User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Underlying heart rhythm, not ICD shocks, drives mortality
Combined data from five implantable cardioverter-defibrillator (ICD) trials suggest that it is the underlying arrhythmic disorder, rather than the ICD therapy itself, that affects mortality in these patients.
Analysis of the MADIT II, MADIT-RISK, MADIT-CRT, MADIT-RIT, and RAID trials showed that the major determinant of mortality in patients receiving a primary prevention ICD was the arrhythmic substrate that leads to occurrence of fast ventricular tachycardia (VT), defined as ≥ 200 bpm, or ventricular fibrillation (VF), not adverse effects of the ICD shock therapy itself.
Patients experiencing an episode of VT had more than a twofold increased risk for death during a follow-up of 2½ years; however, ICD therapies for VT less than 200 bpm and inappropriate ICD shocks were not associated with a higher risk for death.
The findings were published online in the Journal of the American College of Cardiology.
“We know that patients receiving an ICD shock have increased mortality during subsequent follow-up,” first author Mehmet K. Aktas, MD, MBA, University of Rochester (N.Y.), said in an interview.
“There are conflicting data on the impact of ICD shocks on subsequent mortality, and in this study, we aimed to determine whether shocks per se increase subsequent mortality risk or whether the arrhythmic substrate that leads to ICD therapy results in subsequent risk of death,” Dr. Aktas said.
He and his team evaluated the association of ICD therapy with subsequent mortality according to the type of ICD therapy (model I), type of arrhythmia for which ICD therapy was delivered (model II), combined assessment of all arrhythmia and therapy types during follow-up (model III), and incremental risk associated with repeated ICD shocks (model IV).
The study cohort included 5,516 patients. Of these, 1,001 patients (18%) received appropriate ICD therapy and 561 (10%) received inappropriate ICD therapy during an average of 2.4 years.
Patients receiving an appropriate ICD therapy were more likely to be male and to have prior atrial arrhythmia and nonsustained VT compared with those without ICD therapy.
Patients receiving an inappropriate shock were more likely to be younger, to be African American, and to be less likely to have prior nonsustained VT, compared with those without ICD therapy.
Most patients (90%) were receiving beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers regardless of device therapy during follow-up, and 10% of patients were treated with amiodarone.
In model I, at 3 years, the cumulative probability of death following an appropriate ICD shock was 38% compared with no appropriate ICD shock (P < .001). Inappropriate shock alone was not associated with mortality risk.
In model II, which looked at the type of arrhythmia for which ICD therapy was delivered, the cumulative death rate at 3 years following the first occurrence of ICD therapy for VT ≥ 200 beats/min or VF was 27%, compared with 10% in patients not experiencing VT ≥ 200 beats/min or VF (P < .001).
In model III, the highest risk for death was observed following shocks delivered after a failed antitachycardia pacing (ATP) for fast VT (hazard ratio [HR], 3.05), followed by ICD shock for VF (HR, 2.86), ICD shock for fast VT without a prior ATP (HR, 2.83), and ICD shock for slower VT (< 200 beats/min) without a prior ATP (HR, 2.39).
In contrast, other types of appropriate and inappropriate shock or ATP therapies were not associated with a significant risk increase.
In model IV, which assessed the association of shock therapy counts with the risk for death, two or more ICD appropriate shocks were not associated with increased risk after the first appropriate ICD shock.
“Our findings shed light on the mechanisms associated with increased mortality risk in primary prevention ICD recipients,” Dr. Aktas said.
“Studies that evaluate interventions focused on treating and stabilizing the myocardial substrate, which promotes ventricular tachyarrhythmias, such as catheter ablation, are needed to improve survival in heart failure patients,” he added.
Thoughtful study design
In an accompanying editorial, Rajat Deo, MD, and Naga Venkata K. Pothineni, MD, both from the University of Pennsylvania, Philadelphia, praised the researchers for their “thoughtful study design.”
“The take-home message that is most relevant to our clinical practice is clear: Sustained ventricular arrhythmias are a prognostic marker of death and heart failure hospitalization,” they wrote.
The editorialists also commented on the higher rate of inappropriate ICD therapies in African Americans.
“It is concerning to observe that Black patients had a markedly higher rate of inappropriate ICD therapies compared with White patients – and this was in the setting of some of the most respectable, established, and well-funded clinical trials,” they wrote.
Reasons for disparities in outcomes include access to appropriate and affordable medical therapies, access to specialty clinics and caregivers, remote ICD monitoring, and compliance issues.
“Future work will need to understand how the social determinants of health including race affect the treatment and outcomes of our primary prevention ICD population,” they wrote.
Identifying and characterizing the arrhythmic substrate will become a key component of sudden cardiac death risk stratification, the editorialists predicted.
“Concurrently, we must continue to partner with industry colleagues and work with our professional societies to ensure health equity across our patient population,” they concluded.
Dr. Aktas has received research grants from Boston Scientific and Medtronic. Dr. Deo and his coeditorialists report no relevant financial relationships. The MADIT trials were funded by an unrestricted research grant from Boston Scientific to the University of Rochester Medical Center. The RAID trial was funded by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Combined data from five implantable cardioverter-defibrillator (ICD) trials suggest that it is the underlying arrhythmic disorder, rather than the ICD therapy itself, that affects mortality in these patients.
Analysis of the MADIT II, MADIT-RISK, MADIT-CRT, MADIT-RIT, and RAID trials showed that the major determinant of mortality in patients receiving a primary prevention ICD was the arrhythmic substrate that leads to occurrence of fast ventricular tachycardia (VT), defined as ≥ 200 bpm, or ventricular fibrillation (VF), not adverse effects of the ICD shock therapy itself.
Patients experiencing an episode of VT had more than a twofold increased risk for death during a follow-up of 2½ years; however, ICD therapies for VT less than 200 bpm and inappropriate ICD shocks were not associated with a higher risk for death.
The findings were published online in the Journal of the American College of Cardiology.
“We know that patients receiving an ICD shock have increased mortality during subsequent follow-up,” first author Mehmet K. Aktas, MD, MBA, University of Rochester (N.Y.), said in an interview.
“There are conflicting data on the impact of ICD shocks on subsequent mortality, and in this study, we aimed to determine whether shocks per se increase subsequent mortality risk or whether the arrhythmic substrate that leads to ICD therapy results in subsequent risk of death,” Dr. Aktas said.
He and his team evaluated the association of ICD therapy with subsequent mortality according to the type of ICD therapy (model I), type of arrhythmia for which ICD therapy was delivered (model II), combined assessment of all arrhythmia and therapy types during follow-up (model III), and incremental risk associated with repeated ICD shocks (model IV).
The study cohort included 5,516 patients. Of these, 1,001 patients (18%) received appropriate ICD therapy and 561 (10%) received inappropriate ICD therapy during an average of 2.4 years.
Patients receiving an appropriate ICD therapy were more likely to be male and to have prior atrial arrhythmia and nonsustained VT compared with those without ICD therapy.
Patients receiving an inappropriate shock were more likely to be younger, to be African American, and to be less likely to have prior nonsustained VT, compared with those without ICD therapy.
Most patients (90%) were receiving beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers regardless of device therapy during follow-up, and 10% of patients were treated with amiodarone.
In model I, at 3 years, the cumulative probability of death following an appropriate ICD shock was 38% compared with no appropriate ICD shock (P < .001). Inappropriate shock alone was not associated with mortality risk.
In model II, which looked at the type of arrhythmia for which ICD therapy was delivered, the cumulative death rate at 3 years following the first occurrence of ICD therapy for VT ≥ 200 beats/min or VF was 27%, compared with 10% in patients not experiencing VT ≥ 200 beats/min or VF (P < .001).
In model III, the highest risk for death was observed following shocks delivered after a failed antitachycardia pacing (ATP) for fast VT (hazard ratio [HR], 3.05), followed by ICD shock for VF (HR, 2.86), ICD shock for fast VT without a prior ATP (HR, 2.83), and ICD shock for slower VT (< 200 beats/min) without a prior ATP (HR, 2.39).
In contrast, other types of appropriate and inappropriate shock or ATP therapies were not associated with a significant risk increase.
In model IV, which assessed the association of shock therapy counts with the risk for death, two or more ICD appropriate shocks were not associated with increased risk after the first appropriate ICD shock.
“Our findings shed light on the mechanisms associated with increased mortality risk in primary prevention ICD recipients,” Dr. Aktas said.
“Studies that evaluate interventions focused on treating and stabilizing the myocardial substrate, which promotes ventricular tachyarrhythmias, such as catheter ablation, are needed to improve survival in heart failure patients,” he added.
Thoughtful study design
In an accompanying editorial, Rajat Deo, MD, and Naga Venkata K. Pothineni, MD, both from the University of Pennsylvania, Philadelphia, praised the researchers for their “thoughtful study design.”
“The take-home message that is most relevant to our clinical practice is clear: Sustained ventricular arrhythmias are a prognostic marker of death and heart failure hospitalization,” they wrote.
The editorialists also commented on the higher rate of inappropriate ICD therapies in African Americans.
“It is concerning to observe that Black patients had a markedly higher rate of inappropriate ICD therapies compared with White patients – and this was in the setting of some of the most respectable, established, and well-funded clinical trials,” they wrote.
Reasons for disparities in outcomes include access to appropriate and affordable medical therapies, access to specialty clinics and caregivers, remote ICD monitoring, and compliance issues.
“Future work will need to understand how the social determinants of health including race affect the treatment and outcomes of our primary prevention ICD population,” they wrote.
Identifying and characterizing the arrhythmic substrate will become a key component of sudden cardiac death risk stratification, the editorialists predicted.
“Concurrently, we must continue to partner with industry colleagues and work with our professional societies to ensure health equity across our patient population,” they concluded.
Dr. Aktas has received research grants from Boston Scientific and Medtronic. Dr. Deo and his coeditorialists report no relevant financial relationships. The MADIT trials were funded by an unrestricted research grant from Boston Scientific to the University of Rochester Medical Center. The RAID trial was funded by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Combined data from five implantable cardioverter-defibrillator (ICD) trials suggest that it is the underlying arrhythmic disorder, rather than the ICD therapy itself, that affects mortality in these patients.
Analysis of the MADIT II, MADIT-RISK, MADIT-CRT, MADIT-RIT, and RAID trials showed that the major determinant of mortality in patients receiving a primary prevention ICD was the arrhythmic substrate that leads to occurrence of fast ventricular tachycardia (VT), defined as ≥ 200 bpm, or ventricular fibrillation (VF), not adverse effects of the ICD shock therapy itself.
Patients experiencing an episode of VT had more than a twofold increased risk for death during a follow-up of 2½ years; however, ICD therapies for VT less than 200 bpm and inappropriate ICD shocks were not associated with a higher risk for death.
The findings were published online in the Journal of the American College of Cardiology.
“We know that patients receiving an ICD shock have increased mortality during subsequent follow-up,” first author Mehmet K. Aktas, MD, MBA, University of Rochester (N.Y.), said in an interview.
“There are conflicting data on the impact of ICD shocks on subsequent mortality, and in this study, we aimed to determine whether shocks per se increase subsequent mortality risk or whether the arrhythmic substrate that leads to ICD therapy results in subsequent risk of death,” Dr. Aktas said.
He and his team evaluated the association of ICD therapy with subsequent mortality according to the type of ICD therapy (model I), type of arrhythmia for which ICD therapy was delivered (model II), combined assessment of all arrhythmia and therapy types during follow-up (model III), and incremental risk associated with repeated ICD shocks (model IV).
The study cohort included 5,516 patients. Of these, 1,001 patients (18%) received appropriate ICD therapy and 561 (10%) received inappropriate ICD therapy during an average of 2.4 years.
Patients receiving an appropriate ICD therapy were more likely to be male and to have prior atrial arrhythmia and nonsustained VT compared with those without ICD therapy.
Patients receiving an inappropriate shock were more likely to be younger, to be African American, and to be less likely to have prior nonsustained VT, compared with those without ICD therapy.
Most patients (90%) were receiving beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers regardless of device therapy during follow-up, and 10% of patients were treated with amiodarone.
In model I, at 3 years, the cumulative probability of death following an appropriate ICD shock was 38% compared with no appropriate ICD shock (P < .001). Inappropriate shock alone was not associated with mortality risk.
In model II, which looked at the type of arrhythmia for which ICD therapy was delivered, the cumulative death rate at 3 years following the first occurrence of ICD therapy for VT ≥ 200 beats/min or VF was 27%, compared with 10% in patients not experiencing VT ≥ 200 beats/min or VF (P < .001).
