User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Insulin degludec effectively intensifies type 2 diabetes treatment
VIENNA – A hemoglobin A1c of less than 7% was achieved by 78% patients with type 2 diabetes when insulin degludec (IDeg) was added to oral antidiabetic drug therapy in a randomized, double blind, phase III study.
In comparison, 36% of patients treated with the glucagon-like peptide 1 (GLP-1) agonist liraglutide (Victoza, Novo Nordisk) on top of metformin achieved this blood glucose target, with an odds ratio favoring treatment intensification of 7.79.
Furthermore, after about 6 months of treatment, HbA1c was 0.92% lower in the insulin-added arm than in the control arm, with mean end-of-treatment values of 6.5% versus 7.5% (P < .0001), respectively.
“This treatment approach also resulted in lower fasting plasma glucose [FPG] … and overall low rates of hypoglycemia,” Dr. Vanita Aroda of MedStar Health Research Institute in Hyattsville, Md., said at the annual meeting of the European Association for the Study of Diabetes.
IDeg (Tresiba, Novo Nordisk) is an ultra-long-acting basal insulin analogue currently approved for use in Europe and some other countries around the world. A dual, once-daily, single-injection formulation of IDeg and liraglutide (Xultophy, Novo Nordisk) also is under investigation.
Results from the DUAL (Dual Action of Liraglutide and Insulin Degludec in Type 2 Diabetes) program with the fixed dose combination were reported elsewhere at the EASD annual meeting and recently reported (Lancet Diabetes Endocrinol. 2014 Sept. 2 [doi:10.1016/S2213-8587(14)70174-3]).
In the present study, Dr. Aroda and her associates aimed to confirm the efficacy and safety of IDeg given separately but in combination with liraglutide and metformin versus these oral antidiabetic drugs (OADs) plus an injected insulin placebo.
A total of 1,504 patients with diabetes of at least 6 months’ duration, being treated with metformin or other OADs but not insulin, and in need of treatment intensification were screen for possible inclusion in the trial.
After a 15-week run-in period, during which time the dose of liraglutide was titrated up to 1.5 mg, 346 patients were randomized to receive liraglutide plus metformin and either IDeg or an injectable placebo.
Ninety-two percent of the 174 patients randomized to IDeg plus liraglutide and metformin completed the 26-week trial, as did 76% of the 172 randomized to the placebo arm. Baseline characteristics of the two groups of patients were similar, with a mean age of 57 years, 9 years’ diabetes duration, and a starting HbA1c of about 7.5%.
FPG values at baseline were 8.7 mmol/L in the intensified arm and 9.1 mmol/L in the placebo arm. These decreased to 8.8 and 6.1 mmol/L, respectively, with an end-of-treatment difference of –2.55 mmol/L favoring the intensified arm, a highly significant difference.
Daily insulin doses at the end of treatment were 51 units in the intensified arm and the equivalent of 105 units in the placebo arm.
Mean body weight was lower in the IDeg-supplemented arm than in the placebo arm at baseline (90.7 kg vs. 94.2 kg), and there was an average weight gain of 2 kg and weight loss of 1.3 kg in each arm, respectively, over the study period, such that the mean body weight at the end of the trial was the same, at 92.7 kg.
No cases of severe hypoglycemia were reported, and there was no significant difference in the number of confirmed nocturnal hypoglycemia cases (four events in three patients with IDeg versus two events in two patients with placebo). The rate of confirmed hypoglycemia was significantly higher in insulin-treated patients (17.3% vs 4.7%); otherwise, both regimens studied were well tolerated.
Dr. Julio Rosenstock of Dallas Diabetes & Endocrine Center, who chaired the session, said that these results were “impressive” and that the study was much better designed than was a similar study with insulin detemir (Levemir, Novo Nordisk) added to liraglutide.
“This is better because you have a control injectable,” he said. “In the previous study with detemir the A1c came down to 7.1% after 6 months, as reported by DeVries [Diabetes Care 2012;35:1446-54], and to 7.2%, as we reported, after 1 year [J. Diabetes Complications 2013;27:492-500].
“In that previous study the dose of detemir was 39 units, here it was 51 units, so the question is, are the better results because of a higher insulin dose, or because of a difference in the insulin?”
Dr. Rosenstock suggested that a head-to-head trial of insulin detemir and IDeg would be needed to determine the answer. Dr. Aroda agreed.
Dr. Aroda and Dr. Rosenstock disclosed ties with Novo Nordisk, which funded the study, and with other companies that manufacture diabetes medications and devices.
VIENNA – A hemoglobin A1c of less than 7% was achieved by 78% patients with type 2 diabetes when insulin degludec (IDeg) was added to oral antidiabetic drug therapy in a randomized, double blind, phase III study.
In comparison, 36% of patients treated with the glucagon-like peptide 1 (GLP-1) agonist liraglutide (Victoza, Novo Nordisk) on top of metformin achieved this blood glucose target, with an odds ratio favoring treatment intensification of 7.79.
Furthermore, after about 6 months of treatment, HbA1c was 0.92% lower in the insulin-added arm than in the control arm, with mean end-of-treatment values of 6.5% versus 7.5% (P < .0001), respectively.
“This treatment approach also resulted in lower fasting plasma glucose [FPG] … and overall low rates of hypoglycemia,” Dr. Vanita Aroda of MedStar Health Research Institute in Hyattsville, Md., said at the annual meeting of the European Association for the Study of Diabetes.
IDeg (Tresiba, Novo Nordisk) is an ultra-long-acting basal insulin analogue currently approved for use in Europe and some other countries around the world. A dual, once-daily, single-injection formulation of IDeg and liraglutide (Xultophy, Novo Nordisk) also is under investigation.
Results from the DUAL (Dual Action of Liraglutide and Insulin Degludec in Type 2 Diabetes) program with the fixed dose combination were reported elsewhere at the EASD annual meeting and recently reported (Lancet Diabetes Endocrinol. 2014 Sept. 2 [doi:10.1016/S2213-8587(14)70174-3]).
In the present study, Dr. Aroda and her associates aimed to confirm the efficacy and safety of IDeg given separately but in combination with liraglutide and metformin versus these oral antidiabetic drugs (OADs) plus an injected insulin placebo.
A total of 1,504 patients with diabetes of at least 6 months’ duration, being treated with metformin or other OADs but not insulin, and in need of treatment intensification were screen for possible inclusion in the trial.
After a 15-week run-in period, during which time the dose of liraglutide was titrated up to 1.5 mg, 346 patients were randomized to receive liraglutide plus metformin and either IDeg or an injectable placebo.
Ninety-two percent of the 174 patients randomized to IDeg plus liraglutide and metformin completed the 26-week trial, as did 76% of the 172 randomized to the placebo arm. Baseline characteristics of the two groups of patients were similar, with a mean age of 57 years, 9 years’ diabetes duration, and a starting HbA1c of about 7.5%.
FPG values at baseline were 8.7 mmol/L in the intensified arm and 9.1 mmol/L in the placebo arm. These decreased to 8.8 and 6.1 mmol/L, respectively, with an end-of-treatment difference of –2.55 mmol/L favoring the intensified arm, a highly significant difference.
Daily insulin doses at the end of treatment were 51 units in the intensified arm and the equivalent of 105 units in the placebo arm.
Mean body weight was lower in the IDeg-supplemented arm than in the placebo arm at baseline (90.7 kg vs. 94.2 kg), and there was an average weight gain of 2 kg and weight loss of 1.3 kg in each arm, respectively, over the study period, such that the mean body weight at the end of the trial was the same, at 92.7 kg.
No cases of severe hypoglycemia were reported, and there was no significant difference in the number of confirmed nocturnal hypoglycemia cases (four events in three patients with IDeg versus two events in two patients with placebo). The rate of confirmed hypoglycemia was significantly higher in insulin-treated patients (17.3% vs 4.7%); otherwise, both regimens studied were well tolerated.
Dr. Julio Rosenstock of Dallas Diabetes & Endocrine Center, who chaired the session, said that these results were “impressive” and that the study was much better designed than was a similar study with insulin detemir (Levemir, Novo Nordisk) added to liraglutide.
“This is better because you have a control injectable,” he said. “In the previous study with detemir the A1c came down to 7.1% after 6 months, as reported by DeVries [Diabetes Care 2012;35:1446-54], and to 7.2%, as we reported, after 1 year [J. Diabetes Complications 2013;27:492-500].
“In that previous study the dose of detemir was 39 units, here it was 51 units, so the question is, are the better results because of a higher insulin dose, or because of a difference in the insulin?”
Dr. Rosenstock suggested that a head-to-head trial of insulin detemir and IDeg would be needed to determine the answer. Dr. Aroda agreed.
Dr. Aroda and Dr. Rosenstock disclosed ties with Novo Nordisk, which funded the study, and with other companies that manufacture diabetes medications and devices.
VIENNA – A hemoglobin A1c of less than 7% was achieved by 78% patients with type 2 diabetes when insulin degludec (IDeg) was added to oral antidiabetic drug therapy in a randomized, double blind, phase III study.
In comparison, 36% of patients treated with the glucagon-like peptide 1 (GLP-1) agonist liraglutide (Victoza, Novo Nordisk) on top of metformin achieved this blood glucose target, with an odds ratio favoring treatment intensification of 7.79.
Furthermore, after about 6 months of treatment, HbA1c was 0.92% lower in the insulin-added arm than in the control arm, with mean end-of-treatment values of 6.5% versus 7.5% (P < .0001), respectively.
“This treatment approach also resulted in lower fasting plasma glucose [FPG] … and overall low rates of hypoglycemia,” Dr. Vanita Aroda of MedStar Health Research Institute in Hyattsville, Md., said at the annual meeting of the European Association for the Study of Diabetes.
IDeg (Tresiba, Novo Nordisk) is an ultra-long-acting basal insulin analogue currently approved for use in Europe and some other countries around the world. A dual, once-daily, single-injection formulation of IDeg and liraglutide (Xultophy, Novo Nordisk) also is under investigation.
Results from the DUAL (Dual Action of Liraglutide and Insulin Degludec in Type 2 Diabetes) program with the fixed dose combination were reported elsewhere at the EASD annual meeting and recently reported (Lancet Diabetes Endocrinol. 2014 Sept. 2 [doi:10.1016/S2213-8587(14)70174-3]).
In the present study, Dr. Aroda and her associates aimed to confirm the efficacy and safety of IDeg given separately but in combination with liraglutide and metformin versus these oral antidiabetic drugs (OADs) plus an injected insulin placebo.
A total of 1,504 patients with diabetes of at least 6 months’ duration, being treated with metformin or other OADs but not insulin, and in need of treatment intensification were screen for possible inclusion in the trial.
After a 15-week run-in period, during which time the dose of liraglutide was titrated up to 1.5 mg, 346 patients were randomized to receive liraglutide plus metformin and either IDeg or an injectable placebo.
Ninety-two percent of the 174 patients randomized to IDeg plus liraglutide and metformin completed the 26-week trial, as did 76% of the 172 randomized to the placebo arm. Baseline characteristics of the two groups of patients were similar, with a mean age of 57 years, 9 years’ diabetes duration, and a starting HbA1c of about 7.5%.
FPG values at baseline were 8.7 mmol/L in the intensified arm and 9.1 mmol/L in the placebo arm. These decreased to 8.8 and 6.1 mmol/L, respectively, with an end-of-treatment difference of –2.55 mmol/L favoring the intensified arm, a highly significant difference.
Daily insulin doses at the end of treatment were 51 units in the intensified arm and the equivalent of 105 units in the placebo arm.
Mean body weight was lower in the IDeg-supplemented arm than in the placebo arm at baseline (90.7 kg vs. 94.2 kg), and there was an average weight gain of 2 kg and weight loss of 1.3 kg in each arm, respectively, over the study period, such that the mean body weight at the end of the trial was the same, at 92.7 kg.
No cases of severe hypoglycemia were reported, and there was no significant difference in the number of confirmed nocturnal hypoglycemia cases (four events in three patients with IDeg versus two events in two patients with placebo). The rate of confirmed hypoglycemia was significantly higher in insulin-treated patients (17.3% vs 4.7%); otherwise, both regimens studied were well tolerated.
Dr. Julio Rosenstock of Dallas Diabetes & Endocrine Center, who chaired the session, said that these results were “impressive” and that the study was much better designed than was a similar study with insulin detemir (Levemir, Novo Nordisk) added to liraglutide.
“This is better because you have a control injectable,” he said. “In the previous study with detemir the A1c came down to 7.1% after 6 months, as reported by DeVries [Diabetes Care 2012;35:1446-54], and to 7.2%, as we reported, after 1 year [J. Diabetes Complications 2013;27:492-500].
“In that previous study the dose of detemir was 39 units, here it was 51 units, so the question is, are the better results because of a higher insulin dose, or because of a difference in the insulin?”
Dr. Rosenstock suggested that a head-to-head trial of insulin detemir and IDeg would be needed to determine the answer. Dr. Aroda agreed.
Dr. Aroda and Dr. Rosenstock disclosed ties with Novo Nordisk, which funded the study, and with other companies that manufacture diabetes medications and devices.
AT EASD 2014
Key clinical point: Insulin degludec added to liraglutide plus metformin significantly improves glycemic control in type 2 diabetes patients not reaching blood glucose targets.
Major finding: At 26 weeks, end of treatment differences in HbA1c (–0.92%) and fasting plasma glucose (–2.55 mmol/L) favored treatment intensification with insulin degludec.
Data source: Phase III trial of 349 patients with type 2 diabetes on oral antidiabetic therapy.
Disclosures: Dr. Aroda and Dr. Rosenstock disclosed ties with Novo Nordisk, which funded the study, and with other companies that manufacture diabetes medications and devices.
Retinopathy screening during gestational diabetes may be lacking
VIENNA – A concerning number of women with gestational diabetes may not be getting optimal retinal care during their pregnancy.
About 11% of women in an Irish observational cohort study received just one retinal exam of the two that are recommended during pregnancy and 29% received no exam, Dr. Aoife Maria Egan said at the annual meeting of the European Association for the Study of Diabetes.
Women who attended prepregnancy care clinics were most likely to get appropriate screening. “This is probably because they had been made aware of what would be expected for them during pregnancy,” said Dr. Egan of the University Hospital Galway (Ireland).
The study was an offshoot of the ongoing Atlantic Diabetes in Pregnancy (DIP) project, which examines the outcomes of pregnancy for women with type 1 and type 2 diabetes and the factors influencing these outcomes. It was created in 2005 and provides universal screening for gestational diabetes as part of its outcomes research. The retinopathy study covered 2006-2012.
Adequate retinopathy evaluation was considered to be at least two retinal exams conducted in separate trimesters. Each exam consisted of tests of visual acuity, dilation, and opthalmologic exams and retinal images that were reviewed by an accredited retinal grader.
