User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Dupilumab gains off-label uses as clinicians turn to drug for more indications
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
.
The drug, marketed as Dupixent, is currently approved in the United States to treat atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis in adults. Dupilumab is also approved to treat eosinophilic esophagitis in patients aged 12 years and older and atopic dermatitis and asthma in some patients as young as age 6 months.
As the roster of approved and off-label indications grows, skin specialists said, pediatricians and other primary care providers should become familiar with the drug – given the increasing likelihood that their patients may be taking the medication.
The U.S. Food and Drug Administration first approved dupilumab in 2017 for eczema and has continued to add new treatment indications, the most recent being for prurigo nodularis, in 2022. Sanofi, which markets the drug with Regeneron, announced in April 2022 that some 430,000 patients worldwide were taking the drug – a figure it hoped to raise by 1.5 million by 2025.
A well-tolerated – if expensive – drug
Dupilumab, an interleukin-4 (IL-4) receptor alpha-antagonist biologic, blocks both IL-4 and IL-13 signaling, Marlys Fassett, MD, PhD, associate professor of dermatology at the University of California, San Francisco, told this news organization.
Dr. Fassett said she prescribes the drug off label for chronic idiopathic urticaria, including in older patients, and finds that the side effects in older patients are similar to those in younger people. The medication costs $36,000 per year, although some patients can get it more cheaply.
“Dupixent is a super-safe drug because it doesn’t immunosuppress any other part of the immune system, so you still have good antibacterial, antiviral, and antifungal immunity,” she added. “That makes perfect sense as a biological mechanism, and it’s been found safe in clinical trials.”
Case reports of potential adverse reactions to dupilumab have included ocular surface disease, lichen planus, and rash on the face and neck.
“We’re still learning about complications and are watching patients carefully,” said Marissa J. Perman, MD, section chief of dermatology at Children’s Hospital of Philadelphia.
Many people with atopic dermatitis also have other allergic conditions, such as contact dermatitis, asthma, prurigo nodularis, allergic rhinitis, and seasonal allergies. Each of these conditions has a pathway that depends on IL-4 receptors, Dr. Fassett said.
“It’s amazing how many conditions Dupixent improves. Sometimes we prescribe on-label Dupixent for atopic dermatitis, and inadvertently, the drug also improves that patient’s other, off-label conditions,” Dr. Fassett said. “I think that’s the best evidence that Dupixent works in these off-label cases.”
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she uses off-label dupilumab to treat bullous pemphigoid and intense pruritus of unknown etiology.
“And several times I have treated drug reaction with eosinophilia and systemic symptoms, a rare adverse drug reaction that causes a rash and eosinophilia,” Dr. Strowd added.
Tissa Hata, MD, professor of medicine and clinical service chief at the University of California, San Diego, mainly treats elderly patients. She uses dupilumab to treat bullous pemphigoid and chronic pruritus. “There have been reports of using Dupixent to treat adult alopecia areata, chronic urticaria, localized scleroderma, and even keloids,” she told this news organization.
As a pediatric dermatologist, Dr. Perman treats children with atopic dermatitis as young as 3 months of age. She also uses dupilumab for alopecia areata, graft vs. host disease, and pruritus not otherwise specified.
Conjunctivitis and facial redness are two side effects Dr. Fassett sometimes sees with dupilumab. They occur similarly with all conditions and in all age groups. “We don’t know why they occur, and we don’t always know how to alleviate them,” she said. “So a small number of patients stop using Dupixent because they can’t tolerate those two side effects.
“We’re not worried about infection risk,” Dr. Fassett said. “Your patients may have heard of dupilumab as an immunosuppressant, but its immunosuppression is very focused. You can reassure them that they’re not at increased risk for viral or bacterial infections when they’re on this drug.”
“I don’t think there are any different safety signals to watch for with on-label vs. off-label Dupixent use,” Dr. Strowd added. “In general, the medicine is very safe.”
Dr. Hata said she is impressed with dupilumab’s safety in her elderly patients. All her patients older than 85 years who have taken the drug for bullous pemphigoid have tolerated it well, she said.
“Dupixent seems to be a safe alternative for elderly patients with pruritus because they often cannot tolerate sedating antihistamines due to the risk of falling,” Dr. Hata said. “And UV therapy may be difficult for elderly patients due to problems with transport.”
Although some of Dr. Hata’s elderly patients with atopic dermatitis have discontinued use of the drug after developing conjunctivitis, none taking the drug off label have discontinued it because of side effects, she noted.
“Dupixent manages the condition, but it is not a cure,” Dr. Fassett noted. “Based on the current data, we think it’s safe and effective to take long term, potentially for life.”
Making injections less bothersome
Dupilumab is injected subcutaneously from a single-dose prefilled syringe or a prefilled pen (syringe hidden in an opaque sheath), typically in the thigh, arm, abdomen, or buttocks. According to Sanofi and Regeneron, patients receive dupilumab injections every 2 to 4 weeks in doses based on their age and weight.
“The medication is somewhat viscous, so taking the syringe or pen out of the refrigerator ahead of time to warm it up can make the experience less painful,” Dr. Strowd advised. “For pediatric patients, I sometimes prescribe topical lidocaine applied 30 minutes before injection.”
Dr. Hata suggested icing the skin prior to injecting or distracting the patient by tapping a different area of the skin.
For her pediatric patients, Dr. Perman said she uses “lots of distraction, EMLA cream, and having one person hold the child while a second person injects.”
Clinic and pharmacy staff may show patients how to inject properly, Dr. Fassett added; and the product website provides injection tutorials.
Off-label dupixent can be expensive, difficult to obtain
The list price per injection, regardless of dose, is around $1,800. But according to the company’s website, most patients have health insurance or qualify for other assistance, so “very few patients pay the list price.”
Even so, “due to cost and insurance coverage hurdles, obtaining Dupixent for off-label use can be difficult,” Dr. Strowd said.
“In academic medicine, we can obtain drugs for our patients that community doctors may not get approval for,” Dr. Fassett added. “Community doctors can use information in the medical literature and in news articles to press insurance companies to spend money to provide their patients with Dupixent.”
The experts who commented have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Delayed introduction of allergens increases allergy risk
These findings were published in Allergy.
Launched in April 2011, the French ELFE study aims to monitor children from birth to adulthood to better understand the factors from the intrauterine period to adolescence that affect their development, health, social skills, and school career. Thanks to this cohort, a team of scientists has reviewed the relationship between complementary feeding practices and allergies in French children.
The study focused on 6,662 children who had no signs of an allergic reaction before 2 months of age. Data on feeding practices were collected monthly from ages 3 months to 10 months. Their age at complementary feeding introduction was calculated, and a food diversity score was determined at 8 and 10 months. The number of major allergenic foods (out of eggs, fish, wheat, and dairy products) not introduced at 8 and 10 months was also determined. Allergic diseases (food allergy, eczema, asthma, and rhinoconjunctivitis) were reported by parents at 2 months and at 1, 2, 3.5, and 5.5 years.
Initially, scientists determined that just 62% of children began complementary feeding in the recommended age window, which is between ages 4 months and 6 months. They then closely studied the link between delayed introduction of major allergenic foods and the risk of food allergies. They saw that for 1 in 10 children, at least two major allergens, from eggs, fish, wheat, and dairy products, had still not been introduced into the diet of infants by the age of 10 months. Now, these children have a risk of developing a food allergy before the age of 5.5 years that is two times greater than that of children in whom the four major allergens were introduced before the age of 10 months.
These findings therefore confirm the importance of not delaying the introduction of major food allergens to prevent the occurrence of childhood allergic diseases. They provide convincing arguments in support of new recommendations made by the French pediatric and allergy societies as well as those issued by Public Health France.
ELFE: The first cohort to follow children from birth to adulthood
ELFE is the first longitudinal nationwide French study dedicated to monitoring children from birth to adulthood. More than 18,000 children born in metropolitan France in 2011 were included in this study, which represents 1 in 50 children born in 2011. From the time that researchers first met the families in the maternity ward, the parents who agreed to participate in this great scientific adventure have been questioned at regular intervals to better understand how environment, family members, and living conditions affect the development, health, and socialization of children.
This article was translated from the Medscape French Edition. A version of this article first appeared on Medscape.com.
These findings were published in Allergy.
Launched in April 2011, the French ELFE study aims to monitor children from birth to adulthood to better understand the factors from the intrauterine period to adolescence that affect their development, health, social skills, and school career. Thanks to this cohort, a team of scientists has reviewed the relationship between complementary feeding practices and allergies in French children.
The study focused on 6,662 children who had no signs of an allergic reaction before 2 months of age. Data on feeding practices were collected monthly from ages 3 months to 10 months. Their age at complementary feeding introduction was calculated, and a food diversity score was determined at 8 and 10 months. The number of major allergenic foods (out of eggs, fish, wheat, and dairy products) not introduced at 8 and 10 months was also determined. Allergic diseases (food allergy, eczema, asthma, and rhinoconjunctivitis) were reported by parents at 2 months and at 1, 2, 3.5, and 5.5 years.
Initially, scientists determined that just 62% of children began complementary feeding in the recommended age window, which is between ages 4 months and 6 months. They then closely studied the link between delayed introduction of major allergenic foods and the risk of food allergies. They saw that for 1 in 10 children, at least two major allergens, from eggs, fish, wheat, and dairy products, had still not been introduced into the diet of infants by the age of 10 months. Now, these children have a risk of developing a food allergy before the age of 5.5 years that is two times greater than that of children in whom the four major allergens were introduced before the age of 10 months.
These findings therefore confirm the importance of not delaying the introduction of major food allergens to prevent the occurrence of childhood allergic diseases. They provide convincing arguments in support of new recommendations made by the French pediatric and allergy societies as well as those issued by Public Health France.
ELFE: The first cohort to follow children from birth to adulthood
ELFE is the first longitudinal nationwide French study dedicated to monitoring children from birth to adulthood. More than 18,000 children born in metropolitan France in 2011 were included in this study, which represents 1 in 50 children born in 2011. From the time that researchers first met the families in the maternity ward, the parents who agreed to participate in this great scientific adventure have been questioned at regular intervals to better understand how environment, family members, and living conditions affect the development, health, and socialization of children.
This article was translated from the Medscape French Edition. A version of this article first appeared on Medscape.com.
These findings were published in Allergy.
Launched in April 2011, the French ELFE study aims to monitor children from birth to adulthood to better understand the factors from the intrauterine period to adolescence that affect their development, health, social skills, and school career. Thanks to this cohort, a team of scientists has reviewed the relationship between complementary feeding practices and allergies in French children.
The study focused on 6,662 children who had no signs of an allergic reaction before 2 months of age. Data on feeding practices were collected monthly from ages 3 months to 10 months. Their age at complementary feeding introduction was calculated, and a food diversity score was determined at 8 and 10 months. The number of major allergenic foods (out of eggs, fish, wheat, and dairy products) not introduced at 8 and 10 months was also determined. Allergic diseases (food allergy, eczema, asthma, and rhinoconjunctivitis) were reported by parents at 2 months and at 1, 2, 3.5, and 5.5 years.
Initially, scientists determined that just 62% of children began complementary feeding in the recommended age window, which is between ages 4 months and 6 months. They then closely studied the link between delayed introduction of major allergenic foods and the risk of food allergies. They saw that for 1 in 10 children, at least two major allergens, from eggs, fish, wheat, and dairy products, had still not been introduced into the diet of infants by the age of 10 months. Now, these children have a risk of developing a food allergy before the age of 5.5 years that is two times greater than that of children in whom the four major allergens were introduced before the age of 10 months.
