User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Who benefits most from device PFO closure after a stroke?
It has been well established that device closure has, on average, prevented stroke recurrence in people who’ve had patent foramen ovale–associated stroke, but a meta-analysis has drilled down into clinical trials to advance a potentially practice-changing principle: that, while device closure shows an overall benefit, not all patients derive a benefit and some may actually be harmed by the procedure.
What’s more, the researchers developed a scoring system that helps determine which patients are likely to benefit from device closure.
“What was unknown was how to treat individual patients because the decision to close the patent foramen ovale (PFO) is still preference sensitive because the risk of a recurrent stroke is low, and most of the strokes that recur are not terribly severe,” lead study author David M. Kent, MD, MS, said in an interview.
“On top of this,” he said, “it was still suspected that some of the PFOs, even in trials of well-selected patients, may not be causally related to stroke; the stroke may still have another occult cause, such as paroxysmal atrial fibrillation or aortic arch atheroma.” Dr. Kent is a professor of medicine at Tufts University in Boston and director of the Predictive Analytics and Comparative Effectiveness Center there.
The meta-analysis, conducted by the Systematic, Collaborative, PFO Closure Evaluation (SCOPE) consortium, analyzed data from six randomized clinical trials that compared device closure and medical therapy to medical therapy alone in 3,740 patients who had PFO-associated stroke from 2000 to 2017. It was published in JAMA.
Overall, the rate of recurrent ischemic stroke was less than half that in patients who had device closure, compared with those who were on medical therapy: 0.47% (n = 39 of 1,889) vs. 1.09% (n = 82 of 1,851).
The researchers also applied two tools designed to calculate the probability of recurrent stroke in individual patients: Risk of Paradoxical Embolism (RoPE), an index that assigns a score of 0-10 to stratify cryptogenic stroke patients with PFO by the likelihood that the stroke was associated with their PFO; and the PFO-Associated Stroke Causal Likelihood (PASCAL) classification system, which integrates the RoPE score with physiological and anatomical features – namely, the size of the PFO shunt and the presence of an atrial septal aneurysm.
“We came up with a way to more accurately identify those patients who are likely to get the most benefit from PFO closure based on mathematic modeling that estimates an individual’s probability that the PFO is causally related to the stroke,” Dr. Kent said.
Multivariate analysis determines risk
The study used a multivariate classification system that Dr. Kent had been developing to perform subgroup analyses of the clinical trials. It assigned patients to three different risk groups based on the likelihood that the PFO was causally related to their stroke: PASCAL categories of unlikely, possible, and probable.
The PASCAL unlikely group had a risk of stroke recurrence in the first 2 years of 3.4% (95% confidence interval, 1.1%-5.7%) if they were on medical therapy, and 4.1% (95% CI, 1.7%-6.4%) if they had device closure. In the PASCAL possible group, those risks were 3.6% (95% CI, 2.4%-4.9%) and 1.5% (95% CI, 0.7-2.3%), respectively. For the probable group, device closure represents “a near perfect therapy” with a 90% risk reduction, Dr. Kent said. “Moreover,” he said, “adverse events of device closure, such as atrial fibrillation, appear to be concentrated in those patients who fall into the unlikely classification, who appear to get no benefit.”
The ideal patient for device closure is age 60 years or younger and without vascular risk factors such as hypertension, diabetes, a history of smoking, or a prior stroke, but has high-risk PFO features such as a large shunt or atrial septal aneurysm, Dr. Kent said.
“We think these findings should be practice changing now,” Dr. Kent said.
Faisal M. Merchant, MD, director of cardiac electrophysiology at Emory Healthcare in Atlanta, concurred with that statement. “This is in my mind probably as good as any data we’re going to get on this,” he said in an interview. “The results support what’s been a general gestalt in the clinical world, but [also] really provide an evidence base on how to make decisions.”
He noted that guidelines, including those of the American Academy of Neurology, recommend medical therapy or device closure to prevent recurrent stroke in people who’ve had PFO-associated ischemic stroke. “But they hedge a bit,” he said of the guidelines. “We haven’t had data that’s as robust as this. I think this really solidifies those recommendations.”
He also credited the “unique” study design to extract findings from clinical trials and apply them to personalized medicine. “Clinical trial results give you an average treatment effect of the patients included, but who are ones who really benefit? Who are the ones that don’t benefit? Who are the ones who are harmed?” Dr. Merchant said. “It’s rare that you can parse out this nicely between the people who both benefit and are less likely to be harmed and the people who don’t benefit and are more likely to be harmed.”
The study received funding from the Patient-Centered Outcomes Research Institute. Dr. Kent disclosed relationships with PCORI, W.L. Gore and the Canadian Stroke Consortium. Dr. Merchant has no relevant disclosures.
It has been well established that device closure has, on average, prevented stroke recurrence in people who’ve had patent foramen ovale–associated stroke, but a meta-analysis has drilled down into clinical trials to advance a potentially practice-changing principle: that, while device closure shows an overall benefit, not all patients derive a benefit and some may actually be harmed by the procedure.
What’s more, the researchers developed a scoring system that helps determine which patients are likely to benefit from device closure.
“What was unknown was how to treat individual patients because the decision to close the patent foramen ovale (PFO) is still preference sensitive because the risk of a recurrent stroke is low, and most of the strokes that recur are not terribly severe,” lead study author David M. Kent, MD, MS, said in an interview.
“On top of this,” he said, “it was still suspected that some of the PFOs, even in trials of well-selected patients, may not be causally related to stroke; the stroke may still have another occult cause, such as paroxysmal atrial fibrillation or aortic arch atheroma.” Dr. Kent is a professor of medicine at Tufts University in Boston and director of the Predictive Analytics and Comparative Effectiveness Center there.
The meta-analysis, conducted by the Systematic, Collaborative, PFO Closure Evaluation (SCOPE) consortium, analyzed data from six randomized clinical trials that compared device closure and medical therapy to medical therapy alone in 3,740 patients who had PFO-associated stroke from 2000 to 2017. It was published in JAMA.
Overall, the rate of recurrent ischemic stroke was less than half that in patients who had device closure, compared with those who were on medical therapy: 0.47% (n = 39 of 1,889) vs. 1.09% (n = 82 of 1,851).
The researchers also applied two tools designed to calculate the probability of recurrent stroke in individual patients: Risk of Paradoxical Embolism (RoPE), an index that assigns a score of 0-10 to stratify cryptogenic stroke patients with PFO by the likelihood that the stroke was associated with their PFO; and the PFO-Associated Stroke Causal Likelihood (PASCAL) classification system, which integrates the RoPE score with physiological and anatomical features – namely, the size of the PFO shunt and the presence of an atrial septal aneurysm.
“We came up with a way to more accurately identify those patients who are likely to get the most benefit from PFO closure based on mathematic modeling that estimates an individual’s probability that the PFO is causally related to the stroke,” Dr. Kent said.
Multivariate analysis determines risk
The study used a multivariate classification system that Dr. Kent had been developing to perform subgroup analyses of the clinical trials. It assigned patients to three different risk groups based on the likelihood that the PFO was causally related to their stroke: PASCAL categories of unlikely, possible, and probable.
The PASCAL unlikely group had a risk of stroke recurrence in the first 2 years of 3.4% (95% confidence interval, 1.1%-5.7%) if they were on medical therapy, and 4.1% (95% CI, 1.7%-6.4%) if they had device closure. In the PASCAL possible group, those risks were 3.6% (95% CI, 2.4%-4.9%) and 1.5% (95% CI, 0.7-2.3%), respectively. For the probable group, device closure represents “a near perfect therapy” with a 90% risk reduction, Dr. Kent said. “Moreover,” he said, “adverse events of device closure, such as atrial fibrillation, appear to be concentrated in those patients who fall into the unlikely classification, who appear to get no benefit.”
The ideal patient for device closure is age 60 years or younger and without vascular risk factors such as hypertension, diabetes, a history of smoking, or a prior stroke, but has high-risk PFO features such as a large shunt or atrial septal aneurysm, Dr. Kent said.
“We think these findings should be practice changing now,” Dr. Kent said.
Faisal M. Merchant, MD, director of cardiac electrophysiology at Emory Healthcare in Atlanta, concurred with that statement. “This is in my mind probably as good as any data we’re going to get on this,” he said in an interview. “The results support what’s been a general gestalt in the clinical world, but [also] really provide an evidence base on how to make decisions.”
He noted that guidelines, including those of the American Academy of Neurology, recommend medical therapy or device closure to prevent recurrent stroke in people who’ve had PFO-associated ischemic stroke. “But they hedge a bit,” he said of the guidelines. “We haven’t had data that’s as robust as this. I think this really solidifies those recommendations.”
He also credited the “unique” study design to extract findings from clinical trials and apply them to personalized medicine. “Clinical trial results give you an average treatment effect of the patients included, but who are ones who really benefit? Who are the ones that don’t benefit? Who are the ones who are harmed?” Dr. Merchant said. “It’s rare that you can parse out this nicely between the people who both benefit and are less likely to be harmed and the people who don’t benefit and are more likely to be harmed.”
The study received funding from the Patient-Centered Outcomes Research Institute. Dr. Kent disclosed relationships with PCORI, W.L. Gore and the Canadian Stroke Consortium. Dr. Merchant has no relevant disclosures.
It has been well established that device closure has, on average, prevented stroke recurrence in people who’ve had patent foramen ovale–associated stroke, but a meta-analysis has drilled down into clinical trials to advance a potentially practice-changing principle: that, while device closure shows an overall benefit, not all patients derive a benefit and some may actually be harmed by the procedure.
What’s more, the researchers developed a scoring system that helps determine which patients are likely to benefit from device closure.
