User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Blood biomarkers could help predict when athletes recover from concussions
, according to a new study of collegiate athletes and recovery time. “Although preliminary, the current results highlight the potential role of biomarkers in tracking neuronal recovery, which may be associated with duration of [return to sport],” wrote Cassandra L. Pattinson, PhD, of the University of Queensland, Brisbane, Australia, and the National Institutes of Health, Bethesda, Md., along with coauthors. The study was published in JAMA Network Open.
To determine if three specific blood biomarkers – total tau protein, glial fibrillary acidic protein (GFAP), and neurofilament light chain protein (NfL) – can help predict when athletes should return from sports-related concussions, a multicenter, prospective diagnostic study was launched and led by the Advanced Research Core (ARC) of the Concussion Assessment, Research, and Education (CARE) Consortium. The consortium is a joint effort of the National Collegiate Athletics Association (NCAA) and the U.S. Department of Defense.
From among the CARE ARC database, researchers evaluated 127 eligible student athletes who had experienced a sports-related concussion, underwent clinical testing and blood collection before and after their injuries, and returned to their sports. Their average age was 18.9 years old, 76% were men, and 65% were White. Biomarker levels were measured from nonfasting blood samples via ultrasensitive single molecule array technology. As current NCAA guidelines indicate that most athletes will be asymptomatic roughly 2 weeks after a concussion, the study used 14 days as a cutoff period.
Among the 127 athletes, the median return-to-sport time was 14 days; 65 returned to their sports in less than 14 days while 62 returned to their sports in 14 days or more. According to the study’s linear mixed models, athletes with a return-to-sport time of 14 days or longer had significantly higher total tau levels at 24-48 hours post injury (mean difference –0.51 pg/mL, 95% confidence interval, –0.88 to –0.14; P = .008) and when symptoms had resolved (mean difference –0.71 pg/mL, 95% CI, –1.09 to –0.34; P < .001) compared with athletes with a return-to-sport time of less than 14 days. Athletes who returned in 14 days or more also had comparatively lower levels of GFAP postinjury than did those who returned in under 14 days (4.39 pg/mL versus 4.72 pg/mL; P = .04).
Preliminary steps toward an appropriate point-of-care test
“This particular study is one of several emerging studies on what these biomarkers look like,” Brian W. Hainline, MD, chief medical officer of the NCAA, said in an interview. “It’s all still very preliminary – you couldn’t make policy changes based on what we have – but the data is accumulating. Ultimately, we should be able to perform a multivariate analysis of all the different objective biomarkers, looking at repetitive head impact exposure, looking at imaging, looking at these blood-based biomarkers. Then you can say, ‘OK, what can we do? Can we actually predict recovery, who is likely or less likely to do well?’ ”
“It’s not realistic to be taking blood samples all the time,” said Dr. Hainline, who was not involved in the study. “Another goal, once we know which biomarkers are valuable, is to convert to a point-of-care test. You get a finger prick or even a salivary test and we get the result immediately; that’s the direction that all of this is heading. But first, we have to lay out the groundwork. We envision a day, in the not too distant future, where we can get this information much more quickly.”
The authors acknowledged their study’s limitations, including an inability to standardize the time of biomarker collection and the fact that they analyzed a “relatively small number of athletes” who met their specific criteria. That said, they emphasized that their work is based on “the largest prospective sample of sports-related concussions in athletes to date” and that they “anticipate that we will be able to continue to gather a more representative sample” in the future to better generalize to the larger collegiate community.
The study was supported by the Grand Alliance Concussion Assessment, Research, and Education Consortium, which was funded in part by the NCAA and the Department of Defense. The authors disclosed receiving grants and travel reimbursements from – or working as advisers or consultants for – various organizations, college programs, and sports leagues.
SOURCE: Pattinson CL, et al. JAMA Netw Open. 2020 Aug 27. doi: 10.1001/jamanetworkopen.2020.13191.
, according to a new study of collegiate athletes and recovery time. “Although preliminary, the current results highlight the potential role of biomarkers in tracking neuronal recovery, which may be associated with duration of [return to sport],” wrote Cassandra L. Pattinson, PhD, of the University of Queensland, Brisbane, Australia, and the National Institutes of Health, Bethesda, Md., along with coauthors. The study was published in JAMA Network Open.
To determine if three specific blood biomarkers – total tau protein, glial fibrillary acidic protein (GFAP), and neurofilament light chain protein (NfL) – can help predict when athletes should return from sports-related concussions, a multicenter, prospective diagnostic study was launched and led by the Advanced Research Core (ARC) of the Concussion Assessment, Research, and Education (CARE) Consortium. The consortium is a joint effort of the National Collegiate Athletics Association (NCAA) and the U.S. Department of Defense.
From among the CARE ARC database, researchers evaluated 127 eligible student athletes who had experienced a sports-related concussion, underwent clinical testing and blood collection before and after their injuries, and returned to their sports. Their average age was 18.9 years old, 76% were men, and 65% were White. Biomarker levels were measured from nonfasting blood samples via ultrasensitive single molecule array technology. As current NCAA guidelines indicate that most athletes will be asymptomatic roughly 2 weeks after a concussion, the study used 14 days as a cutoff period.
Among the 127 athletes, the median return-to-sport time was 14 days; 65 returned to their sports in less than 14 days while 62 returned to their sports in 14 days or more. According to the study’s linear mixed models, athletes with a return-to-sport time of 14 days or longer had significantly higher total tau levels at 24-48 hours post injury (mean difference –0.51 pg/mL, 95% confidence interval, –0.88 to –0.14; P = .008) and when symptoms had resolved (mean difference –0.71 pg/mL, 95% CI, –1.09 to –0.34; P < .001) compared with athletes with a return-to-sport time of less than 14 days. Athletes who returned in 14 days or more also had comparatively lower levels of GFAP postinjury than did those who returned in under 14 days (4.39 pg/mL versus 4.72 pg/mL; P = .04).
Preliminary steps toward an appropriate point-of-care test
“This particular study is one of several emerging studies on what these biomarkers look like,” Brian W. Hainline, MD, chief medical officer of the NCAA, said in an interview. “It’s all still very preliminary – you couldn’t make policy changes based on what we have – but the data is accumulating. Ultimately, we should be able to perform a multivariate analysis of all the different objective biomarkers, looking at repetitive head impact exposure, looking at imaging, looking at these blood-based biomarkers. Then you can say, ‘OK, what can we do? Can we actually predict recovery, who is likely or less likely to do well?’ ”
“It’s not realistic to be taking blood samples all the time,” said Dr. Hainline, who was not involved in the study. “Another goal, once we know which biomarkers are valuable, is to convert to a point-of-care test. You get a finger prick or even a salivary test and we get the result immediately; that’s the direction that all of this is heading. But first, we have to lay out the groundwork. We envision a day, in the not too distant future, where we can get this information much more quickly.”
The authors acknowledged their study’s limitations, including an inability to standardize the time of biomarker collection and the fact that they analyzed a “relatively small number of athletes” who met their specific criteria. That said, they emphasized that their work is based on “the largest prospective sample of sports-related concussions in athletes to date” and that they “anticipate that we will be able to continue to gather a more representative sample” in the future to better generalize to the larger collegiate community.
The study was supported by the Grand Alliance Concussion Assessment, Research, and Education Consortium, which was funded in part by the NCAA and the Department of Defense. The authors disclosed receiving grants and travel reimbursements from – or working as advisers or consultants for – various organizations, college programs, and sports leagues.
SOURCE: Pattinson CL, et al. JAMA Netw Open. 2020 Aug 27. doi: 10.1001/jamanetworkopen.2020.13191.
, according to a new study of collegiate athletes and recovery time. “Although preliminary, the current results highlight the potential role of biomarkers in tracking neuronal recovery, which may be associated with duration of [return to sport],” wrote Cassandra L. Pattinson, PhD, of the University of Queensland, Brisbane, Australia, and the National Institutes of Health, Bethesda, Md., along with coauthors. The study was published in JAMA Network Open.
To determine if three specific blood biomarkers – total tau protein, glial fibrillary acidic protein (GFAP), and neurofilament light chain protein (NfL) – can help predict when athletes should return from sports-related concussions, a multicenter, prospective diagnostic study was launched and led by the Advanced Research Core (ARC) of the Concussion Assessment, Research, and Education (CARE) Consortium. The consortium is a joint effort of the National Collegiate Athletics Association (NCAA) and the U.S. Department of Defense.
