User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
ADA standards of care 2022: Screen more, personalize, use technology
The American Diabetes Association’s updated clinical recommendations for 2022 call for wider population screening, along with furthering the trends toward individualization of care use of diabetes technology.
The summary of changes from 2021 spans four pages. “Diabetes is a really dynamic field so there is a lot to update which is good. It means progress,” ADA chief science and medical officer Robert A. Gabbay, MD, PhD, told this news organization.
The ADA Standards of Medical Care in Diabetes – 2022 was published Dec. 20, 2021, online as a supplement to Diabetes Care.
Screening widened by age, in pregnancy, and for type 1 diabetes
One dramatic change is a drop in age to begin screening all people for prediabetes and diabetes from 45 years to 35 years, regardless of risk factors such as obesity.
“Sadly, there are increasing numbers of people with diabetes and developing diabetes younger,” Dr. Gabbay said.
In August 2021, the U.S. Preventive Services Task Force dropped its recommended age of diabetes screening from 40 to 35 years for people with overweight or obesity, but not universally, as ADA now has.
The ADA made its recommendation independently, Dr. Gabbay noted.
The recommendation for testing pregnant women early in gestation (<15 weeks) for preexisting diabetes was also expanded, from just those with risk factors to consideration of testing all women for undiagnosed diabetes at the time they’re planning pregnancy, and if not then, at the first prenatal visit. Screening for gestational diabetes is then performed at 24-28 weeks.
Again, this is caused by increasing diabetes onset at younger ages, Dr. Gabbay said. “We’re well aware that the number of women who have diabetes and don’t know it and become pregnant is significant and therefore screening early on is important.”
New guidance regarding autoantibody screening in adults suspected of having type 1 diabetes and genetic testing for those who don’t fit typical criteria for either of the two main types are based on the ADA/European Association for the Study of Diabetes joint consensus statement on type 1 diabetes in adults.
Individualization of care based on comorbidities, other factors
The concept of individualization of care in diabetes has been emphasized for several years now, but continues to be enhanced with new data and newly available management tools.
Regarding management of type 2 diabetes, several charts have been included to help guide decision-making.
One lists drug-specific and patient factors, including comorbidities, to consider when selecting glucose-lowering medications. A new table depicts a building with four “pillars,” for complication risk reduction, including management of blood pressure, lipids, and glucose, as well as use of agents with cardiovascular and kidney benefit.
“On the type 2 side, the choice of therapy is really guided by several factors. We lay them out in a nice diagram. ... A lot of useful information there compares classes of drugs in order to help clinicians make decisions on what would be the appropriate therapy for a given individual,” Dr. Gabbay said.
An algorithm for pharmacologic treatment includes considerations of weight, hypoglycemia, and cost. Tables are also provided listing average wholesale prices of insulins and noninsulin medications.
A section now entitled “Obesity and weight management for the prevention and treatment of type 2 diabetes” has added content regarding the importance of addressing obesity in diabetes, particularly in the context of the COVID-19 pandemic, and the addition of semaglutide as an approved obesity treatment.
“What we hope is that this engenders a shared decision-making process with the patient to identify what the goals are and then choose the appropriate therapy for those goals,” Dr. Gabbay said.
New information has also been added about management of nonalcoholic fatty liver disease. “I think that’s one of the unrecognized and unaddressed complications of diabetes that we’ll see in the future, particularly as new therapies come out,” Dr. Gabbay predicted.
The section on cardiovascular disease and risk management, endorsed for the fourth year in a row by the American College of Cardiology, includes several new recommendations, including diagnosis of hypertension at a single visit if blood pressure is 180/110 mm Hg or greater, and individualization of blood pressure targets.
Chronic kidney disease management has now been separated from other microvascular complications into a standalone section, with several new updates. Retinopathy, neuropathy, and foot care remain combined in one section.
Diabetes technology: Rapidly evolving, access an issue
The new technology section “doubles down on the time in [normal glucose] range (TIR) concept,” but also emphasizes the importance of time below range.
“When we see that, we need to make a therapeutic change. We were concerned that as there’s more and more information and numbers, users might not pick up on what’s important,” Dr. Gabbay noted.
The new standards also provides greater affirmation of the value of continuous glucose monitoring (CGM) for people with both type 1 and type 2 diabetes at any age, with individualized choice of devices.
Access to technology is a “big issue, and something the ADA has really been fighting for, particularly in terms of health disparities,” Dr. Gabbay said, noting that ADA has a new Health Equity Now platform, which includes a “bill of rights” calling for all patients with diabetes to have access to state-of-the-art technologies, including CGM.
Overall, he said, “I think the big picture is diabetes continues to evolve and advance. After careful review of the literature, the standards of care identifies at least four big areas where there are some changes that clinicians need to know about: screening, how to individualize treatment, considerations of comorbidities, and the important role that technology plays.”
Dr. Gabbay is an employee of the ADA.
A version of this article first appeared on Medscape.com.
The American Diabetes Association’s updated clinical recommendations for 2022 call for wider population screening, along with furthering the trends toward individualization of care use of diabetes technology.
The summary of changes from 2021 spans four pages. “Diabetes is a really dynamic field so there is a lot to update which is good. It means progress,” ADA chief science and medical officer Robert A. Gabbay, MD, PhD, told this news organization.
The ADA Standards of Medical Care in Diabetes – 2022 was published Dec. 20, 2021, online as a supplement to Diabetes Care.
Screening widened by age, in pregnancy, and for type 1 diabetes
One dramatic change is a drop in age to begin screening all people for prediabetes and diabetes from 45 years to 35 years, regardless of risk factors such as obesity.
“Sadly, there are increasing numbers of people with diabetes and developing diabetes younger,” Dr. Gabbay said.
In August 2021, the U.S. Preventive Services Task Force dropped its recommended age of diabetes screening from 40 to 35 years for people with overweight or obesity, but not universally, as ADA now has.
The ADA made its recommendation independently, Dr. Gabbay noted.
The recommendation for testing pregnant women early in gestation (<15 weeks) for preexisting diabetes was also expanded, from just those with risk factors to consideration of testing all women for undiagnosed diabetes at the time they’re planning pregnancy, and if not then, at the first prenatal visit. Screening for gestational diabetes is then performed at 24-28 weeks.
Again, this is caused by increasing diabetes onset at younger ages, Dr. Gabbay said. “We’re well aware that the number of women who have diabetes and don’t know it and become pregnant is significant and therefore screening early on is important.”
New guidance regarding autoantibody screening in adults suspected of having type 1 diabetes and genetic testing for those who don’t fit typical criteria for either of the two main types are based on the ADA/European Association for the Study of Diabetes joint consensus statement on type 1 diabetes in adults.
Individualization of care based on comorbidities, other factors
The concept of individualization of care in diabetes has been emphasized for several years now, but continues to be enhanced with new data and newly available management tools.
Regarding management of type 2 diabetes, several charts have been included to help guide decision-making.
One lists drug-specific and patient factors, including comorbidities, to consider when selecting glucose-lowering medications. A new table depicts a building with four “pillars,” for complication risk reduction, including management of blood pressure, lipids, and glucose, as well as use of agents with cardiovascular and kidney benefit.
“On the type 2 side, the choice of therapy is really guided by several factors. We lay them out in a nice diagram. ... A lot of useful information there compares classes of drugs in order to help clinicians make decisions on what would be the appropriate therapy for a given individual,” Dr. Gabbay said.
An algorithm for pharmacologic treatment includes considerations of weight, hypoglycemia, and cost. Tables are also provided listing average wholesale prices of insulins and noninsulin medications.
A section now entitled “Obesity and weight management for the prevention and treatment of type 2 diabetes” has added content regarding the importance of addressing obesity in diabetes, particularly in the context of the COVID-19 pandemic, and the addition of semaglutide as an approved obesity treatment.
“What we hope is that this engenders a shared decision-making process with the patient to identify what the goals are and then choose the appropriate therapy for those goals,” Dr. Gabbay said.
New information has also been added about management of nonalcoholic fatty liver disease. “I think that’s one of the unrecognized and unaddressed complications of diabetes that we’ll see in the future, particularly as new therapies come out,” Dr. Gabbay predicted.
The section on cardiovascular disease and risk management, endorsed for the fourth year in a row by the American College of Cardiology, includes several new recommendations, including diagnosis of hypertension at a single visit if blood pressure is 180/110 mm Hg or greater, and individualization of blood pressure targets.
Chronic kidney disease management has now been separated from other microvascular complications into a standalone section, with several new updates. Retinopathy, neuropathy, and foot care remain combined in one section.
Diabetes technology: Rapidly evolving, access an issue
The new technology section “doubles down on the time in [normal glucose] range (TIR) concept,” but also emphasizes the importance of time below range.
“When we see that, we need to make a therapeutic change. We were concerned that as there’s more and more information and numbers, users might not pick up on what’s important,” Dr. Gabbay noted.
The new standards also provides greater affirmation of the value of continuous glucose monitoring (CGM) for people with both type 1 and type 2 diabetes at any age, with individualized choice of devices.
Access to technology is a “big issue, and something the ADA has really been fighting for, particularly in terms of health disparities,” Dr. Gabbay said, noting that ADA has a new Health Equity Now platform, which includes a “bill of rights” calling for all patients with diabetes to have access to state-of-the-art technologies, including CGM.
Overall, he said, “I think the big picture is diabetes continues to evolve and advance. After careful review of the literature, the standards of care identifies at least four big areas where there are some changes that clinicians need to know about: screening, how to individualize treatment, considerations of comorbidities, and the important role that technology plays.”
Dr. Gabbay is an employee of the ADA.
A version of this article first appeared on Medscape.com.
The American Diabetes Association’s updated clinical recommendations for 2022 call for wider population screening, along with furthering the trends toward individualization of care use of diabetes technology.
The summary of changes from 2021 spans four pages. “Diabetes is a really dynamic field so there is a lot to update which is good. It means progress,” ADA chief science and medical officer Robert A. Gabbay, MD, PhD, told this news organization.
The ADA Standards of Medical Care in Diabetes – 2022 was published Dec. 20, 2021, online as a supplement to Diabetes Care.
Screening widened by age, in pregnancy, and for type 1 diabetes
One dramatic change is a drop in age to begin screening all people for prediabetes and diabetes from 45 years to 35 years, regardless of risk factors such as obesity.
“Sadly, there are increasing numbers of people with diabetes and developing diabetes younger,” Dr. Gabbay said.
In August 2021, the U.S. Preventive Services Task Force dropped its recommended age of diabetes screening from 40 to 35 years for people with overweight or obesity, but not universally, as ADA now has.
The ADA made its recommendation independently, Dr. Gabbay noted.