“What was unknown was how to treat individual patients because the decision to close the patent foramen ovale (PFO) is still preference sensitive because the risk of a recurrent stroke is low, and most of the strokes that recur are not terribly severe,” lead study author David M. Kent, MD, MS, said in an interview.
“On top of this,” he said, “it was still suspected that some of the PFOs, even in trials of well-selected patients, may not be causally related to stroke; the stroke may still have another occult cause, such as paroxysmal atrial fibrillation or aortic arch atheroma.” Dr. Kent is a professor of medicine at Tufts University in Boston and director of the Predictive Analytics and Comparative Effectiveness Center there.
The meta-analysis, conducted by the Systematic, Collaborative, PFO Closure Evaluation (SCOPE) consortium, analyzed data from six randomized clinical trials that compared device closure and medical therapy to medical therapy alone in 3,740 patients who had PFO-associated stroke from 2000 to 2017. It was published in JAMA.
Overall, the rate of recurrent ischemic stroke was less than half that in patients who had device closure, compared with those who were on medical therapy: 0.47% (n = 39 of 1,889) vs. 1.09% (n = 82 of 1,851).
The researchers also applied two tools designed to calculate the probability of recurrent stroke in individual patients: Risk of Paradoxical Embolism (RoPE), an index that assigns a score of 0-10 to stratify cryptogenic stroke patients with PFO by the likelihood that the stroke was associated with their PFO; and the PFO-Associated Stroke Causal Likelihood (PASCAL) classification system, which integrates the RoPE score with physiological and anatomical features – namely, the size of the PFO shunt and the presence of an atrial septal aneurysm.
“We came up with a way to more accurately identify those patients who are likely to get the most benefit from PFO closure based on mathematic modeling that estimates an individual’s probability that the PFO is causally related to the stroke,” Dr. Kent said.
Multivariate analysis determines risk
The study used a multivariate classification system that Dr. Kent had been developing to perform subgroup analyses of the clinical trials. It assigned patients to three different risk groups based on the likelihood that the PFO was causally related to their stroke: PASCAL categories of unlikely, possible, and probable.
The PASCAL unlikely group had a risk of stroke recurrence in the first 2 years of 3.4% (95% confidence interval, 1.1%-5.7%) if they were on medical therapy, and 4.1% (95% CI, 1.7%-6.4%) if they had device closure. In the PASCAL possible group, those risks were 3.6% (95% CI, 2.4%-4.9%) and 1.5% (95% CI, 0.7-2.3%), respectively. For the probable group, device closure represents “a near perfect therapy” with a 90% risk reduction, Dr. Kent said. “Moreover,” he said, “adverse events of device closure, such as atrial fibrillation, appear to be concentrated in those patients who fall into the unlikely classification, who appear to get no benefit.”
The ideal patient for device closure is age 60 years or younger and without vascular risk factors such as hypertension, diabetes, a history of smoking, or a prior stroke, but has high-risk PFO features such as a large shunt or atrial septal aneurysm, Dr. Kent said.
“We think these findings should be practice changing now,” Dr. Kent said.
Faisal M. Merchant, MD, director of cardiac electrophysiology at Emory Healthcare in Atlanta, concurred with that statement. “This is in my mind probably as good as any data we’re going to get on this,” he said in an interview. “The results support what’s been a general gestalt in the clinical world, but [also] really provide an evidence base on how to make decisions.”
He noted that guidelines, including those of the American Academy of Neurology, recommend medical therapy or device closure to prevent recurrent stroke in people who’ve had PFO-associated ischemic stroke. “But they hedge a bit,” he said of the guidelines. “We haven’t had data that’s as robust as this. I think this really solidifies those recommendations.”
He also credited the “unique” study design to extract findings from clinical trials and apply them to personalized medicine. “Clinical trial results give you an average treatment effect of the patients included, but who are ones who really benefit? Who are the ones that don’t benefit? Who are the ones who are harmed?” Dr. Merchant said. “It’s rare that you can parse out this nicely between the people who both benefit and are less likely to be harmed and the people who don’t benefit and are more likely to be harmed.”
The study received funding from the Patient-Centered Outcomes Research Institute. Dr. Kent disclosed relationships with PCORI, W.L. Gore and the Canadian Stroke Consortium. Dr. Merchant has no relevant disclosures.
FROM JAMA
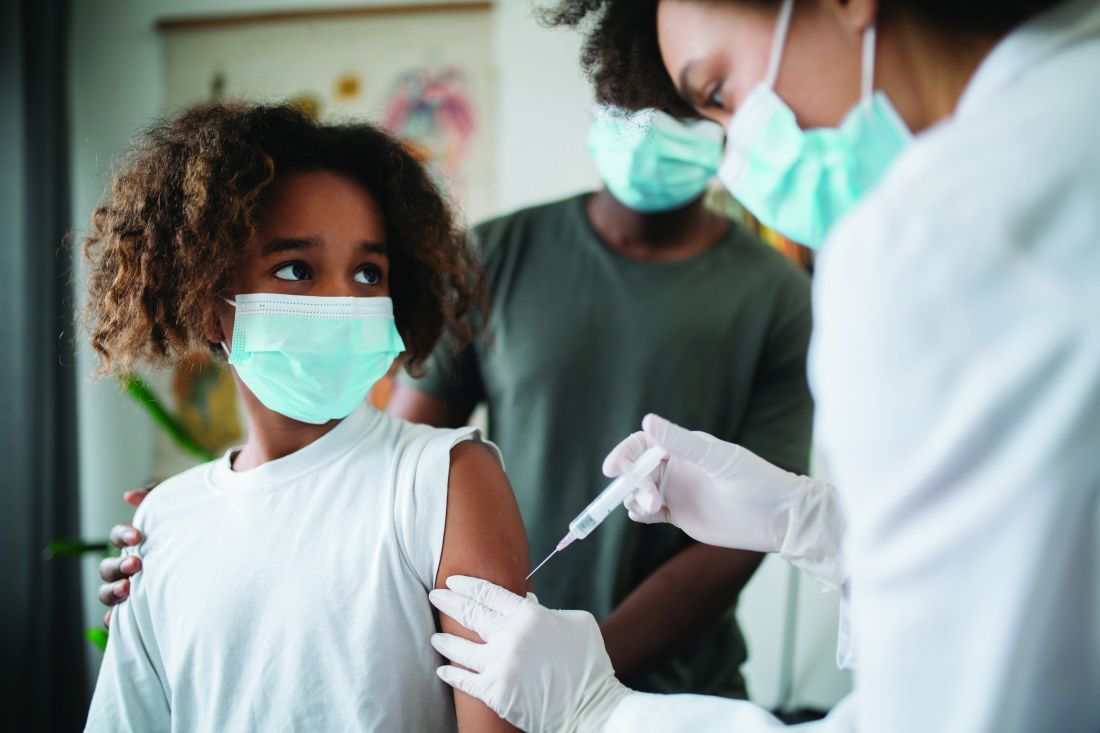
Small myocarditis risk now seen for adenovirus-based COVID-19 vaccine
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
CDC panel backs mRNA COVID vaccines over J&J because of clot risk
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
Califf plans work on opioids, accelerated approvals on return to FDA
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Robert M. Califf, MD, plans to take a close look at federal policies on opioid prescriptions in his expected second turn as the top U.S. regulator of medical products, as well as keep closer tabs on the performance of drugs cleared with accelerated approvals.
Dr. Califf on Tuesday fielded questions at a Senate hearing about his nomination by President Joe Biden to serve as administrator of the U.S. Food and Drug Administration, a role in which he served in the Obama administration. He also spoke about the need to bolster the nation’s ability to maintain an adequate supply of key medical products, including drugs.
Members of the Senate Health, Education, Labor and Pensions Committee, which is handling Dr. Califf’s nomination, were largely cordial and supportive during the hearing. Sen. Patty Murray (D-Wash.), the committee chair, and the panel’s top Republican, Sen. Richard Burr of North Carolina, addressed Dr. Califf during the hearing as if he would soon serve again as the FDA’s leader. Both were among the senators who voted 89-4 to confirm Dr. Califf in a February 2016 vote.
Dr. Califf “was previously confirmed to lead FDA in an overwhelming bipartisan vote, and I look forward to working with him again to ensure FDA continues to protect families across the country, uphold the gold standard of safety and effectiveness, and put science and data first,” Sen. Murray said.
Less enthusiastic about Dr. Califf was Sen. Bernie Sanders (I-VT), who was among the seven senators who did not vote on Dr. Califf’s nomination in 2016.
Sen. Sanders objected in 2016 to Dr. Califf’s ties to the pharmaceutical industry, and he did so again Tuesday. A noted leader in conducting clinical trials, Dr. Califf has worked with many drugmakers. But at the hearing, Dr. Califf said he concurs with Sen. Sanders on an idea strongly opposed by the pharmaceutical industry.
In response to Sen. Sanders’ question, Dr. Califf said he already is “on record as being in favor of Medicare negotiating with the industry on prices.”
The FDA would not take direct part in negotiations, as this work would be handled by the Centers for Medicare & Medicaid Services. Democrats want to give Medicare some negotiating authority through their sweeping Build Back Better Act.
People in the United States are dismayed over both the cost of prescription drugs and the widespread distribution of prescription painkillers that helped fuel the current opioid epidemic, Sen. Sanders told Dr. Califf. Many people will be concerned about an FDA commissioner who has benefited from close ties to the industry, Sen. Sanders said.
“How are they going to believe that you’re going to be an independent and strong voice against this enormously powerful, special interest?” Sen. Sanders asked.
“I’m totally with you on the concept that the price of pharmaceuticals is way too high in this country,” Dr. Califf said in reply.
