User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
New JAK inhibitor study data confirm benefit in alopecia areata
from clinical trials of two drugs presented at a late-breaker research session at the annual meeting of the American Academy of Dermatology.
Based on phase 3 studies that document robust hair growth in about one third of patients, deuruxolitinib (CTP-543), an inhibitor of the JAK1 and JAK2 enzymes, has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received U.S. approval almost 1 year ago.
In his talk on THRIVE-AA2, a phase 3 trial of the investigational medicine deuruxolitinib, the principal investigator, Brett A. King, MD, PhD, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.”
THRIVE-AA2 was the second of two phase 3 studies of deuruxolitinib. King was a principal investigator for both pivotal trials, called THRIVE-AA1 and THRIVE AA-2. He characterized the results of the two THRIVE trials as “comparable.”
Dr. King also was a principal investigator for the trials with baricitinib, called BRAVE-AA1 and BRAVE AA-2, which were published last year in the New England Journal of Medicine. The trials for both drugs had similar designs and endpoints.
Deuruxolitinib and the THRIVE studies
In the THRIVE-AA2 trial, 517 adult patients were enrolled with moderate to severe alopecia areata, defined as a SALT (Severity of Alopecia Tool) score of ≥ 50%, which signifies a hair loss of at least 50%. Like THRIVE-AA1, patients participated at treatment centers in North America and Europe. About two-thirds were female. The mean age was 39 years. The majority of patients had complete or near complete hair loss at baseline.
“Many of these patients are the ones we have historically characterized as having alopecia totalis or universalis,” Dr. King said.
Participating patients were randomly assigned to 8 mg deuruxolitinib twice daily, 12 mg deuruxolitinib twice daily, or placebo. The primary endpoint was a SALT score of ≤ 20% at week 24.
At 24 weeks, almost no patients in the placebo group (1%) vs. 33% and 38% in the 8 mg and 12 mg twice-daily groups, respectively, met the primary endpoint. Each active treatment group was highly significant vs. placebo.
Of the responders, the majority achieved complete or near complete hair growth as defined by a SALT score of ≤ 10%, Dr. King reported.
Based on a graph that showed a relatively steep climb over the entire 24-week study period, deuruxolitinib “had a really fast onset of action,” Dr. King said. By week 8, which was the time of the first assessment, both doses of deuruxolitinib were superior to placebo.
The majority of patients had complete or significant loss of eyebrows and eye lashes at baseline, but more than two-thirds of these patients had regrowth by week 24, Dr. King said. Again, no significant regrowth was observed in the placebo arm.
On the Satisfaction of Hair Patient Reported Outcomes (SPRO), more than half of patients on both doses reported being satisfied or very satisfied with the improvement when evaluated at 24 weeks.
“The patient satisfaction overshot what one would expect by looking at the SALT scores, but a lot of subjects were at the precipice of the primary endpoint, sitting on SALT scores of 21, 25, or 30,” Dr. King said.
High participation in extension trial
More than 90% of the patients assigned to deuruxolitinib completed the trial and have entered an open-label extension (OLE). Dr. King credited the substantial rates of hair growth and the low rate of significant adverse events for the high rate of transition to OLE. Those who experienced the response were motivated to maintain it.
“This is a devastating disease. Patients want to get better,” Dr. King said.
There were no serious treatment-emergent adverse events associated with deuruxolitinib, including no thromboembolic events or other off-target events that have been reported previously with other JAK inhibitors in other disease states, such as rheumatoid arthritis. Although some adverse events, such as nasopharyngitis, were observed more often in those taking deuruxolitinib than placebo, there were “very few” discontinuations because of an adverse event, he said.
The data of THRIVE-AA2 are wholly compatible with the previously reported 706-patient THRIVE-AA1, according to Dr. King. In THRIVE-AA1, the primary endpoint of SALT ≤ 20% was reached by 29.6%, 41.5%, and 0.8% of the 8 mg, 12 mg, and placebo groups, respectively. Patient satisfaction scores, safety, and tolerability were also similar, according to Dr. King.
The experience with deuruxolitinib in the THRIVE-AA phase 3 program is similar to the experience with baricitinib in the BRAVE-AA trials. Although they cannot be compared directly because of potential differences between study populations, the 4-mg dose of baricitinib also achieved SALT score ≤ 20 in about 35% of patients, he said. The proportion was lower in the 2-mg group but was also superior to the placebo group.
“JAK inhibitors are changing the paradigm of alopecia areata,” Dr. King said. Responding to a question about payers reluctant to reimburse therapies for a “cosmetic” condition, Dr. King added that the effective treatments are “changing the landscape of how we think about this disease.” Dr. King believes these kinds of data show that “we are literally transforming lives forever.”
Baricitinib and the BRAVE studies
When baricitinib received regulatory approval for alopecia areata last year, it was not just the first JAK inhibitor approved for this disease, but the first systemic therapy of any kind, according to Maryanne Senna, MD, an assistant professor of dermatology at Harvard Medical School, Boston, and the director of the Lahey Hair Loss Center of Excellence, Burlington, Mass. Dr. Senna was a clinical investigator of BRAVE-AA1, as well as of THRIVE-AA2.
Providing an update on the BRAVE-AA program, Dr. Senna reported 104-week data that appear to support the idea of a life-changing benefit from JAK inhibitor therapy. This is because the effects appear durable.
In the data she presented at the AAD, responders and mixed responders at 52 weeks were followed to 104 weeks. Mixed responders were defined as those without a SALT response of ≤ 20 at week 52 but who had achieved this degree of hair regrowth at some earlier point.
Of the responders, 90% maintained their response at 104 weeks. In addition, many of the mixed responders and patients with a partial response but who never achieved a SALT score ≤ 20% gained additional hair growth, including complete or near complete hair growth, when maintained on treatment over the 2 years of follow-up.
“The follow-up suggests that, if you keep patients on treatment, you can get many of them to a meaningful response,” she said.
Meanwhile, “there have been no new safety signals,” Dr. Senna said. She based this statement not only of the 104-week data but on follow-up of up to 3.6 years among patients who have remained on treatment after participating in previous studies.
According to Dr. Senna, the off-target events that have been reported previously in other diseases with other JAK inhibitors, such as major adverse cardiovascular events and thromboembolic events, have not so far been observed in the BRAVE-AA phase 3 program.
Baricitinib, much like all but one of the JAK inhibitors with dermatologic indications, carries a black box warning that lists multiple risks for drugs in this class, based on a rheumatoid arthritis study.
The Food and Drug Administration has granted deuruxolitinib Breakthrough Therapy designation for the treatment of adult patients with moderate to severe alopecia areata and Fast Track designation for the treatment of alopecia areata, according to its manufacturer Concert Pharmaceuticals.
Dr. King reports financial relationships with more than 15 pharmaceutical companies, including Concert Pharmaceuticals, which provided the funding for the THRIVE-AA trial program, and for Eli Lilly, which provided funding for the BRAVE-AA trial program. Dr. Senna reports financial relationships with Arena pharmaceuticals, Follica, and both Concert Pharmaceuticals and Eli Lilly.
A version of this article originally appeared on Medscape.com.
from clinical trials of two drugs presented at a late-breaker research session at the annual meeting of the American Academy of Dermatology.
Based on phase 3 studies that document robust hair growth in about one third of patients, deuruxolitinib (CTP-543), an inhibitor of the JAK1 and JAK2 enzymes, has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received U.S. approval almost 1 year ago.
In his talk on THRIVE-AA2, a phase 3 trial of the investigational medicine deuruxolitinib, the principal investigator, Brett A. King, MD, PhD, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.”
THRIVE-AA2 was the second of two phase 3 studies of deuruxolitinib. King was a principal investigator for both pivotal trials, called THRIVE-AA1 and THRIVE AA-2. He characterized the results of the two THRIVE trials as “comparable.”
Dr. King also was a principal investigator for the trials with baricitinib, called BRAVE-AA1 and BRAVE AA-2, which were published last year in the New England Journal of Medicine. The trials for both drugs had similar designs and endpoints.
Deuruxolitinib and the THRIVE studies
In the THRIVE-AA2 trial, 517 adult patients were enrolled with moderate to severe alopecia areata, defined as a SALT (Severity of Alopecia Tool) score of ≥ 50%, which signifies a hair loss of at least 50%. Like THRIVE-AA1, patients participated at treatment centers in North America and Europe. About two-thirds were female. The mean age was 39 years. The majority of patients had complete or near complete hair loss at baseline.
“Many of these patients are the ones we have historically characterized as having alopecia totalis or universalis,” Dr. King said.
Participating patients were randomly assigned to 8 mg deuruxolitinib twice daily, 12 mg deuruxolitinib twice daily, or placebo. The primary endpoint was a SALT score of ≤ 20% at week 24.
At 24 weeks, almost no patients in the placebo group (1%) vs. 33% and 38% in the 8 mg and 12 mg twice-daily groups, respectively, met the primary endpoint. Each active treatment group was highly significant vs. placebo.
Of the responders, the majority achieved complete or near complete hair growth as defined by a SALT score of ≤ 10%, Dr. King reported.
Based on a graph that showed a relatively steep climb over the entire 24-week study period, deuruxolitinib “had a really fast onset of action,” Dr. King said. By week 8, which was the time of the first assessment, both doses of deuruxolitinib were superior to placebo.
The majority of patients had complete or significant loss of eyebrows and eye lashes at baseline, but more than two-thirds of these patients had regrowth by week 24, Dr. King said. Again, no significant regrowth was observed in the placebo arm.
On the Satisfaction of Hair Patient Reported Outcomes (SPRO), more than half of patients on both doses reported being satisfied or very satisfied with the improvement when evaluated at 24 weeks.
“The patient satisfaction overshot what one would expect by looking at the SALT scores, but a lot of subjects were at the precipice of the primary endpoint, sitting on SALT scores of 21, 25, or 30,” Dr. King said.
High participation in extension trial
More than 90% of the patients assigned to deuruxolitinib completed the trial and have entered an open-label extension (OLE). Dr. King credited the substantial rates of hair growth and the low rate of significant adverse events for the high rate of transition to OLE. Those who experienced the response were motivated to maintain it.
“This is a devastating disease. Patients want to get better,” Dr. King said.
There were no serious treatment-emergent adverse events associated with deuruxolitinib, including no thromboembolic events or other off-target events that have been reported previously with other JAK inhibitors in other disease states, such as rheumatoid arthritis. Although some adverse events, such as nasopharyngitis, were observed more often in those taking deuruxolitinib than placebo, there were “very few” discontinuations because of an adverse event, he said.
The data of THRIVE-AA2 are wholly compatible with the previously reported 706-patient THRIVE-AA1, according to Dr. King. In THRIVE-AA1, the primary endpoint of SALT ≤ 20% was reached by 29.6%, 41.5%, and 0.8% of the 8 mg, 12 mg, and placebo groups, respectively. Patient satisfaction scores, safety, and tolerability were also similar, according to Dr. King.
The experience with deuruxolitinib in the THRIVE-AA phase 3 program is similar to the experience with baricitinib in the BRAVE-AA trials. Although they cannot be compared directly because of potential differences between study populations, the 4-mg dose of baricitinib also achieved SALT score ≤ 20 in about 35% of patients, he said. The proportion was lower in the 2-mg group but was also superior to the placebo group.
“JAK inhibitors are changing the paradigm of alopecia areata,” Dr. King said. Responding to a question about payers reluctant to reimburse therapies for a “cosmetic” condition, Dr. King added that the effective treatments are “changing the landscape of how we think about this disease.” Dr. King believes these kinds of data show that “we are literally transforming lives forever.”
Baricitinib and the BRAVE studies
When baricitinib received regulatory approval for alopecia areata last year, it was not just the first JAK inhibitor approved for this disease, but the first systemic therapy of any kind, according to Maryanne Senna, MD, an assistant professor of dermatology at Harvard Medical School, Boston, and the director of the Lahey Hair Loss Center of Excellence, Burlington, Mass. Dr. Senna was a clinical investigator of BRAVE-AA1, as well as of THRIVE-AA2.
Providing an update on the BRAVE-AA program, Dr. Senna reported 104-week data that appear to support the idea of a life-changing benefit from JAK inhibitor therapy. This is because the effects appear durable.
In the data she presented at the AAD, responders and mixed responders at 52 weeks were followed to 104 weeks. Mixed responders were defined as those without a SALT response of ≤ 20 at week 52 but who had achieved this degree of hair regrowth at some earlier point.
Of the responders, 90% maintained their response at 104 weeks. In addition, many of the mixed responders and patients with a partial response but who never achieved a SALT score ≤ 20% gained additional hair growth, including complete or near complete hair growth, when maintained on treatment over the 2 years of follow-up.
“The follow-up suggests that, if you keep patients on treatment, you can get many of them to a meaningful response,” she said.
Meanwhile, “there have been no new safety signals,” Dr. Senna said. She based this statement not only of the 104-week data but on follow-up of up to 3.6 years among patients who have remained on treatment after participating in previous studies.
According to Dr. Senna, the off-target events that have been reported previously in other diseases with other JAK inhibitors, such as major adverse cardiovascular events and thromboembolic events, have not so far been observed in the BRAVE-AA phase 3 program.
Baricitinib, much like all but one of the JAK inhibitors with dermatologic indications, carries a black box warning that lists multiple risks for drugs in this class, based on a rheumatoid arthritis study.
The Food and Drug Administration has granted deuruxolitinib Breakthrough Therapy designation for the treatment of adult patients with moderate to severe alopecia areata and Fast Track designation for the treatment of alopecia areata, according to its manufacturer Concert Pharmaceuticals.
Dr. King reports financial relationships with more than 15 pharmaceutical companies, including Concert Pharmaceuticals, which provided the funding for the THRIVE-AA trial program, and for Eli Lilly, which provided funding for the BRAVE-AA trial program. Dr. Senna reports financial relationships with Arena pharmaceuticals, Follica, and both Concert Pharmaceuticals and Eli Lilly.
