User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
‘Substantial’ CVD risks, burden up to a year after COVID-19
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
If you’ve got 3 seconds, then you’ve got time to work out
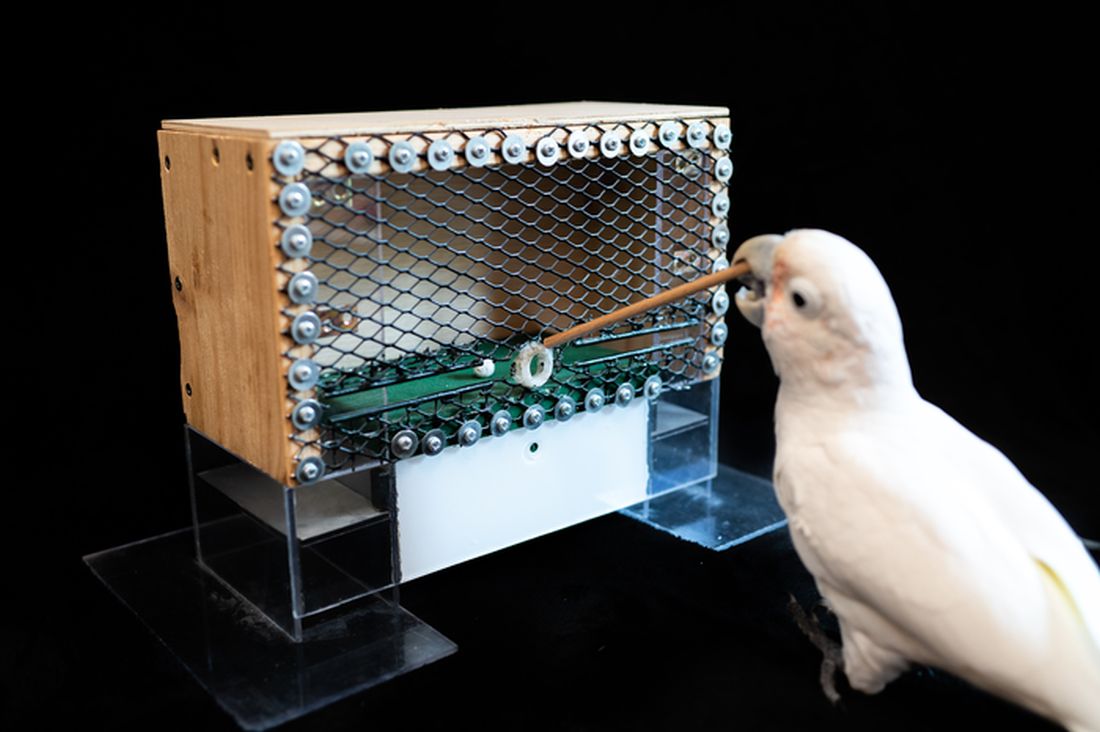
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Goffin’s cockatoo? More like golfin’ cockatoo
Can birds play golf? Of course not; it’s ridiculous. Humans can barely play golf, and we invented the sport. Anyway, moving on to “Brian retraction injury after elective aneurysm clipping.”
Hang on, we’re now hearing that a group of researchers, as part of a large international project comparing children’s innovation and problem-solving skills with those of cockatoos, have in fact taught a group of Goffin’s cockatoos how to play golf. Huh. What an oddly specific project. All right, fine, I guess we’ll go with the golf-playing birds.
Golf may seem very simple at its core. It is, essentially, whacking a ball with a stick. But the Scots who invented the game were undertaking a complex project involving combined usage of multiple tools, and until now, only primates were thought to be capable of utilizing compound tools to play games such as golf.
For this latest research, published in Scientific Reports, our intrepid birds were given a rudimentary form of golf to play (featuring a stick, a ball, and a closed box to get the ball through). Putting the ball through the hole gave the bird a reward. Not every cockatoo was able to hole out, but three did, with each inventing a unique way to manipulate the stick to hit the ball.
As entertaining as it would be to simply teach some birds how to play golf, we do loop back around to medical relevance. While children are perfectly capable of using tools, young children in particular are actually quite bad at using tools to solve novel solutions. Present a 5-year-old with a stick, a ball, and a hole, and that child might not figure out what the cockatoos did. The research really does give insight into the psychology behind the development of complex tools and technology by our ancient ancestors, according to the researchers.
We’re not entirely convinced this isn’t an elaborate ploy to get a bird out onto the PGA Tour. The LOTME staff can see the future headline already: “Painted bunting wins Valspar Championship in epic playoff.”
Work out now, sweat never
Okay, show of hands: Who’s familiar with “Name that tune?” The TV game show got a reboot last year, but some of us are old enough to remember the 1970s version hosted by national treasure Tom Kennedy.
The contestants try to identify a song as quickly as possible, claiming that they “can name that tune in five notes.” Or four notes, or three. Well, welcome to “Name that exercise study.”
Senior author Masatoshi Nakamura, PhD, and associates gathered together 39 students from Niigata (Japan) University of Health and Welfare and had them perform one isometric, concentric, or eccentric bicep curl with a dumbbell for 3 seconds a day at maximum effort for 5 days a week, over 4 weeks. And yes, we did say 3 seconds.
“Lifting the weight sees the bicep in concentric contraction, lowering the weight sees it in eccentric contraction, while holding the weight parallel to the ground is isometric,” they explained in a statement on Eurekalert.
The three exercise groups were compared with a group that did no exercise, and after 4 weeks of rigorous but brief science, the group doing eccentric contractions had the best results, as their overall muscle strength increased by 11.5%. After a total of just 60 seconds of exercise in 4 weeks. That’s 60 seconds. In 4 weeks.
Big news, but maybe we can do better. “Tom, we can do that exercise in 2 seconds.”
And one! And two! Whoa, feel the burn.
Tingling over anxiety
Apparently there are two kinds of people in this world. Those who love ASMR and those who just don’t get it.
ASMR, for those who don’t know, is the autonomous sensory meridian response. An online community has surfaced, with video creators making tapping sounds, whispering, or brushing mannequin hair to elicit “a pleasant tingling sensation originating from the scalp and neck which can spread to the rest of the body” from viewers, Charlotte M. Eid and associates said in PLOS One.
The people who are into these types of videos are more likely to have higher levels of neuroticism than those who aren’t, which gives ASMR the potential to be a nontraditional form of treatment for anxiety and/or neuroticism, they suggested.
The research involved a group of 64 volunteers who watched an ASMR video meant to trigger the tingles and then completed questionnaires to evaluate their levels of neuroticism, trait anxiety, and state anxiety, said Ms. Eid and associates of Northumbria University in Newcastle-upon-Tyne, England.
The people who had a history of producing tingles from ASMR videos in the past had higher levels of anxiety, compared with those who didn’t. Those who responded to triggers also received some benefit from the video in the study, reporting lower levels of neuroticism and anxiety after watching, the investigators found.
Although people who didn’t have a history of tingles didn’t feel any reduction in anxiety after the video, that didn’t stop the people who weren’t familiar with the genre from catching tingles.
So if you find yourself a little high strung or anxious, or if you can’t sleep, consider watching a person pretending to give you a makeover or using fingernails to tap on books for some relaxation. Don’t knock it until you try it!
Living in the past? Not so far-fetched
It’s usually an insult when people tell us to stop living in the past, but the joke’s on them because we really do live in the past. By 15 seconds, to be exact, according to researchers from the University of California, Berkeley.
But wait, did you just read that last sentence 15 seconds ago, even though it feels like real time? Did we just type these words now, or 15 seconds ago?
Think of your brain as a web page you’re constantly refreshing. We are constantly seeing new pictures, images, and colors, and your brain is responsible for keeping everything in chronological order. This new research suggests that our brains show us images from 15 seconds prior. Is your mind blown yet?
“One could say our brain is procrastinating. It’s too much work to constantly update images, so it sticks to the past because the past is a good predictor of the present. We recycle information from the past because it’s faster, more efficient and less work,” senior author David Whitney explained in a statement from the university.
It seems like the 15-second rule helps us not lose our minds by keeping a steady flow of information, but it could be a bit dangerous if someone, such as a surgeon, needs to see things with extreme precision.
And now we are definitely feeling a bit anxious about our upcoming heart/spleen/gallbladder replacement. … Where’s that link to the ASMR video?
Children and COVID: New cases down again, but still ‘extremely high’
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.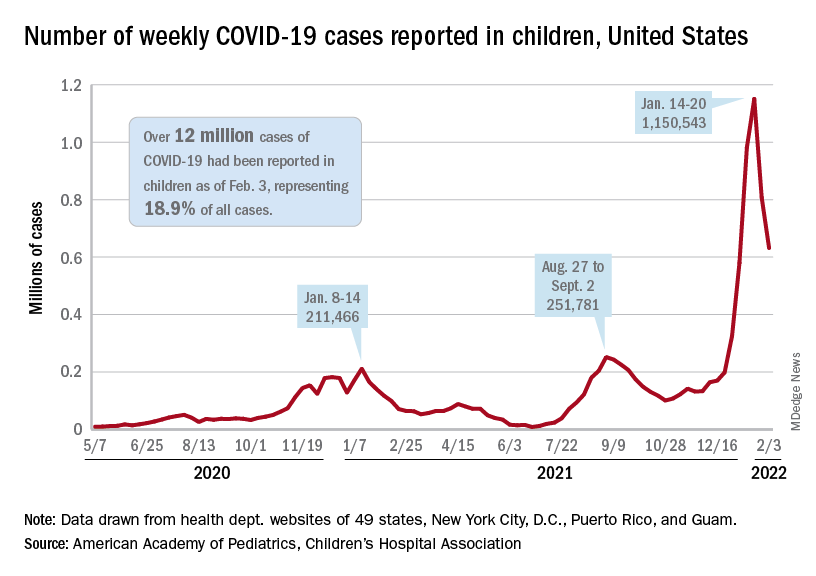
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
The indication of an Omicron decline has become a trend: New cases of COVID-19 in children were down for a second consecutive week in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.
but the nearly 632,000 cases reported were down by 22% from the previous week and by 45% from what appears to be the peak of the Omicron surge during the week of Jan. 14-20, the AAP/CHA data show.