Dr. Califf was paid $2.7 million in salary and bonus by Verily Life Sciences, the biomedical research organization operated by Alphabet, parent company of Google, according to his federal financial disclosure. He also reported holding board positions with pharmaceutical companies AmyriAD and Centessa Pharmaceuticals.
Bloomberg Government reported that Dr. Califf has ties to about 16 other research organizations and biotech companies. Bloomberg Government also said that, in his earlier FDA service, Dr. Califf kept a whiteboard in his office that listed all the activities and projects that required his recusal, citing as a source Howard Sklamberg, who was a deputy commissioner under Dr. Califf.
“He was very, very, very careful,” Mr. Sklamberg, who’s now an attorney at Arnold & Porter LLP, told Bloomberg Government.
‘Work to do’ on opioids
Senators looped back repeatedly to the topic of opioids during Dr. Califf’s hearing, reflecting deep concerns about the FDA’s efforts to warn of the risks of prescription painkillers.
There were an estimated 100,306 drug overdose deaths in the United States in the 12 months ending in April, an increase of 28.5% from the 78,056 deaths during the same period the year before, according to the Centers for Disease Control and Prevention.
Dr. Califf said he plans to focus on what information the FDA conveys to the public about the risks of prescription painkillers, including a look at what the labels for these products say.
“I am committed to do a comprehensive review of the status of opioids, early in my tenure,” Dr. Califf said.
Dr. Califf indicated that physicians are still too quick to provide excess doses of these medicines, despite years of efforts to restrain their use. He said he knows relatives who were given 30-day prescriptions for opioids after minor surgery.
“So I know we have work to do,” Dr. Califf said.
Concerns about the FDA’s previous work in managing opioids has led to protests from a few Democratic senators about the prospect of President Biden nominating the acting FDA commissioner, Janet Woodcock, MD, for the permanent post.
At the hearing, Sen. Ben Ray Luján (D-NM) raised the case of the FDA’s approval of the powerful Zohydro painkiller. The agency approved that drug despite an 11-2 vote against it by the FDA’s Anesthetic and Analgesic Drug Products Advisory Committee.
Sen. Luján asked Dr. Califf what he would do if an FDA advisory committee voted “overwhelmingly” against recommending approval of a medicine, as happened in the Zohydro case.
While not mentioned by Sen. Luján in this exchange during the hearing with Dr. Califf, the FDA staff’s rejection of recommendations of advisory committees has been a growing concern among researchers.
The agency last year approved aducanumab (Aduhelm, Biogen), a drug for Alzheimer’s disease, dismissing the advice of its Peripheral and Central Nervous System Drugs Advisory Committee. That decision triggered the resignation of several members of the panel. The FDA staff also earlier rejected the conclusion the majority of members of the same advisory committee offered in 2016 on eteplirsen (Exondys 51, Sarepta), a drug for Duchenne muscular dystrophy.
Dr. Califf told Sen. Luján he had done recent research into how often the FDA staff does not concur with the recommendations of an advisory committee. He said the FDA takes a different course of action in about 25% of cases. In about three-quarters of those cases, the FDA staff opts for a “more stringent” approach regarding allowing the public access to the drug, as opposed to a more generous one as seen in the Zohydro, Aduhelm, and Exondys 51 cases.
Still, Dr. Califf said that when there’s an 11-2 advisory committee vote against recommendation of a product, “the leaders at FDA really need to take a close look” at what’s happening.
Question on accelerated approvals
The FDA’s approval of aducanumab drew attention to a debate already underway about conditional clearances known as accelerated approvals.
The FDA has used this path since the 1990s to speed access to drugs for serious conditions. The trade-off for early access is that the agency sometimes makes the wrong call based on initial findings, and clears a medicine later found not to benefit patients as expected.
The FDA’s cancer division is in the midst of public efforts to address cases where drugmakers have not been able to deliver studies that support accelerated approvals of their oncology drugs. In addition, the Office of Inspector General of the U.S. Department of Health & Human Services announced in August that it is reviewing the FDA’s handling of the accelerated approval process.
At Tuesday’s hearing, Sen. Burr grilled Dr. Califf about how he would respond to calls to change how the FDA handles the accelerated-approval process.
“Can you commit to me and to patients who may rely on cutting-edge treatments that you will not support efforts to narrow this pathway or raise the bar for drugs to be approved under those pathways?” Burr asked Califf.
Dr. Califf responded by saying he was “a fan of accelerated approval – for the right conditions.”
Earlier, in his opening statement, Dr. Califf had said his mother benefited directly from the accelerated approval of new drugs for multiple myeloma. Dr. Califf told Sen. Burr that he had spent “countless hours with patient groups” and understands the need to speed the approval of medicines for serious diseases.
But the FDA also has to make sure it holds up its end of the bargain struck with accelerated approvals. This involves checking on how these medicines work once they are marketed.
“We’re accepting that there’s more uncertainty,” Dr. Califf said. “That means we’ve got to have a better system to evaluate these products as they’re used on the market. And I think there are ways that we can do that now. Technology is making this possible in ways that it just was not possible before.”
Worries about the medical supply chain
Sen. Susan Collins (R-Maine) asked Dr. Califf about the vulnerability of the U.S. medical system to disruptions of the supply chain. She raised concerns about China’s dominance in antibiotic manufacturing as an example. She asked if Congress could do more to encourage domestic manufacturing of medical supplies, such as by offering tax incentives.
Dr. Califf told Sen. Collins he shared her concern about the U.S. manufacturing of ingredients used in both branded and generic drugs. He said he recently has served on a committee of the National Academy of Medicine that is examining supply chain issues.
This committee will soon release a report with specific recommendations, Dr. Califf said.
“We don’t have enough competitive entities in what’s become sort of a commodity business” of drug manufacturing, Dr. Califf said. “So we need a number of steps to make the system more resilient.”
A version of this article first appeared on Medscape.com.
Even COVID-19 can’t stop a true optimist
Squeezing a little lemonade out of COVID-19
We like to think of ourselves as optimists here at LOTME. A glass is half full, the sky is partly sunny, and our motto is “Always look on the bright side of insanity.” Then again, our motto before that was “LOTME: Where science meets stupid,” so what do we know?
Anyway, it’s that upbeat, can-do attitude that allows us to say something positive – two somethings, actually – about the insanity that is COVID-19.
Our journey to the bright side begins, oddly enough, in the courtroom. Seems that our old friend, the face mask, is something of a lie-detector aid for juries. The authors of a recent literature review of studies on deception “found that facial expressions and other forms of nonverbal behaviour are an unreliable indicator of deceit,” according to a statement from the University of Portsmouth, where the analysis was conducted.
The one study that directly examined the role of face coverings in court proceedings showed that, “by taking away the distraction of nonverbal behaviours, observers had to rely on speech content, which turned out to be better for detecting lies,” the university said.
The second stage of our positivity trek brings us to the National Trends in Disability Employment monthly update, where we see a fourth consecutive month of gains for people with disabilities despite the larger trend of declines among those without disabilities.
Here are some numbers from the Kessler Foundation and the University of New Hampshire’s Institute on Disability to tell the story: From October to November, the employment-to-population ratio increased 4.2% for working-age people with disabilities, compared with 0.4% for people without disabilities. At the same time, the labor force participation rate rose 2.4% for working-age people with disabilities and just 0.1% for working-age people without disabilities.
Both indicators surpassed their historic highs, Andrew Houtenville, PhD, director of the Institute on Disability, said in the update. “These gains suggest that the restructuring resulting from the pandemic may be benefiting people with disabilities. Ironically, it may have taken a pandemic to shake the labor market loose for people with disabilities.”
And that is how a world-class optimist turns one gigantic lemon into lemonade.
Cut the cheese for better sleep
So, we’ve already talked about the TikTok lettuce tea hack that’s supposed to help us sleep better. Well, there’s another food that could have the opposite effect.
According to an article from the BBC, cheese has something of a reputation. Ever since the 1960s, when a researcher noted that one patient’s nightmares stopped after he quit eating an ounce or two of cheddar each night, there’s been speculation that cheese gives you weird dreams. Another study in 2005 suggested certain types of cheese cause certain types of dreams. Blue cheese for vivid dreams and cheddar cheese for celebrity cameos.
But is there any truth to it at all?
Regardless of what we eat, going to bed hungry could cause vivid dreams, according to research by Tore Nielsen, director of the University of Montreal’s dream and nightmare lab. The 2015 study showed that high lactose could have an effect on dreams.
In that study, 17% of participants said their dreams were influenced by what they ate, but the kicker was that dairy products were the foods most reported as causing the weird dreams, the BBC noted.
“It’s likely an indirect effect in that lactose produces symptoms like gas, bloating and diarrhoea and influences dreams, as dreams draw on somatic sources like this. And if you have certain kinds of intolerances, you still may be likely to eat those foods sometimes,” Mr. Nielsen told the BBC.
There’s also the theory that it’s all in the timing of consumption. Are you the type of person to sneak a slice of cheese from the fridge late at night? (Nods.) Same.
“One reason cheese and nightmares come about is that eating later before bed is more likely to disrupt sleep, and cheese can be hard to digest,” said Charlotte Gupta, a research fellow at Central Queensland University in Australia and a coauthor of a 2020 review on how diet affects our sleep.
So as tempting as it is, maybe skip sprinkling Parmesan cheese shreds into your mouth at the open fridge before bed.
Teeing up against Parkinson’s
For the nearly 1 million people in the United States with Parkinson’s disease, tai chi is one of the best ways to alleviate the symptoms. The average Parkinson’s patient, however, is going to be on the older side and more likely to view the martial art as some sort of communist plot. And would you participate in a communist plot? We don’t think so.
