User login
EMERGENCY MEDICINE is a practical, peer-reviewed monthly publication and Web site that meets the educational needs of emergency clinicians and urgent care clinicians for their practice.
Liquid embolism of AVM tied to high cure rate
new observational data suggest. In a prospective, real-world study of more than 100 patients, use of the Onyx system was associated with a cure rate of 86% for cAVMs smaller than 3 cm.
“Endovascular treatment using Onyx is able to achieve, on its own, a very efficient cure rate with a low morbidity and mortality rate,” said investigator Laurent Spelle, MD, PhD, professor of neuroradiology at Paris-Saclay University and chair of NEURI, the Brain Vascular Center, Bicêtre Hospital, also in Paris.
Dr. Spelle presented the findings at the International Stroke Conference sponsored by the American Heart Association.
Prospective, multicenter study
Currently, the main treatment options for cAVM are embolization, neurosurgery, and radiosurgery. The Onyx liquid system, one method of providing embolization, uses a biocompatible ethylene vinyl alcohol copolymer.
It has been used in Europe for 22 years as a curative treatment and as a treatment before radiosurgery or neurosurgery. In the United States, Onyx is indicated for presurgical and preradiotherapy treatment only.
For this analysis, the researchers conducted a prospective, multicenter study to evaluate the long-term safety and efficacy of Onyx for the embolization of cAVM as curative treatment or preoperative preparation.
They enrolled 165 patients in the nonrandomized, observational study, which was conducted at 15 hospitals in France. Eligible participants had an untreated cAVM.
Patients were assigned to one of three groups, according to the hospital’s standard of care. One group underwent embolization with Onyx as curative treatment, one received Onyx as preparation for neurosurgery, and one underwent embolization with Onyx as preparation for radiosurgery.
The study’s safety endpoints were device- and procedure-related serious adverse events at 1 month after each embolization. The efficacy endpoints were recovery at 12 months after the last embolization or neurosurgery, or at a minimum of 36 months after radiosurgery.
The researchers defined morbidity as a worsening of modified Rankin Scale score of 2 or more points for patients presenting with baseline mRS of 0 or 1, or a worsening of 1 or more points for patients with an mRS of 2 or greater at baseline. An independent clinical events committee and core laboratory adjudicated the results.
‘A fantastic result’
In all, 140 patients were prospectively included, and 212 embolization procedures were performed. The population’s mean age was 41.4 years, and 60% of participants were men. About 61% of patients presented with symptoms, the most common of which were progressive neurologic deficit (41.2%) and headache (36.5%).
Approximately 64% of the cAVMs were ruptured. Most (75.7%) were smaller than 3 cm, and the remainder were between 3 and 6 cm. Most patients (59.3%) did not have an aneurysm.
Eight (3.8%) adverse events were associated with the use of Onyx. The rate of procedure-related neurologic serious adverse events was 7.1% within 1 month post embolization. Three deaths occurred (2.1%), one of which was considered device or procedure related.
A total of 87 patients underwent embolization alone, 14 of whom did not complete the study (2 died, 5 were lost to follow-up, and 7 withdrew). Of the 73 who completed the study, 58 (79.5%) had complete occlusion and full recovery at last follow-up. An additional 6.8% had 99% occlusion.
In addition, 3.4% of the population had significant morbidity, and 18.4% presented at baseline with mRS scores of 3-5. Of the latter group, 81.3% had mRS scores of 0-2 at last visit.
Of 21 patients who underwent subsequent neurosurgery, 18 completed follow-up. Of this group, 94.4% had complete occlusion. Of 32 patients receiving subsequent radiosurgery, 54.8% had complete occlusion, which was “a little bit disappointing,” said Dr. Spelle.
Overall, most patients (92.9%) had improved or stable mRS score. The overall mortality rate was 2.9%, and the rate of significant morbidity was 4.3%.
The rate of improved or stable mRS score was 94.3% for patients who underwent embolization alone, 85.7% for patients who also underwent neurosurgery, and 93.75% for patients who also underwent radiosurgery.
The mortality rate was 3.4% for patients who underwent embolization alone, 4.8% for patients who also underwent neurosurgery, and 0% for patients who also underwent radiosurgery.
The rate of significant morbidity was 2.3% for patients who underwent embolization alone, 9.5% for those who also underwent neurosurgery, and 6.25% for those who also underwent radiosurgery.
“We knew that this treatment was very effective, but this effectiveness was only known in a limited number of centers with a very high level of expertise,” said Dr. Spelle. “We were very pleasantly surprised that a larger-scale, multicenter study conducted in 15 different hospitals in France could achieve such a fantastic result.”
The study sites, however, were all departments in university hospitals with great experience in endovascular treatment of cAVM, he added.
Effective in unruptured AVMs?
Commenting on the findings, Mitchell Elkind, MD, professor of neurology and epidemiology, Columbia University, New York, said: “Arteriovenous malformations remain a relatively uncommon but serious cerebrovascular disorder. Any additional tool in the armamentarium to treat these lesions is welcome.”
The study results are encouraging, said Dr. Elkind, who was not involved in the study. They suggest that Onyx embolization can play an important role in the care of these patients. The treatment is associated with “low morbidity and excellent efficacy, particularly in combination with other surgical and radiographic approaches.”
The lack of a direct comparison with alternative embolization materials is a limitation of the study, however. “It is hard to compare Onyx to other agents based on these results,” said Dr. Elkind.
“It is also notable that one-third of the patients in the study had unruptured AVMs, which at least in one randomized trial, ARUBA, were not clearly shown to benefit from an intervention at all,” he continued.
It would have been valuable for the researchers to stratify the study results by ruptured versus unruptured AVMs, Dr. Elkind said.
The study was funded by Medtronic. Dr. Spelle reported receiving honoraria from the company. Dr. Elkind disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new observational data suggest. In a prospective, real-world study of more than 100 patients, use of the Onyx system was associated with a cure rate of 86% for cAVMs smaller than 3 cm.
“Endovascular treatment using Onyx is able to achieve, on its own, a very efficient cure rate with a low morbidity and mortality rate,” said investigator Laurent Spelle, MD, PhD, professor of neuroradiology at Paris-Saclay University and chair of NEURI, the Brain Vascular Center, Bicêtre Hospital, also in Paris.
Dr. Spelle presented the findings at the International Stroke Conference sponsored by the American Heart Association.
Prospective, multicenter study
Currently, the main treatment options for cAVM are embolization, neurosurgery, and radiosurgery. The Onyx liquid system, one method of providing embolization, uses a biocompatible ethylene vinyl alcohol copolymer.
It has been used in Europe for 22 years as a curative treatment and as a treatment before radiosurgery or neurosurgery. In the United States, Onyx is indicated for presurgical and preradiotherapy treatment only.
For this analysis, the researchers conducted a prospective, multicenter study to evaluate the long-term safety and efficacy of Onyx for the embolization of cAVM as curative treatment or preoperative preparation.
They enrolled 165 patients in the nonrandomized, observational study, which was conducted at 15 hospitals in France. Eligible participants had an untreated cAVM.
Patients were assigned to one of three groups, according to the hospital’s standard of care. One group underwent embolization with Onyx as curative treatment, one received Onyx as preparation for neurosurgery, and one underwent embolization with Onyx as preparation for radiosurgery.
The study’s safety endpoints were device- and procedure-related serious adverse events at 1 month after each embolization. The efficacy endpoints were recovery at 12 months after the last embolization or neurosurgery, or at a minimum of 36 months after radiosurgery.
The researchers defined morbidity as a worsening of modified Rankin Scale score of 2 or more points for patients presenting with baseline mRS of 0 or 1, or a worsening of 1 or more points for patients with an mRS of 2 or greater at baseline. An independent clinical events committee and core laboratory adjudicated the results.
‘A fantastic result’
In all, 140 patients were prospectively included, and 212 embolization procedures were performed. The population’s mean age was 41.4 years, and 60% of participants were men. About 61% of patients presented with symptoms, the most common of which were progressive neurologic deficit (41.2%) and headache (36.5%).
Approximately 64% of the cAVMs were ruptured. Most (75.7%) were smaller than 3 cm, and the remainder were between 3 and 6 cm. Most patients (59.3%) did not have an aneurysm.
Eight (3.8%) adverse events were associated with the use of Onyx. The rate of procedure-related neurologic serious adverse events was 7.1% within 1 month post embolization. Three deaths occurred (2.1%), one of which was considered device or procedure related.
A total of 87 patients underwent embolization alone, 14 of whom did not complete the study (2 died, 5 were lost to follow-up, and 7 withdrew). Of the 73 who completed the study, 58 (79.5%) had complete occlusion and full recovery at last follow-up. An additional 6.8% had 99% occlusion.
In addition, 3.4% of the population had significant morbidity, and 18.4% presented at baseline with mRS scores of 3-5. Of the latter group, 81.3% had mRS scores of 0-2 at last visit.
Of 21 patients who underwent subsequent neurosurgery, 18 completed follow-up. Of this group, 94.4% had complete occlusion. Of 32 patients receiving subsequent radiosurgery, 54.8% had complete occlusion, which was “a little bit disappointing,” said Dr. Spelle.
Overall, most patients (92.9%) had improved or stable mRS score. The overall mortality rate was 2.9%, and the rate of significant morbidity was 4.3%.
The rate of improved or stable mRS score was 94.3% for patients who underwent embolization alone, 85.7% for patients who also underwent neurosurgery, and 93.75% for patients who also underwent radiosurgery.
The mortality rate was 3.4% for patients who underwent embolization alone, 4.8% for patients who also underwent neurosurgery, and 0% for patients who also underwent radiosurgery.
The rate of significant morbidity was 2.3% for patients who underwent embolization alone, 9.5% for those who also underwent neurosurgery, and 6.25% for those who also underwent radiosurgery.
“We knew that this treatment was very effective, but this effectiveness was only known in a limited number of centers with a very high level of expertise,” said Dr. Spelle. “We were very pleasantly surprised that a larger-scale, multicenter study conducted in 15 different hospitals in France could achieve such a fantastic result.”
The study sites, however, were all departments in university hospitals with great experience in endovascular treatment of cAVM, he added.
Effective in unruptured AVMs?
Commenting on the findings, Mitchell Elkind, MD, professor of neurology and epidemiology, Columbia University, New York, said: “Arteriovenous malformations remain a relatively uncommon but serious cerebrovascular disorder. Any additional tool in the armamentarium to treat these lesions is welcome.”
The study results are encouraging, said Dr. Elkind, who was not involved in the study. They suggest that Onyx embolization can play an important role in the care of these patients. The treatment is associated with “low morbidity and excellent efficacy, particularly in combination with other surgical and radiographic approaches.”
The lack of a direct comparison with alternative embolization materials is a limitation of the study, however. “It is hard to compare Onyx to other agents based on these results,” said Dr. Elkind.
“It is also notable that one-third of the patients in the study had unruptured AVMs, which at least in one randomized trial, ARUBA, were not clearly shown to benefit from an intervention at all,” he continued.
It would have been valuable for the researchers to stratify the study results by ruptured versus unruptured AVMs, Dr. Elkind said.
The study was funded by Medtronic. Dr. Spelle reported receiving honoraria from the company. Dr. Elkind disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new observational data suggest. In a prospective, real-world study of more than 100 patients, use of the Onyx system was associated with a cure rate of 86% for cAVMs smaller than 3 cm.
“Endovascular treatment using Onyx is able to achieve, on its own, a very efficient cure rate with a low morbidity and mortality rate,” said investigator Laurent Spelle, MD, PhD, professor of neuroradiology at Paris-Saclay University and chair of NEURI, the Brain Vascular Center, Bicêtre Hospital, also in Paris.
Dr. Spelle presented the findings at the International Stroke Conference sponsored by the American Heart Association.
Prospective, multicenter study
Currently, the main treatment options for cAVM are embolization, neurosurgery, and radiosurgery. The Onyx liquid system, one method of providing embolization, uses a biocompatible ethylene vinyl alcohol copolymer.
It has been used in Europe for 22 years as a curative treatment and as a treatment before radiosurgery or neurosurgery. In the United States, Onyx is indicated for presurgical and preradiotherapy treatment only.
For this analysis, the researchers conducted a prospective, multicenter study to evaluate the long-term safety and efficacy of Onyx for the embolization of cAVM as curative treatment or preoperative preparation.
They enrolled 165 patients in the nonrandomized, observational study, which was conducted at 15 hospitals in France. Eligible participants had an untreated cAVM.
Patients were assigned to one of three groups, according to the hospital’s standard of care. One group underwent embolization with Onyx as curative treatment, one received Onyx as preparation for neurosurgery, and one underwent embolization with Onyx as preparation for radiosurgery.
The study’s safety endpoints were device- and procedure-related serious adverse events at 1 month after each embolization. The efficacy endpoints were recovery at 12 months after the last embolization or neurosurgery, or at a minimum of 36 months after radiosurgery.
The researchers defined morbidity as a worsening of modified Rankin Scale score of 2 or more points for patients presenting with baseline mRS of 0 or 1, or a worsening of 1 or more points for patients with an mRS of 2 or greater at baseline. An independent clinical events committee and core laboratory adjudicated the results.
‘A fantastic result’
In all, 140 patients were prospectively included, and 212 embolization procedures were performed. The population’s mean age was 41.4 years, and 60% of participants were men. About 61% of patients presented with symptoms, the most common of which were progressive neurologic deficit (41.2%) and headache (36.5%).
Approximately 64% of the cAVMs were ruptured. Most (75.7%) were smaller than 3 cm, and the remainder were between 3 and 6 cm. Most patients (59.3%) did not have an aneurysm.
Eight (3.8%) adverse events were associated with the use of Onyx. The rate of procedure-related neurologic serious adverse events was 7.1% within 1 month post embolization. Three deaths occurred (2.1%), one of which was considered device or procedure related.
A total of 87 patients underwent embolization alone, 14 of whom did not complete the study (2 died, 5 were lost to follow-up, and 7 withdrew). Of the 73 who completed the study, 58 (79.5%) had complete occlusion and full recovery at last follow-up. An additional 6.8% had 99% occlusion.
In addition, 3.4% of the population had significant morbidity, and 18.4% presented at baseline with mRS scores of 3-5. Of the latter group, 81.3% had mRS scores of 0-2 at last visit.
Of 21 patients who underwent subsequent neurosurgery, 18 completed follow-up. Of this group, 94.4% had complete occlusion. Of 32 patients receiving subsequent radiosurgery, 54.8% had complete occlusion, which was “a little bit disappointing,” said Dr. Spelle.
Overall, most patients (92.9%) had improved or stable mRS score. The overall mortality rate was 2.9%, and the rate of significant morbidity was 4.3%.
The rate of improved or stable mRS score was 94.3% for patients who underwent embolization alone, 85.7% for patients who also underwent neurosurgery, and 93.75% for patients who also underwent radiosurgery.
The mortality rate was 3.4% for patients who underwent embolization alone, 4.8% for patients who also underwent neurosurgery, and 0% for patients who also underwent radiosurgery.
The rate of significant morbidity was 2.3% for patients who underwent embolization alone, 9.5% for those who also underwent neurosurgery, and 6.25% for those who also underwent radiosurgery.
