Williams noted, and other studies have reported how bariatric surgery affects the risk of major adverse cardiovascular events – a composite of stroke, myocardial infarction, coronary artery disease, and all-cause death – including a study presented in the same meeting session.
However, a very large sample size is needed to be able to demonstrate the effect of bariatric surgery on stroke, since stroke is a rare event.
The researchers analyzed data from the Mariner (PearlDiver.) all-payer insurance national claims database of patients in the United States.
They matched 56,514 patients with a body mass index over 35 kg/m2 and comorbidities or a BMI of more than 40 who underwent sleeve gastrectomy or Roux-en-Y gastric bypass during 2010-2019 with 56,514 control patients who did not undergo bariatric surgery.
A year after bariatric surgery, patients in that group had a lower stroke rate than patients in the control group (0.6% vs. 1.2%), and they had close to 50% lower odds of having a stroke (odds ratio, 0.54; 95% CI, 0.47-0.61).
Three years after bariatric surgery, there were 44,948 patients in each group; the rate of stroke was 2.1% in the surgery group and 2.2% in the control group, and there was no significant difference in the odds of having a stroke (OR, 0.96; 95% CI, 0.91-1.00).
Five years after bariatric surgery, there were 27,619 patients in each group; the stroke rate was lower in the bariatric surgery group than in the control group (2.8% vs 3.6%), but reduced odds of stroke was not as great as after 1 year (OR, 0.78; 95% CI, 0.65-0.90).
Dr. Williams has no relevant financial disclosures. Dr. McBride and Dr. Scott disclosed that they are speakers/trainers/faculty advisers for Gore. Dr. Scott is also a consultant for C-SATS (part of Johnson & Johnson).
Patients with obesity who underwent bariatric surgery had 46% lower odds of stroke 1 year later, similar odds of stroke 3 years later, and 22% lower odds of stroke 5 years later, compared with matched control patients, in new research.
Michael D. Williams, MD, presented the study findings (abstract A002) at the annual meeting of the American Society for Metabolic & Bariatric Surgery.
The findings are “very good news,” even though the protection against stroke declined further out from the surgery, John D. Scott, MD, scientific program chair of the ASMBS meeting, told this news organization.
The investigators matched more than 56,000 patients with obesity who had bariatric surgery with an equal number of similar patients who did not have this surgery, from a large national insurance database, in what they believe is the largest study of this to date.
“Any intervention that decreases your risk of [cardiovascular] events is good news,” said Dr. Scott, a clinical professor of surgery at the University of South Carolina, Greenville, and metabolic and bariatric surgery director at Prisma Health in Greenville, S.C. “And having a 22%-45% chance of reduction in stroke risk is a very worthwhile intervention.”
Asked how this would change the way clinicians inform patients of what to expect from bariatric surgery, he said: “I would advise patients that studies like this show that surgery would not increase your risk of having a stroke.
“This is consistent with many studies that show that the risks of all macrovascular events decrease after the comorbidity reductions seen after surgery.”
According to Dr. Scott, “the next steps might include a prospective randomized trial of medical treatment versus surgery alone for [cardiovascular]/stroke outcomes, but this is unlikely.”
Similarly, Dr. Williams told this news organization that “I would tell [patients] that surgery is an effective and durable method for weight loss. It also can improve comorbid conditions, particularly diabetes and hypertension.”
Even with this study, “I’m not sure it’s appropriate to say that bariatric surgery will reduce the risk of stroke,” he cautioned.
“However, as we continue to investigate the effects of bariatric surgery, this study contributes to the greater body of knowledge that suggests that reduction in ischemic stroke risk is yet another benefit of bariatric surgery.”
The assigned discussant, Corrigan L. McBride, MD, MBA wanted to know if the lower odds ratio at 1 year might be because preoperative patient selection might eliminate patients at high risk of poor cardiovascular outcomes.
Dr. Williams, a resident at Rush Medical College, Chicago, replied that it is difficult to eliminate potential selection bias, despite best efforts, but this study shows that he can tell patients: “Having surgery is not going to increases your risk of stroke.”
“This is an important study,” Dr. McBride, professor and chief of minimally invasive surgery and bariatric surgery, University of Nebraska Medical Center, Omaha, told this news organization.
“It is the first large study to show a decreased [or no increased] risk of stroke 1, 3, and 5 years after bariatric surgery compared to matched patients, and it had enough data to look at stroke as a standalone endpoint,” Dr. McBride said. “It is important too, for patients and their physicians to understand that there is a lower chance of them having a stroke if they have surgery than if they do not.”
‘Important,’ ‘good news’ for stroke risk after bariatric surgery
The impact of bariatric surgery on remission of type 2 diabetes is well known, Dr. Williams noted, and other studies have reported how bariatric surgery affects the risk of major adverse cardiovascular events – a composite of stroke, myocardial infarction, coronary artery disease, and all-cause death – including a study presented in the same meeting session.
However, a very large sample size is needed to be able to demonstrate the effect of bariatric surgery on stroke, since stroke is a rare event.
The researchers analyzed data from the Mariner (PearlDiver.) all-payer insurance national claims database of patients in the United States.
They matched 56,514 patients with a body mass index over 35 kg/m2 and comorbidities or a BMI of more than 40 who underwent sleeve gastrectomy or Roux-en-Y gastric bypass during 2010-2019 with 56,514 control patients who did not undergo bariatric surgery.
A year after bariatric surgery, patients in that group had a lower stroke rate than patients in the control group (0.6% vs. 1.2%), and they had close to 50% lower odds of having a stroke (odds ratio, 0.54; 95% CI, 0.47-0.61).
Three years after bariatric surgery, there were 44,948 patients in each group; the rate of stroke was 2.1% in the surgery group and 2.2% in the control group, and there was no significant difference in the odds of having a stroke (OR, 0.96; 95% CI, 0.91-1.00).
Five years after bariatric surgery, there were 27,619 patients in each group; the stroke rate was lower in the bariatric surgery group than in the control group (2.8% vs 3.6%), but reduced odds of stroke was not as great as after 1 year (OR, 0.78; 95% CI, 0.65-0.90).
Dr. Williams has no relevant financial disclosures. Dr. McBride and Dr. Scott disclosed that they are speakers/trainers/faculty advisers for Gore. Dr. Scott is also a consultant for C-SATS (part of Johnson & Johnson).
FROM ASMBS 2021
Prophylactic anticoagulation tied to lower death rate in COVID
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Prophylactic anticoagulation to prevent venous thromboembolism (VTE) was associated with reduced 60-day mortality in patients with COVID-19 who were ill enough to require hospitalization, a new report shows.