These findings therefore confirm the importance of not delaying the introduction of major food allergens to prevent the occurrence of childhood allergic diseases. They provide convincing arguments in support of new recommendations made by the French pediatric and allergy societies as well as those issued by Public Health France.
ELFE: The first cohort to follow children from birth to adulthood
ELFE is the first longitudinal nationwide French study dedicated to monitoring children from birth to adulthood. More than 18,000 children born in metropolitan France in 2011 were included in this study, which represents 1 in 50 children born in 2011. From the time that researchers first met the families in the maternity ward, the parents who agreed to participate in this great scientific adventure have been questioned at regular intervals to better understand how environment, family members, and living conditions affect the development, health, and socialization of children.
This article was translated from the Medscape French Edition. A version of this article first appeared on Medscape.com.
FROM ALLERGY
EMA validates marketing authorization application for delgocitinib cream
The which marks the beginning of the review process for the treatment by the EMA’s Committee for Medicinal Products for Human Use.
Delgocitinib is an investigational topical pan–Janus kinase inhibitor that inhibits activation of the JAK-STAT pathway.
The development follows results reported from two phase 3 clinical trials known as DELTA 1 and DELTA 2, which evaluated the safety and efficacy of delgocitinib cream applications twice per day compared with a vehicle cream in adults with mild to severe chronic hand eczema. Results of DELTA 1 were presented at the 2023 annual meeting of the American Academy of Dermatology. A multisite, open-label extension trial known as DELTA 3 is still in progress.
According to a press release from LEO Pharma, which is developing the product, the efficacy and safety of delgocitinib cream have not been evaluated by any regulatory authority. In 2020, the drug was granted fast-track designation by the Food and Drug Administration for the potential treatment of adults with moderate to severe chronic hand eczema. There are currently no treatment options available in the United States specifically approved for treating the condition.
The which marks the beginning of the review process for the treatment by the EMA’s Committee for Medicinal Products for Human Use.
Delgocitinib is an investigational topical pan–Janus kinase inhibitor that inhibits activation of the JAK-STAT pathway.
The development follows results reported from two phase 3 clinical trials known as DELTA 1 and DELTA 2, which evaluated the safety and efficacy of delgocitinib cream applications twice per day compared with a vehicle cream in adults with mild to severe chronic hand eczema. Results of DELTA 1 were presented at the 2023 annual meeting of the American Academy of Dermatology. A multisite, open-label extension trial known as DELTA 3 is still in progress.
According to a press release from LEO Pharma, which is developing the product, the efficacy and safety of delgocitinib cream have not been evaluated by any regulatory authority. In 2020, the drug was granted fast-track designation by the Food and Drug Administration for the potential treatment of adults with moderate to severe chronic hand eczema. There are currently no treatment options available in the United States specifically approved for treating the condition.
The which marks the beginning of the review process for the treatment by the EMA’s Committee for Medicinal Products for Human Use.
Delgocitinib is an investigational topical pan–Janus kinase inhibitor that inhibits activation of the JAK-STAT pathway.
The development follows results reported from two phase 3 clinical trials known as DELTA 1 and DELTA 2, which evaluated the safety and efficacy of delgocitinib cream applications twice per day compared with a vehicle cream in adults with mild to severe chronic hand eczema. Results of DELTA 1 were presented at the 2023 annual meeting of the American Academy of Dermatology. A multisite, open-label extension trial known as DELTA 3 is still in progress.
According to a press release from LEO Pharma, which is developing the product, the efficacy and safety of delgocitinib cream have not been evaluated by any regulatory authority. In 2020, the drug was granted fast-track designation by the Food and Drug Administration for the potential treatment of adults with moderate to severe chronic hand eczema. There are currently no treatment options available in the United States specifically approved for treating the condition.
Lobbying allowed insurers to charge physicians fees to receive payments online: Report
, according to a new investigation by the nonprofit news organization ProPublica.
The Affordable Care Act requires that health plans give providers the option of being paid electronically to improve efficiency and save money. In 2017, the Centers for Medicare & Medicaid Services issued guidance that prohibited insurers and their payment processing vendors from “engaging in unfair business practices that do not support an efficient healthcare system,” according to a recent Medical Group Management Association position paper.
But that guidance, which appeared to forbid requiring fees to receive payments online, disappeared from the CMS site 6 months later.
According to ProPublica’s reporting, the change was the result of a quiet insurance industry lobbying campaign led by Matthew Albright, a former CMS employee who left government service to work for Zelis, a payment processing company co-owned by private equity giant Bain Capital.
The details of the lobbying effort were discovered by Alex Shteynshlyuger, a New York urologist, who through public records requests received the email correspondence between Mr. Albright and CMS and shared that material with ProPublica.
Mr. Albright had been able to influence CMS policy to protect what ProPublica called a “crucial revenue stream” for payment processors. The fee notice was removed just 3 days after Mr. Albright requested the change, ProPublica found.
When CMS resisted further changes, including eliminating guidance forbidding insurers and payment processors from charging excess fees for online payments, Mr. Albright brought in a law firm. The threat of a lawsuit by deep-pocketed Zelis was enough to bring CMS in line, ProPublica reported. Today, these fees can cost larger medical practices more than $1 million a year, according to the MGMA report.
“It took less than a decade for a new industry of middlemen, owned by private equity funds and giant conglomerates like UnitedHealth Group, to cash in,” writes Cezary Podkul, the author of the ProPublica report.
Predatory practices
It might seem that avoiding the fees would be as simple as requesting to be paid by check. However, a 2021 poll by the MGMA found that 57% of doctors were being charged these fees when they hadn’t agreed to them. According to the ProPublica report, physicians who have requested to be paid by check often find themselves being bounced back to electronic fund transfer (EFT) payments, where they are again charged fees.
In October 2021, more than 90 physician organizations, including the American Medical Association and the MGMA, signed a letter calling on the Biden administration to reinstate guidance to protect physicians’ right to receive EFT payments without paying fees. The letter describes the practice as “outrageous” and analogous to “an employee being required to enroll in a program that would deduct a percentage of each paycheck to receive direct deposit payments from an employer.”
So far, however, the situation remains unchanged. The language on the CMS site has changed, though. In 2022, the guidelines were adjusted to clarify that EFT fees are allowed.
A version of this article first appeared on Medscape.com.
, according to a new investigation by the nonprofit news organization ProPublica.
The Affordable Care Act requires that health plans give providers the option of being paid electronically to improve efficiency and save money. In 2017, the Centers for Medicare & Medicaid Services issued guidance that prohibited insurers and their payment processing vendors from “engaging in unfair business practices that do not support an efficient healthcare system,” according to a recent Medical Group Management Association position paper.
But that guidance, which appeared to forbid requiring fees to receive payments online, disappeared from the CMS site 6 months later.
According to ProPublica’s reporting, the change was the result of a quiet insurance industry lobbying campaign led by Matthew Albright, a former CMS employee who left government service to work for Zelis, a payment processing company co-owned by private equity giant Bain Capital.
The details of the lobbying effort were discovered by Alex Shteynshlyuger, a New York urologist, who through public records requests received the email correspondence between Mr. Albright and CMS and shared that material with ProPublica.
Mr. Albright had been able to influence CMS policy to protect what ProPublica called a “crucial revenue stream” for payment processors. The fee notice was removed just 3 days after Mr. Albright requested the change, ProPublica found.
When CMS resisted further changes, including eliminating guidance forbidding insurers and payment processors from charging excess fees for online payments, Mr. Albright brought in a law firm. The threat of a lawsuit by deep-pocketed Zelis was enough to bring CMS in line, ProPublica reported. Today, these fees can cost larger medical practices more than $1 million a year, according to the MGMA report.
“It took less than a decade for a new industry of middlemen, owned by private equity funds and giant conglomerates like UnitedHealth Group, to cash in,” writes Cezary Podkul, the author of the ProPublica report.
Predatory practices
It might seem that avoiding the fees would be as simple as requesting to be paid by check. However, a 2021 poll by the MGMA found that 57% of doctors were being charged these fees when they hadn’t agreed to them. According to the ProPublica report, physicians who have requested to be paid by check often find themselves being bounced back to electronic fund transfer (EFT) payments, where they are again charged fees.
In October 2021, more than 90 physician organizations, including the American Medical Association and the MGMA, signed a letter calling on the Biden administration to reinstate guidance to protect physicians’ right to receive EFT payments without paying fees. The letter describes the practice as “outrageous” and analogous to “an employee being required to enroll in a program that would deduct a percentage of each paycheck to receive direct deposit payments from an employer.”
So far, however, the situation remains unchanged. The language on the CMS site has changed, though. In 2022, the guidelines were adjusted to clarify that EFT fees are allowed.
A version of this article first appeared on Medscape.com.
, according to a new investigation by the nonprofit news organization ProPublica.
The Affordable Care Act requires that health plans give providers the option of being paid electronically to improve efficiency and save money. In 2017, the Centers for Medicare & Medicaid Services issued guidance that prohibited insurers and their payment processing vendors from “engaging in unfair business practices that do not support an efficient healthcare system,” according to a recent Medical Group Management Association position paper.
But that guidance, which appeared to forbid requiring fees to receive payments online, disappeared from the CMS site 6 months later.
According to ProPublica’s reporting, the change was the result of a quiet insurance industry lobbying campaign led by Matthew Albright, a former CMS employee who left government service to work for Zelis, a payment processing company co-owned by private equity giant Bain Capital.
The details of the lobbying effort were discovered by Alex Shteynshlyuger, a New York urologist, who through public records requests received the email correspondence between Mr. Albright and CMS and shared that material with ProPublica.
Mr. Albright had been able to influence CMS policy to protect what ProPublica called a “crucial revenue stream” for payment processors. The fee notice was removed just 3 days after Mr. Albright requested the change, ProPublica found.
When CMS resisted further changes, including eliminating guidance forbidding insurers and payment processors from charging excess fees for online payments, Mr. Albright brought in a law firm. The threat of a lawsuit by deep-pocketed Zelis was enough to bring CMS in line, ProPublica reported. Today, these fees can cost larger medical practices more than $1 million a year, according to the MGMA report.
“It took less than a decade for a new industry of middlemen, owned by private equity funds and giant conglomerates like UnitedHealth Group, to cash in,” writes Cezary Podkul, the author of the ProPublica report.
Predatory practices
It might seem that avoiding the fees would be as simple as requesting to be paid by check. However, a 2021 poll by the MGMA found that 57% of doctors were being charged these fees when they hadn’t agreed to them. According to the ProPublica report, physicians who have requested to be paid by check often find themselves being bounced back to electronic fund transfer (EFT) payments, where they are again charged fees.
In October 2021, more than 90 physician organizations, including the American Medical Association and the MGMA, signed a letter calling on the Biden administration to reinstate guidance to protect physicians’ right to receive EFT payments without paying fees. The letter describes the practice as “outrageous” and analogous to “an employee being required to enroll in a program that would deduct a percentage of each paycheck to receive direct deposit payments from an employer.”
So far, however, the situation remains unchanged. The language on the CMS site has changed, though. In 2022, the guidelines were adjusted to clarify that EFT fees are allowed.
A version of this article first appeared on Medscape.com.
Structural changes may separate axial psoriatic arthritis from axial spondyloarthritis
Approximately 20% of adults with axial psoriatic arthritis (PsA) show active or structural spinal changes without changes in the sacroiliac joint, based on imaging data from 106 individuals.