From among the CARE ARC database, researchers evaluated 127 eligible student athletes who had experienced a sports-related concussion, underwent clinical testing and blood collection before and after their injuries, and returned to their sports. Their average age was 18.9 years old, 76% were men, and 65% were White. Biomarker levels were measured from nonfasting blood samples via ultrasensitive single molecule array technology. As current NCAA guidelines indicate that most athletes will be asymptomatic roughly 2 weeks after a concussion, the study used 14 days as a cutoff period.
Among the 127 athletes, the median return-to-sport time was 14 days; 65 returned to their sports in less than 14 days while 62 returned to their sports in 14 days or more. According to the study’s linear mixed models, athletes with a return-to-sport time of 14 days or longer had significantly higher total tau levels at 24-48 hours post injury (mean difference –0.51 pg/mL, 95% confidence interval, –0.88 to –0.14; P = .008) and when symptoms had resolved (mean difference –0.71 pg/mL, 95% CI, –1.09 to –0.34; P < .001) compared with athletes with a return-to-sport time of less than 14 days. Athletes who returned in 14 days or more also had comparatively lower levels of GFAP postinjury than did those who returned in under 14 days (4.39 pg/mL versus 4.72 pg/mL; P = .04).
Preliminary steps toward an appropriate point-of-care test
“This particular study is one of several emerging studies on what these biomarkers look like,” Brian W. Hainline, MD, chief medical officer of the NCAA, said in an interview. “It’s all still very preliminary – you couldn’t make policy changes based on what we have – but the data is accumulating. Ultimately, we should be able to perform a multivariate analysis of all the different objective biomarkers, looking at repetitive head impact exposure, looking at imaging, looking at these blood-based biomarkers. Then you can say, ‘OK, what can we do? Can we actually predict recovery, who is likely or less likely to do well?’ ”
“It’s not realistic to be taking blood samples all the time,” said Dr. Hainline, who was not involved in the study. “Another goal, once we know which biomarkers are valuable, is to convert to a point-of-care test. You get a finger prick or even a salivary test and we get the result immediately; that’s the direction that all of this is heading. But first, we have to lay out the groundwork. We envision a day, in the not too distant future, where we can get this information much more quickly.”
The authors acknowledged their study’s limitations, including an inability to standardize the time of biomarker collection and the fact that they analyzed a “relatively small number of athletes” who met their specific criteria. That said, they emphasized that their work is based on “the largest prospective sample of sports-related concussions in athletes to date” and that they “anticipate that we will be able to continue to gather a more representative sample” in the future to better generalize to the larger collegiate community.
The study was supported by the Grand Alliance Concussion Assessment, Research, and Education Consortium, which was funded in part by the NCAA and the Department of Defense. The authors disclosed receiving grants and travel reimbursements from – or working as advisers or consultants for – various organizations, college programs, and sports leagues.
SOURCE: Pattinson CL, et al. JAMA Netw Open. 2020 Aug 27. doi: 10.1001/jamanetworkopen.2020.13191.
FROM JAMA NETWORK OPEN
Asymptomatic children may transmit COVID-19 in communities
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
FROM JAMA PEDIATRICS
Early evolocumab quickly lowers LDL cholesterol after primary PCI
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
Early administration of evolocumab significantly reduced levels of LDL cholesterol in patients with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention, according to data from an open-label randomized trial of 102 adults in Japan.

Data from previous studies have shown that proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors can reduce LDL cholesterol in acute coronary syndrome patients, wrote Tomoaki Okada, MD, of Kagawa (Japan) Prefectural Central Hospital and colleagues.
In particular, “The EVOPACS trial [J Am Coll Cardiol 2019; 74:2452-62] reported that evolocumab therapy initiated at an early phase of ACS showed [LDL cholesterol] level reduction by 4-8 weeks,” they said.
“However, the 4-week efficacy of PCSK9 inhibitor therapy combined with a statin remains unknown,” they said.
In a study presented at the virtual annual congress of the European Society of Cardiology and published simultaneously in JACC: Cardiovascular Interventions, the researchers randomized 52 patients to receive 140 mg of evolocumab subcutaneously within 24 hours of indexed percutaneous coronary intervention and again after 2 weeks. A group of 50 controls received evolocumab after PCI only, but no additional dose after 2 weeks.
The average age of the patients was 65 years, 88% were men, and 26% had a history of statin treatment.
A total of 49 patients in each group were included in the final analysis, with a primary outcome of change in LDL cholesterol levels from baseline to 4 weeks.
Baseline LCL cholesterol levels were 120.8 mg/dL and 124.7 mg/dL in the evolocumab and control groups, respectively. Changes from baseline were significantly greater in the evolocumab group, compared with controls, at –76% and –33%, respectively.
All patients in the evolocumab group and 27% of patients in the control groups achieved LDL cholesterol levels of less than 70 mg/dL at 4 weeks. In addition, 92% and 96% of evolocumab patients achieved LDL cholesterol levels less than 55 mg/dL at 2 weeks and 4 weeks, respectively.
Overall changes in non-HDL cholesterol, HDL cholesterol, and small dense LDL in the evolocumab and control groups were –66.2% and –26.0%; 2.8% and –0.7%; and –67% and –13.8%, respectively. Of these, changes in non-HDL cholesterol and small dense LDL were significantly different between the groups.
In addition, patients in the evolocumab group showed a 3% decrease in lipoprotein, compared with an 82% increase in the control group. This finding suggests the additional benefit of including evolocumab for managing residual risk in patients with high lipoprotein(a) levels” after acute MI, the researchers noted.
Adverse events and serious adverse events were similar between the groups.
‘Early and strong’ LDL cholesterol lowering best for preventing repeat events
“By using the PCSK9 inhibitors, we have the opportunity to lower LDL cholesterol [LDL-C]” both quickly and dramatically, said Heinz Drexel, MD, in an interview.
“This Japanese study shows that very low LDL-C levels can be obtained as fast as within 4 weeks,” he said. “This fits into the concept that risk for future infarctions and strokes is best reduced by early and strong LDL-C lowering,” he explained.
Dr. Drexel said that he was not surprised by the magnitude of the decrease in LDL cholesterol in study findings in light of the EVOPACS study and other research, as well as his own clinical experience.
“The primary message for doctors is that it is now possible to achieve these low levels of LDL-C in a short time,” he said.
“Additional research must prove that this low LDL-C translates to reduction of MIs and strokes, and there is increasing evidence that this will happen,” Dr. Drexel noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Drexel had no financial conflicts to disclose.
SOURCE: Okada T et al. ESC 2020. JACC Cardiovascular Interventions. 2020 Aug 28. doi: 10.1016/j.jcin.2020.08.026.
FROM ESC CONGRESS 2020
LoDoCo2: Added steam for colchicine as secondary prevention
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.
The anti-inflammatory drug colchicine picked up new support as secondary prevention in chronic coronary disease, cutting the risk of cardiovascular events by one-third when added to standard prevention therapies in the double-blind LoDoCo2 study.
Across a median follow up of 29 months in more than 5,000 patients, almost 1 in 10 patients assigned to placebo experienced the primary endpoint of cardiovascular death, myocardial infarction (MI), ischemic stroke, or ischemia-driven coronary revascularization. That risk was 31% lower and resulted in 77 fewer events in those assigned to colchicine (hazard ratio, 0.69; 95% confidence interval, 0.57-0.83).
The beneficial effect of low-dose colchicine 0.5 mg daily was seen early on and accrued over time, extending to five of the eight secondary end points, including a near 30% reduction in the composite of major adverse cardiac events, as well as reductions in the individual endpoints of MI and ischemia-driven revascularization.
“It did that with broadly consistent effects across a range of clinical subgroups, which together speak to the strength of the effect of colchicine on cardiovascular outcomes in the sort of patients we routinely see in our clinics,” primary investigator Mark Nidorf, MD, MBBS, GenesisCare Western Australia, Perth, said at the virtual annual congress of the European Society of Cardiology.
The results were published simultaneously in the New England Journal of Medicine (2020 Aug 31. doi: 10.1056/NEJMoa2021372).
“The totality of evidence from the big three double-blind placebo controlled trials – CANTOS, COLCOT, and LoDoCo2 – are highly consistent and should be practice changing,” Paul Ridker, MD, MPH, director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital in Boston, said in an interview.