“We knew that this treatment was very effective, but this effectiveness was only known in a limited number of centers with a very high level of expertise,” said Dr. Spelle. “We were very pleasantly surprised that a larger-scale, multicenter study conducted in 15 different hospitals in France could achieve such a fantastic result.”
The study sites, however, were all departments in university hospitals with great experience in endovascular treatment of cAVM, he added.
Effective in unruptured AVMs?
Commenting on the findings, Mitchell Elkind, MD, professor of neurology and epidemiology, Columbia University, New York, said: “Arteriovenous malformations remain a relatively uncommon but serious cerebrovascular disorder. Any additional tool in the armamentarium to treat these lesions is welcome.”
The study results are encouraging, said Dr. Elkind, who was not involved in the study. They suggest that Onyx embolization can play an important role in the care of these patients. The treatment is associated with “low morbidity and excellent efficacy, particularly in combination with other surgical and radiographic approaches.”
The lack of a direct comparison with alternative embolization materials is a limitation of the study, however. “It is hard to compare Onyx to other agents based on these results,” said Dr. Elkind.
“It is also notable that one-third of the patients in the study had unruptured AVMs, which at least in one randomized trial, ARUBA, were not clearly shown to benefit from an intervention at all,” he continued.
It would have been valuable for the researchers to stratify the study results by ruptured versus unruptured AVMs, Dr. Elkind said.
The study was funded by Medtronic. Dr. Spelle reported receiving honoraria from the company. Dr. Elkind disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2022
Tenecteplase for stroke thrombolysis up to 24 hours?
, a new trial from China suggests.
The phase 2a CHABLIS trial was presented at the International Stroke Conference by Xin Cheng, MD, associate professor of neurology at the Huashan Hospital of Fudan University and the National Center for Neurological Disorders in Shanghai, China.
“These results are the first to be reported with tenecteplase in the extended time window and suggest that it may be feasible to extend the time window of intravenous thrombolysis to 24 hours after last known well through perfusion imaging selection,” she concluded at the conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Cheng noted that alteplase (tissue plasminogen activator) is the standard of care for thrombolysis in stroke, with a time window of up to 4.5 hours after stroke onset. However, the recent EXTEND trial suggested benefit of alteplase in patients who were between 4.5 and 9 hours of stroke onset and who had hypoperfused but salvageable regions of brain detected on automated perfusion imaging.
Tenecteplase is a genetically modified variant of alteplase. It has received regulatory approval for treatment of myocardial infarction. Dr. Cheng said there is increasing interest in tenecteplase as an alternative to alteplase, mainly because of its practical advantages (single bolus, rather than 1-hour infusion) and its having a number of hypothetical advantages over alteplase, including greater fibrin specificity and lesser likelihood of fibrinogen depletion.
Until now, studies of tenecteplase in stroke have included patients in the traditional time window, which has been no longer than 6 hours from stroke onset, she added.
For the current CHABLIS trial, the Chinese researchers investigated the use of tenecteplase administered to ischemic stroke patients at 4.5-24 hours from time of their being last seen well who were selected by significant penumbral mismatch on perfusion imaging. The trial included 86 patients who had an anterior large-vessel occlusion or severe stenosis identified on head and neck CT angiography and penumbral mismatch on CT perfusion imaging. They were randomized to one of two doses of tenecteplase, 0.25 mg/kg or 0.32 mg/kg.
The primary outcome was the achievement of reperfusion without symptomatic intracranial hemorrhage at 24-48 hours after thrombolysis. This occurred in 32% of the 0.25-mg/kg group versus 23.3% of the 0.32-mg/kg group.
Recanalization at 4-6 hours occurred in 44% of both groups.
In terms of neurologic outcomes, an excellent functional outcome, defined as a Modified Rankin Scale (mRS) score of 0-1 at 90 days, was achieved in 28% of the 0.25-mg/kg group and 49% of the 0.32-mg/kg group. A good functional outcome (mRS, 0-2) occurred in 46% of the 0.25-mg/kg group versus 60% of the 0.32-mg/kg group.
Limitations of the study included a small sample size and the lack of a control group. In addition, the study included only Chinese patients, who are known to have different stroke etiologies in comparison with White patients, Dr. Cheng noted.
In the subset of patients who received tenecteplase and who underwent endovascular therapy, fewer patients (8.8%) reached the primary outcome measure of reperfusion without symptomatic ICH, compared with those who received only tenecteplase (40.4%).
“In our study, tenecteplase seems to be quite effective and safe in patients who do not need endovascular therapy,” Dr. Cheng said. “More research is needed to understand why tenecteplase was less effective in restoring blood flow and more likely to result in symptomatic brain bleeding among those who had endovascular therapy.”
The researchers have now started a phase 2b trial, CHABLIS-2. This is a randomized, multicenter, controlled, open-label study of the 0.25-mg/kg dose of tenecteplase.
Commenting on the current study at an ISC press conference, Tudor G. Jovin, MD, chair of neurology at Cooper University Hospital, Cherry Hill, New Jersey, said: “This is very important study looking at the question of using thrombolysis out to 24 hours, and it does suggest a benefit, but we don’t know the best dose yet.”
He noted that his hospital system has already switched from alteplase to tenecteplase in the treatment of stroke, and several other centers are also making this switch. “In our center, we use the 0.25-mg/kg dose, but we don’t routinely treat patients beyond the 4.5-hour time window,” Dr. Jovin reported.
“The signals are there for a longer treatment window,” he said. “But this study was not aiming to directly answer whether tenecteplase is better than no treatment or alteplase, or its use with endovascular therapy.”
Noting that there are similar randomized trials ongoing in the United States and other countries exploring the same doses of tenecteplase, he said he thought the “dose and approach is applicable to U.S. practice.”
The CHABLIS study was funded by national key research and development program of China from the Science and Technology Ministry. Tenecteplase was provided by Guangzhou Recomgen Biotech.
A version of this article first appeared on Medscape.com.
, a new trial from China suggests.
The phase 2a CHABLIS trial was presented at the International Stroke Conference by Xin Cheng, MD, associate professor of neurology at the Huashan Hospital of Fudan University and the National Center for Neurological Disorders in Shanghai, China.
“These results are the first to be reported with tenecteplase in the extended time window and suggest that it may be feasible to extend the time window of intravenous thrombolysis to 24 hours after last known well through perfusion imaging selection,” she concluded at the conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Cheng noted that alteplase (tissue plasminogen activator) is the standard of care for thrombolysis in stroke, with a time window of up to 4.5 hours after stroke onset. However, the recent EXTEND trial suggested benefit of alteplase in patients who were between 4.5 and 9 hours of stroke onset and who had hypoperfused but salvageable regions of brain detected on automated perfusion imaging.
Tenecteplase is a genetically modified variant of alteplase. It has received regulatory approval for treatment of myocardial infarction. Dr. Cheng said there is increasing interest in tenecteplase as an alternative to alteplase, mainly because of its practical advantages (single bolus, rather than 1-hour infusion) and its having a number of hypothetical advantages over alteplase, including greater fibrin specificity and lesser likelihood of fibrinogen depletion.
Until now, studies of tenecteplase in stroke have included patients in the traditional time window, which has been no longer than 6 hours from stroke onset, she added.
For the current CHABLIS trial, the Chinese researchers investigated the use of tenecteplase administered to ischemic stroke patients at 4.5-24 hours from time of their being last seen well who were selected by significant penumbral mismatch on perfusion imaging. The trial included 86 patients who had an anterior large-vessel occlusion or severe stenosis identified on head and neck CT angiography and penumbral mismatch on CT perfusion imaging. They were randomized to one of two doses of tenecteplase, 0.25 mg/kg or 0.32 mg/kg.
The primary outcome was the achievement of reperfusion without symptomatic intracranial hemorrhage at 24-48 hours after thrombolysis. This occurred in 32% of the 0.25-mg/kg group versus 23.3% of the 0.32-mg/kg group.
Recanalization at 4-6 hours occurred in 44% of both groups.
In terms of neurologic outcomes, an excellent functional outcome, defined as a Modified Rankin Scale (mRS) score of 0-1 at 90 days, was achieved in 28% of the 0.25-mg/kg group and 49% of the 0.32-mg/kg group. A good functional outcome (mRS, 0-2) occurred in 46% of the 0.25-mg/kg group versus 60% of the 0.32-mg/kg group.
Limitations of the study included a small sample size and the lack of a control group. In addition, the study included only Chinese patients, who are known to have different stroke etiologies in comparison with White patients, Dr. Cheng noted.
In the subset of patients who received tenecteplase and who underwent endovascular therapy, fewer patients (8.8%) reached the primary outcome measure of reperfusion without symptomatic ICH, compared with those who received only tenecteplase (40.4%).
“In our study, tenecteplase seems to be quite effective and safe in patients who do not need endovascular therapy,” Dr. Cheng said. “More research is needed to understand why tenecteplase was less effective in restoring blood flow and more likely to result in symptomatic brain bleeding among those who had endovascular therapy.”
The researchers have now started a phase 2b trial, CHABLIS-2. This is a randomized, multicenter, controlled, open-label study of the 0.25-mg/kg dose of tenecteplase.
Commenting on the current study at an ISC press conference, Tudor G. Jovin, MD, chair of neurology at Cooper University Hospital, Cherry Hill, New Jersey, said: “This is very important study looking at the question of using thrombolysis out to 24 hours, and it does suggest a benefit, but we don’t know the best dose yet.”
He noted that his hospital system has already switched from alteplase to tenecteplase in the treatment of stroke, and several other centers are also making this switch. “In our center, we use the 0.25-mg/kg dose, but we don’t routinely treat patients beyond the 4.5-hour time window,” Dr. Jovin reported.
“The signals are there for a longer treatment window,” he said. “But this study was not aiming to directly answer whether tenecteplase is better than no treatment or alteplase, or its use with endovascular therapy.”
Noting that there are similar randomized trials ongoing in the United States and other countries exploring the same doses of tenecteplase, he said he thought the “dose and approach is applicable to U.S. practice.”
The CHABLIS study was funded by national key research and development program of China from the Science and Technology Ministry. Tenecteplase was provided by Guangzhou Recomgen Biotech.
A version of this article first appeared on Medscape.com.
, a new trial from China suggests.
The phase 2a CHABLIS trial was presented at the International Stroke Conference by Xin Cheng, MD, associate professor of neurology at the Huashan Hospital of Fudan University and the National Center for Neurological Disorders in Shanghai, China.
“These results are the first to be reported with tenecteplase in the extended time window and suggest that it may be feasible to extend the time window of intravenous thrombolysis to 24 hours after last known well through perfusion imaging selection,” she concluded at the conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Cheng noted that alteplase (tissue plasminogen activator) is the standard of care for thrombolysis in stroke, with a time window of up to 4.5 hours after stroke onset. However, the recent EXTEND trial suggested benefit of alteplase in patients who were between 4.5 and 9 hours of stroke onset and who had hypoperfused but salvageable regions of brain detected on automated perfusion imaging.
Tenecteplase is a genetically modified variant of alteplase. It has received regulatory approval for treatment of myocardial infarction. Dr. Cheng said there is increasing interest in tenecteplase as an alternative to alteplase, mainly because of its practical advantages (single bolus, rather than 1-hour infusion) and its having a number of hypothetical advantages over alteplase, including greater fibrin specificity and lesser likelihood of fibrinogen depletion.
Until now, studies of tenecteplase in stroke have included patients in the traditional time window, which has been no longer than 6 hours from stroke onset, she added.
For the current CHABLIS trial, the Chinese researchers investigated the use of tenecteplase administered to ischemic stroke patients at 4.5-24 hours from time of their being last seen well who were selected by significant penumbral mismatch on perfusion imaging. The trial included 86 patients who had an anterior large-vessel occlusion or severe stenosis identified on head and neck CT angiography and penumbral mismatch on CT perfusion imaging. They were randomized to one of two doses of tenecteplase, 0.25 mg/kg or 0.32 mg/kg.
The primary outcome was the achievement of reperfusion without symptomatic intracranial hemorrhage at 24-48 hours after thrombolysis. This occurred in 32% of the 0.25-mg/kg group versus 23.3% of the 0.32-mg/kg group.
Recanalization at 4-6 hours occurred in 44% of both groups.
In terms of neurologic outcomes, an excellent functional outcome, defined as a Modified Rankin Scale (mRS) score of 0-1 at 90 days, was achieved in 28% of the 0.25-mg/kg group and 49% of the 0.32-mg/kg group. A good functional outcome (mRS, 0-2) occurred in 46% of the 0.25-mg/kg group versus 60% of the 0.32-mg/kg group.
Limitations of the study included a small sample size and the lack of a control group. In addition, the study included only Chinese patients, who are known to have different stroke etiologies in comparison with White patients, Dr. Cheng noted.
In the subset of patients who received tenecteplase and who underwent endovascular therapy, fewer patients (8.8%) reached the primary outcome measure of reperfusion without symptomatic ICH, compared with those who received only tenecteplase (40.4%).
“In our study, tenecteplase seems to be quite effective and safe in patients who do not need endovascular therapy,” Dr. Cheng said. “More research is needed to understand why tenecteplase was less effective in restoring blood flow and more likely to result in symptomatic brain bleeding among those who had endovascular therapy.”
The researchers have now started a phase 2b trial, CHABLIS-2. This is a randomized, multicenter, controlled, open-label study of the 0.25-mg/kg dose of tenecteplase.
Commenting on the current study at an ISC press conference, Tudor G. Jovin, MD, chair of neurology at Cooper University Hospital, Cherry Hill, New Jersey, said: “This is very important study looking at the question of using thrombolysis out to 24 hours, and it does suggest a benefit, but we don’t know the best dose yet.”
He noted that his hospital system has already switched from alteplase to tenecteplase in the treatment of stroke, and several other centers are also making this switch. “In our center, we use the 0.25-mg/kg dose, but we don’t routinely treat patients beyond the 4.5-hour time window,” Dr. Jovin reported.
“The signals are there for a longer treatment window,” he said. “But this study was not aiming to directly answer whether tenecteplase is better than no treatment or alteplase, or its use with endovascular therapy.”
Noting that there are similar randomized trials ongoing in the United States and other countries exploring the same doses of tenecteplase, he said he thought the “dose and approach is applicable to U.S. practice.”
The CHABLIS study was funded by national key research and development program of China from the Science and Technology Ministry. Tenecteplase was provided by Guangzhou Recomgen Biotech.
A version of this article first appeared on Medscape.com.
FROM ISC 2022
Stroke risk is highest right after COVID infection
, new research shows.
The study among Medicare beneficiaries with COVID-19 also showed that stroke risk is higher for relatively young older adults, those aged 65 to 74 years, and those without a history of stroke.
The study highlights the impact COVID-19 has on the cardiovascular system, said study author Quanhe Yang, PhD, senior scientist, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta.
“Clinicians and patients should understand that stroke might be one of the very important clinical consequences of COVID-19.”
The study was presented during the hybrid International Stroke Conference held in New Orleans and online. The meeting was presented by the American Stroke Association, a division of the American Heart Association.
Stroke is the fifth leading cause of death in the U.S. As an increasing number of people become infected with COVID-19, “it’s important to determine if there’s a relationship between COVID and the risk of stroke,” said Dr. Yang.