One group of researchers saw that patients weren’t keeping up with their therapy and decided to try a different activity, something that older people would be more likely to stick with. Something a bit more stereotypical. No, not shuffleboard. They tried golf.
“Golf is popular – the most popular sport for people over the age of 55 – which might encourage people to try it and stick with it,” study author Anne-Marie A. Wills, MD, of Massachusetts General Hospital, Boston, said in a Study Finds report.
In a small study, the investigators had a group of patients with Parkinson’s regularly go to a driving range for 10 weeks to hit golf balls (all expenses paid too, and that’s a big deal for golf), while another group continued with their tai chi.
At the end of the study, the 8 patients who went to the driving range had significantly better results in a Parkinson’s mobility test than those of the 12 patients in the tai chi group. In addition, the golf-group participants said they were more likely to continue with their therapy than were those who did tai chi.
Despite the small size of the study, the research team said the results certainly warrant further research. After all, the best sort of therapy is the kind that actually gets done. And golf just gets in your head. The eternal quest to add distance, to straighten out that annoying slice, to stop thinning half your chips, to make those annoying 4-footers. ... Maybe that’s just us.
Squeezing a little lemonade out of COVID-19
We like to think of ourselves as optimists here at LOTME. A glass is half full, the sky is partly sunny, and our motto is “Always look on the bright side of insanity.” Then again, our motto before that was “LOTME: Where science meets stupid,” so what do we know?
Anyway, it’s that upbeat, can-do attitude that allows us to say something positive – two somethings, actually – about the insanity that is COVID-19.
Our journey to the bright side begins, oddly enough, in the courtroom. Seems that our old friend, the face mask, is something of a lie-detector aid for juries. The authors of a recent literature review of studies on deception “found that facial expressions and other forms of nonverbal behaviour are an unreliable indicator of deceit,” according to a statement from the University of Portsmouth, where the analysis was conducted.
The one study that directly examined the role of face coverings in court proceedings showed that, “by taking away the distraction of nonverbal behaviours, observers had to rely on speech content, which turned out to be better for detecting lies,” the university said.
The second stage of our positivity trek brings us to the National Trends in Disability Employment monthly update, where we see a fourth consecutive month of gains for people with disabilities despite the larger trend of declines among those without disabilities.
Here are some numbers from the Kessler Foundation and the University of New Hampshire’s Institute on Disability to tell the story: From October to November, the employment-to-population ratio increased 4.2% for working-age people with disabilities, compared with 0.4% for people without disabilities. At the same time, the labor force participation rate rose 2.4% for working-age people with disabilities and just 0.1% for working-age people without disabilities.
Both indicators surpassed their historic highs, Andrew Houtenville, PhD, director of the Institute on Disability, said in the update. “These gains suggest that the restructuring resulting from the pandemic may be benefiting people with disabilities. Ironically, it may have taken a pandemic to shake the labor market loose for people with disabilities.”
And that is how a world-class optimist turns one gigantic lemon into lemonade.
Cut the cheese for better sleep
So, we’ve already talked about the TikTok lettuce tea hack that’s supposed to help us sleep better. Well, there’s another food that could have the opposite effect.
According to an article from the BBC, cheese has something of a reputation. Ever since the 1960s, when a researcher noted that one patient’s nightmares stopped after he quit eating an ounce or two of cheddar each night, there’s been speculation that cheese gives you weird dreams. Another study in 2005 suggested certain types of cheese cause certain types of dreams. Blue cheese for vivid dreams and cheddar cheese for celebrity cameos.
But is there any truth to it at all?
Regardless of what we eat, going to bed hungry could cause vivid dreams, according to research by Tore Nielsen, director of the University of Montreal’s dream and nightmare lab. The 2015 study showed that high lactose could have an effect on dreams.
In that study, 17% of participants said their dreams were influenced by what they ate, but the kicker was that dairy products were the foods most reported as causing the weird dreams, the BBC noted.
“It’s likely an indirect effect in that lactose produces symptoms like gas, bloating and diarrhoea and influences dreams, as dreams draw on somatic sources like this. And if you have certain kinds of intolerances, you still may be likely to eat those foods sometimes,” Mr. Nielsen told the BBC.
There’s also the theory that it’s all in the timing of consumption. Are you the type of person to sneak a slice of cheese from the fridge late at night? (Nods.) Same.
“One reason cheese and nightmares come about is that eating later before bed is more likely to disrupt sleep, and cheese can be hard to digest,” said Charlotte Gupta, a research fellow at Central Queensland University in Australia and a coauthor of a 2020 review on how diet affects our sleep.
So as tempting as it is, maybe skip sprinkling Parmesan cheese shreds into your mouth at the open fridge before bed.
Teeing up against Parkinson’s
For the nearly 1 million people in the United States with Parkinson’s disease, tai chi is one of the best ways to alleviate the symptoms. The average Parkinson’s patient, however, is going to be on the older side and more likely to view the martial art as some sort of communist plot. And would you participate in a communist plot? We don’t think so.
One group of researchers saw that patients weren’t keeping up with their therapy and decided to try a different activity, something that older people would be more likely to stick with. Something a bit more stereotypical. No, not shuffleboard. They tried golf.
“Golf is popular – the most popular sport for people over the age of 55 – which might encourage people to try it and stick with it,” study author Anne-Marie A. Wills, MD, of Massachusetts General Hospital, Boston, said in a Study Finds report.
In a small study, the investigators had a group of patients with Parkinson’s regularly go to a driving range for 10 weeks to hit golf balls (all expenses paid too, and that’s a big deal for golf), while another group continued with their tai chi.
At the end of the study, the 8 patients who went to the driving range had significantly better results in a Parkinson’s mobility test than those of the 12 patients in the tai chi group. In addition, the golf-group participants said they were more likely to continue with their therapy than were those who did tai chi.
Despite the small size of the study, the research team said the results certainly warrant further research. After all, the best sort of therapy is the kind that actually gets done. And golf just gets in your head. The eternal quest to add distance, to straighten out that annoying slice, to stop thinning half your chips, to make those annoying 4-footers. ... Maybe that’s just us.
Squeezing a little lemonade out of COVID-19
We like to think of ourselves as optimists here at LOTME. A glass is half full, the sky is partly sunny, and our motto is “Always look on the bright side of insanity.” Then again, our motto before that was “LOTME: Where science meets stupid,” so what do we know?
Anyway, it’s that upbeat, can-do attitude that allows us to say something positive – two somethings, actually – about the insanity that is COVID-19.
Our journey to the bright side begins, oddly enough, in the courtroom. Seems that our old friend, the face mask, is something of a lie-detector aid for juries. The authors of a recent literature review of studies on deception “found that facial expressions and other forms of nonverbal behaviour are an unreliable indicator of deceit,” according to a statement from the University of Portsmouth, where the analysis was conducted.
The one study that directly examined the role of face coverings in court proceedings showed that, “by taking away the distraction of nonverbal behaviours, observers had to rely on speech content, which turned out to be better for detecting lies,” the university said.
The second stage of our positivity trek brings us to the National Trends in Disability Employment monthly update, where we see a fourth consecutive month of gains for people with disabilities despite the larger trend of declines among those without disabilities.
Here are some numbers from the Kessler Foundation and the University of New Hampshire’s Institute on Disability to tell the story: From October to November, the employment-to-population ratio increased 4.2% for working-age people with disabilities, compared with 0.4% for people without disabilities. At the same time, the labor force participation rate rose 2.4% for working-age people with disabilities and just 0.1% for working-age people without disabilities.
Both indicators surpassed their historic highs, Andrew Houtenville, PhD, director of the Institute on Disability, said in the update. “These gains suggest that the restructuring resulting from the pandemic may be benefiting people with disabilities. Ironically, it may have taken a pandemic to shake the labor market loose for people with disabilities.”
And that is how a world-class optimist turns one gigantic lemon into lemonade.
Cut the cheese for better sleep
So, we’ve already talked about the TikTok lettuce tea hack that’s supposed to help us sleep better. Well, there’s another food that could have the opposite effect.
According to an article from the BBC, cheese has something of a reputation. Ever since the 1960s, when a researcher noted that one patient’s nightmares stopped after he quit eating an ounce or two of cheddar each night, there’s been speculation that cheese gives you weird dreams. Another study in 2005 suggested certain types of cheese cause certain types of dreams. Blue cheese for vivid dreams and cheddar cheese for celebrity cameos.
But is there any truth to it at all?
Regardless of what we eat, going to bed hungry could cause vivid dreams, according to research by Tore Nielsen, director of the University of Montreal’s dream and nightmare lab. The 2015 study showed that high lactose could have an effect on dreams.
In that study, 17% of participants said their dreams were influenced by what they ate, but the kicker was that dairy products were the foods most reported as causing the weird dreams, the BBC noted.
“It’s likely an indirect effect in that lactose produces symptoms like gas, bloating and diarrhoea and influences dreams, as dreams draw on somatic sources like this. And if you have certain kinds of intolerances, you still may be likely to eat those foods sometimes,” Mr. Nielsen told the BBC.
There’s also the theory that it’s all in the timing of consumption. Are you the type of person to sneak a slice of cheese from the fridge late at night? (Nods.) Same.
“One reason cheese and nightmares come about is that eating later before bed is more likely to disrupt sleep, and cheese can be hard to digest,” said Charlotte Gupta, a research fellow at Central Queensland University in Australia and a coauthor of a 2020 review on how diet affects our sleep.
So as tempting as it is, maybe skip sprinkling Parmesan cheese shreds into your mouth at the open fridge before bed.
Teeing up against Parkinson’s
For the nearly 1 million people in the United States with Parkinson’s disease, tai chi is one of the best ways to alleviate the symptoms. The average Parkinson’s patient, however, is going to be on the older side and more likely to view the martial art as some sort of communist plot. And would you participate in a communist plot? We don’t think so.