In model III, the highest risk for death was observed following shocks delivered after a failed antitachycardia pacing (ATP) for fast VT (hazard ratio [HR], 3.05), followed by ICD shock for VF (HR, 2.86), ICD shock for fast VT without a prior ATP (HR, 2.83), and ICD shock for slower VT (< 200 beats/min) without a prior ATP (HR, 2.39).
In contrast, other types of appropriate and inappropriate shock or ATP therapies were not associated with a significant risk increase.
In model IV, which assessed the association of shock therapy counts with the risk for death, two or more ICD appropriate shocks were not associated with increased risk after the first appropriate ICD shock.
“Our findings shed light on the mechanisms associated with increased mortality risk in primary prevention ICD recipients,” Dr. Aktas said.
“Studies that evaluate interventions focused on treating and stabilizing the myocardial substrate, which promotes ventricular tachyarrhythmias, such as catheter ablation, are needed to improve survival in heart failure patients,” he added.
Thoughtful study design
In an accompanying editorial, Rajat Deo, MD, and Naga Venkata K. Pothineni, MD, both from the University of Pennsylvania, Philadelphia, praised the researchers for their “thoughtful study design.”
“The take-home message that is most relevant to our clinical practice is clear: Sustained ventricular arrhythmias are a prognostic marker of death and heart failure hospitalization,” they wrote.
The editorialists also commented on the higher rate of inappropriate ICD therapies in African Americans.
“It is concerning to observe that Black patients had a markedly higher rate of inappropriate ICD therapies compared with White patients – and this was in the setting of some of the most respectable, established, and well-funded clinical trials,” they wrote.
Reasons for disparities in outcomes include access to appropriate and affordable medical therapies, access to specialty clinics and caregivers, remote ICD monitoring, and compliance issues.
“Future work will need to understand how the social determinants of health including race affect the treatment and outcomes of our primary prevention ICD population,” they wrote.
Identifying and characterizing the arrhythmic substrate will become a key component of sudden cardiac death risk stratification, the editorialists predicted.
“Concurrently, we must continue to partner with industry colleagues and work with our professional societies to ensure health equity across our patient population,” they concluded.
Dr. Aktas has received research grants from Boston Scientific and Medtronic. Dr. Deo and his coeditorialists report no relevant financial relationships. The MADIT trials were funded by an unrestricted research grant from Boston Scientific to the University of Rochester Medical Center. The RAID trial was funded by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
A new take on breathing and a performance-enhancing placebo
No ifs, ands, or butt ventilators
Breathing, on most days, is a pretty simple task. You inhale, the oxygen goes in, fills your lungs, becomes carbon dioxide, and is exhaled. But as certain recent events have made very clear, some diseases make this task difficult, which is where ventilators come in. The issue is, some patients can’t really use ventilators.
Enter a new study from Japan, which tested the ability of mice and pigs to absorb oxygen through the rectum. Yes, breathing through the butt. It’s not actually such a far-fetched idea; several aquatic animals such as sea cucumbers and catfish absorb oxygen through their intestines, and as any drunken frat boy can tell you after a good butt chug, other chemicals can absolutely be absorbed by human intestines.
After an initial successful experiment where a group of mice had their intestines scrubbed, had pure oxygen inserted enterally, and were exposed to a hypoxic environment, the researchers decided to step up their game and avoid the exhaustive act of digestive scrubbing by enlisting the aid of something out of science fiction: perfluorocarbon. If you haven’t seen “The Abyss,” this liquid can absorb massive amounts of oxygen, so you can actually breathe it in the same way you do with air.
In part two of the experiment, a group of hypoxic mice and pigs had perfluorocarbon inserted into their anuses, while another group got saline solution. The saline group did not fare well, but the animals that got perfluorocarbon had their hypoxic symptoms relieved within minutes.
The effectiveness of this procedure in humans clearly has yet to be tested, and while it may not be useful in all, or even most, situations, it is always beneficial to have more ways to combat a problem. Just don’t tell the frat boys: They’ll be hooking oxygen tanks up to their butts and chanting: “Breathe! Breathe! Breathe!”
Better, stronger, faster … pinker
Many people, most of whom aren’t even athletes, commit huge amounts of time, effort, and expense to improve their athletic performance. But what if there’s an easier way?
Research conducted at the University of Westminster (England) showed that participants could, with one fairly simple intervention, get on a treadmill and run 212 meters further in 30 minutes, increasing their speed by an average of 4.4%. Not only that, but “feelings of pleasure were also enhanced, meaning participants found running more enjoyable,” according to a statement from the university.
Is this amazing intervention a new wonder drug? No. Is it a super special nutritional supplement? Negatory. An energy drink that “gives you wiiings”? Nope. The latest designer steroid? Nyet.
Like we said, it’s simple, and it’s pink. Literally, the color pink. We will explain.
Each of the 10 study subjects completed two 30-minute trials on the treadmill. For one, they were given a clear, artificially sweetened drink while they were running. For the other, they received the exact same drink colored pink with food dye. Pink did better. So to recap the last month in our column, faster looks pink, and skinny smells like lemons.
Once again, science demonstrates that you can’t go wrong by fooling a brain. Next week, LOTME tries to find out if purple makes you funnier.
Hey … I’m singing here!
Noise pollution has been linked to plenty of negative outcomes, but the latest target is the poor baby zebra finch.
Researchers at the Max Planck Institute of Ornithology in Germany say traffic noise disrupts the timing of vocal development and impairs learning in the flying finches. The noise was also shown to suppress their immune systems, because of lingering stress.
The good news is that the birds with noise-induced stress sang as much as their peers in a control group, so the delay in development “was not due to a lack of vocal practice,” according to researchers. However, one long-term effect could be that zebra finch birdsongs could change over time due to noise-induced copying errors. Imagine a really long game of birdsong telephone – the song at the beginning is unlikely to be the song years from now.
While not mentioned in the study, one could also imagine that due to all that exposure to traffic, young zebra finches could be developing a salty dialect and impatience with fellow finches taking up too much space on the same tree branch. Hopefully, they don’t give others “the bird.”
Slimy soap
Remember at the beginning of the pandemic when it was almost impossible to find sufficient hand-washing supplies? Just when you thought you’d tried everything, there is soap made from snail slime.
Snail slime, surprisingly, has many beneficial properties for humans. The slime has antiaging and skin healing properties and is actually used in some Korean beauty supplies. The snails even use the slime to help fix their shells if they become damaged.
Happily, no snails are harmed in the slime extraction and making of the soap. Snail farmer Damien Desrochers says, “I only touch it with my finger, you see it’s not violent, it’s simple.”
As you can probably imagine, a lot of slime is needed to have a steady supply of this soap, so Mr. Desrochers has systems in place to get enough slime. Approximately 40 snails are needed to make 15 bars of soap, and he hopes to produce about 3,000 bars in the first year.
Nothing really surprises us anymore in the beauty world: People put eggs in their hair and bee venom on their skin, so what’s wrong with a little snail slime?
No ifs, ands, or butt ventilators
Breathing, on most days, is a pretty simple task. You inhale, the oxygen goes in, fills your lungs, becomes carbon dioxide, and is exhaled. But as certain recent events have made very clear, some diseases make this task difficult, which is where ventilators come in. The issue is, some patients can’t really use ventilators.
Enter a new study from Japan, which tested the ability of mice and pigs to absorb oxygen through the rectum. Yes, breathing through the butt. It’s not actually such a far-fetched idea; several aquatic animals such as sea cucumbers and catfish absorb oxygen through their intestines, and as any drunken frat boy can tell you after a good butt chug, other chemicals can absolutely be absorbed by human intestines.
After an initial successful experiment where a group of mice had their intestines scrubbed, had pure oxygen inserted enterally, and were exposed to a hypoxic environment, the researchers decided to step up their game and avoid the exhaustive act of digestive scrubbing by enlisting the aid of something out of science fiction: perfluorocarbon. If you haven’t seen “The Abyss,” this liquid can absorb massive amounts of oxygen, so you can actually breathe it in the same way you do with air.
In part two of the experiment, a group of hypoxic mice and pigs had perfluorocarbon inserted into their anuses, while another group got saline solution. The saline group did not fare well, but the animals that got perfluorocarbon had their hypoxic symptoms relieved within minutes.
The effectiveness of this procedure in humans clearly has yet to be tested, and while it may not be useful in all, or even most, situations, it is always beneficial to have more ways to combat a problem. Just don’t tell the frat boys: They’ll be hooking oxygen tanks up to their butts and chanting: “Breathe! Breathe! Breathe!”
Better, stronger, faster … pinker
Many people, most of whom aren’t even athletes, commit huge amounts of time, effort, and expense to improve their athletic performance. But what if there’s an easier way?
Research conducted at the University of Westminster (England) showed that participants could, with one fairly simple intervention, get on a treadmill and run 212 meters further in 30 minutes, increasing their speed by an average of 4.4%. Not only that, but “feelings of pleasure were also enhanced, meaning participants found running more enjoyable,” according to a statement from the university.
Is this amazing intervention a new wonder drug? No. Is it a super special nutritional supplement? Negatory. An energy drink that “gives you wiiings”? Nope. The latest designer steroid? Nyet.
Like we said, it’s simple, and it’s pink. Literally, the color pink. We will explain.
Each of the 10 study subjects completed two 30-minute trials on the treadmill. For one, they were given a clear, artificially sweetened drink while they were running. For the other, they received the exact same drink colored pink with food dye. Pink did better. So to recap the last month in our column, faster looks pink, and skinny smells like lemons.
Once again, science demonstrates that you can’t go wrong by fooling a brain. Next week, LOTME tries to find out if purple makes you funnier.
Hey … I’m singing here!
Noise pollution has been linked to plenty of negative outcomes, but the latest target is the poor baby zebra finch.
Researchers at the Max Planck Institute of Ornithology in Germany say traffic noise disrupts the timing of vocal development and impairs learning in the flying finches. The noise was also shown to suppress their immune systems, because of lingering stress.
The good news is that the birds with noise-induced stress sang as much as their peers in a control group, so the delay in development “was not due to a lack of vocal practice,” according to researchers. However, one long-term effect could be that zebra finch birdsongs could change over time due to noise-induced copying errors. Imagine a really long game of birdsong telephone – the song at the beginning is unlikely to be the song years from now.
While not mentioned in the study, one could also imagine that due to all that exposure to traffic, young zebra finches could be developing a salty dialect and impatience with fellow finches taking up too much space on the same tree branch. Hopefully, they don’t give others “the bird.”
Slimy soap
Remember at the beginning of the pandemic when it was almost impossible to find sufficient hand-washing supplies? Just when you thought you’d tried everything, there is soap made from snail slime.
Snail slime, surprisingly, has many beneficial properties for humans. The slime has antiaging and skin healing properties and is actually used in some Korean beauty supplies. The snails even use the slime to help fix their shells if they become damaged.
Happily, no snails are harmed in the slime extraction and making of the soap. Snail farmer Damien Desrochers says, “I only touch it with my finger, you see it’s not violent, it’s simple.”
As you can probably imagine, a lot of slime is needed to have a steady supply of this soap, so Mr. Desrochers has systems in place to get enough slime. Approximately 40 snails are needed to make 15 bars of soap, and he hopes to produce about 3,000 bars in the first year.
Nothing really surprises us anymore in the beauty world: People put eggs in their hair and bee venom on their skin, so what’s wrong with a little snail slime?
No ifs, ands, or butt ventilators
Breathing, on most days, is a pretty simple task. You inhale, the oxygen goes in, fills your lungs, becomes carbon dioxide, and is exhaled. But as certain recent events have made very clear, some diseases make this task difficult, which is where ventilators come in. The issue is, some patients can’t really use ventilators.
Enter a new study from Japan, which tested the ability of mice and pigs to absorb oxygen through the rectum. Yes, breathing through the butt. It’s not actually such a far-fetched idea; several aquatic animals such as sea cucumbers and catfish absorb oxygen through their intestines, and as any drunken frat boy can tell you after a good butt chug, other chemicals can absolutely be absorbed by human intestines.
After an initial successful experiment where a group of mice had their intestines scrubbed, had pure oxygen inserted enterally, and were exposed to a hypoxic environment, the researchers decided to step up their game and avoid the exhaustive act of digestive scrubbing by enlisting the aid of something out of science fiction: perfluorocarbon. If you haven’t seen “The Abyss,” this liquid can absorb massive amounts of oxygen, so you can actually breathe it in the same way you do with air.
In part two of the experiment, a group of hypoxic mice and pigs had perfluorocarbon inserted into their anuses, while another group got saline solution. The saline group did not fare well, but the animals that got perfluorocarbon had their hypoxic symptoms relieved within minutes.