To put the effect of the Delta and Omicron variants into some sort of perspective, the total number of COVID-19 cases among children passed 5 million at the beginning of September 2021, about a year and a half into the pandemic. In the last 5 months, the cumulative count has more than doubled and now stands at 12 million, the AAP and CHA said in their weekly COVID report.
Hospital admissions and emergency department visits followed the same downward trend over the last week. The rate of new hospitalizations fell to 0.81 per 100,000 children aged 0-17 years as of Feb. 2 (down from a peak of 1.25 per 100,000 on Jan. 15), and ED visits with diagnosed COVID-19 dropped to 1.8% (peak was 14.1%), 1.9% (peak was 14.3%), and 3.4% (peak was 14%) of all visits for children aged 16-17, 12-15, and 0-11 years, respectively, the Centers for Disease Control and Prevention reported.
The vaccination response
The surge of infections brought about by the Omicron variant, however, did not translate into increased vaccination, at least for the youngest eligible children. Vaccine initiation rose slightly among children aged 5-11 in early and mid-January but, by early February, new vaccinations had declined to their lowest point since approval in early November of 2021, the AAP said in its weekly COVID vaccination report.
As a result, the 5- to 11-year-olds are well behind the pace set by those aged 12-15 for the first 3 months of their vaccination experience. Through the first 13 weeks after the COVID vaccine was approved for children aged 12-15 in early May, 44.5% had received at least one dose and 32.3% were fully vaccinated. Among children aged 5-11, the corresponding figures through 13 weeks were 31% and 22.5%, according to CDC data.
The vaccination reaction to Omicron was somewhat more robust for children aged 12-17, compared with the younger group, but initiations dropped at the same time that new cases began to decline. In terms of total volume, the response among 12- to 17-year-olds was much smaller than that seen in July and August of 2021 as the Delta surge was hitting the United States, the AAP vaccination report shows.
All those vaccinations add up to this: Over 16.8 million children aged 12-17 and almost 9 million aged 5-11 had received at least one dose of vaccine as of Feb. 7, which works out to 66.6% of the older group and 31.2% of the younger cohort. Almost 14.3 million (56.4%) of those aged 12-17 are fully vaccinated, as are 6.6 million (22.9%) of the 5- to 11-year-olds, the CDC said on its COVID Data Tracker.
Two emerging drugs exacerbating opioid crisis
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Motor function restored in three men after complete paralysis from spinal cord injury
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SCI), new research shows.
The study demonstrated that an epidural electrical stimulation (EES) system developed specifically for spinal cord injuries enabled three men with complete paralysis to stand, walk, cycle, swim, and move their torso within 1 day.
“Thanks to this technology, we have been able to target individuals with the most serious spinal cord injury, meaning those with clinically complete spinal cord injury, with no sensation and no movement in the legs,” Grégoire Courtine, PhD, professor of neuroscience and neurotechnology at the Swiss Federal Institute of Technology, University Hospital Lausanne (Switzerland), and the University of Lausanne, told reporters attending a press briefing.
The study was published online Feb. 7, 2022, in Nature Medicine.
More rapid, precise, effective
SCIs involve severed connections between the brain and extremities. To compensate for these lost connections, researchers have investigated stem cell therapy, brain-machine interfaces, and powered exoskeletons.
However, these approaches aren’t yet ready for prime time.
In the meantime, researchers discovered even patients with a “complete” injury may have low-functioning connections and started investigating epidural stimulators designed to treat chronic pain. Recent studies – including three published in 2018 – showed promise for these pain-related stimulators in patients with incomplete SCI.
But using such “repurposed” technology meant the electrode array was relatively narrow and short, “so we could not target all the regions of the spinal cord involving control of leg and trunk movements,” said Dr. Courtine. With the newer technology “we are much more precise, effective, and more rapid in delivering therapy.”
To develop this new approach, the researchers designed a paddle lead with an arrangement of electrodes that targets sacral, lumbar, and low-thoracic dorsal roots involved in leg and trunk movements. They also established a personalized computational framework that allows for optimal surgical placement of this paddle lead.
In addition, they developed software that renders the configuration of individualized activity–dependent stimulation programs rapid, simple, and predictable.
They tested these neurotechnologies in three men with complete sensorimotor paralysis as part of an ongoing clinical trial. The participants, aged 29, 32, and 41 years, suffered an SCI from a motor bike accident 3, 9, and 1 year before enrollment.
All three patients exhibited complete sensorimotor paralysis. They were unable to take any step, and muscles remained quiescent during these attempts.
A neurosurgeon implanted electrodes along the spinal cord of study subjects. Wires from these electrodes were connected to a neurostimulator implanted under the skin in the abdomen.
The men can select different activity-based programs from a tablet that sends signals to the implanted device.
Personalized approach
Within a single day of the surgery, the participants were able to stand, walk, cycle, swim, and control trunk movements.
“It was not perfect at the very beginning, but they could train very early on to have a more fluid gait,” said study investigator neurosurgeon Joceylyne Bloch, MD, associate professor, University of Lausanne and University Hospital Lausanne.
At this stage, not all paralyzed patients are eligible for the procedure. Dr. Bloch explained that at least 6 cm of healthy spinal cord under the lesion is needed to implant the electrodes.
“There’s a huge variability of spinal cord anatomy between individuals. That’s why it’s important to study each person individually and to have individual models in order to be precise.”
Researchers envision having “a library of electrode arrays,” added Dr. Courtine. With preoperative imaging of the individual’s spinal cord, “the neurosurgeon can select the more appropriate electrode array for that specific patient.”
Dr. Courtine noted recovery of sensation with the system differs from one individual to another. One study participant, Michel Roccati, now 30, told the briefing he feels a contraction in his muscle during the stimulation.
Currently, only individuals whose injury is more than a year old are included in the study to ensure patients have “a stable lesion” and reached “a plateau of recovery,” said Dr. Bloch. However, animal models show intervening earlier might boost the benefits.
A patient’s age can influence the outcome, as younger patients are likely in better condition and more motivated than older patients, said Dr. Bloch. However, she noted patients closing in on 50 years have responded well to the therapy.
Such stimulation systems may prove useful in treating conditions typically associated with SCI, such as hypertension and bladder control, and perhaps also in patients with Parkinson’s disease, said Dr. Courtine.
The researchers plan to conduct another study that will include a next-generation pulse generator with features that make the stimulation even more effective and user friendly. A voice recognition system could eventually be connected to the system.
“The next step is a minicomputer that you implant in the body that communicates in real time with an external iPhone,” said Dr. Courtine.
ONWARD Medical, which developed the technology, has received a breakthrough device designation from the Food and Drug Administration. The company is in discussions with the FDA to carry out a clinical trial of the device in the United States.
A ‘huge step forward’
Peter J. Grahn, PhD, assistant professor, department of physical medicine and rehabilitation and department of neurologic surgery, Mayo Clinic, Rochester, Minn., an author of one of the 2018 studies, said this technology “is a huge step forward” and “really pushes the field.”
Compared with the device used in his study that’s designed to treat neuropathic pain, this new system “is much more capable of dynamic stimulation,” said Dr. Grahn. “You can tailor the stimulation based on which area of the spinal cord you want to target during a specific function.”
There has been “a lot of hope and hype” recently around stem cells and biological molecules that were supposed to be “magic pills” to cure spinal cord dysfunction, said Dr. Grahn. “I don’t think this is one of those.”
However, he questioned the researchers’ use of the word “walking.”
“They say independent stepping or walking is restored on day 1, but the graphs show day 1 function is having over 60% of their body weight supported when they’re taking these steps,” he said.
In addition, the “big question” is how this technology can “be distilled down” into an approach “applicable across rehabilitation centers,” said Dr. Grahn.
The study was supported by numerous organizations, including ONWARD Medical. Dr. Courtine and Dr. Bloch hold various patents in relation with the present work. Dr. Courtine is a consultant with ONWARD Medical, and he and Dr. Bloch are shareholders of ONWARD Medical, a company with direct relationships with the presented work. Dr. Grahn reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Updated guidance for COVID vaccination in rheumatology patients arrives amid continued hesitancy
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
FROM ARTHRITIS & RHEUMATOLOGY
Boosted Americans 97 times less likely to die of COVID-19 than unvaccinated
according to a new update from the CDC.
In addition, fully vaccinated Americans — meaning those with up to two doses, but no booster — are 14 times less likely to die from COVID-19 than unvaccinated people.
“These data confirm that vaccination and boosting continues to protect against severe illness and hospitalization, even during the Omicron surge,” Rochelle Walensky, MD, director of the CDC, said during a briefing by the White House COVID-19 Response Team.
“If you are not up to date on your COVID-19 vaccinations, you have not optimized your protection against severe disease and death, and you should get vaccinated and boosted if you are eligible,” she said.
Dr. Walensky presented the latest numbers on Feb. 2 based on reports from 25 jurisdictions in early December. The number of average weekly deaths for those who were unvaccinated was 9.7 per 100,000 people, as compared with 0.7 of those who were vaccinated and 0.1 of those who had received a booster.
“The data are really stunningly obvious why a booster is really very important,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during the briefing.
Dr. Fauci also encouraged vaccination for those who are pregnant and couples who may want to conceive in the near feature. He highlighted two recent studies that found vaccination in either partner didn’t affect fertility, including in vitro fertilization.
Meanwhile, fertility fell temporarily among men who were infected with the coronavirus. Couples were 18% less likely to conceive if the male partner had contracted the coronavirus within 60 days before a menstrual cycle.
“New data adds to previous studies that indicate that COVID-19 vaccination does not negatively impact fertility,” Dr. Fauci said. “Vaccination is recommended for people who are trying to get pregnant now or might become pregnant in the future, as well as their partners.”
