User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
How three cardiac procedures changed in the COVID era
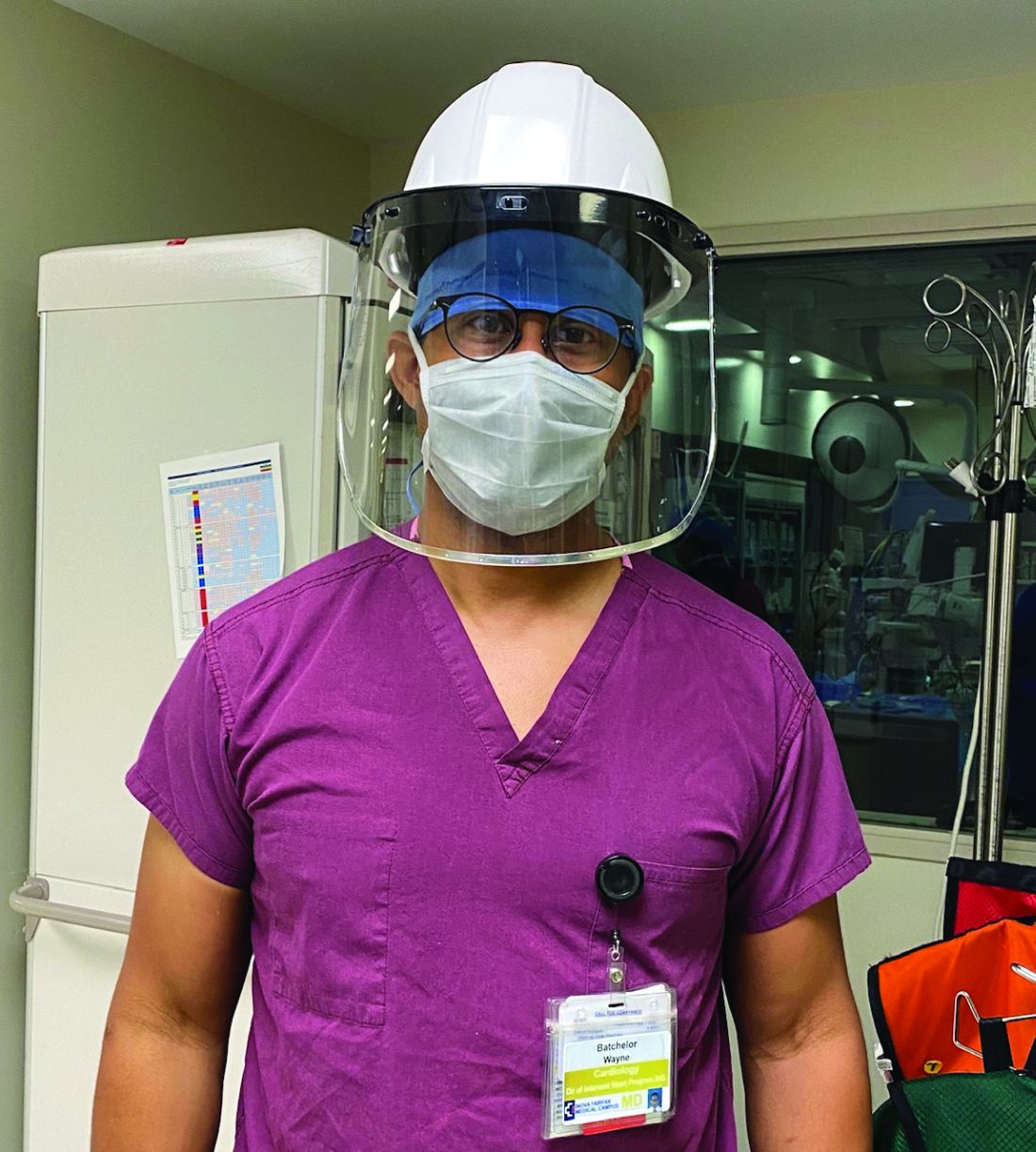
When Virginia’s governor directed the postponement of all elective surgeries in late March, Wayne Batchelor, MD and his colleagues at the Inova Heart and Vascular Institute in Falls Church, Va., canceled about two-thirds of their transcatheter aortic valve replacement (TAVR) procedures.
They then categorized patients by tiers to gauge which procedures could safely be postponed and to guide triaging. And while they did not deviate from the practice of having both an interventional cardiologist and a cardiothoracic surgeon present for TAVR, they slimmed down preprocedural testing when feasible and delayed some 30-day post-TAVR echocardiographic assessments. “It was a delicate dance, very difficult dance. But luckily, we were able to navigate the challenges effectively,” said Dr. Batchelor, the institute’s director of interventional cardiology and interventional cardiology research.
A “system capacity dashboard” that merged bed and staffing data from interventional cardiology spaces with cardiovascular and noncardiovascular ICU beds, operating rooms, and other resources – and daily cross-department meetings – enabled them to proceed with the most urgent TAVR procedures while “keeping a buffer of ICU beds to accommodate an anticipated surge of COVID-19,” he explained.
Such adaptations in cardiac procedures and processes are occurring in hospitals across the country as efforts are made to minimize the risk of COVID-19 exposure for patients and staff. Dr. Batchelor is one of four cardiologists who shared their experiences and advice on common cardiac procedures across three locales: TAVR in Virginia, percutaneous coronary intervention (PCI) in New York City, and atrial fibrillation (AFib) ablation in Kentucky.
More on TAVR in Virginia
Inova’s framework for triaging structural heart disease interventions (largely TAVR and/or percutaneous mitral valve repair) comprised three tiers. Tier 1 captured “emergent cases that had to be done, no questions asked,” Dr. Batchelor said. For TAVR, these were inpatients with severe to critical symptomatic aortic stenosis and advanced congestive heart failure who could not safely be discharged, as well as other patients “with refractory symptoms of heart failure that were compelling.” Many had associated left ventricular systolic dysfunction.
Those who could delay 14-30 days were placed in tier 2, and patients who “we felt were fairly stable and could wait at least 30 days” were placed in tier 3. “For TAVR, a tier 3 patient might be the one … who has severe aortic stenosis but is walking around and doing well at home with only stable exertional symptoms,” he said.
Patients whose procedures were delayed were contacted weekly by the valve clinic’s advanced practice practitioners through video visits or telephone calls, and tier categorization was reevaluated if symptoms worsened. “We had to keep in close contact with them,” Dr. Batchelor said. “These patients can deteriorate quite rapidly and sometimes without much warning.”
Virtual video visits were often used for 30-day postprocedural follow-ups, taking the place of in-person visits during which post-TAVR echocardiographic assessments would normally be performed. “For follow-up, we’d often just do a quick visit to check the vascular access site within 7-10 days, and then, if they were doing okay we’d delay the 30-day echo to a later time frame,” he said.
Preprocedural testing was streamlined to minimize the number of patient-provider interactions, with pulmonary function testing and pre-TAVR catheterization omitted unless absolutely necessary. “A TAVR CT angiogram [performed within the prior year] is the only test you really absolutely need,” Dr. Batchelor said. “We were much less likely to order a heart catheterization unless the patient was having angina and high risk or suspicion for significant coronary artery disease.”
This approach was not associated with any compromise in postprocedural outcomes, he noted. Prior to the pandemic, Inova routinely employed a minimalist approach to TAVR with moderate conscious sedation and avoiding transesophageal echocardiography – steps that were recommended for structural heart procedures in the COVID-19 era in a published review by the heart team at New York-Presbyterian Hospital/Columbia University Irving Medical Center.
The New York review is useful for cardiologists in areas with rising case burdens of COVID-19, Dr. Batchelor said, as is a position statement he coauthored from the American College of Cardiology and the Society for Cardiology and Angiography Interventions (SCAI) on triage considerations for structural heart disease interventions during the pandemic.
TAVR’s resource-heavy nature made the “system capacity dashboard” and daily meetings critical, Dr. Batchelor explained. At one point during the hold on elective procedures, the Falls Church INOVA facility had approximately 300 patients with COVID-19, a significant proportion of whom were in cardiac ICU beds.
“Everyone has to be flexible and learn,” he said. “We trained our cardiologists on managing ventilators in case some of the [critical care] staff got ill or were overwhelmed by the surge.”
More than 2 months after the surge eased and the ban on elective surgery was lifted, Dr. Batchelor and his colleagues are still using the dashboard and continue to meet daily to discuss COVID-19 prevalence in the hospital and the community as they work through the backlog of delayed procedures. They also routinely review the status of COVID-19 testing for inpatients and outpatients and the donning and doffing of personal protective equipment.
“You have to communicate early and often across the whole system of care because you’re competing for the same resources,” he advised. “And you have to be flexible and reassess. A policy that works at the beginning of the pandemic might have to change.”
PCI in New York
Before the pandemic, the cardiac catheterization laboratory at Mount Sinai Morningside Hospital in New York handled a monthly average of 140-150 PCIs, including 6-10 primary PCIs for ST-segment elevation myocardial infarction.
When electives were halted by the hospital in March and the City became the global epicenter for COVID-19, the cath lab went quiet. “Even though we were still able to do urgent cases or emergent cases, the case volume dropped tremendously,” said Jacqueline E. Tamis-Holland, MD, associate director of the cardiac catheterization laboratory and director of the interventional cardiology fellowship. “There weren’t many outpatients in our hospital … and by late March and through April, there wasn’t a single acute infarction.”
She and Tak W. Kwan, MD, director of the cardiac catheterization laboratory and professor of medicine at Icahn School of Medicine at Mount Sinai, New York, were prepared to move true STEMI patients into the cath lab for primary PCI without delay unless the staff or system were overrun.
That primary PCI should remain the first-line treatment for STEMI even in cases of confirmed or suspected COVID-19 was recommended by SCAI guidance issued in March and by a consensus statement released by the SCAI, ACC, and American College of Emergency Physicians in April – and “we were very much of the same frame of mind,” Dr. Tamis-Holland said.
Deciding which elective cases could not be delayed required a completely individualized approach, the cardiologists emphasized. Dr. Tamis-Holland had a few patients scheduled for elective PCI when the hold began, and “we spoke every few days or once a week in the beginning, then transitioned to once every 2 weeks,” she said. “With medical therapy and given that they were relatively sedentary, my patients did okay [with the delays].”
For subsequent patients, she considered their symptoms or stress test results. “If it’s someone who I’d [normally] wait until next week to schedule the cath, then we would wait 2 or 3 more weeks, or a month more with careful monitoring,” she said. “Certainly, there was a decrease in the number of abnormal stress tests that I referred to the cath lab during [the surge period].”
Dr. Kwan described one patient who had new-onset congestive heart failure in late March “with a markedly positive nuclear stress test.” The patient was monitored with twice-weekly telemedicine visits and office visits, and a cardiac catheterization was performed in early May as an urgent elective case. “He had severe three-vessel and left main disease,” he said. “Subsequently, [coronary artery bypass surgery] was done.”
There were no changes in the PCI procedure itself in terms of hospital stay (most elective cases at Mt. Sinai are same-day procedures) or in staffing, other than a ban on visiting students or residents. The most important changes during the surge – in addition to stocking enough personal protective equipment – concerned testing. Patients undergoing elective PCI are tested for the novel coronavirus 72 hours before the procedure, and rapid testing is performed in the emergency room for STEMI patients to determine patient disposition after the procedure.
“Until we have the results back we should treat all patients as if they are a patient under investigation or have COVID,” said Dr. Tamis-Holland, who helped develop emergency guidance on STEMI systems of care during the pandemic for the American Heart Association.
In early May, the hospital freed up additional space for cardiac care, allowing more “urgent-elective” PCIs to be done. Some patients were reluctant to proceed, the cardiologists said, because of a no-visitor policy. In mid-June, the hold on elective procedures was lifted, and around the same time, the hospital shifted to a one-visitor policy. Still, some patients opted to continue longer with medical therapy.
Patients need to feel comfortable, and “there is a lag time from the time everything opens up and when patients get their stress tests and their evaluations, and then arrive for PCI,” said Dr. Tamis-Holland.
By mid-July, the cardiologists were anticipating an increase in complications from infarctions among patients who “waited them out at home” – heart failure or mitral valve regurgitation, for instance – but, in their hospital at least, “we haven’t really seen that,” she added.
AFib ablation in Kentucky
As New York experienced its surge, John Mandrola, MD, and other electrophysiologists across the Baptist Health system in Kentucky reached a consensus on how to categorize their procedures. Electrophysiology interventions were classified urgent, emergent, and truly elective in the event that the state’s relatively low case burden of COVID-19 were to significantly worsen.
There was no doubt where AFib ablation sat. “It’s one of the most elective procedures there is” in terms of scheduling under normal circumstances, and it almost always requires an overnight stay and general anesthesia – factors that upped the ante on an elective classification, said Dr. Mandrola.
All AF ablations were deemed elective unless the patient required immediate hospitalization. For 8-10 weeks during the state’s shutdown of elective care, Dr. Mandrola and his partner successfully monitored patients with phone calls. “To be honest,” he said, “most patients did not want to have their AFib ablation anyway until the pandemic slowed and they knew it was safe.”
In some cases, patients reported that their symptoms were improving: “There are so many things to speculate about. ... Was it that everyone took their foot off the accelerator?” Dr. Mandrola thinks that postpandemic outcomes analyses may drive more scrutiny of the necessity of some AFib ablations and other procedures and tests. AFib ablation “has its place but is probably overused,” he said.
During the pause on electives, “the vast majority of procedures we did were pacemaker procedures,” he said. “We also did some atrial flutter ablations, and ablations for ventricular tachycardia and supraventricular tachycardia.” In mid-July, as the COVID-19 case burden in Kentucky remained relatively low, Dr. Mandrola was “up to 120%” of his pre-COVID electrophysiology volume – but ready to scale back again if needed.
Dr. Batchelor reported consulting fees from Boston Scientific, Abbott Medical, Medtronic, and V-wave. Dr. Kwan, Dr. Mandrola, and Dr. Tamis-Holland reported no relevant financial disclosures.
This article is a collaboration between Medscape and MDedge. A version of it originally appeared on Medscape.com.
When Virginia’s governor directed the postponement of all elective surgeries in late March, Wayne Batchelor, MD and his colleagues at the Inova Heart and Vascular Institute in Falls Church, Va., canceled about two-thirds of their transcatheter aortic valve replacement (TAVR) procedures.
They then categorized patients by tiers to gauge which procedures could safely be postponed and to guide triaging. And while they did not deviate from the practice of having both an interventional cardiologist and a cardiothoracic surgeon present for TAVR, they slimmed down preprocedural testing when feasible and delayed some 30-day post-TAVR echocardiographic assessments. “It was a delicate dance, very difficult dance. But luckily, we were able to navigate the challenges effectively,” said Dr. Batchelor, the institute’s director of interventional cardiology and interventional cardiology research.
A “system capacity dashboard” that merged bed and staffing data from interventional cardiology spaces with cardiovascular and noncardiovascular ICU beds, operating rooms, and other resources – and daily cross-department meetings – enabled them to proceed with the most urgent TAVR procedures while “keeping a buffer of ICU beds to accommodate an anticipated surge of COVID-19,” he explained.
Such adaptations in cardiac procedures and processes are occurring in hospitals across the country as efforts are made to minimize the risk of COVID-19 exposure for patients and staff. Dr. Batchelor is one of four cardiologists who shared their experiences and advice on common cardiac procedures across three locales: TAVR in Virginia, percutaneous coronary intervention (PCI) in New York City, and atrial fibrillation (AFib) ablation in Kentucky.
More on TAVR in Virginia
Inova’s framework for triaging structural heart disease interventions (largely TAVR and/or percutaneous mitral valve repair) comprised three tiers. Tier 1 captured “emergent cases that had to be done, no questions asked,” Dr. Batchelor said. For TAVR, these were inpatients with severe to critical symptomatic aortic stenosis and advanced congestive heart failure who could not safely be discharged, as well as other patients “with refractory symptoms of heart failure that were compelling.” Many had associated left ventricular systolic dysfunction.
Those who could delay 14-30 days were placed in tier 2, and patients who “we felt were fairly stable and could wait at least 30 days” were placed in tier 3. “For TAVR, a tier 3 patient might be the one … who has severe aortic stenosis but is walking around and doing well at home with only stable exertional symptoms,” he said.
Patients whose procedures were delayed were contacted weekly by the valve clinic’s advanced practice practitioners through video visits or telephone calls, and tier categorization was reevaluated if symptoms worsened. “We had to keep in close contact with them,” Dr. Batchelor said. “These patients can deteriorate quite rapidly and sometimes without much warning.”
Virtual video visits were often used for 30-day postprocedural follow-ups, taking the place of in-person visits during which post-TAVR echocardiographic assessments would normally be performed. “For follow-up, we’d often just do a quick visit to check the vascular access site within 7-10 days, and then, if they were doing okay we’d delay the 30-day echo to a later time frame,” he said.
Preprocedural testing was streamlined to minimize the number of patient-provider interactions, with pulmonary function testing and pre-TAVR catheterization omitted unless absolutely necessary. “A TAVR CT angiogram [performed within the prior year] is the only test you really absolutely need,” Dr. Batchelor said. “We were much less likely to order a heart catheterization unless the patient was having angina and high risk or suspicion for significant coronary artery disease.”
This approach was not associated with any compromise in postprocedural outcomes, he noted. Prior to the pandemic, Inova routinely employed a minimalist approach to TAVR with moderate conscious sedation and avoiding transesophageal echocardiography – steps that were recommended for structural heart procedures in the COVID-19 era in a published review by the heart team at New York-Presbyterian Hospital/Columbia University Irving Medical Center.
The New York review is useful for cardiologists in areas with rising case burdens of COVID-19, Dr. Batchelor said, as is a position statement he coauthored from the American College of Cardiology and the Society for Cardiology and Angiography Interventions (SCAI) on triage considerations for structural heart disease interventions during the pandemic.
TAVR’s resource-heavy nature made the “system capacity dashboard” and daily meetings critical, Dr. Batchelor explained. At one point during the hold on elective procedures, the Falls Church INOVA facility had approximately 300 patients with COVID-19, a significant proportion of whom were in cardiac ICU beds.
“Everyone has to be flexible and learn,” he said. “We trained our cardiologists on managing ventilators in case some of the [critical care] staff got ill or were overwhelmed by the surge.”
More than 2 months after the surge eased and the ban on elective surgery was lifted, Dr. Batchelor and his colleagues are still using the dashboard and continue to meet daily to discuss COVID-19 prevalence in the hospital and the community as they work through the backlog of delayed procedures. They also routinely review the status of COVID-19 testing for inpatients and outpatients and the donning and doffing of personal protective equipment.
“You have to communicate early and often across the whole system of care because you’re competing for the same resources,” he advised. “And you have to be flexible and reassess. A policy that works at the beginning of the pandemic might have to change.”
PCI in New York
Before the pandemic, the cardiac catheterization laboratory at Mount Sinai Morningside Hospital in New York handled a monthly average of 140-150 PCIs, including 6-10 primary PCIs for ST-segment elevation myocardial infarction.
When electives were halted by the hospital in March and the City became the global epicenter for COVID-19, the cath lab went quiet. “Even though we were still able to do urgent cases or emergent cases, the case volume dropped tremendously,” said Jacqueline E. Tamis-Holland, MD, associate director of the cardiac catheterization laboratory and director of the interventional cardiology fellowship. “There weren’t many outpatients in our hospital … and by late March and through April, there wasn’t a single acute infarction.”
She and Tak W. Kwan, MD, director of the cardiac catheterization laboratory and professor of medicine at Icahn School of Medicine at Mount Sinai, New York, were prepared to move true STEMI patients into the cath lab for primary PCI without delay unless the staff or system were overrun.
That primary PCI should remain the first-line treatment for STEMI even in cases of confirmed or suspected COVID-19 was recommended by SCAI guidance issued in March and by a consensus statement released by the SCAI, ACC, and American College of Emergency Physicians in April – and “we were very much of the same frame of mind,” Dr. Tamis-Holland said.
Deciding which elective cases could not be delayed required a completely individualized approach, the cardiologists emphasized. Dr. Tamis-Holland had a few patients scheduled for elective PCI when the hold began, and “we spoke every few days or once a week in the beginning, then transitioned to once every 2 weeks,” she said. “With medical therapy and given that they were relatively sedentary, my patients did okay [with the delays].”