One group of researchers saw that patients weren’t keeping up with their therapy and decided to try a different activity, something that older people would be more likely to stick with. Something a bit more stereotypical. No, not shuffleboard. They tried golf.
“Golf is popular – the most popular sport for people over the age of 55 – which might encourage people to try it and stick with it,” study author Anne-Marie A. Wills, MD, of Massachusetts General Hospital, Boston, said in a Study Finds report.
In a small study, the investigators had a group of patients with Parkinson’s regularly go to a driving range for 10 weeks to hit golf balls (all expenses paid too, and that’s a big deal for golf), while another group continued with their tai chi.
At the end of the study, the 8 patients who went to the driving range had significantly better results in a Parkinson’s mobility test than those of the 12 patients in the tai chi group. In addition, the golf-group participants said they were more likely to continue with their therapy than were those who did tai chi.
Despite the small size of the study, the research team said the results certainly warrant further research. After all, the best sort of therapy is the kind that actually gets done. And golf just gets in your head. The eternal quest to add distance, to straighten out that annoying slice, to stop thinning half your chips, to make those annoying 4-footers. ... Maybe that’s just us.
Discharge within 24 hours of PCI can be safe in select STEMI
Highly selected low-risk patients can be safely sent home about 24 hours after successful percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction (STEMI) when supported by intense, multidisciplinary virtual follow-up, a prospective study suggests for the first time.
The risk for major adverse cardiac events (MACE) in STEMI patients following an early hospital discharge (EHD) pathway was similar at 9 months to that seen for propensity-matched historic control subjects who met the same EHD criteria but were discharged later than 48 hours.
The stay in almost half (48%) the early discharge group was 24 hours or less, according to the study, published Dec. 13 in the Journal of the American College of Cardiology.
“We’ve shown that if we use appropriate risk criteria and instigate the appropriate, safe follow-up that it’s safe to select and discharge low-risk patients at an earlier time period, such as 24 hours,” senior author Daniel A. Jones, PhD, Barts Heart Centre, London, this news organization.
“Obviously, it’s one center in one city in the world,” he said. “Whether it’s applicable at other heart site centers, I believe it is, but I think we need more data to be able to change guidelines.”
Current European Society of Cardiology guidelines say that select patients should be considered for early discharge 48 to 72 hours after STEMI, but the COVID-19 pandemic incentivized the team to try and push that window.
“The COVID pandemic essentially brought a focus on resources, on minimizing the risk to our patient population in terms of catching COVID within hospital,” he said. “It became clear that to maintain the heart site service, we probably needed to get people out a bit quicker than we did before, so we came up with this pathway.”
Between March 2020 and June 2021, 600 patients presenting with STEMI were entered into the EHD pathway if they met the following pre-existing criteria for 48- to 72-hour discharge:
- Left ventricular ejection fraction 40% or greater
- Successful primary PCI with TIMI flow grade 3
- Absence of bystander disease requiring inpatient revascularization
- No recurrent ischemic symptoms
- No heart failure
- No significant arrhythmias
- No hemodynamic instability
- No significant comorbidity
- Suitable social circumstances for early discharge
The patients were given cardiac rehabilitation counseling over the phone within 48 hours and blood pressure machines if not available at home. At weeks 2 and 8, they spoke virtually with a dedicated cardiology advanced care practitioner who up-titrated medications and answered any questions. At week 12, they were seen by an interventional cardiologist or at a high-risk prevention clinic.
Their mean age was 59.2 years, 86% were male, the median symptom-to-balloon time was 80 minutes, and median door-to-balloon time was 50 minutes.
The early discharge patients were compared with 700 historic control subjects who met the EHD criteria and were discharged after 48 hours from Oct. 2018 to June 2021 and 560 patients discharged on standard-care pathways between April 2020 and June 2021.
Those discharged after 48 hours were more likely to have an anterior MI, multivessel disease, and multivessel PCI.
Comparable outcomes
The median length of stay was 24.6 hours (minimum 17 hours, maximum 40 hours) for the EHD group, 56.1 hours for historic control subjects, and 78.9 hours for the standard-care group.
The introduction of the EHD pathway significantly reduced the overall length of stay for all STEMI patients compared with the pre-pathway period of Oct. 2018 to March 2020 (median, 3 vs. 2 days; P < .0001).
Length of stay varied among patients; however, 420 patients stayed 1 less night in the hospital with the remaining patients staying about 8 to 12 fewer hours, resulting in approximate savings of £450,000, the authors note.
Over a median follow-up of 271 days, there were no cardiovascular deaths, two deaths from COVID-19, and a MACE rate of 1.2% (two deaths, three unscheduled revascularizations, and two further MI presentations) in the EHD group. That compares with a 0.7% mortality and 1.9% rate of MACE among historic control subjects, neither of which were significantly different.
There was also no difference in mortality (0.34% vs. 0.69%; P = .410) or MACE (1.2% vs. 1.9%; P = .342) among 560 pairs of propensity-matched EHD patients and historic control subjects.
Mortality was 4.1% in the standard-care group; cardiovascular mortality was 2.2%, and the rate of MACE was 8.6%.
When patients were surveyed, 85% were “satisfied” or “very satisfied” with the EHD pathway, whereas 73% of control and standard-care patients were satisfied with their care. Three-fourths of EHD patients also reported saving money and 62.5% saved time off work because of the virtual follow-up.
Judgment calls
“They didn’t really tell us much about the patients who didn’t qualify into this ultra–low-risk group but, obviously, it’s highly selected,” Cindy Grines, MD, Northside Hospital Cardiovascular Institute, Lawrenceville, Georgia, said in an interview. “In the U.S., you don’t get those chest pain onset-to-reperfusion in 80 minutes. So that was really kind of shocking.”
It also suggests that early discharge was applied to patients who may have had minimal myocardial damage from the STEMI, she suggested. “Even in their own hospital system, a lot of patients who met the criteria on paper were kept longer than 48 hours. So a lot of it’s a judgment call.”
Additional red flags where physicians may overrule the early discharge protocol are very late perfusion, advanced age, severe renal insufficiency, profound anemia, cardiac arrest requiring more than brief resuscitation, bleeding complications, or symptomatic coronavirus, Dr. Grines and J. Jeffrey Marshall, MD, also from Northside, observe in an accompanying editorial.
About 60% of patients were suitable for the EHD pathway, Dr. Jones said. “Typically, they are quite low risk, but we still had four in 10 anterior infarct, and about 25% had left ventricular function between 40% and 45%. So even though the majority are low risk, there are patients in there that you would consider to have had a decent infarct.”
“I think this is applicable to patients at most centers, and probably anywhere between a third to a fifth of all patients presenting to heart centers would be suitable for this discharge pathway,” he said.
Dr. Grines said the pathway is “definitely feasible” but there aren’t enough patients studied to know with 100% certainty whether it’s safe. A single observational study also isn’t enough to change guidelines, which in the United States do not comment on length of stay.
“In the ultra-low-risk patients – such as the ones where you got them in very early and you almost aborted the infarct or if it was a very small infarct – you can kind of treat them like an unstable angina patient, where you can do the PCI and potentially discharge them in 24 hours,” Dr. Grines said. “I think most of us might agree on that.”
“The other thing you have to weigh is the risk/benefit ratio,” she said. “If you have no beds available, you end up rationing care to some extent. So if you have a patient that’s otherwise doing well after a very small MI and have an emergency room full of people that need to be admitted and they’re sicker, then you end up making those judgment calls.”
Dr. Jones pointed out that current guidelines are based largely on observational data and that the team is planning to pilot the EHD pathway at five to 10 centers around the United Kingdom or potentially in Europe or the United States.
“This is an area where a [randomized controlled trial] RCT would be expensive, whereas a well-coordinated multicenter registry would probably provide enough information to change guidelines,” he said. “We’re not suggesting that every STEMI patient is suitable, but people that are low risk that you would already be considering for early discharge I think can go a bit quicker.”
Dr. Jones has received funding from the Barts Charity and financial support for blood pressure machines from the Barts Guild. First author Krishnaraj Rathod has received funding from the National Institute for Health and Research in the form of an Academic Clinical Lectureship. All other authors, Dr. Grines, and Dr. Marshall report having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Highly selected low-risk patients can be safely sent home about 24 hours after successful percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction (STEMI) when supported by intense, multidisciplinary virtual follow-up, a prospective study suggests for the first time.
The risk for major adverse cardiac events (MACE) in STEMI patients following an early hospital discharge (EHD) pathway was similar at 9 months to that seen for propensity-matched historic control subjects who met the same EHD criteria but were discharged later than 48 hours.
The stay in almost half (48%) the early discharge group was 24 hours or less, according to the study, published Dec. 13 in the Journal of the American College of Cardiology.
“We’ve shown that if we use appropriate risk criteria and instigate the appropriate, safe follow-up that it’s safe to select and discharge low-risk patients at an earlier time period, such as 24 hours,” senior author Daniel A. Jones, PhD, Barts Heart Centre, London, this news organization.
“Obviously, it’s one center in one city in the world,” he said. “Whether it’s applicable at other heart site centers, I believe it is, but I think we need more data to be able to change guidelines.”
Current European Society of Cardiology guidelines say that select patients should be considered for early discharge 48 to 72 hours after STEMI, but the COVID-19 pandemic incentivized the team to try and push that window.
“The COVID pandemic essentially brought a focus on resources, on minimizing the risk to our patient population in terms of catching COVID within hospital,” he said. “It became clear that to maintain the heart site service, we probably needed to get people out a bit quicker than we did before, so we came up with this pathway.”