Massimo Imazio, MD, the formal discussant for the study and professor of cardiology at the University of Turin, Italy, also called for repurposing the inexpensive gout medication for cardiovascular patients.
“I would like to congratulate the authors for a well-designed, large, randomized trial that in my view provides convincing evidence that colchicine is safe and efficacious for secondary prevention in chronic coronary syndrome, of course if tolerated,” he said.
Dr. Imazio noted that colchicine demonstrated similar benefits in the smaller, open-label LoDoCo trial, but that 1 in 10 patients couldn’t tolerate the drug, largely because of gastrointestinal issues. The LoCoDo2 investigators very wisely opted for a 30-day run-in period for tolerance without a loading dose, and 90% of patients in each arm continued study medication while 3.4% stopped because of perceived effects.
Clinicians should bear in mind the potential for side effects and interactions with other medications, particularly statins, observed Dr. Imazio. “So monitoring of repeat blood tests is indicated, especially blood cell count, transaminase, and [creatine kinase] CK.”
Colchicine can be problematic in patients with chronic kidney disease because it is renally excreted, particularly if patients also take some common antibiotics such as clarithromycin, said Dr. Ridker, who led the landmark CANTOS trial. “So while these data are exciting and confirm the importance of inflammation inhibition in stable coronary disease, colchicine is not for all patients.”
During the discussion of the results, Dr. Nidorf said: “We were very concerned at the outset that there would be an interaction because there is certainly literature there, particularly in renal patients. But as the data showed, the incidence of myotoxicity was decidedly rare.”
Further, myotoxic episodes were independently assessed by a blinded reviewer, and although there was one case of mild rhabdomyolysis in the treatment group, it was considered not primarily caused by colchicine, he said. “So we’re fairly comfortable that you can use colchicine at a low dose quite comfortably with full-dose statins.”
Notably, 94% of patients in both groups were taking statins, and two-thirds were on moderate- or high-dose statins. About one-quarter were on dual-antiplatelet therapy, and 12% were on an anticoagulant.
In all, 5,522 patients aged 35-82 years (mean, 66 years) were randomly assigned to colchicine 0.5 mg once daily or placebo on top of proven secondary prevention therapies, and all but one was available for analysis.
Most were male (85%), one-half had hypertension, 18% had diabetes, and 84% had a history of acute coronary syndrome, with an equal number having undergone revascularization. Patients with advanced renal disease, severe heart failure, or severe valvular heart disease were excluded.
Colchicine, when compared with placebo, was associated with significantly lower incidence rates of the top five ranked secondary endpoints:
- Cardiovascular death, MI, or ischemic stroke (4.2% vs. 5.7%; HR, 0.72).
- MI or ischemia-driven revascularization (5.6% vs. 8.1%; HR, 0.67).
- Cardiovascular death or MI (3.6% vs. 5.0%; HR, 0.71).
- Ischemia-driven revascularization (4.9% vs. 6.4%; HR, 0.75).
- MI (3.0% vs. 4.2%; HR, 0.70).
The incidence rates were similar among the remaining three secondary outcomes: ischemic stroke (0.6% vs. 0.9%), all-cause death (2.6% vs. 2.2%), and CV death (0.7% vs. 0.9%), Dr. Nidorf reported.
The effect of colchicine was consistent in 13 subgroups, including those with and without hypertension, diabetes, or prior acute coronary syndrome. Patients in Australia appeared to do better with colchicine than did those in the Netherlands, which was a bit unexpected but likely caused by the play of chance, Dr. Nidorf said.
“Importantly, the effect when we looked at the predictors of outcome of our patients in this trial, they related to factors such as age and diabetes, which were included in both populations. So we believe the effect of therapy to be universal,” he added.
Session moderator Stephan Achenbach, MD, chair of cardiology at the University of Erlangen (Germany), however, noted that event rates were about 3% per year and many patients had undergone coronary revascularizations for acute coronary syndromes, suggesting this may be a preselected, somewhat higher-risk cohort. “Do you think we can transfer these findings to the just-average patient who comes in with chest pain and gets an elective [percutaneous coronary intervention]?” he asked.
Dr. Nidorf replied that, unlike the patients in COLCOT, who were randomized to colchicine within 30 days of an MI, acute events occurred more than 24 months before randomization in most (68.2%) patients. As such, patients were quite stable, and major adverse cardiac event and cardiovascular death rates were also exceedingly low.
“We did not see them as a particularly high-risk group, which I think is one of the beauties of this study,” Dr. Nidorf said. “It looks at people that are very similar to those who come and meet us in our clinics for regular review and follow-up.”
“And in that regard, I think the next time we’re faced with patients in our rooms, we have to ask the question: Are we doing enough for this patient beyond aspirin and statins? Should we be considering treating the inflammatory axis? And now we have an opportunity to do that,” he said.
Serious adverse effects were similar in the colchicine and placebo groups, including hospitalizations for infection (5.0% vs. 5.2%), pneumonia (1.7% vs. 2.0%), or gastrointestinal reasons (1.9% vs. 1.8%). Myotoxicity occurred in four and three patients, respectively.
Although the signal for increased risk of infection observed in CANTOS and COLCOT was not borne out, Dr. Nidorf observed that chest infections can occur frequently in these patients and echoed cautions about a potential unfavorable interaction between clarithromycin and colchicine.
“If we are to use this drug widely, clinicians will need to learn how to use this drug and what drugs to avoid, and that’s an important teaching point,” he said.
Limitations of the study are the small number of women and lack of routine measurement of C-reactive protein or other inflammatory markers at baseline.
The study was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands. The authors’ disclosures are listed in the article.
A version of this article originally appeared on Medscape.com.
SSRIs risky after intracerebral hemorrhage
SSRIs effectively treat depression following intracerebral hemorrhage (ICH) but also increase risk for recurrent hemorrhagic stroke, particularly in patients at high risk for repeat ICH, new research indicates.
“Clinicians must exercise judgment when weighing the use of SSRIs for ICH survivors in the high risk category – especially those with multiple ICH events,” study investigator Alessandro Biffi, MD, director, Aging and Brain Health Research (ABHR) Group, Departments of Neurology and Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, told Medscape Medical News.
The study was published online August 31 in JAMA Neurology.
Risks and benefits
Depression is common following stroke. SSRIs are generally considered first-line treatment for post-stroke depression but are associated with increased risk for first ICH, most likely owing to their antithrombotic effects. Less is known about SSRI use and recurrent ICH risk.
To investigate, Biffi and colleagues followed 1,279 adults (mean age, 71.3 years) for a median of 53.2 months (4.5 years) following primary ICH; 602 were women, 1049 were White, 89 Black, 77 Hispanic, and 64 were other race/ethnicity.
During follow-up, 128 adults suffered recurrent ICH (annual rate, 4.2%) and 766 (60%) were diagnosed with depression.
(subhazard ratio, 1.53; 95% CI, 1.12-2.09; P = .009).
However, SSRI use was also an independent risk factor for recurrent ICH (SHR, 1.31; 95% CI, 1.08-1.59; P = .006).
High SSRI dose was associated with higher ICH recurrence risk (SHR, 1.61; 95% CI, 1.15-2.25), with a larger effect size (comparison P = .02) than low SSRI dose (SHR, 1.25; 95% CI, 1.01-1.55), but there was no difference in depression remission comparing low vs. high SSRI dose.
Among individuals at high risk for recurrent ICH, SSRI use was associated with further increased risk for ICH recurrence (SHR, 1.79; 95% CI, 1.22 - 2.64) compared with all other survivors of ICH (SHR, 1.20; 95% CI, 1.01-1.42; P = .008 for comparison of effect sizes).
These higher-risk subgroups included carriers of the APOE e2/e4 alleles, patients with lobar ICH, patients with prior ICH, and minority participants.
“Our analyses identified patients for whom the risks are higher, and therefore additional thought is warranted. This approach may in the future lead to personalized/precision medicine approaches to determining whether these patients should receive SSRIs or not,” said Biffi.
Experts weigh in
Commenting on the research for Medscape Medical News, Daniel G. Hackam, MD, division of clinical pharmacology, Western University, London, Ont., said the study is “an important contribution to the literature, as there are to date no data on the risk of ICH in prior ICH survivors in relation to SSRI exposure.”