In a cohort study of more than 1,300 hospitalized patients with COVID-19 infection across 30 hospitals in Michigan, both prophylactic- and therapeutic-dose anticoagulation were associated with reduced in-hospital mortality; however, at 60 days, only prophylactic-dose anticoagulation remained associated with lower mortality.
And adherence was key; nonadherence, or missing 2 days or more of anticoagulation, was linked to more deaths at 60 days.
The findings, which were published online June 11 in JAMA Network Open, are final proof that a prophylactic anticoagulation strategy for the hospitalized COVID population is, indeed, the right one, Valerie M. Vaughn, MD, director of hospital medicine research at the University of Utah, Salt Lake City, said in an interview.
“We’ve probably always known that patients with COVID need prophylaxis for VTE, but we found that early on, unfortunately, that wasn’t being done,” Dr. Vaughn said.
“Now, we see that prophylactic rates have increased. We always knew to use anticoagulation prophylactically in patients who were hospitalized with infection because of their risk for VTE, so this study just drives home that proper adherence to an anticoagulation protocol improves mortality,” she said.
Dr. Vaughn was on the front lines when COVID-19 came to Michigan, where the research was conducted.
“We probably should have been anticoagulating from the get-go, but you have to remember that in the early days of COVID, the hospitals in Michigan were being overwhelmed. They didn’t have PPE. They were taking care of patients outside of their typical hospital beds or setting up field hospitals,” she said. “It was not quite as bad as New York, but at the University of Michigan, we set up four or five ICUs outside of our normal care.”
They also converted the top floor of their pediatric hospital into an ICU to take care of patients with COVID during the first surge, she added. “We didn’t know much about this disease, but faced with this influx of patients, many of whom were dying with blood clots, we had to do something.”
Some hospitals began prophylactically anticoagulating their patients, but others hesitated before adopting the strategy. “But now we feel confident that prophylactic anticoagulation, done according to the right protocol, with no interruptions in the treatment, is beneficial,” Dr. Vaughn said.
The best medication choice is enoxaparin (Lovenox), which can be given once a day, as opposed to heparin, which needs to be given via injection three times a day, she said.
“Prophylactic dose anticoagulation is typically given by an injection under the skin, but a lot of times, I’ve had patients tell me they feel like a human pin cushion and have all these bruises from being stuck with needles every day, which I can totally relate to,” she said.
“It is important for us as clinicians to explain that we’re having to poke our patients because it is good for them and will help them fight COVID,” she added. “Also having the once-a-day option is going to be a lot better for adherence, and adherence to the protocol, not missing any days, is key to the better outcome.”
Dr. Vaughn and her team reviewed the charts of 1,351 patients (48% women, 49% Black, median age 64 [range 52-75]) who were hospitalized throughout Michigan during the first several months of the COVID-19 pandemic, from March to June 2020.
Only 18 patients (1.3%) had a confirmed VTE and 219 patients (16.2%) received treatment-dose anticoagulation.
The researchers noted that use of treatment-dose anticoagulation without imaging ranged from 0% to 29% across hospitals and increased significantly over time.
Of the 1,127 patients who received anticoagulation, 392 (34.8%) missed 2 days or more of prophylaxis.
In addition, there were varying rates of missed prophylaxis among the hospitals, from 11% to 61%, but these rates decreased markedly over time.
Missed doses were associated with a higher 60-day mortality (adjusted hazard ratio, 1.31; 95% confidence interval, 1.03-1.67), but not in-hospital mortality (aHR, 0.97; 95% CI, 0.91-1.03).
Compared with no anticoagulation, receiving any dose of anticoagulation was associated with lower in-hospital mortality.
However, only prophylactic-dose anticoagulation remained associated with lower mortality at 60 days. The adjusted hazard ratio for prophylactic-dose anticoagulation was 0.71 (95% CI, 0.51-0.90), compared with 0.92 (95% CI, 0.63-1.35) for treatment-dose anticoagulation.
Study boosts confidence
Despite its limitations, the study should make clinicians more confident that the use of prophylactic anticoagulation is warranted for hospitalized patients with COVID-19, write Andrew B. Dicks, MD, and Ido Weinberg, MD, from Massachusetts General Hospital, Boston, in an invited commentary.
“Practically, we still lack the granular data we need to help guide us in patient-by-patient decision-making – such as anticoagulation agent choice, dosage, and duration of therapy – especially as dictated by acuity of patient illness,” Dr. Dicks and Dr. Weinberg note.
“While we still await the data from randomized controlled trials to guide the optimal anticoagulation dose and duration, this study adds significant merit to the previously published recommendations from several different medical organizations regarding the use of prophylactic anticoagulation in hospitalized patients with COVID-19,” Dr. Dicks told this news organization.
The study was supported by Blue Cross and Blue Shield of Michigan and Blue Care Network as part of their Value Partnerships program. Dr. Vaughn has reported receiving speaking fees from Thermo Fisher Scientific. Dr. Dicks and Dr. Weinberg have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What’s behind brain fog in treated hypothyroidism?
The phenomenon of brain fog, as described by some patients with hypothyroidism despite treatment, is often associated with fatigue and cognitive symptoms and may be relieved by a variety of pharmacologic and nonpharmacologic approaches, new research suggests.
The findings come from a survey of more than 700 patients with hypothyroidism due to thyroid surgery and/or radioactive iodine therapy (RAI) or Hashimoto’s who reported having brain fog.
The survey results were presented May 29 at the American Association of Clinical Endocrinology Virtual Annual Meeting 2021 by investigators Matthew D. Ettleson, MD, and Ava Raine, of the University of Chicago, Illinois.
Many patients with hypothyroidism continue to experience symptoms despite taking thyroid hormone replacement therapy and having normal thyroid function test results.
These symptoms can include quantifiable cognitive, quality of life, and metabolic abnormalities. However, “some patients also experience vague and difficult to quantify symptoms, which they describe as brain fog,” Ms. Raine said.
The brain fog phenomenon has been described with somewhat varying features in several different chronic conditions, including postural orthostatic tachycardia syndrome, myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia, post-menopausal syndrome, and recently, among people with “long haul” COVID-19 symptoms.
However, brain fog associated with treated hypothyroidism has not been explored in-depth, despite the fact that patients often report it, Ms. Raine noted.
Results will help clinicians assist patients with brain fog
Fatigue was the most prominent brain fog symptom reported in the survey, followed by forgetfulness and difficulty focusing. On the other hand, rest and relaxation were the most reported factors that alleviated symptoms, followed by thyroid hormone adjustment.
“Hopefully these findings will help clinicians to recognize and treat the symptoms of brain fog and shed light on a condition which up until now has not been very well understood,” Dr. Ettleson said.