A version of this article originally appeared on Medscape.com.
from clinical trials of two drugs presented at a late-breaker research session at the annual meeting of the American Academy of Dermatology.
Based on phase 3 studies that document robust hair growth in about one third of patients, deuruxolitinib (CTP-543), an inhibitor of the JAK1 and JAK2 enzymes, has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received U.S. approval almost 1 year ago.
In his talk on THRIVE-AA2, a phase 3 trial of the investigational medicine deuruxolitinib, the principal investigator, Brett A. King, MD, PhD, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.”
THRIVE-AA2 was the second of two phase 3 studies of deuruxolitinib. King was a principal investigator for both pivotal trials, called THRIVE-AA1 and THRIVE AA-2. He characterized the results of the two THRIVE trials as “comparable.”
Dr. King also was a principal investigator for the trials with baricitinib, called BRAVE-AA1 and BRAVE AA-2, which were published last year in the New England Journal of Medicine. The trials for both drugs had similar designs and endpoints.
Deuruxolitinib and the THRIVE studies
In the THRIVE-AA2 trial, 517 adult patients were enrolled with moderate to severe alopecia areata, defined as a SALT (Severity of Alopecia Tool) score of ≥ 50%, which signifies a hair loss of at least 50%. Like THRIVE-AA1, patients participated at treatment centers in North America and Europe. About two-thirds were female. The mean age was 39 years. The majority of patients had complete or near complete hair loss at baseline.
“Many of these patients are the ones we have historically characterized as having alopecia totalis or universalis,” Dr. King said.
Participating patients were randomly assigned to 8 mg deuruxolitinib twice daily, 12 mg deuruxolitinib twice daily, or placebo. The primary endpoint was a SALT score of ≤ 20% at week 24.
At 24 weeks, almost no patients in the placebo group (1%) vs. 33% and 38% in the 8 mg and 12 mg twice-daily groups, respectively, met the primary endpoint. Each active treatment group was highly significant vs. placebo.
Of the responders, the majority achieved complete or near complete hair growth as defined by a SALT score of ≤ 10%, Dr. King reported.
Based on a graph that showed a relatively steep climb over the entire 24-week study period, deuruxolitinib “had a really fast onset of action,” Dr. King said. By week 8, which was the time of the first assessment, both doses of deuruxolitinib were superior to placebo.
The majority of patients had complete or significant loss of eyebrows and eye lashes at baseline, but more than two-thirds of these patients had regrowth by week 24, Dr. King said. Again, no significant regrowth was observed in the placebo arm.
On the Satisfaction of Hair Patient Reported Outcomes (SPRO), more than half of patients on both doses reported being satisfied or very satisfied with the improvement when evaluated at 24 weeks.
“The patient satisfaction overshot what one would expect by looking at the SALT scores, but a lot of subjects were at the precipice of the primary endpoint, sitting on SALT scores of 21, 25, or 30,” Dr. King said.
High participation in extension trial
More than 90% of the patients assigned to deuruxolitinib completed the trial and have entered an open-label extension (OLE). Dr. King credited the substantial rates of hair growth and the low rate of significant adverse events for the high rate of transition to OLE. Those who experienced the response were motivated to maintain it.
“This is a devastating disease. Patients want to get better,” Dr. King said.
There were no serious treatment-emergent adverse events associated with deuruxolitinib, including no thromboembolic events or other off-target events that have been reported previously with other JAK inhibitors in other disease states, such as rheumatoid arthritis. Although some adverse events, such as nasopharyngitis, were observed more often in those taking deuruxolitinib than placebo, there were “very few” discontinuations because of an adverse event, he said.
The data of THRIVE-AA2 are wholly compatible with the previously reported 706-patient THRIVE-AA1, according to Dr. King. In THRIVE-AA1, the primary endpoint of SALT ≤ 20% was reached by 29.6%, 41.5%, and 0.8% of the 8 mg, 12 mg, and placebo groups, respectively. Patient satisfaction scores, safety, and tolerability were also similar, according to Dr. King.
The experience with deuruxolitinib in the THRIVE-AA phase 3 program is similar to the experience with baricitinib in the BRAVE-AA trials. Although they cannot be compared directly because of potential differences between study populations, the 4-mg dose of baricitinib also achieved SALT score ≤ 20 in about 35% of patients, he said. The proportion was lower in the 2-mg group but was also superior to the placebo group.
“JAK inhibitors are changing the paradigm of alopecia areata,” Dr. King said. Responding to a question about payers reluctant to reimburse therapies for a “cosmetic” condition, Dr. King added that the effective treatments are “changing the landscape of how we think about this disease.” Dr. King believes these kinds of data show that “we are literally transforming lives forever.”
Baricitinib and the BRAVE studies
When baricitinib received regulatory approval for alopecia areata last year, it was not just the first JAK inhibitor approved for this disease, but the first systemic therapy of any kind, according to Maryanne Senna, MD, an assistant professor of dermatology at Harvard Medical School, Boston, and the director of the Lahey Hair Loss Center of Excellence, Burlington, Mass. Dr. Senna was a clinical investigator of BRAVE-AA1, as well as of THRIVE-AA2.
Providing an update on the BRAVE-AA program, Dr. Senna reported 104-week data that appear to support the idea of a life-changing benefit from JAK inhibitor therapy. This is because the effects appear durable.
In the data she presented at the AAD, responders and mixed responders at 52 weeks were followed to 104 weeks. Mixed responders were defined as those without a SALT response of ≤ 20 at week 52 but who had achieved this degree of hair regrowth at some earlier point.
Of the responders, 90% maintained their response at 104 weeks. In addition, many of the mixed responders and patients with a partial response but who never achieved a SALT score ≤ 20% gained additional hair growth, including complete or near complete hair growth, when maintained on treatment over the 2 years of follow-up.
“The follow-up suggests that, if you keep patients on treatment, you can get many of them to a meaningful response,” she said.
Meanwhile, “there have been no new safety signals,” Dr. Senna said. She based this statement not only of the 104-week data but on follow-up of up to 3.6 years among patients who have remained on treatment after participating in previous studies.
According to Dr. Senna, the off-target events that have been reported previously in other diseases with other JAK inhibitors, such as major adverse cardiovascular events and thromboembolic events, have not so far been observed in the BRAVE-AA phase 3 program.
Baricitinib, much like all but one of the JAK inhibitors with dermatologic indications, carries a black box warning that lists multiple risks for drugs in this class, based on a rheumatoid arthritis study.
The Food and Drug Administration has granted deuruxolitinib Breakthrough Therapy designation for the treatment of adult patients with moderate to severe alopecia areata and Fast Track designation for the treatment of alopecia areata, according to its manufacturer Concert Pharmaceuticals.
Dr. King reports financial relationships with more than 15 pharmaceutical companies, including Concert Pharmaceuticals, which provided the funding for the THRIVE-AA trial program, and for Eli Lilly, which provided funding for the BRAVE-AA trial program. Dr. Senna reports financial relationships with Arena pharmaceuticals, Follica, and both Concert Pharmaceuticals and Eli Lilly.
A version of this article originally appeared on Medscape.com.
AT AAD 2023
Pilot study evaluates sensitive skin burden in persons of color
NEW ORLEANS – .
Respondents also reported high rates of reactions to skin care products marketed for sensitive skin, and most said they had visited a dermatologist about their condition.
Those are among the key findings of a pilot study designed to assess the prevalence, symptom burden, and behaviors of self-identified persons of color with sensitive skin, which senior author Adam Friedman, MD, and colleagues defined as a subjective syndrome of cutaneous hyperreactivity to otherwise innocuous stimuli. “Improved understanding of sensitive skin is essential, and we encourage additional research into pathophysiology and creating a consensus definition for sensitive skin,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. The findings were also reported online in JAAD International.
In May of 2022, Dr. Friedman, first author Erika McCormick, a 4th-year medical student at George Washington University, and colleagues invited individuals attending a community health fair in an undeserved area of Washington, to complete the Sensitive Scale-10 (SS-10) and to answer other questions after receiving a brief education about sensitive skin. Of the 58 respondents, 78% were female, and 86% self-identified as a person of color.
“Our study population predominantly self-identified as Black, which only represents one piece of those who would be characterized as persons of color,” Dr. Friedman said. “That said, improved representation of both our study population, and furthermore persons of color, in all aspects of dermatology research is crucial to at a minimum ensure generalizability of findings to the U.S. population, and research on sensitive skin is but one component of this.”
Nearly two-thirds of all respondents (63.8%) reported having an underlying skin condition, most commonly acne (21%), eczema (17%), and rosacea (6%). More than half (57%) reported sensitive skin, 27% of whom reported no other skin disease. Individuals with sensitive skin had higher mean SS-10 scores, compared with those with nonsensitive skin (14.61 vs. 4.32; P = .002) and burning was the main symptom among those with sensitive skin (56%), followed by itch (50%), redness (39%), dryness (39%) and pain (17%).
Compared with those who did not meet criteria for sensitive skin, those who did were more likely to report a personal history of allergy (56.25% vs. 8.33%; P = .0002) and were nearly seven times more likely to have seen a dermatologist about their concerns (odds ratio, 6.857; P = .0012).
In other findings limited to respondents with sensitive skin, 72% who reported reactions to general consumer skin care products also reported reacting to products marketed for sensitive skin, and 94% reported reactivity to at least one trigger, most commonly extreme temperatures (34%), stress (34%), sweat (33%), sun exposure (29%), and diet (28%). “We were particularly surprised by the high rates of reactivity to skin care products designed for and marketed to those suffering with sensitive skin,” Ms. McCormick told this news organization. “Importantly, there is currently no federal or legal standard regulating ingredients in products marketed for sensitive skin, and many products lack testing in sensitive skin specifically. Our data suggest an opportunity for improvement of sensitive skin care.”
She acknowledged certain limitations of the study, including its small sample size. “Reconducting this survey in a larger population will help validate our findings,” she said.
The research was supported by two independent research grants from Galderma: one supporting Ms. McCormick with a Sensitive Skin Research Fellowship and the other a Sensitive Skin Research Acceleration Fund. Dr. Friedman reported having no relevant disclosures.
NEW ORLEANS – .
Respondents also reported high rates of reactions to skin care products marketed for sensitive skin, and most said they had visited a dermatologist about their condition.
Those are among the key findings of a pilot study designed to assess the prevalence, symptom burden, and behaviors of self-identified persons of color with sensitive skin, which senior author Adam Friedman, MD, and colleagues defined as a subjective syndrome of cutaneous hyperreactivity to otherwise innocuous stimuli. “Improved understanding of sensitive skin is essential, and we encourage additional research into pathophysiology and creating a consensus definition for sensitive skin,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. The findings were also reported online in JAAD International.
In May of 2022, Dr. Friedman, first author Erika McCormick, a 4th-year medical student at George Washington University, and colleagues invited individuals attending a community health fair in an undeserved area of Washington, to complete the Sensitive Scale-10 (SS-10) and to answer other questions after receiving a brief education about sensitive skin. Of the 58 respondents, 78% were female, and 86% self-identified as a person of color.
“Our study population predominantly self-identified as Black, which only represents one piece of those who would be characterized as persons of color,” Dr. Friedman said. “That said, improved representation of both our study population, and furthermore persons of color, in all aspects of dermatology research is crucial to at a minimum ensure generalizability of findings to the U.S. population, and research on sensitive skin is but one component of this.”
Nearly two-thirds of all respondents (63.8%) reported having an underlying skin condition, most commonly acne (21%), eczema (17%), and rosacea (6%). More than half (57%) reported sensitive skin, 27% of whom reported no other skin disease. Individuals with sensitive skin had higher mean SS-10 scores, compared with those with nonsensitive skin (14.61 vs. 4.32; P = .002) and burning was the main symptom among those with sensitive skin (56%), followed by itch (50%), redness (39%), dryness (39%) and pain (17%).
Compared with those who did not meet criteria for sensitive skin, those who did were more likely to report a personal history of allergy (56.25% vs. 8.33%; P = .0002) and were nearly seven times more likely to have seen a dermatologist about their concerns (odds ratio, 6.857; P = .0012).
In other findings limited to respondents with sensitive skin, 72% who reported reactions to general consumer skin care products also reported reacting to products marketed for sensitive skin, and 94% reported reactivity to at least one trigger, most commonly extreme temperatures (34%), stress (34%), sweat (33%), sun exposure (29%), and diet (28%). “We were particularly surprised by the high rates of reactivity to skin care products designed for and marketed to those suffering with sensitive skin,” Ms. McCormick told this news organization. “Importantly, there is currently no federal or legal standard regulating ingredients in products marketed for sensitive skin, and many products lack testing in sensitive skin specifically. Our data suggest an opportunity for improvement of sensitive skin care.”
She acknowledged certain limitations of the study, including its small sample size. “Reconducting this survey in a larger population will help validate our findings,” she said.
The research was supported by two independent research grants from Galderma: one supporting Ms. McCormick with a Sensitive Skin Research Fellowship and the other a Sensitive Skin Research Acceleration Fund. Dr. Friedman reported having no relevant disclosures.
NEW ORLEANS – .
Respondents also reported high rates of reactions to skin care products marketed for sensitive skin, and most said they had visited a dermatologist about their condition.
Those are among the key findings of a pilot study designed to assess the prevalence, symptom burden, and behaviors of self-identified persons of color with sensitive skin, which senior author Adam Friedman, MD, and colleagues defined as a subjective syndrome of cutaneous hyperreactivity to otherwise innocuous stimuli. “Improved understanding of sensitive skin is essential, and we encourage additional research into pathophysiology and creating a consensus definition for sensitive skin,” Dr. Friedman, professor and chair of dermatology at George Washington University, Washington, said in an interview in advance of the annual meeting of the American Academy of Dermatology, where the study was presented during an e-poster session. The findings were also reported online in JAAD International.
