User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Obesity can cut 19 years of health, 8 years of life
Excess body weight could lower “healthy life-years” by as much as 19 years, in addition to reducing life expectancy in certain demographics by as much as 8 years, new research published in Lancet Diabetes & Endocrinology suggests.
“The pattern is clear: The more an individual weighs and the younger their age, the greater the effect of excess weight on health,” wrote Dr. Steven A. Grover and his coinvestigators at the Research Institute of the McGill University Health Centre, Montreal.
They created a disease-simulation model, and by using data from 3,992 non-Hispanic white participants in the National Health and Nutrition and Examination Survey (NHANES) during 2003-2010, they estimated the annual risk of diabetes, cardiovascular disease, and mortality. They compared people with an ideal body mass index of 18.5 up to 25 kg/m² against overweight people with BMI of 25 up to 30 kg/m², obese participants with a BMI of 30 up to 35 kg/m², and very obese persons with a BMI of 35 kg/m² and higher. Health life-years lost was defined as years free of cardiovascular disease (CVD) and diabetes.
Depending on their age and sex, overweight individuals were estimated to lose 0-3 years of life expectancy, obese individuals could lose 1-6 years of life expectancy, and very obese individuals were estimated to lose 1-8 years. Younger adults with high body fat were generally at a greater risk for developing health problems than those who developed obesity later in life (Lancet Diabetes Endocrinol. 2014: [doi:10.1016/S2213-8587 (14)70229-3]) .The years of life lost for obese men ranged from 0.8 years in those aged 60-79 years to 5.9 years in those aged 20-39 years. Years lost for very obese men ranged from 0.9 years to 8.4 years, respectively. Years of life lost for very obese women females were similar, with 0.9 years lost for those aged 60-79 years and 6.1 years lost for those aged 20-39 years.
As expected, the negative health effects of excess body weight were greatest in patients with extreme obesity, with very obese males at the greatest risk for developing type 2 diabetes and obesity-associated CVD. The researchers further noted that morbidity (healthy life-years lost) can reduce quality of life nearly two to four times as much as the number of years of life lost.
These results might help health professionals to more actively encourage weight loss in their overweight and obese patients, and also provide such patients with additional motivation to adhere to healthier lifestyles, Dr. Grover said.
The study was funded by the Canadian Institutes of Health Research. Dr. Grover is a consultant to Merck, Roche, AstraZeneca, and Amgen. Several coinvestigators have ties to pharmaceutical companies.
As mortality from cardiovascular disease and diabetes decline, it may become more important to give overweight and obese patients a more accurate forecast of the effects of their excess weight. This is what Dr. Grover and his colleagues have done.
However, changing aspects of the epidemiology of obesity and diabetes might make healthy life-years a moving target. For example, the declining mortality from CVD and diabetes making the risk ratios for cardiovascular disease and related mortality associated with diabetes much lower than those used in the model by Dr. Grover and his colleagues.
Even so, efforts to refine estimation of the lifelong effect of obesity and diabetes are important for other reasons. Knowing more about the various clinical courses of these diseases will place a higher premium on decision-making methods that can simultaneously take a life-course perspective, incorporate interventions, and consider individual differences so that clinicians and public health leaders alike can effectively tackle the next phases of the obesity and diabetes epidemics.
Dr. Albert Gregg is a researcher at the National Center for Chronic Disease Prevention and Health Promotion unit of the Centers for Disease Control and Prevention. These comments were taken from an accompanying editorial (Lancet Diabetes Endocrinol. 2014 [doi: 10.1016/S2213-8587(14)70242-6]). He had no conflicts of interest to disclose.
As mortality from cardiovascular disease and diabetes decline, it may become more important to give overweight and obese patients a more accurate forecast of the effects of their excess weight. This is what Dr. Grover and his colleagues have done.
However, changing aspects of the epidemiology of obesity and diabetes might make healthy life-years a moving target. For example, the declining mortality from CVD and diabetes making the risk ratios for cardiovascular disease and related mortality associated with diabetes much lower than those used in the model by Dr. Grover and his colleagues.
Even so, efforts to refine estimation of the lifelong effect of obesity and diabetes are important for other reasons. Knowing more about the various clinical courses of these diseases will place a higher premium on decision-making methods that can simultaneously take a life-course perspective, incorporate interventions, and consider individual differences so that clinicians and public health leaders alike can effectively tackle the next phases of the obesity and diabetes epidemics.
Dr. Albert Gregg is a researcher at the National Center for Chronic Disease Prevention and Health Promotion unit of the Centers for Disease Control and Prevention. These comments were taken from an accompanying editorial (Lancet Diabetes Endocrinol. 2014 [doi: 10.1016/S2213-8587(14)70242-6]). He had no conflicts of interest to disclose.
As mortality from cardiovascular disease and diabetes decline, it may become more important to give overweight and obese patients a more accurate forecast of the effects of their excess weight. This is what Dr. Grover and his colleagues have done.
However, changing aspects of the epidemiology of obesity and diabetes might make healthy life-years a moving target. For example, the declining mortality from CVD and diabetes making the risk ratios for cardiovascular disease and related mortality associated with diabetes much lower than those used in the model by Dr. Grover and his colleagues.
Even so, efforts to refine estimation of the lifelong effect of obesity and diabetes are important for other reasons. Knowing more about the various clinical courses of these diseases will place a higher premium on decision-making methods that can simultaneously take a life-course perspective, incorporate interventions, and consider individual differences so that clinicians and public health leaders alike can effectively tackle the next phases of the obesity and diabetes epidemics.
Dr. Albert Gregg is a researcher at the National Center for Chronic Disease Prevention and Health Promotion unit of the Centers for Disease Control and Prevention. These comments were taken from an accompanying editorial (Lancet Diabetes Endocrinol. 2014 [doi: 10.1016/S2213-8587(14)70242-6]). He had no conflicts of interest to disclose.
Excess body weight could lower “healthy life-years” by as much as 19 years, in addition to reducing life expectancy in certain demographics by as much as 8 years, new research published in Lancet Diabetes & Endocrinology suggests.
“The pattern is clear: The more an individual weighs and the younger their age, the greater the effect of excess weight on health,” wrote Dr. Steven A. Grover and his coinvestigators at the Research Institute of the McGill University Health Centre, Montreal.
They created a disease-simulation model, and by using data from 3,992 non-Hispanic white participants in the National Health and Nutrition and Examination Survey (NHANES) during 2003-2010, they estimated the annual risk of diabetes, cardiovascular disease, and mortality. They compared people with an ideal body mass index of 18.5 up to 25 kg/m² against overweight people with BMI of 25 up to 30 kg/m², obese participants with a BMI of 30 up to 35 kg/m², and very obese persons with a BMI of 35 kg/m² and higher. Health life-years lost was defined as years free of cardiovascular disease (CVD) and diabetes.
Depending on their age and sex, overweight individuals were estimated to lose 0-3 years of life expectancy, obese individuals could lose 1-6 years of life expectancy, and very obese individuals were estimated to lose 1-8 years. Younger adults with high body fat were generally at a greater risk for developing health problems than those who developed obesity later in life (Lancet Diabetes Endocrinol. 2014: [doi:10.1016/S2213-8587 (14)70229-3]) .The years of life lost for obese men ranged from 0.8 years in those aged 60-79 years to 5.9 years in those aged 20-39 years. Years lost for very obese men ranged from 0.9 years to 8.4 years, respectively. Years of life lost for very obese women females were similar, with 0.9 years lost for those aged 60-79 years and 6.1 years lost for those aged 20-39 years.
As expected, the negative health effects of excess body weight were greatest in patients with extreme obesity, with very obese males at the greatest risk for developing type 2 diabetes and obesity-associated CVD. The researchers further noted that morbidity (healthy life-years lost) can reduce quality of life nearly two to four times as much as the number of years of life lost.
These results might help health professionals to more actively encourage weight loss in their overweight and obese patients, and also provide such patients with additional motivation to adhere to healthier lifestyles, Dr. Grover said.
The study was funded by the Canadian Institutes of Health Research. Dr. Grover is a consultant to Merck, Roche, AstraZeneca, and Amgen. Several coinvestigators have ties to pharmaceutical companies.
Excess body weight could lower “healthy life-years” by as much as 19 years, in addition to reducing life expectancy in certain demographics by as much as 8 years, new research published in Lancet Diabetes & Endocrinology suggests.
“The pattern is clear: The more an individual weighs and the younger their age, the greater the effect of excess weight on health,” wrote Dr. Steven A. Grover and his coinvestigators at the Research Institute of the McGill University Health Centre, Montreal.
They created a disease-simulation model, and by using data from 3,992 non-Hispanic white participants in the National Health and Nutrition and Examination Survey (NHANES) during 2003-2010, they estimated the annual risk of diabetes, cardiovascular disease, and mortality. They compared people with an ideal body mass index of 18.5 up to 25 kg/m² against overweight people with BMI of 25 up to 30 kg/m², obese participants with a BMI of 30 up to 35 kg/m², and very obese persons with a BMI of 35 kg/m² and higher. Health life-years lost was defined as years free of cardiovascular disease (CVD) and diabetes.
Depending on their age and sex, overweight individuals were estimated to lose 0-3 years of life expectancy, obese individuals could lose 1-6 years of life expectancy, and very obese individuals were estimated to lose 1-8 years. Younger adults with high body fat were generally at a greater risk for developing health problems than those who developed obesity later in life (Lancet Diabetes Endocrinol. 2014: [doi:10.1016/S2213-8587 (14)70229-3]) .The years of life lost for obese men ranged from 0.8 years in those aged 60-79 years to 5.9 years in those aged 20-39 years. Years lost for very obese men ranged from 0.9 years to 8.4 years, respectively. Years of life lost for very obese women females were similar, with 0.9 years lost for those aged 60-79 years and 6.1 years lost for those aged 20-39 years.
As expected, the negative health effects of excess body weight were greatest in patients with extreme obesity, with very obese males at the greatest risk for developing type 2 diabetes and obesity-associated CVD. The researchers further noted that morbidity (healthy life-years lost) can reduce quality of life nearly two to four times as much as the number of years of life lost.
These results might help health professionals to more actively encourage weight loss in their overweight and obese patients, and also provide such patients with additional motivation to adhere to healthier lifestyles, Dr. Grover said.
The study was funded by the Canadian Institutes of Health Research. Dr. Grover is a consultant to Merck, Roche, AstraZeneca, and Amgen. Several coinvestigators have ties to pharmaceutical companies.
FROM LANCET DIABETES AND ENDOCRINOLOGY
Key clinical point: Excess body weight at a young age lowers life expectancy and years of health more than that at older ages.
Major finding: Problems from excess body weight can lower life expectancy by as much as 8 years and create morbidity problems for as many as 19 years.
Data source: A disease-simulation model using data from 3,992 non-Hispanic white participants aged 20-79 years in the 2003-2010 NHANES.
Disclosures: The study was funded by the Canadian Institutes of Health Research. Dr. Grover is a consultant to Merck, Roche, AstraZeneca, and Amgen. Several coinvestigators have ties to pharmaceutical companies.