Asked to comment, session moderator Jad G. Sfeir, MD, of the Mayo Medical School, Rochester, Minn., told this news organization: “We do see patients complain a lot about this brain fog. The question is how can I help, and what has worked for them in the past?”
“When you have symptoms that are vague, like brain fog, you don’t have a lot of objective tools to [measure], so you can’t really develop a study to see how a certain medication affects the symptoms. Relying on subjective information from patients saying what worked for them and what did not, you can draw a lot of implications to clinical practice.”
The survey results, Dr. Sfeir said, “will help direct clinicians to know what type of questions to ask patients based on the survey responses and how to make some recommendations that may help.”
Fatigue, memory problems, difficulty focusing characterize brain fog
The online survey was distributed to hypothyroidism support groups and through the American Thyroid Association. Of the 5,282 respondents with hypothyroidism and symptoms of brain fog, 46% (2,453) reported having experienced brain fog symptoms prior to their diagnosis of hypothyroidism.
The population analyzed for the study was the 17% (731) who reported experiencing brain fog weeks to months following a diagnosis of hypothyroidism. Of those, 33% had Hashimoto’s, 21% thyroid surgery, 11% RAI therapy, and 15.6% had both thyroid surgery and RAI.
Brain fog symptoms were reported as occurring “frequently” by 44.5% and “all the time” by 37.0%. The composite symptom score was 22.9 out of 30.
Fatigue, or lack of energy, was the most commonly named symptom, reported by over 90% of both the thyroid surgery/RAI and Hashimoto’s groups, and as occurring “all the time” by about half in each group. Others reported by at least half of both groups included memory problems, difficulty focusing, sleep problems, and difficulties with decision-making. Other symptoms frequently cited included confusion, mood disturbance, and anxiety.
“Each ... domain was reported with some frequency by at least 85% of respondents, regardless of etiology of hypothyroidism, so it really was a high symptom burden that we were seeing, even in those whose symptoms were the least frequent,” Ms. Raine noted.
Symptom scores generally correlated with patient satisfaction scores, particularly with those of cognitive signs and difficulty focusing.
Lifting the fog: What do patients say helps them?
The survey asked patients what factors improved or worsened their brain fog symptoms. By far, the most frequent answer was rest/relaxation, endorsed by 58.5%. Another 10.5% listed exercise/outdoor time, but 1.5% said exercise worsened their symptoms.
Unspecified adjustments of thyroid medications were said to improve symptoms for 13.9%. Specific thyroid hormones reported to improve symptoms were liothyronine in 8.8%, desiccated thyroid extract in 3.1%, and levothyroxine in 2.7%. However, another 4.2% said thyroxine worsened their symptoms.
Healthy/nutritious diets were reported to improve symptoms by 6.3%, while consuming gluten, a high-sugar diet, and consuming alcohol were reported to worsen symptoms for 1.3%, 3.2%, and 1.3%, respectively. Caffeine was said to help for 3.1% and to harm by 0.6%.
Small numbers of patients reported improvements in symptoms with vitamins B12 and D, Adderall, or other stimulant medications, antidepressants, naltrexone, sun exposure, and blood glucose stability.
Other factors reported to worsen symptoms included menstruation, infection or other acute illness, pain, and “loud noise.”
Dr. Ettleson pointed out, “For many of these patients [the brain fog] may have nothing to do with their thyroid. We saw a large proportion of patients who said they had symptoms well before they were ever diagnosed with hypothyroidism, and yet many patients have linked these brain fog symptoms to their thyroid.”
Nonetheless, he said, “I think it’s imperative for the clinician to at least engage in these conversations and not just stop when the thyroid function tests are normal. We have many lifestyle suggestions that have emerged from this study that I think physicians can put forward to patients who are dealing with this ... early in the process in addition to thyroid hormone adjustment, which may help some patients.”
Dr. Ettleson, Ms. Raine, and Dr. Sfeir have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The phenomenon of brain fog, as described by some patients with hypothyroidism despite treatment, is often associated with fatigue and cognitive symptoms and may be relieved by a variety of pharmacologic and nonpharmacologic approaches, new research suggests.
The findings come from a survey of more than 700 patients with hypothyroidism due to thyroid surgery and/or radioactive iodine therapy (RAI) or Hashimoto’s who reported having brain fog.
The survey results were presented May 29 at the American Association of Clinical Endocrinology Virtual Annual Meeting 2021 by investigators Matthew D. Ettleson, MD, and Ava Raine, of the University of Chicago, Illinois.
Many patients with hypothyroidism continue to experience symptoms despite taking thyroid hormone replacement therapy and having normal thyroid function test results.
These symptoms can include quantifiable cognitive, quality of life, and metabolic abnormalities. However, “some patients also experience vague and difficult to quantify symptoms, which they describe as brain fog,” Ms. Raine said.
The brain fog phenomenon has been described with somewhat varying features in several different chronic conditions, including postural orthostatic tachycardia syndrome, myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia, post-menopausal syndrome, and recently, among people with “long haul” COVID-19 symptoms.
However, brain fog associated with treated hypothyroidism has not been explored in-depth, despite the fact that patients often report it, Ms. Raine noted.
Results will help clinicians assist patients with brain fog
Fatigue was the most prominent brain fog symptom reported in the survey, followed by forgetfulness and difficulty focusing. On the other hand, rest and relaxation were the most reported factors that alleviated symptoms, followed by thyroid hormone adjustment.
“Hopefully these findings will help clinicians to recognize and treat the symptoms of brain fog and shed light on a condition which up until now has not been very well understood,” Dr. Ettleson said.
Asked to comment, session moderator Jad G. Sfeir, MD, of the Mayo Medical School, Rochester, Minn., told this news organization: “We do see patients complain a lot about this brain fog. The question is how can I help, and what has worked for them in the past?”
“When you have symptoms that are vague, like brain fog, you don’t have a lot of objective tools to [measure], so you can’t really develop a study to see how a certain medication affects the symptoms. Relying on subjective information from patients saying what worked for them and what did not, you can draw a lot of implications to clinical practice.”
The survey results, Dr. Sfeir said, “will help direct clinicians to know what type of questions to ask patients based on the survey responses and how to make some recommendations that may help.”
Fatigue, memory problems, difficulty focusing characterize brain fog
The online survey was distributed to hypothyroidism support groups and through the American Thyroid Association. Of the 5,282 respondents with hypothyroidism and symptoms of brain fog, 46% (2,453) reported having experienced brain fog symptoms prior to their diagnosis of hypothyroidism.