Findings from prior research examining the link between stroke and COVID-19 have been inconsistent, he noted. Some studies found an association while others did not, and in still others, the association was not as strong as expected.
Many factors may contribute to these inconsistent findings, said Dr. Yang, including differences in study design, inclusion criteria, comparison groups, sample sizes, and countries where the research was carried out. Dr. Yang pointed out that many of these studies were done in the early stages of the pandemic or didn’t include older adults, the population most at risk for stroke.
The current study included 19,553 Medicare beneficiaries aged 65 years and older diagnosed with COVID-19 and hospitalized with acute ischemic stroke. The median age at diagnosis of COVID-19 was 80.5 years, 57.5% were women, and more than 75% were non-Hispanic Whites.
To ensure the stroke occurred after a COVID infection, researchers used a self-controlled case series study design, a “within person” comparison between the risk period and the control period.
They divided the study period (Jan. 1, 2019 to Feb. 28, 2021) into the exposure or stroke risk periods after the COVID diagnosis (0-3 days; 4-7 days; 8-15 days; and 15-28 days) and control periods.
Strokes that occurred 7 days before or 28 days after a COVID diagnosis served as a control period. “Any stroke that occurred outside the risk window is in the control period,” explained Dr. Yang.
He added that the control period provides a baseline. “Without COVID-19, this is what I would expect” in terms of the number of strokes.
To estimate the incidence rate ratio (IRR), investigators compared the incidence of acute ischemic stroke in the various risk periods with control periods.
The IRR was 10.97 (95% confidence interval, 10.30-11.68) at 0-3 days. The risk then quickly declined but stayed higher than the control period. The IRRs were: 1.59 (95% CI, 1.35-1.87) at 4-7 days; 1.23 (95% CI, 1.07-1.41) at 8-14 days; and 1.06 (95% CI, 0.95-1.18) at 15-28 days.
The temporary increase in stroke risk early after an infection isn’t novel; the pattern has been observed with influenza, respiratory infections, and shingles, said Dr. Yang. “But COVID-19 appears to be particularly risky.”
Although the mechanism driving the early increased stroke risk isn’t fully understood, it’s likely tied to an “exaggerated inflammatory response,” said Dr. Yang. This can trigger the cascade of events setting the stage for a stroke – a hypercoagulation state leading to the formation of blood clots that then block arteries to the brain, he said.
It’s also possible the infection directly affects endothelial cells, leading to rupture of plaque, again blocking arteries and raising stroke risks, added Dr. Yang.
The association was stronger among younger beneficiaries, aged 65 to 74 years, compared with those 85 years and older, a finding Dr. Yang said was somewhat surprising. But he noted other studies have found stroke patients with COVID are younger than stroke patients without COVID – by some 5 to 6 years.
“If COVID-19 disproportionately affects younger patients, that may explain the stronger association,” said Dr. Yang. “Stroke risk increases tremendously with age, so if you’re a younger age, your baseline stroke risk is lower.”
The association was also stronger among beneficiaries without a history of stroke. Again, this could be related to the stronger association among younger patients who are less likely to have suffered a stroke. The association was largely consistent across sex and race/ethnicities.
Dr. Yang stressed that the findings need to be confirmed with further studies.
The study was carried out before widespread use of vaccinations in the U.S. Once those data are available, Dr. Yang and his colleagues plan to determine if vaccinations modify the association between COVID-19 and stroke risk.
The new results contribute to the mounting evidence that a COVID-19 infection “can actually affect multiple human organs structurally or functionally in addition to the impact on [the] respiratory system,” said Dr. Yang.
Some dates of COVID-19 diagnoses may be incorrect due to limited test availability, particularly early in the pandemic. Another limitation of the study was possible misclassification from the use of Medicare real-time preliminary claims.
In a provided statement, Louise D. McCullough, MD, PhD, chair of the ISC 2022 and professor and chair of neurology, McGovern Medical School, University of Texas Health Science Center at Houston, noted that the study focused on older adults because it was examining Medicare beneficiaries.
“But everyone is likely at risk for stroke after COVID,” she said. “Any infection is linked to stroke risk, probably because any infection will cause inflammation, and inflammation can cause clots or thrombus, which is the cause of stroke.”
There was no outside funding for the study. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
, new research shows.
The study among Medicare beneficiaries with COVID-19 also showed that stroke risk is higher for relatively young older adults, those aged 65 to 74 years, and those without a history of stroke.
The study highlights the impact COVID-19 has on the cardiovascular system, said study author Quanhe Yang, PhD, senior scientist, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta.
“Clinicians and patients should understand that stroke might be one of the very important clinical consequences of COVID-19.”
The study was presented during the hybrid International Stroke Conference held in New Orleans and online. The meeting was presented by the American Stroke Association, a division of the American Heart Association.
Stroke is the fifth leading cause of death in the U.S. As an increasing number of people become infected with COVID-19, “it’s important to determine if there’s a relationship between COVID and the risk of stroke,” said Dr. Yang.
Findings from prior research examining the link between stroke and COVID-19 have been inconsistent, he noted. Some studies found an association while others did not, and in still others, the association was not as strong as expected.
Many factors may contribute to these inconsistent findings, said Dr. Yang, including differences in study design, inclusion criteria, comparison groups, sample sizes, and countries where the research was carried out. Dr. Yang pointed out that many of these studies were done in the early stages of the pandemic or didn’t include older adults, the population most at risk for stroke.
The current study included 19,553 Medicare beneficiaries aged 65 years and older diagnosed with COVID-19 and hospitalized with acute ischemic stroke. The median age at diagnosis of COVID-19 was 80.5 years, 57.5% were women, and more than 75% were non-Hispanic Whites.
To ensure the stroke occurred after a COVID infection, researchers used a self-controlled case series study design, a “within person” comparison between the risk period and the control period.
They divided the study period (Jan. 1, 2019 to Feb. 28, 2021) into the exposure or stroke risk periods after the COVID diagnosis (0-3 days; 4-7 days; 8-15 days; and 15-28 days) and control periods.
Strokes that occurred 7 days before or 28 days after a COVID diagnosis served as a control period. “Any stroke that occurred outside the risk window is in the control period,” explained Dr. Yang.
He added that the control period provides a baseline. “Without COVID-19, this is what I would expect” in terms of the number of strokes.
To estimate the incidence rate ratio (IRR), investigators compared the incidence of acute ischemic stroke in the various risk periods with control periods.
The IRR was 10.97 (95% confidence interval, 10.30-11.68) at 0-3 days. The risk then quickly declined but stayed higher than the control period. The IRRs were: 1.59 (95% CI, 1.35-1.87) at 4-7 days; 1.23 (95% CI, 1.07-1.41) at 8-14 days; and 1.06 (95% CI, 0.95-1.18) at 15-28 days.
The temporary increase in stroke risk early after an infection isn’t novel; the pattern has been observed with influenza, respiratory infections, and shingles, said Dr. Yang. “But COVID-19 appears to be particularly risky.”
Although the mechanism driving the early increased stroke risk isn’t fully understood, it’s likely tied to an “exaggerated inflammatory response,” said Dr. Yang. This can trigger the cascade of events setting the stage for a stroke – a hypercoagulation state leading to the formation of blood clots that then block arteries to the brain, he said.
It’s also possible the infection directly affects endothelial cells, leading to rupture of plaque, again blocking arteries and raising stroke risks, added Dr. Yang.
The association was stronger among younger beneficiaries, aged 65 to 74 years, compared with those 85 years and older, a finding Dr. Yang said was somewhat surprising. But he noted other studies have found stroke patients with COVID are younger than stroke patients without COVID – by some 5 to 6 years.
“If COVID-19 disproportionately affects younger patients, that may explain the stronger association,” said Dr. Yang. “Stroke risk increases tremendously with age, so if you’re a younger age, your baseline stroke risk is lower.”
The association was also stronger among beneficiaries without a history of stroke. Again, this could be related to the stronger association among younger patients who are less likely to have suffered a stroke. The association was largely consistent across sex and race/ethnicities.
Dr. Yang stressed that the findings need to be confirmed with further studies.
The study was carried out before widespread use of vaccinations in the U.S. Once those data are available, Dr. Yang and his colleagues plan to determine if vaccinations modify the association between COVID-19 and stroke risk.
The new results contribute to the mounting evidence that a COVID-19 infection “can actually affect multiple human organs structurally or functionally in addition to the impact on [the] respiratory system,” said Dr. Yang.
Some dates of COVID-19 diagnoses may be incorrect due to limited test availability, particularly early in the pandemic. Another limitation of the study was possible misclassification from the use of Medicare real-time preliminary claims.
In a provided statement, Louise D. McCullough, MD, PhD, chair of the ISC 2022 and professor and chair of neurology, McGovern Medical School, University of Texas Health Science Center at Houston, noted that the study focused on older adults because it was examining Medicare beneficiaries.
“But everyone is likely at risk for stroke after COVID,” she said. “Any infection is linked to stroke risk, probably because any infection will cause inflammation, and inflammation can cause clots or thrombus, which is the cause of stroke.”
There was no outside funding for the study. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
, new research shows.
The study among Medicare beneficiaries with COVID-19 also showed that stroke risk is higher for relatively young older adults, those aged 65 to 74 years, and those without a history of stroke.
The study highlights the impact COVID-19 has on the cardiovascular system, said study author Quanhe Yang, PhD, senior scientist, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta.
“Clinicians and patients should understand that stroke might be one of the very important clinical consequences of COVID-19.”
The study was presented during the hybrid International Stroke Conference held in New Orleans and online. The meeting was presented by the American Stroke Association, a division of the American Heart Association.
Stroke is the fifth leading cause of death in the U.S. As an increasing number of people become infected with COVID-19, “it’s important to determine if there’s a relationship between COVID and the risk of stroke,” said Dr. Yang.
Findings from prior research examining the link between stroke and COVID-19 have been inconsistent, he noted. Some studies found an association while others did not, and in still others, the association was not as strong as expected.
Many factors may contribute to these inconsistent findings, said Dr. Yang, including differences in study design, inclusion criteria, comparison groups, sample sizes, and countries where the research was carried out. Dr. Yang pointed out that many of these studies were done in the early stages of the pandemic or didn’t include older adults, the population most at risk for stroke.
The current study included 19,553 Medicare beneficiaries aged 65 years and older diagnosed with COVID-19 and hospitalized with acute ischemic stroke. The median age at diagnosis of COVID-19 was 80.5 years, 57.5% were women, and more than 75% were non-Hispanic Whites.
To ensure the stroke occurred after a COVID infection, researchers used a self-controlled case series study design, a “within person” comparison between the risk period and the control period.
They divided the study period (Jan. 1, 2019 to Feb. 28, 2021) into the exposure or stroke risk periods after the COVID diagnosis (0-3 days; 4-7 days; 8-15 days; and 15-28 days) and control periods.
Strokes that occurred 7 days before or 28 days after a COVID diagnosis served as a control period. “Any stroke that occurred outside the risk window is in the control period,” explained Dr. Yang.
He added that the control period provides a baseline. “Without COVID-19, this is what I would expect” in terms of the number of strokes.
To estimate the incidence rate ratio (IRR), investigators compared the incidence of acute ischemic stroke in the various risk periods with control periods.
The IRR was 10.97 (95% confidence interval, 10.30-11.68) at 0-3 days. The risk then quickly declined but stayed higher than the control period. The IRRs were: 1.59 (95% CI, 1.35-1.87) at 4-7 days; 1.23 (95% CI, 1.07-1.41) at 8-14 days; and 1.06 (95% CI, 0.95-1.18) at 15-28 days.
The temporary increase in stroke risk early after an infection isn’t novel; the pattern has been observed with influenza, respiratory infections, and shingles, said Dr. Yang. “But COVID-19 appears to be particularly risky.”
Although the mechanism driving the early increased stroke risk isn’t fully understood, it’s likely tied to an “exaggerated inflammatory response,” said Dr. Yang. This can trigger the cascade of events setting the stage for a stroke – a hypercoagulation state leading to the formation of blood clots that then block arteries to the brain, he said.
It’s also possible the infection directly affects endothelial cells, leading to rupture of plaque, again blocking arteries and raising stroke risks, added Dr. Yang.
The association was stronger among younger beneficiaries, aged 65 to 74 years, compared with those 85 years and older, a finding Dr. Yang said was somewhat surprising. But he noted other studies have found stroke patients with COVID are younger than stroke patients without COVID – by some 5 to 6 years.
“If COVID-19 disproportionately affects younger patients, that may explain the stronger association,” said Dr. Yang. “Stroke risk increases tremendously with age, so if you’re a younger age, your baseline stroke risk is lower.”
The association was also stronger among beneficiaries without a history of stroke. Again, this could be related to the stronger association among younger patients who are less likely to have suffered a stroke. The association was largely consistent across sex and race/ethnicities.
Dr. Yang stressed that the findings need to be confirmed with further studies.
The study was carried out before widespread use of vaccinations in the U.S. Once those data are available, Dr. Yang and his colleagues plan to determine if vaccinations modify the association between COVID-19 and stroke risk.
The new results contribute to the mounting evidence that a COVID-19 infection “can actually affect multiple human organs structurally or functionally in addition to the impact on [the] respiratory system,” said Dr. Yang.
Some dates of COVID-19 diagnoses may be incorrect due to limited test availability, particularly early in the pandemic. Another limitation of the study was possible misclassification from the use of Medicare real-time preliminary claims.
In a provided statement, Louise D. McCullough, MD, PhD, chair of the ISC 2022 and professor and chair of neurology, McGovern Medical School, University of Texas Health Science Center at Houston, noted that the study focused on older adults because it was examining Medicare beneficiaries.
“But everyone is likely at risk for stroke after COVID,” she said. “Any infection is linked to stroke risk, probably because any infection will cause inflammation, and inflammation can cause clots or thrombus, which is the cause of stroke.”
There was no outside funding for the study. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
FROM ISC 2022
Two emerging drugs exacerbating opioid crisis
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Two illicit drugs are contributing to a sharp rise in fentanyl-related deaths, a new study from the Centers for Disease Control and Prevention shows.
Para-fluorofentanyl, a schedule I substance often found in heroin packets and counterfeit pills, is making a comeback on the illicit drug market, Jordan Trecki, PhD, and associates reported in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report (2022 Jan 28;71[4]:153-5). U.S. medical examiner reports and national law enforcement seizure data point to a rise in encounters of this drug along with metonitazene, a benzimidazole-opioid, in combination with fentanyl.
On their own, para-fluorofentanyl and metonitazene can kill the user through respiratory depression. Combinations of these substances and other opioids, including fentanyl-related compounds or adulterants, “pose an even greater potential harm to the patient than previously observed,” reported Dr. Trecki, a pharmacologist affiliated with the Drug Enforcement Administration, and colleagues.
Opioids contribute to about 75% of all U.S. drug overdose deaths, which rose by 28.5% during 2020-2021, according to the National Center for Health Statistics. And fentanyl is replacing heroin as the primary drug of use, said addiction specialist Brian Fuehrlein, MD, PhD, in an interview.
“For patients with stimulant use disorder and even cannabis use disorder, fentanyl is becoming more and more common as an adulterant in those substances, often resulting in inadvertent use. Hence, fentanyl and fentanyl-like drugs and fentanyl analogues are becoming increasingly common and important,” said Dr. Fuehrlein, director of the psychiatric emergency room at the VA Connecticut Healthcare System. He was not involved with the MMWR study.