For subsequent patients, she considered their symptoms or stress test results. “If it’s someone who I’d [normally] wait until next week to schedule the cath, then we would wait 2 or 3 more weeks, or a month more with careful monitoring,” she said. “Certainly, there was a decrease in the number of abnormal stress tests that I referred to the cath lab during [the surge period].”
Dr. Kwan described one patient who had new-onset congestive heart failure in late March “with a markedly positive nuclear stress test.” The patient was monitored with twice-weekly telemedicine visits and office visits, and a cardiac catheterization was performed in early May as an urgent elective case. “He had severe three-vessel and left main disease,” he said. “Subsequently, [coronary artery bypass surgery] was done.”
There were no changes in the PCI procedure itself in terms of hospital stay (most elective cases at Mt. Sinai are same-day procedures) or in staffing, other than a ban on visiting students or residents. The most important changes during the surge – in addition to stocking enough personal protective equipment – concerned testing. Patients undergoing elective PCI are tested for the novel coronavirus 72 hours before the procedure, and rapid testing is performed in the emergency room for STEMI patients to determine patient disposition after the procedure.
“Until we have the results back we should treat all patients as if they are a patient under investigation or have COVID,” said Dr. Tamis-Holland, who helped develop emergency guidance on STEMI systems of care during the pandemic for the American Heart Association.
In early May, the hospital freed up additional space for cardiac care, allowing more “urgent-elective” PCIs to be done. Some patients were reluctant to proceed, the cardiologists said, because of a no-visitor policy. In mid-June, the hold on elective procedures was lifted, and around the same time, the hospital shifted to a one-visitor policy. Still, some patients opted to continue longer with medical therapy.
Patients need to feel comfortable, and “there is a lag time from the time everything opens up and when patients get their stress tests and their evaluations, and then arrive for PCI,” said Dr. Tamis-Holland.
By mid-July, the cardiologists were anticipating an increase in complications from infarctions among patients who “waited them out at home” – heart failure or mitral valve regurgitation, for instance – but, in their hospital at least, “we haven’t really seen that,” she added.
AFib ablation in Kentucky
As New York experienced its surge, John Mandrola, MD, and other electrophysiologists across the Baptist Health system in Kentucky reached a consensus on how to categorize their procedures. Electrophysiology interventions were classified urgent, emergent, and truly elective in the event that the state’s relatively low case burden of COVID-19 were to significantly worsen.
There was no doubt where AFib ablation sat. “It’s one of the most elective procedures there is” in terms of scheduling under normal circumstances, and it almost always requires an overnight stay and general anesthesia – factors that upped the ante on an elective classification, said Dr. Mandrola.
All AF ablations were deemed elective unless the patient required immediate hospitalization. For 8-10 weeks during the state’s shutdown of elective care, Dr. Mandrola and his partner successfully monitored patients with phone calls. “To be honest,” he said, “most patients did not want to have their AFib ablation anyway until the pandemic slowed and they knew it was safe.”
In some cases, patients reported that their symptoms were improving: “There are so many things to speculate about. ... Was it that everyone took their foot off the accelerator?” Dr. Mandrola thinks that postpandemic outcomes analyses may drive more scrutiny of the necessity of some AFib ablations and other procedures and tests. AFib ablation “has its place but is probably overused,” he said.
During the pause on electives, “the vast majority of procedures we did were pacemaker procedures,” he said. “We also did some atrial flutter ablations, and ablations for ventricular tachycardia and supraventricular tachycardia.” In mid-July, as the COVID-19 case burden in Kentucky remained relatively low, Dr. Mandrola was “up to 120%” of his pre-COVID electrophysiology volume – but ready to scale back again if needed.
Dr. Batchelor reported consulting fees from Boston Scientific, Abbott Medical, Medtronic, and V-wave. Dr. Kwan, Dr. Mandrola, and Dr. Tamis-Holland reported no relevant financial disclosures.
This article is a collaboration between Medscape and MDedge. A version of it originally appeared on Medscape.com.
When Virginia’s governor directed the postponement of all elective surgeries in late March, Wayne Batchelor, MD and his colleagues at the Inova Heart and Vascular Institute in Falls Church, Va., canceled about two-thirds of their transcatheter aortic valve replacement (TAVR) procedures.
They then categorized patients by tiers to gauge which procedures could safely be postponed and to guide triaging. And while they did not deviate from the practice of having both an interventional cardiologist and a cardiothoracic surgeon present for TAVR, they slimmed down preprocedural testing when feasible and delayed some 30-day post-TAVR echocardiographic assessments. “It was a delicate dance, very difficult dance. But luckily, we were able to navigate the challenges effectively,” said Dr. Batchelor, the institute’s director of interventional cardiology and interventional cardiology research.
A “system capacity dashboard” that merged bed and staffing data from interventional cardiology spaces with cardiovascular and noncardiovascular ICU beds, operating rooms, and other resources – and daily cross-department meetings – enabled them to proceed with the most urgent TAVR procedures while “keeping a buffer of ICU beds to accommodate an anticipated surge of COVID-19,” he explained.
Such adaptations in cardiac procedures and processes are occurring in hospitals across the country as efforts are made to minimize the risk of COVID-19 exposure for patients and staff. Dr. Batchelor is one of four cardiologists who shared their experiences and advice on common cardiac procedures across three locales: TAVR in Virginia, percutaneous coronary intervention (PCI) in New York City, and atrial fibrillation (AFib) ablation in Kentucky.
More on TAVR in Virginia
Inova’s framework for triaging structural heart disease interventions (largely TAVR and/or percutaneous mitral valve repair) comprised three tiers. Tier 1 captured “emergent cases that had to be done, no questions asked,” Dr. Batchelor said. For TAVR, these were inpatients with severe to critical symptomatic aortic stenosis and advanced congestive heart failure who could not safely be discharged, as well as other patients “with refractory symptoms of heart failure that were compelling.” Many had associated left ventricular systolic dysfunction.
Those who could delay 14-30 days were placed in tier 2, and patients who “we felt were fairly stable and could wait at least 30 days” were placed in tier 3. “For TAVR, a tier 3 patient might be the one … who has severe aortic stenosis but is walking around and doing well at home with only stable exertional symptoms,” he said.
Patients whose procedures were delayed were contacted weekly by the valve clinic’s advanced practice practitioners through video visits or telephone calls, and tier categorization was reevaluated if symptoms worsened. “We had to keep in close contact with them,” Dr. Batchelor said. “These patients can deteriorate quite rapidly and sometimes without much warning.”
Virtual video visits were often used for 30-day postprocedural follow-ups, taking the place of in-person visits during which post-TAVR echocardiographic assessments would normally be performed. “For follow-up, we’d often just do a quick visit to check the vascular access site within 7-10 days, and then, if they were doing okay we’d delay the 30-day echo to a later time frame,” he said.
Preprocedural testing was streamlined to minimize the number of patient-provider interactions, with pulmonary function testing and pre-TAVR catheterization omitted unless absolutely necessary. “A TAVR CT angiogram [performed within the prior year] is the only test you really absolutely need,” Dr. Batchelor said. “We were much less likely to order a heart catheterization unless the patient was having angina and high risk or suspicion for significant coronary artery disease.”
This approach was not associated with any compromise in postprocedural outcomes, he noted. Prior to the pandemic, Inova routinely employed a minimalist approach to TAVR with moderate conscious sedation and avoiding transesophageal echocardiography – steps that were recommended for structural heart procedures in the COVID-19 era in a published review by the heart team at New York-Presbyterian Hospital/Columbia University Irving Medical Center.
The New York review is useful for cardiologists in areas with rising case burdens of COVID-19, Dr. Batchelor said, as is a position statement he coauthored from the American College of Cardiology and the Society for Cardiology and Angiography Interventions (SCAI) on triage considerations for structural heart disease interventions during the pandemic.
TAVR’s resource-heavy nature made the “system capacity dashboard” and daily meetings critical, Dr. Batchelor explained. At one point during the hold on elective procedures, the Falls Church INOVA facility had approximately 300 patients with COVID-19, a significant proportion of whom were in cardiac ICU beds.
“Everyone has to be flexible and learn,” he said. “We trained our cardiologists on managing ventilators in case some of the [critical care] staff got ill or were overwhelmed by the surge.”
More than 2 months after the surge eased and the ban on elective surgery was lifted, Dr. Batchelor and his colleagues are still using the dashboard and continue to meet daily to discuss COVID-19 prevalence in the hospital and the community as they work through the backlog of delayed procedures. They also routinely review the status of COVID-19 testing for inpatients and outpatients and the donning and doffing of personal protective equipment.
“You have to communicate early and often across the whole system of care because you’re competing for the same resources,” he advised. “And you have to be flexible and reassess. A policy that works at the beginning of the pandemic might have to change.”
PCI in New York
Before the pandemic, the cardiac catheterization laboratory at Mount Sinai Morningside Hospital in New York handled a monthly average of 140-150 PCIs, including 6-10 primary PCIs for ST-segment elevation myocardial infarction.
When electives were halted by the hospital in March and the City became the global epicenter for COVID-19, the cath lab went quiet. “Even though we were still able to do urgent cases or emergent cases, the case volume dropped tremendously,” said Jacqueline E. Tamis-Holland, MD, associate director of the cardiac catheterization laboratory and director of the interventional cardiology fellowship. “There weren’t many outpatients in our hospital … and by late March and through April, there wasn’t a single acute infarction.”
She and Tak W. Kwan, MD, director of the cardiac catheterization laboratory and professor of medicine at Icahn School of Medicine at Mount Sinai, New York, were prepared to move true STEMI patients into the cath lab for primary PCI without delay unless the staff or system were overrun.
That primary PCI should remain the first-line treatment for STEMI even in cases of confirmed or suspected COVID-19 was recommended by SCAI guidance issued in March and by a consensus statement released by the SCAI, ACC, and American College of Emergency Physicians in April – and “we were very much of the same frame of mind,” Dr. Tamis-Holland said.
Deciding which elective cases could not be delayed required a completely individualized approach, the cardiologists emphasized. Dr. Tamis-Holland had a few patients scheduled for elective PCI when the hold began, and “we spoke every few days or once a week in the beginning, then transitioned to once every 2 weeks,” she said. “With medical therapy and given that they were relatively sedentary, my patients did okay [with the delays].”
For subsequent patients, she considered their symptoms or stress test results. “If it’s someone who I’d [normally] wait until next week to schedule the cath, then we would wait 2 or 3 more weeks, or a month more with careful monitoring,” she said. “Certainly, there was a decrease in the number of abnormal stress tests that I referred to the cath lab during [the surge period].”
Dr. Kwan described one patient who had new-onset congestive heart failure in late March “with a markedly positive nuclear stress test.” The patient was monitored with twice-weekly telemedicine visits and office visits, and a cardiac catheterization was performed in early May as an urgent elective case. “He had severe three-vessel and left main disease,” he said. “Subsequently, [coronary artery bypass surgery] was done.”
There were no changes in the PCI procedure itself in terms of hospital stay (most elective cases at Mt. Sinai are same-day procedures) or in staffing, other than a ban on visiting students or residents. The most important changes during the surge – in addition to stocking enough personal protective equipment – concerned testing. Patients undergoing elective PCI are tested for the novel coronavirus 72 hours before the procedure, and rapid testing is performed in the emergency room for STEMI patients to determine patient disposition after the procedure.
“Until we have the results back we should treat all patients as if they are a patient under investigation or have COVID,” said Dr. Tamis-Holland, who helped develop emergency guidance on STEMI systems of care during the pandemic for the American Heart Association.
In early May, the hospital freed up additional space for cardiac care, allowing more “urgent-elective” PCIs to be done. Some patients were reluctant to proceed, the cardiologists said, because of a no-visitor policy. In mid-June, the hold on elective procedures was lifted, and around the same time, the hospital shifted to a one-visitor policy. Still, some patients opted to continue longer with medical therapy.
Patients need to feel comfortable, and “there is a lag time from the time everything opens up and when patients get their stress tests and their evaluations, and then arrive for PCI,” said Dr. Tamis-Holland.
By mid-July, the cardiologists were anticipating an increase in complications from infarctions among patients who “waited them out at home” – heart failure or mitral valve regurgitation, for instance – but, in their hospital at least, “we haven’t really seen that,” she added.
AFib ablation in Kentucky
As New York experienced its surge, John Mandrola, MD, and other electrophysiologists across the Baptist Health system in Kentucky reached a consensus on how to categorize their procedures. Electrophysiology interventions were classified urgent, emergent, and truly elective in the event that the state’s relatively low case burden of COVID-19 were to significantly worsen.
There was no doubt where AFib ablation sat. “It’s one of the most elective procedures there is” in terms of scheduling under normal circumstances, and it almost always requires an overnight stay and general anesthesia – factors that upped the ante on an elective classification, said Dr. Mandrola.
All AF ablations were deemed elective unless the patient required immediate hospitalization. For 8-10 weeks during the state’s shutdown of elective care, Dr. Mandrola and his partner successfully monitored patients with phone calls. “To be honest,” he said, “most patients did not want to have their AFib ablation anyway until the pandemic slowed and they knew it was safe.”
In some cases, patients reported that their symptoms were improving: “There are so many things to speculate about. ... Was it that everyone took their foot off the accelerator?” Dr. Mandrola thinks that postpandemic outcomes analyses may drive more scrutiny of the necessity of some AFib ablations and other procedures and tests. AFib ablation “has its place but is probably overused,” he said.
During the pause on electives, “the vast majority of procedures we did were pacemaker procedures,” he said. “We also did some atrial flutter ablations, and ablations for ventricular tachycardia and supraventricular tachycardia.” In mid-July, as the COVID-19 case burden in Kentucky remained relatively low, Dr. Mandrola was “up to 120%” of his pre-COVID electrophysiology volume – but ready to scale back again if needed.
Dr. Batchelor reported consulting fees from Boston Scientific, Abbott Medical, Medtronic, and V-wave. Dr. Kwan, Dr. Mandrola, and Dr. Tamis-Holland reported no relevant financial disclosures.
This article is a collaboration between Medscape and MDedge. A version of it originally appeared on Medscape.com.
The best and worst states for health care in 2020
according to the personal finance website WalletHub.
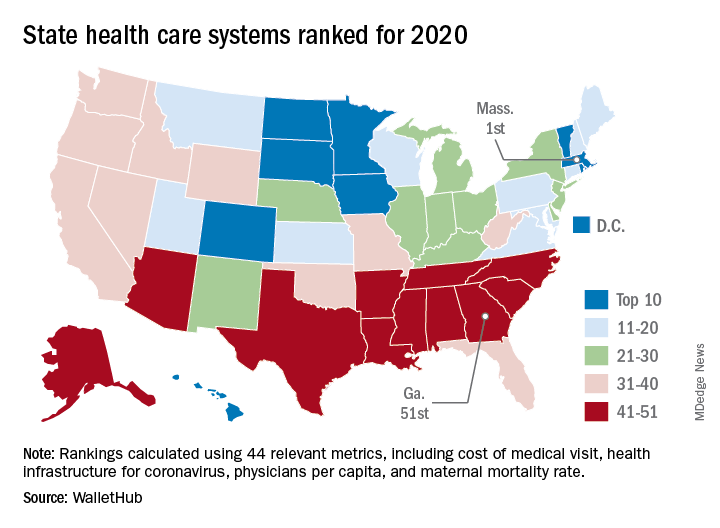
The Bay State finds itself at the top of the company’s annual ranking of state health care systems this year after finishing second in 2019 to Minnesota, which is now ranked second. Rhode Island is third this year, followed by Washington, D.C., and North Dakota, WalletHub reported Aug. 3.
The inclusion of Washington, D.C., allowed Georgia to finish 51st out of 50 states, just below the quartet of Louisiana (50th), Alabama (49th), North Carolina (48th), and Mississippi (47th). Alaska, which occupied the bottom spot in 2019, moved up to 42nd this year, the analysis showed.
The rankings are based on 44 (up from 43 last year) metrics that are grouped into three broad categories: cost (6 metrics), access (24 metrics), and outcomes (14 metrics). The one new measure added for 2020? That would be health infrastructure for coronavirus, which is itself based on a different WalletHub ranking.
Massachusetts’ top finish this year was driven by strong showings in such metrics as average monthly insurance premium (first), physicians per capita (second), insured children (first) and adults (first), and infant mortality rate (fourth). The state was 1st overall in outcomes and 4th in access but only 20th in cost, the company said.
Positive signs among the lowest-ranked states include Louisiana’s 18th-place finish in access, ahead of such top 10 states as Iowa and Hawaii, and Mississippi’s 17th in cost, which is higher than four of the states in the top 10, including Massachusetts, WalletHub said in the report.
Data for the analysis came from 22 different sources, including the Institute for Health Metrics and Evaluation, Centers for Medicare & Medicaid Services, Association of American Medical Colleges, and the American Telemedicine Association.
according to the personal finance website WalletHub.

The Bay State finds itself at the top of the company’s annual ranking of state health care systems this year after finishing second in 2019 to Minnesota, which is now ranked second. Rhode Island is third this year, followed by Washington, D.C., and North Dakota, WalletHub reported Aug. 3.
The inclusion of Washington, D.C., allowed Georgia to finish 51st out of 50 states, just below the quartet of Louisiana (50th), Alabama (49th), North Carolina (48th), and Mississippi (47th). Alaska, which occupied the bottom spot in 2019, moved up to 42nd this year, the analysis showed.
The rankings are based on 44 (up from 43 last year) metrics that are grouped into three broad categories: cost (6 metrics), access (24 metrics), and outcomes (14 metrics). The one new measure added for 2020? That would be health infrastructure for coronavirus, which is itself based on a different WalletHub ranking.
Massachusetts’ top finish this year was driven by strong showings in such metrics as average monthly insurance premium (first), physicians per capita (second), insured children (first) and adults (first), and infant mortality rate (fourth). The state was 1st overall in outcomes and 4th in access but only 20th in cost, the company said.
Positive signs among the lowest-ranked states include Louisiana’s 18th-place finish in access, ahead of such top 10 states as Iowa and Hawaii, and Mississippi’s 17th in cost, which is higher than four of the states in the top 10, including Massachusetts, WalletHub said in the report.
Data for the analysis came from 22 different sources, including the Institute for Health Metrics and Evaluation, Centers for Medicare & Medicaid Services, Association of American Medical Colleges, and the American Telemedicine Association.
according to the personal finance website WalletHub.

The Bay State finds itself at the top of the company’s annual ranking of state health care systems this year after finishing second in 2019 to Minnesota, which is now ranked second. Rhode Island is third this year, followed by Washington, D.C., and North Dakota, WalletHub reported Aug. 3.
The inclusion of Washington, D.C., allowed Georgia to finish 51st out of 50 states, just below the quartet of Louisiana (50th), Alabama (49th), North Carolina (48th), and Mississippi (47th). Alaska, which occupied the bottom spot in 2019, moved up to 42nd this year, the analysis showed.
The rankings are based on 44 (up from 43 last year) metrics that are grouped into three broad categories: cost (6 metrics), access (24 metrics), and outcomes (14 metrics). The one new measure added for 2020? That would be health infrastructure for coronavirus, which is itself based on a different WalletHub ranking.
Massachusetts’ top finish this year was driven by strong showings in such metrics as average monthly insurance premium (first), physicians per capita (second), insured children (first) and adults (first), and infant mortality rate (fourth). The state was 1st overall in outcomes and 4th in access but only 20th in cost, the company said.
Positive signs among the lowest-ranked states include Louisiana’s 18th-place finish in access, ahead of such top 10 states as Iowa and Hawaii, and Mississippi’s 17th in cost, which is higher than four of the states in the top 10, including Massachusetts, WalletHub said in the report.
Data for the analysis came from 22 different sources, including the Institute for Health Metrics and Evaluation, Centers for Medicare & Medicaid Services, Association of American Medical Colleges, and the American Telemedicine Association.
Unexpected rosuvastatin-canagliflozin adverse effect reported
A 76-year-old woman presented recently to a Toronto-area hospital with acute onset muscle pain, limb weakness, difficulty walking, and rhabdomyolysis associated with a sharp spike in her plasma level of rosuvastatin – a drug she had been on uneventfully for more than 5 years, within days of starting for the first time treatment with the SGLT2 inhibitor canagliflozin (Invokana).