“The bottom line is that I would be very cautious about initiating SSRIs in patients with a history of ICH,” said Hackam, who was not involved with the study.
“There are other nonserotonergic antidepressants that could be used instead, which do not inhibit platelet function. There was still a risk even in the lower-risk ICH survivors. ICH is a highly recurrent disease. We already avoid antiplatelets, anticoagulants, and high dose statins in these patients. I would add SSRI’s to that list, based on this study,” said Hackam.
Also weighing in, Amytis Towfighi, MD, associate professor of neurology, University of Southern California, Los Angeles, said this study addresses a “common clinical dilemma: how to manage depression among individuals with ICH, given the high risk of recurrent ICH among ICH survivors and potential for SSRIs to increase that risk. This scenario is common, and a source of debate for practicing clinicians.”
“The authors conducted an elegant study,” said Towfighi, by considering sociodemographic, historical, imaging, and genetic factors.
“One must interpret this study with caution as it is a single-center cohort study. However, it provides the most rigorous information to date regarding the associations between SSRI use and recurrent ICH,” she told Medscape Medical News.
The study was supported by the National Institutes of Health. Biffi, Hackam, and Towfighi have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SSRIs effectively treat depression following intracerebral hemorrhage (ICH) but also increase risk for recurrent hemorrhagic stroke, particularly in patients at high risk for repeat ICH, new research indicates.
“Clinicians must exercise judgment when weighing the use of SSRIs for ICH survivors in the high risk category – especially those with multiple ICH events,” study investigator Alessandro Biffi, MD, director, Aging and Brain Health Research (ABHR) Group, Departments of Neurology and Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, told Medscape Medical News.
The study was published online August 31 in JAMA Neurology.
Risks and benefits
Depression is common following stroke. SSRIs are generally considered first-line treatment for post-stroke depression but are associated with increased risk for first ICH, most likely owing to their antithrombotic effects. Less is known about SSRI use and recurrent ICH risk.
To investigate, Biffi and colleagues followed 1,279 adults (mean age, 71.3 years) for a median of 53.2 months (4.5 years) following primary ICH; 602 were women, 1049 were White, 89 Black, 77 Hispanic, and 64 were other race/ethnicity.
During follow-up, 128 adults suffered recurrent ICH (annual rate, 4.2%) and 766 (60%) were diagnosed with depression.
(subhazard ratio, 1.53; 95% CI, 1.12-2.09; P = .009).
However, SSRI use was also an independent risk factor for recurrent ICH (SHR, 1.31; 95% CI, 1.08-1.59; P = .006).
High SSRI dose was associated with higher ICH recurrence risk (SHR, 1.61; 95% CI, 1.15-2.25), with a larger effect size (comparison P = .02) than low SSRI dose (SHR, 1.25; 95% CI, 1.01-1.55), but there was no difference in depression remission comparing low vs. high SSRI dose.
Among individuals at high risk for recurrent ICH, SSRI use was associated with further increased risk for ICH recurrence (SHR, 1.79; 95% CI, 1.22 - 2.64) compared with all other survivors of ICH (SHR, 1.20; 95% CI, 1.01-1.42; P = .008 for comparison of effect sizes).
These higher-risk subgroups included carriers of the APOE e2/e4 alleles, patients with lobar ICH, patients with prior ICH, and minority participants.
“Our analyses identified patients for whom the risks are higher, and therefore additional thought is warranted. This approach may in the future lead to personalized/precision medicine approaches to determining whether these patients should receive SSRIs or not,” said Biffi.
Experts weigh in
Commenting on the research for Medscape Medical News, Daniel G. Hackam, MD, division of clinical pharmacology, Western University, London, Ont., said the study is “an important contribution to the literature, as there are to date no data on the risk of ICH in prior ICH survivors in relation to SSRI exposure.”
“The bottom line is that I would be very cautious about initiating SSRIs in patients with a history of ICH,” said Hackam, who was not involved with the study.
“There are other nonserotonergic antidepressants that could be used instead, which do not inhibit platelet function. There was still a risk even in the lower-risk ICH survivors. ICH is a highly recurrent disease. We already avoid antiplatelets, anticoagulants, and high dose statins in these patients. I would add SSRI’s to that list, based on this study,” said Hackam.
Also weighing in, Amytis Towfighi, MD, associate professor of neurology, University of Southern California, Los Angeles, said this study addresses a “common clinical dilemma: how to manage depression among individuals with ICH, given the high risk of recurrent ICH among ICH survivors and potential for SSRIs to increase that risk. This scenario is common, and a source of debate for practicing clinicians.”
“The authors conducted an elegant study,” said Towfighi, by considering sociodemographic, historical, imaging, and genetic factors.
“One must interpret this study with caution as it is a single-center cohort study. However, it provides the most rigorous information to date regarding the associations between SSRI use and recurrent ICH,” she told Medscape Medical News.
The study was supported by the National Institutes of Health. Biffi, Hackam, and Towfighi have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SSRIs effectively treat depression following intracerebral hemorrhage (ICH) but also increase risk for recurrent hemorrhagic stroke, particularly in patients at high risk for repeat ICH, new research indicates.
“Clinicians must exercise judgment when weighing the use of SSRIs for ICH survivors in the high risk category – especially those with multiple ICH events,” study investigator Alessandro Biffi, MD, director, Aging and Brain Health Research (ABHR) Group, Departments of Neurology and Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, told Medscape Medical News.
The study was published online August 31 in JAMA Neurology.
Risks and benefits
Depression is common following stroke. SSRIs are generally considered first-line treatment for post-stroke depression but are associated with increased risk for first ICH, most likely owing to their antithrombotic effects. Less is known about SSRI use and recurrent ICH risk.
To investigate, Biffi and colleagues followed 1,279 adults (mean age, 71.3 years) for a median of 53.2 months (4.5 years) following primary ICH; 602 were women, 1049 were White, 89 Black, 77 Hispanic, and 64 were other race/ethnicity.
During follow-up, 128 adults suffered recurrent ICH (annual rate, 4.2%) and 766 (60%) were diagnosed with depression.
(subhazard ratio, 1.53; 95% CI, 1.12-2.09; P = .009).
However, SSRI use was also an independent risk factor for recurrent ICH (SHR, 1.31; 95% CI, 1.08-1.59; P = .006).
High SSRI dose was associated with higher ICH recurrence risk (SHR, 1.61; 95% CI, 1.15-2.25), with a larger effect size (comparison P = .02) than low SSRI dose (SHR, 1.25; 95% CI, 1.01-1.55), but there was no difference in depression remission comparing low vs. high SSRI dose.
Among individuals at high risk for recurrent ICH, SSRI use was associated with further increased risk for ICH recurrence (SHR, 1.79; 95% CI, 1.22 - 2.64) compared with all other survivors of ICH (SHR, 1.20; 95% CI, 1.01-1.42; P = .008 for comparison of effect sizes).
These higher-risk subgroups included carriers of the APOE e2/e4 alleles, patients with lobar ICH, patients with prior ICH, and minority participants.
“Our analyses identified patients for whom the risks are higher, and therefore additional thought is warranted. This approach may in the future lead to personalized/precision medicine approaches to determining whether these patients should receive SSRIs or not,” said Biffi.
Experts weigh in
Commenting on the research for Medscape Medical News, Daniel G. Hackam, MD, division of clinical pharmacology, Western University, London, Ont., said the study is “an important contribution to the literature, as there are to date no data on the risk of ICH in prior ICH survivors in relation to SSRI exposure.”
“The bottom line is that I would be very cautious about initiating SSRIs in patients with a history of ICH,” said Hackam, who was not involved with the study.
“There are other nonserotonergic antidepressants that could be used instead, which do not inhibit platelet function. There was still a risk even in the lower-risk ICH survivors. ICH is a highly recurrent disease. We already avoid antiplatelets, anticoagulants, and high dose statins in these patients. I would add SSRI’s to that list, based on this study,” said Hackam.
Also weighing in, Amytis Towfighi, MD, associate professor of neurology, University of Southern California, Los Angeles, said this study addresses a “common clinical dilemma: how to manage depression among individuals with ICH, given the high risk of recurrent ICH among ICH survivors and potential for SSRIs to increase that risk. This scenario is common, and a source of debate for practicing clinicians.”
“The authors conducted an elegant study,” said Towfighi, by considering sociodemographic, historical, imaging, and genetic factors.
