User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Maskomania: Masks and COVID-19
A comprehensive review
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13
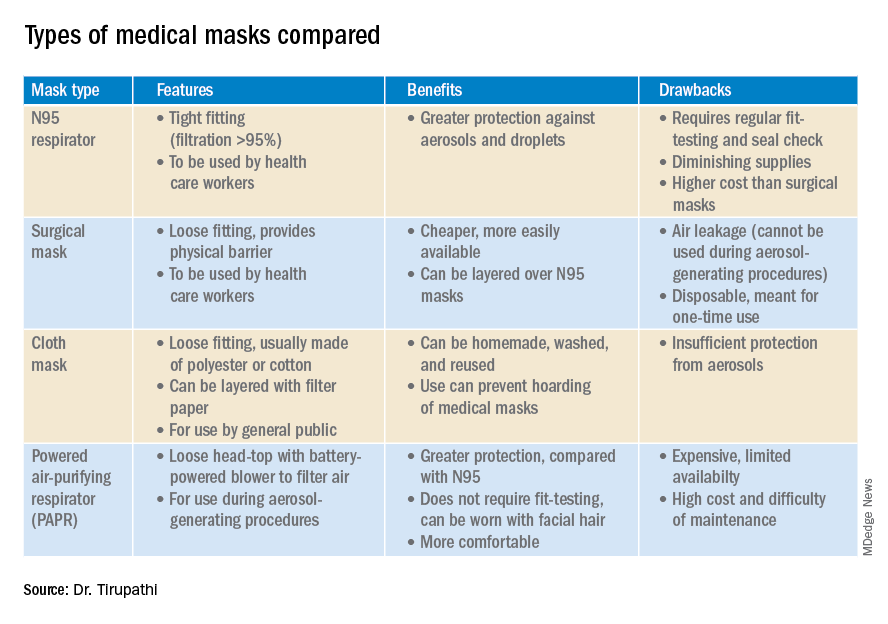
N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
A comprehensive review
A comprehensive review
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13

N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13

N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
Today’s top news highlights: COVID-19 in kids, addiction-related suicide
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 in kids
Children and young adults in all age groups can develop severe illess after SARS-CoV-2 infection, but infants and teens are most likely to be hospitalized, according to retrospective data from 177 children and young adults at a single center. “One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Roberta L. DeBiasi, MD, of Children’s National Hospital, Washington, and colleagues reported in the Journal of Pediatrics. READ MORE
Avoiding ageism in COVID resource allocation
The American Geriatrics Society has issued new policy recommendations for resource allocation during the COVID-19 pandemic that are aimed at protecting seniors for ageism. When allocating scarce resources in an emergency, officials should equally weigh in-hospital survival and severe comorbidities contributing to short-term mortality, the group wrote. “Age per se should never be used as a means for a categorical exclusion from therapeutic interventions that represent the standard of care. ... Likewise, specific age-based cutoffs should not be used in resource allocation strategies,” AGS officials wrote in the statement. READ MORE
Preventing addiction-related suicide
Individuals with substance use disorders are at a significant risk for suicide, but there have been few evidence-based options for their treatment. Now a single intervention is showing promise for this high-risk group. In a large, multicenter randomized effectiveness study, a single 3-hour-long group psychosocial intervention resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up. The intervention to prevent future suicide was designed specifically for patients who were in intensive outpatient programs for addiction treatment. “We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Richard K. Ries, MD, director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center. READ MORE
TNF inhibitors may hamper COVID-19 severity
Early evidence from the COVID-19 Global Rheumatology Alliance Registry has produced an intriguing result: Patients on tumor necrosis factor inhibitors for their rheumatic disease are less likely to require hospitalization when infected with COVID-19. The registry data also show that taking hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization. “A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” said Jinoos Yazdany, MD, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital. READ MORE
Audrey Hepburn’s lessons in pandemic grace
There are a lot of new skills required for praticing medicine during the COVID-19 pandemic. In his latest MDedge column, Jeffrey Benabio, MD, explains that grace is one of them. Dr. Benabio, director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego, looks to Audrey Hepburn for inspiration. “Effort is also required for telephone and video visits,” he writes. “In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.” READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 in kids
Children and young adults in all age groups can develop severe illess after SARS-CoV-2 infection, but infants and teens are most likely to be hospitalized, according to retrospective data from 177 children and young adults at a single center. “One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Roberta L. DeBiasi, MD, of Children’s National Hospital, Washington, and colleagues reported in the Journal of Pediatrics. READ MORE
Avoiding ageism in COVID resource allocation
The American Geriatrics Society has issued new policy recommendations for resource allocation during the COVID-19 pandemic that are aimed at protecting seniors for ageism. When allocating scarce resources in an emergency, officials should equally weigh in-hospital survival and severe comorbidities contributing to short-term mortality, the group wrote. “Age per se should never be used as a means for a categorical exclusion from therapeutic interventions that represent the standard of care. ... Likewise, specific age-based cutoffs should not be used in resource allocation strategies,” AGS officials wrote in the statement. READ MORE
Preventing addiction-related suicide
Individuals with substance use disorders are at a significant risk for suicide, but there have been few evidence-based options for their treatment. Now a single intervention is showing promise for this high-risk group. In a large, multicenter randomized effectiveness study, a single 3-hour-long group psychosocial intervention resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up. The intervention to prevent future suicide was designed specifically for patients who were in intensive outpatient programs for addiction treatment. “We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Richard K. Ries, MD, director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center. READ MORE
TNF inhibitors may hamper COVID-19 severity
Early evidence from the COVID-19 Global Rheumatology Alliance Registry has produced an intriguing result: Patients on tumor necrosis factor inhibitors for their rheumatic disease are less likely to require hospitalization when infected with COVID-19. The registry data also show that taking hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization. “A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” said Jinoos Yazdany, MD, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital. READ MORE
Audrey Hepburn’s lessons in pandemic grace
There are a lot of new skills required for praticing medicine during the COVID-19 pandemic. In his latest MDedge column, Jeffrey Benabio, MD, explains that grace is one of them. Dr. Benabio, director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego, looks to Audrey Hepburn for inspiration. “Effort is also required for telephone and video visits,” he writes. “In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.” READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 in kids
Children and young adults in all age groups can develop severe illess after SARS-CoV-2 infection, but infants and teens are most likely to be hospitalized, according to retrospective data from 177 children and young adults at a single center. “One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Roberta L. DeBiasi, MD, of Children’s National Hospital, Washington, and colleagues reported in the Journal of Pediatrics. READ MORE
Avoiding ageism in COVID resource allocation
The American Geriatrics Society has issued new policy recommendations for resource allocation during the COVID-19 pandemic that are aimed at protecting seniors for ageism. When allocating scarce resources in an emergency, officials should equally weigh in-hospital survival and severe comorbidities contributing to short-term mortality, the group wrote. “Age per se should never be used as a means for a categorical exclusion from therapeutic interventions that represent the standard of care. ... Likewise, specific age-based cutoffs should not be used in resource allocation strategies,” AGS officials wrote in the statement. READ MORE
Preventing addiction-related suicide
Individuals with substance use disorders are at a significant risk for suicide, but there have been few evidence-based options for their treatment. Now a single intervention is showing promise for this high-risk group. In a large, multicenter randomized effectiveness study, a single 3-hour-long group psychosocial intervention resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up. The intervention to prevent future suicide was designed specifically for patients who were in intensive outpatient programs for addiction treatment. “We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Richard K. Ries, MD, director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center. READ MORE
TNF inhibitors may hamper COVID-19 severity
Early evidence from the COVID-19 Global Rheumatology Alliance Registry has produced an intriguing result: Patients on tumor necrosis factor inhibitors for their rheumatic disease are less likely to require hospitalization when infected with COVID-19. The registry data also show that taking hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization. “A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” said Jinoos Yazdany, MD, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital. READ MORE
Audrey Hepburn’s lessons in pandemic grace
There are a lot of new skills required for praticing medicine during the COVID-19 pandemic. In his latest MDedge column, Jeffrey Benabio, MD, explains that grace is one of them. Dr. Benabio, director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego, looks to Audrey Hepburn for inspiration. “Effort is also required for telephone and video visits,” he writes. “In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.” READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Audrey Hepburn’s lessons for a COVID clinic
Queues of patients wait to clear security and enter the sterile area at every medical office. Water bottles are allowed, fevers and visitors are not. Those who fail clearance or who are afraid to be seen in person must be treated virtually. In this context, virtually means by telephone or video, yet, aptly, it also means “nearly or almost,” as in we can nearly or almost treat them these ways. We’ve emerged safely, but we’ve lost sensibility. Because of this, what’s important in the doctor-patient relationships will drift a bit. Clinical acumen and technical skill won’t be enough. Successful practices will also have grace.
If your image of grace is Audrey Hepburn gliding along Fifth Avenue in a long black dress and elbow-length gloves, you’re in the right place. Ms. Hepburn embodied elegance and decorum and there are lessons to be drawn from her. Piling your hair high and donning oversized sunglasses along with your face mask would be to miss the point here though. Ms. Hepburn dressed exquisitely, yes, but her grace came from what wearing a difficult-to-walk-in dress meant to us, not to her. Appearance, self-control, and warmth are what made her charismatic.
To appear urbane requires effort; it’s the effort that we appreciate in someone who is graceful. When you’re thoughtful about how you look, you plan ahead, you work to look polished. In effect, you’re saying: “As my patient, you’re important enough for me to be well dressed.” It is a visible signal of all the unobservable work you’ve done to care for them. This is more critical now that our faces are covered and concern for infection means wearing shabby hospital scrubs rather than shirt and tie.
Effort is also required for telephone and video visits. In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.
Whether in person or by phone, grace, as Ms. Hepburn demonstrated, is physical awareness and body control. She would often be completely still when someone is speaking, showing a countenance of warmth. She’d pause after the other person completed a thought and before replying. In doing so, she conveyed that she was present and engaged in what was being said. It is that confidence and ease of manner we perceived as grace.