The population analyzed for the study was the 17% (731) who reported experiencing brain fog weeks to months following a diagnosis of hypothyroidism. Of those, 33% had Hashimoto’s, 21% thyroid surgery, 11% RAI therapy, and 15.6% had both thyroid surgery and RAI.
Brain fog symptoms were reported as occurring “frequently” by 44.5% and “all the time” by 37.0%. The composite symptom score was 22.9 out of 30.
Fatigue, or lack of energy, was the most commonly named symptom, reported by over 90% of both the thyroid surgery/RAI and Hashimoto’s groups, and as occurring “all the time” by about half in each group. Others reported by at least half of both groups included memory problems, difficulty focusing, sleep problems, and difficulties with decision-making. Other symptoms frequently cited included confusion, mood disturbance, and anxiety.
“Each ... domain was reported with some frequency by at least 85% of respondents, regardless of etiology of hypothyroidism, so it really was a high symptom burden that we were seeing, even in those whose symptoms were the least frequent,” Ms. Raine noted.
Symptom scores generally correlated with patient satisfaction scores, particularly with those of cognitive signs and difficulty focusing.
Lifting the fog: What do patients say helps them?
The survey asked patients what factors improved or worsened their brain fog symptoms. By far, the most frequent answer was rest/relaxation, endorsed by 58.5%. Another 10.5% listed exercise/outdoor time, but 1.5% said exercise worsened their symptoms.
Unspecified adjustments of thyroid medications were said to improve symptoms for 13.9%. Specific thyroid hormones reported to improve symptoms were liothyronine in 8.8%, desiccated thyroid extract in 3.1%, and levothyroxine in 2.7%. However, another 4.2% said thyroxine worsened their symptoms.
Healthy/nutritious diets were reported to improve symptoms by 6.3%, while consuming gluten, a high-sugar diet, and consuming alcohol were reported to worsen symptoms for 1.3%, 3.2%, and 1.3%, respectively. Caffeine was said to help for 3.1% and to harm by 0.6%.
Small numbers of patients reported improvements in symptoms with vitamins B12 and D, Adderall, or other stimulant medications, antidepressants, naltrexone, sun exposure, and blood glucose stability.
Other factors reported to worsen symptoms included menstruation, infection or other acute illness, pain, and “loud noise.”
Dr. Ettleson pointed out, “For many of these patients [the brain fog] may have nothing to do with their thyroid. We saw a large proportion of patients who said they had symptoms well before they were ever diagnosed with hypothyroidism, and yet many patients have linked these brain fog symptoms to their thyroid.”
Nonetheless, he said, “I think it’s imperative for the clinician to at least engage in these conversations and not just stop when the thyroid function tests are normal. We have many lifestyle suggestions that have emerged from this study that I think physicians can put forward to patients who are dealing with this ... early in the process in addition to thyroid hormone adjustment, which may help some patients.”
Dr. Ettleson, Ms. Raine, and Dr. Sfeir have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The phenomenon of brain fog, as described by some patients with hypothyroidism despite treatment, is often associated with fatigue and cognitive symptoms and may be relieved by a variety of pharmacologic and nonpharmacologic approaches, new research suggests.
The findings come from a survey of more than 700 patients with hypothyroidism due to thyroid surgery and/or radioactive iodine therapy (RAI) or Hashimoto’s who reported having brain fog.
The survey results were presented May 29 at the American Association of Clinical Endocrinology Virtual Annual Meeting 2021 by investigators Matthew D. Ettleson, MD, and Ava Raine, of the University of Chicago, Illinois.
Many patients with hypothyroidism continue to experience symptoms despite taking thyroid hormone replacement therapy and having normal thyroid function test results.
These symptoms can include quantifiable cognitive, quality of life, and metabolic abnormalities. However, “some patients also experience vague and difficult to quantify symptoms, which they describe as brain fog,” Ms. Raine said.
The brain fog phenomenon has been described with somewhat varying features in several different chronic conditions, including postural orthostatic tachycardia syndrome, myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia, post-menopausal syndrome, and recently, among people with “long haul” COVID-19 symptoms.
However, brain fog associated with treated hypothyroidism has not been explored in-depth, despite the fact that patients often report it, Ms. Raine noted.
Results will help clinicians assist patients with brain fog
Fatigue was the most prominent brain fog symptom reported in the survey, followed by forgetfulness and difficulty focusing. On the other hand, rest and relaxation were the most reported factors that alleviated symptoms, followed by thyroid hormone adjustment.
“Hopefully these findings will help clinicians to recognize and treat the symptoms of brain fog and shed light on a condition which up until now has not been very well understood,” Dr. Ettleson said.
Asked to comment, session moderator Jad G. Sfeir, MD, of the Mayo Medical School, Rochester, Minn., told this news organization: “We do see patients complain a lot about this brain fog. The question is how can I help, and what has worked for them in the past?”
“When you have symptoms that are vague, like brain fog, you don’t have a lot of objective tools to [measure], so you can’t really develop a study to see how a certain medication affects the symptoms. Relying on subjective information from patients saying what worked for them and what did not, you can draw a lot of implications to clinical practice.”
The survey results, Dr. Sfeir said, “will help direct clinicians to know what type of questions to ask patients based on the survey responses and how to make some recommendations that may help.”
Fatigue, memory problems, difficulty focusing characterize brain fog
The online survey was distributed to hypothyroidism support groups and through the American Thyroid Association. Of the 5,282 respondents with hypothyroidism and symptoms of brain fog, 46% (2,453) reported having experienced brain fog symptoms prior to their diagnosis of hypothyroidism.
The population analyzed for the study was the 17% (731) who reported experiencing brain fog weeks to months following a diagnosis of hypothyroidism. Of those, 33% had Hashimoto’s, 21% thyroid surgery, 11% RAI therapy, and 15.6% had both thyroid surgery and RAI.
Brain fog symptoms were reported as occurring “frequently” by 44.5% and “all the time” by 37.0%. The composite symptom score was 22.9 out of 30.
Fatigue, or lack of energy, was the most commonly named symptom, reported by over 90% of both the thyroid surgery/RAI and Hashimoto’s groups, and as occurring “all the time” by about half in each group. Others reported by at least half of both groups included memory problems, difficulty focusing, sleep problems, and difficulties with decision-making. Other symptoms frequently cited included confusion, mood disturbance, and anxiety.
“Each ... domain was reported with some frequency by at least 85% of respondents, regardless of etiology of hypothyroidism, so it really was a high symptom burden that we were seeing, even in those whose symptoms were the least frequent,” Ms. Raine noted.
Symptom scores generally correlated with patient satisfaction scores, particularly with those of cognitive signs and difficulty focusing.
Lifting the fog: What do patients say helps them?