Tennessee data reflect national problem
Recent data from a medical examiner in Knoxville, Tenn., illustrate what might be happening nationwide with those two emerging substances.
Over the last 2 years, the Knox County Regional Forensic Center has identified para-fluorofentanyl in the toxicology results of drug overdose victims, and metonitazene – either on its own or in combination with fentanyl and para-fluorofentanyl. Fentanyl appeared in 562 or 73% of 770 unintentional drug overdose deaths from November 2020 to August 2021. Forty-eight of these cases involved para-fluorofentanyl, and 26 involved metonitazene.
“Although the percentage of law enforcement encounters with these substances in Tennessee decreased relative to the national total percentage within this time frame, the increase in encounters both within Tennessee and nationally reflect an increased distribution of para-fluorofentanyl and metonitazene throughout the United States,” the authors reported.
How to identify substances, manage overdoses
The authors encouraged physicians, labs, and medical examiners to be on the lookout for these two substances either in the emergency department or when identifying the cause of drug overdose deaths.
They also advised that stronger opioids, such as fentanyl, para-fluorofentanyl, metonitazene, or other benzimidazoles may warrant additional doses of the opioid-reversal drug naloxone.
While he hasn’t personally seen any of these drugs in his practice, “I would assume that these are on the rise due to inexpensive cost to manufacture and potency of effect,” said Dr. Fuehrlein, also an associate professor of psychiatry at Yale University, New Haven, Conn.
The need for additional naloxone to manage acute overdoses is a key takeaway of the MMWR paper, he added. Clinicians should also educate patients about harm reduction strategies to avoid overdose death when using potentially powerful and unknown drugs. “Things like start low and go slow, buy from the same supplier, do not use opioids with alcohol or benzos, have Narcan available, do not use alone, etc.”
Dr. Fuehrlein had no disclosures.
Another winter for our discontent
Here we are. Again. It’s cold and it’s gray. The sun rises late and sets early, so that it feels like midnight by 8 p.m. Indoor venues are risky with the highly contagious Omicron variant, and I feel like we are all pushing the replay button on 2021’s miserable winter.
In some ways, it’s worse: In 2021 we had the hope that vaccines would pull us out of the pandemic and we had guidance on all that we should not be doing. In January, we were gaming the various Internet sites to get a coveted vaccine for ourselves or our family and friends, then lining up to get jabbed. We did not yet know that it wouldn’t be enough – that we’d need boosters, that Delta and Omicron would defy the vaccines. Yes, the vaccines work miracles to prevent severe disease and death, but the worry of passing the virus to someone who is vulnerable or unvaccinated(!), or both, remains – and now we can wonder how we’ll ever get out of this mess with hopeful talk of an endemic, while we wait on the next variant. I like certainty, and this pandemic is one big screaming reminder that certainty about anything is just a pleasant notion, death and taxes excluded, of course.
Kris Lukish, vice president of human resources at Johns Hopkins Hospital in Baltimore, started an update to the hospital employees with: “As we begin 2022, it feels like we are experiencing dejà vu, or ‘Groundhog Day,’ or ‘50 First Dates.’ In ‘50 First Dates,’ Drew Barrymore wakes up each day reliving one specific day. It never changes. I realize our world may seem a little like that right now. We thought we’d turned a corner with COVID, and instead we saw a rapid rise in cases and hospitalizations due to the Omicron variant, higher than in previous surges.”
In 2021, many of us skipped holiday travel and ate outdoors. My morning coffee group moved to Zoom and it wasn’t until late spring, when community rates of COVID nose-dived, that I began seeing patients in my office for the first time in over a year. Since many of my patients are over 60, I tested myself with a home antigen test before going into the office. I changed my schedule so sessions began on the half-hour to be sure the suite’s waiting room would be empty, and I purchased an air purifier, cracked the window open, and figured everyone was as safe as we could reasonably be.
By the first Monday in January 2022, the positivity rate in Maryland was just shy of 30%. Twitter circulated anecdotes about false negatives with the home antigen test kits, and I decided it was safest to return to all-virtual appointments.
Mona Masood, DO, is cofounder of the Physician Support Line, a call-in service for doctors that started in March 2020. She has noted a change in the problems physicians face.
“We’re seeing a lot of empathy fatigue,” Dr. Masood said. “It’s not unexpected with a prolonged situation like this – the trauma has doctors in survival mode and they need to be present for themselves, their families, and their patients. People are emotionally drained, and we’re stretching them to the limit. Now at the front lines, doctors are getting a lot of backlash. There are the conspiracy theories, and people who challenge their knowledge and training and it leads them to ask if they should be doing this work. and these are large decisions that are being made in a specific context.
“The other thing we’re hearing is from trainees – residents and fellows – who are expected to carry a lot of work on the COVID units. Some are being told that they can’t graduate because they haven’t finished their other training requirements. This type of systemic issue produces moral injury.”
Dr. Masood talked about what running the support line has been like for her. “I know people want to give more in a catastrophe, and I was realistic that the enthusiasm might die off. I would go as long as psychiatrists volunteer, and the most incredible thing is that it hasn’t stopped. Some of the original people are no longer with us, but others have come aboard, and it’s been incredible to be a part of this.”
In her Jan. 26, 2022, newsletter, epidemiologist Katelyn Jetelina, PhD, MPH, tried to be reassuring about the future. “In order to know how this will end, we need to look at how other pandemics ended,” Dr. Jetelina wrote. “First, recognize the last part of that sentence ... pandemics end. Every epi curve comes down. This pandemic will end, too. Hold that fact close to you.”
She wrote about the three ways that pandemics end. The SARS pandemic of 2002 lasted 1.5 years as public health measures were effective, in large part because the disease was spread only by symptomatic patients. Vaccines offer a second way to end pandemics, as they have for polio and smallpox. “If the globe works together, we could possibly eradicate SARS-CoV-2 with vaccines. [Now that we have numerous animal reservoirs, though, this is close to impossible.]”
Finally, Dr. Jetelina noted that the 1918 flu changed from a pandemic situation to being endemic. “Over time, the virus attenuated, it became less severe.” Society acclimates to a virus with a low mortality rate. “The vast majority of scientists think an endemic state is the future of SARS-CoV-2. I agree.” And she goes on to define endemic as a steady state, but not the absence of suffering. She likens it to malaria and tuberculosis, illnesses with high global mortality.
“An endemic will come without an announcement or headlines, we won’t know we’re there until well after we’ve arrived.” She wrote of the uncertainty that faces us moving forward: We don’t know how much, or how long, immunity from Omicron infections will last, or if future variants will cause more or less severe disease. She casted her vote for global vaccinations, boosters, masks, better ventilation, communication, empathy, and tolerance to end the pandemic.
In Maryland, hospitalizations and positivity are starting to decline from the postholiday surge. I have figured out that I am not good at predicting what will happen next, and the experts don’t seem to be much better. I’d like a headline ending, the kind we looked to be heading toward last June.
I’ve told my patients who want to come in person that I will reassess in March. We have written our own rules, and mine are somewhere in the middle – I don’t go to public indoor spaces unmasked, but I do see vaccinated family and friends in our homes without masks. I don’t want to be responsible for transmitting a potentially fatal illness to a vulnerable patient. Honestly, this makes no sense, but since there is a video option, I feel I should not risk passing a potentially lethal virus to my patients. I just hope I’m not writing this same article again in January 2023.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins. Dr. Miller has no conflicts of interest.
Here we are. Again. It’s cold and it’s gray. The sun rises late and sets early, so that it feels like midnight by 8 p.m. Indoor venues are risky with the highly contagious Omicron variant, and I feel like we are all pushing the replay button on 2021’s miserable winter.
In some ways, it’s worse: In 2021 we had the hope that vaccines would pull us out of the pandemic and we had guidance on all that we should not be doing. In January, we were gaming the various Internet sites to get a coveted vaccine for ourselves or our family and friends, then lining up to get jabbed. We did not yet know that it wouldn’t be enough – that we’d need boosters, that Delta and Omicron would defy the vaccines. Yes, the vaccines work miracles to prevent severe disease and death, but the worry of passing the virus to someone who is vulnerable or unvaccinated(!), or both, remains – and now we can wonder how we’ll ever get out of this mess with hopeful talk of an endemic, while we wait on the next variant. I like certainty, and this pandemic is one big screaming reminder that certainty about anything is just a pleasant notion, death and taxes excluded, of course.
Kris Lukish, vice president of human resources at Johns Hopkins Hospital in Baltimore, started an update to the hospital employees with: “As we begin 2022, it feels like we are experiencing dejà vu, or ‘Groundhog Day,’ or ‘50 First Dates.’ In ‘50 First Dates,’ Drew Barrymore wakes up each day reliving one specific day. It never changes. I realize our world may seem a little like that right now. We thought we’d turned a corner with COVID, and instead we saw a rapid rise in cases and hospitalizations due to the Omicron variant, higher than in previous surges.”
In 2021, many of us skipped holiday travel and ate outdoors. My morning coffee group moved to Zoom and it wasn’t until late spring, when community rates of COVID nose-dived, that I began seeing patients in my office for the first time in over a year. Since many of my patients are over 60, I tested myself with a home antigen test before going into the office. I changed my schedule so sessions began on the half-hour to be sure the suite’s waiting room would be empty, and I purchased an air purifier, cracked the window open, and figured everyone was as safe as we could reasonably be.
By the first Monday in January 2022, the positivity rate in Maryland was just shy of 30%. Twitter circulated anecdotes about false negatives with the home antigen test kits, and I decided it was safest to return to all-virtual appointments.
Mona Masood, DO, is cofounder of the Physician Support Line, a call-in service for doctors that started in March 2020. She has noted a change in the problems physicians face.
“We’re seeing a lot of empathy fatigue,” Dr. Masood said. “It’s not unexpected with a prolonged situation like this – the trauma has doctors in survival mode and they need to be present for themselves, their families, and their patients. People are emotionally drained, and we’re stretching them to the limit. Now at the front lines, doctors are getting a lot of backlash. There are the conspiracy theories, and people who challenge their knowledge and training and it leads them to ask if they should be doing this work. and these are large decisions that are being made in a specific context.
“The other thing we’re hearing is from trainees – residents and fellows – who are expected to carry a lot of work on the COVID units. Some are being told that they can’t graduate because they haven’t finished their other training requirements. This type of systemic issue produces moral injury.”
Dr. Masood talked about what running the support line has been like for her. “I know people want to give more in a catastrophe, and I was realistic that the enthusiasm might die off. I would go as long as psychiatrists volunteer, and the most incredible thing is that it hasn’t stopped. Some of the original people are no longer with us, but others have come aboard, and it’s been incredible to be a part of this.”
In her Jan. 26, 2022, newsletter, epidemiologist Katelyn Jetelina, PhD, MPH, tried to be reassuring about the future. “In order to know how this will end, we need to look at how other pandemics ended,” Dr. Jetelina wrote. “First, recognize the last part of that sentence ... pandemics end. Every epi curve comes down. This pandemic will end, too. Hold that fact close to you.”
She wrote about the three ways that pandemics end. The SARS pandemic of 2002 lasted 1.5 years as public health measures were effective, in large part because the disease was spread only by symptomatic patients. Vaccines offer a second way to end pandemics, as they have for polio and smallpox. “If the globe works together, we could possibly eradicate SARS-CoV-2 with vaccines. [Now that we have numerous animal reservoirs, though, this is close to impossible.]”
Finally, Dr. Jetelina noted that the 1918 flu changed from a pandemic situation to being endemic. “Over time, the virus attenuated, it became less severe.” Society acclimates to a virus with a low mortality rate. “The vast majority of scientists think an endemic state is the future of SARS-CoV-2. I agree.” And she goes on to define endemic as a steady state, but not the absence of suffering. She likens it to malaria and tuberculosis, illnesses with high global mortality.
“An endemic will come without an announcement or headlines, we won’t know we’re there until well after we’ve arrived.” She wrote of the uncertainty that faces us moving forward: We don’t know how much, or how long, immunity from Omicron infections will last, or if future variants will cause more or less severe disease. She casted her vote for global vaccinations, boosters, masks, better ventilation, communication, empathy, and tolerance to end the pandemic.
In Maryland, hospitalizations and positivity are starting to decline from the postholiday surge. I have figured out that I am not good at predicting what will happen next, and the experts don’t seem to be much better. I’d like a headline ending, the kind we looked to be heading toward last June.
I’ve told my patients who want to come in person that I will reassess in March. We have written our own rules, and mine are somewhere in the middle – I don’t go to public indoor spaces unmasked, but I do see vaccinated family and friends in our homes without masks. I don’t want to be responsible for transmitting a potentially fatal illness to a vulnerable patient. Honestly, this makes no sense, but since there is a video option, I feel I should not risk passing a potentially lethal virus to my patients. I just hope I’m not writing this same article again in January 2023.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins. Dr. Miller has no conflicts of interest.
Here we are. Again. It’s cold and it’s gray. The sun rises late and sets early, so that it feels like midnight by 8 p.m. Indoor venues are risky with the highly contagious Omicron variant, and I feel like we are all pushing the replay button on 2021’s miserable winter.
In some ways, it’s worse: In 2021 we had the hope that vaccines would pull us out of the pandemic and we had guidance on all that we should not be doing. In January, we were gaming the various Internet sites to get a coveted vaccine for ourselves or our family and friends, then lining up to get jabbed. We did not yet know that it wouldn’t be enough – that we’d need boosters, that Delta and Omicron would defy the vaccines. Yes, the vaccines work miracles to prevent severe disease and death, but the worry of passing the virus to someone who is vulnerable or unvaccinated(!), or both, remains – and now we can wonder how we’ll ever get out of this mess with hopeful talk of an endemic, while we wait on the next variant. I like certainty, and this pandemic is one big screaming reminder that certainty about anything is just a pleasant notion, death and taxes excluded, of course.
Kris Lukish, vice president of human resources at Johns Hopkins Hospital in Baltimore, started an update to the hospital employees with: “As we begin 2022, it feels like we are experiencing dejà vu, or ‘Groundhog Day,’ or ‘50 First Dates.’ In ‘50 First Dates,’ Drew Barrymore wakes up each day reliving one specific day. It never changes. I realize our world may seem a little like that right now. We thought we’d turned a corner with COVID, and instead we saw a rapid rise in cases and hospitalizations due to the Omicron variant, higher than in previous surges.”
In 2021, many of us skipped holiday travel and ate outdoors. My morning coffee group moved to Zoom and it wasn’t until late spring, when community rates of COVID nose-dived, that I began seeing patients in my office for the first time in over a year. Since many of my patients are over 60, I tested myself with a home antigen test before going into the office. I changed my schedule so sessions began on the half-hour to be sure the suite’s waiting room would be empty, and I purchased an air purifier, cracked the window open, and figured everyone was as safe as we could reasonably be.
By the first Monday in January 2022, the positivity rate in Maryland was just shy of 30%. Twitter circulated anecdotes about false negatives with the home antigen test kits, and I decided it was safest to return to all-virtual appointments.
Mona Masood, DO, is cofounder of the Physician Support Line, a call-in service for doctors that started in March 2020. She has noted a change in the problems physicians face.