I thought about this the other day during a mixed clinic of telephone and face-to-face visits. I had on my wrinkle-free scrubs (I could do better). I was listening to a patient describe all possible triggers for her hand dermatitis. My urge to interrupt grew with each paragraph of her storytelling. “Be patient,” I thought, “be at ease with her rambling. ... When she stops, thank her as if you were looking her in the eye acknowledging how interesting her observations were.” This is not just good manners, it’s the essence of grace: The art of showing how important others are to you.
Our world needs grace more than ever and what better place to start but with us. In pleasing, assisting, and honoring them, our patients can be reassured that we can and will care for them. Make Ms. Hepburn proud.
“For beautiful eyes, look for the good in others; for beautiful lips, speak only words of kindness; and for poise, walk with the knowledge that you are never alone.” – Audrey Hepburn
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. He has no disclosures related to this column. Write to him at [email protected] .
Queues of patients wait to clear security and enter the sterile area at every medical office. Water bottles are allowed, fevers and visitors are not. Those who fail clearance or who are afraid to be seen in person must be treated virtually. In this context, virtually means by telephone or video, yet, aptly, it also means “nearly or almost,” as in we can nearly or almost treat them these ways. We’ve emerged safely, but we’ve lost sensibility. Because of this, what’s important in the doctor-patient relationships will drift a bit. Clinical acumen and technical skill won’t be enough. Successful practices will also have grace.
If your image of grace is Audrey Hepburn gliding along Fifth Avenue in a long black dress and elbow-length gloves, you’re in the right place. Ms. Hepburn embodied elegance and decorum and there are lessons to be drawn from her. Piling your hair high and donning oversized sunglasses along with your face mask would be to miss the point here though. Ms. Hepburn dressed exquisitely, yes, but her grace came from what wearing a difficult-to-walk-in dress meant to us, not to her. Appearance, self-control, and warmth are what made her charismatic.
To appear urbane requires effort; it’s the effort that we appreciate in someone who is graceful. When you’re thoughtful about how you look, you plan ahead, you work to look polished. In effect, you’re saying: “As my patient, you’re important enough for me to be well dressed.” It is a visible signal of all the unobservable work you’ve done to care for them. This is more critical now that our faces are covered and concern for infection means wearing shabby hospital scrubs rather than shirt and tie.
Effort is also required for telephone and video visits. In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.
Whether in person or by phone, grace, as Ms. Hepburn demonstrated, is physical awareness and body control. She would often be completely still when someone is speaking, showing a countenance of warmth. She’d pause after the other person completed a thought and before replying. In doing so, she conveyed that she was present and engaged in what was being said. It is that confidence and ease of manner we perceived as grace.
I thought about this the other day during a mixed clinic of telephone and face-to-face visits. I had on my wrinkle-free scrubs (I could do better). I was listening to a patient describe all possible triggers for her hand dermatitis. My urge to interrupt grew with each paragraph of her storytelling. “Be patient,” I thought, “be at ease with her rambling. ... When she stops, thank her as if you were looking her in the eye acknowledging how interesting her observations were.” This is not just good manners, it’s the essence of grace: The art of showing how important others are to you.
Our world needs grace more than ever and what better place to start but with us. In pleasing, assisting, and honoring them, our patients can be reassured that we can and will care for them. Make Ms. Hepburn proud.
“For beautiful eyes, look for the good in others; for beautiful lips, speak only words of kindness; and for poise, walk with the knowledge that you are never alone.” – Audrey Hepburn
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. He has no disclosures related to this column. Write to him at [email protected] .
Queues of patients wait to clear security and enter the sterile area at every medical office. Water bottles are allowed, fevers and visitors are not. Those who fail clearance or who are afraid to be seen in person must be treated virtually. In this context, virtually means by telephone or video, yet, aptly, it also means “nearly or almost,” as in we can nearly or almost treat them these ways. We’ve emerged safely, but we’ve lost sensibility. Because of this, what’s important in the doctor-patient relationships will drift a bit. Clinical acumen and technical skill won’t be enough. Successful practices will also have grace.
If your image of grace is Audrey Hepburn gliding along Fifth Avenue in a long black dress and elbow-length gloves, you’re in the right place. Ms. Hepburn embodied elegance and decorum and there are lessons to be drawn from her. Piling your hair high and donning oversized sunglasses along with your face mask would be to miss the point here though. Ms. Hepburn dressed exquisitely, yes, but her grace came from what wearing a difficult-to-walk-in dress meant to us, not to her. Appearance, self-control, and warmth are what made her charismatic.
To appear urbane requires effort; it’s the effort that we appreciate in someone who is graceful. When you’re thoughtful about how you look, you plan ahead, you work to look polished. In effect, you’re saying: “As my patient, you’re important enough for me to be well dressed.” It is a visible signal of all the unobservable work you’ve done to care for them. This is more critical now that our faces are covered and concern for infection means wearing shabby hospital scrubs rather than shirt and tie.
Effort is also required for telephone and video visits. In them, our doctor-patient connection is diminished – no matter how high definition, it’s a virtual affair. Ms. Hepburn would no doubt take the time to ensure she appeared professional, well lit, with a pleasing background. She’d plan for the call to be done in a quiet location and without distraction.
Whether in person or by phone, grace, as Ms. Hepburn demonstrated, is physical awareness and body control. She would often be completely still when someone is speaking, showing a countenance of warmth. She’d pause after the other person completed a thought and before replying. In doing so, she conveyed that she was present and engaged in what was being said. It is that confidence and ease of manner we perceived as grace.
I thought about this the other day during a mixed clinic of telephone and face-to-face visits. I had on my wrinkle-free scrubs (I could do better). I was listening to a patient describe all possible triggers for her hand dermatitis. My urge to interrupt grew with each paragraph of her storytelling. “Be patient,” I thought, “be at ease with her rambling. ... When she stops, thank her as if you were looking her in the eye acknowledging how interesting her observations were.” This is not just good manners, it’s the essence of grace: The art of showing how important others are to you.
Our world needs grace more than ever and what better place to start but with us. In pleasing, assisting, and honoring them, our patients can be reassured that we can and will care for them. Make Ms. Hepburn proud.
“For beautiful eyes, look for the good in others; for beautiful lips, speak only words of kindness; and for poise, walk with the knowledge that you are never alone.” – Audrey Hepburn
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. He has no disclosures related to this column. Write to him at [email protected] .
Novel program for preventing addiction-related suicide
A single 3-hour-long group psychosocial intervention designed specifically for patients in intensive outpatient programs for addiction treatment to prevent future suicide resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up in a large multicenter randomized effectiveness study, reported Richard K. Ries, MD.
There is an enormous unmet need for evidence-based strategies for preventing addiction-related suicide, since people with substance use disorders have a 10-fold increased risk of suicide. Based upon these new study findings, the Preventing Addiction Related Suicide (PARS) program can now be considered the first such evidence-based intervention for this extremely high-risk population, Dr. Ries said at the virtual annual meeting of the American Association of Suicidology.
“We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Dr. Ries, professor of psychiatry at the University of Washington, Seattle, and director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center.
The workbook-based PARS program developed by Dr. Ries and colleagues adapted empirically supported suicide prevention best practices from other settings for use in group-based intensive outpatient addiction treatment, which is the most common form of treatment for chemical dependency in the United States. Dr. Ries recognized the need for a program such as PARS because addiction counselors often feel out of their depth regarding suicide-related issues.
he explained. “We designed the PARS intervention to be integrated right into the counselors’ workflow. They get trained right in their setting in a one-shot deal that takes 2-3 hours. The training was highly rated by counselors as acceptable and effective in their day-to-day work, not ivory tower-type stuff.”
Once the counselors were trained in PARS, they then trained their alcohol- and drug-addicted patients. Elements of the PARS program include information on suicide risk and protective factors, myths and facts about suicide, action steps to take when warning signs of suicide are observed, and local crisis resources.
The effectiveness study was a randomized, stepped-wedge cluster design intervention that included 905 patients in 15 busy community group–based intensive outpatient addiction treatment programs in Western Washington. Patients were randomized to counselor-delivered PARS or treatment as usual, with follow-up assessments at 2 weeks and 1, 3, and 6 months.
There was no attempt to enrich the study population for suicidality by prescreening potential enrollees, since participation in a drug and alcohol treatment program already places an individual in a high-risk group. For example, 74% of study participants indicated they had thought of suicide at least once in the past year, compared with a background rate of 4% in the U.S. general population. And 29% of study participants reported a lifetime history of one or more suicide attempts, versus roughly 5% in the general population.
Dr. Ries’ coinvestigator, Katherine Anne Comtois, PhD, MPH, presented the study results. The three key outcomes were improvement on structured measures of suicide knowledge, attitudes, and help-seeking behavior. The PARS recipients showed statistically significant improvement compared with baseline on two of the three: they displayed greater knowledge about suicide and its close relationship with addiction, and less stigmatization and other maladaptive attitudes toward suicide. Scores on all three measures remained unchanged over time in the control group.
“Overall, we had small to medium effect sizes, comparable to what you might see in studies measuring antidepressant effect sizes. I think these were meaningful improvements in knowledge and attitudes,” said Dr. Comtois of the University of Washington.
The PARS group showed a small increase in the third endpoint – willingness to seek professional help for themselves, friends, or family if depressed or suicidal, but this didn’t achieve statistical significance. However, Dr. Comtois noted that the study outcomes were assessed per protocol using an intent-to-treat analysis. This likely underestimated the true effectiveness of the PARS intervention, given that 40% of patients randomized to PARS didn’t actually attend the intervention session.
“People with drug and alcohol problems have complicated lives,” she said by way of explanation for the high no-show rate.
The investigators are now performing a per-protocol analysis of the data, restricted to those subjects who actually attended their session. A long-term look at suicide events and outcomes in the study population is planned.