DIP considered four grades of retinopathy, which have been defined by the National Screening Committee in the United Kingdom: none (R0), background (R1), preproliferative (R2), and proliferative (R3). Additionally, macular edema is graded as present or absent. Progression was considered to be a change from one retinopathy grade to the next or the development of new macular edema.
The study group consisted of 341 women with gestational diabetes (68% type 1). Most of these (90%) were white. They averaged 31 years of age, with a history of two pregnancies. There were 296 live births (87%). The final analysis included 307 women who delivered after 22 weeks.
Most of the women (60%) did have an adequate evaluation. However, 11% had only one exam and 29% had no exam, Dr. Egan said.
There were a few significant differences between those who had adequate retinal exams and those who did not. Women who had two exams were more likely to have type 1 diabetes (72% vs. 61% without adequate exams) and white (94% vs. 85%). They had a longer duration of diabetics (11 vs. 9 years). More of them had attended a prepregnancy center (56% vs. 17%) and were taking folic acid (70% vs. 54%), she reported.
A multivariate analysis determined that attending the prepregnancy clinic was the strongest predictive factor, increasing the likelihood of adequate screening by more than six times.
Of those who had an adequate exam, 74% did not progress during their pregnancy and 26% did. Of those 48 who progressed, the largest portion (26) went from R0 to R1. R1 to R2 progression occurred in seven patients, and R1/R2 to R3 in six. Six patients developed a new maculopathy and three had worsening maculopathy.
Several significant differences emerged when comparing those who progressed with those who did not. The women with worsening retinopathy had a longer duration of diabetes (14 vs. 10 years) and higher systolic blood pressure at baseline (129 vs. 122 mm Hg).
They also had higher baseline hemoglobin A1c (7.7% vs. 7%) and a greater change in HbA1cduring the pregnancy (1.38% vs. 0.74%). An HbA1creduction between the first and third trimester was associated with a doubling in the risk of progression. Those who had a higher reduction of HbA1cbetween trimesters one and three were more than twice as likely to have retinopathy progression.
This highlights the dilemma clinicians face when trying to balance maternal and fetal risks of glycemic control, Dr. Egan said. Women who present with poor glycemic control should moderate that to optimize fetal health, but lowering HbA1ccan predispose the mother to retinopathic changes.
“We try and target tight control and monitor the ones we know are at risk for retinopathy progression very closely,” she said.
Whether that makes any long-term difference is still an unknown. “Some studies have shown that, while pregnancy might accelerate retinopathy, in the long run women end up even in that regard. Six or seven years down the road, everyone looks the same,” she noted.
The DIP program is funded by the Health Research Board of Ireland. Dr. Egan had no financial disclosures.
On Twitter @alz_gal
VIENNA – A concerning number of women with gestational diabetes may not be getting optimal retinal care during their pregnancy.
About 11% of women in an Irish observational cohort study received just one retinal exam of the two that are recommended during pregnancy and 29% received no exam, Dr. Aoife Maria Egan said at the annual meeting of the European Association for the Study of Diabetes.
Women who attended prepregnancy care clinics were most likely to get appropriate screening. “This is probably because they had been made aware of what would be expected for them during pregnancy,” said Dr. Egan of the University Hospital Galway (Ireland).
The study was an offshoot of the ongoing Atlantic Diabetes in Pregnancy (DIP) project, which examines the outcomes of pregnancy for women with type 1 and type 2 diabetes and the factors influencing these outcomes. It was created in 2005 and provides universal screening for gestational diabetes as part of its outcomes research. The retinopathy study covered 2006-2012.
Adequate retinopathy evaluation was considered to be at least two retinal exams conducted in separate trimesters. Each exam consisted of tests of visual acuity, dilation, and opthalmologic exams and retinal images that were reviewed by an accredited retinal grader.
DIP considered four grades of retinopathy, which have been defined by the National Screening Committee in the United Kingdom: none (R0), background (R1), preproliferative (R2), and proliferative (R3). Additionally, macular edema is graded as present or absent. Progression was considered to be a change from one retinopathy grade to the next or the development of new macular edema.
The study group consisted of 341 women with gestational diabetes (68% type 1). Most of these (90%) were white. They averaged 31 years of age, with a history of two pregnancies. There were 296 live births (87%). The final analysis included 307 women who delivered after 22 weeks.
Most of the women (60%) did have an adequate evaluation. However, 11% had only one exam and 29% had no exam, Dr. Egan said.
There were a few significant differences between those who had adequate retinal exams and those who did not. Women who had two exams were more likely to have type 1 diabetes (72% vs. 61% without adequate exams) and white (94% vs. 85%). They had a longer duration of diabetics (11 vs. 9 years). More of them had attended a prepregnancy center (56% vs. 17%) and were taking folic acid (70% vs. 54%), she reported.
A multivariate analysis determined that attending the prepregnancy clinic was the strongest predictive factor, increasing the likelihood of adequate screening by more than six times.
Of those who had an adequate exam, 74% did not progress during their pregnancy and 26% did. Of those 48 who progressed, the largest portion (26) went from R0 to R1. R1 to R2 progression occurred in seven patients, and R1/R2 to R3 in six. Six patients developed a new maculopathy and three had worsening maculopathy.
Several significant differences emerged when comparing those who progressed with those who did not. The women with worsening retinopathy had a longer duration of diabetes (14 vs. 10 years) and higher systolic blood pressure at baseline (129 vs. 122 mm Hg).
They also had higher baseline hemoglobin A1c (7.7% vs. 7%) and a greater change in HbA1cduring the pregnancy (1.38% vs. 0.74%). An HbA1creduction between the first and third trimester was associated with a doubling in the risk of progression. Those who had a higher reduction of HbA1cbetween trimesters one and three were more than twice as likely to have retinopathy progression.
This highlights the dilemma clinicians face when trying to balance maternal and fetal risks of glycemic control, Dr. Egan said. Women who present with poor glycemic control should moderate that to optimize fetal health, but lowering HbA1ccan predispose the mother to retinopathic changes.
“We try and target tight control and monitor the ones we know are at risk for retinopathy progression very closely,” she said.
Whether that makes any long-term difference is still an unknown. “Some studies have shown that, while pregnancy might accelerate retinopathy, in the long run women end up even in that regard. Six or seven years down the road, everyone looks the same,” she noted.
The DIP program is funded by the Health Research Board of Ireland. Dr. Egan had no financial disclosures.
On Twitter @alz_gal
VIENNA – A concerning number of women with gestational diabetes may not be getting optimal retinal care during their pregnancy.
About 11% of women in an Irish observational cohort study received just one retinal exam of the two that are recommended during pregnancy and 29% received no exam, Dr. Aoife Maria Egan said at the annual meeting of the European Association for the Study of Diabetes.
Women who attended prepregnancy care clinics were most likely to get appropriate screening. “This is probably because they had been made aware of what would be expected for them during pregnancy,” said Dr. Egan of the University Hospital Galway (Ireland).
The study was an offshoot of the ongoing Atlantic Diabetes in Pregnancy (DIP) project, which examines the outcomes of pregnancy for women with type 1 and type 2 diabetes and the factors influencing these outcomes. It was created in 2005 and provides universal screening for gestational diabetes as part of its outcomes research. The retinopathy study covered 2006-2012.
Adequate retinopathy evaluation was considered to be at least two retinal exams conducted in separate trimesters. Each exam consisted of tests of visual acuity, dilation, and opthalmologic exams and retinal images that were reviewed by an accredited retinal grader.
DIP considered four grades of retinopathy, which have been defined by the National Screening Committee in the United Kingdom: none (R0), background (R1), preproliferative (R2), and proliferative (R3). Additionally, macular edema is graded as present or absent. Progression was considered to be a change from one retinopathy grade to the next or the development of new macular edema.
The study group consisted of 341 women with gestational diabetes (68% type 1). Most of these (90%) were white. They averaged 31 years of age, with a history of two pregnancies. There were 296 live births (87%). The final analysis included 307 women who delivered after 22 weeks.
Most of the women (60%) did have an adequate evaluation. However, 11% had only one exam and 29% had no exam, Dr. Egan said.
There were a few significant differences between those who had adequate retinal exams and those who did not. Women who had two exams were more likely to have type 1 diabetes (72% vs. 61% without adequate exams) and white (94% vs. 85%). They had a longer duration of diabetics (11 vs. 9 years). More of them had attended a prepregnancy center (56% vs. 17%) and were taking folic acid (70% vs. 54%), she reported.
A multivariate analysis determined that attending the prepregnancy clinic was the strongest predictive factor, increasing the likelihood of adequate screening by more than six times.
Of those who had an adequate exam, 74% did not progress during their pregnancy and 26% did. Of those 48 who progressed, the largest portion (26) went from R0 to R1. R1 to R2 progression occurred in seven patients, and R1/R2 to R3 in six. Six patients developed a new maculopathy and three had worsening maculopathy.
Several significant differences emerged when comparing those who progressed with those who did not. The women with worsening retinopathy had a longer duration of diabetes (14 vs. 10 years) and higher systolic blood pressure at baseline (129 vs. 122 mm Hg).
They also had higher baseline hemoglobin A1c (7.7% vs. 7%) and a greater change in HbA1cduring the pregnancy (1.38% vs. 0.74%). An HbA1creduction between the first and third trimester was associated with a doubling in the risk of progression. Those who had a higher reduction of HbA1cbetween trimesters one and three were more than twice as likely to have retinopathy progression.
This highlights the dilemma clinicians face when trying to balance maternal and fetal risks of glycemic control, Dr. Egan said. Women who present with poor glycemic control should moderate that to optimize fetal health, but lowering HbA1ccan predispose the mother to retinopathic changes.
“We try and target tight control and monitor the ones we know are at risk for retinopathy progression very closely,” she said.
Whether that makes any long-term difference is still an unknown. “Some studies have shown that, while pregnancy might accelerate retinopathy, in the long run women end up even in that regard. Six or seven years down the road, everyone looks the same,” she noted.
The DIP program is funded by the Health Research Board of Ireland. Dr. Egan had no financial disclosures.
On Twitter @alz_gal
AT EASD 2014
Key clinical point: Women with gestational diabetes may not get adequate evaluation for retinopathy.
Major finding: About 40% of women with gestational diabetes didn’t receive adequate evaluation for retinopathy during their pregnancy.
Data source: The prospective observational cohort comprised 307 women.
Disclosures: The DIP program is funded by the Health Research Board of Ireland. Dr. Egan had no financial disclosures.
GLINT to test metformin’s effect on cardiovascular outcomes
VIENNA – Metformin will be tested for its ability to prevent cardiovascular events in the upcoming Glucose Lowering in Non-diabetic Hyperglycemia Trial (GLINT).
The researchers for the U.K.-based trial plan to recruit 11,834 men and women aged 40 years or older who are at increased risk for type 2 diabetes and have a 20% risk of developing CV disease in the next 10 years.
“Metformin is now the most widely prescribed oral antidiabetic drug in the world,” Dr. Rury Holman, director of the University of Oxford Diabetes Trials Unit (DTU) and one of the driving forces behind the new trial, said during a press briefing held at the annual meeting of the European Association for the Study of Diabetes.
This will be “the first large-scale randomized controlled outcome trial of this drug since the UKPDS [United Kingdom Prospective Diabetes Study], and is looking specifically at cardiovascular disease and cancer,” added Dr. Holman, who also is the chief investigator of the landmark UKPDS trial.
In the original UKPDS trial (Lancet 1998;352:854–65), there were reductions of 39% in heart attacks and 36% in the risk of death in patients treated with metformin versus conventional therapy, Dr. Holman observed. The effect was sustained, albeit slightly reduced, in the 10-year post-trial follow-up (N. Engl. J. Med. 2008;359:1577-89), but only 753 patients were studied in this analysis.
While there have been plenty of observational data to back up the UKPDS findings, no further randomized controlled trials have been conducted, which is why GLINT is now being conducted to resolve the uncertainty. As to why it is has taken so long to do a second study, Dr. Holman observed that metformin is off patent, and raising the funding to perform the much needed trial has taken time.
GLINT will be a pragmatic, primary prevention study conducted in multiple centers in the United Kingdom and jointly coordinated by Dr. Holman at the DTU and Dr. Nicholas Wareham at the University of Cambridge. A feasibility study is underway, recruiting an expected 500 patients. If it is successful, the study population will be expanded to almost 12,000 U.K. individuals, who will be randomly allocated to double-blind treatment with either an extended-release formulation of metformin (Glucophage, Merck Serono) or placebo. Follow-up will continue for 5 and 7 years, with the results likely to be available in 2022.
While the primary purpose is to look at metformin’s effects on cardiovascular outcome reduction versus placebo, the trial will also look at whether the drug can reduce the risk for cancer. Observational data have suggested that metformin may reduce cancer risk (Diabetes Care 2009;32:1620–5), while meta-analyses of randomized controlled trials have suggested that there is no benefit (Diabetologia 2012;55:2593–603).
Metformin has been used to treat type 2 diabetes for more than 50 years, with the first clinical trial published in 1957 leading to its approval for use in England in 1958. However, it took almost another 20 years for the drug to be licensed in Canada in 1972, with the U.S. Food and Drug Administration approving metformin for the treatment of diabetes only in 1994.
“It’s amazing that, more than five decades after it was introduced in the U.K., we remain unclear about the benefits and risks of the most widely used antidiabetic drug,” Dr. Holman said. “Hopefully, GLINT will bring much needed clarity by providing robust evidence for metformin’s effects.”
GLINT is sponsored by the University of Cambridge and funded by the National Institute for Health Research Health Technology Assessment program. Merck Serono is donating the study drug, Glucophage XR. Dr. Holman has received research funding and honoraria from Bayer, BMS, and MSD, and additional honoraria from Amgen, Elcelyx, Janssen, Novartis, and Novo Nordisk.
VIENNA – Metformin will be tested for its ability to prevent cardiovascular events in the upcoming Glucose Lowering in Non-diabetic Hyperglycemia Trial (GLINT).
The researchers for the U.K.-based trial plan to recruit 11,834 men and women aged 40 years or older who are at increased risk for type 2 diabetes and have a 20% risk of developing CV disease in the next 10 years.
“Metformin is now the most widely prescribed oral antidiabetic drug in the world,” Dr. Rury Holman, director of the University of Oxford Diabetes Trials Unit (DTU) and one of the driving forces behind the new trial, said during a press briefing held at the annual meeting of the European Association for the Study of Diabetes.
This will be “the first large-scale randomized controlled outcome trial of this drug since the UKPDS [United Kingdom Prospective Diabetes Study], and is looking specifically at cardiovascular disease and cancer,” added Dr. Holman, who also is the chief investigator of the landmark UKPDS trial.
In the original UKPDS trial (Lancet 1998;352:854–65), there were reductions of 39% in heart attacks and 36% in the risk of death in patients treated with metformin versus conventional therapy, Dr. Holman observed. The effect was sustained, albeit slightly reduced, in the 10-year post-trial follow-up (N. Engl. J. Med. 2008;359:1577-89), but only 753 patients were studied in this analysis.