In May of 2022, Dr. Friedman, first author Erika McCormick, a 4th-year medical student at George Washington University, and colleagues invited individuals attending a community health fair in an undeserved area of Washington, to complete the Sensitive Scale-10 (SS-10) and to answer other questions after receiving a brief education about sensitive skin. Of the 58 respondents, 78% were female, and 86% self-identified as a person of color.
“Our study population predominantly self-identified as Black, which only represents one piece of those who would be characterized as persons of color,” Dr. Friedman said. “That said, improved representation of both our study population, and furthermore persons of color, in all aspects of dermatology research is crucial to at a minimum ensure generalizability of findings to the U.S. population, and research on sensitive skin is but one component of this.”
Nearly two-thirds of all respondents (63.8%) reported having an underlying skin condition, most commonly acne (21%), eczema (17%), and rosacea (6%). More than half (57%) reported sensitive skin, 27% of whom reported no other skin disease. Individuals with sensitive skin had higher mean SS-10 scores, compared with those with nonsensitive skin (14.61 vs. 4.32; P = .002) and burning was the main symptom among those with sensitive skin (56%), followed by itch (50%), redness (39%), dryness (39%) and pain (17%).
Compared with those who did not meet criteria for sensitive skin, those who did were more likely to report a personal history of allergy (56.25% vs. 8.33%; P = .0002) and were nearly seven times more likely to have seen a dermatologist about their concerns (odds ratio, 6.857; P = .0012).
In other findings limited to respondents with sensitive skin, 72% who reported reactions to general consumer skin care products also reported reacting to products marketed for sensitive skin, and 94% reported reactivity to at least one trigger, most commonly extreme temperatures (34%), stress (34%), sweat (33%), sun exposure (29%), and diet (28%). “We were particularly surprised by the high rates of reactivity to skin care products designed for and marketed to those suffering with sensitive skin,” Ms. McCormick told this news organization. “Importantly, there is currently no federal or legal standard regulating ingredients in products marketed for sensitive skin, and many products lack testing in sensitive skin specifically. Our data suggest an opportunity for improvement of sensitive skin care.”
She acknowledged certain limitations of the study, including its small sample size. “Reconducting this survey in a larger population will help validate our findings,” she said.
The research was supported by two independent research grants from Galderma: one supporting Ms. McCormick with a Sensitive Skin Research Fellowship and the other a Sensitive Skin Research Acceleration Fund. Dr. Friedman reported having no relevant disclosures.
AT AAD 2023
Expert shares her tips for diagnosing, treating onychomycosis
NEW ORLEANS – .
“The PAS [periodic acid-Schiff] stain is very popular because it can identify the presence or absence of fungal elements, but a fungal culture will identify the organism living in the nail,” Dr. Elewski, professor and chair of dermatology at the University of Alabama, Birmingham, said at the annual meeting of the American Academy of Dermatology. “You also could do a PCR to identify the organism, with or without a KOH or PAS stain. It is often helpful to know what organism is causing the infection.”
While waiting for lab results, there are three clinical clues to look for – the first being that an infection likely resides in the toenail. “You almost never see dermatophyte onychomycosis in the fingernails without it being in the toenails, too,” Dr. Elewski said.
The presence of tinea pedis is a second clinical clue. “Sometimes it’s subtle, so I will ask the patient, ‘Have you been treating yourself for athlete’s foot?’ If they say ‘no, I’ve never had it,’ put down on your list that it’s unlikely they have onychomycosis. How is the fungus going to jump from the floor into the nail without taking a little vacation on the bottom of the foot? It just isn’t going to happen.”
The presence of dermatophytoma is the third clinical clue. “These are dermatophyte abscesses encased in a biofilm, and they’re really hard to treat,” she said.
Treatments
Clinicians typically turn to one of three oral drugs for treating onychomycosis: terbinafine, itraconazole, and fluconazole, Dr. Elewski noted. Referring to terbinafine as “the gold standard,” she said that she typically writes a prescription for 90 250-mg pills. “When I give terbinafine, I often do baseline liver profiling, depending on the patient’s age, their state of health, their comorbidities, and other medications they’re taking,” she said. “If they’re 18 years old and otherwise healthy, I probably don’t.” While she generally prescribes 90 pills, she added, “keep in mind that 90 pills are not going to cure everybody. I see the patient 4 months later because the drug should stay in the nail for 30 days or more at therapeutic levels after you take that 90-day course.”
Another option is itraconazole, which can be taken at a dose of 200 mg a day for 12 weeks, or at a pulse dose, where patients take 400 mg every day for 1 week, 1 week a month, for 4 consecutive months. “I’ll often do a baseline liver profile with itraconazole, too,” Dr. Elewski said. “I don’t think you have to, but it makes sense if it’s feasible for you. Decide that based on each patient.”
Itraconazole can’t be given concomitantly with statins because of the potential for rhabdomyolysis. For patients taking statins, she consults with their physicians to make sure it’s safe to stop the statin a couple of days before and after their scheduled pulse dose of itraconazole. “This involves 1 week per month of taking itraconazole without the statin,” she said. “Or they could stop statins for the time you treat, if cleared by their doctor.”
As for fluconazole, Dr. Elewski usually prescribes 200 mg once or twice per week until the nail is normal. She offers patients the mnemonic for “Fungal Fridays” or Toesdays” as a way for them to remember which day to take the fluconazole.
According to data in the package inserts, rates of complete and mycologic cures are 38% and 70% for terbinafine, respectively, 14% and 54% for itraconazole, and 37% to 48% and 47% to 62% for fluconazole. “These cures are not 100% based on the standard course [of the drug],” Dr. Elewski noted. “I don’t use the standard course. I believe in treating to terminate. You want to kill the fungus.”
Resistant dermatophytes ‘are coming’
Halting treatment with an oral drug at a particular time point instead of when the nail is fungal-free likely contributes to resistant strains, she added, noting that she has at least two dozen patients in her practice with dermatophyte resistance documented in labs. “We need to be antifungal stewards, because resistant dermatophytes are coming to us,” she said. “They’re here already, and we don’t want it to be endemic in the U.S.”
In a published study from 2020, researchers from India enrolled 200 patients with relapsing tinea corporis, tinea cruris, and tinea faciei and allocated 50 each to treatment with either fluconazole, griseofulvin, itraconazole, or terbinafine. At week 4, all treatment arms had cure rates of less than 8%. At week 8, the cure rates were 42% for fluconazole, 16% for griseofulvin, 28% for terbinafine, and 66% for itraconazole.
Based in part on these study findings, Dr. Elewski said that she has become more aggressive in her therapeutic approach, including treating some of her patients on terbinafine for a minimum of 6 months. “If that’s not enough, I keep treating,” she said. “But, patients may not respond to terbinafine; we see resistance. So, itraconazole may be our best drug going forward for treating onychomycosis. You just have to watch out for side effects of itraconazole, mainly drug-drug interactions.”
Dr. Elewski reported having no relevant financial disclosures related to her presentation.
NEW ORLEANS – .
“The PAS [periodic acid-Schiff] stain is very popular because it can identify the presence or absence of fungal elements, but a fungal culture will identify the organism living in the nail,” Dr. Elewski, professor and chair of dermatology at the University of Alabama, Birmingham, said at the annual meeting of the American Academy of Dermatology. “You also could do a PCR to identify the organism, with or without a KOH or PAS stain. It is often helpful to know what organism is causing the infection.”
While waiting for lab results, there are three clinical clues to look for – the first being that an infection likely resides in the toenail. “You almost never see dermatophyte onychomycosis in the fingernails without it being in the toenails, too,” Dr. Elewski said.
The presence of tinea pedis is a second clinical clue. “Sometimes it’s subtle, so I will ask the patient, ‘Have you been treating yourself for athlete’s foot?’ If they say ‘no, I’ve never had it,’ put down on your list that it’s unlikely they have onychomycosis. How is the fungus going to jump from the floor into the nail without taking a little vacation on the bottom of the foot? It just isn’t going to happen.”
The presence of dermatophytoma is the third clinical clue. “These are dermatophyte abscesses encased in a biofilm, and they’re really hard to treat,” she said.
Treatments
Clinicians typically turn to one of three oral drugs for treating onychomycosis: terbinafine, itraconazole, and fluconazole, Dr. Elewski noted. Referring to terbinafine as “the gold standard,” she said that she typically writes a prescription for 90 250-mg pills. “When I give terbinafine, I often do baseline liver profiling, depending on the patient’s age, their state of health, their comorbidities, and other medications they’re taking,” she said. “If they’re 18 years old and otherwise healthy, I probably don’t.” While she generally prescribes 90 pills, she added, “keep in mind that 90 pills are not going to cure everybody. I see the patient 4 months later because the drug should stay in the nail for 30 days or more at therapeutic levels after you take that 90-day course.”
Another option is itraconazole, which can be taken at a dose of 200 mg a day for 12 weeks, or at a pulse dose, where patients take 400 mg every day for 1 week, 1 week a month, for 4 consecutive months. “I’ll often do a baseline liver profile with itraconazole, too,” Dr. Elewski said. “I don’t think you have to, but it makes sense if it’s feasible for you. Decide that based on each patient.”
Itraconazole can’t be given concomitantly with statins because of the potential for rhabdomyolysis. For patients taking statins, she consults with their physicians to make sure it’s safe to stop the statin a couple of days before and after their scheduled pulse dose of itraconazole. “This involves 1 week per month of taking itraconazole without the statin,” she said. “Or they could stop statins for the time you treat, if cleared by their doctor.”
As for fluconazole, Dr. Elewski usually prescribes 200 mg once or twice per week until the nail is normal. She offers patients the mnemonic for “Fungal Fridays” or Toesdays” as a way for them to remember which day to take the fluconazole.
According to data in the package inserts, rates of complete and mycologic cures are 38% and 70% for terbinafine, respectively, 14% and 54% for itraconazole, and 37% to 48% and 47% to 62% for fluconazole. “These cures are not 100% based on the standard course [of the drug],” Dr. Elewski noted. “I don’t use the standard course. I believe in treating to terminate. You want to kill the fungus.”
Resistant dermatophytes ‘are coming’
Halting treatment with an oral drug at a particular time point instead of when the nail is fungal-free likely contributes to resistant strains, she added, noting that she has at least two dozen patients in her practice with dermatophyte resistance documented in labs. “We need to be antifungal stewards, because resistant dermatophytes are coming to us,” she said. “They’re here already, and we don’t want it to be endemic in the U.S.”
In a published study from 2020, researchers from India enrolled 200 patients with relapsing tinea corporis, tinea cruris, and tinea faciei and allocated 50 each to treatment with either fluconazole, griseofulvin, itraconazole, or terbinafine. At week 4, all treatment arms had cure rates of less than 8%. At week 8, the cure rates were 42% for fluconazole, 16% for griseofulvin, 28% for terbinafine, and 66% for itraconazole.
Based in part on these study findings, Dr. Elewski said that she has become more aggressive in her therapeutic approach, including treating some of her patients on terbinafine for a minimum of 6 months. “If that’s not enough, I keep treating,” she said. “But, patients may not respond to terbinafine; we see resistance. So, itraconazole may be our best drug going forward for treating onychomycosis. You just have to watch out for side effects of itraconazole, mainly drug-drug interactions.”
Dr. Elewski reported having no relevant financial disclosures related to her presentation.
NEW ORLEANS – .
“The PAS [periodic acid-Schiff] stain is very popular because it can identify the presence or absence of fungal elements, but a fungal culture will identify the organism living in the nail,” Dr. Elewski, professor and chair of dermatology at the University of Alabama, Birmingham, said at the annual meeting of the American Academy of Dermatology. “You also could do a PCR to identify the organism, with or without a KOH or PAS stain. It is often helpful to know what organism is causing the infection.”
While waiting for lab results, there are three clinical clues to look for – the first being that an infection likely resides in the toenail. “You almost never see dermatophyte onychomycosis in the fingernails without it being in the toenails, too,” Dr. Elewski said.
The presence of tinea pedis is a second clinical clue. “Sometimes it’s subtle, so I will ask the patient, ‘Have you been treating yourself for athlete’s foot?’ If they say ‘no, I’ve never had it,’ put down on your list that it’s unlikely they have onychomycosis. How is the fungus going to jump from the floor into the nail without taking a little vacation on the bottom of the foot? It just isn’t going to happen.”
The presence of dermatophytoma is the third clinical clue. “These are dermatophyte abscesses encased in a biofilm, and they’re really hard to treat,” she said.
Treatments
Clinicians typically turn to one of three oral drugs for treating onychomycosis: terbinafine, itraconazole, and fluconazole, Dr. Elewski noted. Referring to terbinafine as “the gold standard,” she said that she typically writes a prescription for 90 250-mg pills. “When I give terbinafine, I often do baseline liver profiling, depending on the patient’s age, their state of health, their comorbidities, and other medications they’re taking,” she said. “If they’re 18 years old and otherwise healthy, I probably don’t.” While she generally prescribes 90 pills, she added, “keep in mind that 90 pills are not going to cure everybody. I see the patient 4 months later because the drug should stay in the nail for 30 days or more at therapeutic levels after you take that 90-day course.”
Another option is itraconazole, which can be taken at a dose of 200 mg a day for 12 weeks, or at a pulse dose, where patients take 400 mg every day for 1 week, 1 week a month, for 4 consecutive months. “I’ll often do a baseline liver profile with itraconazole, too,” Dr. Elewski said. “I don’t think you have to, but it makes sense if it’s feasible for you. Decide that based on each patient.”
Itraconazole can’t be given concomitantly with statins because of the potential for rhabdomyolysis. For patients taking statins, she consults with their physicians to make sure it’s safe to stop the statin a couple of days before and after their scheduled pulse dose of itraconazole. “This involves 1 week per month of taking itraconazole without the statin,” she said. “Or they could stop statins for the time you treat, if cleared by their doctor.”