The patient’s Canadian clinicians stopped her treatment with both rosuvastatin and canagliflozin, administered intravenous crystalloid fluids, and within days her pain subsided and her limb weakness gradually improved, allowing her discharge 10 days later while she was ambulating with a walker.
“To our knowledge this is the first published report of a drug interaction between rosuvastatin and canagliflozin,” wrote the authors of the case report (Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549). They cited the importance of the observation given the widespread use today of rosuvastatin for lowering low density lipoprotein cholesterol and exerting pleiotropic effects; and canagliflozin for its modest effects for reducing hyperglycemia, as well as its important role in reducing adverse cardiovascular outcomes, slowing progression of chronic kidney disease, and having a mild but important diuretic effect. “We encourage clinicians to remain vigilant for features of myotoxicity when canagliflozin and rosuvastatin are coprescribed,” they wrote, avoiding discussion of whether this may represent class or drug-specific effects.
“It’s reasonable to be mindful of this risk, but this is not a reason to not use rosuvastatin and canagliflozin in a patient,” nor for the time being to avoid any other combination of a statin and SGLT2 (sodium-glucose cotransporter 2) inhibitor, said David Juurlink, MD, head of the division of clinical pharmacology and toxicology at Sunnybrook Health Sciences Centre in Toronto and lead author of the report. “Few drug interactions have absolute contraindications. The admonition is just to be careful. It’s premature to say they shouldn’t be used together,” he said in an interview.
“We don’t know how much of an outlier this patient is. But it would be important to tell patients” on this or a similar combination to alert their clinicians if they start to have muscle aches, which should be a “red flag” to stop the statin, the SGLT2 inhibitor, or both until the situation can be fully assessed, Dr. Juurlink advised.
Sky high rosuvastatin levels
The linchpin of the observed adverse effects appeared to be a startlingly high elevation of the patient’s plasma rosuvastatin level when she was hospitalized 15 days after starting canagliflozin and 12 days after the onset of her thigh pain and weakness. Testing showed a plasma rosuvastatin concentration of 176 ng/mL, “more than 15-fold higher than the mean value expected” in patients taking 40 mg rosuvastatin daily, the maximum labeled dosage for the drug and what the affected patient had been taking without prior incident for more than 5 years. The patient’s canagliflozin dosage was 100 mg/day, the standard starting dosage according to the drug’s label.
The report’s authors noted that genetic assessment of the patient, a woman originally from the Philippines who was “high functioning,” and diagnosed with type 2 diabetes, showed she was heterozygous for a polymorphism, c.421C>A, which is linked with increased rosuvastatin plasma levels in the plasma. They also cited a report that canagliflozin can interact with proteins involved in hepatic drug uptake.
“We speculate that, in our patient, the addition of canagliflozin enhanced intestinal rosuvastatin absorption, inhibited its hepatocellular uptake, and impaired its excretion into bile canaliculi and the proximal tubule, resulting in rosuvastatin accumulation and leading to hepatotoxicity and myotoxicity,” the clinicians wrote in their report.
“There is little doubt this was a drug interaction, but it does not apply uniformly to everyone.” The severity of the interaction would depend on the dosages, the comorbidities a patient has, and their genetic profile, Dr. Juurlink said.
Concern and skepticism
Other clinicians who regularly prescribe these drugs expressed concern about the observation as well as skepticism about the prevalence of patients who could potentially experience similar effects.
“We don’t know how common are these genetic abnormalities. If this is extremely rare, then it doesn’t have many clinical implications, but if a large portion of the population has this [genetic] abnormality, it’s something we’d need to pay attention to,” Steven E. Nissen, MD, chair of cardiovascular medicine at the Cleveland Clinic Foundation, said in an interview. “It will be important to know the prevalence” of the genetic polymorphism carried by the reported patient, said Dr. Nissen, who has done research on lipid-lowering medications and drug safety.
“This could be important, or a very rare one-off. I can’t say which,” based on what’s currently known, he said. “There are many unanswered questions that make it hard to know how important this will be. It requires further investigation. There is a lot of uncertainty.”
Dr. Nissen particularly endorsed studies that approach this issue by looking at the prevalence rates of the implicated genetic polymorphism rather than pharmacovigilance studies that make epidemiologic assessments of adverse-effect prevalence. Studies that look for adverse-effect associations in large patient populations are “sloppy, and unless the interaction is incredibly intense they are not very sensitive,” he said.
But Dr. Juurlink, a pharmacoepidemiologist whose specialty includes studies of this sort, said that they could be useful if carefully designed. He suggested, for example, comparing in large patient databases the observed incidence of rhabdomyolysis among patients on an SGLT2 inhibitor and also on rosuvastatin with those on pravastatin, a statin with a different metabolic profile. Another approach to further examining the observation would be dosage studies with rosuvastatin and canagliflozin in healthy volunteers, he said.
Dr. Nissen noted that rosuvastatin is a key agent from the statin class because it’s the “most effective” for lowering low density lipoprotein cholesterol. “Rosuvastatin is a go-to drug,” he declared. On the other hand, canagliflozin is “a little less used” than other drugs in the SGLT2 inhibitor class, specifically dapagliflozin (Farxiga) and empagliflozin (Jardiance), he said.
One in a million?
“This was a freak accident. I don’t find it at all concerning. It was definitely one in a million,” Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif., said in an interview. “None of us have seen it” in either the several cardiovascular outcome trials now run on multiple drugs in the SGLT2 inhibitor class that included many patients also taking a statin, or in routine practice, he said. Dr. Handelsman noted that in his practice he had never seen a similar case despite treating “hundreds if not thousands of patients” with type 2 diabetes, virtually all of whom were on a statin and were also treated with an SGLT2 inhibitor, including many with canagliflozin.
Dr. Handelsman cited the notably low estimated glomerular filtration rate in the reported patient, who was described as having a serum creatinine level of 150 mcmol/L (1.7 mg/dL) prior to canagliflozin treatment that then rose to 194 mcmol/L (2.19 mg/dL) at the time of hospitalization, which corresponds to estimated glomerular filtration rates of 29-31 and 21-23 mL/min per 1.73 m2, respectively, depending on the calculator used, rates that were possibly below the labeled minimum rate of 30 mL/min per 1.73 m2 for patients starting canagliflozin treatment. The case report cited the patient as having stage 3B chronic kidney disease, which corresponds to a eGFR of 30-44* mL/min per 1.73 m2.
“I think the patient had acute kidney injury” on starting canagliflozin “that may have affected the [rosuvastatin] metabolism,” Dr. Handelsman suggested. “She had severe kidney dysfunction to start with that fell further with SGLT2 inhibitor treatment,” a well described and usually transient effect of starting drugs in this class because of changes the SGLT2 inhibitors cause in renal blood flow. He noted that the patient had not been receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, which may have contributed to her acute problems with fluid balance. Most similar patients with type 2 diabetes, cardiovascular disease risk, and chronic kidney disease would be on stable treatment with a drug that inhibits the renin-angiotensin system before starting an SGLT2 inhibitor, and not already having a RAS inhibitor on board before starting canagliflozin may have somehow contributed to the observed adverse effects, Dr. Handelsman said.
Dr. Juurlink was skeptical that the kidneys played a major role. “An abrupt change in renal function can influence statin clearance, but this was a 15-fold increase. You can’t explain such a dramatic increase by a transient reduction in renal function,” he said.
Dr. Juurlink and coauthors had no disclosures. Dr. Nissen had no relevant disclosures. Dr. Handelsman has been a consultant to companies that market drugs in the SGLT2 inhibitor class.
SOURCE: Brailovski E et al. Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549.
*Correction: This value was missing from the original article.
A 76-year-old woman presented recently to a Toronto-area hospital with acute onset muscle pain, limb weakness, difficulty walking, and rhabdomyolysis associated with a sharp spike in her plasma level of rosuvastatin – a drug she had been on uneventfully for more than 5 years, within days of starting for the first time treatment with the SGLT2 inhibitor canagliflozin (Invokana).
The patient’s Canadian clinicians stopped her treatment with both rosuvastatin and canagliflozin, administered intravenous crystalloid fluids, and within days her pain subsided and her limb weakness gradually improved, allowing her discharge 10 days later while she was ambulating with a walker.
“To our knowledge this is the first published report of a drug interaction between rosuvastatin and canagliflozin,” wrote the authors of the case report (Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549). They cited the importance of the observation given the widespread use today of rosuvastatin for lowering low density lipoprotein cholesterol and exerting pleiotropic effects; and canagliflozin for its modest effects for reducing hyperglycemia, as well as its important role in reducing adverse cardiovascular outcomes, slowing progression of chronic kidney disease, and having a mild but important diuretic effect. “We encourage clinicians to remain vigilant for features of myotoxicity when canagliflozin and rosuvastatin are coprescribed,” they wrote, avoiding discussion of whether this may represent class or drug-specific effects.
“It’s reasonable to be mindful of this risk, but this is not a reason to not use rosuvastatin and canagliflozin in a patient,” nor for the time being to avoid any other combination of a statin and SGLT2 (sodium-glucose cotransporter 2) inhibitor, said David Juurlink, MD, head of the division of clinical pharmacology and toxicology at Sunnybrook Health Sciences Centre in Toronto and lead author of the report. “Few drug interactions have absolute contraindications. The admonition is just to be careful. It’s premature to say they shouldn’t be used together,” he said in an interview.
“We don’t know how much of an outlier this patient is. But it would be important to tell patients” on this or a similar combination to alert their clinicians if they start to have muscle aches, which should be a “red flag” to stop the statin, the SGLT2 inhibitor, or both until the situation can be fully assessed, Dr. Juurlink advised.
Sky high rosuvastatin levels
The linchpin of the observed adverse effects appeared to be a startlingly high elevation of the patient’s plasma rosuvastatin level when she was hospitalized 15 days after starting canagliflozin and 12 days after the onset of her thigh pain and weakness. Testing showed a plasma rosuvastatin concentration of 176 ng/mL, “more than 15-fold higher than the mean value expected” in patients taking 40 mg rosuvastatin daily, the maximum labeled dosage for the drug and what the affected patient had been taking without prior incident for more than 5 years. The patient’s canagliflozin dosage was 100 mg/day, the standard starting dosage according to the drug’s label.
The report’s authors noted that genetic assessment of the patient, a woman originally from the Philippines who was “high functioning,” and diagnosed with type 2 diabetes, showed she was heterozygous for a polymorphism, c.421C>A, which is linked with increased rosuvastatin plasma levels in the plasma. They also cited a report that canagliflozin can interact with proteins involved in hepatic drug uptake.
“We speculate that, in our patient, the addition of canagliflozin enhanced intestinal rosuvastatin absorption, inhibited its hepatocellular uptake, and impaired its excretion into bile canaliculi and the proximal tubule, resulting in rosuvastatin accumulation and leading to hepatotoxicity and myotoxicity,” the clinicians wrote in their report.
“There is little doubt this was a drug interaction, but it does not apply uniformly to everyone.” The severity of the interaction would depend on the dosages, the comorbidities a patient has, and their genetic profile, Dr. Juurlink said.
Concern and skepticism
Other clinicians who regularly prescribe these drugs expressed concern about the observation as well as skepticism about the prevalence of patients who could potentially experience similar effects.
“We don’t know how common are these genetic abnormalities. If this is extremely rare, then it doesn’t have many clinical implications, but if a large portion of the population has this [genetic] abnormality, it’s something we’d need to pay attention to,” Steven E. Nissen, MD, chair of cardiovascular medicine at the Cleveland Clinic Foundation, said in an interview. “It will be important to know the prevalence” of the genetic polymorphism carried by the reported patient, said Dr. Nissen, who has done research on lipid-lowering medications and drug safety.
“This could be important, or a very rare one-off. I can’t say which,” based on what’s currently known, he said. “There are many unanswered questions that make it hard to know how important this will be. It requires further investigation. There is a lot of uncertainty.”
Dr. Nissen particularly endorsed studies that approach this issue by looking at the prevalence rates of the implicated genetic polymorphism rather than pharmacovigilance studies that make epidemiologic assessments of adverse-effect prevalence. Studies that look for adverse-effect associations in large patient populations are “sloppy, and unless the interaction is incredibly intense they are not very sensitive,” he said.
But Dr. Juurlink, a pharmacoepidemiologist whose specialty includes studies of this sort, said that they could be useful if carefully designed. He suggested, for example, comparing in large patient databases the observed incidence of rhabdomyolysis among patients on an SGLT2 inhibitor and also on rosuvastatin with those on pravastatin, a statin with a different metabolic profile. Another approach to further examining the observation would be dosage studies with rosuvastatin and canagliflozin in healthy volunteers, he said.
Dr. Nissen noted that rosuvastatin is a key agent from the statin class because it’s the “most effective” for lowering low density lipoprotein cholesterol. “Rosuvastatin is a go-to drug,” he declared. On the other hand, canagliflozin is “a little less used” than other drugs in the SGLT2 inhibitor class, specifically dapagliflozin (Farxiga) and empagliflozin (Jardiance), he said.
One in a million?
“This was a freak accident. I don’t find it at all concerning. It was definitely one in a million,” Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif., said in an interview. “None of us have seen it” in either the several cardiovascular outcome trials now run on multiple drugs in the SGLT2 inhibitor class that included many patients also taking a statin, or in routine practice, he said. Dr. Handelsman noted that in his practice he had never seen a similar case despite treating “hundreds if not thousands of patients” with type 2 diabetes, virtually all of whom were on a statin and were also treated with an SGLT2 inhibitor, including many with canagliflozin.
Dr. Handelsman cited the notably low estimated glomerular filtration rate in the reported patient, who was described as having a serum creatinine level of 150 mcmol/L (1.7 mg/dL) prior to canagliflozin treatment that then rose to 194 mcmol/L (2.19 mg/dL) at the time of hospitalization, which corresponds to estimated glomerular filtration rates of 29-31 and 21-23 mL/min per 1.73 m2, respectively, depending on the calculator used, rates that were possibly below the labeled minimum rate of 30 mL/min per 1.73 m2 for patients starting canagliflozin treatment. The case report cited the patient as having stage 3B chronic kidney disease, which corresponds to a eGFR of 30-44* mL/min per 1.73 m2.
“I think the patient had acute kidney injury” on starting canagliflozin “that may have affected the [rosuvastatin] metabolism,” Dr. Handelsman suggested. “She had severe kidney dysfunction to start with that fell further with SGLT2 inhibitor treatment,” a well described and usually transient effect of starting drugs in this class because of changes the SGLT2 inhibitors cause in renal blood flow. He noted that the patient had not been receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, which may have contributed to her acute problems with fluid balance. Most similar patients with type 2 diabetes, cardiovascular disease risk, and chronic kidney disease would be on stable treatment with a drug that inhibits the renin-angiotensin system before starting an SGLT2 inhibitor, and not already having a RAS inhibitor on board before starting canagliflozin may have somehow contributed to the observed adverse effects, Dr. Handelsman said.
Dr. Juurlink was skeptical that the kidneys played a major role. “An abrupt change in renal function can influence statin clearance, but this was a 15-fold increase. You can’t explain such a dramatic increase by a transient reduction in renal function,” he said.
Dr. Juurlink and coauthors had no disclosures. Dr. Nissen had no relevant disclosures. Dr. Handelsman has been a consultant to companies that market drugs in the SGLT2 inhibitor class.
SOURCE: Brailovski E et al. Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549.
*Correction: This value was missing from the original article.
A 76-year-old woman presented recently to a Toronto-area hospital with acute onset muscle pain, limb weakness, difficulty walking, and rhabdomyolysis associated with a sharp spike in her plasma level of rosuvastatin – a drug she had been on uneventfully for more than 5 years, within days of starting for the first time treatment with the SGLT2 inhibitor canagliflozin (Invokana).
The patient’s Canadian clinicians stopped her treatment with both rosuvastatin and canagliflozin, administered intravenous crystalloid fluids, and within days her pain subsided and her limb weakness gradually improved, allowing her discharge 10 days later while she was ambulating with a walker.
“To our knowledge this is the first published report of a drug interaction between rosuvastatin and canagliflozin,” wrote the authors of the case report (Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549). They cited the importance of the observation given the widespread use today of rosuvastatin for lowering low density lipoprotein cholesterol and exerting pleiotropic effects; and canagliflozin for its modest effects for reducing hyperglycemia, as well as its important role in reducing adverse cardiovascular outcomes, slowing progression of chronic kidney disease, and having a mild but important diuretic effect. “We encourage clinicians to remain vigilant for features of myotoxicity when canagliflozin and rosuvastatin are coprescribed,” they wrote, avoiding discussion of whether this may represent class or drug-specific effects.
“It’s reasonable to be mindful of this risk, but this is not a reason to not use rosuvastatin and canagliflozin in a patient,” nor for the time being to avoid any other combination of a statin and SGLT2 (sodium-glucose cotransporter 2) inhibitor, said David Juurlink, MD, head of the division of clinical pharmacology and toxicology at Sunnybrook Health Sciences Centre in Toronto and lead author of the report. “Few drug interactions have absolute contraindications. The admonition is just to be careful. It’s premature to say they shouldn’t be used together,” he said in an interview.
“We don’t know how much of an outlier this patient is. But it would be important to tell patients” on this or a similar combination to alert their clinicians if they start to have muscle aches, which should be a “red flag” to stop the statin, the SGLT2 inhibitor, or both until the situation can be fully assessed, Dr. Juurlink advised.
Sky high rosuvastatin levels
The linchpin of the observed adverse effects appeared to be a startlingly high elevation of the patient’s plasma rosuvastatin level when she was hospitalized 15 days after starting canagliflozin and 12 days after the onset of her thigh pain and weakness. Testing showed a plasma rosuvastatin concentration of 176 ng/mL, “more than 15-fold higher than the mean value expected” in patients taking 40 mg rosuvastatin daily, the maximum labeled dosage for the drug and what the affected patient had been taking without prior incident for more than 5 years. The patient’s canagliflozin dosage was 100 mg/day, the standard starting dosage according to the drug’s label.
The report’s authors noted that genetic assessment of the patient, a woman originally from the Philippines who was “high functioning,” and diagnosed with type 2 diabetes, showed she was heterozygous for a polymorphism, c.421C>A, which is linked with increased rosuvastatin plasma levels in the plasma. They also cited a report that canagliflozin can interact with proteins involved in hepatic drug uptake.
“We speculate that, in our patient, the addition of canagliflozin enhanced intestinal rosuvastatin absorption, inhibited its hepatocellular uptake, and impaired its excretion into bile canaliculi and the proximal tubule, resulting in rosuvastatin accumulation and leading to hepatotoxicity and myotoxicity,” the clinicians wrote in their report.
“There is little doubt this was a drug interaction, but it does not apply uniformly to everyone.” The severity of the interaction would depend on the dosages, the comorbidities a patient has, and their genetic profile, Dr. Juurlink said.
Concern and skepticism
Other clinicians who regularly prescribe these drugs expressed concern about the observation as well as skepticism about the prevalence of patients who could potentially experience similar effects.
“We don’t know how common are these genetic abnormalities. If this is extremely rare, then it doesn’t have many clinical implications, but if a large portion of the population has this [genetic] abnormality, it’s something we’d need to pay attention to,” Steven E. Nissen, MD, chair of cardiovascular medicine at the Cleveland Clinic Foundation, said in an interview. “It will be important to know the prevalence” of the genetic polymorphism carried by the reported patient, said Dr. Nissen, who has done research on lipid-lowering medications and drug safety.
“This could be important, or a very rare one-off. I can’t say which,” based on what’s currently known, he said. “There are many unanswered questions that make it hard to know how important this will be. It requires further investigation. There is a lot of uncertainty.”
Dr. Nissen particularly endorsed studies that approach this issue by looking at the prevalence rates of the implicated genetic polymorphism rather than pharmacovigilance studies that make epidemiologic assessments of adverse-effect prevalence. Studies that look for adverse-effect associations in large patient populations are “sloppy, and unless the interaction is incredibly intense they are not very sensitive,” he said.
But Dr. Juurlink, a pharmacoepidemiologist whose specialty includes studies of this sort, said that they could be useful if carefully designed. He suggested, for example, comparing in large patient databases the observed incidence of rhabdomyolysis among patients on an SGLT2 inhibitor and also on rosuvastatin with those on pravastatin, a statin with a different metabolic profile. Another approach to further examining the observation would be dosage studies with rosuvastatin and canagliflozin in healthy volunteers, he said.
Dr. Nissen noted that rosuvastatin is a key agent from the statin class because it’s the “most effective” for lowering low density lipoprotein cholesterol. “Rosuvastatin is a go-to drug,” he declared. On the other hand, canagliflozin is “a little less used” than other drugs in the SGLT2 inhibitor class, specifically dapagliflozin (Farxiga) and empagliflozin (Jardiance), he said.
One in a million?
“This was a freak accident. I don’t find it at all concerning. It was definitely one in a million,” Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of America in Tarzana, Calif., said in an interview. “None of us have seen it” in either the several cardiovascular outcome trials now run on multiple drugs in the SGLT2 inhibitor class that included many patients also taking a statin, or in routine practice, he said. Dr. Handelsman noted that in his practice he had never seen a similar case despite treating “hundreds if not thousands of patients” with type 2 diabetes, virtually all of whom were on a statin and were also treated with an SGLT2 inhibitor, including many with canagliflozin.
Dr. Handelsman cited the notably low estimated glomerular filtration rate in the reported patient, who was described as having a serum creatinine level of 150 mcmol/L (1.7 mg/dL) prior to canagliflozin treatment that then rose to 194 mcmol/L (2.19 mg/dL) at the time of hospitalization, which corresponds to estimated glomerular filtration rates of 29-31 and 21-23 mL/min per 1.73 m2, respectively, depending on the calculator used, rates that were possibly below the labeled minimum rate of 30 mL/min per 1.73 m2 for patients starting canagliflozin treatment. The case report cited the patient as having stage 3B chronic kidney disease, which corresponds to a eGFR of 30-44* mL/min per 1.73 m2.
“I think the patient had acute kidney injury” on starting canagliflozin “that may have affected the [rosuvastatin] metabolism,” Dr. Handelsman suggested. “She had severe kidney dysfunction to start with that fell further with SGLT2 inhibitor treatment,” a well described and usually transient effect of starting drugs in this class because of changes the SGLT2 inhibitors cause in renal blood flow. He noted that the patient had not been receiving an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker, which may have contributed to her acute problems with fluid balance. Most similar patients with type 2 diabetes, cardiovascular disease risk, and chronic kidney disease would be on stable treatment with a drug that inhibits the renin-angiotensin system before starting an SGLT2 inhibitor, and not already having a RAS inhibitor on board before starting canagliflozin may have somehow contributed to the observed adverse effects, Dr. Handelsman said.
Dr. Juurlink was skeptical that the kidneys played a major role. “An abrupt change in renal function can influence statin clearance, but this was a 15-fold increase. You can’t explain such a dramatic increase by a transient reduction in renal function,” he said.
Dr. Juurlink and coauthors had no disclosures. Dr. Nissen had no relevant disclosures. Dr. Handelsman has been a consultant to companies that market drugs in the SGLT2 inhibitor class.
SOURCE: Brailovski E et al. Ann Intern Med. 2020 Aug 3. doi: 10.7326/L20-0549.
*Correction: This value was missing from the original article.
FROM ANNALS OF INTERNAL MEDICINE
Most younger MI patients wouldn’t get statins under guidelines
Clinical guidelines for cholesterol management may have two blind spots when it comes to heart attack prevention: Most younger adults with premature coronary artery disease who’ve had a myocardial infarction don’t meet guideline criteria for preventative statin therapy, and survivors under age 55 don’t meet the criteria for continuing nonstatin lipid-lowering treatments, a large single-center retrospective study has shown.
“The classic approach we’ve taken to identifying young adults for prevention is inadequate in younger adults,” corresponding author Ann Marie Navar, MD, PhD, of Duke University, Durham, N.C., said in an interview. “While awaiting more definitive research we should at minimum be using all the tools at our disposal, including broader use of coronary artery calcium [CAC] scoring, to identify young people who may benefit from statin therapy.”
The retrospective observational study analyzed records of 6,639 adults who had cardiac catheterization at Duke University Medical Center from 1995 to 2012 for a first myocardial infarction with obstructive coronary artery disease. The study considered those under age 55 years as “younger” patients, comprising 41% of the study group (2,733); 35% were “middle-aged” at 55-65 years (2,324) and 24% were “older,” at 66-75 years (1,582).
The report, published online Aug. 3 in the Journal of the American College of Cardiology, noted that most of the adults with premature CAD did not meet criteria for preventative statin therapy before their first MI based on ACC/American Heart Association clinical guidelines from 2013 and 2018. It also noted that younger MI survivors are also less frequently eligible for secondary prevention with intensive nonstatin lipid-lowering therapies than are older adults despite a much longer potential life span – and opportunity for another MI – for the former.
The researchers sought to evaluate the real-world implications of changes made in the 2018 guideline for adults who develop premature ischemic heart disease, and found that fewer younger patients qualify for preventative statin therapy under the 2018 guidelines.
“Younger individuals with very high-risk criteria are at higher risk of major adverse cardiovascular events, a finding supporting the appropriate implementation of intensive lipid-lowering therapies in these patients,” wrote lead author Michel Zeitouni, MD, MSc, and colleagues.
Key findings
The investigators reported that younger adults were significantly less likely to meet a class I recommendation for statins under the 2013 guideline (42.9%), compared with their middle-aged (70%) and older (82.5%) counterparts; and under the 2018 guideline, at 39.4%, 59.5%, and 77.4%, respectively (both P < .001).
Similarly, when both class I and class IIa recommendations were accounted for, younger patients were significantly less likely than were middle-aged and older patients to be eligible for statins before their index MI under both the 2013 (56.7%, 79.5%, and 85.2%, respectively and 2018 guidelines (46.4%, 73.5%, and 88.2%, respectively (both P < .01).
After their first MI, one in four younger patients (28.3%) met the very high-risk criteria compared with 40% of middle-aged and 81.4% of older patients (P trend < .001). In 8 years of follow-up, patients with very high-risk criteria based on the 2018 guideline had twice the rate of death, nonfatal MI, or stroke (hazard ratio [HR]: 2.15; 95% confidence interval, 1.98-2.33; P < .001).
The researchers acknowledged that the 2018 guideline took the important step of implementing risk enhancers – patient characteristics such as obesity and metabolic syndrome – along with the 10-year atherosclerotic cardiovascular disease (ASCVD) risk score to better identify high-risk young individuals who need statins. However, they also noted that the ability of the guidelines to identify young adults before their first MI “remains suboptimal.”
How to protect younger patients
“The 2018 guidelines will be most effective if we as providers do our best to identify risk enhancers and if we can use CAC scoring more broadly,” Dr. Navar said, noting that although CAC scoring has been shown to improve risk prediction, insurance coverage can be problematic.
“We also need to be careful to screen for the presence of the risk enhancers, such as inflammatory disease, family history, and women-specific risk factors, to make sure we aren’t missing an important high-risk group,” she added.
Other solutions to better identify at-risk younger adults include considering upgrades to the guidelines’ class IIb recommendation to class IIa to emphasize the importance of recognizing lower-risk younger adults, and recommending statins for patients at higher lifetime risk than age- and sex-matched peers, the researchers noted. “In our cohort, young individuals admitted for a first MI had a higher lifetime ASCVD risk score than did patients in the older age categories,” Dr. Zeitouni and colleagues wrote.
Dr. Navar said that these findings are a reminder that guidelines aren’t mandates. “Guidelines are meant to be a starting point for patients and physicians,” she said. “The absence of a recommendation doesn’t mean something isn’t recommended, but that there is not enough data to say one way or another.”
The study “provides important evidence” that the 2018 guidelines exempted about half of the younger adults who had a first MI from preventative statin therapy, Ron Blankstein, MD, and Avinainder Singh, MD, MMSc, noted in an editorial (J Am Coll Cardiol. 2020;76:665-8).
“Data from both the Duke and Young-MI registries should force us to reexamine how we allocate statin use among young individuals,” they noted. Dr. Blankstein is with Brigham and Women’s Hospital, Harvard Medical School, Boston. Dr. Singh is with Yale University, New Haven, Conn.
Dr. Zeitouni reported receiving lecture fees from Bristol-Myers Squibb/Pfizer. Dr. Navar reported financial relationships with Amarin, Janssen, Amgen, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Esperion, Novo Nordisk, Novartis, The Medicine Company, New Amsterdam, Cerner and Pfizer. Dr. Blankstein reported receiving research support from Amgen. Dr. Singh has no relevant financial relationships to report.
SOURCE: M. Zeitouni et al. J Am Coll Cardiol 2020 Aug 3;76:653-64.
Clinical guidelines for cholesterol management may have two blind spots when it comes to heart attack prevention: Most younger adults with premature coronary artery disease who’ve had a myocardial infarction don’t meet guideline criteria for preventative statin therapy, and survivors under age 55 don’t meet the criteria for continuing nonstatin lipid-lowering treatments, a large single-center retrospective study has shown.
“The classic approach we’ve taken to identifying young adults for prevention is inadequate in younger adults,” corresponding author Ann Marie Navar, MD, PhD, of Duke University, Durham, N.C., said in an interview. “While awaiting more definitive research we should at minimum be using all the tools at our disposal, including broader use of coronary artery calcium [CAC] scoring, to identify young people who may benefit from statin therapy.”
The retrospective observational study analyzed records of 6,639 adults who had cardiac catheterization at Duke University Medical Center from 1995 to 2012 for a first myocardial infarction with obstructive coronary artery disease. The study considered those under age 55 years as “younger” patients, comprising 41% of the study group (2,733); 35% were “middle-aged” at 55-65 years (2,324) and 24% were “older,” at 66-75 years (1,582).
The report, published online Aug. 3 in the Journal of the American College of Cardiology, noted that most of the adults with premature CAD did not meet criteria for preventative statin therapy before their first MI based on ACC/American Heart Association clinical guidelines from 2013 and 2018. It also noted that younger MI survivors are also less frequently eligible for secondary prevention with intensive nonstatin lipid-lowering therapies than are older adults despite a much longer potential life span – and opportunity for another MI – for the former.
The researchers sought to evaluate the real-world implications of changes made in the 2018 guideline for adults who develop premature ischemic heart disease, and found that fewer younger patients qualify for preventative statin therapy under the 2018 guidelines.
“Younger individuals with very high-risk criteria are at higher risk of major adverse cardiovascular events, a finding supporting the appropriate implementation of intensive lipid-lowering therapies in these patients,” wrote lead author Michel Zeitouni, MD, MSc, and colleagues.
Key findings
The investigators reported that younger adults were significantly less likely to meet a class I recommendation for statins under the 2013 guideline (42.9%), compared with their middle-aged (70%) and older (82.5%) counterparts; and under the 2018 guideline, at 39.4%, 59.5%, and 77.4%, respectively (both P < .001).
Similarly, when both class I and class IIa recommendations were accounted for, younger patients were significantly less likely than were middle-aged and older patients to be eligible for statins before their index MI under both the 2013 (56.7%, 79.5%, and 85.2%, respectively and 2018 guidelines (46.4%, 73.5%, and 88.2%, respectively (both P < .01).
After their first MI, one in four younger patients (28.3%) met the very high-risk criteria compared with 40% of middle-aged and 81.4% of older patients (P trend < .001). In 8 years of follow-up, patients with very high-risk criteria based on the 2018 guideline had twice the rate of death, nonfatal MI, or stroke (hazard ratio [HR]: 2.15; 95% confidence interval, 1.98-2.33; P < .001).
The researchers acknowledged that the 2018 guideline took the important step of implementing risk enhancers – patient characteristics such as obesity and metabolic syndrome – along with the 10-year atherosclerotic cardiovascular disease (ASCVD) risk score to better identify high-risk young individuals who need statins. However, they also noted that the ability of the guidelines to identify young adults before their first MI “remains suboptimal.”
How to protect younger patients
“The 2018 guidelines will be most effective if we as providers do our best to identify risk enhancers and if we can use CAC scoring more broadly,” Dr. Navar said, noting that although CAC scoring has been shown to improve risk prediction, insurance coverage can be problematic.
“We also need to be careful to screen for the presence of the risk enhancers, such as inflammatory disease, family history, and women-specific risk factors, to make sure we aren’t missing an important high-risk group,” she added.
Other solutions to better identify at-risk younger adults include considering upgrades to the guidelines’ class IIb recommendation to class IIa to emphasize the importance of recognizing lower-risk younger adults, and recommending statins for patients at higher lifetime risk than age- and sex-matched peers, the researchers noted. “In our cohort, young individuals admitted for a first MI had a higher lifetime ASCVD risk score than did patients in the older age categories,” Dr. Zeitouni and colleagues wrote.
Dr. Navar said that these findings are a reminder that guidelines aren’t mandates. “Guidelines are meant to be a starting point for patients and physicians,” she said. “The absence of a recommendation doesn’t mean something isn’t recommended, but that there is not enough data to say one way or another.”
The study “provides important evidence” that the 2018 guidelines exempted about half of the younger adults who had a first MI from preventative statin therapy, Ron Blankstein, MD, and Avinainder Singh, MD, MMSc, noted in an editorial (J Am Coll Cardiol. 2020;76:665-8).
“Data from both the Duke and Young-MI registries should force us to reexamine how we allocate statin use among young individuals,” they noted. Dr. Blankstein is with Brigham and Women’s Hospital, Harvard Medical School, Boston. Dr. Singh is with Yale University, New Haven, Conn.
Dr. Zeitouni reported receiving lecture fees from Bristol-Myers Squibb/Pfizer. Dr. Navar reported financial relationships with Amarin, Janssen, Amgen, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Esperion, Novo Nordisk, Novartis, The Medicine Company, New Amsterdam, Cerner and Pfizer. Dr. Blankstein reported receiving research support from Amgen. Dr. Singh has no relevant financial relationships to report.
SOURCE: M. Zeitouni et al. J Am Coll Cardiol 2020 Aug 3;76:653-64.
Clinical guidelines for cholesterol management may have two blind spots when it comes to heart attack prevention: Most younger adults with premature coronary artery disease who’ve had a myocardial infarction don’t meet guideline criteria for preventative statin therapy, and survivors under age 55 don’t meet the criteria for continuing nonstatin lipid-lowering treatments, a large single-center retrospective study has shown.
“The classic approach we’ve taken to identifying young adults for prevention is inadequate in younger adults,” corresponding author Ann Marie Navar, MD, PhD, of Duke University, Durham, N.C., said in an interview. “While awaiting more definitive research we should at minimum be using all the tools at our disposal, including broader use of coronary artery calcium [CAC] scoring, to identify young people who may benefit from statin therapy.”
The retrospective observational study analyzed records of 6,639 adults who had cardiac catheterization at Duke University Medical Center from 1995 to 2012 for a first myocardial infarction with obstructive coronary artery disease. The study considered those under age 55 years as “younger” patients, comprising 41% of the study group (2,733); 35% were “middle-aged” at 55-65 years (2,324) and 24% were “older,” at 66-75 years (1,582).
The report, published online Aug. 3 in the Journal of the American College of Cardiology, noted that most of the adults with premature CAD did not meet criteria for preventative statin therapy before their first MI based on ACC/American Heart Association clinical guidelines from 2013 and 2018. It also noted that younger MI survivors are also less frequently eligible for secondary prevention with intensive nonstatin lipid-lowering therapies than are older adults despite a much longer potential life span – and opportunity for another MI – for the former.
The researchers sought to evaluate the real-world implications of changes made in the 2018 guideline for adults who develop premature ischemic heart disease, and found that fewer younger patients qualify for preventative statin therapy under the 2018 guidelines.
“Younger individuals with very high-risk criteria are at higher risk of major adverse cardiovascular events, a finding supporting the appropriate implementation of intensive lipid-lowering therapies in these patients,” wrote lead author Michel Zeitouni, MD, MSc, and colleagues.
Key findings
The investigators reported that younger adults were significantly less likely to meet a class I recommendation for statins under the 2013 guideline (42.9%), compared with their middle-aged (70%) and older (82.5%) counterparts; and under the 2018 guideline, at 39.4%, 59.5%, and 77.4%, respectively (both P < .001).
Similarly, when both class I and class IIa recommendations were accounted for, younger patients were significantly less likely than were middle-aged and older patients to be eligible for statins before their index MI under both the 2013 (56.7%, 79.5%, and 85.2%, respectively and 2018 guidelines (46.4%, 73.5%, and 88.2%, respectively (both P < .01).
After their first MI, one in four younger patients (28.3%) met the very high-risk criteria compared with 40% of middle-aged and 81.4% of older patients (P trend < .001). In 8 years of follow-up, patients with very high-risk criteria based on the 2018 guideline had twice the rate of death, nonfatal MI, or stroke (hazard ratio [HR]: 2.15; 95% confidence interval, 1.98-2.33; P < .001).
The researchers acknowledged that the 2018 guideline took the important step of implementing risk enhancers – patient characteristics such as obesity and metabolic syndrome – along with the 10-year atherosclerotic cardiovascular disease (ASCVD) risk score to better identify high-risk young individuals who need statins. However, they also noted that the ability of the guidelines to identify young adults before their first MI “remains suboptimal.”
How to protect younger patients
“The 2018 guidelines will be most effective if we as providers do our best to identify risk enhancers and if we can use CAC scoring more broadly,” Dr. Navar said, noting that although CAC scoring has been shown to improve risk prediction, insurance coverage can be problematic.
“We also need to be careful to screen for the presence of the risk enhancers, such as inflammatory disease, family history, and women-specific risk factors, to make sure we aren’t missing an important high-risk group,” she added.
Other solutions to better identify at-risk younger adults include considering upgrades to the guidelines’ class IIb recommendation to class IIa to emphasize the importance of recognizing lower-risk younger adults, and recommending statins for patients at higher lifetime risk than age- and sex-matched peers, the researchers noted. “In our cohort, young individuals admitted for a first MI had a higher lifetime ASCVD risk score than did patients in the older age categories,” Dr. Zeitouni and colleagues wrote.
Dr. Navar said that these findings are a reminder that guidelines aren’t mandates. “Guidelines are meant to be a starting point for patients and physicians,” she said. “The absence of a recommendation doesn’t mean something isn’t recommended, but that there is not enough data to say one way or another.”
The study “provides important evidence” that the 2018 guidelines exempted about half of the younger adults who had a first MI from preventative statin therapy, Ron Blankstein, MD, and Avinainder Singh, MD, MMSc, noted in an editorial (J Am Coll Cardiol. 2020;76:665-8).
“Data from both the Duke and Young-MI registries should force us to reexamine how we allocate statin use among young individuals,” they noted. Dr. Blankstein is with Brigham and Women’s Hospital, Harvard Medical School, Boston. Dr. Singh is with Yale University, New Haven, Conn.
Dr. Zeitouni reported receiving lecture fees from Bristol-Myers Squibb/Pfizer. Dr. Navar reported financial relationships with Amarin, Janssen, Amgen, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Esperion, Novo Nordisk, Novartis, The Medicine Company, New Amsterdam, Cerner and Pfizer. Dr. Blankstein reported receiving research support from Amgen. Dr. Singh has no relevant financial relationships to report.
SOURCE: M. Zeitouni et al. J Am Coll Cardiol 2020 Aug 3;76:653-64.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Global study to track COVID-19’s impact on the brain
At its annual meeting, the Alzheimer’s Association announced the launch of a global study to examine the impact of COVID-19 on the brain, as well as policy recommendations to better address the COVID-19 crisis in long-term care facilities. The study will be led by researchers at the Alzheimer’s Association and the University of Texas Health, San Antonio, with participation from more than 30 countries and technical guidance from the World Health Organization.
The target sample size is 20,000-40,000 total participants.
Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, announced the study’s launch during a COVID-19–focused panel discussion at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
“To build a strong foundation for this research, we will align with existing studies, such as the Framingham Heart Study, and clinicians from around the world on how the data are going to be collected, obtained, and shared. We are going to have cross-study collaborations to understand the impact of the virus on the brain directly,” said Dr. Carrillo. “We will have some very good data to present next year at AAIC.”
‘Frightening’ headlines
As previously reported, mounting evidence suggests that SARS-CoV-2 invades the central nervous system, causing a wide range of neurologic and neuropsychiatric complications, including stroke, psychosis, altered mental state, and dementia-like syndrome. It’s likely that “dementia does not increase the risk for COVID-19, just like dementia does not increase risk for the flu. But increased age, being in a long-term care setting, and common health conditions that often accompany dementia may increase the risk,” Dr. Carrillo said.
Panel member Beth Kallmyer, MSW, vice president of care and support at the Alzheimer’s Association, spoke about the ongoing challenges long-term care facilities are facing during the pandemic. “You’ve all seen the headlines, and they’re frightening, frankly,” she said. An estimated 59,000 residents and employees of long-term care have died as a result of COVID-19, which is 42% of all U.S. deaths.
The long-term care community is being impacted at “significantly greater rates than the rest of society and yet we don’t have things in place to protect them. We also know that individuals living with dementia make up a large percentage of those that are living in long-term care,” Ms. Kallmyer said.
She noted that infection control is always a challenge in long-term care settings, but infection control during a pandemic “takes it to a whole other level.” Quarantining is hard for anyone, “but when you layer dementia on top of that we have a real challenge.” One long-term care provider told Ms. Kallmyer that “we might be saving them from COVID, but we’re losing them to social isolation and cognitive decline.”
New recommendations
Ms. Kallmyer outlined new policy recommendations from the Alzheimer’s Association to address the COVID-19 crisis in long-term and community-based care settings. They include:
- Testing every resident, employee, and visitor each time they leave and come back, so residents would not need to be confined to their own rooms
- Having a single portal that is easy and efficient for reporting cases
- Developing “surge activation” protocols to respond to hot spots, including the possibility of “strike teams” that go in and help during an outbreak
- Making sure all long-term care providers have full access to all needed personal protective equipment (PPE)
“Five months in and long-term care providers still don’t have adequate PPE. This is unacceptable,” said Ms. Kallmyer. “We have to be able to provide them with PPE.”
Panel member Gregory A. Jicha, MD, PhD, Sanders-Brown Center on Aging, University of Kentucky, Lexington, spoke about the critical need to continue Alzheimer’s disease research during the pandemic, noting that the number of promising targets for Alzheimer’s disease and related dementias has “never been higher or more comprehensive.”
Measures to ensure safety of researchers and participants include screening for symptoms (50% effective), social distancing (93% effective), minimizing exposure time (50% effective), limiting staff to 50% (50% effective), cloth/paper masks (80% effective), and testing (99.25% effective), Dr. Jicha noted.
With no safety measures in place, the risk of getting COVID-19 from a research visit is 1 in 20; when all these safety measures are combined, the risk is 1 in over 1.5 million, so “we can essentially eradicate or minimize the risks for COVID to less that of a lightning strike,” he said.
Dr. Carrillo, Ms. Kallmyer, and Dr. Jicha disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
At its annual meeting, the Alzheimer’s Association announced the launch of a global study to examine the impact of COVID-19 on the brain, as well as policy recommendations to better address the COVID-19 crisis in long-term care facilities. The study will be led by researchers at the Alzheimer’s Association and the University of Texas Health, San Antonio, with participation from more than 30 countries and technical guidance from the World Health Organization.
The target sample size is 20,000-40,000 total participants.
Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, announced the study’s launch during a COVID-19–focused panel discussion at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
“To build a strong foundation for this research, we will align with existing studies, such as the Framingham Heart Study, and clinicians from around the world on how the data are going to be collected, obtained, and shared. We are going to have cross-study collaborations to understand the impact of the virus on the brain directly,” said Dr. Carrillo. “We will have some very good data to present next year at AAIC.”
‘Frightening’ headlines
As previously reported, mounting evidence suggests that SARS-CoV-2 invades the central nervous system, causing a wide range of neurologic and neuropsychiatric complications, including stroke, psychosis, altered mental state, and dementia-like syndrome. It’s likely that “dementia does not increase the risk for COVID-19, just like dementia does not increase risk for the flu. But increased age, being in a long-term care setting, and common health conditions that often accompany dementia may increase the risk,” Dr. Carrillo said.
Panel member Beth Kallmyer, MSW, vice president of care and support at the Alzheimer’s Association, spoke about the ongoing challenges long-term care facilities are facing during the pandemic. “You’ve all seen the headlines, and they’re frightening, frankly,” she said. An estimated 59,000 residents and employees of long-term care have died as a result of COVID-19, which is 42% of all U.S. deaths.
The long-term care community is being impacted at “significantly greater rates than the rest of society and yet we don’t have things in place to protect them. We also know that individuals living with dementia make up a large percentage of those that are living in long-term care,” Ms. Kallmyer said.
She noted that infection control is always a challenge in long-term care settings, but infection control during a pandemic “takes it to a whole other level.” Quarantining is hard for anyone, “but when you layer dementia on top of that we have a real challenge.” One long-term care provider told Ms. Kallmyer that “we might be saving them from COVID, but we’re losing them to social isolation and cognitive decline.”
New recommendations
Ms. Kallmyer outlined new policy recommendations from the Alzheimer’s Association to address the COVID-19 crisis in long-term and community-based care settings. They include:
- Testing every resident, employee, and visitor each time they leave and come back, so residents would not need to be confined to their own rooms
- Having a single portal that is easy and efficient for reporting cases
- Developing “surge activation” protocols to respond to hot spots, including the possibility of “strike teams” that go in and help during an outbreak
- Making sure all long-term care providers have full access to all needed personal protective equipment (PPE)
“Five months in and long-term care providers still don’t have adequate PPE. This is unacceptable,” said Ms. Kallmyer. “We have to be able to provide them with PPE.”
Panel member Gregory A. Jicha, MD, PhD, Sanders-Brown Center on Aging, University of Kentucky, Lexington, spoke about the critical need to continue Alzheimer’s disease research during the pandemic, noting that the number of promising targets for Alzheimer’s disease and related dementias has “never been higher or more comprehensive.”
Measures to ensure safety of researchers and participants include screening for symptoms (50% effective), social distancing (93% effective), minimizing exposure time (50% effective), limiting staff to 50% (50% effective), cloth/paper masks (80% effective), and testing (99.25% effective), Dr. Jicha noted.
With no safety measures in place, the risk of getting COVID-19 from a research visit is 1 in 20; when all these safety measures are combined, the risk is 1 in over 1.5 million, so “we can essentially eradicate or minimize the risks for COVID to less that of a lightning strike,” he said.
Dr. Carrillo, Ms. Kallmyer, and Dr. Jicha disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
At its annual meeting, the Alzheimer’s Association announced the launch of a global study to examine the impact of COVID-19 on the brain, as well as policy recommendations to better address the COVID-19 crisis in long-term care facilities. The study will be led by researchers at the Alzheimer’s Association and the University of Texas Health, San Antonio, with participation from more than 30 countries and technical guidance from the World Health Organization.
The target sample size is 20,000-40,000 total participants.
Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, announced the study’s launch during a COVID-19–focused panel discussion at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
“To build a strong foundation for this research, we will align with existing studies, such as the Framingham Heart Study, and clinicians from around the world on how the data are going to be collected, obtained, and shared. We are going to have cross-study collaborations to understand the impact of the virus on the brain directly,” said Dr. Carrillo. “We will have some very good data to present next year at AAIC.”
‘Frightening’ headlines
As previously reported, mounting evidence suggests that SARS-CoV-2 invades the central nervous system, causing a wide range of neurologic and neuropsychiatric complications, including stroke, psychosis, altered mental state, and dementia-like syndrome. It’s likely that “dementia does not increase the risk for COVID-19, just like dementia does not increase risk for the flu. But increased age, being in a long-term care setting, and common health conditions that often accompany dementia may increase the risk,” Dr. Carrillo said.
Panel member Beth Kallmyer, MSW, vice president of care and support at the Alzheimer’s Association, spoke about the ongoing challenges long-term care facilities are facing during the pandemic. “You’ve all seen the headlines, and they’re frightening, frankly,” she said. An estimated 59,000 residents and employees of long-term care have died as a result of COVID-19, which is 42% of all U.S. deaths.
The long-term care community is being impacted at “significantly greater rates than the rest of society and yet we don’t have things in place to protect them. We also know that individuals living with dementia make up a large percentage of those that are living in long-term care,” Ms. Kallmyer said.
She noted that infection control is always a challenge in long-term care settings, but infection control during a pandemic “takes it to a whole other level.” Quarantining is hard for anyone, “but when you layer dementia on top of that we have a real challenge.” One long-term care provider told Ms. Kallmyer that “we might be saving them from COVID, but we’re losing them to social isolation and cognitive decline.”
New recommendations
Ms. Kallmyer outlined new policy recommendations from the Alzheimer’s Association to address the COVID-19 crisis in long-term and community-based care settings. They include:
- Testing every resident, employee, and visitor each time they leave and come back, so residents would not need to be confined to their own rooms
- Having a single portal that is easy and efficient for reporting cases
- Developing “surge activation” protocols to respond to hot spots, including the possibility of “strike teams” that go in and help during an outbreak
- Making sure all long-term care providers have full access to all needed personal protective equipment (PPE)
“Five months in and long-term care providers still don’t have adequate PPE. This is unacceptable,” said Ms. Kallmyer. “We have to be able to provide them with PPE.”
Panel member Gregory A. Jicha, MD, PhD, Sanders-Brown Center on Aging, University of Kentucky, Lexington, spoke about the critical need to continue Alzheimer’s disease research during the pandemic, noting that the number of promising targets for Alzheimer’s disease and related dementias has “never been higher or more comprehensive.”
Measures to ensure safety of researchers and participants include screening for symptoms (50% effective), social distancing (93% effective), minimizing exposure time (50% effective), limiting staff to 50% (50% effective), cloth/paper masks (80% effective), and testing (99.25% effective), Dr. Jicha noted.
With no safety measures in place, the risk of getting COVID-19 from a research visit is 1 in 20; when all these safety measures are combined, the risk is 1 in over 1.5 million, so “we can essentially eradicate or minimize the risks for COVID to less that of a lightning strike,” he said.
Dr. Carrillo, Ms. Kallmyer, and Dr. Jicha disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAIC 2020
COVID-19 taking financial toll on people in U.S. with diabetes
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
Early palliative care fails to improve QOL in advanced heart failure
A new palliative care intervention for U.S. patients with advanced heart failure did not improve quality of life or mood after 16 weeks of participation in a randomized trial.
“Future analyses and studies will examine both the patient factors and intervention components to find the right palliative care dose, for the right patient, at the right time,” wrote Marie A. Bakitas, DNSc, of the University of Alabama at Birmingham, and coauthors. The study was published in JAMA Internal Medicine.
“My first reaction is disappointment,” Larry Allen, MD, of the University of Colorado in Denver, said in an interview. “We had hoped to see the ENABLE program, which had been successful in cancer, translate to the heart failure setting.”
Improvement of palliative care in heart failure patients might rest on who needs it most
“One thing to note,” Dr. Allen added in an interview, “is that, in this population of patients, some of the measures they were trying to improve were already relatively mild to start with. It may not be that the intervention didn’t help but that they picked a patient population that wasn’t particularly in need. If you treat someone who doesn’t have a problem, it’s hard to make them better.”
In a separate interview, Dr. Bakitas acknowledged a similar sentiment. “We were a little surprised until we looked at our sample,” she said. “We realized that we had recruited all these very high-functioning, good quality-of-life patients. What we then did was look at a subsample of patients who had low quality of life at baseline. Low and behold, the intervention had an effect. The patients who started with a poor quality of life had a statistically and clinically significant benefit. Their KCCQ score increased by over 5 points.”
As for next steps. Dr. Bakitas noted that they’re twofold: “One is refining the patient population who can benefit, and the second is working on the intervention and figuring out which pieces are the ones that provide the most benefit.
“Because of logistics and practical issues, not everyone in the study got all the intervention that they should have. Think of it like a drug trial; if someone misses a pill, they don’t get the full dose that we thought would work. We need to make sure our interventions have the right pieces in place. We don’t want to develop a great intervention that’s not practical for patients.”
Study design and outcomes
To determine the benefits of early palliative care for patients with heart failure, the researchers developed the ENABLE CHF-PC (Educate, Nurture, Advise, Before Life Ends Comprehensive Heartcare for Patients and Caregivers) intervention. This nurse-led program includes an in-person consultant followed by six telehealth nurse coaching sessions lasting 30-40 minutes and then monthly follow-up calls through either 48 weeks or the patient’s death.
To test the effectiveness of their intervention after 16 weeks, the researchers launched a two-site, single-blind randomized clinical trial made up of 415 patients who were 50 years or older with advanced heart failure. Among the patients, 53% were men and the mean age was 64 years; 55% were African American, 26% lived in a rural area, and 46% had a high school education or less. The average length of time since heart failure diagnosis was 5.1 years.
Patients were randomized evenly to receive either the ENABLE CHF-PC intervention (208) or usual care. The primary outcomes were quality of life (QOL), which was measured by the heart failure–specific 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ) and the 14-item Functional Assessment of Chronic Illness Therapy–Palliative-14 (FACIT Pal-14), and mood, which was measured by the 14-item Hospital Anxiety and Depression Scale (HADS). Pain was measured via 3-item pain intensity and 2-item pain interference scales.
Effect size was measured as Cohen d or d-equivalent, where a small effect is 0.2, medium is 0.5, and large is about 0.854.
At baseline, the mean KCCQ score of 52.6 at baseline indicated a “fairly good” QOL across all patients. After 16 weeks, the mean KCCQ score improved 3.9 points in the intervention group, compared with 2.3 points in the usual care group (d = 0.07; [95% confidence interval, –0.09-0.24]). In addition, the mean FACIT-Pal-14 score improved 1.4 points in the intervention group compared to 0.2 points in the usual care group (d = 0.12 [95% CI, –0.03-0.28]). Only small differences were observed between groups regarding anxiety and depression, but pain intensity (difference, –2.8; SE, 0.9; d = –0.26 [95% CI, –0.43-0.09]) and pain interference (difference, –2.3; SE, 1; d = –0.21 [95% CI, –0.40 to –0.02]) demonstrated a statistically significant and clinically important decrease.
As heart failure care evolves, so must palliative care
Though the study and intervention developed by Dr. Bakitas and colleagues is commendable, it is only somewhat surprising that it did not drastically improve patients’ quality of life, Nathan E. Goldstein, MD, of the Icahn School of Medicine at Mount Sinai in New York, wrote in an accompanying editorial.
He noted several reasons for the lack of improvement, including a large proportion of patients still being in the early stages of the disease. Ultimately, however, he wonders if innovation in heart failure care ultimately impacted the study while it was occurring. Medications and technological advancements evolve rapidly in this field, he said, especially over the course of a 3-year study period.
To continue this work and produce real benefits in patients with advanced heart failure, Dr. Goldstein emphasized the need for “dynamic palliative care interventions that can adapt to the constantly changing landscape of the patient’s needs caused by the underlying nature of the disease, as well as the innovations in the field of cardiology.”
The authors acknowledged their study’s limitations, including data attrition at 16 weeks that was higher than expected – a turn of events they attributed to “unique socioeconomic factors … and lack of regular health care appointments” among some participants. In addition, a minority of patients were unable to stick to the study protocol, which has led the researchers to begin investigating video alternatives to in-person consultation.
The study was supported by the National Institutes of Health/National Institutes of Nursing Research. Four of the authors reported received grants from the National Institutes of Nursing Research outside the submitted work or during the study. Dr. Goldstein reported no conflicts of interest.
SOURCE: Bakitas MA et al. JAMA Intern Med. 2020 July 27. doi: 10.1001/jamainternmed.2020.2861.
A new palliative care intervention for U.S. patients with advanced heart failure did not improve quality of life or mood after 16 weeks of participation in a randomized trial.
“Future analyses and studies will examine both the patient factors and intervention components to find the right palliative care dose, for the right patient, at the right time,” wrote Marie A. Bakitas, DNSc, of the University of Alabama at Birmingham, and coauthors. The study was published in JAMA Internal Medicine.
“My first reaction is disappointment,” Larry Allen, MD, of the University of Colorado in Denver, said in an interview. “We had hoped to see the ENABLE program, which had been successful in cancer, translate to the heart failure setting.”
Improvement of palliative care in heart failure patients might rest on who needs it most
“One thing to note,” Dr. Allen added in an interview, “is that, in this population of patients, some of the measures they were trying to improve were already relatively mild to start with. It may not be that the intervention didn’t help but that they picked a patient population that wasn’t particularly in need. If you treat someone who doesn’t have a problem, it’s hard to make them better.”
In a separate interview, Dr. Bakitas acknowledged a similar sentiment. “We were a little surprised until we looked at our sample,” she said. “We realized that we had recruited all these very high-functioning, good quality-of-life patients. What we then did was look at a subsample of patients who had low quality of life at baseline. Low and behold, the intervention had an effect. The patients who started with a poor quality of life had a statistically and clinically significant benefit. Their KCCQ score increased by over 5 points.”
As for next steps. Dr. Bakitas noted that they’re twofold: “One is refining the patient population who can benefit, and the second is working on the intervention and figuring out which pieces are the ones that provide the most benefit.
“Because of logistics and practical issues, not everyone in the study got all the intervention that they should have. Think of it like a drug trial; if someone misses a pill, they don’t get the full dose that we thought would work. We need to make sure our interventions have the right pieces in place. We don’t want to develop a great intervention that’s not practical for patients.”
Study design and outcomes
To determine the benefits of early palliative care for patients with heart failure, the researchers developed the ENABLE CHF-PC (Educate, Nurture, Advise, Before Life Ends Comprehensive Heartcare for Patients and Caregivers) intervention. This nurse-led program includes an in-person consultant followed by six telehealth nurse coaching sessions lasting 30-40 minutes and then monthly follow-up calls through either 48 weeks or the patient’s death.
To test the effectiveness of their intervention after 16 weeks, the researchers launched a two-site, single-blind randomized clinical trial made up of 415 patients who were 50 years or older with advanced heart failure. Among the patients, 53% were men and the mean age was 64 years; 55% were African American, 26% lived in a rural area, and 46% had a high school education or less. The average length of time since heart failure diagnosis was 5.1 years.
Patients were randomized evenly to receive either the ENABLE CHF-PC intervention (208) or usual care. The primary outcomes were quality of life (QOL), which was measured by the heart failure–specific 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ) and the 14-item Functional Assessment of Chronic Illness Therapy–Palliative-14 (FACIT Pal-14), and mood, which was measured by the 14-item Hospital Anxiety and Depression Scale (HADS). Pain was measured via 3-item pain intensity and 2-item pain interference scales.
Effect size was measured as Cohen d or d-equivalent, where a small effect is 0.2, medium is 0.5, and large is about 0.854.
At baseline, the mean KCCQ score of 52.6 at baseline indicated a “fairly good” QOL across all patients. After 16 weeks, the mean KCCQ score improved 3.9 points in the intervention group, compared with 2.3 points in the usual care group (d = 0.07; [95% confidence interval, –0.09-0.24]). In addition, the mean FACIT-Pal-14 score improved 1.4 points in the intervention group compared to 0.2 points in the usual care group (d = 0.12 [95% CI, –0.03-0.28]). Only small differences were observed between groups regarding anxiety and depression, but pain intensity (difference, –2.8; SE, 0.9; d = –0.26 [95% CI, –0.43-0.09]) and pain interference (difference, –2.3; SE, 1; d = –0.21 [95% CI, –0.40 to –0.02]) demonstrated a statistically significant and clinically important decrease.
As heart failure care evolves, so must palliative care
Though the study and intervention developed by Dr. Bakitas and colleagues is commendable, it is only somewhat surprising that it did not drastically improve patients’ quality of life, Nathan E. Goldstein, MD, of the Icahn School of Medicine at Mount Sinai in New York, wrote in an accompanying editorial.
He noted several reasons for the lack of improvement, including a large proportion of patients still being in the early stages of the disease. Ultimately, however, he wonders if innovation in heart failure care ultimately impacted the study while it was occurring. Medications and technological advancements evolve rapidly in this field, he said, especially over the course of a 3-year study period.
To continue this work and produce real benefits in patients with advanced heart failure, Dr. Goldstein emphasized the need for “dynamic palliative care interventions that can adapt to the constantly changing landscape of the patient’s needs caused by the underlying nature of the disease, as well as the innovations in the field of cardiology.”
The authors acknowledged their study’s limitations, including data attrition at 16 weeks that was higher than expected – a turn of events they attributed to “unique socioeconomic factors … and lack of regular health care appointments” among some participants. In addition, a minority of patients were unable to stick to the study protocol, which has led the researchers to begin investigating video alternatives to in-person consultation.
The study was supported by the National Institutes of Health/National Institutes of Nursing Research. Four of the authors reported received grants from the National Institutes of Nursing Research outside the submitted work or during the study. Dr. Goldstein reported no conflicts of interest.
SOURCE: Bakitas MA et al. JAMA Intern Med. 2020 July 27. doi: 10.1001/jamainternmed.2020.2861.
A new palliative care intervention for U.S. patients with advanced heart failure did not improve quality of life or mood after 16 weeks of participation in a randomized trial.
“Future analyses and studies will examine both the patient factors and intervention components to find the right palliative care dose, for the right patient, at the right time,” wrote Marie A. Bakitas, DNSc, of the University of Alabama at Birmingham, and coauthors. The study was published in JAMA Internal Medicine.
“My first reaction is disappointment,” Larry Allen, MD, of the University of Colorado in Denver, said in an interview. “We had hoped to see the ENABLE program, which had been successful in cancer, translate to the heart failure setting.”
Improvement of palliative care in heart failure patients might rest on who needs it most
“One thing to note,” Dr. Allen added in an interview, “is that, in this population of patients, some of the measures they were trying to improve were already relatively mild to start with. It may not be that the intervention didn’t help but that they picked a patient population that wasn’t particularly in need. If you treat someone who doesn’t have a problem, it’s hard to make them better.”
In a separate interview, Dr. Bakitas acknowledged a similar sentiment. “We were a little surprised until we looked at our sample,” she said. “We realized that we had recruited all these very high-functioning, good quality-of-life patients. What we then did was look at a subsample of patients who had low quality of life at baseline. Low and behold, the intervention had an effect. The patients who started with a poor quality of life had a statistically and clinically significant benefit. Their KCCQ score increased by over 5 points.”
As for next steps. Dr. Bakitas noted that they’re twofold: “One is refining the patient population who can benefit, and the second is working on the intervention and figuring out which pieces are the ones that provide the most benefit.
“Because of logistics and practical issues, not everyone in the study got all the intervention that they should have. Think of it like a drug trial; if someone misses a pill, they don’t get the full dose that we thought would work. We need to make sure our interventions have the right pieces in place. We don’t want to develop a great intervention that’s not practical for patients.”
Study design and outcomes
To determine the benefits of early palliative care for patients with heart failure, the researchers developed the ENABLE CHF-PC (Educate, Nurture, Advise, Before Life Ends Comprehensive Heartcare for Patients and Caregivers) intervention. This nurse-led program includes an in-person consultant followed by six telehealth nurse coaching sessions lasting 30-40 minutes and then monthly follow-up calls through either 48 weeks or the patient’s death.
To test the effectiveness of their intervention after 16 weeks, the researchers launched a two-site, single-blind randomized clinical trial made up of 415 patients who were 50 years or older with advanced heart failure. Among the patients, 53% were men and the mean age was 64 years; 55% were African American, 26% lived in a rural area, and 46% had a high school education or less. The average length of time since heart failure diagnosis was 5.1 years.
Patients were randomized evenly to receive either the ENABLE CHF-PC intervention (208) or usual care. The primary outcomes were quality of life (QOL), which was measured by the heart failure–specific 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ) and the 14-item Functional Assessment of Chronic Illness Therapy–Palliative-14 (FACIT Pal-14), and mood, which was measured by the 14-item Hospital Anxiety and Depression Scale (HADS). Pain was measured via 3-item pain intensity and 2-item pain interference scales.
Effect size was measured as Cohen d or d-equivalent, where a small effect is 0.2, medium is 0.5, and large is about 0.854.
At baseline, the mean KCCQ score of 52.6 at baseline indicated a “fairly good” QOL across all patients. After 16 weeks, the mean KCCQ score improved 3.9 points in the intervention group, compared with 2.3 points in the usual care group (d = 0.07; [95% confidence interval, –0.09-0.24]). In addition, the mean FACIT-Pal-14 score improved 1.4 points in the intervention group compared to 0.2 points in the usual care group (d = 0.12 [95% CI, –0.03-0.28]). Only small differences were observed between groups regarding anxiety and depression, but pain intensity (difference, –2.8; SE, 0.9; d = –0.26 [95% CI, –0.43-0.09]) and pain interference (difference, –2.3; SE, 1; d = –0.21 [95% CI, –0.40 to –0.02]) demonstrated a statistically significant and clinically important decrease.
As heart failure care evolves, so must palliative care
Though the study and intervention developed by Dr. Bakitas and colleagues is commendable, it is only somewhat surprising that it did not drastically improve patients’ quality of life, Nathan E. Goldstein, MD, of the Icahn School of Medicine at Mount Sinai in New York, wrote in an accompanying editorial.
He noted several reasons for the lack of improvement, including a large proportion of patients still being in the early stages of the disease. Ultimately, however, he wonders if innovation in heart failure care ultimately impacted the study while it was occurring. Medications and technological advancements evolve rapidly in this field, he said, especially over the course of a 3-year study period.
To continue this work and produce real benefits in patients with advanced heart failure, Dr. Goldstein emphasized the need for “dynamic palliative care interventions that can adapt to the constantly changing landscape of the patient’s needs caused by the underlying nature of the disease, as well as the innovations in the field of cardiology.”
The authors acknowledged their study’s limitations, including data attrition at 16 weeks that was higher than expected – a turn of events they attributed to “unique socioeconomic factors … and lack of regular health care appointments” among some participants. In addition, a minority of patients were unable to stick to the study protocol, which has led the researchers to begin investigating video alternatives to in-person consultation.
The study was supported by the National Institutes of Health/National Institutes of Nursing Research. Four of the authors reported received grants from the National Institutes of Nursing Research outside the submitted work or during the study. Dr. Goldstein reported no conflicts of interest.
SOURCE: Bakitas MA et al. JAMA Intern Med. 2020 July 27. doi: 10.1001/jamainternmed.2020.2861.
FROM JAMA INTERNAL MEDICINE
COVID-19–related skin changes: The hidden racism in documentation
Belatedly, the disproportionate impact of COVID-19 on patients of color is getting attention. By now, we’ve read the headlines. Black people in the United States make up about 13% of the population but account for almost three times (34%) as many deaths. This story repeats – in other countries and in other minority communities.
Early detection is critical both to initiate supportive care and to isolate affected individuals and limit spread. Skin manifestations of COVID-19, especially those that occur early in the disease (eg, vesicular eruptions) or have prognostic significance (livedo, retiform purpura, necrosis), are critical to this goal of early recognition.
In this context, a recent systematic literature review looked at all articles describing skin manifestations associated with COVID-19. The investigators identified 46 articles published between March and May 2020 which included a total of 130 clinical images.
The following findings from this study are striking:
- 92% of the published images of COVID-associated skin manifestations were in I-III.
- Only 6% of COVID skin lesions included in the articles were in patients with skin type IV.
- None showed COVID skin lesions in skin types V or VI.
- Only six of the articles reported race and ethnicity demographics. In those, 91% of the patients were White and 9% were Hispanic.
These results reveal a critical lack of representative clinical images of COVID-associated skin manifestations in patients of color. This deficiency is made all the more egregious given the fact that patients of color, including those who are Black, Latinx, and Native American, have been especially hard hit by the COVID-19 pandemic and suffer disproportionate disease-related morbidity and mortality.
As the study authors point out, skin manifestations in people of color often differ significantly from findings in White skin (for example, look at the figure depicting the rash typical of Kawasaki disease in a dark-skinned child compared with a light-skinned child). It is not a stretch to suggest that skin manifestations associated with COVID-19 may look very different in darker skin.
This isn’t a new phenomenon. Almost half of dermatologists feel that they’ve had insufficient exposure to skin disease in darker skin types. Skin of color remains underrepresented in medical journals.
Like other forms of passive, institutional racism, this deficiency will only be improved if dermatologists and dermatology publications actively seek out COVID-associated skin manifestations in patients of color and prioritize sharing these images. A medical student in the United Kingdom has gotten the ball rolling, compiling a handbook of clinical signs in darker skin types as part of a student-staff partnership at St. George’s Hospital and the University of London. At this time, Mind the Gap is looking for a publisher.
Dr. Lipper is an assistant clinical professor at the University of Vermont, Burlington, and a staff physician in the department of dermatology at Danbury (Conn.) Hospital. He has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Belatedly, the disproportionate impact of COVID-19 on patients of color is getting attention. By now, we’ve read the headlines. Black people in the United States make up about 13% of the population but account for almost three times (34%) as many deaths. This story repeats – in other countries and in other minority communities.
Early detection is critical both to initiate supportive care and to isolate affected individuals and limit spread. Skin manifestations of COVID-19, especially those that occur early in the disease (eg, vesicular eruptions) or have prognostic significance (livedo, retiform purpura, necrosis), are critical to this goal of early recognition.
In this context, a recent systematic literature review looked at all articles describing skin manifestations associated with COVID-19. The investigators identified 46 articles published between March and May 2020 which included a total of 130 clinical images.
The following findings from this study are striking:
- 92% of the published images of COVID-associated skin manifestations were in I-III.
- Only 6% of COVID skin lesions included in the articles were in patients with skin type IV.
- None showed COVID skin lesions in skin types V or VI.
- Only six of the articles reported race and ethnicity demographics. In those, 91% of the patients were White and 9% were Hispanic.
These results reveal a critical lack of representative clinical images of COVID-associated skin manifestations in patients of color. This deficiency is made all the more egregious given the fact that patients of color, including those who are Black, Latinx, and Native American, have been especially hard hit by the COVID-19 pandemic and suffer disproportionate disease-related morbidity and mortality.
As the study authors point out, skin manifestations in people of color often differ significantly from findings in White skin (for example, look at the figure depicting the rash typical of Kawasaki disease in a dark-skinned child compared with a light-skinned child). It is not a stretch to suggest that skin manifestations associated with COVID-19 may look very different in darker skin.
This isn’t a new phenomenon. Almost half of dermatologists feel that they’ve had insufficient exposure to skin disease in darker skin types. Skin of color remains underrepresented in medical journals.
Like other forms of passive, institutional racism, this deficiency will only be improved if dermatologists and dermatology publications actively seek out COVID-associated skin manifestations in patients of color and prioritize sharing these images. A medical student in the United Kingdom has gotten the ball rolling, compiling a handbook of clinical signs in darker skin types as part of a student-staff partnership at St. George’s Hospital and the University of London. At this time, Mind the Gap is looking for a publisher.
Dr. Lipper is an assistant clinical professor at the University of Vermont, Burlington, and a staff physician in the department of dermatology at Danbury (Conn.) Hospital. He has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Belatedly, the disproportionate impact of COVID-19 on patients of color is getting attention. By now, we’ve read the headlines. Black people in the United States make up about 13% of the population but account for almost three times (34%) as many deaths. This story repeats – in other countries and in other minority communities.
Early detection is critical both to initiate supportive care and to isolate affected individuals and limit spread. Skin manifestations of COVID-19, especially those that occur early in the disease (eg, vesicular eruptions) or have prognostic significance (livedo, retiform purpura, necrosis), are critical to this goal of early recognition.
In this context, a recent systematic literature review looked at all articles describing skin manifestations associated with COVID-19. The investigators identified 46 articles published between March and May 2020 which included a total of 130 clinical images.
The following findings from this study are striking:
- 92% of the published images of COVID-associated skin manifestations were in I-III.
- Only 6% of COVID skin lesions included in the articles were in patients with skin type IV.
- None showed COVID skin lesions in skin types V or VI.
- Only six of the articles reported race and ethnicity demographics. In those, 91% of the patients were White and 9% were Hispanic.
These results reveal a critical lack of representative clinical images of COVID-associated skin manifestations in patients of color. This deficiency is made all the more egregious given the fact that patients of color, including those who are Black, Latinx, and Native American, have been especially hard hit by the COVID-19 pandemic and suffer disproportionate disease-related morbidity and mortality.
As the study authors point out, skin manifestations in people of color often differ significantly from findings in White skin (for example, look at the figure depicting the rash typical of Kawasaki disease in a dark-skinned child compared with a light-skinned child). It is not a stretch to suggest that skin manifestations associated with COVID-19 may look very different in darker skin.
This isn’t a new phenomenon. Almost half of dermatologists feel that they’ve had insufficient exposure to skin disease in darker skin types. Skin of color remains underrepresented in medical journals.
Like other forms of passive, institutional racism, this deficiency will only be improved if dermatologists and dermatology publications actively seek out COVID-associated skin manifestations in patients of color and prioritize sharing these images. A medical student in the United Kingdom has gotten the ball rolling, compiling a handbook of clinical signs in darker skin types as part of a student-staff partnership at St. George’s Hospital and the University of London. At this time, Mind the Gap is looking for a publisher.
Dr. Lipper is an assistant clinical professor at the University of Vermont, Burlington, and a staff physician in the department of dermatology at Danbury (Conn.) Hospital. He has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Heart damage even after COVID-19 ‘recovery’ evokes specter of later heart failure
Evidence that the heart can take a major hit in patients hospitalized with COVID-19, especially those already with cardiovascular disease (CV) or its risk factors, has been sadly apparent from the pandemic’s earliest days.
Less clear from case studies and small series to date has been whether SARS-CoV-2 directly attacks the heart and whether acute cardiac effects of the illness may lead to some kind of lingering cardiomyopathy.
The field’s grasp of those issues advanced a bit in two new reports published July 27 in JAMA Cardiology that seem to validate concerns the virus can infect the myocardium, without necessarily causing myocarditis and the possibility that some “recovered” patients may be left with persisting myocardial injury and inflammation that potentially could later manifest as heart failure.
Persisting inflammation by cardiac magnetic resonance
A prospective cohort study with 100 patients recovered from a recent bout of the disease showed evidence of ventricular dysfunction, greater ventricular mass, and in 78% of the cohort, signs of myocardial inflammation by cardiac magnetic resonance (CMR) imaging. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
Two-thirds of the cohort, whose acute COVID-19 severity had “ranged from asymptomatic to minor-to-moderate symptoms,” had recovered at home, whereas the remaining “severely unwell patients” had been hospitalized, wrote the authors, led by Valentina O. Püntmann, MD, PhD, University Hospital Frankfurt (Germany).
None of the patients had a history of heart failure or cardiomyopathy, although some had hypertension, diabetes, or evidence of coronary disease.
“Our findings demonstrate that participants with a relative paucity of preexisting cardiovascular condition and with mostly home-based recovery had frequent cardiac inflammatory involvement, which was similar to the hospitalized subgroup with regards to severity and extent,” the group noted.
“There is a considerable ongoing myocardial inflammation in the heart muscle weeks after recovery from COVID-19 illness. This finding is important because it may herald a considerable burden of heart failure in a few years down the line,” Dr. Püntmann said in an interview.
Early diagnosis would offer “a good chance that early treatment could reduce the relentless course of inflammatory damage or even halt it,” she said.
“The relatively clear onset of COVID-19 illness provides an opportunity, which we often do not have with other conditions, to take a proactive action and to look for heart involvement early, within a few weeks of recovery.”
The study’s CMR evidence of inflammation edema, scarring, and pericardial effusion are among “the major diagnostic criteria for inflammatory and viral myocarditis,” observed Biykem Bozkurt, MD, PhD, from Baylor College of Medicine, Houston, who wasn’t part of either new study.
The findings suggest – consistent with previous evidence – that some patients with recent COVID-19 may be left with ongoing myocardial inflammation, and this study further adds that it could potentially become subacute or even chronic and in some may not be totally reversible, she said in an interview. How long the effects are likely to persist “remains to be determined. We need longer-term outcomes data.”
Viral presence without myocarditis
The accompanying report featured a postmortem analysis of hearts from 39 patients with mostly severe COVID-19 that pointed to a significant SARS-CoV-2 presence and signs that the virus vigorously replicated in the myocardium.
But there was no evidence that the infection led to fulminant myocarditis. Rather, the virus had apparently infiltrated the heart by localizing in interstitial cells or in macrophages that took up in the myocardium without actually entering myocytes, concluded the report’s authors, led by Diana Lindner, PhD, from the University Heart and Vascular Centre, Hamburg (Germany).
The findings suggest “that the presence of SARS-CoV-2 in cardiac tissue does not necessarily cause an inflammatory reaction consistent with clinical myocarditis,” the group wrote.
Previously in the literature, in “cases in which myocardial inflammation was present, there was also evidence of clinical myocarditis, and therefore the current cases underlie a different pathophysiology,” they concluded.
No evidence of the virus was seen in 15 cases, about 61% of the group. In 16 of the remaining 24 hearts, the viral load exceeded 1,000 copies per mcg of RNA, a substantial presence. Those 16 showed increased expression of inflammatory cytokines but no inflammatory cell infiltrates or changes in leukocyte counts, the researchers noted.
“Findings of suggested viral replication in the cases with a very high viral load are showing that we need to do more studies to find out long-term consequences, which we do not know right now,” senior author Dirk Westermann, MD, also from the University Heart and Vascular Centre, Hamburg, said.
Implications for heart failure
The postmortem findings from Dr. Lindner and associates “provide intriguing evidence that COVID-19 is associated with at least some component of myocardial injury, perhaps as the result of direct viral infection of the heart,” wrote Clyde W. Yancy, MD, MSc, from Northwestern University, Chicago, and Gregg C. Fonarow, MD, from the University of California, Los Angeles, in an editorial accompanying both reports.
The CMR study from Dr. Püntmann and colleagues – on the backdrop of earlier COVID-19 observations – suggests the potential for “residual left ventricular dysfunction and ongoing inflammation” in the months following a COVID-19 diagnosis. Both developments may be “of sufficient concern to represent a nidus for new-onset heart failure and other cardiovascular complications,” contend Dr. Yancy and Dr. Fonarow.
“When added to the postmortem pathological findings from Lindner et al, we see the plot thickening and we are inclined to raise a new and very evident concern that cardiomyopathy and heart failure related to COVID-19 may potentially evolve as the natural history of this infection becomes clearer,” they wrote.
Some patients, having recovered from the acute illness, may be left with a chronic inflammatory state that probably puts them at increased risk for future heart failure, agreed Dr. Bozkurt when interviewed. “They could show further decline in cardiac function, and their recovery might take longer than with the usual viral illnesses that we see,” she said.
“There could also be a risk of sudden death. Inflammation sometimes gives rise to sudden death and ventricular arrhythmia, which I would be very worried about, especially if the myocardium is stressed,” Dr. Bozkurt said. “So competitive sports in those patients potentially could be risky.”
COVID-19 cohort vs. matched control subjects
The CMR study from Dr. Püntmann and colleagues prospectively entered 100 patients recently recovered from an acute bout of COVID-19, either at home or at a hospital, who were followed in a registry based at University Hospital Frankfurt. Their median age was 49 years; 47% were female. They were compared with 50 age- and sex-matched control patients and 50 apparently healthy volunteers matched for risk factors, the group noted.
On the same day as the CMR assessment, the recently recovered patients, compared with the healthy control subjects and risk-factor matched control subjects, respectively, showed (P ≤ .001 in each case):
- A reduced left ventricular (LV) ejection fraction: 56% vs. 60% and 61%.
- A higher LV end-diastolic volume index: 86 mL/m2 vs. 80 mL/m2 and 75 mL/m2.
- A greater LV mass index: 51 g/m2 vs. 47 g/m2 and 53 g/m2.
- A higher hs-TnT level: 5.6 pg/mL vs. 3.2 pg/mL and 3.9 pg/mL.
- A greater prevalence of hs-TnT levels 3 pg/mL or more: 71% vs. 11% and 31%.
At CMR, 78% of the recovered COVID-19 patients showed abnormalities that included raised myocardial native T1 and T2 mapping, which is suggestive of fibrosis and edema from inflammation, compared with the two control groups (P < .001 for all differences), “independent of preexisting conditions, severity and overall course of the acute illness, and the time from the original diagnosis,” the group wrote. Native T1 and T2 mapping correlated significantly with hs-TnT.
“We now have the diagnostic means to detect cardiac inflammation early, and we need make every effort to apply them in every day practice,”Dr. Püntmann said in the interview.
“Using cardiac MRI will allow us to raise our game against COVID-19 and proactively develop efficient cardioprotective treatments,” she said. “Until we have effective means of protecting from the infection, that is vaccination, we must act swiftly and within the means at hand.”
The analysis evokes several other ways patients with COVID-19 might be screened for significant myocardial involvement.
“Strategies could include checking troponins, not only at admission but maybe at discharge and perhaps even those individuals who are at home and are not necessarily requiring care,” Dr. Bozkurt said.
“Biomarker profiling and screening for ongoing inflammation probably are going to be important components of COVID-19, especially for those with subclinical risk and disease.”
Dr. Westermann proposed that troponin elevations at discharge “might be a good starting point” for selecting COVID-19 patients for functional testing or imaging to screen for cardiac sequelae. Performing such tests routinely now “would be overwhelming given the massive increase in patients we still see today.”
Dr. Püntmann had no disclosures; statements of potential conflict for the other authors are in the report. Dr. Bozkurt has previously disclosed receiving consultant fees or honoraria from Bayer Healthcare, Bristol-Myers Squibb, Lantheus Medical Imaging, and Respicardia; serving on a data safety monitoring board for LivaNova USA ; and having unspecified relationships with Abbott Laboratories. Dr. Lindner had no disclosures; Dr. Westermann reported receiving personal fees from AstraZeneca, Bayer, Novartis, and Medtronic. Dr. Yancy is a deputy editor and Dr. Fonarow a section editor for JAMA Cardiology. Dr. Yancy had no other disclosures. Dr. Fonarow reported receiving personal fees from Abbott Laboratories, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards Lifesciences, Janssen, Medtronic, Merck, and Novartis.
A version of this article originally appeared on Medscape.com.
Evidence that the heart can take a major hit in patients hospitalized with COVID-19, especially those already with cardiovascular disease (CV) or its risk factors, has been sadly apparent from the pandemic’s earliest days.
Less clear from case studies and small series to date has been whether SARS-CoV-2 directly attacks the heart and whether acute cardiac effects of the illness may lead to some kind of lingering cardiomyopathy.
The field’s grasp of those issues advanced a bit in two new reports published July 27 in JAMA Cardiology that seem to validate concerns the virus can infect the myocardium, without necessarily causing myocarditis and the possibility that some “recovered” patients may be left with persisting myocardial injury and inflammation that potentially could later manifest as heart failure.
Persisting inflammation by cardiac magnetic resonance
A prospective cohort study with 100 patients recovered from a recent bout of the disease showed evidence of ventricular dysfunction, greater ventricular mass, and in 78% of the cohort, signs of myocardial inflammation by cardiac magnetic resonance (CMR) imaging. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
Two-thirds of the cohort, whose acute COVID-19 severity had “ranged from asymptomatic to minor-to-moderate symptoms,” had recovered at home, whereas the remaining “severely unwell patients” had been hospitalized, wrote the authors, led by Valentina O. Püntmann, MD, PhD, University Hospital Frankfurt (Germany).
None of the patients had a history of heart failure or cardiomyopathy, although some had hypertension, diabetes, or evidence of coronary disease.
“Our findings demonstrate that participants with a relative paucity of preexisting cardiovascular condition and with mostly home-based recovery had frequent cardiac inflammatory involvement, which was similar to the hospitalized subgroup with regards to severity and extent,” the group noted.
“There is a considerable ongoing myocardial inflammation in the heart muscle weeks after recovery from COVID-19 illness. This finding is important because it may herald a considerable burden of heart failure in a few years down the line,” Dr. Püntmann said in an interview.
Early diagnosis would offer “a good chance that early treatment could reduce the relentless course of inflammatory damage or even halt it,” she said.
“The relatively clear onset of COVID-19 illness provides an opportunity, which we often do not have with other conditions, to take a proactive action and to look for heart involvement early, within a few weeks of recovery.”
The study’s CMR evidence of inflammation edema, scarring, and pericardial effusion are among “the major diagnostic criteria for inflammatory and viral myocarditis,” observed Biykem Bozkurt, MD, PhD, from Baylor College of Medicine, Houston, who wasn’t part of either new study.
The findings suggest – consistent with previous evidence – that some patients with recent COVID-19 may be left with ongoing myocardial inflammation, and this study further adds that it could potentially become subacute or even chronic and in some may not be totally reversible, she said in an interview. How long the effects are likely to persist “remains to be determined. We need longer-term outcomes data.”
Viral presence without myocarditis
The accompanying report featured a postmortem analysis of hearts from 39 patients with mostly severe COVID-19 that pointed to a significant SARS-CoV-2 presence and signs that the virus vigorously replicated in the myocardium.
But there was no evidence that the infection led to fulminant myocarditis. Rather, the virus had apparently infiltrated the heart by localizing in interstitial cells or in macrophages that took up in the myocardium without actually entering myocytes, concluded the report’s authors, led by Diana Lindner, PhD, from the University Heart and Vascular Centre, Hamburg (Germany).
The findings suggest “that the presence of SARS-CoV-2 in cardiac tissue does not necessarily cause an inflammatory reaction consistent with clinical myocarditis,” the group wrote.
Previously in the literature, in “cases in which myocardial inflammation was present, there was also evidence of clinical myocarditis, and therefore the current cases underlie a different pathophysiology,” they concluded.
No evidence of the virus was seen in 15 cases, about 61% of the group. In 16 of the remaining 24 hearts, the viral load exceeded 1,000 copies per mcg of RNA, a substantial presence. Those 16 showed increased expression of inflammatory cytokines but no inflammatory cell infiltrates or changes in leukocyte counts, the researchers noted.
“Findings of suggested viral replication in the cases with a very high viral load are showing that we need to do more studies to find out long-term consequences, which we do not know right now,” senior author Dirk Westermann, MD, also from the University Heart and Vascular Centre, Hamburg, said.
Implications for heart failure
The postmortem findings from Dr. Lindner and associates “provide intriguing evidence that COVID-19 is associated with at least some component of myocardial injury, perhaps as the result of direct viral infection of the heart,” wrote Clyde W. Yancy, MD, MSc, from Northwestern University, Chicago, and Gregg C. Fonarow, MD, from the University of California, Los Angeles, in an editorial accompanying both reports.
The CMR study from Dr. Püntmann and colleagues – on the backdrop of earlier COVID-19 observations – suggests the potential for “residual left ventricular dysfunction and ongoing inflammation” in the months following a COVID-19 diagnosis. Both developments may be “of sufficient concern to represent a nidus for new-onset heart failure and other cardiovascular complications,” contend Dr. Yancy and Dr. Fonarow.
“When added to the postmortem pathological findings from Lindner et al, we see the plot thickening and we are inclined to raise a new and very evident concern that cardiomyopathy and heart failure related to COVID-19 may potentially evolve as the natural history of this infection becomes clearer,” they wrote.
Some patients, having recovered from the acute illness, may be left with a chronic inflammatory state that probably puts them at increased risk for future heart failure, agreed Dr. Bozkurt when interviewed. “They could show further decline in cardiac function, and their recovery might take longer than with the usual viral illnesses that we see,” she said.
“There could also be a risk of sudden death. Inflammation sometimes gives rise to sudden death and ventricular arrhythmia, which I would be very worried about, especially if the myocardium is stressed,” Dr. Bozkurt said. “So competitive sports in those patients potentially could be risky.”
COVID-19 cohort vs. matched control subjects
The CMR study from Dr. Püntmann and colleagues prospectively entered 100 patients recently recovered from an acute bout of COVID-19, either at home or at a hospital, who were followed in a registry based at University Hospital Frankfurt. Their median age was 49 years; 47% were female. They were compared with 50 age- and sex-matched control patients and 50 apparently healthy volunteers matched for risk factors, the group noted.
On the same day as the CMR assessment, the recently recovered patients, compared with the healthy control subjects and risk-factor matched control subjects, respectively, showed (P ≤ .001 in each case):
- A reduced left ventricular (LV) ejection fraction: 56% vs. 60% and 61%.
- A higher LV end-diastolic volume index: 86 mL/m2 vs. 80 mL/m2 and 75 mL/m2.
- A greater LV mass index: 51 g/m2 vs. 47 g/m2 and 53 g/m2.
- A higher hs-TnT level: 5.6 pg/mL vs. 3.2 pg/mL and 3.9 pg/mL.
- A greater prevalence of hs-TnT levels 3 pg/mL or more: 71% vs. 11% and 31%.
At CMR, 78% of the recovered COVID-19 patients showed abnormalities that included raised myocardial native T1 and T2 mapping, which is suggestive of fibrosis and edema from inflammation, compared with the two control groups (P < .001 for all differences), “independent of preexisting conditions, severity and overall course of the acute illness, and the time from the original diagnosis,” the group wrote. Native T1 and T2 mapping correlated significantly with hs-TnT.
“We now have the diagnostic means to detect cardiac inflammation early, and we need make every effort to apply them in every day practice,”Dr. Püntmann said in the interview.
“Using cardiac MRI will allow us to raise our game against COVID-19 and proactively develop efficient cardioprotective treatments,” she said. “Until we have effective means of protecting from the infection, that is vaccination, we must act swiftly and within the means at hand.”
The analysis evokes several other ways patients with COVID-19 might be screened for significant myocardial involvement.
“Strategies could include checking troponins, not only at admission but maybe at discharge and perhaps even those individuals who are at home and are not necessarily requiring care,” Dr. Bozkurt said.
“Biomarker profiling and screening for ongoing inflammation probably are going to be important components of COVID-19, especially for those with subclinical risk and disease.”
Dr. Westermann proposed that troponin elevations at discharge “might be a good starting point” for selecting COVID-19 patients for functional testing or imaging to screen for cardiac sequelae. Performing such tests routinely now “would be overwhelming given the massive increase in patients we still see today.”
Dr. Püntmann had no disclosures; statements of potential conflict for the other authors are in the report. Dr. Bozkurt has previously disclosed receiving consultant fees or honoraria from Bayer Healthcare, Bristol-Myers Squibb, Lantheus Medical Imaging, and Respicardia; serving on a data safety monitoring board for LivaNova USA ; and having unspecified relationships with Abbott Laboratories. Dr. Lindner had no disclosures; Dr. Westermann reported receiving personal fees from AstraZeneca, Bayer, Novartis, and Medtronic. Dr. Yancy is a deputy editor and Dr. Fonarow a section editor for JAMA Cardiology. Dr. Yancy had no other disclosures. Dr. Fonarow reported receiving personal fees from Abbott Laboratories, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards Lifesciences, Janssen, Medtronic, Merck, and Novartis.
A version of this article originally appeared on Medscape.com.
Evidence that the heart can take a major hit in patients hospitalized with COVID-19, especially those already with cardiovascular disease (CV) or its risk factors, has been sadly apparent from the pandemic’s earliest days.
Less clear from case studies and small series to date has been whether SARS-CoV-2 directly attacks the heart and whether acute cardiac effects of the illness may lead to some kind of lingering cardiomyopathy.
The field’s grasp of those issues advanced a bit in two new reports published July 27 in JAMA Cardiology that seem to validate concerns the virus can infect the myocardium, without necessarily causing myocarditis and the possibility that some “recovered” patients may be left with persisting myocardial injury and inflammation that potentially could later manifest as heart failure.
Persisting inflammation by cardiac magnetic resonance
A prospective cohort study with 100 patients recovered from a recent bout of the disease showed evidence of ventricular dysfunction, greater ventricular mass, and in 78% of the cohort, signs of myocardial inflammation by cardiac magnetic resonance (CMR) imaging. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
Two-thirds of the cohort, whose acute COVID-19 severity had “ranged from asymptomatic to minor-to-moderate symptoms,” had recovered at home, whereas the remaining “severely unwell patients” had been hospitalized, wrote the authors, led by Valentina O. Püntmann, MD, PhD, University Hospital Frankfurt (Germany).
None of the patients had a history of heart failure or cardiomyopathy, although some had hypertension, diabetes, or evidence of coronary disease.
“Our findings demonstrate that participants with a relative paucity of preexisting cardiovascular condition and with mostly home-based recovery had frequent cardiac inflammatory involvement, which was similar to the hospitalized subgroup with regards to severity and extent,” the group noted.
“There is a considerable ongoing myocardial inflammation in the heart muscle weeks after recovery from COVID-19 illness. This finding is important because it may herald a considerable burden of heart failure in a few years down the line,” Dr. Püntmann said in an interview.
Early diagnosis would offer “a good chance that early treatment could reduce the relentless course of inflammatory damage or even halt it,” she said.
“The relatively clear onset of COVID-19 illness provides an opportunity, which we often do not have with other conditions, to take a proactive action and to look for heart involvement early, within a few weeks of recovery.”
The study’s CMR evidence of inflammation edema, scarring, and pericardial effusion are among “the major diagnostic criteria for inflammatory and viral myocarditis,” observed Biykem Bozkurt, MD, PhD, from Baylor College of Medicine, Houston, who wasn’t part of either new study.
The findings suggest – consistent with previous evidence – that some patients with recent COVID-19 may be left with ongoing myocardial inflammation, and this study further adds that it could potentially become subacute or even chronic and in some may not be totally reversible, she said in an interview. How long the effects are likely to persist “remains to be determined. We need longer-term outcomes data.”
Viral presence without myocarditis
The accompanying report featured a postmortem analysis of hearts from 39 patients with mostly severe COVID-19 that pointed to a significant SARS-CoV-2 presence and signs that the virus vigorously replicated in the myocardium.
But there was no evidence that the infection led to fulminant myocarditis. Rather, the virus had apparently infiltrated the heart by localizing in interstitial cells or in macrophages that took up in the myocardium without actually entering myocytes, concluded the report’s authors, led by Diana Lindner, PhD, from the University Heart and Vascular Centre, Hamburg (Germany).
The findings suggest “that the presence of SARS-CoV-2 in cardiac tissue does not necessarily cause an inflammatory reaction consistent with clinical myocarditis,” the group wrote.
Previously in the literature, in “cases in which myocardial inflammation was present, there was also evidence of clinical myocarditis, and therefore the current cases underlie a different pathophysiology,” they concluded.
No evidence of the virus was seen in 15 cases, about 61% of the group. In 16 of the remaining 24 hearts, the viral load exceeded 1,000 copies per mcg of RNA, a substantial presence. Those 16 showed increased expression of inflammatory cytokines but no inflammatory cell infiltrates or changes in leukocyte counts, the researchers noted.
“Findings of suggested viral replication in the cases with a very high viral load are showing that we need to do more studies to find out long-term consequences, which we do not know right now,” senior author Dirk Westermann, MD, also from the University Heart and Vascular Centre, Hamburg, said.
Implications for heart failure
The postmortem findings from Dr. Lindner and associates “provide intriguing evidence that COVID-19 is associated with at least some component of myocardial injury, perhaps as the result of direct viral infection of the heart,” wrote Clyde W. Yancy, MD, MSc, from Northwestern University, Chicago, and Gregg C. Fonarow, MD, from the University of California, Los Angeles, in an editorial accompanying both reports.
The CMR study from Dr. Püntmann and colleagues – on the backdrop of earlier COVID-19 observations – suggests the potential for “residual left ventricular dysfunction and ongoing inflammation” in the months following a COVID-19 diagnosis. Both developments may be “of sufficient concern to represent a nidus for new-onset heart failure and other cardiovascular complications,” contend Dr. Yancy and Dr. Fonarow.
“When added to the postmortem pathological findings from Lindner et al, we see the plot thickening and we are inclined to raise a new and very evident concern that cardiomyopathy and heart failure related to COVID-19 may potentially evolve as the natural history of this infection becomes clearer,” they wrote.
Some patients, having recovered from the acute illness, may be left with a chronic inflammatory state that probably puts them at increased risk for future heart failure, agreed Dr. Bozkurt when interviewed. “They could show further decline in cardiac function, and their recovery might take longer than with the usual viral illnesses that we see,” she said.
“There could also be a risk of sudden death. Inflammation sometimes gives rise to sudden death and ventricular arrhythmia, which I would be very worried about, especially if the myocardium is stressed,” Dr. Bozkurt said. “So competitive sports in those patients potentially could be risky.”
COVID-19 cohort vs. matched control subjects
The CMR study from Dr. Püntmann and colleagues prospectively entered 100 patients recently recovered from an acute bout of COVID-19, either at home or at a hospital, who were followed in a registry based at University Hospital Frankfurt. Their median age was 49 years; 47% were female. They were compared with 50 age- and sex-matched control patients and 50 apparently healthy volunteers matched for risk factors, the group noted.
On the same day as the CMR assessment, the recently recovered patients, compared with the healthy control subjects and risk-factor matched control subjects, respectively, showed (P ≤ .001 in each case):
- A reduced left ventricular (LV) ejection fraction: 56% vs. 60% and 61%.
- A higher LV end-diastolic volume index: 86 mL/m2 vs. 80 mL/m2 and 75 mL/m2.
- A greater LV mass index: 51 g/m2 vs. 47 g/m2 and 53 g/m2.
- A higher hs-TnT level: 5.6 pg/mL vs. 3.2 pg/mL and 3.9 pg/mL.
- A greater prevalence of hs-TnT levels 3 pg/mL or more: 71% vs. 11% and 31%.
At CMR, 78% of the recovered COVID-19 patients showed abnormalities that included raised myocardial native T1 and T2 mapping, which is suggestive of fibrosis and edema from inflammation, compared with the two control groups (P < .001 for all differences), “independent of preexisting conditions, severity and overall course of the acute illness, and the time from the original diagnosis,” the group wrote. Native T1 and T2 mapping correlated significantly with hs-TnT.
“We now have the diagnostic means to detect cardiac inflammation early, and we need make every effort to apply them in every day practice,”Dr. Püntmann said in the interview.
“Using cardiac MRI will allow us to raise our game against COVID-19 and proactively develop efficient cardioprotective treatments,” she said. “Until we have effective means of protecting from the infection, that is vaccination, we must act swiftly and within the means at hand.”
The analysis evokes several other ways patients with COVID-19 might be screened for significant myocardial involvement.
“Strategies could include checking troponins, not only at admission but maybe at discharge and perhaps even those individuals who are at home and are not necessarily requiring care,” Dr. Bozkurt said.
“Biomarker profiling and screening for ongoing inflammation probably are going to be important components of COVID-19, especially for those with subclinical risk and disease.”
Dr. Westermann proposed that troponin elevations at discharge “might be a good starting point” for selecting COVID-19 patients for functional testing or imaging to screen for cardiac sequelae. Performing such tests routinely now “would be overwhelming given the massive increase in patients we still see today.”
Dr. Püntmann had no disclosures; statements of potential conflict for the other authors are in the report. Dr. Bozkurt has previously disclosed receiving consultant fees or honoraria from Bayer Healthcare, Bristol-Myers Squibb, Lantheus Medical Imaging, and Respicardia; serving on a data safety monitoring board for LivaNova USA ; and having unspecified relationships with Abbott Laboratories. Dr. Lindner had no disclosures; Dr. Westermann reported receiving personal fees from AstraZeneca, Bayer, Novartis, and Medtronic. Dr. Yancy is a deputy editor and Dr. Fonarow a section editor for JAMA Cardiology. Dr. Yancy had no other disclosures. Dr. Fonarow reported receiving personal fees from Abbott Laboratories, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards Lifesciences, Janssen, Medtronic, Merck, and Novartis.
A version of this article originally appeared on Medscape.com.
SCD-HeFT 10-year results: Primary-prevention ICD insights in nonischemic heart failure
A 10-year follow-up analysis based on one of cardiology’s most influential trials has shed further light on one of its key issues: how to sharpen selection of patients most likely to benefit from a primary prevention implantable cardioverter-defibrillator (ICD).
In a new report from SCD-HeFT, the survival advantage in patients with heart failure seen 5 years after receiving ICDs, compared with a non-ICD control group, narrowed a bit but remained significant after an additional 5 years. But not all patients with devices shared in that long-term ICD benefit. Patients with either ischemic disease or nonischemic cardiomyopathy (NICM) with devices showed a similar mortality risk reduction in the trial’s previously reported 5-year outcomes. That advantage, compared with non-ICD control patients, persisted throughout the subsequent 5 years for ischemic patients but tapered to nil for those with NICM.
The NICM patients “had what appears to be some accrual of benefit maybe out to about 6 years, and then the curves appear to come together where there’s no apparent further benefit after 6 years,” Jeanne E. Poole, MD, of the University of Washington, Seattle, said in an interview.
In both the 10-year analysis and the earlier results, ICD survival gains went preferentially to patients who enrolled with New York Heart Association (NYHA) functional class II symptoms. Patients who entered in NYHA class III “didn’t appear to have any benefit whatsoever” in either period, Dr. Poole said.
“The simple message is that the same groups of patients that benefited strongly from the ICD in the original SCD-HeFT – the NYHA class 2 patients and those with ischemic cardiomyopathy – were really the ones who benefited the greatest over the long term,” she said.
Dr. Poole is lead author on the SCD-HeFT 10-year analysis, which was published in the July 28 issue of the Journal of the American College of Cardiology.
Why the ICD survival effect disappeared midway in patients with NICM “is hard to sort out,” she said. Many in the control group were offered such devices after the trial concluded. Among those, it’s possible that disproportionately more control patients with NICM, compared with patients with ischemic disease, were fitted with ICDs that were also cardiac resynchronization therapy (CRT) devices, Dr. Poole and her colleagues speculated. That could have shifted their late outcomes to be more in line with patients who had received ICDs when the trial started.
Or “it is possible that the intermediate-term benefit of ICD therapy in NICM is overwhelmed by nonarrhythmic death in extended follow-up” given that ICDs prolong survival only by preventing arrhythmic death, noted an editorial accompanying the new SCD-HeFT publication.
Another possibility: Because NICM is a heterogeneous disorder with many potential causes, perhaps “the absence of long-term mortality benefit among SCD-HeFT participants with NICM was due to an unintended but preferential enrollment of subtypes at relatively lower risk for arrhythmic death in the longer term,” proposed Eric C. Stecker, MD, MPH, Oregon Health & Science University, Portland, and coauthors in their editorial.
“What are the take-away messages from the current analysis by Poole et al?” they asked. “These findings strongly support the clinical efficacy and cost-effectiveness of ICD therapy for the majority of patients with severe but mildly symptomatic ischemic cardiomyopathy who do not have an excessive comorbidity burden.”
But “the implications for patients with NICM are less clear,” they wrote. “Given evidence for intermediate-term benefit and the limitations inherent to assessing longer-term benefit, we do not believe it is appropriate to walk back guideline recommendations regarding ICD implantation for NICM patients.”
The findings in nonischemic patients invite comparison with the randomized DANISH trial, which entered only patients with NICM and, over more than 5 years, saw no primary-prevention ICD advantage for the end point of all-cause mortality.
But patients who received ICDs showed a reduction in arrhythmic death, a secondary end point. And mortality in the trial showed a significant interaction with patient age; survival went up sharply with ICDs for those younger than 60 years.
Also in DANISH, “the ICD treatment effect appears to vary over time, with an earlier phase showing possible survival benefit and a later phase showing attenuation of that benefit,” similar to what was seen long-term in SCD-HeFT, in which the interaction between mortality and time since implantation was significant at P = .0015, observe Dr. Poole and colleagues.
However, Dr. Poole cautioned when interviewed, patient management in DANISH, conducted exclusively in Denmark, may not have been representative of the rest of the world, complicating comparisons with other studies. For example, nearly 60% of all patients in DANISH had defibrillating CRT devices. Virtually everyone was on ACE inhibitors or angiotensin-receptor blockers, and almost 60% were taking aldosterone inhibitors.
“DANISH is an unusually high bar and probably does not reflect all patients with heart failure, and certainly does not reflect patients in the United States in terms of those high levels of guideline-directed medical therapy,” Dr. Poole said. The message from DANISH, she said, seems to be that patients with NICM who are definitely on goal-directed heart failure medications with CRT devices “probably don’t have a meaningful benefit from an ICD, on total mortality, because their sudden death rates are simply so low.”
SCD-HeFT had originally assigned 2,521 patients with heart failure of NYHA class II or III and an left ventricular ejection fraction of less than 35% to receive an ICD, amiodarone without an ICD, or an amiodarone placebo and no ICD; patients in the latter cohorts made up the non-ICD control group.
Those who received an ICD, compared with the non-ICD control patients, showed a 23% drop in all-cause mortality over a median of 45.5 months ending on October 31, 2003, Dr. Poole and colleagues noted in their current report. The trial’s primary results were unveiled 2005.
The current analysis, based on data collected in 2010 and 2011, followed the 1,855 patients alive at the trial’s official conclusion and combined outcomes before and after that time for a median follow-up of 11 years, Dr. Poole and colleagues reported.
In the ICD group, the overall hazard ratio for mortality by intention-to-treat was 0.87 (95% confidence interval, 0.76-0.98; P = .028), compared with the non-ICD control group.
In their report, Poole and associates clarified one of the foremost potential confounders in the current analysis: device implantations after the trial in patients who had been in the non-ICD groups. From partial clinical data collected after the trial, they wrote, the estimated rate of subsequent ICD implantation in non-ICD control patients was about 55%. Such a low number is consistent with clinical practice in the United States, where “a surprisingly low number of patients who are eligible actually end up getting devices,” Dr. Poole said.
Subsequent ICD use in the former non-ICD control patients presumably boosted their survival over the long term, narrowing the gap between their all-cause mortality and that of the original ICD patients, Dr. Poole observed. Despite that, the ICD-group’s late survival advantage remained significant.
SCD-HeFT was sponsored by Medtronic, Wyeth Pharmaceuticals, and the National Heart, Lung, and Blood Institute. The current analysis was partially supported by a grant from St. Jude Medical. Dr. Poole disclosed receiving research support from Medtronic, Biotronik, AtriCure, and Kestra; serving as a speaker for Boston Scientific, Medtronic, and MediaSphere Medical and on an advisory board for Boston Scientific; serving on a committee for Medtronic and on a data and safety monitoring board for EBR Systems; and receiving royalties from Elsevier and compensation from the Heart Rhythm Society for serving as editor in chief for the Heart Rhythm O2 journal. Disclosures for the other authors are in the report. Dr. Stecker and coauthors disclosed that they have no relevant relationships.
A version of this article originally appeared on Medscape.com.
A 10-year follow-up analysis based on one of cardiology’s most influential trials has shed further light on one of its key issues: how to sharpen selection of patients most likely to benefit from a primary prevention implantable cardioverter-defibrillator (ICD).
In a new report from SCD-HeFT, the survival advantage in patients with heart failure seen 5 years after receiving ICDs, compared with a non-ICD control group, narrowed a bit but remained significant after an additional 5 years. But not all patients with devices shared in that long-term ICD benefit. Patients with either ischemic disease or nonischemic cardiomyopathy (NICM) with devices showed a similar mortality risk reduction in the trial’s previously reported 5-year outcomes. That advantage, compared with non-ICD control patients, persisted throughout the subsequent 5 years for ischemic patients but tapered to nil for those with NICM.
The NICM patients “had what appears to be some accrual of benefit maybe out to about 6 years, and then the curves appear to come together where there’s no apparent further benefit after 6 years,” Jeanne E. Poole, MD, of the University of Washington, Seattle, said in an interview.
In both the 10-year analysis and the earlier results, ICD survival gains went preferentially to patients who enrolled with New York Heart Association (NYHA) functional class II symptoms. Patients who entered in NYHA class III “didn’t appear to have any benefit whatsoever” in either period, Dr. Poole said.
“The simple message is that the same groups of patients that benefited strongly from the ICD in the original SCD-HeFT – the NYHA class 2 patients and those with ischemic cardiomyopathy – were really the ones who benefited the greatest over the long term,” she said.
Dr. Poole is lead author on the SCD-HeFT 10-year analysis, which was published in the July 28 issue of the Journal of the American College of Cardiology.
Why the ICD survival effect disappeared midway in patients with NICM “is hard to sort out,” she said. Many in the control group were offered such devices after the trial concluded. Among those, it’s possible that disproportionately more control patients with NICM, compared with patients with ischemic disease, were fitted with ICDs that were also cardiac resynchronization therapy (CRT) devices, Dr. Poole and her colleagues speculated. That could have shifted their late outcomes to be more in line with patients who had received ICDs when the trial started.
Or “it is possible that the intermediate-term benefit of ICD therapy in NICM is overwhelmed by nonarrhythmic death in extended follow-up” given that ICDs prolong survival only by preventing arrhythmic death, noted an editorial accompanying the new SCD-HeFT publication.
Another possibility: Because NICM is a heterogeneous disorder with many potential causes, perhaps “the absence of long-term mortality benefit among SCD-HeFT participants with NICM was due to an unintended but preferential enrollment of subtypes at relatively lower risk for arrhythmic death in the longer term,” proposed Eric C. Stecker, MD, MPH, Oregon Health & Science University, Portland, and coauthors in their editorial.
“What are the take-away messages from the current analysis by Poole et al?” they asked. “These findings strongly support the clinical efficacy and cost-effectiveness of ICD therapy for the majority of patients with severe but mildly symptomatic ischemic cardiomyopathy who do not have an excessive comorbidity burden.”
But “the implications for patients with NICM are less clear,” they wrote. “Given evidence for intermediate-term benefit and the limitations inherent to assessing longer-term benefit, we do not believe it is appropriate to walk back guideline recommendations regarding ICD implantation for NICM patients.”
The findings in nonischemic patients invite comparison with the randomized DANISH trial, which entered only patients with NICM and, over more than 5 years, saw no primary-prevention ICD advantage for the end point of all-cause mortality.
But patients who received ICDs showed a reduction in arrhythmic death, a secondary end point. And mortality in the trial showed a significant interaction with patient age; survival went up sharply with ICDs for those younger than 60 years.
Also in DANISH, “the ICD treatment effect appears to vary over time, with an earlier phase showing possible survival benefit and a later phase showing attenuation of that benefit,” similar to what was seen long-term in SCD-HeFT, in which the interaction between mortality and time since implantation was significant at P = .0015, observe Dr. Poole and colleagues.
However, Dr. Poole cautioned when interviewed, patient management in DANISH, conducted exclusively in Denmark, may not have been representative of the rest of the world, complicating comparisons with other studies. For example, nearly 60% of all patients in DANISH had defibrillating CRT devices. Virtually everyone was on ACE inhibitors or angiotensin-receptor blockers, and almost 60% were taking aldosterone inhibitors.
“DANISH is an unusually high bar and probably does not reflect all patients with heart failure, and certainly does not reflect patients in the United States in terms of those high levels of guideline-directed medical therapy,” Dr. Poole said. The message from DANISH, she said, seems to be that patients with NICM who are definitely on goal-directed heart failure medications with CRT devices “probably don’t have a meaningful benefit from an ICD, on total mortality, because their sudden death rates are simply so low.”
SCD-HeFT had originally assigned 2,521 patients with heart failure of NYHA class II or III and an left ventricular ejection fraction of less than 35% to receive an ICD, amiodarone without an ICD, or an amiodarone placebo and no ICD; patients in the latter cohorts made up the non-ICD control group.
Those who received an ICD, compared with the non-ICD control patients, showed a 23% drop in all-cause mortality over a median of 45.5 months ending on October 31, 2003, Dr. Poole and colleagues noted in their current report. The trial’s primary results were unveiled 2005.
The current analysis, based on data collected in 2010 and 2011, followed the 1,855 patients alive at the trial’s official conclusion and combined outcomes before and after that time for a median follow-up of 11 years, Dr. Poole and colleagues reported.
In the ICD group, the overall hazard ratio for mortality by intention-to-treat was 0.87 (95% confidence interval, 0.76-0.98; P = .028), compared with the non-ICD control group.
In their report, Poole and associates clarified one of the foremost potential confounders in the current analysis: device implantations after the trial in patients who had been in the non-ICD groups. From partial clinical data collected after the trial, they wrote, the estimated rate of subsequent ICD implantation in non-ICD control patients was about 55%. Such a low number is consistent with clinical practice in the United States, where “a surprisingly low number of patients who are eligible actually end up getting devices,” Dr. Poole said.
Subsequent ICD use in the former non-ICD control patients presumably boosted their survival over the long term, narrowing the gap between their all-cause mortality and that of the original ICD patients, Dr. Poole observed. Despite that, the ICD-group’s late survival advantage remained significant.
SCD-HeFT was sponsored by Medtronic, Wyeth Pharmaceuticals, and the National Heart, Lung, and Blood Institute. The current analysis was partially supported by a grant from St. Jude Medical. Dr. Poole disclosed receiving research support from Medtronic, Biotronik, AtriCure, and Kestra; serving as a speaker for Boston Scientific, Medtronic, and MediaSphere Medical and on an advisory board for Boston Scientific; serving on a committee for Medtronic and on a data and safety monitoring board for EBR Systems; and receiving royalties from Elsevier and compensation from the Heart Rhythm Society for serving as editor in chief for the Heart Rhythm O2 journal. Disclosures for the other authors are in the report. Dr. Stecker and coauthors disclosed that they have no relevant relationships.
A version of this article originally appeared on Medscape.com.
A 10-year follow-up analysis based on one of cardiology’s most influential trials has shed further light on one of its key issues: how to sharpen selection of patients most likely to benefit from a primary prevention implantable cardioverter-defibrillator (ICD).
In a new report from SCD-HeFT, the survival advantage in patients with heart failure seen 5 years after receiving ICDs, compared with a non-ICD control group, narrowed a bit but remained significant after an additional 5 years. But not all patients with devices shared in that long-term ICD benefit. Patients with either ischemic disease or nonischemic cardiomyopathy (NICM) with devices showed a similar mortality risk reduction in the trial’s previously reported 5-year outcomes. That advantage, compared with non-ICD control patients, persisted throughout the subsequent 5 years for ischemic patients but tapered to nil for those with NICM.
The NICM patients “had what appears to be some accrual of benefit maybe out to about 6 years, and then the curves appear to come together where there’s no apparent further benefit after 6 years,” Jeanne E. Poole, MD, of the University of Washington, Seattle, said in an interview.
In both the 10-year analysis and the earlier results, ICD survival gains went preferentially to patients who enrolled with New York Heart Association (NYHA) functional class II symptoms. Patients who entered in NYHA class III “didn’t appear to have any benefit whatsoever” in either period, Dr. Poole said.
“The simple message is that the same groups of patients that benefited strongly from the ICD in the original SCD-HeFT – the NYHA class 2 patients and those with ischemic cardiomyopathy – were really the ones who benefited the greatest over the long term,” she said.
Dr. Poole is lead author on the SCD-HeFT 10-year analysis, which was published in the July 28 issue of the Journal of the American College of Cardiology.
Why the ICD survival effect disappeared midway in patients with NICM “is hard to sort out,” she said. Many in the control group were offered such devices after the trial concluded. Among those, it’s possible that disproportionately more control patients with NICM, compared with patients with ischemic disease, were fitted with ICDs that were also cardiac resynchronization therapy (CRT) devices, Dr. Poole and her colleagues speculated. That could have shifted their late outcomes to be more in line with patients who had received ICDs when the trial started.
Or “it is possible that the intermediate-term benefit of ICD therapy in NICM is overwhelmed by nonarrhythmic death in extended follow-up” given that ICDs prolong survival only by preventing arrhythmic death, noted an editorial accompanying the new SCD-HeFT publication.
Another possibility: Because NICM is a heterogeneous disorder with many potential causes, perhaps “the absence of long-term mortality benefit among SCD-HeFT participants with NICM was due to an unintended but preferential enrollment of subtypes at relatively lower risk for arrhythmic death in the longer term,” proposed Eric C. Stecker, MD, MPH, Oregon Health & Science University, Portland, and coauthors in their editorial.
“What are the take-away messages from the current analysis by Poole et al?” they asked. “These findings strongly support the clinical efficacy and cost-effectiveness of ICD therapy for the majority of patients with severe but mildly symptomatic ischemic cardiomyopathy who do not have an excessive comorbidity burden.”
But “the implications for patients with NICM are less clear,” they wrote. “Given evidence for intermediate-term benefit and the limitations inherent to assessing longer-term benefit, we do not believe it is appropriate to walk back guideline recommendations regarding ICD implantation for NICM patients.”
The findings in nonischemic patients invite comparison with the randomized DANISH trial, which entered only patients with NICM and, over more than 5 years, saw no primary-prevention ICD advantage for the end point of all-cause mortality.
But patients who received ICDs showed a reduction in arrhythmic death, a secondary end point. And mortality in the trial showed a significant interaction with patient age; survival went up sharply with ICDs for those younger than 60 years.
Also in DANISH, “the ICD treatment effect appears to vary over time, with an earlier phase showing possible survival benefit and a later phase showing attenuation of that benefit,” similar to what was seen long-term in SCD-HeFT, in which the interaction between mortality and time since implantation was significant at P = .0015, observe Dr. Poole and colleagues.
However, Dr. Poole cautioned when interviewed, patient management in DANISH, conducted exclusively in Denmark, may not have been representative of the rest of the world, complicating comparisons with other studies. For example, nearly 60% of all patients in DANISH had defibrillating CRT devices. Virtually everyone was on ACE inhibitors or angiotensin-receptor blockers, and almost 60% were taking aldosterone inhibitors.
“DANISH is an unusually high bar and probably does not reflect all patients with heart failure, and certainly does not reflect patients in the United States in terms of those high levels of guideline-directed medical therapy,” Dr. Poole said. The message from DANISH, she said, seems to be that patients with NICM who are definitely on goal-directed heart failure medications with CRT devices “probably don’t have a meaningful benefit from an ICD, on total mortality, because their sudden death rates are simply so low.”
SCD-HeFT had originally assigned 2,521 patients with heart failure of NYHA class II or III and an left ventricular ejection fraction of less than 35% to receive an ICD, amiodarone without an ICD, or an amiodarone placebo and no ICD; patients in the latter cohorts made up the non-ICD control group.
Those who received an ICD, compared with the non-ICD control patients, showed a 23% drop in all-cause mortality over a median of 45.5 months ending on October 31, 2003, Dr. Poole and colleagues noted in their current report. The trial’s primary results were unveiled 2005.
The current analysis, based on data collected in 2010 and 2011, followed the 1,855 patients alive at the trial’s official conclusion and combined outcomes before and after that time for a median follow-up of 11 years, Dr. Poole and colleagues reported.
In the ICD group, the overall hazard ratio for mortality by intention-to-treat was 0.87 (95% confidence interval, 0.76-0.98; P = .028), compared with the non-ICD control group.
In their report, Poole and associates clarified one of the foremost potential confounders in the current analysis: device implantations after the trial in patients who had been in the non-ICD groups. From partial clinical data collected after the trial, they wrote, the estimated rate of subsequent ICD implantation in non-ICD control patients was about 55%. Such a low number is consistent with clinical practice in the United States, where “a surprisingly low number of patients who are eligible actually end up getting devices,” Dr. Poole said.
Subsequent ICD use in the former non-ICD control patients presumably boosted their survival over the long term, narrowing the gap between their all-cause mortality and that of the original ICD patients, Dr. Poole observed. Despite that, the ICD-group’s late survival advantage remained significant.
SCD-HeFT was sponsored by Medtronic, Wyeth Pharmaceuticals, and the National Heart, Lung, and Blood Institute. The current analysis was partially supported by a grant from St. Jude Medical. Dr. Poole disclosed receiving research support from Medtronic, Biotronik, AtriCure, and Kestra; serving as a speaker for Boston Scientific, Medtronic, and MediaSphere Medical and on an advisory board for Boston Scientific; serving on a committee for Medtronic and on a data and safety monitoring board for EBR Systems; and receiving royalties from Elsevier and compensation from the Heart Rhythm Society for serving as editor in chief for the Heart Rhythm O2 journal. Disclosures for the other authors are in the report. Dr. Stecker and coauthors disclosed that they have no relevant relationships.
A version of this article originally appeared on Medscape.com.