Dr. Ries and Dr. Comtois reported having no financial conflicts regarding the study, which was funded by a multiyear grant from the National Institute on Drug Abuse.
A single 3-hour-long group psychosocial intervention designed specifically for patients in intensive outpatient programs for addiction treatment to prevent future suicide resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up in a large multicenter randomized effectiveness study, reported Richard K. Ries, MD.
There is an enormous unmet need for evidence-based strategies for preventing addiction-related suicide, since people with substance use disorders have a 10-fold increased risk of suicide. Based upon these new study findings, the Preventing Addiction Related Suicide (PARS) program can now be considered the first such evidence-based intervention for this extremely high-risk population, Dr. Ries said at the virtual annual meeting of the American Association of Suicidology.
“We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Dr. Ries, professor of psychiatry at the University of Washington, Seattle, and director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center.
The workbook-based PARS program developed by Dr. Ries and colleagues adapted empirically supported suicide prevention best practices from other settings for use in group-based intensive outpatient addiction treatment, which is the most common form of treatment for chemical dependency in the United States. Dr. Ries recognized the need for a program such as PARS because addiction counselors often feel out of their depth regarding suicide-related issues.
he explained. “We designed the PARS intervention to be integrated right into the counselors’ workflow. They get trained right in their setting in a one-shot deal that takes 2-3 hours. The training was highly rated by counselors as acceptable and effective in their day-to-day work, not ivory tower-type stuff.”
Once the counselors were trained in PARS, they then trained their alcohol- and drug-addicted patients. Elements of the PARS program include information on suicide risk and protective factors, myths and facts about suicide, action steps to take when warning signs of suicide are observed, and local crisis resources.
The effectiveness study was a randomized, stepped-wedge cluster design intervention that included 905 patients in 15 busy community group–based intensive outpatient addiction treatment programs in Western Washington. Patients were randomized to counselor-delivered PARS or treatment as usual, with follow-up assessments at 2 weeks and 1, 3, and 6 months.
There was no attempt to enrich the study population for suicidality by prescreening potential enrollees, since participation in a drug and alcohol treatment program already places an individual in a high-risk group. For example, 74% of study participants indicated they had thought of suicide at least once in the past year, compared with a background rate of 4% in the U.S. general population. And 29% of study participants reported a lifetime history of one or more suicide attempts, versus roughly 5% in the general population.
Dr. Ries’ coinvestigator, Katherine Anne Comtois, PhD, MPH, presented the study results. The three key outcomes were improvement on structured measures of suicide knowledge, attitudes, and help-seeking behavior. The PARS recipients showed statistically significant improvement compared with baseline on two of the three: they displayed greater knowledge about suicide and its close relationship with addiction, and less stigmatization and other maladaptive attitudes toward suicide. Scores on all three measures remained unchanged over time in the control group.
“Overall, we had small to medium effect sizes, comparable to what you might see in studies measuring antidepressant effect sizes. I think these were meaningful improvements in knowledge and attitudes,” said Dr. Comtois of the University of Washington.
The PARS group showed a small increase in the third endpoint – willingness to seek professional help for themselves, friends, or family if depressed or suicidal, but this didn’t achieve statistical significance. However, Dr. Comtois noted that the study outcomes were assessed per protocol using an intent-to-treat analysis. This likely underestimated the true effectiveness of the PARS intervention, given that 40% of patients randomized to PARS didn’t actually attend the intervention session.
“People with drug and alcohol problems have complicated lives,” she said by way of explanation for the high no-show rate.
The investigators are now performing a per-protocol analysis of the data, restricted to those subjects who actually attended their session. A long-term look at suicide events and outcomes in the study population is planned.
Dr. Ries and Dr. Comtois reported having no financial conflicts regarding the study, which was funded by a multiyear grant from the National Institute on Drug Abuse.
A single 3-hour-long group psychosocial intervention designed specifically for patients in intensive outpatient programs for addiction treatment to prevent future suicide resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up in a large multicenter randomized effectiveness study, reported Richard K. Ries, MD.
There is an enormous unmet need for evidence-based strategies for preventing addiction-related suicide, since people with substance use disorders have a 10-fold increased risk of suicide. Based upon these new study findings, the Preventing Addiction Related Suicide (PARS) program can now be considered the first such evidence-based intervention for this extremely high-risk population, Dr. Ries said at the virtual annual meeting of the American Association of Suicidology.
“We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Dr. Ries, professor of psychiatry at the University of Washington, Seattle, and director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center.
The workbook-based PARS program developed by Dr. Ries and colleagues adapted empirically supported suicide prevention best practices from other settings for use in group-based intensive outpatient addiction treatment, which is the most common form of treatment for chemical dependency in the United States. Dr. Ries recognized the need for a program such as PARS because addiction counselors often feel out of their depth regarding suicide-related issues.
he explained. “We designed the PARS intervention to be integrated right into the counselors’ workflow. They get trained right in their setting in a one-shot deal that takes 2-3 hours. The training was highly rated by counselors as acceptable and effective in their day-to-day work, not ivory tower-type stuff.”
Once the counselors were trained in PARS, they then trained their alcohol- and drug-addicted patients. Elements of the PARS program include information on suicide risk and protective factors, myths and facts about suicide, action steps to take when warning signs of suicide are observed, and local crisis resources.
The effectiveness study was a randomized, stepped-wedge cluster design intervention that included 905 patients in 15 busy community group–based intensive outpatient addiction treatment programs in Western Washington. Patients were randomized to counselor-delivered PARS or treatment as usual, with follow-up assessments at 2 weeks and 1, 3, and 6 months.
There was no attempt to enrich the study population for suicidality by prescreening potential enrollees, since participation in a drug and alcohol treatment program already places an individual in a high-risk group. For example, 74% of study participants indicated they had thought of suicide at least once in the past year, compared with a background rate of 4% in the U.S. general population. And 29% of study participants reported a lifetime history of one or more suicide attempts, versus roughly 5% in the general population.
Dr. Ries’ coinvestigator, Katherine Anne Comtois, PhD, MPH, presented the study results. The three key outcomes were improvement on structured measures of suicide knowledge, attitudes, and help-seeking behavior. The PARS recipients showed statistically significant improvement compared with baseline on two of the three: they displayed greater knowledge about suicide and its close relationship with addiction, and less stigmatization and other maladaptive attitudes toward suicide. Scores on all three measures remained unchanged over time in the control group.
“Overall, we had small to medium effect sizes, comparable to what you might see in studies measuring antidepressant effect sizes. I think these were meaningful improvements in knowledge and attitudes,” said Dr. Comtois of the University of Washington.
The PARS group showed a small increase in the third endpoint – willingness to seek professional help for themselves, friends, or family if depressed or suicidal, but this didn’t achieve statistical significance. However, Dr. Comtois noted that the study outcomes were assessed per protocol using an intent-to-treat analysis. This likely underestimated the true effectiveness of the PARS intervention, given that 40% of patients randomized to PARS didn’t actually attend the intervention session.
“People with drug and alcohol problems have complicated lives,” she said by way of explanation for the high no-show rate.
The investigators are now performing a per-protocol analysis of the data, restricted to those subjects who actually attended their session. A long-term look at suicide events and outcomes in the study population is planned.
Dr. Ries and Dr. Comtois reported having no financial conflicts regarding the study, which was funded by a multiyear grant from the National Institute on Drug Abuse.
FROM AAS20
Vitamin D: A low-hanging fruit in COVID-19?
Mainstream media outlets have been flooded recently with reports speculating on what role, if any, vitamin D may play in reducing the severity of COVID-19 infection.
as well as mortality, with the further suggestion of an effect of vitamin D on the immune response to infection.
But other studies question such a link, including any association between vitamin D concentration and differences in COVID-19 severity by ethnic group.
And while some researchers and clinicians believe people should get tested to see if they have adequate vitamin D levels during this pandemic – in particular frontline health care workers – most doctors say the best way to ensure that people have adequate levels of vitamin D during COVID-19 is to simply take supplements at currently recommended levels.
This is especially important given the fact that, during “lockdown” scenarios, many people are spending more time than usual indoors.
Clifford Rosen, MD, senior scientist at Maine Medical Center’s Research Institute in Scarborough, has been researching vitamin D for 25 years.
“There’s no randomized, controlled trial for sure, and that’s the gold standard,” he said in an interview, and “the observational data are so confounded, it’s difficult to know.”
Whether from diet or supplementation, having adequate vitamin D is important, especially for those at the highest risk of COVID-19, he said. Still, robust data supporting a role of vitamin D in prevention of COVID-19, or as any kind of “therapy” for the infection, are currently lacking.
Rose Anne Kenny, MD, professor of medical gerontology at Trinity College Dublin, recently coauthored an article detailing an inverse association between vitamin D levels and mortality from COVID-19 across countries in Europe.
“At no stage are any of us saying this is a given, but there’s a probability that [vitamin D] – a low-hanging fruit – is a contributory factor and we can do something about it now,” she said in an interview.
Dr. Kenny is calling for the Irish government to formally change their recommendations. “We call on the Irish government to update guidelines as a matter of urgency and encourage all adults to take [vitamin D] supplements during the COVID-19 crisis.” Northern Ireland, part of the United Kingdom, also has not yet made this recommendation, she said.
Meanwhile, Harpreet S. Bajaj, MD, MPH, a practicing endocrinologist from Mount Sinai Hospital, Toronto, said: “Vitamin D could have any of three potential roles in risk for COVID-19 and/or its severity: no role, simply a marker, or a causal factor.”
Dr. Bajaj said – as did Dr. Rosen and Dr. Kenny – that randomized, controlled trials (RCTs) are sorely needed to help ascertain whether there is a specific role of vitamin D.
“Until then, we should continue to follow established public health recommendations for vitamin D supplementation, in addition to following COVID-19 prevention guidance and evolving guidelines for COVID-19 treatment.”
What is the role of vitamin D fortification?