Between March 2020 and June 2021, 600 patients presenting with STEMI were entered into the EHD pathway if they met the following pre-existing criteria for 48- to 72-hour discharge:
- Left ventricular ejection fraction 40% or greater
- Successful primary PCI with TIMI flow grade 3
- Absence of bystander disease requiring inpatient revascularization
- No recurrent ischemic symptoms
- No heart failure
- No significant arrhythmias
- No hemodynamic instability
- No significant comorbidity
- Suitable social circumstances for early discharge
The patients were given cardiac rehabilitation counseling over the phone within 48 hours and blood pressure machines if not available at home. At weeks 2 and 8, they spoke virtually with a dedicated cardiology advanced care practitioner who up-titrated medications and answered any questions. At week 12, they were seen by an interventional cardiologist or at a high-risk prevention clinic.
Their mean age was 59.2 years, 86% were male, the median symptom-to-balloon time was 80 minutes, and median door-to-balloon time was 50 minutes.
The early discharge patients were compared with 700 historic control subjects who met the EHD criteria and were discharged after 48 hours from Oct. 2018 to June 2021 and 560 patients discharged on standard-care pathways between April 2020 and June 2021.
Those discharged after 48 hours were more likely to have an anterior MI, multivessel disease, and multivessel PCI.
Comparable outcomes
The median length of stay was 24.6 hours (minimum 17 hours, maximum 40 hours) for the EHD group, 56.1 hours for historic control subjects, and 78.9 hours for the standard-care group.
The introduction of the EHD pathway significantly reduced the overall length of stay for all STEMI patients compared with the pre-pathway period of Oct. 2018 to March 2020 (median, 3 vs. 2 days; P < .0001).
Length of stay varied among patients; however, 420 patients stayed 1 less night in the hospital with the remaining patients staying about 8 to 12 fewer hours, resulting in approximate savings of £450,000, the authors note.
Over a median follow-up of 271 days, there were no cardiovascular deaths, two deaths from COVID-19, and a MACE rate of 1.2% (two deaths, three unscheduled revascularizations, and two further MI presentations) in the EHD group. That compares with a 0.7% mortality and 1.9% rate of MACE among historic control subjects, neither of which were significantly different.
There was also no difference in mortality (0.34% vs. 0.69%; P = .410) or MACE (1.2% vs. 1.9%; P = .342) among 560 pairs of propensity-matched EHD patients and historic control subjects.
Mortality was 4.1% in the standard-care group; cardiovascular mortality was 2.2%, and the rate of MACE was 8.6%.
When patients were surveyed, 85% were “satisfied” or “very satisfied” with the EHD pathway, whereas 73% of control and standard-care patients were satisfied with their care. Three-fourths of EHD patients also reported saving money and 62.5% saved time off work because of the virtual follow-up.
Judgment calls
“They didn’t really tell us much about the patients who didn’t qualify into this ultra–low-risk group but, obviously, it’s highly selected,” Cindy Grines, MD, Northside Hospital Cardiovascular Institute, Lawrenceville, Georgia, said in an interview. “In the U.S., you don’t get those chest pain onset-to-reperfusion in 80 minutes. So that was really kind of shocking.”
It also suggests that early discharge was applied to patients who may have had minimal myocardial damage from the STEMI, she suggested. “Even in their own hospital system, a lot of patients who met the criteria on paper were kept longer than 48 hours. So a lot of it’s a judgment call.”
Additional red flags where physicians may overrule the early discharge protocol are very late perfusion, advanced age, severe renal insufficiency, profound anemia, cardiac arrest requiring more than brief resuscitation, bleeding complications, or symptomatic coronavirus, Dr. Grines and J. Jeffrey Marshall, MD, also from Northside, observe in an accompanying editorial.
About 60% of patients were suitable for the EHD pathway, Dr. Jones said. “Typically, they are quite low risk, but we still had four in 10 anterior infarct, and about 25% had left ventricular function between 40% and 45%. So even though the majority are low risk, there are patients in there that you would consider to have had a decent infarct.”
“I think this is applicable to patients at most centers, and probably anywhere between a third to a fifth of all patients presenting to heart centers would be suitable for this discharge pathway,” he said.
Dr. Grines said the pathway is “definitely feasible” but there aren’t enough patients studied to know with 100% certainty whether it’s safe. A single observational study also isn’t enough to change guidelines, which in the United States do not comment on length of stay.
“In the ultra-low-risk patients – such as the ones where you got them in very early and you almost aborted the infarct or if it was a very small infarct – you can kind of treat them like an unstable angina patient, where you can do the PCI and potentially discharge them in 24 hours,” Dr. Grines said. “I think most of us might agree on that.”
“The other thing you have to weigh is the risk/benefit ratio,” she said. “If you have no beds available, you end up rationing care to some extent. So if you have a patient that’s otherwise doing well after a very small MI and have an emergency room full of people that need to be admitted and they’re sicker, then you end up making those judgment calls.”
Dr. Jones pointed out that current guidelines are based largely on observational data and that the team is planning to pilot the EHD pathway at five to 10 centers around the United Kingdom or potentially in Europe or the United States.
“This is an area where a [randomized controlled trial] RCT would be expensive, whereas a well-coordinated multicenter registry would probably provide enough information to change guidelines,” he said. “We’re not suggesting that every STEMI patient is suitable, but people that are low risk that you would already be considering for early discharge I think can go a bit quicker.”
Dr. Jones has received funding from the Barts Charity and financial support for blood pressure machines from the Barts Guild. First author Krishnaraj Rathod has received funding from the National Institute for Health and Research in the form of an Academic Clinical Lectureship. All other authors, Dr. Grines, and Dr. Marshall report having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Highly selected low-risk patients can be safely sent home about 24 hours after successful percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction (STEMI) when supported by intense, multidisciplinary virtual follow-up, a prospective study suggests for the first time.
The risk for major adverse cardiac events (MACE) in STEMI patients following an early hospital discharge (EHD) pathway was similar at 9 months to that seen for propensity-matched historic control subjects who met the same EHD criteria but were discharged later than 48 hours.
The stay in almost half (48%) the early discharge group was 24 hours or less, according to the study, published Dec. 13 in the Journal of the American College of Cardiology.
“We’ve shown that if we use appropriate risk criteria and instigate the appropriate, safe follow-up that it’s safe to select and discharge low-risk patients at an earlier time period, such as 24 hours,” senior author Daniel A. Jones, PhD, Barts Heart Centre, London, this news organization.
“Obviously, it’s one center in one city in the world,” he said. “Whether it’s applicable at other heart site centers, I believe it is, but I think we need more data to be able to change guidelines.”
Current European Society of Cardiology guidelines say that select patients should be considered for early discharge 48 to 72 hours after STEMI, but the COVID-19 pandemic incentivized the team to try and push that window.
“The COVID pandemic essentially brought a focus on resources, on minimizing the risk to our patient population in terms of catching COVID within hospital,” he said. “It became clear that to maintain the heart site service, we probably needed to get people out a bit quicker than we did before, so we came up with this pathway.”
Between March 2020 and June 2021, 600 patients presenting with STEMI were entered into the EHD pathway if they met the following pre-existing criteria for 48- to 72-hour discharge:
- Left ventricular ejection fraction 40% or greater
- Successful primary PCI with TIMI flow grade 3
- Absence of bystander disease requiring inpatient revascularization
- No recurrent ischemic symptoms
- No heart failure
- No significant arrhythmias
- No hemodynamic instability
- No significant comorbidity
- Suitable social circumstances for early discharge
The patients were given cardiac rehabilitation counseling over the phone within 48 hours and blood pressure machines if not available at home. At weeks 2 and 8, they spoke virtually with a dedicated cardiology advanced care practitioner who up-titrated medications and answered any questions. At week 12, they were seen by an interventional cardiologist or at a high-risk prevention clinic.
Their mean age was 59.2 years, 86% were male, the median symptom-to-balloon time was 80 minutes, and median door-to-balloon time was 50 minutes.
The early discharge patients were compared with 700 historic control subjects who met the EHD criteria and were discharged after 48 hours from Oct. 2018 to June 2021 and 560 patients discharged on standard-care pathways between April 2020 and June 2021.
Those discharged after 48 hours were more likely to have an anterior MI, multivessel disease, and multivessel PCI.
Comparable outcomes
The median length of stay was 24.6 hours (minimum 17 hours, maximum 40 hours) for the EHD group, 56.1 hours for historic control subjects, and 78.9 hours for the standard-care group.
The introduction of the EHD pathway significantly reduced the overall length of stay for all STEMI patients compared with the pre-pathway period of Oct. 2018 to March 2020 (median, 3 vs. 2 days; P < .0001).
Length of stay varied among patients; however, 420 patients stayed 1 less night in the hospital with the remaining patients staying about 8 to 12 fewer hours, resulting in approximate savings of £450,000, the authors note.
Over a median follow-up of 271 days, there were no cardiovascular deaths, two deaths from COVID-19, and a MACE rate of 1.2% (two deaths, three unscheduled revascularizations, and two further MI presentations) in the EHD group. That compares with a 0.7% mortality and 1.9% rate of MACE among historic control subjects, neither of which were significantly different.
There was also no difference in mortality (0.34% vs. 0.69%; P = .410) or MACE (1.2% vs. 1.9%; P = .342) among 560 pairs of propensity-matched EHD patients and historic control subjects.
Mortality was 4.1% in the standard-care group; cardiovascular mortality was 2.2%, and the rate of MACE was 8.6%.
When patients were surveyed, 85% were “satisfied” or “very satisfied” with the EHD pathway, whereas 73% of control and standard-care patients were satisfied with their care. Three-fourths of EHD patients also reported saving money and 62.5% saved time off work because of the virtual follow-up.