The survey asked patients what factors improved or worsened their brain fog symptoms. By far, the most frequent answer was rest/relaxation, endorsed by 58.5%. Another 10.5% listed exercise/outdoor time, but 1.5% said exercise worsened their symptoms.
Unspecified adjustments of thyroid medications were said to improve symptoms for 13.9%. Specific thyroid hormones reported to improve symptoms were liothyronine in 8.8%, desiccated thyroid extract in 3.1%, and levothyroxine in 2.7%. However, another 4.2% said thyroxine worsened their symptoms.
Healthy/nutritious diets were reported to improve symptoms by 6.3%, while consuming gluten, a high-sugar diet, and consuming alcohol were reported to worsen symptoms for 1.3%, 3.2%, and 1.3%, respectively. Caffeine was said to help for 3.1% and to harm by 0.6%.
Small numbers of patients reported improvements in symptoms with vitamins B12 and D, Adderall, or other stimulant medications, antidepressants, naltrexone, sun exposure, and blood glucose stability.
Other factors reported to worsen symptoms included menstruation, infection or other acute illness, pain, and “loud noise.”
Dr. Ettleson pointed out, “For many of these patients [the brain fog] may have nothing to do with their thyroid. We saw a large proportion of patients who said they had symptoms well before they were ever diagnosed with hypothyroidism, and yet many patients have linked these brain fog symptoms to their thyroid.”
Nonetheless, he said, “I think it’s imperative for the clinician to at least engage in these conversations and not just stop when the thyroid function tests are normal. We have many lifestyle suggestions that have emerged from this study that I think physicians can put forward to patients who are dealing with this ... early in the process in addition to thyroid hormone adjustment, which may help some patients.”
Dr. Ettleson, Ms. Raine, and Dr. Sfeir have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Medically suspect criterion can determine bariatric surgery coverage
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
FROM ASMBS 2021
Hormone pellet safety data ‘not very reassuring at all’ for women
Women who receive pellet hormonal therapy may be significantly more likely to have side effects such as mood swings, anxiety, breast tenderness, hair pattern change, acne, and weight gain, compared with women who receive hormonal treatments that have been approved by the Food and Drug Administration, a study indicates.
In addition, abnormal uterine bleeding may be significantly more common in women who receive pellets than it is in women who receive Food and Drug Administration–approved options, according to the retrospective study, which was published online in Menopause.
Women receiving pellets also were more likely to undergo hysterectomy while on hormonal therapy, and they had higher supraphysiological levels of estradiol and total testosterone during treatment, compared with women on conventional therapy, the study of 539 women shows.
The findings, which had been presented at the North American Menopause Society annual meeting, were highlighted during a lecture at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
The data are “not very reassuring at all,” said Robert P. Kauffman, MD, a professor of obstetrics and gynecology at Texas Tech University, Amarillo, who was not involved in the study.
Dr. Kauffman commented on the research during a review of concerns surrounding non–FDA-approved hormone replacement therapies at the ACOG meeting. Concerns include variations in compounded products, a lack of randomized, controlled trial data supporting their use, and ethical dilemmas that may exist if clinicians have financial incentives to provide compounded bioidentical hormone therapy over FDA-approved treatments.
No peer-reviewed studies show that compounded hormone creams or pellets are safer, more efficacious, or less likely to cause adverse effects, compared with FDA-approved products, Dr. Kauffman said.
Data from Pennsylvania
For the retrospective study, Xuezhi (Daniel) Jiang, MD, PhD, and colleagues identified postmenopausal patients in the Reading Hospital Electronic Medical Record System, including 10,801 on FDA-approved hormonal therapy and 1,061 on pellet hormonal therapy. Their analysis focused on data from the medical records of 384 women on pellet hormonal therapy and 155 women on FDA-approved hormonal therapy. Dr. Jiang is affiliated with the department of obstetrics and gynecology at Reading (Pa.) Hospital and Sidney Kimmel Medical College, Philadelphia.
The researchers examined data from 2005 to 2017 for patients in the pellet therapy group, and from 1985 to 2017 for patients in the conventional therapy group.
Patients in the conventional therapy group received 24 brands of FDA-approved hormone products; 4.5% received testosterone or methyltestosterone in addition to estrogen. Patients in the pellet therapy group had pellets prescribed by clinicians at two private practices in the hospital system that use this treatment approach. Patients in the pellet group received compounded estradiol and testosterone pellets made at a pharmacy in Tennessee.* Almost all of the patients in the pellet group received testosterone and estradiol pellets.
Low libido was listed as a reason why women started treatment for 83.5% of the pellet group versus 4.5% of the conventional therapy group.
In all, 57.6% of patients on pellet therapy had side effects, versus 14.8% on FDA-approved therapy, the researchers found. Patients on pellet hormonal therapy reported higher incidence of mood swings (7% vs. 1.9%), anxiety (18.5% vs. 5.8%), breast tenderness (10.1% vs. 2.6%), hair pattern change (13.5% vs. 2.6%), acne (8.6% vs. 1.3%), and weight gain (34.4% vs 4.5%), relative to patients on FDA-approved options.
Among those with an intact uterus when starting therapy (246 of those on pellets and 133 of those on FDA-approved treatments), abnormal uterine bleeding occurred in 55.3% on pellets, compared with 15.2% on FDA-approved treatments (adjusted odds ratio, 7.9). Hysterectomy secondary to abnormal uterine bleeding occurred in 20.3% of the patients on pellets versus 6.3% on FDA-approved treatments (aOR, 3.2).
In many cases, records show that patients chose to have a hysterectomy so they could continue pellet therapy without worrying about abnormal uterine bleeding, Dr. Jiang said in an interview.
Dr. Kauffman has seen patients on pellet therapy, usually implanted by family physicians, develop postmenopausal bleeding because of high levels of estrogen. “Our experience has been too that, if you have pellets, you are more likely to get a hysterectomy for bleeding issues. And I think these are the safety issues that need to be looked at on a broader scope,” he said in an interview.
Although hysterectomy may stop the bleeding, other safety risks may remain with pellet therapy, noted Sharon Winer, MD, MPH, an obstetrician and gynecologist with a subspecialty in reproductive endocrinology and infertility who practices in Beverly Hills, Calif.
Pellets, which are about the size of a grain of rice, typically are implanted in the hip, lower abdomen, or buttock and release hormones over 3-6 months. The pellets are not retrievable. “The question becomes, what if she has a new breast cancer diagnosis or a diagnosis where estrogen is contraindicated? She has got that estrogen already in her system,” Dr. Winer said.
“The hysterectomy may solve the bleeding problem ... but it doesn’t solve the safety problem overall,” said Dr. Winer, who also is a professor of obstetrics and gynecology and codirector of the reproductive endocrinology and infertility clinic at the University of Southern California, Los Angeles.