In their study in the Irish Medical Journal, Dr. Kenny and colleagues noted that, in Europe, despite being sunny, Spain and Northern Italy had high rates of vitamin D deficiency and have experienced some of the highest COVID-19 infection and mortality rates in the world.
But these countries do not formally fortify foods or recommend supplementation with vitamin D.
Conversely, the northern countries of Norway, Finland, and Sweden had higher vitamin D levels despite less UVB sunlight exposure, as a result of common supplementation and formal fortification of foods. These Nordic countries also had lower levels of COVID-19 infection and mortality.
Overall, the correlation between low vitamin D levels and mortality from COVID-19 was statistically significant (P = .046), the investigators reported.
“Optimizing vitamin D status to recommendations by national and international public health agencies will certainly have ... potential benefits for COVID-19,” they concluded.
“We’re not saying there aren’t any confounders. This can absolutely be the case, but this [finding] needs to be in the mix of evidence,” Dr. Kenny said.
Dr. Kenny also noted that countries in the Southern Hemisphere have been seeing a relatively low mortality from COVID-19, although she acknowledged the explanation could be that the virus spread later to those countries.
Dr. Rosen has doubts on this issue, too.
“Sure, vitamin D supplementation may have worked for [Nordic countries], their COVID-19 has been better controlled, but there’s no causality here; there’s another step to actually prove this. Other factors might be at play,” he said.
“Look at Brazil, it’s at the equator but the disease is devastating the country. Right now, I just don’t believe it.”
Does vitamin D have a role to play in immune modulation?
One theory currently circulating is that, if vitamin D does have any role to play in modulating response to COVID-19, this may be via a blunting of the immune system reaction to the virus.
In a recent preprint study, Ali Daneshkhah, PhD, and colleagues from Northwestern University, Chicago, interrogated hospital data from China, France, Germany, Italy, Iran, South Korea, Spain, Switzerland, the United Kingdom, and the United States.
Specifically, the risk of severe COVID-19 cases among patients with severe vitamin D deficiency was 17.3%, whereas the equivalent figure for patients with normal vitamin D levels was 14.6% (a reduction of 15.6%).
“This potential effect may be attributed to vitamin D’s ability to suppress the adaptive immune system, regulating cytokine levels and thereby reducing the risk of developing severe COVID-19,” said the researchers.
Likewise, JoAnn E. Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital in Boston, in a recent commentary, noted evidence from an observational study from three South Asian hospitals, in which the prevalence of vitamin D deficiency was much higher among those with severe COVID-19 illness compared with those with mild illness.
“We also know that vitamin D has an immune-modulating effect and can lower inflammation, and this may be relevant to the respiratory response during COVID-19 and the cytokine storm that’s been demonstrated,” she noted.
Dr. Rosen said he is willing to listen on the issue of a potential role of vitamin D in immune modulation.
“I’ve been a huge skeptic from the get-go, and loudly criticized the data for doing nothing. I am surprised at myself for saying there might be some effect,” he said.
“Clearly most people don’t get this [cytokine storm] but of those that do, it’s unclear why they do. Maybe if you are vitamin D sufficient, it might have some impact down the road on your response to an infection,” Dr. Rosen said. “Vitamin D may induce proteins important in modulating the function of macrophages of the immune system.”
Ethnic minorities disproportionately affected
It is also well recognized that COVID-19 disproportionately affects black and Asian minority ethnic individuals.
But on the issue of vitamin D in this context, one recent peer-reviewed study using UK Biobank data found no evidence to support a potential role for vitamin D concentration to explain susceptibility to COVID-19 infection either overall or in explaining differences between ethnic groups.
“Vitamin D is unlikely to be the underlying mechanism for the higher risk observed in black and minority ethnic individuals, and vitamin D supplements are unlikely to provide an effective intervention,” Claire Hastie, PhD, of the University of Glasgow and colleagues concluded.
But this hasn’t stopped two endocrinologists from appealing to members of the British Association of Physicians of Indian Origin (BAPIO) to get their vitamin D levels tested.
The black and Asian minority ethnic population, “especially frontline staff, should get their Vitamin D3 levels checked and get appropriate replacement as required,” said Parag Singhal, MD, of Weston General Hospital, Weston-Super-Mare, England, and David C. Anderson, a retired endocrinologist, said in a letter to BAPIO members.
Indeed, they suggested a booster dose of 100,000 IU as a one-off for black and Asian minority ethnic health care staff that should raise vitamin D levels for 2-3 months. They referred to a systematic review that concludes that “single vitamin D3 doses ≥300,000 IU are most effective at improving vitamin D status ... for up to 3 months”.
Commenting on the idea, Dr. Rosen remarked that, in general, the high-dose 50,000-500,000 IU given as a one-off does not confer any greater benefit than a single dose of 1,000 IU per day, except that the blood levels go up quicker and higher.
“Really there is no evidence that getting to super-high levels of vitamin D confer a greater benefit than normal levels,” he said. “So if health care workers suspect vitamin D deficiency, daily doses of 1,000 IU seem reasonable; even if they miss doses, the blood levels are relatively stable.”
On the specific question of vitamin D needs in ethnic minorities, Dr. Rosen said while such individuals do have lower serum levels of vitamin D, the issue is whether there are meaningful clinical implications related to this.
“The real question is whether [ethnic minority individuals] have physiologically adapted for this in other ways because these low levels have been so for thousands of years. In fact, African Americans have lower vitamin D levels but they absolutely have better bones than [whites],” he pointed out.
Testing and governmental recommendations during COVID-19
The U.S. National Institutes of Health in general advises 400 IU to 800 IU per day intake of vitamin D, depending on age, with those over 70 years requiring the highest daily dose. This will result in blood levels that are sufficient to maintain bone health and normal calcium metabolism in healthy people. There are no additional recommendations specific to vitamin D intake during the COVID-19 pandemic, however.
And Dr. Rosen pointed out that there is no evidence for mass screening of vitamin D levels among the U.S. population.
“U.S. public health guidance was pre-COVID, and I think high-risk individuals might want to think about their levels; for example, someone with inflammatory bowel disease or liver or pancreatic disease. These people are at higher risk anyway, and it could be because their vitamin D is low,” he said.
“Skip the test and ensure you are getting adequate levels of vitamin D whether via diet or supplement [400-800 IU per day],” he suggested. “It won’t harm.”
The U.K.’s Public Health England (PHE) clarified its advice on vitamin D supplementation during COVID-19. Alison Tedstone, PhD, chief nutritionist at PHE, said: “Many people are spending more time indoors and may not get all the vitamin D they need from sunlight. To protect their bone and muscle health, they should consider taking a daily supplement containing 10 micrograms [400 IU] of vitamin D.”
However, “there is no sufficient evidence to support recommending Vitamin D for reducing the risk of COVID-19,” she stressed.
Dr. Bajaj is on the advisory board of Medscape Diabetes & Endocrinology. He has ties with Amgen, AstraZeneca Boehringer Ingelheim, Janssen, Merck, Novo Nordisk, Sanofi, Eli Lilly,Valeant, Canadian Collaborative Research Network, CMS Knowledge Translation, Diabetes Canada Scientific Group, LMC Healthcare,mdBriefCase,Medscape, andMeducom. Dr. Kenny, Dr. Rosen, and Dr. Singhal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mainstream media outlets have been flooded recently with reports speculating on what role, if any, vitamin D may play in reducing the severity of COVID-19 infection.
as well as mortality, with the further suggestion of an effect of vitamin D on the immune response to infection.
But other studies question such a link, including any association between vitamin D concentration and differences in COVID-19 severity by ethnic group.
And while some researchers and clinicians believe people should get tested to see if they have adequate vitamin D levels during this pandemic – in particular frontline health care workers – most doctors say the best way to ensure that people have adequate levels of vitamin D during COVID-19 is to simply take supplements at currently recommended levels.
This is especially important given the fact that, during “lockdown” scenarios, many people are spending more time than usual indoors.
Clifford Rosen, MD, senior scientist at Maine Medical Center’s Research Institute in Scarborough, has been researching vitamin D for 25 years.
“There’s no randomized, controlled trial for sure, and that’s the gold standard,” he said in an interview, and “the observational data are so confounded, it’s difficult to know.”
Whether from diet or supplementation, having adequate vitamin D is important, especially for those at the highest risk of COVID-19, he said. Still, robust data supporting a role of vitamin D in prevention of COVID-19, or as any kind of “therapy” for the infection, are currently lacking.
Rose Anne Kenny, MD, professor of medical gerontology at Trinity College Dublin, recently coauthored an article detailing an inverse association between vitamin D levels and mortality from COVID-19 across countries in Europe.
“At no stage are any of us saying this is a given, but there’s a probability that [vitamin D] – a low-hanging fruit – is a contributory factor and we can do something about it now,” she said in an interview.
Dr. Kenny is calling for the Irish government to formally change their recommendations. “We call on the Irish government to update guidelines as a matter of urgency and encourage all adults to take [vitamin D] supplements during the COVID-19 crisis.” Northern Ireland, part of the United Kingdom, also has not yet made this recommendation, she said.
Meanwhile, Harpreet S. Bajaj, MD, MPH, a practicing endocrinologist from Mount Sinai Hospital, Toronto, said: “Vitamin D could have any of three potential roles in risk for COVID-19 and/or its severity: no role, simply a marker, or a causal factor.”
Dr. Bajaj said – as did Dr. Rosen and Dr. Kenny – that randomized, controlled trials (RCTs) are sorely needed to help ascertain whether there is a specific role of vitamin D.
“Until then, we should continue to follow established public health recommendations for vitamin D supplementation, in addition to following COVID-19 prevention guidance and evolving guidelines for COVID-19 treatment.”
What is the role of vitamin D fortification?
In their study in the Irish Medical Journal, Dr. Kenny and colleagues noted that, in Europe, despite being sunny, Spain and Northern Italy had high rates of vitamin D deficiency and have experienced some of the highest COVID-19 infection and mortality rates in the world.