While there have been plenty of observational data to back up the UKPDS findings, no further randomized controlled trials have been conducted, which is why GLINT is now being conducted to resolve the uncertainty. As to why it is has taken so long to do a second study, Dr. Holman observed that metformin is off patent, and raising the funding to perform the much needed trial has taken time.
GLINT will be a pragmatic, primary prevention study conducted in multiple centers in the United Kingdom and jointly coordinated by Dr. Holman at the DTU and Dr. Nicholas Wareham at the University of Cambridge. A feasibility study is underway, recruiting an expected 500 patients. If it is successful, the study population will be expanded to almost 12,000 U.K. individuals, who will be randomly allocated to double-blind treatment with either an extended-release formulation of metformin (Glucophage, Merck Serono) or placebo. Follow-up will continue for 5 and 7 years, with the results likely to be available in 2022.
While the primary purpose is to look at metformin’s effects on cardiovascular outcome reduction versus placebo, the trial will also look at whether the drug can reduce the risk for cancer. Observational data have suggested that metformin may reduce cancer risk (Diabetes Care 2009;32:1620–5), while meta-analyses of randomized controlled trials have suggested that there is no benefit (Diabetologia 2012;55:2593–603).
Metformin has been used to treat type 2 diabetes for more than 50 years, with the first clinical trial published in 1957 leading to its approval for use in England in 1958. However, it took almost another 20 years for the drug to be licensed in Canada in 1972, with the U.S. Food and Drug Administration approving metformin for the treatment of diabetes only in 1994.
“It’s amazing that, more than five decades after it was introduced in the U.K., we remain unclear about the benefits and risks of the most widely used antidiabetic drug,” Dr. Holman said. “Hopefully, GLINT will bring much needed clarity by providing robust evidence for metformin’s effects.”
GLINT is sponsored by the University of Cambridge and funded by the National Institute for Health Research Health Technology Assessment program. Merck Serono is donating the study drug, Glucophage XR. Dr. Holman has received research funding and honoraria from Bayer, BMS, and MSD, and additional honoraria from Amgen, Elcelyx, Janssen, Novartis, and Novo Nordisk.
VIENNA – Metformin will be tested for its ability to prevent cardiovascular events in the upcoming Glucose Lowering in Non-diabetic Hyperglycemia Trial (GLINT).
The researchers for the U.K.-based trial plan to recruit 11,834 men and women aged 40 years or older who are at increased risk for type 2 diabetes and have a 20% risk of developing CV disease in the next 10 years.
“Metformin is now the most widely prescribed oral antidiabetic drug in the world,” Dr. Rury Holman, director of the University of Oxford Diabetes Trials Unit (DTU) and one of the driving forces behind the new trial, said during a press briefing held at the annual meeting of the European Association for the Study of Diabetes.
This will be “the first large-scale randomized controlled outcome trial of this drug since the UKPDS [United Kingdom Prospective Diabetes Study], and is looking specifically at cardiovascular disease and cancer,” added Dr. Holman, who also is the chief investigator of the landmark UKPDS trial.
In the original UKPDS trial (Lancet 1998;352:854–65), there were reductions of 39% in heart attacks and 36% in the risk of death in patients treated with metformin versus conventional therapy, Dr. Holman observed. The effect was sustained, albeit slightly reduced, in the 10-year post-trial follow-up (N. Engl. J. Med. 2008;359:1577-89), but only 753 patients were studied in this analysis.
While there have been plenty of observational data to back up the UKPDS findings, no further randomized controlled trials have been conducted, which is why GLINT is now being conducted to resolve the uncertainty. As to why it is has taken so long to do a second study, Dr. Holman observed that metformin is off patent, and raising the funding to perform the much needed trial has taken time.
GLINT will be a pragmatic, primary prevention study conducted in multiple centers in the United Kingdom and jointly coordinated by Dr. Holman at the DTU and Dr. Nicholas Wareham at the University of Cambridge. A feasibility study is underway, recruiting an expected 500 patients. If it is successful, the study population will be expanded to almost 12,000 U.K. individuals, who will be randomly allocated to double-blind treatment with either an extended-release formulation of metformin (Glucophage, Merck Serono) or placebo. Follow-up will continue for 5 and 7 years, with the results likely to be available in 2022.
While the primary purpose is to look at metformin’s effects on cardiovascular outcome reduction versus placebo, the trial will also look at whether the drug can reduce the risk for cancer. Observational data have suggested that metformin may reduce cancer risk (Diabetes Care 2009;32:1620–5), while meta-analyses of randomized controlled trials have suggested that there is no benefit (Diabetologia 2012;55:2593–603).
Metformin has been used to treat type 2 diabetes for more than 50 years, with the first clinical trial published in 1957 leading to its approval for use in England in 1958. However, it took almost another 20 years for the drug to be licensed in Canada in 1972, with the U.S. Food and Drug Administration approving metformin for the treatment of diabetes only in 1994.
“It’s amazing that, more than five decades after it was introduced in the U.K., we remain unclear about the benefits and risks of the most widely used antidiabetic drug,” Dr. Holman said. “Hopefully, GLINT will bring much needed clarity by providing robust evidence for metformin’s effects.”
GLINT is sponsored by the University of Cambridge and funded by the National Institute for Health Research Health Technology Assessment program. Merck Serono is donating the study drug, Glucophage XR. Dr. Holman has received research funding and honoraria from Bayer, BMS, and MSD, and additional honoraria from Amgen, Elcelyx, Janssen, Novartis, and Novo Nordisk.
AT EASD 2014
Distinct HbA1c and blood pressure trajectories found in type 2 diabetes
VIENNA – Two separate analyses of data from the Dutch-based, longitudinal Diabetes Care System cohort show that there are four distinct, but not necessarily related, subgroups of patients with type 2 diabetes based on hemoglobin A1c and systolic blood pressure over time.
Both analyses showed that, on the whole, most patients with type 2 diabetes are well controlled, with 83% hitting a European guideline–directed HbA1c target of 7% or less and 86% achieving adequate (140 mm Hg or lower) systolic blood pressure (SBP) control, both after a mean follow-up of 5.7 years.
However, there were two distinct subgroups of patients in both analyses who did not achieve good HbA1c or SBP control, with a fourth group showing a delayed response to therapy.
“This is the start of a new analysis,” said Dr. Giel Nijpels of the VU University Medical Center in Amsterdam where the research was coordinated. “We plan to do dynamic prediction models,” he added, with the aim of “more individualized prediction of patient level of risk.”
Patients with type 2 diabetes are at increased risk for both micro- and macrovascular complications, Dr. Nijpels said at the annual meeting of the European Association for the Study of Diabetes. However, “what every doctor knows, especially primary care physicians, is that not every patient with type 2 diabetes has the same risk.”
Guidelines do not take the individual characteristics into account and recommend fixed targets for both glucose and blood pressure control. These targets are based on randomized, controlled trial data, which do not reflect “real world” clinical practice, he observed. Since type 2 diabetes is a heterogeneous disease, and clearly “one size does not fit all,” there was a need to look at the trajectories of both blood glucose and blood pressure control, to see if there are any changes over time that may help to identify patients that may need a little extra help to achieve their personalized targets.
The Diabetes Care System cohort was initiated in 1998 and is a centrally organized diabetes care system. There are currently 9,849 patients with types 2 diabetes registered in the system, who were included anytime from the start of the program until 2012. Patients undergo a physical examination at recruitment and glycemic, blood pressure, and other key parameters are recorded at this baseline. Patients are then checked annually, providing a longitudinal source of real-world data.
For the analysis of blood glucose control, which Dr. Nijpels presented, patients had to have at least two HbA1c follow-up measurements; 5,423 patients were included. For the analysis of blood pressure control, presented by his college Dr. Iris Walraven, at least two SBP follow-up measurements were needed; 5,711 patients fulfilled this criteria.
The four subgroups of patients based on glycemic control were labeled “good glycemic control,” “fast responders,” “reduced glycemic control,” and “nonresponders.” There were 83.1%, 8.2%, 5.2%, and 3.4% in each group, respectively. The good glycemic control group maintained a target HbA1c of 7% or lower throughout the follow-up period, which was up to a maximum of 9 years. As the name suggests, the 8.2% of patients in the fast responders group experienced a rapid drop in HbA1c in the first 2 years of treatment, and then maintained a target HbA1c for the duration of follow-up. The 5.2% of patients with reduced glycemic control exhibited an initial HbA1c decrease very close to target, but this subsequently rose further away from the desired target during follow-up, and the 3.4% who were “nonresponders” failed to achieved a target HbA1c throughout the course of their treatment.
Analysis showed that the fast responders tended to have significantly higher HbA1c values at baseline, compared with the reduced glycemic control or nonresponsive subgroups, with comparable HbA1c to those in the “good glycemic control” subgroup. Patients in the reduced control and nonresponder subgroups tended to be younger (under 60 years of age) and have a longer diabetes duration (more than 1 year), with a higher prevalence of microvascular complications than the good or fast control groups, Dr. Nijpels reported.
In terms of treatment, most patients were “doing well” on metformin alone, sulfonylurea monotherapy, or on both of those, with about a quarter of patients using insulin.
Four subgroups of patients with distinct SBP control over time were also identified, although they were not directly linked to the four glycemic control subgroups, said Dr. Walraven in an interview. She noted that while the majority (85.6%) of patients fell into the “adequate SBP control” group, achieving a guideline-recommend systolic blood pressure of 140 mm Hg or lower, 5.6% were “delayed responders,” 3.4% were “insufficient responders,” and 3.4% were “nonresponders.”
When the delayed responders were compared with the adequate responders, they tended to be older (66 vs. 59 years) and have higher body mass indexes (31 kg/m2 vs. 29 kg/m2). The insufficient responders were also older and had higher body weight, and were also more likely to be female than male (68% vs. 47%), compared with the adequate responders). Nonresponders were also more likely to be women, of older age, and have a longer diabetes duration (2 vs. 0.9 years).
Dr. Walraven reported that patients with insufficient SBP control had almost a twofold increased risk for cardiovascular disease. “Subgroups with inferior SBP control are important to target in order to eventually improve BP management,” she observed.
Patients’ adherence to medication was questioned in the discussions following both presentations. Although this was not directly measured, Dr. Nijpels responded that, certainly with regard to glucose-lowering agents, that he was “convinced that everyone in this cohort took their medication.”
Dr. Nijpels and Dr. Walraven had no conflicts of interest.
VIENNA – Two separate analyses of data from the Dutch-based, longitudinal Diabetes Care System cohort show that there are four distinct, but not necessarily related, subgroups of patients with type 2 diabetes based on hemoglobin A1c and systolic blood pressure over time.
Both analyses showed that, on the whole, most patients with type 2 diabetes are well controlled, with 83% hitting a European guideline–directed HbA1c target of 7% or less and 86% achieving adequate (140 mm Hg or lower) systolic blood pressure (SBP) control, both after a mean follow-up of 5.7 years.
However, there were two distinct subgroups of patients in both analyses who did not achieve good HbA1c or SBP control, with a fourth group showing a delayed response to therapy.
“This is the start of a new analysis,” said Dr. Giel Nijpels of the VU University Medical Center in Amsterdam where the research was coordinated. “We plan to do dynamic prediction models,” he added, with the aim of “more individualized prediction of patient level of risk.”
Patients with type 2 diabetes are at increased risk for both micro- and macrovascular complications, Dr. Nijpels said at the annual meeting of the European Association for the Study of Diabetes. However, “what every doctor knows, especially primary care physicians, is that not every patient with type 2 diabetes has the same risk.”
Guidelines do not take the individual characteristics into account and recommend fixed targets for both glucose and blood pressure control. These targets are based on randomized, controlled trial data, which do not reflect “real world” clinical practice, he observed. Since type 2 diabetes is a heterogeneous disease, and clearly “one size does not fit all,” there was a need to look at the trajectories of both blood glucose and blood pressure control, to see if there are any changes over time that may help to identify patients that may need a little extra help to achieve their personalized targets.
The Diabetes Care System cohort was initiated in 1998 and is a centrally organized diabetes care system. There are currently 9,849 patients with types 2 diabetes registered in the system, who were included anytime from the start of the program until 2012. Patients undergo a physical examination at recruitment and glycemic, blood pressure, and other key parameters are recorded at this baseline. Patients are then checked annually, providing a longitudinal source of real-world data.
For the analysis of blood glucose control, which Dr. Nijpels presented, patients had to have at least two HbA1c follow-up measurements; 5,423 patients were included. For the analysis of blood pressure control, presented by his college Dr. Iris Walraven, at least two SBP follow-up measurements were needed; 5,711 patients fulfilled this criteria.
The four subgroups of patients based on glycemic control were labeled “good glycemic control,” “fast responders,” “reduced glycemic control,” and “nonresponders.” There were 83.1%, 8.2%, 5.2%, and 3.4% in each group, respectively. The good glycemic control group maintained a target HbA1c of 7% or lower throughout the follow-up period, which was up to a maximum of 9 years. As the name suggests, the 8.2% of patients in the fast responders group experienced a rapid drop in HbA1c in the first 2 years of treatment, and then maintained a target HbA1c for the duration of follow-up. The 5.2% of patients with reduced glycemic control exhibited an initial HbA1c decrease very close to target, but this subsequently rose further away from the desired target during follow-up, and the 3.4% who were “nonresponders” failed to achieved a target HbA1c throughout the course of their treatment.
Analysis showed that the fast responders tended to have significantly higher HbA1c values at baseline, compared with the reduced glycemic control or nonresponsive subgroups, with comparable HbA1c to those in the “good glycemic control” subgroup. Patients in the reduced control and nonresponder subgroups tended to be younger (under 60 years of age) and have a longer diabetes duration (more than 1 year), with a higher prevalence of microvascular complications than the good or fast control groups, Dr. Nijpels reported.
In terms of treatment, most patients were “doing well” on metformin alone, sulfonylurea monotherapy, or on both of those, with about a quarter of patients using insulin.
Four subgroups of patients with distinct SBP control over time were also identified, although they were not directly linked to the four glycemic control subgroups, said Dr. Walraven in an interview. She noted that while the majority (85.6%) of patients fell into the “adequate SBP control” group, achieving a guideline-recommend systolic blood pressure of 140 mm Hg or lower, 5.6% were “delayed responders,” 3.4% were “insufficient responders,” and 3.4% were “nonresponders.”
When the delayed responders were compared with the adequate responders, they tended to be older (66 vs. 59 years) and have higher body mass indexes (31 kg/m2 vs. 29 kg/m2). The insufficient responders were also older and had higher body weight, and were also more likely to be female than male (68% vs. 47%), compared with the adequate responders). Nonresponders were also more likely to be women, of older age, and have a longer diabetes duration (2 vs. 0.9 years).
Dr. Walraven reported that patients with insufficient SBP control had almost a twofold increased risk for cardiovascular disease. “Subgroups with inferior SBP control are important to target in order to eventually improve BP management,” she observed.