Axial PsA has been historically grouped with axial spondyloarthritis (axSpA), but it has received more attention in recent years as a condition potentially distinct from axSpA, Henriette Käding, an MD and PhD student in the department of gastroenterology, infectiology, and rheumatology at Charité-Universitätsmedizin Berlin, said in her research presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). She added that the debate persists as to whether these conditions are on the same spectrum or should be separated.
Data from previous studies suggest differences in genetic, clinical, radiographic, and prognostic characteristics between axial PsA and axSpA that may affect patients’ response to available treatments. However, there are relatively little data available on distinguishing imaging and clinical features, and there’s a lack of classification criteria for axial PsA, Ms. Käding said.
Ms. Käding and colleagues prospectively collected data from 106 patients with axial PsA between August 2019 and June 2023 and presented the baseline data of this longitudinal project at the GRAPPA annual meeting in Dublin. At baseline, the researchers conducted clinical assessments of the participants, along with blood sampling, stool samples, and imaging protocols that included MRI of the whole spine and sacroiliac joint (SIJ).
The mean age of the included patients was 44.5 years; 55.7% were female. Inflammatory back pain was present in most of the patients at baseline (78.4%), and 48.1% were positive for HLA-B27, a genetic risk factor for both axSpA and axial PsA. Approximately one-third of the patients had elevated C-reactive protein (> 5 mg/L). In the baseline MRI scans, active inflammatory changes in the sacroiliac joints (SIJ) were seen in 51.9% of the patients and structural changes in 72.1%. MRI spine scans showed active changes in 58.7% of the patients. Notably, active and/or structural changes of the spine without changes in the SIJ appeared in 20% of the patients, Ms. Käding said.
With regard to existing classification criteria, the researchers observed that 92% of the patients met the CASPAR (Classification Criteria for Psoriatic Arthritis) criteria for PsA, 73% met the ASAS (Assessment of Spondyloarthritis International Society) criteria, while 66% of patients met both ASAS and CASPAR criteria.
The study will be the first to include longitudinal MRI scans of the whole spine and SIJ in addition to conventional radiographs, Ms. Käding said.
Better characterization should improve treatment
“Axial involvement in PsA might, on one hand, go unnoticed, but on the other hand, it could also be misdiagnosed in patients with degenerative spinal disease,” Denis Poddubnyy, MD, one of the study coauthors, also of Charité-Universitätsmedizin Berlin, said in an interview.
“By comprehending the unique characteristics, progression, and treatment responses within the axial domain, rheumatologists can customize interventions and therapies to effectively manage the psoriatic disease,” Dr. Poddubnyy said.
“One of the most significant findings [of the current study] is the relatively high frequency of spinal involvement without sacroiliac joint” involvement, Fabian Proft, MD, of Charité-Universitätsmedizin Berlin and senior author of the study, said in an interview. “This finding holds importance as, in primary axial SpA, the disease typically originates in the sacroiliac joints. In contrast, in PsA, the scenario differs, which has implications for the diagnostic approach in clinical practice.”
“In individuals with PsA, spinal involvement can occur independently of sacroiliac joint [involvement]. As a result, imaging studies conducted on patients suspected of having axial PsA should encompass not only the sacroiliac joints but also the spine,” Dr. Poddubnyy explained. “It is important to note, however, that imaging findings such as bony spurs and bone marrow edema might be caused by degeneration or mechanical issues and, therefore, need to be interpreted with caution within the clinical context.”
The study was supported in part by an unrestricted research grant from Novartis. Dr. Poddubnyy and Dr. Proft disclosed receiving research grants and consultancy payments from Novartis and serving on speaker bureaus for the company.
Approximately 20% of adults with axial psoriatic arthritis (PsA) show active or structural spinal changes without changes in the sacroiliac joint, based on imaging data from 106 individuals.
Axial PsA has been historically grouped with axial spondyloarthritis (axSpA), but it has received more attention in recent years as a condition potentially distinct from axSpA, Henriette Käding, an MD and PhD student in the department of gastroenterology, infectiology, and rheumatology at Charité-Universitätsmedizin Berlin, said in her research presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). She added that the debate persists as to whether these conditions are on the same spectrum or should be separated.
Data from previous studies suggest differences in genetic, clinical, radiographic, and prognostic characteristics between axial PsA and axSpA that may affect patients’ response to available treatments. However, there are relatively little data available on distinguishing imaging and clinical features, and there’s a lack of classification criteria for axial PsA, Ms. Käding said.
Ms. Käding and colleagues prospectively collected data from 106 patients with axial PsA between August 2019 and June 2023 and presented the baseline data of this longitudinal project at the GRAPPA annual meeting in Dublin. At baseline, the researchers conducted clinical assessments of the participants, along with blood sampling, stool samples, and imaging protocols that included MRI of the whole spine and sacroiliac joint (SIJ).
The mean age of the included patients was 44.5 years; 55.7% were female. Inflammatory back pain was present in most of the patients at baseline (78.4%), and 48.1% were positive for HLA-B27, a genetic risk factor for both axSpA and axial PsA. Approximately one-third of the patients had elevated C-reactive protein (> 5 mg/L). In the baseline MRI scans, active inflammatory changes in the sacroiliac joints (SIJ) were seen in 51.9% of the patients and structural changes in 72.1%. MRI spine scans showed active changes in 58.7% of the patients. Notably, active and/or structural changes of the spine without changes in the SIJ appeared in 20% of the patients, Ms. Käding said.
With regard to existing classification criteria, the researchers observed that 92% of the patients met the CASPAR (Classification Criteria for Psoriatic Arthritis) criteria for PsA, 73% met the ASAS (Assessment of Spondyloarthritis International Society) criteria, while 66% of patients met both ASAS and CASPAR criteria.
The study will be the first to include longitudinal MRI scans of the whole spine and SIJ in addition to conventional radiographs, Ms. Käding said.
Better characterization should improve treatment
“Axial involvement in PsA might, on one hand, go unnoticed, but on the other hand, it could also be misdiagnosed in patients with degenerative spinal disease,” Denis Poddubnyy, MD, one of the study coauthors, also of Charité-Universitätsmedizin Berlin, said in an interview.
“By comprehending the unique characteristics, progression, and treatment responses within the axial domain, rheumatologists can customize interventions and therapies to effectively manage the psoriatic disease,” Dr. Poddubnyy said.
“One of the most significant findings [of the current study] is the relatively high frequency of spinal involvement without sacroiliac joint” involvement, Fabian Proft, MD, of Charité-Universitätsmedizin Berlin and senior author of the study, said in an interview. “This finding holds importance as, in primary axial SpA, the disease typically originates in the sacroiliac joints. In contrast, in PsA, the scenario differs, which has implications for the diagnostic approach in clinical practice.”
“In individuals with PsA, spinal involvement can occur independently of sacroiliac joint [involvement]. As a result, imaging studies conducted on patients suspected of having axial PsA should encompass not only the sacroiliac joints but also the spine,” Dr. Poddubnyy explained. “It is important to note, however, that imaging findings such as bony spurs and bone marrow edema might be caused by degeneration or mechanical issues and, therefore, need to be interpreted with caution within the clinical context.”
The study was supported in part by an unrestricted research grant from Novartis. Dr. Poddubnyy and Dr. Proft disclosed receiving research grants and consultancy payments from Novartis and serving on speaker bureaus for the company.
Approximately 20% of adults with axial psoriatic arthritis (PsA) show active or structural spinal changes without changes in the sacroiliac joint, based on imaging data from 106 individuals.
Axial PsA has been historically grouped with axial spondyloarthritis (axSpA), but it has received more attention in recent years as a condition potentially distinct from axSpA, Henriette Käding, an MD and PhD student in the department of gastroenterology, infectiology, and rheumatology at Charité-Universitätsmedizin Berlin, said in her research presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). She added that the debate persists as to whether these conditions are on the same spectrum or should be separated.
Data from previous studies suggest differences in genetic, clinical, radiographic, and prognostic characteristics between axial PsA and axSpA that may affect patients’ response to available treatments. However, there are relatively little data available on distinguishing imaging and clinical features, and there’s a lack of classification criteria for axial PsA, Ms. Käding said.
Ms. Käding and colleagues prospectively collected data from 106 patients with axial PsA between August 2019 and June 2023 and presented the baseline data of this longitudinal project at the GRAPPA annual meeting in Dublin. At baseline, the researchers conducted clinical assessments of the participants, along with blood sampling, stool samples, and imaging protocols that included MRI of the whole spine and sacroiliac joint (SIJ).
The mean age of the included patients was 44.5 years; 55.7% were female. Inflammatory back pain was present in most of the patients at baseline (78.4%), and 48.1% were positive for HLA-B27, a genetic risk factor for both axSpA and axial PsA. Approximately one-third of the patients had elevated C-reactive protein (> 5 mg/L). In the baseline MRI scans, active inflammatory changes in the sacroiliac joints (SIJ) were seen in 51.9% of the patients and structural changes in 72.1%. MRI spine scans showed active changes in 58.7% of the patients. Notably, active and/or structural changes of the spine without changes in the SIJ appeared in 20% of the patients, Ms. Käding said.
With regard to existing classification criteria, the researchers observed that 92% of the patients met the CASPAR (Classification Criteria for Psoriatic Arthritis) criteria for PsA, 73% met the ASAS (Assessment of Spondyloarthritis International Society) criteria, while 66% of patients met both ASAS and CASPAR criteria.
The study will be the first to include longitudinal MRI scans of the whole spine and SIJ in addition to conventional radiographs, Ms. Käding said.
Better characterization should improve treatment
“Axial involvement in PsA might, on one hand, go unnoticed, but on the other hand, it could also be misdiagnosed in patients with degenerative spinal disease,” Denis Poddubnyy, MD, one of the study coauthors, also of Charité-Universitätsmedizin Berlin, said in an interview.
“By comprehending the unique characteristics, progression, and treatment responses within the axial domain, rheumatologists can customize interventions and therapies to effectively manage the psoriatic disease,” Dr. Poddubnyy said.
“One of the most significant findings [of the current study] is the relatively high frequency of spinal involvement without sacroiliac joint” involvement, Fabian Proft, MD, of Charité-Universitätsmedizin Berlin and senior author of the study, said in an interview. “This finding holds importance as, in primary axial SpA, the disease typically originates in the sacroiliac joints. In contrast, in PsA, the scenario differs, which has implications for the diagnostic approach in clinical practice.”
“In individuals with PsA, spinal involvement can occur independently of sacroiliac joint [involvement]. As a result, imaging studies conducted on patients suspected of having axial PsA should encompass not only the sacroiliac joints but also the spine,” Dr. Poddubnyy explained. “It is important to note, however, that imaging findings such as bony spurs and bone marrow edema might be caused by degeneration or mechanical issues and, therefore, need to be interpreted with caution within the clinical context.”
The study was supported in part by an unrestricted research grant from Novartis. Dr. Poddubnyy and Dr. Proft disclosed receiving research grants and consultancy payments from Novartis and serving on speaker bureaus for the company.
FROM GRAPPA 2023
Low-dose oral minoxidil for female pattern hair loss: Benefits, impact on BP, heart rate evaluated
results from a small retrospective analysis showed.
“Additionally, few patients experienced hair loss progression while slightly over a third experienced hair regrowth,” the study’s first author, Reese Imhof, MD, a third-year resident in the department of dermatology at Mayo Clinic, Rochester, Minn., said in an interview. The results were published online in JAAD International.
At low doses, oral minoxidil, approved as an antihypertensive over 40 years ago, has become an increasingly popular treatment for hair loss, particularly since an article about its use for hair loss was published in the New York Times in August 2022. (Oral minoxidil is not approved for treating alopecia, and is used off label for this purpose.)