But these countries do not formally fortify foods or recommend supplementation with vitamin D.
Conversely, the northern countries of Norway, Finland, and Sweden had higher vitamin D levels despite less UVB sunlight exposure, as a result of common supplementation and formal fortification of foods. These Nordic countries also had lower levels of COVID-19 infection and mortality.
Overall, the correlation between low vitamin D levels and mortality from COVID-19 was statistically significant (P = .046), the investigators reported.
“Optimizing vitamin D status to recommendations by national and international public health agencies will certainly have ... potential benefits for COVID-19,” they concluded.
“We’re not saying there aren’t any confounders. This can absolutely be the case, but this [finding] needs to be in the mix of evidence,” Dr. Kenny said.
Dr. Kenny also noted that countries in the Southern Hemisphere have been seeing a relatively low mortality from COVID-19, although she acknowledged the explanation could be that the virus spread later to those countries.
Dr. Rosen has doubts on this issue, too.
“Sure, vitamin D supplementation may have worked for [Nordic countries], their COVID-19 has been better controlled, but there’s no causality here; there’s another step to actually prove this. Other factors might be at play,” he said.
“Look at Brazil, it’s at the equator but the disease is devastating the country. Right now, I just don’t believe it.”
Does vitamin D have a role to play in immune modulation?
One theory currently circulating is that, if vitamin D does have any role to play in modulating response to COVID-19, this may be via a blunting of the immune system reaction to the virus.
In a recent preprint study, Ali Daneshkhah, PhD, and colleagues from Northwestern University, Chicago, interrogated hospital data from China, France, Germany, Italy, Iran, South Korea, Spain, Switzerland, the United Kingdom, and the United States.
Specifically, the risk of severe COVID-19 cases among patients with severe vitamin D deficiency was 17.3%, whereas the equivalent figure for patients with normal vitamin D levels was 14.6% (a reduction of 15.6%).
“This potential effect may be attributed to vitamin D’s ability to suppress the adaptive immune system, regulating cytokine levels and thereby reducing the risk of developing severe COVID-19,” said the researchers.
Likewise, JoAnn E. Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital in Boston, in a recent commentary, noted evidence from an observational study from three South Asian hospitals, in which the prevalence of vitamin D deficiency was much higher among those with severe COVID-19 illness compared with those with mild illness.
“We also know that vitamin D has an immune-modulating effect and can lower inflammation, and this may be relevant to the respiratory response during COVID-19 and the cytokine storm that’s been demonstrated,” she noted.
Dr. Rosen said he is willing to listen on the issue of a potential role of vitamin D in immune modulation.
“I’ve been a huge skeptic from the get-go, and loudly criticized the data for doing nothing. I am surprised at myself for saying there might be some effect,” he said.
“Clearly most people don’t get this [cytokine storm] but of those that do, it’s unclear why they do. Maybe if you are vitamin D sufficient, it might have some impact down the road on your response to an infection,” Dr. Rosen said. “Vitamin D may induce proteins important in modulating the function of macrophages of the immune system.”
Ethnic minorities disproportionately affected
It is also well recognized that COVID-19 disproportionately affects black and Asian minority ethnic individuals.
But on the issue of vitamin D in this context, one recent peer-reviewed study using UK Biobank data found no evidence to support a potential role for vitamin D concentration to explain susceptibility to COVID-19 infection either overall or in explaining differences between ethnic groups.
“Vitamin D is unlikely to be the underlying mechanism for the higher risk observed in black and minority ethnic individuals, and vitamin D supplements are unlikely to provide an effective intervention,” Claire Hastie, PhD, of the University of Glasgow and colleagues concluded.
But this hasn’t stopped two endocrinologists from appealing to members of the British Association of Physicians of Indian Origin (BAPIO) to get their vitamin D levels tested.
The black and Asian minority ethnic population, “especially frontline staff, should get their Vitamin D3 levels checked and get appropriate replacement as required,” said Parag Singhal, MD, of Weston General Hospital, Weston-Super-Mare, England, and David C. Anderson, a retired endocrinologist, said in a letter to BAPIO members.
Indeed, they suggested a booster dose of 100,000 IU as a one-off for black and Asian minority ethnic health care staff that should raise vitamin D levels for 2-3 months. They referred to a systematic review that concludes that “single vitamin D3 doses ≥300,000 IU are most effective at improving vitamin D status ... for up to 3 months”.
Commenting on the idea, Dr. Rosen remarked that, in general, the high-dose 50,000-500,000 IU given as a one-off does not confer any greater benefit than a single dose of 1,000 IU per day, except that the blood levels go up quicker and higher.
“Really there is no evidence that getting to super-high levels of vitamin D confer a greater benefit than normal levels,” he said. “So if health care workers suspect vitamin D deficiency, daily doses of 1,000 IU seem reasonable; even if they miss doses, the blood levels are relatively stable.”
On the specific question of vitamin D needs in ethnic minorities, Dr. Rosen said while such individuals do have lower serum levels of vitamin D, the issue is whether there are meaningful clinical implications related to this.
“The real question is whether [ethnic minority individuals] have physiologically adapted for this in other ways because these low levels have been so for thousands of years. In fact, African Americans have lower vitamin D levels but they absolutely have better bones than [whites],” he pointed out.
Testing and governmental recommendations during COVID-19
The U.S. National Institutes of Health in general advises 400 IU to 800 IU per day intake of vitamin D, depending on age, with those over 70 years requiring the highest daily dose. This will result in blood levels that are sufficient to maintain bone health and normal calcium metabolism in healthy people. There are no additional recommendations specific to vitamin D intake during the COVID-19 pandemic, however.
And Dr. Rosen pointed out that there is no evidence for mass screening of vitamin D levels among the U.S. population.
“U.S. public health guidance was pre-COVID, and I think high-risk individuals might want to think about their levels; for example, someone with inflammatory bowel disease or liver or pancreatic disease. These people are at higher risk anyway, and it could be because their vitamin D is low,” he said.
“Skip the test and ensure you are getting adequate levels of vitamin D whether via diet or supplement [400-800 IU per day],” he suggested. “It won’t harm.”
The U.K.’s Public Health England (PHE) clarified its advice on vitamin D supplementation during COVID-19. Alison Tedstone, PhD, chief nutritionist at PHE, said: “Many people are spending more time indoors and may not get all the vitamin D they need from sunlight. To protect their bone and muscle health, they should consider taking a daily supplement containing 10 micrograms [400 IU] of vitamin D.”
However, “there is no sufficient evidence to support recommending Vitamin D for reducing the risk of COVID-19,” she stressed.
Dr. Bajaj is on the advisory board of Medscape Diabetes & Endocrinology. He has ties with Amgen, AstraZeneca Boehringer Ingelheim, Janssen, Merck, Novo Nordisk, Sanofi, Eli Lilly,Valeant, Canadian Collaborative Research Network, CMS Knowledge Translation, Diabetes Canada Scientific Group, LMC Healthcare,mdBriefCase,Medscape, andMeducom. Dr. Kenny, Dr. Rosen, and Dr. Singhal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mainstream media outlets have been flooded recently with reports speculating on what role, if any, vitamin D may play in reducing the severity of COVID-19 infection.
as well as mortality, with the further suggestion of an effect of vitamin D on the immune response to infection.
But other studies question such a link, including any association between vitamin D concentration and differences in COVID-19 severity by ethnic group.
And while some researchers and clinicians believe people should get tested to see if they have adequate vitamin D levels during this pandemic – in particular frontline health care workers – most doctors say the best way to ensure that people have adequate levels of vitamin D during COVID-19 is to simply take supplements at currently recommended levels.
This is especially important given the fact that, during “lockdown” scenarios, many people are spending more time than usual indoors.
Clifford Rosen, MD, senior scientist at Maine Medical Center’s Research Institute in Scarborough, has been researching vitamin D for 25 years.
“There’s no randomized, controlled trial for sure, and that’s the gold standard,” he said in an interview, and “the observational data are so confounded, it’s difficult to know.”
Whether from diet or supplementation, having adequate vitamin D is important, especially for those at the highest risk of COVID-19, he said. Still, robust data supporting a role of vitamin D in prevention of COVID-19, or as any kind of “therapy” for the infection, are currently lacking.
Rose Anne Kenny, MD, professor of medical gerontology at Trinity College Dublin, recently coauthored an article detailing an inverse association between vitamin D levels and mortality from COVID-19 across countries in Europe.
“At no stage are any of us saying this is a given, but there’s a probability that [vitamin D] – a low-hanging fruit – is a contributory factor and we can do something about it now,” she said in an interview.
Dr. Kenny is calling for the Irish government to formally change their recommendations. “We call on the Irish government to update guidelines as a matter of urgency and encourage all adults to take [vitamin D] supplements during the COVID-19 crisis.” Northern Ireland, part of the United Kingdom, also has not yet made this recommendation, she said.
Meanwhile, Harpreet S. Bajaj, MD, MPH, a practicing endocrinologist from Mount Sinai Hospital, Toronto, said: “Vitamin D could have any of three potential roles in risk for COVID-19 and/or its severity: no role, simply a marker, or a causal factor.”
Dr. Bajaj said – as did Dr. Rosen and Dr. Kenny – that randomized, controlled trials (RCTs) are sorely needed to help ascertain whether there is a specific role of vitamin D.
“Until then, we should continue to follow established public health recommendations for vitamin D supplementation, in addition to following COVID-19 prevention guidance and evolving guidelines for COVID-19 treatment.”
What is the role of vitamin D fortification?
In their study in the Irish Medical Journal, Dr. Kenny and colleagues noted that, in Europe, despite being sunny, Spain and Northern Italy had high rates of vitamin D deficiency and have experienced some of the highest COVID-19 infection and mortality rates in the world.
But these countries do not formally fortify foods or recommend supplementation with vitamin D.
Conversely, the northern countries of Norway, Finland, and Sweden had higher vitamin D levels despite less UVB sunlight exposure, as a result of common supplementation and formal fortification of foods. These Nordic countries also had lower levels of COVID-19 infection and mortality.