The effectiveness of this procedure in humans clearly has yet to be tested, and while it may not be useful in all, or even most, situations, it is always beneficial to have more ways to combat a problem. Just don’t tell the frat boys: They’ll be hooking oxygen tanks up to their butts and chanting: “Breathe! Breathe! Breathe!”
Better, stronger, faster … pinker
Many people, most of whom aren’t even athletes, commit huge amounts of time, effort, and expense to improve their athletic performance. But what if there’s an easier way?
Research conducted at the University of Westminster (England) showed that participants could, with one fairly simple intervention, get on a treadmill and run 212 meters further in 30 minutes, increasing their speed by an average of 4.4%. Not only that, but “feelings of pleasure were also enhanced, meaning participants found running more enjoyable,” according to a statement from the university.
Is this amazing intervention a new wonder drug? No. Is it a super special nutritional supplement? Negatory. An energy drink that “gives you wiiings”? Nope. The latest designer steroid? Nyet.
Like we said, it’s simple, and it’s pink. Literally, the color pink. We will explain.
Each of the 10 study subjects completed two 30-minute trials on the treadmill. For one, they were given a clear, artificially sweetened drink while they were running. For the other, they received the exact same drink colored pink with food dye. Pink did better. So to recap the last month in our column, faster looks pink, and skinny smells like lemons.
Once again, science demonstrates that you can’t go wrong by fooling a brain. Next week, LOTME tries to find out if purple makes you funnier.
Hey … I’m singing here!
Noise pollution has been linked to plenty of negative outcomes, but the latest target is the poor baby zebra finch.
Researchers at the Max Planck Institute of Ornithology in Germany say traffic noise disrupts the timing of vocal development and impairs learning in the flying finches. The noise was also shown to suppress their immune systems, because of lingering stress.
The good news is that the birds with noise-induced stress sang as much as their peers in a control group, so the delay in development “was not due to a lack of vocal practice,” according to researchers. However, one long-term effect could be that zebra finch birdsongs could change over time due to noise-induced copying errors. Imagine a really long game of birdsong telephone – the song at the beginning is unlikely to be the song years from now.
While not mentioned in the study, one could also imagine that due to all that exposure to traffic, young zebra finches could be developing a salty dialect and impatience with fellow finches taking up too much space on the same tree branch. Hopefully, they don’t give others “the bird.”
Slimy soap
Remember at the beginning of the pandemic when it was almost impossible to find sufficient hand-washing supplies? Just when you thought you’d tried everything, there is soap made from snail slime.
Snail slime, surprisingly, has many beneficial properties for humans. The slime has antiaging and skin healing properties and is actually used in some Korean beauty supplies. The snails even use the slime to help fix their shells if they become damaged.
Happily, no snails are harmed in the slime extraction and making of the soap. Snail farmer Damien Desrochers says, “I only touch it with my finger, you see it’s not violent, it’s simple.”
As you can probably imagine, a lot of slime is needed to have a steady supply of this soap, so Mr. Desrochers has systems in place to get enough slime. Approximately 40 snails are needed to make 15 bars of soap, and he hopes to produce about 3,000 bars in the first year.
Nothing really surprises us anymore in the beauty world: People put eggs in their hair and bee venom on their skin, so what’s wrong with a little snail slime?
FIDELIO-DKD: Finerenone cuts new-onset AFib in patients with type 2 diabetes and CKD
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
FROM ACC 2021
Dapagliflozin misses as treatment for COVID-19 but leaves intriguing signal for benefit
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
FROM ACC 2021
Novel rehab program fights frailty, boosts capacity in advanced HF
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.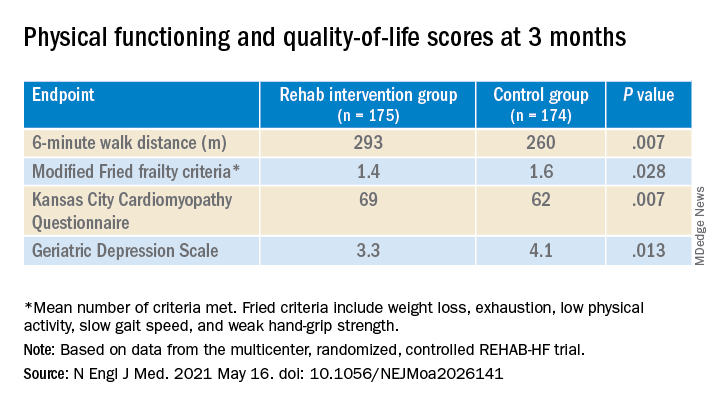
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
Omics analysis links blood type to COVID-19
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Are more naturopaths trying to compete with docs?
Jon Hislop, MD, PhD, hadn’t been in practice very long before patients began coming to him with requests to order tests that their naturopaths had recommended.
The family physician in North Vancouver, British Columbia, knew little about naturopathy but began researching it.
“I was finding that some of what the naturopaths were telling them was a little odd. Some of the tests they were asking for were unnecessary,” Dr. Hislop said.
The more he learned about naturopathy, the more appalled he became. He eventually took to Twitter, where he wages a campaign against naturopathy and alternative medicine.
“There is no alternative medicine,” he said. “There’s medicine and there’s other stuff. We need to stick to medicine and stay away from the other stuff.”
Dr. Hislop is not alone in his criticism of naturopathic medicine. Professional medical societies almost universally oppose naturopathy, but that has not stopped its spread or prevented it from becoming part of some health care systems.
Americans spent $30.2 billion on out-of-pocket complementary health care, according to a 2016 report from the National Institutes of Health. That includes everything from herbal supplements and massage therapy to chiropractic care.
What is naturopathic medicine?
Naturopathy came to the United States from Germany in the 1800s, but some of its practices are thousands of years old. Naturopathic treatments include homeopathy, IV vitamin infusions, acupuncture, Reiki, and herbal supplements.
Naturopathy is based on the belief that the body has an innate ability to heal itself. It discourages drugs and surgery in favor of supplements, herbs, and other so-called natural treatments. Much of it centers around addressing lifestyle issues and counseling patients to improve their diets, quit smoking, exercise more, lose weight, etc., in order to address the root causes of some health problems.
Practitioners are critical of Western medicine for what they regard as an over-reliance on drugs and technology and for treating symptoms rather than the causes of disease.
“We get a lot of people who are at the end of their ropes, people with hard-to-diagnose diseases who know they are sick but whose labs are normal,” said Jaquel Patterson, ND, former president of the American Association of Naturopathic Physicians (AANP) and medical director of a naturopathic practice in Connecticut.
Separate training and licensing
There are major differences among naturopaths.
At one extreme are unlicensed, self-taught “healers,” who can embrace everything from homeopathy to aromatherapy.
At the other end are naturopathic doctors (NDs), who are more likely to become part of health care systems. These caregivers are trained and licensed, though not by the same institutions as traditional physicians.
To be licensed, NDs must graduate from one of seven accredited naturopathic medical schools in the United States and Canada. In addition to a standard medical curriculum, schools require graduates to complete 4 years of training in clinical nutrition, acupuncture, homeopathic medicine, botanical medicine, physical medicine, and counseling. Medical students intern in clinical settings for 2 years.
NDs are eager to distinguish themselves from their uncredentialed counterparts.
“Some people go to a weekend class and call themselves naturopaths. That’s very concerning. I don’t want those people to be licensed either,” said Hallie Armstrong, ND, who practices in Michigan.
In the United States, there are 6,000 practicing NDs and an unknown number of unlicensed naturopathic healers.
Can naturopaths call themselves ‘physicians’?
Twenty-two states, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands have licensing or registration laws for naturopathic doctors. Three states – South Carolina, Tennessee, and Florida – prohibit practicing naturopathic medicine without a license, according to the AANP.
States that license NDs differ in what they permit them to do.
Nine states allow licensed NDs to use the term “physician,” although this is prohibited in seven states. Most licensed states allow naturopathic practitioners some prescribing authority, including the prescribing of many controlled substances, although only a few states permit full prescribing rights. Most states that license NDs allow them to prescribe and administer nonprescription therapeutic substances, drugs, and therapies.
Twelve states and the District of Columbia allow licensed naturopathic doctors to perform some minor procedures, such as stitching up wounds. Additionally, 13 states allow NDs to order diagnostic tests.
Although the AANP lobbies to get licensure in more states and to expand the activities that NDs can perform, the medical establishment in those states nearly always opposes the legislation, as do national organizations, such as the American Academy of Family Physicians and the American College of Physicians.
“They absolutely will not stop until they get licenses. They’ve done a really good job of selling themselves as legitimate health care professionals to state legislatures,” said David Gorski, MD, PhD, FACS, a surgical oncologist and managing editor of Science-Based Medicine, a blog that attacks unproven medical claims and defends traditional medicine. Naturopathy is a favorite target.
Are naturopaths gaining ground anyway?
Despite the opposition of the medical establishment and many individual health care professionals, a growing number of health care systems are adopting alternative medicine.
In 2018, the AANP stated that 28 prominent health systems, hospitals, and cancer treatment centers had one or more licensed NDs on staff. Among them were Cancer Treatment Centers of America, Cedars-Sinai, Columbia University’s Herbert Irving Comprehensive Cancer Center, and the Fred Hutchinson Cancer Research Center.
Other health care systems may not have NDs on staff but provide naturopathic treatments, usually under the heading of “complementary medicine” or “integrative medicine.” For example, the Cleveland Clinic’s Center for Integrative and Lifestyle Medicine offers acupuncture, Chinese herbal medicine, Reiki, yoga, and culinary medicine.
Critics find this appalling.
“I think it’s a mistake to integrate that kind of practice into a science-based health care setting. If we learned anything over the past year, it’s that medicine based on magical thinking is dangerous,” said Timothy Caulfield, LLM, FCAHS, research director at the Health Law Institute of the University of Alberta, Edmonton.
Dr. Gorski added: “I’m not exactly sure why doctors who should know better have become more accepting of practices that aren’t science-based or are outright quackery.”
Becoming part of the system
Beaumont Health, Michigan’s largest health care system, added integrative medicine in 2006 and hired its first naturopathic practitioners a year later.
The integrative practitioners began in oncology, offering such things as massage therapy, acupuncture, guided imagery, and Reiki. “Very quickly, people outside oncology began saying, ‘I’ve got a cardiology patient who would really benefit from this ... I’ve got a GI patient who could benefit from this...,’” said Maureen Anderson, MD, medical director of Beaumont Integrative Medicine.
Beaumont now offers integrative medicine at three locations. They average 20,000 visits a year and work with 50 to 60 practitioners, many of whom work part-time.
Because Michigan does not license NDs, their scope of practice at Beaumont is limited. They take patient histories, provide advice on nutrition, diet, and exercise, and prescribe herbs and supplements. Beaumont operates its own herbal and supplement pharmacy.
NDs work under the medical supervision of Dr. Anderson, an emergency medicine physician who became interested in naturopathy because she thought traditional medicine doesn’t do a good job of providing care for chronic conditions. Any initial skepticism on the part of the medical staff has been overcome by seeing the benefits naturopathy provides, Dr. Anderson said. The claim is echoed by Mr. Armstrong, an ND who works in the system part-time: “As soon as [doctors] understand our schooling and where we’re coming from and understand that we want to do the same things, then they’re very accepting.”
The University of California, Irvine, health care system has one of the largest naturopathic medicine programs in the country, the result of a $200 million donation in 2017 from a couple who champion alternative medicine. The Susan Samueli Integrative Health Institute includes 28 health care professionals, including MDs, NDs, RNs, acupuncturists, dietitians, yoga instructors, and others. It includes a research arm, which is focused primarily on acupuncture.
The alternative medicine offerings benefit the system, said Kim Hecht, DO, medical director of inpatient and ambulatory services at the Samueli Institute.
“I’m not against traditional medicine, because I think everything has a time and a place,” Dr. Hecht said. However, she rejects the idea that MDs can offer the same holistic approach as NDs.
“Medical science likes to say we’re interested in treating the whole person, but if you look at medical school courses, that’s not what’s being taught,” she said.
The chance to work within a traditional health care system was attractive to Arvin Jenab, ND, medical director of naturopathic medicine at the institute.
“It offers the opportunity to refine our medicine and trim the things that aren’t necessary or are controversial and concentrate on the things at the core of what we do,” he said.
UCI Health practices a conservative model of naturopathy that supports traditional practitioners, Mr. Jenab said.
Is there any harm?
Some patients clearly want what naturopathy offers. So what’s the harm?
Health care systems that integrate alternative medicine legitimize it and lower the overall standard of care, Mr. Caulfield said. Most naturopathy claims are not backed by evidence, and making it available to patients amounts to deceiving them, he said.