Elevated levels
Average peak serum estradiol was significantly higher in the pellet treatment group than in the conventional therapy group (237.70 pg/mL vs. 93.45 pg/mL), as was average peak serum testosterone (192.84 ng/dL vs. 15.59 ng/dL), the researchers reported. Patients on FDA-approved treatments were less likely to have had their hormone levels measured. How concentrations of hormone levels correlate with side effects is unclear, Dr. Jiang said.
The study was limited by its single-institution, retrospective design, and some patient characteristics differed between the treatment groups, the authors noted.
Still, “clinicians ought to be mindful of fully counseling patients on side effects identified in the current study,” Dr. Jiang and coauthors concluded. Clinicians also need to discuss potential risks of breast cancer, endometrial cancer, and cardiovascular disease with patients.
Many primary care clinicians rely on outdated information from the Women’s Health Initiative, published in 2002 and 2004, in their understanding of postmenopausal hormonal therapy and its risks and benefits, Dr. Jiang said. And some patients consider custom-compounded hormone therapy to be safer and more natural, “which is totally misleading.”
Pellets and other custom-compounded medicine containing testosterone may make patients feel better and more energetic, Dr. Jiang acknowledged. “That’s a reason why patients ... tend to stay on, even though they have side effects. The only issue is the safety.”
Additional questions remain. The researchers recently started to examine rates of breast cancer and abnormal breast pathology and mammogram results. “It’s a long journey,” he said.
Plenty of approved options
Custom-compounded medicines are not FDA approved and are not recommended by medical menopause societies, Dr. Jiang said. Meanwhile, plenty of approved hormone therapies, including bioidentical treatments, have safety data and are available.
A 2020 consensus study report from the National Academies of Sciences, Engineering, and Medicine that examined the use of compounded hormonal therapy and provides guidance for clinicians is a good start in addressing this major issue, he added.
A committee determined “there is insufficient evidence to support the overall clinical utility of [compounded bioidentical hormone therapies] as treatment for menopause and male hypogonadism symptoms.”
If an FDA-approved option is available, “I would always go with an FDA-approved product before I would go with a compounded product,” Dr. Winer said. A 2012 fungal meningitis outbreak linked to a compounding pharmacy highlighted risks associated with poor quality compounded drugs.
“I think at least now it is recognized that compounding is an issue that has got to be dealt with,” Dr. Winer said. “It is just that it is so widespread and it is sometimes under the radar ... that I think it is really hard for the FDA to get a handle on it.”
Dr. Winer has seen patients on compounded treatments who are underdosed and patients who are overdosed. “I’ve also seen patients who do quite well with it, but I’m not happy continuing it because tomorrow there may be inconsistency in potency or quality resulting in a different clinical response,” she said.
Nevertheless, compounded pharmacies are needed, Dr. Winer said. If she wants to give natural progesterone that is FDA approved but happens to be made with peanut oil, she will have a compounding pharmacy make it with canola oil instead if a patient has a peanut allergy, for example. Other patients need dosages that are so low that they are not available as FDA-approved products.
Dr. Jiang and Dr. Kauffman had no relevant financial disclosures. Dr. Winer has done work with AbbVie (related to endometriosis), TherapeuticsMD (related to a menopause bioidentical hormonal pill and vaginal estrogen product), and Biogix (related to an antioxidant supplement for menopause symptoms).
*This story was updated on 6/22/2021.
Women who receive pellet hormonal therapy may be significantly more likely to have side effects such as mood swings, anxiety, breast tenderness, hair pattern change, acne, and weight gain, compared with women who receive hormonal treatments that have been approved by the Food and Drug Administration, a study indicates.
In addition, abnormal uterine bleeding may be significantly more common in women who receive pellets than it is in women who receive Food and Drug Administration–approved options, according to the retrospective study, which was published online in Menopause.
Women receiving pellets also were more likely to undergo hysterectomy while on hormonal therapy, and they had higher supraphysiological levels of estradiol and total testosterone during treatment, compared with women on conventional therapy, the study of 539 women shows.
The findings, which had been presented at the North American Menopause Society annual meeting, were highlighted during a lecture at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
The data are “not very reassuring at all,” said Robert P. Kauffman, MD, a professor of obstetrics and gynecology at Texas Tech University, Amarillo, who was not involved in the study.
Dr. Kauffman commented on the research during a review of concerns surrounding non–FDA-approved hormone replacement therapies at the ACOG meeting. Concerns include variations in compounded products, a lack of randomized, controlled trial data supporting their use, and ethical dilemmas that may exist if clinicians have financial incentives to provide compounded bioidentical hormone therapy over FDA-approved treatments.
No peer-reviewed studies show that compounded hormone creams or pellets are safer, more efficacious, or less likely to cause adverse effects, compared with FDA-approved products, Dr. Kauffman said.
Data from Pennsylvania
For the retrospective study, Xuezhi (Daniel) Jiang, MD, PhD, and colleagues identified postmenopausal patients in the Reading Hospital Electronic Medical Record System, including 10,801 on FDA-approved hormonal therapy and 1,061 on pellet hormonal therapy. Their analysis focused on data from the medical records of 384 women on pellet hormonal therapy and 155 women on FDA-approved hormonal therapy. Dr. Jiang is affiliated with the department of obstetrics and gynecology at Reading (Pa.) Hospital and Sidney Kimmel Medical College, Philadelphia.
The researchers examined data from 2005 to 2017 for patients in the pellet therapy group, and from 1985 to 2017 for patients in the conventional therapy group.
Patients in the conventional therapy group received 24 brands of FDA-approved hormone products; 4.5% received testosterone or methyltestosterone in addition to estrogen. Patients in the pellet therapy group had pellets prescribed by clinicians at two private practices in the hospital system that use this treatment approach. Patients in the pellet group received compounded estradiol and testosterone pellets made at a pharmacy in Tennessee.* Almost all of the patients in the pellet group received testosterone and estradiol pellets.
Low libido was listed as a reason why women started treatment for 83.5% of the pellet group versus 4.5% of the conventional therapy group.
In all, 57.6% of patients on pellet therapy had side effects, versus 14.8% on FDA-approved therapy, the researchers found. Patients on pellet hormonal therapy reported higher incidence of mood swings (7% vs. 1.9%), anxiety (18.5% vs. 5.8%), breast tenderness (10.1% vs. 2.6%), hair pattern change (13.5% vs. 2.6%), acne (8.6% vs. 1.3%), and weight gain (34.4% vs 4.5%), relative to patients on FDA-approved options.