Overall, the correlation between low vitamin D levels and mortality from COVID-19 was statistically significant (P = .046), the investigators reported.
“Optimizing vitamin D status to recommendations by national and international public health agencies will certainly have ... potential benefits for COVID-19,” they concluded.
“We’re not saying there aren’t any confounders. This can absolutely be the case, but this [finding] needs to be in the mix of evidence,” Dr. Kenny said.
Dr. Kenny also noted that countries in the Southern Hemisphere have been seeing a relatively low mortality from COVID-19, although she acknowledged the explanation could be that the virus spread later to those countries.
Dr. Rosen has doubts on this issue, too.
“Sure, vitamin D supplementation may have worked for [Nordic countries], their COVID-19 has been better controlled, but there’s no causality here; there’s another step to actually prove this. Other factors might be at play,” he said.
“Look at Brazil, it’s at the equator but the disease is devastating the country. Right now, I just don’t believe it.”
Does vitamin D have a role to play in immune modulation?
One theory currently circulating is that, if vitamin D does have any role to play in modulating response to COVID-19, this may be via a blunting of the immune system reaction to the virus.
In a recent preprint study, Ali Daneshkhah, PhD, and colleagues from Northwestern University, Chicago, interrogated hospital data from China, France, Germany, Italy, Iran, South Korea, Spain, Switzerland, the United Kingdom, and the United States.
Specifically, the risk of severe COVID-19 cases among patients with severe vitamin D deficiency was 17.3%, whereas the equivalent figure for patients with normal vitamin D levels was 14.6% (a reduction of 15.6%).
“This potential effect may be attributed to vitamin D’s ability to suppress the adaptive immune system, regulating cytokine levels and thereby reducing the risk of developing severe COVID-19,” said the researchers.
Likewise, JoAnn E. Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital in Boston, in a recent commentary, noted evidence from an observational study from three South Asian hospitals, in which the prevalence of vitamin D deficiency was much higher among those with severe COVID-19 illness compared with those with mild illness.
“We also know that vitamin D has an immune-modulating effect and can lower inflammation, and this may be relevant to the respiratory response during COVID-19 and the cytokine storm that’s been demonstrated,” she noted.
Dr. Rosen said he is willing to listen on the issue of a potential role of vitamin D in immune modulation.
“I’ve been a huge skeptic from the get-go, and loudly criticized the data for doing nothing. I am surprised at myself for saying there might be some effect,” he said.
“Clearly most people don’t get this [cytokine storm] but of those that do, it’s unclear why they do. Maybe if you are vitamin D sufficient, it might have some impact down the road on your response to an infection,” Dr. Rosen said. “Vitamin D may induce proteins important in modulating the function of macrophages of the immune system.”
Ethnic minorities disproportionately affected
It is also well recognized that COVID-19 disproportionately affects black and Asian minority ethnic individuals.
But on the issue of vitamin D in this context, one recent peer-reviewed study using UK Biobank data found no evidence to support a potential role for vitamin D concentration to explain susceptibility to COVID-19 infection either overall or in explaining differences between ethnic groups.
“Vitamin D is unlikely to be the underlying mechanism for the higher risk observed in black and minority ethnic individuals, and vitamin D supplements are unlikely to provide an effective intervention,” Claire Hastie, PhD, of the University of Glasgow and colleagues concluded.
But this hasn’t stopped two endocrinologists from appealing to members of the British Association of Physicians of Indian Origin (BAPIO) to get their vitamin D levels tested.
The black and Asian minority ethnic population, “especially frontline staff, should get their Vitamin D3 levels checked and get appropriate replacement as required,” said Parag Singhal, MD, of Weston General Hospital, Weston-Super-Mare, England, and David C. Anderson, a retired endocrinologist, said in a letter to BAPIO members.
Indeed, they suggested a booster dose of 100,000 IU as a one-off for black and Asian minority ethnic health care staff that should raise vitamin D levels for 2-3 months. They referred to a systematic review that concludes that “single vitamin D3 doses ≥300,000 IU are most effective at improving vitamin D status ... for up to 3 months”.
Commenting on the idea, Dr. Rosen remarked that, in general, the high-dose 50,000-500,000 IU given as a one-off does not confer any greater benefit than a single dose of 1,000 IU per day, except that the blood levels go up quicker and higher.
“Really there is no evidence that getting to super-high levels of vitamin D confer a greater benefit than normal levels,” he said. “So if health care workers suspect vitamin D deficiency, daily doses of 1,000 IU seem reasonable; even if they miss doses, the blood levels are relatively stable.”
On the specific question of vitamin D needs in ethnic minorities, Dr. Rosen said while such individuals do have lower serum levels of vitamin D, the issue is whether there are meaningful clinical implications related to this.
“The real question is whether [ethnic minority individuals] have physiologically adapted for this in other ways because these low levels have been so for thousands of years. In fact, African Americans have lower vitamin D levels but they absolutely have better bones than [whites],” he pointed out.
Testing and governmental recommendations during COVID-19
The U.S. National Institutes of Health in general advises 400 IU to 800 IU per day intake of vitamin D, depending on age, with those over 70 years requiring the highest daily dose. This will result in blood levels that are sufficient to maintain bone health and normal calcium metabolism in healthy people. There are no additional recommendations specific to vitamin D intake during the COVID-19 pandemic, however.
And Dr. Rosen pointed out that there is no evidence for mass screening of vitamin D levels among the U.S. population.
“U.S. public health guidance was pre-COVID, and I think high-risk individuals might want to think about their levels; for example, someone with inflammatory bowel disease or liver or pancreatic disease. These people are at higher risk anyway, and it could be because their vitamin D is low,” he said.
“Skip the test and ensure you are getting adequate levels of vitamin D whether via diet or supplement [400-800 IU per day],” he suggested. “It won’t harm.”
The U.K.’s Public Health England (PHE) clarified its advice on vitamin D supplementation during COVID-19. Alison Tedstone, PhD, chief nutritionist at PHE, said: “Many people are spending more time indoors and may not get all the vitamin D they need from sunlight. To protect their bone and muscle health, they should consider taking a daily supplement containing 10 micrograms [400 IU] of vitamin D.”
However, “there is no sufficient evidence to support recommending Vitamin D for reducing the risk of COVID-19,” she stressed.
Dr. Bajaj is on the advisory board of Medscape Diabetes & Endocrinology. He has ties with Amgen, AstraZeneca Boehringer Ingelheim, Janssen, Merck, Novo Nordisk, Sanofi, Eli Lilly,Valeant, Canadian Collaborative Research Network, CMS Knowledge Translation, Diabetes Canada Scientific Group, LMC Healthcare,mdBriefCase,Medscape, andMeducom. Dr. Kenny, Dr. Rosen, and Dr. Singhal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TNF inhibitors may dampen COVID-19 severity
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
REPORTING FROM SOTA 2020
Antibody testing suggests COVID-19 cases are being missed
The number of COVID-19 infections in the community may be “substantially greater” than totals confirmed by authorities, based on SARS-CoV-2 antibody testing among a random sample of adults in Los Angeles County, Calif.
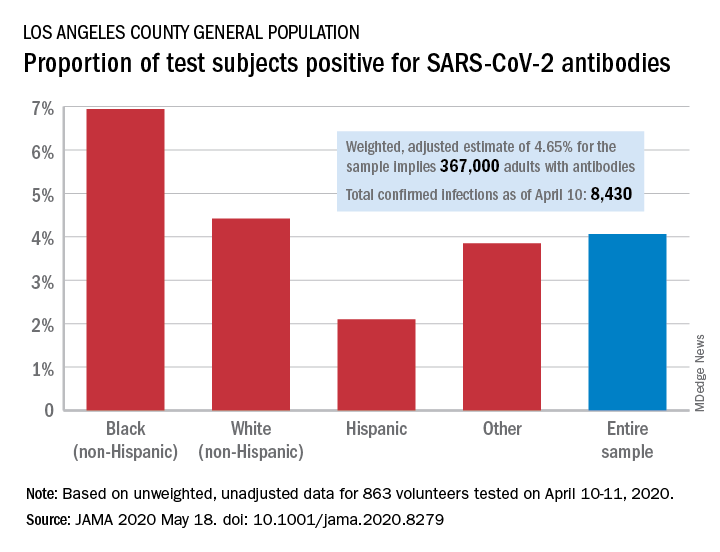
Testing of 863 people on April 10-11 revealed that 35 (4.06%) were positive for SARS-CoV-2–specific antibodies (IgM or IgG), and after adjustment for test sensitivity and specificity, the weighted prevalence for the entire sample was 4.65%, Neeraj Sood, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
The estimate of 4.65% “implies that approximately 367,000 adults [in Los Angeles County] had SARS-CoV-2 antibodies, which is substantially greater than the 8,430 cumulative number of confirmed infections in the county on April 10,” they wrote.
It also suggests that fatality rates based on the larger number of infections may be lower than rates based on confirmed cases. “In addition, contact tracing methods to limit the spread of infection will face considerable challenges,” Dr. Sood and associates said.
Test positivity varied by race/ethnicity, sex, and income. The proportion of non-Hispanic blacks with a positive result was 6.94%, compared with 4.42% for non-Hispanic whites, 2.10% for Hispanics, and 3.85% for others. Men were much more likely than women to be positive for SARS-CoV-2: 5.18% vs. 3.31%, the investigators said.
Household income favored the middle ground. Those individuals making less than $50,000 a year had a positivity rate of 5.14% and those with an income of $100,000 or more had a rate of 4.90%, but only 1.58% of those making $50,000-$99,999 tested positive, they reported.
The authors reported numerous sources of nonprofit organization support.
SOURCE: Sood N et al. JAMA 2020 May 18. doi: 10.1001/jama.2020.8279.