The recommendation for testing pregnant women early in gestation (<15 weeks) for preexisting diabetes was also expanded, from just those with risk factors to consideration of testing all women for undiagnosed diabetes at the time they’re planning pregnancy, and if not then, at the first prenatal visit. Screening for gestational diabetes is then performed at 24-28 weeks.
Again, this is caused by increasing diabetes onset at younger ages, Dr. Gabbay said. “We’re well aware that the number of women who have diabetes and don’t know it and become pregnant is significant and therefore screening early on is important.”
New guidance regarding autoantibody screening in adults suspected of having type 1 diabetes and genetic testing for those who don’t fit typical criteria for either of the two main types are based on the ADA/European Association for the Study of Diabetes joint consensus statement on type 1 diabetes in adults.
Individualization of care based on comorbidities, other factors
The concept of individualization of care in diabetes has been emphasized for several years now, but continues to be enhanced with new data and newly available management tools.
Regarding management of type 2 diabetes, several charts have been included to help guide decision-making.
One lists drug-specific and patient factors, including comorbidities, to consider when selecting glucose-lowering medications. A new table depicts a building with four “pillars,” for complication risk reduction, including management of blood pressure, lipids, and glucose, as well as use of agents with cardiovascular and kidney benefit.
“On the type 2 side, the choice of therapy is really guided by several factors. We lay them out in a nice diagram. ... A lot of useful information there compares classes of drugs in order to help clinicians make decisions on what would be the appropriate therapy for a given individual,” Dr. Gabbay said.
An algorithm for pharmacologic treatment includes considerations of weight, hypoglycemia, and cost. Tables are also provided listing average wholesale prices of insulins and noninsulin medications.
A section now entitled “Obesity and weight management for the prevention and treatment of type 2 diabetes” has added content regarding the importance of addressing obesity in diabetes, particularly in the context of the COVID-19 pandemic, and the addition of semaglutide as an approved obesity treatment.
“What we hope is that this engenders a shared decision-making process with the patient to identify what the goals are and then choose the appropriate therapy for those goals,” Dr. Gabbay said.
New information has also been added about management of nonalcoholic fatty liver disease. “I think that’s one of the unrecognized and unaddressed complications of diabetes that we’ll see in the future, particularly as new therapies come out,” Dr. Gabbay predicted.
The section on cardiovascular disease and risk management, endorsed for the fourth year in a row by the American College of Cardiology, includes several new recommendations, including diagnosis of hypertension at a single visit if blood pressure is 180/110 mm Hg or greater, and individualization of blood pressure targets.
Chronic kidney disease management has now been separated from other microvascular complications into a standalone section, with several new updates. Retinopathy, neuropathy, and foot care remain combined in one section.
Diabetes technology: Rapidly evolving, access an issue
The new technology section “doubles down on the time in [normal glucose] range (TIR) concept,” but also emphasizes the importance of time below range.
“When we see that, we need to make a therapeutic change. We were concerned that as there’s more and more information and numbers, users might not pick up on what’s important,” Dr. Gabbay noted.
The new standards also provides greater affirmation of the value of continuous glucose monitoring (CGM) for people with both type 1 and type 2 diabetes at any age, with individualized choice of devices.
Access to technology is a “big issue, and something the ADA has really been fighting for, particularly in terms of health disparities,” Dr. Gabbay said, noting that ADA has a new Health Equity Now platform, which includes a “bill of rights” calling for all patients with diabetes to have access to state-of-the-art technologies, including CGM.
Overall, he said, “I think the big picture is diabetes continues to evolve and advance. After careful review of the literature, the standards of care identifies at least four big areas where there are some changes that clinicians need to know about: screening, how to individualize treatment, considerations of comorbidities, and the important role that technology plays.”
Dr. Gabbay is an employee of the ADA.
A version of this article first appeared on Medscape.com.
FDA OKs emergency use of Merck pill for COVID-19
Similar to FDA authorization of another antiviral pill regimen – ritonavir plus nirmatrelvir, or Paxlovid – granted to Pfizer on Wednesday, molnupiravir (brand name Lagevrio) should be taken early in the course of COVID-19 illness.
Pfizer’s drug is authorized for anyone aged 12 and up. But Merck’s is only for adults aged 18 and older.
Merck filed an application for emergency use authorization with the FDA in October. The company included results of its phase 3 study showing the treatment could lead to a 50% reduction in COVID-19 hospitalizations. Data later showed this efficacy at closer to a 30% reduction. In November, an FDA advisory panel narrowly recommended the agency grant authorization by a 13-10 vote.
Animal studies found the drug may harm a fetus, so it is not recommended for pregnant people, the FDA says. It may be prescribed to a pregnant person only after their doctor determines the benefits outweigh the risks and the patient is told of those risks.
Women who may get pregnant should use a reliable method of birth control if being treated with molnupiravir and for 4 days after the final dose.
Two weapons against COVID
Two antiviral pills could be better than one, at least in terms of making more COVID-19 treatments available in early 2022. It is yet to be seen if the drugmakers will be able to keep up with demand, which could substantially increase with an expected surge in Omicron variant cases.
Ritonavir and molnupiravir join remdesivir (brand name Veklury) as available antivirals to treat COVID-19. Remdesivir is fully approved by the FDA but is given only through an IV to people in the hospital.
Officials point out that COVID-19 treatments in tablet form are more convenient for patients in the United States and across the globe, particularly where IV infusion services may be limited.
In March 2021, experts accurately predicted that the molnupiravir pill would be available by year’s end.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of SARS-CoV-2 because the agent does not target the virus’s spike protein.
Perhaps in part because of early promising results, the U.S. government announced in November intentions to purchase $1 billion worth of molnupiravir. That new order came on top of $1.2 billion worth of the pills the U.S. ordered in June.
A version of this article first appeared on WebMD.com.
Similar to FDA authorization of another antiviral pill regimen – ritonavir plus nirmatrelvir, or Paxlovid – granted to Pfizer on Wednesday, molnupiravir (brand name Lagevrio) should be taken early in the course of COVID-19 illness.
Pfizer’s drug is authorized for anyone aged 12 and up. But Merck’s is only for adults aged 18 and older.
Merck filed an application for emergency use authorization with the FDA in October. The company included results of its phase 3 study showing the treatment could lead to a 50% reduction in COVID-19 hospitalizations. Data later showed this efficacy at closer to a 30% reduction. In November, an FDA advisory panel narrowly recommended the agency grant authorization by a 13-10 vote.
Animal studies found the drug may harm a fetus, so it is not recommended for pregnant people, the FDA says. It may be prescribed to a pregnant person only after their doctor determines the benefits outweigh the risks and the patient is told of those risks.
Women who may get pregnant should use a reliable method of birth control if being treated with molnupiravir and for 4 days after the final dose.
Two weapons against COVID
Two antiviral pills could be better than one, at least in terms of making more COVID-19 treatments available in early 2022. It is yet to be seen if the drugmakers will be able to keep up with demand, which could substantially increase with an expected surge in Omicron variant cases.
Ritonavir and molnupiravir join remdesivir (brand name Veklury) as available antivirals to treat COVID-19. Remdesivir is fully approved by the FDA but is given only through an IV to people in the hospital.
Officials point out that COVID-19 treatments in tablet form are more convenient for patients in the United States and across the globe, particularly where IV infusion services may be limited.
In March 2021, experts accurately predicted that the molnupiravir pill would be available by year’s end.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of SARS-CoV-2 because the agent does not target the virus’s spike protein.
Perhaps in part because of early promising results, the U.S. government announced in November intentions to purchase $1 billion worth of molnupiravir. That new order came on top of $1.2 billion worth of the pills the U.S. ordered in June.
A version of this article first appeared on WebMD.com.
Similar to FDA authorization of another antiviral pill regimen – ritonavir plus nirmatrelvir, or Paxlovid – granted to Pfizer on Wednesday, molnupiravir (brand name Lagevrio) should be taken early in the course of COVID-19 illness.
Pfizer’s drug is authorized for anyone aged 12 and up. But Merck’s is only for adults aged 18 and older.
Merck filed an application for emergency use authorization with the FDA in October. The company included results of its phase 3 study showing the treatment could lead to a 50% reduction in COVID-19 hospitalizations. Data later showed this efficacy at closer to a 30% reduction. In November, an FDA advisory panel narrowly recommended the agency grant authorization by a 13-10 vote.
Animal studies found the drug may harm a fetus, so it is not recommended for pregnant people, the FDA says. It may be prescribed to a pregnant person only after their doctor determines the benefits outweigh the risks and the patient is told of those risks.
Women who may get pregnant should use a reliable method of birth control if being treated with molnupiravir and for 4 days after the final dose.
Two weapons against COVID
Two antiviral pills could be better than one, at least in terms of making more COVID-19 treatments available in early 2022. It is yet to be seen if the drugmakers will be able to keep up with demand, which could substantially increase with an expected surge in Omicron variant cases.
Ritonavir and molnupiravir join remdesivir (brand name Veklury) as available antivirals to treat COVID-19. Remdesivir is fully approved by the FDA but is given only through an IV to people in the hospital.
Officials point out that COVID-19 treatments in tablet form are more convenient for patients in the United States and across the globe, particularly where IV infusion services may be limited.
In March 2021, experts accurately predicted that the molnupiravir pill would be available by year’s end.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of SARS-CoV-2 because the agent does not target the virus’s spike protein.
Perhaps in part because of early promising results, the U.S. government announced in November intentions to purchase $1 billion worth of molnupiravir. That new order came on top of $1.2 billion worth of the pills the U.S. ordered in June.
A version of this article first appeared on WebMD.com.
New studies suggest Omicron infections are less severe than Delta ones
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
Last call? Moderate alcohol’s health benefits look increasingly doubtful
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
FDA authorizes Pfizer antiviral pill for COVID-19
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
Convalescent plasma cuts COVID-19 hospitalizations in half: Study
A “definitive study” from Johns Hopkins University researchers and others shows that convalescent plasma can cut hospital admissions for COVID-19 by 54% if therapy is administered within 8 days of symptom onset.
In the study of 1,181 adults randomly assigned to high-titer convalescent plasma or placebo, 2.9% of people receiving the therapy were hospitalized, compared with 6.3% who received placebo control plasma.
This translates to a 54% risk reduction for hospitalization with convalescent plasma.
“We have a clear difference,” principal investigator David Sullivan, MD, a professor at Johns Hopkins University, Baltimore, said during a Dec. 21 media briefing.
“This is very good news since we are in the midst of the Omicron surge, which has defeated [some of] our major monocular antibody therapies,” said Arturo Casadevall, MD, chair of the department of molecular microbiology and immunology at Johns Hopkins.
“So we have a new tool to keep people from progressing in their disease and to reduce progression or hospitalization,” Dr. Casadevall said.
The findings were published as a preprint study on Dec. 21, 2021, on medRxiv. The study has not yet been peer reviewed.
Whereas many convalescent plasma studies were done in hospitalized patients, this is one of only a handful performed in outpatients, the researchers noted.