Few newly diagnosed diabetics trained in self-management
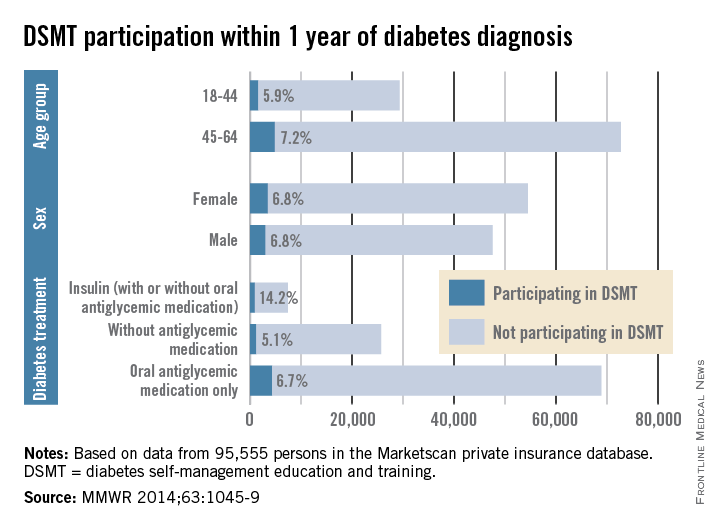
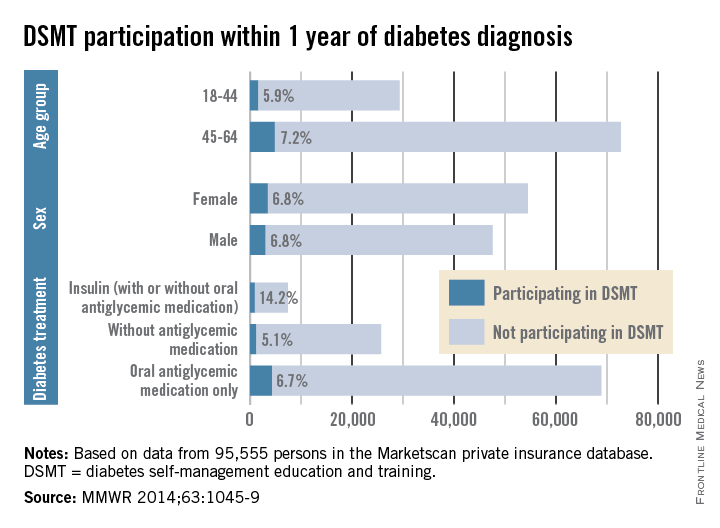
Among insured patients with diabetes, only 6.8% participated in self-management education and training within 1 year of their diagnosis, the Centers for Disease Control and Prevention reported.
There was some variation in the rate of diabetes self-management education and training (DSMT) across various subgroups, but none reached a participation rate higher than 14.2% in a study analyzing 2009-2012 data for 95,555 persons in the Marketscan Commercial Claims and Encounters database (MMWR 2014;63:1045-9).
That 14.2% participation rate was achieved among patients treated with insulin (with or without antiglycemic medication), which was somewhat offset by the small percentage who were using insulin (6.8%). Those taking oral antiglycemics – by far the largest treatment group – had a 6.7% DSMT participation rate and those not taking antiglycemics had a rate of only 5.1%, the CDC reported.
The older of the two age groups in the study, 45- to 69-year-olds, was slightly more likely to participate in DSMT (7.2%) than were those aged 18-44 years (5.9), while men and women were equally likely to participate (6.8%), the Marketscan data showed.
“The finding of low rates of participation in DSMT among privately insured adults with newly diagnosed diabetes underscores the need to identify specific barriers to access and participation in DSMT along with strategies to overcome these barriers,” the CDC investigators wrote.
Among insured patients with diabetes, only 6.8% participated in self-management education and training within 1 year of their diagnosis, the Centers for Disease Control and Prevention reported.
There was some variation in the rate of diabetes self-management education and training (DSMT) across various subgroups, but none reached a participation rate higher than 14.2% in a study analyzing 2009-2012 data for 95,555 persons in the Marketscan Commercial Claims and Encounters database (MMWR 2014;63:1045-9).
That 14.2% participation rate was achieved among patients treated with insulin (with or without antiglycemic medication), which was somewhat offset by the small percentage who were using insulin (6.8%). Those taking oral antiglycemics – by far the largest treatment group – had a 6.7% DSMT participation rate and those not taking antiglycemics had a rate of only 5.1%, the CDC reported.
The older of the two age groups in the study, 45- to 69-year-olds, was slightly more likely to participate in DSMT (7.2%) than were those aged 18-44 years (5.9), while men and women were equally likely to participate (6.8%), the Marketscan data showed.
“The finding of low rates of participation in DSMT among privately insured adults with newly diagnosed diabetes underscores the need to identify specific barriers to access and participation in DSMT along with strategies to overcome these barriers,” the CDC investigators wrote.

Among insured patients with diabetes, only 6.8% participated in self-management education and training within 1 year of their diagnosis, the Centers for Disease Control and Prevention reported.
There was some variation in the rate of diabetes self-management education and training (DSMT) across various subgroups, but none reached a participation rate higher than 14.2% in a study analyzing 2009-2012 data for 95,555 persons in the Marketscan Commercial Claims and Encounters database (MMWR 2014;63:1045-9).
That 14.2% participation rate was achieved among patients treated with insulin (with or without antiglycemic medication), which was somewhat offset by the small percentage who were using insulin (6.8%). Those taking oral antiglycemics – by far the largest treatment group – had a 6.7% DSMT participation rate and those not taking antiglycemics had a rate of only 5.1%, the CDC reported.
The older of the two age groups in the study, 45- to 69-year-olds, was slightly more likely to participate in DSMT (7.2%) than were those aged 18-44 years (5.9), while men and women were equally likely to participate (6.8%), the Marketscan data showed.
“The finding of low rates of participation in DSMT among privately insured adults with newly diagnosed diabetes underscores the need to identify specific barriers to access and participation in DSMT along with strategies to overcome these barriers,” the CDC investigators wrote.

FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Liraglutide lowers HbA1c in diabetic CKD patients
PHILADELPHIA – Once-daily add-on liraglutide reduces HbA1c in patients with type 2 diabetes and moderate renal impairment when compared with placebo, according to findings from the phase III LIRA-RENAL trial.
The effects on blood glucose occurred across glomerular filtration rate (GFR) subgroups, and no worsening of kidney function occurred during the randomized, double-blind, 26-week study, Dr. David Scott reported at Kidney Week 2014.
In 277 patients with stage 3 chronic kidney disease (CKD) who were randomized to receive either an oral daily dose of 1.8 mg of liraglutide or placebo in addition to existing oral antidiabetic agents and/or insulin therapy, active treatment was associated with a significantly greater overall reduction in mean HbA1c from baseline to week 26 (–1.05% vs. –0.38%; estimated treatment difference, –0.66%). Similar reductions were seen in those with stage 3a disease (–1.10% vs. –0.35%; estimated treatment difference, –0.72%), and stage 3b disease (–0.97% vs. –0.40%; estimated treatment difference, –0.57%), Dr. Scott of Clinical Research Development Associates, Springfield Gardens, N.Y., reported at the meeting, which was sponsored by the American Society of Nephrology.
No significant treatment effect was observed on urinary albumin/creatinine ratio in any of the albuminuria subgroups. The estimated treatment ratios (liraglutide/placebo) were 1.09, 0.73, and 0.66 in those with normal (less than 30 mg/g), moderately increased (30-300 mg/g) or severely increased (greater than 300 mg/g) albuminuria levels.
Also, no difference was seen in estimated GFR change from baseline between the liraglutide and placebo groups (–1% vs. +1%, respectively), Dr. Scott said.
Adverse events and serious adverse events occurred in similar numbers of patients in the treatment and placebo groups (76% and 69%, and 10% and 11% of liraglutide and placebo patients, respectively), although those in the treatment group experienced more nausea and vomiting than did those in the placebo group (35.7% vs. 17.5%), he noted.
Confirmed hypoglycemia occurred in 11% and 17% of the liraglutide and placebo patients, respectively, but no real difference was seen between the groups with respect to severe hypoglycemia, he said.
Subjects were adults with body mass index of 20-45 kg/m2 and HbA1c of 7.0%-10.0%. All were on stable diabetes medication and had moderate renal impairment (estimated GFR of 30-59 mL/min/1.73 m2; modification of diet in renal disease).
“I think this is a very important study. It’s important, because as a clinical nephrologist, I’m faced day-to-day with the challenges of how to treat my diabetic patients with CKD. There are therapies that are contraindicated, there are therapies that are not well tolerated,” Dr. Scott said, explaining that treatment options remain limited for patients with diabetes and impaired renal function.
Further, while the incidence of diabetic nephropathy and diabetes has been declining, diabetes remains one of the most prevalent causes of CKD, and the incidence in some populations, including the elderly – who are at greatest risk for complications from therapy – has increased.
Although prior studies demonstrated that liraglutide was safe and well tolerated in patients with mild renal insufficiency, guidelines continue to include specific language suggesting that it is not indicated in the setting of CKD, Dr. Scott said.
The current findings provide additional evidence of the safety and efficacy of liraglutide in patients with type 2 diabetes and moderate renal impairment, he concluded.
The LIRA-RENAL trial was funded by Novo Nordisk. Dr. Scott reported having no disclosures.
PHILADELPHIA – Once-daily add-on liraglutide reduces HbA1c in patients with type 2 diabetes and moderate renal impairment when compared with placebo, according to findings from the phase III LIRA-RENAL trial.
The effects on blood glucose occurred across glomerular filtration rate (GFR) subgroups, and no worsening of kidney function occurred during the randomized, double-blind, 26-week study, Dr. David Scott reported at Kidney Week 2014.
In 277 patients with stage 3 chronic kidney disease (CKD) who were randomized to receive either an oral daily dose of 1.8 mg of liraglutide or placebo in addition to existing oral antidiabetic agents and/or insulin therapy, active treatment was associated with a significantly greater overall reduction in mean HbA1c from baseline to week 26 (–1.05% vs. –0.38%; estimated treatment difference, –0.66%). Similar reductions were seen in those with stage 3a disease (–1.10% vs. –0.35%; estimated treatment difference, –0.72%), and stage 3b disease (–0.97% vs. –0.40%; estimated treatment difference, –0.57%), Dr. Scott of Clinical Research Development Associates, Springfield Gardens, N.Y., reported at the meeting, which was sponsored by the American Society of Nephrology.
No significant treatment effect was observed on urinary albumin/creatinine ratio in any of the albuminuria subgroups. The estimated treatment ratios (liraglutide/placebo) were 1.09, 0.73, and 0.66 in those with normal (less than 30 mg/g), moderately increased (30-300 mg/g) or severely increased (greater than 300 mg/g) albuminuria levels.
Also, no difference was seen in estimated GFR change from baseline between the liraglutide and placebo groups (–1% vs. +1%, respectively), Dr. Scott said.
Adverse events and serious adverse events occurred in similar numbers of patients in the treatment and placebo groups (76% and 69%, and 10% and 11% of liraglutide and placebo patients, respectively), although those in the treatment group experienced more nausea and vomiting than did those in the placebo group (35.7% vs. 17.5%), he noted.
Confirmed hypoglycemia occurred in 11% and 17% of the liraglutide and placebo patients, respectively, but no real difference was seen between the groups with respect to severe hypoglycemia, he said.