“One must interpret this study with caution as it is a single-center cohort study. However, it provides the most rigorous information to date regarding the associations between SSRI use and recurrent ICH,” she told Medscape Medical News.
The study was supported by the National Institutes of Health. Biffi, Hackam, and Towfighi have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Statins linked to reduced mortality in COVID-19
Treatment with statins was associated with a reduced risk of a severe or fatal course of COVID-19 by 30%, a meta-analysis of four published studies has shown.
In the analysis that included almost 9,000 COVID-19 patients, there was a significantly reduced risk for fatal or severe COVID-19 among patients who were users of statins, compared with nonusers (pooled hazard ratio, 0.70; 95% confidence interval, 0.53-0.94).
Based on the findings, “it may be time we shift our focus to statins as the potential therapeutic options in COVID-19 patients,” authors Syed Shahzad Hasan, PhD, University of Huddersfield (England), and Chia Siang Kow, MPharm, International Medical University, Kuala Lumpur, Malaysia, said in an interview.
The study was published online August 11 in The American Journal of Cardiology.
Moderate- to good-quality data
The analysis included four studies published up to July 27 of this year. Eligible studies included those with a cohort or case-control designs, enrolled patients with confirmed COVID-19, and had data available allowing comparison of the risk of severe illness and/or mortality among statin users versus nonusers in adjusted analyses, the authors noted.
The four studies – one of “moderate” quality and three of “good” quality – included a total of 8,990 COVID-19 patients.
In the pooled analysis, there was a significantly reduced risk for fatal or severe COVID-19 with use of statins, compared with non-use of statins (pooled HR, 0.70; 95% CI, 0.53-0.94).
Their findings also “discredited the suggestion of harms with the use of statins in COVID-19 patients,” the authors concluded.
“Since our meta-analysis included a fairly large total number of COVID-19 patients from four studies in which three are large-scale studies that adjusted extensively for multiple potential confounding factors, the findings can be considered reliable,” Dr. Hasan and Mr. Kow wrote in their article.
Based on the results, “moderate- to high-intensity statin therapy is likely to be beneficial” in patients with COVID-19, they said.
However, they cautioned that more data from prospective studies are needed to substantiate the findings and to determine the appropriate regimen for a statin in COVID-19 patients.
Yibin Wang, PhD, of the University of California, Los Angeles, said that “this is a very simple meta-analysis from four published studies which consistently reported a protective or neutral effect of statin usage on mortality or severe complications in COVID-19 patients.”
Although the scope of this meta-analysis was “quite limited, the conclusion was not unexpected, as most of the clinical analysis so far reported supports the benefits or safety of statin usage in COVID-19 patients,” Dr. Wang said in an interview.
Nonetheless, questions remain
While there is “almost no dispute” about the safety of continuing statin therapy in COVID-19 patients, it remains to be determined if statin therapy can be implemented as an adjuvant or independent therapy and a part of the standard care for COVID-19 patients regardless of their hyperlipidemia status, said Dr. Wang, who was not associated with Dr. Hasan’s and Mr. Kow’s research.
“While statin usage is associated with several beneficial effects such as anti-inflammation and cytoprotection, these effects are usually observed from long-term usage rather than short-term/acute administration. Therefore, prospective studies and randomized trials should be conducted to test the efficacy of stain usage for COVID-19 patients with mild to severe symptoms,” he noted.
“Considering the excellent record of statins as a safe and cheap drug, it is certainly a worthwhile effort to consider its broad-based usage for COVID-19 in order to lower the overall death and severe complications,” Dr. Wang concluded.
Guillermo Rodriguez-Nava, MD, department of internal medicine, AMITA Health Saint Francis Hospital, Evanston, Ill., is first author on one of the studies included in this meta-analysis.
The retrospective, single-center study found slower progression to death associated with atorvastatin in older patients with COVID-19 admitted to the ICU.
“Currently, there are hundreds of clinical trials evaluating a wide variety of pharmacological therapies for COVID-19. Unfortunately, these trials take time, and we are getting results in dribs and drabs,” Dr. Rodriguez-Nava said in an interview.
“In the meantime, the best available evidence is observational, and COVID-19 treatment regiments will continue to evolve. Whether atorvastatin is effective against COVID-19 is still under investigation. Nevertheless, clinicians should consider at least continuing them in patients with COVID-19,” he advised.
The study had no specific funding. Dr. Hasan, Mr. Kow, Dr. Wang, and Dr. Rodriguez-Nava disclosed no relationships relevant to this research.
A version of this article originally appeared on Medscape.com.
Treatment with statins was associated with a reduced risk of a severe or fatal course of COVID-19 by 30%, a meta-analysis of four published studies has shown.
In the analysis that included almost 9,000 COVID-19 patients, there was a significantly reduced risk for fatal or severe COVID-19 among patients who were users of statins, compared with nonusers (pooled hazard ratio, 0.70; 95% confidence interval, 0.53-0.94).
Based on the findings, “it may be time we shift our focus to statins as the potential therapeutic options in COVID-19 patients,” authors Syed Shahzad Hasan, PhD, University of Huddersfield (England), and Chia Siang Kow, MPharm, International Medical University, Kuala Lumpur, Malaysia, said in an interview.
The study was published online August 11 in The American Journal of Cardiology.
Moderate- to good-quality data
The analysis included four studies published up to July 27 of this year. Eligible studies included those with a cohort or case-control designs, enrolled patients with confirmed COVID-19, and had data available allowing comparison of the risk of severe illness and/or mortality among statin users versus nonusers in adjusted analyses, the authors noted.
The four studies – one of “moderate” quality and three of “good” quality – included a total of 8,990 COVID-19 patients.
In the pooled analysis, there was a significantly reduced risk for fatal or severe COVID-19 with use of statins, compared with non-use of statins (pooled HR, 0.70; 95% CI, 0.53-0.94).
Their findings also “discredited the suggestion of harms with the use of statins in COVID-19 patients,” the authors concluded.
“Since our meta-analysis included a fairly large total number of COVID-19 patients from four studies in which three are large-scale studies that adjusted extensively for multiple potential confounding factors, the findings can be considered reliable,” Dr. Hasan and Mr. Kow wrote in their article.
Based on the results, “moderate- to high-intensity statin therapy is likely to be beneficial” in patients with COVID-19, they said.
However, they cautioned that more data from prospective studies are needed to substantiate the findings and to determine the appropriate regimen for a statin in COVID-19 patients.
Yibin Wang, PhD, of the University of California, Los Angeles, said that “this is a very simple meta-analysis from four published studies which consistently reported a protective or neutral effect of statin usage on mortality or severe complications in COVID-19 patients.”
Although the scope of this meta-analysis was “quite limited, the conclusion was not unexpected, as most of the clinical analysis so far reported supports the benefits or safety of statin usage in COVID-19 patients,” Dr. Wang said in an interview.
Nonetheless, questions remain
While there is “almost no dispute” about the safety of continuing statin therapy in COVID-19 patients, it remains to be determined if statin therapy can be implemented as an adjuvant or independent therapy and a part of the standard care for COVID-19 patients regardless of their hyperlipidemia status, said Dr. Wang, who was not associated with Dr. Hasan’s and Mr. Kow’s research.
“While statin usage is associated with several beneficial effects such as anti-inflammation and cytoprotection, these effects are usually observed from long-term usage rather than short-term/acute administration. Therefore, prospective studies and randomized trials should be conducted to test the efficacy of stain usage for COVID-19 patients with mild to severe symptoms,” he noted.
“Considering the excellent record of statins as a safe and cheap drug, it is certainly a worthwhile effort to consider its broad-based usage for COVID-19 in order to lower the overall death and severe complications,” Dr. Wang concluded.
Guillermo Rodriguez-Nava, MD, department of internal medicine, AMITA Health Saint Francis Hospital, Evanston, Ill., is first author on one of the studies included in this meta-analysis.
The retrospective, single-center study found slower progression to death associated with atorvastatin in older patients with COVID-19 admitted to the ICU.
“Currently, there are hundreds of clinical trials evaluating a wide variety of pharmacological therapies for COVID-19. Unfortunately, these trials take time, and we are getting results in dribs and drabs,” Dr. Rodriguez-Nava said in an interview.