Patients’ adherence to medication was questioned in the discussions following both presentations. Although this was not directly measured, Dr. Nijpels responded that, certainly with regard to glucose-lowering agents, that he was “convinced that everyone in this cohort took their medication.”
Dr. Nijpels and Dr. Walraven had no conflicts of interest.
VIENNA – Two separate analyses of data from the Dutch-based, longitudinal Diabetes Care System cohort show that there are four distinct, but not necessarily related, subgroups of patients with type 2 diabetes based on hemoglobin A1c and systolic blood pressure over time.
Both analyses showed that, on the whole, most patients with type 2 diabetes are well controlled, with 83% hitting a European guideline–directed HbA1c target of 7% or less and 86% achieving adequate (140 mm Hg or lower) systolic blood pressure (SBP) control, both after a mean follow-up of 5.7 years.
However, there were two distinct subgroups of patients in both analyses who did not achieve good HbA1c or SBP control, with a fourth group showing a delayed response to therapy.
“This is the start of a new analysis,” said Dr. Giel Nijpels of the VU University Medical Center in Amsterdam where the research was coordinated. “We plan to do dynamic prediction models,” he added, with the aim of “more individualized prediction of patient level of risk.”
Patients with type 2 diabetes are at increased risk for both micro- and macrovascular complications, Dr. Nijpels said at the annual meeting of the European Association for the Study of Diabetes. However, “what every doctor knows, especially primary care physicians, is that not every patient with type 2 diabetes has the same risk.”
Guidelines do not take the individual characteristics into account and recommend fixed targets for both glucose and blood pressure control. These targets are based on randomized, controlled trial data, which do not reflect “real world” clinical practice, he observed. Since type 2 diabetes is a heterogeneous disease, and clearly “one size does not fit all,” there was a need to look at the trajectories of both blood glucose and blood pressure control, to see if there are any changes over time that may help to identify patients that may need a little extra help to achieve their personalized targets.
The Diabetes Care System cohort was initiated in 1998 and is a centrally organized diabetes care system. There are currently 9,849 patients with types 2 diabetes registered in the system, who were included anytime from the start of the program until 2012. Patients undergo a physical examination at recruitment and glycemic, blood pressure, and other key parameters are recorded at this baseline. Patients are then checked annually, providing a longitudinal source of real-world data.
For the analysis of blood glucose control, which Dr. Nijpels presented, patients had to have at least two HbA1c follow-up measurements; 5,423 patients were included. For the analysis of blood pressure control, presented by his college Dr. Iris Walraven, at least two SBP follow-up measurements were needed; 5,711 patients fulfilled this criteria.
The four subgroups of patients based on glycemic control were labeled “good glycemic control,” “fast responders,” “reduced glycemic control,” and “nonresponders.” There were 83.1%, 8.2%, 5.2%, and 3.4% in each group, respectively. The good glycemic control group maintained a target HbA1c of 7% or lower throughout the follow-up period, which was up to a maximum of 9 years. As the name suggests, the 8.2% of patients in the fast responders group experienced a rapid drop in HbA1c in the first 2 years of treatment, and then maintained a target HbA1c for the duration of follow-up. The 5.2% of patients with reduced glycemic control exhibited an initial HbA1c decrease very close to target, but this subsequently rose further away from the desired target during follow-up, and the 3.4% who were “nonresponders” failed to achieved a target HbA1c throughout the course of their treatment.
Analysis showed that the fast responders tended to have significantly higher HbA1c values at baseline, compared with the reduced glycemic control or nonresponsive subgroups, with comparable HbA1c to those in the “good glycemic control” subgroup. Patients in the reduced control and nonresponder subgroups tended to be younger (under 60 years of age) and have a longer diabetes duration (more than 1 year), with a higher prevalence of microvascular complications than the good or fast control groups, Dr. Nijpels reported.
In terms of treatment, most patients were “doing well” on metformin alone, sulfonylurea monotherapy, or on both of those, with about a quarter of patients using insulin.
Four subgroups of patients with distinct SBP control over time were also identified, although they were not directly linked to the four glycemic control subgroups, said Dr. Walraven in an interview. She noted that while the majority (85.6%) of patients fell into the “adequate SBP control” group, achieving a guideline-recommend systolic blood pressure of 140 mm Hg or lower, 5.6% were “delayed responders,” 3.4% were “insufficient responders,” and 3.4% were “nonresponders.”
When the delayed responders were compared with the adequate responders, they tended to be older (66 vs. 59 years) and have higher body mass indexes (31 kg/m2 vs. 29 kg/m2). The insufficient responders were also older and had higher body weight, and were also more likely to be female than male (68% vs. 47%), compared with the adequate responders). Nonresponders were also more likely to be women, of older age, and have a longer diabetes duration (2 vs. 0.9 years).
Dr. Walraven reported that patients with insufficient SBP control had almost a twofold increased risk for cardiovascular disease. “Subgroups with inferior SBP control are important to target in order to eventually improve BP management,” she observed.
Patients’ adherence to medication was questioned in the discussions following both presentations. Although this was not directly measured, Dr. Nijpels responded that, certainly with regard to glucose-lowering agents, that he was “convinced that everyone in this cohort took their medication.”
Dr. Nijpels and Dr. Walraven had no conflicts of interest.
AT EASD 2014
Key clinical point: Diabetes is associated with distinct temporal changes in HbA1c and systolic blood pressure.
Major finding: Four unrelated subgroups of patients were each identified with distinct trajectories of HbA1c and SBP over time.
Data source: The Dutch-based Diabetes Care System cohort of 9,849 patients with type 2 diabetes.
Disclosures: Dr. Nijpels and Dr. Walraven had no conflicts of interest.
U.K. model predicts sight-threatening diabetic retinopathy
VIENNA – A risk model based on a single assessment of hemoglobin A1c and other parameters was able to differentiate well between patients who had a low and high risk for progression of sight-threatening diabetic retinopathy, researchers reported at the annual meeting of the European Association for the Study of Diabetes.
Although further validation is needed, the risk model “would be suitable for personalized screening intervals,” said presenting author Dr. Irene Stratton of the Gloucestershire (England) Hospitals NHS Foundation Trust. In England, the current recommendation is to screen everyone with diabetes annually using digital retinal photography.
Because of the increasing numbers of patients that require annual screening, health budgets for diabetic eye screening are being stretched and alternatives are desirable. In England, such screening considers both retinopathy and macropathology, Dr. Stratton said.
The risk model was derived and tested based on data from 14,000 patients with no or mild nonproliferative retinopathy treated in the Gloucestershire area. The investigators looked at HbA1c levels in the 12 months prior to diabetic eye screening to determine if they could predict which patients did and did not develop sight-threatening retinopathy. The risk model also considered the baseline retinopathy status in both eyes of patients; systolic and diastolic blood pressure; measures of kidney function and lipids; the time to develop retinopathy from diagnosis; and the type of diabetes.
“We found that the most important piece of information that went into the model was the grading at the baseline screening episode,” Dr. Stratton said. “So patients who had no retinopathy in either eye were at lowest risk, patients with background retinopathy in one eye had about a doubling of risk, and patients with background retinopathy in both eyes were at a much higher level of risk.”
The next most important parameters were the time since the first mention of a diagnosis of diabetes and HbA1c in the year prior to screening. Total cholesterol in the year prior to screening also was a factor for consideration.
Hazard ratios for the development of diabetic retinopathy were 7.13 for patients with mild retinopathy in both eyes at baseline and 2.56 for those with mild retinopathy in one eye. The hazard ratios increased by 1.28 for every 10-mmol/mol increase in HbA1c in the past 12 months, by 1.20 for every 5-year increase in the duration of diabetes, and by 1.12 for every 1-mmol/L increase in total serum cholesterol.
Three study populations, which altogether comprised almost 20,000 patients with diabetes, were used to validate the model; the largest population included more than 17,000 individuals. The main difference between the screening programs was the ethnic mix, Dr. Stratton highlighted. White patients dominated in the largest screening cohort, at 98%, but to a lesser extent in the other cohorts, at 47% (of 1,223 patients) and 81% (of 1,083). About half the patients in the cohorts were women, and the duration of diabetes ranged from 2.9 to 4.5 years. The majority (95%) of patients screened had type 1 diabetes. HbA1c ranged from 6.3% to 8.2% overall.
The investigators stratified patients according to quintiles of risk using the model, and found that the quintiles correlated very well with the chances of patients developing sight-threatening eye disease in each of the three validation cohorts. Comparing the lowest- with the highest-risk quintiles, the rate of progression to sight-threatening retinopathy was 1-3 and 55-79 per 1,000 per patient-years, respectively. The overall event rate was around 20 per 1,000 patient years.
“Further validation in other screening programs and ethnic groups is required,” Dr. Stratton concluded.
The study was funded by a grant from the U.K. National Institute for Health Research, Health Technology Assessment Programme; and the Gloucestershire Hospitals National Health Service Foundation Trust. Dr. Stratton had no conflicts of interest.
VIENNA – A risk model based on a single assessment of hemoglobin A1c and other parameters was able to differentiate well between patients who had a low and high risk for progression of sight-threatening diabetic retinopathy, researchers reported at the annual meeting of the European Association for the Study of Diabetes.
Although further validation is needed, the risk model “would be suitable for personalized screening intervals,” said presenting author Dr. Irene Stratton of the Gloucestershire (England) Hospitals NHS Foundation Trust. In England, the current recommendation is to screen everyone with diabetes annually using digital retinal photography.
Because of the increasing numbers of patients that require annual screening, health budgets for diabetic eye screening are being stretched and alternatives are desirable. In England, such screening considers both retinopathy and macropathology, Dr. Stratton said.
The risk model was derived and tested based on data from 14,000 patients with no or mild nonproliferative retinopathy treated in the Gloucestershire area. The investigators looked at HbA1c levels in the 12 months prior to diabetic eye screening to determine if they could predict which patients did and did not develop sight-threatening retinopathy. The risk model also considered the baseline retinopathy status in both eyes of patients; systolic and diastolic blood pressure; measures of kidney function and lipids; the time to develop retinopathy from diagnosis; and the type of diabetes.
“We found that the most important piece of information that went into the model was the grading at the baseline screening episode,” Dr. Stratton said. “So patients who had no retinopathy in either eye were at lowest risk, patients with background retinopathy in one eye had about a doubling of risk, and patients with background retinopathy in both eyes were at a much higher level of risk.”
The next most important parameters were the time since the first mention of a diagnosis of diabetes and HbA1c in the year prior to screening. Total cholesterol in the year prior to screening also was a factor for consideration.
Hazard ratios for the development of diabetic retinopathy were 7.13 for patients with mild retinopathy in both eyes at baseline and 2.56 for those with mild retinopathy in one eye. The hazard ratios increased by 1.28 for every 10-mmol/mol increase in HbA1c in the past 12 months, by 1.20 for every 5-year increase in the duration of diabetes, and by 1.12 for every 1-mmol/L increase in total serum cholesterol.
Three study populations, which altogether comprised almost 20,000 patients with diabetes, were used to validate the model; the largest population included more than 17,000 individuals. The main difference between the screening programs was the ethnic mix, Dr. Stratton highlighted. White patients dominated in the largest screening cohort, at 98%, but to a lesser extent in the other cohorts, at 47% (of 1,223 patients) and 81% (of 1,083). About half the patients in the cohorts were women, and the duration of diabetes ranged from 2.9 to 4.5 years. The majority (95%) of patients screened had type 1 diabetes. HbA1c ranged from 6.3% to 8.2% overall.
The investigators stratified patients according to quintiles of risk using the model, and found that the quintiles correlated very well with the chances of patients developing sight-threatening eye disease in each of the three validation cohorts. Comparing the lowest- with the highest-risk quintiles, the rate of progression to sight-threatening retinopathy was 1-3 and 55-79 per 1,000 per patient-years, respectively. The overall event rate was around 20 per 1,000 patient years.
“Further validation in other screening programs and ethnic groups is required,” Dr. Stratton concluded.
The study was funded by a grant from the U.K. National Institute for Health Research, Health Technology Assessment Programme; and the Gloucestershire Hospitals National Health Service Foundation Trust. Dr. Stratton had no conflicts of interest.
VIENNA – A risk model based on a single assessment of hemoglobin A1c and other parameters was able to differentiate well between patients who had a low and high risk for progression of sight-threatening diabetic retinopathy, researchers reported at the annual meeting of the European Association for the Study of Diabetes.
Although further validation is needed, the risk model “would be suitable for personalized screening intervals,” said presenting author Dr. Irene Stratton of the Gloucestershire (England) Hospitals NHS Foundation Trust. In England, the current recommendation is to screen everyone with diabetes annually using digital retinal photography.
Because of the increasing numbers of patients that require annual screening, health budgets for diabetic eye screening are being stretched and alternatives are desirable. In England, such screening considers both retinopathy and macropathology, Dr. Stratton said.
The risk model was derived and tested based on data from 14,000 patients with no or mild nonproliferative retinopathy treated in the Gloucestershire area. The investigators looked at HbA1c levels in the 12 months prior to diabetic eye screening to determine if they could predict which patients did and did not develop sight-threatening retinopathy. The risk model also considered the baseline retinopathy status in both eyes of patients; systolic and diastolic blood pressure; measures of kidney function and lipids; the time to develop retinopathy from diagnosis; and the type of diabetes.
“We found that the most important piece of information that went into the model was the grading at the baseline screening episode,” Dr. Stratton said. “So patients who had no retinopathy in either eye were at lowest risk, patients with background retinopathy in one eye had about a doubling of risk, and patients with background retinopathy in both eyes were at a much higher level of risk.”
The next most important parameters were the time since the first mention of a diagnosis of diabetes and HbA1c in the year prior to screening. Total cholesterol in the year prior to screening also was a factor for consideration.
Hazard ratios for the development of diabetic retinopathy were 7.13 for patients with mild retinopathy in both eyes at baseline and 2.56 for those with mild retinopathy in one eye. The hazard ratios increased by 1.28 for every 10-mmol/mol increase in HbA1c in the past 12 months, by 1.20 for every 5-year increase in the duration of diabetes, and by 1.12 for every 1-mmol/L increase in total serum cholesterol.
Three study populations, which altogether comprised almost 20,000 patients with diabetes, were used to validate the model; the largest population included more than 17,000 individuals. The main difference between the screening programs was the ethnic mix, Dr. Stratton highlighted. White patients dominated in the largest screening cohort, at 98%, but to a lesser extent in the other cohorts, at 47% (of 1,223 patients) and 81% (of 1,083). About half the patients in the cohorts were women, and the duration of diabetes ranged from 2.9 to 4.5 years. The majority (95%) of patients screened had type 1 diabetes. HbA1c ranged from 6.3% to 8.2% overall.