To evaluate the effects of LDOM in female patients with female pattern hair loss, Dr. Imhof, along with colleagues Beija Villalpando, MD, of the department of medicine and Rochelle R. Torgerson, MD, PhD, of the department of dermatology at the Mayo Clinic, reviewed the records of 25 adult women who were evaluated for female pattern hair loss at the Mayo Clinic over a 5-year period that ended on Nov. 27, 2022. Previous studies have looked at the cardiovascular effects of treatment with oral minoxidil and impact on BP in men, but “few studies have reported on female patients receiving LDOM as monotherapy for female pattern hair loss,” the authors noted.
The mean age of the women in their study was 61 years, and they took LDOM for a mean of 6.2 months. Slightly more than half (52%) took a dose of 1.25 mg daily, while 40% took 2.5 mg daily and 8% took 0.625 mg daily.
Of the 25 patients, 10 (40%) had previously tried topical minoxidil but had discontinued it because of local side effects or challenges with adherence. Also, three patients (12%) had previously tried finasteride and spironolactone but discontinued those medications because of adverse side effects.
The researchers noted disease improvement and hair regrowth was observed in nine patients who were treated with LDOM (36%), while three patients (12%) had “unaltered disease progression.” Adverse side effects observed in the cohort included four patients with facial hypertrichosis (16%) and one patient with fluid retention/lower limb edema (4%).
The patients who developed hypertrichosis did not discontinue LDOM, but the patient who developed edema did stop treatment.
At baseline, systolic BP (SBP) ranged from 107-161 mm Hg, diastolic BP (DBP) ranged from 58-88 mm Hg, and heart rate ranged from 54-114 beats per minute. Post treatment, SBP ranged from 102-152 mm Hg, DBP ranged from 63-90 mm Hg, and heart rate ranged from 56 to 105 bpm. “It was surprising how little ambulatory blood pressure and heart rate changed after an average of 6 months of treatment,” Dr. Imhof said in an interview. “On average, SBP decreased by 2.8 mm HG while DBP decreased by 1.4 mm Hg. Heart rate increased an average of 4.4 beats per minute.”
He acknowledged certain limitations of the study, including its small sample size and lack of inclusion of patients who were being treated for hypertension with concomitant antihypertensive medications. “Some unique aspects of our study are that we focused on women, and we had a slightly older cohort than prior studies (61 years old on average) as well as exposure to higher doses of LDOM, with most patients on either 1.25 mg daily or 2.5 mg daily,” Dr. Imhof said.
The researchers reported having no relevant disclosures, and there was no funding source for the study.
results from a small retrospective analysis showed.
“Additionally, few patients experienced hair loss progression while slightly over a third experienced hair regrowth,” the study’s first author, Reese Imhof, MD, a third-year resident in the department of dermatology at Mayo Clinic, Rochester, Minn., said in an interview. The results were published online in JAAD International.
At low doses, oral minoxidil, approved as an antihypertensive over 40 years ago, has become an increasingly popular treatment for hair loss, particularly since an article about its use for hair loss was published in the New York Times in August 2022. (Oral minoxidil is not approved for treating alopecia, and is used off label for this purpose.)
To evaluate the effects of LDOM in female patients with female pattern hair loss, Dr. Imhof, along with colleagues Beija Villalpando, MD, of the department of medicine and Rochelle R. Torgerson, MD, PhD, of the department of dermatology at the Mayo Clinic, reviewed the records of 25 adult women who were evaluated for female pattern hair loss at the Mayo Clinic over a 5-year period that ended on Nov. 27, 2022. Previous studies have looked at the cardiovascular effects of treatment with oral minoxidil and impact on BP in men, but “few studies have reported on female patients receiving LDOM as monotherapy for female pattern hair loss,” the authors noted.
The mean age of the women in their study was 61 years, and they took LDOM for a mean of 6.2 months. Slightly more than half (52%) took a dose of 1.25 mg daily, while 40% took 2.5 mg daily and 8% took 0.625 mg daily.
Of the 25 patients, 10 (40%) had previously tried topical minoxidil but had discontinued it because of local side effects or challenges with adherence. Also, three patients (12%) had previously tried finasteride and spironolactone but discontinued those medications because of adverse side effects.
The researchers noted disease improvement and hair regrowth was observed in nine patients who were treated with LDOM (36%), while three patients (12%) had “unaltered disease progression.” Adverse side effects observed in the cohort included four patients with facial hypertrichosis (16%) and one patient with fluid retention/lower limb edema (4%).
The patients who developed hypertrichosis did not discontinue LDOM, but the patient who developed edema did stop treatment.
At baseline, systolic BP (SBP) ranged from 107-161 mm Hg, diastolic BP (DBP) ranged from 58-88 mm Hg, and heart rate ranged from 54-114 beats per minute. Post treatment, SBP ranged from 102-152 mm Hg, DBP ranged from 63-90 mm Hg, and heart rate ranged from 56 to 105 bpm. “It was surprising how little ambulatory blood pressure and heart rate changed after an average of 6 months of treatment,” Dr. Imhof said in an interview. “On average, SBP decreased by 2.8 mm HG while DBP decreased by 1.4 mm Hg. Heart rate increased an average of 4.4 beats per minute.”
He acknowledged certain limitations of the study, including its small sample size and lack of inclusion of patients who were being treated for hypertension with concomitant antihypertensive medications. “Some unique aspects of our study are that we focused on women, and we had a slightly older cohort than prior studies (61 years old on average) as well as exposure to higher doses of LDOM, with most patients on either 1.25 mg daily or 2.5 mg daily,” Dr. Imhof said.
The researchers reported having no relevant disclosures, and there was no funding source for the study.
results from a small retrospective analysis showed.
“Additionally, few patients experienced hair loss progression while slightly over a third experienced hair regrowth,” the study’s first author, Reese Imhof, MD, a third-year resident in the department of dermatology at Mayo Clinic, Rochester, Minn., said in an interview. The results were published online in JAAD International.
At low doses, oral minoxidil, approved as an antihypertensive over 40 years ago, has become an increasingly popular treatment for hair loss, particularly since an article about its use for hair loss was published in the New York Times in August 2022. (Oral minoxidil is not approved for treating alopecia, and is used off label for this purpose.)
To evaluate the effects of LDOM in female patients with female pattern hair loss, Dr. Imhof, along with colleagues Beija Villalpando, MD, of the department of medicine and Rochelle R. Torgerson, MD, PhD, of the department of dermatology at the Mayo Clinic, reviewed the records of 25 adult women who were evaluated for female pattern hair loss at the Mayo Clinic over a 5-year period that ended on Nov. 27, 2022. Previous studies have looked at the cardiovascular effects of treatment with oral minoxidil and impact on BP in men, but “few studies have reported on female patients receiving LDOM as monotherapy for female pattern hair loss,” the authors noted.
The mean age of the women in their study was 61 years, and they took LDOM for a mean of 6.2 months. Slightly more than half (52%) took a dose of 1.25 mg daily, while 40% took 2.5 mg daily and 8% took 0.625 mg daily.
Of the 25 patients, 10 (40%) had previously tried topical minoxidil but had discontinued it because of local side effects or challenges with adherence. Also, three patients (12%) had previously tried finasteride and spironolactone but discontinued those medications because of adverse side effects.
The researchers noted disease improvement and hair regrowth was observed in nine patients who were treated with LDOM (36%), while three patients (12%) had “unaltered disease progression.” Adverse side effects observed in the cohort included four patients with facial hypertrichosis (16%) and one patient with fluid retention/lower limb edema (4%).
The patients who developed hypertrichosis did not discontinue LDOM, but the patient who developed edema did stop treatment.
At baseline, systolic BP (SBP) ranged from 107-161 mm Hg, diastolic BP (DBP) ranged from 58-88 mm Hg, and heart rate ranged from 54-114 beats per minute. Post treatment, SBP ranged from 102-152 mm Hg, DBP ranged from 63-90 mm Hg, and heart rate ranged from 56 to 105 bpm. “It was surprising how little ambulatory blood pressure and heart rate changed after an average of 6 months of treatment,” Dr. Imhof said in an interview. “On average, SBP decreased by 2.8 mm HG while DBP decreased by 1.4 mm Hg. Heart rate increased an average of 4.4 beats per minute.”
He acknowledged certain limitations of the study, including its small sample size and lack of inclusion of patients who were being treated for hypertension with concomitant antihypertensive medications. “Some unique aspects of our study are that we focused on women, and we had a slightly older cohort than prior studies (61 years old on average) as well as exposure to higher doses of LDOM, with most patients on either 1.25 mg daily or 2.5 mg daily,” Dr. Imhof said.
The researchers reported having no relevant disclosures, and there was no funding source for the study.
FROM JAAD INTERNATIONAL
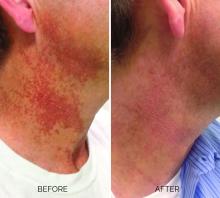
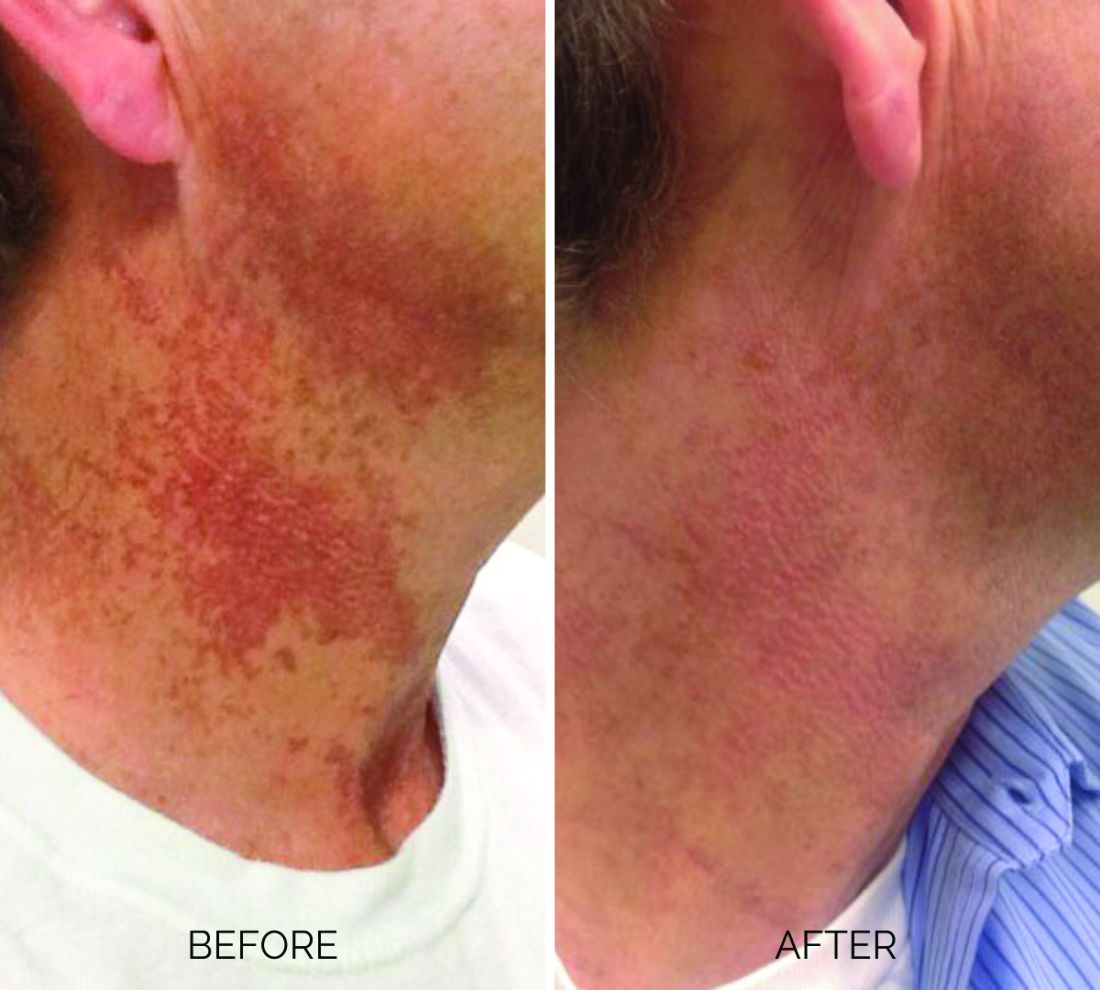
Treating poikiloderma
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at [email protected]. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
Could a malpractice insurer drop you when you need it most?