Subjects were adults with body mass index of 20-45 kg/m2 and HbA1c of 7.0%-10.0%. All were on stable diabetes medication and had moderate renal impairment (estimated GFR of 30-59 mL/min/1.73 m2; modification of diet in renal disease).
“I think this is a very important study. It’s important, because as a clinical nephrologist, I’m faced day-to-day with the challenges of how to treat my diabetic patients with CKD. There are therapies that are contraindicated, there are therapies that are not well tolerated,” Dr. Scott said, explaining that treatment options remain limited for patients with diabetes and impaired renal function.
Further, while the incidence of diabetic nephropathy and diabetes has been declining, diabetes remains one of the most prevalent causes of CKD, and the incidence in some populations, including the elderly – who are at greatest risk for complications from therapy – has increased.
Although prior studies demonstrated that liraglutide was safe and well tolerated in patients with mild renal insufficiency, guidelines continue to include specific language suggesting that it is not indicated in the setting of CKD, Dr. Scott said.
The current findings provide additional evidence of the safety and efficacy of liraglutide in patients with type 2 diabetes and moderate renal impairment, he concluded.
The LIRA-RENAL trial was funded by Novo Nordisk. Dr. Scott reported having no disclosures.
PHILADELPHIA – Once-daily add-on liraglutide reduces HbA1c in patients with type 2 diabetes and moderate renal impairment when compared with placebo, according to findings from the phase III LIRA-RENAL trial.
The effects on blood glucose occurred across glomerular filtration rate (GFR) subgroups, and no worsening of kidney function occurred during the randomized, double-blind, 26-week study, Dr. David Scott reported at Kidney Week 2014.
In 277 patients with stage 3 chronic kidney disease (CKD) who were randomized to receive either an oral daily dose of 1.8 mg of liraglutide or placebo in addition to existing oral antidiabetic agents and/or insulin therapy, active treatment was associated with a significantly greater overall reduction in mean HbA1c from baseline to week 26 (–1.05% vs. –0.38%; estimated treatment difference, –0.66%). Similar reductions were seen in those with stage 3a disease (–1.10% vs. –0.35%; estimated treatment difference, –0.72%), and stage 3b disease (–0.97% vs. –0.40%; estimated treatment difference, –0.57%), Dr. Scott of Clinical Research Development Associates, Springfield Gardens, N.Y., reported at the meeting, which was sponsored by the American Society of Nephrology.
No significant treatment effect was observed on urinary albumin/creatinine ratio in any of the albuminuria subgroups. The estimated treatment ratios (liraglutide/placebo) were 1.09, 0.73, and 0.66 in those with normal (less than 30 mg/g), moderately increased (30-300 mg/g) or severely increased (greater than 300 mg/g) albuminuria levels.
Also, no difference was seen in estimated GFR change from baseline between the liraglutide and placebo groups (–1% vs. +1%, respectively), Dr. Scott said.
Adverse events and serious adverse events occurred in similar numbers of patients in the treatment and placebo groups (76% and 69%, and 10% and 11% of liraglutide and placebo patients, respectively), although those in the treatment group experienced more nausea and vomiting than did those in the placebo group (35.7% vs. 17.5%), he noted.
Confirmed hypoglycemia occurred in 11% and 17% of the liraglutide and placebo patients, respectively, but no real difference was seen between the groups with respect to severe hypoglycemia, he said.
Subjects were adults with body mass index of 20-45 kg/m2 and HbA1c of 7.0%-10.0%. All were on stable diabetes medication and had moderate renal impairment (estimated GFR of 30-59 mL/min/1.73 m2; modification of diet in renal disease).
“I think this is a very important study. It’s important, because as a clinical nephrologist, I’m faced day-to-day with the challenges of how to treat my diabetic patients with CKD. There are therapies that are contraindicated, there are therapies that are not well tolerated,” Dr. Scott said, explaining that treatment options remain limited for patients with diabetes and impaired renal function.
Further, while the incidence of diabetic nephropathy and diabetes has been declining, diabetes remains one of the most prevalent causes of CKD, and the incidence in some populations, including the elderly – who are at greatest risk for complications from therapy – has increased.
Although prior studies demonstrated that liraglutide was safe and well tolerated in patients with mild renal insufficiency, guidelines continue to include specific language suggesting that it is not indicated in the setting of CKD, Dr. Scott said.
The current findings provide additional evidence of the safety and efficacy of liraglutide in patients with type 2 diabetes and moderate renal impairment, he concluded.
The LIRA-RENAL trial was funded by Novo Nordisk. Dr. Scott reported having no disclosures.
Key clinical point: Liraglutide is safe and effective as add-on therapy for lowering blood glucose in patients with type 2 diabetes and CKD.
Major finding: Mean reductions in HbA1c with liraglutide and placebo were –1.05% and –0.38%, respectively.
Data source: The phase III LIRA-RENAL trial in 277 patients.
Disclosures: The LIRA-RENAL trial was funded by Novo Nordisk. Dr. Scott reported having no disclosures.
Cigarette smoking rates among U.S. adults hit all-time low
The rate of cigarette smoking among adults in the United States dropped from 20.9% in 2005 to 17.8% in 2013, the lowest it has been since the Centers for Disease Control and Prevention began recording such data in 1965.
The numbers come from the Nov. 28 issue of the CDC’s Morbidity and Mortality Weekly Report (MMWR 2014;63:1108-12), which also states that the percentage of daily smokers who went through 20-29 cigarettes per day dropped from 34.9% in 2005 to 29.3% in 2013. Conversely, the rate of daily smokers who consumed 10 or fewer cigarettes per day increased from 16.4% in 2005 to 23.3% in 2013.
“Though smokers are smoking fewer cigarettes, cutting back by a few cigarettes a day rather than quitting completely does not produce significant health benefits,” Brian King, Ph.D., a senior scientific advisor with the CDC’s Office on Smoking and Health, said in a statement. “Smokers who quit before they’re 40 years old can get back almost all of the 10 years of life expectancy smoking takes away.”
Despite the strides in cutting down overall smoking among American adults, certain demographics continue to struggle. A total of 42.1 million adults remained smokers in 2013. Smoking rates remain especially high among males, younger individuals, those who are multiracial or American Indian/Alaska Native, have less education, live below the federal poverty level, live in the South or Midwest, have a disability or other limitation, and those who are lesbian, gay, or bisexual.
“There is encouraging news in this study, but we still have much more work to do to help people quit,” Dr. Tim McAfee, director of the CDC’s Office on Smoking and Health, noted in the statement. “We can bring down cigarette smoking rates much further, much faster, if strategies proven to work are put in place like funding tobacco control programs at the CDC-recommended levels, increasing prices of tobacco products, implementing and enforcing comprehensive smoke-free laws, and sustaining hard-hitting media campaigns.”
According to the CDC, cigarette smoking is the leading preventable cause of disease and death in the United States, claiming over 480,000 lives annually. Its impact can also be felt economically, with cigarette smoking costing the United States at least $133 billion in direct medical care for adults and more than $156 billion in lost productivity. Meanwhile, the rates of other forms of tobacco consumption, such as cigars and hookahs, are not declining.
Surveys cited by the CDC estimate that 70% of smokers want to quit.
The rate of cigarette smoking among adults in the United States dropped from 20.9% in 2005 to 17.8% in 2013, the lowest it has been since the Centers for Disease Control and Prevention began recording such data in 1965.
The numbers come from the Nov. 28 issue of the CDC’s Morbidity and Mortality Weekly Report (MMWR 2014;63:1108-12), which also states that the percentage of daily smokers who went through 20-29 cigarettes per day dropped from 34.9% in 2005 to 29.3% in 2013. Conversely, the rate of daily smokers who consumed 10 or fewer cigarettes per day increased from 16.4% in 2005 to 23.3% in 2013.
“Though smokers are smoking fewer cigarettes, cutting back by a few cigarettes a day rather than quitting completely does not produce significant health benefits,” Brian King, Ph.D., a senior scientific advisor with the CDC’s Office on Smoking and Health, said in a statement. “Smokers who quit before they’re 40 years old can get back almost all of the 10 years of life expectancy smoking takes away.”
Despite the strides in cutting down overall smoking among American adults, certain demographics continue to struggle. A total of 42.1 million adults remained smokers in 2013. Smoking rates remain especially high among males, younger individuals, those who are multiracial or American Indian/Alaska Native, have less education, live below the federal poverty level, live in the South or Midwest, have a disability or other limitation, and those who are lesbian, gay, or bisexual.
“There is encouraging news in this study, but we still have much more work to do to help people quit,” Dr. Tim McAfee, director of the CDC’s Office on Smoking and Health, noted in the statement. “We can bring down cigarette smoking rates much further, much faster, if strategies proven to work are put in place like funding tobacco control programs at the CDC-recommended levels, increasing prices of tobacco products, implementing and enforcing comprehensive smoke-free laws, and sustaining hard-hitting media campaigns.”
According to the CDC, cigarette smoking is the leading preventable cause of disease and death in the United States, claiming over 480,000 lives annually. Its impact can also be felt economically, with cigarette smoking costing the United States at least $133 billion in direct medical care for adults and more than $156 billion in lost productivity. Meanwhile, the rates of other forms of tobacco consumption, such as cigars and hookahs, are not declining.
Surveys cited by the CDC estimate that 70% of smokers want to quit.
The rate of cigarette smoking among adults in the United States dropped from 20.9% in 2005 to 17.8% in 2013, the lowest it has been since the Centers for Disease Control and Prevention began recording such data in 1965.
The numbers come from the Nov. 28 issue of the CDC’s Morbidity and Mortality Weekly Report (MMWR 2014;63:1108-12), which also states that the percentage of daily smokers who went through 20-29 cigarettes per day dropped from 34.9% in 2005 to 29.3% in 2013. Conversely, the rate of daily smokers who consumed 10 or fewer cigarettes per day increased from 16.4% in 2005 to 23.3% in 2013.
“Though smokers are smoking fewer cigarettes, cutting back by a few cigarettes a day rather than quitting completely does not produce significant health benefits,” Brian King, Ph.D., a senior scientific advisor with the CDC’s Office on Smoking and Health, said in a statement. “Smokers who quit before they’re 40 years old can get back almost all of the 10 years of life expectancy smoking takes away.”
Despite the strides in cutting down overall smoking among American adults, certain demographics continue to struggle. A total of 42.1 million adults remained smokers in 2013. Smoking rates remain especially high among males, younger individuals, those who are multiracial or American Indian/Alaska Native, have less education, live below the federal poverty level, live in the South or Midwest, have a disability or other limitation, and those who are lesbian, gay, or bisexual.
“There is encouraging news in this study, but we still have much more work to do to help people quit,” Dr. Tim McAfee, director of the CDC’s Office on Smoking and Health, noted in the statement. “We can bring down cigarette smoking rates much further, much faster, if strategies proven to work are put in place like funding tobacco control programs at the CDC-recommended levels, increasing prices of tobacco products, implementing and enforcing comprehensive smoke-free laws, and sustaining hard-hitting media campaigns.”