“If there’s good science behind it, it’s not going to be alternative medicine; it’s going to be medicine,” Mr. Caulfield said.
Family physician Dr. Hislop said that refusing to order naturopath-recommended tests interferes with his relationships with patients and often requires lengthy conversations to explain the problems with naturopathy.
Naturopathic medicine can deter patients from seeking proven conventional treatments, which can put their health at risk, Dr. Gorski said.
Some naturopaths could potentially be harmful.
In 2017, a California woman died after receiving an IV preparation of curcumin, a chemical constituent in the Indian spice turmeric featured in alternative medicine. The U.S. Food and Drug Administration found that the treating ND mixed the curcumin emulsion product with ungraded castor oil that had a warning label stating: “CAUTION: For manufacturing or laboratory use only.”
Because naturopathic care is generally not covered by insurance, it can also be expensive for patients who pay out of pocket.
Ironically, the mainstream health care system helps create the environment in which naturopathic medicine thrives.
It offers patients a more relaxed and personal alternative to rushed visits with harried doctors scrambling to see the required number of patients in a day. By contrast, an initial visit with an ND might last a leisurely 60 minutes, with 30-minute follow-up appointments.
Mr. Caulfield acknowledged that the relaxed naturopathic approach can be more attractive to patients but said the answer is to reform the current system: “You don’t fix a broken arm by acupuncture.”
A version of this article first appeared on Medscape.com.
Jon Hislop, MD, PhD, hadn’t been in practice very long before patients began coming to him with requests to order tests that their naturopaths had recommended.
The family physician in North Vancouver, British Columbia, knew little about naturopathy but began researching it.
“I was finding that some of what the naturopaths were telling them was a little odd. Some of the tests they were asking for were unnecessary,” Dr. Hislop said.
The more he learned about naturopathy, the more appalled he became. He eventually took to Twitter, where he wages a campaign against naturopathy and alternative medicine.
“There is no alternative medicine,” he said. “There’s medicine and there’s other stuff. We need to stick to medicine and stay away from the other stuff.”
Dr. Hislop is not alone in his criticism of naturopathic medicine. Professional medical societies almost universally oppose naturopathy, but that has not stopped its spread or prevented it from becoming part of some health care systems.
Americans spent $30.2 billion on out-of-pocket complementary health care, according to a 2016 report from the National Institutes of Health. That includes everything from herbal supplements and massage therapy to chiropractic care.
What is naturopathic medicine?
Naturopathy came to the United States from Germany in the 1800s, but some of its practices are thousands of years old. Naturopathic treatments include homeopathy, IV vitamin infusions, acupuncture, Reiki, and herbal supplements.
Naturopathy is based on the belief that the body has an innate ability to heal itself. It discourages drugs and surgery in favor of supplements, herbs, and other so-called natural treatments. Much of it centers around addressing lifestyle issues and counseling patients to improve their diets, quit smoking, exercise more, lose weight, etc., in order to address the root causes of some health problems.
Practitioners are critical of Western medicine for what they regard as an over-reliance on drugs and technology and for treating symptoms rather than the causes of disease.
“We get a lot of people who are at the end of their ropes, people with hard-to-diagnose diseases who know they are sick but whose labs are normal,” said Jaquel Patterson, ND, former president of the American Association of Naturopathic Physicians (AANP) and medical director of a naturopathic practice in Connecticut.
Separate training and licensing
There are major differences among naturopaths.
At one extreme are unlicensed, self-taught “healers,” who can embrace everything from homeopathy to aromatherapy.
At the other end are naturopathic doctors (NDs), who are more likely to become part of health care systems. These caregivers are trained and licensed, though not by the same institutions as traditional physicians.
To be licensed, NDs must graduate from one of seven accredited naturopathic medical schools in the United States and Canada. In addition to a standard medical curriculum, schools require graduates to complete 4 years of training in clinical nutrition, acupuncture, homeopathic medicine, botanical medicine, physical medicine, and counseling. Medical students intern in clinical settings for 2 years.
NDs are eager to distinguish themselves from their uncredentialed counterparts.
“Some people go to a weekend class and call themselves naturopaths. That’s very concerning. I don’t want those people to be licensed either,” said Hallie Armstrong, ND, who practices in Michigan.
In the United States, there are 6,000 practicing NDs and an unknown number of unlicensed naturopathic healers.
Can naturopaths call themselves ‘physicians’?
Twenty-two states, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands have licensing or registration laws for naturopathic doctors. Three states – South Carolina, Tennessee, and Florida – prohibit practicing naturopathic medicine without a license, according to the AANP.
States that license NDs differ in what they permit them to do.
Nine states allow licensed NDs to use the term “physician,” although this is prohibited in seven states. Most licensed states allow naturopathic practitioners some prescribing authority, including the prescribing of many controlled substances, although only a few states permit full prescribing rights. Most states that license NDs allow them to prescribe and administer nonprescription therapeutic substances, drugs, and therapies.
Twelve states and the District of Columbia allow licensed naturopathic doctors to perform some minor procedures, such as stitching up wounds. Additionally, 13 states allow NDs to order diagnostic tests.
Although the AANP lobbies to get licensure in more states and to expand the activities that NDs can perform, the medical establishment in those states nearly always opposes the legislation, as do national organizations, such as the American Academy of Family Physicians and the American College of Physicians.
“They absolutely will not stop until they get licenses. They’ve done a really good job of selling themselves as legitimate health care professionals to state legislatures,” said David Gorski, MD, PhD, FACS, a surgical oncologist and managing editor of Science-Based Medicine, a blog that attacks unproven medical claims and defends traditional medicine. Naturopathy is a favorite target.
Are naturopaths gaining ground anyway?
Despite the opposition of the medical establishment and many individual health care professionals, a growing number of health care systems are adopting alternative medicine.
In 2018, the AANP stated that 28 prominent health systems, hospitals, and cancer treatment centers had one or more licensed NDs on staff. Among them were Cancer Treatment Centers of America, Cedars-Sinai, Columbia University’s Herbert Irving Comprehensive Cancer Center, and the Fred Hutchinson Cancer Research Center.
Other health care systems may not have NDs on staff but provide naturopathic treatments, usually under the heading of “complementary medicine” or “integrative medicine.” For example, the Cleveland Clinic’s Center for Integrative and Lifestyle Medicine offers acupuncture, Chinese herbal medicine, Reiki, yoga, and culinary medicine.
Critics find this appalling.
“I think it’s a mistake to integrate that kind of practice into a science-based health care setting. If we learned anything over the past year, it’s that medicine based on magical thinking is dangerous,” said Timothy Caulfield, LLM, FCAHS, research director at the Health Law Institute of the University of Alberta, Edmonton.
Dr. Gorski added: “I’m not exactly sure why doctors who should know better have become more accepting of practices that aren’t science-based or are outright quackery.”
Becoming part of the system
Beaumont Health, Michigan’s largest health care system, added integrative medicine in 2006 and hired its first naturopathic practitioners a year later.
The integrative practitioners began in oncology, offering such things as massage therapy, acupuncture, guided imagery, and Reiki. “Very quickly, people outside oncology began saying, ‘I’ve got a cardiology patient who would really benefit from this ... I’ve got a GI patient who could benefit from this...,’” said Maureen Anderson, MD, medical director of Beaumont Integrative Medicine.
Beaumont now offers integrative medicine at three locations. They average 20,000 visits a year and work with 50 to 60 practitioners, many of whom work part-time.
Because Michigan does not license NDs, their scope of practice at Beaumont is limited. They take patient histories, provide advice on nutrition, diet, and exercise, and prescribe herbs and supplements. Beaumont operates its own herbal and supplement pharmacy.
NDs work under the medical supervision of Dr. Anderson, an emergency medicine physician who became interested in naturopathy because she thought traditional medicine doesn’t do a good job of providing care for chronic conditions. Any initial skepticism on the part of the medical staff has been overcome by seeing the benefits naturopathy provides, Dr. Anderson said. The claim is echoed by Mr. Armstrong, an ND who works in the system part-time: “As soon as [doctors] understand our schooling and where we’re coming from and understand that we want to do the same things, then they’re very accepting.”
The University of California, Irvine, health care system has one of the largest naturopathic medicine programs in the country, the result of a $200 million donation in 2017 from a couple who champion alternative medicine. The Susan Samueli Integrative Health Institute includes 28 health care professionals, including MDs, NDs, RNs, acupuncturists, dietitians, yoga instructors, and others. It includes a research arm, which is focused primarily on acupuncture.
The alternative medicine offerings benefit the system, said Kim Hecht, DO, medical director of inpatient and ambulatory services at the Samueli Institute.
“I’m not against traditional medicine, because I think everything has a time and a place,” Dr. Hecht said. However, she rejects the idea that MDs can offer the same holistic approach as NDs.
“Medical science likes to say we’re interested in treating the whole person, but if you look at medical school courses, that’s not what’s being taught,” she said.
The chance to work within a traditional health care system was attractive to Arvin Jenab, ND, medical director of naturopathic medicine at the institute.
“It offers the opportunity to refine our medicine and trim the things that aren’t necessary or are controversial and concentrate on the things at the core of what we do,” he said.
UCI Health practices a conservative model of naturopathy that supports traditional practitioners, Mr. Jenab said.
Is there any harm?
Some patients clearly want what naturopathy offers. So what’s the harm?
Health care systems that integrate alternative medicine legitimize it and lower the overall standard of care, Mr. Caulfield said. Most naturopathy claims are not backed by evidence, and making it available to patients amounts to deceiving them, he said.
“If there’s good science behind it, it’s not going to be alternative medicine; it’s going to be medicine,” Mr. Caulfield said.
Family physician Dr. Hislop said that refusing to order naturopath-recommended tests interferes with his relationships with patients and often requires lengthy conversations to explain the problems with naturopathy.
Naturopathic medicine can deter patients from seeking proven conventional treatments, which can put their health at risk, Dr. Gorski said.
Some naturopaths could potentially be harmful.
In 2017, a California woman died after receiving an IV preparation of curcumin, a chemical constituent in the Indian spice turmeric featured in alternative medicine. The U.S. Food and Drug Administration found that the treating ND mixed the curcumin emulsion product with ungraded castor oil that had a warning label stating: “CAUTION: For manufacturing or laboratory use only.”
Because naturopathic care is generally not covered by insurance, it can also be expensive for patients who pay out of pocket.
Ironically, the mainstream health care system helps create the environment in which naturopathic medicine thrives.
It offers patients a more relaxed and personal alternative to rushed visits with harried doctors scrambling to see the required number of patients in a day. By contrast, an initial visit with an ND might last a leisurely 60 minutes, with 30-minute follow-up appointments.
Mr. Caulfield acknowledged that the relaxed naturopathic approach can be more attractive to patients but said the answer is to reform the current system: “You don’t fix a broken arm by acupuncture.”
A version of this article first appeared on Medscape.com.
Jon Hislop, MD, PhD, hadn’t been in practice very long before patients began coming to him with requests to order tests that their naturopaths had recommended.
The family physician in North Vancouver, British Columbia, knew little about naturopathy but began researching it.
“I was finding that some of what the naturopaths were telling them was a little odd. Some of the tests they were asking for were unnecessary,” Dr. Hislop said.
The more he learned about naturopathy, the more appalled he became. He eventually took to Twitter, where he wages a campaign against naturopathy and alternative medicine.
“There is no alternative medicine,” he said. “There’s medicine and there’s other stuff. We need to stick to medicine and stay away from the other stuff.”
Dr. Hislop is not alone in his criticism of naturopathic medicine. Professional medical societies almost universally oppose naturopathy, but that has not stopped its spread or prevented it from becoming part of some health care systems.
Americans spent $30.2 billion on out-of-pocket complementary health care, according to a 2016 report from the National Institutes of Health. That includes everything from herbal supplements and massage therapy to chiropractic care.
What is naturopathic medicine?
Naturopathy came to the United States from Germany in the 1800s, but some of its practices are thousands of years old. Naturopathic treatments include homeopathy, IV vitamin infusions, acupuncture, Reiki, and herbal supplements.
Naturopathy is based on the belief that the body has an innate ability to heal itself. It discourages drugs and surgery in favor of supplements, herbs, and other so-called natural treatments. Much of it centers around addressing lifestyle issues and counseling patients to improve their diets, quit smoking, exercise more, lose weight, etc., in order to address the root causes of some health problems.
Practitioners are critical of Western medicine for what they regard as an over-reliance on drugs and technology and for treating symptoms rather than the causes of disease.
“We get a lot of people who are at the end of their ropes, people with hard-to-diagnose diseases who know they are sick but whose labs are normal,” said Jaquel Patterson, ND, former president of the American Association of Naturopathic Physicians (AANP) and medical director of a naturopathic practice in Connecticut.