The investigators stratified patients according to quintiles of risk using the model, and found that the quintiles correlated very well with the chances of patients developing sight-threatening eye disease in each of the three validation cohorts. Comparing the lowest- with the highest-risk quintiles, the rate of progression to sight-threatening retinopathy was 1-3 and 55-79 per 1,000 per patient-years, respectively. The overall event rate was around 20 per 1,000 patient years.
“Further validation in other screening programs and ethnic groups is required,” Dr. Stratton concluded.
The study was funded by a grant from the U.K. National Institute for Health Research, Health Technology Assessment Programme; and the Gloucestershire Hospitals National Health Service Foundation Trust. Dr. Stratton had no conflicts of interest.
AT EASD 2014
Key clinical point: A diabetic retinopathy screening tool based on a single hemoglobin A1c measurement performed well in three validation cohorts.
Major finding: The risk model discriminated between patients with a very low and a very high risk of progression of sight-threatening diabetic retinopathy.
Data source: Three English screening programs with a combined population of almost 20,000 patients with diabetes.
Disclosures: The study was funded by a grant from the U.K. National Institute for Health Research, Health Technology Assessment Programme; and the Gloucestershire Hospitals National Health Service Foundation Trust. Dr. Stratton had no conflicts of interest.
Gout may predispose people, particularly women, to diabetes
Screen for diabetes and aggressively manage risk factors in patients with gout, especially women, investigators concluded from a retrospective, matched cohort study published Oct. 2 in Annals of the Rheumatic Diseases.
They found that women have a 71% greater risk of developing diabetes if they have gout (hazard ratio, 1.71; 95% confidence interval, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
The study “suggests that gout may be independently associated with an increased risk of diabetes. These findings were independent of BMI [body mass index], lifestyle factors, and other known risk factors. The magnitude of excess diabetes risk in gout was significantly larger among women than men, both in risk difference and relative risk, and these findings persisted across all age categories. These findings support aggressive management of risk factors of diabetes in patients with gout,” concluded senior investigator Dr. Hyon K. Choi and his colleagues at Boston University (Ann. Rheum. Dis. 2014 Oct. 2 [doi: 10.1136/annrheumdis-2014-205827]).
Using 15 years’ worth of data from the U.K. Health Improvement Network, which contains the records of about 7.3 million patients, the investigators matched 35,339 patients with newly diagnosed gout with up to 5 control subjects for age, sex, and BMI; they then looked to see who subsequently developed diabetes.
Among patients with gout, there were 10.1 cases of new-onset diabetes in women and 9.5 cases in men per 1,000 person-years. Among the 137,056 controls without gout, there were 5.6 cases of new-onset diabetes in women and 7.2 cases in men per 1,000 person-years.
After adjustment for smoking, alcohol consumption, physician visits, comorbidities, medication use, and BMI as a continuous variable, gout increased the risk of diabetes by 48% in women (HR, 1.48; 95% CI, 1.29-1.68; P less than .001) and by 15% in men (HR, 1.15; 95% CI, 1.06-1.24; P less than .001). The sex difference persisted across age groups.
Gout patients consumed more alcohol, visited their doctor more often, had more health problems, and took steroids and diuretics more frequently than did those who did not have gout. Overall, 72.4% of the gout cases were in men with a mean age of 62.7 years; the rest were in women, but women with gout tended to be a bit older, with a mean age of 67.9 years.
Perhaps, “low-grade inflammation among patients with gout promote[s] the diabetogenic process,” the investigators wrote. “Alternatively, the link may stem from the shared metabolic factors of the two conditions, such as the correlates of the metabolic syndrome or shared genes. Furthermore, the link between hyperuricemia and the risk of type 2 diabetes may originate at the renal level, as insulin resistance and higher insulin levels are known to reduce renal excretion of urate,” they noted.
It’s unclear why women seem to be more affected. “SUA [serum uric acid] levels in men are about 1 mg/dL higher than in women during adulthood, although levels in women increase around natural menopause. Thus, the physiological impact of uric acid levels, which are high enough to cause gout, could be stronger among women than men. Furthermore, female gout patients may have higher SUA levels on average than male gout patients, which could also contribute to a larger association with the risk of diabetes among women,” they suggested.
Dr. Choi previously linked gout to the development of diabetes, but only in men with high cardiovascular risk profiles (Rheumatology 2008;47:1567-70).
The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Screen for diabetes and aggressively manage risk factors in patients with gout, especially women, investigators concluded from a retrospective, matched cohort study published Oct. 2 in Annals of the Rheumatic Diseases.
They found that women have a 71% greater risk of developing diabetes if they have gout (hazard ratio, 1.71; 95% confidence interval, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
The study “suggests that gout may be independently associated with an increased risk of diabetes. These findings were independent of BMI [body mass index], lifestyle factors, and other known risk factors. The magnitude of excess diabetes risk in gout was significantly larger among women than men, both in risk difference and relative risk, and these findings persisted across all age categories. These findings support aggressive management of risk factors of diabetes in patients with gout,” concluded senior investigator Dr. Hyon K. Choi and his colleagues at Boston University (Ann. Rheum. Dis. 2014 Oct. 2 [doi: 10.1136/annrheumdis-2014-205827]).
Using 15 years’ worth of data from the U.K. Health Improvement Network, which contains the records of about 7.3 million patients, the investigators matched 35,339 patients with newly diagnosed gout with up to 5 control subjects for age, sex, and BMI; they then looked to see who subsequently developed diabetes.
Among patients with gout, there were 10.1 cases of new-onset diabetes in women and 9.5 cases in men per 1,000 person-years. Among the 137,056 controls without gout, there were 5.6 cases of new-onset diabetes in women and 7.2 cases in men per 1,000 person-years.
After adjustment for smoking, alcohol consumption, physician visits, comorbidities, medication use, and BMI as a continuous variable, gout increased the risk of diabetes by 48% in women (HR, 1.48; 95% CI, 1.29-1.68; P less than .001) and by 15% in men (HR, 1.15; 95% CI, 1.06-1.24; P less than .001). The sex difference persisted across age groups.
Gout patients consumed more alcohol, visited their doctor more often, had more health problems, and took steroids and diuretics more frequently than did those who did not have gout. Overall, 72.4% of the gout cases were in men with a mean age of 62.7 years; the rest were in women, but women with gout tended to be a bit older, with a mean age of 67.9 years.
Perhaps, “low-grade inflammation among patients with gout promote[s] the diabetogenic process,” the investigators wrote. “Alternatively, the link may stem from the shared metabolic factors of the two conditions, such as the correlates of the metabolic syndrome or shared genes. Furthermore, the link between hyperuricemia and the risk of type 2 diabetes may originate at the renal level, as insulin resistance and higher insulin levels are known to reduce renal excretion of urate,” they noted.
It’s unclear why women seem to be more affected. “SUA [serum uric acid] levels in men are about 1 mg/dL higher than in women during adulthood, although levels in women increase around natural menopause. Thus, the physiological impact of uric acid levels, which are high enough to cause gout, could be stronger among women than men. Furthermore, female gout patients may have higher SUA levels on average than male gout patients, which could also contribute to a larger association with the risk of diabetes among women,” they suggested.
Dr. Choi previously linked gout to the development of diabetes, but only in men with high cardiovascular risk profiles (Rheumatology 2008;47:1567-70).
The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Screen for diabetes and aggressively manage risk factors in patients with gout, especially women, investigators concluded from a retrospective, matched cohort study published Oct. 2 in Annals of the Rheumatic Diseases.
They found that women have a 71% greater risk of developing diabetes if they have gout (hazard ratio, 1.71; 95% confidence interval, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
The study “suggests that gout may be independently associated with an increased risk of diabetes. These findings were independent of BMI [body mass index], lifestyle factors, and other known risk factors. The magnitude of excess diabetes risk in gout was significantly larger among women than men, both in risk difference and relative risk, and these findings persisted across all age categories. These findings support aggressive management of risk factors of diabetes in patients with gout,” concluded senior investigator Dr. Hyon K. Choi and his colleagues at Boston University (Ann. Rheum. Dis. 2014 Oct. 2 [doi: 10.1136/annrheumdis-2014-205827]).
Using 15 years’ worth of data from the U.K. Health Improvement Network, which contains the records of about 7.3 million patients, the investigators matched 35,339 patients with newly diagnosed gout with up to 5 control subjects for age, sex, and BMI; they then looked to see who subsequently developed diabetes.
Among patients with gout, there were 10.1 cases of new-onset diabetes in women and 9.5 cases in men per 1,000 person-years. Among the 137,056 controls without gout, there were 5.6 cases of new-onset diabetes in women and 7.2 cases in men per 1,000 person-years.
After adjustment for smoking, alcohol consumption, physician visits, comorbidities, medication use, and BMI as a continuous variable, gout increased the risk of diabetes by 48% in women (HR, 1.48; 95% CI, 1.29-1.68; P less than .001) and by 15% in men (HR, 1.15; 95% CI, 1.06-1.24; P less than .001). The sex difference persisted across age groups.
Gout patients consumed more alcohol, visited their doctor more often, had more health problems, and took steroids and diuretics more frequently than did those who did not have gout. Overall, 72.4% of the gout cases were in men with a mean age of 62.7 years; the rest were in women, but women with gout tended to be a bit older, with a mean age of 67.9 years.
Perhaps, “low-grade inflammation among patients with gout promote[s] the diabetogenic process,” the investigators wrote. “Alternatively, the link may stem from the shared metabolic factors of the two conditions, such as the correlates of the metabolic syndrome or shared genes. Furthermore, the link between hyperuricemia and the risk of type 2 diabetes may originate at the renal level, as insulin resistance and higher insulin levels are known to reduce renal excretion of urate,” they noted.
It’s unclear why women seem to be more affected. “SUA [serum uric acid] levels in men are about 1 mg/dL higher than in women during adulthood, although levels in women increase around natural menopause. Thus, the physiological impact of uric acid levels, which are high enough to cause gout, could be stronger among women than men. Furthermore, female gout patients may have higher SUA levels on average than male gout patients, which could also contribute to a larger association with the risk of diabetes among women,” they suggested.
Dr. Choi previously linked gout to the development of diabetes, but only in men with high cardiovascular risk profiles (Rheumatology 2008;47:1567-70).
The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Be on the lookout for diabetes in your gout patients.
Major finding: Women have a 71% greater risk of developing diabetes if they have gout (HR, 1.71; 95% CI, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
Data source: Retrospective database study of more than 170,000 patients.
Disclosures: The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Cannula pretreatment reduces hypoglycemia in insulin pump users
VIENNA – Pretreating cannulas with a recombinant human hyaluronidase reduces the risk of hypoglycemia in patients with type 1 diabetes using insulin pumps, according to the results of a phase IV study.
In CONSISTENT-1 (Continuous Subcutaneous Insulin Infusion Study Enrolling Type 1), there were 23% fewer hypoglycemic events, defined as a blood glucose level below 56 mg/dL, and 21% fewer nocturnal hypoglycemic events in patients who received the infusion site pretreatment, compared with those who did not.
Importantly, the primary endpoint of noninferiority for glycemic control after 6 months was achieved, with comparable final mean and mean change from baseline hemoglobin A1c results.
“Current rapid-acting analogue insulin preparations are too slow to mimic the physiologic insulin meal response,” Dr. Jay Skyler, a study investigator with the University of Miami, explained at the annual meeting of the European Association for the Study of Diabetes.
He noted that hyaluronidase has been used for more than 60 years and recombinant human hyaluronidase (rHuPH20) is approved by the Food and Drug Administration as a means of improving the dispersion and absorption of injected or infused drugs. “Previously, we have demonstrated that adding recombinant human hyaluronidase [Halozyme Therapeutics’ Hylex] to rapid-acting insulin preparations accelerates the absorption and action of insulin,” Dr. Skyler said.
In this study, rHuPH20 was given in a single injection into the cannula prior to the placement of a pump for continuous subcutaneous insulin infusion (CSII), but Dr. Skyler observed that it could also be coformulated with insulin for use in pens or vials.
The aim of CONSISTENT-1 was to assess the metabolic outcomes and safety of rHuPH20 after 6 months. The trial was open label, and 456 patients with type 1 diabetes were randomized, in a 3 to 1 ratio, to pretreatment with rHuPH20 or to standard CSII without rHuPH20.
The mean age of participants was 47 years in the rHuPH20 arm and 50 years in the CSII-alone arm. Around a quarter of patients in each group used continuous glucose monitors (CGMs), and 77% had used an insulin pump for more than 5 years. Patients had been using insulin pumps for a mean of about 10 years in both groups.
The number of hypoglycemic events per month was 13.8 in the rHuPH20-pretreated group and 12.1 in the CSII-only group when a blood glucose cutoff of 70 mg/dL or lower was used, representing a nonsignificant 12% reduction. However, when the lower threshold of 56 mg/dL or lower was used, there were fewer hypoglycemia events per month (4.0 vs. 3.1; P = .02).
While the difference in the number of nocturnal hypoglycemic events per month between the rHuPH20 and CSII-only groups was also significant (2.1 vs. 1.7; P = .02), the rate of severe hypoglycemic events per 100 subject-years was only numerically lower (19 vs. 7; P = .08) in the rHuPH20 group.
Subgroup analysis showed that the timing of bolus dosing was important, with fewer blood glucose excursions and hypoglycemic events if insulin was dosed within 15 minutes before a meal than if it was dosed within 15 minutes after a meal.
Looking at CGM profiles for breakfast, lunch, and dinner, there were fewer glucose excursions in the patients who had received rHuPH20, and, stating that it was the reason for performing the study, Dr. Skyler said there were fewer excursions on subsequent days.
In terms of safety, he noted that there was an increase in injection-site reactions in the rHuPH20-pretreated group, with pain, discomfort, or paresthesia in 15.5%, compared with 6.2% in the CSII-only patients. In addition, hematoma, bruising, or hemorrhage occurred in 3.8% of the rHuPH20 group vs. 0.0% of the standard CSII group. There was no “meaningful immunogenicity,” and adverse events were otherwise well balanced between the groups.
Dr. Skyler said that a 1.5-year extension study is in progress and that the current findings “demonstrate that rHuPH20 pretreatment eliminates the systemic variability in glucose control that naturally occurs as infusion sites age.
“This should allow for improved diabetes control since insulin responses will occur more predictably.”
Halozyme supported the study. Dr. Skyler has been an adviser for Halozyme and has consulted for other companies with an interest in insulin pump therapy.
VIENNA – Pretreating cannulas with a recombinant human hyaluronidase reduces the risk of hypoglycemia in patients with type 1 diabetes using insulin pumps, according to the results of a phase IV study.
In CONSISTENT-1 (Continuous Subcutaneous Insulin Infusion Study Enrolling Type 1), there were 23% fewer hypoglycemic events, defined as a blood glucose level below 56 mg/dL, and 21% fewer nocturnal hypoglycemic events in patients who received the infusion site pretreatment, compared with those who did not.
Importantly, the primary endpoint of noninferiority for glycemic control after 6 months was achieved, with comparable final mean and mean change from baseline hemoglobin A1c results.