There is a regulatory catch. The Food and Drug Administration restricted emergency use authorization (EUA) for convalescent plasma in February 2021 to include only high-dose titer plasma and to limit the therapy to hospitalized patients with early disease or for immunocompromised people who cannot mount an adequate antibody response.
Dr. Sullivan and colleagues hoped their findings will prompt the FDA to expand the EUA to include outpatients.
“We have shared this data with both the World Health Organization and the FDA,” study coauthor Kelly Gebo, MD, MPH, said during the media briefing.
“We do believe that this could be scaled up quickly,” added Dr. Gebo, professor of medicine at Johns Hopkins University. Convalescent plasma “could be used as a potential treatment as variants continue to evolve, such as we’ve seen with Omicron.”
Pre-Omicron results
The study was conducted at Johns Hopkins University and 23 other sites nationwide between June 2020 and October 2021. This means researchers enrolled symptomatic adults during circulation of the SARS-CoV-2 ancestral strain and the Alpha and Delta variants.
However, Dr. Sullivan said, “we think that ... plasma with high levels of antibodies can adapt faster to Omicron, although it will take us longer to get an Omicron-specific supply.”
Because of the timing of the study, 80% of participants were unvaccinated. Mean age was 44 years and 57% were women. Black and Hispanic participants each accounted for more than 12% of the study population.
On average, participants received a transfusion within 6 days of the start of symptoms.
In the study, 37 people out of 589 control group participants were hospitalized, compared with 17 of the 592 who received the convalescent plasma.
“We know antibodies work against SARS-CoV-2. The vaccines have been spectacular – producing antibodies that reduce hospitalizations and prevent transmission,” Dr. Sullivan said. “Convalescent plasma provides much of the same antibodies instantly.”
Convalescent and controversial
Convalescent plasma has been one of the controversial treatments for people with COVID-19 – with studies going back and forth on the potential benefits and efficacy. A National Institutes of Health–funded study published in August 2021, for example, showed no significant benefit.
“As you know, convalescent plasma has had a rocky ride,” Dr. Casadevall said.
“It was deployed with great excitement in the terrible, early days of the pandemic. Unfortunately, the early excitement and optimism was dampened with some of the randomized control trials appearing to show no benefit in reducing mortality and hospitalized patients,” he added.
In contrast, the current study shows “where convalescent plasma works using the latest, most rigorous clinical investigation tools available: a double-blinded, randomized, placebo-control trial,” Dr. Casadevall said.
Why a preprint, and why now?
The researchers decided to release their data in recognition of the lag time between reporting of COVID-19 cases and hospitalizations, Dr. Sullivan said. “That’s part of the reason we decided to act now with this knowledge – that it does take a couple of weeks – with cases of Omicron going up.”
Furthermore, “we thought this was actionable data for decision-makers,” he added.
A reporter asked why the Johns Hopkins researchers chose to hold a media briefing for a preprint study.
A preprint is “not so unusual given the SARS-CoV-2 pandemic,” said study senior author Daniel Hanley, MD, division director of brain injury outcomes at Johns Hopkins University.
“The data are the data,” Dr. Casadevall added. “This is not going to change from peer review.”
Peer review may change some of the wording of the manuscript, but not the numbers, he added.
“Now with the Omicron crisis and the fact that we have lost some more main monoclonal antibodies, it is essential to get this information out,” Dr. Casadevall said.
Plasma therapy nothing new
Donation and transfusion of convalescent plasma is highly regulated with strict criteria, said Evan Bloch, MBChB, associate director of the transfusion medicine division at Johns Hopkins University.
If the FDA opts to expand the EUA based on this or other evidence, administration of convalescent plasma could be rolled out fairly quickly, the researchers noted.
Plasma transfusion takes place in hospitals and at infusion centers every day. The infrastructure is in place in many countries, even low- and middle-resource nations, around the world to provide convalescent plasma therapy. The major difference between traditional plasma and SARS-CoV-2 convalescent plasma is the indication, Dr. Bloch added.
In addition, convalescent plasma has a polyclonal composition – a benefit compared with monoclonal antibodies, he added. “It’s more durable or adaptive [compared with] some of the targeted therapies, such as monoclonal antibodies, where we’ve witnessed this diminished efficacy with viral evolution.”
A version of this article first appeared on Medscape.com.
A “definitive study” from Johns Hopkins University researchers and others shows that convalescent plasma can cut hospital admissions for COVID-19 by 54% if therapy is administered within 8 days of symptom onset.
In the study of 1,181 adults randomly assigned to high-titer convalescent plasma or placebo, 2.9% of people receiving the therapy were hospitalized, compared with 6.3% who received placebo control plasma.
This translates to a 54% risk reduction for hospitalization with convalescent plasma.
“We have a clear difference,” principal investigator David Sullivan, MD, a professor at Johns Hopkins University, Baltimore, said during a Dec. 21 media briefing.
“This is very good news since we are in the midst of the Omicron surge, which has defeated [some of] our major monocular antibody therapies,” said Arturo Casadevall, MD, chair of the department of molecular microbiology and immunology at Johns Hopkins.
“So we have a new tool to keep people from progressing in their disease and to reduce progression or hospitalization,” Dr. Casadevall said.
The findings were published as a preprint study on Dec. 21, 2021, on medRxiv. The study has not yet been peer reviewed.
Whereas many convalescent plasma studies were done in hospitalized patients, this is one of only a handful performed in outpatients, the researchers noted.
There is a regulatory catch. The Food and Drug Administration restricted emergency use authorization (EUA) for convalescent plasma in February 2021 to include only high-dose titer plasma and to limit the therapy to hospitalized patients with early disease or for immunocompromised people who cannot mount an adequate antibody response.
Dr. Sullivan and colleagues hoped their findings will prompt the FDA to expand the EUA to include outpatients.
“We have shared this data with both the World Health Organization and the FDA,” study coauthor Kelly Gebo, MD, MPH, said during the media briefing.
“We do believe that this could be scaled up quickly,” added Dr. Gebo, professor of medicine at Johns Hopkins University. Convalescent plasma “could be used as a potential treatment as variants continue to evolve, such as we’ve seen with Omicron.”
Pre-Omicron results
The study was conducted at Johns Hopkins University and 23 other sites nationwide between June 2020 and October 2021. This means researchers enrolled symptomatic adults during circulation of the SARS-CoV-2 ancestral strain and the Alpha and Delta variants.
However, Dr. Sullivan said, “we think that ... plasma with high levels of antibodies can adapt faster to Omicron, although it will take us longer to get an Omicron-specific supply.”
Because of the timing of the study, 80% of participants were unvaccinated. Mean age was 44 years and 57% were women. Black and Hispanic participants each accounted for more than 12% of the study population.
On average, participants received a transfusion within 6 days of the start of symptoms.
In the study, 37 people out of 589 control group participants were hospitalized, compared with 17 of the 592 who received the convalescent plasma.
“We know antibodies work against SARS-CoV-2. The vaccines have been spectacular – producing antibodies that reduce hospitalizations and prevent transmission,” Dr. Sullivan said. “Convalescent plasma provides much of the same antibodies instantly.”
Convalescent and controversial
Convalescent plasma has been one of the controversial treatments for people with COVID-19 – with studies going back and forth on the potential benefits and efficacy. A National Institutes of Health–funded study published in August 2021, for example, showed no significant benefit.
“As you know, convalescent plasma has had a rocky ride,” Dr. Casadevall said.
“It was deployed with great excitement in the terrible, early days of the pandemic. Unfortunately, the early excitement and optimism was dampened with some of the randomized control trials appearing to show no benefit in reducing mortality and hospitalized patients,” he added.
In contrast, the current study shows “where convalescent plasma works using the latest, most rigorous clinical investigation tools available: a double-blinded, randomized, placebo-control trial,” Dr. Casadevall said.
Why a preprint, and why now?
The researchers decided to release their data in recognition of the lag time between reporting of COVID-19 cases and hospitalizations, Dr. Sullivan said. “That’s part of the reason we decided to act now with this knowledge – that it does take a couple of weeks – with cases of Omicron going up.”
Furthermore, “we thought this was actionable data for decision-makers,” he added.
A reporter asked why the Johns Hopkins researchers chose to hold a media briefing for a preprint study.
A preprint is “not so unusual given the SARS-CoV-2 pandemic,” said study senior author Daniel Hanley, MD, division director of brain injury outcomes at Johns Hopkins University.
“The data are the data,” Dr. Casadevall added. “This is not going to change from peer review.”
Peer review may change some of the wording of the manuscript, but not the numbers, he added.
“Now with the Omicron crisis and the fact that we have lost some more main monoclonal antibodies, it is essential to get this information out,” Dr. Casadevall said.
Plasma therapy nothing new
Donation and transfusion of convalescent plasma is highly regulated with strict criteria, said Evan Bloch, MBChB, associate director of the transfusion medicine division at Johns Hopkins University.
If the FDA opts to expand the EUA based on this or other evidence, administration of convalescent plasma could be rolled out fairly quickly, the researchers noted.
Plasma transfusion takes place in hospitals and at infusion centers every day. The infrastructure is in place in many countries, even low- and middle-resource nations, around the world to provide convalescent plasma therapy. The major difference between traditional plasma and SARS-CoV-2 convalescent plasma is the indication, Dr. Bloch added.
In addition, convalescent plasma has a polyclonal composition – a benefit compared with monoclonal antibodies, he added. “It’s more durable or adaptive [compared with] some of the targeted therapies, such as monoclonal antibodies, where we’ve witnessed this diminished efficacy with viral evolution.”
A version of this article first appeared on Medscape.com.
A “definitive study” from Johns Hopkins University researchers and others shows that convalescent plasma can cut hospital admissions for COVID-19 by 54% if therapy is administered within 8 days of symptom onset.
In the study of 1,181 adults randomly assigned to high-titer convalescent plasma or placebo, 2.9% of people receiving the therapy were hospitalized, compared with 6.3% who received placebo control plasma.
This translates to a 54% risk reduction for hospitalization with convalescent plasma.
“We have a clear difference,” principal investigator David Sullivan, MD, a professor at Johns Hopkins University, Baltimore, said during a Dec. 21 media briefing.
“This is very good news since we are in the midst of the Omicron surge, which has defeated [some of] our major monocular antibody therapies,” said Arturo Casadevall, MD, chair of the department of molecular microbiology and immunology at Johns Hopkins.
“So we have a new tool to keep people from progressing in their disease and to reduce progression or hospitalization,” Dr. Casadevall said.
The findings were published as a preprint study on Dec. 21, 2021, on medRxiv. The study has not yet been peer reviewed.
Whereas many convalescent plasma studies were done in hospitalized patients, this is one of only a handful performed in outpatients, the researchers noted.