About 80% of eligible Americans have received at least one vaccine dose, and 68% are fully vaccinated, according to the latest CDC data. About 51% of those who are eligible for a booster dose have received one.
The FDA could authorize the Pfizer vaccine for children under age 5 later this month. When that happens, about 18 million children will qualify for a shot, Jeff Zients, coordinator of the White House COVID-19 Response Team, said during the briefing. The Biden administration is already working on distribution plans for the shot for young kids, he added.
“We’ll be ready to start getting shots in arms soon after FDA and CDC make their decisions,” he said.
A version of this article first appeared on WebMD.com.
according to a new update from the CDC.
In addition, fully vaccinated Americans — meaning those with up to two doses, but no booster — are 14 times less likely to die from COVID-19 than unvaccinated people.
“These data confirm that vaccination and boosting continues to protect against severe illness and hospitalization, even during the Omicron surge,” Rochelle Walensky, MD, director of the CDC, said during a briefing by the White House COVID-19 Response Team.
“If you are not up to date on your COVID-19 vaccinations, you have not optimized your protection against severe disease and death, and you should get vaccinated and boosted if you are eligible,” she said.
Dr. Walensky presented the latest numbers on Feb. 2 based on reports from 25 jurisdictions in early December. The number of average weekly deaths for those who were unvaccinated was 9.7 per 100,000 people, as compared with 0.7 of those who were vaccinated and 0.1 of those who had received a booster.
“The data are really stunningly obvious why a booster is really very important,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during the briefing.
Dr. Fauci also encouraged vaccination for those who are pregnant and couples who may want to conceive in the near feature. He highlighted two recent studies that found vaccination in either partner didn’t affect fertility, including in vitro fertilization.
Meanwhile, fertility fell temporarily among men who were infected with the coronavirus. Couples were 18% less likely to conceive if the male partner had contracted the coronavirus within 60 days before a menstrual cycle.
“New data adds to previous studies that indicate that COVID-19 vaccination does not negatively impact fertility,” Dr. Fauci said. “Vaccination is recommended for people who are trying to get pregnant now or might become pregnant in the future, as well as their partners.”
About 80% of eligible Americans have received at least one vaccine dose, and 68% are fully vaccinated, according to the latest CDC data. About 51% of those who are eligible for a booster dose have received one.
The FDA could authorize the Pfizer vaccine for children under age 5 later this month. When that happens, about 18 million children will qualify for a shot, Jeff Zients, coordinator of the White House COVID-19 Response Team, said during the briefing. The Biden administration is already working on distribution plans for the shot for young kids, he added.
“We’ll be ready to start getting shots in arms soon after FDA and CDC make their decisions,” he said.
A version of this article first appeared on WebMD.com.
according to a new update from the CDC.
In addition, fully vaccinated Americans — meaning those with up to two doses, but no booster — are 14 times less likely to die from COVID-19 than unvaccinated people.
“These data confirm that vaccination and boosting continues to protect against severe illness and hospitalization, even during the Omicron surge,” Rochelle Walensky, MD, director of the CDC, said during a briefing by the White House COVID-19 Response Team.
“If you are not up to date on your COVID-19 vaccinations, you have not optimized your protection against severe disease and death, and you should get vaccinated and boosted if you are eligible,” she said.
Dr. Walensky presented the latest numbers on Feb. 2 based on reports from 25 jurisdictions in early December. The number of average weekly deaths for those who were unvaccinated was 9.7 per 100,000 people, as compared with 0.7 of those who were vaccinated and 0.1 of those who had received a booster.
“The data are really stunningly obvious why a booster is really very important,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during the briefing.
Dr. Fauci also encouraged vaccination for those who are pregnant and couples who may want to conceive in the near feature. He highlighted two recent studies that found vaccination in either partner didn’t affect fertility, including in vitro fertilization.
Meanwhile, fertility fell temporarily among men who were infected with the coronavirus. Couples were 18% less likely to conceive if the male partner had contracted the coronavirus within 60 days before a menstrual cycle.
“New data adds to previous studies that indicate that COVID-19 vaccination does not negatively impact fertility,” Dr. Fauci said. “Vaccination is recommended for people who are trying to get pregnant now or might become pregnant in the future, as well as their partners.”
About 80% of eligible Americans have received at least one vaccine dose, and 68% are fully vaccinated, according to the latest CDC data. About 51% of those who are eligible for a booster dose have received one.
The FDA could authorize the Pfizer vaccine for children under age 5 later this month. When that happens, about 18 million children will qualify for a shot, Jeff Zients, coordinator of the White House COVID-19 Response Team, said during the briefing. The Biden administration is already working on distribution plans for the shot for young kids, he added.
“We’ll be ready to start getting shots in arms soon after FDA and CDC make their decisions,” he said.
A version of this article first appeared on WebMD.com.
VARC-3 TAVR technical failure definition ‘highly clinically relevant’
A new study offers early validation of the recently released Valve Academic Research Consortium 3 (VARC-3) definition of technical success after transcatheter aortic valve replacement (TAVR) and highlights its role in patient prognosis.
Results show that one in 10 patients (11.6%) undergoing TAVR with contemporary devices and techniques experiences technical failure, according to VARC-3.
At 30 days, patients with technical failure had significantly higher rates of the composite of cardiovascular (CV) death or stroke (11.5% vs. 3.5%), CV death (6.0% vs. 1.0%), and stroke (7.2% vs. 2.9%), compared with those with technical success.
Technical failure after TAVR was also independently associated with a twofold higher risk for CV death or stroke at 1 year (20.0% vs. 10.3%; hazard ratio, 2.01; 95% CI, 1.37-2.95).
Other independent predictors were history of peripheral artery disease (HR, 1.97), New York Heart Association III or IV disease (HR, 1.86), baseline moderate or greater mitral regurgitation (HR, 1.48), atrial fibrillation (HR, 1.40), and Society of Thoracic Surgeons predicted mortality risk (HR, 1.04).
“We were expecting that we were getting better over time with device iterations, with more experience, so we weren’t surprised by the result. But I think what is somewhat surprising is how much of an impact it has on the outcome,” senior study author Thomas Pilgrim, MD, Inselspital, University of Bern, Switzerland, told this news organization.
The VARC-3 document, introduced last year to some controversy, features a heavier focus on patient outcomes, as well as composite safety and efficacy endpoints. The definition of technical success after TAVR includes freedom from death; successful access, delivery of the device, and retrieval of the delivery system; correct positioning of a prosthetic heart valve into the proper anatomical location; and freedom from surgery or intervention related to the device or to an access-related or cardiac structural complication.
The composite endpoint is meant to replace the VARC-2 definition of “device success,” which also included freedom from death and correct valve positioning but required echocardiographic evaluation. With VARC-3, there is an “immediate measure” of success without having to wait for echocardiography, observed Dr. Pilgrim.
As reported in the Journal of the American College of Cardiology Cardiovascular Interventions, TAVR was a technical success in 1,435 of 1,624 (88.4%) patients. Technical failure occurred in 189 patients related to either vascular complications (8.6%) or procedural death or cardiac complications (3.0%).
The VARC-2 endpoint of device success was observed in 66.1% of patients. The high rate of device failure was largely attributed to a 28% incidence of prosthesis-patient mismatch.
“If you use the VARC-2 device success [definition], you include this patient–prosthesis mismatch, the [valve] gradients, [and] regurgitation and then device success is always lower,” Dr. Pilgrim said.
Asked whether the VARC-3 definition may be missing case failures, he replied: “At this stage, we don’t know how important these echocardiographic parameters are for hard clinical endpoints. Maybe the VARC-2 endpoint was too sensitive or the VARC-3 endpoint is not sensitive enough. This is something we just don’t know at this stage.”
Marco Barbanti, MD, an interventional cardiologist at Rodolico Polyclinic University Hospital-San Marco, Catania, Italy, and author of an accompanying editorial, said VARC-3 represents a more accurate indicator of immediate success of the procedure.
“It’s a more pertinent definition according to what really has an impact on prognosis, and, according to the results of this paper, actually, the calibration of this new definition is quite good,” Dr. Barbanti said in an interview.
Patients with VARC-3 technical failure were older, had a higher body mass index, and had more advanced heart failure symptoms than those with technical success. There were no significant differences between the two groups in echocardiographic or CT data, anesthetic strategy, valve type or size, or use of pre- or post-dilation.
All patients underwent TAVR with current balloon-expandable (Sapien 3/Sapien Ultra, Edwards Lifesciences) or self-expanding (Evolut R/PRO [Medtronic], Portico [Abbott], Symetis ACURATE/ACURATE neo [Boston Scientific]) devices between March 2012 and December 2019. A transfemoral approach was used in 92.5% of patients.
In a landmark analysis with the landmark set at 30 days, the effect of technical failure on adverse outcome was limited to the first 30 days (composite endpoint 0-30 days: HR, 3.42; P < .001; 30-360 days: HR, 1.36; P = .266; P for interaction = .002).
At 1 year, the composite of CV death and stroke endpoint occurred in 24.1% of patients with cardiac technical failure, in 18.8% of patients with vascular technical failure, and in 10.3% of patients with technical success.
In multivariate analyses, cardiac and vascular technical failures were independently associated with a 2.6-fold and 1.9-fold increased risk, respectively, for the composite of cardiovascular death and stroke at 1 year.
Female sex, larger device landing zone calcium volume, and earlier procedures (March 2012 to July 2016) were associated with a higher risk for cardiac technical failure, whereas, consistent with previous studies, higher body mass index and use of the Prostar/Manta versus the ProGlide closure device predicted vascular technical failure.
The findings “underscore that technical success is highly clinically relevant and may serve as one of the pivotal endpoints to evaluate the improvement of TAVR or for head-to-head comparisons of new devices in future clinical trials,” the authors conclude.