“Current rapid-acting analogue insulin preparations are too slow to mimic the physiologic insulin meal response,” Dr. Jay Skyler, a study investigator with the University of Miami, explained at the annual meeting of the European Association for the Study of Diabetes.
He noted that hyaluronidase has been used for more than 60 years and recombinant human hyaluronidase (rHuPH20) is approved by the Food and Drug Administration as a means of improving the dispersion and absorption of injected or infused drugs. “Previously, we have demonstrated that adding recombinant human hyaluronidase [Halozyme Therapeutics’ Hylex] to rapid-acting insulin preparations accelerates the absorption and action of insulin,” Dr. Skyler said.
In this study, rHuPH20 was given in a single injection into the cannula prior to the placement of a pump for continuous subcutaneous insulin infusion (CSII), but Dr. Skyler observed that it could also be coformulated with insulin for use in pens or vials.
The aim of CONSISTENT-1 was to assess the metabolic outcomes and safety of rHuPH20 after 6 months. The trial was open label, and 456 patients with type 1 diabetes were randomized, in a 3 to 1 ratio, to pretreatment with rHuPH20 or to standard CSII without rHuPH20.
The mean age of participants was 47 years in the rHuPH20 arm and 50 years in the CSII-alone arm. Around a quarter of patients in each group used continuous glucose monitors (CGMs), and 77% had used an insulin pump for more than 5 years. Patients had been using insulin pumps for a mean of about 10 years in both groups.
The number of hypoglycemic events per month was 13.8 in the rHuPH20-pretreated group and 12.1 in the CSII-only group when a blood glucose cutoff of 70 mg/dL or lower was used, representing a nonsignificant 12% reduction. However, when the lower threshold of 56 mg/dL or lower was used, there were fewer hypoglycemia events per month (4.0 vs. 3.1; P = .02).
While the difference in the number of nocturnal hypoglycemic events per month between the rHuPH20 and CSII-only groups was also significant (2.1 vs. 1.7; P = .02), the rate of severe hypoglycemic events per 100 subject-years was only numerically lower (19 vs. 7; P = .08) in the rHuPH20 group.
Subgroup analysis showed that the timing of bolus dosing was important, with fewer blood glucose excursions and hypoglycemic events if insulin was dosed within 15 minutes before a meal than if it was dosed within 15 minutes after a meal.
Looking at CGM profiles for breakfast, lunch, and dinner, there were fewer glucose excursions in the patients who had received rHuPH20, and, stating that it was the reason for performing the study, Dr. Skyler said there were fewer excursions on subsequent days.
In terms of safety, he noted that there was an increase in injection-site reactions in the rHuPH20-pretreated group, with pain, discomfort, or paresthesia in 15.5%, compared with 6.2% in the CSII-only patients. In addition, hematoma, bruising, or hemorrhage occurred in 3.8% of the rHuPH20 group vs. 0.0% of the standard CSII group. There was no “meaningful immunogenicity,” and adverse events were otherwise well balanced between the groups.
Dr. Skyler said that a 1.5-year extension study is in progress and that the current findings “demonstrate that rHuPH20 pretreatment eliminates the systemic variability in glucose control that naturally occurs as infusion sites age.
“This should allow for improved diabetes control since insulin responses will occur more predictably.”
Halozyme supported the study. Dr. Skyler has been an adviser for Halozyme and has consulted for other companies with an interest in insulin pump therapy.
VIENNA – Pretreating cannulas with a recombinant human hyaluronidase reduces the risk of hypoglycemia in patients with type 1 diabetes using insulin pumps, according to the results of a phase IV study.
In CONSISTENT-1 (Continuous Subcutaneous Insulin Infusion Study Enrolling Type 1), there were 23% fewer hypoglycemic events, defined as a blood glucose level below 56 mg/dL, and 21% fewer nocturnal hypoglycemic events in patients who received the infusion site pretreatment, compared with those who did not.
Importantly, the primary endpoint of noninferiority for glycemic control after 6 months was achieved, with comparable final mean and mean change from baseline hemoglobin A1c results.
“Current rapid-acting analogue insulin preparations are too slow to mimic the physiologic insulin meal response,” Dr. Jay Skyler, a study investigator with the University of Miami, explained at the annual meeting of the European Association for the Study of Diabetes.
He noted that hyaluronidase has been used for more than 60 years and recombinant human hyaluronidase (rHuPH20) is approved by the Food and Drug Administration as a means of improving the dispersion and absorption of injected or infused drugs. “Previously, we have demonstrated that adding recombinant human hyaluronidase [Halozyme Therapeutics’ Hylex] to rapid-acting insulin preparations accelerates the absorption and action of insulin,” Dr. Skyler said.
In this study, rHuPH20 was given in a single injection into the cannula prior to the placement of a pump for continuous subcutaneous insulin infusion (CSII), but Dr. Skyler observed that it could also be coformulated with insulin for use in pens or vials.
The aim of CONSISTENT-1 was to assess the metabolic outcomes and safety of rHuPH20 after 6 months. The trial was open label, and 456 patients with type 1 diabetes were randomized, in a 3 to 1 ratio, to pretreatment with rHuPH20 or to standard CSII without rHuPH20.
The mean age of participants was 47 years in the rHuPH20 arm and 50 years in the CSII-alone arm. Around a quarter of patients in each group used continuous glucose monitors (CGMs), and 77% had used an insulin pump for more than 5 years. Patients had been using insulin pumps for a mean of about 10 years in both groups.
The number of hypoglycemic events per month was 13.8 in the rHuPH20-pretreated group and 12.1 in the CSII-only group when a blood glucose cutoff of 70 mg/dL or lower was used, representing a nonsignificant 12% reduction. However, when the lower threshold of 56 mg/dL or lower was used, there were fewer hypoglycemia events per month (4.0 vs. 3.1; P = .02).
While the difference in the number of nocturnal hypoglycemic events per month between the rHuPH20 and CSII-only groups was also significant (2.1 vs. 1.7; P = .02), the rate of severe hypoglycemic events per 100 subject-years was only numerically lower (19 vs. 7; P = .08) in the rHuPH20 group.
Subgroup analysis showed that the timing of bolus dosing was important, with fewer blood glucose excursions and hypoglycemic events if insulin was dosed within 15 minutes before a meal than if it was dosed within 15 minutes after a meal.
Looking at CGM profiles for breakfast, lunch, and dinner, there were fewer glucose excursions in the patients who had received rHuPH20, and, stating that it was the reason for performing the study, Dr. Skyler said there were fewer excursions on subsequent days.
In terms of safety, he noted that there was an increase in injection-site reactions in the rHuPH20-pretreated group, with pain, discomfort, or paresthesia in 15.5%, compared with 6.2% in the CSII-only patients. In addition, hematoma, bruising, or hemorrhage occurred in 3.8% of the rHuPH20 group vs. 0.0% of the standard CSII group. There was no “meaningful immunogenicity,” and adverse events were otherwise well balanced between the groups.
Dr. Skyler said that a 1.5-year extension study is in progress and that the current findings “demonstrate that rHuPH20 pretreatment eliminates the systemic variability in glucose control that naturally occurs as infusion sites age.
“This should allow for improved diabetes control since insulin responses will occur more predictably.”
Halozyme supported the study. Dr. Skyler has been an adviser for Halozyme and has consulted for other companies with an interest in insulin pump therapy.
AT EASD 2014
Key clinical point: Pretreating CSII sites with recombinant human hyaluronidase before unit changeover provides equivalent glycemic control but reduces the likelihood of hypoglycemia.
Major finding: Compared with no pretreatment of cannulas, pretreatment with rHuHP20 yielded a relative risk of 0.77 for any hypoglycemic event (blood glucose level less than 56 mg/dL) and 0.79 for nocturnal hypoglycemic events (both P = .02).
Data source: CONSISTENT-1, a 6-month, open-label study of 456 patients with type 1 diabetes using insulin pumps.
Disclosures: Halozyme supported the study. Dr. Skyler has been an adviser for Halozyme and has consulted for other companies with an interest in insulin pump therapy.
Intensive glycemic control safely cut end-stage renal disease
VIENNA – Five years of intensive glycemic control in patients with type 2 diabetes safely halved long-term rate of end-stage renal disease, compared with placebo, in a multicenter study with about 8,000 patients, a finding that refutes prior suggestions that more intensive glycemic control can harm patients.
Intensive glucose control that produced an average hemoglobin A1c of 6.5% “is important for preventing serious renal complications and does not cause harm in patients with established type 2 diabetes,” Dr. Sophia Zoungas said at the annual meeting of the European Association for the Study of Diabetes.
Following the report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial in 2008 (N. Engl. J. Med. 2008;358;2545-59), which showed increased mortality with intensive glycemic control, compared with standard treatment, many physicians became leery of treating patients to a HbA1c level below 7%. But 5-year follow-up results of patients originally enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial (N. Engl. J. Med. 2008;358:2560-72) has now shown that a prolonged period maintaining patients at a HbA1c of roughly 6.5% produced no excess rates of total mortality, major macrovascular events, or major clinical microvascular events when compared with patients on standard care with an average HbA1c of 7.2%, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
“Whether the excess mortality seen in ACCORD was a true effect or not, there has been concern [about tight glycemic control] that has translated into where HbA1c targets have been set,” Dr. Zoungas said in an interview. “We have been blinded by this signal of increased fatal myocardial infarctions [seen in ACCORD] that many have been a chance effect.”
She suggested that the safety that intense glycemic control showed during 10-year follow-up of patients in the ADVANCE posttrial Observational Study (ADVANCE ON) may be explained by the more gradual HbA1c reductions achieved in the ADVANCE patients, compared with patients in ACCORD. In ADVANCE, patients’ HbA1c levels came down to about 6.5% over the course of a year, compared with a 3-6 month period in ACCORD, Dr. Zoungas noted.
In addition to establishing safety, the new results reported by Dr. Zoungas showed a statistically significant, 46% reduction in the incidence of end-stage renal disease (defined as progression to dialysis or need for kidney transplant) during the entire, 10-year follow-up. Simultaneous with Dr. Zoungas’s report at the meeting the ADVANCE ON results were published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407963]).
“This is the first time study results have shown that maintaining a HbA1cof less than 7% is associated with a long-term reduction in end-stage kidney disease,” she said. “End-stage kidney disease has been hard to study [as an endpoint] because the overall incidence is low, so patients need to be followed for a long time to see enough events.”
ADVANCE ON initially included 8,494 of the roughly 10,000 patients who completed the 5-year ADVANCE intervention trial at 215 centers in 20 countries. ADVANCE tested the impact of an antihypertensive intervention as well as the effect of intensified glycemic control in patients aged 55 years or older with type 2 diabetes and at least one other cardiovascular risk factor. At the time ADVANCE ended, average HbA1c levels were 7.2% in the control patients and 6.5% in those who had been on a more intensive hypoglycemic regimen. However, at the time patients began ADVANCE ON, their average HbA1c was 7.5%, regardless of which arm of ADVANCE they had been in, and the average level remained there through a median of 5 more years of follow-up. A total of 5,131 patients remained under study for the entire 10 years of follow-up. Analysis of the ADVANCE ON findings also showed a significant long-term effect from 5 years of added antihypertensive treatment, results reported in early September at the annual congress of the European Society of Cardiology.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
On Twitter @mitchelzoler
The ADVANCE ON results change our perspective on the medical treatment of patients with type 2 diabetes.
The results provide more evidence for the safety of metformin and sulfonylureas, two of the major drugs used on patients in the study during the intensive-management phase. This is not level I evidence, but it is evidence of safety. The results from ADVANCE ON as well as from other trials also suggest that these drugs reduce microvascular and macrovascular complications. The long-term legacy effect of intensive glycemic control to reduce the incidence of end-stage kidney disease was impressive. The hemoglobin A1c target established by existing management guidelines can remain in place.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Joachim Spranger |
The results also highlight our desperate need for glucose-lowering drugs that can substantially reduce fatal cardiovascular disease.
New drugs being considered for treatment of type 2 diabetes need to outperform metformin, sulfonylureas, and insulin for reducing clinically relevant endpoints. It is not enough for trials to show that new drugs can reduce glucose while causing fewer adverse effects than the older agents. Proven impact on clinical endpoints is also needed. We also must remember that the safety of treatment with three oral agents in a single regimen remains unproven.
Dr. Joachim Spranger is a professor of endocrinology at Charité Medical University in Berlin. He had no disclosures. He made these comments as the invited discussant for ADVANCE ON.
The ADVANCE ON results change our perspective on the medical treatment of patients with type 2 diabetes.
The results provide more evidence for the safety of metformin and sulfonylureas, two of the major drugs used on patients in the study during the intensive-management phase. This is not level I evidence, but it is evidence of safety. The results from ADVANCE ON as well as from other trials also suggest that these drugs reduce microvascular and macrovascular complications. The long-term legacy effect of intensive glycemic control to reduce the incidence of end-stage kidney disease was impressive. The hemoglobin A1c target established by existing management guidelines can remain in place.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Joachim Spranger |
The results also highlight our desperate need for glucose-lowering drugs that can substantially reduce fatal cardiovascular disease.
New drugs being considered for treatment of type 2 diabetes need to outperform metformin, sulfonylureas, and insulin for reducing clinically relevant endpoints. It is not enough for trials to show that new drugs can reduce glucose while causing fewer adverse effects than the older agents. Proven impact on clinical endpoints is also needed. We also must remember that the safety of treatment with three oral agents in a single regimen remains unproven.
Dr. Joachim Spranger is a professor of endocrinology at Charité Medical University in Berlin. He had no disclosures. He made these comments as the invited discussant for ADVANCE ON.
The ADVANCE ON results change our perspective on the medical treatment of patients with type 2 diabetes.
The results provide more evidence for the safety of metformin and sulfonylureas, two of the major drugs used on patients in the study during the intensive-management phase. This is not level I evidence, but it is evidence of safety. The results from ADVANCE ON as well as from other trials also suggest that these drugs reduce microvascular and macrovascular complications. The long-term legacy effect of intensive glycemic control to reduce the incidence of end-stage kidney disease was impressive. The hemoglobin A1c target established by existing management guidelines can remain in place.

|
| Mitchel L. Zoler/Frontline Medical News Dr. Joachim Spranger |
The results also highlight our desperate need for glucose-lowering drugs that can substantially reduce fatal cardiovascular disease.
New drugs being considered for treatment of type 2 diabetes need to outperform metformin, sulfonylureas, and insulin for reducing clinically relevant endpoints. It is not enough for trials to show that new drugs can reduce glucose while causing fewer adverse effects than the older agents. Proven impact on clinical endpoints is also needed. We also must remember that the safety of treatment with three oral agents in a single regimen remains unproven.
Dr. Joachim Spranger is a professor of endocrinology at Charité Medical University in Berlin. He had no disclosures. He made these comments as the invited discussant for ADVANCE ON.