According to the CDC, cigarette smoking is the leading preventable cause of disease and death in the United States, claiming over 480,000 lives annually. Its impact can also be felt economically, with cigarette smoking costing the United States at least $133 billion in direct medical care for adults and more than $156 billion in lost productivity. Meanwhile, the rates of other forms of tobacco consumption, such as cigars and hookahs, are not declining.
Surveys cited by the CDC estimate that 70% of smokers want to quit.
FROM MMWR
Simple risk score predicts dementia risk in type 2 diabetes
BERLIN – The first-ever dementia risk score designed specifically for patients with type 2 diabetes has successfully undergone external validation and is ready for everyday use by clinicians, Rachel A. Whitmer, Ph.D., said at the annual congress of the European College of Neuropsychopharmacology.
Patients with type 2 diabetes are on average twice as likely to develop dementia as are nondiabetics. But within the diabetes population, the magnitude of risk varies enormously. The Diabetes-Specific Dementia Risk Score pins down an individual’s 10-year risk more precisely. The predictive factors are easily obtained from a patient’s medical history, enabling primary care physicians, endocrinologists, psychiatrists, and neurologists to readily calculate an individualized risk score without resorting to cognitive function testing or other labor-intensive measures.
“This is a risk score that can easily be determined in a primary care setting. It can be done by self-report. It can be done using an electronic medical record. This is a way to tell someone what their risk is. Knowing your risk might encourage patients to take steps to reduce that risk. And if their risk is low you can show it to them and say it’s all the more reason to avoid getting diabetes complications – because it’s not just about your diabetes, it’s also for your brain health,” said Dr. Whitmer, a research scientist at the division of research, Kaiser Permanente Northern California, Oakland.
She and her coinvestigators harnessed the Kaiser Permanente Northern California Diabetes Registry to develop the risk score. They evaluated 45 candidate predictors in nearly 30,000 registry participants age 60 or older with type 2 diabetes, 5,173 of whom developed dementia during 10 years of follow-up. Once they’d developed the risk score, which is based upon the eight strongest predictors, they validated it in a cohort of 2,413 type 2 diabetic patients at Group Health of Puget Sound. The validation study was published last year (Lancet Diabetes Endocrinol. 2013;1:183-90).
The predictive variables are age, education, depression, microvascular disease, acute metabolic events, cerebrovascular disease, cardiovascular disease, and having a diabetic foot.
Here’s how the Diabetes-Specific Dementia Risk Score works: A patient gets 0 points for being age 60-64, 3 for being 65-69, and so on up to a maximum of 10 points for being age 85 or more. A history of an acute metabolic event is worth 2 points, as is cerebrovascular disease or depression. Microvascular disease or cardiovascular disease are 1 point each. A college education is worth minus 1 point, a high school education or less gets 0 points. The points awarded for the eight predictors are added up. In the validation study, the 10-year risk of dementia ranged from a low of 5% in patients with a net score of minus 1 to 73% in patients with a score of 12-19.
Dr. Whitmer sees the risk score as being particularly well-suited as a tool for dementia screening of type 2 diabetes patients by primary care physicians. She noted that, today, well-managed diabetic patients have a podiatrist, dietician, and dentist, as well as a physician, all working to prevent the physical complications of diabetes. But there’s not typically anyone looking out for the diabetes patient’s brain health.
“I think it’s time now when we’re taking care of diabetic individuals that we need to think about the brain,” she said.
Numerous earlier studies conducted using the Kaiser Permanente Northern California Diabetes Registry have been instrumental in helping to establish vascular complications, depression, and hypoglycemic episodes as events that markedly elevate the risk of dementia among people with type 2 diabetes.
“The question is, if we can treat depression more effectively and manage glycemic control and prevent vascular complications, can we lower the risk of dementia in these people who are at particularly high risk? That’s where the field is right now. There are trials ongoing in people with diabetes and prediabetes looking at this question,” Dr. Whitmer noted.
The question takes on some urgency, she continued, because the intersection of type 2 diabetes and dementia looms ahead as an enormous public health problem. The International Diabetes Federation estimates that there are now 392 million people worldwide with diabetes, and that by 2035 this figure will climb to 592 million. Meanwhile, other projections are that the worldwide population of individuals with dementia will jump from just under 50 million today to more than 125 million by 2050, with most of the growth coming from low- and middle-income countries where the average lifespan is increasing.
The risk score project was supported by Kaiser Permanente and the National Institutes of Health. Dr. Whitmer reported having no financial conflicts.
BERLIN – The first-ever dementia risk score designed specifically for patients with type 2 diabetes has successfully undergone external validation and is ready for everyday use by clinicians, Rachel A. Whitmer, Ph.D., said at the annual congress of the European College of Neuropsychopharmacology.
Patients with type 2 diabetes are on average twice as likely to develop dementia as are nondiabetics. But within the diabetes population, the magnitude of risk varies enormously. The Diabetes-Specific Dementia Risk Score pins down an individual’s 10-year risk more precisely. The predictive factors are easily obtained from a patient’s medical history, enabling primary care physicians, endocrinologists, psychiatrists, and neurologists to readily calculate an individualized risk score without resorting to cognitive function testing or other labor-intensive measures.
“This is a risk score that can easily be determined in a primary care setting. It can be done by self-report. It can be done using an electronic medical record. This is a way to tell someone what their risk is. Knowing your risk might encourage patients to take steps to reduce that risk. And if their risk is low you can show it to them and say it’s all the more reason to avoid getting diabetes complications – because it’s not just about your diabetes, it’s also for your brain health,” said Dr. Whitmer, a research scientist at the division of research, Kaiser Permanente Northern California, Oakland.
She and her coinvestigators harnessed the Kaiser Permanente Northern California Diabetes Registry to develop the risk score. They evaluated 45 candidate predictors in nearly 30,000 registry participants age 60 or older with type 2 diabetes, 5,173 of whom developed dementia during 10 years of follow-up. Once they’d developed the risk score, which is based upon the eight strongest predictors, they validated it in a cohort of 2,413 type 2 diabetic patients at Group Health of Puget Sound. The validation study was published last year (Lancet Diabetes Endocrinol. 2013;1:183-90).
The predictive variables are age, education, depression, microvascular disease, acute metabolic events, cerebrovascular disease, cardiovascular disease, and having a diabetic foot.
Here’s how the Diabetes-Specific Dementia Risk Score works: A patient gets 0 points for being age 60-64, 3 for being 65-69, and so on up to a maximum of 10 points for being age 85 or more. A history of an acute metabolic event is worth 2 points, as is cerebrovascular disease or depression. Microvascular disease or cardiovascular disease are 1 point each. A college education is worth minus 1 point, a high school education or less gets 0 points. The points awarded for the eight predictors are added up. In the validation study, the 10-year risk of dementia ranged from a low of 5% in patients with a net score of minus 1 to 73% in patients with a score of 12-19.
Dr. Whitmer sees the risk score as being particularly well-suited as a tool for dementia screening of type 2 diabetes patients by primary care physicians. She noted that, today, well-managed diabetic patients have a podiatrist, dietician, and dentist, as well as a physician, all working to prevent the physical complications of diabetes. But there’s not typically anyone looking out for the diabetes patient’s brain health.
“I think it’s time now when we’re taking care of diabetic individuals that we need to think about the brain,” she said.
Numerous earlier studies conducted using the Kaiser Permanente Northern California Diabetes Registry have been instrumental in helping to establish vascular complications, depression, and hypoglycemic episodes as events that markedly elevate the risk of dementia among people with type 2 diabetes.
“The question is, if we can treat depression more effectively and manage glycemic control and prevent vascular complications, can we lower the risk of dementia in these people who are at particularly high risk? That’s where the field is right now. There are trials ongoing in people with diabetes and prediabetes looking at this question,” Dr. Whitmer noted.
The question takes on some urgency, she continued, because the intersection of type 2 diabetes and dementia looms ahead as an enormous public health problem. The International Diabetes Federation estimates that there are now 392 million people worldwide with diabetes, and that by 2035 this figure will climb to 592 million. Meanwhile, other projections are that the worldwide population of individuals with dementia will jump from just under 50 million today to more than 125 million by 2050, with most of the growth coming from low- and middle-income countries where the average lifespan is increasing.
The risk score project was supported by Kaiser Permanente and the National Institutes of Health. Dr. Whitmer reported having no financial conflicts.
BERLIN – The first-ever dementia risk score designed specifically for patients with type 2 diabetes has successfully undergone external validation and is ready for everyday use by clinicians, Rachel A. Whitmer, Ph.D., said at the annual congress of the European College of Neuropsychopharmacology.
Patients with type 2 diabetes are on average twice as likely to develop dementia as are nondiabetics. But within the diabetes population, the magnitude of risk varies enormously. The Diabetes-Specific Dementia Risk Score pins down an individual’s 10-year risk more precisely. The predictive factors are easily obtained from a patient’s medical history, enabling primary care physicians, endocrinologists, psychiatrists, and neurologists to readily calculate an individualized risk score without resorting to cognitive function testing or other labor-intensive measures.
“This is a risk score that can easily be determined in a primary care setting. It can be done by self-report. It can be done using an electronic medical record. This is a way to tell someone what their risk is. Knowing your risk might encourage patients to take steps to reduce that risk. And if their risk is low you can show it to them and say it’s all the more reason to avoid getting diabetes complications – because it’s not just about your diabetes, it’s also for your brain health,” said Dr. Whitmer, a research scientist at the division of research, Kaiser Permanente Northern California, Oakland.
She and her coinvestigators harnessed the Kaiser Permanente Northern California Diabetes Registry to develop the risk score. They evaluated 45 candidate predictors in nearly 30,000 registry participants age 60 or older with type 2 diabetes, 5,173 of whom developed dementia during 10 years of follow-up. Once they’d developed the risk score, which is based upon the eight strongest predictors, they validated it in a cohort of 2,413 type 2 diabetic patients at Group Health of Puget Sound. The validation study was published last year (Lancet Diabetes Endocrinol. 2013;1:183-90).
The predictive variables are age, education, depression, microvascular disease, acute metabolic events, cerebrovascular disease, cardiovascular disease, and having a diabetic foot.
Here’s how the Diabetes-Specific Dementia Risk Score works: A patient gets 0 points for being age 60-64, 3 for being 65-69, and so on up to a maximum of 10 points for being age 85 or more. A history of an acute metabolic event is worth 2 points, as is cerebrovascular disease or depression. Microvascular disease or cardiovascular disease are 1 point each. A college education is worth minus 1 point, a high school education or less gets 0 points. The points awarded for the eight predictors are added up. In the validation study, the 10-year risk of dementia ranged from a low of 5% in patients with a net score of minus 1 to 73% in patients with a score of 12-19.
Dr. Whitmer sees the risk score as being particularly well-suited as a tool for dementia screening of type 2 diabetes patients by primary care physicians. She noted that, today, well-managed diabetic patients have a podiatrist, dietician, and dentist, as well as a physician, all working to prevent the physical complications of diabetes. But there’s not typically anyone looking out for the diabetes patient’s brain health.
“I think it’s time now when we’re taking care of diabetic individuals that we need to think about the brain,” she said.
Numerous earlier studies conducted using the Kaiser Permanente Northern California Diabetes Registry have been instrumental in helping to establish vascular complications, depression, and hypoglycemic episodes as events that markedly elevate the risk of dementia among people with type 2 diabetes.