The findings reflect the experience of a single high-volume center with highly experienced operators in the prospective BERN TAVR registry, however, and may not be generalizable to other heart centers, they note. Although the registry has standardized follow-up, independent analysis of echocardiographic and CT, and independent event adjudication, vascular anatomy was not systematically assessed, and the potential exists for confounding from unmeasured variables.
Dr. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientific, and Biotronik, personal fees from Biotronik and Boston Scientific, and other from HighLife SAS. Dr. Barbanti is a consultant for Edwards Lifesciences and Boston Scientific.
A version of this article first appeared on Medscape.com.
A new study offers early validation of the recently released Valve Academic Research Consortium 3 (VARC-3) definition of technical success after transcatheter aortic valve replacement (TAVR) and highlights its role in patient prognosis.
Results show that one in 10 patients (11.6%) undergoing TAVR with contemporary devices and techniques experiences technical failure, according to VARC-3.
At 30 days, patients with technical failure had significantly higher rates of the composite of cardiovascular (CV) death or stroke (11.5% vs. 3.5%), CV death (6.0% vs. 1.0%), and stroke (7.2% vs. 2.9%), compared with those with technical success.
Technical failure after TAVR was also independently associated with a twofold higher risk for CV death or stroke at 1 year (20.0% vs. 10.3%; hazard ratio, 2.01; 95% CI, 1.37-2.95).
Other independent predictors were history of peripheral artery disease (HR, 1.97), New York Heart Association III or IV disease (HR, 1.86), baseline moderate or greater mitral regurgitation (HR, 1.48), atrial fibrillation (HR, 1.40), and Society of Thoracic Surgeons predicted mortality risk (HR, 1.04).
“We were expecting that we were getting better over time with device iterations, with more experience, so we weren’t surprised by the result. But I think what is somewhat surprising is how much of an impact it has on the outcome,” senior study author Thomas Pilgrim, MD, Inselspital, University of Bern, Switzerland, told this news organization.
The VARC-3 document, introduced last year to some controversy, features a heavier focus on patient outcomes, as well as composite safety and efficacy endpoints. The definition of technical success after TAVR includes freedom from death; successful access, delivery of the device, and retrieval of the delivery system; correct positioning of a prosthetic heart valve into the proper anatomical location; and freedom from surgery or intervention related to the device or to an access-related or cardiac structural complication.
The composite endpoint is meant to replace the VARC-2 definition of “device success,” which also included freedom from death and correct valve positioning but required echocardiographic evaluation. With VARC-3, there is an “immediate measure” of success without having to wait for echocardiography, observed Dr. Pilgrim.
As reported in the Journal of the American College of Cardiology Cardiovascular Interventions, TAVR was a technical success in 1,435 of 1,624 (88.4%) patients. Technical failure occurred in 189 patients related to either vascular complications (8.6%) or procedural death or cardiac complications (3.0%).
The VARC-2 endpoint of device success was observed in 66.1% of patients. The high rate of device failure was largely attributed to a 28% incidence of prosthesis-patient mismatch.
“If you use the VARC-2 device success [definition], you include this patient–prosthesis mismatch, the [valve] gradients, [and] regurgitation and then device success is always lower,” Dr. Pilgrim said.
Asked whether the VARC-3 definition may be missing case failures, he replied: “At this stage, we don’t know how important these echocardiographic parameters are for hard clinical endpoints. Maybe the VARC-2 endpoint was too sensitive or the VARC-3 endpoint is not sensitive enough. This is something we just don’t know at this stage.”
Marco Barbanti, MD, an interventional cardiologist at Rodolico Polyclinic University Hospital-San Marco, Catania, Italy, and author of an accompanying editorial, said VARC-3 represents a more accurate indicator of immediate success of the procedure.
“It’s a more pertinent definition according to what really has an impact on prognosis, and, according to the results of this paper, actually, the calibration of this new definition is quite good,” Dr. Barbanti said in an interview.
Patients with VARC-3 technical failure were older, had a higher body mass index, and had more advanced heart failure symptoms than those with technical success. There were no significant differences between the two groups in echocardiographic or CT data, anesthetic strategy, valve type or size, or use of pre- or post-dilation.
All patients underwent TAVR with current balloon-expandable (Sapien 3/Sapien Ultra, Edwards Lifesciences) or self-expanding (Evolut R/PRO [Medtronic], Portico [Abbott], Symetis ACURATE/ACURATE neo [Boston Scientific]) devices between March 2012 and December 2019. A transfemoral approach was used in 92.5% of patients.
In a landmark analysis with the landmark set at 30 days, the effect of technical failure on adverse outcome was limited to the first 30 days (composite endpoint 0-30 days: HR, 3.42; P < .001; 30-360 days: HR, 1.36; P = .266; P for interaction = .002).
At 1 year, the composite of CV death and stroke endpoint occurred in 24.1% of patients with cardiac technical failure, in 18.8% of patients with vascular technical failure, and in 10.3% of patients with technical success.
In multivariate analyses, cardiac and vascular technical failures were independently associated with a 2.6-fold and 1.9-fold increased risk, respectively, for the composite of cardiovascular death and stroke at 1 year.
Female sex, larger device landing zone calcium volume, and earlier procedures (March 2012 to July 2016) were associated with a higher risk for cardiac technical failure, whereas, consistent with previous studies, higher body mass index and use of the Prostar/Manta versus the ProGlide closure device predicted vascular technical failure.
The findings “underscore that technical success is highly clinically relevant and may serve as one of the pivotal endpoints to evaluate the improvement of TAVR or for head-to-head comparisons of new devices in future clinical trials,” the authors conclude.
The findings reflect the experience of a single high-volume center with highly experienced operators in the prospective BERN TAVR registry, however, and may not be generalizable to other heart centers, they note. Although the registry has standardized follow-up, independent analysis of echocardiographic and CT, and independent event adjudication, vascular anatomy was not systematically assessed, and the potential exists for confounding from unmeasured variables.
Dr. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientific, and Biotronik, personal fees from Biotronik and Boston Scientific, and other from HighLife SAS. Dr. Barbanti is a consultant for Edwards Lifesciences and Boston Scientific.
A version of this article first appeared on Medscape.com.
A new study offers early validation of the recently released Valve Academic Research Consortium 3 (VARC-3) definition of technical success after transcatheter aortic valve replacement (TAVR) and highlights its role in patient prognosis.
Results show that one in 10 patients (11.6%) undergoing TAVR with contemporary devices and techniques experiences technical failure, according to VARC-3.
At 30 days, patients with technical failure had significantly higher rates of the composite of cardiovascular (CV) death or stroke (11.5% vs. 3.5%), CV death (6.0% vs. 1.0%), and stroke (7.2% vs. 2.9%), compared with those with technical success.
Technical failure after TAVR was also independently associated with a twofold higher risk for CV death or stroke at 1 year (20.0% vs. 10.3%; hazard ratio, 2.01; 95% CI, 1.37-2.95).
Other independent predictors were history of peripheral artery disease (HR, 1.97), New York Heart Association III or IV disease (HR, 1.86), baseline moderate or greater mitral regurgitation (HR, 1.48), atrial fibrillation (HR, 1.40), and Society of Thoracic Surgeons predicted mortality risk (HR, 1.04).
“We were expecting that we were getting better over time with device iterations, with more experience, so we weren’t surprised by the result. But I think what is somewhat surprising is how much of an impact it has on the outcome,” senior study author Thomas Pilgrim, MD, Inselspital, University of Bern, Switzerland, told this news organization.
The VARC-3 document, introduced last year to some controversy, features a heavier focus on patient outcomes, as well as composite safety and efficacy endpoints. The definition of technical success after TAVR includes freedom from death; successful access, delivery of the device, and retrieval of the delivery system; correct positioning of a prosthetic heart valve into the proper anatomical location; and freedom from surgery or intervention related to the device or to an access-related or cardiac structural complication.
The composite endpoint is meant to replace the VARC-2 definition of “device success,” which also included freedom from death and correct valve positioning but required echocardiographic evaluation. With VARC-3, there is an “immediate measure” of success without having to wait for echocardiography, observed Dr. Pilgrim.
As reported in the Journal of the American College of Cardiology Cardiovascular Interventions, TAVR was a technical success in 1,435 of 1,624 (88.4%) patients. Technical failure occurred in 189 patients related to either vascular complications (8.6%) or procedural death or cardiac complications (3.0%).
The VARC-2 endpoint of device success was observed in 66.1% of patients. The high rate of device failure was largely attributed to a 28% incidence of prosthesis-patient mismatch.
“If you use the VARC-2 device success [definition], you include this patient–prosthesis mismatch, the [valve] gradients, [and] regurgitation and then device success is always lower,” Dr. Pilgrim said.
Asked whether the VARC-3 definition may be missing case failures, he replied: “At this stage, we don’t know how important these echocardiographic parameters are for hard clinical endpoints. Maybe the VARC-2 endpoint was too sensitive or the VARC-3 endpoint is not sensitive enough. This is something we just don’t know at this stage.”
Marco Barbanti, MD, an interventional cardiologist at Rodolico Polyclinic University Hospital-San Marco, Catania, Italy, and author of an accompanying editorial, said VARC-3 represents a more accurate indicator of immediate success of the procedure.
“It’s a more pertinent definition according to what really has an impact on prognosis, and, according to the results of this paper, actually, the calibration of this new definition is quite good,” Dr. Barbanti said in an interview.
Patients with VARC-3 technical failure were older, had a higher body mass index, and had more advanced heart failure symptoms than those with technical success. There were no significant differences between the two groups in echocardiographic or CT data, anesthetic strategy, valve type or size, or use of pre- or post-dilation.
All patients underwent TAVR with current balloon-expandable (Sapien 3/Sapien Ultra, Edwards Lifesciences) or self-expanding (Evolut R/PRO [Medtronic], Portico [Abbott], Symetis ACURATE/ACURATE neo [Boston Scientific]) devices between March 2012 and December 2019. A transfemoral approach was used in 92.5% of patients.