VIENNA – Five years of intensive glycemic control in patients with type 2 diabetes safely halved long-term rate of end-stage renal disease, compared with placebo, in a multicenter study with about 8,000 patients, a finding that refutes prior suggestions that more intensive glycemic control can harm patients.
Intensive glucose control that produced an average hemoglobin A1c of 6.5% “is important for preventing serious renal complications and does not cause harm in patients with established type 2 diabetes,” Dr. Sophia Zoungas said at the annual meeting of the European Association for the Study of Diabetes.
Following the report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial in 2008 (N. Engl. J. Med. 2008;358;2545-59), which showed increased mortality with intensive glycemic control, compared with standard treatment, many physicians became leery of treating patients to a HbA1c level below 7%. But 5-year follow-up results of patients originally enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial (N. Engl. J. Med. 2008;358:2560-72) has now shown that a prolonged period maintaining patients at a HbA1c of roughly 6.5% produced no excess rates of total mortality, major macrovascular events, or major clinical microvascular events when compared with patients on standard care with an average HbA1c of 7.2%, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
“Whether the excess mortality seen in ACCORD was a true effect or not, there has been concern [about tight glycemic control] that has translated into where HbA1c targets have been set,” Dr. Zoungas said in an interview. “We have been blinded by this signal of increased fatal myocardial infarctions [seen in ACCORD] that many have been a chance effect.”
She suggested that the safety that intense glycemic control showed during 10-year follow-up of patients in the ADVANCE posttrial Observational Study (ADVANCE ON) may be explained by the more gradual HbA1c reductions achieved in the ADVANCE patients, compared with patients in ACCORD. In ADVANCE, patients’ HbA1c levels came down to about 6.5% over the course of a year, compared with a 3-6 month period in ACCORD, Dr. Zoungas noted.
In addition to establishing safety, the new results reported by Dr. Zoungas showed a statistically significant, 46% reduction in the incidence of end-stage renal disease (defined as progression to dialysis or need for kidney transplant) during the entire, 10-year follow-up. Simultaneous with Dr. Zoungas’s report at the meeting the ADVANCE ON results were published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407963]).
“This is the first time study results have shown that maintaining a HbA1cof less than 7% is associated with a long-term reduction in end-stage kidney disease,” she said. “End-stage kidney disease has been hard to study [as an endpoint] because the overall incidence is low, so patients need to be followed for a long time to see enough events.”
ADVANCE ON initially included 8,494 of the roughly 10,000 patients who completed the 5-year ADVANCE intervention trial at 215 centers in 20 countries. ADVANCE tested the impact of an antihypertensive intervention as well as the effect of intensified glycemic control in patients aged 55 years or older with type 2 diabetes and at least one other cardiovascular risk factor. At the time ADVANCE ended, average HbA1c levels were 7.2% in the control patients and 6.5% in those who had been on a more intensive hypoglycemic regimen. However, at the time patients began ADVANCE ON, their average HbA1c was 7.5%, regardless of which arm of ADVANCE they had been in, and the average level remained there through a median of 5 more years of follow-up. A total of 5,131 patients remained under study for the entire 10 years of follow-up. Analysis of the ADVANCE ON findings also showed a significant long-term effect from 5 years of added antihypertensive treatment, results reported in early September at the annual congress of the European Society of Cardiology.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
On Twitter @mitchelzoler
VIENNA – Five years of intensive glycemic control in patients with type 2 diabetes safely halved long-term rate of end-stage renal disease, compared with placebo, in a multicenter study with about 8,000 patients, a finding that refutes prior suggestions that more intensive glycemic control can harm patients.
Intensive glucose control that produced an average hemoglobin A1c of 6.5% “is important for preventing serious renal complications and does not cause harm in patients with established type 2 diabetes,” Dr. Sophia Zoungas said at the annual meeting of the European Association for the Study of Diabetes.
Following the report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial in 2008 (N. Engl. J. Med. 2008;358;2545-59), which showed increased mortality with intensive glycemic control, compared with standard treatment, many physicians became leery of treating patients to a HbA1c level below 7%. But 5-year follow-up results of patients originally enrolled in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial (N. Engl. J. Med. 2008;358:2560-72) has now shown that a prolonged period maintaining patients at a HbA1c of roughly 6.5% produced no excess rates of total mortality, major macrovascular events, or major clinical microvascular events when compared with patients on standard care with an average HbA1c of 7.2%, said Dr. Zoungas, an endocrinologist with the George Institute of the University of Sydney.
“Whether the excess mortality seen in ACCORD was a true effect or not, there has been concern [about tight glycemic control] that has translated into where HbA1c targets have been set,” Dr. Zoungas said in an interview. “We have been blinded by this signal of increased fatal myocardial infarctions [seen in ACCORD] that many have been a chance effect.”
She suggested that the safety that intense glycemic control showed during 10-year follow-up of patients in the ADVANCE posttrial Observational Study (ADVANCE ON) may be explained by the more gradual HbA1c reductions achieved in the ADVANCE patients, compared with patients in ACCORD. In ADVANCE, patients’ HbA1c levels came down to about 6.5% over the course of a year, compared with a 3-6 month period in ACCORD, Dr. Zoungas noted.
In addition to establishing safety, the new results reported by Dr. Zoungas showed a statistically significant, 46% reduction in the incidence of end-stage renal disease (defined as progression to dialysis or need for kidney transplant) during the entire, 10-year follow-up. Simultaneous with Dr. Zoungas’s report at the meeting the ADVANCE ON results were published online (N. Engl. J. Med. 2014 [doi:10.1056/NEJMoa1407963]).
“This is the first time study results have shown that maintaining a HbA1cof less than 7% is associated with a long-term reduction in end-stage kidney disease,” she said. “End-stage kidney disease has been hard to study [as an endpoint] because the overall incidence is low, so patients need to be followed for a long time to see enough events.”
ADVANCE ON initially included 8,494 of the roughly 10,000 patients who completed the 5-year ADVANCE intervention trial at 215 centers in 20 countries. ADVANCE tested the impact of an antihypertensive intervention as well as the effect of intensified glycemic control in patients aged 55 years or older with type 2 diabetes and at least one other cardiovascular risk factor. At the time ADVANCE ended, average HbA1c levels were 7.2% in the control patients and 6.5% in those who had been on a more intensive hypoglycemic regimen. However, at the time patients began ADVANCE ON, their average HbA1c was 7.5%, regardless of which arm of ADVANCE they had been in, and the average level remained there through a median of 5 more years of follow-up. A total of 5,131 patients remained under study for the entire 10 years of follow-up. Analysis of the ADVANCE ON findings also showed a significant long-term effect from 5 years of added antihypertensive treatment, results reported in early September at the annual congress of the European Society of Cardiology.
ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
On Twitter @mitchelzoler
AT EASD 2014
Key clinical point: Intensive glycemic control in patients with type 2 diabetes safely led to a long-term reduction in end-stage kidney disease, compared with standard management.
Major finding: Intensive glycemic-control patients had a 46% reduced rate of end-stage kidney disease, compared with standard-care patients.
Data source: The ADVANCE ON study, which followed 8,494 patients after their participation in an international glycemia-control trial.
Disclosures: ADVANCE ON received partial funding from Servier. Dr. Zoungas has received honoraria from Servier as well as from Merck, Bristol-Myers Squibb/AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Amgen.
Link between diabetes and antidepressants ‘not causal’
VIENNA – Long-term use of antidepressant medication does not appear to cause diabetes, according to findings from a longitudinal study.
The team, from the Institut National de la Santé et de la Recherche Médicale (INSERM) in Villejuif, France, found no association between fasting plasma glucose (FPG) or hemoglobin A1c levels measured over a 9-year study period and the use of antidepressants.
“Several studies have shown an association between antidepressant use and the risk of type 2 diabetes,” reported Marine Azevedo Da Silva at the annual meeting of the European Association for the Study of Diabetes.
The reason for this remains unclear, so the hypothesis for the study was that “if antidepressant use is causally associated with type 2 diabetes, then it should also be associated with glucose dysregulation,” said Ms. Azevedo Da Silva, a doctoral student at the Versailles (France) Saint-Quentin-en-Yvelines University.
While other research teams have examined this hypothesis, the results have been inconsistent, Ms. Azevedo Da Silva added. To solve some of the methodological issues of the prior studies, she and her colleagues used data from an epidemiological study on the insulin resistance syndrome (DESIR). This is a longitudinal cohort of 5,212 men and women without diabetes who were aged 30-60 years at recruitment.
Health assessments, including antidepressant use, FPG, and hemoglobin A1c, were performed at four time points: at recruitment in 1994 to 1996, and then at 3-year intervals between 1997-1999, 2000-2002, and 2003-2005. Data on 4,869 participants who did not have diabetes at baseline were available for analysis. Diabetes was defined as an FPG of less than 7 mmol/L (126 mg/dL) and no use of glucose-lowering medication. The majority (96%) of participants were not using antidepressants at baseline.
Baseline mean FPG and hemoglobin A1c levels were similar among antidepressant and non–antidepressant users, at 5.22 mmol/L (96 mg/dL) and 5.22%, versus 5.32 mmol/L (96 mg/dL) and 5.35%, respectively. Among the 4% of subjects who used antidepressants, there was no difference in these blood glucose measures according to the type of antidepressant used.
There were similar increases in both FPG and hemoglobin A1c in the two groups over time, at 0.02 mmol/L (0.36 mg/dL) per year for FPG in both antidepressant users and nonusers, and 0.01% per year for hemoglobin A1c.
Results had been adjusted for multiple confounding factors, including gender, age, marital status, education, and employment status; and several other health factors, such as alcohol use, smoking, body mass index, family diabetes history, hypertension, and other medication use.
Antidepressant use was self-reported, noted Ms. Azevedo Da Silva, which was one of the main limitations of the study. There was also a lack of information on the actual antidepressants used and the doses taken. In addition, there is the possibility of “confounding depression,” she acknowledged.
Nevertheless, this is a large observational, longitudinal cohort with an extended period of follow-up. FPG and hemoglobin A1c were robust clinical measures, and the team was able to study a large number of participants.
“Our results suggest that the association highlighted in recent studies between antidepressant use and type 2 diabetes may not be causal,” Ms. Azevedo Da Silva concluded.
This study is the fourth to find no association between antidepressant use and glucose dysregulation (Biol. Psych. 2011;70:978-84; Ann. Med. 2012;44:279-88; Psychopharmacology 2013;227:467-77), with only two other studies suggesting that there might be a link (Int. J. Clin. Pharm. 2011;33:484-92; PloS One 2011;6:e21551).
There is now a need to look at other reasons that might be responsible for the purported association, such as insulin resistance, she said.
The Ministry of French Research supported the study. Ms. Azevedo Da Silva had no conflicts of interest.
VIENNA – Long-term use of antidepressant medication does not appear to cause diabetes, according to findings from a longitudinal study.
The team, from the Institut National de la Santé et de la Recherche Médicale (INSERM) in Villejuif, France, found no association between fasting plasma glucose (FPG) or hemoglobin A1c levels measured over a 9-year study period and the use of antidepressants.
“Several studies have shown an association between antidepressant use and the risk of type 2 diabetes,” reported Marine Azevedo Da Silva at the annual meeting of the European Association for the Study of Diabetes.
The reason for this remains unclear, so the hypothesis for the study was that “if antidepressant use is causally associated with type 2 diabetes, then it should also be associated with glucose dysregulation,” said Ms. Azevedo Da Silva, a doctoral student at the Versailles (France) Saint-Quentin-en-Yvelines University.
While other research teams have examined this hypothesis, the results have been inconsistent, Ms. Azevedo Da Silva added. To solve some of the methodological issues of the prior studies, she and her colleagues used data from an epidemiological study on the insulin resistance syndrome (DESIR). This is a longitudinal cohort of 5,212 men and women without diabetes who were aged 30-60 years at recruitment.
Health assessments, including antidepressant use, FPG, and hemoglobin A1c, were performed at four time points: at recruitment in 1994 to 1996, and then at 3-year intervals between 1997-1999, 2000-2002, and 2003-2005. Data on 4,869 participants who did not have diabetes at baseline were available for analysis. Diabetes was defined as an FPG of less than 7 mmol/L (126 mg/dL) and no use of glucose-lowering medication. The majority (96%) of participants were not using antidepressants at baseline.
Baseline mean FPG and hemoglobin A1c levels were similar among antidepressant and non–antidepressant users, at 5.22 mmol/L (96 mg/dL) and 5.22%, versus 5.32 mmol/L (96 mg/dL) and 5.35%, respectively. Among the 4% of subjects who used antidepressants, there was no difference in these blood glucose measures according to the type of antidepressant used.
There were similar increases in both FPG and hemoglobin A1c in the two groups over time, at 0.02 mmol/L (0.36 mg/dL) per year for FPG in both antidepressant users and nonusers, and 0.01% per year for hemoglobin A1c.
Results had been adjusted for multiple confounding factors, including gender, age, marital status, education, and employment status; and several other health factors, such as alcohol use, smoking, body mass index, family diabetes history, hypertension, and other medication use.
Antidepressant use was self-reported, noted Ms. Azevedo Da Silva, which was one of the main limitations of the study. There was also a lack of information on the actual antidepressants used and the doses taken. In addition, there is the possibility of “confounding depression,” she acknowledged.
Nevertheless, this is a large observational, longitudinal cohort with an extended period of follow-up. FPG and hemoglobin A1c were robust clinical measures, and the team was able to study a large number of participants.
“Our results suggest that the association highlighted in recent studies between antidepressant use and type 2 diabetes may not be causal,” Ms. Azevedo Da Silva concluded.
This study is the fourth to find no association between antidepressant use and glucose dysregulation (Biol. Psych. 2011;70:978-84; Ann. Med. 2012;44:279-88; Psychopharmacology 2013;227:467-77), with only two other studies suggesting that there might be a link (Int. J. Clin. Pharm. 2011;33:484-92; PloS One 2011;6:e21551).
There is now a need to look at other reasons that might be responsible for the purported association, such as insulin resistance, she said.
The Ministry of French Research supported the study. Ms. Azevedo Da Silva had no conflicts of interest.
VIENNA – Long-term use of antidepressant medication does not appear to cause diabetes, according to findings from a longitudinal study.
The team, from the Institut National de la Santé et de la Recherche Médicale (INSERM) in Villejuif, France, found no association between fasting plasma glucose (FPG) or hemoglobin A1c levels measured over a 9-year study period and the use of antidepressants.
“Several studies have shown an association between antidepressant use and the risk of type 2 diabetes,” reported Marine Azevedo Da Silva at the annual meeting of the European Association for the Study of Diabetes.
The reason for this remains unclear, so the hypothesis for the study was that “if antidepressant use is causally associated with type 2 diabetes, then it should also be associated with glucose dysregulation,” said Ms. Azevedo Da Silva, a doctoral student at the Versailles (France) Saint-Quentin-en-Yvelines University.