“We’re seeing a lot of empathy fatigue,” Dr. Masood said. “It’s not unexpected with a prolonged situation like this – the trauma has doctors in survival mode and they need to be present for themselves, their families, and their patients. People are emotionally drained, and we’re stretching them to the limit. Now at the front lines, doctors are getting a lot of backlash. There are the conspiracy theories, and people who challenge their knowledge and training and it leads them to ask if they should be doing this work. and these are large decisions that are being made in a specific context.
“The other thing we’re hearing is from trainees – residents and fellows – who are expected to carry a lot of work on the COVID units. Some are being told that they can’t graduate because they haven’t finished their other training requirements. This type of systemic issue produces moral injury.”
Dr. Masood talked about what running the support line has been like for her. “I know people want to give more in a catastrophe, and I was realistic that the enthusiasm might die off. I would go as long as psychiatrists volunteer, and the most incredible thing is that it hasn’t stopped. Some of the original people are no longer with us, but others have come aboard, and it’s been incredible to be a part of this.”
In her Jan. 26, 2022, newsletter, epidemiologist Katelyn Jetelina, PhD, MPH, tried to be reassuring about the future. “In order to know how this will end, we need to look at how other pandemics ended,” Dr. Jetelina wrote. “First, recognize the last part of that sentence ... pandemics end. Every epi curve comes down. This pandemic will end, too. Hold that fact close to you.”
She wrote about the three ways that pandemics end. The SARS pandemic of 2002 lasted 1.5 years as public health measures were effective, in large part because the disease was spread only by symptomatic patients. Vaccines offer a second way to end pandemics, as they have for polio and smallpox. “If the globe works together, we could possibly eradicate SARS-CoV-2 with vaccines. [Now that we have numerous animal reservoirs, though, this is close to impossible.]”
Finally, Dr. Jetelina noted that the 1918 flu changed from a pandemic situation to being endemic. “Over time, the virus attenuated, it became less severe.” Society acclimates to a virus with a low mortality rate. “The vast majority of scientists think an endemic state is the future of SARS-CoV-2. I agree.” And she goes on to define endemic as a steady state, but not the absence of suffering. She likens it to malaria and tuberculosis, illnesses with high global mortality.
“An endemic will come without an announcement or headlines, we won’t know we’re there until well after we’ve arrived.” She wrote of the uncertainty that faces us moving forward: We don’t know how much, or how long, immunity from Omicron infections will last, or if future variants will cause more or less severe disease. She casted her vote for global vaccinations, boosters, masks, better ventilation, communication, empathy, and tolerance to end the pandemic.
In Maryland, hospitalizations and positivity are starting to decline from the postholiday surge. I have figured out that I am not good at predicting what will happen next, and the experts don’t seem to be much better. I’d like a headline ending, the kind we looked to be heading toward last June.
I’ve told my patients who want to come in person that I will reassess in March. We have written our own rules, and mine are somewhere in the middle – I don’t go to public indoor spaces unmasked, but I do see vaccinated family and friends in our homes without masks. I don’t want to be responsible for transmitting a potentially fatal illness to a vulnerable patient. Honestly, this makes no sense, but since there is a video option, I feel I should not risk passing a potentially lethal virus to my patients. I just hope I’m not writing this same article again in January 2023.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins. Dr. Miller has no conflicts of interest.
Ways to make sure 2022 doesn’t stink for docs
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Dramatic increase in driving high after cannabis legislation
Since Canada legalized marijuana in 2018, there has been a dramatic increase in the number of individuals driving while high, new research shows.
Investigators studied over 4,000 drivers treated after a motor vehicle collision in British Columbia trauma centers and found that, before cannabis was legalized, a THC level greater than 0 ng/mL in the blood was present in roughly 10% of drivers. After the drug was legalized this percentage increased to 18%. The percentages of injured drivers with at least 2 ng/mL, the Canadian legal limit, and at least 5 ng/mL more than doubled.
“It’s concerning that we’re seeing such a dramatic increase,” study investigator Jeffrey Brubacher, MD, associate professor, department of emergency medicine, University of British Columbia, Vancouver, said in a press release.
“There are serious risks associated with driving after cannabis use and our findings suggest more [work] is needed to deter this dangerous behavior in light of legalization,” he said.
The study was published online Jan. 12 in the New England Journal of Medicine.
Impact of legalization?
The investigators note that the Canadian government introduced a law aiming to prevent cannabis-impaired driving by establishing penalties and criminal charges for drivers found with a whole-blood THC level of 2 ng/mL, with more severe penalties for those with a THC level of greater than 5 ng/mL or greater than 2.5 ng/mL combined with a blood alcohol level of .05%.
Cannabis use is “associated with cognitive deficits and psychomotor impairment, and there is evidence that it increases the risk of motor vehicle crashes, especially at higher THC levels,” they noted.
“I’m an emergency physician at Vancouver General Hospital’s trauma center. We’ve been measuring drug levels in injured drivers since 2013 here in British Columbia and, in particular, we’ve been measuring THC levels,” Dr. Brubacher said in an interview. “We thought it would be interesting and important to see what would happen after legalization.”
The investigators studied 4,339 drivers – 3,550 whose accident took place before legalization of cannabis, and 789 after legalization – who had been moderately injured in a motor vehicle collision and presented to four British Columbia trauma centers between January 2013 and March 2020.
said Dr. Brubacher. Drivers included in the study had excess blood remaining after the clinical testing had been completed, which was then used for drug analysis.
Insufficient laws
After legalization there was an increased prevalence of drivers with a THC level greater than 0 ng/mL, a TCH level of at least 2 ng/mL, and a THC level of at least 5 ng/mL.
The largest increases in a THC level of at least 2 ng/mL were in drivers 50 years of age or older and among male drivers (adjusted prevalence ratio, 5.18; 95% confidence interval, 2.49-10.78 and aPR, 2.44; 95% CI, 1.60-3.74, respectively).
“There were no significant changes in the prevalence of drivers testing positive for alcohol,” the authors reported.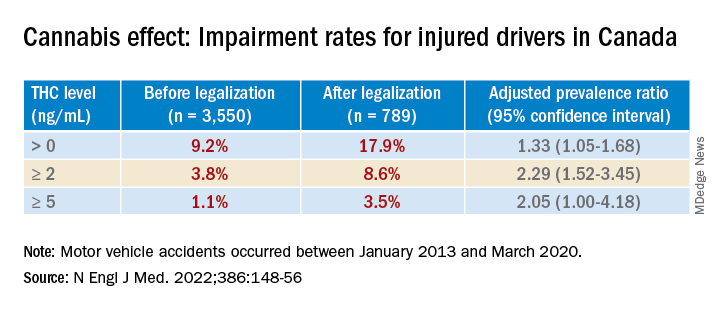
Dr. Brubacher said the evidence suggests these new laws “are not enough to stop everyone from driving after using cannabis.”
The findings have implications for clinicians and patients and for policymakers, he said. “My moderately conservative recommendations are that, if you are going to smoke cannabis, wait at least 4 hours after smoking before you drive. Edibles last longer, and patients should wait least 8 hours after ingesting [edibles] before driving. And of course, if you continue to feel the effects of the THC, you should avoid driving altogether until the time has elapsed and you no longer feel any effects.”
Dr. Brubacher hopes policy makers will use the study’s findings to “design public information campaigns and enforcement measures that encourage drivers, especially older drivers, to separate cannabis use from driving.”
Additionally, “policy makers shouldn’t lose sight of drinking and driving because that’s an even bigger problem than the risk of driving under the influence of cannabis.”
Focus on older adults
In a comment, Anees Bahji, MD, an International Collaborative Addiction Medicine research fellow at the British Columbia Centre on Substance Use, called the study “interesting and relevant.”
He raised several questions regarding the “correlation between the level of a substance in a person’s system and the degree of impairment.” For example, “does the same level of THC in the blood affect us all the same way? And to what extent do the levels detected at the time of the analysis correlate with the level in the person’s system at the time of driving?”
An additional consideration “is for individuals with cannabis use disorder and for those who have developed tolerance to the psychoactive effects of THC: Does it affect their driving skills in the same way as someone who is cannabis naive?” asked Dr. Bahji, a clinical assistant professor at the University of Calgary (Alta.) who was not involved with the study.
Also commenting, Eric Sevigny, PhD, associate professor of criminal justice and criminology at Georgia State University, Atlanta, described it as a “well-designed study that adds yet another data point for considering appropriate road safety policy responses alongside ongoing cannabis liberalization.”
However, the findings “cannot say much about whether cannabis legalization leads to an increase in cannabis-impaired driving, because current research finds little correlation between biological THC concentrations and driving performance,” said Dr. Sevigny, who was not involved with the study.
The finding of “higher THC prevalence among older adults is also relevant for road safety, as this population has a number of concomitant risk factors, such as cognitive decline and prescription drug use,” Dr. Sevigny added.
The study was supported by the Canadian Institutes of Health Research. Dr. Brubacher and Dr. Sevigny disclosed no relevant financial relationships. Dr. Bahji reported receiving research funding from the Canadian Institutes of Health Research, the Calgary Health Trust, the American Psychiatric Association, NIDA, and the University of Calgary.
A version of this article first appeared on Medscape.com.
Since Canada legalized marijuana in 2018, there has been a dramatic increase in the number of individuals driving while high, new research shows.
Investigators studied over 4,000 drivers treated after a motor vehicle collision in British Columbia trauma centers and found that, before cannabis was legalized, a THC level greater than 0 ng/mL in the blood was present in roughly 10% of drivers. After the drug was legalized this percentage increased to 18%. The percentages of injured drivers with at least 2 ng/mL, the Canadian legal limit, and at least 5 ng/mL more than doubled.
“It’s concerning that we’re seeing such a dramatic increase,” study investigator Jeffrey Brubacher, MD, associate professor, department of emergency medicine, University of British Columbia, Vancouver, said in a press release.
“There are serious risks associated with driving after cannabis use and our findings suggest more [work] is needed to deter this dangerous behavior in light of legalization,” he said.
The study was published online Jan. 12 in the New England Journal of Medicine.
Impact of legalization?
The investigators note that the Canadian government introduced a law aiming to prevent cannabis-impaired driving by establishing penalties and criminal charges for drivers found with a whole-blood THC level of 2 ng/mL, with more severe penalties for those with a THC level of greater than 5 ng/mL or greater than 2.5 ng/mL combined with a blood alcohol level of .05%.
Cannabis use is “associated with cognitive deficits and psychomotor impairment, and there is evidence that it increases the risk of motor vehicle crashes, especially at higher THC levels,” they noted.
“I’m an emergency physician at Vancouver General Hospital’s trauma center. We’ve been measuring drug levels in injured drivers since 2013 here in British Columbia and, in particular, we’ve been measuring THC levels,” Dr. Brubacher said in an interview. “We thought it would be interesting and important to see what would happen after legalization.”
The investigators studied 4,339 drivers – 3,550 whose accident took place before legalization of cannabis, and 789 after legalization – who had been moderately injured in a motor vehicle collision and presented to four British Columbia trauma centers between January 2013 and March 2020.
said Dr. Brubacher. Drivers included in the study had excess blood remaining after the clinical testing had been completed, which was then used for drug analysis.
Insufficient laws
After legalization there was an increased prevalence of drivers with a THC level greater than 0 ng/mL, a TCH level of at least 2 ng/mL, and a THC level of at least 5 ng/mL.
The largest increases in a THC level of at least 2 ng/mL were in drivers 50 years of age or older and among male drivers (adjusted prevalence ratio, 5.18; 95% confidence interval, 2.49-10.78 and aPR, 2.44; 95% CI, 1.60-3.74, respectively).
“There were no significant changes in the prevalence of drivers testing positive for alcohol,” the authors reported.
Dr. Brubacher said the evidence suggests these new laws “are not enough to stop everyone from driving after using cannabis.”
The findings have implications for clinicians and patients and for policymakers, he said. “My moderately conservative recommendations are that, if you are going to smoke cannabis, wait at least 4 hours after smoking before you drive. Edibles last longer, and patients should wait least 8 hours after ingesting [edibles] before driving. And of course, if you continue to feel the effects of the THC, you should avoid driving altogether until the time has elapsed and you no longer feel any effects.”
Dr. Brubacher hopes policy makers will use the study’s findings to “design public information campaigns and enforcement measures that encourage drivers, especially older drivers, to separate cannabis use from driving.”
Additionally, “policy makers shouldn’t lose sight of drinking and driving because that’s an even bigger problem than the risk of driving under the influence of cannabis.”
Focus on older adults
In a comment, Anees Bahji, MD, an International Collaborative Addiction Medicine research fellow at the British Columbia Centre on Substance Use, called the study “interesting and relevant.”
He raised several questions regarding the “correlation between the level of a substance in a person’s system and the degree of impairment.” For example, “does the same level of THC in the blood affect us all the same way? And to what extent do the levels detected at the time of the analysis correlate with the level in the person’s system at the time of driving?”
An additional consideration “is for individuals with cannabis use disorder and for those who have developed tolerance to the psychoactive effects of THC: Does it affect their driving skills in the same way as someone who is cannabis naive?” asked Dr. Bahji, a clinical assistant professor at the University of Calgary (Alta.) who was not involved with the study.
Also commenting, Eric Sevigny, PhD, associate professor of criminal justice and criminology at Georgia State University, Atlanta, described it as a “well-designed study that adds yet another data point for considering appropriate road safety policy responses alongside ongoing cannabis liberalization.”
However, the findings “cannot say much about whether cannabis legalization leads to an increase in cannabis-impaired driving, because current research finds little correlation between biological THC concentrations and driving performance,” said Dr. Sevigny, who was not involved with the study.
The finding of “higher THC prevalence among older adults is also relevant for road safety, as this population has a number of concomitant risk factors, such as cognitive decline and prescription drug use,” Dr. Sevigny added.
The study was supported by the Canadian Institutes of Health Research. Dr. Brubacher and Dr. Sevigny disclosed no relevant financial relationships. Dr. Bahji reported receiving research funding from the Canadian Institutes of Health Research, the Calgary Health Trust, the American Psychiatric Association, NIDA, and the University of Calgary.
A version of this article first appeared on Medscape.com.
Since Canada legalized marijuana in 2018, there has been a dramatic increase in the number of individuals driving while high, new research shows.
Investigators studied over 4,000 drivers treated after a motor vehicle collision in British Columbia trauma centers and found that, before cannabis was legalized, a THC level greater than 0 ng/mL in the blood was present in roughly 10% of drivers. After the drug was legalized this percentage increased to 18%. The percentages of injured drivers with at least 2 ng/mL, the Canadian legal limit, and at least 5 ng/mL more than doubled.
“It’s concerning that we’re seeing such a dramatic increase,” study investigator Jeffrey Brubacher, MD, associate professor, department of emergency medicine, University of British Columbia, Vancouver, said in a press release.
“There are serious risks associated with driving after cannabis use and our findings suggest more [work] is needed to deter this dangerous behavior in light of legalization,” he said.
The study was published online Jan. 12 in the New England Journal of Medicine.
Impact of legalization?