As for fluconazole, Dr. Elewski usually prescribes 200 mg once or twice per week until the nail is normal. She offers patients the mnemonic for “Fungal Fridays” or Toesdays” as a way for them to remember which day to take the fluconazole.
According to data in the package inserts, rates of complete and mycologic cures are 38% and 70% for terbinafine, respectively, 14% and 54% for itraconazole, and 37% to 48% and 47% to 62% for fluconazole. “These cures are not 100% based on the standard course [of the drug],” Dr. Elewski noted. “I don’t use the standard course. I believe in treating to terminate. You want to kill the fungus.”
Resistant dermatophytes ‘are coming’
Halting treatment with an oral drug at a particular time point instead of when the nail is fungal-free likely contributes to resistant strains, she added, noting that she has at least two dozen patients in her practice with dermatophyte resistance documented in labs. “We need to be antifungal stewards, because resistant dermatophytes are coming to us,” she said. “They’re here already, and we don’t want it to be endemic in the U.S.”
In a published study from 2020, researchers from India enrolled 200 patients with relapsing tinea corporis, tinea cruris, and tinea faciei and allocated 50 each to treatment with either fluconazole, griseofulvin, itraconazole, or terbinafine. At week 4, all treatment arms had cure rates of less than 8%. At week 8, the cure rates were 42% for fluconazole, 16% for griseofulvin, 28% for terbinafine, and 66% for itraconazole.
Based in part on these study findings, Dr. Elewski said that she has become more aggressive in her therapeutic approach, including treating some of her patients on terbinafine for a minimum of 6 months. “If that’s not enough, I keep treating,” she said. “But, patients may not respond to terbinafine; we see resistance. So, itraconazole may be our best drug going forward for treating onychomycosis. You just have to watch out for side effects of itraconazole, mainly drug-drug interactions.”
Dr. Elewski reported having no relevant financial disclosures related to her presentation.
AT AAD 2023
FDA approves new Merkel cell carcinoma treatment
the agency announced.
This marks the first regulatory approval for the PD-1 inhibitor. The FDA granted accelerated approval for the drug on the basis of tumor response rate and duration of response from the POD1UM-201 trial. Drugmaker Incyte said that “continued approval of Zynyz for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
MCC is a rare and aggressive skin cancer with a high rate of metastatic disease and an estimated 5-year overall survival of just 14% among those who present with metastatic disease. Incidence is rapidly increasing in the United States, particularly among adults older than 65 years, Incyte noted.
“More than a third of patients with MCC present with regional or distant metastases, which are associated with high rates of mortality,” principal author Shailender Bhatia, MD, of the University of Washington and Fred Hutchinson Cancer Center, both in Seattle, said in a news release. “The approval of Zynyz offers health care providers another first-line treatment option against MCC that can result in durable responses in patients with metastatic disease.”
POD1UM-201 was an open-label, single-arm, phase 2 study that evaluated the agent in 65 systemic treatment–naive adults with metastatic or recurrent locally advanced MCC.
Overall, 52% of patients had an objective response rate. A complete response was observed in 12 patients (18%), and a partial response was observed in 22 patients (34%).
Duration of response ranged from 1.1 to 24.9 months; 76% of responders experienced responses of 6 months or longer, and 62% experienced responses of 12 months or longer.
Study participants received a 500-mg dose of retifanlimab every 4 weeks for up to 24 weeks or until disease progression or unacceptable toxicity. Serious adverse events occurred in 22% of patients and most often included fatigue, arrhythmia, and pneumonitis; 11% of patients discontinued treatment because of serious adverse events.
Retifanlimab may cause a severe or life-threatening immune response during treatment or after discontinuation. Patients should be advised to immediately report any new or worsening signs or symptoms to their health care provider. Side effects can also be reported to the FDA.
A version of this article first appeared on Medscape.com.
the agency announced.
This marks the first regulatory approval for the PD-1 inhibitor. The FDA granted accelerated approval for the drug on the basis of tumor response rate and duration of response from the POD1UM-201 trial. Drugmaker Incyte said that “continued approval of Zynyz for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
MCC is a rare and aggressive skin cancer with a high rate of metastatic disease and an estimated 5-year overall survival of just 14% among those who present with metastatic disease. Incidence is rapidly increasing in the United States, particularly among adults older than 65 years, Incyte noted.
“More than a third of patients with MCC present with regional or distant metastases, which are associated with high rates of mortality,” principal author Shailender Bhatia, MD, of the University of Washington and Fred Hutchinson Cancer Center, both in Seattle, said in a news release. “The approval of Zynyz offers health care providers another first-line treatment option against MCC that can result in durable responses in patients with metastatic disease.”
POD1UM-201 was an open-label, single-arm, phase 2 study that evaluated the agent in 65 systemic treatment–naive adults with metastatic or recurrent locally advanced MCC.
Overall, 52% of patients had an objective response rate. A complete response was observed in 12 patients (18%), and a partial response was observed in 22 patients (34%).
Duration of response ranged from 1.1 to 24.9 months; 76% of responders experienced responses of 6 months or longer, and 62% experienced responses of 12 months or longer.
Study participants received a 500-mg dose of retifanlimab every 4 weeks for up to 24 weeks or until disease progression or unacceptable toxicity. Serious adverse events occurred in 22% of patients and most often included fatigue, arrhythmia, and pneumonitis; 11% of patients discontinued treatment because of serious adverse events.
Retifanlimab may cause a severe or life-threatening immune response during treatment or after discontinuation. Patients should be advised to immediately report any new or worsening signs or symptoms to their health care provider. Side effects can also be reported to the FDA.
A version of this article first appeared on Medscape.com.
the agency announced.
This marks the first regulatory approval for the PD-1 inhibitor. The FDA granted accelerated approval for the drug on the basis of tumor response rate and duration of response from the POD1UM-201 trial. Drugmaker Incyte said that “continued approval of Zynyz for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.”
MCC is a rare and aggressive skin cancer with a high rate of metastatic disease and an estimated 5-year overall survival of just 14% among those who present with metastatic disease. Incidence is rapidly increasing in the United States, particularly among adults older than 65 years, Incyte noted.
“More than a third of patients with MCC present with regional or distant metastases, which are associated with high rates of mortality,” principal author Shailender Bhatia, MD, of the University of Washington and Fred Hutchinson Cancer Center, both in Seattle, said in a news release. “The approval of Zynyz offers health care providers another first-line treatment option against MCC that can result in durable responses in patients with metastatic disease.”
POD1UM-201 was an open-label, single-arm, phase 2 study that evaluated the agent in 65 systemic treatment–naive adults with metastatic or recurrent locally advanced MCC.
Overall, 52% of patients had an objective response rate. A complete response was observed in 12 patients (18%), and a partial response was observed in 22 patients (34%).
Duration of response ranged from 1.1 to 24.9 months; 76% of responders experienced responses of 6 months or longer, and 62% experienced responses of 12 months or longer.
Study participants received a 500-mg dose of retifanlimab every 4 weeks for up to 24 weeks or until disease progression or unacceptable toxicity. Serious adverse events occurred in 22% of patients and most often included fatigue, arrhythmia, and pneumonitis; 11% of patients discontinued treatment because of serious adverse events.
Retifanlimab may cause a severe or life-threatening immune response during treatment or after discontinuation. Patients should be advised to immediately report any new or worsening signs or symptoms to their health care provider. Side effects can also be reported to the FDA.
A version of this article first appeared on Medscape.com.
Nurse makes millions selling her licensing exam study sheets
Ms. Beggs, 28, sells one-page study sheets or bundles of sheets, sometimes with colorful drawings, conversation bubbles and underlining, that boil down concepts for particular conditions into easy-to-understand language.
The biggest seller on Ms. Beggs’ online marketplace Etsy site, RNExplained, is a bundle of study guides covering eight core nursing classes. The notes range in price from $2 to $150. More than 70,000 customers have bought the $60 bundle, according to the website.
Ms. Beggs’ business developed in a “very unintentional” way when COVID hit with just months left in her nursing program at Mount Saint Mary’s University, Los Angeles, she told this news organization.
Classes had switched to Zoom, and she had no one to study with as she prepared to take her board exams.
“The best way I know how to study is to teach things out loud. But because I had nobody to teach out loud to, I would literally teach them to the wall,” Ms. Beggs said. “I would record myself so I could play it back and teach myself these topics that were hard for me to understand.”
Just for fun, she says, she posted them on TikTok and the responses started flowing in, with followers asking where she was selling the sheets. She now has more than 660,000 TikTok followers and 9 million likes.
Ms. Beggs said that every sheet highlights a condition, and she has made 308 of them.
Traditional classroom lessons typically teach one medical condition in 5-6 pages, Ms. Beggs said. “I go straight to the point.”
One reviewer on Ms. Beggs’ Etsy site appreciated the handwritten notes, calling them “simplified and concise.” Another commented: “Definitely helped me pass my last exam.”
Ms. Beggs says that her notes may seem simple, but each page represents comprehensive research.
“I have to go through not just one source of information to make sure my information is factual,” Ms. Beggs says. “What you teach in California might be a little different than what you teach in Florida. It’s very meticulous. The lab values will be a little different everywhere you go.”
She acknowledges her competition, noting that there are many other study guides for the NCLEX and nursing courses.
Nursing groups weigh in
Dawn Kappel, spokesperson for the National Council of State Boards of Nursing, which oversees NCLEX, said in an interview that “NCSBN has no issue with the current content of Stephanee Beggs’ business venture.”
For many students, the study guides will be helpful, especially for visual learners, said Carole Kenner, PhD, RN, dean and professor in the School of Nursing and Health Sciences at The College of New Jersey.
But for students “who are less confident in their knowledge, I would want to see a lot more in-depth explanation and rationale,” Dr. Kenner said.
“Since the NCLEX is moving to more cased-based scenarios, the next-gen unfolding cases, you really have to understand a lot of the rationale.”
The notes remind Dr. Kenner of traditional flash cards. “I don’t think it will work for all students, but even the fanciest of onsite review courses are useful to everyone,” she said.
‘Not cutting corners’
As an emergency nurse, Ms. Beggs said, “I have the experience as a nurse to show people that what you are learning will be seen in real life.”
“The way I teach my brand is not to take shortcuts. I love to teach to understand rather than teaching to memorize for an exam.”
She said she sees her guides as a supplement to learning, not a replacement.
“It’s not cutting corners,” she says. “I condense a medical condition that could take a very long time to understand and break it into layman’s terms.”
Ms. Beggs said when people hear about the $2 million, they often ask her whether she plans to give up her shifts in the emergency department for the more lucrative venture.
The answer is no, at least not yet.
“Aside from teaching, I genuinely love being at the bedside,” Ms. Beggs said. “I don’t foresee myself leaving that for good for as long as I can handle both.” She acknowledged, though, that her business now takes up most of her time.
“I love everything about both aspects, so it’s hard for me to choose.”
A version of this article first appeared on Medscape.com.
Ms. Beggs, 28, sells one-page study sheets or bundles of sheets, sometimes with colorful drawings, conversation bubbles and underlining, that boil down concepts for particular conditions into easy-to-understand language.
The biggest seller on Ms. Beggs’ online marketplace Etsy site, RNExplained, is a bundle of study guides covering eight core nursing classes. The notes range in price from $2 to $150. More than 70,000 customers have bought the $60 bundle, according to the website.
Ms. Beggs’ business developed in a “very unintentional” way when COVID hit with just months left in her nursing program at Mount Saint Mary’s University, Los Angeles, she told this news organization.
Classes had switched to Zoom, and she had no one to study with as she prepared to take her board exams.
“The best way I know how to study is to teach things out loud. But because I had nobody to teach out loud to, I would literally teach them to the wall,” Ms. Beggs said. “I would record myself so I could play it back and teach myself these topics that were hard for me to understand.”
Just for fun, she says, she posted them on TikTok and the responses started flowing in, with followers asking where she was selling the sheets. She now has more than 660,000 TikTok followers and 9 million likes.
Ms. Beggs said that every sheet highlights a condition, and she has made 308 of them.
Traditional classroom lessons typically teach one medical condition in 5-6 pages, Ms. Beggs said. “I go straight to the point.”
One reviewer on Ms. Beggs’ Etsy site appreciated the handwritten notes, calling them “simplified and concise.” Another commented: “Definitely helped me pass my last exam.”
Ms. Beggs says that her notes may seem simple, but each page represents comprehensive research.
“I have to go through not just one source of information to make sure my information is factual,” Ms. Beggs says. “What you teach in California might be a little different than what you teach in Florida. It’s very meticulous. The lab values will be a little different everywhere you go.”
She acknowledges her competition, noting that there are many other study guides for the NCLEX and nursing courses.
Nursing groups weigh in
Dawn Kappel, spokesperson for the National Council of State Boards of Nursing, which oversees NCLEX, said in an interview that “NCSBN has no issue with the current content of Stephanee Beggs’ business venture.”
For many students, the study guides will be helpful, especially for visual learners, said Carole Kenner, PhD, RN, dean and professor in the School of Nursing and Health Sciences at The College of New Jersey.
But for students “who are less confident in their knowledge, I would want to see a lot more in-depth explanation and rationale,” Dr. Kenner said.
“Since the NCLEX is moving to more cased-based scenarios, the next-gen unfolding cases, you really have to understand a lot of the rationale.”
The notes remind Dr. Kenner of traditional flash cards. “I don’t think it will work for all students, but even the fanciest of onsite review courses are useful to everyone,” she said.
‘Not cutting corners’
As an emergency nurse, Ms. Beggs said, “I have the experience as a nurse to show people that what you are learning will be seen in real life.”
“The way I teach my brand is not to take shortcuts. I love to teach to understand rather than teaching to memorize for an exam.”
She said she sees her guides as a supplement to learning, not a replacement.
“It’s not cutting corners,” she says. “I condense a medical condition that could take a very long time to understand and break it into layman’s terms.”
Ms. Beggs said when people hear about the $2 million, they often ask her whether she plans to give up her shifts in the emergency department for the more lucrative venture.