There is a regulatory catch. The Food and Drug Administration restricted emergency use authorization (EUA) for convalescent plasma in February 2021 to include only high-dose titer plasma and to limit the therapy to hospitalized patients with early disease or for immunocompromised people who cannot mount an adequate antibody response.
Dr. Sullivan and colleagues hoped their findings will prompt the FDA to expand the EUA to include outpatients.
“We have shared this data with both the World Health Organization and the FDA,” study coauthor Kelly Gebo, MD, MPH, said during the media briefing.
“We do believe that this could be scaled up quickly,” added Dr. Gebo, professor of medicine at Johns Hopkins University. Convalescent plasma “could be used as a potential treatment as variants continue to evolve, such as we’ve seen with Omicron.”
Pre-Omicron results
The study was conducted at Johns Hopkins University and 23 other sites nationwide between June 2020 and October 2021. This means researchers enrolled symptomatic adults during circulation of the SARS-CoV-2 ancestral strain and the Alpha and Delta variants.
However, Dr. Sullivan said, “we think that ... plasma with high levels of antibodies can adapt faster to Omicron, although it will take us longer to get an Omicron-specific supply.”
Because of the timing of the study, 80% of participants were unvaccinated. Mean age was 44 years and 57% were women. Black and Hispanic participants each accounted for more than 12% of the study population.
On average, participants received a transfusion within 6 days of the start of symptoms.
In the study, 37 people out of 589 control group participants were hospitalized, compared with 17 of the 592 who received the convalescent plasma.
“We know antibodies work against SARS-CoV-2. The vaccines have been spectacular – producing antibodies that reduce hospitalizations and prevent transmission,” Dr. Sullivan said. “Convalescent plasma provides much of the same antibodies instantly.”
Convalescent and controversial
Convalescent plasma has been one of the controversial treatments for people with COVID-19 – with studies going back and forth on the potential benefits and efficacy. A National Institutes of Health–funded study published in August 2021, for example, showed no significant benefit.
“As you know, convalescent plasma has had a rocky ride,” Dr. Casadevall said.
“It was deployed with great excitement in the terrible, early days of the pandemic. Unfortunately, the early excitement and optimism was dampened with some of the randomized control trials appearing to show no benefit in reducing mortality and hospitalized patients,” he added.
In contrast, the current study shows “where convalescent plasma works using the latest, most rigorous clinical investigation tools available: a double-blinded, randomized, placebo-control trial,” Dr. Casadevall said.
Why a preprint, and why now?
The researchers decided to release their data in recognition of the lag time between reporting of COVID-19 cases and hospitalizations, Dr. Sullivan said. “That’s part of the reason we decided to act now with this knowledge – that it does take a couple of weeks – with cases of Omicron going up.”
Furthermore, “we thought this was actionable data for decision-makers,” he added.
A reporter asked why the Johns Hopkins researchers chose to hold a media briefing for a preprint study.
A preprint is “not so unusual given the SARS-CoV-2 pandemic,” said study senior author Daniel Hanley, MD, division director of brain injury outcomes at Johns Hopkins University.
“The data are the data,” Dr. Casadevall added. “This is not going to change from peer review.”
Peer review may change some of the wording of the manuscript, but not the numbers, he added.
“Now with the Omicron crisis and the fact that we have lost some more main monoclonal antibodies, it is essential to get this information out,” Dr. Casadevall said.
Plasma therapy nothing new
Donation and transfusion of convalescent plasma is highly regulated with strict criteria, said Evan Bloch, MBChB, associate director of the transfusion medicine division at Johns Hopkins University.
If the FDA opts to expand the EUA based on this or other evidence, administration of convalescent plasma could be rolled out fairly quickly, the researchers noted.
Plasma transfusion takes place in hospitals and at infusion centers every day. The infrastructure is in place in many countries, even low- and middle-resource nations, around the world to provide convalescent plasma therapy. The major difference between traditional plasma and SARS-CoV-2 convalescent plasma is the indication, Dr. Bloch added.
In addition, convalescent plasma has a polyclonal composition – a benefit compared with monoclonal antibodies, he added. “It’s more durable or adaptive [compared with] some of the targeted therapies, such as monoclonal antibodies, where we’ve witnessed this diminished efficacy with viral evolution.”
A version of this article first appeared on Medscape.com.
FROM MEDRXIV
Bamlanivimab’s effects in COVID-19 depend on antibodies
In the randomized controlled trial, in both the group who received bamlanivimab and the group who received placebo, higher antigen and viral RNA levels were associated with a lower proportion of patients achieving recovery.
Other studies have shown that the use of monoclonal antibodies reduces hospitalization risk in outpatients with early COVID-19, and appears to promote viral load decline in the nasopharynx, wrote Jens D. Lundgren, MD, of the University of Copenhagen and colleagues in their article published in the Annals of Internal Medicine. What had been missing prior to this new research was final results from hospitalized patients, the authors said.
In the new study, the researchers randomized 314 adults hospitalized with COVID-19 but without end-organ failure to receive 7,000 mg bamlanivimab (163 patients) or a placebo (151 patients). All patients received study-supplied remdesivir unless contraindicated. The researchers compared the efficacy of bamlanivimab versus placebo, but considered remdesivir the standard of care in this study.
At baseline, 50% of patients overall had antispike endogenous neutralizing antibodies (nAbs), and 50% had SARS-CoV-2 nucleocapsid plasma antigen levels of at least 1,000 ng/L.
The median time to sustained recovery, 19 days, was not significantly different between the bamlanivimab and placebo groups (subhazard ratio, 0.99).
“As hypothesized, among those who were negative for nAb, the difference between bamlanivimab and placebo was more evident if levels of plasma antigen or nasal-swab viral RNA were above the median entry levels,” with subhazard ratios of 1.48 and 1.89, respectively, the researchers explained.
However, the hazard ratio for death for bamlanivimab vs. placebo was 0.45 for patients negative for nAb vs. 3.53 for those positive for nAb. These differences with respect to nAb status were similar across all 90 elements of a composite safety outcome, the researchers said.
Potential benefits remain unclear
The use of neutralizing monoclonal antibodies has been extensively documented as an effective treatment for COVID-19 among ambulatory patients, corresponding author Dr. Lundgren said in an interview.
“Conversely, among admitted patients with COVID-19 pneumonia, the benefit has been questionable,” he said.
The researchers examined a hypothesis that the null finding in hospitalized patients may stem from differences in underlying mechanisms, “either from uncontrolled viral replication – which would be predicted to occur in particular among those not yet been able to mount an endogenous immune response – or from hyperinflammation among those that have mounted such a response,” Dr. Lundgren said.
The study findings supported the stated hypothesis, said Dr. Lundgren. “However, it was surprising that not only was the neutralizing antibody without any benefit among those that had mounted an endogenous immune response, but it actually may have been harmful,” he said.
Bamlanivimab was effective against the viral strain that circulated at the time of enrollment in the study, but subsequent viral strains have appeared to be unaffected by the neutralizing activity of the antibody, said Dr. Lundgren.
From a practical standpoint, “the findings would suggest that use of neutralizing monoclonal antibodies for patients admitted to a hospital with COVID pneumonia should be restricted to those that have not yet mounted an endogenous immune response, as determined by lack of detectable neutralizing antibodies at the time of admission,” Dr. Lundgren said.
Looking ahead, studies are currently underway to examine how the findings translate to vaccinated patients, he added. Other questions to be addressed include whether the benefits and harms apply to some or all neutralizing antibody products, he said.
In addition, “our research consortium is currently doing field testing of several point-of-care test candidates to examine their reliability and functionality,” for how quickly they might identify an endogenous neutralizing antibody response in an admitted COVID pneumonia patient,” Dr. Lundgren noted.
Findings show bamlanivimab’s limits
“Based on the findings of the current study, no clear subgroup of patients could be identified who would benefit from bamlanivimab when hospitalized with COVID-19,” said Suman Pal, MD, of the University of New Mexico, Albuquerque, in an interview.
“The study findings also show possible harm of using bamlanivimab in hospitalized COVID-19 patients who were seropositive for neutralizing antibodies prior to receiving therapy,” Dr. Pal emphasized. “Moreover, the study did not include participants with COVID-19 from variant strains, such as delta and omicron, which currently account for a large number of cases.” “Therefore, the results of this study do not support the use of bamlanivimab in the clinical setting until further evidence is available to guide the selection of patients who may benefit from therapy,” he explained.
“The possible benefit of bamlanivimab does not outweigh the risks in patients hospitalized with COVID-19,” he concluded.
Dr. Pal emphasized the need for larger prospective studies to establish whether bamlanivimab may have benefits in a subgroup of patients, but “well-validated point-of-care tests to identify such patients need to be readily available before this therapy can be considered by clinicians at the bedside,” he concluded.
Diligent screening required before use
Monoclonal antibody treatment has been administered to individuals with diagnosis of COVID-19 infection as outpatients as well as for hospitalized inpatients, said Noel Deep, MD, an internist in Antigo, Wisc., in an interview. “This study is important because it helps physicians and health care institutions to evaluate whether continued use of the monoclonal antibodies would be beneficial and, if so, in what patient populations,” he said.
The findings present interesting implications for the care of COVID-19 patients, said Dr. Deep. “This study indicates that bamlanivimab does not provide the benefit that was initially envisioned when the monoclonal antibody infusions were initially initiated in the treatment of COVID-19 infections. “Serological screening of the patients would help to identify that subgroup of individuals who could benefit from this monoclonal antibody rather than administering it to every COVID-19–positive individual,” he explained.
However, “it is important to note that the emergency use authorization (EUA) for single-agent bamlanivimab has been revoked,” Dr. Deep said.
“The potential benefits of bamlanivimab can be realized only if adequate attention is paid to identifying the appropriate candidates based on serological screening, and administering bamlanivimab to those who are already producing endogenous antibodies could lead to increased risk to those individuals,” he said. Dr. Deep added that he would favor administration of bamlanivimab “in those appropriately screened and eligible candidates, and it is my opinion that the benefits outweigh the risks in those individuals.”
Although the EUA for single-agent bamlanivimab has been revoked, “alternative monoclonal antibody therapies remain available under EUA, including REGEN-COV (casirivimab and imdevimab, administered together), and bamlanivimab and etesevimab administered together, for the same uses as previously authorized for bamlanivimab alone,” Dr. Deep said. “The FDA believes that these alternative monoclonal antibody therapies remain appropriate to treat patients with COVID-19, and I would like to see some data about the benefits and risks of these agents,” he noted.
Limitations, funding, and disclosures
The main limitation of the study was the small size and the fact that it was a subgroup analysis of a trial that ended early because of futility, the researchers wrote. However, the Therapeutics for Inpatients With COVID-19 (TICO) platform will proceed with clinical evaluation of additional COVID-19 treatments, they said.