You’ve practiced medicine for years without issues, but now you are facing a medical malpractice case. No worries – you’ve had professional liability insurance all this time, so surely there’s nothing to be concerned about. Undoubtedly, your medical malpractice insurer will cover the costs of defending you. Or will they? One case casts questions on just this issue.
Professional liability insurance
According to the American Medical Association, almost one in three physicians (31%) have had a medical malpractice lawsuit filed against them at some point in their careers. These numbers only increase the longer a physician practices; almost half of doctors 55 and over have been sued, compared with less than 10% of physicians under 40.
And while the majority of cases are dropped or dismissed, and the small minority of cases that do go to trial are mostly won by the defense, the cost of defending these cases can be extremely high. Physicians have medical malpractice insurance to defray these costs.
Malpractice insurance generally covers the costs of attorney fees, court costs, arbitration, compensatory damages, and settlements related to patient injury or death. Insurance sometimes, but not always, pays for the costs of malpractice lawsuits arising out of Health Insurance Portability and Accountability Act (HIPAA) violations.
But it is what the policies don’t pay for that should be of most interest to practitioners.
Exclusions to medical malpractice insurance
All professional liability insurance policies contain exclusions, and it is essential that you know what they are. While the exclusions may vary by policy, most malpractice insurance policies exclude claims stemming from:
- Reckless or intentional acts.
- Illegal/criminal activities, including theft.
- Misrepresentation, including dishonesty, fraudulent activity, falsification, and misrepresentation on forms.
- Practicing under the influence of alcohol or drugs.
- Altering patient or hospital records.
- Sexual misconduct.
- Cyber security issues, which typically require a separate cyber liability policy to protect against cyber attacks and data breaches affecting patient medical records.
It’s essential to know what your specific policy’s exclusions are, or you may be surprised to find that your malpractice liability insurance doesn’t cover you when you expected that it would. Such was the situation in a recently decided case.
Also essential is knowing what type of coverage your policy provides – claims-made or occurrence-based. Occurrence policies offer lifetime coverage for incidents that occurred during the policy period, no matter when the claim is made. Claims-made policies cover only incidents that occur and are reported within the policy’s time period (unless a “tail” policy is purchased to extend the reporting period).
The case
Dr. P was a neurologist specializing in pain management. He had a professional liability insurance policy with an insurance company. In 2012, Dr. P’s insurance agent saw a television news story about the physician being accused by the state medical board for overprescribing opioids, resulting in the deaths of 17 patients. The next day, the agent obtained copies of documents from the state medical board, including a summary suspension order and a notice of contemplated action.
The notice of contemplated action specified that Dr. P had deviated from the standard of care through injudicious prescribing, leading to approximately 17 patient deaths due to drug toxicity. Because the agent realized that lawsuits could be filed against Dr. P for the deaths, she sent the insurance company the paperwork from the medical board so the insurer would be aware of the potential claims.
However, when the insurer received the information, it did not investigate or seek more information as it was required to do. The insurer failed to get medical records, or specific patient names, and none of the 17 deaths were recorded in the insurance company’s claims system (a failure to follow company procedure). Instead, the insurance company decided to cancel Dr. P’s policy effective the following month.
The company sent Dr. P a cancellation letter advising him that his policy was being terminated due to “license suspension, nature of allegations, and practice profile,” and offered him a tail policy to purchase.
The insurance company did not advise Dr. P that he should ensure all potential claims were reported, including the 17 deaths, before his policy expired. The company also did not advise him that he had a claims-made policy and what that meant regarding future lawsuits that might be filed after his policy period expired.
A year later, Dr. P was sued in two wrongful death lawsuits by the families of two of the 17 opioid-related deaths. When he was served with the papers, he promptly notified the insurance company. The insurance company issued a denial letter, incorrectly asserting that the 17 drug-toxicity deaths that they were aware of did not qualify as claims under Dr. P’s policy.
After his insurance company failed to represent him, Dr. P divorced his wife of 35 years and filed for bankruptcy. The only creditors with claims were the two families who had sued him. The bankruptcy trustee filed a lawsuit against the insurance company on behalf of Dr. P for the insurer’s failure to defend and indemnify Dr. P against the wrongful death lawsuits. In 2017, the bankruptcy trustee settled the two wrongful death cases by paying the families a certain amount of cash and assigning the insurance bad faith lawsuit to them.
Court and jury decide
In 2020, the case against the insurance company ended up in court. By 2022, the court had decided some of the issues and left some for the jury to determine.
The court found that the insurance company had breached its obligation to defend and indemnify Dr. P, committed unfair insurance claims practices, and committed bad faith in failing to defend the physician. The court limited the compensation to the amount of cash that had been paid to settle the two cases, and any fees and costs that Dr. P had incurred defending himself.
However, this still left the jury to decide whether the insurance company had committed bad faith in failing to indemnify (secure a person against legal liability for his/her actions) Dr. P, whether it had violated the state’s Unfair Insurance Practices Act, and whether punitive damages should be levied against the insurer.
The jury trial ended in a stunning $52 million verdict against the insurance company after less than 2 hours of deliberation. The jury found that the insurance company had acted in bad faith and willfully violated the Unfair Insurances Practices Act.
While the jury ultimately decided against the insurance company and sent it a strong message with a large verdict, Dr. P’s career was still over. He had stopped practicing medicine, was bankrupt, and his personal life was in shambles. The litigation had taken about a decade. Sometimes a win isn’t a victory.
Protecting yourself
The best way to protect yourself from a situation in which your insurer will not defend you is to really know and understand your insurance policy. Is it occurrence-based or claims-made insurance? What exactly does it cover? How are claims supposed to be made? Your professional liability insurance can be extremely important if you get sued, so it is equally important to choose it carefully and to really understand what is being covered.
Other ways to protect yourself:
- Know your agent. Your agent is key to explaining your policy as well as helping in the event that you need to make a claim. Dr. P’s agent saw a news story about him on television, which is why she submitted the information to the insurance company. Dr. P would have been far better off calling the agent directly when he was being investigated by the state medical board to explain the situation and seek advice.
- Be aware of exclusions to your policy. Many – such as criminal acts, reckless or intentional acts, or practicing under the influence – were mentioned earlier in this article. Some may be unexpected, so it is extremely important that you understand the specific exclusions to your particular policy.
- Be aware of your state law, and how changes might affect you. For example, in states that have outlawed or criminalized abortion, an insurance company would probably not have to represent a policy holder who was sued for malpractice involving an abortion. On the other hand, be aware that not treating a patient who needs life-saving care because you are afraid of running afoul of the law can also get you in trouble if the patient is harmed by not being treated. (For example, the Centers for Medicare & Medicaid Services is currently investigating two hospitals that failed to provide necessary stabilizing abortion care to a patient with an emergency medication condition resulting from a miscarriage.)
- Know how your policy defines ‘intentional’ acts (which are typically excluded from coverage). This is important. In some jurisdictions, the insured clinician has to merely intend to commit the acts in order for the claim to be excluded. In other jurisdictions, the insured doctor has to intend to cause the resulting damage. This can result in a very different outcome.
- The best thing doctors can do is to really understand what the policy covers and be prepared to make some noise if the company is not covering something that it should. Don’t be afraid to ask questions if you think your insurer is doing something wrong, and if the answers don’t satisfy you, consult an attorney.
The future
In the fall of 2022, at least partially in response to the Dobbs v. Jackson Women’s Health Organization decision regarding abortion, one professional liability company (Physician’s Insurance) launched criminal defense reimbursement coverage for physicians and hospitals to pay for defense costs incurred in responding to criminal allegations arising directly from patient care.
The add-on Criminal Defense Reimbursement Endorsement was made available in Washington State in January 2023, and will be offered in other states pending regulatory approval. It reimburses defense costs up to $250,000 when criminal actions have arisen from direct patient care.
In a press release announcing the new coverage, Physician’s Insurance CEO Bill Cotter explained the company’s reasoning in providing it: “The already challenging environment for physicians and hospitals has been made even more difficult as they now navigate the legal ramifications of increased criminal medical negligence claims as seen in the case of the Nashville nurse at the Vanderbilt University Medical Center, the potential for criminal state claims arising out of the U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization, and the subsequent state criminalization of healthcare practices that have long been the professionally accepted standard of care.”
Expect to see more insurance companies offering new coverage options for physicians in the future as they recognize that physicians may be facing more than just medical malpractice lawsuits arising out of patient care.
A version of this article first appeared on Medscape.com.
You’ve practiced medicine for years without issues, but now you are facing a medical malpractice case. No worries – you’ve had professional liability insurance all this time, so surely there’s nothing to be concerned about. Undoubtedly, your medical malpractice insurer will cover the costs of defending you. Or will they? One case casts questions on just this issue.
Professional liability insurance
According to the American Medical Association, almost one in three physicians (31%) have had a medical malpractice lawsuit filed against them at some point in their careers. These numbers only increase the longer a physician practices; almost half of doctors 55 and over have been sued, compared with less than 10% of physicians under 40.
And while the majority of cases are dropped or dismissed, and the small minority of cases that do go to trial are mostly won by the defense, the cost of defending these cases can be extremely high. Physicians have medical malpractice insurance to defray these costs.
Malpractice insurance generally covers the costs of attorney fees, court costs, arbitration, compensatory damages, and settlements related to patient injury or death. Insurance sometimes, but not always, pays for the costs of malpractice lawsuits arising out of Health Insurance Portability and Accountability Act (HIPAA) violations.
But it is what the policies don’t pay for that should be of most interest to practitioners.
Exclusions to medical malpractice insurance
All professional liability insurance policies contain exclusions, and it is essential that you know what they are. While the exclusions may vary by policy, most malpractice insurance policies exclude claims stemming from:
- Reckless or intentional acts.
- Illegal/criminal activities, including theft.
- Misrepresentation, including dishonesty, fraudulent activity, falsification, and misrepresentation on forms.
- Practicing under the influence of alcohol or drugs.
- Altering patient or hospital records.
- Sexual misconduct.
- Cyber security issues, which typically require a separate cyber liability policy to protect against cyber attacks and data breaches affecting patient medical records.
It’s essential to know what your specific policy’s exclusions are, or you may be surprised to find that your malpractice liability insurance doesn’t cover you when you expected that it would. Such was the situation in a recently decided case.
Also essential is knowing what type of coverage your policy provides – claims-made or occurrence-based. Occurrence policies offer lifetime coverage for incidents that occurred during the policy period, no matter when the claim is made. Claims-made policies cover only incidents that occur and are reported within the policy’s time period (unless a “tail” policy is purchased to extend the reporting period).
