User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
PAS: Mind-body practices benefit teens with chronic illnesses
SAN DIEGO – Teens with chronic illnesses who participated in a program that consisted of mind-body principles and peer support demonstrated statistically significant positive changes in physical and mental well-being, anger, tension, and stress, in a pilot study.
“Young people living with serious chronic illnesses must deal not only with the physical effects and uncomfortable symptoms of their conditions, but must also endure significant psychosocial effects,” Dr. Brittany Blockman said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “As we learn more about the impact of stress not only on mental health but also physical health, it will be important to design interventions and programs that address this vital, yet sometimes intangible area of health.”
Mind-body medicine focuses on the ways in which emotional, mental, social, and spiritual factors can directly impact health, said Dr. Blockman, a resident physician for the University of California, San Francisco’s Pediatric Leadership for the Underserved program. “Mind-body skills can enhance an individual’s sense of control and have been demonstrated to lower sympathetic arousal, decrease anxiety, and improve mood. Some examples of these modalities include meditation, mindfulness practices, breath work, yoga, biofeedback, and guided imagery. There is a growing body of literature reporting the positive physical and psychological health benefits of mind-body group interventions in various adult populations living with chronic illnesses, including improvements in quality of life, mood, pain, stress, coping skills, disease progression, and mortality. However, there is limited research exploring similar mind-body and group support interventions in pediatric and adolescent populations.”
Dr. Blockman and her associates enrolled 10 teens aged 13-18 years with chronic illnesses, and their parents, to study the impact of Communitas, a novel program that provides mind-body skills and peer support. Teens and parents met in separate groups 10 times for 2 hours at a time. Each session consisted of meditation, a lesson on guided imagery or some other mind-body technique, an exercise, and group sharing. The National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS) Global Health Scale, the Profile of Mood States (POMS), the Brief COPE scale, the Mindfulness Attention Awareness Scale (MAAS), the Perceived Stress Scale, and the Resilience Scale were administered before and after the 10 sessions.
The majority of teens (80%) were female; they attended an average of 7.3 sessions. Illnesses represented included juvenile idiopathic arthritis, cerebral palsy, type 1 diabetes, cancer, muscular dystrophy, cystic fibrosis, lung disease, spinal cord injury, and Wegener’s granulomatosis. Of the nine parents who participated, 75% were female; they completed an average of 7.5 sessions.
When Dr. Blockman and her associates compared baseline teen responses with those immediately after the 10-session intervention, they observed statistically significant effects on a number of scales, including the physical well-being subscale of the NIH PROMIS Global Health Scale (a mean increase of 1.56; P = .047); the mental well-being subscale of the NIH PROMIS Global Health Scale (a mean increase of 2.33; P = .025); the anger subscale of the POMS (a mean decrease of .54; P = .039); the tension subscale of the POMS (a mean decrease of .78; P = .006); the distraction/disengagement subscale of the Brief Cope (a mean decrease of .84; P = .035); and the Perceived Stress Scale (a mean decrease of 3.89; P = .005). The results for adults are still being analyzed.
“I was surprised at how willing the adolescents were to participate in the intervention and how engaged they were in the practices that were taught,” Dr. Blockman said. “It is our hope that the results of this study are helpful in thinking about the types of innovative interventions that may be useful in treating, healing, and supporting adolescents living with chronic illness, as well as their family members.”
She acknowledged certain limitations of the study, including the small sample size and the fact that there was no comparison group. “In the future, we plan to test our intervention further in a controlled trial design study, with a larger sample size. Another limitation is we cannot easily evaluate whether our positive results were attributable to the intervention content or the supportive nature of the intervention group. Our qualitative data may help us in teasing this apart.”
Dr. Blockman reported having no relevant financial conflicts.
On Twitter @dougbrunk
SAN DIEGO – Teens with chronic illnesses who participated in a program that consisted of mind-body principles and peer support demonstrated statistically significant positive changes in physical and mental well-being, anger, tension, and stress, in a pilot study.
“Young people living with serious chronic illnesses must deal not only with the physical effects and uncomfortable symptoms of their conditions, but must also endure significant psychosocial effects,” Dr. Brittany Blockman said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “As we learn more about the impact of stress not only on mental health but also physical health, it will be important to design interventions and programs that address this vital, yet sometimes intangible area of health.”
Mind-body medicine focuses on the ways in which emotional, mental, social, and spiritual factors can directly impact health, said Dr. Blockman, a resident physician for the University of California, San Francisco’s Pediatric Leadership for the Underserved program. “Mind-body skills can enhance an individual’s sense of control and have been demonstrated to lower sympathetic arousal, decrease anxiety, and improve mood. Some examples of these modalities include meditation, mindfulness practices, breath work, yoga, biofeedback, and guided imagery. There is a growing body of literature reporting the positive physical and psychological health benefits of mind-body group interventions in various adult populations living with chronic illnesses, including improvements in quality of life, mood, pain, stress, coping skills, disease progression, and mortality. However, there is limited research exploring similar mind-body and group support interventions in pediatric and adolescent populations.”
Dr. Blockman and her associates enrolled 10 teens aged 13-18 years with chronic illnesses, and their parents, to study the impact of Communitas, a novel program that provides mind-body skills and peer support. Teens and parents met in separate groups 10 times for 2 hours at a time. Each session consisted of meditation, a lesson on guided imagery or some other mind-body technique, an exercise, and group sharing. The National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS) Global Health Scale, the Profile of Mood States (POMS), the Brief COPE scale, the Mindfulness Attention Awareness Scale (MAAS), the Perceived Stress Scale, and the Resilience Scale were administered before and after the 10 sessions.
The majority of teens (80%) were female; they attended an average of 7.3 sessions. Illnesses represented included juvenile idiopathic arthritis, cerebral palsy, type 1 diabetes, cancer, muscular dystrophy, cystic fibrosis, lung disease, spinal cord injury, and Wegener’s granulomatosis. Of the nine parents who participated, 75% were female; they completed an average of 7.5 sessions.
When Dr. Blockman and her associates compared baseline teen responses with those immediately after the 10-session intervention, they observed statistically significant effects on a number of scales, including the physical well-being subscale of the NIH PROMIS Global Health Scale (a mean increase of 1.56; P = .047); the mental well-being subscale of the NIH PROMIS Global Health Scale (a mean increase of 2.33; P = .025); the anger subscale of the POMS (a mean decrease of .54; P = .039); the tension subscale of the POMS (a mean decrease of .78; P = .006); the distraction/disengagement subscale of the Brief Cope (a mean decrease of .84; P = .035); and the Perceived Stress Scale (a mean decrease of 3.89; P = .005). The results for adults are still being analyzed.
“I was surprised at how willing the adolescents were to participate in the intervention and how engaged they were in the practices that were taught,” Dr. Blockman said. “It is our hope that the results of this study are helpful in thinking about the types of innovative interventions that may be useful in treating, healing, and supporting adolescents living with chronic illness, as well as their family members.”
She acknowledged certain limitations of the study, including the small sample size and the fact that there was no comparison group. “In the future, we plan to test our intervention further in a controlled trial design study, with a larger sample size. Another limitation is we cannot easily evaluate whether our positive results were attributable to the intervention content or the supportive nature of the intervention group. Our qualitative data may help us in teasing this apart.”
Dr. Blockman reported having no relevant financial conflicts.
On Twitter @dougbrunk
SAN DIEGO – Teens with chronic illnesses who participated in a program that consisted of mind-body principles and peer support demonstrated statistically significant positive changes in physical and mental well-being, anger, tension, and stress, in a pilot study.
“Young people living with serious chronic illnesses must deal not only with the physical effects and uncomfortable symptoms of their conditions, but must also endure significant psychosocial effects,” Dr. Brittany Blockman said in an interview in advance of the annual meeting of the Pediatric Academic Societies. “As we learn more about the impact of stress not only on mental health but also physical health, it will be important to design interventions and programs that address this vital, yet sometimes intangible area of health.”
Mind-body medicine focuses on the ways in which emotional, mental, social, and spiritual factors can directly impact health, said Dr. Blockman, a resident physician for the University of California, San Francisco’s Pediatric Leadership for the Underserved program. “Mind-body skills can enhance an individual’s sense of control and have been demonstrated to lower sympathetic arousal, decrease anxiety, and improve mood. Some examples of these modalities include meditation, mindfulness practices, breath work, yoga, biofeedback, and guided imagery. There is a growing body of literature reporting the positive physical and psychological health benefits of mind-body group interventions in various adult populations living with chronic illnesses, including improvements in quality of life, mood, pain, stress, coping skills, disease progression, and mortality. However, there is limited research exploring similar mind-body and group support interventions in pediatric and adolescent populations.”
Dr. Blockman and her associates enrolled 10 teens aged 13-18 years with chronic illnesses, and their parents, to study the impact of Communitas, a novel program that provides mind-body skills and peer support. Teens and parents met in separate groups 10 times for 2 hours at a time. Each session consisted of meditation, a lesson on guided imagery or some other mind-body technique, an exercise, and group sharing. The National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS) Global Health Scale, the Profile of Mood States (POMS), the Brief COPE scale, the Mindfulness Attention Awareness Scale (MAAS), the Perceived Stress Scale, and the Resilience Scale were administered before and after the 10 sessions.
The majority of teens (80%) were female; they attended an average of 7.3 sessions. Illnesses represented included juvenile idiopathic arthritis, cerebral palsy, type 1 diabetes, cancer, muscular dystrophy, cystic fibrosis, lung disease, spinal cord injury, and Wegener’s granulomatosis. Of the nine parents who participated, 75% were female; they completed an average of 7.5 sessions.
When Dr. Blockman and her associates compared baseline teen responses with those immediately after the 10-session intervention, they observed statistically significant effects on a number of scales, including the physical well-being subscale of the NIH PROMIS Global Health Scale (a mean increase of 1.56; P = .047); the mental well-being subscale of the NIH PROMIS Global Health Scale (a mean increase of 2.33; P = .025); the anger subscale of the POMS (a mean decrease of .54; P = .039); the tension subscale of the POMS (a mean decrease of .78; P = .006); the distraction/disengagement subscale of the Brief Cope (a mean decrease of .84; P = .035); and the Perceived Stress Scale (a mean decrease of 3.89; P = .005). The results for adults are still being analyzed.
“I was surprised at how willing the adolescents were to participate in the intervention and how engaged they were in the practices that were taught,” Dr. Blockman said. “It is our hope that the results of this study are helpful in thinking about the types of innovative interventions that may be useful in treating, healing, and supporting adolescents living with chronic illness, as well as their family members.”
She acknowledged certain limitations of the study, including the small sample size and the fact that there was no comparison group. “In the future, we plan to test our intervention further in a controlled trial design study, with a larger sample size. Another limitation is we cannot easily evaluate whether our positive results were attributable to the intervention content or the supportive nature of the intervention group. Our qualitative data may help us in teasing this apart.”