While other research teams have examined this hypothesis, the results have been inconsistent, Ms. Azevedo Da Silva added. To solve some of the methodological issues of the prior studies, she and her colleagues used data from an epidemiological study on the insulin resistance syndrome (DESIR). This is a longitudinal cohort of 5,212 men and women without diabetes who were aged 30-60 years at recruitment.
Health assessments, including antidepressant use, FPG, and hemoglobin A1c, were performed at four time points: at recruitment in 1994 to 1996, and then at 3-year intervals between 1997-1999, 2000-2002, and 2003-2005. Data on 4,869 participants who did not have diabetes at baseline were available for analysis. Diabetes was defined as an FPG of less than 7 mmol/L (126 mg/dL) and no use of glucose-lowering medication. The majority (96%) of participants were not using antidepressants at baseline.
Baseline mean FPG and hemoglobin A1c levels were similar among antidepressant and non–antidepressant users, at 5.22 mmol/L (96 mg/dL) and 5.22%, versus 5.32 mmol/L (96 mg/dL) and 5.35%, respectively. Among the 4% of subjects who used antidepressants, there was no difference in these blood glucose measures according to the type of antidepressant used.
There were similar increases in both FPG and hemoglobin A1c in the two groups over time, at 0.02 mmol/L (0.36 mg/dL) per year for FPG in both antidepressant users and nonusers, and 0.01% per year for hemoglobin A1c.
Results had been adjusted for multiple confounding factors, including gender, age, marital status, education, and employment status; and several other health factors, such as alcohol use, smoking, body mass index, family diabetes history, hypertension, and other medication use.
Antidepressant use was self-reported, noted Ms. Azevedo Da Silva, which was one of the main limitations of the study. There was also a lack of information on the actual antidepressants used and the doses taken. In addition, there is the possibility of “confounding depression,” she acknowledged.
Nevertheless, this is a large observational, longitudinal cohort with an extended period of follow-up. FPG and hemoglobin A1c were robust clinical measures, and the team was able to study a large number of participants.
“Our results suggest that the association highlighted in recent studies between antidepressant use and type 2 diabetes may not be causal,” Ms. Azevedo Da Silva concluded.
This study is the fourth to find no association between antidepressant use and glucose dysregulation (Biol. Psych. 2011;70:978-84; Ann. Med. 2012;44:279-88; Psychopharmacology 2013;227:467-77), with only two other studies suggesting that there might be a link (Int. J. Clin. Pharm. 2011;33:484-92; PloS One 2011;6:e21551).
There is now a need to look at other reasons that might be responsible for the purported association, such as insulin resistance, she said.
The Ministry of French Research supported the study. Ms. Azevedo Da Silva had no conflicts of interest.
AT EASD 2014
Key clinical point: Antidepressants do not alter glucose levels in the long term.
Major finding: No differences were seen in mean fasting plasma glucose or glycated hemoglobin over 9 years of follow up.
Data source: More than 4,000 individuals without diabetes at baseline enrolled in the French DESIR longitudinal cohort.
Disclosures: The Ministry of French Research supported the study. Ms. Azevedo Da Silva had no conflicts of interest.
Saxagliptin reverses proteinuria in type 2 diabetes
VIENNA – Patients with type 2 diabetes treated with the oral hypoglycemic saxagliptin (Onglyza) benefited from stabilization and, in some cases, reversal of urinary protein levels in a prespecified, secondary analysis of data collected from more than 16,000 patients in a randomized, international trial.
Progression of patients with diabetes from normoalbuminuria to microalbuminuria and then proteinuria tracks along with their deteriorating renal function. Until now, no agent has been identified that could stop or reverse this process, although a similar effect had been documented in results from a few prior, much smaller studies using other drugs from the class of dipeptidyl peptidase-4 (DPP-4) inhibitors, which includes saxagliptin.
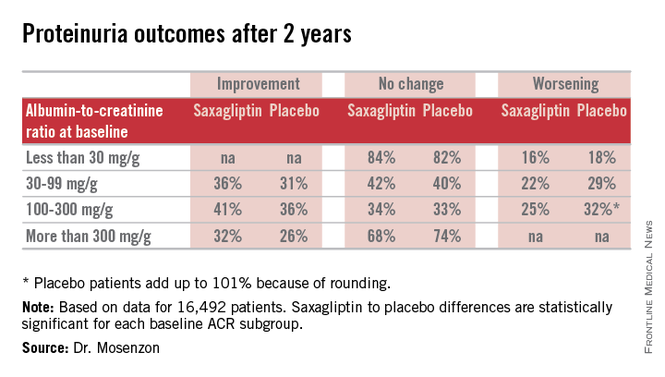
“It’s a very important finding that needs further investigation in longer-term studies so we can see whether the improvement in the [albumin to creatinine ratio (ACR)] will also mean less deterioration of the eGFR [estimated glomerular filtration rate] and thereby prevent end-stage renal disease,” Dr. Ofri Mosenzon said following her report at the annual meeting of the European Association for the Study of Diabetes.
The new analysis also showed that saxagliptin did this safely, without causing any renal damage, and that the effect on microalbuminuria and proteinuria was completely independent of the drug’s glycemic control effect measured by hemoglobin A1c levels. Dr. Mosenzon had no simple explanation for the mechanism by which saxagliptin and other DPP-4 inhibitors might exert this effect, though she suggested that an anti-inflammatory pleiotropic effect of the drug class might be involved.
“We have this clinical result. Now we need to look for an explanation,” said Dr. Mosenzon, a diabetologist at Hadassah Hospital in Jerusalem.
Her analysis used data collected in the the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) – Thrombolysis in Myocardial Infarction (TIMI) 53 trial, which had the primary purpose of assessing the cardiovascular safety of saxagliptin in 16,492 patients with type 2 diabetes and a high risk for cardiovascular events (N. Engl. J. Med. 2013;369:1317-26). The study randomized patients to treatment with either 5 mg saxagliptin daily (2.5 mg daily in patients with impaired renal function at entry) or placebo in addition to whatever other standard medications their physicians prescribed. The secondary analysis of the impact of saxagliptin on proteinuria and renal function used prespecified definitions for evaluating the renal safety and renal-protective efficacy of treatment.
On the safety side, treatment with saxagliptin, compared with placebo linked with no statistically significant differences in the rate or magnitude of serum creatinine increases or in the rates of progression to dialysis or renal transplant.
For efficacy, treatment with saxagliptin, compared with placebo led to a consistent pattern of stabilization and reversal of the severity of microalbuminuria and proteinuria that cut across patients at all baseline levels of urinary protein (see table). For example, among 1,638 categorized as having proteinuria at entry into the study, based on an ACR of more than 300 mg/g, 32% of the patients treated with saxagliptin had improved to daily urinary protein excretions of less than 300 mg/g after an average 2.1 years on treatment, compared with a 26% rate in the placebo-treated control patients, a statistically significant difference. The extent of reversals and progressions prevented were also statistically significant among the 9,696 patients who entered the study with normoalbuminuria and urinary protein levels of less than 30 mg/g and those who entered with microalbuminuria and daily urinary protein levels of 30-300 mg/g.
Expressed another way, when compared with placebo-treated patients those patients treated with saxagliptin for 2 years who entered the study with an eGFR of more than 50 mL/min/1.73 m2 had on average 19 mg less daily protein in their urine, those who entered with an eGFR of 30-50 mL/min/1.73 m2 averaged 105 mg less daily urine protein, and those who entered with an eGFR of less than 30 mL/min/1.73 m2 averaged 245 mg less daily urine protein.
SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.
VIENNA – Patients with type 2 diabetes treated with the oral hypoglycemic saxagliptin (Onglyza) benefited from stabilization and, in some cases, reversal of urinary protein levels in a prespecified, secondary analysis of data collected from more than 16,000 patients in a randomized, international trial.
Progression of patients with diabetes from normoalbuminuria to microalbuminuria and then proteinuria tracks along with their deteriorating renal function. Until now, no agent has been identified that could stop or reverse this process, although a similar effect had been documented in results from a few prior, much smaller studies using other drugs from the class of dipeptidyl peptidase-4 (DPP-4) inhibitors, which includes saxagliptin.

“It’s a very important finding that needs further investigation in longer-term studies so we can see whether the improvement in the [albumin to creatinine ratio (ACR)] will also mean less deterioration of the eGFR [estimated glomerular filtration rate] and thereby prevent end-stage renal disease,” Dr. Ofri Mosenzon said following her report at the annual meeting of the European Association for the Study of Diabetes.
The new analysis also showed that saxagliptin did this safely, without causing any renal damage, and that the effect on microalbuminuria and proteinuria was completely independent of the drug’s glycemic control effect measured by hemoglobin A1c levels. Dr. Mosenzon had no simple explanation for the mechanism by which saxagliptin and other DPP-4 inhibitors might exert this effect, though she suggested that an anti-inflammatory pleiotropic effect of the drug class might be involved.
“We have this clinical result. Now we need to look for an explanation,” said Dr. Mosenzon, a diabetologist at Hadassah Hospital in Jerusalem.
Her analysis used data collected in the the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) – Thrombolysis in Myocardial Infarction (TIMI) 53 trial, which had the primary purpose of assessing the cardiovascular safety of saxagliptin in 16,492 patients with type 2 diabetes and a high risk for cardiovascular events (N. Engl. J. Med. 2013;369:1317-26). The study randomized patients to treatment with either 5 mg saxagliptin daily (2.5 mg daily in patients with impaired renal function at entry) or placebo in addition to whatever other standard medications their physicians prescribed. The secondary analysis of the impact of saxagliptin on proteinuria and renal function used prespecified definitions for evaluating the renal safety and renal-protective efficacy of treatment.
On the safety side, treatment with saxagliptin, compared with placebo linked with no statistically significant differences in the rate or magnitude of serum creatinine increases or in the rates of progression to dialysis or renal transplant.
For efficacy, treatment with saxagliptin, compared with placebo led to a consistent pattern of stabilization and reversal of the severity of microalbuminuria and proteinuria that cut across patients at all baseline levels of urinary protein (see table). For example, among 1,638 categorized as having proteinuria at entry into the study, based on an ACR of more than 300 mg/g, 32% of the patients treated with saxagliptin had improved to daily urinary protein excretions of less than 300 mg/g after an average 2.1 years on treatment, compared with a 26% rate in the placebo-treated control patients, a statistically significant difference. The extent of reversals and progressions prevented were also statistically significant among the 9,696 patients who entered the study with normoalbuminuria and urinary protein levels of less than 30 mg/g and those who entered with microalbuminuria and daily urinary protein levels of 30-300 mg/g.
Expressed another way, when compared with placebo-treated patients those patients treated with saxagliptin for 2 years who entered the study with an eGFR of more than 50 mL/min/1.73 m2 had on average 19 mg less daily protein in their urine, those who entered with an eGFR of 30-50 mL/min/1.73 m2 averaged 105 mg less daily urine protein, and those who entered with an eGFR of less than 30 mL/min/1.73 m2 averaged 245 mg less daily urine protein.
SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.
VIENNA – Patients with type 2 diabetes treated with the oral hypoglycemic saxagliptin (Onglyza) benefited from stabilization and, in some cases, reversal of urinary protein levels in a prespecified, secondary analysis of data collected from more than 16,000 patients in a randomized, international trial.
Progression of patients with diabetes from normoalbuminuria to microalbuminuria and then proteinuria tracks along with their deteriorating renal function. Until now, no agent has been identified that could stop or reverse this process, although a similar effect had been documented in results from a few prior, much smaller studies using other drugs from the class of dipeptidyl peptidase-4 (DPP-4) inhibitors, which includes saxagliptin.

“It’s a very important finding that needs further investigation in longer-term studies so we can see whether the improvement in the [albumin to creatinine ratio (ACR)] will also mean less deterioration of the eGFR [estimated glomerular filtration rate] and thereby prevent end-stage renal disease,” Dr. Ofri Mosenzon said following her report at the annual meeting of the European Association for the Study of Diabetes.
The new analysis also showed that saxagliptin did this safely, without causing any renal damage, and that the effect on microalbuminuria and proteinuria was completely independent of the drug’s glycemic control effect measured by hemoglobin A1c levels. Dr. Mosenzon had no simple explanation for the mechanism by which saxagliptin and other DPP-4 inhibitors might exert this effect, though she suggested that an anti-inflammatory pleiotropic effect of the drug class might be involved.
“We have this clinical result. Now we need to look for an explanation,” said Dr. Mosenzon, a diabetologist at Hadassah Hospital in Jerusalem.
Her analysis used data collected in the the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) – Thrombolysis in Myocardial Infarction (TIMI) 53 trial, which had the primary purpose of assessing the cardiovascular safety of saxagliptin in 16,492 patients with type 2 diabetes and a high risk for cardiovascular events (N. Engl. J. Med. 2013;369:1317-26). The study randomized patients to treatment with either 5 mg saxagliptin daily (2.5 mg daily in patients with impaired renal function at entry) or placebo in addition to whatever other standard medications their physicians prescribed. The secondary analysis of the impact of saxagliptin on proteinuria and renal function used prespecified definitions for evaluating the renal safety and renal-protective efficacy of treatment.
On the safety side, treatment with saxagliptin, compared with placebo linked with no statistically significant differences in the rate or magnitude of serum creatinine increases or in the rates of progression to dialysis or renal transplant.
For efficacy, treatment with saxagliptin, compared with placebo led to a consistent pattern of stabilization and reversal of the severity of microalbuminuria and proteinuria that cut across patients at all baseline levels of urinary protein (see table). For example, among 1,638 categorized as having proteinuria at entry into the study, based on an ACR of more than 300 mg/g, 32% of the patients treated with saxagliptin had improved to daily urinary protein excretions of less than 300 mg/g after an average 2.1 years on treatment, compared with a 26% rate in the placebo-treated control patients, a statistically significant difference. The extent of reversals and progressions prevented were also statistically significant among the 9,696 patients who entered the study with normoalbuminuria and urinary protein levels of less than 30 mg/g and those who entered with microalbuminuria and daily urinary protein levels of 30-300 mg/g.
Expressed another way, when compared with placebo-treated patients those patients treated with saxagliptin for 2 years who entered the study with an eGFR of more than 50 mL/min/1.73 m2 had on average 19 mg less daily protein in their urine, those who entered with an eGFR of 30-50 mL/min/1.73 m2 averaged 105 mg less daily urine protein, and those who entered with an eGFR of less than 30 mL/min/1.73 m2 averaged 245 mg less daily urine protein.
SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.
AT EASD 2014
Key clinical point: Treatment with saxagliptin led to stabilization and in some patients reversal of microalbuminuria and proteinuria in patients with type 2 diabetes.
Major finding: Proteinuria reversed in 32% of patients treated with saxagliptin during 2.1 years, compared with a 26% rate in controls.
Data source: Prespecified, secondary analysis of data from SAVOR-TIMI 53, an international, randomized trial with 16,492 patients with type 2 diabetes..
Disclosures: SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.