“In the meantime, the best available evidence is observational, and COVID-19 treatment regiments will continue to evolve. Whether atorvastatin is effective against COVID-19 is still under investigation. Nevertheless, clinicians should consider at least continuing them in patients with COVID-19,” he advised.
The study had no specific funding. Dr. Hasan, Mr. Kow, Dr. Wang, and Dr. Rodriguez-Nava disclosed no relationships relevant to this research.
A version of this article originally appeared on Medscape.com.
Treatment with statins was associated with a reduced risk of a severe or fatal course of COVID-19 by 30%, a meta-analysis of four published studies has shown.
In the analysis that included almost 9,000 COVID-19 patients, there was a significantly reduced risk for fatal or severe COVID-19 among patients who were users of statins, compared with nonusers (pooled hazard ratio, 0.70; 95% confidence interval, 0.53-0.94).
Based on the findings, “it may be time we shift our focus to statins as the potential therapeutic options in COVID-19 patients,” authors Syed Shahzad Hasan, PhD, University of Huddersfield (England), and Chia Siang Kow, MPharm, International Medical University, Kuala Lumpur, Malaysia, said in an interview.
The study was published online August 11 in The American Journal of Cardiology.
Moderate- to good-quality data
The analysis included four studies published up to July 27 of this year. Eligible studies included those with a cohort or case-control designs, enrolled patients with confirmed COVID-19, and had data available allowing comparison of the risk of severe illness and/or mortality among statin users versus nonusers in adjusted analyses, the authors noted.
The four studies – one of “moderate” quality and three of “good” quality – included a total of 8,990 COVID-19 patients.
In the pooled analysis, there was a significantly reduced risk for fatal or severe COVID-19 with use of statins, compared with non-use of statins (pooled HR, 0.70; 95% CI, 0.53-0.94).
Their findings also “discredited the suggestion of harms with the use of statins in COVID-19 patients,” the authors concluded.
“Since our meta-analysis included a fairly large total number of COVID-19 patients from four studies in which three are large-scale studies that adjusted extensively for multiple potential confounding factors, the findings can be considered reliable,” Dr. Hasan and Mr. Kow wrote in their article.
Based on the results, “moderate- to high-intensity statin therapy is likely to be beneficial” in patients with COVID-19, they said.
However, they cautioned that more data from prospective studies are needed to substantiate the findings and to determine the appropriate regimen for a statin in COVID-19 patients.
Yibin Wang, PhD, of the University of California, Los Angeles, said that “this is a very simple meta-analysis from four published studies which consistently reported a protective or neutral effect of statin usage on mortality or severe complications in COVID-19 patients.”
Although the scope of this meta-analysis was “quite limited, the conclusion was not unexpected, as most of the clinical analysis so far reported supports the benefits or safety of statin usage in COVID-19 patients,” Dr. Wang said in an interview.
Nonetheless, questions remain
While there is “almost no dispute” about the safety of continuing statin therapy in COVID-19 patients, it remains to be determined if statin therapy can be implemented as an adjuvant or independent therapy and a part of the standard care for COVID-19 patients regardless of their hyperlipidemia status, said Dr. Wang, who was not associated with Dr. Hasan’s and Mr. Kow’s research.
“While statin usage is associated with several beneficial effects such as anti-inflammation and cytoprotection, these effects are usually observed from long-term usage rather than short-term/acute administration. Therefore, prospective studies and randomized trials should be conducted to test the efficacy of stain usage for COVID-19 patients with mild to severe symptoms,” he noted.
“Considering the excellent record of statins as a safe and cheap drug, it is certainly a worthwhile effort to consider its broad-based usage for COVID-19 in order to lower the overall death and severe complications,” Dr. Wang concluded.
Guillermo Rodriguez-Nava, MD, department of internal medicine, AMITA Health Saint Francis Hospital, Evanston, Ill., is first author on one of the studies included in this meta-analysis.
The retrospective, single-center study found slower progression to death associated with atorvastatin in older patients with COVID-19 admitted to the ICU.
“Currently, there are hundreds of clinical trials evaluating a wide variety of pharmacological therapies for COVID-19. Unfortunately, these trials take time, and we are getting results in dribs and drabs,” Dr. Rodriguez-Nava said in an interview.
“In the meantime, the best available evidence is observational, and COVID-19 treatment regiments will continue to evolve. Whether atorvastatin is effective against COVID-19 is still under investigation. Nevertheless, clinicians should consider at least continuing them in patients with COVID-19,” he advised.
The study had no specific funding. Dr. Hasan, Mr. Kow, Dr. Wang, and Dr. Rodriguez-Nava disclosed no relationships relevant to this research.
A version of this article originally appeared on Medscape.com.
Who’s better off: Employed or self-employed physicians?
Self-employed physicians have the highest salaries, largest homes, and greatest wealth – yet they feel the least fairly compensated, according to an analysis of data from over 17,000 physicians.
A new examination of survey responses from the Medscape Physician Compensation Report 2020, which included information about income, job satisfaction, and more, compared responses from self-employed physicians, independent contractors, and employed physicians.
Income and wealth, benefits, and job satisfaction were compared. From the results of the questionnaire, self-employed physicians stand out among their peers across all categories: They enjoy greater income, wealth, and benefits and appear to be more satisfied by their choice of practice.
“The survey confirms that self-employed is the most satisfying, although the trend in health care is to take employed positions,” said Robert Scroggins, JD, CPA, certified health care business consultant with ScrogginsGreer, Cincinnati. “Doctors who become employees primarily do that to escape the management responsibilities for the practice. It seems to be more a decision to get away from something than to go toward something.”
The financial and work picture for self-employed physicians
Self-employed physicians reported the largest salaries for 2019 (average, $360,752), followed by independent contractors ($336,005). Employees reported the lowest average salary ($297,332).
The largest percentage of self-employed physicians (46%) work in an office-based group practice, followed by those in office-based solo practices (30%). Almost two-thirds of self-employed respondents are owners and 37% are partners.
Self-employed physicians are more likely to be older than 45 years; 79% fall into that age bracket, compared with 57% of employees and 70% of independent contractors.
Self-employed physicians reported the highest levels of wealth among their peers. About 44% of self-employed respondents declared a net wealth of over $2 million, compared with 25% of employees. Only 6% of contractors and employed physicians reported a net wealth of over $5 million, compared with 13% of self-employed physicians.
Self-employed physicians also managed their personal expenses slightly differently. They were more likely to pool their income with their spouse in a common account used for bills and expenses, regardless of how much they each earned (63% of self-employed respondents, compared with 58% of employees and 50% of independent contractors).
Perhaps unsurprisingly, self-employed physicians also reported having the largest homes, with an average square footage of 3,629 square feet, compared with 3,023 square feet for employees and 2,984 square feet for independent contractors. Self-employed physicians’ mortgages (average, $240,389) were similar to those of employed physicians’ mortgages but were higher than those of independent contractors’ mortgages (average, $213,740).
Self-employed physicians were also most likely to highly appraise their own performance: Half of all self-employed respondents felt “very satisfied” with their job performance, compared with 40% of employees and 44% of independent contractors.
When asked what they consider to be the most rewarding aspect of their job, self-employed physicians were more likely to choose gratitude and patient relationships than their peers (32%, compared with 26% of employees and 19% of independent contractors).
Despite their higher net wealth and larger salaries, self-employed physicians were least likely to feel fairly compensated; 49% of self-employed physicians said they did not feel fairly compensated for their work, compared with 40% of employees and 40% of independent contractors.
“Self-employed physicians may be better compensated than others of the same specialty who are employees, so some of that may be perception,” said Mr. Scroggins. “Or they feel they should be compensated to a far greater degree than those who are employed.”
Self-employed physicians were also more likely to respond that they would choose the same practice setting again, though across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
The financial and work picture for employed physicians
About a third (32%) of employed physician respondents work in hospitals; 28% work in private practices.
Employed physicians were most likely to report a salary increase from 2018 to 2019: 74%, compared with 45% of self-employed and 52% of independent contractors.
As for declines in income, self-employed physicians and independent contractors suffered a comparable loss, with 13% and 12% of them, respectively, reporting salary cuts greater than 10%. Decreases of up to 10% were felt mostly by the self-employed, with 17% experiencing such cuts, compared with 7% of employees and 10% of independent contractors.