Judgment calls
“They didn’t really tell us much about the patients who didn’t qualify into this ultra–low-risk group but, obviously, it’s highly selected,” Cindy Grines, MD, Northside Hospital Cardiovascular Institute, Lawrenceville, Georgia, said in an interview. “In the U.S., you don’t get those chest pain onset-to-reperfusion in 80 minutes. So that was really kind of shocking.”
It also suggests that early discharge was applied to patients who may have had minimal myocardial damage from the STEMI, she suggested. “Even in their own hospital system, a lot of patients who met the criteria on paper were kept longer than 48 hours. So a lot of it’s a judgment call.”
Additional red flags where physicians may overrule the early discharge protocol are very late perfusion, advanced age, severe renal insufficiency, profound anemia, cardiac arrest requiring more than brief resuscitation, bleeding complications, or symptomatic coronavirus, Dr. Grines and J. Jeffrey Marshall, MD, also from Northside, observe in an accompanying editorial.
About 60% of patients were suitable for the EHD pathway, Dr. Jones said. “Typically, they are quite low risk, but we still had four in 10 anterior infarct, and about 25% had left ventricular function between 40% and 45%. So even though the majority are low risk, there are patients in there that you would consider to have had a decent infarct.”
“I think this is applicable to patients at most centers, and probably anywhere between a third to a fifth of all patients presenting to heart centers would be suitable for this discharge pathway,” he said.
Dr. Grines said the pathway is “definitely feasible” but there aren’t enough patients studied to know with 100% certainty whether it’s safe. A single observational study also isn’t enough to change guidelines, which in the United States do not comment on length of stay.
“In the ultra-low-risk patients – such as the ones where you got them in very early and you almost aborted the infarct or if it was a very small infarct – you can kind of treat them like an unstable angina patient, where you can do the PCI and potentially discharge them in 24 hours,” Dr. Grines said. “I think most of us might agree on that.”
“The other thing you have to weigh is the risk/benefit ratio,” she said. “If you have no beds available, you end up rationing care to some extent. So if you have a patient that’s otherwise doing well after a very small MI and have an emergency room full of people that need to be admitted and they’re sicker, then you end up making those judgment calls.”
Dr. Jones pointed out that current guidelines are based largely on observational data and that the team is planning to pilot the EHD pathway at five to 10 centers around the United Kingdom or potentially in Europe or the United States.
“This is an area where a [randomized controlled trial] RCT would be expensive, whereas a well-coordinated multicenter registry would probably provide enough information to change guidelines,” he said. “We’re not suggesting that every STEMI patient is suitable, but people that are low risk that you would already be considering for early discharge I think can go a bit quicker.”
Dr. Jones has received funding from the Barts Charity and financial support for blood pressure machines from the Barts Guild. First author Krishnaraj Rathod has received funding from the National Institute for Health and Research in the form of an Academic Clinical Lectureship. All other authors, Dr. Grines, and Dr. Marshall report having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
CRP elevated in adults with AD and sleep disturbance
and mortality, results from a large cohort analysis showed.
“The implications of these findings are vast,” presenting author Varsha Parthasarathy said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium. “Poor sleep quality is known to be associated with increased inflammatory markers such as IL-6, IL-17, and CRP, so it is interesting to see this reflected in AD patients with versus without sleep disturbance. Additionally, we know that CRP is a driver of inflammation and is strongly associated with cardiovascular complications such as heart attack and stroke. Therefore, CRP may be a useful prognostic marker in AD patients with sleep disturbances.”
To examine the comorbidity burden of sleep disorders in AD patients and associate findings with inflammatory CRP and cardiovascular comorbidities, Mr. Parthasarathy, a medical student and itch fellow in the department of dermatology at the Johns Hopkins University School of Medicine, Baltimore, and colleagues drew from TriNetX, a health care network of approximately 73 million de-identified medical records in 53 organizations. The years of study were 2015 to 2021. The researchers limited the analysis to adults with at least two instances of International Classification of Diseases, Tenth Revision (ICD-10) code L28 for AD, to capture a population with true AD. Controls were adults without AD who presented for general checkup and were matched to AD patients by age, race, and sex.
The study population consisted of 120,480 AD patients and matched controls. Their mean age was 36 years, 61% were female, and 26% were Black. Compared with controls, AD patients had an increased risk of developing general sleep disorders over the 6-year period (relative risk, 1.10), as well as obstructive sleep apnea (RR, 1.13), insomnia (RR, 1.10), hypersomnia (RR, 1.24), sleep-related movement disorders (RR, 1.36), restless legs syndrome (RR, 1.25), sleep deprivation (RR, 1.36), and unspecified sleep disorders (RR, 1.22).
To examine the association of sleep disturbance with the inflammatory biomarker CRP, the researchers measured CRP levels between these patient groups. They found a substantially higher CRP in AD patients compared with controls (21.2 mg/L vs. 7.6 mg/L, respectively; P < .0001). This finding “is suggestive of a higher level of inflammation in these patients,” Mr. Parthasarathy said. Interestingly, he added, they also found a higher CRP level in AD patients with sleep disturbances compared to AD patients without sleep disturbances (23.3 vs. 20.6 mg/L; P = .02), “also pointing to a higher inflammatory burden in AD patients whose sleep was affected.”
Compared to matched AD patients without sleep disorders, AD patients with sleep disorders were more likely to develop obesity (RR, 2.65), hyperlipidemia (RR, 2.18), type 2 diabetes (RR, 2.45), metabolic syndrome (RR, 4.16), atherosclerosis (RR, 2.42), peripheral vascular disease (RR, 2.47), stroke (RR, 2.37), venous thromboembolism (RR, 2.93), and mortality (hazard ratio, 1.24).
“There is a consequence of not treating patients with atopic dermatitis, especially those patients with sleep disturbance,” the study’s primary author, Shawn G. Kwatra, MD, associate professor of dermatology at Johns Hopkins, told this news organization. “Chronic inflammation can lead to the development of comorbidities, so it is important to offer patients early treatment to reduce their overall inflammation.” He said that he was most surprised by the degree of increased inflammation in the blood of AD as compared to healthy controls. “This likely plays a part in the development of several comorbidities,” he said.
Mr. Parthasarathy acknowledged certain limitations of the study, including the inability to infer causal relationships, as uncontrolled factors may be present. “Additionally, sampling of only patients that have had medical encounters limits the generalizability of the findings,” she said. “However, findings in this large cohort study suggest that clinicians should seek to identify sleep disorders in AD patients and screen for cardiac comorbidities secondary to inflammation in this patient population.”
“There is increased data to suggest that adults with AD, particularly those with more severe disease, may be at an increased risk of cardiovascular disease and the results from [this study] further support the concept of AD as systemic disease,” said Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study. She cited the large population-based, retrospective design and use of two instances of ICD codes for AD to confirm diagnosis as key strengths of the research. “However, it is unclear if for each patient CRP levels were measured at one single timepoint,” Dr. Chiesa Fuxench said. “For future studies, it would be interesting to see if these levels fluctuate with time and if persistently elevated levels are associated with worse cardiovascular outcomes in this population. More data is needed to better understand the relationship better atopic dermatitis disease severity, impact on sleep, and how this relates to increased systemic inflammation and worse cardiovascular outcomes in this population.”
Dr. Kwatra disclosed support by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR077073-01A1 and previous funding by the Dermatology Foundation and Skin of Color Society. Dr. Kwatra is also an advisory board member/consultant for AbbVie, Celldex Therapeutics, Galderma, Incyte Corporation, Johnson & Johnson, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Kiniksa Pharmaceuticals and has served as an investigator for Galderma, Pfizer, and Sanofi. Dr. Chiesa Fuxench disclosed research grants from several pharmaceutical companies for work related to AD. She has also served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer.
A version of this article first appeared on Medscape.com.
and mortality, results from a large cohort analysis showed.
“The implications of these findings are vast,” presenting author Varsha Parthasarathy said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium. “Poor sleep quality is known to be associated with increased inflammatory markers such as IL-6, IL-17, and CRP, so it is interesting to see this reflected in AD patients with versus without sleep disturbance. Additionally, we know that CRP is a driver of inflammation and is strongly associated with cardiovascular complications such as heart attack and stroke. Therefore, CRP may be a useful prognostic marker in AD patients with sleep disturbances.”
To examine the comorbidity burden of sleep disorders in AD patients and associate findings with inflammatory CRP and cardiovascular comorbidities, Mr. Parthasarathy, a medical student and itch fellow in the department of dermatology at the Johns Hopkins University School of Medicine, Baltimore, and colleagues drew from TriNetX, a health care network of approximately 73 million de-identified medical records in 53 organizations. The years of study were 2015 to 2021. The researchers limited the analysis to adults with at least two instances of International Classification of Diseases, Tenth Revision (ICD-10) code L28 for AD, to capture a population with true AD. Controls were adults without AD who presented for general checkup and were matched to AD patients by age, race, and sex.
The study population consisted of 120,480 AD patients and matched controls. Their mean age was 36 years, 61% were female, and 26% were Black. Compared with controls, AD patients had an increased risk of developing general sleep disorders over the 6-year period (relative risk, 1.10), as well as obstructive sleep apnea (RR, 1.13), insomnia (RR, 1.10), hypersomnia (RR, 1.24), sleep-related movement disorders (RR, 1.36), restless legs syndrome (RR, 1.25), sleep deprivation (RR, 1.36), and unspecified sleep disorders (RR, 1.22).