Dr. Blockman reported having no relevant financial conflicts.
On Twitter @dougbrunk
AT THE PAS ANNUAL MEETING
Key clinical point: A mind-body and peer support program helps teens with chronic illness.
Major finding: Teens who participated in up to 10 sessions of a mind-body intervention experienced statistically significant effects on the physical well-being subscale of the NIH PROMIS Global Health Scale (a mean increase of 1.56; P = .047), the mental well-being subscale of the NIH PROMIS Global Health Scale (a mean increase of 2.33; P = .025), and the anger subscale of the Profile of Mood States (a mean decrease of .54; P =. 039).
Data source: A study of 10 teens aged 13-18 years with chronic illnesses who attended a program that provides mind-body skills and peer support.
Disclosures:Dr. Blockman reported having no relevant financial conflicts.
ILC: Liraglutide shows NASH benefit in small trial
VIENNA – The oral hypoglycemic liraglutide produced significant histologic resolution of nonalcoholic steatohepatitis after nearly a year of treatment in a phase II randomized trial of 52 patients.
Although liraglutide (Victoza) is already available in the United States and Europe for treating diabetes, its use specifically for treating nonalcoholic steatohepatitis (NASH), advanced fatty liver disease with currently no approved treatment, should await results from a larger, phase III trial, Dr. Matthew J. Armstrong said at the meeting sponsored by the European Association for the Study of the Liver.
“Oral diabetes drugs have been extensively investigated for NASH, but this is the first time a GLP [glucagon-like peptide]-1 analog has been looked at for NASH in a randomized trial with placebo control,” said Dr. Armstrong, a researcher at the Centre for Liver Research of the University of Birmingham, England. “We feel that GLP-1 analogs like liraglutide give you the whole package” of improving lipid levels, weight, and glycemic control while also addressing liver disease.
“One of the biggest killers in patients with NASH is cardiovascular disease. The weight reduction and improved glycemic control and lipids should improve overall cardiovascular outcomes,” Dr. Armstrong said during a press conference at the meeting.
The issue with a drug like liraglutide is “will it have an effect independent of weight loss,” said Dr. Markus Peck-Radosavljevic, vice-chairman at the department of gastroenterology and hepatology at the University of Vienna. “We know that weight loss will help patients with nonalcoholic fatty-liver disease, but the problem is that patients often do not lose weight. What we would like is a drug with a beneficial effect that is independent of weight loss.”
Liraglutide, an analog of the gut satiety hormone GLP-1 but with extended physiologic half-life, “seems to have an effect independent of weight loss,” Dr. Armstrong said. In addition to its documented efficacy for producing weight loss and decreasing glycosylated hemoglobin A1c, liraglutide showed activity in animal and in vitro models for improving liver-enzyme levels, oxidative stress, and hepatic steatosis.
The Liraglutide Efficacy and Action in Nonalcoholic steatohepatitis (LEAN) trial randomized 52 patients aged 18-60 years with biopsy-confirmed NASH to daily liraglutide treatment or placebo for 48 weeks. Researchers at four U.K. centers uptitrated patients on liraglutide to 1.8 mg/day over the study’s first 2 weeks and then kept patents at that dosage. The average age of the patients was 51 years, a majority were men, a third had type 2 diabetes, the average hemoglobin A1c was 6.0%, and the average body mass index was more than 35 kg/m2.
After 48 weeks on treatment, follow-up biopsies on 23 of the patients on liraglutide and 22 on placebo showed 9 liraglutide patients (39%) had resolution of their NASH without worsening fibrosis, the study’s primary endpoint, compared with 2 NASH resolutions (9%) in the control arm, Dr. Armstrong reported.
The 39% efficacy rate for resolutions surpassed the study’s prespecified threshold of 38% to define effective treatment.
Liraglutide-treated patients showed improvements in several other clinical and metabolic parameters including fibrosis and steatosis, as well as an average 5-kg greater weight loss compared with placebo and a 0.45% drop in hemoglobin A1c compared with placebo that fell short of statistical significance.
The safety analysis showed that liraglutide was “well tolerated with an acceptable safety profile in NASH patients,” Dr. Armstrong said. The phase II study was “as much about safety as efficacy,” as the feasibility of treating NASH patients with liraglutide had been uncertain until now.
On Twitter @mitchelzoler
VIENNA – The oral hypoglycemic liraglutide produced significant histologic resolution of nonalcoholic steatohepatitis after nearly a year of treatment in a phase II randomized trial of 52 patients.
Although liraglutide (Victoza) is already available in the United States and Europe for treating diabetes, its use specifically for treating nonalcoholic steatohepatitis (NASH), advanced fatty liver disease with currently no approved treatment, should await results from a larger, phase III trial, Dr. Matthew J. Armstrong said at the meeting sponsored by the European Association for the Study of the Liver.
“Oral diabetes drugs have been extensively investigated for NASH, but this is the first time a GLP [glucagon-like peptide]-1 analog has been looked at for NASH in a randomized trial with placebo control,” said Dr. Armstrong, a researcher at the Centre for Liver Research of the University of Birmingham, England. “We feel that GLP-1 analogs like liraglutide give you the whole package” of improving lipid levels, weight, and glycemic control while also addressing liver disease.
“One of the biggest killers in patients with NASH is cardiovascular disease. The weight reduction and improved glycemic control and lipids should improve overall cardiovascular outcomes,” Dr. Armstrong said during a press conference at the meeting.
The issue with a drug like liraglutide is “will it have an effect independent of weight loss,” said Dr. Markus Peck-Radosavljevic, vice-chairman at the department of gastroenterology and hepatology at the University of Vienna. “We know that weight loss will help patients with nonalcoholic fatty-liver disease, but the problem is that patients often do not lose weight. What we would like is a drug with a beneficial effect that is independent of weight loss.”
Liraglutide, an analog of the gut satiety hormone GLP-1 but with extended physiologic half-life, “seems to have an effect independent of weight loss,” Dr. Armstrong said. In addition to its documented efficacy for producing weight loss and decreasing glycosylated hemoglobin A1c, liraglutide showed activity in animal and in vitro models for improving liver-enzyme levels, oxidative stress, and hepatic steatosis.
The Liraglutide Efficacy and Action in Nonalcoholic steatohepatitis (LEAN) trial randomized 52 patients aged 18-60 years with biopsy-confirmed NASH to daily liraglutide treatment or placebo for 48 weeks. Researchers at four U.K. centers uptitrated patients on liraglutide to 1.8 mg/day over the study’s first 2 weeks and then kept patents at that dosage. The average age of the patients was 51 years, a majority were men, a third had type 2 diabetes, the average hemoglobin A1c was 6.0%, and the average body mass index was more than 35 kg/m2.
After 48 weeks on treatment, follow-up biopsies on 23 of the patients on liraglutide and 22 on placebo showed 9 liraglutide patients (39%) had resolution of their NASH without worsening fibrosis, the study’s primary endpoint, compared with 2 NASH resolutions (9%) in the control arm, Dr. Armstrong reported.
The 39% efficacy rate for resolutions surpassed the study’s prespecified threshold of 38% to define effective treatment.
Liraglutide-treated patients showed improvements in several other clinical and metabolic parameters including fibrosis and steatosis, as well as an average 5-kg greater weight loss compared with placebo and a 0.45% drop in hemoglobin A1c compared with placebo that fell short of statistical significance.
The safety analysis showed that liraglutide was “well tolerated with an acceptable safety profile in NASH patients,” Dr. Armstrong said. The phase II study was “as much about safety as efficacy,” as the feasibility of treating NASH patients with liraglutide had been uncertain until now.
On Twitter @mitchelzoler
VIENNA – The oral hypoglycemic liraglutide produced significant histologic resolution of nonalcoholic steatohepatitis after nearly a year of treatment in a phase II randomized trial of 52 patients.
Although liraglutide (Victoza) is already available in the United States and Europe for treating diabetes, its use specifically for treating nonalcoholic steatohepatitis (NASH), advanced fatty liver disease with currently no approved treatment, should await results from a larger, phase III trial, Dr. Matthew J. Armstrong said at the meeting sponsored by the European Association for the Study of the Liver.
“Oral diabetes drugs have been extensively investigated for NASH, but this is the first time a GLP [glucagon-like peptide]-1 analog has been looked at for NASH in a randomized trial with placebo control,” said Dr. Armstrong, a researcher at the Centre for Liver Research of the University of Birmingham, England. “We feel that GLP-1 analogs like liraglutide give you the whole package” of improving lipid levels, weight, and glycemic control while also addressing liver disease.
“One of the biggest killers in patients with NASH is cardiovascular disease. The weight reduction and improved glycemic control and lipids should improve overall cardiovascular outcomes,” Dr. Armstrong said during a press conference at the meeting.
The issue with a drug like liraglutide is “will it have an effect independent of weight loss,” said Dr. Markus Peck-Radosavljevic, vice-chairman at the department of gastroenterology and hepatology at the University of Vienna. “We know that weight loss will help patients with nonalcoholic fatty-liver disease, but the problem is that patients often do not lose weight. What we would like is a drug with a beneficial effect that is independent of weight loss.”
Liraglutide, an analog of the gut satiety hormone GLP-1 but with extended physiologic half-life, “seems to have an effect independent of weight loss,” Dr. Armstrong said. In addition to its documented efficacy for producing weight loss and decreasing glycosylated hemoglobin A1c, liraglutide showed activity in animal and in vitro models for improving liver-enzyme levels, oxidative stress, and hepatic steatosis.
The Liraglutide Efficacy and Action in Nonalcoholic steatohepatitis (LEAN) trial randomized 52 patients aged 18-60 years with biopsy-confirmed NASH to daily liraglutide treatment or placebo for 48 weeks. Researchers at four U.K. centers uptitrated patients on liraglutide to 1.8 mg/day over the study’s first 2 weeks and then kept patents at that dosage. The average age of the patients was 51 years, a majority were men, a third had type 2 diabetes, the average hemoglobin A1c was 6.0%, and the average body mass index was more than 35 kg/m2.
After 48 weeks on treatment, follow-up biopsies on 23 of the patients on liraglutide and 22 on placebo showed 9 liraglutide patients (39%) had resolution of their NASH without worsening fibrosis, the study’s primary endpoint, compared with 2 NASH resolutions (9%) in the control arm, Dr. Armstrong reported.
The 39% efficacy rate for resolutions surpassed the study’s prespecified threshold of 38% to define effective treatment.
Liraglutide-treated patients showed improvements in several other clinical and metabolic parameters including fibrosis and steatosis, as well as an average 5-kg greater weight loss compared with placebo and a 0.45% drop in hemoglobin A1c compared with placebo that fell short of statistical significance.