In contrast, employees were the least likely of the three categories to have incurred large financial losses over the past year: 77% of employed respondents indicated that they had not experienced any significant financial losses in the past year, compared with 63% of self-employed physicians and 63% of independent contractors. They were also least likely to have made any investments at all over the past year – 21% of employees reported having made none at all in 2019, compared to 11% of self-employed physicians and 16% of independent contractors.
The financial and work picture for independent contractors
Just over half (52%) of all independent contractors who responded to our questionnaire work in hospitals, 15% work in group practices, 9% work in outpatient clinics, and just 2% work in solo practices.
Independent contractors were less likely than their peers to have received employment benefits such as health insurance, malpractice coverage, and paid time off. They were also less likely to be saving for retirement. Almost half (45%) of independent contractors said they received no employment benefits at all, compared to 20% of self-employed physicians and just 8% of employees.
What’s more, 27% of independent contractors do not currently put money into a 401(k) retirement account or tax-deferred college savings account on a regular basis, compared with 16% of self-employed physicians and 8% of employees. Similarly, they were less likely to put money into a taxable savings account (39% responded that they do not, compared with 32% of self-employed physicians and 27% of employees).
“Net worth and retirement funding findings do line up with what I’ve observed,” said Mr. Scroggins. “Those who have independent practices as opposed to working for a hospital do tend to more heavily fund retirement plan accounts, which is typically the biggest driver of building net worth.”
Despite the lack of retirement planning, independent contractors were more likely than their peers to derive satisfaction from making money at a job they like (18%, compared with 12% of employees and 11% of self-employed physicians). They’re also far more likely to be in emergency medicine (22% of independent contractors, compared with 3% of self-employed and 5% of employees) or psychiatry (11% of independent contractors, compared with 5% of self-employed and 6% of employees).
Among the three categories of physicians, independent contractors were least likely to say that they would choose the same practice setting again. Across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
Physicians who are considering leaving their own practice for a hospital setting should do so with caution and fully understand what they are getting into, said Mr. Scroggins. “If they’re just looking at compensation, they also should be looking very carefully at retirement plan benefits. If that’s their main method of saving and building net worth, then that’s a dramatic difference.”
And of course, there’s always the intangible value of feeling connected to a practice and its patients: “Physicians got into this line of work to treat patients and help people become healthier, and in hospitals they end up being more disconnected from their patients,” Mr. Scroggins said. “That’s a big factor as well.”
Editor’s note: Only differences that are statistically significant at a 95% confidence level between categories of employment have been included. Of the 13,893 responses included in this analysis, 3,860 physicians identified as self-employed, 9,262 as employees, and 772 as independent contractors.
A version of this article originally appeared on Medscape.com.
Self-employed physicians have the highest salaries, largest homes, and greatest wealth – yet they feel the least fairly compensated, according to an analysis of data from over 17,000 physicians.
A new examination of survey responses from the Medscape Physician Compensation Report 2020, which included information about income, job satisfaction, and more, compared responses from self-employed physicians, independent contractors, and employed physicians.
Income and wealth, benefits, and job satisfaction were compared. From the results of the questionnaire, self-employed physicians stand out among their peers across all categories: They enjoy greater income, wealth, and benefits and appear to be more satisfied by their choice of practice.
“The survey confirms that self-employed is the most satisfying, although the trend in health care is to take employed positions,” said Robert Scroggins, JD, CPA, certified health care business consultant with ScrogginsGreer, Cincinnati. “Doctors who become employees primarily do that to escape the management responsibilities for the practice. It seems to be more a decision to get away from something than to go toward something.”
The financial and work picture for self-employed physicians
Self-employed physicians reported the largest salaries for 2019 (average, $360,752), followed by independent contractors ($336,005). Employees reported the lowest average salary ($297,332).
The largest percentage of self-employed physicians (46%) work in an office-based group practice, followed by those in office-based solo practices (30%). Almost two-thirds of self-employed respondents are owners and 37% are partners.
Self-employed physicians are more likely to be older than 45 years; 79% fall into that age bracket, compared with 57% of employees and 70% of independent contractors.
Self-employed physicians reported the highest levels of wealth among their peers. About 44% of self-employed respondents declared a net wealth of over $2 million, compared with 25% of employees. Only 6% of contractors and employed physicians reported a net wealth of over $5 million, compared with 13% of self-employed physicians.
Self-employed physicians also managed their personal expenses slightly differently. They were more likely to pool their income with their spouse in a common account used for bills and expenses, regardless of how much they each earned (63% of self-employed respondents, compared with 58% of employees and 50% of independent contractors).
Perhaps unsurprisingly, self-employed physicians also reported having the largest homes, with an average square footage of 3,629 square feet, compared with 3,023 square feet for employees and 2,984 square feet for independent contractors. Self-employed physicians’ mortgages (average, $240,389) were similar to those of employed physicians’ mortgages but were higher than those of independent contractors’ mortgages (average, $213,740).
Self-employed physicians were also most likely to highly appraise their own performance: Half of all self-employed respondents felt “very satisfied” with their job performance, compared with 40% of employees and 44% of independent contractors.
When asked what they consider to be the most rewarding aspect of their job, self-employed physicians were more likely to choose gratitude and patient relationships than their peers (32%, compared with 26% of employees and 19% of independent contractors).
Despite their higher net wealth and larger salaries, self-employed physicians were least likely to feel fairly compensated; 49% of self-employed physicians said they did not feel fairly compensated for their work, compared with 40% of employees and 40% of independent contractors.
“Self-employed physicians may be better compensated than others of the same specialty who are employees, so some of that may be perception,” said Mr. Scroggins. “Or they feel they should be compensated to a far greater degree than those who are employed.”
Self-employed physicians were also more likely to respond that they would choose the same practice setting again, though across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
The financial and work picture for employed physicians
About a third (32%) of employed physician respondents work in hospitals; 28% work in private practices.
Employed physicians were most likely to report a salary increase from 2018 to 2019: 74%, compared with 45% of self-employed and 52% of independent contractors.
As for declines in income, self-employed physicians and independent contractors suffered a comparable loss, with 13% and 12% of them, respectively, reporting salary cuts greater than 10%. Decreases of up to 10% were felt mostly by the self-employed, with 17% experiencing such cuts, compared with 7% of employees and 10% of independent contractors.
In contrast, employees were the least likely of the three categories to have incurred large financial losses over the past year: 77% of employed respondents indicated that they had not experienced any significant financial losses in the past year, compared with 63% of self-employed physicians and 63% of independent contractors. They were also least likely to have made any investments at all over the past year – 21% of employees reported having made none at all in 2019, compared to 11% of self-employed physicians and 16% of independent contractors.
The financial and work picture for independent contractors
Just over half (52%) of all independent contractors who responded to our questionnaire work in hospitals, 15% work in group practices, 9% work in outpatient clinics, and just 2% work in solo practices.
Independent contractors were less likely than their peers to have received employment benefits such as health insurance, malpractice coverage, and paid time off. They were also less likely to be saving for retirement. Almost half (45%) of independent contractors said they received no employment benefits at all, compared to 20% of self-employed physicians and just 8% of employees.
What’s more, 27% of independent contractors do not currently put money into a 401(k) retirement account or tax-deferred college savings account on a regular basis, compared with 16% of self-employed physicians and 8% of employees. Similarly, they were less likely to put money into a taxable savings account (39% responded that they do not, compared with 32% of self-employed physicians and 27% of employees).
“Net worth and retirement funding findings do line up with what I’ve observed,” said Mr. Scroggins. “Those who have independent practices as opposed to working for a hospital do tend to more heavily fund retirement plan accounts, which is typically the biggest driver of building net worth.”
Despite the lack of retirement planning, independent contractors were more likely than their peers to derive satisfaction from making money at a job they like (18%, compared with 12% of employees and 11% of self-employed physicians). They’re also far more likely to be in emergency medicine (22% of independent contractors, compared with 3% of self-employed and 5% of employees) or psychiatry (11% of independent contractors, compared with 5% of self-employed and 6% of employees).
Among the three categories of physicians, independent contractors were least likely to say that they would choose the same practice setting again. Across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
Physicians who are considering leaving their own practice for a hospital setting should do so with caution and fully understand what they are getting into, said Mr. Scroggins. “If they’re just looking at compensation, they also should be looking very carefully at retirement plan benefits. If that’s their main method of saving and building net worth, then that’s a dramatic difference.”