Separate training and licensing
There are major differences among naturopaths.
At one extreme are unlicensed, self-taught “healers,” who can embrace everything from homeopathy to aromatherapy.
At the other end are naturopathic doctors (NDs), who are more likely to become part of health care systems. These caregivers are trained and licensed, though not by the same institutions as traditional physicians.
To be licensed, NDs must graduate from one of seven accredited naturopathic medical schools in the United States and Canada. In addition to a standard medical curriculum, schools require graduates to complete 4 years of training in clinical nutrition, acupuncture, homeopathic medicine, botanical medicine, physical medicine, and counseling. Medical students intern in clinical settings for 2 years.
NDs are eager to distinguish themselves from their uncredentialed counterparts.
“Some people go to a weekend class and call themselves naturopaths. That’s very concerning. I don’t want those people to be licensed either,” said Hallie Armstrong, ND, who practices in Michigan.
In the United States, there are 6,000 practicing NDs and an unknown number of unlicensed naturopathic healers.
Can naturopaths call themselves ‘physicians’?
Twenty-two states, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands have licensing or registration laws for naturopathic doctors. Three states – South Carolina, Tennessee, and Florida – prohibit practicing naturopathic medicine without a license, according to the AANP.
States that license NDs differ in what they permit them to do.
Nine states allow licensed NDs to use the term “physician,” although this is prohibited in seven states. Most licensed states allow naturopathic practitioners some prescribing authority, including the prescribing of many controlled substances, although only a few states permit full prescribing rights. Most states that license NDs allow them to prescribe and administer nonprescription therapeutic substances, drugs, and therapies.
Twelve states and the District of Columbia allow licensed naturopathic doctors to perform some minor procedures, such as stitching up wounds. Additionally, 13 states allow NDs to order diagnostic tests.
Although the AANP lobbies to get licensure in more states and to expand the activities that NDs can perform, the medical establishment in those states nearly always opposes the legislation, as do national organizations, such as the American Academy of Family Physicians and the American College of Physicians.
“They absolutely will not stop until they get licenses. They’ve done a really good job of selling themselves as legitimate health care professionals to state legislatures,” said David Gorski, MD, PhD, FACS, a surgical oncologist and managing editor of Science-Based Medicine, a blog that attacks unproven medical claims and defends traditional medicine. Naturopathy is a favorite target.
Are naturopaths gaining ground anyway?
Despite the opposition of the medical establishment and many individual health care professionals, a growing number of health care systems are adopting alternative medicine.
In 2018, the AANP stated that 28 prominent health systems, hospitals, and cancer treatment centers had one or more licensed NDs on staff. Among them were Cancer Treatment Centers of America, Cedars-Sinai, Columbia University’s Herbert Irving Comprehensive Cancer Center, and the Fred Hutchinson Cancer Research Center.
Other health care systems may not have NDs on staff but provide naturopathic treatments, usually under the heading of “complementary medicine” or “integrative medicine.” For example, the Cleveland Clinic’s Center for Integrative and Lifestyle Medicine offers acupuncture, Chinese herbal medicine, Reiki, yoga, and culinary medicine.
Critics find this appalling.
“I think it’s a mistake to integrate that kind of practice into a science-based health care setting. If we learned anything over the past year, it’s that medicine based on magical thinking is dangerous,” said Timothy Caulfield, LLM, FCAHS, research director at the Health Law Institute of the University of Alberta, Edmonton.
Dr. Gorski added: “I’m not exactly sure why doctors who should know better have become more accepting of practices that aren’t science-based or are outright quackery.”
Becoming part of the system
Beaumont Health, Michigan’s largest health care system, added integrative medicine in 2006 and hired its first naturopathic practitioners a year later.
The integrative practitioners began in oncology, offering such things as massage therapy, acupuncture, guided imagery, and Reiki. “Very quickly, people outside oncology began saying, ‘I’ve got a cardiology patient who would really benefit from this ... I’ve got a GI patient who could benefit from this...,’” said Maureen Anderson, MD, medical director of Beaumont Integrative Medicine.
Beaumont now offers integrative medicine at three locations. They average 20,000 visits a year and work with 50 to 60 practitioners, many of whom work part-time.
Because Michigan does not license NDs, their scope of practice at Beaumont is limited. They take patient histories, provide advice on nutrition, diet, and exercise, and prescribe herbs and supplements. Beaumont operates its own herbal and supplement pharmacy.
NDs work under the medical supervision of Dr. Anderson, an emergency medicine physician who became interested in naturopathy because she thought traditional medicine doesn’t do a good job of providing care for chronic conditions. Any initial skepticism on the part of the medical staff has been overcome by seeing the benefits naturopathy provides, Dr. Anderson said. The claim is echoed by Mr. Armstrong, an ND who works in the system part-time: “As soon as [doctors] understand our schooling and where we’re coming from and understand that we want to do the same things, then they’re very accepting.”
The University of California, Irvine, health care system has one of the largest naturopathic medicine programs in the country, the result of a $200 million donation in 2017 from a couple who champion alternative medicine. The Susan Samueli Integrative Health Institute includes 28 health care professionals, including MDs, NDs, RNs, acupuncturists, dietitians, yoga instructors, and others. It includes a research arm, which is focused primarily on acupuncture.
The alternative medicine offerings benefit the system, said Kim Hecht, DO, medical director of inpatient and ambulatory services at the Samueli Institute.
“I’m not against traditional medicine, because I think everything has a time and a place,” Dr. Hecht said. However, she rejects the idea that MDs can offer the same holistic approach as NDs.
“Medical science likes to say we’re interested in treating the whole person, but if you look at medical school courses, that’s not what’s being taught,” she said.
The chance to work within a traditional health care system was attractive to Arvin Jenab, ND, medical director of naturopathic medicine at the institute.
“It offers the opportunity to refine our medicine and trim the things that aren’t necessary or are controversial and concentrate on the things at the core of what we do,” he said.
UCI Health practices a conservative model of naturopathy that supports traditional practitioners, Mr. Jenab said.
Is there any harm?
Some patients clearly want what naturopathy offers. So what’s the harm?
Health care systems that integrate alternative medicine legitimize it and lower the overall standard of care, Mr. Caulfield said. Most naturopathy claims are not backed by evidence, and making it available to patients amounts to deceiving them, he said.
“If there’s good science behind it, it’s not going to be alternative medicine; it’s going to be medicine,” Mr. Caulfield said.
Family physician Dr. Hislop said that refusing to order naturopath-recommended tests interferes with his relationships with patients and often requires lengthy conversations to explain the problems with naturopathy.
Naturopathic medicine can deter patients from seeking proven conventional treatments, which can put their health at risk, Dr. Gorski said.
Some naturopaths could potentially be harmful.
In 2017, a California woman died after receiving an IV preparation of curcumin, a chemical constituent in the Indian spice turmeric featured in alternative medicine. The U.S. Food and Drug Administration found that the treating ND mixed the curcumin emulsion product with ungraded castor oil that had a warning label stating: “CAUTION: For manufacturing or laboratory use only.”
Because naturopathic care is generally not covered by insurance, it can also be expensive for patients who pay out of pocket.
Ironically, the mainstream health care system helps create the environment in which naturopathic medicine thrives.
It offers patients a more relaxed and personal alternative to rushed visits with harried doctors scrambling to see the required number of patients in a day. By contrast, an initial visit with an ND might last a leisurely 60 minutes, with 30-minute follow-up appointments.
Mr. Caulfield acknowledged that the relaxed naturopathic approach can be more attractive to patients but said the answer is to reform the current system: “You don’t fix a broken arm by acupuncture.”
A version of this article first appeared on Medscape.com.
New STRENGTH analysis reignites debate on omega-3 CV benefits
Questions over the cardiovascular benefits shown in the REDUCE-IT trial with icosapent ethyl, a high-dose eicosapentaenoic acid (EPA) product, have been reignited with a new analysis from the STRENGTH trial showing no benefit of a high-dose combined omega-3 fatty acid product in patients who achieved the highest EPA levels and no harm in those with the highest levels of docosahexaenoic acid (DHA).
STRENGTH investigator Steven Nissen, MD, said these new results add to concerns about the positive result in the previously reported REDUCE-IT trial and suggest that “there is no strong evidence of a benefit of fish oil in preventing major cardiovascular events.”
But Dr. Nissen, who is chair of the department of cardiovascular medicine at the Cleveland Clinic in Ohio, pointed out evidence of harm, with both REDUCE-IT and STRENGTH showing an increase in atrial fibrillation with the high-dose omega-3 fatty acid products.
“Fish oils increase the risk of atrial fibrillation substantially, and there is no solid evidence that they help the heart in any way,” he stated.
The new STRENGTH analysis was presented at the annual scientific sessions of the American College of Cardiology. and was simultaneously published in JAMA Cardiology.
The REDUCE-IT trial showed a large 25% relative-risk reduction in cardiovascular events in patients taking icosapent ethyl (Vascepa, Amarin), a high-dose purified formulation of EPA, compared with patients taking a mineral oil placebo. But a similar trial, STRENGTH, showed no effect of a similar high dose of the mixed EPA/DHA product (Epanova, AstraZeneca), compared with a corn oil placebo.
The different results from these two studies have led to many questions about how the benefits seen in REDUCE-IT were brought about, and why they weren’t replicated in the STRENGTH study.
Dr. Nissen noted that several hypotheses have been proposed. These include a potential adverse effect of the mineral oil placebo in the REDUCE-IT trial, which may have elevated risk in the placebo treatment group and led to a false-positive result for icosapent ethyl. Another possibility is that the moderately higher plasma levels of EPA achieved in REDUCE-IT were responsible for the observed benefits or that the coadministration of DHA in STRENGTH may have counteracted the potential beneficial effects of EPA.
The current post hoc analysis of STRENGTH was conducted to address these latter two possibilities. It aimed to assess the association between cardiovascular outcomes and achieved levels of EPA, DHA, or changes in levels of these fatty acids.
“In our new analysis, among patients treated with fish oil, we found no evidence that EPA is beneficial or that DHA is harmful,” Dr. Nissen said.
Results of the new analysis showed an absence of a benefit from achieving high levels of EPA or harm from achieving high levels of DHA which, the authors say, “strengthens the concerns that the choice of comparator may have influenced the divergent results observed in the two trials.”
“Unlike corn oil, which is inert, mineral oil has major adverse effects, increasing LDL by 10.9% and CRP [C-reactive protein] by 32% in the REDUCE-IT trial,” Dr. Nissen said. “If you give a toxic placebo, then the active drug may falsely look really good.”
The STRENGTH trial randomly assigned 13,078 individuals at high risk for major cardiovascular events to receive 4 g daily of the EPA/DHA combined product (omega-3 carboxylic acid) or corn oil as the placebo. Main results, reported previously, showed no difference between the two groups in terms of the primary outcome – a composite of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or unstable angina requiring hospitalization.
The current analysis, in 10,382 patients with available omega-3 fatty acid levels, looked at event rates according to tertiles of achieved EPA and DHA levels. The median plasma EPA level for patients taking the omega-3 product was 89 mcg/mL, with the top tertile achieving levels of 151 mcg/mL (a 443% increase). Dr. Nissen pointed out that this was higher than the median level of EPA reported in the REDUCE-IT trial (144 mcg/mL).
The median level of DHA was 91 mcg/mL, rising to 118 mcg/mL (a 68% increase) in the top tertile in the STRENGTH analysis.
Results showed no difference in the occurrence of the prespecified primary outcome among patients treated with omega-3 carboxylic acid who were in the top tertile of achieved EPA levels at 1 year (event rate, 11.3%), compared with patients treated with corn oil (11.0%), a nonsignificant difference (hazard ratio, 0.98; P = .81).
For DHA, patients in the top tertile of achieved DHA levels had an event rate of 11.4% vs. 11.0% in the corn oil group, also a nonsignificant difference (HR, 1.02; P = .85)
Sensitivity analyses based on the highest tertile of change in EPA or DHA levels showed similarly neutral results.
Because plasma levels may not reflect tissue levels of EPA or DHA, additional analyses assessed red blood cell EPA and DHA levels, neither of which showed any evidence of benefit or harm.
“These findings suggest that supplementation of omega-3 fatty acids in high-risk cardiovascular patients is neutral even at the highest achieved levels,” Dr. Nissen said. “And, in the context of increased risk of atrial fibrillation in omega-3 trials, they cast uncertainty over whether there is net benefit or harm with any omega-3 preparation,” he concluded.