The safety analysis showed that liraglutide was “well tolerated with an acceptable safety profile in NASH patients,” Dr. Armstrong said. The phase II study was “as much about safety as efficacy,” as the feasibility of treating NASH patients with liraglutide had been uncertain until now.
On Twitter @mitchelzoler
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: Daily liraglutide injections for 48 weeks safely increased resolution of nonalcoholic steatohepatitis.
Major finding: NASH resolved in 39% of liraglutide-treated patients and 9% of controls.
Data source: LEAN, a randomized trial of 52 patients with biopsy-proven NASH enrolled at four U.K. centers.
Disclosures: LEAN and Dr. Armstrong received educational grants and free study medication from Novo Nordisk, the company that markets liraglutide (Victoza).
Cystic fibrosis–related diabetes requires different approach
SAN DIEGO – Cystic fibrosis–related diabetes is a unique disease, and it requires a different mindset on the part of the treating physician.
“The risk of cardiovascular death drives a lot of the recommendations for management of our patients with type 1 and type 2 diabetes, but this doesn’t apply in cystic fibrosis. Patients with cystic fibrosis–related diabetes do not appear to get macrovascular complications. These patients have other, more important concerns – namely, survival. They die from their CF lung disease. Diabetes is important, but we have to remember that in CF, lung function and nutrition come first. It’s our job to work around that,” Dr. Antoinette Moran asserted at the annual meeting of the Pediatric Academic Societies.
Diabetes is the most common comorbidity associated with CF. And it spells big trouble. It’s associated with pancreatic insufficiency, liver dysfunction, requirement for corticosteroids, and prognostically with undernutrition, worse pulmonary function, and early death, noted Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis.
The prevalence of cystic fibrosis–related diabetes (CFRD) is age related. It’s rare in children, but the prevalence climbs to about 15% in adolescents, 40% in 20- to 39-year-olds, and 55% after age 40.
“In fact, more than 80% of CF patients with the most severe mutations have diabetes by the time they’re 40,” according to Dr. Moran, who was lead author of CFRD management guidelines released last year by the International Society for Pediatric and Adolescent Diabetes (Pediatr. Diabetes 2014 Sep;15 Suppl 20:65-76).
CFRD is not an autoimmune disease. Ketones are rare. Glycosylated hemoglobin levels are spuriously low. And the definitive treatment for CFRD is insulin.
“Remember, you’re not just treating hyperglycemia, you’re treating insulin deficiency. Insulin deficiency is really the hallmark of this disease. It is progressive and eventually severe, but not complete – unlike in type 1 diabetes,” she observed. “Treatment of patients in their well state is similar to treating type 1 diabetes in the honeymoon phase. However, during acute illness patients become extremely insulin resistant. It’s a black hole that you can pour insulin into, and sometimes you can’t get them to budge. Then a couple of months later they’re insulin sensitive again.”
Multiple studies have demonstrated that diabetes has a negative impact upon survival in patients with CF. Both hyperglycemia and insulin insufficiency have negative impacts upon the CF lung disease.
Insulin is a potent anabolic hormone that’s necessary for maintenance of body weight and lean body mass, and insulin insufficiency leads to a catabolic state which accelerates pulmonary decline in CF. Studies show that nutritional status and pulmonary function start to decline in CF patients several years before they’re diagnosed with diabetes. Thus, by the time CFRD is diagnosed, patients have already experienced several years of insulin insufficiency, with adverse consequences.
Moreover, when blood glucose levels exceed 144 mg/dL, glucose appears in the airways of CF patients. That’s not good. It probably promotes pulmonary infection. Anecdotal evidence suggests hyperglycemia makes sputum thicker and more difficult to clear as well as boosting bacterial growth. And continuous glucose monitoring studies conducted in patients with CFRD indicate they spend roughly half of each day with a blood glucose in excess of 144 mg/dL.
Aggressive screening and early initiation of insulin therapy help reverse chronic weight loss and reduce mortality in patients with CFRD. The various guidelines recommend annual screening for diabetes in CF patients starting by age 10.
“I personally believe it should begin much earlier than that,” Dr. Moran said, citing a study led by her Minnesota colleague Dr. Katie L. Ode that showed that abnormal glucose tolerance was already present in 41% of children with CF at ages 6-9, and that those children had a high rate of early-onset CFRD (Pediatr. Diabetes 2010 Nov;11:487-92).
The oral glucose tolerance test, performed when the patient is clinically stable, is the screening tool of choice for CFRD.
“It’s not that it’s such a great test – we all know it has problems – but the other tests perform poorly in CF. And a diagnosis based upon an oral glucose tolerance test correlates with prognosis and future outcomes, so you get meaningful data when you do it,” she explained.
Evidence-based guidelines for CFRD put forth jointly by the American Diabetes Association, Cystic Fibrosis Foundation, and Lawson Wilkins Pediatric Endocrinology Society (Diabetes Care 2010;33:2697-2708) emphasize that, unlike in patients without CF, the diagnosis of CFRD can be made while a patient is hospitalized with an acute illness. The criterion is fasting or postprandial hyperglycemia persisting for more than 48 hours after hospitalization.
“Why are we calling this diabetes? These patients have repeated bouts of acute illness. The CF patient you’re seeing today in the hospital may very well be back in 5 months, and again 2 months after that. It’s a frequent event in these patients, and when their diabetes persists for longer than 48 hours it tends to persist for weeks before their need for insulin goes away until the next time they get sick. But most of these patients spend a substantial amount of time each year hyperglycemic. And most importantly, if you use as your date of diagnosis diabetes that’s present at the time of an acute illness, it correlates with microvascular complications and with mortality. So it establishes a meaningful start point for future risk,” Dr. Moran said.
She reported financial relationships with Novo Nordisk and Vertex.
SAN DIEGO – Cystic fibrosis–related diabetes is a unique disease, and it requires a different mindset on the part of the treating physician.
“The risk of cardiovascular death drives a lot of the recommendations for management of our patients with type 1 and type 2 diabetes, but this doesn’t apply in cystic fibrosis. Patients with cystic fibrosis–related diabetes do not appear to get macrovascular complications. These patients have other, more important concerns – namely, survival. They die from their CF lung disease. Diabetes is important, but we have to remember that in CF, lung function and nutrition come first. It’s our job to work around that,” Dr. Antoinette Moran asserted at the annual meeting of the Pediatric Academic Societies.
Diabetes is the most common comorbidity associated with CF. And it spells big trouble. It’s associated with pancreatic insufficiency, liver dysfunction, requirement for corticosteroids, and prognostically with undernutrition, worse pulmonary function, and early death, noted Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis.
The prevalence of cystic fibrosis–related diabetes (CFRD) is age related. It’s rare in children, but the prevalence climbs to about 15% in adolescents, 40% in 20- to 39-year-olds, and 55% after age 40.
“In fact, more than 80% of CF patients with the most severe mutations have diabetes by the time they’re 40,” according to Dr. Moran, who was lead author of CFRD management guidelines released last year by the International Society for Pediatric and Adolescent Diabetes (Pediatr. Diabetes 2014 Sep;15 Suppl 20:65-76).
CFRD is not an autoimmune disease. Ketones are rare. Glycosylated hemoglobin levels are spuriously low. And the definitive treatment for CFRD is insulin.
“Remember, you’re not just treating hyperglycemia, you’re treating insulin deficiency. Insulin deficiency is really the hallmark of this disease. It is progressive and eventually severe, but not complete – unlike in type 1 diabetes,” she observed. “Treatment of patients in their well state is similar to treating type 1 diabetes in the honeymoon phase. However, during acute illness patients become extremely insulin resistant. It’s a black hole that you can pour insulin into, and sometimes you can’t get them to budge. Then a couple of months later they’re insulin sensitive again.”
Multiple studies have demonstrated that diabetes has a negative impact upon survival in patients with CF. Both hyperglycemia and insulin insufficiency have negative impacts upon the CF lung disease.
Insulin is a potent anabolic hormone that’s necessary for maintenance of body weight and lean body mass, and insulin insufficiency leads to a catabolic state which accelerates pulmonary decline in CF. Studies show that nutritional status and pulmonary function start to decline in CF patients several years before they’re diagnosed with diabetes. Thus, by the time CFRD is diagnosed, patients have already experienced several years of insulin insufficiency, with adverse consequences.
Moreover, when blood glucose levels exceed 144 mg/dL, glucose appears in the airways of CF patients. That’s not good. It probably promotes pulmonary infection. Anecdotal evidence suggests hyperglycemia makes sputum thicker and more difficult to clear as well as boosting bacterial growth. And continuous glucose monitoring studies conducted in patients with CFRD indicate they spend roughly half of each day with a blood glucose in excess of 144 mg/dL.
Aggressive screening and early initiation of insulin therapy help reverse chronic weight loss and reduce mortality in patients with CFRD. The various guidelines recommend annual screening for diabetes in CF patients starting by age 10.
“I personally believe it should begin much earlier than that,” Dr. Moran said, citing a study led by her Minnesota colleague Dr. Katie L. Ode that showed that abnormal glucose tolerance was already present in 41% of children with CF at ages 6-9, and that those children had a high rate of early-onset CFRD (Pediatr. Diabetes 2010 Nov;11:487-92).
The oral glucose tolerance test, performed when the patient is clinically stable, is the screening tool of choice for CFRD.
“It’s not that it’s such a great test – we all know it has problems – but the other tests perform poorly in CF. And a diagnosis based upon an oral glucose tolerance test correlates with prognosis and future outcomes, so you get meaningful data when you do it,” she explained.
Evidence-based guidelines for CFRD put forth jointly by the American Diabetes Association, Cystic Fibrosis Foundation, and Lawson Wilkins Pediatric Endocrinology Society (Diabetes Care 2010;33:2697-2708) emphasize that, unlike in patients without CF, the diagnosis of CFRD can be made while a patient is hospitalized with an acute illness. The criterion is fasting or postprandial hyperglycemia persisting for more than 48 hours after hospitalization.
“Why are we calling this diabetes? These patients have repeated bouts of acute illness. The CF patient you’re seeing today in the hospital may very well be back in 5 months, and again 2 months after that. It’s a frequent event in these patients, and when their diabetes persists for longer than 48 hours it tends to persist for weeks before their need for insulin goes away until the next time they get sick. But most of these patients spend a substantial amount of time each year hyperglycemic. And most importantly, if you use as your date of diagnosis diabetes that’s present at the time of an acute illness, it correlates with microvascular complications and with mortality. So it establishes a meaningful start point for future risk,” Dr. Moran said.
She reported financial relationships with Novo Nordisk and Vertex.
SAN DIEGO – Cystic fibrosis–related diabetes is a unique disease, and it requires a different mindset on the part of the treating physician.