And of course, there’s always the intangible value of feeling connected to a practice and its patients: “Physicians got into this line of work to treat patients and help people become healthier, and in hospitals they end up being more disconnected from their patients,” Mr. Scroggins said. “That’s a big factor as well.”
Editor’s note: Only differences that are statistically significant at a 95% confidence level between categories of employment have been included. Of the 13,893 responses included in this analysis, 3,860 physicians identified as self-employed, 9,262 as employees, and 772 as independent contractors.
A version of this article originally appeared on Medscape.com.
Self-employed physicians have the highest salaries, largest homes, and greatest wealth – yet they feel the least fairly compensated, according to an analysis of data from over 17,000 physicians.
A new examination of survey responses from the Medscape Physician Compensation Report 2020, which included information about income, job satisfaction, and more, compared responses from self-employed physicians, independent contractors, and employed physicians.
Income and wealth, benefits, and job satisfaction were compared. From the results of the questionnaire, self-employed physicians stand out among their peers across all categories: They enjoy greater income, wealth, and benefits and appear to be more satisfied by their choice of practice.
“The survey confirms that self-employed is the most satisfying, although the trend in health care is to take employed positions,” said Robert Scroggins, JD, CPA, certified health care business consultant with ScrogginsGreer, Cincinnati. “Doctors who become employees primarily do that to escape the management responsibilities for the practice. It seems to be more a decision to get away from something than to go toward something.”
The financial and work picture for self-employed physicians
Self-employed physicians reported the largest salaries for 2019 (average, $360,752), followed by independent contractors ($336,005). Employees reported the lowest average salary ($297,332).
The largest percentage of self-employed physicians (46%) work in an office-based group practice, followed by those in office-based solo practices (30%). Almost two-thirds of self-employed respondents are owners and 37% are partners.
Self-employed physicians are more likely to be older than 45 years; 79% fall into that age bracket, compared with 57% of employees and 70% of independent contractors.
Self-employed physicians reported the highest levels of wealth among their peers. About 44% of self-employed respondents declared a net wealth of over $2 million, compared with 25% of employees. Only 6% of contractors and employed physicians reported a net wealth of over $5 million, compared with 13% of self-employed physicians.
Self-employed physicians also managed their personal expenses slightly differently. They were more likely to pool their income with their spouse in a common account used for bills and expenses, regardless of how much they each earned (63% of self-employed respondents, compared with 58% of employees and 50% of independent contractors).
Perhaps unsurprisingly, self-employed physicians also reported having the largest homes, with an average square footage of 3,629 square feet, compared with 3,023 square feet for employees and 2,984 square feet for independent contractors. Self-employed physicians’ mortgages (average, $240,389) were similar to those of employed physicians’ mortgages but were higher than those of independent contractors’ mortgages (average, $213,740).
Self-employed physicians were also most likely to highly appraise their own performance: Half of all self-employed respondents felt “very satisfied” with their job performance, compared with 40% of employees and 44% of independent contractors.
When asked what they consider to be the most rewarding aspect of their job, self-employed physicians were more likely to choose gratitude and patient relationships than their peers (32%, compared with 26% of employees and 19% of independent contractors).
Despite their higher net wealth and larger salaries, self-employed physicians were least likely to feel fairly compensated; 49% of self-employed physicians said they did not feel fairly compensated for their work, compared with 40% of employees and 40% of independent contractors.
“Self-employed physicians may be better compensated than others of the same specialty who are employees, so some of that may be perception,” said Mr. Scroggins. “Or they feel they should be compensated to a far greater degree than those who are employed.”
Self-employed physicians were also more likely to respond that they would choose the same practice setting again, though across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
The financial and work picture for employed physicians
About a third (32%) of employed physician respondents work in hospitals; 28% work in private practices.
Employed physicians were most likely to report a salary increase from 2018 to 2019: 74%, compared with 45% of self-employed and 52% of independent contractors.
As for declines in income, self-employed physicians and independent contractors suffered a comparable loss, with 13% and 12% of them, respectively, reporting salary cuts greater than 10%. Decreases of up to 10% were felt mostly by the self-employed, with 17% experiencing such cuts, compared with 7% of employees and 10% of independent contractors.
In contrast, employees were the least likely of the three categories to have incurred large financial losses over the past year: 77% of employed respondents indicated that they had not experienced any significant financial losses in the past year, compared with 63% of self-employed physicians and 63% of independent contractors. They were also least likely to have made any investments at all over the past year – 21% of employees reported having made none at all in 2019, compared to 11% of self-employed physicians and 16% of independent contractors.
The financial and work picture for independent contractors
Just over half (52%) of all independent contractors who responded to our questionnaire work in hospitals, 15% work in group practices, 9% work in outpatient clinics, and just 2% work in solo practices.
Independent contractors were less likely than their peers to have received employment benefits such as health insurance, malpractice coverage, and paid time off. They were also less likely to be saving for retirement. Almost half (45%) of independent contractors said they received no employment benefits at all, compared to 20% of self-employed physicians and just 8% of employees.
What’s more, 27% of independent contractors do not currently put money into a 401(k) retirement account or tax-deferred college savings account on a regular basis, compared with 16% of self-employed physicians and 8% of employees. Similarly, they were less likely to put money into a taxable savings account (39% responded that they do not, compared with 32% of self-employed physicians and 27% of employees).
“Net worth and retirement funding findings do line up with what I’ve observed,” said Mr. Scroggins. “Those who have independent practices as opposed to working for a hospital do tend to more heavily fund retirement plan accounts, which is typically the biggest driver of building net worth.”
Despite the lack of retirement planning, independent contractors were more likely than their peers to derive satisfaction from making money at a job they like (18%, compared with 12% of employees and 11% of self-employed physicians). They’re also far more likely to be in emergency medicine (22% of independent contractors, compared with 3% of self-employed and 5% of employees) or psychiatry (11% of independent contractors, compared with 5% of self-employed and 6% of employees).
Among the three categories of physicians, independent contractors were least likely to say that they would choose the same practice setting again. Across all three categories, fewer than 50% of respondents would do so: 34% of self-employed physicians, compared with 29% of employees and 28% of independent contractors.
Physicians who are considering leaving their own practice for a hospital setting should do so with caution and fully understand what they are getting into, said Mr. Scroggins. “If they’re just looking at compensation, they also should be looking very carefully at retirement plan benefits. If that’s their main method of saving and building net worth, then that’s a dramatic difference.”
And of course, there’s always the intangible value of feeling connected to a practice and its patients: “Physicians got into this line of work to treat patients and help people become healthier, and in hospitals they end up being more disconnected from their patients,” Mr. Scroggins said. “That’s a big factor as well.”
Editor’s note: Only differences that are statistically significant at a 95% confidence level between categories of employment have been included. Of the 13,893 responses included in this analysis, 3,860 physicians identified as self-employed, 9,262 as employees, and 772 as independent contractors.
A version of this article originally appeared on Medscape.com.
High mortality rates reported in large COVID-19 study
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
REALITY trial supports restrictive transfusion in anemic MI
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
REPORTING FROM ESC CONGRESS 2020
Latest report adds almost 44,000 child COVID-19 cases in 1 week
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
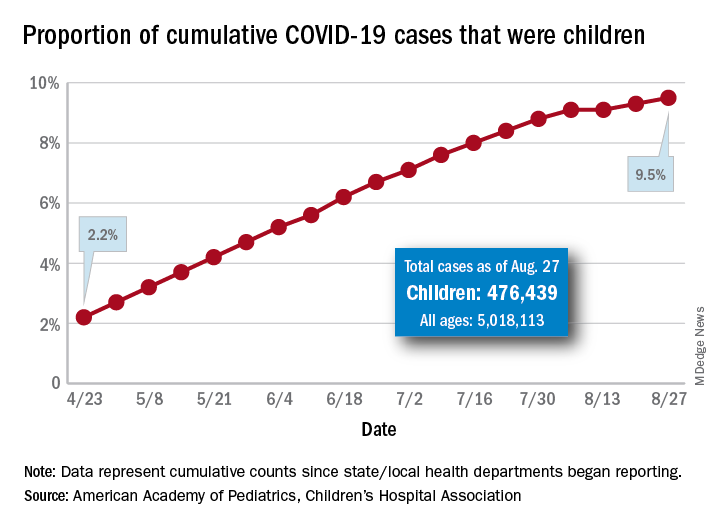
The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.