The answer is no, at least not yet.
“Aside from teaching, I genuinely love being at the bedside,” Ms. Beggs said. “I don’t foresee myself leaving that for good for as long as I can handle both.” She acknowledged, though, that her business now takes up most of her time.
“I love everything about both aspects, so it’s hard for me to choose.”
A version of this article first appeared on Medscape.com.
Ms. Beggs, 28, sells one-page study sheets or bundles of sheets, sometimes with colorful drawings, conversation bubbles and underlining, that boil down concepts for particular conditions into easy-to-understand language.
The biggest seller on Ms. Beggs’ online marketplace Etsy site, RNExplained, is a bundle of study guides covering eight core nursing classes. The notes range in price from $2 to $150. More than 70,000 customers have bought the $60 bundle, according to the website.
Ms. Beggs’ business developed in a “very unintentional” way when COVID hit with just months left in her nursing program at Mount Saint Mary’s University, Los Angeles, she told this news organization.
Classes had switched to Zoom, and she had no one to study with as she prepared to take her board exams.
“The best way I know how to study is to teach things out loud. But because I had nobody to teach out loud to, I would literally teach them to the wall,” Ms. Beggs said. “I would record myself so I could play it back and teach myself these topics that were hard for me to understand.”
Just for fun, she says, she posted them on TikTok and the responses started flowing in, with followers asking where she was selling the sheets. She now has more than 660,000 TikTok followers and 9 million likes.
Ms. Beggs said that every sheet highlights a condition, and she has made 308 of them.
Traditional classroom lessons typically teach one medical condition in 5-6 pages, Ms. Beggs said. “I go straight to the point.”
One reviewer on Ms. Beggs’ Etsy site appreciated the handwritten notes, calling them “simplified and concise.” Another commented: “Definitely helped me pass my last exam.”
Ms. Beggs says that her notes may seem simple, but each page represents comprehensive research.
“I have to go through not just one source of information to make sure my information is factual,” Ms. Beggs says. “What you teach in California might be a little different than what you teach in Florida. It’s very meticulous. The lab values will be a little different everywhere you go.”
She acknowledges her competition, noting that there are many other study guides for the NCLEX and nursing courses.
Nursing groups weigh in
Dawn Kappel, spokesperson for the National Council of State Boards of Nursing, which oversees NCLEX, said in an interview that “NCSBN has no issue with the current content of Stephanee Beggs’ business venture.”
For many students, the study guides will be helpful, especially for visual learners, said Carole Kenner, PhD, RN, dean and professor in the School of Nursing and Health Sciences at The College of New Jersey.
But for students “who are less confident in their knowledge, I would want to see a lot more in-depth explanation and rationale,” Dr. Kenner said.
“Since the NCLEX is moving to more cased-based scenarios, the next-gen unfolding cases, you really have to understand a lot of the rationale.”
The notes remind Dr. Kenner of traditional flash cards. “I don’t think it will work for all students, but even the fanciest of onsite review courses are useful to everyone,” she said.
‘Not cutting corners’
As an emergency nurse, Ms. Beggs said, “I have the experience as a nurse to show people that what you are learning will be seen in real life.”
“The way I teach my brand is not to take shortcuts. I love to teach to understand rather than teaching to memorize for an exam.”
She said she sees her guides as a supplement to learning, not a replacement.
“It’s not cutting corners,” she says. “I condense a medical condition that could take a very long time to understand and break it into layman’s terms.”
Ms. Beggs said when people hear about the $2 million, they often ask her whether she plans to give up her shifts in the emergency department for the more lucrative venture.
The answer is no, at least not yet.
“Aside from teaching, I genuinely love being at the bedside,” Ms. Beggs said. “I don’t foresee myself leaving that for good for as long as I can handle both.” She acknowledged, though, that her business now takes up most of her time.
“I love everything about both aspects, so it’s hard for me to choose.”
A version of this article first appeared on Medscape.com.
Psoriatic arthritis treatment for women falls short, study suggests
Women with psoriatic arthritis (PsA) presented with more severe disease at baseline and were less likely to achieve favorable outcomes after 12 months of treatment with either ustekinumab (Stelara) or a tumor necrosis factor (TNF) inhibitor, compared with men, according to a post hoc analysis of data from nearly 1,000 individuals.
Although data suggest that the overall prevalence of PsA is similar across genders, recent studies have identified differences in various aspects of PsA between men and women, wrote Arno W.R. Van Kuijk, MD, of Amsterdam Rheumatology and Immunology Center, and colleagues wrote in a study published in Rheumatology.
“Accumulating evidence in multiple rheumatic diseases indicates that gender may influence the likelihood of achieving the desired outcome with treatment,” but studies of differences in treatment response according to gender are limited, they said.
The researchers conducted a post hoc analysis of women and men with PsA who were part of PsABio, a noninterventional European study of patients with PsA. All participants were starting first-, second-, or third-line treatment with ustekinumab or a TNF inhibitor. The primary outcome was response to treatment at 12 months. Disease activity was assessed using the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA), the Health Assessment Questionnaire–Disability Index (HAQ-DI), and total score on the 12-item Psoriatic Arthritis Impact of Disease (PsAID-12) questionnaire.
Baseline available data for 512 women and 417 men showed the mean duration of disease was similar between genders (6.7 years for females and 6.9 years for males); body mass index was similar, as was the proportion of male and female patients receiving concomitant conventional synthetic disease-modifying antirheumatic drugs (DMARDs). Females scored significantly worse than males on disease activity assessments at baseline with mean cDAPSA scores of 32.3 and 26.8, respectively.
The final analysis of 895 patients with baseline data and a postbaseline assessment included 439 who started ustekinumab (247 females, 192 males), and 456 who started a TNF inhibitor (248 females, 208 males).
At 12 months, females showed smaller degrees of improvement than males; 57.8% and 80.3%, respectively, achieved low disease activity based on cDAPSA scores, while 33.7% and 55.5% of females and males, respectively, achieved minimal disease activity. Measures of disability were higher in females than males, with HAQ-DI scores of 0.85 versus 0.50. PsAID-12 scores also were higher for females, compared with males (3.5 vs. 2.4).
A total of 81.7% of patients were on their initial biologic DMARD after 12 months, but more females than males who were taking ustekinumab or a TNF inhibitor changed or discontinued treatment.
Treatment persistence was significantly lower in females than males (P = .01), and lack of effectiveness was the main reason for discontinuation regardless of gender.
“The analysis of gender subgroup results of the PsABio study has expanded previously published observations that men and women with PsA have different experiences with the disease activity, clinical manifestations, impact on health-related quality of life, response to [biologic] DMARDs, and drug persistence,” the researchers wrote.
The lack of a medication protocol in the PsABio study limited the conclusions that could be drawn from the post hoc analysis, but the results were strengthened by the relatively large and diverse sample size and the inclusion of responses to more than one medication, the researchers noted.
The study was supported by Janssen. Dr. Van Kuijk disclosed serving as a consultant and receiving grant support from Janssen and other companies; several coauthors also disclosed relationships with Janssen.
Women with psoriatic arthritis (PsA) presented with more severe disease at baseline and were less likely to achieve favorable outcomes after 12 months of treatment with either ustekinumab (Stelara) or a tumor necrosis factor (TNF) inhibitor, compared with men, according to a post hoc analysis of data from nearly 1,000 individuals.
Although data suggest that the overall prevalence of PsA is similar across genders, recent studies have identified differences in various aspects of PsA between men and women, wrote Arno W.R. Van Kuijk, MD, of Amsterdam Rheumatology and Immunology Center, and colleagues wrote in a study published in Rheumatology.
“Accumulating evidence in multiple rheumatic diseases indicates that gender may influence the likelihood of achieving the desired outcome with treatment,” but studies of differences in treatment response according to gender are limited, they said.
The researchers conducted a post hoc analysis of women and men with PsA who were part of PsABio, a noninterventional European study of patients with PsA. All participants were starting first-, second-, or third-line treatment with ustekinumab or a TNF inhibitor. The primary outcome was response to treatment at 12 months. Disease activity was assessed using the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA), the Health Assessment Questionnaire–Disability Index (HAQ-DI), and total score on the 12-item Psoriatic Arthritis Impact of Disease (PsAID-12) questionnaire.
Baseline available data for 512 women and 417 men showed the mean duration of disease was similar between genders (6.7 years for females and 6.9 years for males); body mass index was similar, as was the proportion of male and female patients receiving concomitant conventional synthetic disease-modifying antirheumatic drugs (DMARDs). Females scored significantly worse than males on disease activity assessments at baseline with mean cDAPSA scores of 32.3 and 26.8, respectively.
The final analysis of 895 patients with baseline data and a postbaseline assessment included 439 who started ustekinumab (247 females, 192 males), and 456 who started a TNF inhibitor (248 females, 208 males).
At 12 months, females showed smaller degrees of improvement than males; 57.8% and 80.3%, respectively, achieved low disease activity based on cDAPSA scores, while 33.7% and 55.5% of females and males, respectively, achieved minimal disease activity. Measures of disability were higher in females than males, with HAQ-DI scores of 0.85 versus 0.50. PsAID-12 scores also were higher for females, compared with males (3.5 vs. 2.4).
A total of 81.7% of patients were on their initial biologic DMARD after 12 months, but more females than males who were taking ustekinumab or a TNF inhibitor changed or discontinued treatment.
Treatment persistence was significantly lower in females than males (P = .01), and lack of effectiveness was the main reason for discontinuation regardless of gender.
“The analysis of gender subgroup results of the PsABio study has expanded previously published observations that men and women with PsA have different experiences with the disease activity, clinical manifestations, impact on health-related quality of life, response to [biologic] DMARDs, and drug persistence,” the researchers wrote.
The lack of a medication protocol in the PsABio study limited the conclusions that could be drawn from the post hoc analysis, but the results were strengthened by the relatively large and diverse sample size and the inclusion of responses to more than one medication, the researchers noted.
The study was supported by Janssen. Dr. Van Kuijk disclosed serving as a consultant and receiving grant support from Janssen and other companies; several coauthors also disclosed relationships with Janssen.
Women with psoriatic arthritis (PsA) presented with more severe disease at baseline and were less likely to achieve favorable outcomes after 12 months of treatment with either ustekinumab (Stelara) or a tumor necrosis factor (TNF) inhibitor, compared with men, according to a post hoc analysis of data from nearly 1,000 individuals.
Although data suggest that the overall prevalence of PsA is similar across genders, recent studies have identified differences in various aspects of PsA between men and women, wrote Arno W.R. Van Kuijk, MD, of Amsterdam Rheumatology and Immunology Center, and colleagues wrote in a study published in Rheumatology.
“Accumulating evidence in multiple rheumatic diseases indicates that gender may influence the likelihood of achieving the desired outcome with treatment,” but studies of differences in treatment response according to gender are limited, they said.
The researchers conducted a post hoc analysis of women and men with PsA who were part of PsABio, a noninterventional European study of patients with PsA. All participants were starting first-, second-, or third-line treatment with ustekinumab or a TNF inhibitor. The primary outcome was response to treatment at 12 months. Disease activity was assessed using the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA), the Health Assessment Questionnaire–Disability Index (HAQ-DI), and total score on the 12-item Psoriatic Arthritis Impact of Disease (PsAID-12) questionnaire.
Baseline available data for 512 women and 417 men showed the mean duration of disease was similar between genders (6.7 years for females and 6.9 years for males); body mass index was similar, as was the proportion of male and female patients receiving concomitant conventional synthetic disease-modifying antirheumatic drugs (DMARDs). Females scored significantly worse than males on disease activity assessments at baseline with mean cDAPSA scores of 32.3 and 26.8, respectively.
The final analysis of 895 patients with baseline data and a postbaseline assessment included 439 who started ustekinumab (247 females, 192 males), and 456 who started a TNF inhibitor (248 females, 208 males).
At 12 months, females showed smaller degrees of improvement than males; 57.8% and 80.3%, respectively, achieved low disease activity based on cDAPSA scores, while 33.7% and 55.5% of females and males, respectively, achieved minimal disease activity. Measures of disability were higher in females than males, with HAQ-DI scores of 0.85 versus 0.50. PsAID-12 scores also were higher for females, compared with males (3.5 vs. 2.4).
A total of 81.7% of patients were on their initial biologic DMARD after 12 months, but more females than males who were taking ustekinumab or a TNF inhibitor changed or discontinued treatment.
Treatment persistence was significantly lower in females than males (P = .01), and lack of effectiveness was the main reason for discontinuation regardless of gender.
“The analysis of gender subgroup results of the PsABio study has expanded previously published observations that men and women with PsA have different experiences with the disease activity, clinical manifestations, impact on health-related quality of life, response to [biologic] DMARDs, and drug persistence,” the researchers wrote.
The lack of a medication protocol in the PsABio study limited the conclusions that could be drawn from the post hoc analysis, but the results were strengthened by the relatively large and diverse sample size and the inclusion of responses to more than one medication, the researchers noted.
The study was supported by Janssen. Dr. Van Kuijk disclosed serving as a consultant and receiving grant support from Janssen and other companies; several coauthors also disclosed relationships with Janssen.
FROM RHEUMATOLOGY
The air up there: Oxygen could be a bit overrated
Into thin, but healthy, air
Human civilization has essentially been built on proximity to water. Ancient civilizations in Mesopotamia, Egypt, Greece, China, and India were all intimately connected to either rivers or the ocean. Even today, with all our technology, about a third of Earth’s 8 billion people live within 100 vertical meters of sea level, and the median person lives at an elevation of just 200 meters.
All things considered, one might imagine life is pretty tough for the 2 million people living at an elevation of 4,500 meters (nearly 15,000 feet). Not too many Wal-Marts or McDonalds up there. Oh, and not much air either. And for most of us not named Spongebob, air is good.