The investigators note that the Canadian government introduced a law aiming to prevent cannabis-impaired driving by establishing penalties and criminal charges for drivers found with a whole-blood THC level of 2 ng/mL, with more severe penalties for those with a THC level of greater than 5 ng/mL or greater than 2.5 ng/mL combined with a blood alcohol level of .05%.
Cannabis use is “associated with cognitive deficits and psychomotor impairment, and there is evidence that it increases the risk of motor vehicle crashes, especially at higher THC levels,” they noted.
“I’m an emergency physician at Vancouver General Hospital’s trauma center. We’ve been measuring drug levels in injured drivers since 2013 here in British Columbia and, in particular, we’ve been measuring THC levels,” Dr. Brubacher said in an interview. “We thought it would be interesting and important to see what would happen after legalization.”
The investigators studied 4,339 drivers – 3,550 whose accident took place before legalization of cannabis, and 789 after legalization – who had been moderately injured in a motor vehicle collision and presented to four British Columbia trauma centers between January 2013 and March 2020.
said Dr. Brubacher. Drivers included in the study had excess blood remaining after the clinical testing had been completed, which was then used for drug analysis.
Insufficient laws
After legalization there was an increased prevalence of drivers with a THC level greater than 0 ng/mL, a TCH level of at least 2 ng/mL, and a THC level of at least 5 ng/mL.
The largest increases in a THC level of at least 2 ng/mL were in drivers 50 years of age or older and among male drivers (adjusted prevalence ratio, 5.18; 95% confidence interval, 2.49-10.78 and aPR, 2.44; 95% CI, 1.60-3.74, respectively).
“There were no significant changes in the prevalence of drivers testing positive for alcohol,” the authors reported.
Dr. Brubacher said the evidence suggests these new laws “are not enough to stop everyone from driving after using cannabis.”
The findings have implications for clinicians and patients and for policymakers, he said. “My moderately conservative recommendations are that, if you are going to smoke cannabis, wait at least 4 hours after smoking before you drive. Edibles last longer, and patients should wait least 8 hours after ingesting [edibles] before driving. And of course, if you continue to feel the effects of the THC, you should avoid driving altogether until the time has elapsed and you no longer feel any effects.”
Dr. Brubacher hopes policy makers will use the study’s findings to “design public information campaigns and enforcement measures that encourage drivers, especially older drivers, to separate cannabis use from driving.”
Additionally, “policy makers shouldn’t lose sight of drinking and driving because that’s an even bigger problem than the risk of driving under the influence of cannabis.”
Focus on older adults
In a comment, Anees Bahji, MD, an International Collaborative Addiction Medicine research fellow at the British Columbia Centre on Substance Use, called the study “interesting and relevant.”
He raised several questions regarding the “correlation between the level of a substance in a person’s system and the degree of impairment.” For example, “does the same level of THC in the blood affect us all the same way? And to what extent do the levels detected at the time of the analysis correlate with the level in the person’s system at the time of driving?”
An additional consideration “is for individuals with cannabis use disorder and for those who have developed tolerance to the psychoactive effects of THC: Does it affect their driving skills in the same way as someone who is cannabis naive?” asked Dr. Bahji, a clinical assistant professor at the University of Calgary (Alta.) who was not involved with the study.
Also commenting, Eric Sevigny, PhD, associate professor of criminal justice and criminology at Georgia State University, Atlanta, described it as a “well-designed study that adds yet another data point for considering appropriate road safety policy responses alongside ongoing cannabis liberalization.”
However, the findings “cannot say much about whether cannabis legalization leads to an increase in cannabis-impaired driving, because current research finds little correlation between biological THC concentrations and driving performance,” said Dr. Sevigny, who was not involved with the study.
The finding of “higher THC prevalence among older adults is also relevant for road safety, as this population has a number of concomitant risk factors, such as cognitive decline and prescription drug use,” Dr. Sevigny added.
The study was supported by the Canadian Institutes of Health Research. Dr. Brubacher and Dr. Sevigny disclosed no relevant financial relationships. Dr. Bahji reported receiving research funding from the Canadian Institutes of Health Research, the Calgary Health Trust, the American Psychiatric Association, NIDA, and the University of Calgary.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Similar 10-year survival after CABG, PCI in heavy calcification
Patients with complex coronary artery disease (CAD) – either three-vessel disease and/or left main disease – who also had heavy coronary artery calcification (CAC) had greater all-cause mortality 10 years after revascularization, compared with those without such lesions.
However, perhaps unexpectedly, patients with heavily calcified lesions (HCLs) had similar 10-year survival whether they had undergone coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).
These findings from a post hoc analysis of the SYNTAX Extended Survival (SYNTAXES) study led by Hideyuki Kawashima, MD, PhD, National University of Ireland, Galway, and the University of Amsterdam, were published online Dec. 29, 2021, in JACC: Cardiovascular Interventions.
“There was an apparent lack of benefit at very long-term with CABG versus PCI in the presence of HCL,” Dr. Kawashima and corresponding author Patrick W. Serruys, MD, PhD, National University of Ireland and Imperial College London, summarized in a joint email to this news organization.
“Since HCLs – the final status of atherosclerosis and inflammation – reflect the aging process, complexity, and extensiveness of CAD, and comorbidity, it is possible that the currently available revascularization methods do not provide benefit in the prevention of long-term [10-year] mortality,” they suggested.
In an accompanying editorial, Usman Baber, MD, commented that this study provides a “novel insight.”
Specifically, while patients without HCLs had significantly lower 10-year mortality with CABG versus PCI (18.8% vs. 26.0%; P = .003), an opposite trend was observed among those with HCLs (39.0% vs. 34.0%; P = .26; P int = .005).
The patients with HCLs had higher SYNTAX scores (30.8 vs. 22.4; P < .001) and more complex CAD, so their lack of 10-year mortality benefit with CABG “is somewhat unexpected and warrants further scrutiny,” added Dr. Baber, from the University of Oklahoma Health Sciences Center in Oklahoma City.
Dr. Serruys and Dr. Kawashima agreed that “this study highlights the need for further research on this topic focusing on this specific population with HCLs,” which were 30% of the patients with complex lesions who participated in SYNTAXES.
Consider factors beyond coronary anatomy
The current findings reinforce “the importance of considering not just coronary anatomy, but patient age and other comorbid factors when evaluating mode of revascularization,” said Dr. Baber.
“Coronary calcification is a strong factor in deciding between CABG versus PCI, as multiple studies have shown that CAC increases risk after PCI, even with contemporary safe stent platforms,” he explained in an email.
The current study suggests the adverse prognosis associated with CAC also persists for patients treated with CABG.
Dr. Baber said that, “for patients in whom PCI may not be feasible due to extensive and bulky coronary calcification, it is important to emphasize that the benefits of CABG (versus PCI) may not be as significant or durable.”
“The lack of benefit with CABG,” he added, “is likely due to comorbid factors that tend to increase in prevalence with vascular calcification (older age, peripheral arterial disease, renal impairment, etc).”
This study reinforces “the importance of not just considering coronary complexity, but also additional noncoronary factors that influence long-term prognosis in patients with advanced multivessel CAD,” Dr. Baber stressed.
More aggressive lipid-lowering or antithrombotic therapy may improve the prognosis for such patients, he suggested.
“In general,” Dr. Serruys and Dr. Kawashima similarly noted, “for short-/mid-term outcomes, CABG is preferred to PCI in patients with HCLs because of a higher rate of complete revascularization and less need for repeat revascularization.”
“Our findings at 10 years are in line with the general findings preferring CABG in mid and long term, whereas the benefit of very long-term follow-up might be more complex to capture and comprehend,” they concluded. “Whether HCLs require special consideration when deciding the mode of revascularization beyond their contribution to the SYNTAX score deserves further evaluation.
“Newer PCI technology or CABG methods may become a game-changer in the future,” they speculated.
Worse clinical outcomes
Heavy coronary calcification is associated with worse clinical outcomes after PCI or CABG, but to date, no trial has compared 10-year outcomes after PCI or CABG in patients with complex CAD with versus without HCLs.
To look at this, Dr. Kawashima and colleagues performed a subanalysis of patients in the SYNTAXES study. The original SYNTAX trial had randomized 1,800 patients with complex CAD who were eligible for either PCI or CABG 1:1 to these two treatments, with a 5-year follow-up, and SYNTAXES extended the follow-up to 10 years.
Of the 1,800 patients, 532 (29.6%) had at least one HCL and the rest (70.4%) did not.
The median follow-up in SYNTAXES was 11.2 years overall and 11.9 years in survivors.
At baseline, compared with other patients, those with HCLs were older and had a lower body mass index and higher rates of insulin-treated diabetes, hypertension, previous cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and heart failure.
After adjusting for multiple variables, having a HCL was an independent predictor of greater risk of 10-year mortality (hazard ratio, 1.36; 95% confidence interval, 1.09-1.69; P = .006).
In patients without HCLs, mortality was significantly higher after PCI than CABG (HR, 1.44; 95% CI, 1.14-1.83; P = .003), whereas in those with HCLs, there was no significant difference (HR, 0.85; 95% CI, 0.64-1.13; P = .264).
The location of the HCL did not have any impact on 10-year mortality regardless of the assigned treatment.
Among patients with at least one HCL who underwent CABG, those with at least two HCLs had greater 10-year all-cause mortality than those with one HCL; this difference was not seen among patients with at least one HCL who underwent PCI.
The researchers acknowledge study limitations include that it was a post hoc analysis, so it should be considered hypothesis generating.
In addition, SYNTAX was conducted between 2005 and 2007, when PCI mainly used first-generation paclitaxel drug-eluting stents, so the findings may not be generalizable to current practice.
SYNTAXES was supported by the German Foundation of Heart Research. SYNTAX, during 0- to 5-year follow-up, was funded by Boston Scientific. Dr. Serruys reported receiving personal fees from SMT, Philips/Volcano, Xeltis, Novartis, and Meril Life. Dr. Kawashima reported no relevant financial relationships. Dr. Baber reported receiving honoraria and speaker fees from AstraZeneca, Biotronik, and Amgen.
A version of this article first appeared on Medscape.com.
Patients with complex coronary artery disease (CAD) – either three-vessel disease and/or left main disease – who also had heavy coronary artery calcification (CAC) had greater all-cause mortality 10 years after revascularization, compared with those without such lesions.
However, perhaps unexpectedly, patients with heavily calcified lesions (HCLs) had similar 10-year survival whether they had undergone coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).
These findings from a post hoc analysis of the SYNTAX Extended Survival (SYNTAXES) study led by Hideyuki Kawashima, MD, PhD, National University of Ireland, Galway, and the University of Amsterdam, were published online Dec. 29, 2021, in JACC: Cardiovascular Interventions.
“There was an apparent lack of benefit at very long-term with CABG versus PCI in the presence of HCL,” Dr. Kawashima and corresponding author Patrick W. Serruys, MD, PhD, National University of Ireland and Imperial College London, summarized in a joint email to this news organization.
“Since HCLs – the final status of atherosclerosis and inflammation – reflect the aging process, complexity, and extensiveness of CAD, and comorbidity, it is possible that the currently available revascularization methods do not provide benefit in the prevention of long-term [10-year] mortality,” they suggested.
In an accompanying editorial, Usman Baber, MD, commented that this study provides a “novel insight.”
Specifically, while patients without HCLs had significantly lower 10-year mortality with CABG versus PCI (18.8% vs. 26.0%; P = .003), an opposite trend was observed among those with HCLs (39.0% vs. 34.0%; P = .26; P int = .005).
The patients with HCLs had higher SYNTAX scores (30.8 vs. 22.4; P < .001) and more complex CAD, so their lack of 10-year mortality benefit with CABG “is somewhat unexpected and warrants further scrutiny,” added Dr. Baber, from the University of Oklahoma Health Sciences Center in Oklahoma City.
Dr. Serruys and Dr. Kawashima agreed that “this study highlights the need for further research on this topic focusing on this specific population with HCLs,” which were 30% of the patients with complex lesions who participated in SYNTAXES.
Consider factors beyond coronary anatomy
The current findings reinforce “the importance of considering not just coronary anatomy, but patient age and other comorbid factors when evaluating mode of revascularization,” said Dr. Baber.
“Coronary calcification is a strong factor in deciding between CABG versus PCI, as multiple studies have shown that CAC increases risk after PCI, even with contemporary safe stent platforms,” he explained in an email.
The current study suggests the adverse prognosis associated with CAC also persists for patients treated with CABG.
Dr. Baber said that, “for patients in whom PCI may not be feasible due to extensive and bulky coronary calcification, it is important to emphasize that the benefits of CABG (versus PCI) may not be as significant or durable.”
“The lack of benefit with CABG,” he added, “is likely due to comorbid factors that tend to increase in prevalence with vascular calcification (older age, peripheral arterial disease, renal impairment, etc).”
This study reinforces “the importance of not just considering coronary complexity, but also additional noncoronary factors that influence long-term prognosis in patients with advanced multivessel CAD,” Dr. Baber stressed.
More aggressive lipid-lowering or antithrombotic therapy may improve the prognosis for such patients, he suggested.
“In general,” Dr. Serruys and Dr. Kawashima similarly noted, “for short-/mid-term outcomes, CABG is preferred to PCI in patients with HCLs because of a higher rate of complete revascularization and less need for repeat revascularization.”
“Our findings at 10 years are in line with the general findings preferring CABG in mid and long term, whereas the benefit of very long-term follow-up might be more complex to capture and comprehend,” they concluded. “Whether HCLs require special consideration when deciding the mode of revascularization beyond their contribution to the SYNTAX score deserves further evaluation.
“Newer PCI technology or CABG methods may become a game-changer in the future,” they speculated.
Worse clinical outcomes
Heavy coronary calcification is associated with worse clinical outcomes after PCI or CABG, but to date, no trial has compared 10-year outcomes after PCI or CABG in patients with complex CAD with versus without HCLs.
To look at this, Dr. Kawashima and colleagues performed a subanalysis of patients in the SYNTAXES study. The original SYNTAX trial had randomized 1,800 patients with complex CAD who were eligible for either PCI or CABG 1:1 to these two treatments, with a 5-year follow-up, and SYNTAXES extended the follow-up to 10 years.
Of the 1,800 patients, 532 (29.6%) had at least one HCL and the rest (70.4%) did not.
The median follow-up in SYNTAXES was 11.2 years overall and 11.9 years in survivors.
At baseline, compared with other patients, those with HCLs were older and had a lower body mass index and higher rates of insulin-treated diabetes, hypertension, previous cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and heart failure.
After adjusting for multiple variables, having a HCL was an independent predictor of greater risk of 10-year mortality (hazard ratio, 1.36; 95% confidence interval, 1.09-1.69; P = .006).
In patients without HCLs, mortality was significantly higher after PCI than CABG (HR, 1.44; 95% CI, 1.14-1.83; P = .003), whereas in those with HCLs, there was no significant difference (HR, 0.85; 95% CI, 0.64-1.13; P = .264).
The location of the HCL did not have any impact on 10-year mortality regardless of the assigned treatment.
Among patients with at least one HCL who underwent CABG, those with at least two HCLs had greater 10-year all-cause mortality than those with one HCL; this difference was not seen among patients with at least one HCL who underwent PCI.
The researchers acknowledge study limitations include that it was a post hoc analysis, so it should be considered hypothesis generating.