The study was supported primarily by the U.S. government Operation Warp Speed and the National Institute of Allergy and Infectious Diseases. Other funding sources included the Division of Clinical Research and Leidos Biomedical Research for the INSIGHT (International Network for Strategic Initiatives in Global HIV Trials) Network, as well as an agreement between the National Heart, Lung, and Blood Institute and the Research Triangle Institute for the PETAL (Prevention & Early Treatment of Acute Lung Injury) Network and CTSN (Cardiothoracic Surgical Trials Network). Other support came from the U.S. Department of Veterans Affairs and the governments of Denmark (National Research Foundation), Australia (National Health and Medical Research Council), and the United Kingdom (Medical Research Council).
The medications used in the study were donated by Gilead Sciences and Eli Lilly.
The researchers had no financial conflicts do disclose. Dr. Deep and Dr. Pal had no relevant financial conflicts to disclose.
In the randomized controlled trial, in both the group who received bamlanivimab and the group who received placebo, higher antigen and viral RNA levels were associated with a lower proportion of patients achieving recovery.
Other studies have shown that the use of monoclonal antibodies reduces hospitalization risk in outpatients with early COVID-19, and appears to promote viral load decline in the nasopharynx, wrote Jens D. Lundgren, MD, of the University of Copenhagen and colleagues in their article published in the Annals of Internal Medicine. What had been missing prior to this new research was final results from hospitalized patients, the authors said.
In the new study, the researchers randomized 314 adults hospitalized with COVID-19 but without end-organ failure to receive 7,000 mg bamlanivimab (163 patients) or a placebo (151 patients). All patients received study-supplied remdesivir unless contraindicated. The researchers compared the efficacy of bamlanivimab versus placebo, but considered remdesivir the standard of care in this study.
At baseline, 50% of patients overall had antispike endogenous neutralizing antibodies (nAbs), and 50% had SARS-CoV-2 nucleocapsid plasma antigen levels of at least 1,000 ng/L.
The median time to sustained recovery, 19 days, was not significantly different between the bamlanivimab and placebo groups (subhazard ratio, 0.99).
“As hypothesized, among those who were negative for nAb, the difference between bamlanivimab and placebo was more evident if levels of plasma antigen or nasal-swab viral RNA were above the median entry levels,” with subhazard ratios of 1.48 and 1.89, respectively, the researchers explained.
However, the hazard ratio for death for bamlanivimab vs. placebo was 0.45 for patients negative for nAb vs. 3.53 for those positive for nAb. These differences with respect to nAb status were similar across all 90 elements of a composite safety outcome, the researchers said.
Potential benefits remain unclear
The use of neutralizing monoclonal antibodies has been extensively documented as an effective treatment for COVID-19 among ambulatory patients, corresponding author Dr. Lundgren said in an interview.
“Conversely, among admitted patients with COVID-19 pneumonia, the benefit has been questionable,” he said.
The researchers examined a hypothesis that the null finding in hospitalized patients may stem from differences in underlying mechanisms, “either from uncontrolled viral replication – which would be predicted to occur in particular among those not yet been able to mount an endogenous immune response – or from hyperinflammation among those that have mounted such a response,” Dr. Lundgren said.
The study findings supported the stated hypothesis, said Dr. Lundgren. “However, it was surprising that not only was the neutralizing antibody without any benefit among those that had mounted an endogenous immune response, but it actually may have been harmful,” he said.
Bamlanivimab was effective against the viral strain that circulated at the time of enrollment in the study, but subsequent viral strains have appeared to be unaffected by the neutralizing activity of the antibody, said Dr. Lundgren.
From a practical standpoint, “the findings would suggest that use of neutralizing monoclonal antibodies for patients admitted to a hospital with COVID pneumonia should be restricted to those that have not yet mounted an endogenous immune response, as determined by lack of detectable neutralizing antibodies at the time of admission,” Dr. Lundgren said.
Looking ahead, studies are currently underway to examine how the findings translate to vaccinated patients, he added. Other questions to be addressed include whether the benefits and harms apply to some or all neutralizing antibody products, he said.
In addition, “our research consortium is currently doing field testing of several point-of-care test candidates to examine their reliability and functionality,” for how quickly they might identify an endogenous neutralizing antibody response in an admitted COVID pneumonia patient,” Dr. Lundgren noted.
Findings show bamlanivimab’s limits
“Based on the findings of the current study, no clear subgroup of patients could be identified who would benefit from bamlanivimab when hospitalized with COVID-19,” said Suman Pal, MD, of the University of New Mexico, Albuquerque, in an interview.
“The study findings also show possible harm of using bamlanivimab in hospitalized COVID-19 patients who were seropositive for neutralizing antibodies prior to receiving therapy,” Dr. Pal emphasized. “Moreover, the study did not include participants with COVID-19 from variant strains, such as delta and omicron, which currently account for a large number of cases.” “Therefore, the results of this study do not support the use of bamlanivimab in the clinical setting until further evidence is available to guide the selection of patients who may benefit from therapy,” he explained.
“The possible benefit of bamlanivimab does not outweigh the risks in patients hospitalized with COVID-19,” he concluded.
Dr. Pal emphasized the need for larger prospective studies to establish whether bamlanivimab may have benefits in a subgroup of patients, but “well-validated point-of-care tests to identify such patients need to be readily available before this therapy can be considered by clinicians at the bedside,” he concluded.
Diligent screening required before use
Monoclonal antibody treatment has been administered to individuals with diagnosis of COVID-19 infection as outpatients as well as for hospitalized inpatients, said Noel Deep, MD, an internist in Antigo, Wisc., in an interview. “This study is important because it helps physicians and health care institutions to evaluate whether continued use of the monoclonal antibodies would be beneficial and, if so, in what patient populations,” he said.
The findings present interesting implications for the care of COVID-19 patients, said Dr. Deep. “This study indicates that bamlanivimab does not provide the benefit that was initially envisioned when the monoclonal antibody infusions were initially initiated in the treatment of COVID-19 infections. “Serological screening of the patients would help to identify that subgroup of individuals who could benefit from this monoclonal antibody rather than administering it to every COVID-19–positive individual,” he explained.
However, “it is important to note that the emergency use authorization (EUA) for single-agent bamlanivimab has been revoked,” Dr. Deep said.
“The potential benefits of bamlanivimab can be realized only if adequate attention is paid to identifying the appropriate candidates based on serological screening, and administering bamlanivimab to those who are already producing endogenous antibodies could lead to increased risk to those individuals,” he said. Dr. Deep added that he would favor administration of bamlanivimab “in those appropriately screened and eligible candidates, and it is my opinion that the benefits outweigh the risks in those individuals.”
Although the EUA for single-agent bamlanivimab has been revoked, “alternative monoclonal antibody therapies remain available under EUA, including REGEN-COV (casirivimab and imdevimab, administered together), and bamlanivimab and etesevimab administered together, for the same uses as previously authorized for bamlanivimab alone,” Dr. Deep said. “The FDA believes that these alternative monoclonal antibody therapies remain appropriate to treat patients with COVID-19, and I would like to see some data about the benefits and risks of these agents,” he noted.
Limitations, funding, and disclosures
The main limitation of the study was the small size and the fact that it was a subgroup analysis of a trial that ended early because of futility, the researchers wrote. However, the Therapeutics for Inpatients With COVID-19 (TICO) platform will proceed with clinical evaluation of additional COVID-19 treatments, they said.
The study was supported primarily by the U.S. government Operation Warp Speed and the National Institute of Allergy and Infectious Diseases. Other funding sources included the Division of Clinical Research and Leidos Biomedical Research for the INSIGHT (International Network for Strategic Initiatives in Global HIV Trials) Network, as well as an agreement between the National Heart, Lung, and Blood Institute and the Research Triangle Institute for the PETAL (Prevention & Early Treatment of Acute Lung Injury) Network and CTSN (Cardiothoracic Surgical Trials Network). Other support came from the U.S. Department of Veterans Affairs and the governments of Denmark (National Research Foundation), Australia (National Health and Medical Research Council), and the United Kingdom (Medical Research Council).
The medications used in the study were donated by Gilead Sciences and Eli Lilly.
The researchers had no financial conflicts do disclose. Dr. Deep and Dr. Pal had no relevant financial conflicts to disclose.
In the randomized controlled trial, in both the group who received bamlanivimab and the group who received placebo, higher antigen and viral RNA levels were associated with a lower proportion of patients achieving recovery.
Other studies have shown that the use of monoclonal antibodies reduces hospitalization risk in outpatients with early COVID-19, and appears to promote viral load decline in the nasopharynx, wrote Jens D. Lundgren, MD, of the University of Copenhagen and colleagues in their article published in the Annals of Internal Medicine. What had been missing prior to this new research was final results from hospitalized patients, the authors said.
In the new study, the researchers randomized 314 adults hospitalized with COVID-19 but without end-organ failure to receive 7,000 mg bamlanivimab (163 patients) or a placebo (151 patients). All patients received study-supplied remdesivir unless contraindicated. The researchers compared the efficacy of bamlanivimab versus placebo, but considered remdesivir the standard of care in this study.
At baseline, 50% of patients overall had antispike endogenous neutralizing antibodies (nAbs), and 50% had SARS-CoV-2 nucleocapsid plasma antigen levels of at least 1,000 ng/L.
The median time to sustained recovery, 19 days, was not significantly different between the bamlanivimab and placebo groups (subhazard ratio, 0.99).
“As hypothesized, among those who were negative for nAb, the difference between bamlanivimab and placebo was more evident if levels of plasma antigen or nasal-swab viral RNA were above the median entry levels,” with subhazard ratios of 1.48 and 1.89, respectively, the researchers explained.
However, the hazard ratio for death for bamlanivimab vs. placebo was 0.45 for patients negative for nAb vs. 3.53 for those positive for nAb. These differences with respect to nAb status were similar across all 90 elements of a composite safety outcome, the researchers said.
Potential benefits remain unclear
The use of neutralizing monoclonal antibodies has been extensively documented as an effective treatment for COVID-19 among ambulatory patients, corresponding author Dr. Lundgren said in an interview.
“Conversely, among admitted patients with COVID-19 pneumonia, the benefit has been questionable,” he said.
The researchers examined a hypothesis that the null finding in hospitalized patients may stem from differences in underlying mechanisms, “either from uncontrolled viral replication – which would be predicted to occur in particular among those not yet been able to mount an endogenous immune response – or from hyperinflammation among those that have mounted such a response,” Dr. Lundgren said.
The study findings supported the stated hypothesis, said Dr. Lundgren. “However, it was surprising that not only was the neutralizing antibody without any benefit among those that had mounted an endogenous immune response, but it actually may have been harmful,” he said.
Bamlanivimab was effective against the viral strain that circulated at the time of enrollment in the study, but subsequent viral strains have appeared to be unaffected by the neutralizing activity of the antibody, said Dr. Lundgren.