Among those with an intact uterus when starting therapy (246 of those on pellets and 133 of those on FDA-approved treatments), abnormal uterine bleeding occurred in 55.3% on pellets, compared with 15.2% on FDA-approved treatments (adjusted odds ratio, 7.9). Hysterectomy secondary to abnormal uterine bleeding occurred in 20.3% of the patients on pellets versus 6.3% on FDA-approved treatments (aOR, 3.2).
In many cases, records show that patients chose to have a hysterectomy so they could continue pellet therapy without worrying about abnormal uterine bleeding, Dr. Jiang said in an interview.
Dr. Kauffman has seen patients on pellet therapy, usually implanted by family physicians, develop postmenopausal bleeding because of high levels of estrogen. “Our experience has been too that, if you have pellets, you are more likely to get a hysterectomy for bleeding issues. And I think these are the safety issues that need to be looked at on a broader scope,” he said in an interview.
Although hysterectomy may stop the bleeding, other safety risks may remain with pellet therapy, noted Sharon Winer, MD, MPH, an obstetrician and gynecologist with a subspecialty in reproductive endocrinology and infertility who practices in Beverly Hills, Calif.
Pellets, which are about the size of a grain of rice, typically are implanted in the hip, lower abdomen, or buttock and release hormones over 3-6 months. The pellets are not retrievable. “The question becomes, what if she has a new breast cancer diagnosis or a diagnosis where estrogen is contraindicated? She has got that estrogen already in her system,” Dr. Winer said.
“The hysterectomy may solve the bleeding problem ... but it doesn’t solve the safety problem overall,” said Dr. Winer, who also is a professor of obstetrics and gynecology and codirector of the reproductive endocrinology and infertility clinic at the University of Southern California, Los Angeles.
Elevated levels
Average peak serum estradiol was significantly higher in the pellet treatment group than in the conventional therapy group (237.70 pg/mL vs. 93.45 pg/mL), as was average peak serum testosterone (192.84 ng/dL vs. 15.59 ng/dL), the researchers reported. Patients on FDA-approved treatments were less likely to have had their hormone levels measured. How concentrations of hormone levels correlate with side effects is unclear, Dr. Jiang said.
The study was limited by its single-institution, retrospective design, and some patient characteristics differed between the treatment groups, the authors noted.
Still, “clinicians ought to be mindful of fully counseling patients on side effects identified in the current study,” Dr. Jiang and coauthors concluded. Clinicians also need to discuss potential risks of breast cancer, endometrial cancer, and cardiovascular disease with patients.
Many primary care clinicians rely on outdated information from the Women’s Health Initiative, published in 2002 and 2004, in their understanding of postmenopausal hormonal therapy and its risks and benefits, Dr. Jiang said. And some patients consider custom-compounded hormone therapy to be safer and more natural, “which is totally misleading.”
Pellets and other custom-compounded medicine containing testosterone may make patients feel better and more energetic, Dr. Jiang acknowledged. “That’s a reason why patients ... tend to stay on, even though they have side effects. The only issue is the safety.”
Additional questions remain. The researchers recently started to examine rates of breast cancer and abnormal breast pathology and mammogram results. “It’s a long journey,” he said.
Plenty of approved options
Custom-compounded medicines are not FDA approved and are not recommended by medical menopause societies, Dr. Jiang said. Meanwhile, plenty of approved hormone therapies, including bioidentical treatments, have safety data and are available.
A 2020 consensus study report from the National Academies of Sciences, Engineering, and Medicine that examined the use of compounded hormonal therapy and provides guidance for clinicians is a good start in addressing this major issue, he added.
A committee determined “there is insufficient evidence to support the overall clinical utility of [compounded bioidentical hormone therapies] as treatment for menopause and male hypogonadism symptoms.”
If an FDA-approved option is available, “I would always go with an FDA-approved product before I would go with a compounded product,” Dr. Winer said. A 2012 fungal meningitis outbreak linked to a compounding pharmacy highlighted risks associated with poor quality compounded drugs.
“I think at least now it is recognized that compounding is an issue that has got to be dealt with,” Dr. Winer said. “It is just that it is so widespread and it is sometimes under the radar ... that I think it is really hard for the FDA to get a handle on it.”
Dr. Winer has seen patients on compounded treatments who are underdosed and patients who are overdosed. “I’ve also seen patients who do quite well with it, but I’m not happy continuing it because tomorrow there may be inconsistency in potency or quality resulting in a different clinical response,” she said.
Nevertheless, compounded pharmacies are needed, Dr. Winer said. If she wants to give natural progesterone that is FDA approved but happens to be made with peanut oil, she will have a compounding pharmacy make it with canola oil instead if a patient has a peanut allergy, for example. Other patients need dosages that are so low that they are not available as FDA-approved products.
Dr. Jiang and Dr. Kauffman had no relevant financial disclosures. Dr. Winer has done work with AbbVie (related to endometriosis), TherapeuticsMD (related to a menopause bioidentical hormonal pill and vaginal estrogen product), and Biogix (related to an antioxidant supplement for menopause symptoms).
*This story was updated on 6/22/2021.
Women who receive pellet hormonal therapy may be significantly more likely to have side effects such as mood swings, anxiety, breast tenderness, hair pattern change, acne, and weight gain, compared with women who receive hormonal treatments that have been approved by the Food and Drug Administration, a study indicates.
In addition, abnormal uterine bleeding may be significantly more common in women who receive pellets than it is in women who receive Food and Drug Administration–approved options, according to the retrospective study, which was published online in Menopause.
Women receiving pellets also were more likely to undergo hysterectomy while on hormonal therapy, and they had higher supraphysiological levels of estradiol and total testosterone during treatment, compared with women on conventional therapy, the study of 539 women shows.
The findings, which had been presented at the North American Menopause Society annual meeting, were highlighted during a lecture at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
The data are “not very reassuring at all,” said Robert P. Kauffman, MD, a professor of obstetrics and gynecology at Texas Tech University, Amarillo, who was not involved in the study.
Dr. Kauffman commented on the research during a review of concerns surrounding non–FDA-approved hormone replacement therapies at the ACOG meeting. Concerns include variations in compounded products, a lack of randomized, controlled trial data supporting their use, and ethical dilemmas that may exist if clinicians have financial incentives to provide compounded bioidentical hormone therapy over FDA-approved treatments.
No peer-reviewed studies show that compounded hormone creams or pellets are safer, more efficacious, or less likely to cause adverse effects, compared with FDA-approved products, Dr. Kauffman said.