The number of COVID-19 infections in the community may be “substantially greater” than totals confirmed by authorities, based on SARS-CoV-2 antibody testing among a random sample of adults in Los Angeles County, Calif.

Testing of 863 people on April 10-11 revealed that 35 (4.06%) were positive for SARS-CoV-2–specific antibodies (IgM or IgG), and after adjustment for test sensitivity and specificity, the weighted prevalence for the entire sample was 4.65%, Neeraj Sood, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
The estimate of 4.65% “implies that approximately 367,000 adults [in Los Angeles County] had SARS-CoV-2 antibodies, which is substantially greater than the 8,430 cumulative number of confirmed infections in the county on April 10,” they wrote.
It also suggests that fatality rates based on the larger number of infections may be lower than rates based on confirmed cases. “In addition, contact tracing methods to limit the spread of infection will face considerable challenges,” Dr. Sood and associates said.
Test positivity varied by race/ethnicity, sex, and income. The proportion of non-Hispanic blacks with a positive result was 6.94%, compared with 4.42% for non-Hispanic whites, 2.10% for Hispanics, and 3.85% for others. Men were much more likely than women to be positive for SARS-CoV-2: 5.18% vs. 3.31%, the investigators said.
Household income favored the middle ground. Those individuals making less than $50,000 a year had a positivity rate of 5.14% and those with an income of $100,000 or more had a rate of 4.90%, but only 1.58% of those making $50,000-$99,999 tested positive, they reported.
The authors reported numerous sources of nonprofit organization support.
SOURCE: Sood N et al. JAMA 2020 May 18. doi: 10.1001/jama.2020.8279.
The number of COVID-19 infections in the community may be “substantially greater” than totals confirmed by authorities, based on SARS-CoV-2 antibody testing among a random sample of adults in Los Angeles County, Calif.

Testing of 863 people on April 10-11 revealed that 35 (4.06%) were positive for SARS-CoV-2–specific antibodies (IgM or IgG), and after adjustment for test sensitivity and specificity, the weighted prevalence for the entire sample was 4.65%, Neeraj Sood, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
The estimate of 4.65% “implies that approximately 367,000 adults [in Los Angeles County] had SARS-CoV-2 antibodies, which is substantially greater than the 8,430 cumulative number of confirmed infections in the county on April 10,” they wrote.
It also suggests that fatality rates based on the larger number of infections may be lower than rates based on confirmed cases. “In addition, contact tracing methods to limit the spread of infection will face considerable challenges,” Dr. Sood and associates said.
Test positivity varied by race/ethnicity, sex, and income. The proportion of non-Hispanic blacks with a positive result was 6.94%, compared with 4.42% for non-Hispanic whites, 2.10% for Hispanics, and 3.85% for others. Men were much more likely than women to be positive for SARS-CoV-2: 5.18% vs. 3.31%, the investigators said.
Household income favored the middle ground. Those individuals making less than $50,000 a year had a positivity rate of 5.14% and those with an income of $100,000 or more had a rate of 4.90%, but only 1.58% of those making $50,000-$99,999 tested positive, they reported.
The authors reported numerous sources of nonprofit organization support.
SOURCE: Sood N et al. JAMA 2020 May 18. doi: 10.1001/jama.2020.8279.
FROM JAMA
Leadless pacemaker shown safe in older, sicker patients
A leadless right-ventricular pacemaker continued to show an edge over conventional transvenous pacemakers by triggering a substantially reduced rate of complications during the 6 months following placement in a review of more than 10,000 Medicare patients treated over 2 years.
The “largest leadless pacemaker cohort to date” showed that in propensity score–matched cohorts, the 3,276 patients who received the Micra leadless transcatheter pacemaker during routine management and were followed for 6 months had a 3.3% rate of total complications, compared with a 9.4% rate among 7,256 patients who received a conventional VVI pacemaker with a transvenous lead, a statistically significant 66% relative risk reduction, Jonathan P. Piccini, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
The 66% reduced rate of complications – both acutely and with further follow-up – was similar to the complication reductions seen with Micra, compared with historical controls who received transvenous single-chamber pacemakers in both the pivotal study for the device (Heart Rhythm. 2017 May 1;14[3]:702-9) and in a postapproval registry study (Heart Rhythm. 2018 Dec 1;15[12]:1800-7). However, the newly reported advantage came in a population that was notably older and had significantly more comorbidities than in the prior leadless pacemaker studies, said Dr. Piccini, a cardiac electrophysiologist at Duke University, Durham, N.C.
The new Medicare data “tell us that physicians are reaching for these devices [leadless pacemakers] in patients with more comorbidities and a higher risk for complications to give them a [device with] better safety profile,” he said during a press briefing. “At Duke, and I suspect at other centers, when a patients is eligible for a leadless pacemaker that’s the preferred option.”
However, Dr. Piccini cited three examples of the small proportion of patients who are appropriate for the type of pacing the leadless pacemaker supplies but would be better candidates for a device with a transvenous lead: patients who failed treatment with a initial leadless pacemaker and have no suitable alternative subcutaneous spot to place the replacement device in a stable way, those with severe right ventricular enlargement that interferes with optimal placement, and those who don’t currently meet criteria for biventricular pacing but appear likely to switch to that pacing mode in the near term.
The 66% relative reduction in complications was “impressive; I hope this will be a message,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University, New Orleans. Importantly, this reduced complication rate occurred in a real-world population that was sicker than any patient group previously studied with the device, he noted as a designated discussant for the report.
But the report’s second designated discussant, Roderick Tung, MD, highlighted some caveats when interpreting the lower complication rate with the leadless device compared with historical controls. He cited the absence of any episodes of pneumothorax among the patients reviewed by Dr. Piccini who received a leadless pacemaker, compared with a 5% rate among the control patients who had received a device with a transvenous lead, a major driver of the overall difference in complication rates. This difference “may not be relevant to operators who use either an axillary extrathoracic vein route for lead placement or a cephalic vein approach,” said Dr. Tung, director of cardiac electrophysiology at the University of Chicago. “There should not be a 5% rate of pneumothorax when implanting a VVI device.” The results reported by Dr. Piccini have the advantages of coming from many patients and from real-world practice, he acknowledged, but interpretation is limited by the lack of a randomized control group and the outsized impact of pneumothorax complications on the safety comparison.
The other major component of the 6-month complication tally was device-related events, which were twice as common in the historical controls who received a transvenous lead at a rate of 3.4%. The sole 6-month event more common among the patients who received a leadless pacemaker was pericarditis, at a rate of 1.3% in the Micra group and 0.5% in the transvenous lead controls, Dr. Piccini reported. The 6-month rate of device revisions was 1.7% with the leadless device and 2.8% with transvenous lead pacemakers, a difference that was not statistically significant. The two treatment arms had virtually identical 6-month mortality rates.
The rate of acute complications during the first 30 days after implant was also virtually the same in the two study arms. Patient who received the leadless device had significantly more puncture-site events, at a rate of 1.2%, and significantly more cardiac effusions or perforations, at a rate of 0.8%. The historical control patients who received devices with transvenous leads had significantly more device-related complications after 30 days, a 2.5% rate.
The 30-day cohorts examined had larger numbers of patients than at 6 months, 5,746 leadless pacemaker recipients and 9,662 matched historical controls who had received a transvenous lead pacemaker. The clinical and demographic profile of the 30-day cohort who received the leadless pacemaker highlighted the sicker nature of these patients compared with earlier studies of the device. They were an average age of 79 years, compared with average ages of 76 years in the two prior Micra studies, and they also had double the prevalence of coronary disease, triple the prevalence of heart failure, more than twice the rate of chronic obstructive pulmonary disease, and almost twice the prevalence of diabetes.
During the period examined in this report from Micra CED (Longitudinal Coverage With Evidence Development Study on Micra Leadless Pacemakers), in 2017-2018, the leadless pacemaker’s initial approved indications were for a circumscribed portion of the overall patient population that needs pacing. Essentially, they were elderly patients with persistent atrial fibrillation who only need ventricular pacing, roughly 15% of the overall cohort of pacing candidates. In January 2020, the FDA added an indication for high-grade atrioventricular block, an expanded population of candidates that roughly tripled the number of potentially appropriate recipients, said Larry A. Chinitz, MD, a cardiac electrophysiologist and a coinvestigator on some of the studies that led to the new indication, in an interview at the time of the revised labeling.
The study was sponsored by Medtronic, which markets the Micra leadless pacemaker. Dr. Piccini has received honoraria from Medtronic and several other companies. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster. Dr. Chinitz has received fees and fellowship support from Medtronic, and has also received fees from Abbott, Biosense Webster, Biotronik, and Pfizer.
SOURCE: Piccini JP et al. Heart Rhythm 2020, Abstract D-LBCT04-01.
A leadless right-ventricular pacemaker continued to show an edge over conventional transvenous pacemakers by triggering a substantially reduced rate of complications during the 6 months following placement in a review of more than 10,000 Medicare patients treated over 2 years.
The “largest leadless pacemaker cohort to date” showed that in propensity score–matched cohorts, the 3,276 patients who received the Micra leadless transcatheter pacemaker during routine management and were followed for 6 months had a 3.3% rate of total complications, compared with a 9.4% rate among 7,256 patients who received a conventional VVI pacemaker with a transvenous lead, a statistically significant 66% relative risk reduction, Jonathan P. Piccini, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
The 66% reduced rate of complications – both acutely and with further follow-up – was similar to the complication reductions seen with Micra, compared with historical controls who received transvenous single-chamber pacemakers in both the pivotal study for the device (Heart Rhythm. 2017 May 1;14[3]:702-9) and in a postapproval registry study (Heart Rhythm. 2018 Dec 1;15[12]:1800-7). However, the newly reported advantage came in a population that was notably older and had significantly more comorbidities than in the prior leadless pacemaker studies, said Dr. Piccini, a cardiac electrophysiologist at Duke University, Durham, N.C.