From a practical standpoint, “the findings would suggest that use of neutralizing monoclonal antibodies for patients admitted to a hospital with COVID pneumonia should be restricted to those that have not yet mounted an endogenous immune response, as determined by lack of detectable neutralizing antibodies at the time of admission,” Dr. Lundgren said.
Looking ahead, studies are currently underway to examine how the findings translate to vaccinated patients, he added. Other questions to be addressed include whether the benefits and harms apply to some or all neutralizing antibody products, he said.
In addition, “our research consortium is currently doing field testing of several point-of-care test candidates to examine their reliability and functionality,” for how quickly they might identify an endogenous neutralizing antibody response in an admitted COVID pneumonia patient,” Dr. Lundgren noted.
Findings show bamlanivimab’s limits
“Based on the findings of the current study, no clear subgroup of patients could be identified who would benefit from bamlanivimab when hospitalized with COVID-19,” said Suman Pal, MD, of the University of New Mexico, Albuquerque, in an interview.
“The study findings also show possible harm of using bamlanivimab in hospitalized COVID-19 patients who were seropositive for neutralizing antibodies prior to receiving therapy,” Dr. Pal emphasized. “Moreover, the study did not include participants with COVID-19 from variant strains, such as delta and omicron, which currently account for a large number of cases.” “Therefore, the results of this study do not support the use of bamlanivimab in the clinical setting until further evidence is available to guide the selection of patients who may benefit from therapy,” he explained.
“The possible benefit of bamlanivimab does not outweigh the risks in patients hospitalized with COVID-19,” he concluded.
Dr. Pal emphasized the need for larger prospective studies to establish whether bamlanivimab may have benefits in a subgroup of patients, but “well-validated point-of-care tests to identify such patients need to be readily available before this therapy can be considered by clinicians at the bedside,” he concluded.
Diligent screening required before use
Monoclonal antibody treatment has been administered to individuals with diagnosis of COVID-19 infection as outpatients as well as for hospitalized inpatients, said Noel Deep, MD, an internist in Antigo, Wisc., in an interview. “This study is important because it helps physicians and health care institutions to evaluate whether continued use of the monoclonal antibodies would be beneficial and, if so, in what patient populations,” he said.
The findings present interesting implications for the care of COVID-19 patients, said Dr. Deep. “This study indicates that bamlanivimab does not provide the benefit that was initially envisioned when the monoclonal antibody infusions were initially initiated in the treatment of COVID-19 infections. “Serological screening of the patients would help to identify that subgroup of individuals who could benefit from this monoclonal antibody rather than administering it to every COVID-19–positive individual,” he explained.
However, “it is important to note that the emergency use authorization (EUA) for single-agent bamlanivimab has been revoked,” Dr. Deep said.
“The potential benefits of bamlanivimab can be realized only if adequate attention is paid to identifying the appropriate candidates based on serological screening, and administering bamlanivimab to those who are already producing endogenous antibodies could lead to increased risk to those individuals,” he said. Dr. Deep added that he would favor administration of bamlanivimab “in those appropriately screened and eligible candidates, and it is my opinion that the benefits outweigh the risks in those individuals.”
Although the EUA for single-agent bamlanivimab has been revoked, “alternative monoclonal antibody therapies remain available under EUA, including REGEN-COV (casirivimab and imdevimab, administered together), and bamlanivimab and etesevimab administered together, for the same uses as previously authorized for bamlanivimab alone,” Dr. Deep said. “The FDA believes that these alternative monoclonal antibody therapies remain appropriate to treat patients with COVID-19, and I would like to see some data about the benefits and risks of these agents,” he noted.
Limitations, funding, and disclosures
The main limitation of the study was the small size and the fact that it was a subgroup analysis of a trial that ended early because of futility, the researchers wrote. However, the Therapeutics for Inpatients With COVID-19 (TICO) platform will proceed with clinical evaluation of additional COVID-19 treatments, they said.
The study was supported primarily by the U.S. government Operation Warp Speed and the National Institute of Allergy and Infectious Diseases. Other funding sources included the Division of Clinical Research and Leidos Biomedical Research for the INSIGHT (International Network for Strategic Initiatives in Global HIV Trials) Network, as well as an agreement between the National Heart, Lung, and Blood Institute and the Research Triangle Institute for the PETAL (Prevention & Early Treatment of Acute Lung Injury) Network and CTSN (Cardiothoracic Surgical Trials Network). Other support came from the U.S. Department of Veterans Affairs and the governments of Denmark (National Research Foundation), Australia (National Health and Medical Research Council), and the United Kingdom (Medical Research Council).
The medications used in the study were donated by Gilead Sciences and Eli Lilly.
The researchers had no financial conflicts do disclose. Dr. Deep and Dr. Pal had no relevant financial conflicts to disclose.
FROM ANNALS OF INTERNAL MEDICINE
Children and COVID: New cases up slightly, vaccinations continue to slow
New COVID-19 vaccinations in children were down by almost 24% in the last week as new cases rose by just 3.5%, based on new data.
That fairly low number suggests the latest case count from the American Academy of Pediatrics and the Children’s Hospital Association has not caught up yet to the reality of the Omicron variant, which has sent new cases climbing among all ages and now represents the majority of COVID-19 infections nationwide, the Centers for Disease Control and Prevention said.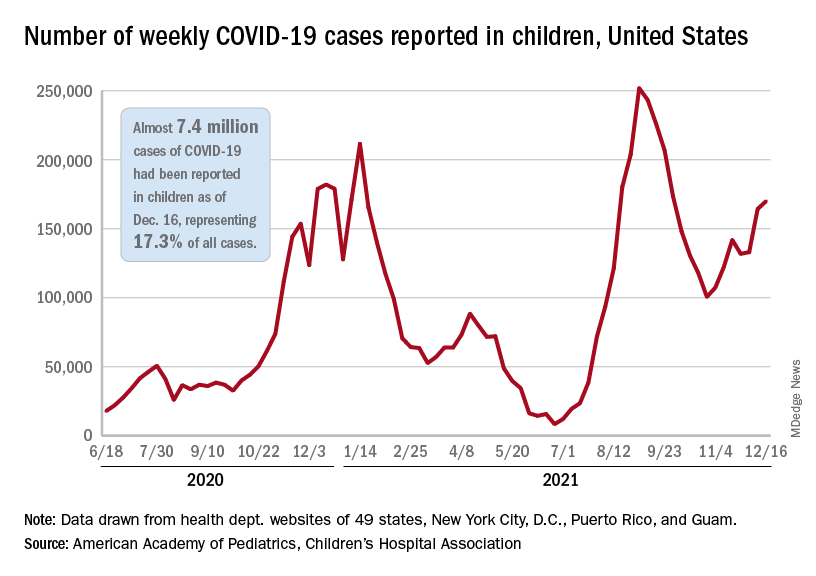
Meanwhile, in the midst of the latest surge, the United States just passed yet another sobering COVID milestone: 1,000 deaths in children aged 17 and under. The total as of Dec. 20 was 1,015, according to the CDC, with the largest share, almost 32%, occurring in children less than 5 years of age.
Regionally, the majority of that increase came in the Northeast, with a small rise in the South and decreases in the Midwest and West, the AAP and CHA said in their weekly COVID report.
At the state level, the largest percent increases in cases over the past 2 weeks were seen in Maine and New Hampshire, as well as Vermont, which has the nation’s highest vaccination rates for children aged 5-11 (51%) and 12-17 (84%), the AAP said in its vaccination trends report.
Nationally, new COVID vaccinations in children continue to trend downward. The number of children aged 5-17 years who had received at least one dose increased by about 498,000 for the week of Dec. 13-19, down from 654,000 (–23.9%) the previous week. Children aged 5-11 years still represented the largest share (22.7%) of all vaccine initiators in the last 2 weeks, but that proportion was 42.8% just before Thanksgiving, according to data from the CDC.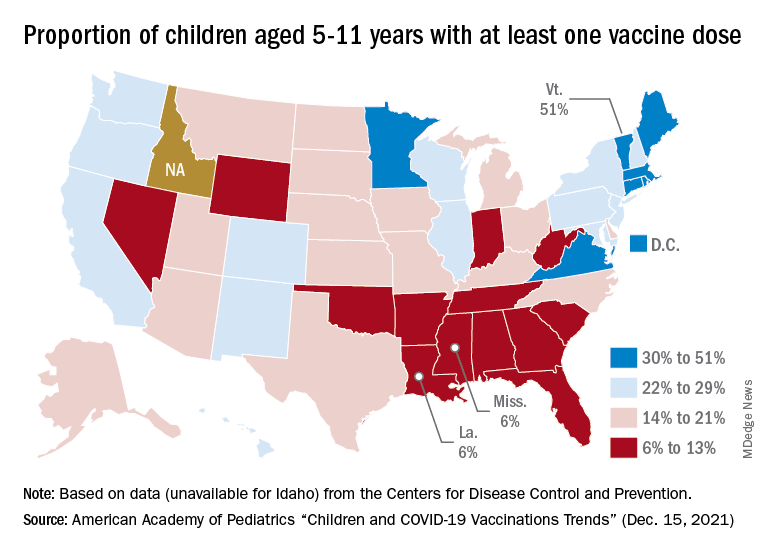
On a more positive note, children aged 5-11 made up 51% of all Americans who completed the vaccine regimen during the 2 weeks ending Dec. 20. The cumulative completion count is 3.6 million in that age group, along with almost 13.4 million children aged 12-17, and the CDC data show that 6.1 million children aged 5-11 and 15.9 million children aged 12-17 have received at least one dose.
On a less positive note, however, that means almost half (47%) of 12- to 17-year-olds still are not fully vaccinated and that over a third (37%) have received no vaccine at all, according to the COVID Data Tracker.
New COVID-19 vaccinations in children were down by almost 24% in the last week as new cases rose by just 3.5%, based on new data.
That fairly low number suggests the latest case count from the American Academy of Pediatrics and the Children’s Hospital Association has not caught up yet to the reality of the Omicron variant, which has sent new cases climbing among all ages and now represents the majority of COVID-19 infections nationwide, the Centers for Disease Control and Prevention said.
Meanwhile, in the midst of the latest surge, the United States just passed yet another sobering COVID milestone: 1,000 deaths in children aged 17 and under. The total as of Dec. 20 was 1,015, according to the CDC, with the largest share, almost 32%, occurring in children less than 5 years of age.
Regionally, the majority of that increase came in the Northeast, with a small rise in the South and decreases in the Midwest and West, the AAP and CHA said in their weekly COVID report.
At the state level, the largest percent increases in cases over the past 2 weeks were seen in Maine and New Hampshire, as well as Vermont, which has the nation’s highest vaccination rates for children aged 5-11 (51%) and 12-17 (84%), the AAP said in its vaccination trends report.