“The risk of cardiovascular death drives a lot of the recommendations for management of our patients with type 1 and type 2 diabetes, but this doesn’t apply in cystic fibrosis. Patients with cystic fibrosis–related diabetes do not appear to get macrovascular complications. These patients have other, more important concerns – namely, survival. They die from their CF lung disease. Diabetes is important, but we have to remember that in CF, lung function and nutrition come first. It’s our job to work around that,” Dr. Antoinette Moran asserted at the annual meeting of the Pediatric Academic Societies.
Diabetes is the most common comorbidity associated with CF. And it spells big trouble. It’s associated with pancreatic insufficiency, liver dysfunction, requirement for corticosteroids, and prognostically with undernutrition, worse pulmonary function, and early death, noted Dr. Moran, professor of pediatrics and chief of the division of pediatric endocrinology and diabetes at the University of Minnesota, Minneapolis.
The prevalence of cystic fibrosis–related diabetes (CFRD) is age related. It’s rare in children, but the prevalence climbs to about 15% in adolescents, 40% in 20- to 39-year-olds, and 55% after age 40.
“In fact, more than 80% of CF patients with the most severe mutations have diabetes by the time they’re 40,” according to Dr. Moran, who was lead author of CFRD management guidelines released last year by the International Society for Pediatric and Adolescent Diabetes (Pediatr. Diabetes 2014 Sep;15 Suppl 20:65-76).
CFRD is not an autoimmune disease. Ketones are rare. Glycosylated hemoglobin levels are spuriously low. And the definitive treatment for CFRD is insulin.
“Remember, you’re not just treating hyperglycemia, you’re treating insulin deficiency. Insulin deficiency is really the hallmark of this disease. It is progressive and eventually severe, but not complete – unlike in type 1 diabetes,” she observed. “Treatment of patients in their well state is similar to treating type 1 diabetes in the honeymoon phase. However, during acute illness patients become extremely insulin resistant. It’s a black hole that you can pour insulin into, and sometimes you can’t get them to budge. Then a couple of months later they’re insulin sensitive again.”
Multiple studies have demonstrated that diabetes has a negative impact upon survival in patients with CF. Both hyperglycemia and insulin insufficiency have negative impacts upon the CF lung disease.
Insulin is a potent anabolic hormone that’s necessary for maintenance of body weight and lean body mass, and insulin insufficiency leads to a catabolic state which accelerates pulmonary decline in CF. Studies show that nutritional status and pulmonary function start to decline in CF patients several years before they’re diagnosed with diabetes. Thus, by the time CFRD is diagnosed, patients have already experienced several years of insulin insufficiency, with adverse consequences.
Moreover, when blood glucose levels exceed 144 mg/dL, glucose appears in the airways of CF patients. That’s not good. It probably promotes pulmonary infection. Anecdotal evidence suggests hyperglycemia makes sputum thicker and more difficult to clear as well as boosting bacterial growth. And continuous glucose monitoring studies conducted in patients with CFRD indicate they spend roughly half of each day with a blood glucose in excess of 144 mg/dL.
Aggressive screening and early initiation of insulin therapy help reverse chronic weight loss and reduce mortality in patients with CFRD. The various guidelines recommend annual screening for diabetes in CF patients starting by age 10.
“I personally believe it should begin much earlier than that,” Dr. Moran said, citing a study led by her Minnesota colleague Dr. Katie L. Ode that showed that abnormal glucose tolerance was already present in 41% of children with CF at ages 6-9, and that those children had a high rate of early-onset CFRD (Pediatr. Diabetes 2010 Nov;11:487-92).
The oral glucose tolerance test, performed when the patient is clinically stable, is the screening tool of choice for CFRD.
“It’s not that it’s such a great test – we all know it has problems – but the other tests perform poorly in CF. And a diagnosis based upon an oral glucose tolerance test correlates with prognosis and future outcomes, so you get meaningful data when you do it,” she explained.
Evidence-based guidelines for CFRD put forth jointly by the American Diabetes Association, Cystic Fibrosis Foundation, and Lawson Wilkins Pediatric Endocrinology Society (Diabetes Care 2010;33:2697-2708) emphasize that, unlike in patients without CF, the diagnosis of CFRD can be made while a patient is hospitalized with an acute illness. The criterion is fasting or postprandial hyperglycemia persisting for more than 48 hours after hospitalization.
“Why are we calling this diabetes? These patients have repeated bouts of acute illness. The CF patient you’re seeing today in the hospital may very well be back in 5 months, and again 2 months after that. It’s a frequent event in these patients, and when their diabetes persists for longer than 48 hours it tends to persist for weeks before their need for insulin goes away until the next time they get sick. But most of these patients spend a substantial amount of time each year hyperglycemic. And most importantly, if you use as your date of diagnosis diabetes that’s present at the time of an acute illness, it correlates with microvascular complications and with mortality. So it establishes a meaningful start point for future risk,” Dr. Moran said.
She reported financial relationships with Novo Nordisk and Vertex.
EXPERT ANALYSIS FROM THE PAS ANNUAL MEETING
Obesity increases risk of bleeding on warfarin
Obese patients on warfarin may be at greater risk of bleeding than those of normal weight, according to a study presented at the American Heart Association’s Arteriosclerosis, Thrombosis, and Vascular Biology/Peripheral Vascular Disease Scientific Sessions 2015.
Researchers followed 863 patients attending an anticoagulation clinic for 1 year and found that obesity (body mass index greater than 30 kg/m2) was associated with a statistically significant 84% increase in the risk of major bleeds, such as gastrointestinal, intracerebral, and retroperitoneal hemorrhage.
The study also showed that increasing obesity increased bleeding risk; there was a 30% increase in bleeding risk for patients with class I obesity but a 93% increase in patients with class III obesity.
“This result suggests that BMI plays a role in bleeding events in patients on warfarin [and] future studies are needed to understand the mechanism by which obesity increases bleeding risk for patients on warfarin, and whether similar risk exists for the novel oral anticoagulants,” said Dr. Adedotun A. Ogunsua of the University of Massachusetts, Worcester, and coauthors.
There were no conflicts of interest disclosed.
Obese patients on warfarin may be at greater risk of bleeding than those of normal weight, according to a study presented at the American Heart Association’s Arteriosclerosis, Thrombosis, and Vascular Biology/Peripheral Vascular Disease Scientific Sessions 2015.
Researchers followed 863 patients attending an anticoagulation clinic for 1 year and found that obesity (body mass index greater than 30 kg/m2) was associated with a statistically significant 84% increase in the risk of major bleeds, such as gastrointestinal, intracerebral, and retroperitoneal hemorrhage.
The study also showed that increasing obesity increased bleeding risk; there was a 30% increase in bleeding risk for patients with class I obesity but a 93% increase in patients with class III obesity.
“This result suggests that BMI plays a role in bleeding events in patients on warfarin [and] future studies are needed to understand the mechanism by which obesity increases bleeding risk for patients on warfarin, and whether similar risk exists for the novel oral anticoagulants,” said Dr. Adedotun A. Ogunsua of the University of Massachusetts, Worcester, and coauthors.
There were no conflicts of interest disclosed.
Obese patients on warfarin may be at greater risk of bleeding than those of normal weight, according to a study presented at the American Heart Association’s Arteriosclerosis, Thrombosis, and Vascular Biology/Peripheral Vascular Disease Scientific Sessions 2015.
Researchers followed 863 patients attending an anticoagulation clinic for 1 year and found that obesity (body mass index greater than 30 kg/m2) was associated with a statistically significant 84% increase in the risk of major bleeds, such as gastrointestinal, intracerebral, and retroperitoneal hemorrhage.
The study also showed that increasing obesity increased bleeding risk; there was a 30% increase in bleeding risk for patients with class I obesity but a 93% increase in patients with class III obesity.
“This result suggests that BMI plays a role in bleeding events in patients on warfarin [and] future studies are needed to understand the mechanism by which obesity increases bleeding risk for patients on warfarin, and whether similar risk exists for the novel oral anticoagulants,” said Dr. Adedotun A. Ogunsua of the University of Massachusetts, Worcester, and coauthors.
There were no conflicts of interest disclosed.
FROM ATVB/PVD 2015
Key clinical point: Obesity is associated with an increased risk of major bleeding in patients taking warfarin.
Major finding: Obese patients on warfarin had an 84% increased incidence of major bleeding.
Data source: Observational study of 863 patients attending an anticoagulation clinic.
Disclosures: No conflicts of interest were disclosed.
Growth in preventive personalized medicine could increase life expectancy
Americans could see improvements in their quality of life and life expectancy if more of them utilize personalized and precision medicine (PPM), according to an opinion piece by Dr. Victor J. Dzau, president of the Institute of Medicine, Washington, D.C., and his colleagues.
“Applications of personalized and precision medicine aimed at prevention have the potential to generate substantial value for society,” the authors wrote.
This opinion is based on the authors’ analysis of an assessment of the benefits and costs of PPM innovations designed to improve screening and risk-factor stratification technologies for identifying presymptomatic individuals at high risk for specific diseases. Dr. Dzau and his associates assumed that the preventive PPM interventions permanently reduced the incidence of cancer, diabetes, heart disease, hypertension, lung disease, and stroke by a fixed percentage beginning in 2012 and needed to be sustained over a lifetime. Benefits were computed by looking at life expectancy and quality-adjusted life expectancy gains during the subsequent 50 years. Values of health were expressed in dollars using $100,000 per quality-adjusted life year.
According to the assessment performed with the Health Economics Medical Innovation Simulation, a PPM innovation that reduced the incidence of the six aforementioned diseases by 10% would generate between $33 billion and $114 billion per disease in the form of longer lives. When the incidence of the diseases was reduced by 50%, the values of health generated ranged from $161 billion to $607 billion. In both scenarios, reductions in heart disease generated the highest number of quality-adjusted life years.
Dr. Dzau and his associates acknowledged that “PPM treatments that might generate less value overall, but provide greater short-term returns” are favored in the current reimbursement environment.
Read the full paper in the Lancet (2015 [doi:10.1016/S0140-6736(15)60722-X]).
Americans could see improvements in their quality of life and life expectancy if more of them utilize personalized and precision medicine (PPM), according to an opinion piece by Dr. Victor J. Dzau, president of the Institute of Medicine, Washington, D.C., and his colleagues.
“Applications of personalized and precision medicine aimed at prevention have the potential to generate substantial value for society,” the authors wrote.