In a landmark analysis with the landmark set at 30 days, the effect of technical failure on adverse outcome was limited to the first 30 days (composite endpoint 0-30 days: HR, 3.42; P < .001; 30-360 days: HR, 1.36; P = .266; P for interaction = .002).
At 1 year, the composite of CV death and stroke endpoint occurred in 24.1% of patients with cardiac technical failure, in 18.8% of patients with vascular technical failure, and in 10.3% of patients with technical success.
In multivariate analyses, cardiac and vascular technical failures were independently associated with a 2.6-fold and 1.9-fold increased risk, respectively, for the composite of cardiovascular death and stroke at 1 year.
Female sex, larger device landing zone calcium volume, and earlier procedures (March 2012 to July 2016) were associated with a higher risk for cardiac technical failure, whereas, consistent with previous studies, higher body mass index and use of the Prostar/Manta versus the ProGlide closure device predicted vascular technical failure.
The findings “underscore that technical success is highly clinically relevant and may serve as one of the pivotal endpoints to evaluate the improvement of TAVR or for head-to-head comparisons of new devices in future clinical trials,” the authors conclude.
The findings reflect the experience of a single high-volume center with highly experienced operators in the prospective BERN TAVR registry, however, and may not be generalizable to other heart centers, they note. Although the registry has standardized follow-up, independent analysis of echocardiographic and CT, and independent event adjudication, vascular anatomy was not systematically assessed, and the potential exists for confounding from unmeasured variables.
Dr. Pilgrim reports research grants to the institution from Edwards Lifesciences, Boston Scientific, and Biotronik, personal fees from Biotronik and Boston Scientific, and other from HighLife SAS. Dr. Barbanti is a consultant for Edwards Lifesciences and Boston Scientific.
A version of this article first appeared on Medscape.com.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Case report: Male with acute new-onset suicidal ideation tied to SARS-CoV-2
An otherwise healthy 55-year-old male, with no previous psychiatric or medical history, sought care with a family medicine physician for the first time in decades.
Medical symptoms began Oct. 9, 2021, with “some leg weakness and mild sniffles.” Since he was going to be at a public event, he decided to take a PCR test for the SARS-CoV-2 virus on Oct. 13. The patient tested positive.
His symptoms continued to worsen, and he experienced severe body fatigue, sleep disturbance, and lethargy. “A few days after my positive test, the cognitive and physical symptoms dramatically ramped up,” the patient recalled.
Because of those worsening symptoms, on Oct. 20, the patient obtained a new patient appointment with a family medicine physician. After a telemedicine evaluation, the family medicine physician began a multifaceted early outpatient COVID-19 treatment protocol,1 as I (C.M.W.) and colleagues wrote about late last year. However, this treatment began late in the course because of the patient’s initial resistance to seek care.
This early outpatient treatment protocol for COVID-19 included vitamin D3 125 mcg (5,000 ICU), N-acetylcysteine (NAC) 600 mg every day x 30 days; acetylsalicylic acid 325 mg every day x 30 days; azithromycin 250 mg b.i.d. before every meal x 10 days; hydroxychloroquine sulfate 200 mg b.i.d. x 10 days; ivermectin 3 mg, 5 pills daily x 10 days; zinc sulfate 220 mg (50 mg elemental) every day x 30 days; and a prednisone taper (30 mg daily x 3 days, tapering down 5 mg every 3 days). Hydroxyzine 50 mg at bedtime as needed was added for sleep. The patient did not comment to the family physician on any of the psychological or psychiatric symptoms and responded appropriately to questions during the Oct. 20 initial evaluation.
However, he later described that around the time the PCR was positive, For example, he was watching a simple YouTube video for work and “everything was confusing me ... it rattled me, and I couldn’t understand it.” He described his COVID-19 mind as: “The words in my head would come out in a jumbled order, like the message from the words in my brain to my mouth would get crossed. I had trouble spelling and texting. Total cognitive breakdown. I couldn’t do simple mathematics.”
Despite his physical exhaustion, he endured a 3-day period of sleep deprivation. During this time, he recalled looking up at the roof and thinking, “I need to jump off the roof” or thinking, “I might want to throw myself under a bus.” He did not initially reveal his suicidal thoughts to his family medicine physician. After beginning COVID-19 treatment, the patient had two nights of sleep and felt notably improved, and his physical symptoms began to remit. However, the sleeplessness quickly returned “with a vengeance” along with “silly suicidal thoughts.” The thoughts took on a more obsessional quality. For example, he repeatedly thought of jumping out of his second-story bedroom to the living room below and was preoccupied by continually looking at people’s roofs and thinking about jumping. Those thoughts intensified and culminated in his “going missing,” leading his wife to call the police. It was discovered that he had driven to a local bridge and was contemplating jumping off.
After that “going missing” incident, the patient and his wife reached out to their family medicine physician. He reevaluated the patient and, given the new information about the psychiatric symptoms, strongly recommended stat crisis and psychiatric consultation. After discussing the case on the same day, both the family medicine physician and the psychiatrist recommended stat hospital emergency department (ED) assessment on Oct. 29. In the ED, a head CT without contrast at the recommendation of both psychiatrist and family physician, routine electrolytes, CBC with differential, and EKG all were within normal limits. The ED initially discharged him home after crisis evaluation, deciding he was not an imminent risk to himself or others.
The next day, the psychiatrist spoke on the phone with the patient, family medicine physician, and the patient’s wife to arrange an initial assessment. At that time, it remained unclear to all whether the obsessional thoughts had resolved to such a degree that the patient could resist acting upon them. Further, the patient’s sleep architecture had not returned to normal. All agreed another emergency ED assessment was indicated. Ultimately, after voluntary re-evaluation and a difficult hold in the crisis unit, the patient was admitted for psychiatric hospitalization on Oct. 29 and discharged on Nov. 4.
In the psychiatric hospital, venlafaxine XR was started and titrated to 75 mg. The patient was discovered to be hypertensive, and hydrochlorothiazide was started. The discharge diagnosis was major depressive disorder, single episode, severe, without psychotic features.
Posthospitalization course
He was seen for his initial psychiatric outpatient assessment postpsychiatric hospitalization on Nov. 9, as he had not yet been formally evaluated by the psychiatrist because of the emergency situation.
Gabapentin 300 mg by mouth at bedtime was started, and his sleep architecture was restored. The initial plan to titrate venlafaxine XR into dual selective norepinephrine reuptake inhibitor dose range was terminated, and his psychiatrist considered tapering and discontinuing the venlafaxine XR. A clinical examination, additional history, and collateral data no longer necessarily pointed to an active major depressive disorder or even unspecified depressive disorder, though to be sure, the patient was taking 75 mg of venlafaxine XR. While there were seasonal stressors, historically, nothing had risen to the level of MDD.
The obsessions driving his thoughts to jump off buildings and bridges had completely remitted. His cognitive ability returned to baseline with an ability to focus and perform the complicated tasks of his high-intensity work by the Dec. 8 psychiatric examination, where he was accompanied by his wife. He described feeling like, “I snapped back to like I was before this crazy stuff happened.” His mood was reported as, “Very good; like my old self” and this was confirmed by his wife. His affect was calmer and less tense. He was now using gabapentin sparingly for sleep. We continued to entertain discontinuing the venlafaxine XR, considering this recent severe episode likely driven by the COVID-19 virus. The decision was made to continue venlafaxine XR through the winter rather than discontinuing, remaining on the conservative side of treatment. The patient’s diagnosis was changed from “MDD, single episode,” to “mood disorder due to known physiologic condition (COVID-19) (F06.31) with depressive features; resolving.” At the patient’s follow-up examination on Jan. 5, 2022, he was continuing to do well, stating, “The whole series of crazy events happened to someone else.” The hydrochlorothiazide had been discontinued, and the patient’s blood pressure and pulse were normal at 119/81 and 69, respectively. He had made strategic changes at work to lessen stressors during the typically difficult months.
Discussion
Literature has discussed neuropsychiatric sequelae of COVID-19.2 The cited example questions whether psychiatric symptoms are tied directly to the viral infection or to the “host’s immune response.” We believe our case represents a direct neurocognitive/neuropsychiatric insult due to the COVID-19 infection.
This case presents a 55-year-old male with no previous psychiatric or medical history with new onset significant and debilitating cognitive impairment and obsessive thoughts of throwing himself from his bedroom balcony ending up at a bridge struggling with an irrational thought of jumping; ultimately requiring psychiatric hospitalization for acute suicidal thoughts. The patient’s psychiatric symptoms arose prior to any and all medication treatment. The obsessive thoughts correlated both with the onset of SARS-CoV-2 infection and a period of sleep deprivation subsequent to the infection. A course of steroid treatment and taper were started after the onset of neurocognitive-psychiatric symptoms, though there is close timing. We submit that the patient experienced, as part of the initial neurocognitive psychiatric initiating cascade, a COVID-19–induced sleep deprivation that was not etiologic but part of the process; since, even when sleep returned to normal, it was still several weeks before full cognitive function returned to baseline.
An argument could be made for possible MDD or unspecified depressive disorder, as historically there had been work-related stressors for the patient at this time of year because of the chronological nature of his work; though previously nothing presented with obsessional suicidal thinking and nothing with any cognitive impairment – let alone to this incapacitating degree.
The patient describes his seasonal work much like an accountant’s work at the beginning of each year. In the patient’s case, the months of September and October are historically “nonstop, working days,” which then slow down in the winter months for a period of recuperation. In gathering his past history of symptoms, he denied neurovegetative symptoms to meet full diagnostic criteria for MDD or unspecified depressive disorder, absent this episode in the presence of SARS-CoV-2 infection.
We could also consider a contributory negative “organic push” by the viral load and prednisone helping to express an underlying unspecified depression or MDD, but for the profound and unusual presentation. There was little prodrome of depressive symptoms (again, he reported his “typical” extraordinary work burden for this time of year, which is common in his industry).