The new Medicare data “tell us that physicians are reaching for these devices [leadless pacemakers] in patients with more comorbidities and a higher risk for complications to give them a [device with] better safety profile,” he said during a press briefing. “At Duke, and I suspect at other centers, when a patients is eligible for a leadless pacemaker that’s the preferred option.”
However, Dr. Piccini cited three examples of the small proportion of patients who are appropriate for the type of pacing the leadless pacemaker supplies but would be better candidates for a device with a transvenous lead: patients who failed treatment with a initial leadless pacemaker and have no suitable alternative subcutaneous spot to place the replacement device in a stable way, those with severe right ventricular enlargement that interferes with optimal placement, and those who don’t currently meet criteria for biventricular pacing but appear likely to switch to that pacing mode in the near term.
The 66% relative reduction in complications was “impressive; I hope this will be a message,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University, New Orleans. Importantly, this reduced complication rate occurred in a real-world population that was sicker than any patient group previously studied with the device, he noted as a designated discussant for the report.
But the report’s second designated discussant, Roderick Tung, MD, highlighted some caveats when interpreting the lower complication rate with the leadless device compared with historical controls. He cited the absence of any episodes of pneumothorax among the patients reviewed by Dr. Piccini who received a leadless pacemaker, compared with a 5% rate among the control patients who had received a device with a transvenous lead, a major driver of the overall difference in complication rates. This difference “may not be relevant to operators who use either an axillary extrathoracic vein route for lead placement or a cephalic vein approach,” said Dr. Tung, director of cardiac electrophysiology at the University of Chicago. “There should not be a 5% rate of pneumothorax when implanting a VVI device.” The results reported by Dr. Piccini have the advantages of coming from many patients and from real-world practice, he acknowledged, but interpretation is limited by the lack of a randomized control group and the outsized impact of pneumothorax complications on the safety comparison.
The other major component of the 6-month complication tally was device-related events, which were twice as common in the historical controls who received a transvenous lead at a rate of 3.4%. The sole 6-month event more common among the patients who received a leadless pacemaker was pericarditis, at a rate of 1.3% in the Micra group and 0.5% in the transvenous lead controls, Dr. Piccini reported. The 6-month rate of device revisions was 1.7% with the leadless device and 2.8% with transvenous lead pacemakers, a difference that was not statistically significant. The two treatment arms had virtually identical 6-month mortality rates.
The rate of acute complications during the first 30 days after implant was also virtually the same in the two study arms. Patient who received the leadless device had significantly more puncture-site events, at a rate of 1.2%, and significantly more cardiac effusions or perforations, at a rate of 0.8%. The historical control patients who received devices with transvenous leads had significantly more device-related complications after 30 days, a 2.5% rate.
The 30-day cohorts examined had larger numbers of patients than at 6 months, 5,746 leadless pacemaker recipients and 9,662 matched historical controls who had received a transvenous lead pacemaker. The clinical and demographic profile of the 30-day cohort who received the leadless pacemaker highlighted the sicker nature of these patients compared with earlier studies of the device. They were an average age of 79 years, compared with average ages of 76 years in the two prior Micra studies, and they also had double the prevalence of coronary disease, triple the prevalence of heart failure, more than twice the rate of chronic obstructive pulmonary disease, and almost twice the prevalence of diabetes.
During the period examined in this report from Micra CED (Longitudinal Coverage With Evidence Development Study on Micra Leadless Pacemakers), in 2017-2018, the leadless pacemaker’s initial approved indications were for a circumscribed portion of the overall patient population that needs pacing. Essentially, they were elderly patients with persistent atrial fibrillation who only need ventricular pacing, roughly 15% of the overall cohort of pacing candidates. In January 2020, the FDA added an indication for high-grade atrioventricular block, an expanded population of candidates that roughly tripled the number of potentially appropriate recipients, said Larry A. Chinitz, MD, a cardiac electrophysiologist and a coinvestigator on some of the studies that led to the new indication, in an interview at the time of the revised labeling.
The study was sponsored by Medtronic, which markets the Micra leadless pacemaker. Dr. Piccini has received honoraria from Medtronic and several other companies. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster. Dr. Chinitz has received fees and fellowship support from Medtronic, and has also received fees from Abbott, Biosense Webster, Biotronik, and Pfizer.
SOURCE: Piccini JP et al. Heart Rhythm 2020, Abstract D-LBCT04-01.
A leadless right-ventricular pacemaker continued to show an edge over conventional transvenous pacemakers by triggering a substantially reduced rate of complications during the 6 months following placement in a review of more than 10,000 Medicare patients treated over 2 years.
The “largest leadless pacemaker cohort to date” showed that in propensity score–matched cohorts, the 3,276 patients who received the Micra leadless transcatheter pacemaker during routine management and were followed for 6 months had a 3.3% rate of total complications, compared with a 9.4% rate among 7,256 patients who received a conventional VVI pacemaker with a transvenous lead, a statistically significant 66% relative risk reduction, Jonathan P. Piccini, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
The 66% reduced rate of complications – both acutely and with further follow-up – was similar to the complication reductions seen with Micra, compared with historical controls who received transvenous single-chamber pacemakers in both the pivotal study for the device (Heart Rhythm. 2017 May 1;14[3]:702-9) and in a postapproval registry study (Heart Rhythm. 2018 Dec 1;15[12]:1800-7). However, the newly reported advantage came in a population that was notably older and had significantly more comorbidities than in the prior leadless pacemaker studies, said Dr. Piccini, a cardiac electrophysiologist at Duke University, Durham, N.C.
The new Medicare data “tell us that physicians are reaching for these devices [leadless pacemakers] in patients with more comorbidities and a higher risk for complications to give them a [device with] better safety profile,” he said during a press briefing. “At Duke, and I suspect at other centers, when a patients is eligible for a leadless pacemaker that’s the preferred option.”
However, Dr. Piccini cited three examples of the small proportion of patients who are appropriate for the type of pacing the leadless pacemaker supplies but would be better candidates for a device with a transvenous lead: patients who failed treatment with a initial leadless pacemaker and have no suitable alternative subcutaneous spot to place the replacement device in a stable way, those with severe right ventricular enlargement that interferes with optimal placement, and those who don’t currently meet criteria for biventricular pacing but appear likely to switch to that pacing mode in the near term.
The 66% relative reduction in complications was “impressive; I hope this will be a message,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University, New Orleans. Importantly, this reduced complication rate occurred in a real-world population that was sicker than any patient group previously studied with the device, he noted as a designated discussant for the report.
But the report’s second designated discussant, Roderick Tung, MD, highlighted some caveats when interpreting the lower complication rate with the leadless device compared with historical controls. He cited the absence of any episodes of pneumothorax among the patients reviewed by Dr. Piccini who received a leadless pacemaker, compared with a 5% rate among the control patients who had received a device with a transvenous lead, a major driver of the overall difference in complication rates. This difference “may not be relevant to operators who use either an axillary extrathoracic vein route for lead placement or a cephalic vein approach,” said Dr. Tung, director of cardiac electrophysiology at the University of Chicago. “There should not be a 5% rate of pneumothorax when implanting a VVI device.” The results reported by Dr. Piccini have the advantages of coming from many patients and from real-world practice, he acknowledged, but interpretation is limited by the lack of a randomized control group and the outsized impact of pneumothorax complications on the safety comparison.
The other major component of the 6-month complication tally was device-related events, which were twice as common in the historical controls who received a transvenous lead at a rate of 3.4%. The sole 6-month event more common among the patients who received a leadless pacemaker was pericarditis, at a rate of 1.3% in the Micra group and 0.5% in the transvenous lead controls, Dr. Piccini reported. The 6-month rate of device revisions was 1.7% with the leadless device and 2.8% with transvenous lead pacemakers, a difference that was not statistically significant. The two treatment arms had virtually identical 6-month mortality rates.
The rate of acute complications during the first 30 days after implant was also virtually the same in the two study arms. Patient who received the leadless device had significantly more puncture-site events, at a rate of 1.2%, and significantly more cardiac effusions or perforations, at a rate of 0.8%. The historical control patients who received devices with transvenous leads had significantly more device-related complications after 30 days, a 2.5% rate.
The 30-day cohorts examined had larger numbers of patients than at 6 months, 5,746 leadless pacemaker recipients and 9,662 matched historical controls who had received a transvenous lead pacemaker. The clinical and demographic profile of the 30-day cohort who received the leadless pacemaker highlighted the sicker nature of these patients compared with earlier studies of the device. They were an average age of 79 years, compared with average ages of 76 years in the two prior Micra studies, and they also had double the prevalence of coronary disease, triple the prevalence of heart failure, more than twice the rate of chronic obstructive pulmonary disease, and almost twice the prevalence of diabetes.
During the period examined in this report from Micra CED (Longitudinal Coverage With Evidence Development Study on Micra Leadless Pacemakers), in 2017-2018, the leadless pacemaker’s initial approved indications were for a circumscribed portion of the overall patient population that needs pacing. Essentially, they were elderly patients with persistent atrial fibrillation who only need ventricular pacing, roughly 15% of the overall cohort of pacing candidates. In January 2020, the FDA added an indication for high-grade atrioventricular block, an expanded population of candidates that roughly tripled the number of potentially appropriate recipients, said Larry A. Chinitz, MD, a cardiac electrophysiologist and a coinvestigator on some of the studies that led to the new indication, in an interview at the time of the revised labeling.
The study was sponsored by Medtronic, which markets the Micra leadless pacemaker. Dr. Piccini has received honoraria from Medtronic and several other companies. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster. Dr. Chinitz has received fees and fellowship support from Medtronic, and has also received fees from Abbott, Biosense Webster, Biotronik, and Pfizer.
SOURCE: Piccini JP et al. Heart Rhythm 2020, Abstract D-LBCT04-01.
FROM HEART RHYTHM 2020
COVID-19 in kids: Severe illness most common in infants, teens
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
FROM THE JOURNAL OF PEDIATRICS
Dermatologic changes with COVID-19: What we know and don’t know
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.