In addition, SYNTAX was conducted between 2005 and 2007, when PCI mainly used first-generation paclitaxel drug-eluting stents, so the findings may not be generalizable to current practice.
SYNTAXES was supported by the German Foundation of Heart Research. SYNTAX, during 0- to 5-year follow-up, was funded by Boston Scientific. Dr. Serruys reported receiving personal fees from SMT, Philips/Volcano, Xeltis, Novartis, and Meril Life. Dr. Kawashima reported no relevant financial relationships. Dr. Baber reported receiving honoraria and speaker fees from AstraZeneca, Biotronik, and Amgen.
A version of this article first appeared on Medscape.com.
Patients with complex coronary artery disease (CAD) – either three-vessel disease and/or left main disease – who also had heavy coronary artery calcification (CAC) had greater all-cause mortality 10 years after revascularization, compared with those without such lesions.
However, perhaps unexpectedly, patients with heavily calcified lesions (HCLs) had similar 10-year survival whether they had undergone coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).
These findings from a post hoc analysis of the SYNTAX Extended Survival (SYNTAXES) study led by Hideyuki Kawashima, MD, PhD, National University of Ireland, Galway, and the University of Amsterdam, were published online Dec. 29, 2021, in JACC: Cardiovascular Interventions.
“There was an apparent lack of benefit at very long-term with CABG versus PCI in the presence of HCL,” Dr. Kawashima and corresponding author Patrick W. Serruys, MD, PhD, National University of Ireland and Imperial College London, summarized in a joint email to this news organization.
“Since HCLs – the final status of atherosclerosis and inflammation – reflect the aging process, complexity, and extensiveness of CAD, and comorbidity, it is possible that the currently available revascularization methods do not provide benefit in the prevention of long-term [10-year] mortality,” they suggested.
In an accompanying editorial, Usman Baber, MD, commented that this study provides a “novel insight.”
Specifically, while patients without HCLs had significantly lower 10-year mortality with CABG versus PCI (18.8% vs. 26.0%; P = .003), an opposite trend was observed among those with HCLs (39.0% vs. 34.0%; P = .26; P int = .005).
The patients with HCLs had higher SYNTAX scores (30.8 vs. 22.4; P < .001) and more complex CAD, so their lack of 10-year mortality benefit with CABG “is somewhat unexpected and warrants further scrutiny,” added Dr. Baber, from the University of Oklahoma Health Sciences Center in Oklahoma City.
Dr. Serruys and Dr. Kawashima agreed that “this study highlights the need for further research on this topic focusing on this specific population with HCLs,” which were 30% of the patients with complex lesions who participated in SYNTAXES.
Consider factors beyond coronary anatomy
The current findings reinforce “the importance of considering not just coronary anatomy, but patient age and other comorbid factors when evaluating mode of revascularization,” said Dr. Baber.
“Coronary calcification is a strong factor in deciding between CABG versus PCI, as multiple studies have shown that CAC increases risk after PCI, even with contemporary safe stent platforms,” he explained in an email.
The current study suggests the adverse prognosis associated with CAC also persists for patients treated with CABG.
Dr. Baber said that, “for patients in whom PCI may not be feasible due to extensive and bulky coronary calcification, it is important to emphasize that the benefits of CABG (versus PCI) may not be as significant or durable.”
“The lack of benefit with CABG,” he added, “is likely due to comorbid factors that tend to increase in prevalence with vascular calcification (older age, peripheral arterial disease, renal impairment, etc).”
This study reinforces “the importance of not just considering coronary complexity, but also additional noncoronary factors that influence long-term prognosis in patients with advanced multivessel CAD,” Dr. Baber stressed.
More aggressive lipid-lowering or antithrombotic therapy may improve the prognosis for such patients, he suggested.
“In general,” Dr. Serruys and Dr. Kawashima similarly noted, “for short-/mid-term outcomes, CABG is preferred to PCI in patients with HCLs because of a higher rate of complete revascularization and less need for repeat revascularization.”
“Our findings at 10 years are in line with the general findings preferring CABG in mid and long term, whereas the benefit of very long-term follow-up might be more complex to capture and comprehend,” they concluded. “Whether HCLs require special consideration when deciding the mode of revascularization beyond their contribution to the SYNTAX score deserves further evaluation.
“Newer PCI technology or CABG methods may become a game-changer in the future,” they speculated.
Worse clinical outcomes
Heavy coronary calcification is associated with worse clinical outcomes after PCI or CABG, but to date, no trial has compared 10-year outcomes after PCI or CABG in patients with complex CAD with versus without HCLs.
To look at this, Dr. Kawashima and colleagues performed a subanalysis of patients in the SYNTAXES study. The original SYNTAX trial had randomized 1,800 patients with complex CAD who were eligible for either PCI or CABG 1:1 to these two treatments, with a 5-year follow-up, and SYNTAXES extended the follow-up to 10 years.
Of the 1,800 patients, 532 (29.6%) had at least one HCL and the rest (70.4%) did not.
The median follow-up in SYNTAXES was 11.2 years overall and 11.9 years in survivors.
At baseline, compared with other patients, those with HCLs were older and had a lower body mass index and higher rates of insulin-treated diabetes, hypertension, previous cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and heart failure.
After adjusting for multiple variables, having a HCL was an independent predictor of greater risk of 10-year mortality (hazard ratio, 1.36; 95% confidence interval, 1.09-1.69; P = .006).
In patients without HCLs, mortality was significantly higher after PCI than CABG (HR, 1.44; 95% CI, 1.14-1.83; P = .003), whereas in those with HCLs, there was no significant difference (HR, 0.85; 95% CI, 0.64-1.13; P = .264).
The location of the HCL did not have any impact on 10-year mortality regardless of the assigned treatment.
Among patients with at least one HCL who underwent CABG, those with at least two HCLs had greater 10-year all-cause mortality than those with one HCL; this difference was not seen among patients with at least one HCL who underwent PCI.
The researchers acknowledge study limitations include that it was a post hoc analysis, so it should be considered hypothesis generating.
In addition, SYNTAX was conducted between 2005 and 2007, when PCI mainly used first-generation paclitaxel drug-eluting stents, so the findings may not be generalizable to current practice.
SYNTAXES was supported by the German Foundation of Heart Research. SYNTAX, during 0- to 5-year follow-up, was funded by Boston Scientific. Dr. Serruys reported receiving personal fees from SMT, Philips/Volcano, Xeltis, Novartis, and Meril Life. Dr. Kawashima reported no relevant financial relationships. Dr. Baber reported receiving honoraria and speaker fees from AstraZeneca, Biotronik, and Amgen.
A version of this article first appeared on Medscape.com.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Freshwater aquarium provides source for melioidosis infection
A Maryland woman came down with a severe tropical infection called melioidosis from her freshwater home aquarium, says a report in Emerging Infectious Diseases describing a new route of transmission. Melioidosis is caused by the bacteria Burkholderia pseudomallei in soil or water.
Until last year, almost all U.S. cases of melioidosis were from people who lived or traveled to disease-endemic areas. It has been a rare infection in the United States.
But this is not the first case of melioidosis from an unusual source. Earlier in 2021, CDC and state epidemiologists traced an outbreak of melioidosis in Georgia, Kansas, Minnesota, and Texas to B pseudomallei in a bottle of “Better Homes & Gardens Lavender & Chamomile Essential Oil Infused Aromatherapy Room Spray with Gemstones.”
In the aquarium case, the patient was a 56-year-old woman with diabetes and rheumatologic disease. She had been on immunosuppressives (methotrexate, azathioprine, and prednisone) until 1 month before she became symptomatic. She was hospitalized for fever and pneumonia.
Multiple blood cultures obtained on days 1-4 grew B. pseudomallei, but she had no evidence of endocarditis or intravascular seeding. Despite weeks of meropenem (Merrem), she developed evidence of a lung abscess, and trimethoprim/sulfamethoxazole (Bactrim) was added. Ultimately, the patient required a 12-week course of antibiotics.
CDC epidemiologist Patrick Dawson, PhD, first author of the report, told this news organization that although outbreak investigators always ask about pet ownership, they have not explicitly asked about fish. In this case, the patient did not volunteer exposure to the fish.
When state epidemiologists visited the patient’s home, “one of the first things they saw was a few aquariums,” Dr. Dawson said. Seeing the water and knowing “that most freshwater tropical fish in the U.S. are imported from Southeast Asia” led them to culture specifically for B. pseudomallei, which can be difficult for the microbiology lab to identify.
From there, Dr. Dawson explained, “The Maryland Department of Health sent a team to the local pet store” but did not find any of the bacteria there. (The patient had bought her fish 6 months earlier.) The investigators then worked with the national brand “to identify where they had actually sourced the fish from.”
Two retailers supply almost all of U.S. guppies and plants. While investigators could not find an exact matching isolate after so many months had elapsed, they found a positive PCR for B. pseudomallei in a water sample from imported fish in Los Angeles.
Dr. Dawson said tropical fish are imported from southeast Asia and typically come from small family fish farms. The fish import industry has “certain products that they add to the water to hopefully kill any bacteria.” He was unaware whether this included antibiotics but suggested, “we would have seen many more cases [of antibiotic resistance] by now” if it did.
In general advice for the public, Dr. Dawson said, “I would recommend washing hands before and after contact with the aquarium. If you have cuts or wounds on your hands, it’s really important to wear gloves if you have to go clean or maintain the aquarium and you’re putting your hands in the water, just for that extra layer of protection. It’s probably a strong idea to just avoid that altogether if someone’s immunocompromised. And not letting young children under 5 years old clean aquariums.” These are the “simplest things to do to protect yourself.”
Stephen A. Smith, DVM, PhD, a professor in the Aquatic Medicine Program at Virginia-Maryland College of Veterinary Medicine, Blacksburg, also stressed the importance of careful hand hygiene when caring for aquariums. He said that the filter, filter floss, biofilm, charcoal, and gravel might have exceptionally high concentrations of bacteria. Dr. Smith also recommended gloves when cleaning aquariums and not doing this task if immunocompromised.
Dr. Smith, who was not involved in the CDC study, shared a broader perspective, noting that “the reason why it’s important to federal regulators is that [B. pseudomallei] is a tier 1 select agent. And so, when that was isolated, it sent up all the red flags.” The far more common Mycobacterium marinum, or fish handler’s disease, is not reportable.
Mycobacterium marinum is another pathogen of concern that can be acquired from aquariums. These infections typically occur as nodular lesions on the arms and require months of therapy.
Dr. Smith stressed the importance of physicians eliciting a careful exposure history as the key to diagnosing zoonoses. For most exotic aquarium animals, he noted, “They’re caught in the wild wherever they are. They’re transported to a major hub to transport to the U.S., and a lot of times, we don’t have quarantine for those animals.”
Dr. Smith said.
Many infections also occur in the course of water sports – or even hiking and getting a cut or abrasion wet from a stream or lake. Aeromonas hydrophila can cause life-threatening infections. Vibrio vulnificus infections from salt-water injuries can cause sepsis and characteristic hemorrhagic bullae – large, discolored blisters filled with body fluid – during the summer. And eating contaminated shellfish has a 50%-60% death rate.
Other exposures to water-loving bacteria happen during fishing or cleaning/preparing fish. For example, Streptococcus iniae has caused cellulitis, arthritis, endocarditis, and meningitis following superficial or puncture injuries, notably from cleaning tilapia.
Other infections from contact with fish include Erysipelothrix rhusiopathiae (primarily skin infections) and gastroenteritis from Plesiomonas shigelloides, Campylobacter spp, and Salmonella spp.
Each of these zoonoses illustrates the importance of a careful exposure history when there’s an atypical presentation or an infection that is not responding promptly to empiric treatment. The aquarium case broadens the differential to include melioidosis, a serious disease from southeast Asia.
Dr. Dawson and Dr. Smith have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A Maryland woman came down with a severe tropical infection called melioidosis from her freshwater home aquarium, says a report in Emerging Infectious Diseases describing a new route of transmission. Melioidosis is caused by the bacteria Burkholderia pseudomallei in soil or water.
Until last year, almost all U.S. cases of melioidosis were from people who lived or traveled to disease-endemic areas. It has been a rare infection in the United States.
But this is not the first case of melioidosis from an unusual source. Earlier in 2021, CDC and state epidemiologists traced an outbreak of melioidosis in Georgia, Kansas, Minnesota, and Texas to B pseudomallei in a bottle of “Better Homes & Gardens Lavender & Chamomile Essential Oil Infused Aromatherapy Room Spray with Gemstones.”
In the aquarium case, the patient was a 56-year-old woman with diabetes and rheumatologic disease. She had been on immunosuppressives (methotrexate, azathioprine, and prednisone) until 1 month before she became symptomatic. She was hospitalized for fever and pneumonia.
Multiple blood cultures obtained on days 1-4 grew B. pseudomallei, but she had no evidence of endocarditis or intravascular seeding. Despite weeks of meropenem (Merrem), she developed evidence of a lung abscess, and trimethoprim/sulfamethoxazole (Bactrim) was added. Ultimately, the patient required a 12-week course of antibiotics.
CDC epidemiologist Patrick Dawson, PhD, first author of the report, told this news organization that although outbreak investigators always ask about pet ownership, they have not explicitly asked about fish. In this case, the patient did not volunteer exposure to the fish.
When state epidemiologists visited the patient’s home, “one of the first things they saw was a few aquariums,” Dr. Dawson said. Seeing the water and knowing “that most freshwater tropical fish in the U.S. are imported from Southeast Asia” led them to culture specifically for B. pseudomallei, which can be difficult for the microbiology lab to identify.
From there, Dr. Dawson explained, “The Maryland Department of Health sent a team to the local pet store” but did not find any of the bacteria there. (The patient had bought her fish 6 months earlier.) The investigators then worked with the national brand “to identify where they had actually sourced the fish from.”
Two retailers supply almost all of U.S. guppies and plants. While investigators could not find an exact matching isolate after so many months had elapsed, they found a positive PCR for B. pseudomallei in a water sample from imported fish in Los Angeles.
Dr. Dawson said tropical fish are imported from southeast Asia and typically come from small family fish farms. The fish import industry has “certain products that they add to the water to hopefully kill any bacteria.” He was unaware whether this included antibiotics but suggested, “we would have seen many more cases [of antibiotic resistance] by now” if it did.
In general advice for the public, Dr. Dawson said, “I would recommend washing hands before and after contact with the aquarium. If you have cuts or wounds on your hands, it’s really important to wear gloves if you have to go clean or maintain the aquarium and you’re putting your hands in the water, just for that extra layer of protection. It’s probably a strong idea to just avoid that altogether if someone’s immunocompromised. And not letting young children under 5 years old clean aquariums.” These are the “simplest things to do to protect yourself.”
Stephen A. Smith, DVM, PhD, a professor in the Aquatic Medicine Program at Virginia-Maryland College of Veterinary Medicine, Blacksburg, also stressed the importance of careful hand hygiene when caring for aquariums. He said that the filter, filter floss, biofilm, charcoal, and gravel might have exceptionally high concentrations of bacteria. Dr. Smith also recommended gloves when cleaning aquariums and not doing this task if immunocompromised.
Dr. Smith, who was not involved in the CDC study, shared a broader perspective, noting that “the reason why it’s important to federal regulators is that [B. pseudomallei] is a tier 1 select agent. And so, when that was isolated, it sent up all the red flags.” The far more common Mycobacterium marinum, or fish handler’s disease, is not reportable.