In this patient, the symptoms have remitted completely. However, the patient is currently taking venlafaxine XR 75 mg. We have considered tapering and discontinuing the venlafaxine – since it is not entirely clear that he needs to be on this medication – so this question remains an open one. We did decide, however, to continue the venlafaxine until after the winter months and to reassess at that time.
Conclusion
The patient presented with new onset psychological and psychiatric symptoms in addition to physiologic symptoms; the former symptoms were not revealed prior to initial family medicine evaluation. As the symptoms worsened, he and his wife sought additional consultation with family physician, psychiatrists, and ED. Steroid treatment may have played a part in exacerbation of symptoms, but the neuropsychiatric cognitive symptoms were present prior to initiation of all pharmacologic and medical treatment. The successful outcome of this case was based upon quick action and collaboration between the family medicine physician, the psychiatrist, and the ED physician. The value of communication, assessment, and action via phone call and text cannot be overstated. Future considerations include further large-scale evaluation of multifaceted early treatment of patients with COVID-19 within the first 72 hours of symptoms to prevent not only hospitalization, morbidity, and mortality, but newly recognized psychological and psychiatric syndromes.3,4
Lastly, fluvoxamine might have been a better choice for adjunctive early treatment of COVID-19.5 As a matter of distinction, if a lingering mood disorder or obsessive-compulsive disorder remain a result of SARS-CoV-2 or if one were to start an antidepressant during the course of illness, it would be reasonable to consider fluvoxamine as a potential first-line agent.
Dr. Kohanski is a fellowship trained forensic psychiatrist and a diplomate of the American Board of Psychiatry & Neurology. She maintains a private practice in Somerset, N.J., and is a frequent media commentator and medical podcaster. Dr. Kohanski has no conflicts of interest. Dr. Wax is a residency-trained osteopathic family medicine physician in independent private practice in Mullica Hill, N.J. He has authored multiple papers over 2 decades on topics such as SARS-CoV-2 and COVID-19 early treatment. He has been a speaker and media host over 2 decades and served on the National Physicians Council on Healthcare Policy’s congressional subcommittee. Dr. Wax has no conflicts of interest.
References
1. Rev Cardiovasc Med. 2020 Dec 30;21(4):517-30.
2. Brain Behav Immun. 2020 Jul;87:34-9.
3. Trav Med Infect Dis. 2020 May-Jun 35;10738.
4. Kirsch S. “Early treatment for COVID is key to better outcomes.” Times of India. 2021 May 21.
5. Lancet. 2022 Jan 1;10(1):E42-E51.
An otherwise healthy 55-year-old male, with no previous psychiatric or medical history, sought care with a family medicine physician for the first time in decades.
Medical symptoms began Oct. 9, 2021, with “some leg weakness and mild sniffles.” Since he was going to be at a public event, he decided to take a PCR test for the SARS-CoV-2 virus on Oct. 13. The patient tested positive.
His symptoms continued to worsen, and he experienced severe body fatigue, sleep disturbance, and lethargy. “A few days after my positive test, the cognitive and physical symptoms dramatically ramped up,” the patient recalled.
Because of those worsening symptoms, on Oct. 20, the patient obtained a new patient appointment with a family medicine physician. After a telemedicine evaluation, the family medicine physician began a multifaceted early outpatient COVID-19 treatment protocol,1 as I (C.M.W.) and colleagues wrote about late last year. However, this treatment began late in the course because of the patient’s initial resistance to seek care.
This early outpatient treatment protocol for COVID-19 included vitamin D3 125 mcg (5,000 ICU), N-acetylcysteine (NAC) 600 mg every day x 30 days; acetylsalicylic acid 325 mg every day x 30 days; azithromycin 250 mg b.i.d. before every meal x 10 days; hydroxychloroquine sulfate 200 mg b.i.d. x 10 days; ivermectin 3 mg, 5 pills daily x 10 days; zinc sulfate 220 mg (50 mg elemental) every day x 30 days; and a prednisone taper (30 mg daily x 3 days, tapering down 5 mg every 3 days). Hydroxyzine 50 mg at bedtime as needed was added for sleep. The patient did not comment to the family physician on any of the psychological or psychiatric symptoms and responded appropriately to questions during the Oct. 20 initial evaluation.
However, he later described that around the time the PCR was positive, For example, he was watching a simple YouTube video for work and “everything was confusing me ... it rattled me, and I couldn’t understand it.” He described his COVID-19 mind as: “The words in my head would come out in a jumbled order, like the message from the words in my brain to my mouth would get crossed. I had trouble spelling and texting. Total cognitive breakdown. I couldn’t do simple mathematics.”
Despite his physical exhaustion, he endured a 3-day period of sleep deprivation. During this time, he recalled looking up at the roof and thinking, “I need to jump off the roof” or thinking, “I might want to throw myself under a bus.” He did not initially reveal his suicidal thoughts to his family medicine physician. After beginning COVID-19 treatment, the patient had two nights of sleep and felt notably improved, and his physical symptoms began to remit. However, the sleeplessness quickly returned “with a vengeance” along with “silly suicidal thoughts.” The thoughts took on a more obsessional quality. For example, he repeatedly thought of jumping out of his second-story bedroom to the living room below and was preoccupied by continually looking at people’s roofs and thinking about jumping. Those thoughts intensified and culminated in his “going missing,” leading his wife to call the police. It was discovered that he had driven to a local bridge and was contemplating jumping off.
After that “going missing” incident, the patient and his wife reached out to their family medicine physician. He reevaluated the patient and, given the new information about the psychiatric symptoms, strongly recommended stat crisis and psychiatric consultation. After discussing the case on the same day, both the family medicine physician and the psychiatrist recommended stat hospital emergency department (ED) assessment on Oct. 29. In the ED, a head CT without contrast at the recommendation of both psychiatrist and family physician, routine electrolytes, CBC with differential, and EKG all were within normal limits. The ED initially discharged him home after crisis evaluation, deciding he was not an imminent risk to himself or others.
The next day, the psychiatrist spoke on the phone with the patient, family medicine physician, and the patient’s wife to arrange an initial assessment. At that time, it remained unclear to all whether the obsessional thoughts had resolved to such a degree that the patient could resist acting upon them. Further, the patient’s sleep architecture had not returned to normal. All agreed another emergency ED assessment was indicated. Ultimately, after voluntary re-evaluation and a difficult hold in the crisis unit, the patient was admitted for psychiatric hospitalization on Oct. 29 and discharged on Nov. 4.
In the psychiatric hospital, venlafaxine XR was started and titrated to 75 mg. The patient was discovered to be hypertensive, and hydrochlorothiazide was started. The discharge diagnosis was major depressive disorder, single episode, severe, without psychotic features.
Posthospitalization course
He was seen for his initial psychiatric outpatient assessment postpsychiatric hospitalization on Nov. 9, as he had not yet been formally evaluated by the psychiatrist because of the emergency situation.
Gabapentin 300 mg by mouth at bedtime was started, and his sleep architecture was restored. The initial plan to titrate venlafaxine XR into dual selective norepinephrine reuptake inhibitor dose range was terminated, and his psychiatrist considered tapering and discontinuing the venlafaxine XR. A clinical examination, additional history, and collateral data no longer necessarily pointed to an active major depressive disorder or even unspecified depressive disorder, though to be sure, the patient was taking 75 mg of venlafaxine XR. While there were seasonal stressors, historically, nothing had risen to the level of MDD.
The obsessions driving his thoughts to jump off buildings and bridges had completely remitted. His cognitive ability returned to baseline with an ability to focus and perform the complicated tasks of his high-intensity work by the Dec. 8 psychiatric examination, where he was accompanied by his wife. He described feeling like, “I snapped back to like I was before this crazy stuff happened.” His mood was reported as, “Very good; like my old self” and this was confirmed by his wife. His affect was calmer and less tense. He was now using gabapentin sparingly for sleep. We continued to entertain discontinuing the venlafaxine XR, considering this recent severe episode likely driven by the COVID-19 virus. The decision was made to continue venlafaxine XR through the winter rather than discontinuing, remaining on the conservative side of treatment. The patient’s diagnosis was changed from “MDD, single episode,” to “mood disorder due to known physiologic condition (COVID-19) (F06.31) with depressive features; resolving.” At the patient’s follow-up examination on Jan. 5, 2022, he was continuing to do well, stating, “The whole series of crazy events happened to someone else.” The hydrochlorothiazide had been discontinued, and the patient’s blood pressure and pulse were normal at 119/81 and 69, respectively. He had made strategic changes at work to lessen stressors during the typically difficult months.
Discussion
Literature has discussed neuropsychiatric sequelae of COVID-19.2 The cited example questions whether psychiatric symptoms are tied directly to the viral infection or to the “host’s immune response.” We believe our case represents a direct neurocognitive/neuropsychiatric insult due to the COVID-19 infection.
This case presents a 55-year-old male with no previous psychiatric or medical history with new onset significant and debilitating cognitive impairment and obsessive thoughts of throwing himself from his bedroom balcony ending up at a bridge struggling with an irrational thought of jumping; ultimately requiring psychiatric hospitalization for acute suicidal thoughts. The patient’s psychiatric symptoms arose prior to any and all medication treatment. The obsessive thoughts correlated both with the onset of SARS-CoV-2 infection and a period of sleep deprivation subsequent to the infection. A course of steroid treatment and taper were started after the onset of neurocognitive-psychiatric symptoms, though there is close timing. We submit that the patient experienced, as part of the initial neurocognitive psychiatric initiating cascade, a COVID-19–induced sleep deprivation that was not etiologic but part of the process; since, even when sleep returned to normal, it was still several weeks before full cognitive function returned to baseline.
An argument could be made for possible MDD or unspecified depressive disorder, as historically there had been work-related stressors for the patient at this time of year because of the chronological nature of his work; though previously nothing presented with obsessional suicidal thinking and nothing with any cognitive impairment – let alone to this incapacitating degree.