Nationally, new COVID vaccinations in children continue to trend downward. The number of children aged 5-17 years who had received at least one dose increased by about 498,000 for the week of Dec. 13-19, down from 654,000 (–23.9%) the previous week. Children aged 5-11 years still represented the largest share (22.7%) of all vaccine initiators in the last 2 weeks, but that proportion was 42.8% just before Thanksgiving, according to data from the CDC.
On a more positive note, children aged 5-11 made up 51% of all Americans who completed the vaccine regimen during the 2 weeks ending Dec. 20. The cumulative completion count is 3.6 million in that age group, along with almost 13.4 million children aged 12-17, and the CDC data show that 6.1 million children aged 5-11 and 15.9 million children aged 12-17 have received at least one dose.
On a less positive note, however, that means almost half (47%) of 12- to 17-year-olds still are not fully vaccinated and that over a third (37%) have received no vaccine at all, according to the COVID Data Tracker.
New COVID-19 vaccinations in children were down by almost 24% in the last week as new cases rose by just 3.5%, based on new data.
That fairly low number suggests the latest case count from the American Academy of Pediatrics and the Children’s Hospital Association has not caught up yet to the reality of the Omicron variant, which has sent new cases climbing among all ages and now represents the majority of COVID-19 infections nationwide, the Centers for Disease Control and Prevention said.
Meanwhile, in the midst of the latest surge, the United States just passed yet another sobering COVID milestone: 1,000 deaths in children aged 17 and under. The total as of Dec. 20 was 1,015, according to the CDC, with the largest share, almost 32%, occurring in children less than 5 years of age.
Regionally, the majority of that increase came in the Northeast, with a small rise in the South and decreases in the Midwest and West, the AAP and CHA said in their weekly COVID report.
At the state level, the largest percent increases in cases over the past 2 weeks were seen in Maine and New Hampshire, as well as Vermont, which has the nation’s highest vaccination rates for children aged 5-11 (51%) and 12-17 (84%), the AAP said in its vaccination trends report.
Nationally, new COVID vaccinations in children continue to trend downward. The number of children aged 5-17 years who had received at least one dose increased by about 498,000 for the week of Dec. 13-19, down from 654,000 (–23.9%) the previous week. Children aged 5-11 years still represented the largest share (22.7%) of all vaccine initiators in the last 2 weeks, but that proportion was 42.8% just before Thanksgiving, according to data from the CDC.
On a more positive note, children aged 5-11 made up 51% of all Americans who completed the vaccine regimen during the 2 weeks ending Dec. 20. The cumulative completion count is 3.6 million in that age group, along with almost 13.4 million children aged 12-17, and the CDC data show that 6.1 million children aged 5-11 and 15.9 million children aged 12-17 have received at least one dose.
On a less positive note, however, that means almost half (47%) of 12- to 17-year-olds still are not fully vaccinated and that over a third (37%) have received no vaccine at all, according to the COVID Data Tracker.
Many clinicians feel ill-prepared for drug overdose deaths
new research suggests.
However, results from a survey study also showed that colleagues were an important source of support in the wake of this type of event.
“A patient overdose death can change clinical decision-making for providers experiencing high levels of stress related to the overdose death,” noted the investigators, led by Amy Yule, MD, director of adolescent addiction psychiatry, Boston Medical Center, and assistant professor of psychiatry at Boston University Medical Center.
The findings were presented by Dr. Yule at the annual meeting of the American Academy of Addiction Psychiatry.
All-time high
As reported by this news organization, there has recently been a record number of drug overdose deaths. And these deaths affect families, communities, and often providers, Dr. Yule told meeting attendees.
Previous research has looked at the impact of drug overdose deaths and the opioid epidemic on first responders and community health workers in the field of overdose prevention.
“But there’s less in the literature to my knowledge that describes the experience of providers and clinicians who are working in more formalized medical settings,” said Dr. Yule.
In December 2020, researchers sent an email to members of the Providers Clinical Support System (PCSS) inviting them to complete an anonymous survey. The PCSS program was created in response to the opioid overdose epidemic to train primary care clinicians in the prevention and treatment of opioid use disorders.
A total of 12,204 members received the email, 1,064 opened the survey link, and 523 completed the survey.
Participants were mostly White and female, with an average age of 52 years. Respondents had been practicing for an average of about 16 years.
The largest responder group was physicians (47%), followed by counselors (29%), nurse practitioners (17%), and nurses (7%).
Among physician respondents, 41% reported having received additional formal training in addiction.
Only 24% of the respondents indicated they received training in “postvention,” which refers to interventions after a suicide to support the bereaved. Such interventions “could be helpful in potentially preparing them for a drug overdose death in their practice,” said Dr. Yule.
Categories of preparedness
The survey inquired about three categories of preparedness: coping with a drug overdose death, providing support to a colleague, and talking with families who have lost a member to a drug overdose.
Overall, 59% said they felt somewhat or fairly well prepared for the first two categories and 55% for the third category.
“I think it’s notable that there is a higher percentage of people who felt not at all prepared to talk with family members (20.5%), compared to those who felt not at all prepared to cope with a drug overdose death (13.8%) or prepared to support a colleague (12%),” Dr. Yule said.
More than half of respondents (55%) indicated a drug overdose death had occurred in their own practice.
The survey also looked at frequency of consultations with colleagues, critical incident debriefing sessions, and interactions with a patient’s family.
Almost half (48%) of the sample said they consulted with a colleague after most patient overdose deaths. Only 24% said they had a critical incidence debriefing session after most of these events, and 20% said they interacted with the patient’s family.
Asked what resources they found helpful for coping with a recent patient drug overdose death, respondents flagged their colleagues and meetings with families.
The survey also examined provider trauma after a patient drug overdose death, using the Impact of Event Scale–R. “If the score is above a certain cutoff level, there is potential concern” for PTSD, Dr. Yule said.
Among the 141 respondents who had a patient drug overdose death in their practice during the previous year, 121 completed this trauma scale. Of these, 18% had “a very elevated” score, Dr. Yule reported.
Sources of support
Commenting on the survey study, Larissa Mooney, MD, associate professor and director of the addiction psychiatry division in the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles, said it is not surprising that many providers do not feel adequately prepared to cope with an overdose death, or how to support a colleague after such an event.
“This is not routinely covered in training, and patient overdose may occur without warning signs,” said Dr. Mooney, who was not involved with the research.
However, these new findings suggest a range of potential sources of support for providers after a patient overdose death that may be helpful, “including colleagues, friends, therapy, supervision, and meeting with the patient’s family,” she said.
The study received funding from the PCSS. Dr. Yule disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
However, results from a survey study also showed that colleagues were an important source of support in the wake of this type of event.
“A patient overdose death can change clinical decision-making for providers experiencing high levels of stress related to the overdose death,” noted the investigators, led by Amy Yule, MD, director of adolescent addiction psychiatry, Boston Medical Center, and assistant professor of psychiatry at Boston University Medical Center.
The findings were presented by Dr. Yule at the annual meeting of the American Academy of Addiction Psychiatry.
All-time high
As reported by this news organization, there has recently been a record number of drug overdose deaths. And these deaths affect families, communities, and often providers, Dr. Yule told meeting attendees.
Previous research has looked at the impact of drug overdose deaths and the opioid epidemic on first responders and community health workers in the field of overdose prevention.
“But there’s less in the literature to my knowledge that describes the experience of providers and clinicians who are working in more formalized medical settings,” said Dr. Yule.
In December 2020, researchers sent an email to members of the Providers Clinical Support System (PCSS) inviting them to complete an anonymous survey. The PCSS program was created in response to the opioid overdose epidemic to train primary care clinicians in the prevention and treatment of opioid use disorders.
A total of 12,204 members received the email, 1,064 opened the survey link, and 523 completed the survey.
Participants were mostly White and female, with an average age of 52 years. Respondents had been practicing for an average of about 16 years.
The largest responder group was physicians (47%), followed by counselors (29%), nurse practitioners (17%), and nurses (7%).
Among physician respondents, 41% reported having received additional formal training in addiction.
Only 24% of the respondents indicated they received training in “postvention,” which refers to interventions after a suicide to support the bereaved. Such interventions “could be helpful in potentially preparing them for a drug overdose death in their practice,” said Dr. Yule.
Categories of preparedness
The survey inquired about three categories of preparedness: coping with a drug overdose death, providing support to a colleague, and talking with families who have lost a member to a drug overdose.
Overall, 59% said they felt somewhat or fairly well prepared for the first two categories and 55% for the third category.
“I think it’s notable that there is a higher percentage of people who felt not at all prepared to talk with family members (20.5%), compared to those who felt not at all prepared to cope with a drug overdose death (13.8%) or prepared to support a colleague (12%),” Dr. Yule said.
More than half of respondents (55%) indicated a drug overdose death had occurred in their own practice.
The survey also looked at frequency of consultations with colleagues, critical incident debriefing sessions, and interactions with a patient’s family.
Almost half (48%) of the sample said they consulted with a colleague after most patient overdose deaths. Only 24% said they had a critical incidence debriefing session after most of these events, and 20% said they interacted with the patient’s family.
Asked what resources they found helpful for coping with a recent patient drug overdose death, respondents flagged their colleagues and meetings with families.
The survey also examined provider trauma after a patient drug overdose death, using the Impact of Event Scale–R. “If the score is above a certain cutoff level, there is potential concern” for PTSD, Dr. Yule said.
Among the 141 respondents who had a patient drug overdose death in their practice during the previous year, 121 completed this trauma scale. Of these, 18% had “a very elevated” score, Dr. Yule reported.
Sources of support
Commenting on the survey study, Larissa Mooney, MD, associate professor and director of the addiction psychiatry division in the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles, said it is not surprising that many providers do not feel adequately prepared to cope with an overdose death, or how to support a colleague after such an event.
“This is not routinely covered in training, and patient overdose may occur without warning signs,” said Dr. Mooney, who was not involved with the research.
However, these new findings suggest a range of potential sources of support for providers after a patient overdose death that may be helpful, “including colleagues, friends, therapy, supervision, and meeting with the patient’s family,” she said.
The study received funding from the PCSS. Dr. Yule disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
However, results from a survey study also showed that colleagues were an important source of support in the wake of this type of event.
“A patient overdose death can change clinical decision-making for providers experiencing high levels of stress related to the overdose death,” noted the investigators, led by Amy Yule, MD, director of adolescent addiction psychiatry, Boston Medical Center, and assistant professor of psychiatry at Boston University Medical Center.
The findings were presented by Dr. Yule at the annual meeting of the American Academy of Addiction Psychiatry.
All-time high
As reported by this news organization, there has recently been a record number of drug overdose deaths. And these deaths affect families, communities, and often providers, Dr. Yule told meeting attendees.