Mycobacterium marinum is another pathogen of concern that can be acquired from aquariums. These infections typically occur as nodular lesions on the arms and require months of therapy.
Dr. Smith stressed the importance of physicians eliciting a careful exposure history as the key to diagnosing zoonoses. For most exotic aquarium animals, he noted, “They’re caught in the wild wherever they are. They’re transported to a major hub to transport to the U.S., and a lot of times, we don’t have quarantine for those animals.”
Dr. Smith said.
Many infections also occur in the course of water sports – or even hiking and getting a cut or abrasion wet from a stream or lake. Aeromonas hydrophila can cause life-threatening infections. Vibrio vulnificus infections from salt-water injuries can cause sepsis and characteristic hemorrhagic bullae – large, discolored blisters filled with body fluid – during the summer. And eating contaminated shellfish has a 50%-60% death rate.
Other exposures to water-loving bacteria happen during fishing or cleaning/preparing fish. For example, Streptococcus iniae has caused cellulitis, arthritis, endocarditis, and meningitis following superficial or puncture injuries, notably from cleaning tilapia.
Other infections from contact with fish include Erysipelothrix rhusiopathiae (primarily skin infections) and gastroenteritis from Plesiomonas shigelloides, Campylobacter spp, and Salmonella spp.
Each of these zoonoses illustrates the importance of a careful exposure history when there’s an atypical presentation or an infection that is not responding promptly to empiric treatment. The aquarium case broadens the differential to include melioidosis, a serious disease from southeast Asia.
Dr. Dawson and Dr. Smith have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A Maryland woman came down with a severe tropical infection called melioidosis from her freshwater home aquarium, says a report in Emerging Infectious Diseases describing a new route of transmission. Melioidosis is caused by the bacteria Burkholderia pseudomallei in soil or water.
Until last year, almost all U.S. cases of melioidosis were from people who lived or traveled to disease-endemic areas. It has been a rare infection in the United States.
But this is not the first case of melioidosis from an unusual source. Earlier in 2021, CDC and state epidemiologists traced an outbreak of melioidosis in Georgia, Kansas, Minnesota, and Texas to B pseudomallei in a bottle of “Better Homes & Gardens Lavender & Chamomile Essential Oil Infused Aromatherapy Room Spray with Gemstones.”
In the aquarium case, the patient was a 56-year-old woman with diabetes and rheumatologic disease. She had been on immunosuppressives (methotrexate, azathioprine, and prednisone) until 1 month before she became symptomatic. She was hospitalized for fever and pneumonia.
Multiple blood cultures obtained on days 1-4 grew B. pseudomallei, but she had no evidence of endocarditis or intravascular seeding. Despite weeks of meropenem (Merrem), she developed evidence of a lung abscess, and trimethoprim/sulfamethoxazole (Bactrim) was added. Ultimately, the patient required a 12-week course of antibiotics.
CDC epidemiologist Patrick Dawson, PhD, first author of the report, told this news organization that although outbreak investigators always ask about pet ownership, they have not explicitly asked about fish. In this case, the patient did not volunteer exposure to the fish.
When state epidemiologists visited the patient’s home, “one of the first things they saw was a few aquariums,” Dr. Dawson said. Seeing the water and knowing “that most freshwater tropical fish in the U.S. are imported from Southeast Asia” led them to culture specifically for B. pseudomallei, which can be difficult for the microbiology lab to identify.
From there, Dr. Dawson explained, “The Maryland Department of Health sent a team to the local pet store” but did not find any of the bacteria there. (The patient had bought her fish 6 months earlier.) The investigators then worked with the national brand “to identify where they had actually sourced the fish from.”
Two retailers supply almost all of U.S. guppies and plants. While investigators could not find an exact matching isolate after so many months had elapsed, they found a positive PCR for B. pseudomallei in a water sample from imported fish in Los Angeles.
Dr. Dawson said tropical fish are imported from southeast Asia and typically come from small family fish farms. The fish import industry has “certain products that they add to the water to hopefully kill any bacteria.” He was unaware whether this included antibiotics but suggested, “we would have seen many more cases [of antibiotic resistance] by now” if it did.
In general advice for the public, Dr. Dawson said, “I would recommend washing hands before and after contact with the aquarium. If you have cuts or wounds on your hands, it’s really important to wear gloves if you have to go clean or maintain the aquarium and you’re putting your hands in the water, just for that extra layer of protection. It’s probably a strong idea to just avoid that altogether if someone’s immunocompromised. And not letting young children under 5 years old clean aquariums.” These are the “simplest things to do to protect yourself.”
Stephen A. Smith, DVM, PhD, a professor in the Aquatic Medicine Program at Virginia-Maryland College of Veterinary Medicine, Blacksburg, also stressed the importance of careful hand hygiene when caring for aquariums. He said that the filter, filter floss, biofilm, charcoal, and gravel might have exceptionally high concentrations of bacteria. Dr. Smith also recommended gloves when cleaning aquariums and not doing this task if immunocompromised.
Dr. Smith, who was not involved in the CDC study, shared a broader perspective, noting that “the reason why it’s important to federal regulators is that [B. pseudomallei] is a tier 1 select agent. And so, when that was isolated, it sent up all the red flags.” The far more common Mycobacterium marinum, or fish handler’s disease, is not reportable.
Mycobacterium marinum is another pathogen of concern that can be acquired from aquariums. These infections typically occur as nodular lesions on the arms and require months of therapy.
Dr. Smith stressed the importance of physicians eliciting a careful exposure history as the key to diagnosing zoonoses. For most exotic aquarium animals, he noted, “They’re caught in the wild wherever they are. They’re transported to a major hub to transport to the U.S., and a lot of times, we don’t have quarantine for those animals.”
Dr. Smith said.
Many infections also occur in the course of water sports – or even hiking and getting a cut or abrasion wet from a stream or lake. Aeromonas hydrophila can cause life-threatening infections. Vibrio vulnificus infections from salt-water injuries can cause sepsis and characteristic hemorrhagic bullae – large, discolored blisters filled with body fluid – during the summer. And eating contaminated shellfish has a 50%-60% death rate.
Other exposures to water-loving bacteria happen during fishing or cleaning/preparing fish. For example, Streptococcus iniae has caused cellulitis, arthritis, endocarditis, and meningitis following superficial or puncture injuries, notably from cleaning tilapia.
Other infections from contact with fish include Erysipelothrix rhusiopathiae (primarily skin infections) and gastroenteritis from Plesiomonas shigelloides, Campylobacter spp, and Salmonella spp.
Each of these zoonoses illustrates the importance of a careful exposure history when there’s an atypical presentation or an infection that is not responding promptly to empiric treatment. The aquarium case broadens the differential to include melioidosis, a serious disease from southeast Asia.
Dr. Dawson and Dr. Smith have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Omega-3 supplementation improves sleep, mood in breast cancer patients on hormone therapy
After 4 weeks of treatment, patients who received omega-3 reported better sleep, depression, and mood outcomes than those who received placebo.
Estrogen-receptor inhibitors are used to treat breast cancer with positive hormone receptors in combination with other therapies. However, the drugs can lead to long-term side effects, including hot flashes, night sweats, and changes to mood and sleep.
These side effects are often treated with selective serotonin reuptake inhibitors and some anticonvulsant drugs. Omega-3 supplements contain various polyunsaturated fatty acids, which influence cell signaling and contribute to the production of bioactive fat mediators that counter inflammation. They are widely used in cardiovascular disease, breast cancer, rheumatoid arthritis, depression, and other cognitive disorders. They also appear to amplify the antitumor efficacy of tamoxifen through the inhibition of proliferative and antiapoptotic pathways that that are influenced by estrogen-receptor signaling.
“This study showed that omega-3 supplementation can improve mood and sleep disorder in women suffering from breast cancer while they (are) managing with antihormone drugs. … this supplement can be proposed for the treatment of these patients,” wrote researchers led by Azadeh Moghaddas, MD, PhD, who is an associate professor of clinical pharmacy and pharmacy practice at Isfahan (Iran) University of Medical Sciences.
The study was made available as a preprint on ResearchSquare and has not yet been peer reviewed. It included 60 patients who were screened for baseline mood disorders using the hospital anxiety and depression scale (HADS), then randomized to 2 mg omega-3 per day for 4 weeks, or placebo.
Studies have shown that omega-3 supplementation improves menopause and mood symptoms in postmenopausal women without cancer.
Omega-3 supplementation has neuroprotective effects and improved brain function and mood in rats, and a 2019 review suggested that the evidence is strong enough to warrant clinical studies.
To determine if the supplement was also safe and effective in women with breast cancer undergoing hormone therapy, the researchers analyzed data from 32 patients in the intervention group and 28 patients in the placebo group.
At 4 weeks of follow-up, patients in the intervention group had significantly lower values on the Center for Epidemiological Studies-Depression scale (mean, 22.8 vs. 30.8; P < .001), Profile of Mood State (mean, 30.8 versus 39.5; P<.001), and Pittsburgh Sleep Quality Index (mean, 4.6 vs. 5.9; P = .04). There were no statistically significant changes in these values in the placebo group.
At 4 weeks, paired samples t-test comparisons between the intervention and the placebo groups revealed lower scores in the intervention group for mean scores in the PSQI subscales subjective sleep quality (0.8 vs. 1.4; P = .002), delay in falling asleep (1.1 vs. 1.6; P = .02), and sleep disturbances (0.8 vs. 1.1; P = .005).
There were no significant adverse reactions in either group.
The study is limited by its small sample size and the short follow-up period.
The study was funded by Isfahan University of Medical Sciences. The authors declare no other conflicts of interest.
After 4 weeks of treatment, patients who received omega-3 reported better sleep, depression, and mood outcomes than those who received placebo.
Estrogen-receptor inhibitors are used to treat breast cancer with positive hormone receptors in combination with other therapies. However, the drugs can lead to long-term side effects, including hot flashes, night sweats, and changes to mood and sleep.
These side effects are often treated with selective serotonin reuptake inhibitors and some anticonvulsant drugs. Omega-3 supplements contain various polyunsaturated fatty acids, which influence cell signaling and contribute to the production of bioactive fat mediators that counter inflammation. They are widely used in cardiovascular disease, breast cancer, rheumatoid arthritis, depression, and other cognitive disorders. They also appear to amplify the antitumor efficacy of tamoxifen through the inhibition of proliferative and antiapoptotic pathways that that are influenced by estrogen-receptor signaling.
“This study showed that omega-3 supplementation can improve mood and sleep disorder in women suffering from breast cancer while they (are) managing with antihormone drugs. … this supplement can be proposed for the treatment of these patients,” wrote researchers led by Azadeh Moghaddas, MD, PhD, who is an associate professor of clinical pharmacy and pharmacy practice at Isfahan (Iran) University of Medical Sciences.
The study was made available as a preprint on ResearchSquare and has not yet been peer reviewed. It included 60 patients who were screened for baseline mood disorders using the hospital anxiety and depression scale (HADS), then randomized to 2 mg omega-3 per day for 4 weeks, or placebo.
Studies have shown that omega-3 supplementation improves menopause and mood symptoms in postmenopausal women without cancer.
Omega-3 supplementation has neuroprotective effects and improved brain function and mood in rats, and a 2019 review suggested that the evidence is strong enough to warrant clinical studies.
To determine if the supplement was also safe and effective in women with breast cancer undergoing hormone therapy, the researchers analyzed data from 32 patients in the intervention group and 28 patients in the placebo group.
At 4 weeks of follow-up, patients in the intervention group had significantly lower values on the Center for Epidemiological Studies-Depression scale (mean, 22.8 vs. 30.8; P < .001), Profile of Mood State (mean, 30.8 versus 39.5; P<.001), and Pittsburgh Sleep Quality Index (mean, 4.6 vs. 5.9; P = .04). There were no statistically significant changes in these values in the placebo group.
At 4 weeks, paired samples t-test comparisons between the intervention and the placebo groups revealed lower scores in the intervention group for mean scores in the PSQI subscales subjective sleep quality (0.8 vs. 1.4; P = .002), delay in falling asleep (1.1 vs. 1.6; P = .02), and sleep disturbances (0.8 vs. 1.1; P = .005).
There were no significant adverse reactions in either group.
The study is limited by its small sample size and the short follow-up period.
The study was funded by Isfahan University of Medical Sciences. The authors declare no other conflicts of interest.
After 4 weeks of treatment, patients who received omega-3 reported better sleep, depression, and mood outcomes than those who received placebo.
Estrogen-receptor inhibitors are used to treat breast cancer with positive hormone receptors in combination with other therapies. However, the drugs can lead to long-term side effects, including hot flashes, night sweats, and changes to mood and sleep.
These side effects are often treated with selective serotonin reuptake inhibitors and some anticonvulsant drugs. Omega-3 supplements contain various polyunsaturated fatty acids, which influence cell signaling and contribute to the production of bioactive fat mediators that counter inflammation. They are widely used in cardiovascular disease, breast cancer, rheumatoid arthritis, depression, and other cognitive disorders. They also appear to amplify the antitumor efficacy of tamoxifen through the inhibition of proliferative and antiapoptotic pathways that that are influenced by estrogen-receptor signaling.
“This study showed that omega-3 supplementation can improve mood and sleep disorder in women suffering from breast cancer while they (are) managing with antihormone drugs. … this supplement can be proposed for the treatment of these patients,” wrote researchers led by Azadeh Moghaddas, MD, PhD, who is an associate professor of clinical pharmacy and pharmacy practice at Isfahan (Iran) University of Medical Sciences.
The study was made available as a preprint on ResearchSquare and has not yet been peer reviewed. It included 60 patients who were screened for baseline mood disorders using the hospital anxiety and depression scale (HADS), then randomized to 2 mg omega-3 per day for 4 weeks, or placebo.
Studies have shown that omega-3 supplementation improves menopause and mood symptoms in postmenopausal women without cancer.
Omega-3 supplementation has neuroprotective effects and improved brain function and mood in rats, and a 2019 review suggested that the evidence is strong enough to warrant clinical studies.
To determine if the supplement was also safe and effective in women with breast cancer undergoing hormone therapy, the researchers analyzed data from 32 patients in the intervention group and 28 patients in the placebo group.
At 4 weeks of follow-up, patients in the intervention group had significantly lower values on the Center for Epidemiological Studies-Depression scale (mean, 22.8 vs. 30.8; P < .001), Profile of Mood State (mean, 30.8 versus 39.5; P<.001), and Pittsburgh Sleep Quality Index (mean, 4.6 vs. 5.9; P = .04). There were no statistically significant changes in these values in the placebo group.
At 4 weeks, paired samples t-test comparisons between the intervention and the placebo groups revealed lower scores in the intervention group for mean scores in the PSQI subscales subjective sleep quality (0.8 vs. 1.4; P = .002), delay in falling asleep (1.1 vs. 1.6; P = .02), and sleep disturbances (0.8 vs. 1.1; P = .005).
There were no significant adverse reactions in either group.
The study is limited by its small sample size and the short follow-up period.
The study was funded by Isfahan University of Medical Sciences. The authors declare no other conflicts of interest.
FROM RESEARCHSQUARE