Or is it? That’s the question posed by a new study. After all, the researchers said, people living at high altitudes, where the air has only 11% effective oxygen instead of the 21% we have at low altitude, have significantly lower rates of metabolic disorders such as diabetes and heart diseases. Maybe breathing isn’t all it’s cracked up to be.
To find out, the researchers placed a group of mice in environments with either 11% oxygen or 8% oxygen. This netted them a bunch of very tired mice. Hey, sudden altitude gain doesn’t go too well for us either, but after 3 weeks, all the mice in the hypoxic environments had regained their normal movement and were behaving as any mouse would.
While the critters seemed normal on the outside, a closer examination found the truth. Their metabolism had been permanently altered, and their blood sugar and weight went down and never bounced back up. Further examination through PET scans showed that the hypoxic mice’s organs showed an increase in glucose metabolism and that brown fat and skeletal muscles reduced the amount of sugar they used.
This goes against the prevailing assumption about hypoxic conditions, the researchers said, since it was previously theorized that the body simply burned more glucose in response to having less oxygen. And while that’s true, our organs also conspicuously use less glucose. Currently, many athletes use hypoxic environments to train, but these new data suggest that people with metabolic disorders also would see benefits from living in low-oxygen environments.
Do you know what this means? All we have to do to stop diabetes is take civilization and push it somewhere else. This can’t possibly end badly.
Sleep survey: The restless majority
Newsflash! This just in: Nobody is sleeping well.
When we go to bed, our goal is to get rest, right? Sorry America, but you’re falling short. In a recent survey conducted by OnePoll for Purple Mattress, almost two-thirds of the 2,011 participants considered themselves restless sleepers.
Not surprised. So what’s keeping us up?
Snoring partners (20%) and anxiety (26%) made the list, but the award for top complaint goes to body pain. Back pain was most prevalent, reported by 36% of respondents, followed by neck pain (33%) and shoulder pain (24%). No wonder, then, that only 10% of the group reported feeling well rested when they woke up.
Do you ever blame your tiredness on sleeping funny? Well, we all kind of sleep funny, and yet we’re still not sleeping well.
The largest proportion of people like to sleep on their side (48%), compared with 18% on their back and 17% on their stomach. The main reasons to choose certain positions were to ease soreness or sleep better, both at 28%. The largest share of participants (47%) reported sleeping in a “yearner” position, while 40% lay on their stomachs in the “free faller” position, and 39% reported using the “soldier” position.
Regardless of the method people use to get to sleep or the position they’re in, the goal is always the same. We’re all just trying to figure out what’s the right one for us.
Seen a UFO recently? Don’t blame COVID
First of all, because we know you’re going to be thinking it in a minute, no, we did not make this up. With COVID-19 still hanging around, there’s no need for fabrication on our part.
The pandemic, clearly, has caused humans to do some strange things over the last 3 years, but what about some of the more, shall we say … eccentric behavior that people were already exhibiting before COVID found its way into our lives?
If, like R. Chase Cockrell, PhD, of the University of Vermont and associates at the Center for UFO Studies, you were wondering if the pandemic affected UFO reporting, then wonder no more. After all, with all that extra time being spent outdoors back in 2020 and all the additional anxiety, surely somebody must have seen something.
The investigators started with the basics by analyzing data from the National UFO Reporting Center and the Mutual UFO Network. Sightings did increase by about 600 in each database during 2020, compared with 2018 and 2019, but not because of the pandemic.
That’s right, we can’t pin this one on our good friend SARS-CoV-2. Further analysis showed that the launches of SpaceX Starlink satellites – sometimes as many as 60 at a time – probably caused the increase in UFO sightings, which means that our favorite billionaire, Elon Musk, is to blame. Yup, the genial Mr. Muskellunge did something that even a global pandemic couldn’t, and yet we vaccinate for COVID.
Next week on tenuous connections: A new study links the 2020 presidential election to increased emergency department visits for external hemorrhoids.
See? That’s fabrication. We made that up.
This article was updated 5/15/23.
Into thin, but healthy, air
Human civilization has essentially been built on proximity to water. Ancient civilizations in Mesopotamia, Egypt, Greece, China, and India were all intimately connected to either rivers or the ocean. Even today, with all our technology, about a third of Earth’s 8 billion people live within 100 vertical meters of sea level, and the median person lives at an elevation of just 200 meters.
All things considered, one might imagine life is pretty tough for the 2 million people living at an elevation of 4,500 meters (nearly 15,000 feet). Not too many Wal-Marts or McDonalds up there. Oh, and not much air either. And for most of us not named Spongebob, air is good.
Or is it? That’s the question posed by a new study. After all, the researchers said, people living at high altitudes, where the air has only 11% effective oxygen instead of the 21% we have at low altitude, have significantly lower rates of metabolic disorders such as diabetes and heart diseases. Maybe breathing isn’t all it’s cracked up to be.
To find out, the researchers placed a group of mice in environments with either 11% oxygen or 8% oxygen. This netted them a bunch of very tired mice. Hey, sudden altitude gain doesn’t go too well for us either, but after 3 weeks, all the mice in the hypoxic environments had regained their normal movement and were behaving as any mouse would.
While the critters seemed normal on the outside, a closer examination found the truth. Their metabolism had been permanently altered, and their blood sugar and weight went down and never bounced back up. Further examination through PET scans showed that the hypoxic mice’s organs showed an increase in glucose metabolism and that brown fat and skeletal muscles reduced the amount of sugar they used.
This goes against the prevailing assumption about hypoxic conditions, the researchers said, since it was previously theorized that the body simply burned more glucose in response to having less oxygen. And while that’s true, our organs also conspicuously use less glucose. Currently, many athletes use hypoxic environments to train, but these new data suggest that people with metabolic disorders also would see benefits from living in low-oxygen environments.
Do you know what this means? All we have to do to stop diabetes is take civilization and push it somewhere else. This can’t possibly end badly.
Sleep survey: The restless majority
Newsflash! This just in: Nobody is sleeping well.
When we go to bed, our goal is to get rest, right? Sorry America, but you’re falling short. In a recent survey conducted by OnePoll for Purple Mattress, almost two-thirds of the 2,011 participants considered themselves restless sleepers.
Not surprised. So what’s keeping us up?
Snoring partners (20%) and anxiety (26%) made the list, but the award for top complaint goes to body pain. Back pain was most prevalent, reported by 36% of respondents, followed by neck pain (33%) and shoulder pain (24%). No wonder, then, that only 10% of the group reported feeling well rested when they woke up.
Do you ever blame your tiredness on sleeping funny? Well, we all kind of sleep funny, and yet we’re still not sleeping well.
The largest proportion of people like to sleep on their side (48%), compared with 18% on their back and 17% on their stomach. The main reasons to choose certain positions were to ease soreness or sleep better, both at 28%. The largest share of participants (47%) reported sleeping in a “yearner” position, while 40% lay on their stomachs in the “free faller” position, and 39% reported using the “soldier” position.
Regardless of the method people use to get to sleep or the position they’re in, the goal is always the same. We’re all just trying to figure out what’s the right one for us.
Seen a UFO recently? Don’t blame COVID
First of all, because we know you’re going to be thinking it in a minute, no, we did not make this up. With COVID-19 still hanging around, there’s no need for fabrication on our part.
The pandemic, clearly, has caused humans to do some strange things over the last 3 years, but what about some of the more, shall we say … eccentric behavior that people were already exhibiting before COVID found its way into our lives?
If, like R. Chase Cockrell, PhD, of the University of Vermont and associates at the Center for UFO Studies, you were wondering if the pandemic affected UFO reporting, then wonder no more. After all, with all that extra time being spent outdoors back in 2020 and all the additional anxiety, surely somebody must have seen something.
The investigators started with the basics by analyzing data from the National UFO Reporting Center and the Mutual UFO Network. Sightings did increase by about 600 in each database during 2020, compared with 2018 and 2019, but not because of the pandemic.
That’s right, we can’t pin this one on our good friend SARS-CoV-2. Further analysis showed that the launches of SpaceX Starlink satellites – sometimes as many as 60 at a time – probably caused the increase in UFO sightings, which means that our favorite billionaire, Elon Musk, is to blame. Yup, the genial Mr. Muskellunge did something that even a global pandemic couldn’t, and yet we vaccinate for COVID.
Next week on tenuous connections: A new study links the 2020 presidential election to increased emergency department visits for external hemorrhoids.
See? That’s fabrication. We made that up.
This article was updated 5/15/23.
Into thin, but healthy, air
Human civilization has essentially been built on proximity to water. Ancient civilizations in Mesopotamia, Egypt, Greece, China, and India were all intimately connected to either rivers or the ocean. Even today, with all our technology, about a third of Earth’s 8 billion people live within 100 vertical meters of sea level, and the median person lives at an elevation of just 200 meters.
All things considered, one might imagine life is pretty tough for the 2 million people living at an elevation of 4,500 meters (nearly 15,000 feet). Not too many Wal-Marts or McDonalds up there. Oh, and not much air either. And for most of us not named Spongebob, air is good.
Or is it? That’s the question posed by a new study. After all, the researchers said, people living at high altitudes, where the air has only 11% effective oxygen instead of the 21% we have at low altitude, have significantly lower rates of metabolic disorders such as diabetes and heart diseases. Maybe breathing isn’t all it’s cracked up to be.
To find out, the researchers placed a group of mice in environments with either 11% oxygen or 8% oxygen. This netted them a bunch of very tired mice. Hey, sudden altitude gain doesn’t go too well for us either, but after 3 weeks, all the mice in the hypoxic environments had regained their normal movement and were behaving as any mouse would.
While the critters seemed normal on the outside, a closer examination found the truth. Their metabolism had been permanently altered, and their blood sugar and weight went down and never bounced back up. Further examination through PET scans showed that the hypoxic mice’s organs showed an increase in glucose metabolism and that brown fat and skeletal muscles reduced the amount of sugar they used.
This goes against the prevailing assumption about hypoxic conditions, the researchers said, since it was previously theorized that the body simply burned more glucose in response to having less oxygen. And while that’s true, our organs also conspicuously use less glucose. Currently, many athletes use hypoxic environments to train, but these new data suggest that people with metabolic disorders also would see benefits from living in low-oxygen environments.
Do you know what this means? All we have to do to stop diabetes is take civilization and push it somewhere else. This can’t possibly end badly.
Sleep survey: The restless majority
Newsflash! This just in: Nobody is sleeping well.
When we go to bed, our goal is to get rest, right? Sorry America, but you’re falling short. In a recent survey conducted by OnePoll for Purple Mattress, almost two-thirds of the 2,011 participants considered themselves restless sleepers.
Not surprised. So what’s keeping us up?
Snoring partners (20%) and anxiety (26%) made the list, but the award for top complaint goes to body pain. Back pain was most prevalent, reported by 36% of respondents, followed by neck pain (33%) and shoulder pain (24%). No wonder, then, that only 10% of the group reported feeling well rested when they woke up.
Do you ever blame your tiredness on sleeping funny? Well, we all kind of sleep funny, and yet we’re still not sleeping well.
The largest proportion of people like to sleep on their side (48%), compared with 18% on their back and 17% on their stomach. The main reasons to choose certain positions were to ease soreness or sleep better, both at 28%. The largest share of participants (47%) reported sleeping in a “yearner” position, while 40% lay on their stomachs in the “free faller” position, and 39% reported using the “soldier” position.
Regardless of the method people use to get to sleep or the position they’re in, the goal is always the same. We’re all just trying to figure out what’s the right one for us.
Seen a UFO recently? Don’t blame COVID
First of all, because we know you’re going to be thinking it in a minute, no, we did not make this up. With COVID-19 still hanging around, there’s no need for fabrication on our part.
The pandemic, clearly, has caused humans to do some strange things over the last 3 years, but what about some of the more, shall we say … eccentric behavior that people were already exhibiting before COVID found its way into our lives?
If, like R. Chase Cockrell, PhD, of the University of Vermont and associates at the Center for UFO Studies, you were wondering if the pandemic affected UFO reporting, then wonder no more. After all, with all that extra time being spent outdoors back in 2020 and all the additional anxiety, surely somebody must have seen something.
The investigators started with the basics by analyzing data from the National UFO Reporting Center and the Mutual UFO Network. Sightings did increase by about 600 in each database during 2020, compared with 2018 and 2019, but not because of the pandemic.
That’s right, we can’t pin this one on our good friend SARS-CoV-2. Further analysis showed that the launches of SpaceX Starlink satellites – sometimes as many as 60 at a time – probably caused the increase in UFO sightings, which means that our favorite billionaire, Elon Musk, is to blame. Yup, the genial Mr. Muskellunge did something that even a global pandemic couldn’t, and yet we vaccinate for COVID.
Next week on tenuous connections: A new study links the 2020 presidential election to increased emergency department visits for external hemorrhoids.
See? That’s fabrication. We made that up.
This article was updated 5/15/23.
Cases of potentially deadly fungus jump 200%: CDC
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
After the Match: Next steps for new residents, unmatched
Medical school graduates around the US took to social media after last week's Match Day to share their joy ― or explore their options if they did not match.
Take this post March 19 on Twitter: “I went unmatched this year; looking for research position at any institute for internal medicine.”
including an international medical graduate who matched into his chosen specialty after multiple disappointments.
“I’ve waited for this email for 8 years,” Sahil Bawa, MD, posted on Twitter on March 13. A few days later, when he learned about his residency position, he posted: “I’m beyond grateful. Will be moving to Alabama soon #familymedicine.”
Dr. Bawa, who matched into UAB Medicine Selma (Ala.), graduated from medical school in India in 2014. He said in an interview that he has visited the United States periodically since then to pass medical tests, obtain letters of recommendation, and participate in research.
Over the years he watched his Indian colleagues give up on becoming American doctors, find alternative careers, or resolve to practice in their native country. But he held onto the few success stories he saw on social media. “There were always one to two every year. It kept me going. If they can do it, I can do it.”