Previous research has looked at the impact of drug overdose deaths and the opioid epidemic on first responders and community health workers in the field of overdose prevention.
“But there’s less in the literature to my knowledge that describes the experience of providers and clinicians who are working in more formalized medical settings,” said Dr. Yule.
In December 2020, researchers sent an email to members of the Providers Clinical Support System (PCSS) inviting them to complete an anonymous survey. The PCSS program was created in response to the opioid overdose epidemic to train primary care clinicians in the prevention and treatment of opioid use disorders.
A total of 12,204 members received the email, 1,064 opened the survey link, and 523 completed the survey.
Participants were mostly White and female, with an average age of 52 years. Respondents had been practicing for an average of about 16 years.
The largest responder group was physicians (47%), followed by counselors (29%), nurse practitioners (17%), and nurses (7%).
Among physician respondents, 41% reported having received additional formal training in addiction.
Only 24% of the respondents indicated they received training in “postvention,” which refers to interventions after a suicide to support the bereaved. Such interventions “could be helpful in potentially preparing them for a drug overdose death in their practice,” said Dr. Yule.
Categories of preparedness
The survey inquired about three categories of preparedness: coping with a drug overdose death, providing support to a colleague, and talking with families who have lost a member to a drug overdose.
Overall, 59% said they felt somewhat or fairly well prepared for the first two categories and 55% for the third category.
“I think it’s notable that there is a higher percentage of people who felt not at all prepared to talk with family members (20.5%), compared to those who felt not at all prepared to cope with a drug overdose death (13.8%) or prepared to support a colleague (12%),” Dr. Yule said.
More than half of respondents (55%) indicated a drug overdose death had occurred in their own practice.
The survey also looked at frequency of consultations with colleagues, critical incident debriefing sessions, and interactions with a patient’s family.
Almost half (48%) of the sample said they consulted with a colleague after most patient overdose deaths. Only 24% said they had a critical incidence debriefing session after most of these events, and 20% said they interacted with the patient’s family.
Asked what resources they found helpful for coping with a recent patient drug overdose death, respondents flagged their colleagues and meetings with families.
The survey also examined provider trauma after a patient drug overdose death, using the Impact of Event Scale–R. “If the score is above a certain cutoff level, there is potential concern” for PTSD, Dr. Yule said.
Among the 141 respondents who had a patient drug overdose death in their practice during the previous year, 121 completed this trauma scale. Of these, 18% had “a very elevated” score, Dr. Yule reported.
Sources of support
Commenting on the survey study, Larissa Mooney, MD, associate professor and director of the addiction psychiatry division in the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles, said it is not surprising that many providers do not feel adequately prepared to cope with an overdose death, or how to support a colleague after such an event.
“This is not routinely covered in training, and patient overdose may occur without warning signs,” said Dr. Mooney, who was not involved with the research.
However, these new findings suggest a range of potential sources of support for providers after a patient overdose death that may be helpful, “including colleagues, friends, therapy, supervision, and meeting with the patient’s family,” she said.
The study received funding from the PCSS. Dr. Yule disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAAP 2021
Emergency docs cite ‘dire’ situation as COVID grows, nurses scarce
With emergency departments straining to keep up with the latest COVID surge, the American College of Emergency Physicians
The organization said that it is “very concerned that nursing shortages in emergency departments can complicate patient access to care and add to incredible levels of stress already on physician-led care teams,” according to a press release.
ACEP President Gillian Schmitz, MD, told this news organization, “The situation is dire in many emergency departments around the country. Emergency physicians are seeing more patients with fewer resources and less staff.
“Emergency physicians in the hardest hit communities are scrambling to locate available experts, exhausting federal support, and doing all they can to adapt to the demands of the current surge – everyone is being stretched to their limit.”
The Emergency Nurses Association (ENA) agrees with ACEP’s call for a team approach to stemming the shortage.
ENA President Ron Kraus, MSN, RN, said in an interview, “The pandemic has only amplified several long-standing issues impacting emergency nurses, such as workplace violence, a healthy work environment, and concerns about staffing shortages and the pipeline of new nurses. That said, we can’t lose focus on what’s most important in these challenging moments – ensuring every patient receives the high quality of care.”
The responsibility falls on the “collaborative effort” of the emergency department with emergency nurses playing a pivotal role, he said. But the stress, fatigue, and burnout driving nurses away from their jobs “should not be viewed as added inconvenience to anyone during a pandemic, but as a long-term threat to our health care system.”
ACEP’s press release stated that with fewer nurses available in the emergency department, team members are clocking extra hours, caring for more patients, and stretched to take on additional clinical and nonclinical duties.
“I am hearing from colleagues from Washington state to Michigan to New York that this is the worst they have seen since the beginning of the pandemic,” Dr. Schmitz said. “Everyone available is filling gaps as best they can, but the current path for many frontline workers is not sustainable,” she said in the release.
Meanwhile, ACEP is also tackling violence in the emergency department and has initiatives to protect the mental health of those working on the front lines, the release states.
“Emergency physicians will continue to do everything necessary to treat patients,” Dr. Schmitz said in the release, “but it will take a collaborative effort with legislators, policymakers and health system leaders to strengthen care teams, improve access and address capacity concerns with solutions that can save lives right now and in the months ahead.”
Dr. Schmitz stated that in Washington state, ICUs are at 97% to 100% capacity and less than 30 pediatric inpatient beds are available in the western part of the state.
“In Michigan and New York, several emergency departments are overflowing, and doctors are being called in to triage people in the waiting room because all of the emergency department beds are holding admissions. There are scenarios where entire hospitals are backing up into the emergency department and waiting room and we are physically running out of space and nursing staff.”
ACEP represents its 40,000 emergency physician members.
A version of this article first appeared on Medscape.com.
With emergency departments straining to keep up with the latest COVID surge, the American College of Emergency Physicians
The organization said that it is “very concerned that nursing shortages in emergency departments can complicate patient access to care and add to incredible levels of stress already on physician-led care teams,” according to a press release.
ACEP President Gillian Schmitz, MD, told this news organization, “The situation is dire in many emergency departments around the country. Emergency physicians are seeing more patients with fewer resources and less staff.
“Emergency physicians in the hardest hit communities are scrambling to locate available experts, exhausting federal support, and doing all they can to adapt to the demands of the current surge – everyone is being stretched to their limit.”
The Emergency Nurses Association (ENA) agrees with ACEP’s call for a team approach to stemming the shortage.
ENA President Ron Kraus, MSN, RN, said in an interview, “The pandemic has only amplified several long-standing issues impacting emergency nurses, such as workplace violence, a healthy work environment, and concerns about staffing shortages and the pipeline of new nurses. That said, we can’t lose focus on what’s most important in these challenging moments – ensuring every patient receives the high quality of care.”
The responsibility falls on the “collaborative effort” of the emergency department with emergency nurses playing a pivotal role, he said. But the stress, fatigue, and burnout driving nurses away from their jobs “should not be viewed as added inconvenience to anyone during a pandemic, but as a long-term threat to our health care system.”
ACEP’s press release stated that with fewer nurses available in the emergency department, team members are clocking extra hours, caring for more patients, and stretched to take on additional clinical and nonclinical duties.
“I am hearing from colleagues from Washington state to Michigan to New York that this is the worst they have seen since the beginning of the pandemic,” Dr. Schmitz said. “Everyone available is filling gaps as best they can, but the current path for many frontline workers is not sustainable,” she said in the release.
Meanwhile, ACEP is also tackling violence in the emergency department and has initiatives to protect the mental health of those working on the front lines, the release states.
“Emergency physicians will continue to do everything necessary to treat patients,” Dr. Schmitz said in the release, “but it will take a collaborative effort with legislators, policymakers and health system leaders to strengthen care teams, improve access and address capacity concerns with solutions that can save lives right now and in the months ahead.”
Dr. Schmitz stated that in Washington state, ICUs are at 97% to 100% capacity and less than 30 pediatric inpatient beds are available in the western part of the state.
“In Michigan and New York, several emergency departments are overflowing, and doctors are being called in to triage people in the waiting room because all of the emergency department beds are holding admissions. There are scenarios where entire hospitals are backing up into the emergency department and waiting room and we are physically running out of space and nursing staff.”
ACEP represents its 40,000 emergency physician members.
A version of this article first appeared on Medscape.com.
With emergency departments straining to keep up with the latest COVID surge, the American College of Emergency Physicians
The organization said that it is “very concerned that nursing shortages in emergency departments can complicate patient access to care and add to incredible levels of stress already on physician-led care teams,” according to a press release.
ACEP President Gillian Schmitz, MD, told this news organization, “The situation is dire in many emergency departments around the country. Emergency physicians are seeing more patients with fewer resources and less staff.
“Emergency physicians in the hardest hit communities are scrambling to locate available experts, exhausting federal support, and doing all they can to adapt to the demands of the current surge – everyone is being stretched to their limit.”
The Emergency Nurses Association (ENA) agrees with ACEP’s call for a team approach to stemming the shortage.
ENA President Ron Kraus, MSN, RN, said in an interview, “The pandemic has only amplified several long-standing issues impacting emergency nurses, such as workplace violence, a healthy work environment, and concerns about staffing shortages and the pipeline of new nurses. That said, we can’t lose focus on what’s most important in these challenging moments – ensuring every patient receives the high quality of care.”
The responsibility falls on the “collaborative effort” of the emergency department with emergency nurses playing a pivotal role, he said. But the stress, fatigue, and burnout driving nurses away from their jobs “should not be viewed as added inconvenience to anyone during a pandemic, but as a long-term threat to our health care system.”
ACEP’s press release stated that with fewer nurses available in the emergency department, team members are clocking extra hours, caring for more patients, and stretched to take on additional clinical and nonclinical duties.
“I am hearing from colleagues from Washington state to Michigan to New York that this is the worst they have seen since the beginning of the pandemic,” Dr. Schmitz said. “Everyone available is filling gaps as best they can, but the current path for many frontline workers is not sustainable,” she said in the release.
Meanwhile, ACEP is also tackling violence in the emergency department and has initiatives to protect the mental health of those working on the front lines, the release states.
“Emergency physicians will continue to do everything necessary to treat patients,” Dr. Schmitz said in the release, “but it will take a collaborative effort with legislators, policymakers and health system leaders to strengthen care teams, improve access and address capacity concerns with solutions that can save lives right now and in the months ahead.”
Dr. Schmitz stated that in Washington state, ICUs are at 97% to 100% capacity and less than 30 pediatric inpatient beds are available in the western part of the state.
“In Michigan and New York, several emergency departments are overflowing, and doctors are being called in to triage people in the waiting room because all of the emergency department beds are holding admissions. There are scenarios where entire hospitals are backing up into the emergency department and waiting room and we are physically running out of space and nursing staff.”
ACEP represents its 40,000 emergency physician members.
A version of this article first appeared on Medscape.com.