To examine the association of sleep disturbance with the inflammatory biomarker CRP, the researchers measured CRP levels between these patient groups. They found a substantially higher CRP in AD patients compared with controls (21.2 mg/L vs. 7.6 mg/L, respectively; P < .0001). This finding “is suggestive of a higher level of inflammation in these patients,” Mr. Parthasarathy said. Interestingly, he added, they also found a higher CRP level in AD patients with sleep disturbances compared to AD patients without sleep disturbances (23.3 vs. 20.6 mg/L; P = .02), “also pointing to a higher inflammatory burden in AD patients whose sleep was affected.”
Compared to matched AD patients without sleep disorders, AD patients with sleep disorders were more likely to develop obesity (RR, 2.65), hyperlipidemia (RR, 2.18), type 2 diabetes (RR, 2.45), metabolic syndrome (RR, 4.16), atherosclerosis (RR, 2.42), peripheral vascular disease (RR, 2.47), stroke (RR, 2.37), venous thromboembolism (RR, 2.93), and mortality (hazard ratio, 1.24).
“There is a consequence of not treating patients with atopic dermatitis, especially those patients with sleep disturbance,” the study’s primary author, Shawn G. Kwatra, MD, associate professor of dermatology at Johns Hopkins, told this news organization. “Chronic inflammation can lead to the development of comorbidities, so it is important to offer patients early treatment to reduce their overall inflammation.” He said that he was most surprised by the degree of increased inflammation in the blood of AD as compared to healthy controls. “This likely plays a part in the development of several comorbidities,” he said.
Mr. Parthasarathy acknowledged certain limitations of the study, including the inability to infer causal relationships, as uncontrolled factors may be present. “Additionally, sampling of only patients that have had medical encounters limits the generalizability of the findings,” she said. “However, findings in this large cohort study suggest that clinicians should seek to identify sleep disorders in AD patients and screen for cardiac comorbidities secondary to inflammation in this patient population.”
“There is increased data to suggest that adults with AD, particularly those with more severe disease, may be at an increased risk of cardiovascular disease and the results from [this study] further support the concept of AD as systemic disease,” said Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study. She cited the large population-based, retrospective design and use of two instances of ICD codes for AD to confirm diagnosis as key strengths of the research. “However, it is unclear if for each patient CRP levels were measured at one single timepoint,” Dr. Chiesa Fuxench said. “For future studies, it would be interesting to see if these levels fluctuate with time and if persistently elevated levels are associated with worse cardiovascular outcomes in this population. More data is needed to better understand the relationship better atopic dermatitis disease severity, impact on sleep, and how this relates to increased systemic inflammation and worse cardiovascular outcomes in this population.”
Dr. Kwatra disclosed support by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR077073-01A1 and previous funding by the Dermatology Foundation and Skin of Color Society. Dr. Kwatra is also an advisory board member/consultant for AbbVie, Celldex Therapeutics, Galderma, Incyte Corporation, Johnson & Johnson, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Kiniksa Pharmaceuticals and has served as an investigator for Galderma, Pfizer, and Sanofi. Dr. Chiesa Fuxench disclosed research grants from several pharmaceutical companies for work related to AD. She has also served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer.
A version of this article first appeared on Medscape.com.
and mortality, results from a large cohort analysis showed.
“The implications of these findings are vast,” presenting author Varsha Parthasarathy said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium. “Poor sleep quality is known to be associated with increased inflammatory markers such as IL-6, IL-17, and CRP, so it is interesting to see this reflected in AD patients with versus without sleep disturbance. Additionally, we know that CRP is a driver of inflammation and is strongly associated with cardiovascular complications such as heart attack and stroke. Therefore, CRP may be a useful prognostic marker in AD patients with sleep disturbances.”
To examine the comorbidity burden of sleep disorders in AD patients and associate findings with inflammatory CRP and cardiovascular comorbidities, Mr. Parthasarathy, a medical student and itch fellow in the department of dermatology at the Johns Hopkins University School of Medicine, Baltimore, and colleagues drew from TriNetX, a health care network of approximately 73 million de-identified medical records in 53 organizations. The years of study were 2015 to 2021. The researchers limited the analysis to adults with at least two instances of International Classification of Diseases, Tenth Revision (ICD-10) code L28 for AD, to capture a population with true AD. Controls were adults without AD who presented for general checkup and were matched to AD patients by age, race, and sex.
The study population consisted of 120,480 AD patients and matched controls. Their mean age was 36 years, 61% were female, and 26% were Black. Compared with controls, AD patients had an increased risk of developing general sleep disorders over the 6-year period (relative risk, 1.10), as well as obstructive sleep apnea (RR, 1.13), insomnia (RR, 1.10), hypersomnia (RR, 1.24), sleep-related movement disorders (RR, 1.36), restless legs syndrome (RR, 1.25), sleep deprivation (RR, 1.36), and unspecified sleep disorders (RR, 1.22).
To examine the association of sleep disturbance with the inflammatory biomarker CRP, the researchers measured CRP levels between these patient groups. They found a substantially higher CRP in AD patients compared with controls (21.2 mg/L vs. 7.6 mg/L, respectively; P < .0001). This finding “is suggestive of a higher level of inflammation in these patients,” Mr. Parthasarathy said. Interestingly, he added, they also found a higher CRP level in AD patients with sleep disturbances compared to AD patients without sleep disturbances (23.3 vs. 20.6 mg/L; P = .02), “also pointing to a higher inflammatory burden in AD patients whose sleep was affected.”
Compared to matched AD patients without sleep disorders, AD patients with sleep disorders were more likely to develop obesity (RR, 2.65), hyperlipidemia (RR, 2.18), type 2 diabetes (RR, 2.45), metabolic syndrome (RR, 4.16), atherosclerosis (RR, 2.42), peripheral vascular disease (RR, 2.47), stroke (RR, 2.37), venous thromboembolism (RR, 2.93), and mortality (hazard ratio, 1.24).
“There is a consequence of not treating patients with atopic dermatitis, especially those patients with sleep disturbance,” the study’s primary author, Shawn G. Kwatra, MD, associate professor of dermatology at Johns Hopkins, told this news organization. “Chronic inflammation can lead to the development of comorbidities, so it is important to offer patients early treatment to reduce their overall inflammation.” He said that he was most surprised by the degree of increased inflammation in the blood of AD as compared to healthy controls. “This likely plays a part in the development of several comorbidities,” he said.
Mr. Parthasarathy acknowledged certain limitations of the study, including the inability to infer causal relationships, as uncontrolled factors may be present. “Additionally, sampling of only patients that have had medical encounters limits the generalizability of the findings,” she said. “However, findings in this large cohort study suggest that clinicians should seek to identify sleep disorders in AD patients and screen for cardiac comorbidities secondary to inflammation in this patient population.”
“There is increased data to suggest that adults with AD, particularly those with more severe disease, may be at an increased risk of cardiovascular disease and the results from [this study] further support the concept of AD as systemic disease,” said Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study. She cited the large population-based, retrospective design and use of two instances of ICD codes for AD to confirm diagnosis as key strengths of the research. “However, it is unclear if for each patient CRP levels were measured at one single timepoint,” Dr. Chiesa Fuxench said. “For future studies, it would be interesting to see if these levels fluctuate with time and if persistently elevated levels are associated with worse cardiovascular outcomes in this population. More data is needed to better understand the relationship better atopic dermatitis disease severity, impact on sleep, and how this relates to increased systemic inflammation and worse cardiovascular outcomes in this population.”
Dr. Kwatra disclosed support by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR077073-01A1 and previous funding by the Dermatology Foundation and Skin of Color Society. Dr. Kwatra is also an advisory board member/consultant for AbbVie, Celldex Therapeutics, Galderma, Incyte Corporation, Johnson & Johnson, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Kiniksa Pharmaceuticals and has served as an investigator for Galderma, Pfizer, and Sanofi. Dr. Chiesa Fuxench disclosed research grants from several pharmaceutical companies for work related to AD. She has also served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer.
A version of this article first appeared on Medscape.com.
More Americans skipping medical care because of cost, survey says
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.
That’s the highest reported number since the pandemic began and a tripling from March to October.
Even 20% of the country’s highest-income households – earning more than $120,000 per year – said they’ve also skipped care. That’s an increase of about seven times for higher-income families since March.
“Americans tend to think there is a group of lower-income people, and they have worse health care than the rest of us, and the rest of us, we’re okay,” Tim Lash, chief strategy officer for West Health, a nonprofit focused on lowering health care costs, told CBS News.
“What we are seeing now in this survey is this group of people who are identifying themselves as struggling with health care costs is growing,” he said.
As part of the 2021 Healthcare in America Report, researchers surveyed more than 6,000 people in September and October about their concerns and experiences with affording health care and treatment. About half of respondents said health care in America has gotten worse because of the pandemic, and more than half said they’re more worried about medical costs than before.
What’s more, many Americans put off routine doctor visits at the beginning of the pandemic, and now that they’re beginning to schedule appointments again, they’re facing major costs, the survey found. Some expenses have increased in the past year, including prescription medications.
The rising costs have led many people to skip care or treatment, which can have major consequences. About 1 in 20 adults said they know a friend or family member who died during the past year because they couldn’t afford medical care, the survey found. And about 20% of adults said they or someone in their household had a health issue that grew worse after postponing care because of price.
About 23% of survey respondents said that paying for health care represents a major financial burden, which increases to a third of respondents who earn less than $48,000 per year. Out-of-pocket costs such as deductibles and insurance premiums have increased, which have taken up larger portions of people’s budgets.
“We often overlook the side effect of costs, and it’s quite toxic – there is a financial toxicity that exists in health care,” Mr. Lash said. “We know when you skip treatment, that can have an impact on mortality.”
A version of this article first appeared on WebMD.com.
COVID-19 asymptomatic infection rate remains high
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Appendicitis: Up-front antibiotics OK in select patients
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA