User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
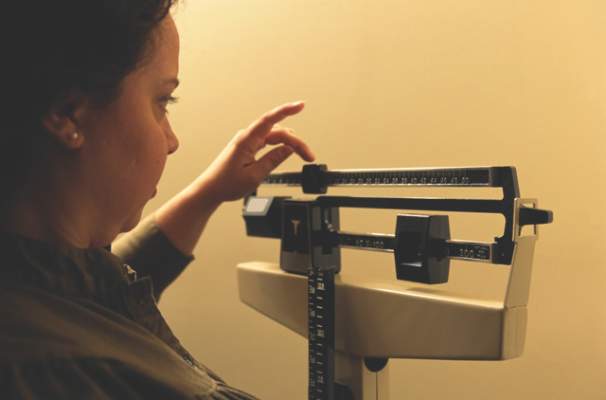
Internet program spurs 5.5-kg weight loss
An online behavioral intervention helped overweight and obese patients lose an average of 5.5 kilograms in 3 months and maintain that loss at 6 months, according to a prospective randomized study. The results were published online Nov. 17 in Diabetes Care.
The almost fully automated program “is an effective, potentially low-cost option that physicians could use with their overweight and obese patients to reduce the risk of type 2 diabetes and other weight-related diseases,” said John G. Thomas, Ph.D., of Brown University, Providence, R.I.
At 3 months, 41 (53%) of program participants had lost at least 5% of their body weight, and 37 (48%) met that target at 6 months, the researchers said. The control group lost an average of 1.3 kilograms. The findings show that to lose weight, patients need more than physician advice and basic information about diet and exercise, wrote Dr. Thomas and his associates (Diabetes Care 2014 Nov. 17 [doi: 10.2337/dc14-1474]).
The study comprised 154 patients aged 18-70 years whose body mass index was between 25 and 45 kg per m2. Eighty percent were women, and most were non-Hispanic whites.
Every week, the intervention group was asked to view a 10- to 15-minute interactive video on topics such as eating in restaurants, changing the home environment, and social support. These patients also used a website to report their daily calorie intake, exercise, and body weight, and received automated feedback based on their progress. In contrast, the control group was asked to view weekly online newsletters about the benefits of healthy eating, weight loss, and exercise, the investigators noted.
Compared with controls, the intervention group reported significantly more weeks in which they had cut their calorie and fat intake, consumed more fruits and vegetables, and exercised more than at baseline, the researchers said. Differences for the calorie and fat measures remained significant at 6 months, and the others trended toward significance, they reported. “Given that about 80% of U.S. adults use the Internet, this approach may provide a cost-effective alternative or complement to the face-to-face counseling models of obesity treatment, including that which is currently reimbursable by the Centers for Medicare & Medicaid Services,” they added.
Participants in behavioral weight loss programs tend to initially lose weight and then slowly regain it, but the researchers noted that they did not follow patients at the time point when they would be most likely to regain weight. The study design required patients to visit an academic medical center several times, and that could have influenced the results, the researchers added.
The National Heart, Lung, and Blood Institute funded the study. The authors reported having no conflicts of interest.
An online behavioral intervention helped overweight and obese patients lose an average of 5.5 kilograms in 3 months and maintain that loss at 6 months, according to a prospective randomized study. The results were published online Nov. 17 in Diabetes Care.
The almost fully automated program “is an effective, potentially low-cost option that physicians could use with their overweight and obese patients to reduce the risk of type 2 diabetes and other weight-related diseases,” said John G. Thomas, Ph.D., of Brown University, Providence, R.I.
At 3 months, 41 (53%) of program participants had lost at least 5% of their body weight, and 37 (48%) met that target at 6 months, the researchers said. The control group lost an average of 1.3 kilograms. The findings show that to lose weight, patients need more than physician advice and basic information about diet and exercise, wrote Dr. Thomas and his associates (Diabetes Care 2014 Nov. 17 [doi: 10.2337/dc14-1474]).
The study comprised 154 patients aged 18-70 years whose body mass index was between 25 and 45 kg per m2. Eighty percent were women, and most were non-Hispanic whites.
Every week, the intervention group was asked to view a 10- to 15-minute interactive video on topics such as eating in restaurants, changing the home environment, and social support. These patients also used a website to report their daily calorie intake, exercise, and body weight, and received automated feedback based on their progress. In contrast, the control group was asked to view weekly online newsletters about the benefits of healthy eating, weight loss, and exercise, the investigators noted.
Compared with controls, the intervention group reported significantly more weeks in which they had cut their calorie and fat intake, consumed more fruits and vegetables, and exercised more than at baseline, the researchers said. Differences for the calorie and fat measures remained significant at 6 months, and the others trended toward significance, they reported. “Given that about 80% of U.S. adults use the Internet, this approach may provide a cost-effective alternative or complement to the face-to-face counseling models of obesity treatment, including that which is currently reimbursable by the Centers for Medicare & Medicaid Services,” they added.
Participants in behavioral weight loss programs tend to initially lose weight and then slowly regain it, but the researchers noted that they did not follow patients at the time point when they would be most likely to regain weight. The study design required patients to visit an academic medical center several times, and that could have influenced the results, the researchers added.
The National Heart, Lung, and Blood Institute funded the study. The authors reported having no conflicts of interest.
An online behavioral intervention helped overweight and obese patients lose an average of 5.5 kilograms in 3 months and maintain that loss at 6 months, according to a prospective randomized study. The results were published online Nov. 17 in Diabetes Care.
The almost fully automated program “is an effective, potentially low-cost option that physicians could use with their overweight and obese patients to reduce the risk of type 2 diabetes and other weight-related diseases,” said John G. Thomas, Ph.D., of Brown University, Providence, R.I.
At 3 months, 41 (53%) of program participants had lost at least 5% of their body weight, and 37 (48%) met that target at 6 months, the researchers said. The control group lost an average of 1.3 kilograms. The findings show that to lose weight, patients need more than physician advice and basic information about diet and exercise, wrote Dr. Thomas and his associates (Diabetes Care 2014 Nov. 17 [doi: 10.2337/dc14-1474]).
The study comprised 154 patients aged 18-70 years whose body mass index was between 25 and 45 kg per m2. Eighty percent were women, and most were non-Hispanic whites.
Every week, the intervention group was asked to view a 10- to 15-minute interactive video on topics such as eating in restaurants, changing the home environment, and social support. These patients also used a website to report their daily calorie intake, exercise, and body weight, and received automated feedback based on their progress. In contrast, the control group was asked to view weekly online newsletters about the benefits of healthy eating, weight loss, and exercise, the investigators noted.
Compared with controls, the intervention group reported significantly more weeks in which they had cut their calorie and fat intake, consumed more fruits and vegetables, and exercised more than at baseline, the researchers said. Differences for the calorie and fat measures remained significant at 6 months, and the others trended toward significance, they reported. “Given that about 80% of U.S. adults use the Internet, this approach may provide a cost-effective alternative or complement to the face-to-face counseling models of obesity treatment, including that which is currently reimbursable by the Centers for Medicare & Medicaid Services,” they added.
Participants in behavioral weight loss programs tend to initially lose weight and then slowly regain it, but the researchers noted that they did not follow patients at the time point when they would be most likely to regain weight. The study design required patients to visit an academic medical center several times, and that could have influenced the results, the researchers added.
The National Heart, Lung, and Blood Institute funded the study. The authors reported having no conflicts of interest.
Key clinical point: Patients achieved clinically significant weight loss with an automated, Internet-based behavioral program.
Major finding: At 3 months, the intervention group had lost an average of 5.5 kg, compared with 1.3 kg for the control group.
Data source: Prospective randomized, controlled trial of 154 overweight or obese patients.
Disclosures: The National Heart, Lung, and Blood Institute funded the study. The authors reported having no conflicts of interest.
Digital resource provides tips on diabetes management, treatment
More than a dozen federal agencies have banded together to create the Guiding Principles for the Care of People With or at Risk for Diabetes, a digital resource center aimed at providing clinically relevant information on diabetes management to clinicians and health care professionals.
The tool, created by the National Diabetes Education Program, is based on areas of general agreement among existing diabetes management protocols, can better inform primary care providers and health care teams on how to deliver quality care to adults with or at risk of diabetes.
The guidelines are not intended as a definitive resource for diabetes management and do not provide information on specific clinical management of diabetes.
Supporters of the guidelines include the American Diabetes Association, the Academy of Nutrition and Dietetics, the American Association of Nurse Practitioners, the Office of Minority Health, and the Agency for Healthcare Research and Quality.
The resource guide has information about the following topics:
• Identify undiagnosed diabetes and prediabetes.
• Manage prediabetes.
• Provide self-management education and support.
• Provide individualized nutrition therapy.
• Encourage regular physical activity.
• Control blood glucose.
• Reduce cardiovascular disease risk.
• Detect and monitor microvascular complications.
• Consider special populations.
• Provide patient-centered care.
To learn more about the Guiding Principles, check out the website here.
More than a dozen federal agencies have banded together to create the Guiding Principles for the Care of People With or at Risk for Diabetes, a digital resource center aimed at providing clinically relevant information on diabetes management to clinicians and health care professionals.
The tool, created by the National Diabetes Education Program, is based on areas of general agreement among existing diabetes management protocols, can better inform primary care providers and health care teams on how to deliver quality care to adults with or at risk of diabetes.
The guidelines are not intended as a definitive resource for diabetes management and do not provide information on specific clinical management of diabetes.
Supporters of the guidelines include the American Diabetes Association, the Academy of Nutrition and Dietetics, the American Association of Nurse Practitioners, the Office of Minority Health, and the Agency for Healthcare Research and Quality.
The resource guide has information about the following topics:
• Identify undiagnosed diabetes and prediabetes.
• Manage prediabetes.
• Provide self-management education and support.
• Provide individualized nutrition therapy.
• Encourage regular physical activity.
• Control blood glucose.
• Reduce cardiovascular disease risk.
• Detect and monitor microvascular complications.
• Consider special populations.
• Provide patient-centered care.
To learn more about the Guiding Principles, check out the website here.
More than a dozen federal agencies have banded together to create the Guiding Principles for the Care of People With or at Risk for Diabetes, a digital resource center aimed at providing clinically relevant information on diabetes management to clinicians and health care professionals.
The tool, created by the National Diabetes Education Program, is based on areas of general agreement among existing diabetes management protocols, can better inform primary care providers and health care teams on how to deliver quality care to adults with or at risk of diabetes.
The guidelines are not intended as a definitive resource for diabetes management and do not provide information on specific clinical management of diabetes.
Supporters of the guidelines include the American Diabetes Association, the Academy of Nutrition and Dietetics, the American Association of Nurse Practitioners, the Office of Minority Health, and the Agency for Healthcare Research and Quality.
The resource guide has information about the following topics:
• Identify undiagnosed diabetes and prediabetes.
• Manage prediabetes.
• Provide self-management education and support.
• Provide individualized nutrition therapy.
• Encourage regular physical activity.
• Control blood glucose.
• Reduce cardiovascular disease risk.
• Detect and monitor microvascular complications.
• Consider special populations.
• Provide patient-centered care.
To learn more about the Guiding Principles, check out the website here.
Obesity, diabetes spur on group A strep infections
PHILADELPHIA – Obesity and diabetes are independent risk factors for invasive group A Streptococcus infections, with the highest risk among those with diabetes and morbid obesity.
The relative risk of invasive group A Streptococcus (iGAS) infection was 15 times higher in adults with diabetes and grade 3 morbid obesity (body mass index ≥ 40 kg/m2) than in those of normal weight, Dr. Gayle Langley reported at Infectious Diseases Week 2014.
Moreover, grades 1-2 obesity and morbid obesity increased the odds of ICU admission and death from an iGAS infection in multivariable logistic regression.
The adjusted odds ratios for ICU admission and death were 1.46 and 1.55 for patients with grades 1-2 obesity (BMI 30 < 40 kg/m2) and 2.07 and 1.62 for those with morbid obesity, she said.
Diabetes was not associated with an increased risk for either ICU admission (odds ratio, 1.06) or death (OR, 0.77) in the analysis.
Skin and soft tissue infections, which were significantly more common in obese and diabetic patients, appear to be driving the increased risk for iGAS in these groups, Dr. Langley of the Centers for Disease Control and Prevention said in an interview.
“Based on the literature, I’m not all that surprised because we definitely know that they are at increased risk for these skin and soft tissue infections, and that is a common manifestation of strep,” she said. “The mortality is slightly increased, but not to the extent of the incidence.”
The study was prompted by the limited number of prevention strategies for what Langley described as a “really serious infection” and by the rise in obesity and diabetes in the United States.
Data from select counties in 10 of the CDC’s Active Bacterial Core surveillance sites were used to identify 2,927 cases of 2010-2012 community onset iGAS. Cases were defined by isolation of GAS from a normally sterile site or from a wound in a patient with necrotizing fasciitis or streptococcal toxic shock syndrome in a resident in the surveillance area. Clinical data were obtained from medical records, with height and weight used to calculate BMI.
Diabetes was present in 859 patients (29.3%), grades 1-2 obesity in 743 (25.4%), grade 3 obesity in 399 patients (13.6%), and skin/soft tissue infections in 976 (33.3%). Most patients were aged 18-49 years (37.6%) and male (55%).
Among diabetic patients, the relative risk of an iGAS infection was 4.09 for those who were underweight (BMI < 18.5), 1.23 for the overweight (BMI 25 < 30), 3.14 for those with grade 1-2 obesity, and 15.6 for the morbidly obese. Among nondiabetics, the corresponding relative risks were 3.29, 0.87, 1.15, and 2.95, the authors reported in the poster presentation.
Dr. Langley said the next step is to look at treatment options and whether antibiotic dosing is important and “Long term, if we have a vaccine, this might be a group that needs to be targeted for vaccination.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
PHILADELPHIA – Obesity and diabetes are independent risk factors for invasive group A Streptococcus infections, with the highest risk among those with diabetes and morbid obesity.
The relative risk of invasive group A Streptococcus (iGAS) infection was 15 times higher in adults with diabetes and grade 3 morbid obesity (body mass index ≥ 40 kg/m2) than in those of normal weight, Dr. Gayle Langley reported at Infectious Diseases Week 2014.
Moreover, grades 1-2 obesity and morbid obesity increased the odds of ICU admission and death from an iGAS infection in multivariable logistic regression.
The adjusted odds ratios for ICU admission and death were 1.46 and 1.55 for patients with grades 1-2 obesity (BMI 30 < 40 kg/m2) and 2.07 and 1.62 for those with morbid obesity, she said.
Diabetes was not associated with an increased risk for either ICU admission (odds ratio, 1.06) or death (OR, 0.77) in the analysis.
Skin and soft tissue infections, which were significantly more common in obese and diabetic patients, appear to be driving the increased risk for iGAS in these groups, Dr. Langley of the Centers for Disease Control and Prevention said in an interview.
“Based on the literature, I’m not all that surprised because we definitely know that they are at increased risk for these skin and soft tissue infections, and that is a common manifestation of strep,” she said. “The mortality is slightly increased, but not to the extent of the incidence.”
The study was prompted by the limited number of prevention strategies for what Langley described as a “really serious infection” and by the rise in obesity and diabetes in the United States.
Data from select counties in 10 of the CDC’s Active Bacterial Core surveillance sites were used to identify 2,927 cases of 2010-2012 community onset iGAS. Cases were defined by isolation of GAS from a normally sterile site or from a wound in a patient with necrotizing fasciitis or streptococcal toxic shock syndrome in a resident in the surveillance area. Clinical data were obtained from medical records, with height and weight used to calculate BMI.
Diabetes was present in 859 patients (29.3%), grades 1-2 obesity in 743 (25.4%), grade 3 obesity in 399 patients (13.6%), and skin/soft tissue infections in 976 (33.3%). Most patients were aged 18-49 years (37.6%) and male (55%).
Among diabetic patients, the relative risk of an iGAS infection was 4.09 for those who were underweight (BMI < 18.5), 1.23 for the overweight (BMI 25 < 30), 3.14 for those with grade 1-2 obesity, and 15.6 for the morbidly obese. Among nondiabetics, the corresponding relative risks were 3.29, 0.87, 1.15, and 2.95, the authors reported in the poster presentation.
Dr. Langley said the next step is to look at treatment options and whether antibiotic dosing is important and “Long term, if we have a vaccine, this might be a group that needs to be targeted for vaccination.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
PHILADELPHIA – Obesity and diabetes are independent risk factors for invasive group A Streptococcus infections, with the highest risk among those with diabetes and morbid obesity.
The relative risk of invasive group A Streptococcus (iGAS) infection was 15 times higher in adults with diabetes and grade 3 morbid obesity (body mass index ≥ 40 kg/m2) than in those of normal weight, Dr. Gayle Langley reported at Infectious Diseases Week 2014.
Moreover, grades 1-2 obesity and morbid obesity increased the odds of ICU admission and death from an iGAS infection in multivariable logistic regression.
The adjusted odds ratios for ICU admission and death were 1.46 and 1.55 for patients with grades 1-2 obesity (BMI 30 < 40 kg/m2) and 2.07 and 1.62 for those with morbid obesity, she said.
Diabetes was not associated with an increased risk for either ICU admission (odds ratio, 1.06) or death (OR, 0.77) in the analysis.
Skin and soft tissue infections, which were significantly more common in obese and diabetic patients, appear to be driving the increased risk for iGAS in these groups, Dr. Langley of the Centers for Disease Control and Prevention said in an interview.
“Based on the literature, I’m not all that surprised because we definitely know that they are at increased risk for these skin and soft tissue infections, and that is a common manifestation of strep,” she said. “The mortality is slightly increased, but not to the extent of the incidence.”
The study was prompted by the limited number of prevention strategies for what Langley described as a “really serious infection” and by the rise in obesity and diabetes in the United States.
Data from select counties in 10 of the CDC’s Active Bacterial Core surveillance sites were used to identify 2,927 cases of 2010-2012 community onset iGAS. Cases were defined by isolation of GAS from a normally sterile site or from a wound in a patient with necrotizing fasciitis or streptococcal toxic shock syndrome in a resident in the surveillance area. Clinical data were obtained from medical records, with height and weight used to calculate BMI.
Diabetes was present in 859 patients (29.3%), grades 1-2 obesity in 743 (25.4%), grade 3 obesity in 399 patients (13.6%), and skin/soft tissue infections in 976 (33.3%). Most patients were aged 18-49 years (37.6%) and male (55%).
Among diabetic patients, the relative risk of an iGAS infection was 4.09 for those who were underweight (BMI < 18.5), 1.23 for the overweight (BMI 25 < 30), 3.14 for those with grade 1-2 obesity, and 15.6 for the morbidly obese. Among nondiabetics, the corresponding relative risks were 3.29, 0.87, 1.15, and 2.95, the authors reported in the poster presentation.
Dr. Langley said the next step is to look at treatment options and whether antibiotic dosing is important and “Long term, if we have a vaccine, this might be a group that needs to be targeted for vaccination.”
ID Week is a joint meeting of the Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and Pediatric Infectious Diseases Society.
AT ID WEEK 2014
Key clinical point: Patients with diabetes and obesity are at increased risk for invasive group A Streptococcus infection.
Major finding: The relative risk of invasive group A Streptococcus (iGAS) infection was 15.6 in diabetics with grade 3 morbid obesity vs. normal-weight diabetics.
Data source: Active surveillance data from 2,927 cases of iGAS.
Disclosures: Dr. Langley reported having no financial disclosures. The CDC funds the ABC surveillance sites.
Higher-dose liraglutide effective for weight loss in nondiabetic patients
BOSTON – Further parsing of data from the SCALE clinical trials reinforces the finding that an investigational dose of liraglutide, combined with diet and exercise, is linked with more weight loss in obese diabetic and nondiabetic adults than diet and exercise alone, albeit with significant GI adverse effects.
Among patients who completed the 56-week SCALE Obesity and Prediabetes trial, obese and prediabetic adults treated with an investigational 3-mg injectable daily dose of liraglutide plus calorie restriction and exercise lost a mean of 9.2% of their body weight, compared with 3.5% for patients who injected themselves with placebo and also dieted and exercised.
“Liraglutide 3 mg, in obese patients with prediabetes and without prediabetes, gave significantly more weight loss than in the placebo group, and it was independent of whether or not patients had prediabetes, or their body mass index,” Dr. Frank Greenway of the Pennington Biomedical Research Center in Baton Rouge, La., said at Obesity Week 2014, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Patients with a baseline BMI of up to 29.9 kg/m2 lost an average of 7.5% of body weight, those with a BMI of 30-34.9 lost 8.3%, those with a BMI of 35-39.9% dropped 9.3% on average, and those with BMIs of 40 or over shed 7.4%. In each category, the difference between the liraglutide and placebo groups was significant (P < .0001).
Liraglutide is a glucagon-like peptide-1 (GLP-1) analogue approved in the United States as a 1.2-mg and 1.8-mg daily subcutaneous injection as an adjunct to diet and exercise in patients with type 2 diabetes. It is not recommended as first-line therapy for patients with diabetes that is not adequately controlled with diet and exercise. On the basis of the results of the SCALE trials and others, advisers to the Food and Drug Administration in September recommended that the 3-mg dose be approved for weight management in adults. At press time, no decision on the application by the manufacturer, Novo Nordisk, had been made by the FDA.
The SCALE trial enrolled 3,731 patients and randomized them on a 2:1 basis to liraglutide (2,487) or placebo (1,244).
GI adverse events common
The most common adverse event with liraglutide over the course of the study was nausea, which occurred in 40% of patients who injected the drug, compared with 15% of patients who injected a placebo. Diarrhea occurred in 21% and 9%., respectively, and vomiting occurred in 16% and 4%.
Despite these adverse events, which tended to peak by the 6th to 8th week of the study, more patients in the placebo group dropped out of the study (28.1% vs. 35.6%), said Dr. Ken Fujioka of the department of nutrition and metabolic research at the Scripps Clinic in La Jolla, Calif.
“This is a high rate of nausea,” Dr. Fujioka said. He noted that the trial protocol required an upward dose titration that could not be reversed without causing the patient to exit the study, which could partially explain the high rate of gastrointestinal distress seen with the drug.
Dr. Fujioka reported health-related quality of life data from the trial, based on scores in the Short Form–36 (SF-36) and Impact of Weight on Quality of Life–Lite (IWQoL-Lite) questionnaires, which showed better, albeit modest, gains over baselines in patients on liraglutide compared with placebo.
Liraglutide in diabetes patients
Dr. Robert F. Kushner of Northwestern University, Chicago, reported results from a separate trial from the SCALE clinical trial series, SCALE-Diabetes, looking at health-related quality of life in 846 overweight or obese patients with type 2 diabetes treated with liraglutide at the investigational 3-mg dose, the approved 1.8-mg dose, or placebo. All patients also engaged in calorie restriction and exercise.
At week 56, the mean body weight percentage lost from baseline was 5.9% in the 3-mg group, 4.6% in the 1.8 mg group, and 2% in the placebo group.
Compared with placebo, there was a significantly better mean total improvement in the IWQoL-Lite score with the 3-mg dose but not with the 1.8-mg dose. Gastrointestinal adverse events also were common in this trial, with nausea occurring in 33% on the 3-mg dose, 31% on the 1.8-mg dose, and 14% of patients on placebo. Diarrhea occurred in 26%, 18%, and 13%, respectively, and constipation in 16%, 10%, and 6%.
“The nausea that is seen in this trial primarily occurs from the first 12-16 weeks and then settles down, and there is not that much difference thereafter,” Dr. Kushner said.
Too expensive?
An obesity researcher who was not involved in the study noted that liraglutide is not yet reimbursed for weight loss, and has an adverse event profile that may discourage some patients from remaining on the drug.
“I think that for those people who can tolerate the medication and who can afford it, it’s better than placebo. If you can have access to medication it’s great, but a lot of people are not able to stick to it for any duration of time, and that’s the downside of it. If you don’t stick to it, the weight comes back,” Dr. Ranjan Sudan, a surgeon at Duke University, Durham, N.C., and chair of the ASMBS Research Committee, said in an interview.
“If this medication, for instance, could show that down the road it prevented or prolonged the onset of diabetes, that would be a whole different thing, and there may be some justification for its use. But for the moment, being primarily for weight loss purposes and not to treat comorbidities makes it a little bit of a hard sell,” he said.
If approved, liraglutide 3 mg will be marketed as Saxenda. Liraglutide 1.8 mg was approved in 2010 and is marketed as Victoza.
The studies were supported by Novo Nordisk. Dr. Greenway and Dr. Kushner disclosed serving on the company’s advisory board. Dr. Fujioka disclosed receiving research support and consulting with the company.
BOSTON – Further parsing of data from the SCALE clinical trials reinforces the finding that an investigational dose of liraglutide, combined with diet and exercise, is linked with more weight loss in obese diabetic and nondiabetic adults than diet and exercise alone, albeit with significant GI adverse effects.
Among patients who completed the 56-week SCALE Obesity and Prediabetes trial, obese and prediabetic adults treated with an investigational 3-mg injectable daily dose of liraglutide plus calorie restriction and exercise lost a mean of 9.2% of their body weight, compared with 3.5% for patients who injected themselves with placebo and also dieted and exercised.
“Liraglutide 3 mg, in obese patients with prediabetes and without prediabetes, gave significantly more weight loss than in the placebo group, and it was independent of whether or not patients had prediabetes, or their body mass index,” Dr. Frank Greenway of the Pennington Biomedical Research Center in Baton Rouge, La., said at Obesity Week 2014, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Patients with a baseline BMI of up to 29.9 kg/m2 lost an average of 7.5% of body weight, those with a BMI of 30-34.9 lost 8.3%, those with a BMI of 35-39.9% dropped 9.3% on average, and those with BMIs of 40 or over shed 7.4%. In each category, the difference between the liraglutide and placebo groups was significant (P < .0001).
Liraglutide is a glucagon-like peptide-1 (GLP-1) analogue approved in the United States as a 1.2-mg and 1.8-mg daily subcutaneous injection as an adjunct to diet and exercise in patients with type 2 diabetes. It is not recommended as first-line therapy for patients with diabetes that is not adequately controlled with diet and exercise. On the basis of the results of the SCALE trials and others, advisers to the Food and Drug Administration in September recommended that the 3-mg dose be approved for weight management in adults. At press time, no decision on the application by the manufacturer, Novo Nordisk, had been made by the FDA.
The SCALE trial enrolled 3,731 patients and randomized them on a 2:1 basis to liraglutide (2,487) or placebo (1,244).
GI adverse events common
The most common adverse event with liraglutide over the course of the study was nausea, which occurred in 40% of patients who injected the drug, compared with 15% of patients who injected a placebo. Diarrhea occurred in 21% and 9%., respectively, and vomiting occurred in 16% and 4%.
Despite these adverse events, which tended to peak by the 6th to 8th week of the study, more patients in the placebo group dropped out of the study (28.1% vs. 35.6%), said Dr. Ken Fujioka of the department of nutrition and metabolic research at the Scripps Clinic in La Jolla, Calif.
“This is a high rate of nausea,” Dr. Fujioka said. He noted that the trial protocol required an upward dose titration that could not be reversed without causing the patient to exit the study, which could partially explain the high rate of gastrointestinal distress seen with the drug.
Dr. Fujioka reported health-related quality of life data from the trial, based on scores in the Short Form–36 (SF-36) and Impact of Weight on Quality of Life–Lite (IWQoL-Lite) questionnaires, which showed better, albeit modest, gains over baselines in patients on liraglutide compared with placebo.
Liraglutide in diabetes patients
Dr. Robert F. Kushner of Northwestern University, Chicago, reported results from a separate trial from the SCALE clinical trial series, SCALE-Diabetes, looking at health-related quality of life in 846 overweight or obese patients with type 2 diabetes treated with liraglutide at the investigational 3-mg dose, the approved 1.8-mg dose, or placebo. All patients also engaged in calorie restriction and exercise.
At week 56, the mean body weight percentage lost from baseline was 5.9% in the 3-mg group, 4.6% in the 1.8 mg group, and 2% in the placebo group.
Compared with placebo, there was a significantly better mean total improvement in the IWQoL-Lite score with the 3-mg dose but not with the 1.8-mg dose. Gastrointestinal adverse events also were common in this trial, with nausea occurring in 33% on the 3-mg dose, 31% on the 1.8-mg dose, and 14% of patients on placebo. Diarrhea occurred in 26%, 18%, and 13%, respectively, and constipation in 16%, 10%, and 6%.
“The nausea that is seen in this trial primarily occurs from the first 12-16 weeks and then settles down, and there is not that much difference thereafter,” Dr. Kushner said.
Too expensive?
An obesity researcher who was not involved in the study noted that liraglutide is not yet reimbursed for weight loss, and has an adverse event profile that may discourage some patients from remaining on the drug.
“I think that for those people who can tolerate the medication and who can afford it, it’s better than placebo. If you can have access to medication it’s great, but a lot of people are not able to stick to it for any duration of time, and that’s the downside of it. If you don’t stick to it, the weight comes back,” Dr. Ranjan Sudan, a surgeon at Duke University, Durham, N.C., and chair of the ASMBS Research Committee, said in an interview.
“If this medication, for instance, could show that down the road it prevented or prolonged the onset of diabetes, that would be a whole different thing, and there may be some justification for its use. But for the moment, being primarily for weight loss purposes and not to treat comorbidities makes it a little bit of a hard sell,” he said.
If approved, liraglutide 3 mg will be marketed as Saxenda. Liraglutide 1.8 mg was approved in 2010 and is marketed as Victoza.
The studies were supported by Novo Nordisk. Dr. Greenway and Dr. Kushner disclosed serving on the company’s advisory board. Dr. Fujioka disclosed receiving research support and consulting with the company.
BOSTON – Further parsing of data from the SCALE clinical trials reinforces the finding that an investigational dose of liraglutide, combined with diet and exercise, is linked with more weight loss in obese diabetic and nondiabetic adults than diet and exercise alone, albeit with significant GI adverse effects.
Among patients who completed the 56-week SCALE Obesity and Prediabetes trial, obese and prediabetic adults treated with an investigational 3-mg injectable daily dose of liraglutide plus calorie restriction and exercise lost a mean of 9.2% of their body weight, compared with 3.5% for patients who injected themselves with placebo and also dieted and exercised.
“Liraglutide 3 mg, in obese patients with prediabetes and without prediabetes, gave significantly more weight loss than in the placebo group, and it was independent of whether or not patients had prediabetes, or their body mass index,” Dr. Frank Greenway of the Pennington Biomedical Research Center in Baton Rouge, La., said at Obesity Week 2014, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Patients with a baseline BMI of up to 29.9 kg/m2 lost an average of 7.5% of body weight, those with a BMI of 30-34.9 lost 8.3%, those with a BMI of 35-39.9% dropped 9.3% on average, and those with BMIs of 40 or over shed 7.4%. In each category, the difference between the liraglutide and placebo groups was significant (P < .0001).
Liraglutide is a glucagon-like peptide-1 (GLP-1) analogue approved in the United States as a 1.2-mg and 1.8-mg daily subcutaneous injection as an adjunct to diet and exercise in patients with type 2 diabetes. It is not recommended as first-line therapy for patients with diabetes that is not adequately controlled with diet and exercise. On the basis of the results of the SCALE trials and others, advisers to the Food and Drug Administration in September recommended that the 3-mg dose be approved for weight management in adults. At press time, no decision on the application by the manufacturer, Novo Nordisk, had been made by the FDA.
The SCALE trial enrolled 3,731 patients and randomized them on a 2:1 basis to liraglutide (2,487) or placebo (1,244).
GI adverse events common
The most common adverse event with liraglutide over the course of the study was nausea, which occurred in 40% of patients who injected the drug, compared with 15% of patients who injected a placebo. Diarrhea occurred in 21% and 9%., respectively, and vomiting occurred in 16% and 4%.
Despite these adverse events, which tended to peak by the 6th to 8th week of the study, more patients in the placebo group dropped out of the study (28.1% vs. 35.6%), said Dr. Ken Fujioka of the department of nutrition and metabolic research at the Scripps Clinic in La Jolla, Calif.
“This is a high rate of nausea,” Dr. Fujioka said. He noted that the trial protocol required an upward dose titration that could not be reversed without causing the patient to exit the study, which could partially explain the high rate of gastrointestinal distress seen with the drug.
Dr. Fujioka reported health-related quality of life data from the trial, based on scores in the Short Form–36 (SF-36) and Impact of Weight on Quality of Life–Lite (IWQoL-Lite) questionnaires, which showed better, albeit modest, gains over baselines in patients on liraglutide compared with placebo.
Liraglutide in diabetes patients
Dr. Robert F. Kushner of Northwestern University, Chicago, reported results from a separate trial from the SCALE clinical trial series, SCALE-Diabetes, looking at health-related quality of life in 846 overweight or obese patients with type 2 diabetes treated with liraglutide at the investigational 3-mg dose, the approved 1.8-mg dose, or placebo. All patients also engaged in calorie restriction and exercise.
At week 56, the mean body weight percentage lost from baseline was 5.9% in the 3-mg group, 4.6% in the 1.8 mg group, and 2% in the placebo group.
Compared with placebo, there was a significantly better mean total improvement in the IWQoL-Lite score with the 3-mg dose but not with the 1.8-mg dose. Gastrointestinal adverse events also were common in this trial, with nausea occurring in 33% on the 3-mg dose, 31% on the 1.8-mg dose, and 14% of patients on placebo. Diarrhea occurred in 26%, 18%, and 13%, respectively, and constipation in 16%, 10%, and 6%.
“The nausea that is seen in this trial primarily occurs from the first 12-16 weeks and then settles down, and there is not that much difference thereafter,” Dr. Kushner said.
Too expensive?
An obesity researcher who was not involved in the study noted that liraglutide is not yet reimbursed for weight loss, and has an adverse event profile that may discourage some patients from remaining on the drug.
“I think that for those people who can tolerate the medication and who can afford it, it’s better than placebo. If you can have access to medication it’s great, but a lot of people are not able to stick to it for any duration of time, and that’s the downside of it. If you don’t stick to it, the weight comes back,” Dr. Ranjan Sudan, a surgeon at Duke University, Durham, N.C., and chair of the ASMBS Research Committee, said in an interview.
“If this medication, for instance, could show that down the road it prevented or prolonged the onset of diabetes, that would be a whole different thing, and there may be some justification for its use. But for the moment, being primarily for weight loss purposes and not to treat comorbidities makes it a little bit of a hard sell,” he said.
If approved, liraglutide 3 mg will be marketed as Saxenda. Liraglutide 1.8 mg was approved in 2010 and is marketed as Victoza.
The studies were supported by Novo Nordisk. Dr. Greenway and Dr. Kushner disclosed serving on the company’s advisory board. Dr. Fujioka disclosed receiving research support and consulting with the company.
Key clinical point: The antidiabetic drug liraglutide combined with diet and exercise helped nondiabetic overweight and obese patients lose more weight than did placebo.
Major finding: Obese and prediabetic adults treated with an investigational 3-mg injectable daily dose of liraglutide (Victoza) plus calorie restriction and exercise lost a mean of 9.2% of their body weight, compared with 3.5% for those on placebo.
Data source: Randomized clinical trial comparing weight loss and safety parameters in 3,731 overweight and obese adults.
Disclosures:The study was supported by Novo Nordisk. Dr. Greenway and Dr. Kushner disclosed serving on the company’s advisory board. Dr. Fujioka disclosed receiving research support and consulting with the company.
Data lacking for intensive weight-loss counseling in primary care
No studies have yet assessed the efficacy of intensive behavioral weight-loss counseling delivered in the primary care setting in accordance with Medicare/Medicaid requirements, according to a systematic review of the literature published online Nov. 4 in JAMA.
The U.S. Preventive Services Task Force recommends that all primary care physicians screen all their adult patients for obesity and offer the affected patients such intensive counseling, either by providing it themselves or by referral, said Thomas A. Wadden, Ph.D., of the Center for Weight and Eating Disorders, University of Pennsylvania, Philadelphia, and his associates.
In 2011, the Centers for Medicare & Medicaid Services (CMS) approved the provision of intensive behavioral counseling for obese beneficiaries seen in primary care practices for approximately 14 face-to-face sessions over 6 months when delivered by physicians and other select practitioners. While the CMS covers the costs of such counseling, it does so only when counseling meets specifics on treatment intensity, who may provide it, and where it may be provided.
In their systematic review, the investigators identified 3,304 articles on the topic, and winnowed their detailed review to 12 good-quality trials involving 3,893 participants. They found that to date, not a single study has assessed even one real-world primary care practice that complies with all the CMS requirements.
Data from two of the reviewed clinical trials supported the “treatment intensity” requirement, which is that intensive behavioral weight-loss counseling must include approximately 14 face-to-face sessions during the initial 6 months: weekly sessions for the first month, every-other-week sessions for months 2-6, and monthly sessions during months 7-12. In these two studies, participants attended three sessions with the primary care physician and eight with a trained interventionist for 6 months, and achieved mean weight losses of 4.4 kg and 3.5 kg, respectively.
However, one of these trials substituted brief telephone sessions with trained interventionists at a call center for face-to-face interviews, with good results. “A growing literature suggests that telephone-delivered counseling is generally as effective as traditional face-to-face contact, potentially is more convenient and less costly for patients, and can reach more individuals in underserved areas,” Dr. Wadden and his associates said (JAMA 2014 Nov. 4 [doi: 10.1001/jama.2014.14173]).
Similarly, data from three trials supported the requirement that counseling be provided by trained medical assistants in collaboration with primary care physicians. Weight loss achieved with this approach was far greater than that achieved with counseling provided by trained interventionists who had limited or no collaboration with the primary care physicians. The results of several trials confirmed the requirement that this counseling must be comprehensive, incorporating a dietary component, an exercise component, and a behavioral component to maximize results. In particular, interventions that provided specific goals for energy restriction and expenditure were successful, whereas those that did not were unsuccessful, the investigators said.
This study was funded in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Wadden and his associates reported ties to Novo Nordisk, Nutrisystem, Orexigen, Shire Pharmaceuticals, and Weight Watchers.
No studies have yet assessed the efficacy of intensive behavioral weight-loss counseling delivered in the primary care setting in accordance with Medicare/Medicaid requirements, according to a systematic review of the literature published online Nov. 4 in JAMA.
The U.S. Preventive Services Task Force recommends that all primary care physicians screen all their adult patients for obesity and offer the affected patients such intensive counseling, either by providing it themselves or by referral, said Thomas A. Wadden, Ph.D., of the Center for Weight and Eating Disorders, University of Pennsylvania, Philadelphia, and his associates.
In 2011, the Centers for Medicare & Medicaid Services (CMS) approved the provision of intensive behavioral counseling for obese beneficiaries seen in primary care practices for approximately 14 face-to-face sessions over 6 months when delivered by physicians and other select practitioners. While the CMS covers the costs of such counseling, it does so only when counseling meets specifics on treatment intensity, who may provide it, and where it may be provided.
In their systematic review, the investigators identified 3,304 articles on the topic, and winnowed their detailed review to 12 good-quality trials involving 3,893 participants. They found that to date, not a single study has assessed even one real-world primary care practice that complies with all the CMS requirements.
Data from two of the reviewed clinical trials supported the “treatment intensity” requirement, which is that intensive behavioral weight-loss counseling must include approximately 14 face-to-face sessions during the initial 6 months: weekly sessions for the first month, every-other-week sessions for months 2-6, and monthly sessions during months 7-12. In these two studies, participants attended three sessions with the primary care physician and eight with a trained interventionist for 6 months, and achieved mean weight losses of 4.4 kg and 3.5 kg, respectively.
However, one of these trials substituted brief telephone sessions with trained interventionists at a call center for face-to-face interviews, with good results. “A growing literature suggests that telephone-delivered counseling is generally as effective as traditional face-to-face contact, potentially is more convenient and less costly for patients, and can reach more individuals in underserved areas,” Dr. Wadden and his associates said (JAMA 2014 Nov. 4 [doi: 10.1001/jama.2014.14173]).
Similarly, data from three trials supported the requirement that counseling be provided by trained medical assistants in collaboration with primary care physicians. Weight loss achieved with this approach was far greater than that achieved with counseling provided by trained interventionists who had limited or no collaboration with the primary care physicians. The results of several trials confirmed the requirement that this counseling must be comprehensive, incorporating a dietary component, an exercise component, and a behavioral component to maximize results. In particular, interventions that provided specific goals for energy restriction and expenditure were successful, whereas those that did not were unsuccessful, the investigators said.
This study was funded in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Wadden and his associates reported ties to Novo Nordisk, Nutrisystem, Orexigen, Shire Pharmaceuticals, and Weight Watchers.
No studies have yet assessed the efficacy of intensive behavioral weight-loss counseling delivered in the primary care setting in accordance with Medicare/Medicaid requirements, according to a systematic review of the literature published online Nov. 4 in JAMA.
The U.S. Preventive Services Task Force recommends that all primary care physicians screen all their adult patients for obesity and offer the affected patients such intensive counseling, either by providing it themselves or by referral, said Thomas A. Wadden, Ph.D., of the Center for Weight and Eating Disorders, University of Pennsylvania, Philadelphia, and his associates.
In 2011, the Centers for Medicare & Medicaid Services (CMS) approved the provision of intensive behavioral counseling for obese beneficiaries seen in primary care practices for approximately 14 face-to-face sessions over 6 months when delivered by physicians and other select practitioners. While the CMS covers the costs of such counseling, it does so only when counseling meets specifics on treatment intensity, who may provide it, and where it may be provided.
In their systematic review, the investigators identified 3,304 articles on the topic, and winnowed their detailed review to 12 good-quality trials involving 3,893 participants. They found that to date, not a single study has assessed even one real-world primary care practice that complies with all the CMS requirements.
Data from two of the reviewed clinical trials supported the “treatment intensity” requirement, which is that intensive behavioral weight-loss counseling must include approximately 14 face-to-face sessions during the initial 6 months: weekly sessions for the first month, every-other-week sessions for months 2-6, and monthly sessions during months 7-12. In these two studies, participants attended three sessions with the primary care physician and eight with a trained interventionist for 6 months, and achieved mean weight losses of 4.4 kg and 3.5 kg, respectively.
However, one of these trials substituted brief telephone sessions with trained interventionists at a call center for face-to-face interviews, with good results. “A growing literature suggests that telephone-delivered counseling is generally as effective as traditional face-to-face contact, potentially is more convenient and less costly for patients, and can reach more individuals in underserved areas,” Dr. Wadden and his associates said (JAMA 2014 Nov. 4 [doi: 10.1001/jama.2014.14173]).
Similarly, data from three trials supported the requirement that counseling be provided by trained medical assistants in collaboration with primary care physicians. Weight loss achieved with this approach was far greater than that achieved with counseling provided by trained interventionists who had limited or no collaboration with the primary care physicians. The results of several trials confirmed the requirement that this counseling must be comprehensive, incorporating a dietary component, an exercise component, and a behavioral component to maximize results. In particular, interventions that provided specific goals for energy restriction and expenditure were successful, whereas those that did not were unsuccessful, the investigators said.
This study was funded in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Wadden and his associates reported ties to Novo Nordisk, Nutrisystem, Orexigen, Shire Pharmaceuticals, and Weight Watchers.
Key clinical point: No studies have assessed the efficacy of intensive behavioral weight-loss counseling delivered in the primary care setting according to CMS guidelines.
Major finding: Data from two of the reviewed clinical trials supported the “treatment intensity” requirement, finding that participants who attended three counseling sessions with the primary care physician and eight with a trained interventionist for 6 months achieved mean weight losses of 4.4 kg and 3.5 kg, respectively.
Data source: A systematic review summarizing the findings of 12 randomized controlled trials involving 3,893 overweight or obese participants treated with intensive behavioral weight-loss counseling in the primary care setting.
Disclosures: This study was funded in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Wadden and his associates reported ties to Novo Nordisk, Nutrisystem, Orexigen, Shire Pharmaceuticals, and Weight Watchers.
Dapagliflozin-metformin combination tablet approved by FDA
A combination oral tablet containing dapagliflozin and extended-release metformin was approved by the Food and Drug Administration for treating adults with type 2 diabetes, the manufacturer, AstraZeneca, announced on Oct. 30.
The dosage strengths approved include 5 mg and 10 mg of dapagliflozin with 500 mg or 1,000 mg of extended-release metformin. The approved indication is as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes when treatment with both dapagliflozin and metformin is appropriate.
This is the first product combing a sodium-glucose cotransporter 2 (SGLT2) inhibitor (dapagliflozin) with a biguanide (metformin) in one tablet administered once a day, the company said in a statement.
Approval was based on four phase III studies evaluating the drugs as combination therapy, but not with the actual combined tablet formulation, the statement said.
The FDA’s approval letter says that as part of its postmarketing requirements, the company should conduct a study evaluating whether pediatric patients with type 2 diabetes or healthy children aged 10-17 years can safely swallow the combination tablets. The letter also describes a randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety, and tolerability of dapagliflozin for the treatment of children and adolescents aged 10-17 years with type 2 diabetes as add-on therapy to metformin or as monotherapy, a required postmarketing study for the approval of dapagliflozin in January 2014.
AstraZeneca is marketing the combined product as Xigduo XR. It is also marketed in Australia under the same trade name. In the European Union, a combination of dapagliflozin and immediate-release metformin is marketed as Xigduo.
A combination oral tablet containing dapagliflozin and extended-release metformin was approved by the Food and Drug Administration for treating adults with type 2 diabetes, the manufacturer, AstraZeneca, announced on Oct. 30.
The dosage strengths approved include 5 mg and 10 mg of dapagliflozin with 500 mg or 1,000 mg of extended-release metformin. The approved indication is as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes when treatment with both dapagliflozin and metformin is appropriate.
This is the first product combing a sodium-glucose cotransporter 2 (SGLT2) inhibitor (dapagliflozin) with a biguanide (metformin) in one tablet administered once a day, the company said in a statement.
Approval was based on four phase III studies evaluating the drugs as combination therapy, but not with the actual combined tablet formulation, the statement said.
The FDA’s approval letter says that as part of its postmarketing requirements, the company should conduct a study evaluating whether pediatric patients with type 2 diabetes or healthy children aged 10-17 years can safely swallow the combination tablets. The letter also describes a randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety, and tolerability of dapagliflozin for the treatment of children and adolescents aged 10-17 years with type 2 diabetes as add-on therapy to metformin or as monotherapy, a required postmarketing study for the approval of dapagliflozin in January 2014.
AstraZeneca is marketing the combined product as Xigduo XR. It is also marketed in Australia under the same trade name. In the European Union, a combination of dapagliflozin and immediate-release metformin is marketed as Xigduo.
A combination oral tablet containing dapagliflozin and extended-release metformin was approved by the Food and Drug Administration for treating adults with type 2 diabetes, the manufacturer, AstraZeneca, announced on Oct. 30.
The dosage strengths approved include 5 mg and 10 mg of dapagliflozin with 500 mg or 1,000 mg of extended-release metformin. The approved indication is as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes when treatment with both dapagliflozin and metformin is appropriate.
This is the first product combing a sodium-glucose cotransporter 2 (SGLT2) inhibitor (dapagliflozin) with a biguanide (metformin) in one tablet administered once a day, the company said in a statement.
Approval was based on four phase III studies evaluating the drugs as combination therapy, but not with the actual combined tablet formulation, the statement said.
The FDA’s approval letter says that as part of its postmarketing requirements, the company should conduct a study evaluating whether pediatric patients with type 2 diabetes or healthy children aged 10-17 years can safely swallow the combination tablets. The letter also describes a randomized, double-blind, placebo-controlled study to evaluate the efficacy, safety, and tolerability of dapagliflozin for the treatment of children and adolescents aged 10-17 years with type 2 diabetes as add-on therapy to metformin or as monotherapy, a required postmarketing study for the approval of dapagliflozin in January 2014.
AstraZeneca is marketing the combined product as Xigduo XR. It is also marketed in Australia under the same trade name. In the European Union, a combination of dapagliflozin and immediate-release metformin is marketed as Xigduo.
Metformin linked to less intensification of diabetes treatment
First-line treatment with metformin for diabetes is associated with significantly less intensification of treatment, and fewer short-term adverse outcomes such as cardiovascular events, emergency department visits, and hypoglycemia, compared with other oral glucose-lowering medications.
A retrospective cohort study of 15,516 patients prescribed an oral glucose-lowering medication found that 25% of patients initially prescribed metformin required a second oral agent, compared with 37% of patients prescribed a sulfonylurea, 40% of those prescribed a thiazolidinedione, and 36% of those prescribed a dipeptidyl peptidase–4 inhibitor, according to a retrospective study published online Oct. 27 (JAMA Intern. Med. 2014 Oct. 27 [doi: 10.1001/jamainternmed.2014.5294]).
While numerous guidelines recommend metformin as the first-line choice of glucose-lowering agents, only 58% of patients started therapy with metformin, with a sulfonylurea the second-most-popular choice, despite its increased risk of adverse cardiovascular events.
“Because underuse of metformin may lead to important harms and costs in the treatment of patients with diabetes, multilevel interventions to increase prescribing quality may be needed,” wrote Dr. Seth A. Berkowitz of Harvard University, Boston, and his colleagues.
The study was supported by an unrestricted grant from CVS Health to Brigham and Women’s Hospital, Boston. One author declared funding from an Institutional National Research Service Award, the Ryoichi Sasakawa Fellowship Fund, and Massachusetts General Hospital. There were no other conflicts of interest declared.
Although the superiority of metformin as a first-line monotherapy is not news, the study investigators’ choice of treatment intensification as its primary outcome was novel, and highlighted something of greater significance to patients than to physicians.
Patients consider treatment intensification as a result of failure. Reframing the addition of medication as a necessary step for wellness and health maintenance may go a long way toward patient acceptance of intensification as an unfortunate but necessary part of good self-care.
Dr. Jodi B. Segal and Dr. Nisa M. Maruthur are with the department of medicine at Johns Hopkins University, Baltimore. These comments are taken from their editorial accompanying Dr. Berkowitz’s article (JAMA Intern. Med. 2014 [doi: 10.1001/jamainternmed.2014.4296]). They reported having no relevant disclosures.
Although the superiority of metformin as a first-line monotherapy is not news, the study investigators’ choice of treatment intensification as its primary outcome was novel, and highlighted something of greater significance to patients than to physicians.
Patients consider treatment intensification as a result of failure. Reframing the addition of medication as a necessary step for wellness and health maintenance may go a long way toward patient acceptance of intensification as an unfortunate but necessary part of good self-care.
Dr. Jodi B. Segal and Dr. Nisa M. Maruthur are with the department of medicine at Johns Hopkins University, Baltimore. These comments are taken from their editorial accompanying Dr. Berkowitz’s article (JAMA Intern. Med. 2014 [doi: 10.1001/jamainternmed.2014.4296]). They reported having no relevant disclosures.
Although the superiority of metformin as a first-line monotherapy is not news, the study investigators’ choice of treatment intensification as its primary outcome was novel, and highlighted something of greater significance to patients than to physicians.
Patients consider treatment intensification as a result of failure. Reframing the addition of medication as a necessary step for wellness and health maintenance may go a long way toward patient acceptance of intensification as an unfortunate but necessary part of good self-care.
Dr. Jodi B. Segal and Dr. Nisa M. Maruthur are with the department of medicine at Johns Hopkins University, Baltimore. These comments are taken from their editorial accompanying Dr. Berkowitz’s article (JAMA Intern. Med. 2014 [doi: 10.1001/jamainternmed.2014.4296]). They reported having no relevant disclosures.
First-line treatment with metformin for diabetes is associated with significantly less intensification of treatment, and fewer short-term adverse outcomes such as cardiovascular events, emergency department visits, and hypoglycemia, compared with other oral glucose-lowering medications.
A retrospective cohort study of 15,516 patients prescribed an oral glucose-lowering medication found that 25% of patients initially prescribed metformin required a second oral agent, compared with 37% of patients prescribed a sulfonylurea, 40% of those prescribed a thiazolidinedione, and 36% of those prescribed a dipeptidyl peptidase–4 inhibitor, according to a retrospective study published online Oct. 27 (JAMA Intern. Med. 2014 Oct. 27 [doi: 10.1001/jamainternmed.2014.5294]).
While numerous guidelines recommend metformin as the first-line choice of glucose-lowering agents, only 58% of patients started therapy with metformin, with a sulfonylurea the second-most-popular choice, despite its increased risk of adverse cardiovascular events.
“Because underuse of metformin may lead to important harms and costs in the treatment of patients with diabetes, multilevel interventions to increase prescribing quality may be needed,” wrote Dr. Seth A. Berkowitz of Harvard University, Boston, and his colleagues.
The study was supported by an unrestricted grant from CVS Health to Brigham and Women’s Hospital, Boston. One author declared funding from an Institutional National Research Service Award, the Ryoichi Sasakawa Fellowship Fund, and Massachusetts General Hospital. There were no other conflicts of interest declared.
First-line treatment with metformin for diabetes is associated with significantly less intensification of treatment, and fewer short-term adverse outcomes such as cardiovascular events, emergency department visits, and hypoglycemia, compared with other oral glucose-lowering medications.
A retrospective cohort study of 15,516 patients prescribed an oral glucose-lowering medication found that 25% of patients initially prescribed metformin required a second oral agent, compared with 37% of patients prescribed a sulfonylurea, 40% of those prescribed a thiazolidinedione, and 36% of those prescribed a dipeptidyl peptidase–4 inhibitor, according to a retrospective study published online Oct. 27 (JAMA Intern. Med. 2014 Oct. 27 [doi: 10.1001/jamainternmed.2014.5294]).
While numerous guidelines recommend metformin as the first-line choice of glucose-lowering agents, only 58% of patients started therapy with metformin, with a sulfonylurea the second-most-popular choice, despite its increased risk of adverse cardiovascular events.
“Because underuse of metformin may lead to important harms and costs in the treatment of patients with diabetes, multilevel interventions to increase prescribing quality may be needed,” wrote Dr. Seth A. Berkowitz of Harvard University, Boston, and his colleagues.
The study was supported by an unrestricted grant from CVS Health to Brigham and Women’s Hospital, Boston. One author declared funding from an Institutional National Research Service Award, the Ryoichi Sasakawa Fellowship Fund, and Massachusetts General Hospital. There were no other conflicts of interest declared.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Metformin as first-line therapy is associated with lower rates of progression, compared with other oral glucose-lowering medications.
Major finding: Around one-quarter of patients started on metformin progressed to a second agent, compared with 37% of patients prescribed sulfonylurea.
Data source: Retrospective cohort study of 15,516 patients prescribed an oral glucose-lowering agent.
Disclosures: The study was supported by an unrestricted grant from CVS Health to Brigham and Women’s Hospital, Boston. One author declared funding from an Institutional National Research Service Award, the Ryoichi Sasakawa Fellowship Fund, and Massachusetts General Hospital. There were no other conflicts of interest declared.
VIDEO: Experts call for tighter regulation of glucose monitoring devices
WASHINGTON – Congress should back better regulation of glucose-monitoring technologies and expanded access to continuous glucose monitoring for patients with either type 1 or insulin-dependent type 2 diabetes, according to two endocrinology organizations.
At a consensus conference on glucose monitoring, hosted by the American Association of Clinical Endocrinologists and the American College of Endocrinology, advocates pushed for federal legislation that would increase the role endocrinology experts play in setting policy and reimbursement for glucose monitoring.
Inaccurate glucose level readings can have devastating effects, ranging from seizures to coma or even death. Self-test glucose monitoring kits are currently considered the standard of care for people with type 1 diabetes or with insulin-dependent type-2 diabetes.
However, the reliability of the kits has come into question over the years – although knowing which kits are defective is difficult, endocrinology experts noted. That’s in part because adverse event reporting by manufacturers is voluntary, and any data that are collected are not well reviewed.
“What this impressed upon me is the inherent unfairness in the playing field right now,” said Dr. Daniel Einhorn, past president of the AACE and medical director for the Scripps Whittier Diabetes Institute in La Jolla, Calif. “It’s possible to be a company that is less ethical and yet still make it in the market once cleared [by the FDA], because it’s hard to remove it.”
Abbott, Dexcom, Johnson and Johnson, Medtronic, Merck, and several other medical manufacturers supported the conference. Dr. Einhorn disclosed financial relationships with Janssen, Lilly, and Sanofi.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
WASHINGTON – Congress should back better regulation of glucose-monitoring technologies and expanded access to continuous glucose monitoring for patients with either type 1 or insulin-dependent type 2 diabetes, according to two endocrinology organizations.
At a consensus conference on glucose monitoring, hosted by the American Association of Clinical Endocrinologists and the American College of Endocrinology, advocates pushed for federal legislation that would increase the role endocrinology experts play in setting policy and reimbursement for glucose monitoring.
Inaccurate glucose level readings can have devastating effects, ranging from seizures to coma or even death. Self-test glucose monitoring kits are currently considered the standard of care for people with type 1 diabetes or with insulin-dependent type-2 diabetes.
However, the reliability of the kits has come into question over the years – although knowing which kits are defective is difficult, endocrinology experts noted. That’s in part because adverse event reporting by manufacturers is voluntary, and any data that are collected are not well reviewed.
“What this impressed upon me is the inherent unfairness in the playing field right now,” said Dr. Daniel Einhorn, past president of the AACE and medical director for the Scripps Whittier Diabetes Institute in La Jolla, Calif. “It’s possible to be a company that is less ethical and yet still make it in the market once cleared [by the FDA], because it’s hard to remove it.”
Abbott, Dexcom, Johnson and Johnson, Medtronic, Merck, and several other medical manufacturers supported the conference. Dr. Einhorn disclosed financial relationships with Janssen, Lilly, and Sanofi.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
WASHINGTON – Congress should back better regulation of glucose-monitoring technologies and expanded access to continuous glucose monitoring for patients with either type 1 or insulin-dependent type 2 diabetes, according to two endocrinology organizations.
At a consensus conference on glucose monitoring, hosted by the American Association of Clinical Endocrinologists and the American College of Endocrinology, advocates pushed for federal legislation that would increase the role endocrinology experts play in setting policy and reimbursement for glucose monitoring.
Inaccurate glucose level readings can have devastating effects, ranging from seizures to coma or even death. Self-test glucose monitoring kits are currently considered the standard of care for people with type 1 diabetes or with insulin-dependent type-2 diabetes.
However, the reliability of the kits has come into question over the years – although knowing which kits are defective is difficult, endocrinology experts noted. That’s in part because adverse event reporting by manufacturers is voluntary, and any data that are collected are not well reviewed.
“What this impressed upon me is the inherent unfairness in the playing field right now,” said Dr. Daniel Einhorn, past president of the AACE and medical director for the Scripps Whittier Diabetes Institute in La Jolla, Calif. “It’s possible to be a company that is less ethical and yet still make it in the market once cleared [by the FDA], because it’s hard to remove it.”
Abbott, Dexcom, Johnson and Johnson, Medtronic, Merck, and several other medical manufacturers supported the conference. Dr. Einhorn disclosed financial relationships with Janssen, Lilly, and Sanofi.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
AT AN AACE/ACE CONFERENCE
Insulin pumps associated with reduced risk of cardiovascular disease
VIENNA – Treatment with an insulin pump rather than insulin injections may help protect patients with type 2 diabetes from heart disease, a large registry study has determined.
Over a mean follow-up of about 7 years, the pump was associated with a 44% decrease in the risk of fatal cardiovascular disease. It also conferred a 29% decrease in the risk of death overall, Dr. Soffia Gudbjornsdottir said at the annual meeting of the European Association for the Study of Diabetes.
She used data extracted from the Swedish National Diabetes Registry, which was founded in 2005. It contains information on 95% of the country’s type 1 diabetes patients and is linked with national inpatient and death registries.
The study group comprised 18,168 patients, 2,441 of whom were using an insulin pump. There were some significant baseline differences between the pump users and the users of injectable insulin.
Pump users were younger (age at baseline 38 vs. 41 years), and more likely to be women. Those treated with the pump had better measures of blood pressure and lipids. They were more likely to exercise and less likely to smoke. Insulin injection users had higher rates of prior cardiovascular disease. However, a propensity matching score eliminated these differences and balanced the group, Dr. Gudbjornsdottir noted.
By 7 years, there had been few cardiovascular events or deaths – an expected finding, since the cohort was young. Fatal/nonfatal coronary heart disease had developed in 7% of the insulin injection users and 4% of the of pump users, a nonsignificant difference. Neither were there significant differences in fatal or nonfatal cardiovascular disease (8% vs. 5%) or noncardiovascular mortality (4% vs. 2%).
However, significant differences did appear in two other endpoints: fatal cardiovascular disease (3% vs. 1%) and total mortality (7% vs. 3%).
A multivariate regression model found several significant risk reductions associated with pump use, including fatal cardiovascular disease (hazard ratio, 0.56), and all-cause mortality (HR, 0.71).
There were no significant differences in the risks for fatal/nonfatal coronary heart disease, fatal/nonfatal cardiovascular disease, or noncardiovascular death.
During the discussion, Dr. Gudbjornsdottir addressed the concern of a treatment allocation bias due to some unknown variable in the group. “If we had such an unknown covariate, with, for example, a hazard ratio of 1.3, it would have had to be present in at least 80% of the insulin group and in none of the pump group to invalidate the results, so I would say our study findings are quite robust.”
She had no financial disclosures.
On Twitter @alz_gal
VIENNA – Treatment with an insulin pump rather than insulin injections may help protect patients with type 2 diabetes from heart disease, a large registry study has determined.
Over a mean follow-up of about 7 years, the pump was associated with a 44% decrease in the risk of fatal cardiovascular disease. It also conferred a 29% decrease in the risk of death overall, Dr. Soffia Gudbjornsdottir said at the annual meeting of the European Association for the Study of Diabetes.
She used data extracted from the Swedish National Diabetes Registry, which was founded in 2005. It contains information on 95% of the country’s type 1 diabetes patients and is linked with national inpatient and death registries.
The study group comprised 18,168 patients, 2,441 of whom were using an insulin pump. There were some significant baseline differences between the pump users and the users of injectable insulin.
Pump users were younger (age at baseline 38 vs. 41 years), and more likely to be women. Those treated with the pump had better measures of blood pressure and lipids. They were more likely to exercise and less likely to smoke. Insulin injection users had higher rates of prior cardiovascular disease. However, a propensity matching score eliminated these differences and balanced the group, Dr. Gudbjornsdottir noted.
By 7 years, there had been few cardiovascular events or deaths – an expected finding, since the cohort was young. Fatal/nonfatal coronary heart disease had developed in 7% of the insulin injection users and 4% of the of pump users, a nonsignificant difference. Neither were there significant differences in fatal or nonfatal cardiovascular disease (8% vs. 5%) or noncardiovascular mortality (4% vs. 2%).
However, significant differences did appear in two other endpoints: fatal cardiovascular disease (3% vs. 1%) and total mortality (7% vs. 3%).
A multivariate regression model found several significant risk reductions associated with pump use, including fatal cardiovascular disease (hazard ratio, 0.56), and all-cause mortality (HR, 0.71).
There were no significant differences in the risks for fatal/nonfatal coronary heart disease, fatal/nonfatal cardiovascular disease, or noncardiovascular death.
During the discussion, Dr. Gudbjornsdottir addressed the concern of a treatment allocation bias due to some unknown variable in the group. “If we had such an unknown covariate, with, for example, a hazard ratio of 1.3, it would have had to be present in at least 80% of the insulin group and in none of the pump group to invalidate the results, so I would say our study findings are quite robust.”
She had no financial disclosures.
On Twitter @alz_gal
VIENNA – Treatment with an insulin pump rather than insulin injections may help protect patients with type 2 diabetes from heart disease, a large registry study has determined.
Over a mean follow-up of about 7 years, the pump was associated with a 44% decrease in the risk of fatal cardiovascular disease. It also conferred a 29% decrease in the risk of death overall, Dr. Soffia Gudbjornsdottir said at the annual meeting of the European Association for the Study of Diabetes.
She used data extracted from the Swedish National Diabetes Registry, which was founded in 2005. It contains information on 95% of the country’s type 1 diabetes patients and is linked with national inpatient and death registries.
The study group comprised 18,168 patients, 2,441 of whom were using an insulin pump. There were some significant baseline differences between the pump users and the users of injectable insulin.
Pump users were younger (age at baseline 38 vs. 41 years), and more likely to be women. Those treated with the pump had better measures of blood pressure and lipids. They were more likely to exercise and less likely to smoke. Insulin injection users had higher rates of prior cardiovascular disease. However, a propensity matching score eliminated these differences and balanced the group, Dr. Gudbjornsdottir noted.
By 7 years, there had been few cardiovascular events or deaths – an expected finding, since the cohort was young. Fatal/nonfatal coronary heart disease had developed in 7% of the insulin injection users and 4% of the of pump users, a nonsignificant difference. Neither were there significant differences in fatal or nonfatal cardiovascular disease (8% vs. 5%) or noncardiovascular mortality (4% vs. 2%).
However, significant differences did appear in two other endpoints: fatal cardiovascular disease (3% vs. 1%) and total mortality (7% vs. 3%).
A multivariate regression model found several significant risk reductions associated with pump use, including fatal cardiovascular disease (hazard ratio, 0.56), and all-cause mortality (HR, 0.71).
There were no significant differences in the risks for fatal/nonfatal coronary heart disease, fatal/nonfatal cardiovascular disease, or noncardiovascular death.
During the discussion, Dr. Gudbjornsdottir addressed the concern of a treatment allocation bias due to some unknown variable in the group. “If we had such an unknown covariate, with, for example, a hazard ratio of 1.3, it would have had to be present in at least 80% of the insulin group and in none of the pump group to invalidate the results, so I would say our study findings are quite robust.”
She had no financial disclosures.
On Twitter @alz_gal
FROM EASD 2014
Key clinical point: Insulin pumps may moderate the risk of cardiovascular disease in patients with type 1 diabetes.
Major finding: Compared to injected insulin, treatment with an insulin pump conferred a 44% reduction in the risk of cardiovascular disease death.
Data source: Data were drawn from a Swedish registry of patients with type 1 diabetes.
Disclosures: Dr. Gudbjornsdottir had no financial disclosures.
Diabetes-related increased cancer risk may be statistical artifact
VIENNA – Most of the increased risk of cancer associated with diabetes appears in the first year or so after diabetes diagnosis, suggesting that it’s mainly attributable to increased medical surveillance.
Three studies presented at the annual meeting of the European Association for the Study of Diabetes came to similar conclusions, which seemed to hold for both genders, for obesity-driven and non–obesity-driven cancers, and for patients with type 1 as well as type 2 diabetes – a new finding, according to Mr. Bendix Carstensen.
His large, population-based study found no overall excess risk of cancers among type 1 diabetes patients, compared with the general population.
“Based on this, we can exclude a major carcinogenic effect of exogenous insulin among those with type 1 diabetes, because if there was such an effect we would see some substantial increases in at least some cancers,” said Mr. Carstensen, an epidemiologist at the Steno Diabetes Center in Gentofte, Denmark.
Cancer and type 1 diabetes
The study examined cancer rates in patients with type 1 diabetes from five countries: Australia, Denmark, Finland, Scotland, and Sweden. Type 1 diabetes was defined as a diabetes diagnosis that occurred before age 30. In general, these registries contained patients who were still younger than 60 years. Despite the very large 4.6 million person-years of follow-up, the databases represent a population that does not exhibit the typical age-related increase in cancer risk – a slight limitation of the study, Mr. Carstensen noted.
The databases identified 9,369 cases of cancer among patients with type 1 diabetes. They were classified by gender, age, date of cancer diagnosis, and cancer rate, compared with that of the country’s entire population stratified by the same variables.
The crude rate of cancers in all the diabetes patients combined was no different from that of the general populations, with a risk ratio of 1.00 for men and 1.04 for women.
When cancers were examined by site and gender, some significant differences did arise between the diabetic and nondiabetic groups. Stomach cancer was 19% more likely in men and 75% more likely in women. The risk of pancreatic cancer was 70% increased in men and 36% in women. The risk of liver cancer was about doubled in each gender, and for kidney cancer, the risk was 29% in men and 42% in women. For women, there was a 53% increase in the risk of endometrial cancer.
In the time-dependent analysis, Mr. Carstensen found that almost all of the cancers diagnoses were made in the first year after diabetes was diagnosed and dropped rapidly thereafter. The extended time curve showed no lasting impact of diabetes on overall cancer incidence.
A more specific analysis looked at the time-dependent rate ratios of prostate and colorectal cancers for men, and breast, endometrial, and colorectal cancers in women. Each curve showed the high rate ratio in the first few years, followed by a drop-off and no lasting impact.
There were also no lasting impacts of diabetes on lung cancer, melanoma, or non-Hodgkins lymphoma in either gender.
“We did see an elevated site-specific cancer pattern in patients with type 1 diabetes, but no overall excess,” he said. “For these patients, the total cancer occurrence is not really different from population rates.”
Detection bias in diabetes and obesity
Another large, population-based study came to a similar conclusion for those with type 2 diabetes. In fact, “Detection bias may be the main cause of the increased cancer incidence among patients with diabetes,” Dr. Kirstin De Bruijn said.
She presented a subanalysis of the ongoing Rotterdam Study. The Rotterdam Study, launched in 1990, is investigating determinants of disease in residents aged 55 years and older. It now comprises about 11,000 subjects. Dr. De Bruijn of Erasmus University, Rotterdam, the Netherlands, investigated the association of cancer and diabetes in 10,181 patients. Of these, 906 had an incident type 2 diabetes diagnosis, and 2,238 had an incident cancer diagnosis during the mean follow-up period of 11 years. She looked at the incidence of breast, prostate, pancreatic, lung, and colorectal cancers.
In the overall analysis, the risk of any cancer was 30% increased in the patients with diabetes. This risk was attenuated, but remained statistically significant, in the fully adjusted model (hazard ratio, 1.2). In a cancer-specific analysis, however, only the risk of pancreatic cancer was significantly elevated (HR, 3.6 in the adjusted model). The risk of prostate cancer was 30% lower than that of the general population, but that was not a significant finding, she added.
She then looked at a time-dependent model that split the follow-up period into epochs according to time since diabetes diagnosis (up to 3 months, 3 months to 5 years, and more than 5 years).
The overall risk of a cancer diagnosis was more than three times higher in the first 3 months. It remained significantly elevated, though less so, in the first 5 years (HR, 1.5), and then fell off.
When the individual cancers were considered, only pancreatic cancer was significantly more common among the diabetes patients, and was almost 29 times more likely to be diagnosed in the first 3 months. However, Dr. De Bruijn noted, it’s tough to tease out that particular relationship, since the obesity that accompanies type 2 diabetes can also drive the development of pancreatic cancer.
Ellena Badrick, a researcher at the University of Manchester (England), also examined the idea of detection bias in a population database, exploring the time-dependent relationships for obesity-related cancers, compared with those not related to obesity.
She found 10,315 patients who were diagnosed with type 2 diabetes from 1995 to 2010. These were compared with 20,630 controls chosen from the same period. Obesity-related cancers were considered to be breast, endometrial, ovarian, renal, esophageal, pancreatic, and gallbladder.
There were 1,349 cancers among the patients with diabetes, of which 323 were related to obesity. Among the controls, there were 3,218 cancers, of which 634 were obesity related.
She also split her follow-up period into epochs since diabetes diagnosis: up to 6 months, 6-12 months, 12-24 months, 24 months-5 years, and beyond. Cancer incidence was reported as cases per 1,000 person/epoch.
There was a much higher detection rate overall in the first 6 months after diagnosis – more than 100 cases per 1,000 person-epoch. After 6 months, this dropped to less than 20 cases per 1,000 person-epoch. The incidence did increase over the follow-up period, but she said that reflected the expected age-related pattern.
The same pattern emerged when looking at obesity- vs. non–obesity-related cancers. In the first 6 months, the incidence of obesity-related cancer hovered around 30 per 1,000 person-epoch. By 1 year this had dropped to near zero. For non–obesity-related cancers, the incidence rate was much higher in the first 6 months, at nearly 80 per person/epoch. But this also dropped to near zero by 1 year. Both incidence curves followed the same slow increase as the subjects aged.
Over the entire follow-up, 27% of all the obesity-related cancers and 73% of the non–obesity-related cancers were diagnosed within the first year after a diagnosis of type 2 diabetes.
When cancers diagnosed during the first 2 years were excluded, patients with type 2 diabetes had a 43% increase in the risk of developing an obesity-related cancer during the study. There was no increased risk, however, in developing a cancer not related to obesity.
“This suggests that in patients with type 2 diabetes, cancer risk-reduction strategies should be targeted against obesity-related cancers,” Ms. Badrick said.
Mr. Carstensen is an employee of the Steno Diabetes Center, which is owned by Novo Nordisk. He disclosed that he owns stock in the company. Dr. De Bruijn and Ms. Badrick had no financial disclosures.
On Twitter @alz_gal
VIENNA – Most of the increased risk of cancer associated with diabetes appears in the first year or so after diabetes diagnosis, suggesting that it’s mainly attributable to increased medical surveillance.
Three studies presented at the annual meeting of the European Association for the Study of Diabetes came to similar conclusions, which seemed to hold for both genders, for obesity-driven and non–obesity-driven cancers, and for patients with type 1 as well as type 2 diabetes – a new finding, according to Mr. Bendix Carstensen.
His large, population-based study found no overall excess risk of cancers among type 1 diabetes patients, compared with the general population.
“Based on this, we can exclude a major carcinogenic effect of exogenous insulin among those with type 1 diabetes, because if there was such an effect we would see some substantial increases in at least some cancers,” said Mr. Carstensen, an epidemiologist at the Steno Diabetes Center in Gentofte, Denmark.
Cancer and type 1 diabetes
The study examined cancer rates in patients with type 1 diabetes from five countries: Australia, Denmark, Finland, Scotland, and Sweden. Type 1 diabetes was defined as a diabetes diagnosis that occurred before age 30. In general, these registries contained patients who were still younger than 60 years. Despite the very large 4.6 million person-years of follow-up, the databases represent a population that does not exhibit the typical age-related increase in cancer risk – a slight limitation of the study, Mr. Carstensen noted.
The databases identified 9,369 cases of cancer among patients with type 1 diabetes. They were classified by gender, age, date of cancer diagnosis, and cancer rate, compared with that of the country’s entire population stratified by the same variables.
The crude rate of cancers in all the diabetes patients combined was no different from that of the general populations, with a risk ratio of 1.00 for men and 1.04 for women.
When cancers were examined by site and gender, some significant differences did arise between the diabetic and nondiabetic groups. Stomach cancer was 19% more likely in men and 75% more likely in women. The risk of pancreatic cancer was 70% increased in men and 36% in women. The risk of liver cancer was about doubled in each gender, and for kidney cancer, the risk was 29% in men and 42% in women. For women, there was a 53% increase in the risk of endometrial cancer.
In the time-dependent analysis, Mr. Carstensen found that almost all of the cancers diagnoses were made in the first year after diabetes was diagnosed and dropped rapidly thereafter. The extended time curve showed no lasting impact of diabetes on overall cancer incidence.
A more specific analysis looked at the time-dependent rate ratios of prostate and colorectal cancers for men, and breast, endometrial, and colorectal cancers in women. Each curve showed the high rate ratio in the first few years, followed by a drop-off and no lasting impact.
There were also no lasting impacts of diabetes on lung cancer, melanoma, or non-Hodgkins lymphoma in either gender.
“We did see an elevated site-specific cancer pattern in patients with type 1 diabetes, but no overall excess,” he said. “For these patients, the total cancer occurrence is not really different from population rates.”
Detection bias in diabetes and obesity
Another large, population-based study came to a similar conclusion for those with type 2 diabetes. In fact, “Detection bias may be the main cause of the increased cancer incidence among patients with diabetes,” Dr. Kirstin De Bruijn said.
She presented a subanalysis of the ongoing Rotterdam Study. The Rotterdam Study, launched in 1990, is investigating determinants of disease in residents aged 55 years and older. It now comprises about 11,000 subjects. Dr. De Bruijn of Erasmus University, Rotterdam, the Netherlands, investigated the association of cancer and diabetes in 10,181 patients. Of these, 906 had an incident type 2 diabetes diagnosis, and 2,238 had an incident cancer diagnosis during the mean follow-up period of 11 years. She looked at the incidence of breast, prostate, pancreatic, lung, and colorectal cancers.
In the overall analysis, the risk of any cancer was 30% increased in the patients with diabetes. This risk was attenuated, but remained statistically significant, in the fully adjusted model (hazard ratio, 1.2). In a cancer-specific analysis, however, only the risk of pancreatic cancer was significantly elevated (HR, 3.6 in the adjusted model). The risk of prostate cancer was 30% lower than that of the general population, but that was not a significant finding, she added.
She then looked at a time-dependent model that split the follow-up period into epochs according to time since diabetes diagnosis (up to 3 months, 3 months to 5 years, and more than 5 years).
The overall risk of a cancer diagnosis was more than three times higher in the first 3 months. It remained significantly elevated, though less so, in the first 5 years (HR, 1.5), and then fell off.
When the individual cancers were considered, only pancreatic cancer was significantly more common among the diabetes patients, and was almost 29 times more likely to be diagnosed in the first 3 months. However, Dr. De Bruijn noted, it’s tough to tease out that particular relationship, since the obesity that accompanies type 2 diabetes can also drive the development of pancreatic cancer.
Ellena Badrick, a researcher at the University of Manchester (England), also examined the idea of detection bias in a population database, exploring the time-dependent relationships for obesity-related cancers, compared with those not related to obesity.
She found 10,315 patients who were diagnosed with type 2 diabetes from 1995 to 2010. These were compared with 20,630 controls chosen from the same period. Obesity-related cancers were considered to be breast, endometrial, ovarian, renal, esophageal, pancreatic, and gallbladder.
There were 1,349 cancers among the patients with diabetes, of which 323 were related to obesity. Among the controls, there were 3,218 cancers, of which 634 were obesity related.
She also split her follow-up period into epochs since diabetes diagnosis: up to 6 months, 6-12 months, 12-24 months, 24 months-5 years, and beyond. Cancer incidence was reported as cases per 1,000 person/epoch.
There was a much higher detection rate overall in the first 6 months after diagnosis – more than 100 cases per 1,000 person-epoch. After 6 months, this dropped to less than 20 cases per 1,000 person-epoch. The incidence did increase over the follow-up period, but she said that reflected the expected age-related pattern.
The same pattern emerged when looking at obesity- vs. non–obesity-related cancers. In the first 6 months, the incidence of obesity-related cancer hovered around 30 per 1,000 person-epoch. By 1 year this had dropped to near zero. For non–obesity-related cancers, the incidence rate was much higher in the first 6 months, at nearly 80 per person/epoch. But this also dropped to near zero by 1 year. Both incidence curves followed the same slow increase as the subjects aged.
Over the entire follow-up, 27% of all the obesity-related cancers and 73% of the non–obesity-related cancers were diagnosed within the first year after a diagnosis of type 2 diabetes.
When cancers diagnosed during the first 2 years were excluded, patients with type 2 diabetes had a 43% increase in the risk of developing an obesity-related cancer during the study. There was no increased risk, however, in developing a cancer not related to obesity.
“This suggests that in patients with type 2 diabetes, cancer risk-reduction strategies should be targeted against obesity-related cancers,” Ms. Badrick said.
Mr. Carstensen is an employee of the Steno Diabetes Center, which is owned by Novo Nordisk. He disclosed that he owns stock in the company. Dr. De Bruijn and Ms. Badrick had no financial disclosures.
On Twitter @alz_gal
VIENNA – Most of the increased risk of cancer associated with diabetes appears in the first year or so after diabetes diagnosis, suggesting that it’s mainly attributable to increased medical surveillance.
Three studies presented at the annual meeting of the European Association for the Study of Diabetes came to similar conclusions, which seemed to hold for both genders, for obesity-driven and non–obesity-driven cancers, and for patients with type 1 as well as type 2 diabetes – a new finding, according to Mr. Bendix Carstensen.
His large, population-based study found no overall excess risk of cancers among type 1 diabetes patients, compared with the general population.
“Based on this, we can exclude a major carcinogenic effect of exogenous insulin among those with type 1 diabetes, because if there was such an effect we would see some substantial increases in at least some cancers,” said Mr. Carstensen, an epidemiologist at the Steno Diabetes Center in Gentofte, Denmark.
Cancer and type 1 diabetes
The study examined cancer rates in patients with type 1 diabetes from five countries: Australia, Denmark, Finland, Scotland, and Sweden. Type 1 diabetes was defined as a diabetes diagnosis that occurred before age 30. In general, these registries contained patients who were still younger than 60 years. Despite the very large 4.6 million person-years of follow-up, the databases represent a population that does not exhibit the typical age-related increase in cancer risk – a slight limitation of the study, Mr. Carstensen noted.
The databases identified 9,369 cases of cancer among patients with type 1 diabetes. They were classified by gender, age, date of cancer diagnosis, and cancer rate, compared with that of the country’s entire population stratified by the same variables.
The crude rate of cancers in all the diabetes patients combined was no different from that of the general populations, with a risk ratio of 1.00 for men and 1.04 for women.
When cancers were examined by site and gender, some significant differences did arise between the diabetic and nondiabetic groups. Stomach cancer was 19% more likely in men and 75% more likely in women. The risk of pancreatic cancer was 70% increased in men and 36% in women. The risk of liver cancer was about doubled in each gender, and for kidney cancer, the risk was 29% in men and 42% in women. For women, there was a 53% increase in the risk of endometrial cancer.
In the time-dependent analysis, Mr. Carstensen found that almost all of the cancers diagnoses were made in the first year after diabetes was diagnosed and dropped rapidly thereafter. The extended time curve showed no lasting impact of diabetes on overall cancer incidence.
A more specific analysis looked at the time-dependent rate ratios of prostate and colorectal cancers for men, and breast, endometrial, and colorectal cancers in women. Each curve showed the high rate ratio in the first few years, followed by a drop-off and no lasting impact.
There were also no lasting impacts of diabetes on lung cancer, melanoma, or non-Hodgkins lymphoma in either gender.
“We did see an elevated site-specific cancer pattern in patients with type 1 diabetes, but no overall excess,” he said. “For these patients, the total cancer occurrence is not really different from population rates.”
Detection bias in diabetes and obesity
Another large, population-based study came to a similar conclusion for those with type 2 diabetes. In fact, “Detection bias may be the main cause of the increased cancer incidence among patients with diabetes,” Dr. Kirstin De Bruijn said.
She presented a subanalysis of the ongoing Rotterdam Study. The Rotterdam Study, launched in 1990, is investigating determinants of disease in residents aged 55 years and older. It now comprises about 11,000 subjects. Dr. De Bruijn of Erasmus University, Rotterdam, the Netherlands, investigated the association of cancer and diabetes in 10,181 patients. Of these, 906 had an incident type 2 diabetes diagnosis, and 2,238 had an incident cancer diagnosis during the mean follow-up period of 11 years. She looked at the incidence of breast, prostate, pancreatic, lung, and colorectal cancers.
In the overall analysis, the risk of any cancer was 30% increased in the patients with diabetes. This risk was attenuated, but remained statistically significant, in the fully adjusted model (hazard ratio, 1.2). In a cancer-specific analysis, however, only the risk of pancreatic cancer was significantly elevated (HR, 3.6 in the adjusted model). The risk of prostate cancer was 30% lower than that of the general population, but that was not a significant finding, she added.
She then looked at a time-dependent model that split the follow-up period into epochs according to time since diabetes diagnosis (up to 3 months, 3 months to 5 years, and more than 5 years).
The overall risk of a cancer diagnosis was more than three times higher in the first 3 months. It remained significantly elevated, though less so, in the first 5 years (HR, 1.5), and then fell off.
When the individual cancers were considered, only pancreatic cancer was significantly more common among the diabetes patients, and was almost 29 times more likely to be diagnosed in the first 3 months. However, Dr. De Bruijn noted, it’s tough to tease out that particular relationship, since the obesity that accompanies type 2 diabetes can also drive the development of pancreatic cancer.
Ellena Badrick, a researcher at the University of Manchester (England), also examined the idea of detection bias in a population database, exploring the time-dependent relationships for obesity-related cancers, compared with those not related to obesity.
She found 10,315 patients who were diagnosed with type 2 diabetes from 1995 to 2010. These were compared with 20,630 controls chosen from the same period. Obesity-related cancers were considered to be breast, endometrial, ovarian, renal, esophageal, pancreatic, and gallbladder.
There were 1,349 cancers among the patients with diabetes, of which 323 were related to obesity. Among the controls, there were 3,218 cancers, of which 634 were obesity related.
She also split her follow-up period into epochs since diabetes diagnosis: up to 6 months, 6-12 months, 12-24 months, 24 months-5 years, and beyond. Cancer incidence was reported as cases per 1,000 person/epoch.
There was a much higher detection rate overall in the first 6 months after diagnosis – more than 100 cases per 1,000 person-epoch. After 6 months, this dropped to less than 20 cases per 1,000 person-epoch. The incidence did increase over the follow-up period, but she said that reflected the expected age-related pattern.
The same pattern emerged when looking at obesity- vs. non–obesity-related cancers. In the first 6 months, the incidence of obesity-related cancer hovered around 30 per 1,000 person-epoch. By 1 year this had dropped to near zero. For non–obesity-related cancers, the incidence rate was much higher in the first 6 months, at nearly 80 per person/epoch. But this also dropped to near zero by 1 year. Both incidence curves followed the same slow increase as the subjects aged.
Over the entire follow-up, 27% of all the obesity-related cancers and 73% of the non–obesity-related cancers were diagnosed within the first year after a diagnosis of type 2 diabetes.
When cancers diagnosed during the first 2 years were excluded, patients with type 2 diabetes had a 43% increase in the risk of developing an obesity-related cancer during the study. There was no increased risk, however, in developing a cancer not related to obesity.
“This suggests that in patients with type 2 diabetes, cancer risk-reduction strategies should be targeted against obesity-related cancers,” Ms. Badrick said.
Mr. Carstensen is an employee of the Steno Diabetes Center, which is owned by Novo Nordisk. He disclosed that he owns stock in the company. Dr. De Bruijn and Ms. Badrick had no financial disclosures.
On Twitter @alz_gal
AT EASD 2014
Key clinical point: Most cancers are diagnosed shortly after a diabetes diagnosis, suggesting they’re found during a period of increased medical attention.
Major finding: The overall cancer risk was about 30% elevated in three studies, but that risk was stacked almost exclusively in the first year after diabetes diagnosis.
Data source: Three population-based studies examined time-dependent cancer incidence.
Disclosures: Mr. Carstensen is an employee of the Steno Diabetes Center, owned by Novo Nordisk. Neither Dr. Bruijn nor Ms. Badrick had any financial disclosures.