Data from Pennsylvania
For the retrospective study, Xuezhi (Daniel) Jiang, MD, PhD, and colleagues identified postmenopausal patients in the Reading Hospital Electronic Medical Record System, including 10,801 on FDA-approved hormonal therapy and 1,061 on pellet hormonal therapy. Their analysis focused on data from the medical records of 384 women on pellet hormonal therapy and 155 women on FDA-approved hormonal therapy. Dr. Jiang is affiliated with the department of obstetrics and gynecology at Reading (Pa.) Hospital and Sidney Kimmel Medical College, Philadelphia.
The researchers examined data from 2005 to 2017 for patients in the pellet therapy group, and from 1985 to 2017 for patients in the conventional therapy group.
Patients in the conventional therapy group received 24 brands of FDA-approved hormone products; 4.5% received testosterone or methyltestosterone in addition to estrogen. Patients in the pellet therapy group had pellets prescribed by clinicians at two private practices in the hospital system that use this treatment approach. Patients in the pellet group received compounded estradiol and testosterone pellets made at a pharmacy in Tennessee.* Almost all of the patients in the pellet group received testosterone and estradiol pellets.
Low libido was listed as a reason why women started treatment for 83.5% of the pellet group versus 4.5% of the conventional therapy group.
In all, 57.6% of patients on pellet therapy had side effects, versus 14.8% on FDA-approved therapy, the researchers found. Patients on pellet hormonal therapy reported higher incidence of mood swings (7% vs. 1.9%), anxiety (18.5% vs. 5.8%), breast tenderness (10.1% vs. 2.6%), hair pattern change (13.5% vs. 2.6%), acne (8.6% vs. 1.3%), and weight gain (34.4% vs 4.5%), relative to patients on FDA-approved options.
Among those with an intact uterus when starting therapy (246 of those on pellets and 133 of those on FDA-approved treatments), abnormal uterine bleeding occurred in 55.3% on pellets, compared with 15.2% on FDA-approved treatments (adjusted odds ratio, 7.9). Hysterectomy secondary to abnormal uterine bleeding occurred in 20.3% of the patients on pellets versus 6.3% on FDA-approved treatments (aOR, 3.2).
In many cases, records show that patients chose to have a hysterectomy so they could continue pellet therapy without worrying about abnormal uterine bleeding, Dr. Jiang said in an interview.
Dr. Kauffman has seen patients on pellet therapy, usually implanted by family physicians, develop postmenopausal bleeding because of high levels of estrogen. “Our experience has been too that, if you have pellets, you are more likely to get a hysterectomy for bleeding issues. And I think these are the safety issues that need to be looked at on a broader scope,” he said in an interview.
Although hysterectomy may stop the bleeding, other safety risks may remain with pellet therapy, noted Sharon Winer, MD, MPH, an obstetrician and gynecologist with a subspecialty in reproductive endocrinology and infertility who practices in Beverly Hills, Calif.
Pellets, which are about the size of a grain of rice, typically are implanted in the hip, lower abdomen, or buttock and release hormones over 3-6 months. The pellets are not retrievable. “The question becomes, what if she has a new breast cancer diagnosis or a diagnosis where estrogen is contraindicated? She has got that estrogen already in her system,” Dr. Winer said.
“The hysterectomy may solve the bleeding problem ... but it doesn’t solve the safety problem overall,” said Dr. Winer, who also is a professor of obstetrics and gynecology and codirector of the reproductive endocrinology and infertility clinic at the University of Southern California, Los Angeles.
Elevated levels
Average peak serum estradiol was significantly higher in the pellet treatment group than in the conventional therapy group (237.70 pg/mL vs. 93.45 pg/mL), as was average peak serum testosterone (192.84 ng/dL vs. 15.59 ng/dL), the researchers reported. Patients on FDA-approved treatments were less likely to have had their hormone levels measured. How concentrations of hormone levels correlate with side effects is unclear, Dr. Jiang said.
The study was limited by its single-institution, retrospective design, and some patient characteristics differed between the treatment groups, the authors noted.
Still, “clinicians ought to be mindful of fully counseling patients on side effects identified in the current study,” Dr. Jiang and coauthors concluded. Clinicians also need to discuss potential risks of breast cancer, endometrial cancer, and cardiovascular disease with patients.
Many primary care clinicians rely on outdated information from the Women’s Health Initiative, published in 2002 and 2004, in their understanding of postmenopausal hormonal therapy and its risks and benefits, Dr. Jiang said. And some patients consider custom-compounded hormone therapy to be safer and more natural, “which is totally misleading.”
Pellets and other custom-compounded medicine containing testosterone may make patients feel better and more energetic, Dr. Jiang acknowledged. “That’s a reason why patients ... tend to stay on, even though they have side effects. The only issue is the safety.”
Additional questions remain. The researchers recently started to examine rates of breast cancer and abnormal breast pathology and mammogram results. “It’s a long journey,” he said.
Plenty of approved options
Custom-compounded medicines are not FDA approved and are not recommended by medical menopause societies, Dr. Jiang said. Meanwhile, plenty of approved hormone therapies, including bioidentical treatments, have safety data and are available.
A 2020 consensus study report from the National Academies of Sciences, Engineering, and Medicine that examined the use of compounded hormonal therapy and provides guidance for clinicians is a good start in addressing this major issue, he added.
A committee determined “there is insufficient evidence to support the overall clinical utility of [compounded bioidentical hormone therapies] as treatment for menopause and male hypogonadism symptoms.”
If an FDA-approved option is available, “I would always go with an FDA-approved product before I would go with a compounded product,” Dr. Winer said. A 2012 fungal meningitis outbreak linked to a compounding pharmacy highlighted risks associated with poor quality compounded drugs.
“I think at least now it is recognized that compounding is an issue that has got to be dealt with,” Dr. Winer said. “It is just that it is so widespread and it is sometimes under the radar ... that I think it is really hard for the FDA to get a handle on it.”
Dr. Winer has seen patients on compounded treatments who are underdosed and patients who are overdosed. “I’ve also seen patients who do quite well with it, but I’m not happy continuing it because tomorrow there may be inconsistency in potency or quality resulting in a different clinical response,” she said.
Nevertheless, compounded pharmacies are needed, Dr. Winer said. If she wants to give natural progesterone that is FDA approved but happens to be made with peanut oil, she will have a compounding pharmacy make it with canola oil instead if a patient has a peanut allergy, for example. Other patients need dosages that are so low that they are not available as FDA-approved products.
Dr. Jiang and Dr. Kauffman had no relevant financial disclosures. Dr. Winer has done work with AbbVie (related to endometriosis), TherapeuticsMD (related to a menopause bioidentical hormonal pill and vaginal estrogen product), and Biogix (related to an antioxidant supplement for menopause symptoms).
*This story was updated on 6/22/2021.
FROM ACOG 2021