International medical graduates (IMGs) like Dr. Bawa applied in record numbers to Match2023, according to the National Resident Matching Program (NRMP), which announced the results on March 13 of its main residency match and the Supplemental Offer and Acceptance Program (SOAP) for unfilled positions or unmatched applicants.
Overall, 48,156 total applicants registered for the match, which was driven by the increase of non-U.S. IMG applicants and U.S. DO seniors over the past year, NRMP stated in its release. U.S. MD seniors had a match rate of nearly 94%, and U.S. DO seniors, nearly 92%. U.S. IMGs had a match rate of nearly 68%, an “all-time high,” and non-U.S. IMGs, nearly 60%, NRMP stated.
Three specialties that filled all of their 30 or more available positions were orthopedic surgery, plastic surgery (integrated), radiology – diagnostic, and thoracic surgery. Specialties with 30 or more positions that filled with the highest percentage of U.S. MD and DO seniors were plastic surgery (integrated), internal medicine-pediatrics, ob.gyn., and orthopedic surgery.
The number of available primary care positions increased slightly, NRMP reported. Considering “a serious and growing shortage of primary care physicians across the U.S.,” there were 571 more primary care positions than 2022. That’s an increase of about 3% over last year and 17% over the past 5 years. Primary care positions filled at a rate of 94%, which remained steady from 2022.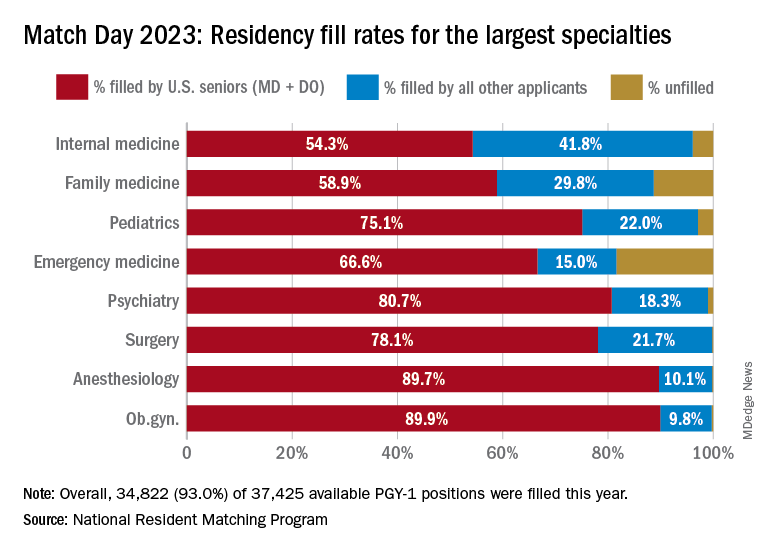
NRMP also pointed out specialties with increases in the number of positions filled by U.S. MD seniors of more than 10% and 10 positions in the past 5 years: anesthesiology, child neurology, interventional radiology, neurology, pathology, physical medicine and rehabilitation, plastic surgery (integrated), psychiatry, radiology-diagnostic, transitional year, and vascular surgery.
Bryan Carmody, MD, MPH, a pediatric nephrologist known for his medical school commentaries, said in an interview that the most competitive specialties he noted in 2023 were radiology, pathology, and neurology.
“The surgical specialties are always competitive, so it wasn’t a surprise that orthopedics, plastic surgery, and thoracic surgery filled all of their positions. But I was surprised to see diagnostic radiology fill every single one of their positions in the match. And although pathology and neurology aren’t typically considered extremely competitive specialties, they filled over 99% of their positions in the Match this year.”
On Dr. Carmody’s blog about the winners and losers of Match Day, he said that despite the record number of primary care positions offered, family medicine programs suffered. “Only 89% of family medicine programs filled in the Match, and graduating U.S. MD and DO students only filled a little more than half of all the available positions,” he wrote.
For a record number of applicants that match each year, and “the most favorable ratio in the past 2 decades” of applicants-to-positions in 2023, there are still a lot unmatched, Dr. Carmody said. “It’s a tough thing to talk about. The reality is the number of residency positions should be determined by the number of physicians needed.”
One student, Asim Ansari, didn’t match into a traditional residency or through SOAP. It was his fifth attempt. He was serving a transitional-year residency at Merit Health Wesley in Hattiesburg, Miss., and when he didn’t match, he accepted a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City.
He said he was “relieved and excited” to have found a program in his chosen specialty. Still, in 2 years, Mr. Ansari must again try to match into a traditional psychiatry residency.
Meanwhile, Dr. Bawa will prepare for his 3-year residency in Alabama after completing his interim research year in the surgery department at Wayne State University, Detroit, in May.
Despite his years in limbo, Dr. Bawa said, “I have no regrets, no complaints. I am still very happy.”
A version of this article originally appeared on Medscape.com.
Medical school graduates around the US took to social media after last week's Match Day to share their joy ― or explore their options if they did not match.
Take this post March 19 on Twitter: “I went unmatched this year; looking for research position at any institute for internal medicine.”
including an international medical graduate who matched into his chosen specialty after multiple disappointments.
“I’ve waited for this email for 8 years,” Sahil Bawa, MD, posted on Twitter on March 13. A few days later, when he learned about his residency position, he posted: “I’m beyond grateful. Will be moving to Alabama soon #familymedicine.”
Dr. Bawa, who matched into UAB Medicine Selma (Ala.), graduated from medical school in India in 2014. He said in an interview that he has visited the United States periodically since then to pass medical tests, obtain letters of recommendation, and participate in research.
Over the years he watched his Indian colleagues give up on becoming American doctors, find alternative careers, or resolve to practice in their native country. But he held onto the few success stories he saw on social media. “There were always one to two every year. It kept me going. If they can do it, I can do it.”
International medical graduates (IMGs) like Dr. Bawa applied in record numbers to Match2023, according to the National Resident Matching Program (NRMP), which announced the results on March 13 of its main residency match and the Supplemental Offer and Acceptance Program (SOAP) for unfilled positions or unmatched applicants.
Overall, 48,156 total applicants registered for the match, which was driven by the increase of non-U.S. IMG applicants and U.S. DO seniors over the past year, NRMP stated in its release. U.S. MD seniors had a match rate of nearly 94%, and U.S. DO seniors, nearly 92%. U.S. IMGs had a match rate of nearly 68%, an “all-time high,” and non-U.S. IMGs, nearly 60%, NRMP stated.
Three specialties that filled all of their 30 or more available positions were orthopedic surgery, plastic surgery (integrated), radiology – diagnostic, and thoracic surgery. Specialties with 30 or more positions that filled with the highest percentage of U.S. MD and DO seniors were plastic surgery (integrated), internal medicine-pediatrics, ob.gyn., and orthopedic surgery.
The number of available primary care positions increased slightly, NRMP reported. Considering “a serious and growing shortage of primary care physicians across the U.S.,” there were 571 more primary care positions than 2022. That’s an increase of about 3% over last year and 17% over the past 5 years. Primary care positions filled at a rate of 94%, which remained steady from 2022.
NRMP also pointed out specialties with increases in the number of positions filled by U.S. MD seniors of more than 10% and 10 positions in the past 5 years: anesthesiology, child neurology, interventional radiology, neurology, pathology, physical medicine and rehabilitation, plastic surgery (integrated), psychiatry, radiology-diagnostic, transitional year, and vascular surgery.
Bryan Carmody, MD, MPH, a pediatric nephrologist known for his medical school commentaries, said in an interview that the most competitive specialties he noted in 2023 were radiology, pathology, and neurology.
“The surgical specialties are always competitive, so it wasn’t a surprise that orthopedics, plastic surgery, and thoracic surgery filled all of their positions. But I was surprised to see diagnostic radiology fill every single one of their positions in the match. And although pathology and neurology aren’t typically considered extremely competitive specialties, they filled over 99% of their positions in the Match this year.”
On Dr. Carmody’s blog about the winners and losers of Match Day, he said that despite the record number of primary care positions offered, family medicine programs suffered. “Only 89% of family medicine programs filled in the Match, and graduating U.S. MD and DO students only filled a little more than half of all the available positions,” he wrote.
For a record number of applicants that match each year, and “the most favorable ratio in the past 2 decades” of applicants-to-positions in 2023, there are still a lot unmatched, Dr. Carmody said. “It’s a tough thing to talk about. The reality is the number of residency positions should be determined by the number of physicians needed.”
One student, Asim Ansari, didn’t match into a traditional residency or through SOAP. It was his fifth attempt. He was serving a transitional-year residency at Merit Health Wesley in Hattiesburg, Miss., and when he didn’t match, he accepted a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City.
He said he was “relieved and excited” to have found a program in his chosen specialty. Still, in 2 years, Mr. Ansari must again try to match into a traditional psychiatry residency.
Meanwhile, Dr. Bawa will prepare for his 3-year residency in Alabama after completing his interim research year in the surgery department at Wayne State University, Detroit, in May.
Despite his years in limbo, Dr. Bawa said, “I have no regrets, no complaints. I am still very happy.”
A version of this article originally appeared on Medscape.com.
Medical school graduates around the US took to social media after last week's Match Day to share their joy ― or explore their options if they did not match.
Take this post March 19 on Twitter: “I went unmatched this year; looking for research position at any institute for internal medicine.”
including an international medical graduate who matched into his chosen specialty after multiple disappointments.
“I’ve waited for this email for 8 years,” Sahil Bawa, MD, posted on Twitter on March 13. A few days later, when he learned about his residency position, he posted: “I’m beyond grateful. Will be moving to Alabama soon #familymedicine.”
Dr. Bawa, who matched into UAB Medicine Selma (Ala.), graduated from medical school in India in 2014. He said in an interview that he has visited the United States periodically since then to pass medical tests, obtain letters of recommendation, and participate in research.
Over the years he watched his Indian colleagues give up on becoming American doctors, find alternative careers, or resolve to practice in their native country. But he held onto the few success stories he saw on social media. “There were always one to two every year. It kept me going. If they can do it, I can do it.”
International medical graduates (IMGs) like Dr. Bawa applied in record numbers to Match2023, according to the National Resident Matching Program (NRMP), which announced the results on March 13 of its main residency match and the Supplemental Offer and Acceptance Program (SOAP) for unfilled positions or unmatched applicants.
Overall, 48,156 total applicants registered for the match, which was driven by the increase of non-U.S. IMG applicants and U.S. DO seniors over the past year, NRMP stated in its release. U.S. MD seniors had a match rate of nearly 94%, and U.S. DO seniors, nearly 92%. U.S. IMGs had a match rate of nearly 68%, an “all-time high,” and non-U.S. IMGs, nearly 60%, NRMP stated.
Three specialties that filled all of their 30 or more available positions were orthopedic surgery, plastic surgery (integrated), radiology – diagnostic, and thoracic surgery. Specialties with 30 or more positions that filled with the highest percentage of U.S. MD and DO seniors were plastic surgery (integrated), internal medicine-pediatrics, ob.gyn., and orthopedic surgery.
The number of available primary care positions increased slightly, NRMP reported. Considering “a serious and growing shortage of primary care physicians across the U.S.,” there were 571 more primary care positions than 2022. That’s an increase of about 3% over last year and 17% over the past 5 years. Primary care positions filled at a rate of 94%, which remained steady from 2022.
NRMP also pointed out specialties with increases in the number of positions filled by U.S. MD seniors of more than 10% and 10 positions in the past 5 years: anesthesiology, child neurology, interventional radiology, neurology, pathology, physical medicine and rehabilitation, plastic surgery (integrated), psychiatry, radiology-diagnostic, transitional year, and vascular surgery.
Bryan Carmody, MD, MPH, a pediatric nephrologist known for his medical school commentaries, said in an interview that the most competitive specialties he noted in 2023 were radiology, pathology, and neurology.
“The surgical specialties are always competitive, so it wasn’t a surprise that orthopedics, plastic surgery, and thoracic surgery filled all of their positions. But I was surprised to see diagnostic radiology fill every single one of their positions in the match. And although pathology and neurology aren’t typically considered extremely competitive specialties, they filled over 99% of their positions in the Match this year.”
On Dr. Carmody’s blog about the winners and losers of Match Day, he said that despite the record number of primary care positions offered, family medicine programs suffered. “Only 89% of family medicine programs filled in the Match, and graduating U.S. MD and DO students only filled a little more than half of all the available positions,” he wrote.
For a record number of applicants that match each year, and “the most favorable ratio in the past 2 decades” of applicants-to-positions in 2023, there are still a lot unmatched, Dr. Carmody said. “It’s a tough thing to talk about. The reality is the number of residency positions should be determined by the number of physicians needed.”
One student, Asim Ansari, didn’t match into a traditional residency or through SOAP. It was his fifth attempt. He was serving a transitional-year residency at Merit Health Wesley in Hattiesburg, Miss., and when he didn’t match, he accepted a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City.
He said he was “relieved and excited” to have found a program in his chosen specialty. Still, in 2 years, Mr. Ansari must again try to match into a traditional psychiatry residency.
Meanwhile, Dr. Bawa will prepare for his 3-year residency in Alabama after completing his interim research year in the surgery department at Wayne State University, Detroit, in May.
Despite his years in limbo, Dr. Bawa said, “I have no regrets, no complaints. I am still very happy.”
A version of this article originally appeared on Medscape.com.
Phase 3 prurigo nodularis trial shows positive results for nemolizumab
NEW ORLEANS – demonstrated.
Nemolizumab is a first-in-class investigational monoclonal antibody directed against the interleukin-31 receptor alpha that blocks signaling from IL-31. “From prior studies we know that it modulates pruritus, but also alters keratinocyte differentiation, inflammation, and fibrosis,” one of the investigators, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
OLYMPIA 2 was a phase 3, multicenter, double-blind study in adults with PN presenting with 20 or more nodules, and Investigator’s Global Assessment (IGA) score of 3 or more, and the Peak Pruritus Numerical Rating Scale (PP-NRS) score of 7 or more. Exclusion criteria included chronic pruritus resulting from an active condition other than PN, such as neuropathic and psychogenic pruritus and active atopic dermatitis. In addition, the use of topical steroids, considered a rescue therapy, was not allowed in the trial, Dr. Kwatra said.