The case
Dr. P was a neurologist specializing in pain management. He had a professional liability insurance policy with an insurance company. In 2012, Dr. P’s insurance agent saw a television news story about the physician being accused by the state medical board for overprescribing opioids, resulting in the deaths of 17 patients. The next day, the agent obtained copies of documents from the state medical board, including a summary suspension order and a notice of contemplated action.
The notice of contemplated action specified that Dr. P had deviated from the standard of care through injudicious prescribing, leading to approximately 17 patient deaths due to drug toxicity. Because the agent realized that lawsuits could be filed against Dr. P for the deaths, she sent the insurance company the paperwork from the medical board so the insurer would be aware of the potential claims.
However, when the insurer received the information, it did not investigate or seek more information as it was required to do. The insurer failed to get medical records, or specific patient names, and none of the 17 deaths were recorded in the insurance company’s claims system (a failure to follow company procedure). Instead, the insurance company decided to cancel Dr. P’s policy effective the following month.
The company sent Dr. P a cancellation letter advising him that his policy was being terminated due to “license suspension, nature of allegations, and practice profile,” and offered him a tail policy to purchase.
The insurance company did not advise Dr. P that he should ensure all potential claims were reported, including the 17 deaths, before his policy expired. The company also did not advise him that he had a claims-made policy and what that meant regarding future lawsuits that might be filed after his policy period expired.
A year later, Dr. P was sued in two wrongful death lawsuits by the families of two of the 17 opioid-related deaths. When he was served with the papers, he promptly notified the insurance company. The insurance company issued a denial letter, incorrectly asserting that the 17 drug-toxicity deaths that they were aware of did not qualify as claims under Dr. P’s policy.
After his insurance company failed to represent him, Dr. P divorced his wife of 35 years and filed for bankruptcy. The only creditors with claims were the two families who had sued him. The bankruptcy trustee filed a lawsuit against the insurance company on behalf of Dr. P for the insurer’s failure to defend and indemnify Dr. P against the wrongful death lawsuits. In 2017, the bankruptcy trustee settled the two wrongful death cases by paying the families a certain amount of cash and assigning the insurance bad faith lawsuit to them.
Court and jury decide
In 2020, the case against the insurance company ended up in court. By 2022, the court had decided some of the issues and left some for the jury to determine.
The court found that the insurance company had breached its obligation to defend and indemnify Dr. P, committed unfair insurance claims practices, and committed bad faith in failing to defend the physician. The court limited the compensation to the amount of cash that had been paid to settle the two cases, and any fees and costs that Dr. P had incurred defending himself.
However, this still left the jury to decide whether the insurance company had committed bad faith in failing to indemnify (secure a person against legal liability for his/her actions) Dr. P, whether it had violated the state’s Unfair Insurance Practices Act, and whether punitive damages should be levied against the insurer.
The jury trial ended in a stunning $52 million verdict against the insurance company after less than 2 hours of deliberation. The jury found that the insurance company had acted in bad faith and willfully violated the Unfair Insurances Practices Act.
While the jury ultimately decided against the insurance company and sent it a strong message with a large verdict, Dr. P’s career was still over. He had stopped practicing medicine, was bankrupt, and his personal life was in shambles. The litigation had taken about a decade. Sometimes a win isn’t a victory.
Protecting yourself
The best way to protect yourself from a situation in which your insurer will not defend you is to really know and understand your insurance policy. Is it occurrence-based or claims-made insurance? What exactly does it cover? How are claims supposed to be made? Your professional liability insurance can be extremely important if you get sued, so it is equally important to choose it carefully and to really understand what is being covered.
Other ways to protect yourself:
- Know your agent. Your agent is key to explaining your policy as well as helping in the event that you need to make a claim. Dr. P’s agent saw a news story about him on television, which is why she submitted the information to the insurance company. Dr. P would have been far better off calling the agent directly when he was being investigated by the state medical board to explain the situation and seek advice.
- Be aware of exclusions to your policy. Many – such as criminal acts, reckless or intentional acts, or practicing under the influence – were mentioned earlier in this article. Some may be unexpected, so it is extremely important that you understand the specific exclusions to your particular policy.
- Be aware of your state law, and how changes might affect you. For example, in states that have outlawed or criminalized abortion, an insurance company would probably not have to represent a policy holder who was sued for malpractice involving an abortion. On the other hand, be aware that not treating a patient who needs life-saving care because you are afraid of running afoul of the law can also get you in trouble if the patient is harmed by not being treated. (For example, the Centers for Medicare & Medicaid Services is currently investigating two hospitals that failed to provide necessary stabilizing abortion care to a patient with an emergency medication condition resulting from a miscarriage.)
- Know how your policy defines ‘intentional’ acts (which are typically excluded from coverage). This is important. In some jurisdictions, the insured clinician has to merely intend to commit the acts in order for the claim to be excluded. In other jurisdictions, the insured doctor has to intend to cause the resulting damage. This can result in a very different outcome.
- The best thing doctors can do is to really understand what the policy covers and be prepared to make some noise if the company is not covering something that it should. Don’t be afraid to ask questions if you think your insurer is doing something wrong, and if the answers don’t satisfy you, consult an attorney.
The future
In the fall of 2022, at least partially in response to the Dobbs v. Jackson Women’s Health Organization decision regarding abortion, one professional liability company (Physician’s Insurance) launched criminal defense reimbursement coverage for physicians and hospitals to pay for defense costs incurred in responding to criminal allegations arising directly from patient care.
The add-on Criminal Defense Reimbursement Endorsement was made available in Washington State in January 2023, and will be offered in other states pending regulatory approval. It reimburses defense costs up to $250,000 when criminal actions have arisen from direct patient care.
In a press release announcing the new coverage, Physician’s Insurance CEO Bill Cotter explained the company’s reasoning in providing it: “The already challenging environment for physicians and hospitals has been made even more difficult as they now navigate the legal ramifications of increased criminal medical negligence claims as seen in the case of the Nashville nurse at the Vanderbilt University Medical Center, the potential for criminal state claims arising out of the U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization, and the subsequent state criminalization of healthcare practices that have long been the professionally accepted standard of care.”
Expect to see more insurance companies offering new coverage options for physicians in the future as they recognize that physicians may be facing more than just medical malpractice lawsuits arising out of patient care.
A version of this article first appeared on Medscape.com.
You’ve practiced medicine for years without issues, but now you are facing a medical malpractice case. No worries – you’ve had professional liability insurance all this time, so surely there’s nothing to be concerned about. Undoubtedly, your medical malpractice insurer will cover the costs of defending you. Or will they? One case casts questions on just this issue.
Professional liability insurance
According to the American Medical Association, almost one in three physicians (31%) have had a medical malpractice lawsuit filed against them at some point in their careers. These numbers only increase the longer a physician practices; almost half of doctors 55 and over have been sued, compared with less than 10% of physicians under 40.
And while the majority of cases are dropped or dismissed, and the small minority of cases that do go to trial are mostly won by the defense, the cost of defending these cases can be extremely high. Physicians have medical malpractice insurance to defray these costs.
Malpractice insurance generally covers the costs of attorney fees, court costs, arbitration, compensatory damages, and settlements related to patient injury or death. Insurance sometimes, but not always, pays for the costs of malpractice lawsuits arising out of Health Insurance Portability and Accountability Act (HIPAA) violations.
But it is what the policies don’t pay for that should be of most interest to practitioners.
Exclusions to medical malpractice insurance
All professional liability insurance policies contain exclusions, and it is essential that you know what they are. While the exclusions may vary by policy, most malpractice insurance policies exclude claims stemming from:
- Reckless or intentional acts.
- Illegal/criminal activities, including theft.
- Misrepresentation, including dishonesty, fraudulent activity, falsification, and misrepresentation on forms.
- Practicing under the influence of alcohol or drugs.
- Altering patient or hospital records.
- Sexual misconduct.
- Cyber security issues, which typically require a separate cyber liability policy to protect against cyber attacks and data breaches affecting patient medical records.
It’s essential to know what your specific policy’s exclusions are, or you may be surprised to find that your malpractice liability insurance doesn’t cover you when you expected that it would. Such was the situation in a recently decided case.
Also essential is knowing what type of coverage your policy provides – claims-made or occurrence-based. Occurrence policies offer lifetime coverage for incidents that occurred during the policy period, no matter when the claim is made. Claims-made policies cover only incidents that occur and are reported within the policy’s time period (unless a “tail” policy is purchased to extend the reporting period).
The case
Dr. P was a neurologist specializing in pain management. He had a professional liability insurance policy with an insurance company. In 2012, Dr. P’s insurance agent saw a television news story about the physician being accused by the state medical board for overprescribing opioids, resulting in the deaths of 17 patients. The next day, the agent obtained copies of documents from the state medical board, including a summary suspension order and a notice of contemplated action.
The notice of contemplated action specified that Dr. P had deviated from the standard of care through injudicious prescribing, leading to approximately 17 patient deaths due to drug toxicity. Because the agent realized that lawsuits could be filed against Dr. P for the deaths, she sent the insurance company the paperwork from the medical board so the insurer would be aware of the potential claims.
However, when the insurer received the information, it did not investigate or seek more information as it was required to do. The insurer failed to get medical records, or specific patient names, and none of the 17 deaths were recorded in the insurance company’s claims system (a failure to follow company procedure). Instead, the insurance company decided to cancel Dr. P’s policy effective the following month.
The company sent Dr. P a cancellation letter advising him that his policy was being terminated due to “license suspension, nature of allegations, and practice profile,” and offered him a tail policy to purchase.
The insurance company did not advise Dr. P that he should ensure all potential claims were reported, including the 17 deaths, before his policy expired. The company also did not advise him that he had a claims-made policy and what that meant regarding future lawsuits that might be filed after his policy period expired.
A year later, Dr. P was sued in two wrongful death lawsuits by the families of two of the 17 opioid-related deaths. When he was served with the papers, he promptly notified the insurance company. The insurance company issued a denial letter, incorrectly asserting that the 17 drug-toxicity deaths that they were aware of did not qualify as claims under Dr. P’s policy.
After his insurance company failed to represent him, Dr. P divorced his wife of 35 years and filed for bankruptcy. The only creditors with claims were the two families who had sued him. The bankruptcy trustee filed a lawsuit against the insurance company on behalf of Dr. P for the insurer’s failure to defend and indemnify Dr. P against the wrongful death lawsuits. In 2017, the bankruptcy trustee settled the two wrongful death cases by paying the families a certain amount of cash and assigning the insurance bad faith lawsuit to them.
Court and jury decide
In 2020, the case against the insurance company ended up in court. By 2022, the court had decided some of the issues and left some for the jury to determine.
The court found that the insurance company had breached its obligation to defend and indemnify Dr. P, committed unfair insurance claims practices, and committed bad faith in failing to defend the physician. The court limited the compensation to the amount of cash that had been paid to settle the two cases, and any fees and costs that Dr. P had incurred defending himself.
However, this still left the jury to decide whether the insurance company had committed bad faith in failing to indemnify (secure a person against legal liability for his/her actions) Dr. P, whether it had violated the state’s Unfair Insurance Practices Act, and whether punitive damages should be levied against the insurer.
The jury trial ended in a stunning $52 million verdict against the insurance company after less than 2 hours of deliberation. The jury found that the insurance company had acted in bad faith and willfully violated the Unfair Insurances Practices Act.