“The question is, if we can treat depression more effectively and manage glycemic control and prevent vascular complications, can we lower the risk of dementia in these people who are at particularly high risk? That’s where the field is right now. There are trials ongoing in people with diabetes and prediabetes looking at this question,” Dr. Whitmer noted.
The question takes on some urgency, she continued, because the intersection of type 2 diabetes and dementia looms ahead as an enormous public health problem. The International Diabetes Federation estimates that there are now 392 million people worldwide with diabetes, and that by 2035 this figure will climb to 592 million. Meanwhile, other projections are that the worldwide population of individuals with dementia will jump from just under 50 million today to more than 125 million by 2050, with most of the growth coming from low- and middle-income countries where the average lifespan is increasing.
The risk score project was supported by Kaiser Permanente and the National Institutes of Health. Dr. Whitmer reported having no financial conflicts.
AT THE ECNP CONGRESS
Key clinical point: It’s now readily possible to predict the 10-year dementia risk for individuals with type 2 diabetes.
Major finding: The 10-year risk of dementia in patients with type 2 diabetes ranged from 5% to 73% depending upon their Diabetes-Specific Dementia Risk Score, on the basis of eight variables easily obtained from a patient’s medical history.
Data source: The risk score was developed by evaluating 45 candidate predictive variables in a longitudinal cohort of 29,961 type 2 diabetes patients, 5,173 of whom developed dementia during 10 years of follow-up.
Disclosures: The study was funded by Kaiser Permanente and the National Institutes of Health. The presenter reported having no financial conflicts.
ZS-9 promotes normokalemia in patients with diabetes and CKD
PHILADELPHIA – ZS-9, an investigational cation exchanger designed to trap potassium in the gut, effectively and rapidly restores and maintains normokalemia in hyperkalemic patients with diabetes and significant renal impairment, according to a subgroup analysis of data from a randomized, placebo-controlled phase III trial.
Treatment with the first-in-class insoluble, nonabsorbed zirconium silicate, which was designed and engineered to have a three-dimensional crystalline lattice structure that preferentially traps potassium ions, was shown to have clinically and statistically significant benefit at daily oral doses of 5 mg and 10 mg, compared with placebo in the overall study population of 753 patients with hyperkalemia and underlying diabetes, heart failure, or chronic kidney disease.
The current analysis included 366 patients who had diabetes and an estimated glomerular filtration rate less than 60 mL/min/1.73m2. The patients, who had baseline potassium levels of 5.0-6.5 mEq/L (mean of 5.3 mEq/L) were randomized to oral ZS-9 at doses of 1.25, 2.5, 5, or 10 g or placebo given three times daily for the first 48 hours. Following this acute phase, those with a potassium level of 3.5-5.0 mEq/L were rerandomized to either the same ZS-9 acute-phase dose once daily for days 3-15 or placebo.
In the subgroup, ZS-9 treatment was associated with a significant decrease in potassium at 48 hours (decreases of -0.45, -0.59, and -0.81 mEq/L in the 2.5-, 5-, and 10-g groups, respectively, compared with +0.24 mEq/L in the placebo group, Dr. Bhupinder Singh reported at the meeting sponsored by the American Society of Nephrology.
During the maintenance phase, patients receiving a 10-g dose of ZS-9 maintained normokalemia (4.5 mEq/L at day 3 vs. 4.6 mEq/L at day 15) vs. placebo (4.4 mEq/L at day 3, vs. 5.1 mEq/L at day 15). Similar results were seen in the 5-mg treatment group, Dr. Singh of Apex Research, Riverside, Calif. said.
“Hyperkalemia is a common and serious complication in patients with diabetes who have chronic kidney disease, particularly those who are treated with renin-angiotensin-aldosterone system (RAAS) inhibitors. Even mild to moderate hyperkalemia has been recently shown to be associated with increased mortality,” Dr. Singh said, noting that concerns about the seriousness of hyperkalemia have likely led to under-prescription of RAAS inhibitors, which have been proven to have cardio- and renoprotective action, but which are associated with hyperkalemia.
In head-to-head in vitro studies, ZS-9 has been shown to be more than 125 times more selective than sodium polystyrene sulfonate (SPS) – the current mainstay of treatment for hyperkalemia), and to have more than 9 times the potassium-binding capacity.
In the current analysis, ZS-9 was associated with a “fairly acute” effect, reducing potassium levels significantly within 1 hour, and to less than 5 mEq/L within 4 hours. The study also was published Nov. 21 in the New England Journal of Medicine (doi:10.1056/NEJMoa1411487).
In both the overall study population and the subgroup with diabetes and chronic kidney disease, ZS-9 discontinuation at the end of the study was associated with recurrence of hyperkalemia, indicating a need for continued use. The adverse event profile was similar in the treatment and placebo groups in both studies.
The findings suggest that long-term treatment with ZS-9 may allow continuation of chronic RAAS inhibition in patients with diabetes and chronic kidney disease, Dr. Sing said, adding that long-term treatment studies are underway.
This study was funded by ZS Pharma Inc., which is developing ZS-9. Dr. Singh is a consultant to ZS Pharma Inc.
PHILADELPHIA – ZS-9, an investigational cation exchanger designed to trap potassium in the gut, effectively and rapidly restores and maintains normokalemia in hyperkalemic patients with diabetes and significant renal impairment, according to a subgroup analysis of data from a randomized, placebo-controlled phase III trial.
Treatment with the first-in-class insoluble, nonabsorbed zirconium silicate, which was designed and engineered to have a three-dimensional crystalline lattice structure that preferentially traps potassium ions, was shown to have clinically and statistically significant benefit at daily oral doses of 5 mg and 10 mg, compared with placebo in the overall study population of 753 patients with hyperkalemia and underlying diabetes, heart failure, or chronic kidney disease.
The current analysis included 366 patients who had diabetes and an estimated glomerular filtration rate less than 60 mL/min/1.73m2. The patients, who had baseline potassium levels of 5.0-6.5 mEq/L (mean of 5.3 mEq/L) were randomized to oral ZS-9 at doses of 1.25, 2.5, 5, or 10 g or placebo given three times daily for the first 48 hours. Following this acute phase, those with a potassium level of 3.5-5.0 mEq/L were rerandomized to either the same ZS-9 acute-phase dose once daily for days 3-15 or placebo.
In the subgroup, ZS-9 treatment was associated with a significant decrease in potassium at 48 hours (decreases of -0.45, -0.59, and -0.81 mEq/L in the 2.5-, 5-, and 10-g groups, respectively, compared with +0.24 mEq/L in the placebo group, Dr. Bhupinder Singh reported at the meeting sponsored by the American Society of Nephrology.
During the maintenance phase, patients receiving a 10-g dose of ZS-9 maintained normokalemia (4.5 mEq/L at day 3 vs. 4.6 mEq/L at day 15) vs. placebo (4.4 mEq/L at day 3, vs. 5.1 mEq/L at day 15). Similar results were seen in the 5-mg treatment group, Dr. Singh of Apex Research, Riverside, Calif. said.
“Hyperkalemia is a common and serious complication in patients with diabetes who have chronic kidney disease, particularly those who are treated with renin-angiotensin-aldosterone system (RAAS) inhibitors. Even mild to moderate hyperkalemia has been recently shown to be associated with increased mortality,” Dr. Singh said, noting that concerns about the seriousness of hyperkalemia have likely led to under-prescription of RAAS inhibitors, which have been proven to have cardio- and renoprotective action, but which are associated with hyperkalemia.
In head-to-head in vitro studies, ZS-9 has been shown to be more than 125 times more selective than sodium polystyrene sulfonate (SPS) – the current mainstay of treatment for hyperkalemia), and to have more than 9 times the potassium-binding capacity.
In the current analysis, ZS-9 was associated with a “fairly acute” effect, reducing potassium levels significantly within 1 hour, and to less than 5 mEq/L within 4 hours. The study also was published Nov. 21 in the New England Journal of Medicine (doi:10.1056/NEJMoa1411487).
In both the overall study population and the subgroup with diabetes and chronic kidney disease, ZS-9 discontinuation at the end of the study was associated with recurrence of hyperkalemia, indicating a need for continued use. The adverse event profile was similar in the treatment and placebo groups in both studies.
The findings suggest that long-term treatment with ZS-9 may allow continuation of chronic RAAS inhibition in patients with diabetes and chronic kidney disease, Dr. Sing said, adding that long-term treatment studies are underway.
This study was funded by ZS Pharma Inc., which is developing ZS-9. Dr. Singh is a consultant to ZS Pharma Inc.
PHILADELPHIA – ZS-9, an investigational cation exchanger designed to trap potassium in the gut, effectively and rapidly restores and maintains normokalemia in hyperkalemic patients with diabetes and significant renal impairment, according to a subgroup analysis of data from a randomized, placebo-controlled phase III trial.
Treatment with the first-in-class insoluble, nonabsorbed zirconium silicate, which was designed and engineered to have a three-dimensional crystalline lattice structure that preferentially traps potassium ions, was shown to have clinically and statistically significant benefit at daily oral doses of 5 mg and 10 mg, compared with placebo in the overall study population of 753 patients with hyperkalemia and underlying diabetes, heart failure, or chronic kidney disease.
The current analysis included 366 patients who had diabetes and an estimated glomerular filtration rate less than 60 mL/min/1.73m2. The patients, who had baseline potassium levels of 5.0-6.5 mEq/L (mean of 5.3 mEq/L) were randomized to oral ZS-9 at doses of 1.25, 2.5, 5, or 10 g or placebo given three times daily for the first 48 hours. Following this acute phase, those with a potassium level of 3.5-5.0 mEq/L were rerandomized to either the same ZS-9 acute-phase dose once daily for days 3-15 or placebo.
In the subgroup, ZS-9 treatment was associated with a significant decrease in potassium at 48 hours (decreases of -0.45, -0.59, and -0.81 mEq/L in the 2.5-, 5-, and 10-g groups, respectively, compared with +0.24 mEq/L in the placebo group, Dr. Bhupinder Singh reported at the meeting sponsored by the American Society of Nephrology.
During the maintenance phase, patients receiving a 10-g dose of ZS-9 maintained normokalemia (4.5 mEq/L at day 3 vs. 4.6 mEq/L at day 15) vs. placebo (4.4 mEq/L at day 3, vs. 5.1 mEq/L at day 15). Similar results were seen in the 5-mg treatment group, Dr. Singh of Apex Research, Riverside, Calif. said.
“Hyperkalemia is a common and serious complication in patients with diabetes who have chronic kidney disease, particularly those who are treated with renin-angiotensin-aldosterone system (RAAS) inhibitors. Even mild to moderate hyperkalemia has been recently shown to be associated with increased mortality,” Dr. Singh said, noting that concerns about the seriousness of hyperkalemia have likely led to under-prescription of RAAS inhibitors, which have been proven to have cardio- and renoprotective action, but which are associated with hyperkalemia.
In head-to-head in vitro studies, ZS-9 has been shown to be more than 125 times more selective than sodium polystyrene sulfonate (SPS) – the current mainstay of treatment for hyperkalemia), and to have more than 9 times the potassium-binding capacity.