This opinion is based on the authors’ analysis of an assessment of the benefits and costs of PPM innovations designed to improve screening and risk-factor stratification technologies for identifying presymptomatic individuals at high risk for specific diseases. Dr. Dzau and his associates assumed that the preventive PPM interventions permanently reduced the incidence of cancer, diabetes, heart disease, hypertension, lung disease, and stroke by a fixed percentage beginning in 2012 and needed to be sustained over a lifetime. Benefits were computed by looking at life expectancy and quality-adjusted life expectancy gains during the subsequent 50 years. Values of health were expressed in dollars using $100,000 per quality-adjusted life year.
According to the assessment performed with the Health Economics Medical Innovation Simulation, a PPM innovation that reduced the incidence of the six aforementioned diseases by 10% would generate between $33 billion and $114 billion per disease in the form of longer lives. When the incidence of the diseases was reduced by 50%, the values of health generated ranged from $161 billion to $607 billion. In both scenarios, reductions in heart disease generated the highest number of quality-adjusted life years.
Dr. Dzau and his associates acknowledged that “PPM treatments that might generate less value overall, but provide greater short-term returns” are favored in the current reimbursement environment.
Read the full paper in the Lancet (2015 [doi:10.1016/S0140-6736(15)60722-X]).
Americans could see improvements in their quality of life and life expectancy if more of them utilize personalized and precision medicine (PPM), according to an opinion piece by Dr. Victor J. Dzau, president of the Institute of Medicine, Washington, D.C., and his colleagues.
“Applications of personalized and precision medicine aimed at prevention have the potential to generate substantial value for society,” the authors wrote.
This opinion is based on the authors’ analysis of an assessment of the benefits and costs of PPM innovations designed to improve screening and risk-factor stratification technologies for identifying presymptomatic individuals at high risk for specific diseases. Dr. Dzau and his associates assumed that the preventive PPM interventions permanently reduced the incidence of cancer, diabetes, heart disease, hypertension, lung disease, and stroke by a fixed percentage beginning in 2012 and needed to be sustained over a lifetime. Benefits were computed by looking at life expectancy and quality-adjusted life expectancy gains during the subsequent 50 years. Values of health were expressed in dollars using $100,000 per quality-adjusted life year.
According to the assessment performed with the Health Economics Medical Innovation Simulation, a PPM innovation that reduced the incidence of the six aforementioned diseases by 10% would generate between $33 billion and $114 billion per disease in the form of longer lives. When the incidence of the diseases was reduced by 50%, the values of health generated ranged from $161 billion to $607 billion. In both scenarios, reductions in heart disease generated the highest number of quality-adjusted life years.
Dr. Dzau and his associates acknowledged that “PPM treatments that might generate less value overall, but provide greater short-term returns” are favored in the current reimbursement environment.
Read the full paper in the Lancet (2015 [doi:10.1016/S0140-6736(15)60722-X]).
Avoiding metformin in renal insufficiency
A 47-year-old obese male with type 2 diabetes has been on metformin for the last 2 years with good effect (hemoglobin A1c of 6.8), and with exercise has been able to lose 5-10 pounds. His last two blood tests show creatinine levels of 1.5 and 1.6. What do you recommend?
A) Continue with metformin.
B) Stop metformin, start sulfonylurea.
C) Stop metformin, begin glargine.
D) Stop metformin, begin pioglitazone.
Myth: Metformin should not be used in patients with mild to moderate renal insufficiency because of an increased risk of lactic acidosis.
Metformin is the most commonly used oral agent for the treatment of type 2 diabetes in the United States, but the FDA-approved drug label states that it is contraindicated in patients with an abnormal creatinine clearance or serum creatinine of 1.4 in women and 1.5 in men.<sup/>The concern is for development of lactic acidosis in patients because the renally excreted metformin may build up as a result of decreased renal function.
Metformin was approved for use in the United States in 1995, many years after the drug was introduced in Europe. The first drug in its class, phenformin, was removed from the United States and most European markets in 1977 because of a high incidence of lactic acidosis occurring at therapeutic doses. One in 4,000 patients taking phenformin develops lactic acidosis (J. Emerg. Med. 1998;16:881-6). Phenformin has been shown to cause type B lactic acidosis, without evidence of hypoxia or hypoperfusion, and lactic acidosis because of phenformin carried a 50% mortality rate.
Deep concern for the possibility of a similar problem with metformin played an important role in its delay of availability in the United States. It isn’t clear, however, that diabetes patients on metformin have a higher risk of developing lactic acidosis than diabetes patients who are not on metformin.
A Cochrane review of 347 studies, including 70,490 person-years of metformin use, compared with 55,451 person-years in the nonmetformin group, showed no cases of fatal or nonfatal lactic acidosis in either group (Cochrane Database Syst. Rev. 2010 Apr 14:CD002967). More than half the studies included (53%) allowed for the inclusion of patients with creatinine levels greater than 1.5. There were no differences in lactate levels between metformin-treated patients and patients who did not receive metformin.
In a study using the Saskatchewan Health administrative database, which involved 11,797 patients with 22,296 years of metformin exposure, there were two cases of lactic acidosis (Diabetes Care 1999;22:925-7). This calculates to a rate of 9 cases per 100,000 person years, the same rate as in patients with diabetes who are not taking metformin (9.7 cases per 100,000) (Diabetes Care 1998;21:1659-63).
A recent study looked at the incidence of lactic acidosis in patients taking metformin with and without abnormalities in renal function (Diabetes Care 2014;37:2291-5). There was no statistically significant difference in the rates of lactic acidosis in patients who were on metformin with normal renal function, compared with those with varying degrees of renal insufficiency. The overall rate of lactic acidosis was 10.3 per 100,000 patient years, which is almost identical to the rates in the other studies mentioned, and there were no fatalities.
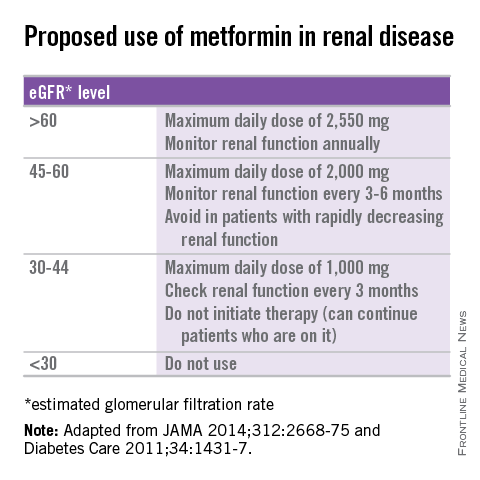
Several recommendations for using metformin in patients with renal insufficiency have been published (see table) (JAMA 2014;312:2668-75; Diabetes Care 2011;34:1431-7). Metformin has shown cardiovascular mortality benefits, compared with sulfonylureas, in the treatment of diabetes (Diabetes Care 2013;36:1304-11). Avoiding its use in patients with mild to moderate renal insufficiency in favor of other treatments that may not be as beneficial and may well lead to worse outcomes.
There is no evidence that metformin increases the lactic acidosis risk in patients with diabetes, but until there is a change in the FDA labeling, physicians will likely continue to be hesitant to use it in patients with renal insufficiency.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is the Rathmann Family Foundation Chair in Patient-Centered Clinical Education. Contact Dr. Paauw at [email protected].
A 47-year-old obese male with type 2 diabetes has been on metformin for the last 2 years with good effect (hemoglobin A1c of 6.8), and with exercise has been able to lose 5-10 pounds. His last two blood tests show creatinine levels of 1.5 and 1.6. What do you recommend?
A) Continue with metformin.
B) Stop metformin, start sulfonylurea.
C) Stop metformin, begin glargine.
D) Stop metformin, begin pioglitazone.
Myth: Metformin should not be used in patients with mild to moderate renal insufficiency because of an increased risk of lactic acidosis.
Metformin is the most commonly used oral agent for the treatment of type 2 diabetes in the United States, but the FDA-approved drug label states that it is contraindicated in patients with an abnormal creatinine clearance or serum creatinine of 1.4 in women and 1.5 in men.<sup/>The concern is for development of lactic acidosis in patients because the renally excreted metformin may build up as a result of decreased renal function.
Metformin was approved for use in the United States in 1995, many years after the drug was introduced in Europe. The first drug in its class, phenformin, was removed from the United States and most European markets in 1977 because of a high incidence of lactic acidosis occurring at therapeutic doses. One in 4,000 patients taking phenformin develops lactic acidosis (J. Emerg. Med. 1998;16:881-6). Phenformin has been shown to cause type B lactic acidosis, without evidence of hypoxia or hypoperfusion, and lactic acidosis because of phenformin carried a 50% mortality rate.
Deep concern for the possibility of a similar problem with metformin played an important role in its delay of availability in the United States. It isn’t clear, however, that diabetes patients on metformin have a higher risk of developing lactic acidosis than diabetes patients who are not on metformin.
A Cochrane review of 347 studies, including 70,490 person-years of metformin use, compared with 55,451 person-years in the nonmetformin group, showed no cases of fatal or nonfatal lactic acidosis in either group (Cochrane Database Syst. Rev. 2010 Apr 14:CD002967). More than half the studies included (53%) allowed for the inclusion of patients with creatinine levels greater than 1.5. There were no differences in lactate levels between metformin-treated patients and patients who did not receive metformin.
In a study using the Saskatchewan Health administrative database, which involved 11,797 patients with 22,296 years of metformin exposure, there were two cases of lactic acidosis (Diabetes Care 1999;22:925-7). This calculates to a rate of 9 cases per 100,000 person years, the same rate as in patients with diabetes who are not taking metformin (9.7 cases per 100,000) (Diabetes Care 1998;21:1659-63).
A recent study looked at the incidence of lactic acidosis in patients taking metformin with and without abnormalities in renal function (Diabetes Care 2014;37:2291-5). There was no statistically significant difference in the rates of lactic acidosis in patients who were on metformin with normal renal function, compared with those with varying degrees of renal insufficiency. The overall rate of lactic acidosis was 10.3 per 100,000 patient years, which is almost identical to the rates in the other studies mentioned, and there were no fatalities.
Several recommendations for using metformin in patients with renal insufficiency have been published (see table) (JAMA 2014;312:2668-75; Diabetes Care 2011;34:1431-7). Metformin has shown cardiovascular mortality benefits, compared with sulfonylureas, in the treatment of diabetes (Diabetes Care 2013;36:1304-11). Avoiding its use in patients with mild to moderate renal insufficiency in favor of other treatments that may not be as beneficial and may well lead to worse outcomes.
There is no evidence that metformin increases the lactic acidosis risk in patients with diabetes, but until there is a change in the FDA labeling, physicians will likely continue to be hesitant to use it in patients with renal insufficiency.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is the Rathmann Family Foundation Chair in Patient-Centered Clinical Education. Contact Dr. Paauw at [email protected].
A 47-year-old obese male with type 2 diabetes has been on metformin for the last 2 years with good effect (hemoglobin A1c of 6.8), and with exercise has been able to lose 5-10 pounds. His last two blood tests show creatinine levels of 1.5 and 1.6. What do you recommend?