After an initial screening period, 274 patients at 73 sites in nine countries were randomized 2:1 either to the nemolizumab monotherapy or placebo. Following an initial 60-mg subcutaneous dose, patients received 30 mg or 60 mg (depending on their baseline weight) every 4 weeks for 16 weeks. The primary endpoint was the proportion of patients with a 4-point or greater improvement in the PP-NRS from baseline at week 16 and the proportion of patients with IGA success at week 16.
Selected key secondary endpoints included the proportion of patients with a 4 point or greater improvement from baseline in the PP-NRS at week 4, the Sleep Disturbance Numerical Rating Scale at week 4, and the SD-NRS at week 16. Safety endpoints included the incidence and severity of all adverse events.
Of the 274 patients randomized, 183 received nemolizumab and 91 received placebo. A total of 174 patients in the nemolizumab group completed the study, compared with 88 in the placebo group. The mean age of study participants was 53 years, 61% were women, 79% were White, 14% were Asian, and the rest were from other racial groups. More than half (57%) had IGA category 3 disease (moderate) and the remainder had IGA category 4 disease (severe); 63% had 20-100 lesions, and the remainder had more than 100. About one-third of study enrollees (32%) had a history of atopy.
Primary, secondary endpoint results
Dr. Kwatra reported that 56.3% of the patients in the nemolizumab group achieved a 4-point or greater improvement in the PP-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001), while 37.7% of those in the nemolizumab group achieved IGA success at week 16, compared with 11% of those in the placebo group (P < .0001).
As for secondary endpoints, 41% of patients in the nemolizumab group achieved a 4-point or greater improvement in PP-NRS at week 4, compared with 7.7% of those in the placebo group (P < .0001); and 37.2% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 4, compared with 9.9% of those in the placebo group (P < .0001). Almost 52% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001); and 9.8% of those in the nemolizumab group achieved IGA success at week 4, compared with 1.1% of those in the placebo group (P < .0074).
Adverse events
Treatment-emergent adverse events occurred in 61.2% of subjects in the nemolizumab group, compared with 52.7% of those in the placebo group. “There were no imbalances overall, [including] no injection-related reactions in either group,” Dr. Kwatra said. There was one case of newly diagnosed asthma in the placebo arm, and none in the treatment arm.
The researchers observed a slightly increased onset of atopic dermatitis in the treatment arm, compared with the placebo arm (5.5% vs. 0%). “Seven out of those 10 patients actually had a history of atopic dermatitis or high IgE [levels] and they were mostly managed with topical steroids without study drug discontinuation,” Dr. Kwatra added. Neurodermatitis, or worsening of PN, occurred in 3.8% of patients in the nemolizumab group, compared with 11% of those in the placebo group.
“The results of this study extend the efficacy and safety findings from the phase 2 study of nemolizumab in patients with PN,” Dr. Kwatra concluded. “I think they also help to usher in a new era of PN [treatment] in prime time.”
Kenneth B. Gordon, MD, who chairs the department of dermatology at the Medical College of Wisconsin, Milwaukee, and was asked to comment on the study, was impressed with nemolizumab’s propensity for blocking IL-31. “To be able to treat PN effectively by simply blocking the itch and not having a significant inflammatory function is really interesting,” he said in an interview at the meeting. If approved, nemolizumab “gives us another treatment option for a disease that is really debilitating. It’s very promising and we hope [the drug] will be available to us in the near future.”
Nemolizumab is being developed by Galderma. According to a press release from the company, nemolizumab was granted Breakthrough Therapy designation by the Food and Drug Administration in December 2019 for the treatment of pruritus associated with PN, a status that was reconfirmed in February 2023.
Dr. Kwatra disclosed that he is an advisory board member/consultant for Galderma, AbbVie, Amgen, Arcutis, ASLAN Pharmaceuticals, Cara Therapeutics, Castle Biosciences, Celldex, Incyte, Johnson and Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Gordon disclosed that he is a consultant to, an investigator for, and/or a member of the advisory board for several pharmaceutical companies, but not Galderma.
NEW ORLEANS – demonstrated.
Nemolizumab is a first-in-class investigational monoclonal antibody directed against the interleukin-31 receptor alpha that blocks signaling from IL-31. “From prior studies we know that it modulates pruritus, but also alters keratinocyte differentiation, inflammation, and fibrosis,” one of the investigators, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
OLYMPIA 2 was a phase 3, multicenter, double-blind study in adults with PN presenting with 20 or more nodules, and Investigator’s Global Assessment (IGA) score of 3 or more, and the Peak Pruritus Numerical Rating Scale (PP-NRS) score of 7 or more. Exclusion criteria included chronic pruritus resulting from an active condition other than PN, such as neuropathic and psychogenic pruritus and active atopic dermatitis. In addition, the use of topical steroids, considered a rescue therapy, was not allowed in the trial, Dr. Kwatra said.
After an initial screening period, 274 patients at 73 sites in nine countries were randomized 2:1 either to the nemolizumab monotherapy or placebo. Following an initial 60-mg subcutaneous dose, patients received 30 mg or 60 mg (depending on their baseline weight) every 4 weeks for 16 weeks. The primary endpoint was the proportion of patients with a 4-point or greater improvement in the PP-NRS from baseline at week 16 and the proportion of patients with IGA success at week 16.
Selected key secondary endpoints included the proportion of patients with a 4 point or greater improvement from baseline in the PP-NRS at week 4, the Sleep Disturbance Numerical Rating Scale at week 4, and the SD-NRS at week 16. Safety endpoints included the incidence and severity of all adverse events.
Of the 274 patients randomized, 183 received nemolizumab and 91 received placebo. A total of 174 patients in the nemolizumab group completed the study, compared with 88 in the placebo group. The mean age of study participants was 53 years, 61% were women, 79% were White, 14% were Asian, and the rest were from other racial groups. More than half (57%) had IGA category 3 disease (moderate) and the remainder had IGA category 4 disease (severe); 63% had 20-100 lesions, and the remainder had more than 100. About one-third of study enrollees (32%) had a history of atopy.
Primary, secondary endpoint results
Dr. Kwatra reported that 56.3% of the patients in the nemolizumab group achieved a 4-point or greater improvement in the PP-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001), while 37.7% of those in the nemolizumab group achieved IGA success at week 16, compared with 11% of those in the placebo group (P < .0001).
As for secondary endpoints, 41% of patients in the nemolizumab group achieved a 4-point or greater improvement in PP-NRS at week 4, compared with 7.7% of those in the placebo group (P < .0001); and 37.2% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 4, compared with 9.9% of those in the placebo group (P < .0001). Almost 52% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001); and 9.8% of those in the nemolizumab group achieved IGA success at week 4, compared with 1.1% of those in the placebo group (P < .0074).
Adverse events
Treatment-emergent adverse events occurred in 61.2% of subjects in the nemolizumab group, compared with 52.7% of those in the placebo group. “There were no imbalances overall, [including] no injection-related reactions in either group,” Dr. Kwatra said. There was one case of newly diagnosed asthma in the placebo arm, and none in the treatment arm.
The researchers observed a slightly increased onset of atopic dermatitis in the treatment arm, compared with the placebo arm (5.5% vs. 0%). “Seven out of those 10 patients actually had a history of atopic dermatitis or high IgE [levels] and they were mostly managed with topical steroids without study drug discontinuation,” Dr. Kwatra added. Neurodermatitis, or worsening of PN, occurred in 3.8% of patients in the nemolizumab group, compared with 11% of those in the placebo group.
“The results of this study extend the efficacy and safety findings from the phase 2 study of nemolizumab in patients with PN,” Dr. Kwatra concluded. “I think they also help to usher in a new era of PN [treatment] in prime time.”
Kenneth B. Gordon, MD, who chairs the department of dermatology at the Medical College of Wisconsin, Milwaukee, and was asked to comment on the study, was impressed with nemolizumab’s propensity for blocking IL-31. “To be able to treat PN effectively by simply blocking the itch and not having a significant inflammatory function is really interesting,” he said in an interview at the meeting. If approved, nemolizumab “gives us another treatment option for a disease that is really debilitating. It’s very promising and we hope [the drug] will be available to us in the near future.”
Nemolizumab is being developed by Galderma. According to a press release from the company, nemolizumab was granted Breakthrough Therapy designation by the Food and Drug Administration in December 2019 for the treatment of pruritus associated with PN, a status that was reconfirmed in February 2023.
Dr. Kwatra disclosed that he is an advisory board member/consultant for Galderma, AbbVie, Amgen, Arcutis, ASLAN Pharmaceuticals, Cara Therapeutics, Castle Biosciences, Celldex, Incyte, Johnson and Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Gordon disclosed that he is a consultant to, an investigator for, and/or a member of the advisory board for several pharmaceutical companies, but not Galderma.
NEW ORLEANS – demonstrated.
Nemolizumab is a first-in-class investigational monoclonal antibody directed against the interleukin-31 receptor alpha that blocks signaling from IL-31. “From prior studies we know that it modulates pruritus, but also alters keratinocyte differentiation, inflammation, and fibrosis,” one of the investigators, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said during a late-breaking research session at the annual meeting of the American Academy of Dermatology.
OLYMPIA 2 was a phase 3, multicenter, double-blind study in adults with PN presenting with 20 or more nodules, and Investigator’s Global Assessment (IGA) score of 3 or more, and the Peak Pruritus Numerical Rating Scale (PP-NRS) score of 7 or more. Exclusion criteria included chronic pruritus resulting from an active condition other than PN, such as neuropathic and psychogenic pruritus and active atopic dermatitis. In addition, the use of topical steroids, considered a rescue therapy, was not allowed in the trial, Dr. Kwatra said.
After an initial screening period, 274 patients at 73 sites in nine countries were randomized 2:1 either to the nemolizumab monotherapy or placebo. Following an initial 60-mg subcutaneous dose, patients received 30 mg or 60 mg (depending on their baseline weight) every 4 weeks for 16 weeks. The primary endpoint was the proportion of patients with a 4-point or greater improvement in the PP-NRS from baseline at week 16 and the proportion of patients with IGA success at week 16.
Selected key secondary endpoints included the proportion of patients with a 4 point or greater improvement from baseline in the PP-NRS at week 4, the Sleep Disturbance Numerical Rating Scale at week 4, and the SD-NRS at week 16. Safety endpoints included the incidence and severity of all adverse events.
Of the 274 patients randomized, 183 received nemolizumab and 91 received placebo. A total of 174 patients in the nemolizumab group completed the study, compared with 88 in the placebo group. The mean age of study participants was 53 years, 61% were women, 79% were White, 14% were Asian, and the rest were from other racial groups. More than half (57%) had IGA category 3 disease (moderate) and the remainder had IGA category 4 disease (severe); 63% had 20-100 lesions, and the remainder had more than 100. About one-third of study enrollees (32%) had a history of atopy.
Primary, secondary endpoint results
Dr. Kwatra reported that 56.3% of the patients in the nemolizumab group achieved a 4-point or greater improvement in the PP-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001), while 37.7% of those in the nemolizumab group achieved IGA success at week 16, compared with 11% of those in the placebo group (P < .0001).
As for secondary endpoints, 41% of patients in the nemolizumab group achieved a 4-point or greater improvement in PP-NRS at week 4, compared with 7.7% of those in the placebo group (P < .0001); and 37.2% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 4, compared with 9.9% of those in the placebo group (P < .0001). Almost 52% of patients in the nemolizumab group achieved a 4-point or greater improvement in SD-NRS at week 16, compared with 20.9% of those in the placebo group (P < .0001); and 9.8% of those in the nemolizumab group achieved IGA success at week 4, compared with 1.1% of those in the placebo group (P < .0074).
Adverse events
Treatment-emergent adverse events occurred in 61.2% of subjects in the nemolizumab group, compared with 52.7% of those in the placebo group. “There were no imbalances overall, [including] no injection-related reactions in either group,” Dr. Kwatra said. There was one case of newly diagnosed asthma in the placebo arm, and none in the treatment arm.
The researchers observed a slightly increased onset of atopic dermatitis in the treatment arm, compared with the placebo arm (5.5% vs. 0%). “Seven out of those 10 patients actually had a history of atopic dermatitis or high IgE [levels] and they were mostly managed with topical steroids without study drug discontinuation,” Dr. Kwatra added. Neurodermatitis, or worsening of PN, occurred in 3.8% of patients in the nemolizumab group, compared with 11% of those in the placebo group.
“The results of this study extend the efficacy and safety findings from the phase 2 study of nemolizumab in patients with PN,” Dr. Kwatra concluded. “I think they also help to usher in a new era of PN [treatment] in prime time.”
Kenneth B. Gordon, MD, who chairs the department of dermatology at the Medical College of Wisconsin, Milwaukee, and was asked to comment on the study, was impressed with nemolizumab’s propensity for blocking IL-31. “To be able to treat PN effectively by simply blocking the itch and not having a significant inflammatory function is really interesting,” he said in an interview at the meeting. If approved, nemolizumab “gives us another treatment option for a disease that is really debilitating. It’s very promising and we hope [the drug] will be available to us in the near future.”
Nemolizumab is being developed by Galderma. According to a press release from the company, nemolizumab was granted Breakthrough Therapy designation by the Food and Drug Administration in December 2019 for the treatment of pruritus associated with PN, a status that was reconfirmed in February 2023.
Dr. Kwatra disclosed that he is an advisory board member/consultant for Galderma, AbbVie, Amgen, Arcutis, ASLAN Pharmaceuticals, Cara Therapeutics, Castle Biosciences, Celldex, Incyte, Johnson and Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Gordon disclosed that he is a consultant to, an investigator for, and/or a member of the advisory board for several pharmaceutical companies, but not Galderma.
AT AAD 2023