While the jury ultimately decided against the insurance company and sent it a strong message with a large verdict, Dr. P’s career was still over. He had stopped practicing medicine, was bankrupt, and his personal life was in shambles. The litigation had taken about a decade. Sometimes a win isn’t a victory.
Protecting yourself
The best way to protect yourself from a situation in which your insurer will not defend you is to really know and understand your insurance policy. Is it occurrence-based or claims-made insurance? What exactly does it cover? How are claims supposed to be made? Your professional liability insurance can be extremely important if you get sued, so it is equally important to choose it carefully and to really understand what is being covered.
Other ways to protect yourself:
- Know your agent. Your agent is key to explaining your policy as well as helping in the event that you need to make a claim. Dr. P’s agent saw a news story about him on television, which is why she submitted the information to the insurance company. Dr. P would have been far better off calling the agent directly when he was being investigated by the state medical board to explain the situation and seek advice.
- Be aware of exclusions to your policy. Many – such as criminal acts, reckless or intentional acts, or practicing under the influence – were mentioned earlier in this article. Some may be unexpected, so it is extremely important that you understand the specific exclusions to your particular policy.
- Be aware of your state law, and how changes might affect you. For example, in states that have outlawed or criminalized abortion, an insurance company would probably not have to represent a policy holder who was sued for malpractice involving an abortion. On the other hand, be aware that not treating a patient who needs life-saving care because you are afraid of running afoul of the law can also get you in trouble if the patient is harmed by not being treated. (For example, the Centers for Medicare & Medicaid Services is currently investigating two hospitals that failed to provide necessary stabilizing abortion care to a patient with an emergency medication condition resulting from a miscarriage.)
- Know how your policy defines ‘intentional’ acts (which are typically excluded from coverage). This is important. In some jurisdictions, the insured clinician has to merely intend to commit the acts in order for the claim to be excluded. In other jurisdictions, the insured doctor has to intend to cause the resulting damage. This can result in a very different outcome.
- The best thing doctors can do is to really understand what the policy covers and be prepared to make some noise if the company is not covering something that it should. Don’t be afraid to ask questions if you think your insurer is doing something wrong, and if the answers don’t satisfy you, consult an attorney.
The future
In the fall of 2022, at least partially in response to the Dobbs v. Jackson Women’s Health Organization decision regarding abortion, one professional liability company (Physician’s Insurance) launched criminal defense reimbursement coverage for physicians and hospitals to pay for defense costs incurred in responding to criminal allegations arising directly from patient care.
The add-on Criminal Defense Reimbursement Endorsement was made available in Washington State in January 2023, and will be offered in other states pending regulatory approval. It reimburses defense costs up to $250,000 when criminal actions have arisen from direct patient care.
In a press release announcing the new coverage, Physician’s Insurance CEO Bill Cotter explained the company’s reasoning in providing it: “The already challenging environment for physicians and hospitals has been made even more difficult as they now navigate the legal ramifications of increased criminal medical negligence claims as seen in the case of the Nashville nurse at the Vanderbilt University Medical Center, the potential for criminal state claims arising out of the U.S. Supreme Court decision in Dobbs v. Jackson Women’s Health Organization, and the subsequent state criminalization of healthcare practices that have long been the professionally accepted standard of care.”
Expect to see more insurance companies offering new coverage options for physicians in the future as they recognize that physicians may be facing more than just medical malpractice lawsuits arising out of patient care.
A version of this article first appeared on Medscape.com.
Can this common herb help grow hair?
If you’re looking to grow hair, you might just have a solution in your kitchen cabinet – if TikTok and some dermatologists are correct.
The herb might also protect hair from the sun, pollution, and other environmental elements, according to an article in Insider.
The study published in Skinmed found that rosemary oil was similar to the effectiveness of minoxidil for regrowing hair in men with androgenetic alopecia. The scalp was also less itchy after 3-6 months of use.
The study included only men.
Still, dermatologist Shilpi Khetarpal, MD, told the Cleveland Clinic that it seems to work.
“The study really prompted people to look at rosemary oil for hair growth,” she said. “It became much more common in over-the-counter products after that, too.”
The Cleveland Clinic also reports that rosemary oil might help against dandruff and premature graying.
Dr. Khetarpal suggested massaging rosemary oil into the scalp, letting it soak overnight, and then washing it out. This should be done two or three times a week.
She also noted that only a few drops of rosemary oil are needed, and that the focus should be on the scalp rather than the hair, which rosemary oil makes look greasy.
It may take 6 months for “meaningful improvement,” Dr. Khetarpal said.
Meanwhile, TikTok users love hyping the oil’s hair care qualities. On the social media platform, videos with the hashtag #rosemaryoil have been viewed more than 2 billion times.
A version of this article appeared on WebMD.com.
If you’re looking to grow hair, you might just have a solution in your kitchen cabinet – if TikTok and some dermatologists are correct.
The herb might also protect hair from the sun, pollution, and other environmental elements, according to an article in Insider.
The study published in Skinmed found that rosemary oil was similar to the effectiveness of minoxidil for regrowing hair in men with androgenetic alopecia. The scalp was also less itchy after 3-6 months of use.
The study included only men.
Still, dermatologist Shilpi Khetarpal, MD, told the Cleveland Clinic that it seems to work.
“The study really prompted people to look at rosemary oil for hair growth,” she said. “It became much more common in over-the-counter products after that, too.”
The Cleveland Clinic also reports that rosemary oil might help against dandruff and premature graying.
Dr. Khetarpal suggested massaging rosemary oil into the scalp, letting it soak overnight, and then washing it out. This should be done two or three times a week.
She also noted that only a few drops of rosemary oil are needed, and that the focus should be on the scalp rather than the hair, which rosemary oil makes look greasy.
It may take 6 months for “meaningful improvement,” Dr. Khetarpal said.
Meanwhile, TikTok users love hyping the oil’s hair care qualities. On the social media platform, videos with the hashtag #rosemaryoil have been viewed more than 2 billion times.
A version of this article appeared on WebMD.com.
If you’re looking to grow hair, you might just have a solution in your kitchen cabinet – if TikTok and some dermatologists are correct.
The herb might also protect hair from the sun, pollution, and other environmental elements, according to an article in Insider.
The study published in Skinmed found that rosemary oil was similar to the effectiveness of minoxidil for regrowing hair in men with androgenetic alopecia. The scalp was also less itchy after 3-6 months of use.
The study included only men.
Still, dermatologist Shilpi Khetarpal, MD, told the Cleveland Clinic that it seems to work.
“The study really prompted people to look at rosemary oil for hair growth,” she said. “It became much more common in over-the-counter products after that, too.”
The Cleveland Clinic also reports that rosemary oil might help against dandruff and premature graying.
Dr. Khetarpal suggested massaging rosemary oil into the scalp, letting it soak overnight, and then washing it out. This should be done two or three times a week.
She also noted that only a few drops of rosemary oil are needed, and that the focus should be on the scalp rather than the hair, which rosemary oil makes look greasy.
It may take 6 months for “meaningful improvement,” Dr. Khetarpal said.
Meanwhile, TikTok users love hyping the oil’s hair care qualities. On the social media platform, videos with the hashtag #rosemaryoil have been viewed more than 2 billion times.
A version of this article appeared on WebMD.com.
Docs using AI? Some love it, most remain wary
When OpenAI released ChatGPT-3 publicly last November, some doctors decided to try out the free AI tool that learns language and writes human-like text. Some physicians found the chatbot made mistakes and stopped using it, while others were happy with the results and plan to use it more often.
“We’ve played around with it. It was very early on in AI and we noticed it gave us incorrect information with regards to clinical guidance,” said Monalisa Tailor, MD, an internal medicine physician at Norton Health Care in Louisville, Ky. “We decided not to pursue it further,” she said.
Orthopedic spine surgeon Daniel Choi, MD, who owns a small medical/surgical practice in Long Island, New York, tested the chatbot’s performance with a few administrative tasks, including writing a job listing for an administrator and prior authorization letters.
He was enthusiastic. “A well-polished job posting that would usually take me 2-3 hours to write was done in 5 minutes,” Dr. Choi said. “I was blown away by the writing – it was much better than anything I could write.”
The chatbot can also automate administrative tasks in doctors’ practices from appointment scheduling and billing to clinical documentation, saving doctors time and money, experts say.
Most physicians are proceeding cautiously. About 10% of more than 500 medical group leaders, responding to a March poll by the Medical Group Management Association, said their practices regularly use AI tools.
More than half of the respondents not using AI said they first want more evidence that the technology works as intended.
“None of them work as advertised,” said one respondent.
MGMA practice management consultant Dawn Plested acknowledges that many of the physician practices she’s worked with are still wary. “I have yet to encounter a practice that is using any AI tool, even something as low-risk as appointment scheduling,” she said.
Physician groups may be concerned about the costs and logistics of integrating ChatGPT with their electronic health record systems (EHRs) and how that would work, said Ms. Plested.
Doctors may also be skeptical of AI based on their experience with EHRs, she said.
“They were promoted as a panacea to many problems; they were supposed to automate business practice, reduce staff and clinician’s work, and improve billing/coding/documentation. Unfortunately, they have become a major source of frustration for doctors,” said Ms. Plested.
Drawing the line at patient care
Patients are worried about their doctors relying on AI for their care, according to a Pew Research Center poll released in February. About 60% of U.S. adults say they would feel uncomfortable if their own health care professional relied on artificial intelligence to do things like diagnose disease and recommend treatments; about 40% say they would feel comfortable with this.
“We have not yet gone into using ChatGPT for clinical purposes and will be very cautious with these types of applications due to concerns about inaccuracies,” Dr. Choi said.
Practice leaders reported in the MGMA poll that the most common uses of AI were nonclinical, such as:
- Patient communications, including call center answering service to help triage calls, to sort/distribute incoming fax messages, and outreach such as appointment reminders and marketing materials.
- Capturing clinical documentation, often with natural language processing or speech recognition platforms to help virtually scribe.
- Improving billing operations and predictive analytics.
Some doctors told The New York Times that ChatGPT helped them communicate with patients in a more compassionate way.
They used chatbots “to find words to break bad news and express concerns about a patient’s suffering, or to just more clearly explain medical recommendations,” the story noted.
Is regulation needed?
Some legal scholars and medical groups say that AI should be regulated to protect patients and doctors from risks, including medical errors, that could harm patients.
“It’s very important to evaluate the accuracy, safety, and privacy of language learning models (LLMs) before integrating them into the medical system. The same should be true of any new medical tool,” said Mason Marks, MD, JD, a health law professor at the Florida State University College of Law in Tallahassee.
In mid-June, the American Medical Association approved two resolutions calling for greater government oversight of AI. The AMA will develop proposed state and federal regulations and work with the federal government and other organizations to protect patients from false or misleading AI-generated medical advice.
Dr. Marks pointed to existing federal rules that apply to AI. “The Federal Trade Commission already has regulation that can potentially be used to combat unfair or deceptive trade practices associated with chatbots,” he said.
In addition, “the U.S. Food and Drug Administration can also regulate these tools, but it needs to update how it approaches risk when it comes to AI. The FDA has an outdated view of risk as physical harm, for instance, from traditional medical devices. That view of risk needs to be updated and expanded to encompass the unique harms of AI,” Dr. Marks said.
There should also be more transparency about how LLM software is used in medicine, he said. “That could be a norm implemented by the LLM developers and it could also be enforced by federal agencies. For instance, the FDA could require developers to be more transparent regarding training data and methods, and the FTC could require greater transparency regarding how consumer data might be used and opportunities to opt out of certain uses,” said Dr. Marks.