The patient describes his seasonal work much like an accountant’s work at the beginning of each year. In the patient’s case, the months of September and October are historically “nonstop, working days,” which then slow down in the winter months for a period of recuperation. In gathering his past history of symptoms, he denied neurovegetative symptoms to meet full diagnostic criteria for MDD or unspecified depressive disorder, absent this episode in the presence of SARS-CoV-2 infection.
We could also consider a contributory negative “organic push” by the viral load and prednisone helping to express an underlying unspecified depression or MDD, but for the profound and unusual presentation. There was little prodrome of depressive symptoms (again, he reported his “typical” extraordinary work burden for this time of year, which is common in his industry).
In this patient, the symptoms have remitted completely. However, the patient is currently taking venlafaxine XR 75 mg. We have considered tapering and discontinuing the venlafaxine – since it is not entirely clear that he needs to be on this medication – so this question remains an open one. We did decide, however, to continue the venlafaxine until after the winter months and to reassess at that time.
Conclusion
The patient presented with new onset psychological and psychiatric symptoms in addition to physiologic symptoms; the former symptoms were not revealed prior to initial family medicine evaluation. As the symptoms worsened, he and his wife sought additional consultation with family physician, psychiatrists, and ED. Steroid treatment may have played a part in exacerbation of symptoms, but the neuropsychiatric cognitive symptoms were present prior to initiation of all pharmacologic and medical treatment. The successful outcome of this case was based upon quick action and collaboration between the family medicine physician, the psychiatrist, and the ED physician. The value of communication, assessment, and action via phone call and text cannot be overstated. Future considerations include further large-scale evaluation of multifaceted early treatment of patients with COVID-19 within the first 72 hours of symptoms to prevent not only hospitalization, morbidity, and mortality, but newly recognized psychological and psychiatric syndromes.3,4
Lastly, fluvoxamine might have been a better choice for adjunctive early treatment of COVID-19.5 As a matter of distinction, if a lingering mood disorder or obsessive-compulsive disorder remain a result of SARS-CoV-2 or if one were to start an antidepressant during the course of illness, it would be reasonable to consider fluvoxamine as a potential first-line agent.
Dr. Kohanski is a fellowship trained forensic psychiatrist and a diplomate of the American Board of Psychiatry & Neurology. She maintains a private practice in Somerset, N.J., and is a frequent media commentator and medical podcaster. Dr. Kohanski has no conflicts of interest. Dr. Wax is a residency-trained osteopathic family medicine physician in independent private practice in Mullica Hill, N.J. He has authored multiple papers over 2 decades on topics such as SARS-CoV-2 and COVID-19 early treatment. He has been a speaker and media host over 2 decades and served on the National Physicians Council on Healthcare Policy’s congressional subcommittee. Dr. Wax has no conflicts of interest.
References
1. Rev Cardiovasc Med. 2020 Dec 30;21(4):517-30.
2. Brain Behav Immun. 2020 Jul;87:34-9.
3. Trav Med Infect Dis. 2020 May-Jun 35;10738.
4. Kirsch S. “Early treatment for COVID is key to better outcomes.” Times of India. 2021 May 21.
5. Lancet. 2022 Jan 1;10(1):E42-E51.
An otherwise healthy 55-year-old male, with no previous psychiatric or medical history, sought care with a family medicine physician for the first time in decades.
Medical symptoms began Oct. 9, 2021, with “some leg weakness and mild sniffles.” Since he was going to be at a public event, he decided to take a PCR test for the SARS-CoV-2 virus on Oct. 13. The patient tested positive.
His symptoms continued to worsen, and he experienced severe body fatigue, sleep disturbance, and lethargy. “A few days after my positive test, the cognitive and physical symptoms dramatically ramped up,” the patient recalled.
Because of those worsening symptoms, on Oct. 20, the patient obtained a new patient appointment with a family medicine physician. After a telemedicine evaluation, the family medicine physician began a multifaceted early outpatient COVID-19 treatment protocol,1 as I (C.M.W.) and colleagues wrote about late last year. However, this treatment began late in the course because of the patient’s initial resistance to seek care.
This early outpatient treatment protocol for COVID-19 included vitamin D3 125 mcg (5,000 ICU), N-acetylcysteine (NAC) 600 mg every day x 30 days; acetylsalicylic acid 325 mg every day x 30 days; azithromycin 250 mg b.i.d. before every meal x 10 days; hydroxychloroquine sulfate 200 mg b.i.d. x 10 days; ivermectin 3 mg, 5 pills daily x 10 days; zinc sulfate 220 mg (50 mg elemental) every day x 30 days; and a prednisone taper (30 mg daily x 3 days, tapering down 5 mg every 3 days). Hydroxyzine 50 mg at bedtime as needed was added for sleep. The patient did not comment to the family physician on any of the psychological or psychiatric symptoms and responded appropriately to questions during the Oct. 20 initial evaluation.
However, he later described that around the time the PCR was positive, For example, he was watching a simple YouTube video for work and “everything was confusing me ... it rattled me, and I couldn’t understand it.” He described his COVID-19 mind as: “The words in my head would come out in a jumbled order, like the message from the words in my brain to my mouth would get crossed. I had trouble spelling and texting. Total cognitive breakdown. I couldn’t do simple mathematics.”
Despite his physical exhaustion, he endured a 3-day period of sleep deprivation. During this time, he recalled looking up at the roof and thinking, “I need to jump off the roof” or thinking, “I might want to throw myself under a bus.” He did not initially reveal his suicidal thoughts to his family medicine physician. After beginning COVID-19 treatment, the patient had two nights of sleep and felt notably improved, and his physical symptoms began to remit. However, the sleeplessness quickly returned “with a vengeance” along with “silly suicidal thoughts.” The thoughts took on a more obsessional quality. For example, he repeatedly thought of jumping out of his second-story bedroom to the living room below and was preoccupied by continually looking at people’s roofs and thinking about jumping. Those thoughts intensified and culminated in his “going missing,” leading his wife to call the police. It was discovered that he had driven to a local bridge and was contemplating jumping off.
After that “going missing” incident, the patient and his wife reached out to their family medicine physician. He reevaluated the patient and, given the new information about the psychiatric symptoms, strongly recommended stat crisis and psychiatric consultation. After discussing the case on the same day, both the family medicine physician and the psychiatrist recommended stat hospital emergency department (ED) assessment on Oct. 29. In the ED, a head CT without contrast at the recommendation of both psychiatrist and family physician, routine electrolytes, CBC with differential, and EKG all were within normal limits. The ED initially discharged him home after crisis evaluation, deciding he was not an imminent risk to himself or others.
The next day, the psychiatrist spoke on the phone with the patient, family medicine physician, and the patient’s wife to arrange an initial assessment. At that time, it remained unclear to all whether the obsessional thoughts had resolved to such a degree that the patient could resist acting upon them. Further, the patient’s sleep architecture had not returned to normal. All agreed another emergency ED assessment was indicated. Ultimately, after voluntary re-evaluation and a difficult hold in the crisis unit, the patient was admitted for psychiatric hospitalization on Oct. 29 and discharged on Nov. 4.
In the psychiatric hospital, venlafaxine XR was started and titrated to 75 mg. The patient was discovered to be hypertensive, and hydrochlorothiazide was started. The discharge diagnosis was major depressive disorder, single episode, severe, without psychotic features.
Posthospitalization course
He was seen for his initial psychiatric outpatient assessment postpsychiatric hospitalization on Nov. 9, as he had not yet been formally evaluated by the psychiatrist because of the emergency situation.
Gabapentin 300 mg by mouth at bedtime was started, and his sleep architecture was restored. The initial plan to titrate venlafaxine XR into dual selective norepinephrine reuptake inhibitor dose range was terminated, and his psychiatrist considered tapering and discontinuing the venlafaxine XR. A clinical examination, additional history, and collateral data no longer necessarily pointed to an active major depressive disorder or even unspecified depressive disorder, though to be sure, the patient was taking 75 mg of venlafaxine XR. While there were seasonal stressors, historically, nothing had risen to the level of MDD.
The obsessions driving his thoughts to jump off buildings and bridges had completely remitted. His cognitive ability returned to baseline with an ability to focus and perform the complicated tasks of his high-intensity work by the Dec. 8 psychiatric examination, where he was accompanied by his wife. He described feeling like, “I snapped back to like I was before this crazy stuff happened.” His mood was reported as, “Very good; like my old self” and this was confirmed by his wife. His affect was calmer and less tense. He was now using gabapentin sparingly for sleep. We continued to entertain discontinuing the venlafaxine XR, considering this recent severe episode likely driven by the COVID-19 virus. The decision was made to continue venlafaxine XR through the winter rather than discontinuing, remaining on the conservative side of treatment. The patient’s diagnosis was changed from “MDD, single episode,” to “mood disorder due to known physiologic condition (COVID-19) (F06.31) with depressive features; resolving.” At the patient’s follow-up examination on Jan. 5, 2022, he was continuing to do well, stating, “The whole series of crazy events happened to someone else.” The hydrochlorothiazide had been discontinued, and the patient’s blood pressure and pulse were normal at 119/81 and 69, respectively. He had made strategic changes at work to lessen stressors during the typically difficult months.
Discussion
Literature has discussed neuropsychiatric sequelae of COVID-19.2 The cited example questions whether psychiatric symptoms are tied directly to the viral infection or to the “host’s immune response.” We believe our case represents a direct neurocognitive/neuropsychiatric insult due to the COVID-19 infection.
This case presents a 55-year-old male with no previous psychiatric or medical history with new onset significant and debilitating cognitive impairment and obsessive thoughts of throwing himself from his bedroom balcony ending up at a bridge struggling with an irrational thought of jumping; ultimately requiring psychiatric hospitalization for acute suicidal thoughts. The patient’s psychiatric symptoms arose prior to any and all medication treatment. The obsessive thoughts correlated both with the onset of SARS-CoV-2 infection and a period of sleep deprivation subsequent to the infection. A course of steroid treatment and taper were started after the onset of neurocognitive-psychiatric symptoms, though there is close timing. We submit that the patient experienced, as part of the initial neurocognitive psychiatric initiating cascade, a COVID-19–induced sleep deprivation that was not etiologic but part of the process; since, even when sleep returned to normal, it was still several weeks before full cognitive function returned to baseline.