A) Continue with metformin.
B) Stop metformin, start sulfonylurea.
C) Stop metformin, begin glargine.
D) Stop metformin, begin pioglitazone.
Myth: Metformin should not be used in patients with mild to moderate renal insufficiency because of an increased risk of lactic acidosis.
Metformin is the most commonly used oral agent for the treatment of type 2 diabetes in the United States, but the FDA-approved drug label states that it is contraindicated in patients with an abnormal creatinine clearance or serum creatinine of 1.4 in women and 1.5 in men.<sup/>The concern is for development of lactic acidosis in patients because the renally excreted metformin may build up as a result of decreased renal function.
Metformin was approved for use in the United States in 1995, many years after the drug was introduced in Europe. The first drug in its class, phenformin, was removed from the United States and most European markets in 1977 because of a high incidence of lactic acidosis occurring at therapeutic doses. One in 4,000 patients taking phenformin develops lactic acidosis (J. Emerg. Med. 1998;16:881-6). Phenformin has been shown to cause type B lactic acidosis, without evidence of hypoxia or hypoperfusion, and lactic acidosis because of phenformin carried a 50% mortality rate.
Deep concern for the possibility of a similar problem with metformin played an important role in its delay of availability in the United States. It isn’t clear, however, that diabetes patients on metformin have a higher risk of developing lactic acidosis than diabetes patients who are not on metformin.
A Cochrane review of 347 studies, including 70,490 person-years of metformin use, compared with 55,451 person-years in the nonmetformin group, showed no cases of fatal or nonfatal lactic acidosis in either group (Cochrane Database Syst. Rev. 2010 Apr 14:CD002967). More than half the studies included (53%) allowed for the inclusion of patients with creatinine levels greater than 1.5. There were no differences in lactate levels between metformin-treated patients and patients who did not receive metformin.
In a study using the Saskatchewan Health administrative database, which involved 11,797 patients with 22,296 years of metformin exposure, there were two cases of lactic acidosis (Diabetes Care 1999;22:925-7). This calculates to a rate of 9 cases per 100,000 person years, the same rate as in patients with diabetes who are not taking metformin (9.7 cases per 100,000) (Diabetes Care 1998;21:1659-63).
A recent study looked at the incidence of lactic acidosis in patients taking metformin with and without abnormalities in renal function (Diabetes Care 2014;37:2291-5). There was no statistically significant difference in the rates of lactic acidosis in patients who were on metformin with normal renal function, compared with those with varying degrees of renal insufficiency. The overall rate of lactic acidosis was 10.3 per 100,000 patient years, which is almost identical to the rates in the other studies mentioned, and there were no fatalities.
Several recommendations for using metformin in patients with renal insufficiency have been published (see table) (JAMA 2014;312:2668-75; Diabetes Care 2011;34:1431-7). Metformin has shown cardiovascular mortality benefits, compared with sulfonylureas, in the treatment of diabetes (Diabetes Care 2013;36:1304-11). Avoiding its use in patients with mild to moderate renal insufficiency in favor of other treatments that may not be as beneficial and may well lead to worse outcomes.
There is no evidence that metformin increases the lactic acidosis risk in patients with diabetes, but until there is a change in the FDA labeling, physicians will likely continue to be hesitant to use it in patients with renal insufficiency.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is the Rathmann Family Foundation Chair in Patient-Centered Clinical Education. Contact Dr. Paauw at [email protected].
Expanded genetic score improves risk prediction for type 2 diabetes
Use of an expanded genetic score in conjunction with the Framingham risk score improved risk prediction for type 2 diabetes, according to findings recently published in the journal Diabetes.
In an analysis of seven cohorts of European patients, a 65-SNP (single nucleotide polymorphism) genetic score alone detected 19.9% of incident cases with a 10% false-positive rate, compared with the Framingham risk model, which detected 30.7% (95% confidence interval, 27.5-33.8) of cases.
When combined, the two measures detected 37.3% (95% CI, 33.9-40.6) of cases, reported Dr. Philippa J. Talmud of the Centre for Cardiovascular Genetics at the Institute of Cardiovascular Science, University College London, and her associates.
The addition of a 65-SNP gene score improved correct classification of patients into higher risk disease categories by 6.2%.
Of the 13,294 patients included in the study, 804 developed type 2 diabetes during the study period. Patients were classified as having type 2 diabetes according to self-report, medical record review, prescription of glucose-lowering drugs, or a recorded fasting glucose of 7 mmol/L or greater, the authors said.
Genotyping was performed using MetaboChip array, which includes nearly 200,000 SNP loci for cardiometabolic disease and their genetic variants.
Area under the curve values for genetic score, Framingham risk model, and combined score were 0.60 (95% CI, 0.58-0.62), 0.75 (95% CI, 0.73-0.77), and 0.76 (95% CI, 0.75-0.78), respectively. The combined risk score net reclassification improvement (NRI) was 8.1% (P = 3.31 × 10–7), the investigators reported (Diabetes 2015;64:1830-40).
The findings suggest that common variants of small effects in combination may help improve risk prediction for type 2 diabetes, Dr. Talmud and her coauthors said.
“For a gene score to be effective, it should improve the reclassification of individuals with [type 2 diabetes] into a more accurate risk category over and above the phenotypic risk score. The 65 SNP–weighted gene score did this,” they said.
“In actual terms, at a 10% [false-positive rate], the combined phenotypic and genetic risk score led to the correct identification of an additional 53 (6.6%) of the 804 cases,” they added.
This slight improvement demonstrates that the addition of a gene score to a phenotyping risk model could be a “potentially clinically important improvement” in disease discrimination and classification, Dr. Talmud and her associates concluded.
The study authors reported several sources of funding, including the British Heart Foundation, the Stroke Association, the National Heart, Lung, and Blood Institute, and others.
Though some at-risk populations may benefit from genetic risk scores in type 2 diabetes, “there are major implementation challenges ahead,” wrote Brendan J. Keating, Ph.D., in an editorial published with the study.
Potential challenges to the integration of genetic risk scores include implementation of clinical genotyping and sequencing; consent between patients, physicians, and the health care system; and the use of clinical decision support models, Dr. Keating noted.
Although type 2 diabetes genetic risk scores “will likely improve incrementally, the clinical utility remains to be determined at national scales, although it is likely that benefits will be reaped by at-risk populations such as lean individuals with [type 2 diabetes] who may not present to primary care with later stages of disease manifestation,” he wrote (Diabetes 2015;64:1495-7).
Dr. Keating is with the division of genetics at the Children’s Hospital of Philadelphia. He did not report any financial disclosures.
Though some at-risk populations may benefit from genetic risk scores in type 2 diabetes, “there are major implementation challenges ahead,” wrote Brendan J. Keating, Ph.D., in an editorial published with the study.
Potential challenges to the integration of genetic risk scores include implementation of clinical genotyping and sequencing; consent between patients, physicians, and the health care system; and the use of clinical decision support models, Dr. Keating noted.
Although type 2 diabetes genetic risk scores “will likely improve incrementally, the clinical utility remains to be determined at national scales, although it is likely that benefits will be reaped by at-risk populations such as lean individuals with [type 2 diabetes] who may not present to primary care with later stages of disease manifestation,” he wrote (Diabetes 2015;64:1495-7).
Dr. Keating is with the division of genetics at the Children’s Hospital of Philadelphia. He did not report any financial disclosures.
Though some at-risk populations may benefit from genetic risk scores in type 2 diabetes, “there are major implementation challenges ahead,” wrote Brendan J. Keating, Ph.D., in an editorial published with the study.
Potential challenges to the integration of genetic risk scores include implementation of clinical genotyping and sequencing; consent between patients, physicians, and the health care system; and the use of clinical decision support models, Dr. Keating noted.
Although type 2 diabetes genetic risk scores “will likely improve incrementally, the clinical utility remains to be determined at national scales, although it is likely that benefits will be reaped by at-risk populations such as lean individuals with [type 2 diabetes] who may not present to primary care with later stages of disease manifestation,” he wrote (Diabetes 2015;64:1495-7).
Dr. Keating is with the division of genetics at the Children’s Hospital of Philadelphia. He did not report any financial disclosures.
Use of an expanded genetic score in conjunction with the Framingham risk score improved risk prediction for type 2 diabetes, according to findings recently published in the journal Diabetes.
In an analysis of seven cohorts of European patients, a 65-SNP (single nucleotide polymorphism) genetic score alone detected 19.9% of incident cases with a 10% false-positive rate, compared with the Framingham risk model, which detected 30.7% (95% confidence interval, 27.5-33.8) of cases.
When combined, the two measures detected 37.3% (95% CI, 33.9-40.6) of cases, reported Dr. Philippa J. Talmud of the Centre for Cardiovascular Genetics at the Institute of Cardiovascular Science, University College London, and her associates.
The addition of a 65-SNP gene score improved correct classification of patients into higher risk disease categories by 6.2%.
Of the 13,294 patients included in the study, 804 developed type 2 diabetes during the study period. Patients were classified as having type 2 diabetes according to self-report, medical record review, prescription of glucose-lowering drugs, or a recorded fasting glucose of 7 mmol/L or greater, the authors said.
Genotyping was performed using MetaboChip array, which includes nearly 200,000 SNP loci for cardiometabolic disease and their genetic variants.
Area under the curve values for genetic score, Framingham risk model, and combined score were 0.60 (95% CI, 0.58-0.62), 0.75 (95% CI, 0.73-0.77), and 0.76 (95% CI, 0.75-0.78), respectively. The combined risk score net reclassification improvement (NRI) was 8.1% (P = 3.31 × 10–7), the investigators reported (Diabetes 2015;64:1830-40).
The findings suggest that common variants of small effects in combination may help improve risk prediction for type 2 diabetes, Dr. Talmud and her coauthors said.
“For a gene score to be effective, it should improve the reclassification of individuals with [type 2 diabetes] into a more accurate risk category over and above the phenotypic risk score. The 65 SNP–weighted gene score did this,” they said.
“In actual terms, at a 10% [false-positive rate], the combined phenotypic and genetic risk score led to the correct identification of an additional 53 (6.6%) of the 804 cases,” they added.
This slight improvement demonstrates that the addition of a gene score to a phenotyping risk model could be a “potentially clinically important improvement” in disease discrimination and classification, Dr. Talmud and her associates concluded.
The study authors reported several sources of funding, including the British Heart Foundation, the Stroke Association, the National Heart, Lung, and Blood Institute, and others.
Use of an expanded genetic score in conjunction with the Framingham risk score improved risk prediction for type 2 diabetes, according to findings recently published in the journal Diabetes.