What should doctors do?
Dr. Marks advised doctors to be cautious when using ChatGPT and other LLMs, especially for medical advice. “The same would apply to any new medical tool, but we know that the current generation of LLMs [is] particularly prone to making things up, which could lead to medical errors if relied on in clinical settings,” he said.
There is also potential for breaches of patient confidentiality if doctors input clinical information. ChatGPT and OpenAI-enabled tools may not be compliant with the Health Insurance Portability and Accountability Act, which set national standards to protect individuals’ medical records and individually identifiable health information.
“The best approach is to use chatbots cautiously and with skepticism. Don’t input patient information, confirm the accuracy of information produced, and don’t use them as replacements for professional judgment,” Dr. Marks recommended.
Ms. Plested suggested that doctors who want to experiment with AI start with a low-risk tool such as appointment reminders that could save staff time and money. “I never recommend they start with something as high-stakes as coding/billing,” she said.
A version of this article appeared on Medscape.com.
When OpenAI released ChatGPT-3 publicly last November, some doctors decided to try out the free AI tool that learns language and writes human-like text. Some physicians found the chatbot made mistakes and stopped using it, while others were happy with the results and plan to use it more often.
“We’ve played around with it. It was very early on in AI and we noticed it gave us incorrect information with regards to clinical guidance,” said Monalisa Tailor, MD, an internal medicine physician at Norton Health Care in Louisville, Ky. “We decided not to pursue it further,” she said.
Orthopedic spine surgeon Daniel Choi, MD, who owns a small medical/surgical practice in Long Island, New York, tested the chatbot’s performance with a few administrative tasks, including writing a job listing for an administrator and prior authorization letters.
He was enthusiastic. “A well-polished job posting that would usually take me 2-3 hours to write was done in 5 minutes,” Dr. Choi said. “I was blown away by the writing – it was much better than anything I could write.”
The chatbot can also automate administrative tasks in doctors’ practices from appointment scheduling and billing to clinical documentation, saving doctors time and money, experts say.
Most physicians are proceeding cautiously. About 10% of more than 500 medical group leaders, responding to a March poll by the Medical Group Management Association, said their practices regularly use AI tools.
More than half of the respondents not using AI said they first want more evidence that the technology works as intended.
“None of them work as advertised,” said one respondent.
MGMA practice management consultant Dawn Plested acknowledges that many of the physician practices she’s worked with are still wary. “I have yet to encounter a practice that is using any AI tool, even something as low-risk as appointment scheduling,” she said.
Physician groups may be concerned about the costs and logistics of integrating ChatGPT with their electronic health record systems (EHRs) and how that would work, said Ms. Plested.
Doctors may also be skeptical of AI based on their experience with EHRs, she said.
“They were promoted as a panacea to many problems; they were supposed to automate business practice, reduce staff and clinician’s work, and improve billing/coding/documentation. Unfortunately, they have become a major source of frustration for doctors,” said Ms. Plested.
Drawing the line at patient care
Patients are worried about their doctors relying on AI for their care, according to a Pew Research Center poll released in February. About 60% of U.S. adults say they would feel uncomfortable if their own health care professional relied on artificial intelligence to do things like diagnose disease and recommend treatments; about 40% say they would feel comfortable with this.
“We have not yet gone into using ChatGPT for clinical purposes and will be very cautious with these types of applications due to concerns about inaccuracies,” Dr. Choi said.
Practice leaders reported in the MGMA poll that the most common uses of AI were nonclinical, such as:
- Patient communications, including call center answering service to help triage calls, to sort/distribute incoming fax messages, and outreach such as appointment reminders and marketing materials.
- Capturing clinical documentation, often with natural language processing or speech recognition platforms to help virtually scribe.
- Improving billing operations and predictive analytics.
Some doctors told The New York Times that ChatGPT helped them communicate with patients in a more compassionate way.
They used chatbots “to find words to break bad news and express concerns about a patient’s suffering, or to just more clearly explain medical recommendations,” the story noted.
Is regulation needed?
Some legal scholars and medical groups say that AI should be regulated to protect patients and doctors from risks, including medical errors, that could harm patients.
“It’s very important to evaluate the accuracy, safety, and privacy of language learning models (LLMs) before integrating them into the medical system. The same should be true of any new medical tool,” said Mason Marks, MD, JD, a health law professor at the Florida State University College of Law in Tallahassee.
In mid-June, the American Medical Association approved two resolutions calling for greater government oversight of AI. The AMA will develop proposed state and federal regulations and work with the federal government and other organizations to protect patients from false or misleading AI-generated medical advice.
Dr. Marks pointed to existing federal rules that apply to AI. “The Federal Trade Commission already has regulation that can potentially be used to combat unfair or deceptive trade practices associated with chatbots,” he said.
In addition, “the U.S. Food and Drug Administration can also regulate these tools, but it needs to update how it approaches risk when it comes to AI. The FDA has an outdated view of risk as physical harm, for instance, from traditional medical devices. That view of risk needs to be updated and expanded to encompass the unique harms of AI,” Dr. Marks said.
There should also be more transparency about how LLM software is used in medicine, he said. “That could be a norm implemented by the LLM developers and it could also be enforced by federal agencies. For instance, the FDA could require developers to be more transparent regarding training data and methods, and the FTC could require greater transparency regarding how consumer data might be used and opportunities to opt out of certain uses,” said Dr. Marks.
What should doctors do?
Dr. Marks advised doctors to be cautious when using ChatGPT and other LLMs, especially for medical advice. “The same would apply to any new medical tool, but we know that the current generation of LLMs [is] particularly prone to making things up, which could lead to medical errors if relied on in clinical settings,” he said.
There is also potential for breaches of patient confidentiality if doctors input clinical information. ChatGPT and OpenAI-enabled tools may not be compliant with the Health Insurance Portability and Accountability Act, which set national standards to protect individuals’ medical records and individually identifiable health information.
“The best approach is to use chatbots cautiously and with skepticism. Don’t input patient information, confirm the accuracy of information produced, and don’t use them as replacements for professional judgment,” Dr. Marks recommended.
Ms. Plested suggested that doctors who want to experiment with AI start with a low-risk tool such as appointment reminders that could save staff time and money. “I never recommend they start with something as high-stakes as coding/billing,” she said.
A version of this article appeared on Medscape.com.
When OpenAI released ChatGPT-3 publicly last November, some doctors decided to try out the free AI tool that learns language and writes human-like text. Some physicians found the chatbot made mistakes and stopped using it, while others were happy with the results and plan to use it more often.
“We’ve played around with it. It was very early on in AI and we noticed it gave us incorrect information with regards to clinical guidance,” said Monalisa Tailor, MD, an internal medicine physician at Norton Health Care in Louisville, Ky. “We decided not to pursue it further,” she said.
Orthopedic spine surgeon Daniel Choi, MD, who owns a small medical/surgical practice in Long Island, New York, tested the chatbot’s performance with a few administrative tasks, including writing a job listing for an administrator and prior authorization letters.
He was enthusiastic. “A well-polished job posting that would usually take me 2-3 hours to write was done in 5 minutes,” Dr. Choi said. “I was blown away by the writing – it was much better than anything I could write.”
The chatbot can also automate administrative tasks in doctors’ practices from appointment scheduling and billing to clinical documentation, saving doctors time and money, experts say.
Most physicians are proceeding cautiously. About 10% of more than 500 medical group leaders, responding to a March poll by the Medical Group Management Association, said their practices regularly use AI tools.
More than half of the respondents not using AI said they first want more evidence that the technology works as intended.
“None of them work as advertised,” said one respondent.
MGMA practice management consultant Dawn Plested acknowledges that many of the physician practices she’s worked with are still wary. “I have yet to encounter a practice that is using any AI tool, even something as low-risk as appointment scheduling,” she said.
Physician groups may be concerned about the costs and logistics of integrating ChatGPT with their electronic health record systems (EHRs) and how that would work, said Ms. Plested.
Doctors may also be skeptical of AI based on their experience with EHRs, she said.
“They were promoted as a panacea to many problems; they were supposed to automate business practice, reduce staff and clinician’s work, and improve billing/coding/documentation. Unfortunately, they have become a major source of frustration for doctors,” said Ms. Plested.
Drawing the line at patient care
Patients are worried about their doctors relying on AI for their care, according to a Pew Research Center poll released in February. About 60% of U.S. adults say they would feel uncomfortable if their own health care professional relied on artificial intelligence to do things like diagnose disease and recommend treatments; about 40% say they would feel comfortable with this.
“We have not yet gone into using ChatGPT for clinical purposes and will be very cautious with these types of applications due to concerns about inaccuracies,” Dr. Choi said.
Practice leaders reported in the MGMA poll that the most common uses of AI were nonclinical, such as:
- Patient communications, including call center answering service to help triage calls, to sort/distribute incoming fax messages, and outreach such as appointment reminders and marketing materials.
- Capturing clinical documentation, often with natural language processing or speech recognition platforms to help virtually scribe.
- Improving billing operations and predictive analytics.
Some doctors told The New York Times that ChatGPT helped them communicate with patients in a more compassionate way.
They used chatbots “to find words to break bad news and express concerns about a patient’s suffering, or to just more clearly explain medical recommendations,” the story noted.
Is regulation needed?
Some legal scholars and medical groups say that AI should be regulated to protect patients and doctors from risks, including medical errors, that could harm patients.
“It’s very important to evaluate the accuracy, safety, and privacy of language learning models (LLMs) before integrating them into the medical system. The same should be true of any new medical tool,” said Mason Marks, MD, JD, a health law professor at the Florida State University College of Law in Tallahassee.
In mid-June, the American Medical Association approved two resolutions calling for greater government oversight of AI. The AMA will develop proposed state and federal regulations and work with the federal government and other organizations to protect patients from false or misleading AI-generated medical advice.
Dr. Marks pointed to existing federal rules that apply to AI. “The Federal Trade Commission already has regulation that can potentially be used to combat unfair or deceptive trade practices associated with chatbots,” he said.
In addition, “the U.S. Food and Drug Administration can also regulate these tools, but it needs to update how it approaches risk when it comes to AI. The FDA has an outdated view of risk as physical harm, for instance, from traditional medical devices. That view of risk needs to be updated and expanded to encompass the unique harms of AI,” Dr. Marks said.
There should also be more transparency about how LLM software is used in medicine, he said. “That could be a norm implemented by the LLM developers and it could also be enforced by federal agencies. For instance, the FDA could require developers to be more transparent regarding training data and methods, and the FTC could require greater transparency regarding how consumer data might be used and opportunities to opt out of certain uses,” said Dr. Marks.
What should doctors do?
Dr. Marks advised doctors to be cautious when using ChatGPT and other LLMs, especially for medical advice. “The same would apply to any new medical tool, but we know that the current generation of LLMs [is] particularly prone to making things up, which could lead to medical errors if relied on in clinical settings,” he said.
There is also potential for breaches of patient confidentiality if doctors input clinical information. ChatGPT and OpenAI-enabled tools may not be compliant with the Health Insurance Portability and Accountability Act, which set national standards to protect individuals’ medical records and individually identifiable health information.
“The best approach is to use chatbots cautiously and with skepticism. Don’t input patient information, confirm the accuracy of information produced, and don’t use them as replacements for professional judgment,” Dr. Marks recommended.
Ms. Plested suggested that doctors who want to experiment with AI start with a low-risk tool such as appointment reminders that could save staff time and money. “I never recommend they start with something as high-stakes as coding/billing,” she said.
A version of this article appeared on Medscape.com.