In the current analysis, ZS-9 was associated with a “fairly acute” effect, reducing potassium levels significantly within 1 hour, and to less than 5 mEq/L within 4 hours. The study also was published Nov. 21 in the New England Journal of Medicine (doi:10.1056/NEJMoa1411487).
In both the overall study population and the subgroup with diabetes and chronic kidney disease, ZS-9 discontinuation at the end of the study was associated with recurrence of hyperkalemia, indicating a need for continued use. The adverse event profile was similar in the treatment and placebo groups in both studies.
The findings suggest that long-term treatment with ZS-9 may allow continuation of chronic RAAS inhibition in patients with diabetes and chronic kidney disease, Dr. Sing said, adding that long-term treatment studies are underway.
This study was funded by ZS Pharma Inc., which is developing ZS-9. Dr. Singh is a consultant to ZS Pharma Inc.
Key clinical point: ZS-9 may facilitate safe administration of RAAS inhibition in patients with diabetes and significant renal impairment.
Major finding: A daily 10-g dose of ZS-9 maintained normokalemia (4.5 mEq/L at day 3 vs. 4.6 mEq/L at day 15) vs. placebo (4.4 mEq/L at day 3, vs. 5.1 mEq/L at day 15).
Data source: A subgroup analysis of data from 366 patients in a randomized, placebo-controlled phase III trial.
Disclosures: This study was funded by ZS Pharma Inc., which is developing ZS-9. Dr. Singh is a consultant to ZS Pharma Inc.
On-target glycemic control does not lessen excess mortality
Adults with type 1 diabetes who had on-target glycemic control still showed twice the risk of death from any cause and death from cardiovascular causes as did the general population, according to findings from a Swedish study involving virtually every affected patient in that country that was reported online Nov. 20.
The excess mortality despite good glycemic control was attributed almost entirely to diabetes itself (ketoacidosis or hypoglycemia) or to cardiovascular disease (CVD), which is particularly puzzling because adults with type 1 diabetes “generally do not have excess rates of obesity, hypertension, or hypercholesterolemia.” Moreover, the diabetes patients in this study were four to five times more likely than controls to be taking cardioprotective drugs such as statins or renin-angiotensin-aldosterone system inhibitors, said Dr. Marcus Lind of Uddevalla Hospital and the University of Gothenburg in Sweden, and his associates.
The investigators assessed mortality risks in patients who had varying degrees of glycemic control using data from the Swedish National Diabetes Register and national mortality databases during a 14-year period. They matched 33,915 patients for age, sex, and region of residence with 169,249 adults in the general population. The mean duration of diabetes was 20 years, and the mean glycosylated hemoglobin level at baseline was 8.2%.
Overall mortality for patients with type 1 diabetes was 8% (9.97 per 1,000 observation-years), compared with 2.9% (3.45 per 1,000 observation-years) for controls. After the data were adjusted to account for education level and other possible confounding factors, hazard ratios for patients vs. controls were still high at 3.52 for death from any cause and 4.60 for death from CVD causes, the investigators said (N. Engl. J. Med. 2014 November 20 [doi:10.1056/NEJMoa1408214]).
Even patients who had good glycemic control, with a mean glycosylated hemoglobin level of 6.9% or lower, had an hazard ratio for death from any cause of 2.36 and an HR for cardiovascular death of 2.92, in relation to controls. Mortality increased as mean glycosylated hemoglobin level increased, so that patients with a mean glycosylated hemoglobin level of 9.7% or higher were 8 times more likely to die from any cause and 10 times more likely to die from cardiovascular causes, in relation to controls.
Adults with type 1 diabetes who had on-target glycemic control still showed twice the risk of death from any cause and death from cardiovascular causes as did the general population, according to findings from a Swedish study involving virtually every affected patient in that country that was reported online Nov. 20.
The excess mortality despite good glycemic control was attributed almost entirely to diabetes itself (ketoacidosis or hypoglycemia) or to cardiovascular disease (CVD), which is particularly puzzling because adults with type 1 diabetes “generally do not have excess rates of obesity, hypertension, or hypercholesterolemia.” Moreover, the diabetes patients in this study were four to five times more likely than controls to be taking cardioprotective drugs such as statins or renin-angiotensin-aldosterone system inhibitors, said Dr. Marcus Lind of Uddevalla Hospital and the University of Gothenburg in Sweden, and his associates.
The investigators assessed mortality risks in patients who had varying degrees of glycemic control using data from the Swedish National Diabetes Register and national mortality databases during a 14-year period. They matched 33,915 patients for age, sex, and region of residence with 169,249 adults in the general population. The mean duration of diabetes was 20 years, and the mean glycosylated hemoglobin level at baseline was 8.2%.
Overall mortality for patients with type 1 diabetes was 8% (9.97 per 1,000 observation-years), compared with 2.9% (3.45 per 1,000 observation-years) for controls. After the data were adjusted to account for education level and other possible confounding factors, hazard ratios for patients vs. controls were still high at 3.52 for death from any cause and 4.60 for death from CVD causes, the investigators said (N. Engl. J. Med. 2014 November 20 [doi:10.1056/NEJMoa1408214]).
Even patients who had good glycemic control, with a mean glycosylated hemoglobin level of 6.9% or lower, had an hazard ratio for death from any cause of 2.36 and an HR for cardiovascular death of 2.92, in relation to controls. Mortality increased as mean glycosylated hemoglobin level increased, so that patients with a mean glycosylated hemoglobin level of 9.7% or higher were 8 times more likely to die from any cause and 10 times more likely to die from cardiovascular causes, in relation to controls.
Adults with type 1 diabetes who had on-target glycemic control still showed twice the risk of death from any cause and death from cardiovascular causes as did the general population, according to findings from a Swedish study involving virtually every affected patient in that country that was reported online Nov. 20.
The excess mortality despite good glycemic control was attributed almost entirely to diabetes itself (ketoacidosis or hypoglycemia) or to cardiovascular disease (CVD), which is particularly puzzling because adults with type 1 diabetes “generally do not have excess rates of obesity, hypertension, or hypercholesterolemia.” Moreover, the diabetes patients in this study were four to five times more likely than controls to be taking cardioprotective drugs such as statins or renin-angiotensin-aldosterone system inhibitors, said Dr. Marcus Lind of Uddevalla Hospital and the University of Gothenburg in Sweden, and his associates.
The investigators assessed mortality risks in patients who had varying degrees of glycemic control using data from the Swedish National Diabetes Register and national mortality databases during a 14-year period. They matched 33,915 patients for age, sex, and region of residence with 169,249 adults in the general population. The mean duration of diabetes was 20 years, and the mean glycosylated hemoglobin level at baseline was 8.2%.
Overall mortality for patients with type 1 diabetes was 8% (9.97 per 1,000 observation-years), compared with 2.9% (3.45 per 1,000 observation-years) for controls. After the data were adjusted to account for education level and other possible confounding factors, hazard ratios for patients vs. controls were still high at 3.52 for death from any cause and 4.60 for death from CVD causes, the investigators said (N. Engl. J. Med. 2014 November 20 [doi:10.1056/NEJMoa1408214]).
Even patients who had good glycemic control, with a mean glycosylated hemoglobin level of 6.9% or lower, had an hazard ratio for death from any cause of 2.36 and an HR for cardiovascular death of 2.92, in relation to controls. Mortality increased as mean glycosylated hemoglobin level increased, so that patients with a mean glycosylated hemoglobin level of 9.7% or higher were 8 times more likely to die from any cause and 10 times more likely to die from cardiovascular causes, in relation to controls.
Key clinical point: Adults with type 1 diabetes who have good glycemic control still have twice the all-cause and CVD mortality as the general population.
Major finding: Even patients who had good glycemic control, with a mean glycosylated hemoglobin level of 6.9% or lower, had an HR for death from any cause of 2.36 and an HR for cardiovascular death of 2.92, in relation to controls.
Data source: An observational analysis of glycemic control and mortality data involving 33,915 adults with type 1 diabetes and 169,249 controls in the general population matched for age, sex, and area of residence in Sweden.
Disclosures: This study was supported by the Swedish government, the Swedish Society of Medicine, the Regional Vastra Gotaland Executive Board’s Health and Medical Care Committee, the Swedish Heart and Lung Foundation, Diabetes Wellness, and the Swedish Research Council. Dr. Lind reported receiving honoraria and grant support from AstraZeneca, Novo Nordisk, Pfizer, Medtronic, Abbott, and Dexcom; his associates reported ties to numerous industry sources.
CABG beats PCI for revascularization of diabetics
Coronary artery bypass grafting is the preferred long-term revascularization technique for diabetics, based on data from a meta-analysis published in the Annals of Internal Medicine (2014;161:724-32 [doi:10.7326/M14-0808]).
“With more than 1 million revascularization procedures done annually in the United States alone, assessing the risks and benefits of these techniques in this subgroup is a public health priority,” said Dr. Benny Tu of Greenslopes Private Hospital in Queensland, Australia, and his colleagues. “In particular, deciding on an optimal revascularization strategy is a crucial element of clinical decision making,” the researchers wrote.
The researchers used a Bayesian network meta-analysis to combine 40 studies from English-language publications such as PubMed, the Cochrane Central Register of Controlled Trials, Ovid, and EMBASE that took place between Jan. 1, 1990, and June 1, 2014. Each of these studies was a randomized, controlled trial comparing the effects of percutaneous coronary intervention (PCI), including PCIs with bare-metal stents (PCI-BMS) and those with drug-eluding stents (PCI-DES), with coronary artery bypass grafting (CABG) in adult diabetics with either multivessel or left main coronary artery disease.
The primary outcome combination of stroke, nonfatal MI, and all-cause mortality was 33% more likely in patients who underwent PCI (odds ratio, 1.33) as opposed to CABG. PCI also was associated with significant increase of 44% in mortality (OR, 1.44), and a 44% decrease in stroke.
“The largest advantage of CABG is in avoiding repeated revascularization,” the researchers noted; the need for repeated revascularization was 137% higher with PCI than with CABG.
Researchers extracted data relevant to study design, quality, patient characteristics, length of postprocedure follow-ups, and overall outcomes to determine which procedure proved most effective at mitigating mortality and the need for repeated revascularization. For duplicate publications, outcomes were obtained from the publication with the longest follow-up.
“This trial showed that CABG significantly decreased mortality in patients with diabetes at 5 years compared with percutaneous transluminal coronary angioplasty,” the researchers noted, adding that the findings confirm the CABG endorsement published in the 1997 Bypass Angioplasty Revascularization Investigation.
However, as techniques for both PCI and CABG have improved considerably between 1990 and 2014, the researchers advised physicians to review each option on a case-by-case basis, and to consider that PCI may in fact be the preferred option for some high-risk patients.
“Although CABG may generally be preferred, there are individual clinical situations in which PCI may be a reasonable alternative,” they wrote. “For example, it might be preferred for patients at high risk for perioperative stroke or whose long-term survival is compromised because of noncardiac factors.”
The study’s primary source of funding was the Fonds de recherche du Québec-Santé, but none of the researchers reported relevant financial disclosures.
Coronary artery bypass grafting is the preferred long-term revascularization technique for diabetics, based on data from a meta-analysis published in the Annals of Internal Medicine (2014;161:724-32 [doi:10.7326/M14-0808]).