In an analysis of seven cohorts of European patients, a 65-SNP (single nucleotide polymorphism) genetic score alone detected 19.9% of incident cases with a 10% false-positive rate, compared with the Framingham risk model, which detected 30.7% (95% confidence interval, 27.5-33.8) of cases.
When combined, the two measures detected 37.3% (95% CI, 33.9-40.6) of cases, reported Dr. Philippa J. Talmud of the Centre for Cardiovascular Genetics at the Institute of Cardiovascular Science, University College London, and her associates.
The addition of a 65-SNP gene score improved correct classification of patients into higher risk disease categories by 6.2%.
Of the 13,294 patients included in the study, 804 developed type 2 diabetes during the study period. Patients were classified as having type 2 diabetes according to self-report, medical record review, prescription of glucose-lowering drugs, or a recorded fasting glucose of 7 mmol/L or greater, the authors said.
Genotyping was performed using MetaboChip array, which includes nearly 200,000 SNP loci for cardiometabolic disease and their genetic variants.
Area under the curve values for genetic score, Framingham risk model, and combined score were 0.60 (95% CI, 0.58-0.62), 0.75 (95% CI, 0.73-0.77), and 0.76 (95% CI, 0.75-0.78), respectively. The combined risk score net reclassification improvement (NRI) was 8.1% (P = 3.31 × 10–7), the investigators reported (Diabetes 2015;64:1830-40).
The findings suggest that common variants of small effects in combination may help improve risk prediction for type 2 diabetes, Dr. Talmud and her coauthors said.
“For a gene score to be effective, it should improve the reclassification of individuals with [type 2 diabetes] into a more accurate risk category over and above the phenotypic risk score. The 65 SNP–weighted gene score did this,” they said.
“In actual terms, at a 10% [false-positive rate], the combined phenotypic and genetic risk score led to the correct identification of an additional 53 (6.6%) of the 804 cases,” they added.
This slight improvement demonstrates that the addition of a gene score to a phenotyping risk model could be a “potentially clinically important improvement” in disease discrimination and classification, Dr. Talmud and her associates concluded.
The study authors reported several sources of funding, including the British Heart Foundation, the Stroke Association, the National Heart, Lung, and Blood Institute, and others.
FROM DIABETES
Key clinical point: When combined with the Framingham risk score, use of an expanded gene score improved risk prediction for type 2 diabetes.
Major finding: With a 10% false-positive rate, a 65-SNP genetic score alone detected 19.9% of incident cases, compared with the Framingham risk model, which detected 30.7% (95% CI; 27.5-33.8) of cases; when combined, the two measures detected 37.3% (95% CI; 33.9-40.6) of cases.
Data source: An analysis of 13,294 European patients, 804 of whom developed type 2 diabetes over the course of study.
Disclosures: The study authors reported several sources of funding, including the British Heart Foundation, the Stroke Association, the National Heart, Lung, and Blood Institute, and others.
New ACOG president puts focus on smoking, obesity
SAN FRANCISCO – The new president of the American College of Obstetricians and Gynecologists wants ob.gyns. to step up their efforts to help patients with their overall wellness, including smoking cessation, help with weight control, and cardiac screening.
For many women, the ob.gyn. is their primary physician and if that physician isn’t counseling and screening for these health issues, it won’t be done at all, Dr. Mark S. DeFrancesco, the incoming president of ACOG, said during a press briefing at the organization’s annual meeting.
Dr. DeFrancesco, an ob.gyn. in Waterbury, Conn., and assistant clinical professor at the University of Connecticut, was installed as the new ACOG president on May 6. He also will serve as president of the American Congress of Obstetricians and Gynecologists.
He said that he plans to focus on population health, specifically encouraging ob.gyns. to do more to address significant preventable causes of death such as smoking and obesity, during his year-long term in office.
The idea isn’t that ob.gyns. should be treating hypertension in their offices but that they should be looking for it during the well woman visit and making appropriate referrals.
“These things should not happen on our watch,” Dr. DeFrancesco said.
He said that he plans to work with the ACOG staff to develop a toolkit for ob.gyns. that will make it easier to address these population health issues. He also plans to work with insurers to convince them to pay physicians for the time spent on this counseling.
Dr. John C. Jennings, the outgoing president of ACOG, said that Dr. DeFrancesco’s focus on population health will complement the work that ACOG has been doing over the past year in encouraging a team approach to women’s health care. For instance, if a patient needs help losing weight, physicians could partner with a dietitian. Physicians shouldn’t be expected to conquer complex health issues such as smoking and obesity on their own, he said.
“You can’t tackle those [issues] with just one physician,” Dr. Jennings said.
On Twitter @maryellenny
SAN FRANCISCO – The new president of the American College of Obstetricians and Gynecologists wants ob.gyns. to step up their efforts to help patients with their overall wellness, including smoking cessation, help with weight control, and cardiac screening.
For many women, the ob.gyn. is their primary physician and if that physician isn’t counseling and screening for these health issues, it won’t be done at all, Dr. Mark S. DeFrancesco, the incoming president of ACOG, said during a press briefing at the organization’s annual meeting.
Dr. DeFrancesco, an ob.gyn. in Waterbury, Conn., and assistant clinical professor at the University of Connecticut, was installed as the new ACOG president on May 6. He also will serve as president of the American Congress of Obstetricians and Gynecologists.
He said that he plans to focus on population health, specifically encouraging ob.gyns. to do more to address significant preventable causes of death such as smoking and obesity, during his year-long term in office.
The idea isn’t that ob.gyns. should be treating hypertension in their offices but that they should be looking for it during the well woman visit and making appropriate referrals.
“These things should not happen on our watch,” Dr. DeFrancesco said.
He said that he plans to work with the ACOG staff to develop a toolkit for ob.gyns. that will make it easier to address these population health issues. He also plans to work with insurers to convince them to pay physicians for the time spent on this counseling.
Dr. John C. Jennings, the outgoing president of ACOG, said that Dr. DeFrancesco’s focus on population health will complement the work that ACOG has been doing over the past year in encouraging a team approach to women’s health care. For instance, if a patient needs help losing weight, physicians could partner with a dietitian. Physicians shouldn’t be expected to conquer complex health issues such as smoking and obesity on their own, he said.
“You can’t tackle those [issues] with just one physician,” Dr. Jennings said.
On Twitter @maryellenny
SAN FRANCISCO – The new president of the American College of Obstetricians and Gynecologists wants ob.gyns. to step up their efforts to help patients with their overall wellness, including smoking cessation, help with weight control, and cardiac screening.
For many women, the ob.gyn. is their primary physician and if that physician isn’t counseling and screening for these health issues, it won’t be done at all, Dr. Mark S. DeFrancesco, the incoming president of ACOG, said during a press briefing at the organization’s annual meeting.
Dr. DeFrancesco, an ob.gyn. in Waterbury, Conn., and assistant clinical professor at the University of Connecticut, was installed as the new ACOG president on May 6. He also will serve as president of the American Congress of Obstetricians and Gynecologists.
He said that he plans to focus on population health, specifically encouraging ob.gyns. to do more to address significant preventable causes of death such as smoking and obesity, during his year-long term in office.
The idea isn’t that ob.gyns. should be treating hypertension in their offices but that they should be looking for it during the well woman visit and making appropriate referrals.
“These things should not happen on our watch,” Dr. DeFrancesco said.
He said that he plans to work with the ACOG staff to develop a toolkit for ob.gyns. that will make it easier to address these population health issues. He also plans to work with insurers to convince them to pay physicians for the time spent on this counseling.
Dr. John C. Jennings, the outgoing president of ACOG, said that Dr. DeFrancesco’s focus on population health will complement the work that ACOG has been doing over the past year in encouraging a team approach to women’s health care. For instance, if a patient needs help losing weight, physicians could partner with a dietitian. Physicians shouldn’t be expected to conquer complex health issues such as smoking and obesity on their own, he said.
“You can’t tackle those [issues] with just one physician,” Dr. Jennings said.
On Twitter @maryellenny
AT THE ACOG ANNUAL CLINICAL MEETING
Overweight, but not obese, diabetes patients had lower mortality risks
For type 2 diabetes patients, being overweight or obese was associated with a higher risk for nonfatal cardiovascular events, but overweight was associated with lower mortality, according to research published in Annals of Internal Medicine.
Dr. Pierluigi Costanzo of the University of Hull in Kingston upon Hull, England, and his associates reported results of a prospective cohort study of the “diabetes paradox” in 10,568 type 2 patients without cardiovascular disease who were followed for a median of 10.6 years (median baseline body mass index was 29.0 kg/m2).
Overweight (BMI, 25-29.9 kg/m2) was associated with a lower mortality risk, whereas obesity (BMI >30 kg/m2) had a mortality risk similar to that of normal-weight persons. The precise mechanisms behind the survival advantage in overweight but not obese patients is still unknown. The investigators noted that they did not have patient information on fitness levels, medication use, or cause of death.
Overweight or obese patients (BMI >25 kg/m2) also had a higher rate of cardiac events than did patients of normal weight (BMI, 18.5-24.9 kg/m2).
“The obesity paradox is open to several interpretations that should be addressed by further research rather than promoting preconceptions about the ideal BMI. These results should not discourage patients from adopting a healthy lifestyle,” the investigators wrote.
Read the full article here: Ann Intern Med. 2015;162:610-618 (doi:10.7326/M14-1551)
For type 2 diabetes patients, being overweight or obese was associated with a higher risk for nonfatal cardiovascular events, but overweight was associated with lower mortality, according to research published in Annals of Internal Medicine.
Dr. Pierluigi Costanzo of the University of Hull in Kingston upon Hull, England, and his associates reported results of a prospective cohort study of the “diabetes paradox” in 10,568 type 2 patients without cardiovascular disease who were followed for a median of 10.6 years (median baseline body mass index was 29.0 kg/m2).
Overweight (BMI, 25-29.9 kg/m2) was associated with a lower mortality risk, whereas obesity (BMI >30 kg/m2) had a mortality risk similar to that of normal-weight persons. The precise mechanisms behind the survival advantage in overweight but not obese patients is still unknown. The investigators noted that they did not have patient information on fitness levels, medication use, or cause of death.
Overweight or obese patients (BMI >25 kg/m2) also had a higher rate of cardiac events than did patients of normal weight (BMI, 18.5-24.9 kg/m2).
“The obesity paradox is open to several interpretations that should be addressed by further research rather than promoting preconceptions about the ideal BMI. These results should not discourage patients from adopting a healthy lifestyle,” the investigators wrote.
Read the full article here: Ann Intern Med. 2015;162:610-618 (doi:10.7326/M14-1551)
For type 2 diabetes patients, being overweight or obese was associated with a higher risk for nonfatal cardiovascular events, but overweight was associated with lower mortality, according to research published in Annals of Internal Medicine.