He suggested that the choice of placebo comparator may play an important role in determining outcome for trials of omega-3 products, adding that further research is needed with trials specifically designed to compare corn oil with mineral oil and compare purified EPA with other formulations of omega-3 fatty acids.
At an press conference, Dr. Nissen said he could not recommend use of omega-3 fatty acid products for cardiovascular risk reduction given the uncertainty over the benefit in REDUCE-IT.
“We need replication, and the problem is STRENGTH did not replicate REDUCE-IT,” he stated.
REDUCE-IT investigator responds
The discussant of the STRENGTH analysis at the ACC presentation, Deepak L. Bhatt, MD, who was lead investigator of the REDUCE-IT trial, suggested that one conclusion could be that “an absence of a relationship in a negative trial doesn’t tell us that much other than that specific drug doesn’t work.”
Dr. Bhatt, who is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that comparisons should not be made between different trials using different products.
“I commend the STRENGTH investigators on a well-conducted trial that provided a definitive answer about the specific drug they studied, finding no benefit. But in a completely negative trial, I wouldn’t necessarily expect to see a relationship between any biomarker and outcome,” he said.
“With respect to icosapent ethyl (pure EPA), every cardiovascular trial to date has been positive: REDUCE-IT (randomized, placebo-controlled), JELIS (randomized, no placebo), EVAPORATE (randomized, placebo-controlled), CHERRY (randomized, no placebo), and some smaller ones,” Dr. Bhatt added. “Both REDUCE-IT and JELIS found associations between higher levels of EPA and lower rates of cardiovascular events, suggesting that higher EPA levels attained specifically with icosapent ethyl are beneficial.”
Pointing out that all the glucagonlike peptide–1 agonists lower glucose, for example, but not all reduce cardiovascular events, Dr. Bhatt said it was best to focus on clinical trial results and not overly focus on biomarker changes.
“Yes, the drug in STRENGTH raised EPA (and raised DHA, as well as lowering triglycerides), but the drug in REDUCE-IT and JELIS raised EPA much more, without raising DHA – and more importantly, the increase in EPA was via a totally different drug, with many different properties,” he added.
In his discussion of the study at the ACC presentation, Dr. Bhatt pointed out that in the STRENGTH trial overall there was no reduction in major adverse cardiovascular events despite a 19% reduction in triglycerides, which he said was a “very interesting disconnect.” He asked Dr. Nissen what he thought the reason was for the observation in this analysis of no relationship between EPA or DHA level and triglyceride reduction.
Dr. Nissen said that was an interesting point. “When we look at the two trials, they both reduced triglyceride levels by an almost identical amount, 19%, but we don’t see a relationship with that and EPA levels achieved.” He suggested this may be because of different threshold levels.
Dr. Bhatt also noted that high-intensity statin use was lower in the patients with higher EPA levels in the STRENGTH analysis, but Dr. Nissen countered: “I don’t think that was enough of a difference to explain the lack of an effect.”
Invited commentator on the new analysis at an ACC press conference, Eileen Handberg, PhD, said it was important to try to understand the reasons behind the different results of the STRENGTH and REDUCE-IT trials. “These new findings are important because they explain potentially why these outcomes are different,” she stated.
Dr. Handberg, who is professor of medicine at the University of Florida, Gainesville, said she hoped the additional research called for by Dr. Nissen would go ahead as a head-to-head study of the two omega-3 products or of the two different placebo oils.
The STRENGTH trial was sponsored by Astra Zeneca. Dr. Nissen reports research grants from AbbVie, Amgen, Astra Zeneca, Eli Lilly, Esperion Therapeutics, MEDTRONIC, MyoKardia, Novartis, Novo Nordisk, Pfizer, and Silence Therapeutics. Dr. Bhatt reports constant fees/honoraria from CellProthera, Elsevier Practice Update Cardiology, K2P, Level Ex, Medtelligence, MJH Life Sciences, and WebMD; data safety monitoring board activities with Contego; other roles with TobeSoft, Belvoir Publications, Cardax, Cereno Scientific, Clinical Cardiology, Elsevier, HMP Global, Janssen Pharmaceuticals, Journal of Invasive Cardiology, Medscape Cardiology, Merck, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Slack Publications/Cardiology Research Foundation; and research grants from Abbott, Afimmune, Amarin, Amgen, Astra Zeneca, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Cardax, Chiesi, Eisai, Eli Lilly, Ethicon, FlowCo, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, MEDTRONIC, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, Takeda, and The Medicines Company.
A version of this article first appeared on Medscape.com.
Questions over the cardiovascular benefits shown in the REDUCE-IT trial with icosapent ethyl, a high-dose eicosapentaenoic acid (EPA) product, have been reignited with a new analysis from the STRENGTH trial showing no benefit of a high-dose combined omega-3 fatty acid product in patients who achieved the highest EPA levels and no harm in those with the highest levels of docosahexaenoic acid (DHA).
STRENGTH investigator Steven Nissen, MD, said these new results add to concerns about the positive result in the previously reported REDUCE-IT trial and suggest that “there is no strong evidence of a benefit of fish oil in preventing major cardiovascular events.”
But Dr. Nissen, who is chair of the department of cardiovascular medicine at the Cleveland Clinic in Ohio, pointed out evidence of harm, with both REDUCE-IT and STRENGTH showing an increase in atrial fibrillation with the high-dose omega-3 fatty acid products.
“Fish oils increase the risk of atrial fibrillation substantially, and there is no solid evidence that they help the heart in any way,” he stated.
The new STRENGTH analysis was presented at the annual scientific sessions of the American College of Cardiology. and was simultaneously published in JAMA Cardiology.
The REDUCE-IT trial showed a large 25% relative-risk reduction in cardiovascular events in patients taking icosapent ethyl (Vascepa, Amarin), a high-dose purified formulation of EPA, compared with patients taking a mineral oil placebo. But a similar trial, STRENGTH, showed no effect of a similar high dose of the mixed EPA/DHA product (Epanova, AstraZeneca), compared with a corn oil placebo.
The different results from these two studies have led to many questions about how the benefits seen in REDUCE-IT were brought about, and why they weren’t replicated in the STRENGTH study.
Dr. Nissen noted that several hypotheses have been proposed. These include a potential adverse effect of the mineral oil placebo in the REDUCE-IT trial, which may have elevated risk in the placebo treatment group and led to a false-positive result for icosapent ethyl. Another possibility is that the moderately higher plasma levels of EPA achieved in REDUCE-IT were responsible for the observed benefits or that the coadministration of DHA in STRENGTH may have counteracted the potential beneficial effects of EPA.
The current post hoc analysis of STRENGTH was conducted to address these latter two possibilities. It aimed to assess the association between cardiovascular outcomes and achieved levels of EPA, DHA, or changes in levels of these fatty acids.
“In our new analysis, among patients treated with fish oil, we found no evidence that EPA is beneficial or that DHA is harmful,” Dr. Nissen said.
Results of the new analysis showed an absence of a benefit from achieving high levels of EPA or harm from achieving high levels of DHA which, the authors say, “strengthens the concerns that the choice of comparator may have influenced the divergent results observed in the two trials.”
“Unlike corn oil, which is inert, mineral oil has major adverse effects, increasing LDL by 10.9% and CRP [C-reactive protein] by 32% in the REDUCE-IT trial,” Dr. Nissen said. “If you give a toxic placebo, then the active drug may falsely look really good.”
The STRENGTH trial randomly assigned 13,078 individuals at high risk for major cardiovascular events to receive 4 g daily of the EPA/DHA combined product (omega-3 carboxylic acid) or corn oil as the placebo. Main results, reported previously, showed no difference between the two groups in terms of the primary outcome – a composite of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or unstable angina requiring hospitalization.
The current analysis, in 10,382 patients with available omega-3 fatty acid levels, looked at event rates according to tertiles of achieved EPA and DHA levels. The median plasma EPA level for patients taking the omega-3 product was 89 mcg/mL, with the top tertile achieving levels of 151 mcg/mL (a 443% increase). Dr. Nissen pointed out that this was higher than the median level of EPA reported in the REDUCE-IT trial (144 mcg/mL).
The median level of DHA was 91 mcg/mL, rising to 118 mcg/mL (a 68% increase) in the top tertile in the STRENGTH analysis.
Results showed no difference in the occurrence of the prespecified primary outcome among patients treated with omega-3 carboxylic acid who were in the top tertile of achieved EPA levels at 1 year (event rate, 11.3%), compared with patients treated with corn oil (11.0%), a nonsignificant difference (hazard ratio, 0.98; P = .81).
For DHA, patients in the top tertile of achieved DHA levels had an event rate of 11.4% vs. 11.0% in the corn oil group, also a nonsignificant difference (HR, 1.02; P = .85)
Sensitivity analyses based on the highest tertile of change in EPA or DHA levels showed similarly neutral results.
Because plasma levels may not reflect tissue levels of EPA or DHA, additional analyses assessed red blood cell EPA and DHA levels, neither of which showed any evidence of benefit or harm.
“These findings suggest that supplementation of omega-3 fatty acids in high-risk cardiovascular patients is neutral even at the highest achieved levels,” Dr. Nissen said. “And, in the context of increased risk of atrial fibrillation in omega-3 trials, they cast uncertainty over whether there is net benefit or harm with any omega-3 preparation,” he concluded.
He suggested that the choice of placebo comparator may play an important role in determining outcome for trials of omega-3 products, adding that further research is needed with trials specifically designed to compare corn oil with mineral oil and compare purified EPA with other formulations of omega-3 fatty acids.
At an press conference, Dr. Nissen said he could not recommend use of omega-3 fatty acid products for cardiovascular risk reduction given the uncertainty over the benefit in REDUCE-IT.
“We need replication, and the problem is STRENGTH did not replicate REDUCE-IT,” he stated.
REDUCE-IT investigator responds
The discussant of the STRENGTH analysis at the ACC presentation, Deepak L. Bhatt, MD, who was lead investigator of the REDUCE-IT trial, suggested that one conclusion could be that “an absence of a relationship in a negative trial doesn’t tell us that much other than that specific drug doesn’t work.”
Dr. Bhatt, who is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that comparisons should not be made between different trials using different products.
“I commend the STRENGTH investigators on a well-conducted trial that provided a definitive answer about the specific drug they studied, finding no benefit. But in a completely negative trial, I wouldn’t necessarily expect to see a relationship between any biomarker and outcome,” he said.
“With respect to icosapent ethyl (pure EPA), every cardiovascular trial to date has been positive: REDUCE-IT (randomized, placebo-controlled), JELIS (randomized, no placebo), EVAPORATE (randomized, placebo-controlled), CHERRY (randomized, no placebo), and some smaller ones,” Dr. Bhatt added. “Both REDUCE-IT and JELIS found associations between higher levels of EPA and lower rates of cardiovascular events, suggesting that higher EPA levels attained specifically with icosapent ethyl are beneficial.”
Pointing out that all the glucagonlike peptide–1 agonists lower glucose, for example, but not all reduce cardiovascular events, Dr. Bhatt said it was best to focus on clinical trial results and not overly focus on biomarker changes.
“Yes, the drug in STRENGTH raised EPA (and raised DHA, as well as lowering triglycerides), but the drug in REDUCE-IT and JELIS raised EPA much more, without raising DHA – and more importantly, the increase in EPA was via a totally different drug, with many different properties,” he added.
In his discussion of the study at the ACC presentation, Dr. Bhatt pointed out that in the STRENGTH trial overall there was no reduction in major adverse cardiovascular events despite a 19% reduction in triglycerides, which he said was a “very interesting disconnect.” He asked Dr. Nissen what he thought the reason was for the observation in this analysis of no relationship between EPA or DHA level and triglyceride reduction.
Dr. Nissen said that was an interesting point. “When we look at the two trials, they both reduced triglyceride levels by an almost identical amount, 19%, but we don’t see a relationship with that and EPA levels achieved.” He suggested this may be because of different threshold levels.
Dr. Bhatt also noted that high-intensity statin use was lower in the patients with higher EPA levels in the STRENGTH analysis, but Dr. Nissen countered: “I don’t think that was enough of a difference to explain the lack of an effect.”
Invited commentator on the new analysis at an ACC press conference, Eileen Handberg, PhD, said it was important to try to understand the reasons behind the different results of the STRENGTH and REDUCE-IT trials. “These new findings are important because they explain potentially why these outcomes are different,” she stated.
Dr. Handberg, who is professor of medicine at the University of Florida, Gainesville, said she hoped the additional research called for by Dr. Nissen would go ahead as a head-to-head study of the two omega-3 products or of the two different placebo oils.