“With more than 1 million revascularization procedures done annually in the United States alone, assessing the risks and benefits of these techniques in this subgroup is a public health priority,” said Dr. Benny Tu of Greenslopes Private Hospital in Queensland, Australia, and his colleagues. “In particular, deciding on an optimal revascularization strategy is a crucial element of clinical decision making,” the researchers wrote.
The researchers used a Bayesian network meta-analysis to combine 40 studies from English-language publications such as PubMed, the Cochrane Central Register of Controlled Trials, Ovid, and EMBASE that took place between Jan. 1, 1990, and June 1, 2014. Each of these studies was a randomized, controlled trial comparing the effects of percutaneous coronary intervention (PCI), including PCIs with bare-metal stents (PCI-BMS) and those with drug-eluding stents (PCI-DES), with coronary artery bypass grafting (CABG) in adult diabetics with either multivessel or left main coronary artery disease.
The primary outcome combination of stroke, nonfatal MI, and all-cause mortality was 33% more likely in patients who underwent PCI (odds ratio, 1.33) as opposed to CABG. PCI also was associated with significant increase of 44% in mortality (OR, 1.44), and a 44% decrease in stroke.
“The largest advantage of CABG is in avoiding repeated revascularization,” the researchers noted; the need for repeated revascularization was 137% higher with PCI than with CABG.
Researchers extracted data relevant to study design, quality, patient characteristics, length of postprocedure follow-ups, and overall outcomes to determine which procedure proved most effective at mitigating mortality and the need for repeated revascularization. For duplicate publications, outcomes were obtained from the publication with the longest follow-up.
“This trial showed that CABG significantly decreased mortality in patients with diabetes at 5 years compared with percutaneous transluminal coronary angioplasty,” the researchers noted, adding that the findings confirm the CABG endorsement published in the 1997 Bypass Angioplasty Revascularization Investigation.
However, as techniques for both PCI and CABG have improved considerably between 1990 and 2014, the researchers advised physicians to review each option on a case-by-case basis, and to consider that PCI may in fact be the preferred option for some high-risk patients.
“Although CABG may generally be preferred, there are individual clinical situations in which PCI may be a reasonable alternative,” they wrote. “For example, it might be preferred for patients at high risk for perioperative stroke or whose long-term survival is compromised because of noncardiac factors.”
The study’s primary source of funding was the Fonds de recherche du Québec-Santé, but none of the researchers reported relevant financial disclosures.
Coronary artery bypass grafting is the preferred long-term revascularization technique for diabetics, based on data from a meta-analysis published in the Annals of Internal Medicine (2014;161:724-32 [doi:10.7326/M14-0808]).
“With more than 1 million revascularization procedures done annually in the United States alone, assessing the risks and benefits of these techniques in this subgroup is a public health priority,” said Dr. Benny Tu of Greenslopes Private Hospital in Queensland, Australia, and his colleagues. “In particular, deciding on an optimal revascularization strategy is a crucial element of clinical decision making,” the researchers wrote.
The researchers used a Bayesian network meta-analysis to combine 40 studies from English-language publications such as PubMed, the Cochrane Central Register of Controlled Trials, Ovid, and EMBASE that took place between Jan. 1, 1990, and June 1, 2014. Each of these studies was a randomized, controlled trial comparing the effects of percutaneous coronary intervention (PCI), including PCIs with bare-metal stents (PCI-BMS) and those with drug-eluding stents (PCI-DES), with coronary artery bypass grafting (CABG) in adult diabetics with either multivessel or left main coronary artery disease.
The primary outcome combination of stroke, nonfatal MI, and all-cause mortality was 33% more likely in patients who underwent PCI (odds ratio, 1.33) as opposed to CABG. PCI also was associated with significant increase of 44% in mortality (OR, 1.44), and a 44% decrease in stroke.
“The largest advantage of CABG is in avoiding repeated revascularization,” the researchers noted; the need for repeated revascularization was 137% higher with PCI than with CABG.
Researchers extracted data relevant to study design, quality, patient characteristics, length of postprocedure follow-ups, and overall outcomes to determine which procedure proved most effective at mitigating mortality and the need for repeated revascularization. For duplicate publications, outcomes were obtained from the publication with the longest follow-up.
“This trial showed that CABG significantly decreased mortality in patients with diabetes at 5 years compared with percutaneous transluminal coronary angioplasty,” the researchers noted, adding that the findings confirm the CABG endorsement published in the 1997 Bypass Angioplasty Revascularization Investigation.
However, as techniques for both PCI and CABG have improved considerably between 1990 and 2014, the researchers advised physicians to review each option on a case-by-case basis, and to consider that PCI may in fact be the preferred option for some high-risk patients.
“Although CABG may generally be preferred, there are individual clinical situations in which PCI may be a reasonable alternative,” they wrote. “For example, it might be preferred for patients at high risk for perioperative stroke or whose long-term survival is compromised because of noncardiac factors.”
The study’s primary source of funding was the Fonds de recherche du Québec-Santé, but none of the researchers reported relevant financial disclosures.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point: CABG is the preferred long-term revascularization technique for diabetics.
Major finding: Diabetic patients with multivessel disease or left main coronary artery disease who underwent PCI were 33% more likely to experience stroke, nonfatal heart attack, or death than were those who underwent CABG.
Data source: Bayesian network meta-analysis of 40 studies.
Disclosures: The authors disclosed that the study’s primary source of funding was the Fonds de recherche du Québec-Santé but reported no other relevant financial disclosures.
Presurgical BMI doesn’t predict diabetes remission
A patient’s body mass index before bariatric surgery is unrelated to remission of his or her diabetes afterward, according to a report published online in Annals of Surgery.
In a meta-analysis of 94 observational and interventional clinical studies, the efficacy of the surgery as a metabolic procedure had nothing to do with baseline body mass index (BMI) and instead depended on the degree of diabetic compromise at baseline. “We believe therefore that the use of baseline BMIs to gauge the metabolic effect of surgery is misleading and should be avoided,” said Simona Panunzi, Ph.D., of the Italian National Council of Research-Institute of Systems Analysis and Computer Sciences, Biomathematics Laboratory, Rome, and her associates.
At present, baseline BMI is considered the only valid selection criterion for bariatric surgery in diabetic adults, even though numerous studies have reported that neither BMI nor body weight are good predictors of diabetes remission. In contrast, many other studies have reported that body fat distribution, and visceral obesity in particular, directly affects glycemic control, insulin resistance, and the metabolic syndrome, the investigators commented (Ann. Surg. 2014 Oct. 30 [E-pub ahead of print]).
To assess which factors best predict diabetes remission after bariatric surgery, Dr. Panunzi and her associates performed a systematic review and meta-analysis of the literature. They screened 1,437 articles and focused on 94: 35 studies included patients with a baseline BMI of less than 35 kg/m2, 56 studies included patients with a baseline BMI of 35 kg/m2 or more, and 3 studies included patients with any baseline BMI. There were a total of 94,579 patients, of whom 4,944 had type 2 diabetes.
The mean age of the study participants was approximately 46 years. Bariatric procedures included sleeve gastrectomy, Roux-en-Y gastric bypass, biliopancreatic diversion, laparoscopic gastric banding, and duodenal-jejunal bypass. Across all the studies, the percent reduction in mean BMI after surgery was 17% for patients with a baseline BMI of less than 35 kg/m2 (group 1) and 35% for those with a baseline BMI of 35 kg/m2 or more (group 2).
Diabetes remitted in 72% of the patients in group 1 and 71% of those in group 2, a nonsignificant difference. The only factor found to predict remission, defined as the normalization of HbA1c, was the severity of diabetes as measured by fasting levels of glucose, insulin, and HbA1c at baseline, the investigators said.
“The appropriateness of the BMI criterion proposed by the National Institutes of Health for determining the eligibility of patients with diabetes for bariatric surgery has already been challenged by other authors. However, until now there was no direct evidence of its lack of relevance. It seems clear from the results of [our] study that baseline BMI does not carry prognostic information for diabetes resolution after bariatric surgery,” they added.
This meta-analysis was limited in that few of the reviewed studies reported diabetes duration, a well-known predictor of remission. And the definition of diabetes remission varied widely among the studies, from simple withdrawal of diabetes medications to various specific plasma glucose or HbA1c levels, the investigators noted.
A patient’s body mass index before bariatric surgery is unrelated to remission of his or her diabetes afterward, according to a report published online in Annals of Surgery.
In a meta-analysis of 94 observational and interventional clinical studies, the efficacy of the surgery as a metabolic procedure had nothing to do with baseline body mass index (BMI) and instead depended on the degree of diabetic compromise at baseline. “We believe therefore that the use of baseline BMIs to gauge the metabolic effect of surgery is misleading and should be avoided,” said Simona Panunzi, Ph.D., of the Italian National Council of Research-Institute of Systems Analysis and Computer Sciences, Biomathematics Laboratory, Rome, and her associates.
At present, baseline BMI is considered the only valid selection criterion for bariatric surgery in diabetic adults, even though numerous studies have reported that neither BMI nor body weight are good predictors of diabetes remission. In contrast, many other studies have reported that body fat distribution, and visceral obesity in particular, directly affects glycemic control, insulin resistance, and the metabolic syndrome, the investigators commented (Ann. Surg. 2014 Oct. 30 [E-pub ahead of print]).
To assess which factors best predict diabetes remission after bariatric surgery, Dr. Panunzi and her associates performed a systematic review and meta-analysis of the literature. They screened 1,437 articles and focused on 94: 35 studies included patients with a baseline BMI of less than 35 kg/m2, 56 studies included patients with a baseline BMI of 35 kg/m2 or more, and 3 studies included patients with any baseline BMI. There were a total of 94,579 patients, of whom 4,944 had type 2 diabetes.
The mean age of the study participants was approximately 46 years. Bariatric procedures included sleeve gastrectomy, Roux-en-Y gastric bypass, biliopancreatic diversion, laparoscopic gastric banding, and duodenal-jejunal bypass. Across all the studies, the percent reduction in mean BMI after surgery was 17% for patients with a baseline BMI of less than 35 kg/m2 (group 1) and 35% for those with a baseline BMI of 35 kg/m2 or more (group 2).
Diabetes remitted in 72% of the patients in group 1 and 71% of those in group 2, a nonsignificant difference. The only factor found to predict remission, defined as the normalization of HbA1c, was the severity of diabetes as measured by fasting levels of glucose, insulin, and HbA1c at baseline, the investigators said.
“The appropriateness of the BMI criterion proposed by the National Institutes of Health for determining the eligibility of patients with diabetes for bariatric surgery has already been challenged by other authors. However, until now there was no direct evidence of its lack of relevance. It seems clear from the results of [our] study that baseline BMI does not carry prognostic information for diabetes resolution after bariatric surgery,” they added.
This meta-analysis was limited in that few of the reviewed studies reported diabetes duration, a well-known predictor of remission. And the definition of diabetes remission varied widely among the studies, from simple withdrawal of diabetes medications to various specific plasma glucose or HbA1c levels, the investigators noted.
A patient’s body mass index before bariatric surgery is unrelated to remission of his or her diabetes afterward, according to a report published online in Annals of Surgery.