An argument could be made for possible MDD or unspecified depressive disorder, as historically there had been work-related stressors for the patient at this time of year because of the chronological nature of his work; though previously nothing presented with obsessional suicidal thinking and nothing with any cognitive impairment – let alone to this incapacitating degree.
The patient describes his seasonal work much like an accountant’s work at the beginning of each year. In the patient’s case, the months of September and October are historically “nonstop, working days,” which then slow down in the winter months for a period of recuperation. In gathering his past history of symptoms, he denied neurovegetative symptoms to meet full diagnostic criteria for MDD or unspecified depressive disorder, absent this episode in the presence of SARS-CoV-2 infection.
We could also consider a contributory negative “organic push” by the viral load and prednisone helping to express an underlying unspecified depression or MDD, but for the profound and unusual presentation. There was little prodrome of depressive symptoms (again, he reported his “typical” extraordinary work burden for this time of year, which is common in his industry).
In this patient, the symptoms have remitted completely. However, the patient is currently taking venlafaxine XR 75 mg. We have considered tapering and discontinuing the venlafaxine – since it is not entirely clear that he needs to be on this medication – so this question remains an open one. We did decide, however, to continue the venlafaxine until after the winter months and to reassess at that time.
Conclusion
The patient presented with new onset psychological and psychiatric symptoms in addition to physiologic symptoms; the former symptoms were not revealed prior to initial family medicine evaluation. As the symptoms worsened, he and his wife sought additional consultation with family physician, psychiatrists, and ED. Steroid treatment may have played a part in exacerbation of symptoms, but the neuropsychiatric cognitive symptoms were present prior to initiation of all pharmacologic and medical treatment. The successful outcome of this case was based upon quick action and collaboration between the family medicine physician, the psychiatrist, and the ED physician. The value of communication, assessment, and action via phone call and text cannot be overstated. Future considerations include further large-scale evaluation of multifaceted early treatment of patients with COVID-19 within the first 72 hours of symptoms to prevent not only hospitalization, morbidity, and mortality, but newly recognized psychological and psychiatric syndromes.3,4
Lastly, fluvoxamine might have been a better choice for adjunctive early treatment of COVID-19.5 As a matter of distinction, if a lingering mood disorder or obsessive-compulsive disorder remain a result of SARS-CoV-2 or if one were to start an antidepressant during the course of illness, it would be reasonable to consider fluvoxamine as a potential first-line agent.
Dr. Kohanski is a fellowship trained forensic psychiatrist and a diplomate of the American Board of Psychiatry & Neurology. She maintains a private practice in Somerset, N.J., and is a frequent media commentator and medical podcaster. Dr. Kohanski has no conflicts of interest. Dr. Wax is a residency-trained osteopathic family medicine physician in independent private practice in Mullica Hill, N.J. He has authored multiple papers over 2 decades on topics such as SARS-CoV-2 and COVID-19 early treatment. He has been a speaker and media host over 2 decades and served on the National Physicians Council on Healthcare Policy’s congressional subcommittee. Dr. Wax has no conflicts of interest.
References
1. Rev Cardiovasc Med. 2020 Dec 30;21(4):517-30.
2. Brain Behav Immun. 2020 Jul;87:34-9.
3. Trav Med Infect Dis. 2020 May-Jun 35;10738.
4. Kirsch S. “Early treatment for COVID is key to better outcomes.” Times of India. 2021 May 21.
5. Lancet. 2022 Jan 1;10(1):E42-E51.
Topline data for aficamten positive in obstructive HCM
The investigational, next-generation cardiac myosin inhibitor aficamten (previously CK-274, Cytokinetics) continues to show promise as a potential treatment for hypertrophic cardiomyopathy (HCM).
Today, the company announced positive topline results from cohort 3 of the REDWOOD-HCM phase 2 clinical trial, which included 13 patients with symptomatic obstructive HCM and a resting or post-Valsalva left ventricular outflow tract pressure gradient (LVOT-G) of 50 mm Hg or greater whose background therapy included disopyramide.
Treatment with aficamten led to substantial reductions in the average resting LVOT-G, as well as the post-Valsalva LVOT-G (defined as resting gradient less than 30 mm Hg and post-Valsalva gradient less than 50 mm Hg), the company reported.
These “clinically relevant” decreases in pressure gradients were achieved with only modest decreases in average left ventricular ejection fraction (LVEF), the company said.
In no patient did LVEF fall below the prespecified safety threshold of 50%.
New York Heart Association (NYHA) functional class was improved in most patients.
The safety and tolerability of aficamten in cohort 3 were consistent with previous experience in the REDWOOD-HCM trial, with no treatment interruptions and no serious treatment-related adverse events.
The pharmacokinetic data from cohort 3 are similar to those observed in REDWOOD-HCM cohorts 1 and 2, which included HCM patients taking background medications exclusive of disopyramide, as reported previously by this news organization.
“We are encouraged by the clinically relevant reductions in the LVOT gradient observed in these medically refractory patients and are pleased with the safety profile of aficamten when administered in combination with disopyramide,” Fady Malik, MD, PhD, Cytokinetics’ executive vice president of research and development, said in a news release.
“These results represent the first report of patients with obstructive HCM treated with a combination of a cardiac myosin inhibitor and disopyramide and support our plan to include this patient population in SEQUOIA-HCM, our phase 3 trial, which is important, given these patients have exhausted other available medical therapies,” Dr. Malik said.
The results from cohort 3 of the REDWOOD-HCM trial will be presented at the upcoming American College of Cardiology Annual Meeting in April.
A version of this article first appeared on Medscape.com.
The investigational, next-generation cardiac myosin inhibitor aficamten (previously CK-274, Cytokinetics) continues to show promise as a potential treatment for hypertrophic cardiomyopathy (HCM).
Today, the company announced positive topline results from cohort 3 of the REDWOOD-HCM phase 2 clinical trial, which included 13 patients with symptomatic obstructive HCM and a resting or post-Valsalva left ventricular outflow tract pressure gradient (LVOT-G) of 50 mm Hg or greater whose background therapy included disopyramide.
Treatment with aficamten led to substantial reductions in the average resting LVOT-G, as well as the post-Valsalva LVOT-G (defined as resting gradient less than 30 mm Hg and post-Valsalva gradient less than 50 mm Hg), the company reported.
These “clinically relevant” decreases in pressure gradients were achieved with only modest decreases in average left ventricular ejection fraction (LVEF), the company said.
In no patient did LVEF fall below the prespecified safety threshold of 50%.
New York Heart Association (NYHA) functional class was improved in most patients.
The safety and tolerability of aficamten in cohort 3 were consistent with previous experience in the REDWOOD-HCM trial, with no treatment interruptions and no serious treatment-related adverse events.
The pharmacokinetic data from cohort 3 are similar to those observed in REDWOOD-HCM cohorts 1 and 2, which included HCM patients taking background medications exclusive of disopyramide, as reported previously by this news organization.
“We are encouraged by the clinically relevant reductions in the LVOT gradient observed in these medically refractory patients and are pleased with the safety profile of aficamten when administered in combination with disopyramide,” Fady Malik, MD, PhD, Cytokinetics’ executive vice president of research and development, said in a news release.
“These results represent the first report of patients with obstructive HCM treated with a combination of a cardiac myosin inhibitor and disopyramide and support our plan to include this patient population in SEQUOIA-HCM, our phase 3 trial, which is important, given these patients have exhausted other available medical therapies,” Dr. Malik said.
The results from cohort 3 of the REDWOOD-HCM trial will be presented at the upcoming American College of Cardiology Annual Meeting in April.
A version of this article first appeared on Medscape.com.
The investigational, next-generation cardiac myosin inhibitor aficamten (previously CK-274, Cytokinetics) continues to show promise as a potential treatment for hypertrophic cardiomyopathy (HCM).
Today, the company announced positive topline results from cohort 3 of the REDWOOD-HCM phase 2 clinical trial, which included 13 patients with symptomatic obstructive HCM and a resting or post-Valsalva left ventricular outflow tract pressure gradient (LVOT-G) of 50 mm Hg or greater whose background therapy included disopyramide.
Treatment with aficamten led to substantial reductions in the average resting LVOT-G, as well as the post-Valsalva LVOT-G (defined as resting gradient less than 30 mm Hg and post-Valsalva gradient less than 50 mm Hg), the company reported.
These “clinically relevant” decreases in pressure gradients were achieved with only modest decreases in average left ventricular ejection fraction (LVEF), the company said.
In no patient did LVEF fall below the prespecified safety threshold of 50%.
New York Heart Association (NYHA) functional class was improved in most patients.
The safety and tolerability of aficamten in cohort 3 were consistent with previous experience in the REDWOOD-HCM trial, with no treatment interruptions and no serious treatment-related adverse events.
The pharmacokinetic data from cohort 3 are similar to those observed in REDWOOD-HCM cohorts 1 and 2, which included HCM patients taking background medications exclusive of disopyramide, as reported previously by this news organization.
“We are encouraged by the clinically relevant reductions in the LVOT gradient observed in these medically refractory patients and are pleased with the safety profile of aficamten when administered in combination with disopyramide,” Fady Malik, MD, PhD, Cytokinetics’ executive vice president of research and development, said in a news release.
“These results represent the first report of patients with obstructive HCM treated with a combination of a cardiac myosin inhibitor and disopyramide and support our plan to include this patient population in SEQUOIA-HCM, our phase 3 trial, which is important, given these patients have exhausted other available medical therapies,” Dr. Malik said.
The results from cohort 3 of the REDWOOD-HCM trial will be presented at the upcoming American College of Cardiology Annual Meeting in April.
A version of this article first appeared on Medscape.com.