The STRENGTH trial was sponsored by Astra Zeneca. Dr. Nissen reports research grants from AbbVie, Amgen, Astra Zeneca, Eli Lilly, Esperion Therapeutics, MEDTRONIC, MyoKardia, Novartis, Novo Nordisk, Pfizer, and Silence Therapeutics. Dr. Bhatt reports constant fees/honoraria from CellProthera, Elsevier Practice Update Cardiology, K2P, Level Ex, Medtelligence, MJH Life Sciences, and WebMD; data safety monitoring board activities with Contego; other roles with TobeSoft, Belvoir Publications, Cardax, Cereno Scientific, Clinical Cardiology, Elsevier, HMP Global, Janssen Pharmaceuticals, Journal of Invasive Cardiology, Medscape Cardiology, Merck, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Slack Publications/Cardiology Research Foundation; and research grants from Abbott, Afimmune, Amarin, Amgen, Astra Zeneca, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Cardax, Chiesi, Eisai, Eli Lilly, Ethicon, FlowCo, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, MEDTRONIC, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, Takeda, and The Medicines Company.
A version of this article first appeared on Medscape.com.
Questions over the cardiovascular benefits shown in the REDUCE-IT trial with icosapent ethyl, a high-dose eicosapentaenoic acid (EPA) product, have been reignited with a new analysis from the STRENGTH trial showing no benefit of a high-dose combined omega-3 fatty acid product in patients who achieved the highest EPA levels and no harm in those with the highest levels of docosahexaenoic acid (DHA).
STRENGTH investigator Steven Nissen, MD, said these new results add to concerns about the positive result in the previously reported REDUCE-IT trial and suggest that “there is no strong evidence of a benefit of fish oil in preventing major cardiovascular events.”
But Dr. Nissen, who is chair of the department of cardiovascular medicine at the Cleveland Clinic in Ohio, pointed out evidence of harm, with both REDUCE-IT and STRENGTH showing an increase in atrial fibrillation with the high-dose omega-3 fatty acid products.
“Fish oils increase the risk of atrial fibrillation substantially, and there is no solid evidence that they help the heart in any way,” he stated.
The new STRENGTH analysis was presented at the annual scientific sessions of the American College of Cardiology. and was simultaneously published in JAMA Cardiology.
The REDUCE-IT trial showed a large 25% relative-risk reduction in cardiovascular events in patients taking icosapent ethyl (Vascepa, Amarin), a high-dose purified formulation of EPA, compared with patients taking a mineral oil placebo. But a similar trial, STRENGTH, showed no effect of a similar high dose of the mixed EPA/DHA product (Epanova, AstraZeneca), compared with a corn oil placebo.
The different results from these two studies have led to many questions about how the benefits seen in REDUCE-IT were brought about, and why they weren’t replicated in the STRENGTH study.
Dr. Nissen noted that several hypotheses have been proposed. These include a potential adverse effect of the mineral oil placebo in the REDUCE-IT trial, which may have elevated risk in the placebo treatment group and led to a false-positive result for icosapent ethyl. Another possibility is that the moderately higher plasma levels of EPA achieved in REDUCE-IT were responsible for the observed benefits or that the coadministration of DHA in STRENGTH may have counteracted the potential beneficial effects of EPA.
The current post hoc analysis of STRENGTH was conducted to address these latter two possibilities. It aimed to assess the association between cardiovascular outcomes and achieved levels of EPA, DHA, or changes in levels of these fatty acids.
“In our new analysis, among patients treated with fish oil, we found no evidence that EPA is beneficial or that DHA is harmful,” Dr. Nissen said.
Results of the new analysis showed an absence of a benefit from achieving high levels of EPA or harm from achieving high levels of DHA which, the authors say, “strengthens the concerns that the choice of comparator may have influenced the divergent results observed in the two trials.”
“Unlike corn oil, which is inert, mineral oil has major adverse effects, increasing LDL by 10.9% and CRP [C-reactive protein] by 32% in the REDUCE-IT trial,” Dr. Nissen said. “If you give a toxic placebo, then the active drug may falsely look really good.”
The STRENGTH trial randomly assigned 13,078 individuals at high risk for major cardiovascular events to receive 4 g daily of the EPA/DHA combined product (omega-3 carboxylic acid) or corn oil as the placebo. Main results, reported previously, showed no difference between the two groups in terms of the primary outcome – a composite of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or unstable angina requiring hospitalization.
The current analysis, in 10,382 patients with available omega-3 fatty acid levels, looked at event rates according to tertiles of achieved EPA and DHA levels. The median plasma EPA level for patients taking the omega-3 product was 89 mcg/mL, with the top tertile achieving levels of 151 mcg/mL (a 443% increase). Dr. Nissen pointed out that this was higher than the median level of EPA reported in the REDUCE-IT trial (144 mcg/mL).
The median level of DHA was 91 mcg/mL, rising to 118 mcg/mL (a 68% increase) in the top tertile in the STRENGTH analysis.
Results showed no difference in the occurrence of the prespecified primary outcome among patients treated with omega-3 carboxylic acid who were in the top tertile of achieved EPA levels at 1 year (event rate, 11.3%), compared with patients treated with corn oil (11.0%), a nonsignificant difference (hazard ratio, 0.98; P = .81).
For DHA, patients in the top tertile of achieved DHA levels had an event rate of 11.4% vs. 11.0% in the corn oil group, also a nonsignificant difference (HR, 1.02; P = .85)
Sensitivity analyses based on the highest tertile of change in EPA or DHA levels showed similarly neutral results.
Because plasma levels may not reflect tissue levels of EPA or DHA, additional analyses assessed red blood cell EPA and DHA levels, neither of which showed any evidence of benefit or harm.
“These findings suggest that supplementation of omega-3 fatty acids in high-risk cardiovascular patients is neutral even at the highest achieved levels,” Dr. Nissen said. “And, in the context of increased risk of atrial fibrillation in omega-3 trials, they cast uncertainty over whether there is net benefit or harm with any omega-3 preparation,” he concluded.
He suggested that the choice of placebo comparator may play an important role in determining outcome for trials of omega-3 products, adding that further research is needed with trials specifically designed to compare corn oil with mineral oil and compare purified EPA with other formulations of omega-3 fatty acids.
At an press conference, Dr. Nissen said he could not recommend use of omega-3 fatty acid products for cardiovascular risk reduction given the uncertainty over the benefit in REDUCE-IT.
“We need replication, and the problem is STRENGTH did not replicate REDUCE-IT,” he stated.
REDUCE-IT investigator responds
The discussant of the STRENGTH analysis at the ACC presentation, Deepak L. Bhatt, MD, who was lead investigator of the REDUCE-IT trial, suggested that one conclusion could be that “an absence of a relationship in a negative trial doesn’t tell us that much other than that specific drug doesn’t work.”
Dr. Bhatt, who is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that comparisons should not be made between different trials using different products.
“I commend the STRENGTH investigators on a well-conducted trial that provided a definitive answer about the specific drug they studied, finding no benefit. But in a completely negative trial, I wouldn’t necessarily expect to see a relationship between any biomarker and outcome,” he said.
“With respect to icosapent ethyl (pure EPA), every cardiovascular trial to date has been positive: REDUCE-IT (randomized, placebo-controlled), JELIS (randomized, no placebo), EVAPORATE (randomized, placebo-controlled), CHERRY (randomized, no placebo), and some smaller ones,” Dr. Bhatt added. “Both REDUCE-IT and JELIS found associations between higher levels of EPA and lower rates of cardiovascular events, suggesting that higher EPA levels attained specifically with icosapent ethyl are beneficial.”
Pointing out that all the glucagonlike peptide–1 agonists lower glucose, for example, but not all reduce cardiovascular events, Dr. Bhatt said it was best to focus on clinical trial results and not overly focus on biomarker changes.
“Yes, the drug in STRENGTH raised EPA (and raised DHA, as well as lowering triglycerides), but the drug in REDUCE-IT and JELIS raised EPA much more, without raising DHA – and more importantly, the increase in EPA was via a totally different drug, with many different properties,” he added.
In his discussion of the study at the ACC presentation, Dr. Bhatt pointed out that in the STRENGTH trial overall there was no reduction in major adverse cardiovascular events despite a 19% reduction in triglycerides, which he said was a “very interesting disconnect.” He asked Dr. Nissen what he thought the reason was for the observation in this analysis of no relationship between EPA or DHA level and triglyceride reduction.
Dr. Nissen said that was an interesting point. “When we look at the two trials, they both reduced triglyceride levels by an almost identical amount, 19%, but we don’t see a relationship with that and EPA levels achieved.” He suggested this may be because of different threshold levels.
Dr. Bhatt also noted that high-intensity statin use was lower in the patients with higher EPA levels in the STRENGTH analysis, but Dr. Nissen countered: “I don’t think that was enough of a difference to explain the lack of an effect.”
Invited commentator on the new analysis at an ACC press conference, Eileen Handberg, PhD, said it was important to try to understand the reasons behind the different results of the STRENGTH and REDUCE-IT trials. “These new findings are important because they explain potentially why these outcomes are different,” she stated.
Dr. Handberg, who is professor of medicine at the University of Florida, Gainesville, said she hoped the additional research called for by Dr. Nissen would go ahead as a head-to-head study of the two omega-3 products or of the two different placebo oils.
The STRENGTH trial was sponsored by Astra Zeneca. Dr. Nissen reports research grants from AbbVie, Amgen, Astra Zeneca, Eli Lilly, Esperion Therapeutics, MEDTRONIC, MyoKardia, Novartis, Novo Nordisk, Pfizer, and Silence Therapeutics. Dr. Bhatt reports constant fees/honoraria from CellProthera, Elsevier Practice Update Cardiology, K2P, Level Ex, Medtelligence, MJH Life Sciences, and WebMD; data safety monitoring board activities with Contego; other roles with TobeSoft, Belvoir Publications, Cardax, Cereno Scientific, Clinical Cardiology, Elsevier, HMP Global, Janssen Pharmaceuticals, Journal of Invasive Cardiology, Medscape Cardiology, Merck, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Slack Publications/Cardiology Research Foundation; and research grants from Abbott, Afimmune, Amarin, Amgen, Astra Zeneca, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Cardax, Chiesi, Eisai, Eli Lilly, Ethicon, FlowCo, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, MEDTRONIC, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, Takeda, and The Medicines Company.
A version of this article first appeared on Medscape.com.
FROM ACC 2021
Ultrasound renal denervation drops BP in patients on triple therapy
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
FROM ACC 2021
FLOWER-MI: FFR-guided complete revascularization shows no advantage in STEMI
For patients with ST-elevated myocardial infarction (STEMI) undergoing complete revascularization, percutaneous coronary interventions (PCI) guided by fractional flow reserve (FFR) relative to angiography-guided PCI do not result in significantly lower risk of death or events, according to data from the randomized FLOWER-MI trial.
Rather, the events at 1 year were numerically lower among those randomized to the angiography-guided approach, according to the principal investigator of the trial, Etienne Puymirat, MD, PhD.
Prior studies showing an advantage for FFR-guided PCI in patients with coronary syndromes provided the hypothesis that FFR-guided PCI would also be superior for guiding PCI in STEMI patients. In the multicenter FAME trial, for example, FFR-guided PCI for patients with multivessel disease was associated with fewer stent placements (P < .001) and a nearly 30% lower rate of events at 1 year (P = .02).
While the advantage of complete revascularization, meaning PCI treatment of nonculprit as well as culprit lesions, has already been shown to be a better strategy than treatment of culprit lesions alone, FLOWER-MI is the first large study to compare FFR to angiography for guiding this approach to STEMI patients with multivessel disease, said Dr. Puymirat of Hôpital Européen George Pompidou, Paris, at the annual scientific sessions of the American College of Cardiology.
In this trial, involving multiple centers in France, STEMI patients were eligible for randomization if they had successful PCI of a culprit lesion and 50% or greater stenosis in at least one additional nonculprit lesion. The complete revascularization, whether patients were randomized to PCI guided by angiography or FFR, was performed during the index hospital admission. Patient management and follow-up was otherwise the same.
After a small number of exclusions, the intention-to-treat populations were 577 patients in the angiography-guided group and 586 in the FFR-guided group. The characteristics of the groups were well matched with an average age of about 62 years and similar rates of risk factors, such as hypertension and diabetes.
Angiography guidance just as good
The primary outcome was a composite of all-cause mortality, nonfatal MI, and unplanned revascularization. By hazard ratio, the risk of having one of these events within 1 year of PCI was numerically greater, at 32 in the FFR-guided group and 24 in the angiography-guided group, but the difference was not statistically significant (1.32; P = .31).
However, the total rate of events was low (5.5% vs. 4.2% for the angiography-guided and FFR-guided groups, respectively) and the confidence intervals were wide (95% CI, 0.78-2.23). This was also true of the components of the primary outcome.
No signal for a difference between strategies could be derived from these components, which included a higher rate of MI in the FFR-guided group (3.1% vs. 1.7%) but a lower rate of death (1.5% vs. 1.7%).
Unplanned hospitalizations leading to revascularization rates were also low (1.9% and 2.6% for angiography-guided and FFR-guided PCI, respectively), although it was reported that the rate of revascularization for nonculprit lesions was about twice as high in the FFR group (53.3% vs. 27.3%).
At 1 year, there were also low rates and no significant differences in a list of secondary outcomes that included hospitalization for recurrent ischemia or heart failure, stent thrombosis, and revascularization. As within the primary composite outcome, no pattern could be seen in the secondary events, some of which were numerically more common in the FFR-guided group and some numerically lower.
In a cost-efficacy analysis, the median per-patient cost of the FFR-guided strategy was about 500 Euros ($607) greater (8,832 vs. 8,322; P < .01), leading Dr. Puymirat to conclude that “the use of FFR for nonculprit lesions appears to be less effective but more expensive,” at least by costs derived in France.
Lack of statistical power limits interpretation
The conclusion of FLOWER-MI is that FFR-guided PCI in complete revascularization of nonculprit lesions in STEMI patients is not superior to an angiography-guided approach, but Dr. Puymirat cautioned that the low number of events precludes a definitive message.
William Fearon, MD, professor of cardiovascular medicine at Stanford (Calif.) University Medical Center, agreed. Based on his calculations, the trial was substantially underpowered. Evaluating the details of treatment in the FFR group, Dr. Fearon pointed out that a nonculprit lesion with a FFR of 0.80 or less was identified in about 55% of patients. Ultimately, 66% in the FFR group received PCI, eliminating the key distinction between strategies for the majority of patients enrolled.
“Only about one-third of the FFR-guided patients, or about 200 patients, did not receive nonculprit PCI, and therefore only in this small group could we expect a difference in outcomes from the angio-guided group,” Dr. Fearon said.
Fewer stents were placed in the FFR-guided than angiography-guided group (1.01 vs. 1.5), but Dr. Fearon suggested that it would be very difficult to show a difference in risk of events in a study of this size when event rates at 1 year reached only about 5%.
In response, Dr. Puymirat acknowledged that the rate of events for this trial, which was designed in 2015, were lower than expected. In recalculating the power needed based on the rate of events observed in FLOWER-MI, he estimated that about 8,000 patients would have been needed to show a meaningful difference in these PCI strategies.
Dr. Puymirat reports financial relationships with more than a dozen pharmaceutical companies, including Abbott, which provided some of the funding for this trial. Dr. Fearon reports financial relationships with Abbott, CathWorks, HeartFlow, and Medtronic.
For patients with ST-elevated myocardial infarction (STEMI) undergoing complete revascularization, percutaneous coronary interventions (PCI) guided by fractional flow reserve (FFR) relative to angiography-guided PCI do not result in significantly lower risk of death or events, according to data from the randomized FLOWER-MI trial.
Rather, the events at 1 year were numerically lower among those randomized to the angiography-guided approach, according to the principal investigator of the trial, Etienne Puymirat, MD, PhD.
Prior studies showing an advantage for FFR-guided PCI in patients with coronary syndromes provided the hypothesis that FFR-guided PCI would also be superior for guiding PCI in STEMI patients. In the multicenter FAME trial, for example, FFR-guided PCI for patients with multivessel disease was associated with fewer stent placements (P < .001) and a nearly 30% lower rate of events at 1 year (P = .02).
While the advantage of complete revascularization, meaning PCI treatment of nonculprit as well as culprit lesions, has already been shown to be a better strategy than treatment of culprit lesions alone, FLOWER-MI is the first large study to compare FFR to angiography for guiding this approach to STEMI patients with multivessel disease, said Dr. Puymirat of Hôpital Européen George Pompidou, Paris, at the annual scientific sessions of the American College of Cardiology.
In this trial, involving multiple centers in France, STEMI patients were eligible for randomization if they had successful PCI of a culprit lesion and 50% or greater stenosis in at least one additional nonculprit lesion. The complete revascularization, whether patients were randomized to PCI guided by angiography or FFR, was performed during the index hospital admission. Patient management and follow-up was otherwise the same.
After a small number of exclusions, the intention-to-treat populations were 577 patients in the angiography-guided group and 586 in the FFR-guided group. The characteristics of the groups were well matched with an average age of about 62 years and similar rates of risk factors, such as hypertension and diabetes.
Angiography guidance just as good
The primary outcome was a composite of all-cause mortality, nonfatal MI, and unplanned revascularization. By hazard ratio, the risk of having one of these events within 1 year of PCI was numerically greater, at 32 in the FFR-guided group and 24 in the angiography-guided group, but the difference was not statistically significant (1.32; P = .31).
However, the total rate of events was low (5.5% vs. 4.2% for the angiography-guided and FFR-guided groups, respectively) and the confidence intervals were wide (95% CI, 0.78-2.23). This was also true of the components of the primary outcome.
No signal for a difference between strategies could be derived from these components, which included a higher rate of MI in the FFR-guided group (3.1% vs. 1.7%) but a lower rate of death (1.5% vs. 1.7%).
Unplanned hospitalizations leading to revascularization rates were also low (1.9% and 2.6% for angiography-guided and FFR-guided PCI, respectively), although it was reported that the rate of revascularization for nonculprit lesions was about twice as high in the FFR group (53.3% vs. 27.3%).
At 1 year, there were also low rates and no significant differences in a list of secondary outcomes that included hospitalization for recurrent ischemia or heart failure, stent thrombosis, and revascularization. As within the primary composite outcome, no pattern could be seen in the secondary events, some of which were numerically more common in the FFR-guided group and some numerically lower.
In a cost-efficacy analysis, the median per-patient cost of the FFR-guided strategy was about 500 Euros ($607) greater (8,832 vs. 8,322; P < .01), leading Dr. Puymirat to conclude that “the use of FFR for nonculprit lesions appears to be less effective but more expensive,” at least by costs derived in France.
Lack of statistical power limits interpretation
The conclusion of FLOWER-MI is that FFR-guided PCI in complete revascularization of nonculprit lesions in STEMI patients is not superior to an angiography-guided approach, but Dr. Puymirat cautioned that the low number of events precludes a definitive message.
William Fearon, MD, professor of cardiovascular medicine at Stanford (Calif.) University Medical Center, agreed. Based on his calculations, the trial was substantially underpowered. Evaluating the details of treatment in the FFR group, Dr. Fearon pointed out that a nonculprit lesion with a FFR of 0.80 or less was identified in about 55% of patients. Ultimately, 66% in the FFR group received PCI, eliminating the key distinction between strategies for the majority of patients enrolled.
“Only about one-third of the FFR-guided patients, or about 200 patients, did not receive nonculprit PCI, and therefore only in this small group could we expect a difference in outcomes from the angio-guided group,” Dr. Fearon said.
Fewer stents were placed in the FFR-guided than angiography-guided group (1.01 vs. 1.5), but Dr. Fearon suggested that it would be very difficult to show a difference in risk of events in a study of this size when event rates at 1 year reached only about 5%.
In response, Dr. Puymirat acknowledged that the rate of events for this trial, which was designed in 2015, were lower than expected. In recalculating the power needed based on the rate of events observed in FLOWER-MI, he estimated that about 8,000 patients would have been needed to show a meaningful difference in these PCI strategies.
Dr. Puymirat reports financial relationships with more than a dozen pharmaceutical companies, including Abbott, which provided some of the funding for this trial. Dr. Fearon reports financial relationships with Abbott, CathWorks, HeartFlow, and Medtronic.
For patients with ST-elevated myocardial infarction (STEMI) undergoing complete revascularization, percutaneous coronary interventions (PCI) guided by fractional flow reserve (FFR) relative to angiography-guided PCI do not result in significantly lower risk of death or events, according to data from the randomized FLOWER-MI trial.
Rather, the events at 1 year were numerically lower among those randomized to the angiography-guided approach, according to the principal investigator of the trial, Etienne Puymirat, MD, PhD.
Prior studies showing an advantage for FFR-guided PCI in patients with coronary syndromes provided the hypothesis that FFR-guided PCI would also be superior for guiding PCI in STEMI patients. In the multicenter FAME trial, for example, FFR-guided PCI for patients with multivessel disease was associated with fewer stent placements (P < .001) and a nearly 30% lower rate of events at 1 year (P = .02).
While the advantage of complete revascularization, meaning PCI treatment of nonculprit as well as culprit lesions, has already been shown to be a better strategy than treatment of culprit lesions alone, FLOWER-MI is the first large study to compare FFR to angiography for guiding this approach to STEMI patients with multivessel disease, said Dr. Puymirat of Hôpital Européen George Pompidou, Paris, at the annual scientific sessions of the American College of Cardiology.
In this trial, involving multiple centers in France, STEMI patients were eligible for randomization if they had successful PCI of a culprit lesion and 50% or greater stenosis in at least one additional nonculprit lesion. The complete revascularization, whether patients were randomized to PCI guided by angiography or FFR, was performed during the index hospital admission. Patient management and follow-up was otherwise the same.
After a small number of exclusions, the intention-to-treat populations were 577 patients in the angiography-guided group and 586 in the FFR-guided group. The characteristics of the groups were well matched with an average age of about 62 years and similar rates of risk factors, such as hypertension and diabetes.
Angiography guidance just as good
The primary outcome was a composite of all-cause mortality, nonfatal MI, and unplanned revascularization. By hazard ratio, the risk of having one of these events within 1 year of PCI was numerically greater, at 32 in the FFR-guided group and 24 in the angiography-guided group, but the difference was not statistically significant (1.32; P = .31).
However, the total rate of events was low (5.5% vs. 4.2% for the angiography-guided and FFR-guided groups, respectively) and the confidence intervals were wide (95% CI, 0.78-2.23). This was also true of the components of the primary outcome.
No signal for a difference between strategies could be derived from these components, which included a higher rate of MI in the FFR-guided group (3.1% vs. 1.7%) but a lower rate of death (1.5% vs. 1.7%).
Unplanned hospitalizations leading to revascularization rates were also low (1.9% and 2.6% for angiography-guided and FFR-guided PCI, respectively), although it was reported that the rate of revascularization for nonculprit lesions was about twice as high in the FFR group (53.3% vs. 27.3%).
At 1 year, there were also low rates and no significant differences in a list of secondary outcomes that included hospitalization for recurrent ischemia or heart failure, stent thrombosis, and revascularization. As within the primary composite outcome, no pattern could be seen in the secondary events, some of which were numerically more common in the FFR-guided group and some numerically lower.
In a cost-efficacy analysis, the median per-patient cost of the FFR-guided strategy was about 500 Euros ($607) greater (8,832 vs. 8,322; P < .01), leading Dr. Puymirat to conclude that “the use of FFR for nonculprit lesions appears to be less effective but more expensive,” at least by costs derived in France.
Lack of statistical power limits interpretation
The conclusion of FLOWER-MI is that FFR-guided PCI in complete revascularization of nonculprit lesions in STEMI patients is not superior to an angiography-guided approach, but Dr. Puymirat cautioned that the low number of events precludes a definitive message.
William Fearon, MD, professor of cardiovascular medicine at Stanford (Calif.) University Medical Center, agreed. Based on his calculations, the trial was substantially underpowered. Evaluating the details of treatment in the FFR group, Dr. Fearon pointed out that a nonculprit lesion with a FFR of 0.80 or less was identified in about 55% of patients. Ultimately, 66% in the FFR group received PCI, eliminating the key distinction between strategies for the majority of patients enrolled.
“Only about one-third of the FFR-guided patients, or about 200 patients, did not receive nonculprit PCI, and therefore only in this small group could we expect a difference in outcomes from the angio-guided group,” Dr. Fearon said.
Fewer stents were placed in the FFR-guided than angiography-guided group (1.01 vs. 1.5), but Dr. Fearon suggested that it would be very difficult to show a difference in risk of events in a study of this size when event rates at 1 year reached only about 5%.
In response, Dr. Puymirat acknowledged that the rate of events for this trial, which was designed in 2015, were lower than expected. In recalculating the power needed based on the rate of events observed in FLOWER-MI, he estimated that about 8,000 patients would have been needed to show a meaningful difference in these PCI strategies.
Dr. Puymirat reports financial relationships with more than a dozen pharmaceutical companies, including Abbott, which provided some of the funding for this trial. Dr. Fearon reports financial relationships with Abbott, CathWorks, HeartFlow, and Medtronic.
FROM ACC 2021