Dr. Pierluigi Costanzo of the University of Hull in Kingston upon Hull, England, and his associates reported results of a prospective cohort study of the “diabetes paradox” in 10,568 type 2 patients without cardiovascular disease who were followed for a median of 10.6 years (median baseline body mass index was 29.0 kg/m2).
Overweight (BMI, 25-29.9 kg/m2) was associated with a lower mortality risk, whereas obesity (BMI >30 kg/m2) had a mortality risk similar to that of normal-weight persons. The precise mechanisms behind the survival advantage in overweight but not obese patients is still unknown. The investigators noted that they did not have patient information on fitness levels, medication use, or cause of death.
Overweight or obese patients (BMI >25 kg/m2) also had a higher rate of cardiac events than did patients of normal weight (BMI, 18.5-24.9 kg/m2).
“The obesity paradox is open to several interpretations that should be addressed by further research rather than promoting preconceptions about the ideal BMI. These results should not discourage patients from adopting a healthy lifestyle,” the investigators wrote.
Read the full article here: Ann Intern Med. 2015;162:610-618 (doi:10.7326/M14-1551)
PAS: Early antibiotics linked to later overweight
SAN DIEGO – The use of oral antibiotics before 4 years of age was associated with a significantly increased risk of later overweight in a large cohort study.
“Antibiotics may provide a physician-modifiable risk factor for obesity prevention in early childhood,” Dr. Elizabeth Dawson-Hahn said at the annual meeting of the Pediatric Academic Societies.
The study included 4,938 children born at Group Health Cooperative, the Seattle-based integrated health care system. Their electronic medical records indicated that 3,533 of them, or 72%, filled one or more prescriptions for oral antibiotics at ages 0-47 months. In this group, 53% were less than 12 months old at the time of their first oral antibiotic exposure, while the rest were 12-47 months old.
At ages 48-59 months, roughly 12% of all study participants were overweight, as defined by a body mass index at or above the 85th percentile. The prevalence was similar regardless of whether a child was exposed to early oral antibiotics. However, the groups with and without early antibiotic exposure differed in key ways, including a more than threefold higher prevalence of childhood asthma in those with early antibiotic exposure.
In a prespecified logistic regression analysis adjusted for sex, Medicaid status, childhood asthma, race, maternal antibiotic exposure during pregnancy, delivery type, and birth weight, each course of oral antibiotics a child received up to 47 months of age was associated with a 3% increased likelihood of being overweight at 48-59 months – and children who got early oral antibiotics received a mean of 3.7 courses by age 47 months. Thus, early antibiotic exposure was associated on average with a significant 11% increased risk of later overweight (P = .005), reported Dr. Dawson-Hahn, a general pediatrics fellow at the University of Washington, Seattle.
The increased risk of overweight related to early antibiotics was concentrated in the children who received the medications in infancy. In another logistic regression analysis, children who got oral antibiotics prior to age 12 months were at an adjusted 20% increased risk of being overweight at ages 48-59 months, compared with children who didn’t receive oral antibiotics before 48 months of age. In contrast, the risk of overweight in kids who received oral antibiotics at ages 12-47 months wasn’t significantly different from the risk in the unexposed group.
Future studies should explore the mechanism of the observed relationship between early antibiotic exposure and later overweight, Dr. Dawson-Hahn said. One leading hypothesis is that the early exposure alters the composition of the developing gut microbiome. Antibiotics have been used for decades as a means of boosting the weight of livestock, she noted.
Dr. Dawson-Hahn said she is planning to reanalyze the data set to see if weight gain differed depending upon the type of antibiotic involved in an early exposure. More than 80% of children with early antibiotic exposure received amoxicillin.
One audience member noted that just because the health plan’s electronic pharmacy records show a prescription for oral antibiotics was filled doesn’t mean the child took the full course. Dr. Dawson-Hahn agreed, but noted that her study findings are consistent with the results of prior studies by other investigators in the past 2 years, which typically relied upon parental recall of antibiotic exposure.
“We thought documented prescription fill would be an improvement over that,” she added.
Dr. Dawson-Hahn’s study was funded by a Hearst Foundation Fellowship Award and a Ruth L. Kirschstein National Research Service Award. She reported having no relevant financial conflicts.
SAN DIEGO – The use of oral antibiotics before 4 years of age was associated with a significantly increased risk of later overweight in a large cohort study.
“Antibiotics may provide a physician-modifiable risk factor for obesity prevention in early childhood,” Dr. Elizabeth Dawson-Hahn said at the annual meeting of the Pediatric Academic Societies.
The study included 4,938 children born at Group Health Cooperative, the Seattle-based integrated health care system. Their electronic medical records indicated that 3,533 of them, or 72%, filled one or more prescriptions for oral antibiotics at ages 0-47 months. In this group, 53% were less than 12 months old at the time of their first oral antibiotic exposure, while the rest were 12-47 months old.
At ages 48-59 months, roughly 12% of all study participants were overweight, as defined by a body mass index at or above the 85th percentile. The prevalence was similar regardless of whether a child was exposed to early oral antibiotics. However, the groups with and without early antibiotic exposure differed in key ways, including a more than threefold higher prevalence of childhood asthma in those with early antibiotic exposure.
In a prespecified logistic regression analysis adjusted for sex, Medicaid status, childhood asthma, race, maternal antibiotic exposure during pregnancy, delivery type, and birth weight, each course of oral antibiotics a child received up to 47 months of age was associated with a 3% increased likelihood of being overweight at 48-59 months – and children who got early oral antibiotics received a mean of 3.7 courses by age 47 months. Thus, early antibiotic exposure was associated on average with a significant 11% increased risk of later overweight (P = .005), reported Dr. Dawson-Hahn, a general pediatrics fellow at the University of Washington, Seattle.
The increased risk of overweight related to early antibiotics was concentrated in the children who received the medications in infancy. In another logistic regression analysis, children who got oral antibiotics prior to age 12 months were at an adjusted 20% increased risk of being overweight at ages 48-59 months, compared with children who didn’t receive oral antibiotics before 48 months of age. In contrast, the risk of overweight in kids who received oral antibiotics at ages 12-47 months wasn’t significantly different from the risk in the unexposed group.
Future studies should explore the mechanism of the observed relationship between early antibiotic exposure and later overweight, Dr. Dawson-Hahn said. One leading hypothesis is that the early exposure alters the composition of the developing gut microbiome. Antibiotics have been used for decades as a means of boosting the weight of livestock, she noted.
Dr. Dawson-Hahn said she is planning to reanalyze the data set to see if weight gain differed depending upon the type of antibiotic involved in an early exposure. More than 80% of children with early antibiotic exposure received amoxicillin.
One audience member noted that just because the health plan’s electronic pharmacy records show a prescription for oral antibiotics was filled doesn’t mean the child took the full course. Dr. Dawson-Hahn agreed, but noted that her study findings are consistent with the results of prior studies by other investigators in the past 2 years, which typically relied upon parental recall of antibiotic exposure.
“We thought documented prescription fill would be an improvement over that,” she added.
Dr. Dawson-Hahn’s study was funded by a Hearst Foundation Fellowship Award and a Ruth L. Kirschstein National Research Service Award. She reported having no relevant financial conflicts.
SAN DIEGO – The use of oral antibiotics before 4 years of age was associated with a significantly increased risk of later overweight in a large cohort study.
“Antibiotics may provide a physician-modifiable risk factor for obesity prevention in early childhood,” Dr. Elizabeth Dawson-Hahn said at the annual meeting of the Pediatric Academic Societies.
The study included 4,938 children born at Group Health Cooperative, the Seattle-based integrated health care system. Their electronic medical records indicated that 3,533 of them, or 72%, filled one or more prescriptions for oral antibiotics at ages 0-47 months. In this group, 53% were less than 12 months old at the time of their first oral antibiotic exposure, while the rest were 12-47 months old.
At ages 48-59 months, roughly 12% of all study participants were overweight, as defined by a body mass index at or above the 85th percentile. The prevalence was similar regardless of whether a child was exposed to early oral antibiotics. However, the groups with and without early antibiotic exposure differed in key ways, including a more than threefold higher prevalence of childhood asthma in those with early antibiotic exposure.
In a prespecified logistic regression analysis adjusted for sex, Medicaid status, childhood asthma, race, maternal antibiotic exposure during pregnancy, delivery type, and birth weight, each course of oral antibiotics a child received up to 47 months of age was associated with a 3% increased likelihood of being overweight at 48-59 months – and children who got early oral antibiotics received a mean of 3.7 courses by age 47 months. Thus, early antibiotic exposure was associated on average with a significant 11% increased risk of later overweight (P = .005), reported Dr. Dawson-Hahn, a general pediatrics fellow at the University of Washington, Seattle.
The increased risk of overweight related to early antibiotics was concentrated in the children who received the medications in infancy. In another logistic regression analysis, children who got oral antibiotics prior to age 12 months were at an adjusted 20% increased risk of being overweight at ages 48-59 months, compared with children who didn’t receive oral antibiotics before 48 months of age. In contrast, the risk of overweight in kids who received oral antibiotics at ages 12-47 months wasn’t significantly different from the risk in the unexposed group.
Future studies should explore the mechanism of the observed relationship between early antibiotic exposure and later overweight, Dr. Dawson-Hahn said. One leading hypothesis is that the early exposure alters the composition of the developing gut microbiome. Antibiotics have been used for decades as a means of boosting the weight of livestock, she noted.
Dr. Dawson-Hahn said she is planning to reanalyze the data set to see if weight gain differed depending upon the type of antibiotic involved in an early exposure. More than 80% of children with early antibiotic exposure received amoxicillin.
One audience member noted that just because the health plan’s electronic pharmacy records show a prescription for oral antibiotics was filled doesn’t mean the child took the full course. Dr. Dawson-Hahn agreed, but noted that her study findings are consistent with the results of prior studies by other investigators in the past 2 years, which typically relied upon parental recall of antibiotic exposure.
“We thought documented prescription fill would be an improvement over that,” she added.
Dr. Dawson-Hahn’s study was funded by a Hearst Foundation Fellowship Award and a Ruth L. Kirschstein National Research Service Award. She reported having no relevant financial conflicts.
AT THE PAS ANNUAL MEETING
Key clinical point: Children who received oral antibiotics prior to 48 months of age were more likely to be overweight at ages 48-59 months.
Major finding: For each course of oral antibiotics a child received during 0-47 months of age, the odds of being at or above the 85th percentile for body mass index at 48-59 months increased by 3%.
Data source: A retrospective cohort study including 4,938 children whose families belonged to a large integrated health plan.
Disclosures: Dr. Dawson-Hahn’s study was funded by a Hearst Foundation Fellowship Award and a Ruth L. Kirschstein National Research Service Award. She reported having no relevant financial conflicts.