In a meta-analysis of 94 observational and interventional clinical studies, the efficacy of the surgery as a metabolic procedure had nothing to do with baseline body mass index (BMI) and instead depended on the degree of diabetic compromise at baseline. “We believe therefore that the use of baseline BMIs to gauge the metabolic effect of surgery is misleading and should be avoided,” said Simona Panunzi, Ph.D., of the Italian National Council of Research-Institute of Systems Analysis and Computer Sciences, Biomathematics Laboratory, Rome, and her associates.
At present, baseline BMI is considered the only valid selection criterion for bariatric surgery in diabetic adults, even though numerous studies have reported that neither BMI nor body weight are good predictors of diabetes remission. In contrast, many other studies have reported that body fat distribution, and visceral obesity in particular, directly affects glycemic control, insulin resistance, and the metabolic syndrome, the investigators commented (Ann. Surg. 2014 Oct. 30 [E-pub ahead of print]).
To assess which factors best predict diabetes remission after bariatric surgery, Dr. Panunzi and her associates performed a systematic review and meta-analysis of the literature. They screened 1,437 articles and focused on 94: 35 studies included patients with a baseline BMI of less than 35 kg/m2, 56 studies included patients with a baseline BMI of 35 kg/m2 or more, and 3 studies included patients with any baseline BMI. There were a total of 94,579 patients, of whom 4,944 had type 2 diabetes.
The mean age of the study participants was approximately 46 years. Bariatric procedures included sleeve gastrectomy, Roux-en-Y gastric bypass, biliopancreatic diversion, laparoscopic gastric banding, and duodenal-jejunal bypass. Across all the studies, the percent reduction in mean BMI after surgery was 17% for patients with a baseline BMI of less than 35 kg/m2 (group 1) and 35% for those with a baseline BMI of 35 kg/m2 or more (group 2).
Diabetes remitted in 72% of the patients in group 1 and 71% of those in group 2, a nonsignificant difference. The only factor found to predict remission, defined as the normalization of HbA1c, was the severity of diabetes as measured by fasting levels of glucose, insulin, and HbA1c at baseline, the investigators said.
“The appropriateness of the BMI criterion proposed by the National Institutes of Health for determining the eligibility of patients with diabetes for bariatric surgery has already been challenged by other authors. However, until now there was no direct evidence of its lack of relevance. It seems clear from the results of [our] study that baseline BMI does not carry prognostic information for diabetes resolution after bariatric surgery,” they added.
This meta-analysis was limited in that few of the reviewed studies reported diabetes duration, a well-known predictor of remission. And the definition of diabetes remission varied widely among the studies, from simple withdrawal of diabetes medications to various specific plasma glucose or HbA1c levels, the investigators noted.
FROM ANNALS OF SURGERY
Key clinical point: BMI before bariatric surgery is unrelated to diabetes remission afterward.
Major finding: Diabetes remitted in 72% of the patients with a baseline BMI under 35 kg/m2 and 71% of those with a baseline BMI of 35 kg/m2 or more.
Data source: A meta-analysis of 94 clinical studies reporting bariatric surgery results for 94,579 overweight/obese patients, including 4,944 who had diabetes.
Disclosures: This study received no financial support from external sponsors. Dr. Panunzi and her associates reported having no financial disclosures.
Functional MRI shows diabetes-induced cognitive deficits in elderly
Elderly patients with type 2 diabetes had diminished frontal brain activity on functional magnetic resonance imaging that correlated with deficits in working memory and executive function, investigators have reported. The results were published online Nov. 17.
“To our knowledge, this study is the first to detect the specific brain mechanisms related to diabetes-induced working memory dysfunction,” wrote Dr. Yaojing Chen of Beijing Normal University, China.
Patients with diabetes also exhibited varying frontal brain deficits depending on the difficulty of the working memory tasks they were asked to perform. The left inferior frontal gyrus had reduced brain activation during easier tasks, while the middle frontal gyrus and the superior frontal gyrus were affected during more difficult tasks (Diabetes Care 2014 Nov. 17 [doi: 10.2337/dc14-1683]).
Type 2 diabetes is known to expedite brain aging and increase the risk of Alzheimer’s disease. Affected patients can develop deficits in executive functioning, attention, memory, and visuospatial abilities, the researchers noted. To visualize the specific structures affected, the researchers performed functional magnetic resonance imaging (fMRI) of 67 patients from the Beijing Aging Brain Rejuvenation Initiative, a longitudinal study of aging and cognitive impairment in the urban elderly population of Beijing.
Patients in the study had no history of dementia or psychiatric illness, coronary artery disease, nephritis, cancer, or gastrointestinal disease.
Compared with 37 controls, the patients with diabetes performed significantly worse on several working memory tasks, including the backward digit span, the digit span, and the Stroop Color and Word Test, which is an executive function task, the researchers reported.
Patients with diabetes also had less fMRI activation in several frontal brain areas, including the right superior frontal gyrus, the bilateral medial frontal gyrus, and the inferior frontal gyrus. Furthermore, the diabetes group had lower fMRI activation of the left inferior frontal gyrus (Brodmann area 13) in the 1-back versus 0-back (working memory) condition, and had less activation of the left middle frontal gyrus and the superior frontal gyrus in the 2-back versus 0-back comparison, they added. “These findings indicate that with increasing task difficulty, additional frontal brain regions might have been recruited to play compensatory roles to complete the harder task,” they said.
Larger longitudinal studies would be needed to confirm the findings and explore their potential for predicting early cognitive changes in patients with type 2 diabetes, they noted.
The research was funded by the Beijing New Medical Discipline Based Group, the Natural Science Foundation of China, the Institute of Basic Research in Clinical Medicine, the China Academy of Chinese Medical Sciences, and the program for New Century Excellent Talents in University. The researchers declared no conflicts of interest.
Elderly patients with type 2 diabetes had diminished frontal brain activity on functional magnetic resonance imaging that correlated with deficits in working memory and executive function, investigators have reported. The results were published online Nov. 17.
“To our knowledge, this study is the first to detect the specific brain mechanisms related to diabetes-induced working memory dysfunction,” wrote Dr. Yaojing Chen of Beijing Normal University, China.
Patients with diabetes also exhibited varying frontal brain deficits depending on the difficulty of the working memory tasks they were asked to perform. The left inferior frontal gyrus had reduced brain activation during easier tasks, while the middle frontal gyrus and the superior frontal gyrus were affected during more difficult tasks (Diabetes Care 2014 Nov. 17 [doi: 10.2337/dc14-1683]).
Type 2 diabetes is known to expedite brain aging and increase the risk of Alzheimer’s disease. Affected patients can develop deficits in executive functioning, attention, memory, and visuospatial abilities, the researchers noted. To visualize the specific structures affected, the researchers performed functional magnetic resonance imaging (fMRI) of 67 patients from the Beijing Aging Brain Rejuvenation Initiative, a longitudinal study of aging and cognitive impairment in the urban elderly population of Beijing.
Patients in the study had no history of dementia or psychiatric illness, coronary artery disease, nephritis, cancer, or gastrointestinal disease.
Compared with 37 controls, the patients with diabetes performed significantly worse on several working memory tasks, including the backward digit span, the digit span, and the Stroop Color and Word Test, which is an executive function task, the researchers reported.
Patients with diabetes also had less fMRI activation in several frontal brain areas, including the right superior frontal gyrus, the bilateral medial frontal gyrus, and the inferior frontal gyrus. Furthermore, the diabetes group had lower fMRI activation of the left inferior frontal gyrus (Brodmann area 13) in the 1-back versus 0-back (working memory) condition, and had less activation of the left middle frontal gyrus and the superior frontal gyrus in the 2-back versus 0-back comparison, they added. “These findings indicate that with increasing task difficulty, additional frontal brain regions might have been recruited to play compensatory roles to complete the harder task,” they said.
Larger longitudinal studies would be needed to confirm the findings and explore their potential for predicting early cognitive changes in patients with type 2 diabetes, they noted.
The research was funded by the Beijing New Medical Discipline Based Group, the Natural Science Foundation of China, the Institute of Basic Research in Clinical Medicine, the China Academy of Chinese Medical Sciences, and the program for New Century Excellent Talents in University. The researchers declared no conflicts of interest.
Elderly patients with type 2 diabetes had diminished frontal brain activity on functional magnetic resonance imaging that correlated with deficits in working memory and executive function, investigators have reported. The results were published online Nov. 17.
“To our knowledge, this study is the first to detect the specific brain mechanisms related to diabetes-induced working memory dysfunction,” wrote Dr. Yaojing Chen of Beijing Normal University, China.
Patients with diabetes also exhibited varying frontal brain deficits depending on the difficulty of the working memory tasks they were asked to perform. The left inferior frontal gyrus had reduced brain activation during easier tasks, while the middle frontal gyrus and the superior frontal gyrus were affected during more difficult tasks (Diabetes Care 2014 Nov. 17 [doi: 10.2337/dc14-1683]).
Type 2 diabetes is known to expedite brain aging and increase the risk of Alzheimer’s disease. Affected patients can develop deficits in executive functioning, attention, memory, and visuospatial abilities, the researchers noted. To visualize the specific structures affected, the researchers performed functional magnetic resonance imaging (fMRI) of 67 patients from the Beijing Aging Brain Rejuvenation Initiative, a longitudinal study of aging and cognitive impairment in the urban elderly population of Beijing.
Patients in the study had no history of dementia or psychiatric illness, coronary artery disease, nephritis, cancer, or gastrointestinal disease.
Compared with 37 controls, the patients with diabetes performed significantly worse on several working memory tasks, including the backward digit span, the digit span, and the Stroop Color and Word Test, which is an executive function task, the researchers reported.
Patients with diabetes also had less fMRI activation in several frontal brain areas, including the right superior frontal gyrus, the bilateral medial frontal gyrus, and the inferior frontal gyrus. Furthermore, the diabetes group had lower fMRI activation of the left inferior frontal gyrus (Brodmann area 13) in the 1-back versus 0-back (working memory) condition, and had less activation of the left middle frontal gyrus and the superior frontal gyrus in the 2-back versus 0-back comparison, they added. “These findings indicate that with increasing task difficulty, additional frontal brain regions might have been recruited to play compensatory roles to complete the harder task,” they said.
Larger longitudinal studies would be needed to confirm the findings and explore their potential for predicting early cognitive changes in patients with type 2 diabetes, they noted.
The research was funded by the Beijing New Medical Discipline Based Group, the Natural Science Foundation of China, the Institute of Basic Research in Clinical Medicine, the China Academy of Chinese Medical Sciences, and the program for New Century Excellent Talents in University. The researchers declared no conflicts of interest.
Key clinical point: Elderly patients with type 2 diabetes had diminished frontal brain activity that correlated with memory and executive function deficits.
Major finding: Compared with controls, patients with diabetes performed significantly worse on the backward digit span, the digit span, and the Stroop Color and Word Test, and had less fMRI activation in several frontal brain areas.
Data source: Cross-sectional study of 30 elderly patients with type 2 diabetes and 37 healthy controls.
Disclosures: The research was funded by the Beijing New Medical Discipline Based Group, the Natural Science Foundation of China, the Institute of Basic Research in Clinical Medicine, the China Academy of Chinese Medical Sciences, and the New Century Excellent Talents in University. The researchers declared no conflicts of interest.